User login
The perimenopausal period and the benefits of progestin IUDs
Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
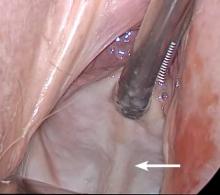
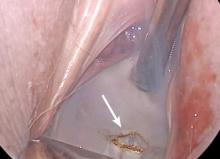
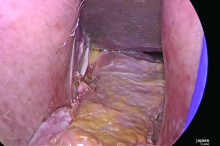
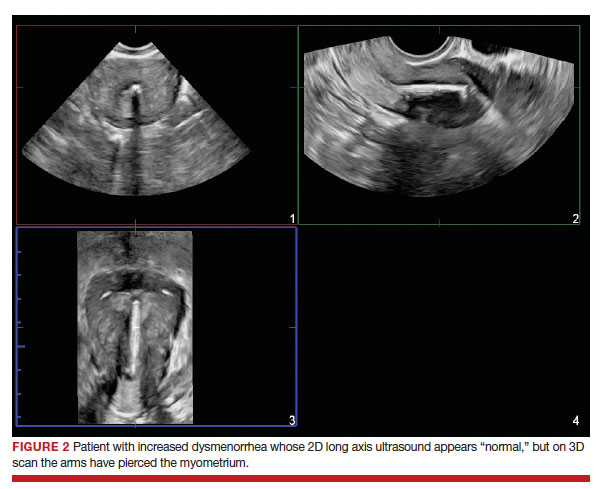
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.
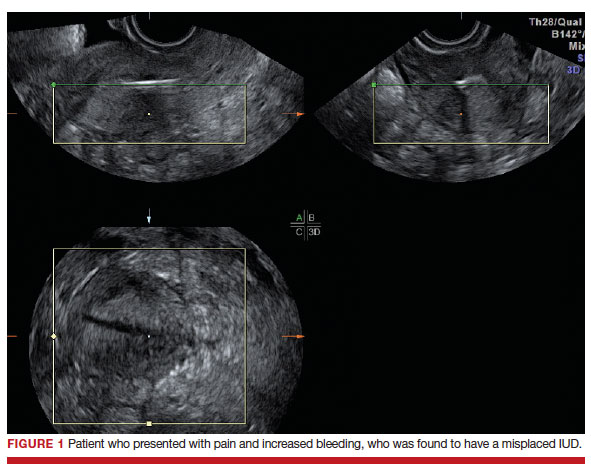
It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
Changes required for gynecologic surgeons to achieve greater pay equity
In a recent commentary published in Obstetrics & Gynecology, Katie L. Watson, JD, and Louise P. King, MD, JD, describe the issue of “double discrimination” in gynecologic surgery. The authors outlined how lower pay in a specialty where a majority of the surgeons and all of the patients are women may impact quality of care.
The commentary raises a number of concerns in gynecologic surgery that are important to discuss. Ob.gyn. as a whole is underpaid, as are many nonprocedural specialties such as family medicine and internal medicine. When ob.gyns. were predominantly men, the same situation existed – ob.gyns. were paid less than many other procedural specialties. While we’ve come a long way from the relative value unit (RVU) originally determined from the Harvard studies 30 years ago, there is room for additional improvement.
Several rationales were proposed by the authors to explain the disparities in pay between gynecologic surgery and those in urology: patient gender, surgeon gender, and length of training for gynecologic surgeons. The authors cited comparisons between urology and gynecology regarding “anatomically similar, sex-specific procedures” which require closer examination. Many of the code pairs selected were not actually comparable services. For example, management of Peyronie’s disease is a highly complex treatment performed by urologists that is not comparable with vaginectomy, yet this is an example of two codes used in the reference cited by the authors to conclude that surgeries on women are undervalued.
The overall RVUs for a procedure are also dependent upon the global period. The Centers for Medicare & Medicaid Services designated RVUs as the total amount of work before, during, and after a procedure. If a surgery has a 90-day global period, all the work for 90 days thereafter is bundled into the value, whereas if something is a zero-day global, only that day’s work is counted. A gynecologic surgeon who sees a patient back two or three times is coding and billing for those encounters in addition to that initial procedure.
Many of the code comparisons used in the analysis of gender in RVUs compared services with different global periods. Finally, some of the services that were compared had vastly different utilization. Some of the services and codes that were compared are performed extremely rarely and for that reason have not had their values reassessed over the years. There may be inequities in the RVUs for these services, but they will account for extremely little in overall compensation.
As a former chair of the American Medical Association’s RVS Update Committee (RUC), I spent years attempting to revalue ob.gyn. procedures. CMS assigns work RVUs based on physician work, practice expense, and professional liability insurance. The work is calculated using total physician time and intensity based on surveys completed by the specialty. The American College of Obstetrician and Gynecologist’s Committee on Health Economics and Coding, and the AMA RUC have worked diligently over many years to reassess potentially misvalued services. The ultimate RVUs assigned by CMS for gynecologic surgery are determined by the surveys completed by ACOG members. One issue we encountered with reexamining some procedures under RBRVS is that they have become so low volume that it has been difficult to justify the cost and effort to revalue them.
Lack of ob.gyn. training isn’t the full story
On average, ob.gyns. have between 18 and 24 months of surgical training, which is significantly less than other specialties. Lack of training in gynecologic surgery was proposed as another explanation for reduced compensation among female gynecologic surgeons. This is a complex issue not adequately explained by training time for gynecologic surgeons alone. While the number of trained ob.gyns. has increased in recent decades, the surgical volume has diminished and the workload of gynecologic surgery is far lower than it used to be. Surgical volume during and after training was much higher 35 years ago, prior to the advancements of procedures like endometrial ablation or tubal ligation. Women who had finished childbearing often underwent vaginal hysterectomies to manage contraception along with various other conditions.
With the advent of minimally invasive surgery, laparoscopic sterilization became possible, which has reduced the number of hysterectomies performed. Endometrial ablation is an office-based, noninvasive procedure. The development of the levonorgestrel IUD has helped manage abnormal bleeding, further reducing the need for hysterectomy.
This reduction in surgical volume does have an impact on quality of care. The model of tracking surgical outcomes at Kaiser Health System, as mentioned by the authors, could work well in some, but not all centers. A more approachable solution to address surgical volume for the average ob.gyn. would be to implement a mentoring and coaching process whereby recently trained ob.gyns. assist their senior partner(s) in surgery. This was the model years ago: I was trained by an ob.gyn. who was trained as a general surgeon. It was through the experience of assisting on each one of his cases – and him assisting on each one of my cases – that I received incredibly thorough surgical training.
These changes in practice, however, do not impact reimbursement. Rather than discrimination based on the gender of the surgeon, lower salaries in ob.gyn. are more likely to be the result of these and other factors.
The wage and quality gap in ob.gyn.
As a predominantly female surgical specialty, some of the disparity between gynecology and urology could be explained by how each specialty values its work. Here, gender plays a role in that when ob.gyns. are surveyed during the RUC process they may undervalue their work by reporting they can perform a procedure (and the before and after care) faster than what a urologist reports. The survey results may then result in lower RVUs.
Ob.gyn. is an overpopulated specialty for the number of surgeons needed to manage the volume of gynecologic surgery. When a health system wants to hire a general ob.gyn., it doesn’t have trouble finding one, while urologists are more challenging to recruit. This is not because of the structure of resource-based relative value scale (RBRVS) – despite the overall RVUs for gynecologic surgery, gynecologic oncologists are often paid well because health systems need them – but rather to the market economy of hiring physicians in specialty areas where there is demand.
Women are also chronically undervalued for the hours that we spend with patients. Data show that we spend more time with patients, which does not generate as many RVUs, but it generates better outcomes for patients. Evidence shows that women doctors in internal medicine and family medicine have better outcomes than doctors who are men.
On Jan. 1, 2021, Medicare and other payers implemented a new structure to reporting the level of office visit based on either medical decision-making or time spent on the date of encounter. Time spent with patients will now be rewarded – increased RVUs for increased time.
Part of the solution is value-based medicine and moving away from counting RVUs. This is also an opportunity to look at where time is spent in general ob.gyn. training and redistribute it, focusing on what trainees need for their education and not what hospitals need to service labor and delivery. We should step back and look creatively at optimizing the education and the training of ob.gyns., and where possible utilize other health care professionals such as nurse practitioners and midwives to address the uncomplicated obstetric needs of the hospital which could free up ob.gyn. trainees to obtain further surgical education.
To be clear, gender discrimination in compensation is prevalent and a persistent problem in medicine – ob.gyn. is no exception. Many ob.gyns. are employed by large health systems with payment structures and incentives that don’t align with those of the physician or the patient. There is definite misalignment in the way salaries are determined. Transparency on salaries is a critical component of addressing the pay gap that exists between women and men in medicine and in other industries.
The pay gap as it relates to reimbursement for gynecologic surgery, however, is a more complex matter that relates to how the RBRVS system was developed nearly 30 years ago when gynecologic surgery was not predominantly performed by women.
Dr. Levy is a voluntary clinical professor in the department of obstetrics, gynecology, and reproductive sciences at University of California San Diego Health, the former vice president of health policy at ACOG, past chair of the AMA/RUC, and current voting member of the AMA CPT editorial panel. She reported no relevant financial disclosures.
In a recent commentary published in Obstetrics & Gynecology, Katie L. Watson, JD, and Louise P. King, MD, JD, describe the issue of “double discrimination” in gynecologic surgery. The authors outlined how lower pay in a specialty where a majority of the surgeons and all of the patients are women may impact quality of care.
The commentary raises a number of concerns in gynecologic surgery that are important to discuss. Ob.gyn. as a whole is underpaid, as are many nonprocedural specialties such as family medicine and internal medicine. When ob.gyns. were predominantly men, the same situation existed – ob.gyns. were paid less than many other procedural specialties. While we’ve come a long way from the relative value unit (RVU) originally determined from the Harvard studies 30 years ago, there is room for additional improvement.
Several rationales were proposed by the authors to explain the disparities in pay between gynecologic surgery and those in urology: patient gender, surgeon gender, and length of training for gynecologic surgeons. The authors cited comparisons between urology and gynecology regarding “anatomically similar, sex-specific procedures” which require closer examination. Many of the code pairs selected were not actually comparable services. For example, management of Peyronie’s disease is a highly complex treatment performed by urologists that is not comparable with vaginectomy, yet this is an example of two codes used in the reference cited by the authors to conclude that surgeries on women are undervalued.
The overall RVUs for a procedure are also dependent upon the global period. The Centers for Medicare & Medicaid Services designated RVUs as the total amount of work before, during, and after a procedure. If a surgery has a 90-day global period, all the work for 90 days thereafter is bundled into the value, whereas if something is a zero-day global, only that day’s work is counted. A gynecologic surgeon who sees a patient back two or three times is coding and billing for those encounters in addition to that initial procedure.
Many of the code comparisons used in the analysis of gender in RVUs compared services with different global periods. Finally, some of the services that were compared had vastly different utilization. Some of the services and codes that were compared are performed extremely rarely and for that reason have not had their values reassessed over the years. There may be inequities in the RVUs for these services, but they will account for extremely little in overall compensation.
As a former chair of the American Medical Association’s RVS Update Committee (RUC), I spent years attempting to revalue ob.gyn. procedures. CMS assigns work RVUs based on physician work, practice expense, and professional liability insurance. The work is calculated using total physician time and intensity based on surveys completed by the specialty. The American College of Obstetrician and Gynecologist’s Committee on Health Economics and Coding, and the AMA RUC have worked diligently over many years to reassess potentially misvalued services. The ultimate RVUs assigned by CMS for gynecologic surgery are determined by the surveys completed by ACOG members. One issue we encountered with reexamining some procedures under RBRVS is that they have become so low volume that it has been difficult to justify the cost and effort to revalue them.
Lack of ob.gyn. training isn’t the full story
On average, ob.gyns. have between 18 and 24 months of surgical training, which is significantly less than other specialties. Lack of training in gynecologic surgery was proposed as another explanation for reduced compensation among female gynecologic surgeons. This is a complex issue not adequately explained by training time for gynecologic surgeons alone. While the number of trained ob.gyns. has increased in recent decades, the surgical volume has diminished and the workload of gynecologic surgery is far lower than it used to be. Surgical volume during and after training was much higher 35 years ago, prior to the advancements of procedures like endometrial ablation or tubal ligation. Women who had finished childbearing often underwent vaginal hysterectomies to manage contraception along with various other conditions.
With the advent of minimally invasive surgery, laparoscopic sterilization became possible, which has reduced the number of hysterectomies performed. Endometrial ablation is an office-based, noninvasive procedure. The development of the levonorgestrel IUD has helped manage abnormal bleeding, further reducing the need for hysterectomy.
This reduction in surgical volume does have an impact on quality of care. The model of tracking surgical outcomes at Kaiser Health System, as mentioned by the authors, could work well in some, but not all centers. A more approachable solution to address surgical volume for the average ob.gyn. would be to implement a mentoring and coaching process whereby recently trained ob.gyns. assist their senior partner(s) in surgery. This was the model years ago: I was trained by an ob.gyn. who was trained as a general surgeon. It was through the experience of assisting on each one of his cases – and him assisting on each one of my cases – that I received incredibly thorough surgical training.
These changes in practice, however, do not impact reimbursement. Rather than discrimination based on the gender of the surgeon, lower salaries in ob.gyn. are more likely to be the result of these and other factors.
The wage and quality gap in ob.gyn.
As a predominantly female surgical specialty, some of the disparity between gynecology and urology could be explained by how each specialty values its work. Here, gender plays a role in that when ob.gyns. are surveyed during the RUC process they may undervalue their work by reporting they can perform a procedure (and the before and after care) faster than what a urologist reports. The survey results may then result in lower RVUs.
Ob.gyn. is an overpopulated specialty for the number of surgeons needed to manage the volume of gynecologic surgery. When a health system wants to hire a general ob.gyn., it doesn’t have trouble finding one, while urologists are more challenging to recruit. This is not because of the structure of resource-based relative value scale (RBRVS) – despite the overall RVUs for gynecologic surgery, gynecologic oncologists are often paid well because health systems need them – but rather to the market economy of hiring physicians in specialty areas where there is demand.
Women are also chronically undervalued for the hours that we spend with patients. Data show that we spend more time with patients, which does not generate as many RVUs, but it generates better outcomes for patients. Evidence shows that women doctors in internal medicine and family medicine have better outcomes than doctors who are men.
On Jan. 1, 2021, Medicare and other payers implemented a new structure to reporting the level of office visit based on either medical decision-making or time spent on the date of encounter. Time spent with patients will now be rewarded – increased RVUs for increased time.
Part of the solution is value-based medicine and moving away from counting RVUs. This is also an opportunity to look at where time is spent in general ob.gyn. training and redistribute it, focusing on what trainees need for their education and not what hospitals need to service labor and delivery. We should step back and look creatively at optimizing the education and the training of ob.gyns., and where possible utilize other health care professionals such as nurse practitioners and midwives to address the uncomplicated obstetric needs of the hospital which could free up ob.gyn. trainees to obtain further surgical education.
To be clear, gender discrimination in compensation is prevalent and a persistent problem in medicine – ob.gyn. is no exception. Many ob.gyns. are employed by large health systems with payment structures and incentives that don’t align with those of the physician or the patient. There is definite misalignment in the way salaries are determined. Transparency on salaries is a critical component of addressing the pay gap that exists between women and men in medicine and in other industries.
The pay gap as it relates to reimbursement for gynecologic surgery, however, is a more complex matter that relates to how the RBRVS system was developed nearly 30 years ago when gynecologic surgery was not predominantly performed by women.
Dr. Levy is a voluntary clinical professor in the department of obstetrics, gynecology, and reproductive sciences at University of California San Diego Health, the former vice president of health policy at ACOG, past chair of the AMA/RUC, and current voting member of the AMA CPT editorial panel. She reported no relevant financial disclosures.
In a recent commentary published in Obstetrics & Gynecology, Katie L. Watson, JD, and Louise P. King, MD, JD, describe the issue of “double discrimination” in gynecologic surgery. The authors outlined how lower pay in a specialty where a majority of the surgeons and all of the patients are women may impact quality of care.
The commentary raises a number of concerns in gynecologic surgery that are important to discuss. Ob.gyn. as a whole is underpaid, as are many nonprocedural specialties such as family medicine and internal medicine. When ob.gyns. were predominantly men, the same situation existed – ob.gyns. were paid less than many other procedural specialties. While we’ve come a long way from the relative value unit (RVU) originally determined from the Harvard studies 30 years ago, there is room for additional improvement.
Several rationales were proposed by the authors to explain the disparities in pay between gynecologic surgery and those in urology: patient gender, surgeon gender, and length of training for gynecologic surgeons. The authors cited comparisons between urology and gynecology regarding “anatomically similar, sex-specific procedures” which require closer examination. Many of the code pairs selected were not actually comparable services. For example, management of Peyronie’s disease is a highly complex treatment performed by urologists that is not comparable with vaginectomy, yet this is an example of two codes used in the reference cited by the authors to conclude that surgeries on women are undervalued.
The overall RVUs for a procedure are also dependent upon the global period. The Centers for Medicare & Medicaid Services designated RVUs as the total amount of work before, during, and after a procedure. If a surgery has a 90-day global period, all the work for 90 days thereafter is bundled into the value, whereas if something is a zero-day global, only that day’s work is counted. A gynecologic surgeon who sees a patient back two or three times is coding and billing for those encounters in addition to that initial procedure.
Many of the code comparisons used in the analysis of gender in RVUs compared services with different global periods. Finally, some of the services that were compared had vastly different utilization. Some of the services and codes that were compared are performed extremely rarely and for that reason have not had their values reassessed over the years. There may be inequities in the RVUs for these services, but they will account for extremely little in overall compensation.
As a former chair of the American Medical Association’s RVS Update Committee (RUC), I spent years attempting to revalue ob.gyn. procedures. CMS assigns work RVUs based on physician work, practice expense, and professional liability insurance. The work is calculated using total physician time and intensity based on surveys completed by the specialty. The American College of Obstetrician and Gynecologist’s Committee on Health Economics and Coding, and the AMA RUC have worked diligently over many years to reassess potentially misvalued services. The ultimate RVUs assigned by CMS for gynecologic surgery are determined by the surveys completed by ACOG members. One issue we encountered with reexamining some procedures under RBRVS is that they have become so low volume that it has been difficult to justify the cost and effort to revalue them.
Lack of ob.gyn. training isn’t the full story
On average, ob.gyns. have between 18 and 24 months of surgical training, which is significantly less than other specialties. Lack of training in gynecologic surgery was proposed as another explanation for reduced compensation among female gynecologic surgeons. This is a complex issue not adequately explained by training time for gynecologic surgeons alone. While the number of trained ob.gyns. has increased in recent decades, the surgical volume has diminished and the workload of gynecologic surgery is far lower than it used to be. Surgical volume during and after training was much higher 35 years ago, prior to the advancements of procedures like endometrial ablation or tubal ligation. Women who had finished childbearing often underwent vaginal hysterectomies to manage contraception along with various other conditions.
With the advent of minimally invasive surgery, laparoscopic sterilization became possible, which has reduced the number of hysterectomies performed. Endometrial ablation is an office-based, noninvasive procedure. The development of the levonorgestrel IUD has helped manage abnormal bleeding, further reducing the need for hysterectomy.
This reduction in surgical volume does have an impact on quality of care. The model of tracking surgical outcomes at Kaiser Health System, as mentioned by the authors, could work well in some, but not all centers. A more approachable solution to address surgical volume for the average ob.gyn. would be to implement a mentoring and coaching process whereby recently trained ob.gyns. assist their senior partner(s) in surgery. This was the model years ago: I was trained by an ob.gyn. who was trained as a general surgeon. It was through the experience of assisting on each one of his cases – and him assisting on each one of my cases – that I received incredibly thorough surgical training.
These changes in practice, however, do not impact reimbursement. Rather than discrimination based on the gender of the surgeon, lower salaries in ob.gyn. are more likely to be the result of these and other factors.
The wage and quality gap in ob.gyn.
As a predominantly female surgical specialty, some of the disparity between gynecology and urology could be explained by how each specialty values its work. Here, gender plays a role in that when ob.gyns. are surveyed during the RUC process they may undervalue their work by reporting they can perform a procedure (and the before and after care) faster than what a urologist reports. The survey results may then result in lower RVUs.
Ob.gyn. is an overpopulated specialty for the number of surgeons needed to manage the volume of gynecologic surgery. When a health system wants to hire a general ob.gyn., it doesn’t have trouble finding one, while urologists are more challenging to recruit. This is not because of the structure of resource-based relative value scale (RBRVS) – despite the overall RVUs for gynecologic surgery, gynecologic oncologists are often paid well because health systems need them – but rather to the market economy of hiring physicians in specialty areas where there is demand.
Women are also chronically undervalued for the hours that we spend with patients. Data show that we spend more time with patients, which does not generate as many RVUs, but it generates better outcomes for patients. Evidence shows that women doctors in internal medicine and family medicine have better outcomes than doctors who are men.
On Jan. 1, 2021, Medicare and other payers implemented a new structure to reporting the level of office visit based on either medical decision-making or time spent on the date of encounter. Time spent with patients will now be rewarded – increased RVUs for increased time.
Part of the solution is value-based medicine and moving away from counting RVUs. This is also an opportunity to look at where time is spent in general ob.gyn. training and redistribute it, focusing on what trainees need for their education and not what hospitals need to service labor and delivery. We should step back and look creatively at optimizing the education and the training of ob.gyns., and where possible utilize other health care professionals such as nurse practitioners and midwives to address the uncomplicated obstetric needs of the hospital which could free up ob.gyn. trainees to obtain further surgical education.
To be clear, gender discrimination in compensation is prevalent and a persistent problem in medicine – ob.gyn. is no exception. Many ob.gyns. are employed by large health systems with payment structures and incentives that don’t align with those of the physician or the patient. There is definite misalignment in the way salaries are determined. Transparency on salaries is a critical component of addressing the pay gap that exists between women and men in medicine and in other industries.
The pay gap as it relates to reimbursement for gynecologic surgery, however, is a more complex matter that relates to how the RBRVS system was developed nearly 30 years ago when gynecologic surgery was not predominantly performed by women.
Dr. Levy is a voluntary clinical professor in the department of obstetrics, gynecology, and reproductive sciences at University of California San Diego Health, the former vice president of health policy at ACOG, past chair of the AMA/RUC, and current voting member of the AMA CPT editorial panel. She reported no relevant financial disclosures.
2019 Update on female sexual dysfunction
Hypoactive sexual desire disorder (HSDD) is the most prevalent sexual health problem in women of all ages, with population-based studies showing that about 36% to 39% of women report low sexual desire, and 8% to 10% meet the diagnostic criteria of low sexual desire and associated distress.1,2 An expanded definition of HSDD may include3:
- lack of motivation for sexual activity (reduced or absent spontaneous desire or responsive desire to erotic cues and stimulation; inability to maintain desire or interest through sexual activity)
- loss of desire to initiate or participate in sexual activity (including avoiding situations that could lead to sexual activity) combined with significant personal distress (frustration, loss, sadness, worry) (FIGURE).4
Despite the high prevalence of HSDD, patients often are uncomfortable and reluctant to voice concerns about low sexual desire to their ObGyn. Further, clinicians may feel ill equipped to diagnose and treat patients with HSDD. ObGyns, however, are well positioned to initiate a general discussion about sexual concerns with patients and use screening tools, such as the Decreased Sexual Desire Screener (DSDS), to facilitate a discussion and clarify a diagnosis of generalized acquired HSDD (TABLES 1 and 2).5 Helpful guidance on HSDD is available from the American College of Obstetricians and Gynecologists and the International Society for the Study of Women’s Sexual Health.6-8
Importantly, clinicians have a new treatment option they can offer to patients with HSDD. Bremelanotide was approved by the US Food and Drug Administration (FDA) on June 21, 2019, to treat acquired, generalized HSDD in premenopausal women. Up until this approval, flibanserin (approved in 2015) was the only drug FDA approved for the treatment of HSDD.
Assessing and treating HSDD today can be likened to managing depression 30 years ago, before selective serotonin receptor inhibitors were available. ObGyns would refer patients with depression to other health care providers, or not even ask patients about depressive symptoms because we had so little to offer. Once safe and effective antidepressants became available, knowing we could provide pharmacologic options made inquiring about depressive symptoms and the use of screening tools more readily incorporated into standard clinical practice. Depression is now recognized as a medical condition with biologic underpinnings, just like HSDD, and treatment options are available for both disorders.
For this Update, I had the opportunity to discuss the clinical trial experience with bremelanotide for HSDD with Dr. Sheryl Kingsberg, including efficacy and safety, dosage and administration, contraindications, and adverse events. She also details an ideal patient for treatment with bremelanotide, and we review pertinent aspects of flibanserin for comparative purposes.

Bremelanotide: A new therapeutic option
According to the product labeling for bremelanotide, the drug is indicated for the treatment of premenopausal women with acquired, generalized HSDD (low sexual desire that causes marked distress or interpersonal difficulty).9 This means that the HSDD developed in a woman who previously did not have problems with sexual desire, and that it occurred regardless of the type of stimulation, situation, or partner. In addition, the HSDD should not result from a coexisting medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug substance.
Flibanserin also is indicated for the treatment of premenopausal women with HSDD. While both bremelanotide and flibanserin have indications only for premenopausal women, 2 studies of flibanserin in postmenopausal women have been published.10,11 Results from these studies in naturally menopausal women suggest that flibanserin may be efficacious in this population, with improvement in sexual desire, reduced distress associated with low desire, and improvement in the number of satisfying sexual events (SSEs).
No trials of bremelanotide in postmenopausal women have been published, but since this drug acts on central nervous system receptors, as does flibanserin, it may have similar effectiveness in postmenopausal women as well.

Continue to: Clinical trials show bremelanotide improves desire, reduces distress...
Clinical trials show bremelanotide improves desire, reduces distress
Two phase 3 clinical trials, dubbed the Reconnect studies, demonstrated that, compared with placebo, bremelanotide was associated with statistically significant improvements in sexual desire and levels of distress regarding sexual desire.
The 2 identical, randomized, placebo-controlled multicenter trials included 1,247 premenopausal women with HSDD of at least 6 months' duration.9,12 Bremelanotide 1.75 mg (or placebo) was self-administered subcutaneously with an autoinjector on an as-desired basis. The 24-week double-blind treatment period was followed by a 52-week open-label extension study.
The co-primary efficacy end points were the change from baseline to end-of-study (week 24 of the double-blind treatment period) in the 1) Female Sexual Function Index (FSFI) desire domain score and 2) feeling bothered by low sexual desire as measured by Question 13 on the Female Sexual Distress Scale (FSDS). An increase in the FSFI desire domain score over time denotes improvement in sexual desire, while a decrease in the FSDS Question 13 score over time indicates improvement in the level of distress associated with low sexual desire.
In the 2 clinical studies, the mean change from baseline (SD) in the FSFI desire domain score, which ranged from 1.2 to 6.0 at study outset (higher scores indicate greater desire), was:
- study 1: 0.5 (1.1) in the bremelanotide-treated women and 0.2 (1.0) in the placebo-treated women (P = .0002)
- study 2: 0.6 (1.0) in the bremelanotide group versus 0.2 (0.9) in the placebo group (P<.0001).
For FSDS Question 13, for which the score range was 0 to 4 (higher scores indicate greater bother), the mean change from baseline score was:
- study 1: -0.7 (1.2) in the bremelanotide-treated group compared with -0.4 (1.1) in the placebo-treated group (P<.0001)
- study 2: -0.7 (1.1) in the bremelanotide group and -0.4 (1.1) in the placebo group (P = .0053).
It should be noted that, in the past, SSEs were used as a primary end point in clinical studies. However, we have shifted from SSEs to desire and distress as an end point because SSEs have little to do with desire. Women worry about and are distressed by the fact that they no longer have sexual appetite. They no longer "want to want" even though their body will be responsive and they can have an orgasm. That is exemplified by the woman in our case scenario (see box, page 18), who very much wants the experience of being able to anticipate with pleasure the idea of having an enjoyable connection with her partner.
Continue to: Physiologic target: The melanocortin receptor...
Physiologic target: The melanocortin receptor
Bremelanotide's theorized mechanism of action is that it works to rebalance neurotransmitters that are implicated in causing HSDD, acting as an agonist on the melanocortin receptor to promote dopamine release and allow women to perceive sexual cues as rewarding. They can then respond to those cues the way they used to and therefore experience desire. Flibanserin has affinity for serotonin (5-hydroxytryptamine [5-HT]) receptors, with agonist and antagonist activity, as well as moderate antagonist activity on some dopamine receptors.
The bottom line is that we now have treatments to address the underlying biologic aspect of HSDD, which is a biopsychosocial disorder. Again, this has parallels to depression and its biologic mechanism, for which we have effective treatments.
Dosing is an as-needed injection
Unlike the daily nighttime oral dose required with flibanserin, bremelanotide is a 1.75-mg dose administered as a subcutaneous injection (in either the thigh or the abdomen) with a pen-like autoinjector, on an as-needed basis. It should be administered at least 45 minutes before anticipated sexual activity. That is a benefit for many women who do not want to take a daily pill when they know that their "desire to desire" may be once per week or once every other week.
Regarding the drug delivery mode, nobody dropped out of the bremelanotide clinical trials because of having to take an injection with an autoinjector, which employs a very thin needle and is virtually painless. A small number of bremelanotide-treated women, about 13%, had injection site reactions (compared with 8% in the placebo group), which is common with subcutaneous injection. Even in the phase 2 clinical trial, in which a syringe was used to administer the drug, no participants discontinued the study because of the injection mode.
There are no clear pharmacokinetic data on how long bremelanotide's effects last, but it may be anywhere from 8 to 16 hours. Patients should not take more than 1 dose within 24 hours--but since the effect may last up to 16 hours that should not be a problem--and use of more than 8 doses per month is not recommended.
While bremelanotide improves desire, certainly better than placebo, there is also some peripheral improvement in arousal, although women in the trials had only HSDD. We do not know whether bremelanotide would treat arousal disorder, but it will help women with or without arousal difficulties associated with their HSDD, as shown in a subgroup analysis in the trials.13
Counsel patients on treatment potentialities
Clinicians should be aware of several precautions with bremelanotide use.
Blood pressure increases. After each dose of bremelanotide, transient increases in blood pressure (6 mm Hg in systolic and 3 mm Hg in diastolic blood pressure) and reductions in heart rate (up to 5 beats per minute) occur; these measurements return to baseline usually within 12 hours postdose.9 When you think about whether having sexual desire will increase blood pressure, this may be physiologic. It is similar to walking up a flight of stairs.
The drug is not recommended, however, for use in patients at high risk for cardiovascular disease, and it is contraindicated in women with uncontrolled hypertension or known cardiovascular disease. Blood pressure should be well controlled before bremelanotide is initiated--use of antihypertensive agents is not contraindicated with bremelanotide as the drugs do not interact.
Clinicians are not required to participate in a Risk Evaluation and Mitigation Strategy (REMS) program to prescribe bremelanotide as they are with flibanserin (because of the increased risk of severe hypotension and syncope due to flibanserin's interaction with alcohol).
Drug interactions. Bremelanotide is a melanocortin receptor agonist--a unique compound. Antidepressants, other psychoactive medications, and oral contraceptives are not contraindicated with bremelanotide as there are no known interactions. Alcohol use also is not a contraindication or caution, in contrast to flibanserin. (In April, the FDA issued a labeling change order for flibanserin, specifying that alcohol does not have to be avoided completely when taking flibanserin, but that women should discontinue drinking alcohol at least 2 hours before taking the drug at bedtime, or skip the flibanserin dose that evening.14) Bremelanotide may slow gastric emptying, though, so when a patient is taking oral drugs that require threshold concentrations for efficacy, such as antibiotics, they should avoid bremelanotide. In addition, some drugs, such as indomethacin, may have a delayed onset of action with concomitant bremelanotide use.9
Importantly, patients should avoid using bremelanotide if they are taking an oral naltrexone product for treatment of alcohol or opioid addiction, because bremelanotide may decrease systemic exposure of oral naltrexone. That would potentially lead to naltrexone treatment failure and its consequences.9
Skin pigmentation changes. Hyperpigmentation occurred with bremelanotide use on the face, gingiva, and breasts, as reported in the clinical trials, in 1% of treated patients who received up to 8 doses per month, compared with no such occurrences in placebo-treated patients. In addition, 38% of patients who received bremelanotide daily for 8 days developed focal hyperpigmentation. It was not confirmed in all patients whether the hyperpigmentation resolved. Women with dark skin were more likely to develop hyperpigmentation.9
Common adverse reactions. The most common adverse reactions with bremelanotide treatment are nausea, flushing, injection site reactions, and headache, with most events being mild to moderate in intensity. In the clinical trials, 40% of the bremelanotide-treated women experienced nausea (compared with 1% of placebo-treated women), with most occurrences being mild; for most participants nausea improved with the second dose. Women had nausea that either went away or was intermittent, or it was mild enough that the drug benefits outweighed the tolerability costs--of women who experienced nausea, 92% continued in the trial, and 8% dropped out because of nausea.9
The following scenario describes the experience of HSDD in one of Dr. Kingsberg's patients.
CASE Woman avoids sex because of low desire; marriage is suffering
A 40-year-old woman, Sandra, who has been married for 19 years and has fraternal twins aged 8, presented to the behavioral medicine clinic with distressing symptoms of low sexual desire. For several years into the marriage the patient experienced excellent sex drive. After 6 to 7 years, she noticed that her desire had declined and that she was starting to avoid sex. She was irritated when her husband initiated sex, and she would make excuses as to why it was not the right time.
Her husband felt hurt, frustrated, and rejected. The couple was close to divorce because he was angry and resentful. Sandra recognized there was a problem but did not know how to fix it. She could not understand why her interest had waned since she still loved her husband and considered him objectively very attractive.
Sandra came to see Dr. Kingsberg at the behavioral medicine clinic. Using the 5-item validated diagnostic tool called the Decreased Sexual Desire Screener, Dr. Kingsberg diagnosed hypoactive sexual desire disorder (HSDD), a term Sandra had never heard of and did not know was a condition. The patient was relieved to know that she was one of several million women affected by HSDD and that the problem was not just that she was a "bad wife" or that she had some kind of psychological block. She emphasized how much she loved her husband and how she wanted desperately to "want to want desire," as she recalled feeling previously.
Sandra was treated with counseling and psychotherapy in which we addressed the relationship issues, the avoidance of sex, the comfort with being sexual, and the recognition that responsive desire can be helpful (as she was able to have arousal and orgasm and have a satisfying sexual event). The issue was that she had no motivation to seek out sex and had no interest in experiencing that pleasure. In subsequent couple's therapy, the husband recognized that his wife was not intentionally rejecting him, but that she had a real medical condition.
Although Sandra's relationship was now more stable and she and her husband were both working toward finding a solution to Sandra's loss of desire, she was still very distressed by her lack of desire. Sandra tried flibanserin for 3 months but unfortunately did not respond. Sandra heard about the recent approval of bremelanotide and is looking forward to the drug being available so that she can try it.
Final considerations
Asking patients about sexual function and using sexual function screening tools can help clinicians identify patients with the decreased sexual desire and associated distress characteristic of HSDD. ObGyns are the appropriate clinicians to treat these women and soon will have 2 pharmacologic options--bremelanotide (anticipated to be available in Fall 2019) and flibanserin--to offer patients with this biopsychosocial disorder that can adversely impact well-being and quality of life. Clinicians should individualize treatment, which may include psychotherapeutic counseling, and counsel patients on appropriate drug use and potential adverse effects.
AMAG Pharmaceuticals, Inc. has announced that they will have a copay assistance program for bremelanotide, where the first prescription of four autoinjectors will be a $0 copay, followed by a $99 copay or less for refills.15
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- West SL, D'Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441-1449.
- Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions: part II. J Sex Med. 2016;13:1888-1906.
- Basson R. Using a different model for female sexual response to address women's problematic low sexual desire. J Sex Marital Ther. 2001;27:395-403.
- Clayton AH, Goldfischer ER, Goldstein I, et al. Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med. 2009;6:730-738.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction. Obstet Gynecol. 2019;134:e1-e18.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114-128.
- Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women's Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin Proc. 2018;93:467-487.
- Vyleesi [package insert]. Waltham, MA: AMAG Pharmaceuticals; 2019.
- Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21;633-640.
- Portman DJ, Brown L, Yuan, et al. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834-842.
- Kingsberg SA, Clayton AH, Portman D, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol. Forthcoming.
- Clayton AH, Lucas J, Jordon R, et al. Efficacy of the Investigational drug bremelanotide in the Reconnect studies. Poster presented at: 30th ECNP Congress of Applied and Translational Neuroscience; September 2-5, 2017, Paris, France.
- US Food and Drug Administration. FDA orders important safety labeling changes for Addyi [press release]. April 11, 2019. https://www.fda.gov/news-events/press-announcements/fda-orders-important-safety-labeling-changes-addyi. Accessed July 17, 2019.
- Vyleesi website (https://vyleesipro.com). Accessed August 5, 2019.
Hypoactive sexual desire disorder (HSDD) is the most prevalent sexual health problem in women of all ages, with population-based studies showing that about 36% to 39% of women report low sexual desire, and 8% to 10% meet the diagnostic criteria of low sexual desire and associated distress.1,2 An expanded definition of HSDD may include3:
- lack of motivation for sexual activity (reduced or absent spontaneous desire or responsive desire to erotic cues and stimulation; inability to maintain desire or interest through sexual activity)
- loss of desire to initiate or participate in sexual activity (including avoiding situations that could lead to sexual activity) combined with significant personal distress (frustration, loss, sadness, worry) (FIGURE).4

Despite the high prevalence of HSDD, patients often are uncomfortable and reluctant to voice concerns about low sexual desire to their ObGyn. Further, clinicians may feel ill equipped to diagnose and treat patients with HSDD. ObGyns, however, are well positioned to initiate a general discussion about sexual concerns with patients and use screening tools, such as the Decreased Sexual Desire Screener (DSDS), to facilitate a discussion and clarify a diagnosis of generalized acquired HSDD (TABLES 1 and 2).5 Helpful guidance on HSDD is available from the American College of Obstetricians and Gynecologists and the International Society for the Study of Women’s Sexual Health.6-8
Importantly, clinicians have a new treatment option they can offer to patients with HSDD. Bremelanotide was approved by the US Food and Drug Administration (FDA) on June 21, 2019, to treat acquired, generalized HSDD in premenopausal women. Up until this approval, flibanserin (approved in 2015) was the only drug FDA approved for the treatment of HSDD.
Assessing and treating HSDD today can be likened to managing depression 30 years ago, before selective serotonin receptor inhibitors were available. ObGyns would refer patients with depression to other health care providers, or not even ask patients about depressive symptoms because we had so little to offer. Once safe and effective antidepressants became available, knowing we could provide pharmacologic options made inquiring about depressive symptoms and the use of screening tools more readily incorporated into standard clinical practice. Depression is now recognized as a medical condition with biologic underpinnings, just like HSDD, and treatment options are available for both disorders.
For this Update, I had the opportunity to discuss the clinical trial experience with bremelanotide for HSDD with Dr. Sheryl Kingsberg, including efficacy and safety, dosage and administration, contraindications, and adverse events. She also details an ideal patient for treatment with bremelanotide, and we review pertinent aspects of flibanserin for comparative purposes.

Bremelanotide: A new therapeutic option
According to the product labeling for bremelanotide, the drug is indicated for the treatment of premenopausal women with acquired, generalized HSDD (low sexual desire that causes marked distress or interpersonal difficulty).9 This means that the HSDD developed in a woman who previously did not have problems with sexual desire, and that it occurred regardless of the type of stimulation, situation, or partner. In addition, the HSDD should not result from a coexisting medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug substance.
Flibanserin also is indicated for the treatment of premenopausal women with HSDD. While both bremelanotide and flibanserin have indications only for premenopausal women, 2 studies of flibanserin in postmenopausal women have been published.10,11 Results from these studies in naturally menopausal women suggest that flibanserin may be efficacious in this population, with improvement in sexual desire, reduced distress associated with low desire, and improvement in the number of satisfying sexual events (SSEs).
No trials of bremelanotide in postmenopausal women have been published, but since this drug acts on central nervous system receptors, as does flibanserin, it may have similar effectiveness in postmenopausal women as well.

Continue to: Clinical trials show bremelanotide improves desire, reduces distress...
Clinical trials show bremelanotide improves desire, reduces distress
Two phase 3 clinical trials, dubbed the Reconnect studies, demonstrated that, compared with placebo, bremelanotide was associated with statistically significant improvements in sexual desire and levels of distress regarding sexual desire.
The 2 identical, randomized, placebo-controlled multicenter trials included 1,247 premenopausal women with HSDD of at least 6 months' duration.9,12 Bremelanotide 1.75 mg (or placebo) was self-administered subcutaneously with an autoinjector on an as-desired basis. The 24-week double-blind treatment period was followed by a 52-week open-label extension study.
The co-primary efficacy end points were the change from baseline to end-of-study (week 24 of the double-blind treatment period) in the 1) Female Sexual Function Index (FSFI) desire domain score and 2) feeling bothered by low sexual desire as measured by Question 13 on the Female Sexual Distress Scale (FSDS). An increase in the FSFI desire domain score over time denotes improvement in sexual desire, while a decrease in the FSDS Question 13 score over time indicates improvement in the level of distress associated with low sexual desire.
In the 2 clinical studies, the mean change from baseline (SD) in the FSFI desire domain score, which ranged from 1.2 to 6.0 at study outset (higher scores indicate greater desire), was:
- study 1: 0.5 (1.1) in the bremelanotide-treated women and 0.2 (1.0) in the placebo-treated women (P = .0002)
- study 2: 0.6 (1.0) in the bremelanotide group versus 0.2 (0.9) in the placebo group (P<.0001).
For FSDS Question 13, for which the score range was 0 to 4 (higher scores indicate greater bother), the mean change from baseline score was:
- study 1: -0.7 (1.2) in the bremelanotide-treated group compared with -0.4 (1.1) in the placebo-treated group (P<.0001)
- study 2: -0.7 (1.1) in the bremelanotide group and -0.4 (1.1) in the placebo group (P = .0053).
It should be noted that, in the past, SSEs were used as a primary end point in clinical studies. However, we have shifted from SSEs to desire and distress as an end point because SSEs have little to do with desire. Women worry about and are distressed by the fact that they no longer have sexual appetite. They no longer "want to want" even though their body will be responsive and they can have an orgasm. That is exemplified by the woman in our case scenario (see box, page 18), who very much wants the experience of being able to anticipate with pleasure the idea of having an enjoyable connection with her partner.
Continue to: Physiologic target: The melanocortin receptor...
Physiologic target: The melanocortin receptor
Bremelanotide's theorized mechanism of action is that it works to rebalance neurotransmitters that are implicated in causing HSDD, acting as an agonist on the melanocortin receptor to promote dopamine release and allow women to perceive sexual cues as rewarding. They can then respond to those cues the way they used to and therefore experience desire. Flibanserin has affinity for serotonin (5-hydroxytryptamine [5-HT]) receptors, with agonist and antagonist activity, as well as moderate antagonist activity on some dopamine receptors.
The bottom line is that we now have treatments to address the underlying biologic aspect of HSDD, which is a biopsychosocial disorder. Again, this has parallels to depression and its biologic mechanism, for which we have effective treatments.
Dosing is an as-needed injection
Unlike the daily nighttime oral dose required with flibanserin, bremelanotide is a 1.75-mg dose administered as a subcutaneous injection (in either the thigh or the abdomen) with a pen-like autoinjector, on an as-needed basis. It should be administered at least 45 minutes before anticipated sexual activity. That is a benefit for many women who do not want to take a daily pill when they know that their "desire to desire" may be once per week or once every other week.
Regarding the drug delivery mode, nobody dropped out of the bremelanotide clinical trials because of having to take an injection with an autoinjector, which employs a very thin needle and is virtually painless. A small number of bremelanotide-treated women, about 13%, had injection site reactions (compared with 8% in the placebo group), which is common with subcutaneous injection. Even in the phase 2 clinical trial, in which a syringe was used to administer the drug, no participants discontinued the study because of the injection mode.
There are no clear pharmacokinetic data on how long bremelanotide's effects last, but it may be anywhere from 8 to 16 hours. Patients should not take more than 1 dose within 24 hours--but since the effect may last up to 16 hours that should not be a problem--and use of more than 8 doses per month is not recommended.
While bremelanotide improves desire, certainly better than placebo, there is also some peripheral improvement in arousal, although women in the trials had only HSDD. We do not know whether bremelanotide would treat arousal disorder, but it will help women with or without arousal difficulties associated with their HSDD, as shown in a subgroup analysis in the trials.13
Counsel patients on treatment potentialities
Clinicians should be aware of several precautions with bremelanotide use.
Blood pressure increases. After each dose of bremelanotide, transient increases in blood pressure (6 mm Hg in systolic and 3 mm Hg in diastolic blood pressure) and reductions in heart rate (up to 5 beats per minute) occur; these measurements return to baseline usually within 12 hours postdose.9 When you think about whether having sexual desire will increase blood pressure, this may be physiologic. It is similar to walking up a flight of stairs.
The drug is not recommended, however, for use in patients at high risk for cardiovascular disease, and it is contraindicated in women with uncontrolled hypertension or known cardiovascular disease. Blood pressure should be well controlled before bremelanotide is initiated--use of antihypertensive agents is not contraindicated with bremelanotide as the drugs do not interact.
Clinicians are not required to participate in a Risk Evaluation and Mitigation Strategy (REMS) program to prescribe bremelanotide as they are with flibanserin (because of the increased risk of severe hypotension and syncope due to flibanserin's interaction with alcohol).
Drug interactions. Bremelanotide is a melanocortin receptor agonist--a unique compound. Antidepressants, other psychoactive medications, and oral contraceptives are not contraindicated with bremelanotide as there are no known interactions. Alcohol use also is not a contraindication or caution, in contrast to flibanserin. (In April, the FDA issued a labeling change order for flibanserin, specifying that alcohol does not have to be avoided completely when taking flibanserin, but that women should discontinue drinking alcohol at least 2 hours before taking the drug at bedtime, or skip the flibanserin dose that evening.14) Bremelanotide may slow gastric emptying, though, so when a patient is taking oral drugs that require threshold concentrations for efficacy, such as antibiotics, they should avoid bremelanotide. In addition, some drugs, such as indomethacin, may have a delayed onset of action with concomitant bremelanotide use.9
Importantly, patients should avoid using bremelanotide if they are taking an oral naltrexone product for treatment of alcohol or opioid addiction, because bremelanotide may decrease systemic exposure of oral naltrexone. That would potentially lead to naltrexone treatment failure and its consequences.9
Skin pigmentation changes. Hyperpigmentation occurred with bremelanotide use on the face, gingiva, and breasts, as reported in the clinical trials, in 1% of treated patients who received up to 8 doses per month, compared with no such occurrences in placebo-treated patients. In addition, 38% of patients who received bremelanotide daily for 8 days developed focal hyperpigmentation. It was not confirmed in all patients whether the hyperpigmentation resolved. Women with dark skin were more likely to develop hyperpigmentation.9
Common adverse reactions. The most common adverse reactions with bremelanotide treatment are nausea, flushing, injection site reactions, and headache, with most events being mild to moderate in intensity. In the clinical trials, 40% of the bremelanotide-treated women experienced nausea (compared with 1% of placebo-treated women), with most occurrences being mild; for most participants nausea improved with the second dose. Women had nausea that either went away or was intermittent, or it was mild enough that the drug benefits outweighed the tolerability costs--of women who experienced nausea, 92% continued in the trial, and 8% dropped out because of nausea.9
The following scenario describes the experience of HSDD in one of Dr. Kingsberg's patients.
CASE Woman avoids sex because of low desire; marriage is suffering
A 40-year-old woman, Sandra, who has been married for 19 years and has fraternal twins aged 8, presented to the behavioral medicine clinic with distressing symptoms of low sexual desire. For several years into the marriage the patient experienced excellent sex drive. After 6 to 7 years, she noticed that her desire had declined and that she was starting to avoid sex. She was irritated when her husband initiated sex, and she would make excuses as to why it was not the right time.
Her husband felt hurt, frustrated, and rejected. The couple was close to divorce because he was angry and resentful. Sandra recognized there was a problem but did not know how to fix it. She could not understand why her interest had waned since she still loved her husband and considered him objectively very attractive.
Sandra came to see Dr. Kingsberg at the behavioral medicine clinic. Using the 5-item validated diagnostic tool called the Decreased Sexual Desire Screener, Dr. Kingsberg diagnosed hypoactive sexual desire disorder (HSDD), a term Sandra had never heard of and did not know was a condition. The patient was relieved to know that she was one of several million women affected by HSDD and that the problem was not just that she was a "bad wife" or that she had some kind of psychological block. She emphasized how much she loved her husband and how she wanted desperately to "want to want desire," as she recalled feeling previously.
Sandra was treated with counseling and psychotherapy in which we addressed the relationship issues, the avoidance of sex, the comfort with being sexual, and the recognition that responsive desire can be helpful (as she was able to have arousal and orgasm and have a satisfying sexual event). The issue was that she had no motivation to seek out sex and had no interest in experiencing that pleasure. In subsequent couple's therapy, the husband recognized that his wife was not intentionally rejecting him, but that she had a real medical condition.
Although Sandra's relationship was now more stable and she and her husband were both working toward finding a solution to Sandra's loss of desire, she was still very distressed by her lack of desire. Sandra tried flibanserin for 3 months but unfortunately did not respond. Sandra heard about the recent approval of bremelanotide and is looking forward to the drug being available so that she can try it.
Final considerations
Asking patients about sexual function and using sexual function screening tools can help clinicians identify patients with the decreased sexual desire and associated distress characteristic of HSDD. ObGyns are the appropriate clinicians to treat these women and soon will have 2 pharmacologic options--bremelanotide (anticipated to be available in Fall 2019) and flibanserin--to offer patients with this biopsychosocial disorder that can adversely impact well-being and quality of life. Clinicians should individualize treatment, which may include psychotherapeutic counseling, and counsel patients on appropriate drug use and potential adverse effects.
AMAG Pharmaceuticals, Inc. has announced that they will have a copay assistance program for bremelanotide, where the first prescription of four autoinjectors will be a $0 copay, followed by a $99 copay or less for refills.15
Hypoactive sexual desire disorder (HSDD) is the most prevalent sexual health problem in women of all ages, with population-based studies showing that about 36% to 39% of women report low sexual desire, and 8% to 10% meet the diagnostic criteria of low sexual desire and associated distress.1,2 An expanded definition of HSDD may include3:
- lack of motivation for sexual activity (reduced or absent spontaneous desire or responsive desire to erotic cues and stimulation; inability to maintain desire or interest through sexual activity)
- loss of desire to initiate or participate in sexual activity (including avoiding situations that could lead to sexual activity) combined with significant personal distress (frustration, loss, sadness, worry) (FIGURE).4

Despite the high prevalence of HSDD, patients often are uncomfortable and reluctant to voice concerns about low sexual desire to their ObGyn. Further, clinicians may feel ill equipped to diagnose and treat patients with HSDD. ObGyns, however, are well positioned to initiate a general discussion about sexual concerns with patients and use screening tools, such as the Decreased Sexual Desire Screener (DSDS), to facilitate a discussion and clarify a diagnosis of generalized acquired HSDD (TABLES 1 and 2).5 Helpful guidance on HSDD is available from the American College of Obstetricians and Gynecologists and the International Society for the Study of Women’s Sexual Health.6-8
Importantly, clinicians have a new treatment option they can offer to patients with HSDD. Bremelanotide was approved by the US Food and Drug Administration (FDA) on June 21, 2019, to treat acquired, generalized HSDD in premenopausal women. Up until this approval, flibanserin (approved in 2015) was the only drug FDA approved for the treatment of HSDD.
Assessing and treating HSDD today can be likened to managing depression 30 years ago, before selective serotonin receptor inhibitors were available. ObGyns would refer patients with depression to other health care providers, or not even ask patients about depressive symptoms because we had so little to offer. Once safe and effective antidepressants became available, knowing we could provide pharmacologic options made inquiring about depressive symptoms and the use of screening tools more readily incorporated into standard clinical practice. Depression is now recognized as a medical condition with biologic underpinnings, just like HSDD, and treatment options are available for both disorders.
For this Update, I had the opportunity to discuss the clinical trial experience with bremelanotide for HSDD with Dr. Sheryl Kingsberg, including efficacy and safety, dosage and administration, contraindications, and adverse events. She also details an ideal patient for treatment with bremelanotide, and we review pertinent aspects of flibanserin for comparative purposes.

Bremelanotide: A new therapeutic option
According to the product labeling for bremelanotide, the drug is indicated for the treatment of premenopausal women with acquired, generalized HSDD (low sexual desire that causes marked distress or interpersonal difficulty).9 This means that the HSDD developed in a woman who previously did not have problems with sexual desire, and that it occurred regardless of the type of stimulation, situation, or partner. In addition, the HSDD should not result from a coexisting medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug substance.
Flibanserin also is indicated for the treatment of premenopausal women with HSDD. While both bremelanotide and flibanserin have indications only for premenopausal women, 2 studies of flibanserin in postmenopausal women have been published.10,11 Results from these studies in naturally menopausal women suggest that flibanserin may be efficacious in this population, with improvement in sexual desire, reduced distress associated with low desire, and improvement in the number of satisfying sexual events (SSEs).
No trials of bremelanotide in postmenopausal women have been published, but since this drug acts on central nervous system receptors, as does flibanserin, it may have similar effectiveness in postmenopausal women as well.

Continue to: Clinical trials show bremelanotide improves desire, reduces distress...
Clinical trials show bremelanotide improves desire, reduces distress
Two phase 3 clinical trials, dubbed the Reconnect studies, demonstrated that, compared with placebo, bremelanotide was associated with statistically significant improvements in sexual desire and levels of distress regarding sexual desire.
The 2 identical, randomized, placebo-controlled multicenter trials included 1,247 premenopausal women with HSDD of at least 6 months' duration.9,12 Bremelanotide 1.75 mg (or placebo) was self-administered subcutaneously with an autoinjector on an as-desired basis. The 24-week double-blind treatment period was followed by a 52-week open-label extension study.
The co-primary efficacy end points were the change from baseline to end-of-study (week 24 of the double-blind treatment period) in the 1) Female Sexual Function Index (FSFI) desire domain score and 2) feeling bothered by low sexual desire as measured by Question 13 on the Female Sexual Distress Scale (FSDS). An increase in the FSFI desire domain score over time denotes improvement in sexual desire, while a decrease in the FSDS Question 13 score over time indicates improvement in the level of distress associated with low sexual desire.
In the 2 clinical studies, the mean change from baseline (SD) in the FSFI desire domain score, which ranged from 1.2 to 6.0 at study outset (higher scores indicate greater desire), was:
- study 1: 0.5 (1.1) in the bremelanotide-treated women and 0.2 (1.0) in the placebo-treated women (P = .0002)
- study 2: 0.6 (1.0) in the bremelanotide group versus 0.2 (0.9) in the placebo group (P<.0001).
For FSDS Question 13, for which the score range was 0 to 4 (higher scores indicate greater bother), the mean change from baseline score was:
- study 1: -0.7 (1.2) in the bremelanotide-treated group compared with -0.4 (1.1) in the placebo-treated group (P<.0001)
- study 2: -0.7 (1.1) in the bremelanotide group and -0.4 (1.1) in the placebo group (P = .0053).
It should be noted that, in the past, SSEs were used as a primary end point in clinical studies. However, we have shifted from SSEs to desire and distress as an end point because SSEs have little to do with desire. Women worry about and are distressed by the fact that they no longer have sexual appetite. They no longer "want to want" even though their body will be responsive and they can have an orgasm. That is exemplified by the woman in our case scenario (see box, page 18), who very much wants the experience of being able to anticipate with pleasure the idea of having an enjoyable connection with her partner.
Continue to: Physiologic target: The melanocortin receptor...
Physiologic target: The melanocortin receptor
Bremelanotide's theorized mechanism of action is that it works to rebalance neurotransmitters that are implicated in causing HSDD, acting as an agonist on the melanocortin receptor to promote dopamine release and allow women to perceive sexual cues as rewarding. They can then respond to those cues the way they used to and therefore experience desire. Flibanserin has affinity for serotonin (5-hydroxytryptamine [5-HT]) receptors, with agonist and antagonist activity, as well as moderate antagonist activity on some dopamine receptors.
The bottom line is that we now have treatments to address the underlying biologic aspect of HSDD, which is a biopsychosocial disorder. Again, this has parallels to depression and its biologic mechanism, for which we have effective treatments.
Dosing is an as-needed injection
Unlike the daily nighttime oral dose required with flibanserin, bremelanotide is a 1.75-mg dose administered as a subcutaneous injection (in either the thigh or the abdomen) with a pen-like autoinjector, on an as-needed basis. It should be administered at least 45 minutes before anticipated sexual activity. That is a benefit for many women who do not want to take a daily pill when they know that their "desire to desire" may be once per week or once every other week.
Regarding the drug delivery mode, nobody dropped out of the bremelanotide clinical trials because of having to take an injection with an autoinjector, which employs a very thin needle and is virtually painless. A small number of bremelanotide-treated women, about 13%, had injection site reactions (compared with 8% in the placebo group), which is common with subcutaneous injection. Even in the phase 2 clinical trial, in which a syringe was used to administer the drug, no participants discontinued the study because of the injection mode.
There are no clear pharmacokinetic data on how long bremelanotide's effects last, but it may be anywhere from 8 to 16 hours. Patients should not take more than 1 dose within 24 hours--but since the effect may last up to 16 hours that should not be a problem--and use of more than 8 doses per month is not recommended.
While bremelanotide improves desire, certainly better than placebo, there is also some peripheral improvement in arousal, although women in the trials had only HSDD. We do not know whether bremelanotide would treat arousal disorder, but it will help women with or without arousal difficulties associated with their HSDD, as shown in a subgroup analysis in the trials.13
Counsel patients on treatment potentialities
Clinicians should be aware of several precautions with bremelanotide use.
Blood pressure increases. After each dose of bremelanotide, transient increases in blood pressure (6 mm Hg in systolic and 3 mm Hg in diastolic blood pressure) and reductions in heart rate (up to 5 beats per minute) occur; these measurements return to baseline usually within 12 hours postdose.9 When you think about whether having sexual desire will increase blood pressure, this may be physiologic. It is similar to walking up a flight of stairs.
The drug is not recommended, however, for use in patients at high risk for cardiovascular disease, and it is contraindicated in women with uncontrolled hypertension or known cardiovascular disease. Blood pressure should be well controlled before bremelanotide is initiated--use of antihypertensive agents is not contraindicated with bremelanotide as the drugs do not interact.
Clinicians are not required to participate in a Risk Evaluation and Mitigation Strategy (REMS) program to prescribe bremelanotide as they are with flibanserin (because of the increased risk of severe hypotension and syncope due to flibanserin's interaction with alcohol).
Drug interactions. Bremelanotide is a melanocortin receptor agonist--a unique compound. Antidepressants, other psychoactive medications, and oral contraceptives are not contraindicated with bremelanotide as there are no known interactions. Alcohol use also is not a contraindication or caution, in contrast to flibanserin. (In April, the FDA issued a labeling change order for flibanserin, specifying that alcohol does not have to be avoided completely when taking flibanserin, but that women should discontinue drinking alcohol at least 2 hours before taking the drug at bedtime, or skip the flibanserin dose that evening.14) Bremelanotide may slow gastric emptying, though, so when a patient is taking oral drugs that require threshold concentrations for efficacy, such as antibiotics, they should avoid bremelanotide. In addition, some drugs, such as indomethacin, may have a delayed onset of action with concomitant bremelanotide use.9
Importantly, patients should avoid using bremelanotide if they are taking an oral naltrexone product for treatment of alcohol or opioid addiction, because bremelanotide may decrease systemic exposure of oral naltrexone. That would potentially lead to naltrexone treatment failure and its consequences.9
Skin pigmentation changes. Hyperpigmentation occurred with bremelanotide use on the face, gingiva, and breasts, as reported in the clinical trials, in 1% of treated patients who received up to 8 doses per month, compared with no such occurrences in placebo-treated patients. In addition, 38% of patients who received bremelanotide daily for 8 days developed focal hyperpigmentation. It was not confirmed in all patients whether the hyperpigmentation resolved. Women with dark skin were more likely to develop hyperpigmentation.9
Common adverse reactions. The most common adverse reactions with bremelanotide treatment are nausea, flushing, injection site reactions, and headache, with most events being mild to moderate in intensity. In the clinical trials, 40% of the bremelanotide-treated women experienced nausea (compared with 1% of placebo-treated women), with most occurrences being mild; for most participants nausea improved with the second dose. Women had nausea that either went away or was intermittent, or it was mild enough that the drug benefits outweighed the tolerability costs--of women who experienced nausea, 92% continued in the trial, and 8% dropped out because of nausea.9
The following scenario describes the experience of HSDD in one of Dr. Kingsberg's patients.
CASE Woman avoids sex because of low desire; marriage is suffering
A 40-year-old woman, Sandra, who has been married for 19 years and has fraternal twins aged 8, presented to the behavioral medicine clinic with distressing symptoms of low sexual desire. For several years into the marriage the patient experienced excellent sex drive. After 6 to 7 years, she noticed that her desire had declined and that she was starting to avoid sex. She was irritated when her husband initiated sex, and she would make excuses as to why it was not the right time.
Her husband felt hurt, frustrated, and rejected. The couple was close to divorce because he was angry and resentful. Sandra recognized there was a problem but did not know how to fix it. She could not understand why her interest had waned since she still loved her husband and considered him objectively very attractive.
Sandra came to see Dr. Kingsberg at the behavioral medicine clinic. Using the 5-item validated diagnostic tool called the Decreased Sexual Desire Screener, Dr. Kingsberg diagnosed hypoactive sexual desire disorder (HSDD), a term Sandra had never heard of and did not know was a condition. The patient was relieved to know that she was one of several million women affected by HSDD and that the problem was not just that she was a "bad wife" or that she had some kind of psychological block. She emphasized how much she loved her husband and how she wanted desperately to "want to want desire," as she recalled feeling previously.
Sandra was treated with counseling and psychotherapy in which we addressed the relationship issues, the avoidance of sex, the comfort with being sexual, and the recognition that responsive desire can be helpful (as she was able to have arousal and orgasm and have a satisfying sexual event). The issue was that she had no motivation to seek out sex and had no interest in experiencing that pleasure. In subsequent couple's therapy, the husband recognized that his wife was not intentionally rejecting him, but that she had a real medical condition.
Although Sandra's relationship was now more stable and she and her husband were both working toward finding a solution to Sandra's loss of desire, she was still very distressed by her lack of desire. Sandra tried flibanserin for 3 months but unfortunately did not respond. Sandra heard about the recent approval of bremelanotide and is looking forward to the drug being available so that she can try it.
Final considerations
Asking patients about sexual function and using sexual function screening tools can help clinicians identify patients with the decreased sexual desire and associated distress characteristic of HSDD. ObGyns are the appropriate clinicians to treat these women and soon will have 2 pharmacologic options--bremelanotide (anticipated to be available in Fall 2019) and flibanserin--to offer patients with this biopsychosocial disorder that can adversely impact well-being and quality of life. Clinicians should individualize treatment, which may include psychotherapeutic counseling, and counsel patients on appropriate drug use and potential adverse effects.
AMAG Pharmaceuticals, Inc. has announced that they will have a copay assistance program for bremelanotide, where the first prescription of four autoinjectors will be a $0 copay, followed by a $99 copay or less for refills.15
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- West SL, D'Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441-1449.
- Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions: part II. J Sex Med. 2016;13:1888-1906.
- Basson R. Using a different model for female sexual response to address women's problematic low sexual desire. J Sex Marital Ther. 2001;27:395-403.
- Clayton AH, Goldfischer ER, Goldstein I, et al. Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med. 2009;6:730-738.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction. Obstet Gynecol. 2019;134:e1-e18.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114-128.
- Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women's Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin Proc. 2018;93:467-487.
- Vyleesi [package insert]. Waltham, MA: AMAG Pharmaceuticals; 2019.
- Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21;633-640.
- Portman DJ, Brown L, Yuan, et al. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834-842.
- Kingsberg SA, Clayton AH, Portman D, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol. Forthcoming.
- Clayton AH, Lucas J, Jordon R, et al. Efficacy of the Investigational drug bremelanotide in the Reconnect studies. Poster presented at: 30th ECNP Congress of Applied and Translational Neuroscience; September 2-5, 2017, Paris, France.
- US Food and Drug Administration. FDA orders important safety labeling changes for Addyi [press release]. April 11, 2019. https://www.fda.gov/news-events/press-announcements/fda-orders-important-safety-labeling-changes-addyi. Accessed July 17, 2019.
- Vyleesi website (https://vyleesipro.com). Accessed August 5, 2019.
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- West SL, D'Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441-1449.
- Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions: part II. J Sex Med. 2016;13:1888-1906.
- Basson R. Using a different model for female sexual response to address women's problematic low sexual desire. J Sex Marital Ther. 2001;27:395-403.
- Clayton AH, Goldfischer ER, Goldstein I, et al. Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med. 2009;6:730-738.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction. Obstet Gynecol. 2019;134:e1-e18.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114-128.
- Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women's Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin Proc. 2018;93:467-487.
- Vyleesi [package insert]. Waltham, MA: AMAG Pharmaceuticals; 2019.
- Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21;633-640.
- Portman DJ, Brown L, Yuan, et al. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834-842.
- Kingsberg SA, Clayton AH, Portman D, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol. Forthcoming.
- Clayton AH, Lucas J, Jordon R, et al. Efficacy of the Investigational drug bremelanotide in the Reconnect studies. Poster presented at: 30th ECNP Congress of Applied and Translational Neuroscience; September 2-5, 2017, Paris, France.
- US Food and Drug Administration. FDA orders important safety labeling changes for Addyi [press release]. April 11, 2019. https://www.fda.gov/news-events/press-announcements/fda-orders-important-safety-labeling-changes-addyi. Accessed July 17, 2019.
- Vyleesi website (https://vyleesipro.com). Accessed August 5, 2019.
2017 Update on female sexual dysfunction
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
Fast Tracks
- Although not fully understood how, estradiol can improve sexual desire, progesterone tends to dampen sexual desire, and testosterone has a neutral effect in premenopausal women
- Newly available since July 2017, prasterone is a once-daily intravaginal agent that treats moderate to severe dyspareunia and has costs similar to topical estrogens
- Estrogen therapy may be considered in a breast cancer mutation carrier who has undergone prophylactic mastectomies and bilateral salpingo-oophorectomy
It is time for HPV vaccination to be considered part of routine preventive health care
The recognition that human papillomavirus (HPV) oncogenic viruses cause cervical carcinoma remains one of the most game-changing medical discoveries of the last century. Improvements in screening options for detecting cervical cancer precursors followed. We now have the ability to detect high-risk HPV subtypes in routine specimens. Finally, a highly effective vaccine was developed that targets HPV types 16 and 18, which are responsible for causing approximately 70% of all cases of cervical carcinoma.
In one of the original vaccines HPV types 6 and 11, responsible for 90% of all genital warts, were also targeted. In 2014, a 9-valent vaccine incorporating an additional 5 HPV strains (31, 33, 45, 52, and 58) was approved and is set to replace all previous vaccine versions. Together, these 7 oncogenic HPV types are responsible for approximately 90% of HPV-related cancers, including cervical, anal, oropharyngeal, vaginal, and vulvar cancer.
By vaccinating boys and girls between ages 9 and 21 (for males) and 9 and 26 (for females), we could effectively eliminate 90% of genital warts and 90% of all HPV-related cancers. So why have we not capitalized on this extraordinary discovery? In 2016, why were only 40% of teenage girls and less than 25% of teenage boys vaccinated against HPV when we are immunizing 80% to 90% of these populations with tetanus, diphtheria, and acellular pertusis (Tdap) and meningococcal vaccines?
Related article:
2016 Update on cervical disease
Barriers to HPV vaccination
When the first HPV vaccine was approved in 2006, cost was a significant factor. Many health insurance plans did not cover this “discretionary” vaccine, which was viewed as a prevention for sexually transmitted infections rather than as a valuable intervention for the prevention of cervical and other cancers. At well over $125 per dose with 3 doses required for a full series, ObGyns were reluctant to stock and provide these expensive vaccines without assurance of reimbursement. The logistics of recalling patients for their subsequent vaccine doses were challenging for offices that were not accustomed to seeing patients for preventive care activities more than once a year. In addition, the office infrastructure required to maintain the vaccine stock and manage the necessary paperwork could be daunting. Finally, the requirement that patients be observed for 15 to 30 minutes in the office after vaccine administration created efficiency and rooming problems in busy, active practices.
Over time, almost all payers covered the HPV vaccines, but the logistical issues in ObGyn practices remain. Pediatric practices, on the other hand, are ideally suited for vaccine administration. Unfortunately, our colleagues delivering preventive care to young teens have persisted in considering the HPV vaccine as an optional adjunct to routine vaccination despite the advice of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC), which for many years has recommended the HPV vaccine for girls. In 2011, the ACIP extended the HPV vaccine recommendation to include boys beginning at ages 11 to 12.
New 2-dose HPV vaccine schedule for children <15 years
In October 2016, 10 years after the first HPV vaccine approval, the ACIP and the CDC approved a reduced, 2-dose schedule for those younger than 15.1 The first dose can be administered simultaneously with other recommended vaccines for 11- to 12-year-olds (the meningococcal and Tdap vaccines) and the second dose, 6 or 12 months later.2 The 12-month interval would allow administration, once again, of all required vaccines at the annual visit.
Pivotal immunogenicity study
The new recommendation is based on robust multinational data (52 sites in 15 countries, N = 1,518) from an open-label trial.3 Immunogenicity of 2 doses of the 9-valent HPV vaccine in girls and boys ages 9 to 14 was compared with that of a standard 3-dose regimen in adolescents and young women ages 16 to 26. Five cohorts were studied: boys 9 to 14 given 2 doses at 6-month intervals; girls 9 to 14 given 2 doses at 6-month intervals; boys and girls 9 to 14 given 2 doses at a 12-month interval; girls 9 to 14 given the standard 3-dose regimen; and girls and young women 16 to 26 receiving 3 doses over 6 months.
The authors assessed the antibody responses against each HPV subtype 1 month after the final vaccine dose. Data from 1,377 participants (90.7% of the original cohort) were analyzed. Prespecified antibody titers were set conservatively to ensure adequate immunogenicity. Noninferiority criteria had to be met for all 9 HPV types.
Trial results. The immune responses for the 9- to 14-year-olds were consistently higher than those for the 16- to 26-year-old age group regardless of the regimen—not a surprising finding since the initial trials for HPV vaccine demonstrated a greater response among younger vaccine recipients. In this trial, higher antibody responses were found for the 12-month dosing interval than for the 6-month interval, although both regimens produced an adequate response.
Immunogenicity remained at 6 months. Antibody levels were retested 6 months after the last dose of HPV vaccine in a post hoc analysis. In all groups the antib
Related article:
2015 Update on cervical disease: New ammo for HPV prevention and screening
Simplified dosing may help increase vaccination rates
What does this new dosing regimen mean for practice? It will be simpler to incorporate HPV vaccination routinely into the standard vaccine regimen for preadolescent boys and girls. In addition, counseling for HPV vaccine administration can be combined with counseling for the meningococcal vaccine and routine Tdap booster.
Notably, primary care physicians have reported perceiving HPV vaccine discussions with parents as burdensome, and they tend to discuss it last after conversations about Tdap and meningococcal vaccines.4 Brewer and colleagues5 documented a 5% increase in first HPV vaccine doses among patients in practices in which the providers were taught to “announce” the need for HPV vaccine along with other routine vaccines. There was no increase in HPV vaccine uptake among practices in which providers were taught to “discuss” HPV with parents and to address their concerns, or in control practices. Therefore, less conversation about HPV and the HPV vaccine, as distinct from any other recommended vaccines, is better.
With the new 2-dose regimen, it should be easier to convey that the HPV vaccine is another necessary, routine intervention for children’s health. We should be able to achieve 90% vaccination rates for HPV—similar to rates for Tdap.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. CDC recommends only two HPV shots for younger adolescents. https://www.cdc.gov/media/releases/2016/p1020-hpv-shots.html. Published October 19, 2016. Accessed February 22, 2017.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. Morbid Mortal Weekly Rep MMWR. 2016;65(49)1405–1408.
- Iverson OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411–2421.
- Gilkey MB, Moss JL, Coyne-Beasley T, Hall ME, Shah PH, Brewer NT. Physician communication about adolescent vaccination: how is human papillomavirus vaccine different? Prev Med. 2015;77:181–185.
The recognition that human papillomavirus (HPV) oncogenic viruses cause cervical carcinoma remains one of the most game-changing medical discoveries of the last century. Improvements in screening options for detecting cervical cancer precursors followed. We now have the ability to detect high-risk HPV subtypes in routine specimens. Finally, a highly effective vaccine was developed that targets HPV types 16 and 18, which are responsible for causing approximately 70% of all cases of cervical carcinoma.
In one of the original vaccines HPV types 6 and 11, responsible for 90% of all genital warts, were also targeted. In 2014, a 9-valent vaccine incorporating an additional 5 HPV strains (31, 33, 45, 52, and 58) was approved and is set to replace all previous vaccine versions. Together, these 7 oncogenic HPV types are responsible for approximately 90% of HPV-related cancers, including cervical, anal, oropharyngeal, vaginal, and vulvar cancer.
By vaccinating boys and girls between ages 9 and 21 (for males) and 9 and 26 (for females), we could effectively eliminate 90% of genital warts and 90% of all HPV-related cancers. So why have we not capitalized on this extraordinary discovery? In 2016, why were only 40% of teenage girls and less than 25% of teenage boys vaccinated against HPV when we are immunizing 80% to 90% of these populations with tetanus, diphtheria, and acellular pertusis (Tdap) and meningococcal vaccines?
Related article:
2016 Update on cervical disease
Barriers to HPV vaccination
When the first HPV vaccine was approved in 2006, cost was a significant factor. Many health insurance plans did not cover this “discretionary” vaccine, which was viewed as a prevention for sexually transmitted infections rather than as a valuable intervention for the prevention of cervical and other cancers. At well over $125 per dose with 3 doses required for a full series, ObGyns were reluctant to stock and provide these expensive vaccines without assurance of reimbursement. The logistics of recalling patients for their subsequent vaccine doses were challenging for offices that were not accustomed to seeing patients for preventive care activities more than once a year. In addition, the office infrastructure required to maintain the vaccine stock and manage the necessary paperwork could be daunting. Finally, the requirement that patients be observed for 15 to 30 minutes in the office after vaccine administration created efficiency and rooming problems in busy, active practices.
Over time, almost all payers covered the HPV vaccines, but the logistical issues in ObGyn practices remain. Pediatric practices, on the other hand, are ideally suited for vaccine administration. Unfortunately, our colleagues delivering preventive care to young teens have persisted in considering the HPV vaccine as an optional adjunct to routine vaccination despite the advice of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC), which for many years has recommended the HPV vaccine for girls. In 2011, the ACIP extended the HPV vaccine recommendation to include boys beginning at ages 11 to 12.
New 2-dose HPV vaccine schedule for children <15 years
In October 2016, 10 years after the first HPV vaccine approval, the ACIP and the CDC approved a reduced, 2-dose schedule for those younger than 15.1 The first dose can be administered simultaneously with other recommended vaccines for 11- to 12-year-olds (the meningococcal and Tdap vaccines) and the second dose, 6 or 12 months later.2 The 12-month interval would allow administration, once again, of all required vaccines at the annual visit.
Pivotal immunogenicity study
The new recommendation is based on robust multinational data (52 sites in 15 countries, N = 1,518) from an open-label trial.3 Immunogenicity of 2 doses of the 9-valent HPV vaccine in girls and boys ages 9 to 14 was compared with that of a standard 3-dose regimen in adolescents and young women ages 16 to 26. Five cohorts were studied: boys 9 to 14 given 2 doses at 6-month intervals; girls 9 to 14 given 2 doses at 6-month intervals; boys and girls 9 to 14 given 2 doses at a 12-month interval; girls 9 to 14 given the standard 3-dose regimen; and girls and young women 16 to 26 receiving 3 doses over 6 months.
The authors assessed the antibody responses against each HPV subtype 1 month after the final vaccine dose. Data from 1,377 participants (90.7% of the original cohort) were analyzed. Prespecified antibody titers were set conservatively to ensure adequate immunogenicity. Noninferiority criteria had to be met for all 9 HPV types.
Trial results. The immune responses for the 9- to 14-year-olds were consistently higher than those for the 16- to 26-year-old age group regardless of the regimen—not a surprising finding since the initial trials for HPV vaccine demonstrated a greater response among younger vaccine recipients. In this trial, higher antibody responses were found for the 12-month dosing interval than for the 6-month interval, although both regimens produced an adequate response.
Immunogenicity remained at 6 months. Antibody levels were retested 6 months after the last dose of HPV vaccine in a post hoc analysis. In all groups the antib
Related article:
2015 Update on cervical disease: New ammo for HPV prevention and screening
Simplified dosing may help increase vaccination rates
What does this new dosing regimen mean for practice? It will be simpler to incorporate HPV vaccination routinely into the standard vaccine regimen for preadolescent boys and girls. In addition, counseling for HPV vaccine administration can be combined with counseling for the meningococcal vaccine and routine Tdap booster.
Notably, primary care physicians have reported perceiving HPV vaccine discussions with parents as burdensome, and they tend to discuss it last after conversations about Tdap and meningococcal vaccines.4 Brewer and colleagues5 documented a 5% increase in first HPV vaccine doses among patients in practices in which the providers were taught to “announce” the need for HPV vaccine along with other routine vaccines. There was no increase in HPV vaccine uptake among practices in which providers were taught to “discuss” HPV with parents and to address their concerns, or in control practices. Therefore, less conversation about HPV and the HPV vaccine, as distinct from any other recommended vaccines, is better.
With the new 2-dose regimen, it should be easier to convey that the HPV vaccine is another necessary, routine intervention for children’s health. We should be able to achieve 90% vaccination rates for HPV—similar to rates for Tdap.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The recognition that human papillomavirus (HPV) oncogenic viruses cause cervical carcinoma remains one of the most game-changing medical discoveries of the last century. Improvements in screening options for detecting cervical cancer precursors followed. We now have the ability to detect high-risk HPV subtypes in routine specimens. Finally, a highly effective vaccine was developed that targets HPV types 16 and 18, which are responsible for causing approximately 70% of all cases of cervical carcinoma.
In one of the original vaccines HPV types 6 and 11, responsible for 90% of all genital warts, were also targeted. In 2014, a 9-valent vaccine incorporating an additional 5 HPV strains (31, 33, 45, 52, and 58) was approved and is set to replace all previous vaccine versions. Together, these 7 oncogenic HPV types are responsible for approximately 90% of HPV-related cancers, including cervical, anal, oropharyngeal, vaginal, and vulvar cancer.
By vaccinating boys and girls between ages 9 and 21 (for males) and 9 and 26 (for females), we could effectively eliminate 90% of genital warts and 90% of all HPV-related cancers. So why have we not capitalized on this extraordinary discovery? In 2016, why were only 40% of teenage girls and less than 25% of teenage boys vaccinated against HPV when we are immunizing 80% to 90% of these populations with tetanus, diphtheria, and acellular pertusis (Tdap) and meningococcal vaccines?
Related article:
2016 Update on cervical disease
Barriers to HPV vaccination
When the first HPV vaccine was approved in 2006, cost was a significant factor. Many health insurance plans did not cover this “discretionary” vaccine, which was viewed as a prevention for sexually transmitted infections rather than as a valuable intervention for the prevention of cervical and other cancers. At well over $125 per dose with 3 doses required for a full series, ObGyns were reluctant to stock and provide these expensive vaccines without assurance of reimbursement. The logistics of recalling patients for their subsequent vaccine doses were challenging for offices that were not accustomed to seeing patients for preventive care activities more than once a year. In addition, the office infrastructure required to maintain the vaccine stock and manage the necessary paperwork could be daunting. Finally, the requirement that patients be observed for 15 to 30 minutes in the office after vaccine administration created efficiency and rooming problems in busy, active practices.
Over time, almost all payers covered the HPV vaccines, but the logistical issues in ObGyn practices remain. Pediatric practices, on the other hand, are ideally suited for vaccine administration. Unfortunately, our colleagues delivering preventive care to young teens have persisted in considering the HPV vaccine as an optional adjunct to routine vaccination despite the advice of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC), which for many years has recommended the HPV vaccine for girls. In 2011, the ACIP extended the HPV vaccine recommendation to include boys beginning at ages 11 to 12.
New 2-dose HPV vaccine schedule for children <15 years
In October 2016, 10 years after the first HPV vaccine approval, the ACIP and the CDC approved a reduced, 2-dose schedule for those younger than 15.1 The first dose can be administered simultaneously with other recommended vaccines for 11- to 12-year-olds (the meningococcal and Tdap vaccines) and the second dose, 6 or 12 months later.2 The 12-month interval would allow administration, once again, of all required vaccines at the annual visit.
Pivotal immunogenicity study
The new recommendation is based on robust multinational data (52 sites in 15 countries, N = 1,518) from an open-label trial.3 Immunogenicity of 2 doses of the 9-valent HPV vaccine in girls and boys ages 9 to 14 was compared with that of a standard 3-dose regimen in adolescents and young women ages 16 to 26. Five cohorts were studied: boys 9 to 14 given 2 doses at 6-month intervals; girls 9 to 14 given 2 doses at 6-month intervals; boys and girls 9 to 14 given 2 doses at a 12-month interval; girls 9 to 14 given the standard 3-dose regimen; and girls and young women 16 to 26 receiving 3 doses over 6 months.
The authors assessed the antibody responses against each HPV subtype 1 month after the final vaccine dose. Data from 1,377 participants (90.7% of the original cohort) were analyzed. Prespecified antibody titers were set conservatively to ensure adequate immunogenicity. Noninferiority criteria had to be met for all 9 HPV types.
Trial results. The immune responses for the 9- to 14-year-olds were consistently higher than those for the 16- to 26-year-old age group regardless of the regimen—not a surprising finding since the initial trials for HPV vaccine demonstrated a greater response among younger vaccine recipients. In this trial, higher antibody responses were found for the 12-month dosing interval than for the 6-month interval, although both regimens produced an adequate response.
Immunogenicity remained at 6 months. Antibody levels were retested 6 months after the last dose of HPV vaccine in a post hoc analysis. In all groups the antib
Related article:
2015 Update on cervical disease: New ammo for HPV prevention and screening
Simplified dosing may help increase vaccination rates
What does this new dosing regimen mean for practice? It will be simpler to incorporate HPV vaccination routinely into the standard vaccine regimen for preadolescent boys and girls. In addition, counseling for HPV vaccine administration can be combined with counseling for the meningococcal vaccine and routine Tdap booster.
Notably, primary care physicians have reported perceiving HPV vaccine discussions with parents as burdensome, and they tend to discuss it last after conversations about Tdap and meningococcal vaccines.4 Brewer and colleagues5 documented a 5% increase in first HPV vaccine doses among patients in practices in which the providers were taught to “announce” the need for HPV vaccine along with other routine vaccines. There was no increase in HPV vaccine uptake among practices in which providers were taught to “discuss” HPV with parents and to address their concerns, or in control practices. Therefore, less conversation about HPV and the HPV vaccine, as distinct from any other recommended vaccines, is better.
With the new 2-dose regimen, it should be easier to convey that the HPV vaccine is another necessary, routine intervention for children’s health. We should be able to achieve 90% vaccination rates for HPV—similar to rates for Tdap.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. CDC recommends only two HPV shots for younger adolescents. https://www.cdc.gov/media/releases/2016/p1020-hpv-shots.html. Published October 19, 2016. Accessed February 22, 2017.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. Morbid Mortal Weekly Rep MMWR. 2016;65(49)1405–1408.
- Iverson OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411–2421.
- Gilkey MB, Moss JL, Coyne-Beasley T, Hall ME, Shah PH, Brewer NT. Physician communication about adolescent vaccination: how is human papillomavirus vaccine different? Prev Med. 2015;77:181–185.
- Centers for Disease Control and Prevention. CDC recommends only two HPV shots for younger adolescents. https://www.cdc.gov/media/releases/2016/p1020-hpv-shots.html. Published October 19, 2016. Accessed February 22, 2017.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. Morbid Mortal Weekly Rep MMWR. 2016;65(49)1405–1408.
- Iverson OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411–2421.
- Gilkey MB, Moss JL, Coyne-Beasley T, Hall ME, Shah PH, Brewer NT. Physician communication about adolescent vaccination: how is human papillomavirus vaccine different? Prev Med. 2015;77:181–185.
2016 Update on female sexual dysfunction
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.
Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.

Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.

Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
In This Article
- This roundtable's expert panel
- Dyspareunia and low sexual desire in a breast cancer survivor
- Laser treatment and vaginal health
Vaginal hysterectomy with basic instrumentation
In the United States, gynecologic surgeons remove approximately one uterus every minute of the year.1 That rate translates to more than 525,000 hysterectomies annually in this country alone. Yet, despite the widespread availability of information on the benefits of a vaginal approach to hysterectomy, the great majority of these operations—close to 50%—are still performed via an open abdominal approach.2
As I pointed out last month in my “Update on Vaginal Hysterectomy,” the vaginal approach not only is more cosmetically pleasing than laparoscopic and robot-assisted hysterectomy (not to mention open abdominal surgery) but also has a lower complication rate.3
As I also noted, one reason for the low rate of vaginal hysterectomy may be the assumption, on the part of many gynecologic surgeons, that the techniques and tools they learned to use during training are still the only options available today. That assumption is wrong.
In this article, I describe the technique for vaginal hysterectomy using basic instru mentation. This article is based on a master class in vaginal hysterectomy produced by the AAGL and co-sponsored by the Am erican College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. This master class offers continuing medical education credits and is avail able at http://www.aagl.org/vaghystwebinar.
For a look at innovative tools for this procedure, see my “Update on Vaginal Hysterectomy” in the September 2015 issue of this journal at obgmanagement.com.
Vaginal hysterectomy has few contraindications
Many commonly cited contraindications to the vaginal approach are not, in fact, absolute contraindications. An open or laparoscopic approach is preferred when the patient has a known cancer, of course, and when deep infiltrating endometriosis is present at the rectovaginal septum. However, previous pelvic surgery, nulliparity, an enlarged uterus, or lack of a prior vaginal delivery need not exclude the vaginal approach. Nor does a narrow introitus necessarily mandate a laparoscopic or open abdominal approach. In fact, in this article, I describe my basic technique in a patient (a cadaver) with a very narrow pubic arch, and I offer strategies for gaining some needed mobility and avoiding complications (TABLES 1 and 2).
Next month, in the November issue of OBG Management, John B. Gebhart, MD, will describe his vaginal technique for right salpingectomy with ovarian preservation, as well as his technique for right salpingo-oophorectomy.
Proper patient positioning is key
You can simplify the operation by positioning the patient so that her buttocks are over the edge of the table fairly far—at least 1 inch beyond the edge of the table for optimal exposure and greater access. If the patient is thin, it then becomes important to pad the sacrum because, when she is positioned that far off the table, all her weight comes to rest on the sacrum. In overweight patients, this is not an issue, but for thin patients, I place a bit of egg crate or gel beneath the sacrum.
For the procedure, I prefer to place my instruments on a tray that is kept on my lap. This arrangement frees the scrub technician from having to hand tools over my shoulder—and it saves time. I use a narrow, covered Mayo stand, and I place a stepstool beneath my feet to keep my knees at right angles so that things don’t slip during the operation.
Surgical technique
Choose an appropriate retractor
In a woman with a narrow introitus, I find that a posterior weighted speculum takes up too much space. Once I place a clamp on the cervix with that speculum in place, I don’t have much room to work. However, if I substitute a small Deaver retractor, which is narrower, I gain more workspace.
Inject the uterosacral ligaments
Grasp the cervix using a Jacobs vulsellum tenaculum. Use of a single tenaculum allows for much more movement than the use of instruments placed anteriorly and posteriorly. The Jacobs tenaculum obtains a better purchase on the tissue than a single tooth and is considerably less likely to tear through the tissue.
Before beginning the hysterectomy, locate the uterosacral ligaments and inject each one at its junction with the cervix, aspirating slightly before infiltrating the ligament with 0.25% to 0.50% bupivacaine with epinephrine, with dilute vasopressin mixed in. (I place 1 unit in 20 mL of the local solution.) Injection of this solution achieves 2 goals:
- improved intraoperative hemostasis
- postoperative pain relief.
Use a short needle with a needle extender attached to a control syringe rather than a spinal needle for greater control.
Enter the posterior peritoneal cavity
Before entering the peritoneal cavity, create a right angle with the Jacobs tenaculum and Deaver retractor in relation to the surgical field (FIGURE 1). This right angle is difficult to achieve when you are using a weighted speculum in a tight vagina. Once you have a right angle, tent the vaginal tissue in the midline (FIGURE 2).
In a nulliparous patient or a woman with a tight pelvis, you may discover that the peritoneum is pulled up between the uterosacral ligaments. One common pitfall arises when the surgeon, having dissected the vaginal epithelium, continues cutting into the vaginal epithelium instead of reaching into the peritoneal cavity. Palpate the tissue to ensure that there is no bowel in the way and stay at right angles while confidently grasping the peritoneum with a toothed forceps.
I like to use a bit of electrosurgery to incise the vaginal wall. I don’t begin at the cervix but incise more distally into the vaginal epithelium approximately 2 cm from the cervicovaginal junction. This strategy prevents dissection into the cervix and/or rectovaginal septum rather than the posterior
cul-de-sac (FIGURE 3).
Once the incision is made, it is possible to feel the posterior peritoneum. And as you tent the peritoneum, you can then very confidently extend the incision and enter the cavity posteriorly.
In a patient with significant adhesions such as this one, I feel around posteriorly to determine exactly where I am. One tactic I use is to release the tenaculum and regrasp the cervix with it. This allows for improved visualization and movement of the cervix as the procedure progresses. Depending on the case, it may be necessary to insert a sponge to hold bowel out of the way.
Avoid the bladder
Move the Deaver retractor to the anterior position, switch the Jacobs clamp to the anterior cervix, and pull straight down. Now that you have incised the vaginal epithelium posteriorly, the length of the cervix should be apparent to you, and you can easily determine the location of the bladder reflection.
Keep in mind that, in a postmenopausal patient, there will be fewer vaginal rugae to guide you. Place the Jacobs tenaculum as close to the midline as possible so that you can confidently grab the tissue without fear of grabbing the bladder. If you tilt the Jacobs clamp, you can feel the edge of the bladder reflection. Remember that postmenopausal patients with prolapse (or, occasionally, obese patients with cervical elongation but little actual descensus) may have altered anatomy.
You can create a bit more space in which to dissect by injecting the bupivacaine/ epinephrine solution into the vaginal epithelium. This technique also ensures that the vaginal epithelial incisions won’t bleed.
Now, tilt the Jacobs tenaculum downward and push the junction of the cervix with the bladder reflection toward you so that you have a good sense of how deeply to incise.
Once you’ve made the incision, reclamp the Jacobs tenaculum so that it holds all of that tissue, and repeat the maneuver, tilting the clamp downward and pushing the junction toward you. In this way, you create traction and countertraction, sweeping the tissue out of your way.
Always use sharp dissection. When adhesions are present, surgeons often get into trouble using blunt dissection and may inadvertently enter the bladder if they use a sponge-covered digit for dissection, because adhesions can be much denser than normal tissue. In such cases, the bladder tears open rather than the adhesions being swept away.
Consider this: You don’t need to enter the peritoneal cavity anteriorly in order to continue working on the procedure. You can safely protect the bladder throughout the case, until the very end, if necessary, in patients who have undergone multiple previous surgeries or cesarean deliveries.
Rather than enter the anterior peritoneum, I dissect as much of the vaginal epithelium as possible and place a second Deaver retractor posteriorly.
I massage the uterosacral ligament for about 10 seconds to lengthen it and create more descensus, then place a Ballantine Heaney clamp on the ligament.
Next, I cut the pedicle and suture it, maintaining a clamp on the uterosacral ligament suture so that I can use it later for repair of the vaginal cuff.
I recommend a vessel-sealing device to secure the major blood supply, but I do suture the uterosacral and round ligaments for attachment to the apex at the conclusion of the hysterectomy. I suggest that you place straight clamps to hold the uterosacral ligament sutures and curved clamps on the round ligament ties to help you keep track of what you’re doing.
I generally prefer to use a smaller vessel-sealing device, such as the LigaSure Max (Covidien), because it allows me to take very small bites of tissue. It is also less expensive because it uses a disposable electrode within a reusable Heaney-type clamp.
Many people have argued that we need to teach surgeons to suture vaginally and, for that reason, should avoid vessel sealing. My response: Why wouldn’t we want to use the very best technology available? Randomized trials have demonstrated a 50% reduction in pain relief postoperatively when we use vessel sealing.4 Less foreign material is left in the pelvis, lowering the risk of infection. And it really doesn’t matter which vessel-sealing technology you use, as long as you’re familiar with the specifics of the system you choose. Another advantage: There is no need to pass needles back and forth.
Take small bites of tissue
Because this patient has a very small uterus, a small bite of tissue will get you close to where you want to be. When you take a bite with the vessel sealer, try to protect the vaginal epithelium and vulva from the steam that is emitted. The clamp itself does not heat up, but the steam that is released from the tissue is 100° C, so place a finger between the clamp and the sidewall for protection. It is preferable to burn your own finger than to burn the patient.
Because you haven’t entered the peritoneal cavity anteriorly, it is important to ensure that you don’t take too big of a bite with the vessel sealer. Rather, stay where you know you’ve done your dissection, where things are safe.
One cardinal principle of surgery is that you shouldn’t operate where you can’t feel or see. One of the common errors in vaginal surgery is that surgeons start dissecting higher than they can see. It’s easy to get into trouble when you start pushing tissue or dissecting tissue that you can’t visualize.
At this point, the anterior Deaver retractor is not essential, so I remove it. If you don’t need it, don’t use it. I try to avoid metal when I can.
If I were using suture rather than vessel sealing, I would place a Heaney clamp on the uterosacral ligament and cut. Using a clamp-cut-tie technique, I would pull on the pedicle and cut just beyond the tip of the clamp to ensure that the suture will be secure (FIGURE 4). This approach would not be appropriate during use of a vessel sealer. In that case, you would want to cut to but not beyond the tip of the clamp.
One of the skills helpful in suturing is learning to move your elbow and wrist to achieve the proper angle. Determine where you want the suture to exit the tissue, and then angle your elbow and wrist so that the suture comes out where you want it. It’s easy to lose track of the needle tip, especially when you’re working in a limited space under the pubic symphysis, so use your shoulder, elbow, and wrist to control
suture placement.
Protect the anterior epithelium
Because you have not yet entered the peritoneal cavity anteriorly, it is important to protect the anterior epithelium and bladder. Reinsert a narrow Deaver retractor anteriorly, remove the Jacobs clamp, and replace the clamp laterally so that the cervix can be pulled off to the side (FIGURE 5).
One nice thing about some vessel sealers is that the surgeon can twist them in any direction. It isn’t necessary to move your hand; you simply move the device itself.
Once you have taken at least the descending branch of the uterine artery, remove the posterior retractor and pull downward on the Jacobs tenaculum. You should have reached just about to the level of the uterine fundus, with the anatomy well visualized (FIGURE 6). Next, open the anterior peritoneum.
Pay attention to the surgical field
Now that you have entered the peritoneum anteriorly as well as posteriorly, identify the broad ligament, keeping in mind that the ureter is retroperitoneal, not intraperitoneal. If you were to place a clamp from the posterior leaf of the broad ligament across to the anterior leaf of the broad ligament, you would be grasping all the vessels but not the ureter. In fact, the anterior Deaver retractor is lifting both ureters up and out of the way. If you pull the cervix off to the opposite side, you create an additional couple of centimeters—a safe space for the vessel sealer
(FIGURE 7).
In placing the vessel sealer, there is no need to move out laterally, as there is no need for space to place suture. Instead, hug the uterus. At this point, the main concern is the risk of damaging any small bowel behind the uterine fundus that might be coming down into the surgical field, obscured from vision. And because there may be steam emitted at the tip of the vessel-sealing clamp, keep a finger back there to protect anything that might be in the field.
Last steps
Before taking the last bite of tissue on the right-hand side, place the round ligament in a Heaney clamp. Now that the round and utero-ovarian ligaments have been skeletonized, you can grasp the pedicle in a clamp. If the pedicle is especially thick, it may be beneficial to close the clamp, leave it on for a few seconds, and then reapply it. In that way, you obtain a better purchase.
Next, free the rest of the tissue with a vessel sealer, or cut it. I prefer to use a vessel sealer, and I again protect the adjacent tissue with my fingers anteriorly and posteriorly.
With the clamp remaining on the round ligament and utero-ovarian ligament
(FIGURE 8), which will be sutured, push the uterine tissue out of the way, back into the pelvis, to make room for suturing.
Because a postmenopausal vulva may be cut by the suture, it’s important to take pains not to abrade that tissue. Once you have finished suturing the round/utero-ovarian pedicle, leave the needle on the suture so that you can reconnect the round ligament to the anterior pubocervical ring to reconstruct the vaginal apex. For safekeeping, clamp the needle out of the way and tuck it beneath the drape.
Switching to the other side, use a Lahey clamp to flip the uterus, then clamp the pedicle and use the vessel sealer to separate it, again protecting the tissue beneath and ahead of the clamp. Sometimes, with an especially thick pedicle, the vessel sealer will signal that the tissue hasn’t been completely sealed. In that case, get another purchase of the pedicle, protect the adjacent tissue, and seal again.
Once the uterus has been removed completely, suture the utero-ovarian and round ligament on this side.
One tip to aid in the placement of suture is to move your clamped tissue in such a way as to prevent inadvertent suturing of other tissue (FIGURE 9).
An additional strategy for pain relief at this point is to infiltrate the round ligaments with local anesthetic. We know that we’re working with higher-level fibers—T10 to T12—through the round ligaments. By infiltrating them with anesthetic, you achieve denser pain relief for post- operative management.
Uterine reduction strategies facilitate vaginal removal of tissue
When the uterus is too large to remove intact through the vagina, there are a number of techniques for coring, wedging, and morcellating the tissue. As always, a complete knowledge of anatomy is essential, as well as an understanding that fibroids can frequently distort the uterus, twisting it to the left or right. It is important to anticipate such distortion to avoid the inadvertent destruction of anatomic landmarks or damage to the adnexae.
One straight-forward strategy is to debulk the uterus using a knife to core it, removing the central portion. In cases in which you need to keep the entire endometrial cavity intact, you can core the central portion of the uterus while grasping the cervix so that you can remove the endometrium intact for the pathologist (FIGURE).
For this strategy it is important to protect the vaginal sidewalls with metal. You can use another retractor to do that, pulling down on the cervix and beginning the morcellation. I generally prefer to use a short knife handle only because I want to be sure I’m not tempted to cut any higher than I can see.
For more on coring and wedging techniques, see the introductory video for the ACOG/SGS/AAGL master class on vaginal hysterectomy at http://www.aagl.org/vaghystwebinar.
Close the vaginal cuff
The reconstruction of the vaginal cuff is a critical component of any hysterectomy. My approach is to reattach the uterosacral ligaments to the posterior cuff and the round ligaments to the anterior cuff, thereby re- creating an intact pubocervical ring. It is not necessary to include the peritoneum in the cuff closure. In fact, kinking of the ureters is more likely when the peritoneum is closed.
Attach one uterosacral ligament, then place a running, full-thickness stitch across the posterior cuff, and attach the uterosacral ligament on the opposite side. Use the needle you left attached to the round ligament to bring the right pedicle to the anterior cuff at 10 o’clock (be sure you grasp the full thickness of the vaginal epithelium without compromising the bladder). Attach the left round-ligament pedicle at the 2 o’clock position. Then close the cuff side to side down to the uterosacral ligaments. This completely reconstructs the pubocervical ring and provides excellent support at the apex.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www .brighamandwomens.org/Departments_and_Services/obgyn /services/mininvgynsurg/mininvoptions/hysterectomy.aspx. Published October 3, 2014. Accessed September 2, 2015.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009; 114(5):1156–1158.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
In the United States, gynecologic surgeons remove approximately one uterus every minute of the year.1 That rate translates to more than 525,000 hysterectomies annually in this country alone. Yet, despite the widespread availability of information on the benefits of a vaginal approach to hysterectomy, the great majority of these operations—close to 50%—are still performed via an open abdominal approach.2
As I pointed out last month in my “Update on Vaginal Hysterectomy,” the vaginal approach not only is more cosmetically pleasing than laparoscopic and robot-assisted hysterectomy (not to mention open abdominal surgery) but also has a lower complication rate.3
As I also noted, one reason for the low rate of vaginal hysterectomy may be the assumption, on the part of many gynecologic surgeons, that the techniques and tools they learned to use during training are still the only options available today. That assumption is wrong.
In this article, I describe the technique for vaginal hysterectomy using basic instru mentation. This article is based on a master class in vaginal hysterectomy produced by the AAGL and co-sponsored by the Am erican College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. This master class offers continuing medical education credits and is avail able at http://www.aagl.org/vaghystwebinar.
For a look at innovative tools for this procedure, see my “Update on Vaginal Hysterectomy” in the September 2015 issue of this journal at obgmanagement.com.
Vaginal hysterectomy has few contraindications
Many commonly cited contraindications to the vaginal approach are not, in fact, absolute contraindications. An open or laparoscopic approach is preferred when the patient has a known cancer, of course, and when deep infiltrating endometriosis is present at the rectovaginal septum. However, previous pelvic surgery, nulliparity, an enlarged uterus, or lack of a prior vaginal delivery need not exclude the vaginal approach. Nor does a narrow introitus necessarily mandate a laparoscopic or open abdominal approach. In fact, in this article, I describe my basic technique in a patient (a cadaver) with a very narrow pubic arch, and I offer strategies for gaining some needed mobility and avoiding complications (TABLES 1 and 2).
Next month, in the November issue of OBG Management, John B. Gebhart, MD, will describe his vaginal technique for right salpingectomy with ovarian preservation, as well as his technique for right salpingo-oophorectomy.
Proper patient positioning is key
You can simplify the operation by positioning the patient so that her buttocks are over the edge of the table fairly far—at least 1 inch beyond the edge of the table for optimal exposure and greater access. If the patient is thin, it then becomes important to pad the sacrum because, when she is positioned that far off the table, all her weight comes to rest on the sacrum. In overweight patients, this is not an issue, but for thin patients, I place a bit of egg crate or gel beneath the sacrum.
For the procedure, I prefer to place my instruments on a tray that is kept on my lap. This arrangement frees the scrub technician from having to hand tools over my shoulder—and it saves time. I use a narrow, covered Mayo stand, and I place a stepstool beneath my feet to keep my knees at right angles so that things don’t slip during the operation.
Surgical technique
Choose an appropriate retractor
In a woman with a narrow introitus, I find that a posterior weighted speculum takes up too much space. Once I place a clamp on the cervix with that speculum in place, I don’t have much room to work. However, if I substitute a small Deaver retractor, which is narrower, I gain more workspace.
Inject the uterosacral ligaments
Grasp the cervix using a Jacobs vulsellum tenaculum. Use of a single tenaculum allows for much more movement than the use of instruments placed anteriorly and posteriorly. The Jacobs tenaculum obtains a better purchase on the tissue than a single tooth and is considerably less likely to tear through the tissue.
Before beginning the hysterectomy, locate the uterosacral ligaments and inject each one at its junction with the cervix, aspirating slightly before infiltrating the ligament with 0.25% to 0.50% bupivacaine with epinephrine, with dilute vasopressin mixed in. (I place 1 unit in 20 mL of the local solution.) Injection of this solution achieves 2 goals:
- improved intraoperative hemostasis
- postoperative pain relief.
Use a short needle with a needle extender attached to a control syringe rather than a spinal needle for greater control.
Enter the posterior peritoneal cavity
Before entering the peritoneal cavity, create a right angle with the Jacobs tenaculum and Deaver retractor in relation to the surgical field (FIGURE 1). This right angle is difficult to achieve when you are using a weighted speculum in a tight vagina. Once you have a right angle, tent the vaginal tissue in the midline (FIGURE 2).
In a nulliparous patient or a woman with a tight pelvis, you may discover that the peritoneum is pulled up between the uterosacral ligaments. One common pitfall arises when the surgeon, having dissected the vaginal epithelium, continues cutting into the vaginal epithelium instead of reaching into the peritoneal cavity. Palpate the tissue to ensure that there is no bowel in the way and stay at right angles while confidently grasping the peritoneum with a toothed forceps.
I like to use a bit of electrosurgery to incise the vaginal wall. I don’t begin at the cervix but incise more distally into the vaginal epithelium approximately 2 cm from the cervicovaginal junction. This strategy prevents dissection into the cervix and/or rectovaginal septum rather than the posterior
cul-de-sac (FIGURE 3).
Once the incision is made, it is possible to feel the posterior peritoneum. And as you tent the peritoneum, you can then very confidently extend the incision and enter the cavity posteriorly.
In a patient with significant adhesions such as this one, I feel around posteriorly to determine exactly where I am. One tactic I use is to release the tenaculum and regrasp the cervix with it. This allows for improved visualization and movement of the cervix as the procedure progresses. Depending on the case, it may be necessary to insert a sponge to hold bowel out of the way.
Avoid the bladder
Move the Deaver retractor to the anterior position, switch the Jacobs clamp to the anterior cervix, and pull straight down. Now that you have incised the vaginal epithelium posteriorly, the length of the cervix should be apparent to you, and you can easily determine the location of the bladder reflection.
Keep in mind that, in a postmenopausal patient, there will be fewer vaginal rugae to guide you. Place the Jacobs tenaculum as close to the midline as possible so that you can confidently grab the tissue without fear of grabbing the bladder. If you tilt the Jacobs clamp, you can feel the edge of the bladder reflection. Remember that postmenopausal patients with prolapse (or, occasionally, obese patients with cervical elongation but little actual descensus) may have altered anatomy.
You can create a bit more space in which to dissect by injecting the bupivacaine/ epinephrine solution into the vaginal epithelium. This technique also ensures that the vaginal epithelial incisions won’t bleed.
Now, tilt the Jacobs tenaculum downward and push the junction of the cervix with the bladder reflection toward you so that you have a good sense of how deeply to incise.
Once you’ve made the incision, reclamp the Jacobs tenaculum so that it holds all of that tissue, and repeat the maneuver, tilting the clamp downward and pushing the junction toward you. In this way, you create traction and countertraction, sweeping the tissue out of your way.
Always use sharp dissection. When adhesions are present, surgeons often get into trouble using blunt dissection and may inadvertently enter the bladder if they use a sponge-covered digit for dissection, because adhesions can be much denser than normal tissue. In such cases, the bladder tears open rather than the adhesions being swept away.
Consider this: You don’t need to enter the peritoneal cavity anteriorly in order to continue working on the procedure. You can safely protect the bladder throughout the case, until the very end, if necessary, in patients who have undergone multiple previous surgeries or cesarean deliveries.
Rather than enter the anterior peritoneum, I dissect as much of the vaginal epithelium as possible and place a second Deaver retractor posteriorly.
I massage the uterosacral ligament for about 10 seconds to lengthen it and create more descensus, then place a Ballantine Heaney clamp on the ligament.
Next, I cut the pedicle and suture it, maintaining a clamp on the uterosacral ligament suture so that I can use it later for repair of the vaginal cuff.
I recommend a vessel-sealing device to secure the major blood supply, but I do suture the uterosacral and round ligaments for attachment to the apex at the conclusion of the hysterectomy. I suggest that you place straight clamps to hold the uterosacral ligament sutures and curved clamps on the round ligament ties to help you keep track of what you’re doing.
I generally prefer to use a smaller vessel-sealing device, such as the LigaSure Max (Covidien), because it allows me to take very small bites of tissue. It is also less expensive because it uses a disposable electrode within a reusable Heaney-type clamp.
Many people have argued that we need to teach surgeons to suture vaginally and, for that reason, should avoid vessel sealing. My response: Why wouldn’t we want to use the very best technology available? Randomized trials have demonstrated a 50% reduction in pain relief postoperatively when we use vessel sealing.4 Less foreign material is left in the pelvis, lowering the risk of infection. And it really doesn’t matter which vessel-sealing technology you use, as long as you’re familiar with the specifics of the system you choose. Another advantage: There is no need to pass needles back and forth.
Take small bites of tissue
Because this patient has a very small uterus, a small bite of tissue will get you close to where you want to be. When you take a bite with the vessel sealer, try to protect the vaginal epithelium and vulva from the steam that is emitted. The clamp itself does not heat up, but the steam that is released from the tissue is 100° C, so place a finger between the clamp and the sidewall for protection. It is preferable to burn your own finger than to burn the patient.
Because you haven’t entered the peritoneal cavity anteriorly, it is important to ensure that you don’t take too big of a bite with the vessel sealer. Rather, stay where you know you’ve done your dissection, where things are safe.
One cardinal principle of surgery is that you shouldn’t operate where you can’t feel or see. One of the common errors in vaginal surgery is that surgeons start dissecting higher than they can see. It’s easy to get into trouble when you start pushing tissue or dissecting tissue that you can’t visualize.
At this point, the anterior Deaver retractor is not essential, so I remove it. If you don’t need it, don’t use it. I try to avoid metal when I can.
If I were using suture rather than vessel sealing, I would place a Heaney clamp on the uterosacral ligament and cut. Using a clamp-cut-tie technique, I would pull on the pedicle and cut just beyond the tip of the clamp to ensure that the suture will be secure (FIGURE 4). This approach would not be appropriate during use of a vessel sealer. In that case, you would want to cut to but not beyond the tip of the clamp.
One of the skills helpful in suturing is learning to move your elbow and wrist to achieve the proper angle. Determine where you want the suture to exit the tissue, and then angle your elbow and wrist so that the suture comes out where you want it. It’s easy to lose track of the needle tip, especially when you’re working in a limited space under the pubic symphysis, so use your shoulder, elbow, and wrist to control
suture placement.
Protect the anterior epithelium
Because you have not yet entered the peritoneal cavity anteriorly, it is important to protect the anterior epithelium and bladder. Reinsert a narrow Deaver retractor anteriorly, remove the Jacobs clamp, and replace the clamp laterally so that the cervix can be pulled off to the side (FIGURE 5).
One nice thing about some vessel sealers is that the surgeon can twist them in any direction. It isn’t necessary to move your hand; you simply move the device itself.
Once you have taken at least the descending branch of the uterine artery, remove the posterior retractor and pull downward on the Jacobs tenaculum. You should have reached just about to the level of the uterine fundus, with the anatomy well visualized (FIGURE 6). Next, open the anterior peritoneum.
Pay attention to the surgical field
Now that you have entered the peritoneum anteriorly as well as posteriorly, identify the broad ligament, keeping in mind that the ureter is retroperitoneal, not intraperitoneal. If you were to place a clamp from the posterior leaf of the broad ligament across to the anterior leaf of the broad ligament, you would be grasping all the vessels but not the ureter. In fact, the anterior Deaver retractor is lifting both ureters up and out of the way. If you pull the cervix off to the opposite side, you create an additional couple of centimeters—a safe space for the vessel sealer
(FIGURE 7).
In placing the vessel sealer, there is no need to move out laterally, as there is no need for space to place suture. Instead, hug the uterus. At this point, the main concern is the risk of damaging any small bowel behind the uterine fundus that might be coming down into the surgical field, obscured from vision. And because there may be steam emitted at the tip of the vessel-sealing clamp, keep a finger back there to protect anything that might be in the field.
Last steps
Before taking the last bite of tissue on the right-hand side, place the round ligament in a Heaney clamp. Now that the round and utero-ovarian ligaments have been skeletonized, you can grasp the pedicle in a clamp. If the pedicle is especially thick, it may be beneficial to close the clamp, leave it on for a few seconds, and then reapply it. In that way, you obtain a better purchase.
Next, free the rest of the tissue with a vessel sealer, or cut it. I prefer to use a vessel sealer, and I again protect the adjacent tissue with my fingers anteriorly and posteriorly.
With the clamp remaining on the round ligament and utero-ovarian ligament
(FIGURE 8), which will be sutured, push the uterine tissue out of the way, back into the pelvis, to make room for suturing.
Because a postmenopausal vulva may be cut by the suture, it’s important to take pains not to abrade that tissue. Once you have finished suturing the round/utero-ovarian pedicle, leave the needle on the suture so that you can reconnect the round ligament to the anterior pubocervical ring to reconstruct the vaginal apex. For safekeeping, clamp the needle out of the way and tuck it beneath the drape.
Switching to the other side, use a Lahey clamp to flip the uterus, then clamp the pedicle and use the vessel sealer to separate it, again protecting the tissue beneath and ahead of the clamp. Sometimes, with an especially thick pedicle, the vessel sealer will signal that the tissue hasn’t been completely sealed. In that case, get another purchase of the pedicle, protect the adjacent tissue, and seal again.
Once the uterus has been removed completely, suture the utero-ovarian and round ligament on this side.
One tip to aid in the placement of suture is to move your clamped tissue in such a way as to prevent inadvertent suturing of other tissue (FIGURE 9).
An additional strategy for pain relief at this point is to infiltrate the round ligaments with local anesthetic. We know that we’re working with higher-level fibers—T10 to T12—through the round ligaments. By infiltrating them with anesthetic, you achieve denser pain relief for post- operative management.
Uterine reduction strategies facilitate vaginal removal of tissue
When the uterus is too large to remove intact through the vagina, there are a number of techniques for coring, wedging, and morcellating the tissue. As always, a complete knowledge of anatomy is essential, as well as an understanding that fibroids can frequently distort the uterus, twisting it to the left or right. It is important to anticipate such distortion to avoid the inadvertent destruction of anatomic landmarks or damage to the adnexae.
One straight-forward strategy is to debulk the uterus using a knife to core it, removing the central portion. In cases in which you need to keep the entire endometrial cavity intact, you can core the central portion of the uterus while grasping the cervix so that you can remove the endometrium intact for the pathologist (FIGURE).
For this strategy it is important to protect the vaginal sidewalls with metal. You can use another retractor to do that, pulling down on the cervix and beginning the morcellation. I generally prefer to use a short knife handle only because I want to be sure I’m not tempted to cut any higher than I can see.
For more on coring and wedging techniques, see the introductory video for the ACOG/SGS/AAGL master class on vaginal hysterectomy at http://www.aagl.org/vaghystwebinar.
Close the vaginal cuff
The reconstruction of the vaginal cuff is a critical component of any hysterectomy. My approach is to reattach the uterosacral ligaments to the posterior cuff and the round ligaments to the anterior cuff, thereby re- creating an intact pubocervical ring. It is not necessary to include the peritoneum in the cuff closure. In fact, kinking of the ureters is more likely when the peritoneum is closed.
Attach one uterosacral ligament, then place a running, full-thickness stitch across the posterior cuff, and attach the uterosacral ligament on the opposite side. Use the needle you left attached to the round ligament to bring the right pedicle to the anterior cuff at 10 o’clock (be sure you grasp the full thickness of the vaginal epithelium without compromising the bladder). Attach the left round-ligament pedicle at the 2 o’clock position. Then close the cuff side to side down to the uterosacral ligaments. This completely reconstructs the pubocervical ring and provides excellent support at the apex.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In the United States, gynecologic surgeons remove approximately one uterus every minute of the year.1 That rate translates to more than 525,000 hysterectomies annually in this country alone. Yet, despite the widespread availability of information on the benefits of a vaginal approach to hysterectomy, the great majority of these operations—close to 50%—are still performed via an open abdominal approach.2
As I pointed out last month in my “Update on Vaginal Hysterectomy,” the vaginal approach not only is more cosmetically pleasing than laparoscopic and robot-assisted hysterectomy (not to mention open abdominal surgery) but also has a lower complication rate.3
As I also noted, one reason for the low rate of vaginal hysterectomy may be the assumption, on the part of many gynecologic surgeons, that the techniques and tools they learned to use during training are still the only options available today. That assumption is wrong.
In this article, I describe the technique for vaginal hysterectomy using basic instru mentation. This article is based on a master class in vaginal hysterectomy produced by the AAGL and co-sponsored by the Am erican College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. This master class offers continuing medical education credits and is avail able at http://www.aagl.org/vaghystwebinar.
For a look at innovative tools for this procedure, see my “Update on Vaginal Hysterectomy” in the September 2015 issue of this journal at obgmanagement.com.
Vaginal hysterectomy has few contraindications
Many commonly cited contraindications to the vaginal approach are not, in fact, absolute contraindications. An open or laparoscopic approach is preferred when the patient has a known cancer, of course, and when deep infiltrating endometriosis is present at the rectovaginal septum. However, previous pelvic surgery, nulliparity, an enlarged uterus, or lack of a prior vaginal delivery need not exclude the vaginal approach. Nor does a narrow introitus necessarily mandate a laparoscopic or open abdominal approach. In fact, in this article, I describe my basic technique in a patient (a cadaver) with a very narrow pubic arch, and I offer strategies for gaining some needed mobility and avoiding complications (TABLES 1 and 2).
Next month, in the November issue of OBG Management, John B. Gebhart, MD, will describe his vaginal technique for right salpingectomy with ovarian preservation, as well as his technique for right salpingo-oophorectomy.
Proper patient positioning is key
You can simplify the operation by positioning the patient so that her buttocks are over the edge of the table fairly far—at least 1 inch beyond the edge of the table for optimal exposure and greater access. If the patient is thin, it then becomes important to pad the sacrum because, when she is positioned that far off the table, all her weight comes to rest on the sacrum. In overweight patients, this is not an issue, but for thin patients, I place a bit of egg crate or gel beneath the sacrum.
For the procedure, I prefer to place my instruments on a tray that is kept on my lap. This arrangement frees the scrub technician from having to hand tools over my shoulder—and it saves time. I use a narrow, covered Mayo stand, and I place a stepstool beneath my feet to keep my knees at right angles so that things don’t slip during the operation.
Surgical technique
Choose an appropriate retractor
In a woman with a narrow introitus, I find that a posterior weighted speculum takes up too much space. Once I place a clamp on the cervix with that speculum in place, I don’t have much room to work. However, if I substitute a small Deaver retractor, which is narrower, I gain more workspace.
Inject the uterosacral ligaments
Grasp the cervix using a Jacobs vulsellum tenaculum. Use of a single tenaculum allows for much more movement than the use of instruments placed anteriorly and posteriorly. The Jacobs tenaculum obtains a better purchase on the tissue than a single tooth and is considerably less likely to tear through the tissue.
Before beginning the hysterectomy, locate the uterosacral ligaments and inject each one at its junction with the cervix, aspirating slightly before infiltrating the ligament with 0.25% to 0.50% bupivacaine with epinephrine, with dilute vasopressin mixed in. (I place 1 unit in 20 mL of the local solution.) Injection of this solution achieves 2 goals:
- improved intraoperative hemostasis
- postoperative pain relief.
Use a short needle with a needle extender attached to a control syringe rather than a spinal needle for greater control.
Enter the posterior peritoneal cavity
Before entering the peritoneal cavity, create a right angle with the Jacobs tenaculum and Deaver retractor in relation to the surgical field (FIGURE 1). This right angle is difficult to achieve when you are using a weighted speculum in a tight vagina. Once you have a right angle, tent the vaginal tissue in the midline (FIGURE 2).
In a nulliparous patient or a woman with a tight pelvis, you may discover that the peritoneum is pulled up between the uterosacral ligaments. One common pitfall arises when the surgeon, having dissected the vaginal epithelium, continues cutting into the vaginal epithelium instead of reaching into the peritoneal cavity. Palpate the tissue to ensure that there is no bowel in the way and stay at right angles while confidently grasping the peritoneum with a toothed forceps.
I like to use a bit of electrosurgery to incise the vaginal wall. I don’t begin at the cervix but incise more distally into the vaginal epithelium approximately 2 cm from the cervicovaginal junction. This strategy prevents dissection into the cervix and/or rectovaginal septum rather than the posterior
cul-de-sac (FIGURE 3).
Once the incision is made, it is possible to feel the posterior peritoneum. And as you tent the peritoneum, you can then very confidently extend the incision and enter the cavity posteriorly.
In a patient with significant adhesions such as this one, I feel around posteriorly to determine exactly where I am. One tactic I use is to release the tenaculum and regrasp the cervix with it. This allows for improved visualization and movement of the cervix as the procedure progresses. Depending on the case, it may be necessary to insert a sponge to hold bowel out of the way.
Avoid the bladder
Move the Deaver retractor to the anterior position, switch the Jacobs clamp to the anterior cervix, and pull straight down. Now that you have incised the vaginal epithelium posteriorly, the length of the cervix should be apparent to you, and you can easily determine the location of the bladder reflection.
Keep in mind that, in a postmenopausal patient, there will be fewer vaginal rugae to guide you. Place the Jacobs tenaculum as close to the midline as possible so that you can confidently grab the tissue without fear of grabbing the bladder. If you tilt the Jacobs clamp, you can feel the edge of the bladder reflection. Remember that postmenopausal patients with prolapse (or, occasionally, obese patients with cervical elongation but little actual descensus) may have altered anatomy.
You can create a bit more space in which to dissect by injecting the bupivacaine/ epinephrine solution into the vaginal epithelium. This technique also ensures that the vaginal epithelial incisions won’t bleed.
Now, tilt the Jacobs tenaculum downward and push the junction of the cervix with the bladder reflection toward you so that you have a good sense of how deeply to incise.
Once you’ve made the incision, reclamp the Jacobs tenaculum so that it holds all of that tissue, and repeat the maneuver, tilting the clamp downward and pushing the junction toward you. In this way, you create traction and countertraction, sweeping the tissue out of your way.
Always use sharp dissection. When adhesions are present, surgeons often get into trouble using blunt dissection and may inadvertently enter the bladder if they use a sponge-covered digit for dissection, because adhesions can be much denser than normal tissue. In such cases, the bladder tears open rather than the adhesions being swept away.
Consider this: You don’t need to enter the peritoneal cavity anteriorly in order to continue working on the procedure. You can safely protect the bladder throughout the case, until the very end, if necessary, in patients who have undergone multiple previous surgeries or cesarean deliveries.
Rather than enter the anterior peritoneum, I dissect as much of the vaginal epithelium as possible and place a second Deaver retractor posteriorly.
I massage the uterosacral ligament for about 10 seconds to lengthen it and create more descensus, then place a Ballantine Heaney clamp on the ligament.
Next, I cut the pedicle and suture it, maintaining a clamp on the uterosacral ligament suture so that I can use it later for repair of the vaginal cuff.
I recommend a vessel-sealing device to secure the major blood supply, but I do suture the uterosacral and round ligaments for attachment to the apex at the conclusion of the hysterectomy. I suggest that you place straight clamps to hold the uterosacral ligament sutures and curved clamps on the round ligament ties to help you keep track of what you’re doing.
I generally prefer to use a smaller vessel-sealing device, such as the LigaSure Max (Covidien), because it allows me to take very small bites of tissue. It is also less expensive because it uses a disposable electrode within a reusable Heaney-type clamp.
Many people have argued that we need to teach surgeons to suture vaginally and, for that reason, should avoid vessel sealing. My response: Why wouldn’t we want to use the very best technology available? Randomized trials have demonstrated a 50% reduction in pain relief postoperatively when we use vessel sealing.4 Less foreign material is left in the pelvis, lowering the risk of infection. And it really doesn’t matter which vessel-sealing technology you use, as long as you’re familiar with the specifics of the system you choose. Another advantage: There is no need to pass needles back and forth.
Take small bites of tissue
Because this patient has a very small uterus, a small bite of tissue will get you close to where you want to be. When you take a bite with the vessel sealer, try to protect the vaginal epithelium and vulva from the steam that is emitted. The clamp itself does not heat up, but the steam that is released from the tissue is 100° C, so place a finger between the clamp and the sidewall for protection. It is preferable to burn your own finger than to burn the patient.
Because you haven’t entered the peritoneal cavity anteriorly, it is important to ensure that you don’t take too big of a bite with the vessel sealer. Rather, stay where you know you’ve done your dissection, where things are safe.
One cardinal principle of surgery is that you shouldn’t operate where you can’t feel or see. One of the common errors in vaginal surgery is that surgeons start dissecting higher than they can see. It’s easy to get into trouble when you start pushing tissue or dissecting tissue that you can’t visualize.
At this point, the anterior Deaver retractor is not essential, so I remove it. If you don’t need it, don’t use it. I try to avoid metal when I can.
If I were using suture rather than vessel sealing, I would place a Heaney clamp on the uterosacral ligament and cut. Using a clamp-cut-tie technique, I would pull on the pedicle and cut just beyond the tip of the clamp to ensure that the suture will be secure (FIGURE 4). This approach would not be appropriate during use of a vessel sealer. In that case, you would want to cut to but not beyond the tip of the clamp.
One of the skills helpful in suturing is learning to move your elbow and wrist to achieve the proper angle. Determine where you want the suture to exit the tissue, and then angle your elbow and wrist so that the suture comes out where you want it. It’s easy to lose track of the needle tip, especially when you’re working in a limited space under the pubic symphysis, so use your shoulder, elbow, and wrist to control
suture placement.
Protect the anterior epithelium
Because you have not yet entered the peritoneal cavity anteriorly, it is important to protect the anterior epithelium and bladder. Reinsert a narrow Deaver retractor anteriorly, remove the Jacobs clamp, and replace the clamp laterally so that the cervix can be pulled off to the side (FIGURE 5).
One nice thing about some vessel sealers is that the surgeon can twist them in any direction. It isn’t necessary to move your hand; you simply move the device itself.
Once you have taken at least the descending branch of the uterine artery, remove the posterior retractor and pull downward on the Jacobs tenaculum. You should have reached just about to the level of the uterine fundus, with the anatomy well visualized (FIGURE 6). Next, open the anterior peritoneum.
Pay attention to the surgical field
Now that you have entered the peritoneum anteriorly as well as posteriorly, identify the broad ligament, keeping in mind that the ureter is retroperitoneal, not intraperitoneal. If you were to place a clamp from the posterior leaf of the broad ligament across to the anterior leaf of the broad ligament, you would be grasping all the vessels but not the ureter. In fact, the anterior Deaver retractor is lifting both ureters up and out of the way. If you pull the cervix off to the opposite side, you create an additional couple of centimeters—a safe space for the vessel sealer
(FIGURE 7).
In placing the vessel sealer, there is no need to move out laterally, as there is no need for space to place suture. Instead, hug the uterus. At this point, the main concern is the risk of damaging any small bowel behind the uterine fundus that might be coming down into the surgical field, obscured from vision. And because there may be steam emitted at the tip of the vessel-sealing clamp, keep a finger back there to protect anything that might be in the field.
Last steps
Before taking the last bite of tissue on the right-hand side, place the round ligament in a Heaney clamp. Now that the round and utero-ovarian ligaments have been skeletonized, you can grasp the pedicle in a clamp. If the pedicle is especially thick, it may be beneficial to close the clamp, leave it on for a few seconds, and then reapply it. In that way, you obtain a better purchase.
Next, free the rest of the tissue with a vessel sealer, or cut it. I prefer to use a vessel sealer, and I again protect the adjacent tissue with my fingers anteriorly and posteriorly.
With the clamp remaining on the round ligament and utero-ovarian ligament
(FIGURE 8), which will be sutured, push the uterine tissue out of the way, back into the pelvis, to make room for suturing.
Because a postmenopausal vulva may be cut by the suture, it’s important to take pains not to abrade that tissue. Once you have finished suturing the round/utero-ovarian pedicle, leave the needle on the suture so that you can reconnect the round ligament to the anterior pubocervical ring to reconstruct the vaginal apex. For safekeeping, clamp the needle out of the way and tuck it beneath the drape.
Switching to the other side, use a Lahey clamp to flip the uterus, then clamp the pedicle and use the vessel sealer to separate it, again protecting the tissue beneath and ahead of the clamp. Sometimes, with an especially thick pedicle, the vessel sealer will signal that the tissue hasn’t been completely sealed. In that case, get another purchase of the pedicle, protect the adjacent tissue, and seal again.
Once the uterus has been removed completely, suture the utero-ovarian and round ligament on this side.
One tip to aid in the placement of suture is to move your clamped tissue in such a way as to prevent inadvertent suturing of other tissue (FIGURE 9).
An additional strategy for pain relief at this point is to infiltrate the round ligaments with local anesthetic. We know that we’re working with higher-level fibers—T10 to T12—through the round ligaments. By infiltrating them with anesthetic, you achieve denser pain relief for post- operative management.
Uterine reduction strategies facilitate vaginal removal of tissue
When the uterus is too large to remove intact through the vagina, there are a number of techniques for coring, wedging, and morcellating the tissue. As always, a complete knowledge of anatomy is essential, as well as an understanding that fibroids can frequently distort the uterus, twisting it to the left or right. It is important to anticipate such distortion to avoid the inadvertent destruction of anatomic landmarks or damage to the adnexae.
One straight-forward strategy is to debulk the uterus using a knife to core it, removing the central portion. In cases in which you need to keep the entire endometrial cavity intact, you can core the central portion of the uterus while grasping the cervix so that you can remove the endometrium intact for the pathologist (FIGURE).
For this strategy it is important to protect the vaginal sidewalls with metal. You can use another retractor to do that, pulling down on the cervix and beginning the morcellation. I generally prefer to use a short knife handle only because I want to be sure I’m not tempted to cut any higher than I can see.
For more on coring and wedging techniques, see the introductory video for the ACOG/SGS/AAGL master class on vaginal hysterectomy at http://www.aagl.org/vaghystwebinar.
Close the vaginal cuff
The reconstruction of the vaginal cuff is a critical component of any hysterectomy. My approach is to reattach the uterosacral ligaments to the posterior cuff and the round ligaments to the anterior cuff, thereby re- creating an intact pubocervical ring. It is not necessary to include the peritoneum in the cuff closure. In fact, kinking of the ureters is more likely when the peritoneum is closed.
Attach one uterosacral ligament, then place a running, full-thickness stitch across the posterior cuff, and attach the uterosacral ligament on the opposite side. Use the needle you left attached to the round ligament to bring the right pedicle to the anterior cuff at 10 o’clock (be sure you grasp the full thickness of the vaginal epithelium without compromising the bladder). Attach the left round-ligament pedicle at the 2 o’clock position. Then close the cuff side to side down to the uterosacral ligaments. This completely reconstructs the pubocervical ring and provides excellent support at the apex.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www .brighamandwomens.org/Departments_and_Services/obgyn /services/mininvgynsurg/mininvoptions/hysterectomy.aspx. Published October 3, 2014. Accessed September 2, 2015.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009; 114(5):1156–1158.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www .brighamandwomens.org/Departments_and_Services/obgyn /services/mininvgynsurg/mininvoptions/hysterectomy.aspx. Published October 3, 2014. Accessed September 2, 2015.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009; 114(5):1156–1158.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
In this Article
- 5 solutions to difficult vaginal access
- The need to take small bites of tissue
- Uterine reduction strategies
2015 Update on vaginal hysterectomy
We’ve come a long way since Conrad Langebeck performed the first vaginal hysterectomy in 1813. For the inaugural surgery, Langebeck used no anesthesia, gloves, or other sterilization strategies, and he held the suture in his teeth at one point during the operation! (The patient survived.)1
Despite our dramatic progress since then, too many of us still perform benign hysterectomy by an approach other than vaginal. And too many of us still perform vaginal hysterectomy the way it was taught in the 1950s—frequently a backbreaking, frustrating undertaking.
That approach is unnecessary. In recent years, the technological world has developed many useful tools for minimally invasive gynecologic surgery, some of which greatly facilitate vaginal hysterectomy. In this Update, I focus on 3 of them:
- vessel-sealing devices
- a unique visualization system
- a lighted suction irrigator.
It is my hope that you will incorporate these tools into your vaginal hysterectomy cases and gain some of the significant benefits they have to offer. The revival of vaginal hysterectomy and vaginal surgery in general is all about using the best tools that we have available and using them well, cost- effectively, and thoughtfully to improve the experience of both surgeon and patient.
Why you should default to vaginal hysterectomy
Not only is vaginal hysterectomy more cosmetically pleasing but it also has a lower complication rate than laparoscopic, robot-assisted, or laparotomic hysterectomy, requiring no incisions through the abdominal wall. The original natural orifice translumenal endoscopic surgery (NOTES) procedure also is less expensive than laparoscopic or robot-assisted hysterectomy. Vaginal hysterectomy has so much to recommend it, in fact, that the biggest barrier to widespread use may simply be the lack of industry support.
According to the latest committee opinion from the American College of Obstetricians and Gynecologists (ACOG), “When choosing the route and method of hysterectomy, the physician should take into consideration how the procedure may be performed most safely and cost-effectively to fulfill the medical needs of the patient. Most literature supports the opinion that, when feasible, vaginal hysterectomy is the safest and most cost-effective route by which to remove the uterus.”2
A 2009 Cochrane review of surgical approaches to hysterectomy found that vaginal hysterectomy should be performed in preference to abdominal hysterectomy whenever possible.3 Yet data from 2008 indicate that almost 50% of all hysterectomies were still being performed using an open abdominal approach, and laparoscopic hysterectomy made up almost another 25%.4
To address the disparity between the evidence and practice, ACOG has joined forces with the AAGL and the Society for Gynecologic Surgeons (SGS) to present an online master course on vaginal hysterectomy, available at http://www.aagl.org/vaghystwebinar. This course features videos and live demonstrations on cadaveric models and is free to physicians, with continuing medical education (CME) credits available.
Vessel sealing offers real benefits over suturing
In any surgery, the need to achieve reliable hemostasis is critical. In vaginal hysterectomy, this goal traditionally has been attained by clamping and suturing of the vessels. In many respects, vaginal surgeons seem to have gotten trapped in the mindset that we need to suture during vaginal surgery—and train residents to suture, too. When it comes to laparoscopic surgery, however, the reverse is true. In that setting, vessel-sealing devices are used to seal blood vessels with “supraphysiologic burst pressure equal to that of previously used surgical clips or ligatures.”5
Why is vessel sealing necessarily better than suturing?
It’s safer, for one thing, eliminating the need to pass needles back and forth. It also frees the scrub technician to become the surgical assistant because there are no needles to load and unload. In order for suture to hold around a pedicle, it is necessary to have tissue adjacent to it. The surgeon ties and cuts but must have something beyond the suture or the suture won’t hold. That something is dead, devascularized tissue. Before healing can occur, all this tissue must be absorbed by the body. That is not the case with vessel sealing, which fuses the walls of the blood vessels, leaving less foreign material and dead tissue behind.
What the data show
The literature offers several randomized comparisons of bipolar vessel sealing and suturing during vaginal hysterectomy, and all of them find increased benefits for the vessel-sealing approach.
For example, in 2003, I published a randomized comparison looking specifically at blood loss and operative time.6 Sixty women in a single surgical practice were randomly allocated to vessel sealing or sutures for hemostasis during vaginal hysterectomy. In the vessel-sealing group, the mean operative time was 39.1 minutes (range, 22–93), compared with 53.6 minutes in the suturing group (range, 37–160; P = .003). Mean estimated blood loss also was significantly lower with vessel sealing, at 68.9 mL (range, 20–200) versus 126.7 mL for suturing (range, 25–600; P = .005). Complication rates and length of stay were similar between groups.6
In another randomized trial of vessel sealing versus suturing involving 68 women undergoing vaginal hysterectomy, pain was markedly reduced in the vessel-sealing group (median score, 4 vs 6; P<.0001). Operative time again was shorter with vessel sealing than with suturing (median of 32 vs 40 minutes; P = .003), but there were no differences in blood loss and hospitalization.7
Silva-Filho and colleagues randomly allocated 90 women to bipolar vessel sealing or suturing during vaginal hysterectomy.8 Vessel sealing provided reduced postoperative pain (pain score [SD] of 1.6 [0.4] vs 3.6 [0.4]; P<.001), shorter operative time (mean of 29.2 [2.1] vs 75.2 [5] minutes; P<.001), less blood loss (mean of 84 [5.9] vs 136.4 [89.1] mL; P = .001), and a shorter hospital stay (mean of 25.6 [0.9] vs 33.2 [1.7] hours; P<.001).8
A systematic review and meta-analysis by Kroft and Selk found that vessel sealing reduced: operative time by a mean of 17.2 minutes (95% confidence interval [CI], 7.5–27.0); blood loss by a mean of 47.7 mL (95% CI, 15.5–79.9); and hospital stay by a mean of 0.25 days (95% CI, 0.13–0.37) during vaginal hysterectomy.9
And in a randomized controlled trial from the Netherlands, women undergoing vaginal hysterectomy reported significantly less pain the evening after surgery in the vessel-sealing group, compared with the suturing group (pain score of 4.5 vs 5.7 on a scale of 1 to 10; P = .03).10 They also had a shorter operative time than women in the suturing group (60 vs 71 minutes; P = .05). Blood loss and hospital stays did not differ between groups, and there were no major differences in cost.
A reduction in pain is an especially important indicator of surgical success. In an interesting twist, Candiani and colleagues compared laparoscopic and vaginal hysterectomy for a number of variables, including pain, for benign pathology.11 They found less postoperative pain the day of surgery and a reduced number of days of analgesic request in the laparoscopic group, compared with vaginal hysterectomy. One reason: Hemostasis was achieved via vessel sealing in the laparoscopic group, compared with clamping and suturing in the vaginal group.11
Lighted suction irrigator facilitates visualization “around corners”
Many years ago, I conducted some informal studies for industry that showed—as one might guess intuitively—that the ability to see well during surgery cuts operative time. We all know that light is good. One useful lighting aid I’ve adopted of late is the Vital Vue (Covidien/Medtronic) suction irrigator. It has a disposable tip like all suction devices, but it includes 3 channels: one for a fiber optic cord, another for fluid, and the third for suction (FIGURE 1). It plugs into a regular suction machine, with a reusable box that provides the fiber optic light.
Because the suction tip is curved, the device makes it possible to illuminate the surgical field “around corners” if need be. Any bleeding can be irrigated to clear the field.
How to choose a vessel sealer
When selecting a vessel-sealing device for vaginal hysterectomy, keep in mind a number of factors:
- size of the vessels that will need to be controlled. Most devices on the market today control vessels 7 mm in size or smaller.
- amount of steam it releases, which can damage adjacent tissue
- overall size of the device
- size of the pedicles that will need to be controlled
- overall space required for use
- cost of the device.
In other words, to choose an appropriate device, you will need to think in advance about the specifics of the case you are planning, as not all hysterectomies are alike. The type of vessel sealer best for the surgery will vary with these details.
Both bipolar electrosurgical and ultrasonic devices now provide consistent hemostasis, increased functionality, and greater efficiency. What’s more, they cause minimal to no damage to surrounding tissue.
External scope offers visualization of vaginal procedures to entire OR
Designed for open surgeries, the VITOM system (Karl Storz) is an innovative tool for displaying procedures in which surgical access is limited. It’s an external telescope, or “exoscope,” with a 90° lens. It clips onto the table, providing visualization for the entire operative team (FIGURE 2).
As we all know, the advent of the camera made an enormous difference in laparoscopic procedures and in teaching because it enabled the assistant to see what the surgeon was doing and anticipate his or her needs. This device offers the same advantages for vaginal hysterectomy. In my opinion, it’s a game changer.
The VITOM system provides outstanding image quality and depth of view. It is placed at a distance of 25 cm to 75 cm from the surgical field and thus does not impinge on the surgeon’s workspace. Because it is compact, it facilitates the use of long instruments, if necessary. In addition, because it can be sterilized, the VITOM system can be manipulated directly by the surgeon or assistant.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www.brighamandwomens.org/Departments_and_Services/obgyn/ser vices/mininvgynsurg/mininvoptions/hysterectomy.aspx. Updated October 3, 2014. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- Nezhat C, Lewis M, King LP. Laparoscopic vessel sealing devices. Society of Laparoendoscopic Surgeons. http://laparoscopy.blogs.com/prevention_management_3/2010/10/laparoscopic-vessel-sealing-devices.html. Published 2010. Accessed August 6, 2015.
- Levy B, Emery L. Randomized trial of suture versus electrosurgical bipolar vessel sealing in vaginal hysterectomy. Obstet Gynecol. 2003;102(1):147–151.
- Cronjé HS, de Coning EC. Electrosurgical bipolar vessel sealing during vaginal hysterectomy. Int J Gynaecol Obstet. 2005;91(3):243–245.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
- Kroft J, Selk A. Energy-based vessel sealing in vaginal hysterectomy: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
- Lakeman MM, The S, Schellart RP, et al. Electrosurgical bipolar vessel sealing versus conventional clamping and suturing for vaginal hysterectomy: a randomised controlled trial. BJOG. 2012;119(12):1473–1482.
- Candiani M, Izzo S, Bulfoni A, Riparini J, Ronzoni S, Marconi A. Laparoscopic vs vaginal hysterectomy for benign pathology. Am J Obstet Gynecol. 2009;200(4):368.e1–e7.
We’ve come a long way since Conrad Langebeck performed the first vaginal hysterectomy in 1813. For the inaugural surgery, Langebeck used no anesthesia, gloves, or other sterilization strategies, and he held the suture in his teeth at one point during the operation! (The patient survived.)1
Despite our dramatic progress since then, too many of us still perform benign hysterectomy by an approach other than vaginal. And too many of us still perform vaginal hysterectomy the way it was taught in the 1950s—frequently a backbreaking, frustrating undertaking.
That approach is unnecessary. In recent years, the technological world has developed many useful tools for minimally invasive gynecologic surgery, some of which greatly facilitate vaginal hysterectomy. In this Update, I focus on 3 of them:
- vessel-sealing devices
- a unique visualization system
- a lighted suction irrigator.
It is my hope that you will incorporate these tools into your vaginal hysterectomy cases and gain some of the significant benefits they have to offer. The revival of vaginal hysterectomy and vaginal surgery in general is all about using the best tools that we have available and using them well, cost- effectively, and thoughtfully to improve the experience of both surgeon and patient.
Why you should default to vaginal hysterectomy
Not only is vaginal hysterectomy more cosmetically pleasing but it also has a lower complication rate than laparoscopic, robot-assisted, or laparotomic hysterectomy, requiring no incisions through the abdominal wall. The original natural orifice translumenal endoscopic surgery (NOTES) procedure also is less expensive than laparoscopic or robot-assisted hysterectomy. Vaginal hysterectomy has so much to recommend it, in fact, that the biggest barrier to widespread use may simply be the lack of industry support.
According to the latest committee opinion from the American College of Obstetricians and Gynecologists (ACOG), “When choosing the route and method of hysterectomy, the physician should take into consideration how the procedure may be performed most safely and cost-effectively to fulfill the medical needs of the patient. Most literature supports the opinion that, when feasible, vaginal hysterectomy is the safest and most cost-effective route by which to remove the uterus.”2
A 2009 Cochrane review of surgical approaches to hysterectomy found that vaginal hysterectomy should be performed in preference to abdominal hysterectomy whenever possible.3 Yet data from 2008 indicate that almost 50% of all hysterectomies were still being performed using an open abdominal approach, and laparoscopic hysterectomy made up almost another 25%.4
To address the disparity between the evidence and practice, ACOG has joined forces with the AAGL and the Society for Gynecologic Surgeons (SGS) to present an online master course on vaginal hysterectomy, available at http://www.aagl.org/vaghystwebinar. This course features videos and live demonstrations on cadaveric models and is free to physicians, with continuing medical education (CME) credits available.
Vessel sealing offers real benefits over suturing
In any surgery, the need to achieve reliable hemostasis is critical. In vaginal hysterectomy, this goal traditionally has been attained by clamping and suturing of the vessels. In many respects, vaginal surgeons seem to have gotten trapped in the mindset that we need to suture during vaginal surgery—and train residents to suture, too. When it comes to laparoscopic surgery, however, the reverse is true. In that setting, vessel-sealing devices are used to seal blood vessels with “supraphysiologic burst pressure equal to that of previously used surgical clips or ligatures.”5
Why is vessel sealing necessarily better than suturing?
It’s safer, for one thing, eliminating the need to pass needles back and forth. It also frees the scrub technician to become the surgical assistant because there are no needles to load and unload. In order for suture to hold around a pedicle, it is necessary to have tissue adjacent to it. The surgeon ties and cuts but must have something beyond the suture or the suture won’t hold. That something is dead, devascularized tissue. Before healing can occur, all this tissue must be absorbed by the body. That is not the case with vessel sealing, which fuses the walls of the blood vessels, leaving less foreign material and dead tissue behind.
What the data show
The literature offers several randomized comparisons of bipolar vessel sealing and suturing during vaginal hysterectomy, and all of them find increased benefits for the vessel-sealing approach.
For example, in 2003, I published a randomized comparison looking specifically at blood loss and operative time.6 Sixty women in a single surgical practice were randomly allocated to vessel sealing or sutures for hemostasis during vaginal hysterectomy. In the vessel-sealing group, the mean operative time was 39.1 minutes (range, 22–93), compared with 53.6 minutes in the suturing group (range, 37–160; P = .003). Mean estimated blood loss also was significantly lower with vessel sealing, at 68.9 mL (range, 20–200) versus 126.7 mL for suturing (range, 25–600; P = .005). Complication rates and length of stay were similar between groups.6
In another randomized trial of vessel sealing versus suturing involving 68 women undergoing vaginal hysterectomy, pain was markedly reduced in the vessel-sealing group (median score, 4 vs 6; P<.0001). Operative time again was shorter with vessel sealing than with suturing (median of 32 vs 40 minutes; P = .003), but there were no differences in blood loss and hospitalization.7
Silva-Filho and colleagues randomly allocated 90 women to bipolar vessel sealing or suturing during vaginal hysterectomy.8 Vessel sealing provided reduced postoperative pain (pain score [SD] of 1.6 [0.4] vs 3.6 [0.4]; P<.001), shorter operative time (mean of 29.2 [2.1] vs 75.2 [5] minutes; P<.001), less blood loss (mean of 84 [5.9] vs 136.4 [89.1] mL; P = .001), and a shorter hospital stay (mean of 25.6 [0.9] vs 33.2 [1.7] hours; P<.001).8
A systematic review and meta-analysis by Kroft and Selk found that vessel sealing reduced: operative time by a mean of 17.2 minutes (95% confidence interval [CI], 7.5–27.0); blood loss by a mean of 47.7 mL (95% CI, 15.5–79.9); and hospital stay by a mean of 0.25 days (95% CI, 0.13–0.37) during vaginal hysterectomy.9
And in a randomized controlled trial from the Netherlands, women undergoing vaginal hysterectomy reported significantly less pain the evening after surgery in the vessel-sealing group, compared with the suturing group (pain score of 4.5 vs 5.7 on a scale of 1 to 10; P = .03).10 They also had a shorter operative time than women in the suturing group (60 vs 71 minutes; P = .05). Blood loss and hospital stays did not differ between groups, and there were no major differences in cost.
A reduction in pain is an especially important indicator of surgical success. In an interesting twist, Candiani and colleagues compared laparoscopic and vaginal hysterectomy for a number of variables, including pain, for benign pathology.11 They found less postoperative pain the day of surgery and a reduced number of days of analgesic request in the laparoscopic group, compared with vaginal hysterectomy. One reason: Hemostasis was achieved via vessel sealing in the laparoscopic group, compared with clamping and suturing in the vaginal group.11
Lighted suction irrigator facilitates visualization “around corners”
Many years ago, I conducted some informal studies for industry that showed—as one might guess intuitively—that the ability to see well during surgery cuts operative time. We all know that light is good. One useful lighting aid I’ve adopted of late is the Vital Vue (Covidien/Medtronic) suction irrigator. It has a disposable tip like all suction devices, but it includes 3 channels: one for a fiber optic cord, another for fluid, and the third for suction (FIGURE 1). It plugs into a regular suction machine, with a reusable box that provides the fiber optic light.
Because the suction tip is curved, the device makes it possible to illuminate the surgical field “around corners” if need be. Any bleeding can be irrigated to clear the field.
How to choose a vessel sealer
When selecting a vessel-sealing device for vaginal hysterectomy, keep in mind a number of factors:
- size of the vessels that will need to be controlled. Most devices on the market today control vessels 7 mm in size or smaller.
- amount of steam it releases, which can damage adjacent tissue
- overall size of the device
- size of the pedicles that will need to be controlled
- overall space required for use
- cost of the device.
In other words, to choose an appropriate device, you will need to think in advance about the specifics of the case you are planning, as not all hysterectomies are alike. The type of vessel sealer best for the surgery will vary with these details.
Both bipolar electrosurgical and ultrasonic devices now provide consistent hemostasis, increased functionality, and greater efficiency. What’s more, they cause minimal to no damage to surrounding tissue.
External scope offers visualization of vaginal procedures to entire OR
Designed for open surgeries, the VITOM system (Karl Storz) is an innovative tool for displaying procedures in which surgical access is limited. It’s an external telescope, or “exoscope,” with a 90° lens. It clips onto the table, providing visualization for the entire operative team (FIGURE 2).
As we all know, the advent of the camera made an enormous difference in laparoscopic procedures and in teaching because it enabled the assistant to see what the surgeon was doing and anticipate his or her needs. This device offers the same advantages for vaginal hysterectomy. In my opinion, it’s a game changer.
The VITOM system provides outstanding image quality and depth of view. It is placed at a distance of 25 cm to 75 cm from the surgical field and thus does not impinge on the surgeon’s workspace. Because it is compact, it facilitates the use of long instruments, if necessary. In addition, because it can be sterilized, the VITOM system can be manipulated directly by the surgeon or assistant.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
We’ve come a long way since Conrad Langebeck performed the first vaginal hysterectomy in 1813. For the inaugural surgery, Langebeck used no anesthesia, gloves, or other sterilization strategies, and he held the suture in his teeth at one point during the operation! (The patient survived.)1
Despite our dramatic progress since then, too many of us still perform benign hysterectomy by an approach other than vaginal. And too many of us still perform vaginal hysterectomy the way it was taught in the 1950s—frequently a backbreaking, frustrating undertaking.
That approach is unnecessary. In recent years, the technological world has developed many useful tools for minimally invasive gynecologic surgery, some of which greatly facilitate vaginal hysterectomy. In this Update, I focus on 3 of them:
- vessel-sealing devices
- a unique visualization system
- a lighted suction irrigator.
It is my hope that you will incorporate these tools into your vaginal hysterectomy cases and gain some of the significant benefits they have to offer. The revival of vaginal hysterectomy and vaginal surgery in general is all about using the best tools that we have available and using them well, cost- effectively, and thoughtfully to improve the experience of both surgeon and patient.
Why you should default to vaginal hysterectomy
Not only is vaginal hysterectomy more cosmetically pleasing but it also has a lower complication rate than laparoscopic, robot-assisted, or laparotomic hysterectomy, requiring no incisions through the abdominal wall. The original natural orifice translumenal endoscopic surgery (NOTES) procedure also is less expensive than laparoscopic or robot-assisted hysterectomy. Vaginal hysterectomy has so much to recommend it, in fact, that the biggest barrier to widespread use may simply be the lack of industry support.
According to the latest committee opinion from the American College of Obstetricians and Gynecologists (ACOG), “When choosing the route and method of hysterectomy, the physician should take into consideration how the procedure may be performed most safely and cost-effectively to fulfill the medical needs of the patient. Most literature supports the opinion that, when feasible, vaginal hysterectomy is the safest and most cost-effective route by which to remove the uterus.”2
A 2009 Cochrane review of surgical approaches to hysterectomy found that vaginal hysterectomy should be performed in preference to abdominal hysterectomy whenever possible.3 Yet data from 2008 indicate that almost 50% of all hysterectomies were still being performed using an open abdominal approach, and laparoscopic hysterectomy made up almost another 25%.4
To address the disparity between the evidence and practice, ACOG has joined forces with the AAGL and the Society for Gynecologic Surgeons (SGS) to present an online master course on vaginal hysterectomy, available at http://www.aagl.org/vaghystwebinar. This course features videos and live demonstrations on cadaveric models and is free to physicians, with continuing medical education (CME) credits available.
Vessel sealing offers real benefits over suturing
In any surgery, the need to achieve reliable hemostasis is critical. In vaginal hysterectomy, this goal traditionally has been attained by clamping and suturing of the vessels. In many respects, vaginal surgeons seem to have gotten trapped in the mindset that we need to suture during vaginal surgery—and train residents to suture, too. When it comes to laparoscopic surgery, however, the reverse is true. In that setting, vessel-sealing devices are used to seal blood vessels with “supraphysiologic burst pressure equal to that of previously used surgical clips or ligatures.”5
Why is vessel sealing necessarily better than suturing?
It’s safer, for one thing, eliminating the need to pass needles back and forth. It also frees the scrub technician to become the surgical assistant because there are no needles to load and unload. In order for suture to hold around a pedicle, it is necessary to have tissue adjacent to it. The surgeon ties and cuts but must have something beyond the suture or the suture won’t hold. That something is dead, devascularized tissue. Before healing can occur, all this tissue must be absorbed by the body. That is not the case with vessel sealing, which fuses the walls of the blood vessels, leaving less foreign material and dead tissue behind.
What the data show
The literature offers several randomized comparisons of bipolar vessel sealing and suturing during vaginal hysterectomy, and all of them find increased benefits for the vessel-sealing approach.
For example, in 2003, I published a randomized comparison looking specifically at blood loss and operative time.6 Sixty women in a single surgical practice were randomly allocated to vessel sealing or sutures for hemostasis during vaginal hysterectomy. In the vessel-sealing group, the mean operative time was 39.1 minutes (range, 22–93), compared with 53.6 minutes in the suturing group (range, 37–160; P = .003). Mean estimated blood loss also was significantly lower with vessel sealing, at 68.9 mL (range, 20–200) versus 126.7 mL for suturing (range, 25–600; P = .005). Complication rates and length of stay were similar between groups.6
In another randomized trial of vessel sealing versus suturing involving 68 women undergoing vaginal hysterectomy, pain was markedly reduced in the vessel-sealing group (median score, 4 vs 6; P<.0001). Operative time again was shorter with vessel sealing than with suturing (median of 32 vs 40 minutes; P = .003), but there were no differences in blood loss and hospitalization.7
Silva-Filho and colleagues randomly allocated 90 women to bipolar vessel sealing or suturing during vaginal hysterectomy.8 Vessel sealing provided reduced postoperative pain (pain score [SD] of 1.6 [0.4] vs 3.6 [0.4]; P<.001), shorter operative time (mean of 29.2 [2.1] vs 75.2 [5] minutes; P<.001), less blood loss (mean of 84 [5.9] vs 136.4 [89.1] mL; P = .001), and a shorter hospital stay (mean of 25.6 [0.9] vs 33.2 [1.7] hours; P<.001).8
A systematic review and meta-analysis by Kroft and Selk found that vessel sealing reduced: operative time by a mean of 17.2 minutes (95% confidence interval [CI], 7.5–27.0); blood loss by a mean of 47.7 mL (95% CI, 15.5–79.9); and hospital stay by a mean of 0.25 days (95% CI, 0.13–0.37) during vaginal hysterectomy.9
And in a randomized controlled trial from the Netherlands, women undergoing vaginal hysterectomy reported significantly less pain the evening after surgery in the vessel-sealing group, compared with the suturing group (pain score of 4.5 vs 5.7 on a scale of 1 to 10; P = .03).10 They also had a shorter operative time than women in the suturing group (60 vs 71 minutes; P = .05). Blood loss and hospital stays did not differ between groups, and there were no major differences in cost.
A reduction in pain is an especially important indicator of surgical success. In an interesting twist, Candiani and colleagues compared laparoscopic and vaginal hysterectomy for a number of variables, including pain, for benign pathology.11 They found less postoperative pain the day of surgery and a reduced number of days of analgesic request in the laparoscopic group, compared with vaginal hysterectomy. One reason: Hemostasis was achieved via vessel sealing in the laparoscopic group, compared with clamping and suturing in the vaginal group.11
Lighted suction irrigator facilitates visualization “around corners”
Many years ago, I conducted some informal studies for industry that showed—as one might guess intuitively—that the ability to see well during surgery cuts operative time. We all know that light is good. One useful lighting aid I’ve adopted of late is the Vital Vue (Covidien/Medtronic) suction irrigator. It has a disposable tip like all suction devices, but it includes 3 channels: one for a fiber optic cord, another for fluid, and the third for suction (FIGURE 1). It plugs into a regular suction machine, with a reusable box that provides the fiber optic light.
Because the suction tip is curved, the device makes it possible to illuminate the surgical field “around corners” if need be. Any bleeding can be irrigated to clear the field.
How to choose a vessel sealer
When selecting a vessel-sealing device for vaginal hysterectomy, keep in mind a number of factors:
- size of the vessels that will need to be controlled. Most devices on the market today control vessels 7 mm in size or smaller.
- amount of steam it releases, which can damage adjacent tissue
- overall size of the device
- size of the pedicles that will need to be controlled
- overall space required for use
- cost of the device.
In other words, to choose an appropriate device, you will need to think in advance about the specifics of the case you are planning, as not all hysterectomies are alike. The type of vessel sealer best for the surgery will vary with these details.
Both bipolar electrosurgical and ultrasonic devices now provide consistent hemostasis, increased functionality, and greater efficiency. What’s more, they cause minimal to no damage to surrounding tissue.
External scope offers visualization of vaginal procedures to entire OR
Designed for open surgeries, the VITOM system (Karl Storz) is an innovative tool for displaying procedures in which surgical access is limited. It’s an external telescope, or “exoscope,” with a 90° lens. It clips onto the table, providing visualization for the entire operative team (FIGURE 2).
As we all know, the advent of the camera made an enormous difference in laparoscopic procedures and in teaching because it enabled the assistant to see what the surgeon was doing and anticipate his or her needs. This device offers the same advantages for vaginal hysterectomy. In my opinion, it’s a game changer.
The VITOM system provides outstanding image quality and depth of view. It is placed at a distance of 25 cm to 75 cm from the surgical field and thus does not impinge on the surgeon’s workspace. Because it is compact, it facilitates the use of long instruments, if necessary. In addition, because it can be sterilized, the VITOM system can be manipulated directly by the surgeon or assistant.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www.brighamandwomens.org/Departments_and_Services/obgyn/ser vices/mininvgynsurg/mininvoptions/hysterectomy.aspx. Updated October 3, 2014. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- Nezhat C, Lewis M, King LP. Laparoscopic vessel sealing devices. Society of Laparoendoscopic Surgeons. http://laparoscopy.blogs.com/prevention_management_3/2010/10/laparoscopic-vessel-sealing-devices.html. Published 2010. Accessed August 6, 2015.
- Levy B, Emery L. Randomized trial of suture versus electrosurgical bipolar vessel sealing in vaginal hysterectomy. Obstet Gynecol. 2003;102(1):147–151.
- Cronjé HS, de Coning EC. Electrosurgical bipolar vessel sealing during vaginal hysterectomy. Int J Gynaecol Obstet. 2005;91(3):243–245.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
- Kroft J, Selk A. Energy-based vessel sealing in vaginal hysterectomy: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
- Lakeman MM, The S, Schellart RP, et al. Electrosurgical bipolar vessel sealing versus conventional clamping and suturing for vaginal hysterectomy: a randomised controlled trial. BJOG. 2012;119(12):1473–1482.
- Candiani M, Izzo S, Bulfoni A, Riparini J, Ronzoni S, Marconi A. Laparoscopic vs vaginal hysterectomy for benign pathology. Am J Obstet Gynecol. 2009;200(4):368.e1–e7.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www.brighamandwomens.org/Departments_and_Services/obgyn/ser vices/mininvgynsurg/mininvoptions/hysterectomy.aspx. Updated October 3, 2014. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- Nezhat C, Lewis M, King LP. Laparoscopic vessel sealing devices. Society of Laparoendoscopic Surgeons. http://laparoscopy.blogs.com/prevention_management_3/2010/10/laparoscopic-vessel-sealing-devices.html. Published 2010. Accessed August 6, 2015.
- Levy B, Emery L. Randomized trial of suture versus electrosurgical bipolar vessel sealing in vaginal hysterectomy. Obstet Gynecol. 2003;102(1):147–151.
- Cronjé HS, de Coning EC. Electrosurgical bipolar vessel sealing during vaginal hysterectomy. Int J Gynaecol Obstet. 2005;91(3):243–245.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
- Kroft J, Selk A. Energy-based vessel sealing in vaginal hysterectomy: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
- Lakeman MM, The S, Schellart RP, et al. Electrosurgical bipolar vessel sealing versus conventional clamping and suturing for vaginal hysterectomy: a randomised controlled trial. BJOG. 2012;119(12):1473–1482.
- Candiani M, Izzo S, Bulfoni A, Riparini J, Ronzoni S, Marconi A. Laparoscopic vs vaginal hysterectomy for benign pathology. Am J Obstet Gynecol. 2009;200(4):368.e1–e7.
In this Article
- Benefits of vessel sealing over suturing
- Lighted suction irrigator: visualization “around corners”
- External scope offers optimal visualization to the entire team
Update on Sexual Dysfunction
Recently, three new drugs have been added to the armamentarium for menopausal symptoms and dyspareunia:
• Paroxetine 7.5 mg
• Conjugated estrogens and bazedoxifene
• Ospemifene.
In this article, I present a case-based approach to incorporating these drugs into practice and restoring sexual function in the setting of vulvovaginal atrophy and dyspareunia. As is often the case, decision-making requires sifting through multiple layers of information.
CASE: LOW DESIRE AND DISCOMFORT DURING INTERCOURSE
A 58-year-old patient mentions during her annual visit that she’s not that interested in sex anymore. Her children are grown, she’s been happily married for 28 years, and she enjoys her job and denies any symptoms of depression. She says her relationship with her husband is good and, aside from her low desire, she has no worries about the marriage. Her only medication is paroxetine 7.5 mg/d for management of her moderate hot flashes, which she initiated at her last annual visit. She reports improvement in her sleep and menopausal symptoms as a result. She has an intact uterus.
You perform a pelvic exam and find atrophic vulva and vagina with mild erythema and thinned epithelium. When you ask if she has experienced any discomfort, she reports that she needs to use lubrication for intercourse and that, even with lubrication, she has pain upon penetration and a burning sensation that continues throughout intercourse. She also reports that it seems to take her much longer to achieve arousal than in the past, and she often fails to reach orgasm.
How would you manage this patient?
Continued on next page >>
AS ALWAYS, BEGIN WITH THE HISTORY
The transition to menopause creates multiple layers of potential symptoms and problems for our patients, and sometimes medical therapy can generate additional ones.
In a patient reporting the onset of low desire and dyspareunia, you would want to first consider her medication history, despite the clear evidence of vaginal atrophy. Begin by asking whether she is taking any new medications prescribed by another provider. In some cases, antihypertensive drugs, psychotropic agents, and other medications can affect sexual function.
This patient has been taking paroxetine for one year and is happy with its effect on her sleep and hot flashes. Simon and colleagues found this nonhormonal agent for moderate to severe vasomotor symptoms produced no notable effects on weight, libido, or sleep, compared with placebo.
Nevertheless, in this case, because selective serotonin reuptake inhibitors such as paroxetine can affect arousal and orgasm, it is unclear whether the ultra-low dose of paroxetine she is taking is contributing to her problems. If you were to discontinue the drug to find out, her vasomotor symptoms and sleep disruption would likely recur.
Your decision-making is important here and should involve the patient in an extensive discussion. If there is not enough time for this discussion at the current visit, schedule a follow-up to address her concerns fully.
VULVOVAGINAL ATROPHY HAS ITS OWN TIMELINE
In many cases, vasomotor symptoms such as hot flashes occur years before the skin begins to atrophy in the vulva and vagina, particularly in women who enter menopause naturally. Among menopausal women who continue to have intercourse on a regular basis, however, these skin changes often are much less troublesome than they are for women who have sex more rarely.
In this patient, one possible scenario is that paroxetine caused a slight reduction in sexual interest, and the frequency of intercourse went down as a result. In women who have little or no intercourse, the vagina begins to shrink and the tissues lose elasticity. This patient may have been undergoing the natural process of menopause, and that process may have been compounded by a decrease in the frequency of sex.
If you were to discontinue the paroxetine, it would still be necessary to treat the vulvovaginal skin and work on manual techniques to gently dilate the introitus.
Option 1: Systemic hormone therapy >>
Option 1: Systemic hormone therapy
Systemic estrogen is the most effective treatment for menopausal vasomotor symptoms, reducing hot flashes by 50% to 100% within four weeks of initiation. However, because our patient has an intact uterus, any systemic estrogen she opts to use must be opposed by a progestin for safety reasons.
In terms of estrogen, her options are oral or nonoral formulations. Not only would estrogen manage our patient’s hot flashes but, over time, it would improve her sexual problems and atrophy, which might or might not improve her current complaint of low desire. You likely would need to add a short regimen of topical estrogen and perhaps even a dilator to restore her sexual function completely, however.
Since our patient chose the nonhormonal agent paroxetine to manage her menopausal symptoms, she may be worried about the increased risk for breast cancer associated with use of a progestin in combination with estrogen. One hormonal option now available that eliminates the need for a progestin is conjugated estrogens and bazedoxefine. Bazedoxefine is a third-generation selective estrogen receptor modulator. This drug has estrogen-like effects on bone and antiestrogen effects on the uterus.
Conjugated estrogens/bazedoxifene is indicated for use in women with a uterus for treatment of
• Moderate to severe vasomotor symptoms of menopause
• Prevention of postmenopausal osteoporosis.
Among the risks are an increased risk for venous thromboembolism (VTE) and stroke. It is not approved specifically for the treatment of dyspareunia.
Another hormonal option is ospemifene, an estrogen agonist/antagonist indicated for the treatment of moderate to severe dyspareunia in menopausal women. Among the drugs in its class, such as tamoxifen and raloxifene, ospemifene is the only agent that maintains a full estrogenic effect on vaginal tissues. Its risks include VTE and stroke.
Although the labeling includes a warning about the risk for endometrial hyperplasia associated with its use, Goldstein and colleagues found no significant difference in the rate of endometrial thickening greater than 5 mm between women taking ospemifene and those taking placebo after one year of daily oral treatment. No carcinomas were found in either group.
Option 2: Local estrogen
If our patient declines all systemic hormone therapy, the topical approach should resolve her vulvovaginal symptoms, and she could continue taking paroxetine for her menopausal symptoms. Vaginal estrogen would address the skin problems, provided the patient applies it correctly. Many women are afraid to use estrogen creams and compensate by applying them only to the vulva, thinking that, by limiting their use to external tissues, they are avoiding any associated risks.
If she opts for the local approach, this patient should be encouraged to use transvaginal estrogen in small doses to increase the elasticity of the vulvovaginal tissue, even though it may require daily use for a week or two to improve her symptoms, after which once- or twice-weekly administration should suffice.
The use of low-dose vaginal cream for a short duration is unlikely to increase her risks in any way. Local estrogen is available as a tablet, cream, or ring.
Option 3: A nonhormonal approach >>
Option 3: A nonhormonal approach
If the patient refuses any hormonal agent—even topical estrogen—I would recommend the use of silicone-based lubricants and a dilator and prescribe more frequent penetration to increase elasticity and reduce pain. Paroxetine could be continued to address her menopausal symptoms.
DON'T OVERLOOK BEHAVIORAL TECHNIQUES
Before this patient leaves your office with the option of her choice, a bit of counseling is necessary to instruct her about methods of restoring full sexual function.
Pain is a powerful aversive stimulus. This patient clearly states that she has had less frequent intercourse as a result of dyspareunia. It is not unusual for patients to develop a “habit” of avoidance in response to the behavior that causes their pain.
One recommendation is to talk to this patient about putting sex back into her life by encouraging her to increase sexual activity without penetration until she begins to arouse easily again. Arousal produces physiologic effects, increasing the caliber and length of the vagina as well as lubrication. The use of fingers or dilators may help restore caliber.
The patient can be encouraged to engage in snuggling and cuddling to regain those activities without the fear of pain associated with penetration. Follow-up after two weeks of this therapy can confirm the restoration of tissue elasticity, and the green light can be given for penetration to begin again. Couples can be encouraged to plan a “honeymoon weekend” and put some fun back into their sex lives so that this phase of healing doesn’t become an onerous task.
CASE RESOLVED
After a discussion of her options, the patient chooses to stick with paroxetine and use behavioral therapy alone to resolve her dyspareunia. At her follow-up visit two weeks later, she reports that she has enjoyed the period of pain-free “sex” and feels ready to add penetration into her activities.
You encourage her to continue sexual intercourse on a regular, relatively frequent basis to prevent a recurrence of dyspareunia. She continues to use silicone-based lubricants.
Recently, three new drugs have been added to the armamentarium for menopausal symptoms and dyspareunia:
• Paroxetine 7.5 mg
• Conjugated estrogens and bazedoxifene
• Ospemifene.
In this article, I present a case-based approach to incorporating these drugs into practice and restoring sexual function in the setting of vulvovaginal atrophy and dyspareunia. As is often the case, decision-making requires sifting through multiple layers of information.
CASE: LOW DESIRE AND DISCOMFORT DURING INTERCOURSE
A 58-year-old patient mentions during her annual visit that she’s not that interested in sex anymore. Her children are grown, she’s been happily married for 28 years, and she enjoys her job and denies any symptoms of depression. She says her relationship with her husband is good and, aside from her low desire, she has no worries about the marriage. Her only medication is paroxetine 7.5 mg/d for management of her moderate hot flashes, which she initiated at her last annual visit. She reports improvement in her sleep and menopausal symptoms as a result. She has an intact uterus.
You perform a pelvic exam and find atrophic vulva and vagina with mild erythema and thinned epithelium. When you ask if she has experienced any discomfort, she reports that she needs to use lubrication for intercourse and that, even with lubrication, she has pain upon penetration and a burning sensation that continues throughout intercourse. She also reports that it seems to take her much longer to achieve arousal than in the past, and she often fails to reach orgasm.
How would you manage this patient?
Continued on next page >>
AS ALWAYS, BEGIN WITH THE HISTORY
The transition to menopause creates multiple layers of potential symptoms and problems for our patients, and sometimes medical therapy can generate additional ones.
In a patient reporting the onset of low desire and dyspareunia, you would want to first consider her medication history, despite the clear evidence of vaginal atrophy. Begin by asking whether she is taking any new medications prescribed by another provider. In some cases, antihypertensive drugs, psychotropic agents, and other medications can affect sexual function.
This patient has been taking paroxetine for one year and is happy with its effect on her sleep and hot flashes. Simon and colleagues found this nonhormonal agent for moderate to severe vasomotor symptoms produced no notable effects on weight, libido, or sleep, compared with placebo.
Nevertheless, in this case, because selective serotonin reuptake inhibitors such as paroxetine can affect arousal and orgasm, it is unclear whether the ultra-low dose of paroxetine she is taking is contributing to her problems. If you were to discontinue the drug to find out, her vasomotor symptoms and sleep disruption would likely recur.
Your decision-making is important here and should involve the patient in an extensive discussion. If there is not enough time for this discussion at the current visit, schedule a follow-up to address her concerns fully.
VULVOVAGINAL ATROPHY HAS ITS OWN TIMELINE
In many cases, vasomotor symptoms such as hot flashes occur years before the skin begins to atrophy in the vulva and vagina, particularly in women who enter menopause naturally. Among menopausal women who continue to have intercourse on a regular basis, however, these skin changes often are much less troublesome than they are for women who have sex more rarely.
In this patient, one possible scenario is that paroxetine caused a slight reduction in sexual interest, and the frequency of intercourse went down as a result. In women who have little or no intercourse, the vagina begins to shrink and the tissues lose elasticity. This patient may have been undergoing the natural process of menopause, and that process may have been compounded by a decrease in the frequency of sex.
If you were to discontinue the paroxetine, it would still be necessary to treat the vulvovaginal skin and work on manual techniques to gently dilate the introitus.
Option 1: Systemic hormone therapy >>
Option 1: Systemic hormone therapy
Systemic estrogen is the most effective treatment for menopausal vasomotor symptoms, reducing hot flashes by 50% to 100% within four weeks of initiation. However, because our patient has an intact uterus, any systemic estrogen she opts to use must be opposed by a progestin for safety reasons.
In terms of estrogen, her options are oral or nonoral formulations. Not only would estrogen manage our patient’s hot flashes but, over time, it would improve her sexual problems and atrophy, which might or might not improve her current complaint of low desire. You likely would need to add a short regimen of topical estrogen and perhaps even a dilator to restore her sexual function completely, however.
Since our patient chose the nonhormonal agent paroxetine to manage her menopausal symptoms, she may be worried about the increased risk for breast cancer associated with use of a progestin in combination with estrogen. One hormonal option now available that eliminates the need for a progestin is conjugated estrogens and bazedoxefine. Bazedoxefine is a third-generation selective estrogen receptor modulator. This drug has estrogen-like effects on bone and antiestrogen effects on the uterus.
Conjugated estrogens/bazedoxifene is indicated for use in women with a uterus for treatment of
• Moderate to severe vasomotor symptoms of menopause
• Prevention of postmenopausal osteoporosis.
Among the risks are an increased risk for venous thromboembolism (VTE) and stroke. It is not approved specifically for the treatment of dyspareunia.
Another hormonal option is ospemifene, an estrogen agonist/antagonist indicated for the treatment of moderate to severe dyspareunia in menopausal women. Among the drugs in its class, such as tamoxifen and raloxifene, ospemifene is the only agent that maintains a full estrogenic effect on vaginal tissues. Its risks include VTE and stroke.
Although the labeling includes a warning about the risk for endometrial hyperplasia associated with its use, Goldstein and colleagues found no significant difference in the rate of endometrial thickening greater than 5 mm between women taking ospemifene and those taking placebo after one year of daily oral treatment. No carcinomas were found in either group.
Option 2: Local estrogen
If our patient declines all systemic hormone therapy, the topical approach should resolve her vulvovaginal symptoms, and she could continue taking paroxetine for her menopausal symptoms. Vaginal estrogen would address the skin problems, provided the patient applies it correctly. Many women are afraid to use estrogen creams and compensate by applying them only to the vulva, thinking that, by limiting their use to external tissues, they are avoiding any associated risks.
If she opts for the local approach, this patient should be encouraged to use transvaginal estrogen in small doses to increase the elasticity of the vulvovaginal tissue, even though it may require daily use for a week or two to improve her symptoms, after which once- or twice-weekly administration should suffice.
The use of low-dose vaginal cream for a short duration is unlikely to increase her risks in any way. Local estrogen is available as a tablet, cream, or ring.
Option 3: A nonhormonal approach >>
Option 3: A nonhormonal approach
If the patient refuses any hormonal agent—even topical estrogen—I would recommend the use of silicone-based lubricants and a dilator and prescribe more frequent penetration to increase elasticity and reduce pain. Paroxetine could be continued to address her menopausal symptoms.
DON'T OVERLOOK BEHAVIORAL TECHNIQUES
Before this patient leaves your office with the option of her choice, a bit of counseling is necessary to instruct her about methods of restoring full sexual function.
Pain is a powerful aversive stimulus. This patient clearly states that she has had less frequent intercourse as a result of dyspareunia. It is not unusual for patients to develop a “habit” of avoidance in response to the behavior that causes their pain.
One recommendation is to talk to this patient about putting sex back into her life by encouraging her to increase sexual activity without penetration until she begins to arouse easily again. Arousal produces physiologic effects, increasing the caliber and length of the vagina as well as lubrication. The use of fingers or dilators may help restore caliber.
The patient can be encouraged to engage in snuggling and cuddling to regain those activities without the fear of pain associated with penetration. Follow-up after two weeks of this therapy can confirm the restoration of tissue elasticity, and the green light can be given for penetration to begin again. Couples can be encouraged to plan a “honeymoon weekend” and put some fun back into their sex lives so that this phase of healing doesn’t become an onerous task.
CASE RESOLVED
After a discussion of her options, the patient chooses to stick with paroxetine and use behavioral therapy alone to resolve her dyspareunia. At her follow-up visit two weeks later, she reports that she has enjoyed the period of pain-free “sex” and feels ready to add penetration into her activities.
You encourage her to continue sexual intercourse on a regular, relatively frequent basis to prevent a recurrence of dyspareunia. She continues to use silicone-based lubricants.
Recently, three new drugs have been added to the armamentarium for menopausal symptoms and dyspareunia:
• Paroxetine 7.5 mg
• Conjugated estrogens and bazedoxifene
• Ospemifene.
In this article, I present a case-based approach to incorporating these drugs into practice and restoring sexual function in the setting of vulvovaginal atrophy and dyspareunia. As is often the case, decision-making requires sifting through multiple layers of information.
CASE: LOW DESIRE AND DISCOMFORT DURING INTERCOURSE
A 58-year-old patient mentions during her annual visit that she’s not that interested in sex anymore. Her children are grown, she’s been happily married for 28 years, and she enjoys her job and denies any symptoms of depression. She says her relationship with her husband is good and, aside from her low desire, she has no worries about the marriage. Her only medication is paroxetine 7.5 mg/d for management of her moderate hot flashes, which she initiated at her last annual visit. She reports improvement in her sleep and menopausal symptoms as a result. She has an intact uterus.
You perform a pelvic exam and find atrophic vulva and vagina with mild erythema and thinned epithelium. When you ask if she has experienced any discomfort, she reports that she needs to use lubrication for intercourse and that, even with lubrication, she has pain upon penetration and a burning sensation that continues throughout intercourse. She also reports that it seems to take her much longer to achieve arousal than in the past, and she often fails to reach orgasm.
How would you manage this patient?
Continued on next page >>
AS ALWAYS, BEGIN WITH THE HISTORY
The transition to menopause creates multiple layers of potential symptoms and problems for our patients, and sometimes medical therapy can generate additional ones.
In a patient reporting the onset of low desire and dyspareunia, you would want to first consider her medication history, despite the clear evidence of vaginal atrophy. Begin by asking whether she is taking any new medications prescribed by another provider. In some cases, antihypertensive drugs, psychotropic agents, and other medications can affect sexual function.
This patient has been taking paroxetine for one year and is happy with its effect on her sleep and hot flashes. Simon and colleagues found this nonhormonal agent for moderate to severe vasomotor symptoms produced no notable effects on weight, libido, or sleep, compared with placebo.
Nevertheless, in this case, because selective serotonin reuptake inhibitors such as paroxetine can affect arousal and orgasm, it is unclear whether the ultra-low dose of paroxetine she is taking is contributing to her problems. If you were to discontinue the drug to find out, her vasomotor symptoms and sleep disruption would likely recur.
Your decision-making is important here and should involve the patient in an extensive discussion. If there is not enough time for this discussion at the current visit, schedule a follow-up to address her concerns fully.
VULVOVAGINAL ATROPHY HAS ITS OWN TIMELINE
In many cases, vasomotor symptoms such as hot flashes occur years before the skin begins to atrophy in the vulva and vagina, particularly in women who enter menopause naturally. Among menopausal women who continue to have intercourse on a regular basis, however, these skin changes often are much less troublesome than they are for women who have sex more rarely.
In this patient, one possible scenario is that paroxetine caused a slight reduction in sexual interest, and the frequency of intercourse went down as a result. In women who have little or no intercourse, the vagina begins to shrink and the tissues lose elasticity. This patient may have been undergoing the natural process of menopause, and that process may have been compounded by a decrease in the frequency of sex.
If you were to discontinue the paroxetine, it would still be necessary to treat the vulvovaginal skin and work on manual techniques to gently dilate the introitus.
Option 1: Systemic hormone therapy >>
Option 1: Systemic hormone therapy
Systemic estrogen is the most effective treatment for menopausal vasomotor symptoms, reducing hot flashes by 50% to 100% within four weeks of initiation. However, because our patient has an intact uterus, any systemic estrogen she opts to use must be opposed by a progestin for safety reasons.
In terms of estrogen, her options are oral or nonoral formulations. Not only would estrogen manage our patient’s hot flashes but, over time, it would improve her sexual problems and atrophy, which might or might not improve her current complaint of low desire. You likely would need to add a short regimen of topical estrogen and perhaps even a dilator to restore her sexual function completely, however.
Since our patient chose the nonhormonal agent paroxetine to manage her menopausal symptoms, she may be worried about the increased risk for breast cancer associated with use of a progestin in combination with estrogen. One hormonal option now available that eliminates the need for a progestin is conjugated estrogens and bazedoxefine. Bazedoxefine is a third-generation selective estrogen receptor modulator. This drug has estrogen-like effects on bone and antiestrogen effects on the uterus.
Conjugated estrogens/bazedoxifene is indicated for use in women with a uterus for treatment of
• Moderate to severe vasomotor symptoms of menopause
• Prevention of postmenopausal osteoporosis.
Among the risks are an increased risk for venous thromboembolism (VTE) and stroke. It is not approved specifically for the treatment of dyspareunia.
Another hormonal option is ospemifene, an estrogen agonist/antagonist indicated for the treatment of moderate to severe dyspareunia in menopausal women. Among the drugs in its class, such as tamoxifen and raloxifene, ospemifene is the only agent that maintains a full estrogenic effect on vaginal tissues. Its risks include VTE and stroke.
Although the labeling includes a warning about the risk for endometrial hyperplasia associated with its use, Goldstein and colleagues found no significant difference in the rate of endometrial thickening greater than 5 mm between women taking ospemifene and those taking placebo after one year of daily oral treatment. No carcinomas were found in either group.
Option 2: Local estrogen
If our patient declines all systemic hormone therapy, the topical approach should resolve her vulvovaginal symptoms, and she could continue taking paroxetine for her menopausal symptoms. Vaginal estrogen would address the skin problems, provided the patient applies it correctly. Many women are afraid to use estrogen creams and compensate by applying them only to the vulva, thinking that, by limiting their use to external tissues, they are avoiding any associated risks.
If she opts for the local approach, this patient should be encouraged to use transvaginal estrogen in small doses to increase the elasticity of the vulvovaginal tissue, even though it may require daily use for a week or two to improve her symptoms, after which once- or twice-weekly administration should suffice.
The use of low-dose vaginal cream for a short duration is unlikely to increase her risks in any way. Local estrogen is available as a tablet, cream, or ring.
Option 3: A nonhormonal approach >>
Option 3: A nonhormonal approach
If the patient refuses any hormonal agent—even topical estrogen—I would recommend the use of silicone-based lubricants and a dilator and prescribe more frequent penetration to increase elasticity and reduce pain. Paroxetine could be continued to address her menopausal symptoms.
DON'T OVERLOOK BEHAVIORAL TECHNIQUES
Before this patient leaves your office with the option of her choice, a bit of counseling is necessary to instruct her about methods of restoring full sexual function.
Pain is a powerful aversive stimulus. This patient clearly states that she has had less frequent intercourse as a result of dyspareunia. It is not unusual for patients to develop a “habit” of avoidance in response to the behavior that causes their pain.
One recommendation is to talk to this patient about putting sex back into her life by encouraging her to increase sexual activity without penetration until she begins to arouse easily again. Arousal produces physiologic effects, increasing the caliber and length of the vagina as well as lubrication. The use of fingers or dilators may help restore caliber.
The patient can be encouraged to engage in snuggling and cuddling to regain those activities without the fear of pain associated with penetration. Follow-up after two weeks of this therapy can confirm the restoration of tissue elasticity, and the green light can be given for penetration to begin again. Couples can be encouraged to plan a “honeymoon weekend” and put some fun back into their sex lives so that this phase of healing doesn’t become an onerous task.
CASE RESOLVED
After a discussion of her options, the patient chooses to stick with paroxetine and use behavioral therapy alone to resolve her dyspareunia. At her follow-up visit two weeks later, she reports that she has enjoyed the period of pain-free “sex” and feels ready to add penetration into her activities.
You encourage her to continue sexual intercourse on a regular, relatively frequent basis to prevent a recurrence of dyspareunia. She continues to use silicone-based lubricants.
The economics of gynecologic surgery: 13 coding tips to ensure fair payment
The payment structure for physicians is changing. Our government, the American public, purchasers, and employers are unhappy with the fee-for-service system as it currently exists, and are pushing to drive the system into what is called “value-based purchasing.”
But what is value?
One way to define it is quality divided by cost—but how do we measure quality?
At present, insurers are measuring your quality based on some nebulous definition created at United Healthcare or Blue Cross Blue Shield—looking specifically at your “efficiency,” based on the costs attributed to you, as revealed in the codes you and others submit to payers.
Let’s say you perform minimally invasive surgery, and the referring physician ordered a lot of tests before sending the patient to you. Are you aware that all of those costs may be attributed to you in an administrative system?
ACOG is working hard to establish clinical systems rather than administrative ones to determine the true cost of care. We may want to think of obstetrics and gynecology as primary care and take advantage of advanced payment models and the opportunities afforded to accountable care organizations, but the truth is, insurers frequently do not consider us primary care. Although some of us may develop medical homes for women’s health care, we are unlikely to collect a per-patient, per-month income like primary care physicians do. That means that we need to be more assertive in negotiating contracts with insurers.
In this article, I offer recommendations for such negotiations and explain how to determine what you can and cannot accept in terms of payment.
You are the responsible party
Some of us do our own coding and some of us do not. However, if that coding is inaccurate, it is the physician who goes to jail, not the coder. You are personally responsible and liable for the coding submitted under your provider number.
Clearly, we need to do a better job of advocating for ourselves. We need to lobby. Legislators and bureaucrats are less likely to target people who have strong lobbyists working consistently on their behalf.
Accountable care organizations may have some leverage in negotiating lower prices, and some market forces may come into play in large systems. It remains to be seen which models will succeed as new payment structures develop. The overarching question: What can we do today to optimize our payments, given the system that we have? Here are 13 tactics that can enhance your bottom line.
1. Know the rules
To play the game, you must know the rules. You need to know what systems payers are using to determine your reimbursement—and you have to understand those systems as well as, or better than, the payers do. Then you’ll be able to use them to your advantage.
Payers are well aware that we don’t like to focus on this end of practice, that what we really want to do is spend the day practicing medicine. However, we need to learn these details because we’re leaving money on the table every single day.
2. Educate yourself
With the change to the International Classification of Diseases (ICD) scheduled to take effect on October 1, 2015, many of us are worried that payers are going to reject our claims because of our lack of familiarity with ICD-10.
Rest assured. There are crosswalks from ICD-9 to ICD-10. ACOG has published an information sheet for both obstetrics and gynecology that pairs typically used ICD-9 codes with their ICD-10 counterparts. And because it is published by ACOG, payers will find it hard to claim that it’s inaccurate.
ACOG also offers half-day courses on ICD-10 coding for both physicians and staff.
3. Record your decision-making process
When I audit medical charts, I often discover that this process has been neglected. Instead, the coder has relied on documentation from the electronic health record and a basic description of the treatment plan. But a plan is just that—what someone intends to do. It doesn’t convey the decision-making that underlies it. What was the differential diagnosis? What did you discuss with the patient? These details are critical for appropriate coding of the level of service—whether it’s high, intermediate, or low.
4. Refine your approach to coding
Recognize that the system is currently set up to pay physicians for the services we provide—and that service must be justified by the appropriate diagnosis code. Tougher cases, or high-risk patients, tend to have longer surgeries and hospital stays, and their outcomes often are not as good as those of more typical patients. They may have more complications because they’re obese or have severe diabetes, for example. If so, it is critical that these other conditions—obesity and severe diabetes—be included with the principal diagnosis code so that risk stratification is possible. Otherwise, we will be held to the same standard as someone treating a routine, low-risk case.
Risk stratification is being performed according to algorithms in the payers’ software—and payers are unlikely to share the details with us. However, the only real data payers have to run through these algorithms come from diagnosis coding. Even though you’re not required to code for variables such as obesity and diabetes in order to get paid for what you do, you do need to use those additional codes to make risk stratification possible—so that you don’t get inappropriately placed into a group of low-risk providers when you are treating a higher-risk cohort.
5. Develop an understanding of RVUs
Another variable that changes regularly is relative value units (RVUs) under Medicare rules. ACOG’s Committee on Health Economics and Coding—which enjoys the participation of AAGL, the American Urogynecologic Society (AUGS), the Society of Gynecologic Surgeons (SGS), and the Society of Gynecologic Oncology (SGO), as well as other organizations—tries to maintain the RVUs as up to date and appropriate as possible relative to other services in the fee schedule.
For example, about 10 years ago many urogynecologic procedures were getting bundled together when they were performed at the same time. We had only one or two ICD-9 codes to describe prolapse, with no separate codes to describe whether it affected the anterior, apical, or posterior compartment, even though we performed different procedures in the individual compartments. Payers were mapping all prolapse procedures to the same diagnosis code. So ACOG went to the National Center for Health Statistics, where ICD-9 coding was done—and developed a series of about 10 codes to describe the different areas that prolapse could affect.
That kind of nuanced coding is continuing today. In fact, we have a long list of areas to go forward with now that ICD-10 is scheduled to take effect. A good example involves new Pap smear guidelines, which recommend testing every 3 or 5 years except for patients who have undergone hysterectomy for benign disease. How do you code for a patient who has had a hysterectomy? There was no code for a woman with an absent cervix, so we created a “V-code,” a code classification for factors that influence health status, so that it is possible to explain why a Pap smear was not performed.
As we go forward into a value-based system, specialists like us likely will be negotiating contracts according to RVU-based payments. That’s why it’s important for you to understand the resource-based relative value scale (RBRVS). It has three components: a work component, which makes up about 52% of the total RVUs; a practice expense, which makes up more than 45% of total RVUS; and, finally, a malpractice component, a small percentage. There also is a geographic adjustment and a uniform conversion factor.
When you hear about the sustainable growth rate (SGR) fix, and the fact that we’re going to see a 20% or 24% reduction in payment, that talk is referring to a reduction in the conversion factor. Each component of the RVU is adjusted for geography and then multiplied by the dollar conversion factor to calculate the total RVUs. The work, practice, and malpractice components vary by where the service is provided.
Let’s use placement of Essure inserts as an example. If you perform the procedure in the hospital, then the hospital buys the equipment, including the hysteroscope and light source. The hospital also pays for the room and staff and manages equipment sterilization. If, on the other hand, you perform the procedure in your office, all those responsibilities are yours. If it’s done in your office, you get paid more but it also costs you more.
The Relative Value Update Committee, or RUC, plays a major role in determining RVUs. This committee is composed of 31 clinicians, including nonphysician providers, psychologists, and nurses who deliver services under the Medicare fee schedule. The RUC makes recommendations to the Centers for Medicare and Medicaid Services (CMS), but it is the Secretary of Health and Human Services who determines the final rule on RVUs.
Approximately 75% to 95% of the recommendations of the RUC are accepted by the Secretary and become law. So it’s not the RUC or the American Medical Association (AMA) that determines RVUs; in the long run, it is CMS and the Secretary of Health and Human Services. We are fortunate that, when CMS assigns RVUs we’re not happy with, we have an opportunity to appeal.
Under Medicare, all physician payments are based on the same conversion factor, regardless of specialty. That’s not necessarily true for other payers, who may, essentially, do whatever they wish. These other payers frequently will contract at higher or lower rates, depending on how prevalent a specialist is in the community. Sometimes they use a higher conversion factor for surgical specialists than they use for primary care.
6. Find out which RVUs the payer is using
When you negotiate contracts with payers, and you are in private practice or part of a medical practice, it’s important to know what year’s RVUs the payer is using, as RVUs vary from year to year. For example, if the payer is using the RBRVS from 2002, it is paying you less than you should be getting. So when you look at a contract, you should determine not only whether the payer is anchoring your payment to the RBRVS but also whether it is keeping up with current RVUs as well. What dollar conversion factor is the payer using? What global periods—the same as CMS, or something different?
7. Determine what global period is in play
Some private payers use 6 postoperative weeks as the global period for a surgical procedure, whereas Medicare uses 90 days. You need to know which period is in play so that you don’t leave money on the table if you see the patient within 90 days but more than 6 weeks postoperatively.
Current Procedural Terminology (CPT) has global surgical packages that include a 10-day or 90-day period. But those periods do not include services provided more than 24 hours before the procedure. They don’t include the administration of anesthesia or conscious sedation. And they don’t include management of complications, exacerbations, or recurrences. Nor do they include additional services that might be necessary due to the presence of another disease or injury.
Under Medicare, the rules are different. Medicare preoperative services begin 1 day before surgery. However, any preoperative intervention is included whether it’s performed 1 day or 1 week before surgery. If it’s simply a preoperative physical examination for the patient and you aren’t performing significant evaluation and management, it’s included in the global package, along with all the intraoperative work. In addition, under Medicare, you don’t get paid for the management of complications unless a return to the operating room is required.
8. Learn to use modifiers
As ObGyns, we often see patients for multiple conditions or problem reports, so you need to be aware that if a patient is within a global period and you do not submit a bill with a modifier to indicate special circumstances, the intervention will be bundled into the global and you will not get paid for it. Modifiers are two-digit codes that describe these separate services. They provide critical information to payers so that their computer programs separate these services out for payment.
Major surgical procedures don’t include unrelated procedures that are performed at the same time of surgery. Nor do they include visits that take place during the global period that are unrelated to the original surgery. For example, if a patient presents with a breast lump after you performed a hysterectomy, and you do a work-up, you deserve full payment for that evaluation and management service. If you don’t use a modifier, however, you won’t get that payment.
9. Don’t be passive when payers won’t pay
Let’s say you contract with HMOs or independent practice associations (IPAs), and they’re not compensating you for the extra things you’re doing and are failing to recognize surgical modifiers. What can you do about it?
You need to develop a profile of your typical patient. Because these organizations are individualizing it—they are saying that, in a typical scenario, this is the type of work you do. So these organizations offer a different kind of contract. Nevertheless, you can use your coding to help you determine what a fair payment should be, by going through your billing to determine what you’ve spent.
10. Analyze payer bundling
Medicare put in place a correct coding initiative (CCI) that lists services typically provided by the same person on the same day of service. The aim: to prevent separate payment for these services. These are “bundled” services. The CCI bundles are revised every quarter. They are listed on the ACOG Web site under “practice management.”
On October 1, 2014, the CCI inappropriately bundled pelvic organ prolapse repair procedures into the vaginal hysterectomy codes. ACOG, AUGS, SGS, and AAGL are arguing vehemently as this article is going to press to ensure that these damaging bundles are rescinded.
Private payers can bundle anything, and it may or may not make sense or be fair. One ACOG resource is the book Ob/Gyn Coding Manual: Components of Correct Procedural Coding, which is revised every year. It has a tear-out page for every procedure code and will help you determine whether or not a bundle is appropriate.
You need to know about bundling and dispute resolution. Why? Because it is possible to insert clauses into your contract that give you some rights. Insurers have all the clout and you have nothing unless you fight for it.
You may see clauses such as “the company reserves the right to re-bundle to the primary procedure....” You shouldn’t tolerate that. Rather, you want to say, “the company will use CCI bundled rules” so that you at least know what the rules are.
11. Don’t be afraid to revise a contract
If we have to hold a payer harmless, the payer should hold us harmless as well. If we consult an insurer’s Web site to confirm that a patient is covered, and we take her to surgery because we have evidence she has insurance, the insurer shouldn’t be able to rescind payment 6 months later because the patient didn’t pay for her insurance that month. That’s not fair. The company told you she was covered, and you deserve to get paid for that surgery because you are relying on information from the company itself. So when you sign a contract, you need to ensure that you are being held harmless as well as the insurer.
12. Calculate your own RVUs
Use your claims software for data. Consult the Federal Register or ACOG to determine the total number of RVUs for a given CPT code. Multiply the RVUs by the quantity for each code. Let’s say it’s an evaluation and management visit, code 99213, and you’ve done 50 this month. That’s 50 multiplied by 1.3 RVUs. Add all the codes together, then use your monthly profit and loss statement to determine what your expenses are. Divide your total expenses by the total number of RVUs to determine your practice cost per RVU. You then can decide on a conversion factor you can tolerate, and you can use this information when contracting with IPAs, HMOs, and other insurers.
13. Spend money to make money
There are many coding resources available to you. Coding is well worth what you spend on it because you can get it back in a heartbeat.
This information may not be easy to master, but it’s critically important for your economic survival—to get what’s rightfully yours and get paid fairly for what you do.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The payment structure for physicians is changing. Our government, the American public, purchasers, and employers are unhappy with the fee-for-service system as it currently exists, and are pushing to drive the system into what is called “value-based purchasing.”
But what is value?
One way to define it is quality divided by cost—but how do we measure quality?
At present, insurers are measuring your quality based on some nebulous definition created at United Healthcare or Blue Cross Blue Shield—looking specifically at your “efficiency,” based on the costs attributed to you, as revealed in the codes you and others submit to payers.
Let’s say you perform minimally invasive surgery, and the referring physician ordered a lot of tests before sending the patient to you. Are you aware that all of those costs may be attributed to you in an administrative system?
ACOG is working hard to establish clinical systems rather than administrative ones to determine the true cost of care. We may want to think of obstetrics and gynecology as primary care and take advantage of advanced payment models and the opportunities afforded to accountable care organizations, but the truth is, insurers frequently do not consider us primary care. Although some of us may develop medical homes for women’s health care, we are unlikely to collect a per-patient, per-month income like primary care physicians do. That means that we need to be more assertive in negotiating contracts with insurers.
In this article, I offer recommendations for such negotiations and explain how to determine what you can and cannot accept in terms of payment.
You are the responsible party
Some of us do our own coding and some of us do not. However, if that coding is inaccurate, it is the physician who goes to jail, not the coder. You are personally responsible and liable for the coding submitted under your provider number.
Clearly, we need to do a better job of advocating for ourselves. We need to lobby. Legislators and bureaucrats are less likely to target people who have strong lobbyists working consistently on their behalf.
Accountable care organizations may have some leverage in negotiating lower prices, and some market forces may come into play in large systems. It remains to be seen which models will succeed as new payment structures develop. The overarching question: What can we do today to optimize our payments, given the system that we have? Here are 13 tactics that can enhance your bottom line.
1. Know the rules
To play the game, you must know the rules. You need to know what systems payers are using to determine your reimbursement—and you have to understand those systems as well as, or better than, the payers do. Then you’ll be able to use them to your advantage.
Payers are well aware that we don’t like to focus on this end of practice, that what we really want to do is spend the day practicing medicine. However, we need to learn these details because we’re leaving money on the table every single day.
2. Educate yourself
With the change to the International Classification of Diseases (ICD) scheduled to take effect on October 1, 2015, many of us are worried that payers are going to reject our claims because of our lack of familiarity with ICD-10.
Rest assured. There are crosswalks from ICD-9 to ICD-10. ACOG has published an information sheet for both obstetrics and gynecology that pairs typically used ICD-9 codes with their ICD-10 counterparts. And because it is published by ACOG, payers will find it hard to claim that it’s inaccurate.
ACOG also offers half-day courses on ICD-10 coding for both physicians and staff.
3. Record your decision-making process
When I audit medical charts, I often discover that this process has been neglected. Instead, the coder has relied on documentation from the electronic health record and a basic description of the treatment plan. But a plan is just that—what someone intends to do. It doesn’t convey the decision-making that underlies it. What was the differential diagnosis? What did you discuss with the patient? These details are critical for appropriate coding of the level of service—whether it’s high, intermediate, or low.
4. Refine your approach to coding
Recognize that the system is currently set up to pay physicians for the services we provide—and that service must be justified by the appropriate diagnosis code. Tougher cases, or high-risk patients, tend to have longer surgeries and hospital stays, and their outcomes often are not as good as those of more typical patients. They may have more complications because they’re obese or have severe diabetes, for example. If so, it is critical that these other conditions—obesity and severe diabetes—be included with the principal diagnosis code so that risk stratification is possible. Otherwise, we will be held to the same standard as someone treating a routine, low-risk case.
Risk stratification is being performed according to algorithms in the payers’ software—and payers are unlikely to share the details with us. However, the only real data payers have to run through these algorithms come from diagnosis coding. Even though you’re not required to code for variables such as obesity and diabetes in order to get paid for what you do, you do need to use those additional codes to make risk stratification possible—so that you don’t get inappropriately placed into a group of low-risk providers when you are treating a higher-risk cohort.
5. Develop an understanding of RVUs
Another variable that changes regularly is relative value units (RVUs) under Medicare rules. ACOG’s Committee on Health Economics and Coding—which enjoys the participation of AAGL, the American Urogynecologic Society (AUGS), the Society of Gynecologic Surgeons (SGS), and the Society of Gynecologic Oncology (SGO), as well as other organizations—tries to maintain the RVUs as up to date and appropriate as possible relative to other services in the fee schedule.
For example, about 10 years ago many urogynecologic procedures were getting bundled together when they were performed at the same time. We had only one or two ICD-9 codes to describe prolapse, with no separate codes to describe whether it affected the anterior, apical, or posterior compartment, even though we performed different procedures in the individual compartments. Payers were mapping all prolapse procedures to the same diagnosis code. So ACOG went to the National Center for Health Statistics, where ICD-9 coding was done—and developed a series of about 10 codes to describe the different areas that prolapse could affect.
That kind of nuanced coding is continuing today. In fact, we have a long list of areas to go forward with now that ICD-10 is scheduled to take effect. A good example involves new Pap smear guidelines, which recommend testing every 3 or 5 years except for patients who have undergone hysterectomy for benign disease. How do you code for a patient who has had a hysterectomy? There was no code for a woman with an absent cervix, so we created a “V-code,” a code classification for factors that influence health status, so that it is possible to explain why a Pap smear was not performed.
As we go forward into a value-based system, specialists like us likely will be negotiating contracts according to RVU-based payments. That’s why it’s important for you to understand the resource-based relative value scale (RBRVS). It has three components: a work component, which makes up about 52% of the total RVUs; a practice expense, which makes up more than 45% of total RVUS; and, finally, a malpractice component, a small percentage. There also is a geographic adjustment and a uniform conversion factor.
When you hear about the sustainable growth rate (SGR) fix, and the fact that we’re going to see a 20% or 24% reduction in payment, that talk is referring to a reduction in the conversion factor. Each component of the RVU is adjusted for geography and then multiplied by the dollar conversion factor to calculate the total RVUs. The work, practice, and malpractice components vary by where the service is provided.
Let’s use placement of Essure inserts as an example. If you perform the procedure in the hospital, then the hospital buys the equipment, including the hysteroscope and light source. The hospital also pays for the room and staff and manages equipment sterilization. If, on the other hand, you perform the procedure in your office, all those responsibilities are yours. If it’s done in your office, you get paid more but it also costs you more.
The Relative Value Update Committee, or RUC, plays a major role in determining RVUs. This committee is composed of 31 clinicians, including nonphysician providers, psychologists, and nurses who deliver services under the Medicare fee schedule. The RUC makes recommendations to the Centers for Medicare and Medicaid Services (CMS), but it is the Secretary of Health and Human Services who determines the final rule on RVUs.
Approximately 75% to 95% of the recommendations of the RUC are accepted by the Secretary and become law. So it’s not the RUC or the American Medical Association (AMA) that determines RVUs; in the long run, it is CMS and the Secretary of Health and Human Services. We are fortunate that, when CMS assigns RVUs we’re not happy with, we have an opportunity to appeal.
Under Medicare, all physician payments are based on the same conversion factor, regardless of specialty. That’s not necessarily true for other payers, who may, essentially, do whatever they wish. These other payers frequently will contract at higher or lower rates, depending on how prevalent a specialist is in the community. Sometimes they use a higher conversion factor for surgical specialists than they use for primary care.
6. Find out which RVUs the payer is using
When you negotiate contracts with payers, and you are in private practice or part of a medical practice, it’s important to know what year’s RVUs the payer is using, as RVUs vary from year to year. For example, if the payer is using the RBRVS from 2002, it is paying you less than you should be getting. So when you look at a contract, you should determine not only whether the payer is anchoring your payment to the RBRVS but also whether it is keeping up with current RVUs as well. What dollar conversion factor is the payer using? What global periods—the same as CMS, or something different?
7. Determine what global period is in play
Some private payers use 6 postoperative weeks as the global period for a surgical procedure, whereas Medicare uses 90 days. You need to know which period is in play so that you don’t leave money on the table if you see the patient within 90 days but more than 6 weeks postoperatively.
Current Procedural Terminology (CPT) has global surgical packages that include a 10-day or 90-day period. But those periods do not include services provided more than 24 hours before the procedure. They don’t include the administration of anesthesia or conscious sedation. And they don’t include management of complications, exacerbations, or recurrences. Nor do they include additional services that might be necessary due to the presence of another disease or injury.
Under Medicare, the rules are different. Medicare preoperative services begin 1 day before surgery. However, any preoperative intervention is included whether it’s performed 1 day or 1 week before surgery. If it’s simply a preoperative physical examination for the patient and you aren’t performing significant evaluation and management, it’s included in the global package, along with all the intraoperative work. In addition, under Medicare, you don’t get paid for the management of complications unless a return to the operating room is required.
8. Learn to use modifiers
As ObGyns, we often see patients for multiple conditions or problem reports, so you need to be aware that if a patient is within a global period and you do not submit a bill with a modifier to indicate special circumstances, the intervention will be bundled into the global and you will not get paid for it. Modifiers are two-digit codes that describe these separate services. They provide critical information to payers so that their computer programs separate these services out for payment.
Major surgical procedures don’t include unrelated procedures that are performed at the same time of surgery. Nor do they include visits that take place during the global period that are unrelated to the original surgery. For example, if a patient presents with a breast lump after you performed a hysterectomy, and you do a work-up, you deserve full payment for that evaluation and management service. If you don’t use a modifier, however, you won’t get that payment.
9. Don’t be passive when payers won’t pay
Let’s say you contract with HMOs or independent practice associations (IPAs), and they’re not compensating you for the extra things you’re doing and are failing to recognize surgical modifiers. What can you do about it?
You need to develop a profile of your typical patient. Because these organizations are individualizing it—they are saying that, in a typical scenario, this is the type of work you do. So these organizations offer a different kind of contract. Nevertheless, you can use your coding to help you determine what a fair payment should be, by going through your billing to determine what you’ve spent.
10. Analyze payer bundling
Medicare put in place a correct coding initiative (CCI) that lists services typically provided by the same person on the same day of service. The aim: to prevent separate payment for these services. These are “bundled” services. The CCI bundles are revised every quarter. They are listed on the ACOG Web site under “practice management.”
On October 1, 2014, the CCI inappropriately bundled pelvic organ prolapse repair procedures into the vaginal hysterectomy codes. ACOG, AUGS, SGS, and AAGL are arguing vehemently as this article is going to press to ensure that these damaging bundles are rescinded.
Private payers can bundle anything, and it may or may not make sense or be fair. One ACOG resource is the book Ob/Gyn Coding Manual: Components of Correct Procedural Coding, which is revised every year. It has a tear-out page for every procedure code and will help you determine whether or not a bundle is appropriate.
You need to know about bundling and dispute resolution. Why? Because it is possible to insert clauses into your contract that give you some rights. Insurers have all the clout and you have nothing unless you fight for it.
You may see clauses such as “the company reserves the right to re-bundle to the primary procedure....” You shouldn’t tolerate that. Rather, you want to say, “the company will use CCI bundled rules” so that you at least know what the rules are.
11. Don’t be afraid to revise a contract
If we have to hold a payer harmless, the payer should hold us harmless as well. If we consult an insurer’s Web site to confirm that a patient is covered, and we take her to surgery because we have evidence she has insurance, the insurer shouldn’t be able to rescind payment 6 months later because the patient didn’t pay for her insurance that month. That’s not fair. The company told you she was covered, and you deserve to get paid for that surgery because you are relying on information from the company itself. So when you sign a contract, you need to ensure that you are being held harmless as well as the insurer.
12. Calculate your own RVUs
Use your claims software for data. Consult the Federal Register or ACOG to determine the total number of RVUs for a given CPT code. Multiply the RVUs by the quantity for each code. Let’s say it’s an evaluation and management visit, code 99213, and you’ve done 50 this month. That’s 50 multiplied by 1.3 RVUs. Add all the codes together, then use your monthly profit and loss statement to determine what your expenses are. Divide your total expenses by the total number of RVUs to determine your practice cost per RVU. You then can decide on a conversion factor you can tolerate, and you can use this information when contracting with IPAs, HMOs, and other insurers.
13. Spend money to make money
There are many coding resources available to you. Coding is well worth what you spend on it because you can get it back in a heartbeat.
This information may not be easy to master, but it’s critically important for your economic survival—to get what’s rightfully yours and get paid fairly for what you do.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The payment structure for physicians is changing. Our government, the American public, purchasers, and employers are unhappy with the fee-for-service system as it currently exists, and are pushing to drive the system into what is called “value-based purchasing.”
But what is value?
One way to define it is quality divided by cost—but how do we measure quality?
At present, insurers are measuring your quality based on some nebulous definition created at United Healthcare or Blue Cross Blue Shield—looking specifically at your “efficiency,” based on the costs attributed to you, as revealed in the codes you and others submit to payers.
Let’s say you perform minimally invasive surgery, and the referring physician ordered a lot of tests before sending the patient to you. Are you aware that all of those costs may be attributed to you in an administrative system?
ACOG is working hard to establish clinical systems rather than administrative ones to determine the true cost of care. We may want to think of obstetrics and gynecology as primary care and take advantage of advanced payment models and the opportunities afforded to accountable care organizations, but the truth is, insurers frequently do not consider us primary care. Although some of us may develop medical homes for women’s health care, we are unlikely to collect a per-patient, per-month income like primary care physicians do. That means that we need to be more assertive in negotiating contracts with insurers.
In this article, I offer recommendations for such negotiations and explain how to determine what you can and cannot accept in terms of payment.
You are the responsible party
Some of us do our own coding and some of us do not. However, if that coding is inaccurate, it is the physician who goes to jail, not the coder. You are personally responsible and liable for the coding submitted under your provider number.
Clearly, we need to do a better job of advocating for ourselves. We need to lobby. Legislators and bureaucrats are less likely to target people who have strong lobbyists working consistently on their behalf.
Accountable care organizations may have some leverage in negotiating lower prices, and some market forces may come into play in large systems. It remains to be seen which models will succeed as new payment structures develop. The overarching question: What can we do today to optimize our payments, given the system that we have? Here are 13 tactics that can enhance your bottom line.
1. Know the rules
To play the game, you must know the rules. You need to know what systems payers are using to determine your reimbursement—and you have to understand those systems as well as, or better than, the payers do. Then you’ll be able to use them to your advantage.
Payers are well aware that we don’t like to focus on this end of practice, that what we really want to do is spend the day practicing medicine. However, we need to learn these details because we’re leaving money on the table every single day.
2. Educate yourself
With the change to the International Classification of Diseases (ICD) scheduled to take effect on October 1, 2015, many of us are worried that payers are going to reject our claims because of our lack of familiarity with ICD-10.
Rest assured. There are crosswalks from ICD-9 to ICD-10. ACOG has published an information sheet for both obstetrics and gynecology that pairs typically used ICD-9 codes with their ICD-10 counterparts. And because it is published by ACOG, payers will find it hard to claim that it’s inaccurate.
ACOG also offers half-day courses on ICD-10 coding for both physicians and staff.
3. Record your decision-making process
When I audit medical charts, I often discover that this process has been neglected. Instead, the coder has relied on documentation from the electronic health record and a basic description of the treatment plan. But a plan is just that—what someone intends to do. It doesn’t convey the decision-making that underlies it. What was the differential diagnosis? What did you discuss with the patient? These details are critical for appropriate coding of the level of service—whether it’s high, intermediate, or low.
4. Refine your approach to coding
Recognize that the system is currently set up to pay physicians for the services we provide—and that service must be justified by the appropriate diagnosis code. Tougher cases, or high-risk patients, tend to have longer surgeries and hospital stays, and their outcomes often are not as good as those of more typical patients. They may have more complications because they’re obese or have severe diabetes, for example. If so, it is critical that these other conditions—obesity and severe diabetes—be included with the principal diagnosis code so that risk stratification is possible. Otherwise, we will be held to the same standard as someone treating a routine, low-risk case.
Risk stratification is being performed according to algorithms in the payers’ software—and payers are unlikely to share the details with us. However, the only real data payers have to run through these algorithms come from diagnosis coding. Even though you’re not required to code for variables such as obesity and diabetes in order to get paid for what you do, you do need to use those additional codes to make risk stratification possible—so that you don’t get inappropriately placed into a group of low-risk providers when you are treating a higher-risk cohort.
5. Develop an understanding of RVUs
Another variable that changes regularly is relative value units (RVUs) under Medicare rules. ACOG’s Committee on Health Economics and Coding—which enjoys the participation of AAGL, the American Urogynecologic Society (AUGS), the Society of Gynecologic Surgeons (SGS), and the Society of Gynecologic Oncology (SGO), as well as other organizations—tries to maintain the RVUs as up to date and appropriate as possible relative to other services in the fee schedule.
For example, about 10 years ago many urogynecologic procedures were getting bundled together when they were performed at the same time. We had only one or two ICD-9 codes to describe prolapse, with no separate codes to describe whether it affected the anterior, apical, or posterior compartment, even though we performed different procedures in the individual compartments. Payers were mapping all prolapse procedures to the same diagnosis code. So ACOG went to the National Center for Health Statistics, where ICD-9 coding was done—and developed a series of about 10 codes to describe the different areas that prolapse could affect.
That kind of nuanced coding is continuing today. In fact, we have a long list of areas to go forward with now that ICD-10 is scheduled to take effect. A good example involves new Pap smear guidelines, which recommend testing every 3 or 5 years except for patients who have undergone hysterectomy for benign disease. How do you code for a patient who has had a hysterectomy? There was no code for a woman with an absent cervix, so we created a “V-code,” a code classification for factors that influence health status, so that it is possible to explain why a Pap smear was not performed.
As we go forward into a value-based system, specialists like us likely will be negotiating contracts according to RVU-based payments. That’s why it’s important for you to understand the resource-based relative value scale (RBRVS). It has three components: a work component, which makes up about 52% of the total RVUs; a practice expense, which makes up more than 45% of total RVUS; and, finally, a malpractice component, a small percentage. There also is a geographic adjustment and a uniform conversion factor.
When you hear about the sustainable growth rate (SGR) fix, and the fact that we’re going to see a 20% or 24% reduction in payment, that talk is referring to a reduction in the conversion factor. Each component of the RVU is adjusted for geography and then multiplied by the dollar conversion factor to calculate the total RVUs. The work, practice, and malpractice components vary by where the service is provided.
Let’s use placement of Essure inserts as an example. If you perform the procedure in the hospital, then the hospital buys the equipment, including the hysteroscope and light source. The hospital also pays for the room and staff and manages equipment sterilization. If, on the other hand, you perform the procedure in your office, all those responsibilities are yours. If it’s done in your office, you get paid more but it also costs you more.
The Relative Value Update Committee, or RUC, plays a major role in determining RVUs. This committee is composed of 31 clinicians, including nonphysician providers, psychologists, and nurses who deliver services under the Medicare fee schedule. The RUC makes recommendations to the Centers for Medicare and Medicaid Services (CMS), but it is the Secretary of Health and Human Services who determines the final rule on RVUs.
Approximately 75% to 95% of the recommendations of the RUC are accepted by the Secretary and become law. So it’s not the RUC or the American Medical Association (AMA) that determines RVUs; in the long run, it is CMS and the Secretary of Health and Human Services. We are fortunate that, when CMS assigns RVUs we’re not happy with, we have an opportunity to appeal.
Under Medicare, all physician payments are based on the same conversion factor, regardless of specialty. That’s not necessarily true for other payers, who may, essentially, do whatever they wish. These other payers frequently will contract at higher or lower rates, depending on how prevalent a specialist is in the community. Sometimes they use a higher conversion factor for surgical specialists than they use for primary care.
6. Find out which RVUs the payer is using
When you negotiate contracts with payers, and you are in private practice or part of a medical practice, it’s important to know what year’s RVUs the payer is using, as RVUs vary from year to year. For example, if the payer is using the RBRVS from 2002, it is paying you less than you should be getting. So when you look at a contract, you should determine not only whether the payer is anchoring your payment to the RBRVS but also whether it is keeping up with current RVUs as well. What dollar conversion factor is the payer using? What global periods—the same as CMS, or something different?
7. Determine what global period is in play
Some private payers use 6 postoperative weeks as the global period for a surgical procedure, whereas Medicare uses 90 days. You need to know which period is in play so that you don’t leave money on the table if you see the patient within 90 days but more than 6 weeks postoperatively.
Current Procedural Terminology (CPT) has global surgical packages that include a 10-day or 90-day period. But those periods do not include services provided more than 24 hours before the procedure. They don’t include the administration of anesthesia or conscious sedation. And they don’t include management of complications, exacerbations, or recurrences. Nor do they include additional services that might be necessary due to the presence of another disease or injury.
Under Medicare, the rules are different. Medicare preoperative services begin 1 day before surgery. However, any preoperative intervention is included whether it’s performed 1 day or 1 week before surgery. If it’s simply a preoperative physical examination for the patient and you aren’t performing significant evaluation and management, it’s included in the global package, along with all the intraoperative work. In addition, under Medicare, you don’t get paid for the management of complications unless a return to the operating room is required.
8. Learn to use modifiers
As ObGyns, we often see patients for multiple conditions or problem reports, so you need to be aware that if a patient is within a global period and you do not submit a bill with a modifier to indicate special circumstances, the intervention will be bundled into the global and you will not get paid for it. Modifiers are two-digit codes that describe these separate services. They provide critical information to payers so that their computer programs separate these services out for payment.
Major surgical procedures don’t include unrelated procedures that are performed at the same time of surgery. Nor do they include visits that take place during the global period that are unrelated to the original surgery. For example, if a patient presents with a breast lump after you performed a hysterectomy, and you do a work-up, you deserve full payment for that evaluation and management service. If you don’t use a modifier, however, you won’t get that payment.
9. Don’t be passive when payers won’t pay
Let’s say you contract with HMOs or independent practice associations (IPAs), and they’re not compensating you for the extra things you’re doing and are failing to recognize surgical modifiers. What can you do about it?
You need to develop a profile of your typical patient. Because these organizations are individualizing it—they are saying that, in a typical scenario, this is the type of work you do. So these organizations offer a different kind of contract. Nevertheless, you can use your coding to help you determine what a fair payment should be, by going through your billing to determine what you’ve spent.
10. Analyze payer bundling
Medicare put in place a correct coding initiative (CCI) that lists services typically provided by the same person on the same day of service. The aim: to prevent separate payment for these services. These are “bundled” services. The CCI bundles are revised every quarter. They are listed on the ACOG Web site under “practice management.”
On October 1, 2014, the CCI inappropriately bundled pelvic organ prolapse repair procedures into the vaginal hysterectomy codes. ACOG, AUGS, SGS, and AAGL are arguing vehemently as this article is going to press to ensure that these damaging bundles are rescinded.
Private payers can bundle anything, and it may or may not make sense or be fair. One ACOG resource is the book Ob/Gyn Coding Manual: Components of Correct Procedural Coding, which is revised every year. It has a tear-out page for every procedure code and will help you determine whether or not a bundle is appropriate.
You need to know about bundling and dispute resolution. Why? Because it is possible to insert clauses into your contract that give you some rights. Insurers have all the clout and you have nothing unless you fight for it.
You may see clauses such as “the company reserves the right to re-bundle to the primary procedure....” You shouldn’t tolerate that. Rather, you want to say, “the company will use CCI bundled rules” so that you at least know what the rules are.
11. Don’t be afraid to revise a contract
If we have to hold a payer harmless, the payer should hold us harmless as well. If we consult an insurer’s Web site to confirm that a patient is covered, and we take her to surgery because we have evidence she has insurance, the insurer shouldn’t be able to rescind payment 6 months later because the patient didn’t pay for her insurance that month. That’s not fair. The company told you she was covered, and you deserve to get paid for that surgery because you are relying on information from the company itself. So when you sign a contract, you need to ensure that you are being held harmless as well as the insurer.
12. Calculate your own RVUs
Use your claims software for data. Consult the Federal Register or ACOG to determine the total number of RVUs for a given CPT code. Multiply the RVUs by the quantity for each code. Let’s say it’s an evaluation and management visit, code 99213, and you’ve done 50 this month. That’s 50 multiplied by 1.3 RVUs. Add all the codes together, then use your monthly profit and loss statement to determine what your expenses are. Divide your total expenses by the total number of RVUs to determine your practice cost per RVU. You then can decide on a conversion factor you can tolerate, and you can use this information when contracting with IPAs, HMOs, and other insurers.
13. Spend money to make money
There are many coding resources available to you. Coding is well worth what you spend on it because you can get it back in a heartbeat.
This information may not be easy to master, but it’s critically important for your economic survival—to get what’s rightfully yours and get paid fairly for what you do.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.