User login
Consider drug holidays for BCC patients on hedgehog inhibitors
KAUAI, HAWAII – according to Kishwer Nehal, MD, director of Mohs micrographic and dermatologic surgery at Memorial Sloan Kettering Cancer Center, New York.
That’s important because, although some patients have a good response to vismodegib, more – about 80% – have side effects that make it necessary to stop treatment, including muscle spasms and weight loss, among other problems. Side effects often come on quickly and can become intolerable after a few months of treatment, so physicians have looked for alternative dosing regimens to hold them off, with some success.
Compared with those on continuous dosing, fewer patients on intermittent dosing discontinued treatment for adverse events (23% versus 31%). Patients on intermittent dosing also experienced fewer grade 3 adverse events (31% versus 44%) and were on treatment for a longer period of time (a median of 71.4 weeks versus 37.6 weeks).
Meanwhile, among those on intermittent dosing, the number of BCCs was reduced in more than half of the patients in both interrupted treatment groups, but more so in the 12-weeks-on/8-weeks-off group (Lancet Oncol. 2017 Mar; 18[3]:404-12).
Other treatment options are being explored for vismodegib, as well as for sonidegib (Odomzo), another hedgehog signaling pathway inhibitor approved for advanced BCC. Ongoing trials are looking at the use of hedgehog inhibitors with radiation, and for shrinking tumors before surgery, Dr. Nehal said
For now, however, surgery remains the mainstay of treatment for BCC; both biologics are indicated for when other treatments fail or are not feasible. For high-risk BCC (meaning high risk for recurrence, based on infiltrative or poorly defined histology, perineural or bony involvement, or location on the face, for instance), “surgery with clear margins remains the goal and is the most effective treatment. For a high-risk [BCC], you pretty much need surgery,” she said.
Recurrence is less likely with Mohs surgery than with standard excision. When Mohs isn’t available, “you should wait for the pathology report before reconstruction,” she said.
“Radiation for high-risk [BCC] is really reserved for nonsurgical candidates,” Dr. Nehal commented. There are only two scenarios to consider radiation in high-risk BCC, “and they really have no proven benefit in any sort of prospective trial. One is if you cannot, after exhaustive surgery, clear your very high risk [BCC].” The other is if there is “really large nerve involvement, greater than 0.1 mm, or such extensive perineural involvement that surgery is unlikely to be successful,” she said.
Dr. Nehal had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – according to Kishwer Nehal, MD, director of Mohs micrographic and dermatologic surgery at Memorial Sloan Kettering Cancer Center, New York.
That’s important because, although some patients have a good response to vismodegib, more – about 80% – have side effects that make it necessary to stop treatment, including muscle spasms and weight loss, among other problems. Side effects often come on quickly and can become intolerable after a few months of treatment, so physicians have looked for alternative dosing regimens to hold them off, with some success.
Compared with those on continuous dosing, fewer patients on intermittent dosing discontinued treatment for adverse events (23% versus 31%). Patients on intermittent dosing also experienced fewer grade 3 adverse events (31% versus 44%) and were on treatment for a longer period of time (a median of 71.4 weeks versus 37.6 weeks).
Meanwhile, among those on intermittent dosing, the number of BCCs was reduced in more than half of the patients in both interrupted treatment groups, but more so in the 12-weeks-on/8-weeks-off group (Lancet Oncol. 2017 Mar; 18[3]:404-12).
Other treatment options are being explored for vismodegib, as well as for sonidegib (Odomzo), another hedgehog signaling pathway inhibitor approved for advanced BCC. Ongoing trials are looking at the use of hedgehog inhibitors with radiation, and for shrinking tumors before surgery, Dr. Nehal said
For now, however, surgery remains the mainstay of treatment for BCC; both biologics are indicated for when other treatments fail or are not feasible. For high-risk BCC (meaning high risk for recurrence, based on infiltrative or poorly defined histology, perineural or bony involvement, or location on the face, for instance), “surgery with clear margins remains the goal and is the most effective treatment. For a high-risk [BCC], you pretty much need surgery,” she said.
Recurrence is less likely with Mohs surgery than with standard excision. When Mohs isn’t available, “you should wait for the pathology report before reconstruction,” she said.
“Radiation for high-risk [BCC] is really reserved for nonsurgical candidates,” Dr. Nehal commented. There are only two scenarios to consider radiation in high-risk BCC, “and they really have no proven benefit in any sort of prospective trial. One is if you cannot, after exhaustive surgery, clear your very high risk [BCC].” The other is if there is “really large nerve involvement, greater than 0.1 mm, or such extensive perineural involvement that surgery is unlikely to be successful,” she said.
Dr. Nehal had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – according to Kishwer Nehal, MD, director of Mohs micrographic and dermatologic surgery at Memorial Sloan Kettering Cancer Center, New York.
That’s important because, although some patients have a good response to vismodegib, more – about 80% – have side effects that make it necessary to stop treatment, including muscle spasms and weight loss, among other problems. Side effects often come on quickly and can become intolerable after a few months of treatment, so physicians have looked for alternative dosing regimens to hold them off, with some success.
Compared with those on continuous dosing, fewer patients on intermittent dosing discontinued treatment for adverse events (23% versus 31%). Patients on intermittent dosing also experienced fewer grade 3 adverse events (31% versus 44%) and were on treatment for a longer period of time (a median of 71.4 weeks versus 37.6 weeks).
Meanwhile, among those on intermittent dosing, the number of BCCs was reduced in more than half of the patients in both interrupted treatment groups, but more so in the 12-weeks-on/8-weeks-off group (Lancet Oncol. 2017 Mar; 18[3]:404-12).
Other treatment options are being explored for vismodegib, as well as for sonidegib (Odomzo), another hedgehog signaling pathway inhibitor approved for advanced BCC. Ongoing trials are looking at the use of hedgehog inhibitors with radiation, and for shrinking tumors before surgery, Dr. Nehal said
For now, however, surgery remains the mainstay of treatment for BCC; both biologics are indicated for when other treatments fail or are not feasible. For high-risk BCC (meaning high risk for recurrence, based on infiltrative or poorly defined histology, perineural or bony involvement, or location on the face, for instance), “surgery with clear margins remains the goal and is the most effective treatment. For a high-risk [BCC], you pretty much need surgery,” she said.
Recurrence is less likely with Mohs surgery than with standard excision. When Mohs isn’t available, “you should wait for the pathology report before reconstruction,” she said.
“Radiation for high-risk [BCC] is really reserved for nonsurgical candidates,” Dr. Nehal commented. There are only two scenarios to consider radiation in high-risk BCC, “and they really have no proven benefit in any sort of prospective trial. One is if you cannot, after exhaustive surgery, clear your very high risk [BCC].” The other is if there is “really large nerve involvement, greater than 0.1 mm, or such extensive perineural involvement that surgery is unlikely to be successful,” she said.
Dr. Nehal had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: Bioimpedance provides accurate assessment of Mohs surgical margins
SAN DIEGO – In assessing tumor-free margins during Mohs micrographic surgery for skin cancer, of histologic sections, in a single-center, pilot study of bioimpedance in 151 specimens from 50 consecutive patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If the finding of high diagnostic accuracy using bioimpedance spectroscopy is confirmed in larger numbers of patients and specimens run at multiple sites, this approach could “potentially revolutionize what happens with the way Mohs sections are processed in the future” by potentially shaving many minutes off the duration of a standard procedure, Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology.
Usually, it takes 10-20 minutes to process and examine Mohs specimens at each stage of the surgical procedure to determine whether additional excision must remove residual cancer cells, said Dr. Rigel, a dermatologist at New York University. In contrast, assessment for residual cancer cells in the surgical field takes less than a minute using bioimpedance spectroscopy, which relies on differences in electrical conductivity between benign and cancerous cells to identify cancer cells remaining at the surgical margins.
The results of the study were presented in a poster at the meeting, by a research associate of Dr. Rigel’s, Ryan Svoboda, MD, of the National Society for Cutaneous Medicine, New York.
The researchers used a bioimpedance spectroscopy device made by NovaScan to assess 151 histology slides prepared during Mohs micrographic surgery on patients with nonmelanoma skin cancer, and compared the findings against the gold standard of histological slide examination. By this criterion, bioimpedance spectroscopy identified 105 true negatives and 2 false negatives, and 43 true positives and 1 false positive. Calculations showed that this equated to 95.6% sensitivity, 99.1% specificity, a 97.7% positive predictive value, and a 98.1% negative predictive value.
These may be underestimates of the accuracy of bioimpedance spectroscopy because the calculations presume that conventional histology is always correct, but Dr. Rigel noted that sometimes the histological diagnosis is wrong.
SOURCE: Svoboda R et al. Poster 7304.
SAN DIEGO – In assessing tumor-free margins during Mohs micrographic surgery for skin cancer, of histologic sections, in a single-center, pilot study of bioimpedance in 151 specimens from 50 consecutive patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If the finding of high diagnostic accuracy using bioimpedance spectroscopy is confirmed in larger numbers of patients and specimens run at multiple sites, this approach could “potentially revolutionize what happens with the way Mohs sections are processed in the future” by potentially shaving many minutes off the duration of a standard procedure, Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology.
Usually, it takes 10-20 minutes to process and examine Mohs specimens at each stage of the surgical procedure to determine whether additional excision must remove residual cancer cells, said Dr. Rigel, a dermatologist at New York University. In contrast, assessment for residual cancer cells in the surgical field takes less than a minute using bioimpedance spectroscopy, which relies on differences in electrical conductivity between benign and cancerous cells to identify cancer cells remaining at the surgical margins.
The results of the study were presented in a poster at the meeting, by a research associate of Dr. Rigel’s, Ryan Svoboda, MD, of the National Society for Cutaneous Medicine, New York.
The researchers used a bioimpedance spectroscopy device made by NovaScan to assess 151 histology slides prepared during Mohs micrographic surgery on patients with nonmelanoma skin cancer, and compared the findings against the gold standard of histological slide examination. By this criterion, bioimpedance spectroscopy identified 105 true negatives and 2 false negatives, and 43 true positives and 1 false positive. Calculations showed that this equated to 95.6% sensitivity, 99.1% specificity, a 97.7% positive predictive value, and a 98.1% negative predictive value.
These may be underestimates of the accuracy of bioimpedance spectroscopy because the calculations presume that conventional histology is always correct, but Dr. Rigel noted that sometimes the histological diagnosis is wrong.
SOURCE: Svoboda R et al. Poster 7304.
SAN DIEGO – In assessing tumor-free margins during Mohs micrographic surgery for skin cancer, of histologic sections, in a single-center, pilot study of bioimpedance in 151 specimens from 50 consecutive patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If the finding of high diagnostic accuracy using bioimpedance spectroscopy is confirmed in larger numbers of patients and specimens run at multiple sites, this approach could “potentially revolutionize what happens with the way Mohs sections are processed in the future” by potentially shaving many minutes off the duration of a standard procedure, Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology.
Usually, it takes 10-20 minutes to process and examine Mohs specimens at each stage of the surgical procedure to determine whether additional excision must remove residual cancer cells, said Dr. Rigel, a dermatologist at New York University. In contrast, assessment for residual cancer cells in the surgical field takes less than a minute using bioimpedance spectroscopy, which relies on differences in electrical conductivity between benign and cancerous cells to identify cancer cells remaining at the surgical margins.
The results of the study were presented in a poster at the meeting, by a research associate of Dr. Rigel’s, Ryan Svoboda, MD, of the National Society for Cutaneous Medicine, New York.
The researchers used a bioimpedance spectroscopy device made by NovaScan to assess 151 histology slides prepared during Mohs micrographic surgery on patients with nonmelanoma skin cancer, and compared the findings against the gold standard of histological slide examination. By this criterion, bioimpedance spectroscopy identified 105 true negatives and 2 false negatives, and 43 true positives and 1 false positive. Calculations showed that this equated to 95.6% sensitivity, 99.1% specificity, a 97.7% positive predictive value, and a 98.1% negative predictive value.
These may be underestimates of the accuracy of bioimpedance spectroscopy because the calculations presume that conventional histology is always correct, but Dr. Rigel noted that sometimes the histological diagnosis is wrong.
SOURCE: Svoboda R et al. Poster 7304.
REPORTING FROM AAD 18
Key clinical point: Bioimpedance spectroscopy showed excellent diagnostic accuracy for cancer cells on Mohs surgical margins.
Major finding: Bioimpedance spectroscopy had a sensitivity of 95.6% and specificity of 99.1% compared with Mohs histology.
Study details: A single-center pilot study with 151 Mohs surgical specimens taken from 50 patients.
Disclosures: The study was funded by NovaScan, the company developing the device tested in the study. Dr. Rigel has been a consultant to NovaScan and to Castle Biosciences, DermTech, Ferndale, Myriad, and Neutrogena, and has received research support from Castle and Neutrogena.
Source: Svoboda R et al. Poster 7304.
Evaluating Dermatology Apps for Patient Education
Study: Test for PD-L1 amplification in solid tumors
SAN FRANCISCO – Amplification of programmed death-ligand 1 (PD-L1), also known as cluster of differentiation 274 (CD274), is rare in most solid tumors, but findings from an analysis in which a majority of patients with the alteration experienced durable responses to PD-1/PD-L1 blockade suggest that testing for it may be warranted.
Of 117,344 deidentified cancer patient samples from a large database, only 0.7% had PD-L1 amplification, which was defined as 6 or more copy number alterations (CNAs). The CNAs were found across more than 100 tumor histologies, Aaron Goodman, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
Of a subset of 2,039 clinically annotated patients from the database, who were seen at the University of California, San Diego (UCSD) Center for Personalized Cancer Therapy, 13 (0.6%) had PD-L1 CNAs, and 9 were treated with immune checkpoint blockade, either alone or in combination with another immunotherapeutic or targeted therapy, after a median of four prior systemic therapies.
The PD-1/PD-L1 blockade response rate in those nine patients was 67%, and median progression-free survival was 15.2 months; three objective responses were ongoing for at least 15 months, said Dr. Goodman of UCSD.
The findings are notable, because in unselected patients, the rates of response to immune checkpoint blockade range from 10% to 20%.
Lessons from cHL and solid tumors
“Over the past few years, investigators have identified numerous biomarkers that can select subgroups of patients with increased likelihoods of responding to PD-1 blockade,” he said, adding that biomarkers include PD-L1 expression by immunohistochemistry, microsatellite instability – with microsatellite instability–high tumors responding extremely well to immunotherapy, tumor mutational burden measured by whole exome sequencing and next generation sequencing, and possibly PD-L1 amplification.
Of note, response rates are high in patients with classical Hodgkin lymphoma (cHL). In general, cHL patients respond well to treatment, with the majority being cured by way of multiagent chemotherapy and radiation.
“But for the subpopulation that fails to respond to chemotherapy or relapses, outcomes still remain suboptimal. Remarkably, in the relapsed/refractory population of Hodgkin lymphoma ... response rates to single agent nivolumab and pembrolizumab were 65% to 87% [in recent studies],” he said. “Long-term follow-up demonstrates that the majority of these responses were durable and lasted over a year.”
The question is why relapsed/refractory cHL patients treated with immune checkpoint blockade have such a higher response rate than is typically seen in patients with solid tumors.
One answer might lie in the recent finding that nearly 100% of cHL tumors harbor amplification of 9p24.1; the 9p24.1 amplicon encodes the genes PD-L1, PD-L2, and JAK2, (and thus is also known as the PDJ amplicon), he explained, adding that “through gene dose-dependent increased expression of PD-L1 ligand on the Hodgkin lymphoma Reed-Sternberg cells, there is also JAK-STAT mediation of further expression of PD-L1 on the Reed-Sternberg cells.
An encounter with a patient with metastatic basal cell carcinoma – a “relatively unusual situation, as the majority of patients are cured with local therapy”– led to interest in looking at 9p24.1 alterations in solid tumors.
The patient had extensive metastatic disease, and had progressed through multiple therapies. Given his limited treatment options, next generation sequencing was performed on a biopsy from his tumor, and it revealed the PTCH1 alteration typical in basal cell carcinoma, as well as amplification of 9p24.1 with PD-L1, PD-L2, and JAK2 amplification. Nivolumab monotherapy was initiated.
“Within 2 months, he had an excellent partial response to therapy, and I’m pleased to say that he’s in an ongoing complete response 2 years later,” Dr. Goodman said.
It was that case that sparked the idea for the current study.
9p24.1 alterations and checkpoint blockade
“With my interest in hematologic malignancies, I was unaware that [9p24.1] amplification could occur in solid tumors, so the first aim was to determine the prevalence of chromosome 9p24.1 alterations in solid tumors. The next was to determine if patients with solid tumors and chromosome 9p24.1 alterations respond to PD-1/PD-L1 checkpoint blockade.
“What is astounding is [that PD-L1 amplification] was found in over 100 unique tumor histologies, although rare in most histologies,” Dr. Goodman said, noting that histologies with a statistically increased prevalence of PD-L1 amplification included breast cancer, head and neck squamous cell carcinoma, lung squamous cell carcinoma, and soft tissue sarcoma.
There also were some rare histologies with increased prevalence of PD-L1 amplification, including nasopharyngeal carcinoma, renal sarcomatoid carcinoma, bladder squamous cell carcinoma, and liver mixed hepatocellular cholangiocarcinoma, he said.
Tumors with a paucity of PD-L1 amplification included colorectal cancer, pancreatic cancer, and cutaneous melanoma, although even these still harbored a few patients with amplification, he said.
A closer look at the mutational burden in amplified vs. unamplified tumors showed a median of 7.4 vs. 3.6 mut/mb, but in the PD-L1 amplified group, 85% still had a low-to intermediate mutational burden of 1-20 mut/mb.
“Microsatellite instability and PD-L1 amplification were not mutually exclusive, but a rare event. Five of the 821 cases with PD-L1 amplification were microsatellite high; these included three carcinomas of unknown origin and two cases of gastrointestinal cancer,” he noted.
Treatment outcomes
In the 13 UCSD patients with PD-L1 amplification, nine different malignancies were identified, and all patients had advanced or metastatic disease and were heavily pretreated. Of the nine treated patients, five received anti-PD-1 monotherapy, one received anti-CTLA4/anti-PD-1 combination therapy, and three received a PD-1/PD-L1 inhibitor plus an investigational agent, which was immunotherapeutic, Dr. Goodman said.
The 67% overall response rate was similar to that seen in Hodgkin lymphoma, and many of the responses were durable; median overall survival was not reached.
Of note, genomic analysis in the 13 UCSD patients found to have PD-L1 amplification showed there were 143 total alterations in 70 different genes. All but one patient had amplification of PD-L1, PD-L2, and JAK2, and that one had amplification of PD-L1 and PD-L2.
Of six tumors with tissue available to test for PD-L1 expression by immunohistochemistry, four (67%) tested positive. None were microsatellite high, and tumor-infiltrating lymphocytes were present in five cases.
The tumors that tested negative for PD-L1 expression were from the patient with the rare basal cell cancer, and another with glioblastoma. Both responded to anti-PD1/PD-L1 therapy.
The glioblastoma patient was a 40-year-old man with progressive disease, who underwent standard surgical debulking followed by concurrent radiation therapy plus temozolomide. He progressed soon after completing the concurrent chemoradiation therapy, and genomic profiling revealed 12 alterations, including 9p24.1 amplification, Dr. Goodman said, adding that nivolumab therapy was initiated.
“By week 12, much of the tumor mass had started to resolve, and by week 26 it continued to decrease further. He continues to be in an ongoing partial response at 5.2 months,” he said.
Recommendations
The findings of this study demonstrate that PD-Ll amplification is rare in solid tumors.
“However, PD-L1 amplification appears to be tissue agnostic, as we have seen in over 100 tumor histologies. We also noted that PD-L1 amplification was enriched in many rare tumors with limited treatment options, including anaplastic thyroid cancer, sarcomatoid carcinoma, and some sarcomas. We believe testing for PD-L1 amplification may be warranted given the frequent responses that were durable and seemed to be independent of mutational burden,” he concluded.
Ravindra Uppaluri, MD, session chair and discussant for Dr. Goodman’s presentation, said that Dr. Goodman’s findings should be considered in the context of “the complex biology [of PD-L1/PD-L2] that has evolved over the last few years.”
He specifically mentioned the two patients without PD-L1 expression despite amplification, but with response to immune checkpoint blockade, and noted that “there are several things going on here ... and we really want to look at all these things.”
The PDJ amplicon, especially given “the ability to look at this with the targeted gene panels that many patients are getting,” is clearly contributing to biomarker stratification, said Dr. Uppaluri of Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston.
However, it should be assessed as part of a “global biomarker” that includes tumor-infiltrating lymphocytes and tumor mutational burden, he said.
Dr. Goodman reported having no disclosures. Dr. Uppaluri has received grant/research support from NIH/NIDCR, Merck, and V Foundation, and has received honoraria from Merck.
sworcester@frontlinemedcom.com
SOURCE: Goodman A et al. ASCO-SITC, Abstract 47
SAN FRANCISCO – Amplification of programmed death-ligand 1 (PD-L1), also known as cluster of differentiation 274 (CD274), is rare in most solid tumors, but findings from an analysis in which a majority of patients with the alteration experienced durable responses to PD-1/PD-L1 blockade suggest that testing for it may be warranted.
Of 117,344 deidentified cancer patient samples from a large database, only 0.7% had PD-L1 amplification, which was defined as 6 or more copy number alterations (CNAs). The CNAs were found across more than 100 tumor histologies, Aaron Goodman, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
Of a subset of 2,039 clinically annotated patients from the database, who were seen at the University of California, San Diego (UCSD) Center for Personalized Cancer Therapy, 13 (0.6%) had PD-L1 CNAs, and 9 were treated with immune checkpoint blockade, either alone or in combination with another immunotherapeutic or targeted therapy, after a median of four prior systemic therapies.
The PD-1/PD-L1 blockade response rate in those nine patients was 67%, and median progression-free survival was 15.2 months; three objective responses were ongoing for at least 15 months, said Dr. Goodman of UCSD.
The findings are notable, because in unselected patients, the rates of response to immune checkpoint blockade range from 10% to 20%.
Lessons from cHL and solid tumors
“Over the past few years, investigators have identified numerous biomarkers that can select subgroups of patients with increased likelihoods of responding to PD-1 blockade,” he said, adding that biomarkers include PD-L1 expression by immunohistochemistry, microsatellite instability – with microsatellite instability–high tumors responding extremely well to immunotherapy, tumor mutational burden measured by whole exome sequencing and next generation sequencing, and possibly PD-L1 amplification.
Of note, response rates are high in patients with classical Hodgkin lymphoma (cHL). In general, cHL patients respond well to treatment, with the majority being cured by way of multiagent chemotherapy and radiation.
“But for the subpopulation that fails to respond to chemotherapy or relapses, outcomes still remain suboptimal. Remarkably, in the relapsed/refractory population of Hodgkin lymphoma ... response rates to single agent nivolumab and pembrolizumab were 65% to 87% [in recent studies],” he said. “Long-term follow-up demonstrates that the majority of these responses were durable and lasted over a year.”
The question is why relapsed/refractory cHL patients treated with immune checkpoint blockade have such a higher response rate than is typically seen in patients with solid tumors.
One answer might lie in the recent finding that nearly 100% of cHL tumors harbor amplification of 9p24.1; the 9p24.1 amplicon encodes the genes PD-L1, PD-L2, and JAK2, (and thus is also known as the PDJ amplicon), he explained, adding that “through gene dose-dependent increased expression of PD-L1 ligand on the Hodgkin lymphoma Reed-Sternberg cells, there is also JAK-STAT mediation of further expression of PD-L1 on the Reed-Sternberg cells.
An encounter with a patient with metastatic basal cell carcinoma – a “relatively unusual situation, as the majority of patients are cured with local therapy”– led to interest in looking at 9p24.1 alterations in solid tumors.
The patient had extensive metastatic disease, and had progressed through multiple therapies. Given his limited treatment options, next generation sequencing was performed on a biopsy from his tumor, and it revealed the PTCH1 alteration typical in basal cell carcinoma, as well as amplification of 9p24.1 with PD-L1, PD-L2, and JAK2 amplification. Nivolumab monotherapy was initiated.
“Within 2 months, he had an excellent partial response to therapy, and I’m pleased to say that he’s in an ongoing complete response 2 years later,” Dr. Goodman said.
It was that case that sparked the idea for the current study.
9p24.1 alterations and checkpoint blockade
“With my interest in hematologic malignancies, I was unaware that [9p24.1] amplification could occur in solid tumors, so the first aim was to determine the prevalence of chromosome 9p24.1 alterations in solid tumors. The next was to determine if patients with solid tumors and chromosome 9p24.1 alterations respond to PD-1/PD-L1 checkpoint blockade.
“What is astounding is [that PD-L1 amplification] was found in over 100 unique tumor histologies, although rare in most histologies,” Dr. Goodman said, noting that histologies with a statistically increased prevalence of PD-L1 amplification included breast cancer, head and neck squamous cell carcinoma, lung squamous cell carcinoma, and soft tissue sarcoma.
There also were some rare histologies with increased prevalence of PD-L1 amplification, including nasopharyngeal carcinoma, renal sarcomatoid carcinoma, bladder squamous cell carcinoma, and liver mixed hepatocellular cholangiocarcinoma, he said.
Tumors with a paucity of PD-L1 amplification included colorectal cancer, pancreatic cancer, and cutaneous melanoma, although even these still harbored a few patients with amplification, he said.
A closer look at the mutational burden in amplified vs. unamplified tumors showed a median of 7.4 vs. 3.6 mut/mb, but in the PD-L1 amplified group, 85% still had a low-to intermediate mutational burden of 1-20 mut/mb.
“Microsatellite instability and PD-L1 amplification were not mutually exclusive, but a rare event. Five of the 821 cases with PD-L1 amplification were microsatellite high; these included three carcinomas of unknown origin and two cases of gastrointestinal cancer,” he noted.
Treatment outcomes
In the 13 UCSD patients with PD-L1 amplification, nine different malignancies were identified, and all patients had advanced or metastatic disease and were heavily pretreated. Of the nine treated patients, five received anti-PD-1 monotherapy, one received anti-CTLA4/anti-PD-1 combination therapy, and three received a PD-1/PD-L1 inhibitor plus an investigational agent, which was immunotherapeutic, Dr. Goodman said.
The 67% overall response rate was similar to that seen in Hodgkin lymphoma, and many of the responses were durable; median overall survival was not reached.
Of note, genomic analysis in the 13 UCSD patients found to have PD-L1 amplification showed there were 143 total alterations in 70 different genes. All but one patient had amplification of PD-L1, PD-L2, and JAK2, and that one had amplification of PD-L1 and PD-L2.
Of six tumors with tissue available to test for PD-L1 expression by immunohistochemistry, four (67%) tested positive. None were microsatellite high, and tumor-infiltrating lymphocytes were present in five cases.
The tumors that tested negative for PD-L1 expression were from the patient with the rare basal cell cancer, and another with glioblastoma. Both responded to anti-PD1/PD-L1 therapy.
The glioblastoma patient was a 40-year-old man with progressive disease, who underwent standard surgical debulking followed by concurrent radiation therapy plus temozolomide. He progressed soon after completing the concurrent chemoradiation therapy, and genomic profiling revealed 12 alterations, including 9p24.1 amplification, Dr. Goodman said, adding that nivolumab therapy was initiated.
“By week 12, much of the tumor mass had started to resolve, and by week 26 it continued to decrease further. He continues to be in an ongoing partial response at 5.2 months,” he said.
Recommendations
The findings of this study demonstrate that PD-Ll amplification is rare in solid tumors.
“However, PD-L1 amplification appears to be tissue agnostic, as we have seen in over 100 tumor histologies. We also noted that PD-L1 amplification was enriched in many rare tumors with limited treatment options, including anaplastic thyroid cancer, sarcomatoid carcinoma, and some sarcomas. We believe testing for PD-L1 amplification may be warranted given the frequent responses that were durable and seemed to be independent of mutational burden,” he concluded.
Ravindra Uppaluri, MD, session chair and discussant for Dr. Goodman’s presentation, said that Dr. Goodman’s findings should be considered in the context of “the complex biology [of PD-L1/PD-L2] that has evolved over the last few years.”
He specifically mentioned the two patients without PD-L1 expression despite amplification, but with response to immune checkpoint blockade, and noted that “there are several things going on here ... and we really want to look at all these things.”
The PDJ amplicon, especially given “the ability to look at this with the targeted gene panels that many patients are getting,” is clearly contributing to biomarker stratification, said Dr. Uppaluri of Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston.
However, it should be assessed as part of a “global biomarker” that includes tumor-infiltrating lymphocytes and tumor mutational burden, he said.
Dr. Goodman reported having no disclosures. Dr. Uppaluri has received grant/research support from NIH/NIDCR, Merck, and V Foundation, and has received honoraria from Merck.
sworcester@frontlinemedcom.com
SOURCE: Goodman A et al. ASCO-SITC, Abstract 47
SAN FRANCISCO – Amplification of programmed death-ligand 1 (PD-L1), also known as cluster of differentiation 274 (CD274), is rare in most solid tumors, but findings from an analysis in which a majority of patients with the alteration experienced durable responses to PD-1/PD-L1 blockade suggest that testing for it may be warranted.
Of 117,344 deidentified cancer patient samples from a large database, only 0.7% had PD-L1 amplification, which was defined as 6 or more copy number alterations (CNAs). The CNAs were found across more than 100 tumor histologies, Aaron Goodman, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
Of a subset of 2,039 clinically annotated patients from the database, who were seen at the University of California, San Diego (UCSD) Center for Personalized Cancer Therapy, 13 (0.6%) had PD-L1 CNAs, and 9 were treated with immune checkpoint blockade, either alone or in combination with another immunotherapeutic or targeted therapy, after a median of four prior systemic therapies.
The PD-1/PD-L1 blockade response rate in those nine patients was 67%, and median progression-free survival was 15.2 months; three objective responses were ongoing for at least 15 months, said Dr. Goodman of UCSD.
The findings are notable, because in unselected patients, the rates of response to immune checkpoint blockade range from 10% to 20%.
Lessons from cHL and solid tumors
“Over the past few years, investigators have identified numerous biomarkers that can select subgroups of patients with increased likelihoods of responding to PD-1 blockade,” he said, adding that biomarkers include PD-L1 expression by immunohistochemistry, microsatellite instability – with microsatellite instability–high tumors responding extremely well to immunotherapy, tumor mutational burden measured by whole exome sequencing and next generation sequencing, and possibly PD-L1 amplification.
Of note, response rates are high in patients with classical Hodgkin lymphoma (cHL). In general, cHL patients respond well to treatment, with the majority being cured by way of multiagent chemotherapy and radiation.
“But for the subpopulation that fails to respond to chemotherapy or relapses, outcomes still remain suboptimal. Remarkably, in the relapsed/refractory population of Hodgkin lymphoma ... response rates to single agent nivolumab and pembrolizumab were 65% to 87% [in recent studies],” he said. “Long-term follow-up demonstrates that the majority of these responses were durable and lasted over a year.”
The question is why relapsed/refractory cHL patients treated with immune checkpoint blockade have such a higher response rate than is typically seen in patients with solid tumors.
One answer might lie in the recent finding that nearly 100% of cHL tumors harbor amplification of 9p24.1; the 9p24.1 amplicon encodes the genes PD-L1, PD-L2, and JAK2, (and thus is also known as the PDJ amplicon), he explained, adding that “through gene dose-dependent increased expression of PD-L1 ligand on the Hodgkin lymphoma Reed-Sternberg cells, there is also JAK-STAT mediation of further expression of PD-L1 on the Reed-Sternberg cells.
An encounter with a patient with metastatic basal cell carcinoma – a “relatively unusual situation, as the majority of patients are cured with local therapy”– led to interest in looking at 9p24.1 alterations in solid tumors.
The patient had extensive metastatic disease, and had progressed through multiple therapies. Given his limited treatment options, next generation sequencing was performed on a biopsy from his tumor, and it revealed the PTCH1 alteration typical in basal cell carcinoma, as well as amplification of 9p24.1 with PD-L1, PD-L2, and JAK2 amplification. Nivolumab monotherapy was initiated.
“Within 2 months, he had an excellent partial response to therapy, and I’m pleased to say that he’s in an ongoing complete response 2 years later,” Dr. Goodman said.
It was that case that sparked the idea for the current study.
9p24.1 alterations and checkpoint blockade
“With my interest in hematologic malignancies, I was unaware that [9p24.1] amplification could occur in solid tumors, so the first aim was to determine the prevalence of chromosome 9p24.1 alterations in solid tumors. The next was to determine if patients with solid tumors and chromosome 9p24.1 alterations respond to PD-1/PD-L1 checkpoint blockade.
“What is astounding is [that PD-L1 amplification] was found in over 100 unique tumor histologies, although rare in most histologies,” Dr. Goodman said, noting that histologies with a statistically increased prevalence of PD-L1 amplification included breast cancer, head and neck squamous cell carcinoma, lung squamous cell carcinoma, and soft tissue sarcoma.
There also were some rare histologies with increased prevalence of PD-L1 amplification, including nasopharyngeal carcinoma, renal sarcomatoid carcinoma, bladder squamous cell carcinoma, and liver mixed hepatocellular cholangiocarcinoma, he said.
Tumors with a paucity of PD-L1 amplification included colorectal cancer, pancreatic cancer, and cutaneous melanoma, although even these still harbored a few patients with amplification, he said.
A closer look at the mutational burden in amplified vs. unamplified tumors showed a median of 7.4 vs. 3.6 mut/mb, but in the PD-L1 amplified group, 85% still had a low-to intermediate mutational burden of 1-20 mut/mb.
“Microsatellite instability and PD-L1 amplification were not mutually exclusive, but a rare event. Five of the 821 cases with PD-L1 amplification were microsatellite high; these included three carcinomas of unknown origin and two cases of gastrointestinal cancer,” he noted.
Treatment outcomes
In the 13 UCSD patients with PD-L1 amplification, nine different malignancies were identified, and all patients had advanced or metastatic disease and were heavily pretreated. Of the nine treated patients, five received anti-PD-1 monotherapy, one received anti-CTLA4/anti-PD-1 combination therapy, and three received a PD-1/PD-L1 inhibitor plus an investigational agent, which was immunotherapeutic, Dr. Goodman said.
The 67% overall response rate was similar to that seen in Hodgkin lymphoma, and many of the responses were durable; median overall survival was not reached.
Of note, genomic analysis in the 13 UCSD patients found to have PD-L1 amplification showed there were 143 total alterations in 70 different genes. All but one patient had amplification of PD-L1, PD-L2, and JAK2, and that one had amplification of PD-L1 and PD-L2.
Of six tumors with tissue available to test for PD-L1 expression by immunohistochemistry, four (67%) tested positive. None were microsatellite high, and tumor-infiltrating lymphocytes were present in five cases.
The tumors that tested negative for PD-L1 expression were from the patient with the rare basal cell cancer, and another with glioblastoma. Both responded to anti-PD1/PD-L1 therapy.
The glioblastoma patient was a 40-year-old man with progressive disease, who underwent standard surgical debulking followed by concurrent radiation therapy plus temozolomide. He progressed soon after completing the concurrent chemoradiation therapy, and genomic profiling revealed 12 alterations, including 9p24.1 amplification, Dr. Goodman said, adding that nivolumab therapy was initiated.
“By week 12, much of the tumor mass had started to resolve, and by week 26 it continued to decrease further. He continues to be in an ongoing partial response at 5.2 months,” he said.
Recommendations
The findings of this study demonstrate that PD-Ll amplification is rare in solid tumors.
“However, PD-L1 amplification appears to be tissue agnostic, as we have seen in over 100 tumor histologies. We also noted that PD-L1 amplification was enriched in many rare tumors with limited treatment options, including anaplastic thyroid cancer, sarcomatoid carcinoma, and some sarcomas. We believe testing for PD-L1 amplification may be warranted given the frequent responses that were durable and seemed to be independent of mutational burden,” he concluded.
Ravindra Uppaluri, MD, session chair and discussant for Dr. Goodman’s presentation, said that Dr. Goodman’s findings should be considered in the context of “the complex biology [of PD-L1/PD-L2] that has evolved over the last few years.”
He specifically mentioned the two patients without PD-L1 expression despite amplification, but with response to immune checkpoint blockade, and noted that “there are several things going on here ... and we really want to look at all these things.”
The PDJ amplicon, especially given “the ability to look at this with the targeted gene panels that many patients are getting,” is clearly contributing to biomarker stratification, said Dr. Uppaluri of Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston.
However, it should be assessed as part of a “global biomarker” that includes tumor-infiltrating lymphocytes and tumor mutational burden, he said.
Dr. Goodman reported having no disclosures. Dr. Uppaluri has received grant/research support from NIH/NIDCR, Merck, and V Foundation, and has received honoraria from Merck.
sworcester@frontlinemedcom.com
SOURCE: Goodman A et al. ASCO-SITC, Abstract 47
REPORTING FROM THE CLINICAL IMMUNO-ONCOLOGY SYMPOSIUM
Key clinical point: Solid tumor patients with PD-L1 amplification had durable responses to PD-1/PD-L1 blockade.
Major finding: The overall response rate was 67% in nine patients treated with PD-1/PD-L1 blockade.
Study details: An analysis of more than 117,000 patient samples.
Disclosures: Dr. Goodman reported having no disclosures. Dr. Uppaluri has received grant/research support from NIH/NIDCR, Merck, and V Foundation, and has received honoraria from Merck.
Source: Goodman A et al. ASCO-SITC, Abstract 47.
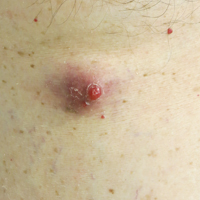
Enlarging Red Papulonodule on the Chest
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.
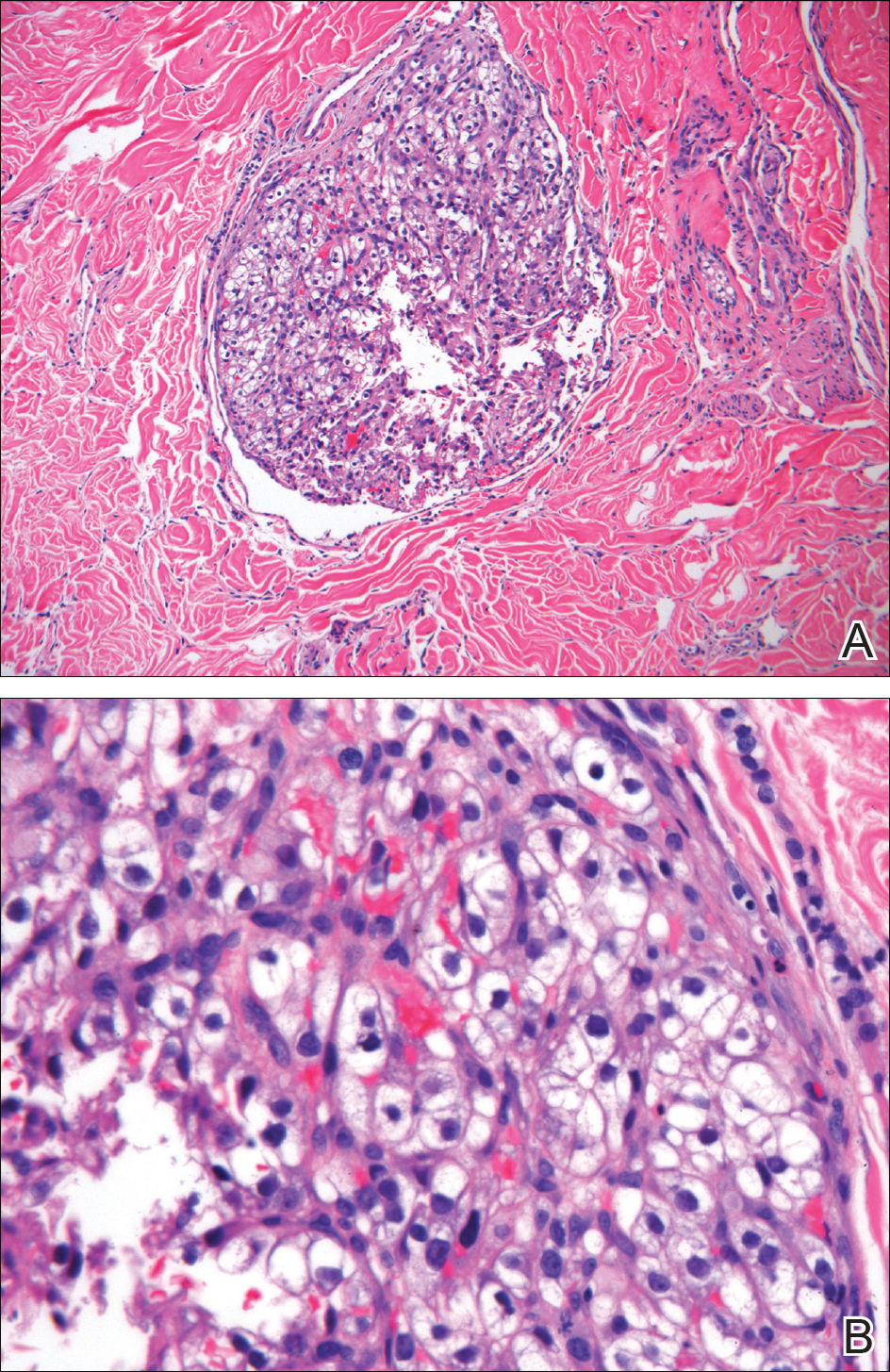
Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.

Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.

Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
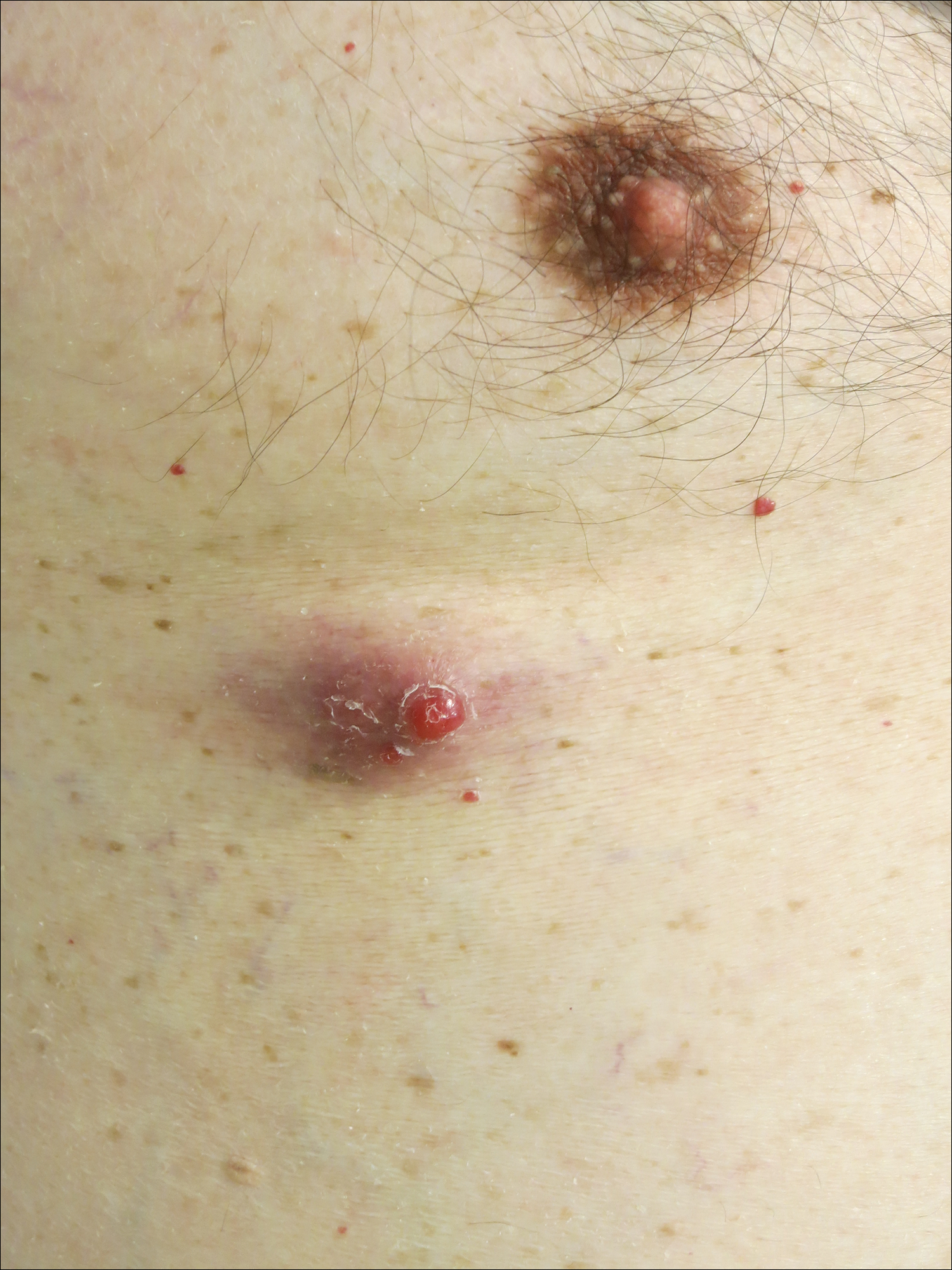
A man in his 60s presented with a subcutaneous nodule on the right side of the chest. Due to impaired mental status, he was unable to describe the precise age of the lesion, but his wife reported it had been present at least several weeks. She recently noted a new, bright red growth on top of the nodule. The lesion was asymptomatic but seemed to be growing in size. Physical examination revealed a 3-cm firm fixed nodule on the right side of the chest with an overlying, exophytic bright red papule. No similar lesions were found elsewhere on physical examination. A punch biopsy of the lesion was performed.
Mobile Medical Apps for Patient Education: A Graded Review of Available Dermatology Apps
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
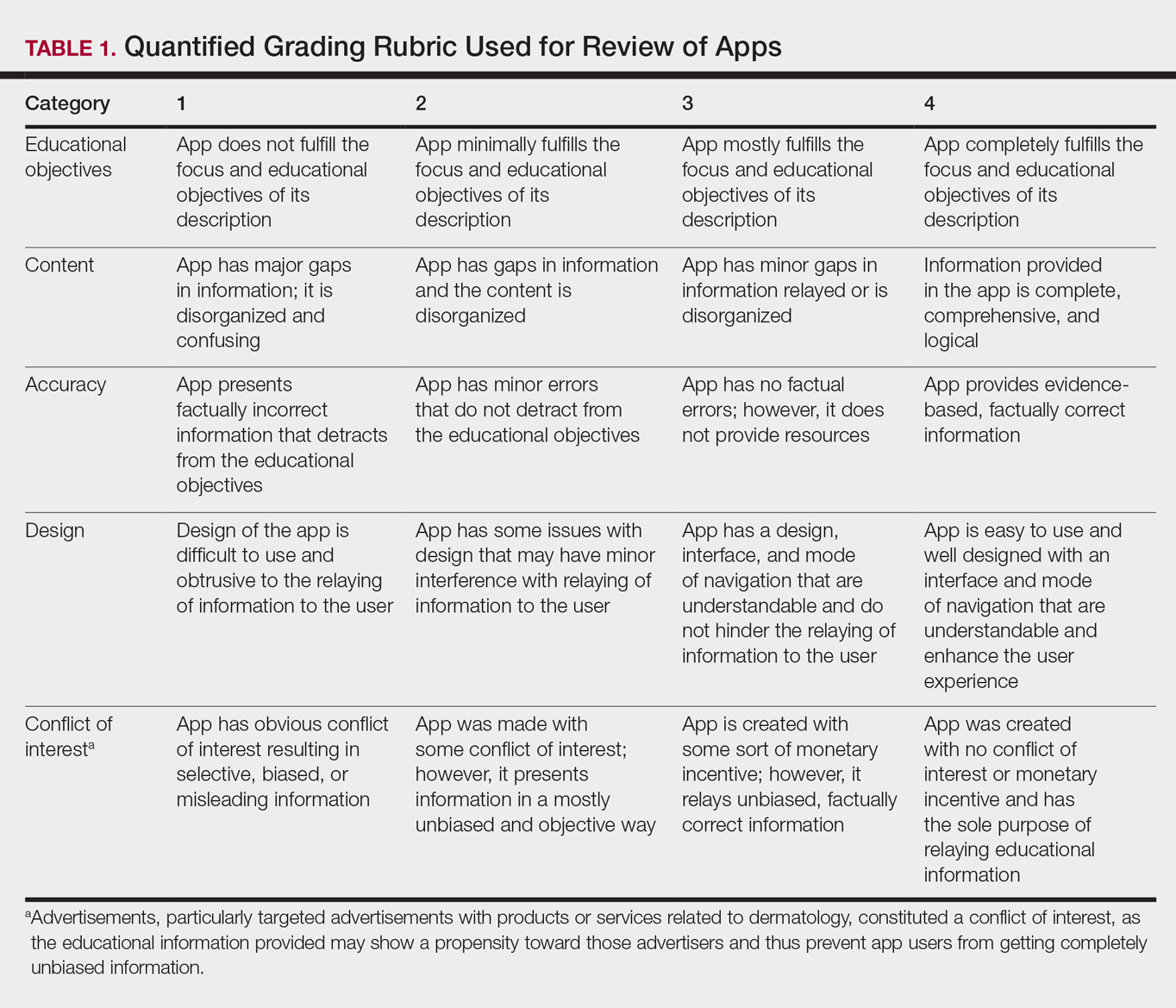
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.
Results
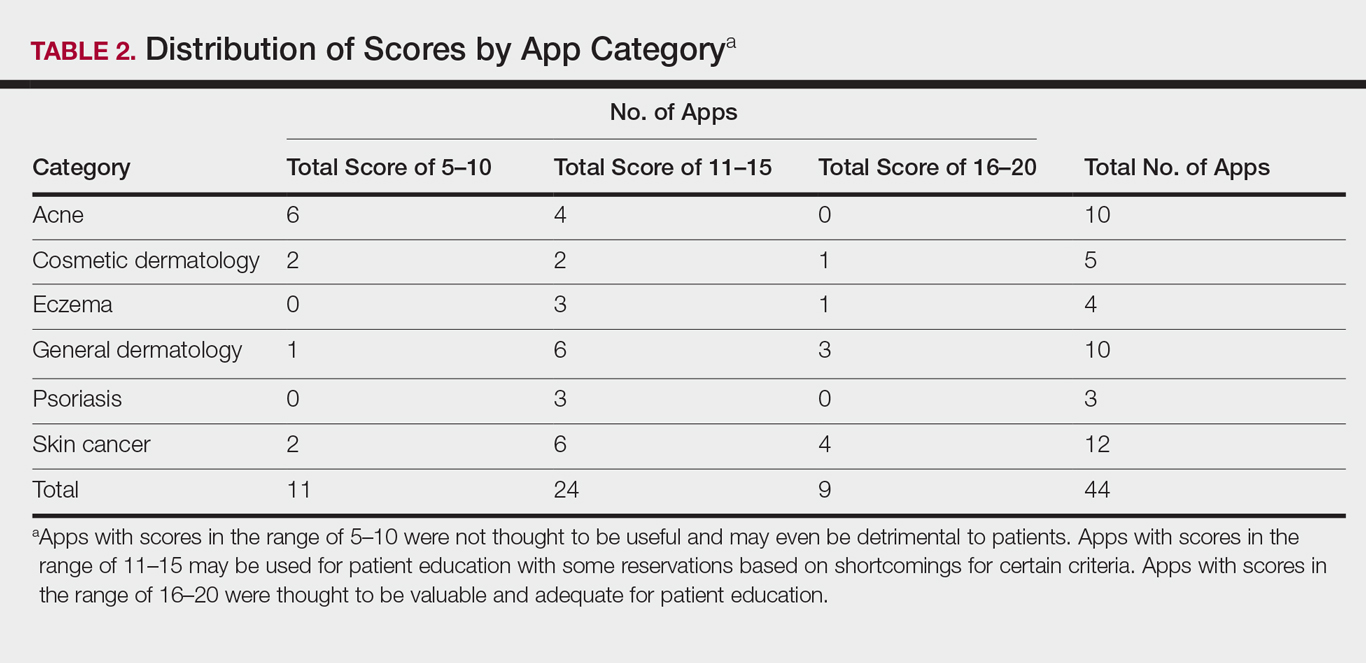
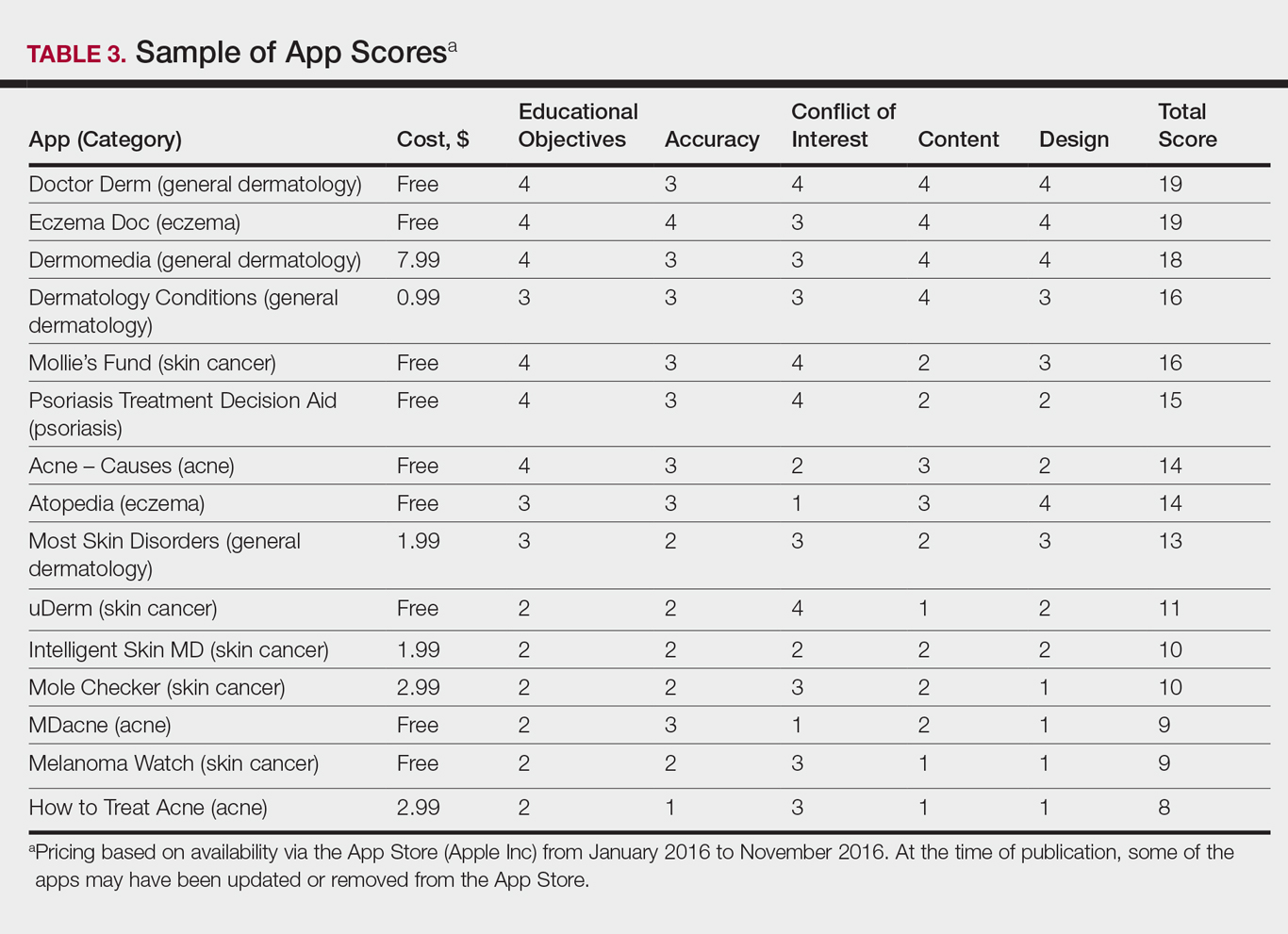
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.
Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.
Results
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.
Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.
Results
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.
Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
Practice Points
- Mobile dermatology apps for educational purposes should be objectively reviewed before being used by patients.
- In our study, only 9 (20.5%) of the 44 dermatology apps evaluated were considered adequate for patient information based on our grading criteria.
Primary Cutaneous Follicle Center Lymphoma Mimicking Folliculitis
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.
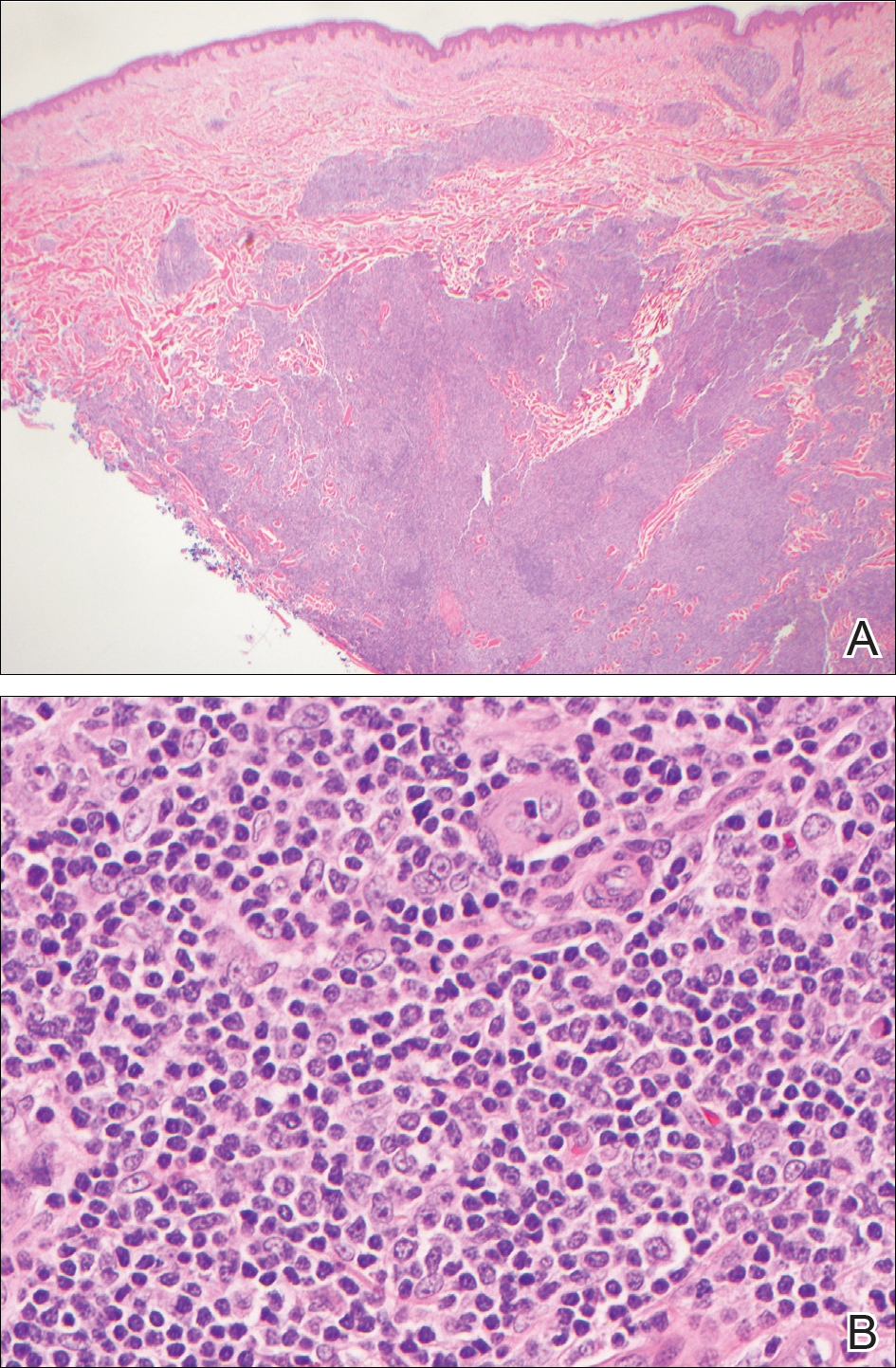
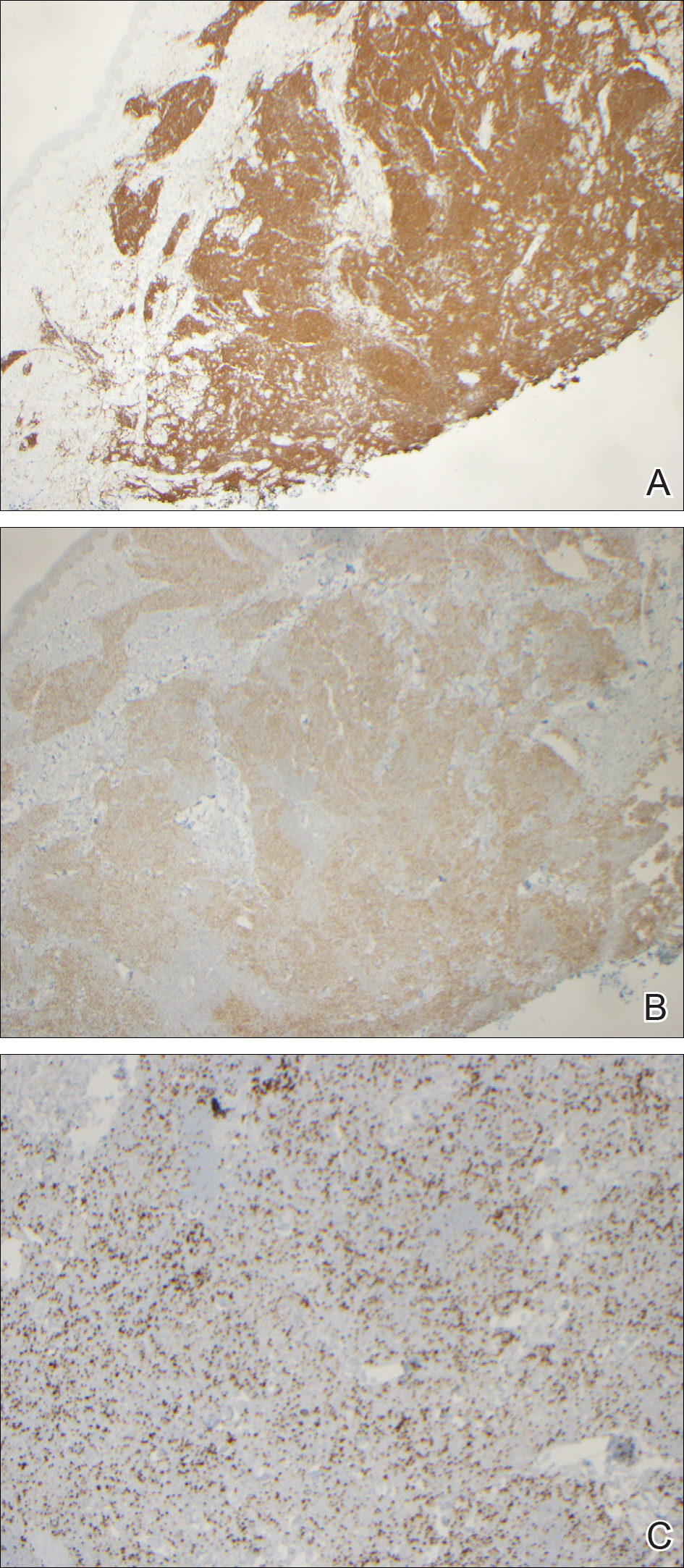
Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.



Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.



Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
Practice Points
- Atypical or unresponsive folliculitis should be biopsied.
- Primary cutaneous follicle center lymphoma can mimic folliculitis or Grover disease.
Complete Remission of Metastatic Merkel Cell Carcinoma in a Patient With Severe Psoriasis
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.
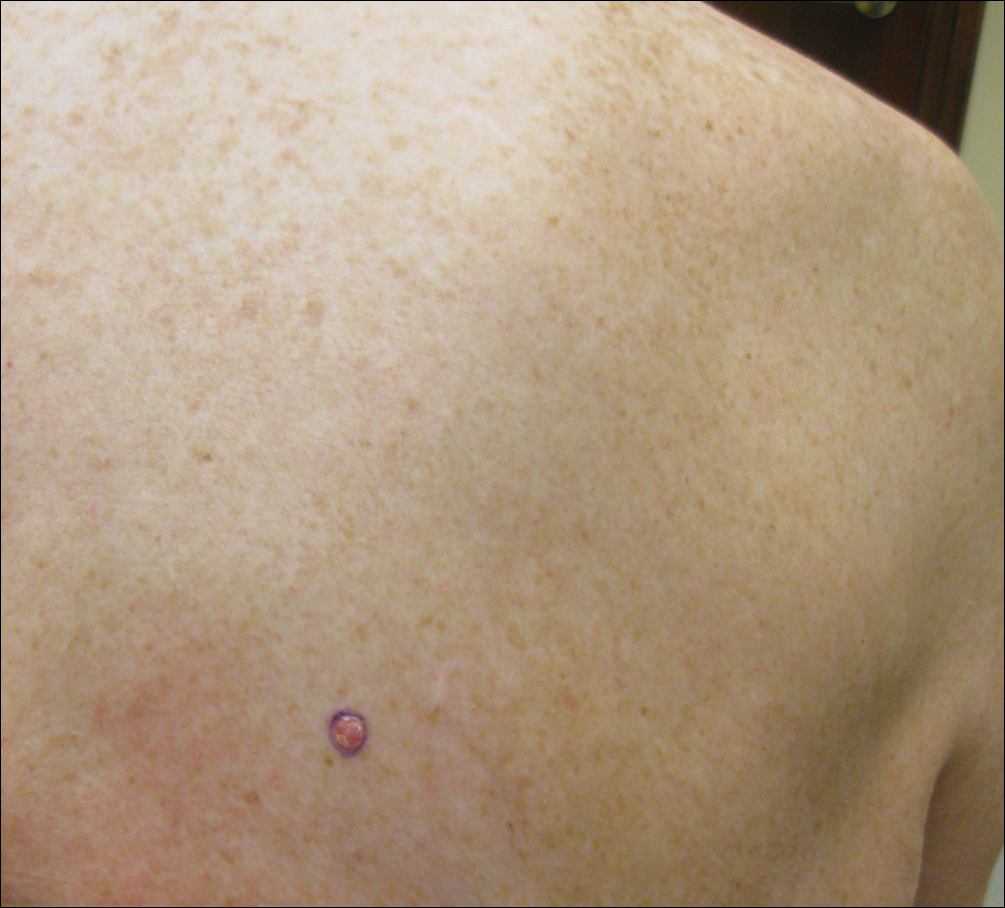
A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.
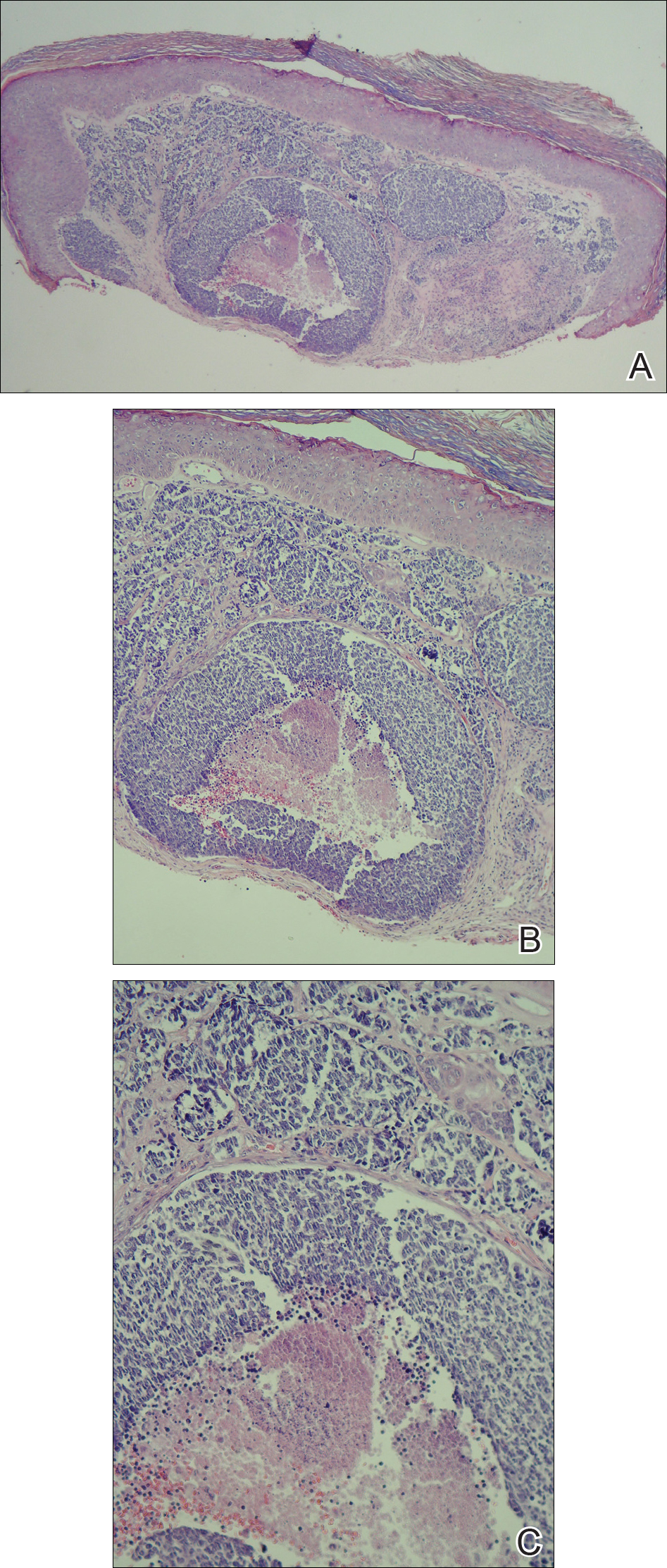
The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.

A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.

The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.

A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.

The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
Practice Points
- Merkel cell carcinoma (MCC) has remarkable metastatic potential.
- Initial evaluation of patients with MCC should include clinical examination to detect cutaneous, lymph node, and distant metastasis.
- Risk factors associated with the development of MCC include immunosuppression, older age, and UV-exposed fair skin.
Best practices address latest trends in PDT, skin cancer treatment
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
REPORTING FROM ODAC 2018
Study IDs predictors of nonmelanoma skin cancer in IBD
LAS VEGAS – , a large national analysis showed.
“Some studies have shown that patients with IBD may be at increased risk for nonmelanoma skin cancer (NMSC) because of the immunomodulators that they take for the management of their disease,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “But these are mostly small, single-center studies.” In an effort to determine the epidemiology of NMSC in patients hospitalized with IBD, Dr. Khan, associate chief resident in the internal medicine department at the University of Toledo (Ohio) Medical Center and his associates analyzed the National Inpatient Sample (NIS) database for all subjects who had a primary or secondary discharge diagnosis of IBD during 2002-2014. Next, they used ICD-9 codes to identify the rate of NMSC in this population.
Compared with IBD patients without NMSC, most of the IBD patients with NMSC were males (54% vs. 42%; P less than 0.001), covered by Medicare (65% vs. 37%), were white (96% vs. 81%; P less than .001), lived in the Midwest or Western United States (27% and 26% vs. 22% and 17%), were admitted to urban teaching hospitals (57% vs. 51%; P less than .001), were discharged to skilled nursing facilities (16% vs. 10%; P less than .001), required home health care (17% vs. 11%), and were admitted electively (27% vs. 20%). The researchers observed no significant difference in mortality among IBD patients with and without NMSC (1.61% vs. 1.53%; P = .22).
Multivariate analysis revealed that the following factors were predictive of NMSC in IBD: comorbid diagnosis of rheumatoid arthritis, collagen vasculature diseases, male sex, and white race. “Patients with those risk factors should be made more aware of their risk for developing NMSC,” Dr. Khan said. “They shouldn’t be taken lightly.”
Dr. Khan reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan Z et al. Crohn’s & Colitis Congress, Poster 209.
LAS VEGAS – , a large national analysis showed.
“Some studies have shown that patients with IBD may be at increased risk for nonmelanoma skin cancer (NMSC) because of the immunomodulators that they take for the management of their disease,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “But these are mostly small, single-center studies.” In an effort to determine the epidemiology of NMSC in patients hospitalized with IBD, Dr. Khan, associate chief resident in the internal medicine department at the University of Toledo (Ohio) Medical Center and his associates analyzed the National Inpatient Sample (NIS) database for all subjects who had a primary or secondary discharge diagnosis of IBD during 2002-2014. Next, they used ICD-9 codes to identify the rate of NMSC in this population.
Compared with IBD patients without NMSC, most of the IBD patients with NMSC were males (54% vs. 42%; P less than 0.001), covered by Medicare (65% vs. 37%), were white (96% vs. 81%; P less than .001), lived in the Midwest or Western United States (27% and 26% vs. 22% and 17%), were admitted to urban teaching hospitals (57% vs. 51%; P less than .001), were discharged to skilled nursing facilities (16% vs. 10%; P less than .001), required home health care (17% vs. 11%), and were admitted electively (27% vs. 20%). The researchers observed no significant difference in mortality among IBD patients with and without NMSC (1.61% vs. 1.53%; P = .22).
Multivariate analysis revealed that the following factors were predictive of NMSC in IBD: comorbid diagnosis of rheumatoid arthritis, collagen vasculature diseases, male sex, and white race. “Patients with those risk factors should be made more aware of their risk for developing NMSC,” Dr. Khan said. “They shouldn’t be taken lightly.”
Dr. Khan reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan Z et al. Crohn’s & Colitis Congress, Poster 209.
LAS VEGAS – , a large national analysis showed.
“Some studies have shown that patients with IBD may be at increased risk for nonmelanoma skin cancer (NMSC) because of the immunomodulators that they take for the management of their disease,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “But these are mostly small, single-center studies.” In an effort to determine the epidemiology of NMSC in patients hospitalized with IBD, Dr. Khan, associate chief resident in the internal medicine department at the University of Toledo (Ohio) Medical Center and his associates analyzed the National Inpatient Sample (NIS) database for all subjects who had a primary or secondary discharge diagnosis of IBD during 2002-2014. Next, they used ICD-9 codes to identify the rate of NMSC in this population.
Compared with IBD patients without NMSC, most of the IBD patients with NMSC were males (54% vs. 42%; P less than 0.001), covered by Medicare (65% vs. 37%), were white (96% vs. 81%; P less than .001), lived in the Midwest or Western United States (27% and 26% vs. 22% and 17%), were admitted to urban teaching hospitals (57% vs. 51%; P less than .001), were discharged to skilled nursing facilities (16% vs. 10%; P less than .001), required home health care (17% vs. 11%), and were admitted electively (27% vs. 20%). The researchers observed no significant difference in mortality among IBD patients with and without NMSC (1.61% vs. 1.53%; P = .22).
Multivariate analysis revealed that the following factors were predictive of NMSC in IBD: comorbid diagnosis of rheumatoid arthritis, collagen vasculature diseases, male sex, and white race. “Patients with those risk factors should be made more aware of their risk for developing NMSC,” Dr. Khan said. “They shouldn’t be taken lightly.”
Dr. Khan reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan Z et al. Crohn’s & Colitis Congress, Poster 209.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Key clinical point: Many factors predict which IBD patients are at risk for developing nonmelanoma skin cancer.
Major finding: Compared with IBD patients without NMSC, most of the IBD patients with NMSC were males (54% vs. 42%; P less than .001) and white (96% vs. 81%; P less than .001).
Study details: An analysis of 22,620 patients who had a primary or secondary discharge diagnosis of IBD during 2002-2014.
Disclosures: Dr. Khan reported having no financial disclosures.
Source: Khan Z et al. Crohn’s & Colitis Congress, Poster 209.