User login
Medication treatment of opioid use disorder in primary care practice: Opportunities and limitations
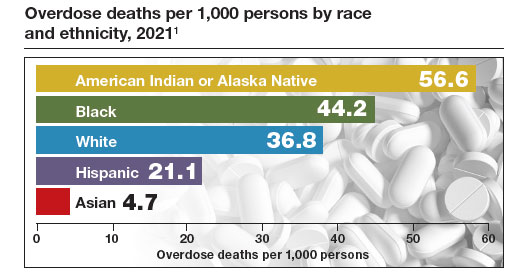
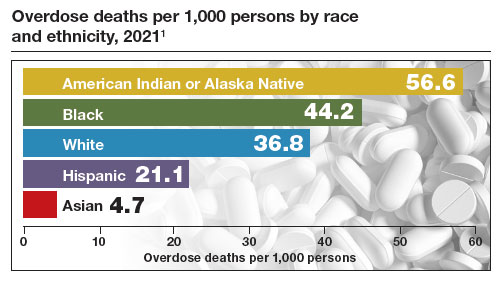
The Centers for Disease Control and Prevention (CDC) reported 106,699 deaths in 2021 from drug overdose, with the majority being linked to synthetic opioids, including fentanyl and tramadol.1 This number compares with 42,795 deaths due to motor vehicle accidents and 48,183 deaths due to suicide in 2021.2,3 Most of the opioid overdose deaths occurred among people aged 25 to 64 years, the peak age of patients cared for by obstetrician-gynecologists. Among pregnant and postpartum persons, mortality due to drug overdose has increased by 81% between 2017 and 2020.4
Among pregnant and postpartum patients, drug overdose death is more common than suicide, and the risk for drug overdose death appears to be greatest in the year following delivery.5,6 In many cases, postpartum patients with OUD have had multiple contacts with the health care system prior to their death, showing that there is an opportunity for therapeutic intervention before the death occurred.7 Medication-assisted recovery for OUD involves a comprehensive array of interventions including medication, counseling, and social support. Medication treatment of OUD with BUP or methadone reduces the risk for death but is underutilized among patients with OUD.6,8 Recent federal legislation has removed restrictions on the use of BUP, increasing the opportunity for primary care clinicians to prescribe it for the treatment of OUD.9
Screening and diagnosis of OUD
Screening for OUD is recommended for patients who are at risk for opioid misuse (ie, those who are taking/have taken opioid medications). The OWLS (Overuse, Worrying, Losing interest, and feeling Slowed down, sluggish, or sedated) screening tool is used to detect prescription medication OUD and has 4 questions10:
1. In the past 3 months did you use your opioid medicines for other purposes—for example, to help you sleep or to help with stress or worry?
2. In the past 3 months did opioid medicines cause you to feel slowed down, sluggish, or sedated?
3. In the past 3 months did opioid medicines cause you to lose interest in your usual activities?
4. In the past 3 months did you worry about your use of opioid medicines?
Patient agreement with 3 or 4 questions indicates a positive screening test.
If the patient has a positive screening test, a formal diagnosis of OUD can be made using the 11 symptoms outlined in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.11 The diagnosis of mild (2 to 3 symptoms), moderate (4 to 5 symptoms), or severe OUD (6 or more symptoms) is made based on the number of symptoms the patient reports.
Buprenorphine treatment of OUD in primary care
The role of primary care clinicians in the medication treatment of OUD is increasing. Using a nationwide system that tracks prescription medications, investigators reported that, in 2004, psychiatrists wrote 32.2% of all BUP prescriptions; in 2021, however, only 10% of such prescriptions were provided by psychiatrists, with most prescriptions written by non-psychiatrist physicians, nurse practitioners, and physician assistants that year.12 Innovative telehealth approaches to consultation and medication treatment of OUD are now available—one example is QuickMD.13 Such sites are designed to remove barriers to initiating medication treatment of OUD.
The role of primary care clinicians in the management of OUD using BUP and buprenorphine-naloxone (BUP-NAL) has increased due to many factors, including:
- the removal of US Food and Drug Administration (FDA) barriers to prescribing BUP
- the epidemic of OUD and the small size of the addiction specialist workforce, necessitating that primary care clinicians become engaged in the treatment of OUD
- an increase in unobserved initiation of BUP among ambulatory patients, and a parallel decrease in cases of observed initiation in addiction center settings
- the reframing of OUD as a chronic medical problem, with many similarities to diabetes, obesity, dyslipidemia, and hypertension.
Similar to other diseases managed by primary care clinicians, OUD requires long-term chronic treatment with a medicine that, if taken as directed, provides excellent outcomes. Primary care clinicians who prescribe BUP also can optimize longitudinal care for comorbid disorders such as hypertension and diabetes, which are prevalent in people with OUD.
In 2019, New Jersey implemented new guidelines for the treatment of OUD, removing prior authorization barriers, increasing reimbursement for office-based OUD treatment, and establishing regional centers of excellence. The implementation of the new guidelines was followed by a marked increase in BUP prescribers among primary care clinicians, emergency medicine physicians, and advanced practice clinicians.14
To estimate the public health impact of BUP prescribing by primary care clinicians, investigators simulated patient outcomes in 3 scenarios15:
1. primary care clinicians refer patients to addiction specialists for OUD treatment
2. primary care clinicians provide BUP services in their practice
3. primary care clinicians provide BUP and harm reduction kits containing syringes and wound care supplies in their practice.
Strategies 2 and 3 resulted in 14% fewer deaths due to opioid overdose, an increased life expectancy of approximately 2.7 years, and reduced hospital costs. For strategy 3, the incremental cost per life-year saved was $34,400. The investigators noted that prescribing BUP in primary care practice increases practice costs.15
Treatment with BUP reduces death from opioid overdose, improves patient health, decreases use of illicit opioids, and reduces patient cravings for opioids. BUP is a safe medication and is associated with fewer adverse effects than insulin or warfarin.16
Continue to: Methadone treatment of OUD...
Methadone treatment of OUD
Methadone is a full opioid agonist approved by the FDA for the treatment of severe pain or OUD. Methadone treatment of OUD is strictly regulated and typically is ordered and administered at an opioid treatment program that is federally licensed. Methadone for OUD treatment cannot be prescribed by a physician to a pharmacy, limiting its use in primary care practice. Methadone used to treat OUD is ordered and dispensed at opioid-treatment programs. Take-home doses of methadone may be available to patients after adherence to the regimen has been established. When used long-term, higher doses of methadone are associated with better adherence, but these higher doses can cause respiratory depression. In a study of 189 pregnant patients taking methadone to treat OUD, daily doses of 60 mg or greater were associated with better treatment retention at delivery and 60 days postpartum, as well as less use of nonprescription opioids.17 Under limited circumstances methadone can be ordered and dispensed for hospitalized patients with OUD.
Methadone is a pure opioid receptor agonist. Naloxone (NAL) is an opioid receptor antagonist. Buprenorphine (BUP) is a partial opioid receptor agonist-antagonist, which limits overdose risk. BUP often is combined with NAL as a combination formulation, which is thought to reduce the repurposing of BUP for non-prescribed uses. At appropriate treatment dosages, both methadone (≥60 mg) and BUP (≥ 16 mg) are highly effective for the treatment of OUD.1 For patients with health insurance, pharmacy benefits often provide some coverage for preferred products but no coverage for other products. Not all pharmacies carry BUP products. In a study of more than 5,000 pharmacies, approximately 60% reported that they carry and can dispense BUP medications.2
BUP monotherapy is available as generic sublingual tablets, buccal films (Belbuca), formulations for injection (Sublocade), and subcutaneous implants (Probuphine). BUPNAL is available as buccal films (Bunavail), sublingual films (Suboxone), and sublingual tablets (Zubsolv). For BUP-NAL combination productions, the following dose combinations have been reported to have similar effects: BUP-NAL 8 mg/2 mg sublingual film, BUP-NAL 5.7 mg/1.4 mg sublingual tablet, and BUP-NAL 4.2 mg/0.7 mg buccal film.3
When initiating BUP-monotherapy or BUP-NAL treatment for OUD, one approach for unobserved initiation is to instruct the patient to discontinue using opioid agonist drugs and wait for the onset of mild to moderate withdrawal symptoms. The purpose of this step is to avoid precipitating severe withdrawal symptoms caused by giving BUP or BUP-NAL to a patient who has recently used opioid drugs.
If BUP-NAL sublingual films (Suboxone) are prescribed following the onset of mild to moderate withdrawal symptoms, the patient can initiate therapy with a dose of 2 mg BUP/0.5 mg NAL or 4 mg BUP/1 mg NAL. At 60 to 120 minutes following the initial dose, if withdrawal symptoms persist, an additional dose of 4 mg BUP/1 mg NAL can be given. Thereafter, symptoms can be assessed every 60 to 120 minutes and additional doses administered to control symptoms. On the second day of therapy, a maximum of 16 mg of BUP is administered. Over the following days and weeks, if symptoms and cravings persist at a BUP dose of 16 mg, the total daily dose of BUP can be titrated up to 24 mg. For long-term treatment, a commonly prescribed daily dose is 16 mg BUP/4 mg NAL or 24 mg BUP/6 mg NAL. An absolute contraindication to BUP or BUP/NAL treatment is an allergy to the medication, and a relative contraindication is liver failure.
One potential complication of transmucosal BUP or BUP-NAL treatment is a dry mouth (xerostomia), which may contribute to dental disease.4 However, some experts question the quality of the data that contributed to the warning.5,6 Potential dental complications might be prevented by regular oral health examinations, daily flossing and teeth brushing, and stimulation of saliva by sugar-free gum or lozenges.
Primary care clinicians who initiate BUP or BUPNAL treatment for OUD often have a weekly visit with the patient during the initial phase of treatment and then every 3 to 4 weeks during maintenance therapy. Most patients need long-term treatment to achieve the goals of therapy, which include prevention of opioid overdose, reduction of cravings for nonprescription narcotics, and improvement in overall health. BUP and BUP-NAL treatment are effective without formal counseling, but counseling and social work support improve long-term adherence with treatment. Primary care clinicians who have experience with medication treatment of OUD report that their experience convinces them that medication treatment of OUD has similarities to the long-term treatment of diabetes, with antihyperglycemia medicines or the treatment of HIV infection with antiviral medications.
References
1. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;CD002207.
2. Weiner SG, Qato DM, Faust JS, et al. Pharmacy availability of buprenorphine for opioid use disorder treatment in the U.S. JAMA Netw Open. 2023;6:E2316089.
3. Substance Abuse and Mental Health Services Administration (SAMHSA). Medications for opioid use disorder. SAMHSA website. Accessed August 21, 2023. https ://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP 21-02-01-002.pdf
4. FDA warns about dental problems with buprenorphine medicines dissolved in the mouth. FDA website. Accessed August 21, 2023. https ://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-dental-problems-buprenorphine-medicines-dissolved-mouth-treat-opioiduse-disorder#:~:text=What%20did%20FDA%20find%3F,medicines%20 dissolved%20in%20the%20mouth
5. Watson DP, Etmian S, Gastala N. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1223-1224.
6. Brothers TD, Lewer D, Bonn M. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1224.
Medication treatment of OUD in obstetrics
In the United States, the prevalence of OUD among pregnant patients hospitalized for delivery more than quadrupled from 1999 through 2014.18 BUP and methadone commonly are used to treat OUD during pregnancy.19 Among pregnant patients about 5% of buprenorphine prescriptions are written by obstetricians.20 An innovative approach to initiating BUP for pregnant patients with OUD is to use unobserved initiation, which involves outpatient discontinuation of nonprescription opioids to induce mild to moderate withdrawal symptoms followed by initiation of BUP treatment. In one cohort study, 55 pregnant patients used an unobserved outpatient protocol to initiate BUP treatment; 80% of the patients previously had used methadone or BUP. No patient experienced a precipitated withdrawal and 96% of patients returned for their office visit 1 week after initiation of treatment. Eighty-six percent of patients remained in treatment 3 months following initiation of BUP.21
Compared with methadone, BUP treatment during pregnancy may result in lower rates of neonatal abstinence syndrome. In one study of pregnant patients who were using methadone (n = 5,056) or BUP (n = 11,272) in late pregnancy, neonatal abstinence syndrome was diagnosed in 69.2% and 52.0% of newborns, respectively (adjusted relative risk, 0.73; 95% confidence interval, 0.71–0.75).22 In addition, compared with methadone, the use of BUP was associated with a reduced risk for low birth weight (14.9% vs 8.3%) and a lower risk for preterm birth (24.9% vs 14.4%). In this study, there were no differences in maternal obstetric outcomes when comparing BUP versus methadone treatment. Similar results have been reported in a meta-analysis analyzing the use of methadone and BUP during pregnancy.23 Studies performed to date have not shown an increased risk of congenital anomalies with the use of BUP-NAL during pregnancy.24,25
Although there may be differences in newborn outcomes with BUP and methadone, the American College of Obstetricians and Gynecologists does not recommend switching from methadone to BUP during pregnancy because precipitated withdrawal may occur.26 Based on recent studies, the American Society of Addiction Medicine has advised that it is safe to prescribe pregnant patients either BUP or BUP-NAL.27,28
Medication treatment of OUD with or without intensive counseling
The FDA recently reviewed literature related to the advantages and challenges of combining intensive counseling with medication treatment of OUD.29 The FDA noted that treatment saves lives and encouraged clinicians to initiate medication treatment of OUD or refer the patient to an appropriate clinician or treatment center. Combining medication treatment of OUD with intensive counseling is associated with greater treatment adherence and reduced health care costs. For example, in one study of 4,987 patients with OUD, initiation of counseling within 8 weeks of the start of medication treatment and a BUP dose of 16 mg or greater daily were associated with increased adherence to treatment.30 For patients receiving a BUP dose of less than 16 mg daily, treatment adherence with and without counseling was approximately 325 and 230 days, respectively. When the dose of BUP was 16 mg or greater, treatment adherence with and without counseling was approximately 405 and 320 days, respectively.30
Counseling should always be offered to patients initiating medication treatment of OUD. It should be noted that counseling alone is not a highly effective treatment for OUD.31 The FDA recently advised that the lack of availability of intensive counseling should not prevent clinicians from initiating BUP for the treatment of OUD.29 OUD is associated with a high mortalityrate and if counseling is not possible, medication treatment should be initiated. Substantial evidence demonstrates that medication treatment of OUD is associated with many benefits.16 The FDA advisory committee concluded that OUD treatment decisions should use shared decision making and be supportive and patient centered.29
The opportunities for medication treatment of OUD in primary care practice have expanded due to the recent FDA removal of restrictions on the use of BUP and heightened awareness of the positive public health impact of medication treatment. Challenges to the medication treatment of OUD remain, including stigmatization of OUD, barriers to insurance coverage for BUP, practice costs of treating OUD, and gaps in clinical education. For many pregnant patients, their main point of contact with health care is their obstetrician. By incorporating OUD treatment in pregnancy care, obstetricians will improve the health of the mother and newborn, contributing to the well-being of current and future generations. ●
Experts have recommended several interventions that may help reduce opioid overdose death.1 A consensus recommendation is that people who use drugs should be provided naloxone rescue medication and educated on the proper use of naloxone. Naloxone rescue medication is available in formulations for nasal or parenteral administration. The US Food and Drug Administration (FDA) recently has approved naloxone for over-the-counter status. The American Medical Association has provided a short web video on how to administer nasal naloxone.2 In a small pilot study, obstetricians offered every postpartum patient with naloxone administration education and a 2-dose nasal naloxone pack, with 76% of patients accepting the nasal naloxone pack.3
Many experts recommend that people who use drugs should be advised to never use them alone and to test a small amount of the drug to assess its potency. Many patients who use opioid drugs also take benzodiazepines, which can contribute to respiratory depression.4 Patients should avoid mixing drugs (eg, opioids and benzodiazepines). Some experts recommend that patients who use drugs should be provided take-home fentanyl test strips so they can evaluate their drugs for the presence of fentanyl, a medication that suppresses respiration and contributes to many overdose deaths. In addition, people who use drugs and are interested in reducing their use of drugs or managing overdose risk can be offered initiation of medication treatment of OUD.1
References
1. Wood E, Solomon ED, Hadland SE. Universal precautions for people at risk of opioid overdose in North America. JAMA Int Med. 2023;183:401-402.
2. How to administer Naloxone. AMA website. Accessed August 28, 2023. https://www.ama-assn.org /delivering-care/overdose-epidemic/how-administer-naloxone
3. Naliboff JA, Tharpe N. Universal postpartum naloxone provision: a harm reduction quality improvement project. J Addict Med. 2022;17:360-362.
4. Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
- Spencer MR, Miniño AM, Warner M. Drug overdose deaths in the United States, 20012021. NCHS Data Brief no 457. Hyattsville, MD, National Center for Health Statistics. 2022. NCHS Data Brief No. 457. Published December 2022. Accessed August 21, 2023. https://www.cdc.gov /nchs/products/databriefs/db457.htm
- US traffic deaths drop slightly in 2022 but still a ‘crisis.’ AP News website. Published April 20, 2023. Accessed August 21, 2023. https://apnews.com /article/traffic-deaths-distracted-driving-crisis -6db6471e273b275920b6c4f9eb7e493b
- Suicide statistics. American Foundation for Suicide Prevention website. Accessed August 21, 2023. https://afsp.org/suicide-statistics/
- Bruzelius E, Martins SS. US Trends in drug overdose mortality among pregnant and postpartum persons, 2017-2020. JAMA. 2022;328:2159-2161.
- Metz TD, Rovner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Schiff DM, Nielsen T, Terplan M, et al. Fatal and nonfatal overdose among pregnant and postpartum women in Massachusetts. Obstet Gynecol. 2018;132:466-474.
- Goldman-Mellor S, Margerison CE. Maternal drug-related death and suicide are leading causes of postpartum death in California. Am J Obstet Gynecol. 2019;221:489.e1-489.e9.
- Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550.
- Waiver elimination (MAT Act). SAMHSA website. Accessed August 21, 2023. https://www .samhsa.gov/medications-substance-use- disorders/removal-data-waiver-requirement
- Picco L, Middleton M, Bruno R, et al. Validation of the OWLS, a Screening Tool for Measuring Prescription Opioid Use Disorder in Primary Care. Pain Med. 2020;21:2757-2764.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Creedon TB, Ali MM, Schuman-Olivier Z. Trends in buprenorphine prescribing for opioid use disorder by psychiatrists in the US from 2003 to 2021. JAMA Health Forum. 2023;4:E230221.
- Quick MD website. Accessed August 21, 2023. https://quick.md/
- Treitler P, Nowels M, Samples H, et al. BUP utilization and prescribing among New Jersey Medicaid beneficiaries after adoption of initiatives designed to improve treatment access. JAMA Netw Open. 2023;6:E2312030.
- Jawa R, Tin Y, Nall S, et al. Estimated clinical outcomes and cost-effectiveness associated with provision of addiction treatment in US primary care clinics. JAMA Netw Open. 2023;6:E237888.
- Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways of opioid use disorder. JAMA Netw Open. 2020;3:E1920622.
- Wilder CM, Hosta D, Winhusen T. Association of methadone dose with substance use and treatment retention in pregnant and postpartum women with opioid use disorder. J Subst Abuse Treat. 2017;80:33-36.
- Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67:845-849.
- Xu KY, Jones HE, Schiff DM, et al. Initiation and treatment discontinuation of medications for opioid use disorder in pregnant people compared with nonpregnant people. Obstet Gynecol. 2023;141:845-853.
- Kelly D, Krans EE. Medical specialty of buprenorphine prescribers for pregnant women with opioid use disorder. Am J Obstet Gynecol. 2019;220:502-503.
- Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
- Suarez EA, Huybrechts KF, Straub L, et al. Buprenorphine versus methadone for opioid use disorder in pregnancy. N Engl J Med. 2022;387:2033-2044.
- Kinsella M, Halliday LO, Shaw M, et al. Buprenorphine compared with methadone in pregnancy: a systematic review and meta-analysis. Subst Use Misuse. 2022;57:1400-1416.
- Jumah NA, Edwards C, Balfour-Boehm J, et al. Observational study of the safety of buprenorphine-naloxone in pregnancy in a rural and remote population. BMJ Open. 2016;6:E011774.
- Mullins N, Galvin SL, Ramage M, et al. Buprenorphine and naloxone versus buprenorphine for opioid use disorder in pregnancy: a cohort study. J Addict Med. 2020;14:185-192.
- Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:E81-E94.
- The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update. J Addict Med. 2020;14(2S suppl 1):1-91.
- Link HM, Jones H, Miller L, et al. Buprenorphinenaloxone use in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100179.
- Delphin-Rittmon ME, Cavazzoni P. US Food and Drug Administration website. https://www.fda .gov/media/168027/download
- Eren K, Schuster J, Herschell A, et al. Association of Counseling and Psychotherapy on retention in medication for addiction treatment within a large Medicaid population. J Addict Med. 2022;16:346353.
- Kakko J, Dybrandt Svanborg K, Kreek MJ, et al. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomized, placebo-controlled trial. Lancet. 2003;361:662-668.
The Centers for Disease Control and Prevention (CDC) reported 106,699 deaths in 2021 from drug overdose, with the majority being linked to synthetic opioids, including fentanyl and tramadol.1 This number compares with 42,795 deaths due to motor vehicle accidents and 48,183 deaths due to suicide in 2021.2,3 Most of the opioid overdose deaths occurred among people aged 25 to 64 years, the peak age of patients cared for by obstetrician-gynecologists. Among pregnant and postpartum persons, mortality due to drug overdose has increased by 81% between 2017 and 2020.4
Among pregnant and postpartum patients, drug overdose death is more common than suicide, and the risk for drug overdose death appears to be greatest in the year following delivery.5,6 In many cases, postpartum patients with OUD have had multiple contacts with the health care system prior to their death, showing that there is an opportunity for therapeutic intervention before the death occurred.7 Medication-assisted recovery for OUD involves a comprehensive array of interventions including medication, counseling, and social support. Medication treatment of OUD with BUP or methadone reduces the risk for death but is underutilized among patients with OUD.6,8 Recent federal legislation has removed restrictions on the use of BUP, increasing the opportunity for primary care clinicians to prescribe it for the treatment of OUD.9
Screening and diagnosis of OUD
Screening for OUD is recommended for patients who are at risk for opioid misuse (ie, those who are taking/have taken opioid medications). The OWLS (Overuse, Worrying, Losing interest, and feeling Slowed down, sluggish, or sedated) screening tool is used to detect prescription medication OUD and has 4 questions10:
1. In the past 3 months did you use your opioid medicines for other purposes—for example, to help you sleep or to help with stress or worry?
2. In the past 3 months did opioid medicines cause you to feel slowed down, sluggish, or sedated?
3. In the past 3 months did opioid medicines cause you to lose interest in your usual activities?
4. In the past 3 months did you worry about your use of opioid medicines?
Patient agreement with 3 or 4 questions indicates a positive screening test.
If the patient has a positive screening test, a formal diagnosis of OUD can be made using the 11 symptoms outlined in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.11 The diagnosis of mild (2 to 3 symptoms), moderate (4 to 5 symptoms), or severe OUD (6 or more symptoms) is made based on the number of symptoms the patient reports.

Buprenorphine treatment of OUD in primary care
The role of primary care clinicians in the medication treatment of OUD is increasing. Using a nationwide system that tracks prescription medications, investigators reported that, in 2004, psychiatrists wrote 32.2% of all BUP prescriptions; in 2021, however, only 10% of such prescriptions were provided by psychiatrists, with most prescriptions written by non-psychiatrist physicians, nurse practitioners, and physician assistants that year.12 Innovative telehealth approaches to consultation and medication treatment of OUD are now available—one example is QuickMD.13 Such sites are designed to remove barriers to initiating medication treatment of OUD.
The role of primary care clinicians in the management of OUD using BUP and buprenorphine-naloxone (BUP-NAL) has increased due to many factors, including:
- the removal of US Food and Drug Administration (FDA) barriers to prescribing BUP
- the epidemic of OUD and the small size of the addiction specialist workforce, necessitating that primary care clinicians become engaged in the treatment of OUD
- an increase in unobserved initiation of BUP among ambulatory patients, and a parallel decrease in cases of observed initiation in addiction center settings
- the reframing of OUD as a chronic medical problem, with many similarities to diabetes, obesity, dyslipidemia, and hypertension.
Similar to other diseases managed by primary care clinicians, OUD requires long-term chronic treatment with a medicine that, if taken as directed, provides excellent outcomes. Primary care clinicians who prescribe BUP also can optimize longitudinal care for comorbid disorders such as hypertension and diabetes, which are prevalent in people with OUD.
In 2019, New Jersey implemented new guidelines for the treatment of OUD, removing prior authorization barriers, increasing reimbursement for office-based OUD treatment, and establishing regional centers of excellence. The implementation of the new guidelines was followed by a marked increase in BUP prescribers among primary care clinicians, emergency medicine physicians, and advanced practice clinicians.14
To estimate the public health impact of BUP prescribing by primary care clinicians, investigators simulated patient outcomes in 3 scenarios15:
1. primary care clinicians refer patients to addiction specialists for OUD treatment
2. primary care clinicians provide BUP services in their practice
3. primary care clinicians provide BUP and harm reduction kits containing syringes and wound care supplies in their practice.
Strategies 2 and 3 resulted in 14% fewer deaths due to opioid overdose, an increased life expectancy of approximately 2.7 years, and reduced hospital costs. For strategy 3, the incremental cost per life-year saved was $34,400. The investigators noted that prescribing BUP in primary care practice increases practice costs.15
Treatment with BUP reduces death from opioid overdose, improves patient health, decreases use of illicit opioids, and reduces patient cravings for opioids. BUP is a safe medication and is associated with fewer adverse effects than insulin or warfarin.16
Continue to: Methadone treatment of OUD...
Methadone treatment of OUD
Methadone is a full opioid agonist approved by the FDA for the treatment of severe pain or OUD. Methadone treatment of OUD is strictly regulated and typically is ordered and administered at an opioid treatment program that is federally licensed. Methadone for OUD treatment cannot be prescribed by a physician to a pharmacy, limiting its use in primary care practice. Methadone used to treat OUD is ordered and dispensed at opioid-treatment programs. Take-home doses of methadone may be available to patients after adherence to the regimen has been established. When used long-term, higher doses of methadone are associated with better adherence, but these higher doses can cause respiratory depression. In a study of 189 pregnant patients taking methadone to treat OUD, daily doses of 60 mg or greater were associated with better treatment retention at delivery and 60 days postpartum, as well as less use of nonprescription opioids.17 Under limited circumstances methadone can be ordered and dispensed for hospitalized patients with OUD.
Methadone is a pure opioid receptor agonist. Naloxone (NAL) is an opioid receptor antagonist. Buprenorphine (BUP) is a partial opioid receptor agonist-antagonist, which limits overdose risk. BUP often is combined with NAL as a combination formulation, which is thought to reduce the repurposing of BUP for non-prescribed uses. At appropriate treatment dosages, both methadone (≥60 mg) and BUP (≥ 16 mg) are highly effective for the treatment of OUD.1 For patients with health insurance, pharmacy benefits often provide some coverage for preferred products but no coverage for other products. Not all pharmacies carry BUP products. In a study of more than 5,000 pharmacies, approximately 60% reported that they carry and can dispense BUP medications.2
BUP monotherapy is available as generic sublingual tablets, buccal films (Belbuca), formulations for injection (Sublocade), and subcutaneous implants (Probuphine). BUPNAL is available as buccal films (Bunavail), sublingual films (Suboxone), and sublingual tablets (Zubsolv). For BUP-NAL combination productions, the following dose combinations have been reported to have similar effects: BUP-NAL 8 mg/2 mg sublingual film, BUP-NAL 5.7 mg/1.4 mg sublingual tablet, and BUP-NAL 4.2 mg/0.7 mg buccal film.3
When initiating BUP-monotherapy or BUP-NAL treatment for OUD, one approach for unobserved initiation is to instruct the patient to discontinue using opioid agonist drugs and wait for the onset of mild to moderate withdrawal symptoms. The purpose of this step is to avoid precipitating severe withdrawal symptoms caused by giving BUP or BUP-NAL to a patient who has recently used opioid drugs.
If BUP-NAL sublingual films (Suboxone) are prescribed following the onset of mild to moderate withdrawal symptoms, the patient can initiate therapy with a dose of 2 mg BUP/0.5 mg NAL or 4 mg BUP/1 mg NAL. At 60 to 120 minutes following the initial dose, if withdrawal symptoms persist, an additional dose of 4 mg BUP/1 mg NAL can be given. Thereafter, symptoms can be assessed every 60 to 120 minutes and additional doses administered to control symptoms. On the second day of therapy, a maximum of 16 mg of BUP is administered. Over the following days and weeks, if symptoms and cravings persist at a BUP dose of 16 mg, the total daily dose of BUP can be titrated up to 24 mg. For long-term treatment, a commonly prescribed daily dose is 16 mg BUP/4 mg NAL or 24 mg BUP/6 mg NAL. An absolute contraindication to BUP or BUP/NAL treatment is an allergy to the medication, and a relative contraindication is liver failure.
One potential complication of transmucosal BUP or BUP-NAL treatment is a dry mouth (xerostomia), which may contribute to dental disease.4 However, some experts question the quality of the data that contributed to the warning.5,6 Potential dental complications might be prevented by regular oral health examinations, daily flossing and teeth brushing, and stimulation of saliva by sugar-free gum or lozenges.
Primary care clinicians who initiate BUP or BUPNAL treatment for OUD often have a weekly visit with the patient during the initial phase of treatment and then every 3 to 4 weeks during maintenance therapy. Most patients need long-term treatment to achieve the goals of therapy, which include prevention of opioid overdose, reduction of cravings for nonprescription narcotics, and improvement in overall health. BUP and BUP-NAL treatment are effective without formal counseling, but counseling and social work support improve long-term adherence with treatment. Primary care clinicians who have experience with medication treatment of OUD report that their experience convinces them that medication treatment of OUD has similarities to the long-term treatment of diabetes, with antihyperglycemia medicines or the treatment of HIV infection with antiviral medications.
References
1. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;CD002207.
2. Weiner SG, Qato DM, Faust JS, et al. Pharmacy availability of buprenorphine for opioid use disorder treatment in the U.S. JAMA Netw Open. 2023;6:E2316089.
3. Substance Abuse and Mental Health Services Administration (SAMHSA). Medications for opioid use disorder. SAMHSA website. Accessed August 21, 2023. https ://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP 21-02-01-002.pdf
4. FDA warns about dental problems with buprenorphine medicines dissolved in the mouth. FDA website. Accessed August 21, 2023. https ://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-dental-problems-buprenorphine-medicines-dissolved-mouth-treat-opioiduse-disorder#:~:text=What%20did%20FDA%20find%3F,medicines%20 dissolved%20in%20the%20mouth
5. Watson DP, Etmian S, Gastala N. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1223-1224.
6. Brothers TD, Lewer D, Bonn M. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1224.
Medication treatment of OUD in obstetrics
In the United States, the prevalence of OUD among pregnant patients hospitalized for delivery more than quadrupled from 1999 through 2014.18 BUP and methadone commonly are used to treat OUD during pregnancy.19 Among pregnant patients about 5% of buprenorphine prescriptions are written by obstetricians.20 An innovative approach to initiating BUP for pregnant patients with OUD is to use unobserved initiation, which involves outpatient discontinuation of nonprescription opioids to induce mild to moderate withdrawal symptoms followed by initiation of BUP treatment. In one cohort study, 55 pregnant patients used an unobserved outpatient protocol to initiate BUP treatment; 80% of the patients previously had used methadone or BUP. No patient experienced a precipitated withdrawal and 96% of patients returned for their office visit 1 week after initiation of treatment. Eighty-six percent of patients remained in treatment 3 months following initiation of BUP.21
Compared with methadone, BUP treatment during pregnancy may result in lower rates of neonatal abstinence syndrome. In one study of pregnant patients who were using methadone (n = 5,056) or BUP (n = 11,272) in late pregnancy, neonatal abstinence syndrome was diagnosed in 69.2% and 52.0% of newborns, respectively (adjusted relative risk, 0.73; 95% confidence interval, 0.71–0.75).22 In addition, compared with methadone, the use of BUP was associated with a reduced risk for low birth weight (14.9% vs 8.3%) and a lower risk for preterm birth (24.9% vs 14.4%). In this study, there were no differences in maternal obstetric outcomes when comparing BUP versus methadone treatment. Similar results have been reported in a meta-analysis analyzing the use of methadone and BUP during pregnancy.23 Studies performed to date have not shown an increased risk of congenital anomalies with the use of BUP-NAL during pregnancy.24,25
Although there may be differences in newborn outcomes with BUP and methadone, the American College of Obstetricians and Gynecologists does not recommend switching from methadone to BUP during pregnancy because precipitated withdrawal may occur.26 Based on recent studies, the American Society of Addiction Medicine has advised that it is safe to prescribe pregnant patients either BUP or BUP-NAL.27,28
Medication treatment of OUD with or without intensive counseling
The FDA recently reviewed literature related to the advantages and challenges of combining intensive counseling with medication treatment of OUD.29 The FDA noted that treatment saves lives and encouraged clinicians to initiate medication treatment of OUD or refer the patient to an appropriate clinician or treatment center. Combining medication treatment of OUD with intensive counseling is associated with greater treatment adherence and reduced health care costs. For example, in one study of 4,987 patients with OUD, initiation of counseling within 8 weeks of the start of medication treatment and a BUP dose of 16 mg or greater daily were associated with increased adherence to treatment.30 For patients receiving a BUP dose of less than 16 mg daily, treatment adherence with and without counseling was approximately 325 and 230 days, respectively. When the dose of BUP was 16 mg or greater, treatment adherence with and without counseling was approximately 405 and 320 days, respectively.30
Counseling should always be offered to patients initiating medication treatment of OUD. It should be noted that counseling alone is not a highly effective treatment for OUD.31 The FDA recently advised that the lack of availability of intensive counseling should not prevent clinicians from initiating BUP for the treatment of OUD.29 OUD is associated with a high mortalityrate and if counseling is not possible, medication treatment should be initiated. Substantial evidence demonstrates that medication treatment of OUD is associated with many benefits.16 The FDA advisory committee concluded that OUD treatment decisions should use shared decision making and be supportive and patient centered.29
The opportunities for medication treatment of OUD in primary care practice have expanded due to the recent FDA removal of restrictions on the use of BUP and heightened awareness of the positive public health impact of medication treatment. Challenges to the medication treatment of OUD remain, including stigmatization of OUD, barriers to insurance coverage for BUP, practice costs of treating OUD, and gaps in clinical education. For many pregnant patients, their main point of contact with health care is their obstetrician. By incorporating OUD treatment in pregnancy care, obstetricians will improve the health of the mother and newborn, contributing to the well-being of current and future generations. ●
Experts have recommended several interventions that may help reduce opioid overdose death.1 A consensus recommendation is that people who use drugs should be provided naloxone rescue medication and educated on the proper use of naloxone. Naloxone rescue medication is available in formulations for nasal or parenteral administration. The US Food and Drug Administration (FDA) recently has approved naloxone for over-the-counter status. The American Medical Association has provided a short web video on how to administer nasal naloxone.2 In a small pilot study, obstetricians offered every postpartum patient with naloxone administration education and a 2-dose nasal naloxone pack, with 76% of patients accepting the nasal naloxone pack.3
Many experts recommend that people who use drugs should be advised to never use them alone and to test a small amount of the drug to assess its potency. Many patients who use opioid drugs also take benzodiazepines, which can contribute to respiratory depression.4 Patients should avoid mixing drugs (eg, opioids and benzodiazepines). Some experts recommend that patients who use drugs should be provided take-home fentanyl test strips so they can evaluate their drugs for the presence of fentanyl, a medication that suppresses respiration and contributes to many overdose deaths. In addition, people who use drugs and are interested in reducing their use of drugs or managing overdose risk can be offered initiation of medication treatment of OUD.1
References
1. Wood E, Solomon ED, Hadland SE. Universal precautions for people at risk of opioid overdose in North America. JAMA Int Med. 2023;183:401-402.
2. How to administer Naloxone. AMA website. Accessed August 28, 2023. https://www.ama-assn.org /delivering-care/overdose-epidemic/how-administer-naloxone
3. Naliboff JA, Tharpe N. Universal postpartum naloxone provision: a harm reduction quality improvement project. J Addict Med. 2022;17:360-362.
4. Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
The Centers for Disease Control and Prevention (CDC) reported 106,699 deaths in 2021 from drug overdose, with the majority being linked to synthetic opioids, including fentanyl and tramadol.1 This number compares with 42,795 deaths due to motor vehicle accidents and 48,183 deaths due to suicide in 2021.2,3 Most of the opioid overdose deaths occurred among people aged 25 to 64 years, the peak age of patients cared for by obstetrician-gynecologists. Among pregnant and postpartum persons, mortality due to drug overdose has increased by 81% between 2017 and 2020.4
Among pregnant and postpartum patients, drug overdose death is more common than suicide, and the risk for drug overdose death appears to be greatest in the year following delivery.5,6 In many cases, postpartum patients with OUD have had multiple contacts with the health care system prior to their death, showing that there is an opportunity for therapeutic intervention before the death occurred.7 Medication-assisted recovery for OUD involves a comprehensive array of interventions including medication, counseling, and social support. Medication treatment of OUD with BUP or methadone reduces the risk for death but is underutilized among patients with OUD.6,8 Recent federal legislation has removed restrictions on the use of BUP, increasing the opportunity for primary care clinicians to prescribe it for the treatment of OUD.9
Screening and diagnosis of OUD
Screening for OUD is recommended for patients who are at risk for opioid misuse (ie, those who are taking/have taken opioid medications). The OWLS (Overuse, Worrying, Losing interest, and feeling Slowed down, sluggish, or sedated) screening tool is used to detect prescription medication OUD and has 4 questions10:
1. In the past 3 months did you use your opioid medicines for other purposes—for example, to help you sleep or to help with stress or worry?
2. In the past 3 months did opioid medicines cause you to feel slowed down, sluggish, or sedated?
3. In the past 3 months did opioid medicines cause you to lose interest in your usual activities?
4. In the past 3 months did you worry about your use of opioid medicines?
Patient agreement with 3 or 4 questions indicates a positive screening test.
If the patient has a positive screening test, a formal diagnosis of OUD can be made using the 11 symptoms outlined in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.11 The diagnosis of mild (2 to 3 symptoms), moderate (4 to 5 symptoms), or severe OUD (6 or more symptoms) is made based on the number of symptoms the patient reports.

Buprenorphine treatment of OUD in primary care
The role of primary care clinicians in the medication treatment of OUD is increasing. Using a nationwide system that tracks prescription medications, investigators reported that, in 2004, psychiatrists wrote 32.2% of all BUP prescriptions; in 2021, however, only 10% of such prescriptions were provided by psychiatrists, with most prescriptions written by non-psychiatrist physicians, nurse practitioners, and physician assistants that year.12 Innovative telehealth approaches to consultation and medication treatment of OUD are now available—one example is QuickMD.13 Such sites are designed to remove barriers to initiating medication treatment of OUD.
The role of primary care clinicians in the management of OUD using BUP and buprenorphine-naloxone (BUP-NAL) has increased due to many factors, including:
- the removal of US Food and Drug Administration (FDA) barriers to prescribing BUP
- the epidemic of OUD and the small size of the addiction specialist workforce, necessitating that primary care clinicians become engaged in the treatment of OUD
- an increase in unobserved initiation of BUP among ambulatory patients, and a parallel decrease in cases of observed initiation in addiction center settings
- the reframing of OUD as a chronic medical problem, with many similarities to diabetes, obesity, dyslipidemia, and hypertension.
Similar to other diseases managed by primary care clinicians, OUD requires long-term chronic treatment with a medicine that, if taken as directed, provides excellent outcomes. Primary care clinicians who prescribe BUP also can optimize longitudinal care for comorbid disorders such as hypertension and diabetes, which are prevalent in people with OUD.
In 2019, New Jersey implemented new guidelines for the treatment of OUD, removing prior authorization barriers, increasing reimbursement for office-based OUD treatment, and establishing regional centers of excellence. The implementation of the new guidelines was followed by a marked increase in BUP prescribers among primary care clinicians, emergency medicine physicians, and advanced practice clinicians.14
To estimate the public health impact of BUP prescribing by primary care clinicians, investigators simulated patient outcomes in 3 scenarios15:
1. primary care clinicians refer patients to addiction specialists for OUD treatment
2. primary care clinicians provide BUP services in their practice
3. primary care clinicians provide BUP and harm reduction kits containing syringes and wound care supplies in their practice.
Strategies 2 and 3 resulted in 14% fewer deaths due to opioid overdose, an increased life expectancy of approximately 2.7 years, and reduced hospital costs. For strategy 3, the incremental cost per life-year saved was $34,400. The investigators noted that prescribing BUP in primary care practice increases practice costs.15
Treatment with BUP reduces death from opioid overdose, improves patient health, decreases use of illicit opioids, and reduces patient cravings for opioids. BUP is a safe medication and is associated with fewer adverse effects than insulin or warfarin.16
Continue to: Methadone treatment of OUD...
Methadone treatment of OUD
Methadone is a full opioid agonist approved by the FDA for the treatment of severe pain or OUD. Methadone treatment of OUD is strictly regulated and typically is ordered and administered at an opioid treatment program that is federally licensed. Methadone for OUD treatment cannot be prescribed by a physician to a pharmacy, limiting its use in primary care practice. Methadone used to treat OUD is ordered and dispensed at opioid-treatment programs. Take-home doses of methadone may be available to patients after adherence to the regimen has been established. When used long-term, higher doses of methadone are associated with better adherence, but these higher doses can cause respiratory depression. In a study of 189 pregnant patients taking methadone to treat OUD, daily doses of 60 mg or greater were associated with better treatment retention at delivery and 60 days postpartum, as well as less use of nonprescription opioids.17 Under limited circumstances methadone can be ordered and dispensed for hospitalized patients with OUD.
Methadone is a pure opioid receptor agonist. Naloxone (NAL) is an opioid receptor antagonist. Buprenorphine (BUP) is a partial opioid receptor agonist-antagonist, which limits overdose risk. BUP often is combined with NAL as a combination formulation, which is thought to reduce the repurposing of BUP for non-prescribed uses. At appropriate treatment dosages, both methadone (≥60 mg) and BUP (≥ 16 mg) are highly effective for the treatment of OUD.1 For patients with health insurance, pharmacy benefits often provide some coverage for preferred products but no coverage for other products. Not all pharmacies carry BUP products. In a study of more than 5,000 pharmacies, approximately 60% reported that they carry and can dispense BUP medications.2
BUP monotherapy is available as generic sublingual tablets, buccal films (Belbuca), formulations for injection (Sublocade), and subcutaneous implants (Probuphine). BUPNAL is available as buccal films (Bunavail), sublingual films (Suboxone), and sublingual tablets (Zubsolv). For BUP-NAL combination productions, the following dose combinations have been reported to have similar effects: BUP-NAL 8 mg/2 mg sublingual film, BUP-NAL 5.7 mg/1.4 mg sublingual tablet, and BUP-NAL 4.2 mg/0.7 mg buccal film.3
When initiating BUP-monotherapy or BUP-NAL treatment for OUD, one approach for unobserved initiation is to instruct the patient to discontinue using opioid agonist drugs and wait for the onset of mild to moderate withdrawal symptoms. The purpose of this step is to avoid precipitating severe withdrawal symptoms caused by giving BUP or BUP-NAL to a patient who has recently used opioid drugs.
If BUP-NAL sublingual films (Suboxone) are prescribed following the onset of mild to moderate withdrawal symptoms, the patient can initiate therapy with a dose of 2 mg BUP/0.5 mg NAL or 4 mg BUP/1 mg NAL. At 60 to 120 minutes following the initial dose, if withdrawal symptoms persist, an additional dose of 4 mg BUP/1 mg NAL can be given. Thereafter, symptoms can be assessed every 60 to 120 minutes and additional doses administered to control symptoms. On the second day of therapy, a maximum of 16 mg of BUP is administered. Over the following days and weeks, if symptoms and cravings persist at a BUP dose of 16 mg, the total daily dose of BUP can be titrated up to 24 mg. For long-term treatment, a commonly prescribed daily dose is 16 mg BUP/4 mg NAL or 24 mg BUP/6 mg NAL. An absolute contraindication to BUP or BUP/NAL treatment is an allergy to the medication, and a relative contraindication is liver failure.
One potential complication of transmucosal BUP or BUP-NAL treatment is a dry mouth (xerostomia), which may contribute to dental disease.4 However, some experts question the quality of the data that contributed to the warning.5,6 Potential dental complications might be prevented by regular oral health examinations, daily flossing and teeth brushing, and stimulation of saliva by sugar-free gum or lozenges.
Primary care clinicians who initiate BUP or BUPNAL treatment for OUD often have a weekly visit with the patient during the initial phase of treatment and then every 3 to 4 weeks during maintenance therapy. Most patients need long-term treatment to achieve the goals of therapy, which include prevention of opioid overdose, reduction of cravings for nonprescription narcotics, and improvement in overall health. BUP and BUP-NAL treatment are effective without formal counseling, but counseling and social work support improve long-term adherence with treatment. Primary care clinicians who have experience with medication treatment of OUD report that their experience convinces them that medication treatment of OUD has similarities to the long-term treatment of diabetes, with antihyperglycemia medicines or the treatment of HIV infection with antiviral medications.
References
1. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;CD002207.
2. Weiner SG, Qato DM, Faust JS, et al. Pharmacy availability of buprenorphine for opioid use disorder treatment in the U.S. JAMA Netw Open. 2023;6:E2316089.
3. Substance Abuse and Mental Health Services Administration (SAMHSA). Medications for opioid use disorder. SAMHSA website. Accessed August 21, 2023. https ://store.samhsa.gov/sites/default/files/SAMHSA_Digital_Download/PEP 21-02-01-002.pdf
4. FDA warns about dental problems with buprenorphine medicines dissolved in the mouth. FDA website. Accessed August 21, 2023. https ://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-dental-problems-buprenorphine-medicines-dissolved-mouth-treat-opioiduse-disorder#:~:text=What%20did%20FDA%20find%3F,medicines%20 dissolved%20in%20the%20mouth
5. Watson DP, Etmian S, Gastala N. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1223-1224.
6. Brothers TD, Lewer D, Bonn M. Sublingual buprenorphine-naloxone exposure and dental disease. JAMA. 2023;329:1224.
Medication treatment of OUD in obstetrics
In the United States, the prevalence of OUD among pregnant patients hospitalized for delivery more than quadrupled from 1999 through 2014.18 BUP and methadone commonly are used to treat OUD during pregnancy.19 Among pregnant patients about 5% of buprenorphine prescriptions are written by obstetricians.20 An innovative approach to initiating BUP for pregnant patients with OUD is to use unobserved initiation, which involves outpatient discontinuation of nonprescription opioids to induce mild to moderate withdrawal symptoms followed by initiation of BUP treatment. In one cohort study, 55 pregnant patients used an unobserved outpatient protocol to initiate BUP treatment; 80% of the patients previously had used methadone or BUP. No patient experienced a precipitated withdrawal and 96% of patients returned for their office visit 1 week after initiation of treatment. Eighty-six percent of patients remained in treatment 3 months following initiation of BUP.21
Compared with methadone, BUP treatment during pregnancy may result in lower rates of neonatal abstinence syndrome. In one study of pregnant patients who were using methadone (n = 5,056) or BUP (n = 11,272) in late pregnancy, neonatal abstinence syndrome was diagnosed in 69.2% and 52.0% of newborns, respectively (adjusted relative risk, 0.73; 95% confidence interval, 0.71–0.75).22 In addition, compared with methadone, the use of BUP was associated with a reduced risk for low birth weight (14.9% vs 8.3%) and a lower risk for preterm birth (24.9% vs 14.4%). In this study, there were no differences in maternal obstetric outcomes when comparing BUP versus methadone treatment. Similar results have been reported in a meta-analysis analyzing the use of methadone and BUP during pregnancy.23 Studies performed to date have not shown an increased risk of congenital anomalies with the use of BUP-NAL during pregnancy.24,25
Although there may be differences in newborn outcomes with BUP and methadone, the American College of Obstetricians and Gynecologists does not recommend switching from methadone to BUP during pregnancy because precipitated withdrawal may occur.26 Based on recent studies, the American Society of Addiction Medicine has advised that it is safe to prescribe pregnant patients either BUP or BUP-NAL.27,28
Medication treatment of OUD with or without intensive counseling
The FDA recently reviewed literature related to the advantages and challenges of combining intensive counseling with medication treatment of OUD.29 The FDA noted that treatment saves lives and encouraged clinicians to initiate medication treatment of OUD or refer the patient to an appropriate clinician or treatment center. Combining medication treatment of OUD with intensive counseling is associated with greater treatment adherence and reduced health care costs. For example, in one study of 4,987 patients with OUD, initiation of counseling within 8 weeks of the start of medication treatment and a BUP dose of 16 mg or greater daily were associated with increased adherence to treatment.30 For patients receiving a BUP dose of less than 16 mg daily, treatment adherence with and without counseling was approximately 325 and 230 days, respectively. When the dose of BUP was 16 mg or greater, treatment adherence with and without counseling was approximately 405 and 320 days, respectively.30
Counseling should always be offered to patients initiating medication treatment of OUD. It should be noted that counseling alone is not a highly effective treatment for OUD.31 The FDA recently advised that the lack of availability of intensive counseling should not prevent clinicians from initiating BUP for the treatment of OUD.29 OUD is associated with a high mortalityrate and if counseling is not possible, medication treatment should be initiated. Substantial evidence demonstrates that medication treatment of OUD is associated with many benefits.16 The FDA advisory committee concluded that OUD treatment decisions should use shared decision making and be supportive and patient centered.29
The opportunities for medication treatment of OUD in primary care practice have expanded due to the recent FDA removal of restrictions on the use of BUP and heightened awareness of the positive public health impact of medication treatment. Challenges to the medication treatment of OUD remain, including stigmatization of OUD, barriers to insurance coverage for BUP, practice costs of treating OUD, and gaps in clinical education. For many pregnant patients, their main point of contact with health care is their obstetrician. By incorporating OUD treatment in pregnancy care, obstetricians will improve the health of the mother and newborn, contributing to the well-being of current and future generations. ●
Experts have recommended several interventions that may help reduce opioid overdose death.1 A consensus recommendation is that people who use drugs should be provided naloxone rescue medication and educated on the proper use of naloxone. Naloxone rescue medication is available in formulations for nasal or parenteral administration. The US Food and Drug Administration (FDA) recently has approved naloxone for over-the-counter status. The American Medical Association has provided a short web video on how to administer nasal naloxone.2 In a small pilot study, obstetricians offered every postpartum patient with naloxone administration education and a 2-dose nasal naloxone pack, with 76% of patients accepting the nasal naloxone pack.3
Many experts recommend that people who use drugs should be advised to never use them alone and to test a small amount of the drug to assess its potency. Many patients who use opioid drugs also take benzodiazepines, which can contribute to respiratory depression.4 Patients should avoid mixing drugs (eg, opioids and benzodiazepines). Some experts recommend that patients who use drugs should be provided take-home fentanyl test strips so they can evaluate their drugs for the presence of fentanyl, a medication that suppresses respiration and contributes to many overdose deaths. In addition, people who use drugs and are interested in reducing their use of drugs or managing overdose risk can be offered initiation of medication treatment of OUD.1
References
1. Wood E, Solomon ED, Hadland SE. Universal precautions for people at risk of opioid overdose in North America. JAMA Int Med. 2023;183:401-402.
2. How to administer Naloxone. AMA website. Accessed August 28, 2023. https://www.ama-assn.org /delivering-care/overdose-epidemic/how-administer-naloxone
3. Naliboff JA, Tharpe N. Universal postpartum naloxone provision: a harm reduction quality improvement project. J Addict Med. 2022;17:360-362.
4. Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
- Spencer MR, Miniño AM, Warner M. Drug overdose deaths in the United States, 20012021. NCHS Data Brief no 457. Hyattsville, MD, National Center for Health Statistics. 2022. NCHS Data Brief No. 457. Published December 2022. Accessed August 21, 2023. https://www.cdc.gov /nchs/products/databriefs/db457.htm
- US traffic deaths drop slightly in 2022 but still a ‘crisis.’ AP News website. Published April 20, 2023. Accessed August 21, 2023. https://apnews.com /article/traffic-deaths-distracted-driving-crisis -6db6471e273b275920b6c4f9eb7e493b
- Suicide statistics. American Foundation for Suicide Prevention website. Accessed August 21, 2023. https://afsp.org/suicide-statistics/
- Bruzelius E, Martins SS. US Trends in drug overdose mortality among pregnant and postpartum persons, 2017-2020. JAMA. 2022;328:2159-2161.
- Metz TD, Rovner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Schiff DM, Nielsen T, Terplan M, et al. Fatal and nonfatal overdose among pregnant and postpartum women in Massachusetts. Obstet Gynecol. 2018;132:466-474.
- Goldman-Mellor S, Margerison CE. Maternal drug-related death and suicide are leading causes of postpartum death in California. Am J Obstet Gynecol. 2019;221:489.e1-489.e9.
- Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550.
- Waiver elimination (MAT Act). SAMHSA website. Accessed August 21, 2023. https://www .samhsa.gov/medications-substance-use- disorders/removal-data-waiver-requirement
- Picco L, Middleton M, Bruno R, et al. Validation of the OWLS, a Screening Tool for Measuring Prescription Opioid Use Disorder in Primary Care. Pain Med. 2020;21:2757-2764.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Creedon TB, Ali MM, Schuman-Olivier Z. Trends in buprenorphine prescribing for opioid use disorder by psychiatrists in the US from 2003 to 2021. JAMA Health Forum. 2023;4:E230221.
- Quick MD website. Accessed August 21, 2023. https://quick.md/
- Treitler P, Nowels M, Samples H, et al. BUP utilization and prescribing among New Jersey Medicaid beneficiaries after adoption of initiatives designed to improve treatment access. JAMA Netw Open. 2023;6:E2312030.
- Jawa R, Tin Y, Nall S, et al. Estimated clinical outcomes and cost-effectiveness associated with provision of addiction treatment in US primary care clinics. JAMA Netw Open. 2023;6:E237888.
- Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways of opioid use disorder. JAMA Netw Open. 2020;3:E1920622.
- Wilder CM, Hosta D, Winhusen T. Association of methadone dose with substance use and treatment retention in pregnant and postpartum women with opioid use disorder. J Subst Abuse Treat. 2017;80:33-36.
- Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67:845-849.
- Xu KY, Jones HE, Schiff DM, et al. Initiation and treatment discontinuation of medications for opioid use disorder in pregnant people compared with nonpregnant people. Obstet Gynecol. 2023;141:845-853.
- Kelly D, Krans EE. Medical specialty of buprenorphine prescribers for pregnant women with opioid use disorder. Am J Obstet Gynecol. 2019;220:502-503.
- Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
- Suarez EA, Huybrechts KF, Straub L, et al. Buprenorphine versus methadone for opioid use disorder in pregnancy. N Engl J Med. 2022;387:2033-2044.
- Kinsella M, Halliday LO, Shaw M, et al. Buprenorphine compared with methadone in pregnancy: a systematic review and meta-analysis. Subst Use Misuse. 2022;57:1400-1416.
- Jumah NA, Edwards C, Balfour-Boehm J, et al. Observational study of the safety of buprenorphine-naloxone in pregnancy in a rural and remote population. BMJ Open. 2016;6:E011774.
- Mullins N, Galvin SL, Ramage M, et al. Buprenorphine and naloxone versus buprenorphine for opioid use disorder in pregnancy: a cohort study. J Addict Med. 2020;14:185-192.
- Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:E81-E94.
- The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update. J Addict Med. 2020;14(2S suppl 1):1-91.
- Link HM, Jones H, Miller L, et al. Buprenorphinenaloxone use in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100179.
- Delphin-Rittmon ME, Cavazzoni P. US Food and Drug Administration website. https://www.fda .gov/media/168027/download
- Eren K, Schuster J, Herschell A, et al. Association of Counseling and Psychotherapy on retention in medication for addiction treatment within a large Medicaid population. J Addict Med. 2022;16:346353.
- Kakko J, Dybrandt Svanborg K, Kreek MJ, et al. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomized, placebo-controlled trial. Lancet. 2003;361:662-668.
- Spencer MR, Miniño AM, Warner M. Drug overdose deaths in the United States, 20012021. NCHS Data Brief no 457. Hyattsville, MD, National Center for Health Statistics. 2022. NCHS Data Brief No. 457. Published December 2022. Accessed August 21, 2023. https://www.cdc.gov /nchs/products/databriefs/db457.htm
- US traffic deaths drop slightly in 2022 but still a ‘crisis.’ AP News website. Published April 20, 2023. Accessed August 21, 2023. https://apnews.com /article/traffic-deaths-distracted-driving-crisis -6db6471e273b275920b6c4f9eb7e493b
- Suicide statistics. American Foundation for Suicide Prevention website. Accessed August 21, 2023. https://afsp.org/suicide-statistics/
- Bruzelius E, Martins SS. US Trends in drug overdose mortality among pregnant and postpartum persons, 2017-2020. JAMA. 2022;328:2159-2161.
- Metz TD, Rovner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Schiff DM, Nielsen T, Terplan M, et al. Fatal and nonfatal overdose among pregnant and postpartum women in Massachusetts. Obstet Gynecol. 2018;132:466-474.
- Goldman-Mellor S, Margerison CE. Maternal drug-related death and suicide are leading causes of postpartum death in California. Am J Obstet Gynecol. 2019;221:489.e1-489.e9.
- Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550.
- Waiver elimination (MAT Act). SAMHSA website. Accessed August 21, 2023. https://www .samhsa.gov/medications-substance-use- disorders/removal-data-waiver-requirement
- Picco L, Middleton M, Bruno R, et al. Validation of the OWLS, a Screening Tool for Measuring Prescription Opioid Use Disorder in Primary Care. Pain Med. 2020;21:2757-2764.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Creedon TB, Ali MM, Schuman-Olivier Z. Trends in buprenorphine prescribing for opioid use disorder by psychiatrists in the US from 2003 to 2021. JAMA Health Forum. 2023;4:E230221.
- Quick MD website. Accessed August 21, 2023. https://quick.md/
- Treitler P, Nowels M, Samples H, et al. BUP utilization and prescribing among New Jersey Medicaid beneficiaries after adoption of initiatives designed to improve treatment access. JAMA Netw Open. 2023;6:E2312030.
- Jawa R, Tin Y, Nall S, et al. Estimated clinical outcomes and cost-effectiveness associated with provision of addiction treatment in US primary care clinics. JAMA Netw Open. 2023;6:E237888.
- Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways of opioid use disorder. JAMA Netw Open. 2020;3:E1920622.
- Wilder CM, Hosta D, Winhusen T. Association of methadone dose with substance use and treatment retention in pregnant and postpartum women with opioid use disorder. J Subst Abuse Treat. 2017;80:33-36.
- Haight SC, Ko JY, Tong VT, et al. Opioid use disorder documented at delivery hospitalization - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67:845-849.
- Xu KY, Jones HE, Schiff DM, et al. Initiation and treatment discontinuation of medications for opioid use disorder in pregnant people compared with nonpregnant people. Obstet Gynecol. 2023;141:845-853.
- Kelly D, Krans EE. Medical specialty of buprenorphine prescribers for pregnant women with opioid use disorder. Am J Obstet Gynecol. 2019;220:502-503.
- Kelly JC, Raghuraman N, Stout MJ, et al. Home induction of buprenorphine for treatment of opioid use disorder in pregnancy. Obstet Gynecol. 2021;138:655-659.
- Suarez EA, Huybrechts KF, Straub L, et al. Buprenorphine versus methadone for opioid use disorder in pregnancy. N Engl J Med. 2022;387:2033-2044.
- Kinsella M, Halliday LO, Shaw M, et al. Buprenorphine compared with methadone in pregnancy: a systematic review and meta-analysis. Subst Use Misuse. 2022;57:1400-1416.
- Jumah NA, Edwards C, Balfour-Boehm J, et al. Observational study of the safety of buprenorphine-naloxone in pregnancy in a rural and remote population. BMJ Open. 2016;6:E011774.
- Mullins N, Galvin SL, Ramage M, et al. Buprenorphine and naloxone versus buprenorphine for opioid use disorder in pregnancy: a cohort study. J Addict Med. 2020;14:185-192.
- Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:E81-E94.
- The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update. J Addict Med. 2020;14(2S suppl 1):1-91.
- Link HM, Jones H, Miller L, et al. Buprenorphinenaloxone use in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100179.
- Delphin-Rittmon ME, Cavazzoni P. US Food and Drug Administration website. https://www.fda .gov/media/168027/download
- Eren K, Schuster J, Herschell A, et al. Association of Counseling and Psychotherapy on retention in medication for addiction treatment within a large Medicaid population. J Addict Med. 2022;16:346353.
- Kakko J, Dybrandt Svanborg K, Kreek MJ, et al. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomized, placebo-controlled trial. Lancet. 2003;361:662-668.
Thoughts on the CDC update on opioid prescribing guidelines
The media is filled with stories about the opioid crisis. We have all heard the horror stories of addiction and overdose, as well as “pill mill” doctors. In fact, more than 932,000 people have died of drug overdose since 1999 and, in recent years, approximately 75% of drug overdoses involved opioids.
Yet, they still have their place in the treatment of pain.
The CDC updated the 2016 guidelines for prescribing opioids for pain in 2022. They cover when to initiate prescribing of opioids, selecting appropriate opioids and doses, and deciding the duration of therapy. The guidelines do a great job providing evidence-based recommendations while at the same time keeping the problems with opioids in the picture.
For primary care doctors, pain is one of the most common complaints we see – from broken bones to low back pain to cancer pain. It is important to note that the current guidelines exclude pain from sickle cell disease, cancer-related pain, palliative care, and end-of-life care. The guidelines apply to acute, subacute, and chronic pain. Pain is a complex symptom and often needs a multipronged approach. We make a mistake if we just prescribe a pain medication without understanding the root cause of the pain.
The guidelines suggest starting with nonopioid medications and incorporating nonmedicinal modes of treatments, such as physical therapy, as well. Opioids should be started at the lowest dose and for the shortest duration. Immediate-release medications are preferred over long-acting or extended-release ones. The patient should always be informed of the risks and benefits.
While the guidelines do a great job recommending how to prescribe opioids, they do not go into any depth discussing other treatment options. Perhaps knowledge of other treatment modalities would help primary care physicians avoid opioid prescribing. When treating our patients, it is important to educate them on how to manage their own symptoms.
The guidelines also advise tapering patients who may have been on high-dose opioids for long periods of time. Doctors know this is a very difficult task. However, resources to help with this are often lacking. For example, rehab may not be covered under a patient’s insurance, or it may be cheaper to take an opioid than to go to physical therapy. Although the recommendation is to taper, community assets may not support this. Guidelines are one thing, but the rest of the health care system needs to catch up to them and make them practical.
Primary care doctors often utilize our physical medicine, rehabilitation, and pain management specialists to assist in managing our patients’ pain. Here too, access to this resource is often difficult to come by. Depending on a patient’s insurance, it can take months to get an appointment.
In general, the current guidelines offer 12 key recommendations when prescribing opioids. They are a great reference; however, we need more real-life tools. For many of us in primary care, these guidelines support what we’ve been doing all along.
Primary care doctors will surely play a huge role in addressing the opioid crisis. We can prescribe opioids appropriately, but it doesn’t erase the problems of those patients who were overprescribed in the past. Many still seek out these medications whether for monetary reasons or just for the high. It is often easy to blame the patient but the one in control is the one with the prescription pad. Yet, it is important to remember that many of these patients are in real pain and need help.
Often, it is simpler to just prescribe a pain medication than it is to explain why one is not appropriate. As primary care doctors, we need to be effective ambassadors of appropriate opioid prescribing and often that means doing the hard thing and saying no to a patient.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
fpnews@mdedge.com
The media is filled with stories about the opioid crisis. We have all heard the horror stories of addiction and overdose, as well as “pill mill” doctors. In fact, more than 932,000 people have died of drug overdose since 1999 and, in recent years, approximately 75% of drug overdoses involved opioids.
Yet, they still have their place in the treatment of pain.
The CDC updated the 2016 guidelines for prescribing opioids for pain in 2022. They cover when to initiate prescribing of opioids, selecting appropriate opioids and doses, and deciding the duration of therapy. The guidelines do a great job providing evidence-based recommendations while at the same time keeping the problems with opioids in the picture.
For primary care doctors, pain is one of the most common complaints we see – from broken bones to low back pain to cancer pain. It is important to note that the current guidelines exclude pain from sickle cell disease, cancer-related pain, palliative care, and end-of-life care. The guidelines apply to acute, subacute, and chronic pain. Pain is a complex symptom and often needs a multipronged approach. We make a mistake if we just prescribe a pain medication without understanding the root cause of the pain.
The guidelines suggest starting with nonopioid medications and incorporating nonmedicinal modes of treatments, such as physical therapy, as well. Opioids should be started at the lowest dose and for the shortest duration. Immediate-release medications are preferred over long-acting or extended-release ones. The patient should always be informed of the risks and benefits.
While the guidelines do a great job recommending how to prescribe opioids, they do not go into any depth discussing other treatment options. Perhaps knowledge of other treatment modalities would help primary care physicians avoid opioid prescribing. When treating our patients, it is important to educate them on how to manage their own symptoms.
The guidelines also advise tapering patients who may have been on high-dose opioids for long periods of time. Doctors know this is a very difficult task. However, resources to help with this are often lacking. For example, rehab may not be covered under a patient’s insurance, or it may be cheaper to take an opioid than to go to physical therapy. Although the recommendation is to taper, community assets may not support this. Guidelines are one thing, but the rest of the health care system needs to catch up to them and make them practical.
Primary care doctors often utilize our physical medicine, rehabilitation, and pain management specialists to assist in managing our patients’ pain. Here too, access to this resource is often difficult to come by. Depending on a patient’s insurance, it can take months to get an appointment.
In general, the current guidelines offer 12 key recommendations when prescribing opioids. They are a great reference; however, we need more real-life tools. For many of us in primary care, these guidelines support what we’ve been doing all along.
Primary care doctors will surely play a huge role in addressing the opioid crisis. We can prescribe opioids appropriately, but it doesn’t erase the problems of those patients who were overprescribed in the past. Many still seek out these medications whether for monetary reasons or just for the high. It is often easy to blame the patient but the one in control is the one with the prescription pad. Yet, it is important to remember that many of these patients are in real pain and need help.
Often, it is simpler to just prescribe a pain medication than it is to explain why one is not appropriate. As primary care doctors, we need to be effective ambassadors of appropriate opioid prescribing and often that means doing the hard thing and saying no to a patient.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
fpnews@mdedge.com
The media is filled with stories about the opioid crisis. We have all heard the horror stories of addiction and overdose, as well as “pill mill” doctors. In fact, more than 932,000 people have died of drug overdose since 1999 and, in recent years, approximately 75% of drug overdoses involved opioids.
Yet, they still have their place in the treatment of pain.
The CDC updated the 2016 guidelines for prescribing opioids for pain in 2022. They cover when to initiate prescribing of opioids, selecting appropriate opioids and doses, and deciding the duration of therapy. The guidelines do a great job providing evidence-based recommendations while at the same time keeping the problems with opioids in the picture.
For primary care doctors, pain is one of the most common complaints we see – from broken bones to low back pain to cancer pain. It is important to note that the current guidelines exclude pain from sickle cell disease, cancer-related pain, palliative care, and end-of-life care. The guidelines apply to acute, subacute, and chronic pain. Pain is a complex symptom and often needs a multipronged approach. We make a mistake if we just prescribe a pain medication without understanding the root cause of the pain.
The guidelines suggest starting with nonopioid medications and incorporating nonmedicinal modes of treatments, such as physical therapy, as well. Opioids should be started at the lowest dose and for the shortest duration. Immediate-release medications are preferred over long-acting or extended-release ones. The patient should always be informed of the risks and benefits.
While the guidelines do a great job recommending how to prescribe opioids, they do not go into any depth discussing other treatment options. Perhaps knowledge of other treatment modalities would help primary care physicians avoid opioid prescribing. When treating our patients, it is important to educate them on how to manage their own symptoms.
The guidelines also advise tapering patients who may have been on high-dose opioids for long periods of time. Doctors know this is a very difficult task. However, resources to help with this are often lacking. For example, rehab may not be covered under a patient’s insurance, or it may be cheaper to take an opioid than to go to physical therapy. Although the recommendation is to taper, community assets may not support this. Guidelines are one thing, but the rest of the health care system needs to catch up to them and make them practical.
Primary care doctors often utilize our physical medicine, rehabilitation, and pain management specialists to assist in managing our patients’ pain. Here too, access to this resource is often difficult to come by. Depending on a patient’s insurance, it can take months to get an appointment.
In general, the current guidelines offer 12 key recommendations when prescribing opioids. They are a great reference; however, we need more real-life tools. For many of us in primary care, these guidelines support what we’ve been doing all along.
Primary care doctors will surely play a huge role in addressing the opioid crisis. We can prescribe opioids appropriately, but it doesn’t erase the problems of those patients who were overprescribed in the past. Many still seek out these medications whether for monetary reasons or just for the high. It is often easy to blame the patient but the one in control is the one with the prescription pad. Yet, it is important to remember that many of these patients are in real pain and need help.
Often, it is simpler to just prescribe a pain medication than it is to explain why one is not appropriate. As primary care doctors, we need to be effective ambassadors of appropriate opioid prescribing and often that means doing the hard thing and saying no to a patient.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
fpnews@mdedge.com
First target doesn’t affect survival in NSCLC with brain metastases
“The findings of our study highlight the importance of adopting a personalized, case-based approach when treating each patient” instead of always treating the brain or lung first, lead author Arvind Kumar, a medical student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
The study was released at European Lung Cancer Congress 2023.
According to the author, current guidelines recommend treating the brain first in patients with non–small cell lung cancer and a tumor that has spread to the brain.
“Determining whether the brain or body gets treated first depends on where the symptoms are coming from, how severe the symptoms are, how bulky the disease is, and how long the treatment to each is expected to take,” radiation oncologist Henry S. Park, MD, MPH, chief of the thoracic radiotherapy program at Yale University, New Haven, Conn., said in an interview. “Often the brain is treated first since surgery is used for both diagnosis of metastatic disease as well as removal of the brain metastasis, especially if it is causing symptoms. The radiosurgery that follows tends to occur within a day or a few days.”
However, he said, “if the brain disease is small and not causing symptoms, and the lung disease is more problematic, then we will often treat the body first and fit in the brain treatment later.”
For the new study, researchers identified 1,044 patients in the National Cancer Database with non–small cell lung cancer and brain metastases who received systemic therapy plus surgery, brain stereotactic radiosurgery, or lung radiation. All were treated from 2010 to 2019; 79.0% received brain treatment first, and the other 21.0% received lung treatment first.
There was no statistically significant difference in overall survival between those whose brains were treated first and those whose lungs were treated first (hazard ratio, 1.24, 95% confidence interval [CI], 0.91-1.70, P = .17). A propensity score–matched analysis turned up no difference in 5-year survival (38.2% of those whose brains were treated first, 95% CI, 27.5-34.4, vs. 38.0% of those whose lungs were treated first, 95% CI, 29.9-44.7, P = .32.)
“These results were consistent regardless of which combination of treatment modalities the patient received – neurosurgery versus brain stereotactic radiosurgery, thoracic surgery versus thoracic radiation,” the author said.
He cautioned that “our study only included patients who were considered candidates for either surgery or radiation to both the brain and lung. The results of our study should therefore be cautiously interpreted for patients who may have contraindications to such treatment.”
Dr. Park, who didn’t take part in the study, said “the results are consistent with what I would generally expect.”
He added: “The take-home message for clinicians should be that there is no one correct answer in how to manage non–small cell lung cancer with synchronous limited metastatic disease in only the brain. If the brain disease is bulky and/or causes symptoms while the body disease isn’t – or if a biopsy or surgery is required to prove that the patient in fact has metastatic disease – then the brain disease should be treated first. On the other hand, if the body disease is bulky and/or causing symptoms while the brain disease isn’t – and there is no need for surgery but rather only a biopsy of the brain – then the body disease can be treated first.”
No funding was reported. The study authors and Dr. Park reported no financial conflicts or other disclosures.
“The findings of our study highlight the importance of adopting a personalized, case-based approach when treating each patient” instead of always treating the brain or lung first, lead author Arvind Kumar, a medical student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
The study was released at European Lung Cancer Congress 2023.
According to the author, current guidelines recommend treating the brain first in patients with non–small cell lung cancer and a tumor that has spread to the brain.
“Determining whether the brain or body gets treated first depends on where the symptoms are coming from, how severe the symptoms are, how bulky the disease is, and how long the treatment to each is expected to take,” radiation oncologist Henry S. Park, MD, MPH, chief of the thoracic radiotherapy program at Yale University, New Haven, Conn., said in an interview. “Often the brain is treated first since surgery is used for both diagnosis of metastatic disease as well as removal of the brain metastasis, especially if it is causing symptoms. The radiosurgery that follows tends to occur within a day or a few days.”
However, he said, “if the brain disease is small and not causing symptoms, and the lung disease is more problematic, then we will often treat the body first and fit in the brain treatment later.”
For the new study, researchers identified 1,044 patients in the National Cancer Database with non–small cell lung cancer and brain metastases who received systemic therapy plus surgery, brain stereotactic radiosurgery, or lung radiation. All were treated from 2010 to 2019; 79.0% received brain treatment first, and the other 21.0% received lung treatment first.
There was no statistically significant difference in overall survival between those whose brains were treated first and those whose lungs were treated first (hazard ratio, 1.24, 95% confidence interval [CI], 0.91-1.70, P = .17). A propensity score–matched analysis turned up no difference in 5-year survival (38.2% of those whose brains were treated first, 95% CI, 27.5-34.4, vs. 38.0% of those whose lungs were treated first, 95% CI, 29.9-44.7, P = .32.)
“These results were consistent regardless of which combination of treatment modalities the patient received – neurosurgery versus brain stereotactic radiosurgery, thoracic surgery versus thoracic radiation,” the author said.
He cautioned that “our study only included patients who were considered candidates for either surgery or radiation to both the brain and lung. The results of our study should therefore be cautiously interpreted for patients who may have contraindications to such treatment.”
Dr. Park, who didn’t take part in the study, said “the results are consistent with what I would generally expect.”
He added: “The take-home message for clinicians should be that there is no one correct answer in how to manage non–small cell lung cancer with synchronous limited metastatic disease in only the brain. If the brain disease is bulky and/or causes symptoms while the body disease isn’t – or if a biopsy or surgery is required to prove that the patient in fact has metastatic disease – then the brain disease should be treated first. On the other hand, if the body disease is bulky and/or causing symptoms while the brain disease isn’t – and there is no need for surgery but rather only a biopsy of the brain – then the body disease can be treated first.”
No funding was reported. The study authors and Dr. Park reported no financial conflicts or other disclosures.
“The findings of our study highlight the importance of adopting a personalized, case-based approach when treating each patient” instead of always treating the brain or lung first, lead author Arvind Kumar, a medical student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
The study was released at European Lung Cancer Congress 2023.
According to the author, current guidelines recommend treating the brain first in patients with non–small cell lung cancer and a tumor that has spread to the brain.
“Determining whether the brain or body gets treated first depends on where the symptoms are coming from, how severe the symptoms are, how bulky the disease is, and how long the treatment to each is expected to take,” radiation oncologist Henry S. Park, MD, MPH, chief of the thoracic radiotherapy program at Yale University, New Haven, Conn., said in an interview. “Often the brain is treated first since surgery is used for both diagnosis of metastatic disease as well as removal of the brain metastasis, especially if it is causing symptoms. The radiosurgery that follows tends to occur within a day or a few days.”
However, he said, “if the brain disease is small and not causing symptoms, and the lung disease is more problematic, then we will often treat the body first and fit in the brain treatment later.”
For the new study, researchers identified 1,044 patients in the National Cancer Database with non–small cell lung cancer and brain metastases who received systemic therapy plus surgery, brain stereotactic radiosurgery, or lung radiation. All were treated from 2010 to 2019; 79.0% received brain treatment first, and the other 21.0% received lung treatment first.
There was no statistically significant difference in overall survival between those whose brains were treated first and those whose lungs were treated first (hazard ratio, 1.24, 95% confidence interval [CI], 0.91-1.70, P = .17). A propensity score–matched analysis turned up no difference in 5-year survival (38.2% of those whose brains were treated first, 95% CI, 27.5-34.4, vs. 38.0% of those whose lungs were treated first, 95% CI, 29.9-44.7, P = .32.)
“These results were consistent regardless of which combination of treatment modalities the patient received – neurosurgery versus brain stereotactic radiosurgery, thoracic surgery versus thoracic radiation,” the author said.
He cautioned that “our study only included patients who were considered candidates for either surgery or radiation to both the brain and lung. The results of our study should therefore be cautiously interpreted for patients who may have contraindications to such treatment.”
Dr. Park, who didn’t take part in the study, said “the results are consistent with what I would generally expect.”
He added: “The take-home message for clinicians should be that there is no one correct answer in how to manage non–small cell lung cancer with synchronous limited metastatic disease in only the brain. If the brain disease is bulky and/or causes symptoms while the body disease isn’t – or if a biopsy or surgery is required to prove that the patient in fact has metastatic disease – then the brain disease should be treated first. On the other hand, if the body disease is bulky and/or causing symptoms while the brain disease isn’t – and there is no need for surgery but rather only a biopsy of the brain – then the body disease can be treated first.”
No funding was reported. The study authors and Dr. Park reported no financial conflicts or other disclosures.
FROM ELCC 2023
Watch for buprenorphine ‘spiking’ in urine drug tests
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Urine drug testing can be valuable for monitoring patients undergoing treatment with buprenorphine for opioid use disorder (OUD). However, some patients alter their test results by adding buprenorphine directly to their urine sample to imply adherence, a new study shows.
“I anticipate a much-needed increase” in the number of people gaining access to buprenorphine therapy, given elimination of the X waiver, first author Jarratt D. Pytell, MD, with University of Colorado at Denver, Aurora, said in a statement.
“New prescribers of buprenorphine will need to learn how to conduct the increasingly complex initiation of treatment and then gauge whether it is successful or not,” added Dr. Pytell, a general internist and addiction medicine specialist.
“Spiking suggests that treatment is not working – especially in patients continuing illicit drug use. Detecting spiking allows clinicians to adjust or intensify the treatment plan,” Dr. Pytell said in an interview.
The study was published online in JAMA Psychiatry.
A sign of elevated patient risk
In a cross-sectional study using Millennium Health’s proprietary urine drug test (UDT) database, researchers analyzed 507,735 urine specimens from 58,476 OUD patients collected between January 2017 and April 2022.
A total of 9546 (1.9%) specimens from 4,550 patients (7.6%) were suggestive of spiking.
UDT specimens suggestive of spiking had two times the odds of being positive for other opioids (fentanyl or heroin), compared with opioid negative samples.
UDT specimens obtained from primary care clinics, from patients aged 35-44 years, and from patients living in the South Atlantic region of the United States were also more likely to be suggestive of buprenorphine spiking.
“Our study demonstrated that a buprenorphine to norbuprenorphine ratio of less than 0.02 indicates the possibility of spiking,” Dr. Pytell said in an interview.
“Nevertheless, it is important to note that this cutoff is not a definitive standard and further controlled studies are necessary to determine its predictive value for spiking. But recognizing possible spiking is very important since it demonstrates a point of elevated risk for the patient and the treatment approach should be reconsidered,” Dr. Pytell said.
“At Millennium Health, we have been tracking the enormity of the drug use crisis. This study suggests that spiking is an important patient safety issue, and it is not uncommon,” study coauthor Eric Dawson, PharmD, vice president of clinical affairs, Millennium Health, said in a statement.
“Detection of spiking requires definitive drug testing. Immunoassay-based, point-of-care tests cannot detect spiking because they are generally incapable of quantitative analysis and differentiating buprenorphine from norbuprenorphine,” Dr. Dawson said.
Best practices?
“We need to develop best practices specific for this situation where a patient has added buprenorphine to the urine drug test specimen,” said Dr. Pytell.
“As with all unexpected findings, it is crucial for clinicians to approach this finding in a nonjudgmental manner and work with the patient to understand what might have motivated them to alter their urine specimen,” he added.
Dr. Pytell said a common reaction for clinicians might be to discontinue treatment. However, this is actually a time to try and engage with the patient.
“Clinicians should work collaboratively with patients to identify potential reasons for spiking and determine what changes may need to be made to better support the patient’s recovery,” Dr. Pytell said.
“This could include more frequent monitoring or referral to a higher level of care. In addition, clinicians should be aware that patients who engage in spiking may be experiencing other challenges that impact their ability to adhere to treatment, such as inadequate housing, mental health issues, or financial strain. Addressing these underlying issues may help patients overcome barriers to treatment adherence and reduce the likelihood of future spiking,” Dr. Pytell said.
This study was supported by Millennium Health. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Opioid overdose is an important cause of postpartum death
(OUD), according to research published in Obstetrics and Gynecology.
Opioid overdose deaths account for up to 10% of pregnancy-associated deaths in the United States, and 75% of the deliveries of women with OUD are covered by Medicaid, according to lead author Elizabeth Suarez, PhD, MPH, with the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues.
Nearly 5 million deliveries studied
Researchers studied claims data from Medicaid and the National Death Index database in the United States from 2006 to 2013 for 4,972,061 deliveries. They also identified a subgroup of women with a documented history of OUD in the 3 months before delivery.
They found the incidence of postpartum opioid overdose deaths was 5.4 per 100,000 deliveries (95% confidence interval, 4.5-6.4) among all in the study and 118 per 100,000 (95% CI, 84-163) among individuals with OUD.
Incidence of all-cause postpartum death was six times higher in women with OUD than in all the women studied. Common causes of death of those with OUD were other drug- and alcohol-related deaths (47/100,000); suicide (26/100,000); and other injuries, including accidents and falls (33/100,000).
Risk factors strongly linked with postpartum opioid overdose death included mental health and other substance use disorders.
Medication significantly lowers death risk
The authors also documented the benefit of buprenorphine or methadone for OUD.
For women with OUD who used medication to treat OUD post partum, odds of opioid overdose death were 60% lower (odds ratio, 0.4; 95% CI 0.1-0.9).
As important as use of medication, Marcela Smid, MD, MS, writes in an accompanying editorial, is noting that 80% of the women in this study who died of opioid overdoses had contact with a health care provider before death.
“Both of these results indicate that we have the means and opportunity to prevent these deaths,” writes Dr. Smid, with the division of maternal fetal medicine, University of Utah Health in Salt Lake City.
Dismal numbers on ob.gyns. trained to prescribe medications
She points out some barriers, however. Most clinicians, she notes, lack time and training to prescribe buprenorphine, and in 2019, fewer than 2% of ob.gyns. who accept Medicaid were able to prescribe it.
Her charge to ob.gyns.: “We need to help identify individuals who are at high risk of OUD or opioid overdose by screening.” A validated screening tool should be used at prenatal and postpartum appointments.
On a bigger scale, she urges Medicaid to be expanded for a full year post partum through the American Rescue Act’s State Plan Amendment, something only 28 states and Washington, D.C., have done so far.
Dr. Smid points out some good news, however: President Joe Biden signed the Consolidated Appropriations Act 2023, which eliminated the “X” waiver.
Now all clinicians who have a Drug Enforcement Administration registration that includes Schedule III authority can prescribe buprenorphine for OUD if applicable state law allows it.
But that calls for medical schools and residency programs to prioritize addiction medicine as a core competency, Dr. Smid says.
Getting naloxone to patients, families
One of the potential interventions the study authors suggest is providing naloxone prescriptions and training to pregnant and postpartum women who have a substance use history and to their partners and significant others.
However, Mishka Terplan, MD, MPH, told this publication, “It’s one thing to write a prescription; it’s another thing for the person to actually get the medication.” He is medical director of the Friends Research Institute in Baltimore, an ob.gyn. who specializes in addiction medicine.
“What can we do?” We can think about how to get naloxone into people’s hands at discharge from the hospital after they give birth, instead of prescribing. That would mean that health systems need to prioritize this, he said. “We give people discharge medications all the time.”
Still, naloxone can’t be seen as the answer, he said.
He compares it to defibrillators in public places, which are for rescues, not reversing a population problem.
“Some people think that naloxone reversals are doing something about OUD. It’s doing about as much about OUD as defibrillators do for cardiovascular disease,” he said.
The best help, he says, will be continuation of treatment.
“Addiction is a chronic condition,” he says, “but often we only provide episodic care. We see that particularly in pregnancy. Once the pregnancy is finished, there’s not categorical continuation of insurance.”
Even if you do have insurance, it’s hard to find a clinic that’s family friendly, he notes. “You might not feel comfortable taking your newborn and standing in line in the morning to get your daily methodone dose. We have to make those environments more welcoming.”
Problem probably understated
He also says that though the study was well done given the data available, he’s frustrated that researchers still have to depend on billing data and can’t capture factors such as child care availability, living wages, and continuation of health insurance. Additionally, not everyone is coded correctly for OUD.
“It’s all Medicaid, so it’s only people who continued with care,” he pointed out. That means these numbers may actually underrepresent the problem.
Still, he says it’s important to realize the magnitude of deaths this study does highlight in this population.
In people with OUD in the postpartum period, the deaths are more than 1 in 1,000.
“That should be alarming,” Dr. Terplan said. “That’s a very big number from a public health perspective.”
Coauthor Kathryn J. Gray received payment from Aetion Inc., Roche, and BillionToOne. Funds were paid to the University of Utah for Dr. Smid from Alydia Inc. for being the site principal investigator for a study of the JADA device, and from Gilead for Dr. Smid’s study of hepatitis C in pregnancy; she was also a consultant for Organon and Rhia Ventures. Dr. Terplan reports no relevant financial relationships.
(OUD), according to research published in Obstetrics and Gynecology.
Opioid overdose deaths account for up to 10% of pregnancy-associated deaths in the United States, and 75% of the deliveries of women with OUD are covered by Medicaid, according to lead author Elizabeth Suarez, PhD, MPH, with the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues.
Nearly 5 million deliveries studied
Researchers studied claims data from Medicaid and the National Death Index database in the United States from 2006 to 2013 for 4,972,061 deliveries. They also identified a subgroup of women with a documented history of OUD in the 3 months before delivery.
They found the incidence of postpartum opioid overdose deaths was 5.4 per 100,000 deliveries (95% confidence interval, 4.5-6.4) among all in the study and 118 per 100,000 (95% CI, 84-163) among individuals with OUD.
Incidence of all-cause postpartum death was six times higher in women with OUD than in all the women studied. Common causes of death of those with OUD were other drug- and alcohol-related deaths (47/100,000); suicide (26/100,000); and other injuries, including accidents and falls (33/100,000).
Risk factors strongly linked with postpartum opioid overdose death included mental health and other substance use disorders.
Medication significantly lowers death risk
The authors also documented the benefit of buprenorphine or methadone for OUD.
For women with OUD who used medication to treat OUD post partum, odds of opioid overdose death were 60% lower (odds ratio, 0.4; 95% CI 0.1-0.9).
As important as use of medication, Marcela Smid, MD, MS, writes in an accompanying editorial, is noting that 80% of the women in this study who died of opioid overdoses had contact with a health care provider before death.
“Both of these results indicate that we have the means and opportunity to prevent these deaths,” writes Dr. Smid, with the division of maternal fetal medicine, University of Utah Health in Salt Lake City.
Dismal numbers on ob.gyns. trained to prescribe medications
She points out some barriers, however. Most clinicians, she notes, lack time and training to prescribe buprenorphine, and in 2019, fewer than 2% of ob.gyns. who accept Medicaid were able to prescribe it.
Her charge to ob.gyns.: “We need to help identify individuals who are at high risk of OUD or opioid overdose by screening.” A validated screening tool should be used at prenatal and postpartum appointments.
On a bigger scale, she urges Medicaid to be expanded for a full year post partum through the American Rescue Act’s State Plan Amendment, something only 28 states and Washington, D.C., have done so far.
Dr. Smid points out some good news, however: President Joe Biden signed the Consolidated Appropriations Act 2023, which eliminated the “X” waiver.
Now all clinicians who have a Drug Enforcement Administration registration that includes Schedule III authority can prescribe buprenorphine for OUD if applicable state law allows it.
But that calls for medical schools and residency programs to prioritize addiction medicine as a core competency, Dr. Smid says.
Getting naloxone to patients, families
One of the potential interventions the study authors suggest is providing naloxone prescriptions and training to pregnant and postpartum women who have a substance use history and to their partners and significant others.
However, Mishka Terplan, MD, MPH, told this publication, “It’s one thing to write a prescription; it’s another thing for the person to actually get the medication.” He is medical director of the Friends Research Institute in Baltimore, an ob.gyn. who specializes in addiction medicine.
“What can we do?” We can think about how to get naloxone into people’s hands at discharge from the hospital after they give birth, instead of prescribing. That would mean that health systems need to prioritize this, he said. “We give people discharge medications all the time.”
Still, naloxone can’t be seen as the answer, he said.
He compares it to defibrillators in public places, which are for rescues, not reversing a population problem.
“Some people think that naloxone reversals are doing something about OUD. It’s doing about as much about OUD as defibrillators do for cardiovascular disease,” he said.
The best help, he says, will be continuation of treatment.
“Addiction is a chronic condition,” he says, “but often we only provide episodic care. We see that particularly in pregnancy. Once the pregnancy is finished, there’s not categorical continuation of insurance.”
Even if you do have insurance, it’s hard to find a clinic that’s family friendly, he notes. “You might not feel comfortable taking your newborn and standing in line in the morning to get your daily methodone dose. We have to make those environments more welcoming.”
Problem probably understated
He also says that though the study was well done given the data available, he’s frustrated that researchers still have to depend on billing data and can’t capture factors such as child care availability, living wages, and continuation of health insurance. Additionally, not everyone is coded correctly for OUD.
“It’s all Medicaid, so it’s only people who continued with care,” he pointed out. That means these numbers may actually underrepresent the problem.
Still, he says it’s important to realize the magnitude of deaths this study does highlight in this population.
In people with OUD in the postpartum period, the deaths are more than 1 in 1,000.
“That should be alarming,” Dr. Terplan said. “That’s a very big number from a public health perspective.”
Coauthor Kathryn J. Gray received payment from Aetion Inc., Roche, and BillionToOne. Funds were paid to the University of Utah for Dr. Smid from Alydia Inc. for being the site principal investigator for a study of the JADA device, and from Gilead for Dr. Smid’s study of hepatitis C in pregnancy; she was also a consultant for Organon and Rhia Ventures. Dr. Terplan reports no relevant financial relationships.
(OUD), according to research published in Obstetrics and Gynecology.
Opioid overdose deaths account for up to 10% of pregnancy-associated deaths in the United States, and 75% of the deliveries of women with OUD are covered by Medicaid, according to lead author Elizabeth Suarez, PhD, MPH, with the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston, and colleagues.
Nearly 5 million deliveries studied
Researchers studied claims data from Medicaid and the National Death Index database in the United States from 2006 to 2013 for 4,972,061 deliveries. They also identified a subgroup of women with a documented history of OUD in the 3 months before delivery.
They found the incidence of postpartum opioid overdose deaths was 5.4 per 100,000 deliveries (95% confidence interval, 4.5-6.4) among all in the study and 118 per 100,000 (95% CI, 84-163) among individuals with OUD.
Incidence of all-cause postpartum death was six times higher in women with OUD than in all the women studied. Common causes of death of those with OUD were other drug- and alcohol-related deaths (47/100,000); suicide (26/100,000); and other injuries, including accidents and falls (33/100,000).
Risk factors strongly linked with postpartum opioid overdose death included mental health and other substance use disorders.
Medication significantly lowers death risk
The authors also documented the benefit of buprenorphine or methadone for OUD.
For women with OUD who used medication to treat OUD post partum, odds of opioid overdose death were 60% lower (odds ratio, 0.4; 95% CI 0.1-0.9).
As important as use of medication, Marcela Smid, MD, MS, writes in an accompanying editorial, is noting that 80% of the women in this study who died of opioid overdoses had contact with a health care provider before death.
“Both of these results indicate that we have the means and opportunity to prevent these deaths,” writes Dr. Smid, with the division of maternal fetal medicine, University of Utah Health in Salt Lake City.
Dismal numbers on ob.gyns. trained to prescribe medications
She points out some barriers, however. Most clinicians, she notes, lack time and training to prescribe buprenorphine, and in 2019, fewer than 2% of ob.gyns. who accept Medicaid were able to prescribe it.
Her charge to ob.gyns.: “We need to help identify individuals who are at high risk of OUD or opioid overdose by screening.” A validated screening tool should be used at prenatal and postpartum appointments.
On a bigger scale, she urges Medicaid to be expanded for a full year post partum through the American Rescue Act’s State Plan Amendment, something only 28 states and Washington, D.C., have done so far.
Dr. Smid points out some good news, however: President Joe Biden signed the Consolidated Appropriations Act 2023, which eliminated the “X” waiver.
Now all clinicians who have a Drug Enforcement Administration registration that includes Schedule III authority can prescribe buprenorphine for OUD if applicable state law allows it.
But that calls for medical schools and residency programs to prioritize addiction medicine as a core competency, Dr. Smid says.
Getting naloxone to patients, families
One of the potential interventions the study authors suggest is providing naloxone prescriptions and training to pregnant and postpartum women who have a substance use history and to their partners and significant others.
However, Mishka Terplan, MD, MPH, told this publication, “It’s one thing to write a prescription; it’s another thing for the person to actually get the medication.” He is medical director of the Friends Research Institute in Baltimore, an ob.gyn. who specializes in addiction medicine.
“What can we do?” We can think about how to get naloxone into people’s hands at discharge from the hospital after they give birth, instead of prescribing. That would mean that health systems need to prioritize this, he said. “We give people discharge medications all the time.”
Still, naloxone can’t be seen as the answer, he said.
He compares it to defibrillators in public places, which are for rescues, not reversing a population problem.
“Some people think that naloxone reversals are doing something about OUD. It’s doing about as much about OUD as defibrillators do for cardiovascular disease,” he said.
The best help, he says, will be continuation of treatment.
“Addiction is a chronic condition,” he says, “but often we only provide episodic care. We see that particularly in pregnancy. Once the pregnancy is finished, there’s not categorical continuation of insurance.”
Even if you do have insurance, it’s hard to find a clinic that’s family friendly, he notes. “You might not feel comfortable taking your newborn and standing in line in the morning to get your daily methodone dose. We have to make those environments more welcoming.”
Problem probably understated
He also says that though the study was well done given the data available, he’s frustrated that researchers still have to depend on billing data and can’t capture factors such as child care availability, living wages, and continuation of health insurance. Additionally, not everyone is coded correctly for OUD.
“It’s all Medicaid, so it’s only people who continued with care,” he pointed out. That means these numbers may actually underrepresent the problem.
Still, he says it’s important to realize the magnitude of deaths this study does highlight in this population.
In people with OUD in the postpartum period, the deaths are more than 1 in 1,000.
“That should be alarming,” Dr. Terplan said. “That’s a very big number from a public health perspective.”
Coauthor Kathryn J. Gray received payment from Aetion Inc., Roche, and BillionToOne. Funds were paid to the University of Utah for Dr. Smid from Alydia Inc. for being the site principal investigator for a study of the JADA device, and from Gilead for Dr. Smid’s study of hepatitis C in pregnancy; she was also a consultant for Organon and Rhia Ventures. Dr. Terplan reports no relevant financial relationships.
FROM OBSTETRICS AND GYNECOLOGY
FDA moves to stop the spread of illicit ‘tranq’ in the U.S.
The agency issued an import alert, which gives it the power to detain raw ingredients or bulk finished product if the shipments are suspected to be in violation of the law. Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals.
It is increasingly being detected and is usually mixed with fentanyl, cocaine, methamphetamine, and other illicit drugs. A January 2023 study by Nashville-based testing company Aegis Sciences found xylazine in 413 of about 60,000 urine samples and in 25 of 39 states that submitted tests. The vast majority of xylazine-positive samples also tested positive for fentanyl.
The FDA said it would continue to ensure the availability of xylazine for veterinary use, and the American Veterinary Medicine Association said in a statement that it “supports such efforts to combat illicit drug use.”
FDA Commissioner Robert M. Califf, MD, said in a statement that the agency “remains concerned about the increasing prevalence of xylazine mixed with illicit drugs, and this action is one part of broader efforts the agency is undertaking to address this issue.”
In November, the agency warned health care providers that because xylazine is not an opioid, the overdose reversal agent naloxone would not be effective. Xylazine acts as a central alpha-2-adrenergic receptor agonist in the brainstem, causing a rapid decrease in the release of norepinephrine and dopamine in the central nervous system. Its use can lead to central nervous system and respiratory depression, said the FDA.
Clinicians have scrambled to treat severe necrotic skin ulcerations that develop at injection sites.
Xylazine is relatively cheap and easy to access, said the Drug Enforcement Administration and Department of Justice in a November joint report. The drug is “readily available for purchase on other Internet sites in liquid and powder form, often with no association to the veterinary profession nor requirements to prove legitimate need,” said the Justice Department. A buyer can purchase xylazine powder online from Chinese suppliers for $6-$20 per kilogram, according to the report.
In 2021, xylazine-positive overdoses were highest in the South, which experienced a 1,127% increase from 2020, the Justice Department reported. The same year, there were 1,281 overdoses involving the substance in the Northeast and 351 in the Midwest.
There were just 34 overdoses involving xylazine in the West in 2021, but its use appears to be growing. The San Francisco Department of Public Health said it had detected low levels of xylazine in four people who died of overdoses in December and January.
“Identifying xylazine in San Francisco is concerning,” said the department in a statement, adding that it had not yet seen evidence of skin wounds in injection drug users in the city.
In late February, the Los Angeles County Department of Public Health issued a warning to first responders and health care professionals that xylazine had been detected in the area’s illicit drug supply.
The department said it will “work closely with other partners to understand the extent of the possible xylazine contamination in the illicit drug supply to increase awareness and education to the public.”
The FDA commissioner said the agency will coordinate with public health officials to more closely track xylazine.
“We will continue to use all tools at our disposal and partner with the Drug Enforcement Administration and other federal, state, local agencies, and stakeholders as appropriate to stem these illicit activities and protect public health,” said Dr. Califf.
A version of this article first appeared on Medscape.com.
The agency issued an import alert, which gives it the power to detain raw ingredients or bulk finished product if the shipments are suspected to be in violation of the law. Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals.
It is increasingly being detected and is usually mixed with fentanyl, cocaine, methamphetamine, and other illicit drugs. A January 2023 study by Nashville-based testing company Aegis Sciences found xylazine in 413 of about 60,000 urine samples and in 25 of 39 states that submitted tests. The vast majority of xylazine-positive samples also tested positive for fentanyl.
The FDA said it would continue to ensure the availability of xylazine for veterinary use, and the American Veterinary Medicine Association said in a statement that it “supports such efforts to combat illicit drug use.”
FDA Commissioner Robert M. Califf, MD, said in a statement that the agency “remains concerned about the increasing prevalence of xylazine mixed with illicit drugs, and this action is one part of broader efforts the agency is undertaking to address this issue.”
In November, the agency warned health care providers that because xylazine is not an opioid, the overdose reversal agent naloxone would not be effective. Xylazine acts as a central alpha-2-adrenergic receptor agonist in the brainstem, causing a rapid decrease in the release of norepinephrine and dopamine in the central nervous system. Its use can lead to central nervous system and respiratory depression, said the FDA.
Clinicians have scrambled to treat severe necrotic skin ulcerations that develop at injection sites.
Xylazine is relatively cheap and easy to access, said the Drug Enforcement Administration and Department of Justice in a November joint report. The drug is “readily available for purchase on other Internet sites in liquid and powder form, often with no association to the veterinary profession nor requirements to prove legitimate need,” said the Justice Department. A buyer can purchase xylazine powder online from Chinese suppliers for $6-$20 per kilogram, according to the report.
In 2021, xylazine-positive overdoses were highest in the South, which experienced a 1,127% increase from 2020, the Justice Department reported. The same year, there were 1,281 overdoses involving the substance in the Northeast and 351 in the Midwest.
There were just 34 overdoses involving xylazine in the West in 2021, but its use appears to be growing. The San Francisco Department of Public Health said it had detected low levels of xylazine in four people who died of overdoses in December and January.
“Identifying xylazine in San Francisco is concerning,” said the department in a statement, adding that it had not yet seen evidence of skin wounds in injection drug users in the city.
In late February, the Los Angeles County Department of Public Health issued a warning to first responders and health care professionals that xylazine had been detected in the area’s illicit drug supply.
The department said it will “work closely with other partners to understand the extent of the possible xylazine contamination in the illicit drug supply to increase awareness and education to the public.”
The FDA commissioner said the agency will coordinate with public health officials to more closely track xylazine.
“We will continue to use all tools at our disposal and partner with the Drug Enforcement Administration and other federal, state, local agencies, and stakeholders as appropriate to stem these illicit activities and protect public health,” said Dr. Califf.
A version of this article first appeared on Medscape.com.
The agency issued an import alert, which gives it the power to detain raw ingredients or bulk finished product if the shipments are suspected to be in violation of the law. Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals.
It is increasingly being detected and is usually mixed with fentanyl, cocaine, methamphetamine, and other illicit drugs. A January 2023 study by Nashville-based testing company Aegis Sciences found xylazine in 413 of about 60,000 urine samples and in 25 of 39 states that submitted tests. The vast majority of xylazine-positive samples also tested positive for fentanyl.
The FDA said it would continue to ensure the availability of xylazine for veterinary use, and the American Veterinary Medicine Association said in a statement that it “supports such efforts to combat illicit drug use.”
FDA Commissioner Robert M. Califf, MD, said in a statement that the agency “remains concerned about the increasing prevalence of xylazine mixed with illicit drugs, and this action is one part of broader efforts the agency is undertaking to address this issue.”
In November, the agency warned health care providers that because xylazine is not an opioid, the overdose reversal agent naloxone would not be effective. Xylazine acts as a central alpha-2-adrenergic receptor agonist in the brainstem, causing a rapid decrease in the release of norepinephrine and dopamine in the central nervous system. Its use can lead to central nervous system and respiratory depression, said the FDA.
Clinicians have scrambled to treat severe necrotic skin ulcerations that develop at injection sites.
Xylazine is relatively cheap and easy to access, said the Drug Enforcement Administration and Department of Justice in a November joint report. The drug is “readily available for purchase on other Internet sites in liquid and powder form, often with no association to the veterinary profession nor requirements to prove legitimate need,” said the Justice Department. A buyer can purchase xylazine powder online from Chinese suppliers for $6-$20 per kilogram, according to the report.
In 2021, xylazine-positive overdoses were highest in the South, which experienced a 1,127% increase from 2020, the Justice Department reported. The same year, there were 1,281 overdoses involving the substance in the Northeast and 351 in the Midwest.
There were just 34 overdoses involving xylazine in the West in 2021, but its use appears to be growing. The San Francisco Department of Public Health said it had detected low levels of xylazine in four people who died of overdoses in December and January.
“Identifying xylazine in San Francisco is concerning,” said the department in a statement, adding that it had not yet seen evidence of skin wounds in injection drug users in the city.
In late February, the Los Angeles County Department of Public Health issued a warning to first responders and health care professionals that xylazine had been detected in the area’s illicit drug supply.
The department said it will “work closely with other partners to understand the extent of the possible xylazine contamination in the illicit drug supply to increase awareness and education to the public.”
The FDA commissioner said the agency will coordinate with public health officials to more closely track xylazine.
“We will continue to use all tools at our disposal and partner with the Drug Enforcement Administration and other federal, state, local agencies, and stakeholders as appropriate to stem these illicit activities and protect public health,” said Dr. Califf.
A version of this article first appeared on Medscape.com.
DEA proposals on telehealth for controlled substances draw fire
The proposed rules – one for Schedule III-V substances, and the other for buprenorphine – are due to go into effect on May 11, when the COVID-19 public health emergency (PHE), and temporary flexibilities, end.
Essentially, both proposals would allow providers to prescribe a 30-day supply of a controlled substance or buprenorphine, but then require a face-to-face meeting for patients to receive additional prescriptions.
The DEA says that the rules are aimed at preventing abuse and diversion of the substances, but clinicians claim they are creating unnecessary hurdles that will probably lead to some patients dropping out of treatment.
“We were happy to see that there is ongoing flexibility to be able to initiate buprenorphine through telehealth, but we were disappointed to see that the DEA set an arbitrary time frame, in this case, a 30-day time frame after which the patient would have to be seen in person before ongoing care with buprenorphine for opioid use disorder could be provided,” Brian Hurley, MD, MBA, the president-elect of the American Society of Addiction Medicine told this news organization.
Dr. Hurley agreed that it is best practice to see patients in person for ongoing care, but he noted they have many reasons why they might not be able to make it into an office every month.
“What this rule would do if instituted as written is prevent me from continuing care for patients unless I can get them in in person,” he said. “And while I’d make every effort as a clinician, it’s not always feasible to do so.”
The addiction specialist noted that only about 20% of Americans with opioid use disorder have access to medications for the disorder. “I would posit that untreated opioid use disorder is a bigger threat to public safety currently than the risk of diversion,” he said.
The DEA is also proposing to allow state laws to supersede its regulations, which concerns Dr. Hurley and other clinicians because some states are more restrictive. “Our position is that state laws that restrict access to medications for opioid use disorder through telehealth means are inconsistent with our policy recommendation. I certainly hope that the DEA hears our concerns and amends the proposal,” said Dr. Hurley.
A potential ‘telehealth cliff’
Shabana Khan, MD, chair of the American Psychiatric Association’s telepsychiatry committee, said that “because of potential overlap with state rules that may be more stringent than these new regulations, APA is concerned that the proposed rules will create a telehealth cliff for those in most need of critical psychiatric and opioid use disorder treatment, particularly in communities where this specialty care is limited or nonexistent.”
Dr. Khan noted that “clarification is necessary on how patients who started treatment during the PHE can continue treatment with a prescribing provider, if at all, through an in-person evaluation with a DEA-registered provider referral.”
Telehealth companies were also disappointed in the DEA proposals.
“The continuity of care for countless Americans will be severed, potentially leaving these patients to fall through the cracks of our health care system without access to needed medications,” said Kyle Zebley, the American Telemedicine Association’s senior vice president of public policy, in a statement.
“Requiring every patient who has initiated treatment via telemedicine during the pandemic to now visit a provider in person clearly falls on the side of being overly restrictive,” Mr. Zebley added.
The DEA is proposing to allow patients who have been receiving telehealth over the past 3 years to continue to do so for 180 days after the PHE ends.
But the American Telemedicine Association and others said that they still want to see a change in the proposal as written. “Our hope is that the DEA works with us to avoid unnecessary and inappropriate restrictions on the prescription of essential medications for these vulnerable and underserved populations,” Mr. Zebley said in the statement.
DEA Administrator Anne Milgram said in a statement that the agency believes that “the telemedicine regulations would continue to expand access to buprenorphine for patients with opioid use disorder,” and that the DEA “is committed to the expansion of telemedicine with guardrails that prevent the online overprescribing of controlled medications that can cause harm.”
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said in a statement that “This proposed rule builds on President Biden’s historic move to eliminate the X-waiver that prevented many prescribers from treating patients with buprenorphine.” He added, “Thanks to these changes, millions of Americans will be able to access the lifesaving care they need.”
The DEA estimated that there were 15.7 million prescriptions for buprenorphine in 2021 and that about 67,000 were for initial prescriptions.
Ketamine confusion
The rule on controlled substances has also caused some consternation, especially given that it does not differentiate between racemic ketamine and esketamine, said Lisa Marie Harding, MD, vice president of the board of the American Society of Ketamine Physicians, Psychotherapists & Practitioners.
Esketamine (Spravato) is approved by the Food and Drug Administration and, under a Risk Evaluation and Mitigation Strategy, can only be administered in FDA-monitored treatment facilities. Racemic ketamine is being prescribed – often for home use – with almost no regulatory oversight.
Dr. Harding, who is an approved Spravato provider and also administers intravenous ketamine in her practice, does not believe that ketamine should be used at home without supervision.
“I had a patient who had a very powerful dissociative experience in my office earlier this week,” Dr. Harding said in an interview. One of her staff asked what would happen if the patient had experienced that at home. “We don’t know. Nor do we want this to happen,” said Dr. Harding.
However, the DEA proposal would continue to allow for home use, at least initially. “If it’s open to interpretation, those people that prescribe ketamine for home use can use that leeway to then continue to do it,” she said. “That is not safe.”
Dr. Harding approves of the proposed DEA requirement for face-to-face visits. “It’s good patient care,” she said. But she wants the administration to adjust the rules to make it harder to offer home ketamine therapy.
“Lots of people are using racemic ketamine off-label for treating depression with success but doing it in treatment settings that are appropriate,” said Dr. Harding.
Dr. Hurley and Dr. Harding report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The proposed rules – one for Schedule III-V substances, and the other for buprenorphine – are due to go into effect on May 11, when the COVID-19 public health emergency (PHE), and temporary flexibilities, end.
Essentially, both proposals would allow providers to prescribe a 30-day supply of a controlled substance or buprenorphine, but then require a face-to-face meeting for patients to receive additional prescriptions.
The DEA says that the rules are aimed at preventing abuse and diversion of the substances, but clinicians claim they are creating unnecessary hurdles that will probably lead to some patients dropping out of treatment.
“We were happy to see that there is ongoing flexibility to be able to initiate buprenorphine through telehealth, but we were disappointed to see that the DEA set an arbitrary time frame, in this case, a 30-day time frame after which the patient would have to be seen in person before ongoing care with buprenorphine for opioid use disorder could be provided,” Brian Hurley, MD, MBA, the president-elect of the American Society of Addiction Medicine told this news organization.
Dr. Hurley agreed that it is best practice to see patients in person for ongoing care, but he noted they have many reasons why they might not be able to make it into an office every month.
“What this rule would do if instituted as written is prevent me from continuing care for patients unless I can get them in in person,” he said. “And while I’d make every effort as a clinician, it’s not always feasible to do so.”
The addiction specialist noted that only about 20% of Americans with opioid use disorder have access to medications for the disorder. “I would posit that untreated opioid use disorder is a bigger threat to public safety currently than the risk of diversion,” he said.
The DEA is also proposing to allow state laws to supersede its regulations, which concerns Dr. Hurley and other clinicians because some states are more restrictive. “Our position is that state laws that restrict access to medications for opioid use disorder through telehealth means are inconsistent with our policy recommendation. I certainly hope that the DEA hears our concerns and amends the proposal,” said Dr. Hurley.
A potential ‘telehealth cliff’
Shabana Khan, MD, chair of the American Psychiatric Association’s telepsychiatry committee, said that “because of potential overlap with state rules that may be more stringent than these new regulations, APA is concerned that the proposed rules will create a telehealth cliff for those in most need of critical psychiatric and opioid use disorder treatment, particularly in communities where this specialty care is limited or nonexistent.”
Dr. Khan noted that “clarification is necessary on how patients who started treatment during the PHE can continue treatment with a prescribing provider, if at all, through an in-person evaluation with a DEA-registered provider referral.”
Telehealth companies were also disappointed in the DEA proposals.
“The continuity of care for countless Americans will be severed, potentially leaving these patients to fall through the cracks of our health care system without access to needed medications,” said Kyle Zebley, the American Telemedicine Association’s senior vice president of public policy, in a statement.
“Requiring every patient who has initiated treatment via telemedicine during the pandemic to now visit a provider in person clearly falls on the side of being overly restrictive,” Mr. Zebley added.
The DEA is proposing to allow patients who have been receiving telehealth over the past 3 years to continue to do so for 180 days after the PHE ends.
But the American Telemedicine Association and others said that they still want to see a change in the proposal as written. “Our hope is that the DEA works with us to avoid unnecessary and inappropriate restrictions on the prescription of essential medications for these vulnerable and underserved populations,” Mr. Zebley said in the statement.
DEA Administrator Anne Milgram said in a statement that the agency believes that “the telemedicine regulations would continue to expand access to buprenorphine for patients with opioid use disorder,” and that the DEA “is committed to the expansion of telemedicine with guardrails that prevent the online overprescribing of controlled medications that can cause harm.”
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said in a statement that “This proposed rule builds on President Biden’s historic move to eliminate the X-waiver that prevented many prescribers from treating patients with buprenorphine.” He added, “Thanks to these changes, millions of Americans will be able to access the lifesaving care they need.”
The DEA estimated that there were 15.7 million prescriptions for buprenorphine in 2021 and that about 67,000 were for initial prescriptions.
Ketamine confusion
The rule on controlled substances has also caused some consternation, especially given that it does not differentiate between racemic ketamine and esketamine, said Lisa Marie Harding, MD, vice president of the board of the American Society of Ketamine Physicians, Psychotherapists & Practitioners.
Esketamine (Spravato) is approved by the Food and Drug Administration and, under a Risk Evaluation and Mitigation Strategy, can only be administered in FDA-monitored treatment facilities. Racemic ketamine is being prescribed – often for home use – with almost no regulatory oversight.
Dr. Harding, who is an approved Spravato provider and also administers intravenous ketamine in her practice, does not believe that ketamine should be used at home without supervision.
“I had a patient who had a very powerful dissociative experience in my office earlier this week,” Dr. Harding said in an interview. One of her staff asked what would happen if the patient had experienced that at home. “We don’t know. Nor do we want this to happen,” said Dr. Harding.
However, the DEA proposal would continue to allow for home use, at least initially. “If it’s open to interpretation, those people that prescribe ketamine for home use can use that leeway to then continue to do it,” she said. “That is not safe.”
Dr. Harding approves of the proposed DEA requirement for face-to-face visits. “It’s good patient care,” she said. But she wants the administration to adjust the rules to make it harder to offer home ketamine therapy.
“Lots of people are using racemic ketamine off-label for treating depression with success but doing it in treatment settings that are appropriate,” said Dr. Harding.
Dr. Hurley and Dr. Harding report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The proposed rules – one for Schedule III-V substances, and the other for buprenorphine – are due to go into effect on May 11, when the COVID-19 public health emergency (PHE), and temporary flexibilities, end.
Essentially, both proposals would allow providers to prescribe a 30-day supply of a controlled substance or buprenorphine, but then require a face-to-face meeting for patients to receive additional prescriptions.
The DEA says that the rules are aimed at preventing abuse and diversion of the substances, but clinicians claim they are creating unnecessary hurdles that will probably lead to some patients dropping out of treatment.
“We were happy to see that there is ongoing flexibility to be able to initiate buprenorphine through telehealth, but we were disappointed to see that the DEA set an arbitrary time frame, in this case, a 30-day time frame after which the patient would have to be seen in person before ongoing care with buprenorphine for opioid use disorder could be provided,” Brian Hurley, MD, MBA, the president-elect of the American Society of Addiction Medicine told this news organization.
Dr. Hurley agreed that it is best practice to see patients in person for ongoing care, but he noted they have many reasons why they might not be able to make it into an office every month.
“What this rule would do if instituted as written is prevent me from continuing care for patients unless I can get them in in person,” he said. “And while I’d make every effort as a clinician, it’s not always feasible to do so.”
The addiction specialist noted that only about 20% of Americans with opioid use disorder have access to medications for the disorder. “I would posit that untreated opioid use disorder is a bigger threat to public safety currently than the risk of diversion,” he said.
The DEA is also proposing to allow state laws to supersede its regulations, which concerns Dr. Hurley and other clinicians because some states are more restrictive. “Our position is that state laws that restrict access to medications for opioid use disorder through telehealth means are inconsistent with our policy recommendation. I certainly hope that the DEA hears our concerns and amends the proposal,” said Dr. Hurley.
A potential ‘telehealth cliff’
Shabana Khan, MD, chair of the American Psychiatric Association’s telepsychiatry committee, said that “because of potential overlap with state rules that may be more stringent than these new regulations, APA is concerned that the proposed rules will create a telehealth cliff for those in most need of critical psychiatric and opioid use disorder treatment, particularly in communities where this specialty care is limited or nonexistent.”
Dr. Khan noted that “clarification is necessary on how patients who started treatment during the PHE can continue treatment with a prescribing provider, if at all, through an in-person evaluation with a DEA-registered provider referral.”
Telehealth companies were also disappointed in the DEA proposals.
“The continuity of care for countless Americans will be severed, potentially leaving these patients to fall through the cracks of our health care system without access to needed medications,” said Kyle Zebley, the American Telemedicine Association’s senior vice president of public policy, in a statement.
“Requiring every patient who has initiated treatment via telemedicine during the pandemic to now visit a provider in person clearly falls on the side of being overly restrictive,” Mr. Zebley added.
The DEA is proposing to allow patients who have been receiving telehealth over the past 3 years to continue to do so for 180 days after the PHE ends.
But the American Telemedicine Association and others said that they still want to see a change in the proposal as written. “Our hope is that the DEA works with us to avoid unnecessary and inappropriate restrictions on the prescription of essential medications for these vulnerable and underserved populations,” Mr. Zebley said in the statement.
DEA Administrator Anne Milgram said in a statement that the agency believes that “the telemedicine regulations would continue to expand access to buprenorphine for patients with opioid use disorder,” and that the DEA “is committed to the expansion of telemedicine with guardrails that prevent the online overprescribing of controlled medications that can cause harm.”
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said in a statement that “This proposed rule builds on President Biden’s historic move to eliminate the X-waiver that prevented many prescribers from treating patients with buprenorphine.” He added, “Thanks to these changes, millions of Americans will be able to access the lifesaving care they need.”
The DEA estimated that there were 15.7 million prescriptions for buprenorphine in 2021 and that about 67,000 were for initial prescriptions.
Ketamine confusion
The rule on controlled substances has also caused some consternation, especially given that it does not differentiate between racemic ketamine and esketamine, said Lisa Marie Harding, MD, vice president of the board of the American Society of Ketamine Physicians, Psychotherapists & Practitioners.
Esketamine (Spravato) is approved by the Food and Drug Administration and, under a Risk Evaluation and Mitigation Strategy, can only be administered in FDA-monitored treatment facilities. Racemic ketamine is being prescribed – often for home use – with almost no regulatory oversight.
Dr. Harding, who is an approved Spravato provider and also administers intravenous ketamine in her practice, does not believe that ketamine should be used at home without supervision.
“I had a patient who had a very powerful dissociative experience in my office earlier this week,” Dr. Harding said in an interview. One of her staff asked what would happen if the patient had experienced that at home. “We don’t know. Nor do we want this to happen,” said Dr. Harding.
However, the DEA proposal would continue to allow for home use, at least initially. “If it’s open to interpretation, those people that prescribe ketamine for home use can use that leeway to then continue to do it,” she said. “That is not safe.”
Dr. Harding approves of the proposed DEA requirement for face-to-face visits. “It’s good patient care,” she said. But she wants the administration to adjust the rules to make it harder to offer home ketamine therapy.
“Lots of people are using racemic ketamine off-label for treating depression with success but doing it in treatment settings that are appropriate,” said Dr. Harding.
Dr. Hurley and Dr. Harding report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Buprenorphine proves effective for fentanyl users in the ED
based on data from nearly 900 individuals.
California EDs include a facilitation program known as CA Bridge for the treatment of opioid use disorder. Guidelines for CA Bridge call for high-dose buprenorphine to treat patients in drug withdrawal, with doses starting at 8-16 mg, Hannah Snyder, MD, of the University of California, San Francisco, and colleagues wrote.
“Buprenorphine has been repeatedly shown to save lives and prevent overdoses,” Dr. Snyder said in an interview. “We know that emergency department–initiated buprenorphine is an essential tool for increasing access. In the era of fentanyl, both patients and providers have expressed concerns that buprenorphine may not work as well as it did when patients were more likely to be using heroin or opioid pills.
“This retrospective cohort study provides additional information about emergency department buprenorphine as fentanyl becomes increasingly prevalent.”
In a research letter published in JAMA Network Open, the investigators reviewed data from the electronic health records of 896 patients who presented with opioid use disorder (OUD) at 16 CA Bridge EDs between Jan. 1, 2020, and April 30, 2020. All patients with OUD were included regardless of chief concern, current treatment, treatment desires, or withdrawal. A total of 87 individuals reported fentanyl use; if no fentanyl use was reported, the patient was classified as not using fentanyl. The median age of the patients was 35 years, two thirds were male, approximately 46% were White and non-Hispanic, and 30% had unstable housing.
The primary outcome was follow-up engagement at 7-14 days and 25-37 days.
A total of 492 patients received buprenorphine, including 44 fentanyl users, and 439 initiated high doses of 8-32 mg. At a 30-day follow-up, eight patients had precipitated withdrawal, including two cases in fentanyl users; none of these cases required hospital admission.
The follow-up engagement was similar for both groups, with adjusted odds ratios of 0.60 for administered buprenorphine at the initial ED encounter, 1.09 for 7-day follow-up, and 1.33 for 30-day follow-up.
The findings were limited by the retrospective design and use of clinical documentation, which likely resulted in underreporting of fentanyl use and follow-up, the researchers noted. However, the results supported the effectiveness of buprenorphine for ED patients in withdrawal with a history of fentanyl exposure.
“We were pleased to see that precipitated withdrawal was relatively uncommon in this study, and that patients who did and did not use fentanyl followed up at similar rates,” said Dr. Snyder. “This aligns with our clinical experience and prior research showing that emergency department buprenorphine starts continue to be an essential tool.”
The message for clinicians: “If a patient presents to the emergency department in objective opioid withdrawal and desires buprenorphine, they should be offered treatment in that moment,” Dr. Snyder said. “Treatment protocols used by hospitals in this study are available online. Emergency departments can offer compassionate and evidence-based treatment initiation 24 hours a day, 7 days a week, 365 days a year.”
More data needed on dosing strategies
“We need additional research to determine best practices for patients who use fentanyl and want to start buprenorphine, but are not yet in withdrawal,” Dr. Snyder said. “Doses of buprenorphine like those in this study are only appropriate for patients who are in withdrawal with objective signs, so some patients may struggle to wait long enough after their last use to go into sufficient withdrawal.”
Precipitated withdrawal does occur in some cases, said Dr. Snyder. “If it does, the emergency department is a very good place to manage it. We need additional research to determine best practices in management to make patients as comfortable as possible, including additional high-dose buprenorphine as well as additional adjunctive agents.”
Findings support buprenorphine
“The classic approach to buprenorphine initiation, which emerged from psychiatry outpatient office visits, is to start with very small doses of buprenorphine [2-4 mg] and titrate up slowly,” Reuben J. Strayer, MD, said in an interview.
“This dose range turns out to be the ‘sour spot’ most likely to cause the most important complication around buprenorphine initiation–precipitated withdrawal,” said Dr. Strayer, the director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York.
“One of the current focus areas of OUD treatment research is determining how to initiate buprenorphine without entailing a period of spontaneous withdrawal and without causing precipitated withdrawal,” Strayer explained. “The two primary strategies are low-dose buprenorphine initiation [LDBI, less than 2 mg, sometimes called microdosing] and high-dose [HDBI, ≥ 16 mg] buprenorphine initiation. HDBI is attractive because the primary treatment of buprenorphine-precipitated withdrawal is more buprenorphine.
“Additionally, using a high dose up front immediately transitions the patient to therapeutic blood levels, which protects the patient from withdrawal, cravings, and overdose from dangerous opioids (heroin, fentanyl, oxycodone).”
However, “the contamination and now replacement of heroin with fentanyl in the street drug supply has challenged buprenorphine initiation, because fentanyl, when used chronically, accumulates in the body and leaks into the bloodstream slowly over time, preventing the opioid washout that is required to eliminate the risk of precipitated withdrawal when buprenorphine is administered,” said Dr. Strayer.
The current study demonstrates that patients who are initiated with a first dose of 8-16 mg buprenorphine are unlikely to experience precipitated withdrawal and are successfully transitioned to buprenorphine maintenance and clinic follow-up, Dr. Snyder said, but he was surprised by the low rate of precipitated withdrawal in the current study, “which is discordant with what is being anecdotally reported across the country.”
However, the take-home message for clinicians is the support for the initiation of buprenorphine in emergency department settings at a starting dose of 8-16 mg, regardless of reported fentanyl use, he said. “Given the huge impact buprenorphine therapy has on OUD-related mortality, clinicians should make every effort to initiate buprenorphine for OUD patients at every opportunity, and precipitated withdrawal is very unlikely in appropriately selected patients.
“Many clinicians remain reluctant to initiate buprenorphine in ED settings for unfamiliarity with the drug, fear of precipitated withdrawal, or concerns around the certainty of outpatient follow-up,” Dr. Snyder said. “Education, encouragement, systems programming, such as including decision support within the electronic health record, and role-modeling from local champions will promote wider adoption of this lifesaving practice.”
Looking ahead, “more research, including prospective research, is needed to refine best practices around buprenorphine administration,” said Dr. Snyder. Questions to address include which patients are most at risk for precipitated withdrawal and whether there are alternatives to standard initiation dosing that are sufficiently unlikely to cause precipitated withdrawal. “Possibly effective alternatives include buprenorphine initiation by administration of long-acting injectable depot buprenorphine, which accumulates slowly, potentially avoiding precipitated withdrawal, as well as a slow intravenous buprenorphine infusion such as 9 mg given over 12 hours.”
The study received no outside funding. Dr. Snyder disclosed grants from the Substance Abuse and Mental Health Services Administration and the California Department of Health Care Services during the study. Dr. Strayer reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
based on data from nearly 900 individuals.
California EDs include a facilitation program known as CA Bridge for the treatment of opioid use disorder. Guidelines for CA Bridge call for high-dose buprenorphine to treat patients in drug withdrawal, with doses starting at 8-16 mg, Hannah Snyder, MD, of the University of California, San Francisco, and colleagues wrote.
“Buprenorphine has been repeatedly shown to save lives and prevent overdoses,” Dr. Snyder said in an interview. “We know that emergency department–initiated buprenorphine is an essential tool for increasing access. In the era of fentanyl, both patients and providers have expressed concerns that buprenorphine may not work as well as it did when patients were more likely to be using heroin or opioid pills.
“This retrospective cohort study provides additional information about emergency department buprenorphine as fentanyl becomes increasingly prevalent.”
In a research letter published in JAMA Network Open, the investigators reviewed data from the electronic health records of 896 patients who presented with opioid use disorder (OUD) at 16 CA Bridge EDs between Jan. 1, 2020, and April 30, 2020. All patients with OUD were included regardless of chief concern, current treatment, treatment desires, or withdrawal. A total of 87 individuals reported fentanyl use; if no fentanyl use was reported, the patient was classified as not using fentanyl. The median age of the patients was 35 years, two thirds were male, approximately 46% were White and non-Hispanic, and 30% had unstable housing.
The primary outcome was follow-up engagement at 7-14 days and 25-37 days.
A total of 492 patients received buprenorphine, including 44 fentanyl users, and 439 initiated high doses of 8-32 mg. At a 30-day follow-up, eight patients had precipitated withdrawal, including two cases in fentanyl users; none of these cases required hospital admission.
The follow-up engagement was similar for both groups, with adjusted odds ratios of 0.60 for administered buprenorphine at the initial ED encounter, 1.09 for 7-day follow-up, and 1.33 for 30-day follow-up.
The findings were limited by the retrospective design and use of clinical documentation, which likely resulted in underreporting of fentanyl use and follow-up, the researchers noted. However, the results supported the effectiveness of buprenorphine for ED patients in withdrawal with a history of fentanyl exposure.
“We were pleased to see that precipitated withdrawal was relatively uncommon in this study, and that patients who did and did not use fentanyl followed up at similar rates,” said Dr. Snyder. “This aligns with our clinical experience and prior research showing that emergency department buprenorphine starts continue to be an essential tool.”
The message for clinicians: “If a patient presents to the emergency department in objective opioid withdrawal and desires buprenorphine, they should be offered treatment in that moment,” Dr. Snyder said. “Treatment protocols used by hospitals in this study are available online. Emergency departments can offer compassionate and evidence-based treatment initiation 24 hours a day, 7 days a week, 365 days a year.”
More data needed on dosing strategies
“We need additional research to determine best practices for patients who use fentanyl and want to start buprenorphine, but are not yet in withdrawal,” Dr. Snyder said. “Doses of buprenorphine like those in this study are only appropriate for patients who are in withdrawal with objective signs, so some patients may struggle to wait long enough after their last use to go into sufficient withdrawal.”
Precipitated withdrawal does occur in some cases, said Dr. Snyder. “If it does, the emergency department is a very good place to manage it. We need additional research to determine best practices in management to make patients as comfortable as possible, including additional high-dose buprenorphine as well as additional adjunctive agents.”
Findings support buprenorphine
“The classic approach to buprenorphine initiation, which emerged from psychiatry outpatient office visits, is to start with very small doses of buprenorphine [2-4 mg] and titrate up slowly,” Reuben J. Strayer, MD, said in an interview.
“This dose range turns out to be the ‘sour spot’ most likely to cause the most important complication around buprenorphine initiation–precipitated withdrawal,” said Dr. Strayer, the director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York.
“One of the current focus areas of OUD treatment research is determining how to initiate buprenorphine without entailing a period of spontaneous withdrawal and without causing precipitated withdrawal,” Strayer explained. “The two primary strategies are low-dose buprenorphine initiation [LDBI, less than 2 mg, sometimes called microdosing] and high-dose [HDBI, ≥ 16 mg] buprenorphine initiation. HDBI is attractive because the primary treatment of buprenorphine-precipitated withdrawal is more buprenorphine.
“Additionally, using a high dose up front immediately transitions the patient to therapeutic blood levels, which protects the patient from withdrawal, cravings, and overdose from dangerous opioids (heroin, fentanyl, oxycodone).”
However, “the contamination and now replacement of heroin with fentanyl in the street drug supply has challenged buprenorphine initiation, because fentanyl, when used chronically, accumulates in the body and leaks into the bloodstream slowly over time, preventing the opioid washout that is required to eliminate the risk of precipitated withdrawal when buprenorphine is administered,” said Dr. Strayer.
The current study demonstrates that patients who are initiated with a first dose of 8-16 mg buprenorphine are unlikely to experience precipitated withdrawal and are successfully transitioned to buprenorphine maintenance and clinic follow-up, Dr. Snyder said, but he was surprised by the low rate of precipitated withdrawal in the current study, “which is discordant with what is being anecdotally reported across the country.”
However, the take-home message for clinicians is the support for the initiation of buprenorphine in emergency department settings at a starting dose of 8-16 mg, regardless of reported fentanyl use, he said. “Given the huge impact buprenorphine therapy has on OUD-related mortality, clinicians should make every effort to initiate buprenorphine for OUD patients at every opportunity, and precipitated withdrawal is very unlikely in appropriately selected patients.
“Many clinicians remain reluctant to initiate buprenorphine in ED settings for unfamiliarity with the drug, fear of precipitated withdrawal, or concerns around the certainty of outpatient follow-up,” Dr. Snyder said. “Education, encouragement, systems programming, such as including decision support within the electronic health record, and role-modeling from local champions will promote wider adoption of this lifesaving practice.”
Looking ahead, “more research, including prospective research, is needed to refine best practices around buprenorphine administration,” said Dr. Snyder. Questions to address include which patients are most at risk for precipitated withdrawal and whether there are alternatives to standard initiation dosing that are sufficiently unlikely to cause precipitated withdrawal. “Possibly effective alternatives include buprenorphine initiation by administration of long-acting injectable depot buprenorphine, which accumulates slowly, potentially avoiding precipitated withdrawal, as well as a slow intravenous buprenorphine infusion such as 9 mg given over 12 hours.”
The study received no outside funding. Dr. Snyder disclosed grants from the Substance Abuse and Mental Health Services Administration and the California Department of Health Care Services during the study. Dr. Strayer reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
based on data from nearly 900 individuals.
California EDs include a facilitation program known as CA Bridge for the treatment of opioid use disorder. Guidelines for CA Bridge call for high-dose buprenorphine to treat patients in drug withdrawal, with doses starting at 8-16 mg, Hannah Snyder, MD, of the University of California, San Francisco, and colleagues wrote.
“Buprenorphine has been repeatedly shown to save lives and prevent overdoses,” Dr. Snyder said in an interview. “We know that emergency department–initiated buprenorphine is an essential tool for increasing access. In the era of fentanyl, both patients and providers have expressed concerns that buprenorphine may not work as well as it did when patients were more likely to be using heroin or opioid pills.
“This retrospective cohort study provides additional information about emergency department buprenorphine as fentanyl becomes increasingly prevalent.”
In a research letter published in JAMA Network Open, the investigators reviewed data from the electronic health records of 896 patients who presented with opioid use disorder (OUD) at 16 CA Bridge EDs between Jan. 1, 2020, and April 30, 2020. All patients with OUD were included regardless of chief concern, current treatment, treatment desires, or withdrawal. A total of 87 individuals reported fentanyl use; if no fentanyl use was reported, the patient was classified as not using fentanyl. The median age of the patients was 35 years, two thirds were male, approximately 46% were White and non-Hispanic, and 30% had unstable housing.
The primary outcome was follow-up engagement at 7-14 days and 25-37 days.
A total of 492 patients received buprenorphine, including 44 fentanyl users, and 439 initiated high doses of 8-32 mg. At a 30-day follow-up, eight patients had precipitated withdrawal, including two cases in fentanyl users; none of these cases required hospital admission.
The follow-up engagement was similar for both groups, with adjusted odds ratios of 0.60 for administered buprenorphine at the initial ED encounter, 1.09 for 7-day follow-up, and 1.33 for 30-day follow-up.
The findings were limited by the retrospective design and use of clinical documentation, which likely resulted in underreporting of fentanyl use and follow-up, the researchers noted. However, the results supported the effectiveness of buprenorphine for ED patients in withdrawal with a history of fentanyl exposure.
“We were pleased to see that precipitated withdrawal was relatively uncommon in this study, and that patients who did and did not use fentanyl followed up at similar rates,” said Dr. Snyder. “This aligns with our clinical experience and prior research showing that emergency department buprenorphine starts continue to be an essential tool.”
The message for clinicians: “If a patient presents to the emergency department in objective opioid withdrawal and desires buprenorphine, they should be offered treatment in that moment,” Dr. Snyder said. “Treatment protocols used by hospitals in this study are available online. Emergency departments can offer compassionate and evidence-based treatment initiation 24 hours a day, 7 days a week, 365 days a year.”
More data needed on dosing strategies
“We need additional research to determine best practices for patients who use fentanyl and want to start buprenorphine, but are not yet in withdrawal,” Dr. Snyder said. “Doses of buprenorphine like those in this study are only appropriate for patients who are in withdrawal with objective signs, so some patients may struggle to wait long enough after their last use to go into sufficient withdrawal.”
Precipitated withdrawal does occur in some cases, said Dr. Snyder. “If it does, the emergency department is a very good place to manage it. We need additional research to determine best practices in management to make patients as comfortable as possible, including additional high-dose buprenorphine as well as additional adjunctive agents.”
Findings support buprenorphine
“The classic approach to buprenorphine initiation, which emerged from psychiatry outpatient office visits, is to start with very small doses of buprenorphine [2-4 mg] and titrate up slowly,” Reuben J. Strayer, MD, said in an interview.
“This dose range turns out to be the ‘sour spot’ most likely to cause the most important complication around buprenorphine initiation–precipitated withdrawal,” said Dr. Strayer, the director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York.
“One of the current focus areas of OUD treatment research is determining how to initiate buprenorphine without entailing a period of spontaneous withdrawal and without causing precipitated withdrawal,” Strayer explained. “The two primary strategies are low-dose buprenorphine initiation [LDBI, less than 2 mg, sometimes called microdosing] and high-dose [HDBI, ≥ 16 mg] buprenorphine initiation. HDBI is attractive because the primary treatment of buprenorphine-precipitated withdrawal is more buprenorphine.
“Additionally, using a high dose up front immediately transitions the patient to therapeutic blood levels, which protects the patient from withdrawal, cravings, and overdose from dangerous opioids (heroin, fentanyl, oxycodone).”
However, “the contamination and now replacement of heroin with fentanyl in the street drug supply has challenged buprenorphine initiation, because fentanyl, when used chronically, accumulates in the body and leaks into the bloodstream slowly over time, preventing the opioid washout that is required to eliminate the risk of precipitated withdrawal when buprenorphine is administered,” said Dr. Strayer.
The current study demonstrates that patients who are initiated with a first dose of 8-16 mg buprenorphine are unlikely to experience precipitated withdrawal and are successfully transitioned to buprenorphine maintenance and clinic follow-up, Dr. Snyder said, but he was surprised by the low rate of precipitated withdrawal in the current study, “which is discordant with what is being anecdotally reported across the country.”
However, the take-home message for clinicians is the support for the initiation of buprenorphine in emergency department settings at a starting dose of 8-16 mg, regardless of reported fentanyl use, he said. “Given the huge impact buprenorphine therapy has on OUD-related mortality, clinicians should make every effort to initiate buprenorphine for OUD patients at every opportunity, and precipitated withdrawal is very unlikely in appropriately selected patients.
“Many clinicians remain reluctant to initiate buprenorphine in ED settings for unfamiliarity with the drug, fear of precipitated withdrawal, or concerns around the certainty of outpatient follow-up,” Dr. Snyder said. “Education, encouragement, systems programming, such as including decision support within the electronic health record, and role-modeling from local champions will promote wider adoption of this lifesaving practice.”
Looking ahead, “more research, including prospective research, is needed to refine best practices around buprenorphine administration,” said Dr. Snyder. Questions to address include which patients are most at risk for precipitated withdrawal and whether there are alternatives to standard initiation dosing that are sufficiently unlikely to cause precipitated withdrawal. “Possibly effective alternatives include buprenorphine initiation by administration of long-acting injectable depot buprenorphine, which accumulates slowly, potentially avoiding precipitated withdrawal, as well as a slow intravenous buprenorphine infusion such as 9 mg given over 12 hours.”
The study received no outside funding. Dr. Snyder disclosed grants from the Substance Abuse and Mental Health Services Administration and the California Department of Health Care Services during the study. Dr. Strayer reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Which nonopioid meds are best for easing acute low back pain?
based on data from more than 3,000 individuals.
Acute low back pain (LBP) remains a common cause of disability worldwide, with a high socioeconomic burden, write Alice Baroncini, MD, of RWTH University Hospital, Aachen, Germany, and colleagues.
In an analysis published in the Journal of Orthopaedic Research, a team of investigators from Germany examined which nonopioid drugs are best for treating LBP.
The researchers identified 18 studies totaling 3,478 patients with acute low back pain of less than 12 weeks’ duration. They selected studies that only investigated the lumbar spine, and studies involving opioids were excluded. The mean age of the patients across all the studies was 42.5 years, and 54% were women. The mean duration of symptoms before treatment was 15.1 days.
Overall, muscle relaxants and NSAIDs demonstrated effectiveness in reducing pain and disability for acute LBP patients after about 1 week of use.
In addition, studies of a combination of NSAIDs and paracetamol (also known as acetaminophen) showed a greater improvement than NSAIDs alone, but paracetamol/acetaminophen alone had no significant impact on LBP.
Most patients with acute LBP experience spontaneous recovery and reduction of symptoms, thus the real impact of most medications is uncertain, the researchers write in their discussion. The lack of a placebo effect in the selected studies reinforces the hypothesis that nonopioid medications improve LBP symptoms, they say.
However, “while this work only focuses on the pharmacological management of acute LBP, it is fundamental to highlight that the use of drugs should always be a second-line strategy once other nonpharmacological, noninvasive therapies have proved to be insufficient,” the researchers write.
The study findings were limited by several factors, including the inability to distinguish among different NSAID classes, the inability to conduct a subanalysis of the best drug or treatment protocol for a given drug class, and the short follow-up period for the included studies, the researchers note.
More research is needed to address the effects of different drugs on LBP recurrence, they add.
However, the results support the current opinion that NSAIDs can be effectively used for LBP, strengthened by the large number of studies and relatively low risk of bias, the researchers conclude.
The current study addresses a common cause of morbidity among patients and highlights alternatives to opioid analgesics for its management, Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview.
Dr. Pal said he was not surprised by the results. “The findings of the study mirror prior studies,” he said. “However, the lack of benefit of paracetamol alone needs to be highlighted as important to clinical practice.”
A key message for clinicians is the role of NSAIDs in LBP, Dr. Pal said. “NSAIDs, either alone or in combination with paracetamol or myorelaxants, can be effective therapy for select patients with acute LBP.” However, “further research is needed to better identify which patients would derive most benefit from this approach,” he said.
Other research needs include more evidence to better understand the appropriate duration of therapy, given the potential for adverse effects with chronic NSAID use, Dr. Pal said.
The study received no outside funding. The researchers and Dr. Pal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
based on data from more than 3,000 individuals.
Acute low back pain (LBP) remains a common cause of disability worldwide, with a high socioeconomic burden, write Alice Baroncini, MD, of RWTH University Hospital, Aachen, Germany, and colleagues.
In an analysis published in the Journal of Orthopaedic Research, a team of investigators from Germany examined which nonopioid drugs are best for treating LBP.
The researchers identified 18 studies totaling 3,478 patients with acute low back pain of less than 12 weeks’ duration. They selected studies that only investigated the lumbar spine, and studies involving opioids were excluded. The mean age of the patients across all the studies was 42.5 years, and 54% were women. The mean duration of symptoms before treatment was 15.1 days.
Overall, muscle relaxants and NSAIDs demonstrated effectiveness in reducing pain and disability for acute LBP patients after about 1 week of use.
In addition, studies of a combination of NSAIDs and paracetamol (also known as acetaminophen) showed a greater improvement than NSAIDs alone, but paracetamol/acetaminophen alone had no significant impact on LBP.
Most patients with acute LBP experience spontaneous recovery and reduction of symptoms, thus the real impact of most medications is uncertain, the researchers write in their discussion. The lack of a placebo effect in the selected studies reinforces the hypothesis that nonopioid medications improve LBP symptoms, they say.
However, “while this work only focuses on the pharmacological management of acute LBP, it is fundamental to highlight that the use of drugs should always be a second-line strategy once other nonpharmacological, noninvasive therapies have proved to be insufficient,” the researchers write.
The study findings were limited by several factors, including the inability to distinguish among different NSAID classes, the inability to conduct a subanalysis of the best drug or treatment protocol for a given drug class, and the short follow-up period for the included studies, the researchers note.
More research is needed to address the effects of different drugs on LBP recurrence, they add.
However, the results support the current opinion that NSAIDs can be effectively used for LBP, strengthened by the large number of studies and relatively low risk of bias, the researchers conclude.
The current study addresses a common cause of morbidity among patients and highlights alternatives to opioid analgesics for its management, Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview.
Dr. Pal said he was not surprised by the results. “The findings of the study mirror prior studies,” he said. “However, the lack of benefit of paracetamol alone needs to be highlighted as important to clinical practice.”
A key message for clinicians is the role of NSAIDs in LBP, Dr. Pal said. “NSAIDs, either alone or in combination with paracetamol or myorelaxants, can be effective therapy for select patients with acute LBP.” However, “further research is needed to better identify which patients would derive most benefit from this approach,” he said.
Other research needs include more evidence to better understand the appropriate duration of therapy, given the potential for adverse effects with chronic NSAID use, Dr. Pal said.
The study received no outside funding. The researchers and Dr. Pal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
based on data from more than 3,000 individuals.
Acute low back pain (LBP) remains a common cause of disability worldwide, with a high socioeconomic burden, write Alice Baroncini, MD, of RWTH University Hospital, Aachen, Germany, and colleagues.
In an analysis published in the Journal of Orthopaedic Research, a team of investigators from Germany examined which nonopioid drugs are best for treating LBP.
The researchers identified 18 studies totaling 3,478 patients with acute low back pain of less than 12 weeks’ duration. They selected studies that only investigated the lumbar spine, and studies involving opioids were excluded. The mean age of the patients across all the studies was 42.5 years, and 54% were women. The mean duration of symptoms before treatment was 15.1 days.
Overall, muscle relaxants and NSAIDs demonstrated effectiveness in reducing pain and disability for acute LBP patients after about 1 week of use.
In addition, studies of a combination of NSAIDs and paracetamol (also known as acetaminophen) showed a greater improvement than NSAIDs alone, but paracetamol/acetaminophen alone had no significant impact on LBP.
Most patients with acute LBP experience spontaneous recovery and reduction of symptoms, thus the real impact of most medications is uncertain, the researchers write in their discussion. The lack of a placebo effect in the selected studies reinforces the hypothesis that nonopioid medications improve LBP symptoms, they say.
However, “while this work only focuses on the pharmacological management of acute LBP, it is fundamental to highlight that the use of drugs should always be a second-line strategy once other nonpharmacological, noninvasive therapies have proved to be insufficient,” the researchers write.
The study findings were limited by several factors, including the inability to distinguish among different NSAID classes, the inability to conduct a subanalysis of the best drug or treatment protocol for a given drug class, and the short follow-up period for the included studies, the researchers note.
More research is needed to address the effects of different drugs on LBP recurrence, they add.
However, the results support the current opinion that NSAIDs can be effectively used for LBP, strengthened by the large number of studies and relatively low risk of bias, the researchers conclude.
The current study addresses a common cause of morbidity among patients and highlights alternatives to opioid analgesics for its management, Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview.
Dr. Pal said he was not surprised by the results. “The findings of the study mirror prior studies,” he said. “However, the lack of benefit of paracetamol alone needs to be highlighted as important to clinical practice.”
A key message for clinicians is the role of NSAIDs in LBP, Dr. Pal said. “NSAIDs, either alone or in combination with paracetamol or myorelaxants, can be effective therapy for select patients with acute LBP.” However, “further research is needed to better identify which patients would derive most benefit from this approach,” he said.
Other research needs include more evidence to better understand the appropriate duration of therapy, given the potential for adverse effects with chronic NSAID use, Dr. Pal said.
The study received no outside funding. The researchers and Dr. Pal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ORTHOPAEDIC RESEARCH
Physician pleads guilty to 52 counts in opioid scheme
Jeffrey B. Sutton, DO, a neuromuscular medicine specialist, pled guilty on January 30 in federal court to 31 counts of illegally prescribing opioids and other controlled substances, 1 count of illegally distributing controlled substances, and 20 counts of health care fraud.
Prosecutors said Dr. Sutton admitted that he ignored warnings from prescription drug management organizations, insurers, and state authorities that he was prescribing excessively high dosages of opioids.
Dr. Sutton also admitted to ignoring patient requests to lower dosages and that he also ignored signs that patients were selling prescribed medications or otherwise engaging in illicit activity, including violations of a “pain management agreement” that he required them to sign.
The fraud counts pertained to Dr. Sutton billing Medicare, Medicaid, and other insurers for medically unnecessary visits that he required of patients so that he could prescribe inappropriate or unnecessary opioids.
In the charging document shared with this news organization, prosecutors said Dr. Sutton had sex with at least three patients, including during office visits and outside of the office. Occasionally, the physician would give opioids or other controlled substances – often benzodiazepines – to these patients, without a prescription or valid medical need.
Dr. Sutton escalated the dosage for one of those patients, even as the subjective pain score did not improve and when the patient’s urine tests showed the presence of THC and buprenorphine, but not any of the prescribed medications.
Another patient came to Dr. Sutton in 2007 with a warning that she had a history of “narcotic-seeking” behavior and diagnoses of depression, anxiety, paranoid schizophrenia, and obsessive-compulsive disorder.
The patient was hospitalized in 2018 for complications from benzodiazepine use (prescribed by Dr. Sutton). She weighed 80 pounds at the time. Dr. Sutton continued to prescribe benzodiazepines and extreme doses of opioids – in excess of 2,000 morphine equivalent dose – “despite recognizing and documenting repeated instances of noncompliance with treatment for psychiatric conditions, and despite the known contraindications of long-term opioid use for patients with these mental illnesses,” according to the charging document.
Dr. Sutton continued to prescribe opioids despite two hospitalizations for overdoses, more than 20 failed urine drug screens that showed presence of illicit drugs such as cocaine, and documented excessive use of alprazolam (Xanax) and methadone.
The physician surrendered his Drug Enforcement Administration Certificate of Registration of Controlled Substances Privileges in February 2022 “as an indication of your good faith in desiring to remedy any incorrect or unlawful practices on your part,” according to a letter to Dr. Sutton from the State Medical Board of Ohio. In that September 2022 letter, the Board notified Dr. Sutton of its intention to possibly suspend or revoke his license.
Dr. Sutton did not request a hearing, and the Board permanently revoked his medical license on January 16.
The court will sentence Dr. Sutton on May 23, according to a report by WFMJ.
A version of this article originally appeared on Medscape.com.
Jeffrey B. Sutton, DO, a neuromuscular medicine specialist, pled guilty on January 30 in federal court to 31 counts of illegally prescribing opioids and other controlled substances, 1 count of illegally distributing controlled substances, and 20 counts of health care fraud.
Prosecutors said Dr. Sutton admitted that he ignored warnings from prescription drug management organizations, insurers, and state authorities that he was prescribing excessively high dosages of opioids.
Dr. Sutton also admitted to ignoring patient requests to lower dosages and that he also ignored signs that patients were selling prescribed medications or otherwise engaging in illicit activity, including violations of a “pain management agreement” that he required them to sign.
The fraud counts pertained to Dr. Sutton billing Medicare, Medicaid, and other insurers for medically unnecessary visits that he required of patients so that he could prescribe inappropriate or unnecessary opioids.
In the charging document shared with this news organization, prosecutors said Dr. Sutton had sex with at least three patients, including during office visits and outside of the office. Occasionally, the physician would give opioids or other controlled substances – often benzodiazepines – to these patients, without a prescription or valid medical need.
Dr. Sutton escalated the dosage for one of those patients, even as the subjective pain score did not improve and when the patient’s urine tests showed the presence of THC and buprenorphine, but not any of the prescribed medications.
Another patient came to Dr. Sutton in 2007 with a warning that she had a history of “narcotic-seeking” behavior and diagnoses of depression, anxiety, paranoid schizophrenia, and obsessive-compulsive disorder.
The patient was hospitalized in 2018 for complications from benzodiazepine use (prescribed by Dr. Sutton). She weighed 80 pounds at the time. Dr. Sutton continued to prescribe benzodiazepines and extreme doses of opioids – in excess of 2,000 morphine equivalent dose – “despite recognizing and documenting repeated instances of noncompliance with treatment for psychiatric conditions, and despite the known contraindications of long-term opioid use for patients with these mental illnesses,” according to the charging document.
Dr. Sutton continued to prescribe opioids despite two hospitalizations for overdoses, more than 20 failed urine drug screens that showed presence of illicit drugs such as cocaine, and documented excessive use of alprazolam (Xanax) and methadone.
The physician surrendered his Drug Enforcement Administration Certificate of Registration of Controlled Substances Privileges in February 2022 “as an indication of your good faith in desiring to remedy any incorrect or unlawful practices on your part,” according to a letter to Dr. Sutton from the State Medical Board of Ohio. In that September 2022 letter, the Board notified Dr. Sutton of its intention to possibly suspend or revoke his license.
Dr. Sutton did not request a hearing, and the Board permanently revoked his medical license on January 16.
The court will sentence Dr. Sutton on May 23, according to a report by WFMJ.
A version of this article originally appeared on Medscape.com.
Jeffrey B. Sutton, DO, a neuromuscular medicine specialist, pled guilty on January 30 in federal court to 31 counts of illegally prescribing opioids and other controlled substances, 1 count of illegally distributing controlled substances, and 20 counts of health care fraud.
Prosecutors said Dr. Sutton admitted that he ignored warnings from prescription drug management organizations, insurers, and state authorities that he was prescribing excessively high dosages of opioids.
Dr. Sutton also admitted to ignoring patient requests to lower dosages and that he also ignored signs that patients were selling prescribed medications or otherwise engaging in illicit activity, including violations of a “pain management agreement” that he required them to sign.
The fraud counts pertained to Dr. Sutton billing Medicare, Medicaid, and other insurers for medically unnecessary visits that he required of patients so that he could prescribe inappropriate or unnecessary opioids.
In the charging document shared with this news organization, prosecutors said Dr. Sutton had sex with at least three patients, including during office visits and outside of the office. Occasionally, the physician would give opioids or other controlled substances – often benzodiazepines – to these patients, without a prescription or valid medical need.
Dr. Sutton escalated the dosage for one of those patients, even as the subjective pain score did not improve and when the patient’s urine tests showed the presence of THC and buprenorphine, but not any of the prescribed medications.
Another patient came to Dr. Sutton in 2007 with a warning that she had a history of “narcotic-seeking” behavior and diagnoses of depression, anxiety, paranoid schizophrenia, and obsessive-compulsive disorder.
The patient was hospitalized in 2018 for complications from benzodiazepine use (prescribed by Dr. Sutton). She weighed 80 pounds at the time. Dr. Sutton continued to prescribe benzodiazepines and extreme doses of opioids – in excess of 2,000 morphine equivalent dose – “despite recognizing and documenting repeated instances of noncompliance with treatment for psychiatric conditions, and despite the known contraindications of long-term opioid use for patients with these mental illnesses,” according to the charging document.
Dr. Sutton continued to prescribe opioids despite two hospitalizations for overdoses, more than 20 failed urine drug screens that showed presence of illicit drugs such as cocaine, and documented excessive use of alprazolam (Xanax) and methadone.
The physician surrendered his Drug Enforcement Administration Certificate of Registration of Controlled Substances Privileges in February 2022 “as an indication of your good faith in desiring to remedy any incorrect or unlawful practices on your part,” according to a letter to Dr. Sutton from the State Medical Board of Ohio. In that September 2022 letter, the Board notified Dr. Sutton of its intention to possibly suspend or revoke his license.
Dr. Sutton did not request a hearing, and the Board permanently revoked his medical license on January 16.
The court will sentence Dr. Sutton on May 23, according to a report by WFMJ.
A version of this article originally appeared on Medscape.com.