User login
First recommendations for cancer screening in myositis issued
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
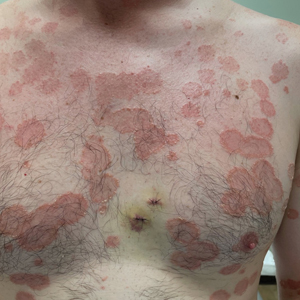
Painful and Pruritic Eruptions on the Entire Body
The Diagnosis: IgA Pemphigus
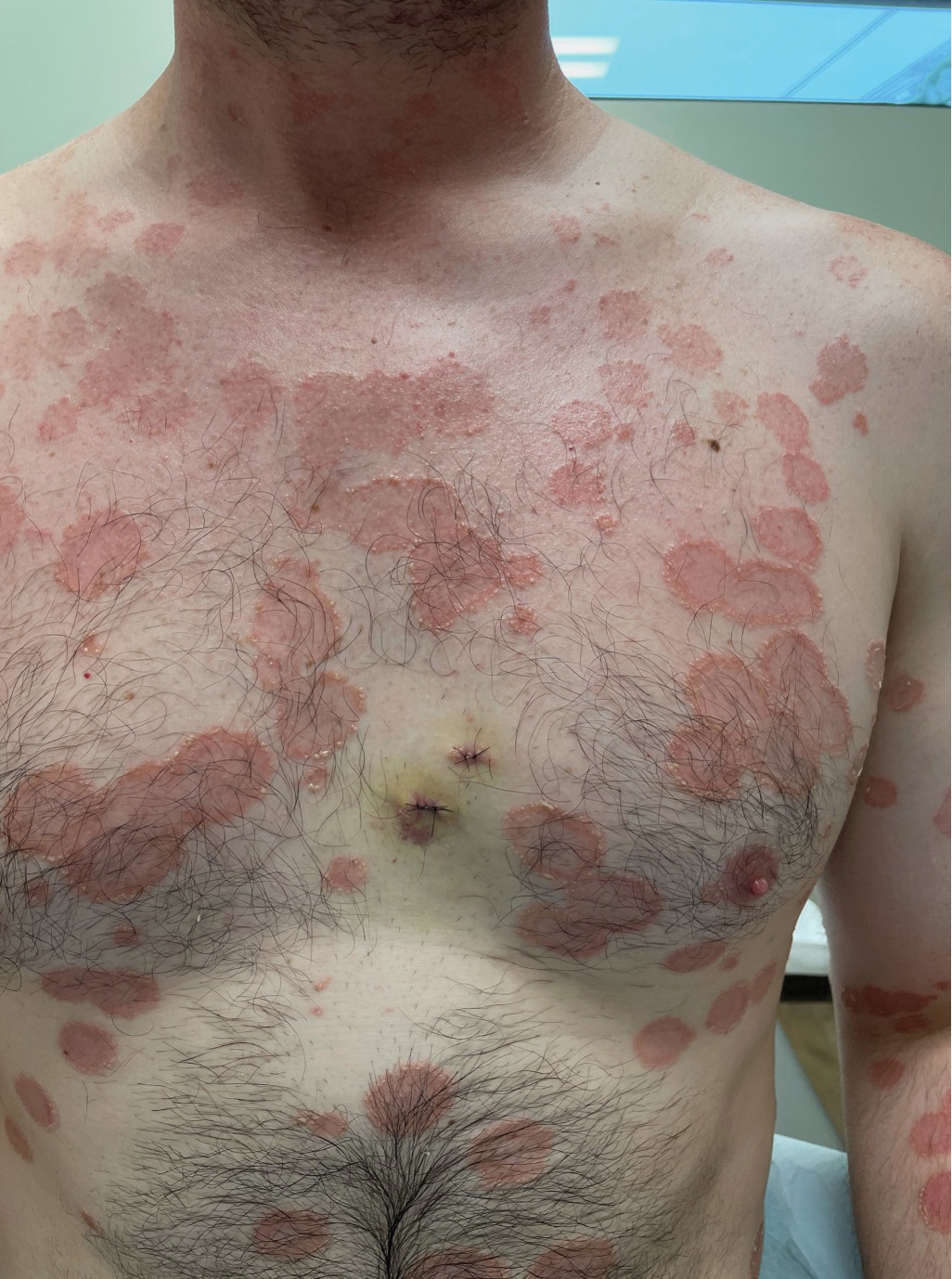
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
A 36-year-old man presented with painful tender blisters and rashes on the entire body, including the ears and tongue. The rash began as a few pinpointed red dots on the abdomen, which subsequently increased in size and spread over the last week. He initially felt red and flushed and noticed new lesions appearing throughout the day. He did not attempt any specific treatment for these lesions. The patient tested positive for COVID-19 four months prior to the skin eruption. He denied systemic symptoms, smoking, or recent travel. He had no history of skin cancer, skin disorders, HIV, or hepatitis. He had no known medication allergies. Physical examination revealed multiple disseminated pustules on the ears, superficial ulcerations on the tongue, and blisters on the right lip. Few lesions were tender to the touch and drained clear fluid. Bacterial, viral, HIV, herpes, and rapid plasma reagin culture and laboratory screenings were negative. He was started on valaciclovir and cephalexin; however, no improvement was noticed. Punch biopsies were taken from the blisters on the chest and perilesional area.
Atypical Localized Scleroderma Development During Nivolumab Therapy for Metastatic Lung Adenocarcinoma
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
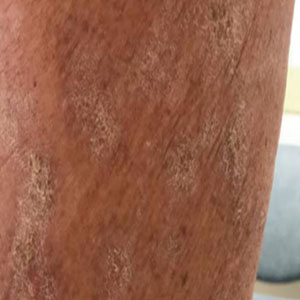
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.
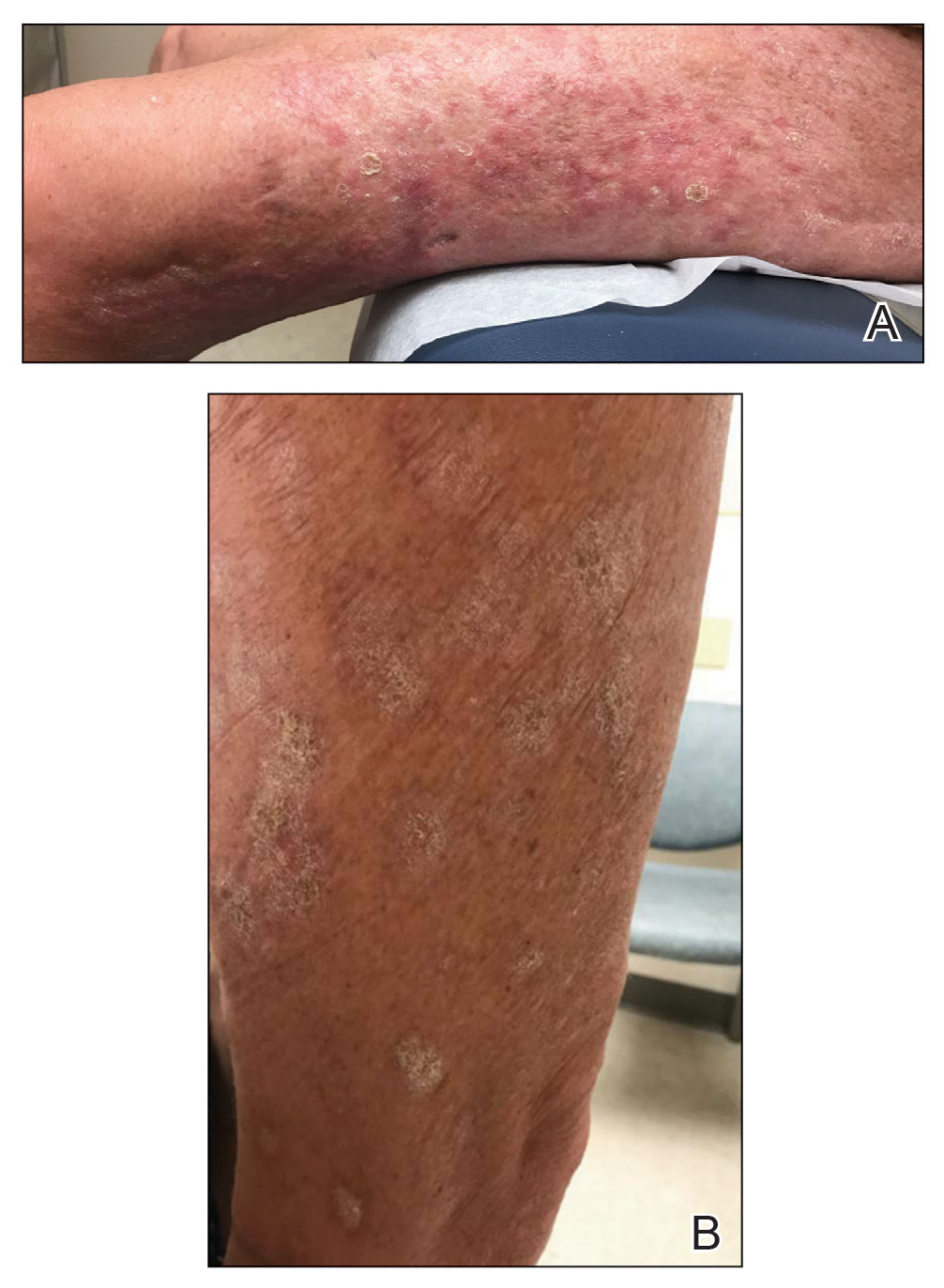
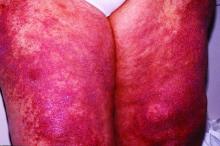
Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.
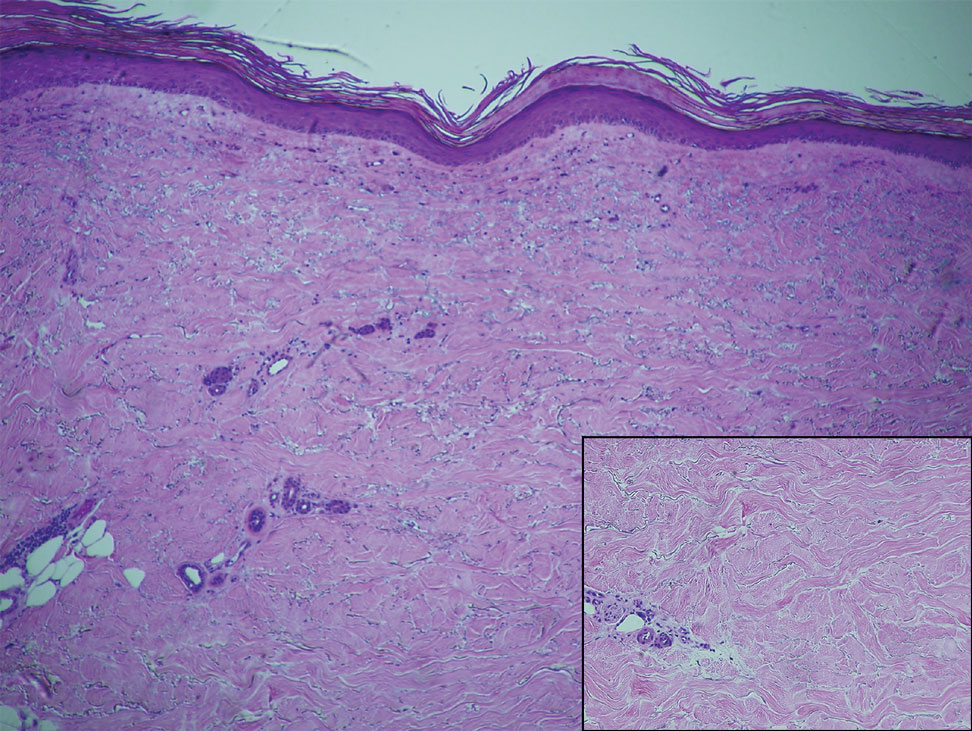
Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.

Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.

Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.

Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.

Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793.
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79.
- Badea I, Taylor M, Rosenberg A, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology (Oxford). 2009;48:213-221.
- Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- Villadolid J, Asim A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70-80.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed celldeath 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128-1136.
- Torok KS, Kurzinski K, Kelsey C, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45:284-293.
- Guo X, Higgs BW, Bay-Jensen AC, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. 2015;135:2402-2409.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumor necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238.
- Barbosa NS, Wetter DA, Wieland CN, et al. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92:1158-1163.
- Cheng MW, Hisaw LD, Bernet L. Generalized morphea in the setting of pembrolizumab. Int J Dermatol. 2019;58:736-738.
- Alegre-Sánchez A, Fonda-Pascual P, Saceda-Corralo D, et al. Relapse of morphea during nivolumab therapy for lung adenocarcinoma. Actas Dermosifiliogr. 2017;108:69-70.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345-361.
Practice Points
- Immune checkpoint inhibitors such as nivolumab, a programmed cell death protein 1 (PD-1) inhibitor, are associated with immune-related adverse events (irAEs) such as skin toxicity.
- Scleroderma should be considered in the differential diagnosis of patients who develop cutaneous eruptions during treatment with PD-1 inhibitors.
- To ensure prompt recognition and treatment, health care providers should maintain a high index of suspicion for development of cutaneous irAEs in patients using checkpoint inhibitors.
Dermatologists fear effects of Dobbs decision for patients on isotretinoin, methotrexate
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
IVIG proves effective for dermatomyositis in phase 3 trial
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Cell-killing cancer therapy treats lupus successfully
In a first-of-its-kind clinical trial, researchers in Germany used a cancer-killing cell therapy to successfully treat lupus in a small number of patients.
Their study, published online in Nature Medicine, included five patients with systemic lupus erythematosus (SLE). All of the patients were treated with chimeric antigen receptor (CAR) T-cell therapy, a treatment regularly used to kill cancer cells. Researchers harvested the patients’ immune cells and engineered them to destroy dysfunctional cells when infused back into the body.
The five patients – all of whom had an aggressive form of the autoimmune disease – underwent a single infusion of the experimental treatment. All five patients were able to stop their standard treatments for as long as 17 months following the therapy, the study found. The patients also stopped experiencing severe symptoms such as lung inflammation, fibrosis of the heart valves, arthritis, and fatigue. The patients have not relapsed.
“Our data reveal unexpected insights for a role of CAR T cells in nonmalignant diseases that could provide new opportunities for the treatment of autoimmune disease,” the study authors wrote.
Lupus is a chronic inflammatory disease in which the immune system attacks the body’s own cells. Both antibody-producing B and T cells in individuals with lupus become overactive, which can lead to a flare of symptoms that range from mild pain and fatigue to life-threatening inflammation and tissue damage. They are often treated with medications that deplete their B cells or change the way they function to help wipe out infected cells.
The approach used by the study researchers is similar to monoclonal antibody therapies that destroy dysfunctional B cells, such as rituximab (Rituxan and biosimilars) and obinutuzumab (Gazyva), according to Michael Belmont, MD, codirector of New York University’s Lupus Center and medical director of Bellevue Hospital Lupus Clinic, also in New York.
“Previously, this has been accomplished with monoclonal antibodies that target surface markers on B cells and results in their removal,” said Dr. Belmont, who was not connected to the study. “The report describes a novel approach that harnesses a patient’s own T cells, another type of white blood cell, to eliminate that patient’s own B cells.”
Preclinical studies involving mice previously showed that CAR T-cell therapy could help to reset the immune system. However, this latest study also found that patients did not need to continue any of their previous therapies, even after they regained their B cells about 4 months after the therapy.
“A deep depletion of CD19+ B cells and plasmablasts in the tissues could trigger an immune reset in SLE that could allow the cessation of immunosuppressive treatment,” said Mehrnaz Hojjati, MD, a rheumatologist and director of rheumatology operations at Loma Linda (Calif.) University Health. Dr. Hojjati was not affiliated with the study.
While the single-treatment therapy is promising, transfused T cells do carry risks. Some of the patients in the study experienced fever and muscle pain following the procedure, the authors noted. Dr. Belmont said more serious risks for this kind of therapy may include organ injury.
“This treatment can [also] increase incidence, for example, of pneumonia or shingles,” he said.
The study authors initially documented their work in a correspondence published in August 2021 in the New England Journal of Medicine. At that time, they reported that a 20-year-old woman with a severe refractory SLE went into remission following the treatment.
The five patients in the current study – four women and one man – were aged 18-24 years. All of the patients had previously been treated with several immunosuppressive medications, the study authors noted.
“This is an exciting approach, but many more patients need to be treated to really understand the efficacy and safety,” Dr. Belmont said.
Experts, including Dr. Belmont, also said the procedure is also costly and requires access to labs that can engineer a patient’s own T cells after they’ve been donated.
“The entire process must maintain sterility to avoid contamination, which would be harmful when reinfused into the patient,” he said.
According to Arthur Kavanaugh, MD, professor of medicine at UC San Diego Health, this form of therapy may be an option for severe refractory patients who have not responded well to other more established therapies.
“[It’s] exciting data, but very intense and so not likely to be something for an average patient in the near future,” said Dr. Kavanaugh, who was not affiliated with the study.
The study authors say they intend to create a larger trial to further explore which type of patient may benefit the most from this treatment, and for how long.
The study was supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union, and the Innovative Medicines Initiative–funded project, Rheuma Tolerance for Cure. The study received no commercial funding, and the authors said they had no competing interests related to the study. None of the experts interviewed reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a first-of-its-kind clinical trial, researchers in Germany used a cancer-killing cell therapy to successfully treat lupus in a small number of patients.
Their study, published online in Nature Medicine, included five patients with systemic lupus erythematosus (SLE). All of the patients were treated with chimeric antigen receptor (CAR) T-cell therapy, a treatment regularly used to kill cancer cells. Researchers harvested the patients’ immune cells and engineered them to destroy dysfunctional cells when infused back into the body.
The five patients – all of whom had an aggressive form of the autoimmune disease – underwent a single infusion of the experimental treatment. All five patients were able to stop their standard treatments for as long as 17 months following the therapy, the study found. The patients also stopped experiencing severe symptoms such as lung inflammation, fibrosis of the heart valves, arthritis, and fatigue. The patients have not relapsed.
“Our data reveal unexpected insights for a role of CAR T cells in nonmalignant diseases that could provide new opportunities for the treatment of autoimmune disease,” the study authors wrote.
Lupus is a chronic inflammatory disease in which the immune system attacks the body’s own cells. Both antibody-producing B and T cells in individuals with lupus become overactive, which can lead to a flare of symptoms that range from mild pain and fatigue to life-threatening inflammation and tissue damage. They are often treated with medications that deplete their B cells or change the way they function to help wipe out infected cells.
The approach used by the study researchers is similar to monoclonal antibody therapies that destroy dysfunctional B cells, such as rituximab (Rituxan and biosimilars) and obinutuzumab (Gazyva), according to Michael Belmont, MD, codirector of New York University’s Lupus Center and medical director of Bellevue Hospital Lupus Clinic, also in New York.
“Previously, this has been accomplished with monoclonal antibodies that target surface markers on B cells and results in their removal,” said Dr. Belmont, who was not connected to the study. “The report describes a novel approach that harnesses a patient’s own T cells, another type of white blood cell, to eliminate that patient’s own B cells.”
Preclinical studies involving mice previously showed that CAR T-cell therapy could help to reset the immune system. However, this latest study also found that patients did not need to continue any of their previous therapies, even after they regained their B cells about 4 months after the therapy.
“A deep depletion of CD19+ B cells and plasmablasts in the tissues could trigger an immune reset in SLE that could allow the cessation of immunosuppressive treatment,” said Mehrnaz Hojjati, MD, a rheumatologist and director of rheumatology operations at Loma Linda (Calif.) University Health. Dr. Hojjati was not affiliated with the study.
While the single-treatment therapy is promising, transfused T cells do carry risks. Some of the patients in the study experienced fever and muscle pain following the procedure, the authors noted. Dr. Belmont said more serious risks for this kind of therapy may include organ injury.
“This treatment can [also] increase incidence, for example, of pneumonia or shingles,” he said.
The study authors initially documented their work in a correspondence published in August 2021 in the New England Journal of Medicine. At that time, they reported that a 20-year-old woman with a severe refractory SLE went into remission following the treatment.
The five patients in the current study – four women and one man – were aged 18-24 years. All of the patients had previously been treated with several immunosuppressive medications, the study authors noted.
“This is an exciting approach, but many more patients need to be treated to really understand the efficacy and safety,” Dr. Belmont said.
Experts, including Dr. Belmont, also said the procedure is also costly and requires access to labs that can engineer a patient’s own T cells after they’ve been donated.
“The entire process must maintain sterility to avoid contamination, which would be harmful when reinfused into the patient,” he said.
According to Arthur Kavanaugh, MD, professor of medicine at UC San Diego Health, this form of therapy may be an option for severe refractory patients who have not responded well to other more established therapies.
“[It’s] exciting data, but very intense and so not likely to be something for an average patient in the near future,” said Dr. Kavanaugh, who was not affiliated with the study.
The study authors say they intend to create a larger trial to further explore which type of patient may benefit the most from this treatment, and for how long.
The study was supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union, and the Innovative Medicines Initiative–funded project, Rheuma Tolerance for Cure. The study received no commercial funding, and the authors said they had no competing interests related to the study. None of the experts interviewed reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a first-of-its-kind clinical trial, researchers in Germany used a cancer-killing cell therapy to successfully treat lupus in a small number of patients.
Their study, published online in Nature Medicine, included five patients with systemic lupus erythematosus (SLE). All of the patients were treated with chimeric antigen receptor (CAR) T-cell therapy, a treatment regularly used to kill cancer cells. Researchers harvested the patients’ immune cells and engineered them to destroy dysfunctional cells when infused back into the body.
The five patients – all of whom had an aggressive form of the autoimmune disease – underwent a single infusion of the experimental treatment. All five patients were able to stop their standard treatments for as long as 17 months following the therapy, the study found. The patients also stopped experiencing severe symptoms such as lung inflammation, fibrosis of the heart valves, arthritis, and fatigue. The patients have not relapsed.
“Our data reveal unexpected insights for a role of CAR T cells in nonmalignant diseases that could provide new opportunities for the treatment of autoimmune disease,” the study authors wrote.
Lupus is a chronic inflammatory disease in which the immune system attacks the body’s own cells. Both antibody-producing B and T cells in individuals with lupus become overactive, which can lead to a flare of symptoms that range from mild pain and fatigue to life-threatening inflammation and tissue damage. They are often treated with medications that deplete their B cells or change the way they function to help wipe out infected cells.
The approach used by the study researchers is similar to monoclonal antibody therapies that destroy dysfunctional B cells, such as rituximab (Rituxan and biosimilars) and obinutuzumab (Gazyva), according to Michael Belmont, MD, codirector of New York University’s Lupus Center and medical director of Bellevue Hospital Lupus Clinic, also in New York.
“Previously, this has been accomplished with monoclonal antibodies that target surface markers on B cells and results in their removal,” said Dr. Belmont, who was not connected to the study. “The report describes a novel approach that harnesses a patient’s own T cells, another type of white blood cell, to eliminate that patient’s own B cells.”
Preclinical studies involving mice previously showed that CAR T-cell therapy could help to reset the immune system. However, this latest study also found that patients did not need to continue any of their previous therapies, even after they regained their B cells about 4 months after the therapy.
“A deep depletion of CD19+ B cells and plasmablasts in the tissues could trigger an immune reset in SLE that could allow the cessation of immunosuppressive treatment,” said Mehrnaz Hojjati, MD, a rheumatologist and director of rheumatology operations at Loma Linda (Calif.) University Health. Dr. Hojjati was not affiliated with the study.
While the single-treatment therapy is promising, transfused T cells do carry risks. Some of the patients in the study experienced fever and muscle pain following the procedure, the authors noted. Dr. Belmont said more serious risks for this kind of therapy may include organ injury.
“This treatment can [also] increase incidence, for example, of pneumonia or shingles,” he said.
The study authors initially documented their work in a correspondence published in August 2021 in the New England Journal of Medicine. At that time, they reported that a 20-year-old woman with a severe refractory SLE went into remission following the treatment.
The five patients in the current study – four women and one man – were aged 18-24 years. All of the patients had previously been treated with several immunosuppressive medications, the study authors noted.
“This is an exciting approach, but many more patients need to be treated to really understand the efficacy and safety,” Dr. Belmont said.
Experts, including Dr. Belmont, also said the procedure is also costly and requires access to labs that can engineer a patient’s own T cells after they’ve been donated.
“The entire process must maintain sterility to avoid contamination, which would be harmful when reinfused into the patient,” he said.
According to Arthur Kavanaugh, MD, professor of medicine at UC San Diego Health, this form of therapy may be an option for severe refractory patients who have not responded well to other more established therapies.
“[It’s] exciting data, but very intense and so not likely to be something for an average patient in the near future,” said Dr. Kavanaugh, who was not affiliated with the study.
The study authors say they intend to create a larger trial to further explore which type of patient may benefit the most from this treatment, and for how long.
The study was supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union, and the Innovative Medicines Initiative–funded project, Rheuma Tolerance for Cure. The study received no commercial funding, and the authors said they had no competing interests related to the study. None of the experts interviewed reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
One in three MS patients reports chronic itch
, according to investigators.
Itch is historically underrecognized as a symptom of MS, but physicians should know that it is common and may negatively impact quality of life, reported lead author Giuseppe Ingrasci, MD, a dermatology research fellow at the University of Miami, Miller School of Medicine, and colleagues.
While previous publications suggest that pruritus occurs in just 2%-6% of patients with MS, principal author Gil Yosipovitch, MD, professor, Stiefel Chair of Medical Dermatology, and director of the Miami Itch Center in the Dr. Phillip Frost department of dermatology and cutaneous surgery at the University of Miami Miller School of Medicine, encountered itch in enough patients with MS that he presented his observations to a group of neurologists.
Most of them dismissed him, he recalled in an interview: “The neurologists said, ‘Very interesting, but we don’t really see it.’ ”
One of those neurologists, however, decided to take a closer look.
Andrew Brown, MD, assistant professor of clinical neurology and chief of the general neurology division at the University of Miami, Miller School of Medicine, began asking his patients with MS if they were experiencing itch and soon found that it was “a very common problem,” according to Dr. Yosipovitch.
Dr. Yosipovitch, who was the first to report pruritus in patients with psoriasis, launched the present investigation with Dr. Brown to determine if itch is also a blind spot in the world of MS. Their results, and their uphill battle to publication, suggest that it very well could be.
After being rejected from six neurology journals, with one editor suggesting that itch is “not relevant at all to neurology,” their findings were published in the Journal of the European Academy of Dermatology & Venereology.
A common problem that may indicate more severe disease
At the Multiple Sclerosis Center of Excellence in Miami, 27 out of 79 outpatients with MS (35%) reported pruritus, with an average severity of 5.42 out of 10. Among those with itch, the extremities were affected in about half of the patients, while the face, scalp, and trunk were affected in about one-third of the patients. Many described paroxysmal itch that was aggravated by heat, and about half experienced itch on a weekly basis.
Further investigation showed that itch was associated with more severe MS. Compared with patients not experiencing itch, those with itch were significantly more likely to report fatigue (77% vs. 44%), anxiety or depression (48% vs. 16%), and cognitive impairment (62% vs. 26%).
MRI findings backed up these clinical results. Compared with patients not experiencing itch, patients with itch had significantly more T2 hyperintensities in the posterior cervical cord (74.1% vs. 46.0%) and anterior pons/ventromedial medulla (62% vs. 26%). These hyperintensities in the medulla were also associated with an 11-fold increased rate of itch on the face or scalp (odds ratio, 11.3; 95% confidence interval, 1.6-78.6, P = 0.025).
“Health care providers should be aware of episodes of localized, neuropathic itch in MS patients, as they appear to be more prevalent than previously thought and may impair these patients’ quality of life,” the investigators concluded.
Challenges with symptom characterization, management
“This is an important study for both patients and clinicians,” said Justin Abbatemarco, MD, of Cleveland Clinic’s Mellen Center for Multiple Sclerosis, in a written comment. “As the authors mention, many of our patients experience transient symptoms, including many different types of sensory disturbance (that is, pins & needles, burning, electrical shocks, and itching). These symptoms can be really distressing for patients and their caregivers.”
While Dr. Abbatemarco has encountered severe itching in “several patients” with MS, he maintained that it is “relatively uncommon” and noted that MS symptomatology is an inherently cloudy subject.
“I think it is difficult to be definite in any opinion on this topic,” Dr. Abbatemarco said. “How patients experience these symptoms is very subjective and can be difficult to describe/characterize.”
Dr. Abbatemarco emphasized that transient symptoms “do not usually represent MS relapse/flare or new inflammatory disease activity. Instead, we believe these symptoms are related to old areas of injury or demyelination.”
Symptom management can be challenging, he added. He recommended setting realistic expectations, and in the case of pruritus, asking dermatologists to rule out other causes of itch, and to offer “unique treatment approaches.”
Cool the itch?
Noting how heat appears to aggravate itch in patients with MS, Dr. Yosipovitch suggested that one of those unique – and simple – treatment approaches may be cooling itchy areas. Alternatively, clinicians may consider oral agents, like gabapentin to dampen neural transmission, or compounded formulations applied to the skin to reduce neural sensitivity, such as topical ketamine. Finally, Dr. Yosipovitch speculated that newer antibody agents for MS could potentially reduce itch.
All these treatment suggestions are purely hypothetical, he said, and require further investigation before they can be recommended with confidence.
The investigators disclosed relationships with Galderma, Pfizer, Novartis, and others. Dr. Abbatemarco disclosed no conflicts of interest.
Correction, 9/19/22: An earlier version of this article misidentified the photo of Dr. Justin Abbatemarco.
, according to investigators.
Itch is historically underrecognized as a symptom of MS, but physicians should know that it is common and may negatively impact quality of life, reported lead author Giuseppe Ingrasci, MD, a dermatology research fellow at the University of Miami, Miller School of Medicine, and colleagues.
While previous publications suggest that pruritus occurs in just 2%-6% of patients with MS, principal author Gil Yosipovitch, MD, professor, Stiefel Chair of Medical Dermatology, and director of the Miami Itch Center in the Dr. Phillip Frost department of dermatology and cutaneous surgery at the University of Miami Miller School of Medicine, encountered itch in enough patients with MS that he presented his observations to a group of neurologists.
Most of them dismissed him, he recalled in an interview: “The neurologists said, ‘Very interesting, but we don’t really see it.’ ”
One of those neurologists, however, decided to take a closer look.
Andrew Brown, MD, assistant professor of clinical neurology and chief of the general neurology division at the University of Miami, Miller School of Medicine, began asking his patients with MS if they were experiencing itch and soon found that it was “a very common problem,” according to Dr. Yosipovitch.
Dr. Yosipovitch, who was the first to report pruritus in patients with psoriasis, launched the present investigation with Dr. Brown to determine if itch is also a blind spot in the world of MS. Their results, and their uphill battle to publication, suggest that it very well could be.
After being rejected from six neurology journals, with one editor suggesting that itch is “not relevant at all to neurology,” their findings were published in the Journal of the European Academy of Dermatology & Venereology.
A common problem that may indicate more severe disease
At the Multiple Sclerosis Center of Excellence in Miami, 27 out of 79 outpatients with MS (35%) reported pruritus, with an average severity of 5.42 out of 10. Among those with itch, the extremities were affected in about half of the patients, while the face, scalp, and trunk were affected in about one-third of the patients. Many described paroxysmal itch that was aggravated by heat, and about half experienced itch on a weekly basis.
Further investigation showed that itch was associated with more severe MS. Compared with patients not experiencing itch, those with itch were significantly more likely to report fatigue (77% vs. 44%), anxiety or depression (48% vs. 16%), and cognitive impairment (62% vs. 26%).
MRI findings backed up these clinical results. Compared with patients not experiencing itch, patients with itch had significantly more T2 hyperintensities in the posterior cervical cord (74.1% vs. 46.0%) and anterior pons/ventromedial medulla (62% vs. 26%). These hyperintensities in the medulla were also associated with an 11-fold increased rate of itch on the face or scalp (odds ratio, 11.3; 95% confidence interval, 1.6-78.6, P = 0.025).
“Health care providers should be aware of episodes of localized, neuropathic itch in MS patients, as they appear to be more prevalent than previously thought and may impair these patients’ quality of life,” the investigators concluded.
Challenges with symptom characterization, management
“This is an important study for both patients and clinicians,” said Justin Abbatemarco, MD, of Cleveland Clinic’s Mellen Center for Multiple Sclerosis, in a written comment. “As the authors mention, many of our patients experience transient symptoms, including many different types of sensory disturbance (that is, pins & needles, burning, electrical shocks, and itching). These symptoms can be really distressing for patients and their caregivers.”
While Dr. Abbatemarco has encountered severe itching in “several patients” with MS, he maintained that it is “relatively uncommon” and noted that MS symptomatology is an inherently cloudy subject.
“I think it is difficult to be definite in any opinion on this topic,” Dr. Abbatemarco said. “How patients experience these symptoms is very subjective and can be difficult to describe/characterize.”
Dr. Abbatemarco emphasized that transient symptoms “do not usually represent MS relapse/flare or new inflammatory disease activity. Instead, we believe these symptoms are related to old areas of injury or demyelination.”
Symptom management can be challenging, he added. He recommended setting realistic expectations, and in the case of pruritus, asking dermatologists to rule out other causes of itch, and to offer “unique treatment approaches.”
Cool the itch?
Noting how heat appears to aggravate itch in patients with MS, Dr. Yosipovitch suggested that one of those unique – and simple – treatment approaches may be cooling itchy areas. Alternatively, clinicians may consider oral agents, like gabapentin to dampen neural transmission, or compounded formulations applied to the skin to reduce neural sensitivity, such as topical ketamine. Finally, Dr. Yosipovitch speculated that newer antibody agents for MS could potentially reduce itch.
All these treatment suggestions are purely hypothetical, he said, and require further investigation before they can be recommended with confidence.
The investigators disclosed relationships with Galderma, Pfizer, Novartis, and others. Dr. Abbatemarco disclosed no conflicts of interest.
Correction, 9/19/22: An earlier version of this article misidentified the photo of Dr. Justin Abbatemarco.
, according to investigators.
Itch is historically underrecognized as a symptom of MS, but physicians should know that it is common and may negatively impact quality of life, reported lead author Giuseppe Ingrasci, MD, a dermatology research fellow at the University of Miami, Miller School of Medicine, and colleagues.
While previous publications suggest that pruritus occurs in just 2%-6% of patients with MS, principal author Gil Yosipovitch, MD, professor, Stiefel Chair of Medical Dermatology, and director of the Miami Itch Center in the Dr. Phillip Frost department of dermatology and cutaneous surgery at the University of Miami Miller School of Medicine, encountered itch in enough patients with MS that he presented his observations to a group of neurologists.
Most of them dismissed him, he recalled in an interview: “The neurologists said, ‘Very interesting, but we don’t really see it.’ ”
One of those neurologists, however, decided to take a closer look.
Andrew Brown, MD, assistant professor of clinical neurology and chief of the general neurology division at the University of Miami, Miller School of Medicine, began asking his patients with MS if they were experiencing itch and soon found that it was “a very common problem,” according to Dr. Yosipovitch.
Dr. Yosipovitch, who was the first to report pruritus in patients with psoriasis, launched the present investigation with Dr. Brown to determine if itch is also a blind spot in the world of MS. Their results, and their uphill battle to publication, suggest that it very well could be.
After being rejected from six neurology journals, with one editor suggesting that itch is “not relevant at all to neurology,” their findings were published in the Journal of the European Academy of Dermatology & Venereology.
A common problem that may indicate more severe disease
At the Multiple Sclerosis Center of Excellence in Miami, 27 out of 79 outpatients with MS (35%) reported pruritus, with an average severity of 5.42 out of 10. Among those with itch, the extremities were affected in about half of the patients, while the face, scalp, and trunk were affected in about one-third of the patients. Many described paroxysmal itch that was aggravated by heat, and about half experienced itch on a weekly basis.
Further investigation showed that itch was associated with more severe MS. Compared with patients not experiencing itch, those with itch were significantly more likely to report fatigue (77% vs. 44%), anxiety or depression (48% vs. 16%), and cognitive impairment (62% vs. 26%).
MRI findings backed up these clinical results. Compared with patients not experiencing itch, patients with itch had significantly more T2 hyperintensities in the posterior cervical cord (74.1% vs. 46.0%) and anterior pons/ventromedial medulla (62% vs. 26%). These hyperintensities in the medulla were also associated with an 11-fold increased rate of itch on the face or scalp (odds ratio, 11.3; 95% confidence interval, 1.6-78.6, P = 0.025).
“Health care providers should be aware of episodes of localized, neuropathic itch in MS patients, as they appear to be more prevalent than previously thought and may impair these patients’ quality of life,” the investigators concluded.
Challenges with symptom characterization, management
“This is an important study for both patients and clinicians,” said Justin Abbatemarco, MD, of Cleveland Clinic’s Mellen Center for Multiple Sclerosis, in a written comment. “As the authors mention, many of our patients experience transient symptoms, including many different types of sensory disturbance (that is, pins & needles, burning, electrical shocks, and itching). These symptoms can be really distressing for patients and their caregivers.”
While Dr. Abbatemarco has encountered severe itching in “several patients” with MS, he maintained that it is “relatively uncommon” and noted that MS symptomatology is an inherently cloudy subject.
“I think it is difficult to be definite in any opinion on this topic,” Dr. Abbatemarco said. “How patients experience these symptoms is very subjective and can be difficult to describe/characterize.”
Dr. Abbatemarco emphasized that transient symptoms “do not usually represent MS relapse/flare or new inflammatory disease activity. Instead, we believe these symptoms are related to old areas of injury or demyelination.”
Symptom management can be challenging, he added. He recommended setting realistic expectations, and in the case of pruritus, asking dermatologists to rule out other causes of itch, and to offer “unique treatment approaches.”
Cool the itch?
Noting how heat appears to aggravate itch in patients with MS, Dr. Yosipovitch suggested that one of those unique – and simple – treatment approaches may be cooling itchy areas. Alternatively, clinicians may consider oral agents, like gabapentin to dampen neural transmission, or compounded formulations applied to the skin to reduce neural sensitivity, such as topical ketamine. Finally, Dr. Yosipovitch speculated that newer antibody agents for MS could potentially reduce itch.
All these treatment suggestions are purely hypothetical, he said, and require further investigation before they can be recommended with confidence.
The investigators disclosed relationships with Galderma, Pfizer, Novartis, and others. Dr. Abbatemarco disclosed no conflicts of interest.
Correction, 9/19/22: An earlier version of this article misidentified the photo of Dr. Justin Abbatemarco.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY & VENEREOLOGY
Litifilimab meets primary endpoint in phase 2 lupus trial
Treatment with the humanized monoclonal antibody litifilimab for patients with systemic lupus erythematosus (SLE) led to greater improvements in joint manifestations than did placebo in an international phase 2 trial that reflects keen interest in targeting type 1 interferon and the innate immune system.
Litifilimab was associated with an approximately three-joint reduction in the number of swollen and tender joints, compared with placebo, over 24 weeks in the study, which was published in The New England Journal of Medicine.
The study was the first part of the LILAC trial, a two-part, phase 2 study. The second part involved cutaneous lupus erythematosus (CLE) with or without systemic manifestations. Treatment led to improvements in skin disease, as measured by Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores. It was published in the New England Journal of Medicine.
The investigational drug targets blood dendritic cell antigen 2 (BDCA2). The antigen is expressed solely on plasmacytoid dendritic cells (pDCs), which accumulate in skin lesions and organs of patients with SLE. When the antibody binds to BDCA2, “the synthesis of a variety of cytokines is shut down – type 1 interferons, type 3 interferons, TNF [tumor necrosis factor], and [other cytokines and chemokines] made by the pDCs,” Richard A. Furie, MD, lead author of the article, said in an interview.
In a phase 1 trial involving patients with SLE and CLE, the drug’s biologic activity was shown by a dampened interferon signature in blood and modulated type 1 interferon-induced proteins in the skin, he and his coinvestigators noted.
Dr. Furie is chief of rheumatology at Northwell Health and professor of medicine at the Feinstein Institutes for Medical Research at Northwell and at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York.
Impact on the joints
The primary analysis in the SLE trial involved 102 patients who had SLE, arthritis, and active skin disease. The patients received litifilimab 450 mg or placebo, administered subcutaneously, at weeks 0, 2, 4, 8, 12, 16, and 20. The patients were required to have at least four tender joints and at least four swollen joints, and these active joints had to be those classically involved in lupus arthritis.
The mean (± standard deviation) baseline number of active joints was 19 ± 8.4 in the litifilimab group and 21.6 ± 8.5 in placebo group. From baseline to week 24, the least-squares mean (± standard deviation) change in the total number of active joints was –15.0 ± 1.2 with litifilimab and –11.6 ± 1.3 with placebo (mean difference, –3.4; 95% confidence interval, –6.7 to -0.2; P = .04).
Most of the secondary endpoints did not support the results of the primary analysis. However, improvement was seen in the SLE Responder Index (SRI-4) – a three-component global index that Dr. Furie and others developed in 2009 using data from the phase 2 SLE trial of belimumab (Benlysta).
The composite index, used in the phase 3 trial of belimumab, captures improvement in disease activity without a worsening of the condition overall or new significant disease activity in other domains. “It’s a dichotomous measure – either you’re a responder or not,” Dr. Furie said in the interview.
Response on the SRI-4 was defined as a reduction of at least 4 points from baseline in the SLEDAI-2K score (the Systemic Lupus Erythematosus Disease Activity Index), no new disease activity as measured by one score of A (severe) or more than one score of B (moderate) on the BILAG (British Isles Lupus Assessment Group) index, and no increase of 0.3 points or more on the Physician’s Global Assessment.
A total of 56% of the patients in the litifilimab group showed responses on the SRI-4 at week 24, compared with 29% in the placebo group (least-squares mean difference, 26.4%; 95% confidence interval [CI], 9.5-43.2). This is “a robust response” that is much greater than the effect size seen in the phase 3 trial of belimumab or in research on anifrolumab (Saphnelo). Both of those drugs are approved for SLE, Dr. Furie said. “We’ll need to see if it’s reproduced in phase 3.”
There’s “little question that litifilimab works for the skin,” Dr. Furie noted. In the second part of the LILAC study, which focused on CLE, litifilimab demonstrated efficacy, and the SLE trial lends more support. Among several secondary endpoints evaluating skin-related disease activity, a reduction of at least 7 points from baseline in the CLASI-A score (a clinically relevant threshold) occurred in 56% of the litifilimab group and 34% of the placebo group.
The trial was conducted at 55 centers in Asia, Europe, Latin America, and the United States. The SLE part of the study began as a dose-ranging study aimed at evaluating cutaneous lupus activity, but owing to “slow enrollment and to allow an assessment of the effect of litifilimab on arthritis in SLE,” the protocol and primary endpoint were amended before the trial data were unblinded to evaluate only the 450-mg dose among participants with active arthritis and skin disease (at least one active skin lesion), the investigators explained.
Background therapy for SLE was allowed if the therapy was initiated at least 12 weeks before randomization and if dose levels were stable through the trial period. Glucocorticoids had to be tapered to ≤ 10 mg/day according to a specified regimen.
Making progress for lupus
Jane E. Salmon, MD, director of the Lupus and APS Center of Excellence and codirector of the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery in New York, who was not involved in the research, said in an email that she is “cautiously optimistic, because in SLE, successful phase 2 trials too often are followed by unsuccessful phase 3 trials.”
Blocking the production of type 1 interferon by pDCs implicated in SLE pathogenesis has the theoretical advantage of preserving type 1 interferon critical to protection from viruses, she noted. Herpes infections were reported among patients who received litifilimab, but rates were not increased, compared with placebo.
Diversity is an important priority in further research, Dr. Salmon said.
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center in Los Angeles, similarly pointed out in an editorial that accompanied the SLE phase 2 trial that while Black patients make up one-third of the U.S. population with lupus, only about 10% of study participants whose race and ethnicity was reported were Black). (Race was not reported by sites in Europe.)
The results of the LILAC trials “encourage further exploration of interventions that affect upstream lupus inflammatory pathways in the innate immune system in lupus,” Dr. Wallace wrote. He noted that lupus has “lagged behind its rheumatic cousins,” such as rheumatoid arthritis and vasculitis, in drug development.
Developing endpoints and study designs for SLE trials has been challenging, at least partly because it is a multisystem disease, Dr. Furie said. “But we’re making progress.”
Anifrolumab, a type 1 interferon receptor monoclonal antibody that was approved for SLE in July 2021, “may have a broader effect on type 1 interferons,” he noted, while litifilimab “may have a broader effect on proinflammatory cytokines, at least those expressed by pDCs.”
Biogen, the sponsor of the LILAC trial, is currently enrolling patients in phase 3 studies – TOPAZ-1 and TOPAZ-2 – to evaluate litifilimab in SLE over a 52-week period. The company also plans to start a pivotal study of the drug in CLE later this year, according to a press release.
Six coauthors are employees of Biogen; five, including Dr. Furie, reported serving as a consultant to the company; one served on a data and safety monitoring board for Biogen; and Dr. Salmon owns stock in the company.
A version of this article first appeared on Medscape.com.
Treatment with the humanized monoclonal antibody litifilimab for patients with systemic lupus erythematosus (SLE) led to greater improvements in joint manifestations than did placebo in an international phase 2 trial that reflects keen interest in targeting type 1 interferon and the innate immune system.
Litifilimab was associated with an approximately three-joint reduction in the number of swollen and tender joints, compared with placebo, over 24 weeks in the study, which was published in The New England Journal of Medicine.
The study was the first part of the LILAC trial, a two-part, phase 2 study. The second part involved cutaneous lupus erythematosus (CLE) with or without systemic manifestations. Treatment led to improvements in skin disease, as measured by Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores. It was published in the New England Journal of Medicine.
The investigational drug targets blood dendritic cell antigen 2 (BDCA2). The antigen is expressed solely on plasmacytoid dendritic cells (pDCs), which accumulate in skin lesions and organs of patients with SLE. When the antibody binds to BDCA2, “the synthesis of a variety of cytokines is shut down – type 1 interferons, type 3 interferons, TNF [tumor necrosis factor], and [other cytokines and chemokines] made by the pDCs,” Richard A. Furie, MD, lead author of the article, said in an interview.
In a phase 1 trial involving patients with SLE and CLE, the drug’s biologic activity was shown by a dampened interferon signature in blood and modulated type 1 interferon-induced proteins in the skin, he and his coinvestigators noted.
Dr. Furie is chief of rheumatology at Northwell Health and professor of medicine at the Feinstein Institutes for Medical Research at Northwell and at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York.
Impact on the joints
The primary analysis in the SLE trial involved 102 patients who had SLE, arthritis, and active skin disease. The patients received litifilimab 450 mg or placebo, administered subcutaneously, at weeks 0, 2, 4, 8, 12, 16, and 20. The patients were required to have at least four tender joints and at least four swollen joints, and these active joints had to be those classically involved in lupus arthritis.
The mean (± standard deviation) baseline number of active joints was 19 ± 8.4 in the litifilimab group and 21.6 ± 8.5 in placebo group. From baseline to week 24, the least-squares mean (± standard deviation) change in the total number of active joints was –15.0 ± 1.2 with litifilimab and –11.6 ± 1.3 with placebo (mean difference, –3.4; 95% confidence interval, –6.7 to -0.2; P = .04).
Most of the secondary endpoints did not support the results of the primary analysis. However, improvement was seen in the SLE Responder Index (SRI-4) – a three-component global index that Dr. Furie and others developed in 2009 using data from the phase 2 SLE trial of belimumab (Benlysta).
The composite index, used in the phase 3 trial of belimumab, captures improvement in disease activity without a worsening of the condition overall or new significant disease activity in other domains. “It’s a dichotomous measure – either you’re a responder or not,” Dr. Furie said in the interview.
Response on the SRI-4 was defined as a reduction of at least 4 points from baseline in the SLEDAI-2K score (the Systemic Lupus Erythematosus Disease Activity Index), no new disease activity as measured by one score of A (severe) or more than one score of B (moderate) on the BILAG (British Isles Lupus Assessment Group) index, and no increase of 0.3 points or more on the Physician’s Global Assessment.
A total of 56% of the patients in the litifilimab group showed responses on the SRI-4 at week 24, compared with 29% in the placebo group (least-squares mean difference, 26.4%; 95% confidence interval [CI], 9.5-43.2). This is “a robust response” that is much greater than the effect size seen in the phase 3 trial of belimumab or in research on anifrolumab (Saphnelo). Both of those drugs are approved for SLE, Dr. Furie said. “We’ll need to see if it’s reproduced in phase 3.”
There’s “little question that litifilimab works for the skin,” Dr. Furie noted. In the second part of the LILAC study, which focused on CLE, litifilimab demonstrated efficacy, and the SLE trial lends more support. Among several secondary endpoints evaluating skin-related disease activity, a reduction of at least 7 points from baseline in the CLASI-A score (a clinically relevant threshold) occurred in 56% of the litifilimab group and 34% of the placebo group.
The trial was conducted at 55 centers in Asia, Europe, Latin America, and the United States. The SLE part of the study began as a dose-ranging study aimed at evaluating cutaneous lupus activity, but owing to “slow enrollment and to allow an assessment of the effect of litifilimab on arthritis in SLE,” the protocol and primary endpoint were amended before the trial data were unblinded to evaluate only the 450-mg dose among participants with active arthritis and skin disease (at least one active skin lesion), the investigators explained.
Background therapy for SLE was allowed if the therapy was initiated at least 12 weeks before randomization and if dose levels were stable through the trial period. Glucocorticoids had to be tapered to ≤ 10 mg/day according to a specified regimen.
Making progress for lupus
Jane E. Salmon, MD, director of the Lupus and APS Center of Excellence and codirector of the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery in New York, who was not involved in the research, said in an email that she is “cautiously optimistic, because in SLE, successful phase 2 trials too often are followed by unsuccessful phase 3 trials.”
Blocking the production of type 1 interferon by pDCs implicated in SLE pathogenesis has the theoretical advantage of preserving type 1 interferon critical to protection from viruses, she noted. Herpes infections were reported among patients who received litifilimab, but rates were not increased, compared with placebo.
Diversity is an important priority in further research, Dr. Salmon said.
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center in Los Angeles, similarly pointed out in an editorial that accompanied the SLE phase 2 trial that while Black patients make up one-third of the U.S. population with lupus, only about 10% of study participants whose race and ethnicity was reported were Black). (Race was not reported by sites in Europe.)
The results of the LILAC trials “encourage further exploration of interventions that affect upstream lupus inflammatory pathways in the innate immune system in lupus,” Dr. Wallace wrote. He noted that lupus has “lagged behind its rheumatic cousins,” such as rheumatoid arthritis and vasculitis, in drug development.
Developing endpoints and study designs for SLE trials has been challenging, at least partly because it is a multisystem disease, Dr. Furie said. “But we’re making progress.”
Anifrolumab, a type 1 interferon receptor monoclonal antibody that was approved for SLE in July 2021, “may have a broader effect on type 1 interferons,” he noted, while litifilimab “may have a broader effect on proinflammatory cytokines, at least those expressed by pDCs.”
Biogen, the sponsor of the LILAC trial, is currently enrolling patients in phase 3 studies – TOPAZ-1 and TOPAZ-2 – to evaluate litifilimab in SLE over a 52-week period. The company also plans to start a pivotal study of the drug in CLE later this year, according to a press release.
Six coauthors are employees of Biogen; five, including Dr. Furie, reported serving as a consultant to the company; one served on a data and safety monitoring board for Biogen; and Dr. Salmon owns stock in the company.
A version of this article first appeared on Medscape.com.
Treatment with the humanized monoclonal antibody litifilimab for patients with systemic lupus erythematosus (SLE) led to greater improvements in joint manifestations than did placebo in an international phase 2 trial that reflects keen interest in targeting type 1 interferon and the innate immune system.
Litifilimab was associated with an approximately three-joint reduction in the number of swollen and tender joints, compared with placebo, over 24 weeks in the study, which was published in The New England Journal of Medicine.
The study was the first part of the LILAC trial, a two-part, phase 2 study. The second part involved cutaneous lupus erythematosus (CLE) with or without systemic manifestations. Treatment led to improvements in skin disease, as measured by Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores. It was published in the New England Journal of Medicine.
The investigational drug targets blood dendritic cell antigen 2 (BDCA2). The antigen is expressed solely on plasmacytoid dendritic cells (pDCs), which accumulate in skin lesions and organs of patients with SLE. When the antibody binds to BDCA2, “the synthesis of a variety of cytokines is shut down – type 1 interferons, type 3 interferons, TNF [tumor necrosis factor], and [other cytokines and chemokines] made by the pDCs,” Richard A. Furie, MD, lead author of the article, said in an interview.
In a phase 1 trial involving patients with SLE and CLE, the drug’s biologic activity was shown by a dampened interferon signature in blood and modulated type 1 interferon-induced proteins in the skin, he and his coinvestigators noted.
Dr. Furie is chief of rheumatology at Northwell Health and professor of medicine at the Feinstein Institutes for Medical Research at Northwell and at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York.
Impact on the joints
The primary analysis in the SLE trial involved 102 patients who had SLE, arthritis, and active skin disease. The patients received litifilimab 450 mg or placebo, administered subcutaneously, at weeks 0, 2, 4, 8, 12, 16, and 20. The patients were required to have at least four tender joints and at least four swollen joints, and these active joints had to be those classically involved in lupus arthritis.
The mean (± standard deviation) baseline number of active joints was 19 ± 8.4 in the litifilimab group and 21.6 ± 8.5 in placebo group. From baseline to week 24, the least-squares mean (± standard deviation) change in the total number of active joints was –15.0 ± 1.2 with litifilimab and –11.6 ± 1.3 with placebo (mean difference, –3.4; 95% confidence interval, –6.7 to -0.2; P = .04).
Most of the secondary endpoints did not support the results of the primary analysis. However, improvement was seen in the SLE Responder Index (SRI-4) – a three-component global index that Dr. Furie and others developed in 2009 using data from the phase 2 SLE trial of belimumab (Benlysta).
The composite index, used in the phase 3 trial of belimumab, captures improvement in disease activity without a worsening of the condition overall or new significant disease activity in other domains. “It’s a dichotomous measure – either you’re a responder or not,” Dr. Furie said in the interview.
Response on the SRI-4 was defined as a reduction of at least 4 points from baseline in the SLEDAI-2K score (the Systemic Lupus Erythematosus Disease Activity Index), no new disease activity as measured by one score of A (severe) or more than one score of B (moderate) on the BILAG (British Isles Lupus Assessment Group) index, and no increase of 0.3 points or more on the Physician’s Global Assessment.
A total of 56% of the patients in the litifilimab group showed responses on the SRI-4 at week 24, compared with 29% in the placebo group (least-squares mean difference, 26.4%; 95% confidence interval [CI], 9.5-43.2). This is “a robust response” that is much greater than the effect size seen in the phase 3 trial of belimumab or in research on anifrolumab (Saphnelo). Both of those drugs are approved for SLE, Dr. Furie said. “We’ll need to see if it’s reproduced in phase 3.”
There’s “little question that litifilimab works for the skin,” Dr. Furie noted. In the second part of the LILAC study, which focused on CLE, litifilimab demonstrated efficacy, and the SLE trial lends more support. Among several secondary endpoints evaluating skin-related disease activity, a reduction of at least 7 points from baseline in the CLASI-A score (a clinically relevant threshold) occurred in 56% of the litifilimab group and 34% of the placebo group.
The trial was conducted at 55 centers in Asia, Europe, Latin America, and the United States. The SLE part of the study began as a dose-ranging study aimed at evaluating cutaneous lupus activity, but owing to “slow enrollment and to allow an assessment of the effect of litifilimab on arthritis in SLE,” the protocol and primary endpoint were amended before the trial data were unblinded to evaluate only the 450-mg dose among participants with active arthritis and skin disease (at least one active skin lesion), the investigators explained.
Background therapy for SLE was allowed if the therapy was initiated at least 12 weeks before randomization and if dose levels were stable through the trial period. Glucocorticoids had to be tapered to ≤ 10 mg/day according to a specified regimen.
Making progress for lupus
Jane E. Salmon, MD, director of the Lupus and APS Center of Excellence and codirector of the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery in New York, who was not involved in the research, said in an email that she is “cautiously optimistic, because in SLE, successful phase 2 trials too often are followed by unsuccessful phase 3 trials.”
Blocking the production of type 1 interferon by pDCs implicated in SLE pathogenesis has the theoretical advantage of preserving type 1 interferon critical to protection from viruses, she noted. Herpes infections were reported among patients who received litifilimab, but rates were not increased, compared with placebo.
Diversity is an important priority in further research, Dr. Salmon said.
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center in Los Angeles, similarly pointed out in an editorial that accompanied the SLE phase 2 trial that while Black patients make up one-third of the U.S. population with lupus, only about 10% of study participants whose race and ethnicity was reported were Black). (Race was not reported by sites in Europe.)
The results of the LILAC trials “encourage further exploration of interventions that affect upstream lupus inflammatory pathways in the innate immune system in lupus,” Dr. Wallace wrote. He noted that lupus has “lagged behind its rheumatic cousins,” such as rheumatoid arthritis and vasculitis, in drug development.
Developing endpoints and study designs for SLE trials has been challenging, at least partly because it is a multisystem disease, Dr. Furie said. “But we’re making progress.”
Anifrolumab, a type 1 interferon receptor monoclonal antibody that was approved for SLE in July 2021, “may have a broader effect on type 1 interferons,” he noted, while litifilimab “may have a broader effect on proinflammatory cytokines, at least those expressed by pDCs.”
Biogen, the sponsor of the LILAC trial, is currently enrolling patients in phase 3 studies – TOPAZ-1 and TOPAZ-2 – to evaluate litifilimab in SLE over a 52-week period. The company also plans to start a pivotal study of the drug in CLE later this year, according to a press release.
Six coauthors are employees of Biogen; five, including Dr. Furie, reported serving as a consultant to the company; one served on a data and safety monitoring board for Biogen; and Dr. Salmon owns stock in the company.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Hidradenitis Suppurativa
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
Novel study offers clues to sex bias in lupus
Systemic lupus erythematosus (SLE), or lupus, shows a marked sex bias, affecting about nine females for every one male, according to Susan Kovats, PhD, who studies sex differences in immunity at the Oklahoma Medical Research Foundation in Oklahoma City. This characteristic of lupus suggests that hormones are involved in the pathogenesis of the disease. It also suggests, Dr. Kovats said, that the X chromosome might play a role.
Though studies since the 1970s have indicated a significant role for hormones, the issue is still complex and not well understood, and relatively little research has been done on the molecular mechanisms that might be responsible. This may be because of difficulties with influencing the immune system in vitro, said George A. Robinson, PhD, of University College London’s Centre for Rheumatology.
But Dr. Robinson and his team found a unique way of investigating the role of sex chromosomes and hormones in the inflammatory profiles across subjects of different sex, gender, age, and disease status. In research published online in The Lancet Rheumatology, Dr. Robinson and his team looked at immune cells taken from both cisgender men and women and transgender men and women, and thus were able to “get a more physiological view of what sex hormones are doing to the immune system,” he said.
Dr. Kovats agreed that it was a useful approach. “The transgender people provided an opportunity to effectively separate sex hormone levels from chromosome content,” she said in an interview.
Methods and findings
Peripheral blood mononuclear cell (PBMC) samples were taken from cisgender individuals with and without juvenile-onset lupus and assessed for 28 immune-cell subsets, including different T-cell, B-cell, and monotype subsets. Subjects included 39 postpubertal cisgender men and women (17 men and 22 women) who did not have juvenile-onset lupus, and 35 postpubertal cisgender men and women (12 men and 23 women) who did have juvenile onset lupus. All were aged 16-25 years. The transgender group included five transgender men and five transgender women (aged 18-19) who were undergoing gender-affirming sex hormone treatment.
The analysis found that one of the key differences between young postpubertal cisgender men and age-matched cisgender women was that the men had significantly elevated frequencies of regulatory T cells (T-reg cells), and the T-reg cells from young cisgender men had greater suppressive capacity in vitro than did those from cisgender women. In addition, RNA sequencing data from isolated T-reg cells showed the transcriptomic signature of the cisgender men’s T-regs were significantly enriched for genes in the P13K-AKT signaling pathway. The frequency of T-reg cells was not influenced by sex hormones, but their transcriptomic profile was affected.
“These results are beginning to give us an indication of which genes might be differentially regulated by sex hormones and how these are associated with autoimmunity,” Dr. Robinson said. “We’ve also found that, depending on whether you’re a cisgender man or woman, you may have a different pathogenic process to developing lupus. It’s not necessarily that one mechanism drives the disease across both sexes.”
New approaches, better insights
Dr. Kovats was particularly impressed by the methods of this study. “It was a natural study, the kind of thing we can usually do only in mice,” she said.
“One problem with studies on the effects of hormones in disease is that historically researchers have not paid that much attention to the actual hormone levels in the humans they studied. They might look at 100 women and 100 men, roughly between the ages of 20 and 50. We’re starting to see more, but there aren’t a lot of studies correlating numbers of cells in blood with actual hormone levels in the person. And as we know, just because someone’s a certain age doesn’t mean that they have a textbook hormone level. Early menopause, birth-control pills, many things can affect those levels.”
The researchers hope that these findings will shed light on the mechanisms that create sexual bias in autoimmune diseases, particularly lupus, as well as help researchers to better understand the innate and adaptive immunological differences between men and women. It could also be useful in the clinical setting, Dr. Robinson said. Because of the extreme sex bias in lupus, doctors see far more women with the illness than men. When they do see men with lupus, they need to be able to consider how the patient’s sex affects the development and course of the disease. “I think that people need to start looking at patients as clinically different, depending on their sex and gender,” he said. Information like that analyzed in this study could help with that. This could be especially important because as Dr. Kovats pointed out, although men get lupus far less often than women, when they do have it, they tend to have more severe disease.
Help from machines
This study was groundbreaking in another area as well. The researchers used machine learning to analyze the data. “We’ve started working a lot more with these analysis methods to try to answer as much as we can with these smaller data sets,” Dr. Robinson said. “Rather than the conventional analysis that we would typically perform, we’re able to use machine learning and artificial intelligence to try and learn from the data and increase the numbers that we’re working with by using a training data set. This allows us to interrogate the data with a lot more precision.”
The authors declared no competing interests.
Systemic lupus erythematosus (SLE), or lupus, shows a marked sex bias, affecting about nine females for every one male, according to Susan Kovats, PhD, who studies sex differences in immunity at the Oklahoma Medical Research Foundation in Oklahoma City. This characteristic of lupus suggests that hormones are involved in the pathogenesis of the disease. It also suggests, Dr. Kovats said, that the X chromosome might play a role.
Though studies since the 1970s have indicated a significant role for hormones, the issue is still complex and not well understood, and relatively little research has been done on the molecular mechanisms that might be responsible. This may be because of difficulties with influencing the immune system in vitro, said George A. Robinson, PhD, of University College London’s Centre for Rheumatology.
But Dr. Robinson and his team found a unique way of investigating the role of sex chromosomes and hormones in the inflammatory profiles across subjects of different sex, gender, age, and disease status. In research published online in The Lancet Rheumatology, Dr. Robinson and his team looked at immune cells taken from both cisgender men and women and transgender men and women, and thus were able to “get a more physiological view of what sex hormones are doing to the immune system,” he said.
Dr. Kovats agreed that it was a useful approach. “The transgender people provided an opportunity to effectively separate sex hormone levels from chromosome content,” she said in an interview.
Methods and findings
Peripheral blood mononuclear cell (PBMC) samples were taken from cisgender individuals with and without juvenile-onset lupus and assessed for 28 immune-cell subsets, including different T-cell, B-cell, and monotype subsets. Subjects included 39 postpubertal cisgender men and women (17 men and 22 women) who did not have juvenile-onset lupus, and 35 postpubertal cisgender men and women (12 men and 23 women) who did have juvenile onset lupus. All were aged 16-25 years. The transgender group included five transgender men and five transgender women (aged 18-19) who were undergoing gender-affirming sex hormone treatment.
The analysis found that one of the key differences between young postpubertal cisgender men and age-matched cisgender women was that the men had significantly elevated frequencies of regulatory T cells (T-reg cells), and the T-reg cells from young cisgender men had greater suppressive capacity in vitro than did those from cisgender women. In addition, RNA sequencing data from isolated T-reg cells showed the transcriptomic signature of the cisgender men’s T-regs were significantly enriched for genes in the P13K-AKT signaling pathway. The frequency of T-reg cells was not influenced by sex hormones, but their transcriptomic profile was affected.
“These results are beginning to give us an indication of which genes might be differentially regulated by sex hormones and how these are associated with autoimmunity,” Dr. Robinson said. “We’ve also found that, depending on whether you’re a cisgender man or woman, you may have a different pathogenic process to developing lupus. It’s not necessarily that one mechanism drives the disease across both sexes.”
New approaches, better insights
Dr. Kovats was particularly impressed by the methods of this study. “It was a natural study, the kind of thing we can usually do only in mice,” she said.
“One problem with studies on the effects of hormones in disease is that historically researchers have not paid that much attention to the actual hormone levels in the humans they studied. They might look at 100 women and 100 men, roughly between the ages of 20 and 50. We’re starting to see more, but there aren’t a lot of studies correlating numbers of cells in blood with actual hormone levels in the person. And as we know, just because someone’s a certain age doesn’t mean that they have a textbook hormone level. Early menopause, birth-control pills, many things can affect those levels.”
The researchers hope that these findings will shed light on the mechanisms that create sexual bias in autoimmune diseases, particularly lupus, as well as help researchers to better understand the innate and adaptive immunological differences between men and women. It could also be useful in the clinical setting, Dr. Robinson said. Because of the extreme sex bias in lupus, doctors see far more women with the illness than men. When they do see men with lupus, they need to be able to consider how the patient’s sex affects the development and course of the disease. “I think that people need to start looking at patients as clinically different, depending on their sex and gender,” he said. Information like that analyzed in this study could help with that. This could be especially important because as Dr. Kovats pointed out, although men get lupus far less often than women, when they do have it, they tend to have more severe disease.
Help from machines
This study was groundbreaking in another area as well. The researchers used machine learning to analyze the data. “We’ve started working a lot more with these analysis methods to try to answer as much as we can with these smaller data sets,” Dr. Robinson said. “Rather than the conventional analysis that we would typically perform, we’re able to use machine learning and artificial intelligence to try and learn from the data and increase the numbers that we’re working with by using a training data set. This allows us to interrogate the data with a lot more precision.”
The authors declared no competing interests.
Systemic lupus erythematosus (SLE), or lupus, shows a marked sex bias, affecting about nine females for every one male, according to Susan Kovats, PhD, who studies sex differences in immunity at the Oklahoma Medical Research Foundation in Oklahoma City. This characteristic of lupus suggests that hormones are involved in the pathogenesis of the disease. It also suggests, Dr. Kovats said, that the X chromosome might play a role.
Though studies since the 1970s have indicated a significant role for hormones, the issue is still complex and not well understood, and relatively little research has been done on the molecular mechanisms that might be responsible. This may be because of difficulties with influencing the immune system in vitro, said George A. Robinson, PhD, of University College London’s Centre for Rheumatology.
But Dr. Robinson and his team found a unique way of investigating the role of sex chromosomes and hormones in the inflammatory profiles across subjects of different sex, gender, age, and disease status. In research published online in The Lancet Rheumatology, Dr. Robinson and his team looked at immune cells taken from both cisgender men and women and transgender men and women, and thus were able to “get a more physiological view of what sex hormones are doing to the immune system,” he said.
Dr. Kovats agreed that it was a useful approach. “The transgender people provided an opportunity to effectively separate sex hormone levels from chromosome content,” she said in an interview.
Methods and findings
Peripheral blood mononuclear cell (PBMC) samples were taken from cisgender individuals with and without juvenile-onset lupus and assessed for 28 immune-cell subsets, including different T-cell, B-cell, and monotype subsets. Subjects included 39 postpubertal cisgender men and women (17 men and 22 women) who did not have juvenile-onset lupus, and 35 postpubertal cisgender men and women (12 men and 23 women) who did have juvenile onset lupus. All were aged 16-25 years. The transgender group included five transgender men and five transgender women (aged 18-19) who were undergoing gender-affirming sex hormone treatment.
The analysis found that one of the key differences between young postpubertal cisgender men and age-matched cisgender women was that the men had significantly elevated frequencies of regulatory T cells (T-reg cells), and the T-reg cells from young cisgender men had greater suppressive capacity in vitro than did those from cisgender women. In addition, RNA sequencing data from isolated T-reg cells showed the transcriptomic signature of the cisgender men’s T-regs were significantly enriched for genes in the P13K-AKT signaling pathway. The frequency of T-reg cells was not influenced by sex hormones, but their transcriptomic profile was affected.
“These results are beginning to give us an indication of which genes might be differentially regulated by sex hormones and how these are associated with autoimmunity,” Dr. Robinson said. “We’ve also found that, depending on whether you’re a cisgender man or woman, you may have a different pathogenic process to developing lupus. It’s not necessarily that one mechanism drives the disease across both sexes.”
New approaches, better insights
Dr. Kovats was particularly impressed by the methods of this study. “It was a natural study, the kind of thing we can usually do only in mice,” she said.
“One problem with studies on the effects of hormones in disease is that historically researchers have not paid that much attention to the actual hormone levels in the humans they studied. They might look at 100 women and 100 men, roughly between the ages of 20 and 50. We’re starting to see more, but there aren’t a lot of studies correlating numbers of cells in blood with actual hormone levels in the person. And as we know, just because someone’s a certain age doesn’t mean that they have a textbook hormone level. Early menopause, birth-control pills, many things can affect those levels.”
The researchers hope that these findings will shed light on the mechanisms that create sexual bias in autoimmune diseases, particularly lupus, as well as help researchers to better understand the innate and adaptive immunological differences between men and women. It could also be useful in the clinical setting, Dr. Robinson said. Because of the extreme sex bias in lupus, doctors see far more women with the illness than men. When they do see men with lupus, they need to be able to consider how the patient’s sex affects the development and course of the disease. “I think that people need to start looking at patients as clinically different, depending on their sex and gender,” he said. Information like that analyzed in this study could help with that. This could be especially important because as Dr. Kovats pointed out, although men get lupus far less often than women, when they do have it, they tend to have more severe disease.
Help from machines
This study was groundbreaking in another area as well. The researchers used machine learning to analyze the data. “We’ve started working a lot more with these analysis methods to try to answer as much as we can with these smaller data sets,” Dr. Robinson said. “Rather than the conventional analysis that we would typically perform, we’re able to use machine learning and artificial intelligence to try and learn from the data and increase the numbers that we’re working with by using a training data set. This allows us to interrogate the data with a lot more precision.”
The authors declared no competing interests.
FROM THE LANCET RHEUMATOLOGY