User login
New influx of Humira biosimilars may not drive immediate change
Gastroenterologists in 2023 will have more tools in their arsenal to treat patients with Crohn’s disease or ulcerative colitis. As many as 8-10 adalimumab biosimilars are anticipated to come on the market this year, giving mainstay drug Humira some vigorous competition.
Three scenarios will drive adalimumab biosimilar initiation: Insurance preference for the initial treatment of a newly diagnosed condition, a change in a patient’s insurance plan, or an insurance-mandated switch, said Edward C. Oldfield IV, MD, assistant professor at Eastern Virginia Medical School’s division of gastroenterology in Norfolk.
“Outside of these scenarios, I would encourage patients to remain on their current biologic so long as cost and accessibility remain stable,” said Dr. Oldfield.
Many factors will contribute to the success of biosimilars. Will physicians be prescribing them? How are biosimilars placed on formularies and will they be given preferred status? How will manufacturers price their biosimilars? “We have to wait and see to get the answers to these questions,” said Steven Newmark, JD, MPA, chief legal officer and director of policy, Global Healthy Living Foundation/CreakyJoints, a nonprofit advocacy organization based in New York.
Prescribing biosimilars is no different than prescribing originator biologics, so providers should know how to use them, said Mr. Newmark. “Most important will be the availability of patient-friendly resources that providers can share with their patients to provide education about and confidence in using biosimilars,” he added.
Overall, biosimilars are a good thing, said Dr. Oldfield. “In the long run they should bring down costs and increase access to medications for our patients.”
Others are skeptical that the adalimumab biosimilars will save patients much money.
Biosimilar laws were created to lower costs. However, if a patient with insurance pays only $5 a month out of pocket for Humira – a drug that normally costs $7,000 without coverage – it’s unlikely they would want to switch unless there’s comparable savings from the biosimilar, said Stephen B. Hanauer, MD, medical director of the Digestive Health Center and professor of medicine at Northwestern Medicine, Northwestern University, Evanston, Ill.
Like generics, Humira biosimilars may face some initial backlash, said Dr. Hanauer.
2023 broadens scope of adalimumab treatments
The American Gastroenterological Association describes a biosimilar as something that’s “highly similar to, but not an exact copy of, a biologic reference product already approved” by the Food and Drug Administration. Congress under the 2010 Affordable Care Act created a special, abbreviated pathway to approval for biosimilars.
AbbVie’s Humira, the global revenue for which exceeded $20 billion in 2021, has long dominated the U.S. market on injectable treatments for autoimmune diseases. The popular drug faces some competition in 2023, however, following a series of legal settlements that allowed AbbVie competitors to release their own adalimumab biosimilars.
“So far, we haven’t seen biosimilars live up to their potential in the U.S. in the inflammatory space,” said Mr. Newmark. This may change, however. Previously, biosimilars have required infusion, which demanded more time, commitment, and travel from patients. “The new set of forthcoming Humira biosimilars are injectables, an administration method preferred by patients,” he said.
The FDA will approve a biosimilar if it determines that the biological product is highly similar to the reference product, and that there are no clinically meaningful differences between the biological and reference product in terms of the safety, purity, and potency of the product.
The agency to date has approved 8 adalimumab biosimilars. These include: Idacio (adalimumab-aacf, Fresenius Kabi); Amjevita (adalimumab-atto, Amgen); Hadlima (adalimumab-bwwd, Organon); Cyltezo (adalimumab-adbm, Boehringer Ingelheim); Yusimry (adalimumab-aqvh from Coherus BioSciences); Hulio (adalimumab-fkjp; Mylan/Fujifilm Kyowa Kirin Biologics); Hyrimoz (adalimumab-adaz, Sandoz), and Abrilada (adalimumab-afzb, Pfizer).
“While FDA doesn’t formally track when products come to market, we know based on published reports that application holders for many of the currently FDA-approved biosimilars plan to market this year, starting with Amjevita being the first adalimumab biosimilar launched” in January, said Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars at the agency.
At press time, two other companies (Celltrion and Alvotech/Teva) were awaiting FDA approval for their adalimumab biosimilar drugs.
Among the eight approved drugs, Cyltezo is the only one that has a designation for interchangeability with Humira.
An interchangeable biosimilar may be substituted at the pharmacy without the intervention of the prescriber – much like generics are substituted, depending on state laws, said Dr. Yim. “However, in terms of safety and effectiveness, FDA’s standards for approval mean that biosimilar or interchangeable biosimilar products can be used in place of the reference product they were compared to.”
FDA-approved biosimilars undergo a rigorous evaluation for safety, effectiveness, and quality for their approved conditions of use, she continued. “Therefore, patients and health care providers can rely on a biosimilar to be as safe and effective for its approved uses as the original biological product.”
Remicade as a yard stick
Gastroenterologists dealt with this situation once before, when Remicade (infliximab) biosimilars came on the market in 2016, noted Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic.
Remicade and Humira are both tumor necrosis factor inhibitors with the same mechanism of action and many of the same indications. “We already had that experience with Remicade and biosimilar switch 2 or 3 years ago. Now we’re talking about Humira,” said Dr. Regueiro.
Most GI doctors have prescribed one of the more common infliximab biosimilars (Inflectra or Renflexis), noted Dr. Oldfield.
Cardinal Health, which recently surveyed 300 gastroenterologists, rheumatologists, and dermatologists about adalimumab biosimilars, found that gastroenterologists had the highest comfort level in prescribing them. Their top concern, however, was changing a patient from adalimumab to an adalimumab biosimilar.
For most patients, Dr. Oldfield sees the Humira reference biologic and biosimilar as equivalent.
However, he said he would change a patient’s drug only if there were a good reason or if his hand was forced by insurance. He would not make the change for a patient who recently began induction with the reference biologic or a patient with highly active clinical disease.
“While there is limited data to support this, I would also have some qualms about changing a patient from reference biologic to a biosimilar if they previously had immune-mediated pharmacokinetic failure due to antibody development with a biologic and were currently doing well on their new biologic,” he said.
Those with a new ulcerative colitis or Crohn’s diagnosis who are initiating a biologic for the first time might consider a biosimilar. If a patient is transitioning from a reference biologic to a biosimilar, “I would want to make that change during a time of stable remission and with the recognition that the switch is not a temporary switch, but a long-term switch,” he continued.
A paper that reviewed 23 observational studies of adalimumab and other biosimilars found that switching biosimilars was safe and effective. But if possible, patients should minimize the number of switches until more robust long-term data are available, added Dr. Oldfield.
If a patient is apprehensive about switching to a new therapy, “one may need to be cognizant of the ‘nocebo’ effect in which there is an unexplained or unfavorable therapeutic effect after switching,” he said.
Other gastroenterologists voiced similar reservations about switching. “I won’t use an adalimumab biosimilar unless the patient requests it, the insurance requires it, or there is a cost advantage for the patient such that they prefer it,” said Doug Wolf, MD, an Atlanta gastroenterologist.
“There is no medical treatment advantage to a biosimilar, especially if switching from Humira,” added Dr. Wolf.
Insurance will guide treatment
Once a drug is approved for use by the FDA, that drug will be available in all 50 states. “Different private insurance formularies, as well as state Medicaid formularies, might affect the actual ability of patients to receive such drugs,” said Mr. Newmark.
Patients should consult with their providers and insurance companies to see what therapies are available, he advised.
Dr. Hanauer anticipates some headaches arising for patients and doctors alike when negotiating for a specific drug.
Cyltezo may be the only biosimilar interchangeable with Humira, but the third-party pharmacy benefit manager (PBM) could negotiate for one of the noninterchangeable ones. “On a yearly basis they could switch their preference,” said Dr. Hanauer.
In the Cardinal Health survey, more than 60% of respondents said they would feel comfortable prescribing an adalimumab biosimilar only with an interchangeability designation.
A PBM may offer a patient Cyltezo if it’s cheaper than Humira. If the patient insists on staying on Humira, then they’ll have to pay more for that drug on their payer’s formulary, said Dr. Hanauer. In a worst-case scenario, a physician may have to appeal on a patient’s behalf to get Humira if the insurer offers only the biosimilar.
Taking that step to appeal is a major hassle for the physician, and leads to extra back door costs as well, said Dr. Hanauer.
Humira manufacturer AbbVie, in turn, may offer discounts and rebates to the PBMs to put Humira on their formulary. “That’s the AbbVie negotiating power. It’s not that the cost is going to be that much different. It’s going to be that there are rebates and discounts that are going to make the cost different,” he added.
As a community physician, Dr. Oldfield has specific concerns about accessibility.
The ever-increasing burden of insurance documentation and prior authorization means it can take weeks or months to get these medications approved. “The addition of new biosimilars is a welcome entrance if it can get patients the medications they need when they need it,” he said.
When it comes to prescribing biologics, many physicians rely on ancillary staff for assistance. It’s a team effort to sift through all the paperwork, observed Dr. Oldfield.
“While many community GI practices have specialized staff to deal with prior authorizations, they are still a far cry from the IBD [inflammatory bowel disease] academic centers where there are often pharmacists, nursing specialists, and home-monitoring programs to check in on patients,” he explained.
Landscape on cost is uncertain
At present, little is known about the cost of the biosimilars and impact on future drug pricing, said Dr. Oldfield.
At least for Medicare, Humira biosimilars will be considered Medicare Part D drugs if used for a medically accepted indication, said a spokesperson for the Centers for Medicare and Medicaid Services.
Part D sponsors (pharmacy and therapeutic committees) “will make the determination as to whether Amjevita and other products will be added to their formularies,” said the spokesperson.
Patients never saw a significant cost savings with Remicade biosimilars. “I imagine the same would be true with biosimilars for Humira,” said Dr. Regueiro. Patients may see greater access to these drugs, however, because the insurance plan or the pharmacy plan will make them more readily available, he added.
The hope is that, as biosimilars are introduced, the price of the originator biologic will go down, said Mr. Newmark. “Therefore, we can expect Humira to be offered at a lower price as it faces competition. Where it will sit in comparison to the forthcoming biosimilars will depend on how much biosimilar companies drop their price and how much pressure will be on PBMs and insurers to cover the lowest list price drug,” he said.
AbbVie did not respond to several requests for comment.
Charitable patient assistance programs for biosimilars or biologics can help offset the price of copayments, Mr. Newmark offered.
Ideally, insurers will offer designated biosimilars at a reduced or even no out-of-pocket expense on their formularies. This should lead to a decreased administrative burden for approval with streamlined (or even removal) of prior authorizations for certain medications, said Dr. Oldfield.
Without insurance or medication assistance programs, the cost of biosimilars is prohibitively expensive, he added.
“Biosimilars have higher research, development, and manufacturing costs than what people conventionally think of [for] a generic medication.”
Educating, advising patients
Dr. Oldfield advised that gastroenterologists refer to biologics by the generic name rather than branded name when initiating therapy unless there is a very specific reason not to. “This approach should make the process more streamlined and less subjected to quick denials for brand-only requests as biosimilars start to assume a larger market share,” he said.
Uptake of the Humira biosimilars also will depend on proper education of physicians and patients and their comfort level with the biosimilars, said Dr. Regueiro. Cleveland Clinic uses a team approach to educate on this topic, relying on pharmacists, clinicians, and nurses to explain that there’s no real difference between the reference drug and its biosimilars, based on efficacy and safety data.
Physicians can also direct patients to patient-friendly resources, said Mr. Newmark. “By starting the conversation early, it ensures that when/if the time comes that your patient is switched to or chooses a biosimilar they will feel more confident because they have the knowledge to make decisions about their care.”
The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars , is a free resource for patients, he added.
It’s important that doctors also understand these products so they can explain to their patients what to expect, said the FDA’s Dr. Yim. The FDA provides educational materials on its website, including a comprehensive curriculum toolkit.
Dr. Hanauer has served as a consultant for AbbVie, Amgen, American College of Gastroenterology, GlaxoSmithKline, American Gastroenterological Association, Pfizer, and a host of other companies . Dr. Regueiro has served on advisory boards and as a consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET Pharma Solutions,Trellis, and Boehringer Ingelheim Pharmaceuticals. Dr. Wolf, Dr. Yim, Dr. Oldfield, and Mr. Newmark have no financial conflicts of interest.
Gastroenterologists in 2023 will have more tools in their arsenal to treat patients with Crohn’s disease or ulcerative colitis. As many as 8-10 adalimumab biosimilars are anticipated to come on the market this year, giving mainstay drug Humira some vigorous competition.
Three scenarios will drive adalimumab biosimilar initiation: Insurance preference for the initial treatment of a newly diagnosed condition, a change in a patient’s insurance plan, or an insurance-mandated switch, said Edward C. Oldfield IV, MD, assistant professor at Eastern Virginia Medical School’s division of gastroenterology in Norfolk.
“Outside of these scenarios, I would encourage patients to remain on their current biologic so long as cost and accessibility remain stable,” said Dr. Oldfield.
Many factors will contribute to the success of biosimilars. Will physicians be prescribing them? How are biosimilars placed on formularies and will they be given preferred status? How will manufacturers price their biosimilars? “We have to wait and see to get the answers to these questions,” said Steven Newmark, JD, MPA, chief legal officer and director of policy, Global Healthy Living Foundation/CreakyJoints, a nonprofit advocacy organization based in New York.
Prescribing biosimilars is no different than prescribing originator biologics, so providers should know how to use them, said Mr. Newmark. “Most important will be the availability of patient-friendly resources that providers can share with their patients to provide education about and confidence in using biosimilars,” he added.
Overall, biosimilars are a good thing, said Dr. Oldfield. “In the long run they should bring down costs and increase access to medications for our patients.”
Others are skeptical that the adalimumab biosimilars will save patients much money.
Biosimilar laws were created to lower costs. However, if a patient with insurance pays only $5 a month out of pocket for Humira – a drug that normally costs $7,000 without coverage – it’s unlikely they would want to switch unless there’s comparable savings from the biosimilar, said Stephen B. Hanauer, MD, medical director of the Digestive Health Center and professor of medicine at Northwestern Medicine, Northwestern University, Evanston, Ill.
Like generics, Humira biosimilars may face some initial backlash, said Dr. Hanauer.
2023 broadens scope of adalimumab treatments
The American Gastroenterological Association describes a biosimilar as something that’s “highly similar to, but not an exact copy of, a biologic reference product already approved” by the Food and Drug Administration. Congress under the 2010 Affordable Care Act created a special, abbreviated pathway to approval for biosimilars.
AbbVie’s Humira, the global revenue for which exceeded $20 billion in 2021, has long dominated the U.S. market on injectable treatments for autoimmune diseases. The popular drug faces some competition in 2023, however, following a series of legal settlements that allowed AbbVie competitors to release their own adalimumab biosimilars.
“So far, we haven’t seen biosimilars live up to their potential in the U.S. in the inflammatory space,” said Mr. Newmark. This may change, however. Previously, biosimilars have required infusion, which demanded more time, commitment, and travel from patients. “The new set of forthcoming Humira biosimilars are injectables, an administration method preferred by patients,” he said.
The FDA will approve a biosimilar if it determines that the biological product is highly similar to the reference product, and that there are no clinically meaningful differences between the biological and reference product in terms of the safety, purity, and potency of the product.
The agency to date has approved 8 adalimumab biosimilars. These include: Idacio (adalimumab-aacf, Fresenius Kabi); Amjevita (adalimumab-atto, Amgen); Hadlima (adalimumab-bwwd, Organon); Cyltezo (adalimumab-adbm, Boehringer Ingelheim); Yusimry (adalimumab-aqvh from Coherus BioSciences); Hulio (adalimumab-fkjp; Mylan/Fujifilm Kyowa Kirin Biologics); Hyrimoz (adalimumab-adaz, Sandoz), and Abrilada (adalimumab-afzb, Pfizer).
“While FDA doesn’t formally track when products come to market, we know based on published reports that application holders for many of the currently FDA-approved biosimilars plan to market this year, starting with Amjevita being the first adalimumab biosimilar launched” in January, said Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars at the agency.
At press time, two other companies (Celltrion and Alvotech/Teva) were awaiting FDA approval for their adalimumab biosimilar drugs.
Among the eight approved drugs, Cyltezo is the only one that has a designation for interchangeability with Humira.
An interchangeable biosimilar may be substituted at the pharmacy without the intervention of the prescriber – much like generics are substituted, depending on state laws, said Dr. Yim. “However, in terms of safety and effectiveness, FDA’s standards for approval mean that biosimilar or interchangeable biosimilar products can be used in place of the reference product they were compared to.”
FDA-approved biosimilars undergo a rigorous evaluation for safety, effectiveness, and quality for their approved conditions of use, she continued. “Therefore, patients and health care providers can rely on a biosimilar to be as safe and effective for its approved uses as the original biological product.”
Remicade as a yard stick
Gastroenterologists dealt with this situation once before, when Remicade (infliximab) biosimilars came on the market in 2016, noted Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic.
Remicade and Humira are both tumor necrosis factor inhibitors with the same mechanism of action and many of the same indications. “We already had that experience with Remicade and biosimilar switch 2 or 3 years ago. Now we’re talking about Humira,” said Dr. Regueiro.
Most GI doctors have prescribed one of the more common infliximab biosimilars (Inflectra or Renflexis), noted Dr. Oldfield.
Cardinal Health, which recently surveyed 300 gastroenterologists, rheumatologists, and dermatologists about adalimumab biosimilars, found that gastroenterologists had the highest comfort level in prescribing them. Their top concern, however, was changing a patient from adalimumab to an adalimumab biosimilar.
For most patients, Dr. Oldfield sees the Humira reference biologic and biosimilar as equivalent.
However, he said he would change a patient’s drug only if there were a good reason or if his hand was forced by insurance. He would not make the change for a patient who recently began induction with the reference biologic or a patient with highly active clinical disease.
“While there is limited data to support this, I would also have some qualms about changing a patient from reference biologic to a biosimilar if they previously had immune-mediated pharmacokinetic failure due to antibody development with a biologic and were currently doing well on their new biologic,” he said.
Those with a new ulcerative colitis or Crohn’s diagnosis who are initiating a biologic for the first time might consider a biosimilar. If a patient is transitioning from a reference biologic to a biosimilar, “I would want to make that change during a time of stable remission and with the recognition that the switch is not a temporary switch, but a long-term switch,” he continued.
A paper that reviewed 23 observational studies of adalimumab and other biosimilars found that switching biosimilars was safe and effective. But if possible, patients should minimize the number of switches until more robust long-term data are available, added Dr. Oldfield.
If a patient is apprehensive about switching to a new therapy, “one may need to be cognizant of the ‘nocebo’ effect in which there is an unexplained or unfavorable therapeutic effect after switching,” he said.
Other gastroenterologists voiced similar reservations about switching. “I won’t use an adalimumab biosimilar unless the patient requests it, the insurance requires it, or there is a cost advantage for the patient such that they prefer it,” said Doug Wolf, MD, an Atlanta gastroenterologist.
“There is no medical treatment advantage to a biosimilar, especially if switching from Humira,” added Dr. Wolf.
Insurance will guide treatment
Once a drug is approved for use by the FDA, that drug will be available in all 50 states. “Different private insurance formularies, as well as state Medicaid formularies, might affect the actual ability of patients to receive such drugs,” said Mr. Newmark.
Patients should consult with their providers and insurance companies to see what therapies are available, he advised.
Dr. Hanauer anticipates some headaches arising for patients and doctors alike when negotiating for a specific drug.
Cyltezo may be the only biosimilar interchangeable with Humira, but the third-party pharmacy benefit manager (PBM) could negotiate for one of the noninterchangeable ones. “On a yearly basis they could switch their preference,” said Dr. Hanauer.
In the Cardinal Health survey, more than 60% of respondents said they would feel comfortable prescribing an adalimumab biosimilar only with an interchangeability designation.
A PBM may offer a patient Cyltezo if it’s cheaper than Humira. If the patient insists on staying on Humira, then they’ll have to pay more for that drug on their payer’s formulary, said Dr. Hanauer. In a worst-case scenario, a physician may have to appeal on a patient’s behalf to get Humira if the insurer offers only the biosimilar.
Taking that step to appeal is a major hassle for the physician, and leads to extra back door costs as well, said Dr. Hanauer.
Humira manufacturer AbbVie, in turn, may offer discounts and rebates to the PBMs to put Humira on their formulary. “That’s the AbbVie negotiating power. It’s not that the cost is going to be that much different. It’s going to be that there are rebates and discounts that are going to make the cost different,” he added.
As a community physician, Dr. Oldfield has specific concerns about accessibility.
The ever-increasing burden of insurance documentation and prior authorization means it can take weeks or months to get these medications approved. “The addition of new biosimilars is a welcome entrance if it can get patients the medications they need when they need it,” he said.
When it comes to prescribing biologics, many physicians rely on ancillary staff for assistance. It’s a team effort to sift through all the paperwork, observed Dr. Oldfield.
“While many community GI practices have specialized staff to deal with prior authorizations, they are still a far cry from the IBD [inflammatory bowel disease] academic centers where there are often pharmacists, nursing specialists, and home-monitoring programs to check in on patients,” he explained.
Landscape on cost is uncertain
At present, little is known about the cost of the biosimilars and impact on future drug pricing, said Dr. Oldfield.
At least for Medicare, Humira biosimilars will be considered Medicare Part D drugs if used for a medically accepted indication, said a spokesperson for the Centers for Medicare and Medicaid Services.
Part D sponsors (pharmacy and therapeutic committees) “will make the determination as to whether Amjevita and other products will be added to their formularies,” said the spokesperson.
Patients never saw a significant cost savings with Remicade biosimilars. “I imagine the same would be true with biosimilars for Humira,” said Dr. Regueiro. Patients may see greater access to these drugs, however, because the insurance plan or the pharmacy plan will make them more readily available, he added.
The hope is that, as biosimilars are introduced, the price of the originator biologic will go down, said Mr. Newmark. “Therefore, we can expect Humira to be offered at a lower price as it faces competition. Where it will sit in comparison to the forthcoming biosimilars will depend on how much biosimilar companies drop their price and how much pressure will be on PBMs and insurers to cover the lowest list price drug,” he said.
AbbVie did not respond to several requests for comment.
Charitable patient assistance programs for biosimilars or biologics can help offset the price of copayments, Mr. Newmark offered.
Ideally, insurers will offer designated biosimilars at a reduced or even no out-of-pocket expense on their formularies. This should lead to a decreased administrative burden for approval with streamlined (or even removal) of prior authorizations for certain medications, said Dr. Oldfield.
Without insurance or medication assistance programs, the cost of biosimilars is prohibitively expensive, he added.
“Biosimilars have higher research, development, and manufacturing costs than what people conventionally think of [for] a generic medication.”
Educating, advising patients
Dr. Oldfield advised that gastroenterologists refer to biologics by the generic name rather than branded name when initiating therapy unless there is a very specific reason not to. “This approach should make the process more streamlined and less subjected to quick denials for brand-only requests as biosimilars start to assume a larger market share,” he said.
Uptake of the Humira biosimilars also will depend on proper education of physicians and patients and their comfort level with the biosimilars, said Dr. Regueiro. Cleveland Clinic uses a team approach to educate on this topic, relying on pharmacists, clinicians, and nurses to explain that there’s no real difference between the reference drug and its biosimilars, based on efficacy and safety data.
Physicians can also direct patients to patient-friendly resources, said Mr. Newmark. “By starting the conversation early, it ensures that when/if the time comes that your patient is switched to or chooses a biosimilar they will feel more confident because they have the knowledge to make decisions about their care.”
The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars , is a free resource for patients, he added.
It’s important that doctors also understand these products so they can explain to their patients what to expect, said the FDA’s Dr. Yim. The FDA provides educational materials on its website, including a comprehensive curriculum toolkit.
Dr. Hanauer has served as a consultant for AbbVie, Amgen, American College of Gastroenterology, GlaxoSmithKline, American Gastroenterological Association, Pfizer, and a host of other companies . Dr. Regueiro has served on advisory boards and as a consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET Pharma Solutions,Trellis, and Boehringer Ingelheim Pharmaceuticals. Dr. Wolf, Dr. Yim, Dr. Oldfield, and Mr. Newmark have no financial conflicts of interest.
Gastroenterologists in 2023 will have more tools in their arsenal to treat patients with Crohn’s disease or ulcerative colitis. As many as 8-10 adalimumab biosimilars are anticipated to come on the market this year, giving mainstay drug Humira some vigorous competition.
Three scenarios will drive adalimumab biosimilar initiation: Insurance preference for the initial treatment of a newly diagnosed condition, a change in a patient’s insurance plan, or an insurance-mandated switch, said Edward C. Oldfield IV, MD, assistant professor at Eastern Virginia Medical School’s division of gastroenterology in Norfolk.
“Outside of these scenarios, I would encourage patients to remain on their current biologic so long as cost and accessibility remain stable,” said Dr. Oldfield.
Many factors will contribute to the success of biosimilars. Will physicians be prescribing them? How are biosimilars placed on formularies and will they be given preferred status? How will manufacturers price their biosimilars? “We have to wait and see to get the answers to these questions,” said Steven Newmark, JD, MPA, chief legal officer and director of policy, Global Healthy Living Foundation/CreakyJoints, a nonprofit advocacy organization based in New York.
Prescribing biosimilars is no different than prescribing originator biologics, so providers should know how to use them, said Mr. Newmark. “Most important will be the availability of patient-friendly resources that providers can share with their patients to provide education about and confidence in using biosimilars,” he added.
Overall, biosimilars are a good thing, said Dr. Oldfield. “In the long run they should bring down costs and increase access to medications for our patients.”
Others are skeptical that the adalimumab biosimilars will save patients much money.
Biosimilar laws were created to lower costs. However, if a patient with insurance pays only $5 a month out of pocket for Humira – a drug that normally costs $7,000 without coverage – it’s unlikely they would want to switch unless there’s comparable savings from the biosimilar, said Stephen B. Hanauer, MD, medical director of the Digestive Health Center and professor of medicine at Northwestern Medicine, Northwestern University, Evanston, Ill.
Like generics, Humira biosimilars may face some initial backlash, said Dr. Hanauer.
2023 broadens scope of adalimumab treatments
The American Gastroenterological Association describes a biosimilar as something that’s “highly similar to, but not an exact copy of, a biologic reference product already approved” by the Food and Drug Administration. Congress under the 2010 Affordable Care Act created a special, abbreviated pathway to approval for biosimilars.
AbbVie’s Humira, the global revenue for which exceeded $20 billion in 2021, has long dominated the U.S. market on injectable treatments for autoimmune diseases. The popular drug faces some competition in 2023, however, following a series of legal settlements that allowed AbbVie competitors to release their own adalimumab biosimilars.
“So far, we haven’t seen biosimilars live up to their potential in the U.S. in the inflammatory space,” said Mr. Newmark. This may change, however. Previously, biosimilars have required infusion, which demanded more time, commitment, and travel from patients. “The new set of forthcoming Humira biosimilars are injectables, an administration method preferred by patients,” he said.
The FDA will approve a biosimilar if it determines that the biological product is highly similar to the reference product, and that there are no clinically meaningful differences between the biological and reference product in terms of the safety, purity, and potency of the product.
The agency to date has approved 8 adalimumab biosimilars. These include: Idacio (adalimumab-aacf, Fresenius Kabi); Amjevita (adalimumab-atto, Amgen); Hadlima (adalimumab-bwwd, Organon); Cyltezo (adalimumab-adbm, Boehringer Ingelheim); Yusimry (adalimumab-aqvh from Coherus BioSciences); Hulio (adalimumab-fkjp; Mylan/Fujifilm Kyowa Kirin Biologics); Hyrimoz (adalimumab-adaz, Sandoz), and Abrilada (adalimumab-afzb, Pfizer).
“While FDA doesn’t formally track when products come to market, we know based on published reports that application holders for many of the currently FDA-approved biosimilars plan to market this year, starting with Amjevita being the first adalimumab biosimilar launched” in January, said Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars at the agency.
At press time, two other companies (Celltrion and Alvotech/Teva) were awaiting FDA approval for their adalimumab biosimilar drugs.
Among the eight approved drugs, Cyltezo is the only one that has a designation for interchangeability with Humira.
An interchangeable biosimilar may be substituted at the pharmacy without the intervention of the prescriber – much like generics are substituted, depending on state laws, said Dr. Yim. “However, in terms of safety and effectiveness, FDA’s standards for approval mean that biosimilar or interchangeable biosimilar products can be used in place of the reference product they were compared to.”
FDA-approved biosimilars undergo a rigorous evaluation for safety, effectiveness, and quality for their approved conditions of use, she continued. “Therefore, patients and health care providers can rely on a biosimilar to be as safe and effective for its approved uses as the original biological product.”
Remicade as a yard stick
Gastroenterologists dealt with this situation once before, when Remicade (infliximab) biosimilars came on the market in 2016, noted Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic.
Remicade and Humira are both tumor necrosis factor inhibitors with the same mechanism of action and many of the same indications. “We already had that experience with Remicade and biosimilar switch 2 or 3 years ago. Now we’re talking about Humira,” said Dr. Regueiro.
Most GI doctors have prescribed one of the more common infliximab biosimilars (Inflectra or Renflexis), noted Dr. Oldfield.
Cardinal Health, which recently surveyed 300 gastroenterologists, rheumatologists, and dermatologists about adalimumab biosimilars, found that gastroenterologists had the highest comfort level in prescribing them. Their top concern, however, was changing a patient from adalimumab to an adalimumab biosimilar.
For most patients, Dr. Oldfield sees the Humira reference biologic and biosimilar as equivalent.
However, he said he would change a patient’s drug only if there were a good reason or if his hand was forced by insurance. He would not make the change for a patient who recently began induction with the reference biologic or a patient with highly active clinical disease.
“While there is limited data to support this, I would also have some qualms about changing a patient from reference biologic to a biosimilar if they previously had immune-mediated pharmacokinetic failure due to antibody development with a biologic and were currently doing well on their new biologic,” he said.
Those with a new ulcerative colitis or Crohn’s diagnosis who are initiating a biologic for the first time might consider a biosimilar. If a patient is transitioning from a reference biologic to a biosimilar, “I would want to make that change during a time of stable remission and with the recognition that the switch is not a temporary switch, but a long-term switch,” he continued.
A paper that reviewed 23 observational studies of adalimumab and other biosimilars found that switching biosimilars was safe and effective. But if possible, patients should minimize the number of switches until more robust long-term data are available, added Dr. Oldfield.
If a patient is apprehensive about switching to a new therapy, “one may need to be cognizant of the ‘nocebo’ effect in which there is an unexplained or unfavorable therapeutic effect after switching,” he said.
Other gastroenterologists voiced similar reservations about switching. “I won’t use an adalimumab biosimilar unless the patient requests it, the insurance requires it, or there is a cost advantage for the patient such that they prefer it,” said Doug Wolf, MD, an Atlanta gastroenterologist.
“There is no medical treatment advantage to a biosimilar, especially if switching from Humira,” added Dr. Wolf.
Insurance will guide treatment
Once a drug is approved for use by the FDA, that drug will be available in all 50 states. “Different private insurance formularies, as well as state Medicaid formularies, might affect the actual ability of patients to receive such drugs,” said Mr. Newmark.
Patients should consult with their providers and insurance companies to see what therapies are available, he advised.
Dr. Hanauer anticipates some headaches arising for patients and doctors alike when negotiating for a specific drug.
Cyltezo may be the only biosimilar interchangeable with Humira, but the third-party pharmacy benefit manager (PBM) could negotiate for one of the noninterchangeable ones. “On a yearly basis they could switch their preference,” said Dr. Hanauer.
In the Cardinal Health survey, more than 60% of respondents said they would feel comfortable prescribing an adalimumab biosimilar only with an interchangeability designation.
A PBM may offer a patient Cyltezo if it’s cheaper than Humira. If the patient insists on staying on Humira, then they’ll have to pay more for that drug on their payer’s formulary, said Dr. Hanauer. In a worst-case scenario, a physician may have to appeal on a patient’s behalf to get Humira if the insurer offers only the biosimilar.
Taking that step to appeal is a major hassle for the physician, and leads to extra back door costs as well, said Dr. Hanauer.
Humira manufacturer AbbVie, in turn, may offer discounts and rebates to the PBMs to put Humira on their formulary. “That’s the AbbVie negotiating power. It’s not that the cost is going to be that much different. It’s going to be that there are rebates and discounts that are going to make the cost different,” he added.
As a community physician, Dr. Oldfield has specific concerns about accessibility.
The ever-increasing burden of insurance documentation and prior authorization means it can take weeks or months to get these medications approved. “The addition of new biosimilars is a welcome entrance if it can get patients the medications they need when they need it,” he said.
When it comes to prescribing biologics, many physicians rely on ancillary staff for assistance. It’s a team effort to sift through all the paperwork, observed Dr. Oldfield.
“While many community GI practices have specialized staff to deal with prior authorizations, they are still a far cry from the IBD [inflammatory bowel disease] academic centers where there are often pharmacists, nursing specialists, and home-monitoring programs to check in on patients,” he explained.
Landscape on cost is uncertain
At present, little is known about the cost of the biosimilars and impact on future drug pricing, said Dr. Oldfield.
At least for Medicare, Humira biosimilars will be considered Medicare Part D drugs if used for a medically accepted indication, said a spokesperson for the Centers for Medicare and Medicaid Services.
Part D sponsors (pharmacy and therapeutic committees) “will make the determination as to whether Amjevita and other products will be added to their formularies,” said the spokesperson.
Patients never saw a significant cost savings with Remicade biosimilars. “I imagine the same would be true with biosimilars for Humira,” said Dr. Regueiro. Patients may see greater access to these drugs, however, because the insurance plan or the pharmacy plan will make them more readily available, he added.
The hope is that, as biosimilars are introduced, the price of the originator biologic will go down, said Mr. Newmark. “Therefore, we can expect Humira to be offered at a lower price as it faces competition. Where it will sit in comparison to the forthcoming biosimilars will depend on how much biosimilar companies drop their price and how much pressure will be on PBMs and insurers to cover the lowest list price drug,” he said.
AbbVie did not respond to several requests for comment.
Charitable patient assistance programs for biosimilars or biologics can help offset the price of copayments, Mr. Newmark offered.
Ideally, insurers will offer designated biosimilars at a reduced or even no out-of-pocket expense on their formularies. This should lead to a decreased administrative burden for approval with streamlined (or even removal) of prior authorizations for certain medications, said Dr. Oldfield.
Without insurance or medication assistance programs, the cost of biosimilars is prohibitively expensive, he added.
“Biosimilars have higher research, development, and manufacturing costs than what people conventionally think of [for] a generic medication.”
Educating, advising patients
Dr. Oldfield advised that gastroenterologists refer to biologics by the generic name rather than branded name when initiating therapy unless there is a very specific reason not to. “This approach should make the process more streamlined and less subjected to quick denials for brand-only requests as biosimilars start to assume a larger market share,” he said.
Uptake of the Humira biosimilars also will depend on proper education of physicians and patients and their comfort level with the biosimilars, said Dr. Regueiro. Cleveland Clinic uses a team approach to educate on this topic, relying on pharmacists, clinicians, and nurses to explain that there’s no real difference between the reference drug and its biosimilars, based on efficacy and safety data.
Physicians can also direct patients to patient-friendly resources, said Mr. Newmark. “By starting the conversation early, it ensures that when/if the time comes that your patient is switched to or chooses a biosimilar they will feel more confident because they have the knowledge to make decisions about their care.”
The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars , is a free resource for patients, he added.
It’s important that doctors also understand these products so they can explain to their patients what to expect, said the FDA’s Dr. Yim. The FDA provides educational materials on its website, including a comprehensive curriculum toolkit.
Dr. Hanauer has served as a consultant for AbbVie, Amgen, American College of Gastroenterology, GlaxoSmithKline, American Gastroenterological Association, Pfizer, and a host of other companies . Dr. Regueiro has served on advisory boards and as a consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET Pharma Solutions,Trellis, and Boehringer Ingelheim Pharmaceuticals. Dr. Wolf, Dr. Yim, Dr. Oldfield, and Mr. Newmark have no financial conflicts of interest.
New tool better estimates cardiovascular risk in people with lupus
Current risk estimators are inaccurate
A tool that incorporates lupus-related variables with traditional risk factors provides a much more accurate assessment of cardiovascular (CV) risk in patients with systemic lupus erythematosus (SLE), according to data presented at the annual meeting of the Canadian Rheumatology Association.
In the initial clinical assessment of this tool, called the SLECRISK, “it identified high-risk lupus patients who would otherwise be missed by traditional methods of CV risk assessment,” reported May Y. Choi, MD, associate director of translational research at the University of Calgary’s (Alta.) Lupus Centre of Excellence.
It is well known that patients with SLE face an increased risk of CV events starting at an age long before risk begins climbing in the general population, according to Dr. Choi. She cited one study that showed women aged 35-44 years have a 50-fold greater risk of myocardial infarction than healthy individuals.
All major guidelines recognize this increased risk and recommend CV risk assessment in patients with SLE, even though Dr. Choi pointed out that traditional tools, such as the American College of Cardiology atherosclerotic cardiovascular disease (ASCVD) risk calculator or the Framingham Risk Score (FRS) have a limited ability to detect the patients with SLE who are most likely to have an event.
In SLE, current tools are inadequate
“These risk assessment tools perform poorly in SLE patients because they do not capture SLE-related inflammation,” Dr. Choi said. Of several examples, Dr. Choi cited a study showing “seven times more MIs and strokes observed than expected in SLE patients on the basis of the FRS.”
The disparity between expected and observed MIs and strokes is worse with increasing severity of SLE. In a study she presented 3 years ago, rates of CV events were 12 times higher in those with inactive or mild SLE, rising to a 16-fold increase among those with moderate disease and jumping to a 32-fold increase in those with severe SLE.
The SLECRISK tool was developed from the Brigham and Women’s Hospital SLE Registry, which was initiated in 1992. Patients without a history of CV disease were evaluated for traditional CV risk factors and for SLE-specific characteristics such as disease activity, levels of the complement proteins C3 and C4, kidney function, the presence of nephritis, and SLE duration. The value of these characteristics as predictors of CV events were then assessed over a 10-year follow-up period before being assembled into the SLECRISK tool.
In an example of the risk equation, Dr. Choi described a 50-year-old patient with SLE and a 5% 10-year ASCVD risk score, which is low. After adjustment for SLE risks, which included 10 years disease duration, high disease activity, elevated creatinine, and positive anti–double stranded DNA status, the 10-year CV risk score climbed to 16.2%, which is moderate.
The performance of the SLECRISK was evaluated in 1,243 patients providing 8,946.51 person-years of follow-up. During this period, there were 90 major adverse cardiac events (MACE), of which 82% were adjudicated by cardiologists, and 211 secondary events.
Relative to the ASCVD risk score, the SLECRISK identified about twice as many patients with SLE as having moderate risk and 3.5-fold more patients as having high risk. Among patients who experienced CV events, traditional CV risk factors were more common but so were SLE-specific risk factors, including greater disease severity, a greater likelihood of lupus nephritis, increased complement levels, and greater exposure to glucocorticoids, according to Dr. Choi.
Specificities for CV events higher on SLECRISK
In predicting CV events, the differences in specificities were in the same general range, although somewhat higher for the ASCVD risk score in regard to predicting MACE (83% vs. 72%) and MACE plus secondary events (90% vs. 79%). However, the sensitivities were much higher for SLECRISK relative to the ASCVD risk score for MACE alone (64% vs. 41%) and for MACE plus secondary events (58% vs. 35%).
When comparing those who had an MI or stroke, the ASCVD risk score identified 8 (7%) patients missed by SLECRISK, whereas SLECRISK identified 89 (73%) missed by the ASCVD risk score. The remaining 25 patients (20%) were identified by both. The advantage of SLECRISK was similar for MACE plus secondary outcomes.
Dr. Choi noted that all of the SLE-specific variables in SLECRISK are readily obtained and often already available in patient charts. She said that there is a plan to validate the tool in larger groups, but with a goal of creating a tool available online for clinicians and their patients to use. There is also an even more ambitious plan for the future.
“We have funding to look at machine learning to evaluate predictive variables in SLE patients,” Dr. Choi said. Rather than adding SLE-specific variables to traditional risks, the plan is to “start from scratch,” letting artificial intelligence assemble predictors without prejudice to what might or might not be relevant.
A SLE-specific tool for evaluating CV risk is an important “unmet need,” according to Karen H. Costenbader, MD, professor in the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. In an interview, she reiterated that measuring CV risk in SLE is already guideline recommended, but conventional tools have been shown to be inaccurate.
“I can envision it being used in clinical encounters to help guide shared decision-making with patients,” explained Dr. Costenbader, who was not involved in the presentation at the CRA meeting but worked with Dr. Choi in developing SLECRISK. “It would give us more precise estimates, allowing us to risk stratify our patients and informing us as to which modifiable SLE-specific and nonspecific factors are contributing most to CV risk.’
The problem of using conventional risk assessments in SLE has been well recognized. Of those who have written on this subject, Maureen McMahon, MD, site director of the Lupus Clinical Trials Network at the University of California, Los Angeles, said: “There is a critical need for the development of SLE-specific risk assessment tools like SLECRISK.”
Author of several studies looking at alternatives for CV risk assessment in SLE, including a study looking at a panel of biomarkers that was published in ACR Open Rheumatology, Dr. McMahon said in an interview that CV risk in SLE is high but conventional risk assessments are flawed.
“Multiple previous studies have demonstrated that these currently available calculators are not adequate for identifying risk in the lupus patient population,” she said. According to Dr. McMahon, the fact that rheumatologists remain “dependent upon [these conventional] cardiovascular risk calculators” is a well-recognized problem that needs resolution.
Dr. Choi has financial relationships with AstraZeneca, GlaxoSmithKline, Mallinckrodt. MitogenDx, Organon, and Werfen International. Dr. Costenbader reports no potential conflicts of interest. Dr. McMahon has financial relationships with AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, and GlaxoSmithKline.
Current risk estimators are inaccurate
Current risk estimators are inaccurate
A tool that incorporates lupus-related variables with traditional risk factors provides a much more accurate assessment of cardiovascular (CV) risk in patients with systemic lupus erythematosus (SLE), according to data presented at the annual meeting of the Canadian Rheumatology Association.
In the initial clinical assessment of this tool, called the SLECRISK, “it identified high-risk lupus patients who would otherwise be missed by traditional methods of CV risk assessment,” reported May Y. Choi, MD, associate director of translational research at the University of Calgary’s (Alta.) Lupus Centre of Excellence.
It is well known that patients with SLE face an increased risk of CV events starting at an age long before risk begins climbing in the general population, according to Dr. Choi. She cited one study that showed women aged 35-44 years have a 50-fold greater risk of myocardial infarction than healthy individuals.
All major guidelines recognize this increased risk and recommend CV risk assessment in patients with SLE, even though Dr. Choi pointed out that traditional tools, such as the American College of Cardiology atherosclerotic cardiovascular disease (ASCVD) risk calculator or the Framingham Risk Score (FRS) have a limited ability to detect the patients with SLE who are most likely to have an event.
In SLE, current tools are inadequate
“These risk assessment tools perform poorly in SLE patients because they do not capture SLE-related inflammation,” Dr. Choi said. Of several examples, Dr. Choi cited a study showing “seven times more MIs and strokes observed than expected in SLE patients on the basis of the FRS.”
The disparity between expected and observed MIs and strokes is worse with increasing severity of SLE. In a study she presented 3 years ago, rates of CV events were 12 times higher in those with inactive or mild SLE, rising to a 16-fold increase among those with moderate disease and jumping to a 32-fold increase in those with severe SLE.
The SLECRISK tool was developed from the Brigham and Women’s Hospital SLE Registry, which was initiated in 1992. Patients without a history of CV disease were evaluated for traditional CV risk factors and for SLE-specific characteristics such as disease activity, levels of the complement proteins C3 and C4, kidney function, the presence of nephritis, and SLE duration. The value of these characteristics as predictors of CV events were then assessed over a 10-year follow-up period before being assembled into the SLECRISK tool.
In an example of the risk equation, Dr. Choi described a 50-year-old patient with SLE and a 5% 10-year ASCVD risk score, which is low. After adjustment for SLE risks, which included 10 years disease duration, high disease activity, elevated creatinine, and positive anti–double stranded DNA status, the 10-year CV risk score climbed to 16.2%, which is moderate.
The performance of the SLECRISK was evaluated in 1,243 patients providing 8,946.51 person-years of follow-up. During this period, there were 90 major adverse cardiac events (MACE), of which 82% were adjudicated by cardiologists, and 211 secondary events.
Relative to the ASCVD risk score, the SLECRISK identified about twice as many patients with SLE as having moderate risk and 3.5-fold more patients as having high risk. Among patients who experienced CV events, traditional CV risk factors were more common but so were SLE-specific risk factors, including greater disease severity, a greater likelihood of lupus nephritis, increased complement levels, and greater exposure to glucocorticoids, according to Dr. Choi.
Specificities for CV events higher on SLECRISK
In predicting CV events, the differences in specificities were in the same general range, although somewhat higher for the ASCVD risk score in regard to predicting MACE (83% vs. 72%) and MACE plus secondary events (90% vs. 79%). However, the sensitivities were much higher for SLECRISK relative to the ASCVD risk score for MACE alone (64% vs. 41%) and for MACE plus secondary events (58% vs. 35%).
When comparing those who had an MI or stroke, the ASCVD risk score identified 8 (7%) patients missed by SLECRISK, whereas SLECRISK identified 89 (73%) missed by the ASCVD risk score. The remaining 25 patients (20%) were identified by both. The advantage of SLECRISK was similar for MACE plus secondary outcomes.
Dr. Choi noted that all of the SLE-specific variables in SLECRISK are readily obtained and often already available in patient charts. She said that there is a plan to validate the tool in larger groups, but with a goal of creating a tool available online for clinicians and their patients to use. There is also an even more ambitious plan for the future.
“We have funding to look at machine learning to evaluate predictive variables in SLE patients,” Dr. Choi said. Rather than adding SLE-specific variables to traditional risks, the plan is to “start from scratch,” letting artificial intelligence assemble predictors without prejudice to what might or might not be relevant.
A SLE-specific tool for evaluating CV risk is an important “unmet need,” according to Karen H. Costenbader, MD, professor in the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. In an interview, she reiterated that measuring CV risk in SLE is already guideline recommended, but conventional tools have been shown to be inaccurate.
“I can envision it being used in clinical encounters to help guide shared decision-making with patients,” explained Dr. Costenbader, who was not involved in the presentation at the CRA meeting but worked with Dr. Choi in developing SLECRISK. “It would give us more precise estimates, allowing us to risk stratify our patients and informing us as to which modifiable SLE-specific and nonspecific factors are contributing most to CV risk.’
The problem of using conventional risk assessments in SLE has been well recognized. Of those who have written on this subject, Maureen McMahon, MD, site director of the Lupus Clinical Trials Network at the University of California, Los Angeles, said: “There is a critical need for the development of SLE-specific risk assessment tools like SLECRISK.”
Author of several studies looking at alternatives for CV risk assessment in SLE, including a study looking at a panel of biomarkers that was published in ACR Open Rheumatology, Dr. McMahon said in an interview that CV risk in SLE is high but conventional risk assessments are flawed.
“Multiple previous studies have demonstrated that these currently available calculators are not adequate for identifying risk in the lupus patient population,” she said. According to Dr. McMahon, the fact that rheumatologists remain “dependent upon [these conventional] cardiovascular risk calculators” is a well-recognized problem that needs resolution.
Dr. Choi has financial relationships with AstraZeneca, GlaxoSmithKline, Mallinckrodt. MitogenDx, Organon, and Werfen International. Dr. Costenbader reports no potential conflicts of interest. Dr. McMahon has financial relationships with AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, and GlaxoSmithKline.
A tool that incorporates lupus-related variables with traditional risk factors provides a much more accurate assessment of cardiovascular (CV) risk in patients with systemic lupus erythematosus (SLE), according to data presented at the annual meeting of the Canadian Rheumatology Association.
In the initial clinical assessment of this tool, called the SLECRISK, “it identified high-risk lupus patients who would otherwise be missed by traditional methods of CV risk assessment,” reported May Y. Choi, MD, associate director of translational research at the University of Calgary’s (Alta.) Lupus Centre of Excellence.
It is well known that patients with SLE face an increased risk of CV events starting at an age long before risk begins climbing in the general population, according to Dr. Choi. She cited one study that showed women aged 35-44 years have a 50-fold greater risk of myocardial infarction than healthy individuals.
All major guidelines recognize this increased risk and recommend CV risk assessment in patients with SLE, even though Dr. Choi pointed out that traditional tools, such as the American College of Cardiology atherosclerotic cardiovascular disease (ASCVD) risk calculator or the Framingham Risk Score (FRS) have a limited ability to detect the patients with SLE who are most likely to have an event.
In SLE, current tools are inadequate
“These risk assessment tools perform poorly in SLE patients because they do not capture SLE-related inflammation,” Dr. Choi said. Of several examples, Dr. Choi cited a study showing “seven times more MIs and strokes observed than expected in SLE patients on the basis of the FRS.”
The disparity between expected and observed MIs and strokes is worse with increasing severity of SLE. In a study she presented 3 years ago, rates of CV events were 12 times higher in those with inactive or mild SLE, rising to a 16-fold increase among those with moderate disease and jumping to a 32-fold increase in those with severe SLE.
The SLECRISK tool was developed from the Brigham and Women’s Hospital SLE Registry, which was initiated in 1992. Patients without a history of CV disease were evaluated for traditional CV risk factors and for SLE-specific characteristics such as disease activity, levels of the complement proteins C3 and C4, kidney function, the presence of nephritis, and SLE duration. The value of these characteristics as predictors of CV events were then assessed over a 10-year follow-up period before being assembled into the SLECRISK tool.
In an example of the risk equation, Dr. Choi described a 50-year-old patient with SLE and a 5% 10-year ASCVD risk score, which is low. After adjustment for SLE risks, which included 10 years disease duration, high disease activity, elevated creatinine, and positive anti–double stranded DNA status, the 10-year CV risk score climbed to 16.2%, which is moderate.
The performance of the SLECRISK was evaluated in 1,243 patients providing 8,946.51 person-years of follow-up. During this period, there were 90 major adverse cardiac events (MACE), of which 82% were adjudicated by cardiologists, and 211 secondary events.
Relative to the ASCVD risk score, the SLECRISK identified about twice as many patients with SLE as having moderate risk and 3.5-fold more patients as having high risk. Among patients who experienced CV events, traditional CV risk factors were more common but so were SLE-specific risk factors, including greater disease severity, a greater likelihood of lupus nephritis, increased complement levels, and greater exposure to glucocorticoids, according to Dr. Choi.
Specificities for CV events higher on SLECRISK
In predicting CV events, the differences in specificities were in the same general range, although somewhat higher for the ASCVD risk score in regard to predicting MACE (83% vs. 72%) and MACE plus secondary events (90% vs. 79%). However, the sensitivities were much higher for SLECRISK relative to the ASCVD risk score for MACE alone (64% vs. 41%) and for MACE plus secondary events (58% vs. 35%).
When comparing those who had an MI or stroke, the ASCVD risk score identified 8 (7%) patients missed by SLECRISK, whereas SLECRISK identified 89 (73%) missed by the ASCVD risk score. The remaining 25 patients (20%) were identified by both. The advantage of SLECRISK was similar for MACE plus secondary outcomes.
Dr. Choi noted that all of the SLE-specific variables in SLECRISK are readily obtained and often already available in patient charts. She said that there is a plan to validate the tool in larger groups, but with a goal of creating a tool available online for clinicians and their patients to use. There is also an even more ambitious plan for the future.
“We have funding to look at machine learning to evaluate predictive variables in SLE patients,” Dr. Choi said. Rather than adding SLE-specific variables to traditional risks, the plan is to “start from scratch,” letting artificial intelligence assemble predictors without prejudice to what might or might not be relevant.
A SLE-specific tool for evaluating CV risk is an important “unmet need,” according to Karen H. Costenbader, MD, professor in the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. In an interview, she reiterated that measuring CV risk in SLE is already guideline recommended, but conventional tools have been shown to be inaccurate.
“I can envision it being used in clinical encounters to help guide shared decision-making with patients,” explained Dr. Costenbader, who was not involved in the presentation at the CRA meeting but worked with Dr. Choi in developing SLECRISK. “It would give us more precise estimates, allowing us to risk stratify our patients and informing us as to which modifiable SLE-specific and nonspecific factors are contributing most to CV risk.’
The problem of using conventional risk assessments in SLE has been well recognized. Of those who have written on this subject, Maureen McMahon, MD, site director of the Lupus Clinical Trials Network at the University of California, Los Angeles, said: “There is a critical need for the development of SLE-specific risk assessment tools like SLECRISK.”
Author of several studies looking at alternatives for CV risk assessment in SLE, including a study looking at a panel of biomarkers that was published in ACR Open Rheumatology, Dr. McMahon said in an interview that CV risk in SLE is high but conventional risk assessments are flawed.
“Multiple previous studies have demonstrated that these currently available calculators are not adequate for identifying risk in the lupus patient population,” she said. According to Dr. McMahon, the fact that rheumatologists remain “dependent upon [these conventional] cardiovascular risk calculators” is a well-recognized problem that needs resolution.
Dr. Choi has financial relationships with AstraZeneca, GlaxoSmithKline, Mallinckrodt. MitogenDx, Organon, and Werfen International. Dr. Costenbader reports no potential conflicts of interest. Dr. McMahon has financial relationships with AstraZeneca, Aurinia Pharmaceuticals, Eli Lilly, and GlaxoSmithKline.
FROM CRA 2023
Health plans get very poor scores for access to autoimmune drugs
Both public and private health plans score poorly when it comes to providing access to autoimmune medication, according to a report commissioned by the Autoimmune Association and Let My Doctors Decide, a national partnership of health care professionals. The analysis, published Jan. 26, found that 75% of insurers in the United States have policies that can limit coverage for Food and Drug Administration–approved medications for Crohn’s disease, lupus nephritis, multiple sclerosis, psoriasis, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis.
“Choice among health plans is a hallmark of the American health insurance system, yet this analysis shows that people living with autoimmune conditions have few, if any, coverage choices that do not involve significant to severe access restrictions,” the authors wrote.
The study looked at three common utilization management policies by health plans that can limit coverage of certain medications: step therapy, formulary/tier placement, and prior authorization. To compare health plans, researchers weighted these policies using a point system. Each medication indicated for each condition was given a score of 0-4 based on access restrictions in a health plan. If a plan used step therapy, it received one point, and requiring prior authorization added an additional point. They also added points based on where a drug appeared on a plan’s formulary. A lower total score meant fewer access barriers. The numbers were then added, and each health plan received a grade of A, B, C, or F based on their average score. The datasets and analysis were provided and performed by the data analytics firm MMIT.
Nearly 9 in 10 Medicare plans received a C or worse for coverage of medication received via mail order or the pharmacy. In commercial plans, the majority of plans scored Cs or Fs for six of the seven conditions, excluding lupus nephritis, where 67% of all commercial health plans scored a B for access to these medications.
Physician-administered medications tended to receive poorer coverage than drugs received via pharmacy. Across all conditions, 65% of Medicare Advantage plans scored an F for physician-administered medication access. For both psoriasis and multiple sclerosis, at least 80% of Medicare plans earned failing scores because of these restrictions. Coverage was poorer on both commercial and health exchange plans, where across all conditions, 83% achieved failing scores. Two exceptions were the Southern and Northern California PPO plans by the Kaiser Foundation Health Plan. Out of the largest 25 health plans in the United States, these two plans earned As in coverage for physician-administered medications across all seven autoimmune conditions.
The report shows “a growing disconnect between science and health insurance benefit designs that were developed in the 1960s and 1970s,” Kenneth Thorpe, PhD, of Emory University, Atlanta, said in an interview. Insurers originally designed these benefits to prevent excessive utilization in a population of mostly acutely ill patients, he said, whereas now, 90% of healthcare spending is linked to chronic conditions. For these patients, research shows that incentivizing patients to adhere to medications results in fewer hospitalizations and, therefore, more cost savings, Thorpe noted. These plans also do not consider that there is no average patient, he said, and healthcare providers should be able to match each patient to the best treatment option for them rather than trying out other less expensive medications first. “To the extent that physicians can have the flexibility to provide medications and treatments to patients that are going to have the best clinical response, that’s better outcomes at lower cost,” Dr. Thorpe said. While research shows heterogeneity in patient outcomes with different medication, “benefit designs from the past just don’t recognize that.”
Neither America’s Health Insurance Plans nor Pharmaceutical Care Management Association responded to a request for comment.
Quardricos Driskell, executive director of Let My Doctors Decide and vice president of government relations and public policy at the Autoimmune Association, hopes the study will spur action by policy makers and health plans to improve access to medications for the people who need them. Another larger point of the report is to “uphold the sanctity of protecting the doctor and patient relationship,” he said in an interview, adding “that decisions fundamentally need to be made not by insurance plans or middleman pharmacy benefit managers, but by the provider and patient.”
Mr. Driskell and Dr. Thorpe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both public and private health plans score poorly when it comes to providing access to autoimmune medication, according to a report commissioned by the Autoimmune Association and Let My Doctors Decide, a national partnership of health care professionals. The analysis, published Jan. 26, found that 75% of insurers in the United States have policies that can limit coverage for Food and Drug Administration–approved medications for Crohn’s disease, lupus nephritis, multiple sclerosis, psoriasis, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis.
“Choice among health plans is a hallmark of the American health insurance system, yet this analysis shows that people living with autoimmune conditions have few, if any, coverage choices that do not involve significant to severe access restrictions,” the authors wrote.
The study looked at three common utilization management policies by health plans that can limit coverage of certain medications: step therapy, formulary/tier placement, and prior authorization. To compare health plans, researchers weighted these policies using a point system. Each medication indicated for each condition was given a score of 0-4 based on access restrictions in a health plan. If a plan used step therapy, it received one point, and requiring prior authorization added an additional point. They also added points based on where a drug appeared on a plan’s formulary. A lower total score meant fewer access barriers. The numbers were then added, and each health plan received a grade of A, B, C, or F based on their average score. The datasets and analysis were provided and performed by the data analytics firm MMIT.
Nearly 9 in 10 Medicare plans received a C or worse for coverage of medication received via mail order or the pharmacy. In commercial plans, the majority of plans scored Cs or Fs for six of the seven conditions, excluding lupus nephritis, where 67% of all commercial health plans scored a B for access to these medications.
Physician-administered medications tended to receive poorer coverage than drugs received via pharmacy. Across all conditions, 65% of Medicare Advantage plans scored an F for physician-administered medication access. For both psoriasis and multiple sclerosis, at least 80% of Medicare plans earned failing scores because of these restrictions. Coverage was poorer on both commercial and health exchange plans, where across all conditions, 83% achieved failing scores. Two exceptions were the Southern and Northern California PPO plans by the Kaiser Foundation Health Plan. Out of the largest 25 health plans in the United States, these two plans earned As in coverage for physician-administered medications across all seven autoimmune conditions.
The report shows “a growing disconnect between science and health insurance benefit designs that were developed in the 1960s and 1970s,” Kenneth Thorpe, PhD, of Emory University, Atlanta, said in an interview. Insurers originally designed these benefits to prevent excessive utilization in a population of mostly acutely ill patients, he said, whereas now, 90% of healthcare spending is linked to chronic conditions. For these patients, research shows that incentivizing patients to adhere to medications results in fewer hospitalizations and, therefore, more cost savings, Thorpe noted. These plans also do not consider that there is no average patient, he said, and healthcare providers should be able to match each patient to the best treatment option for them rather than trying out other less expensive medications first. “To the extent that physicians can have the flexibility to provide medications and treatments to patients that are going to have the best clinical response, that’s better outcomes at lower cost,” Dr. Thorpe said. While research shows heterogeneity in patient outcomes with different medication, “benefit designs from the past just don’t recognize that.”
Neither America’s Health Insurance Plans nor Pharmaceutical Care Management Association responded to a request for comment.
Quardricos Driskell, executive director of Let My Doctors Decide and vice president of government relations and public policy at the Autoimmune Association, hopes the study will spur action by policy makers and health plans to improve access to medications for the people who need them. Another larger point of the report is to “uphold the sanctity of protecting the doctor and patient relationship,” he said in an interview, adding “that decisions fundamentally need to be made not by insurance plans or middleman pharmacy benefit managers, but by the provider and patient.”
Mr. Driskell and Dr. Thorpe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both public and private health plans score poorly when it comes to providing access to autoimmune medication, according to a report commissioned by the Autoimmune Association and Let My Doctors Decide, a national partnership of health care professionals. The analysis, published Jan. 26, found that 75% of insurers in the United States have policies that can limit coverage for Food and Drug Administration–approved medications for Crohn’s disease, lupus nephritis, multiple sclerosis, psoriasis, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis.
“Choice among health plans is a hallmark of the American health insurance system, yet this analysis shows that people living with autoimmune conditions have few, if any, coverage choices that do not involve significant to severe access restrictions,” the authors wrote.
The study looked at three common utilization management policies by health plans that can limit coverage of certain medications: step therapy, formulary/tier placement, and prior authorization. To compare health plans, researchers weighted these policies using a point system. Each medication indicated for each condition was given a score of 0-4 based on access restrictions in a health plan. If a plan used step therapy, it received one point, and requiring prior authorization added an additional point. They also added points based on where a drug appeared on a plan’s formulary. A lower total score meant fewer access barriers. The numbers were then added, and each health plan received a grade of A, B, C, or F based on their average score. The datasets and analysis were provided and performed by the data analytics firm MMIT.
Nearly 9 in 10 Medicare plans received a C or worse for coverage of medication received via mail order or the pharmacy. In commercial plans, the majority of plans scored Cs or Fs for six of the seven conditions, excluding lupus nephritis, where 67% of all commercial health plans scored a B for access to these medications.
Physician-administered medications tended to receive poorer coverage than drugs received via pharmacy. Across all conditions, 65% of Medicare Advantage plans scored an F for physician-administered medication access. For both psoriasis and multiple sclerosis, at least 80% of Medicare plans earned failing scores because of these restrictions. Coverage was poorer on both commercial and health exchange plans, where across all conditions, 83% achieved failing scores. Two exceptions were the Southern and Northern California PPO plans by the Kaiser Foundation Health Plan. Out of the largest 25 health plans in the United States, these two plans earned As in coverage for physician-administered medications across all seven autoimmune conditions.
The report shows “a growing disconnect between science and health insurance benefit designs that were developed in the 1960s and 1970s,” Kenneth Thorpe, PhD, of Emory University, Atlanta, said in an interview. Insurers originally designed these benefits to prevent excessive utilization in a population of mostly acutely ill patients, he said, whereas now, 90% of healthcare spending is linked to chronic conditions. For these patients, research shows that incentivizing patients to adhere to medications results in fewer hospitalizations and, therefore, more cost savings, Thorpe noted. These plans also do not consider that there is no average patient, he said, and healthcare providers should be able to match each patient to the best treatment option for them rather than trying out other less expensive medications first. “To the extent that physicians can have the flexibility to provide medications and treatments to patients that are going to have the best clinical response, that’s better outcomes at lower cost,” Dr. Thorpe said. While research shows heterogeneity in patient outcomes with different medication, “benefit designs from the past just don’t recognize that.”
Neither America’s Health Insurance Plans nor Pharmaceutical Care Management Association responded to a request for comment.
Quardricos Driskell, executive director of Let My Doctors Decide and vice president of government relations and public policy at the Autoimmune Association, hopes the study will spur action by policy makers and health plans to improve access to medications for the people who need them. Another larger point of the report is to “uphold the sanctity of protecting the doctor and patient relationship,” he said in an interview, adding “that decisions fundamentally need to be made not by insurance plans or middleman pharmacy benefit managers, but by the provider and patient.”
Mr. Driskell and Dr. Thorpe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Topical CBD oil study suggests benefits for pain, healing in systemic sclerosis digital ulcers
Topical cannabidiol (CBD) oil appeared to lower pain scores and reduce painkiller use vs. standard treatment in patients with digital ulcers due to systemic sclerosis, a small new study finds. Patients who received the treatment also showed signs of more healing.
The study, published in Advances in Skin & Wound Care, is far from definitive since it’s retrospective and tracked only 45 patients. But the findings add to other research suggesting dermatologic benefits from the topical use of CBD, an ingredient in cannabis that’s widely available and doesn’t cause people to become high or become addicted.
“This is a good first step in trying to address scleroderma digital ulcer pain and healing,” University of Colorado rheumatologist Melissa Griffith, MD, said in an interview. “Digital ulcers cause great impact on quality of life, daily activities of living, and pain, so we are always looking for new, effective tools.”
According to Dr. Griffith, digital ulcers occur in scleroderma due to Raynaud’s phenomenon with reversible vasospasm. “Unlike patients with primary Raynaud’s phenomenon, patients will develop ischemia of digits, leading to digital ulcers due to the vasculopathy or vascular remodeling that occurs in scleroderma,” she said.
Current treatments include removal of causative drugs/toxins and warmth, rest, and pain control, although “no trials exist to compare the scleroderma digital ulcer or digital ischemia treatments to each other,” Dr. Griffith said.
Therapy for vasospasm begins with calcium channel blockers such as amlodipine and nifedipine, she said, followed by phosphodiesterase type 5 inhibitors such as sildenafil or endothelin receptor antagonist medications such as bosentan. “If these fail, we use an IV option – epoprostenol. Other options are sympathectomy surgery, Botox, digital nerve blocks, biofeedback, and SSRIs,” she said. “These treatments work fairly well in most patients, but there are patients who break through these therapies and have ongoing digital ischemia, leading to digital ulcers, pain, infections, acro-osteolysis, and auto-amputation. There is definitely room to improve on our current treatment paradigm.”
For the new study, researchers in Italy led by Amelia Spinella, MD, PhD, of University Hospital of Modena, retrospectively tracked 45 patients with systemic sclerosis and at least one digital ulcer (40 women; average age, 53 years) who were treated in 2019. All patients’ ulcers were resistant to opioid therapy at the maximum tolerated dose, and all had undergone periodic iloprost infusion every 30-40 days. Based on each patient’s clinical situation, they had received calcium-channel blockers, phosphodiesterase type 5 inhibitors (sildenafil), and/or endothelin receptor antagonists (bosentan or macitentan). The researchers noted that all patients underwent surgical debridement regularly following wound bed preparation procedures and received advanced dressings (alginate, hydrocolloid, hydrofiber, hydrogel, and polyurethane foam or film). Of the 45 patients, 25 treated their wounds daily over the course of a month by administering four drops of a preparation of 10% CBD oil in acidic form and 90% hemp oil over the wound bed and perilesional skin and then covering it with a nonadhesive cloth.
“Basal wound-related pain NRS [numeric rating scale] scores decreased from 8.4 (standard deviation [SD], 0.8) at the baseline (T0) to 6.0 (SD, 0.82) after 1 month of CBD treatment (T1; P < .0001),” the researchers reported. “Across the same time period, volitional incident pain NRS scores decreased from 9.32 (SD, 0.75; T0) to 6.8 (SD, 1.12; T1; P < .0001). In addition, mean total hours of sleep per night increased from 2.56 (SD, 1.28) to 5.67 (SD, 0.85) hours (P < .0001).” Twelve of the 25 needed additional painkiller therapy.
Complete digital ulcer healing occurred by the end of the study in 18 of 25 (72%) CBD-treated patients, compared with 6 of 20 (30%) control patients.
In contrast, the control group didn’t see any significant improvement in wound-related pain, volitional incident pain, or sleep. All needed additional painkiller therapy. Six developed ulcer infections and received antibiotics.
No significant adverse effects were reported, although 28% of those in the CBD oil group said they had mild effects such as itch and perilesional erythema.
The authors of the new study called for larger, randomized controlled, multicenter trials to confirm the benefit of CBD topical treatment.
In recent years, researchers have devoted more attention to topical CBD as a treatment for skin conditions. While limited, the evidence suggests they “may be effective for the treatment of various inflammatory skin disorders,” researchers wrote in a 2022 report. “Although promising, additional research is necessary to evaluate efficacy and to determine dosing, safety, and long-term treatment guidelines.”
Dr. Griffith, who did not take part in the new study but is familiar with its findings, said she was especially surprised by the hint that topical CBD improves healing in addition to relieving symptoms. “I thought only pain would be affected. This is a great outcome if it can be replicated.”
As for future research, she said “there are difficulties with reproducing this at a big scale in the U.S. given CBD commercial variability. The big issue is the standardization of CBD extraction and production. It is really hard for us as physicians to know what patients are getting. Some online CBD orders contain THC [the major psychoactive ingredient of cannabis] > 0.3% or no CBD at all.”
Still, she said, “physicians and patients may consider this when standard therapies are not working or causing too many adverse effects,” especially since “the downsides here seem fairly minimal – at worst itchiness and redness that did not prevent patients from continuing in the study.”
No details about study funding were provided. The authors and Dr. Griffith report no disclosures.
Topical cannabidiol (CBD) oil appeared to lower pain scores and reduce painkiller use vs. standard treatment in patients with digital ulcers due to systemic sclerosis, a small new study finds. Patients who received the treatment also showed signs of more healing.
The study, published in Advances in Skin & Wound Care, is far from definitive since it’s retrospective and tracked only 45 patients. But the findings add to other research suggesting dermatologic benefits from the topical use of CBD, an ingredient in cannabis that’s widely available and doesn’t cause people to become high or become addicted.
“This is a good first step in trying to address scleroderma digital ulcer pain and healing,” University of Colorado rheumatologist Melissa Griffith, MD, said in an interview. “Digital ulcers cause great impact on quality of life, daily activities of living, and pain, so we are always looking for new, effective tools.”
According to Dr. Griffith, digital ulcers occur in scleroderma due to Raynaud’s phenomenon with reversible vasospasm. “Unlike patients with primary Raynaud’s phenomenon, patients will develop ischemia of digits, leading to digital ulcers due to the vasculopathy or vascular remodeling that occurs in scleroderma,” she said.
Current treatments include removal of causative drugs/toxins and warmth, rest, and pain control, although “no trials exist to compare the scleroderma digital ulcer or digital ischemia treatments to each other,” Dr. Griffith said.
Therapy for vasospasm begins with calcium channel blockers such as amlodipine and nifedipine, she said, followed by phosphodiesterase type 5 inhibitors such as sildenafil or endothelin receptor antagonist medications such as bosentan. “If these fail, we use an IV option – epoprostenol. Other options are sympathectomy surgery, Botox, digital nerve blocks, biofeedback, and SSRIs,” she said. “These treatments work fairly well in most patients, but there are patients who break through these therapies and have ongoing digital ischemia, leading to digital ulcers, pain, infections, acro-osteolysis, and auto-amputation. There is definitely room to improve on our current treatment paradigm.”
For the new study, researchers in Italy led by Amelia Spinella, MD, PhD, of University Hospital of Modena, retrospectively tracked 45 patients with systemic sclerosis and at least one digital ulcer (40 women; average age, 53 years) who were treated in 2019. All patients’ ulcers were resistant to opioid therapy at the maximum tolerated dose, and all had undergone periodic iloprost infusion every 30-40 days. Based on each patient’s clinical situation, they had received calcium-channel blockers, phosphodiesterase type 5 inhibitors (sildenafil), and/or endothelin receptor antagonists (bosentan or macitentan). The researchers noted that all patients underwent surgical debridement regularly following wound bed preparation procedures and received advanced dressings (alginate, hydrocolloid, hydrofiber, hydrogel, and polyurethane foam or film). Of the 45 patients, 25 treated their wounds daily over the course of a month by administering four drops of a preparation of 10% CBD oil in acidic form and 90% hemp oil over the wound bed and perilesional skin and then covering it with a nonadhesive cloth.
“Basal wound-related pain NRS [numeric rating scale] scores decreased from 8.4 (standard deviation [SD], 0.8) at the baseline (T0) to 6.0 (SD, 0.82) after 1 month of CBD treatment (T1; P < .0001),” the researchers reported. “Across the same time period, volitional incident pain NRS scores decreased from 9.32 (SD, 0.75; T0) to 6.8 (SD, 1.12; T1; P < .0001). In addition, mean total hours of sleep per night increased from 2.56 (SD, 1.28) to 5.67 (SD, 0.85) hours (P < .0001).” Twelve of the 25 needed additional painkiller therapy.
Complete digital ulcer healing occurred by the end of the study in 18 of 25 (72%) CBD-treated patients, compared with 6 of 20 (30%) control patients.
In contrast, the control group didn’t see any significant improvement in wound-related pain, volitional incident pain, or sleep. All needed additional painkiller therapy. Six developed ulcer infections and received antibiotics.
No significant adverse effects were reported, although 28% of those in the CBD oil group said they had mild effects such as itch and perilesional erythema.
The authors of the new study called for larger, randomized controlled, multicenter trials to confirm the benefit of CBD topical treatment.
In recent years, researchers have devoted more attention to topical CBD as a treatment for skin conditions. While limited, the evidence suggests they “may be effective for the treatment of various inflammatory skin disorders,” researchers wrote in a 2022 report. “Although promising, additional research is necessary to evaluate efficacy and to determine dosing, safety, and long-term treatment guidelines.”
Dr. Griffith, who did not take part in the new study but is familiar with its findings, said she was especially surprised by the hint that topical CBD improves healing in addition to relieving symptoms. “I thought only pain would be affected. This is a great outcome if it can be replicated.”
As for future research, she said “there are difficulties with reproducing this at a big scale in the U.S. given CBD commercial variability. The big issue is the standardization of CBD extraction and production. It is really hard for us as physicians to know what patients are getting. Some online CBD orders contain THC [the major psychoactive ingredient of cannabis] > 0.3% or no CBD at all.”
Still, she said, “physicians and patients may consider this when standard therapies are not working or causing too many adverse effects,” especially since “the downsides here seem fairly minimal – at worst itchiness and redness that did not prevent patients from continuing in the study.”
No details about study funding were provided. The authors and Dr. Griffith report no disclosures.
Topical cannabidiol (CBD) oil appeared to lower pain scores and reduce painkiller use vs. standard treatment in patients with digital ulcers due to systemic sclerosis, a small new study finds. Patients who received the treatment also showed signs of more healing.
The study, published in Advances in Skin & Wound Care, is far from definitive since it’s retrospective and tracked only 45 patients. But the findings add to other research suggesting dermatologic benefits from the topical use of CBD, an ingredient in cannabis that’s widely available and doesn’t cause people to become high or become addicted.
“This is a good first step in trying to address scleroderma digital ulcer pain and healing,” University of Colorado rheumatologist Melissa Griffith, MD, said in an interview. “Digital ulcers cause great impact on quality of life, daily activities of living, and pain, so we are always looking for new, effective tools.”
According to Dr. Griffith, digital ulcers occur in scleroderma due to Raynaud’s phenomenon with reversible vasospasm. “Unlike patients with primary Raynaud’s phenomenon, patients will develop ischemia of digits, leading to digital ulcers due to the vasculopathy or vascular remodeling that occurs in scleroderma,” she said.
Current treatments include removal of causative drugs/toxins and warmth, rest, and pain control, although “no trials exist to compare the scleroderma digital ulcer or digital ischemia treatments to each other,” Dr. Griffith said.
Therapy for vasospasm begins with calcium channel blockers such as amlodipine and nifedipine, she said, followed by phosphodiesterase type 5 inhibitors such as sildenafil or endothelin receptor antagonist medications such as bosentan. “If these fail, we use an IV option – epoprostenol. Other options are sympathectomy surgery, Botox, digital nerve blocks, biofeedback, and SSRIs,” she said. “These treatments work fairly well in most patients, but there are patients who break through these therapies and have ongoing digital ischemia, leading to digital ulcers, pain, infections, acro-osteolysis, and auto-amputation. There is definitely room to improve on our current treatment paradigm.”
For the new study, researchers in Italy led by Amelia Spinella, MD, PhD, of University Hospital of Modena, retrospectively tracked 45 patients with systemic sclerosis and at least one digital ulcer (40 women; average age, 53 years) who were treated in 2019. All patients’ ulcers were resistant to opioid therapy at the maximum tolerated dose, and all had undergone periodic iloprost infusion every 30-40 days. Based on each patient’s clinical situation, they had received calcium-channel blockers, phosphodiesterase type 5 inhibitors (sildenafil), and/or endothelin receptor antagonists (bosentan or macitentan). The researchers noted that all patients underwent surgical debridement regularly following wound bed preparation procedures and received advanced dressings (alginate, hydrocolloid, hydrofiber, hydrogel, and polyurethane foam or film). Of the 45 patients, 25 treated their wounds daily over the course of a month by administering four drops of a preparation of 10% CBD oil in acidic form and 90% hemp oil over the wound bed and perilesional skin and then covering it with a nonadhesive cloth.
“Basal wound-related pain NRS [numeric rating scale] scores decreased from 8.4 (standard deviation [SD], 0.8) at the baseline (T0) to 6.0 (SD, 0.82) after 1 month of CBD treatment (T1; P < .0001),” the researchers reported. “Across the same time period, volitional incident pain NRS scores decreased from 9.32 (SD, 0.75; T0) to 6.8 (SD, 1.12; T1; P < .0001). In addition, mean total hours of sleep per night increased from 2.56 (SD, 1.28) to 5.67 (SD, 0.85) hours (P < .0001).” Twelve of the 25 needed additional painkiller therapy.
Complete digital ulcer healing occurred by the end of the study in 18 of 25 (72%) CBD-treated patients, compared with 6 of 20 (30%) control patients.
In contrast, the control group didn’t see any significant improvement in wound-related pain, volitional incident pain, or sleep. All needed additional painkiller therapy. Six developed ulcer infections and received antibiotics.
No significant adverse effects were reported, although 28% of those in the CBD oil group said they had mild effects such as itch and perilesional erythema.
The authors of the new study called for larger, randomized controlled, multicenter trials to confirm the benefit of CBD topical treatment.
In recent years, researchers have devoted more attention to topical CBD as a treatment for skin conditions. While limited, the evidence suggests they “may be effective for the treatment of various inflammatory skin disorders,” researchers wrote in a 2022 report. “Although promising, additional research is necessary to evaluate efficacy and to determine dosing, safety, and long-term treatment guidelines.”
Dr. Griffith, who did not take part in the new study but is familiar with its findings, said she was especially surprised by the hint that topical CBD improves healing in addition to relieving symptoms. “I thought only pain would be affected. This is a great outcome if it can be replicated.”
As for future research, she said “there are difficulties with reproducing this at a big scale in the U.S. given CBD commercial variability. The big issue is the standardization of CBD extraction and production. It is really hard for us as physicians to know what patients are getting. Some online CBD orders contain THC [the major psychoactive ingredient of cannabis] > 0.3% or no CBD at all.”
Still, she said, “physicians and patients may consider this when standard therapies are not working or causing too many adverse effects,” especially since “the downsides here seem fairly minimal – at worst itchiness and redness that did not prevent patients from continuing in the study.”
No details about study funding were provided. The authors and Dr. Griffith report no disclosures.
FROM ADVANCED IN SKIN & WOUND CARE
Best estimates made for hydroxychloroquine retinopathy risk
A new study likely makes the best estimate yet of the degree of retinopathy risk that patients who take the antimalarial drug hydroxychloroquine (HCQ) can expect, deriving mainly from the cumulative dose taken during the first 5 years of use, according to a study published in Annals of Internal Medicine.
HCQ works to decrease activity in a patient’s immune system, which is effective in many cases of systemic lupus erythematosus, one of the most common indications for the drug. However, an adverse outcome of treatment can be HCQ retinopathy, a progressive form of vision loss in patients taking HCQ over an extended period (mostly for longer than 5 years). The disease is often asymptomatic, although some patients do present a paracentral scotoma and a decrease in color vision. Patients may also notice flashing shapes in their vision and find that they have difficulty reading. Eventually, HCQ retinopathy can lead to loss of visual acuity, loss of peripheral vision, and loss of night vision.
Researchers from Kaiser Permanente Northern California and Harvard Medical School analyzed 3,325 persons who received HCQ for 5 or more years between 2004 and 2020. Their goal was to both characterize the long-term risk for incident HCQ retinopathy and examine the degree to which average HCQ dose within the first 5 years of treatment serves as a prediction of the risk.
The researchers then estimated the risk for developing retinopathy after 15 years, according to patients’ average dosing levels during the first 5 years of therapy. Overall, 81 participants developed HCQ retinopathy with overall cumulative incidences of 2.5% after 10 years and 8.6% after 15 years; the risk was greater for those given a higher dose during the first 5 years of treatment.
The mechanism of how HCQ toxicity may occur is still not completely known. There is evidence that toxicity happens because HCQ binds to melanin in both the retinal pigment epithelium and uvea in high concentrations. HCQ can interfere with lysosomal function, leading to oxidation and accumulation of lysosomes, which can cause dysfunction of the retinal pigment epithelium.
Progressive retinopathy can continue even after the drug is stopped. “It’s thought to be a very mild but important risk,” said Nilanjana Bose, MD, MBA, a rheumatologist with Memorial Hermann Health System in Houston. “Patients taking HCQ must be screened for retinal issues, most certainly elderly patients and patients with any kind of comorbidities.”
A 2021 joint position statement from the American College of Rheumatology, American Academy of Dermatology, the Rheumatologic Dermatology Society, and the American Academy of Ophthalmology recommends a baseline eye exam within a few months after starting therapy, then additional screening at 5 years on HCQ and annually thereafter.
“Early detection of retinopathy is important in overall visual prognosis, because toxicity can continue even after discontinuation of the medication,” said Rukhsana G. Mirza, MD, professor of ophthalmology and medical education at Northwestern University in Chicago.
“Examination alone is not sufficient to evaluate early changes, and specialized testing must be done. These include color photos, visual field tests, optical coherence tomography, fundus autofluorescence and in some cases, multifocal electroretinogram. Also, the AAO [American Academy of Ophthalmology] has specific recommendations related to Asian patients as they may have a different pattern of retinopathy that must also be considered.”
More accurate risk measurements
This news organization asked study coauthor April Jorge, MD, assistant professor of medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, Boston, to discuss the study, how it correlates to past research, and what it adds that’s new and useful to rheumatologists and ophthalmologists:
Question: Your research found that a higher dose of HCQ in the first 5 years of treatment led to a greater risk of retinopathy. Is there any indication that a lower dose given more frequently, either within that 5-year period or longer, would pose a similar risk?
Answer: In our study, we assessed the HCQ dose in the first 5 years of use but followed patients who continued the medication longer than 5 years, through up to 15 years of use. Therefore, we compared the risk of HCQ retinopathy associated with different HCQ dosages but for the same duration of use. We found that for any dose of HCQ, the risk of retinopathy increases the longer the medication is used. However, patients who used a higher dose of HCQ had a higher risk of developing retinopathy over time.
Although current guidelines recommend avoiding any HCQ dose over 5 mg/kg per day to reduce the risk of retinopathy, we found a higher risk of retinopathy associated with dosing over 6 mg/kg per day than between 5 and 6 mg/kg per day and the lowest risk with dosing under 5 mg/kg per day.
Q: How does your study align with and/or expand upon previous research regarding HCQ risk?
A: An important prior study of hydroxychloroquine retinopathy was the 2014 study by Ronald B. Melles, MD, and Michael F. Marmor, MD, published in JAMA Ophthalmology. Prior to our present study, that was the largest study to use the modern screening method (optical coherence tomography) to detect HCQ retinopathy. That screening tool is more sensitive than older methods, so it can detect early/mild cases of retinopathy that are typically asymptomatic. Compared to older studies, that 2014 study found a much higher risk of HCQ retinopathy than was previously appreciated.
However, that 2014 study did have some key limitations that could affect the risk estimates, such as using prevalent cases. A key feature of our present study is that we took several important steps to generate more accurate risk estimates. This included using an incident user cohort and detecting incident retinopathy cases through serial review of optical coherence tomography (screening) studies.
To achieve a high degree of methodologic rigor in correctly identifying retinopathy outcomes, we had expert ophthalmologists perform masked adjudication of all screening studies, and we assessed the intra-rater reliability of these study interpretations. Therefore, our study adds to the literature more accurate estimates of retinopathy risk. We found a lower cumulative incidence of retinopathy than was identified in the 2014 study, but the risk is still noteworthy.
Also unique to our study, we graded the severity of HCQ retinopathy outcomes. This was important, as we found that the majority of retinopathy cases detected through routine screening are mild and presumed to be asymptomatic. This will likely be reassuring news for patients that we can screen for this adverse event to detect it early and prevent vision loss.
Another important difference was that we assessed the risk of retinopathy associated with using over 6 mg/kg per day, between 5 and 6 mg/kg per day, and less than 5 mg/kg per day, whereas the highest dosing group assessed in the 2014 study included all patients using over 5 mg/kg per day. The risk was considerably higher in the > 6 mg/kg per day group than in the 5-6 mg/kg per day group.
Q: How can rheumatologists and ophthalmologists use this new information specifically to better treat their patients?
A: Our study provides more accurate estimates of the risk of HCQ retinopathy than in prior studies. These risk estimates can be used when rheumatologists (and other clinicians who prescribe HCQ) consider the risks and benefits of this otherwise important and well-tolerated medication. The risk associated with different dose ranges could also inform dosing decisions, since dosing over 6 mg/kg per day may be more of a concern than using doses in the 5-6 mg/kg range. Ophthalmologists can also use these new risk estimates to counsel patients of the importance of HCQ retinopathy screening and can also hopefully provide some reassurance to patients that the risk of severe retinopathy is low as long as they are being monitored.
The study authors were supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rheumatology Research Foundation. The authors report no relevant financial relationships. Dr. Bose and Dr. Mirza had no relevant disclosures.
A version of this article first appeared on Medscape.com.
A new study likely makes the best estimate yet of the degree of retinopathy risk that patients who take the antimalarial drug hydroxychloroquine (HCQ) can expect, deriving mainly from the cumulative dose taken during the first 5 years of use, according to a study published in Annals of Internal Medicine.
HCQ works to decrease activity in a patient’s immune system, which is effective in many cases of systemic lupus erythematosus, one of the most common indications for the drug. However, an adverse outcome of treatment can be HCQ retinopathy, a progressive form of vision loss in patients taking HCQ over an extended period (mostly for longer than 5 years). The disease is often asymptomatic, although some patients do present a paracentral scotoma and a decrease in color vision. Patients may also notice flashing shapes in their vision and find that they have difficulty reading. Eventually, HCQ retinopathy can lead to loss of visual acuity, loss of peripheral vision, and loss of night vision.
Researchers from Kaiser Permanente Northern California and Harvard Medical School analyzed 3,325 persons who received HCQ for 5 or more years between 2004 and 2020. Their goal was to both characterize the long-term risk for incident HCQ retinopathy and examine the degree to which average HCQ dose within the first 5 years of treatment serves as a prediction of the risk.
The researchers then estimated the risk for developing retinopathy after 15 years, according to patients’ average dosing levels during the first 5 years of therapy. Overall, 81 participants developed HCQ retinopathy with overall cumulative incidences of 2.5% after 10 years and 8.6% after 15 years; the risk was greater for those given a higher dose during the first 5 years of treatment.
The mechanism of how HCQ toxicity may occur is still not completely known. There is evidence that toxicity happens because HCQ binds to melanin in both the retinal pigment epithelium and uvea in high concentrations. HCQ can interfere with lysosomal function, leading to oxidation and accumulation of lysosomes, which can cause dysfunction of the retinal pigment epithelium.
Progressive retinopathy can continue even after the drug is stopped. “It’s thought to be a very mild but important risk,” said Nilanjana Bose, MD, MBA, a rheumatologist with Memorial Hermann Health System in Houston. “Patients taking HCQ must be screened for retinal issues, most certainly elderly patients and patients with any kind of comorbidities.”
A 2021 joint position statement from the American College of Rheumatology, American Academy of Dermatology, the Rheumatologic Dermatology Society, and the American Academy of Ophthalmology recommends a baseline eye exam within a few months after starting therapy, then additional screening at 5 years on HCQ and annually thereafter.
“Early detection of retinopathy is important in overall visual prognosis, because toxicity can continue even after discontinuation of the medication,” said Rukhsana G. Mirza, MD, professor of ophthalmology and medical education at Northwestern University in Chicago.
“Examination alone is not sufficient to evaluate early changes, and specialized testing must be done. These include color photos, visual field tests, optical coherence tomography, fundus autofluorescence and in some cases, multifocal electroretinogram. Also, the AAO [American Academy of Ophthalmology] has specific recommendations related to Asian patients as they may have a different pattern of retinopathy that must also be considered.”
More accurate risk measurements
This news organization asked study coauthor April Jorge, MD, assistant professor of medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, Boston, to discuss the study, how it correlates to past research, and what it adds that’s new and useful to rheumatologists and ophthalmologists:
Question: Your research found that a higher dose of HCQ in the first 5 years of treatment led to a greater risk of retinopathy. Is there any indication that a lower dose given more frequently, either within that 5-year period or longer, would pose a similar risk?
Answer: In our study, we assessed the HCQ dose in the first 5 years of use but followed patients who continued the medication longer than 5 years, through up to 15 years of use. Therefore, we compared the risk of HCQ retinopathy associated with different HCQ dosages but for the same duration of use. We found that for any dose of HCQ, the risk of retinopathy increases the longer the medication is used. However, patients who used a higher dose of HCQ had a higher risk of developing retinopathy over time.
Although current guidelines recommend avoiding any HCQ dose over 5 mg/kg per day to reduce the risk of retinopathy, we found a higher risk of retinopathy associated with dosing over 6 mg/kg per day than between 5 and 6 mg/kg per day and the lowest risk with dosing under 5 mg/kg per day.
Q: How does your study align with and/or expand upon previous research regarding HCQ risk?
A: An important prior study of hydroxychloroquine retinopathy was the 2014 study by Ronald B. Melles, MD, and Michael F. Marmor, MD, published in JAMA Ophthalmology. Prior to our present study, that was the largest study to use the modern screening method (optical coherence tomography) to detect HCQ retinopathy. That screening tool is more sensitive than older methods, so it can detect early/mild cases of retinopathy that are typically asymptomatic. Compared to older studies, that 2014 study found a much higher risk of HCQ retinopathy than was previously appreciated.
However, that 2014 study did have some key limitations that could affect the risk estimates, such as using prevalent cases. A key feature of our present study is that we took several important steps to generate more accurate risk estimates. This included using an incident user cohort and detecting incident retinopathy cases through serial review of optical coherence tomography (screening) studies.
To achieve a high degree of methodologic rigor in correctly identifying retinopathy outcomes, we had expert ophthalmologists perform masked adjudication of all screening studies, and we assessed the intra-rater reliability of these study interpretations. Therefore, our study adds to the literature more accurate estimates of retinopathy risk. We found a lower cumulative incidence of retinopathy than was identified in the 2014 study, but the risk is still noteworthy.
Also unique to our study, we graded the severity of HCQ retinopathy outcomes. This was important, as we found that the majority of retinopathy cases detected through routine screening are mild and presumed to be asymptomatic. This will likely be reassuring news for patients that we can screen for this adverse event to detect it early and prevent vision loss.
Another important difference was that we assessed the risk of retinopathy associated with using over 6 mg/kg per day, between 5 and 6 mg/kg per day, and less than 5 mg/kg per day, whereas the highest dosing group assessed in the 2014 study included all patients using over 5 mg/kg per day. The risk was considerably higher in the > 6 mg/kg per day group than in the 5-6 mg/kg per day group.
Q: How can rheumatologists and ophthalmologists use this new information specifically to better treat their patients?
A: Our study provides more accurate estimates of the risk of HCQ retinopathy than in prior studies. These risk estimates can be used when rheumatologists (and other clinicians who prescribe HCQ) consider the risks and benefits of this otherwise important and well-tolerated medication. The risk associated with different dose ranges could also inform dosing decisions, since dosing over 6 mg/kg per day may be more of a concern than using doses in the 5-6 mg/kg range. Ophthalmologists can also use these new risk estimates to counsel patients of the importance of HCQ retinopathy screening and can also hopefully provide some reassurance to patients that the risk of severe retinopathy is low as long as they are being monitored.
The study authors were supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rheumatology Research Foundation. The authors report no relevant financial relationships. Dr. Bose and Dr. Mirza had no relevant disclosures.
A version of this article first appeared on Medscape.com.
A new study likely makes the best estimate yet of the degree of retinopathy risk that patients who take the antimalarial drug hydroxychloroquine (HCQ) can expect, deriving mainly from the cumulative dose taken during the first 5 years of use, according to a study published in Annals of Internal Medicine.
HCQ works to decrease activity in a patient’s immune system, which is effective in many cases of systemic lupus erythematosus, one of the most common indications for the drug. However, an adverse outcome of treatment can be HCQ retinopathy, a progressive form of vision loss in patients taking HCQ over an extended period (mostly for longer than 5 years). The disease is often asymptomatic, although some patients do present a paracentral scotoma and a decrease in color vision. Patients may also notice flashing shapes in their vision and find that they have difficulty reading. Eventually, HCQ retinopathy can lead to loss of visual acuity, loss of peripheral vision, and loss of night vision.
Researchers from Kaiser Permanente Northern California and Harvard Medical School analyzed 3,325 persons who received HCQ for 5 or more years between 2004 and 2020. Their goal was to both characterize the long-term risk for incident HCQ retinopathy and examine the degree to which average HCQ dose within the first 5 years of treatment serves as a prediction of the risk.
The researchers then estimated the risk for developing retinopathy after 15 years, according to patients’ average dosing levels during the first 5 years of therapy. Overall, 81 participants developed HCQ retinopathy with overall cumulative incidences of 2.5% after 10 years and 8.6% after 15 years; the risk was greater for those given a higher dose during the first 5 years of treatment.
The mechanism of how HCQ toxicity may occur is still not completely known. There is evidence that toxicity happens because HCQ binds to melanin in both the retinal pigment epithelium and uvea in high concentrations. HCQ can interfere with lysosomal function, leading to oxidation and accumulation of lysosomes, which can cause dysfunction of the retinal pigment epithelium.
Progressive retinopathy can continue even after the drug is stopped. “It’s thought to be a very mild but important risk,” said Nilanjana Bose, MD, MBA, a rheumatologist with Memorial Hermann Health System in Houston. “Patients taking HCQ must be screened for retinal issues, most certainly elderly patients and patients with any kind of comorbidities.”
A 2021 joint position statement from the American College of Rheumatology, American Academy of Dermatology, the Rheumatologic Dermatology Society, and the American Academy of Ophthalmology recommends a baseline eye exam within a few months after starting therapy, then additional screening at 5 years on HCQ and annually thereafter.
“Early detection of retinopathy is important in overall visual prognosis, because toxicity can continue even after discontinuation of the medication,” said Rukhsana G. Mirza, MD, professor of ophthalmology and medical education at Northwestern University in Chicago.
“Examination alone is not sufficient to evaluate early changes, and specialized testing must be done. These include color photos, visual field tests, optical coherence tomography, fundus autofluorescence and in some cases, multifocal electroretinogram. Also, the AAO [American Academy of Ophthalmology] has specific recommendations related to Asian patients as they may have a different pattern of retinopathy that must also be considered.”
More accurate risk measurements
This news organization asked study coauthor April Jorge, MD, assistant professor of medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, Boston, to discuss the study, how it correlates to past research, and what it adds that’s new and useful to rheumatologists and ophthalmologists:
Question: Your research found that a higher dose of HCQ in the first 5 years of treatment led to a greater risk of retinopathy. Is there any indication that a lower dose given more frequently, either within that 5-year period or longer, would pose a similar risk?
Answer: In our study, we assessed the HCQ dose in the first 5 years of use but followed patients who continued the medication longer than 5 years, through up to 15 years of use. Therefore, we compared the risk of HCQ retinopathy associated with different HCQ dosages but for the same duration of use. We found that for any dose of HCQ, the risk of retinopathy increases the longer the medication is used. However, patients who used a higher dose of HCQ had a higher risk of developing retinopathy over time.
Although current guidelines recommend avoiding any HCQ dose over 5 mg/kg per day to reduce the risk of retinopathy, we found a higher risk of retinopathy associated with dosing over 6 mg/kg per day than between 5 and 6 mg/kg per day and the lowest risk with dosing under 5 mg/kg per day.
Q: How does your study align with and/or expand upon previous research regarding HCQ risk?
A: An important prior study of hydroxychloroquine retinopathy was the 2014 study by Ronald B. Melles, MD, and Michael F. Marmor, MD, published in JAMA Ophthalmology. Prior to our present study, that was the largest study to use the modern screening method (optical coherence tomography) to detect HCQ retinopathy. That screening tool is more sensitive than older methods, so it can detect early/mild cases of retinopathy that are typically asymptomatic. Compared to older studies, that 2014 study found a much higher risk of HCQ retinopathy than was previously appreciated.
However, that 2014 study did have some key limitations that could affect the risk estimates, such as using prevalent cases. A key feature of our present study is that we took several important steps to generate more accurate risk estimates. This included using an incident user cohort and detecting incident retinopathy cases through serial review of optical coherence tomography (screening) studies.
To achieve a high degree of methodologic rigor in correctly identifying retinopathy outcomes, we had expert ophthalmologists perform masked adjudication of all screening studies, and we assessed the intra-rater reliability of these study interpretations. Therefore, our study adds to the literature more accurate estimates of retinopathy risk. We found a lower cumulative incidence of retinopathy than was identified in the 2014 study, but the risk is still noteworthy.
Also unique to our study, we graded the severity of HCQ retinopathy outcomes. This was important, as we found that the majority of retinopathy cases detected through routine screening are mild and presumed to be asymptomatic. This will likely be reassuring news for patients that we can screen for this adverse event to detect it early and prevent vision loss.
Another important difference was that we assessed the risk of retinopathy associated with using over 6 mg/kg per day, between 5 and 6 mg/kg per day, and less than 5 mg/kg per day, whereas the highest dosing group assessed in the 2014 study included all patients using over 5 mg/kg per day. The risk was considerably higher in the > 6 mg/kg per day group than in the 5-6 mg/kg per day group.
Q: How can rheumatologists and ophthalmologists use this new information specifically to better treat their patients?
A: Our study provides more accurate estimates of the risk of HCQ retinopathy than in prior studies. These risk estimates can be used when rheumatologists (and other clinicians who prescribe HCQ) consider the risks and benefits of this otherwise important and well-tolerated medication. The risk associated with different dose ranges could also inform dosing decisions, since dosing over 6 mg/kg per day may be more of a concern than using doses in the 5-6 mg/kg range. Ophthalmologists can also use these new risk estimates to counsel patients of the importance of HCQ retinopathy screening and can also hopefully provide some reassurance to patients that the risk of severe retinopathy is low as long as they are being monitored.
The study authors were supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rheumatology Research Foundation. The authors report no relevant financial relationships. Dr. Bose and Dr. Mirza had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Belimumab for pregnant women with lupus: B-cell concerns remain
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
FROM ANNALS OF THE RHEUMATIC DISEASES
Rituximab Treatment and Improvement of Health-Related Quality of Life in Patients With Pemphigus
Pemphigus is a group of autoimmune blistering diseases characterized by the development of painful and flaccid blisters on the skin and/or mucous membranes. Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are 2 major subtypes and can be distinguished by the location of blister formation or the specificity of autoantibodies directed against different desmogleins.1,2 Although rare, pemphigus is considered a serious and life-threatening condition with a great impact on quality of life (QOL) due to disease symptoms (eg, painful lesions, physical appearance of skin lesions) as well as treatment complications (eg, adverse drug effects, cost of treatment).3-6 Moreover, the physical and psychological effects can lead to marked functional morbidity and work-related disability during patients’ productive years.7 Therefore, affected individuals usually have a remarkably compromised health-related quality of life (HRQOL).8 Effective treatments may considerably improve the QOL of patients with pemphigus.6
Despite the available treatment options, finding the best regimen for pemphigus remains a challenge. Corticosteroids are assumed to be the main treatment, though they have considerable side effects.9,10 Adjuvant therapies are used to suppress or modulate immune responses, leading to remission with the least possible need for corticosteroids. Finding an optimal steroid-sparing agent has been the aim of research, and biologic agents seem to be the best option.8 Rituximab (RTX), an anti-CD20 monoclonal antibody, has shown great promise in several studies of its clinical efficacy and has become a first-line treatment in new guidelines.11-14 Rituximab treatment has been associated with notable improvement in physician-assessed outcome measures with a favorable safety profile in patients with pemphigus.11-15 However, it is important to assess response to treatment from a patient’s perspective through the use of outcome-assessment measures that encompass patient-reported outcomes to reflect the complete patient experience and establish the overall impact of RTX as well as its likelihood of acceptance by patients with pemphigus.
In our study, we compared clinical outcomes and HRQOL through the use of disease-specific measures as well as comprehensive generic health status measures among patients with PV and PF who received RTX treatment 3 months earlier and those who received RTX in the last 2 weeks. The clinical relevance of the patient-reported outcomes is discussed.
MATERIALS AND METHODS
Study Design
We conducted a single-center cross-sectional study of 96 patients with pemphigus aged 18 to 65 years of either sex who were willing to participate in this study. Patients with a confirmed diagnosis of PV or PF who received RTX 3 months earlier or in the last 2 weeks were enrolled in the study. Patients were identified using Dermatry.ir, an archiving software that contains patients’ medical data. Exclusion criteria included lack of sufficient knowledge of the concepts of the questionnaires as well as age younger than 16 years. The study was conducted from October 2019 to April 2020 by the Autoimmune Bullous Disease Research Center at Razi Hospital in Tehran, Iran, which is the main dermatology-specific center and teaching hospital of Iran. The study protocol was approved by the relevant ethics committee.
Patients were categorized into 2 groups: (1) those who received RTX 3 months earlier (3M group); and (2) those who received RTX in the last 2 weeks (R group).
After an explanation of the study to participants, informed written consent was signed by each patient, and their personal data (eg, age, sex, education, marital status, smoking status), as well as clinical data (eg, type of pemphigus, duration of disease, site of onset, prednisolone dosage, presence of Nikolsky sign, anti-DSG1 and anti-DSG3 values, Pemphigus Disease Area Index [PDAI] score, RTX treatment protocol); any known comorbidities such as hypertension, diabetes mellitus, or morbid obesity; and any chronic pulmonary, cardiac, endocrinologic, renal, or hepatic condition, were collected and recorded in a predefined Case Record.
Patient-Reported Outcome Measures
The effect of RTX on QOL in patients with pemphigus was assessed using 2 HRQOL instruments: (1) a general health status indicator, the 36-Item Short Form Survey (SF-36), and (2) a validated, Persian version of a dermatology-specific questionnaire, Dermatology Life Quality Index (DLQI). The questionnaires were completed by each patient or by an assistant if needed.
The SF-36 is a widely used 36-item questionnaire measuring functional health and well-being across 8 domains—mental health, pain, physical function, role emotional, role physical, social functioning, vitality, and general health perception—with scores for each ranging from 0 to 100. The physical component scores (PCSs) and mental component scores (MCSs) were derived from these 8 subscales, each ranging from 0 to 400, with higher scores indicating better health status.6
The DLQI, one of the most frequently used QOL measures in dermatology, contains 10 questions, each referring to the prior week and classified in the following 6 subscales: symptoms and feelings, daily activities, leisure, personal relationships, work and school, and treatment.16 The total score ranges from 0 (no impact) to 30 (very high impact), with a higher score indicating a lower QOL (eTable 1). The minimal clinically important difference (MCD) for the DLQI was considered to be 2- to 5-point changes in prior studies.17,18 In this study, we used an MCD of a 5-point change or more between study groups.
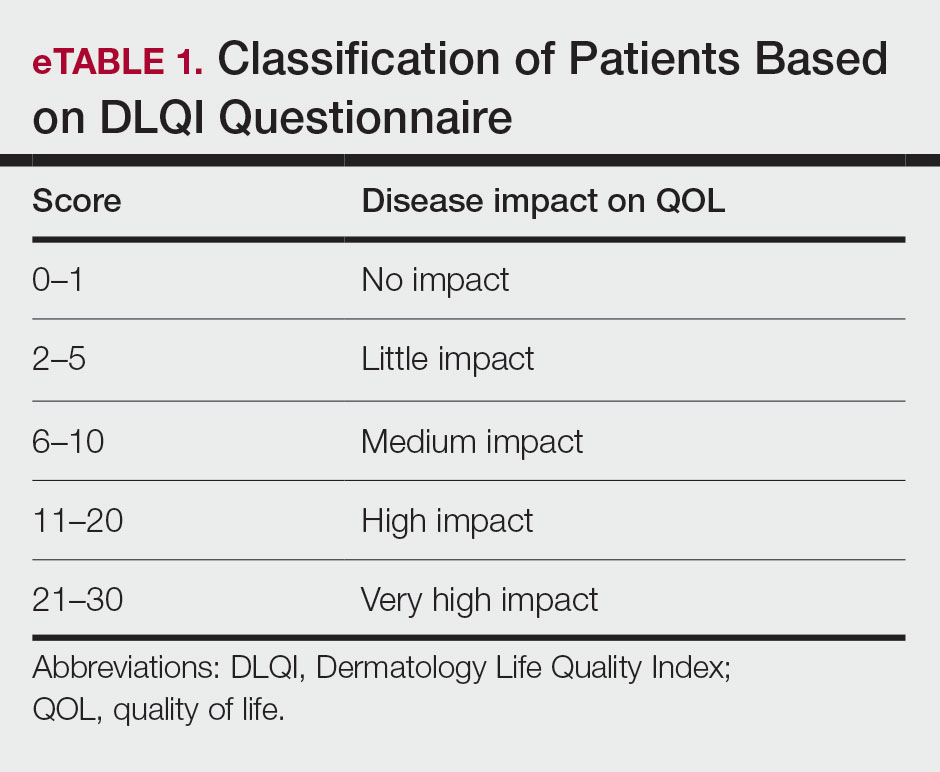
Moreover, the patient general assessment (PGA) of disease severity was identified using a 3-point scale (1=mild, 2=moderate, 3=severe).
Statistical Analysis
Data were analyzed using SPSS Statistics version 23. P≤.05 was considered significant. Mean and SD were calculated for descriptive data. The t test, Fisher exact test, analysis of variance, multiple regression analysis, and logistic regression analysis were used to identify the relationship between variables.
RESULTS
Patient Characteristics
A total of 96 patients were enrolled in this study. The mean (SD) age of participants was 41.42 (15.1) years (range, 18–58 years). Of 96 patients whose data were included, 55 (57.29%) patients had received RTX 3 months earlier (3M group) and 41 (42.71%) received RTX in the last 2 weeks (R group). A summary of study patient characteristics in each group is provided in eTable 2. There was no significant difference between the 2 groups in terms of age, sex, type of pemphigus, marital status, education, positive Nikolsky sign, smoking status, existence of comorbidities, site of lesions, and RTX treatment protocol. However, a significant difference was found for duration of disease (P=.0124) and mean prednisolone dosage (P=.001) as well as severity of disease measured by PDAI score (P=.003) and anti-DSG1 (P=.003) and anti-DSG3 (P=.021) values.
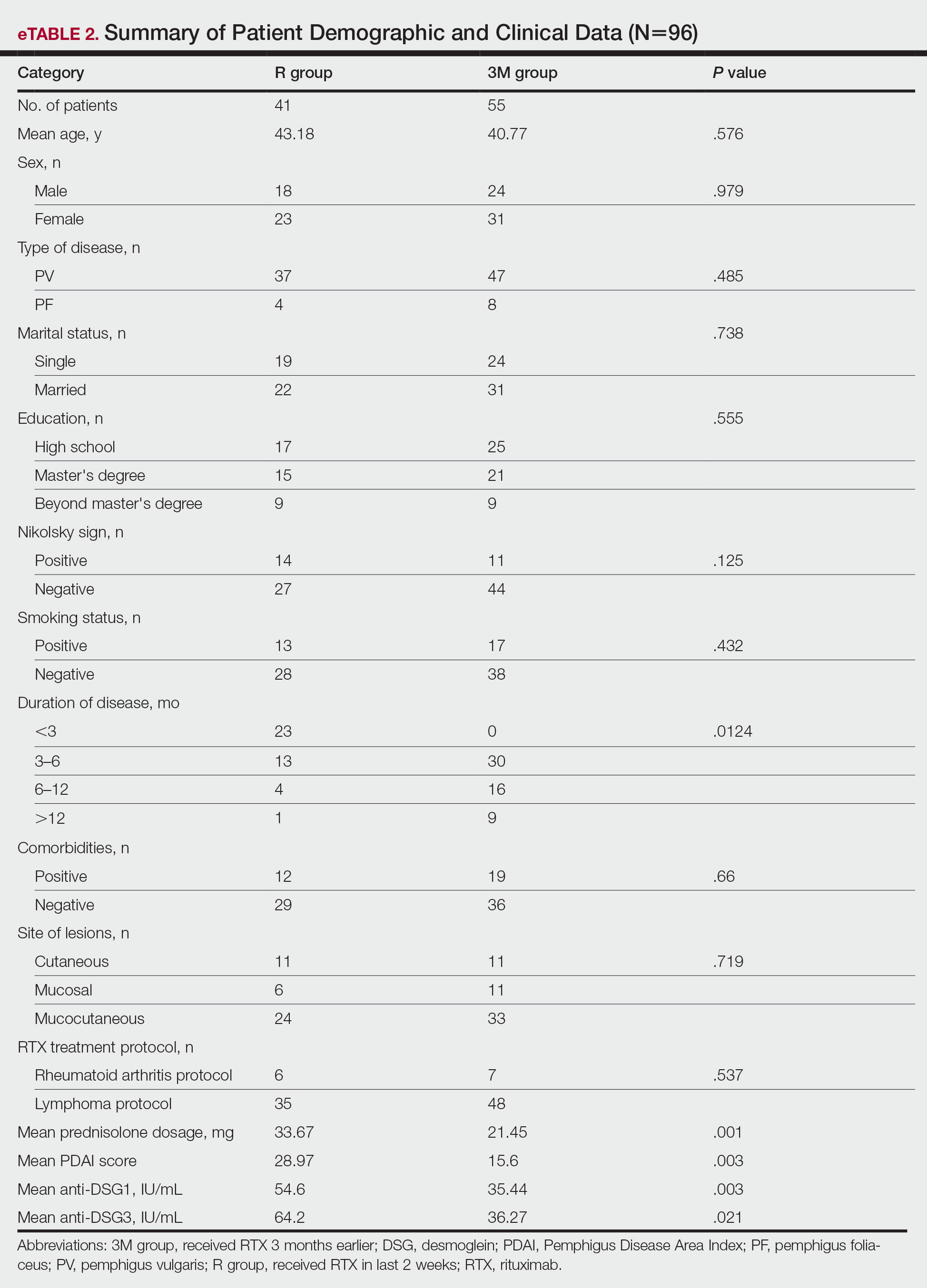
Patient-Reported Outcomes
Physical and mental component scores are summarized in eTable 3. Generally, SF-36 scores were improved with RTX treatment in all dimensions except for mental health, though these differences were not statistically significant, with the greatest mean improvement in the role physical index (75.45 in the 3M group vs 53.04 in the R group; P=.009). Mean SF-36 PCS and MCS scores were higher in the 3M group vs the R group, though the difference in MCS score did not reach the level of significance (eTable 3).
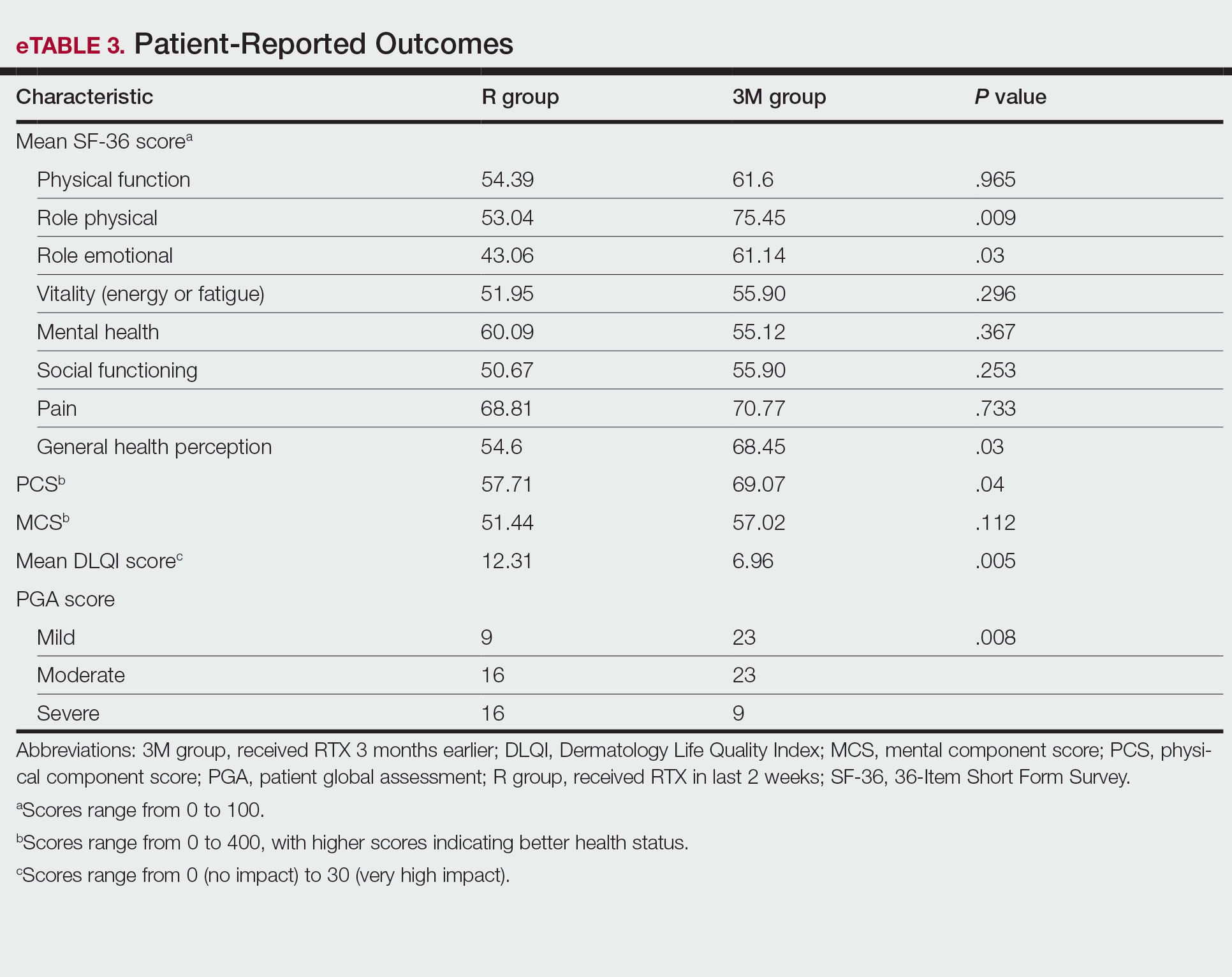
Mean DLQI scores in the R and 3M groups were 12.31 and 6.96, respectively, indicating a considerable burden on HRQOL in both groups. However, a statistically significant difference between these values was seen that also was clinically meaningful, indicating a significant improvement of QOL in patients receiving RTX 3 months earlier (P=.005)(eTable 3).
The PGA scores indicated that patients in the 3M group were significantly more likely to report less severe disease vs the R group (P=.008)(eTable 3).
Multivariate Analysis—Effect of the patient characteristics and some disease features on indices of QOL was evaluated using the multiple linear regression model. eTable 4 shows the P values of those analyses.
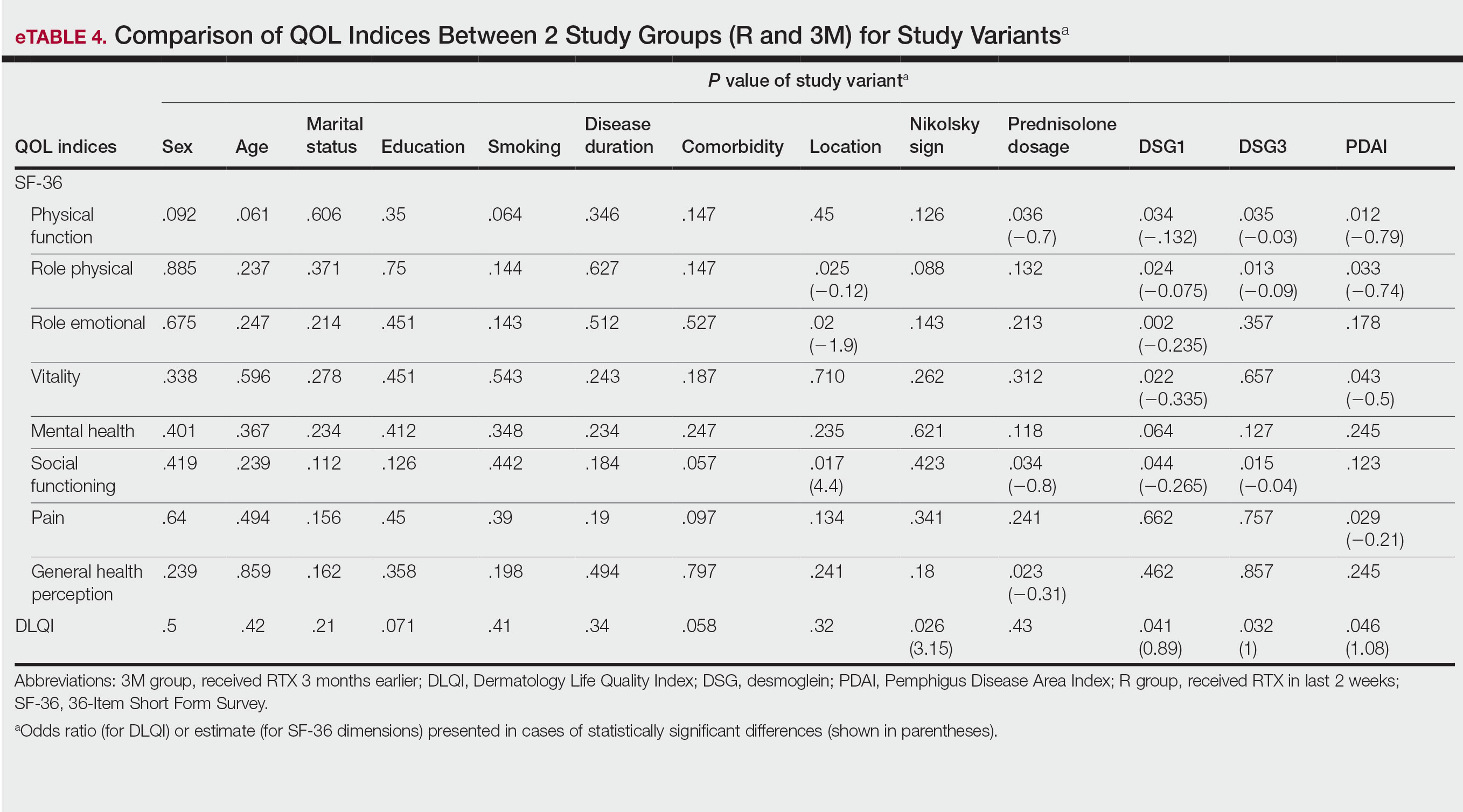
COMMENT
Pemphigus is a chronic disabling disease with notable QOL impairment due to disease burden as well as the need for long-term use of immunosuppressive agents during the disease course. To study the effect of RTX on QOL of patients with pemphigus, we compared 2 sets of patients. Prior studies have shown that clinically significant effects of RTX take 4 to 12 weeks to appear.19,20 Therefore, we selected patients who received RTX 3 months earlier to measure their HRQOL indices and compare them with patients who had received RTX in the last 2 weeks as a control group to investigate the effect of RTX intrinsically, as this was the focus of this study.
In our study, one of the research tools was the DLQI. Healthy patients typically have an average score of 0.5.21 The mean DLQI score of the patients in R group was 12.31, which was similar to prior analysis8 and reflects a substantial burden of disease comparable to atopic dermatitis and psoriasis.21,22 In patients in the 3M group, the mean DLQI score was lower than the R group (6.96 vs 12.31), indicating a significant (P=.005) and clinically meaningful improvement in QOL of patients due to the dramatic therapeutic effect of RTX. However, this score indicated a moderate effect on HRQOL, even in the context of clinical improvement due to RTX treatment, which may reflect that the short duration of treatment in the 3M group was a limitation of this study. Although the 12-week treatment duration was comparable with other studies19,20 and major differences in objective measures of treatment efficacy were found in PDAI as well as anti-DSG1 and anti-DSG3 values, longer treatment duration may be needed for a more comprehensive assessment of the benefit of RTX on HRQOL indices in patients with pemphigus.
Based on results of the SF-36 questionnaire, PCS and MCS scores were not substantially impaired in the R group considering the fact that a mean score of 50 has been articulated as a normative value for all scales.23 These data demonstrated the importance of using a dermatologic-specific instrument such as the DLQI instead of a general questionnaire to assess QOL in patients with pemphigus. However, better indices were reported with RTX treatment in the 3 SF-36 domains—role physical (P=.009), role emotional (P=.03), and general health perception (P=.03)—with the role physical showing the greatest magnitude of mean change (75.45 in the 3M group vs 53.04 in the R group). Notably, PCS was impaired to a greater extent than MCS in patients in the R group and showed a greater magnitude of improvement after 3 months of treatment. These results could be explained by the fact that MCS can be largely changed in diseases with a direct effect on the central nervous system.23
Our results also revealed that the dose of corticosteroid correlated to HRQOL of patients with pemphigus who recently received RTX therapy. Indeed, it is more likely that patients on lower-dose prednisolone have a higher QOL, especially on physical function and social function dimensions of SF-36. This finding is highly expectable by less severe disease due to RTX treatment and also lower potential dose-dependent adverse effects of long-term steroid therapy.
One of the most striking findings of this study was the correlation of location of lesions to QOL indices. We found that the mucocutaneous phenotype was significantly correlated to greater improvement in role emotional, role physical, and social functioning scores due to RTX treatment compared with cutaneous or mucosal types (P=.02, P=.025, and P=.017, respectively). Although mucosal involvement of the disease can be the most burdensome feature because of its large impact on essential activities such as eating and speaking, cutaneous lesions with unpleasant appearance and undesirable symptoms may have a similar impact on QOL. Therefore, having both mucosal and cutaneous lesions causes a worsened QOL and decreased treatment efficacy vs having only one area involved. This may explain the greater improvement in some QOL indices with RTX treatment.
Limitations—Given the cross-sectional design of this study in which patients were observed at a single time point during their treatment course, it is not possible to establish a clear cause-effect relationship between variables. Moreover, we did not evaluate the impact of RTX or prednisolone adverse effects on QOL. Therefore, further prospective studies with longer treatment durations may help to validate our findings. In addition, MCDs for DLQI and SF-36 in pemphigus need to be determined and validated in future studies.
CONCLUSION
The results of our study demonstrated that patients with pemphigus may benefit from taking RTX, not only in terms of clinical improvement of their disease measured by objective indices such as PDAI and anti-DSG1 and anti-DSG3 values but also in several domains that are important to patients, including physical and mental health status (SF-36), HRQOL (DLQI), and overall disease severity (PGA). Rituximab administration in patients with pemphigus can lead to rapid and significant improvement in HRQOL as well as patient- and physician-assessed measures. Its favorable safety profile along with its impact on patients’ daily lives and mental health makes RTX a suitable treatment option for patients with pemphigus. Moreover, we recommend taking QOL indices into account while evaluating the efficacy of new medications to improve our insight into the patient experience and provide better patient adherence to treatment, which is an important issue for optimal control of chronic disorders.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:175-197.
- Kasperkiewicz M, Ellebrecht CT, Takahashi H, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris, result from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005;3:431-435.
- Terrab Z, Benckikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034.
- Paradisi A, Sampogna F, Di Pietro, C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269.
- Heelan K, Hitzig SL, Knowles S, et al. Loss of work productivity and quality of life in patients with autoimmune bullous dermatoses. J Cutan Med Surg. 2015;19:546-554.
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J Dermatol. 2012;39:141-144.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:2343.
- Mohammad-Javad N, Parvaneh H, Maryam G, et al. Randomized trial of tacrolimus 0.1% ointment versus triamcinolone acetonide 0.1% paste in the treatment of oral pemphigus vulgaris. Iranian J Dermatol. 2012;15:42-46.
- Lunardon L, Tsai KJ, Propert KJ, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031-1036.
- Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30.
- Heelan K, Al-Mohammedi F, Smith MJ, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040
- Aryanian Z, Balighi K, Daneshpazhooh M, et al. Rituximab exhibits a better safety profile when used as a first line of treatment for pemphigus vulgaris: a retrospective study. Int Immunopharmacol. 2021;96:107755.
- Aghai S, Sodaifi M, Jafari P, et al. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8.
- Schünemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: the clinician’s perspective. Health Qual Life Outcomes. 2006;4:62.
- Quality of life questionnaires. Cardiff University website. Accessed December 16, 2022. http://sites.cardiff.ac.uk/dermatology/quality-oflife/dermatology-quality-of-life-index-dlqi/dlqi-instructions-foruse-and-scoring/
- Kanwar AJ, Tsuruta D, Vinay K, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27:E17-E23.
- Ingen-Housz-Oro S, Valeyrie-Allanore L, Cosnes A, et al. First-line treatment of pemphigus vulgaris with a combination of rituximab and high-potency topical corticosteroids. JAMA Dermatol. 2015;151:200-203.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Aghaei S, Moradi A, Ardekani GS. Impact of psoriasis on quality of life in Iran. Indian J Dermatol Venereol Leprol. 2009;75:220.
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). 1. conceptual framework and item selection. Med Care. 1992;30:473-483.
Pemphigus is a group of autoimmune blistering diseases characterized by the development of painful and flaccid blisters on the skin and/or mucous membranes. Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are 2 major subtypes and can be distinguished by the location of blister formation or the specificity of autoantibodies directed against different desmogleins.1,2 Although rare, pemphigus is considered a serious and life-threatening condition with a great impact on quality of life (QOL) due to disease symptoms (eg, painful lesions, physical appearance of skin lesions) as well as treatment complications (eg, adverse drug effects, cost of treatment).3-6 Moreover, the physical and psychological effects can lead to marked functional morbidity and work-related disability during patients’ productive years.7 Therefore, affected individuals usually have a remarkably compromised health-related quality of life (HRQOL).8 Effective treatments may considerably improve the QOL of patients with pemphigus.6
Despite the available treatment options, finding the best regimen for pemphigus remains a challenge. Corticosteroids are assumed to be the main treatment, though they have considerable side effects.9,10 Adjuvant therapies are used to suppress or modulate immune responses, leading to remission with the least possible need for corticosteroids. Finding an optimal steroid-sparing agent has been the aim of research, and biologic agents seem to be the best option.8 Rituximab (RTX), an anti-CD20 monoclonal antibody, has shown great promise in several studies of its clinical efficacy and has become a first-line treatment in new guidelines.11-14 Rituximab treatment has been associated with notable improvement in physician-assessed outcome measures with a favorable safety profile in patients with pemphigus.11-15 However, it is important to assess response to treatment from a patient’s perspective through the use of outcome-assessment measures that encompass patient-reported outcomes to reflect the complete patient experience and establish the overall impact of RTX as well as its likelihood of acceptance by patients with pemphigus.
In our study, we compared clinical outcomes and HRQOL through the use of disease-specific measures as well as comprehensive generic health status measures among patients with PV and PF who received RTX treatment 3 months earlier and those who received RTX in the last 2 weeks. The clinical relevance of the patient-reported outcomes is discussed.
MATERIALS AND METHODS
Study Design
We conducted a single-center cross-sectional study of 96 patients with pemphigus aged 18 to 65 years of either sex who were willing to participate in this study. Patients with a confirmed diagnosis of PV or PF who received RTX 3 months earlier or in the last 2 weeks were enrolled in the study. Patients were identified using Dermatry.ir, an archiving software that contains patients’ medical data. Exclusion criteria included lack of sufficient knowledge of the concepts of the questionnaires as well as age younger than 16 years. The study was conducted from October 2019 to April 2020 by the Autoimmune Bullous Disease Research Center at Razi Hospital in Tehran, Iran, which is the main dermatology-specific center and teaching hospital of Iran. The study protocol was approved by the relevant ethics committee.
Patients were categorized into 2 groups: (1) those who received RTX 3 months earlier (3M group); and (2) those who received RTX in the last 2 weeks (R group).
After an explanation of the study to participants, informed written consent was signed by each patient, and their personal data (eg, age, sex, education, marital status, smoking status), as well as clinical data (eg, type of pemphigus, duration of disease, site of onset, prednisolone dosage, presence of Nikolsky sign, anti-DSG1 and anti-DSG3 values, Pemphigus Disease Area Index [PDAI] score, RTX treatment protocol); any known comorbidities such as hypertension, diabetes mellitus, or morbid obesity; and any chronic pulmonary, cardiac, endocrinologic, renal, or hepatic condition, were collected and recorded in a predefined Case Record.
Patient-Reported Outcome Measures
The effect of RTX on QOL in patients with pemphigus was assessed using 2 HRQOL instruments: (1) a general health status indicator, the 36-Item Short Form Survey (SF-36), and (2) a validated, Persian version of a dermatology-specific questionnaire, Dermatology Life Quality Index (DLQI). The questionnaires were completed by each patient or by an assistant if needed.
The SF-36 is a widely used 36-item questionnaire measuring functional health and well-being across 8 domains—mental health, pain, physical function, role emotional, role physical, social functioning, vitality, and general health perception—with scores for each ranging from 0 to 100. The physical component scores (PCSs) and mental component scores (MCSs) were derived from these 8 subscales, each ranging from 0 to 400, with higher scores indicating better health status.6
The DLQI, one of the most frequently used QOL measures in dermatology, contains 10 questions, each referring to the prior week and classified in the following 6 subscales: symptoms and feelings, daily activities, leisure, personal relationships, work and school, and treatment.16 The total score ranges from 0 (no impact) to 30 (very high impact), with a higher score indicating a lower QOL (eTable 1). The minimal clinically important difference (MCD) for the DLQI was considered to be 2- to 5-point changes in prior studies.17,18 In this study, we used an MCD of a 5-point change or more between study groups.

Moreover, the patient general assessment (PGA) of disease severity was identified using a 3-point scale (1=mild, 2=moderate, 3=severe).
Statistical Analysis
Data were analyzed using SPSS Statistics version 23. P≤.05 was considered significant. Mean and SD were calculated for descriptive data. The t test, Fisher exact test, analysis of variance, multiple regression analysis, and logistic regression analysis were used to identify the relationship between variables.
RESULTS
Patient Characteristics
A total of 96 patients were enrolled in this study. The mean (SD) age of participants was 41.42 (15.1) years (range, 18–58 years). Of 96 patients whose data were included, 55 (57.29%) patients had received RTX 3 months earlier (3M group) and 41 (42.71%) received RTX in the last 2 weeks (R group). A summary of study patient characteristics in each group is provided in eTable 2. There was no significant difference between the 2 groups in terms of age, sex, type of pemphigus, marital status, education, positive Nikolsky sign, smoking status, existence of comorbidities, site of lesions, and RTX treatment protocol. However, a significant difference was found for duration of disease (P=.0124) and mean prednisolone dosage (P=.001) as well as severity of disease measured by PDAI score (P=.003) and anti-DSG1 (P=.003) and anti-DSG3 (P=.021) values.

Patient-Reported Outcomes
Physical and mental component scores are summarized in eTable 3. Generally, SF-36 scores were improved with RTX treatment in all dimensions except for mental health, though these differences were not statistically significant, with the greatest mean improvement in the role physical index (75.45 in the 3M group vs 53.04 in the R group; P=.009). Mean SF-36 PCS and MCS scores were higher in the 3M group vs the R group, though the difference in MCS score did not reach the level of significance (eTable 3).

Mean DLQI scores in the R and 3M groups were 12.31 and 6.96, respectively, indicating a considerable burden on HRQOL in both groups. However, a statistically significant difference between these values was seen that also was clinically meaningful, indicating a significant improvement of QOL in patients receiving RTX 3 months earlier (P=.005)(eTable 3).
The PGA scores indicated that patients in the 3M group were significantly more likely to report less severe disease vs the R group (P=.008)(eTable 3).
Multivariate Analysis—Effect of the patient characteristics and some disease features on indices of QOL was evaluated using the multiple linear regression model. eTable 4 shows the P values of those analyses.

COMMENT
Pemphigus is a chronic disabling disease with notable QOL impairment due to disease burden as well as the need for long-term use of immunosuppressive agents during the disease course. To study the effect of RTX on QOL of patients with pemphigus, we compared 2 sets of patients. Prior studies have shown that clinically significant effects of RTX take 4 to 12 weeks to appear.19,20 Therefore, we selected patients who received RTX 3 months earlier to measure their HRQOL indices and compare them with patients who had received RTX in the last 2 weeks as a control group to investigate the effect of RTX intrinsically, as this was the focus of this study.
In our study, one of the research tools was the DLQI. Healthy patients typically have an average score of 0.5.21 The mean DLQI score of the patients in R group was 12.31, which was similar to prior analysis8 and reflects a substantial burden of disease comparable to atopic dermatitis and psoriasis.21,22 In patients in the 3M group, the mean DLQI score was lower than the R group (6.96 vs 12.31), indicating a significant (P=.005) and clinically meaningful improvement in QOL of patients due to the dramatic therapeutic effect of RTX. However, this score indicated a moderate effect on HRQOL, even in the context of clinical improvement due to RTX treatment, which may reflect that the short duration of treatment in the 3M group was a limitation of this study. Although the 12-week treatment duration was comparable with other studies19,20 and major differences in objective measures of treatment efficacy were found in PDAI as well as anti-DSG1 and anti-DSG3 values, longer treatment duration may be needed for a more comprehensive assessment of the benefit of RTX on HRQOL indices in patients with pemphigus.
Based on results of the SF-36 questionnaire, PCS and MCS scores were not substantially impaired in the R group considering the fact that a mean score of 50 has been articulated as a normative value for all scales.23 These data demonstrated the importance of using a dermatologic-specific instrument such as the DLQI instead of a general questionnaire to assess QOL in patients with pemphigus. However, better indices were reported with RTX treatment in the 3 SF-36 domains—role physical (P=.009), role emotional (P=.03), and general health perception (P=.03)—with the role physical showing the greatest magnitude of mean change (75.45 in the 3M group vs 53.04 in the R group). Notably, PCS was impaired to a greater extent than MCS in patients in the R group and showed a greater magnitude of improvement after 3 months of treatment. These results could be explained by the fact that MCS can be largely changed in diseases with a direct effect on the central nervous system.23
Our results also revealed that the dose of corticosteroid correlated to HRQOL of patients with pemphigus who recently received RTX therapy. Indeed, it is more likely that patients on lower-dose prednisolone have a higher QOL, especially on physical function and social function dimensions of SF-36. This finding is highly expectable by less severe disease due to RTX treatment and also lower potential dose-dependent adverse effects of long-term steroid therapy.
One of the most striking findings of this study was the correlation of location of lesions to QOL indices. We found that the mucocutaneous phenotype was significantly correlated to greater improvement in role emotional, role physical, and social functioning scores due to RTX treatment compared with cutaneous or mucosal types (P=.02, P=.025, and P=.017, respectively). Although mucosal involvement of the disease can be the most burdensome feature because of its large impact on essential activities such as eating and speaking, cutaneous lesions with unpleasant appearance and undesirable symptoms may have a similar impact on QOL. Therefore, having both mucosal and cutaneous lesions causes a worsened QOL and decreased treatment efficacy vs having only one area involved. This may explain the greater improvement in some QOL indices with RTX treatment.
Limitations—Given the cross-sectional design of this study in which patients were observed at a single time point during their treatment course, it is not possible to establish a clear cause-effect relationship between variables. Moreover, we did not evaluate the impact of RTX or prednisolone adverse effects on QOL. Therefore, further prospective studies with longer treatment durations may help to validate our findings. In addition, MCDs for DLQI and SF-36 in pemphigus need to be determined and validated in future studies.
CONCLUSION
The results of our study demonstrated that patients with pemphigus may benefit from taking RTX, not only in terms of clinical improvement of their disease measured by objective indices such as PDAI and anti-DSG1 and anti-DSG3 values but also in several domains that are important to patients, including physical and mental health status (SF-36), HRQOL (DLQI), and overall disease severity (PGA). Rituximab administration in patients with pemphigus can lead to rapid and significant improvement in HRQOL as well as patient- and physician-assessed measures. Its favorable safety profile along with its impact on patients’ daily lives and mental health makes RTX a suitable treatment option for patients with pemphigus. Moreover, we recommend taking QOL indices into account while evaluating the efficacy of new medications to improve our insight into the patient experience and provide better patient adherence to treatment, which is an important issue for optimal control of chronic disorders.
Pemphigus is a group of autoimmune blistering diseases characterized by the development of painful and flaccid blisters on the skin and/or mucous membranes. Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are 2 major subtypes and can be distinguished by the location of blister formation or the specificity of autoantibodies directed against different desmogleins.1,2 Although rare, pemphigus is considered a serious and life-threatening condition with a great impact on quality of life (QOL) due to disease symptoms (eg, painful lesions, physical appearance of skin lesions) as well as treatment complications (eg, adverse drug effects, cost of treatment).3-6 Moreover, the physical and psychological effects can lead to marked functional morbidity and work-related disability during patients’ productive years.7 Therefore, affected individuals usually have a remarkably compromised health-related quality of life (HRQOL).8 Effective treatments may considerably improve the QOL of patients with pemphigus.6
Despite the available treatment options, finding the best regimen for pemphigus remains a challenge. Corticosteroids are assumed to be the main treatment, though they have considerable side effects.9,10 Adjuvant therapies are used to suppress or modulate immune responses, leading to remission with the least possible need for corticosteroids. Finding an optimal steroid-sparing agent has been the aim of research, and biologic agents seem to be the best option.8 Rituximab (RTX), an anti-CD20 monoclonal antibody, has shown great promise in several studies of its clinical efficacy and has become a first-line treatment in new guidelines.11-14 Rituximab treatment has been associated with notable improvement in physician-assessed outcome measures with a favorable safety profile in patients with pemphigus.11-15 However, it is important to assess response to treatment from a patient’s perspective through the use of outcome-assessment measures that encompass patient-reported outcomes to reflect the complete patient experience and establish the overall impact of RTX as well as its likelihood of acceptance by patients with pemphigus.
In our study, we compared clinical outcomes and HRQOL through the use of disease-specific measures as well as comprehensive generic health status measures among patients with PV and PF who received RTX treatment 3 months earlier and those who received RTX in the last 2 weeks. The clinical relevance of the patient-reported outcomes is discussed.
MATERIALS AND METHODS
Study Design
We conducted a single-center cross-sectional study of 96 patients with pemphigus aged 18 to 65 years of either sex who were willing to participate in this study. Patients with a confirmed diagnosis of PV or PF who received RTX 3 months earlier or in the last 2 weeks were enrolled in the study. Patients were identified using Dermatry.ir, an archiving software that contains patients’ medical data. Exclusion criteria included lack of sufficient knowledge of the concepts of the questionnaires as well as age younger than 16 years. The study was conducted from October 2019 to April 2020 by the Autoimmune Bullous Disease Research Center at Razi Hospital in Tehran, Iran, which is the main dermatology-specific center and teaching hospital of Iran. The study protocol was approved by the relevant ethics committee.
Patients were categorized into 2 groups: (1) those who received RTX 3 months earlier (3M group); and (2) those who received RTX in the last 2 weeks (R group).
After an explanation of the study to participants, informed written consent was signed by each patient, and their personal data (eg, age, sex, education, marital status, smoking status), as well as clinical data (eg, type of pemphigus, duration of disease, site of onset, prednisolone dosage, presence of Nikolsky sign, anti-DSG1 and anti-DSG3 values, Pemphigus Disease Area Index [PDAI] score, RTX treatment protocol); any known comorbidities such as hypertension, diabetes mellitus, or morbid obesity; and any chronic pulmonary, cardiac, endocrinologic, renal, or hepatic condition, were collected and recorded in a predefined Case Record.
Patient-Reported Outcome Measures
The effect of RTX on QOL in patients with pemphigus was assessed using 2 HRQOL instruments: (1) a general health status indicator, the 36-Item Short Form Survey (SF-36), and (2) a validated, Persian version of a dermatology-specific questionnaire, Dermatology Life Quality Index (DLQI). The questionnaires were completed by each patient or by an assistant if needed.
The SF-36 is a widely used 36-item questionnaire measuring functional health and well-being across 8 domains—mental health, pain, physical function, role emotional, role physical, social functioning, vitality, and general health perception—with scores for each ranging from 0 to 100. The physical component scores (PCSs) and mental component scores (MCSs) were derived from these 8 subscales, each ranging from 0 to 400, with higher scores indicating better health status.6
The DLQI, one of the most frequently used QOL measures in dermatology, contains 10 questions, each referring to the prior week and classified in the following 6 subscales: symptoms and feelings, daily activities, leisure, personal relationships, work and school, and treatment.16 The total score ranges from 0 (no impact) to 30 (very high impact), with a higher score indicating a lower QOL (eTable 1). The minimal clinically important difference (MCD) for the DLQI was considered to be 2- to 5-point changes in prior studies.17,18 In this study, we used an MCD of a 5-point change or more between study groups.

Moreover, the patient general assessment (PGA) of disease severity was identified using a 3-point scale (1=mild, 2=moderate, 3=severe).
Statistical Analysis
Data were analyzed using SPSS Statistics version 23. P≤.05 was considered significant. Mean and SD were calculated for descriptive data. The t test, Fisher exact test, analysis of variance, multiple regression analysis, and logistic regression analysis were used to identify the relationship between variables.
RESULTS
Patient Characteristics
A total of 96 patients were enrolled in this study. The mean (SD) age of participants was 41.42 (15.1) years (range, 18–58 years). Of 96 patients whose data were included, 55 (57.29%) patients had received RTX 3 months earlier (3M group) and 41 (42.71%) received RTX in the last 2 weeks (R group). A summary of study patient characteristics in each group is provided in eTable 2. There was no significant difference between the 2 groups in terms of age, sex, type of pemphigus, marital status, education, positive Nikolsky sign, smoking status, existence of comorbidities, site of lesions, and RTX treatment protocol. However, a significant difference was found for duration of disease (P=.0124) and mean prednisolone dosage (P=.001) as well as severity of disease measured by PDAI score (P=.003) and anti-DSG1 (P=.003) and anti-DSG3 (P=.021) values.

Patient-Reported Outcomes
Physical and mental component scores are summarized in eTable 3. Generally, SF-36 scores were improved with RTX treatment in all dimensions except for mental health, though these differences were not statistically significant, with the greatest mean improvement in the role physical index (75.45 in the 3M group vs 53.04 in the R group; P=.009). Mean SF-36 PCS and MCS scores were higher in the 3M group vs the R group, though the difference in MCS score did not reach the level of significance (eTable 3).

Mean DLQI scores in the R and 3M groups were 12.31 and 6.96, respectively, indicating a considerable burden on HRQOL in both groups. However, a statistically significant difference between these values was seen that also was clinically meaningful, indicating a significant improvement of QOL in patients receiving RTX 3 months earlier (P=.005)(eTable 3).
The PGA scores indicated that patients in the 3M group were significantly more likely to report less severe disease vs the R group (P=.008)(eTable 3).
Multivariate Analysis—Effect of the patient characteristics and some disease features on indices of QOL was evaluated using the multiple linear regression model. eTable 4 shows the P values of those analyses.

COMMENT
Pemphigus is a chronic disabling disease with notable QOL impairment due to disease burden as well as the need for long-term use of immunosuppressive agents during the disease course. To study the effect of RTX on QOL of patients with pemphigus, we compared 2 sets of patients. Prior studies have shown that clinically significant effects of RTX take 4 to 12 weeks to appear.19,20 Therefore, we selected patients who received RTX 3 months earlier to measure their HRQOL indices and compare them with patients who had received RTX in the last 2 weeks as a control group to investigate the effect of RTX intrinsically, as this was the focus of this study.
In our study, one of the research tools was the DLQI. Healthy patients typically have an average score of 0.5.21 The mean DLQI score of the patients in R group was 12.31, which was similar to prior analysis8 and reflects a substantial burden of disease comparable to atopic dermatitis and psoriasis.21,22 In patients in the 3M group, the mean DLQI score was lower than the R group (6.96 vs 12.31), indicating a significant (P=.005) and clinically meaningful improvement in QOL of patients due to the dramatic therapeutic effect of RTX. However, this score indicated a moderate effect on HRQOL, even in the context of clinical improvement due to RTX treatment, which may reflect that the short duration of treatment in the 3M group was a limitation of this study. Although the 12-week treatment duration was comparable with other studies19,20 and major differences in objective measures of treatment efficacy were found in PDAI as well as anti-DSG1 and anti-DSG3 values, longer treatment duration may be needed for a more comprehensive assessment of the benefit of RTX on HRQOL indices in patients with pemphigus.
Based on results of the SF-36 questionnaire, PCS and MCS scores were not substantially impaired in the R group considering the fact that a mean score of 50 has been articulated as a normative value for all scales.23 These data demonstrated the importance of using a dermatologic-specific instrument such as the DLQI instead of a general questionnaire to assess QOL in patients with pemphigus. However, better indices were reported with RTX treatment in the 3 SF-36 domains—role physical (P=.009), role emotional (P=.03), and general health perception (P=.03)—with the role physical showing the greatest magnitude of mean change (75.45 in the 3M group vs 53.04 in the R group). Notably, PCS was impaired to a greater extent than MCS in patients in the R group and showed a greater magnitude of improvement after 3 months of treatment. These results could be explained by the fact that MCS can be largely changed in diseases with a direct effect on the central nervous system.23
Our results also revealed that the dose of corticosteroid correlated to HRQOL of patients with pemphigus who recently received RTX therapy. Indeed, it is more likely that patients on lower-dose prednisolone have a higher QOL, especially on physical function and social function dimensions of SF-36. This finding is highly expectable by less severe disease due to RTX treatment and also lower potential dose-dependent adverse effects of long-term steroid therapy.
One of the most striking findings of this study was the correlation of location of lesions to QOL indices. We found that the mucocutaneous phenotype was significantly correlated to greater improvement in role emotional, role physical, and social functioning scores due to RTX treatment compared with cutaneous or mucosal types (P=.02, P=.025, and P=.017, respectively). Although mucosal involvement of the disease can be the most burdensome feature because of its large impact on essential activities such as eating and speaking, cutaneous lesions with unpleasant appearance and undesirable symptoms may have a similar impact on QOL. Therefore, having both mucosal and cutaneous lesions causes a worsened QOL and decreased treatment efficacy vs having only one area involved. This may explain the greater improvement in some QOL indices with RTX treatment.
Limitations—Given the cross-sectional design of this study in which patients were observed at a single time point during their treatment course, it is not possible to establish a clear cause-effect relationship between variables. Moreover, we did not evaluate the impact of RTX or prednisolone adverse effects on QOL. Therefore, further prospective studies with longer treatment durations may help to validate our findings. In addition, MCDs for DLQI and SF-36 in pemphigus need to be determined and validated in future studies.
CONCLUSION
The results of our study demonstrated that patients with pemphigus may benefit from taking RTX, not only in terms of clinical improvement of their disease measured by objective indices such as PDAI and anti-DSG1 and anti-DSG3 values but also in several domains that are important to patients, including physical and mental health status (SF-36), HRQOL (DLQI), and overall disease severity (PGA). Rituximab administration in patients with pemphigus can lead to rapid and significant improvement in HRQOL as well as patient- and physician-assessed measures. Its favorable safety profile along with its impact on patients’ daily lives and mental health makes RTX a suitable treatment option for patients with pemphigus. Moreover, we recommend taking QOL indices into account while evaluating the efficacy of new medications to improve our insight into the patient experience and provide better patient adherence to treatment, which is an important issue for optimal control of chronic disorders.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:175-197.
- Kasperkiewicz M, Ellebrecht CT, Takahashi H, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris, result from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005;3:431-435.
- Terrab Z, Benckikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034.
- Paradisi A, Sampogna F, Di Pietro, C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269.
- Heelan K, Hitzig SL, Knowles S, et al. Loss of work productivity and quality of life in patients with autoimmune bullous dermatoses. J Cutan Med Surg. 2015;19:546-554.
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J Dermatol. 2012;39:141-144.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:2343.
- Mohammad-Javad N, Parvaneh H, Maryam G, et al. Randomized trial of tacrolimus 0.1% ointment versus triamcinolone acetonide 0.1% paste in the treatment of oral pemphigus vulgaris. Iranian J Dermatol. 2012;15:42-46.
- Lunardon L, Tsai KJ, Propert KJ, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031-1036.
- Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30.
- Heelan K, Al-Mohammedi F, Smith MJ, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040
- Aryanian Z, Balighi K, Daneshpazhooh M, et al. Rituximab exhibits a better safety profile when used as a first line of treatment for pemphigus vulgaris: a retrospective study. Int Immunopharmacol. 2021;96:107755.
- Aghai S, Sodaifi M, Jafari P, et al. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8.
- Schünemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: the clinician’s perspective. Health Qual Life Outcomes. 2006;4:62.
- Quality of life questionnaires. Cardiff University website. Accessed December 16, 2022. http://sites.cardiff.ac.uk/dermatology/quality-oflife/dermatology-quality-of-life-index-dlqi/dlqi-instructions-foruse-and-scoring/
- Kanwar AJ, Tsuruta D, Vinay K, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27:E17-E23.
- Ingen-Housz-Oro S, Valeyrie-Allanore L, Cosnes A, et al. First-line treatment of pemphigus vulgaris with a combination of rituximab and high-potency topical corticosteroids. JAMA Dermatol. 2015;151:200-203.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Aghaei S, Moradi A, Ardekani GS. Impact of psoriasis on quality of life in Iran. Indian J Dermatol Venereol Leprol. 2009;75:220.
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). 1. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:175-197.
- Kasperkiewicz M, Ellebrecht CT, Takahashi H, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris, result from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005;3:431-435.
- Terrab Z, Benckikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034.
- Paradisi A, Sampogna F, Di Pietro, C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269.
- Heelan K, Hitzig SL, Knowles S, et al. Loss of work productivity and quality of life in patients with autoimmune bullous dermatoses. J Cutan Med Surg. 2015;19:546-554.
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J Dermatol. 2012;39:141-144.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:2343.
- Mohammad-Javad N, Parvaneh H, Maryam G, et al. Randomized trial of tacrolimus 0.1% ointment versus triamcinolone acetonide 0.1% paste in the treatment of oral pemphigus vulgaris. Iranian J Dermatol. 2012;15:42-46.
- Lunardon L, Tsai KJ, Propert KJ, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031-1036.
- Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30.
- Heelan K, Al-Mohammedi F, Smith MJ, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040
- Aryanian Z, Balighi K, Daneshpazhooh M, et al. Rituximab exhibits a better safety profile when used as a first line of treatment for pemphigus vulgaris: a retrospective study. Int Immunopharmacol. 2021;96:107755.
- Aghai S, Sodaifi M, Jafari P, et al. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8.
- Schünemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: the clinician’s perspective. Health Qual Life Outcomes. 2006;4:62.
- Quality of life questionnaires. Cardiff University website. Accessed December 16, 2022. http://sites.cardiff.ac.uk/dermatology/quality-oflife/dermatology-quality-of-life-index-dlqi/dlqi-instructions-foruse-and-scoring/
- Kanwar AJ, Tsuruta D, Vinay K, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27:E17-E23.
- Ingen-Housz-Oro S, Valeyrie-Allanore L, Cosnes A, et al. First-line treatment of pemphigus vulgaris with a combination of rituximab and high-potency topical corticosteroids. JAMA Dermatol. 2015;151:200-203.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Aghaei S, Moradi A, Ardekani GS. Impact of psoriasis on quality of life in Iran. Indian J Dermatol Venereol Leprol. 2009;75:220.
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). 1. conceptual framework and item selection. Med Care. 1992;30:473-483.
PRACTICE POINTS
- Pemphigus is an autoimmune blistering disease that can negatively affect patients’ lives.
- Assessing the impact of treatment from a patient’s perspective using outcome assessment measures is important and relevant in trials of new pemphigus treatments including rituximab.
- Rituximab administration in pemphigus patients led to rapid and notable improvement in health-related quality of life and patient-assessed measures.
Juvenile Dermatomyositis–Associated Panniculitis
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.
A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.
Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
Practice Points
- Juvenile dermatomyositis is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin.
- Juvenile dermatomyositis has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant, dermatomyositis (DM).
- Panniculitis is a rare but severe complication of DM, and this subset of DM may be challenging to treat, requiring aggressive therapy.
Combination therapy shows mixed results for scleroderma-related lung disease
PHILADELPHIA – Combining the immunomodulatory agent mycophenolate with the antifibrotic pirfenidone led to more rapid improvement and showed a trend to be more effective than mycophenolate mofetil alone for treating the signs and symptoms of scleroderma-related interstitial lung disease, but the combination therapy came with an increase in side effects, according to results from the Scleroderma Lung Study III.
Dinesh Khanna, MBBS, MSc, of the University of Michigan, Ann Arbor, presented the results at the annual meeting of the American College of Rheumatology. He noted some problems with the study – namely its small size, enrolling only 51 patients, about one-third of its original goal. But he also said it showed a potential signal for efficacy and that the study itself could serve as a “template” for future studies of combination mycophenolate mofetil (MMF) plus pirfenidone therapy for scleroderma-related interstitial lung disease (SSc-ILD).
“The pirfenidone patients had quite a bit more GI side effects and photosensitivity, and those are known side effects,” Dr. Khanna said in an interview. “So the combination therapy had more side effects but trends to higher efficacy.”
The design of SLS-III, a phase 2 clinical trial, was a challenge, Dr. Khanna explained. The goal was to enroll 150 SSc-ILD patients who hadn’t had any previous treatment for their disease. Finding those patients proved difficult. “In fact, if you look at the recent history, 70% of the patients with early diffuse scleroderma are on MMF,” he said in his presentation. Compounding low study enrollment was the intervening COVID-19 pandemic, he added.
Testing a faster-acting combination
Nonetheless, the trial managed to enroll 27 patients in the combination therapy group and 24 in the MMF-plus-placebo group and compared their outcomes over 18 months. Study dosing was 1,500 mg MMF twice daily and pirfenidone 801 mg three times daily, titrated to the tolerable dose.
Despite the study’s being underpowered, Dr. Khanna said, it still reported some notable outcomes that merit further investigation. “I think what was intriguing in the study was the long-term benefit in the patient-reported outcomes and the structural changes,” he said in the interview.
Among those notable outcomes was a clinically significant change in forced vital capacity (FVC) percentage for the combination vs. the placebo groups: 2.24% vs. 2.09%. He also noted that the combination group saw a somewhat more robust improvement in FVC at six months: 2.59% (± 0.98%) vs. 0.92% (± 1.1%) in the placebo group.
The combination group showed greater improvements in high-resolution computed tomography-evaluated lung involvement and lung fibrosis and patient-reported outcomes, including a statistically significant 3.67-point greater improvement in PROMIS-29 physical function score (4.42 vs. 0.75).
The patients on combination therapy had higher rates of serious adverse events (SAEs), and seven discontinued one or both study drugs early, all in the combined arm. Four combination therapy patients had six SAEs, compared to two placebo patients with three SAEs. In the combination group, SAEs included chest pain, herpes zoster ophthalmicus, nodular basal cell cancer, marginal zone B cell lymphoma, renal crisis, and dyspnea. SAEs in the placebo group were colitis, COVID-19 and hypoxic respiratory failure.
Study design challenges
Nonetheless, Dr. Khanna said the SLS-III data are consistent with the SLS-II findings, with mean improvements in FVC of 2.24% and 2.1%, respectively.
“The next study may be able to replicate what we tried to do, keeping in mind that there are really no MMF-naive patients who are walking around,” Dr. Khanna said. “So the challenge is about the feasibility of recruiting within a trial vs. trying to show a statistical difference between the drug and placebo.”
This study could serve as a foundation for future studies of MMF in patients with SSc-ILD, Robert Spiera, MD, of the Hospital for Special Surgery in New York, said in an interview. “There are lessons to be learned both from the study but also from prior studies looking at MMF use in the background in patients treated with other drugs in clinical trials,” he said.
Dr. Spiera noted that the study had other challenges besides the difficulty in recruiting patients who hadn’t been on MMF therapy. “A great challenge is that the benefit with regard to the impact on the lungs from MMF seems most prominent in the first 6 months to a year to even 2 years that somebody is on the drug,” he said.
The other challenge with this study is that a large proportion of patients had limited systemic disease and relatively lower levels of skin disease compared with other studies of patients on MMF, Dr. Spiera said.
“The optimal treatment of scleroderma-associated lung disease remains a very important and not-adequately met need,” he said. “Particularly, we’re looking for drugs that are tolerable in a patient population that are very prone to GI side effects in general. This study and others have taught us a lot about trial design, and I think more globally this will allow us to move this field forward.”
Dr. Khanna disclosed relationships with Actelion, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Horizon Therapeutics USA, Janssen Global Services, Prometheus Biosciences, Mitsubishi Tanabe Pharma Corp., Genentech/Roche, Theraly, and Pfizer. Genentech provided funding for the study and pirfenidone and placebo drugs at no cost.
Dr. Spiera disclosed relationships with GlaxoSmithKline, Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche and Vera.
PHILADELPHIA – Combining the immunomodulatory agent mycophenolate with the antifibrotic pirfenidone led to more rapid improvement and showed a trend to be more effective than mycophenolate mofetil alone for treating the signs and symptoms of scleroderma-related interstitial lung disease, but the combination therapy came with an increase in side effects, according to results from the Scleroderma Lung Study III.
Dinesh Khanna, MBBS, MSc, of the University of Michigan, Ann Arbor, presented the results at the annual meeting of the American College of Rheumatology. He noted some problems with the study – namely its small size, enrolling only 51 patients, about one-third of its original goal. But he also said it showed a potential signal for efficacy and that the study itself could serve as a “template” for future studies of combination mycophenolate mofetil (MMF) plus pirfenidone therapy for scleroderma-related interstitial lung disease (SSc-ILD).
“The pirfenidone patients had quite a bit more GI side effects and photosensitivity, and those are known side effects,” Dr. Khanna said in an interview. “So the combination therapy had more side effects but trends to higher efficacy.”
The design of SLS-III, a phase 2 clinical trial, was a challenge, Dr. Khanna explained. The goal was to enroll 150 SSc-ILD patients who hadn’t had any previous treatment for their disease. Finding those patients proved difficult. “In fact, if you look at the recent history, 70% of the patients with early diffuse scleroderma are on MMF,” he said in his presentation. Compounding low study enrollment was the intervening COVID-19 pandemic, he added.
Testing a faster-acting combination
Nonetheless, the trial managed to enroll 27 patients in the combination therapy group and 24 in the MMF-plus-placebo group and compared their outcomes over 18 months. Study dosing was 1,500 mg MMF twice daily and pirfenidone 801 mg three times daily, titrated to the tolerable dose.
Despite the study’s being underpowered, Dr. Khanna said, it still reported some notable outcomes that merit further investigation. “I think what was intriguing in the study was the long-term benefit in the patient-reported outcomes and the structural changes,” he said in the interview.
Among those notable outcomes was a clinically significant change in forced vital capacity (FVC) percentage for the combination vs. the placebo groups: 2.24% vs. 2.09%. He also noted that the combination group saw a somewhat more robust improvement in FVC at six months: 2.59% (± 0.98%) vs. 0.92% (± 1.1%) in the placebo group.
The combination group showed greater improvements in high-resolution computed tomography-evaluated lung involvement and lung fibrosis and patient-reported outcomes, including a statistically significant 3.67-point greater improvement in PROMIS-29 physical function score (4.42 vs. 0.75).
The patients on combination therapy had higher rates of serious adverse events (SAEs), and seven discontinued one or both study drugs early, all in the combined arm. Four combination therapy patients had six SAEs, compared to two placebo patients with three SAEs. In the combination group, SAEs included chest pain, herpes zoster ophthalmicus, nodular basal cell cancer, marginal zone B cell lymphoma, renal crisis, and dyspnea. SAEs in the placebo group were colitis, COVID-19 and hypoxic respiratory failure.
Study design challenges
Nonetheless, Dr. Khanna said the SLS-III data are consistent with the SLS-II findings, with mean improvements in FVC of 2.24% and 2.1%, respectively.
“The next study may be able to replicate what we tried to do, keeping in mind that there are really no MMF-naive patients who are walking around,” Dr. Khanna said. “So the challenge is about the feasibility of recruiting within a trial vs. trying to show a statistical difference between the drug and placebo.”
This study could serve as a foundation for future studies of MMF in patients with SSc-ILD, Robert Spiera, MD, of the Hospital for Special Surgery in New York, said in an interview. “There are lessons to be learned both from the study but also from prior studies looking at MMF use in the background in patients treated with other drugs in clinical trials,” he said.
Dr. Spiera noted that the study had other challenges besides the difficulty in recruiting patients who hadn’t been on MMF therapy. “A great challenge is that the benefit with regard to the impact on the lungs from MMF seems most prominent in the first 6 months to a year to even 2 years that somebody is on the drug,” he said.
The other challenge with this study is that a large proportion of patients had limited systemic disease and relatively lower levels of skin disease compared with other studies of patients on MMF, Dr. Spiera said.
“The optimal treatment of scleroderma-associated lung disease remains a very important and not-adequately met need,” he said. “Particularly, we’re looking for drugs that are tolerable in a patient population that are very prone to GI side effects in general. This study and others have taught us a lot about trial design, and I think more globally this will allow us to move this field forward.”
Dr. Khanna disclosed relationships with Actelion, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Horizon Therapeutics USA, Janssen Global Services, Prometheus Biosciences, Mitsubishi Tanabe Pharma Corp., Genentech/Roche, Theraly, and Pfizer. Genentech provided funding for the study and pirfenidone and placebo drugs at no cost.
Dr. Spiera disclosed relationships with GlaxoSmithKline, Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche and Vera.
PHILADELPHIA – Combining the immunomodulatory agent mycophenolate with the antifibrotic pirfenidone led to more rapid improvement and showed a trend to be more effective than mycophenolate mofetil alone for treating the signs and symptoms of scleroderma-related interstitial lung disease, but the combination therapy came with an increase in side effects, according to results from the Scleroderma Lung Study III.
Dinesh Khanna, MBBS, MSc, of the University of Michigan, Ann Arbor, presented the results at the annual meeting of the American College of Rheumatology. He noted some problems with the study – namely its small size, enrolling only 51 patients, about one-third of its original goal. But he also said it showed a potential signal for efficacy and that the study itself could serve as a “template” for future studies of combination mycophenolate mofetil (MMF) plus pirfenidone therapy for scleroderma-related interstitial lung disease (SSc-ILD).
“The pirfenidone patients had quite a bit more GI side effects and photosensitivity, and those are known side effects,” Dr. Khanna said in an interview. “So the combination therapy had more side effects but trends to higher efficacy.”
The design of SLS-III, a phase 2 clinical trial, was a challenge, Dr. Khanna explained. The goal was to enroll 150 SSc-ILD patients who hadn’t had any previous treatment for their disease. Finding those patients proved difficult. “In fact, if you look at the recent history, 70% of the patients with early diffuse scleroderma are on MMF,” he said in his presentation. Compounding low study enrollment was the intervening COVID-19 pandemic, he added.
Testing a faster-acting combination
Nonetheless, the trial managed to enroll 27 patients in the combination therapy group and 24 in the MMF-plus-placebo group and compared their outcomes over 18 months. Study dosing was 1,500 mg MMF twice daily and pirfenidone 801 mg three times daily, titrated to the tolerable dose.
Despite the study’s being underpowered, Dr. Khanna said, it still reported some notable outcomes that merit further investigation. “I think what was intriguing in the study was the long-term benefit in the patient-reported outcomes and the structural changes,” he said in the interview.
Among those notable outcomes was a clinically significant change in forced vital capacity (FVC) percentage for the combination vs. the placebo groups: 2.24% vs. 2.09%. He also noted that the combination group saw a somewhat more robust improvement in FVC at six months: 2.59% (± 0.98%) vs. 0.92% (± 1.1%) in the placebo group.
The combination group showed greater improvements in high-resolution computed tomography-evaluated lung involvement and lung fibrosis and patient-reported outcomes, including a statistically significant 3.67-point greater improvement in PROMIS-29 physical function score (4.42 vs. 0.75).
The patients on combination therapy had higher rates of serious adverse events (SAEs), and seven discontinued one or both study drugs early, all in the combined arm. Four combination therapy patients had six SAEs, compared to two placebo patients with three SAEs. In the combination group, SAEs included chest pain, herpes zoster ophthalmicus, nodular basal cell cancer, marginal zone B cell lymphoma, renal crisis, and dyspnea. SAEs in the placebo group were colitis, COVID-19 and hypoxic respiratory failure.
Study design challenges
Nonetheless, Dr. Khanna said the SLS-III data are consistent with the SLS-II findings, with mean improvements in FVC of 2.24% and 2.1%, respectively.
“The next study may be able to replicate what we tried to do, keeping in mind that there are really no MMF-naive patients who are walking around,” Dr. Khanna said. “So the challenge is about the feasibility of recruiting within a trial vs. trying to show a statistical difference between the drug and placebo.”
This study could serve as a foundation for future studies of MMF in patients with SSc-ILD, Robert Spiera, MD, of the Hospital for Special Surgery in New York, said in an interview. “There are lessons to be learned both from the study but also from prior studies looking at MMF use in the background in patients treated with other drugs in clinical trials,” he said.
Dr. Spiera noted that the study had other challenges besides the difficulty in recruiting patients who hadn’t been on MMF therapy. “A great challenge is that the benefit with regard to the impact on the lungs from MMF seems most prominent in the first 6 months to a year to even 2 years that somebody is on the drug,” he said.
The other challenge with this study is that a large proportion of patients had limited systemic disease and relatively lower levels of skin disease compared with other studies of patients on MMF, Dr. Spiera said.
“The optimal treatment of scleroderma-associated lung disease remains a very important and not-adequately met need,” he said. “Particularly, we’re looking for drugs that are tolerable in a patient population that are very prone to GI side effects in general. This study and others have taught us a lot about trial design, and I think more globally this will allow us to move this field forward.”
Dr. Khanna disclosed relationships with Actelion, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Horizon Therapeutics USA, Janssen Global Services, Prometheus Biosciences, Mitsubishi Tanabe Pharma Corp., Genentech/Roche, Theraly, and Pfizer. Genentech provided funding for the study and pirfenidone and placebo drugs at no cost.
Dr. Spiera disclosed relationships with GlaxoSmithKline, Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche and Vera.
AT ACR 2022
Lower hydroxychloroquine dose for lupus tied to hospitalizations for flares
PHILADELPHIA – Patients with systemic lupus erythematosus treated with lower doses of hydroxychloroquine (HCQ) had an increased risk for hospitalization for flares, according to study results presented during a press conference at the annual meeting of the American College of Rheumatology.
HCQ is a cornerstone treatment for SLE as it has been shown to increase survival and decrease disease flares.
Doses decreased with changing guidelines
Guidelines over the years have recommended decreasing doses of HCQ. In 2011, ophthalmology guidelines recommended limiting HCQ dosing to 6.5 mg/kg per day or less of ideal body weight to reduce the chance of retinopathy. For many patients, this required a dose lower than 400 mg/day, an amount frequently used to treat lupus.
In 2016, updated guidelines further lowered the dosage of HCQ, recommending 5 mg/kg or less of patient’s actual body weight.
The effects that lower dosing has had on SLE-associated hospitalizations was unknown, which inspired Dr. Nestor’s research.
The team conducted a case-crossover study within the Mass General Brigham SLE cohort.
Hospitalizations studied over a decade
Dr. Nestor and colleagues identified patients with SLE (via electronic health records) who had at least one visit for SLE and were prescribed HCQ between January 2011 and December 2021, the period over which the recommendations were made.
They identified patients who had been hospitalized during that decade with SLE as the primary discharge diagnosis.
Patients were excluded if they had non-SLE indications, such as kidney transplant or infection without a concomitant SLE flare.
Of 2,971 patients with SLE who used HCQ, 576 had at least one hospitalization with primary discharge diagnosis of SLE.
Of these, 108 were hospitalized for an SLE flare and had used HCQ prior to that hospitalization and had at least one control period with HCQ use during the study period.
All of the patients in the study had to have a case period and a control period, Dr. Nestor explained. The case period was 6 months on HCQ ending in hospitalization for lupus and the control period was 6 months on HCQ that did not end in hospitalization for lupus.
Significantly increased hospitalizations
Low-dose HCQ by weight-based dose (≤ 5 vs. > 5 mg/kg per day) and by non–weight-based dose (< 400 vs. 400 mg per day) were both associated with significantly increased hospitalizations for SLE (adjusted odds ratio, 4.41; 95% confidence interval, 1.50-12.98; and AOR, 3.48; 95% CI, 1.33-9.13, respectively).
The average age of the hospitalized group was 36 years. Most patients (92%) were female, 43.5% were White, and 32.4% were Black.
In calling for reassessment of the dosing, Dr. Nestor said, “We are protecting our patients against a very long-term side effect of hydroxychloroquine retinopathy. [It] typically takes 10-20 years to develop in our patients. But by doing that, we’re missing many of the short-term benefits from hydroxychloroquine in our patients, leading to more lupus flares, which leads to more end-organ damage.”
She said patients taking HCQ for lupus are asked to see an ophthalmologist once a year to monitor for the side effect, adding that rheumatologists and ophthalmologists could work together to adjust the guidelines.
Dr. Nestor suggested it’s possible that patients need higher doses of HCQ earlier in their disease and lower doses later. “Perhaps it’s just the patients who are particularly active who need the higher doses,” she said.
“I don’t think this question is settled,” he told this news organization. “The 5 mg/kg dose recommendation was based on terms of safety but not of effectiveness. We don’t know what the effective dose of HCQ is, and this study shows that low dose is less effective.”
He agreed there needs to be a risk/benefit balance, but noted, “HCQ retinopathy is very rare and we have great tools to screen for it.”
Study limitations include incomplete information on whether patients adhered to treatment plans and reasons for using lower-dose HCQ.
The study authors and Dr. Duarte Garcia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Patients with systemic lupus erythematosus treated with lower doses of hydroxychloroquine (HCQ) had an increased risk for hospitalization for flares, according to study results presented during a press conference at the annual meeting of the American College of Rheumatology.
HCQ is a cornerstone treatment for SLE as it has been shown to increase survival and decrease disease flares.
Doses decreased with changing guidelines
Guidelines over the years have recommended decreasing doses of HCQ. In 2011, ophthalmology guidelines recommended limiting HCQ dosing to 6.5 mg/kg per day or less of ideal body weight to reduce the chance of retinopathy. For many patients, this required a dose lower than 400 mg/day, an amount frequently used to treat lupus.
In 2016, updated guidelines further lowered the dosage of HCQ, recommending 5 mg/kg or less of patient’s actual body weight.
The effects that lower dosing has had on SLE-associated hospitalizations was unknown, which inspired Dr. Nestor’s research.
The team conducted a case-crossover study within the Mass General Brigham SLE cohort.
Hospitalizations studied over a decade
Dr. Nestor and colleagues identified patients with SLE (via electronic health records) who had at least one visit for SLE and were prescribed HCQ between January 2011 and December 2021, the period over which the recommendations were made.
They identified patients who had been hospitalized during that decade with SLE as the primary discharge diagnosis.
Patients were excluded if they had non-SLE indications, such as kidney transplant or infection without a concomitant SLE flare.
Of 2,971 patients with SLE who used HCQ, 576 had at least one hospitalization with primary discharge diagnosis of SLE.
Of these, 108 were hospitalized for an SLE flare and had used HCQ prior to that hospitalization and had at least one control period with HCQ use during the study period.
All of the patients in the study had to have a case period and a control period, Dr. Nestor explained. The case period was 6 months on HCQ ending in hospitalization for lupus and the control period was 6 months on HCQ that did not end in hospitalization for lupus.
Significantly increased hospitalizations
Low-dose HCQ by weight-based dose (≤ 5 vs. > 5 mg/kg per day) and by non–weight-based dose (< 400 vs. 400 mg per day) were both associated with significantly increased hospitalizations for SLE (adjusted odds ratio, 4.41; 95% confidence interval, 1.50-12.98; and AOR, 3.48; 95% CI, 1.33-9.13, respectively).
The average age of the hospitalized group was 36 years. Most patients (92%) were female, 43.5% were White, and 32.4% were Black.
In calling for reassessment of the dosing, Dr. Nestor said, “We are protecting our patients against a very long-term side effect of hydroxychloroquine retinopathy. [It] typically takes 10-20 years to develop in our patients. But by doing that, we’re missing many of the short-term benefits from hydroxychloroquine in our patients, leading to more lupus flares, which leads to more end-organ damage.”
She said patients taking HCQ for lupus are asked to see an ophthalmologist once a year to monitor for the side effect, adding that rheumatologists and ophthalmologists could work together to adjust the guidelines.
Dr. Nestor suggested it’s possible that patients need higher doses of HCQ earlier in their disease and lower doses later. “Perhaps it’s just the patients who are particularly active who need the higher doses,” she said.
“I don’t think this question is settled,” he told this news organization. “The 5 mg/kg dose recommendation was based on terms of safety but not of effectiveness. We don’t know what the effective dose of HCQ is, and this study shows that low dose is less effective.”
He agreed there needs to be a risk/benefit balance, but noted, “HCQ retinopathy is very rare and we have great tools to screen for it.”
Study limitations include incomplete information on whether patients adhered to treatment plans and reasons for using lower-dose HCQ.
The study authors and Dr. Duarte Garcia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Patients with systemic lupus erythematosus treated with lower doses of hydroxychloroquine (HCQ) had an increased risk for hospitalization for flares, according to study results presented during a press conference at the annual meeting of the American College of Rheumatology.
HCQ is a cornerstone treatment for SLE as it has been shown to increase survival and decrease disease flares.
Doses decreased with changing guidelines
Guidelines over the years have recommended decreasing doses of HCQ. In 2011, ophthalmology guidelines recommended limiting HCQ dosing to 6.5 mg/kg per day or less of ideal body weight to reduce the chance of retinopathy. For many patients, this required a dose lower than 400 mg/day, an amount frequently used to treat lupus.
In 2016, updated guidelines further lowered the dosage of HCQ, recommending 5 mg/kg or less of patient’s actual body weight.
The effects that lower dosing has had on SLE-associated hospitalizations was unknown, which inspired Dr. Nestor’s research.
The team conducted a case-crossover study within the Mass General Brigham SLE cohort.
Hospitalizations studied over a decade
Dr. Nestor and colleagues identified patients with SLE (via electronic health records) who had at least one visit for SLE and were prescribed HCQ between January 2011 and December 2021, the period over which the recommendations were made.
They identified patients who had been hospitalized during that decade with SLE as the primary discharge diagnosis.
Patients were excluded if they had non-SLE indications, such as kidney transplant or infection without a concomitant SLE flare.
Of 2,971 patients with SLE who used HCQ, 576 had at least one hospitalization with primary discharge diagnosis of SLE.
Of these, 108 were hospitalized for an SLE flare and had used HCQ prior to that hospitalization and had at least one control period with HCQ use during the study period.
All of the patients in the study had to have a case period and a control period, Dr. Nestor explained. The case period was 6 months on HCQ ending in hospitalization for lupus and the control period was 6 months on HCQ that did not end in hospitalization for lupus.
Significantly increased hospitalizations
Low-dose HCQ by weight-based dose (≤ 5 vs. > 5 mg/kg per day) and by non–weight-based dose (< 400 vs. 400 mg per day) were both associated with significantly increased hospitalizations for SLE (adjusted odds ratio, 4.41; 95% confidence interval, 1.50-12.98; and AOR, 3.48; 95% CI, 1.33-9.13, respectively).
The average age of the hospitalized group was 36 years. Most patients (92%) were female, 43.5% were White, and 32.4% were Black.
In calling for reassessment of the dosing, Dr. Nestor said, “We are protecting our patients against a very long-term side effect of hydroxychloroquine retinopathy. [It] typically takes 10-20 years to develop in our patients. But by doing that, we’re missing many of the short-term benefits from hydroxychloroquine in our patients, leading to more lupus flares, which leads to more end-organ damage.”
She said patients taking HCQ for lupus are asked to see an ophthalmologist once a year to monitor for the side effect, adding that rheumatologists and ophthalmologists could work together to adjust the guidelines.
Dr. Nestor suggested it’s possible that patients need higher doses of HCQ earlier in their disease and lower doses later. “Perhaps it’s just the patients who are particularly active who need the higher doses,” she said.
“I don’t think this question is settled,” he told this news organization. “The 5 mg/kg dose recommendation was based on terms of safety but not of effectiveness. We don’t know what the effective dose of HCQ is, and this study shows that low dose is less effective.”
He agreed there needs to be a risk/benefit balance, but noted, “HCQ retinopathy is very rare and we have great tools to screen for it.”
Study limitations include incomplete information on whether patients adhered to treatment plans and reasons for using lower-dose HCQ.
The study authors and Dr. Duarte Garcia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2022