User login
Surgeon gender not associated with maternal morbidity and hemorrhage after C-section
Surgeon gender was not associated with maternal morbidity or severe blood loss after cesarean delivery, a large prospective cohort study from France reports. The results have important implications for the promotion of gender equality among surgeons, obstetricians in particular, wrote a team led by Hanane Bouchghoul, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital. The report is in JAMA Surgery.
“Our findings are significant in that they add substantially to the string of studies contradicting the age-old dogma that men are better surgeons than women,” the authors wrote. Previous research has suggested slightly better outcomes with female surgeons or higher complication rates with male surgeons.
The results support those of a recent Canadian retrospective analysis suggesting that patients treated by male or female surgeons for various elective indications experience similar surgical outcomes but with a slight, statistically significant decrease in 30-day mortality when treated by female surgeons.
“Policy makers need to combat prejudice against women in surgical careers, particularly in obstetrics and gynecology, so that women no longer experience conscious or unconscious barriers or difficulties in their professional choices, training, and relationships with colleagues or patients,” study corresponding author Loïc Sentilhes, MD, PhD, of Bordeaux University Hospital, said in an interview.
Facing such barriers, women may doubt their ability to be surgeons, their legitimacy as surgeons, and may not consider this type of career, he continued. “Moreover a teacher may not be as involved in teaching young female surgeons as young male surgeons, or the doctor-patient relationship may be more complicated in the event of complications if the patient thinks that a female surgeon has less competence than a male surgeon.”
The analysis drew on data from the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery 2 trial, a multicenter, randomized, placebo-controlled study conducted from March 2018 through January 2020 in mothers from 27 French maternity hospitals.
Eligible participants had a cesarean delivery before or during labor at or after 34 weeks’ gestation. The primary endpoint was the incidence of a composite maternal morbidity variable, and the secondary endpoint was the incidence of postpartum hemorrhage, defined by a calculated estimated blood loss exceeding 1,000 mL or transfusion by day 2.
Among the 4,244 women included, male surgeons performed 943 cesarean deliveries (22.2%) and female surgeons performed 3,301 (77.8%). The percentage who were attending obstetricians was higher for men at 441 of 929 (47.5%) than women at 687 of 3,239 (21.2%).
The observed risk of maternal morbidity did not differ between male and female surgeons: 119 of 837 (14.2%) vs. 476 of 2,928 (16.3%), for an adjusted risk ratio (aRR) of 0.92 (95% confidence interval [CI], 0.77-1.13). Interaction between surgeon gender and level of experience with the risk of maternal morbidity was not statistically significant; nor did the groups differ specifically by risk for postpartum hemorrhage: aRR, 0.98 (95% CI, 0.85-1.13).
Despite the longstanding stereotype that men perform surgery better than women, and the traditional preponderance of male surgeons, the authors noted, postoperative morbidity and mortality may be lower after various surgeries performed by women.
The TRAAP2 trial
In an accompanying editorial, Amanda Fader, MD, of the department of obstetrics and gynecology at Johns Hopkins School of Medicine in Baltimore, and colleagues caution that the French study’s methodology may not fully account for the complex intersection of surgeon volume, experience, gender, clinical decision-making skills, and patient-level and clinical factors affecting outcomes.
That said, appraising surgical outcomes based on gender may be an essential step toward reducing implicit bias and dispelling engendered perceptions regarding gender and technical proficiency, the commentators stated. “To definitively dispel archaic, gender-based notions about performance in clinical or surgical settings, efforts must go beyond peer-reviewed research,” Dr. Fader said in an interview. “Medical institutions and leaders of clinical departments must make concerted efforts to recruit, mentor, support, and promote women and persons of all genders in medicine – as well as confront any discriminatory perceptions and experiences concerning sex, race and ethnicity, sexual orientation, or economic class.”
This study was supported by the French Ministry of Health under its Clinical Research Hospital Program. Dr. Sentilhes reported financial relationships with Dilafor, Bayer, GlaxoSmithKline, Sigvaris, and Ferring Pharmaceuticals. The editorial commentators disclosed no funding for their commentary or conflicts of interest.
Surgeon gender was not associated with maternal morbidity or severe blood loss after cesarean delivery, a large prospective cohort study from France reports. The results have important implications for the promotion of gender equality among surgeons, obstetricians in particular, wrote a team led by Hanane Bouchghoul, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital. The report is in JAMA Surgery.
“Our findings are significant in that they add substantially to the string of studies contradicting the age-old dogma that men are better surgeons than women,” the authors wrote. Previous research has suggested slightly better outcomes with female surgeons or higher complication rates with male surgeons.
The results support those of a recent Canadian retrospective analysis suggesting that patients treated by male or female surgeons for various elective indications experience similar surgical outcomes but with a slight, statistically significant decrease in 30-day mortality when treated by female surgeons.
“Policy makers need to combat prejudice against women in surgical careers, particularly in obstetrics and gynecology, so that women no longer experience conscious or unconscious barriers or difficulties in their professional choices, training, and relationships with colleagues or patients,” study corresponding author Loïc Sentilhes, MD, PhD, of Bordeaux University Hospital, said in an interview.
Facing such barriers, women may doubt their ability to be surgeons, their legitimacy as surgeons, and may not consider this type of career, he continued. “Moreover a teacher may not be as involved in teaching young female surgeons as young male surgeons, or the doctor-patient relationship may be more complicated in the event of complications if the patient thinks that a female surgeon has less competence than a male surgeon.”
The analysis drew on data from the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery 2 trial, a multicenter, randomized, placebo-controlled study conducted from March 2018 through January 2020 in mothers from 27 French maternity hospitals.
Eligible participants had a cesarean delivery before or during labor at or after 34 weeks’ gestation. The primary endpoint was the incidence of a composite maternal morbidity variable, and the secondary endpoint was the incidence of postpartum hemorrhage, defined by a calculated estimated blood loss exceeding 1,000 mL or transfusion by day 2.
Among the 4,244 women included, male surgeons performed 943 cesarean deliveries (22.2%) and female surgeons performed 3,301 (77.8%). The percentage who were attending obstetricians was higher for men at 441 of 929 (47.5%) than women at 687 of 3,239 (21.2%).
The observed risk of maternal morbidity did not differ between male and female surgeons: 119 of 837 (14.2%) vs. 476 of 2,928 (16.3%), for an adjusted risk ratio (aRR) of 0.92 (95% confidence interval [CI], 0.77-1.13). Interaction between surgeon gender and level of experience with the risk of maternal morbidity was not statistically significant; nor did the groups differ specifically by risk for postpartum hemorrhage: aRR, 0.98 (95% CI, 0.85-1.13).
Despite the longstanding stereotype that men perform surgery better than women, and the traditional preponderance of male surgeons, the authors noted, postoperative morbidity and mortality may be lower after various surgeries performed by women.
The TRAAP2 trial
In an accompanying editorial, Amanda Fader, MD, of the department of obstetrics and gynecology at Johns Hopkins School of Medicine in Baltimore, and colleagues caution that the French study’s methodology may not fully account for the complex intersection of surgeon volume, experience, gender, clinical decision-making skills, and patient-level and clinical factors affecting outcomes.
That said, appraising surgical outcomes based on gender may be an essential step toward reducing implicit bias and dispelling engendered perceptions regarding gender and technical proficiency, the commentators stated. “To definitively dispel archaic, gender-based notions about performance in clinical or surgical settings, efforts must go beyond peer-reviewed research,” Dr. Fader said in an interview. “Medical institutions and leaders of clinical departments must make concerted efforts to recruit, mentor, support, and promote women and persons of all genders in medicine – as well as confront any discriminatory perceptions and experiences concerning sex, race and ethnicity, sexual orientation, or economic class.”
This study was supported by the French Ministry of Health under its Clinical Research Hospital Program. Dr. Sentilhes reported financial relationships with Dilafor, Bayer, GlaxoSmithKline, Sigvaris, and Ferring Pharmaceuticals. The editorial commentators disclosed no funding for their commentary or conflicts of interest.
Surgeon gender was not associated with maternal morbidity or severe blood loss after cesarean delivery, a large prospective cohort study from France reports. The results have important implications for the promotion of gender equality among surgeons, obstetricians in particular, wrote a team led by Hanane Bouchghoul, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital. The report is in JAMA Surgery.
“Our findings are significant in that they add substantially to the string of studies contradicting the age-old dogma that men are better surgeons than women,” the authors wrote. Previous research has suggested slightly better outcomes with female surgeons or higher complication rates with male surgeons.
The results support those of a recent Canadian retrospective analysis suggesting that patients treated by male or female surgeons for various elective indications experience similar surgical outcomes but with a slight, statistically significant decrease in 30-day mortality when treated by female surgeons.
“Policy makers need to combat prejudice against women in surgical careers, particularly in obstetrics and gynecology, so that women no longer experience conscious or unconscious barriers or difficulties in their professional choices, training, and relationships with colleagues or patients,” study corresponding author Loïc Sentilhes, MD, PhD, of Bordeaux University Hospital, said in an interview.
Facing such barriers, women may doubt their ability to be surgeons, their legitimacy as surgeons, and may not consider this type of career, he continued. “Moreover a teacher may not be as involved in teaching young female surgeons as young male surgeons, or the doctor-patient relationship may be more complicated in the event of complications if the patient thinks that a female surgeon has less competence than a male surgeon.”
The analysis drew on data from the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery 2 trial, a multicenter, randomized, placebo-controlled study conducted from March 2018 through January 2020 in mothers from 27 French maternity hospitals.
Eligible participants had a cesarean delivery before or during labor at or after 34 weeks’ gestation. The primary endpoint was the incidence of a composite maternal morbidity variable, and the secondary endpoint was the incidence of postpartum hemorrhage, defined by a calculated estimated blood loss exceeding 1,000 mL or transfusion by day 2.
Among the 4,244 women included, male surgeons performed 943 cesarean deliveries (22.2%) and female surgeons performed 3,301 (77.8%). The percentage who were attending obstetricians was higher for men at 441 of 929 (47.5%) than women at 687 of 3,239 (21.2%).
The observed risk of maternal morbidity did not differ between male and female surgeons: 119 of 837 (14.2%) vs. 476 of 2,928 (16.3%), for an adjusted risk ratio (aRR) of 0.92 (95% confidence interval [CI], 0.77-1.13). Interaction between surgeon gender and level of experience with the risk of maternal morbidity was not statistically significant; nor did the groups differ specifically by risk for postpartum hemorrhage: aRR, 0.98 (95% CI, 0.85-1.13).
Despite the longstanding stereotype that men perform surgery better than women, and the traditional preponderance of male surgeons, the authors noted, postoperative morbidity and mortality may be lower after various surgeries performed by women.
The TRAAP2 trial
In an accompanying editorial, Amanda Fader, MD, of the department of obstetrics and gynecology at Johns Hopkins School of Medicine in Baltimore, and colleagues caution that the French study’s methodology may not fully account for the complex intersection of surgeon volume, experience, gender, clinical decision-making skills, and patient-level and clinical factors affecting outcomes.
That said, appraising surgical outcomes based on gender may be an essential step toward reducing implicit bias and dispelling engendered perceptions regarding gender and technical proficiency, the commentators stated. “To definitively dispel archaic, gender-based notions about performance in clinical or surgical settings, efforts must go beyond peer-reviewed research,” Dr. Fader said in an interview. “Medical institutions and leaders of clinical departments must make concerted efforts to recruit, mentor, support, and promote women and persons of all genders in medicine – as well as confront any discriminatory perceptions and experiences concerning sex, race and ethnicity, sexual orientation, or economic class.”
This study was supported by the French Ministry of Health under its Clinical Research Hospital Program. Dr. Sentilhes reported financial relationships with Dilafor, Bayer, GlaxoSmithKline, Sigvaris, and Ferring Pharmaceuticals. The editorial commentators disclosed no funding for their commentary or conflicts of interest.
FROM JAMA SURGERY
Update on secondary cytoreduction in recurrent ovarian cancer
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.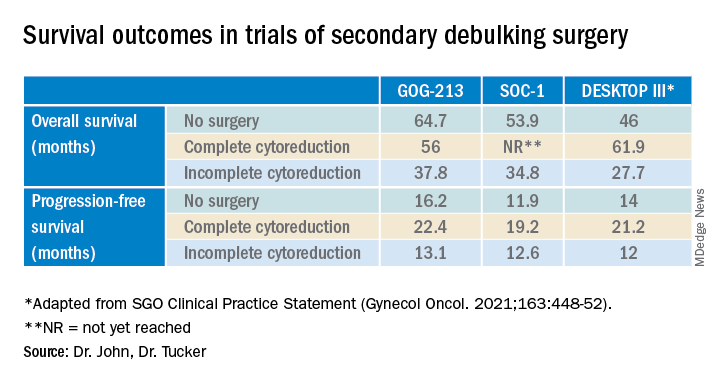
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Screen all patients for cannabis use before surgery: Guideline
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
All patients who undergo procedures that require regional or general anesthesia should be asked if, how often, and in what forms they use the drug, according to recommendations from the American Society of Regional Anesthesia and Pain Medicine.
One reason: Patients who regularly use cannabis may experience worse pain and nausea after surgery and may require more opioid analgesia, the group said.
The society’s recommendations – published in Regional Anesthesia and Pain Medicine – are the first guidelines in the United States to cover cannabis use as it relates to surgery, the group said.
Possible interactions
Use of cannabis has increased in recent years, and researchers have been concerned that the drug may interact with anesthesia and complicate pain management. Few studies have evaluated interactions between cannabis and anesthetic agents, however, according to the authors of the new guidelines.
“With the rising prevalence of both medical and recreational cannabis use in the general population, anesthesiologists, surgeons, and perioperative physicians must have an understanding of the effects of cannabis on physiology in order to provide safe perioperative care,” the guideline said.
“Before surgery, anesthesiologists should ask patients if they use cannabis – whether medicinally or recreationally – and be prepared to possibly change the anesthesia plan or delay the procedure in certain situations,” Samer Narouze, MD, PhD, ASRA president and senior author of the guidelines, said in a news release about the recommendations.
Although some patients may use cannabis to relieve pain, research shows that “regular users may have more pain and nausea after surgery, not less, and may need more medications, including opioids, to manage the discomfort,” said Dr. Narouze, chairman of the Center for Pain Medicine at Western Reserve Hospital in Cuyahoga Falls, Ohio.
Risks for vomiting, heart attack
The new recommendations were created by a committee of 13 experts, including anesthesiologists, chronic pain physicians, and a patient advocate. Shalini Shah, MD, vice chair of anesthesiology at the University of California, Irvine, was lead author of the document.
Four of 21 recommendations were classified as grade A, meaning that following them would be expected to provide substantial benefits. Those recommendations are to screen all patients before surgery; postpone elective surgery for patients who have altered mental status or impaired decision-making capacity at the time of surgery; counsel frequent, heavy users about the potential for cannabis use to impair postoperative pain control; and counsel pregnant patients about the risks of cannabis use to unborn children.
The authors cited studies to support their recommendations, including one showing that long-term cannabis use was associated with a 20% increase in the incidence of postoperative nausea and vomiting, a leading complaint of surgery patients. Other research has shown that cannabis use is linked to more pain and use of opioids after surgery.
Other recommendations include delaying elective surgery for at least 2 hours after a patient has smoked cannabis, owing to an increased risk for heart attack, and considering adjustment of ventilation settings during surgery for regular smokers of cannabis. Research has shown that smoking cannabis may be a rare trigger for myocardial infarction and is associated with airway inflammation and self-reported respiratory symptoms.
Nevertheless, doctors should not conduct universal toxicology screening, given a lack of evidence supporting this practice, the guideline stated.
The authors did not have enough information to make recommendations about reducing cannabis use before surgery or adjusting opioid prescriptions after surgery for patients who use cannabis, they said.
Kenneth Finn, MD, president of the American Board of Pain Medicine, welcomed the publication of the new guidelines. Dr. Finn, who practices at Springs Rehabilitation in Colorado Springs, has edited a textbook about cannabis in medicine and founded the International Academy on the Science and Impact of Cannabis.
“The vast majority of medical providers really have no idea about cannabis and what its impacts are on the human body,” Dr. Finn said.
For one, it can interact with numerous other drugs, including warfarin.
Guideline coauthor Eugene R. Viscusi, MD, professor of anesthesiology at the Sidney Kimmel Medical College, Philadelphia, emphasized that, while cannabis may be perceived as “natural,” it should not be considered differently from manufactured drugs.
Cannabis and cannabinoids represent “a class of very potent and pharmacologically active compounds,” Dr. Viscusi said in an interview. While researchers continue to assess possible medically beneficial effects of cannabis compounds, clinicians also need to be aware of the risks.
“The literature continues to emerge, and while we are always hopeful for good news, as physicians, we need to be very well versed on potential risks, especially in a high-risk situation like surgery,” he said.
Dr. Shah has consulted for companies that develop medical devices and drugs. Dr. Finn is the editor of the textbook, “Cannabis in Medicine: An Evidence-Based Approach” (Springer: New York, 2020), for which he receives royalties.
A version of this article first appeared on Medscape.com.
FROM REGIONAL ANETHESIA AND MEDICINE
Findings question value of pessary for pelvic organ prolapse
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA
Docs used permanent, not temporary stitches; lawsuits result
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
Poor evidence for vaginal laser therapy
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
FROM NAMS 2022
Models stratify hysterectomy risk with benign conditions
New models can help predict whether women having a hysterectomy for benign conditions are likely to have major complications, according to researchers.
The models, which use routinely collected data, are meant to aid surgeons in counseling women before surgery and help guide shared decision-making. The tools may lead to referrals for centers with greater surgical experience or may result in seeking nonsurgical treatment options, the researchers indicate.
The tools are not applicable to patients having hysterectomy for malignant disease.
Findings of the study, led by Krupa Madhvani, MD, of Barts and the London School of Medicine and Dentistry in London, are published online in the Canadian Medical Association Journal.
Calculators complement surgeons’ intuition
“Our aim was to generate prediction models that can be used in conjunction with a surgeon’s intuition to enhance preoperative patient counseling and match the advances made in the technical aspects of surgery,” the authors write.
“Internal–external cross-validation and external validation showed moderate discrimination,” they note.
The study included 68,599 patients who had laparoscopic hysterectomies and 125,971 patients who had an abdominal hysterectomy, all English National Health System patients between 2011 and 2018.
Among their findings were that major complications occurred in 4.4% of laparoscopic and 4.9% of abdominal hysterectomies. Major complications in this study included ureteric, gastrointestinal, and vascular injury and wound complications.
Adhesions biggest predictors of complications
Adhesions were most predictive of complications – with double the odds – in both models (laparoscopic: odds ratio, 1.92; 95% confidence interval, 1.73-2.13; abdominal: OR, 2.46; 95% CI, 2.27-2.66). That finding was consistent with previous literature.
“Adhesions should be suspected if there is a previous history of laparotomy, cesarean section, pelvic infection, or endometriosis, and can be reliably diagnosed preoperatively using ultrasonography,” the authors write. “As the global rate of cesarean sections continues to rise, this will undoubtedly remain a key determinant of major complications.”
Other factors that best predicted complications included adenomyosis in the laparoscopic model, and Asian ethnicity and diabetes in the abdominal model. Diabetes was not a predictive factor for complications in laparoscopic hysterectomy as it was in a previous study.
Obesity was not a significant predictor of major complications for either form of hysterectomy.
Factors protective against major complications included younger age and diagnosed menstrual disorders or benign adnexal mass (both models) and diagnosis of fibroids in the abdominal model.
Models miss surgeon experience
Jon Ivar Einarsson MD, PhD, MPH, founder of the division of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston, said it’s good to have these models to estimate risk as “there’s possibly a tendency to underestimate the risk by the surgeon.”
However, he told this publication that, though these models are based on a very large data set, the models are missing some key variables – often a problem with database studies – that are more indicative of complications. The most important factor missing, he said, is surgeon experience.
“We’ve shown in our publications that there’s a correlation between that and the risk of complications,“ Dr. Einarsson said.
Among other variables missing, he noted, are some that the authors list when acknowledging the limitations: severity of endometriosis and severity of adhesions.
He said his team wouldn’t use such models because they rely on their own data for gauging risk. He encourages other surgeons to track their own data and outcomes as well.
“I think the external validity here is nonexistent because we’re dealing with a different patient population in a different country with different surgeons [who] have various degrees of expertise,” Dr. Einarsson said.
“But if surgeons have not collected their own data, then this could be useful,” he said.
Links to online calculators
The online calculator can be found at www.evidencio.com (laparoscopic, www.evidencio.com/models/show/2551; abdominal, www.evidencio.com/models/show/2552).
The large, national multi-institutional database helps with generalizability of findings, the authors write. Additionally, patients had a unique identifier number so if patients were admitted to a different hospital after surgery, they were not lost to follow-up.
Limitations, in addition to those mentioned, include gaps in detailed clinical information, such as exact body mass index, and location, type, and size of leiomyoma, the authors write.
“Further research should focus on improving the discriminatory ability of these tools by including factors other than patient characteristics, including surgeon volume, as this has been shown to reduce complications,” they write.
Dr. Madhvani has received article-processing fees from Elly Charity (East London International Women’s Health Charity). No other competing interests were declared. Dr. Einarsson reports no relevant financial relationships. The acquisition of the data was funded by the British Society for Gynaecological Endoscopy. They were not involved in the study design, analysis, interpretation of data, the writing of the report, or the decision to submit the article for publication. Coauthor Khalid Khan, MD is a distinguished investigator funded by the Beatriz Galindo Program grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Government of Spain.
New models can help predict whether women having a hysterectomy for benign conditions are likely to have major complications, according to researchers.
The models, which use routinely collected data, are meant to aid surgeons in counseling women before surgery and help guide shared decision-making. The tools may lead to referrals for centers with greater surgical experience or may result in seeking nonsurgical treatment options, the researchers indicate.
The tools are not applicable to patients having hysterectomy for malignant disease.
Findings of the study, led by Krupa Madhvani, MD, of Barts and the London School of Medicine and Dentistry in London, are published online in the Canadian Medical Association Journal.
Calculators complement surgeons’ intuition
“Our aim was to generate prediction models that can be used in conjunction with a surgeon’s intuition to enhance preoperative patient counseling and match the advances made in the technical aspects of surgery,” the authors write.
“Internal–external cross-validation and external validation showed moderate discrimination,” they note.
The study included 68,599 patients who had laparoscopic hysterectomies and 125,971 patients who had an abdominal hysterectomy, all English National Health System patients between 2011 and 2018.
Among their findings were that major complications occurred in 4.4% of laparoscopic and 4.9% of abdominal hysterectomies. Major complications in this study included ureteric, gastrointestinal, and vascular injury and wound complications.
Adhesions biggest predictors of complications
Adhesions were most predictive of complications – with double the odds – in both models (laparoscopic: odds ratio, 1.92; 95% confidence interval, 1.73-2.13; abdominal: OR, 2.46; 95% CI, 2.27-2.66). That finding was consistent with previous literature.
“Adhesions should be suspected if there is a previous history of laparotomy, cesarean section, pelvic infection, or endometriosis, and can be reliably diagnosed preoperatively using ultrasonography,” the authors write. “As the global rate of cesarean sections continues to rise, this will undoubtedly remain a key determinant of major complications.”
Other factors that best predicted complications included adenomyosis in the laparoscopic model, and Asian ethnicity and diabetes in the abdominal model. Diabetes was not a predictive factor for complications in laparoscopic hysterectomy as it was in a previous study.
Obesity was not a significant predictor of major complications for either form of hysterectomy.
Factors protective against major complications included younger age and diagnosed menstrual disorders or benign adnexal mass (both models) and diagnosis of fibroids in the abdominal model.
Models miss surgeon experience
Jon Ivar Einarsson MD, PhD, MPH, founder of the division of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston, said it’s good to have these models to estimate risk as “there’s possibly a tendency to underestimate the risk by the surgeon.”
However, he told this publication that, though these models are based on a very large data set, the models are missing some key variables – often a problem with database studies – that are more indicative of complications. The most important factor missing, he said, is surgeon experience.
“We’ve shown in our publications that there’s a correlation between that and the risk of complications,“ Dr. Einarsson said.
Among other variables missing, he noted, are some that the authors list when acknowledging the limitations: severity of endometriosis and severity of adhesions.
He said his team wouldn’t use such models because they rely on their own data for gauging risk. He encourages other surgeons to track their own data and outcomes as well.
“I think the external validity here is nonexistent because we’re dealing with a different patient population in a different country with different surgeons [who] have various degrees of expertise,” Dr. Einarsson said.
“But if surgeons have not collected their own data, then this could be useful,” he said.
Links to online calculators
The online calculator can be found at www.evidencio.com (laparoscopic, www.evidencio.com/models/show/2551; abdominal, www.evidencio.com/models/show/2552).
The large, national multi-institutional database helps with generalizability of findings, the authors write. Additionally, patients had a unique identifier number so if patients were admitted to a different hospital after surgery, they were not lost to follow-up.
Limitations, in addition to those mentioned, include gaps in detailed clinical information, such as exact body mass index, and location, type, and size of leiomyoma, the authors write.
“Further research should focus on improving the discriminatory ability of these tools by including factors other than patient characteristics, including surgeon volume, as this has been shown to reduce complications,” they write.
Dr. Madhvani has received article-processing fees from Elly Charity (East London International Women’s Health Charity). No other competing interests were declared. Dr. Einarsson reports no relevant financial relationships. The acquisition of the data was funded by the British Society for Gynaecological Endoscopy. They were not involved in the study design, analysis, interpretation of data, the writing of the report, or the decision to submit the article for publication. Coauthor Khalid Khan, MD is a distinguished investigator funded by the Beatriz Galindo Program grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Government of Spain.
New models can help predict whether women having a hysterectomy for benign conditions are likely to have major complications, according to researchers.
The models, which use routinely collected data, are meant to aid surgeons in counseling women before surgery and help guide shared decision-making. The tools may lead to referrals for centers with greater surgical experience or may result in seeking nonsurgical treatment options, the researchers indicate.
The tools are not applicable to patients having hysterectomy for malignant disease.
Findings of the study, led by Krupa Madhvani, MD, of Barts and the London School of Medicine and Dentistry in London, are published online in the Canadian Medical Association Journal.
Calculators complement surgeons’ intuition
“Our aim was to generate prediction models that can be used in conjunction with a surgeon’s intuition to enhance preoperative patient counseling and match the advances made in the technical aspects of surgery,” the authors write.
“Internal–external cross-validation and external validation showed moderate discrimination,” they note.
The study included 68,599 patients who had laparoscopic hysterectomies and 125,971 patients who had an abdominal hysterectomy, all English National Health System patients between 2011 and 2018.
Among their findings were that major complications occurred in 4.4% of laparoscopic and 4.9% of abdominal hysterectomies. Major complications in this study included ureteric, gastrointestinal, and vascular injury and wound complications.
Adhesions biggest predictors of complications
Adhesions were most predictive of complications – with double the odds – in both models (laparoscopic: odds ratio, 1.92; 95% confidence interval, 1.73-2.13; abdominal: OR, 2.46; 95% CI, 2.27-2.66). That finding was consistent with previous literature.
“Adhesions should be suspected if there is a previous history of laparotomy, cesarean section, pelvic infection, or endometriosis, and can be reliably diagnosed preoperatively using ultrasonography,” the authors write. “As the global rate of cesarean sections continues to rise, this will undoubtedly remain a key determinant of major complications.”
Other factors that best predicted complications included adenomyosis in the laparoscopic model, and Asian ethnicity and diabetes in the abdominal model. Diabetes was not a predictive factor for complications in laparoscopic hysterectomy as it was in a previous study.
Obesity was not a significant predictor of major complications for either form of hysterectomy.
Factors protective against major complications included younger age and diagnosed menstrual disorders or benign adnexal mass (both models) and diagnosis of fibroids in the abdominal model.
Models miss surgeon experience
Jon Ivar Einarsson MD, PhD, MPH, founder of the division of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston, said it’s good to have these models to estimate risk as “there’s possibly a tendency to underestimate the risk by the surgeon.”
However, he told this publication that, though these models are based on a very large data set, the models are missing some key variables – often a problem with database studies – that are more indicative of complications. The most important factor missing, he said, is surgeon experience.
“We’ve shown in our publications that there’s a correlation between that and the risk of complications,“ Dr. Einarsson said.
Among other variables missing, he noted, are some that the authors list when acknowledging the limitations: severity of endometriosis and severity of adhesions.
He said his team wouldn’t use such models because they rely on their own data for gauging risk. He encourages other surgeons to track their own data and outcomes as well.
“I think the external validity here is nonexistent because we’re dealing with a different patient population in a different country with different surgeons [who] have various degrees of expertise,” Dr. Einarsson said.
“But if surgeons have not collected their own data, then this could be useful,” he said.
Links to online calculators
The online calculator can be found at www.evidencio.com (laparoscopic, www.evidencio.com/models/show/2551; abdominal, www.evidencio.com/models/show/2552).
The large, national multi-institutional database helps with generalizability of findings, the authors write. Additionally, patients had a unique identifier number so if patients were admitted to a different hospital after surgery, they were not lost to follow-up.
Limitations, in addition to those mentioned, include gaps in detailed clinical information, such as exact body mass index, and location, type, and size of leiomyoma, the authors write.
“Further research should focus on improving the discriminatory ability of these tools by including factors other than patient characteristics, including surgeon volume, as this has been shown to reduce complications,” they write.
Dr. Madhvani has received article-processing fees from Elly Charity (East London International Women’s Health Charity). No other competing interests were declared. Dr. Einarsson reports no relevant financial relationships. The acquisition of the data was funded by the British Society for Gynaecological Endoscopy. They were not involved in the study design, analysis, interpretation of data, the writing of the report, or the decision to submit the article for publication. Coauthor Khalid Khan, MD is a distinguished investigator funded by the Beatriz Galindo Program grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Government of Spain.
FROM CANADIAN MEDICAL ASSOCIATION JOURNAL
Postpartum sexual enjoyment: Does mode of delivery matter?
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Increased risk of dyspareunia following cesarean section
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.