User login
Dying in the Hospital: A Necessary Choice?
More than a third of all patients with cancer die in hospitals, a figure that has increased slightly in recent years, while deaths at home have decreased. These findings come from a recent study published in Cancer Epidemiology, which analyzed data on the different places in Italy where end of life occurs.
“Place of death is relevant both for individuals and for the society. Home is universally considered the optimal place of death, while dying in a hospital may be a signal of inappropriate end-of-life care,” wrote the authors, led by Gianmauro Numico, MD, head of the Oncology Department at the Santa Croce e Carle General Hospital in Cuneo, Italy.
“Despite the general trend toward strengthening community-based networks and the increasing number of hospice and long-term care facilities, we oncologists are facing an opposite trend, with many patients spending their last days in the hospital,” Numico explained to Univadis Italy. This observation led to the questions that prompted the study: Is this only a perception among doctors, or is it a real phenomenon? If the latter, why is it happening?
What’s Preferable
For their analysis, Numico and colleagues relied on death certificates published by the Italian National Institute of Statistics from 2015 to 2019, excluding data from the pandemic years to avoid potential biases.
The analysis of data pertaining to cancer deaths revealed that approximately 35% of Italian patients with cancer die in hospitals, with a slight increase over the study period. Of the patients who die elsewhere, 40% die at home and 20% die in hospice or other long-term care facilities. Home deaths have decreased by 3.09%, while those in hospices and long-term care facilities have increased by 2.71%, and hospital deaths have risen by 0.3%.
The study also highlighted notable geographical differences: Hospital deaths are more frequent in the north, while in the south, home deaths remain predominant, although hospital admissions are on the rise. “These differences reflect not only access to facilities but also cultural and social variables,” explained Numico. “Some end-of-life issues with cancer patients are more straightforward, while others are difficult to manage outside the hospital,” he said, recalling that many family members and caregivers are afraid they won’t be able to care for their loved ones properly without the support of an appropriate facility and skilled personnel.
Social factors also contribute to the increased use of hospitals for end-of-life care: Without a social and family network, it is often impossible to manage the final stages of life at home. “We cannot guarantee that dying at home is better for everyone; in some cases, the home cannot provide the necessary care and emotional support,” Numico added.
Attitudes Need Change
Looking beyond Italy, it is clear that this trend exists in other countries as well. For example, in the Netherlands — where community-based care is highly developed and includes practices such as euthanasia — hospital death rates are higher than those in Italy. In the United States, the trend is different, but this is largely due to the structure of the US healthcare system, where patients bear much of the financial burden of hospital admissions.
“The basic requests of patients and families are clear: They want a safe place that is adequately staffed and where the patient won’t suffer,” said Numico, questioning whether the home is truly the best place to die. “In reality, this is not always the case, and it’s important to focus on the quality of care in the final days rather than just the place of care,” he added.
Ruling out hospitals a priori as a place to die is not a winning strategy, according to the expert. Instead of trying to reverse the trend, he suggests integrating the hospital into a care network that prioritizes the patient’s well-being, regardless of the setting. “Our goal should not be to eliminate hospital deaths — a common request from hospital administrations — but rather to ensure that end-of-life care in hospitals is a dignified experience that respects the needs of the dying and their loved ones,” Numico said. “We must ensure that, wherever the end-of-life process occurs, it should happen in the best way possible, and the hospital must be a part of this overall framework,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
More than a third of all patients with cancer die in hospitals, a figure that has increased slightly in recent years, while deaths at home have decreased. These findings come from a recent study published in Cancer Epidemiology, which analyzed data on the different places in Italy where end of life occurs.
“Place of death is relevant both for individuals and for the society. Home is universally considered the optimal place of death, while dying in a hospital may be a signal of inappropriate end-of-life care,” wrote the authors, led by Gianmauro Numico, MD, head of the Oncology Department at the Santa Croce e Carle General Hospital in Cuneo, Italy.
“Despite the general trend toward strengthening community-based networks and the increasing number of hospice and long-term care facilities, we oncologists are facing an opposite trend, with many patients spending their last days in the hospital,” Numico explained to Univadis Italy. This observation led to the questions that prompted the study: Is this only a perception among doctors, or is it a real phenomenon? If the latter, why is it happening?
What’s Preferable
For their analysis, Numico and colleagues relied on death certificates published by the Italian National Institute of Statistics from 2015 to 2019, excluding data from the pandemic years to avoid potential biases.
The analysis of data pertaining to cancer deaths revealed that approximately 35% of Italian patients with cancer die in hospitals, with a slight increase over the study period. Of the patients who die elsewhere, 40% die at home and 20% die in hospice or other long-term care facilities. Home deaths have decreased by 3.09%, while those in hospices and long-term care facilities have increased by 2.71%, and hospital deaths have risen by 0.3%.
The study also highlighted notable geographical differences: Hospital deaths are more frequent in the north, while in the south, home deaths remain predominant, although hospital admissions are on the rise. “These differences reflect not only access to facilities but also cultural and social variables,” explained Numico. “Some end-of-life issues with cancer patients are more straightforward, while others are difficult to manage outside the hospital,” he said, recalling that many family members and caregivers are afraid they won’t be able to care for their loved ones properly without the support of an appropriate facility and skilled personnel.
Social factors also contribute to the increased use of hospitals for end-of-life care: Without a social and family network, it is often impossible to manage the final stages of life at home. “We cannot guarantee that dying at home is better for everyone; in some cases, the home cannot provide the necessary care and emotional support,” Numico added.
Attitudes Need Change
Looking beyond Italy, it is clear that this trend exists in other countries as well. For example, in the Netherlands — where community-based care is highly developed and includes practices such as euthanasia — hospital death rates are higher than those in Italy. In the United States, the trend is different, but this is largely due to the structure of the US healthcare system, where patients bear much of the financial burden of hospital admissions.
“The basic requests of patients and families are clear: They want a safe place that is adequately staffed and where the patient won’t suffer,” said Numico, questioning whether the home is truly the best place to die. “In reality, this is not always the case, and it’s important to focus on the quality of care in the final days rather than just the place of care,” he added.
Ruling out hospitals a priori as a place to die is not a winning strategy, according to the expert. Instead of trying to reverse the trend, he suggests integrating the hospital into a care network that prioritizes the patient’s well-being, regardless of the setting. “Our goal should not be to eliminate hospital deaths — a common request from hospital administrations — but rather to ensure that end-of-life care in hospitals is a dignified experience that respects the needs of the dying and their loved ones,” Numico said. “We must ensure that, wherever the end-of-life process occurs, it should happen in the best way possible, and the hospital must be a part of this overall framework,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
More than a third of all patients with cancer die in hospitals, a figure that has increased slightly in recent years, while deaths at home have decreased. These findings come from a recent study published in Cancer Epidemiology, which analyzed data on the different places in Italy where end of life occurs.
“Place of death is relevant both for individuals and for the society. Home is universally considered the optimal place of death, while dying in a hospital may be a signal of inappropriate end-of-life care,” wrote the authors, led by Gianmauro Numico, MD, head of the Oncology Department at the Santa Croce e Carle General Hospital in Cuneo, Italy.
“Despite the general trend toward strengthening community-based networks and the increasing number of hospice and long-term care facilities, we oncologists are facing an opposite trend, with many patients spending their last days in the hospital,” Numico explained to Univadis Italy. This observation led to the questions that prompted the study: Is this only a perception among doctors, or is it a real phenomenon? If the latter, why is it happening?
What’s Preferable
For their analysis, Numico and colleagues relied on death certificates published by the Italian National Institute of Statistics from 2015 to 2019, excluding data from the pandemic years to avoid potential biases.
The analysis of data pertaining to cancer deaths revealed that approximately 35% of Italian patients with cancer die in hospitals, with a slight increase over the study period. Of the patients who die elsewhere, 40% die at home and 20% die in hospice or other long-term care facilities. Home deaths have decreased by 3.09%, while those in hospices and long-term care facilities have increased by 2.71%, and hospital deaths have risen by 0.3%.
The study also highlighted notable geographical differences: Hospital deaths are more frequent in the north, while in the south, home deaths remain predominant, although hospital admissions are on the rise. “These differences reflect not only access to facilities but also cultural and social variables,” explained Numico. “Some end-of-life issues with cancer patients are more straightforward, while others are difficult to manage outside the hospital,” he said, recalling that many family members and caregivers are afraid they won’t be able to care for their loved ones properly without the support of an appropriate facility and skilled personnel.
Social factors also contribute to the increased use of hospitals for end-of-life care: Without a social and family network, it is often impossible to manage the final stages of life at home. “We cannot guarantee that dying at home is better for everyone; in some cases, the home cannot provide the necessary care and emotional support,” Numico added.
Attitudes Need Change
Looking beyond Italy, it is clear that this trend exists in other countries as well. For example, in the Netherlands — where community-based care is highly developed and includes practices such as euthanasia — hospital death rates are higher than those in Italy. In the United States, the trend is different, but this is largely due to the structure of the US healthcare system, where patients bear much of the financial burden of hospital admissions.
“The basic requests of patients and families are clear: They want a safe place that is adequately staffed and where the patient won’t suffer,” said Numico, questioning whether the home is truly the best place to die. “In reality, this is not always the case, and it’s important to focus on the quality of care in the final days rather than just the place of care,” he added.
Ruling out hospitals a priori as a place to die is not a winning strategy, according to the expert. Instead of trying to reverse the trend, he suggests integrating the hospital into a care network that prioritizes the patient’s well-being, regardless of the setting. “Our goal should not be to eliminate hospital deaths — a common request from hospital administrations — but rather to ensure that end-of-life care in hospitals is a dignified experience that respects the needs of the dying and their loved ones,” Numico said. “We must ensure that, wherever the end-of-life process occurs, it should happen in the best way possible, and the hospital must be a part of this overall framework,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
Chronic pain is one of the most prevalent public health concerns in the United States, affecting > 51 million adults with about $500 billion in health care costs.1 Military veterans are among the most vulnerable subpopulations, with 65% of veterans reporting chronic pain in the last 3 months.2 Chronic pain is complex, affecting the biopsychosocial-spiritual levels of human health, and requires multimodal and comprehensive treatment approaches.3 Hence, chronic pain treatment can be best delivered via interdisciplinary teams (IDTs) that use a patient-centered approach.4,5
The Veterans Healthcare Administration (VHA) is a leader in developing and delivering interdisciplinary pain care.6,7 VHA Directive 2009-053 requires every US Department of Veterans Affairs (VA) medical center to offer an IDT for chronic pain. However, VHA and non-VHA IDT programs vary significantly.8-11 A recent systematic review found a median of 5 disciplines included on IDTs (range, 2-8), and program content often included exercise and education; only 11% of included IDTs met simultaneously with patients.11 The heterogeneity of IDT programs has made determining best practices challenging.8,11 The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has denoted several core measures and measurement domains that were critical for determining the success of pain management interventions, including patient satisfaction.12,13 Nevertheless, the association of IDTs with high patient satisfaction and improvement in pain measures has been documented.5,11
The VHA has worked to implement the Whole Health System into health care, which considers well-being across physical, behavioral, spiritual, and socioeconomic domains. As such, the Whole Health System involves an interpersonal, team-based approach, “anchored in trusting longitudinal relationships to promote resilience, prevent disease, and restore health.”14 It aligns with the patient’s mission, aspiration, and purpose. Surgeon General VADM Vivek H. Murthy, MD, MBA, recently endorsed this approach.15,16 Other health care systems adopting whole health tend to have higher patient satisfaction, increased access to care, and improved patient-reported outcomes.15 Within the VHA, the Whole Health System has shifted the conversation between clinicians and patients from “What is the matter with you?” to “What is important to you?” while emphasizing a proactive and personalized approach to health care.17 Rather than emphasizing passive modalities such as medications and clinician-led services (eg, interventional pain service), the Whole Health System highlights self-care.3,17 Initial research findings within the VHA have been promising.18-21 Whole health peer coaching calls appear to be an effective approach for veterans diagnosed with PTSD, and the use of whole health services is associated with a decrease in opioid use.19,22 However, there are negligible data on patient experiences after meeting with a whole health-focused pain IDT, and studies to date have focused on urban populations.23 One approach to IDT that has shown promise for other health issues involves a patient meeting simultaneously with all members of the IDT.24-27 With the integration of the Whole Health System and the VHA priorities to provide veterans with the “soonest and best care,” more data are needed on the experiences of patients and support persons with various approaches to IDT pain care.28 This study aimed to evaluate patient and support person experiences with a whole health-focused pain IDT that met simultaneously with the patient and support person during an initial evaluation. This study was approved by the institutional review board at the Salem VA Health Care System (SVAHCS) in Virginia.
Methods
The PREVAIL IDT Track is a clinical program offered at SVAHCS with a whole health-focused approach that involves patients and their support persons meeting simultaneously with a pain IDT. PREVAIL IDT Track is designed to help veterans more effectively self-manage chronic pain (Table 1).6,29 Health care practitioners (HCPs) at SVAHCS recommended that veterans with pain persisting for > 3 months participate in PREVAIL IDT Track. After meeting with an advanced practice clinician for an intake, veterans elected to participate in the PREVAIL IDT Track program and completed the initial 6 weeks of pain education. Veterans were then invited to be evaluated by the pain IDT. A team including HCPs from interventional pain, psychology, pharmacy, nutrition, and physical therapy services met with the veteran for 60 minutes. Veterans were also invited to bring a support person to the IDT initial evaluation.
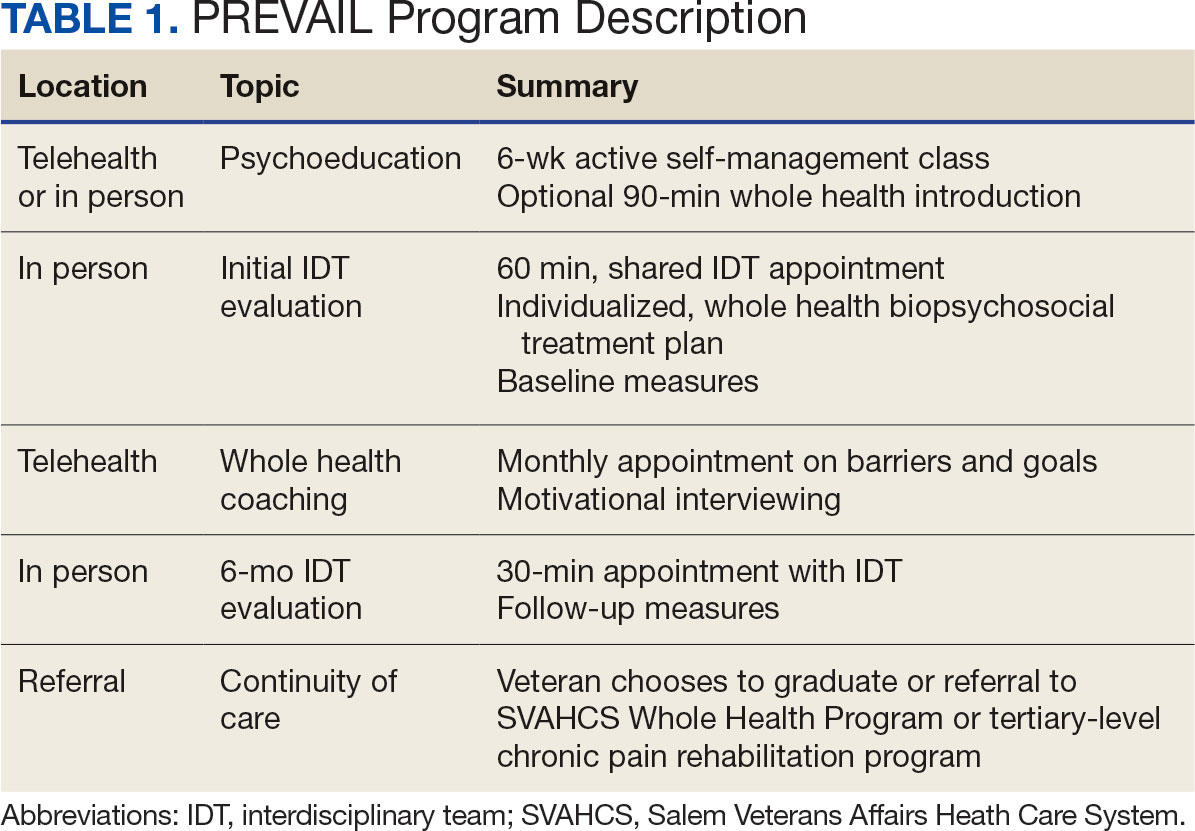
During the IDT initial evaluation, HCPs inquired about the patient’s mission, aspiration, and purpose (“If you were in less pain, what would you be doing more of?”) and about whole health self-care and wellness factors that may contribute to their chronic pain using the Personal Health Inventory.30,31 Veterans were then invited to select 3 whole health self-care areas to focus on during the 6-month program.3 The IDT HCPs worked with the veteran to establish the treatment plan for the first month in the areas of self-care selected by the patient and made recommendations for additional treatments. If the veteran brought a support person to the IDT initial evaluation, their feedback was elicited throughout and at the end to ensure the final treatment plan and first month’s goals were realistic. At the end of the appointment, the veteran and their support person were asked to complete a program-specific satisfaction survey. The HCPs on the team and the veteran executed the treatment plan developed during the appointment, except for medication prescribing. Recommendations for medication changes are included in clinical notes. Veterans then received 5 monthly coaching calls from a nurse navigator with training in whole health and a 6-month follow-up appointment with the IDT HCPs to discuss a plan for continuity of care.
Participant demographic information was not collected, and participants were not compensated for completing the survey. Veterans in PREVAIL IDT Track are predominantly residents of central Appalachia, White, male, unemployed, have ≥ 1 mental and physical health comorbidity, and have a history of mental health treatment.32 Veterans participating in PREVAIL IDT had a mean age of 57 years, and about 1 in 3 have opioid prescriptions.32
A program-specific 17-question satisfaction survey was developed, which included questions related to satisfaction with previous SVAHCS pain care and staff interactions. To assess the overall impression of the IDT initial evaluation, 3 yes/no questions and a 0 to 10-point scale were used. The 5 remaining open-ended questions allowed participants to give feedback about the IDT initial evaluation.
Data Analysis
A convergent mixed-methods approach was used to evaluate participant satisfaction with the initial IDT evaluation. The study team collected and analyzed quantitative and qualitative survey data and triangulated the findings.33 For quantitative responses, frequencies and means were calculated using Python. For qualitative responses, thematic data analysis was conducted by systematic coding, using inductive methods and allowing themes to emerge. Study team members performed a line-by-line analysis of responses using NVivo to identify important codes and reach a consensus. This study adhered to the Consolidated Criteria for Reporting Qualitative Research and followed the National Institute for Health Care Excellence checklist.34,35
Results
Quantitative Responses
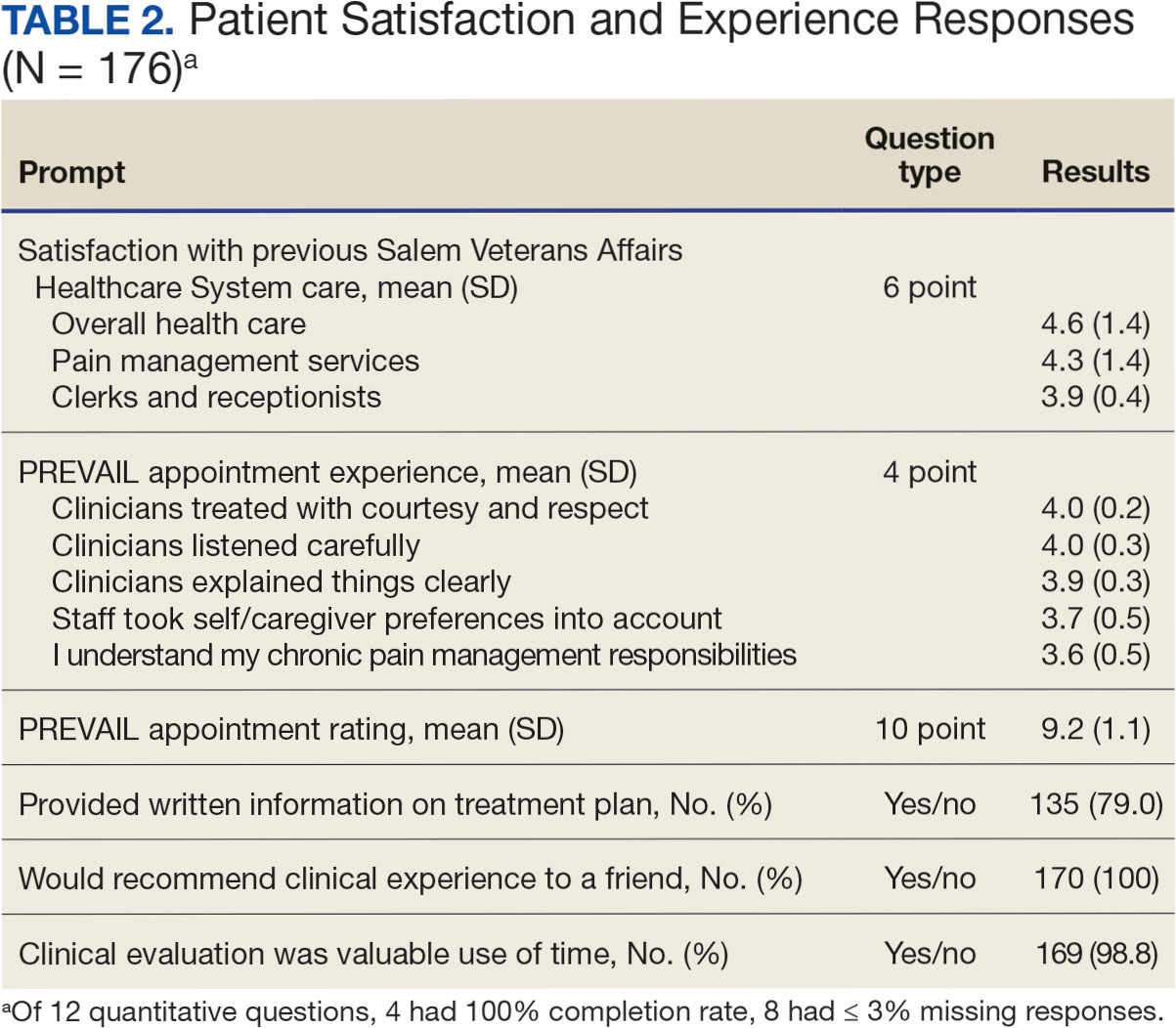
In 2022, 168 veterans completed the initial IDT evaluation, and 144 (85.2%) completed the satisfaction survey and were included in this study. Thirty-two support persons who attended the initial IDT evaluation and completed the survey also were included. Of the 12 quantitative questions, 4 had a 100% completion rate, while 8 had ≤ 3% missing responses. When describing care prior to participating in PREVAIL, participants indicated a mean (SD) response of 4.6 (1.4) with the health care they received at SVAHCS and 4.3 (1.4) with SVAHCS pain management services, both on 6-point scales. All but 2 participants (98.9%) reported always being treated with courtesy and respect by PREVAIL HCPs during the initial IDT evaluation, with a mean (SD) score of 4.0 (0.2) on a 4-point scale. Most respondents (96.6%) reported that PREVAIL HCPs always listened carefully during the initial IDT evaluation, with a mean (SD) 4.0 (0.3) on a 4-point scale. Similarly, 92.6% reported that PREVAIL HCPs explained things clearly during the initial IDT evaluation, with a mean (SD) 3.9 (0.3) on a 4-point scale.
All respondents agreed that PREVAIL HCPs considered veteran preferences and those of their support persons in deciding their health care needs during the initial IDT evaluation, with a mean (SD) 3.7 (0.5) on a 4-point scale. Most respondents left the appointment with a good understanding of their responsibilities for chronic pain management with 99.4% (n = 169) strongly agreeing or agreeing (mean [SD] 3.6 [0.5]). A total of 135 respondents (79.4%) reported they left appointments with written information on their treatment plan. All 170 respondents reported that they would recommend PREVAIL to a friend, and 169 respondents (98.8%) felt that the initial PREVAIL IDT evaluation was a valuable use of time. Eighty-seven respondents (50.9%) rated the initial IDT evaluation as the “best clinical experience possible” with a mean (SD) score of 9.2 (1.1) on a 10-point scale (Table 2).
Qualitative Responses
Respondents provided complementary feedback on the program, with many participants stating that they enjoyed every aspect (eAppendix, available at doi:10.12788/fp.0503). In terms of positive aspects of the program, several themes emerged: participants appreciated meeting as an IDT, feeling cared for and listened to, learning more about their pain and ways to manage it, and specific services offered. Thirty-three of 144 respondents wanted longer appointment times. Twenty-two respondents suggested logistics improvements (eg, meeting in a larger room, having a written plan at the end, sending paperwork ahead of time, and later appointment times).
Discussion
Veterans and support persons were satisfied with the initial IDT evaluation for the PREVAIL whole health-focused pain clinical program for veterans predominantly residing in central Appalachia. These satisfaction findings are noteworthy since 20% of this same sample reported dissatisfaction with prior pain services, which could affect engagement and outcomes in pain care. In addition to high satisfaction levels, the PREVAIL IDT model may benefit veterans with limited resources. Rather than needing to attend several individual appointments, the PREVAIL IDT Track provides a 1-stop shop approach that decreases patient burden and barriers to care (eg, travel, transportation, and time) as well as health care system burden. For instance, schedulers need only to make 1 appointment for the veteran rather than several. This approach was highly acceptable to veterans served at SVAHCS and may increase the reach and impact of VHA IDT pain care.
The PREVAIL model may foster rapport with HCPs and encourage an active role in self-managing pain.36,37 Participants noted that their preferences were considered and that they had a good understanding of their responsibilities for managing their chronic pain. This patient-centered approach, emphasizing an active role for the patient, is a hallmark of the VHA Whole Health System and aligns with the overarching PREVAIL IDT Track goal to enhance self-management skills, thus improving functioning through decreased pain interference.14,38-41
Participants in PREVAIL provided substantial open-ended feedback that has contributed to the program’s improvement and may provide information into preferred components of pain IDT programs, particularly for rural veterans. When asked about their favorite component of the initial IDT evaluation, the most emergent theme was meeting simultaneously with HCPs on the IDT. This finding is significant, given that only 11% of IDTs involve direct patient interaction.
Furthermore, unlike most IDTs, PREVAIL IDT includes a dietitian.11,42 IDT programs may benefit from dietitian involvement given the importance of the anti-inflammatory diet on chronic pain.43-46 Participants recommended improvements, (eg, changes to the location and timing, adding a written treatment plan at the end of the appointment, and completing paperwork prior to the appointment) many of which have been addressed. The program now uses validated measures to track progress and comprehensive assessments of pain in response to calls for measurement-based care.13,47 These process improvement suggestions may be instructive for other VA medical centers with rural populations.
Limitations
This study used a program-specific satisfaction survey with open-ended questions to allow for rich responses; however, the survey has not been validated. It also sought to minimize bias by asking participants to give completed surveys to staff members who were not HCPs on the IDT. However, participants’ responses may still have been influenced by this process. Response rate and demographics for support persons were impossible to determine. The results analyzed the responses of veterans and support persons together, which may have skewed the data. Future studies of pain IDT programs should consider analyzing responses from veterans and their support persons separately and identifying factors (eg, demographics or clinical characteristics) that influence the patients’ experiences while participating.
Conclusions
The initial PREVAIL IDT evaluation at SVAHCS is associated with high-levels of satisfaction. These veterans living in rural Appalachia, similar to the 4.4 million rural US veterans, are more likely to encounter barriers to care (eg, drive time, or transportation concerns) and be prescribed opioids.48 These veterans are also at high risk of chronic physical and mental health comorbidities, drug misuse, overdose, and suicide.49,50 Providing veterans in rural communities the opportunity to attend a single appointment with a pain IDT instead of requiring several individual appointments could improve the reach of evidence-based pain care.
This model of meeting simultaneously with all HCPs on the pain IDT may connect all veterans to the most available and best care, something prioritized by the VHA.28 The initial PREVAIL IDT evaluation also utilizes the Personal Health Inventory and the VHA Whole Health System Circle of Health to design patient-centered treatment plans. Integration of the Whole Health System is currently a high priority within VHA. The PREVAIL IDT Track model warrants additional efficacy research.
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults - United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385. doi:10.15585/mmwr.mm7215a1
- Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Mackey SC, Pearl RG. Pain management: optimizing patient care through comprehensive, interdisciplinary models and continuous innovations. Anesthesiol Clin. 2023;41(2):xv-xvii. doi:10.1016/j.anclin.2023.03.011
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130. doi:10.1037/a0035514
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the veterans administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi:10.1136/bmj.h444
- Waterschoot FPC, Dijkstra PU, Hollak N, De Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155(1):179-189. doi:10.1016/j.pain.2013.10.006
- Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678. doi:10.1093/rheumatology/ken021
- Elbers S, Wittink H, Konings S, et al. Longitudinal outcome evaluations of interdisciplinary multimodal pain Treatment programmes for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2022;26(2):310-335. doi:10.1002/ejp.1875
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337-345. doi:10.1016/j.pain.2003.08.001
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi:10.1016/j.pain.2004.09.012
- Kligler B, Hyde J, Gantt C, Bokhour B. The whole health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?”. Med Care. 2022;60(5):387-391. doi:10.1097/mlr.0000000000001706
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Transforming Health Care to Create Whole Health: Strategies to Assess, Scale, and Spread the Whole Person Approach to Health, Meisnere M, SouthPaul J, Krist AH, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. National Academies Press (US); February 15, 2023.
- The time Is now for a whole-person health approach to public health. Public Health Rep. 2023;138(4):561-564. doi:10.1177/00333549231154583
- Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12 Suppl 5):S5-S8. doi:10.1097/mlr.0000000000000226
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the whole health system of care. Health Serv Res. 2022;57 Suppl 1(Suppl 1):53-65. doi:10.1111/1475-6773.13938
- Zeliadt SB, Douglas JH, Gelman H, et al. Effectiveness of a whole health model of care emphasizing complementary and integrative health on reducing opioid use among patients with chronic pain. BMC Health Serv Res. 2022;22(1):1053. doi:10.1186/s12913-022-08388-2
- Reed DE 2nd, Bokhour BG, Gaj L, et al. Whole health use and interest across veterans with cooccurring chronic pain and PTSD: an examination of the 18 VA medical center flagship sites. Glob Adv Health Med. 2022;11:21649561211065374. doi:10.1177/21649561211065374
- Etingen B, Smith BM, Zeliadt SB, et al. VHA whole health services and complementary and integrative health therapies: a gateway to evidence-based mental health treatment. J Gen Intern Med. 2023;38(14):3144-3151. doi:10.1007/s11606-023-08296-z
- Johnson EM, Possemato K, Khan S, Chinman M, Maisto SA. Engagement, experience, and satisfaction with peerdelivered whole health coaching for veterans with PTSD: a mixed methods process evaluation. Psychol Serv. 2021;19(2):305-316. doi:10.1037/ser0000529
- Purcell N, Zamora K, Gibson C, et al. Patient experiences with integrated pain care: a qualitative evaluation of one VA’s biopsychosocial approach to chronic pain treatment and opioid safety. Glob Adv Health Med. 2019;8:2164956119838845. doi:10.1177/2164956119838845
- Will KK, Johnson ML, Lamb G. Team-based care and patient satisfaction in the hospital setting: a systematic review. J Patient Cent Res Rev. 2019;6(2):158-171. doi:10.17294/2330-0698.1695
- van Dongen JJJ, Habets IGJ, Beurskens A, van Bokhoven MA. Successful participation of patients in interprofessional team meetings: a qualitative study. Health Expect. 2017;20(4):724-733. doi:10.1111/hex.12511
- Oliver DP, Albright DL, Kruse RL, Wittenberg-Lyles E, Washington K, Demiris G. Caregiver evaluation of the ACTIVE intervention: “it was like we were sitting at the table with everyone.” Am J Hosp Palliat Care. 2014;31(4):444-453. doi:10.1177/1049909113490823
- Ansmann L, Heuser C, Diekmann A, et al. Patient participation in multidisciplinary tumor conferences: how is it implemented? What is the patients’ role? What are patients’ experiences? Cancer Med. 2021;10(19):6714-6724. doi:10.1002/cam4.4213
- US Department of Veterans Affairs, Veterans Health Administration. Updated March 20, 2023. Accessed June 11, 2024. https://www.va.gov/health/priorities/index.asp
- Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res (Lausanne). 2023;4:1223172. doi:10.3389/fpain.2023.1223172
- Kligler B. Whole health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Howe RJ, Poulin LM, Federman DG. The personal health inventory: current use, perceived barriers, and benefits. Fed Pract. 2017;34(5):23-26. doi:10.1177/2164957X221077214
- Hicks N, Harden S, Oursler KA, Courtney RE. Determining the representativeness of participants in a whole health interdisciplinary chronic pain program (PREVAIL) in a VA medical center: who did we reach? Presented at: PAINWeek 2022; September 6-9, 2022; Las Vegas, Nevada. Accessed September 10, 2024. https://www.tandfonline.com/doi/full/10.1080/00325481.2022.2116839
- Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. SAGE Publications; 2018.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual in Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, 3rd edition. Published September 26, 2012. Accessed June 11, 2024. https://www.nice.org.uk/process/pmg4/chapter/introduction
- Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient-physician role relationships and patient activation among individuals with chronic illness. Health Serv Res. 2012;47(3 PART 1):1201-1223. doi:10.1111/j.1475-6773.2011.01354.x
- Fu Y, Yu G, McNichol E, Marczewski K, Closs SJ. The association between patient-professional partnerships and self-management of chronic back pain: a mixed methods study. Eur J Pain. 2018;22(7):1229-1244. doi:10.1002/ejp.1210
- Nicholas MK, Asghari A, Blyth FM, et al. Self-management intervention for chronic pain in older adults: a randomised controlled trial. Pain. 2013;154(6):824-835. doi:10.1016/j.pain.2013.02.009
- Nøst TH, Steinsbekk A, Bratås O, Grønning K. Twelvemonth effect of chronic pain self-management intervention delivered in an easily accessible primary healthcare service - a randomised controlled trial. BMC Health Serv Res. 2018;18(1):1012. doi:10.1186/s12913-018-3843-x
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Selfmanagement of chronic pain: a population-based study. Pain. 2005;113(3):285-292. doi:10.1016/j.pain.2004.12.004
- Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(7):1070-1078. doi:10.1002/ejp.830
- Murphy JL, Palyo SA, Schmidt ZS, et al. The resurrection of interdisciplinary pain rehabilitation: outcomes across a veterans affairs collaborative. Pain Med. 2021;22(2):430- 443. doi:10.1093/pm/pnaa417
- Brain K, Burrows TL, Bruggink L, et al. Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med. 2021;10(21):5203. doi:10.3390/jcm10215203
- Field R, Pourkazemi F, Turton J, Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Med). 2021;22(3):694-714. doi:10.1093/pm/pnaa378
- Bjørklund G, Aaseth J, Do§a MD, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153-165. doi:10.1016/j.nut.2019.04.007
- Kaushik AS, Strath LJ, Sorge RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9(2):487-498. doi:10.1007/s40122-020-00200-5
- Boswell JF, Hepner KA, Lysell K, et al. The need for a measurement-based care professional practice guideline. Psychotherapy (Chic). 2023;60(1):1-16. doi:10.1037/pst0000439
- Lund BC, Ohl ME, Hadlandsmyth K, Mosher HJ. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894- 900. doi:10.1093/milmed/usz104
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated May 14, 2024. Accessed June 11, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- McCarthy JF, Blow FC, Ignacio R V., Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs health system: rural-urban differences in rates, risks, and methods. Am J Public Health. 2012;102 Suppl 1(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
Chronic pain is one of the most prevalent public health concerns in the United States, affecting > 51 million adults with about $500 billion in health care costs.1 Military veterans are among the most vulnerable subpopulations, with 65% of veterans reporting chronic pain in the last 3 months.2 Chronic pain is complex, affecting the biopsychosocial-spiritual levels of human health, and requires multimodal and comprehensive treatment approaches.3 Hence, chronic pain treatment can be best delivered via interdisciplinary teams (IDTs) that use a patient-centered approach.4,5
The Veterans Healthcare Administration (VHA) is a leader in developing and delivering interdisciplinary pain care.6,7 VHA Directive 2009-053 requires every US Department of Veterans Affairs (VA) medical center to offer an IDT for chronic pain. However, VHA and non-VHA IDT programs vary significantly.8-11 A recent systematic review found a median of 5 disciplines included on IDTs (range, 2-8), and program content often included exercise and education; only 11% of included IDTs met simultaneously with patients.11 The heterogeneity of IDT programs has made determining best practices challenging.8,11 The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has denoted several core measures and measurement domains that were critical for determining the success of pain management interventions, including patient satisfaction.12,13 Nevertheless, the association of IDTs with high patient satisfaction and improvement in pain measures has been documented.5,11
The VHA has worked to implement the Whole Health System into health care, which considers well-being across physical, behavioral, spiritual, and socioeconomic domains. As such, the Whole Health System involves an interpersonal, team-based approach, “anchored in trusting longitudinal relationships to promote resilience, prevent disease, and restore health.”14 It aligns with the patient’s mission, aspiration, and purpose. Surgeon General VADM Vivek H. Murthy, MD, MBA, recently endorsed this approach.15,16 Other health care systems adopting whole health tend to have higher patient satisfaction, increased access to care, and improved patient-reported outcomes.15 Within the VHA, the Whole Health System has shifted the conversation between clinicians and patients from “What is the matter with you?” to “What is important to you?” while emphasizing a proactive and personalized approach to health care.17 Rather than emphasizing passive modalities such as medications and clinician-led services (eg, interventional pain service), the Whole Health System highlights self-care.3,17 Initial research findings within the VHA have been promising.18-21 Whole health peer coaching calls appear to be an effective approach for veterans diagnosed with PTSD, and the use of whole health services is associated with a decrease in opioid use.19,22 However, there are negligible data on patient experiences after meeting with a whole health-focused pain IDT, and studies to date have focused on urban populations.23 One approach to IDT that has shown promise for other health issues involves a patient meeting simultaneously with all members of the IDT.24-27 With the integration of the Whole Health System and the VHA priorities to provide veterans with the “soonest and best care,” more data are needed on the experiences of patients and support persons with various approaches to IDT pain care.28 This study aimed to evaluate patient and support person experiences with a whole health-focused pain IDT that met simultaneously with the patient and support person during an initial evaluation. This study was approved by the institutional review board at the Salem VA Health Care System (SVAHCS) in Virginia.
Methods
The PREVAIL IDT Track is a clinical program offered at SVAHCS with a whole health-focused approach that involves patients and their support persons meeting simultaneously with a pain IDT. PREVAIL IDT Track is designed to help veterans more effectively self-manage chronic pain (Table 1).6,29 Health care practitioners (HCPs) at SVAHCS recommended that veterans with pain persisting for > 3 months participate in PREVAIL IDT Track. After meeting with an advanced practice clinician for an intake, veterans elected to participate in the PREVAIL IDT Track program and completed the initial 6 weeks of pain education. Veterans were then invited to be evaluated by the pain IDT. A team including HCPs from interventional pain, psychology, pharmacy, nutrition, and physical therapy services met with the veteran for 60 minutes. Veterans were also invited to bring a support person to the IDT initial evaluation.

During the IDT initial evaluation, HCPs inquired about the patient’s mission, aspiration, and purpose (“If you were in less pain, what would you be doing more of?”) and about whole health self-care and wellness factors that may contribute to their chronic pain using the Personal Health Inventory.30,31 Veterans were then invited to select 3 whole health self-care areas to focus on during the 6-month program.3 The IDT HCPs worked with the veteran to establish the treatment plan for the first month in the areas of self-care selected by the patient and made recommendations for additional treatments. If the veteran brought a support person to the IDT initial evaluation, their feedback was elicited throughout and at the end to ensure the final treatment plan and first month’s goals were realistic. At the end of the appointment, the veteran and their support person were asked to complete a program-specific satisfaction survey. The HCPs on the team and the veteran executed the treatment plan developed during the appointment, except for medication prescribing. Recommendations for medication changes are included in clinical notes. Veterans then received 5 monthly coaching calls from a nurse navigator with training in whole health and a 6-month follow-up appointment with the IDT HCPs to discuss a plan for continuity of care.
Participant demographic information was not collected, and participants were not compensated for completing the survey. Veterans in PREVAIL IDT Track are predominantly residents of central Appalachia, White, male, unemployed, have ≥ 1 mental and physical health comorbidity, and have a history of mental health treatment.32 Veterans participating in PREVAIL IDT had a mean age of 57 years, and about 1 in 3 have opioid prescriptions.32
A program-specific 17-question satisfaction survey was developed, which included questions related to satisfaction with previous SVAHCS pain care and staff interactions. To assess the overall impression of the IDT initial evaluation, 3 yes/no questions and a 0 to 10-point scale were used. The 5 remaining open-ended questions allowed participants to give feedback about the IDT initial evaluation.
Data Analysis
A convergent mixed-methods approach was used to evaluate participant satisfaction with the initial IDT evaluation. The study team collected and analyzed quantitative and qualitative survey data and triangulated the findings.33 For quantitative responses, frequencies and means were calculated using Python. For qualitative responses, thematic data analysis was conducted by systematic coding, using inductive methods and allowing themes to emerge. Study team members performed a line-by-line analysis of responses using NVivo to identify important codes and reach a consensus. This study adhered to the Consolidated Criteria for Reporting Qualitative Research and followed the National Institute for Health Care Excellence checklist.34,35
Results
Quantitative Responses
In 2022, 168 veterans completed the initial IDT evaluation, and 144 (85.2%) completed the satisfaction survey and were included in this study. Thirty-two support persons who attended the initial IDT evaluation and completed the survey also were included. Of the 12 quantitative questions, 4 had a 100% completion rate, while 8 had ≤ 3% missing responses. When describing care prior to participating in PREVAIL, participants indicated a mean (SD) response of 4.6 (1.4) with the health care they received at SVAHCS and 4.3 (1.4) with SVAHCS pain management services, both on 6-point scales. All but 2 participants (98.9%) reported always being treated with courtesy and respect by PREVAIL HCPs during the initial IDT evaluation, with a mean (SD) score of 4.0 (0.2) on a 4-point scale. Most respondents (96.6%) reported that PREVAIL HCPs always listened carefully during the initial IDT evaluation, with a mean (SD) 4.0 (0.3) on a 4-point scale. Similarly, 92.6% reported that PREVAIL HCPs explained things clearly during the initial IDT evaluation, with a mean (SD) 3.9 (0.3) on a 4-point scale.
All respondents agreed that PREVAIL HCPs considered veteran preferences and those of their support persons in deciding their health care needs during the initial IDT evaluation, with a mean (SD) 3.7 (0.5) on a 4-point scale. Most respondents left the appointment with a good understanding of their responsibilities for chronic pain management with 99.4% (n = 169) strongly agreeing or agreeing (mean [SD] 3.6 [0.5]). A total of 135 respondents (79.4%) reported they left appointments with written information on their treatment plan. All 170 respondents reported that they would recommend PREVAIL to a friend, and 169 respondents (98.8%) felt that the initial PREVAIL IDT evaluation was a valuable use of time. Eighty-seven respondents (50.9%) rated the initial IDT evaluation as the “best clinical experience possible” with a mean (SD) score of 9.2 (1.1) on a 10-point scale (Table 2).

Qualitative Responses
Respondents provided complementary feedback on the program, with many participants stating that they enjoyed every aspect (eAppendix, available at doi:10.12788/fp.0503). In terms of positive aspects of the program, several themes emerged: participants appreciated meeting as an IDT, feeling cared for and listened to, learning more about their pain and ways to manage it, and specific services offered. Thirty-three of 144 respondents wanted longer appointment times. Twenty-two respondents suggested logistics improvements (eg, meeting in a larger room, having a written plan at the end, sending paperwork ahead of time, and later appointment times).
Discussion
Veterans and support persons were satisfied with the initial IDT evaluation for the PREVAIL whole health-focused pain clinical program for veterans predominantly residing in central Appalachia. These satisfaction findings are noteworthy since 20% of this same sample reported dissatisfaction with prior pain services, which could affect engagement and outcomes in pain care. In addition to high satisfaction levels, the PREVAIL IDT model may benefit veterans with limited resources. Rather than needing to attend several individual appointments, the PREVAIL IDT Track provides a 1-stop shop approach that decreases patient burden and barriers to care (eg, travel, transportation, and time) as well as health care system burden. For instance, schedulers need only to make 1 appointment for the veteran rather than several. This approach was highly acceptable to veterans served at SVAHCS and may increase the reach and impact of VHA IDT pain care.
The PREVAIL model may foster rapport with HCPs and encourage an active role in self-managing pain.36,37 Participants noted that their preferences were considered and that they had a good understanding of their responsibilities for managing their chronic pain. This patient-centered approach, emphasizing an active role for the patient, is a hallmark of the VHA Whole Health System and aligns with the overarching PREVAIL IDT Track goal to enhance self-management skills, thus improving functioning through decreased pain interference.14,38-41
Participants in PREVAIL provided substantial open-ended feedback that has contributed to the program’s improvement and may provide information into preferred components of pain IDT programs, particularly for rural veterans. When asked about their favorite component of the initial IDT evaluation, the most emergent theme was meeting simultaneously with HCPs on the IDT. This finding is significant, given that only 11% of IDTs involve direct patient interaction.
Furthermore, unlike most IDTs, PREVAIL IDT includes a dietitian.11,42 IDT programs may benefit from dietitian involvement given the importance of the anti-inflammatory diet on chronic pain.43-46 Participants recommended improvements, (eg, changes to the location and timing, adding a written treatment plan at the end of the appointment, and completing paperwork prior to the appointment) many of which have been addressed. The program now uses validated measures to track progress and comprehensive assessments of pain in response to calls for measurement-based care.13,47 These process improvement suggestions may be instructive for other VA medical centers with rural populations.
Limitations
This study used a program-specific satisfaction survey with open-ended questions to allow for rich responses; however, the survey has not been validated. It also sought to minimize bias by asking participants to give completed surveys to staff members who were not HCPs on the IDT. However, participants’ responses may still have been influenced by this process. Response rate and demographics for support persons were impossible to determine. The results analyzed the responses of veterans and support persons together, which may have skewed the data. Future studies of pain IDT programs should consider analyzing responses from veterans and their support persons separately and identifying factors (eg, demographics or clinical characteristics) that influence the patients’ experiences while participating.
Conclusions
The initial PREVAIL IDT evaluation at SVAHCS is associated with high-levels of satisfaction. These veterans living in rural Appalachia, similar to the 4.4 million rural US veterans, are more likely to encounter barriers to care (eg, drive time, or transportation concerns) and be prescribed opioids.48 These veterans are also at high risk of chronic physical and mental health comorbidities, drug misuse, overdose, and suicide.49,50 Providing veterans in rural communities the opportunity to attend a single appointment with a pain IDT instead of requiring several individual appointments could improve the reach of evidence-based pain care.
This model of meeting simultaneously with all HCPs on the pain IDT may connect all veterans to the most available and best care, something prioritized by the VHA.28 The initial PREVAIL IDT evaluation also utilizes the Personal Health Inventory and the VHA Whole Health System Circle of Health to design patient-centered treatment plans. Integration of the Whole Health System is currently a high priority within VHA. The PREVAIL IDT Track model warrants additional efficacy research.
Chronic pain is one of the most prevalent public health concerns in the United States, affecting > 51 million adults with about $500 billion in health care costs.1 Military veterans are among the most vulnerable subpopulations, with 65% of veterans reporting chronic pain in the last 3 months.2 Chronic pain is complex, affecting the biopsychosocial-spiritual levels of human health, and requires multimodal and comprehensive treatment approaches.3 Hence, chronic pain treatment can be best delivered via interdisciplinary teams (IDTs) that use a patient-centered approach.4,5
The Veterans Healthcare Administration (VHA) is a leader in developing and delivering interdisciplinary pain care.6,7 VHA Directive 2009-053 requires every US Department of Veterans Affairs (VA) medical center to offer an IDT for chronic pain. However, VHA and non-VHA IDT programs vary significantly.8-11 A recent systematic review found a median of 5 disciplines included on IDTs (range, 2-8), and program content often included exercise and education; only 11% of included IDTs met simultaneously with patients.11 The heterogeneity of IDT programs has made determining best practices challenging.8,11 The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has denoted several core measures and measurement domains that were critical for determining the success of pain management interventions, including patient satisfaction.12,13 Nevertheless, the association of IDTs with high patient satisfaction and improvement in pain measures has been documented.5,11
The VHA has worked to implement the Whole Health System into health care, which considers well-being across physical, behavioral, spiritual, and socioeconomic domains. As such, the Whole Health System involves an interpersonal, team-based approach, “anchored in trusting longitudinal relationships to promote resilience, prevent disease, and restore health.”14 It aligns with the patient’s mission, aspiration, and purpose. Surgeon General VADM Vivek H. Murthy, MD, MBA, recently endorsed this approach.15,16 Other health care systems adopting whole health tend to have higher patient satisfaction, increased access to care, and improved patient-reported outcomes.15 Within the VHA, the Whole Health System has shifted the conversation between clinicians and patients from “What is the matter with you?” to “What is important to you?” while emphasizing a proactive and personalized approach to health care.17 Rather than emphasizing passive modalities such as medications and clinician-led services (eg, interventional pain service), the Whole Health System highlights self-care.3,17 Initial research findings within the VHA have been promising.18-21 Whole health peer coaching calls appear to be an effective approach for veterans diagnosed with PTSD, and the use of whole health services is associated with a decrease in opioid use.19,22 However, there are negligible data on patient experiences after meeting with a whole health-focused pain IDT, and studies to date have focused on urban populations.23 One approach to IDT that has shown promise for other health issues involves a patient meeting simultaneously with all members of the IDT.24-27 With the integration of the Whole Health System and the VHA priorities to provide veterans with the “soonest and best care,” more data are needed on the experiences of patients and support persons with various approaches to IDT pain care.28 This study aimed to evaluate patient and support person experiences with a whole health-focused pain IDT that met simultaneously with the patient and support person during an initial evaluation. This study was approved by the institutional review board at the Salem VA Health Care System (SVAHCS) in Virginia.
Methods
The PREVAIL IDT Track is a clinical program offered at SVAHCS with a whole health-focused approach that involves patients and their support persons meeting simultaneously with a pain IDT. PREVAIL IDT Track is designed to help veterans more effectively self-manage chronic pain (Table 1).6,29 Health care practitioners (HCPs) at SVAHCS recommended that veterans with pain persisting for > 3 months participate in PREVAIL IDT Track. After meeting with an advanced practice clinician for an intake, veterans elected to participate in the PREVAIL IDT Track program and completed the initial 6 weeks of pain education. Veterans were then invited to be evaluated by the pain IDT. A team including HCPs from interventional pain, psychology, pharmacy, nutrition, and physical therapy services met with the veteran for 60 minutes. Veterans were also invited to bring a support person to the IDT initial evaluation.

During the IDT initial evaluation, HCPs inquired about the patient’s mission, aspiration, and purpose (“If you were in less pain, what would you be doing more of?”) and about whole health self-care and wellness factors that may contribute to their chronic pain using the Personal Health Inventory.30,31 Veterans were then invited to select 3 whole health self-care areas to focus on during the 6-month program.3 The IDT HCPs worked with the veteran to establish the treatment plan for the first month in the areas of self-care selected by the patient and made recommendations for additional treatments. If the veteran brought a support person to the IDT initial evaluation, their feedback was elicited throughout and at the end to ensure the final treatment plan and first month’s goals were realistic. At the end of the appointment, the veteran and their support person were asked to complete a program-specific satisfaction survey. The HCPs on the team and the veteran executed the treatment plan developed during the appointment, except for medication prescribing. Recommendations for medication changes are included in clinical notes. Veterans then received 5 monthly coaching calls from a nurse navigator with training in whole health and a 6-month follow-up appointment with the IDT HCPs to discuss a plan for continuity of care.
Participant demographic information was not collected, and participants were not compensated for completing the survey. Veterans in PREVAIL IDT Track are predominantly residents of central Appalachia, White, male, unemployed, have ≥ 1 mental and physical health comorbidity, and have a history of mental health treatment.32 Veterans participating in PREVAIL IDT had a mean age of 57 years, and about 1 in 3 have opioid prescriptions.32
A program-specific 17-question satisfaction survey was developed, which included questions related to satisfaction with previous SVAHCS pain care and staff interactions. To assess the overall impression of the IDT initial evaluation, 3 yes/no questions and a 0 to 10-point scale were used. The 5 remaining open-ended questions allowed participants to give feedback about the IDT initial evaluation.
Data Analysis
A convergent mixed-methods approach was used to evaluate participant satisfaction with the initial IDT evaluation. The study team collected and analyzed quantitative and qualitative survey data and triangulated the findings.33 For quantitative responses, frequencies and means were calculated using Python. For qualitative responses, thematic data analysis was conducted by systematic coding, using inductive methods and allowing themes to emerge. Study team members performed a line-by-line analysis of responses using NVivo to identify important codes and reach a consensus. This study adhered to the Consolidated Criteria for Reporting Qualitative Research and followed the National Institute for Health Care Excellence checklist.34,35
Results
Quantitative Responses
In 2022, 168 veterans completed the initial IDT evaluation, and 144 (85.2%) completed the satisfaction survey and were included in this study. Thirty-two support persons who attended the initial IDT evaluation and completed the survey also were included. Of the 12 quantitative questions, 4 had a 100% completion rate, while 8 had ≤ 3% missing responses. When describing care prior to participating in PREVAIL, participants indicated a mean (SD) response of 4.6 (1.4) with the health care they received at SVAHCS and 4.3 (1.4) with SVAHCS pain management services, both on 6-point scales. All but 2 participants (98.9%) reported always being treated with courtesy and respect by PREVAIL HCPs during the initial IDT evaluation, with a mean (SD) score of 4.0 (0.2) on a 4-point scale. Most respondents (96.6%) reported that PREVAIL HCPs always listened carefully during the initial IDT evaluation, with a mean (SD) 4.0 (0.3) on a 4-point scale. Similarly, 92.6% reported that PREVAIL HCPs explained things clearly during the initial IDT evaluation, with a mean (SD) 3.9 (0.3) on a 4-point scale.
All respondents agreed that PREVAIL HCPs considered veteran preferences and those of their support persons in deciding their health care needs during the initial IDT evaluation, with a mean (SD) 3.7 (0.5) on a 4-point scale. Most respondents left the appointment with a good understanding of their responsibilities for chronic pain management with 99.4% (n = 169) strongly agreeing or agreeing (mean [SD] 3.6 [0.5]). A total of 135 respondents (79.4%) reported they left appointments with written information on their treatment plan. All 170 respondents reported that they would recommend PREVAIL to a friend, and 169 respondents (98.8%) felt that the initial PREVAIL IDT evaluation was a valuable use of time. Eighty-seven respondents (50.9%) rated the initial IDT evaluation as the “best clinical experience possible” with a mean (SD) score of 9.2 (1.1) on a 10-point scale (Table 2).

Qualitative Responses
Respondents provided complementary feedback on the program, with many participants stating that they enjoyed every aspect (eAppendix, available at doi:10.12788/fp.0503). In terms of positive aspects of the program, several themes emerged: participants appreciated meeting as an IDT, feeling cared for and listened to, learning more about their pain and ways to manage it, and specific services offered. Thirty-three of 144 respondents wanted longer appointment times. Twenty-two respondents suggested logistics improvements (eg, meeting in a larger room, having a written plan at the end, sending paperwork ahead of time, and later appointment times).
Discussion
Veterans and support persons were satisfied with the initial IDT evaluation for the PREVAIL whole health-focused pain clinical program for veterans predominantly residing in central Appalachia. These satisfaction findings are noteworthy since 20% of this same sample reported dissatisfaction with prior pain services, which could affect engagement and outcomes in pain care. In addition to high satisfaction levels, the PREVAIL IDT model may benefit veterans with limited resources. Rather than needing to attend several individual appointments, the PREVAIL IDT Track provides a 1-stop shop approach that decreases patient burden and barriers to care (eg, travel, transportation, and time) as well as health care system burden. For instance, schedulers need only to make 1 appointment for the veteran rather than several. This approach was highly acceptable to veterans served at SVAHCS and may increase the reach and impact of VHA IDT pain care.
The PREVAIL model may foster rapport with HCPs and encourage an active role in self-managing pain.36,37 Participants noted that their preferences were considered and that they had a good understanding of their responsibilities for managing their chronic pain. This patient-centered approach, emphasizing an active role for the patient, is a hallmark of the VHA Whole Health System and aligns with the overarching PREVAIL IDT Track goal to enhance self-management skills, thus improving functioning through decreased pain interference.14,38-41
Participants in PREVAIL provided substantial open-ended feedback that has contributed to the program’s improvement and may provide information into preferred components of pain IDT programs, particularly for rural veterans. When asked about their favorite component of the initial IDT evaluation, the most emergent theme was meeting simultaneously with HCPs on the IDT. This finding is significant, given that only 11% of IDTs involve direct patient interaction.
Furthermore, unlike most IDTs, PREVAIL IDT includes a dietitian.11,42 IDT programs may benefit from dietitian involvement given the importance of the anti-inflammatory diet on chronic pain.43-46 Participants recommended improvements, (eg, changes to the location and timing, adding a written treatment plan at the end of the appointment, and completing paperwork prior to the appointment) many of which have been addressed. The program now uses validated measures to track progress and comprehensive assessments of pain in response to calls for measurement-based care.13,47 These process improvement suggestions may be instructive for other VA medical centers with rural populations.
Limitations
This study used a program-specific satisfaction survey with open-ended questions to allow for rich responses; however, the survey has not been validated. It also sought to minimize bias by asking participants to give completed surveys to staff members who were not HCPs on the IDT. However, participants’ responses may still have been influenced by this process. Response rate and demographics for support persons were impossible to determine. The results analyzed the responses of veterans and support persons together, which may have skewed the data. Future studies of pain IDT programs should consider analyzing responses from veterans and their support persons separately and identifying factors (eg, demographics or clinical characteristics) that influence the patients’ experiences while participating.
Conclusions
The initial PREVAIL IDT evaluation at SVAHCS is associated with high-levels of satisfaction. These veterans living in rural Appalachia, similar to the 4.4 million rural US veterans, are more likely to encounter barriers to care (eg, drive time, or transportation concerns) and be prescribed opioids.48 These veterans are also at high risk of chronic physical and mental health comorbidities, drug misuse, overdose, and suicide.49,50 Providing veterans in rural communities the opportunity to attend a single appointment with a pain IDT instead of requiring several individual appointments could improve the reach of evidence-based pain care.
This model of meeting simultaneously with all HCPs on the pain IDT may connect all veterans to the most available and best care, something prioritized by the VHA.28 The initial PREVAIL IDT evaluation also utilizes the Personal Health Inventory and the VHA Whole Health System Circle of Health to design patient-centered treatment plans. Integration of the Whole Health System is currently a high priority within VHA. The PREVAIL IDT Track model warrants additional efficacy research.
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults - United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385. doi:10.15585/mmwr.mm7215a1
- Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Mackey SC, Pearl RG. Pain management: optimizing patient care through comprehensive, interdisciplinary models and continuous innovations. Anesthesiol Clin. 2023;41(2):xv-xvii. doi:10.1016/j.anclin.2023.03.011
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130. doi:10.1037/a0035514
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the veterans administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi:10.1136/bmj.h444
- Waterschoot FPC, Dijkstra PU, Hollak N, De Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155(1):179-189. doi:10.1016/j.pain.2013.10.006
- Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678. doi:10.1093/rheumatology/ken021
- Elbers S, Wittink H, Konings S, et al. Longitudinal outcome evaluations of interdisciplinary multimodal pain Treatment programmes for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2022;26(2):310-335. doi:10.1002/ejp.1875
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337-345. doi:10.1016/j.pain.2003.08.001
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi:10.1016/j.pain.2004.09.012
- Kligler B, Hyde J, Gantt C, Bokhour B. The whole health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?”. Med Care. 2022;60(5):387-391. doi:10.1097/mlr.0000000000001706
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Transforming Health Care to Create Whole Health: Strategies to Assess, Scale, and Spread the Whole Person Approach to Health, Meisnere M, SouthPaul J, Krist AH, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. National Academies Press (US); February 15, 2023.
- The time Is now for a whole-person health approach to public health. Public Health Rep. 2023;138(4):561-564. doi:10.1177/00333549231154583
- Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12 Suppl 5):S5-S8. doi:10.1097/mlr.0000000000000226
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the whole health system of care. Health Serv Res. 2022;57 Suppl 1(Suppl 1):53-65. doi:10.1111/1475-6773.13938
- Zeliadt SB, Douglas JH, Gelman H, et al. Effectiveness of a whole health model of care emphasizing complementary and integrative health on reducing opioid use among patients with chronic pain. BMC Health Serv Res. 2022;22(1):1053. doi:10.1186/s12913-022-08388-2
- Reed DE 2nd, Bokhour BG, Gaj L, et al. Whole health use and interest across veterans with cooccurring chronic pain and PTSD: an examination of the 18 VA medical center flagship sites. Glob Adv Health Med. 2022;11:21649561211065374. doi:10.1177/21649561211065374
- Etingen B, Smith BM, Zeliadt SB, et al. VHA whole health services and complementary and integrative health therapies: a gateway to evidence-based mental health treatment. J Gen Intern Med. 2023;38(14):3144-3151. doi:10.1007/s11606-023-08296-z
- Johnson EM, Possemato K, Khan S, Chinman M, Maisto SA. Engagement, experience, and satisfaction with peerdelivered whole health coaching for veterans with PTSD: a mixed methods process evaluation. Psychol Serv. 2021;19(2):305-316. doi:10.1037/ser0000529
- Purcell N, Zamora K, Gibson C, et al. Patient experiences with integrated pain care: a qualitative evaluation of one VA’s biopsychosocial approach to chronic pain treatment and opioid safety. Glob Adv Health Med. 2019;8:2164956119838845. doi:10.1177/2164956119838845
- Will KK, Johnson ML, Lamb G. Team-based care and patient satisfaction in the hospital setting: a systematic review. J Patient Cent Res Rev. 2019;6(2):158-171. doi:10.17294/2330-0698.1695
- van Dongen JJJ, Habets IGJ, Beurskens A, van Bokhoven MA. Successful participation of patients in interprofessional team meetings: a qualitative study. Health Expect. 2017;20(4):724-733. doi:10.1111/hex.12511
- Oliver DP, Albright DL, Kruse RL, Wittenberg-Lyles E, Washington K, Demiris G. Caregiver evaluation of the ACTIVE intervention: “it was like we were sitting at the table with everyone.” Am J Hosp Palliat Care. 2014;31(4):444-453. doi:10.1177/1049909113490823
- Ansmann L, Heuser C, Diekmann A, et al. Patient participation in multidisciplinary tumor conferences: how is it implemented? What is the patients’ role? What are patients’ experiences? Cancer Med. 2021;10(19):6714-6724. doi:10.1002/cam4.4213
- US Department of Veterans Affairs, Veterans Health Administration. Updated March 20, 2023. Accessed June 11, 2024. https://www.va.gov/health/priorities/index.asp
- Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res (Lausanne). 2023;4:1223172. doi:10.3389/fpain.2023.1223172
- Kligler B. Whole health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Howe RJ, Poulin LM, Federman DG. The personal health inventory: current use, perceived barriers, and benefits. Fed Pract. 2017;34(5):23-26. doi:10.1177/2164957X221077214
- Hicks N, Harden S, Oursler KA, Courtney RE. Determining the representativeness of participants in a whole health interdisciplinary chronic pain program (PREVAIL) in a VA medical center: who did we reach? Presented at: PAINWeek 2022; September 6-9, 2022; Las Vegas, Nevada. Accessed September 10, 2024. https://www.tandfonline.com/doi/full/10.1080/00325481.2022.2116839
- Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. SAGE Publications; 2018.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual in Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, 3rd edition. Published September 26, 2012. Accessed June 11, 2024. https://www.nice.org.uk/process/pmg4/chapter/introduction
- Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient-physician role relationships and patient activation among individuals with chronic illness. Health Serv Res. 2012;47(3 PART 1):1201-1223. doi:10.1111/j.1475-6773.2011.01354.x
- Fu Y, Yu G, McNichol E, Marczewski K, Closs SJ. The association between patient-professional partnerships and self-management of chronic back pain: a mixed methods study. Eur J Pain. 2018;22(7):1229-1244. doi:10.1002/ejp.1210
- Nicholas MK, Asghari A, Blyth FM, et al. Self-management intervention for chronic pain in older adults: a randomised controlled trial. Pain. 2013;154(6):824-835. doi:10.1016/j.pain.2013.02.009
- Nøst TH, Steinsbekk A, Bratås O, Grønning K. Twelvemonth effect of chronic pain self-management intervention delivered in an easily accessible primary healthcare service - a randomised controlled trial. BMC Health Serv Res. 2018;18(1):1012. doi:10.1186/s12913-018-3843-x
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Selfmanagement of chronic pain: a population-based study. Pain. 2005;113(3):285-292. doi:10.1016/j.pain.2004.12.004
- Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(7):1070-1078. doi:10.1002/ejp.830
- Murphy JL, Palyo SA, Schmidt ZS, et al. The resurrection of interdisciplinary pain rehabilitation: outcomes across a veterans affairs collaborative. Pain Med. 2021;22(2):430- 443. doi:10.1093/pm/pnaa417
- Brain K, Burrows TL, Bruggink L, et al. Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med. 2021;10(21):5203. doi:10.3390/jcm10215203
- Field R, Pourkazemi F, Turton J, Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Med). 2021;22(3):694-714. doi:10.1093/pm/pnaa378
- Bjørklund G, Aaseth J, Do§a MD, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153-165. doi:10.1016/j.nut.2019.04.007
- Kaushik AS, Strath LJ, Sorge RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9(2):487-498. doi:10.1007/s40122-020-00200-5
- Boswell JF, Hepner KA, Lysell K, et al. The need for a measurement-based care professional practice guideline. Psychotherapy (Chic). 2023;60(1):1-16. doi:10.1037/pst0000439
- Lund BC, Ohl ME, Hadlandsmyth K, Mosher HJ. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894- 900. doi:10.1093/milmed/usz104
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated May 14, 2024. Accessed June 11, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- McCarthy JF, Blow FC, Ignacio R V., Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs health system: rural-urban differences in rates, risks, and methods. Am J Public Health. 2012;102 Suppl 1(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults - United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385. doi:10.15585/mmwr.mm7215a1
- Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Mackey SC, Pearl RG. Pain management: optimizing patient care through comprehensive, interdisciplinary models and continuous innovations. Anesthesiol Clin. 2023;41(2):xv-xvii. doi:10.1016/j.anclin.2023.03.011
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130. doi:10.1037/a0035514
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the veterans administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi:10.1136/bmj.h444
- Waterschoot FPC, Dijkstra PU, Hollak N, De Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155(1):179-189. doi:10.1016/j.pain.2013.10.006
- Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678. doi:10.1093/rheumatology/ken021
- Elbers S, Wittink H, Konings S, et al. Longitudinal outcome evaluations of interdisciplinary multimodal pain Treatment programmes for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2022;26(2):310-335. doi:10.1002/ejp.1875
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337-345. doi:10.1016/j.pain.2003.08.001
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi:10.1016/j.pain.2004.09.012
- Kligler B, Hyde J, Gantt C, Bokhour B. The whole health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?”. Med Care. 2022;60(5):387-391. doi:10.1097/mlr.0000000000001706
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Transforming Health Care to Create Whole Health: Strategies to Assess, Scale, and Spread the Whole Person Approach to Health, Meisnere M, SouthPaul J, Krist AH, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. National Academies Press (US); February 15, 2023.
- The time Is now for a whole-person health approach to public health. Public Health Rep. 2023;138(4):561-564. doi:10.1177/00333549231154583
- Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12 Suppl 5):S5-S8. doi:10.1097/mlr.0000000000000226
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the whole health system of care. Health Serv Res. 2022;57 Suppl 1(Suppl 1):53-65. doi:10.1111/1475-6773.13938
- Zeliadt SB, Douglas JH, Gelman H, et al. Effectiveness of a whole health model of care emphasizing complementary and integrative health on reducing opioid use among patients with chronic pain. BMC Health Serv Res. 2022;22(1):1053. doi:10.1186/s12913-022-08388-2
- Reed DE 2nd, Bokhour BG, Gaj L, et al. Whole health use and interest across veterans with cooccurring chronic pain and PTSD: an examination of the 18 VA medical center flagship sites. Glob Adv Health Med. 2022;11:21649561211065374. doi:10.1177/21649561211065374
- Etingen B, Smith BM, Zeliadt SB, et al. VHA whole health services and complementary and integrative health therapies: a gateway to evidence-based mental health treatment. J Gen Intern Med. 2023;38(14):3144-3151. doi:10.1007/s11606-023-08296-z
- Johnson EM, Possemato K, Khan S, Chinman M, Maisto SA. Engagement, experience, and satisfaction with peerdelivered whole health coaching for veterans with PTSD: a mixed methods process evaluation. Psychol Serv. 2021;19(2):305-316. doi:10.1037/ser0000529
- Purcell N, Zamora K, Gibson C, et al. Patient experiences with integrated pain care: a qualitative evaluation of one VA’s biopsychosocial approach to chronic pain treatment and opioid safety. Glob Adv Health Med. 2019;8:2164956119838845. doi:10.1177/2164956119838845
- Will KK, Johnson ML, Lamb G. Team-based care and patient satisfaction in the hospital setting: a systematic review. J Patient Cent Res Rev. 2019;6(2):158-171. doi:10.17294/2330-0698.1695
- van Dongen JJJ, Habets IGJ, Beurskens A, van Bokhoven MA. Successful participation of patients in interprofessional team meetings: a qualitative study. Health Expect. 2017;20(4):724-733. doi:10.1111/hex.12511
- Oliver DP, Albright DL, Kruse RL, Wittenberg-Lyles E, Washington K, Demiris G. Caregiver evaluation of the ACTIVE intervention: “it was like we were sitting at the table with everyone.” Am J Hosp Palliat Care. 2014;31(4):444-453. doi:10.1177/1049909113490823
- Ansmann L, Heuser C, Diekmann A, et al. Patient participation in multidisciplinary tumor conferences: how is it implemented? What is the patients’ role? What are patients’ experiences? Cancer Med. 2021;10(19):6714-6724. doi:10.1002/cam4.4213
- US Department of Veterans Affairs, Veterans Health Administration. Updated March 20, 2023. Accessed June 11, 2024. https://www.va.gov/health/priorities/index.asp
- Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res (Lausanne). 2023;4:1223172. doi:10.3389/fpain.2023.1223172
- Kligler B. Whole health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Howe RJ, Poulin LM, Federman DG. The personal health inventory: current use, perceived barriers, and benefits. Fed Pract. 2017;34(5):23-26. doi:10.1177/2164957X221077214
- Hicks N, Harden S, Oursler KA, Courtney RE. Determining the representativeness of participants in a whole health interdisciplinary chronic pain program (PREVAIL) in a VA medical center: who did we reach? Presented at: PAINWeek 2022; September 6-9, 2022; Las Vegas, Nevada. Accessed September 10, 2024. https://www.tandfonline.com/doi/full/10.1080/00325481.2022.2116839
- Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. SAGE Publications; 2018.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual in Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, 3rd edition. Published September 26, 2012. Accessed June 11, 2024. https://www.nice.org.uk/process/pmg4/chapter/introduction
- Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient-physician role relationships and patient activation among individuals with chronic illness. Health Serv Res. 2012;47(3 PART 1):1201-1223. doi:10.1111/j.1475-6773.2011.01354.x
- Fu Y, Yu G, McNichol E, Marczewski K, Closs SJ. The association between patient-professional partnerships and self-management of chronic back pain: a mixed methods study. Eur J Pain. 2018;22(7):1229-1244. doi:10.1002/ejp.1210
- Nicholas MK, Asghari A, Blyth FM, et al. Self-management intervention for chronic pain in older adults: a randomised controlled trial. Pain. 2013;154(6):824-835. doi:10.1016/j.pain.2013.02.009
- Nøst TH, Steinsbekk A, Bratås O, Grønning K. Twelvemonth effect of chronic pain self-management intervention delivered in an easily accessible primary healthcare service - a randomised controlled trial. BMC Health Serv Res. 2018;18(1):1012. doi:10.1186/s12913-018-3843-x
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Selfmanagement of chronic pain: a population-based study. Pain. 2005;113(3):285-292. doi:10.1016/j.pain.2004.12.004
- Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(7):1070-1078. doi:10.1002/ejp.830
- Murphy JL, Palyo SA, Schmidt ZS, et al. The resurrection of interdisciplinary pain rehabilitation: outcomes across a veterans affairs collaborative. Pain Med. 2021;22(2):430- 443. doi:10.1093/pm/pnaa417
- Brain K, Burrows TL, Bruggink L, et al. Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med. 2021;10(21):5203. doi:10.3390/jcm10215203
- Field R, Pourkazemi F, Turton J, Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Med). 2021;22(3):694-714. doi:10.1093/pm/pnaa378
- Bjørklund G, Aaseth J, Do§a MD, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153-165. doi:10.1016/j.nut.2019.04.007
- Kaushik AS, Strath LJ, Sorge RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9(2):487-498. doi:10.1007/s40122-020-00200-5
- Boswell JF, Hepner KA, Lysell K, et al. The need for a measurement-based care professional practice guideline. Psychotherapy (Chic). 2023;60(1):1-16. doi:10.1037/pst0000439
- Lund BC, Ohl ME, Hadlandsmyth K, Mosher HJ. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894- 900. doi:10.1093/milmed/usz104
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated May 14, 2024. Accessed June 11, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- McCarthy JF, Blow FC, Ignacio R V., Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs health system: rural-urban differences in rates, risks, and methods. Am J Public Health. 2012;102 Suppl 1(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
High-Fiber Diet Linked to Improved Stem Cell Transplant, GvHD Outcomes
Importantly, the findings suggest standard recommendations for patients of a low-fiber diet following allo-HCT may run counter to the potential benefits.
“Significant decrease of fiber intake during transplantation is detrimental. It’s a lost opportunity to promote a healthy gut microbiome, recover from treatment-related microbiota injury, and protect against GVHD,” first author Jenny Paredes, PhD, a staff scientist at City of Hope National Medical Center in Duarte, California, said in a press statement for the study presented at the American Society of Hematology (ASH) 2024 Annual Meeting.
Although the health benefits of dietary fiber on the gut microbiome are well-documented, the effects have recently been shown to extend to outcomes after allo-HCT in general, with researchers finding increased overall survival when there is higher diversity in the gut microbiome, including a higher abundance of butyrate producers and lower abundance of enterococcus, explained Paredes when presenting the findings.
Acute GvHD, a common and potentially life-threatening complication of allo-HCT, can have symptoms that mimic irritable bowel disease (IBD), including abdominal pain or cramps, nausea, vomiting, and diarrhea. The low-fiber diet recommendations, including avoidance of raw vegetables and fruits before and after the allo-HCT procedure, are designed to counter those effects, as well as reduce exposure to bacteria.
However, with data suggesting the potential benefits of dietary fiber could extend to the prevention of GvHD, Paredes and colleagues further investigated.
For the observational study, they evaluated all dietary data on 173 allo-HCT recipients at Memorial Sloan Kettering Cancer Center (MSKCC) from 10 days prior to transplantation to 30 days post-transplantation, representing 3837 patient-days in total.
Data collected from the patients also included rRNA sequencing of fecal samples and fecal short-chain fatty acid concentration.
Participants had a median age of 60, and 45% were female. The most common diseases being treated were leukemia (50%), myelodysplastic syndrome (25%), and non-Hodgkin’s lymphoma (8.7%).
After stratifying patients based on high- or low-fiber intake, those with high-fiber intake were found to have significantly higher rates of microbial α-diversity (P = .009), a higher abundance of butyrate producers (P = .03), and a higher concentration of butyrate (P = .02), a short-chain fatty acid that plays a key role in gut health.
Furthermore, the high-fiber group had significantly higher overall survival in an analysis extending to 24 months relative to day 12 of the study (P = .04).
Focusing on GvHD outcomes, the authors further evaluated data on 101 non-T-cell–depleted patients, and identified 29 patients without GvHD and 24 who developed lower gastrointestinal (GI) GvHD.
Patients with lower GI GvHD had significantly lower fecal concentrations of butyrate (P = .03) and acetate (P = .02).
However, patients among those in the high-fiber intake group had a significantly lower cumulative incidence of developing GvHD at day 100 (P = .034) and a lower incidence of lower GI GvHD (P = .04).
A separate preclinical analysis of a mouse model with GvHD further showed that a fiber-rich diet (12% cellulose) significantly increased the expression of genes associated with reduced GvHD, including IDO1 and CEACAM1, and those associated with enrichment of the bile acid pathway.
The findings suggest an opportunity to improve outcomes with relatively small dietary changes, Paredes said.
“Strategies to increase the fiber concentration in these diets paired with the safety that these patients need is what makes this study exciting,” she said in an interview.
“Increasing the fiber intake by 10 to 20 grams/day could potentially increase the microbiome diversity and abundance of butyrate producers, which have been correlated with higher overall survival rates post allo-HCT,” she continued.
“[For instance], that could be an avocado per day, or it could be a small salad per day, or a small vegetable soup per day,” she added. “I would encourage institutions to re-evaluate their menu planning and see how to include more fiber into the meals in a safe way.”
Ultimately, “I think that a dietary intervention outweighs the risks of a pharmacological intervention,” Paredes added.
The necessary duration of a high-fiber diet to produce the beneficial effects on allo-HCT outcomes would likely be over the course of the pre- and post-transplant periods, Paredes added.
“With the survival analysis extending from 5 days before transplantation to 12 days post, we are looking at an intervention that potentially could be around 20 days,” she said.
“We would love to take advantage of the pretransplantation window, in particular, and we can see that just increasing the fiber intake by about 20 grams during this window was shown to improve overall survival after 24 months,” Paredes added.
Importantly, however, some patients may not be appropriate for high-fiber dietary changes, Paredes cautioned.
“Patients that have developed IBD-like symptoms and severe GvHD patients, for example, or with lower GI-GvHD grades 3 and 4 would be not appropriate candidates for a high-fiber diet,” she said.
High-Fiber Diet Slows MM Disease Progression?
The potential important benefits of a high-fiber diet in blood diseases were further demonstrated in a separate study also by MSKCC researchers presented at the meeting, which showed encouraging signs that a plant-based diet rich in fiber could potentially slow disease progression in multiple myeloma (MM).
NUTRIVENTION included 20 patients with the two precancerous MM conditions, monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM), which can last for years without progressing to MM and which researchers have speculated could be a potential opportunity to intervene to prevent progression to cancer.
Patients were provided with a 12-week controlled diet plus health coaching for another 3 months; no meals or coaching were provided for the rest of the 1-year study period. Participants had a median age of 62 and, with being overweight/obesity a risk factor for MM, had a body mass index (BMI) of 25 kg/m2 or higher.
The trial met its endpoint of feasibility, with 91% adherence in the first 3 months. The rate of consumption of unprocessed plant foods increased from 20% at baseline to 92% on the intervention. Overall adherence was 58%. Insulin and anti-inflammatory markers also improved and, despite no calorie restriction, there was a 7% sustained reduction in BMI.
Notably, two patients in the study had stabilization of disease progression.
“We saw improvements in all spheres, including metabolism, microbiome, and immune system markers, and we also saw that two patients with progressive disease had the progression stabilize and slow down on the intervention,” principal investigator Urvi A. Shah, MD, said in a press statement.
“Even though it’s just two cases, to our knowledge, it has not been shown before in an intervention setting that you can improve diet and lifestyle and actually slow or change the trajectory of the disease,” she noted.
The researchers caution that findings in mice do not necessarily translate to humans but note another experiment in mice with SMM that showed animals fed a normal diet had progression to MM after a median of 12 weeks, compared with a median of 30 weeks among those fed a high-fiber diet.
Notably, all mice in the normal-diet group progressed to MM, whereas 40% of mice in the high-fiber group did not.
“We found that a high-fiber plant-based diet can improve BMI, improve insulin resistance [and] the microbiome through diversity and butyrate producers, and with the production of short-chain fatty acids, can have effects on inflammation, immunity, innate and adaptive antitumor immunity, and tumor cells or plasma cells,” Shah said during her presentation.
The study was supported by funding from the National Cancer Institute and private foundations. Paredes has reported no relevant financial relationships. Shah has reported relationships with Sanofi, Bristol Myers Squibb, and Janssen.
A version of this article first appeared on Medscape.com.
Importantly, the findings suggest standard recommendations for patients of a low-fiber diet following allo-HCT may run counter to the potential benefits.
“Significant decrease of fiber intake during transplantation is detrimental. It’s a lost opportunity to promote a healthy gut microbiome, recover from treatment-related microbiota injury, and protect against GVHD,” first author Jenny Paredes, PhD, a staff scientist at City of Hope National Medical Center in Duarte, California, said in a press statement for the study presented at the American Society of Hematology (ASH) 2024 Annual Meeting.
Although the health benefits of dietary fiber on the gut microbiome are well-documented, the effects have recently been shown to extend to outcomes after allo-HCT in general, with researchers finding increased overall survival when there is higher diversity in the gut microbiome, including a higher abundance of butyrate producers and lower abundance of enterococcus, explained Paredes when presenting the findings.
Acute GvHD, a common and potentially life-threatening complication of allo-HCT, can have symptoms that mimic irritable bowel disease (IBD), including abdominal pain or cramps, nausea, vomiting, and diarrhea. The low-fiber diet recommendations, including avoidance of raw vegetables and fruits before and after the allo-HCT procedure, are designed to counter those effects, as well as reduce exposure to bacteria.
However, with data suggesting the potential benefits of dietary fiber could extend to the prevention of GvHD, Paredes and colleagues further investigated.
For the observational study, they evaluated all dietary data on 173 allo-HCT recipients at Memorial Sloan Kettering Cancer Center (MSKCC) from 10 days prior to transplantation to 30 days post-transplantation, representing 3837 patient-days in total.
Data collected from the patients also included rRNA sequencing of fecal samples and fecal short-chain fatty acid concentration.
Participants had a median age of 60, and 45% were female. The most common diseases being treated were leukemia (50%), myelodysplastic syndrome (25%), and non-Hodgkin’s lymphoma (8.7%).
After stratifying patients based on high- or low-fiber intake, those with high-fiber intake were found to have significantly higher rates of microbial α-diversity (P = .009), a higher abundance of butyrate producers (P = .03), and a higher concentration of butyrate (P = .02), a short-chain fatty acid that plays a key role in gut health.
Furthermore, the high-fiber group had significantly higher overall survival in an analysis extending to 24 months relative to day 12 of the study (P = .04).
Focusing on GvHD outcomes, the authors further evaluated data on 101 non-T-cell–depleted patients, and identified 29 patients without GvHD and 24 who developed lower gastrointestinal (GI) GvHD.
Patients with lower GI GvHD had significantly lower fecal concentrations of butyrate (P = .03) and acetate (P = .02).
However, patients among those in the high-fiber intake group had a significantly lower cumulative incidence of developing GvHD at day 100 (P = .034) and a lower incidence of lower GI GvHD (P = .04).
A separate preclinical analysis of a mouse model with GvHD further showed that a fiber-rich diet (12% cellulose) significantly increased the expression of genes associated with reduced GvHD, including IDO1 and CEACAM1, and those associated with enrichment of the bile acid pathway.
The findings suggest an opportunity to improve outcomes with relatively small dietary changes, Paredes said.
“Strategies to increase the fiber concentration in these diets paired with the safety that these patients need is what makes this study exciting,” she said in an interview.
“Increasing the fiber intake by 10 to 20 grams/day could potentially increase the microbiome diversity and abundance of butyrate producers, which have been correlated with higher overall survival rates post allo-HCT,” she continued.
“[For instance], that could be an avocado per day, or it could be a small salad per day, or a small vegetable soup per day,” she added. “I would encourage institutions to re-evaluate their menu planning and see how to include more fiber into the meals in a safe way.”
Ultimately, “I think that a dietary intervention outweighs the risks of a pharmacological intervention,” Paredes added.
The necessary duration of a high-fiber diet to produce the beneficial effects on allo-HCT outcomes would likely be over the course of the pre- and post-transplant periods, Paredes added.
“With the survival analysis extending from 5 days before transplantation to 12 days post, we are looking at an intervention that potentially could be around 20 days,” she said.
“We would love to take advantage of the pretransplantation window, in particular, and we can see that just increasing the fiber intake by about 20 grams during this window was shown to improve overall survival after 24 months,” Paredes added.
Importantly, however, some patients may not be appropriate for high-fiber dietary changes, Paredes cautioned.
“Patients that have developed IBD-like symptoms and severe GvHD patients, for example, or with lower GI-GvHD grades 3 and 4 would be not appropriate candidates for a high-fiber diet,” she said.
High-Fiber Diet Slows MM Disease Progression?
The potential important benefits of a high-fiber diet in blood diseases were further demonstrated in a separate study also by MSKCC researchers presented at the meeting, which showed encouraging signs that a plant-based diet rich in fiber could potentially slow disease progression in multiple myeloma (MM).
NUTRIVENTION included 20 patients with the two precancerous MM conditions, monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM), which can last for years without progressing to MM and which researchers have speculated could be a potential opportunity to intervene to prevent progression to cancer.
Patients were provided with a 12-week controlled diet plus health coaching for another 3 months; no meals or coaching were provided for the rest of the 1-year study period. Participants had a median age of 62 and, with being overweight/obesity a risk factor for MM, had a body mass index (BMI) of 25 kg/m2 or higher.
The trial met its endpoint of feasibility, with 91% adherence in the first 3 months. The rate of consumption of unprocessed plant foods increased from 20% at baseline to 92% on the intervention. Overall adherence was 58%. Insulin and anti-inflammatory markers also improved and, despite no calorie restriction, there was a 7% sustained reduction in BMI.
Notably, two patients in the study had stabilization of disease progression.
“We saw improvements in all spheres, including metabolism, microbiome, and immune system markers, and we also saw that two patients with progressive disease had the progression stabilize and slow down on the intervention,” principal investigator Urvi A. Shah, MD, said in a press statement.
“Even though it’s just two cases, to our knowledge, it has not been shown before in an intervention setting that you can improve diet and lifestyle and actually slow or change the trajectory of the disease,” she noted.
The researchers caution that findings in mice do not necessarily translate to humans but note another experiment in mice with SMM that showed animals fed a normal diet had progression to MM after a median of 12 weeks, compared with a median of 30 weeks among those fed a high-fiber diet.
Notably, all mice in the normal-diet group progressed to MM, whereas 40% of mice in the high-fiber group did not.
“We found that a high-fiber plant-based diet can improve BMI, improve insulin resistance [and] the microbiome through diversity and butyrate producers, and with the production of short-chain fatty acids, can have effects on inflammation, immunity, innate and adaptive antitumor immunity, and tumor cells or plasma cells,” Shah said during her presentation.
The study was supported by funding from the National Cancer Institute and private foundations. Paredes has reported no relevant financial relationships. Shah has reported relationships with Sanofi, Bristol Myers Squibb, and Janssen.
A version of this article first appeared on Medscape.com.
Importantly, the findings suggest standard recommendations for patients of a low-fiber diet following allo-HCT may run counter to the potential benefits.
“Significant decrease of fiber intake during transplantation is detrimental. It’s a lost opportunity to promote a healthy gut microbiome, recover from treatment-related microbiota injury, and protect against GVHD,” first author Jenny Paredes, PhD, a staff scientist at City of Hope National Medical Center in Duarte, California, said in a press statement for the study presented at the American Society of Hematology (ASH) 2024 Annual Meeting.
Although the health benefits of dietary fiber on the gut microbiome are well-documented, the effects have recently been shown to extend to outcomes after allo-HCT in general, with researchers finding increased overall survival when there is higher diversity in the gut microbiome, including a higher abundance of butyrate producers and lower abundance of enterococcus, explained Paredes when presenting the findings.
Acute GvHD, a common and potentially life-threatening complication of allo-HCT, can have symptoms that mimic irritable bowel disease (IBD), including abdominal pain or cramps, nausea, vomiting, and diarrhea. The low-fiber diet recommendations, including avoidance of raw vegetables and fruits before and after the allo-HCT procedure, are designed to counter those effects, as well as reduce exposure to bacteria.
However, with data suggesting the potential benefits of dietary fiber could extend to the prevention of GvHD, Paredes and colleagues further investigated.
For the observational study, they evaluated all dietary data on 173 allo-HCT recipients at Memorial Sloan Kettering Cancer Center (MSKCC) from 10 days prior to transplantation to 30 days post-transplantation, representing 3837 patient-days in total.
Data collected from the patients also included rRNA sequencing of fecal samples and fecal short-chain fatty acid concentration.
Participants had a median age of 60, and 45% were female. The most common diseases being treated were leukemia (50%), myelodysplastic syndrome (25%), and non-Hodgkin’s lymphoma (8.7%).
After stratifying patients based on high- or low-fiber intake, those with high-fiber intake were found to have significantly higher rates of microbial α-diversity (P = .009), a higher abundance of butyrate producers (P = .03), and a higher concentration of butyrate (P = .02), a short-chain fatty acid that plays a key role in gut health.
Furthermore, the high-fiber group had significantly higher overall survival in an analysis extending to 24 months relative to day 12 of the study (P = .04).
Focusing on GvHD outcomes, the authors further evaluated data on 101 non-T-cell–depleted patients, and identified 29 patients without GvHD and 24 who developed lower gastrointestinal (GI) GvHD.
Patients with lower GI GvHD had significantly lower fecal concentrations of butyrate (P = .03) and acetate (P = .02).
However, patients among those in the high-fiber intake group had a significantly lower cumulative incidence of developing GvHD at day 100 (P = .034) and a lower incidence of lower GI GvHD (P = .04).
A separate preclinical analysis of a mouse model with GvHD further showed that a fiber-rich diet (12% cellulose) significantly increased the expression of genes associated with reduced GvHD, including IDO1 and CEACAM1, and those associated with enrichment of the bile acid pathway.
The findings suggest an opportunity to improve outcomes with relatively small dietary changes, Paredes said.
“Strategies to increase the fiber concentration in these diets paired with the safety that these patients need is what makes this study exciting,” she said in an interview.
“Increasing the fiber intake by 10 to 20 grams/day could potentially increase the microbiome diversity and abundance of butyrate producers, which have been correlated with higher overall survival rates post allo-HCT,” she continued.
“[For instance], that could be an avocado per day, or it could be a small salad per day, or a small vegetable soup per day,” she added. “I would encourage institutions to re-evaluate their menu planning and see how to include more fiber into the meals in a safe way.”
Ultimately, “I think that a dietary intervention outweighs the risks of a pharmacological intervention,” Paredes added.
The necessary duration of a high-fiber diet to produce the beneficial effects on allo-HCT outcomes would likely be over the course of the pre- and post-transplant periods, Paredes added.
“With the survival analysis extending from 5 days before transplantation to 12 days post, we are looking at an intervention that potentially could be around 20 days,” she said.
“We would love to take advantage of the pretransplantation window, in particular, and we can see that just increasing the fiber intake by about 20 grams during this window was shown to improve overall survival after 24 months,” Paredes added.
Importantly, however, some patients may not be appropriate for high-fiber dietary changes, Paredes cautioned.
“Patients that have developed IBD-like symptoms and severe GvHD patients, for example, or with lower GI-GvHD grades 3 and 4 would be not appropriate candidates for a high-fiber diet,” she said.
High-Fiber Diet Slows MM Disease Progression?
The potential important benefits of a high-fiber diet in blood diseases were further demonstrated in a separate study also by MSKCC researchers presented at the meeting, which showed encouraging signs that a plant-based diet rich in fiber could potentially slow disease progression in multiple myeloma (MM).
NUTRIVENTION included 20 patients with the two precancerous MM conditions, monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM), which can last for years without progressing to MM and which researchers have speculated could be a potential opportunity to intervene to prevent progression to cancer.
Patients were provided with a 12-week controlled diet plus health coaching for another 3 months; no meals or coaching were provided for the rest of the 1-year study period. Participants had a median age of 62 and, with being overweight/obesity a risk factor for MM, had a body mass index (BMI) of 25 kg/m2 or higher.
The trial met its endpoint of feasibility, with 91% adherence in the first 3 months. The rate of consumption of unprocessed plant foods increased from 20% at baseline to 92% on the intervention. Overall adherence was 58%. Insulin and anti-inflammatory markers also improved and, despite no calorie restriction, there was a 7% sustained reduction in BMI.
Notably, two patients in the study had stabilization of disease progression.
“We saw improvements in all spheres, including metabolism, microbiome, and immune system markers, and we also saw that two patients with progressive disease had the progression stabilize and slow down on the intervention,” principal investigator Urvi A. Shah, MD, said in a press statement.
“Even though it’s just two cases, to our knowledge, it has not been shown before in an intervention setting that you can improve diet and lifestyle and actually slow or change the trajectory of the disease,” she noted.
The researchers caution that findings in mice do not necessarily translate to humans but note another experiment in mice with SMM that showed animals fed a normal diet had progression to MM after a median of 12 weeks, compared with a median of 30 weeks among those fed a high-fiber diet.
Notably, all mice in the normal-diet group progressed to MM, whereas 40% of mice in the high-fiber group did not.
“We found that a high-fiber plant-based diet can improve BMI, improve insulin resistance [and] the microbiome through diversity and butyrate producers, and with the production of short-chain fatty acids, can have effects on inflammation, immunity, innate and adaptive antitumor immunity, and tumor cells or plasma cells,” Shah said during her presentation.
The study was supported by funding from the National Cancer Institute and private foundations. Paredes has reported no relevant financial relationships. Shah has reported relationships with Sanofi, Bristol Myers Squibb, and Janssen.
A version of this article first appeared on Medscape.com.
FROM ASH 2024
24-Hour Urine Testing in Multiple Myeloma: Time to Stop?
Overall, evaluating patients’ responses using urine-free and traditional criteria led to nearly identical assessments. When comparing the two criteria, only 7 of 645 patients evaluated had discordant results.
The findings, presented at the American Society of Hematology (ASH) 2024 Annual Meeting, add weight to the push to drop the requirement to perform routine urine tests from International Myeloma Working Group (IMWG) response criteria for multiple myeloma, said the study’s lead author, Rahul Banerjee, MD, from Fred Hutch Cancer Center, University of Washington School of Medicine, Seattle.
“International guidelines for multiple myeloma, which haven’t been updated in almost a decade, currently recommend these refrigerated 24-hour urine assessments, which are cumbersome for patients and can create substantial disparities,” Banerjee said in an interview.
“The international community is actually in the midst of updating its guidelines (I am part of this effort), and our work will hopefully help lead the way for future guidelines that de-emphasize the need for 24-hour urine testing to only a few rare scenarios, such as AL amyloidosis,” Banerjee added.
Urine tests can help detect the presence of abnormal proteins, which can indicate the level of myeloma tumor burden. Performing these tests routinely can help physicians monitor the effectiveness of patients’ treatment in practice and clinical trials.
Some recent data, however, suggest that dropping urine testing from the response criteria would change the response assessment in fewer than 5% of patients. Still, it’s not clear how urine-free criteria would impact assessments of progression free survival.
In the current study, Banerjee and colleagues performed a secondary analysis of the STaMINA trial. In the original trial, patients were randomized to lenalidomide maintenance, tandem autologous hematopoietic cell transplantation followed by lenalidomide maintenance, or consolidation therapy (lenalidomide, bortezomib, and dexamethasone) followed by lenalidomide maintenance until disease progression.
The secondary analysis included 645 patients from the original trial who were evaluable 56 days following autologous hematopoietic cell transplantation. The analysis looked at patients across all groups, but excluded those with progressive disease, and compared patients’ responses using traditional IMWG criteria, which includes 24-hour urine assessments, and urine-free criteria. Response measurements included complete response, very good partial response, partial response, and stable disease.
Patients were a median age of 56 years, 41% were female, 17% were Black, and 7% were Hispanic; 26% had light-chain only disease. About half (49%) had received lenalidomide alone, 28% had received post-autologous stem cell transplantation consolidation followed by lenalidomide, and 24% had received tandem transplantation followed by lenalidomide.
The analysis showed that “urine-free response criteria worked just fine in terms of their prognostic value,” Banerjee said while presenting the findings.
Specifically, the complete response rate was 29.4% using the traditional criteria vs 29.7% using the urine-free criteria. The very good partial response rate was 37.0% with the traditional approach vs 36.6% with the urine-free approach. The partial response rate was 30.7% for both and the stable disease rate was 3.0% for both.
Achieving a complete response based on the urine-free criteria was highly prognostic for progression-free survival (P = .005) while achieving a very good partial response by either criterion was borderline prognostic for progression-free survival (P = .102).
Only 1.1% of patients — seven patients altogether — had discordant responses between traditional and urine-free response criteria, Banerjee noted. One patient, for instance, was downgraded from a very good partial response with traditional criteria to a partial response with urine-free criteria “because current response criteria rate urine [as] more important than serum-free light chains,” Banerjee explained. Two other patients who met all other stringent criteria for a complete response but still had urine paraprotein at Day 56 were classified as having a very good partial response using traditional criteria but as a complete response with the urine-free criteria.
The other four patients with discordant results were the most important, Banerjee said. These patients were missing urine protein electrophoresis values, which made them non-evaluable using traditional criteria, but became evaluable when using urine-free criteria. “This is, I think, the bane of our existence, right? We ask our patients to put their blood, soul, sweat, and tears into being in a clinical trial, and then they’re not evaluable,” he said.
Overall, these results strongly support the de-emphasis of 24-hour urine requirements in updated IMWG response criteria, said Banerjee. However, he noted, 24-hour urine testing still has a very important place in the screening process and in patients with monoclonal gammopathy of renal significance or AL amyloidosis.
“This study provides reassurance to those of us already not repeating urine tests that urine testing is unnecessary for tracking responses,” said Manni Mohyuddin, MD, from the Multiple Myeloma Program at Huntsman Cancer Institute and assistant professor at the University of Utah, Salt Lake City. “These assessments aren’t done consistently in practice outside of trials anyway, and I hope that this study will lead to a formal change in criteria and the omission of urine assessments in clinical trials.”
Funding for the study was provided by the National Heart, Lung, and Blood Institute; National Cancer Institute; Alliance for Clinical Trials in Oncology; ECOG-ACRIN Cancer Research Group; and SWOG; and contributions were provided by Celgene and Millennium Pharmaceuticals. Banerjee has reported consulting for Adaptive Biotechnologies, Bristol-Myers Squibb, Caribou Biosciences, Genentech, GSK, Johnson & Johnson/Janssen, Karyopharm, Legend Biotech, Pfizer, Sanofi, and SparkCures, and receiving research funding from AbbVie, Johnson & Johnson, Novartis, Pack Health, Prothena, and Sanofi. Mohyuddin has disclosed no personal payments and no consultation for industry. His institution has received research funding from Janssen for his role as a principal investigator on a trial.
A version of this article first appeared on Medscape.com.
Overall, evaluating patients’ responses using urine-free and traditional criteria led to nearly identical assessments. When comparing the two criteria, only 7 of 645 patients evaluated had discordant results.
The findings, presented at the American Society of Hematology (ASH) 2024 Annual Meeting, add weight to the push to drop the requirement to perform routine urine tests from International Myeloma Working Group (IMWG) response criteria for multiple myeloma, said the study’s lead author, Rahul Banerjee, MD, from Fred Hutch Cancer Center, University of Washington School of Medicine, Seattle.
“International guidelines for multiple myeloma, which haven’t been updated in almost a decade, currently recommend these refrigerated 24-hour urine assessments, which are cumbersome for patients and can create substantial disparities,” Banerjee said in an interview.
“The international community is actually in the midst of updating its guidelines (I am part of this effort), and our work will hopefully help lead the way for future guidelines that de-emphasize the need for 24-hour urine testing to only a few rare scenarios, such as AL amyloidosis,” Banerjee added.
Urine tests can help detect the presence of abnormal proteins, which can indicate the level of myeloma tumor burden. Performing these tests routinely can help physicians monitor the effectiveness of patients’ treatment in practice and clinical trials.
Some recent data, however, suggest that dropping urine testing from the response criteria would change the response assessment in fewer than 5% of patients. Still, it’s not clear how urine-free criteria would impact assessments of progression free survival.
In the current study, Banerjee and colleagues performed a secondary analysis of the STaMINA trial. In the original trial, patients were randomized to lenalidomide maintenance, tandem autologous hematopoietic cell transplantation followed by lenalidomide maintenance, or consolidation therapy (lenalidomide, bortezomib, and dexamethasone) followed by lenalidomide maintenance until disease progression.
The secondary analysis included 645 patients from the original trial who were evaluable 56 days following autologous hematopoietic cell transplantation. The analysis looked at patients across all groups, but excluded those with progressive disease, and compared patients’ responses using traditional IMWG criteria, which includes 24-hour urine assessments, and urine-free criteria. Response measurements included complete response, very good partial response, partial response, and stable disease.
Patients were a median age of 56 years, 41% were female, 17% were Black, and 7% were Hispanic; 26% had light-chain only disease. About half (49%) had received lenalidomide alone, 28% had received post-autologous stem cell transplantation consolidation followed by lenalidomide, and 24% had received tandem transplantation followed by lenalidomide.
The analysis showed that “urine-free response criteria worked just fine in terms of their prognostic value,” Banerjee said while presenting the findings.
Specifically, the complete response rate was 29.4% using the traditional criteria vs 29.7% using the urine-free criteria. The very good partial response rate was 37.0% with the traditional approach vs 36.6% with the urine-free approach. The partial response rate was 30.7% for both and the stable disease rate was 3.0% for both.
Achieving a complete response based on the urine-free criteria was highly prognostic for progression-free survival (P = .005) while achieving a very good partial response by either criterion was borderline prognostic for progression-free survival (P = .102).
Only 1.1% of patients — seven patients altogether — had discordant responses between traditional and urine-free response criteria, Banerjee noted. One patient, for instance, was downgraded from a very good partial response with traditional criteria to a partial response with urine-free criteria “because current response criteria rate urine [as] more important than serum-free light chains,” Banerjee explained. Two other patients who met all other stringent criteria for a complete response but still had urine paraprotein at Day 56 were classified as having a very good partial response using traditional criteria but as a complete response with the urine-free criteria.
The other four patients with discordant results were the most important, Banerjee said. These patients were missing urine protein electrophoresis values, which made them non-evaluable using traditional criteria, but became evaluable when using urine-free criteria. “This is, I think, the bane of our existence, right? We ask our patients to put their blood, soul, sweat, and tears into being in a clinical trial, and then they’re not evaluable,” he said.
Overall, these results strongly support the de-emphasis of 24-hour urine requirements in updated IMWG response criteria, said Banerjee. However, he noted, 24-hour urine testing still has a very important place in the screening process and in patients with monoclonal gammopathy of renal significance or AL amyloidosis.
“This study provides reassurance to those of us already not repeating urine tests that urine testing is unnecessary for tracking responses,” said Manni Mohyuddin, MD, from the Multiple Myeloma Program at Huntsman Cancer Institute and assistant professor at the University of Utah, Salt Lake City. “These assessments aren’t done consistently in practice outside of trials anyway, and I hope that this study will lead to a formal change in criteria and the omission of urine assessments in clinical trials.”
Funding for the study was provided by the National Heart, Lung, and Blood Institute; National Cancer Institute; Alliance for Clinical Trials in Oncology; ECOG-ACRIN Cancer Research Group; and SWOG; and contributions were provided by Celgene and Millennium Pharmaceuticals. Banerjee has reported consulting for Adaptive Biotechnologies, Bristol-Myers Squibb, Caribou Biosciences, Genentech, GSK, Johnson & Johnson/Janssen, Karyopharm, Legend Biotech, Pfizer, Sanofi, and SparkCures, and receiving research funding from AbbVie, Johnson & Johnson, Novartis, Pack Health, Prothena, and Sanofi. Mohyuddin has disclosed no personal payments and no consultation for industry. His institution has received research funding from Janssen for his role as a principal investigator on a trial.
A version of this article first appeared on Medscape.com.
Overall, evaluating patients’ responses using urine-free and traditional criteria led to nearly identical assessments. When comparing the two criteria, only 7 of 645 patients evaluated had discordant results.
The findings, presented at the American Society of Hematology (ASH) 2024 Annual Meeting, add weight to the push to drop the requirement to perform routine urine tests from International Myeloma Working Group (IMWG) response criteria for multiple myeloma, said the study’s lead author, Rahul Banerjee, MD, from Fred Hutch Cancer Center, University of Washington School of Medicine, Seattle.
“International guidelines for multiple myeloma, which haven’t been updated in almost a decade, currently recommend these refrigerated 24-hour urine assessments, which are cumbersome for patients and can create substantial disparities,” Banerjee said in an interview.
“The international community is actually in the midst of updating its guidelines (I am part of this effort), and our work will hopefully help lead the way for future guidelines that de-emphasize the need for 24-hour urine testing to only a few rare scenarios, such as AL amyloidosis,” Banerjee added.
Urine tests can help detect the presence of abnormal proteins, which can indicate the level of myeloma tumor burden. Performing these tests routinely can help physicians monitor the effectiveness of patients’ treatment in practice and clinical trials.
Some recent data, however, suggest that dropping urine testing from the response criteria would change the response assessment in fewer than 5% of patients. Still, it’s not clear how urine-free criteria would impact assessments of progression free survival.
In the current study, Banerjee and colleagues performed a secondary analysis of the STaMINA trial. In the original trial, patients were randomized to lenalidomide maintenance, tandem autologous hematopoietic cell transplantation followed by lenalidomide maintenance, or consolidation therapy (lenalidomide, bortezomib, and dexamethasone) followed by lenalidomide maintenance until disease progression.
The secondary analysis included 645 patients from the original trial who were evaluable 56 days following autologous hematopoietic cell transplantation. The analysis looked at patients across all groups, but excluded those with progressive disease, and compared patients’ responses using traditional IMWG criteria, which includes 24-hour urine assessments, and urine-free criteria. Response measurements included complete response, very good partial response, partial response, and stable disease.
Patients were a median age of 56 years, 41% were female, 17% were Black, and 7% were Hispanic; 26% had light-chain only disease. About half (49%) had received lenalidomide alone, 28% had received post-autologous stem cell transplantation consolidation followed by lenalidomide, and 24% had received tandem transplantation followed by lenalidomide.
The analysis showed that “urine-free response criteria worked just fine in terms of their prognostic value,” Banerjee said while presenting the findings.
Specifically, the complete response rate was 29.4% using the traditional criteria vs 29.7% using the urine-free criteria. The very good partial response rate was 37.0% with the traditional approach vs 36.6% with the urine-free approach. The partial response rate was 30.7% for both and the stable disease rate was 3.0% for both.
Achieving a complete response based on the urine-free criteria was highly prognostic for progression-free survival (P = .005) while achieving a very good partial response by either criterion was borderline prognostic for progression-free survival (P = .102).
Only 1.1% of patients — seven patients altogether — had discordant responses between traditional and urine-free response criteria, Banerjee noted. One patient, for instance, was downgraded from a very good partial response with traditional criteria to a partial response with urine-free criteria “because current response criteria rate urine [as] more important than serum-free light chains,” Banerjee explained. Two other patients who met all other stringent criteria for a complete response but still had urine paraprotein at Day 56 were classified as having a very good partial response using traditional criteria but as a complete response with the urine-free criteria.
The other four patients with discordant results were the most important, Banerjee said. These patients were missing urine protein electrophoresis values, which made them non-evaluable using traditional criteria, but became evaluable when using urine-free criteria. “This is, I think, the bane of our existence, right? We ask our patients to put their blood, soul, sweat, and tears into being in a clinical trial, and then they’re not evaluable,” he said.
Overall, these results strongly support the de-emphasis of 24-hour urine requirements in updated IMWG response criteria, said Banerjee. However, he noted, 24-hour urine testing still has a very important place in the screening process and in patients with monoclonal gammopathy of renal significance or AL amyloidosis.
“This study provides reassurance to those of us already not repeating urine tests that urine testing is unnecessary for tracking responses,” said Manni Mohyuddin, MD, from the Multiple Myeloma Program at Huntsman Cancer Institute and assistant professor at the University of Utah, Salt Lake City. “These assessments aren’t done consistently in practice outside of trials anyway, and I hope that this study will lead to a formal change in criteria and the omission of urine assessments in clinical trials.”
Funding for the study was provided by the National Heart, Lung, and Blood Institute; National Cancer Institute; Alliance for Clinical Trials in Oncology; ECOG-ACRIN Cancer Research Group; and SWOG; and contributions were provided by Celgene and Millennium Pharmaceuticals. Banerjee has reported consulting for Adaptive Biotechnologies, Bristol-Myers Squibb, Caribou Biosciences, Genentech, GSK, Johnson & Johnson/Janssen, Karyopharm, Legend Biotech, Pfizer, Sanofi, and SparkCures, and receiving research funding from AbbVie, Johnson & Johnson, Novartis, Pack Health, Prothena, and Sanofi. Mohyuddin has disclosed no personal payments and no consultation for industry. His institution has received research funding from Janssen for his role as a principal investigator on a trial.
A version of this article first appeared on Medscape.com.
FROM ASH 2024
LBCL: Bispecific Antibodies Fare Less Well in Real-World Analysis
In a presentation at the American Society of Hematology (ASH) 2024 Annual Meeting, researchers reported that of 172 patients treated with the drugs who had evaluable responses over a median follow-up of 5 months, median progression-free survival was 2.7 months (95% CI, 2.0-3.9) and median overall survival was 7.2 months (95% CI, 6.1–not reached).
It’s important to consider the real-world nature of the study’s patient population, said first author Taylor R. Brooks, MD, of Cleveland Clinic, Ohio, in an interview. “Compared to pivotal trials, our cohort was enriched for patients with high-risk features, with almost three quarters having some comorbidity that would’ve excluded them from one of the [earlier] studies.”
He added that “though individuals eligible to receive these medicines may be more sick with high-risk disease, a sizable fraction will respond, and some will maintain remissions.’”
According to Brooks, about one third of patients with diffuse LBCL relapse after standard front-line R-CHOP therapy. “The prognosis is poor for patients who are not candidates for aggressive salvage chemotherapy and for those who relapse after two or more lines,” Brooks said. “T cell–engaging bispecific antibodies have emerged as a promising option for patients with relapsed or refractory large B-cell lymphoma, given their favorable rates and duration of responses as well as their manageable rates of toxicities.”
The Food and Drug Administration (FDA) granted accelerated approval for epcoritamab and glofitamab in 2023.
“With increasing uptake into clinical practice following the FDA approvals, there is increasing interest in assessing the efficacy and safety of these drugs in real-world, nontrial settings,” Brooks said. “The goal of our study was to investigate outcomes and identify clinical factors associated with outcomes.”
The multicenter, retrospective, observational REALBiTE study tracked 209 patients with relapsed/refractory diffuse LBCL at 19 US centers (epcoritamab, n = 139; glofitamab, n = 70; median age at start of treatment, 67 years [58-76]; 62.2% male; 74.2% diffuse LBCL). The median number of lines of therapy was three (range, 1-12).
“Patients who received epcoritamab tended to be slightly older, were more likely to have a history of indolent non-Hodgkin lymphoma prior to their diagnosis of aggressive B-cell lymphoma and were more likely to have an elevated International Prognostic Index score at the start of bispecific therapy, suggesting that these patients may have been slightly older with higher-risk disease compared to those who received glofitamab,” Brooks said.
In total, 172 patients were response-evaluable. The overall response rate was 50.6% (complete response, 23.8%; partial response, 26.7%; stable disease, 5.8%; progressive disease, 43.6%).
The overall and complete response rates were “somewhat lower that what has been published in the pivotal trials of these medicines,” Brooks said. The low progression-free and overall survival rates “highlight the difficulty in managing this group of patients.”
Cytokine release syndrome (CRS) of any grade occurred in 39.2% of patients: 51% in the epcoritamab group and 28.6% in the glofitamab group. Grade ≥3 CRS occurred in 4.3% of patients, who were all taking epcoritamab.
“For epcoritamab, CRS was almost entirely of low grade, and most CRS events occurred around administration of the first full dose of the drug on day 15,” Brooks said. “Similarly, the CRS events for glofitamab were mostly of low grade, though events were observed to occur throughout the step-up dosing. Tocilizumab was administered in about one fifth of the patients.”
In addition, Brooks said, “we found that, among the 19 individuals with paired biopsy samples before and after bispecific therapy, nearly all — 89% — were found to have lost CD20 expression. We expected some patients to experience loss of this important target, but the rate at which we found this to be the case was surprisingly high.”
Brooks added that “clinicians should be acquainted with CRS, ICANS [immune effector cell-associated neurotoxicity syndrome], and mitigation strategies if they are prescribing these medicines. Appropriate and timely management using tocilizumab, steroids, and other adjunctive measures can effectively manage these complications and hopefully allow for the continued delivery of therapy.”
In an interview, Matthew Lunning, DO, associate professor at the University of Nebraska Medical Center/Fred & Pamela Buffett Cancer Center, Omaha, who didn’t take part in the new study, said the findings aren’t bad news. Instead, they’re “practical news,” because they offer insight into how the drugs work.
“The big lesson from this and other trials is the importance of assessing for CD20 expression prior to taking a bispecific off the shelf, “ he said. “These are learnings that often come after approval.”
He added that it’s clear that, “in more heavily pretreated patients, more disease led to less optimal results and higher risk for toxicities.”
Lunning also noted that both epcoritamab and glofitamab “entered into a crowded and chaotic relapsed/refractory LBCL space based high complete response rates with the opportunity for durability in those complete responses.”
Academic institutions were especially interested, as they can manage CRS and ICANS, but “significantly less enthusiasm has been seen in community practices that expect CRS/ICANS to be in the rear-view mirror if they are going to deliver any bispecific,” he said. “It is not that they don’t have the clinical acumen to manage CRS/ICANS. I believe it is the perception of the lack of supportive infrastructure necessary to manage these toxicities.”
There was no study funding. Brooks has reported no disclosures. Other authors have reported various disclosures including relationships with Novartis, AbbVie, Genentech, Genmab, Biogen, Amgen, and others. Lunning has disclosed ties with AbbVie, Genmab, Kite, Bristol-Myers Squibb, Regeneron, and ADC Therapeutics.
A version of this article first appeared on Medscape.com.
In a presentation at the American Society of Hematology (ASH) 2024 Annual Meeting, researchers reported that of 172 patients treated with the drugs who had evaluable responses over a median follow-up of 5 months, median progression-free survival was 2.7 months (95% CI, 2.0-3.9) and median overall survival was 7.2 months (95% CI, 6.1–not reached).
It’s important to consider the real-world nature of the study’s patient population, said first author Taylor R. Brooks, MD, of Cleveland Clinic, Ohio, in an interview. “Compared to pivotal trials, our cohort was enriched for patients with high-risk features, with almost three quarters having some comorbidity that would’ve excluded them from one of the [earlier] studies.”
He added that “though individuals eligible to receive these medicines may be more sick with high-risk disease, a sizable fraction will respond, and some will maintain remissions.’”
According to Brooks, about one third of patients with diffuse LBCL relapse after standard front-line R-CHOP therapy. “The prognosis is poor for patients who are not candidates for aggressive salvage chemotherapy and for those who relapse after two or more lines,” Brooks said. “T cell–engaging bispecific antibodies have emerged as a promising option for patients with relapsed or refractory large B-cell lymphoma, given their favorable rates and duration of responses as well as their manageable rates of toxicities.”
The Food and Drug Administration (FDA) granted accelerated approval for epcoritamab and glofitamab in 2023.
“With increasing uptake into clinical practice following the FDA approvals, there is increasing interest in assessing the efficacy and safety of these drugs in real-world, nontrial settings,” Brooks said. “The goal of our study was to investigate outcomes and identify clinical factors associated with outcomes.”
The multicenter, retrospective, observational REALBiTE study tracked 209 patients with relapsed/refractory diffuse LBCL at 19 US centers (epcoritamab, n = 139; glofitamab, n = 70; median age at start of treatment, 67 years [58-76]; 62.2% male; 74.2% diffuse LBCL). The median number of lines of therapy was three (range, 1-12).
“Patients who received epcoritamab tended to be slightly older, were more likely to have a history of indolent non-Hodgkin lymphoma prior to their diagnosis of aggressive B-cell lymphoma and were more likely to have an elevated International Prognostic Index score at the start of bispecific therapy, suggesting that these patients may have been slightly older with higher-risk disease compared to those who received glofitamab,” Brooks said.
In total, 172 patients were response-evaluable. The overall response rate was 50.6% (complete response, 23.8%; partial response, 26.7%; stable disease, 5.8%; progressive disease, 43.6%).
The overall and complete response rates were “somewhat lower that what has been published in the pivotal trials of these medicines,” Brooks said. The low progression-free and overall survival rates “highlight the difficulty in managing this group of patients.”
Cytokine release syndrome (CRS) of any grade occurred in 39.2% of patients: 51% in the epcoritamab group and 28.6% in the glofitamab group. Grade ≥3 CRS occurred in 4.3% of patients, who were all taking epcoritamab.
“For epcoritamab, CRS was almost entirely of low grade, and most CRS events occurred around administration of the first full dose of the drug on day 15,” Brooks said. “Similarly, the CRS events for glofitamab were mostly of low grade, though events were observed to occur throughout the step-up dosing. Tocilizumab was administered in about one fifth of the patients.”
In addition, Brooks said, “we found that, among the 19 individuals with paired biopsy samples before and after bispecific therapy, nearly all — 89% — were found to have lost CD20 expression. We expected some patients to experience loss of this important target, but the rate at which we found this to be the case was surprisingly high.”
Brooks added that “clinicians should be acquainted with CRS, ICANS [immune effector cell-associated neurotoxicity syndrome], and mitigation strategies if they are prescribing these medicines. Appropriate and timely management using tocilizumab, steroids, and other adjunctive measures can effectively manage these complications and hopefully allow for the continued delivery of therapy.”
In an interview, Matthew Lunning, DO, associate professor at the University of Nebraska Medical Center/Fred & Pamela Buffett Cancer Center, Omaha, who didn’t take part in the new study, said the findings aren’t bad news. Instead, they’re “practical news,” because they offer insight into how the drugs work.
“The big lesson from this and other trials is the importance of assessing for CD20 expression prior to taking a bispecific off the shelf, “ he said. “These are learnings that often come after approval.”
He added that it’s clear that, “in more heavily pretreated patients, more disease led to less optimal results and higher risk for toxicities.”
Lunning also noted that both epcoritamab and glofitamab “entered into a crowded and chaotic relapsed/refractory LBCL space based high complete response rates with the opportunity for durability in those complete responses.”
Academic institutions were especially interested, as they can manage CRS and ICANS, but “significantly less enthusiasm has been seen in community practices that expect CRS/ICANS to be in the rear-view mirror if they are going to deliver any bispecific,” he said. “It is not that they don’t have the clinical acumen to manage CRS/ICANS. I believe it is the perception of the lack of supportive infrastructure necessary to manage these toxicities.”
There was no study funding. Brooks has reported no disclosures. Other authors have reported various disclosures including relationships with Novartis, AbbVie, Genentech, Genmab, Biogen, Amgen, and others. Lunning has disclosed ties with AbbVie, Genmab, Kite, Bristol-Myers Squibb, Regeneron, and ADC Therapeutics.
A version of this article first appeared on Medscape.com.
In a presentation at the American Society of Hematology (ASH) 2024 Annual Meeting, researchers reported that of 172 patients treated with the drugs who had evaluable responses over a median follow-up of 5 months, median progression-free survival was 2.7 months (95% CI, 2.0-3.9) and median overall survival was 7.2 months (95% CI, 6.1–not reached).
It’s important to consider the real-world nature of the study’s patient population, said first author Taylor R. Brooks, MD, of Cleveland Clinic, Ohio, in an interview. “Compared to pivotal trials, our cohort was enriched for patients with high-risk features, with almost three quarters having some comorbidity that would’ve excluded them from one of the [earlier] studies.”
He added that “though individuals eligible to receive these medicines may be more sick with high-risk disease, a sizable fraction will respond, and some will maintain remissions.’”
According to Brooks, about one third of patients with diffuse LBCL relapse after standard front-line R-CHOP therapy. “The prognosis is poor for patients who are not candidates for aggressive salvage chemotherapy and for those who relapse after two or more lines,” Brooks said. “T cell–engaging bispecific antibodies have emerged as a promising option for patients with relapsed or refractory large B-cell lymphoma, given their favorable rates and duration of responses as well as their manageable rates of toxicities.”
The Food and Drug Administration (FDA) granted accelerated approval for epcoritamab and glofitamab in 2023.
“With increasing uptake into clinical practice following the FDA approvals, there is increasing interest in assessing the efficacy and safety of these drugs in real-world, nontrial settings,” Brooks said. “The goal of our study was to investigate outcomes and identify clinical factors associated with outcomes.”
The multicenter, retrospective, observational REALBiTE study tracked 209 patients with relapsed/refractory diffuse LBCL at 19 US centers (epcoritamab, n = 139; glofitamab, n = 70; median age at start of treatment, 67 years [58-76]; 62.2% male; 74.2% diffuse LBCL). The median number of lines of therapy was three (range, 1-12).
“Patients who received epcoritamab tended to be slightly older, were more likely to have a history of indolent non-Hodgkin lymphoma prior to their diagnosis of aggressive B-cell lymphoma and were more likely to have an elevated International Prognostic Index score at the start of bispecific therapy, suggesting that these patients may have been slightly older with higher-risk disease compared to those who received glofitamab,” Brooks said.
In total, 172 patients were response-evaluable. The overall response rate was 50.6% (complete response, 23.8%; partial response, 26.7%; stable disease, 5.8%; progressive disease, 43.6%).
The overall and complete response rates were “somewhat lower that what has been published in the pivotal trials of these medicines,” Brooks said. The low progression-free and overall survival rates “highlight the difficulty in managing this group of patients.”
Cytokine release syndrome (CRS) of any grade occurred in 39.2% of patients: 51% in the epcoritamab group and 28.6% in the glofitamab group. Grade ≥3 CRS occurred in 4.3% of patients, who were all taking epcoritamab.
“For epcoritamab, CRS was almost entirely of low grade, and most CRS events occurred around administration of the first full dose of the drug on day 15,” Brooks said. “Similarly, the CRS events for glofitamab were mostly of low grade, though events were observed to occur throughout the step-up dosing. Tocilizumab was administered in about one fifth of the patients.”
In addition, Brooks said, “we found that, among the 19 individuals with paired biopsy samples before and after bispecific therapy, nearly all — 89% — were found to have lost CD20 expression. We expected some patients to experience loss of this important target, but the rate at which we found this to be the case was surprisingly high.”
Brooks added that “clinicians should be acquainted with CRS, ICANS [immune effector cell-associated neurotoxicity syndrome], and mitigation strategies if they are prescribing these medicines. Appropriate and timely management using tocilizumab, steroids, and other adjunctive measures can effectively manage these complications and hopefully allow for the continued delivery of therapy.”
In an interview, Matthew Lunning, DO, associate professor at the University of Nebraska Medical Center/Fred & Pamela Buffett Cancer Center, Omaha, who didn’t take part in the new study, said the findings aren’t bad news. Instead, they’re “practical news,” because they offer insight into how the drugs work.
“The big lesson from this and other trials is the importance of assessing for CD20 expression prior to taking a bispecific off the shelf, “ he said. “These are learnings that often come after approval.”
He added that it’s clear that, “in more heavily pretreated patients, more disease led to less optimal results and higher risk for toxicities.”
Lunning also noted that both epcoritamab and glofitamab “entered into a crowded and chaotic relapsed/refractory LBCL space based high complete response rates with the opportunity for durability in those complete responses.”
Academic institutions were especially interested, as they can manage CRS and ICANS, but “significantly less enthusiasm has been seen in community practices that expect CRS/ICANS to be in the rear-view mirror if they are going to deliver any bispecific,” he said. “It is not that they don’t have the clinical acumen to manage CRS/ICANS. I believe it is the perception of the lack of supportive infrastructure necessary to manage these toxicities.”
There was no study funding. Brooks has reported no disclosures. Other authors have reported various disclosures including relationships with Novartis, AbbVie, Genentech, Genmab, Biogen, Amgen, and others. Lunning has disclosed ties with AbbVie, Genmab, Kite, Bristol-Myers Squibb, Regeneron, and ADC Therapeutics.
A version of this article first appeared on Medscape.com.
FROM ASH 2024
Intratumoral Dendritic Cell Therapy Shows Promise in Early-Stage ERBB2-Positive Breast Cancer
TOPLINE:
The higher dose (100 million cells) shows enhanced immune effector recruitment and significant tumor regression before chemotherapy initiation.
METHODOLOGY:
- ERBB2-positive breast cancer survival has improved with anti-ERBB2 antibodies trastuzumab and pertuzumab, but for a pathologic complete response, chemotherapy remains necessary, which comes with significant toxic effects.
- A phase 1, nonrandomized clinical trial enrolled 12 patients with early-stage ERBB2-positive breast cancer in Tampa, Florida, from October 2021 to October 2022.
- Participants received intratumoral (IT) cDC1 injections weekly for 6 weeks at two dose levels (50 million cells for dose level 1 and 100 million cells for dose level 2), with six patients in each group.
- Starting from day 1 of the cDC1 injections, treatment included trastuzumab (8-mg/kg loading dose, then 6 mg/kg) and pertuzumab (840-mg loading dose, then 420 mg) administered intravenously every 3 weeks for six cycles, followed by paclitaxel (80 mg/m2) weekly for 12 weeks and surgery with lumpectomy or mastectomy.
- Primary outcomes measured safety and immune response of increasing doses of cDC1 combined with anti-ERBB2 antibodies before neoadjuvant chemotherapy; secondary outcomes assessed antitumor efficacy through breast MRI and residual cancer burden at surgery.
TAKEAWAY:
- IT delivery of ERBB2 cDC1 was safe and not associated with any dose-limiting toxic effects. The most frequent adverse events attributed to cDC1 were grade 1-2 chills (50%), fatigue (41.7%), headache (33%), and injection-site reactions (33%).
- Dose level 2 showed enhanced recruitment of adaptive CD3, CD4, and CD8 T cells and B cells within the tumor microenvironment (TME), along with increased innate gamma delta T cells and natural killer T cells.
- Breast MRI revealed nine objective responses, including six partial responses and three complete responses, with three cases of stable disease.
- Following surgery, 7 of 12 patients (58%) achieved a pathologic complete response, including all 3 hormone receptor–negative patients and 4 of the 9 hormone receptor–positive patients.
IN PRACTICE:
“Overall, the clinical data shown here demonstrate the effects of combining ERBB2 antibodies with IT [intratumoral] delivery of targeted cDC1 to enhance immune cell infiltration within the TME [tumor microenvironment] and subsequently induce tumor regression before chemotherapy,” wrote the authors, who noted they will be testing the higher dose for an ongoing phase 2 trial with an additional 41 patients.
SOURCE:
The study was led by Hyo S. Han, MD, of H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida. It was published online on December 5, 2024, in JAMA Oncology.
LIMITATIONS:
Because only two dose levels of cDC1 were tested, it remains unclear whether higher doses or different administration schedules could further enhance immune response. Additionally, the nonrandomized design prevents definitive conclusions about whether the clinical benefits were solely from the anti-ERBB2 antibodies. The small sample size also makes it difficult to determine if the pathologic complete responses were primarily due to the 12 weeks of trastuzumab/pertuzumab/paclitaxel treatment.
DISCLOSURES:
This study was funded by the Moffitt Breast Cancer Research Fund, Shula Fund, and Pennies in Action. Several authors reported research support and personal and consulting fees from US funding agencies and multiple pharmaceutical companies outside of the submitted work, as well as related intellectual property and patents.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The higher dose (100 million cells) shows enhanced immune effector recruitment and significant tumor regression before chemotherapy initiation.
METHODOLOGY:
- ERBB2-positive breast cancer survival has improved with anti-ERBB2 antibodies trastuzumab and pertuzumab, but for a pathologic complete response, chemotherapy remains necessary, which comes with significant toxic effects.
- A phase 1, nonrandomized clinical trial enrolled 12 patients with early-stage ERBB2-positive breast cancer in Tampa, Florida, from October 2021 to October 2022.
- Participants received intratumoral (IT) cDC1 injections weekly for 6 weeks at two dose levels (50 million cells for dose level 1 and 100 million cells for dose level 2), with six patients in each group.
- Starting from day 1 of the cDC1 injections, treatment included trastuzumab (8-mg/kg loading dose, then 6 mg/kg) and pertuzumab (840-mg loading dose, then 420 mg) administered intravenously every 3 weeks for six cycles, followed by paclitaxel (80 mg/m2) weekly for 12 weeks and surgery with lumpectomy or mastectomy.
- Primary outcomes measured safety and immune response of increasing doses of cDC1 combined with anti-ERBB2 antibodies before neoadjuvant chemotherapy; secondary outcomes assessed antitumor efficacy through breast MRI and residual cancer burden at surgery.
TAKEAWAY:
- IT delivery of ERBB2 cDC1 was safe and not associated with any dose-limiting toxic effects. The most frequent adverse events attributed to cDC1 were grade 1-2 chills (50%), fatigue (41.7%), headache (33%), and injection-site reactions (33%).
- Dose level 2 showed enhanced recruitment of adaptive CD3, CD4, and CD8 T cells and B cells within the tumor microenvironment (TME), along with increased innate gamma delta T cells and natural killer T cells.
- Breast MRI revealed nine objective responses, including six partial responses and three complete responses, with three cases of stable disease.
- Following surgery, 7 of 12 patients (58%) achieved a pathologic complete response, including all 3 hormone receptor–negative patients and 4 of the 9 hormone receptor–positive patients.
IN PRACTICE:
“Overall, the clinical data shown here demonstrate the effects of combining ERBB2 antibodies with IT [intratumoral] delivery of targeted cDC1 to enhance immune cell infiltration within the TME [tumor microenvironment] and subsequently induce tumor regression before chemotherapy,” wrote the authors, who noted they will be testing the higher dose for an ongoing phase 2 trial with an additional 41 patients.
SOURCE:
The study was led by Hyo S. Han, MD, of H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida. It was published online on December 5, 2024, in JAMA Oncology.
LIMITATIONS:
Because only two dose levels of cDC1 were tested, it remains unclear whether higher doses or different administration schedules could further enhance immune response. Additionally, the nonrandomized design prevents definitive conclusions about whether the clinical benefits were solely from the anti-ERBB2 antibodies. The small sample size also makes it difficult to determine if the pathologic complete responses were primarily due to the 12 weeks of trastuzumab/pertuzumab/paclitaxel treatment.
DISCLOSURES:
This study was funded by the Moffitt Breast Cancer Research Fund, Shula Fund, and Pennies in Action. Several authors reported research support and personal and consulting fees from US funding agencies and multiple pharmaceutical companies outside of the submitted work, as well as related intellectual property and patents.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The higher dose (100 million cells) shows enhanced immune effector recruitment and significant tumor regression before chemotherapy initiation.
METHODOLOGY:
- ERBB2-positive breast cancer survival has improved with anti-ERBB2 antibodies trastuzumab and pertuzumab, but for a pathologic complete response, chemotherapy remains necessary, which comes with significant toxic effects.
- A phase 1, nonrandomized clinical trial enrolled 12 patients with early-stage ERBB2-positive breast cancer in Tampa, Florida, from October 2021 to October 2022.
- Participants received intratumoral (IT) cDC1 injections weekly for 6 weeks at two dose levels (50 million cells for dose level 1 and 100 million cells for dose level 2), with six patients in each group.
- Starting from day 1 of the cDC1 injections, treatment included trastuzumab (8-mg/kg loading dose, then 6 mg/kg) and pertuzumab (840-mg loading dose, then 420 mg) administered intravenously every 3 weeks for six cycles, followed by paclitaxel (80 mg/m2) weekly for 12 weeks and surgery with lumpectomy or mastectomy.
- Primary outcomes measured safety and immune response of increasing doses of cDC1 combined with anti-ERBB2 antibodies before neoadjuvant chemotherapy; secondary outcomes assessed antitumor efficacy through breast MRI and residual cancer burden at surgery.
TAKEAWAY:
- IT delivery of ERBB2 cDC1 was safe and not associated with any dose-limiting toxic effects. The most frequent adverse events attributed to cDC1 were grade 1-2 chills (50%), fatigue (41.7%), headache (33%), and injection-site reactions (33%).
- Dose level 2 showed enhanced recruitment of adaptive CD3, CD4, and CD8 T cells and B cells within the tumor microenvironment (TME), along with increased innate gamma delta T cells and natural killer T cells.
- Breast MRI revealed nine objective responses, including six partial responses and three complete responses, with three cases of stable disease.
- Following surgery, 7 of 12 patients (58%) achieved a pathologic complete response, including all 3 hormone receptor–negative patients and 4 of the 9 hormone receptor–positive patients.
IN PRACTICE:
“Overall, the clinical data shown here demonstrate the effects of combining ERBB2 antibodies with IT [intratumoral] delivery of targeted cDC1 to enhance immune cell infiltration within the TME [tumor microenvironment] and subsequently induce tumor regression before chemotherapy,” wrote the authors, who noted they will be testing the higher dose for an ongoing phase 2 trial with an additional 41 patients.
SOURCE:
The study was led by Hyo S. Han, MD, of H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida. It was published online on December 5, 2024, in JAMA Oncology.
LIMITATIONS:
Because only two dose levels of cDC1 were tested, it remains unclear whether higher doses or different administration schedules could further enhance immune response. Additionally, the nonrandomized design prevents definitive conclusions about whether the clinical benefits were solely from the anti-ERBB2 antibodies. The small sample size also makes it difficult to determine if the pathologic complete responses were primarily due to the 12 weeks of trastuzumab/pertuzumab/paclitaxel treatment.
DISCLOSURES:
This study was funded by the Moffitt Breast Cancer Research Fund, Shula Fund, and Pennies in Action. Several authors reported research support and personal and consulting fees from US funding agencies and multiple pharmaceutical companies outside of the submitted work, as well as related intellectual property and patents.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA Approves Durvalumab for Limited-Stage SCLC
The Food and Drug Administration approval makes the monoclonal antibody — which is already approved for multiple tumor types — the first immunotherapy regimen approved in this setting, AstraZeneca noted in a press release.
“Durvalumab is the first and only systemic treatment following curative-intent, platinum-based chemoradiotherapy to show improved survival for patients with this aggressive form of lung cancer,” international coordinating investigator on the trial, Suresh Senan, PhD, stated in the press release. “This finding represents the first advance for this disease in 4 decades.”
Approval, which followed Priority Review and Breakthrough Therapy Designation, was based on findings from the phase 3 ADRIATIC trial showing a 27% reduction in the risk for death with durvalumab vs placebo.
Findings from the trial were reported during a plenary session at the 2024 American Society of Clinical Oncology conference, and subsequently published in The New England Journal of Medicine.
In 730 patients with LS-SCLC who were randomized 1:1:1 to receive single-agent durvalumab, durvalumab in combination with tremelimumab, or placebo, overall survival (OS) and progression-free survival (PFS) were significantly improved with durvalumab alone vs placebo (hazard ratio, 0.73 and 0.76, for OS and PFS, respectively). Median OS was 55.9 months vs 33.4 months with durvalumab vs placebo, and PFS was 16.6 vs 9.2 months, respectively.
Senan, a professor of clinical experimental radiotherapy at the Amsterdam University Medical Center in the Netherlands, noted in the press release that 57% of patients were still alive at 3 years after being treated with durvalumab, which underscores the practice-changing potential of this medicine in this setting.
“This new treatment option is a game changer for patients with limited-stage small cell lung cancer, a disease known for its high rate of recurrence,” Dusty Donaldson, founder and executive director of the nonprofit advocacy organization LiveLung, stated in the release. “Historically, more often than not, clinical trials to identify new treatment options for this type of cancer have failed to show benefit. We are therefore so excited that many more people will now have the opportunity to access this immunotherapy treatment that holds the potential to significantly improve outcomes.”
Adverse reactions occurring in at least 20% of patients in the ADRIATIC trial included pneumonitis or radiation pneumonitis and fatigue.
The recommended durvalumab dose, according to prescribing information, is 1500 mg every 4 weeks for patients weighing at least 30 kg and 20 mg/kg every 4 weeks for those weighing less than 30 kg, until disease progression or unacceptable toxicity or a maximum of 24 months.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration approval makes the monoclonal antibody — which is already approved for multiple tumor types — the first immunotherapy regimen approved in this setting, AstraZeneca noted in a press release.
“Durvalumab is the first and only systemic treatment following curative-intent, platinum-based chemoradiotherapy to show improved survival for patients with this aggressive form of lung cancer,” international coordinating investigator on the trial, Suresh Senan, PhD, stated in the press release. “This finding represents the first advance for this disease in 4 decades.”
Approval, which followed Priority Review and Breakthrough Therapy Designation, was based on findings from the phase 3 ADRIATIC trial showing a 27% reduction in the risk for death with durvalumab vs placebo.
Findings from the trial were reported during a plenary session at the 2024 American Society of Clinical Oncology conference, and subsequently published in The New England Journal of Medicine.
In 730 patients with LS-SCLC who were randomized 1:1:1 to receive single-agent durvalumab, durvalumab in combination with tremelimumab, or placebo, overall survival (OS) and progression-free survival (PFS) were significantly improved with durvalumab alone vs placebo (hazard ratio, 0.73 and 0.76, for OS and PFS, respectively). Median OS was 55.9 months vs 33.4 months with durvalumab vs placebo, and PFS was 16.6 vs 9.2 months, respectively.
Senan, a professor of clinical experimental radiotherapy at the Amsterdam University Medical Center in the Netherlands, noted in the press release that 57% of patients were still alive at 3 years after being treated with durvalumab, which underscores the practice-changing potential of this medicine in this setting.
“This new treatment option is a game changer for patients with limited-stage small cell lung cancer, a disease known for its high rate of recurrence,” Dusty Donaldson, founder and executive director of the nonprofit advocacy organization LiveLung, stated in the release. “Historically, more often than not, clinical trials to identify new treatment options for this type of cancer have failed to show benefit. We are therefore so excited that many more people will now have the opportunity to access this immunotherapy treatment that holds the potential to significantly improve outcomes.”
Adverse reactions occurring in at least 20% of patients in the ADRIATIC trial included pneumonitis or radiation pneumonitis and fatigue.
The recommended durvalumab dose, according to prescribing information, is 1500 mg every 4 weeks for patients weighing at least 30 kg and 20 mg/kg every 4 weeks for those weighing less than 30 kg, until disease progression or unacceptable toxicity or a maximum of 24 months.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration approval makes the monoclonal antibody — which is already approved for multiple tumor types — the first immunotherapy regimen approved in this setting, AstraZeneca noted in a press release.
“Durvalumab is the first and only systemic treatment following curative-intent, platinum-based chemoradiotherapy to show improved survival for patients with this aggressive form of lung cancer,” international coordinating investigator on the trial, Suresh Senan, PhD, stated in the press release. “This finding represents the first advance for this disease in 4 decades.”
Approval, which followed Priority Review and Breakthrough Therapy Designation, was based on findings from the phase 3 ADRIATIC trial showing a 27% reduction in the risk for death with durvalumab vs placebo.
Findings from the trial were reported during a plenary session at the 2024 American Society of Clinical Oncology conference, and subsequently published in The New England Journal of Medicine.
In 730 patients with LS-SCLC who were randomized 1:1:1 to receive single-agent durvalumab, durvalumab in combination with tremelimumab, or placebo, overall survival (OS) and progression-free survival (PFS) were significantly improved with durvalumab alone vs placebo (hazard ratio, 0.73 and 0.76, for OS and PFS, respectively). Median OS was 55.9 months vs 33.4 months with durvalumab vs placebo, and PFS was 16.6 vs 9.2 months, respectively.
Senan, a professor of clinical experimental radiotherapy at the Amsterdam University Medical Center in the Netherlands, noted in the press release that 57% of patients were still alive at 3 years after being treated with durvalumab, which underscores the practice-changing potential of this medicine in this setting.
“This new treatment option is a game changer for patients with limited-stage small cell lung cancer, a disease known for its high rate of recurrence,” Dusty Donaldson, founder and executive director of the nonprofit advocacy organization LiveLung, stated in the release. “Historically, more often than not, clinical trials to identify new treatment options for this type of cancer have failed to show benefit. We are therefore so excited that many more people will now have the opportunity to access this immunotherapy treatment that holds the potential to significantly improve outcomes.”
Adverse reactions occurring in at least 20% of patients in the ADRIATIC trial included pneumonitis or radiation pneumonitis and fatigue.
The recommended durvalumab dose, according to prescribing information, is 1500 mg every 4 weeks for patients weighing at least 30 kg and 20 mg/kg every 4 weeks for those weighing less than 30 kg, until disease progression or unacceptable toxicity or a maximum of 24 months.
A version of this article first appeared on Medscape.com.
New Cancer Vaccines on the Horizon: Renewed Hope or Hype?
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
FDA Approves Bizengri for NSCLC and Pancreatic Cancers Harboring NRG1 Gene Fusion
Specifically, the systemic agent was approved for those with advanced, unresectable, or metastatic NSCLC or pancreatic adenocarcinoma harboring a neuregulin 1 (NRG1) gene fusion who progress on or after prior systemic therapy, according to the FDA.
The approval, based on findings from the multicenter, open-label eNRGy study, is the first from the FDA for a systemic therapy in this setting. In the multicohort study, treatment was associated with an overall response rate of 33% and 40% in 64 patients with NSCLC and 40 patients with pancreatic adenocarcinoma, respectively. Median duration of response was 7.4 months in the NSCLC patients and ranged from 3.7 to 16.6 months in those with pancreatic adenocarcinoma.
Adverse reactions occurring in at least 10% of patients included diarrhea, musculoskeletal pain, fatigue, nausea, infusion-related reactions, dyspnea, rash, constipation, vomiting, abdominal pain, and edema. Grade 3 or 4 laboratory abnormalities occurring in at least 10% of patients included increased gamma-glutamyl transferase and decreased hemoglobin, sodium, and platelets.
“The Personalized Medicine Coalition applauds the approval of BIZENGRI®,” Edward Abrahams, president of the Personalized Medicine Coalition, a Washington-based education and advocacy organization, stated in a press release from Merus. “In keeping with the growing number of personalized medicines on the market today, BIZENGRI® offers the only approved NRG1+ therapy for patients with these difficult-to-treat cancers.”
The agent is expected to be available for use in the “coming weeks,” according to Merus.
“The FDA approval of BIZENGRI® marks an important milestone for patients with pancreatic adenocarcinoma or NSCLC that is advanced unresectable or metastatic and harbors the NRG1 gene fusion,” noted Alison Schram, MD, an attending medical oncologist in the Early Drug Development Service at Memorial Sloan Kettering Cancer Center, New York City, and a principal investigator for the ongoing eNRGy trial. “I have seen firsthand how treatment with BIZENGRI® can deliver clinically meaningful outcomes for patients.”
Prescribing information for zenocutuzumab-zbco includes a Boxed Warning for embryo-fetal toxicity. The recommended treatment dose is 750 mg every 2 weeks until disease progression or unacceptable toxicity.
A version of this article first appeared on Medscape.com.
Specifically, the systemic agent was approved for those with advanced, unresectable, or metastatic NSCLC or pancreatic adenocarcinoma harboring a neuregulin 1 (NRG1) gene fusion who progress on or after prior systemic therapy, according to the FDA.
The approval, based on findings from the multicenter, open-label eNRGy study, is the first from the FDA for a systemic therapy in this setting. In the multicohort study, treatment was associated with an overall response rate of 33% and 40% in 64 patients with NSCLC and 40 patients with pancreatic adenocarcinoma, respectively. Median duration of response was 7.4 months in the NSCLC patients and ranged from 3.7 to 16.6 months in those with pancreatic adenocarcinoma.
Adverse reactions occurring in at least 10% of patients included diarrhea, musculoskeletal pain, fatigue, nausea, infusion-related reactions, dyspnea, rash, constipation, vomiting, abdominal pain, and edema. Grade 3 or 4 laboratory abnormalities occurring in at least 10% of patients included increased gamma-glutamyl transferase and decreased hemoglobin, sodium, and platelets.
“The Personalized Medicine Coalition applauds the approval of BIZENGRI®,” Edward Abrahams, president of the Personalized Medicine Coalition, a Washington-based education and advocacy organization, stated in a press release from Merus. “In keeping with the growing number of personalized medicines on the market today, BIZENGRI® offers the only approved NRG1+ therapy for patients with these difficult-to-treat cancers.”
The agent is expected to be available for use in the “coming weeks,” according to Merus.
“The FDA approval of BIZENGRI® marks an important milestone for patients with pancreatic adenocarcinoma or NSCLC that is advanced unresectable or metastatic and harbors the NRG1 gene fusion,” noted Alison Schram, MD, an attending medical oncologist in the Early Drug Development Service at Memorial Sloan Kettering Cancer Center, New York City, and a principal investigator for the ongoing eNRGy trial. “I have seen firsthand how treatment with BIZENGRI® can deliver clinically meaningful outcomes for patients.”
Prescribing information for zenocutuzumab-zbco includes a Boxed Warning for embryo-fetal toxicity. The recommended treatment dose is 750 mg every 2 weeks until disease progression or unacceptable toxicity.
A version of this article first appeared on Medscape.com.
Specifically, the systemic agent was approved for those with advanced, unresectable, or metastatic NSCLC or pancreatic adenocarcinoma harboring a neuregulin 1 (NRG1) gene fusion who progress on or after prior systemic therapy, according to the FDA.
The approval, based on findings from the multicenter, open-label eNRGy study, is the first from the FDA for a systemic therapy in this setting. In the multicohort study, treatment was associated with an overall response rate of 33% and 40% in 64 patients with NSCLC and 40 patients with pancreatic adenocarcinoma, respectively. Median duration of response was 7.4 months in the NSCLC patients and ranged from 3.7 to 16.6 months in those with pancreatic adenocarcinoma.
Adverse reactions occurring in at least 10% of patients included diarrhea, musculoskeletal pain, fatigue, nausea, infusion-related reactions, dyspnea, rash, constipation, vomiting, abdominal pain, and edema. Grade 3 or 4 laboratory abnormalities occurring in at least 10% of patients included increased gamma-glutamyl transferase and decreased hemoglobin, sodium, and platelets.
“The Personalized Medicine Coalition applauds the approval of BIZENGRI®,” Edward Abrahams, president of the Personalized Medicine Coalition, a Washington-based education and advocacy organization, stated in a press release from Merus. “In keeping with the growing number of personalized medicines on the market today, BIZENGRI® offers the only approved NRG1+ therapy for patients with these difficult-to-treat cancers.”
The agent is expected to be available for use in the “coming weeks,” according to Merus.
“The FDA approval of BIZENGRI® marks an important milestone for patients with pancreatic adenocarcinoma or NSCLC that is advanced unresectable or metastatic and harbors the NRG1 gene fusion,” noted Alison Schram, MD, an attending medical oncologist in the Early Drug Development Service at Memorial Sloan Kettering Cancer Center, New York City, and a principal investigator for the ongoing eNRGy trial. “I have seen firsthand how treatment with BIZENGRI® can deliver clinically meaningful outcomes for patients.”
Prescribing information for zenocutuzumab-zbco includes a Boxed Warning for embryo-fetal toxicity. The recommended treatment dose is 750 mg every 2 weeks until disease progression or unacceptable toxicity.
A version of this article first appeared on Medscape.com.
Identifying the Best Upfront Regimen for Unresectable CRC Liver Metastasis
A new report demonstrated why patients benefit most from starting on a two-drug chemotherapy regimen — FOLFOX or FOLFIRI — instead of a three-drug regimen — FOLFOXIRI.
The CAIRO5 trial compared overall survival among 294 patients with right sided tumors and/or RAS/BRAF mutations who received FOLFOXIRI (5-fluorouracil [FU], oxaliplatin, irinotecan, plus folinic acid as an enhancer) or investigators’ choice of FOLFOX (5-FU, oxaliplatin, and folinic acid) or FOLFIRI (5-FU, irinotecan, and folinic acid). All patients also received bevacizumab.
In a post hoc analysis, researchers found no overall survival benefit among patients receiving the three-drug regimen. At a median follow-up of just over 5 years, the median overall survival was 23.6 months with FOLFOX or FOLFIRI vs 24.1 months with FOLFOXIRI (P = .44).
The finding means that patients can avoid the extra toxicity associated with combining oxaliplatin and irinotecan without compromising overall survival, Alan P. Venook, MD, a gastrointestinal medical oncologist at the University of California San Francisco, told Medscape Medical News.
The analysis did not stop there in defining the optimal upfront therapy for this patient population.
In a second arm of the analysis, researchers looked at whether swapping in panitumumab for bevacizumab offered any benefit in 236 patients with left-sided tumors and wild-type RAS/BRAF who received either of the two-drug regimens.
Here, the authors also found no benefit of using panitumumab with FOLFOX or FOLFIRI instead of bevacizumab, reporting a median overall survival of 38.3 months with panitumumab vs 39.9 months with bevacizumab.
In addition to avoiding upfront FOLFOXIRI, patients can also avoid the skin reactions and other toxicities associated with panitumumab, including “horrible acne,” Venook said.
Overall, the results support the use of FOLFOX or FOLFIRI with bevacizumab “irrespective of RAS/BRAFV600E status and tumor sidedness” as the initial treatment for CRC with liver-only metastases, concluded the study investigators, from the University Medical Center Utrecht in the Netherlands.
Why Does This Clarity Matter?
The study confirms the standard practice in the United States of starting patients on a two-drug chemotherapy with bevacizumab for the indication and highlights “why we don’t go all in right at the beginning” with a three-drug regimen, Venook said.
In short, more drugs upfront isn’t going to change patients’ long-term survival outcome. Plus, using FOLFOXIRI upfront means “you’ve really pretty much used up all your guns for early treatment,” Venook said.
As for bevacizumab vs panitumumab, most practitioners in the United States favor bevacizumab because of the rash many patients on epidermal growth factor receptor blockers like panitumumab and cetuximab get, Venook said.
Because FOLFOX and FOLFIRI are equally effective on the overall survival front, the decision between them comes down to a balance between patient comorbidities and side effect profiles. Neuropathy, for instance, is more common with FOLFOX, whereas diarrhea is more likely with FOLFIRI, Venook said.
Venook favors FOLFIRI because “almost every patient will develop neuropathy” after seven or eight doses of FOLFOX, which limits its use. “You’re expecting that first treatment to give you the most mileage,” so starting with a treatment “you’re going to get limited use out of ... never made sense to me,” he said.
Venook noted that the results apply only to the older patients studied in CAIRO5 and not necessarily to the ever-growing population of younger people with CRC. Patients in the trial had a median age of 62 years with a performance status of 0-1, a median of 12 liver lesions with no metastases outside the liver, and no contraindications to local or systemic treatment.
The CAIRO5 analysis also looked at what happens after upfront chemotherapy, with the goal being to shrink liver lesions so the lesions can be surgically removed or treated with thermal ablation.
Almost half the patients ultimately underwent resection or ablation, and 39% of those in the FOLFOX or FOLFIRI plus bevacizumab group and 49% in the FOLFOX or FOLFIRI plus panitumumab group then went on to receive adjuvant chemotherapy (ACT) to reduce the risk for recurrence. ACT was recommended in the study, but not required, and consisted of chemotherapy minus bevacizumab or panitumumab.
Overall survival was longest among patients who had complete local treatment without recurrences for at least 6 months (64.3 months) or who had salvage local treatment after early recurrence (58.9 months). Median overall survival was 30.5 months for patients with complete local treatment without salvage after early recurrence, and 28.7 months for patients with incomplete local treatment. Overall survival was worst in patients who remained unresectable (18.3 months).
ACT was associated with improved overall and relapse-free survival, justifying discussing the option with patients who have completed local treatment, the study team said.
CAIRO5 was funded by Roche and Amgen, makers of bevacizumab and panitumumab, respectively. Bond and Venook didn’t have any disclosures.
A version of this article first appeared on Medscape.com.
A new report demonstrated why patients benefit most from starting on a two-drug chemotherapy regimen — FOLFOX or FOLFIRI — instead of a three-drug regimen — FOLFOXIRI.
The CAIRO5 trial compared overall survival among 294 patients with right sided tumors and/or RAS/BRAF mutations who received FOLFOXIRI (5-fluorouracil [FU], oxaliplatin, irinotecan, plus folinic acid as an enhancer) or investigators’ choice of FOLFOX (5-FU, oxaliplatin, and folinic acid) or FOLFIRI (5-FU, irinotecan, and folinic acid). All patients also received bevacizumab.
In a post hoc analysis, researchers found no overall survival benefit among patients receiving the three-drug regimen. At a median follow-up of just over 5 years, the median overall survival was 23.6 months with FOLFOX or FOLFIRI vs 24.1 months with FOLFOXIRI (P = .44).
The finding means that patients can avoid the extra toxicity associated with combining oxaliplatin and irinotecan without compromising overall survival, Alan P. Venook, MD, a gastrointestinal medical oncologist at the University of California San Francisco, told Medscape Medical News.
The analysis did not stop there in defining the optimal upfront therapy for this patient population.
In a second arm of the analysis, researchers looked at whether swapping in panitumumab for bevacizumab offered any benefit in 236 patients with left-sided tumors and wild-type RAS/BRAF who received either of the two-drug regimens.
Here, the authors also found no benefit of using panitumumab with FOLFOX or FOLFIRI instead of bevacizumab, reporting a median overall survival of 38.3 months with panitumumab vs 39.9 months with bevacizumab.
In addition to avoiding upfront FOLFOXIRI, patients can also avoid the skin reactions and other toxicities associated with panitumumab, including “horrible acne,” Venook said.
Overall, the results support the use of FOLFOX or FOLFIRI with bevacizumab “irrespective of RAS/BRAFV600E status and tumor sidedness” as the initial treatment for CRC with liver-only metastases, concluded the study investigators, from the University Medical Center Utrecht in the Netherlands.
Why Does This Clarity Matter?
The study confirms the standard practice in the United States of starting patients on a two-drug chemotherapy with bevacizumab for the indication and highlights “why we don’t go all in right at the beginning” with a three-drug regimen, Venook said.
In short, more drugs upfront isn’t going to change patients’ long-term survival outcome. Plus, using FOLFOXIRI upfront means “you’ve really pretty much used up all your guns for early treatment,” Venook said.
As for bevacizumab vs panitumumab, most practitioners in the United States favor bevacizumab because of the rash many patients on epidermal growth factor receptor blockers like panitumumab and cetuximab get, Venook said.
Because FOLFOX and FOLFIRI are equally effective on the overall survival front, the decision between them comes down to a balance between patient comorbidities and side effect profiles. Neuropathy, for instance, is more common with FOLFOX, whereas diarrhea is more likely with FOLFIRI, Venook said.
Venook favors FOLFIRI because “almost every patient will develop neuropathy” after seven or eight doses of FOLFOX, which limits its use. “You’re expecting that first treatment to give you the most mileage,” so starting with a treatment “you’re going to get limited use out of ... never made sense to me,” he said.
Venook noted that the results apply only to the older patients studied in CAIRO5 and not necessarily to the ever-growing population of younger people with CRC. Patients in the trial had a median age of 62 years with a performance status of 0-1, a median of 12 liver lesions with no metastases outside the liver, and no contraindications to local or systemic treatment.
The CAIRO5 analysis also looked at what happens after upfront chemotherapy, with the goal being to shrink liver lesions so the lesions can be surgically removed or treated with thermal ablation.
Almost half the patients ultimately underwent resection or ablation, and 39% of those in the FOLFOX or FOLFIRI plus bevacizumab group and 49% in the FOLFOX or FOLFIRI plus panitumumab group then went on to receive adjuvant chemotherapy (ACT) to reduce the risk for recurrence. ACT was recommended in the study, but not required, and consisted of chemotherapy minus bevacizumab or panitumumab.
Overall survival was longest among patients who had complete local treatment without recurrences for at least 6 months (64.3 months) or who had salvage local treatment after early recurrence (58.9 months). Median overall survival was 30.5 months for patients with complete local treatment without salvage after early recurrence, and 28.7 months for patients with incomplete local treatment. Overall survival was worst in patients who remained unresectable (18.3 months).
ACT was associated with improved overall and relapse-free survival, justifying discussing the option with patients who have completed local treatment, the study team said.
CAIRO5 was funded by Roche and Amgen, makers of bevacizumab and panitumumab, respectively. Bond and Venook didn’t have any disclosures.
A version of this article first appeared on Medscape.com.
A new report demonstrated why patients benefit most from starting on a two-drug chemotherapy regimen — FOLFOX or FOLFIRI — instead of a three-drug regimen — FOLFOXIRI.
The CAIRO5 trial compared overall survival among 294 patients with right sided tumors and/or RAS/BRAF mutations who received FOLFOXIRI (5-fluorouracil [FU], oxaliplatin, irinotecan, plus folinic acid as an enhancer) or investigators’ choice of FOLFOX (5-FU, oxaliplatin, and folinic acid) or FOLFIRI (5-FU, irinotecan, and folinic acid). All patients also received bevacizumab.
In a post hoc analysis, researchers found no overall survival benefit among patients receiving the three-drug regimen. At a median follow-up of just over 5 years, the median overall survival was 23.6 months with FOLFOX or FOLFIRI vs 24.1 months with FOLFOXIRI (P = .44).
The finding means that patients can avoid the extra toxicity associated with combining oxaliplatin and irinotecan without compromising overall survival, Alan P. Venook, MD, a gastrointestinal medical oncologist at the University of California San Francisco, told Medscape Medical News.
The analysis did not stop there in defining the optimal upfront therapy for this patient population.
In a second arm of the analysis, researchers looked at whether swapping in panitumumab for bevacizumab offered any benefit in 236 patients with left-sided tumors and wild-type RAS/BRAF who received either of the two-drug regimens.
Here, the authors also found no benefit of using panitumumab with FOLFOX or FOLFIRI instead of bevacizumab, reporting a median overall survival of 38.3 months with panitumumab vs 39.9 months with bevacizumab.
In addition to avoiding upfront FOLFOXIRI, patients can also avoid the skin reactions and other toxicities associated with panitumumab, including “horrible acne,” Venook said.
Overall, the results support the use of FOLFOX or FOLFIRI with bevacizumab “irrespective of RAS/BRAFV600E status and tumor sidedness” as the initial treatment for CRC with liver-only metastases, concluded the study investigators, from the University Medical Center Utrecht in the Netherlands.
Why Does This Clarity Matter?
The study confirms the standard practice in the United States of starting patients on a two-drug chemotherapy with bevacizumab for the indication and highlights “why we don’t go all in right at the beginning” with a three-drug regimen, Venook said.
In short, more drugs upfront isn’t going to change patients’ long-term survival outcome. Plus, using FOLFOXIRI upfront means “you’ve really pretty much used up all your guns for early treatment,” Venook said.
As for bevacizumab vs panitumumab, most practitioners in the United States favor bevacizumab because of the rash many patients on epidermal growth factor receptor blockers like panitumumab and cetuximab get, Venook said.
Because FOLFOX and FOLFIRI are equally effective on the overall survival front, the decision between them comes down to a balance between patient comorbidities and side effect profiles. Neuropathy, for instance, is more common with FOLFOX, whereas diarrhea is more likely with FOLFIRI, Venook said.
Venook favors FOLFIRI because “almost every patient will develop neuropathy” after seven or eight doses of FOLFOX, which limits its use. “You’re expecting that first treatment to give you the most mileage,” so starting with a treatment “you’re going to get limited use out of ... never made sense to me,” he said.
Venook noted that the results apply only to the older patients studied in CAIRO5 and not necessarily to the ever-growing population of younger people with CRC. Patients in the trial had a median age of 62 years with a performance status of 0-1, a median of 12 liver lesions with no metastases outside the liver, and no contraindications to local or systemic treatment.
The CAIRO5 analysis also looked at what happens after upfront chemotherapy, with the goal being to shrink liver lesions so the lesions can be surgically removed or treated with thermal ablation.
Almost half the patients ultimately underwent resection or ablation, and 39% of those in the FOLFOX or FOLFIRI plus bevacizumab group and 49% in the FOLFOX or FOLFIRI plus panitumumab group then went on to receive adjuvant chemotherapy (ACT) to reduce the risk for recurrence. ACT was recommended in the study, but not required, and consisted of chemotherapy minus bevacizumab or panitumumab.
Overall survival was longest among patients who had complete local treatment without recurrences for at least 6 months (64.3 months) or who had salvage local treatment after early recurrence (58.9 months). Median overall survival was 30.5 months for patients with complete local treatment without salvage after early recurrence, and 28.7 months for patients with incomplete local treatment. Overall survival was worst in patients who remained unresectable (18.3 months).
ACT was associated with improved overall and relapse-free survival, justifying discussing the option with patients who have completed local treatment, the study team said.
CAIRO5 was funded by Roche and Amgen, makers of bevacizumab and panitumumab, respectively. Bond and Venook didn’t have any disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY