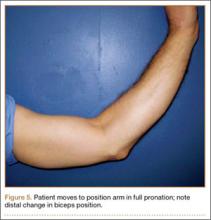
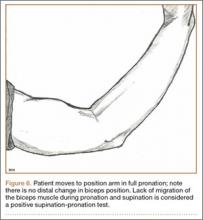
User login
Biceps Tenodesis and Superior Labrum Anterior to Posterior (SLAP) Tears
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
Does Arthritis Contribute to Higher Rates of Poverty In Women?
Developing arthritis increases a person’s risk of falling into poverty, especially for women, according to a study published online ahead of print September 8 in Arthritis & Rheumatology.
For this study of more than 4,000 Australian adults, females who developed arthritis were 51% more likely to fall into income poverty than nonarthritic women. In men, having arthritis was associated with a 22% increased risk of poverty.
Women with arthritis also were 87% more likely to fall into “multidimensional poverty,” which includes income, health, and education attainment, while the arthritis-related risk in men was 29%. The investigators noted that given the high prevalence of arthritis, the condition is an overlooked driver of poverty.
“With population ageing occurring in most of the developed nations around the world, health conditions such as arthritis will become increasingly common. That developing arthritis has such a pronounced impact on the risk of falling into poverty should flag to policy makers in welfare departments the influence of the condition on national living standards,” said lead author Emily Callander, PhD, from the Faculty of Pharmacy at the University of Sydney in Australia.
Researchers utilized survey data from the Household Income and Labour Dynamics in Australia (HILDA) Survey. Survival analysis using Cox regression models were applied to the nationally representative, longitudinal data between the years 2007 and 2012 for Australian adults ages 21 and older.
The hazard ratio for falling into income poverty for females who develop arthritis was 1.51, and for males the hazard ratio for falling into income poverty was 1.22, compared with people who never developed arthritis. The hazard ratio for falling into multidimensional poverty for females who develop arthritis was 1.87 and for males the hazard ratio was 1.29.
According to Dr. Callander, “The high risk of poverty should be kept in mind by clinicians seeking the most appropriate treatment for their patients with arthritis, as affordability of out-of-pocket costs may be an important factor.”
Suggested Reading
Callander EJ, Schofield DJ. Arthritis and the risk of falling into poverty: a survival analysis using Australian data. Arthritis Rheumatol. 2015 Sep 8. [Epub ahead of print].
Developing arthritis increases a person’s risk of falling into poverty, especially for women, according to a study published online ahead of print September 8 in Arthritis & Rheumatology.
For this study of more than 4,000 Australian adults, females who developed arthritis were 51% more likely to fall into income poverty than nonarthritic women. In men, having arthritis was associated with a 22% increased risk of poverty.
Women with arthritis also were 87% more likely to fall into “multidimensional poverty,” which includes income, health, and education attainment, while the arthritis-related risk in men was 29%. The investigators noted that given the high prevalence of arthritis, the condition is an overlooked driver of poverty.
“With population ageing occurring in most of the developed nations around the world, health conditions such as arthritis will become increasingly common. That developing arthritis has such a pronounced impact on the risk of falling into poverty should flag to policy makers in welfare departments the influence of the condition on national living standards,” said lead author Emily Callander, PhD, from the Faculty of Pharmacy at the University of Sydney in Australia.
Researchers utilized survey data from the Household Income and Labour Dynamics in Australia (HILDA) Survey. Survival analysis using Cox regression models were applied to the nationally representative, longitudinal data between the years 2007 and 2012 for Australian adults ages 21 and older.
The hazard ratio for falling into income poverty for females who develop arthritis was 1.51, and for males the hazard ratio for falling into income poverty was 1.22, compared with people who never developed arthritis. The hazard ratio for falling into multidimensional poverty for females who develop arthritis was 1.87 and for males the hazard ratio was 1.29.
According to Dr. Callander, “The high risk of poverty should be kept in mind by clinicians seeking the most appropriate treatment for their patients with arthritis, as affordability of out-of-pocket costs may be an important factor.”
Developing arthritis increases a person’s risk of falling into poverty, especially for women, according to a study published online ahead of print September 8 in Arthritis & Rheumatology.
For this study of more than 4,000 Australian adults, females who developed arthritis were 51% more likely to fall into income poverty than nonarthritic women. In men, having arthritis was associated with a 22% increased risk of poverty.
Women with arthritis also were 87% more likely to fall into “multidimensional poverty,” which includes income, health, and education attainment, while the arthritis-related risk in men was 29%. The investigators noted that given the high prevalence of arthritis, the condition is an overlooked driver of poverty.
“With population ageing occurring in most of the developed nations around the world, health conditions such as arthritis will become increasingly common. That developing arthritis has such a pronounced impact on the risk of falling into poverty should flag to policy makers in welfare departments the influence of the condition on national living standards,” said lead author Emily Callander, PhD, from the Faculty of Pharmacy at the University of Sydney in Australia.
Researchers utilized survey data from the Household Income and Labour Dynamics in Australia (HILDA) Survey. Survival analysis using Cox regression models were applied to the nationally representative, longitudinal data between the years 2007 and 2012 for Australian adults ages 21 and older.
The hazard ratio for falling into income poverty for females who develop arthritis was 1.51, and for males the hazard ratio for falling into income poverty was 1.22, compared with people who never developed arthritis. The hazard ratio for falling into multidimensional poverty for females who develop arthritis was 1.87 and for males the hazard ratio was 1.29.
According to Dr. Callander, “The high risk of poverty should be kept in mind by clinicians seeking the most appropriate treatment for their patients with arthritis, as affordability of out-of-pocket costs may be an important factor.”
Suggested Reading
Callander EJ, Schofield DJ. Arthritis and the risk of falling into poverty: a survival analysis using Australian data. Arthritis Rheumatol. 2015 Sep 8. [Epub ahead of print].
Suggested Reading
Callander EJ, Schofield DJ. Arthritis and the risk of falling into poverty: a survival analysis using Australian data. Arthritis Rheumatol. 2015 Sep 8. [Epub ahead of print].
Changing Paradigms in Short Stay Total Joint Arthroplasty
Failure of the Stem-Condyle Junction of a Modular Femoral Stem in Revision Total Knee Arthroplasty
Revision total knee arthroplasty (TKA) is frequently complicated by bone loss and ligament instability, necessitating specialized implants to increase constraint and transmit forces away from the joint surface. Femoral stems are commonly used to enhance fixation and distribute force from the condyles to the metaphysis or diaphysis, to higher-quality bone capable of sustaining the forces at the knee joint.
Modular implants are now commonplace in revision surgery, because they allow intraoperative customization of the implant to the patient’s anatomy, degree of bone loss, and need for metaphyseal or diaphyseal fixation. However, these advantages are not without a downside. The modular junction introduces potential weaknesses in the implant, which may lead to early failure.
We report a case of loosening of a Triathlon TS (Stryker) femoral component that was not evident on preoperative radiographs. To our knowledge, this complication has not been reported with this particular revision knee system. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman underwent 2-stage revision left TKA secondary to infection at an outside institution. She had undergone 17 prior knee surgeries with multiple revisions prior to this most recent revision surgery. A constrained implant was used at her last reimplantation secondary to ligamentous laxity after extensive débridement for infection. A Triathlon TS revision knee system with cemented stemmed tibial and femoral components was implanted; stems designed for uncemented fixation were cemented. She had a history of a quadriceps tendon tear, which was repaired prior to her revision, and quadricepsplasty was performed at the time of revision.
Seven years after this revision surgery, the patient presented to our clinic with progressive global instability, occasional effusions, and 2 documented episodes of frank dislocation. On examination, she was unstable in flexion and extension. Her extensor mechanism was intact, although with 7º active lag. She had a palpable quadriceps tendon defect. Her passive range of motion was 0º to 130º. Her active range of motion was 7º to 130º. Her erythrocyte sedimentation rate and C-reactive protein levels were within normal limits, and aspiration was negative for infection. Radiographs showed apparently well-fixed components with cemented femoral and tibial stems (Figures 1A, 1B).
The patient underwent revision surgery for global instability with the surgical goal to upsize the polyethylene insert and advance the quadriceps to improve stability. In the operating room, a defect in the quadriceps mechanism was seen between the vastus medialis obliquus (VMO) and the patella, as well as a large effusion. Upon removal of the polyethylene insert, the tibial and patellar components were examined and found to be well fixed. The femoral component was grossly loose. On closer inspection, the condylar portion was found to be rotating in the axial plane freely on the well-fixed cemented stem in the femoral canal (Figures 2A-2D). The entire femoral component was removed with some difficulty because the well-fixed uncemented stem design was cemented in place. This required a small, anterior episiotomy of the femur. Reconstruction of the femur was performed using a trabecular metal cone, a cemented stem, and condylar component with distal and posterior augments (Figures 3A, 3B). A shorter, thinner stem was implanted and cemented into the previous cement mantle. A 19-mm constrained polyethylene liner was selected (the prior liner was 13 mm), which gave adequate stability with range of motion 0º to 130º. The VMO was advanced approximately 1.5 cm at the time of closure of the arthrotomy. The patient was implanted with the same Triathlon TS system, because the tibial component was well fixed, well positioned, and did not require revision.
Discussion
The need and use of stemmed, modular femoral components for revision TKA is neither questioned nor a novel concept in arthroplasty.1 Femoral bone defects encountered in revision arthroplasty generally lack sufficient cortical integrity to support an unstemmed component. Biomechanical analyses have reliably demonstrated improved initial stability and reduced relative motion provided by femoral stem extension.2,3 Correspondingly, significant translational and rotational movements of the femoral component when disconnected from the stem presumably correspond with clinical observations of instability.3 We report a unique case of failure of the modular junction of a stemmed femoral component in revision TKA that was not readily apparent on plain radiographs.
Dissociation of a cemented stem from the condylar portion of the component has been described at our institution with a different implant design.4 To our knowledge, we describe the first report of failure at the modular junction of the Triathlon TS femoral component.
Interestingly, relative motion has been shown to increase with increasing flexion in a biomechanical study2 using the same Triathlon TS system. The authors of that study found they were unable to complete testing at flexion greater than 30º because, absent the stabilizing influence of surrounding ligament and muscle, the sample deformation was so significant that it caused fracture.2 In the case of our patient, the incompetence of her extensor mechanism likely resulted in increased forces transmitted through the implant than might be expected in more physiologic circumstances. This higher stress may account in part for the failure of the implant at the known weakest point, the stem-condyle modular junction.
Modular implants are routinely used, given the variability of scenarios encountered in revision surgery and the need for customization to provide the best approximation of physiologic functioning of the joint. However, modular components introduce junctional points, which are potential points of failure. Stresses on the femoral component occur in multiple dimensions besides the axial loading and medial-lateral, anterior-posterior rocking seen with the tibial component. The maximum stress is observed at the distal-most aspect of the stiffest or most well-fixed components, in this case, the articulation between the cemented stem and the cemented condylar component. Poor distal femoral fixation compounds the problem.
Numerous case reports have documented such failures in other knee systems. Issack and colleagues5 described 2 cases of fracture through the taper lock between the femoral component and the stem extension in the Optetrak stemmed-constrained condylar knee prosthesis (Exactech). Westrich and colleagues6 reported disengagement of the locking bolt of the Insall-Burstein II Constrained Condylar Knee (Zimmer) leading to failure. Lim and colleagues4 reported stem-condyle junctional failure of the Total Condylar III (DePuy, Johnson & Johnson) due to locking-screw failure. Butt and colleagues7 reported a case of failure at the femoral component–stem junction caused by screw breakage. All of these cases involved failure at the condylar-stem junction that was readily apparent on routine preoperative imaging.
Our case is noteworthy because there was no preoperative radiographic evidence that the components were loose or the junction had failed. As with many revision systems observed by Fehring and colleagues,8 determination of fixation is often based on the appearance of the stem because the distal femoral interfaces may be obscured by the intercondylar box. This suggests that a loose component at the stem-condylar junction could easily be overlooked and not appropriately revised based on imaging alone. A solution for achieving stability at the time of revision surgery is to obtain good distal bone apposition and fixation. In this case, a cemented stem with a metaphyseal cone was used for femoral fixation (Figures 3A, 3B).
While long-term, abnormally high stress transmitted through the modular junction may account for the implant’s failure, to our knowledge, this is the first report of its kind related to this particular implant. If quadriceps weakness contributed to this failure, it is worth considering that quadriceps weakness is common after TKA and may persist without appropriate rehabilitation and activity. Furthermore, the lack of evidence on plain radiographs makes this particular form of failure very difficult to screen. A high degree of suspicion for loosening should be maintained in patients with pain and instability after revision TKA with this implant as well as with other modular revision knee systems.
1. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
2. Conlisk N, Gray H, Pankaj P, Howie CR. The influence of stem length and fixation on initial femoral component stability in revision total knee replacement. Bone Joint Res. 2012;1(11):281-288.
3. van Loon CJ, Kyriazopoulos A, Verdonschot N, de Waal Malefijt MC, Huiskes R, Buma P. The role of femoral stem extension in total knee arthroplasty. Clin Orthop Relat Res. 2000;(378):282-289.
4. Lim LA, Trousdale RT, Berry DJ, Hanssen AD. Failure of the stem-condyle junction of a modular femoral stem in revision total knee arthroplasty: a report of five cases. J Arthroplasty. 2001;16(1):128-132.
5. Issack PS, Cottrell JM, Delgado S, Wright TM, Sculco TP, Su EP. Failure at the taper lock of a modular stemmed femoral implant in revision knee arthroplasty. A report of two cases and a retrieval analysis. J Bone Joint Surg Am. 2007;89(10):2271-2274.
6. Westrich GH, Hidaka C, Windsor RE. Disengagement of a locking screw from a modular stem in revision total knee arthroplasty. A report of three cases. J Bone Joint Surg Am. 1997;79(2):254-258.
7. Butt AJ, Shaikh AH, Cameron HU. Coupling failure between stem and femoral component in a constrained revision total knee arthroplasty. J Coll Physicians Surg Pak. 2013;23(2):162-163.
8. Fehring TK, Odum S, Olekson C, Griffin WL, Mason JB, McCoy TH. Stem fixation in revision total knee arthroplasty: a comparative analysis. Clin Orthop Relat Res. 2003;(416):217-224.
Revision total knee arthroplasty (TKA) is frequently complicated by bone loss and ligament instability, necessitating specialized implants to increase constraint and transmit forces away from the joint surface. Femoral stems are commonly used to enhance fixation and distribute force from the condyles to the metaphysis or diaphysis, to higher-quality bone capable of sustaining the forces at the knee joint.
Modular implants are now commonplace in revision surgery, because they allow intraoperative customization of the implant to the patient’s anatomy, degree of bone loss, and need for metaphyseal or diaphyseal fixation. However, these advantages are not without a downside. The modular junction introduces potential weaknesses in the implant, which may lead to early failure.
We report a case of loosening of a Triathlon TS (Stryker) femoral component that was not evident on preoperative radiographs. To our knowledge, this complication has not been reported with this particular revision knee system. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman underwent 2-stage revision left TKA secondary to infection at an outside institution. She had undergone 17 prior knee surgeries with multiple revisions prior to this most recent revision surgery. A constrained implant was used at her last reimplantation secondary to ligamentous laxity after extensive débridement for infection. A Triathlon TS revision knee system with cemented stemmed tibial and femoral components was implanted; stems designed for uncemented fixation were cemented. She had a history of a quadriceps tendon tear, which was repaired prior to her revision, and quadricepsplasty was performed at the time of revision.
Seven years after this revision surgery, the patient presented to our clinic with progressive global instability, occasional effusions, and 2 documented episodes of frank dislocation. On examination, she was unstable in flexion and extension. Her extensor mechanism was intact, although with 7º active lag. She had a palpable quadriceps tendon defect. Her passive range of motion was 0º to 130º. Her active range of motion was 7º to 130º. Her erythrocyte sedimentation rate and C-reactive protein levels were within normal limits, and aspiration was negative for infection. Radiographs showed apparently well-fixed components with cemented femoral and tibial stems (Figures 1A, 1B).
The patient underwent revision surgery for global instability with the surgical goal to upsize the polyethylene insert and advance the quadriceps to improve stability. In the operating room, a defect in the quadriceps mechanism was seen between the vastus medialis obliquus (VMO) and the patella, as well as a large effusion. Upon removal of the polyethylene insert, the tibial and patellar components were examined and found to be well fixed. The femoral component was grossly loose. On closer inspection, the condylar portion was found to be rotating in the axial plane freely on the well-fixed cemented stem in the femoral canal (Figures 2A-2D). The entire femoral component was removed with some difficulty because the well-fixed uncemented stem design was cemented in place. This required a small, anterior episiotomy of the femur. Reconstruction of the femur was performed using a trabecular metal cone, a cemented stem, and condylar component with distal and posterior augments (Figures 3A, 3B). A shorter, thinner stem was implanted and cemented into the previous cement mantle. A 19-mm constrained polyethylene liner was selected (the prior liner was 13 mm), which gave adequate stability with range of motion 0º to 130º. The VMO was advanced approximately 1.5 cm at the time of closure of the arthrotomy. The patient was implanted with the same Triathlon TS system, because the tibial component was well fixed, well positioned, and did not require revision.
Discussion
The need and use of stemmed, modular femoral components for revision TKA is neither questioned nor a novel concept in arthroplasty.1 Femoral bone defects encountered in revision arthroplasty generally lack sufficient cortical integrity to support an unstemmed component. Biomechanical analyses have reliably demonstrated improved initial stability and reduced relative motion provided by femoral stem extension.2,3 Correspondingly, significant translational and rotational movements of the femoral component when disconnected from the stem presumably correspond with clinical observations of instability.3 We report a unique case of failure of the modular junction of a stemmed femoral component in revision TKA that was not readily apparent on plain radiographs.
Dissociation of a cemented stem from the condylar portion of the component has been described at our institution with a different implant design.4 To our knowledge, we describe the first report of failure at the modular junction of the Triathlon TS femoral component.
Interestingly, relative motion has been shown to increase with increasing flexion in a biomechanical study2 using the same Triathlon TS system. The authors of that study found they were unable to complete testing at flexion greater than 30º because, absent the stabilizing influence of surrounding ligament and muscle, the sample deformation was so significant that it caused fracture.2 In the case of our patient, the incompetence of her extensor mechanism likely resulted in increased forces transmitted through the implant than might be expected in more physiologic circumstances. This higher stress may account in part for the failure of the implant at the known weakest point, the stem-condyle modular junction.
Modular implants are routinely used, given the variability of scenarios encountered in revision surgery and the need for customization to provide the best approximation of physiologic functioning of the joint. However, modular components introduce junctional points, which are potential points of failure. Stresses on the femoral component occur in multiple dimensions besides the axial loading and medial-lateral, anterior-posterior rocking seen with the tibial component. The maximum stress is observed at the distal-most aspect of the stiffest or most well-fixed components, in this case, the articulation between the cemented stem and the cemented condylar component. Poor distal femoral fixation compounds the problem.
Numerous case reports have documented such failures in other knee systems. Issack and colleagues5 described 2 cases of fracture through the taper lock between the femoral component and the stem extension in the Optetrak stemmed-constrained condylar knee prosthesis (Exactech). Westrich and colleagues6 reported disengagement of the locking bolt of the Insall-Burstein II Constrained Condylar Knee (Zimmer) leading to failure. Lim and colleagues4 reported stem-condyle junctional failure of the Total Condylar III (DePuy, Johnson & Johnson) due to locking-screw failure. Butt and colleagues7 reported a case of failure at the femoral component–stem junction caused by screw breakage. All of these cases involved failure at the condylar-stem junction that was readily apparent on routine preoperative imaging.
Our case is noteworthy because there was no preoperative radiographic evidence that the components were loose or the junction had failed. As with many revision systems observed by Fehring and colleagues,8 determination of fixation is often based on the appearance of the stem because the distal femoral interfaces may be obscured by the intercondylar box. This suggests that a loose component at the stem-condylar junction could easily be overlooked and not appropriately revised based on imaging alone. A solution for achieving stability at the time of revision surgery is to obtain good distal bone apposition and fixation. In this case, a cemented stem with a metaphyseal cone was used for femoral fixation (Figures 3A, 3B).
While long-term, abnormally high stress transmitted through the modular junction may account for the implant’s failure, to our knowledge, this is the first report of its kind related to this particular implant. If quadriceps weakness contributed to this failure, it is worth considering that quadriceps weakness is common after TKA and may persist without appropriate rehabilitation and activity. Furthermore, the lack of evidence on plain radiographs makes this particular form of failure very difficult to screen. A high degree of suspicion for loosening should be maintained in patients with pain and instability after revision TKA with this implant as well as with other modular revision knee systems.
Revision total knee arthroplasty (TKA) is frequently complicated by bone loss and ligament instability, necessitating specialized implants to increase constraint and transmit forces away from the joint surface. Femoral stems are commonly used to enhance fixation and distribute force from the condyles to the metaphysis or diaphysis, to higher-quality bone capable of sustaining the forces at the knee joint.
Modular implants are now commonplace in revision surgery, because they allow intraoperative customization of the implant to the patient’s anatomy, degree of bone loss, and need for metaphyseal or diaphyseal fixation. However, these advantages are not without a downside. The modular junction introduces potential weaknesses in the implant, which may lead to early failure.
We report a case of loosening of a Triathlon TS (Stryker) femoral component that was not evident on preoperative radiographs. To our knowledge, this complication has not been reported with this particular revision knee system. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman underwent 2-stage revision left TKA secondary to infection at an outside institution. She had undergone 17 prior knee surgeries with multiple revisions prior to this most recent revision surgery. A constrained implant was used at her last reimplantation secondary to ligamentous laxity after extensive débridement for infection. A Triathlon TS revision knee system with cemented stemmed tibial and femoral components was implanted; stems designed for uncemented fixation were cemented. She had a history of a quadriceps tendon tear, which was repaired prior to her revision, and quadricepsplasty was performed at the time of revision.
Seven years after this revision surgery, the patient presented to our clinic with progressive global instability, occasional effusions, and 2 documented episodes of frank dislocation. On examination, she was unstable in flexion and extension. Her extensor mechanism was intact, although with 7º active lag. She had a palpable quadriceps tendon defect. Her passive range of motion was 0º to 130º. Her active range of motion was 7º to 130º. Her erythrocyte sedimentation rate and C-reactive protein levels were within normal limits, and aspiration was negative for infection. Radiographs showed apparently well-fixed components with cemented femoral and tibial stems (Figures 1A, 1B).
The patient underwent revision surgery for global instability with the surgical goal to upsize the polyethylene insert and advance the quadriceps to improve stability. In the operating room, a defect in the quadriceps mechanism was seen between the vastus medialis obliquus (VMO) and the patella, as well as a large effusion. Upon removal of the polyethylene insert, the tibial and patellar components were examined and found to be well fixed. The femoral component was grossly loose. On closer inspection, the condylar portion was found to be rotating in the axial plane freely on the well-fixed cemented stem in the femoral canal (Figures 2A-2D). The entire femoral component was removed with some difficulty because the well-fixed uncemented stem design was cemented in place. This required a small, anterior episiotomy of the femur. Reconstruction of the femur was performed using a trabecular metal cone, a cemented stem, and condylar component with distal and posterior augments (Figures 3A, 3B). A shorter, thinner stem was implanted and cemented into the previous cement mantle. A 19-mm constrained polyethylene liner was selected (the prior liner was 13 mm), which gave adequate stability with range of motion 0º to 130º. The VMO was advanced approximately 1.5 cm at the time of closure of the arthrotomy. The patient was implanted with the same Triathlon TS system, because the tibial component was well fixed, well positioned, and did not require revision.
Discussion
The need and use of stemmed, modular femoral components for revision TKA is neither questioned nor a novel concept in arthroplasty.1 Femoral bone defects encountered in revision arthroplasty generally lack sufficient cortical integrity to support an unstemmed component. Biomechanical analyses have reliably demonstrated improved initial stability and reduced relative motion provided by femoral stem extension.2,3 Correspondingly, significant translational and rotational movements of the femoral component when disconnected from the stem presumably correspond with clinical observations of instability.3 We report a unique case of failure of the modular junction of a stemmed femoral component in revision TKA that was not readily apparent on plain radiographs.
Dissociation of a cemented stem from the condylar portion of the component has been described at our institution with a different implant design.4 To our knowledge, we describe the first report of failure at the modular junction of the Triathlon TS femoral component.
Interestingly, relative motion has been shown to increase with increasing flexion in a biomechanical study2 using the same Triathlon TS system. The authors of that study found they were unable to complete testing at flexion greater than 30º because, absent the stabilizing influence of surrounding ligament and muscle, the sample deformation was so significant that it caused fracture.2 In the case of our patient, the incompetence of her extensor mechanism likely resulted in increased forces transmitted through the implant than might be expected in more physiologic circumstances. This higher stress may account in part for the failure of the implant at the known weakest point, the stem-condyle modular junction.
Modular implants are routinely used, given the variability of scenarios encountered in revision surgery and the need for customization to provide the best approximation of physiologic functioning of the joint. However, modular components introduce junctional points, which are potential points of failure. Stresses on the femoral component occur in multiple dimensions besides the axial loading and medial-lateral, anterior-posterior rocking seen with the tibial component. The maximum stress is observed at the distal-most aspect of the stiffest or most well-fixed components, in this case, the articulation between the cemented stem and the cemented condylar component. Poor distal femoral fixation compounds the problem.
Numerous case reports have documented such failures in other knee systems. Issack and colleagues5 described 2 cases of fracture through the taper lock between the femoral component and the stem extension in the Optetrak stemmed-constrained condylar knee prosthesis (Exactech). Westrich and colleagues6 reported disengagement of the locking bolt of the Insall-Burstein II Constrained Condylar Knee (Zimmer) leading to failure. Lim and colleagues4 reported stem-condyle junctional failure of the Total Condylar III (DePuy, Johnson & Johnson) due to locking-screw failure. Butt and colleagues7 reported a case of failure at the femoral component–stem junction caused by screw breakage. All of these cases involved failure at the condylar-stem junction that was readily apparent on routine preoperative imaging.
Our case is noteworthy because there was no preoperative radiographic evidence that the components were loose or the junction had failed. As with many revision systems observed by Fehring and colleagues,8 determination of fixation is often based on the appearance of the stem because the distal femoral interfaces may be obscured by the intercondylar box. This suggests that a loose component at the stem-condylar junction could easily be overlooked and not appropriately revised based on imaging alone. A solution for achieving stability at the time of revision surgery is to obtain good distal bone apposition and fixation. In this case, a cemented stem with a metaphyseal cone was used for femoral fixation (Figures 3A, 3B).
While long-term, abnormally high stress transmitted through the modular junction may account for the implant’s failure, to our knowledge, this is the first report of its kind related to this particular implant. If quadriceps weakness contributed to this failure, it is worth considering that quadriceps weakness is common after TKA and may persist without appropriate rehabilitation and activity. Furthermore, the lack of evidence on plain radiographs makes this particular form of failure very difficult to screen. A high degree of suspicion for loosening should be maintained in patients with pain and instability after revision TKA with this implant as well as with other modular revision knee systems.
1. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
2. Conlisk N, Gray H, Pankaj P, Howie CR. The influence of stem length and fixation on initial femoral component stability in revision total knee replacement. Bone Joint Res. 2012;1(11):281-288.
3. van Loon CJ, Kyriazopoulos A, Verdonschot N, de Waal Malefijt MC, Huiskes R, Buma P. The role of femoral stem extension in total knee arthroplasty. Clin Orthop Relat Res. 2000;(378):282-289.
4. Lim LA, Trousdale RT, Berry DJ, Hanssen AD. Failure of the stem-condyle junction of a modular femoral stem in revision total knee arthroplasty: a report of five cases. J Arthroplasty. 2001;16(1):128-132.
5. Issack PS, Cottrell JM, Delgado S, Wright TM, Sculco TP, Su EP. Failure at the taper lock of a modular stemmed femoral implant in revision knee arthroplasty. A report of two cases and a retrieval analysis. J Bone Joint Surg Am. 2007;89(10):2271-2274.
6. Westrich GH, Hidaka C, Windsor RE. Disengagement of a locking screw from a modular stem in revision total knee arthroplasty. A report of three cases. J Bone Joint Surg Am. 1997;79(2):254-258.
7. Butt AJ, Shaikh AH, Cameron HU. Coupling failure between stem and femoral component in a constrained revision total knee arthroplasty. J Coll Physicians Surg Pak. 2013;23(2):162-163.
8. Fehring TK, Odum S, Olekson C, Griffin WL, Mason JB, McCoy TH. Stem fixation in revision total knee arthroplasty: a comparative analysis. Clin Orthop Relat Res. 2003;(416):217-224.
1. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
2. Conlisk N, Gray H, Pankaj P, Howie CR. The influence of stem length and fixation on initial femoral component stability in revision total knee replacement. Bone Joint Res. 2012;1(11):281-288.
3. van Loon CJ, Kyriazopoulos A, Verdonschot N, de Waal Malefijt MC, Huiskes R, Buma P. The role of femoral stem extension in total knee arthroplasty. Clin Orthop Relat Res. 2000;(378):282-289.
4. Lim LA, Trousdale RT, Berry DJ, Hanssen AD. Failure of the stem-condyle junction of a modular femoral stem in revision total knee arthroplasty: a report of five cases. J Arthroplasty. 2001;16(1):128-132.
5. Issack PS, Cottrell JM, Delgado S, Wright TM, Sculco TP, Su EP. Failure at the taper lock of a modular stemmed femoral implant in revision knee arthroplasty. A report of two cases and a retrieval analysis. J Bone Joint Surg Am. 2007;89(10):2271-2274.
6. Westrich GH, Hidaka C, Windsor RE. Disengagement of a locking screw from a modular stem in revision total knee arthroplasty. A report of three cases. J Bone Joint Surg Am. 1997;79(2):254-258.
7. Butt AJ, Shaikh AH, Cameron HU. Coupling failure between stem and femoral component in a constrained revision total knee arthroplasty. J Coll Physicians Surg Pak. 2013;23(2):162-163.
8. Fehring TK, Odum S, Olekson C, Griffin WL, Mason JB, McCoy TH. Stem fixation in revision total knee arthroplasty: a comparative analysis. Clin Orthop Relat Res. 2003;(416):217-224.
Gout Causing Isolated Sesamoid Destruction Mimicking a Neoplastic Process
The sesamoid bones are a major contributor to normal gait, with more than 50% of body weight transmitted through the hallux metatarsophalangeal joint (MTPJ) complex. There are varying amounts of stress on the sesamoids, dependent on the gait cycle.1,2 The sesamoids act as a fulcrum to increase the mechanical force of the flexor hallucis brevis tendon.3 Sesamoid pathology can be a source of significant morbidity in patients, especially young athletes or laborers who spend long hours on their feet. More common causes of isolated sesamoid discomfort include sesamoiditis, fracture, and avascular necrosis, with neoplastic, infectious, and inflammatory conditions rarely isolated to the sesamoid.
Gout is a systemic disorder of uric acid metabolism characterized by deposition of monosodium urate crystals in soft tissues and joints.1 This deposition leads to tophus formation with an accompanying inflammatory response. Gout progresses through 3 stages, beginning with acute gout, which may end with chronic, recurrent, and tophaceous gouty arthritis. The hallux MTPJ is the most common joint affected by gout, with few case reports of primary sesamoid gout.1-2,4 We present a case of gout, with radiographic findings isolated to the medial sesamoid, that mimicked a neoplastic process in a patient with no known history of gout. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old laborer presented for evaluation of a right sesamoid injury he sustained 4 months earlier when he fell off a ladder and had acute onset plantar hallux MTPJ pain and swelling. He was treated by an outside physician for a presumptive diagnosis of a medial sesamoid fracture with rest and controlled ankle movement (CAM) boot immobilization that resulted in slowly improving symptoms. In discussion of the patient’s history, he reported that 1 year earlier he had a traumatic event with similar symptoms of MTPJ pain and swelling. At that time, treatment with a CAM boot resulted in complete resolution of pain. His outside physician performed a hematologic workup for gout, which showed a normal uric acid level.
On examination, the patient presented with edema to the right hallux MTPJ and mild tenderness to palpation of the medial sesamoid. He had no pain with motion of the hallux MTPJ or with palpation of the lateral sesamoid. His radiographs showed a bipartite versus fractured sesamoid (Figures 1A, 1B) and serial magnetic resonance imaging (MRI) showed an MTPJ effusion and hyperintense signal in the medial sesamoid, but no erosive findings or soft-tissue masses (Figures 2A, 2B).
The patient was treated with wedge-sandal forefoot offloading, leading to resolution of symptoms over 6 weeks, at which point he was transitioned to normal shoe wear and allowed to progress in his activity as dictated by his symptoms. He presented for reevaluation approximately 2 weeks later with acute, atraumatic onset of plantar left hallux pain and swelling. His examination showed diffuse hallux MTPJ swelling and tenderness isolated to the medial sesamoid. An attempt at aspiration of the MTPJ yielded no fluid, and the patient again was placed in a forefoot-offloading sandal.
Radiographs of the left foot showed an expansile destructive lesion of the medial sesamoid with interval change from his previous imaging approximately 3 months earlier, obtained as part of his contralateral foot evaluation (Figure 3). MRI with and without contrast showed an expansile process isolated to the medial sesamoid with cortical thinning and marrow replacement (Figures 4A-4D).
Because of continued discomfort and lack of a definitive diagnosis, an excisional biopsy of the sesamoid was performed. Intraoperatively, the sesamoid was extensively fragmented with near complete replacement by a chalky tophus, as well as chalky deposition throughout the hallux MTPJ. No significant degenerative changes were observed. Surgical pathology showed bone and fibroconnective tissue with deposits of negative birefringement needle-shaped crystals consistent with monosodium urate deposition and foreign body histocytic reaction, as well as repair reaction of bone (Figures 5A, 5B).
Postoperatively, the patient was again placed in a forefoot-offloading wedge sandal for 6 weeks, followed by progression of activity as dictated by his symptoms. He was also evaluated by a rheumatologist and started on medical treatment for gout, with complete resolution of his bilateral hallux pain. He has been able to return to his previous employment.
Discussion
The sesamoid bones are an important component of the hallux MTPJ complex, giving a mechanical advantage to the flexor hallucis brevis tendons in plantar flexion of the hallux.5 Many pathologic conditions have been well described in the literature, including fracture, sesamoiditis, nonunion, avascular necrosis, and plantar keratosis. There is also a 10% incidence of bipartite sesamoids, most commonly isolated to the medial sesamoid, with up to 25% of patients presenting with bilateral bipartite sesamoids.5 Neoplastic processes of the sesamoid are rare, with a paucity of reports in the literature.6,7 Gout is a condition in which hyperuricemia, due to an imbalance in uric acid production and excretion, leads to deposition of monosodium urate crystals in joints, bones, and soft tissues, causing an inflammatory reaction. Risk factors for gout are male sex, advanced age, and ethnicity, as well as obesity, high protein diet, alcohol use, hypertension, and certain medications. Precipitation of acute attacks has been associated with acute trauma, and the first MTPJ is the most common location for an acute attack.8
Isolated sesamoid lesions are rare, with few isolated case reports in the literature. Benign and malignant lesions appear most often in the metatarsals, with the calcaneus being the second most commonly afflicted site.9 The typical differential diagnosis for isolated lytic bone lesions includes fibrous dysplasia, osteoblastoma, giant cell tumor, metastatic lesion, multiple myeloma, aneurysmal bone cyst, chondroblastoma, brown tumor, infection, eosinophilic granuloma, enchondroma, and bone cyst, with no reports in the literature to our knowledge of these entities presenting in the hallux MTPJ sesamoid. In contrast, gout typically begins with normal radiographic findings, and later leads to erosive, “punched out” lesions on either side of the MTPJ.2
Hyperuricemia is an essential part of the pathophysiology of gout, but not all patients with an acute gouty attack have elevated uric acid levels and, in contrast, may actually have normal or low levels in 12% to 43% of cases.8 The most accurate time frame for assessment of serum uric acid levels is 2 weeks or more after subsidence of an acute event.8 The normal uric acid levels seen in our patient were most likely due to the fact that the workup was undertaken during an acute attack. The difficulty with establishing the diagnosis was compounded by bilateral involvement, history of trauma, negative joint aspiration, and atypical radiographic findings. A number of reports have described patients with tophus deposits prior to or in the absence of gouty arthritis or a gouty attack.10 Risk factors for this presentation include female sex, the predominant or exclusive involvement of fingers, chronic kidney disease, and treatment with a diuretic or anti-inflammatory drug.10
Conclusion
Our case report illustrates the difficulty in diagnosing an acute gouty attack in a patient with a history of trauma and atypical radiographic findings. The hallux MTPJ is the most common location of acute gouty attacks, but the medial sesamoid as an isolated location is a rare site of presentation. The combination of pain isolated to palpation of the sesamoid and radiographs that showed an aggressive and rapidly expansile lesion of the medial sesamoid raised concerns about a neoplastic lesion. Practitioners should consider acute gout in patients with sesamoid pain and with radiographs showing an expansile sesamoid lesion.
1. Mair SD, Coogan AC, Speer KP, Hall RL. Gout as a source of sesamoid pain. Foot Ankle Int. 1995;16(10):613-616.
2. Reber PU, Patel AG, Noesberger B. Gout: rare cause of hallucal sesamoid pain: a case report. Foot Ankle Int. 1997;12(18):818-820.
3. Van Hal ME, Kenne JS, Lange TA, Clancy WG Jr. Stress fractures of the great toe sesamoids. Am J Sports Med. 1982;10(2):122-128.
4. Liu S-Z, Yeh L, Chou Y, Chen CK, Pan HB. Isolated intraosseous gout in hallux sesamoid mimicking a bone tumor in a teenaged patient. Skeletal Radiol. 2003;32(11):647-650.
5. Cohen BE. Hallux sesamoid disorders. Foot Ankle Clin. 2009;14(1):91-104.
6. Harty JA, Kelly P, Niall D, O’Keane JC, Stephens MM. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) of the sesamoid: a case report. Foot Ankle Int. 2000;21(5):408-412.
7. Noguchi M, Ikoma K, Matsumoto N, Nagasawa K. Bizarre parosteal osteochondromatous proliferation of the sesamoid: an unusual hallux valgus deformity. Foot Ankle Int. 2004;25(7):503-506.
8. Becker MA. Clinical manifestations and diagnosis of gout. Up to Date website. http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-gout. Updated June 20, 2015. Accessed August 19, 2015.
9. Bos GD, Esther RJ, Woll TS. Foot tumors: diagnosis and treatment. J Am Acad Orthop Surg. 2002;10(4):259-270.
10. Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. Report of six cases and review of the literature. Arch Intern Med. 1992;152(4):873-876.
The sesamoid bones are a major contributor to normal gait, with more than 50% of body weight transmitted through the hallux metatarsophalangeal joint (MTPJ) complex. There are varying amounts of stress on the sesamoids, dependent on the gait cycle.1,2 The sesamoids act as a fulcrum to increase the mechanical force of the flexor hallucis brevis tendon.3 Sesamoid pathology can be a source of significant morbidity in patients, especially young athletes or laborers who spend long hours on their feet. More common causes of isolated sesamoid discomfort include sesamoiditis, fracture, and avascular necrosis, with neoplastic, infectious, and inflammatory conditions rarely isolated to the sesamoid.
Gout is a systemic disorder of uric acid metabolism characterized by deposition of monosodium urate crystals in soft tissues and joints.1 This deposition leads to tophus formation with an accompanying inflammatory response. Gout progresses through 3 stages, beginning with acute gout, which may end with chronic, recurrent, and tophaceous gouty arthritis. The hallux MTPJ is the most common joint affected by gout, with few case reports of primary sesamoid gout.1-2,4 We present a case of gout, with radiographic findings isolated to the medial sesamoid, that mimicked a neoplastic process in a patient with no known history of gout. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old laborer presented for evaluation of a right sesamoid injury he sustained 4 months earlier when he fell off a ladder and had acute onset plantar hallux MTPJ pain and swelling. He was treated by an outside physician for a presumptive diagnosis of a medial sesamoid fracture with rest and controlled ankle movement (CAM) boot immobilization that resulted in slowly improving symptoms. In discussion of the patient’s history, he reported that 1 year earlier he had a traumatic event with similar symptoms of MTPJ pain and swelling. At that time, treatment with a CAM boot resulted in complete resolution of pain. His outside physician performed a hematologic workup for gout, which showed a normal uric acid level.
On examination, the patient presented with edema to the right hallux MTPJ and mild tenderness to palpation of the medial sesamoid. He had no pain with motion of the hallux MTPJ or with palpation of the lateral sesamoid. His radiographs showed a bipartite versus fractured sesamoid (Figures 1A, 1B) and serial magnetic resonance imaging (MRI) showed an MTPJ effusion and hyperintense signal in the medial sesamoid, but no erosive findings or soft-tissue masses (Figures 2A, 2B).
The patient was treated with wedge-sandal forefoot offloading, leading to resolution of symptoms over 6 weeks, at which point he was transitioned to normal shoe wear and allowed to progress in his activity as dictated by his symptoms. He presented for reevaluation approximately 2 weeks later with acute, atraumatic onset of plantar left hallux pain and swelling. His examination showed diffuse hallux MTPJ swelling and tenderness isolated to the medial sesamoid. An attempt at aspiration of the MTPJ yielded no fluid, and the patient again was placed in a forefoot-offloading sandal.
Radiographs of the left foot showed an expansile destructive lesion of the medial sesamoid with interval change from his previous imaging approximately 3 months earlier, obtained as part of his contralateral foot evaluation (Figure 3). MRI with and without contrast showed an expansile process isolated to the medial sesamoid with cortical thinning and marrow replacement (Figures 4A-4D).
Because of continued discomfort and lack of a definitive diagnosis, an excisional biopsy of the sesamoid was performed. Intraoperatively, the sesamoid was extensively fragmented with near complete replacement by a chalky tophus, as well as chalky deposition throughout the hallux MTPJ. No significant degenerative changes were observed. Surgical pathology showed bone and fibroconnective tissue with deposits of negative birefringement needle-shaped crystals consistent with monosodium urate deposition and foreign body histocytic reaction, as well as repair reaction of bone (Figures 5A, 5B).
Postoperatively, the patient was again placed in a forefoot-offloading wedge sandal for 6 weeks, followed by progression of activity as dictated by his symptoms. He was also evaluated by a rheumatologist and started on medical treatment for gout, with complete resolution of his bilateral hallux pain. He has been able to return to his previous employment.
Discussion
The sesamoid bones are an important component of the hallux MTPJ complex, giving a mechanical advantage to the flexor hallucis brevis tendons in plantar flexion of the hallux.5 Many pathologic conditions have been well described in the literature, including fracture, sesamoiditis, nonunion, avascular necrosis, and plantar keratosis. There is also a 10% incidence of bipartite sesamoids, most commonly isolated to the medial sesamoid, with up to 25% of patients presenting with bilateral bipartite sesamoids.5 Neoplastic processes of the sesamoid are rare, with a paucity of reports in the literature.6,7 Gout is a condition in which hyperuricemia, due to an imbalance in uric acid production and excretion, leads to deposition of monosodium urate crystals in joints, bones, and soft tissues, causing an inflammatory reaction. Risk factors for gout are male sex, advanced age, and ethnicity, as well as obesity, high protein diet, alcohol use, hypertension, and certain medications. Precipitation of acute attacks has been associated with acute trauma, and the first MTPJ is the most common location for an acute attack.8
Isolated sesamoid lesions are rare, with few isolated case reports in the literature. Benign and malignant lesions appear most often in the metatarsals, with the calcaneus being the second most commonly afflicted site.9 The typical differential diagnosis for isolated lytic bone lesions includes fibrous dysplasia, osteoblastoma, giant cell tumor, metastatic lesion, multiple myeloma, aneurysmal bone cyst, chondroblastoma, brown tumor, infection, eosinophilic granuloma, enchondroma, and bone cyst, with no reports in the literature to our knowledge of these entities presenting in the hallux MTPJ sesamoid. In contrast, gout typically begins with normal radiographic findings, and later leads to erosive, “punched out” lesions on either side of the MTPJ.2
Hyperuricemia is an essential part of the pathophysiology of gout, but not all patients with an acute gouty attack have elevated uric acid levels and, in contrast, may actually have normal or low levels in 12% to 43% of cases.8 The most accurate time frame for assessment of serum uric acid levels is 2 weeks or more after subsidence of an acute event.8 The normal uric acid levels seen in our patient were most likely due to the fact that the workup was undertaken during an acute attack. The difficulty with establishing the diagnosis was compounded by bilateral involvement, history of trauma, negative joint aspiration, and atypical radiographic findings. A number of reports have described patients with tophus deposits prior to or in the absence of gouty arthritis or a gouty attack.10 Risk factors for this presentation include female sex, the predominant or exclusive involvement of fingers, chronic kidney disease, and treatment with a diuretic or anti-inflammatory drug.10
Conclusion
Our case report illustrates the difficulty in diagnosing an acute gouty attack in a patient with a history of trauma and atypical radiographic findings. The hallux MTPJ is the most common location of acute gouty attacks, but the medial sesamoid as an isolated location is a rare site of presentation. The combination of pain isolated to palpation of the sesamoid and radiographs that showed an aggressive and rapidly expansile lesion of the medial sesamoid raised concerns about a neoplastic lesion. Practitioners should consider acute gout in patients with sesamoid pain and with radiographs showing an expansile sesamoid lesion.
The sesamoid bones are a major contributor to normal gait, with more than 50% of body weight transmitted through the hallux metatarsophalangeal joint (MTPJ) complex. There are varying amounts of stress on the sesamoids, dependent on the gait cycle.1,2 The sesamoids act as a fulcrum to increase the mechanical force of the flexor hallucis brevis tendon.3 Sesamoid pathology can be a source of significant morbidity in patients, especially young athletes or laborers who spend long hours on their feet. More common causes of isolated sesamoid discomfort include sesamoiditis, fracture, and avascular necrosis, with neoplastic, infectious, and inflammatory conditions rarely isolated to the sesamoid.
Gout is a systemic disorder of uric acid metabolism characterized by deposition of monosodium urate crystals in soft tissues and joints.1 This deposition leads to tophus formation with an accompanying inflammatory response. Gout progresses through 3 stages, beginning with acute gout, which may end with chronic, recurrent, and tophaceous gouty arthritis. The hallux MTPJ is the most common joint affected by gout, with few case reports of primary sesamoid gout.1-2,4 We present a case of gout, with radiographic findings isolated to the medial sesamoid, that mimicked a neoplastic process in a patient with no known history of gout. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old laborer presented for evaluation of a right sesamoid injury he sustained 4 months earlier when he fell off a ladder and had acute onset plantar hallux MTPJ pain and swelling. He was treated by an outside physician for a presumptive diagnosis of a medial sesamoid fracture with rest and controlled ankle movement (CAM) boot immobilization that resulted in slowly improving symptoms. In discussion of the patient’s history, he reported that 1 year earlier he had a traumatic event with similar symptoms of MTPJ pain and swelling. At that time, treatment with a CAM boot resulted in complete resolution of pain. His outside physician performed a hematologic workup for gout, which showed a normal uric acid level.
On examination, the patient presented with edema to the right hallux MTPJ and mild tenderness to palpation of the medial sesamoid. He had no pain with motion of the hallux MTPJ or with palpation of the lateral sesamoid. His radiographs showed a bipartite versus fractured sesamoid (Figures 1A, 1B) and serial magnetic resonance imaging (MRI) showed an MTPJ effusion and hyperintense signal in the medial sesamoid, but no erosive findings or soft-tissue masses (Figures 2A, 2B).
The patient was treated with wedge-sandal forefoot offloading, leading to resolution of symptoms over 6 weeks, at which point he was transitioned to normal shoe wear and allowed to progress in his activity as dictated by his symptoms. He presented for reevaluation approximately 2 weeks later with acute, atraumatic onset of plantar left hallux pain and swelling. His examination showed diffuse hallux MTPJ swelling and tenderness isolated to the medial sesamoid. An attempt at aspiration of the MTPJ yielded no fluid, and the patient again was placed in a forefoot-offloading sandal.
Radiographs of the left foot showed an expansile destructive lesion of the medial sesamoid with interval change from his previous imaging approximately 3 months earlier, obtained as part of his contralateral foot evaluation (Figure 3). MRI with and without contrast showed an expansile process isolated to the medial sesamoid with cortical thinning and marrow replacement (Figures 4A-4D).
Because of continued discomfort and lack of a definitive diagnosis, an excisional biopsy of the sesamoid was performed. Intraoperatively, the sesamoid was extensively fragmented with near complete replacement by a chalky tophus, as well as chalky deposition throughout the hallux MTPJ. No significant degenerative changes were observed. Surgical pathology showed bone and fibroconnective tissue with deposits of negative birefringement needle-shaped crystals consistent with monosodium urate deposition and foreign body histocytic reaction, as well as repair reaction of bone (Figures 5A, 5B).
Postoperatively, the patient was again placed in a forefoot-offloading wedge sandal for 6 weeks, followed by progression of activity as dictated by his symptoms. He was also evaluated by a rheumatologist and started on medical treatment for gout, with complete resolution of his bilateral hallux pain. He has been able to return to his previous employment.
Discussion
The sesamoid bones are an important component of the hallux MTPJ complex, giving a mechanical advantage to the flexor hallucis brevis tendons in plantar flexion of the hallux.5 Many pathologic conditions have been well described in the literature, including fracture, sesamoiditis, nonunion, avascular necrosis, and plantar keratosis. There is also a 10% incidence of bipartite sesamoids, most commonly isolated to the medial sesamoid, with up to 25% of patients presenting with bilateral bipartite sesamoids.5 Neoplastic processes of the sesamoid are rare, with a paucity of reports in the literature.6,7 Gout is a condition in which hyperuricemia, due to an imbalance in uric acid production and excretion, leads to deposition of monosodium urate crystals in joints, bones, and soft tissues, causing an inflammatory reaction. Risk factors for gout are male sex, advanced age, and ethnicity, as well as obesity, high protein diet, alcohol use, hypertension, and certain medications. Precipitation of acute attacks has been associated with acute trauma, and the first MTPJ is the most common location for an acute attack.8
Isolated sesamoid lesions are rare, with few isolated case reports in the literature. Benign and malignant lesions appear most often in the metatarsals, with the calcaneus being the second most commonly afflicted site.9 The typical differential diagnosis for isolated lytic bone lesions includes fibrous dysplasia, osteoblastoma, giant cell tumor, metastatic lesion, multiple myeloma, aneurysmal bone cyst, chondroblastoma, brown tumor, infection, eosinophilic granuloma, enchondroma, and bone cyst, with no reports in the literature to our knowledge of these entities presenting in the hallux MTPJ sesamoid. In contrast, gout typically begins with normal radiographic findings, and later leads to erosive, “punched out” lesions on either side of the MTPJ.2
Hyperuricemia is an essential part of the pathophysiology of gout, but not all patients with an acute gouty attack have elevated uric acid levels and, in contrast, may actually have normal or low levels in 12% to 43% of cases.8 The most accurate time frame for assessment of serum uric acid levels is 2 weeks or more after subsidence of an acute event.8 The normal uric acid levels seen in our patient were most likely due to the fact that the workup was undertaken during an acute attack. The difficulty with establishing the diagnosis was compounded by bilateral involvement, history of trauma, negative joint aspiration, and atypical radiographic findings. A number of reports have described patients with tophus deposits prior to or in the absence of gouty arthritis or a gouty attack.10 Risk factors for this presentation include female sex, the predominant or exclusive involvement of fingers, chronic kidney disease, and treatment with a diuretic or anti-inflammatory drug.10
Conclusion
Our case report illustrates the difficulty in diagnosing an acute gouty attack in a patient with a history of trauma and atypical radiographic findings. The hallux MTPJ is the most common location of acute gouty attacks, but the medial sesamoid as an isolated location is a rare site of presentation. The combination of pain isolated to palpation of the sesamoid and radiographs that showed an aggressive and rapidly expansile lesion of the medial sesamoid raised concerns about a neoplastic lesion. Practitioners should consider acute gout in patients with sesamoid pain and with radiographs showing an expansile sesamoid lesion.
1. Mair SD, Coogan AC, Speer KP, Hall RL. Gout as a source of sesamoid pain. Foot Ankle Int. 1995;16(10):613-616.
2. Reber PU, Patel AG, Noesberger B. Gout: rare cause of hallucal sesamoid pain: a case report. Foot Ankle Int. 1997;12(18):818-820.
3. Van Hal ME, Kenne JS, Lange TA, Clancy WG Jr. Stress fractures of the great toe sesamoids. Am J Sports Med. 1982;10(2):122-128.
4. Liu S-Z, Yeh L, Chou Y, Chen CK, Pan HB. Isolated intraosseous gout in hallux sesamoid mimicking a bone tumor in a teenaged patient. Skeletal Radiol. 2003;32(11):647-650.
5. Cohen BE. Hallux sesamoid disorders. Foot Ankle Clin. 2009;14(1):91-104.
6. Harty JA, Kelly P, Niall D, O’Keane JC, Stephens MM. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) of the sesamoid: a case report. Foot Ankle Int. 2000;21(5):408-412.
7. Noguchi M, Ikoma K, Matsumoto N, Nagasawa K. Bizarre parosteal osteochondromatous proliferation of the sesamoid: an unusual hallux valgus deformity. Foot Ankle Int. 2004;25(7):503-506.
8. Becker MA. Clinical manifestations and diagnosis of gout. Up to Date website. http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-gout. Updated June 20, 2015. Accessed August 19, 2015.
9. Bos GD, Esther RJ, Woll TS. Foot tumors: diagnosis and treatment. J Am Acad Orthop Surg. 2002;10(4):259-270.
10. Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. Report of six cases and review of the literature. Arch Intern Med. 1992;152(4):873-876.
1. Mair SD, Coogan AC, Speer KP, Hall RL. Gout as a source of sesamoid pain. Foot Ankle Int. 1995;16(10):613-616.
2. Reber PU, Patel AG, Noesberger B. Gout: rare cause of hallucal sesamoid pain: a case report. Foot Ankle Int. 1997;12(18):818-820.
3. Van Hal ME, Kenne JS, Lange TA, Clancy WG Jr. Stress fractures of the great toe sesamoids. Am J Sports Med. 1982;10(2):122-128.
4. Liu S-Z, Yeh L, Chou Y, Chen CK, Pan HB. Isolated intraosseous gout in hallux sesamoid mimicking a bone tumor in a teenaged patient. Skeletal Radiol. 2003;32(11):647-650.
5. Cohen BE. Hallux sesamoid disorders. Foot Ankle Clin. 2009;14(1):91-104.
6. Harty JA, Kelly P, Niall D, O’Keane JC, Stephens MM. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) of the sesamoid: a case report. Foot Ankle Int. 2000;21(5):408-412.
7. Noguchi M, Ikoma K, Matsumoto N, Nagasawa K. Bizarre parosteal osteochondromatous proliferation of the sesamoid: an unusual hallux valgus deformity. Foot Ankle Int. 2004;25(7):503-506.
8. Becker MA. Clinical manifestations and diagnosis of gout. Up to Date website. http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-gout. Updated June 20, 2015. Accessed August 19, 2015.
9. Bos GD, Esther RJ, Woll TS. Foot tumors: diagnosis and treatment. J Am Acad Orthop Surg. 2002;10(4):259-270.
10. Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. Report of six cases and review of the literature. Arch Intern Med. 1992;152(4):873-876.
Avascular Necrosis of Trochlea After Supracondylar Humerus Fractures in Children
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
Invasive Compartment Pressure Testing for Chronic Exertional Compartment Syndrome: A Survey of Clinical Practice Among Military Orthopedic Surgeons
Chronic exertional compartment syndrome (CECS) is a common cause of leg pain during exertion in athletic and active-duty populations.1 It is caused by an increase in intramuscular pressure to a point that the tissues within the involved compartment become ischemic because of a decrease in arteriolar blood flow.2 This relative ischemia causes pain and may also be associated with neurologic symptoms. By definition, the pain associated with CECS resolves with rest. Patients typically describe a feeling of fullness or tightness, which eventually evolves into pain as they continue exercising. Pain onset is usually predictable and reproducible after a finite amount of time and/or intensity of exercise.
The differential diagnosis of leg pain during exercise includes CECS, medial tibial stress syndrome, popliteal entrapment syndrome, myopathy, peripheral nerve entrapment syndromes, stress fracture, and effort-induced rhabdomyolysis.3 CECS can be differentiated from other causes of leg pain with measurement of compartment pressures (the standard recommendation).4 Compartment pressure measurement, however, is invasive, time-consuming, and painful and may be associated with bleeding risk, infection, and nerve injury. Noninvasive means of testing for CECS (eg, magnetic resonance imaging [MRI], near-infrared spectroscopy [NIRS], thallium stress testing) remain experimental and expensive and are not easily accessible at all institutions.5-8 While invasive compartment pressure (ICP) testing remains an important tool in the diagnosis of CECS, its criteria and execution vary considerably. Aweid and colleagues4 performed a meta-analysis of use of ICP testing in the diagnosis of CECS and concluded that, though elevated ICP measurements are accepted as the gold standard for diagnosing CECS, the criteria outlined for a positive test lack high-level supporting evidence. In addition, how the test is performed has been inconsistent across studies—further clouding the literature.4
The review by Aweid and colleagues4 highlights the deficiencies in diagnosing CECS by ICP testing. In clinical practice, ICP testing is challenging for both the patient and physician. As other validated, less-invasive tests are lacking, emphasis should remain on the history and the physical examination. Although all athletic populations are at risk for CECS, the active-duty military population is at particularly high risk because of the physical requirements and demands of military service.1,9
We surveyed military orthopedic surgeons to investigate the clinical practice of performing ICP testing in patients with suspected CECS. We hypothesized that the rate of ICP testing among military orthopedic surgeons would not be 100% for patients with the typical signs and symptoms of CECS.
Materials and Methods
This study was approved by the institutional review board at Wright-Patterson Medical Center at Wright-Patterson Air Force Base in Ohio. A link to an online survey was distributed by email to members of the Society of Military Orthopaedic Surgeons. The anonymous survey polled the surgeons regarding basic demographic data and clinical practice as it pertains to the evaluation and treatment of CECS. No patient-protected health information was obtained. Survey results were compiled in a Microsoft Excel file for analysis.
Results
The survey was distributed to 606 email accounts; the response rate was 19% (114/606). Ninety-one surgeons (80%) indicated they have patients with CECS in their practice (Figure 1). Surgeons were asked how many CECS patients they see per year (responses are summarized in Figure 2) and how many years they have been in practice (Table).
Ninety-three percent of the respondents agreed or strongly agreed that ICP testing is unpleasant for the patient (Figure 3), and 90% would prefer a less-invasive test for confirmatory testing for CECS (Figure 4). Only 13% of respondents indicated they actually use noninvasive modalities (eg, MRI, NIRS) to confirm the diagnosis of CECS (Figure 5).
Respondents were asked about the practice of using ICP testing in the diagnosis of CECS (responses are summarized in Figures 6, 7). Although 85% of respondents agreed or strongly agreed with always confirming the diagnosis of CECS with ICP testing, 39% stated they would recommend surgical treatment without ICP testing if they were confident about the diagnosis based on clinical examination findings.
To better understand the apparent discrepancy between the percentage of surgeons who agreed or strongly agreed with always recommending ICP testing (85%) and the percentage who would recommend treatment without testing (39%), responses were stratified by clinical experience. Surgeons in practice more than 11 years (n = 35) were compared with those in practice 5 years or less (n = 31) (Table). Although the vast majority (85%) of respondents from both groups agreed or strongly agreed with always recommending ICP testing, 49% of those in practice more than 11 years and 29% in practice 5 years or less indicated they would recommend surgical treatment for CECS based solely on clinical examination findings (Figures 8, 9).
Responses were also stratified by number of CECS patients seen by each surgeon per year. Twenty-eight respondents saw 1 or 2 patients per year, and 12 saw more than 8 patients per year—31% and 13% of the total number of respondents, respectively. Of the respondents who saw 1 or 2 patients, 86% (24/28) agreed or strongly agreed with always recommending ICP testing—comparable to the 75% (9/12) who saw more than 8 patients (Figure 10). However, of the respondents who saw 1 or 2 patients, 36% (10/28) indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% (9/12) who saw more than 8 patients (Figure 11).
Discussion
CECS is a common cause of leg pain and a significant cause of disability among the active-duty military population. This was illustrated in 2 recent studies by Waterman and colleagues.1,9 The first1 investigated failure rates and disability after surgery for CECS among those on active duty. The authors showed that CECS is a substantial contributor to lower extremity disability in the military population and that there is a substantial risk for persistent symptoms despite surgical treatment. Nearly 1 in 5 patients experienced surgical failure after elective fasciotomy, and about 28% of patients were unable to return to the full activity required in the military. The second, more recent study9 was an epidemiologic study of risk factors associated with CECS in a physically active military population. The authors identified 4100 cases diagnosed between 2006 and 2011—representing an overall annual incidence of 0.49 per 1000 at-risk person-years, or about 683 cases per year; the authors also showed the incidence increased during the time period studied.
The diagnosis of CECS remains imperfect. A clinical history of exercise-induced lower leg pain that is relieved with rest suggests the diagnosis, but a confirmatory test is needed to distinguish CECS from other causes of exercise-induced leg pain. Although direct measurement of compartment pressures is the test used most often, it is invasive and time-consuming, can be uncomfortable for the patient, and may be associated with bleeding risk, infection, and nerve injury. Pedowitz and colleagues10 described the ICP testing criteria now generally used in the diagnosis of CECS. Unfortunately, there is little objective evidence supporting these criteria.4 Although less invasive tests (eg, MRI, NIRS) have been described,5-8 they may not be readily available across institutions, and further study is needed to validate their use in diagnosing CECS.
While an objective, validated test or measurement for confirming the diagnosis of CECS remains elusive, the outcomes after surgical treatment of CECS also remain imperfect. Surgery consists of both open and endoscopically assisted fasciotomy of the involved compartments.2,11-17 Reports of improvement after treatment range from 81% to 100%3; however, symptom relief does not come for all patients, particularly those in the military. Waterman and colleagues1 found a failure rate of about 20% among an active-duty military population. Packer and colleagues18 examined civilians with CECS, treated both operatively and nonoperatively. Patients in this series were diagnosed with CECS based on clinical symptoms as well as compartment pressure measurements according to the Pedowitz criteria. Although overall outcomes were better for operatively treated patients than for nonoperatively treated patients, only 47% of patients were completely pain-free, and 21% were unable to return to full activity.
More recent studies have explored use of other nonoperative treatment modalities. Diebal and colleagues19,20 used a running retraining program to treat patients with CECS. They based this treatment on the hypothesis that a heel-strike running pattern is associated with increased anterior compartment pressures.21 CECS patients who underwent a 6-week systematic treatment program focused on forefoot running, stride shortening, and hamstring activation during push-off experienced a decrease in clinical symptoms and posttreatment intracompartmental pressures.20 The improvements in clinical scores were maintained at 1-year follow-up. Another nonoperative intervention, recently described by Isner-Horobeti and colleagues,22 involves injecting botulinum toxin A (BoNT-A) into the anterior and lateral compartments of the leg. Sixteen patients with CECS received BoNT-A injections. On average, intracompartmental pressures were lower after injection than they were before injection. In addition, exertional pain was eliminated in 15 patients at an average follow-up of 4.4 months.
This survey-based study examined the practice patterns of military orthopedic surgeons who performed ICP testing for cases of suspected CECS. Our hypothesis was that, though ICP testing is the most commonly accepted method for confirming the diagnosis of CECS, the ICP testing rate would not be 100% among those surveyed.
The results of our study uncover an apparent inconsistency in survey responses among physicians who evaluate and treat patients with CECS. About 85% of respondents stated they would always recommend confirming the diagnosis of CECS with ICP testing. However, about 40% stated they would recommend surgical treatment without confirmatory testing if they were confident about the diagnosis based on clinical findings. In other words, only 60% of the respondents disagreed or strongly disagreed with pursuing surgical treatment without testing. One would expect a closer correlation between respondents who would always recommend ICP testing and those who disagreed with recommending surgical treatment without ICP testing. This raises the question of what actually occurs when CECS is suspected in clinical practice.
To better understand the apparent discrepancy between respondents who agreed or strongly agreed with always recommending ICP testing and respondents who would recommend treatment without testing, we grouped responses by clinical experience. Although 85% of respondents (no matter the number of years in practice) agreed or strongly agreed with the statement, “I always recommend confirming the diagnosis of CECS with ICP measurements,” 49% of those in practice more than 11 years (vs. 29% of those in practice 5 years or less) agreed or strongly agreed with recommending surgery without testing if they were 100% confident about the diagnosis of CECS based solely on clinical findings. This may suggest that, though most agreed that the gold standard for confirming the diagnosis of CECS remains ICP testing, those with more clinical experience were more comfortable forgoing this diagnostic measure and recommending treatment without testing.
Another measure of clinical experience used in this survey was based on number of CECS patients seen per year. Responses of surgeons who saw 1 or 2 patients with CECS per year were compared with responses of surgeons who saw more than 8 patients with CECS per year. Of the respondents who saw 1 or 2 patients, 86% agreed or strongly agreed with always recommending ICP testing to confirm CECS—comparable to the 75% who saw more than 8 patients. However, of the respondents who saw 1 or 2 patients, 36% indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% who saw more than 8 patients.
Responses regarding the absolute of always recommending ICP testing and the absolute of being 100% confident about the clinical diagnosis of CECS highlight differences between the surgeons with more experience (>11 years in practice, >8 CECS patients per year) and those with less experience (≤5 years in practice, 1 or 2 CECS patients per year). Surgeons in practice longer, and surgeons who saw more patients with suspected CECS per year, were more likely to recommend surgical treatment based solely on clinical findings.
Conclusion
CECS can cause debilitating activity-related leg pain in both civilian and military populations. Treatment with fasciotomy is often curative, but a significant number of patients may continue to have pain and disability. As the incidence of treatment failures may be higher in the military than in civilians, proper evaluation of patients with suspected CECS is particularly important for military orthopedic surgeons. The diagnosis of CECS can be challenging to both the clinician and patient, and diagnostic modalities remain imperfect. The results of this study highlight this, revealing less than 100% agreement regarding use of ICP testing (the gold standard) for diagnosis of CECS.
This study also highlights the need for an improved method of diagnosing CECS since 93% of respondents agreed or strongly agreed that ICP testing is unpleasant for the patient, and 90% would prefer a less-invasive test. In addition, the ICP testing criteria for establishing the diagnosis of CECS remain inconsistent. If a reliable, consistent, and less-invasive test were available, perhaps there would be less variability in practitioners’ evaluations of patients with CECS.
This study shows an inconsistency among military orthopedic surgeons evaluating and treating patients with CECS. As testing modalities for CECS remain imperfect, clinical acumen and experience assume an important role in the assessment of patients with suspected CECS.
1. Waterman BR, Laughlin M, Kilcoyne K, Cameron KL, Owens BD. Surgical treatment of chronic exertional compartment syndrome of the leg: failure rates and postoperative disability in an active patient population. J Bone Joint Surg Am. 2013;95(7):592-596.
2. Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36-40.
3. Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11(4):268-276.
4. Aweid O, Del Buono A, Malliaras P, et al. Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med. 2012;22(4):356-370.
5. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42(3):385-392.
6. van den Brand JG, Verleisdonk EJ, van der Werken C. Near infrared spectroscopy in the diagnosis of chronic exertional compartment syndrome. Am J Sports Med. 2004;32(2):452-456.
7. van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33(5):699-704.
8. Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30(6):321-325.
9. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
10. Pedowitz RA, Hargens AR, Mubarek SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18(1):35-40.1. Rorabeck CH, Bourne RB, Fowler PJ. The surgical treatment of exertional compartment syndrome in athletes. J Bone Joint Surg Am. 1983;65(9):1245-1251.
12. Rorabeck CH, Fowler PJ, Nott L. The results of fasciotomy in the management of chronic exertional compartment syndrome. Am J Sports Med. 1988;16(3):224-227.
13. Detmer DE, Sharpe K, Sufit RL, Girdley FM. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13(3):162-170.
14. Stein DA, Sennett BJ. One-portal endoscopically assisted fasciotomy for exertional compartment syndrome. Arthroscopy. 2005;21(1):108-112.
15. Fronek J, Mubarak SJ, Hargens AR, et al. Management of chronic exertional anterior compartment syndrome of the lower extremity. Clin Orthop Relat Res. 1989;(220):217-227.
16. Leversedge FJ, Casey PJ, Seiler JG 3rd, Xerogeanes JW. Endoscopically assisted fasciotomy: description of technique and in vitro assessment of lower-leg compartment decompression. Am J Sports Med. 2002;30(2):272-278.
17. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21(6):811-817.
18. Packer JD, Day MS, Nguyen JT, Hobart SJ, Hannafin JA, Metzl JD. Functional outcomes and patient satisfaction after fasciotomy for chronic exertional compartment syndrome. Am J Sports Med. 2013;41(2):430-436.
19. Diebal AR, Gregory R, Alitz C, Gerber JP. Effects of forefoot running on chronic exertional compartment syndrome: a case series. Int J Sports Phys Ther. 2011;6(4):312-321.
20. Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated with chronic exertional compartment syndrome. Am J Sports Med. 2012;40(5):1060-1067.
21. Kirby RL, McDermott AG. Anterior tibial compartment pressures during running with rearfoot and forefoot landing styles. Arch Phys Med Rehabil. 1983;64(7):296-299.
22. Isner-Horobeti ME, Dufour SP, Blaes C, Lecocq J. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41(11):2558-2566.
Chronic exertional compartment syndrome (CECS) is a common cause of leg pain during exertion in athletic and active-duty populations.1 It is caused by an increase in intramuscular pressure to a point that the tissues within the involved compartment become ischemic because of a decrease in arteriolar blood flow.2 This relative ischemia causes pain and may also be associated with neurologic symptoms. By definition, the pain associated with CECS resolves with rest. Patients typically describe a feeling of fullness or tightness, which eventually evolves into pain as they continue exercising. Pain onset is usually predictable and reproducible after a finite amount of time and/or intensity of exercise.
The differential diagnosis of leg pain during exercise includes CECS, medial tibial stress syndrome, popliteal entrapment syndrome, myopathy, peripheral nerve entrapment syndromes, stress fracture, and effort-induced rhabdomyolysis.3 CECS can be differentiated from other causes of leg pain with measurement of compartment pressures (the standard recommendation).4 Compartment pressure measurement, however, is invasive, time-consuming, and painful and may be associated with bleeding risk, infection, and nerve injury. Noninvasive means of testing for CECS (eg, magnetic resonance imaging [MRI], near-infrared spectroscopy [NIRS], thallium stress testing) remain experimental and expensive and are not easily accessible at all institutions.5-8 While invasive compartment pressure (ICP) testing remains an important tool in the diagnosis of CECS, its criteria and execution vary considerably. Aweid and colleagues4 performed a meta-analysis of use of ICP testing in the diagnosis of CECS and concluded that, though elevated ICP measurements are accepted as the gold standard for diagnosing CECS, the criteria outlined for a positive test lack high-level supporting evidence. In addition, how the test is performed has been inconsistent across studies—further clouding the literature.4
The review by Aweid and colleagues4 highlights the deficiencies in diagnosing CECS by ICP testing. In clinical practice, ICP testing is challenging for both the patient and physician. As other validated, less-invasive tests are lacking, emphasis should remain on the history and the physical examination. Although all athletic populations are at risk for CECS, the active-duty military population is at particularly high risk because of the physical requirements and demands of military service.1,9
We surveyed military orthopedic surgeons to investigate the clinical practice of performing ICP testing in patients with suspected CECS. We hypothesized that the rate of ICP testing among military orthopedic surgeons would not be 100% for patients with the typical signs and symptoms of CECS.
Materials and Methods
This study was approved by the institutional review board at Wright-Patterson Medical Center at Wright-Patterson Air Force Base in Ohio. A link to an online survey was distributed by email to members of the Society of Military Orthopaedic Surgeons. The anonymous survey polled the surgeons regarding basic demographic data and clinical practice as it pertains to the evaluation and treatment of CECS. No patient-protected health information was obtained. Survey results were compiled in a Microsoft Excel file for analysis.
Results
The survey was distributed to 606 email accounts; the response rate was 19% (114/606). Ninety-one surgeons (80%) indicated they have patients with CECS in their practice (Figure 1). Surgeons were asked how many CECS patients they see per year (responses are summarized in Figure 2) and how many years they have been in practice (Table).
Ninety-three percent of the respondents agreed or strongly agreed that ICP testing is unpleasant for the patient (Figure 3), and 90% would prefer a less-invasive test for confirmatory testing for CECS (Figure 4). Only 13% of respondents indicated they actually use noninvasive modalities (eg, MRI, NIRS) to confirm the diagnosis of CECS (Figure 5).
Respondents were asked about the practice of using ICP testing in the diagnosis of CECS (responses are summarized in Figures 6, 7). Although 85% of respondents agreed or strongly agreed with always confirming the diagnosis of CECS with ICP testing, 39% stated they would recommend surgical treatment without ICP testing if they were confident about the diagnosis based on clinical examination findings.
To better understand the apparent discrepancy between the percentage of surgeons who agreed or strongly agreed with always recommending ICP testing (85%) and the percentage who would recommend treatment without testing (39%), responses were stratified by clinical experience. Surgeons in practice more than 11 years (n = 35) were compared with those in practice 5 years or less (n = 31) (Table). Although the vast majority (85%) of respondents from both groups agreed or strongly agreed with always recommending ICP testing, 49% of those in practice more than 11 years and 29% in practice 5 years or less indicated they would recommend surgical treatment for CECS based solely on clinical examination findings (Figures 8, 9).
Responses were also stratified by number of CECS patients seen by each surgeon per year. Twenty-eight respondents saw 1 or 2 patients per year, and 12 saw more than 8 patients per year—31% and 13% of the total number of respondents, respectively. Of the respondents who saw 1 or 2 patients, 86% (24/28) agreed or strongly agreed with always recommending ICP testing—comparable to the 75% (9/12) who saw more than 8 patients (Figure 10). However, of the respondents who saw 1 or 2 patients, 36% (10/28) indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% (9/12) who saw more than 8 patients (Figure 11).
Discussion
CECS is a common cause of leg pain and a significant cause of disability among the active-duty military population. This was illustrated in 2 recent studies by Waterman and colleagues.1,9 The first1 investigated failure rates and disability after surgery for CECS among those on active duty. The authors showed that CECS is a substantial contributor to lower extremity disability in the military population and that there is a substantial risk for persistent symptoms despite surgical treatment. Nearly 1 in 5 patients experienced surgical failure after elective fasciotomy, and about 28% of patients were unable to return to the full activity required in the military. The second, more recent study9 was an epidemiologic study of risk factors associated with CECS in a physically active military population. The authors identified 4100 cases diagnosed between 2006 and 2011—representing an overall annual incidence of 0.49 per 1000 at-risk person-years, or about 683 cases per year; the authors also showed the incidence increased during the time period studied.
The diagnosis of CECS remains imperfect. A clinical history of exercise-induced lower leg pain that is relieved with rest suggests the diagnosis, but a confirmatory test is needed to distinguish CECS from other causes of exercise-induced leg pain. Although direct measurement of compartment pressures is the test used most often, it is invasive and time-consuming, can be uncomfortable for the patient, and may be associated with bleeding risk, infection, and nerve injury. Pedowitz and colleagues10 described the ICP testing criteria now generally used in the diagnosis of CECS. Unfortunately, there is little objective evidence supporting these criteria.4 Although less invasive tests (eg, MRI, NIRS) have been described,5-8 they may not be readily available across institutions, and further study is needed to validate their use in diagnosing CECS.
While an objective, validated test or measurement for confirming the diagnosis of CECS remains elusive, the outcomes after surgical treatment of CECS also remain imperfect. Surgery consists of both open and endoscopically assisted fasciotomy of the involved compartments.2,11-17 Reports of improvement after treatment range from 81% to 100%3; however, symptom relief does not come for all patients, particularly those in the military. Waterman and colleagues1 found a failure rate of about 20% among an active-duty military population. Packer and colleagues18 examined civilians with CECS, treated both operatively and nonoperatively. Patients in this series were diagnosed with CECS based on clinical symptoms as well as compartment pressure measurements according to the Pedowitz criteria. Although overall outcomes were better for operatively treated patients than for nonoperatively treated patients, only 47% of patients were completely pain-free, and 21% were unable to return to full activity.
More recent studies have explored use of other nonoperative treatment modalities. Diebal and colleagues19,20 used a running retraining program to treat patients with CECS. They based this treatment on the hypothesis that a heel-strike running pattern is associated with increased anterior compartment pressures.21 CECS patients who underwent a 6-week systematic treatment program focused on forefoot running, stride shortening, and hamstring activation during push-off experienced a decrease in clinical symptoms and posttreatment intracompartmental pressures.20 The improvements in clinical scores were maintained at 1-year follow-up. Another nonoperative intervention, recently described by Isner-Horobeti and colleagues,22 involves injecting botulinum toxin A (BoNT-A) into the anterior and lateral compartments of the leg. Sixteen patients with CECS received BoNT-A injections. On average, intracompartmental pressures were lower after injection than they were before injection. In addition, exertional pain was eliminated in 15 patients at an average follow-up of 4.4 months.
This survey-based study examined the practice patterns of military orthopedic surgeons who performed ICP testing for cases of suspected CECS. Our hypothesis was that, though ICP testing is the most commonly accepted method for confirming the diagnosis of CECS, the ICP testing rate would not be 100% among those surveyed.
The results of our study uncover an apparent inconsistency in survey responses among physicians who evaluate and treat patients with CECS. About 85% of respondents stated they would always recommend confirming the diagnosis of CECS with ICP testing. However, about 40% stated they would recommend surgical treatment without confirmatory testing if they were confident about the diagnosis based on clinical findings. In other words, only 60% of the respondents disagreed or strongly disagreed with pursuing surgical treatment without testing. One would expect a closer correlation between respondents who would always recommend ICP testing and those who disagreed with recommending surgical treatment without ICP testing. This raises the question of what actually occurs when CECS is suspected in clinical practice.
To better understand the apparent discrepancy between respondents who agreed or strongly agreed with always recommending ICP testing and respondents who would recommend treatment without testing, we grouped responses by clinical experience. Although 85% of respondents (no matter the number of years in practice) agreed or strongly agreed with the statement, “I always recommend confirming the diagnosis of CECS with ICP measurements,” 49% of those in practice more than 11 years (vs. 29% of those in practice 5 years or less) agreed or strongly agreed with recommending surgery without testing if they were 100% confident about the diagnosis of CECS based solely on clinical findings. This may suggest that, though most agreed that the gold standard for confirming the diagnosis of CECS remains ICP testing, those with more clinical experience were more comfortable forgoing this diagnostic measure and recommending treatment without testing.
Another measure of clinical experience used in this survey was based on number of CECS patients seen per year. Responses of surgeons who saw 1 or 2 patients with CECS per year were compared with responses of surgeons who saw more than 8 patients with CECS per year. Of the respondents who saw 1 or 2 patients, 86% agreed or strongly agreed with always recommending ICP testing to confirm CECS—comparable to the 75% who saw more than 8 patients. However, of the respondents who saw 1 or 2 patients, 36% indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% who saw more than 8 patients.
Responses regarding the absolute of always recommending ICP testing and the absolute of being 100% confident about the clinical diagnosis of CECS highlight differences between the surgeons with more experience (>11 years in practice, >8 CECS patients per year) and those with less experience (≤5 years in practice, 1 or 2 CECS patients per year). Surgeons in practice longer, and surgeons who saw more patients with suspected CECS per year, were more likely to recommend surgical treatment based solely on clinical findings.
Conclusion
CECS can cause debilitating activity-related leg pain in both civilian and military populations. Treatment with fasciotomy is often curative, but a significant number of patients may continue to have pain and disability. As the incidence of treatment failures may be higher in the military than in civilians, proper evaluation of patients with suspected CECS is particularly important for military orthopedic surgeons. The diagnosis of CECS can be challenging to both the clinician and patient, and diagnostic modalities remain imperfect. The results of this study highlight this, revealing less than 100% agreement regarding use of ICP testing (the gold standard) for diagnosis of CECS.
This study also highlights the need for an improved method of diagnosing CECS since 93% of respondents agreed or strongly agreed that ICP testing is unpleasant for the patient, and 90% would prefer a less-invasive test. In addition, the ICP testing criteria for establishing the diagnosis of CECS remain inconsistent. If a reliable, consistent, and less-invasive test were available, perhaps there would be less variability in practitioners’ evaluations of patients with CECS.
This study shows an inconsistency among military orthopedic surgeons evaluating and treating patients with CECS. As testing modalities for CECS remain imperfect, clinical acumen and experience assume an important role in the assessment of patients with suspected CECS.
Chronic exertional compartment syndrome (CECS) is a common cause of leg pain during exertion in athletic and active-duty populations.1 It is caused by an increase in intramuscular pressure to a point that the tissues within the involved compartment become ischemic because of a decrease in arteriolar blood flow.2 This relative ischemia causes pain and may also be associated with neurologic symptoms. By definition, the pain associated with CECS resolves with rest. Patients typically describe a feeling of fullness or tightness, which eventually evolves into pain as they continue exercising. Pain onset is usually predictable and reproducible after a finite amount of time and/or intensity of exercise.
The differential diagnosis of leg pain during exercise includes CECS, medial tibial stress syndrome, popliteal entrapment syndrome, myopathy, peripheral nerve entrapment syndromes, stress fracture, and effort-induced rhabdomyolysis.3 CECS can be differentiated from other causes of leg pain with measurement of compartment pressures (the standard recommendation).4 Compartment pressure measurement, however, is invasive, time-consuming, and painful and may be associated with bleeding risk, infection, and nerve injury. Noninvasive means of testing for CECS (eg, magnetic resonance imaging [MRI], near-infrared spectroscopy [NIRS], thallium stress testing) remain experimental and expensive and are not easily accessible at all institutions.5-8 While invasive compartment pressure (ICP) testing remains an important tool in the diagnosis of CECS, its criteria and execution vary considerably. Aweid and colleagues4 performed a meta-analysis of use of ICP testing in the diagnosis of CECS and concluded that, though elevated ICP measurements are accepted as the gold standard for diagnosing CECS, the criteria outlined for a positive test lack high-level supporting evidence. In addition, how the test is performed has been inconsistent across studies—further clouding the literature.4
The review by Aweid and colleagues4 highlights the deficiencies in diagnosing CECS by ICP testing. In clinical practice, ICP testing is challenging for both the patient and physician. As other validated, less-invasive tests are lacking, emphasis should remain on the history and the physical examination. Although all athletic populations are at risk for CECS, the active-duty military population is at particularly high risk because of the physical requirements and demands of military service.1,9
We surveyed military orthopedic surgeons to investigate the clinical practice of performing ICP testing in patients with suspected CECS. We hypothesized that the rate of ICP testing among military orthopedic surgeons would not be 100% for patients with the typical signs and symptoms of CECS.
Materials and Methods
This study was approved by the institutional review board at Wright-Patterson Medical Center at Wright-Patterson Air Force Base in Ohio. A link to an online survey was distributed by email to members of the Society of Military Orthopaedic Surgeons. The anonymous survey polled the surgeons regarding basic demographic data and clinical practice as it pertains to the evaluation and treatment of CECS. No patient-protected health information was obtained. Survey results were compiled in a Microsoft Excel file for analysis.
Results
The survey was distributed to 606 email accounts; the response rate was 19% (114/606). Ninety-one surgeons (80%) indicated they have patients with CECS in their practice (Figure 1). Surgeons were asked how many CECS patients they see per year (responses are summarized in Figure 2) and how many years they have been in practice (Table).
Ninety-three percent of the respondents agreed or strongly agreed that ICP testing is unpleasant for the patient (Figure 3), and 90% would prefer a less-invasive test for confirmatory testing for CECS (Figure 4). Only 13% of respondents indicated they actually use noninvasive modalities (eg, MRI, NIRS) to confirm the diagnosis of CECS (Figure 5).
Respondents were asked about the practice of using ICP testing in the diagnosis of CECS (responses are summarized in Figures 6, 7). Although 85% of respondents agreed or strongly agreed with always confirming the diagnosis of CECS with ICP testing, 39% stated they would recommend surgical treatment without ICP testing if they were confident about the diagnosis based on clinical examination findings.
To better understand the apparent discrepancy between the percentage of surgeons who agreed or strongly agreed with always recommending ICP testing (85%) and the percentage who would recommend treatment without testing (39%), responses were stratified by clinical experience. Surgeons in practice more than 11 years (n = 35) were compared with those in practice 5 years or less (n = 31) (Table). Although the vast majority (85%) of respondents from both groups agreed or strongly agreed with always recommending ICP testing, 49% of those in practice more than 11 years and 29% in practice 5 years or less indicated they would recommend surgical treatment for CECS based solely on clinical examination findings (Figures 8, 9).
Responses were also stratified by number of CECS patients seen by each surgeon per year. Twenty-eight respondents saw 1 or 2 patients per year, and 12 saw more than 8 patients per year—31% and 13% of the total number of respondents, respectively. Of the respondents who saw 1 or 2 patients, 86% (24/28) agreed or strongly agreed with always recommending ICP testing—comparable to the 75% (9/12) who saw more than 8 patients (Figure 10). However, of the respondents who saw 1 or 2 patients, 36% (10/28) indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% (9/12) who saw more than 8 patients (Figure 11).
Discussion
CECS is a common cause of leg pain and a significant cause of disability among the active-duty military population. This was illustrated in 2 recent studies by Waterman and colleagues.1,9 The first1 investigated failure rates and disability after surgery for CECS among those on active duty. The authors showed that CECS is a substantial contributor to lower extremity disability in the military population and that there is a substantial risk for persistent symptoms despite surgical treatment. Nearly 1 in 5 patients experienced surgical failure after elective fasciotomy, and about 28% of patients were unable to return to the full activity required in the military. The second, more recent study9 was an epidemiologic study of risk factors associated with CECS in a physically active military population. The authors identified 4100 cases diagnosed between 2006 and 2011—representing an overall annual incidence of 0.49 per 1000 at-risk person-years, or about 683 cases per year; the authors also showed the incidence increased during the time period studied.
The diagnosis of CECS remains imperfect. A clinical history of exercise-induced lower leg pain that is relieved with rest suggests the diagnosis, but a confirmatory test is needed to distinguish CECS from other causes of exercise-induced leg pain. Although direct measurement of compartment pressures is the test used most often, it is invasive and time-consuming, can be uncomfortable for the patient, and may be associated with bleeding risk, infection, and nerve injury. Pedowitz and colleagues10 described the ICP testing criteria now generally used in the diagnosis of CECS. Unfortunately, there is little objective evidence supporting these criteria.4 Although less invasive tests (eg, MRI, NIRS) have been described,5-8 they may not be readily available across institutions, and further study is needed to validate their use in diagnosing CECS.
While an objective, validated test or measurement for confirming the diagnosis of CECS remains elusive, the outcomes after surgical treatment of CECS also remain imperfect. Surgery consists of both open and endoscopically assisted fasciotomy of the involved compartments.2,11-17 Reports of improvement after treatment range from 81% to 100%3; however, symptom relief does not come for all patients, particularly those in the military. Waterman and colleagues1 found a failure rate of about 20% among an active-duty military population. Packer and colleagues18 examined civilians with CECS, treated both operatively and nonoperatively. Patients in this series were diagnosed with CECS based on clinical symptoms as well as compartment pressure measurements according to the Pedowitz criteria. Although overall outcomes were better for operatively treated patients than for nonoperatively treated patients, only 47% of patients were completely pain-free, and 21% were unable to return to full activity.
More recent studies have explored use of other nonoperative treatment modalities. Diebal and colleagues19,20 used a running retraining program to treat patients with CECS. They based this treatment on the hypothesis that a heel-strike running pattern is associated with increased anterior compartment pressures.21 CECS patients who underwent a 6-week systematic treatment program focused on forefoot running, stride shortening, and hamstring activation during push-off experienced a decrease in clinical symptoms and posttreatment intracompartmental pressures.20 The improvements in clinical scores were maintained at 1-year follow-up. Another nonoperative intervention, recently described by Isner-Horobeti and colleagues,22 involves injecting botulinum toxin A (BoNT-A) into the anterior and lateral compartments of the leg. Sixteen patients with CECS received BoNT-A injections. On average, intracompartmental pressures were lower after injection than they were before injection. In addition, exertional pain was eliminated in 15 patients at an average follow-up of 4.4 months.
This survey-based study examined the practice patterns of military orthopedic surgeons who performed ICP testing for cases of suspected CECS. Our hypothesis was that, though ICP testing is the most commonly accepted method for confirming the diagnosis of CECS, the ICP testing rate would not be 100% among those surveyed.
The results of our study uncover an apparent inconsistency in survey responses among physicians who evaluate and treat patients with CECS. About 85% of respondents stated they would always recommend confirming the diagnosis of CECS with ICP testing. However, about 40% stated they would recommend surgical treatment without confirmatory testing if they were confident about the diagnosis based on clinical findings. In other words, only 60% of the respondents disagreed or strongly disagreed with pursuing surgical treatment without testing. One would expect a closer correlation between respondents who would always recommend ICP testing and those who disagreed with recommending surgical treatment without ICP testing. This raises the question of what actually occurs when CECS is suspected in clinical practice.
To better understand the apparent discrepancy between respondents who agreed or strongly agreed with always recommending ICP testing and respondents who would recommend treatment without testing, we grouped responses by clinical experience. Although 85% of respondents (no matter the number of years in practice) agreed or strongly agreed with the statement, “I always recommend confirming the diagnosis of CECS with ICP measurements,” 49% of those in practice more than 11 years (vs. 29% of those in practice 5 years or less) agreed or strongly agreed with recommending surgery without testing if they were 100% confident about the diagnosis of CECS based solely on clinical findings. This may suggest that, though most agreed that the gold standard for confirming the diagnosis of CECS remains ICP testing, those with more clinical experience were more comfortable forgoing this diagnostic measure and recommending treatment without testing.
Another measure of clinical experience used in this survey was based on number of CECS patients seen per year. Responses of surgeons who saw 1 or 2 patients with CECS per year were compared with responses of surgeons who saw more than 8 patients with CECS per year. Of the respondents who saw 1 or 2 patients, 86% agreed or strongly agreed with always recommending ICP testing to confirm CECS—comparable to the 75% who saw more than 8 patients. However, of the respondents who saw 1 or 2 patients, 36% indicated they would recommend surgical treatment, without ICP testing, if they were confident about the clinical diagnosis of CECS—in contrast to the 75% who saw more than 8 patients.
Responses regarding the absolute of always recommending ICP testing and the absolute of being 100% confident about the clinical diagnosis of CECS highlight differences between the surgeons with more experience (>11 years in practice, >8 CECS patients per year) and those with less experience (≤5 years in practice, 1 or 2 CECS patients per year). Surgeons in practice longer, and surgeons who saw more patients with suspected CECS per year, were more likely to recommend surgical treatment based solely on clinical findings.
Conclusion
CECS can cause debilitating activity-related leg pain in both civilian and military populations. Treatment with fasciotomy is often curative, but a significant number of patients may continue to have pain and disability. As the incidence of treatment failures may be higher in the military than in civilians, proper evaluation of patients with suspected CECS is particularly important for military orthopedic surgeons. The diagnosis of CECS can be challenging to both the clinician and patient, and diagnostic modalities remain imperfect. The results of this study highlight this, revealing less than 100% agreement regarding use of ICP testing (the gold standard) for diagnosis of CECS.
This study also highlights the need for an improved method of diagnosing CECS since 93% of respondents agreed or strongly agreed that ICP testing is unpleasant for the patient, and 90% would prefer a less-invasive test. In addition, the ICP testing criteria for establishing the diagnosis of CECS remain inconsistent. If a reliable, consistent, and less-invasive test were available, perhaps there would be less variability in practitioners’ evaluations of patients with CECS.
This study shows an inconsistency among military orthopedic surgeons evaluating and treating patients with CECS. As testing modalities for CECS remain imperfect, clinical acumen and experience assume an important role in the assessment of patients with suspected CECS.
1. Waterman BR, Laughlin M, Kilcoyne K, Cameron KL, Owens BD. Surgical treatment of chronic exertional compartment syndrome of the leg: failure rates and postoperative disability in an active patient population. J Bone Joint Surg Am. 2013;95(7):592-596.
2. Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36-40.
3. Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11(4):268-276.
4. Aweid O, Del Buono A, Malliaras P, et al. Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med. 2012;22(4):356-370.
5. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42(3):385-392.
6. van den Brand JG, Verleisdonk EJ, van der Werken C. Near infrared spectroscopy in the diagnosis of chronic exertional compartment syndrome. Am J Sports Med. 2004;32(2):452-456.
7. van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33(5):699-704.
8. Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30(6):321-325.
9. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
10. Pedowitz RA, Hargens AR, Mubarek SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18(1):35-40.1. Rorabeck CH, Bourne RB, Fowler PJ. The surgical treatment of exertional compartment syndrome in athletes. J Bone Joint Surg Am. 1983;65(9):1245-1251.
12. Rorabeck CH, Fowler PJ, Nott L. The results of fasciotomy in the management of chronic exertional compartment syndrome. Am J Sports Med. 1988;16(3):224-227.
13. Detmer DE, Sharpe K, Sufit RL, Girdley FM. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13(3):162-170.
14. Stein DA, Sennett BJ. One-portal endoscopically assisted fasciotomy for exertional compartment syndrome. Arthroscopy. 2005;21(1):108-112.
15. Fronek J, Mubarak SJ, Hargens AR, et al. Management of chronic exertional anterior compartment syndrome of the lower extremity. Clin Orthop Relat Res. 1989;(220):217-227.
16. Leversedge FJ, Casey PJ, Seiler JG 3rd, Xerogeanes JW. Endoscopically assisted fasciotomy: description of technique and in vitro assessment of lower-leg compartment decompression. Am J Sports Med. 2002;30(2):272-278.
17. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21(6):811-817.
18. Packer JD, Day MS, Nguyen JT, Hobart SJ, Hannafin JA, Metzl JD. Functional outcomes and patient satisfaction after fasciotomy for chronic exertional compartment syndrome. Am J Sports Med. 2013;41(2):430-436.
19. Diebal AR, Gregory R, Alitz C, Gerber JP. Effects of forefoot running on chronic exertional compartment syndrome: a case series. Int J Sports Phys Ther. 2011;6(4):312-321.
20. Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated with chronic exertional compartment syndrome. Am J Sports Med. 2012;40(5):1060-1067.
21. Kirby RL, McDermott AG. Anterior tibial compartment pressures during running with rearfoot and forefoot landing styles. Arch Phys Med Rehabil. 1983;64(7):296-299.
22. Isner-Horobeti ME, Dufour SP, Blaes C, Lecocq J. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41(11):2558-2566.
1. Waterman BR, Laughlin M, Kilcoyne K, Cameron KL, Owens BD. Surgical treatment of chronic exertional compartment syndrome of the leg: failure rates and postoperative disability in an active patient population. J Bone Joint Surg Am. 2013;95(7):592-596.
2. Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36-40.
3. Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11(4):268-276.
4. Aweid O, Del Buono A, Malliaras P, et al. Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med. 2012;22(4):356-370.
5. Ringler MD, Litwiller DV, Felmlee JP, et al. MRI accurately detects chronic exertional compartment syndrome: a validation study. Skeletal Radiol. 2013;42(3):385-392.
6. van den Brand JG, Verleisdonk EJ, van der Werken C. Near infrared spectroscopy in the diagnosis of chronic exertional compartment syndrome. Am J Sports Med. 2004;32(2):452-456.
7. van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33(5):699-704.
8. Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30(6):321-325.
9. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
10. Pedowitz RA, Hargens AR, Mubarek SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18(1):35-40.1. Rorabeck CH, Bourne RB, Fowler PJ. The surgical treatment of exertional compartment syndrome in athletes. J Bone Joint Surg Am. 1983;65(9):1245-1251.
12. Rorabeck CH, Fowler PJ, Nott L. The results of fasciotomy in the management of chronic exertional compartment syndrome. Am J Sports Med. 1988;16(3):224-227.
13. Detmer DE, Sharpe K, Sufit RL, Girdley FM. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13(3):162-170.
14. Stein DA, Sennett BJ. One-portal endoscopically assisted fasciotomy for exertional compartment syndrome. Arthroscopy. 2005;21(1):108-112.
15. Fronek J, Mubarak SJ, Hargens AR, et al. Management of chronic exertional anterior compartment syndrome of the lower extremity. Clin Orthop Relat Res. 1989;(220):217-227.
16. Leversedge FJ, Casey PJ, Seiler JG 3rd, Xerogeanes JW. Endoscopically assisted fasciotomy: description of technique and in vitro assessment of lower-leg compartment decompression. Am J Sports Med. 2002;30(2):272-278.
17. Schepsis AA, Martini D, Corbett M. Surgical management of exertional compartment syndrome of the lower leg. Long-term followup. Am J Sports Med. 1993;21(6):811-817.
18. Packer JD, Day MS, Nguyen JT, Hobart SJ, Hannafin JA, Metzl JD. Functional outcomes and patient satisfaction after fasciotomy for chronic exertional compartment syndrome. Am J Sports Med. 2013;41(2):430-436.
19. Diebal AR, Gregory R, Alitz C, Gerber JP. Effects of forefoot running on chronic exertional compartment syndrome: a case series. Int J Sports Phys Ther. 2011;6(4):312-321.
20. Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated with chronic exertional compartment syndrome. Am J Sports Med. 2012;40(5):1060-1067.
21. Kirby RL, McDermott AG. Anterior tibial compartment pressures during running with rearfoot and forefoot landing styles. Arch Phys Med Rehabil. 1983;64(7):296-299.
22. Isner-Horobeti ME, Dufour SP, Blaes C, Lecocq J. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41(11):2558-2566.
Current Evidence Does Not Support Medicare’s 3-Day Rule in Primary Total Joint Arthroplasty
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
The Supination-Pronation Test for Distal Biceps Tendon Rupture
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Commentary to "Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty"
In this month’s issue of The American Journal of Orthopedics, Tannenbaum and colleagues present a “5 Points” article on “Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty.” This is an excellent article that summarizes the authors’ methodology of determining not only the overall cost of hospital care for shoulder replacement but a detailed analysis of many components contributing to that cost.
The steps are fairly straightforward: identify the various components of the cost, gather the data contributing to those costs, and then analyze what are the major expenditures that contribute to the overall cost. Sounds simple, but, in practice, it is anything but!
As health care expenditures in the United States continue to increase and approach 20% of the gross domestic product, every sector of the health care industry is searching for ways to curtail and eventually decrease the cost of health care. However, one cannot control costs without accurate data that defines those costs. In this article, Tannenbaum and colleagues have provided a methodology to help both hospital administrators and surgeons determine the overall cost of shoulder arthroplasty, but their principles of analysis can be applied to all aspects of hospital care.
Such efforts are gaining the attention of many leaders of the health care industry. For example, in the September 8, 2015, edition of The New York Times, I was very interested to read the article “What are a Hospital’s Costs? Utah System Is Trying to Learn.”1 The article reviewed the efforts of Dr. Vivian Lee, chief executive at University of Utah Health Care, to determine the actual cost of all care provided by the university hospital, the same goal as the present 5 Points article on shoulder arthroplasty but on a vastly greater scale. Analyzing those costs guided Dr. Lee and her colleagues to alter clinical programs, which led to a decrease of 30% in hospital expenditures and fewer complications.1
We are all indebted to Mr. Tannenbaum and his coauthors for providing the journal’s readers with a clear map that we can use to both understand and navigate the current maze of hospital costs. Using such a guide, we will be able to gather information that not only saves money, but will improve care by directing resources to services that actually benefit our patients.
Reference
1. Kolata G. What are a hospital’s costs? Utah system is trying to learn. New York Times. September 8, 2015:A1. http://www.nytimes.com/2015/09/08/health/what-are-a-hospitals-costs-utah-system-is-trying-to-learn.html. Accessed September 17, 2015.
In this month’s issue of The American Journal of Orthopedics, Tannenbaum and colleagues present a “5 Points” article on “Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty.” This is an excellent article that summarizes the authors’ methodology of determining not only the overall cost of hospital care for shoulder replacement but a detailed analysis of many components contributing to that cost.
The steps are fairly straightforward: identify the various components of the cost, gather the data contributing to those costs, and then analyze what are the major expenditures that contribute to the overall cost. Sounds simple, but, in practice, it is anything but!
As health care expenditures in the United States continue to increase and approach 20% of the gross domestic product, every sector of the health care industry is searching for ways to curtail and eventually decrease the cost of health care. However, one cannot control costs without accurate data that defines those costs. In this article, Tannenbaum and colleagues have provided a methodology to help both hospital administrators and surgeons determine the overall cost of shoulder arthroplasty, but their principles of analysis can be applied to all aspects of hospital care.
Such efforts are gaining the attention of many leaders of the health care industry. For example, in the September 8, 2015, edition of The New York Times, I was very interested to read the article “What are a Hospital’s Costs? Utah System Is Trying to Learn.”1 The article reviewed the efforts of Dr. Vivian Lee, chief executive at University of Utah Health Care, to determine the actual cost of all care provided by the university hospital, the same goal as the present 5 Points article on shoulder arthroplasty but on a vastly greater scale. Analyzing those costs guided Dr. Lee and her colleagues to alter clinical programs, which led to a decrease of 30% in hospital expenditures and fewer complications.1
We are all indebted to Mr. Tannenbaum and his coauthors for providing the journal’s readers with a clear map that we can use to both understand and navigate the current maze of hospital costs. Using such a guide, we will be able to gather information that not only saves money, but will improve care by directing resources to services that actually benefit our patients.
In this month’s issue of The American Journal of Orthopedics, Tannenbaum and colleagues present a “5 Points” article on “Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty.” This is an excellent article that summarizes the authors’ methodology of determining not only the overall cost of hospital care for shoulder replacement but a detailed analysis of many components contributing to that cost.
The steps are fairly straightforward: identify the various components of the cost, gather the data contributing to those costs, and then analyze what are the major expenditures that contribute to the overall cost. Sounds simple, but, in practice, it is anything but!
As health care expenditures in the United States continue to increase and approach 20% of the gross domestic product, every sector of the health care industry is searching for ways to curtail and eventually decrease the cost of health care. However, one cannot control costs without accurate data that defines those costs. In this article, Tannenbaum and colleagues have provided a methodology to help both hospital administrators and surgeons determine the overall cost of shoulder arthroplasty, but their principles of analysis can be applied to all aspects of hospital care.
Such efforts are gaining the attention of many leaders of the health care industry. For example, in the September 8, 2015, edition of The New York Times, I was very interested to read the article “What are a Hospital’s Costs? Utah System Is Trying to Learn.”1 The article reviewed the efforts of Dr. Vivian Lee, chief executive at University of Utah Health Care, to determine the actual cost of all care provided by the university hospital, the same goal as the present 5 Points article on shoulder arthroplasty but on a vastly greater scale. Analyzing those costs guided Dr. Lee and her colleagues to alter clinical programs, which led to a decrease of 30% in hospital expenditures and fewer complications.1
We are all indebted to Mr. Tannenbaum and his coauthors for providing the journal’s readers with a clear map that we can use to both understand and navigate the current maze of hospital costs. Using such a guide, we will be able to gather information that not only saves money, but will improve care by directing resources to services that actually benefit our patients.
Reference
1. Kolata G. What are a hospital’s costs? Utah system is trying to learn. New York Times. September 8, 2015:A1. http://www.nytimes.com/2015/09/08/health/what-are-a-hospitals-costs-utah-system-is-trying-to-learn.html. Accessed September 17, 2015.
Reference
1. Kolata G. What are a hospital’s costs? Utah system is trying to learn. New York Times. September 8, 2015:A1. http://www.nytimes.com/2015/09/08/health/what-are-a-hospitals-costs-utah-system-is-trying-to-learn.html. Accessed September 17, 2015.