User login
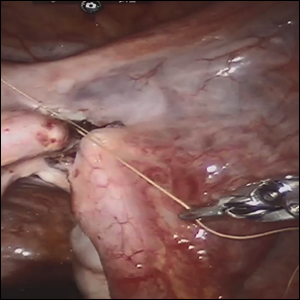
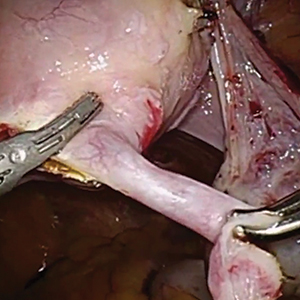
Robot-assisted laparoscopic tubal anastomosis following sterilization

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
Are women seeking short-acting contraception satisfied with LARC after giving it a try?
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
Should return to fertility be a concern for nulliparous patients using an IUD?
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
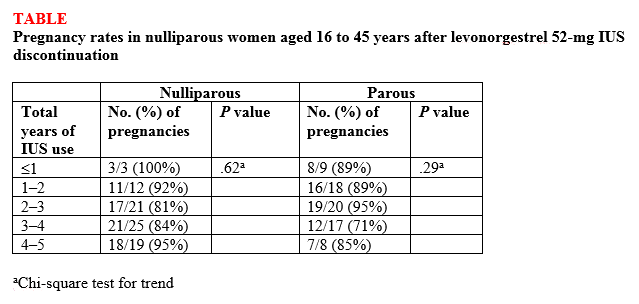
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1
“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
Abortion, the travel ban, and other top Supreme Court rulings affecting your practice
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
Agrees that OC use clearly reduces mortality
Agrees that OC use clearly reduces mortality
Recent evidence from long-term observations of hundreds of thousands of women, in 10 European countries, clearly demonstrated that the use of oral contraceptives (OCs) reduced mortality by roughly 10%.1,2 Newer OCs increase women’s overall survival.
In comparison, reducing obesity by 5 body mass index points would reduce mortality by only 5%, from 1.05 to 1.3
Dr. Stavros Saripanidis
Thessaloniki, Greece
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition: a cohort study. BMC Med. 2015;13:252.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–580.e9.
- Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156.
Agrees that OC use clearly reduces mortality
Recent evidence from long-term observations of hundreds of thousands of women, in 10 European countries, clearly demonstrated that the use of oral contraceptives (OCs) reduced mortality by roughly 10%.1,2 Newer OCs increase women’s overall survival.
In comparison, reducing obesity by 5 body mass index points would reduce mortality by only 5%, from 1.05 to 1.3
Dr. Stavros Saripanidis
Thessaloniki, Greece
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Agrees that OC use clearly reduces mortality
Recent evidence from long-term observations of hundreds of thousands of women, in 10 European countries, clearly demonstrated that the use of oral contraceptives (OCs) reduced mortality by roughly 10%.1,2 Newer OCs increase women’s overall survival.
In comparison, reducing obesity by 5 body mass index points would reduce mortality by only 5%, from 1.05 to 1.3
Dr. Stavros Saripanidis
Thessaloniki, Greece
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition: a cohort study. BMC Med. 2015;13:252.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–580.e9.
- Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition: a cohort study. BMC Med. 2015;13:252.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–580.e9.
- Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156.
No significant VTE risk for women taking noncyclic COCs
Women who use combined oral contraceptives (COC) without hormone-free or low-dose hormone intervals have a slightly elevated, but not statistically significant, risk of venous thromboembolism (VTE), compared with women who use cyclic COCs, according to research published in JAMA Internal Medicine.
Jie Li, PhD, from the Center for Drug Evaluation and Research, and colleagues performed a retrospective cohort study of 733,007 women aged 18-50 years in the Sentinel Distributed Database from 2007 to 2015 who received low-dose extended and continuous cycle (210,691 women; mean age, 30 years) COCs or cyclic COCs (522,316 women; mean age, 29 years). Continuous cycle COCs were defined as an 84/7 cycle or a 365/0 cycle, while cyclic COCs were 21/7 cycles.
The researchers noted some baseline differences between the two groups, with gynecologic conditions occurring in 40% of the noncyclic group, compared with 32% in the cyclic group; cardiovascular and metabolic conditions occurring in 7% of noncyclic women, compared with 5% of cyclic women; inflammatory disease occurring in 3% of noncyclic women, compared with 2% of cyclic women; and a slightly higher rate of health care services use in the noncyclic group, compared with the cyclic group.
Dr. Li and associates found 228 cases of VTE in the noncyclic group and 297 cases in the cyclic group, with an incidence rate of 1.54 (95% confidence interval, 1.34-1.74) per 1,000 person-years for noncyclic users and 0.83 (95% CI, 0.74-0.93) per 1,000 person-years for cyclic users (crude hazard ratio, 1.84; 95% CI, 1.53-2.21).
However, propensity score matching lowered the incidence rate to 1.44 (95% CI, 1.24-1.64) per 1,000 person-years for the noncyclic group and raised it to 1.09 (95% CI, 0.92-1.27) per 1,000 person-years for the cyclic group, for an adjusted hazard ratio of 1.32 (95% CI, 1.07-1.64), which does not show “strong evidence” of VTE risk based on a small absolute risk difference of 0.27 cases per 1,000 persons, the researchers said. They added that there might be residual or unmeasured confounding, perhaps for potential concurrent medication use or incompletely measured covariates.
“Accordingly, we do not recommend selective prescribing of COCs based on the cyclic and continuous/extended type,” Dr. Li and colleagues wrote. “Clinicians should prescribe COCs based on patients’ individual risk factors and preferences.”
The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
SOURCE: Li J et al. JAMA Intern Med. 2018 Oct 1. doi: 10.1001/jamainternmed.2018.4251.
Women who use combined oral contraceptives (COC) without hormone-free or low-dose hormone intervals have a slightly elevated, but not statistically significant, risk of venous thromboembolism (VTE), compared with women who use cyclic COCs, according to research published in JAMA Internal Medicine.
Jie Li, PhD, from the Center for Drug Evaluation and Research, and colleagues performed a retrospective cohort study of 733,007 women aged 18-50 years in the Sentinel Distributed Database from 2007 to 2015 who received low-dose extended and continuous cycle (210,691 women; mean age, 30 years) COCs or cyclic COCs (522,316 women; mean age, 29 years). Continuous cycle COCs were defined as an 84/7 cycle or a 365/0 cycle, while cyclic COCs were 21/7 cycles.
The researchers noted some baseline differences between the two groups, with gynecologic conditions occurring in 40% of the noncyclic group, compared with 32% in the cyclic group; cardiovascular and metabolic conditions occurring in 7% of noncyclic women, compared with 5% of cyclic women; inflammatory disease occurring in 3% of noncyclic women, compared with 2% of cyclic women; and a slightly higher rate of health care services use in the noncyclic group, compared with the cyclic group.
Dr. Li and associates found 228 cases of VTE in the noncyclic group and 297 cases in the cyclic group, with an incidence rate of 1.54 (95% confidence interval, 1.34-1.74) per 1,000 person-years for noncyclic users and 0.83 (95% CI, 0.74-0.93) per 1,000 person-years for cyclic users (crude hazard ratio, 1.84; 95% CI, 1.53-2.21).
However, propensity score matching lowered the incidence rate to 1.44 (95% CI, 1.24-1.64) per 1,000 person-years for the noncyclic group and raised it to 1.09 (95% CI, 0.92-1.27) per 1,000 person-years for the cyclic group, for an adjusted hazard ratio of 1.32 (95% CI, 1.07-1.64), which does not show “strong evidence” of VTE risk based on a small absolute risk difference of 0.27 cases per 1,000 persons, the researchers said. They added that there might be residual or unmeasured confounding, perhaps for potential concurrent medication use or incompletely measured covariates.
“Accordingly, we do not recommend selective prescribing of COCs based on the cyclic and continuous/extended type,” Dr. Li and colleagues wrote. “Clinicians should prescribe COCs based on patients’ individual risk factors and preferences.”
The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
SOURCE: Li J et al. JAMA Intern Med. 2018 Oct 1. doi: 10.1001/jamainternmed.2018.4251.
Women who use combined oral contraceptives (COC) without hormone-free or low-dose hormone intervals have a slightly elevated, but not statistically significant, risk of venous thromboembolism (VTE), compared with women who use cyclic COCs, according to research published in JAMA Internal Medicine.
Jie Li, PhD, from the Center for Drug Evaluation and Research, and colleagues performed a retrospective cohort study of 733,007 women aged 18-50 years in the Sentinel Distributed Database from 2007 to 2015 who received low-dose extended and continuous cycle (210,691 women; mean age, 30 years) COCs or cyclic COCs (522,316 women; mean age, 29 years). Continuous cycle COCs were defined as an 84/7 cycle or a 365/0 cycle, while cyclic COCs were 21/7 cycles.
The researchers noted some baseline differences between the two groups, with gynecologic conditions occurring in 40% of the noncyclic group, compared with 32% in the cyclic group; cardiovascular and metabolic conditions occurring in 7% of noncyclic women, compared with 5% of cyclic women; inflammatory disease occurring in 3% of noncyclic women, compared with 2% of cyclic women; and a slightly higher rate of health care services use in the noncyclic group, compared with the cyclic group.
Dr. Li and associates found 228 cases of VTE in the noncyclic group and 297 cases in the cyclic group, with an incidence rate of 1.54 (95% confidence interval, 1.34-1.74) per 1,000 person-years for noncyclic users and 0.83 (95% CI, 0.74-0.93) per 1,000 person-years for cyclic users (crude hazard ratio, 1.84; 95% CI, 1.53-2.21).
However, propensity score matching lowered the incidence rate to 1.44 (95% CI, 1.24-1.64) per 1,000 person-years for the noncyclic group and raised it to 1.09 (95% CI, 0.92-1.27) per 1,000 person-years for the cyclic group, for an adjusted hazard ratio of 1.32 (95% CI, 1.07-1.64), which does not show “strong evidence” of VTE risk based on a small absolute risk difference of 0.27 cases per 1,000 persons, the researchers said. They added that there might be residual or unmeasured confounding, perhaps for potential concurrent medication use or incompletely measured covariates.
“Accordingly, we do not recommend selective prescribing of COCs based on the cyclic and continuous/extended type,” Dr. Li and colleagues wrote. “Clinicians should prescribe COCs based on patients’ individual risk factors and preferences.”
The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
SOURCE: Li J et al. JAMA Intern Med. 2018 Oct 1. doi: 10.1001/jamainternmed.2018.4251.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Continuous or extended cycle combined oral contraceptive (COC) use was associated with a slightly elevated, but not statistically significant, risk of venous thromboembolism.
Major finding: The adjusted hazard ratio for women taking continuous/extended COCs was 1.32 (95% confidence interval, 1.07-1.74), compared with women taking noncyclic COCs, but the absolute risk difference between the two groups was low (0.27 per 1,000 persons).
Study details: A retrospective cohort study of 210,691 women with continuous/extended COC use and 522,316 women with cyclic COC use.
Disclosures: The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
Source: Li J et al. JAMA Intern Med. 2018 Oct 1. doi:10.1001/jamainternmed.2018.4251.
Ulipristal reduces bleeding with contraceptive implant
Ulipristal acetate reduces breakthrough bleeding in women with etonogestrel implants, according to new findings.
After 30 days, patients treated with ulipristal were more satisfied with their bleeding profiles than were those given placebo, reported Rachel E. Zigler, MD, of the department of obstetrics and gynecology at Washington University in St. Louis and her colleagues.
About half of women experience unscheduled bleeding within the first 6 months of etonogestrel implantation, causing many to discontinue treatment. The etiology of this phenomenon is poorly understood.
“One leading theory is that sustained exposure to a progestin can lead to endometrial angiogenesis disruption, resulting in the development of a dense venous network that is fragile and prone to bleeding,” the researchers wrote in Obstetrics & Gynecology.
Ulipristal acetate is a selective progesterone receptor modulator approved for emergency contraception in the United States. Outside the United States, it is used to treat abnormal uterine bleeding in cases of uterine leiomyoma. Ulipristal acts directly upon myometrial and endometrial tissue and “may also displace local progestin within the uterus to counteract bleeding secondary to … a dense, fragile venous network.”
The double-blind, placebo-controlled study included 65 women aged 18-45 years with etonogestrel implants. Eligibility required that implants be in place for more than 90 days and less than 3 years and that participants experienced more than one bleeding episode over a 24-day time frame.
Patients received either 15 mg ulipristal (n = 32) or placebo (n = 33) daily for 7 days. From the first day of treatment until 30 days, patients self-reported bleeding events. Weekly phone questionnaires also were conducted to determine satisfaction with medication and side effects.
Ten days after starting treatment, bleeding resolved in 34% of patients treated with ulipristal versus 10% of patients given placebo (P = .03).
The ulipristal group reported a median of 5 fewer bleeding days, compared with the placebo group, over the month-long evaluation (7 vs. 12; P = .002). Treatment satisfaction rates were also better in the ulipristal group, with nearly three-quarters (72%) “very happy” with results versus about one-quarter (27%) of women who received placebo.
Consequently, more ulipristal patients desired to keep their implants, compared with placebo patients. All patients receiving ulipristal would consider ulipristal for breakthrough bleeding in the future, compared with two-thirds of patients in the placebo group.
Side effects were uncommon for both groups; the most common side effect was headache, reported in 9% in the ulipristal group and 19% in the placebo group.
“Increased satisfaction with the etonogestrel implant may lead to a decrease in discontinuation rates and potentially a decrease in unintended pregnancy rates in this population,” the researchers wrote.
Study funding was provided by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences. The authors reported financial disclosures related to Bayer and Merck.
SOURCE: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
The recent trial by Zigler et al. showed that ulipristal acetate may reduce bleeding days in contraceptive implant users, but larger studies are needed, and concerns about logistics, dose, and toxicity must be addressed before clinical roll out, according to Eve Espey, MD.
The FDA halted the present trial after another ulipristal study overseas detected liver toxicity. The overseas study (for uterine leiomyoma) involved daily administration of ulipristal (5 mg) for 3 months, which is significantly longer than the present study for breakthrough bleeding. The European Medicines Agency has since determined that women with liver issues should not receive ulipristal and that others should have close liver monitoring before, during, and after ulipristal therapy.
Despite the above concerns, ulipristal still holds promise for a common clinical problem.
“This study contributes to the literature on management of bothersome bleeding with the contraceptive implant,” Dr. Espey said. “It is an important area because bothersome bleeding leads both to dissatisfaction and method discontinuation. As a recent Cochrane review pointed out, although several different medications have been used, studies are small and not yet conclusive. Despite these caveats, the findings were promising, similar to findings of prior work with a similar compound, mifepristone. Future directions would include clinical trials utilizing ulipristal acetate in a larger population powered for discontinuation.”
Dr. Espey is a professor in and chair of the department of obstetrics and gynecology and the family planning fellowship director at the University of New Mexico, Albuquerque. These comments are adapted from an interview. Dr. Espey said she had no relevant financial disclosures.
The recent trial by Zigler et al. showed that ulipristal acetate may reduce bleeding days in contraceptive implant users, but larger studies are needed, and concerns about logistics, dose, and toxicity must be addressed before clinical roll out, according to Eve Espey, MD.
The FDA halted the present trial after another ulipristal study overseas detected liver toxicity. The overseas study (for uterine leiomyoma) involved daily administration of ulipristal (5 mg) for 3 months, which is significantly longer than the present study for breakthrough bleeding. The European Medicines Agency has since determined that women with liver issues should not receive ulipristal and that others should have close liver monitoring before, during, and after ulipristal therapy.
Despite the above concerns, ulipristal still holds promise for a common clinical problem.
“This study contributes to the literature on management of bothersome bleeding with the contraceptive implant,” Dr. Espey said. “It is an important area because bothersome bleeding leads both to dissatisfaction and method discontinuation. As a recent Cochrane review pointed out, although several different medications have been used, studies are small and not yet conclusive. Despite these caveats, the findings were promising, similar to findings of prior work with a similar compound, mifepristone. Future directions would include clinical trials utilizing ulipristal acetate in a larger population powered for discontinuation.”
Dr. Espey is a professor in and chair of the department of obstetrics and gynecology and the family planning fellowship director at the University of New Mexico, Albuquerque. These comments are adapted from an interview. Dr. Espey said she had no relevant financial disclosures.
The recent trial by Zigler et al. showed that ulipristal acetate may reduce bleeding days in contraceptive implant users, but larger studies are needed, and concerns about logistics, dose, and toxicity must be addressed before clinical roll out, according to Eve Espey, MD.
The FDA halted the present trial after another ulipristal study overseas detected liver toxicity. The overseas study (for uterine leiomyoma) involved daily administration of ulipristal (5 mg) for 3 months, which is significantly longer than the present study for breakthrough bleeding. The European Medicines Agency has since determined that women with liver issues should not receive ulipristal and that others should have close liver monitoring before, during, and after ulipristal therapy.
Despite the above concerns, ulipristal still holds promise for a common clinical problem.
“This study contributes to the literature on management of bothersome bleeding with the contraceptive implant,” Dr. Espey said. “It is an important area because bothersome bleeding leads both to dissatisfaction and method discontinuation. As a recent Cochrane review pointed out, although several different medications have been used, studies are small and not yet conclusive. Despite these caveats, the findings were promising, similar to findings of prior work with a similar compound, mifepristone. Future directions would include clinical trials utilizing ulipristal acetate in a larger population powered for discontinuation.”
Dr. Espey is a professor in and chair of the department of obstetrics and gynecology and the family planning fellowship director at the University of New Mexico, Albuquerque. These comments are adapted from an interview. Dr. Espey said she had no relevant financial disclosures.
Ulipristal acetate reduces breakthrough bleeding in women with etonogestrel implants, according to new findings.
After 30 days, patients treated with ulipristal were more satisfied with their bleeding profiles than were those given placebo, reported Rachel E. Zigler, MD, of the department of obstetrics and gynecology at Washington University in St. Louis and her colleagues.
About half of women experience unscheduled bleeding within the first 6 months of etonogestrel implantation, causing many to discontinue treatment. The etiology of this phenomenon is poorly understood.
“One leading theory is that sustained exposure to a progestin can lead to endometrial angiogenesis disruption, resulting in the development of a dense venous network that is fragile and prone to bleeding,” the researchers wrote in Obstetrics & Gynecology.
Ulipristal acetate is a selective progesterone receptor modulator approved for emergency contraception in the United States. Outside the United States, it is used to treat abnormal uterine bleeding in cases of uterine leiomyoma. Ulipristal acts directly upon myometrial and endometrial tissue and “may also displace local progestin within the uterus to counteract bleeding secondary to … a dense, fragile venous network.”
The double-blind, placebo-controlled study included 65 women aged 18-45 years with etonogestrel implants. Eligibility required that implants be in place for more than 90 days and less than 3 years and that participants experienced more than one bleeding episode over a 24-day time frame.
Patients received either 15 mg ulipristal (n = 32) or placebo (n = 33) daily for 7 days. From the first day of treatment until 30 days, patients self-reported bleeding events. Weekly phone questionnaires also were conducted to determine satisfaction with medication and side effects.
Ten days after starting treatment, bleeding resolved in 34% of patients treated with ulipristal versus 10% of patients given placebo (P = .03).
The ulipristal group reported a median of 5 fewer bleeding days, compared with the placebo group, over the month-long evaluation (7 vs. 12; P = .002). Treatment satisfaction rates were also better in the ulipristal group, with nearly three-quarters (72%) “very happy” with results versus about one-quarter (27%) of women who received placebo.
Consequently, more ulipristal patients desired to keep their implants, compared with placebo patients. All patients receiving ulipristal would consider ulipristal for breakthrough bleeding in the future, compared with two-thirds of patients in the placebo group.
Side effects were uncommon for both groups; the most common side effect was headache, reported in 9% in the ulipristal group and 19% in the placebo group.
“Increased satisfaction with the etonogestrel implant may lead to a decrease in discontinuation rates and potentially a decrease in unintended pregnancy rates in this population,” the researchers wrote.
Study funding was provided by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences. The authors reported financial disclosures related to Bayer and Merck.
SOURCE: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
Ulipristal acetate reduces breakthrough bleeding in women with etonogestrel implants, according to new findings.
After 30 days, patients treated with ulipristal were more satisfied with their bleeding profiles than were those given placebo, reported Rachel E. Zigler, MD, of the department of obstetrics and gynecology at Washington University in St. Louis and her colleagues.
About half of women experience unscheduled bleeding within the first 6 months of etonogestrel implantation, causing many to discontinue treatment. The etiology of this phenomenon is poorly understood.
“One leading theory is that sustained exposure to a progestin can lead to endometrial angiogenesis disruption, resulting in the development of a dense venous network that is fragile and prone to bleeding,” the researchers wrote in Obstetrics & Gynecology.
Ulipristal acetate is a selective progesterone receptor modulator approved for emergency contraception in the United States. Outside the United States, it is used to treat abnormal uterine bleeding in cases of uterine leiomyoma. Ulipristal acts directly upon myometrial and endometrial tissue and “may also displace local progestin within the uterus to counteract bleeding secondary to … a dense, fragile venous network.”
The double-blind, placebo-controlled study included 65 women aged 18-45 years with etonogestrel implants. Eligibility required that implants be in place for more than 90 days and less than 3 years and that participants experienced more than one bleeding episode over a 24-day time frame.
Patients received either 15 mg ulipristal (n = 32) or placebo (n = 33) daily for 7 days. From the first day of treatment until 30 days, patients self-reported bleeding events. Weekly phone questionnaires also were conducted to determine satisfaction with medication and side effects.
Ten days after starting treatment, bleeding resolved in 34% of patients treated with ulipristal versus 10% of patients given placebo (P = .03).
The ulipristal group reported a median of 5 fewer bleeding days, compared with the placebo group, over the month-long evaluation (7 vs. 12; P = .002). Treatment satisfaction rates were also better in the ulipristal group, with nearly three-quarters (72%) “very happy” with results versus about one-quarter (27%) of women who received placebo.
Consequently, more ulipristal patients desired to keep their implants, compared with placebo patients. All patients receiving ulipristal would consider ulipristal for breakthrough bleeding in the future, compared with two-thirds of patients in the placebo group.
Side effects were uncommon for both groups; the most common side effect was headache, reported in 9% in the ulipristal group and 19% in the placebo group.
“Increased satisfaction with the etonogestrel implant may lead to a decrease in discontinuation rates and potentially a decrease in unintended pregnancy rates in this population,” the researchers wrote.
Study funding was provided by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences. The authors reported financial disclosures related to Bayer and Merck.
SOURCE: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Treatment with ulipristal was associated with 5 fewer bleeding days per month, compared with placebo (P = .002).
Study details: The double-blind, placebo-controlled trial involved 65 women with etonogestrel implants who reported more than one bleeding episode in a 24-day time frame.
Disclosures: The study was funded by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences (NCATS). The authors reported financial disclosures related to Bayer and Merck.
Source: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
Hormonal contraceptive use linked to leukemia risk in offspring
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
FROM LANCET ONCOLOGY
Key clinical point:
Major finding: Recent maternal hormonal contraceptive use was linked to one additional case of leukemia per 47,170 children.
Study details: Danish nationwide cohort study in 1,185,157 children.
Disclosures: The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
Source: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
2018 Update on contraception
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.
Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).
At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.
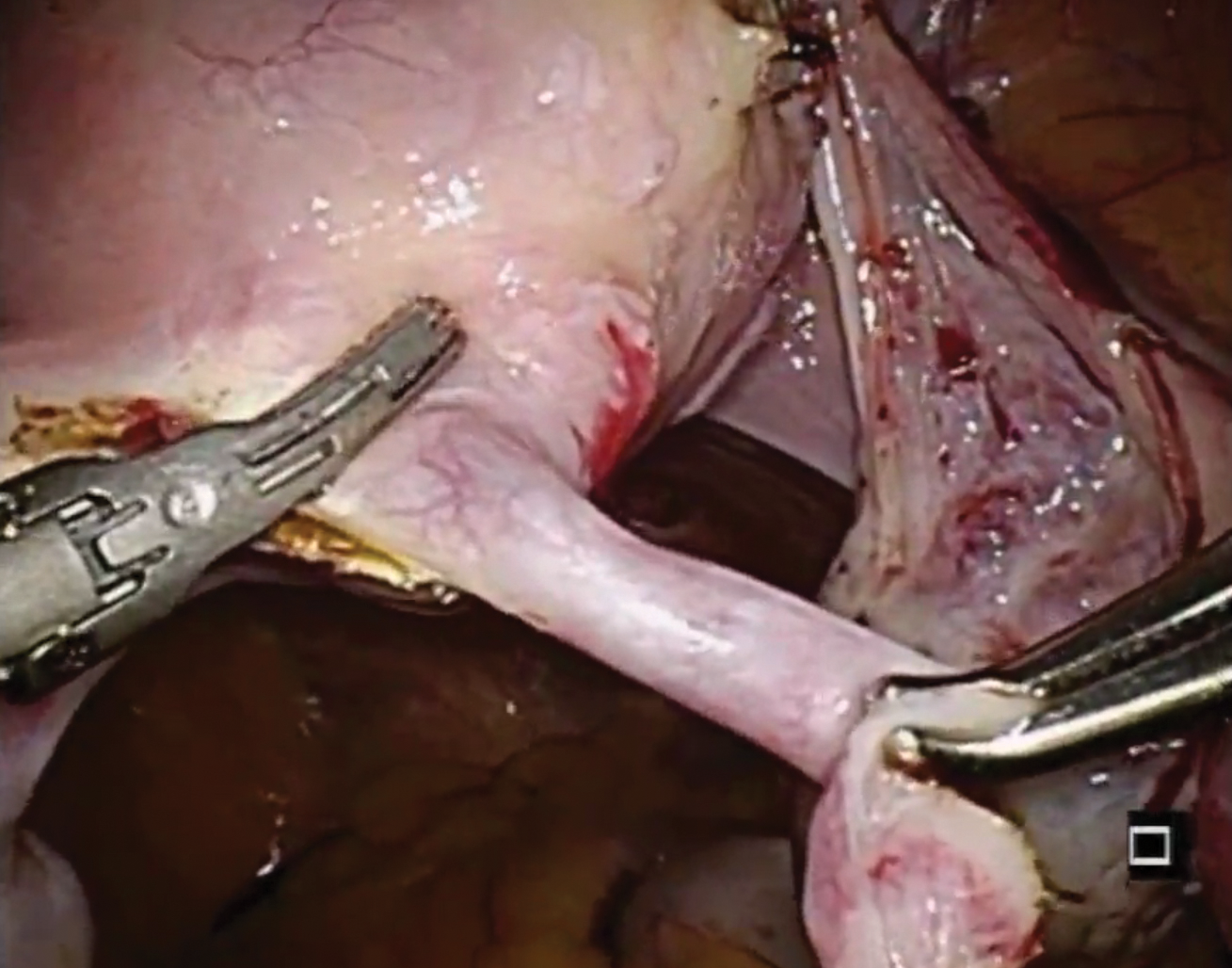
Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.
Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).
At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.

Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.
Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).
At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.

Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
Is the most effective emergency contraception easily obtained at US pharmacies?
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.
According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.

According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Although it is available only by prescription, ulipristal acetate provides emergency contraception that is more effective than the emergency contraception provided by levonorgestrel (LNG), which is available without a prescription (TABLE). In addition, ulipristal acetate appears more effective than LNG in obese and overweight women.1,2 Package labeling for ulipristal acetate indicates that a single 30-mg tablet should be taken orally within 5 days of unprotected sex.

According to a survey of pharmacy availability of ulipristal acetate in Hawaii, 2.6% of retail pharmacies had the drug immediately available, compared with 82.4% for LNG, and 22.8% reported the ability to order it.3 To assess pharmacy availability of ulipristal acetate on a nationwide scale, Shigesato and colleagues conducted a national “secret shopper” telephone survey in 10 cities (each with a population of at least 500,000) in all major regions of the United States.
Details of the study
Independent pharmacies (defined as having fewer than 5 locations within the city) and chain pharmacies were included in the survey. The survey callers, representing themselves as uninsured 18-year-old women attempting to fill a prescription for ulipristal acetate, followed a semistructured questionnaire and recorded the responses. They asked about the immediate availability of ulipristal acetate and LNG, the pharmacy’s ability to order ulipristal acetate if not immediately available, out-of-pocket costs, instructions for use, and the differences between ulipristal acetate and LNG. Questions were directed to whichever pharmacy staff member answered the phone; callers did not specifically ask to speak to a pharmacist.
Of the 344 pharmacies included in this analysis, 10% (33) indicated that they could fill a prescription for ulipristal acetate immediately. While availability did not vary by region, there was a difference in immediate availability by city.
Almost three-quarters of pharmacies without immediate drug availability indicated that they could order ulipristal acetate, with a median predicted time for availability of 24 hours. Of the chain pharmacies, 81% (167 of 205) reported the ability to order ulipristal acetate, compared with 55% (57 of 106) of independent pharmacies.
When asked if ulipristal acetate was different from LNG, more than one-third of pharmacy personnel contacted stated either that there was no difference between ulipristal acetate and LNG or that they were not sure of a difference.
Study strengths and weaknesses
The authors noted that the secret shopper methodology, along with having callers speak to the pharmacy staff person who answered the call (rather than asking for the pharmacist), provided data that closely approximates real-world patient experiences.
Since more pharmacies than anticipated met exclusion criteria for the study, the estimate of ulipristal acetate immediate availability was less precise than the power analysis predicted. Further, results from the 10 large, geographically diverse cities may not be representative of all similarly sized cities nationally or all areas of the United States.
As the authors point out, a low prevalence of pharmacies stock ulipristal acetate, and more than 25% are not able to order this emergency contraception. This underscores the fact that access to the most effective oral emergency contraception is limited for US women. I agree with the authors’ speculation that access to ulipristal acetate may be even lower in rural areas. In many European countries, ulipristal acetate is available without a prescription. Clinicians caring for women who may benefit from emergency contraception, particularly those using short-acting or less effective contraceptives, may wish to prescribe ulipristal acetate in advance of need.
—Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.
- Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Bullock H, Steele S, Kurata N, et al. Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454.