User login
Today’s psychiatric neuroscience advances were science fiction during my residency
During my residency training years, I had many rosy and bold dreams about the future of psychiatry, hoping for many breakthroughs.
Early on, I decided to pursue an academic career, and specifically to focus on the neurobiology of schizophrenia, bipolar disorder, and other psychoses. I secured a neuroscience mentor, conducted a research project, and presented my findings at the American Psychiatric Association Annual Meeting. Although at the time everyone used the term “functional” to describe mental illnesses, I was convinced that they were all neurologic conditions, with prominent psychiatric manifestations. And I have been proven right.
After my residency, I eagerly pursued a neuroscience fellowship at the National Institutes of Health. My fantasy was that during my career as a psychiatric neuroscientist, brain exploration would uncover the many mysteries of psychiatric disorders. I was insightful enough to recognize that what I envisioned for the future of psychiatry qualified as science fiction, but I never stopped dreaming.
Today, the advances in psychiatric neuroscience that were unimaginable during my residency have become dazzling discoveries. My journey as a psychiatric neuroscientist has been more thrilling than I ever imagined. I recall doing postmortem research on the brains of hundreds of deceased psychiatric patients, noticing sulci widening and ventricular dilatation, and wondering whether one day we would be able to detect those atrophic changes while the patients were alive. Although I measured those changes in postmortem brains, I was cognizant that due to preservation artifacts, such measurements were less reliable than measurements of living brains.
And then the advent of neuroimaging fulfilled my fantasies. This began towards the end of my fellowship, and has exploded with neurobiologic findings throughout my academic career. Then came dramatic methodologies to probe brain molecular and cellular pathologies, followed by breakthrough clinical advances. Entirely new vistas of research into psychiatric brain disorders are opening every day. The exhilaration will never end!
From science fiction to clinical reality
Here is a quick outline of some of the “science fiction” of psychiatry that has come true since my training days. Back then, these discoveries were completely absent from the radar screen of psychiatry, when it was still a fledgling medical specialty struggling to emerge from the dominant yet nonempirical era of psychoanalysis.
Brain exploration methods. Unprecedented breakthroughs in computer technology have allowed psychiatric neuroscientists to create a new field of neuroimaging research that includes:
- cerebral blood flow (CBF)
- position emission tomography (PET)
- single photon emission computed tomography (SPECT).
Continue to: These functional neuroimaging...
These functional neuroimaging methods (using ionizing radiation) have enabled clinicians to see abnormal blood flow patterns in the brains of living patients. One of the earliest findings was hypofrontality in patients with schizophrenia, implicating frontal pathology in this severe brain disorder. PET was also used for dopamine and serotonin receptor imaging.
Computerized axia tomography. Compared with skull X-rays, CT (“CAT”) scans provided a more detailed view of brain tissue, and began a structural neuroimaging revolution that enriched psychiatric research, but also was applied to organs other than the brain.
Magnetic resonance imaging (MRI) became the “big kahuna” of neuroimaging when arrived in the early 1980s and quickly supplanted CT research because it is safer (no ionizing radiation, and it can be repeated multiple times with or without tasks). It also provided exquisite neuroanatomical details of brain tissue with stunning fidelity. Subsequently, several MRI techniques/software programs were developed that advanced research in psychiatry to multiple new frontiers, including:
- Morphological neuroimaging with MRI
- Magnetic resonance spectroscopy (MRS), which acts like a living, noninvasive biopsy of several chemicals (such as choline, lactate, glutamine, adenosine triphosphate, and the neuronal marker N-acetylcysteine) in a small volume (≤1 cc) of neural tissue in various regions
- Functional MRI (fMRI), which measures blood flow changes during actual or imagined tasks in the brains of patients vs healthy controls
- Diffusion tensor imaging (DTI), which evaluates the integrity of white matter (60% of brain volume, including 137,000 miles of myelinated fibers) by measuring the flow of water inside myelinated fibers (anisotropy and diffusivity). DTI of the corpus callosum, the largest brain commissure that is comprised of 200 million interhemispheric fibers, has revealed many abnormalities. This was one of the structures I investigated during my fellowship, including a histopathological study.1
All 4 of these neuroimaging techniques continue to generate a wealth of data about brain structure and function in psychosis, mood disorders, anxiety disorders, borderline personality disorder, obsessive-compulsive disorder, eating disorders, and substance use disorders. All these discoveries were utterly impossible to predict during my residency. I am proud to have published the first reports in the literature of ventricular enlargement in patients with bipolar disorder,2 cortical atrophy in schizophrenia and mania,3 reductions of hippocampal volume in patients with schizophrenia using MRS,4 and progressive brain atrophy in patients with schizophrenia.5 It is especially gratifying that I played a small role in translating my science fiction fantasies into clinical reality!
Other breakthrough methodologies that are advancing psychiatric neuroscience today but were science fiction during my residency days include:
- Pluripotent stem cells, which enable the de-differentiation of adult skin cells and then re-differentiating them into any type of cell, including neurons. This allows researchers to conduct studies on any patient’s brain cells without needing to do an invasive, high-risk brain biopsy. As a young resident, I would never have predicted that this virtual brain biopsy would be possible!
- Optogenetics, which enables controlling cell behavior using light and genetically encoded light-sensitive proteins. This triggered a cornucopia of neuroscience discoveries by using optogenetics to modulate cell-signaling cascades to understand cellular biology. Halorhodopsin and bacteriorhodopsin are used as tools to turn neurons off or on rapidly and safely.
- Genome-wide association studies (GWAS) have revolutionized the field of molecular neurogenetics and are enabling clinicians to detect risk genes by comparing the DNA samples of thousands of psychiatric patients with thousands of healthy controls. This is how several hundred risk genes have been identified for schizophrenia, bipolar disorder, autism spectrum disorder, and more to come.
- Clustered regularly interspaced short palindromic repeats (CRISPR) is a remarkable genetic “scissors” (that earned its inventors the 2020 Nobel Prize) that allows splicing out a disease gene and splicing in a normal gene. This will have an enormous future application in preventing an adulthood illness at its roots during fetal life. The future medical implications for psychiatric disorders are prodigious!
Continue to: Clinical advances
Clinical advances. Many therapies or approaches that did not exist during my residency (and how I dreamed about them back then!) are available to today’s clinicians. These include:
- Rapid-acting antidepressants that reverse severe and chronic depression and suicidal urges within a few hours or a couple of days. As a resident, I waited for weeks or months to see patients with depression reach the full remission that is now achieved practically the same day with IV ketamine, intranasal esketamine, IV scopolamine, and inhalable nitrous oxide. During my residency, the closest thing we had to a rapid-acting treatment for depression was electroconvulsive therapy (ECT), but that usually took 2 to 3 weeks. Psychiatric clinicians should never cease to appreciate how an intractable, treatment-refractory depression can rapidly be turned off like a light switch, restoring normal mood to desperately ill persons.
- Neuromodulation techniques are flourishing. Beyond ECT, transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), deep brain stimulation (DBS), low field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near-infrared radiation (NIR), and focused ultrasound (FUS) are approved or under development, offering millions of patients with various neuropsychiatric disorders potential recovery not with pharmacotherapy, but via a brain-targeted approach.
- Telepsychiatry. Now taken for granted during the COVID-19 pandemic, telepsychiatry was completely unimaginable during my residency. Yes, we had phones, but not smartphones! The only “zoom” we knew was the furious sound of a sports car engine! To be able to see and evaluate a patient literally anywhere in the world was science fiction personified! Increased remote access to psychiatric care by patients everywhere is a truly remarkable advance that helped avoid a disastrous lack of psychiatric treatment during the current pandemic that brought in-person interactions between psychiatric physicians and their patients to a screeching halt.
- Neurobiologic effects of psychotherapy. Viewing psychotherapy as a neurobiologic treatment was totally unknown and unimaginable during my residency. I was heavily trained in various types of psychotherapies, but not once did any of my supervisors mention experiential neuroplasticity as a brain-altering process, or that psychotherapy changes brain structure, induces experimental neuroplasticity, and induces billions of dendritic spines in patients’ cortex and limbic structures, helping them connect the dots and develop new insights. No one knew that psychotherapy can mimic the neural effects of pharmacotherapy.
- Immunomodulatory effects of psychotherapy. It was completely unknown that psychotherapies such as cognitive-behavioral therapy can lower levels of inflammatory biomarkers in patients’ CSF and serum. Back then, no one imagined that psychotherapy had immunomodulatory effects. These discoveries are revolutionary for us psychiatrists and confirm the neurobiologic mechanisms of psychotherapy for every patient we treat.
- Epigenetics. This was rarely, if ever, mentioned when I was a resident. We knew from clinical studies that children who were abused or neglected often develop severe mood or psychotic disorders in adulthood. But we did not know that trauma modifies some genes via under- or overexpression, and that such epigenetic changes alter brain development towards psychopathology. The mysteries of psychiatric brain disorders generated by childhood trauma have been clarified by advances in epigenetics.
Aspirational, futuristic therapies. Even now, as a seasoned psychiatric neuroscientist, I continue to dream. Research is providing many clues for potentially radical psychiatric treatments that go beyond standard antipsychotics, antidepressants, mood stabilizers, or anxiolytics. But today, I fully expect that scientific dreams eventually come true through research. For example, the following neuroscientific therapeutics strategies may someday become routine in clinical practice:
- microglia inhibition
- mitochondria repair
- anti-apoptotic therapy
- white matter connectivity restoration
- neuroprotection (enhancing neurogenesis, increasing neurotropic factors, and enhancing synaptogenesis)
- reverse glutamate N-methyl-
d -aspartate hypofunction - prevent amyloid formation.
Data analysis breakthroughs. Side-by-side with the explosion of new findings and amassing mountains of data in psychiatric neuroscience, unprecedented and revolutionary data-management techniques have emerged to facilitate the herculean task of data analysis to extract the mythical needle in a haystack and derive the overall impact of masses of data. These techniques, whose names were not in our vocabulary during my residency days, include:
- machine learning
- artificial intelligence
- deep learning
- big data.
With the help of powerful computers and ingenious software, discovering critical nuggets of knowledge about the brain and predicting the best approaches to healing dysfunctional brains are now possible. Those powerful methods of analyzing massive data are the vehicles for transforming science fiction to reality by assembling the jigsaw puzzle(s) of the human brain, arguably the last frontier in medical science.
My life experiences as a psychiatric neuroscientist have convinced me that nothing is beyond the reach of scientific research. Unraveling the divine brain’s complexities will eventually become reality. So, let us never stop dreaming and fantasizing!
1. Nasrallah HA, McCalley-Whitters M, Bigelow LB, et al. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8(4):251-260.
2. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affect Disord. 1982;4(1):15-19.
3. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43(11):439-441.
4. Nasrallah HA, Skinner TE, Schmalbrock P, et al. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994;165(4):481-485.
5. Nasrallah HA, Olson SC, McCalley-Whitters M, et al. Cerebral ventricular enlargement in schizophrenia. A preliminary follow-up study. Arch Gen Psychiatry. 1986;43(2):157-159.
During my residency training years, I had many rosy and bold dreams about the future of psychiatry, hoping for many breakthroughs.
Early on, I decided to pursue an academic career, and specifically to focus on the neurobiology of schizophrenia, bipolar disorder, and other psychoses. I secured a neuroscience mentor, conducted a research project, and presented my findings at the American Psychiatric Association Annual Meeting. Although at the time everyone used the term “functional” to describe mental illnesses, I was convinced that they were all neurologic conditions, with prominent psychiatric manifestations. And I have been proven right.
After my residency, I eagerly pursued a neuroscience fellowship at the National Institutes of Health. My fantasy was that during my career as a psychiatric neuroscientist, brain exploration would uncover the many mysteries of psychiatric disorders. I was insightful enough to recognize that what I envisioned for the future of psychiatry qualified as science fiction, but I never stopped dreaming.
Today, the advances in psychiatric neuroscience that were unimaginable during my residency have become dazzling discoveries. My journey as a psychiatric neuroscientist has been more thrilling than I ever imagined. I recall doing postmortem research on the brains of hundreds of deceased psychiatric patients, noticing sulci widening and ventricular dilatation, and wondering whether one day we would be able to detect those atrophic changes while the patients were alive. Although I measured those changes in postmortem brains, I was cognizant that due to preservation artifacts, such measurements were less reliable than measurements of living brains.
And then the advent of neuroimaging fulfilled my fantasies. This began towards the end of my fellowship, and has exploded with neurobiologic findings throughout my academic career. Then came dramatic methodologies to probe brain molecular and cellular pathologies, followed by breakthrough clinical advances. Entirely new vistas of research into psychiatric brain disorders are opening every day. The exhilaration will never end!
From science fiction to clinical reality
Here is a quick outline of some of the “science fiction” of psychiatry that has come true since my training days. Back then, these discoveries were completely absent from the radar screen of psychiatry, when it was still a fledgling medical specialty struggling to emerge from the dominant yet nonempirical era of psychoanalysis.
Brain exploration methods. Unprecedented breakthroughs in computer technology have allowed psychiatric neuroscientists to create a new field of neuroimaging research that includes:
- cerebral blood flow (CBF)
- position emission tomography (PET)
- single photon emission computed tomography (SPECT).
Continue to: These functional neuroimaging...
These functional neuroimaging methods (using ionizing radiation) have enabled clinicians to see abnormal blood flow patterns in the brains of living patients. One of the earliest findings was hypofrontality in patients with schizophrenia, implicating frontal pathology in this severe brain disorder. PET was also used for dopamine and serotonin receptor imaging.
Computerized axia tomography. Compared with skull X-rays, CT (“CAT”) scans provided a more detailed view of brain tissue, and began a structural neuroimaging revolution that enriched psychiatric research, but also was applied to organs other than the brain.
Magnetic resonance imaging (MRI) became the “big kahuna” of neuroimaging when arrived in the early 1980s and quickly supplanted CT research because it is safer (no ionizing radiation, and it can be repeated multiple times with or without tasks). It also provided exquisite neuroanatomical details of brain tissue with stunning fidelity. Subsequently, several MRI techniques/software programs were developed that advanced research in psychiatry to multiple new frontiers, including:
- Morphological neuroimaging with MRI
- Magnetic resonance spectroscopy (MRS), which acts like a living, noninvasive biopsy of several chemicals (such as choline, lactate, glutamine, adenosine triphosphate, and the neuronal marker N-acetylcysteine) in a small volume (≤1 cc) of neural tissue in various regions
- Functional MRI (fMRI), which measures blood flow changes during actual or imagined tasks in the brains of patients vs healthy controls
- Diffusion tensor imaging (DTI), which evaluates the integrity of white matter (60% of brain volume, including 137,000 miles of myelinated fibers) by measuring the flow of water inside myelinated fibers (anisotropy and diffusivity). DTI of the corpus callosum, the largest brain commissure that is comprised of 200 million interhemispheric fibers, has revealed many abnormalities. This was one of the structures I investigated during my fellowship, including a histopathological study.1
All 4 of these neuroimaging techniques continue to generate a wealth of data about brain structure and function in psychosis, mood disorders, anxiety disorders, borderline personality disorder, obsessive-compulsive disorder, eating disorders, and substance use disorders. All these discoveries were utterly impossible to predict during my residency. I am proud to have published the first reports in the literature of ventricular enlargement in patients with bipolar disorder,2 cortical atrophy in schizophrenia and mania,3 reductions of hippocampal volume in patients with schizophrenia using MRS,4 and progressive brain atrophy in patients with schizophrenia.5 It is especially gratifying that I played a small role in translating my science fiction fantasies into clinical reality!
Other breakthrough methodologies that are advancing psychiatric neuroscience today but were science fiction during my residency days include:
- Pluripotent stem cells, which enable the de-differentiation of adult skin cells and then re-differentiating them into any type of cell, including neurons. This allows researchers to conduct studies on any patient’s brain cells without needing to do an invasive, high-risk brain biopsy. As a young resident, I would never have predicted that this virtual brain biopsy would be possible!
- Optogenetics, which enables controlling cell behavior using light and genetically encoded light-sensitive proteins. This triggered a cornucopia of neuroscience discoveries by using optogenetics to modulate cell-signaling cascades to understand cellular biology. Halorhodopsin and bacteriorhodopsin are used as tools to turn neurons off or on rapidly and safely.
- Genome-wide association studies (GWAS) have revolutionized the field of molecular neurogenetics and are enabling clinicians to detect risk genes by comparing the DNA samples of thousands of psychiatric patients with thousands of healthy controls. This is how several hundred risk genes have been identified for schizophrenia, bipolar disorder, autism spectrum disorder, and more to come.
- Clustered regularly interspaced short palindromic repeats (CRISPR) is a remarkable genetic “scissors” (that earned its inventors the 2020 Nobel Prize) that allows splicing out a disease gene and splicing in a normal gene. This will have an enormous future application in preventing an adulthood illness at its roots during fetal life. The future medical implications for psychiatric disorders are prodigious!
Continue to: Clinical advances
Clinical advances. Many therapies or approaches that did not exist during my residency (and how I dreamed about them back then!) are available to today’s clinicians. These include:
- Rapid-acting antidepressants that reverse severe and chronic depression and suicidal urges within a few hours or a couple of days. As a resident, I waited for weeks or months to see patients with depression reach the full remission that is now achieved practically the same day with IV ketamine, intranasal esketamine, IV scopolamine, and inhalable nitrous oxide. During my residency, the closest thing we had to a rapid-acting treatment for depression was electroconvulsive therapy (ECT), but that usually took 2 to 3 weeks. Psychiatric clinicians should never cease to appreciate how an intractable, treatment-refractory depression can rapidly be turned off like a light switch, restoring normal mood to desperately ill persons.
- Neuromodulation techniques are flourishing. Beyond ECT, transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), deep brain stimulation (DBS), low field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near-infrared radiation (NIR), and focused ultrasound (FUS) are approved or under development, offering millions of patients with various neuropsychiatric disorders potential recovery not with pharmacotherapy, but via a brain-targeted approach.
- Telepsychiatry. Now taken for granted during the COVID-19 pandemic, telepsychiatry was completely unimaginable during my residency. Yes, we had phones, but not smartphones! The only “zoom” we knew was the furious sound of a sports car engine! To be able to see and evaluate a patient literally anywhere in the world was science fiction personified! Increased remote access to psychiatric care by patients everywhere is a truly remarkable advance that helped avoid a disastrous lack of psychiatric treatment during the current pandemic that brought in-person interactions between psychiatric physicians and their patients to a screeching halt.
- Neurobiologic effects of psychotherapy. Viewing psychotherapy as a neurobiologic treatment was totally unknown and unimaginable during my residency. I was heavily trained in various types of psychotherapies, but not once did any of my supervisors mention experiential neuroplasticity as a brain-altering process, or that psychotherapy changes brain structure, induces experimental neuroplasticity, and induces billions of dendritic spines in patients’ cortex and limbic structures, helping them connect the dots and develop new insights. No one knew that psychotherapy can mimic the neural effects of pharmacotherapy.
- Immunomodulatory effects of psychotherapy. It was completely unknown that psychotherapies such as cognitive-behavioral therapy can lower levels of inflammatory biomarkers in patients’ CSF and serum. Back then, no one imagined that psychotherapy had immunomodulatory effects. These discoveries are revolutionary for us psychiatrists and confirm the neurobiologic mechanisms of psychotherapy for every patient we treat.
- Epigenetics. This was rarely, if ever, mentioned when I was a resident. We knew from clinical studies that children who were abused or neglected often develop severe mood or psychotic disorders in adulthood. But we did not know that trauma modifies some genes via under- or overexpression, and that such epigenetic changes alter brain development towards psychopathology. The mysteries of psychiatric brain disorders generated by childhood trauma have been clarified by advances in epigenetics.
Aspirational, futuristic therapies. Even now, as a seasoned psychiatric neuroscientist, I continue to dream. Research is providing many clues for potentially radical psychiatric treatments that go beyond standard antipsychotics, antidepressants, mood stabilizers, or anxiolytics. But today, I fully expect that scientific dreams eventually come true through research. For example, the following neuroscientific therapeutics strategies may someday become routine in clinical practice:
- microglia inhibition
- mitochondria repair
- anti-apoptotic therapy
- white matter connectivity restoration
- neuroprotection (enhancing neurogenesis, increasing neurotropic factors, and enhancing synaptogenesis)
- reverse glutamate N-methyl-
d -aspartate hypofunction - prevent amyloid formation.
Data analysis breakthroughs. Side-by-side with the explosion of new findings and amassing mountains of data in psychiatric neuroscience, unprecedented and revolutionary data-management techniques have emerged to facilitate the herculean task of data analysis to extract the mythical needle in a haystack and derive the overall impact of masses of data. These techniques, whose names were not in our vocabulary during my residency days, include:
- machine learning
- artificial intelligence
- deep learning
- big data.
With the help of powerful computers and ingenious software, discovering critical nuggets of knowledge about the brain and predicting the best approaches to healing dysfunctional brains are now possible. Those powerful methods of analyzing massive data are the vehicles for transforming science fiction to reality by assembling the jigsaw puzzle(s) of the human brain, arguably the last frontier in medical science.
My life experiences as a psychiatric neuroscientist have convinced me that nothing is beyond the reach of scientific research. Unraveling the divine brain’s complexities will eventually become reality. So, let us never stop dreaming and fantasizing!
During my residency training years, I had many rosy and bold dreams about the future of psychiatry, hoping for many breakthroughs.
Early on, I decided to pursue an academic career, and specifically to focus on the neurobiology of schizophrenia, bipolar disorder, and other psychoses. I secured a neuroscience mentor, conducted a research project, and presented my findings at the American Psychiatric Association Annual Meeting. Although at the time everyone used the term “functional” to describe mental illnesses, I was convinced that they were all neurologic conditions, with prominent psychiatric manifestations. And I have been proven right.
After my residency, I eagerly pursued a neuroscience fellowship at the National Institutes of Health. My fantasy was that during my career as a psychiatric neuroscientist, brain exploration would uncover the many mysteries of psychiatric disorders. I was insightful enough to recognize that what I envisioned for the future of psychiatry qualified as science fiction, but I never stopped dreaming.
Today, the advances in psychiatric neuroscience that were unimaginable during my residency have become dazzling discoveries. My journey as a psychiatric neuroscientist has been more thrilling than I ever imagined. I recall doing postmortem research on the brains of hundreds of deceased psychiatric patients, noticing sulci widening and ventricular dilatation, and wondering whether one day we would be able to detect those atrophic changes while the patients were alive. Although I measured those changes in postmortem brains, I was cognizant that due to preservation artifacts, such measurements were less reliable than measurements of living brains.
And then the advent of neuroimaging fulfilled my fantasies. This began towards the end of my fellowship, and has exploded with neurobiologic findings throughout my academic career. Then came dramatic methodologies to probe brain molecular and cellular pathologies, followed by breakthrough clinical advances. Entirely new vistas of research into psychiatric brain disorders are opening every day. The exhilaration will never end!
From science fiction to clinical reality
Here is a quick outline of some of the “science fiction” of psychiatry that has come true since my training days. Back then, these discoveries were completely absent from the radar screen of psychiatry, when it was still a fledgling medical specialty struggling to emerge from the dominant yet nonempirical era of psychoanalysis.
Brain exploration methods. Unprecedented breakthroughs in computer technology have allowed psychiatric neuroscientists to create a new field of neuroimaging research that includes:
- cerebral blood flow (CBF)
- position emission tomography (PET)
- single photon emission computed tomography (SPECT).
Continue to: These functional neuroimaging...
These functional neuroimaging methods (using ionizing radiation) have enabled clinicians to see abnormal blood flow patterns in the brains of living patients. One of the earliest findings was hypofrontality in patients with schizophrenia, implicating frontal pathology in this severe brain disorder. PET was also used for dopamine and serotonin receptor imaging.
Computerized axia tomography. Compared with skull X-rays, CT (“CAT”) scans provided a more detailed view of brain tissue, and began a structural neuroimaging revolution that enriched psychiatric research, but also was applied to organs other than the brain.
Magnetic resonance imaging (MRI) became the “big kahuna” of neuroimaging when arrived in the early 1980s and quickly supplanted CT research because it is safer (no ionizing radiation, and it can be repeated multiple times with or without tasks). It also provided exquisite neuroanatomical details of brain tissue with stunning fidelity. Subsequently, several MRI techniques/software programs were developed that advanced research in psychiatry to multiple new frontiers, including:
- Morphological neuroimaging with MRI
- Magnetic resonance spectroscopy (MRS), which acts like a living, noninvasive biopsy of several chemicals (such as choline, lactate, glutamine, adenosine triphosphate, and the neuronal marker N-acetylcysteine) in a small volume (≤1 cc) of neural tissue in various regions
- Functional MRI (fMRI), which measures blood flow changes during actual or imagined tasks in the brains of patients vs healthy controls
- Diffusion tensor imaging (DTI), which evaluates the integrity of white matter (60% of brain volume, including 137,000 miles of myelinated fibers) by measuring the flow of water inside myelinated fibers (anisotropy and diffusivity). DTI of the corpus callosum, the largest brain commissure that is comprised of 200 million interhemispheric fibers, has revealed many abnormalities. This was one of the structures I investigated during my fellowship, including a histopathological study.1
All 4 of these neuroimaging techniques continue to generate a wealth of data about brain structure and function in psychosis, mood disorders, anxiety disorders, borderline personality disorder, obsessive-compulsive disorder, eating disorders, and substance use disorders. All these discoveries were utterly impossible to predict during my residency. I am proud to have published the first reports in the literature of ventricular enlargement in patients with bipolar disorder,2 cortical atrophy in schizophrenia and mania,3 reductions of hippocampal volume in patients with schizophrenia using MRS,4 and progressive brain atrophy in patients with schizophrenia.5 It is especially gratifying that I played a small role in translating my science fiction fantasies into clinical reality!
Other breakthrough methodologies that are advancing psychiatric neuroscience today but were science fiction during my residency days include:
- Pluripotent stem cells, which enable the de-differentiation of adult skin cells and then re-differentiating them into any type of cell, including neurons. This allows researchers to conduct studies on any patient’s brain cells without needing to do an invasive, high-risk brain biopsy. As a young resident, I would never have predicted that this virtual brain biopsy would be possible!
- Optogenetics, which enables controlling cell behavior using light and genetically encoded light-sensitive proteins. This triggered a cornucopia of neuroscience discoveries by using optogenetics to modulate cell-signaling cascades to understand cellular biology. Halorhodopsin and bacteriorhodopsin are used as tools to turn neurons off or on rapidly and safely.
- Genome-wide association studies (GWAS) have revolutionized the field of molecular neurogenetics and are enabling clinicians to detect risk genes by comparing the DNA samples of thousands of psychiatric patients with thousands of healthy controls. This is how several hundred risk genes have been identified for schizophrenia, bipolar disorder, autism spectrum disorder, and more to come.
- Clustered regularly interspaced short palindromic repeats (CRISPR) is a remarkable genetic “scissors” (that earned its inventors the 2020 Nobel Prize) that allows splicing out a disease gene and splicing in a normal gene. This will have an enormous future application in preventing an adulthood illness at its roots during fetal life. The future medical implications for psychiatric disorders are prodigious!
Continue to: Clinical advances
Clinical advances. Many therapies or approaches that did not exist during my residency (and how I dreamed about them back then!) are available to today’s clinicians. These include:
- Rapid-acting antidepressants that reverse severe and chronic depression and suicidal urges within a few hours or a couple of days. As a resident, I waited for weeks or months to see patients with depression reach the full remission that is now achieved practically the same day with IV ketamine, intranasal esketamine, IV scopolamine, and inhalable nitrous oxide. During my residency, the closest thing we had to a rapid-acting treatment for depression was electroconvulsive therapy (ECT), but that usually took 2 to 3 weeks. Psychiatric clinicians should never cease to appreciate how an intractable, treatment-refractory depression can rapidly be turned off like a light switch, restoring normal mood to desperately ill persons.
- Neuromodulation techniques are flourishing. Beyond ECT, transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), transcranial direct current stimulation (tDCS), deep brain stimulation (DBS), low field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near-infrared radiation (NIR), and focused ultrasound (FUS) are approved or under development, offering millions of patients with various neuropsychiatric disorders potential recovery not with pharmacotherapy, but via a brain-targeted approach.
- Telepsychiatry. Now taken for granted during the COVID-19 pandemic, telepsychiatry was completely unimaginable during my residency. Yes, we had phones, but not smartphones! The only “zoom” we knew was the furious sound of a sports car engine! To be able to see and evaluate a patient literally anywhere in the world was science fiction personified! Increased remote access to psychiatric care by patients everywhere is a truly remarkable advance that helped avoid a disastrous lack of psychiatric treatment during the current pandemic that brought in-person interactions between psychiatric physicians and their patients to a screeching halt.
- Neurobiologic effects of psychotherapy. Viewing psychotherapy as a neurobiologic treatment was totally unknown and unimaginable during my residency. I was heavily trained in various types of psychotherapies, but not once did any of my supervisors mention experiential neuroplasticity as a brain-altering process, or that psychotherapy changes brain structure, induces experimental neuroplasticity, and induces billions of dendritic spines in patients’ cortex and limbic structures, helping them connect the dots and develop new insights. No one knew that psychotherapy can mimic the neural effects of pharmacotherapy.
- Immunomodulatory effects of psychotherapy. It was completely unknown that psychotherapies such as cognitive-behavioral therapy can lower levels of inflammatory biomarkers in patients’ CSF and serum. Back then, no one imagined that psychotherapy had immunomodulatory effects. These discoveries are revolutionary for us psychiatrists and confirm the neurobiologic mechanisms of psychotherapy for every patient we treat.
- Epigenetics. This was rarely, if ever, mentioned when I was a resident. We knew from clinical studies that children who were abused or neglected often develop severe mood or psychotic disorders in adulthood. But we did not know that trauma modifies some genes via under- or overexpression, and that such epigenetic changes alter brain development towards psychopathology. The mysteries of psychiatric brain disorders generated by childhood trauma have been clarified by advances in epigenetics.
Aspirational, futuristic therapies. Even now, as a seasoned psychiatric neuroscientist, I continue to dream. Research is providing many clues for potentially radical psychiatric treatments that go beyond standard antipsychotics, antidepressants, mood stabilizers, or anxiolytics. But today, I fully expect that scientific dreams eventually come true through research. For example, the following neuroscientific therapeutics strategies may someday become routine in clinical practice:
- microglia inhibition
- mitochondria repair
- anti-apoptotic therapy
- white matter connectivity restoration
- neuroprotection (enhancing neurogenesis, increasing neurotropic factors, and enhancing synaptogenesis)
- reverse glutamate N-methyl-
d -aspartate hypofunction - prevent amyloid formation.
Data analysis breakthroughs. Side-by-side with the explosion of new findings and amassing mountains of data in psychiatric neuroscience, unprecedented and revolutionary data-management techniques have emerged to facilitate the herculean task of data analysis to extract the mythical needle in a haystack and derive the overall impact of masses of data. These techniques, whose names were not in our vocabulary during my residency days, include:
- machine learning
- artificial intelligence
- deep learning
- big data.
With the help of powerful computers and ingenious software, discovering critical nuggets of knowledge about the brain and predicting the best approaches to healing dysfunctional brains are now possible. Those powerful methods of analyzing massive data are the vehicles for transforming science fiction to reality by assembling the jigsaw puzzle(s) of the human brain, arguably the last frontier in medical science.
My life experiences as a psychiatric neuroscientist have convinced me that nothing is beyond the reach of scientific research. Unraveling the divine brain’s complexities will eventually become reality. So, let us never stop dreaming and fantasizing!
1. Nasrallah HA, McCalley-Whitters M, Bigelow LB, et al. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8(4):251-260.
2. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affect Disord. 1982;4(1):15-19.
3. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43(11):439-441.
4. Nasrallah HA, Skinner TE, Schmalbrock P, et al. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994;165(4):481-485.
5. Nasrallah HA, Olson SC, McCalley-Whitters M, et al. Cerebral ventricular enlargement in schizophrenia. A preliminary follow-up study. Arch Gen Psychiatry. 1986;43(2):157-159.
1. Nasrallah HA, McCalley-Whitters M, Bigelow LB, et al. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8(4):251-260.
2. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affect Disord. 1982;4(1):15-19.
3. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43(11):439-441.
4. Nasrallah HA, Skinner TE, Schmalbrock P, et al. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994;165(4):481-485.
5. Nasrallah HA, Olson SC, McCalley-Whitters M, et al. Cerebral ventricular enlargement in schizophrenia. A preliminary follow-up study. Arch Gen Psychiatry. 1986;43(2):157-159.
Myocardial injury seen on MRI in 54% of recovered COVID-19 patients
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.
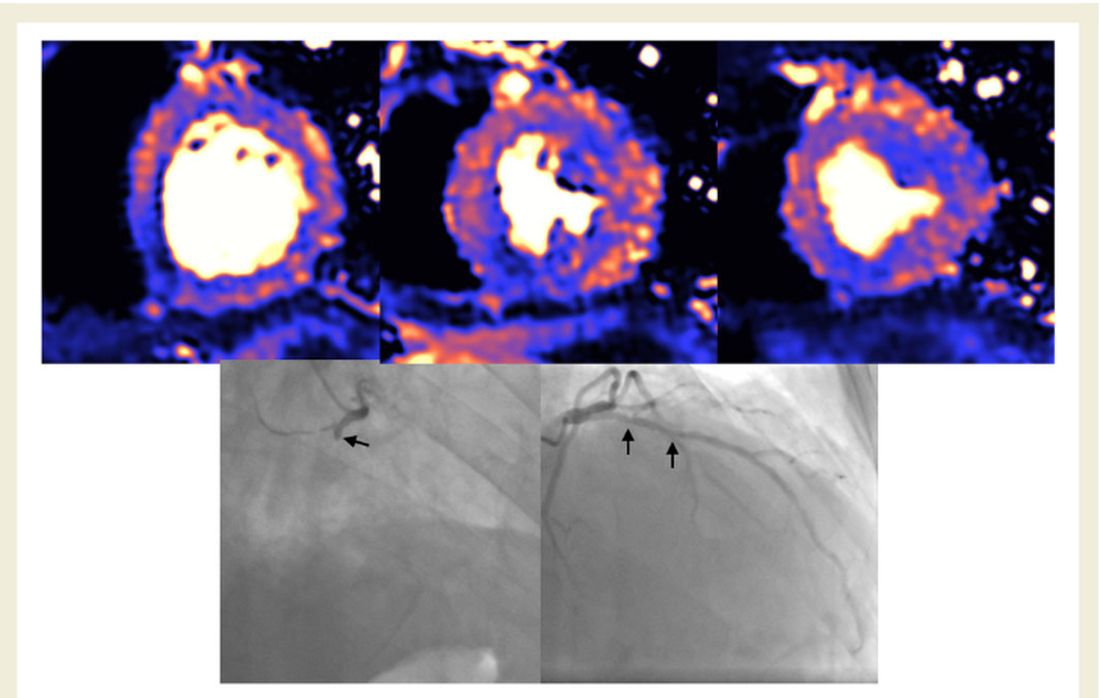
No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
A Preoperative Transthoracic Echocardiography Protocol to Reduce Time to Hip Fracture Surgery
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
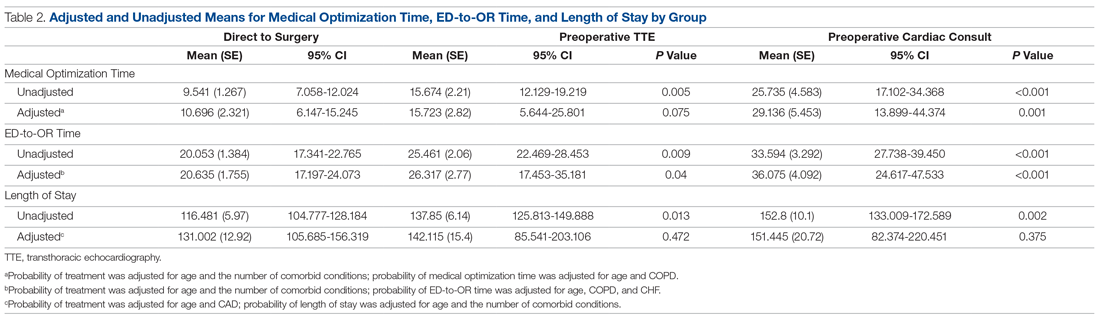
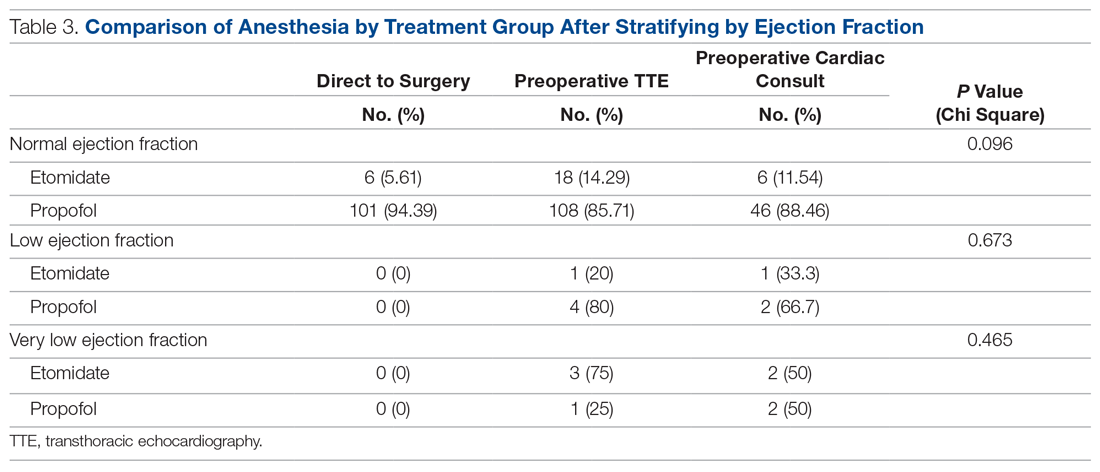
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.
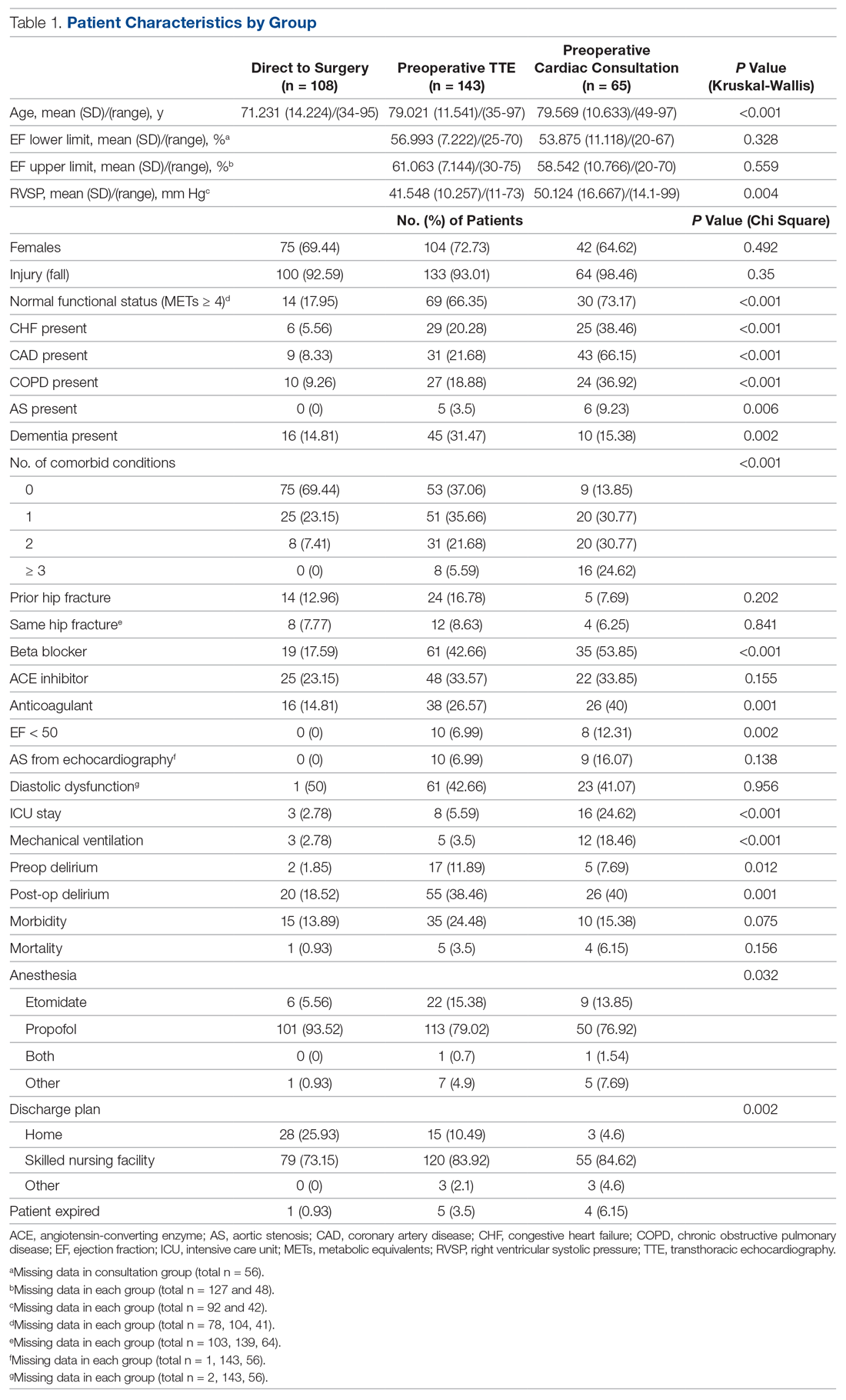
As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).
When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).
Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; robert.skully@ohiohealth.com.
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).
When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).
Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; robert.skully@ohiohealth.com.
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
From Dignity Health Methodist Hospital of Sacramento Family Medicine Residency Program, Sacramento, CA (Dr. Oldach); Nationwide Children’s Hospital, Columbus, OH (Dr. Irwin); OhioHealth Research Institute, Columbus, OH (Dr. Pershing); Department of Clinical Transformation, OhioHealth, Columbus, OH (Dr. Zigmont and Dr. Gascon); and Department of Geriatrics, OhioHealth, Columbus, OH (Dr. Skully).
Abstract
Objective: An interdisciplinary committee was formed to identify factors contributing to surgical delays in urgent hip fracture repair at an urban, level 1 trauma center, with the goal of reducing preoperative time to less than 24 hours. Surgical optimization was identified as a primary, modifiable factor, as surgeons were reluctant to clear patients for surgery without cardiac consultation. Preoperative transthoracic echocardiogram (TTE) was recommended as a safe alternative to cardiac consultation in most patients.
Methods: A retrospective review was conducted for patients who underwent urgent hip fracture repair between January 2010 and April 2014 (n = 316). Time to medical optimization, time to surgery, hospital length of stay, and anesthesia induction were compared for 3 patient groups of interest: those who received (1) neither TTE nor cardiology consultation (ie, direct to surgery); (2) a preoperative TTE; or (3) preoperative cardiac consultation.
Results: There were significant between-group differences in medical optimization time (P = 0.001) and mean time to surgery (P < 0.001) when comparing the 3 groups of interest. Patients in the preoperative cardiac consult group had the longest times, followed by the TTE and direct-to-surgery groups. There were no differences in the type of induction agent used across treatment groups when stratifying by ejection fraction.
Conclusion: Preoperative TTE allows for decreased preoperative time compared to a cardiology consultation. It provides an easily implemented inter-departmental, intra-institutional intervention to decrease preoperative time in patients presenting with hip fractures.
Keywords: surgical delay; preoperative risk stratification; process improvement.
Hip fractures are common, expensive, and associated with poor outcomes.1,2 Ample literature suggests that morbidity, mortality, and cost of care may be reduced by minimizing surgical delays.3-5 While individual reports indicate mixed evidence, in a 2010 meta-analysis, surgery within 72 hours was associated with significant reductions in pneumonia and pressure sores, as well as a 19% reduction in all-cause mortality through 1 year.6 Additional reviews suggest evidence of improved patient outcomes (pain, length of stay, non-union, and/or mortality) when surgery occurs early, within 12 to 72 hours after injury.4,6,7 Regardless of the definition of “early surgery” used, surgical delay remains a challenge, often due to organizational factors, including admission day of the week and hospital staffing, and patient characteristics, such as comorbidities, echocardiographic findings, age, and insurance status.7-9
Among factors that contribute to surgical delays, the need for preoperative cardiovascular risk stratification is significantly modifiable.10 The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force risk stratification framework for preoperative cardiac testing assists clinicians in determining surgical urgency, active cardiac conditions, cardiovascular risk factors, and functional capacity of each patient, and is well established for low- or intermediate-risk patients.11 Specifically, metabolic equivalents (METs) measurements are used to identify medically stable patients with good or excellent functional capacity versus poor or unknown functional status. Patients with ≥ 4 METs may proceed to surgery without further testing; patients with < 4 METs may either proceed with planned surgery or undergo additional testing. Patients with a perceived increased risk profile who require urgent or semi-urgent hip fracture repair may be confounded by disagreement about required preoperative cardiac testing.
At OhioHealth Grant Medical Center (GMC), an urban, level 1 trauma center, the consideration of further preoperative noninvasive testing frequently contributed to surgical delays. In 2009, hip fracture patients arriving to the emergency department (ED) waited an average of 51 hours before being transferred to the operating room (OR) for surgery. Presuming prompt surgery is both desirable and feasible, the Grant Hip Fracture Management Committee (GHFMC) was developed in order to expedite surgeries in hip fracture patients. The GHFMC recommended a preoperative hip fracture protocol, and the outcomes from protocol implementation are described in this article.
Methods
This study was approved by the OhioHealth Institutional Review Board, with a waiver of the informed consent requirement. Medical records from patients treated at GMC during the time period between January 2010 and April 2014 (ie, following implementation of GHFMC recommendations) were retrospectively reviewed to identify the extent to which the use of preoperative transthoracic echocardiography (TTE) reduced average time to surgery and total length of stay, compared to cardiac consultation. This chart review included 316 participants and was used to identify primary induction agent utilized, time to medical optimization, time to surgery, and total length of hospital stay.
Intervention
The GHFMC conducted a 9-month quality improvement project to decrease ED-to-OR time to less than 24 hours for hip fracture patients. The multidisciplinary committee consisted of physicians from orthopedic surgery, anesthesia, hospital medicine, and geriatrics, along with key administrators and nurse outcomes managers. While there is lack of complete clarity surrounding optimal surgical timing, the committee decided that surgery within 24 hours would be beneficial for the majority of patients and therefore was considered a prudent goal.
Based on identified barriers that contributed to surgical delays, several process improvement strategies were implemented, including admitting patients to the hospitalist service, engaging the orthopedic trauma team, and implementing pre- and postoperative protocols and order sets (eg, ED and pain management order sets). Specific emphasis was placed on establishing guidelines for determining medical optimization. In the absence of established guidelines, medical optimization was determined at the discretion of the attending physician. The necessity of preoperative cardiac assessment was based, in part, on physician concerns about determining safe anesthesia protocols and hemodynamically managing patients who may have occult heart disease, specifically those patients with low functional capacity (< 4 METs) and/or inability to accurately communicate their medical history.
Many hip fractures result from a fall, and it may be unclear whether the fall causing a fracture was purely mechanical or indicative of a distinct acute or chronic illness. As a result, many patients received cardiac consultations, with or without pharmacologic stress testing, adding another 24 to 36 hours to preoperative time. As invasive preoperative cardiac procedures generally result in surgical delays without improving outcomes,11 the committee recommended that clinicians reserve preoperative cardiac consultation for patients with active cardiac conditions.
In lieu of cardiac consultation, the committee suggested preoperative TTE. While use of TTE has not been shown to improve preoperative risk stratification in routine noncardiac surgeries, it has been shown to provide clinically useful information in patients at high risk for cardiac complications.11 There was consensus for incorporating preoperative TTE for several reasons: (1) the patients with hip fractures were not “routine,” and often did not have a reliable medical history; (2) a large percentage of patients had cardiac risk factors; (3) patients with undiagnosed aortic stenosis, severe left ventricular dysfunction, or severe pulmonary hypertension would likely have altered intraoperative fluid management; and (4) in supplanting cardiac consultations, TTE would likely expedite patients’ ED-to-OR times. Therefore, the GHFMC created a recommendation of ordering urgent TTE for patients who were unable to exercise at ≥ 4 METs but needed urgent hip fracture surgery.
In order to evaluate the success of the new protocol, the ED-to-OR times were calculated for a cohort of patients who underwent surgery for hip fracture following algorithm implementation.
Participants
A chart review was conducted for patients admitted to GMC between January 2010 and April 2014 for operative treatment of a hip fracture. Exclusion criteria included lack of radiologist-diagnosed hip fracture, periprosthetic hip fracture, or multiple traumas. Electronic patient charts were reviewed by investigators (KI and BO) using a standardized, electronic abstraction form for 3 groups of patients who (1) proceeded directly to planned surgery without TTE or cardiac consultation (direct-to-surgery group); (2) received preoperative TTE but not a cardiac consultation (TTE-only group); or (3) received preoperative cardiac consultation (cardiac consult group).
Measures
Demographics, comorbid conditions, MET score, anesthesia protocol, and in-hospital morbidity and mortality were extracted from medical charts. Medical optimization time was determined by the latest time stamp of 1 of the following: time that the final consulting specialist stated that the patient was stable for surgery; time that the hospitalist described the patient as being ready for surgery; time that the TTE report was certified by the reading cardiologist; or time that the hospitalist described the outcome of completed preoperative risk stratification. Time elapsed prior to medical optimization, surgery, and discharge were calculated using differences between the patient’s arrival date and time at the ED, first recorded time of medical optimization, surgical start time (from the surgical report), and discharge time, respectively.
To assess whether the TTE protocol may have affected anesthesia selection, the induction agent (etomidate or propofol) was abstracted from anesthesia reports and stratified by the ejection fraction of each patient: very low (≤ 35%), low (36%–50%), or normal (> 50%). Patients without an echocardiogram report were assumed to have a normal ejection fraction for this analysis.
Analysis
Descriptive statistics were produced using mean and standard deviation (SD) for continuous variables and frequency and percentage for categorical variables. To determine whether statistically significant differences existed between the 3 groups, the Kruskal-Wallis test was used to compare skewed continuous variables, and Pearson’s chi-square test was used to compare categorical variables. Due to differences in baseline patient characteristics across the 3 treatment groups, inverse probability weights were used to adjust for group differences (using a multinomial logit treatment model) while comparing differences in outcome variables. This modeling strategy does not rely on any assumptions for the distribution of the outcome variable. Covariates were considered for inclusion in the treatment or outcome model if they were significantly associated (P < 0.05) with the group variable. Additionally, anesthetic agent (etomidate or propofol) was compared across the treatment groups after stratifying by ejection fraction to identify whether any differences existed in anesthesia regimen. Patients who were prescribed more than 1 anesthetic agent (n = 2) or an agent that was not of interest were removed from the analysis (n = 13). Stata (version 14) was used for analysis. All other missing data with respect to the tested variables were omitted in the analysis for that variable. Any disagreements about abstraction were resolved through consensus between the investigators.
Results
A total of 316 cases met inclusion criteria, including 108 direct-to-surgery patients, 143 preoperative TTE patients, and 65 cardiac consult patients. Patient demographics and preoperative characteristics are shown in Table 1. The average age for all patients was 76.5 years of age (SD, 12.89; IQR, 34-97); however, direct-to-surgery patients were significantly (P < 0.001) younger (71.2 years; SD, 14.2; interquartile range [IQR], 34-95 years) than TTE-only patients (79.0 years; SD, 11.5; IQR, 35-97 years) and cardiac consult patients (79.57 years; SD, 10.63; IQR, 49-97 years). The majority of patients were female (69.9%) and experienced a fall prior to admission (94%). Almost three-fourths of patients had 1 or more cardiac risk factors (73.7%), including history of congestive heart failure (CHF; 19%), coronary artery disease (CAD; 26.3%), chronic obstructive pulmonary disease (COPD; 19.3%), or aortic stenosis (AS; 3.5%). Due to between-group differences in these comorbid conditions, confounding factors were adjusted for in subsequent analyses.

As shown in Table 2, before adjustment for confounding factors, there were significant between-group differences in medical optimization time for patients in all 3 groups. After adjustment for treatment differences using age and number of comorbid diseases, and medical optimization time differences using age and COPD, fewer between-group differences were statistically significant. Patients who received a cardiac consult had an 18.44-hour longer medical optimization time compared to patients who went directly to surgery (29.136 vs 10.696 hours; P = 0.001). Optimization remained approximately 5 hours longer for the TTE-only group than for the direct-to-surgery group; however, this difference was not significant (P = 0.075).
When comparing differences in ED-to-OR time for the 3 groups after adjusting the probability of treatment for age and the number of comorbid conditions, and adjusting the probability of ED-to-OR time for age, COPD, and CHF, significant differences remained in ED-to-OR times across all groups. Specifically, patients in the direct-to-surgery group experienced the shortest time (mean, 20.64 hours), compared to patients in the TTE-only group (mean, 26.32; P = 0.04) or patients in the cardiac consult group (mean, 36.08; P < 0.001). TTE-only patients had a longer time of 5.68 hours, compared to the direct-to-surgery group, and patients in the preoperative cardiac consult group were on average 15.44 hours longer than the direct-to-surgery group.
When comparing differences in the length of stay for the 3 groups before statistical adjustments, differences were observed; however, after removing the confounding factors related to treatment (age and CAD) and the outcome (age and the number of comorbid conditions), there were no statistically significant differences in the length of stay for the 3 groups. Average length of stay was 131 hours for direct-to-surgery patients, 142 hours for TTE-only patients, and 141 hours for cardiac consult patients.
The use of different anesthetic agents was compared for patients in the 3 groups. The majority of patients in the study (87.7%) were given propofol, and there were no differences after stratifying by ejection fraction (Table 3).
Discussion
The GHFMC was created to reduce surgical delays for hip fracture. Medical optimization was considered a primary, modifiable factor given that surgeons were reluctant to proceed without a cardiac consult. To address this gap, the committee recommended a preoperative TTE for patients with low or unknown functional status. This threshold provides a quick and easy method for stratifying patients who previously required risk stratification by a cardiologist, which often resulted in surgery delays.
In their recommendations for implementation of hip fracture quality improvement projects, the Geriatric Fracture Center emphasizes the importance of multidisciplinary physician leadership along with standardization of approach across patients.12 This recommendation is supported by increasing evidence that orthogeriatric collaborations are associated with decreased mortality and length of stay.13 The GHFMC and subsequent interventions reflect this approach, allowing for collaboration to identify cross-disciplinary procedural barriers to care. In our institution, addressing identified procedural barriers to care was associated with a reduction in the average time to surgery from 51 hours to 25.3 hours.
Multiple approaches have been attempted to decrease presurgical time in hip fracture patients in various settings. Prehospital interventions, such as providing ambulances with checklists and ability to bypass the ED, have not been shown to decrease time to surgery for hip fracture patients, though similar strategies have been successful in other conditions, such as stroke.14,15 In-hospital procedures, such as implementation of a hip fracture protocol and reduction of preoperative interventions, have more consistently been found to decrease time to surgery and in-hospital mortality.16,17 However, reduced delays have not been found universally. Luttrell and Nana found that preoperative TTE resulted in approximately 30.8-hour delays from the ED to OR, compared to patients who did not receive a preoperative TTE.18 However, in that study hospitalists used TTE at their own discretion, and there may have been confounding factors contributing to delays. When used as part of a protocol targeting patients with poor or unknown functional capacity, we believe that preoperative TTE results in modest surgical delays yet provides clinically useful information about each patient.
ACC/AHA preoperative guidelines were updated after we implemented our intervention and now recommend that patients with poor or unknown functional capacity in whom stress testing will not influence care proceed to surgery “according to guideline-directed medical care.”11 While routine use of preoperative evaluation of left ventricular function is not recommended, assessing left ventricular function may be reasonable for patients with heart failure with a change in clinical status. Guidelines also recommend that patients with clinically suspected valvular stenosis undergo preoperative echocardiography.11
Limitations
This study has several limitations. First, due to resource limitations, a substantial period of time elapsed between implementation of the new protocol and the analysis of the data set. That is, the hip fracture protocol assessed in this paper occurred from January 2010 through April 2014, and final analysis of the data set occurred in April 2020. This limitation precludes our ability to formally assess any pre- or post-protocol changes in patient outcomes. Second, randomization was not used to create groups that were balanced in differing health characteristics (ie, patients with noncardiac-related surgeries, patients in different age groups); however, the use of inverse probability treatment regression analysis was a way to statistically address these between-group differences. Moreover, this study is limited by the factors that were measured; unmeasured factors cannot be accounted for. Third, health care providers working at the hospital during this time were aware of the goal to decrease presurgical time, possibly creating exaggerated effects compared to a blinded trial. Finally, although this intervention is likely translatable to other centers, these results represent the experiences of a single level 1 trauma center and may not be replicable elsewhere.
Conclusion
Preoperative TTE in lieu of cardiac consultation has several advantages. First, it requires interdepartmental collaboration for implementation, but can be implemented through a single hospital or hospital system. Unlike prehospital interventions, preoperative urgent TTE for patients with low functional capacity does not require the support of emergency medical technicians, ambulance services, or other hospitals in the region. Second, while costs are associated with TTE, they are offset by a reduction in expensive consultations with specialists, surgical delays, and longer lengths of stay. Third, despite likely increased ED-to-OR times compared to no intervention, urgent TTE decreases time to surgery compared with cardiology consultation. Prior to the GHFMC, the ED-to-OR time at our institution was 51 hours. In contrast, the mean time following the GHFMC-led protocol was less than half that, at 25.3 hours (SD, 19.1 hours). In fact, nearly two-thirds (65.2%) of the patients evaluated in this study underwent surgery within 24 hours of admission. This improvement in presurgical time was attributed, in part, to the implementation of preoperative TTE over cardiology consultations.
Acknowledgments: The authors thank Jenny Williams, RN, who was instrumental in obtaining the data set for analysis, and Shauna Ayres, MPH, from the OhioHealth Research Institute, who provided writing and technical assistance.
Corresponding author: Robert Skully, MD, OhioHealth Family Medicine Grant, 290 East Town St., Columbus, OH 43215; robert.skully@ohiohealth.com.
Funding: This work was supported by the OhioHealth Summer Research Externship Program.
Financial disclosures: None.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
2. Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States 2002 to 2015. Osteoporos Int. 2018;29:717-722.
3. Colais P, Di Martino M, Fusco D, et al. The effect of early surgery after hip fracture on 1-year mortality. BMC Geriatr. 2015;15:141.
4. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality after proximal femoral fracture: a retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg Am. 2015;97:1333-1339.
5. Judd KT, Christianson E. Expedited operative care of hip fractures results in significantly lower cost of treatment. Iowa Orthop J. 2015;35:62-64.
6. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182:1609-1616.
7. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29:343-348.
8. Ricci WM, Brandt A, McAndrew C, Gardner MJ. Factors affecting delay to surgery and length of stay for patients with hip fracture. J Orthop Trauma. 2015;29:e109-e114.
9. Hagino T, Ochiai S, Senga S, et al. Efficacy of early surgery and causes of surgical delay in patients with hip fracture. J Orthop. 2015;12:142-146.
10. Rafiq A, Sklyar E, Bella JN. Cardiac evaluation and monitoring of patients undergoing noncardiac surgery. Health Serv Insights. 2017;9:1178632916686074.
11. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
12. Basu N, Natour M, Mounasamy V, Kates SL. Geriatric hip fracture management: keys to providing a successful program. Eur J Trauma Emerg Surg. 2016;42:565-569.
13. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28:e49-e55.
14. Tai YJ, Yan B. Minimising time to treatment: targeted strategies to minimise time to thrombolysis for acute ischaemic stroke. Intern Med J. 2013;43:1176-1182.
15. Larsson G, Stromberg RU, Rogmark C, Nilsdotter A. Prehospital fast track care for patients with hip fracture: Impact on time to surgery, hospital stay, post-operative complications and mortality a randomised, controlled trial. Injury. 2016;47:881-886.
16. Bohm E, Loucks L, Wittmeier K, et al. Reduced time to surgery improves mortality and length of stay following hip fracture: results from an intervention study in a Canadian health authority. Can J Surg. 2015;58:257-263.
17. Ventura C, Trombetti S, Pioli G, et al. Impact of multidisciplinary hip fracture program on timing of surgery in elderly patients. Osteoporos Int J. 2014;25:2591-2597.
18. Luttrell K, Nana A. Effect of preoperative transthoracic echocardiogram on mortality and surgical timing in elderly adults with hip fracture. J Am Geriatr Soc. 2015;63:2505-2509.
PCPs play a small part in low-value care spending
according to a brief report published online Jan. 18 in Annals of Internal Medicine.
However, one expert said there are better ways to curb low-value care than focusing on which specialties are guilty of the practice.
Analyzing a 20% random sample of Medicare Part B claims, Aaron Baum, PhD, with the Icahn School of Medicine at Mount Sinai, New York, and colleagues found that the services primary care physicians performed or ordered made up on average 8.3% of the low-value care their patients received (interquartile range, 3.9%-15.1%; 95th percentile, 35.6%) and their referrals made up 15.4% (IQR, 6.3%-26.4%; 95th percentile, 44.6%).
By specialty, cardiology had the worst record with 27% of all spending on low-value services ($1.8 billion) attributed to that specialty. Yet, of the 25 highest-spending specialties in the report, 12 of them were associated with 1% or less than 1% each of all low-value spending, indicating the waste was widely distributed.
Dr. Baum said in an interview that though there are some PCPs guilty of high spending on low-value services, overall, most primary care physicians’ low-value services add up to only 0.3% of Part B spending. He noted that Part B spending is about one-third of all Medicare spending.
Primary care is often thought to be at the core of care management and spending and PCPs are often seen as the gatekeepers, but this analysis suggests that efforts to make big differences in curtailing low-value spending might be more effective elsewhere.
“There’s only so much spending you can reduce by changing primary care physicians’ services that they directly perform,” Dr. Baum said.
Low-value care is costly, can be harmful
Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, said in an interview that the report adds confirmation to previous research that has consistently shown low-value care is “extremely common, very costly, and provided by primary care providers and specialists alike.” He noted that it can also be harmful.
“The math is simple,” he said. “If we want to improve coverage and lower patient costs for essential services like visits, diagnostic tests, and drugs, we have to reduce spending on those services that do not make Americans any healthier.”
The study ranked 31 clinical services judged to be low value by physician societies, Medicare and clinical guidelines, and their use among beneficiaries enrolled between 2007 and 2014. Here’s how the top six low-value services compare.
Dr. Fendrick said a weakness of the paper is the years of the data (2007-2014). Some of the criteria around low-value care have changed since then. The age that a prostate-specific antigen test becomes low-value is now 70 years, for instance, instead of 75. He added that some of the figures attributed to non-PCP providers appear out of date.
Dr. Fendrick said, “I understand that there are Medicare patients who end up at a gastroenterologist or surgeon’s office to get colorectal cancer screening, but it would be very hard for me to believe that half of stress tests and over half of colon cancer screening over [age] 85 [years] and half of PSA for people over 75 did not have some type of referring clinicians involved. I certainly don’t think that would be the case in 2020-2021.”
Dr. Baum said those years were the latest years available for the data points needed for this analysis, but he and his colleagues were working to update the data for future publication.
Dr. Fendrick said not much has changed in recent years in terms of waste on low-value care, even with campaigns such as Choosing Wisely dedicated to identifying low-value services or procedures in each specialty.
“I believe there’s not a particular group of clinicians one way or the other who are actually doing any better now than they were 7 years ago,” he said. He would rather focus less on which specialties are associated with the most low-value care and more on the underlying policies that encourage low-value care.
“If you’re going to get paid for doing a stress test and get paid nothing or significantly less if you don’t, the incentives are in the wrong direction,” he said.
Dr. Fendrick said the pandemic era provides an opportunity to eliminate low-value care because use of those services has dropped drastically as resources have been diverted to COVID-19 patients and many services have been delayed or canceled.
He said he has been pushing an approach that providers should be paid more after the pandemic “to do the things we want them to do.”
As an example, he said, instead of paying $886 million on colonoscopies for people over the age of 85, “why don’t we put a policy in place that would make it better for patients by lowering cost sharing and better for providers by paying them more to do the service on the people who need it as opposed to the people who don’t?”
The research was funded by the American Board of Family Medicine Foundation. Dr. Baum and a coauthor reported receiving personal fees from American Board of Family Medicine Foundation during the conduct of the study. Another coauthor reported receiving personal fees from Collective Health, HealthRight 360, PLOS Medicine, and the New England Journal of Medicine, outside the submitted work. Dr. Fendrick disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a brief report published online Jan. 18 in Annals of Internal Medicine.
However, one expert said there are better ways to curb low-value care than focusing on which specialties are guilty of the practice.
Analyzing a 20% random sample of Medicare Part B claims, Aaron Baum, PhD, with the Icahn School of Medicine at Mount Sinai, New York, and colleagues found that the services primary care physicians performed or ordered made up on average 8.3% of the low-value care their patients received (interquartile range, 3.9%-15.1%; 95th percentile, 35.6%) and their referrals made up 15.4% (IQR, 6.3%-26.4%; 95th percentile, 44.6%).
By specialty, cardiology had the worst record with 27% of all spending on low-value services ($1.8 billion) attributed to that specialty. Yet, of the 25 highest-spending specialties in the report, 12 of them were associated with 1% or less than 1% each of all low-value spending, indicating the waste was widely distributed.
Dr. Baum said in an interview that though there are some PCPs guilty of high spending on low-value services, overall, most primary care physicians’ low-value services add up to only 0.3% of Part B spending. He noted that Part B spending is about one-third of all Medicare spending.
Primary care is often thought to be at the core of care management and spending and PCPs are often seen as the gatekeepers, but this analysis suggests that efforts to make big differences in curtailing low-value spending might be more effective elsewhere.
“There’s only so much spending you can reduce by changing primary care physicians’ services that they directly perform,” Dr. Baum said.
Low-value care is costly, can be harmful
Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, said in an interview that the report adds confirmation to previous research that has consistently shown low-value care is “extremely common, very costly, and provided by primary care providers and specialists alike.” He noted that it can also be harmful.
“The math is simple,” he said. “If we want to improve coverage and lower patient costs for essential services like visits, diagnostic tests, and drugs, we have to reduce spending on those services that do not make Americans any healthier.”
The study ranked 31 clinical services judged to be low value by physician societies, Medicare and clinical guidelines, and their use among beneficiaries enrolled between 2007 and 2014. Here’s how the top six low-value services compare.
Dr. Fendrick said a weakness of the paper is the years of the data (2007-2014). Some of the criteria around low-value care have changed since then. The age that a prostate-specific antigen test becomes low-value is now 70 years, for instance, instead of 75. He added that some of the figures attributed to non-PCP providers appear out of date.
Dr. Fendrick said, “I understand that there are Medicare patients who end up at a gastroenterologist or surgeon’s office to get colorectal cancer screening, but it would be very hard for me to believe that half of stress tests and over half of colon cancer screening over [age] 85 [years] and half of PSA for people over 75 did not have some type of referring clinicians involved. I certainly don’t think that would be the case in 2020-2021.”
Dr. Baum said those years were the latest years available for the data points needed for this analysis, but he and his colleagues were working to update the data for future publication.
Dr. Fendrick said not much has changed in recent years in terms of waste on low-value care, even with campaigns such as Choosing Wisely dedicated to identifying low-value services or procedures in each specialty.
“I believe there’s not a particular group of clinicians one way or the other who are actually doing any better now than they were 7 years ago,” he said. He would rather focus less on which specialties are associated with the most low-value care and more on the underlying policies that encourage low-value care.
“If you’re going to get paid for doing a stress test and get paid nothing or significantly less if you don’t, the incentives are in the wrong direction,” he said.
Dr. Fendrick said the pandemic era provides an opportunity to eliminate low-value care because use of those services has dropped drastically as resources have been diverted to COVID-19 patients and many services have been delayed or canceled.
He said he has been pushing an approach that providers should be paid more after the pandemic “to do the things we want them to do.”
As an example, he said, instead of paying $886 million on colonoscopies for people over the age of 85, “why don’t we put a policy in place that would make it better for patients by lowering cost sharing and better for providers by paying them more to do the service on the people who need it as opposed to the people who don’t?”
The research was funded by the American Board of Family Medicine Foundation. Dr. Baum and a coauthor reported receiving personal fees from American Board of Family Medicine Foundation during the conduct of the study. Another coauthor reported receiving personal fees from Collective Health, HealthRight 360, PLOS Medicine, and the New England Journal of Medicine, outside the submitted work. Dr. Fendrick disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a brief report published online Jan. 18 in Annals of Internal Medicine.
However, one expert said there are better ways to curb low-value care than focusing on which specialties are guilty of the practice.
Analyzing a 20% random sample of Medicare Part B claims, Aaron Baum, PhD, with the Icahn School of Medicine at Mount Sinai, New York, and colleagues found that the services primary care physicians performed or ordered made up on average 8.3% of the low-value care their patients received (interquartile range, 3.9%-15.1%; 95th percentile, 35.6%) and their referrals made up 15.4% (IQR, 6.3%-26.4%; 95th percentile, 44.6%).
By specialty, cardiology had the worst record with 27% of all spending on low-value services ($1.8 billion) attributed to that specialty. Yet, of the 25 highest-spending specialties in the report, 12 of them were associated with 1% or less than 1% each of all low-value spending, indicating the waste was widely distributed.
Dr. Baum said in an interview that though there are some PCPs guilty of high spending on low-value services, overall, most primary care physicians’ low-value services add up to only 0.3% of Part B spending. He noted that Part B spending is about one-third of all Medicare spending.
Primary care is often thought to be at the core of care management and spending and PCPs are often seen as the gatekeepers, but this analysis suggests that efforts to make big differences in curtailing low-value spending might be more effective elsewhere.
“There’s only so much spending you can reduce by changing primary care physicians’ services that they directly perform,” Dr. Baum said.
Low-value care is costly, can be harmful
Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, said in an interview that the report adds confirmation to previous research that has consistently shown low-value care is “extremely common, very costly, and provided by primary care providers and specialists alike.” He noted that it can also be harmful.
“The math is simple,” he said. “If we want to improve coverage and lower patient costs for essential services like visits, diagnostic tests, and drugs, we have to reduce spending on those services that do not make Americans any healthier.”
The study ranked 31 clinical services judged to be low value by physician societies, Medicare and clinical guidelines, and their use among beneficiaries enrolled between 2007 and 2014. Here’s how the top six low-value services compare.
Dr. Fendrick said a weakness of the paper is the years of the data (2007-2014). Some of the criteria around low-value care have changed since then. The age that a prostate-specific antigen test becomes low-value is now 70 years, for instance, instead of 75. He added that some of the figures attributed to non-PCP providers appear out of date.
Dr. Fendrick said, “I understand that there are Medicare patients who end up at a gastroenterologist or surgeon’s office to get colorectal cancer screening, but it would be very hard for me to believe that half of stress tests and over half of colon cancer screening over [age] 85 [years] and half of PSA for people over 75 did not have some type of referring clinicians involved. I certainly don’t think that would be the case in 2020-2021.”
Dr. Baum said those years were the latest years available for the data points needed for this analysis, but he and his colleagues were working to update the data for future publication.
Dr. Fendrick said not much has changed in recent years in terms of waste on low-value care, even with campaigns such as Choosing Wisely dedicated to identifying low-value services or procedures in each specialty.
“I believe there’s not a particular group of clinicians one way or the other who are actually doing any better now than they were 7 years ago,” he said. He would rather focus less on which specialties are associated with the most low-value care and more on the underlying policies that encourage low-value care.
“If you’re going to get paid for doing a stress test and get paid nothing or significantly less if you don’t, the incentives are in the wrong direction,” he said.
Dr. Fendrick said the pandemic era provides an opportunity to eliminate low-value care because use of those services has dropped drastically as resources have been diverted to COVID-19 patients and many services have been delayed or canceled.
He said he has been pushing an approach that providers should be paid more after the pandemic “to do the things we want them to do.”
As an example, he said, instead of paying $886 million on colonoscopies for people over the age of 85, “why don’t we put a policy in place that would make it better for patients by lowering cost sharing and better for providers by paying them more to do the service on the people who need it as opposed to the people who don’t?”
The research was funded by the American Board of Family Medicine Foundation. Dr. Baum and a coauthor reported receiving personal fees from American Board of Family Medicine Foundation during the conduct of the study. Another coauthor reported receiving personal fees from Collective Health, HealthRight 360, PLOS Medicine, and the New England Journal of Medicine, outside the submitted work. Dr. Fendrick disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CVD deaths rose, imaging declined during pandemic
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Elite soccer players have big hearts and that’s okay
Elite American soccer players have, on average, larger, thicker, and heavier hearts than the general population, according to a new study that provides clinicians with normative echocardiogram and electrocardiogram (ECG) cutoffs to use when assessing the heart health of competitive athletes.
To provide these age- and sex-specific reference values, a team from Massachusetts General Hospital, Boston, led by Timothy W. Churchill, MD, and Aaron L. Baggish, MD, analyzed data from 122 female and 116 male soccer players from the American national teams preparing for World Cup play and undergoing FIFA-mandated preparticipation screening.
The athletes frequently exceeded normal echocardiographic ranges for left ventricular (LV) mass, volume, and wall thickness – structural cardiac parameters responsive to exercise-induced remodeling – but with none showing pathologic findings that might indicate the need to restrict their participation in the sport.
Almost one-third (30%) of female athletes and 41% of male athletes exceeded the American Society of Echocardiography’s upper limit of normal for LV wall thickness, with a measure greater than 12 mm seen in 12% of men and 1% of women.
The majority (51% of females and 59% of males) exceeded normal ranges for body surface area–indexed LV mass, with 77% and 68%, respectively, having LV volumes above the normal range.
Dr. Baggish stressed in an interview, however, that these data tell a story about healthy hearts, not at-risk hearts.
“These are the healthiest, highest-performing elite soccer players that we have in the United States, and this is really a look at how adaptive the heart can be, how much it can grow and change in size, shape, structure, and function in response to sport,” said Dr. Baggish.
The mean age of screened athletes was 20 years (range, 15-40 years). The majority of the female players were White (71%), whereas the male players were more evenly divided between Black (34%), Hispanic (33%), and White (32%).
Screening was performed at U.S. Soccer training sites by experienced clinicians affiliated with the Massachusetts General Hospital cardiovascular performance program.
Interestingly, the study debunks the idea that women, on average, have smaller chamber sizes. “When we did body-size correction, the men and women actually looked pretty similar with respect to their ability to adapt to strenuous exercise,” noted Dr. Baggish.
They did see, however, that women were more likely than men to have abnormal ECG findings. Male athletes showed a higher prevalence of “normal” training-related ECG findings, whereas female athletes were more likely to have abnormal ECG patterns (11.5% vs. 0.0% in the male cohort), most often pathologic T-wave inversions (TWI) confined to the anterior precordial lead distribution.
“This is important because ECGs are the most common screening tool used and we wanted to alert people to the fact that these women who showed some abnormalities on ECG went on to have a total healthy-looking echo, so a false-positive ECG is something to consider,” said Dr. Baggish.
This excess in anterior TWIs has been seen in previous studies and is thought to be benign, although the mechanism remains unclear. Four of the nine female athletes with abnormal ECG findings on initial evaluation had normalized on repeat testing 2-4 years later. Serial data were available in only a subset of athletes.
Clarity needed after COVID
The data, published recently in JAMA Cardiology, are particularly valuable these days given concern over the effects of COVID-19 on the heart and return-to-play recommendations.
“Athletes who have had COVID are being sent for echocardiograms before they can return to play to check for COVID-induced heart disease – which is real – but what we’re seeing is that there’s confusion out there in terms of what is a COVID-related abnormality and what is a normal, adapted athletic heart,” said Dr. Baggish.
“In this paper, we provide a dataset of normal values – generated before COVID was on anyone’s radar – to let cardiologists know what’s ‘big good’ and not ‘big bad.’ ”
More sport-specific data needed
“Although these numbers are still small, this dataset is an important step forward in our understanding of athletic adaptations,” said Matthew Martinez, MD, in an interview. “Many factors impact physiologic athletic changes, and the study aids in our understanding of gender- and sport-specific changes in athletes.”
Dr. Martinez, who is the director of sports cardiology at Atlantic Health–Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, also in Morristown, and the chair of Sports and Exercise Cardiology Section Leadership Council for the American College of Cardiology, noted the relatively young mean age of screened athletes.
“The data represent collegiate-age athletes with some older groups mixed in, but it does not represent older established elite athlete changes,” he said.
Mean age was 21 years in the female players but only 18 years in the males because the men’s senior national team failed to qualify for the World Cup during the study period and was therefore not screened. The authors acknowledged the “dearth of older men in the cohort.”
There is, overall, little age-, sport-, and sex-specific normative data for differentiating training-related cardiovascular adaptations from potentially pathologic phenotypes, wrote the authors.
It exists for men playing in the National Football League and for both sexes participating in the National Basketball Association, but most other studies have mixed the sports and focused mainly on men. That said, Dr. Baggish does not consider these data to be applicable to all elite athletes.
“Soccer is kind of in a league of its own with respect to the mixed amount of explosive or resistant and aerobic work that these athletes have to do, and also it’s the most popular sport in the world, so we really wanted to focus on them,” said Dr. Baggish.
Although the findings are perhaps applicable to athletes from other team sports characterized by explosive spurts of high-intensity activity – like hockey, lacrosse, and field hockey – he would not suggest they be applied to, say, long-distance runners, cyclists, or other sports that require a similar type of aerobic output.
Dr. Baggish reported no relevant conflict of interest. Dr. Martinez is league cardiologist for Major League Soccer.
A version of this article first appeared on Medscape.com.
Elite American soccer players have, on average, larger, thicker, and heavier hearts than the general population, according to a new study that provides clinicians with normative echocardiogram and electrocardiogram (ECG) cutoffs to use when assessing the heart health of competitive athletes.
To provide these age- and sex-specific reference values, a team from Massachusetts General Hospital, Boston, led by Timothy W. Churchill, MD, and Aaron L. Baggish, MD, analyzed data from 122 female and 116 male soccer players from the American national teams preparing for World Cup play and undergoing FIFA-mandated preparticipation screening.
The athletes frequently exceeded normal echocardiographic ranges for left ventricular (LV) mass, volume, and wall thickness – structural cardiac parameters responsive to exercise-induced remodeling – but with none showing pathologic findings that might indicate the need to restrict their participation in the sport.
Almost one-third (30%) of female athletes and 41% of male athletes exceeded the American Society of Echocardiography’s upper limit of normal for LV wall thickness, with a measure greater than 12 mm seen in 12% of men and 1% of women.
The majority (51% of females and 59% of males) exceeded normal ranges for body surface area–indexed LV mass, with 77% and 68%, respectively, having LV volumes above the normal range.
Dr. Baggish stressed in an interview, however, that these data tell a story about healthy hearts, not at-risk hearts.
“These are the healthiest, highest-performing elite soccer players that we have in the United States, and this is really a look at how adaptive the heart can be, how much it can grow and change in size, shape, structure, and function in response to sport,” said Dr. Baggish.
The mean age of screened athletes was 20 years (range, 15-40 years). The majority of the female players were White (71%), whereas the male players were more evenly divided between Black (34%), Hispanic (33%), and White (32%).
Screening was performed at U.S. Soccer training sites by experienced clinicians affiliated with the Massachusetts General Hospital cardiovascular performance program.
Interestingly, the study debunks the idea that women, on average, have smaller chamber sizes. “When we did body-size correction, the men and women actually looked pretty similar with respect to their ability to adapt to strenuous exercise,” noted Dr. Baggish.
They did see, however, that women were more likely than men to have abnormal ECG findings. Male athletes showed a higher prevalence of “normal” training-related ECG findings, whereas female athletes were more likely to have abnormal ECG patterns (11.5% vs. 0.0% in the male cohort), most often pathologic T-wave inversions (TWI) confined to the anterior precordial lead distribution.
“This is important because ECGs are the most common screening tool used and we wanted to alert people to the fact that these women who showed some abnormalities on ECG went on to have a total healthy-looking echo, so a false-positive ECG is something to consider,” said Dr. Baggish.
This excess in anterior TWIs has been seen in previous studies and is thought to be benign, although the mechanism remains unclear. Four of the nine female athletes with abnormal ECG findings on initial evaluation had normalized on repeat testing 2-4 years later. Serial data were available in only a subset of athletes.
Clarity needed after COVID
The data, published recently in JAMA Cardiology, are particularly valuable these days given concern over the effects of COVID-19 on the heart and return-to-play recommendations.
“Athletes who have had COVID are being sent for echocardiograms before they can return to play to check for COVID-induced heart disease – which is real – but what we’re seeing is that there’s confusion out there in terms of what is a COVID-related abnormality and what is a normal, adapted athletic heart,” said Dr. Baggish.
“In this paper, we provide a dataset of normal values – generated before COVID was on anyone’s radar – to let cardiologists know what’s ‘big good’ and not ‘big bad.’ ”
More sport-specific data needed
“Although these numbers are still small, this dataset is an important step forward in our understanding of athletic adaptations,” said Matthew Martinez, MD, in an interview. “Many factors impact physiologic athletic changes, and the study aids in our understanding of gender- and sport-specific changes in athletes.”
Dr. Martinez, who is the director of sports cardiology at Atlantic Health–Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, also in Morristown, and the chair of Sports and Exercise Cardiology Section Leadership Council for the American College of Cardiology, noted the relatively young mean age of screened athletes.
“The data represent collegiate-age athletes with some older groups mixed in, but it does not represent older established elite athlete changes,” he said.
Mean age was 21 years in the female players but only 18 years in the males because the men’s senior national team failed to qualify for the World Cup during the study period and was therefore not screened. The authors acknowledged the “dearth of older men in the cohort.”
There is, overall, little age-, sport-, and sex-specific normative data for differentiating training-related cardiovascular adaptations from potentially pathologic phenotypes, wrote the authors.
It exists for men playing in the National Football League and for both sexes participating in the National Basketball Association, but most other studies have mixed the sports and focused mainly on men. That said, Dr. Baggish does not consider these data to be applicable to all elite athletes.
“Soccer is kind of in a league of its own with respect to the mixed amount of explosive or resistant and aerobic work that these athletes have to do, and also it’s the most popular sport in the world, so we really wanted to focus on them,” said Dr. Baggish.
Although the findings are perhaps applicable to athletes from other team sports characterized by explosive spurts of high-intensity activity – like hockey, lacrosse, and field hockey – he would not suggest they be applied to, say, long-distance runners, cyclists, or other sports that require a similar type of aerobic output.
Dr. Baggish reported no relevant conflict of interest. Dr. Martinez is league cardiologist for Major League Soccer.
A version of this article first appeared on Medscape.com.
Elite American soccer players have, on average, larger, thicker, and heavier hearts than the general population, according to a new study that provides clinicians with normative echocardiogram and electrocardiogram (ECG) cutoffs to use when assessing the heart health of competitive athletes.
To provide these age- and sex-specific reference values, a team from Massachusetts General Hospital, Boston, led by Timothy W. Churchill, MD, and Aaron L. Baggish, MD, analyzed data from 122 female and 116 male soccer players from the American national teams preparing for World Cup play and undergoing FIFA-mandated preparticipation screening.
The athletes frequently exceeded normal echocardiographic ranges for left ventricular (LV) mass, volume, and wall thickness – structural cardiac parameters responsive to exercise-induced remodeling – but with none showing pathologic findings that might indicate the need to restrict their participation in the sport.
Almost one-third (30%) of female athletes and 41% of male athletes exceeded the American Society of Echocardiography’s upper limit of normal for LV wall thickness, with a measure greater than 12 mm seen in 12% of men and 1% of women.
The majority (51% of females and 59% of males) exceeded normal ranges for body surface area–indexed LV mass, with 77% and 68%, respectively, having LV volumes above the normal range.
Dr. Baggish stressed in an interview, however, that these data tell a story about healthy hearts, not at-risk hearts.
“These are the healthiest, highest-performing elite soccer players that we have in the United States, and this is really a look at how adaptive the heart can be, how much it can grow and change in size, shape, structure, and function in response to sport,” said Dr. Baggish.
The mean age of screened athletes was 20 years (range, 15-40 years). The majority of the female players were White (71%), whereas the male players were more evenly divided between Black (34%), Hispanic (33%), and White (32%).
Screening was performed at U.S. Soccer training sites by experienced clinicians affiliated with the Massachusetts General Hospital cardiovascular performance program.
Interestingly, the study debunks the idea that women, on average, have smaller chamber sizes. “When we did body-size correction, the men and women actually looked pretty similar with respect to their ability to adapt to strenuous exercise,” noted Dr. Baggish.
They did see, however, that women were more likely than men to have abnormal ECG findings. Male athletes showed a higher prevalence of “normal” training-related ECG findings, whereas female athletes were more likely to have abnormal ECG patterns (11.5% vs. 0.0% in the male cohort), most often pathologic T-wave inversions (TWI) confined to the anterior precordial lead distribution.
“This is important because ECGs are the most common screening tool used and we wanted to alert people to the fact that these women who showed some abnormalities on ECG went on to have a total healthy-looking echo, so a false-positive ECG is something to consider,” said Dr. Baggish.
This excess in anterior TWIs has been seen in previous studies and is thought to be benign, although the mechanism remains unclear. Four of the nine female athletes with abnormal ECG findings on initial evaluation had normalized on repeat testing 2-4 years later. Serial data were available in only a subset of athletes.
Clarity needed after COVID
The data, published recently in JAMA Cardiology, are particularly valuable these days given concern over the effects of COVID-19 on the heart and return-to-play recommendations.
“Athletes who have had COVID are being sent for echocardiograms before they can return to play to check for COVID-induced heart disease – which is real – but what we’re seeing is that there’s confusion out there in terms of what is a COVID-related abnormality and what is a normal, adapted athletic heart,” said Dr. Baggish.
“In this paper, we provide a dataset of normal values – generated before COVID was on anyone’s radar – to let cardiologists know what’s ‘big good’ and not ‘big bad.’ ”
More sport-specific data needed
“Although these numbers are still small, this dataset is an important step forward in our understanding of athletic adaptations,” said Matthew Martinez, MD, in an interview. “Many factors impact physiologic athletic changes, and the study aids in our understanding of gender- and sport-specific changes in athletes.”
Dr. Martinez, who is the director of sports cardiology at Atlantic Health–Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, also in Morristown, and the chair of Sports and Exercise Cardiology Section Leadership Council for the American College of Cardiology, noted the relatively young mean age of screened athletes.
“The data represent collegiate-age athletes with some older groups mixed in, but it does not represent older established elite athlete changes,” he said.
Mean age was 21 years in the female players but only 18 years in the males because the men’s senior national team failed to qualify for the World Cup during the study period and was therefore not screened. The authors acknowledged the “dearth of older men in the cohort.”
There is, overall, little age-, sport-, and sex-specific normative data for differentiating training-related cardiovascular adaptations from potentially pathologic phenotypes, wrote the authors.
It exists for men playing in the National Football League and for both sexes participating in the National Basketball Association, but most other studies have mixed the sports and focused mainly on men. That said, Dr. Baggish does not consider these data to be applicable to all elite athletes.
“Soccer is kind of in a league of its own with respect to the mixed amount of explosive or resistant and aerobic work that these athletes have to do, and also it’s the most popular sport in the world, so we really wanted to focus on them,” said Dr. Baggish.
Although the findings are perhaps applicable to athletes from other team sports characterized by explosive spurts of high-intensity activity – like hockey, lacrosse, and field hockey – he would not suggest they be applied to, say, long-distance runners, cyclists, or other sports that require a similar type of aerobic output.
Dr. Baggish reported no relevant conflict of interest. Dr. Martinez is league cardiologist for Major League Soccer.
A version of this article first appeared on Medscape.com.
FDA safety alert: Face masks with metal can burn during MRI
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
SCAPIS: Simple questionnaire can identify silent atherosclerosis
Individuals in the general population with high levels of silent coronary atherosclerosis can be successfully identified with a simple questionnaire that they can complete themselves at home, a new study suggests.
The Swedish CardioPulmonary BioImage Study (SCAPIS) found that 40% of middle-aged adults without known heart disease had evidence of coronary atherosclerosis on coronary CT angiography (CCTA), and 13% had extensive atherosclerotic disease.
The authors found that the screening questionnaire could identify individuals who had extensive coronary atherosclerosis with a reasonably high predictive value.
Initial results from the study were presented today at the virtual American Heart Association (AHA) Scientific Sessions 2020.
“Our study is looking to see if we can estimate how many people in the general population have significant coronary atherosclerosis and therefore could benefit from preventative treatment,” lead author, Göran Bergström, MD, explained to Medscape Medical News.
Bergström, who is professor and lead physician at Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden, said there are no good data on this as yet. “There are studies of atherosclerosis burden in patients who have had a cardiovascular event, but our study was conducted in a random selection of the middle-aged general population who did not have symptoms of heart disease.”
“Our study also suggests that in future we may be able to identify these people with an online questionnaire, and those that reached a certain score could be referred for an imaging test,” he added.
SCAPIS included more than 30,000 men and women, age 50 to 64 years, who had no history of cardiovascular events or cardiac intervention. They were asked questions about sex, age, lifestyle, smoking, body measurements, cholesterol medication, and blood pressure to predict their risk for coronary artery disease.
Researchers then used CCTA images to examine patients’ arteries for the presence of plaque. More than 25,000 individuals from the original sample were successfully imaged.
Results showed that 40% of the middle-aged population had some coronary atherosclerosis and 5% had severe atherosclerosis, defined as the presence of a stenosis blocking 50% or more of blood flow in one of the coronary arteries.
A second aim of the study was to use data from the questionnaire to develop a prediction model to identify people with widespread atherosclerosis — those with any type of stenosis in four different segments of their coronary arteries, who made up 13% of the population.
The questionnaire included data on 120 different variables. Of these variables, around 100 could be assessed by the patients themselves and another 20 measurements could be performed in the clinic, such as blood pressure and cholesterol levels.
The researchers then used artificial intelligence to assess which variables were associated with widespread atherosclerosis. This had an area under the curve (AUC, a measure of the predictive value) of 0.8.
“An AUC of 1.0 would show a perfect prediction, and a value of 0.5 shows no value. A result of 0.8 shows reasonable predictive potential. This is an encouraging result and suggests this strategy could work,” Bergström said.
“We know silent atherosclerosis is a big problem and causes sudden cardiac events in people who have not shown symptoms,” he said.
The goal is to identify these patients before they have an event and offer them preventive treatments. “At present we try and identify patients at high risk of cardiovascular events by using cholesterol and blood pressure measurements and cardiovascular risk scores such as Framingham. But this is not so effective,” Bergström explained.
“Using imaging such as CCTA, where you can actually see atherosclerotic plaque, could be better for prediction, but we can’t image everyone. So, we wanted to see whether we could narrow down the population who should receive imaging with a detailed questionnaire, and it looks like we can.”
The study found that including clinical measurements such as blood pressure and cholesterol did not add much to the predictive value for identifying people with extensive coronary atherosclerosis, a result that Bergström said was surprising.
Which population to target?
Discussant of the study, Pamela Douglas, MD, professor of research in cardiovascular diseases at Duke University, Durham, North Carolina, congratulated the SCAPIS investigators on creating “a very rich data set for current and future study.”
“The SCAPIS study has already yielded novel data on the prevalence of coronary artery disease in the general population, and will address many critical questions over the long term,” she said.
But Douglas suggested that individuals with extensive coronary atherosclerosis were not the most appropriate target population to identify.
“The rationale for choosing this cutpoint is unclear as clinical risk/mortality is higher in all nonobstructive coronary artery disease, starting at one-vessel involvement,” she noted. “Therefore, effective preventive strategies likely need to start with detection and treatment of patients with even minimal plaque.”
Responding to Medscape Medical News, Bergström said this was a valid argument. “We plan to reanalyze our results with different populations as the target — that is something that we can do in the future.
But targeting everyone with just one coronary plaque is going to identify a large group — it was 40% of the population in our study. This will be too many people in whom to perform confirmatory CCTA imaging. It would be impractical to try and conduct cardiac imaging on that many people.”
Bergström noted that more data are needed on the danger of various levels of coronary atherosclerosis in this population who have not had any symptoms.
“We don’t have this information at present, but we are continuing to follow our population and we will have data on cardiac events in a few years’ time. Then we will know which level of atherosclerosis we need to target. It will probably be somewhere in between the extensive levels we used in this first analysis (which occurred in 13% of people) and the 40% of people who showed just one area of plaque.”
This study is the first report from SCAPIS, a collaborative project between six Swedish universities with the following vision statement: to “reduce the risk of cardiovascular and respiratory diseases for generations to come.”
The SCAPIS project is funded by the Swedish Heart and Lung Foundation. Bergström reports no disclosures.
This article first appeared on Medscape.com.
Individuals in the general population with high levels of silent coronary atherosclerosis can be successfully identified with a simple questionnaire that they can complete themselves at home, a new study suggests.
The Swedish CardioPulmonary BioImage Study (SCAPIS) found that 40% of middle-aged adults without known heart disease had evidence of coronary atherosclerosis on coronary CT angiography (CCTA), and 13% had extensive atherosclerotic disease.
The authors found that the screening questionnaire could identify individuals who had extensive coronary atherosclerosis with a reasonably high predictive value.
Initial results from the study were presented today at the virtual American Heart Association (AHA) Scientific Sessions 2020.
“Our study is looking to see if we can estimate how many people in the general population have significant coronary atherosclerosis and therefore could benefit from preventative treatment,” lead author, Göran Bergström, MD, explained to Medscape Medical News.
Bergström, who is professor and lead physician at Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden, said there are no good data on this as yet. “There are studies of atherosclerosis burden in patients who have had a cardiovascular event, but our study was conducted in a random selection of the middle-aged general population who did not have symptoms of heart disease.”
“Our study also suggests that in future we may be able to identify these people with an online questionnaire, and those that reached a certain score could be referred for an imaging test,” he added.
SCAPIS included more than 30,000 men and women, age 50 to 64 years, who had no history of cardiovascular events or cardiac intervention. They were asked questions about sex, age, lifestyle, smoking, body measurements, cholesterol medication, and blood pressure to predict their risk for coronary artery disease.
Researchers then used CCTA images to examine patients’ arteries for the presence of plaque. More than 25,000 individuals from the original sample were successfully imaged.
Results showed that 40% of the middle-aged population had some coronary atherosclerosis and 5% had severe atherosclerosis, defined as the presence of a stenosis blocking 50% or more of blood flow in one of the coronary arteries.
A second aim of the study was to use data from the questionnaire to develop a prediction model to identify people with widespread atherosclerosis — those with any type of stenosis in four different segments of their coronary arteries, who made up 13% of the population.
The questionnaire included data on 120 different variables. Of these variables, around 100 could be assessed by the patients themselves and another 20 measurements could be performed in the clinic, such as blood pressure and cholesterol levels.
The researchers then used artificial intelligence to assess which variables were associated with widespread atherosclerosis. This had an area under the curve (AUC, a measure of the predictive value) of 0.8.
“An AUC of 1.0 would show a perfect prediction, and a value of 0.5 shows no value. A result of 0.8 shows reasonable predictive potential. This is an encouraging result and suggests this strategy could work,” Bergström said.
“We know silent atherosclerosis is a big problem and causes sudden cardiac events in people who have not shown symptoms,” he said.
The goal is to identify these patients before they have an event and offer them preventive treatments. “At present we try and identify patients at high risk of cardiovascular events by using cholesterol and blood pressure measurements and cardiovascular risk scores such as Framingham. But this is not so effective,” Bergström explained.
“Using imaging such as CCTA, where you can actually see atherosclerotic plaque, could be better for prediction, but we can’t image everyone. So, we wanted to see whether we could narrow down the population who should receive imaging with a detailed questionnaire, and it looks like we can.”
The study found that including clinical measurements such as blood pressure and cholesterol did not add much to the predictive value for identifying people with extensive coronary atherosclerosis, a result that Bergström said was surprising.
Which population to target?
Discussant of the study, Pamela Douglas, MD, professor of research in cardiovascular diseases at Duke University, Durham, North Carolina, congratulated the SCAPIS investigators on creating “a very rich data set for current and future study.”
“The SCAPIS study has already yielded novel data on the prevalence of coronary artery disease in the general population, and will address many critical questions over the long term,” she said.
But Douglas suggested that individuals with extensive coronary atherosclerosis were not the most appropriate target population to identify.
“The rationale for choosing this cutpoint is unclear as clinical risk/mortality is higher in all nonobstructive coronary artery disease, starting at one-vessel involvement,” she noted. “Therefore, effective preventive strategies likely need to start with detection and treatment of patients with even minimal plaque.”
Responding to Medscape Medical News, Bergström said this was a valid argument. “We plan to reanalyze our results with different populations as the target — that is something that we can do in the future.
But targeting everyone with just one coronary plaque is going to identify a large group — it was 40% of the population in our study. This will be too many people in whom to perform confirmatory CCTA imaging. It would be impractical to try and conduct cardiac imaging on that many people.”
Bergström noted that more data are needed on the danger of various levels of coronary atherosclerosis in this population who have not had any symptoms.
“We don’t have this information at present, but we are continuing to follow our population and we will have data on cardiac events in a few years’ time. Then we will know which level of atherosclerosis we need to target. It will probably be somewhere in between the extensive levels we used in this first analysis (which occurred in 13% of people) and the 40% of people who showed just one area of plaque.”
This study is the first report from SCAPIS, a collaborative project between six Swedish universities with the following vision statement: to “reduce the risk of cardiovascular and respiratory diseases for generations to come.”
The SCAPIS project is funded by the Swedish Heart and Lung Foundation. Bergström reports no disclosures.
This article first appeared on Medscape.com.
Individuals in the general population with high levels of silent coronary atherosclerosis can be successfully identified with a simple questionnaire that they can complete themselves at home, a new study suggests.
The Swedish CardioPulmonary BioImage Study (SCAPIS) found that 40% of middle-aged adults without known heart disease had evidence of coronary atherosclerosis on coronary CT angiography (CCTA), and 13% had extensive atherosclerotic disease.
The authors found that the screening questionnaire could identify individuals who had extensive coronary atherosclerosis with a reasonably high predictive value.
Initial results from the study were presented today at the virtual American Heart Association (AHA) Scientific Sessions 2020.
“Our study is looking to see if we can estimate how many people in the general population have significant coronary atherosclerosis and therefore could benefit from preventative treatment,” lead author, Göran Bergström, MD, explained to Medscape Medical News.
Bergström, who is professor and lead physician at Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden, said there are no good data on this as yet. “There are studies of atherosclerosis burden in patients who have had a cardiovascular event, but our study was conducted in a random selection of the middle-aged general population who did not have symptoms of heart disease.”
“Our study also suggests that in future we may be able to identify these people with an online questionnaire, and those that reached a certain score could be referred for an imaging test,” he added.
SCAPIS included more than 30,000 men and women, age 50 to 64 years, who had no history of cardiovascular events or cardiac intervention. They were asked questions about sex, age, lifestyle, smoking, body measurements, cholesterol medication, and blood pressure to predict their risk for coronary artery disease.
Researchers then used CCTA images to examine patients’ arteries for the presence of plaque. More than 25,000 individuals from the original sample were successfully imaged.
Results showed that 40% of the middle-aged population had some coronary atherosclerosis and 5% had severe atherosclerosis, defined as the presence of a stenosis blocking 50% or more of blood flow in one of the coronary arteries.
A second aim of the study was to use data from the questionnaire to develop a prediction model to identify people with widespread atherosclerosis — those with any type of stenosis in four different segments of their coronary arteries, who made up 13% of the population.
The questionnaire included data on 120 different variables. Of these variables, around 100 could be assessed by the patients themselves and another 20 measurements could be performed in the clinic, such as blood pressure and cholesterol levels.
The researchers then used artificial intelligence to assess which variables were associated with widespread atherosclerosis. This had an area under the curve (AUC, a measure of the predictive value) of 0.8.
“An AUC of 1.0 would show a perfect prediction, and a value of 0.5 shows no value. A result of 0.8 shows reasonable predictive potential. This is an encouraging result and suggests this strategy could work,” Bergström said.
“We know silent atherosclerosis is a big problem and causes sudden cardiac events in people who have not shown symptoms,” he said.
The goal is to identify these patients before they have an event and offer them preventive treatments. “At present we try and identify patients at high risk of cardiovascular events by using cholesterol and blood pressure measurements and cardiovascular risk scores such as Framingham. But this is not so effective,” Bergström explained.
“Using imaging such as CCTA, where you can actually see atherosclerotic plaque, could be better for prediction, but we can’t image everyone. So, we wanted to see whether we could narrow down the population who should receive imaging with a detailed questionnaire, and it looks like we can.”
The study found that including clinical measurements such as blood pressure and cholesterol did not add much to the predictive value for identifying people with extensive coronary atherosclerosis, a result that Bergström said was surprising.
Which population to target?
Discussant of the study, Pamela Douglas, MD, professor of research in cardiovascular diseases at Duke University, Durham, North Carolina, congratulated the SCAPIS investigators on creating “a very rich data set for current and future study.”
“The SCAPIS study has already yielded novel data on the prevalence of coronary artery disease in the general population, and will address many critical questions over the long term,” she said.
But Douglas suggested that individuals with extensive coronary atherosclerosis were not the most appropriate target population to identify.
“The rationale for choosing this cutpoint is unclear as clinical risk/mortality is higher in all nonobstructive coronary artery disease, starting at one-vessel involvement,” she noted. “Therefore, effective preventive strategies likely need to start with detection and treatment of patients with even minimal plaque.”
Responding to Medscape Medical News, Bergström said this was a valid argument. “We plan to reanalyze our results with different populations as the target — that is something that we can do in the future.
But targeting everyone with just one coronary plaque is going to identify a large group — it was 40% of the population in our study. This will be too many people in whom to perform confirmatory CCTA imaging. It would be impractical to try and conduct cardiac imaging on that many people.”
Bergström noted that more data are needed on the danger of various levels of coronary atherosclerosis in this population who have not had any symptoms.
“We don’t have this information at present, but we are continuing to follow our population and we will have data on cardiac events in a few years’ time. Then we will know which level of atherosclerosis we need to target. It will probably be somewhere in between the extensive levels we used in this first analysis (which occurred in 13% of people) and the 40% of people who showed just one area of plaque.”
This study is the first report from SCAPIS, a collaborative project between six Swedish universities with the following vision statement: to “reduce the risk of cardiovascular and respiratory diseases for generations to come.”
The SCAPIS project is funded by the Swedish Heart and Lung Foundation. Bergström reports no disclosures.
This article first appeared on Medscape.com.
Combined OCT, cardiac MRI unravels root cause in most MINOCA
Optical CT (OCT) plus cardiac MRI (CMR) provides a more specific diagnosis in the majority of women presenting with myocardial infarction with nonobstructive coronary arteries (MINOCA).
The multimodal imaging strategy identified the underlying cause of MINOCA in 85% of women in the HARP-MINOCA study. Overall, 64% of women had a true MI and 21% had an alternate nonischemic diagnosis, most commonly myocarditis.
“OCTCMR findings correlated well with OCT culprit lesions, demonstrating that nonobstructive culprit lesions frequently cause MINOCA,” said study author Harmony Reynolds, MD, director of New York University Langone’s Sarah Ross Soter Center for Women’s Cardiovascular Research.
The results were presented at the virtual American Heart Association (AHA) Scientific Sessions 2020 and published simultaneously in Circulation.
MINOCA occurs in up to 15% of patients with MI and is defined as MI meeting the universal definition but with less than 50% stenosis in all major epicardial arteries on angiography and no specific alternate diagnosis to explain the presentation.
It is three times more common in women than in men and also disproportionately affects Black, Hispanic, Maori, and Pacific persons. MINOCA has several causes, leading to uncertainty in diagnostic testing and treatment.
“Different doctors tell patients different messages about MINOCA and may incorrectly say the event wasn’t a heart attack,” Dr. Reynolds said in an earlier press briefing. “I had a patient who was told ‘your arteries are open,’ and they gave her Xanax.”
As part of the Women’s Heart Attack Research Program (HARP), researchers enrolled 301 women with a clinical diagnosis of MI, of whom 170 were diagnosed with MINOCA during angiography and underwent OCT at that time, followed by CMR within 1 week of the acute presentation.
All images were interpreted by an independent core laboratory blinded to results of the other tests and clinical information. The final cohort included 145 women with interpretable OCT images.
Their median age was 60 years, 49.7% were white non-Hispanic, and 97% presented with a provisional diagnosis of non–ST-segment MI. Their median peak troponin level was 0.94 ng/mL.
OCT identified a definite or probable culprit lesion in 46% of women, most commonly atherosclerosis or thrombosis. On multivariable analysis, having a culprit lesion was associated with older age, abnormal angiography findings at the site, and diabetes, but not peak troponin level or severity of angiographic stenosis.
CMR available in 116 women showed evidence of infarction or regional injury in 69%. Multivariate predictors of an abnormal CMR were higher peak troponin and diastolic blood pressure but not an OCT culprit lesion or angiographic stenosis severity.
When the OCT and CMR results were combined, a cause of MINOCA was identified in 84.5% of women. Three-fourths of the causes were ischemic (64% MI) and one-quarter were nonischemic (15% myocarditis, 3% Takotsubo syndrome, and 3% nonischemic cardiomyopathy). In the remaining 15%, no cause of MINOCA was identified.
To emphasize the effect multimodal imaging can have on treatment, Dr. Reynolds highlighted a 44-year-old woman with no risk factors for coronary artery disease who had chest pain in the context of heavy menstrual bleeding, a low hemoglobin level, and peak troponin level of 3.25 ng/mL.
Unexpectedly, imaging revealed a left anterior descending (LAD) plaque rupture in a thin-cap fibroatheroma, causing a small transmural infarction at the terminus of the LAD.
“Without this diagnosis, it’s unlikely she would have received antiplatelet therapy or statins and might have been given a diagnosis of supply/demand mismatch, when the real diagnosis was MI,” Dr. Reynolds observed.
“Finally we can say this is not just crazy women. There is really something going on,” said panelist Roxana Mehran, MD, of the Icahn School of Medicine at Mount Sinai in New York. “You have now told us this is most likely atherosclerosis for pretty much 85% of the cases. So make the diagnosis and, of course, make sure you treat these patients accordingly for risk factor modification, really thinking about a ruptured plaque.”
Combining OCT and MRI may result in a more specific diagnosis and better treatment but also raises costs and logistical considerations.
“Implementation challenges are that not every form of testing is available in every medical center,” Dr. Reynolds said in an interview. “Many centers have cardiac MRI,” whereas “OCT is not currently available at most medical centers where heart attack patients are treated but is available at specialized centers.”
Asked during the session about the use of CT angiography, invited discussant Martha Gulati, MD, president-elect of the American Society for Preventive Cardiology, said, “For me, CT is helpful when I’m not sure if there’s any plaque because the angiogram looked really normal and there was no opportunity to do intracoronary imaging. And sometimes that will help me, in particular, if a patient doesn’t want to take a statin.”
Dr. Gulati pointed out that the European Society of Cardiology MINOCA guidelines recommend OCT and CMR, whereas the 2019 AHA statement on MINOCA, which she coauthored, also recommends OCT and CMR, but almost as one or the other.
“We already said that you should do cardiac MR to try to make a diagnosis, but I think the combination of the two needs to be emphasized when we next draft these guidelines. It really will help,” Dr. Gulati said in an interview.
“But using OCT, particularly, needs to be in the setting of the MI. I don’t think you want to do a procedure again,” she said. “So we really need it to become more widely available because at the time of an MI, you won’t necessarily know that you’re not going to find an obstructive lesion.”
Dr. Gulati pointed out several unanswered questions, including whether the diagnosis was missed in some patients, because OCT of all three vessels was available in only 59%, and how the use of high-sensitivity troponin, which was left up to the individual institution, might affect the usefulness of OCT and CMR.
It’s also unknown whether the mechanism is different for ST-segment elevation MI, as the trial included very few cases, although MINOCA often occurs in this setting. Future OCT/CMR studies will also need to enroll men to determine potential sex differences, if any.
Commenting on the study, B. Hadley Wilson, MD, Sanger Heart & Vascular Institute in Charlotte, N.C., said, “There would need to be further justification of this invasive interventional procedure to be sure that the benefit outweighed the risk of putting a wire and an OCT catheter down patients without any significant angiographic blockage and to assure interventional cardiologists of its value here.”
He pointed out that noninvasive CMR appears helpful in the diagnosis of nearly three-quarters of these patients and perhaps could be done first to direct which of those with an ischemic cause might benefit from invasive OCT at catheterization. This seems most pertinent in patients with a high suspicion of coronary artery disease or recurrent MINOCA.
“Overall, we need to consider the expense, logistics, and small risk of these combined modalities, particularly in everyday practice, before making recommendations,” Dr. Wilson said. “ Since OCT is much less available than intravascular ultrasound, it would require a challenging marketplace paradigm shift to implement this multimodality imaging strategy regionally and locally in the U.S., including the added costs. However, further study to direct the more judicious use of either CMR and/or combined with OCT is warranted in these patients.”
The study was funded by the AHA through a grant from the Go Red for Women Strategically Focused Research Network. Dr. Reynolds reported in-kind donations from Abbott Vascular and Siemens related to the study and nonfinancial support from BioTelemetry outside the study. Dr. Gulati and Dr. Wilson reported having no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Optical CT (OCT) plus cardiac MRI (CMR) provides a more specific diagnosis in the majority of women presenting with myocardial infarction with nonobstructive coronary arteries (MINOCA).
The multimodal imaging strategy identified the underlying cause of MINOCA in 85% of women in the HARP-MINOCA study. Overall, 64% of women had a true MI and 21% had an alternate nonischemic diagnosis, most commonly myocarditis.
“OCTCMR findings correlated well with OCT culprit lesions, demonstrating that nonobstructive culprit lesions frequently cause MINOCA,” said study author Harmony Reynolds, MD, director of New York University Langone’s Sarah Ross Soter Center for Women’s Cardiovascular Research.
The results were presented at the virtual American Heart Association (AHA) Scientific Sessions 2020 and published simultaneously in Circulation.
MINOCA occurs in up to 15% of patients with MI and is defined as MI meeting the universal definition but with less than 50% stenosis in all major epicardial arteries on angiography and no specific alternate diagnosis to explain the presentation.
It is three times more common in women than in men and also disproportionately affects Black, Hispanic, Maori, and Pacific persons. MINOCA has several causes, leading to uncertainty in diagnostic testing and treatment.
“Different doctors tell patients different messages about MINOCA and may incorrectly say the event wasn’t a heart attack,” Dr. Reynolds said in an earlier press briefing. “I had a patient who was told ‘your arteries are open,’ and they gave her Xanax.”
As part of the Women’s Heart Attack Research Program (HARP), researchers enrolled 301 women with a clinical diagnosis of MI, of whom 170 were diagnosed with MINOCA during angiography and underwent OCT at that time, followed by CMR within 1 week of the acute presentation.
All images were interpreted by an independent core laboratory blinded to results of the other tests and clinical information. The final cohort included 145 women with interpretable OCT images.
Their median age was 60 years, 49.7% were white non-Hispanic, and 97% presented with a provisional diagnosis of non–ST-segment MI. Their median peak troponin level was 0.94 ng/mL.
OCT identified a definite or probable culprit lesion in 46% of women, most commonly atherosclerosis or thrombosis. On multivariable analysis, having a culprit lesion was associated with older age, abnormal angiography findings at the site, and diabetes, but not peak troponin level or severity of angiographic stenosis.
CMR available in 116 women showed evidence of infarction or regional injury in 69%. Multivariate predictors of an abnormal CMR were higher peak troponin and diastolic blood pressure but not an OCT culprit lesion or angiographic stenosis severity.
When the OCT and CMR results were combined, a cause of MINOCA was identified in 84.5% of women. Three-fourths of the causes were ischemic (64% MI) and one-quarter were nonischemic (15% myocarditis, 3% Takotsubo syndrome, and 3% nonischemic cardiomyopathy). In the remaining 15%, no cause of MINOCA was identified.
To emphasize the effect multimodal imaging can have on treatment, Dr. Reynolds highlighted a 44-year-old woman with no risk factors for coronary artery disease who had chest pain in the context of heavy menstrual bleeding, a low hemoglobin level, and peak troponin level of 3.25 ng/mL.
Unexpectedly, imaging revealed a left anterior descending (LAD) plaque rupture in a thin-cap fibroatheroma, causing a small transmural infarction at the terminus of the LAD.
“Without this diagnosis, it’s unlikely she would have received antiplatelet therapy or statins and might have been given a diagnosis of supply/demand mismatch, when the real diagnosis was MI,” Dr. Reynolds observed.
“Finally we can say this is not just crazy women. There is really something going on,” said panelist Roxana Mehran, MD, of the Icahn School of Medicine at Mount Sinai in New York. “You have now told us this is most likely atherosclerosis for pretty much 85% of the cases. So make the diagnosis and, of course, make sure you treat these patients accordingly for risk factor modification, really thinking about a ruptured plaque.”
Combining OCT and MRI may result in a more specific diagnosis and better treatment but also raises costs and logistical considerations.
“Implementation challenges are that not every form of testing is available in every medical center,” Dr. Reynolds said in an interview. “Many centers have cardiac MRI,” whereas “OCT is not currently available at most medical centers where heart attack patients are treated but is available at specialized centers.”
Asked during the session about the use of CT angiography, invited discussant Martha Gulati, MD, president-elect of the American Society for Preventive Cardiology, said, “For me, CT is helpful when I’m not sure if there’s any plaque because the angiogram looked really normal and there was no opportunity to do intracoronary imaging. And sometimes that will help me, in particular, if a patient doesn’t want to take a statin.”
Dr. Gulati pointed out that the European Society of Cardiology MINOCA guidelines recommend OCT and CMR, whereas the 2019 AHA statement on MINOCA, which she coauthored, also recommends OCT and CMR, but almost as one or the other.
“We already said that you should do cardiac MR to try to make a diagnosis, but I think the combination of the two needs to be emphasized when we next draft these guidelines. It really will help,” Dr. Gulati said in an interview.
“But using OCT, particularly, needs to be in the setting of the MI. I don’t think you want to do a procedure again,” she said. “So we really need it to become more widely available because at the time of an MI, you won’t necessarily know that you’re not going to find an obstructive lesion.”
Dr. Gulati pointed out several unanswered questions, including whether the diagnosis was missed in some patients, because OCT of all three vessels was available in only 59%, and how the use of high-sensitivity troponin, which was left up to the individual institution, might affect the usefulness of OCT and CMR.
It’s also unknown whether the mechanism is different for ST-segment elevation MI, as the trial included very few cases, although MINOCA often occurs in this setting. Future OCT/CMR studies will also need to enroll men to determine potential sex differences, if any.
Commenting on the study, B. Hadley Wilson, MD, Sanger Heart & Vascular Institute in Charlotte, N.C., said, “There would need to be further justification of this invasive interventional procedure to be sure that the benefit outweighed the risk of putting a wire and an OCT catheter down patients without any significant angiographic blockage and to assure interventional cardiologists of its value here.”
He pointed out that noninvasive CMR appears helpful in the diagnosis of nearly three-quarters of these patients and perhaps could be done first to direct which of those with an ischemic cause might benefit from invasive OCT at catheterization. This seems most pertinent in patients with a high suspicion of coronary artery disease or recurrent MINOCA.
“Overall, we need to consider the expense, logistics, and small risk of these combined modalities, particularly in everyday practice, before making recommendations,” Dr. Wilson said. “ Since OCT is much less available than intravascular ultrasound, it would require a challenging marketplace paradigm shift to implement this multimodality imaging strategy regionally and locally in the U.S., including the added costs. However, further study to direct the more judicious use of either CMR and/or combined with OCT is warranted in these patients.”
The study was funded by the AHA through a grant from the Go Red for Women Strategically Focused Research Network. Dr. Reynolds reported in-kind donations from Abbott Vascular and Siemens related to the study and nonfinancial support from BioTelemetry outside the study. Dr. Gulati and Dr. Wilson reported having no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Optical CT (OCT) plus cardiac MRI (CMR) provides a more specific diagnosis in the majority of women presenting with myocardial infarction with nonobstructive coronary arteries (MINOCA).
The multimodal imaging strategy identified the underlying cause of MINOCA in 85% of women in the HARP-MINOCA study. Overall, 64% of women had a true MI and 21% had an alternate nonischemic diagnosis, most commonly myocarditis.
“OCTCMR findings correlated well with OCT culprit lesions, demonstrating that nonobstructive culprit lesions frequently cause MINOCA,” said study author Harmony Reynolds, MD, director of New York University Langone’s Sarah Ross Soter Center for Women’s Cardiovascular Research.
The results were presented at the virtual American Heart Association (AHA) Scientific Sessions 2020 and published simultaneously in Circulation.
MINOCA occurs in up to 15% of patients with MI and is defined as MI meeting the universal definition but with less than 50% stenosis in all major epicardial arteries on angiography and no specific alternate diagnosis to explain the presentation.
It is three times more common in women than in men and also disproportionately affects Black, Hispanic, Maori, and Pacific persons. MINOCA has several causes, leading to uncertainty in diagnostic testing and treatment.
“Different doctors tell patients different messages about MINOCA and may incorrectly say the event wasn’t a heart attack,” Dr. Reynolds said in an earlier press briefing. “I had a patient who was told ‘your arteries are open,’ and they gave her Xanax.”
As part of the Women’s Heart Attack Research Program (HARP), researchers enrolled 301 women with a clinical diagnosis of MI, of whom 170 were diagnosed with MINOCA during angiography and underwent OCT at that time, followed by CMR within 1 week of the acute presentation.
All images were interpreted by an independent core laboratory blinded to results of the other tests and clinical information. The final cohort included 145 women with interpretable OCT images.
Their median age was 60 years, 49.7% were white non-Hispanic, and 97% presented with a provisional diagnosis of non–ST-segment MI. Their median peak troponin level was 0.94 ng/mL.
OCT identified a definite or probable culprit lesion in 46% of women, most commonly atherosclerosis or thrombosis. On multivariable analysis, having a culprit lesion was associated with older age, abnormal angiography findings at the site, and diabetes, but not peak troponin level or severity of angiographic stenosis.
CMR available in 116 women showed evidence of infarction or regional injury in 69%. Multivariate predictors of an abnormal CMR were higher peak troponin and diastolic blood pressure but not an OCT culprit lesion or angiographic stenosis severity.
When the OCT and CMR results were combined, a cause of MINOCA was identified in 84.5% of women. Three-fourths of the causes were ischemic (64% MI) and one-quarter were nonischemic (15% myocarditis, 3% Takotsubo syndrome, and 3% nonischemic cardiomyopathy). In the remaining 15%, no cause of MINOCA was identified.
To emphasize the effect multimodal imaging can have on treatment, Dr. Reynolds highlighted a 44-year-old woman with no risk factors for coronary artery disease who had chest pain in the context of heavy menstrual bleeding, a low hemoglobin level, and peak troponin level of 3.25 ng/mL.
Unexpectedly, imaging revealed a left anterior descending (LAD) plaque rupture in a thin-cap fibroatheroma, causing a small transmural infarction at the terminus of the LAD.
“Without this diagnosis, it’s unlikely she would have received antiplatelet therapy or statins and might have been given a diagnosis of supply/demand mismatch, when the real diagnosis was MI,” Dr. Reynolds observed.
“Finally we can say this is not just crazy women. There is really something going on,” said panelist Roxana Mehran, MD, of the Icahn School of Medicine at Mount Sinai in New York. “You have now told us this is most likely atherosclerosis for pretty much 85% of the cases. So make the diagnosis and, of course, make sure you treat these patients accordingly for risk factor modification, really thinking about a ruptured plaque.”
Combining OCT and MRI may result in a more specific diagnosis and better treatment but also raises costs and logistical considerations.
“Implementation challenges are that not every form of testing is available in every medical center,” Dr. Reynolds said in an interview. “Many centers have cardiac MRI,” whereas “OCT is not currently available at most medical centers where heart attack patients are treated but is available at specialized centers.”
Asked during the session about the use of CT angiography, invited discussant Martha Gulati, MD, president-elect of the American Society for Preventive Cardiology, said, “For me, CT is helpful when I’m not sure if there’s any plaque because the angiogram looked really normal and there was no opportunity to do intracoronary imaging. And sometimes that will help me, in particular, if a patient doesn’t want to take a statin.”
Dr. Gulati pointed out that the European Society of Cardiology MINOCA guidelines recommend OCT and CMR, whereas the 2019 AHA statement on MINOCA, which she coauthored, also recommends OCT and CMR, but almost as one or the other.
“We already said that you should do cardiac MR to try to make a diagnosis, but I think the combination of the two needs to be emphasized when we next draft these guidelines. It really will help,” Dr. Gulati said in an interview.
“But using OCT, particularly, needs to be in the setting of the MI. I don’t think you want to do a procedure again,” she said. “So we really need it to become more widely available because at the time of an MI, you won’t necessarily know that you’re not going to find an obstructive lesion.”
Dr. Gulati pointed out several unanswered questions, including whether the diagnosis was missed in some patients, because OCT of all three vessels was available in only 59%, and how the use of high-sensitivity troponin, which was left up to the individual institution, might affect the usefulness of OCT and CMR.
It’s also unknown whether the mechanism is different for ST-segment elevation MI, as the trial included very few cases, although MINOCA often occurs in this setting. Future OCT/CMR studies will also need to enroll men to determine potential sex differences, if any.
Commenting on the study, B. Hadley Wilson, MD, Sanger Heart & Vascular Institute in Charlotte, N.C., said, “There would need to be further justification of this invasive interventional procedure to be sure that the benefit outweighed the risk of putting a wire and an OCT catheter down patients without any significant angiographic blockage and to assure interventional cardiologists of its value here.”
He pointed out that noninvasive CMR appears helpful in the diagnosis of nearly three-quarters of these patients and perhaps could be done first to direct which of those with an ischemic cause might benefit from invasive OCT at catheterization. This seems most pertinent in patients with a high suspicion of coronary artery disease or recurrent MINOCA.
“Overall, we need to consider the expense, logistics, and small risk of these combined modalities, particularly in everyday practice, before making recommendations,” Dr. Wilson said. “ Since OCT is much less available than intravascular ultrasound, it would require a challenging marketplace paradigm shift to implement this multimodality imaging strategy regionally and locally in the U.S., including the added costs. However, further study to direct the more judicious use of either CMR and/or combined with OCT is warranted in these patients.”
The study was funded by the AHA through a grant from the Go Red for Women Strategically Focused Research Network. Dr. Reynolds reported in-kind donations from Abbott Vascular and Siemens related to the study and nonfinancial support from BioTelemetry outside the study. Dr. Gulati and Dr. Wilson reported having no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Radiotherapy planning scans reveal breast cancer patients’ CVD risk
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
FROM EBCC-12 VIRTUAL CONFERENCE