User login
Hidradenitis suppurativa packs mighty QOL impact
KAUAI, HAWAII – Anyone who has treated patients with hidradenitis suppurativa (HS) recognizes that this can be a debilitating disease. Helping put that into fuller perspective, recent evidence has shown that the quality of life effects of moderate to severe HS are objectively worse than those of moderate to severe psoriasis, according to Iltefat H. Hamzavi, MD, president of the Hidradenitis Suppurativa Foundation and a dermatologist at Henry Ford Hospital in Detroit.
He was lead author of a study in which he and his coinvestigators compared weighted averages of a variety of quality of life measures in patients with moderate to severe HS or psoriasis who participated in five AbbVie-sponsored randomized clinical trials of biologic agents (J Am Acad Dermatol. 2017 Dec;77[6]:1038-46).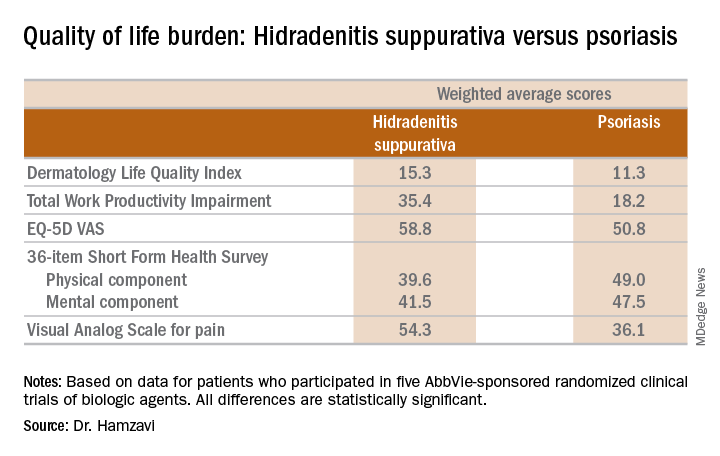
“The number of HS patients who experience downward drift – losing their job and their health insurance and ultimately being unable to move out of a lower socioeconomic group – is staggering,” the dermatologist said.
which underscores the importance of a psychiatric evaluation as part of routine care for patients with this dermatologic disease. “Suicide is much more common in the HS population than in almost any other dermatologic disease,” Dr. Hamzavi added.
He reported serving as a consultant for AbbVie, Incyte, and UCB.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Anyone who has treated patients with hidradenitis suppurativa (HS) recognizes that this can be a debilitating disease. Helping put that into fuller perspective, recent evidence has shown that the quality of life effects of moderate to severe HS are objectively worse than those of moderate to severe psoriasis, according to Iltefat H. Hamzavi, MD, president of the Hidradenitis Suppurativa Foundation and a dermatologist at Henry Ford Hospital in Detroit.
He was lead author of a study in which he and his coinvestigators compared weighted averages of a variety of quality of life measures in patients with moderate to severe HS or psoriasis who participated in five AbbVie-sponsored randomized clinical trials of biologic agents (J Am Acad Dermatol. 2017 Dec;77[6]:1038-46).
“The number of HS patients who experience downward drift – losing their job and their health insurance and ultimately being unable to move out of a lower socioeconomic group – is staggering,” the dermatologist said.
which underscores the importance of a psychiatric evaluation as part of routine care for patients with this dermatologic disease. “Suicide is much more common in the HS population than in almost any other dermatologic disease,” Dr. Hamzavi added.
He reported serving as a consultant for AbbVie, Incyte, and UCB.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Anyone who has treated patients with hidradenitis suppurativa (HS) recognizes that this can be a debilitating disease. Helping put that into fuller perspective, recent evidence has shown that the quality of life effects of moderate to severe HS are objectively worse than those of moderate to severe psoriasis, according to Iltefat H. Hamzavi, MD, president of the Hidradenitis Suppurativa Foundation and a dermatologist at Henry Ford Hospital in Detroit.
He was lead author of a study in which he and his coinvestigators compared weighted averages of a variety of quality of life measures in patients with moderate to severe HS or psoriasis who participated in five AbbVie-sponsored randomized clinical trials of biologic agents (J Am Acad Dermatol. 2017 Dec;77[6]:1038-46).
“The number of HS patients who experience downward drift – losing their job and their health insurance and ultimately being unable to move out of a lower socioeconomic group – is staggering,” the dermatologist said.
which underscores the importance of a psychiatric evaluation as part of routine care for patients with this dermatologic disease. “Suicide is much more common in the HS population than in almost any other dermatologic disease,” Dr. Hamzavi added.
He reported serving as a consultant for AbbVie, Incyte, and UCB.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Psoriasis duration reflects cardiovascular event risk
KAUAI, HAWAII – The recent report that the risk of a major adverse cardiovascular event increases by 1% more than in the general population for each additional year of psoriasis duration is sobering news for physicians who treat pediatric psoriasis.
“If I have a 16-year-old who has a 5-year history of psoriasis, what does that mean for when she’s 30 or 40? And should we be intervening more aggressively?” Lawrence F. Eichenfield, MD, asked at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even though there’s not a great deal of evidence, there’s some evidence to rationalize early screening in psoriasis,” according to Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
Psoriasis develops during childhood in almost one-third of patients.
The pediatric psoriasis screening guidelines describe a simple routine screening program and timeline for early identification of overweight or obesity, type 2 diabetes, hypertension, nonalcoholic fatty liver disease, anxiety, depression, substance abuse, inflammatory bowel disease, and quality of life issues, all of which are encountered with increased frequency in pediatric psoriasis patients. A fasting lipid panel is recommended in children aged 9-11 years with psoriasis and again at age 17-21 years.
“Don’t forget arthritis. For a kid with psoriasis, at every office visit, I ask about morning stiffness or limp. Those are probably the two most sensitive questions in screening for psoriatic arthritis,” according to Dr. Eichenfield.
It has been clear for some time that the skin is not the only organ affected by psoriatic inflammation. The study that quantified the relationship between psoriasis duration and cardiovascular risk – a 1% increase for each year of psoriasis – was a collaboration between investigators at the University of Copenhagen and the University of Pennsylvania, Philadelphia.
The two-part project included aortal imaging of 190 psoriasis patients using fludeoxyglucose F 18 PET/CT scan, which showed a strong relationship between duration of psoriasis and the degree of vascular inflammation. This was bolstered by a population-based study using Danish national registry data on 87,161 psoriasis patients and 4.2 million controls from the general Danish population (J Am Acad Dermatol. 2017 Oct;77[4]:650-56.e3).
Dr. Eichenfield reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The recent report that the risk of a major adverse cardiovascular event increases by 1% more than in the general population for each additional year of psoriasis duration is sobering news for physicians who treat pediatric psoriasis.
“If I have a 16-year-old who has a 5-year history of psoriasis, what does that mean for when she’s 30 or 40? And should we be intervening more aggressively?” Lawrence F. Eichenfield, MD, asked at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even though there’s not a great deal of evidence, there’s some evidence to rationalize early screening in psoriasis,” according to Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
Psoriasis develops during childhood in almost one-third of patients.
The pediatric psoriasis screening guidelines describe a simple routine screening program and timeline for early identification of overweight or obesity, type 2 diabetes, hypertension, nonalcoholic fatty liver disease, anxiety, depression, substance abuse, inflammatory bowel disease, and quality of life issues, all of which are encountered with increased frequency in pediatric psoriasis patients. A fasting lipid panel is recommended in children aged 9-11 years with psoriasis and again at age 17-21 years.
“Don’t forget arthritis. For a kid with psoriasis, at every office visit, I ask about morning stiffness or limp. Those are probably the two most sensitive questions in screening for psoriatic arthritis,” according to Dr. Eichenfield.
It has been clear for some time that the skin is not the only organ affected by psoriatic inflammation. The study that quantified the relationship between psoriasis duration and cardiovascular risk – a 1% increase for each year of psoriasis – was a collaboration between investigators at the University of Copenhagen and the University of Pennsylvania, Philadelphia.
The two-part project included aortal imaging of 190 psoriasis patients using fludeoxyglucose F 18 PET/CT scan, which showed a strong relationship between duration of psoriasis and the degree of vascular inflammation. This was bolstered by a population-based study using Danish national registry data on 87,161 psoriasis patients and 4.2 million controls from the general Danish population (J Am Acad Dermatol. 2017 Oct;77[4]:650-56.e3).
Dr. Eichenfield reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The recent report that the risk of a major adverse cardiovascular event increases by 1% more than in the general population for each additional year of psoriasis duration is sobering news for physicians who treat pediatric psoriasis.
“If I have a 16-year-old who has a 5-year history of psoriasis, what does that mean for when she’s 30 or 40? And should we be intervening more aggressively?” Lawrence F. Eichenfield, MD, asked at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even though there’s not a great deal of evidence, there’s some evidence to rationalize early screening in psoriasis,” according to Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego.
Psoriasis develops during childhood in almost one-third of patients.
The pediatric psoriasis screening guidelines describe a simple routine screening program and timeline for early identification of overweight or obesity, type 2 diabetes, hypertension, nonalcoholic fatty liver disease, anxiety, depression, substance abuse, inflammatory bowel disease, and quality of life issues, all of which are encountered with increased frequency in pediatric psoriasis patients. A fasting lipid panel is recommended in children aged 9-11 years with psoriasis and again at age 17-21 years.
“Don’t forget arthritis. For a kid with psoriasis, at every office visit, I ask about morning stiffness or limp. Those are probably the two most sensitive questions in screening for psoriatic arthritis,” according to Dr. Eichenfield.
It has been clear for some time that the skin is not the only organ affected by psoriatic inflammation. The study that quantified the relationship between psoriasis duration and cardiovascular risk – a 1% increase for each year of psoriasis – was a collaboration between investigators at the University of Copenhagen and the University of Pennsylvania, Philadelphia.
The two-part project included aortal imaging of 190 psoriasis patients using fludeoxyglucose F 18 PET/CT scan, which showed a strong relationship between duration of psoriasis and the degree of vascular inflammation. This was bolstered by a population-based study using Danish national registry data on 87,161 psoriasis patients and 4.2 million controls from the general Danish population (J Am Acad Dermatol. 2017 Oct;77[4]:650-56.e3).
Dr. Eichenfield reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Is PASI 100 the new benchmark in psoriasis?
KAUAI, HAWAII – I think we should just do away with PASI 90 [90% improvement in Psoriasis Area and Severity Index score] and look at how well our drugs do against the metric of PASI 100. The whole ball of wax. Let’s just go for complete clearance,” Craig L. Leonardi, MD, declared in a provocative presentation at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
He advocates using number needed to treat (NNT) as a performance yardstick. He finds it helpful in translating sometimes-arcane clinical trial results into useful information to guide everyday practice. The NNT is the average number of patients who need to be treated with a drug or procedure in order to achieve one additional good outcome, compared with a control intervention or placebo. It’s the inverse of the absolute risk reduction. The lower the NNT, the better an intervention is performing.
He presented a chart that summarized the NNTs to achieve a PASI 100 response for various systemic agents commonly used in treating moderate to severe psoriasis. He obtained the data from Food and Drug Administration–regulated product labeling and phase 3 clinical trials.
Dr. Leonardi drew attention to the worst performers on the list: methotrexate, with an NNT of 25 to achieve a PASI 100 response, and etanercept, with an NNT of 23.3.
“Methotrexate is a drug that the insurance industry says we have to flow through on our way to biologic drugs. But if complete clearance is your goal, this is an exercise in futility. These patients will never, ever get to complete clearance – or it’s at least very unlikely. We shouldn’t be asked to go through methotrexate on our way to anything. We shouldn’t be asked to use methotrexate at all. We should be bypassing it. And some of us are working on this,” he said.
Ustekinumab and adalimumab are the current market leaders in biologic therapy for psoriasis, but they don’t stack up so well when viewed through the filter of PASI 100 response, with NNTs of 9.2 and 5.3, respectively.
“These market leaders may not be the most relevant drugs in the current era,” according to the dermatologist.
In contrast, the high-performance biologics – the interleukin-17 inhibitors secukinumab, ixekizumab, and brodalumab and the interleukin-23 antagonist guselkumab – have impressively low NNTs of 2.4-3.6 in order to achieve complete clearance.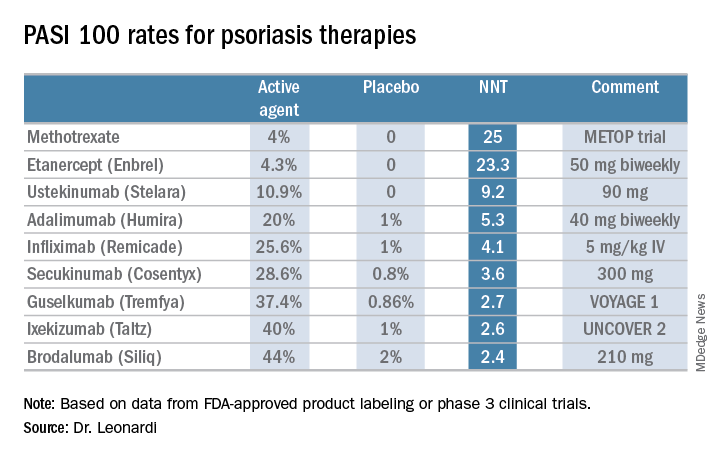
“But our IL-17 and IL-23 antagonists are markedly different from all other therapies, with NNTs of 1.3-1.1. With an NNT of 1.1, if you treated 11 patients with ixekizumab, 10 of them would achieve a PASI 75,” he explained.
“This is really quite remarkable,” Dr. Leonardi commented. “Our first drug back in 2002 was alefacept, and that drug was a ‘twenty-one percenter’: 21% of patients achieved a PASI 75. And quite frankly, we thought that was rocking voodoo science back in the day. Well, we’re really out there now. This is utterly amazing data: a PASI 75 of 81.6% for secukinumab, 86% for brodalumab, 90% for ixekizumab, and 91.2% for guselkumab. This is why we’re publishing this stuff in the best medical journals, because these results are absolutely amazing. So many different medical specialties are interested in what we’re doing with these drugs.”
He reported serving as a consultant to AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly, Janssen, Leo, Pfizer, Sandoz, and UCB and receiving research funding from 21 pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – I think we should just do away with PASI 90 [90% improvement in Psoriasis Area and Severity Index score] and look at how well our drugs do against the metric of PASI 100. The whole ball of wax. Let’s just go for complete clearance,” Craig L. Leonardi, MD, declared in a provocative presentation at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
He advocates using number needed to treat (NNT) as a performance yardstick. He finds it helpful in translating sometimes-arcane clinical trial results into useful information to guide everyday practice. The NNT is the average number of patients who need to be treated with a drug or procedure in order to achieve one additional good outcome, compared with a control intervention or placebo. It’s the inverse of the absolute risk reduction. The lower the NNT, the better an intervention is performing.
He presented a chart that summarized the NNTs to achieve a PASI 100 response for various systemic agents commonly used in treating moderate to severe psoriasis. He obtained the data from Food and Drug Administration–regulated product labeling and phase 3 clinical trials.
Dr. Leonardi drew attention to the worst performers on the list: methotrexate, with an NNT of 25 to achieve a PASI 100 response, and etanercept, with an NNT of 23.3.
“Methotrexate is a drug that the insurance industry says we have to flow through on our way to biologic drugs. But if complete clearance is your goal, this is an exercise in futility. These patients will never, ever get to complete clearance – or it’s at least very unlikely. We shouldn’t be asked to go through methotrexate on our way to anything. We shouldn’t be asked to use methotrexate at all. We should be bypassing it. And some of us are working on this,” he said.
Ustekinumab and adalimumab are the current market leaders in biologic therapy for psoriasis, but they don’t stack up so well when viewed through the filter of PASI 100 response, with NNTs of 9.2 and 5.3, respectively.
“These market leaders may not be the most relevant drugs in the current era,” according to the dermatologist.
In contrast, the high-performance biologics – the interleukin-17 inhibitors secukinumab, ixekizumab, and brodalumab and the interleukin-23 antagonist guselkumab – have impressively low NNTs of 2.4-3.6 in order to achieve complete clearance.
“But our IL-17 and IL-23 antagonists are markedly different from all other therapies, with NNTs of 1.3-1.1. With an NNT of 1.1, if you treated 11 patients with ixekizumab, 10 of them would achieve a PASI 75,” he explained.
“This is really quite remarkable,” Dr. Leonardi commented. “Our first drug back in 2002 was alefacept, and that drug was a ‘twenty-one percenter’: 21% of patients achieved a PASI 75. And quite frankly, we thought that was rocking voodoo science back in the day. Well, we’re really out there now. This is utterly amazing data: a PASI 75 of 81.6% for secukinumab, 86% for brodalumab, 90% for ixekizumab, and 91.2% for guselkumab. This is why we’re publishing this stuff in the best medical journals, because these results are absolutely amazing. So many different medical specialties are interested in what we’re doing with these drugs.”
He reported serving as a consultant to AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly, Janssen, Leo, Pfizer, Sandoz, and UCB and receiving research funding from 21 pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – I think we should just do away with PASI 90 [90% improvement in Psoriasis Area and Severity Index score] and look at how well our drugs do against the metric of PASI 100. The whole ball of wax. Let’s just go for complete clearance,” Craig L. Leonardi, MD, declared in a provocative presentation at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
He advocates using number needed to treat (NNT) as a performance yardstick. He finds it helpful in translating sometimes-arcane clinical trial results into useful information to guide everyday practice. The NNT is the average number of patients who need to be treated with a drug or procedure in order to achieve one additional good outcome, compared with a control intervention or placebo. It’s the inverse of the absolute risk reduction. The lower the NNT, the better an intervention is performing.
He presented a chart that summarized the NNTs to achieve a PASI 100 response for various systemic agents commonly used in treating moderate to severe psoriasis. He obtained the data from Food and Drug Administration–regulated product labeling and phase 3 clinical trials.
Dr. Leonardi drew attention to the worst performers on the list: methotrexate, with an NNT of 25 to achieve a PASI 100 response, and etanercept, with an NNT of 23.3.
“Methotrexate is a drug that the insurance industry says we have to flow through on our way to biologic drugs. But if complete clearance is your goal, this is an exercise in futility. These patients will never, ever get to complete clearance – or it’s at least very unlikely. We shouldn’t be asked to go through methotrexate on our way to anything. We shouldn’t be asked to use methotrexate at all. We should be bypassing it. And some of us are working on this,” he said.
Ustekinumab and adalimumab are the current market leaders in biologic therapy for psoriasis, but they don’t stack up so well when viewed through the filter of PASI 100 response, with NNTs of 9.2 and 5.3, respectively.
“These market leaders may not be the most relevant drugs in the current era,” according to the dermatologist.
In contrast, the high-performance biologics – the interleukin-17 inhibitors secukinumab, ixekizumab, and brodalumab and the interleukin-23 antagonist guselkumab – have impressively low NNTs of 2.4-3.6 in order to achieve complete clearance.
“But our IL-17 and IL-23 antagonists are markedly different from all other therapies, with NNTs of 1.3-1.1. With an NNT of 1.1, if you treated 11 patients with ixekizumab, 10 of them would achieve a PASI 75,” he explained.
“This is really quite remarkable,” Dr. Leonardi commented. “Our first drug back in 2002 was alefacept, and that drug was a ‘twenty-one percenter’: 21% of patients achieved a PASI 75. And quite frankly, we thought that was rocking voodoo science back in the day. Well, we’re really out there now. This is utterly amazing data: a PASI 75 of 81.6% for secukinumab, 86% for brodalumab, 90% for ixekizumab, and 91.2% for guselkumab. This is why we’re publishing this stuff in the best medical journals, because these results are absolutely amazing. So many different medical specialties are interested in what we’re doing with these drugs.”
He reported serving as a consultant to AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly, Janssen, Leo, Pfizer, Sandoz, and UCB and receiving research funding from 21 pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Keloids: Cutting is never enough
KAUAI, HAWAII – The truth about keloids is that most affected patients are not surgical candidates and thus, need to be convinced to pursue nonsurgical options, according to Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in Morristown, N.J.
“Virtually all patients arrive saying, ‘I want this cut off. I want this gone today, or even better, yesterday,’ ” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Removal without adjunctive therapy is a guaranteed failure – about 100% of the time in my experience. ‘I don’t want injections’ is not the answer. They always say they don’t want injections, but regardless of what else I do, they’re going to get shots,” stressed Dr. Baldwin, who is also a dermatologist at the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
With earlobe keloid surgery alone, the recurrence rate is less than 50%. With surgery, followed by a program of corticosteroid injections, the recurrence rate plummets to 1%-3%. And, with surgery followed by adjunctive radiotherapy, the rate is close to zero.
In contrast, keloid surgery at sites other than the earlobe has roughly a 50% recurrence rate if followed up with corticosteroid injections and 20% with radiotherapy. Patients need to understand this upfront. They also need to be told that, while treatment can improve appearance, the site will never look normal.
Pedunculated lesions are quite amenable to surgery. They are often mushroom shaped, with a narrow base that doesn’t contain keloidal tissue. “Pedunculated lesions are the maximum benefit with least risk scenario,” Dr. Baldwin commented.
Mature brownish keloids are less likely to recur than younger red ones. “There are no data for that, just my experience,” she continued. Keloids on the jaw, upper back, mid-chest, and deltoid are the ones most likely to recur.
During her presentation, Dr. Baldwin provided the following points about different treatments:
- Postsurgical adjunctive therapy. The options include corticosteroid injections, injectable interferon, and pressure dressings. Which to chose? Urge patients to opt for all of them. “Go for the whole kit and caboodle. There’s no reason to stop at just one. I can tell you that if you do all of these things on an earlobe keloid, no matter how big it is, that sucker’s not coming back. On the body, sometimes yes, sometimes no. That’s a much harder area to treat,” Dr. Baldwin said.
- Corticosteroids. Her personal recipe is 40 mg/cc of triamcinolone injected to the base and walls of the excision site immediately postsurgically and again every 2 weeks for 2 months, regardless of the site’s clinical appearance. “If they come in absolutely dead flat, I don’t care. I’m injecting it anyway. I have no data behind this, but I can tell you that when I do it that way, the chances of recurrence are much less,” she noted. After the first 2 months of steroid injections, she switches to a once-monthly schedule for another 6 months, with the dose and concentration selected based upon appearance.
- Radiation therapy. “Many of you don’t use it and many patients refuse it, but if you’ve got a patient who’ll do it, it’s by far the single best adjunctive therapy for the treatment of keloids,” Dr. Baldwin said. This is ineffective on existing keloids, and she advises to cut first, then irradiate the base. All forms of radiotherapy are effective, she added, so she leaves the details to the radiotherapist. “I say, ‘Here’s the patient, do your thing.’ Most of them do it immediately postop, others do it a day or 2 or a week later,” she said.
- Interferon. The regimen is 1.5 million U/linear cm injected into the base and walls of the excision site on days 1 and 7. The maximum is 5 million U/session in order to minimize the interferon-induced flulike syndrome. Pretreatment with 1 g of acetaminophen before the interferon injections and every 4 hours for 24 hours posttreatment is also helpful in preventing the flulike symptoms, which can sometimes be formidable. “I tell patients to be able to take the next day off from work if they have to and also not to have any obligations that night for child or elderly care,” Dr. Baldwin noted.
- Pressure dressings. For these to be effective, they have to exert considerable pressure, and they must be worn 24/7 for 4-6 months. The only location in which pressure dressings make sense is on the earlobes. Dr. Baldwin mentioned two producers: Delasco makes silicone pressure earrings, and NBN Products makes a variety of the devices.
- The nonsurgical alternatives. For the many patients who aren’t good surgical candidates, the workhorse therapy for existing keloids is intralesional triamcinolone at 40 mg/cc. This works best for sessile lesions. Dr. Baldwin doesn’t hesitate to give 50-100 mg/treatment session. “Ask, ‘Where do you want to start?’ We do a tiny area with a huge amount of triamcinolone and then that spot will get better. If you spread it around over a large area, nothing’s going get better,” she advised. 5-fluorouracil is a less attractive alternative. It’s painful, less effective, and it can cause ulceration.
“I’ve found [5-fluorouracil] to be of limited use. I think corticosteroid is the heavy lifter. I’d prefer not to be injecting an antimetabolite into somebody if I can help it,” Dr. Baldwin said.
She reported receiving research funding from Dermira, Galderma, La Roche-Posay, Novan, and Valeant; and serving as a consultant and/or on a speakers’ bureau for Allergan, Bayer, Encore, Johnson & Johnson, Mayne, and Sun.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The truth about keloids is that most affected patients are not surgical candidates and thus, need to be convinced to pursue nonsurgical options, according to Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in Morristown, N.J.
“Virtually all patients arrive saying, ‘I want this cut off. I want this gone today, or even better, yesterday,’ ” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Removal without adjunctive therapy is a guaranteed failure – about 100% of the time in my experience. ‘I don’t want injections’ is not the answer. They always say they don’t want injections, but regardless of what else I do, they’re going to get shots,” stressed Dr. Baldwin, who is also a dermatologist at the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
With earlobe keloid surgery alone, the recurrence rate is less than 50%. With surgery, followed by a program of corticosteroid injections, the recurrence rate plummets to 1%-3%. And, with surgery followed by adjunctive radiotherapy, the rate is close to zero.
In contrast, keloid surgery at sites other than the earlobe has roughly a 50% recurrence rate if followed up with corticosteroid injections and 20% with radiotherapy. Patients need to understand this upfront. They also need to be told that, while treatment can improve appearance, the site will never look normal.
Pedunculated lesions are quite amenable to surgery. They are often mushroom shaped, with a narrow base that doesn’t contain keloidal tissue. “Pedunculated lesions are the maximum benefit with least risk scenario,” Dr. Baldwin commented.
Mature brownish keloids are less likely to recur than younger red ones. “There are no data for that, just my experience,” she continued. Keloids on the jaw, upper back, mid-chest, and deltoid are the ones most likely to recur.
During her presentation, Dr. Baldwin provided the following points about different treatments:
- Postsurgical adjunctive therapy. The options include corticosteroid injections, injectable interferon, and pressure dressings. Which to chose? Urge patients to opt for all of them. “Go for the whole kit and caboodle. There’s no reason to stop at just one. I can tell you that if you do all of these things on an earlobe keloid, no matter how big it is, that sucker’s not coming back. On the body, sometimes yes, sometimes no. That’s a much harder area to treat,” Dr. Baldwin said.
- Corticosteroids. Her personal recipe is 40 mg/cc of triamcinolone injected to the base and walls of the excision site immediately postsurgically and again every 2 weeks for 2 months, regardless of the site’s clinical appearance. “If they come in absolutely dead flat, I don’t care. I’m injecting it anyway. I have no data behind this, but I can tell you that when I do it that way, the chances of recurrence are much less,” she noted. After the first 2 months of steroid injections, she switches to a once-monthly schedule for another 6 months, with the dose and concentration selected based upon appearance.
- Radiation therapy. “Many of you don’t use it and many patients refuse it, but if you’ve got a patient who’ll do it, it’s by far the single best adjunctive therapy for the treatment of keloids,” Dr. Baldwin said. This is ineffective on existing keloids, and she advises to cut first, then irradiate the base. All forms of radiotherapy are effective, she added, so she leaves the details to the radiotherapist. “I say, ‘Here’s the patient, do your thing.’ Most of them do it immediately postop, others do it a day or 2 or a week later,” she said.
- Interferon. The regimen is 1.5 million U/linear cm injected into the base and walls of the excision site on days 1 and 7. The maximum is 5 million U/session in order to minimize the interferon-induced flulike syndrome. Pretreatment with 1 g of acetaminophen before the interferon injections and every 4 hours for 24 hours posttreatment is also helpful in preventing the flulike symptoms, which can sometimes be formidable. “I tell patients to be able to take the next day off from work if they have to and also not to have any obligations that night for child or elderly care,” Dr. Baldwin noted.
- Pressure dressings. For these to be effective, they have to exert considerable pressure, and they must be worn 24/7 for 4-6 months. The only location in which pressure dressings make sense is on the earlobes. Dr. Baldwin mentioned two producers: Delasco makes silicone pressure earrings, and NBN Products makes a variety of the devices.
- The nonsurgical alternatives. For the many patients who aren’t good surgical candidates, the workhorse therapy for existing keloids is intralesional triamcinolone at 40 mg/cc. This works best for sessile lesions. Dr. Baldwin doesn’t hesitate to give 50-100 mg/treatment session. “Ask, ‘Where do you want to start?’ We do a tiny area with a huge amount of triamcinolone and then that spot will get better. If you spread it around over a large area, nothing’s going get better,” she advised. 5-fluorouracil is a less attractive alternative. It’s painful, less effective, and it can cause ulceration.
“I’ve found [5-fluorouracil] to be of limited use. I think corticosteroid is the heavy lifter. I’d prefer not to be injecting an antimetabolite into somebody if I can help it,” Dr. Baldwin said.
She reported receiving research funding from Dermira, Galderma, La Roche-Posay, Novan, and Valeant; and serving as a consultant and/or on a speakers’ bureau for Allergan, Bayer, Encore, Johnson & Johnson, Mayne, and Sun.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – The truth about keloids is that most affected patients are not surgical candidates and thus, need to be convinced to pursue nonsurgical options, according to Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in Morristown, N.J.
“Virtually all patients arrive saying, ‘I want this cut off. I want this gone today, or even better, yesterday,’ ” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Removal without adjunctive therapy is a guaranteed failure – about 100% of the time in my experience. ‘I don’t want injections’ is not the answer. They always say they don’t want injections, but regardless of what else I do, they’re going to get shots,” stressed Dr. Baldwin, who is also a dermatologist at the Rutgers Robert Wood Johnson Medical School in New Brunswick, N.J.
With earlobe keloid surgery alone, the recurrence rate is less than 50%. With surgery, followed by a program of corticosteroid injections, the recurrence rate plummets to 1%-3%. And, with surgery followed by adjunctive radiotherapy, the rate is close to zero.
In contrast, keloid surgery at sites other than the earlobe has roughly a 50% recurrence rate if followed up with corticosteroid injections and 20% with radiotherapy. Patients need to understand this upfront. They also need to be told that, while treatment can improve appearance, the site will never look normal.
Pedunculated lesions are quite amenable to surgery. They are often mushroom shaped, with a narrow base that doesn’t contain keloidal tissue. “Pedunculated lesions are the maximum benefit with least risk scenario,” Dr. Baldwin commented.
Mature brownish keloids are less likely to recur than younger red ones. “There are no data for that, just my experience,” she continued. Keloids on the jaw, upper back, mid-chest, and deltoid are the ones most likely to recur.
During her presentation, Dr. Baldwin provided the following points about different treatments:
- Postsurgical adjunctive therapy. The options include corticosteroid injections, injectable interferon, and pressure dressings. Which to chose? Urge patients to opt for all of them. “Go for the whole kit and caboodle. There’s no reason to stop at just one. I can tell you that if you do all of these things on an earlobe keloid, no matter how big it is, that sucker’s not coming back. On the body, sometimes yes, sometimes no. That’s a much harder area to treat,” Dr. Baldwin said.
- Corticosteroids. Her personal recipe is 40 mg/cc of triamcinolone injected to the base and walls of the excision site immediately postsurgically and again every 2 weeks for 2 months, regardless of the site’s clinical appearance. “If they come in absolutely dead flat, I don’t care. I’m injecting it anyway. I have no data behind this, but I can tell you that when I do it that way, the chances of recurrence are much less,” she noted. After the first 2 months of steroid injections, she switches to a once-monthly schedule for another 6 months, with the dose and concentration selected based upon appearance.
- Radiation therapy. “Many of you don’t use it and many patients refuse it, but if you’ve got a patient who’ll do it, it’s by far the single best adjunctive therapy for the treatment of keloids,” Dr. Baldwin said. This is ineffective on existing keloids, and she advises to cut first, then irradiate the base. All forms of radiotherapy are effective, she added, so she leaves the details to the radiotherapist. “I say, ‘Here’s the patient, do your thing.’ Most of them do it immediately postop, others do it a day or 2 or a week later,” she said.
- Interferon. The regimen is 1.5 million U/linear cm injected into the base and walls of the excision site on days 1 and 7. The maximum is 5 million U/session in order to minimize the interferon-induced flulike syndrome. Pretreatment with 1 g of acetaminophen before the interferon injections and every 4 hours for 24 hours posttreatment is also helpful in preventing the flulike symptoms, which can sometimes be formidable. “I tell patients to be able to take the next day off from work if they have to and also not to have any obligations that night for child or elderly care,” Dr. Baldwin noted.
- Pressure dressings. For these to be effective, they have to exert considerable pressure, and they must be worn 24/7 for 4-6 months. The only location in which pressure dressings make sense is on the earlobes. Dr. Baldwin mentioned two producers: Delasco makes silicone pressure earrings, and NBN Products makes a variety of the devices.
- The nonsurgical alternatives. For the many patients who aren’t good surgical candidates, the workhorse therapy for existing keloids is intralesional triamcinolone at 40 mg/cc. This works best for sessile lesions. Dr. Baldwin doesn’t hesitate to give 50-100 mg/treatment session. “Ask, ‘Where do you want to start?’ We do a tiny area with a huge amount of triamcinolone and then that spot will get better. If you spread it around over a large area, nothing’s going get better,” she advised. 5-fluorouracil is a less attractive alternative. It’s painful, less effective, and it can cause ulceration.
“I’ve found [5-fluorouracil] to be of limited use. I think corticosteroid is the heavy lifter. I’d prefer not to be injecting an antimetabolite into somebody if I can help it,” Dr. Baldwin said.
She reported receiving research funding from Dermira, Galderma, La Roche-Posay, Novan, and Valeant; and serving as a consultant and/or on a speakers’ bureau for Allergan, Bayer, Encore, Johnson & Johnson, Mayne, and Sun.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
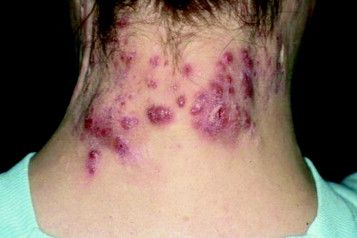
Surgical excision essential in severe hidradenitis suppurativa
KAUAI, HAWAII – Medical therapy alone is never sufficient in Hurley stage III hidradenitis suppurativa (HS), Iltefat H. Hamzavi, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even with the advances in biologics and antibiotic therapy, you still have to excise once you’re in full-blown Hurley stage III disease. Surgery has to be part of your protocol,” according to Dr. Hamzavi, a dermatologist at Henry Ford Hospital in Detroit, which runs one of the nation’s largest hidradenitis suppurativa clinics, with roughly 1,600 patients.
“Of course we’re biased. But until the data can set us free, you’re stuck with me,” the dermatologist quipped.
A core principle of the Henry Ford algorithm is this: “Medical therapy [for patients with advanced HS] stabilizes them and reduces their draining and pain, then you try to bring them back to a lower stage with surgical options,” he explained.
Although other HS staging systems exist, Dr. Hamzavi and his colleagues rely on the Hurley staging system to guide their treatment. Basically, Hurley stage I consists of follicular nodules and abscesses. When the nodules connect to form sinus tracts with scarring, that’s stage II. And if the sinus tracts interconnect throughout an entire area, that’s stage III.
Hurley stage I
First-line treatment of localized Hurley stage I disease at Henry Ford is a 10% topical benzoyl peroxide wash left on for 5 minutes before bathing, followed by postbathing topical clindamycin 1% lotion or solution applied to the nodules. If this maintenance regimen isn’t sufficient to prevent formation of new and worsening nodules, Dr. Hamzavi supplements it with up to three once-monthly 1064-nm Nd:YAG laser sessions aimed at follicular ablation. It’s a laser application he and his colleagues pioneered (Dermatol Surg. 2009 Aug;35[8]:1188-98). They subsequently documented the histopathologic basis of the procedure’s efficacy, which entails selective thermolysis of follicles, destruction of inflammatory lesions in the superficial to mid-dermis, followed by fibrosis and scarring (Arch Dermatol. 2011 Jan;147[1]:21-8).
In generalized Hurley stage I HS, the Henry Ford approach is to supplement the topical regimen and laser sessions with oral doxycycline at 100-150 mg daily for 1-6 months.
“The theory here is this is a dysbiotic event. The antibiotics reduce commensal bacteria, which ultimately reduces the reactive inflammatory response. But when you stop the antibiotics, the inflammatory response returns. So antibiotics can help stabilize the disease state but really can’t reverse the disease state. For that we have to turn to ablative treatment options: laser, surgery,” the dermatologist continued.
Hurley stage II
“At this point you’re looking at procedures,” according to Dr. Hamzavi. “Once you have sinus tracts it’s critical to remove them.”
The treatment backbone in stage II disease is 8-10 weeks of oral clindamycin and rifampin, both at 300 mg twice daily.
“This is one of the fundamental building blocks of HS clinics throughout the world,” he noted.
Clostridium difficile infection is exceedingly rare in HS patients on this regimen, for reasons still unclear.
If this dual-antibiotic regimen doesn’t dramatically reduce drainage and pain, he adds levofloxacin at 500 mg twice daily for up to 2 weeks in an effort to calm down unstable, decompensating disease.
Dapsone at 50-150 mg/day for up to 12 weeks is an additional option. It’s most useful in patients with nodules that are disproportionately painful, in Dr. Hamzavi’s experience.
Deroofing is a simple procedure that should be considered for all sinus tracts. It entails numbing the area with a ring block then introducing a curette or surgical probe into the sinus tract to open it up and get rid of the gelatinous material within. Dutch investigators have detailed the technique (J Am Acad Dermatol. 2010 Sep;63[3]:475-80).
Tumor necrosis factor–inhibitor therapy has been a major advance in Hurley stage II and III disease. “It doesn’t work in everybody, but a lot of patients can be stabilized,” Dr. Hamzavi observed.
Efficacy has been amply demonstrated for adalimumab (Humira) and infliximab (Remicade). In Dr. Hamzavi’s experience infliximab works better, probably because it offers more dosing options.
Assuming medical therapy has resulted in disease stabilization, CO2 laser excision of sinus tracts under local anesthesia can then be employed as an office procedure to turn back the clock and return to an earlier stage of disease. Dermatologists at the Cleveland Clinic have described the technique in detail (Dermatol Surg. 2010 Feb;36[2]:208-13).
Hurley stage III
If biologic therapy doesn’t bring disease stabilization, the patient is likely headed for surgical excision using the CO2 laser. The Henry Ford team favors a specific regimen of surgical preparation using wide-spectrum antibiotics. The program begins with 6 weeks of IV ertapenem at 1 g/day delivered by a peripherally inserted central catheter managed by infectious disease colleagues.
“IV ertapenem is a drug you may not know much about. We find it works really well as a great way to bridge patients towards surgery,” the dermatologist explained.
The IV ertapenem is followed by 6 weeks of oral triple therapy with rifampin, moxifloxacin, and metronidazole then another 6 weeks of rifampin plus moxifloxacin. Next it’s surgical excision time.
Lifestyle modification
Lifestyle modification deserves to be a major priority in all HS patients, regardless of Hurley stage. Smoking cessation results in significantly greater likelihood of favorable response to first-line therapy. In obese patients, greater than 15% weight loss has been associated with significant reduction in disease severity. A sartorial shift to loose-fitting clothing can quiet down skin lesions through decreased friction and pressure. And proper utilization of warm compression will rapidly decrease acute lesional pain.
Dr. Hamzavi and his coinvestigators have described the Henry Ford Hospital treatment algorithm in a review of HS published in an open-access journal meant to serve as a resource for patients and physicians alike (F1000Res. 2017 Jul 28;6:1272. doi: 10.12688/f1000research.11337.1. eCollection 2017).
He reported serving as a consultant to AbbVie, Incyte, and UCB.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – Medical therapy alone is never sufficient in Hurley stage III hidradenitis suppurativa (HS), Iltefat H. Hamzavi, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even with the advances in biologics and antibiotic therapy, you still have to excise once you’re in full-blown Hurley stage III disease. Surgery has to be part of your protocol,” according to Dr. Hamzavi, a dermatologist at Henry Ford Hospital in Detroit, which runs one of the nation’s largest hidradenitis suppurativa clinics, with roughly 1,600 patients.
“Of course we’re biased. But until the data can set us free, you’re stuck with me,” the dermatologist quipped.
A core principle of the Henry Ford algorithm is this: “Medical therapy [for patients with advanced HS] stabilizes them and reduces their draining and pain, then you try to bring them back to a lower stage with surgical options,” he explained.
Although other HS staging systems exist, Dr. Hamzavi and his colleagues rely on the Hurley staging system to guide their treatment. Basically, Hurley stage I consists of follicular nodules and abscesses. When the nodules connect to form sinus tracts with scarring, that’s stage II. And if the sinus tracts interconnect throughout an entire area, that’s stage III.
Hurley stage I
First-line treatment of localized Hurley stage I disease at Henry Ford is a 10% topical benzoyl peroxide wash left on for 5 minutes before bathing, followed by postbathing topical clindamycin 1% lotion or solution applied to the nodules. If this maintenance regimen isn’t sufficient to prevent formation of new and worsening nodules, Dr. Hamzavi supplements it with up to three once-monthly 1064-nm Nd:YAG laser sessions aimed at follicular ablation. It’s a laser application he and his colleagues pioneered (Dermatol Surg. 2009 Aug;35[8]:1188-98). They subsequently documented the histopathologic basis of the procedure’s efficacy, which entails selective thermolysis of follicles, destruction of inflammatory lesions in the superficial to mid-dermis, followed by fibrosis and scarring (Arch Dermatol. 2011 Jan;147[1]:21-8).
In generalized Hurley stage I HS, the Henry Ford approach is to supplement the topical regimen and laser sessions with oral doxycycline at 100-150 mg daily for 1-6 months.
“The theory here is this is a dysbiotic event. The antibiotics reduce commensal bacteria, which ultimately reduces the reactive inflammatory response. But when you stop the antibiotics, the inflammatory response returns. So antibiotics can help stabilize the disease state but really can’t reverse the disease state. For that we have to turn to ablative treatment options: laser, surgery,” the dermatologist continued.
Hurley stage II
“At this point you’re looking at procedures,” according to Dr. Hamzavi. “Once you have sinus tracts it’s critical to remove them.”
The treatment backbone in stage II disease is 8-10 weeks of oral clindamycin and rifampin, both at 300 mg twice daily.
“This is one of the fundamental building blocks of HS clinics throughout the world,” he noted.
Clostridium difficile infection is exceedingly rare in HS patients on this regimen, for reasons still unclear.
If this dual-antibiotic regimen doesn’t dramatically reduce drainage and pain, he adds levofloxacin at 500 mg twice daily for up to 2 weeks in an effort to calm down unstable, decompensating disease.
Dapsone at 50-150 mg/day for up to 12 weeks is an additional option. It’s most useful in patients with nodules that are disproportionately painful, in Dr. Hamzavi’s experience.
Deroofing is a simple procedure that should be considered for all sinus tracts. It entails numbing the area with a ring block then introducing a curette or surgical probe into the sinus tract to open it up and get rid of the gelatinous material within. Dutch investigators have detailed the technique (J Am Acad Dermatol. 2010 Sep;63[3]:475-80).
Tumor necrosis factor–inhibitor therapy has been a major advance in Hurley stage II and III disease. “It doesn’t work in everybody, but a lot of patients can be stabilized,” Dr. Hamzavi observed.
Efficacy has been amply demonstrated for adalimumab (Humira) and infliximab (Remicade). In Dr. Hamzavi’s experience infliximab works better, probably because it offers more dosing options.
Assuming medical therapy has resulted in disease stabilization, CO2 laser excision of sinus tracts under local anesthesia can then be employed as an office procedure to turn back the clock and return to an earlier stage of disease. Dermatologists at the Cleveland Clinic have described the technique in detail (Dermatol Surg. 2010 Feb;36[2]:208-13).
Hurley stage III
If biologic therapy doesn’t bring disease stabilization, the patient is likely headed for surgical excision using the CO2 laser. The Henry Ford team favors a specific regimen of surgical preparation using wide-spectrum antibiotics. The program begins with 6 weeks of IV ertapenem at 1 g/day delivered by a peripherally inserted central catheter managed by infectious disease colleagues.
“IV ertapenem is a drug you may not know much about. We find it works really well as a great way to bridge patients towards surgery,” the dermatologist explained.
The IV ertapenem is followed by 6 weeks of oral triple therapy with rifampin, moxifloxacin, and metronidazole then another 6 weeks of rifampin plus moxifloxacin. Next it’s surgical excision time.
Lifestyle modification
Lifestyle modification deserves to be a major priority in all HS patients, regardless of Hurley stage. Smoking cessation results in significantly greater likelihood of favorable response to first-line therapy. In obese patients, greater than 15% weight loss has been associated with significant reduction in disease severity. A sartorial shift to loose-fitting clothing can quiet down skin lesions through decreased friction and pressure. And proper utilization of warm compression will rapidly decrease acute lesional pain.
Dr. Hamzavi and his coinvestigators have described the Henry Ford Hospital treatment algorithm in a review of HS published in an open-access journal meant to serve as a resource for patients and physicians alike (F1000Res. 2017 Jul 28;6:1272. doi: 10.12688/f1000research.11337.1. eCollection 2017).
He reported serving as a consultant to AbbVie, Incyte, and UCB.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – Medical therapy alone is never sufficient in Hurley stage III hidradenitis suppurativa (HS), Iltefat H. Hamzavi, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even with the advances in biologics and antibiotic therapy, you still have to excise once you’re in full-blown Hurley stage III disease. Surgery has to be part of your protocol,” according to Dr. Hamzavi, a dermatologist at Henry Ford Hospital in Detroit, which runs one of the nation’s largest hidradenitis suppurativa clinics, with roughly 1,600 patients.
“Of course we’re biased. But until the data can set us free, you’re stuck with me,” the dermatologist quipped.
A core principle of the Henry Ford algorithm is this: “Medical therapy [for patients with advanced HS] stabilizes them and reduces their draining and pain, then you try to bring them back to a lower stage with surgical options,” he explained.
Although other HS staging systems exist, Dr. Hamzavi and his colleagues rely on the Hurley staging system to guide their treatment. Basically, Hurley stage I consists of follicular nodules and abscesses. When the nodules connect to form sinus tracts with scarring, that’s stage II. And if the sinus tracts interconnect throughout an entire area, that’s stage III.
Hurley stage I
First-line treatment of localized Hurley stage I disease at Henry Ford is a 10% topical benzoyl peroxide wash left on for 5 minutes before bathing, followed by postbathing topical clindamycin 1% lotion or solution applied to the nodules. If this maintenance regimen isn’t sufficient to prevent formation of new and worsening nodules, Dr. Hamzavi supplements it with up to three once-monthly 1064-nm Nd:YAG laser sessions aimed at follicular ablation. It’s a laser application he and his colleagues pioneered (Dermatol Surg. 2009 Aug;35[8]:1188-98). They subsequently documented the histopathologic basis of the procedure’s efficacy, which entails selective thermolysis of follicles, destruction of inflammatory lesions in the superficial to mid-dermis, followed by fibrosis and scarring (Arch Dermatol. 2011 Jan;147[1]:21-8).
In generalized Hurley stage I HS, the Henry Ford approach is to supplement the topical regimen and laser sessions with oral doxycycline at 100-150 mg daily for 1-6 months.
“The theory here is this is a dysbiotic event. The antibiotics reduce commensal bacteria, which ultimately reduces the reactive inflammatory response. But when you stop the antibiotics, the inflammatory response returns. So antibiotics can help stabilize the disease state but really can’t reverse the disease state. For that we have to turn to ablative treatment options: laser, surgery,” the dermatologist continued.
Hurley stage II
“At this point you’re looking at procedures,” according to Dr. Hamzavi. “Once you have sinus tracts it’s critical to remove them.”
The treatment backbone in stage II disease is 8-10 weeks of oral clindamycin and rifampin, both at 300 mg twice daily.
“This is one of the fundamental building blocks of HS clinics throughout the world,” he noted.
Clostridium difficile infection is exceedingly rare in HS patients on this regimen, for reasons still unclear.
If this dual-antibiotic regimen doesn’t dramatically reduce drainage and pain, he adds levofloxacin at 500 mg twice daily for up to 2 weeks in an effort to calm down unstable, decompensating disease.
Dapsone at 50-150 mg/day for up to 12 weeks is an additional option. It’s most useful in patients with nodules that are disproportionately painful, in Dr. Hamzavi’s experience.
Deroofing is a simple procedure that should be considered for all sinus tracts. It entails numbing the area with a ring block then introducing a curette or surgical probe into the sinus tract to open it up and get rid of the gelatinous material within. Dutch investigators have detailed the technique (J Am Acad Dermatol. 2010 Sep;63[3]:475-80).
Tumor necrosis factor–inhibitor therapy has been a major advance in Hurley stage II and III disease. “It doesn’t work in everybody, but a lot of patients can be stabilized,” Dr. Hamzavi observed.
Efficacy has been amply demonstrated for adalimumab (Humira) and infliximab (Remicade). In Dr. Hamzavi’s experience infliximab works better, probably because it offers more dosing options.
Assuming medical therapy has resulted in disease stabilization, CO2 laser excision of sinus tracts under local anesthesia can then be employed as an office procedure to turn back the clock and return to an earlier stage of disease. Dermatologists at the Cleveland Clinic have described the technique in detail (Dermatol Surg. 2010 Feb;36[2]:208-13).
Hurley stage III
If biologic therapy doesn’t bring disease stabilization, the patient is likely headed for surgical excision using the CO2 laser. The Henry Ford team favors a specific regimen of surgical preparation using wide-spectrum antibiotics. The program begins with 6 weeks of IV ertapenem at 1 g/day delivered by a peripherally inserted central catheter managed by infectious disease colleagues.
“IV ertapenem is a drug you may not know much about. We find it works really well as a great way to bridge patients towards surgery,” the dermatologist explained.
The IV ertapenem is followed by 6 weeks of oral triple therapy with rifampin, moxifloxacin, and metronidazole then another 6 weeks of rifampin plus moxifloxacin. Next it’s surgical excision time.
Lifestyle modification
Lifestyle modification deserves to be a major priority in all HS patients, regardless of Hurley stage. Smoking cessation results in significantly greater likelihood of favorable response to first-line therapy. In obese patients, greater than 15% weight loss has been associated with significant reduction in disease severity. A sartorial shift to loose-fitting clothing can quiet down skin lesions through decreased friction and pressure. And proper utilization of warm compression will rapidly decrease acute lesional pain.
Dr. Hamzavi and his coinvestigators have described the Henry Ford Hospital treatment algorithm in a review of HS published in an open-access journal meant to serve as a resource for patients and physicians alike (F1000Res. 2017 Jul 28;6:1272. doi: 10.12688/f1000research.11337.1. eCollection 2017).
He reported serving as a consultant to AbbVie, Incyte, and UCB.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
How to avoid severe diarrhea from apremilast
KAUAI, HAWAII – Physicians have become much more cognizant of severe diarrhea and nausea as potential side effects of apremilast since the Food and Drug Administration–approved change in the warnings and precautions section of the drug’s labeling in June 2017. Jashin J. Wu, MD, director of the psoriasis clinic at Kaiser Permanente Los Angeles Medical Center, has a tip for avoiding these problems: Delay up-titrating.
“In my opinion, that may be too quick of an up-titration. I tell patients that, if they feel the GI issues are still a problem for them on day 6, they should take 30 mg just once a day for the first 1-2 months. After that we’ll see how they’re doing, and if they feel they can make the jump to twice a day, then they can go for it. Of course, I also tell them that maybe their psoriasis will not clear as well as if they’d been on apremilast twice a day right from day 6, but if they’re able to tolerate it and can continue to take it, they can improve while they’re on it,” the dermatologist said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Wu presented an update on recent developments regarding the newest oral drugs for psoriasis and one of the oldest: apremilast and methotrexate, respectively.
Apremilast
The revised warning label highlighting the risks of severe diarrhea and nausea associated with the oral phosphodiesterase-4 inhibitor says that most such events have occurred within the first few weeks of therapy. The guidance also notes that patients who reduced the dosage or discontinued treatment outright generally improved rapidly.
“I see this in a lot of my patients. They have to go to the bathroom pretty often. It’s actually unusual for me for a patient not to have any GI issues at all,” according to Dr. Wu.
He shared a number of other fresh insights into apremilast’s safety and efficacy derived from recent studies.
Efficacy appears to increase at least out to 1 year
A report from the phase 3b, randomized, placebo-controlled, 250-patient LIBERATE trial showed that the week 16 PASI 75 response rate was 39.8% with apremilast, 48.2% with etanercept (Enbrel), and 11.9% with placebo. After week 16, everyone switched to apremilast. The PASI 75 rate in patients on apremilast all along climbed from 39.8% at week 16 to 52.7% at week 52. That result was in the same ballpark as the 57% rate in those switched from etanercept to apremilast and the 53.4% PASI 75 rate at week 52 in patients switched from placebo to apremilast (J Eur Acad Dermatol Venereol. 2017 Mar;31[3]:507-17).
“It was interesting to see that, as the study continued on for 1 year, the PASI 75 rate continued to improve. That’s worth noting: In general, I tell patients you have to be on a drug for about 3 months before we’re going to say if it worked or not, and that’s true even with drugs for other conditions, like doxycycline for acne. But this study seems to indicate that you have much better improvement at the 1-year point, and that’s not so much true for the biologics,” the dermatologist observed.
Safety to 3 years looks reassuringly good: 3-year follow-up of the 1,184-patient, phase 3, randomized, controlled ESTEEM 1 and 2 trials provided by far the longest look to date at apremilast’s safety. There were no surprises, no serious opportunistic infections, and no significant changes in laboratory values (J Am Acad Dermatol. 2017 Aug;77[2]:310-17.e1).
Of note, 21.9% of patients on apremilast lost more than 5% of their baseline body weight. Most of the weight loss occurred during year 1 of treatment and mostly in patients with a higher baseline body mass index.
“It seems like apremilast is definitely a good option if patients can tolerate the GI upset,” Dr. Wu said.
Apremilast can safely and effectively be combined with other psoriasis therapies: Dermatologists at the University of Toronto reported on a retrospective analysis of 81 biologic-naive psoriasis patients treated with apremilast in combination with methotrexate, acitretin (Soriatane), cyclosporine, narrowband UVB, etanercept, infliximab (Remicade), adalimumab (Humira), and/or ustekinumab (Stelara). Of these patients, 81% achieved a PASI 75 response at week 12 (J Cutan Med Surg. 2016 Jul;20[4]:313-6).
“That’s pretty good. It’s certainly better than apremilast by itself. So if you can get the payer to cover a combination of apremilast and something else, it may help get to PASI 75,” Dr. Wu noted.
Session chair Craig L. Leonardi, MD, said he hasn’t had any luck in going that route.
“The insurance industry just won’t give me apremilast in combination with a biologic drug. Even though it makes complete sense to use it in place of methotrexate with a biologic, I just can’t get it,” according to Dr. Leonardi, a dermatologist at Saint Louis University.
“I don’t have those limitations at Kaiser,” according to Dr. Wu. “I personally have only used apremilast and methotrexate and apremilast and acitretin in combination. I just want to be kind to Kaiser and not give two branded medications to a patient, but I certainly think it’s a feasible option.”
Methotrexate
A simple response prediction rule: Kenneth B. Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin in Milwaukee, and his coinvestigators at AbbVie developed a methotrexate response/nonresponse prediction rule using data on 110 participants in the phase 3 CHAMPION trial. Then they validated the rule in the phase 3 M10-255 trial. They found that a PASI 25 response to methotrexate at week 4 was associated with an 8.9-fold increased likelihood of a week-16 PASI 75 response. Patients with a predicted response probability of less than 30% were asked to discontinue the drug; their week 16 PASI 75 rate was only 21.1%, compared with a 65.8% response rate in patients with a prediction rating (J Am Acad Dermatol. 2017 Dec;77[6]:1030-7).
“Four weeks of methotrexate may be sufficient to determine the long-term response. It may not be necessary to put them on the drug for 3 months,” Dr. Wu commented.
Subcutaneous methotrexate: European investigators demonstrated that an intensified dosing schedule of subcutaneous methotrexate was safe and effective for treatment of moderate to severe plaque psoriasis in the 52-week, phase 3, randomized, 16-center, double-blind, 120-patient, placebo-controlled METOP study.
The intensified subcutaneous regimen consisted of 17.5 mg/week initially, escalated to 22.5 mg/week after 8 weeks if a patient hadn’t achieved at least a PASI 50 response at that point. The primary outcome, the PASI 75 response at week 16, was 41% in the subcutaneous methotrexate group and 10% in controls, with a maximum PASI 75 rate of 51% seen beginning at week 24. The week 4 and 8 PASI 50 rates were 50% and 58%, respectively, with methotrexate versus 3% and 17% in placebo-treated controls. The subcutaneous regimen was generally well tolerated, with no serious infections or malignancies arising during 52 weeks (Lancet. 2017 Feb 4;389[10068]:528-37).
Dr. Wu reported receiving research funding from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Regeneron.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – Physicians have become much more cognizant of severe diarrhea and nausea as potential side effects of apremilast since the Food and Drug Administration–approved change in the warnings and precautions section of the drug’s labeling in June 2017. Jashin J. Wu, MD, director of the psoriasis clinic at Kaiser Permanente Los Angeles Medical Center, has a tip for avoiding these problems: Delay up-titrating.
“In my opinion, that may be too quick of an up-titration. I tell patients that, if they feel the GI issues are still a problem for them on day 6, they should take 30 mg just once a day for the first 1-2 months. After that we’ll see how they’re doing, and if they feel they can make the jump to twice a day, then they can go for it. Of course, I also tell them that maybe their psoriasis will not clear as well as if they’d been on apremilast twice a day right from day 6, but if they’re able to tolerate it and can continue to take it, they can improve while they’re on it,” the dermatologist said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Wu presented an update on recent developments regarding the newest oral drugs for psoriasis and one of the oldest: apremilast and methotrexate, respectively.
Apremilast
The revised warning label highlighting the risks of severe diarrhea and nausea associated with the oral phosphodiesterase-4 inhibitor says that most such events have occurred within the first few weeks of therapy. The guidance also notes that patients who reduced the dosage or discontinued treatment outright generally improved rapidly.
“I see this in a lot of my patients. They have to go to the bathroom pretty often. It’s actually unusual for me for a patient not to have any GI issues at all,” according to Dr. Wu.
He shared a number of other fresh insights into apremilast’s safety and efficacy derived from recent studies.
Efficacy appears to increase at least out to 1 year
A report from the phase 3b, randomized, placebo-controlled, 250-patient LIBERATE trial showed that the week 16 PASI 75 response rate was 39.8% with apremilast, 48.2% with etanercept (Enbrel), and 11.9% with placebo. After week 16, everyone switched to apremilast. The PASI 75 rate in patients on apremilast all along climbed from 39.8% at week 16 to 52.7% at week 52. That result was in the same ballpark as the 57% rate in those switched from etanercept to apremilast and the 53.4% PASI 75 rate at week 52 in patients switched from placebo to apremilast (J Eur Acad Dermatol Venereol. 2017 Mar;31[3]:507-17).
“It was interesting to see that, as the study continued on for 1 year, the PASI 75 rate continued to improve. That’s worth noting: In general, I tell patients you have to be on a drug for about 3 months before we’re going to say if it worked or not, and that’s true even with drugs for other conditions, like doxycycline for acne. But this study seems to indicate that you have much better improvement at the 1-year point, and that’s not so much true for the biologics,” the dermatologist observed.
Safety to 3 years looks reassuringly good: 3-year follow-up of the 1,184-patient, phase 3, randomized, controlled ESTEEM 1 and 2 trials provided by far the longest look to date at apremilast’s safety. There were no surprises, no serious opportunistic infections, and no significant changes in laboratory values (J Am Acad Dermatol. 2017 Aug;77[2]:310-17.e1).
Of note, 21.9% of patients on apremilast lost more than 5% of their baseline body weight. Most of the weight loss occurred during year 1 of treatment and mostly in patients with a higher baseline body mass index.
“It seems like apremilast is definitely a good option if patients can tolerate the GI upset,” Dr. Wu said.
Apremilast can safely and effectively be combined with other psoriasis therapies: Dermatologists at the University of Toronto reported on a retrospective analysis of 81 biologic-naive psoriasis patients treated with apremilast in combination with methotrexate, acitretin (Soriatane), cyclosporine, narrowband UVB, etanercept, infliximab (Remicade), adalimumab (Humira), and/or ustekinumab (Stelara). Of these patients, 81% achieved a PASI 75 response at week 12 (J Cutan Med Surg. 2016 Jul;20[4]:313-6).
“That’s pretty good. It’s certainly better than apremilast by itself. So if you can get the payer to cover a combination of apremilast and something else, it may help get to PASI 75,” Dr. Wu noted.
Session chair Craig L. Leonardi, MD, said he hasn’t had any luck in going that route.
“The insurance industry just won’t give me apremilast in combination with a biologic drug. Even though it makes complete sense to use it in place of methotrexate with a biologic, I just can’t get it,” according to Dr. Leonardi, a dermatologist at Saint Louis University.
“I don’t have those limitations at Kaiser,” according to Dr. Wu. “I personally have only used apremilast and methotrexate and apremilast and acitretin in combination. I just want to be kind to Kaiser and not give two branded medications to a patient, but I certainly think it’s a feasible option.”
Methotrexate
A simple response prediction rule: Kenneth B. Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin in Milwaukee, and his coinvestigators at AbbVie developed a methotrexate response/nonresponse prediction rule using data on 110 participants in the phase 3 CHAMPION trial. Then they validated the rule in the phase 3 M10-255 trial. They found that a PASI 25 response to methotrexate at week 4 was associated with an 8.9-fold increased likelihood of a week-16 PASI 75 response. Patients with a predicted response probability of less than 30% were asked to discontinue the drug; their week 16 PASI 75 rate was only 21.1%, compared with a 65.8% response rate in patients with a prediction rating (J Am Acad Dermatol. 2017 Dec;77[6]:1030-7).
“Four weeks of methotrexate may be sufficient to determine the long-term response. It may not be necessary to put them on the drug for 3 months,” Dr. Wu commented.
Subcutaneous methotrexate: European investigators demonstrated that an intensified dosing schedule of subcutaneous methotrexate was safe and effective for treatment of moderate to severe plaque psoriasis in the 52-week, phase 3, randomized, 16-center, double-blind, 120-patient, placebo-controlled METOP study.
The intensified subcutaneous regimen consisted of 17.5 mg/week initially, escalated to 22.5 mg/week after 8 weeks if a patient hadn’t achieved at least a PASI 50 response at that point. The primary outcome, the PASI 75 response at week 16, was 41% in the subcutaneous methotrexate group and 10% in controls, with a maximum PASI 75 rate of 51% seen beginning at week 24. The week 4 and 8 PASI 50 rates were 50% and 58%, respectively, with methotrexate versus 3% and 17% in placebo-treated controls. The subcutaneous regimen was generally well tolerated, with no serious infections or malignancies arising during 52 weeks (Lancet. 2017 Feb 4;389[10068]:528-37).
Dr. Wu reported receiving research funding from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Regeneron.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – Physicians have become much more cognizant of severe diarrhea and nausea as potential side effects of apremilast since the Food and Drug Administration–approved change in the warnings and precautions section of the drug’s labeling in June 2017. Jashin J. Wu, MD, director of the psoriasis clinic at Kaiser Permanente Los Angeles Medical Center, has a tip for avoiding these problems: Delay up-titrating.
“In my opinion, that may be too quick of an up-titration. I tell patients that, if they feel the GI issues are still a problem for them on day 6, they should take 30 mg just once a day for the first 1-2 months. After that we’ll see how they’re doing, and if they feel they can make the jump to twice a day, then they can go for it. Of course, I also tell them that maybe their psoriasis will not clear as well as if they’d been on apremilast twice a day right from day 6, but if they’re able to tolerate it and can continue to take it, they can improve while they’re on it,” the dermatologist said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Wu presented an update on recent developments regarding the newest oral drugs for psoriasis and one of the oldest: apremilast and methotrexate, respectively.
Apremilast
The revised warning label highlighting the risks of severe diarrhea and nausea associated with the oral phosphodiesterase-4 inhibitor says that most such events have occurred within the first few weeks of therapy. The guidance also notes that patients who reduced the dosage or discontinued treatment outright generally improved rapidly.
“I see this in a lot of my patients. They have to go to the bathroom pretty often. It’s actually unusual for me for a patient not to have any GI issues at all,” according to Dr. Wu.
He shared a number of other fresh insights into apremilast’s safety and efficacy derived from recent studies.
Efficacy appears to increase at least out to 1 year
A report from the phase 3b, randomized, placebo-controlled, 250-patient LIBERATE trial showed that the week 16 PASI 75 response rate was 39.8% with apremilast, 48.2% with etanercept (Enbrel), and 11.9% with placebo. After week 16, everyone switched to apremilast. The PASI 75 rate in patients on apremilast all along climbed from 39.8% at week 16 to 52.7% at week 52. That result was in the same ballpark as the 57% rate in those switched from etanercept to apremilast and the 53.4% PASI 75 rate at week 52 in patients switched from placebo to apremilast (J Eur Acad Dermatol Venereol. 2017 Mar;31[3]:507-17).
“It was interesting to see that, as the study continued on for 1 year, the PASI 75 rate continued to improve. That’s worth noting: In general, I tell patients you have to be on a drug for about 3 months before we’re going to say if it worked or not, and that’s true even with drugs for other conditions, like doxycycline for acne. But this study seems to indicate that you have much better improvement at the 1-year point, and that’s not so much true for the biologics,” the dermatologist observed.
Safety to 3 years looks reassuringly good: 3-year follow-up of the 1,184-patient, phase 3, randomized, controlled ESTEEM 1 and 2 trials provided by far the longest look to date at apremilast’s safety. There were no surprises, no serious opportunistic infections, and no significant changes in laboratory values (J Am Acad Dermatol. 2017 Aug;77[2]:310-17.e1).
Of note, 21.9% of patients on apremilast lost more than 5% of their baseline body weight. Most of the weight loss occurred during year 1 of treatment and mostly in patients with a higher baseline body mass index.
“It seems like apremilast is definitely a good option if patients can tolerate the GI upset,” Dr. Wu said.
Apremilast can safely and effectively be combined with other psoriasis therapies: Dermatologists at the University of Toronto reported on a retrospective analysis of 81 biologic-naive psoriasis patients treated with apremilast in combination with methotrexate, acitretin (Soriatane), cyclosporine, narrowband UVB, etanercept, infliximab (Remicade), adalimumab (Humira), and/or ustekinumab (Stelara). Of these patients, 81% achieved a PASI 75 response at week 12 (J Cutan Med Surg. 2016 Jul;20[4]:313-6).
“That’s pretty good. It’s certainly better than apremilast by itself. So if you can get the payer to cover a combination of apremilast and something else, it may help get to PASI 75,” Dr. Wu noted.
Session chair Craig L. Leonardi, MD, said he hasn’t had any luck in going that route.
“The insurance industry just won’t give me apremilast in combination with a biologic drug. Even though it makes complete sense to use it in place of methotrexate with a biologic, I just can’t get it,” according to Dr. Leonardi, a dermatologist at Saint Louis University.
“I don’t have those limitations at Kaiser,” according to Dr. Wu. “I personally have only used apremilast and methotrexate and apremilast and acitretin in combination. I just want to be kind to Kaiser and not give two branded medications to a patient, but I certainly think it’s a feasible option.”
Methotrexate
A simple response prediction rule: Kenneth B. Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin in Milwaukee, and his coinvestigators at AbbVie developed a methotrexate response/nonresponse prediction rule using data on 110 participants in the phase 3 CHAMPION trial. Then they validated the rule in the phase 3 M10-255 trial. They found that a PASI 25 response to methotrexate at week 4 was associated with an 8.9-fold increased likelihood of a week-16 PASI 75 response. Patients with a predicted response probability of less than 30% were asked to discontinue the drug; their week 16 PASI 75 rate was only 21.1%, compared with a 65.8% response rate in patients with a prediction rating (J Am Acad Dermatol. 2017 Dec;77[6]:1030-7).
“Four weeks of methotrexate may be sufficient to determine the long-term response. It may not be necessary to put them on the drug for 3 months,” Dr. Wu commented.
Subcutaneous methotrexate: European investigators demonstrated that an intensified dosing schedule of subcutaneous methotrexate was safe and effective for treatment of moderate to severe plaque psoriasis in the 52-week, phase 3, randomized, 16-center, double-blind, 120-patient, placebo-controlled METOP study.
The intensified subcutaneous regimen consisted of 17.5 mg/week initially, escalated to 22.5 mg/week after 8 weeks if a patient hadn’t achieved at least a PASI 50 response at that point. The primary outcome, the PASI 75 response at week 16, was 41% in the subcutaneous methotrexate group and 10% in controls, with a maximum PASI 75 rate of 51% seen beginning at week 24. The week 4 and 8 PASI 50 rates were 50% and 58%, respectively, with methotrexate versus 3% and 17% in placebo-treated controls. The subcutaneous regimen was generally well tolerated, with no serious infections or malignancies arising during 52 weeks (Lancet. 2017 Feb 4;389[10068]:528-37).
Dr. Wu reported receiving research funding from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Regeneron.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Hormonal contraception boosts breast cancer risk
KAUAI, HAWAII – Current or recent use of hormonal contraception is associated with an increased risk of breast cancer, although in response to the Danish study findings.
“The data looks clear. It’s not a big risk, but it’s clearly a higher risk,” Lawrence F. Eichenfield, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Investigators at the University of Copenhagen conducted a prospective cohort study among all 1.8 million Danish women aged 15-49 years who had not had cancer, venous thromboembolism, or treatment for infertility. Comprehensive nationwide registries were used to follow the women for an average of 10.9 years, which represents 19.6 million person-years of follow-up.
During that time, 11,517 cases of invasive breast cancer occurred in the study population. The relative risk was 1.2-fold greater in current or recent users of hormonal contraception, compared with never users of oral estrogen/progestin contraceptives or progestin-containing intrauterine devices. The risk rose with duration of use: from 1.09-fold with less than 1 year of exposure to 1.38-fold with more than 10 years of hormonal contraception.
From a population standpoint, there were 13 additional breast cancers diagnosed per 100,000 person-years of hormonal contraception use. That worked out to one extra breast cancer for every 7,690 women using hormonal contraception for 1 year (N Engl J Med. 2017 Dec 7;377[23]:2228-39).
Dr. Eichenfield pointed out that among Danish patients who used an oral contraceptive for less than 5 years, the excess risk of breast cancer went away within a year of stopping the medication. If patients used hormonal contraception for more than 5 years, the excess breast cancer risk lasted for 5 years after stopping.
He noted that the antiandrogen spironolactone, widely used off-label to treat adult female acne, has been shown to be free of any increased breast cancer risk.
He reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company..
KAUAI, HAWAII – Current or recent use of hormonal contraception is associated with an increased risk of breast cancer, although in response to the Danish study findings.
“The data looks clear. It’s not a big risk, but it’s clearly a higher risk,” Lawrence F. Eichenfield, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Investigators at the University of Copenhagen conducted a prospective cohort study among all 1.8 million Danish women aged 15-49 years who had not had cancer, venous thromboembolism, or treatment for infertility. Comprehensive nationwide registries were used to follow the women for an average of 10.9 years, which represents 19.6 million person-years of follow-up.
During that time, 11,517 cases of invasive breast cancer occurred in the study population. The relative risk was 1.2-fold greater in current or recent users of hormonal contraception, compared with never users of oral estrogen/progestin contraceptives or progestin-containing intrauterine devices. The risk rose with duration of use: from 1.09-fold with less than 1 year of exposure to 1.38-fold with more than 10 years of hormonal contraception.
From a population standpoint, there were 13 additional breast cancers diagnosed per 100,000 person-years of hormonal contraception use. That worked out to one extra breast cancer for every 7,690 women using hormonal contraception for 1 year (N Engl J Med. 2017 Dec 7;377[23]:2228-39).
Dr. Eichenfield pointed out that among Danish patients who used an oral contraceptive for less than 5 years, the excess risk of breast cancer went away within a year of stopping the medication. If patients used hormonal contraception for more than 5 years, the excess breast cancer risk lasted for 5 years after stopping.
He noted that the antiandrogen spironolactone, widely used off-label to treat adult female acne, has been shown to be free of any increased breast cancer risk.
He reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company..
KAUAI, HAWAII – Current or recent use of hormonal contraception is associated with an increased risk of breast cancer, although in response to the Danish study findings.
“The data looks clear. It’s not a big risk, but it’s clearly a higher risk,” Lawrence F. Eichenfield, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Investigators at the University of Copenhagen conducted a prospective cohort study among all 1.8 million Danish women aged 15-49 years who had not had cancer, venous thromboembolism, or treatment for infertility. Comprehensive nationwide registries were used to follow the women for an average of 10.9 years, which represents 19.6 million person-years of follow-up.
During that time, 11,517 cases of invasive breast cancer occurred in the study population. The relative risk was 1.2-fold greater in current or recent users of hormonal contraception, compared with never users of oral estrogen/progestin contraceptives or progestin-containing intrauterine devices. The risk rose with duration of use: from 1.09-fold with less than 1 year of exposure to 1.38-fold with more than 10 years of hormonal contraception.
From a population standpoint, there were 13 additional breast cancers diagnosed per 100,000 person-years of hormonal contraception use. That worked out to one extra breast cancer for every 7,690 women using hormonal contraception for 1 year (N Engl J Med. 2017 Dec 7;377[23]:2228-39).
Dr. Eichenfield pointed out that among Danish patients who used an oral contraceptive for less than 5 years, the excess risk of breast cancer went away within a year of stopping the medication. If patients used hormonal contraception for more than 5 years, the excess breast cancer risk lasted for 5 years after stopping.
He noted that the antiandrogen spironolactone, widely used off-label to treat adult female acne, has been shown to be free of any increased breast cancer risk.
He reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company..
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Dermatology practice gaps: improving medication management
KAUAI, HAWAII – Dermatologists don’t ordinarily peruse the ophthalmology literature. So they may be unaware that the American Academy of Ophthalmology has issued Erik J. Stratman, MD, noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Most dermatologists routinely dose hydroxychloroquine at 400 mg/day, regardless of body weight. The former AAO recommendation, which dates back to 2011, called for dosing at up to 6.5 mg/kg of ideal body weight or 400 mg/day, whichever is lower. However, the AAO recommendation has changed in light of a large, retrospective case-control study that suggested this practice may be overdosing thin patients – thereby exposing them to increased risk of retinal toxicity and other drug-related adverse events – while at the same time possibly underdosing some obese patients, said Dr. Stratman, chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
This was one of two dermatology practice gaps he highlighted involving suboptimal medication management, the other being most dermatologists’ failure to protect their patients’ gut when prescribing prednisone.
“I think the push over the last 5 years has been ‘protect the bones, protect the bones, protect the bones.’ We’ve done better and better about protecting the bones and getting that into our conversations with patients on prednisone. But we’re not thinking so much about the gut,” the dermatologist said.
Hydroxychloroquine dosing
The former AAO recommendation was revised in response to a retrospective case-control study of retinal toxicity rates in 2,361 patients on the drug continuously for longer than 5 years. The study demonstrated that the risk of retinopathy jumped 5.7-fold with daily consumption of hydroxychloroquine at more than 5.0 mg/kg (JAMA Ophthalmol. 2014 Dec;132[12]:1453-60).
The current AAO recommendation (Ophthalmology. 2016 Jun;123[6]:1386-94) is to dose hydroxychloroquine at a daily maximum of 5.0 mg/kg of real weight, which correlated better with retinopathy risk in the case-control study than did ideal body weight. Hydroxychloroquine doesn’t accumulate well in fat.
Until now, most dermatologists have not routinely measured patients’ body weight in the office or calculated their body mass index. But Dr. Stratman advised against reliance upon a patient’s self-reported body weight, which may diverge substantially from reality. “Get yourself a good office scale – they’re not that expensive – and use it when prescribing drugs with a tight therapeutic window,” he urged.
Another key to minimizing retinopathy risk in patients on hydroxychloroquine is to pay careful attention to how long they’ve been on the drug. As the years go by in patients being treated for cutaneous lupus or other dermatologic disorders where decades-long therapy is often a mainstay, it’s important to check with patients and make sure they’re getting annual ophthalmologic screening for irreversible retinal toxicity by both threshold visual fields and spectral domain optical coherence tomography. In the large, practice-changing retrospective study, patients on hydroxychloroquine at 4.0-5.0 mg/kg daily had a prevalence of retinopathy of less than 2% during the first 10 years of therapy, but the rate shot up to nearly 20% after 20 years of use, Dr. Stratman observed.
He highlighted as helpful an updated review of the use of hydroxychloroquine in dermatology recently published by Anthony P. Fernandez, MD, PhD, of the department of dermatology at the Cleveland Clinic (J Am Acad Dermatol. 2017 Jun;76[6]:1176-82).
Dr. Fernandez recommends following the AAO guidance to dose the drug at 5.0 mg/kg or less of actual body weight in thin or normal-weight patients; however, he departed from the ophthalmologists with regard to treatment of obese patients. Because dosing based on actual weight could potentially lead to relative overdosing in obese patients, in that growing population he recommends calculating the dose based upon 5.0 mg/kg of actual body weight, as well as the dose based on 6.5 mg/kg of ideal body weight, then prescribing the lower of the two, up to a maximum of 400 mg/day.
“The current recommendation is really about not overdosing thin patients. Basically, dosing is not so difficult for obese people because if you weigh more than 175 pounds, you’re going to get 400 mg/day,” Dr. Stratman explained.
That 400 mg/day ceiling is not cast in stone, he continued. The guideline recommends that, if a patient is a nonresponder to several months of hydroxychloroquine at 400 mg/day, it’s worthwhile to order a drug blood level. If it’s not above the efficacy threshold of more than 750 ng/mL, it’s appropriate to titrate up.
Protecting against prednisone-induced gastritis
“We underprotect the gut,” Dr. Stratman asserted.
He referred to a recent comprehensive dermatologic review of the prevention and management of glucocorticoid-related side effects, especially the part on peptic ulcer disease (J Am Acad Dermatol. 2017 Jan;76[1]:11-6). This is an issue that heretofore hadn’t been much emphasized in the dermatology literature.
“I read this and thought, ‘Gosh, I’m not really having a conversation with my patients about a review of systems for gut protection as I should. And I certainly haven’t been thinking about prescribing PPIs [proton pump inhibitors] for my patients,’” he recalled.
Dr. Stratman polled his Hawaii Dermatology Seminar audience as to who had ever prescribed a PPI. Most indicated with their electronic clickers that they had never done so.
“This is what a practice gap is,” he commented. “You read the literature and you say, ‘Oh, I guess that makes sense. Maybe I should be doing that more often, or making sure it gets done.’”
“I don’t want to come across as saying, ‘For everybody we put on prednisone we should be giving vitamin D, calcium, and a PPI.’ That’s not the message. The message is, assess your patient – or make sure your patient is being assessed – for risk of peptic ulcer disease. And if you don’t feel comfortable prescribing a PPI, please get the patient connected with their primary care provider, who should,” Dr. Stratman said.
The authors of the dermatology review made a case for screening for GI risk factors in every patient who is going to receive an oral glucocorticoid. The ones who absolutely should be prescribed a PPI unless contraindicated include patients who are taking daily aspirin or NSAIDs for an essential reason, such as cardiovascular protection or significant arthritic pain. The authors suggest consideration of a PPI in patients with other, less potent risk factors for peptic ulcer disease, including a history of ulcer disease, gastroesophageal reflux disease, Barrett’s esophagus, heavy smoking, heavy alcohol consumption, age greater than 65, and concomitant use of other medications with an associated risk of peptic ulcer disease – such as bisphosphonates, “which you may have just put them on to protect their bones,” Dr. Stratman noted.
Of course, PPIs come with side effects of their own, including increased fracture risk, Clostridium difficile infections, and rebound acid secretion.
Dr. Stratman reported having no financial conflicts regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Dermatologists don’t ordinarily peruse the ophthalmology literature. So they may be unaware that the American Academy of Ophthalmology has issued Erik J. Stratman, MD, noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Most dermatologists routinely dose hydroxychloroquine at 400 mg/day, regardless of body weight. The former AAO recommendation, which dates back to 2011, called for dosing at up to 6.5 mg/kg of ideal body weight or 400 mg/day, whichever is lower. However, the AAO recommendation has changed in light of a large, retrospective case-control study that suggested this practice may be overdosing thin patients – thereby exposing them to increased risk of retinal toxicity and other drug-related adverse events – while at the same time possibly underdosing some obese patients, said Dr. Stratman, chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
This was one of two dermatology practice gaps he highlighted involving suboptimal medication management, the other being most dermatologists’ failure to protect their patients’ gut when prescribing prednisone.
“I think the push over the last 5 years has been ‘protect the bones, protect the bones, protect the bones.’ We’ve done better and better about protecting the bones and getting that into our conversations with patients on prednisone. But we’re not thinking so much about the gut,” the dermatologist said.
Hydroxychloroquine dosing
The former AAO recommendation was revised in response to a retrospective case-control study of retinal toxicity rates in 2,361 patients on the drug continuously for longer than 5 years. The study demonstrated that the risk of retinopathy jumped 5.7-fold with daily consumption of hydroxychloroquine at more than 5.0 mg/kg (JAMA Ophthalmol. 2014 Dec;132[12]:1453-60).
The current AAO recommendation (Ophthalmology. 2016 Jun;123[6]:1386-94) is to dose hydroxychloroquine at a daily maximum of 5.0 mg/kg of real weight, which correlated better with retinopathy risk in the case-control study than did ideal body weight. Hydroxychloroquine doesn’t accumulate well in fat.
Until now, most dermatologists have not routinely measured patients’ body weight in the office or calculated their body mass index. But Dr. Stratman advised against reliance upon a patient’s self-reported body weight, which may diverge substantially from reality. “Get yourself a good office scale – they’re not that expensive – and use it when prescribing drugs with a tight therapeutic window,” he urged.
Another key to minimizing retinopathy risk in patients on hydroxychloroquine is to pay careful attention to how long they’ve been on the drug. As the years go by in patients being treated for cutaneous lupus or other dermatologic disorders where decades-long therapy is often a mainstay, it’s important to check with patients and make sure they’re getting annual ophthalmologic screening for irreversible retinal toxicity by both threshold visual fields and spectral domain optical coherence tomography. In the large, practice-changing retrospective study, patients on hydroxychloroquine at 4.0-5.0 mg/kg daily had a prevalence of retinopathy of less than 2% during the first 10 years of therapy, but the rate shot up to nearly 20% after 20 years of use, Dr. Stratman observed.
He highlighted as helpful an updated review of the use of hydroxychloroquine in dermatology recently published by Anthony P. Fernandez, MD, PhD, of the department of dermatology at the Cleveland Clinic (J Am Acad Dermatol. 2017 Jun;76[6]:1176-82).
Dr. Fernandez recommends following the AAO guidance to dose the drug at 5.0 mg/kg or less of actual body weight in thin or normal-weight patients; however, he departed from the ophthalmologists with regard to treatment of obese patients. Because dosing based on actual weight could potentially lead to relative overdosing in obese patients, in that growing population he recommends calculating the dose based upon 5.0 mg/kg of actual body weight, as well as the dose based on 6.5 mg/kg of ideal body weight, then prescribing the lower of the two, up to a maximum of 400 mg/day.
“The current recommendation is really about not overdosing thin patients. Basically, dosing is not so difficult for obese people because if you weigh more than 175 pounds, you’re going to get 400 mg/day,” Dr. Stratman explained.
That 400 mg/day ceiling is not cast in stone, he continued. The guideline recommends that, if a patient is a nonresponder to several months of hydroxychloroquine at 400 mg/day, it’s worthwhile to order a drug blood level. If it’s not above the efficacy threshold of more than 750 ng/mL, it’s appropriate to titrate up.
Protecting against prednisone-induced gastritis
“We underprotect the gut,” Dr. Stratman asserted.
He referred to a recent comprehensive dermatologic review of the prevention and management of glucocorticoid-related side effects, especially the part on peptic ulcer disease (J Am Acad Dermatol. 2017 Jan;76[1]:11-6). This is an issue that heretofore hadn’t been much emphasized in the dermatology literature.
“I read this and thought, ‘Gosh, I’m not really having a conversation with my patients about a review of systems for gut protection as I should. And I certainly haven’t been thinking about prescribing PPIs [proton pump inhibitors] for my patients,’” he recalled.
Dr. Stratman polled his Hawaii Dermatology Seminar audience as to who had ever prescribed a PPI. Most indicated with their electronic clickers that they had never done so.
“This is what a practice gap is,” he commented. “You read the literature and you say, ‘Oh, I guess that makes sense. Maybe I should be doing that more often, or making sure it gets done.’”
“I don’t want to come across as saying, ‘For everybody we put on prednisone we should be giving vitamin D, calcium, and a PPI.’ That’s not the message. The message is, assess your patient – or make sure your patient is being assessed – for risk of peptic ulcer disease. And if you don’t feel comfortable prescribing a PPI, please get the patient connected with their primary care provider, who should,” Dr. Stratman said.
The authors of the dermatology review made a case for screening for GI risk factors in every patient who is going to receive an oral glucocorticoid. The ones who absolutely should be prescribed a PPI unless contraindicated include patients who are taking daily aspirin or NSAIDs for an essential reason, such as cardiovascular protection or significant arthritic pain. The authors suggest consideration of a PPI in patients with other, less potent risk factors for peptic ulcer disease, including a history of ulcer disease, gastroesophageal reflux disease, Barrett’s esophagus, heavy smoking, heavy alcohol consumption, age greater than 65, and concomitant use of other medications with an associated risk of peptic ulcer disease – such as bisphosphonates, “which you may have just put them on to protect their bones,” Dr. Stratman noted.
Of course, PPIs come with side effects of their own, including increased fracture risk, Clostridium difficile infections, and rebound acid secretion.
Dr. Stratman reported having no financial conflicts regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Dermatologists don’t ordinarily peruse the ophthalmology literature. So they may be unaware that the American Academy of Ophthalmology has issued Erik J. Stratman, MD, noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Most dermatologists routinely dose hydroxychloroquine at 400 mg/day, regardless of body weight. The former AAO recommendation, which dates back to 2011, called for dosing at up to 6.5 mg/kg of ideal body weight or 400 mg/day, whichever is lower. However, the AAO recommendation has changed in light of a large, retrospective case-control study that suggested this practice may be overdosing thin patients – thereby exposing them to increased risk of retinal toxicity and other drug-related adverse events – while at the same time possibly underdosing some obese patients, said Dr. Stratman, chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
This was one of two dermatology practice gaps he highlighted involving suboptimal medication management, the other being most dermatologists’ failure to protect their patients’ gut when prescribing prednisone.
“I think the push over the last 5 years has been ‘protect the bones, protect the bones, protect the bones.’ We’ve done better and better about protecting the bones and getting that into our conversations with patients on prednisone. But we’re not thinking so much about the gut,” the dermatologist said.
Hydroxychloroquine dosing
The former AAO recommendation was revised in response to a retrospective case-control study of retinal toxicity rates in 2,361 patients on the drug continuously for longer than 5 years. The study demonstrated that the risk of retinopathy jumped 5.7-fold with daily consumption of hydroxychloroquine at more than 5.0 mg/kg (JAMA Ophthalmol. 2014 Dec;132[12]:1453-60).
The current AAO recommendation (Ophthalmology. 2016 Jun;123[6]:1386-94) is to dose hydroxychloroquine at a daily maximum of 5.0 mg/kg of real weight, which correlated better with retinopathy risk in the case-control study than did ideal body weight. Hydroxychloroquine doesn’t accumulate well in fat.
Until now, most dermatologists have not routinely measured patients’ body weight in the office or calculated their body mass index. But Dr. Stratman advised against reliance upon a patient’s self-reported body weight, which may diverge substantially from reality. “Get yourself a good office scale – they’re not that expensive – and use it when prescribing drugs with a tight therapeutic window,” he urged.
Another key to minimizing retinopathy risk in patients on hydroxychloroquine is to pay careful attention to how long they’ve been on the drug. As the years go by in patients being treated for cutaneous lupus or other dermatologic disorders where decades-long therapy is often a mainstay, it’s important to check with patients and make sure they’re getting annual ophthalmologic screening for irreversible retinal toxicity by both threshold visual fields and spectral domain optical coherence tomography. In the large, practice-changing retrospective study, patients on hydroxychloroquine at 4.0-5.0 mg/kg daily had a prevalence of retinopathy of less than 2% during the first 10 years of therapy, but the rate shot up to nearly 20% after 20 years of use, Dr. Stratman observed.
He highlighted as helpful an updated review of the use of hydroxychloroquine in dermatology recently published by Anthony P. Fernandez, MD, PhD, of the department of dermatology at the Cleveland Clinic (J Am Acad Dermatol. 2017 Jun;76[6]:1176-82).
Dr. Fernandez recommends following the AAO guidance to dose the drug at 5.0 mg/kg or less of actual body weight in thin or normal-weight patients; however, he departed from the ophthalmologists with regard to treatment of obese patients. Because dosing based on actual weight could potentially lead to relative overdosing in obese patients, in that growing population he recommends calculating the dose based upon 5.0 mg/kg of actual body weight, as well as the dose based on 6.5 mg/kg of ideal body weight, then prescribing the lower of the two, up to a maximum of 400 mg/day.
“The current recommendation is really about not overdosing thin patients. Basically, dosing is not so difficult for obese people because if you weigh more than 175 pounds, you’re going to get 400 mg/day,” Dr. Stratman explained.
That 400 mg/day ceiling is not cast in stone, he continued. The guideline recommends that, if a patient is a nonresponder to several months of hydroxychloroquine at 400 mg/day, it’s worthwhile to order a drug blood level. If it’s not above the efficacy threshold of more than 750 ng/mL, it’s appropriate to titrate up.
Protecting against prednisone-induced gastritis
“We underprotect the gut,” Dr. Stratman asserted.
He referred to a recent comprehensive dermatologic review of the prevention and management of glucocorticoid-related side effects, especially the part on peptic ulcer disease (J Am Acad Dermatol. 2017 Jan;76[1]:11-6). This is an issue that heretofore hadn’t been much emphasized in the dermatology literature.
“I read this and thought, ‘Gosh, I’m not really having a conversation with my patients about a review of systems for gut protection as I should. And I certainly haven’t been thinking about prescribing PPIs [proton pump inhibitors] for my patients,’” he recalled.
Dr. Stratman polled his Hawaii Dermatology Seminar audience as to who had ever prescribed a PPI. Most indicated with their electronic clickers that they had never done so.
“This is what a practice gap is,” he commented. “You read the literature and you say, ‘Oh, I guess that makes sense. Maybe I should be doing that more often, or making sure it gets done.’”
“I don’t want to come across as saying, ‘For everybody we put on prednisone we should be giving vitamin D, calcium, and a PPI.’ That’s not the message. The message is, assess your patient – or make sure your patient is being assessed – for risk of peptic ulcer disease. And if you don’t feel comfortable prescribing a PPI, please get the patient connected with their primary care provider, who should,” Dr. Stratman said.
The authors of the dermatology review made a case for screening for GI risk factors in every patient who is going to receive an oral glucocorticoid. The ones who absolutely should be prescribed a PPI unless contraindicated include patients who are taking daily aspirin or NSAIDs for an essential reason, such as cardiovascular protection or significant arthritic pain. The authors suggest consideration of a PPI in patients with other, less potent risk factors for peptic ulcer disease, including a history of ulcer disease, gastroesophageal reflux disease, Barrett’s esophagus, heavy smoking, heavy alcohol consumption, age greater than 65, and concomitant use of other medications with an associated risk of peptic ulcer disease – such as bisphosphonates, “which you may have just put them on to protect their bones,” Dr. Stratman noted.
Of course, PPIs come with side effects of their own, including increased fracture risk, Clostridium difficile infections, and rebound acid secretion.
Dr. Stratman reported having no financial conflicts regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
What’s new in the latest melanoma guidelines
KAUAI, HAWAII – Melanoma , resulting in an evidence-based improved prognosis for many of them, Laura Korb Ferris, MD, PhD, said at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
Dr. Ferris, of the department of dermatology, University of Pittsburgh, highlighted some of the key changes in the eighth edition of the AJCC staging manual, which is now in effect. She also described the clinical implications of important updates introduced in the 2018 National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and management of melanoma.
The AJCC eighth edition
The eighth edition is built upon an AJCC database of more than 46,000 patients with stage I-III melanoma diagnosed since 1998 at 10 academic medical centers. The AJCC panel made no changes in stage IV melanoma guidance because the newer targeted therapies have rapidly changed treatment outcomes in that setting and longer follow-up is needed to assess the full impact.
The current edition of the AJCC melanoma staging manual creates a new subcategory within pathologic stage III. In the melanoma staging world, that’s exciting news, especially because this change has important implications for prognosis.
This fourth subcategory, stage IIID, is for melanomas, which in the Tumor, Nodes, Metastasis (TNM) classification scheme, are primary tumor stage T4b, meaning greater than 4.0 mm in thickness and with ulceration; regional lymph node N3a, b, or c, based upon the number of metastatic nodes involved and whether they were clinically occult nodal metastases detected by sentinel lymph node biopsy (SLNB) or clinically detected; and M0, meaning no distant metastatic disease. In the 8th edition, the AJCC staging system can be applied in patients with T2 through T4 primary melanoma only if they have undergone SLNB.
This new approach to stage III disease makes for more homogeneous patient subgroups, which in turn provides much better stratification of prognosis than was possible in the seventh edition of the AJCC staging manual, which dates back to 2010. Most strikingly, the 5-year melanoma-specific survival rate for patients with stage IIIA disease was 78% in the seventh edition of AJCC, but it climbs to 93% in the eighth edition. For patients with stage IIIB melanoma, 5-year melanoma-specific survival improved from 59% in the seventh edition to 83% in the current iteration, while in stage IIIC, the jump is from 40% to 69%. All this is made possible because the eighth edition separates out patients with the new stage IIID, whose 5-year melanoma-specific survival is only 32%, Dr. Ferris explained.
Among the other key points to remember about the eighth edition of AJCC:
- Tumor thickness is now measured to the nearest 0.1 mm rather than to the nearest 0.01 mm, as previously. Thus, a 0.75-mm-thick melanoma is now rounded up to 0.8 mm, while a 0.74-mm melanoma becomes a 0.7-mm tumor.
- Based upon recent evidence, tumors that are 0.8-1.0 mm thick, with or without ulceration, are now classified at T1b. So are ulcerated lesions that are less than 0.8 mm.
- Dermal mitotic rate is no longer used in staging T1 tumors, although it’s still supposed to be included in pathology reports.
- The T category definitions of primary tumors have been clarified in the eighth edition. A tumor is now classified as T0 only if there is no evidence of a primary tumor. Tx is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained by curettage. Tis is utilized for melanoma in situ.
- The N subcategory definitions of regional nodal status have been revised. Microsatellites, clinical satellites, and in-transit metastases are now categorized as N1c, N2c, or N3c based upon the number of tumor-involved regional lymph nodes. These features are no longer defined by their size or distance from the primary tumor.
2018 NCCN melanoma guidelines
The guidelines have been revised to recommend against SLNB if a patient’s pretest probability of finding a positive SLN is less than 5%. This includes patients who have a clinical stage IA/T1a melanoma with a Breslow thickness of less than 0.8 mm without ulceration.
There is to be no SLNB in patients with microsatellites, clinical satellites, or in-transit metastases because SLN status has no prognostic significance in this situation.
Routine ordering of prognostic genetic tests for BRAF or the multigene test panels that are now commercially available is not recommended except to guide systemic therapy or to determine if a patient is a candidate for a specific clinical trial. “Basically, there is not a place to use this information in the NCCN guidelines,” according to the dermatologist.
What about completion lymphadenectomy in the SLN-positive melanoma patient?
Completion lymph node dissection looks increasingly like a procedure in search of an indication. Results of the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–II (MSLT-II) demonstrated not even a hint of a difference in 3-year melanoma-specific survival in 1,934 melanoma patients with sentinel lymph node metastases regardless of whether they were randomized to immediate completion lymph node dissection or ultrasound-based nodal monitoring. Moreover, completion lymphadenectomy was associated with significant morbidity: a 24.1% incidence of lymphedema, compared with a 6.3% rate in the observation group (N Engl J Med. 2017 Jun 8;376[23]:2211-22).
On the other hand, Dr. Ferris noted that many newer drugs are being approved for the treatment of stage III melanoma, and in all the pivotal clinical trials, patients had to have undergone completion lymph node dissection as a condition of participation. So the surgery becomes a consideration if physicians want to use the newer agents the way they were used successfully in the trials.
The full eighth edition of the AJCC cancer staging manual is available for purchase. For physicians with a specific interest in melanoma, Dr. Ferris recommended as an extremely useful alternative the AJCC expert writing panel’s free downloadable summary of the evidence-based changes made in melanoma staging (CA Cancer J Clin. 2017 Nov;67[6]:472-92). The 2018 NCCN guidelines (Melanoma. Version 1.2018 Oct. 11, 2017) are available for free (www.NCCN.org).
Dr. Ferris reported serving as a consultant to DermTech.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Melanoma , resulting in an evidence-based improved prognosis for many of them, Laura Korb Ferris, MD, PhD, said at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
Dr. Ferris, of the department of dermatology, University of Pittsburgh, highlighted some of the key changes in the eighth edition of the AJCC staging manual, which is now in effect. She also described the clinical implications of important updates introduced in the 2018 National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and management of melanoma.
The AJCC eighth edition
The eighth edition is built upon an AJCC database of more than 46,000 patients with stage I-III melanoma diagnosed since 1998 at 10 academic medical centers. The AJCC panel made no changes in stage IV melanoma guidance because the newer targeted therapies have rapidly changed treatment outcomes in that setting and longer follow-up is needed to assess the full impact.
The current edition of the AJCC melanoma staging manual creates a new subcategory within pathologic stage III. In the melanoma staging world, that’s exciting news, especially because this change has important implications for prognosis.
This fourth subcategory, stage IIID, is for melanomas, which in the Tumor, Nodes, Metastasis (TNM) classification scheme, are primary tumor stage T4b, meaning greater than 4.0 mm in thickness and with ulceration; regional lymph node N3a, b, or c, based upon the number of metastatic nodes involved and whether they were clinically occult nodal metastases detected by sentinel lymph node biopsy (SLNB) or clinically detected; and M0, meaning no distant metastatic disease. In the 8th edition, the AJCC staging system can be applied in patients with T2 through T4 primary melanoma only if they have undergone SLNB.
This new approach to stage III disease makes for more homogeneous patient subgroups, which in turn provides much better stratification of prognosis than was possible in the seventh edition of the AJCC staging manual, which dates back to 2010. Most strikingly, the 5-year melanoma-specific survival rate for patients with stage IIIA disease was 78% in the seventh edition of AJCC, but it climbs to 93% in the eighth edition. For patients with stage IIIB melanoma, 5-year melanoma-specific survival improved from 59% in the seventh edition to 83% in the current iteration, while in stage IIIC, the jump is from 40% to 69%. All this is made possible because the eighth edition separates out patients with the new stage IIID, whose 5-year melanoma-specific survival is only 32%, Dr. Ferris explained.
Among the other key points to remember about the eighth edition of AJCC:
- Tumor thickness is now measured to the nearest 0.1 mm rather than to the nearest 0.01 mm, as previously. Thus, a 0.75-mm-thick melanoma is now rounded up to 0.8 mm, while a 0.74-mm melanoma becomes a 0.7-mm tumor.
- Based upon recent evidence, tumors that are 0.8-1.0 mm thick, with or without ulceration, are now classified at T1b. So are ulcerated lesions that are less than 0.8 mm.
- Dermal mitotic rate is no longer used in staging T1 tumors, although it’s still supposed to be included in pathology reports.
- The T category definitions of primary tumors have been clarified in the eighth edition. A tumor is now classified as T0 only if there is no evidence of a primary tumor. Tx is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained by curettage. Tis is utilized for melanoma in situ.
- The N subcategory definitions of regional nodal status have been revised. Microsatellites, clinical satellites, and in-transit metastases are now categorized as N1c, N2c, or N3c based upon the number of tumor-involved regional lymph nodes. These features are no longer defined by their size or distance from the primary tumor.
2018 NCCN melanoma guidelines
The guidelines have been revised to recommend against SLNB if a patient’s pretest probability of finding a positive SLN is less than 5%. This includes patients who have a clinical stage IA/T1a melanoma with a Breslow thickness of less than 0.8 mm without ulceration.
There is to be no SLNB in patients with microsatellites, clinical satellites, or in-transit metastases because SLN status has no prognostic significance in this situation.
Routine ordering of prognostic genetic tests for BRAF or the multigene test panels that are now commercially available is not recommended except to guide systemic therapy or to determine if a patient is a candidate for a specific clinical trial. “Basically, there is not a place to use this information in the NCCN guidelines,” according to the dermatologist.
What about completion lymphadenectomy in the SLN-positive melanoma patient?
Completion lymph node dissection looks increasingly like a procedure in search of an indication. Results of the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–II (MSLT-II) demonstrated not even a hint of a difference in 3-year melanoma-specific survival in 1,934 melanoma patients with sentinel lymph node metastases regardless of whether they were randomized to immediate completion lymph node dissection or ultrasound-based nodal monitoring. Moreover, completion lymphadenectomy was associated with significant morbidity: a 24.1% incidence of lymphedema, compared with a 6.3% rate in the observation group (N Engl J Med. 2017 Jun 8;376[23]:2211-22).
On the other hand, Dr. Ferris noted that many newer drugs are being approved for the treatment of stage III melanoma, and in all the pivotal clinical trials, patients had to have undergone completion lymph node dissection as a condition of participation. So the surgery becomes a consideration if physicians want to use the newer agents the way they were used successfully in the trials.
The full eighth edition of the AJCC cancer staging manual is available for purchase. For physicians with a specific interest in melanoma, Dr. Ferris recommended as an extremely useful alternative the AJCC expert writing panel’s free downloadable summary of the evidence-based changes made in melanoma staging (CA Cancer J Clin. 2017 Nov;67[6]:472-92). The 2018 NCCN guidelines (Melanoma. Version 1.2018 Oct. 11, 2017) are available for free (www.NCCN.org).
Dr. Ferris reported serving as a consultant to DermTech.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Melanoma , resulting in an evidence-based improved prognosis for many of them, Laura Korb Ferris, MD, PhD, said at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
Dr. Ferris, of the department of dermatology, University of Pittsburgh, highlighted some of the key changes in the eighth edition of the AJCC staging manual, which is now in effect. She also described the clinical implications of important updates introduced in the 2018 National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and management of melanoma.
The AJCC eighth edition
The eighth edition is built upon an AJCC database of more than 46,000 patients with stage I-III melanoma diagnosed since 1998 at 10 academic medical centers. The AJCC panel made no changes in stage IV melanoma guidance because the newer targeted therapies have rapidly changed treatment outcomes in that setting and longer follow-up is needed to assess the full impact.
The current edition of the AJCC melanoma staging manual creates a new subcategory within pathologic stage III. In the melanoma staging world, that’s exciting news, especially because this change has important implications for prognosis.
This fourth subcategory, stage IIID, is for melanomas, which in the Tumor, Nodes, Metastasis (TNM) classification scheme, are primary tumor stage T4b, meaning greater than 4.0 mm in thickness and with ulceration; regional lymph node N3a, b, or c, based upon the number of metastatic nodes involved and whether they were clinically occult nodal metastases detected by sentinel lymph node biopsy (SLNB) or clinically detected; and M0, meaning no distant metastatic disease. In the 8th edition, the AJCC staging system can be applied in patients with T2 through T4 primary melanoma only if they have undergone SLNB.
This new approach to stage III disease makes for more homogeneous patient subgroups, which in turn provides much better stratification of prognosis than was possible in the seventh edition of the AJCC staging manual, which dates back to 2010. Most strikingly, the 5-year melanoma-specific survival rate for patients with stage IIIA disease was 78% in the seventh edition of AJCC, but it climbs to 93% in the eighth edition. For patients with stage IIIB melanoma, 5-year melanoma-specific survival improved from 59% in the seventh edition to 83% in the current iteration, while in stage IIIC, the jump is from 40% to 69%. All this is made possible because the eighth edition separates out patients with the new stage IIID, whose 5-year melanoma-specific survival is only 32%, Dr. Ferris explained.
Among the other key points to remember about the eighth edition of AJCC:
- Tumor thickness is now measured to the nearest 0.1 mm rather than to the nearest 0.01 mm, as previously. Thus, a 0.75-mm-thick melanoma is now rounded up to 0.8 mm, while a 0.74-mm melanoma becomes a 0.7-mm tumor.
- Based upon recent evidence, tumors that are 0.8-1.0 mm thick, with or without ulceration, are now classified at T1b. So are ulcerated lesions that are less than 0.8 mm.
- Dermal mitotic rate is no longer used in staging T1 tumors, although it’s still supposed to be included in pathology reports.
- The T category definitions of primary tumors have been clarified in the eighth edition. A tumor is now classified as T0 only if there is no evidence of a primary tumor. Tx is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained by curettage. Tis is utilized for melanoma in situ.
- The N subcategory definitions of regional nodal status have been revised. Microsatellites, clinical satellites, and in-transit metastases are now categorized as N1c, N2c, or N3c based upon the number of tumor-involved regional lymph nodes. These features are no longer defined by their size or distance from the primary tumor.
2018 NCCN melanoma guidelines
The guidelines have been revised to recommend against SLNB if a patient’s pretest probability of finding a positive SLN is less than 5%. This includes patients who have a clinical stage IA/T1a melanoma with a Breslow thickness of less than 0.8 mm without ulceration.
There is to be no SLNB in patients with microsatellites, clinical satellites, or in-transit metastases because SLN status has no prognostic significance in this situation.
Routine ordering of prognostic genetic tests for BRAF or the multigene test panels that are now commercially available is not recommended except to guide systemic therapy or to determine if a patient is a candidate for a specific clinical trial. “Basically, there is not a place to use this information in the NCCN guidelines,” according to the dermatologist.
What about completion lymphadenectomy in the SLN-positive melanoma patient?
Completion lymph node dissection looks increasingly like a procedure in search of an indication. Results of the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–II (MSLT-II) demonstrated not even a hint of a difference in 3-year melanoma-specific survival in 1,934 melanoma patients with sentinel lymph node metastases regardless of whether they were randomized to immediate completion lymph node dissection or ultrasound-based nodal monitoring. Moreover, completion lymphadenectomy was associated with significant morbidity: a 24.1% incidence of lymphedema, compared with a 6.3% rate in the observation group (N Engl J Med. 2017 Jun 8;376[23]:2211-22).
On the other hand, Dr. Ferris noted that many newer drugs are being approved for the treatment of stage III melanoma, and in all the pivotal clinical trials, patients had to have undergone completion lymph node dissection as a condition of participation. So the surgery becomes a consideration if physicians want to use the newer agents the way they were used successfully in the trials.
The full eighth edition of the AJCC cancer staging manual is available for purchase. For physicians with a specific interest in melanoma, Dr. Ferris recommended as an extremely useful alternative the AJCC expert writing panel’s free downloadable summary of the evidence-based changes made in melanoma staging (CA Cancer J Clin. 2017 Nov;67[6]:472-92). The 2018 NCCN guidelines (Melanoma. Version 1.2018 Oct. 11, 2017) are available for free (www.NCCN.org).
Dr. Ferris reported serving as a consultant to DermTech.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Cancer-related clinical pearls from pediatric dermatology
KAUAI, HAWAII – Every child diagnosed with medulloblastoma deserves a careful dermatologic evaluation for possible comorbid basal cell nevus syndrome, according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital and Harvard Medical School.
“Medulloblastoma occurs in 10%-20% of patients with basal cell nevus syndrome and can be the presenting sign. So if a patient with basal cell nevus syndrome gets medulloblastoma, it usually occurs within the first year of life – and it can be the first thing you see,” she said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Huang presented a series of pediatric dermatology clinical pearls focused not only on basal cell nevus syndrome (BCNS) and medulloblastoma, but also on the implications of skin-limited Langerhans cell histiocytosis, how to recognize and treat drug-induced follicular eruptions in pediatric patients on targeted anticancer therapies, and when to suspect Demodex folliculitis in immunosuppressed patients.
Skin-limited Langerhans cell histiocytosis
Around 10%-20% of patients with Langerhans cell histiocytosis (LCH) have the skin-limited form of the malignancy. These are patients who, after a thorough workup, have a normal CBC, skeletal survey, and liver function tests; essentially, no evidence of multisystem disease.
“It’s very rare for patients who present with skin-limited LCH alone to develop multisystem disease and to require chemotherapy or other more aggressive treatment,” Dr. Huang said. “I think that skin-limited LCH is probably a separate entity with its own natural history distinct from multisystem disease. We can see that with current genomic testing: in multisystem LCH, BRAF mutations are identified in at least half of patients, but very few with skin-limited disease express those mutations.
“The clinical pearl here is if you have a patient with skin-limited LCH it very rarely progresses to multisystem involvement. It’s associated with a good prognosis. That doesn’t mean you shouldn’t monitor them, but I think it can be reassuring information for the family,” she said.
Basal cell nevus syndrome and medulloblastoma
“Half of cases of medulloblastoma are associated with mutations in the sonic hedgehog pathway – and a subset of that group has basal cell nevus syndrome,” Dr. Huang said.
BCNS is not a diagnosis frequently made by oncologists, who typically dismiss the multitude of lesions as skin tags, which they often mimic in both appearance and location, particularly on the neck and intertriginous areas. So it’s useful for dermatologists to establish a good referral relationship with their local oncologists.
“As dermatologists it’s really important to recognize not only the major features of basal cell nevus syndrome, but also the associated findings because we can really help in making this diagnosis early,” Dr. Huang stressed.
Early diagnosis of BCNS is a high priority for two reasons: to start treatment aimed at reducing development of basal cell carcinomas, and because radiation therapy for their medulloblastoma is contraindicated in patients with BCNS because it boosts their skin cancer burden.
BCNS is caused by mutations in the PTCH (Patched) gene found on chromosome arm 9q. The major features of BCNS include odontogenic keratocysts, palmoplantar pits, ectopic calcification, and, of course, basal cell carcinomas. The associated findings in BCNS, in addition to medulloblastoma, include macrocephaly and dysmorphic features such as cleft lip or palate, frontal bossing, and hypertelorism.
“I’ve treated a hundred at a time. It’s incredibly successful. It’s locally destructive. It leaves a little bit of hypopigmentation but no scar, which the CO2 laser will do in this instance. It’s actually a pretty cool modality,” said Dr. Eichenfield, professor of dermatology and pediatrics at Rady Children’s Hospital and the University of California, San Diego.
Follicular eruptions in cancer patients on MAPK inhibitors
Cutaneous reactions to anticancer drugs aimed at inhibiting the key MAPK (mitogen-activated protein kinase) pathway in children are common and diverse. Dr. Huang focused on the most common one: follicular eruptions, which occur in up to 80% of pediatric cancer patients on targeted therapy. These eruptions can express themselves in a variety of ways and are easily mistaken for comedonal acne, varicella zoster infection, herpes simplex, or bacterial folliculitis.
The key clues are highly suggestive that a follicular eruption in a child on targeted anticancer therapy is caused by the drug and not something else are the eruption’s symmetric distribution, that it’s truly follicular upon close inspection, and the timing: The eruption typically begins 2-3 weeks after initiation of therapy or within a week after a dose escalation.
Anti-inflammatory agents are the treatment mainstay. Treatment of the cutaneous eruption often is successful without need to discontinue the patient’s MAPK inhibitor.
“Even though some of these eruptions look comedonal, they’re not. It’s not a follicular plugging disorder, it’s an inflammatory condition. Topical steroids, oral tetracyclines, and dilute bleach baths all work pretty well. I haven’t had good experiences with keratolytics like tretinoin cream and benzoyl peroxide; they’re less effective. Dose reduction is the last resort for these patients. Often they are very sick. They need the drug and I think the last thing we want to do is take them off it,” Dr. Huang said.
She has observed that prepubertal children are more likely to have an eczematous reaction to their targeted anticancer therapy than a follicular eruption.
D. folliculitis in immunocompromised patients
“The clinical pearl here is to strongly consider the diagnosis of Demodex folliculitis in an immunosuppresed patient with an itchy acneiform eruption,” Dr. Huang said.
Demodex is a human mite which is part of the normal skin flora. She called it “a great mimicker”: It can cause dermatoses mistaken for rosacea, acne, seborrheic dermatitis, perioral facial dermatitis, blepharitis, and acute graft-versus-host disease.
In the setting of a young, immunosuppressed patient who develops an acneiform eruption, the differential diagnosis is lengthy and includes steroid-induced acne, a cutaneous reaction to targeted anticancer therapy, gram-negative folliculitis secondary to long-term antibiotic therapy, and Pityrosporum folliculitis, as well as D. folliculitis.
Demodex and P. folliculitis are the two acneiform dermatoses where itch figures prominently. A couple of clues are helpful in differentiating the two conditions: P. folliculitis often involves the chest and back, while D. folliculitis generally spares the trunk and is focused on the face and neck. And D. folliculitis typically arises when immunosuppression is weaned. Overgrowth of the mites occurs during immunosuppression, then as the immunosuppression is lifted a prominent inflammatory response with an acne-like appearance occurs.
Dr. Huang usually sticks with topical therapies for D. folliculitis. These include topical sulfur 5%, permethrin 5%, metronidazole, and/or ivermectin. If a young patient is unresponsive to this panoply of topical agents, she resorts to a single dose of oral ivermectin at 0.2 mg/kg, usually with good effect.
Dr. Huang reported having no financial conflicts of interest regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Every child diagnosed with medulloblastoma deserves a careful dermatologic evaluation for possible comorbid basal cell nevus syndrome, according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital and Harvard Medical School.
“Medulloblastoma occurs in 10%-20% of patients with basal cell nevus syndrome and can be the presenting sign. So if a patient with basal cell nevus syndrome gets medulloblastoma, it usually occurs within the first year of life – and it can be the first thing you see,” she said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Huang presented a series of pediatric dermatology clinical pearls focused not only on basal cell nevus syndrome (BCNS) and medulloblastoma, but also on the implications of skin-limited Langerhans cell histiocytosis, how to recognize and treat drug-induced follicular eruptions in pediatric patients on targeted anticancer therapies, and when to suspect Demodex folliculitis in immunosuppressed patients.
Skin-limited Langerhans cell histiocytosis
Around 10%-20% of patients with Langerhans cell histiocytosis (LCH) have the skin-limited form of the malignancy. These are patients who, after a thorough workup, have a normal CBC, skeletal survey, and liver function tests; essentially, no evidence of multisystem disease.
“It’s very rare for patients who present with skin-limited LCH alone to develop multisystem disease and to require chemotherapy or other more aggressive treatment,” Dr. Huang said. “I think that skin-limited LCH is probably a separate entity with its own natural history distinct from multisystem disease. We can see that with current genomic testing: in multisystem LCH, BRAF mutations are identified in at least half of patients, but very few with skin-limited disease express those mutations.
“The clinical pearl here is if you have a patient with skin-limited LCH it very rarely progresses to multisystem involvement. It’s associated with a good prognosis. That doesn’t mean you shouldn’t monitor them, but I think it can be reassuring information for the family,” she said.
Basal cell nevus syndrome and medulloblastoma
“Half of cases of medulloblastoma are associated with mutations in the sonic hedgehog pathway – and a subset of that group has basal cell nevus syndrome,” Dr. Huang said.
BCNS is not a diagnosis frequently made by oncologists, who typically dismiss the multitude of lesions as skin tags, which they often mimic in both appearance and location, particularly on the neck and intertriginous areas. So it’s useful for dermatologists to establish a good referral relationship with their local oncologists.
“As dermatologists it’s really important to recognize not only the major features of basal cell nevus syndrome, but also the associated findings because we can really help in making this diagnosis early,” Dr. Huang stressed.
Early diagnosis of BCNS is a high priority for two reasons: to start treatment aimed at reducing development of basal cell carcinomas, and because radiation therapy for their medulloblastoma is contraindicated in patients with BCNS because it boosts their skin cancer burden.
BCNS is caused by mutations in the PTCH (Patched) gene found on chromosome arm 9q. The major features of BCNS include odontogenic keratocysts, palmoplantar pits, ectopic calcification, and, of course, basal cell carcinomas. The associated findings in BCNS, in addition to medulloblastoma, include macrocephaly and dysmorphic features such as cleft lip or palate, frontal bossing, and hypertelorism.
“I’ve treated a hundred at a time. It’s incredibly successful. It’s locally destructive. It leaves a little bit of hypopigmentation but no scar, which the CO2 laser will do in this instance. It’s actually a pretty cool modality,” said Dr. Eichenfield, professor of dermatology and pediatrics at Rady Children’s Hospital and the University of California, San Diego.
Follicular eruptions in cancer patients on MAPK inhibitors
Cutaneous reactions to anticancer drugs aimed at inhibiting the key MAPK (mitogen-activated protein kinase) pathway in children are common and diverse. Dr. Huang focused on the most common one: follicular eruptions, which occur in up to 80% of pediatric cancer patients on targeted therapy. These eruptions can express themselves in a variety of ways and are easily mistaken for comedonal acne, varicella zoster infection, herpes simplex, or bacterial folliculitis.
The key clues are highly suggestive that a follicular eruption in a child on targeted anticancer therapy is caused by the drug and not something else are the eruption’s symmetric distribution, that it’s truly follicular upon close inspection, and the timing: The eruption typically begins 2-3 weeks after initiation of therapy or within a week after a dose escalation.
Anti-inflammatory agents are the treatment mainstay. Treatment of the cutaneous eruption often is successful without need to discontinue the patient’s MAPK inhibitor.
“Even though some of these eruptions look comedonal, they’re not. It’s not a follicular plugging disorder, it’s an inflammatory condition. Topical steroids, oral tetracyclines, and dilute bleach baths all work pretty well. I haven’t had good experiences with keratolytics like tretinoin cream and benzoyl peroxide; they’re less effective. Dose reduction is the last resort for these patients. Often they are very sick. They need the drug and I think the last thing we want to do is take them off it,” Dr. Huang said.
She has observed that prepubertal children are more likely to have an eczematous reaction to their targeted anticancer therapy than a follicular eruption.
D. folliculitis in immunocompromised patients
“The clinical pearl here is to strongly consider the diagnosis of Demodex folliculitis in an immunosuppresed patient with an itchy acneiform eruption,” Dr. Huang said.
Demodex is a human mite which is part of the normal skin flora. She called it “a great mimicker”: It can cause dermatoses mistaken for rosacea, acne, seborrheic dermatitis, perioral facial dermatitis, blepharitis, and acute graft-versus-host disease.
In the setting of a young, immunosuppressed patient who develops an acneiform eruption, the differential diagnosis is lengthy and includes steroid-induced acne, a cutaneous reaction to targeted anticancer therapy, gram-negative folliculitis secondary to long-term antibiotic therapy, and Pityrosporum folliculitis, as well as D. folliculitis.
Demodex and P. folliculitis are the two acneiform dermatoses where itch figures prominently. A couple of clues are helpful in differentiating the two conditions: P. folliculitis often involves the chest and back, while D. folliculitis generally spares the trunk and is focused on the face and neck. And D. folliculitis typically arises when immunosuppression is weaned. Overgrowth of the mites occurs during immunosuppression, then as the immunosuppression is lifted a prominent inflammatory response with an acne-like appearance occurs.
Dr. Huang usually sticks with topical therapies for D. folliculitis. These include topical sulfur 5%, permethrin 5%, metronidazole, and/or ivermectin. If a young patient is unresponsive to this panoply of topical agents, she resorts to a single dose of oral ivermectin at 0.2 mg/kg, usually with good effect.
Dr. Huang reported having no financial conflicts of interest regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Every child diagnosed with medulloblastoma deserves a careful dermatologic evaluation for possible comorbid basal cell nevus syndrome, according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital and Harvard Medical School.
“Medulloblastoma occurs in 10%-20% of patients with basal cell nevus syndrome and can be the presenting sign. So if a patient with basal cell nevus syndrome gets medulloblastoma, it usually occurs within the first year of life – and it can be the first thing you see,” she said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Huang presented a series of pediatric dermatology clinical pearls focused not only on basal cell nevus syndrome (BCNS) and medulloblastoma, but also on the implications of skin-limited Langerhans cell histiocytosis, how to recognize and treat drug-induced follicular eruptions in pediatric patients on targeted anticancer therapies, and when to suspect Demodex folliculitis in immunosuppressed patients.
Skin-limited Langerhans cell histiocytosis
Around 10%-20% of patients with Langerhans cell histiocytosis (LCH) have the skin-limited form of the malignancy. These are patients who, after a thorough workup, have a normal CBC, skeletal survey, and liver function tests; essentially, no evidence of multisystem disease.
“It’s very rare for patients who present with skin-limited LCH alone to develop multisystem disease and to require chemotherapy or other more aggressive treatment,” Dr. Huang said. “I think that skin-limited LCH is probably a separate entity with its own natural history distinct from multisystem disease. We can see that with current genomic testing: in multisystem LCH, BRAF mutations are identified in at least half of patients, but very few with skin-limited disease express those mutations.
“The clinical pearl here is if you have a patient with skin-limited LCH it very rarely progresses to multisystem involvement. It’s associated with a good prognosis. That doesn’t mean you shouldn’t monitor them, but I think it can be reassuring information for the family,” she said.
Basal cell nevus syndrome and medulloblastoma
“Half of cases of medulloblastoma are associated with mutations in the sonic hedgehog pathway – and a subset of that group has basal cell nevus syndrome,” Dr. Huang said.
BCNS is not a diagnosis frequently made by oncologists, who typically dismiss the multitude of lesions as skin tags, which they often mimic in both appearance and location, particularly on the neck and intertriginous areas. So it’s useful for dermatologists to establish a good referral relationship with their local oncologists.
“As dermatologists it’s really important to recognize not only the major features of basal cell nevus syndrome, but also the associated findings because we can really help in making this diagnosis early,” Dr. Huang stressed.
Early diagnosis of BCNS is a high priority for two reasons: to start treatment aimed at reducing development of basal cell carcinomas, and because radiation therapy for their medulloblastoma is contraindicated in patients with BCNS because it boosts their skin cancer burden.
BCNS is caused by mutations in the PTCH (Patched) gene found on chromosome arm 9q. The major features of BCNS include odontogenic keratocysts, palmoplantar pits, ectopic calcification, and, of course, basal cell carcinomas. The associated findings in BCNS, in addition to medulloblastoma, include macrocephaly and dysmorphic features such as cleft lip or palate, frontal bossing, and hypertelorism.
“I’ve treated a hundred at a time. It’s incredibly successful. It’s locally destructive. It leaves a little bit of hypopigmentation but no scar, which the CO2 laser will do in this instance. It’s actually a pretty cool modality,” said Dr. Eichenfield, professor of dermatology and pediatrics at Rady Children’s Hospital and the University of California, San Diego.
Follicular eruptions in cancer patients on MAPK inhibitors
Cutaneous reactions to anticancer drugs aimed at inhibiting the key MAPK (mitogen-activated protein kinase) pathway in children are common and diverse. Dr. Huang focused on the most common one: follicular eruptions, which occur in up to 80% of pediatric cancer patients on targeted therapy. These eruptions can express themselves in a variety of ways and are easily mistaken for comedonal acne, varicella zoster infection, herpes simplex, or bacterial folliculitis.
The key clues are highly suggestive that a follicular eruption in a child on targeted anticancer therapy is caused by the drug and not something else are the eruption’s symmetric distribution, that it’s truly follicular upon close inspection, and the timing: The eruption typically begins 2-3 weeks after initiation of therapy or within a week after a dose escalation.
Anti-inflammatory agents are the treatment mainstay. Treatment of the cutaneous eruption often is successful without need to discontinue the patient’s MAPK inhibitor.
“Even though some of these eruptions look comedonal, they’re not. It’s not a follicular plugging disorder, it’s an inflammatory condition. Topical steroids, oral tetracyclines, and dilute bleach baths all work pretty well. I haven’t had good experiences with keratolytics like tretinoin cream and benzoyl peroxide; they’re less effective. Dose reduction is the last resort for these patients. Often they are very sick. They need the drug and I think the last thing we want to do is take them off it,” Dr. Huang said.
She has observed that prepubertal children are more likely to have an eczematous reaction to their targeted anticancer therapy than a follicular eruption.
D. folliculitis in immunocompromised patients
“The clinical pearl here is to strongly consider the diagnosis of Demodex folliculitis in an immunosuppresed patient with an itchy acneiform eruption,” Dr. Huang said.
Demodex is a human mite which is part of the normal skin flora. She called it “a great mimicker”: It can cause dermatoses mistaken for rosacea, acne, seborrheic dermatitis, perioral facial dermatitis, blepharitis, and acute graft-versus-host disease.
In the setting of a young, immunosuppressed patient who develops an acneiform eruption, the differential diagnosis is lengthy and includes steroid-induced acne, a cutaneous reaction to targeted anticancer therapy, gram-negative folliculitis secondary to long-term antibiotic therapy, and Pityrosporum folliculitis, as well as D. folliculitis.
Demodex and P. folliculitis are the two acneiform dermatoses where itch figures prominently. A couple of clues are helpful in differentiating the two conditions: P. folliculitis often involves the chest and back, while D. folliculitis generally spares the trunk and is focused on the face and neck. And D. folliculitis typically arises when immunosuppression is weaned. Overgrowth of the mites occurs during immunosuppression, then as the immunosuppression is lifted a prominent inflammatory response with an acne-like appearance occurs.
Dr. Huang usually sticks with topical therapies for D. folliculitis. These include topical sulfur 5%, permethrin 5%, metronidazole, and/or ivermectin. If a young patient is unresponsive to this panoply of topical agents, she resorts to a single dose of oral ivermectin at 0.2 mg/kg, usually with good effect.
Dr. Huang reported having no financial conflicts of interest regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR