User login
Analysis of Predictors and Outcomes of Allogeneic Blood Transfusion After Shoulder Arthroplasty
In shoulder arthroplasty, it is not uncommon for patients to receive postoperative blood transfusions; rates range from 7% to 43%.1-6 Allogeneic blood transfusions (ABTs) are costly and not entirely free of risks.7 The risk for infection has decreased because of improved screening and risk reduction strategies, but there are still significant risks associated with ABTs, such as clerical errors, acute and delayed hemolytic reactions, graft-versus-host reactions, transfusion-related acute lung injury, and anaphylaxis.8-10 As use of shoulder arthroplasty continues to increase, the importance of minimizing unnecessary transfusions is growing as well.7
Predictive factors for ABT have been explored in other orthopedic settings, yet little has been done in shoulder arthroplasty.1-6,11-15 Previous shoulder arthroplasty studies have shown that low preoperative hemoglobin (Hb) levels are independent risk factors for postoperative blood transfusion. However, there is debate over the significance of other variables, such as procedure type, age, sex, and medical comorbidities. Further, prior studies were limited by relatively small samples from single institutions; the largest series included fewer than 600 patients.1-6
We conducted a study to determine predictors of ABT in a large cohort of patients admitted to US hospitals for shoulder arthroplasty. We also wanted to evaluate the effect of ABT on postoperative outcomes, including inpatient mortality, adverse events, prolonged hospital stay, and nonroutine discharge. According to the null hypothesis, in shoulder arthroplasty there will be no difference in risk factors between patients who require ABT and those who did not, after accounting for confounding variables.
Materials and Methods
This study was exempt from institutional review board approval, as all data were appropriately deidentified before use in this project. We used the Nationwide Inpatient Sample (NIS) to retrospectively study the period 2002–2011, from which all demographic, clinical, and resource use data were derived.16 NIS, an annual survey conducted by the Agency for Healthcare Research and Quality (AHRQ) since 1988, has generated a huge amount of data, forming the largest all-payer inpatient care database in the United States. Yearly samples contain discharge data from about 8 million hospital stays at more than 1000 hospitals across 46 states, approximating a 20% random sample of all hospital discharges at participating institutions.17 These data are then weighted to generate statistically valid national estimates.
The NIS database uses International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes to identify 15 medical diagnoses up to the year 2008 and a maximum of 25 medical diagnoses and 15 procedures thereafter. In addition, the database includes information on patient and hospital characteristics as well as inpatient outcomes such as length of stay, total hospitalization charges, and discharge disposition.18,19 Given its large sample size and data volume, NIS is a powerful tool in the analysis of data associated with a multitude of medical diagnoses and procedures.20
We used the NIS database to study a population of 422,371 patients (age, >18 years) who underwent total shoulder arthroplasty (TSA) or hemiarthroplasty (HSA) between 2002 and 2011. ICD-9-CM procedure codes for TSA (81.80, 81.88) and HSA (81.81) were used to identify this population. We also analyzed data for reverse TSA for the year 2011. Then we divided our target population into 2 different cohorts: patients who did not receive any blood transfusion products and patients who received a transfusion of allogeneic packed cells (ICD-9-CM code 99.04 was used to identify the latter cohort).
In this study, normal distribution of the dataset was assumed, given the large sample size. The 2 cohorts were evaluated through bivariate analysis using the Pearson χ2 test for categorical data and the independent-samples t test for continuous data. The extent to which diagnosis, age, race, sex, and medical comorbidities were predictive of blood transfusion after TSA or HSA was evaluated through multivariate binary logistic regression analysis. Statistical significance was set at P < .05. All statistical analyses and data modeling were performed with SPSS Version 22.0.
Results
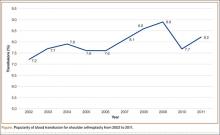
Using the NIS database, we stratified an estimated 422,371 patients who presented for shoulder arthroplasty between January 1, 2002, and December 31, 2011, into a TSA cohort (59.3%) and an HSA cohort (40.7%). Eight percent (33,889) of all patients received an ABT; the proportion of patients who received ABT was higher (P < .001) for the HSA cohort (55.6%) than the TSA cohort (39.4%). Further, the rate of ABT after shoulder arthroplasty showed an upward inclination (Figure).
Demographically, patients who received ABT tended (P < .001) to be older (74±11 years vs 68±11 years) and of a minority race (black or Hispanic) and to fall in either the lowest range of median household income (21.5% vs 20.7%; ≤$38,999) or the highest (27.3% vs 25.4%; ≥$63,000). Shoulder arthroplasty with ABT occurred more often (P < .001) at hospitals that were urban (13.3% vs 11.3%), medium in size (27.3% vs 23.4%), and nonteaching (56.2% vs 54.3%). In addition, ABT was used more often (P < .001) in patients with a primary diagnosis of fracture (43.1% vs 14.3%) or fracture nonunion (4.4% vs 2.1%). These groups also had a longer (P < .001) hospital stay (5.0±4.3 days vs 2.5±2.2 days). Table 1 summarizes these findings.
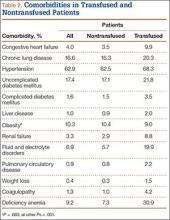
The 2 cohorts were then analyzed for presence of medical comorbidities (Table 2). Patients who required ABT during shoulder arthroplasty had a significantly (P < .001) higher prevalence of congestive heart failure, chronic lung disease, hypertension, uncomplicated and complicated diabetes mellitus, liver disease, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, weight loss, coagulopathy, and deficiency anemia.
In multivariate regression modeling (Table 3), demographic predictors of ABT (P < .001) included increasing age (odds ratio [OR], 1.03 per year; 95% confidence interval [95% CI], 1.03-1.03), female sex (OR, 1.55; 95% CI, 1.51-1.60), and minority race (black or Hispanic). Odds of requiring ABT were higher for patients with Medicare (OR, 1.25; 95% CI, 1.20-1.30) and patients with Medicaid (OR, 1.63; 95% CI, 1.51-1.77) than for patients with private insurance.
ABT was more likely to be required (P < .001) in patients with a primary diagnosis of fracture (OR, 4.49; 95% CI, 4.34-4.65), avascular necrosis (OR, 2.06; 95% CI, 1.91-2.22), rheumatoid arthritis (OR, 1.91; 95% CI, 1.72-2.12), fracture nonunion (OR, 3.55; 95% CI, 3.33-3.79), or rotator cuff arthropathy (OR, 1.47; 95% CI, 1.41-1.54) than for patients with osteoarthritis. Moreover, compared with patients having HSA, patients having TSA were more likely to require ABT (OR, 1.20; 95% CI, 1.17-1.24). According to the analysis restricted to the year 2011, compared with patients having anatomical TSAs, patients having reverse TSAs were 1.6 times more likely (P < .001) to require ABT (OR, 1.63; 95% CI, 1.50-1.79).
With the exception of obesity, all comorbidities were significant (P < .001) independent predictors of ABT after shoulder arthroplasty: deficiency anemia (OR, 3.42; 95% CI, 3.32-3.52), coagulopathy (OR, 2.54; 95% CI, 2.36-2.73), fluid and electrolyte disorders (OR, 1.91; 95% CI, 1.84-1.97), and weight loss (OR, 1.78; 95% CI, 1.58-2.00).
Patients who received ABT were more likely to experience adverse events (OR, 1.74; 95% CI, 1.68-1.81), prolonged hospital stay (OR, 3.21; 95% CI, 3.12-3.30), and nonroutine discharge (OR, 1.77; 95% CI, 1.72-1.82) (Table 4). There was no difference in mortality between the 2 cohorts.
Discussion
There is an abundance of literature on blood transfusions in hip and knee arthroplasty, but there are few articles on ABT in shoulder arthroplasty, and they all report data from single institutions with relatively low caseloads.1,2,11-13,15,21 In the present study, we investigated ABT in shoulder arthroplasty from the perspective of a multi-institutional database with a caseload of more than 400,000. Given the rapidly increasing rates of shoulder arthroplasty, it is important to further examine this issue to minimize unnecessary blood transfusion and its associated risks and costs.7
We found that 8% of patients who had shoulder arthroplasty received ABT, which is consistent with previously reported transfusion rates (range, 7%-43%).1-6 Rates of ABT after shoulder arthroplasty have continued to rise. The exception, a decrease during the year 2010, can be explained by increased efforts to more rigidly follow transfusion indication guidelines to reduce the number of potentially unnecessary ABTs.21-24 Our study also identified numerous significant independent predictors of ABT in shoulder arthroplasty: age, sex, race, insurance status, procedure type, primary diagnoses, and multiple medical comorbidities.
Demographics
According to our analysis, more than 80% of patients who received ABT were over age 65 years, which aligns with what several other studies have demonstrated: Increasing age is a predictor of ABT, despite higher rates of comorbidities and lower preoperative Hb levels in this population.1,2,4,5,25-27 Consistent with previous work, female sex was predictive of ABT.2,5 It has been suggested that females are more likely predisposed to ABT because of lower preoperative Hb and smaller blood mass.2,5,28 Interestingly, our study showed a higher likelihood of ABT in both black and Hispanic populations. Further, patients with Medicare or Medicaid were more likely to receive ABT.
Primary Diagnosis
Although patients with a primary diagnosis of osteoarthritis constitute the majority of patients who undergo shoulder arthroplasty, our analysis showed that patients with a diagnosis of proximal humerus fracture were more likely to receive ABT. This finding is reasonable given studies showing the high prevalence of proximal humerus fractures in elderly women.29,30 Similarly, patients with a humerus fracture nonunion were more likely to receive a blood transfusion, which is unsurprising given the increased complexity associated with arthroplasty in this predominately elderly population.31 Interestingly, compared with patients with osteoarthritis, patients with any one of the other primary diagnoses were more likely to require a transfusion—proximal humerus fracture being the most significant, followed by humerus fracture nonunion, avascular necrosis, rheumatoid arthritis, and rotator cuff arthropathy.
Type of Arthroplasty
Bivariate analysis revealed that 55.6% of the patients who received ABT underwent HSA; the other 44.4% underwent TSA. The effect of primary diagnosis on procedure choice likely played a role in this finding. HSA indications include humerus fracture, which has been associated with increased ABT, whereas patients with osteoarthritis requiring TSA are significantly less likely to require ABT, as reflected in this analysis.7,32-34 Previous studies have failed to show a difference in blood transfusion rates between TSA and HSA.2,4-6,35 Conversely, with confounding factors controlled for, multivariate logistic regression analysis showed that TSA was 1.2 times more likely than HSA to require ABT, which could be explained by the increased operative time, case complexity, and blood loss that may be associated with the glenoid exposure.36,37 With analysis restricted to the year 2011, patients with reverse TSAs were 1.6 times more likely than patients with anatomical TSAs to receive a blood transfusion (OR, 1.63; 95% CI, 1.50-1.79). Although this finding differs from what was previously reported, it fits given that patients having reverse TSAs are often older and may present with a more significant comorbidity profile.3 In addition, there are the increased technical surgical aspects associated with “salvage surgery” for challenging indications such as cuff arthropathy and failed previous arthroplasty.38-41
Medical Comorbidities
Patients who received ABT were more likely to present with numerous medical comorbidities. Previous studies have indicated that the presence of multiple medical comorbidities significantly increased blood transfusion rates, possibly by working synergistically.42 All studies of blood transfusion in shoulder arthroplasty concluded that lower preoperative Hb was an independent predictor.1-6 Schumer and colleagues4 reported a 4-fold increase in likelihood of blood transfusion in patients with a preoperative Hb level less than 12.5 g/dL. In addition, Millett and colleagues6 showed a 20-fold increase in likelihood of transfusion in patients with a preoperative Hb level less than 11.0 g/dL compared with patients with a level higher than 13.0 g/dL. Patients with a Hb level between 11.0 and 13.0 g/dL showed a 5-fold increase in likelihood of transfusion.6 We should note that correction of preoperative anemia through various pharmacologic methods (eg, erythropoietin, intravenous iron supplementation) has been shown to decrease postoperative transfusion rates.43,44 Although we could not include preoperative Hb levels in the present study, given inherent limitations in using NIS, our multivariate analysis showed that preoperative deficiency anemia and coagulopathy were the most significant predictors of ABT.
In addition, the multivariate logistic regression model showed that both cardiac disease and diabetes were independent predictors of ABT, confirming data reported by Ahmadi and colleagues.1 Although not as well characterized in other studies, in the current analysis multiple other medical comorbidities, including fluid and electrolyte abnormalities, weight loss, liver disease, renal failure, and chronic lung disease, had significant predictive value. Contrarily, obesity significantly decreased the odds of ABT, likely because of higher baseline blood volume in obese patients.
Patient Outcomes
Patients who undergo shoulder arthroplasty with ABT are more likely to experience adverse events or a prolonged hospital stay and are more often discharged to a nursing home or an extended-care facility. In this population, however, deaths did not occur at a significantly higher rate—similar to what was found for patients who underwent hip or knee arthroplasty with blood transfusions.45
Little has been done to investigate the effect of pharmacologic agents on the need for perioperative ABT for orthopedic shoulder procedures. Aprotinin, tranexamic acid, epoetin-α, and aminocaproic acid have all been effective in limiting ABT during the perioperative period in various orthopedic hip, knee, and spine procedures.9,46-53 Given the increased morbidity associated with ABT, it may be beneficial to use similar methods to limit blood loss in high-risk patients undergoing shoulder arthroplasty.
Study Limitations
NIS has intrinsic limitations. Given its massive volume, it is subject to errors in both data entry and clinical coding. Moreover, the database lacks data that would have been useful in our study: preoperative Hb levels, intraoperative course, number of units transfused, total blood loss, use of blood conservation techniques, transfusion protocols, and severity of comorbidities. Reverse TSA was given a unique ICD-9-CM code in October 2010, so 2011 was the only year we were able to examine the relationship between reverse TSA and transfusions. Further, our analysis was unable to identify any medications, including chronic anticoagulants or postoperative prophylaxis, that have been shown to significantly affect blood transfusion rates.54 Yet, there are obvious advantages to using the NIS database, as previously outlined across the medical landscape.
Conclusion
Our results confirmed previous findings and identified new predictors of ABT in shoulder arthroplasty in a large cohort. We examined demographics and perioperative complications while identifying predictors of ABT use. Patients who received ABT were older, female, and nonwhite and were covered by Medicare or Medicaid insurance, and many had a primary diagnosis of proximal humerus fracture. The ABT cohort had numerous medical comorbidities, including deficiency anemia and coagulopathy. Identifying this patient population is a prerequisite to educating patients while minimizing unnecessary risks and costs.
Using NIS data on a population of 422,371 patients who underwent shoulder arthroplasty, we identified the 5 likeliest predictors of ABT: fracture, fracture nonunion, deficiency anemia, coagulopathy, and avascular necrosis. Of the identified variables associated with ABT, deficiency anemia may be the most amenable to treatment; therefore, there may be benefit in delaying elective shoulder arthroplasty in this cohort. Given these findings, it is important to identify at-risk patients before surgery, with the intent to provide education and minimize risk.
1. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
2. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
3. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
6. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
7. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
8. Ceccherini-Nelli L, Filipponi F, Mosca F, Campa M. The risk of contracting an infectious disease from blood transfusion. Transplantation Proc. 2004;36(3):680-682.
9. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278.
10. Hatzidakis AM, Mendlick RM, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty. An analysis of risk factors for allogenic transfusion. J Bone Joint Surg Am. 2000;82(1):89-100.
11. Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J. Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am. 2013;95(19):1777-1783.
12. Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86(7):970-973.
13. Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 suppl):34-37.
14. Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion in the United States, 2004–2009. Spine. 2014;39(4):304-310.
15. Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961-967.
16. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
17. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
18. Pereira BM, Chan PH, Weinstein PR, Fishman RA. Cerebral protection during reperfusion with superoxide dismutase in focal cerebral ischemia. Adv Neurol. 1990;52:97-103.
19. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
20. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
21. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86(7):1512-1518.
22. Martinez V, Monsaingeon-Lion A, Cherif K, Judet T, Chauvin M, Fletcher D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. 2007;99(6):794-800.
23. Helm AT, Karski MT, Parsons SJ, Sampath JS, Bale RS. A strategy for reducing blood-transfusion requirements in elective orthopaedic surgery. Audit of an algorithm for arthroplasty of the lower limb. J Bone Joint Surg Br. 2003;85(4):484-489.
24. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
25. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268.
26. Rogers MA, Blumberg N, Heal JM, Langa KM. Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710-718.
27. Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Med. 2007;17(1):1-15.
28. Fosco M, Di Fiore M. Factors predicting blood transfusion in different surgical procedures for degenerative spine disease. Eur Rev Med Pharmacol Sci. 2012;16(13):1853-1858.
29. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
30. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706.
31. Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46-50.
32. Chambers L, Dines JS, Lorich DG, Dines DM. Hemiarthroplasty for proximal humerus fractures. Curr Rev Musculoskeletal Med. 2013;6(1):57-62.
33. Jain NB, Hocker S, Pietrobon R, Guller U, Bathia N, Higgins LD. Total arthroplasty versus hemiarthroplasty for glenohumeral osteoarthritis: role of provider volume. J Shoulder Elbow Surg. 2005;14(4):361-367.
34. Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
35. Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453.
36. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
37. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23(8):1187-1194.
38. Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):388-394.
39. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
40. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
41. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
42. Pola E, Papaleo P, Santoliquido A, Gasparini G, Aulisa L, De Santis E. Clinical factors associated with an increased risk of perioperative blood transfusion in nonanemic patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2004;86(1):57-61.
43. Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfusion Med Rev. 2013;27(4):221-234.
44. Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54(2):289-299.
45. Danninger T, Rasul R, Poeran J, et al. Blood transfusions in total hip and knee arthroplasty: an analysis of outcomes. ScientificWorldJournal. 2014;2014:623460.
46. Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO. Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine. 2010;35(2):235-239.
47. Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552-1557.
48. Delasotta LA, Orozco F, Jafari SM, Blair JL, Ong A. Should we use preoperative epoetin-alpha in the mildly anemic patient undergoing simultaneous total knee arthroplasty? Open Orthop J. 2013;7:47-50.
49. Delasotta LA, Rangavajjula A, Frank ML, Blair J, Orozco F, Ong A. The use of preoperative epoetin-alpha in revision hip arthroplasty. Open Orthop J. 2012;6:179-183.
50. Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014;54(1):26-30.
51. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
52. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008(3):CD006883.
53. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
54. Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281-287.
In shoulder arthroplasty, it is not uncommon for patients to receive postoperative blood transfusions; rates range from 7% to 43%.1-6 Allogeneic blood transfusions (ABTs) are costly and not entirely free of risks.7 The risk for infection has decreased because of improved screening and risk reduction strategies, but there are still significant risks associated with ABTs, such as clerical errors, acute and delayed hemolytic reactions, graft-versus-host reactions, transfusion-related acute lung injury, and anaphylaxis.8-10 As use of shoulder arthroplasty continues to increase, the importance of minimizing unnecessary transfusions is growing as well.7
Predictive factors for ABT have been explored in other orthopedic settings, yet little has been done in shoulder arthroplasty.1-6,11-15 Previous shoulder arthroplasty studies have shown that low preoperative hemoglobin (Hb) levels are independent risk factors for postoperative blood transfusion. However, there is debate over the significance of other variables, such as procedure type, age, sex, and medical comorbidities. Further, prior studies were limited by relatively small samples from single institutions; the largest series included fewer than 600 patients.1-6
We conducted a study to determine predictors of ABT in a large cohort of patients admitted to US hospitals for shoulder arthroplasty. We also wanted to evaluate the effect of ABT on postoperative outcomes, including inpatient mortality, adverse events, prolonged hospital stay, and nonroutine discharge. According to the null hypothesis, in shoulder arthroplasty there will be no difference in risk factors between patients who require ABT and those who did not, after accounting for confounding variables.
Materials and Methods
This study was exempt from institutional review board approval, as all data were appropriately deidentified before use in this project. We used the Nationwide Inpatient Sample (NIS) to retrospectively study the period 2002–2011, from which all demographic, clinical, and resource use data were derived.16 NIS, an annual survey conducted by the Agency for Healthcare Research and Quality (AHRQ) since 1988, has generated a huge amount of data, forming the largest all-payer inpatient care database in the United States. Yearly samples contain discharge data from about 8 million hospital stays at more than 1000 hospitals across 46 states, approximating a 20% random sample of all hospital discharges at participating institutions.17 These data are then weighted to generate statistically valid national estimates.
The NIS database uses International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes to identify 15 medical diagnoses up to the year 2008 and a maximum of 25 medical diagnoses and 15 procedures thereafter. In addition, the database includes information on patient and hospital characteristics as well as inpatient outcomes such as length of stay, total hospitalization charges, and discharge disposition.18,19 Given its large sample size and data volume, NIS is a powerful tool in the analysis of data associated with a multitude of medical diagnoses and procedures.20
We used the NIS database to study a population of 422,371 patients (age, >18 years) who underwent total shoulder arthroplasty (TSA) or hemiarthroplasty (HSA) between 2002 and 2011. ICD-9-CM procedure codes for TSA (81.80, 81.88) and HSA (81.81) were used to identify this population. We also analyzed data for reverse TSA for the year 2011. Then we divided our target population into 2 different cohorts: patients who did not receive any blood transfusion products and patients who received a transfusion of allogeneic packed cells (ICD-9-CM code 99.04 was used to identify the latter cohort).
In this study, normal distribution of the dataset was assumed, given the large sample size. The 2 cohorts were evaluated through bivariate analysis using the Pearson χ2 test for categorical data and the independent-samples t test for continuous data. The extent to which diagnosis, age, race, sex, and medical comorbidities were predictive of blood transfusion after TSA or HSA was evaluated through multivariate binary logistic regression analysis. Statistical significance was set at P < .05. All statistical analyses and data modeling were performed with SPSS Version 22.0.
Results
Using the NIS database, we stratified an estimated 422,371 patients who presented for shoulder arthroplasty between January 1, 2002, and December 31, 2011, into a TSA cohort (59.3%) and an HSA cohort (40.7%). Eight percent (33,889) of all patients received an ABT; the proportion of patients who received ABT was higher (P < .001) for the HSA cohort (55.6%) than the TSA cohort (39.4%). Further, the rate of ABT after shoulder arthroplasty showed an upward inclination (Figure).
Demographically, patients who received ABT tended (P < .001) to be older (74±11 years vs 68±11 years) and of a minority race (black or Hispanic) and to fall in either the lowest range of median household income (21.5% vs 20.7%; ≤$38,999) or the highest (27.3% vs 25.4%; ≥$63,000). Shoulder arthroplasty with ABT occurred more often (P < .001) at hospitals that were urban (13.3% vs 11.3%), medium in size (27.3% vs 23.4%), and nonteaching (56.2% vs 54.3%). In addition, ABT was used more often (P < .001) in patients with a primary diagnosis of fracture (43.1% vs 14.3%) or fracture nonunion (4.4% vs 2.1%). These groups also had a longer (P < .001) hospital stay (5.0±4.3 days vs 2.5±2.2 days). Table 1 summarizes these findings.
The 2 cohorts were then analyzed for presence of medical comorbidities (Table 2). Patients who required ABT during shoulder arthroplasty had a significantly (P < .001) higher prevalence of congestive heart failure, chronic lung disease, hypertension, uncomplicated and complicated diabetes mellitus, liver disease, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, weight loss, coagulopathy, and deficiency anemia.
In multivariate regression modeling (Table 3), demographic predictors of ABT (P < .001) included increasing age (odds ratio [OR], 1.03 per year; 95% confidence interval [95% CI], 1.03-1.03), female sex (OR, 1.55; 95% CI, 1.51-1.60), and minority race (black or Hispanic). Odds of requiring ABT were higher for patients with Medicare (OR, 1.25; 95% CI, 1.20-1.30) and patients with Medicaid (OR, 1.63; 95% CI, 1.51-1.77) than for patients with private insurance.
ABT was more likely to be required (P < .001) in patients with a primary diagnosis of fracture (OR, 4.49; 95% CI, 4.34-4.65), avascular necrosis (OR, 2.06; 95% CI, 1.91-2.22), rheumatoid arthritis (OR, 1.91; 95% CI, 1.72-2.12), fracture nonunion (OR, 3.55; 95% CI, 3.33-3.79), or rotator cuff arthropathy (OR, 1.47; 95% CI, 1.41-1.54) than for patients with osteoarthritis. Moreover, compared with patients having HSA, patients having TSA were more likely to require ABT (OR, 1.20; 95% CI, 1.17-1.24). According to the analysis restricted to the year 2011, compared with patients having anatomical TSAs, patients having reverse TSAs were 1.6 times more likely (P < .001) to require ABT (OR, 1.63; 95% CI, 1.50-1.79).
With the exception of obesity, all comorbidities were significant (P < .001) independent predictors of ABT after shoulder arthroplasty: deficiency anemia (OR, 3.42; 95% CI, 3.32-3.52), coagulopathy (OR, 2.54; 95% CI, 2.36-2.73), fluid and electrolyte disorders (OR, 1.91; 95% CI, 1.84-1.97), and weight loss (OR, 1.78; 95% CI, 1.58-2.00).
Patients who received ABT were more likely to experience adverse events (OR, 1.74; 95% CI, 1.68-1.81), prolonged hospital stay (OR, 3.21; 95% CI, 3.12-3.30), and nonroutine discharge (OR, 1.77; 95% CI, 1.72-1.82) (Table 4). There was no difference in mortality between the 2 cohorts.
Discussion
There is an abundance of literature on blood transfusions in hip and knee arthroplasty, but there are few articles on ABT in shoulder arthroplasty, and they all report data from single institutions with relatively low caseloads.1,2,11-13,15,21 In the present study, we investigated ABT in shoulder arthroplasty from the perspective of a multi-institutional database with a caseload of more than 400,000. Given the rapidly increasing rates of shoulder arthroplasty, it is important to further examine this issue to minimize unnecessary blood transfusion and its associated risks and costs.7
We found that 8% of patients who had shoulder arthroplasty received ABT, which is consistent with previously reported transfusion rates (range, 7%-43%).1-6 Rates of ABT after shoulder arthroplasty have continued to rise. The exception, a decrease during the year 2010, can be explained by increased efforts to more rigidly follow transfusion indication guidelines to reduce the number of potentially unnecessary ABTs.21-24 Our study also identified numerous significant independent predictors of ABT in shoulder arthroplasty: age, sex, race, insurance status, procedure type, primary diagnoses, and multiple medical comorbidities.
Demographics
According to our analysis, more than 80% of patients who received ABT were over age 65 years, which aligns with what several other studies have demonstrated: Increasing age is a predictor of ABT, despite higher rates of comorbidities and lower preoperative Hb levels in this population.1,2,4,5,25-27 Consistent with previous work, female sex was predictive of ABT.2,5 It has been suggested that females are more likely predisposed to ABT because of lower preoperative Hb and smaller blood mass.2,5,28 Interestingly, our study showed a higher likelihood of ABT in both black and Hispanic populations. Further, patients with Medicare or Medicaid were more likely to receive ABT.
Primary Diagnosis
Although patients with a primary diagnosis of osteoarthritis constitute the majority of patients who undergo shoulder arthroplasty, our analysis showed that patients with a diagnosis of proximal humerus fracture were more likely to receive ABT. This finding is reasonable given studies showing the high prevalence of proximal humerus fractures in elderly women.29,30 Similarly, patients with a humerus fracture nonunion were more likely to receive a blood transfusion, which is unsurprising given the increased complexity associated with arthroplasty in this predominately elderly population.31 Interestingly, compared with patients with osteoarthritis, patients with any one of the other primary diagnoses were more likely to require a transfusion—proximal humerus fracture being the most significant, followed by humerus fracture nonunion, avascular necrosis, rheumatoid arthritis, and rotator cuff arthropathy.
Type of Arthroplasty
Bivariate analysis revealed that 55.6% of the patients who received ABT underwent HSA; the other 44.4% underwent TSA. The effect of primary diagnosis on procedure choice likely played a role in this finding. HSA indications include humerus fracture, which has been associated with increased ABT, whereas patients with osteoarthritis requiring TSA are significantly less likely to require ABT, as reflected in this analysis.7,32-34 Previous studies have failed to show a difference in blood transfusion rates between TSA and HSA.2,4-6,35 Conversely, with confounding factors controlled for, multivariate logistic regression analysis showed that TSA was 1.2 times more likely than HSA to require ABT, which could be explained by the increased operative time, case complexity, and blood loss that may be associated with the glenoid exposure.36,37 With analysis restricted to the year 2011, patients with reverse TSAs were 1.6 times more likely than patients with anatomical TSAs to receive a blood transfusion (OR, 1.63; 95% CI, 1.50-1.79). Although this finding differs from what was previously reported, it fits given that patients having reverse TSAs are often older and may present with a more significant comorbidity profile.3 In addition, there are the increased technical surgical aspects associated with “salvage surgery” for challenging indications such as cuff arthropathy and failed previous arthroplasty.38-41
Medical Comorbidities
Patients who received ABT were more likely to present with numerous medical comorbidities. Previous studies have indicated that the presence of multiple medical comorbidities significantly increased blood transfusion rates, possibly by working synergistically.42 All studies of blood transfusion in shoulder arthroplasty concluded that lower preoperative Hb was an independent predictor.1-6 Schumer and colleagues4 reported a 4-fold increase in likelihood of blood transfusion in patients with a preoperative Hb level less than 12.5 g/dL. In addition, Millett and colleagues6 showed a 20-fold increase in likelihood of transfusion in patients with a preoperative Hb level less than 11.0 g/dL compared with patients with a level higher than 13.0 g/dL. Patients with a Hb level between 11.0 and 13.0 g/dL showed a 5-fold increase in likelihood of transfusion.6 We should note that correction of preoperative anemia through various pharmacologic methods (eg, erythropoietin, intravenous iron supplementation) has been shown to decrease postoperative transfusion rates.43,44 Although we could not include preoperative Hb levels in the present study, given inherent limitations in using NIS, our multivariate analysis showed that preoperative deficiency anemia and coagulopathy were the most significant predictors of ABT.
In addition, the multivariate logistic regression model showed that both cardiac disease and diabetes were independent predictors of ABT, confirming data reported by Ahmadi and colleagues.1 Although not as well characterized in other studies, in the current analysis multiple other medical comorbidities, including fluid and electrolyte abnormalities, weight loss, liver disease, renal failure, and chronic lung disease, had significant predictive value. Contrarily, obesity significantly decreased the odds of ABT, likely because of higher baseline blood volume in obese patients.
Patient Outcomes
Patients who undergo shoulder arthroplasty with ABT are more likely to experience adverse events or a prolonged hospital stay and are more often discharged to a nursing home or an extended-care facility. In this population, however, deaths did not occur at a significantly higher rate—similar to what was found for patients who underwent hip or knee arthroplasty with blood transfusions.45
Little has been done to investigate the effect of pharmacologic agents on the need for perioperative ABT for orthopedic shoulder procedures. Aprotinin, tranexamic acid, epoetin-α, and aminocaproic acid have all been effective in limiting ABT during the perioperative period in various orthopedic hip, knee, and spine procedures.9,46-53 Given the increased morbidity associated with ABT, it may be beneficial to use similar methods to limit blood loss in high-risk patients undergoing shoulder arthroplasty.
Study Limitations
NIS has intrinsic limitations. Given its massive volume, it is subject to errors in both data entry and clinical coding. Moreover, the database lacks data that would have been useful in our study: preoperative Hb levels, intraoperative course, number of units transfused, total blood loss, use of blood conservation techniques, transfusion protocols, and severity of comorbidities. Reverse TSA was given a unique ICD-9-CM code in October 2010, so 2011 was the only year we were able to examine the relationship between reverse TSA and transfusions. Further, our analysis was unable to identify any medications, including chronic anticoagulants or postoperative prophylaxis, that have been shown to significantly affect blood transfusion rates.54 Yet, there are obvious advantages to using the NIS database, as previously outlined across the medical landscape.
Conclusion
Our results confirmed previous findings and identified new predictors of ABT in shoulder arthroplasty in a large cohort. We examined demographics and perioperative complications while identifying predictors of ABT use. Patients who received ABT were older, female, and nonwhite and were covered by Medicare or Medicaid insurance, and many had a primary diagnosis of proximal humerus fracture. The ABT cohort had numerous medical comorbidities, including deficiency anemia and coagulopathy. Identifying this patient population is a prerequisite to educating patients while minimizing unnecessary risks and costs.
Using NIS data on a population of 422,371 patients who underwent shoulder arthroplasty, we identified the 5 likeliest predictors of ABT: fracture, fracture nonunion, deficiency anemia, coagulopathy, and avascular necrosis. Of the identified variables associated with ABT, deficiency anemia may be the most amenable to treatment; therefore, there may be benefit in delaying elective shoulder arthroplasty in this cohort. Given these findings, it is important to identify at-risk patients before surgery, with the intent to provide education and minimize risk.
In shoulder arthroplasty, it is not uncommon for patients to receive postoperative blood transfusions; rates range from 7% to 43%.1-6 Allogeneic blood transfusions (ABTs) are costly and not entirely free of risks.7 The risk for infection has decreased because of improved screening and risk reduction strategies, but there are still significant risks associated with ABTs, such as clerical errors, acute and delayed hemolytic reactions, graft-versus-host reactions, transfusion-related acute lung injury, and anaphylaxis.8-10 As use of shoulder arthroplasty continues to increase, the importance of minimizing unnecessary transfusions is growing as well.7
Predictive factors for ABT have been explored in other orthopedic settings, yet little has been done in shoulder arthroplasty.1-6,11-15 Previous shoulder arthroplasty studies have shown that low preoperative hemoglobin (Hb) levels are independent risk factors for postoperative blood transfusion. However, there is debate over the significance of other variables, such as procedure type, age, sex, and medical comorbidities. Further, prior studies were limited by relatively small samples from single institutions; the largest series included fewer than 600 patients.1-6
We conducted a study to determine predictors of ABT in a large cohort of patients admitted to US hospitals for shoulder arthroplasty. We also wanted to evaluate the effect of ABT on postoperative outcomes, including inpatient mortality, adverse events, prolonged hospital stay, and nonroutine discharge. According to the null hypothesis, in shoulder arthroplasty there will be no difference in risk factors between patients who require ABT and those who did not, after accounting for confounding variables.
Materials and Methods
This study was exempt from institutional review board approval, as all data were appropriately deidentified before use in this project. We used the Nationwide Inpatient Sample (NIS) to retrospectively study the period 2002–2011, from which all demographic, clinical, and resource use data were derived.16 NIS, an annual survey conducted by the Agency for Healthcare Research and Quality (AHRQ) since 1988, has generated a huge amount of data, forming the largest all-payer inpatient care database in the United States. Yearly samples contain discharge data from about 8 million hospital stays at more than 1000 hospitals across 46 states, approximating a 20% random sample of all hospital discharges at participating institutions.17 These data are then weighted to generate statistically valid national estimates.
The NIS database uses International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes to identify 15 medical diagnoses up to the year 2008 and a maximum of 25 medical diagnoses and 15 procedures thereafter. In addition, the database includes information on patient and hospital characteristics as well as inpatient outcomes such as length of stay, total hospitalization charges, and discharge disposition.18,19 Given its large sample size and data volume, NIS is a powerful tool in the analysis of data associated with a multitude of medical diagnoses and procedures.20
We used the NIS database to study a population of 422,371 patients (age, >18 years) who underwent total shoulder arthroplasty (TSA) or hemiarthroplasty (HSA) between 2002 and 2011. ICD-9-CM procedure codes for TSA (81.80, 81.88) and HSA (81.81) were used to identify this population. We also analyzed data for reverse TSA for the year 2011. Then we divided our target population into 2 different cohorts: patients who did not receive any blood transfusion products and patients who received a transfusion of allogeneic packed cells (ICD-9-CM code 99.04 was used to identify the latter cohort).
In this study, normal distribution of the dataset was assumed, given the large sample size. The 2 cohorts were evaluated through bivariate analysis using the Pearson χ2 test for categorical data and the independent-samples t test for continuous data. The extent to which diagnosis, age, race, sex, and medical comorbidities were predictive of blood transfusion after TSA or HSA was evaluated through multivariate binary logistic regression analysis. Statistical significance was set at P < .05. All statistical analyses and data modeling were performed with SPSS Version 22.0.
Results
Using the NIS database, we stratified an estimated 422,371 patients who presented for shoulder arthroplasty between January 1, 2002, and December 31, 2011, into a TSA cohort (59.3%) and an HSA cohort (40.7%). Eight percent (33,889) of all patients received an ABT; the proportion of patients who received ABT was higher (P < .001) for the HSA cohort (55.6%) than the TSA cohort (39.4%). Further, the rate of ABT after shoulder arthroplasty showed an upward inclination (Figure).
Demographically, patients who received ABT tended (P < .001) to be older (74±11 years vs 68±11 years) and of a minority race (black or Hispanic) and to fall in either the lowest range of median household income (21.5% vs 20.7%; ≤$38,999) or the highest (27.3% vs 25.4%; ≥$63,000). Shoulder arthroplasty with ABT occurred more often (P < .001) at hospitals that were urban (13.3% vs 11.3%), medium in size (27.3% vs 23.4%), and nonteaching (56.2% vs 54.3%). In addition, ABT was used more often (P < .001) in patients with a primary diagnosis of fracture (43.1% vs 14.3%) or fracture nonunion (4.4% vs 2.1%). These groups also had a longer (P < .001) hospital stay (5.0±4.3 days vs 2.5±2.2 days). Table 1 summarizes these findings.
The 2 cohorts were then analyzed for presence of medical comorbidities (Table 2). Patients who required ABT during shoulder arthroplasty had a significantly (P < .001) higher prevalence of congestive heart failure, chronic lung disease, hypertension, uncomplicated and complicated diabetes mellitus, liver disease, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, weight loss, coagulopathy, and deficiency anemia.
In multivariate regression modeling (Table 3), demographic predictors of ABT (P < .001) included increasing age (odds ratio [OR], 1.03 per year; 95% confidence interval [95% CI], 1.03-1.03), female sex (OR, 1.55; 95% CI, 1.51-1.60), and minority race (black or Hispanic). Odds of requiring ABT were higher for patients with Medicare (OR, 1.25; 95% CI, 1.20-1.30) and patients with Medicaid (OR, 1.63; 95% CI, 1.51-1.77) than for patients with private insurance.
ABT was more likely to be required (P < .001) in patients with a primary diagnosis of fracture (OR, 4.49; 95% CI, 4.34-4.65), avascular necrosis (OR, 2.06; 95% CI, 1.91-2.22), rheumatoid arthritis (OR, 1.91; 95% CI, 1.72-2.12), fracture nonunion (OR, 3.55; 95% CI, 3.33-3.79), or rotator cuff arthropathy (OR, 1.47; 95% CI, 1.41-1.54) than for patients with osteoarthritis. Moreover, compared with patients having HSA, patients having TSA were more likely to require ABT (OR, 1.20; 95% CI, 1.17-1.24). According to the analysis restricted to the year 2011, compared with patients having anatomical TSAs, patients having reverse TSAs were 1.6 times more likely (P < .001) to require ABT (OR, 1.63; 95% CI, 1.50-1.79).
With the exception of obesity, all comorbidities were significant (P < .001) independent predictors of ABT after shoulder arthroplasty: deficiency anemia (OR, 3.42; 95% CI, 3.32-3.52), coagulopathy (OR, 2.54; 95% CI, 2.36-2.73), fluid and electrolyte disorders (OR, 1.91; 95% CI, 1.84-1.97), and weight loss (OR, 1.78; 95% CI, 1.58-2.00).
Patients who received ABT were more likely to experience adverse events (OR, 1.74; 95% CI, 1.68-1.81), prolonged hospital stay (OR, 3.21; 95% CI, 3.12-3.30), and nonroutine discharge (OR, 1.77; 95% CI, 1.72-1.82) (Table 4). There was no difference in mortality between the 2 cohorts.
Discussion
There is an abundance of literature on blood transfusions in hip and knee arthroplasty, but there are few articles on ABT in shoulder arthroplasty, and they all report data from single institutions with relatively low caseloads.1,2,11-13,15,21 In the present study, we investigated ABT in shoulder arthroplasty from the perspective of a multi-institutional database with a caseload of more than 400,000. Given the rapidly increasing rates of shoulder arthroplasty, it is important to further examine this issue to minimize unnecessary blood transfusion and its associated risks and costs.7
We found that 8% of patients who had shoulder arthroplasty received ABT, which is consistent with previously reported transfusion rates (range, 7%-43%).1-6 Rates of ABT after shoulder arthroplasty have continued to rise. The exception, a decrease during the year 2010, can be explained by increased efforts to more rigidly follow transfusion indication guidelines to reduce the number of potentially unnecessary ABTs.21-24 Our study also identified numerous significant independent predictors of ABT in shoulder arthroplasty: age, sex, race, insurance status, procedure type, primary diagnoses, and multiple medical comorbidities.
Demographics
According to our analysis, more than 80% of patients who received ABT were over age 65 years, which aligns with what several other studies have demonstrated: Increasing age is a predictor of ABT, despite higher rates of comorbidities and lower preoperative Hb levels in this population.1,2,4,5,25-27 Consistent with previous work, female sex was predictive of ABT.2,5 It has been suggested that females are more likely predisposed to ABT because of lower preoperative Hb and smaller blood mass.2,5,28 Interestingly, our study showed a higher likelihood of ABT in both black and Hispanic populations. Further, patients with Medicare or Medicaid were more likely to receive ABT.
Primary Diagnosis
Although patients with a primary diagnosis of osteoarthritis constitute the majority of patients who undergo shoulder arthroplasty, our analysis showed that patients with a diagnosis of proximal humerus fracture were more likely to receive ABT. This finding is reasonable given studies showing the high prevalence of proximal humerus fractures in elderly women.29,30 Similarly, patients with a humerus fracture nonunion were more likely to receive a blood transfusion, which is unsurprising given the increased complexity associated with arthroplasty in this predominately elderly population.31 Interestingly, compared with patients with osteoarthritis, patients with any one of the other primary diagnoses were more likely to require a transfusion—proximal humerus fracture being the most significant, followed by humerus fracture nonunion, avascular necrosis, rheumatoid arthritis, and rotator cuff arthropathy.
Type of Arthroplasty
Bivariate analysis revealed that 55.6% of the patients who received ABT underwent HSA; the other 44.4% underwent TSA. The effect of primary diagnosis on procedure choice likely played a role in this finding. HSA indications include humerus fracture, which has been associated with increased ABT, whereas patients with osteoarthritis requiring TSA are significantly less likely to require ABT, as reflected in this analysis.7,32-34 Previous studies have failed to show a difference in blood transfusion rates between TSA and HSA.2,4-6,35 Conversely, with confounding factors controlled for, multivariate logistic regression analysis showed that TSA was 1.2 times more likely than HSA to require ABT, which could be explained by the increased operative time, case complexity, and blood loss that may be associated with the glenoid exposure.36,37 With analysis restricted to the year 2011, patients with reverse TSAs were 1.6 times more likely than patients with anatomical TSAs to receive a blood transfusion (OR, 1.63; 95% CI, 1.50-1.79). Although this finding differs from what was previously reported, it fits given that patients having reverse TSAs are often older and may present with a more significant comorbidity profile.3 In addition, there are the increased technical surgical aspects associated with “salvage surgery” for challenging indications such as cuff arthropathy and failed previous arthroplasty.38-41
Medical Comorbidities
Patients who received ABT were more likely to present with numerous medical comorbidities. Previous studies have indicated that the presence of multiple medical comorbidities significantly increased blood transfusion rates, possibly by working synergistically.42 All studies of blood transfusion in shoulder arthroplasty concluded that lower preoperative Hb was an independent predictor.1-6 Schumer and colleagues4 reported a 4-fold increase in likelihood of blood transfusion in patients with a preoperative Hb level less than 12.5 g/dL. In addition, Millett and colleagues6 showed a 20-fold increase in likelihood of transfusion in patients with a preoperative Hb level less than 11.0 g/dL compared with patients with a level higher than 13.0 g/dL. Patients with a Hb level between 11.0 and 13.0 g/dL showed a 5-fold increase in likelihood of transfusion.6 We should note that correction of preoperative anemia through various pharmacologic methods (eg, erythropoietin, intravenous iron supplementation) has been shown to decrease postoperative transfusion rates.43,44 Although we could not include preoperative Hb levels in the present study, given inherent limitations in using NIS, our multivariate analysis showed that preoperative deficiency anemia and coagulopathy were the most significant predictors of ABT.
In addition, the multivariate logistic regression model showed that both cardiac disease and diabetes were independent predictors of ABT, confirming data reported by Ahmadi and colleagues.1 Although not as well characterized in other studies, in the current analysis multiple other medical comorbidities, including fluid and electrolyte abnormalities, weight loss, liver disease, renal failure, and chronic lung disease, had significant predictive value. Contrarily, obesity significantly decreased the odds of ABT, likely because of higher baseline blood volume in obese patients.
Patient Outcomes
Patients who undergo shoulder arthroplasty with ABT are more likely to experience adverse events or a prolonged hospital stay and are more often discharged to a nursing home or an extended-care facility. In this population, however, deaths did not occur at a significantly higher rate—similar to what was found for patients who underwent hip or knee arthroplasty with blood transfusions.45
Little has been done to investigate the effect of pharmacologic agents on the need for perioperative ABT for orthopedic shoulder procedures. Aprotinin, tranexamic acid, epoetin-α, and aminocaproic acid have all been effective in limiting ABT during the perioperative period in various orthopedic hip, knee, and spine procedures.9,46-53 Given the increased morbidity associated with ABT, it may be beneficial to use similar methods to limit blood loss in high-risk patients undergoing shoulder arthroplasty.
Study Limitations
NIS has intrinsic limitations. Given its massive volume, it is subject to errors in both data entry and clinical coding. Moreover, the database lacks data that would have been useful in our study: preoperative Hb levels, intraoperative course, number of units transfused, total blood loss, use of blood conservation techniques, transfusion protocols, and severity of comorbidities. Reverse TSA was given a unique ICD-9-CM code in October 2010, so 2011 was the only year we were able to examine the relationship between reverse TSA and transfusions. Further, our analysis was unable to identify any medications, including chronic anticoagulants or postoperative prophylaxis, that have been shown to significantly affect blood transfusion rates.54 Yet, there are obvious advantages to using the NIS database, as previously outlined across the medical landscape.
Conclusion
Our results confirmed previous findings and identified new predictors of ABT in shoulder arthroplasty in a large cohort. We examined demographics and perioperative complications while identifying predictors of ABT use. Patients who received ABT were older, female, and nonwhite and were covered by Medicare or Medicaid insurance, and many had a primary diagnosis of proximal humerus fracture. The ABT cohort had numerous medical comorbidities, including deficiency anemia and coagulopathy. Identifying this patient population is a prerequisite to educating patients while minimizing unnecessary risks and costs.
Using NIS data on a population of 422,371 patients who underwent shoulder arthroplasty, we identified the 5 likeliest predictors of ABT: fracture, fracture nonunion, deficiency anemia, coagulopathy, and avascular necrosis. Of the identified variables associated with ABT, deficiency anemia may be the most amenable to treatment; therefore, there may be benefit in delaying elective shoulder arthroplasty in this cohort. Given these findings, it is important to identify at-risk patients before surgery, with the intent to provide education and minimize risk.
1. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
2. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
3. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
6. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
7. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
8. Ceccherini-Nelli L, Filipponi F, Mosca F, Campa M. The risk of contracting an infectious disease from blood transfusion. Transplantation Proc. 2004;36(3):680-682.
9. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278.
10. Hatzidakis AM, Mendlick RM, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty. An analysis of risk factors for allogenic transfusion. J Bone Joint Surg Am. 2000;82(1):89-100.
11. Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J. Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am. 2013;95(19):1777-1783.
12. Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86(7):970-973.
13. Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 suppl):34-37.
14. Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion in the United States, 2004–2009. Spine. 2014;39(4):304-310.
15. Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961-967.
16. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
17. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
18. Pereira BM, Chan PH, Weinstein PR, Fishman RA. Cerebral protection during reperfusion with superoxide dismutase in focal cerebral ischemia. Adv Neurol. 1990;52:97-103.
19. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
20. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
21. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86(7):1512-1518.
22. Martinez V, Monsaingeon-Lion A, Cherif K, Judet T, Chauvin M, Fletcher D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. 2007;99(6):794-800.
23. Helm AT, Karski MT, Parsons SJ, Sampath JS, Bale RS. A strategy for reducing blood-transfusion requirements in elective orthopaedic surgery. Audit of an algorithm for arthroplasty of the lower limb. J Bone Joint Surg Br. 2003;85(4):484-489.
24. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
25. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268.
26. Rogers MA, Blumberg N, Heal JM, Langa KM. Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710-718.
27. Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Med. 2007;17(1):1-15.
28. Fosco M, Di Fiore M. Factors predicting blood transfusion in different surgical procedures for degenerative spine disease. Eur Rev Med Pharmacol Sci. 2012;16(13):1853-1858.
29. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
30. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706.
31. Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46-50.
32. Chambers L, Dines JS, Lorich DG, Dines DM. Hemiarthroplasty for proximal humerus fractures. Curr Rev Musculoskeletal Med. 2013;6(1):57-62.
33. Jain NB, Hocker S, Pietrobon R, Guller U, Bathia N, Higgins LD. Total arthroplasty versus hemiarthroplasty for glenohumeral osteoarthritis: role of provider volume. J Shoulder Elbow Surg. 2005;14(4):361-367.
34. Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
35. Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453.
36. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
37. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23(8):1187-1194.
38. Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):388-394.
39. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
40. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
41. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
42. Pola E, Papaleo P, Santoliquido A, Gasparini G, Aulisa L, De Santis E. Clinical factors associated with an increased risk of perioperative blood transfusion in nonanemic patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2004;86(1):57-61.
43. Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfusion Med Rev. 2013;27(4):221-234.
44. Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54(2):289-299.
45. Danninger T, Rasul R, Poeran J, et al. Blood transfusions in total hip and knee arthroplasty: an analysis of outcomes. ScientificWorldJournal. 2014;2014:623460.
46. Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO. Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine. 2010;35(2):235-239.
47. Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552-1557.
48. Delasotta LA, Orozco F, Jafari SM, Blair JL, Ong A. Should we use preoperative epoetin-alpha in the mildly anemic patient undergoing simultaneous total knee arthroplasty? Open Orthop J. 2013;7:47-50.
49. Delasotta LA, Rangavajjula A, Frank ML, Blair J, Orozco F, Ong A. The use of preoperative epoetin-alpha in revision hip arthroplasty. Open Orthop J. 2012;6:179-183.
50. Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014;54(1):26-30.
51. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
52. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008(3):CD006883.
53. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
54. Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281-287.
1. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
2. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
3. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
6. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
7. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
8. Ceccherini-Nelli L, Filipponi F, Mosca F, Campa M. The risk of contracting an infectious disease from blood transfusion. Transplantation Proc. 2004;36(3):680-682.
9. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278.
10. Hatzidakis AM, Mendlick RM, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty. An analysis of risk factors for allogenic transfusion. J Bone Joint Surg Am. 2000;82(1):89-100.
11. Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J. Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am. 2013;95(19):1777-1783.
12. Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86(7):970-973.
13. Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 suppl):34-37.
14. Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion in the United States, 2004–2009. Spine. 2014;39(4):304-310.
15. Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961-967.
16. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
17. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
18. Pereira BM, Chan PH, Weinstein PR, Fishman RA. Cerebral protection during reperfusion with superoxide dismutase in focal cerebral ischemia. Adv Neurol. 1990;52:97-103.
19. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
20. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
21. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86(7):1512-1518.
22. Martinez V, Monsaingeon-Lion A, Cherif K, Judet T, Chauvin M, Fletcher D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. 2007;99(6):794-800.
23. Helm AT, Karski MT, Parsons SJ, Sampath JS, Bale RS. A strategy for reducing blood-transfusion requirements in elective orthopaedic surgery. Audit of an algorithm for arthroplasty of the lower limb. J Bone Joint Surg Br. 2003;85(4):484-489.
24. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
25. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268.
26. Rogers MA, Blumberg N, Heal JM, Langa KM. Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710-718.
27. Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfusion Med. 2007;17(1):1-15.
28. Fosco M, Di Fiore M. Factors predicting blood transfusion in different surgical procedures for degenerative spine disease. Eur Rev Med Pharmacol Sci. 2012;16(13):1853-1858.
29. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
30. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706.
31. Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46-50.
32. Chambers L, Dines JS, Lorich DG, Dines DM. Hemiarthroplasty for proximal humerus fractures. Curr Rev Musculoskeletal Med. 2013;6(1):57-62.
33. Jain NB, Hocker S, Pietrobon R, Guller U, Bathia N, Higgins LD. Total arthroplasty versus hemiarthroplasty for glenohumeral osteoarthritis: role of provider volume. J Shoulder Elbow Surg. 2005;14(4):361-367.
34. Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
35. Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453.
36. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
37. Singh A, Yian EH, Dillon MT, Takayanagi M, Burke MF, Navarro RA. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23(8):1187-1194.
38. Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):388-394.
39. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
40. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
41. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
42. Pola E, Papaleo P, Santoliquido A, Gasparini G, Aulisa L, De Santis E. Clinical factors associated with an increased risk of perioperative blood transfusion in nonanemic patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2004;86(1):57-61.
43. Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfusion Med Rev. 2013;27(4):221-234.
44. Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54(2):289-299.
45. Danninger T, Rasul R, Poeran J, et al. Blood transfusions in total hip and knee arthroplasty: an analysis of outcomes. ScientificWorldJournal. 2014;2014:623460.
46. Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO. Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine. 2010;35(2):235-239.
47. Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552-1557.
48. Delasotta LA, Orozco F, Jafari SM, Blair JL, Ong A. Should we use preoperative epoetin-alpha in the mildly anemic patient undergoing simultaneous total knee arthroplasty? Open Orthop J. 2013;7:47-50.
49. Delasotta LA, Rangavajjula A, Frank ML, Blair J, Orozco F, Ong A. The use of preoperative epoetin-alpha in revision hip arthroplasty. Open Orthop J. 2012;6:179-183.
50. Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014;54(1):26-30.
51. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
52. Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008(3):CD006883.
53. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
54. Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281-287.
Orthopedic Practice Patterns Relating to Anterior Cruciate Ligament Reconstruction in Elite Athletes
National Hockey League (NHL), Major League Soccer (MLS), and US Olympic/World Cup Ski/Snowboard (Olympic) athletes receive orthopedic care from a select group of surgeons. There are 30 NHL teams, 19 MLS teams, 1 Olympic ski team, and 1 Olympic snowboard team, for a total of 51 teams and a rough total of 2229 athletes (1500 NHL, 570 MLS, 159 Olympic).1
Studies have shown that MLS athletes and X-Game skiers and snowboarders have performed well on return to sport (RTS) after anterior cruciate ligament (ACL) reconstruction.2,3 However, the techniques, graft choices, and rehabilitation protocols used to return these elite athletes to their preinjury level of performance have not been elucidated. It is unclear if the treatment given to these elite athletes differs from that given to recreational athletes and nonathletes. Bradley and colleagues4 examined how 32 NFL team orthopedists treated ACL tears, and Erickson and colleagues5 recently surveyed NFL and National Collegiate Athletic Association (NCAA) team physicians to determine practice patterns (eg, surgical techniques, graft choices, postoperative protocols) in treating ACL tears. Until now, however, no one has examined NHL, MLS, or Olympic team orthopedic surgeons’ practice patterns as they relate to ACL reconstruction.
We conducted an online survey of NHL, MLS, and Olympic team orthopedic surgeons to determine practice patterns relating to ACL reconstruction in elite athletes. Given the practice patterns of surgeons in our practice, we hypothesized that the surveyed surgeons treating these elite athletes would most commonly use bone–patellar tendon–bone (BPTB) autograft with a single-bundle technique. We also hypothesized that they would permit RTS without a brace at a minimum of 6 months after surgery, with a normal physical examination, and after successful completion of a structured battery of RTS tests.
Materials and Methods
On the SurveyMonkey website (http://www.surveymonkey.com), we created a 7-question base survey, with other questions added for the NHL and MLS surveys (Figure 1). We sent this survey to 94 team orthopedic surgeons (41 NHL, 26 MLS, 27 Olympic) identified through Internet searches and direct contact with team public relations departments. The survey was approved by MLS and NHL research committees. In 2013, each survey was sent out 5 times. The response rates for each round are shown in Figure 2. All responses remained confidential; we did not learn surgeons’ identities. Data were collected and analyzed through the SurveyMonkey website. Each surgeon was instructed to respond to all relevant questions in the survey. The survey was designed such that the participant could not submit the survey without answering all the questions. Descriptive statistics were calculated for each study and parameter analyzed. Continuous variable data are reported as means and standard deviations (weighted means where applicable). Categorical data are reported as frequencies with percentages.
Results
Of the 94 team orthopedic surgeons surveyed, 47 (50%) responded (NHL, 49%; MLS, 50%; Olympic, 52%). Mean (SD) experience as a team physician was 7.73 (5.33) years (range, 2-20 years) for NHL, 6.77 (6.64) years (range, 2-20 years) for MLS, and 1.14 (0.36) years (range, 1-10 years) for Olympic. Mean (SD) number of ACL reconstructions performed in 2012 was 101 (51) for NHL (range, 50-200), 78 (38) for MLS (range, 20-150), and 110 (105) for Olympic (range, 25-175) (Table 1). Of the 47 surgeons, 42 (89.4%) used autograft in the treatment of elite athletes, and 5 (10.6%) used allograft. Autograft choices were BPTB (n = 33; 70.2%), 4-strand semitendinosus (n = 7; 14.9%), and quadriceps (n = 2; 4.3%); allograft choices were 4-strand semitendinosus (n = 4; 8.5%) and BPTB (n = 1; 2.1%) (Table 2).
Of the 40 surgeons (85.1%) who indicated they would use autograft in 25-year-old recreational athletes, 25 (53.2%) would use BPTB, 13 (27.7%) would use 4-strand semitendinosus, and 2 (4.3%) would use quadriceps; of the 7 who indicated they would use allograft, 4 (8.5%) would use 4-strand semitendinosus, and 3 (6.4%) would use BPTB. In the NHL and MLS surveys, 19 surgeons (57.6%) indicated they would use autograft (6 would use BPTB, 13 would use 4-strand semitendinosus), and 14 (42.4%) would use allograft (7 would use BPTB, 5 would use Achilles, and 2 would use tibialis anterior) in 35-year-old recreational athletes.
Twenty-one surgeons (44.7%) were drilling the femoral tunnel through a transtibial portal, 36.2% through an anteromedial portal, and 12.8% using a 2-incision technique. All surgeons indicated they were using a single-bundle technique in ACL reconstruction. Thirty-three surgeons (70.2%) did not recommend a brace for their elite athletes on RTS. Olympic team surgeons had the highest rate of brace wear in RTS (50%, both skiers and snowboarders); NHL and MLS surgeons had significantly lower rates (25% and 15.4%, respectively) (Table 3).
Twenty (60.6%) of the NHL and MLS surgeons recommended waiting at least 6 months before RTS; 2 (6.1%) recommended waiting at least 9 months; no surgeon recommended waiting at least 12 months; and the others did not have a specific time frame for RTS. Twenty-seven surgeons (81.8%) recommended RTS after an athlete passed a series of RTS tests (eg, Vail, single-leg hop). Nineteen surgeons (57.6%) recommended waiting until the athlete had full range of motion, no pain, full strength, and subjective stability in the knee. Physicians could choose more than one answer for the previous question, allowing for a total percentage higher than 100%.
Discussion
The goal of this study was to determine how NHL, MLS, and Olympic team orthopedic surgeons manage ACL tears in elite and recreational athletes. Our study hypotheses were confirmed, as 70.2% of those surveyed used BPTB autograft for elite athletes, 100% used the single-bundle technique, 70.2% did not require a brace on RTS, 81.8% recommended RTS after the athlete passed a series of RTS tests (eg, Vail, single-leg hop), and 60.6% waited at least 6 months after surgery.
As soccer and skiing are the top 2 sports in which participants sustain ACL tears, it is necessary to report how surgeons obtain successful results in these patient populations.6 Using the US and Norwegian ACL reconstruction registries, Granan and colleagues6 found that, over a 7-year period, 5760 ACL tears occurred during soccer, and 2030 occurred during skiing. The scope of ACL injuries is broad, and treatment patterns must be elucidated. Although most surgeons do not treat elite athletes, many high school and college athletes compete at very high levels. Therefore, replicating the methods of the surgeons who treat elite athletes may be warranted.
In our survey, autograft (89.4%), particularly BPTB autograft (70.2%), was the most common graft choice for elite athletes. The rate of allograft use (42.4%) was higher for 35-year-old recreational athletes. As BPTB autograft produces reliable long-term results, this graft type is a reasonable choice.7 However, only 18% of our surveyed orthopedic surgeons indicated they would use BPTB autograft in older, recreational athletes. This stark difference is likely related to the more than 40% long-term side effects of anterior knee pain and graft harvest site morbidity with BPTB autograft as opposed to allograft and other types of autograft.8,9 Younger patients may be more willing to accept some anterior knee pain to ensure bone-to-bone healing with BPTB autograft. This shift in graft choice may also reflect the desire to minimize skin incisions and their resulting scars, especially in female recreational athletes.
In a meta-analysis of more than 5000 patients, Kraeutler and colleagues7 found that BPTB autograft outperformed allograft according to several knee scores, including Lysholm and Tegner, and had a lower re-rupture rate (4.3% vs 12.7%). However, despite the superior performance of BPTB autograft, graft choice cannot overcome surgeon error in graft placement.10 BPTB autograft appears to remain the gold standard for ACL reconstruction for many reasons, including low failure rates and decreased costs.11 Recently, investigators have tried to challenge the superiority of BPTB autograft. In a retrospective case–control study, Mascarenhas and colleagues12 found that hamstring autograft afforded patients better extension and higher subjective outcome scores. Bourke and colleagues13 found a higher rate of contralateral ACL rupture in patients treated with BPTB autograft compared with hamstring autograft.
According to this survey, 44.7% of surgeons indicated they drilled the femoral tunnel through a transtibial portal, 36.2% used an anteromedial portal, and 12.8% used the 2-incision technique. These methods were recently evaluated to determine if any is superior to the others, but the study results were not definitive.14 Franceschi and colleagues15 found improved rotational and anterior stability of the knee with use of an anteromedial approach, but their findings were not clinically or functionally significant. Wang and colleagues16 found an extension loss in the late-stance phase of gait with the anteromedial approach; the transtibial approach was correlated with inferior anterior-posterior stability during the stance phase of gait. Therefore, our results parallel those in the current literature in that the surveyed population is split on which technique to use and likely bases its practice on comfort level and residency/fellowship training.
Limitations
This study had several limitations. First, it provided level V evidence of team physicians in 3 major sports. Although some of these physicians were also treating athletes in other sports, our survey targeted NHL, MLS, and Olympic athletes. It did not address all ages and both sexes—which is significant, given the higher rate of ACL tears in females. All NHL and MLS players are male, and there was a high rate of BPTB graft use in these sports. However, recreational athletes include both males and females, and the fact that some surgeons would choose a hamstring graft for a female for cosmetic reasons must not be overlooked. Conversely, that there was no difference in the number of BPTB autografts chosen between NHL and MLS surgeons versus Olympic surgeons, where females are included (all chose about 60% BPTB autografts for their elite athletes), disputes this limitation. Our survey response rate was 50%. Other studies have had similar rates in relation to ACL practices,17 especially elite team physicians’ practices,5 and recent literature has confirmed that lower response rates in surveys did not alter results and may in fact have improved results.18,19 This percentage could be falsely low if some of our email addresses were incorrect. This rate also raises the possibility of selection bias, as surgeons who routinely used allograft in their athlete population may not have wanted to admit this. It is possible that some NHL, MLS, and Olympic athletes were treated by surgeons not included in this survey (in some cases, a non–team surgeon may have performed the athlete’s surgery). This survey did not address concomitant knee pathology or cover all possible technique variables.
Conclusion
Most of the NHL, MLS, and Olympic team orthopedic surgeons who were surveyed perform their ACL reconstructions using BPTB autograft, using a single-bundle technique, through a transtibial portal, and do not require bracing for their athletes returning to sport. Most required their athletes to complete a series of RTS tests before resuming competitive play.
1. Team USA. 2013. US Olympic Committee website. http://www.teamusa.org/athletes?pg=1&seasonId=%7BCF2DC66A-C2B3-44A8-ABB8-A486F3FBFDDF%7D&ngbId=%7BB36167A0-2AC8-4B0F-876F-93D0A44DF60A%7D. Accessed October 23, 2015.
2. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):1-8.
3. Erickson BJ, Harris JD, Fillingham YA, et al. Performance and return to sport after anterior cruciate ligament reconstruction in X-Games skiers and snowboarders. Orthop J Sports Med. 2013;1(6):1-5.
4. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
5. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
6. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
7. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
8. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
9. Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2-8.
10. Boszotta H. Arthroscopic anterior cruciate ligament reconstruction using a patellar tendon graft in press-fit technique: surgical technique and follow-up. Arthroscopy. 1997;13(3):332-339.
11. Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone–patellar tendon–bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc Rev. 2009;17(4):242-246.
12. Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone–patellar tendon–bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1520-1527.
13. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985-1992.
14. Chalmers PN, Mall NA, Cole BJ, Verma NN, Bush-Joseph CA, Bach BR Jr. Anteromedial versus transtibial tunnel drilling in anterior cruciate ligament reconstructions: a systematic review. Arthroscopy. 2013;29(7):1235-1242.
15. Franceschi F, Papalia R, Rizzello G, Del Buono A, Maffulli N, Denaro V. Anteromedial portal versus transtibial drilling techniques in anterior cruciate ligament reconstruction: any clinical relevance? A retrospective comparative study. Arthroscopy. 2013;29(8):1330-1337.
16. Wang H, Fleischli JE, Zheng NN. Transtibial versus anteromedial portal technique in single-bundle anterior cruciate ligament reconstruction: outcomes of knee joint kinematics during walking. Am J Sports Med. 2013;41(8):1847-1856.
17. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
18. Keeter S, Miller C, Kohut A, Groves RM, Presser S. Consequences of reducing nonresponse in a national telephone survey. Public Opin Q. 2000;64(2):125-148.
19. Curtin R, Presser S, Singer E. The effects of response rate changes on the index of consumer sentiment. Public Opin Q. 2000;64(4):413-428.
National Hockey League (NHL), Major League Soccer (MLS), and US Olympic/World Cup Ski/Snowboard (Olympic) athletes receive orthopedic care from a select group of surgeons. There are 30 NHL teams, 19 MLS teams, 1 Olympic ski team, and 1 Olympic snowboard team, for a total of 51 teams and a rough total of 2229 athletes (1500 NHL, 570 MLS, 159 Olympic).1
Studies have shown that MLS athletes and X-Game skiers and snowboarders have performed well on return to sport (RTS) after anterior cruciate ligament (ACL) reconstruction.2,3 However, the techniques, graft choices, and rehabilitation protocols used to return these elite athletes to their preinjury level of performance have not been elucidated. It is unclear if the treatment given to these elite athletes differs from that given to recreational athletes and nonathletes. Bradley and colleagues4 examined how 32 NFL team orthopedists treated ACL tears, and Erickson and colleagues5 recently surveyed NFL and National Collegiate Athletic Association (NCAA) team physicians to determine practice patterns (eg, surgical techniques, graft choices, postoperative protocols) in treating ACL tears. Until now, however, no one has examined NHL, MLS, or Olympic team orthopedic surgeons’ practice patterns as they relate to ACL reconstruction.
We conducted an online survey of NHL, MLS, and Olympic team orthopedic surgeons to determine practice patterns relating to ACL reconstruction in elite athletes. Given the practice patterns of surgeons in our practice, we hypothesized that the surveyed surgeons treating these elite athletes would most commonly use bone–patellar tendon–bone (BPTB) autograft with a single-bundle technique. We also hypothesized that they would permit RTS without a brace at a minimum of 6 months after surgery, with a normal physical examination, and after successful completion of a structured battery of RTS tests.
Materials and Methods
On the SurveyMonkey website (http://www.surveymonkey.com), we created a 7-question base survey, with other questions added for the NHL and MLS surveys (Figure 1). We sent this survey to 94 team orthopedic surgeons (41 NHL, 26 MLS, 27 Olympic) identified through Internet searches and direct contact with team public relations departments. The survey was approved by MLS and NHL research committees. In 2013, each survey was sent out 5 times. The response rates for each round are shown in Figure 2. All responses remained confidential; we did not learn surgeons’ identities. Data were collected and analyzed through the SurveyMonkey website. Each surgeon was instructed to respond to all relevant questions in the survey. The survey was designed such that the participant could not submit the survey without answering all the questions. Descriptive statistics were calculated for each study and parameter analyzed. Continuous variable data are reported as means and standard deviations (weighted means where applicable). Categorical data are reported as frequencies with percentages.
Results
Of the 94 team orthopedic surgeons surveyed, 47 (50%) responded (NHL, 49%; MLS, 50%; Olympic, 52%). Mean (SD) experience as a team physician was 7.73 (5.33) years (range, 2-20 years) for NHL, 6.77 (6.64) years (range, 2-20 years) for MLS, and 1.14 (0.36) years (range, 1-10 years) for Olympic. Mean (SD) number of ACL reconstructions performed in 2012 was 101 (51) for NHL (range, 50-200), 78 (38) for MLS (range, 20-150), and 110 (105) for Olympic (range, 25-175) (Table 1). Of the 47 surgeons, 42 (89.4%) used autograft in the treatment of elite athletes, and 5 (10.6%) used allograft. Autograft choices were BPTB (n = 33; 70.2%), 4-strand semitendinosus (n = 7; 14.9%), and quadriceps (n = 2; 4.3%); allograft choices were 4-strand semitendinosus (n = 4; 8.5%) and BPTB (n = 1; 2.1%) (Table 2).
Of the 40 surgeons (85.1%) who indicated they would use autograft in 25-year-old recreational athletes, 25 (53.2%) would use BPTB, 13 (27.7%) would use 4-strand semitendinosus, and 2 (4.3%) would use quadriceps; of the 7 who indicated they would use allograft, 4 (8.5%) would use 4-strand semitendinosus, and 3 (6.4%) would use BPTB. In the NHL and MLS surveys, 19 surgeons (57.6%) indicated they would use autograft (6 would use BPTB, 13 would use 4-strand semitendinosus), and 14 (42.4%) would use allograft (7 would use BPTB, 5 would use Achilles, and 2 would use tibialis anterior) in 35-year-old recreational athletes.
Twenty-one surgeons (44.7%) were drilling the femoral tunnel through a transtibial portal, 36.2% through an anteromedial portal, and 12.8% using a 2-incision technique. All surgeons indicated they were using a single-bundle technique in ACL reconstruction. Thirty-three surgeons (70.2%) did not recommend a brace for their elite athletes on RTS. Olympic team surgeons had the highest rate of brace wear in RTS (50%, both skiers and snowboarders); NHL and MLS surgeons had significantly lower rates (25% and 15.4%, respectively) (Table 3).
Twenty (60.6%) of the NHL and MLS surgeons recommended waiting at least 6 months before RTS; 2 (6.1%) recommended waiting at least 9 months; no surgeon recommended waiting at least 12 months; and the others did not have a specific time frame for RTS. Twenty-seven surgeons (81.8%) recommended RTS after an athlete passed a series of RTS tests (eg, Vail, single-leg hop). Nineteen surgeons (57.6%) recommended waiting until the athlete had full range of motion, no pain, full strength, and subjective stability in the knee. Physicians could choose more than one answer for the previous question, allowing for a total percentage higher than 100%.
Discussion
The goal of this study was to determine how NHL, MLS, and Olympic team orthopedic surgeons manage ACL tears in elite and recreational athletes. Our study hypotheses were confirmed, as 70.2% of those surveyed used BPTB autograft for elite athletes, 100% used the single-bundle technique, 70.2% did not require a brace on RTS, 81.8% recommended RTS after the athlete passed a series of RTS tests (eg, Vail, single-leg hop), and 60.6% waited at least 6 months after surgery.
As soccer and skiing are the top 2 sports in which participants sustain ACL tears, it is necessary to report how surgeons obtain successful results in these patient populations.6 Using the US and Norwegian ACL reconstruction registries, Granan and colleagues6 found that, over a 7-year period, 5760 ACL tears occurred during soccer, and 2030 occurred during skiing. The scope of ACL injuries is broad, and treatment patterns must be elucidated. Although most surgeons do not treat elite athletes, many high school and college athletes compete at very high levels. Therefore, replicating the methods of the surgeons who treat elite athletes may be warranted.
In our survey, autograft (89.4%), particularly BPTB autograft (70.2%), was the most common graft choice for elite athletes. The rate of allograft use (42.4%) was higher for 35-year-old recreational athletes. As BPTB autograft produces reliable long-term results, this graft type is a reasonable choice.7 However, only 18% of our surveyed orthopedic surgeons indicated they would use BPTB autograft in older, recreational athletes. This stark difference is likely related to the more than 40% long-term side effects of anterior knee pain and graft harvest site morbidity with BPTB autograft as opposed to allograft and other types of autograft.8,9 Younger patients may be more willing to accept some anterior knee pain to ensure bone-to-bone healing with BPTB autograft. This shift in graft choice may also reflect the desire to minimize skin incisions and their resulting scars, especially in female recreational athletes.
In a meta-analysis of more than 5000 patients, Kraeutler and colleagues7 found that BPTB autograft outperformed allograft according to several knee scores, including Lysholm and Tegner, and had a lower re-rupture rate (4.3% vs 12.7%). However, despite the superior performance of BPTB autograft, graft choice cannot overcome surgeon error in graft placement.10 BPTB autograft appears to remain the gold standard for ACL reconstruction for many reasons, including low failure rates and decreased costs.11 Recently, investigators have tried to challenge the superiority of BPTB autograft. In a retrospective case–control study, Mascarenhas and colleagues12 found that hamstring autograft afforded patients better extension and higher subjective outcome scores. Bourke and colleagues13 found a higher rate of contralateral ACL rupture in patients treated with BPTB autograft compared with hamstring autograft.
According to this survey, 44.7% of surgeons indicated they drilled the femoral tunnel through a transtibial portal, 36.2% used an anteromedial portal, and 12.8% used the 2-incision technique. These methods were recently evaluated to determine if any is superior to the others, but the study results were not definitive.14 Franceschi and colleagues15 found improved rotational and anterior stability of the knee with use of an anteromedial approach, but their findings were not clinically or functionally significant. Wang and colleagues16 found an extension loss in the late-stance phase of gait with the anteromedial approach; the transtibial approach was correlated with inferior anterior-posterior stability during the stance phase of gait. Therefore, our results parallel those in the current literature in that the surveyed population is split on which technique to use and likely bases its practice on comfort level and residency/fellowship training.
Limitations
This study had several limitations. First, it provided level V evidence of team physicians in 3 major sports. Although some of these physicians were also treating athletes in other sports, our survey targeted NHL, MLS, and Olympic athletes. It did not address all ages and both sexes—which is significant, given the higher rate of ACL tears in females. All NHL and MLS players are male, and there was a high rate of BPTB graft use in these sports. However, recreational athletes include both males and females, and the fact that some surgeons would choose a hamstring graft for a female for cosmetic reasons must not be overlooked. Conversely, that there was no difference in the number of BPTB autografts chosen between NHL and MLS surgeons versus Olympic surgeons, where females are included (all chose about 60% BPTB autografts for their elite athletes), disputes this limitation. Our survey response rate was 50%. Other studies have had similar rates in relation to ACL practices,17 especially elite team physicians’ practices,5 and recent literature has confirmed that lower response rates in surveys did not alter results and may in fact have improved results.18,19 This percentage could be falsely low if some of our email addresses were incorrect. This rate also raises the possibility of selection bias, as surgeons who routinely used allograft in their athlete population may not have wanted to admit this. It is possible that some NHL, MLS, and Olympic athletes were treated by surgeons not included in this survey (in some cases, a non–team surgeon may have performed the athlete’s surgery). This survey did not address concomitant knee pathology or cover all possible technique variables.
Conclusion
Most of the NHL, MLS, and Olympic team orthopedic surgeons who were surveyed perform their ACL reconstructions using BPTB autograft, using a single-bundle technique, through a transtibial portal, and do not require bracing for their athletes returning to sport. Most required their athletes to complete a series of RTS tests before resuming competitive play.
National Hockey League (NHL), Major League Soccer (MLS), and US Olympic/World Cup Ski/Snowboard (Olympic) athletes receive orthopedic care from a select group of surgeons. There are 30 NHL teams, 19 MLS teams, 1 Olympic ski team, and 1 Olympic snowboard team, for a total of 51 teams and a rough total of 2229 athletes (1500 NHL, 570 MLS, 159 Olympic).1
Studies have shown that MLS athletes and X-Game skiers and snowboarders have performed well on return to sport (RTS) after anterior cruciate ligament (ACL) reconstruction.2,3 However, the techniques, graft choices, and rehabilitation protocols used to return these elite athletes to their preinjury level of performance have not been elucidated. It is unclear if the treatment given to these elite athletes differs from that given to recreational athletes and nonathletes. Bradley and colleagues4 examined how 32 NFL team orthopedists treated ACL tears, and Erickson and colleagues5 recently surveyed NFL and National Collegiate Athletic Association (NCAA) team physicians to determine practice patterns (eg, surgical techniques, graft choices, postoperative protocols) in treating ACL tears. Until now, however, no one has examined NHL, MLS, or Olympic team orthopedic surgeons’ practice patterns as they relate to ACL reconstruction.
We conducted an online survey of NHL, MLS, and Olympic team orthopedic surgeons to determine practice patterns relating to ACL reconstruction in elite athletes. Given the practice patterns of surgeons in our practice, we hypothesized that the surveyed surgeons treating these elite athletes would most commonly use bone–patellar tendon–bone (BPTB) autograft with a single-bundle technique. We also hypothesized that they would permit RTS without a brace at a minimum of 6 months after surgery, with a normal physical examination, and after successful completion of a structured battery of RTS tests.
Materials and Methods
On the SurveyMonkey website (http://www.surveymonkey.com), we created a 7-question base survey, with other questions added for the NHL and MLS surveys (Figure 1). We sent this survey to 94 team orthopedic surgeons (41 NHL, 26 MLS, 27 Olympic) identified through Internet searches and direct contact with team public relations departments. The survey was approved by MLS and NHL research committees. In 2013, each survey was sent out 5 times. The response rates for each round are shown in Figure 2. All responses remained confidential; we did not learn surgeons’ identities. Data were collected and analyzed through the SurveyMonkey website. Each surgeon was instructed to respond to all relevant questions in the survey. The survey was designed such that the participant could not submit the survey without answering all the questions. Descriptive statistics were calculated for each study and parameter analyzed. Continuous variable data are reported as means and standard deviations (weighted means where applicable). Categorical data are reported as frequencies with percentages.
Results
Of the 94 team orthopedic surgeons surveyed, 47 (50%) responded (NHL, 49%; MLS, 50%; Olympic, 52%). Mean (SD) experience as a team physician was 7.73 (5.33) years (range, 2-20 years) for NHL, 6.77 (6.64) years (range, 2-20 years) for MLS, and 1.14 (0.36) years (range, 1-10 years) for Olympic. Mean (SD) number of ACL reconstructions performed in 2012 was 101 (51) for NHL (range, 50-200), 78 (38) for MLS (range, 20-150), and 110 (105) for Olympic (range, 25-175) (Table 1). Of the 47 surgeons, 42 (89.4%) used autograft in the treatment of elite athletes, and 5 (10.6%) used allograft. Autograft choices were BPTB (n = 33; 70.2%), 4-strand semitendinosus (n = 7; 14.9%), and quadriceps (n = 2; 4.3%); allograft choices were 4-strand semitendinosus (n = 4; 8.5%) and BPTB (n = 1; 2.1%) (Table 2).
Of the 40 surgeons (85.1%) who indicated they would use autograft in 25-year-old recreational athletes, 25 (53.2%) would use BPTB, 13 (27.7%) would use 4-strand semitendinosus, and 2 (4.3%) would use quadriceps; of the 7 who indicated they would use allograft, 4 (8.5%) would use 4-strand semitendinosus, and 3 (6.4%) would use BPTB. In the NHL and MLS surveys, 19 surgeons (57.6%) indicated they would use autograft (6 would use BPTB, 13 would use 4-strand semitendinosus), and 14 (42.4%) would use allograft (7 would use BPTB, 5 would use Achilles, and 2 would use tibialis anterior) in 35-year-old recreational athletes.
Twenty-one surgeons (44.7%) were drilling the femoral tunnel through a transtibial portal, 36.2% through an anteromedial portal, and 12.8% using a 2-incision technique. All surgeons indicated they were using a single-bundle technique in ACL reconstruction. Thirty-three surgeons (70.2%) did not recommend a brace for their elite athletes on RTS. Olympic team surgeons had the highest rate of brace wear in RTS (50%, both skiers and snowboarders); NHL and MLS surgeons had significantly lower rates (25% and 15.4%, respectively) (Table 3).
Twenty (60.6%) of the NHL and MLS surgeons recommended waiting at least 6 months before RTS; 2 (6.1%) recommended waiting at least 9 months; no surgeon recommended waiting at least 12 months; and the others did not have a specific time frame for RTS. Twenty-seven surgeons (81.8%) recommended RTS after an athlete passed a series of RTS tests (eg, Vail, single-leg hop). Nineteen surgeons (57.6%) recommended waiting until the athlete had full range of motion, no pain, full strength, and subjective stability in the knee. Physicians could choose more than one answer for the previous question, allowing for a total percentage higher than 100%.
Discussion
The goal of this study was to determine how NHL, MLS, and Olympic team orthopedic surgeons manage ACL tears in elite and recreational athletes. Our study hypotheses were confirmed, as 70.2% of those surveyed used BPTB autograft for elite athletes, 100% used the single-bundle technique, 70.2% did not require a brace on RTS, 81.8% recommended RTS after the athlete passed a series of RTS tests (eg, Vail, single-leg hop), and 60.6% waited at least 6 months after surgery.
As soccer and skiing are the top 2 sports in which participants sustain ACL tears, it is necessary to report how surgeons obtain successful results in these patient populations.6 Using the US and Norwegian ACL reconstruction registries, Granan and colleagues6 found that, over a 7-year period, 5760 ACL tears occurred during soccer, and 2030 occurred during skiing. The scope of ACL injuries is broad, and treatment patterns must be elucidated. Although most surgeons do not treat elite athletes, many high school and college athletes compete at very high levels. Therefore, replicating the methods of the surgeons who treat elite athletes may be warranted.
In our survey, autograft (89.4%), particularly BPTB autograft (70.2%), was the most common graft choice for elite athletes. The rate of allograft use (42.4%) was higher for 35-year-old recreational athletes. As BPTB autograft produces reliable long-term results, this graft type is a reasonable choice.7 However, only 18% of our surveyed orthopedic surgeons indicated they would use BPTB autograft in older, recreational athletes. This stark difference is likely related to the more than 40% long-term side effects of anterior knee pain and graft harvest site morbidity with BPTB autograft as opposed to allograft and other types of autograft.8,9 Younger patients may be more willing to accept some anterior knee pain to ensure bone-to-bone healing with BPTB autograft. This shift in graft choice may also reflect the desire to minimize skin incisions and their resulting scars, especially in female recreational athletes.
In a meta-analysis of more than 5000 patients, Kraeutler and colleagues7 found that BPTB autograft outperformed allograft according to several knee scores, including Lysholm and Tegner, and had a lower re-rupture rate (4.3% vs 12.7%). However, despite the superior performance of BPTB autograft, graft choice cannot overcome surgeon error in graft placement.10 BPTB autograft appears to remain the gold standard for ACL reconstruction for many reasons, including low failure rates and decreased costs.11 Recently, investigators have tried to challenge the superiority of BPTB autograft. In a retrospective case–control study, Mascarenhas and colleagues12 found that hamstring autograft afforded patients better extension and higher subjective outcome scores. Bourke and colleagues13 found a higher rate of contralateral ACL rupture in patients treated with BPTB autograft compared with hamstring autograft.
According to this survey, 44.7% of surgeons indicated they drilled the femoral tunnel through a transtibial portal, 36.2% used an anteromedial portal, and 12.8% used the 2-incision technique. These methods were recently evaluated to determine if any is superior to the others, but the study results were not definitive.14 Franceschi and colleagues15 found improved rotational and anterior stability of the knee with use of an anteromedial approach, but their findings were not clinically or functionally significant. Wang and colleagues16 found an extension loss in the late-stance phase of gait with the anteromedial approach; the transtibial approach was correlated with inferior anterior-posterior stability during the stance phase of gait. Therefore, our results parallel those in the current literature in that the surveyed population is split on which technique to use and likely bases its practice on comfort level and residency/fellowship training.
Limitations
This study had several limitations. First, it provided level V evidence of team physicians in 3 major sports. Although some of these physicians were also treating athletes in other sports, our survey targeted NHL, MLS, and Olympic athletes. It did not address all ages and both sexes—which is significant, given the higher rate of ACL tears in females. All NHL and MLS players are male, and there was a high rate of BPTB graft use in these sports. However, recreational athletes include both males and females, and the fact that some surgeons would choose a hamstring graft for a female for cosmetic reasons must not be overlooked. Conversely, that there was no difference in the number of BPTB autografts chosen between NHL and MLS surgeons versus Olympic surgeons, where females are included (all chose about 60% BPTB autografts for their elite athletes), disputes this limitation. Our survey response rate was 50%. Other studies have had similar rates in relation to ACL practices,17 especially elite team physicians’ practices,5 and recent literature has confirmed that lower response rates in surveys did not alter results and may in fact have improved results.18,19 This percentage could be falsely low if some of our email addresses were incorrect. This rate also raises the possibility of selection bias, as surgeons who routinely used allograft in their athlete population may not have wanted to admit this. It is possible that some NHL, MLS, and Olympic athletes were treated by surgeons not included in this survey (in some cases, a non–team surgeon may have performed the athlete’s surgery). This survey did not address concomitant knee pathology or cover all possible technique variables.
Conclusion
Most of the NHL, MLS, and Olympic team orthopedic surgeons who were surveyed perform their ACL reconstructions using BPTB autograft, using a single-bundle technique, through a transtibial portal, and do not require bracing for their athletes returning to sport. Most required their athletes to complete a series of RTS tests before resuming competitive play.
1. Team USA. 2013. US Olympic Committee website. http://www.teamusa.org/athletes?pg=1&seasonId=%7BCF2DC66A-C2B3-44A8-ABB8-A486F3FBFDDF%7D&ngbId=%7BB36167A0-2AC8-4B0F-876F-93D0A44DF60A%7D. Accessed October 23, 2015.
2. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):1-8.
3. Erickson BJ, Harris JD, Fillingham YA, et al. Performance and return to sport after anterior cruciate ligament reconstruction in X-Games skiers and snowboarders. Orthop J Sports Med. 2013;1(6):1-5.
4. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
5. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
6. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
7. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
8. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
9. Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2-8.
10. Boszotta H. Arthroscopic anterior cruciate ligament reconstruction using a patellar tendon graft in press-fit technique: surgical technique and follow-up. Arthroscopy. 1997;13(3):332-339.
11. Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone–patellar tendon–bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc Rev. 2009;17(4):242-246.
12. Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone–patellar tendon–bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1520-1527.
13. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985-1992.
14. Chalmers PN, Mall NA, Cole BJ, Verma NN, Bush-Joseph CA, Bach BR Jr. Anteromedial versus transtibial tunnel drilling in anterior cruciate ligament reconstructions: a systematic review. Arthroscopy. 2013;29(7):1235-1242.
15. Franceschi F, Papalia R, Rizzello G, Del Buono A, Maffulli N, Denaro V. Anteromedial portal versus transtibial drilling techniques in anterior cruciate ligament reconstruction: any clinical relevance? A retrospective comparative study. Arthroscopy. 2013;29(8):1330-1337.
16. Wang H, Fleischli JE, Zheng NN. Transtibial versus anteromedial portal technique in single-bundle anterior cruciate ligament reconstruction: outcomes of knee joint kinematics during walking. Am J Sports Med. 2013;41(8):1847-1856.
17. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
18. Keeter S, Miller C, Kohut A, Groves RM, Presser S. Consequences of reducing nonresponse in a national telephone survey. Public Opin Q. 2000;64(2):125-148.
19. Curtin R, Presser S, Singer E. The effects of response rate changes on the index of consumer sentiment. Public Opin Q. 2000;64(4):413-428.
1. Team USA. 2013. US Olympic Committee website. http://www.teamusa.org/athletes?pg=1&seasonId=%7BCF2DC66A-C2B3-44A8-ABB8-A486F3FBFDDF%7D&ngbId=%7BB36167A0-2AC8-4B0F-876F-93D0A44DF60A%7D. Accessed October 23, 2015.
2. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):1-8.
3. Erickson BJ, Harris JD, Fillingham YA, et al. Performance and return to sport after anterior cruciate ligament reconstruction in X-Games skiers and snowboarders. Orthop J Sports Med. 2013;1(6):1-5.
4. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
5. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
6. Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(12):2814-2818.
7. Kraeutler MJ, Bravman JT, McCarty EC. Bone–patellar tendon–bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
8. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
9. Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2-8.
10. Boszotta H. Arthroscopic anterior cruciate ligament reconstruction using a patellar tendon graft in press-fit technique: surgical technique and follow-up. Arthroscopy. 1997;13(3):332-339.
11. Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone–patellar tendon–bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc Rev. 2009;17(4):242-246.
12. Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone–patellar tendon–bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2-10 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1520-1527.
13. Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985-1992.
14. Chalmers PN, Mall NA, Cole BJ, Verma NN, Bush-Joseph CA, Bach BR Jr. Anteromedial versus transtibial tunnel drilling in anterior cruciate ligament reconstructions: a systematic review. Arthroscopy. 2013;29(7):1235-1242.
15. Franceschi F, Papalia R, Rizzello G, Del Buono A, Maffulli N, Denaro V. Anteromedial portal versus transtibial drilling techniques in anterior cruciate ligament reconstruction: any clinical relevance? A retrospective comparative study. Arthroscopy. 2013;29(8):1330-1337.
16. Wang H, Fleischli JE, Zheng NN. Transtibial versus anteromedial portal technique in single-bundle anterior cruciate ligament reconstruction: outcomes of knee joint kinematics during walking. Am J Sports Med. 2013;41(8):1847-1856.
17. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
18. Keeter S, Miller C, Kohut A, Groves RM, Presser S. Consequences of reducing nonresponse in a national telephone survey. Public Opin Q. 2000;64(2):125-148.
19. Curtin R, Presser S, Singer E. The effects of response rate changes on the index of consumer sentiment. Public Opin Q. 2000;64(4):413-428.
Magnetic Resonance Imaging of Complications of Anterior Cruciate Ligament Reconstruction
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
Orthopedic Implant Waste: Analysis and Quantification
The cost of health care in the United States is increasing at an unsustainable rate.1-3 To decrease or even reverse this trend, we must decrease the cost of care without adversely affecting quality. Porter4 defined value as the quality of care divided by its cost. The economics of total joint arthroplasty (TJA) has received a great deal of attention because of both increasing demand and increasing cost.5-9 About 33% of all orthopedic surgeries and the majority of TJAs are paid for by Medicare.9 In recent years, the rate of reimbursement for orthopedic cases has steadily declined while the cost of implants has increased.3,10,11 Given the significant cost of implants, health care providers in some subspecialties have focused on implant costs as a potential area for cost reduction.12 For example, in TJA this has proved effective in reducing the overall cost, as has decreasing length of stay after surgery.8,10,13-16
With little evidence suggesting any specific orthopedic implant has outcomes superior to those of others, with the exception of select poorly performing outliers, we must increase value of care by lowering the cost when considering these devices.17,18 In addition, some experts have suggested that intraoperative waste is a significant factor in TJA cost, and it does contribute to the average implant cost for a TJA case.6,19 Using data collected from 72 institutions, Zywiel and colleagues19 estimated the annual cost of wasted hip and knee arthroplasty implants to be more than $36 million in the United States.
However, considering the aging US population, TJA is not the only orthopedic surgery with increased demand. An estimated 600,000 spine surgeries are performed each year in the United States.20 Between 1992 and 2003, Medicare spending for lumbar spinal fusion increased 500%.21 In addition, in a 15-month observational study of incidence of intraoperative waste in spine surgery, Soroceanu and colleagues22 reported waste occurring in 20% of spine procedures.
Although these studies have described implant waste in TJA and spine surgeries, little has been published on the cost of wasted implants in a center performing the full range of orthopedic procedures. In this article, we detail the implant waste costs incurred by surgeons for all orthopedic subspecialties at a single orthopedic specialty hospital over a 1-year period. Our study goals were to identify types of implants wasted, and incidence and cost of implant waste, for all total hip arthroplasties (THAs), total knee arthroplasties (TKAs), and lumbar spinal fusions performed at the hospital and to determine whether case volume or years in surgical practice affect the rate or cost of implants wasted.
Methods
We performed a retrospective economic analysis of 1 year of administrative implant data from our institution. Collected data were quantified and analyzed for factors that might explain any variance in implant waste among surgeons. We were granted exempt institutional review board status, as no patient information was involved in this study.
We reviewed the administrative implant data for the 12-month period beginning June 2012 and ending May 2013. For that period, number of cases in which an implant was used and number of cases in which an implant was wasted were recorded. For each instance of waste, type and cost of the wasted implant were entered into the administrative database. In addition, overall cost of implants for the year and cost of wasted implants were determined. Data were available for 81 surgeons across 8 orthopedic divisions (subspecialties). From this information, we determined percentage of cases in which waste occurred, percentage of total implant cost wasted, average cost of waste per case, and most commonly wasted implants. All 3 variables were also calculated for THAs, TKAs, and lumbar spinal fusion procedures.
Statistical Analysis
The data were analyzed to determine if surgeon case volume or years in surgical practice affected implant waste. All analyses were performed at department, division (subspecialty), and surgeon levels. Case volume was analyzed in 3 groups: top 25%, middle 50%, and lower 25%. Number of years in surgical practice was analyzed in 3 groups: fewer than 10 years, 10 to 19 years, and 20 years or more. Normality assumption of variables was tested using the Shapiro-Wilk test (P < .05). For between-group differences, 1-way analysis of variance and the Tukey honestly significant difference post hoc test were performed for variables with a normal distribution, and the Kruskal-Wallis and Mann-Whitney tests were performed for variables without a normal distribution.
For the subspecialty-level analyses, only the Adult Reconstruction, Sports Medicine, and Spine divisions were analyzed for the effects of volume, and only the Sports Medicine and Spine divisions were analyzed for the effect of surgical experience, as surgeon numbers were insufficient for adequate grouping(s).
Data are presented as means with corresponding 95% confidence intervals (CIs). Categorical variables are presented as counts with percentages. All statistical analyses were performed with SPSS Version 21.0 (IBM SPSS) statistical software. Statistical significance was set at .05.
Results
During the 1-year period, 8954 department cases involved an implant of any type. Waste occurred in 12% (1072) of these cases. The rate ranged from 8% in the Adult Reconstruction division to 30% in the Trauma division (Table 1), and the rate for individual surgeons ranged from 3% to 100%, though the surgeon with 100% performed only 1 case, and the next highest rate was 50%.
Total implant cost for our hospital during the period was $34,340,607. Of that total cost, 1.8% ($634,668) was lost because of implant waste. Percentage of total implant cost wasted ranged from 1.6% in the Adult Reconstruction division to 4.7% in the Sports Medicine division (Table 1). Percentage of total implant cost wasted for individual surgeons ranged from 0.2% to 16.1%. Tables 2 and 3 list the most commonly wasted implants by count and cost, respectively.
When total cost of wasted implants was averaged over all implant cases performed during the period, the loss resulting from waste amounted to $71 per case for the department and ranged from $21 per case for the Hand division to $105 per case for the Pediatric division (Table 1). For individual surgeons, the loss ranged from $4 to $250 per case.
During the period studied, an implant was wasted in 9% (100) of the 1076 primary THAs performed, 4% (42) of the 1003 primary TKAs, and 14% (30) of the 217 lumbar spinal fusions (Tables 4, 5).
There was no significant difference between groups for department (P = .46) or for the Adult Reconstruction (P = .83), Spine (P = .10), or Sports Medicine (P = .69) division. Analyzing for variance by years in surgical practice, we found a significant difference for department (P = .01) but not for the Adult Reconstruction (P = .12) or Spine (P = .14) division. The department difference resulted from a significant difference (P = .001; 95% CI, 1.112-17.408) between surgeons (<10 years of surgical practice) who wasted implants in 12.8% of their cases and surgeons (>20 years of surgical practice) who wasted implants in 9% of their cases (Table 4).
There was no significant difference between groups for department (P = .83) or for the Adult Reconstruction (P = .29) or Spine (P = .41) division when analyzed by years in surgical practice. Analyzing by case volume, we found a significant difference for the Sports Medicine division (P = .004): Percentage of total implant waste was significantly higher (P = .003; 95% CI, –12.61 to –2.97) for surgeons with the lower 25% of case volume (9.8%) than for surgeons with the middle 50% of case volume (3.5%) (Table 5). No other significant difference was found for department (P = .52) or for the Adult Reconstruction (P = .69) or Spine (P = .45) division.
Analyzing by case volume and years in surgical practice, we found no significant difference for department (case volume, P = .76; years in surgical practice, P = .07), Adult Reconstruction division (case volume, P = .47; years in surgical practice, P = .78), Spine division (case volume, P = .11; years in surgical practice, P = .15), or Sports Medicine division (case volume, P = .08).
Selected Procedures
Total Hip Arthroplasty. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .072; years in practice, P = .076), percentage of total implant cost wasted (volume, P = .074; years in practice, P = .12), cost of waste per case (volume, P = .075; years in practice, P = .32).
Total Knee Arthroplasty. Regarding variance by years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (P = .38), percentage of total implant cost wasted (P = .50), cost of waste per case (P = .50). Regarding variance by volume, there was no significant difference for percentage of cases with waste (P = .70) or cost of waste per case (P = .05), but we found a significant difference for percentage of total implant cost wasted (P = .038). That difference was caused by an outlier: One surgeon with the lower 25% of case volume wasted an implant in the only TKA he performed that year. Correction for the outlier removed the significance.
Posterior Lumbar Spinal Fusion. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .36; years in surgical practice, P = .22), percentage of total implant cost wasted (volume, P = .33; years in surgical practice, P = .41), cost of waste per case (volume, P = .34; years in practice, P = .15).
Discussion
The steadily increasing demand for orthopedic surgeries and declining rates of reimbursement by Medicare and other insurance providers have led many hospitals to look for ways to control the cost of these surgeries. Reducing operating room costs, lowering implant prices, and shortening hospital stays have all proved successful.6,15,20,23 One area that has not been thoroughly explored is the cost burden of wasted implants. Our findings suggest implant waste contributes significantly to the cost of orthopedic surgeries.
One weakness of this study is that its data, though encompassing all orthopedic subspecialties and procedures, come from a single teaching institution and therefore are less representative of all orthopedic departments across the United States. However, the findings are useful in that the analysis was performed across multiple specialties at a high-volume institution and may be applied to similar institutions. Another weakness of this study is that the data cover only 1 year. Collecting data over a longer period could improve the magnitude and power of the analysis. Nonetheless, 1 year of data is a good starting point in identifying the issues and guiding the initiation of measures to address them. Last, we did not explore the reason for each instance of waste during the period reviewed. Knowing the reason for implant waste would be helpful in developing strategies to reduce implant waste.
Our study results showed that, in 1 year, implant waste occurred in 1.8% of procedures that required an implant—representing a loss of $634,000. Other studies have quantified implant waste for selected procedures or single departments, but to our knowledge none has quantified implant waste for an entire orthopedic department or hospital. It is therefore difficult to compare our institutional results with other results. For instance, definitions of waste differ. A study that found waste in 20% of spine surgery cases22 included all intraoperative waste, whereas our 11% of spine cases were implant waste only. Similarly, though rates of implant waste in trauma cases differed significantly between a multi-institution study by Zywiel and colleagues24 (0.6%) and our institution (30%), their study excluded arthroplasty cases from the trauma subset and reported implant waste for a single vendor, whereas we included arthroplasty cases and a wide array of implant vendors. In addition, costs cannot be directly compared because, in our study, implants wasted may have differed. Although the Trauma division had the highest incidence of waste (30%) in our analysis, it did not have the highest waste-related costs. Instead, the Adult Reconstruction division, with waste in 8% of cases, had the highest waste cost, $214,869. The cost difference is certainly the result of the difference in type of implants wasted. The implants most commonly wasted in the Trauma division were screws, which cost between $17 and $150; a single femoral stem, though wasted less often, cost significantly more, $2000 to $6000.
Our results showed a combined implant waste incidence of 6.8% for primary THA and primary TKA cases over the year. In their multi-institution study, Zywiel and colleagues19 reported a combined incidence of implant waste in 2% of THA and TKA cases. The difference is that Zywiel and colleagues19 reported data from a single implant vendor and included revision surgeries, hip hemiarthroplasties, and unicondylar knee arthroplasties. Another study reported implant waste in 5.7% of all TKA cases but did not specify whether revision or unicondylar arthroplasties were included.25 For lumbar spinal fusion, we found an implant waste incidence of 14%. Given the lack of studies in this area, we cannot make a comparison of results.
To our knowledge, there has been no other study of the effects of case volume and years in surgical practice on implant waste. Our analysis showed that waste incidence was not related to surgeon case volume but was related to years in surgical practice. Incidence of waste was significantly lower among surgeons practicing 20 years or more than among surgeons practicing fewer than 10 years. The difference may be a reflection that case volume during a single year is not totally indicative of a surgeon’s lifetime case volume. For example, several surgeons with many years of experience and a significant lifetime case volume had an annual case volume in the lower 25% of the department because they were approaching retirement or had only recently joined the institution. More rigorous prospective studies are needed to further understand this relationship.
Conclusions
Our study demonstrated significant costs related to implant waste. These costs are important to consider not only for traditional cases, such as total joint and spine procedures, in which implant costs are routinely scrutinized, but for all subspecialties, such as sports medicine, in which the majority of cases are performed on an outpatient basis. Considering the estimated $36 million wasted during THAs and TKAs and $126 million wasted on spine surgeries in the United States annually, and the significant waste we observed in other orthopedic subspecialties, decreasing the rate of intraoperative waste during orthopedic surgeries represents another area that could provide significant cost reduction through implant cost savings.19,22 A few successful programs have been reported. Soroceanu and colleagues22 found an almost 50% decrease in intraoperative waste during spine surgery after an educational program was used to address such waste. Elsewhere, use of a computer-based system (e.Label and Compatibility) led to an estimated cost reduction of $75,000 in implant waste.25 Efforts to develop and implement other programs to reduce implant waste are needed and should be part of any orthopedic operating room cost reduction strategy.
1. Alhassani A, Chandra A, Chernew ME. The sources of the SGR “hole.” N Engl J Med. 2012;366(4):289-291.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Keehan SP, Sisko AM, Truffer CJ, et al. National health spending projections through 2020: economic recovery and reform drive faster spending growth. Health Aff. 2011;30(8):1594-1605.
4. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481.
5. Belatti DA, Phisitkul P. Trends in orthopedics: an analysis of Medicare claims, 2000–2010. Orthopedics. 2013;36(3):e366-e372.
6. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
7. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(5):23-27.
8. Mendenhall S. 2003 hip and knee implant review. Orthop Network News. 2003;14(3):2.
9. Mendenhall S. 2008 hip and knee implant review. Orthop Network News. 2008;19(3):20.
10. Healy WL, Rana AJ, Iorio R. Hospital economics of primary total knee arthroplasty at a teaching hospital. Clin Orthop Relat Res. 2011;469(1):87-94.
11. Mendenhall S. 2007 hip and knee implant review. Orthop Network News. 2007;18(3):16.
12. Iorio R, Davis CM 3rd, Healy WL, Fehring TK, O’Connor MI, York S. Impact of the economic downturn on adult reconstruction surgery: a survey of the American Association of Hip and Knee Surgeons. J Arthroplasty. 2010;25(7):1005-1014.
13. Healy WL, Iorio R, Ko J, Appleby D, Lemos DW. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am. 2002;84(3):348-353.
14. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
15. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop Relat Res. 2011;469(2):355-361.
16. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
17. de Steiger RN, Miller LN, Davidson DC, Ryan P, Graves SE. Joint registry approach for identification of outlier prostheses. Acta Orthop. 2013;84(4):348-352.
18. Havelin LI, Fenstad AM, Salomonsson R, et al. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop. 2009;80(4):393-401.
19. Zywiel MG, Ulrich SD, Suda AJ, Duncan JL, McGrath MS, Mont MA. Incidence and cost of intraoperative waste of hip and knee arthroplasty implants. J Arthroplasty. 2010;25(4):558-562.
20. Kim P, Kurokawa R, Itoki K. Technical advancements and utilization of spine surgery—international disparities in trend-dynamics between Japan, Korea, and the USA. Neurol Med Chir. 2010;50(9):853-858.
21. Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31(23):2707-2714.
22. Soroceanu A, Canacari E, Brown E, Robinson A, McGuire KJ. Intraoperative waste in spine surgery: incidence, cost, and effectiveness of an educational program. Spine. 2011;36(19):E1270-E1273.
23. Bosco JA, Alvarado CM, Slover JD, Iorio R, Hutzler LH. Decreasing total joint implant costs and physician specific cost variation through negotiation. J Arthroplasty. 2014;29(4):678-680.
24. Zywiel MG, Delanois RE, McGrath MS, Ulrich SD, Duncan JL, Mont MA. Intraoperative waste of trauma implants: a cost burden to hospitals worth addressing? J Orthop Trauma. 2009;23(10):710-715.
25. Ast MP, Mayman DJ, Su EP, Gonzalez Della Valle AM, Parks ML, Haas SB. The reduction of implant-related errors and waste in total knee arthroplasty using a novel, computer based, e.Label and Compatibility system. J Arthroplasty. 2014;29(1):132-136.
The cost of health care in the United States is increasing at an unsustainable rate.1-3 To decrease or even reverse this trend, we must decrease the cost of care without adversely affecting quality. Porter4 defined value as the quality of care divided by its cost. The economics of total joint arthroplasty (TJA) has received a great deal of attention because of both increasing demand and increasing cost.5-9 About 33% of all orthopedic surgeries and the majority of TJAs are paid for by Medicare.9 In recent years, the rate of reimbursement for orthopedic cases has steadily declined while the cost of implants has increased.3,10,11 Given the significant cost of implants, health care providers in some subspecialties have focused on implant costs as a potential area for cost reduction.12 For example, in TJA this has proved effective in reducing the overall cost, as has decreasing length of stay after surgery.8,10,13-16
With little evidence suggesting any specific orthopedic implant has outcomes superior to those of others, with the exception of select poorly performing outliers, we must increase value of care by lowering the cost when considering these devices.17,18 In addition, some experts have suggested that intraoperative waste is a significant factor in TJA cost, and it does contribute to the average implant cost for a TJA case.6,19 Using data collected from 72 institutions, Zywiel and colleagues19 estimated the annual cost of wasted hip and knee arthroplasty implants to be more than $36 million in the United States.
However, considering the aging US population, TJA is not the only orthopedic surgery with increased demand. An estimated 600,000 spine surgeries are performed each year in the United States.20 Between 1992 and 2003, Medicare spending for lumbar spinal fusion increased 500%.21 In addition, in a 15-month observational study of incidence of intraoperative waste in spine surgery, Soroceanu and colleagues22 reported waste occurring in 20% of spine procedures.
Although these studies have described implant waste in TJA and spine surgeries, little has been published on the cost of wasted implants in a center performing the full range of orthopedic procedures. In this article, we detail the implant waste costs incurred by surgeons for all orthopedic subspecialties at a single orthopedic specialty hospital over a 1-year period. Our study goals were to identify types of implants wasted, and incidence and cost of implant waste, for all total hip arthroplasties (THAs), total knee arthroplasties (TKAs), and lumbar spinal fusions performed at the hospital and to determine whether case volume or years in surgical practice affect the rate or cost of implants wasted.
Methods
We performed a retrospective economic analysis of 1 year of administrative implant data from our institution. Collected data were quantified and analyzed for factors that might explain any variance in implant waste among surgeons. We were granted exempt institutional review board status, as no patient information was involved in this study.
We reviewed the administrative implant data for the 12-month period beginning June 2012 and ending May 2013. For that period, number of cases in which an implant was used and number of cases in which an implant was wasted were recorded. For each instance of waste, type and cost of the wasted implant were entered into the administrative database. In addition, overall cost of implants for the year and cost of wasted implants were determined. Data were available for 81 surgeons across 8 orthopedic divisions (subspecialties). From this information, we determined percentage of cases in which waste occurred, percentage of total implant cost wasted, average cost of waste per case, and most commonly wasted implants. All 3 variables were also calculated for THAs, TKAs, and lumbar spinal fusion procedures.
Statistical Analysis
The data were analyzed to determine if surgeon case volume or years in surgical practice affected implant waste. All analyses were performed at department, division (subspecialty), and surgeon levels. Case volume was analyzed in 3 groups: top 25%, middle 50%, and lower 25%. Number of years in surgical practice was analyzed in 3 groups: fewer than 10 years, 10 to 19 years, and 20 years or more. Normality assumption of variables was tested using the Shapiro-Wilk test (P < .05). For between-group differences, 1-way analysis of variance and the Tukey honestly significant difference post hoc test were performed for variables with a normal distribution, and the Kruskal-Wallis and Mann-Whitney tests were performed for variables without a normal distribution.
For the subspecialty-level analyses, only the Adult Reconstruction, Sports Medicine, and Spine divisions were analyzed for the effects of volume, and only the Sports Medicine and Spine divisions were analyzed for the effect of surgical experience, as surgeon numbers were insufficient for adequate grouping(s).
Data are presented as means with corresponding 95% confidence intervals (CIs). Categorical variables are presented as counts with percentages. All statistical analyses were performed with SPSS Version 21.0 (IBM SPSS) statistical software. Statistical significance was set at .05.
Results
During the 1-year period, 8954 department cases involved an implant of any type. Waste occurred in 12% (1072) of these cases. The rate ranged from 8% in the Adult Reconstruction division to 30% in the Trauma division (Table 1), and the rate for individual surgeons ranged from 3% to 100%, though the surgeon with 100% performed only 1 case, and the next highest rate was 50%.
Total implant cost for our hospital during the period was $34,340,607. Of that total cost, 1.8% ($634,668) was lost because of implant waste. Percentage of total implant cost wasted ranged from 1.6% in the Adult Reconstruction division to 4.7% in the Sports Medicine division (Table 1). Percentage of total implant cost wasted for individual surgeons ranged from 0.2% to 16.1%. Tables 2 and 3 list the most commonly wasted implants by count and cost, respectively.
When total cost of wasted implants was averaged over all implant cases performed during the period, the loss resulting from waste amounted to $71 per case for the department and ranged from $21 per case for the Hand division to $105 per case for the Pediatric division (Table 1). For individual surgeons, the loss ranged from $4 to $250 per case.
During the period studied, an implant was wasted in 9% (100) of the 1076 primary THAs performed, 4% (42) of the 1003 primary TKAs, and 14% (30) of the 217 lumbar spinal fusions (Tables 4, 5).
There was no significant difference between groups for department (P = .46) or for the Adult Reconstruction (P = .83), Spine (P = .10), or Sports Medicine (P = .69) division. Analyzing for variance by years in surgical practice, we found a significant difference for department (P = .01) but not for the Adult Reconstruction (P = .12) or Spine (P = .14) division. The department difference resulted from a significant difference (P = .001; 95% CI, 1.112-17.408) between surgeons (<10 years of surgical practice) who wasted implants in 12.8% of their cases and surgeons (>20 years of surgical practice) who wasted implants in 9% of their cases (Table 4).
There was no significant difference between groups for department (P = .83) or for the Adult Reconstruction (P = .29) or Spine (P = .41) division when analyzed by years in surgical practice. Analyzing by case volume, we found a significant difference for the Sports Medicine division (P = .004): Percentage of total implant waste was significantly higher (P = .003; 95% CI, –12.61 to –2.97) for surgeons with the lower 25% of case volume (9.8%) than for surgeons with the middle 50% of case volume (3.5%) (Table 5). No other significant difference was found for department (P = .52) or for the Adult Reconstruction (P = .69) or Spine (P = .45) division.
Analyzing by case volume and years in surgical practice, we found no significant difference for department (case volume, P = .76; years in surgical practice, P = .07), Adult Reconstruction division (case volume, P = .47; years in surgical practice, P = .78), Spine division (case volume, P = .11; years in surgical practice, P = .15), or Sports Medicine division (case volume, P = .08).
Selected Procedures
Total Hip Arthroplasty. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .072; years in practice, P = .076), percentage of total implant cost wasted (volume, P = .074; years in practice, P = .12), cost of waste per case (volume, P = .075; years in practice, P = .32).
Total Knee Arthroplasty. Regarding variance by years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (P = .38), percentage of total implant cost wasted (P = .50), cost of waste per case (P = .50). Regarding variance by volume, there was no significant difference for percentage of cases with waste (P = .70) or cost of waste per case (P = .05), but we found a significant difference for percentage of total implant cost wasted (P = .038). That difference was caused by an outlier: One surgeon with the lower 25% of case volume wasted an implant in the only TKA he performed that year. Correction for the outlier removed the significance.
Posterior Lumbar Spinal Fusion. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .36; years in surgical practice, P = .22), percentage of total implant cost wasted (volume, P = .33; years in surgical practice, P = .41), cost of waste per case (volume, P = .34; years in practice, P = .15).
Discussion
The steadily increasing demand for orthopedic surgeries and declining rates of reimbursement by Medicare and other insurance providers have led many hospitals to look for ways to control the cost of these surgeries. Reducing operating room costs, lowering implant prices, and shortening hospital stays have all proved successful.6,15,20,23 One area that has not been thoroughly explored is the cost burden of wasted implants. Our findings suggest implant waste contributes significantly to the cost of orthopedic surgeries.
One weakness of this study is that its data, though encompassing all orthopedic subspecialties and procedures, come from a single teaching institution and therefore are less representative of all orthopedic departments across the United States. However, the findings are useful in that the analysis was performed across multiple specialties at a high-volume institution and may be applied to similar institutions. Another weakness of this study is that the data cover only 1 year. Collecting data over a longer period could improve the magnitude and power of the analysis. Nonetheless, 1 year of data is a good starting point in identifying the issues and guiding the initiation of measures to address them. Last, we did not explore the reason for each instance of waste during the period reviewed. Knowing the reason for implant waste would be helpful in developing strategies to reduce implant waste.
Our study results showed that, in 1 year, implant waste occurred in 1.8% of procedures that required an implant—representing a loss of $634,000. Other studies have quantified implant waste for selected procedures or single departments, but to our knowledge none has quantified implant waste for an entire orthopedic department or hospital. It is therefore difficult to compare our institutional results with other results. For instance, definitions of waste differ. A study that found waste in 20% of spine surgery cases22 included all intraoperative waste, whereas our 11% of spine cases were implant waste only. Similarly, though rates of implant waste in trauma cases differed significantly between a multi-institution study by Zywiel and colleagues24 (0.6%) and our institution (30%), their study excluded arthroplasty cases from the trauma subset and reported implant waste for a single vendor, whereas we included arthroplasty cases and a wide array of implant vendors. In addition, costs cannot be directly compared because, in our study, implants wasted may have differed. Although the Trauma division had the highest incidence of waste (30%) in our analysis, it did not have the highest waste-related costs. Instead, the Adult Reconstruction division, with waste in 8% of cases, had the highest waste cost, $214,869. The cost difference is certainly the result of the difference in type of implants wasted. The implants most commonly wasted in the Trauma division were screws, which cost between $17 and $150; a single femoral stem, though wasted less often, cost significantly more, $2000 to $6000.
Our results showed a combined implant waste incidence of 6.8% for primary THA and primary TKA cases over the year. In their multi-institution study, Zywiel and colleagues19 reported a combined incidence of implant waste in 2% of THA and TKA cases. The difference is that Zywiel and colleagues19 reported data from a single implant vendor and included revision surgeries, hip hemiarthroplasties, and unicondylar knee arthroplasties. Another study reported implant waste in 5.7% of all TKA cases but did not specify whether revision or unicondylar arthroplasties were included.25 For lumbar spinal fusion, we found an implant waste incidence of 14%. Given the lack of studies in this area, we cannot make a comparison of results.
To our knowledge, there has been no other study of the effects of case volume and years in surgical practice on implant waste. Our analysis showed that waste incidence was not related to surgeon case volume but was related to years in surgical practice. Incidence of waste was significantly lower among surgeons practicing 20 years or more than among surgeons practicing fewer than 10 years. The difference may be a reflection that case volume during a single year is not totally indicative of a surgeon’s lifetime case volume. For example, several surgeons with many years of experience and a significant lifetime case volume had an annual case volume in the lower 25% of the department because they were approaching retirement or had only recently joined the institution. More rigorous prospective studies are needed to further understand this relationship.
Conclusions
Our study demonstrated significant costs related to implant waste. These costs are important to consider not only for traditional cases, such as total joint and spine procedures, in which implant costs are routinely scrutinized, but for all subspecialties, such as sports medicine, in which the majority of cases are performed on an outpatient basis. Considering the estimated $36 million wasted during THAs and TKAs and $126 million wasted on spine surgeries in the United States annually, and the significant waste we observed in other orthopedic subspecialties, decreasing the rate of intraoperative waste during orthopedic surgeries represents another area that could provide significant cost reduction through implant cost savings.19,22 A few successful programs have been reported. Soroceanu and colleagues22 found an almost 50% decrease in intraoperative waste during spine surgery after an educational program was used to address such waste. Elsewhere, use of a computer-based system (e.Label and Compatibility) led to an estimated cost reduction of $75,000 in implant waste.25 Efforts to develop and implement other programs to reduce implant waste are needed and should be part of any orthopedic operating room cost reduction strategy.
The cost of health care in the United States is increasing at an unsustainable rate.1-3 To decrease or even reverse this trend, we must decrease the cost of care without adversely affecting quality. Porter4 defined value as the quality of care divided by its cost. The economics of total joint arthroplasty (TJA) has received a great deal of attention because of both increasing demand and increasing cost.5-9 About 33% of all orthopedic surgeries and the majority of TJAs are paid for by Medicare.9 In recent years, the rate of reimbursement for orthopedic cases has steadily declined while the cost of implants has increased.3,10,11 Given the significant cost of implants, health care providers in some subspecialties have focused on implant costs as a potential area for cost reduction.12 For example, in TJA this has proved effective in reducing the overall cost, as has decreasing length of stay after surgery.8,10,13-16
With little evidence suggesting any specific orthopedic implant has outcomes superior to those of others, with the exception of select poorly performing outliers, we must increase value of care by lowering the cost when considering these devices.17,18 In addition, some experts have suggested that intraoperative waste is a significant factor in TJA cost, and it does contribute to the average implant cost for a TJA case.6,19 Using data collected from 72 institutions, Zywiel and colleagues19 estimated the annual cost of wasted hip and knee arthroplasty implants to be more than $36 million in the United States.
However, considering the aging US population, TJA is not the only orthopedic surgery with increased demand. An estimated 600,000 spine surgeries are performed each year in the United States.20 Between 1992 and 2003, Medicare spending for lumbar spinal fusion increased 500%.21 In addition, in a 15-month observational study of incidence of intraoperative waste in spine surgery, Soroceanu and colleagues22 reported waste occurring in 20% of spine procedures.
Although these studies have described implant waste in TJA and spine surgeries, little has been published on the cost of wasted implants in a center performing the full range of orthopedic procedures. In this article, we detail the implant waste costs incurred by surgeons for all orthopedic subspecialties at a single orthopedic specialty hospital over a 1-year period. Our study goals were to identify types of implants wasted, and incidence and cost of implant waste, for all total hip arthroplasties (THAs), total knee arthroplasties (TKAs), and lumbar spinal fusions performed at the hospital and to determine whether case volume or years in surgical practice affect the rate or cost of implants wasted.
Methods
We performed a retrospective economic analysis of 1 year of administrative implant data from our institution. Collected data were quantified and analyzed for factors that might explain any variance in implant waste among surgeons. We were granted exempt institutional review board status, as no patient information was involved in this study.
We reviewed the administrative implant data for the 12-month period beginning June 2012 and ending May 2013. For that period, number of cases in which an implant was used and number of cases in which an implant was wasted were recorded. For each instance of waste, type and cost of the wasted implant were entered into the administrative database. In addition, overall cost of implants for the year and cost of wasted implants were determined. Data were available for 81 surgeons across 8 orthopedic divisions (subspecialties). From this information, we determined percentage of cases in which waste occurred, percentage of total implant cost wasted, average cost of waste per case, and most commonly wasted implants. All 3 variables were also calculated for THAs, TKAs, and lumbar spinal fusion procedures.
Statistical Analysis
The data were analyzed to determine if surgeon case volume or years in surgical practice affected implant waste. All analyses were performed at department, division (subspecialty), and surgeon levels. Case volume was analyzed in 3 groups: top 25%, middle 50%, and lower 25%. Number of years in surgical practice was analyzed in 3 groups: fewer than 10 years, 10 to 19 years, and 20 years or more. Normality assumption of variables was tested using the Shapiro-Wilk test (P < .05). For between-group differences, 1-way analysis of variance and the Tukey honestly significant difference post hoc test were performed for variables with a normal distribution, and the Kruskal-Wallis and Mann-Whitney tests were performed for variables without a normal distribution.
For the subspecialty-level analyses, only the Adult Reconstruction, Sports Medicine, and Spine divisions were analyzed for the effects of volume, and only the Sports Medicine and Spine divisions were analyzed for the effect of surgical experience, as surgeon numbers were insufficient for adequate grouping(s).
Data are presented as means with corresponding 95% confidence intervals (CIs). Categorical variables are presented as counts with percentages. All statistical analyses were performed with SPSS Version 21.0 (IBM SPSS) statistical software. Statistical significance was set at .05.
Results
During the 1-year period, 8954 department cases involved an implant of any type. Waste occurred in 12% (1072) of these cases. The rate ranged from 8% in the Adult Reconstruction division to 30% in the Trauma division (Table 1), and the rate for individual surgeons ranged from 3% to 100%, though the surgeon with 100% performed only 1 case, and the next highest rate was 50%.
Total implant cost for our hospital during the period was $34,340,607. Of that total cost, 1.8% ($634,668) was lost because of implant waste. Percentage of total implant cost wasted ranged from 1.6% in the Adult Reconstruction division to 4.7% in the Sports Medicine division (Table 1). Percentage of total implant cost wasted for individual surgeons ranged from 0.2% to 16.1%. Tables 2 and 3 list the most commonly wasted implants by count and cost, respectively.
When total cost of wasted implants was averaged over all implant cases performed during the period, the loss resulting from waste amounted to $71 per case for the department and ranged from $21 per case for the Hand division to $105 per case for the Pediatric division (Table 1). For individual surgeons, the loss ranged from $4 to $250 per case.
During the period studied, an implant was wasted in 9% (100) of the 1076 primary THAs performed, 4% (42) of the 1003 primary TKAs, and 14% (30) of the 217 lumbar spinal fusions (Tables 4, 5).
There was no significant difference between groups for department (P = .46) or for the Adult Reconstruction (P = .83), Spine (P = .10), or Sports Medicine (P = .69) division. Analyzing for variance by years in surgical practice, we found a significant difference for department (P = .01) but not for the Adult Reconstruction (P = .12) or Spine (P = .14) division. The department difference resulted from a significant difference (P = .001; 95% CI, 1.112-17.408) between surgeons (<10 years of surgical practice) who wasted implants in 12.8% of their cases and surgeons (>20 years of surgical practice) who wasted implants in 9% of their cases (Table 4).
There was no significant difference between groups for department (P = .83) or for the Adult Reconstruction (P = .29) or Spine (P = .41) division when analyzed by years in surgical practice. Analyzing by case volume, we found a significant difference for the Sports Medicine division (P = .004): Percentage of total implant waste was significantly higher (P = .003; 95% CI, –12.61 to –2.97) for surgeons with the lower 25% of case volume (9.8%) than for surgeons with the middle 50% of case volume (3.5%) (Table 5). No other significant difference was found for department (P = .52) or for the Adult Reconstruction (P = .69) or Spine (P = .45) division.
Analyzing by case volume and years in surgical practice, we found no significant difference for department (case volume, P = .76; years in surgical practice, P = .07), Adult Reconstruction division (case volume, P = .47; years in surgical practice, P = .78), Spine division (case volume, P = .11; years in surgical practice, P = .15), or Sports Medicine division (case volume, P = .08).
Selected Procedures
Total Hip Arthroplasty. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .072; years in practice, P = .076), percentage of total implant cost wasted (volume, P = .074; years in practice, P = .12), cost of waste per case (volume, P = .075; years in practice, P = .32).
Total Knee Arthroplasty. Regarding variance by years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (P = .38), percentage of total implant cost wasted (P = .50), cost of waste per case (P = .50). Regarding variance by volume, there was no significant difference for percentage of cases with waste (P = .70) or cost of waste per case (P = .05), but we found a significant difference for percentage of total implant cost wasted (P = .038). That difference was caused by an outlier: One surgeon with the lower 25% of case volume wasted an implant in the only TKA he performed that year. Correction for the outlier removed the significance.
Posterior Lumbar Spinal Fusion. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .36; years in surgical practice, P = .22), percentage of total implant cost wasted (volume, P = .33; years in surgical practice, P = .41), cost of waste per case (volume, P = .34; years in practice, P = .15).
Discussion
The steadily increasing demand for orthopedic surgeries and declining rates of reimbursement by Medicare and other insurance providers have led many hospitals to look for ways to control the cost of these surgeries. Reducing operating room costs, lowering implant prices, and shortening hospital stays have all proved successful.6,15,20,23 One area that has not been thoroughly explored is the cost burden of wasted implants. Our findings suggest implant waste contributes significantly to the cost of orthopedic surgeries.
One weakness of this study is that its data, though encompassing all orthopedic subspecialties and procedures, come from a single teaching institution and therefore are less representative of all orthopedic departments across the United States. However, the findings are useful in that the analysis was performed across multiple specialties at a high-volume institution and may be applied to similar institutions. Another weakness of this study is that the data cover only 1 year. Collecting data over a longer period could improve the magnitude and power of the analysis. Nonetheless, 1 year of data is a good starting point in identifying the issues and guiding the initiation of measures to address them. Last, we did not explore the reason for each instance of waste during the period reviewed. Knowing the reason for implant waste would be helpful in developing strategies to reduce implant waste.
Our study results showed that, in 1 year, implant waste occurred in 1.8% of procedures that required an implant—representing a loss of $634,000. Other studies have quantified implant waste for selected procedures or single departments, but to our knowledge none has quantified implant waste for an entire orthopedic department or hospital. It is therefore difficult to compare our institutional results with other results. For instance, definitions of waste differ. A study that found waste in 20% of spine surgery cases22 included all intraoperative waste, whereas our 11% of spine cases were implant waste only. Similarly, though rates of implant waste in trauma cases differed significantly between a multi-institution study by Zywiel and colleagues24 (0.6%) and our institution (30%), their study excluded arthroplasty cases from the trauma subset and reported implant waste for a single vendor, whereas we included arthroplasty cases and a wide array of implant vendors. In addition, costs cannot be directly compared because, in our study, implants wasted may have differed. Although the Trauma division had the highest incidence of waste (30%) in our analysis, it did not have the highest waste-related costs. Instead, the Adult Reconstruction division, with waste in 8% of cases, had the highest waste cost, $214,869. The cost difference is certainly the result of the difference in type of implants wasted. The implants most commonly wasted in the Trauma division were screws, which cost between $17 and $150; a single femoral stem, though wasted less often, cost significantly more, $2000 to $6000.
Our results showed a combined implant waste incidence of 6.8% for primary THA and primary TKA cases over the year. In their multi-institution study, Zywiel and colleagues19 reported a combined incidence of implant waste in 2% of THA and TKA cases. The difference is that Zywiel and colleagues19 reported data from a single implant vendor and included revision surgeries, hip hemiarthroplasties, and unicondylar knee arthroplasties. Another study reported implant waste in 5.7% of all TKA cases but did not specify whether revision or unicondylar arthroplasties were included.25 For lumbar spinal fusion, we found an implant waste incidence of 14%. Given the lack of studies in this area, we cannot make a comparison of results.
To our knowledge, there has been no other study of the effects of case volume and years in surgical practice on implant waste. Our analysis showed that waste incidence was not related to surgeon case volume but was related to years in surgical practice. Incidence of waste was significantly lower among surgeons practicing 20 years or more than among surgeons practicing fewer than 10 years. The difference may be a reflection that case volume during a single year is not totally indicative of a surgeon’s lifetime case volume. For example, several surgeons with many years of experience and a significant lifetime case volume had an annual case volume in the lower 25% of the department because they were approaching retirement or had only recently joined the institution. More rigorous prospective studies are needed to further understand this relationship.
Conclusions
Our study demonstrated significant costs related to implant waste. These costs are important to consider not only for traditional cases, such as total joint and spine procedures, in which implant costs are routinely scrutinized, but for all subspecialties, such as sports medicine, in which the majority of cases are performed on an outpatient basis. Considering the estimated $36 million wasted during THAs and TKAs and $126 million wasted on spine surgeries in the United States annually, and the significant waste we observed in other orthopedic subspecialties, decreasing the rate of intraoperative waste during orthopedic surgeries represents another area that could provide significant cost reduction through implant cost savings.19,22 A few successful programs have been reported. Soroceanu and colleagues22 found an almost 50% decrease in intraoperative waste during spine surgery after an educational program was used to address such waste. Elsewhere, use of a computer-based system (e.Label and Compatibility) led to an estimated cost reduction of $75,000 in implant waste.25 Efforts to develop and implement other programs to reduce implant waste are needed and should be part of any orthopedic operating room cost reduction strategy.
1. Alhassani A, Chandra A, Chernew ME. The sources of the SGR “hole.” N Engl J Med. 2012;366(4):289-291.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Keehan SP, Sisko AM, Truffer CJ, et al. National health spending projections through 2020: economic recovery and reform drive faster spending growth. Health Aff. 2011;30(8):1594-1605.
4. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481.
5. Belatti DA, Phisitkul P. Trends in orthopedics: an analysis of Medicare claims, 2000–2010. Orthopedics. 2013;36(3):e366-e372.
6. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
7. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(5):23-27.
8. Mendenhall S. 2003 hip and knee implant review. Orthop Network News. 2003;14(3):2.
9. Mendenhall S. 2008 hip and knee implant review. Orthop Network News. 2008;19(3):20.
10. Healy WL, Rana AJ, Iorio R. Hospital economics of primary total knee arthroplasty at a teaching hospital. Clin Orthop Relat Res. 2011;469(1):87-94.
11. Mendenhall S. 2007 hip and knee implant review. Orthop Network News. 2007;18(3):16.
12. Iorio R, Davis CM 3rd, Healy WL, Fehring TK, O’Connor MI, York S. Impact of the economic downturn on adult reconstruction surgery: a survey of the American Association of Hip and Knee Surgeons. J Arthroplasty. 2010;25(7):1005-1014.
13. Healy WL, Iorio R, Ko J, Appleby D, Lemos DW. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am. 2002;84(3):348-353.
14. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
15. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop Relat Res. 2011;469(2):355-361.
16. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
17. de Steiger RN, Miller LN, Davidson DC, Ryan P, Graves SE. Joint registry approach for identification of outlier prostheses. Acta Orthop. 2013;84(4):348-352.
18. Havelin LI, Fenstad AM, Salomonsson R, et al. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop. 2009;80(4):393-401.
19. Zywiel MG, Ulrich SD, Suda AJ, Duncan JL, McGrath MS, Mont MA. Incidence and cost of intraoperative waste of hip and knee arthroplasty implants. J Arthroplasty. 2010;25(4):558-562.
20. Kim P, Kurokawa R, Itoki K. Technical advancements and utilization of spine surgery—international disparities in trend-dynamics between Japan, Korea, and the USA. Neurol Med Chir. 2010;50(9):853-858.
21. Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31(23):2707-2714.
22. Soroceanu A, Canacari E, Brown E, Robinson A, McGuire KJ. Intraoperative waste in spine surgery: incidence, cost, and effectiveness of an educational program. Spine. 2011;36(19):E1270-E1273.
23. Bosco JA, Alvarado CM, Slover JD, Iorio R, Hutzler LH. Decreasing total joint implant costs and physician specific cost variation through negotiation. J Arthroplasty. 2014;29(4):678-680.
24. Zywiel MG, Delanois RE, McGrath MS, Ulrich SD, Duncan JL, Mont MA. Intraoperative waste of trauma implants: a cost burden to hospitals worth addressing? J Orthop Trauma. 2009;23(10):710-715.
25. Ast MP, Mayman DJ, Su EP, Gonzalez Della Valle AM, Parks ML, Haas SB. The reduction of implant-related errors and waste in total knee arthroplasty using a novel, computer based, e.Label and Compatibility system. J Arthroplasty. 2014;29(1):132-136.
1. Alhassani A, Chandra A, Chernew ME. The sources of the SGR “hole.” N Engl J Med. 2012;366(4):289-291.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Keehan SP, Sisko AM, Truffer CJ, et al. National health spending projections through 2020: economic recovery and reform drive faster spending growth. Health Aff. 2011;30(8):1594-1605.
4. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481.
5. Belatti DA, Phisitkul P. Trends in orthopedics: an analysis of Medicare claims, 2000–2010. Orthopedics. 2013;36(3):e366-e372.
6. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
7. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(5):23-27.
8. Mendenhall S. 2003 hip and knee implant review. Orthop Network News. 2003;14(3):2.
9. Mendenhall S. 2008 hip and knee implant review. Orthop Network News. 2008;19(3):20.
10. Healy WL, Rana AJ, Iorio R. Hospital economics of primary total knee arthroplasty at a teaching hospital. Clin Orthop Relat Res. 2011;469(1):87-94.
11. Mendenhall S. 2007 hip and knee implant review. Orthop Network News. 2007;18(3):16.
12. Iorio R, Davis CM 3rd, Healy WL, Fehring TK, O’Connor MI, York S. Impact of the economic downturn on adult reconstruction surgery: a survey of the American Association of Hip and Knee Surgeons. J Arthroplasty. 2010;25(7):1005-1014.
13. Healy WL, Iorio R, Ko J, Appleby D, Lemos DW. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am. 2002;84(3):348-353.
14. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
15. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop Relat Res. 2011;469(2):355-361.
16. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
17. de Steiger RN, Miller LN, Davidson DC, Ryan P, Graves SE. Joint registry approach for identification of outlier prostheses. Acta Orthop. 2013;84(4):348-352.
18. Havelin LI, Fenstad AM, Salomonsson R, et al. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop. 2009;80(4):393-401.
19. Zywiel MG, Ulrich SD, Suda AJ, Duncan JL, McGrath MS, Mont MA. Incidence and cost of intraoperative waste of hip and knee arthroplasty implants. J Arthroplasty. 2010;25(4):558-562.
20. Kim P, Kurokawa R, Itoki K. Technical advancements and utilization of spine surgery—international disparities in trend-dynamics between Japan, Korea, and the USA. Neurol Med Chir. 2010;50(9):853-858.
21. Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31(23):2707-2714.
22. Soroceanu A, Canacari E, Brown E, Robinson A, McGuire KJ. Intraoperative waste in spine surgery: incidence, cost, and effectiveness of an educational program. Spine. 2011;36(19):E1270-E1273.
23. Bosco JA, Alvarado CM, Slover JD, Iorio R, Hutzler LH. Decreasing total joint implant costs and physician specific cost variation through negotiation. J Arthroplasty. 2014;29(4):678-680.
24. Zywiel MG, Delanois RE, McGrath MS, Ulrich SD, Duncan JL, Mont MA. Intraoperative waste of trauma implants: a cost burden to hospitals worth addressing? J Orthop Trauma. 2009;23(10):710-715.
25. Ast MP, Mayman DJ, Su EP, Gonzalez Della Valle AM, Parks ML, Haas SB. The reduction of implant-related errors and waste in total knee arthroplasty using a novel, computer based, e.Label and Compatibility system. J Arthroplasty. 2014;29(1):132-136.
Patients With Sacroiliac Joint Pain Helped With Implant Procedure
A minimally invasive implant procedure is highly effective in reducing pain and disability for patients with sacroiliac joint dysfunction (SIJ), according to a study published in the November issue of Neurosurgery. The randomized controlled trial showed superior outcomes in patients undergoing minimally invasive sacroiliac joint fusion using triangular titanium implants, compared with nonsurgical management, according to lead author David W. Polly, MD, a professor in the Departments of Orthopedic Surgery and Neurosurgery at the University of Minnesota in Minneapolis.
This study included 148 patients with low back pain caused by confirmed SIJ dysfunction, treated at 19 spine surgery clinics in the United States. SIJ disruption, also known as osteoarthritis, is estimated to cause 15% to 23% of cases of chronic low back pain.
Study participants had severe SIJ pain, with an average pain score of 82 on a 0-to-100-point scale. Average pain duration was longer than 6 years, and about two-thirds of subjects were taking opioid medications. Many study participants had previously received nonsurgical SIJ treatments and many had a history of prior spinal surgery.
Two-thirds of subjects were randomly assigned to undergo minimally invasive SIJ fusion. In this procedure, triangular titanium implants were placed through a small incision to stabilize and fuse the SIJ. Procedures were unilateral in most cases, but some subjects underwent bilateral treatment. The remaining subjects received nonsurgical treatments, such as physical therapy, steroid injections, and/or radiofrequency ablation of sacral nerve root lateral branches.
Pain and other outcomes were compared at baseline and at 1, 3, 6, and 12 months. At 6 months, subjects in the nonsurgical group had the option to “cross over” to the implant procedure.
Based on reduction in pain and absence of complications at 6 months, treatment was rated successful in 81% of subjects assigned to the SIJ implant procedure, compared with 26% of people with nonsurgical treatment. The average pain score decreased to 30 in the surgical group compared with 72 in the nonsurgical group. A total of 73% of subjects undergoing the implant procedure had “clinically significant” reduction in disability scores, compared with 14% in the nonsurgical group.
After 1 year, subjects assigned to SIJ fusion still had significant reductions in pain and disability, as well as improved quality of life. Thirty-five subjects from the nonsurgical group opted to undergo the implant procedure, with similarly good results.
The minimally invasive SIJ implant approach that was evaluated in this trial has been cleared by the FDA. The study is the first randomized controlled trial to directly compare the results of surgical and nonsurgical treatment for SIJ dysfunction.
The results show “clinically and statistically important” improvements in clinical outcomes for patients undergoing the SIJ implant procedure, according to Dr. Polly and colleagues, with “profound differences” between the surgical and nonsurgical groups. The implant procedure is minimally invasive, has few complications, and produces significant and lasting improvements in pain, disability, and quality of life.
The study authors noted some important limitations of their trial, including the lack of long-term outcomes in the nonsurgical group due to the high crossover rate.
Investigators plan further analyses, including 2-year follow-up CT scans and a cost-effectiveness comparison of SIJ fusion versus nonsurgical treatment.
Suggested Reading
Polly DW, Cher DJ, Wine KD, et al. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-691.
A minimally invasive implant procedure is highly effective in reducing pain and disability for patients with sacroiliac joint dysfunction (SIJ), according to a study published in the November issue of Neurosurgery. The randomized controlled trial showed superior outcomes in patients undergoing minimally invasive sacroiliac joint fusion using triangular titanium implants, compared with nonsurgical management, according to lead author David W. Polly, MD, a professor in the Departments of Orthopedic Surgery and Neurosurgery at the University of Minnesota in Minneapolis.
This study included 148 patients with low back pain caused by confirmed SIJ dysfunction, treated at 19 spine surgery clinics in the United States. SIJ disruption, also known as osteoarthritis, is estimated to cause 15% to 23% of cases of chronic low back pain.
Study participants had severe SIJ pain, with an average pain score of 82 on a 0-to-100-point scale. Average pain duration was longer than 6 years, and about two-thirds of subjects were taking opioid medications. Many study participants had previously received nonsurgical SIJ treatments and many had a history of prior spinal surgery.
Two-thirds of subjects were randomly assigned to undergo minimally invasive SIJ fusion. In this procedure, triangular titanium implants were placed through a small incision to stabilize and fuse the SIJ. Procedures were unilateral in most cases, but some subjects underwent bilateral treatment. The remaining subjects received nonsurgical treatments, such as physical therapy, steroid injections, and/or radiofrequency ablation of sacral nerve root lateral branches.
Pain and other outcomes were compared at baseline and at 1, 3, 6, and 12 months. At 6 months, subjects in the nonsurgical group had the option to “cross over” to the implant procedure.
Based on reduction in pain and absence of complications at 6 months, treatment was rated successful in 81% of subjects assigned to the SIJ implant procedure, compared with 26% of people with nonsurgical treatment. The average pain score decreased to 30 in the surgical group compared with 72 in the nonsurgical group. A total of 73% of subjects undergoing the implant procedure had “clinically significant” reduction in disability scores, compared with 14% in the nonsurgical group.
After 1 year, subjects assigned to SIJ fusion still had significant reductions in pain and disability, as well as improved quality of life. Thirty-five subjects from the nonsurgical group opted to undergo the implant procedure, with similarly good results.
The minimally invasive SIJ implant approach that was evaluated in this trial has been cleared by the FDA. The study is the first randomized controlled trial to directly compare the results of surgical and nonsurgical treatment for SIJ dysfunction.
The results show “clinically and statistically important” improvements in clinical outcomes for patients undergoing the SIJ implant procedure, according to Dr. Polly and colleagues, with “profound differences” between the surgical and nonsurgical groups. The implant procedure is minimally invasive, has few complications, and produces significant and lasting improvements in pain, disability, and quality of life.
The study authors noted some important limitations of their trial, including the lack of long-term outcomes in the nonsurgical group due to the high crossover rate.
Investigators plan further analyses, including 2-year follow-up CT scans and a cost-effectiveness comparison of SIJ fusion versus nonsurgical treatment.
A minimally invasive implant procedure is highly effective in reducing pain and disability for patients with sacroiliac joint dysfunction (SIJ), according to a study published in the November issue of Neurosurgery. The randomized controlled trial showed superior outcomes in patients undergoing minimally invasive sacroiliac joint fusion using triangular titanium implants, compared with nonsurgical management, according to lead author David W. Polly, MD, a professor in the Departments of Orthopedic Surgery and Neurosurgery at the University of Minnesota in Minneapolis.
This study included 148 patients with low back pain caused by confirmed SIJ dysfunction, treated at 19 spine surgery clinics in the United States. SIJ disruption, also known as osteoarthritis, is estimated to cause 15% to 23% of cases of chronic low back pain.
Study participants had severe SIJ pain, with an average pain score of 82 on a 0-to-100-point scale. Average pain duration was longer than 6 years, and about two-thirds of subjects were taking opioid medications. Many study participants had previously received nonsurgical SIJ treatments and many had a history of prior spinal surgery.
Two-thirds of subjects were randomly assigned to undergo minimally invasive SIJ fusion. In this procedure, triangular titanium implants were placed through a small incision to stabilize and fuse the SIJ. Procedures were unilateral in most cases, but some subjects underwent bilateral treatment. The remaining subjects received nonsurgical treatments, such as physical therapy, steroid injections, and/or radiofrequency ablation of sacral nerve root lateral branches.
Pain and other outcomes were compared at baseline and at 1, 3, 6, and 12 months. At 6 months, subjects in the nonsurgical group had the option to “cross over” to the implant procedure.
Based on reduction in pain and absence of complications at 6 months, treatment was rated successful in 81% of subjects assigned to the SIJ implant procedure, compared with 26% of people with nonsurgical treatment. The average pain score decreased to 30 in the surgical group compared with 72 in the nonsurgical group. A total of 73% of subjects undergoing the implant procedure had “clinically significant” reduction in disability scores, compared with 14% in the nonsurgical group.
After 1 year, subjects assigned to SIJ fusion still had significant reductions in pain and disability, as well as improved quality of life. Thirty-five subjects from the nonsurgical group opted to undergo the implant procedure, with similarly good results.
The minimally invasive SIJ implant approach that was evaluated in this trial has been cleared by the FDA. The study is the first randomized controlled trial to directly compare the results of surgical and nonsurgical treatment for SIJ dysfunction.
The results show “clinically and statistically important” improvements in clinical outcomes for patients undergoing the SIJ implant procedure, according to Dr. Polly and colleagues, with “profound differences” between the surgical and nonsurgical groups. The implant procedure is minimally invasive, has few complications, and produces significant and lasting improvements in pain, disability, and quality of life.
The study authors noted some important limitations of their trial, including the lack of long-term outcomes in the nonsurgical group due to the high crossover rate.
Investigators plan further analyses, including 2-year follow-up CT scans and a cost-effectiveness comparison of SIJ fusion versus nonsurgical treatment.
Suggested Reading
Polly DW, Cher DJ, Wine KD, et al. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-691.
Suggested Reading
Polly DW, Cher DJ, Wine KD, et al. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-691.
Irisin Increases Cortical Bone Mass
A recently identified molecule produced by skeletal muscle in response to exercise has been shown to increase bone mass, according to a study published online ahead of print September 29 in the Proceedings of the National Academy of Sciences. “This is a novel finding, and offers promise in the lab, and in the clinic,” said co-lead study author Mone Zaidi, MD, PhD, Professor of Medicine and of Structural and Chemical Biology at the Icahn School of Medicine at Mount Sinai, and Director of the Mount Sinai Bone Program in New York. “It establishes for the first time [that] a molecule released from muscle during exercise can act directly on long bones to increase their strength. These are the bones utilized during exercise, and also the ones most likely to break.”
In this study, young male mice, chosen because researchers could best see bone accrual at this age, were injected with irisin. In the injected mice, researchers saw significant increases in bone mass and strength, specifically cortical bone. The action of the recently identified signaling molecule, irisin, was mediated primarily through bone growth.
The study suggests irisin is fundamental to muscle–bone communication, and likely translates the well-known skeletal anabolic action of exercise by directly stimulating new bone synthesis by osteoblasts.
According to the study authors, identifying irisin as a molecule responsible for muscle-bone connectivity during exercise could lead to the development of future therapies for sarcopenia and osteoporosis.
“These diseases often occur together, and both muscle and bone loss are common medical problems in the elderly that cause significant disability. Understanding this molecular connection between muscle and bone gives us hope for treating age-related bone and muscle loss at the same time, with the same agent,” said Dr. Zaidi.
Suggested Reading
Colaianni G, Cuscito C, Mongelli T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA. 2015;112(39):12157-12162. [Epub ahead of print].
A recently identified molecule produced by skeletal muscle in response to exercise has been shown to increase bone mass, according to a study published online ahead of print September 29 in the Proceedings of the National Academy of Sciences. “This is a novel finding, and offers promise in the lab, and in the clinic,” said co-lead study author Mone Zaidi, MD, PhD, Professor of Medicine and of Structural and Chemical Biology at the Icahn School of Medicine at Mount Sinai, and Director of the Mount Sinai Bone Program in New York. “It establishes for the first time [that] a molecule released from muscle during exercise can act directly on long bones to increase their strength. These are the bones utilized during exercise, and also the ones most likely to break.”
In this study, young male mice, chosen because researchers could best see bone accrual at this age, were injected with irisin. In the injected mice, researchers saw significant increases in bone mass and strength, specifically cortical bone. The action of the recently identified signaling molecule, irisin, was mediated primarily through bone growth.
The study suggests irisin is fundamental to muscle–bone communication, and likely translates the well-known skeletal anabolic action of exercise by directly stimulating new bone synthesis by osteoblasts.
According to the study authors, identifying irisin as a molecule responsible for muscle-bone connectivity during exercise could lead to the development of future therapies for sarcopenia and osteoporosis.
“These diseases often occur together, and both muscle and bone loss are common medical problems in the elderly that cause significant disability. Understanding this molecular connection between muscle and bone gives us hope for treating age-related bone and muscle loss at the same time, with the same agent,” said Dr. Zaidi.
A recently identified molecule produced by skeletal muscle in response to exercise has been shown to increase bone mass, according to a study published online ahead of print September 29 in the Proceedings of the National Academy of Sciences. “This is a novel finding, and offers promise in the lab, and in the clinic,” said co-lead study author Mone Zaidi, MD, PhD, Professor of Medicine and of Structural and Chemical Biology at the Icahn School of Medicine at Mount Sinai, and Director of the Mount Sinai Bone Program in New York. “It establishes for the first time [that] a molecule released from muscle during exercise can act directly on long bones to increase their strength. These are the bones utilized during exercise, and also the ones most likely to break.”
In this study, young male mice, chosen because researchers could best see bone accrual at this age, were injected with irisin. In the injected mice, researchers saw significant increases in bone mass and strength, specifically cortical bone. The action of the recently identified signaling molecule, irisin, was mediated primarily through bone growth.
The study suggests irisin is fundamental to muscle–bone communication, and likely translates the well-known skeletal anabolic action of exercise by directly stimulating new bone synthesis by osteoblasts.
According to the study authors, identifying irisin as a molecule responsible for muscle-bone connectivity during exercise could lead to the development of future therapies for sarcopenia and osteoporosis.
“These diseases often occur together, and both muscle and bone loss are common medical problems in the elderly that cause significant disability. Understanding this molecular connection between muscle and bone gives us hope for treating age-related bone and muscle loss at the same time, with the same agent,” said Dr. Zaidi.
Suggested Reading
Colaianni G, Cuscito C, Mongelli T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA. 2015;112(39):12157-12162. [Epub ahead of print].
Suggested Reading
Colaianni G, Cuscito C, Mongelli T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA. 2015;112(39):12157-12162. [Epub ahead of print].
Surgical Management of Gorham-Stout Disease of the Pelvis Refractory to Medical and Radiation Therapy
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
Coracoid Fracture After Reverse Total Shoulder Arthroplasty: A Report of 2 Cases
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
Total Shoulder Arthroplasty Outcome for Treatment of Osteoarthritis: A Multicenter Study Using a Contemporary Implant
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
Collagenase Enzymatic Fasciotomy for Dupuytren Contracture in Patients on Chronic Immunosuppression
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.