User login
500-mg Calcium Pill Protects Against Preeclampsia, Researchers Say
Taking 500 mg of calcium a day reduces the likelihood of pregnant women developing preeclampsia in pregnant women as much as higher doses, according to a study in The New England Journal of Medicine published on January 11.
The study also shows that the lower dose of calcium protects against preterm birth almost as well as the higher dose recommended by the World Health Organization (WHO).
The US Centers for Disease Control and Prevention estimates that preeclampsia occurs in about 1 in 25 pregnancies, and Black women are 60% more likely to develop the condition than are White women.
Calcium supplementation reduces the risks for preeclampsia, per WHO guidelines.
The WHO has recommended between 1500 mg and 2000 mg of calcium supplementation daily along with one 30- to 60-mg iron pill for pregnant women who receive insufficient calcium in their diets, which the WHO says generally occurs in lower-income nations and not wealthier nations, such as the United States.
This dosage amounts to a minimum of three calcium pills per day because the dietary supplements generally come from suppliers in 500-mg doses. Researchers say the supplements are too expensive for many health authorities of low- and middle-income nations to provide, and that taking so many pills presents a barrier to use even if they were plentiful. In countries such as Tanzania and India, governments generally distribute supplements like calcium for free at health clinics, said Christopher Sudfeld, ScD, associate professor of global health and nutrition at Harvard University’s T.H. Chan School of Public Health, in Boston.
The WHO recommendation is “not implemented many places,” Dr. Sudfeld said. Due to the cost, Tanzania has never implemented WHO’s calcium recommendation, providing only the iron pill, he said.
The randomized double-blinded study included 11,000 pregnant women in Tanzania and 11,000 pregnant women in India. None had yet given birth, which increased their risk for preeclampsia. All participants were older than 18 years and were less than 20 weeks pregnant, according to their most recent menstrual date. Half of participants received the three daily 500-mg calcium pills recommended by WHO; the other half received a single calcium pill and two placebo pills.
Researchers measured blood pressure and urine protein levels starting at 20 weeks of gestation, at delivery, and 6 weeks after giving birth.
Regardless of how much calcium people consumed daily, preeclampsia occurred approximately 3% of both the 500-mg and 1500-mg groups. Similar rates of preterm births occurred in both groups, although in Tanzanian, women in the 500-mg arm were somewhat more likely to give birth early.
“We’re working with governments but we’re also going to disseminate the results to WHO, so that they can do their process for the next antenatal care guidelines, to potentially change the global guidelines,” to support a lower calcium supplement target, Dr. Sudfeld said.
Does Calcium Actually Prevent Preeclampsia?
But Ahizechukwu Eke, MD, PhD, MPH, a pharmacologist who practices maternal fetal medicine at Johns Hopkins Medicine in Baltimore, questioned whether calcium really works to prevent preeclampsia.
Eke said that the causes of preeclampsia are multifactorial, and researchers have yet to definitively demonstrate the mechanism of action by which calcium works to prevent the condition. One hypothesis is that calcium reduces the amount of contractions in a woman’s uterus, thereby lowering blood pressure.
Low-dose aspirin is also used to prevent preeclampsia, and Dr. Eke said that the pharmacokinetic pathway by which this drug inhibits preeclampsia is more clear.
“I’m not saying we should stop using calcium, far from it,” Dr. Eke said. But as calcium supplementation to prevent preeclampsia continues, Dr. Eke called for pharmacokinetic studies to explore whether and how calcium works.
Dr. Sudfeld and Dr. Eke report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Taking 500 mg of calcium a day reduces the likelihood of pregnant women developing preeclampsia in pregnant women as much as higher doses, according to a study in The New England Journal of Medicine published on January 11.
The study also shows that the lower dose of calcium protects against preterm birth almost as well as the higher dose recommended by the World Health Organization (WHO).
The US Centers for Disease Control and Prevention estimates that preeclampsia occurs in about 1 in 25 pregnancies, and Black women are 60% more likely to develop the condition than are White women.
Calcium supplementation reduces the risks for preeclampsia, per WHO guidelines.
The WHO has recommended between 1500 mg and 2000 mg of calcium supplementation daily along with one 30- to 60-mg iron pill for pregnant women who receive insufficient calcium in their diets, which the WHO says generally occurs in lower-income nations and not wealthier nations, such as the United States.
This dosage amounts to a minimum of three calcium pills per day because the dietary supplements generally come from suppliers in 500-mg doses. Researchers say the supplements are too expensive for many health authorities of low- and middle-income nations to provide, and that taking so many pills presents a barrier to use even if they were plentiful. In countries such as Tanzania and India, governments generally distribute supplements like calcium for free at health clinics, said Christopher Sudfeld, ScD, associate professor of global health and nutrition at Harvard University’s T.H. Chan School of Public Health, in Boston.
The WHO recommendation is “not implemented many places,” Dr. Sudfeld said. Due to the cost, Tanzania has never implemented WHO’s calcium recommendation, providing only the iron pill, he said.
The randomized double-blinded study included 11,000 pregnant women in Tanzania and 11,000 pregnant women in India. None had yet given birth, which increased their risk for preeclampsia. All participants were older than 18 years and were less than 20 weeks pregnant, according to their most recent menstrual date. Half of participants received the three daily 500-mg calcium pills recommended by WHO; the other half received a single calcium pill and two placebo pills.
Researchers measured blood pressure and urine protein levels starting at 20 weeks of gestation, at delivery, and 6 weeks after giving birth.
Regardless of how much calcium people consumed daily, preeclampsia occurred approximately 3% of both the 500-mg and 1500-mg groups. Similar rates of preterm births occurred in both groups, although in Tanzanian, women in the 500-mg arm were somewhat more likely to give birth early.
“We’re working with governments but we’re also going to disseminate the results to WHO, so that they can do their process for the next antenatal care guidelines, to potentially change the global guidelines,” to support a lower calcium supplement target, Dr. Sudfeld said.
Does Calcium Actually Prevent Preeclampsia?
But Ahizechukwu Eke, MD, PhD, MPH, a pharmacologist who practices maternal fetal medicine at Johns Hopkins Medicine in Baltimore, questioned whether calcium really works to prevent preeclampsia.
Eke said that the causes of preeclampsia are multifactorial, and researchers have yet to definitively demonstrate the mechanism of action by which calcium works to prevent the condition. One hypothesis is that calcium reduces the amount of contractions in a woman’s uterus, thereby lowering blood pressure.
Low-dose aspirin is also used to prevent preeclampsia, and Dr. Eke said that the pharmacokinetic pathway by which this drug inhibits preeclampsia is more clear.
“I’m not saying we should stop using calcium, far from it,” Dr. Eke said. But as calcium supplementation to prevent preeclampsia continues, Dr. Eke called for pharmacokinetic studies to explore whether and how calcium works.
Dr. Sudfeld and Dr. Eke report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Taking 500 mg of calcium a day reduces the likelihood of pregnant women developing preeclampsia in pregnant women as much as higher doses, according to a study in The New England Journal of Medicine published on January 11.
The study also shows that the lower dose of calcium protects against preterm birth almost as well as the higher dose recommended by the World Health Organization (WHO).
The US Centers for Disease Control and Prevention estimates that preeclampsia occurs in about 1 in 25 pregnancies, and Black women are 60% more likely to develop the condition than are White women.
Calcium supplementation reduces the risks for preeclampsia, per WHO guidelines.
The WHO has recommended between 1500 mg and 2000 mg of calcium supplementation daily along with one 30- to 60-mg iron pill for pregnant women who receive insufficient calcium in their diets, which the WHO says generally occurs in lower-income nations and not wealthier nations, such as the United States.
This dosage amounts to a minimum of three calcium pills per day because the dietary supplements generally come from suppliers in 500-mg doses. Researchers say the supplements are too expensive for many health authorities of low- and middle-income nations to provide, and that taking so many pills presents a barrier to use even if they were plentiful. In countries such as Tanzania and India, governments generally distribute supplements like calcium for free at health clinics, said Christopher Sudfeld, ScD, associate professor of global health and nutrition at Harvard University’s T.H. Chan School of Public Health, in Boston.
The WHO recommendation is “not implemented many places,” Dr. Sudfeld said. Due to the cost, Tanzania has never implemented WHO’s calcium recommendation, providing only the iron pill, he said.
The randomized double-blinded study included 11,000 pregnant women in Tanzania and 11,000 pregnant women in India. None had yet given birth, which increased their risk for preeclampsia. All participants were older than 18 years and were less than 20 weeks pregnant, according to their most recent menstrual date. Half of participants received the three daily 500-mg calcium pills recommended by WHO; the other half received a single calcium pill and two placebo pills.
Researchers measured blood pressure and urine protein levels starting at 20 weeks of gestation, at delivery, and 6 weeks after giving birth.
Regardless of how much calcium people consumed daily, preeclampsia occurred approximately 3% of both the 500-mg and 1500-mg groups. Similar rates of preterm births occurred in both groups, although in Tanzanian, women in the 500-mg arm were somewhat more likely to give birth early.
“We’re working with governments but we’re also going to disseminate the results to WHO, so that they can do their process for the next antenatal care guidelines, to potentially change the global guidelines,” to support a lower calcium supplement target, Dr. Sudfeld said.
Does Calcium Actually Prevent Preeclampsia?
But Ahizechukwu Eke, MD, PhD, MPH, a pharmacologist who practices maternal fetal medicine at Johns Hopkins Medicine in Baltimore, questioned whether calcium really works to prevent preeclampsia.
Eke said that the causes of preeclampsia are multifactorial, and researchers have yet to definitively demonstrate the mechanism of action by which calcium works to prevent the condition. One hypothesis is that calcium reduces the amount of contractions in a woman’s uterus, thereby lowering blood pressure.
Low-dose aspirin is also used to prevent preeclampsia, and Dr. Eke said that the pharmacokinetic pathway by which this drug inhibits preeclampsia is more clear.
“I’m not saying we should stop using calcium, far from it,” Dr. Eke said. But as calcium supplementation to prevent preeclampsia continues, Dr. Eke called for pharmacokinetic studies to explore whether and how calcium works.
Dr. Sudfeld and Dr. Eke report no relevant financial relationships.
A version of this article appeared on Medscape.com.
The Knowns and Unknowns About Delivery Timing in Diabetes
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FROM DPSG-NA 2023
A Tale of Two Babies and the ‘Family Tragedy’ of Congenital Syphilis
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Impact of Pregnancy on Rosacea Unpredictable, Study Suggests
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
Perinatal Psychiatry in 2024: Helping More Patients Access Care
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at obnews@mdedge.com.
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at obnews@mdedge.com.
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at obnews@mdedge.com.
Bariatric surgery tied to less pregnancy weight gain
TOPLINE:
Pregnancy weight gain is lower in women with a history of gastric bypass or sleeve gastrectomy than in those without such a history, especially when the interval between surgery and conception is shorter, new data suggest.
METHODOLOGY:
- Using Swedish national registers, researchers investigated the association of pregnancy weight gain with history in 12,776 pregnancies — 6388 in women with a history of bariatric surgery and 6388 in women without such a history.
- Pregnancies were propensity score matched to patients’ early-pregnancy body mass index (BMI), prepregnancy diabetes, , smoking status, education, height, country of birth, and delivery year.
- Post-gastric bypass pregnancies were matched to post-sleeve gastrectomy pregnancies using the same matching strategy.
- Time from surgery to conception was also assessed.
TAKEAWAY:
- Across all early-pregnancy BMI strata, women with a history of bariatric surgery had lower pregnancy weight gain than matched controls.
- The magnitude of difference was largest for women with normal weight or overweight early-pregnancy BMI status (adjusted mean difference in z score, −0.33), which then decreased stepwise within the subclasses (−0.21, −0.16, and −0.08 for obesity classes I, II, and III, respectively).
- Pregnancy weight gain did not differ by surgery type, but lower pregnancy weight gain was associated with a shorter surgery-to-conception interval (particularly within 1 year) or lower surgery-to-conception weight loss.
IN PRACTICE:
“The highest proportion of weight gain below the recommendations was found among women with a normal weight status. Hence, clinical attention to women with history of bariatric surgery and a normal weight status in early pregnancy might be warranted,” the authors advised.
SOURCE:
The study, with the first author Huiling Xu, MD, MSc, Karolinska Institutet, Stockholm, Sweden, was published online in JAMA Network Open.
LIMITATIONS:
Despite rigorous matching, residual confounding was possible. The sample size was limited for some subgroups, possibly affecting statistical power. Although the study provides an overview of pregnancy outcomes within surgery-to-conception interval and pregnancy weight gain z scores, a more in-depth investigation is needed to understand the associations among bariatric surgery, pregnancy weight gain, and pregnancy outcomes.
DISCLOSURES:
Research for this study was supported by the Swedish Research Council for Health, Working Life and Welfare, and the Swedish Research Council. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Pregnancy weight gain is lower in women with a history of gastric bypass or sleeve gastrectomy than in those without such a history, especially when the interval between surgery and conception is shorter, new data suggest.
METHODOLOGY:
- Using Swedish national registers, researchers investigated the association of pregnancy weight gain with history in 12,776 pregnancies — 6388 in women with a history of bariatric surgery and 6388 in women without such a history.
- Pregnancies were propensity score matched to patients’ early-pregnancy body mass index (BMI), prepregnancy diabetes, , smoking status, education, height, country of birth, and delivery year.
- Post-gastric bypass pregnancies were matched to post-sleeve gastrectomy pregnancies using the same matching strategy.
- Time from surgery to conception was also assessed.
TAKEAWAY:
- Across all early-pregnancy BMI strata, women with a history of bariatric surgery had lower pregnancy weight gain than matched controls.
- The magnitude of difference was largest for women with normal weight or overweight early-pregnancy BMI status (adjusted mean difference in z score, −0.33), which then decreased stepwise within the subclasses (−0.21, −0.16, and −0.08 for obesity classes I, II, and III, respectively).
- Pregnancy weight gain did not differ by surgery type, but lower pregnancy weight gain was associated with a shorter surgery-to-conception interval (particularly within 1 year) or lower surgery-to-conception weight loss.
IN PRACTICE:
“The highest proportion of weight gain below the recommendations was found among women with a normal weight status. Hence, clinical attention to women with history of bariatric surgery and a normal weight status in early pregnancy might be warranted,” the authors advised.
SOURCE:
The study, with the first author Huiling Xu, MD, MSc, Karolinska Institutet, Stockholm, Sweden, was published online in JAMA Network Open.
LIMITATIONS:
Despite rigorous matching, residual confounding was possible. The sample size was limited for some subgroups, possibly affecting statistical power. Although the study provides an overview of pregnancy outcomes within surgery-to-conception interval and pregnancy weight gain z scores, a more in-depth investigation is needed to understand the associations among bariatric surgery, pregnancy weight gain, and pregnancy outcomes.
DISCLOSURES:
Research for this study was supported by the Swedish Research Council for Health, Working Life and Welfare, and the Swedish Research Council. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Pregnancy weight gain is lower in women with a history of gastric bypass or sleeve gastrectomy than in those without such a history, especially when the interval between surgery and conception is shorter, new data suggest.
METHODOLOGY:
- Using Swedish national registers, researchers investigated the association of pregnancy weight gain with history in 12,776 pregnancies — 6388 in women with a history of bariatric surgery and 6388 in women without such a history.
- Pregnancies were propensity score matched to patients’ early-pregnancy body mass index (BMI), prepregnancy diabetes, , smoking status, education, height, country of birth, and delivery year.
- Post-gastric bypass pregnancies were matched to post-sleeve gastrectomy pregnancies using the same matching strategy.
- Time from surgery to conception was also assessed.
TAKEAWAY:
- Across all early-pregnancy BMI strata, women with a history of bariatric surgery had lower pregnancy weight gain than matched controls.
- The magnitude of difference was largest for women with normal weight or overweight early-pregnancy BMI status (adjusted mean difference in z score, −0.33), which then decreased stepwise within the subclasses (−0.21, −0.16, and −0.08 for obesity classes I, II, and III, respectively).
- Pregnancy weight gain did not differ by surgery type, but lower pregnancy weight gain was associated with a shorter surgery-to-conception interval (particularly within 1 year) or lower surgery-to-conception weight loss.
IN PRACTICE:
“The highest proportion of weight gain below the recommendations was found among women with a normal weight status. Hence, clinical attention to women with history of bariatric surgery and a normal weight status in early pregnancy might be warranted,” the authors advised.
SOURCE:
The study, with the first author Huiling Xu, MD, MSc, Karolinska Institutet, Stockholm, Sweden, was published online in JAMA Network Open.
LIMITATIONS:
Despite rigorous matching, residual confounding was possible. The sample size was limited for some subgroups, possibly affecting statistical power. Although the study provides an overview of pregnancy outcomes within surgery-to-conception interval and pregnancy weight gain z scores, a more in-depth investigation is needed to understand the associations among bariatric surgery, pregnancy weight gain, and pregnancy outcomes.
DISCLOSURES:
Research for this study was supported by the Swedish Research Council for Health, Working Life and Welfare, and the Swedish Research Council. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
Fivefold Increase in Vaping During Adolescent Pregnancies
TOPLINE:
, according to research published online on December 13 in JAMA Network Open.
METHODOLOGY:
- Researchers analyzed data from the 2016-2021 Pregnancy Risk Assessment Monitoring System.
- They focused on 10,428 adolescents aged 10-19 years who had had a singleton birth and provided information about their use of e-cigarettes or cigarettes.
TAKEAWAY:
- Whereas the researchers found a roughly fivefold increase in the exclusive use of e-cigarettes, the percentage of patients using only cigarettes decreased from 9.2% in 2017 to 3.2% in 2021.
- The percentage of patients who both vaped and smoked fluctuated between 0.6% and 1.6%.
- The rate of small-for-gestational-age (SGA) births for adolescents who did not smoke or vape (12.9%) did not differ significantly from that among adolescents who exclusively used e-cigarettes (16.8%) or those who used both cigarettes and e-cigarettes (17.6%).
- The researchers found use of cigarettes only was associated with a significantly higher rate of SGA births: 24.6%.
IN PRACTICE:
“Exclusive e-cigarette use and dual use of cigarettes and e-cigarettes did not seem to be statistically significantly associated with SGA birth in our analysis, but this finding should be interpreted with caution given the low prevalence of use and the limited sample size,” the study authors wrote.
SOURCE:
Xiaozhong Wen, MD, PhD, with the Jacobs School of Medicine and Biomedical Sciences at the State University of New York at Buffalo, was the corresponding author of the study.
LIMITATIONS:
Participants may have underreported their use of e-cigarettes and cigarettes because of fears of social stigma. The researchers lacked information about vaping in the first and second trimesters, exposure to secondhand smoke, cannabis use, and diet.
DISCLOSURES:
The research was supported by the National Institute on Drug Abuse; the Food and Drug Administration Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the American Heart Association. A study coauthor has received grants from Pfizer and personal fees from Johnson & Johnson, the World Health Organization, and the Campaign for Tobacco-Free Kids.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to research published online on December 13 in JAMA Network Open.
METHODOLOGY:
- Researchers analyzed data from the 2016-2021 Pregnancy Risk Assessment Monitoring System.
- They focused on 10,428 adolescents aged 10-19 years who had had a singleton birth and provided information about their use of e-cigarettes or cigarettes.
TAKEAWAY:
- Whereas the researchers found a roughly fivefold increase in the exclusive use of e-cigarettes, the percentage of patients using only cigarettes decreased from 9.2% in 2017 to 3.2% in 2021.
- The percentage of patients who both vaped and smoked fluctuated between 0.6% and 1.6%.
- The rate of small-for-gestational-age (SGA) births for adolescents who did not smoke or vape (12.9%) did not differ significantly from that among adolescents who exclusively used e-cigarettes (16.8%) or those who used both cigarettes and e-cigarettes (17.6%).
- The researchers found use of cigarettes only was associated with a significantly higher rate of SGA births: 24.6%.
IN PRACTICE:
“Exclusive e-cigarette use and dual use of cigarettes and e-cigarettes did not seem to be statistically significantly associated with SGA birth in our analysis, but this finding should be interpreted with caution given the low prevalence of use and the limited sample size,” the study authors wrote.
SOURCE:
Xiaozhong Wen, MD, PhD, with the Jacobs School of Medicine and Biomedical Sciences at the State University of New York at Buffalo, was the corresponding author of the study.
LIMITATIONS:
Participants may have underreported their use of e-cigarettes and cigarettes because of fears of social stigma. The researchers lacked information about vaping in the first and second trimesters, exposure to secondhand smoke, cannabis use, and diet.
DISCLOSURES:
The research was supported by the National Institute on Drug Abuse; the Food and Drug Administration Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the American Heart Association. A study coauthor has received grants from Pfizer and personal fees from Johnson & Johnson, the World Health Organization, and the Campaign for Tobacco-Free Kids.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to research published online on December 13 in JAMA Network Open.
METHODOLOGY:
- Researchers analyzed data from the 2016-2021 Pregnancy Risk Assessment Monitoring System.
- They focused on 10,428 adolescents aged 10-19 years who had had a singleton birth and provided information about their use of e-cigarettes or cigarettes.
TAKEAWAY:
- Whereas the researchers found a roughly fivefold increase in the exclusive use of e-cigarettes, the percentage of patients using only cigarettes decreased from 9.2% in 2017 to 3.2% in 2021.
- The percentage of patients who both vaped and smoked fluctuated between 0.6% and 1.6%.
- The rate of small-for-gestational-age (SGA) births for adolescents who did not smoke or vape (12.9%) did not differ significantly from that among adolescents who exclusively used e-cigarettes (16.8%) or those who used both cigarettes and e-cigarettes (17.6%).
- The researchers found use of cigarettes only was associated with a significantly higher rate of SGA births: 24.6%.
IN PRACTICE:
“Exclusive e-cigarette use and dual use of cigarettes and e-cigarettes did not seem to be statistically significantly associated with SGA birth in our analysis, but this finding should be interpreted with caution given the low prevalence of use and the limited sample size,” the study authors wrote.
SOURCE:
Xiaozhong Wen, MD, PhD, with the Jacobs School of Medicine and Biomedical Sciences at the State University of New York at Buffalo, was the corresponding author of the study.
LIMITATIONS:
Participants may have underreported their use of e-cigarettes and cigarettes because of fears of social stigma. The researchers lacked information about vaping in the first and second trimesters, exposure to secondhand smoke, cannabis use, and diet.
DISCLOSURES:
The research was supported by the National Institute on Drug Abuse; the Food and Drug Administration Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the American Heart Association. A study coauthor has received grants from Pfizer and personal fees from Johnson & Johnson, the World Health Organization, and the Campaign for Tobacco-Free Kids.
A version of this article appeared on Medscape.com.
Toward a better framework for postmarketing reproductive safety surveillance of medications
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at obnews@mdedge.com.
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at obnews@mdedge.com.
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at obnews@mdedge.com.
1 in 3 women have lasting health problems after giving birth: Study
Those problems include pain during sexual intercourse (35%), low back pain (32%), urinary incontinence (8% to 31%), anxiety (9% to 24%), anal incontinence (19%), depression (11% to 17%), fear of childbirth (6% to 15%), perineal pain (11%), and secondary infertility (11%).
Other problems included pelvic organ prolapse, posttraumatic stress disorder, thyroid dysfunction, mastitis, HIV seroconversion (when the body begins to produce detectable levels of HIV antibodies), nerve injury, and psychosis.
The study says most women see a doctor 6 to 12 weeks after birth and then rarely talk to doctors about these nagging health problems. Many of the problems don’t show up until 6 or more weeks after birth.
“To comprehensively address these conditions, broader and more comprehensive health service opportunities are needed, which should extend beyond 6 weeks postpartum and embrace multidisciplinary models of care,” the study says. “This approach can ensure that these conditions are promptly identified and given the attention that they deserve.”
The study is part of a series organized by the United Nation’s Special Program on Human Reproduction, the World Health Organization, and the U.S. Agency for International Development. The authors said most of the data came from high-income nations. There was little data from low-income and middle-income countries except for postpartum depression, anxiety, and psychosis.
“Many postpartum conditions cause considerable suffering in women’s daily life long after birth, both emotionally and physically, and yet they are largely underappreciated, underrecognized, and underreported,” Pascale Allotey, MD, director of Sexual and Reproductive Health and Research at WHO, said in a statement.
“Throughout their lives, and beyond motherhood, women need access to a range of services from health-care providers who listen to their concerns and meet their needs — so they not only survive childbirth but can enjoy good health and quality of life.”
A version of this article appeared on WebMD.com.
Those problems include pain during sexual intercourse (35%), low back pain (32%), urinary incontinence (8% to 31%), anxiety (9% to 24%), anal incontinence (19%), depression (11% to 17%), fear of childbirth (6% to 15%), perineal pain (11%), and secondary infertility (11%).
Other problems included pelvic organ prolapse, posttraumatic stress disorder, thyroid dysfunction, mastitis, HIV seroconversion (when the body begins to produce detectable levels of HIV antibodies), nerve injury, and psychosis.
The study says most women see a doctor 6 to 12 weeks after birth and then rarely talk to doctors about these nagging health problems. Many of the problems don’t show up until 6 or more weeks after birth.
“To comprehensively address these conditions, broader and more comprehensive health service opportunities are needed, which should extend beyond 6 weeks postpartum and embrace multidisciplinary models of care,” the study says. “This approach can ensure that these conditions are promptly identified and given the attention that they deserve.”
The study is part of a series organized by the United Nation’s Special Program on Human Reproduction, the World Health Organization, and the U.S. Agency for International Development. The authors said most of the data came from high-income nations. There was little data from low-income and middle-income countries except for postpartum depression, anxiety, and psychosis.
“Many postpartum conditions cause considerable suffering in women’s daily life long after birth, both emotionally and physically, and yet they are largely underappreciated, underrecognized, and underreported,” Pascale Allotey, MD, director of Sexual and Reproductive Health and Research at WHO, said in a statement.
“Throughout their lives, and beyond motherhood, women need access to a range of services from health-care providers who listen to their concerns and meet their needs — so they not only survive childbirth but can enjoy good health and quality of life.”
A version of this article appeared on WebMD.com.
Those problems include pain during sexual intercourse (35%), low back pain (32%), urinary incontinence (8% to 31%), anxiety (9% to 24%), anal incontinence (19%), depression (11% to 17%), fear of childbirth (6% to 15%), perineal pain (11%), and secondary infertility (11%).
Other problems included pelvic organ prolapse, posttraumatic stress disorder, thyroid dysfunction, mastitis, HIV seroconversion (when the body begins to produce detectable levels of HIV antibodies), nerve injury, and psychosis.
The study says most women see a doctor 6 to 12 weeks after birth and then rarely talk to doctors about these nagging health problems. Many of the problems don’t show up until 6 or more weeks after birth.
“To comprehensively address these conditions, broader and more comprehensive health service opportunities are needed, which should extend beyond 6 weeks postpartum and embrace multidisciplinary models of care,” the study says. “This approach can ensure that these conditions are promptly identified and given the attention that they deserve.”
The study is part of a series organized by the United Nation’s Special Program on Human Reproduction, the World Health Organization, and the U.S. Agency for International Development. The authors said most of the data came from high-income nations. There was little data from low-income and middle-income countries except for postpartum depression, anxiety, and psychosis.
“Many postpartum conditions cause considerable suffering in women’s daily life long after birth, both emotionally and physically, and yet they are largely underappreciated, underrecognized, and underreported,” Pascale Allotey, MD, director of Sexual and Reproductive Health and Research at WHO, said in a statement.
“Throughout their lives, and beyond motherhood, women need access to a range of services from health-care providers who listen to their concerns and meet their needs — so they not only survive childbirth but can enjoy good health and quality of life.”
A version of this article appeared on WebMD.com.
FROM THE LANCET GLOBAL HEALTH
2023 Update on minimally invasive gynecologic surgery
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15
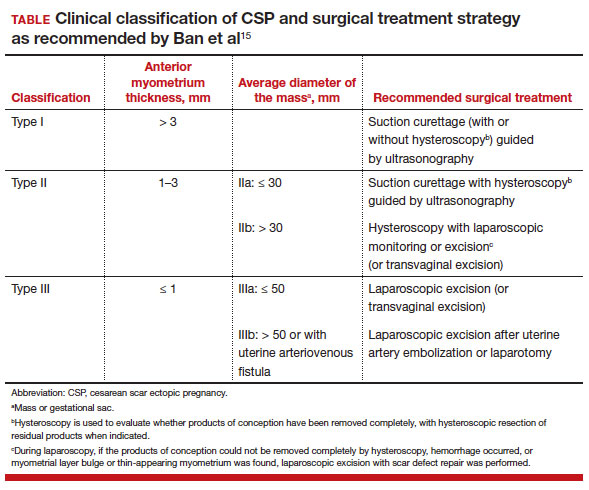
Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15

Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15

Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147



