User login
News & Perspectives from Ob.Gyn. News
NEWS FROM THE FDA/CDC
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
https://www.mdedge.com/obgyn/news-fda/cdc
FEATURE
U.S. mammogram update sparks concern, reignites debates
A recent update to the U.S. recommendations for breast cancer screening is raising concerns about the costs associated with potential follow-up tests, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screen-ing mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
https://www.mdedge.com/obgyn/article/264198/breast-cancer/us-mammogram-update-sparks-concern-reignites-debates
Continue to: GENDER-AFFIRMING GYNECOLOGY...
GENDER-AFFIRMING GYNECOLOGY
Updates on pregnancy outcomes in transgender men
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologistsand midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately, compared with the surgical, hormonal, and mental health aspects of gender-affirming care, fertility and pregnancy in transgender men is still a relatively new and under-researched topic.
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
REPRODUCTIVE ROUNDS
Affordable IVF—Are we there yet?
The price for an in vitro fertilization (IVF) cycle continues to increase annually by many clinics, particularly because of “add-ons” of dubious value.
The initial application of IVF was for tubal factor infertility. Over the decades since 1981, the year of the first successful live birth in the United States, indications for IVF have dramatically expanded—ovulation dysfunction, unexplained infertility, male factor, advanced stage endometriosis, unexplained infertility, embryo testing to avoid an inherited genetic disease from the intended parents carrying the same mutation, and family balancing for gender, along with fertility preservation, including before potentially gonadotoxic treatment and “elective” planned oocyte cryopreservation.
The cost of IVF remains a significant, and possibly leading, stumbling block for women, couples, and men who lack insurance coverage. From RESOLVE.org, the National Infertility Association: “As of June 2022, 20 states have passed fertility insurance coverage laws, 14 of those laws include IVF coverage, and 12 states have fertility preservation laws for iatrogenic (medically induced) infertility.” Consequently, “affordable IVF” is paramount to providing equal access for patients.
https://www.mdedge.com/obgyn/reproductive-rounds
CONFERENCE COVERAGE
‘Artificial pancreas’ for all type 1 diabetes pregnancies?
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
The percentage of time spent in the pregnancy-specific target blood glucose range of 63-140 mg/dL (3.5-7.8 mmol/L) from 16 weeks’ gestation to delivery was significantly higher in women in the AID group.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
NEWS FROM THE FDA/CDC
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
https://www.mdedge.com/obgyn/news-fda/cdc
FEATURE
U.S. mammogram update sparks concern, reignites debates
A recent update to the U.S. recommendations for breast cancer screening is raising concerns about the costs associated with potential follow-up tests, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screen-ing mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
https://www.mdedge.com/obgyn/article/264198/breast-cancer/us-mammogram-update-sparks-concern-reignites-debates
Continue to: GENDER-AFFIRMING GYNECOLOGY...
GENDER-AFFIRMING GYNECOLOGY
Updates on pregnancy outcomes in transgender men
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologistsand midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately, compared with the surgical, hormonal, and mental health aspects of gender-affirming care, fertility and pregnancy in transgender men is still a relatively new and under-researched topic.
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
REPRODUCTIVE ROUNDS
Affordable IVF—Are we there yet?
The price for an in vitro fertilization (IVF) cycle continues to increase annually by many clinics, particularly because of “add-ons” of dubious value.
The initial application of IVF was for tubal factor infertility. Over the decades since 1981, the year of the first successful live birth in the United States, indications for IVF have dramatically expanded—ovulation dysfunction, unexplained infertility, male factor, advanced stage endometriosis, unexplained infertility, embryo testing to avoid an inherited genetic disease from the intended parents carrying the same mutation, and family balancing for gender, along with fertility preservation, including before potentially gonadotoxic treatment and “elective” planned oocyte cryopreservation.
The cost of IVF remains a significant, and possibly leading, stumbling block for women, couples, and men who lack insurance coverage. From RESOLVE.org, the National Infertility Association: “As of June 2022, 20 states have passed fertility insurance coverage laws, 14 of those laws include IVF coverage, and 12 states have fertility preservation laws for iatrogenic (medically induced) infertility.” Consequently, “affordable IVF” is paramount to providing equal access for patients.
https://www.mdedge.com/obgyn/reproductive-rounds
CONFERENCE COVERAGE
‘Artificial pancreas’ for all type 1 diabetes pregnancies?
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
The percentage of time spent in the pregnancy-specific target blood glucose range of 63-140 mg/dL (3.5-7.8 mmol/L) from 16 weeks’ gestation to delivery was significantly higher in women in the AID group.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
NEWS FROM THE FDA/CDC
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
https://www.mdedge.com/obgyn/news-fda/cdc
FEATURE
U.S. mammogram update sparks concern, reignites debates
A recent update to the U.S. recommendations for breast cancer screening is raising concerns about the costs associated with potential follow-up tests, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screen-ing mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
https://www.mdedge.com/obgyn/article/264198/breast-cancer/us-mammogram-update-sparks-concern-reignites-debates
Continue to: GENDER-AFFIRMING GYNECOLOGY...
GENDER-AFFIRMING GYNECOLOGY
Updates on pregnancy outcomes in transgender men
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologistsand midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately, compared with the surgical, hormonal, and mental health aspects of gender-affirming care, fertility and pregnancy in transgender men is still a relatively new and under-researched topic.
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
REPRODUCTIVE ROUNDS
Affordable IVF—Are we there yet?
The price for an in vitro fertilization (IVF) cycle continues to increase annually by many clinics, particularly because of “add-ons” of dubious value.
The initial application of IVF was for tubal factor infertility. Over the decades since 1981, the year of the first successful live birth in the United States, indications for IVF have dramatically expanded—ovulation dysfunction, unexplained infertility, male factor, advanced stage endometriosis, unexplained infertility, embryo testing to avoid an inherited genetic disease from the intended parents carrying the same mutation, and family balancing for gender, along with fertility preservation, including before potentially gonadotoxic treatment and “elective” planned oocyte cryopreservation.
The cost of IVF remains a significant, and possibly leading, stumbling block for women, couples, and men who lack insurance coverage. From RESOLVE.org, the National Infertility Association: “As of June 2022, 20 states have passed fertility insurance coverage laws, 14 of those laws include IVF coverage, and 12 states have fertility preservation laws for iatrogenic (medically induced) infertility.” Consequently, “affordable IVF” is paramount to providing equal access for patients.
https://www.mdedge.com/obgyn/reproductive-rounds
CONFERENCE COVERAGE
‘Artificial pancreas’ for all type 1 diabetes pregnancies?
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
The percentage of time spent in the pregnancy-specific target blood glucose range of 63-140 mg/dL (3.5-7.8 mmol/L) from 16 weeks’ gestation to delivery was significantly higher in women in the AID group.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
Don’t skip contraception talk for women with complex health conditions
.
In an installment of the American College of Physicians’ In the Clinic series, Rachel Cannon, MD, Kelly Treder, MD, and Elisabeth J. Woodhams, MD, all of Boston Medical Center, presented an article on the complex topic of contraception for patients with chronic illness.
“Many patients with chronic illness or complex medical issues interact with a primary care provider on a frequent basis, which provides a great access point for contraceptive counseling with a provider they trust and know,” said Dr. Cannon and Dr. Treder in a joint interview. “We wanted to create a ‘go to’ resource for primary care physicians to review contraceptive options and counseling best practices for all of their patients. Contraceptive care is part of overall health care and should be included in the primary care encounter.”
The authors discussed the types of contraception, as well as risks and benefits, and offered guidance for choosing a contraceptive method for medically complex patients.
“In recent years, there has been a shift in contraceptive counseling toward shared decision-making, a counseling strategy that honors the patient as the expert in their body and their life experiences and emphasizes their autonomy and values,” the authors said. “For providers, this translates to understanding that contraceptive efficacy is not the only important characteristic to patients, and that many other important factors contribute to an individual’s decision to use a particular method or not use birth control at all,” they said.
Start the conversation
Start by assessing a patient’s interest in and readiness for pregnancy, if applicable, the authors said. One example of a screen, the PATH questionnaire (Parent/Pregnancy Attitudes, Timing, and How important), is designed for patients in any demographic, and includes questions about the timing and desire for pregnancy and feelings about birth control, as well as options for patients to express uncertainty or ambivalence about pregnancy and contraception.
Some patients may derive benefits from hormonal contraceptives beyond pregnancy prevention, the authors wrote. Combined hormonal contraceptives (CHCs) may improve menorrhagia, and data suggest that CHC use also may reduce risk for some cancer types, including endometrial and ovarian cancers, they said.
Overall, contraceptive counseling should include discussions of safety, efficacy, and the patient’s lived experience.
Clinical considerations and contraindications
Medically complex patients who desire contraception may consider hormonal or nonhormonal methods based on their preferences and medical conditions, but clinicians need to consider comorbidities and contraindications, the authors wrote.
When a woman of childbearing age with any complex medical issue starts a new medication or receives a new diagnosis, contraception and pregnancy planning should be part of the discussion, the authors said. Safe and successful pregnancies are possible for women with complex medical issues when underlying health concerns are identified and addressed in advance, they added. Alternatively, for patients seeking to avoid pregnancy permanently, options for sterilization can be part of an informed discussion.
The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use offers clinicians detailed information about the risks of both contraceptives and pregnancy for patients with various medical conditions, according to the authors.
The CDC document lists medical conditions associated with an increased risk for adverse health events if the individual becomes pregnant. These conditions include breast cancer, complicated valvular heart disease, cystic fibrosis, diabetes, endometrial or ovarian cancer, epilepsy, hypertension, bariatric surgery within 2 years of the pregnancy, HIV, ischemic heart disease, severe cirrhosis, stroke, lupus, solid organ transplant within 2 years of the pregnancy, and tuberculosis. Women with these and other conditions associated with increased risk of adverse events if pregnancy occurs should be advised of the high failure rate of barrier and behavior-based contraceptive methods, and informed about options for long-acting contraceptives, according to the CDC.
Risks, benefits, and balance
“It is important to remember that the alternative to contraception for many patients is pregnancy – for many patients with complex medical conditions, pregnancy is far more dangerous than any contraceptive method,” Dr. Cannon and Dr. Treder said in an interview. “This is important to consider when thinking about relative contraindications to a certain method or when thinking about ‘less effective’ contraception methods. The most effective method is a method the patient will actually continue to use,” they said.
The recent approval of the over-the-counter minipill is “a huge win for reproductive health care,” said Dr. Cannon and Dr. Treder. The minipill has very few contraindications, and it is the most effective over-the-counter contraceptive now available, they said.
“An over-the-counter contraceptive pill can increase access to contraception without having to see a physician in the clinic, freeing patients from many of the challenges of navigating the health care system,” the authors added.
As for additional research, the establishment of a long-term safety record may help support other OTC contraceptive methods in the future, the authors said.
Contraceptive counseling is everyone’s specialty
In an accompanying editorial, Amy A. Sarma, MD, a cardiologist at Massachusetts General Hospital, Boston, shared an example of the importance of contraceptive discussions with medically complex patients outside of an ob.gyn. setting. A young woman with a family history of myocardial infarction had neglected her own primary care until an MI of her own sent her to the hospital. While hospitalized, the patient was diagnosed with diabetes, hypertension, and hyperlipidemia.
“Her cardiology care team made every effort to optimize her cardiac care, but no one considered that she was also a woman of childbearing potential despite the teratogenic potential of several of her prescribed medications,” Dr. Sarma wrote. When the patient visited Dr. Sarma to discuss prevention of future MIs, Dr. Sarma took the opportunity to discuss the cardiovascular risks of pregnancy and the risks for this patient not only because of her recent MI, but also because of her chronic health conditions.
As it happened, the woman did not want a high-risk pregnancy and was interested in contraceptive methods. Dr. Sarma pointed out that, had the woman been engaged in routine primary care, these issues would have arisen in that setting, but like many younger women with cardiovascular disease, she did not make her own primary care a priority, and had missed out on other opportunities to discuss contraception. “Her MI opened a window of opportunity to help prevent an unintended and high-risk pregnancy,” Dr. Sarma noted.
Dr. Sarma’s patient anecdote illustrated the point of the In the Clinic review: that any clinician can discuss pregnancy and contraception with patients of childbearing age who have medical comorbidities that could affect a pregnancy. “All clinicians who care for patients of reproductive potential should become comfortable discussing pregnancy intent, preconception risk assessment, and contraceptive counseling,” Dr. Sarma said.
The research for this article was funded by the American College of Physicians. The review authors had no financial conflicts to disclose. Dr. Sarma had no financial conflicts to disclose.
.
In an installment of the American College of Physicians’ In the Clinic series, Rachel Cannon, MD, Kelly Treder, MD, and Elisabeth J. Woodhams, MD, all of Boston Medical Center, presented an article on the complex topic of contraception for patients with chronic illness.
“Many patients with chronic illness or complex medical issues interact with a primary care provider on a frequent basis, which provides a great access point for contraceptive counseling with a provider they trust and know,” said Dr. Cannon and Dr. Treder in a joint interview. “We wanted to create a ‘go to’ resource for primary care physicians to review contraceptive options and counseling best practices for all of their patients. Contraceptive care is part of overall health care and should be included in the primary care encounter.”
The authors discussed the types of contraception, as well as risks and benefits, and offered guidance for choosing a contraceptive method for medically complex patients.
“In recent years, there has been a shift in contraceptive counseling toward shared decision-making, a counseling strategy that honors the patient as the expert in their body and their life experiences and emphasizes their autonomy and values,” the authors said. “For providers, this translates to understanding that contraceptive efficacy is not the only important characteristic to patients, and that many other important factors contribute to an individual’s decision to use a particular method or not use birth control at all,” they said.
Start the conversation
Start by assessing a patient’s interest in and readiness for pregnancy, if applicable, the authors said. One example of a screen, the PATH questionnaire (Parent/Pregnancy Attitudes, Timing, and How important), is designed for patients in any demographic, and includes questions about the timing and desire for pregnancy and feelings about birth control, as well as options for patients to express uncertainty or ambivalence about pregnancy and contraception.
Some patients may derive benefits from hormonal contraceptives beyond pregnancy prevention, the authors wrote. Combined hormonal contraceptives (CHCs) may improve menorrhagia, and data suggest that CHC use also may reduce risk for some cancer types, including endometrial and ovarian cancers, they said.
Overall, contraceptive counseling should include discussions of safety, efficacy, and the patient’s lived experience.
Clinical considerations and contraindications
Medically complex patients who desire contraception may consider hormonal or nonhormonal methods based on their preferences and medical conditions, but clinicians need to consider comorbidities and contraindications, the authors wrote.
When a woman of childbearing age with any complex medical issue starts a new medication or receives a new diagnosis, contraception and pregnancy planning should be part of the discussion, the authors said. Safe and successful pregnancies are possible for women with complex medical issues when underlying health concerns are identified and addressed in advance, they added. Alternatively, for patients seeking to avoid pregnancy permanently, options for sterilization can be part of an informed discussion.
The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use offers clinicians detailed information about the risks of both contraceptives and pregnancy for patients with various medical conditions, according to the authors.
The CDC document lists medical conditions associated with an increased risk for adverse health events if the individual becomes pregnant. These conditions include breast cancer, complicated valvular heart disease, cystic fibrosis, diabetes, endometrial or ovarian cancer, epilepsy, hypertension, bariatric surgery within 2 years of the pregnancy, HIV, ischemic heart disease, severe cirrhosis, stroke, lupus, solid organ transplant within 2 years of the pregnancy, and tuberculosis. Women with these and other conditions associated with increased risk of adverse events if pregnancy occurs should be advised of the high failure rate of barrier and behavior-based contraceptive methods, and informed about options for long-acting contraceptives, according to the CDC.
Risks, benefits, and balance
“It is important to remember that the alternative to contraception for many patients is pregnancy – for many patients with complex medical conditions, pregnancy is far more dangerous than any contraceptive method,” Dr. Cannon and Dr. Treder said in an interview. “This is important to consider when thinking about relative contraindications to a certain method or when thinking about ‘less effective’ contraception methods. The most effective method is a method the patient will actually continue to use,” they said.
The recent approval of the over-the-counter minipill is “a huge win for reproductive health care,” said Dr. Cannon and Dr. Treder. The minipill has very few contraindications, and it is the most effective over-the-counter contraceptive now available, they said.
“An over-the-counter contraceptive pill can increase access to contraception without having to see a physician in the clinic, freeing patients from many of the challenges of navigating the health care system,” the authors added.
As for additional research, the establishment of a long-term safety record may help support other OTC contraceptive methods in the future, the authors said.
Contraceptive counseling is everyone’s specialty
In an accompanying editorial, Amy A. Sarma, MD, a cardiologist at Massachusetts General Hospital, Boston, shared an example of the importance of contraceptive discussions with medically complex patients outside of an ob.gyn. setting. A young woman with a family history of myocardial infarction had neglected her own primary care until an MI of her own sent her to the hospital. While hospitalized, the patient was diagnosed with diabetes, hypertension, and hyperlipidemia.
“Her cardiology care team made every effort to optimize her cardiac care, but no one considered that she was also a woman of childbearing potential despite the teratogenic potential of several of her prescribed medications,” Dr. Sarma wrote. When the patient visited Dr. Sarma to discuss prevention of future MIs, Dr. Sarma took the opportunity to discuss the cardiovascular risks of pregnancy and the risks for this patient not only because of her recent MI, but also because of her chronic health conditions.
As it happened, the woman did not want a high-risk pregnancy and was interested in contraceptive methods. Dr. Sarma pointed out that, had the woman been engaged in routine primary care, these issues would have arisen in that setting, but like many younger women with cardiovascular disease, she did not make her own primary care a priority, and had missed out on other opportunities to discuss contraception. “Her MI opened a window of opportunity to help prevent an unintended and high-risk pregnancy,” Dr. Sarma noted.
Dr. Sarma’s patient anecdote illustrated the point of the In the Clinic review: that any clinician can discuss pregnancy and contraception with patients of childbearing age who have medical comorbidities that could affect a pregnancy. “All clinicians who care for patients of reproductive potential should become comfortable discussing pregnancy intent, preconception risk assessment, and contraceptive counseling,” Dr. Sarma said.
The research for this article was funded by the American College of Physicians. The review authors had no financial conflicts to disclose. Dr. Sarma had no financial conflicts to disclose.
.
In an installment of the American College of Physicians’ In the Clinic series, Rachel Cannon, MD, Kelly Treder, MD, and Elisabeth J. Woodhams, MD, all of Boston Medical Center, presented an article on the complex topic of contraception for patients with chronic illness.
“Many patients with chronic illness or complex medical issues interact with a primary care provider on a frequent basis, which provides a great access point for contraceptive counseling with a provider they trust and know,” said Dr. Cannon and Dr. Treder in a joint interview. “We wanted to create a ‘go to’ resource for primary care physicians to review contraceptive options and counseling best practices for all of their patients. Contraceptive care is part of overall health care and should be included in the primary care encounter.”
The authors discussed the types of contraception, as well as risks and benefits, and offered guidance for choosing a contraceptive method for medically complex patients.
“In recent years, there has been a shift in contraceptive counseling toward shared decision-making, a counseling strategy that honors the patient as the expert in their body and their life experiences and emphasizes their autonomy and values,” the authors said. “For providers, this translates to understanding that contraceptive efficacy is not the only important characteristic to patients, and that many other important factors contribute to an individual’s decision to use a particular method or not use birth control at all,” they said.
Start the conversation
Start by assessing a patient’s interest in and readiness for pregnancy, if applicable, the authors said. One example of a screen, the PATH questionnaire (Parent/Pregnancy Attitudes, Timing, and How important), is designed for patients in any demographic, and includes questions about the timing and desire for pregnancy and feelings about birth control, as well as options for patients to express uncertainty or ambivalence about pregnancy and contraception.
Some patients may derive benefits from hormonal contraceptives beyond pregnancy prevention, the authors wrote. Combined hormonal contraceptives (CHCs) may improve menorrhagia, and data suggest that CHC use also may reduce risk for some cancer types, including endometrial and ovarian cancers, they said.
Overall, contraceptive counseling should include discussions of safety, efficacy, and the patient’s lived experience.
Clinical considerations and contraindications
Medically complex patients who desire contraception may consider hormonal or nonhormonal methods based on their preferences and medical conditions, but clinicians need to consider comorbidities and contraindications, the authors wrote.
When a woman of childbearing age with any complex medical issue starts a new medication or receives a new diagnosis, contraception and pregnancy planning should be part of the discussion, the authors said. Safe and successful pregnancies are possible for women with complex medical issues when underlying health concerns are identified and addressed in advance, they added. Alternatively, for patients seeking to avoid pregnancy permanently, options for sterilization can be part of an informed discussion.
The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use offers clinicians detailed information about the risks of both contraceptives and pregnancy for patients with various medical conditions, according to the authors.
The CDC document lists medical conditions associated with an increased risk for adverse health events if the individual becomes pregnant. These conditions include breast cancer, complicated valvular heart disease, cystic fibrosis, diabetes, endometrial or ovarian cancer, epilepsy, hypertension, bariatric surgery within 2 years of the pregnancy, HIV, ischemic heart disease, severe cirrhosis, stroke, lupus, solid organ transplant within 2 years of the pregnancy, and tuberculosis. Women with these and other conditions associated with increased risk of adverse events if pregnancy occurs should be advised of the high failure rate of barrier and behavior-based contraceptive methods, and informed about options for long-acting contraceptives, according to the CDC.
Risks, benefits, and balance
“It is important to remember that the alternative to contraception for many patients is pregnancy – for many patients with complex medical conditions, pregnancy is far more dangerous than any contraceptive method,” Dr. Cannon and Dr. Treder said in an interview. “This is important to consider when thinking about relative contraindications to a certain method or when thinking about ‘less effective’ contraception methods. The most effective method is a method the patient will actually continue to use,” they said.
The recent approval of the over-the-counter minipill is “a huge win for reproductive health care,” said Dr. Cannon and Dr. Treder. The minipill has very few contraindications, and it is the most effective over-the-counter contraceptive now available, they said.
“An over-the-counter contraceptive pill can increase access to contraception without having to see a physician in the clinic, freeing patients from many of the challenges of navigating the health care system,” the authors added.
As for additional research, the establishment of a long-term safety record may help support other OTC contraceptive methods in the future, the authors said.
Contraceptive counseling is everyone’s specialty
In an accompanying editorial, Amy A. Sarma, MD, a cardiologist at Massachusetts General Hospital, Boston, shared an example of the importance of contraceptive discussions with medically complex patients outside of an ob.gyn. setting. A young woman with a family history of myocardial infarction had neglected her own primary care until an MI of her own sent her to the hospital. While hospitalized, the patient was diagnosed with diabetes, hypertension, and hyperlipidemia.
“Her cardiology care team made every effort to optimize her cardiac care, but no one considered that she was also a woman of childbearing potential despite the teratogenic potential of several of her prescribed medications,” Dr. Sarma wrote. When the patient visited Dr. Sarma to discuss prevention of future MIs, Dr. Sarma took the opportunity to discuss the cardiovascular risks of pregnancy and the risks for this patient not only because of her recent MI, but also because of her chronic health conditions.
As it happened, the woman did not want a high-risk pregnancy and was interested in contraceptive methods. Dr. Sarma pointed out that, had the woman been engaged in routine primary care, these issues would have arisen in that setting, but like many younger women with cardiovascular disease, she did not make her own primary care a priority, and had missed out on other opportunities to discuss contraception. “Her MI opened a window of opportunity to help prevent an unintended and high-risk pregnancy,” Dr. Sarma noted.
Dr. Sarma’s patient anecdote illustrated the point of the In the Clinic review: that any clinician can discuss pregnancy and contraception with patients of childbearing age who have medical comorbidities that could affect a pregnancy. “All clinicians who care for patients of reproductive potential should become comfortable discussing pregnancy intent, preconception risk assessment, and contraceptive counseling,” Dr. Sarma said.
The research for this article was funded by the American College of Physicians. The review authors had no financial conflicts to disclose. Dr. Sarma had no financial conflicts to disclose.
FROM THE ANNALS OF INTERNAL MEDICINE
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
“It’s a huge win for patients who need better access to contraception,” said Dr. Gray, who is also a spokesperson for the American College of Obstetricians and Gynecologists.
Women who want hormonal birth control but live in areas without convenient access to a doctor, women who cannot easily take time off of work to see a doctor and get a prescription filled, and women without insurance are examples of people who will benefit, she said.
The Catholic Medical Association, in contrast, expressed “deep concern and disappointment” after an FDA advisory committee’s unanimous vote on May 11 recommending the drug be available over the counter. In a statement after the vote, the group cited “extensive medical studies demonstrating the risks and adverse effects of hormonal contraceptives,” adding that “the social impact of [full approval] would be dramatic.”
But doctors largely disagreed.
“It is definitely a huge win for reproductive autonomy. I’m glad that the FDA is prioritizing patient safety and well-being over politics,” said Catherine Cansino, MD, MPH, an ob.gyn. and clinical professor in the University of California Davis department of obstetrics and gynecology. She said the FDA approved the over-the-counter version because the medication is safe.
While opponents like the Catholic Medical Association cite safety concerns and believe doctors should screen all women before prescribing hormonal contraception, Dr. Gray disagreed. “There’s a lot of evidence that patients can figure out if a progestin-only pill is right for them and safe for them. Medical professionals don’t have to be the gatekeepers for contraception,” she said.
Pricing unknown
Whether insurance companies will pay for Opill now that it will be available without a prescription remains unknown. For some medications, paying a copay through insurance can be less expensive than buying at a retail price.
“Although pricing issues will be relevant, the FDA’s decision will enhance women’s access to hormonal birth control,” said Andrew M. Kaunitz, MD, a professor and associate chairman in the department of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville.
The drugmaker, Perrigo, based in Ireland, has not yet announced how much the pill will cost. The price tag could affect how widely available this form of birth control is. The drug has been shown to be as much as 93% effective for pregnancy prevention. Perrigo says it plans to make the pill available at low or no cost to some women.
Caveats to consider
There are some women for whom hormonal contraceptives have always carried greater risks. For example, women who have breast cancer or a history of breast cancer should not use hormonal contraceptives, the FDA said in a news release announcing the approval. Women with other types of cancer should check with their doctors first, the agency noted.
Women who smoke, who take some medications to lower blood pressure, or who have migraines should also take caution, Dr. Cansino said. “People with migraines may not be suitable for over-the-counter oral contraceptives. But a simple screening through a provider can identify whether you are truly eligible or not.”
Irregular bleeding, headaches, dizziness, nausea, increased appetite, belly pain, cramps, or bloating are the most common side effects of Opill, the FDA said.
The Opill is a progestin-only birth control pill. Similar pills have been available in the United Kingdiom for about 2 years, often referred to as “mini pills” because they contain a single hormone. In contrast, prescription birth control pills in the United States and elsewhere contain more than one hormone, estrogen and progestin, to prevent pregnancy.
Prescription pill packs for combination contraception often feature a week of placebo pills without an active ingredient. While skipping a placebo pill might not make a difference in pregnancy prevention, Opill is different. Every pill in the packet will contain medication, Gray said. “So it’s important to take the pill the same time every day for it to be most effective.”
Even though this may mean one less visit to your doctor, Dr. Kaunitz hopes women will stay up to date on their other medical checkups. “One of our challenges as providers of care to women will be to encourage them to continue to receive important services, including cancer screening and vaccinations, even while they can initiate and continue hormonal contraception without contact with a provider.”
Just the beginning?
The American Medical Association hopes this approval signals more to come.
“While we applaud this move, the AMA continues to urge the FDA and HHS to consider a variety of oral contraceptive options for over-the-counter use,” the association, which has more than 250,000 doctor members, said in a statement. “It is important patients have options when choosing which type of birth control works best for them,”
The American College of Obstetricians and Gynecologists said the FDA’s decision will help many women. “We are glad that more patients will now be empowered to choose when and where they obtain a safe method of contraception without having to wait for a medical appointment or for a prescription to be filled,” Verda J. Hicks, MD, the group’s president, and Christopher M. Zahn, MD, interim chief executive officer, said in a statement.
“Allowing individuals to access birth control at their local pharmacy or drug store will eliminate some barriers,” they said.
A version of this article first appeared on WebMD.com.
This article was updated 7/13/23.
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
“It’s a huge win for patients who need better access to contraception,” said Dr. Gray, who is also a spokesperson for the American College of Obstetricians and Gynecologists.
Women who want hormonal birth control but live in areas without convenient access to a doctor, women who cannot easily take time off of work to see a doctor and get a prescription filled, and women without insurance are examples of people who will benefit, she said.
The Catholic Medical Association, in contrast, expressed “deep concern and disappointment” after an FDA advisory committee’s unanimous vote on May 11 recommending the drug be available over the counter. In a statement after the vote, the group cited “extensive medical studies demonstrating the risks and adverse effects of hormonal contraceptives,” adding that “the social impact of [full approval] would be dramatic.”
But doctors largely disagreed.
“It is definitely a huge win for reproductive autonomy. I’m glad that the FDA is prioritizing patient safety and well-being over politics,” said Catherine Cansino, MD, MPH, an ob.gyn. and clinical professor in the University of California Davis department of obstetrics and gynecology. She said the FDA approved the over-the-counter version because the medication is safe.
While opponents like the Catholic Medical Association cite safety concerns and believe doctors should screen all women before prescribing hormonal contraception, Dr. Gray disagreed. “There’s a lot of evidence that patients can figure out if a progestin-only pill is right for them and safe for them. Medical professionals don’t have to be the gatekeepers for contraception,” she said.
Pricing unknown
Whether insurance companies will pay for Opill now that it will be available without a prescription remains unknown. For some medications, paying a copay through insurance can be less expensive than buying at a retail price.
“Although pricing issues will be relevant, the FDA’s decision will enhance women’s access to hormonal birth control,” said Andrew M. Kaunitz, MD, a professor and associate chairman in the department of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville.
The drugmaker, Perrigo, based in Ireland, has not yet announced how much the pill will cost. The price tag could affect how widely available this form of birth control is. The drug has been shown to be as much as 93% effective for pregnancy prevention. Perrigo says it plans to make the pill available at low or no cost to some women.
Caveats to consider
There are some women for whom hormonal contraceptives have always carried greater risks. For example, women who have breast cancer or a history of breast cancer should not use hormonal contraceptives, the FDA said in a news release announcing the approval. Women with other types of cancer should check with their doctors first, the agency noted.
Women who smoke, who take some medications to lower blood pressure, or who have migraines should also take caution, Dr. Cansino said. “People with migraines may not be suitable for over-the-counter oral contraceptives. But a simple screening through a provider can identify whether you are truly eligible or not.”
Irregular bleeding, headaches, dizziness, nausea, increased appetite, belly pain, cramps, or bloating are the most common side effects of Opill, the FDA said.
The Opill is a progestin-only birth control pill. Similar pills have been available in the United Kingdiom for about 2 years, often referred to as “mini pills” because they contain a single hormone. In contrast, prescription birth control pills in the United States and elsewhere contain more than one hormone, estrogen and progestin, to prevent pregnancy.
Prescription pill packs for combination contraception often feature a week of placebo pills without an active ingredient. While skipping a placebo pill might not make a difference in pregnancy prevention, Opill is different. Every pill in the packet will contain medication, Gray said. “So it’s important to take the pill the same time every day for it to be most effective.”
Even though this may mean one less visit to your doctor, Dr. Kaunitz hopes women will stay up to date on their other medical checkups. “One of our challenges as providers of care to women will be to encourage them to continue to receive important services, including cancer screening and vaccinations, even while they can initiate and continue hormonal contraception without contact with a provider.”
Just the beginning?
The American Medical Association hopes this approval signals more to come.
“While we applaud this move, the AMA continues to urge the FDA and HHS to consider a variety of oral contraceptive options for over-the-counter use,” the association, which has more than 250,000 doctor members, said in a statement. “It is important patients have options when choosing which type of birth control works best for them,”
The American College of Obstetricians and Gynecologists said the FDA’s decision will help many women. “We are glad that more patients will now be empowered to choose when and where they obtain a safe method of contraception without having to wait for a medical appointment or for a prescription to be filled,” Verda J. Hicks, MD, the group’s president, and Christopher M. Zahn, MD, interim chief executive officer, said in a statement.
“Allowing individuals to access birth control at their local pharmacy or drug store will eliminate some barriers,” they said.
A version of this article first appeared on WebMD.com.
This article was updated 7/13/23.
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
“It’s a huge win for patients who need better access to contraception,” said Dr. Gray, who is also a spokesperson for the American College of Obstetricians and Gynecologists.
Women who want hormonal birth control but live in areas without convenient access to a doctor, women who cannot easily take time off of work to see a doctor and get a prescription filled, and women without insurance are examples of people who will benefit, she said.
The Catholic Medical Association, in contrast, expressed “deep concern and disappointment” after an FDA advisory committee’s unanimous vote on May 11 recommending the drug be available over the counter. In a statement after the vote, the group cited “extensive medical studies demonstrating the risks and adverse effects of hormonal contraceptives,” adding that “the social impact of [full approval] would be dramatic.”
But doctors largely disagreed.
“It is definitely a huge win for reproductive autonomy. I’m glad that the FDA is prioritizing patient safety and well-being over politics,” said Catherine Cansino, MD, MPH, an ob.gyn. and clinical professor in the University of California Davis department of obstetrics and gynecology. She said the FDA approved the over-the-counter version because the medication is safe.
While opponents like the Catholic Medical Association cite safety concerns and believe doctors should screen all women before prescribing hormonal contraception, Dr. Gray disagreed. “There’s a lot of evidence that patients can figure out if a progestin-only pill is right for them and safe for them. Medical professionals don’t have to be the gatekeepers for contraception,” she said.
Pricing unknown
Whether insurance companies will pay for Opill now that it will be available without a prescription remains unknown. For some medications, paying a copay through insurance can be less expensive than buying at a retail price.
“Although pricing issues will be relevant, the FDA’s decision will enhance women’s access to hormonal birth control,” said Andrew M. Kaunitz, MD, a professor and associate chairman in the department of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville.
The drugmaker, Perrigo, based in Ireland, has not yet announced how much the pill will cost. The price tag could affect how widely available this form of birth control is. The drug has been shown to be as much as 93% effective for pregnancy prevention. Perrigo says it plans to make the pill available at low or no cost to some women.
Caveats to consider
There are some women for whom hormonal contraceptives have always carried greater risks. For example, women who have breast cancer or a history of breast cancer should not use hormonal contraceptives, the FDA said in a news release announcing the approval. Women with other types of cancer should check with their doctors first, the agency noted.
Women who smoke, who take some medications to lower blood pressure, or who have migraines should also take caution, Dr. Cansino said. “People with migraines may not be suitable for over-the-counter oral contraceptives. But a simple screening through a provider can identify whether you are truly eligible or not.”
Irregular bleeding, headaches, dizziness, nausea, increased appetite, belly pain, cramps, or bloating are the most common side effects of Opill, the FDA said.
The Opill is a progestin-only birth control pill. Similar pills have been available in the United Kingdiom for about 2 years, often referred to as “mini pills” because they contain a single hormone. In contrast, prescription birth control pills in the United States and elsewhere contain more than one hormone, estrogen and progestin, to prevent pregnancy.
Prescription pill packs for combination contraception often feature a week of placebo pills without an active ingredient. While skipping a placebo pill might not make a difference in pregnancy prevention, Opill is different. Every pill in the packet will contain medication, Gray said. “So it’s important to take the pill the same time every day for it to be most effective.”
Even though this may mean one less visit to your doctor, Dr. Kaunitz hopes women will stay up to date on their other medical checkups. “One of our challenges as providers of care to women will be to encourage them to continue to receive important services, including cancer screening and vaccinations, even while they can initiate and continue hormonal contraception without contact with a provider.”
Just the beginning?
The American Medical Association hopes this approval signals more to come.
“While we applaud this move, the AMA continues to urge the FDA and HHS to consider a variety of oral contraceptive options for over-the-counter use,” the association, which has more than 250,000 doctor members, said in a statement. “It is important patients have options when choosing which type of birth control works best for them,”
The American College of Obstetricians and Gynecologists said the FDA’s decision will help many women. “We are glad that more patients will now be empowered to choose when and where they obtain a safe method of contraception without having to wait for a medical appointment or for a prescription to be filled,” Verda J. Hicks, MD, the group’s president, and Christopher M. Zahn, MD, interim chief executive officer, said in a statement.
“Allowing individuals to access birth control at their local pharmacy or drug store will eliminate some barriers,” they said.
A version of this article first appeared on WebMD.com.
This article was updated 7/13/23.
Do oral contraceptives increase depression risk?
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EPIDEMIOLOGY AND PSYCHIATRIC SCIENCES
Postpartum IUD insertion: Best practices
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12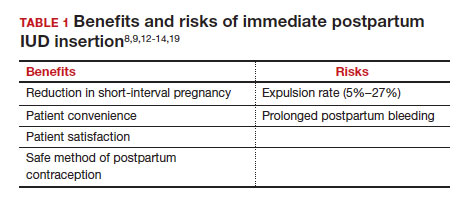
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21
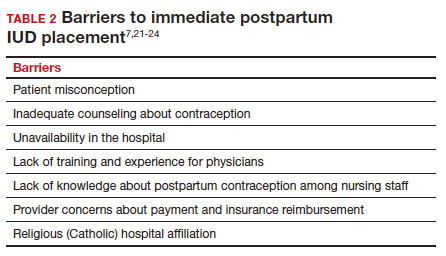
System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).
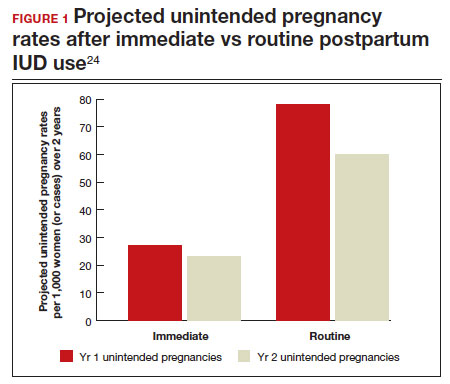
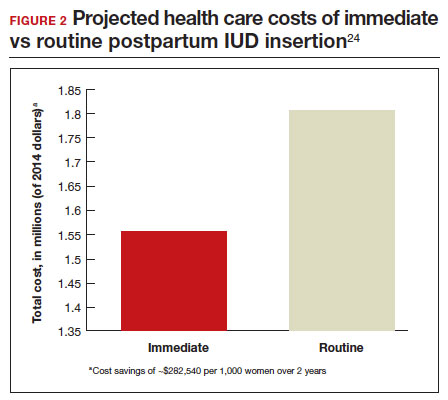
Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
The perimenopausal period and the benefits of progestin IUDs
Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.
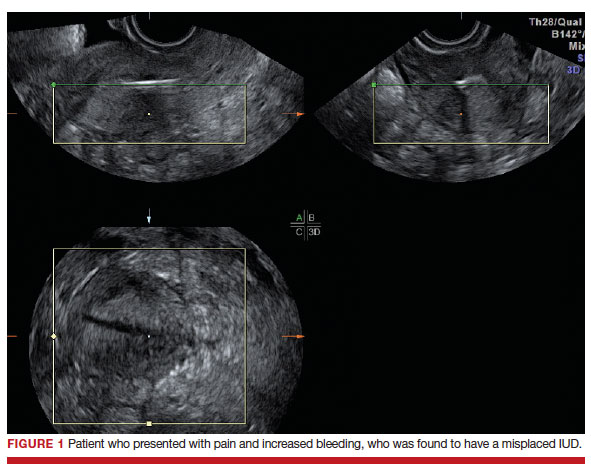
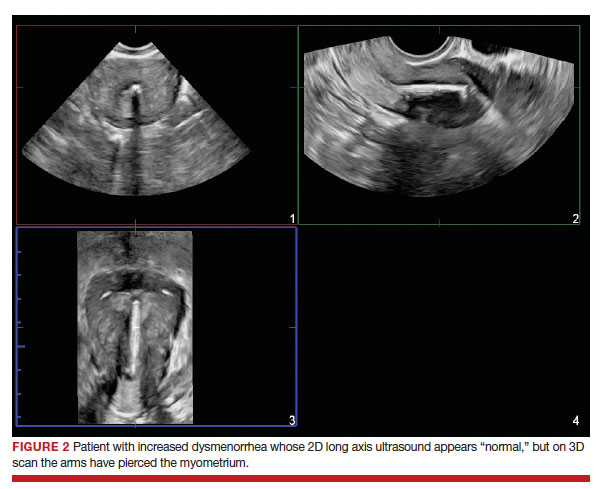
It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
Youth-led sexual health program improves teen knowledge, autonomy
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
While the small pilot study focused primarily on assessing feasibility and effectiveness, the results suggest potential for scaling the program up to reach a larger audience and assessing the knowledge disseminated from direct youth participants.
“The good thing about this subject is that not a lot of it has to be context-specific,” Saumya Sao, a clinical researcher in gynecology and obstetrics at the Johns Hopkins University, Baltimore, and the study’s lead author, said in an interview. “A lot of it is just baseline information that everybody needs and doesn’t get.”
Jaime Friedman, MD, a pediatrician and director of marketing at Children’s Primary Care Medical Group in San Diego, was not involved in the study but was impressed with the program’s objectives and results so far.
“While education is massively important, teens don’t always want to hear it from their parents or other adults,” Dr. Friedman said in an interview. “Learning from their peers is one way to overcome this hurdle.”
Given the high rate of sexually transmitted infections and unintended pregnancies in youth, paired with low sexual and reproductive health literacy in this population, the researchers sought to learn whether a program focused on peer-to-peer health education on these topics was feasible. The goal was to increase youth sexual and reproductive health knowledge, self-efficacy, and autonomy using a youth-led intervention.
The researchers hosted nine monthly, interactive, youth-led sessions that lasted 2 hours over Zoom or in person. Incorporated into the meetings were principles from Youth Participatory Action Research (YPAR) and Positive Youth Development (PYD).
The major topics included the following: Use of social media, values and goal-setting, anatomy and menstrual health, risk factors of sexual activities , STI and HIV prevention, contraceptive methods, healthy relationships and consent, practice responding to unhealthy behavior, gender and sexuality, and social media and body image.
The 24 participants were provided with transportation to the study site at the researchers’ institution and received financial compensation for their participation. They were an average 15.8 years old, lived in the greater Baltimore area, and mostly self-identified as female. Eight percent identified as non-binary and half (50%) identified as LGBTQIA+. Just over half the participants (52%) were Black/African American, 28% were Asian/Asian American, 12% were White, and 8% were Hispanic. The participants attended an average 88% of the sessions throughout the full intervention.
For each of the nine sessions, more than 50% of participants reported that they “learned a lot,” and only one participant reported for one session (session 5) that they “didn’t learn” anything. The researchers assessed participants’ knowledge, self-efficacy, and sense of autonomy at baseline and after completion of the intervention. Significant improvements occurred across all areas.
The average score improved by 31% in sexual and reproductive health knowledge (P < .001), 33% in sexual and reproductive health services awareness (P = .002), 46% in advocacy and empowerment (P < .001), 16% in general perceived efficacy (P = .002), and 22% personal sexuality empowerment (P = .006).
Ms. Sao said she was very pleased to see that the improvements were significant in every domain they measured, which she attributed largely to the incorporation of YPAR and PYD into the program.
“We approached it using these two frameworks that really do focus on involving youth in the teaching themselves, so I think that’s what increased their general perceived efficacy and advocacy empowerment without us necessarily having to emphasize, ‘You are advocates,’” Ms. Sao said. “Those frameworks ask the youth for their opinions and then give the youth an opportunity in every single session to be teachers themselves, and I think that lends itself well to all of the domains.”
Ms. Sao was also pleasantly surprised at the high level of retention across the 9 months.
“Every single session was slotted for 2 hours, but they would want to stay for 3 hours. Eventually, we actually started meeting with them twice a month, just adding an extra session,” she said. “As they gained confidence, they were so excited to be peer educators and realized, ‘I can really do this. I can teach my peers. We’re not getting this from anywhere else.’ ”
Ms. Sao and another study author, Maclaine Barré-Quick, an undergraduate research assistant at Johns Hopkins University, said the participants quickly discovered how easy it was to have a non-stigmatizing conversation about many of the topics once a subject was brought up.
“They’re actively looking for that opportunity,” Ms. Barré-Quick said in an interview.
Dr. Friedman agreed that this type of program provides what many adolescents need in a way that they may welcome more than through other methods.
“Adolescents’ bodies are approaching adulthood and function like adults, but their brains are still developing. They don’t have the worldly experience and education of adults, but they think they know everything,” Dr. Friedman said. “They are a population known for their high risk behavior due to their natural impulsivity. This can be a scary combination, especially when it comes to sexual health.”
But if teens don’t want to hear some of the information they need from adults, they may be more open to hearing it from other teens, Dr. Friedman said.
“Using an evidence-based approach ensures the desired outcome of healthier habits, decreased STIs and decreased teen pregnancy,” Dr. Friedman said. “It also adds weight to the argument against abstinence-only education. Teens deserve accurate and evidence-based education about their own bodies.”
Ms. Sao said the next steps will be exploring ways to scale the program up, such as putting the curriculum resources into a bundle available to other educators. They’re also looking at ways to put it into an online platform that’s self-paced, though that requires solving the challenge of having synchronous meetings for youth-led discussion.
“There are certain kinks that we have to work out because there were some activities where I think the students really benefited from having those open discussions with each other, so [we need to determine] how to replicate that in an online format,” Ms. Sao said.
Dr. Friedman agreed that scalability appears to be the biggest challenge, along with funding programs. But if those obstacles can be overcome, such programs would complement and expand on the education she does currently with families.
“I don’t have time for a full sex ed course at each visit,” Dr. Friedman said. “I would like to be able to direct them to a program that I know works and would be easy for them to complete. Even better, this would be an amazing program to ‘sell’ to practices interested in hosting these sessions themselves.”
Ms. Sao said they also hope to assess the impact of the intervention on the participants’ peers to see how well the knowledge and self-efficacy spread through the youths’ teaching.
No external funding was noted. One author reported research support from Hologic and Merck. Dr. Friedman had no disclosures.
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
While the small pilot study focused primarily on assessing feasibility and effectiveness, the results suggest potential for scaling the program up to reach a larger audience and assessing the knowledge disseminated from direct youth participants.
“The good thing about this subject is that not a lot of it has to be context-specific,” Saumya Sao, a clinical researcher in gynecology and obstetrics at the Johns Hopkins University, Baltimore, and the study’s lead author, said in an interview. “A lot of it is just baseline information that everybody needs and doesn’t get.”
Jaime Friedman, MD, a pediatrician and director of marketing at Children’s Primary Care Medical Group in San Diego, was not involved in the study but was impressed with the program’s objectives and results so far.
“While education is massively important, teens don’t always want to hear it from their parents or other adults,” Dr. Friedman said in an interview. “Learning from their peers is one way to overcome this hurdle.”
Given the high rate of sexually transmitted infections and unintended pregnancies in youth, paired with low sexual and reproductive health literacy in this population, the researchers sought to learn whether a program focused on peer-to-peer health education on these topics was feasible. The goal was to increase youth sexual and reproductive health knowledge, self-efficacy, and autonomy using a youth-led intervention.
The researchers hosted nine monthly, interactive, youth-led sessions that lasted 2 hours over Zoom or in person. Incorporated into the meetings were principles from Youth Participatory Action Research (YPAR) and Positive Youth Development (PYD).
The major topics included the following: Use of social media, values and goal-setting, anatomy and menstrual health, risk factors of sexual activities , STI and HIV prevention, contraceptive methods, healthy relationships and consent, practice responding to unhealthy behavior, gender and sexuality, and social media and body image.
The 24 participants were provided with transportation to the study site at the researchers’ institution and received financial compensation for their participation. They were an average 15.8 years old, lived in the greater Baltimore area, and mostly self-identified as female. Eight percent identified as non-binary and half (50%) identified as LGBTQIA+. Just over half the participants (52%) were Black/African American, 28% were Asian/Asian American, 12% were White, and 8% were Hispanic. The participants attended an average 88% of the sessions throughout the full intervention.
For each of the nine sessions, more than 50% of participants reported that they “learned a lot,” and only one participant reported for one session (session 5) that they “didn’t learn” anything. The researchers assessed participants’ knowledge, self-efficacy, and sense of autonomy at baseline and after completion of the intervention. Significant improvements occurred across all areas.
The average score improved by 31% in sexual and reproductive health knowledge (P < .001), 33% in sexual and reproductive health services awareness (P = .002), 46% in advocacy and empowerment (P < .001), 16% in general perceived efficacy (P = .002), and 22% personal sexuality empowerment (P = .006).
Ms. Sao said she was very pleased to see that the improvements were significant in every domain they measured, which she attributed largely to the incorporation of YPAR and PYD into the program.
“We approached it using these two frameworks that really do focus on involving youth in the teaching themselves, so I think that’s what increased their general perceived efficacy and advocacy empowerment without us necessarily having to emphasize, ‘You are advocates,’” Ms. Sao said. “Those frameworks ask the youth for their opinions and then give the youth an opportunity in every single session to be teachers themselves, and I think that lends itself well to all of the domains.”
Ms. Sao was also pleasantly surprised at the high level of retention across the 9 months.
“Every single session was slotted for 2 hours, but they would want to stay for 3 hours. Eventually, we actually started meeting with them twice a month, just adding an extra session,” she said. “As they gained confidence, they were so excited to be peer educators and realized, ‘I can really do this. I can teach my peers. We’re not getting this from anywhere else.’ ”
Ms. Sao and another study author, Maclaine Barré-Quick, an undergraduate research assistant at Johns Hopkins University, said the participants quickly discovered how easy it was to have a non-stigmatizing conversation about many of the topics once a subject was brought up.
“They’re actively looking for that opportunity,” Ms. Barré-Quick said in an interview.
Dr. Friedman agreed that this type of program provides what many adolescents need in a way that they may welcome more than through other methods.
“Adolescents’ bodies are approaching adulthood and function like adults, but their brains are still developing. They don’t have the worldly experience and education of adults, but they think they know everything,” Dr. Friedman said. “They are a population known for their high risk behavior due to their natural impulsivity. This can be a scary combination, especially when it comes to sexual health.”
But if teens don’t want to hear some of the information they need from adults, they may be more open to hearing it from other teens, Dr. Friedman said.
“Using an evidence-based approach ensures the desired outcome of healthier habits, decreased STIs and decreased teen pregnancy,” Dr. Friedman said. “It also adds weight to the argument against abstinence-only education. Teens deserve accurate and evidence-based education about their own bodies.”
Ms. Sao said the next steps will be exploring ways to scale the program up, such as putting the curriculum resources into a bundle available to other educators. They’re also looking at ways to put it into an online platform that’s self-paced, though that requires solving the challenge of having synchronous meetings for youth-led discussion.
“There are certain kinks that we have to work out because there were some activities where I think the students really benefited from having those open discussions with each other, so [we need to determine] how to replicate that in an online format,” Ms. Sao said.
Dr. Friedman agreed that scalability appears to be the biggest challenge, along with funding programs. But if those obstacles can be overcome, such programs would complement and expand on the education she does currently with families.
“I don’t have time for a full sex ed course at each visit,” Dr. Friedman said. “I would like to be able to direct them to a program that I know works and would be easy for them to complete. Even better, this would be an amazing program to ‘sell’ to practices interested in hosting these sessions themselves.”
Ms. Sao said they also hope to assess the impact of the intervention on the participants’ peers to see how well the knowledge and self-efficacy spread through the youths’ teaching.
No external funding was noted. One author reported research support from Hologic and Merck. Dr. Friedman had no disclosures.
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
While the small pilot study focused primarily on assessing feasibility and effectiveness, the results suggest potential for scaling the program up to reach a larger audience and assessing the knowledge disseminated from direct youth participants.
“The good thing about this subject is that not a lot of it has to be context-specific,” Saumya Sao, a clinical researcher in gynecology and obstetrics at the Johns Hopkins University, Baltimore, and the study’s lead author, said in an interview. “A lot of it is just baseline information that everybody needs and doesn’t get.”
Jaime Friedman, MD, a pediatrician and director of marketing at Children’s Primary Care Medical Group in San Diego, was not involved in the study but was impressed with the program’s objectives and results so far.
“While education is massively important, teens don’t always want to hear it from their parents or other adults,” Dr. Friedman said in an interview. “Learning from their peers is one way to overcome this hurdle.”
Given the high rate of sexually transmitted infections and unintended pregnancies in youth, paired with low sexual and reproductive health literacy in this population, the researchers sought to learn whether a program focused on peer-to-peer health education on these topics was feasible. The goal was to increase youth sexual and reproductive health knowledge, self-efficacy, and autonomy using a youth-led intervention.
The researchers hosted nine monthly, interactive, youth-led sessions that lasted 2 hours over Zoom or in person. Incorporated into the meetings were principles from Youth Participatory Action Research (YPAR) and Positive Youth Development (PYD).
The major topics included the following: Use of social media, values and goal-setting, anatomy and menstrual health, risk factors of sexual activities , STI and HIV prevention, contraceptive methods, healthy relationships and consent, practice responding to unhealthy behavior, gender and sexuality, and social media and body image.
The 24 participants were provided with transportation to the study site at the researchers’ institution and received financial compensation for their participation. They were an average 15.8 years old, lived in the greater Baltimore area, and mostly self-identified as female. Eight percent identified as non-binary and half (50%) identified as LGBTQIA+. Just over half the participants (52%) were Black/African American, 28% were Asian/Asian American, 12% were White, and 8% were Hispanic. The participants attended an average 88% of the sessions throughout the full intervention.
For each of the nine sessions, more than 50% of participants reported that they “learned a lot,” and only one participant reported for one session (session 5) that they “didn’t learn” anything. The researchers assessed participants’ knowledge, self-efficacy, and sense of autonomy at baseline and after completion of the intervention. Significant improvements occurred across all areas.
The average score improved by 31% in sexual and reproductive health knowledge (P < .001), 33% in sexual and reproductive health services awareness (P = .002), 46% in advocacy and empowerment (P < .001), 16% in general perceived efficacy (P = .002), and 22% personal sexuality empowerment (P = .006).
Ms. Sao said she was very pleased to see that the improvements were significant in every domain they measured, which she attributed largely to the incorporation of YPAR and PYD into the program.
“We approached it using these two frameworks that really do focus on involving youth in the teaching themselves, so I think that’s what increased their general perceived efficacy and advocacy empowerment without us necessarily having to emphasize, ‘You are advocates,’” Ms. Sao said. “Those frameworks ask the youth for their opinions and then give the youth an opportunity in every single session to be teachers themselves, and I think that lends itself well to all of the domains.”
Ms. Sao was also pleasantly surprised at the high level of retention across the 9 months.
“Every single session was slotted for 2 hours, but they would want to stay for 3 hours. Eventually, we actually started meeting with them twice a month, just adding an extra session,” she said. “As they gained confidence, they were so excited to be peer educators and realized, ‘I can really do this. I can teach my peers. We’re not getting this from anywhere else.’ ”
Ms. Sao and another study author, Maclaine Barré-Quick, an undergraduate research assistant at Johns Hopkins University, said the participants quickly discovered how easy it was to have a non-stigmatizing conversation about many of the topics once a subject was brought up.
“They’re actively looking for that opportunity,” Ms. Barré-Quick said in an interview.
Dr. Friedman agreed that this type of program provides what many adolescents need in a way that they may welcome more than through other methods.
“Adolescents’ bodies are approaching adulthood and function like adults, but their brains are still developing. They don’t have the worldly experience and education of adults, but they think they know everything,” Dr. Friedman said. “They are a population known for their high risk behavior due to their natural impulsivity. This can be a scary combination, especially when it comes to sexual health.”
But if teens don’t want to hear some of the information they need from adults, they may be more open to hearing it from other teens, Dr. Friedman said.
“Using an evidence-based approach ensures the desired outcome of healthier habits, decreased STIs and decreased teen pregnancy,” Dr. Friedman said. “It also adds weight to the argument against abstinence-only education. Teens deserve accurate and evidence-based education about their own bodies.”
Ms. Sao said the next steps will be exploring ways to scale the program up, such as putting the curriculum resources into a bundle available to other educators. They’re also looking at ways to put it into an online platform that’s self-paced, though that requires solving the challenge of having synchronous meetings for youth-led discussion.
“There are certain kinks that we have to work out because there were some activities where I think the students really benefited from having those open discussions with each other, so [we need to determine] how to replicate that in an online format,” Ms. Sao said.
Dr. Friedman agreed that scalability appears to be the biggest challenge, along with funding programs. But if those obstacles can be overcome, such programs would complement and expand on the education she does currently with families.
“I don’t have time for a full sex ed course at each visit,” Dr. Friedman said. “I would like to be able to direct them to a program that I know works and would be easy for them to complete. Even better, this would be an amazing program to ‘sell’ to practices interested in hosting these sessions themselves.”
Ms. Sao said they also hope to assess the impact of the intervention on the participants’ peers to see how well the knowledge and self-efficacy spread through the youths’ teaching.
No external funding was noted. One author reported research support from Hologic and Merck. Dr. Friedman had no disclosures.
AT ACOG 2023
Scheduled bleeding may boost tolerability of hormone implants
BALTIMORE – The bleeding causes some women to have the device removed, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a randomized, double-blinded, placebo-controlled trial of 51 patients desiring the implants – which suppress ovulation by releasing progestin over a 3-year period – taking norethindrone acetate for 1 week every 4 weeks led to 80% of participants in the treatment group reporting satisfactory bleeding patterns with the etonogestrel implants in place.
Rates of early discontinuation have been variable, according to published literature, ranging from 13% to 21.1%, said Jordan Gray, MD, a fourth-year resident in ob.gyn. at Baylor Scott and White Medical Center, Temple, Tex., who helped conduct the new study. Reasons included bothersome bleeding. Dr. Gray and colleagues found that 24% of women in the placebo group requested removal of the implant, compared with 9% of those in the treatment group. Among these women, none requested removal for bothersome bleeding but rather for reasons such as wanting to get pregnant. One person requested removal because she did not like amenorrhea.
While the results of the study did not achieve statistical significance, owing to its size and noncompliance among some participants, it does indicate that norethindrone acetate may be helpful, Dr. Gray said.
During the study, participants in the treatment group (n = 22) received a monthly treatment regimen of 5 mg of oral norethindrone acetate daily for 7 days each month for the first 6 months after placement of an etonogestrel implant. The placebo group (n = 29) was given inert tablets prescribed in the same regimen. Both groups received products from a mail-order pharmacy.
Participants were women aged 18-48 years who desired an implant or those aged 14 years who had permission from a parent or guardian to receive the contraceptive. The study excluded people with known or suspected pregnancy, those less than 8 weeks’ post partum, those who experienced menarche less than 2 years ago, those with body mass index greater than 40, and those who received depot medroxyprogesterone acetate within the previous 12 weeks. Excessive bleeding was defined as bleeding or spotting on more than 7 consecutive days or a fifth episode of bleeding in 90 days.
Overall, 11 patients (38%) in the placebo group and 10 (45%) in the treatment arm withdrew from the study. Reasons included wanting to get pregnant, mood changes, or noncompliance with study parameters, which included not responding or returning bleeding diaries, Dr. Gray said.
A limitation of the study was that compliance was less than expected. In addition, there were challenges with rates of responses, Dr. Gray said. The study was conducted during the COVID-19 pandemic, when all in-person visits were transitioned to telehealth. Although the investigators offered payment to participants, not all returned text-message surveys. The researchers had intended to enroll 124 participants but curtailed the study early, owing to the limited number of participants.
Given that there is no standard approach to treating prolonged or excessive bleeding with etonogestrel implants, Dr. Gray said, “Our data suggests that this regimen is a simple and acceptable method to treat bothersome bleeding and that predictable bleeding may be more satisfactory than unpredictable bleeding.”
Veronica Maria Pimentel, MD, moderator of the session and a maternal-fetal medicine specialist and director of research for the ob.gyn. residency program at St. Francis Hospital, part of Trinity Health of New England in Hartford, Conn., praised the researchers for a well-designed study.
“However, unfortunately, they were not able to recruit the number of patients that they needed in order to achieve the power to show the difference [between treatment arms], so another study would have to be done to show if there is a difference,” Dr. Pimentel said.
Dr. Pimentel complimented Dr. Gray following her presentation, congratulating her for conducting a randomized, controlled trial: “That’s not easy, as you have shown, but it’s also a good try, so you can actually see how hard it is to obtain quality data from research.”
The study was supported in part by a research grant from the Investigator-Initiated Studies Program of Organon. Dr. Gray is a consultant for Johnson & Johnson. Dr. Pimentel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BALTIMORE – The bleeding causes some women to have the device removed, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a randomized, double-blinded, placebo-controlled trial of 51 patients desiring the implants – which suppress ovulation by releasing progestin over a 3-year period – taking norethindrone acetate for 1 week every 4 weeks led to 80% of participants in the treatment group reporting satisfactory bleeding patterns with the etonogestrel implants in place.
Rates of early discontinuation have been variable, according to published literature, ranging from 13% to 21.1%, said Jordan Gray, MD, a fourth-year resident in ob.gyn. at Baylor Scott and White Medical Center, Temple, Tex., who helped conduct the new study. Reasons included bothersome bleeding. Dr. Gray and colleagues found that 24% of women in the placebo group requested removal of the implant, compared with 9% of those in the treatment group. Among these women, none requested removal for bothersome bleeding but rather for reasons such as wanting to get pregnant. One person requested removal because she did not like amenorrhea.
While the results of the study did not achieve statistical significance, owing to its size and noncompliance among some participants, it does indicate that norethindrone acetate may be helpful, Dr. Gray said.
During the study, participants in the treatment group (n = 22) received a monthly treatment regimen of 5 mg of oral norethindrone acetate daily for 7 days each month for the first 6 months after placement of an etonogestrel implant. The placebo group (n = 29) was given inert tablets prescribed in the same regimen. Both groups received products from a mail-order pharmacy.
Participants were women aged 18-48 years who desired an implant or those aged 14 years who had permission from a parent or guardian to receive the contraceptive. The study excluded people with known or suspected pregnancy, those less than 8 weeks’ post partum, those who experienced menarche less than 2 years ago, those with body mass index greater than 40, and those who received depot medroxyprogesterone acetate within the previous 12 weeks. Excessive bleeding was defined as bleeding or spotting on more than 7 consecutive days or a fifth episode of bleeding in 90 days.
Overall, 11 patients (38%) in the placebo group and 10 (45%) in the treatment arm withdrew from the study. Reasons included wanting to get pregnant, mood changes, or noncompliance with study parameters, which included not responding or returning bleeding diaries, Dr. Gray said.
A limitation of the study was that compliance was less than expected. In addition, there were challenges with rates of responses, Dr. Gray said. The study was conducted during the COVID-19 pandemic, when all in-person visits were transitioned to telehealth. Although the investigators offered payment to participants, not all returned text-message surveys. The researchers had intended to enroll 124 participants but curtailed the study early, owing to the limited number of participants.
Given that there is no standard approach to treating prolonged or excessive bleeding with etonogestrel implants, Dr. Gray said, “Our data suggests that this regimen is a simple and acceptable method to treat bothersome bleeding and that predictable bleeding may be more satisfactory than unpredictable bleeding.”
Veronica Maria Pimentel, MD, moderator of the session and a maternal-fetal medicine specialist and director of research for the ob.gyn. residency program at St. Francis Hospital, part of Trinity Health of New England in Hartford, Conn., praised the researchers for a well-designed study.
“However, unfortunately, they were not able to recruit the number of patients that they needed in order to achieve the power to show the difference [between treatment arms], so another study would have to be done to show if there is a difference,” Dr. Pimentel said.
Dr. Pimentel complimented Dr. Gray following her presentation, congratulating her for conducting a randomized, controlled trial: “That’s not easy, as you have shown, but it’s also a good try, so you can actually see how hard it is to obtain quality data from research.”
The study was supported in part by a research grant from the Investigator-Initiated Studies Program of Organon. Dr. Gray is a consultant for Johnson & Johnson. Dr. Pimentel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BALTIMORE – The bleeding causes some women to have the device removed, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a randomized, double-blinded, placebo-controlled trial of 51 patients desiring the implants – which suppress ovulation by releasing progestin over a 3-year period – taking norethindrone acetate for 1 week every 4 weeks led to 80% of participants in the treatment group reporting satisfactory bleeding patterns with the etonogestrel implants in place.
Rates of early discontinuation have been variable, according to published literature, ranging from 13% to 21.1%, said Jordan Gray, MD, a fourth-year resident in ob.gyn. at Baylor Scott and White Medical Center, Temple, Tex., who helped conduct the new study. Reasons included bothersome bleeding. Dr. Gray and colleagues found that 24% of women in the placebo group requested removal of the implant, compared with 9% of those in the treatment group. Among these women, none requested removal for bothersome bleeding but rather for reasons such as wanting to get pregnant. One person requested removal because she did not like amenorrhea.
While the results of the study did not achieve statistical significance, owing to its size and noncompliance among some participants, it does indicate that norethindrone acetate may be helpful, Dr. Gray said.
During the study, participants in the treatment group (n = 22) received a monthly treatment regimen of 5 mg of oral norethindrone acetate daily for 7 days each month for the first 6 months after placement of an etonogestrel implant. The placebo group (n = 29) was given inert tablets prescribed in the same regimen. Both groups received products from a mail-order pharmacy.
Participants were women aged 18-48 years who desired an implant or those aged 14 years who had permission from a parent or guardian to receive the contraceptive. The study excluded people with known or suspected pregnancy, those less than 8 weeks’ post partum, those who experienced menarche less than 2 years ago, those with body mass index greater than 40, and those who received depot medroxyprogesterone acetate within the previous 12 weeks. Excessive bleeding was defined as bleeding or spotting on more than 7 consecutive days or a fifth episode of bleeding in 90 days.
Overall, 11 patients (38%) in the placebo group and 10 (45%) in the treatment arm withdrew from the study. Reasons included wanting to get pregnant, mood changes, or noncompliance with study parameters, which included not responding or returning bleeding diaries, Dr. Gray said.
A limitation of the study was that compliance was less than expected. In addition, there were challenges with rates of responses, Dr. Gray said. The study was conducted during the COVID-19 pandemic, when all in-person visits were transitioned to telehealth. Although the investigators offered payment to participants, not all returned text-message surveys. The researchers had intended to enroll 124 participants but curtailed the study early, owing to the limited number of participants.
Given that there is no standard approach to treating prolonged or excessive bleeding with etonogestrel implants, Dr. Gray said, “Our data suggests that this regimen is a simple and acceptable method to treat bothersome bleeding and that predictable bleeding may be more satisfactory than unpredictable bleeding.”
Veronica Maria Pimentel, MD, moderator of the session and a maternal-fetal medicine specialist and director of research for the ob.gyn. residency program at St. Francis Hospital, part of Trinity Health of New England in Hartford, Conn., praised the researchers for a well-designed study.
“However, unfortunately, they were not able to recruit the number of patients that they needed in order to achieve the power to show the difference [between treatment arms], so another study would have to be done to show if there is a difference,” Dr. Pimentel said.
Dr. Pimentel complimented Dr. Gray following her presentation, congratulating her for conducting a randomized, controlled trial: “That’s not easy, as you have shown, but it’s also a good try, so you can actually see how hard it is to obtain quality data from research.”
The study was supported in part by a research grant from the Investigator-Initiated Studies Program of Organon. Dr. Gray is a consultant for Johnson & Johnson. Dr. Pimentel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACOG 2023
Cycle timing may reduce hormonal dosage for contraception
Progesterone and estrogen are often used for contraception by preventing ovulation, but the adverse effects associated with large doses of these hormones remain a concern, wrote Brenda Lyn A. Gavina, a PhD candidate at the University of the Philippines Diliman, Quezon City, and colleagues.
In a study published in PLoS Computational Biology, the researchers examined how the timing of hormone administration during a cycle might impact the amount of hormones needed for contraception. Previous research shown that combining hormones can reduce the dosage needed, but the impact of timing on further dose reduction has not been well studied, they said.
The researchers applied optimal control theory in a mathematical model to show the contraceptive effect of estrogen and/or progesterone at different times in the menstrual cycle. The model was based on a normal menstrual cycle with pituitary and ovarian phases. The model assumed that synthesis of luteinizing hormone and follicle-stimulating hormone occurs in the pituitary, that LH and FSH are held in reserve before release into the bloodstream, and that the follicular/luteal mass goes through nine ovarian stages of development. The model also included the activity of ovarian hormones estradiol (E2), progesterone (P4), and inhibin (Inh), in a normal cycle. In the model, LH, FSH, and E2 peaked in the late follicular phase, and P4 and Inh peaked in the luteal phase.
The pituitary model predicted the synthesis, release, and clearance of LH and FSH, and the response of the pituitary to E2, P4, and Inh. The ovarian model predicted the response of E2, P4, and Inh as functions of LH and FSH.
The researchers simulated a constant dose of exogenous progesterone monotherapy and combined exogenous estrogen/progesterone. They determined that a P4 peak of 4.99 ng/mL was taken as the optimum constant dosage for progesterone monotherapy, and for combination estrogen/progesterone.
The researchers then assessed the impact of time on dosage. They found that estrogen administration starting on the first day of a normal cycle preventing FHS from reaching maximum value, and that the low level of FHS in the follicular phase and additional P4 inhibition slowed follicular growth, and use of combination estrogen/progesterone caused similar inhibition at a later follicular stage.
“The combination therapy suggests that time-varying doses of estrogen and progesterone given simultaneously from the start to the end of the 28-day period, only requires a surge in estrogen dose around the 12th day of the cycle (a delayed administration compared to the estrogen monotherapy),” they noted.
With attention to timing, the maximum progesterone levels throughout a menstrual cycle were 4.43 ng/mL, 4.66 ng/mL, and 4.31 ng/mL for estrogen monotherapy, progesterone monotherapy, and combination therapy, respectively. Total doses of the optimal exogenous hormone were 77.76 pg/mL and 48.84 ng/mL for estrogen and progesterone monotherapy, respectively, and 35.58 pg/mL and 21.67 ng/mL for estrogen and progesterone in combination.
The findings were limited by the use of a standard model that does not account for variations in cycle length, the researchers noted. However, the results reflect other studies of hormonal activity, and the model can be used in future studies of the effect of hormones on cycle length, they said.
Overall, the researchers determined that timing dosage with estrogen monotherapy based on their model could provide effective contraception with about 92% of the minimum total constant dosage, while progesterone monotherapy would be effective with approximately 43% of the total constant dose.
Although more work is needed, the current study results may guide clinicians in experimenting with the optimal treatment regimen for anovulation, the researchers said.
“The results presented here give insights on construction of timed devices that give contraception at certain parts of the menstrual cycle,” they concluded.
Model aims to improve women’s control of contraception
“Aside from wanting to contribute to controlling population growth, we aim to empower women more by giving them more control on when to conceive and start motherhood,” and be in control of contraception in a safer way, said lead author Ms. Gavina, in an interview. In addition, studies are showing the noncontraceptive benefits of suppressing ovulation for managing premenstrual syndromes such as breast tenderness and irritability, and for managing diseases such as endometriosis, she said. “Anovulation also lowers the risk of ACL injuries in female athletes,” she added.
Ms. Gavina said that she was surprised primarily by the optimal control result for estrogen monotherapy. “It was surprising that, theoretically, our mathematical model, with the simplifying assumptions, showed that as low as 10% of the total dose in constant administration could achieve contraception as long as the administration of this dosage is perfectly timed, and the timing was also shown in our optimization result,” she said.
“Our model does not capture all factors in contraception, since the reproductive function in women is a very complex multiscale dynamical system highly dependent on both endogenous and exogenous hormones,” Ms. Gavina told this news organization. However, “with the emergence of more data, it can be refined to address other contraception issues. Further, although the results of this study are not directly translatable to the clinical setting, we hope that these results may aid clinicians in identifying the minimum dose and treatment schedule for contraception,” she said.
Future research directions include examining within and between women’s variabilities and adding a pharmacokinetics model to account for the effects of specific drugs, she said. “We also hope to expand or modify the current model to investigate reproductive health concerns in women, such as [polycystic ovary syndrome] and ovarian cysts,” she added.
Ms. Gavina disclosed support from the University of the Philippines Office of International Linkages, a Continuous Operational and Outcomes-based Partnership for Excellence in Research and Academic Training Enhancement grant, and a Commission on Higher Education Faculty Development Program-II scholarship.
Progesterone and estrogen are often used for contraception by preventing ovulation, but the adverse effects associated with large doses of these hormones remain a concern, wrote Brenda Lyn A. Gavina, a PhD candidate at the University of the Philippines Diliman, Quezon City, and colleagues.
In a study published in PLoS Computational Biology, the researchers examined how the timing of hormone administration during a cycle might impact the amount of hormones needed for contraception. Previous research shown that combining hormones can reduce the dosage needed, but the impact of timing on further dose reduction has not been well studied, they said.
The researchers applied optimal control theory in a mathematical model to show the contraceptive effect of estrogen and/or progesterone at different times in the menstrual cycle. The model was based on a normal menstrual cycle with pituitary and ovarian phases. The model assumed that synthesis of luteinizing hormone and follicle-stimulating hormone occurs in the pituitary, that LH and FSH are held in reserve before release into the bloodstream, and that the follicular/luteal mass goes through nine ovarian stages of development. The model also included the activity of ovarian hormones estradiol (E2), progesterone (P4), and inhibin (Inh), in a normal cycle. In the model, LH, FSH, and E2 peaked in the late follicular phase, and P4 and Inh peaked in the luteal phase.
The pituitary model predicted the synthesis, release, and clearance of LH and FSH, and the response of the pituitary to E2, P4, and Inh. The ovarian model predicted the response of E2, P4, and Inh as functions of LH and FSH.
The researchers simulated a constant dose of exogenous progesterone monotherapy and combined exogenous estrogen/progesterone. They determined that a P4 peak of 4.99 ng/mL was taken as the optimum constant dosage for progesterone monotherapy, and for combination estrogen/progesterone.
The researchers then assessed the impact of time on dosage. They found that estrogen administration starting on the first day of a normal cycle preventing FHS from reaching maximum value, and that the low level of FHS in the follicular phase and additional P4 inhibition slowed follicular growth, and use of combination estrogen/progesterone caused similar inhibition at a later follicular stage.
“The combination therapy suggests that time-varying doses of estrogen and progesterone given simultaneously from the start to the end of the 28-day period, only requires a surge in estrogen dose around the 12th day of the cycle (a delayed administration compared to the estrogen monotherapy),” they noted.
With attention to timing, the maximum progesterone levels throughout a menstrual cycle were 4.43 ng/mL, 4.66 ng/mL, and 4.31 ng/mL for estrogen monotherapy, progesterone monotherapy, and combination therapy, respectively. Total doses of the optimal exogenous hormone were 77.76 pg/mL and 48.84 ng/mL for estrogen and progesterone monotherapy, respectively, and 35.58 pg/mL and 21.67 ng/mL for estrogen and progesterone in combination.
The findings were limited by the use of a standard model that does not account for variations in cycle length, the researchers noted. However, the results reflect other studies of hormonal activity, and the model can be used in future studies of the effect of hormones on cycle length, they said.
Overall, the researchers determined that timing dosage with estrogen monotherapy based on their model could provide effective contraception with about 92% of the minimum total constant dosage, while progesterone monotherapy would be effective with approximately 43% of the total constant dose.
Although more work is needed, the current study results may guide clinicians in experimenting with the optimal treatment regimen for anovulation, the researchers said.
“The results presented here give insights on construction of timed devices that give contraception at certain parts of the menstrual cycle,” they concluded.
Model aims to improve women’s control of contraception
“Aside from wanting to contribute to controlling population growth, we aim to empower women more by giving them more control on when to conceive and start motherhood,” and be in control of contraception in a safer way, said lead author Ms. Gavina, in an interview. In addition, studies are showing the noncontraceptive benefits of suppressing ovulation for managing premenstrual syndromes such as breast tenderness and irritability, and for managing diseases such as endometriosis, she said. “Anovulation also lowers the risk of ACL injuries in female athletes,” she added.
Ms. Gavina said that she was surprised primarily by the optimal control result for estrogen monotherapy. “It was surprising that, theoretically, our mathematical model, with the simplifying assumptions, showed that as low as 10% of the total dose in constant administration could achieve contraception as long as the administration of this dosage is perfectly timed, and the timing was also shown in our optimization result,” she said.
“Our model does not capture all factors in contraception, since the reproductive function in women is a very complex multiscale dynamical system highly dependent on both endogenous and exogenous hormones,” Ms. Gavina told this news organization. However, “with the emergence of more data, it can be refined to address other contraception issues. Further, although the results of this study are not directly translatable to the clinical setting, we hope that these results may aid clinicians in identifying the minimum dose and treatment schedule for contraception,” she said.
Future research directions include examining within and between women’s variabilities and adding a pharmacokinetics model to account for the effects of specific drugs, she said. “We also hope to expand or modify the current model to investigate reproductive health concerns in women, such as [polycystic ovary syndrome] and ovarian cysts,” she added.
Ms. Gavina disclosed support from the University of the Philippines Office of International Linkages, a Continuous Operational and Outcomes-based Partnership for Excellence in Research and Academic Training Enhancement grant, and a Commission on Higher Education Faculty Development Program-II scholarship.
Progesterone and estrogen are often used for contraception by preventing ovulation, but the adverse effects associated with large doses of these hormones remain a concern, wrote Brenda Lyn A. Gavina, a PhD candidate at the University of the Philippines Diliman, Quezon City, and colleagues.
In a study published in PLoS Computational Biology, the researchers examined how the timing of hormone administration during a cycle might impact the amount of hormones needed for contraception. Previous research shown that combining hormones can reduce the dosage needed, but the impact of timing on further dose reduction has not been well studied, they said.
The researchers applied optimal control theory in a mathematical model to show the contraceptive effect of estrogen and/or progesterone at different times in the menstrual cycle. The model was based on a normal menstrual cycle with pituitary and ovarian phases. The model assumed that synthesis of luteinizing hormone and follicle-stimulating hormone occurs in the pituitary, that LH and FSH are held in reserve before release into the bloodstream, and that the follicular/luteal mass goes through nine ovarian stages of development. The model also included the activity of ovarian hormones estradiol (E2), progesterone (P4), and inhibin (Inh), in a normal cycle. In the model, LH, FSH, and E2 peaked in the late follicular phase, and P4 and Inh peaked in the luteal phase.
The pituitary model predicted the synthesis, release, and clearance of LH and FSH, and the response of the pituitary to E2, P4, and Inh. The ovarian model predicted the response of E2, P4, and Inh as functions of LH and FSH.
The researchers simulated a constant dose of exogenous progesterone monotherapy and combined exogenous estrogen/progesterone. They determined that a P4 peak of 4.99 ng/mL was taken as the optimum constant dosage for progesterone monotherapy, and for combination estrogen/progesterone.
The researchers then assessed the impact of time on dosage. They found that estrogen administration starting on the first day of a normal cycle preventing FHS from reaching maximum value, and that the low level of FHS in the follicular phase and additional P4 inhibition slowed follicular growth, and use of combination estrogen/progesterone caused similar inhibition at a later follicular stage.
“The combination therapy suggests that time-varying doses of estrogen and progesterone given simultaneously from the start to the end of the 28-day period, only requires a surge in estrogen dose around the 12th day of the cycle (a delayed administration compared to the estrogen monotherapy),” they noted.
With attention to timing, the maximum progesterone levels throughout a menstrual cycle were 4.43 ng/mL, 4.66 ng/mL, and 4.31 ng/mL for estrogen monotherapy, progesterone monotherapy, and combination therapy, respectively. Total doses of the optimal exogenous hormone were 77.76 pg/mL and 48.84 ng/mL for estrogen and progesterone monotherapy, respectively, and 35.58 pg/mL and 21.67 ng/mL for estrogen and progesterone in combination.
The findings were limited by the use of a standard model that does not account for variations in cycle length, the researchers noted. However, the results reflect other studies of hormonal activity, and the model can be used in future studies of the effect of hormones on cycle length, they said.
Overall, the researchers determined that timing dosage with estrogen monotherapy based on their model could provide effective contraception with about 92% of the minimum total constant dosage, while progesterone monotherapy would be effective with approximately 43% of the total constant dose.
Although more work is needed, the current study results may guide clinicians in experimenting with the optimal treatment regimen for anovulation, the researchers said.
“The results presented here give insights on construction of timed devices that give contraception at certain parts of the menstrual cycle,” they concluded.
Model aims to improve women’s control of contraception
“Aside from wanting to contribute to controlling population growth, we aim to empower women more by giving them more control on when to conceive and start motherhood,” and be in control of contraception in a safer way, said lead author Ms. Gavina, in an interview. In addition, studies are showing the noncontraceptive benefits of suppressing ovulation for managing premenstrual syndromes such as breast tenderness and irritability, and for managing diseases such as endometriosis, she said. “Anovulation also lowers the risk of ACL injuries in female athletes,” she added.
Ms. Gavina said that she was surprised primarily by the optimal control result for estrogen monotherapy. “It was surprising that, theoretically, our mathematical model, with the simplifying assumptions, showed that as low as 10% of the total dose in constant administration could achieve contraception as long as the administration of this dosage is perfectly timed, and the timing was also shown in our optimization result,” she said.
“Our model does not capture all factors in contraception, since the reproductive function in women is a very complex multiscale dynamical system highly dependent on both endogenous and exogenous hormones,” Ms. Gavina told this news organization. However, “with the emergence of more data, it can be refined to address other contraception issues. Further, although the results of this study are not directly translatable to the clinical setting, we hope that these results may aid clinicians in identifying the minimum dose and treatment schedule for contraception,” she said.
Future research directions include examining within and between women’s variabilities and adding a pharmacokinetics model to account for the effects of specific drugs, she said. “We also hope to expand or modify the current model to investigate reproductive health concerns in women, such as [polycystic ovary syndrome] and ovarian cysts,” she added.
Ms. Gavina disclosed support from the University of the Philippines Office of International Linkages, a Continuous Operational and Outcomes-based Partnership for Excellence in Research and Academic Training Enhancement grant, and a Commission on Higher Education Faculty Development Program-II scholarship.
FROM PLOS COMPUTATIONAL BIOLOGY
Neuropsychiatric side effects of hormonal contraceptives: More common than you think!
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at obnews@mdedge.com.
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at obnews@mdedge.com.
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at obnews@mdedge.com.