User login
Adherence to oral contraceptive protocols prevents pregnancy
Combined oral contraceptives (COCs) remain a popular method of pregnancy prevention worldwide, but efficacy and failure rates can be difficult to determine, as real-word use does not always mirror clinical trials, wrote Mitchell D. Creinin, MD, of the University of California, Davis, and colleagues. Clinical trials include perfect use or method-failure rates, but data on pregnancy risk based on reported adherence alone are lacking, they said.
To assess the effects of missed pills on COC efficacy, the researchers reviewed data from a pair of parallel phase 3 trials, focusing only on adherence to the pill dosing regimen. The findings were published in Obstetrics & Gynecology.
The study population included 1,864 individuals from the United States and Canada, and 1,553 from Europe and Russia.
The participants were healthy, sexually active adults aged 16-50 years in monogamous relationships from 2016 through 2018 who agreed to used estetrol 15 mg and drospirenone 3 mg for up to 13 28-day cycles as their only contraceptive method. Condom use was permitted for protection against sexually transmitted infections if needed. The 28-day COCs included 24 hormonal tablets and 4 placebo tablets. Participants received written instructions for what to do it they missed pills.
The primary outcome was the relationship between missed pills and pregnancies.
A total of 31 pregnancies occurred across both studies; none of these occurred during cycles in which other contraception was used. Of 22 pregnancies in participants who reported taking all pills, 21 reported daily pill use during the cycle in which pregnancy occurred. One participant reported not taking one pill and one participant reported not taking two pills; neither correctly followed the instructions for missed pills.
Pregnancies occurred in .09% of cycles in which participants reported taking all pills, and in 0.25%, 0.83%, and 1.6% of cycles in which participants reported missing one pill, two pills, or more than two pills, respectively.
“Pregnancy rates exceeded 1% only in participants who did not correctly follow missed-pill instructions,” the researchers noted.
Pregnancy rates per cycle ranged from 0% to 0.21%, and 48.4% of the pregnancies occurred during the first four cycles of COC use. Approximately one-third (32.3%) of pregnancies occurred within the first week of a new pill pack.
“Fertilization does not appear to be related to the timing of missed pills within the cycle because pregnancy did not occur more frequently earlier in the cycle (after the placebo pills),” the researchers wrote in their discussion. This finding contradicts previous research suggesting that contraceptive failure rates decrease over the first year of use, they said. In addition, the formulation of the pill used may affect pregnancy rates when pills are missed, as some hormones have longer half-lives, they noted.
The study findings were limited by several factors, including the lack of adjustment for outcomes based on reported sexual activity per cycle, and by the reliance on self-reports.
However, the results were strengthened by the use of the clinical outcomes of pregnancy as the primary outcome, rather than characteristics and predictors of participants who missed pills, the researchers said.
The cycle-based methodology used in the current study may provide insight on the relationship between COC adherence and pregnancy risk that can inform future studies, they concluded.
Findings highlight the importance of options
“With increasing restrictions on abortion care, offering more contraceptive options for people is critical,” Lauren Owens, MD, associate professor of obstetrics and gynecology at the University of Washington, Seattle, said in an interview. “That’s not to say that having another pill option makes up for the harm people are experiencing as they navigate abortion bans and legal interference in their health care, but no one pill works for all people, and having more options is helpful,” she said.
Dr. Owens noted that the rates of pregnancy in the current study were lower than she traditionally associates with COCs, “although I usually discuss annual failure rates with patients, not failure rates per cycle, and the latter will clearly be lower.” In the current study, “The authors hypothesize some of this may be due to the longer half-life that estetrol has compared to ethinyl estradiol, the estrogen form more commonly found in oral contraceptive pills,” she said.
From a clinical standpoint, “I appreciated the linkage between number of missed pills and pregnancies occurring,” Dr. Owens said. “This is a good reminder to clinicians to talk to patients ahead of time about what to do when missed pills occur and to provide resources in advance that patients can reference when needed,” she said.
“The authors published other studies on this pill in the last year and it seems to work well and have a reasonable safety profile,” Dr. Owens told this news organization. However, “We still need to broaden the methods available to patients, particularly methods that people producing sperm can use. In the face of ongoing and escalating attacks on access to contraceptive care and abortion care, it’s more important than ever to do what we can to improve options for patients,” she said.
The study was supported by Estetra SRL, an affiliate company of Mithra Pharmaceuticals. Dr. Creinin disclosed relationships with multiple companies including Gedeon Richter, Mayne, and Organon. He disclosed serving on the advisory boards for Evofem, Fuji Pharma, Gedeon Richter, GlaxoSmithKline, Mayne, Merck, OLIC, Organon, and Searchlight, and serving as a consultant for Estetra SRL (including the current study), Libbs, Mayne, and Medicines360; his university department receives contraceptive research funding from Chemo Research SL, Evofem, HRA Pharma, Medicines360, Merck, and Sebela. Dr. Owens had no relevant financial conflicts to disclose.
Combined oral contraceptives (COCs) remain a popular method of pregnancy prevention worldwide, but efficacy and failure rates can be difficult to determine, as real-word use does not always mirror clinical trials, wrote Mitchell D. Creinin, MD, of the University of California, Davis, and colleagues. Clinical trials include perfect use or method-failure rates, but data on pregnancy risk based on reported adherence alone are lacking, they said.
To assess the effects of missed pills on COC efficacy, the researchers reviewed data from a pair of parallel phase 3 trials, focusing only on adherence to the pill dosing regimen. The findings were published in Obstetrics & Gynecology.
The study population included 1,864 individuals from the United States and Canada, and 1,553 from Europe and Russia.
The participants were healthy, sexually active adults aged 16-50 years in monogamous relationships from 2016 through 2018 who agreed to used estetrol 15 mg and drospirenone 3 mg for up to 13 28-day cycles as their only contraceptive method. Condom use was permitted for protection against sexually transmitted infections if needed. The 28-day COCs included 24 hormonal tablets and 4 placebo tablets. Participants received written instructions for what to do it they missed pills.
The primary outcome was the relationship between missed pills and pregnancies.
A total of 31 pregnancies occurred across both studies; none of these occurred during cycles in which other contraception was used. Of 22 pregnancies in participants who reported taking all pills, 21 reported daily pill use during the cycle in which pregnancy occurred. One participant reported not taking one pill and one participant reported not taking two pills; neither correctly followed the instructions for missed pills.
Pregnancies occurred in .09% of cycles in which participants reported taking all pills, and in 0.25%, 0.83%, and 1.6% of cycles in which participants reported missing one pill, two pills, or more than two pills, respectively.
“Pregnancy rates exceeded 1% only in participants who did not correctly follow missed-pill instructions,” the researchers noted.
Pregnancy rates per cycle ranged from 0% to 0.21%, and 48.4% of the pregnancies occurred during the first four cycles of COC use. Approximately one-third (32.3%) of pregnancies occurred within the first week of a new pill pack.
“Fertilization does not appear to be related to the timing of missed pills within the cycle because pregnancy did not occur more frequently earlier in the cycle (after the placebo pills),” the researchers wrote in their discussion. This finding contradicts previous research suggesting that contraceptive failure rates decrease over the first year of use, they said. In addition, the formulation of the pill used may affect pregnancy rates when pills are missed, as some hormones have longer half-lives, they noted.
The study findings were limited by several factors, including the lack of adjustment for outcomes based on reported sexual activity per cycle, and by the reliance on self-reports.
However, the results were strengthened by the use of the clinical outcomes of pregnancy as the primary outcome, rather than characteristics and predictors of participants who missed pills, the researchers said.
The cycle-based methodology used in the current study may provide insight on the relationship between COC adherence and pregnancy risk that can inform future studies, they concluded.
Findings highlight the importance of options
“With increasing restrictions on abortion care, offering more contraceptive options for people is critical,” Lauren Owens, MD, associate professor of obstetrics and gynecology at the University of Washington, Seattle, said in an interview. “That’s not to say that having another pill option makes up for the harm people are experiencing as they navigate abortion bans and legal interference in their health care, but no one pill works for all people, and having more options is helpful,” she said.
Dr. Owens noted that the rates of pregnancy in the current study were lower than she traditionally associates with COCs, “although I usually discuss annual failure rates with patients, not failure rates per cycle, and the latter will clearly be lower.” In the current study, “The authors hypothesize some of this may be due to the longer half-life that estetrol has compared to ethinyl estradiol, the estrogen form more commonly found in oral contraceptive pills,” she said.
From a clinical standpoint, “I appreciated the linkage between number of missed pills and pregnancies occurring,” Dr. Owens said. “This is a good reminder to clinicians to talk to patients ahead of time about what to do when missed pills occur and to provide resources in advance that patients can reference when needed,” she said.
“The authors published other studies on this pill in the last year and it seems to work well and have a reasonable safety profile,” Dr. Owens told this news organization. However, “We still need to broaden the methods available to patients, particularly methods that people producing sperm can use. In the face of ongoing and escalating attacks on access to contraceptive care and abortion care, it’s more important than ever to do what we can to improve options for patients,” she said.
The study was supported by Estetra SRL, an affiliate company of Mithra Pharmaceuticals. Dr. Creinin disclosed relationships with multiple companies including Gedeon Richter, Mayne, and Organon. He disclosed serving on the advisory boards for Evofem, Fuji Pharma, Gedeon Richter, GlaxoSmithKline, Mayne, Merck, OLIC, Organon, and Searchlight, and serving as a consultant for Estetra SRL (including the current study), Libbs, Mayne, and Medicines360; his university department receives contraceptive research funding from Chemo Research SL, Evofem, HRA Pharma, Medicines360, Merck, and Sebela. Dr. Owens had no relevant financial conflicts to disclose.
Combined oral contraceptives (COCs) remain a popular method of pregnancy prevention worldwide, but efficacy and failure rates can be difficult to determine, as real-word use does not always mirror clinical trials, wrote Mitchell D. Creinin, MD, of the University of California, Davis, and colleagues. Clinical trials include perfect use or method-failure rates, but data on pregnancy risk based on reported adherence alone are lacking, they said.
To assess the effects of missed pills on COC efficacy, the researchers reviewed data from a pair of parallel phase 3 trials, focusing only on adherence to the pill dosing regimen. The findings were published in Obstetrics & Gynecology.
The study population included 1,864 individuals from the United States and Canada, and 1,553 from Europe and Russia.
The participants were healthy, sexually active adults aged 16-50 years in monogamous relationships from 2016 through 2018 who agreed to used estetrol 15 mg and drospirenone 3 mg for up to 13 28-day cycles as their only contraceptive method. Condom use was permitted for protection against sexually transmitted infections if needed. The 28-day COCs included 24 hormonal tablets and 4 placebo tablets. Participants received written instructions for what to do it they missed pills.
The primary outcome was the relationship between missed pills and pregnancies.
A total of 31 pregnancies occurred across both studies; none of these occurred during cycles in which other contraception was used. Of 22 pregnancies in participants who reported taking all pills, 21 reported daily pill use during the cycle in which pregnancy occurred. One participant reported not taking one pill and one participant reported not taking two pills; neither correctly followed the instructions for missed pills.
Pregnancies occurred in .09% of cycles in which participants reported taking all pills, and in 0.25%, 0.83%, and 1.6% of cycles in which participants reported missing one pill, two pills, or more than two pills, respectively.
“Pregnancy rates exceeded 1% only in participants who did not correctly follow missed-pill instructions,” the researchers noted.
Pregnancy rates per cycle ranged from 0% to 0.21%, and 48.4% of the pregnancies occurred during the first four cycles of COC use. Approximately one-third (32.3%) of pregnancies occurred within the first week of a new pill pack.
“Fertilization does not appear to be related to the timing of missed pills within the cycle because pregnancy did not occur more frequently earlier in the cycle (after the placebo pills),” the researchers wrote in their discussion. This finding contradicts previous research suggesting that contraceptive failure rates decrease over the first year of use, they said. In addition, the formulation of the pill used may affect pregnancy rates when pills are missed, as some hormones have longer half-lives, they noted.
The study findings were limited by several factors, including the lack of adjustment for outcomes based on reported sexual activity per cycle, and by the reliance on self-reports.
However, the results were strengthened by the use of the clinical outcomes of pregnancy as the primary outcome, rather than characteristics and predictors of participants who missed pills, the researchers said.
The cycle-based methodology used in the current study may provide insight on the relationship between COC adherence and pregnancy risk that can inform future studies, they concluded.
Findings highlight the importance of options
“With increasing restrictions on abortion care, offering more contraceptive options for people is critical,” Lauren Owens, MD, associate professor of obstetrics and gynecology at the University of Washington, Seattle, said in an interview. “That’s not to say that having another pill option makes up for the harm people are experiencing as they navigate abortion bans and legal interference in their health care, but no one pill works for all people, and having more options is helpful,” she said.
Dr. Owens noted that the rates of pregnancy in the current study were lower than she traditionally associates with COCs, “although I usually discuss annual failure rates with patients, not failure rates per cycle, and the latter will clearly be lower.” In the current study, “The authors hypothesize some of this may be due to the longer half-life that estetrol has compared to ethinyl estradiol, the estrogen form more commonly found in oral contraceptive pills,” she said.
From a clinical standpoint, “I appreciated the linkage between number of missed pills and pregnancies occurring,” Dr. Owens said. “This is a good reminder to clinicians to talk to patients ahead of time about what to do when missed pills occur and to provide resources in advance that patients can reference when needed,” she said.
“The authors published other studies on this pill in the last year and it seems to work well and have a reasonable safety profile,” Dr. Owens told this news organization. However, “We still need to broaden the methods available to patients, particularly methods that people producing sperm can use. In the face of ongoing and escalating attacks on access to contraceptive care and abortion care, it’s more important than ever to do what we can to improve options for patients,” she said.
The study was supported by Estetra SRL, an affiliate company of Mithra Pharmaceuticals. Dr. Creinin disclosed relationships with multiple companies including Gedeon Richter, Mayne, and Organon. He disclosed serving on the advisory boards for Evofem, Fuji Pharma, Gedeon Richter, GlaxoSmithKline, Mayne, Merck, OLIC, Organon, and Searchlight, and serving as a consultant for Estetra SRL (including the current study), Libbs, Mayne, and Medicines360; his university department receives contraceptive research funding from Chemo Research SL, Evofem, HRA Pharma, Medicines360, Merck, and Sebela. Dr. Owens had no relevant financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
Frustration over iPLEDGE evident at FDA meeting
During 2 days of after the chaotic rollout of the new REMS platform at the end of 2021.
On March 29, at the end of the FDA’s joint meeting of two advisory committees that addressed ways to improve the iPLEDGE program, most panelists voted to change the 19-day lockout period for patients who can become pregnant, and the requirement that every month, providers must document counseling of those who cannot get pregnant and are taking the drug for acne.
However, there was no consensus on whether there should be a lockout at all or for how long, and what an appropriate interval for counseling those who cannot get pregnant would be, if not monthly. Those voting on the questions repeatedly cited a lack of data to make well-informed decisions.
The meeting of the two panels, the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee, was held March 28-29, to discuss proposed changes to iPLEDGE requirements, to minimize the program’s burden on patients, prescribers, and pharmacies – while maintaining safe use of the highly teratogenic drug.
Lockout based on outdated reasoning
John S. Barbieri, MD, a dermatologist and epidemiologist, and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital in Boston, speaking as deputy chair of the American Academy of Dermatology Association (AADA) iPLEDGE work group, described the burden of getting the drug to patients. He was not on the panel, but spoke during the open public hearing.
“Compared to other acne medications, the time it takes to successfully go from prescribed (isotretinoin) to when the patient actually has it in their hands is 5- to 10-fold higher,” he said.
Among the barriers is the 19-day lockout period for people who can get pregnant and miss the 7-day window for picking up their prescriptions. They must then wait 19 days to get a pregnancy test to clear them for receiving the medication.
Gregory Wedin, PharmD, pharmacovigilance and risk management director of Upsher-Smith Laboratories, who spoke on behalf of the Isotretinoin Products Manufacturer Group (IPMG), which manages iPLEDGE, said, “The rationale for the 19-day wait is to ensure the next confirmatory pregnancy test is completed after the most fertile period of the menstrual cycle is passed.”
Many don’t have a monthly cycle
But Dr. Barbieri said that reasoning is outdated.
“The current program’s focus on the menstrual cycle is really an antiquated approach,” he said. “Many patients do not have a monthly cycle due to medical conditions like polycystic ovarian syndrome, or due to [certain kinds of] contraception.”
He added, “By removing this 19-day lockout and, really, the archaic timing around the menstrual cycle in general in this program, we can simplify the program, improve it, and better align it with the real-world biology of our patients.” He added that patients are often missing the 7-day window for picking up their prescriptions through no fault of their own. Speakers at the hearing also mentioned insurance hassles and ordering delays.
Communication with IPMG
Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and outgoing chair of the AADA iPLEDGE work group, cited difficulty in working with IPMG on modifications as another barrier. She also spoke during the open public hearing.
“Despite many, many attempts to work with the IPMG, we are not aware of any organizational structure or key leaders to communicate with. Instead we have been given repeatedly a generic email address for trying to establish a working relationship and we believe this may explain the inaction of the IPMG since our proposals 4 years ago in 2019.”
Among those proposals, she said, were allowing telemedicine visits as part of the iPLEDGE REMS program and reducing counseling attestation to every 6 months instead of monthly for those who cannot become pregnant.
She pointed to the chaotic rollout of modifications to the iPLEDGE program on a new website at the end of 2021.
In 2021, she said, “despite 6 months of notification, no prescriber input was solicited before revamping the website. This lack of transparency and accountability has been a major hurdle in improving iPLEDGE.”
Dr. Barbieri called the rollout “a debacle” that could have been mitigated with communication with IPMG. “We warned about every issue that happened and talked about ways to mitigate it and were largely ignored,” he said.
“By including dermatologists and key stakeholders in these discussions, as we move forward with changes to improve this program, we can make sure that it’s patient-centered.”
IPMG did not address the specific complaints about the working relationship with the AADA workgroup at the meeting.
Monthly attestation for counseling patients who cannot get pregnant
Dr. Barbieri said the monthly requirement to counsel patients who cannot get pregnant and document that counseling unfairly burdens clinicians and patients. “We’re essentially asking patients to come in monthly just to tell them not to share their drugs [or] donate blood,” he said.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among the panel members voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
IPMG representative Dr. Wedin, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while an extension to 120 days would reduce burden on prescribers, it comes with the risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Dr. Wedin said.
Home pregnancy testing
The advisory groups were also tasked with discussing whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most committee members and those in the public hearing who spoke on the issue agreed that home tests should continue in an effort to increase access and decrease burden.
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory.
Lindsey Crist, PharmD, a risk management analyst at the FDA, who presented the FDA review committee’s analysis, said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” Dr. Crist said.
Dr. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Workaround to avoid falsification
Advisory committee member Brian P. Green, DO, associate professor of dermatology at Penn State University, Hershey, Pa., spoke in support of home pregnancy tests.
“What we have people do for telemedicine is take the stick, write their name, write the date on it, and send a picture of that the same day as their visit,” he said. “That way we have the pregnancy test the same day. Allowing this to continue to happen at home is important. Bringing people in is burdensome and costly.”
Emmy Graber, MD, a dermatologist who practices in Boston, and a director of the American Acne and Rosacea Society (AARS), relayed an example of the burden for a patient using isotretinoin who lives 1.5 hours away from the dermatology office. She is able to meet the requirements of iPLEDGE only through telehealth.
“Home pregnancy tests are highly sensitive, equal to the ones done in CLIA-certified labs, and highly accurate when interpreted by a dermatology provider,” said Dr. Graber, who spoke on behalf of the AARS during the open public hearing.
“Notably, CLIA [Clinical Laboratory Improvement Amendments] certification is not required by other REMS programs” for teratogenic drugs, she added.
Dr. Graber said it’s important to note that in the time the pandemic exceptions have been made for isotretinoin patients, “there has been no reported spike in pregnancy in the past three years.
“We do have some data to show that it is not imposing additional harms,” she said.
Suggestions for improvement
At the end of the hearing, advisory committee members were asked to propose improvements to the iPLEDGE REMS program.
Dr. Green advocated for the addition of an iPLEDGE mobile app.
“Most people go to their phones rather than their computers, particularly teenagers and younger people,” he noted.
Advisory committee member Megha M. Tollefson, MD, professor of dermatology and pediatric and adolescent medicine at Mayo Clinic in Rochester, Minn., echoed the need for an iPLEDGE app.
The young patients getting isotretinoin “don’t respond to email, they don’t necessarily go onto web pages. If we’re going to be as effective as possible, it’s going to have to be through an app-based system.”
Dr. Tollefson said she would like to see patient counseling standardized through the app. “I think there’s a lot of variability in what counseling is given when it’s left to the individual prescriber or practice,” she said.
Exceptions for long-acting contraceptives?
Advisory committee member Abbey B. Berenson, MD, PhD, professor of obstetrics and gynecology at University of Texas Medical Branch in Galveston, said that patients taking long-acting reversible contraceptives (LARCs) may need to be considered differently when deciding the intervals for attestation or whether to have a lockout period.
“LARC methods’ rate of failure is extremely low,” she said. “While it is true, as it has been pointed out, that all methods can fail, when they’re over 99% effective, I think that we can treat those methods differently than we treat methods such as birth control pills or abstinence that fail far more often. That is one way we could minimize burden on the providers and the patients.”
She also suggested using members of the health care team other than physicians to complete counseling, such as a nurse or pharmacist.
Prescriptions for emergency contraception
Advisory committee member Sascha Dublin, MD, PhD, senior scientific investigator for Kaiser Permanente Washington Health Research Institute in Seattle, said most patients taking the drug who can get pregnant should get a prescription for emergency contraception at the time of the first isotretinoin prescription.
“They don’t have to buy it, but to make it available at the very beginning sets the expectation that it would be good to have in your medicine cabinet, particularly if the [contraception] choice is abstinence or birth control pills.”
Dr. Dublin also called for better transparency surrounding the role of IPMG.
She said IPMG should be expected to collect data in a way that allows examination of health disparities, including by race and ethnicity and insurance status. Dr. Dublin added that she was concerned about the poor communication between dermatological societies and IPMG.
“The FDA should really require that IPMG hold periodic, regularly scheduled stakeholder forums,” she said. “There has to be a mechanism in place for IPMG to listen to those concerns in real time and respond.”
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
During 2 days of after the chaotic rollout of the new REMS platform at the end of 2021.
On March 29, at the end of the FDA’s joint meeting of two advisory committees that addressed ways to improve the iPLEDGE program, most panelists voted to change the 19-day lockout period for patients who can become pregnant, and the requirement that every month, providers must document counseling of those who cannot get pregnant and are taking the drug for acne.
However, there was no consensus on whether there should be a lockout at all or for how long, and what an appropriate interval for counseling those who cannot get pregnant would be, if not monthly. Those voting on the questions repeatedly cited a lack of data to make well-informed decisions.
The meeting of the two panels, the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee, was held March 28-29, to discuss proposed changes to iPLEDGE requirements, to minimize the program’s burden on patients, prescribers, and pharmacies – while maintaining safe use of the highly teratogenic drug.
Lockout based on outdated reasoning
John S. Barbieri, MD, a dermatologist and epidemiologist, and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital in Boston, speaking as deputy chair of the American Academy of Dermatology Association (AADA) iPLEDGE work group, described the burden of getting the drug to patients. He was not on the panel, but spoke during the open public hearing.
“Compared to other acne medications, the time it takes to successfully go from prescribed (isotretinoin) to when the patient actually has it in their hands is 5- to 10-fold higher,” he said.
Among the barriers is the 19-day lockout period for people who can get pregnant and miss the 7-day window for picking up their prescriptions. They must then wait 19 days to get a pregnancy test to clear them for receiving the medication.
Gregory Wedin, PharmD, pharmacovigilance and risk management director of Upsher-Smith Laboratories, who spoke on behalf of the Isotretinoin Products Manufacturer Group (IPMG), which manages iPLEDGE, said, “The rationale for the 19-day wait is to ensure the next confirmatory pregnancy test is completed after the most fertile period of the menstrual cycle is passed.”
Many don’t have a monthly cycle
But Dr. Barbieri said that reasoning is outdated.
“The current program’s focus on the menstrual cycle is really an antiquated approach,” he said. “Many patients do not have a monthly cycle due to medical conditions like polycystic ovarian syndrome, or due to [certain kinds of] contraception.”
He added, “By removing this 19-day lockout and, really, the archaic timing around the menstrual cycle in general in this program, we can simplify the program, improve it, and better align it with the real-world biology of our patients.” He added that patients are often missing the 7-day window for picking up their prescriptions through no fault of their own. Speakers at the hearing also mentioned insurance hassles and ordering delays.
Communication with IPMG
Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and outgoing chair of the AADA iPLEDGE work group, cited difficulty in working with IPMG on modifications as another barrier. She also spoke during the open public hearing.
“Despite many, many attempts to work with the IPMG, we are not aware of any organizational structure or key leaders to communicate with. Instead we have been given repeatedly a generic email address for trying to establish a working relationship and we believe this may explain the inaction of the IPMG since our proposals 4 years ago in 2019.”
Among those proposals, she said, were allowing telemedicine visits as part of the iPLEDGE REMS program and reducing counseling attestation to every 6 months instead of monthly for those who cannot become pregnant.
She pointed to the chaotic rollout of modifications to the iPLEDGE program on a new website at the end of 2021.
In 2021, she said, “despite 6 months of notification, no prescriber input was solicited before revamping the website. This lack of transparency and accountability has been a major hurdle in improving iPLEDGE.”
Dr. Barbieri called the rollout “a debacle” that could have been mitigated with communication with IPMG. “We warned about every issue that happened and talked about ways to mitigate it and were largely ignored,” he said.
“By including dermatologists and key stakeholders in these discussions, as we move forward with changes to improve this program, we can make sure that it’s patient-centered.”
IPMG did not address the specific complaints about the working relationship with the AADA workgroup at the meeting.
Monthly attestation for counseling patients who cannot get pregnant
Dr. Barbieri said the monthly requirement to counsel patients who cannot get pregnant and document that counseling unfairly burdens clinicians and patients. “We’re essentially asking patients to come in monthly just to tell them not to share their drugs [or] donate blood,” he said.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among the panel members voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
IPMG representative Dr. Wedin, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while an extension to 120 days would reduce burden on prescribers, it comes with the risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Dr. Wedin said.
Home pregnancy testing
The advisory groups were also tasked with discussing whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most committee members and those in the public hearing who spoke on the issue agreed that home tests should continue in an effort to increase access and decrease burden.
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory.
Lindsey Crist, PharmD, a risk management analyst at the FDA, who presented the FDA review committee’s analysis, said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” Dr. Crist said.
Dr. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Workaround to avoid falsification
Advisory committee member Brian P. Green, DO, associate professor of dermatology at Penn State University, Hershey, Pa., spoke in support of home pregnancy tests.
“What we have people do for telemedicine is take the stick, write their name, write the date on it, and send a picture of that the same day as their visit,” he said. “That way we have the pregnancy test the same day. Allowing this to continue to happen at home is important. Bringing people in is burdensome and costly.”
Emmy Graber, MD, a dermatologist who practices in Boston, and a director of the American Acne and Rosacea Society (AARS), relayed an example of the burden for a patient using isotretinoin who lives 1.5 hours away from the dermatology office. She is able to meet the requirements of iPLEDGE only through telehealth.
“Home pregnancy tests are highly sensitive, equal to the ones done in CLIA-certified labs, and highly accurate when interpreted by a dermatology provider,” said Dr. Graber, who spoke on behalf of the AARS during the open public hearing.
“Notably, CLIA [Clinical Laboratory Improvement Amendments] certification is not required by other REMS programs” for teratogenic drugs, she added.
Dr. Graber said it’s important to note that in the time the pandemic exceptions have been made for isotretinoin patients, “there has been no reported spike in pregnancy in the past three years.
“We do have some data to show that it is not imposing additional harms,” she said.
Suggestions for improvement
At the end of the hearing, advisory committee members were asked to propose improvements to the iPLEDGE REMS program.
Dr. Green advocated for the addition of an iPLEDGE mobile app.
“Most people go to their phones rather than their computers, particularly teenagers and younger people,” he noted.
Advisory committee member Megha M. Tollefson, MD, professor of dermatology and pediatric and adolescent medicine at Mayo Clinic in Rochester, Minn., echoed the need for an iPLEDGE app.
The young patients getting isotretinoin “don’t respond to email, they don’t necessarily go onto web pages. If we’re going to be as effective as possible, it’s going to have to be through an app-based system.”
Dr. Tollefson said she would like to see patient counseling standardized through the app. “I think there’s a lot of variability in what counseling is given when it’s left to the individual prescriber or practice,” she said.
Exceptions for long-acting contraceptives?
Advisory committee member Abbey B. Berenson, MD, PhD, professor of obstetrics and gynecology at University of Texas Medical Branch in Galveston, said that patients taking long-acting reversible contraceptives (LARCs) may need to be considered differently when deciding the intervals for attestation or whether to have a lockout period.
“LARC methods’ rate of failure is extremely low,” she said. “While it is true, as it has been pointed out, that all methods can fail, when they’re over 99% effective, I think that we can treat those methods differently than we treat methods such as birth control pills or abstinence that fail far more often. That is one way we could minimize burden on the providers and the patients.”
She also suggested using members of the health care team other than physicians to complete counseling, such as a nurse or pharmacist.
Prescriptions for emergency contraception
Advisory committee member Sascha Dublin, MD, PhD, senior scientific investigator for Kaiser Permanente Washington Health Research Institute in Seattle, said most patients taking the drug who can get pregnant should get a prescription for emergency contraception at the time of the first isotretinoin prescription.
“They don’t have to buy it, but to make it available at the very beginning sets the expectation that it would be good to have in your medicine cabinet, particularly if the [contraception] choice is abstinence or birth control pills.”
Dr. Dublin also called for better transparency surrounding the role of IPMG.
She said IPMG should be expected to collect data in a way that allows examination of health disparities, including by race and ethnicity and insurance status. Dr. Dublin added that she was concerned about the poor communication between dermatological societies and IPMG.
“The FDA should really require that IPMG hold periodic, regularly scheduled stakeholder forums,” she said. “There has to be a mechanism in place for IPMG to listen to those concerns in real time and respond.”
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
During 2 days of after the chaotic rollout of the new REMS platform at the end of 2021.
On March 29, at the end of the FDA’s joint meeting of two advisory committees that addressed ways to improve the iPLEDGE program, most panelists voted to change the 19-day lockout period for patients who can become pregnant, and the requirement that every month, providers must document counseling of those who cannot get pregnant and are taking the drug for acne.
However, there was no consensus on whether there should be a lockout at all or for how long, and what an appropriate interval for counseling those who cannot get pregnant would be, if not monthly. Those voting on the questions repeatedly cited a lack of data to make well-informed decisions.
The meeting of the two panels, the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee, was held March 28-29, to discuss proposed changes to iPLEDGE requirements, to minimize the program’s burden on patients, prescribers, and pharmacies – while maintaining safe use of the highly teratogenic drug.
Lockout based on outdated reasoning
John S. Barbieri, MD, a dermatologist and epidemiologist, and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital in Boston, speaking as deputy chair of the American Academy of Dermatology Association (AADA) iPLEDGE work group, described the burden of getting the drug to patients. He was not on the panel, but spoke during the open public hearing.
“Compared to other acne medications, the time it takes to successfully go from prescribed (isotretinoin) to when the patient actually has it in their hands is 5- to 10-fold higher,” he said.
Among the barriers is the 19-day lockout period for people who can get pregnant and miss the 7-day window for picking up their prescriptions. They must then wait 19 days to get a pregnancy test to clear them for receiving the medication.
Gregory Wedin, PharmD, pharmacovigilance and risk management director of Upsher-Smith Laboratories, who spoke on behalf of the Isotretinoin Products Manufacturer Group (IPMG), which manages iPLEDGE, said, “The rationale for the 19-day wait is to ensure the next confirmatory pregnancy test is completed after the most fertile period of the menstrual cycle is passed.”
Many don’t have a monthly cycle
But Dr. Barbieri said that reasoning is outdated.
“The current program’s focus on the menstrual cycle is really an antiquated approach,” he said. “Many patients do not have a monthly cycle due to medical conditions like polycystic ovarian syndrome, or due to [certain kinds of] contraception.”
He added, “By removing this 19-day lockout and, really, the archaic timing around the menstrual cycle in general in this program, we can simplify the program, improve it, and better align it with the real-world biology of our patients.” He added that patients are often missing the 7-day window for picking up their prescriptions through no fault of their own. Speakers at the hearing also mentioned insurance hassles and ordering delays.
Communication with IPMG
Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and outgoing chair of the AADA iPLEDGE work group, cited difficulty in working with IPMG on modifications as another barrier. She also spoke during the open public hearing.
“Despite many, many attempts to work with the IPMG, we are not aware of any organizational structure or key leaders to communicate with. Instead we have been given repeatedly a generic email address for trying to establish a working relationship and we believe this may explain the inaction of the IPMG since our proposals 4 years ago in 2019.”
Among those proposals, she said, were allowing telemedicine visits as part of the iPLEDGE REMS program and reducing counseling attestation to every 6 months instead of monthly for those who cannot become pregnant.
She pointed to the chaotic rollout of modifications to the iPLEDGE program on a new website at the end of 2021.
In 2021, she said, “despite 6 months of notification, no prescriber input was solicited before revamping the website. This lack of transparency and accountability has been a major hurdle in improving iPLEDGE.”
Dr. Barbieri called the rollout “a debacle” that could have been mitigated with communication with IPMG. “We warned about every issue that happened and talked about ways to mitigate it and were largely ignored,” he said.
“By including dermatologists and key stakeholders in these discussions, as we move forward with changes to improve this program, we can make sure that it’s patient-centered.”
IPMG did not address the specific complaints about the working relationship with the AADA workgroup at the meeting.
Monthly attestation for counseling patients who cannot get pregnant
Dr. Barbieri said the monthly requirement to counsel patients who cannot get pregnant and document that counseling unfairly burdens clinicians and patients. “We’re essentially asking patients to come in monthly just to tell them not to share their drugs [or] donate blood,” he said.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among the panel members voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
IPMG representative Dr. Wedin, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while an extension to 120 days would reduce burden on prescribers, it comes with the risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Dr. Wedin said.
Home pregnancy testing
The advisory groups were also tasked with discussing whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most committee members and those in the public hearing who spoke on the issue agreed that home tests should continue in an effort to increase access and decrease burden.
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory.
Lindsey Crist, PharmD, a risk management analyst at the FDA, who presented the FDA review committee’s analysis, said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” Dr. Crist said.
Dr. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Workaround to avoid falsification
Advisory committee member Brian P. Green, DO, associate professor of dermatology at Penn State University, Hershey, Pa., spoke in support of home pregnancy tests.
“What we have people do for telemedicine is take the stick, write their name, write the date on it, and send a picture of that the same day as their visit,” he said. “That way we have the pregnancy test the same day. Allowing this to continue to happen at home is important. Bringing people in is burdensome and costly.”
Emmy Graber, MD, a dermatologist who practices in Boston, and a director of the American Acne and Rosacea Society (AARS), relayed an example of the burden for a patient using isotretinoin who lives 1.5 hours away from the dermatology office. She is able to meet the requirements of iPLEDGE only through telehealth.
“Home pregnancy tests are highly sensitive, equal to the ones done in CLIA-certified labs, and highly accurate when interpreted by a dermatology provider,” said Dr. Graber, who spoke on behalf of the AARS during the open public hearing.
“Notably, CLIA [Clinical Laboratory Improvement Amendments] certification is not required by other REMS programs” for teratogenic drugs, she added.
Dr. Graber said it’s important to note that in the time the pandemic exceptions have been made for isotretinoin patients, “there has been no reported spike in pregnancy in the past three years.
“We do have some data to show that it is not imposing additional harms,” she said.
Suggestions for improvement
At the end of the hearing, advisory committee members were asked to propose improvements to the iPLEDGE REMS program.
Dr. Green advocated for the addition of an iPLEDGE mobile app.
“Most people go to their phones rather than their computers, particularly teenagers and younger people,” he noted.
Advisory committee member Megha M. Tollefson, MD, professor of dermatology and pediatric and adolescent medicine at Mayo Clinic in Rochester, Minn., echoed the need for an iPLEDGE app.
The young patients getting isotretinoin “don’t respond to email, they don’t necessarily go onto web pages. If we’re going to be as effective as possible, it’s going to have to be through an app-based system.”
Dr. Tollefson said she would like to see patient counseling standardized through the app. “I think there’s a lot of variability in what counseling is given when it’s left to the individual prescriber or practice,” she said.
Exceptions for long-acting contraceptives?
Advisory committee member Abbey B. Berenson, MD, PhD, professor of obstetrics and gynecology at University of Texas Medical Branch in Galveston, said that patients taking long-acting reversible contraceptives (LARCs) may need to be considered differently when deciding the intervals for attestation or whether to have a lockout period.
“LARC methods’ rate of failure is extremely low,” she said. “While it is true, as it has been pointed out, that all methods can fail, when they’re over 99% effective, I think that we can treat those methods differently than we treat methods such as birth control pills or abstinence that fail far more often. That is one way we could minimize burden on the providers and the patients.”
She also suggested using members of the health care team other than physicians to complete counseling, such as a nurse or pharmacist.
Prescriptions for emergency contraception
Advisory committee member Sascha Dublin, MD, PhD, senior scientific investigator for Kaiser Permanente Washington Health Research Institute in Seattle, said most patients taking the drug who can get pregnant should get a prescription for emergency contraception at the time of the first isotretinoin prescription.
“They don’t have to buy it, but to make it available at the very beginning sets the expectation that it would be good to have in your medicine cabinet, particularly if the [contraception] choice is abstinence or birth control pills.”
Dr. Dublin also called for better transparency surrounding the role of IPMG.
She said IPMG should be expected to collect data in a way that allows examination of health disparities, including by race and ethnicity and insurance status. Dr. Dublin added that she was concerned about the poor communication between dermatological societies and IPMG.
“The FDA should really require that IPMG hold periodic, regularly scheduled stakeholder forums,” she said. “There has to be a mechanism in place for IPMG to listen to those concerns in real time and respond.”
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
FDA Advisory panels consider easing isotretinoin requirements
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
Risk of expulsion low after early postpartum IUD placement
Intrauterine device (IUD) placement at 2-4 weeks postpartum was noninferior to placement at 6-8 weeks postpartum for complete expulsion, and carried only a slightly higher risk of partial expulsion. A randomized study of expulsion rates reports the risk of expulsion at these points may help patients and clinicians make informed choices about the timing of IUD insertion, wrote the study authors, led by Sarah H. Averbach, MD, MAS, an obstetrician-gynecologist at the University of California, San Diego. “We found that the risk of complete IUD expulsion was low at 2% after early IUD placement 2-4 weeks after delivery, and was noninferior to interval placement 6-8 weeks after delivery at 0%,” Dr. Averbach said in an interview.
Although the risks of partial expulsion and malposition were modestly greater after early placement, “the possibility of a small increase in the risk of IUD expulsion or malposition with early IUD placement should be weighed against the risk of undesired pregnancy and short-interval pregnancy by delaying placement.”
The timing of IUD placement in the postpartum period should be guided by patients’ goals and preferences, she added. The early postpartum period 2-4 weeks after birth has the advantage of convenience since it coincides with early-postpartum or well-baby visits. The absolute risk differences observed between early and interval placement were small for both complete or partial expulsion at 3.8%, and the rate for complete expulsion after early placement was much lower than historical expulsion rates for immediate postpartum placement within in few days of delivery.
Last year, a large study showed an increase in expulsion risk with IUD insertion within 3 days of delivery. Current guidelines, however, support immediate insertion as a safe practice.
The study
Enrolling 404 participants from diverse settings during the period of 2018 to July 2021, researchers for the noninferiority trial randomly assigned 203 to early IUD placement 14-28 days postpartum and 201 to standard-interval placement at 42-56 days. Patients had a mean age of 29.9 years, 11.4% were Black, 56.4% were White, and 43.3% were Hispanic (some Hispanic participants self-identified as White and some as Black). By 6 months postpartum, 73% of the cohort had received an IUD and completed 6-months of follow-up, while 13% had never received an IUD and 14% were lost to follow-up. Complete expulsion rates were 3 of 149, or 2.0% (95% confidence interval [CI], 0.4-5.8) in the early group and 0 of 145, or 0% (95% CI, 0.0-2.5) in the standard group, for a between-group difference of 2.0 percentage points (95% CI, −0.5 to 5.7, P = .04). Two women chose to replace their IUDs.
Partial expulsion occurred in 14, or 9.4% (95% CI, 5.2-15.3) of patients in the early group and 11, or 7.6% (95% CI, 3.9-13.2) in the standard-interval group, for a between-group difference of 1.8 (95% CI, −4.8 to 8.6) percentage points (P = .22).
The small absolute increase in risk of partial expulsion in the early arm did not meet the prespecified criterion for noninferiority of 6%. Three pelvic infections occurred in the early placement arm.
There were 42 IUD removals: 25 in the early placement group and 17 in the standard interval group. Thirteen participants had their IUDs removed for symptoms such as cramping and bothersome vaginal bleeding.
No perforations were identified in either group at 6 months, suggesting that the rate of uterine perforations is low when IUDs are placed in the early and standard-interval postpartum periods. IUD use at 6 months remained comparable between arms: 69.5% in the early group vs. 67.2% in the standard-interval group.
Commenting on the trial but not involved in it, Maureen K. Baldwin, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland, said it provides further data on the prevalence of expulsion and malposition after placements using ultrasonography as needed. While two failures occurred with asymptomatic malposition, she added, “It should be noted that IUD position can change as a result of pregnancy, so it was not determined that malposition occurred prior to contraceptive failure.”
According to Dr. Baldwin, one strategy to reduce concerns is to use transvaginal ultrasonography at a later time or in the presence of unusual symptoms.
Overall, the study establishes that postpartum placement is an option equivalent to standard timing and it should be incorporated into patient preferences, she said. “Pain may be lowest at early placement compared to other timings, particularly for those who had vaginal birth.”
The study was supported by the Society of Family Planning research fund and the National Institutes of Health - National Institute of Child Health and Human Development. Dr. Averbach reported personal fees from Bayer Pharmaceuticals for advice on postpartum IUD placement as well as grants from the NIH outside of the submitted work. Dr. Baldwin disclosed no potential conflicts of interest with regard to her comments.
Intrauterine device (IUD) placement at 2-4 weeks postpartum was noninferior to placement at 6-8 weeks postpartum for complete expulsion, and carried only a slightly higher risk of partial expulsion. A randomized study of expulsion rates reports the risk of expulsion at these points may help patients and clinicians make informed choices about the timing of IUD insertion, wrote the study authors, led by Sarah H. Averbach, MD, MAS, an obstetrician-gynecologist at the University of California, San Diego. “We found that the risk of complete IUD expulsion was low at 2% after early IUD placement 2-4 weeks after delivery, and was noninferior to interval placement 6-8 weeks after delivery at 0%,” Dr. Averbach said in an interview.
Although the risks of partial expulsion and malposition were modestly greater after early placement, “the possibility of a small increase in the risk of IUD expulsion or malposition with early IUD placement should be weighed against the risk of undesired pregnancy and short-interval pregnancy by delaying placement.”
The timing of IUD placement in the postpartum period should be guided by patients’ goals and preferences, she added. The early postpartum period 2-4 weeks after birth has the advantage of convenience since it coincides with early-postpartum or well-baby visits. The absolute risk differences observed between early and interval placement were small for both complete or partial expulsion at 3.8%, and the rate for complete expulsion after early placement was much lower than historical expulsion rates for immediate postpartum placement within in few days of delivery.
Last year, a large study showed an increase in expulsion risk with IUD insertion within 3 days of delivery. Current guidelines, however, support immediate insertion as a safe practice.
The study
Enrolling 404 participants from diverse settings during the period of 2018 to July 2021, researchers for the noninferiority trial randomly assigned 203 to early IUD placement 14-28 days postpartum and 201 to standard-interval placement at 42-56 days. Patients had a mean age of 29.9 years, 11.4% were Black, 56.4% were White, and 43.3% were Hispanic (some Hispanic participants self-identified as White and some as Black). By 6 months postpartum, 73% of the cohort had received an IUD and completed 6-months of follow-up, while 13% had never received an IUD and 14% were lost to follow-up. Complete expulsion rates were 3 of 149, or 2.0% (95% confidence interval [CI], 0.4-5.8) in the early group and 0 of 145, or 0% (95% CI, 0.0-2.5) in the standard group, for a between-group difference of 2.0 percentage points (95% CI, −0.5 to 5.7, P = .04). Two women chose to replace their IUDs.
Partial expulsion occurred in 14, or 9.4% (95% CI, 5.2-15.3) of patients in the early group and 11, or 7.6% (95% CI, 3.9-13.2) in the standard-interval group, for a between-group difference of 1.8 (95% CI, −4.8 to 8.6) percentage points (P = .22).
The small absolute increase in risk of partial expulsion in the early arm did not meet the prespecified criterion for noninferiority of 6%. Three pelvic infections occurred in the early placement arm.
There were 42 IUD removals: 25 in the early placement group and 17 in the standard interval group. Thirteen participants had their IUDs removed for symptoms such as cramping and bothersome vaginal bleeding.
No perforations were identified in either group at 6 months, suggesting that the rate of uterine perforations is low when IUDs are placed in the early and standard-interval postpartum periods. IUD use at 6 months remained comparable between arms: 69.5% in the early group vs. 67.2% in the standard-interval group.
Commenting on the trial but not involved in it, Maureen K. Baldwin, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland, said it provides further data on the prevalence of expulsion and malposition after placements using ultrasonography as needed. While two failures occurred with asymptomatic malposition, she added, “It should be noted that IUD position can change as a result of pregnancy, so it was not determined that malposition occurred prior to contraceptive failure.”
According to Dr. Baldwin, one strategy to reduce concerns is to use transvaginal ultrasonography at a later time or in the presence of unusual symptoms.
Overall, the study establishes that postpartum placement is an option equivalent to standard timing and it should be incorporated into patient preferences, she said. “Pain may be lowest at early placement compared to other timings, particularly for those who had vaginal birth.”
The study was supported by the Society of Family Planning research fund and the National Institutes of Health - National Institute of Child Health and Human Development. Dr. Averbach reported personal fees from Bayer Pharmaceuticals for advice on postpartum IUD placement as well as grants from the NIH outside of the submitted work. Dr. Baldwin disclosed no potential conflicts of interest with regard to her comments.
Intrauterine device (IUD) placement at 2-4 weeks postpartum was noninferior to placement at 6-8 weeks postpartum for complete expulsion, and carried only a slightly higher risk of partial expulsion. A randomized study of expulsion rates reports the risk of expulsion at these points may help patients and clinicians make informed choices about the timing of IUD insertion, wrote the study authors, led by Sarah H. Averbach, MD, MAS, an obstetrician-gynecologist at the University of California, San Diego. “We found that the risk of complete IUD expulsion was low at 2% after early IUD placement 2-4 weeks after delivery, and was noninferior to interval placement 6-8 weeks after delivery at 0%,” Dr. Averbach said in an interview.
Although the risks of partial expulsion and malposition were modestly greater after early placement, “the possibility of a small increase in the risk of IUD expulsion or malposition with early IUD placement should be weighed against the risk of undesired pregnancy and short-interval pregnancy by delaying placement.”
The timing of IUD placement in the postpartum period should be guided by patients’ goals and preferences, she added. The early postpartum period 2-4 weeks after birth has the advantage of convenience since it coincides with early-postpartum or well-baby visits. The absolute risk differences observed between early and interval placement were small for both complete or partial expulsion at 3.8%, and the rate for complete expulsion after early placement was much lower than historical expulsion rates for immediate postpartum placement within in few days of delivery.
Last year, a large study showed an increase in expulsion risk with IUD insertion within 3 days of delivery. Current guidelines, however, support immediate insertion as a safe practice.
The study
Enrolling 404 participants from diverse settings during the period of 2018 to July 2021, researchers for the noninferiority trial randomly assigned 203 to early IUD placement 14-28 days postpartum and 201 to standard-interval placement at 42-56 days. Patients had a mean age of 29.9 years, 11.4% were Black, 56.4% were White, and 43.3% were Hispanic (some Hispanic participants self-identified as White and some as Black). By 6 months postpartum, 73% of the cohort had received an IUD and completed 6-months of follow-up, while 13% had never received an IUD and 14% were lost to follow-up. Complete expulsion rates were 3 of 149, or 2.0% (95% confidence interval [CI], 0.4-5.8) in the early group and 0 of 145, or 0% (95% CI, 0.0-2.5) in the standard group, for a between-group difference of 2.0 percentage points (95% CI, −0.5 to 5.7, P = .04). Two women chose to replace their IUDs.
Partial expulsion occurred in 14, or 9.4% (95% CI, 5.2-15.3) of patients in the early group and 11, or 7.6% (95% CI, 3.9-13.2) in the standard-interval group, for a between-group difference of 1.8 (95% CI, −4.8 to 8.6) percentage points (P = .22).
The small absolute increase in risk of partial expulsion in the early arm did not meet the prespecified criterion for noninferiority of 6%. Three pelvic infections occurred in the early placement arm.
There were 42 IUD removals: 25 in the early placement group and 17 in the standard interval group. Thirteen participants had their IUDs removed for symptoms such as cramping and bothersome vaginal bleeding.
No perforations were identified in either group at 6 months, suggesting that the rate of uterine perforations is low when IUDs are placed in the early and standard-interval postpartum periods. IUD use at 6 months remained comparable between arms: 69.5% in the early group vs. 67.2% in the standard-interval group.
Commenting on the trial but not involved in it, Maureen K. Baldwin, MD, MPH, associate professor of obstetrics and gynecology at Oregon Health & Science University in Portland, said it provides further data on the prevalence of expulsion and malposition after placements using ultrasonography as needed. While two failures occurred with asymptomatic malposition, she added, “It should be noted that IUD position can change as a result of pregnancy, so it was not determined that malposition occurred prior to contraceptive failure.”
According to Dr. Baldwin, one strategy to reduce concerns is to use transvaginal ultrasonography at a later time or in the presence of unusual symptoms.
Overall, the study establishes that postpartum placement is an option equivalent to standard timing and it should be incorporated into patient preferences, she said. “Pain may be lowest at early placement compared to other timings, particularly for those who had vaginal birth.”
The study was supported by the Society of Family Planning research fund and the National Institutes of Health - National Institute of Child Health and Human Development. Dr. Averbach reported personal fees from Bayer Pharmaceuticals for advice on postpartum IUD placement as well as grants from the NIH outside of the submitted work. Dr. Baldwin disclosed no potential conflicts of interest with regard to her comments.
FROM JAMA
Ectopic pregnancy risk and levonorgestrel-releasing IUDs
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
Researchers report that use of any levonorgestrel-releasing intrauterine system was associated with a significantly increased risk of ectopic pregnancy, compared with other hormonal contraceptives, in a study published in JAMA.
A national health database analysis headed by Amani Meaidi, MD, PhD, of the Danish Cancer Society Research Center, Cancer Surveillance and Pharmacoepidemiology, in Copenhagen, compared the 13.5-mg with the 19.5-mg and 52-mg dosages of levonorgestrel-releasing intrauterine systems (IUSs).
The hormone content in levonorgestrel-releasing IUSs must be high enough to maintain optimal contraceptive effect but sufficiently low to minimize progestin-related adverse events, Dr. Meaidi and colleagues noted; they advised using the middle dosage of 19.5 mg. All dosages are recommended for contraception, with the highest dosage also recommended for heavy menstrual bleeding.
“If 10,000 women using the hormonal IUD for 1 year were given the 19.5-mg hormonal IUD instead of the 13.5-mg hormonal IUD, around nine ectopic pregnancies would be avoided,” Dr. Meaidi said in an interview.
“Ectopic pregnancy is an acknowledged adverse event of hormonal IUD use. Although a rare event, it is a serious one, and a difference in ectopic pregnancy safety between the two low-dose hormonal IUDs would impact my recommendations to women.”
The study
Dr. Meaidi’s group followed 963,964 women for 7.8 million person-years. For users of levonorgestrel IUS dosages 52 mg, 19.5 mg, and 13.5 mg, and other hormonal contraceptives, the median ages were 24, 22, 22, and 21 years, respectively.
Eligible women were nulliparous with no previous ectopic pregnancy, abdominal or pelvic surgery, infertility treatment, endometriosis, or use of a levonorgestrel IUS. They were followed from Jan. 1, 2001, or their 15th birthday, until July 1, 2021, age 35, pregnancy, death, emigration, or the occurrence of any exclusion criterion.
During the study period, the cohort registered 2,925 ectopic pregnancies, including 35 at 52 mg, 32 at 19.5 mg, and 80 at 13.5 mg of levonorgestrel. For all other types of hormonal contraception, there were 763 ectopic pregnancies.
In terms of adjusted absolute rates of ectopic pregnancy per 10,000 person-years, compared with other hormonal contraceptives (rate = 2.4), these were 7.7 with 52 mg levonorgestrel IUS, 7.1 with 19.5 mg, and 15.7 with 13.5 mg. They translated to comparative differences of 5.3 (95% confidence interval, 1.9-8.7), 4.8 (95% CI, 1.5-8.0), and 13.4 (95% CI, 8.8-18.1), respectively.
Corresponding adjusted relative rate ratios were 3.4, 4.1, and 7.9. For each levonorgestrel IUS dosage; the ectopic pregnancy rate increased with duration of use.
The adjusted ectopic pregnancy rate difference per 10,000 person-years between the 19.5-mg and 52-mg levonorgestrel dosages was −0.6 , and between the 13.5-mg and 52-mg doses, 8.0, with a rate ratio of 2.3. The rate difference between the 13.5-mg and 19.5-mg levonorgestrel IUS was 8.6, with a rate ratio of 1.9.
An outsider’s perspective
Offering an outsider’s perspective on the study, Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital in New York, said these data should spark further evaluation of risk of ectopic pregnancy with levonorgestrel-releasing IUDs. “The best advice for clinicians is to individualize the choice of which contraceptive to use, and when levonorgestrel IUD is selected, to individualize the appropriate dose and timing of placement,” he said in an interview.
Several additional factors may determine the best choice, Dr. Bornstein added, including medical conditions that contraindicate other contraceptives and those conditions that justify avoidance of pregnancy, as well as uterine myomas or malformation, the ability of the patient to comply with other options, and informed patient choice. “It is important to remember the potential risk for expulsion and ectopic pregnancy, maintain alertness, and use ultrasound to exclude these potential complications if suspected,” he said.
Dr. Meaidi said the mechanism of ectopic pregnancy with hormonal IUDs is unclear, but in vitro and animal studies have observed that levonorgestrel reduces the ciliary beat frequency in the fallopian tubes. “Thus, it could be hypothesized that if a woman was unfortunate enough to become pregnant using a hormonal IUD, the hormone could inhibit or slow down the movement of the zygote into the uterus for rightful intrauterine implantation and thereby increase the risk of ectopic pregnancy.”
Two coauthors of the study reported financial support from private-sector companies. Dr. Meaidi had no conflicts of interest. Dr. Bornstein disclosed no competing interests.
FROM JAMA
Is self-administered DMPA an answer to contraception access in the post-Roe era?
ILLUSTRATIVE CASE
A 32-year-old woman with a history of migraine with aura presents to your office for contraception management. She works full-time, has 2 children, and has transportation barriers. She previously used injectable DMPA (administered every 3 months at a health care facility) and would like to restart it. However, because she had to reschedule her last appointment due to a lack of transportation, she missed her injection window and subsequently became pregnant with her second child. She would still prefer injectable DMPA over the other contraceptive options offered—etonogestrel implant, oral contraceptive, or intrauterine device (IUD)—given her migraine history. However, she’s concerned she may have difficulty coming to the office every 3 months for her injection. What alternative injectable option can you offer?
When not pregnant or seeking to become pregnant, women may spend a significant amount of their lives trying to avoid pregnancy, and almost all women use contraception at some point.2 During the childbearing years of 15 to 49, 65% of women report using contraception.2 Although DMPA is a safe and effective option, only 2% of women report using it for contraception.2
For patients who have migraine with aura, there are fewer contraception options because their risk for ischemic stroke is increased 2- to 4-fold if they use combined hormonal contraceptives in pill, patch, or vaginal ring form.3 Safe options for these patients include the copper IUD, levonorgestrel-releasing intrauterine system, progestin implant, and DMPA injection.3
DMPA is a progestogen-only contraceptive approved by the US Food and Drug Administration to prevent pregnancy. It is available in an intramuscular formulation (DMPA-IM; 150 mg/mL every 13 weeks) and a subcutaneous formulation (DMPA-SC; 104 mg/0.65 mL every 12-14 weeks). DMPA-IM is administered by a health care provider and thus requires patients to present every 3 months for an injection. About 6% of DMPA-IM users have an unintended pregnancy in the first year due to inconsistent or incorrect use or late receipt of injection.4 DMPA-SC is produced as a prefilled needle that can be self-injected by patients.
Barriers to access are a growing concern. During the COVID-19 pandemic, one-third of women surveyed by the Guttmacher Institute (n = 2009) reported delaying or canceling a health care visit or having difficulty obtaining their contraception. Barriers to health care and contraception access were more common among Black and Hispanic women (vs White women), queer women (vs straight women), and low-income women (vs higher-income women).5
Following the overturning of Roe v Wade in June 2022, abortion access is now limited in parts of the United States. Given this significant policy change, physicians have an increasingly important role in providing contraception care and reducing barriers to contraception access. Since the SC forms of injectable contraception can be administered at home rather than in the health care setting, both the World Health Organization and the Centers for Disease Control and Prevention have recommended that self-administered injectable contraception be made widely available to expand access to contraception.6,7
STUDY SUMMARY
Higher contraceptive continuation rates with comparable safety and efficacy
This 2019 systematic review and meta-analysis evaluated the outcomes associated with use of self-administered DMPA-SC vs provider-administered DMPA in 5 countries.1 The authors searched several electronic databases for peer-reviewed studies of women who chose the option to self-administer DMPA-SC vs those who received DMPA injections from a health care provider.
Continue to: Outcomes included pregnancy
Outcomes included pregnancy; adverse effects or events (bleeding, injection site reactions, mental health concerns); initial use of injectable contraception (contraception uptake); and continuation rate of injectable contraception. Two reviewers extracted the data and assessed trials for bias. The authors used random-effects models to calculate pooled relative risk (RR) for studies with the same outcomes.
The analysis included a total of 6 trials (N = 3851): 3 RCTs (n = 1263) and 3 controlled cohort studies (n = 2588), conducted in the United States (2 trials), Malawi, Scotland, Uganda, and Senegal. All studies compared 12-month continuation rates of self-injected DMPA-SC vs provider-administered DMPA-SC or DMPA-IM every 3 months (12-13 weeks, with a window for early and late injections). Participants were at least 15 years of age (mean range, 26 to 29 years). In some studies, reminders (eg, texts, emails, calendar notifications) were provided to either the self-injection cohort only or to both cohorts of the trial. The RCTs were generally graded as having a low risk for bias, except for nonblinding of participants and personnel, given the nature of the interventions. The authors reported no evidence of significant heterogeneity in the studies.
The meta-analysis found higher continuation rates at 12 months with self-administrated DMPA compared with provider administration in the RCTs (RR = 1.27; 95% CI, 1.16-1.39) and in the observational cohort studies (RR = 1.18; 95% CI, 1.10-1.26). Pregnancy outcomes were reported in 4 studies, with the meta-analysis finding no significant difference in pregnancy rates in 2 RCTs (RR = 0.58; 95% CI, 0.15-2.22) or 2 observational cohort studies (RR = 1.1; 95% CI, 0.23-5.26).
Adverse effects or events were reported in 4 studies: 2 cohort studies reported increased injection site reactions with self-administration, and 1 RCT reported increased injection site pain or irritation with self-administration at 3 and 9 months. No other reported adverse effects occurred at higher rates with self-administration vs provider administration.
WHAT’S NEW
Demonstrated effectiveness of self-administered formulation
This systematic review and meta-analysis demonstrated that self-administration of DMPA-SC leads to higher contraception continuation rates at 12 months, without notable increased pregnancy rates or adverse effects, when compared with provider-administered DMPA.
Continue to: CAVEATS
CAVEATS
Outcome data limited to 12 months
Although self-administered DMPA-SC has the theoretical risk for user error and incorrect administration, this study did not find increased rates of pregnancy despite administration outside a health care center. However, the total number of pregnancies in each of the 4 studies measuring this outcome was low (< 5), and thus the authors noted that the effect size estimates may not be accurate.
Currently, there are no data on long-term outcomes beyond 12 months. Additionally, the health care visits for provider-administered DMPA every 3 months may afford other benefits, such as regular discussion of reproductive health concerns or testing for sexually transmitted infections, which must be weighed against the benefit of increased contraception access with self-administration. However, using the DMPA-SC self-administered formulation at home would not inhibit women from making separate health care visits as needed.
CHALLENGES TO IMPLEMENTATION
Limited resources to teach patients how to self-inject
Barriers to implementation include limited experience with prescribing DMPA-SC and changing practice culture to offer it to patients. Additionally, successful implementation of self-administered DMPA-SC is reliant on providing patients with appropriate information and training on self-injection, which requires knowledge, time, and other resources that may be limited in practices. Another potential barrier is product access, as not all insurers cover DMPA-SC and some pharmacies do not carry it.
1. Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
2. Daniels K, Abma J. Current contraceptive status among women aged 15-49: United States, 2017-2019. NCHS Data Brief. 2020;(388):1-8.
3. Paradise SL, Landis CA, Klein DA. Evidence-based contraception: common questions and answers. Am Fam Physician. 2022;106:251-259.
4. Marx M. Evidence‐based guidance for self‐administration of injectable contraception. J Midwifery Womens Health. 2021;66:108-112. doi: 10.1111/jmwh.13190
5. Lindberg LD, VandeVusse A, Mueller J, et al. Early Impacts of the COVID-19 Pandemic: Findings from the 2020 Guttmacher Survey of Reproductive Health Experiences. Guttmacher Institute; 2020. Accessed October 25, 2022. www.guttmacher.org/report/early-impacts-covid-19-pandemic-findings-2020-guttmacher-survey-reproductive-health
6. World Health Organization. WHO consolidated guidance on self-care interventions for health: sexual and reproductive health and rights. Published 2019. Accessed February 14, 2023. https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf
7. Curtis KM, Nguyen A, Reeves JA, et al. Update to US selected practice recommendations for contraceptive use: self-administration of subcutaneous depot medroxyprogesterone acetate. MMWR Morb Mortal Wkly Rep. 2021;70:739-743. doi: 10.15585/mmwr.mm7020a2
ILLUSTRATIVE CASE
A 32-year-old woman with a history of migraine with aura presents to your office for contraception management. She works full-time, has 2 children, and has transportation barriers. She previously used injectable DMPA (administered every 3 months at a health care facility) and would like to restart it. However, because she had to reschedule her last appointment due to a lack of transportation, she missed her injection window and subsequently became pregnant with her second child. She would still prefer injectable DMPA over the other contraceptive options offered—etonogestrel implant, oral contraceptive, or intrauterine device (IUD)—given her migraine history. However, she’s concerned she may have difficulty coming to the office every 3 months for her injection. What alternative injectable option can you offer?
When not pregnant or seeking to become pregnant, women may spend a significant amount of their lives trying to avoid pregnancy, and almost all women use contraception at some point.2 During the childbearing years of 15 to 49, 65% of women report using contraception.2 Although DMPA is a safe and effective option, only 2% of women report using it for contraception.2
For patients who have migraine with aura, there are fewer contraception options because their risk for ischemic stroke is increased 2- to 4-fold if they use combined hormonal contraceptives in pill, patch, or vaginal ring form.3 Safe options for these patients include the copper IUD, levonorgestrel-releasing intrauterine system, progestin implant, and DMPA injection.3
DMPA is a progestogen-only contraceptive approved by the US Food and Drug Administration to prevent pregnancy. It is available in an intramuscular formulation (DMPA-IM; 150 mg/mL every 13 weeks) and a subcutaneous formulation (DMPA-SC; 104 mg/0.65 mL every 12-14 weeks). DMPA-IM is administered by a health care provider and thus requires patients to present every 3 months for an injection. About 6% of DMPA-IM users have an unintended pregnancy in the first year due to inconsistent or incorrect use or late receipt of injection.4 DMPA-SC is produced as a prefilled needle that can be self-injected by patients.
Barriers to access are a growing concern. During the COVID-19 pandemic, one-third of women surveyed by the Guttmacher Institute (n = 2009) reported delaying or canceling a health care visit or having difficulty obtaining their contraception. Barriers to health care and contraception access were more common among Black and Hispanic women (vs White women), queer women (vs straight women), and low-income women (vs higher-income women).5
Following the overturning of Roe v Wade in June 2022, abortion access is now limited in parts of the United States. Given this significant policy change, physicians have an increasingly important role in providing contraception care and reducing barriers to contraception access. Since the SC forms of injectable contraception can be administered at home rather than in the health care setting, both the World Health Organization and the Centers for Disease Control and Prevention have recommended that self-administered injectable contraception be made widely available to expand access to contraception.6,7
STUDY SUMMARY
Higher contraceptive continuation rates with comparable safety and efficacy
This 2019 systematic review and meta-analysis evaluated the outcomes associated with use of self-administered DMPA-SC vs provider-administered DMPA in 5 countries.1 The authors searched several electronic databases for peer-reviewed studies of women who chose the option to self-administer DMPA-SC vs those who received DMPA injections from a health care provider.
Continue to: Outcomes included pregnancy
Outcomes included pregnancy; adverse effects or events (bleeding, injection site reactions, mental health concerns); initial use of injectable contraception (contraception uptake); and continuation rate of injectable contraception. Two reviewers extracted the data and assessed trials for bias. The authors used random-effects models to calculate pooled relative risk (RR) for studies with the same outcomes.
The analysis included a total of 6 trials (N = 3851): 3 RCTs (n = 1263) and 3 controlled cohort studies (n = 2588), conducted in the United States (2 trials), Malawi, Scotland, Uganda, and Senegal. All studies compared 12-month continuation rates of self-injected DMPA-SC vs provider-administered DMPA-SC or DMPA-IM every 3 months (12-13 weeks, with a window for early and late injections). Participants were at least 15 years of age (mean range, 26 to 29 years). In some studies, reminders (eg, texts, emails, calendar notifications) were provided to either the self-injection cohort only or to both cohorts of the trial. The RCTs were generally graded as having a low risk for bias, except for nonblinding of participants and personnel, given the nature of the interventions. The authors reported no evidence of significant heterogeneity in the studies.
The meta-analysis found higher continuation rates at 12 months with self-administrated DMPA compared with provider administration in the RCTs (RR = 1.27; 95% CI, 1.16-1.39) and in the observational cohort studies (RR = 1.18; 95% CI, 1.10-1.26). Pregnancy outcomes were reported in 4 studies, with the meta-analysis finding no significant difference in pregnancy rates in 2 RCTs (RR = 0.58; 95% CI, 0.15-2.22) or 2 observational cohort studies (RR = 1.1; 95% CI, 0.23-5.26).
Adverse effects or events were reported in 4 studies: 2 cohort studies reported increased injection site reactions with self-administration, and 1 RCT reported increased injection site pain or irritation with self-administration at 3 and 9 months. No other reported adverse effects occurred at higher rates with self-administration vs provider administration.
WHAT’S NEW
Demonstrated effectiveness of self-administered formulation
This systematic review and meta-analysis demonstrated that self-administration of DMPA-SC leads to higher contraception continuation rates at 12 months, without notable increased pregnancy rates or adverse effects, when compared with provider-administered DMPA.
Continue to: CAVEATS
CAVEATS
Outcome data limited to 12 months
Although self-administered DMPA-SC has the theoretical risk for user error and incorrect administration, this study did not find increased rates of pregnancy despite administration outside a health care center. However, the total number of pregnancies in each of the 4 studies measuring this outcome was low (< 5), and thus the authors noted that the effect size estimates may not be accurate.
Currently, there are no data on long-term outcomes beyond 12 months. Additionally, the health care visits for provider-administered DMPA every 3 months may afford other benefits, such as regular discussion of reproductive health concerns or testing for sexually transmitted infections, which must be weighed against the benefit of increased contraception access with self-administration. However, using the DMPA-SC self-administered formulation at home would not inhibit women from making separate health care visits as needed.
CHALLENGES TO IMPLEMENTATION
Limited resources to teach patients how to self-inject
Barriers to implementation include limited experience with prescribing DMPA-SC and changing practice culture to offer it to patients. Additionally, successful implementation of self-administered DMPA-SC is reliant on providing patients with appropriate information and training on self-injection, which requires knowledge, time, and other resources that may be limited in practices. Another potential barrier is product access, as not all insurers cover DMPA-SC and some pharmacies do not carry it.
ILLUSTRATIVE CASE
A 32-year-old woman with a history of migraine with aura presents to your office for contraception management. She works full-time, has 2 children, and has transportation barriers. She previously used injectable DMPA (administered every 3 months at a health care facility) and would like to restart it. However, because she had to reschedule her last appointment due to a lack of transportation, she missed her injection window and subsequently became pregnant with her second child. She would still prefer injectable DMPA over the other contraceptive options offered—etonogestrel implant, oral contraceptive, or intrauterine device (IUD)—given her migraine history. However, she’s concerned she may have difficulty coming to the office every 3 months for her injection. What alternative injectable option can you offer?
When not pregnant or seeking to become pregnant, women may spend a significant amount of their lives trying to avoid pregnancy, and almost all women use contraception at some point.2 During the childbearing years of 15 to 49, 65% of women report using contraception.2 Although DMPA is a safe and effective option, only 2% of women report using it for contraception.2
For patients who have migraine with aura, there are fewer contraception options because their risk for ischemic stroke is increased 2- to 4-fold if they use combined hormonal contraceptives in pill, patch, or vaginal ring form.3 Safe options for these patients include the copper IUD, levonorgestrel-releasing intrauterine system, progestin implant, and DMPA injection.3
DMPA is a progestogen-only contraceptive approved by the US Food and Drug Administration to prevent pregnancy. It is available in an intramuscular formulation (DMPA-IM; 150 mg/mL every 13 weeks) and a subcutaneous formulation (DMPA-SC; 104 mg/0.65 mL every 12-14 weeks). DMPA-IM is administered by a health care provider and thus requires patients to present every 3 months for an injection. About 6% of DMPA-IM users have an unintended pregnancy in the first year due to inconsistent or incorrect use or late receipt of injection.4 DMPA-SC is produced as a prefilled needle that can be self-injected by patients.
Barriers to access are a growing concern. During the COVID-19 pandemic, one-third of women surveyed by the Guttmacher Institute (n = 2009) reported delaying or canceling a health care visit or having difficulty obtaining their contraception. Barriers to health care and contraception access were more common among Black and Hispanic women (vs White women), queer women (vs straight women), and low-income women (vs higher-income women).5
Following the overturning of Roe v Wade in June 2022, abortion access is now limited in parts of the United States. Given this significant policy change, physicians have an increasingly important role in providing contraception care and reducing barriers to contraception access. Since the SC forms of injectable contraception can be administered at home rather than in the health care setting, both the World Health Organization and the Centers for Disease Control and Prevention have recommended that self-administered injectable contraception be made widely available to expand access to contraception.6,7
STUDY SUMMARY
Higher contraceptive continuation rates with comparable safety and efficacy
This 2019 systematic review and meta-analysis evaluated the outcomes associated with use of self-administered DMPA-SC vs provider-administered DMPA in 5 countries.1 The authors searched several electronic databases for peer-reviewed studies of women who chose the option to self-administer DMPA-SC vs those who received DMPA injections from a health care provider.
Continue to: Outcomes included pregnancy
Outcomes included pregnancy; adverse effects or events (bleeding, injection site reactions, mental health concerns); initial use of injectable contraception (contraception uptake); and continuation rate of injectable contraception. Two reviewers extracted the data and assessed trials for bias. The authors used random-effects models to calculate pooled relative risk (RR) for studies with the same outcomes.
The analysis included a total of 6 trials (N = 3851): 3 RCTs (n = 1263) and 3 controlled cohort studies (n = 2588), conducted in the United States (2 trials), Malawi, Scotland, Uganda, and Senegal. All studies compared 12-month continuation rates of self-injected DMPA-SC vs provider-administered DMPA-SC or DMPA-IM every 3 months (12-13 weeks, with a window for early and late injections). Participants were at least 15 years of age (mean range, 26 to 29 years). In some studies, reminders (eg, texts, emails, calendar notifications) were provided to either the self-injection cohort only or to both cohorts of the trial. The RCTs were generally graded as having a low risk for bias, except for nonblinding of participants and personnel, given the nature of the interventions. The authors reported no evidence of significant heterogeneity in the studies.
The meta-analysis found higher continuation rates at 12 months with self-administrated DMPA compared with provider administration in the RCTs (RR = 1.27; 95% CI, 1.16-1.39) and in the observational cohort studies (RR = 1.18; 95% CI, 1.10-1.26). Pregnancy outcomes were reported in 4 studies, with the meta-analysis finding no significant difference in pregnancy rates in 2 RCTs (RR = 0.58; 95% CI, 0.15-2.22) or 2 observational cohort studies (RR = 1.1; 95% CI, 0.23-5.26).
Adverse effects or events were reported in 4 studies: 2 cohort studies reported increased injection site reactions with self-administration, and 1 RCT reported increased injection site pain or irritation with self-administration at 3 and 9 months. No other reported adverse effects occurred at higher rates with self-administration vs provider administration.
WHAT’S NEW
Demonstrated effectiveness of self-administered formulation
This systematic review and meta-analysis demonstrated that self-administration of DMPA-SC leads to higher contraception continuation rates at 12 months, without notable increased pregnancy rates or adverse effects, when compared with provider-administered DMPA.
Continue to: CAVEATS
CAVEATS
Outcome data limited to 12 months
Although self-administered DMPA-SC has the theoretical risk for user error and incorrect administration, this study did not find increased rates of pregnancy despite administration outside a health care center. However, the total number of pregnancies in each of the 4 studies measuring this outcome was low (< 5), and thus the authors noted that the effect size estimates may not be accurate.
Currently, there are no data on long-term outcomes beyond 12 months. Additionally, the health care visits for provider-administered DMPA every 3 months may afford other benefits, such as regular discussion of reproductive health concerns or testing for sexually transmitted infections, which must be weighed against the benefit of increased contraception access with self-administration. However, using the DMPA-SC self-administered formulation at home would not inhibit women from making separate health care visits as needed.
CHALLENGES TO IMPLEMENTATION
Limited resources to teach patients how to self-inject
Barriers to implementation include limited experience with prescribing DMPA-SC and changing practice culture to offer it to patients. Additionally, successful implementation of self-administered DMPA-SC is reliant on providing patients with appropriate information and training on self-injection, which requires knowledge, time, and other resources that may be limited in practices. Another potential barrier is product access, as not all insurers cover DMPA-SC and some pharmacies do not carry it.
1. Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
2. Daniels K, Abma J. Current contraceptive status among women aged 15-49: United States, 2017-2019. NCHS Data Brief. 2020;(388):1-8.
3. Paradise SL, Landis CA, Klein DA. Evidence-based contraception: common questions and answers. Am Fam Physician. 2022;106:251-259.
4. Marx M. Evidence‐based guidance for self‐administration of injectable contraception. J Midwifery Womens Health. 2021;66:108-112. doi: 10.1111/jmwh.13190
5. Lindberg LD, VandeVusse A, Mueller J, et al. Early Impacts of the COVID-19 Pandemic: Findings from the 2020 Guttmacher Survey of Reproductive Health Experiences. Guttmacher Institute; 2020. Accessed October 25, 2022. www.guttmacher.org/report/early-impacts-covid-19-pandemic-findings-2020-guttmacher-survey-reproductive-health
6. World Health Organization. WHO consolidated guidance on self-care interventions for health: sexual and reproductive health and rights. Published 2019. Accessed February 14, 2023. https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf
7. Curtis KM, Nguyen A, Reeves JA, et al. Update to US selected practice recommendations for contraceptive use: self-administration of subcutaneous depot medroxyprogesterone acetate. MMWR Morb Mortal Wkly Rep. 2021;70:739-743. doi: 10.15585/mmwr.mm7020a2
1. Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
2. Daniels K, Abma J. Current contraceptive status among women aged 15-49: United States, 2017-2019. NCHS Data Brief. 2020;(388):1-8.
3. Paradise SL, Landis CA, Klein DA. Evidence-based contraception: common questions and answers. Am Fam Physician. 2022;106:251-259.
4. Marx M. Evidence‐based guidance for self‐administration of injectable contraception. J Midwifery Womens Health. 2021;66:108-112. doi: 10.1111/jmwh.13190
5. Lindberg LD, VandeVusse A, Mueller J, et al. Early Impacts of the COVID-19 Pandemic: Findings from the 2020 Guttmacher Survey of Reproductive Health Experiences. Guttmacher Institute; 2020. Accessed October 25, 2022. www.guttmacher.org/report/early-impacts-covid-19-pandemic-findings-2020-guttmacher-survey-reproductive-health
6. World Health Organization. WHO consolidated guidance on self-care interventions for health: sexual and reproductive health and rights. Published 2019. Accessed February 14, 2023. https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf
7. Curtis KM, Nguyen A, Reeves JA, et al. Update to US selected practice recommendations for contraceptive use: self-administration of subcutaneous depot medroxyprogesterone acetate. MMWR Morb Mortal Wkly Rep. 2021;70:739-743. doi: 10.15585/mmwr.mm7020a2
PRACTICE CHANGER
Consider prescribing self-administered subcutaneous depot medroxyprogesterone acetate (DMPA) for contraception instead of provider-administered DMPA. Self-administration improves contraception continuation rates without notable increases in pregnancy or adverse effects.
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials (RCTs) and cohort studies.1
Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
Comment & Controversy
Drospirenone vs norethindrone progestin-only pills. Is there a clear winner?
ROBERT L. BARBIERI, MD (FEBRUARY 2022)
Contraception queries
Dr. Barbieri, addressing your editorial on drospirenone and norethindrone pills, can you tell me why there are 4 placebo pills in Slynd? In addition, why did Exeltis choose a 24/4 regimen instead of a continuous regimen? And are there data on bleeding patterns with continuous drospirenone versus 24/4?
Meredith S. Cassidy, MD
Colorado Springs, Colorado
Dr. Barbieri responds
I thank Dr. Cassidy for the excellent question! The purpose of the 4 placebo pills in the Slynd (drospirenone 4 mg) 24/4 progestin-only contraceptive is to induce scheduled bleeding and reduce the number of days of unscheduled uterine bleeding. In a study of 858 patients, compared with a continuous progestin-only desogestrel contraceptive, Slynd with 4 placebo pills, was associated with significantly fewer days of unscheduled bleeding, 22 days versus 35 days (P<.0003) over 8 months of contraceptive use.1
The norethindrone progestin-only pill (POP) , which is available in the United States has very weak anti-ovulatory properties. If there were 4 placebo pills in the norethindrone POP, ovulation rates would increase, leading to reduced contraceptive efficacy. In contrast, Slynd with 4 placebo pills has excellent anti-ovulatory efficacy.
Reference
1. Palacios S, Colli E, Regidor PA. Bleeding profile of women using a drospirenone-ony 4 mg over nine cycles in comparison with desogestrel 0.075 mg. PLoS ONE. 2020;15:e0231856.
Should every scheduled cesarean birth use an Enhanced Recovery After Surgery (ERAS) pathway?
ROBERT L. BARBIERI, MD (NOVEMBER 2022)
ERAS for all cesarean deliveries
In Dr. Barbieri’s editorial “Should every scheduled cesarean birth use an Enhanced Recovery After Surgery (ERAS) pathway?”, he and Dr. Schantz-Dunn outline several reasons why the answer is a resounding, “Yes!”
I would suggest that ERAS principles should be used for all cesarean deliveries (CDs), not only scheduled ones. Many components of CD ERAS pathways are equally applicable to scheduled and unscheduled CDs, specifically those components that apply to intraoperative care (antibiotic prophylaxis, skin preparation, surgical technique, uterotonic administration, normothermia, and multimodal anesthesia) and postoperative care (VTE prophylaxis, gum chewing, early oral intake, early ambulation, early removal of bladder catheter, predischarge patient education, scheduled analgesic prophylaxis with acetaminophen, and NSAIDS). Although scheduled CDs have the additional advantage of the pre-hospital components (breastfeeding education, shortened fasting interval, carbohydrate loading, anemia prevention, and physiologic optimization), most of the benefit of ERAS for CD is likely attributable to the intraoperative and postoperative components.
For example, in our CD ERAS program, the median postoperative opioid consumption was reduced from a baseline of more than 100 morphine mg equivalents (MME) in both scheduled CDs (23 MME, interquartile range [IQR], 0-70) and unscheduled CDs (23 MME, IQR, 0-75).1 Remarkably, 29% of patients in the ERAS pathway used no postoperative opioids at all, a testament to the efficacy of neuraxial morphine and postoperative acetaminophen and NSAIDS. In another program, ERAS was associated with decreased postpartum length of stay and reduced direct costs in both scheduled and unscheduled CDs.2
References
- Combs CA, Robinson T, Mekis C, et al. Enhanced recovery after cesarean: impact on postoperative opioid use and length of stay. Am J Obstet Gynecol. 2021;224:237-239.
- Fay EE, Hitti JE, Delgado CM, et al. An enhanced recovery after surgery pathway for cesarean delivery decreases hospital stay and cost. Am J Obstet Gynecol. 2019;221:349.e1-e9.
C. Andrew Combs, MD, PhD
Sunrise, Florida
Dr. Barbieri responds
I am grateful to Dr. Combs’ advocacy for applying ERAS principles to all CD births, including scheduled and unscheduled operations. Dr. Combs notes that the intraoperative and postoperative components of ERAS can be used for both scheduled and unscheduled CD births. Of particular note is the marked reduction in opioid medication use achieved among Dr. Combs’ patients who were on an ERAS pathway. Hopefully, due to Dr. Combs clinical and research leadership many more patients will benefit from the use of an ERAS pathway.
ObGyns united in a divided post-Dobbs America
ERIN TRACY BRADLEY, MD, MPH, AND MEGAN L. EVANS,MD, MPH (DECEMBER 2022)
ObGyns are not united on this issue
I just finished reading the article by Drs. Bradley and Evans in the December edition of
The unborn seem not to have advocates like Drs. Bradley and Evans. In fact, those who hold pro-life opinions are regularly silenced in publications and on social media. The Facebooks and Twitters of the world tend to hold us in derision when they are not silencing us. There used to be a detente in our field where we each respected the viewpoint of the other, but now it is nonstop advocacy for abortion. Some authors want to accelerate and intensify that advocacy. I suspect that the pro-life views like mine will continue to be silenced. I just want the authors to know that we are not united in this post-Dobbs world. Many of us want appropriate limits on termination. We are not in favor of the unlimited right to abort a fetus up to the moment of delivery.
Steven G. Nelson
Phoenix, Arizona
Drospirenone vs norethindrone progestin-only pills. Is there a clear winner?
ROBERT L. BARBIERI, MD (FEBRUARY 2022)
Contraception queries
Dr. Barbieri, addressing your editorial on drospirenone and norethindrone pills, can you tell me why there are 4 placebo pills in Slynd? In addition, why did Exeltis choose a 24/4 regimen instead of a continuous regimen? And are there data on bleeding patterns with continuous drospirenone versus 24/4?
Meredith S. Cassidy, MD
Colorado Springs, Colorado
Dr. Barbieri responds
I thank Dr. Cassidy for the excellent question! The purpose of the 4 placebo pills in the Slynd (drospirenone 4 mg) 24/4 progestin-only contraceptive is to induce scheduled bleeding and reduce the number of days of unscheduled uterine bleeding. In a study of 858 patients, compared with a continuous progestin-only desogestrel contraceptive, Slynd with 4 placebo pills, was associated with significantly fewer days of unscheduled bleeding, 22 days versus 35 days (P<.0003) over 8 months of contraceptive use.1
The norethindrone progestin-only pill (POP) , which is available in the United States has very weak anti-ovulatory properties. If there were 4 placebo pills in the norethindrone POP, ovulation rates would increase, leading to reduced contraceptive efficacy. In contrast, Slynd with 4 placebo pills has excellent anti-ovulatory efficacy.
Reference
1. Palacios S, Colli E, Regidor PA. Bleeding profile of women using a drospirenone-ony 4 mg over nine cycles in comparison with desogestrel 0.075 mg. PLoS ONE. 2020;15:e0231856.
Should every scheduled cesarean birth use an Enhanced Recovery After Surgery (ERAS) pathway?
ROBERT L. BARBIERI, MD (NOVEMBER 2022)
ERAS for all cesarean deliveries
In Dr. Barbieri’s editorial “Should every scheduled cesarean birth use an Enhanced Recovery After Surgery (ERAS) pathway?”, he and Dr. Schantz-Dunn outline several reasons why the answer is a resounding, “Yes!”
I would suggest that ERAS principles should be used for all cesarean deliveries (CDs), not only scheduled ones. Many components of CD ERAS pathways are equally applicable to scheduled and unscheduled CDs, specifically those components that apply to intraoperative care (antibiotic prophylaxis, skin preparation, surgical technique, uterotonic administration, normothermia, and multimodal anesthesia) and postoperative care (VTE prophylaxis, gum chewing, early oral intake, early ambulation, early removal of bladder catheter, predischarge patient education, scheduled analgesic prophylaxis with acetaminophen, and NSAIDS). Although scheduled CDs have the additional advantage of the pre-hospital components (breastfeeding education, shortened fasting interval, carbohydrate loading, anemia prevention, and physiologic optimization), most of the benefit of ERAS for CD is likely attributable to the intraoperative and postoperative components.
For example, in our CD ERAS program, the median postoperative opioid consumption was reduced from a baseline of more than 100 morphine mg equivalents (MME) in both scheduled CDs (23 MME, interquartile range [IQR], 0-70) and unscheduled CDs (23 MME, IQR, 0-75).1 Remarkably, 29% of patients in the ERAS pathway used no postoperative opioids at all, a testament to the efficacy of neuraxial morphine and postoperative acetaminophen and NSAIDS. In another program, ERAS was associated with decreased postpartum length of stay and reduced direct costs in both scheduled and unscheduled CDs.2
References
- Combs CA, Robinson T, Mekis C, et al. Enhanced recovery after cesarean: impact on postoperative opioid use and length of stay. Am J Obstet Gynecol. 2021;224:237-239.
- Fay EE, Hitti JE, Delgado CM, et al. An enhanced recovery after surgery pathway for cesarean delivery decreases hospital stay and cost. Am J Obstet Gynecol. 2019;221:349.e1-e9.
C. Andrew Combs, MD, PhD
Sunrise, Florida
Dr. Barbieri responds
I am grateful to Dr. Combs’ advocacy for applying ERAS principles to all CD births, including scheduled and unscheduled operations. Dr. Combs notes that the intraoperative and postoperative components of ERAS can be used for both scheduled and unscheduled CD births. Of particular note is the marked reduction in opioid medication use achieved among Dr. Combs’ patients who were on an ERAS pathway. Hopefully, due to Dr. Combs clinical and research leadership many more patients will benefit from the use of an ERAS pathway.
ObGyns united in a divided post-Dobbs America
ERIN TRACY BRADLEY, MD, MPH, AND MEGAN L. EVANS,MD, MPH (DECEMBER 2022)
ObGyns are not united on this issue
I just finished reading the article by Drs. Bradley and Evans in the December edition of
The unborn seem not to have advocates like Drs. Bradley and Evans. In fact, those who hold pro-life opinions are regularly silenced in publications and on social media. The Facebooks and Twitters of the world tend to hold us in derision when they are not silencing us. There used to be a detente in our field where we each respected the viewpoint of the other, but now it is nonstop advocacy for abortion. Some authors want to accelerate and intensify that advocacy. I suspect that the pro-life views like mine will continue to be silenced. I just want the authors to know that we are not united in this post-Dobbs world. Many of us want appropriate limits on termination. We are not in favor of the unlimited right to abort a fetus up to the moment of delivery.
Steven G. Nelson
Phoenix, Arizona
Drospirenone vs norethindrone progestin-only pills. Is there a clear winner?
ROBERT L. BARBIERI, MD (FEBRUARY 2022)
Contraception queries
Dr. Barbieri, addressing your editorial on drospirenone and norethindrone pills, can you tell me why there are 4 placebo pills in Slynd? In addition, why did Exeltis choose a 24/4 regimen instead of a continuous regimen? And are there data on bleeding patterns with continuous drospirenone versus 24/4?
Meredith S. Cassidy, MD
Colorado Springs, Colorado
Dr. Barbieri responds
I thank Dr. Cassidy for the excellent question! The purpose of the 4 placebo pills in the Slynd (drospirenone 4 mg) 24/4 progestin-only contraceptive is to induce scheduled bleeding and reduce the number of days of unscheduled uterine bleeding. In a study of 858 patients, compared with a continuous progestin-only desogestrel contraceptive, Slynd with 4 placebo pills, was associated with significantly fewer days of unscheduled bleeding, 22 days versus 35 days (P<.0003) over 8 months of contraceptive use.1
The norethindrone progestin-only pill (POP) , which is available in the United States has very weak anti-ovulatory properties. If there were 4 placebo pills in the norethindrone POP, ovulation rates would increase, leading to reduced contraceptive efficacy. In contrast, Slynd with 4 placebo pills has excellent anti-ovulatory efficacy.
Reference
1. Palacios S, Colli E, Regidor PA. Bleeding profile of women using a drospirenone-ony 4 mg over nine cycles in comparison with desogestrel 0.075 mg. PLoS ONE. 2020;15:e0231856.
Should every scheduled cesarean birth use an Enhanced Recovery After Surgery (ERAS) pathway?
ROBERT L. BARBIERI, MD (NOVEMBER 2022)
ERAS for all cesarean deliveries
In Dr. Barbieri’s editorial “Should every scheduled cesarean birth use an Enhanced Recovery After Surgery (ERAS) pathway?”, he and Dr. Schantz-Dunn outline several reasons why the answer is a resounding, “Yes!”
I would suggest that ERAS principles should be used for all cesarean deliveries (CDs), not only scheduled ones. Many components of CD ERAS pathways are equally applicable to scheduled and unscheduled CDs, specifically those components that apply to intraoperative care (antibiotic prophylaxis, skin preparation, surgical technique, uterotonic administration, normothermia, and multimodal anesthesia) and postoperative care (VTE prophylaxis, gum chewing, early oral intake, early ambulation, early removal of bladder catheter, predischarge patient education, scheduled analgesic prophylaxis with acetaminophen, and NSAIDS). Although scheduled CDs have the additional advantage of the pre-hospital components (breastfeeding education, shortened fasting interval, carbohydrate loading, anemia prevention, and physiologic optimization), most of the benefit of ERAS for CD is likely attributable to the intraoperative and postoperative components.
For example, in our CD ERAS program, the median postoperative opioid consumption was reduced from a baseline of more than 100 morphine mg equivalents (MME) in both scheduled CDs (23 MME, interquartile range [IQR], 0-70) and unscheduled CDs (23 MME, IQR, 0-75).1 Remarkably, 29% of patients in the ERAS pathway used no postoperative opioids at all, a testament to the efficacy of neuraxial morphine and postoperative acetaminophen and NSAIDS. In another program, ERAS was associated with decreased postpartum length of stay and reduced direct costs in both scheduled and unscheduled CDs.2
References
- Combs CA, Robinson T, Mekis C, et al. Enhanced recovery after cesarean: impact on postoperative opioid use and length of stay. Am J Obstet Gynecol. 2021;224:237-239.
- Fay EE, Hitti JE, Delgado CM, et al. An enhanced recovery after surgery pathway for cesarean delivery decreases hospital stay and cost. Am J Obstet Gynecol. 2019;221:349.e1-e9.
C. Andrew Combs, MD, PhD
Sunrise, Florida
Dr. Barbieri responds
I am grateful to Dr. Combs’ advocacy for applying ERAS principles to all CD births, including scheduled and unscheduled operations. Dr. Combs notes that the intraoperative and postoperative components of ERAS can be used for both scheduled and unscheduled CD births. Of particular note is the marked reduction in opioid medication use achieved among Dr. Combs’ patients who were on an ERAS pathway. Hopefully, due to Dr. Combs clinical and research leadership many more patients will benefit from the use of an ERAS pathway.
ObGyns united in a divided post-Dobbs America
ERIN TRACY BRADLEY, MD, MPH, AND MEGAN L. EVANS,MD, MPH (DECEMBER 2022)
ObGyns are not united on this issue
I just finished reading the article by Drs. Bradley and Evans in the December edition of
The unborn seem not to have advocates like Drs. Bradley and Evans. In fact, those who hold pro-life opinions are regularly silenced in publications and on social media. The Facebooks and Twitters of the world tend to hold us in derision when they are not silencing us. There used to be a detente in our field where we each respected the viewpoint of the other, but now it is nonstop advocacy for abortion. Some authors want to accelerate and intensify that advocacy. I suspect that the pro-life views like mine will continue to be silenced. I just want the authors to know that we are not united in this post-Dobbs world. Many of us want appropriate limits on termination. We are not in favor of the unlimited right to abort a fetus up to the moment of delivery.
Steven G. Nelson
Phoenix, Arizona
Ninety-four women allege a Utah doctor sexually assaulted them. Here’s why a judge threw out their case
This article was produced for ProPublica’s Local Reporting Network in partnership with The Salt Lake Tribune.
At 19 years old and about to be married, Stephanie Mateer went to an ob.gyn. within walking distance of her student housing near Brigham Young University in Provo, Utah.
She wanted to start using birth control, and she was looking for guidance about having sex for the first time on her 2008 wedding night.
Ms. Mateer was shocked, she said, when David Broadbent, MD, reached under her gown to grab and squeeze her breasts, started a vaginal exam without warning, then followed it with an extremely painful examination of her rectum.
She felt disgusted and violated, but doubt also crept in. She told herself she must have misinterpreted his actions, or that she should have known that he would do a rectal exam. Raised as a member of The Church of Jesus Christ of Latter-day Saints, she said she was taught to defer to men in leadership.
“I viewed him as being a man in authority,” Ms. Mateer said. “He’s a doctor.”
It was years, she said, before she learned that her experience was in a sharp contrast to the conduct called for in professional standards, including that doctors use only their fingertips during a breast exam and communicate clearly what they are doing in advance, to gain the consent of their patients. Eventually, she gave her experience another name: sexual assault.
Utah judges, however, have called it health care.
And that legal distinction means Utahns like Ms. Mateer who decide to sue a health care provider for alleged sexual abuse are treated more harshly by the court system than plaintiffs who say they were harmed in other settings.
The chance to go to civil court for damages is an important option for survivors, experts say. While a criminal conviction can provide a sense of justice, winning a lawsuit can help victims pay for the therapy and additional support they need to heal after trauma.
Ms. Mateer laid out her allegations in a lawsuit that she and 93 other women filed against Dr. Broadbent last year. But they quickly learned they would be treated differently than other sexual assault survivors.
Filing their case, which alleged the Utah County doctor sexually assaulted them over the span of his 47-year career, was an empowering moment, Ms. Mateer said. But a judge threw out the lawsuit without even considering the merits, determining that because their alleged assailant is a doctor, the case must be governed by medical malpractice rules rather than those that apply to cases of sexual assault.
Under Utah’s rules of medical malpractice, claims made by victims who allege a health care worker sexually assaulted them are literally worth less than lawsuits brought by someone who was assaulted in other settings – even if a jury rules in their favor, a judge is required to limit how much money they receive. And they must meet a shorter filing deadline.
“It’s just crazy that a doctor can sexually assault women and then be protected by the white coat,” Ms. Mateer said. “It’s just a really scary precedent to be calling sexual assault ‘health care.’ ”
Because of the judge’s ruling that leaves them with a shorter window in which to file, some of Dr. Broadbent’s accusers stand to lose their chance to sue. Others were already past that deadline but had hoped to take advantage of an exception that allows plaintiffs to sue if they can prove that the person who harmed them had covered up the wrongdoing and if they discovered they had been hurt within the previous year.
As a group, the women are appealing the ruling to the Utah Supreme Court, which has agreed to hear the case. This decision will set a precedent for future sexual assault victims in Utah.
Dr. Broadbent’s attorney, Chris Nelson, declined an interview request but wrote in an email: “We believe that the allegations against Dr. Broadbent are without merit and will present our case in court. Given that this is an active legal matter, we will not be sharing any details outside the courtroom.”
States have varying legal definitions of medical malpractice, but it’s generally described as treatment that falls short of accepted standards of care. That includes mistakes, such as a surgeon leaving a piece of gauze inside a patient.
The Utah Supreme Court has ruled that a teenage boy was receiving health care when he was allowed to climb a steep, snow-dusted rock outcrop as part of wilderness therapy. When he broke his leg, he could only sue for medical malpractice, so the case faced shorter filing deadlines and lower monetary caps. Similarly, the court has ruled that a boy harmed by another child while in foster care was also bound by medical malpractice law.
Despite these state Supreme Court rulings, Utah legislators have so far not moved to narrow the wording of the malpractice act.
The lawsuit against Dr. Broadbent – and the questions it raises about the broadness of Utah’s medical malpractice laws – comes during a national reckoning with how sexual assault survivors are treated by the law. Legislators in several states have been rewriting laws to give sexual assault victims more time to sue their attackers, in response to the growing cultural understanding of the impact of trauma and the barriers to reporting. Even in Utah, those who were sexually abused as children now have no deadline to file suits against their abusers.
That isn’t true for sexual abuse in a medical setting, where cases must be filed within 2 years of the assault.
These higher hurdles should not exist in Utah, said state Sen. Mike K. McKell, a Utah County Republican who works as a personal injury attorney. He is trying to change state law to ensure that sexual assault lawsuits do not fall under Utah’s Health Care Malpractice Act, a law designed to cover negligence and poor care, not necessarily deliberate actions like an assault.
“Sexual assault, to me, is not medical care. Period,” he said. “It’s sad that we need to clarify that sexual assault is not medical care. But trying to tie sexual assault to a medical malpractice [filing deadline] – it’s just wrong.”
‘Your husband is a lucky man’
Ms. Mateer had gone to Dr. Broadbent in 2008 for a premarital exam, a uniquely Utah visit often scheduled by young women who are members of The Church of Jesus Christ of Latter-day Saints.
Leaders of the faith, which is predominant in Utah, focus on chastity when speaking to young, unmarried people about sex, and public schools have typically focused on abstinence-based sex education. So for some, these visits are the first place they learn about sexual health.
Young women who get premarital exams are typically given a birth control prescription, but the appointments can include care that’s less common for healthy women in other states – such as doctors giving them vaginal dilators to stretch their tissues before their wedding nights.
That’s what Ms. Mateer was expecting when she visited Dr. Broadbent’s office. The ob.gyn. had been practicing for decades in his Provo clinic nestled between student housing apartments across the street from Brigham Young University, which is owned by The Church of Jesus Christ of Latter-day Saints.
So Ms. Mateer was “just totally taken aback,” she said, by the painful examination and by Dr. Broadbent snapping off his gloves after the exam and saying, “Your husband is a lucky man.”
She repeated that remark in her legal filing, along with the doctor’s advice for her: If she bled during intercourse, “just do what the Boy Scouts do and apply pressure.”
“The whole thing was like I’m some object for my husband to enjoy and let him do whatever he wants,” Ms. Mateer said. “It was just very violating and not a great way to start my sexual relationship with my new husband, with these ideas in mind.”
Ms. Mateer thought back to that visit over the years, particularly when she went to other ob.gyns. for health care. Her subsequent doctors, she said, never performed a rectal exam and always explained to her what they were doing and how it would feel, and asked for her consent.
She thought about Dr. Broadbent again in 2017, as the #MeToo movement gained momentum, and looked him up online. Ms. Mateer found reviews from other women who described Dr. Broadbent doing rough examinations without warning that left them feeling the same way she had years before.
Then in December 2021, she spoke out on “Mormon Stories,” a podcast where people who have left or have questioned their Latter-day Saint faith share their life stories. In the episode, she described the painful way he examined her, how it left her feeling traumatized, and her discovery of the reviews that echoed her experience.
“He’s on University Avenue, in Provo, giving these exams to who knows how many naive Mormon 18-year-old, 19-year-old girls who are getting married. … They are naive and they don’t know what to expect,” she said on the podcast. “His name is Dr. David Broadbent.”
After the podcast aired, Ms. Mateer was flooded with messages from women who heard the episode and reached out to tell her that Dr. Broadbent had harmed them, too.
Ms. Mateer and three other women decided to sue the ob.gyn., and in the following weeks and months, 90 additional women joined the lawsuit they filed in Provo. Many of the women allege Dr. Broadbent inappropriately touched their breasts, vaginas and rectums, hurting them, without warning or explanation. Some said he used his bare hand – instead of using a speculum or gloves – during exams. One alleged that she saw he had an erection while he was touching her.
Dr. Broadbent’s actions were not medically necessary, the women allege, and were instead “performed for no other reason than his own sexual gratification.”
The lawsuit also named as defendants two hospitals where Dr. Broadbent had delivered babies and where some of the women allege they were assaulted. The suit accused hospital administrators of knowing about Dr. Broadbent’s inappropriate behavior and doing nothing about it.
After he was sued, the ob.gyn. quickly lost his privileges at the hospitals where he worked. Dr. Broadbent, now 75, has also voluntarily put his medical license in Utah on hold while police investigate 29 reports of sexual assault made against him.
Prosecutors are still considering whether to criminally prosecute Dr. Broadbent. Provo police forwarded more than a dozen reports to the Utah County attorney’s office in November, which are still being reviewed by a local prosecutor.
A spokesperson for Intermountain Health, the nonprofit health system that owns Utah Valley Hospital, where some of the women in the suit were treated, did not respond to specific questions. The spokesperson emphasized in an email that Dr. Broadbent was an “independent physician” who was not employed by Utah Valley Hospital, adding that most of the alleged incidents took place at Dr. Broadbent’s medical office.
A representative for MountainStar Healthcare, another hospital chain named as a defendant, denied knowledge of any allegations of inappropriate conduct reported to its hospital and also emphasized that Dr. Broadbent worked independently, not as an employee.
“Our position since this lawsuit was filed has been that we were inappropriately named in this suit,” said Brittany Glas, the communications director for MountainStar.
Debating whether sexual abuse is health care
For the women who sued Dr. Broadbent, their case boiled down to a key question: Were the sexual assaults they say they experienced part of their health care? There was a lot hanging on the answer.
If their case was considered medical malpractice, they would be limited in how much money they could receive in damages for their pain and suffering. If a jury awarded them millions of dollars, a judge would be required by law to cut that down to $450,000. There’s no cap on these monetary awards for victims sexually assaulted in other settings.
They would also be required to go before a panel, which includes a doctor, a lawyer and a community member, that decides whether their claims have merit. This step, aimed at resolving disputes out of court, does not block anyone from suing afterward. But it does add cost and delay, and for sexual assault victims who’ve gone through this step, it has been another time they were required to describe their experiences and hope they were believed.
The shorter, 2-year filing deadline for medical malpractice cases can also be a particular challenge for those who have been sexually abused because research shows that it’s common to delay reporting such assaults.
Nationwide, these kinds of malpractice reforms were adopted in the 1970s amid concerns – largely driven by insurance companies – that the cost of health care was rising because of frivolous lawsuits and “runaway juries” doling out multimillion-dollar payouts.
Restricting the size of malpractice awards and imposing other limits, many argued, were effective ways to balance compensating injured patients with protecting everyone’s access to health care.
State laws are generally silent on whether sexual assault lawsuits should be covered by malpractice laws, leaving courts to grapple with that question and leading to different conclusions across the country. The Tribune and ProPublica identified at least six cases in which state appellate judges sharply distinguished between assault and health care in considering whether malpractice laws should apply to sexual assault–related cases.
An appellate court in Wisconsin, for example, ruled in 1993 that a physician having an erection and groping a patient was a purposeful harm, not medical malpractice.
Florida’s law is similar to Utah’s, defining allegations “arising” out of medical care as malpractice. While an earlier ruling did treat sexual assault in a health care setting as medical malpractice, appellate rulings in the last decade have moved away from that interpretation. In 2005, an appellate court affirmed a lower-court ruling that when a dentist “stopped providing dental treatment to the victim and began sexually assaulting her, his professional services ended.”
Similarly, a federal judge in Iowa in 1995 weighed in on the meaning of “arising” out of health care: “Rape is not patient care activity,” he wrote.
But Utah’s malpractice law is so broad that judges have been interpreting it as covering any act performed by a health care provider during medical care. The law was passed in 1976 and is popular with doctors and other health care providers, who have lobbied to keep it in place – and who use it to get lawsuits dismissed.
One precedent-setting case in Utah shows the law’s power to safeguard health care providers and was an important test of how Utah defines medical malpractice. Jacob Scott sued WinGate Wilderness Therapy after the teen broke his leg in 2015 when a hiking guide from the center allowed him to climb up and down a steep outcrop in Utah’s red rock desert.
His parents are both lawyers, and after they found that Utah had a 4-year deadline for filing a personal injury lawsuit, court records said, they decided to prioritize “getting Jacob better” for the first 2 years after the accident. But when Mr. Scott’s suit was filed, WinGate argued it was too late – based on the shorter, 2-year deadline for medical malpractice claims.
Mr. Scott’s attorneys scoffed. “Interacting with nature,” his attorneys argued, “is not health care even under the broadest interpretation of … the Utah Health Care Malpractice Act.”
A judge disagreed and threw out Mr. Scott’s case. The Utah Supreme Court unanimously upheld that ruling in 2021.
“We agree with WinGate,” the justices wrote, “that it was acting as a ‘health care provider’ and providing ‘health care’ when Jacob was hiking and rock climbing.”
Last summer, the women who had sued Dr. Broadbent and the two hospitals watched online as lawyers debated whether the abuse they allegedly suffered was health care.
At the hearing, attorneys for Dr. Broadbent and the hospitals argued that the women should have pursued a medical malpractice case, which required them to first notify Dr. Broadbent and the hospitals that they wanted to sue. They also argued to Judge Robert Lunnen that the case couldn’t move forward because the women hadn’t gone before a prelitigation panel.
Attorneys for Dr. Broadbent and the hospitals argued, one after the other, that the painful and traumatic exams the women described arose out of health care treatments.
“Accepting the allegations of the complaint as true – as we must for purposes of this proceeding – we have to assume that [Broadbent] did something that was medically unnecessary, medically inappropriate,” argued David Jordan, a lawyer for Intermountain Health.
“But it doesn’t change the fact that it’s an act performed to a patient, during the patient’s treatment,” he said. “Because that’s what the patient is doing in the doctor’s office. They’re there for treatment.”
The attorney team for the women pushed back. Terry Rooney argued that if Dr. Broadbent’s actions fell under medical malpractice laws, many women would be knocked out of the case because of the age of their claims, and those who remained would be limited in the amount of money in damages they could receive.
“That’s really what this is about,” he argued. “And so it’s troubling – quite frankly it’s shocking to me – that we’re debating heavily the question of whether sexual abuse is health care.”
The judge mulled the issue for months. Judge Lunnen wrote in a September ruling that if the allegations were true, Dr. Broadbent’s treatment of his patients was “insensitive, disrespectful and degrading.”
But Utah law is clear, he said. Malpractice law covers any act or treatment performed by any health care provider during the patient’s medical care. The women had all been seeking health care, Judge Lunnen wrote, and Dr. Broadbent was providing that when the alleged assaults happened.
Their lawsuit was dismissed.
‘I felt defeated’
Brooke, another plaintiff who alleges Dr. Broadbent groped her, remembers feeling sick on the June day she watched the attorneys arguing. She asked to be identified by only her first name for this story.
She alleges Dr. Broadbent violated her in December 2008 while she was hospitalized after experiencing complications with her first pregnancy.
The nearest hospital to her rural town didn’t have a special unit to take care of premature babies, and her doctors feared she might need to deliver her son 6 weeks early. So Brooke had been rushed by ambulance over a mountain pass in a snowstorm to Utah Valley Hospital.
Brooke and her husband were terrified, she said, when they arrived at the Provo hospital. Dr. Broadbent happened to be the doctor on call. With Brooke’s husband and brother-in-law in the room, Dr. Broadbent examined her late that evening, she said, listening to her chest with a stethoscope.
The doctor then suddenly grabbed her breasts, she recalled – his movements causing her hospital gown to fall to expose her chest. She recounted this experience in her lawsuit, saying it was nothing like the breast exams she has had since.
“It was really traumatizing,” she said. “I was mortified. My husband and brother-in-law – we just didn’t say anything about it because it was so uncomfortable.”
Brooke voiced concerns to the nurse manager, and she was assigned a new doctor.
She gave birth to a healthy baby a little more than a month later, at the hospital near her home.
Hearing the judge’s ruling 14 years later, Brooke felt the decision revealed how Utah’s laws are broken.
“I was frustrated,” she said, “and I felt defeated. … I thought justice is not on our side with this.”
If the Utah Supreme Court rules that these alleged sexual assaults should legally be considered health care, the women will likely refile their claims as a medical malpractice lawsuit, said their attorney, Adam Sorensen. But it would be a challenge to keep all 94 women in the case, he said, due to the shorter filing window. Only two women in the lawsuit allege that they were harmed within the last 2 years.
The legal team for the women would have to convince a judge that their claims should still be allowed because they only recently discovered they were harmed. But based on previous rulings, Mr. Sorensen believes the women will have a better chance to win that argument if the civil suit remained a sexual assault case.
Regardless of what happens in their legal case, the decision by Brooke and the other women to come forward could help change state law for victims who come after them.
Recently, Mr. McKell, the state senator, introduced legislation to clarify that civil lawsuits alleging sexual assault by a health care worker do not fall under Utah’s Health Care Malpractice Act.
“I don’t think it’s a close call. Sexual assault is not medical care,” he said. “I know we’ve got some bizarre rulings that have come down through our courts in Utah.”
Both an association of Utah trial lawyers and the Utah Medical Association, which lobbies on behalf of the state’s physicians, support this reform.
“We support the fact that sexual assault should not be part of health care medical malpractice,” said Michelle McOmber, the CEO for the Utah Medical Association. “Sexual assault should be sexual assault, regardless of where it happens or who’s doing it. Sexual assault should be in that category, which is separate from actual health care. Because it’s not health care.”
MountainStar doesn’t have a position on the bill, Ms. Glas said. “If the laws were to change via new legislation and/or interpretation by the courts, we would abide by and comply with those new laws.”
But lawmakers are running out of time. With only a short time left in Utah’s legislative session, state senate and house leaders have so far prioritized passing new laws banning gender-affirming health care for transgender youths and creating a controversial school voucher program that will provide taxpayer funds for students to attend private school.
Utah lawmakers were also expected to consider a dramatic change for other sexual assault victims: a bill that would remove filing deadlines for civil lawsuits brought by people abused as adults. But that bill stalled before it could be debated.
Brooke had been eager to share her story, she said, in hopes it would help the first four women who’d come forward bolster their lawsuit against Dr. Broadbent. She later joined the case as a plaintiff. She read in their lawsuit about one woman who complained about him to the same hospital 7 years before she did, and about another woman who said Dr. Broadbent similarly molested her 2 days after Brooke had expressed her own concern.
“That bothered me so much,” she said. “It didn’t have to happen to all these women.”
Brooke doubts she’ll get vindication in a courtroom. Justice for her, she suspects, won’t come in the form of a legal ruling or a settlement against the doctor she says hurt her years ago.
Instead, she said, “maybe justice looks like changing the laws for future women.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
This article was produced for ProPublica’s Local Reporting Network in partnership with The Salt Lake Tribune.
At 19 years old and about to be married, Stephanie Mateer went to an ob.gyn. within walking distance of her student housing near Brigham Young University in Provo, Utah.
She wanted to start using birth control, and she was looking for guidance about having sex for the first time on her 2008 wedding night.
Ms. Mateer was shocked, she said, when David Broadbent, MD, reached under her gown to grab and squeeze her breasts, started a vaginal exam without warning, then followed it with an extremely painful examination of her rectum.
She felt disgusted and violated, but doubt also crept in. She told herself she must have misinterpreted his actions, or that she should have known that he would do a rectal exam. Raised as a member of The Church of Jesus Christ of Latter-day Saints, she said she was taught to defer to men in leadership.
“I viewed him as being a man in authority,” Ms. Mateer said. “He’s a doctor.”
It was years, she said, before she learned that her experience was in a sharp contrast to the conduct called for in professional standards, including that doctors use only their fingertips during a breast exam and communicate clearly what they are doing in advance, to gain the consent of their patients. Eventually, she gave her experience another name: sexual assault.
Utah judges, however, have called it health care.
And that legal distinction means Utahns like Ms. Mateer who decide to sue a health care provider for alleged sexual abuse are treated more harshly by the court system than plaintiffs who say they were harmed in other settings.
The chance to go to civil court for damages is an important option for survivors, experts say. While a criminal conviction can provide a sense of justice, winning a lawsuit can help victims pay for the therapy and additional support they need to heal after trauma.
Ms. Mateer laid out her allegations in a lawsuit that she and 93 other women filed against Dr. Broadbent last year. But they quickly learned they would be treated differently than other sexual assault survivors.
Filing their case, which alleged the Utah County doctor sexually assaulted them over the span of his 47-year career, was an empowering moment, Ms. Mateer said. But a judge threw out the lawsuit without even considering the merits, determining that because their alleged assailant is a doctor, the case must be governed by medical malpractice rules rather than those that apply to cases of sexual assault.
Under Utah’s rules of medical malpractice, claims made by victims who allege a health care worker sexually assaulted them are literally worth less than lawsuits brought by someone who was assaulted in other settings – even if a jury rules in their favor, a judge is required to limit how much money they receive. And they must meet a shorter filing deadline.
“It’s just crazy that a doctor can sexually assault women and then be protected by the white coat,” Ms. Mateer said. “It’s just a really scary precedent to be calling sexual assault ‘health care.’ ”
Because of the judge’s ruling that leaves them with a shorter window in which to file, some of Dr. Broadbent’s accusers stand to lose their chance to sue. Others were already past that deadline but had hoped to take advantage of an exception that allows plaintiffs to sue if they can prove that the person who harmed them had covered up the wrongdoing and if they discovered they had been hurt within the previous year.
As a group, the women are appealing the ruling to the Utah Supreme Court, which has agreed to hear the case. This decision will set a precedent for future sexual assault victims in Utah.
Dr. Broadbent’s attorney, Chris Nelson, declined an interview request but wrote in an email: “We believe that the allegations against Dr. Broadbent are without merit and will present our case in court. Given that this is an active legal matter, we will not be sharing any details outside the courtroom.”
States have varying legal definitions of medical malpractice, but it’s generally described as treatment that falls short of accepted standards of care. That includes mistakes, such as a surgeon leaving a piece of gauze inside a patient.
The Utah Supreme Court has ruled that a teenage boy was receiving health care when he was allowed to climb a steep, snow-dusted rock outcrop as part of wilderness therapy. When he broke his leg, he could only sue for medical malpractice, so the case faced shorter filing deadlines and lower monetary caps. Similarly, the court has ruled that a boy harmed by another child while in foster care was also bound by medical malpractice law.
Despite these state Supreme Court rulings, Utah legislators have so far not moved to narrow the wording of the malpractice act.
The lawsuit against Dr. Broadbent – and the questions it raises about the broadness of Utah’s medical malpractice laws – comes during a national reckoning with how sexual assault survivors are treated by the law. Legislators in several states have been rewriting laws to give sexual assault victims more time to sue their attackers, in response to the growing cultural understanding of the impact of trauma and the barriers to reporting. Even in Utah, those who were sexually abused as children now have no deadline to file suits against their abusers.
That isn’t true for sexual abuse in a medical setting, where cases must be filed within 2 years of the assault.
These higher hurdles should not exist in Utah, said state Sen. Mike K. McKell, a Utah County Republican who works as a personal injury attorney. He is trying to change state law to ensure that sexual assault lawsuits do not fall under Utah’s Health Care Malpractice Act, a law designed to cover negligence and poor care, not necessarily deliberate actions like an assault.
“Sexual assault, to me, is not medical care. Period,” he said. “It’s sad that we need to clarify that sexual assault is not medical care. But trying to tie sexual assault to a medical malpractice [filing deadline] – it’s just wrong.”
‘Your husband is a lucky man’
Ms. Mateer had gone to Dr. Broadbent in 2008 for a premarital exam, a uniquely Utah visit often scheduled by young women who are members of The Church of Jesus Christ of Latter-day Saints.
Leaders of the faith, which is predominant in Utah, focus on chastity when speaking to young, unmarried people about sex, and public schools have typically focused on abstinence-based sex education. So for some, these visits are the first place they learn about sexual health.
Young women who get premarital exams are typically given a birth control prescription, but the appointments can include care that’s less common for healthy women in other states – such as doctors giving them vaginal dilators to stretch their tissues before their wedding nights.
That’s what Ms. Mateer was expecting when she visited Dr. Broadbent’s office. The ob.gyn. had been practicing for decades in his Provo clinic nestled between student housing apartments across the street from Brigham Young University, which is owned by The Church of Jesus Christ of Latter-day Saints.
So Ms. Mateer was “just totally taken aback,” she said, by the painful examination and by Dr. Broadbent snapping off his gloves after the exam and saying, “Your husband is a lucky man.”
She repeated that remark in her legal filing, along with the doctor’s advice for her: If she bled during intercourse, “just do what the Boy Scouts do and apply pressure.”
“The whole thing was like I’m some object for my husband to enjoy and let him do whatever he wants,” Ms. Mateer said. “It was just very violating and not a great way to start my sexual relationship with my new husband, with these ideas in mind.”
Ms. Mateer thought back to that visit over the years, particularly when she went to other ob.gyns. for health care. Her subsequent doctors, she said, never performed a rectal exam and always explained to her what they were doing and how it would feel, and asked for her consent.
She thought about Dr. Broadbent again in 2017, as the #MeToo movement gained momentum, and looked him up online. Ms. Mateer found reviews from other women who described Dr. Broadbent doing rough examinations without warning that left them feeling the same way she had years before.
Then in December 2021, she spoke out on “Mormon Stories,” a podcast where people who have left or have questioned their Latter-day Saint faith share their life stories. In the episode, she described the painful way he examined her, how it left her feeling traumatized, and her discovery of the reviews that echoed her experience.
“He’s on University Avenue, in Provo, giving these exams to who knows how many naive Mormon 18-year-old, 19-year-old girls who are getting married. … They are naive and they don’t know what to expect,” she said on the podcast. “His name is Dr. David Broadbent.”
After the podcast aired, Ms. Mateer was flooded with messages from women who heard the episode and reached out to tell her that Dr. Broadbent had harmed them, too.
Ms. Mateer and three other women decided to sue the ob.gyn., and in the following weeks and months, 90 additional women joined the lawsuit they filed in Provo. Many of the women allege Dr. Broadbent inappropriately touched their breasts, vaginas and rectums, hurting them, without warning or explanation. Some said he used his bare hand – instead of using a speculum or gloves – during exams. One alleged that she saw he had an erection while he was touching her.
Dr. Broadbent’s actions were not medically necessary, the women allege, and were instead “performed for no other reason than his own sexual gratification.”
The lawsuit also named as defendants two hospitals where Dr. Broadbent had delivered babies and where some of the women allege they were assaulted. The suit accused hospital administrators of knowing about Dr. Broadbent’s inappropriate behavior and doing nothing about it.
After he was sued, the ob.gyn. quickly lost his privileges at the hospitals where he worked. Dr. Broadbent, now 75, has also voluntarily put his medical license in Utah on hold while police investigate 29 reports of sexual assault made against him.
Prosecutors are still considering whether to criminally prosecute Dr. Broadbent. Provo police forwarded more than a dozen reports to the Utah County attorney’s office in November, which are still being reviewed by a local prosecutor.
A spokesperson for Intermountain Health, the nonprofit health system that owns Utah Valley Hospital, where some of the women in the suit were treated, did not respond to specific questions. The spokesperson emphasized in an email that Dr. Broadbent was an “independent physician” who was not employed by Utah Valley Hospital, adding that most of the alleged incidents took place at Dr. Broadbent’s medical office.
A representative for MountainStar Healthcare, another hospital chain named as a defendant, denied knowledge of any allegations of inappropriate conduct reported to its hospital and also emphasized that Dr. Broadbent worked independently, not as an employee.
“Our position since this lawsuit was filed has been that we were inappropriately named in this suit,” said Brittany Glas, the communications director for MountainStar.
Debating whether sexual abuse is health care
For the women who sued Dr. Broadbent, their case boiled down to a key question: Were the sexual assaults they say they experienced part of their health care? There was a lot hanging on the answer.
If their case was considered medical malpractice, they would be limited in how much money they could receive in damages for their pain and suffering. If a jury awarded them millions of dollars, a judge would be required by law to cut that down to $450,000. There’s no cap on these monetary awards for victims sexually assaulted in other settings.
They would also be required to go before a panel, which includes a doctor, a lawyer and a community member, that decides whether their claims have merit. This step, aimed at resolving disputes out of court, does not block anyone from suing afterward. But it does add cost and delay, and for sexual assault victims who’ve gone through this step, it has been another time they were required to describe their experiences and hope they were believed.
The shorter, 2-year filing deadline for medical malpractice cases can also be a particular challenge for those who have been sexually abused because research shows that it’s common to delay reporting such assaults.
Nationwide, these kinds of malpractice reforms were adopted in the 1970s amid concerns – largely driven by insurance companies – that the cost of health care was rising because of frivolous lawsuits and “runaway juries” doling out multimillion-dollar payouts.
Restricting the size of malpractice awards and imposing other limits, many argued, were effective ways to balance compensating injured patients with protecting everyone’s access to health care.
State laws are generally silent on whether sexual assault lawsuits should be covered by malpractice laws, leaving courts to grapple with that question and leading to different conclusions across the country. The Tribune and ProPublica identified at least six cases in which state appellate judges sharply distinguished between assault and health care in considering whether malpractice laws should apply to sexual assault–related cases.
An appellate court in Wisconsin, for example, ruled in 1993 that a physician having an erection and groping a patient was a purposeful harm, not medical malpractice.
Florida’s law is similar to Utah’s, defining allegations “arising” out of medical care as malpractice. While an earlier ruling did treat sexual assault in a health care setting as medical malpractice, appellate rulings in the last decade have moved away from that interpretation. In 2005, an appellate court affirmed a lower-court ruling that when a dentist “stopped providing dental treatment to the victim and began sexually assaulting her, his professional services ended.”
Similarly, a federal judge in Iowa in 1995 weighed in on the meaning of “arising” out of health care: “Rape is not patient care activity,” he wrote.
But Utah’s malpractice law is so broad that judges have been interpreting it as covering any act performed by a health care provider during medical care. The law was passed in 1976 and is popular with doctors and other health care providers, who have lobbied to keep it in place – and who use it to get lawsuits dismissed.
One precedent-setting case in Utah shows the law’s power to safeguard health care providers and was an important test of how Utah defines medical malpractice. Jacob Scott sued WinGate Wilderness Therapy after the teen broke his leg in 2015 when a hiking guide from the center allowed him to climb up and down a steep outcrop in Utah’s red rock desert.
His parents are both lawyers, and after they found that Utah had a 4-year deadline for filing a personal injury lawsuit, court records said, they decided to prioritize “getting Jacob better” for the first 2 years after the accident. But when Mr. Scott’s suit was filed, WinGate argued it was too late – based on the shorter, 2-year deadline for medical malpractice claims.
Mr. Scott’s attorneys scoffed. “Interacting with nature,” his attorneys argued, “is not health care even under the broadest interpretation of … the Utah Health Care Malpractice Act.”
A judge disagreed and threw out Mr. Scott’s case. The Utah Supreme Court unanimously upheld that ruling in 2021.
“We agree with WinGate,” the justices wrote, “that it was acting as a ‘health care provider’ and providing ‘health care’ when Jacob was hiking and rock climbing.”
Last summer, the women who had sued Dr. Broadbent and the two hospitals watched online as lawyers debated whether the abuse they allegedly suffered was health care.
At the hearing, attorneys for Dr. Broadbent and the hospitals argued that the women should have pursued a medical malpractice case, which required them to first notify Dr. Broadbent and the hospitals that they wanted to sue. They also argued to Judge Robert Lunnen that the case couldn’t move forward because the women hadn’t gone before a prelitigation panel.
Attorneys for Dr. Broadbent and the hospitals argued, one after the other, that the painful and traumatic exams the women described arose out of health care treatments.
“Accepting the allegations of the complaint as true – as we must for purposes of this proceeding – we have to assume that [Broadbent] did something that was medically unnecessary, medically inappropriate,” argued David Jordan, a lawyer for Intermountain Health.
“But it doesn’t change the fact that it’s an act performed to a patient, during the patient’s treatment,” he said. “Because that’s what the patient is doing in the doctor’s office. They’re there for treatment.”
The attorney team for the women pushed back. Terry Rooney argued that if Dr. Broadbent’s actions fell under medical malpractice laws, many women would be knocked out of the case because of the age of their claims, and those who remained would be limited in the amount of money in damages they could receive.
“That’s really what this is about,” he argued. “And so it’s troubling – quite frankly it’s shocking to me – that we’re debating heavily the question of whether sexual abuse is health care.”
The judge mulled the issue for months. Judge Lunnen wrote in a September ruling that if the allegations were true, Dr. Broadbent’s treatment of his patients was “insensitive, disrespectful and degrading.”
But Utah law is clear, he said. Malpractice law covers any act or treatment performed by any health care provider during the patient’s medical care. The women had all been seeking health care, Judge Lunnen wrote, and Dr. Broadbent was providing that when the alleged assaults happened.
Their lawsuit was dismissed.
‘I felt defeated’
Brooke, another plaintiff who alleges Dr. Broadbent groped her, remembers feeling sick on the June day she watched the attorneys arguing. She asked to be identified by only her first name for this story.
She alleges Dr. Broadbent violated her in December 2008 while she was hospitalized after experiencing complications with her first pregnancy.
The nearest hospital to her rural town didn’t have a special unit to take care of premature babies, and her doctors feared she might need to deliver her son 6 weeks early. So Brooke had been rushed by ambulance over a mountain pass in a snowstorm to Utah Valley Hospital.
Brooke and her husband were terrified, she said, when they arrived at the Provo hospital. Dr. Broadbent happened to be the doctor on call. With Brooke’s husband and brother-in-law in the room, Dr. Broadbent examined her late that evening, she said, listening to her chest with a stethoscope.
The doctor then suddenly grabbed her breasts, she recalled – his movements causing her hospital gown to fall to expose her chest. She recounted this experience in her lawsuit, saying it was nothing like the breast exams she has had since.
“It was really traumatizing,” she said. “I was mortified. My husband and brother-in-law – we just didn’t say anything about it because it was so uncomfortable.”
Brooke voiced concerns to the nurse manager, and she was assigned a new doctor.
She gave birth to a healthy baby a little more than a month later, at the hospital near her home.
Hearing the judge’s ruling 14 years later, Brooke felt the decision revealed how Utah’s laws are broken.
“I was frustrated,” she said, “and I felt defeated. … I thought justice is not on our side with this.”
If the Utah Supreme Court rules that these alleged sexual assaults should legally be considered health care, the women will likely refile their claims as a medical malpractice lawsuit, said their attorney, Adam Sorensen. But it would be a challenge to keep all 94 women in the case, he said, due to the shorter filing window. Only two women in the lawsuit allege that they were harmed within the last 2 years.
The legal team for the women would have to convince a judge that their claims should still be allowed because they only recently discovered they were harmed. But based on previous rulings, Mr. Sorensen believes the women will have a better chance to win that argument if the civil suit remained a sexual assault case.
Regardless of what happens in their legal case, the decision by Brooke and the other women to come forward could help change state law for victims who come after them.
Recently, Mr. McKell, the state senator, introduced legislation to clarify that civil lawsuits alleging sexual assault by a health care worker do not fall under Utah’s Health Care Malpractice Act.
“I don’t think it’s a close call. Sexual assault is not medical care,” he said. “I know we’ve got some bizarre rulings that have come down through our courts in Utah.”
Both an association of Utah trial lawyers and the Utah Medical Association, which lobbies on behalf of the state’s physicians, support this reform.
“We support the fact that sexual assault should not be part of health care medical malpractice,” said Michelle McOmber, the CEO for the Utah Medical Association. “Sexual assault should be sexual assault, regardless of where it happens or who’s doing it. Sexual assault should be in that category, which is separate from actual health care. Because it’s not health care.”
MountainStar doesn’t have a position on the bill, Ms. Glas said. “If the laws were to change via new legislation and/or interpretation by the courts, we would abide by and comply with those new laws.”
But lawmakers are running out of time. With only a short time left in Utah’s legislative session, state senate and house leaders have so far prioritized passing new laws banning gender-affirming health care for transgender youths and creating a controversial school voucher program that will provide taxpayer funds for students to attend private school.
Utah lawmakers were also expected to consider a dramatic change for other sexual assault victims: a bill that would remove filing deadlines for civil lawsuits brought by people abused as adults. But that bill stalled before it could be debated.
Brooke had been eager to share her story, she said, in hopes it would help the first four women who’d come forward bolster their lawsuit against Dr. Broadbent. She later joined the case as a plaintiff. She read in their lawsuit about one woman who complained about him to the same hospital 7 years before she did, and about another woman who said Dr. Broadbent similarly molested her 2 days after Brooke had expressed her own concern.
“That bothered me so much,” she said. “It didn’t have to happen to all these women.”
Brooke doubts she’ll get vindication in a courtroom. Justice for her, she suspects, won’t come in the form of a legal ruling or a settlement against the doctor she says hurt her years ago.
Instead, she said, “maybe justice looks like changing the laws for future women.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
This article was produced for ProPublica’s Local Reporting Network in partnership with The Salt Lake Tribune.
At 19 years old and about to be married, Stephanie Mateer went to an ob.gyn. within walking distance of her student housing near Brigham Young University in Provo, Utah.
She wanted to start using birth control, and she was looking for guidance about having sex for the first time on her 2008 wedding night.
Ms. Mateer was shocked, she said, when David Broadbent, MD, reached under her gown to grab and squeeze her breasts, started a vaginal exam without warning, then followed it with an extremely painful examination of her rectum.
She felt disgusted and violated, but doubt also crept in. She told herself she must have misinterpreted his actions, or that she should have known that he would do a rectal exam. Raised as a member of The Church of Jesus Christ of Latter-day Saints, she said she was taught to defer to men in leadership.
“I viewed him as being a man in authority,” Ms. Mateer said. “He’s a doctor.”
It was years, she said, before she learned that her experience was in a sharp contrast to the conduct called for in professional standards, including that doctors use only their fingertips during a breast exam and communicate clearly what they are doing in advance, to gain the consent of their patients. Eventually, she gave her experience another name: sexual assault.
Utah judges, however, have called it health care.
And that legal distinction means Utahns like Ms. Mateer who decide to sue a health care provider for alleged sexual abuse are treated more harshly by the court system than plaintiffs who say they were harmed in other settings.
The chance to go to civil court for damages is an important option for survivors, experts say. While a criminal conviction can provide a sense of justice, winning a lawsuit can help victims pay for the therapy and additional support they need to heal after trauma.
Ms. Mateer laid out her allegations in a lawsuit that she and 93 other women filed against Dr. Broadbent last year. But they quickly learned they would be treated differently than other sexual assault survivors.
Filing their case, which alleged the Utah County doctor sexually assaulted them over the span of his 47-year career, was an empowering moment, Ms. Mateer said. But a judge threw out the lawsuit without even considering the merits, determining that because their alleged assailant is a doctor, the case must be governed by medical malpractice rules rather than those that apply to cases of sexual assault.
Under Utah’s rules of medical malpractice, claims made by victims who allege a health care worker sexually assaulted them are literally worth less than lawsuits brought by someone who was assaulted in other settings – even if a jury rules in their favor, a judge is required to limit how much money they receive. And they must meet a shorter filing deadline.
“It’s just crazy that a doctor can sexually assault women and then be protected by the white coat,” Ms. Mateer said. “It’s just a really scary precedent to be calling sexual assault ‘health care.’ ”
Because of the judge’s ruling that leaves them with a shorter window in which to file, some of Dr. Broadbent’s accusers stand to lose their chance to sue. Others were already past that deadline but had hoped to take advantage of an exception that allows plaintiffs to sue if they can prove that the person who harmed them had covered up the wrongdoing and if they discovered they had been hurt within the previous year.
As a group, the women are appealing the ruling to the Utah Supreme Court, which has agreed to hear the case. This decision will set a precedent for future sexual assault victims in Utah.
Dr. Broadbent’s attorney, Chris Nelson, declined an interview request but wrote in an email: “We believe that the allegations against Dr. Broadbent are without merit and will present our case in court. Given that this is an active legal matter, we will not be sharing any details outside the courtroom.”
States have varying legal definitions of medical malpractice, but it’s generally described as treatment that falls short of accepted standards of care. That includes mistakes, such as a surgeon leaving a piece of gauze inside a patient.
The Utah Supreme Court has ruled that a teenage boy was receiving health care when he was allowed to climb a steep, snow-dusted rock outcrop as part of wilderness therapy. When he broke his leg, he could only sue for medical malpractice, so the case faced shorter filing deadlines and lower monetary caps. Similarly, the court has ruled that a boy harmed by another child while in foster care was also bound by medical malpractice law.
Despite these state Supreme Court rulings, Utah legislators have so far not moved to narrow the wording of the malpractice act.
The lawsuit against Dr. Broadbent – and the questions it raises about the broadness of Utah’s medical malpractice laws – comes during a national reckoning with how sexual assault survivors are treated by the law. Legislators in several states have been rewriting laws to give sexual assault victims more time to sue their attackers, in response to the growing cultural understanding of the impact of trauma and the barriers to reporting. Even in Utah, those who were sexually abused as children now have no deadline to file suits against their abusers.
That isn’t true for sexual abuse in a medical setting, where cases must be filed within 2 years of the assault.
These higher hurdles should not exist in Utah, said state Sen. Mike K. McKell, a Utah County Republican who works as a personal injury attorney. He is trying to change state law to ensure that sexual assault lawsuits do not fall under Utah’s Health Care Malpractice Act, a law designed to cover negligence and poor care, not necessarily deliberate actions like an assault.
“Sexual assault, to me, is not medical care. Period,” he said. “It’s sad that we need to clarify that sexual assault is not medical care. But trying to tie sexual assault to a medical malpractice [filing deadline] – it’s just wrong.”
‘Your husband is a lucky man’
Ms. Mateer had gone to Dr. Broadbent in 2008 for a premarital exam, a uniquely Utah visit often scheduled by young women who are members of The Church of Jesus Christ of Latter-day Saints.
Leaders of the faith, which is predominant in Utah, focus on chastity when speaking to young, unmarried people about sex, and public schools have typically focused on abstinence-based sex education. So for some, these visits are the first place they learn about sexual health.
Young women who get premarital exams are typically given a birth control prescription, but the appointments can include care that’s less common for healthy women in other states – such as doctors giving them vaginal dilators to stretch their tissues before their wedding nights.
That’s what Ms. Mateer was expecting when she visited Dr. Broadbent’s office. The ob.gyn. had been practicing for decades in his Provo clinic nestled between student housing apartments across the street from Brigham Young University, which is owned by The Church of Jesus Christ of Latter-day Saints.
So Ms. Mateer was “just totally taken aback,” she said, by the painful examination and by Dr. Broadbent snapping off his gloves after the exam and saying, “Your husband is a lucky man.”
She repeated that remark in her legal filing, along with the doctor’s advice for her: If she bled during intercourse, “just do what the Boy Scouts do and apply pressure.”
“The whole thing was like I’m some object for my husband to enjoy and let him do whatever he wants,” Ms. Mateer said. “It was just very violating and not a great way to start my sexual relationship with my new husband, with these ideas in mind.”
Ms. Mateer thought back to that visit over the years, particularly when she went to other ob.gyns. for health care. Her subsequent doctors, she said, never performed a rectal exam and always explained to her what they were doing and how it would feel, and asked for her consent.
She thought about Dr. Broadbent again in 2017, as the #MeToo movement gained momentum, and looked him up online. Ms. Mateer found reviews from other women who described Dr. Broadbent doing rough examinations without warning that left them feeling the same way she had years before.
Then in December 2021, she spoke out on “Mormon Stories,” a podcast where people who have left or have questioned their Latter-day Saint faith share their life stories. In the episode, she described the painful way he examined her, how it left her feeling traumatized, and her discovery of the reviews that echoed her experience.
“He’s on University Avenue, in Provo, giving these exams to who knows how many naive Mormon 18-year-old, 19-year-old girls who are getting married. … They are naive and they don’t know what to expect,” she said on the podcast. “His name is Dr. David Broadbent.”
After the podcast aired, Ms. Mateer was flooded with messages from women who heard the episode and reached out to tell her that Dr. Broadbent had harmed them, too.
Ms. Mateer and three other women decided to sue the ob.gyn., and in the following weeks and months, 90 additional women joined the lawsuit they filed in Provo. Many of the women allege Dr. Broadbent inappropriately touched their breasts, vaginas and rectums, hurting them, without warning or explanation. Some said he used his bare hand – instead of using a speculum or gloves – during exams. One alleged that she saw he had an erection while he was touching her.
Dr. Broadbent’s actions were not medically necessary, the women allege, and were instead “performed for no other reason than his own sexual gratification.”
The lawsuit also named as defendants two hospitals where Dr. Broadbent had delivered babies and where some of the women allege they were assaulted. The suit accused hospital administrators of knowing about Dr. Broadbent’s inappropriate behavior and doing nothing about it.
After he was sued, the ob.gyn. quickly lost his privileges at the hospitals where he worked. Dr. Broadbent, now 75, has also voluntarily put his medical license in Utah on hold while police investigate 29 reports of sexual assault made against him.
Prosecutors are still considering whether to criminally prosecute Dr. Broadbent. Provo police forwarded more than a dozen reports to the Utah County attorney’s office in November, which are still being reviewed by a local prosecutor.
A spokesperson for Intermountain Health, the nonprofit health system that owns Utah Valley Hospital, where some of the women in the suit were treated, did not respond to specific questions. The spokesperson emphasized in an email that Dr. Broadbent was an “independent physician” who was not employed by Utah Valley Hospital, adding that most of the alleged incidents took place at Dr. Broadbent’s medical office.
A representative for MountainStar Healthcare, another hospital chain named as a defendant, denied knowledge of any allegations of inappropriate conduct reported to its hospital and also emphasized that Dr. Broadbent worked independently, not as an employee.
“Our position since this lawsuit was filed has been that we were inappropriately named in this suit,” said Brittany Glas, the communications director for MountainStar.
Debating whether sexual abuse is health care
For the women who sued Dr. Broadbent, their case boiled down to a key question: Were the sexual assaults they say they experienced part of their health care? There was a lot hanging on the answer.
If their case was considered medical malpractice, they would be limited in how much money they could receive in damages for their pain and suffering. If a jury awarded them millions of dollars, a judge would be required by law to cut that down to $450,000. There’s no cap on these monetary awards for victims sexually assaulted in other settings.
They would also be required to go before a panel, which includes a doctor, a lawyer and a community member, that decides whether their claims have merit. This step, aimed at resolving disputes out of court, does not block anyone from suing afterward. But it does add cost and delay, and for sexual assault victims who’ve gone through this step, it has been another time they were required to describe their experiences and hope they were believed.
The shorter, 2-year filing deadline for medical malpractice cases can also be a particular challenge for those who have been sexually abused because research shows that it’s common to delay reporting such assaults.
Nationwide, these kinds of malpractice reforms were adopted in the 1970s amid concerns – largely driven by insurance companies – that the cost of health care was rising because of frivolous lawsuits and “runaway juries” doling out multimillion-dollar payouts.
Restricting the size of malpractice awards and imposing other limits, many argued, were effective ways to balance compensating injured patients with protecting everyone’s access to health care.
State laws are generally silent on whether sexual assault lawsuits should be covered by malpractice laws, leaving courts to grapple with that question and leading to different conclusions across the country. The Tribune and ProPublica identified at least six cases in which state appellate judges sharply distinguished between assault and health care in considering whether malpractice laws should apply to sexual assault–related cases.
An appellate court in Wisconsin, for example, ruled in 1993 that a physician having an erection and groping a patient was a purposeful harm, not medical malpractice.
Florida’s law is similar to Utah’s, defining allegations “arising” out of medical care as malpractice. While an earlier ruling did treat sexual assault in a health care setting as medical malpractice, appellate rulings in the last decade have moved away from that interpretation. In 2005, an appellate court affirmed a lower-court ruling that when a dentist “stopped providing dental treatment to the victim and began sexually assaulting her, his professional services ended.”
Similarly, a federal judge in Iowa in 1995 weighed in on the meaning of “arising” out of health care: “Rape is not patient care activity,” he wrote.
But Utah’s malpractice law is so broad that judges have been interpreting it as covering any act performed by a health care provider during medical care. The law was passed in 1976 and is popular with doctors and other health care providers, who have lobbied to keep it in place – and who use it to get lawsuits dismissed.
One precedent-setting case in Utah shows the law’s power to safeguard health care providers and was an important test of how Utah defines medical malpractice. Jacob Scott sued WinGate Wilderness Therapy after the teen broke his leg in 2015 when a hiking guide from the center allowed him to climb up and down a steep outcrop in Utah’s red rock desert.
His parents are both lawyers, and after they found that Utah had a 4-year deadline for filing a personal injury lawsuit, court records said, they decided to prioritize “getting Jacob better” for the first 2 years after the accident. But when Mr. Scott’s suit was filed, WinGate argued it was too late – based on the shorter, 2-year deadline for medical malpractice claims.
Mr. Scott’s attorneys scoffed. “Interacting with nature,” his attorneys argued, “is not health care even under the broadest interpretation of … the Utah Health Care Malpractice Act.”
A judge disagreed and threw out Mr. Scott’s case. The Utah Supreme Court unanimously upheld that ruling in 2021.
“We agree with WinGate,” the justices wrote, “that it was acting as a ‘health care provider’ and providing ‘health care’ when Jacob was hiking and rock climbing.”
Last summer, the women who had sued Dr. Broadbent and the two hospitals watched online as lawyers debated whether the abuse they allegedly suffered was health care.
At the hearing, attorneys for Dr. Broadbent and the hospitals argued that the women should have pursued a medical malpractice case, which required them to first notify Dr. Broadbent and the hospitals that they wanted to sue. They also argued to Judge Robert Lunnen that the case couldn’t move forward because the women hadn’t gone before a prelitigation panel.
Attorneys for Dr. Broadbent and the hospitals argued, one after the other, that the painful and traumatic exams the women described arose out of health care treatments.
“Accepting the allegations of the complaint as true – as we must for purposes of this proceeding – we have to assume that [Broadbent] did something that was medically unnecessary, medically inappropriate,” argued David Jordan, a lawyer for Intermountain Health.
“But it doesn’t change the fact that it’s an act performed to a patient, during the patient’s treatment,” he said. “Because that’s what the patient is doing in the doctor’s office. They’re there for treatment.”
The attorney team for the women pushed back. Terry Rooney argued that if Dr. Broadbent’s actions fell under medical malpractice laws, many women would be knocked out of the case because of the age of their claims, and those who remained would be limited in the amount of money in damages they could receive.
“That’s really what this is about,” he argued. “And so it’s troubling – quite frankly it’s shocking to me – that we’re debating heavily the question of whether sexual abuse is health care.”
The judge mulled the issue for months. Judge Lunnen wrote in a September ruling that if the allegations were true, Dr. Broadbent’s treatment of his patients was “insensitive, disrespectful and degrading.”
But Utah law is clear, he said. Malpractice law covers any act or treatment performed by any health care provider during the patient’s medical care. The women had all been seeking health care, Judge Lunnen wrote, and Dr. Broadbent was providing that when the alleged assaults happened.
Their lawsuit was dismissed.
‘I felt defeated’
Brooke, another plaintiff who alleges Dr. Broadbent groped her, remembers feeling sick on the June day she watched the attorneys arguing. She asked to be identified by only her first name for this story.
She alleges Dr. Broadbent violated her in December 2008 while she was hospitalized after experiencing complications with her first pregnancy.
The nearest hospital to her rural town didn’t have a special unit to take care of premature babies, and her doctors feared she might need to deliver her son 6 weeks early. So Brooke had been rushed by ambulance over a mountain pass in a snowstorm to Utah Valley Hospital.
Brooke and her husband were terrified, she said, when they arrived at the Provo hospital. Dr. Broadbent happened to be the doctor on call. With Brooke’s husband and brother-in-law in the room, Dr. Broadbent examined her late that evening, she said, listening to her chest with a stethoscope.
The doctor then suddenly grabbed her breasts, she recalled – his movements causing her hospital gown to fall to expose her chest. She recounted this experience in her lawsuit, saying it was nothing like the breast exams she has had since.
“It was really traumatizing,” she said. “I was mortified. My husband and brother-in-law – we just didn’t say anything about it because it was so uncomfortable.”
Brooke voiced concerns to the nurse manager, and she was assigned a new doctor.
She gave birth to a healthy baby a little more than a month later, at the hospital near her home.
Hearing the judge’s ruling 14 years later, Brooke felt the decision revealed how Utah’s laws are broken.
“I was frustrated,” she said, “and I felt defeated. … I thought justice is not on our side with this.”
If the Utah Supreme Court rules that these alleged sexual assaults should legally be considered health care, the women will likely refile their claims as a medical malpractice lawsuit, said their attorney, Adam Sorensen. But it would be a challenge to keep all 94 women in the case, he said, due to the shorter filing window. Only two women in the lawsuit allege that they were harmed within the last 2 years.
The legal team for the women would have to convince a judge that their claims should still be allowed because they only recently discovered they were harmed. But based on previous rulings, Mr. Sorensen believes the women will have a better chance to win that argument if the civil suit remained a sexual assault case.
Regardless of what happens in their legal case, the decision by Brooke and the other women to come forward could help change state law for victims who come after them.
Recently, Mr. McKell, the state senator, introduced legislation to clarify that civil lawsuits alleging sexual assault by a health care worker do not fall under Utah’s Health Care Malpractice Act.
“I don’t think it’s a close call. Sexual assault is not medical care,” he said. “I know we’ve got some bizarre rulings that have come down through our courts in Utah.”
Both an association of Utah trial lawyers and the Utah Medical Association, which lobbies on behalf of the state’s physicians, support this reform.
“We support the fact that sexual assault should not be part of health care medical malpractice,” said Michelle McOmber, the CEO for the Utah Medical Association. “Sexual assault should be sexual assault, regardless of where it happens or who’s doing it. Sexual assault should be in that category, which is separate from actual health care. Because it’s not health care.”
MountainStar doesn’t have a position on the bill, Ms. Glas said. “If the laws were to change via new legislation and/or interpretation by the courts, we would abide by and comply with those new laws.”
But lawmakers are running out of time. With only a short time left in Utah’s legislative session, state senate and house leaders have so far prioritized passing new laws banning gender-affirming health care for transgender youths and creating a controversial school voucher program that will provide taxpayer funds for students to attend private school.
Utah lawmakers were also expected to consider a dramatic change for other sexual assault victims: a bill that would remove filing deadlines for civil lawsuits brought by people abused as adults. But that bill stalled before it could be debated.
Brooke had been eager to share her story, she said, in hopes it would help the first four women who’d come forward bolster their lawsuit against Dr. Broadbent. She later joined the case as a plaintiff. She read in their lawsuit about one woman who complained about him to the same hospital 7 years before she did, and about another woman who said Dr. Broadbent similarly molested her 2 days after Brooke had expressed her own concern.
“That bothered me so much,” she said. “It didn’t have to happen to all these women.”
Brooke doubts she’ll get vindication in a courtroom. Justice for her, she suspects, won’t come in the form of a legal ruling or a settlement against the doctor she says hurt her years ago.
Instead, she said, “maybe justice looks like changing the laws for future women.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
How to place an IUD with minimal patient discomfort
CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8
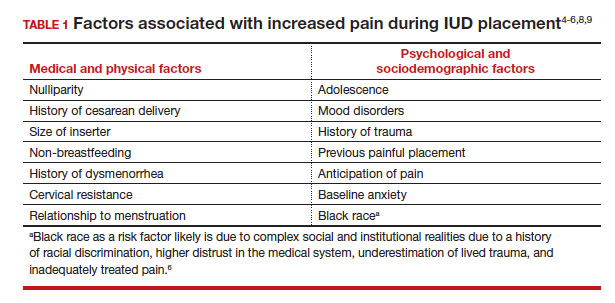
It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10
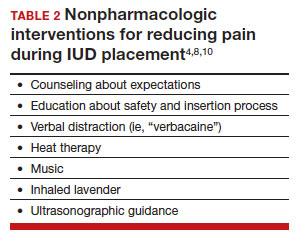
Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).
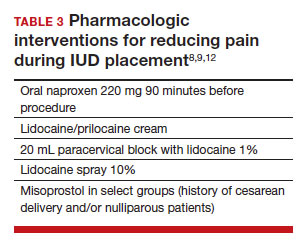
Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai andcolleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai andcolleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai andcolleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
Embattled iPLEDGE program: Changes ahead?
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.