User login
CO2 laser guided by confocal microscopy effectively treated superficial BCC
DALLAS – The use of CO2 laser ablation guided by reflectance confocal microscopy is an effective, minimally invasive treatment for superficial and early nodular basal cell carcinoma (BCC), according to results from an ongoing study.
“While surgery is the gold standard for many basal cell carcinomas, nonsurgical therapies may be a good option for the superficial and early nodular subtypes,” lead study author Anthony M. Rossi, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “Laser ablation was used many years ago, so this is not a novel concept, but we’re bringing it back and we’re trying to use confocal microscopy to hone in on the basal cell and selectively target the tumor.”
For the current analysis, he and his associates used with a mean age of 55 years. Of the 20 lesions, 18 were located on the limbs and trunk, while two were on the head and neck. The median lesion diameter was 7 mm. Prior to laser ablation, the researchers performed reflectance confocal microscopy to define lateral and deep margins and define the laser parameters.
The median number of laser passes was three, and ranged from two to eight, delivered at a fluence of 7.5 J/cm2. Reflectance confocal microscopy was repeated immediately after the laser treatment to the skin wound margins and deep margins, and it was performed every 3-6 months thereafter. “If you do confocal microscopy too early, you’ll see mainly inflammation and you may see residual tumor that hasn’t been fully resolved yet,” Dr. Rossi said.
As for future directions, he and his colleagues are developing contrast agents to enhance the ability to detect BCC tumors in vivo, to highlight tumor islands, and to differentiate sebaceous glands and hair follicles. Dr. Rossi reported having no relevant disclosures.
DALLAS – The use of CO2 laser ablation guided by reflectance confocal microscopy is an effective, minimally invasive treatment for superficial and early nodular basal cell carcinoma (BCC), according to results from an ongoing study.
“While surgery is the gold standard for many basal cell carcinomas, nonsurgical therapies may be a good option for the superficial and early nodular subtypes,” lead study author Anthony M. Rossi, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “Laser ablation was used many years ago, so this is not a novel concept, but we’re bringing it back and we’re trying to use confocal microscopy to hone in on the basal cell and selectively target the tumor.”
For the current analysis, he and his associates used with a mean age of 55 years. Of the 20 lesions, 18 were located on the limbs and trunk, while two were on the head and neck. The median lesion diameter was 7 mm. Prior to laser ablation, the researchers performed reflectance confocal microscopy to define lateral and deep margins and define the laser parameters.
The median number of laser passes was three, and ranged from two to eight, delivered at a fluence of 7.5 J/cm2. Reflectance confocal microscopy was repeated immediately after the laser treatment to the skin wound margins and deep margins, and it was performed every 3-6 months thereafter. “If you do confocal microscopy too early, you’ll see mainly inflammation and you may see residual tumor that hasn’t been fully resolved yet,” Dr. Rossi said.
As for future directions, he and his colleagues are developing contrast agents to enhance the ability to detect BCC tumors in vivo, to highlight tumor islands, and to differentiate sebaceous glands and hair follicles. Dr. Rossi reported having no relevant disclosures.
DALLAS – The use of CO2 laser ablation guided by reflectance confocal microscopy is an effective, minimally invasive treatment for superficial and early nodular basal cell carcinoma (BCC), according to results from an ongoing study.
“While surgery is the gold standard for many basal cell carcinomas, nonsurgical therapies may be a good option for the superficial and early nodular subtypes,” lead study author Anthony M. Rossi, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “Laser ablation was used many years ago, so this is not a novel concept, but we’re bringing it back and we’re trying to use confocal microscopy to hone in on the basal cell and selectively target the tumor.”
For the current analysis, he and his associates used with a mean age of 55 years. Of the 20 lesions, 18 were located on the limbs and trunk, while two were on the head and neck. The median lesion diameter was 7 mm. Prior to laser ablation, the researchers performed reflectance confocal microscopy to define lateral and deep margins and define the laser parameters.
The median number of laser passes was three, and ranged from two to eight, delivered at a fluence of 7.5 J/cm2. Reflectance confocal microscopy was repeated immediately after the laser treatment to the skin wound margins and deep margins, and it was performed every 3-6 months thereafter. “If you do confocal microscopy too early, you’ll see mainly inflammation and you may see residual tumor that hasn’t been fully resolved yet,” Dr. Rossi said.
As for future directions, he and his colleagues are developing contrast agents to enhance the ability to detect BCC tumors in vivo, to highlight tumor islands, and to differentiate sebaceous glands and hair follicles. Dr. Rossi reported having no relevant disclosures.
REPORTING FROM ASLMS 2018
Key clinical point: Reflectance confocal microscopy-guided CO2 laser ablation of basal cell carcinoma (BCC) was found to be effective.
Major finding: After an average follow-up of 17 months, no recurrence of BCC has been detected clinically, dermoscopically, or by reflectance confocal microscopy.
Study details: A clinical analysis of seven adults with superficial BCC who were treated with a CO2 laser guided by confocal microscopy.
Disclosures: Dr. Rossi reported having no financial disclosures.
Tanning is the new tobacco
I was driving to work the other day, perched up in my pickup truck (somehow you knew that) and noticed a fancy race car in front of me with a vanity tag. It read HRTATTK 4. Well, I thought after four heart attacks maybe I would splurge on a special car too (more likely a newer truck). Then I noticed smoke coming out of the driver’s window, and I could see this guy in his side view mirror, presumably Mr. “Heart Attack 4,” puffing away on a cigarette. Wow.
Then I got to work and saw my secretary, who works with her oxygen on, out back puffing a cigarette. Wow.
It turns out that cigarette smoke contains substances that act as a monoamine oxidase (MAO) A inhibitor, prolonging the dopamine high in the brain (Proc Natl Acad Sci U S A. 1996 Nov 26;93[24]:14065-9). Makes sense and may explain the above smoking behavior. I truly believe cigarettes are as or more addictive than any other dopamine enhancing drug.
More than 50 years ago, a national campaign against smoking was launched after the 1964 Surgeon General’s report concluded that smoking was a major health hazard. (Looking back, one of the few losses of not having to pull journal articles from the stacks in the library, is that medical students and residents can’t shake their heads in wonder at the cigarette ads in old medical journals.) The impact of the national antismoking campaign has been dramatic, but smoking remains the leading preventable cause of death in the United States and globally, according to the Centers for Disease Control and Prevention.
in 2006, from 1992 (Arch Dermatol. 2010;146[3]:283-7), dermatologists had good footing on which to start a major prevention campaign. The American Cancer Society got on board, and in 2014, acting surgeon general Boris Lushniak, MD, issued a call to action to prevent skin cancer along with Howard Koh, MD, the assistant secretary of health, in “The Surgeon General’s Call to Action to Prevent Skin Cancer” in 2014, and the campaign was on.
Well, I am delighted to pass on a report from Leonard Lichtenfeld, MD, deputy chief medical officer for the American Cancer Society, who recently described in his March 2018 blog what may the first signs of the effectiveness of efforts to promote protection from ultraviolet ray exposure (JAMA Dermatol. 2018;154[3]:361-2). He writes: “In young white women ages 15 to 24, the incidence of melanoma has declined an average of 5.5% per year from January 2005 through December 2014. Not 5.5% over those ten years but 5.5 % PER YEAR. That’s remarkable, to say the least.”
As for the reasons behind these trends, he says, “no one can say for certain,” but he refers to national data indicating that indoor tanning has decreased in the past few years, especially among adolescents and young adults.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
I was driving to work the other day, perched up in my pickup truck (somehow you knew that) and noticed a fancy race car in front of me with a vanity tag. It read HRTATTK 4. Well, I thought after four heart attacks maybe I would splurge on a special car too (more likely a newer truck). Then I noticed smoke coming out of the driver’s window, and I could see this guy in his side view mirror, presumably Mr. “Heart Attack 4,” puffing away on a cigarette. Wow.
Then I got to work and saw my secretary, who works with her oxygen on, out back puffing a cigarette. Wow.
It turns out that cigarette smoke contains substances that act as a monoamine oxidase (MAO) A inhibitor, prolonging the dopamine high in the brain (Proc Natl Acad Sci U S A. 1996 Nov 26;93[24]:14065-9). Makes sense and may explain the above smoking behavior. I truly believe cigarettes are as or more addictive than any other dopamine enhancing drug.
More than 50 years ago, a national campaign against smoking was launched after the 1964 Surgeon General’s report concluded that smoking was a major health hazard. (Looking back, one of the few losses of not having to pull journal articles from the stacks in the library, is that medical students and residents can’t shake their heads in wonder at the cigarette ads in old medical journals.) The impact of the national antismoking campaign has been dramatic, but smoking remains the leading preventable cause of death in the United States and globally, according to the Centers for Disease Control and Prevention.
in 2006, from 1992 (Arch Dermatol. 2010;146[3]:283-7), dermatologists had good footing on which to start a major prevention campaign. The American Cancer Society got on board, and in 2014, acting surgeon general Boris Lushniak, MD, issued a call to action to prevent skin cancer along with Howard Koh, MD, the assistant secretary of health, in “The Surgeon General’s Call to Action to Prevent Skin Cancer” in 2014, and the campaign was on.
Well, I am delighted to pass on a report from Leonard Lichtenfeld, MD, deputy chief medical officer for the American Cancer Society, who recently described in his March 2018 blog what may the first signs of the effectiveness of efforts to promote protection from ultraviolet ray exposure (JAMA Dermatol. 2018;154[3]:361-2). He writes: “In young white women ages 15 to 24, the incidence of melanoma has declined an average of 5.5% per year from January 2005 through December 2014. Not 5.5% over those ten years but 5.5 % PER YEAR. That’s remarkable, to say the least.”
As for the reasons behind these trends, he says, “no one can say for certain,” but he refers to national data indicating that indoor tanning has decreased in the past few years, especially among adolescents and young adults.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
I was driving to work the other day, perched up in my pickup truck (somehow you knew that) and noticed a fancy race car in front of me with a vanity tag. It read HRTATTK 4. Well, I thought after four heart attacks maybe I would splurge on a special car too (more likely a newer truck). Then I noticed smoke coming out of the driver’s window, and I could see this guy in his side view mirror, presumably Mr. “Heart Attack 4,” puffing away on a cigarette. Wow.
Then I got to work and saw my secretary, who works with her oxygen on, out back puffing a cigarette. Wow.
It turns out that cigarette smoke contains substances that act as a monoamine oxidase (MAO) A inhibitor, prolonging the dopamine high in the brain (Proc Natl Acad Sci U S A. 1996 Nov 26;93[24]:14065-9). Makes sense and may explain the above smoking behavior. I truly believe cigarettes are as or more addictive than any other dopamine enhancing drug.
More than 50 years ago, a national campaign against smoking was launched after the 1964 Surgeon General’s report concluded that smoking was a major health hazard. (Looking back, one of the few losses of not having to pull journal articles from the stacks in the library, is that medical students and residents can’t shake their heads in wonder at the cigarette ads in old medical journals.) The impact of the national antismoking campaign has been dramatic, but smoking remains the leading preventable cause of death in the United States and globally, according to the Centers for Disease Control and Prevention.
in 2006, from 1992 (Arch Dermatol. 2010;146[3]:283-7), dermatologists had good footing on which to start a major prevention campaign. The American Cancer Society got on board, and in 2014, acting surgeon general Boris Lushniak, MD, issued a call to action to prevent skin cancer along with Howard Koh, MD, the assistant secretary of health, in “The Surgeon General’s Call to Action to Prevent Skin Cancer” in 2014, and the campaign was on.
Well, I am delighted to pass on a report from Leonard Lichtenfeld, MD, deputy chief medical officer for the American Cancer Society, who recently described in his March 2018 blog what may the first signs of the effectiveness of efforts to promote protection from ultraviolet ray exposure (JAMA Dermatol. 2018;154[3]:361-2). He writes: “In young white women ages 15 to 24, the incidence of melanoma has declined an average of 5.5% per year from January 2005 through December 2014. Not 5.5% over those ten years but 5.5 % PER YEAR. That’s remarkable, to say the least.”
As for the reasons behind these trends, he says, “no one can say for certain,” but he refers to national data indicating that indoor tanning has decreased in the past few years, especially among adolescents and young adults.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Cancer-related clinical pearls from pediatric dermatology
KAUAI, HAWAII – Every child diagnosed with medulloblastoma deserves a careful dermatologic evaluation for possible comorbid basal cell nevus syndrome, according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital and Harvard Medical School.
“Medulloblastoma occurs in 10%-20% of patients with basal cell nevus syndrome and can be the presenting sign. So if a patient with basal cell nevus syndrome gets medulloblastoma, it usually occurs within the first year of life – and it can be the first thing you see,” she said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Huang presented a series of pediatric dermatology clinical pearls focused not only on basal cell nevus syndrome (BCNS) and medulloblastoma, but also on the implications of skin-limited Langerhans cell histiocytosis, how to recognize and treat drug-induced follicular eruptions in pediatric patients on targeted anticancer therapies, and when to suspect Demodex folliculitis in immunosuppressed patients.
Skin-limited Langerhans cell histiocytosis
Around 10%-20% of patients with Langerhans cell histiocytosis (LCH) have the skin-limited form of the malignancy. These are patients who, after a thorough workup, have a normal CBC, skeletal survey, and liver function tests; essentially, no evidence of multisystem disease.
“It’s very rare for patients who present with skin-limited LCH alone to develop multisystem disease and to require chemotherapy or other more aggressive treatment,” Dr. Huang said. “I think that skin-limited LCH is probably a separate entity with its own natural history distinct from multisystem disease. We can see that with current genomic testing: in multisystem LCH, BRAF mutations are identified in at least half of patients, but very few with skin-limited disease express those mutations.
“The clinical pearl here is if you have a patient with skin-limited LCH it very rarely progresses to multisystem involvement. It’s associated with a good prognosis. That doesn’t mean you shouldn’t monitor them, but I think it can be reassuring information for the family,” she said.
Basal cell nevus syndrome and medulloblastoma
“Half of cases of medulloblastoma are associated with mutations in the sonic hedgehog pathway – and a subset of that group has basal cell nevus syndrome,” Dr. Huang said.
BCNS is not a diagnosis frequently made by oncologists, who typically dismiss the multitude of lesions as skin tags, which they often mimic in both appearance and location, particularly on the neck and intertriginous areas. So it’s useful for dermatologists to establish a good referral relationship with their local oncologists.
“As dermatologists it’s really important to recognize not only the major features of basal cell nevus syndrome, but also the associated findings because we can really help in making this diagnosis early,” Dr. Huang stressed.
Early diagnosis of BCNS is a high priority for two reasons: to start treatment aimed at reducing development of basal cell carcinomas, and because radiation therapy for their medulloblastoma is contraindicated in patients with BCNS because it boosts their skin cancer burden.
BCNS is caused by mutations in the PTCH (Patched) gene found on chromosome arm 9q. The major features of BCNS include odontogenic keratocysts, palmoplantar pits, ectopic calcification, and, of course, basal cell carcinomas. The associated findings in BCNS, in addition to medulloblastoma, include macrocephaly and dysmorphic features such as cleft lip or palate, frontal bossing, and hypertelorism.
“I’ve treated a hundred at a time. It’s incredibly successful. It’s locally destructive. It leaves a little bit of hypopigmentation but no scar, which the CO2 laser will do in this instance. It’s actually a pretty cool modality,” said Dr. Eichenfield, professor of dermatology and pediatrics at Rady Children’s Hospital and the University of California, San Diego.
Follicular eruptions in cancer patients on MAPK inhibitors
Cutaneous reactions to anticancer drugs aimed at inhibiting the key MAPK (mitogen-activated protein kinase) pathway in children are common and diverse. Dr. Huang focused on the most common one: follicular eruptions, which occur in up to 80% of pediatric cancer patients on targeted therapy. These eruptions can express themselves in a variety of ways and are easily mistaken for comedonal acne, varicella zoster infection, herpes simplex, or bacterial folliculitis.
The key clues are highly suggestive that a follicular eruption in a child on targeted anticancer therapy is caused by the drug and not something else are the eruption’s symmetric distribution, that it’s truly follicular upon close inspection, and the timing: The eruption typically begins 2-3 weeks after initiation of therapy or within a week after a dose escalation.
Anti-inflammatory agents are the treatment mainstay. Treatment of the cutaneous eruption often is successful without need to discontinue the patient’s MAPK inhibitor.
“Even though some of these eruptions look comedonal, they’re not. It’s not a follicular plugging disorder, it’s an inflammatory condition. Topical steroids, oral tetracyclines, and dilute bleach baths all work pretty well. I haven’t had good experiences with keratolytics like tretinoin cream and benzoyl peroxide; they’re less effective. Dose reduction is the last resort for these patients. Often they are very sick. They need the drug and I think the last thing we want to do is take them off it,” Dr. Huang said.
She has observed that prepubertal children are more likely to have an eczematous reaction to their targeted anticancer therapy than a follicular eruption.
D. folliculitis in immunocompromised patients
“The clinical pearl here is to strongly consider the diagnosis of Demodex folliculitis in an immunosuppresed patient with an itchy acneiform eruption,” Dr. Huang said.
Demodex is a human mite which is part of the normal skin flora. She called it “a great mimicker”: It can cause dermatoses mistaken for rosacea, acne, seborrheic dermatitis, perioral facial dermatitis, blepharitis, and acute graft-versus-host disease.
In the setting of a young, immunosuppressed patient who develops an acneiform eruption, the differential diagnosis is lengthy and includes steroid-induced acne, a cutaneous reaction to targeted anticancer therapy, gram-negative folliculitis secondary to long-term antibiotic therapy, and Pityrosporum folliculitis, as well as D. folliculitis.
Demodex and P. folliculitis are the two acneiform dermatoses where itch figures prominently. A couple of clues are helpful in differentiating the two conditions: P. folliculitis often involves the chest and back, while D. folliculitis generally spares the trunk and is focused on the face and neck. And D. folliculitis typically arises when immunosuppression is weaned. Overgrowth of the mites occurs during immunosuppression, then as the immunosuppression is lifted a prominent inflammatory response with an acne-like appearance occurs.
Dr. Huang usually sticks with topical therapies for D. folliculitis. These include topical sulfur 5%, permethrin 5%, metronidazole, and/or ivermectin. If a young patient is unresponsive to this panoply of topical agents, she resorts to a single dose of oral ivermectin at 0.2 mg/kg, usually with good effect.
Dr. Huang reported having no financial conflicts of interest regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Every child diagnosed with medulloblastoma deserves a careful dermatologic evaluation for possible comorbid basal cell nevus syndrome, according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital and Harvard Medical School.
“Medulloblastoma occurs in 10%-20% of patients with basal cell nevus syndrome and can be the presenting sign. So if a patient with basal cell nevus syndrome gets medulloblastoma, it usually occurs within the first year of life – and it can be the first thing you see,” she said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Huang presented a series of pediatric dermatology clinical pearls focused not only on basal cell nevus syndrome (BCNS) and medulloblastoma, but also on the implications of skin-limited Langerhans cell histiocytosis, how to recognize and treat drug-induced follicular eruptions in pediatric patients on targeted anticancer therapies, and when to suspect Demodex folliculitis in immunosuppressed patients.
Skin-limited Langerhans cell histiocytosis
Around 10%-20% of patients with Langerhans cell histiocytosis (LCH) have the skin-limited form of the malignancy. These are patients who, after a thorough workup, have a normal CBC, skeletal survey, and liver function tests; essentially, no evidence of multisystem disease.
“It’s very rare for patients who present with skin-limited LCH alone to develop multisystem disease and to require chemotherapy or other more aggressive treatment,” Dr. Huang said. “I think that skin-limited LCH is probably a separate entity with its own natural history distinct from multisystem disease. We can see that with current genomic testing: in multisystem LCH, BRAF mutations are identified in at least half of patients, but very few with skin-limited disease express those mutations.
“The clinical pearl here is if you have a patient with skin-limited LCH it very rarely progresses to multisystem involvement. It’s associated with a good prognosis. That doesn’t mean you shouldn’t monitor them, but I think it can be reassuring information for the family,” she said.
Basal cell nevus syndrome and medulloblastoma
“Half of cases of medulloblastoma are associated with mutations in the sonic hedgehog pathway – and a subset of that group has basal cell nevus syndrome,” Dr. Huang said.
BCNS is not a diagnosis frequently made by oncologists, who typically dismiss the multitude of lesions as skin tags, which they often mimic in both appearance and location, particularly on the neck and intertriginous areas. So it’s useful for dermatologists to establish a good referral relationship with their local oncologists.
“As dermatologists it’s really important to recognize not only the major features of basal cell nevus syndrome, but also the associated findings because we can really help in making this diagnosis early,” Dr. Huang stressed.
Early diagnosis of BCNS is a high priority for two reasons: to start treatment aimed at reducing development of basal cell carcinomas, and because radiation therapy for their medulloblastoma is contraindicated in patients with BCNS because it boosts their skin cancer burden.
BCNS is caused by mutations in the PTCH (Patched) gene found on chromosome arm 9q. The major features of BCNS include odontogenic keratocysts, palmoplantar pits, ectopic calcification, and, of course, basal cell carcinomas. The associated findings in BCNS, in addition to medulloblastoma, include macrocephaly and dysmorphic features such as cleft lip or palate, frontal bossing, and hypertelorism.
“I’ve treated a hundred at a time. It’s incredibly successful. It’s locally destructive. It leaves a little bit of hypopigmentation but no scar, which the CO2 laser will do in this instance. It’s actually a pretty cool modality,” said Dr. Eichenfield, professor of dermatology and pediatrics at Rady Children’s Hospital and the University of California, San Diego.
Follicular eruptions in cancer patients on MAPK inhibitors
Cutaneous reactions to anticancer drugs aimed at inhibiting the key MAPK (mitogen-activated protein kinase) pathway in children are common and diverse. Dr. Huang focused on the most common one: follicular eruptions, which occur in up to 80% of pediatric cancer patients on targeted therapy. These eruptions can express themselves in a variety of ways and are easily mistaken for comedonal acne, varicella zoster infection, herpes simplex, or bacterial folliculitis.
The key clues are highly suggestive that a follicular eruption in a child on targeted anticancer therapy is caused by the drug and not something else are the eruption’s symmetric distribution, that it’s truly follicular upon close inspection, and the timing: The eruption typically begins 2-3 weeks after initiation of therapy or within a week after a dose escalation.
Anti-inflammatory agents are the treatment mainstay. Treatment of the cutaneous eruption often is successful without need to discontinue the patient’s MAPK inhibitor.
“Even though some of these eruptions look comedonal, they’re not. It’s not a follicular plugging disorder, it’s an inflammatory condition. Topical steroids, oral tetracyclines, and dilute bleach baths all work pretty well. I haven’t had good experiences with keratolytics like tretinoin cream and benzoyl peroxide; they’re less effective. Dose reduction is the last resort for these patients. Often they are very sick. They need the drug and I think the last thing we want to do is take them off it,” Dr. Huang said.
She has observed that prepubertal children are more likely to have an eczematous reaction to their targeted anticancer therapy than a follicular eruption.
D. folliculitis in immunocompromised patients
“The clinical pearl here is to strongly consider the diagnosis of Demodex folliculitis in an immunosuppresed patient with an itchy acneiform eruption,” Dr. Huang said.
Demodex is a human mite which is part of the normal skin flora. She called it “a great mimicker”: It can cause dermatoses mistaken for rosacea, acne, seborrheic dermatitis, perioral facial dermatitis, blepharitis, and acute graft-versus-host disease.
In the setting of a young, immunosuppressed patient who develops an acneiform eruption, the differential diagnosis is lengthy and includes steroid-induced acne, a cutaneous reaction to targeted anticancer therapy, gram-negative folliculitis secondary to long-term antibiotic therapy, and Pityrosporum folliculitis, as well as D. folliculitis.
Demodex and P. folliculitis are the two acneiform dermatoses where itch figures prominently. A couple of clues are helpful in differentiating the two conditions: P. folliculitis often involves the chest and back, while D. folliculitis generally spares the trunk and is focused on the face and neck. And D. folliculitis typically arises when immunosuppression is weaned. Overgrowth of the mites occurs during immunosuppression, then as the immunosuppression is lifted a prominent inflammatory response with an acne-like appearance occurs.
Dr. Huang usually sticks with topical therapies for D. folliculitis. These include topical sulfur 5%, permethrin 5%, metronidazole, and/or ivermectin. If a young patient is unresponsive to this panoply of topical agents, she resorts to a single dose of oral ivermectin at 0.2 mg/kg, usually with good effect.
Dr. Huang reported having no financial conflicts of interest regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Every child diagnosed with medulloblastoma deserves a careful dermatologic evaluation for possible comorbid basal cell nevus syndrome, according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital and Harvard Medical School.
“Medulloblastoma occurs in 10%-20% of patients with basal cell nevus syndrome and can be the presenting sign. So if a patient with basal cell nevus syndrome gets medulloblastoma, it usually occurs within the first year of life – and it can be the first thing you see,” she said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Huang presented a series of pediatric dermatology clinical pearls focused not only on basal cell nevus syndrome (BCNS) and medulloblastoma, but also on the implications of skin-limited Langerhans cell histiocytosis, how to recognize and treat drug-induced follicular eruptions in pediatric patients on targeted anticancer therapies, and when to suspect Demodex folliculitis in immunosuppressed patients.
Skin-limited Langerhans cell histiocytosis
Around 10%-20% of patients with Langerhans cell histiocytosis (LCH) have the skin-limited form of the malignancy. These are patients who, after a thorough workup, have a normal CBC, skeletal survey, and liver function tests; essentially, no evidence of multisystem disease.
“It’s very rare for patients who present with skin-limited LCH alone to develop multisystem disease and to require chemotherapy or other more aggressive treatment,” Dr. Huang said. “I think that skin-limited LCH is probably a separate entity with its own natural history distinct from multisystem disease. We can see that with current genomic testing: in multisystem LCH, BRAF mutations are identified in at least half of patients, but very few with skin-limited disease express those mutations.
“The clinical pearl here is if you have a patient with skin-limited LCH it very rarely progresses to multisystem involvement. It’s associated with a good prognosis. That doesn’t mean you shouldn’t monitor them, but I think it can be reassuring information for the family,” she said.
Basal cell nevus syndrome and medulloblastoma
“Half of cases of medulloblastoma are associated with mutations in the sonic hedgehog pathway – and a subset of that group has basal cell nevus syndrome,” Dr. Huang said.
BCNS is not a diagnosis frequently made by oncologists, who typically dismiss the multitude of lesions as skin tags, which they often mimic in both appearance and location, particularly on the neck and intertriginous areas. So it’s useful for dermatologists to establish a good referral relationship with their local oncologists.
“As dermatologists it’s really important to recognize not only the major features of basal cell nevus syndrome, but also the associated findings because we can really help in making this diagnosis early,” Dr. Huang stressed.
Early diagnosis of BCNS is a high priority for two reasons: to start treatment aimed at reducing development of basal cell carcinomas, and because radiation therapy for their medulloblastoma is contraindicated in patients with BCNS because it boosts their skin cancer burden.
BCNS is caused by mutations in the PTCH (Patched) gene found on chromosome arm 9q. The major features of BCNS include odontogenic keratocysts, palmoplantar pits, ectopic calcification, and, of course, basal cell carcinomas. The associated findings in BCNS, in addition to medulloblastoma, include macrocephaly and dysmorphic features such as cleft lip or palate, frontal bossing, and hypertelorism.
“I’ve treated a hundred at a time. It’s incredibly successful. It’s locally destructive. It leaves a little bit of hypopigmentation but no scar, which the CO2 laser will do in this instance. It’s actually a pretty cool modality,” said Dr. Eichenfield, professor of dermatology and pediatrics at Rady Children’s Hospital and the University of California, San Diego.
Follicular eruptions in cancer patients on MAPK inhibitors
Cutaneous reactions to anticancer drugs aimed at inhibiting the key MAPK (mitogen-activated protein kinase) pathway in children are common and diverse. Dr. Huang focused on the most common one: follicular eruptions, which occur in up to 80% of pediatric cancer patients on targeted therapy. These eruptions can express themselves in a variety of ways and are easily mistaken for comedonal acne, varicella zoster infection, herpes simplex, or bacterial folliculitis.
The key clues are highly suggestive that a follicular eruption in a child on targeted anticancer therapy is caused by the drug and not something else are the eruption’s symmetric distribution, that it’s truly follicular upon close inspection, and the timing: The eruption typically begins 2-3 weeks after initiation of therapy or within a week after a dose escalation.
Anti-inflammatory agents are the treatment mainstay. Treatment of the cutaneous eruption often is successful without need to discontinue the patient’s MAPK inhibitor.
“Even though some of these eruptions look comedonal, they’re not. It’s not a follicular plugging disorder, it’s an inflammatory condition. Topical steroids, oral tetracyclines, and dilute bleach baths all work pretty well. I haven’t had good experiences with keratolytics like tretinoin cream and benzoyl peroxide; they’re less effective. Dose reduction is the last resort for these patients. Often they are very sick. They need the drug and I think the last thing we want to do is take them off it,” Dr. Huang said.
She has observed that prepubertal children are more likely to have an eczematous reaction to their targeted anticancer therapy than a follicular eruption.
D. folliculitis in immunocompromised patients
“The clinical pearl here is to strongly consider the diagnosis of Demodex folliculitis in an immunosuppresed patient with an itchy acneiform eruption,” Dr. Huang said.
Demodex is a human mite which is part of the normal skin flora. She called it “a great mimicker”: It can cause dermatoses mistaken for rosacea, acne, seborrheic dermatitis, perioral facial dermatitis, blepharitis, and acute graft-versus-host disease.
In the setting of a young, immunosuppressed patient who develops an acneiform eruption, the differential diagnosis is lengthy and includes steroid-induced acne, a cutaneous reaction to targeted anticancer therapy, gram-negative folliculitis secondary to long-term antibiotic therapy, and Pityrosporum folliculitis, as well as D. folliculitis.
Demodex and P. folliculitis are the two acneiform dermatoses where itch figures prominently. A couple of clues are helpful in differentiating the two conditions: P. folliculitis often involves the chest and back, while D. folliculitis generally spares the trunk and is focused on the face and neck. And D. folliculitis typically arises when immunosuppression is weaned. Overgrowth of the mites occurs during immunosuppression, then as the immunosuppression is lifted a prominent inflammatory response with an acne-like appearance occurs.
Dr. Huang usually sticks with topical therapies for D. folliculitis. These include topical sulfur 5%, permethrin 5%, metronidazole, and/or ivermectin. If a young patient is unresponsive to this panoply of topical agents, she resorts to a single dose of oral ivermectin at 0.2 mg/kg, usually with good effect.
Dr. Huang reported having no financial conflicts of interest regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Spontaneous Regression of Merkel Cell Carcinoma
Merkel cell carcinoma (MCC) is a rare, rapidly growing, aggressive neoplasm with a generally poor prognosis. The cells of origin are highly anaplastic and share structural and immunohistochemical features with various neuroectodermally derived cells. Although Merkel cells, which are slow-acting cutaneous mechanoreceptors located in the basal layer of the epidermis, and MCC share immunohistochemical and ultrastructural features, there is limited evidence of a direct histogenetic relationship between the two.1,2 Additionally, some extracutaneous neuroendocrine tumors have features similar to MCC; therefore, although it may be more accurate and perhaps more practical to describe these lesions as primary neuroendocrine carcinomas of the skin, the term MCC is more commonly used both in the literature and in clinical practice.1,2
Merkel cell carcinoma typically presents in the head and neck region in white patients older than 70 years of age and in the immunocompromised population.3-6 The mean age of diagnosis is 76 years for women and 74 years for men.7 The incidence of MCC in the United States tripled over a 15-year period, and there are approximately 1500 new cases of MCC diagnosed each year, making it about 40 times less common than melanoma.8 The 5-year survival rate for patients without lymph node involvement is 75%, whereas the 5-year survival rate for patients with distant metastases is 25%.9
Merkel cell carcinoma is thought to develop through 1 of 2 distinct pathways. In a virally mediated pathway, which represents at least 80% of cases, the Merkel cell polyomavirus (MCV) monoclonally integrates into the host genome and promotes oncogenesis via altered p53 and retinoblastoma protein expression.10-12 The remainder of cases are believed to develop via a nonvirally mediated pathway in which genetic anomalies, immune status, and environmental factors influence oncogenesis.10-13
Due to the similarity between MCC and metastatic neuroendocrine neoplasms, especially small-cell lung carcinomas, immunohistochemistry is important in making the diagnosis. Cytokeratin 20 and neuron-specific enolase positivity and thyroid transcription factor 1 negativity are the most useful markers in identifying MCC.
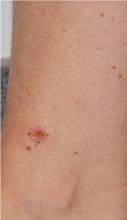
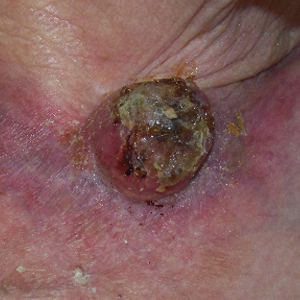
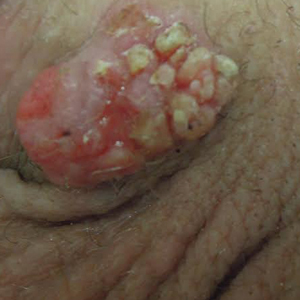
Regression of MCC is a very rare and poorly understood event. A 2010 review of the literature described 22 cases of spontaneous regression.14 We report a rare case of rapid and complete regression of MCC following punch biopsy in a 96-year-old woman.
Case Report

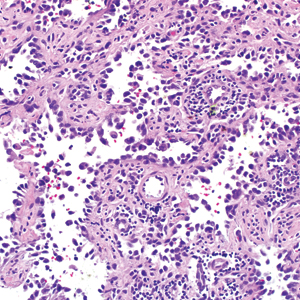
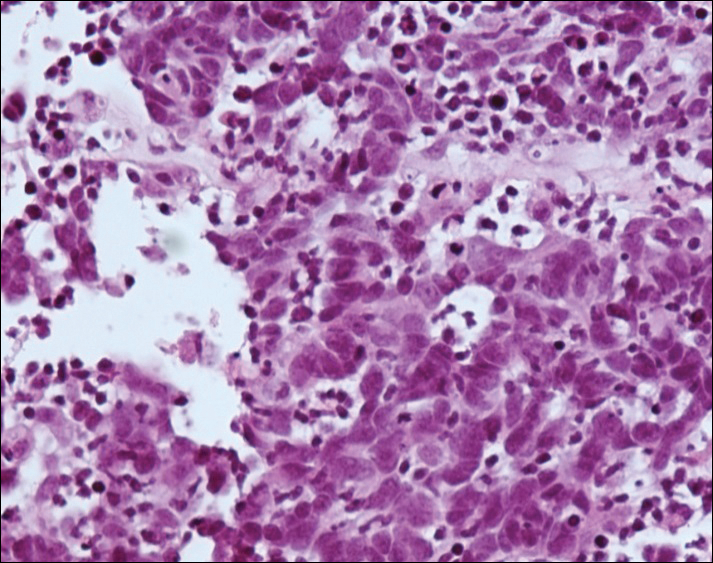
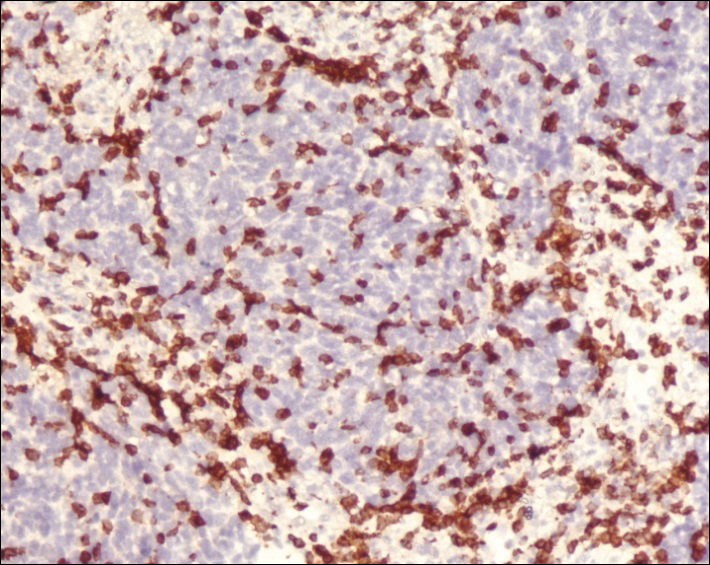
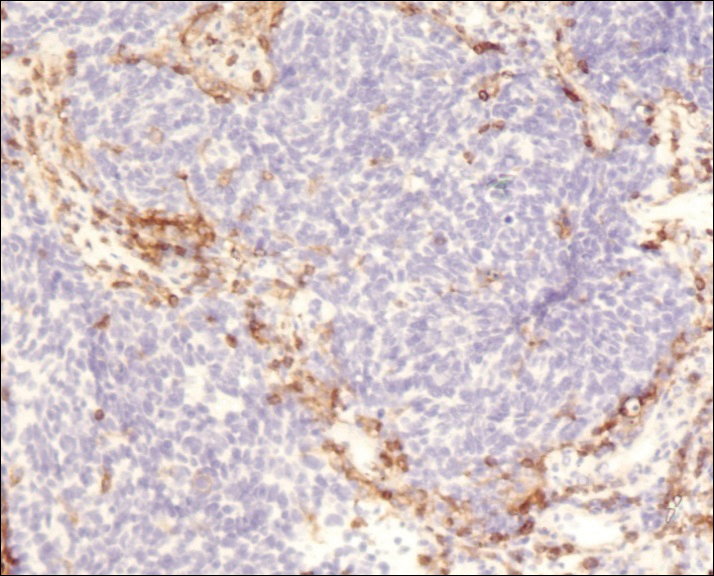
A 4-mm punch biopsy was obtained at a follow-up visit 4 weeks later (12 weeks after the reported onset of the lesion). Hematoxylin and eosin staining showed a small-cell neoplasm with stippled nuclei and scant cytoplasm forming a nested and somewhat trabecular pattern. Mitotic activity, apoptosis, and nuclear molding also were present (Figure 2). The tumor cells were positive for cytokeratin 20 with a dotlike, paranuclear pattern (Figure 3). Staining for CAM 5.2 also was positive. Cytokeratin 5/6, human melanoma black 45, and leukocyte common antigen were negative. The immunophenotyping of the lymphocytic response to the tumor showed that the majority of intratumoral lymphocytes were CD8 positive (Figure 4). CD4-positive lymphocytes were predominantly seen at the periphery of the tumor nests without tumor infiltration (Figure 5). Based on these findings, a diagnosis of MCC was made. The patient’s family declined treatment based on her advanced age and current health status, which included advanced dementia.

Two weeks after the punch biopsy, the lesion had noticeably decreased in size and lost its dome-shaped appearance. Within 8 weeks after biopsy (20 weeks since the lesion first appeared), the lesion had completely resolved (Figure 6). The patient was lost to follow-up months later, but no recurrence of the lesion was reported.

Comment
Spontaneous regression is not unique to MCC, as this phenomenon also has been reported in keratoacanthoma, lymphoma, basal cell carcinoma, and melanoma.15 Complete spontaneous regression is defined as occurring in the absence of therapy that is intended to have a treatment effect.15,16 Spontaneous regression is estimated to occur in malignant neoplasms at a rate of 1 case per 60,000 to 100,000 (approximately 0.0013% of all malignant neoplasms).17 Considering the reported prevalence of MCC and the number of cases that have been known to regress, the estimated incidence of complete spontaneous regression may be as high as 1.5%.14 Though spontaneous regression of MCC is more prevalent than expected, it still is considered a rare phenomenon. A 2010 review of the literature yielded 22 cases of complete spontaneous regression of MCC.14 No recurrences have been observed; however, follow-up was relatively short in some cases.
In a unique report by Bertolotti et al,18 a patient with MCC on the nasal tip presented 4 weeks after biopsy with complete spontaneous regression of the tumor, which was associated with bilateral cervical lymph node involvement as noted by hypermetabolic uptake on positron emission tomography scanning. The patient underwent radiation therapy and was disease free at 12 months’ follow-up.18
Complete spontaneous regression has been described in MCC patients with local disease, regional recurrences, and metastatic disease.19 In
The histopathologic features observed in our case, specifically intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells, were similar to the findings in other reported cases. In one series of 2 cases, the one case showed scar tissue with a moderate, predominantly T-lymphocytic infiltrate and no tumor cells, and the second showed cellular proliferation in the deep dermis with dense lymphocytic infiltrates primarily composed of CD3-positive T cells.14 Other studies of regression of both localized and metastatic MCC demonstrated infiltration by CD4-positive, CD8-positive, and CD3-positive lymphocytes and foamy macrophages.21-23
The discovery of the MCV was one of the most important advances in elucidating the pathogenesis of MCC.10,24-26 Merkel cell polyomavirus DNA has been detected in a majority of MCC cases.25,27 Viral integration has been shown to take place early, prior to tumor clonal expansion.10 Importantly, not all cases of MCC show MCV infection, and MCV infection is not exclusive to MCC.28 Merkel cell polyomavirus is considered to be part of the normal human flora, and asymptomatic infection is quite common.29 It has been identified in 80% of adults older than 50 years of age and, interestingly, in 35% of children by 13 years of age or younger.30,31 It remains unclear what role the presence of MCV plays in determining MCC prognosis. Several reports have demonstrated lower disease-specific mortality associated with MCV-positive MCC.32-35 In contrast, Schrama et al36 correlated the MCV status of 174 MCC tumors and found no difference in clinical behavior or prognosis between MCV-positive and MCV-negative MCCs.
Immunosuppression also may play a role in the development of MCC.5,25 There is increased prevalence of MCC in the human immunodeficiency virus–positive population, as well as in organ-transplant recipients and patients with leukemia. Chronic lymphocytic leukemia seems to be the most frequent neoplasia associated with development of MCC.37
The mechanism of MCC regression remains unclear, but many investigators emphasize the importance of T-cell–mediated immunity.16,21-23,38,39 Apoptosis also has been shown to play an important role.40 Our case showed tumor-infiltrating CD8-positive lymphocytes and CD4-positive lymphocytes present predominantly at the periphery of the tumor, with close proximity to the tumor nests but with no tumor infiltration (Figure 3). This distribution was consistently present in multiple sections of the tumor. These findings are consistent with prior reports of both CD4-positive and CD8-positive T lymphocytes associated with MCC regression. Our findings confirm that immune response may play an important role in spontaneous regression of MCC.
There is much speculation regarding the initial biopsy of an MCC lesion (or other traumatic event) and its role in tumor regression. Koba et al41 examined the effect of biopsy on CD8-positive lymphocytic infiltration of MCC tumor cells and found that biopsy does not commonly alter intratumoral CD8-positive infiltration. These findings suggest trauma does not directly induce immunologic recognition of this cancer.
Conclusion
We report a case of complete spontaneous regression of a localized MCC following a punch biopsy. The histopathology showed a brisk T-lymphocyte response with intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells. The age and clinical profile of our patient as well as the clinicopathologic characteristics of the tumor regression are similar to other reported cases. Further research is needed to elucidate the mechanism of MCC regression, and a better understanding of this fascinating phenomenon could help in development of new immunotherapeutic approaches.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Sibley RK, Dahl D. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. an immunocytochemical study of 21 cases. Am J Surg Pathol. 1985;9:109-116.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Gooptu C, Woolloons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immunosuppression. Br J Dermatol. 1997;137:637-641.
- Plunkett TA, Harris AJ, Ogg CS, et al. The treatment of Merkel cell carcinoma and its association with immunosuppression. Br J Dermatol. 1998;139:345-346.
- Calder KB, Smoller BR. New insights into Merkel cell carcinoma. Adv Anat Pathol. 2010;17:155-161.
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
- Decaprio JA. Does detection of Merkel cell polyomavirus in Merkel cell carcinoma provide prognostic information? J Natl Cancer Inst. 2009;101:905-907.
- Popp S, Waltering S, Herbst C, et al. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99:352-360.
- Ciudad C, Avilés JA, Alfageme F, et al. Spontaneous regression in Merkel cell carcinoma: report of two cases with description of dermoscopic features and review of literature. Dermatol Surg. 2010;36:687-693.
- O’Rourke MGE, Bell JR. Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol. 1986;12:994-997.
- Connelly TJ, Cribier B, Brown TJ, et al. Complete spontaneous regression of Merkel cell carcinoma: a review of 10 reported cases. Dermatol Surg. 2000;26:853-856.
- Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201-209.
- Bertolotti A, Conte H, Francois L, et al. Merkel cell carcinoma: complete clinical remission associated with disease progression. JAMA Dermatol. 2013;149:501-502.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Richetta AG, Mancini M, Torroni A, et al. Total spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg. 2008;34:815-822.
- Vesely MJ, Murray DJ, Neligan PC, et al. Complete spontaneous regression in Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2008;61:165-171.
- Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991;127:550-553.
- Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma-a case showing replacement of tumour cells by foamy cells. Br J Dermatol. 2000;142:1184-1189.
- Duncavage E, Zehnbauer B, Pfeifer J. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of unique deletion in the VP1 gene. Cancer Res. 2008;68:5009-5013.
- Becker J, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2009;66:1-8.
- Haitz KA, Rady PL, Nguyen HP, et al. Merkel cell polyomavirus DNA detection in a patient with Merkel cell carcinoma and multiple other skin cancers. Int J Dermatol. 2012;51:442-444.
- Andres C, Puchta U, Sander CA, et al. Prevalence of Merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. 2010;32:593-598.
- Showalter RM, Pastrana DV, Pumphrey KA, et al. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509-515.
- Tolstov YL, Pastrana DV, Feng H, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250-1256.
- Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125-129.
- Laude HC, Jonchère B, Maubec E, et al. Distinct Merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with Merkel cell carcinoma. PLoS Pathog. 2010;6:e1001076.
- Waltari M, Sihto H, Kukko H, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129:619-628.
- Sihto H, Kukko H, Koljonen V, et al. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938-945.
- Paulson KG, Lemos BD, Feng B, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547-1555.
- Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Invest Dermatol. 2011;131:1631-1638.
- Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250-256.
- Wooff J, Trites JR, Walsh NM, et al. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:614-617.
- Turk TO, Smoljan I, Nacinovic A, et al. Spontaneous regression of Merkel cell carcinoma in a patient with chronic lymphocytic leukemia: a case report. J Med Case Rep. 2009;3:7270.
- Mori Y, Tanaka K, Cui CY, et al. A study of apoptosis in Merkel cell carcinoma. an immunohistochemical, ultrasctructural, DNA ladder and TUNEL labeling study. Am J Dermatopathol. 2001;23:16-23.
- Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS ONE. 2012;7:e41465.
Merkel cell carcinoma (MCC) is a rare, rapidly growing, aggressive neoplasm with a generally poor prognosis. The cells of origin are highly anaplastic and share structural and immunohistochemical features with various neuroectodermally derived cells. Although Merkel cells, which are slow-acting cutaneous mechanoreceptors located in the basal layer of the epidermis, and MCC share immunohistochemical and ultrastructural features, there is limited evidence of a direct histogenetic relationship between the two.1,2 Additionally, some extracutaneous neuroendocrine tumors have features similar to MCC; therefore, although it may be more accurate and perhaps more practical to describe these lesions as primary neuroendocrine carcinomas of the skin, the term MCC is more commonly used both in the literature and in clinical practice.1,2
Merkel cell carcinoma typically presents in the head and neck region in white patients older than 70 years of age and in the immunocompromised population.3-6 The mean age of diagnosis is 76 years for women and 74 years for men.7 The incidence of MCC in the United States tripled over a 15-year period, and there are approximately 1500 new cases of MCC diagnosed each year, making it about 40 times less common than melanoma.8 The 5-year survival rate for patients without lymph node involvement is 75%, whereas the 5-year survival rate for patients with distant metastases is 25%.9
Merkel cell carcinoma is thought to develop through 1 of 2 distinct pathways. In a virally mediated pathway, which represents at least 80% of cases, the Merkel cell polyomavirus (MCV) monoclonally integrates into the host genome and promotes oncogenesis via altered p53 and retinoblastoma protein expression.10-12 The remainder of cases are believed to develop via a nonvirally mediated pathway in which genetic anomalies, immune status, and environmental factors influence oncogenesis.10-13
Due to the similarity between MCC and metastatic neuroendocrine neoplasms, especially small-cell lung carcinomas, immunohistochemistry is important in making the diagnosis. Cytokeratin 20 and neuron-specific enolase positivity and thyroid transcription factor 1 negativity are the most useful markers in identifying MCC.
Regression of MCC is a very rare and poorly understood event. A 2010 review of the literature described 22 cases of spontaneous regression.14 We report a rare case of rapid and complete regression of MCC following punch biopsy in a 96-year-old woman.
Case Report

A 4-mm punch biopsy was obtained at a follow-up visit 4 weeks later (12 weeks after the reported onset of the lesion). Hematoxylin and eosin staining showed a small-cell neoplasm with stippled nuclei and scant cytoplasm forming a nested and somewhat trabecular pattern. Mitotic activity, apoptosis, and nuclear molding also were present (Figure 2). The tumor cells were positive for cytokeratin 20 with a dotlike, paranuclear pattern (Figure 3). Staining for CAM 5.2 also was positive. Cytokeratin 5/6, human melanoma black 45, and leukocyte common antigen were negative. The immunophenotyping of the lymphocytic response to the tumor showed that the majority of intratumoral lymphocytes were CD8 positive (Figure 4). CD4-positive lymphocytes were predominantly seen at the periphery of the tumor nests without tumor infiltration (Figure 5). Based on these findings, a diagnosis of MCC was made. The patient’s family declined treatment based on her advanced age and current health status, which included advanced dementia.




Two weeks after the punch biopsy, the lesion had noticeably decreased in size and lost its dome-shaped appearance. Within 8 weeks after biopsy (20 weeks since the lesion first appeared), the lesion had completely resolved (Figure 6). The patient was lost to follow-up months later, but no recurrence of the lesion was reported.

Comment
Spontaneous regression is not unique to MCC, as this phenomenon also has been reported in keratoacanthoma, lymphoma, basal cell carcinoma, and melanoma.15 Complete spontaneous regression is defined as occurring in the absence of therapy that is intended to have a treatment effect.15,16 Spontaneous regression is estimated to occur in malignant neoplasms at a rate of 1 case per 60,000 to 100,000 (approximately 0.0013% of all malignant neoplasms).17 Considering the reported prevalence of MCC and the number of cases that have been known to regress, the estimated incidence of complete spontaneous regression may be as high as 1.5%.14 Though spontaneous regression of MCC is more prevalent than expected, it still is considered a rare phenomenon. A 2010 review of the literature yielded 22 cases of complete spontaneous regression of MCC.14 No recurrences have been observed; however, follow-up was relatively short in some cases.
In a unique report by Bertolotti et al,18 a patient with MCC on the nasal tip presented 4 weeks after biopsy with complete spontaneous regression of the tumor, which was associated with bilateral cervical lymph node involvement as noted by hypermetabolic uptake on positron emission tomography scanning. The patient underwent radiation therapy and was disease free at 12 months’ follow-up.18
Complete spontaneous regression has been described in MCC patients with local disease, regional recurrences, and metastatic disease.19 In
The histopathologic features observed in our case, specifically intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells, were similar to the findings in other reported cases. In one series of 2 cases, the one case showed scar tissue with a moderate, predominantly T-lymphocytic infiltrate and no tumor cells, and the second showed cellular proliferation in the deep dermis with dense lymphocytic infiltrates primarily composed of CD3-positive T cells.14 Other studies of regression of both localized and metastatic MCC demonstrated infiltration by CD4-positive, CD8-positive, and CD3-positive lymphocytes and foamy macrophages.21-23
The discovery of the MCV was one of the most important advances in elucidating the pathogenesis of MCC.10,24-26 Merkel cell polyomavirus DNA has been detected in a majority of MCC cases.25,27 Viral integration has been shown to take place early, prior to tumor clonal expansion.10 Importantly, not all cases of MCC show MCV infection, and MCV infection is not exclusive to MCC.28 Merkel cell polyomavirus is considered to be part of the normal human flora, and asymptomatic infection is quite common.29 It has been identified in 80% of adults older than 50 years of age and, interestingly, in 35% of children by 13 years of age or younger.30,31 It remains unclear what role the presence of MCV plays in determining MCC prognosis. Several reports have demonstrated lower disease-specific mortality associated with MCV-positive MCC.32-35 In contrast, Schrama et al36 correlated the MCV status of 174 MCC tumors and found no difference in clinical behavior or prognosis between MCV-positive and MCV-negative MCCs.
Immunosuppression also may play a role in the development of MCC.5,25 There is increased prevalence of MCC in the human immunodeficiency virus–positive population, as well as in organ-transplant recipients and patients with leukemia. Chronic lymphocytic leukemia seems to be the most frequent neoplasia associated with development of MCC.37
The mechanism of MCC regression remains unclear, but many investigators emphasize the importance of T-cell–mediated immunity.16,21-23,38,39 Apoptosis also has been shown to play an important role.40 Our case showed tumor-infiltrating CD8-positive lymphocytes and CD4-positive lymphocytes present predominantly at the periphery of the tumor, with close proximity to the tumor nests but with no tumor infiltration (Figure 3). This distribution was consistently present in multiple sections of the tumor. These findings are consistent with prior reports of both CD4-positive and CD8-positive T lymphocytes associated with MCC regression. Our findings confirm that immune response may play an important role in spontaneous regression of MCC.
There is much speculation regarding the initial biopsy of an MCC lesion (or other traumatic event) and its role in tumor regression. Koba et al41 examined the effect of biopsy on CD8-positive lymphocytic infiltration of MCC tumor cells and found that biopsy does not commonly alter intratumoral CD8-positive infiltration. These findings suggest trauma does not directly induce immunologic recognition of this cancer.
Conclusion
We report a case of complete spontaneous regression of a localized MCC following a punch biopsy. The histopathology showed a brisk T-lymphocyte response with intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells. The age and clinical profile of our patient as well as the clinicopathologic characteristics of the tumor regression are similar to other reported cases. Further research is needed to elucidate the mechanism of MCC regression, and a better understanding of this fascinating phenomenon could help in development of new immunotherapeutic approaches.
Merkel cell carcinoma (MCC) is a rare, rapidly growing, aggressive neoplasm with a generally poor prognosis. The cells of origin are highly anaplastic and share structural and immunohistochemical features with various neuroectodermally derived cells. Although Merkel cells, which are slow-acting cutaneous mechanoreceptors located in the basal layer of the epidermis, and MCC share immunohistochemical and ultrastructural features, there is limited evidence of a direct histogenetic relationship between the two.1,2 Additionally, some extracutaneous neuroendocrine tumors have features similar to MCC; therefore, although it may be more accurate and perhaps more practical to describe these lesions as primary neuroendocrine carcinomas of the skin, the term MCC is more commonly used both in the literature and in clinical practice.1,2
Merkel cell carcinoma typically presents in the head and neck region in white patients older than 70 years of age and in the immunocompromised population.3-6 The mean age of diagnosis is 76 years for women and 74 years for men.7 The incidence of MCC in the United States tripled over a 15-year period, and there are approximately 1500 new cases of MCC diagnosed each year, making it about 40 times less common than melanoma.8 The 5-year survival rate for patients without lymph node involvement is 75%, whereas the 5-year survival rate for patients with distant metastases is 25%.9
Merkel cell carcinoma is thought to develop through 1 of 2 distinct pathways. In a virally mediated pathway, which represents at least 80% of cases, the Merkel cell polyomavirus (MCV) monoclonally integrates into the host genome and promotes oncogenesis via altered p53 and retinoblastoma protein expression.10-12 The remainder of cases are believed to develop via a nonvirally mediated pathway in which genetic anomalies, immune status, and environmental factors influence oncogenesis.10-13
Due to the similarity between MCC and metastatic neuroendocrine neoplasms, especially small-cell lung carcinomas, immunohistochemistry is important in making the diagnosis. Cytokeratin 20 and neuron-specific enolase positivity and thyroid transcription factor 1 negativity are the most useful markers in identifying MCC.
Regression of MCC is a very rare and poorly understood event. A 2010 review of the literature described 22 cases of spontaneous regression.14 We report a rare case of rapid and complete regression of MCC following punch biopsy in a 96-year-old woman.
Case Report

A 4-mm punch biopsy was obtained at a follow-up visit 4 weeks later (12 weeks after the reported onset of the lesion). Hematoxylin and eosin staining showed a small-cell neoplasm with stippled nuclei and scant cytoplasm forming a nested and somewhat trabecular pattern. Mitotic activity, apoptosis, and nuclear molding also were present (Figure 2). The tumor cells were positive for cytokeratin 20 with a dotlike, paranuclear pattern (Figure 3). Staining for CAM 5.2 also was positive. Cytokeratin 5/6, human melanoma black 45, and leukocyte common antigen were negative. The immunophenotyping of the lymphocytic response to the tumor showed that the majority of intratumoral lymphocytes were CD8 positive (Figure 4). CD4-positive lymphocytes were predominantly seen at the periphery of the tumor nests without tumor infiltration (Figure 5). Based on these findings, a diagnosis of MCC was made. The patient’s family declined treatment based on her advanced age and current health status, which included advanced dementia.




Two weeks after the punch biopsy, the lesion had noticeably decreased in size and lost its dome-shaped appearance. Within 8 weeks after biopsy (20 weeks since the lesion first appeared), the lesion had completely resolved (Figure 6). The patient was lost to follow-up months later, but no recurrence of the lesion was reported.

Comment
Spontaneous regression is not unique to MCC, as this phenomenon also has been reported in keratoacanthoma, lymphoma, basal cell carcinoma, and melanoma.15 Complete spontaneous regression is defined as occurring in the absence of therapy that is intended to have a treatment effect.15,16 Spontaneous regression is estimated to occur in malignant neoplasms at a rate of 1 case per 60,000 to 100,000 (approximately 0.0013% of all malignant neoplasms).17 Considering the reported prevalence of MCC and the number of cases that have been known to regress, the estimated incidence of complete spontaneous regression may be as high as 1.5%.14 Though spontaneous regression of MCC is more prevalent than expected, it still is considered a rare phenomenon. A 2010 review of the literature yielded 22 cases of complete spontaneous regression of MCC.14 No recurrences have been observed; however, follow-up was relatively short in some cases.
In a unique report by Bertolotti et al,18 a patient with MCC on the nasal tip presented 4 weeks after biopsy with complete spontaneous regression of the tumor, which was associated with bilateral cervical lymph node involvement as noted by hypermetabolic uptake on positron emission tomography scanning. The patient underwent radiation therapy and was disease free at 12 months’ follow-up.18
Complete spontaneous regression has been described in MCC patients with local disease, regional recurrences, and metastatic disease.19 In
The histopathologic features observed in our case, specifically intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells, were similar to the findings in other reported cases. In one series of 2 cases, the one case showed scar tissue with a moderate, predominantly T-lymphocytic infiltrate and no tumor cells, and the second showed cellular proliferation in the deep dermis with dense lymphocytic infiltrates primarily composed of CD3-positive T cells.14 Other studies of regression of both localized and metastatic MCC demonstrated infiltration by CD4-positive, CD8-positive, and CD3-positive lymphocytes and foamy macrophages.21-23
The discovery of the MCV was one of the most important advances in elucidating the pathogenesis of MCC.10,24-26 Merkel cell polyomavirus DNA has been detected in a majority of MCC cases.25,27 Viral integration has been shown to take place early, prior to tumor clonal expansion.10 Importantly, not all cases of MCC show MCV infection, and MCV infection is not exclusive to MCC.28 Merkel cell polyomavirus is considered to be part of the normal human flora, and asymptomatic infection is quite common.29 It has been identified in 80% of adults older than 50 years of age and, interestingly, in 35% of children by 13 years of age or younger.30,31 It remains unclear what role the presence of MCV plays in determining MCC prognosis. Several reports have demonstrated lower disease-specific mortality associated with MCV-positive MCC.32-35 In contrast, Schrama et al36 correlated the MCV status of 174 MCC tumors and found no difference in clinical behavior or prognosis between MCV-positive and MCV-negative MCCs.
Immunosuppression also may play a role in the development of MCC.5,25 There is increased prevalence of MCC in the human immunodeficiency virus–positive population, as well as in organ-transplant recipients and patients with leukemia. Chronic lymphocytic leukemia seems to be the most frequent neoplasia associated with development of MCC.37
The mechanism of MCC regression remains unclear, but many investigators emphasize the importance of T-cell–mediated immunity.16,21-23,38,39 Apoptosis also has been shown to play an important role.40 Our case showed tumor-infiltrating CD8-positive lymphocytes and CD4-positive lymphocytes present predominantly at the periphery of the tumor, with close proximity to the tumor nests but with no tumor infiltration (Figure 3). This distribution was consistently present in multiple sections of the tumor. These findings are consistent with prior reports of both CD4-positive and CD8-positive T lymphocytes associated with MCC regression. Our findings confirm that immune response may play an important role in spontaneous regression of MCC.
There is much speculation regarding the initial biopsy of an MCC lesion (or other traumatic event) and its role in tumor regression. Koba et al41 examined the effect of biopsy on CD8-positive lymphocytic infiltration of MCC tumor cells and found that biopsy does not commonly alter intratumoral CD8-positive infiltration. These findings suggest trauma does not directly induce immunologic recognition of this cancer.
Conclusion
We report a case of complete spontaneous regression of a localized MCC following a punch biopsy. The histopathology showed a brisk T-lymphocyte response with intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells. The age and clinical profile of our patient as well as the clinicopathologic characteristics of the tumor regression are similar to other reported cases. Further research is needed to elucidate the mechanism of MCC regression, and a better understanding of this fascinating phenomenon could help in development of new immunotherapeutic approaches.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Sibley RK, Dahl D. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. an immunocytochemical study of 21 cases. Am J Surg Pathol. 1985;9:109-116.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Gooptu C, Woolloons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immunosuppression. Br J Dermatol. 1997;137:637-641.
- Plunkett TA, Harris AJ, Ogg CS, et al. The treatment of Merkel cell carcinoma and its association with immunosuppression. Br J Dermatol. 1998;139:345-346.
- Calder KB, Smoller BR. New insights into Merkel cell carcinoma. Adv Anat Pathol. 2010;17:155-161.
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
- Decaprio JA. Does detection of Merkel cell polyomavirus in Merkel cell carcinoma provide prognostic information? J Natl Cancer Inst. 2009;101:905-907.
- Popp S, Waltering S, Herbst C, et al. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99:352-360.
- Ciudad C, Avilés JA, Alfageme F, et al. Spontaneous regression in Merkel cell carcinoma: report of two cases with description of dermoscopic features and review of literature. Dermatol Surg. 2010;36:687-693.
- O’Rourke MGE, Bell JR. Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol. 1986;12:994-997.
- Connelly TJ, Cribier B, Brown TJ, et al. Complete spontaneous regression of Merkel cell carcinoma: a review of 10 reported cases. Dermatol Surg. 2000;26:853-856.
- Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201-209.
- Bertolotti A, Conte H, Francois L, et al. Merkel cell carcinoma: complete clinical remission associated with disease progression. JAMA Dermatol. 2013;149:501-502.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Richetta AG, Mancini M, Torroni A, et al. Total spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg. 2008;34:815-822.
- Vesely MJ, Murray DJ, Neligan PC, et al. Complete spontaneous regression in Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2008;61:165-171.
- Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991;127:550-553.
- Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma-a case showing replacement of tumour cells by foamy cells. Br J Dermatol. 2000;142:1184-1189.
- Duncavage E, Zehnbauer B, Pfeifer J. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of unique deletion in the VP1 gene. Cancer Res. 2008;68:5009-5013.
- Becker J, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2009;66:1-8.
- Haitz KA, Rady PL, Nguyen HP, et al. Merkel cell polyomavirus DNA detection in a patient with Merkel cell carcinoma and multiple other skin cancers. Int J Dermatol. 2012;51:442-444.
- Andres C, Puchta U, Sander CA, et al. Prevalence of Merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. 2010;32:593-598.
- Showalter RM, Pastrana DV, Pumphrey KA, et al. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509-515.
- Tolstov YL, Pastrana DV, Feng H, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250-1256.
- Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125-129.
- Laude HC, Jonchère B, Maubec E, et al. Distinct Merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with Merkel cell carcinoma. PLoS Pathog. 2010;6:e1001076.
- Waltari M, Sihto H, Kukko H, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129:619-628.
- Sihto H, Kukko H, Koljonen V, et al. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938-945.
- Paulson KG, Lemos BD, Feng B, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547-1555.
- Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Invest Dermatol. 2011;131:1631-1638.
- Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250-256.
- Wooff J, Trites JR, Walsh NM, et al. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:614-617.
- Turk TO, Smoljan I, Nacinovic A, et al. Spontaneous regression of Merkel cell carcinoma in a patient with chronic lymphocytic leukemia: a case report. J Med Case Rep. 2009;3:7270.
- Mori Y, Tanaka K, Cui CY, et al. A study of apoptosis in Merkel cell carcinoma. an immunohistochemical, ultrasctructural, DNA ladder and TUNEL labeling study. Am J Dermatopathol. 2001;23:16-23.
- Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS ONE. 2012;7:e41465.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Sibley RK, Dahl D. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. an immunocytochemical study of 21 cases. Am J Surg Pathol. 1985;9:109-116.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Gooptu C, Woolloons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immunosuppression. Br J Dermatol. 1997;137:637-641.
- Plunkett TA, Harris AJ, Ogg CS, et al. The treatment of Merkel cell carcinoma and its association with immunosuppression. Br J Dermatol. 1998;139:345-346.
- Calder KB, Smoller BR. New insights into Merkel cell carcinoma. Adv Anat Pathol. 2010;17:155-161.
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
- Decaprio JA. Does detection of Merkel cell polyomavirus in Merkel cell carcinoma provide prognostic information? J Natl Cancer Inst. 2009;101:905-907.
- Popp S, Waltering S, Herbst C, et al. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99:352-360.
- Ciudad C, Avilés JA, Alfageme F, et al. Spontaneous regression in Merkel cell carcinoma: report of two cases with description of dermoscopic features and review of literature. Dermatol Surg. 2010;36:687-693.
- O’Rourke MGE, Bell JR. Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol. 1986;12:994-997.
- Connelly TJ, Cribier B, Brown TJ, et al. Complete spontaneous regression of Merkel cell carcinoma: a review of 10 reported cases. Dermatol Surg. 2000;26:853-856.
- Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201-209.
- Bertolotti A, Conte H, Francois L, et al. Merkel cell carcinoma: complete clinical remission associated with disease progression. JAMA Dermatol. 2013;149:501-502.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Richetta AG, Mancini M, Torroni A, et al. Total spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg. 2008;34:815-822.
- Vesely MJ, Murray DJ, Neligan PC, et al. Complete spontaneous regression in Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2008;61:165-171.
- Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991;127:550-553.
- Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma-a case showing replacement of tumour cells by foamy cells. Br J Dermatol. 2000;142:1184-1189.
- Duncavage E, Zehnbauer B, Pfeifer J. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of unique deletion in the VP1 gene. Cancer Res. 2008;68:5009-5013.
- Becker J, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2009;66:1-8.
- Haitz KA, Rady PL, Nguyen HP, et al. Merkel cell polyomavirus DNA detection in a patient with Merkel cell carcinoma and multiple other skin cancers. Int J Dermatol. 2012;51:442-444.
- Andres C, Puchta U, Sander CA, et al. Prevalence of Merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. 2010;32:593-598.
- Showalter RM, Pastrana DV, Pumphrey KA, et al. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509-515.
- Tolstov YL, Pastrana DV, Feng H, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250-1256.
- Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125-129.
- Laude HC, Jonchère B, Maubec E, et al. Distinct Merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with Merkel cell carcinoma. PLoS Pathog. 2010;6:e1001076.
- Waltari M, Sihto H, Kukko H, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129:619-628.
- Sihto H, Kukko H, Koljonen V, et al. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938-945.
- Paulson KG, Lemos BD, Feng B, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547-1555.
- Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Invest Dermatol. 2011;131:1631-1638.
- Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250-256.
- Wooff J, Trites JR, Walsh NM, et al. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:614-617.
- Turk TO, Smoljan I, Nacinovic A, et al. Spontaneous regression of Merkel cell carcinoma in a patient with chronic lymphocytic leukemia: a case report. J Med Case Rep. 2009;3:7270.
- Mori Y, Tanaka K, Cui CY, et al. A study of apoptosis in Merkel cell carcinoma. an immunohistochemical, ultrasctructural, DNA ladder and TUNEL labeling study. Am J Dermatopathol. 2001;23:16-23.
- Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS ONE. 2012;7:e41465.
Practice Points
- Merkel cell carcinoma (MCC) is a rare malignancy with a high rate of metastasis and poor prognosis.
- T-cell mediated immunity appears to play an important role in tumor regression in MCC.
- Merkel cell polyomavirus appears to play a role in the pathogenesis of MCC and may be associated with a better prognosis.
- A better understanding of spontaneous regression of MCC could help in the development of new immunotherapeutic approaches to this malignancy.
Gone Fishing: A Unique Histologic Pattern in Cutaneous Angiosarcoma
Cutaneous angiosarcoma is a rare malignant tumor of vascular endothelial cells that has the propensity to arise in various clinical settings. This tumor predominantly occurs in the head and neck region in elderly patients, but it also has been reported to develop postradiotherapy or in the setting of chronic lymphedema in the extremities.1-3 In all settings, the diagnosis carries a very poor prognosis with a high likelihood of local recurrence and rapid dissemination. The mortality rate typically is 80% or higher.2,4-6
Making the correct clinical diagnosis of cutaneous angiosarcoma may be difficult given the variety of patient symptoms and clinical appearances that can be demonstrated on presentation. Lesions can appear as bluish or violaceous plaques, macules, or nodules, and ulceration may be present in some advanced cases.5,7 Clinical misdiagnosis is common, as cutaneous angiosarcomas may be mistaken for infectious processes, benign vascular malformations, and other cutaneous malignancies.1 Biopsy often is delayed given the initial benign appearance of the lesions, and this frequently results in aggressive and extensive disease at the time of diagnosis, which is unfortunate given that small tumor size has been shown to be one of the only favorable prognostic indicators in cutaneous angiosarcoma.1,2,6,8
Microscopically, diagnosis of cutaneous angiosarcoma can present a challenge, as the histology varies between a well-differentiated vascular neoplasm and a considerably anaplastic and poorly differentiated malignancy. On low power, some areas may appear as benign hemangiomas with other areas showing frank sarcomatous features.9 As a result, these tumors can be mistaken for a variety of other diseases including melanomas, carcinomas, or other vascular tumors.6,8,9 Previously, electron microscopy has been utilized on undifferentiated tumors to help distinguish cutaneous angiosarcomas from other potential diagnoses. The atypical tumor cells of cutaneous angiosarcoma display common features of endothelial cells (eg, pinocytotic vesicles, tubulated bodies).7 Historically, it has been noted that the histologic findings and tumor grade provide little evidence regarding the aggressiveness of the tumor, and all cutaneous angiosarcoma diagnoses receive a poor prognosis.6,8
Classically, the histologic findings of cutaneous angiosarcoma include a highly infiltrative neoplasm forming irregular vascular channels that penetrate through the cutaneous soft tissues and frequently extend into the subcutaneous fat. The vascular spaces are lined by hyperchromatic endothelial cells with varying degrees of atypia.1,2,4,6,7,10 Occasionally, prominent endothelial cells lining a papillary structure within the lumen of the neoformed vessel may also be observed. Currently, immunohistochemical staining for MYC, Ki-67, D2-40, and various other markers complement the histologic findings to aid in the diagnosis of cutaneous angiosarcoma.11,12 An additional diagnostic clue that has been described in cases of postirradiation cutaneous angiosarcoma shows free-floating or tufted pleomorphic spindle cells within the vascular lumen (Figure). This finding has been described as “fish in the creek.”11 In this study, we aimed to determine the frequency and subsequent diagnostic utility of the fish-in-the-creek finding in cases of cutaneous angiosarcoma.

Methods
A natural language search of our institutional archives over a 20-year period (1997–2017) using the term angiosarcoma was performed. Fifteen cases of cutaneous angiosarcoma were identified. Fifteen additional benign and malignant vascular tumors with cutaneous angiosarco
Results
The histologic pattern of fish in the creek was identified in all 15 cases of cutaneous angiosarcoma and was absent in the other 15 malignancies examined in this study. This finding shows the potential for the fish-in-the-creek pattern to be used as an additional diagnostic tool for dermatopathologists.
Comment
Cutaneous angiosarcoma is a rare but aggressive malignancy that proves difficult to diagnose both clinically and histologically as well as to treat effectively.1,5-8 Our results indicate that fish in the creek may be a useful and salient histologic feature in cutaneous angiosarcoma. It is important to recognize, however, that this finding should not be the sole feature upon which a diagnosis of cutaneous angiosarcoma is made, as it requires corroboration with positivity of MYC and D2-40 as well as a high Ki-67 proliferation index (>20%).11,12 Finding a fish-in-the-creek pattern should prompt dermatopathologists to consider a diagnosis of cutaneous angiosarcoma in the appropriate clinical and histologic settings.
The chief limitation of this study was the small sample size, with only 15 cases of cutaneous angiosarcoma available in the last 20 years at our institution. The limited sample size did not allow us to make claims on sensitivity and specificity regarding this histologic feature; however, with a larger sample size, the true diagnostic potential could be elucidated. Although the pathologists were blinded to the original diagnoses as they examined it for fish in the creek, it is possible they were able to make the correct diagnosis based on other histopathologic clues and therefore were biased.
Although the fish-in-the-creek pattern is present in cutaneous angiosarcoma, there may be other mimickers to consider. Intraluminal papillary projections lined by endothelial cells may be sectioned in a manner imitating this finding.3 In such a case, these endothelial cells must be differentiated from the free-floating or tufted spindle cells in order to have a positive finding for fish in the creek. There can be confusion if the biopsy cuts through a section of spindled cells, resulting in difficulty differentiating cutaneous angiosarcoma from other spindle tumors such as spindle cell melanoma or spindle cell squamous cell carcinoma.6 In such cases, immunohistochemistry may be helpful, as spindle cell melanoma would stain positive for S100 and SOX10 and spindle cell squamous cell carcinoma would stain positive for p63 and cytokeratin.
Various treatment strategies for cutaneous angiosarcoma have been employed, with the majority still resulting in poor outcomes.2,4-6 The recommended treatment is radical surgical excision of the primary tumor with lymph node clearance if possible. Following excision, the patient should undergo high-dose, wide-field radiotherapy to the region.5,8 Cutaneous angiosarcomas also have the ability to spread extensively through the dermis and can result in subclinical or clinically obvious widespread disease with multifocal or satellite lesions present. Distant metastases occur most frequently in the cervical lymph nodes and lungs.7 In cases where the disease is too extensive for surgery, palliative radiation monotherapy can be used.5,6
As atypical vascular lesions are considered to be a precursor to cutaneous angiosarcoma, it is important to note that the fish-in-the-creek feature was absent in all 6 of the atypical vascular lesions observed in the study. The differentiation generally is made based on MYC, which is present in cutaneous angiosarcomas and absent in atypical vascular lesions.10 The feature of fish in the creek may now be an additional clue for dermatopathologists to differentiate between angiosarcomas and other similar-appearing tumors.
Conclusion
Our study aimed to highlight an important histologic feature of cutaneous angiosarcomas that can aid in the diagnosis of this deceptive malignancy. Our findings warrant further study of the fish-in-the-creek histologic pattern in a larger sample size to determine its success as a diagnostic tool for cutaneous angiosarcomas. As noted previously, tumor grade does not impact survival outcome, but small tumor size has been one of the only features found to result in a more favorable prognosis.1,6,8 Future studies to identify a correlation between the histologic finding of fish in the creek and disease outcome in cutaneous angiosarcoma may be helpful to determine if these histologic findings provide prognostic significance in cases of cutaneous angiosarcoma.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Woodward AH, Ivins JC, Soule EH. Lymphangiosarcoma arising in chronic lymphedematous extremities. Cancer. 1972;30:562-572.
- Calonje E, Brenn T, McKee PH, et al. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier Saunders; 2012.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Rosai J, Sumner HW, Kostianovsky M, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Pawlik TM, Paulino AF, Mcginn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. Edinburgh, Scotland: Saunders Elsevier; 2014.
- Requena L, Kutzner H. Cutaneous Soft Tissue Tumors. Philadelphia, PA: Wolters Kluwer; 2015.
- Cuda J, Mirzamani N, Kantipudi R, et al. Diagnostic utility of Fli-1 and D2-40 in distinguishing atypical fibroxanthoma from angiosarcoma. Am J Dermatopathol. 2013;35:316-318.
Cutaneous angiosarcoma is a rare malignant tumor of vascular endothelial cells that has the propensity to arise in various clinical settings. This tumor predominantly occurs in the head and neck region in elderly patients, but it also has been reported to develop postradiotherapy or in the setting of chronic lymphedema in the extremities.1-3 In all settings, the diagnosis carries a very poor prognosis with a high likelihood of local recurrence and rapid dissemination. The mortality rate typically is 80% or higher.2,4-6
Making the correct clinical diagnosis of cutaneous angiosarcoma may be difficult given the variety of patient symptoms and clinical appearances that can be demonstrated on presentation. Lesions can appear as bluish or violaceous plaques, macules, or nodules, and ulceration may be present in some advanced cases.5,7 Clinical misdiagnosis is common, as cutaneous angiosarcomas may be mistaken for infectious processes, benign vascular malformations, and other cutaneous malignancies.1 Biopsy often is delayed given the initial benign appearance of the lesions, and this frequently results in aggressive and extensive disease at the time of diagnosis, which is unfortunate given that small tumor size has been shown to be one of the only favorable prognostic indicators in cutaneous angiosarcoma.1,2,6,8
Microscopically, diagnosis of cutaneous angiosarcoma can present a challenge, as the histology varies between a well-differentiated vascular neoplasm and a considerably anaplastic and poorly differentiated malignancy. On low power, some areas may appear as benign hemangiomas with other areas showing frank sarcomatous features.9 As a result, these tumors can be mistaken for a variety of other diseases including melanomas, carcinomas, or other vascular tumors.6,8,9 Previously, electron microscopy has been utilized on undifferentiated tumors to help distinguish cutaneous angiosarcomas from other potential diagnoses. The atypical tumor cells of cutaneous angiosarcoma display common features of endothelial cells (eg, pinocytotic vesicles, tubulated bodies).7 Historically, it has been noted that the histologic findings and tumor grade provide little evidence regarding the aggressiveness of the tumor, and all cutaneous angiosarcoma diagnoses receive a poor prognosis.6,8
Classically, the histologic findings of cutaneous angiosarcoma include a highly infiltrative neoplasm forming irregular vascular channels that penetrate through the cutaneous soft tissues and frequently extend into the subcutaneous fat. The vascular spaces are lined by hyperchromatic endothelial cells with varying degrees of atypia.1,2,4,6,7,10 Occasionally, prominent endothelial cells lining a papillary structure within the lumen of the neoformed vessel may also be observed. Currently, immunohistochemical staining for MYC, Ki-67, D2-40, and various other markers complement the histologic findings to aid in the diagnosis of cutaneous angiosarcoma.11,12 An additional diagnostic clue that has been described in cases of postirradiation cutaneous angiosarcoma shows free-floating or tufted pleomorphic spindle cells within the vascular lumen (Figure). This finding has been described as “fish in the creek.”11 In this study, we aimed to determine the frequency and subsequent diagnostic utility of the fish-in-the-creek finding in cases of cutaneous angiosarcoma.

Methods
A natural language search of our institutional archives over a 20-year period (1997–2017) using the term angiosarcoma was performed. Fifteen cases of cutaneous angiosarcoma were identified. Fifteen additional benign and malignant vascular tumors with cutaneous angiosarco
Results
The histologic pattern of fish in the creek was identified in all 15 cases of cutaneous angiosarcoma and was absent in the other 15 malignancies examined in this study. This finding shows the potential for the fish-in-the-creek pattern to be used as an additional diagnostic tool for dermatopathologists.
Comment
Cutaneous angiosarcoma is a rare but aggressive malignancy that proves difficult to diagnose both clinically and histologically as well as to treat effectively.1,5-8 Our results indicate that fish in the creek may be a useful and salient histologic feature in cutaneous angiosarcoma. It is important to recognize, however, that this finding should not be the sole feature upon which a diagnosis of cutaneous angiosarcoma is made, as it requires corroboration with positivity of MYC and D2-40 as well as a high Ki-67 proliferation index (>20%).11,12 Finding a fish-in-the-creek pattern should prompt dermatopathologists to consider a diagnosis of cutaneous angiosarcoma in the appropriate clinical and histologic settings.
The chief limitation of this study was the small sample size, with only 15 cases of cutaneous angiosarcoma available in the last 20 years at our institution. The limited sample size did not allow us to make claims on sensitivity and specificity regarding this histologic feature; however, with a larger sample size, the true diagnostic potential could be elucidated. Although the pathologists were blinded to the original diagnoses as they examined it for fish in the creek, it is possible they were able to make the correct diagnosis based on other histopathologic clues and therefore were biased.
Although the fish-in-the-creek pattern is present in cutaneous angiosarcoma, there may be other mimickers to consider. Intraluminal papillary projections lined by endothelial cells may be sectioned in a manner imitating this finding.3 In such a case, these endothelial cells must be differentiated from the free-floating or tufted spindle cells in order to have a positive finding for fish in the creek. There can be confusion if the biopsy cuts through a section of spindled cells, resulting in difficulty differentiating cutaneous angiosarcoma from other spindle tumors such as spindle cell melanoma or spindle cell squamous cell carcinoma.6 In such cases, immunohistochemistry may be helpful, as spindle cell melanoma would stain positive for S100 and SOX10 and spindle cell squamous cell carcinoma would stain positive for p63 and cytokeratin.
Various treatment strategies for cutaneous angiosarcoma have been employed, with the majority still resulting in poor outcomes.2,4-6 The recommended treatment is radical surgical excision of the primary tumor with lymph node clearance if possible. Following excision, the patient should undergo high-dose, wide-field radiotherapy to the region.5,8 Cutaneous angiosarcomas also have the ability to spread extensively through the dermis and can result in subclinical or clinically obvious widespread disease with multifocal or satellite lesions present. Distant metastases occur most frequently in the cervical lymph nodes and lungs.7 In cases where the disease is too extensive for surgery, palliative radiation monotherapy can be used.5,6
As atypical vascular lesions are considered to be a precursor to cutaneous angiosarcoma, it is important to note that the fish-in-the-creek feature was absent in all 6 of the atypical vascular lesions observed in the study. The differentiation generally is made based on MYC, which is present in cutaneous angiosarcomas and absent in atypical vascular lesions.10 The feature of fish in the creek may now be an additional clue for dermatopathologists to differentiate between angiosarcomas and other similar-appearing tumors.
Conclusion
Our study aimed to highlight an important histologic feature of cutaneous angiosarcomas that can aid in the diagnosis of this deceptive malignancy. Our findings warrant further study of the fish-in-the-creek histologic pattern in a larger sample size to determine its success as a diagnostic tool for cutaneous angiosarcomas. As noted previously, tumor grade does not impact survival outcome, but small tumor size has been one of the only features found to result in a more favorable prognosis.1,6,8 Future studies to identify a correlation between the histologic finding of fish in the creek and disease outcome in cutaneous angiosarcoma may be helpful to determine if these histologic findings provide prognostic significance in cases of cutaneous angiosarcoma.
Cutaneous angiosarcoma is a rare malignant tumor of vascular endothelial cells that has the propensity to arise in various clinical settings. This tumor predominantly occurs in the head and neck region in elderly patients, but it also has been reported to develop postradiotherapy or in the setting of chronic lymphedema in the extremities.1-3 In all settings, the diagnosis carries a very poor prognosis with a high likelihood of local recurrence and rapid dissemination. The mortality rate typically is 80% or higher.2,4-6
Making the correct clinical diagnosis of cutaneous angiosarcoma may be difficult given the variety of patient symptoms and clinical appearances that can be demonstrated on presentation. Lesions can appear as bluish or violaceous plaques, macules, or nodules, and ulceration may be present in some advanced cases.5,7 Clinical misdiagnosis is common, as cutaneous angiosarcomas may be mistaken for infectious processes, benign vascular malformations, and other cutaneous malignancies.1 Biopsy often is delayed given the initial benign appearance of the lesions, and this frequently results in aggressive and extensive disease at the time of diagnosis, which is unfortunate given that small tumor size has been shown to be one of the only favorable prognostic indicators in cutaneous angiosarcoma.1,2,6,8
Microscopically, diagnosis of cutaneous angiosarcoma can present a challenge, as the histology varies between a well-differentiated vascular neoplasm and a considerably anaplastic and poorly differentiated malignancy. On low power, some areas may appear as benign hemangiomas with other areas showing frank sarcomatous features.9 As a result, these tumors can be mistaken for a variety of other diseases including melanomas, carcinomas, or other vascular tumors.6,8,9 Previously, electron microscopy has been utilized on undifferentiated tumors to help distinguish cutaneous angiosarcomas from other potential diagnoses. The atypical tumor cells of cutaneous angiosarcoma display common features of endothelial cells (eg, pinocytotic vesicles, tubulated bodies).7 Historically, it has been noted that the histologic findings and tumor grade provide little evidence regarding the aggressiveness of the tumor, and all cutaneous angiosarcoma diagnoses receive a poor prognosis.6,8
Classically, the histologic findings of cutaneous angiosarcoma include a highly infiltrative neoplasm forming irregular vascular channels that penetrate through the cutaneous soft tissues and frequently extend into the subcutaneous fat. The vascular spaces are lined by hyperchromatic endothelial cells with varying degrees of atypia.1,2,4,6,7,10 Occasionally, prominent endothelial cells lining a papillary structure within the lumen of the neoformed vessel may also be observed. Currently, immunohistochemical staining for MYC, Ki-67, D2-40, and various other markers complement the histologic findings to aid in the diagnosis of cutaneous angiosarcoma.11,12 An additional diagnostic clue that has been described in cases of postirradiation cutaneous angiosarcoma shows free-floating or tufted pleomorphic spindle cells within the vascular lumen (Figure). This finding has been described as “fish in the creek.”11 In this study, we aimed to determine the frequency and subsequent diagnostic utility of the fish-in-the-creek finding in cases of cutaneous angiosarcoma.

Methods
A natural language search of our institutional archives over a 20-year period (1997–2017) using the term angiosarcoma was performed. Fifteen cases of cutaneous angiosarcoma were identified. Fifteen additional benign and malignant vascular tumors with cutaneous angiosarco
Results
The histologic pattern of fish in the creek was identified in all 15 cases of cutaneous angiosarcoma and was absent in the other 15 malignancies examined in this study. This finding shows the potential for the fish-in-the-creek pattern to be used as an additional diagnostic tool for dermatopathologists.
Comment
Cutaneous angiosarcoma is a rare but aggressive malignancy that proves difficult to diagnose both clinically and histologically as well as to treat effectively.1,5-8 Our results indicate that fish in the creek may be a useful and salient histologic feature in cutaneous angiosarcoma. It is important to recognize, however, that this finding should not be the sole feature upon which a diagnosis of cutaneous angiosarcoma is made, as it requires corroboration with positivity of MYC and D2-40 as well as a high Ki-67 proliferation index (>20%).11,12 Finding a fish-in-the-creek pattern should prompt dermatopathologists to consider a diagnosis of cutaneous angiosarcoma in the appropriate clinical and histologic settings.
The chief limitation of this study was the small sample size, with only 15 cases of cutaneous angiosarcoma available in the last 20 years at our institution. The limited sample size did not allow us to make claims on sensitivity and specificity regarding this histologic feature; however, with a larger sample size, the true diagnostic potential could be elucidated. Although the pathologists were blinded to the original diagnoses as they examined it for fish in the creek, it is possible they were able to make the correct diagnosis based on other histopathologic clues and therefore were biased.
Although the fish-in-the-creek pattern is present in cutaneous angiosarcoma, there may be other mimickers to consider. Intraluminal papillary projections lined by endothelial cells may be sectioned in a manner imitating this finding.3 In such a case, these endothelial cells must be differentiated from the free-floating or tufted spindle cells in order to have a positive finding for fish in the creek. There can be confusion if the biopsy cuts through a section of spindled cells, resulting in difficulty differentiating cutaneous angiosarcoma from other spindle tumors such as spindle cell melanoma or spindle cell squamous cell carcinoma.6 In such cases, immunohistochemistry may be helpful, as spindle cell melanoma would stain positive for S100 and SOX10 and spindle cell squamous cell carcinoma would stain positive for p63 and cytokeratin.
Various treatment strategies for cutaneous angiosarcoma have been employed, with the majority still resulting in poor outcomes.2,4-6 The recommended treatment is radical surgical excision of the primary tumor with lymph node clearance if possible. Following excision, the patient should undergo high-dose, wide-field radiotherapy to the region.5,8 Cutaneous angiosarcomas also have the ability to spread extensively through the dermis and can result in subclinical or clinically obvious widespread disease with multifocal or satellite lesions present. Distant metastases occur most frequently in the cervical lymph nodes and lungs.7 In cases where the disease is too extensive for surgery, palliative radiation monotherapy can be used.5,6
As atypical vascular lesions are considered to be a precursor to cutaneous angiosarcoma, it is important to note that the fish-in-the-creek feature was absent in all 6 of the atypical vascular lesions observed in the study. The differentiation generally is made based on MYC, which is present in cutaneous angiosarcomas and absent in atypical vascular lesions.10 The feature of fish in the creek may now be an additional clue for dermatopathologists to differentiate between angiosarcomas and other similar-appearing tumors.
Conclusion
Our study aimed to highlight an important histologic feature of cutaneous angiosarcomas that can aid in the diagnosis of this deceptive malignancy. Our findings warrant further study of the fish-in-the-creek histologic pattern in a larger sample size to determine its success as a diagnostic tool for cutaneous angiosarcomas. As noted previously, tumor grade does not impact survival outcome, but small tumor size has been one of the only features found to result in a more favorable prognosis.1,6,8 Future studies to identify a correlation between the histologic finding of fish in the creek and disease outcome in cutaneous angiosarcoma may be helpful to determine if these histologic findings provide prognostic significance in cases of cutaneous angiosarcoma.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Woodward AH, Ivins JC, Soule EH. Lymphangiosarcoma arising in chronic lymphedematous extremities. Cancer. 1972;30:562-572.
- Calonje E, Brenn T, McKee PH, et al. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier Saunders; 2012.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Rosai J, Sumner HW, Kostianovsky M, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Pawlik TM, Paulino AF, Mcginn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. Edinburgh, Scotland: Saunders Elsevier; 2014.
- Requena L, Kutzner H. Cutaneous Soft Tissue Tumors. Philadelphia, PA: Wolters Kluwer; 2015.
- Cuda J, Mirzamani N, Kantipudi R, et al. Diagnostic utility of Fli-1 and D2-40 in distinguishing atypical fibroxanthoma from angiosarcoma. Am J Dermatopathol. 2013;35:316-318.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Woodward AH, Ivins JC, Soule EH. Lymphangiosarcoma arising in chronic lymphedematous extremities. Cancer. 1972;30:562-572.
- Calonje E, Brenn T, McKee PH, et al. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier Saunders; 2012.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Rosai J, Sumner HW, Kostianovsky M, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Pawlik TM, Paulino AF, Mcginn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. Edinburgh, Scotland: Saunders Elsevier; 2014.
- Requena L, Kutzner H. Cutaneous Soft Tissue Tumors. Philadelphia, PA: Wolters Kluwer; 2015.
- Cuda J, Mirzamani N, Kantipudi R, et al. Diagnostic utility of Fli-1 and D2-40 in distinguishing atypical fibroxanthoma from angiosarcoma. Am J Dermatopathol. 2013;35:316-318.
Practice Points
- The histologic finding of “fish in the creek” is characterized by free-floating or tufted pleomorphic spindle cells within the vascular lumen.
- Fish in the creek has only been demonstrated in cutaneous angiosarcoma when compared to histologic findings of other similar vascular malignancies.
- The fish-in-the-creek finding may be an additional diagnostic tool in cases of cutaneous angiosarcoma.
Posttransplant skin conditions vary widely by ethnicity
SAN DIEGO – A new study finds that the risk of skin cancers in organ transplant recipients may vary widely by ethnicity.
“The most important findings from our study are the high rates of keratinocyte neoplasms observed in our white Northern European patients, but also in those of Far East Asian descent. Dermatologists should also appreciate the high risk of Kaposi’s sarcoma (KS) in patients originating from Sub-Saharan Africa,” Jonathan Kentley, MBBS, of Royal London Hospital, said in an interview. He presented the study findings at the annual meeting of the American Academy of Dermatology.
For the study, Dr. Kentley and colleagues sought to better understand .
They analyzed an organ transplant center database for the years 1989-2016, and tracked 1,304 consecutive patients – which included 1,125 with skin problems. The overall population was 64% male with a median age in the early 40s, and almost all (1,276) had undergone renal transplants. A relative handful underwent liver, lung, heart, and pancreas transplants.
The majority of patients (885) were white Northern Europeans, but there were also significant numbers of people with South Asian (202), black African/Caribbean (131) and white/Mediterranean (52) heritage. A small number were Far East Asian (26) and Middle Eastern (8). The median follow-up time for the ethnic groups varied from about 5 years to about 12 years.
The researchers found that basal cell carcinoma was most common in white Northern European patients, at nearly 25%, with other groups under 10%. SCC was common in white Northern European patients and Far East Asians, both at nearly 25%.
By far, KS was the most common in black African/Caribbean patients, at nearly 11%. According to Dr. Kentley, researchers found the number of KS cases to be surprisingly high in this group, “compounded by the fact that we have had a number of additional cases in the past year after we had collected the data for this study.” He attributes the higher number of KS cases in these patients to an increased seroprevalence of its causative agent, human herpesvirus-8, in Sub-Saharan Africa. The rate of KS in the second most commonly affected group – white Mediterranean patients – was almost 2%.
Viral warts were common in most groups, with the rate in both white groups (white Northern European and white Mediterranean) at nearly 60%, and Far East Asians at about 65%. Porokeratosis was by far the most common in white Norther Europeans, at nearly 8%, and sebaceous hyperplasia was common in all groups (more than 20% to about 27%) except in the black African/Caribbean and South Asian groups.
All these results were statistically significant with P values less than .05.
“Our study has confirmed the increased risk of keratinocyte cancers in patients of white Northern European descent, as well as providing more information on the increased risk in patients of Far East Asian descent,” Dr. Kentley said. “We have also confirmed the propensity of black African/Caribbean patients to develop Kaposi’s sarcoma in the first 5 years post transplant and highlighted that white Mediterranean patients are also at high risk. Beyond this, we have been able to review the prevalence of rare malignancies, such as Merkel cell carcinoma and appendageal tumors, and highlight that white Northern European patients remain at high risk of developing these conditions.”
As for the impact on clinical practice, “the patterns of skin disease susceptibility we have identified have important implications for rational design of transplant skin surveillance programs, targeted patient (and provider) education, and optimized clinical management,” Dr. Kentley said. “Ultimately, this is likely to have a significant impact on strategic deployment of limited dermatology health care resources.”
Specifically, the study suggests that all organ transplant patients receive a baseline skin assessment visit and nurse-led targeted education. Black African/Caribbean patients should be followed up for at least 5 years after transplant.
In the United States, at least 724,000 people have undergone organ transplants since 1988, with most getting kidney transplants, according to the United Network for Organ Sharing (UNOS).
No study funding was reported. The authors had no disclosures.
SOURCE: Kentley J et al. AAD 2018, Session F055.
SAN DIEGO – A new study finds that the risk of skin cancers in organ transplant recipients may vary widely by ethnicity.
“The most important findings from our study are the high rates of keratinocyte neoplasms observed in our white Northern European patients, but also in those of Far East Asian descent. Dermatologists should also appreciate the high risk of Kaposi’s sarcoma (KS) in patients originating from Sub-Saharan Africa,” Jonathan Kentley, MBBS, of Royal London Hospital, said in an interview. He presented the study findings at the annual meeting of the American Academy of Dermatology.
For the study, Dr. Kentley and colleagues sought to better understand .
They analyzed an organ transplant center database for the years 1989-2016, and tracked 1,304 consecutive patients – which included 1,125 with skin problems. The overall population was 64% male with a median age in the early 40s, and almost all (1,276) had undergone renal transplants. A relative handful underwent liver, lung, heart, and pancreas transplants.
The majority of patients (885) were white Northern Europeans, but there were also significant numbers of people with South Asian (202), black African/Caribbean (131) and white/Mediterranean (52) heritage. A small number were Far East Asian (26) and Middle Eastern (8). The median follow-up time for the ethnic groups varied from about 5 years to about 12 years.
The researchers found that basal cell carcinoma was most common in white Northern European patients, at nearly 25%, with other groups under 10%. SCC was common in white Northern European patients and Far East Asians, both at nearly 25%.
By far, KS was the most common in black African/Caribbean patients, at nearly 11%. According to Dr. Kentley, researchers found the number of KS cases to be surprisingly high in this group, “compounded by the fact that we have had a number of additional cases in the past year after we had collected the data for this study.” He attributes the higher number of KS cases in these patients to an increased seroprevalence of its causative agent, human herpesvirus-8, in Sub-Saharan Africa. The rate of KS in the second most commonly affected group – white Mediterranean patients – was almost 2%.
Viral warts were common in most groups, with the rate in both white groups (white Northern European and white Mediterranean) at nearly 60%, and Far East Asians at about 65%. Porokeratosis was by far the most common in white Norther Europeans, at nearly 8%, and sebaceous hyperplasia was common in all groups (more than 20% to about 27%) except in the black African/Caribbean and South Asian groups.
All these results were statistically significant with P values less than .05.
“Our study has confirmed the increased risk of keratinocyte cancers in patients of white Northern European descent, as well as providing more information on the increased risk in patients of Far East Asian descent,” Dr. Kentley said. “We have also confirmed the propensity of black African/Caribbean patients to develop Kaposi’s sarcoma in the first 5 years post transplant and highlighted that white Mediterranean patients are also at high risk. Beyond this, we have been able to review the prevalence of rare malignancies, such as Merkel cell carcinoma and appendageal tumors, and highlight that white Northern European patients remain at high risk of developing these conditions.”
As for the impact on clinical practice, “the patterns of skin disease susceptibility we have identified have important implications for rational design of transplant skin surveillance programs, targeted patient (and provider) education, and optimized clinical management,” Dr. Kentley said. “Ultimately, this is likely to have a significant impact on strategic deployment of limited dermatology health care resources.”
Specifically, the study suggests that all organ transplant patients receive a baseline skin assessment visit and nurse-led targeted education. Black African/Caribbean patients should be followed up for at least 5 years after transplant.
In the United States, at least 724,000 people have undergone organ transplants since 1988, with most getting kidney transplants, according to the United Network for Organ Sharing (UNOS).
No study funding was reported. The authors had no disclosures.
SOURCE: Kentley J et al. AAD 2018, Session F055.
SAN DIEGO – A new study finds that the risk of skin cancers in organ transplant recipients may vary widely by ethnicity.
“The most important findings from our study are the high rates of keratinocyte neoplasms observed in our white Northern European patients, but also in those of Far East Asian descent. Dermatologists should also appreciate the high risk of Kaposi’s sarcoma (KS) in patients originating from Sub-Saharan Africa,” Jonathan Kentley, MBBS, of Royal London Hospital, said in an interview. He presented the study findings at the annual meeting of the American Academy of Dermatology.
For the study, Dr. Kentley and colleagues sought to better understand .
They analyzed an organ transplant center database for the years 1989-2016, and tracked 1,304 consecutive patients – which included 1,125 with skin problems. The overall population was 64% male with a median age in the early 40s, and almost all (1,276) had undergone renal transplants. A relative handful underwent liver, lung, heart, and pancreas transplants.
The majority of patients (885) were white Northern Europeans, but there were also significant numbers of people with South Asian (202), black African/Caribbean (131) and white/Mediterranean (52) heritage. A small number were Far East Asian (26) and Middle Eastern (8). The median follow-up time for the ethnic groups varied from about 5 years to about 12 years.
The researchers found that basal cell carcinoma was most common in white Northern European patients, at nearly 25%, with other groups under 10%. SCC was common in white Northern European patients and Far East Asians, both at nearly 25%.
By far, KS was the most common in black African/Caribbean patients, at nearly 11%. According to Dr. Kentley, researchers found the number of KS cases to be surprisingly high in this group, “compounded by the fact that we have had a number of additional cases in the past year after we had collected the data for this study.” He attributes the higher number of KS cases in these patients to an increased seroprevalence of its causative agent, human herpesvirus-8, in Sub-Saharan Africa. The rate of KS in the second most commonly affected group – white Mediterranean patients – was almost 2%.
Viral warts were common in most groups, with the rate in both white groups (white Northern European and white Mediterranean) at nearly 60%, and Far East Asians at about 65%. Porokeratosis was by far the most common in white Norther Europeans, at nearly 8%, and sebaceous hyperplasia was common in all groups (more than 20% to about 27%) except in the black African/Caribbean and South Asian groups.
All these results were statistically significant with P values less than .05.
“Our study has confirmed the increased risk of keratinocyte cancers in patients of white Northern European descent, as well as providing more information on the increased risk in patients of Far East Asian descent,” Dr. Kentley said. “We have also confirmed the propensity of black African/Caribbean patients to develop Kaposi’s sarcoma in the first 5 years post transplant and highlighted that white Mediterranean patients are also at high risk. Beyond this, we have been able to review the prevalence of rare malignancies, such as Merkel cell carcinoma and appendageal tumors, and highlight that white Northern European patients remain at high risk of developing these conditions.”
As for the impact on clinical practice, “the patterns of skin disease susceptibility we have identified have important implications for rational design of transplant skin surveillance programs, targeted patient (and provider) education, and optimized clinical management,” Dr. Kentley said. “Ultimately, this is likely to have a significant impact on strategic deployment of limited dermatology health care resources.”
Specifically, the study suggests that all organ transplant patients receive a baseline skin assessment visit and nurse-led targeted education. Black African/Caribbean patients should be followed up for at least 5 years after transplant.
In the United States, at least 724,000 people have undergone organ transplants since 1988, with most getting kidney transplants, according to the United Network for Organ Sharing (UNOS).
No study funding was reported. The authors had no disclosures.
SOURCE: Kentley J et al. AAD 2018, Session F055.
REPORTING FROM AAD 18
Key clinical point: Skin disorders after organ transplant differ widely by ethnicity.
Major finding: Posttransplant basal cell and squamous cell carcinomas are most common in white Northern Europeans (at nearly 25%), while Kaposi’s sarcoma was higher than expected (nearly 10%) in black African/Caribbean patients.
Study details: Analysis of 1,125 patients from a single transplant center who received organ transplants and developed skin problems over a median follow-up time of 5 to more than 12 years, depending on ethnicity.
Disclosures: No study funding was reported. The authors had no disclosures.
Source: Kentley J et al. AAD 2018, Session F055.
Lenalidomide yields responses in a rare cutaneous lymphoma
The oral immunomodulatory drug lenalidomide is active and may provide prolonged responses in certain patients with a rare and aggressive subtype of primary cutaneous lymphoma, according to results of a phase 2 study.
In the study, which comprised 19 patients with primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL, LT), 5 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months. The findings were reported in the Journal of Investigative Dermatology.
In an exploratory analysis, reducing the dose of lenalidomide was associated with prolonged response and improved survival, noted lead author Marie Beylot-Barry, MD, of the dermatology department, Hôpital Saint-André, CHU Bordeaux, France, and her colleagues.
“Lenalidomide at reduced doses may allow prolonged responses in few patients, and represents a therapeutic option in relapsing/refractory PCDLBCL, LT,” the researchers wrote.
Found mostly on the lower limbs of elderly patients, PCDLBCL, LT exhibits aggressive behavior and is associated with a high rate of skin recurrences. First-line therapy for the cutaneous lymphoma is typically rituximab and chemotherapy, regardless of clinical stage or patient age, the researchers wrote, though primary resistance or recurrence after treatment occurs in about half of patients. “In such relapsing or refractory cases, no treatment has demonstrated a sustained benefit thus far,” they noted.
Lenalidomide has already demonstrated efficacy in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and it induces inhibition of cell signaling, engaging NF-kappaB signaling. PCDLBCL, LT is marked by genetic alterations leading to the NF-kappaB pathway, which represents a therapeutic target.
Dr. Beylot-Barry and her colleagues initiated a multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT. Median progression-free survival in the trial was 4.9 months. The 6-month overall response rate – the primary endpoint of the trial – was 26.3%, which was not significantly superior to a prespecified 20% minimal response rate, according to the researchers.
“However, it was a stringent goal, and other secondary evaluations have to be considered in this context, such as a 6-month disease control rate at 42%,” they wrote.
Reduced doses were associated with improved outcomes, they added. Comparing the nine patients who had lenalidomide dose reductions to those who did not, there was a higher likelihood of 6- to 11-month overall response rate (44.4% vs. 10.0%; P = .11) and lower risk of disease progression or death (hazard ratio, 0.54; 95% confidence interval, 0.19-1.59; P = .27).
Grade 3 adverse events were primarily hematologic, and two deaths occurred (pulmonary embolism and sepsis).
Taken together, the encouraging results at reduced doses, the advanced age of the patients, and the high rate of adverse events suggests a role for lenalidomide as a part of combination treatment for PCDLBCL, LT in future trials, the researchers concluded.
The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
SOURCE: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
The oral immunomodulatory drug lenalidomide is active and may provide prolonged responses in certain patients with a rare and aggressive subtype of primary cutaneous lymphoma, according to results of a phase 2 study.
In the study, which comprised 19 patients with primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL, LT), 5 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months. The findings were reported in the Journal of Investigative Dermatology.
In an exploratory analysis, reducing the dose of lenalidomide was associated with prolonged response and improved survival, noted lead author Marie Beylot-Barry, MD, of the dermatology department, Hôpital Saint-André, CHU Bordeaux, France, and her colleagues.
“Lenalidomide at reduced doses may allow prolonged responses in few patients, and represents a therapeutic option in relapsing/refractory PCDLBCL, LT,” the researchers wrote.
Found mostly on the lower limbs of elderly patients, PCDLBCL, LT exhibits aggressive behavior and is associated with a high rate of skin recurrences. First-line therapy for the cutaneous lymphoma is typically rituximab and chemotherapy, regardless of clinical stage or patient age, the researchers wrote, though primary resistance or recurrence after treatment occurs in about half of patients. “In such relapsing or refractory cases, no treatment has demonstrated a sustained benefit thus far,” they noted.
Lenalidomide has already demonstrated efficacy in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and it induces inhibition of cell signaling, engaging NF-kappaB signaling. PCDLBCL, LT is marked by genetic alterations leading to the NF-kappaB pathway, which represents a therapeutic target.
Dr. Beylot-Barry and her colleagues initiated a multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT. Median progression-free survival in the trial was 4.9 months. The 6-month overall response rate – the primary endpoint of the trial – was 26.3%, which was not significantly superior to a prespecified 20% minimal response rate, according to the researchers.
“However, it was a stringent goal, and other secondary evaluations have to be considered in this context, such as a 6-month disease control rate at 42%,” they wrote.
Reduced doses were associated with improved outcomes, they added. Comparing the nine patients who had lenalidomide dose reductions to those who did not, there was a higher likelihood of 6- to 11-month overall response rate (44.4% vs. 10.0%; P = .11) and lower risk of disease progression or death (hazard ratio, 0.54; 95% confidence interval, 0.19-1.59; P = .27).
Grade 3 adverse events were primarily hematologic, and two deaths occurred (pulmonary embolism and sepsis).
Taken together, the encouraging results at reduced doses, the advanced age of the patients, and the high rate of adverse events suggests a role for lenalidomide as a part of combination treatment for PCDLBCL, LT in future trials, the researchers concluded.
The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
SOURCE: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
The oral immunomodulatory drug lenalidomide is active and may provide prolonged responses in certain patients with a rare and aggressive subtype of primary cutaneous lymphoma, according to results of a phase 2 study.
In the study, which comprised 19 patients with primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL, LT), 5 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months. The findings were reported in the Journal of Investigative Dermatology.
In an exploratory analysis, reducing the dose of lenalidomide was associated with prolonged response and improved survival, noted lead author Marie Beylot-Barry, MD, of the dermatology department, Hôpital Saint-André, CHU Bordeaux, France, and her colleagues.
“Lenalidomide at reduced doses may allow prolonged responses in few patients, and represents a therapeutic option in relapsing/refractory PCDLBCL, LT,” the researchers wrote.
Found mostly on the lower limbs of elderly patients, PCDLBCL, LT exhibits aggressive behavior and is associated with a high rate of skin recurrences. First-line therapy for the cutaneous lymphoma is typically rituximab and chemotherapy, regardless of clinical stage or patient age, the researchers wrote, though primary resistance or recurrence after treatment occurs in about half of patients. “In such relapsing or refractory cases, no treatment has demonstrated a sustained benefit thus far,” they noted.
Lenalidomide has already demonstrated efficacy in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and it induces inhibition of cell signaling, engaging NF-kappaB signaling. PCDLBCL, LT is marked by genetic alterations leading to the NF-kappaB pathway, which represents a therapeutic target.
Dr. Beylot-Barry and her colleagues initiated a multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT. Median progression-free survival in the trial was 4.9 months. The 6-month overall response rate – the primary endpoint of the trial – was 26.3%, which was not significantly superior to a prespecified 20% minimal response rate, according to the researchers.
“However, it was a stringent goal, and other secondary evaluations have to be considered in this context, such as a 6-month disease control rate at 42%,” they wrote.
Reduced doses were associated with improved outcomes, they added. Comparing the nine patients who had lenalidomide dose reductions to those who did not, there was a higher likelihood of 6- to 11-month overall response rate (44.4% vs. 10.0%; P = .11) and lower risk of disease progression or death (hazard ratio, 0.54; 95% confidence interval, 0.19-1.59; P = .27).
Grade 3 adverse events were primarily hematologic, and two deaths occurred (pulmonary embolism and sepsis).
Taken together, the encouraging results at reduced doses, the advanced age of the patients, and the high rate of adverse events suggests a role for lenalidomide as a part of combination treatment for PCDLBCL, LT in future trials, the researchers concluded.
The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
SOURCE: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point:
Major finding: Five of 19 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months.
Study details: A multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT.
Disclosures: The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
Source: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
Counsel children and young adults on skin cancer prevention
(USPSTF). The recommendations, published online March 20 in JAMA, advise clinicians to counsel young adults, children, and parents of young children who are aged 6 months to 24 years and have fair skin types about skin cancer prevention. Counseling for individuals aged 24 years and older should be based on a clinician’s assessment of patient risk.
The recommendations target asymptomatic individuals with no history of skin cancer who might be likely to sunburn easily, wrote David C. Grossman, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, the corresponding author of the USPSTF recommendation statement, and his associates.
The task force found adequate (grade B) evidence to support behavioral counseling for children and young adults aged 6 months to 24 years with no notable risk of harm from this intervention. The task force gave a grade C recommendation for routine skin cancer counseling for adults older than 24 years, citing a small net benefit. In addition, the USPSTF found insufficient evidence (I statement) to evaluate the risks versus benefits of counseling adults about skin self-examination as a way to reduce skin cancer risk.
In the evidence report, lead author Nora B. Henrikson, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues addressed five topics: the effects of skin cancer prevention counseling on short- and long-term outcomes, the effects of primary care counseling interventions on skin cancer prevention behavior, the association between skin self-examination and skin cancer outcomes, the potential harms of counseling interventions, and the potential harms of skin self-examinations.
“Small to moderate effects of behavioral interventions on increased sun protection behaviors were observed in studies of all age groups, though overall, adult trial results were mixed and fewer studies demonstrated an intervention effect,” the researchers said.
The evidence review was limited by several factors including a focus on primary care intervention only and an exclusion of skin cancer survivors, the researchers noted. Although evidence does not show that sunburns are less frequent as a result of interventions, behavioral intervention can improve sun protection behavior, they said. However, intervention in adults “may lead to increased skin procedures without detecting additional atypical nevi or skin cancers,” they noted.
The recommendations are consistent with the draft recommendations published in 2017 and expand the recommendations from 2012 that advised counseling for individuals aged 10-24 years.
The research was funded by the Agency for Healthcare Research and Quality. The authors had no financial conflicts to disclose.
SOURCE: Grossman DC et al. JAMA. 2018;319(11):1134-42.
The term “fair skin types” as used in the USPSTF recommendations is not necessarily helpful in identifying individuals who could benefit from skin cancer prevention counseling, June K. Robinson, MD, and Nina G. Jablonski, PhD, wrote in an accompanying editorial (JAMA. 2018;319[11]:1101-2). Hair and eye color do not predict sun sensitivity, and in general, men and individuals with darker skin don’t think they are at risk for skin cancer even when they sunburn, they noted.
“The terminology that is used by investigators and then incorporated into the USPSTF evidence base needs to evolve to include all persons at risk, without disenfranchising portions of the diverse U.S. population,” they said. In addition to skin type, physicians need to evaluate a patient’s melanoma risk based on lifestyle factors, such as time spent outdoors, photosensitizing medications, and sun protection habits, they added, but primary care clinicians often lack the time to offer personalized sun protection counseling.
“It would be better to encourage people to check the UV Index daily – or consider a mobile application that automatically provides it – and plan outdoor activities, especially physical activities, to be sun safe,” they said. In addition, individuals may be more likely to manage skin cancer risk with a mix of supportive messages via social media to augment in-person counseling from a clinician; furthermore, “normative approval by friends and peers can have a strong reinforcing influence on sun safety behaviors, particularly among youth, who are at a vulnerable age for acquiring melanoma risk,” they emphasized.
Dr. Robinson is a research professor of dermatology at Northwestern University, Chicago, and is the editor of JAMA Dermatology. She is supported in part by the National Cancer Institute. Dr. Jablonski is a professor of anthropology at Pennsylvania State University, University Park. Dr. Robinson had no financial conflicts to disclose; Dr. Jablonski has served on the scientific advisory board of the L’Oreal Group.
The term “fair skin types” as used in the USPSTF recommendations is not necessarily helpful in identifying individuals who could benefit from skin cancer prevention counseling, June K. Robinson, MD, and Nina G. Jablonski, PhD, wrote in an accompanying editorial (JAMA. 2018;319[11]:1101-2). Hair and eye color do not predict sun sensitivity, and in general, men and individuals with darker skin don’t think they are at risk for skin cancer even when they sunburn, they noted.
“The terminology that is used by investigators and then incorporated into the USPSTF evidence base needs to evolve to include all persons at risk, without disenfranchising portions of the diverse U.S. population,” they said. In addition to skin type, physicians need to evaluate a patient’s melanoma risk based on lifestyle factors, such as time spent outdoors, photosensitizing medications, and sun protection habits, they added, but primary care clinicians often lack the time to offer personalized sun protection counseling.
“It would be better to encourage people to check the UV Index daily – or consider a mobile application that automatically provides it – and plan outdoor activities, especially physical activities, to be sun safe,” they said. In addition, individuals may be more likely to manage skin cancer risk with a mix of supportive messages via social media to augment in-person counseling from a clinician; furthermore, “normative approval by friends and peers can have a strong reinforcing influence on sun safety behaviors, particularly among youth, who are at a vulnerable age for acquiring melanoma risk,” they emphasized.
Dr. Robinson is a research professor of dermatology at Northwestern University, Chicago, and is the editor of JAMA Dermatology. She is supported in part by the National Cancer Institute. Dr. Jablonski is a professor of anthropology at Pennsylvania State University, University Park. Dr. Robinson had no financial conflicts to disclose; Dr. Jablonski has served on the scientific advisory board of the L’Oreal Group.
The term “fair skin types” as used in the USPSTF recommendations is not necessarily helpful in identifying individuals who could benefit from skin cancer prevention counseling, June K. Robinson, MD, and Nina G. Jablonski, PhD, wrote in an accompanying editorial (JAMA. 2018;319[11]:1101-2). Hair and eye color do not predict sun sensitivity, and in general, men and individuals with darker skin don’t think they are at risk for skin cancer even when they sunburn, they noted.
“The terminology that is used by investigators and then incorporated into the USPSTF evidence base needs to evolve to include all persons at risk, without disenfranchising portions of the diverse U.S. population,” they said. In addition to skin type, physicians need to evaluate a patient’s melanoma risk based on lifestyle factors, such as time spent outdoors, photosensitizing medications, and sun protection habits, they added, but primary care clinicians often lack the time to offer personalized sun protection counseling.
“It would be better to encourage people to check the UV Index daily – or consider a mobile application that automatically provides it – and plan outdoor activities, especially physical activities, to be sun safe,” they said. In addition, individuals may be more likely to manage skin cancer risk with a mix of supportive messages via social media to augment in-person counseling from a clinician; furthermore, “normative approval by friends and peers can have a strong reinforcing influence on sun safety behaviors, particularly among youth, who are at a vulnerable age for acquiring melanoma risk,” they emphasized.
Dr. Robinson is a research professor of dermatology at Northwestern University, Chicago, and is the editor of JAMA Dermatology. She is supported in part by the National Cancer Institute. Dr. Jablonski is a professor of anthropology at Pennsylvania State University, University Park. Dr. Robinson had no financial conflicts to disclose; Dr. Jablonski has served on the scientific advisory board of the L’Oreal Group.
(USPSTF). The recommendations, published online March 20 in JAMA, advise clinicians to counsel young adults, children, and parents of young children who are aged 6 months to 24 years and have fair skin types about skin cancer prevention. Counseling for individuals aged 24 years and older should be based on a clinician’s assessment of patient risk.
The recommendations target asymptomatic individuals with no history of skin cancer who might be likely to sunburn easily, wrote David C. Grossman, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, the corresponding author of the USPSTF recommendation statement, and his associates.
The task force found adequate (grade B) evidence to support behavioral counseling for children and young adults aged 6 months to 24 years with no notable risk of harm from this intervention. The task force gave a grade C recommendation for routine skin cancer counseling for adults older than 24 years, citing a small net benefit. In addition, the USPSTF found insufficient evidence (I statement) to evaluate the risks versus benefits of counseling adults about skin self-examination as a way to reduce skin cancer risk.
In the evidence report, lead author Nora B. Henrikson, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues addressed five topics: the effects of skin cancer prevention counseling on short- and long-term outcomes, the effects of primary care counseling interventions on skin cancer prevention behavior, the association between skin self-examination and skin cancer outcomes, the potential harms of counseling interventions, and the potential harms of skin self-examinations.
“Small to moderate effects of behavioral interventions on increased sun protection behaviors were observed in studies of all age groups, though overall, adult trial results were mixed and fewer studies demonstrated an intervention effect,” the researchers said.
The evidence review was limited by several factors including a focus on primary care intervention only and an exclusion of skin cancer survivors, the researchers noted. Although evidence does not show that sunburns are less frequent as a result of interventions, behavioral intervention can improve sun protection behavior, they said. However, intervention in adults “may lead to increased skin procedures without detecting additional atypical nevi or skin cancers,” they noted.
The recommendations are consistent with the draft recommendations published in 2017 and expand the recommendations from 2012 that advised counseling for individuals aged 10-24 years.
The research was funded by the Agency for Healthcare Research and Quality. The authors had no financial conflicts to disclose.
SOURCE: Grossman DC et al. JAMA. 2018;319(11):1134-42.
(USPSTF). The recommendations, published online March 20 in JAMA, advise clinicians to counsel young adults, children, and parents of young children who are aged 6 months to 24 years and have fair skin types about skin cancer prevention. Counseling for individuals aged 24 years and older should be based on a clinician’s assessment of patient risk.
The recommendations target asymptomatic individuals with no history of skin cancer who might be likely to sunburn easily, wrote David C. Grossman, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, the corresponding author of the USPSTF recommendation statement, and his associates.
The task force found adequate (grade B) evidence to support behavioral counseling for children and young adults aged 6 months to 24 years with no notable risk of harm from this intervention. The task force gave a grade C recommendation for routine skin cancer counseling for adults older than 24 years, citing a small net benefit. In addition, the USPSTF found insufficient evidence (I statement) to evaluate the risks versus benefits of counseling adults about skin self-examination as a way to reduce skin cancer risk.
In the evidence report, lead author Nora B. Henrikson, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues addressed five topics: the effects of skin cancer prevention counseling on short- and long-term outcomes, the effects of primary care counseling interventions on skin cancer prevention behavior, the association between skin self-examination and skin cancer outcomes, the potential harms of counseling interventions, and the potential harms of skin self-examinations.
“Small to moderate effects of behavioral interventions on increased sun protection behaviors were observed in studies of all age groups, though overall, adult trial results were mixed and fewer studies demonstrated an intervention effect,” the researchers said.
The evidence review was limited by several factors including a focus on primary care intervention only and an exclusion of skin cancer survivors, the researchers noted. Although evidence does not show that sunburns are less frequent as a result of interventions, behavioral intervention can improve sun protection behavior, they said. However, intervention in adults “may lead to increased skin procedures without detecting additional atypical nevi or skin cancers,” they noted.
The recommendations are consistent with the draft recommendations published in 2017 and expand the recommendations from 2012 that advised counseling for individuals aged 10-24 years.
The research was funded by the Agency for Healthcare Research and Quality. The authors had no financial conflicts to disclose.
SOURCE: Grossman DC et al. JAMA. 2018;319(11):1134-42.
FROM JAMA
Key clinical point: Moderate evidence supports behavioral counseling to help reduce skin cancer risk in children and young adults.
Major finding: One trial that included 1,356 adults showed no difference in the number of skin cancers and atypical nevi between a control group and patients who received counseling to encourage skin examination.
Study details: The evidence review included 21 trials in 27 publications for a total of 20,561 individuals.
Disclosures: The review was funded by the Agency for Healthcare Research and Quality.
Source: Grossman DC et al. JAMA. 2018;319(11):1134-42.
cSCC staging systems poorly determine metastasis risk
Current staging systems for cutaneous squamous cell carcinoma (cSCC) poorly discerned between patients with and without metastases, according to researchers.
In a population-based case-control study of 6,721 cSCC patients, the American Joint Committee on Cancer, 7th edition (AJCC 7) staging system had the lowest rate of correctly classified cases (61.8%), followed by the AJCC 8 (68.2%), the Brigham and Women’s Hospital (BWH) system (72.3%), and the Breuninger system (76.2%). The Breuninger system performed best, with sensitivity of 77.3%, specificity of 75%, correctly classified tumor rate of 76.2%, and concordance index (C-index) of 0.81, reported Ingrid Roscher, MD, of the department of dermatology at Oslo University Hospital, and her coauthors. The report was published in JAMA Dermatology.
Investigators used data from the Cancer Registry of Norway to identify 6,721 patients with a first cSCC diagnosis between Jan. 1, 2000, and Dec. 31, 2004. Metastasis status was split into one of two categories: no metastases (local disease only) and metastasis (regional lymph node or distant metastasis).
Within 5 years follow-up, 112 patients had developed metastasis, and 112 patients without metastasis were selected at random as controls. Tumor tissue was collected for all 224 patients and checked by a pathologist. Tumors were classified under all four staging systems, and a chi-squared test was performed to compare patients with and without metastasis. Relative risk of metastasis was calculated via logistic regression analyses.
Sensitivity, specificity, and correctly identified cases were used to evaluate performance of the staging systems, and C-index was used to measure discriminatory ability, wrote Dr. Roscher and her colleagues.
AJCC 7 had a sensitivity of 85.6%, specificity of 33.3%, and C-index value of 0.59; compared with AJCC scores of 67.1%, 69.6%, and 0.70 for sensitivity, specificity, and C-index, respectively. BWH had a sensitivity of 68.9%, specificity of 76.5%, and C-index of 0.75.
Within the AJCC 7 system, 85.6% of patients with metastasis and 66.7% without metastasis fell into the T2 category, and no patients were grouped into T3 or T4 (P = .003). Under the BWH system, patients without metastasis fell mostly into the T1 category, while those with metastasis were about equally distributed among T1, T2a, and T2b (P less than .001). With the Breuninger system, more patients with metastasis than without metastasis fell into the high-risk categories for tumor diameter and depth (P less than .001). Lastly, under AJCC 8, 10% of all patients fell into the T2 category, while less than 20% of patients without metastasis and more than 50% of patients with metastasis fell into the T3 category (P less than .001).
Risk of metastasis for T2 patients was greater than for T1 patients under the AJCC 7 system (odds ratio = 2.96; 95% confidence interval, 1.43-6.15). Under BWH, OR for metastasis were 4.6 (95% CI, 2.23-9.49) for T2a patients and 21.31 (95% CI, 6.07-74.88) for T2b patients. Under the Breuninger system, tumor diameter greater than 2 cm (OR = 5.92; 95% CI, 2.18-16.07) and depth of invasion greater than 6 mm (OR = 9.00; 95% CI, 3.51-32.31) increased risk of metastasis. The AJCC 8 system showed increased metastasis risk for T2 (OR = 2.00; 95% CI, 0.62-6.44) and T3 (OR = 6.14; 95% CI, 0.41-1.09) patients, the authors reported.
The results suggest that the current staging systems “distinguished poorly to moderately between patients who developed metastases and those who did not,” Dr. Roscher and her coauthors wrote. Moreover, “the poorest results were found for the AJCC 7 system, which is most widely used,” they added.
“Our findings indicate a need for a more reliable, easy-to-perform, and clinically useful staging system than those presently available,” they concluded.
Oslo University Hospital and the Cancer Registry of Norway funded the study. No other disclosures were reported.
SOURCE: Roscher et al. JAMA Dermatol. 2018 March 7 doi: 10.1001/jamadermatol.2017.6428.
Current staging systems for cutaneous squamous cell carcinoma (cSCC) poorly discerned between patients with and without metastases, according to researchers.
In a population-based case-control study of 6,721 cSCC patients, the American Joint Committee on Cancer, 7th edition (AJCC 7) staging system had the lowest rate of correctly classified cases (61.8%), followed by the AJCC 8 (68.2%), the Brigham and Women’s Hospital (BWH) system (72.3%), and the Breuninger system (76.2%). The Breuninger system performed best, with sensitivity of 77.3%, specificity of 75%, correctly classified tumor rate of 76.2%, and concordance index (C-index) of 0.81, reported Ingrid Roscher, MD, of the department of dermatology at Oslo University Hospital, and her coauthors. The report was published in JAMA Dermatology.
Investigators used data from the Cancer Registry of Norway to identify 6,721 patients with a first cSCC diagnosis between Jan. 1, 2000, and Dec. 31, 2004. Metastasis status was split into one of two categories: no metastases (local disease only) and metastasis (regional lymph node or distant metastasis).
Within 5 years follow-up, 112 patients had developed metastasis, and 112 patients without metastasis were selected at random as controls. Tumor tissue was collected for all 224 patients and checked by a pathologist. Tumors were classified under all four staging systems, and a chi-squared test was performed to compare patients with and without metastasis. Relative risk of metastasis was calculated via logistic regression analyses.
Sensitivity, specificity, and correctly identified cases were used to evaluate performance of the staging systems, and C-index was used to measure discriminatory ability, wrote Dr. Roscher and her colleagues.
AJCC 7 had a sensitivity of 85.6%, specificity of 33.3%, and C-index value of 0.59; compared with AJCC scores of 67.1%, 69.6%, and 0.70 for sensitivity, specificity, and C-index, respectively. BWH had a sensitivity of 68.9%, specificity of 76.5%, and C-index of 0.75.
Within the AJCC 7 system, 85.6% of patients with metastasis and 66.7% without metastasis fell into the T2 category, and no patients were grouped into T3 or T4 (P = .003). Under the BWH system, patients without metastasis fell mostly into the T1 category, while those with metastasis were about equally distributed among T1, T2a, and T2b (P less than .001). With the Breuninger system, more patients with metastasis than without metastasis fell into the high-risk categories for tumor diameter and depth (P less than .001). Lastly, under AJCC 8, 10% of all patients fell into the T2 category, while less than 20% of patients without metastasis and more than 50% of patients with metastasis fell into the T3 category (P less than .001).
Risk of metastasis for T2 patients was greater than for T1 patients under the AJCC 7 system (odds ratio = 2.96; 95% confidence interval, 1.43-6.15). Under BWH, OR for metastasis were 4.6 (95% CI, 2.23-9.49) for T2a patients and 21.31 (95% CI, 6.07-74.88) for T2b patients. Under the Breuninger system, tumor diameter greater than 2 cm (OR = 5.92; 95% CI, 2.18-16.07) and depth of invasion greater than 6 mm (OR = 9.00; 95% CI, 3.51-32.31) increased risk of metastasis. The AJCC 8 system showed increased metastasis risk for T2 (OR = 2.00; 95% CI, 0.62-6.44) and T3 (OR = 6.14; 95% CI, 0.41-1.09) patients, the authors reported.
The results suggest that the current staging systems “distinguished poorly to moderately between patients who developed metastases and those who did not,” Dr. Roscher and her coauthors wrote. Moreover, “the poorest results were found for the AJCC 7 system, which is most widely used,” they added.
“Our findings indicate a need for a more reliable, easy-to-perform, and clinically useful staging system than those presently available,” they concluded.
Oslo University Hospital and the Cancer Registry of Norway funded the study. No other disclosures were reported.
SOURCE: Roscher et al. JAMA Dermatol. 2018 March 7 doi: 10.1001/jamadermatol.2017.6428.
Current staging systems for cutaneous squamous cell carcinoma (cSCC) poorly discerned between patients with and without metastases, according to researchers.
In a population-based case-control study of 6,721 cSCC patients, the American Joint Committee on Cancer, 7th edition (AJCC 7) staging system had the lowest rate of correctly classified cases (61.8%), followed by the AJCC 8 (68.2%), the Brigham and Women’s Hospital (BWH) system (72.3%), and the Breuninger system (76.2%). The Breuninger system performed best, with sensitivity of 77.3%, specificity of 75%, correctly classified tumor rate of 76.2%, and concordance index (C-index) of 0.81, reported Ingrid Roscher, MD, of the department of dermatology at Oslo University Hospital, and her coauthors. The report was published in JAMA Dermatology.
Investigators used data from the Cancer Registry of Norway to identify 6,721 patients with a first cSCC diagnosis between Jan. 1, 2000, and Dec. 31, 2004. Metastasis status was split into one of two categories: no metastases (local disease only) and metastasis (regional lymph node or distant metastasis).
Within 5 years follow-up, 112 patients had developed metastasis, and 112 patients without metastasis were selected at random as controls. Tumor tissue was collected for all 224 patients and checked by a pathologist. Tumors were classified under all four staging systems, and a chi-squared test was performed to compare patients with and without metastasis. Relative risk of metastasis was calculated via logistic regression analyses.
Sensitivity, specificity, and correctly identified cases were used to evaluate performance of the staging systems, and C-index was used to measure discriminatory ability, wrote Dr. Roscher and her colleagues.
AJCC 7 had a sensitivity of 85.6%, specificity of 33.3%, and C-index value of 0.59; compared with AJCC scores of 67.1%, 69.6%, and 0.70 for sensitivity, specificity, and C-index, respectively. BWH had a sensitivity of 68.9%, specificity of 76.5%, and C-index of 0.75.
Within the AJCC 7 system, 85.6% of patients with metastasis and 66.7% without metastasis fell into the T2 category, and no patients were grouped into T3 or T4 (P = .003). Under the BWH system, patients without metastasis fell mostly into the T1 category, while those with metastasis were about equally distributed among T1, T2a, and T2b (P less than .001). With the Breuninger system, more patients with metastasis than without metastasis fell into the high-risk categories for tumor diameter and depth (P less than .001). Lastly, under AJCC 8, 10% of all patients fell into the T2 category, while less than 20% of patients without metastasis and more than 50% of patients with metastasis fell into the T3 category (P less than .001).
Risk of metastasis for T2 patients was greater than for T1 patients under the AJCC 7 system (odds ratio = 2.96; 95% confidence interval, 1.43-6.15). Under BWH, OR for metastasis were 4.6 (95% CI, 2.23-9.49) for T2a patients and 21.31 (95% CI, 6.07-74.88) for T2b patients. Under the Breuninger system, tumor diameter greater than 2 cm (OR = 5.92; 95% CI, 2.18-16.07) and depth of invasion greater than 6 mm (OR = 9.00; 95% CI, 3.51-32.31) increased risk of metastasis. The AJCC 8 system showed increased metastasis risk for T2 (OR = 2.00; 95% CI, 0.62-6.44) and T3 (OR = 6.14; 95% CI, 0.41-1.09) patients, the authors reported.
The results suggest that the current staging systems “distinguished poorly to moderately between patients who developed metastases and those who did not,” Dr. Roscher and her coauthors wrote. Moreover, “the poorest results were found for the AJCC 7 system, which is most widely used,” they added.
“Our findings indicate a need for a more reliable, easy-to-perform, and clinically useful staging system than those presently available,” they concluded.
Oslo University Hospital and the Cancer Registry of Norway funded the study. No other disclosures were reported.
SOURCE: Roscher et al. JAMA Dermatol. 2018 March 7 doi: 10.1001/jamadermatol.2017.6428.
FROM JAMA DERMATOLOGY
Key clinical point: The four cSCC staging systems, especially the AJCC 7, poorly identified metastasis risk.
Major finding: The AJCC 7 staging system had the lowest rate of correctly classified cases (61.8%), followed by the AJCC 8 (68.2%), the Brigham and Women’s Hospital (BWH) system (72.3%), and the Breuninger system (76.2%).
Data source: A population-based case-control study of 6,721 patients with cSCC.
Disclosures: Oslo University Hospital and the Cancer Registry of Norway funded the study.
Source: Roscher et al. JAMA Dermatol. 2018 Mar 7. doi: 10.1001/jamadermatol.2017.6428.
Invasive Penile Squamous Cell Carcinoma
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.
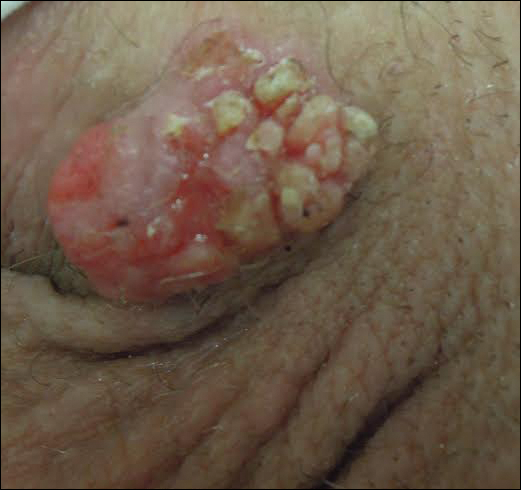
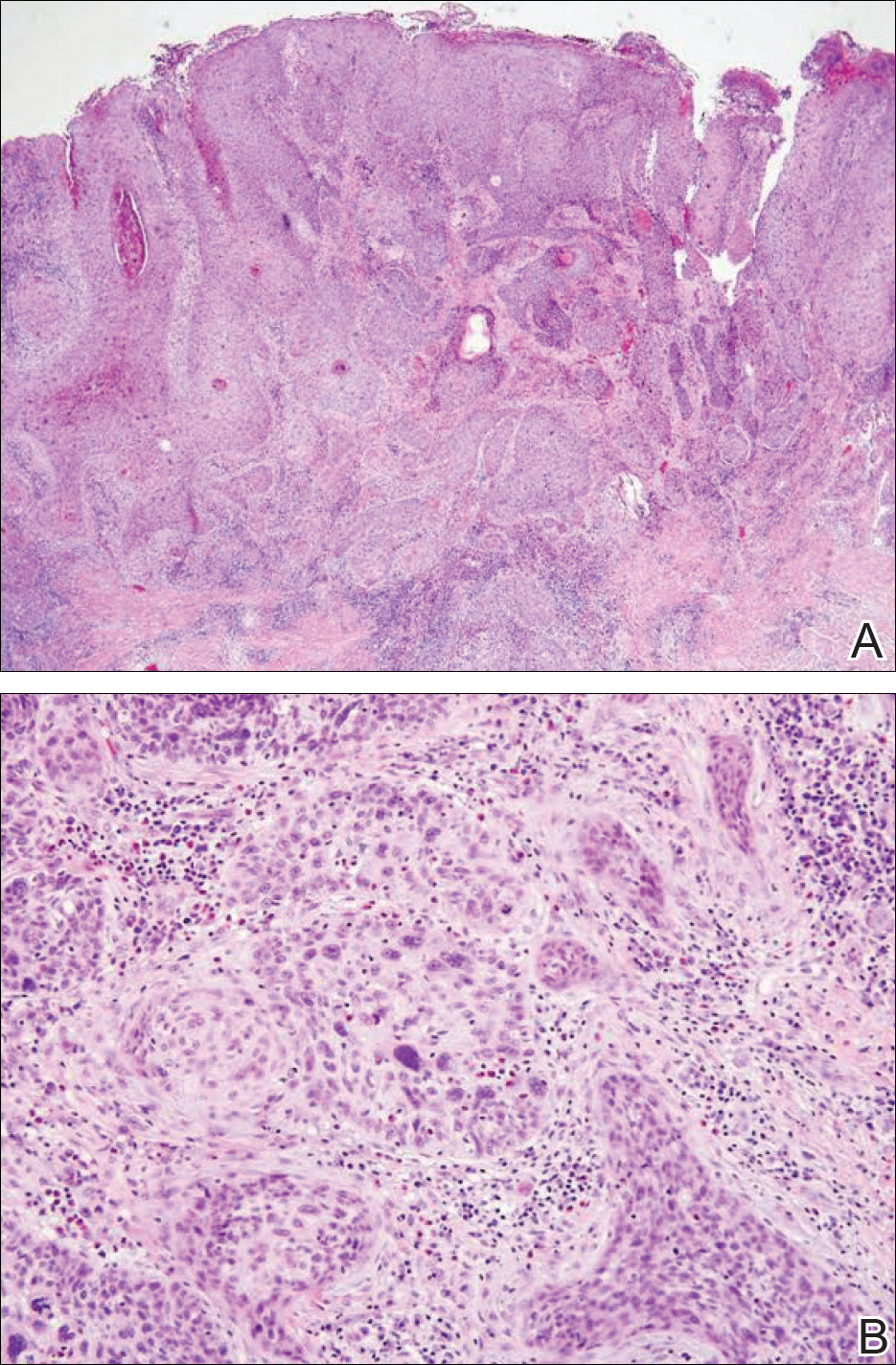
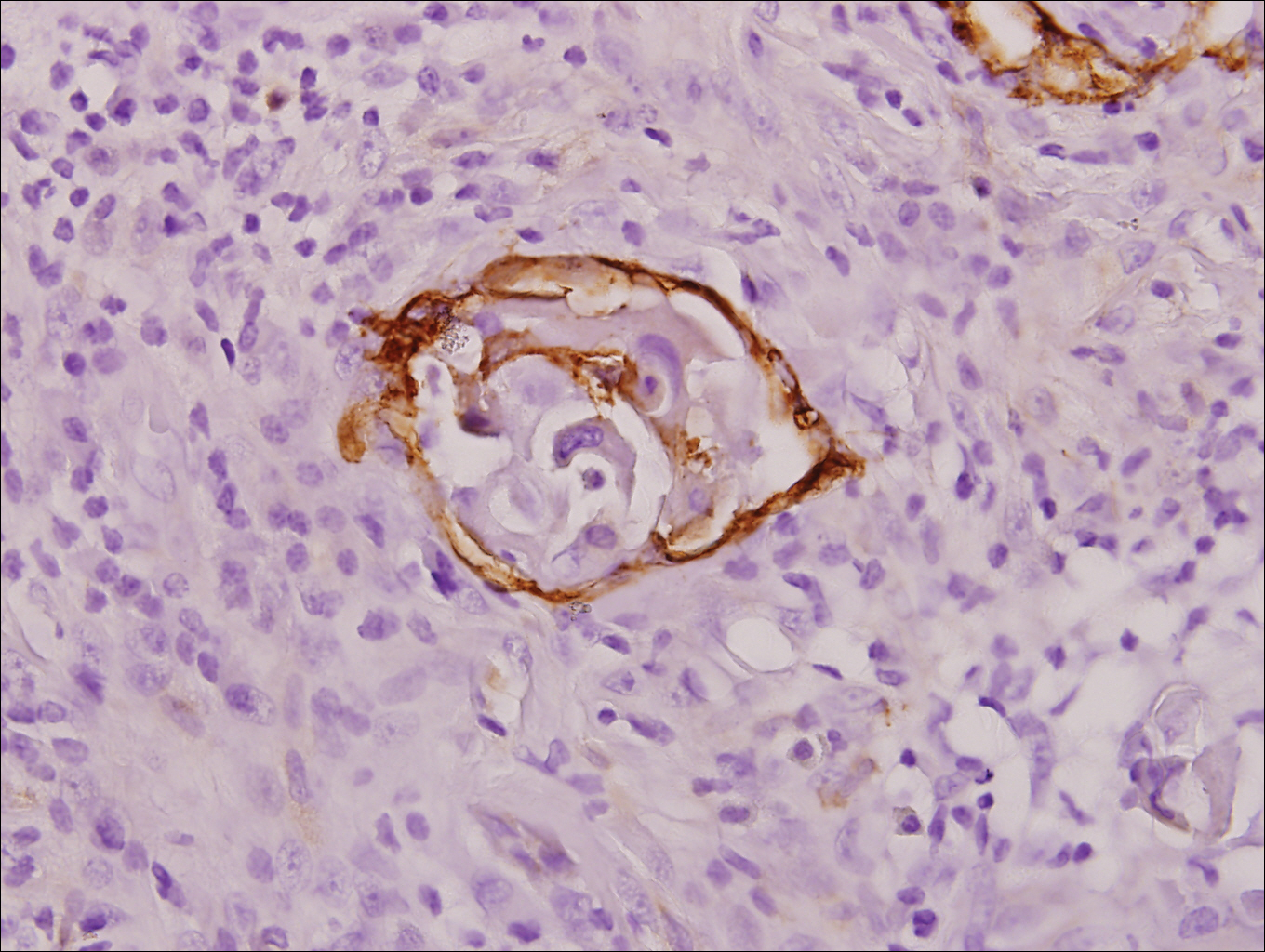
Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Practice Points
- Invasive penile squamous cell carcinoma (PSCC) is a rare malignancy with considerable morbidity and mortality that typically does not present in young men.
- Delayed or incorrect diagnosis of PSCC can have a devastating outcome; therefore, physicians should maintain a high index of clinical suspicion for PSCC in patients presenting with penile lesions, particularly in young or middle-aged patients.