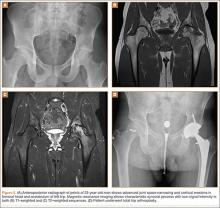
User login
Risk-Stratified VTE Prophylaxis Following Total Joint Replacement Leads to Significant Hospital Cost Reductions and Prevents Deep Vein Thrombosis
DALLAS—Medical Compression Systems, Inc. (Concord, Massachusetts), announced new data that further validates the use of their ActiveCare deep vein thrombosis prophylaxis compression system following total joint replacement procedures. The study results demonstrate that a risk-stratification protocol using a synchronized mobile compression and an aspirin regimen is associated with low rates of venous thromboembolism, lower rates of adverse events, and reduced overall costs compared with a group treated with aggressive anticoagulant agents. Data were presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
“We’ve established through previous studies that prophylactic treatment with mobile compression and aspirin following total joint replacement can reduce the occurrence of venous thromboembolism and decrease adverse events, infections, and bleeding complications in patients undergoing total joint replacement,” said Richard Iorio, MD, primary study author and Professor of Orthopedic Surgery at NYU School of Medicine in New York.
The study was designed to determine if utilizing a risk-based venous thromboembolism chemoprophylaxis protocol would improve prevention of deep vein thrombosis and pulmonary embolism, quality metrics, and bleeding-related complications in patients undergoing total joint arthroplasty.
The retrospective review evaluated 2,611 patients that were divided into 2 cohorts. Cohort 1 included 1,203 patients who were previously treated with standard aggressive chemoprophylaxis agents (Enoxaparin, Rivaroxaban, Warfarin). Cohort 2 consisted of a risk-stratified group of patients either undergoing treatment with prophylactic synchronized mobile compression and aspirin (n=843) or aggressive prophylaxis (n=565).
Results demonstrated that patients in the risk-stratified protocol had a lower incidence of venous thromboembolism than the group treated with anticoagulation. Patients in this group also experienced fewer adverse events, readmissions, infections, and bleeding-related complications. Hospital costs were significantly lower in the synchronized mobile compression and aspirin subgroup of cohort 2 and overall costs were lower in the risk-stratified cohort, though they did not reach statistical significance.
“These results are significant in that they represent a large study population of more than 2,600 patients and are the first to demonstrate significant reductions in hospital costs, which support the hypothesis that a risk stratification protocol can advance patient-specific therapy and enhance the delivery of value-based care,” Dr. Iorio said.
DALLAS—Medical Compression Systems, Inc. (Concord, Massachusetts), announced new data that further validates the use of their ActiveCare deep vein thrombosis prophylaxis compression system following total joint replacement procedures. The study results demonstrate that a risk-stratification protocol using a synchronized mobile compression and an aspirin regimen is associated with low rates of venous thromboembolism, lower rates of adverse events, and reduced overall costs compared with a group treated with aggressive anticoagulant agents. Data were presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
“We’ve established through previous studies that prophylactic treatment with mobile compression and aspirin following total joint replacement can reduce the occurrence of venous thromboembolism and decrease adverse events, infections, and bleeding complications in patients undergoing total joint replacement,” said Richard Iorio, MD, primary study author and Professor of Orthopedic Surgery at NYU School of Medicine in New York.
The study was designed to determine if utilizing a risk-based venous thromboembolism chemoprophylaxis protocol would improve prevention of deep vein thrombosis and pulmonary embolism, quality metrics, and bleeding-related complications in patients undergoing total joint arthroplasty.
The retrospective review evaluated 2,611 patients that were divided into 2 cohorts. Cohort 1 included 1,203 patients who were previously treated with standard aggressive chemoprophylaxis agents (Enoxaparin, Rivaroxaban, Warfarin). Cohort 2 consisted of a risk-stratified group of patients either undergoing treatment with prophylactic synchronized mobile compression and aspirin (n=843) or aggressive prophylaxis (n=565).
Results demonstrated that patients in the risk-stratified protocol had a lower incidence of venous thromboembolism than the group treated with anticoagulation. Patients in this group also experienced fewer adverse events, readmissions, infections, and bleeding-related complications. Hospital costs were significantly lower in the synchronized mobile compression and aspirin subgroup of cohort 2 and overall costs were lower in the risk-stratified cohort, though they did not reach statistical significance.
“These results are significant in that they represent a large study population of more than 2,600 patients and are the first to demonstrate significant reductions in hospital costs, which support the hypothesis that a risk stratification protocol can advance patient-specific therapy and enhance the delivery of value-based care,” Dr. Iorio said.
DALLAS—Medical Compression Systems, Inc. (Concord, Massachusetts), announced new data that further validates the use of their ActiveCare deep vein thrombosis prophylaxis compression system following total joint replacement procedures. The study results demonstrate that a risk-stratification protocol using a synchronized mobile compression and an aspirin regimen is associated with low rates of venous thromboembolism, lower rates of adverse events, and reduced overall costs compared with a group treated with aggressive anticoagulant agents. Data were presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
“We’ve established through previous studies that prophylactic treatment with mobile compression and aspirin following total joint replacement can reduce the occurrence of venous thromboembolism and decrease adverse events, infections, and bleeding complications in patients undergoing total joint replacement,” said Richard Iorio, MD, primary study author and Professor of Orthopedic Surgery at NYU School of Medicine in New York.
The study was designed to determine if utilizing a risk-based venous thromboembolism chemoprophylaxis protocol would improve prevention of deep vein thrombosis and pulmonary embolism, quality metrics, and bleeding-related complications in patients undergoing total joint arthroplasty.
The retrospective review evaluated 2,611 patients that were divided into 2 cohorts. Cohort 1 included 1,203 patients who were previously treated with standard aggressive chemoprophylaxis agents (Enoxaparin, Rivaroxaban, Warfarin). Cohort 2 consisted of a risk-stratified group of patients either undergoing treatment with prophylactic synchronized mobile compression and aspirin (n=843) or aggressive prophylaxis (n=565).
Results demonstrated that patients in the risk-stratified protocol had a lower incidence of venous thromboembolism than the group treated with anticoagulation. Patients in this group also experienced fewer adverse events, readmissions, infections, and bleeding-related complications. Hospital costs were significantly lower in the synchronized mobile compression and aspirin subgroup of cohort 2 and overall costs were lower in the risk-stratified cohort, though they did not reach statistical significance.
“These results are significant in that they represent a large study population of more than 2,600 patients and are the first to demonstrate significant reductions in hospital costs, which support the hypothesis that a risk stratification protocol can advance patient-specific therapy and enhance the delivery of value-based care,” Dr. Iorio said.
Web Page Content and Quality Assessed for Shoulder Replacement
The Internet is becoming a primary source for obtaining medical information. This growing trend may have serious implications for the medical field. As patients increasingly regard the Internet as an essential tool for obtaining health-related information, questions have been raised regarding the quality of medical information available on the Internet.1 Studies have shown that health-related sites often present inaccurate, inconsistent, and outdated information that may have a negative impact on health care decisions made by patients.2
According to the US Census Bureau, 71.7% of American households report having access to the Internet.3 Of those who have access to Internet, approximately 72% have sought health information online over the last year.4 Among people older than age 65 years living in the United States, there has been a growing trend toward using the Internet, from 14% in 2000 to almost 60% in 2013, according to the Pew Research Internet Project.5 Most medical websites are viewed for information on diseases and treatment options.6 Since most patients want to be informed about treatment options, as well as risks and benefits for each treatment, access to credible information is essential for proper decision-making.7
To assess the quality of information on the Internet, we used DISCERN, a standardized questionnaire to aid consumers in judging Internet content.8 The DISCERN instrument, available at www.discern.org.uk, was designed by an expert group in the United Kingdom. First, an expert panel developed and tested the instrument, and then health care providers and self-help group members tested it further.8,9 The questionnaire had been found to have good interrater reliability, regardless of use by health professionals or consumers.8-10
More than 53,000 shoulder arthroplasties are performed in the United States annually, and the number is growing, with the main goal of pain relief from glenohumeral degenerative joint disease.11,12 The Internet has become a quasi–second opinion for patients trying to participate in their care. Given the prevalence of shoulder-related surgeries, it is critical to analyze and become familiar with the quality of information that patients read online in order to direct them to nonbiased, all-inclusive websites. In this study, we provide a summary assessment and comparison of the quality of online information pertaining to shoulder replacement, using medical (total shoulder replacement) and nontechnical (shoulder replacement) search terms.
Methods
Websites were identified using 3 search engines (Google, Yahoo, and Bing) and 2 search terms, shoulder replacement (SR) and total shoulder arthroplasty (TSA), on January 17, 2014. These 3 search engines were used because 77% of health care–related information online searches begin through a search engine (Google, Bing, Yahoo); only 13% begin at a health care–specialized website.4 These search terms were used after consulting with orthopedic residents and attending physicians in a focus group regarding the terminology used with patients. The first 30 websites in each search engine were identified consecutively and evaluated for category and quality of information using the DISCERN instrument.
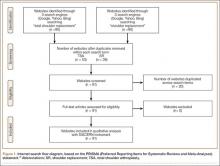
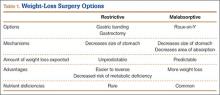
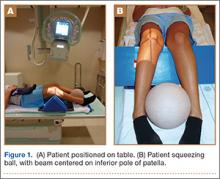
A total of 180 websites (90 per search term) were reviewed. Each website was evaluated independently by 3 medical students. In the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, we recorded how websites were identified, screened, and included (Figure 1).13 Websites that were duplicated within each search term and those that were inaccessible were used to determine the total number of noncommercial versus commercial websites, but were excluded from the final analysis. The first part of the analysis involved determining the type of website (eg, commercial vs noncommercial) based upon the html endings. All .com endings were classified as commercial websites; noncommercial included .gov, .org, .edu, and .net endings. Next, each website was categorized based on the target audience. Websites were grouped into health professional–oriented information, patient-oriented, advertisement, or “other.” These classifications were based on those described in previous works.14,15 The “other” category included images, YouTube videos, another search engine, and open forums, which were also excluded from the final analysis because they were not easily evaluable with the DISCERN instrument. Websites were considered health professional–oriented if they included journal articles, scholarly articles, and/or rehabilitation protocols. Patient-directed websites clearly stated the information was directed to patients or provided a general overview. Advertisement included sites that displayed ads or products for sale. Websites were evaluated for quality using the DISCERN instrument (Figure 2).
DISCERN has 3 subdivision scores: the reliable score (composed of the first 8 questions), the treatment options (the next 7 questions), and 1 final question that addresses the overall quality of the website and is rated independently of the first 15 questions. DISCERN uses 2 scales, a binary scale anchored on both extremes with the number 1 equaling complete absence of the criteria being measured, and the number 5 at the upper extreme, representing completeness of the quality being assessed. In between 1 and 5 is a partial ordinal scale measuring from 2 to 4, which indicates the information is present to some extent but not complete. The ordinal scale allows ranking of the criteria being assessed. Summarizing values from each of the 2 scales poses some concern: the scale is not a true binary scale because of the ordinal scale of the middle numbers (2-4), and as such, is not amenable to being an interval scale to calculate arithmetic means. To summarize the values from the 2 scales, we calculated the harmonic mean, the arithmetic mean, the geometric mean, and the median. The means were empirically compared with the median, and we used the harmonic mean to summarize scale values because it was the best approximation of the medians.
Results
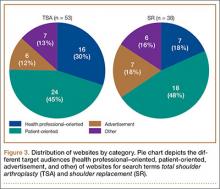
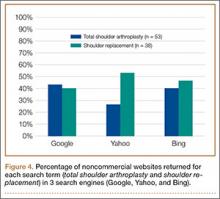
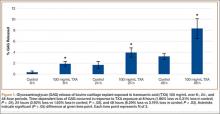
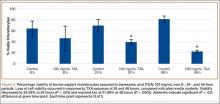
A total of 90 websites were assessed with the search term total shoulder arthroplasty and another 90 with shoulder replacement. When 37 duplicate websites for TSA and 52 for SR were eliminated, 53 (59%) and 38 (42%) unique websites were evaluated for each search term, respectively (Figure 1). (These unique websites are included in the Appendix.) Between the 2 search terms, 20 websites were duplicated. Figure 3 shows the distribution of websites by category. Total shoulder arthroplasty provided the highest percentage of health professional–oriented information; SR had the greatest percentage of patient-oriented information. Both TSA and SR had nearly the same number of advertisements and websites labeled “other.” The percentage of noncommercial websites from each search engine is represented in Figure 4. For SR, Google had 40% (12/30) noncommercial websites compared with Yahoo at 53% (16/30) and Bing at 46% (14/30). Total shoulder arthroplasty had 43% (13/30) noncommercial websites on Google, 27% (8/30) on Yahoo, and 40% (12/30) on Bing. In total, SR had more noncommercial websites, 47% (42/90), compared with 37% (33/90) for TSA.
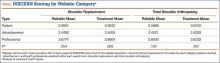
The mean of all 3 raters for reliablity (DISCERN questions 1-8) and treatment options (DISCERN questions 9-15) is represented in the Table. For both search terms, we found that websites identified as health professional–oriented had the highest reliable mean scores, followed by patient-oriented, and advertisement at the lowest (SR: P = .054; TSA: P = .134). For SR, treatment mean scores demonstrated similar results with health professional–oriented websites receiving the highest, followed by patient-oriented and advertisement (P = .005). However, the treatment mean scores for TSA differed with patient-oriented websites receiving higher scores than health professional–oriented websites, but this was not statistically significant (P= .407). Regarding search terms, there were no significant differences between mean reliable and treatment scores across all categories.
The average overall DISCERN score for TSA websites was 2.5 (range, 1-5), compared with 2.3 (range, 1-5) for SR websites. The overall reliable score (DISCERN questions 1-8) for TSA websites was 2.6 and 2.5 for SR websites (P < .001). For TSA websites, 38% (20/53) were classified as good, having an overall DISCERN score ≥3, versus 26% (10/38) of SR websites. The overall DISCERN score for health professional–oriented websites was 2.7, patient-oriented websites received a score of 2.6, and advertisements had the lowest score at 2.4.
Discussion
Both patients and health professionals obtain information on health care subjects through the Internet, which has become the primary resource for patients.15,16 However, there are no strict regulations of the content being written. This creates a challenge for the typical user to find credible and evidence-based information, which is important because misleading information could cause undue anxiety, among other effects.17,18 The aims of this study were to determine the quality of Internet information for shoulder replacement surgeries using the medical terminology total shoulder arthroplasty (TSA) and the nontechnical term shoulder replacement (SR), and to compare the results.
After analyzing the types of websites returned for both total shoulder arthroplasty and shoulder replacement (Figure 4), it was interesting to find that using nonmedical terminology as the search term provided more noncommercial websites compared with total shoulder arthroplasty. Furthermore, Yahoo provided the highest yield of noncommercial websites at 16, with Bing at 14, when using SR as the search term. We believe the increase in noncommercial websites returned for SR was greater than for TSA because SR yielded more patient-oriented websites, which usually had html endings of .edu and .org, as shown in Figure 3 (48% of SR websites offered patient-oriented information).
Although there were more noncommercial websites for SR, the majority of the DISCERN values between the 2 search terms did not differ significantly. This is a direct result of the number of sites (20) that were duplicated across both search terms. However as seen in the Table, TSA had similar reliable mean scores for advertisements and patient-oriented websites but a slightly higher reliable score for health professional–oriented websites. We correlated this with the increased number of health professional–oriented websites returned when using TSA as the search term (Figure 3). The health professional–oriented websites explained their aims and cited their sources more consistently than did patient-oriented sites and advertisements, resulting in higher reliable scores. Although patient-oriented websites frequently lacked citations, they provided information about multiple treatment options, which were more relevant to consumers. This resulted in nearly equivalent reliable scores. Treatment means for advertisements in both SR and TSA were similar. However, treatment means for professional-oriented websites in TSA were lower than those for SR because health professional–oriented websites often were only moderately relevant to consumers, with their focus usually on 1 treatment option or on rehabilitation protocols. Although the DISCERN scores were similar between the search terms, total shoulder arthroplasty provided more websites (20) classified as good—overall DISCERN score, ≥3—than SR did (10). Advertisement websites had similar overall DISCERN scores, which we anticipated because most of the advertisements were duplicated across the search terms.
Using the 2 search terms, academic websites and commercial websites, such as WebMD, consistently received higher reliable and overall DISCERN scores. Advertisement websites, which need to deliver a clear message, frequently scored high on explicitly stating their aims and relevance to consumers, but focused on their products without discussing the benefits of other treatment options. This is significant because Internet search engines, such as Google, offer sponsor links for which organizations pay to appear at the top of the search results. This creates the potential for consumers to receive biased information because most individuals only visit the top 10 websites generated by a search engine.19
We concluded that the quality of online information relating to SR and TSA was highly variable and frequently of moderate-to-poor quality, with most overall DISCERN scores <3. The quality of information found online for this study using the DISCERN instrument is consistent with those studies using DISCERN to evaluate other medical conditions (eg, bunions, chronic pain, general anesthesia, and anterior cruciate ligament reconstruction).2,9,15,19 These studies also concluded that online information varies tremendously in quality and completeness.
This study has several limitations. Websites were searched at a single time point and, because Internet resources are frequently updated, the results of this study could vary. Furthermore, although Google, Yahoo, and Bing are 3 of the most popular search engines, these are not the only resources patients use when searching the Internet for health-related information. Other search engines, such as Pubmed.gov and MSN.com, could provide additional websites for Internet users. Lastly, although DISCERN is validated to address the quality of information available online, it does not evaluate the accuracy of the information.8 Our use of DISCERN involves 2 scales, a binary yes/no (ratings, 1 and 5) and an ordinal scale (ratings, 2-4). As such, a single mean summary statistic cannot be calculated.
Conclusion
The information available on the Internet pertaining to TSA and SR is highly variable and provides mostly moderate-to-poor quality information based on the DISCERN instrument. Many websites failed to describe the benefits and the risks of different treatment options, including nonoperative management. Health care professionals should be aware that patients often refer to the Internet as a primary resource for obtaining medical information. It is important to direct patients to websites that provide accurate information, because patients who educate themselves about their conditions and actively participate in decision-making may have improved health outcomes.20-22 Overall, academic websites and commercial websites, such as WebMD and OrthoInfo, generally had higher DISCERN scores when using either search term. Of major concern is the potential for misleading advertisements or incorrect information that can negatively affect health outcomes. This study found that using nonmedical terminology (SR) provided more noncommercial and patient-oriented websites, especially through Yahoo. This study highlights the need for more comprehensive online information pertaining to shoulder replacement that can better serve as a resource for Internet users.
1. Eysenbach G, Powell J, Kuss O, Sa ER. Empirical studies assessing the quality of health information for consumers on the world wide web: a systematic review. JAMA. 2002;287(20):2691-2700.
2. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the internet. Arthroscopy. 2013;29(6):1095-1100.
3. Computer and internet use in the United States: population characteristics. US Census Bureau website. http://www.census.gov/hhes/computer/. Accessed December 11, 2015.
4. Fox S, Duggan M. Health online 2013. Pew Research Center website. http://pewinternet.org/Reports/2013/Health-online.aspx. Published January 15, 2013. Accessed November 24, 2015.
5. Smith A. Older adults and technology use. Pew Research Center website. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use. Published April 3, 2014. Accessed November 24, 2015.
6. Shuyler KS, Knight KM. What are patients seeking when they turn to the internet? Qualitative content analysis of questions asked by visitors to an orthopaedics web site. J Med Internet Res. 2003;5(4):e24.
7. Meredith P, Emberton M, Wood C, Smith J. Comparison of patients’ needs for information on prostate surgery with printed materials provided by surgeons. Qual Health Care. 1995;4(1):18-23.
8. Charnock D, Shepperd S, Needham G, Gann R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J Epidemiol Community Health. 1999;53(2):105-111.
9. Kaicker J, Debono VB, Dang W, Buckley N, Thabane L. Assessment of the quality and variability of health information on chronic pain websites using the DISCERN instrument. BMC Med. 2010;8(1):59.
10. Griffiths KM, Christensen H. Website quality indicators for consumers. J Med Internet Res. 2005;7(5):e55.
11. Wiater JM. Shoulder joint replacement. American Academy of Orthopedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Updated December 2011. Accessed November 24, 2015.
12. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the united states. J Bone Joint Surg Am. 2011;93(24):2249-2254.
13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94.
14. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Bernstein J, Ahn J, Veillette C. The future of orthopaedic information management. J Bone Joint Surg Am. 2012;94(13):e95.
17. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
18. Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575-580.
19. Chong YM, Fraval A, Chandrananth J, Plunkett V, Tran P. Assessment of the quality of web-based information on bunions. Foot Ankle Int. 2013;34(8):1134-1139.
20. Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care. J Gen Intern Med. 1989;4(6):506-511.
21. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528.
22. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3 suppl):S110-S127.
The Internet is becoming a primary source for obtaining medical information. This growing trend may have serious implications for the medical field. As patients increasingly regard the Internet as an essential tool for obtaining health-related information, questions have been raised regarding the quality of medical information available on the Internet.1 Studies have shown that health-related sites often present inaccurate, inconsistent, and outdated information that may have a negative impact on health care decisions made by patients.2
According to the US Census Bureau, 71.7% of American households report having access to the Internet.3 Of those who have access to Internet, approximately 72% have sought health information online over the last year.4 Among people older than age 65 years living in the United States, there has been a growing trend toward using the Internet, from 14% in 2000 to almost 60% in 2013, according to the Pew Research Internet Project.5 Most medical websites are viewed for information on diseases and treatment options.6 Since most patients want to be informed about treatment options, as well as risks and benefits for each treatment, access to credible information is essential for proper decision-making.7
To assess the quality of information on the Internet, we used DISCERN, a standardized questionnaire to aid consumers in judging Internet content.8 The DISCERN instrument, available at www.discern.org.uk, was designed by an expert group in the United Kingdom. First, an expert panel developed and tested the instrument, and then health care providers and self-help group members tested it further.8,9 The questionnaire had been found to have good interrater reliability, regardless of use by health professionals or consumers.8-10
More than 53,000 shoulder arthroplasties are performed in the United States annually, and the number is growing, with the main goal of pain relief from glenohumeral degenerative joint disease.11,12 The Internet has become a quasi–second opinion for patients trying to participate in their care. Given the prevalence of shoulder-related surgeries, it is critical to analyze and become familiar with the quality of information that patients read online in order to direct them to nonbiased, all-inclusive websites. In this study, we provide a summary assessment and comparison of the quality of online information pertaining to shoulder replacement, using medical (total shoulder replacement) and nontechnical (shoulder replacement) search terms.
Methods
Websites were identified using 3 search engines (Google, Yahoo, and Bing) and 2 search terms, shoulder replacement (SR) and total shoulder arthroplasty (TSA), on January 17, 2014. These 3 search engines were used because 77% of health care–related information online searches begin through a search engine (Google, Bing, Yahoo); only 13% begin at a health care–specialized website.4 These search terms were used after consulting with orthopedic residents and attending physicians in a focus group regarding the terminology used with patients. The first 30 websites in each search engine were identified consecutively and evaluated for category and quality of information using the DISCERN instrument.
A total of 180 websites (90 per search term) were reviewed. Each website was evaluated independently by 3 medical students. In the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, we recorded how websites were identified, screened, and included (Figure 1).13 Websites that were duplicated within each search term and those that were inaccessible were used to determine the total number of noncommercial versus commercial websites, but were excluded from the final analysis. The first part of the analysis involved determining the type of website (eg, commercial vs noncommercial) based upon the html endings. All .com endings were classified as commercial websites; noncommercial included .gov, .org, .edu, and .net endings. Next, each website was categorized based on the target audience. Websites were grouped into health professional–oriented information, patient-oriented, advertisement, or “other.” These classifications were based on those described in previous works.14,15 The “other” category included images, YouTube videos, another search engine, and open forums, which were also excluded from the final analysis because they were not easily evaluable with the DISCERN instrument. Websites were considered health professional–oriented if they included journal articles, scholarly articles, and/or rehabilitation protocols. Patient-directed websites clearly stated the information was directed to patients or provided a general overview. Advertisement included sites that displayed ads or products for sale. Websites were evaluated for quality using the DISCERN instrument (Figure 2).
DISCERN has 3 subdivision scores: the reliable score (composed of the first 8 questions), the treatment options (the next 7 questions), and 1 final question that addresses the overall quality of the website and is rated independently of the first 15 questions. DISCERN uses 2 scales, a binary scale anchored on both extremes with the number 1 equaling complete absence of the criteria being measured, and the number 5 at the upper extreme, representing completeness of the quality being assessed. In between 1 and 5 is a partial ordinal scale measuring from 2 to 4, which indicates the information is present to some extent but not complete. The ordinal scale allows ranking of the criteria being assessed. Summarizing values from each of the 2 scales poses some concern: the scale is not a true binary scale because of the ordinal scale of the middle numbers (2-4), and as such, is not amenable to being an interval scale to calculate arithmetic means. To summarize the values from the 2 scales, we calculated the harmonic mean, the arithmetic mean, the geometric mean, and the median. The means were empirically compared with the median, and we used the harmonic mean to summarize scale values because it was the best approximation of the medians.
Results
A total of 90 websites were assessed with the search term total shoulder arthroplasty and another 90 with shoulder replacement. When 37 duplicate websites for TSA and 52 for SR were eliminated, 53 (59%) and 38 (42%) unique websites were evaluated for each search term, respectively (Figure 1). (These unique websites are included in the Appendix.) Between the 2 search terms, 20 websites were duplicated. Figure 3 shows the distribution of websites by category. Total shoulder arthroplasty provided the highest percentage of health professional–oriented information; SR had the greatest percentage of patient-oriented information. Both TSA and SR had nearly the same number of advertisements and websites labeled “other.” The percentage of noncommercial websites from each search engine is represented in Figure 4. For SR, Google had 40% (12/30) noncommercial websites compared with Yahoo at 53% (16/30) and Bing at 46% (14/30). Total shoulder arthroplasty had 43% (13/30) noncommercial websites on Google, 27% (8/30) on Yahoo, and 40% (12/30) on Bing. In total, SR had more noncommercial websites, 47% (42/90), compared with 37% (33/90) for TSA.
The mean of all 3 raters for reliablity (DISCERN questions 1-8) and treatment options (DISCERN questions 9-15) is represented in the Table. For both search terms, we found that websites identified as health professional–oriented had the highest reliable mean scores, followed by patient-oriented, and advertisement at the lowest (SR: P = .054; TSA: P = .134). For SR, treatment mean scores demonstrated similar results with health professional–oriented websites receiving the highest, followed by patient-oriented and advertisement (P = .005). However, the treatment mean scores for TSA differed with patient-oriented websites receiving higher scores than health professional–oriented websites, but this was not statistically significant (P= .407). Regarding search terms, there were no significant differences between mean reliable and treatment scores across all categories.
The average overall DISCERN score for TSA websites was 2.5 (range, 1-5), compared with 2.3 (range, 1-5) for SR websites. The overall reliable score (DISCERN questions 1-8) for TSA websites was 2.6 and 2.5 for SR websites (P < .001). For TSA websites, 38% (20/53) were classified as good, having an overall DISCERN score ≥3, versus 26% (10/38) of SR websites. The overall DISCERN score for health professional–oriented websites was 2.7, patient-oriented websites received a score of 2.6, and advertisements had the lowest score at 2.4.
Discussion
Both patients and health professionals obtain information on health care subjects through the Internet, which has become the primary resource for patients.15,16 However, there are no strict regulations of the content being written. This creates a challenge for the typical user to find credible and evidence-based information, which is important because misleading information could cause undue anxiety, among other effects.17,18 The aims of this study were to determine the quality of Internet information for shoulder replacement surgeries using the medical terminology total shoulder arthroplasty (TSA) and the nontechnical term shoulder replacement (SR), and to compare the results.
After analyzing the types of websites returned for both total shoulder arthroplasty and shoulder replacement (Figure 4), it was interesting to find that using nonmedical terminology as the search term provided more noncommercial websites compared with total shoulder arthroplasty. Furthermore, Yahoo provided the highest yield of noncommercial websites at 16, with Bing at 14, when using SR as the search term. We believe the increase in noncommercial websites returned for SR was greater than for TSA because SR yielded more patient-oriented websites, which usually had html endings of .edu and .org, as shown in Figure 3 (48% of SR websites offered patient-oriented information).
Although there were more noncommercial websites for SR, the majority of the DISCERN values between the 2 search terms did not differ significantly. This is a direct result of the number of sites (20) that were duplicated across both search terms. However as seen in the Table, TSA had similar reliable mean scores for advertisements and patient-oriented websites but a slightly higher reliable score for health professional–oriented websites. We correlated this with the increased number of health professional–oriented websites returned when using TSA as the search term (Figure 3). The health professional–oriented websites explained their aims and cited their sources more consistently than did patient-oriented sites and advertisements, resulting in higher reliable scores. Although patient-oriented websites frequently lacked citations, they provided information about multiple treatment options, which were more relevant to consumers. This resulted in nearly equivalent reliable scores. Treatment means for advertisements in both SR and TSA were similar. However, treatment means for professional-oriented websites in TSA were lower than those for SR because health professional–oriented websites often were only moderately relevant to consumers, with their focus usually on 1 treatment option or on rehabilitation protocols. Although the DISCERN scores were similar between the search terms, total shoulder arthroplasty provided more websites (20) classified as good—overall DISCERN score, ≥3—than SR did (10). Advertisement websites had similar overall DISCERN scores, which we anticipated because most of the advertisements were duplicated across the search terms.
Using the 2 search terms, academic websites and commercial websites, such as WebMD, consistently received higher reliable and overall DISCERN scores. Advertisement websites, which need to deliver a clear message, frequently scored high on explicitly stating their aims and relevance to consumers, but focused on their products without discussing the benefits of other treatment options. This is significant because Internet search engines, such as Google, offer sponsor links for which organizations pay to appear at the top of the search results. This creates the potential for consumers to receive biased information because most individuals only visit the top 10 websites generated by a search engine.19
We concluded that the quality of online information relating to SR and TSA was highly variable and frequently of moderate-to-poor quality, with most overall DISCERN scores <3. The quality of information found online for this study using the DISCERN instrument is consistent with those studies using DISCERN to evaluate other medical conditions (eg, bunions, chronic pain, general anesthesia, and anterior cruciate ligament reconstruction).2,9,15,19 These studies also concluded that online information varies tremendously in quality and completeness.
This study has several limitations. Websites were searched at a single time point and, because Internet resources are frequently updated, the results of this study could vary. Furthermore, although Google, Yahoo, and Bing are 3 of the most popular search engines, these are not the only resources patients use when searching the Internet for health-related information. Other search engines, such as Pubmed.gov and MSN.com, could provide additional websites for Internet users. Lastly, although DISCERN is validated to address the quality of information available online, it does not evaluate the accuracy of the information.8 Our use of DISCERN involves 2 scales, a binary yes/no (ratings, 1 and 5) and an ordinal scale (ratings, 2-4). As such, a single mean summary statistic cannot be calculated.
Conclusion
The information available on the Internet pertaining to TSA and SR is highly variable and provides mostly moderate-to-poor quality information based on the DISCERN instrument. Many websites failed to describe the benefits and the risks of different treatment options, including nonoperative management. Health care professionals should be aware that patients often refer to the Internet as a primary resource for obtaining medical information. It is important to direct patients to websites that provide accurate information, because patients who educate themselves about their conditions and actively participate in decision-making may have improved health outcomes.20-22 Overall, academic websites and commercial websites, such as WebMD and OrthoInfo, generally had higher DISCERN scores when using either search term. Of major concern is the potential for misleading advertisements or incorrect information that can negatively affect health outcomes. This study found that using nonmedical terminology (SR) provided more noncommercial and patient-oriented websites, especially through Yahoo. This study highlights the need for more comprehensive online information pertaining to shoulder replacement that can better serve as a resource for Internet users.
The Internet is becoming a primary source for obtaining medical information. This growing trend may have serious implications for the medical field. As patients increasingly regard the Internet as an essential tool for obtaining health-related information, questions have been raised regarding the quality of medical information available on the Internet.1 Studies have shown that health-related sites often present inaccurate, inconsistent, and outdated information that may have a negative impact on health care decisions made by patients.2
According to the US Census Bureau, 71.7% of American households report having access to the Internet.3 Of those who have access to Internet, approximately 72% have sought health information online over the last year.4 Among people older than age 65 years living in the United States, there has been a growing trend toward using the Internet, from 14% in 2000 to almost 60% in 2013, according to the Pew Research Internet Project.5 Most medical websites are viewed for information on diseases and treatment options.6 Since most patients want to be informed about treatment options, as well as risks and benefits for each treatment, access to credible information is essential for proper decision-making.7
To assess the quality of information on the Internet, we used DISCERN, a standardized questionnaire to aid consumers in judging Internet content.8 The DISCERN instrument, available at www.discern.org.uk, was designed by an expert group in the United Kingdom. First, an expert panel developed and tested the instrument, and then health care providers and self-help group members tested it further.8,9 The questionnaire had been found to have good interrater reliability, regardless of use by health professionals or consumers.8-10
More than 53,000 shoulder arthroplasties are performed in the United States annually, and the number is growing, with the main goal of pain relief from glenohumeral degenerative joint disease.11,12 The Internet has become a quasi–second opinion for patients trying to participate in their care. Given the prevalence of shoulder-related surgeries, it is critical to analyze and become familiar with the quality of information that patients read online in order to direct them to nonbiased, all-inclusive websites. In this study, we provide a summary assessment and comparison of the quality of online information pertaining to shoulder replacement, using medical (total shoulder replacement) and nontechnical (shoulder replacement) search terms.
Methods
Websites were identified using 3 search engines (Google, Yahoo, and Bing) and 2 search terms, shoulder replacement (SR) and total shoulder arthroplasty (TSA), on January 17, 2014. These 3 search engines were used because 77% of health care–related information online searches begin through a search engine (Google, Bing, Yahoo); only 13% begin at a health care–specialized website.4 These search terms were used after consulting with orthopedic residents and attending physicians in a focus group regarding the terminology used with patients. The first 30 websites in each search engine were identified consecutively and evaluated for category and quality of information using the DISCERN instrument.
A total of 180 websites (90 per search term) were reviewed. Each website was evaluated independently by 3 medical students. In the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, we recorded how websites were identified, screened, and included (Figure 1).13 Websites that were duplicated within each search term and those that were inaccessible were used to determine the total number of noncommercial versus commercial websites, but were excluded from the final analysis. The first part of the analysis involved determining the type of website (eg, commercial vs noncommercial) based upon the html endings. All .com endings were classified as commercial websites; noncommercial included .gov, .org, .edu, and .net endings. Next, each website was categorized based on the target audience. Websites were grouped into health professional–oriented information, patient-oriented, advertisement, or “other.” These classifications were based on those described in previous works.14,15 The “other” category included images, YouTube videos, another search engine, and open forums, which were also excluded from the final analysis because they were not easily evaluable with the DISCERN instrument. Websites were considered health professional–oriented if they included journal articles, scholarly articles, and/or rehabilitation protocols. Patient-directed websites clearly stated the information was directed to patients or provided a general overview. Advertisement included sites that displayed ads or products for sale. Websites were evaluated for quality using the DISCERN instrument (Figure 2).
DISCERN has 3 subdivision scores: the reliable score (composed of the first 8 questions), the treatment options (the next 7 questions), and 1 final question that addresses the overall quality of the website and is rated independently of the first 15 questions. DISCERN uses 2 scales, a binary scale anchored on both extremes with the number 1 equaling complete absence of the criteria being measured, and the number 5 at the upper extreme, representing completeness of the quality being assessed. In between 1 and 5 is a partial ordinal scale measuring from 2 to 4, which indicates the information is present to some extent but not complete. The ordinal scale allows ranking of the criteria being assessed. Summarizing values from each of the 2 scales poses some concern: the scale is not a true binary scale because of the ordinal scale of the middle numbers (2-4), and as such, is not amenable to being an interval scale to calculate arithmetic means. To summarize the values from the 2 scales, we calculated the harmonic mean, the arithmetic mean, the geometric mean, and the median. The means were empirically compared with the median, and we used the harmonic mean to summarize scale values because it was the best approximation of the medians.
Results
A total of 90 websites were assessed with the search term total shoulder arthroplasty and another 90 with shoulder replacement. When 37 duplicate websites for TSA and 52 for SR were eliminated, 53 (59%) and 38 (42%) unique websites were evaluated for each search term, respectively (Figure 1). (These unique websites are included in the Appendix.) Between the 2 search terms, 20 websites were duplicated. Figure 3 shows the distribution of websites by category. Total shoulder arthroplasty provided the highest percentage of health professional–oriented information; SR had the greatest percentage of patient-oriented information. Both TSA and SR had nearly the same number of advertisements and websites labeled “other.” The percentage of noncommercial websites from each search engine is represented in Figure 4. For SR, Google had 40% (12/30) noncommercial websites compared with Yahoo at 53% (16/30) and Bing at 46% (14/30). Total shoulder arthroplasty had 43% (13/30) noncommercial websites on Google, 27% (8/30) on Yahoo, and 40% (12/30) on Bing. In total, SR had more noncommercial websites, 47% (42/90), compared with 37% (33/90) for TSA.
The mean of all 3 raters for reliablity (DISCERN questions 1-8) and treatment options (DISCERN questions 9-15) is represented in the Table. For both search terms, we found that websites identified as health professional–oriented had the highest reliable mean scores, followed by patient-oriented, and advertisement at the lowest (SR: P = .054; TSA: P = .134). For SR, treatment mean scores demonstrated similar results with health professional–oriented websites receiving the highest, followed by patient-oriented and advertisement (P = .005). However, the treatment mean scores for TSA differed with patient-oriented websites receiving higher scores than health professional–oriented websites, but this was not statistically significant (P= .407). Regarding search terms, there were no significant differences between mean reliable and treatment scores across all categories.
The average overall DISCERN score for TSA websites was 2.5 (range, 1-5), compared with 2.3 (range, 1-5) for SR websites. The overall reliable score (DISCERN questions 1-8) for TSA websites was 2.6 and 2.5 for SR websites (P < .001). For TSA websites, 38% (20/53) were classified as good, having an overall DISCERN score ≥3, versus 26% (10/38) of SR websites. The overall DISCERN score for health professional–oriented websites was 2.7, patient-oriented websites received a score of 2.6, and advertisements had the lowest score at 2.4.
Discussion
Both patients and health professionals obtain information on health care subjects through the Internet, which has become the primary resource for patients.15,16 However, there are no strict regulations of the content being written. This creates a challenge for the typical user to find credible and evidence-based information, which is important because misleading information could cause undue anxiety, among other effects.17,18 The aims of this study were to determine the quality of Internet information for shoulder replacement surgeries using the medical terminology total shoulder arthroplasty (TSA) and the nontechnical term shoulder replacement (SR), and to compare the results.
After analyzing the types of websites returned for both total shoulder arthroplasty and shoulder replacement (Figure 4), it was interesting to find that using nonmedical terminology as the search term provided more noncommercial websites compared with total shoulder arthroplasty. Furthermore, Yahoo provided the highest yield of noncommercial websites at 16, with Bing at 14, when using SR as the search term. We believe the increase in noncommercial websites returned for SR was greater than for TSA because SR yielded more patient-oriented websites, which usually had html endings of .edu and .org, as shown in Figure 3 (48% of SR websites offered patient-oriented information).
Although there were more noncommercial websites for SR, the majority of the DISCERN values between the 2 search terms did not differ significantly. This is a direct result of the number of sites (20) that were duplicated across both search terms. However as seen in the Table, TSA had similar reliable mean scores for advertisements and patient-oriented websites but a slightly higher reliable score for health professional–oriented websites. We correlated this with the increased number of health professional–oriented websites returned when using TSA as the search term (Figure 3). The health professional–oriented websites explained their aims and cited their sources more consistently than did patient-oriented sites and advertisements, resulting in higher reliable scores. Although patient-oriented websites frequently lacked citations, they provided information about multiple treatment options, which were more relevant to consumers. This resulted in nearly equivalent reliable scores. Treatment means for advertisements in both SR and TSA were similar. However, treatment means for professional-oriented websites in TSA were lower than those for SR because health professional–oriented websites often were only moderately relevant to consumers, with their focus usually on 1 treatment option or on rehabilitation protocols. Although the DISCERN scores were similar between the search terms, total shoulder arthroplasty provided more websites (20) classified as good—overall DISCERN score, ≥3—than SR did (10). Advertisement websites had similar overall DISCERN scores, which we anticipated because most of the advertisements were duplicated across the search terms.
Using the 2 search terms, academic websites and commercial websites, such as WebMD, consistently received higher reliable and overall DISCERN scores. Advertisement websites, which need to deliver a clear message, frequently scored high on explicitly stating their aims and relevance to consumers, but focused on their products without discussing the benefits of other treatment options. This is significant because Internet search engines, such as Google, offer sponsor links for which organizations pay to appear at the top of the search results. This creates the potential for consumers to receive biased information because most individuals only visit the top 10 websites generated by a search engine.19
We concluded that the quality of online information relating to SR and TSA was highly variable and frequently of moderate-to-poor quality, with most overall DISCERN scores <3. The quality of information found online for this study using the DISCERN instrument is consistent with those studies using DISCERN to evaluate other medical conditions (eg, bunions, chronic pain, general anesthesia, and anterior cruciate ligament reconstruction).2,9,15,19 These studies also concluded that online information varies tremendously in quality and completeness.
This study has several limitations. Websites were searched at a single time point and, because Internet resources are frequently updated, the results of this study could vary. Furthermore, although Google, Yahoo, and Bing are 3 of the most popular search engines, these are not the only resources patients use when searching the Internet for health-related information. Other search engines, such as Pubmed.gov and MSN.com, could provide additional websites for Internet users. Lastly, although DISCERN is validated to address the quality of information available online, it does not evaluate the accuracy of the information.8 Our use of DISCERN involves 2 scales, a binary yes/no (ratings, 1 and 5) and an ordinal scale (ratings, 2-4). As such, a single mean summary statistic cannot be calculated.
Conclusion
The information available on the Internet pertaining to TSA and SR is highly variable and provides mostly moderate-to-poor quality information based on the DISCERN instrument. Many websites failed to describe the benefits and the risks of different treatment options, including nonoperative management. Health care professionals should be aware that patients often refer to the Internet as a primary resource for obtaining medical information. It is important to direct patients to websites that provide accurate information, because patients who educate themselves about their conditions and actively participate in decision-making may have improved health outcomes.20-22 Overall, academic websites and commercial websites, such as WebMD and OrthoInfo, generally had higher DISCERN scores when using either search term. Of major concern is the potential for misleading advertisements or incorrect information that can negatively affect health outcomes. This study found that using nonmedical terminology (SR) provided more noncommercial and patient-oriented websites, especially through Yahoo. This study highlights the need for more comprehensive online information pertaining to shoulder replacement that can better serve as a resource for Internet users.
1. Eysenbach G, Powell J, Kuss O, Sa ER. Empirical studies assessing the quality of health information for consumers on the world wide web: a systematic review. JAMA. 2002;287(20):2691-2700.
2. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the internet. Arthroscopy. 2013;29(6):1095-1100.
3. Computer and internet use in the United States: population characteristics. US Census Bureau website. http://www.census.gov/hhes/computer/. Accessed December 11, 2015.
4. Fox S, Duggan M. Health online 2013. Pew Research Center website. http://pewinternet.org/Reports/2013/Health-online.aspx. Published January 15, 2013. Accessed November 24, 2015.
5. Smith A. Older adults and technology use. Pew Research Center website. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use. Published April 3, 2014. Accessed November 24, 2015.
6. Shuyler KS, Knight KM. What are patients seeking when they turn to the internet? Qualitative content analysis of questions asked by visitors to an orthopaedics web site. J Med Internet Res. 2003;5(4):e24.
7. Meredith P, Emberton M, Wood C, Smith J. Comparison of patients’ needs for information on prostate surgery with printed materials provided by surgeons. Qual Health Care. 1995;4(1):18-23.
8. Charnock D, Shepperd S, Needham G, Gann R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J Epidemiol Community Health. 1999;53(2):105-111.
9. Kaicker J, Debono VB, Dang W, Buckley N, Thabane L. Assessment of the quality and variability of health information on chronic pain websites using the DISCERN instrument. BMC Med. 2010;8(1):59.
10. Griffiths KM, Christensen H. Website quality indicators for consumers. J Med Internet Res. 2005;7(5):e55.
11. Wiater JM. Shoulder joint replacement. American Academy of Orthopedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Updated December 2011. Accessed November 24, 2015.
12. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the united states. J Bone Joint Surg Am. 2011;93(24):2249-2254.
13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94.
14. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Bernstein J, Ahn J, Veillette C. The future of orthopaedic information management. J Bone Joint Surg Am. 2012;94(13):e95.
17. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
18. Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575-580.
19. Chong YM, Fraval A, Chandrananth J, Plunkett V, Tran P. Assessment of the quality of web-based information on bunions. Foot Ankle Int. 2013;34(8):1134-1139.
20. Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care. J Gen Intern Med. 1989;4(6):506-511.
21. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528.
22. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3 suppl):S110-S127.
1. Eysenbach G, Powell J, Kuss O, Sa ER. Empirical studies assessing the quality of health information for consumers on the world wide web: a systematic review. JAMA. 2002;287(20):2691-2700.
2. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the internet. Arthroscopy. 2013;29(6):1095-1100.
3. Computer and internet use in the United States: population characteristics. US Census Bureau website. http://www.census.gov/hhes/computer/. Accessed December 11, 2015.
4. Fox S, Duggan M. Health online 2013. Pew Research Center website. http://pewinternet.org/Reports/2013/Health-online.aspx. Published January 15, 2013. Accessed November 24, 2015.
5. Smith A. Older adults and technology use. Pew Research Center website. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use. Published April 3, 2014. Accessed November 24, 2015.
6. Shuyler KS, Knight KM. What are patients seeking when they turn to the internet? Qualitative content analysis of questions asked by visitors to an orthopaedics web site. J Med Internet Res. 2003;5(4):e24.
7. Meredith P, Emberton M, Wood C, Smith J. Comparison of patients’ needs for information on prostate surgery with printed materials provided by surgeons. Qual Health Care. 1995;4(1):18-23.
8. Charnock D, Shepperd S, Needham G, Gann R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J Epidemiol Community Health. 1999;53(2):105-111.
9. Kaicker J, Debono VB, Dang W, Buckley N, Thabane L. Assessment of the quality and variability of health information on chronic pain websites using the DISCERN instrument. BMC Med. 2010;8(1):59.
10. Griffiths KM, Christensen H. Website quality indicators for consumers. J Med Internet Res. 2005;7(5):e55.
11. Wiater JM. Shoulder joint replacement. American Academy of Orthopedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Updated December 2011. Accessed November 24, 2015.
12. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the united states. J Bone Joint Surg Am. 2011;93(24):2249-2254.
13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94.
14. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Bernstein J, Ahn J, Veillette C. The future of orthopaedic information management. J Bone Joint Surg Am. 2012;94(13):e95.
17. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
18. Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575-580.
19. Chong YM, Fraval A, Chandrananth J, Plunkett V, Tran P. Assessment of the quality of web-based information on bunions. Foot Ankle Int. 2013;34(8):1134-1139.
20. Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care. J Gen Intern Med. 1989;4(6):506-511.
21. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528.
22. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3 suppl):S110-S127.
Incidence, Risk Factors, and Outcome Trends of Acute Kidney Injury in Elective Total Hip and Knee Arthroplasty
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
A Bariatric Surgery Primer for Orthopedic Surgeons
An estimated 220,000 bariatric surgeries are performed annually in the United States and Canada, and 344,221 procedures worldwide.1 Not only are orthopedic surgeons seeing more patients who have had bariatric surgery, they are also referring morbidly obese patients to bariatric surgeons before elective procedures.2 Patients with body mass index (BMI) over 40 kg/m2 are candidates for surgical treatment of obesity. Comorbid conditions directly related to obesity, including diabetes, respiratory insufficiency, and pseudotumor cerebri, decrease the BMI of eligibility to 35 kg/m2. Other considerations are failure of nonsurgical weight-loss methods, such as dietary programs for weight reduction, behavioral modification programs, and pharmacotherapy. Patients’ psychological stability is extremely important given the rigorous dietary changes required after surgery.3 Although weight-loss surgery can eliminate many of the complications of obesity, bariatric patients even with weight loss have increased operative and postoperative risks, likely because of alterations in nutrient absorption. Knowledge of the pathophysiology associated with bariatric surgery can assist orthopedic surgeons in optimizing medical and surgical management of patients’ musculoskeletal issues.
Bariatric Surgery
Surgically induced weight loss works by reducing quantity of food consumed and absorption of calories. Jejunoileal bypass, one of the first procedures used, significantly decreased the absorptive area for nutrients, which led to complications such as diarrhea, cirrhosis, and nephrolithiasis.4 This surgery is no longer performed, and current procedures try to minimize the risks of malabsorption.5
The 2 types of bariatric surgeries now available in the United States are gastroplasty and gastric bypass, both of which are performed laparoscopically.6 Gastroplasties are purely restrictive procedures, which reduce stomach volume. In gastric banding, the most common gastroplasty, a silicone band is placed around the proximal stomach to create a 15-mL pouch in the cardia. Sleeve gastrectomy also reduces stomach volume, to about 25%, by stapling along the greater curvature. In both procedures, consumed calories are restricted, but the gastrointestinal tract is left in continuity, and essential nutrients are properly absorbed.7 However, failure rates are higher, and weight loss more variable, than with gastric bypass procedures.8
Gastric bypass uses both restriction and malabsorption to increase weight loss.7 A gastric pouch (15-30 mL) is created by stapling across the cardia of the stomach. The jejunum is then divided, and the distal portion of the divided jejunum anastomosed to the small proximal stomach pouch. This creates the roux limb where food passes. The duodenum is excluded, and the proximal portion of the jejunum is attached to the roux limb to provide a conduit for biliary and pancreatic digestive secretions. Weight loss is caused by both reduction in stomach size and malabsorption of calories owing to the diversion of digestive enzymes and the decrease in absorptive surface area. Only 28% of ingested fat and 57% of ingested protein are absorbed9 (Table 1).
Metabolic Consequences
Nutrient deficiencies are seen more often in the malabsorptive procedures; however, patients with restrictive procedures may have poor eating habits and are therefore also at risk.10 In fact, many patients have nutritional deficiencies predating their bariatric surgery, as obesity creates a chronic inflammatory state that leads to anemia of chronic disease. Schweiger and colleagues11 assessed the nutritional status of bariatric surgery candidates and noted a high incidence of iron and folic acid deficiencies with corresponding anemia. They concluded these deficiencies stemmed from calorie-dense diets high in carbohydrates and fats. Although patients may improve their diet after surgery with concomitant nutritional counseling, deficiencies in iron, calcium, vitamin B12, folate, and vitamin D are common12 (Table 2).
Iron deficiency continues after bariatric surgery because dietary iron must be converted to its absorbable form by hydrochloric acid secreted from the stomach. As stomach volume is reduced, there is a corresponding decrease in acid secretion. The result is that iron deficiency occurs in both restrictive and malabsorptive procedures.13 Moreover, with the diversion from the duodenum and the proximal jejunum in bypass surgery, the main areas of absorption are excluded.10 Patients may require intravenous therapy for iron-deficiency anemia—or oral supplementation combined with ascorbic acid to increase stomach acidity.
As calcium is absorbed mainly in the duodenum and the jejunum, patients who undergo malabsorptive procedures can absorb only 20% of the amount ingested.14 Restrictive procedures do not have the same effect on calcium absorption; however, patients may have reduced dietary lactose intake and be at risk for deficiency.
A study by Ducloux and colleagues15 found that 96% of bariatric surgery patients had vitamin D deficiency before the procedure. After malabsorptive procedures, the decrease in bile salts leads to an inability to break down fat-soluble vitamins and to uncoordinated mixing of food and bile secretions.16 Restrictive procedures do not carry this risk, though many patients still require supplementation because of their underlying deficiency.
The decrease in stomach size causes a decrease in intrinsic factor from parietal cells, with subsequent inability to appropriately transport vitamin B12. Exclusion of the duodenum also eliminates the site of absorption; therefore, B12 should be replaced orally.11 Megaloblastic anemia is a rarely reported sequela.17,18 Folate deficiency is less common because it can take place in the entire intestine after surgery, even though absorption occurs primarily in the proximal portion of the small intestine.10
Protein deficiency can result in loss of muscle mass and subsequent muscle weakness, edema, and anomalies of the skin, mucosa, and nails.12 It is seen after both types of procedures because of decreased dietary intake from intolerance. Malabsorptive procedures also decrease pepsinogen secretion and reduce the intestinal absorption surface.
Considerations for Orthopedic Surgeons
Wound Healing
Much of our knowledge of the effects of bariatric surgery on skin and wound healing has been gleaned from samples obtained from patients during abdominoplasty or other body-contouring procedures. These samples have all shown a decrease in hydroxyproline, the major constituent of collagen and the main factor in determining the tensile strength of a wound.19 D’Ettorre and colleagues20 performed biopsies of abdominal skin before and after biliopancreatic diversion and noted that tissue proteins, including hydroxyproline, were significantly reduced. Histologic examination revealed disorganized dermal elastic and collagen fibers. In addition, all patients involved in the study had wound-healing problems, with delayed healing of 25 days, compared with 12 days in nonbariatric patients. Deficiencies in vitamins B12, D, and E, as well as folate and total tissue protein, were implicated as causative factors.
Effects on Bone
Malabsorptive procedures decrease bone mineral density (BMD) through their effects on calcium and vitamin D. BMD is also decreased because these procedures lower levels of plasma leptin and ghrelin, increase adiponectin, and reduce estrogen in women.21 The BMD decline correlates with amount of weight lost.22 This complication is not seen in restrictive procedures, even though patients may have decreased calcium and vitamin D levels.23 The exact effect on BMD and on subsequent risk for osteopenia and osteoporosis is difficult to quantify, as obese patients have higher BMD than age-matched controls do, because of increased mechanical loading. In a prospective study, Vilarrasa and colleagues24 found a 10.9% decrease in femoral neck BMD in women 1 year after Roux-en-Y with 34% weight loss, despite supplementation with 800 IU of vitamin D and 1200 mg of calcium daily.
Fracture Healing
Although BMD is decreased in patients after gastric bypass surgery, there has been only 1 recorded case of fracture nonunion after bariatric surgery—involving a distal radius fracture in a patient who had undergone jejunoileal bypass surgery.25 Hypovitaminosis has a detrimental effect on bone repair and BMD, increasing the risk for fracture from minor trauma; however, delayed union and nonunion have not been reported as consequences.26
Pharmacology
Both restrictive and malabsorptive procedures decrease drug bioavailabilty from tablet preparations by shortening the surface area available for absorption and diminishing stomach acidity.27 These consequences pose a problem particularly for extended-release formulations, as these formulations are not given enough time to dissolve and reach therapeutic concentrations.28 Also affected is warfarin, which requires a larger dose to maintain therapeutic international normalized ratio. Antibiotics may have reduced bioavailability because of decreased transit time. Therefore, liquid preparations are preferred, as they need not be dissolved.
As there is no reported change in intravenous bioavailability with preoperative and postoperative antimicrobial prophylaxis, this is the preferred administration method.29 However, obese patients in general may have altered pharmacokinetics, including increased glomerular filtration rate, and in most cases they should be treated with higher levels of antibiotics.30
Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided in all patients. The acidic composition of NSAIDs causes direct injury to the gastric pouch. NSAIDs also injure the gastrointestinal lining by inhibiting prostaglandin synthesis, which thins the mucosa. In turn, erosions and ulcers may form in the epithelial layer.31 Acetaminophen or a centrally acting agent (eg, tramadol) is recommended instead. Aspirin has a chemical structure similar to that of NSAIDs and should not be used either. Alendronate causes esophageal ulceration; however, no such complication has been reported with teriparatide32 (Table 3).
Preoperative Evaluation
As already discussed, patients who undergo weight-loss surgery are at higher risk for wound-healing complications because of nutritional deficiencies. Total protein, albumin, and prealbumin levels and total lymphocyte count should be used to identify protein deficiency, which can decrease the likelihood of organized collagen formation. Huang and colleagues33 noted a statistically significant increase in complications after total knee arthroplasty (TKA) in patients with a prealbumin level under 3.5 mg/dL or a transferrin level under 200 mg/dL. Rates of prosthetic joint infection and development of hematoma or seroma requiring operative management were much higher, as were rates of postoperative neurovascular, renal, and cardiovascular complications.
Serum levels of vitamin A, folate, vitamin B12, and vitamin C should also be ordered, as many patients are deficient. Transferrin levels should be checked before surgery, as iron-deficiency anemia is common. Naghshineh and colleagues34 noted an anecdotal decrease in wound-healing complications in body-contouring surgery after correction of subclinical and clinical deficiencies in protein, arginine, glutamine, vitamin A, vitamin B12, vitamin C, folate, thiamine, iron, zinc, and selenium. Zinc deficiency was similarly implicated in wound-healing complications by Zorrilla and colleagues,35,36 who found a statistically significant delay in wound healing in patients with serum zinc levels under 95 mg/dL after total hip arthroplasty (THA)35 and hip hemiarthroplasty.36 To facilitate bone healing, physicians should give patients a thorough workup of levels of serum and urine calcium, 24-hydroxyvitamin D, and alkaline phosphatase. Osteomalacia typically presents with high alkaline phosphatase levels37 and secondary hyperparathyroidism. Therefore, physicians should monitor for these conditions. Although nonunion and aseptic loosening have not been reported as consequences of bariatric surgery, bone health should nevertheless be optimized when possible (Table 4).
Elective Orthopedic Surgery
Little is known about the true effect of weight-loss surgery on subsequent orthopedic procedures. Few investigators have explored the effect of surgery on arthrodesis, and the only recommendation for orthopedic surgeons is to be prepared for poor bone healing and the possibility of nonunion.38 Hidalgo and colleagues39 studied laparoscopic sleeve gastrectomy performed a minimum of 6 months before another elective surgery. Two patients had lumbar laminectomies, 2 had lumbar discectomies, 1 had a cervical discectomy, and 1 had a rotator cuff repair. By most recent follow-up, there were no complications of any of the orthopedic procedures, and all patients had healed.
There is no recommended amount of time between bariatric surgery and elective orthopedic surgery. Maximum weight loss and stabilization are typically achieved 2 years after surgery.40 However, elective orthopedic surgery has been performed as early as 6 months after bariatric surgery. Inacio and colleagues41 studied 3 groups of patients who underwent total joint arthroplasty (TJA): those who had it less than 2 years after bariatric surgery, those who had it more than 2 years after bariatric surgery, and those who were obese but did not have bariatric surgery. Complications of TJA occurred within the first year in 2.9% of the patients who had it more than 2 years after bariatric surgery, in 5.9% of the patients who had it less than 2 years after bariatric surgery, and in 4.1% of the patients who did not undergo bariatric surgery. Similarly, Severson and colleagues2 evaluated intraoperative and postoperative complications of TKA in 3 groups of obese patients: those who had TKA before bariatric surgery, those who had TKA less than 2 years after bariatric surgery, and those who had TKA more than 2 years after bariatric surgery. Gastroplasty and bypass patients were included. BMI was statistically significantly higher in the preoperative group than in the other 2 groups, though mean BMI for all groups was above 35 kg/m2. Operative time and tourniquet time were reduced in patients who had TKA more than 2 years after bariatric surgery, but there was no significant difference in anesthesia time. There was also no difference in 90-day complication rates between patients who had TKA before bariatric surgery and those who had it afterward. Severson and colleagues2 recommended communicating the risks to all obese patients, whether they undergo weight-loss surgery or not.
Arthroplasty
Obese patients have a higher rate of complications after arthroplasty—hence the referrals to bariatric surgeons. Bariatric surgery and its associated weight loss might improve joint pain and delay the need for arthroplasty in some cases.42 Obese people are prone to joint degeneration from the excess weight, and from altered gait patterns (eg, exaggerated step width, slower walking speed, increased time in double-limb stance).43 Gait changes are reversible after weight loss.44 Hooper and colleagues45 found a 37% decrease in lower extremity complaints after surgical weight loss, even though mean BMI at final follow-up was still in the obese range.
Obesity itself is a risk factor for ligamentous instability, but it is unclear whether the risk is mitigated by bariatric surgery. Disruption of the anterior fibers of the medial collateral ligament is more common in obese patients, as are complications involving the extensor mechanism (eg, patellofemoral dislocation). As a result, slower postoperative rehabilitation is recommended.46 Although there is no recorded link between bariatric surgery and the development of ligamentous laxity, surgeons should be aware of the potential for medial collateral ligament avulsion in obese and formerly obese patients and have appropriate implants available.
Kulkarni and colleagues47 compared the rates of hip and knee arthroplasty complications in patients who were obese before bariatric surgery and patients who were still obese after bariatric surgery. Gastroplasty and bypass patients were included. Data on superficial wound infections were excluded; however, the bariatric surgery group’s deep wound infection rate was 3.5 times lower, and its 30-day readmission rate was 7 times lower. There was no difference in dislocation and hip revision rates at 1 year. Although 1 patient in the bariatric surgery group died of an unknown cause 9 days after surgery, Kulkarni and colleagues47 concluded it is safer to operate on obese patients after versus before bariatric surgery. However, their study did not include mean BMI, so no conclusion can be drawn about the risk of operating on patients who were still obese after bariatric surgery.
Studies of weight loss in primary TJA patients have had conflicting findings.48 Trofa and colleagues49 reported that 15 patients who underwent arthroplasty a mean of 42.4 months after bariatric surgery lost 27.9% more of their original BMI compared with patients who underwent bariatric surgery but not arthroplasty. This relationship between arthroplasty and weight loss was strongest in patients who underwent knee arthroplasty, with an average of 43.9% more BMI lost compared to patients who did not undergo TKA. There was no significant change in BMI in patients who underwent THA and bariatric surgery compared with patients who underwent bariatric surgery but not THA.
Parvizi and colleagues50 assessed the results of 20 arthroplasties (8 THAs, 12 TKAs) performed in 14 patients a mean of 23 months after bariatric surgery (2 gastroplasties, 12 bypass surgeries). Mean BMI was 29 kg/m2. At final follow-up, 1 patient required revision THA for aseptic loosening, but all the others showed no evidence of radiographic loosening or wear. One patient had a superficial wound infection, and 1 had a deep wound infection. Parvizi and colleagues50 reported that arthroplasty after bariatric surgery is a viable option and is preferable to operating on morbidly obese patients.
Summary
Orthopedic surgeons are increasingly performing elective hip and knee arthroplasties on patients who have undergone bariatric surgery. Although bariatric surgery may alleviate some of the complications associated with surgery on morbidly obese patients, it should be approached with caution. Studies have shown that bariatric surgery patients are at increased risk for wound-healing and other complications, often caused by unrecognized preoperative nutrient deficiencies. In addition, patients are often unable to tolerate commonly used medications. The exact timing of bariatric surgery relative to elective orthopedic procedures is unclear. Surgeons should perform a preoperative evaluation based on type of bariatric surgery in order to reduce the likelihood of adverse events. Such preemptive therapy may improve the short- and long-term results of major reconstructive surgery. Further research is needed to determine the true effect of bariatric surgery on orthopedic procedures.
1. Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19(12):1605-1611.
2. Severson EP, Singh JA, Browne JA, Trousdale RT, Sarr MG, Lewallen DG. Total knee arthroplasty in morbidly obese patients treated with bariatric surgery. J Arthroplasty. 2012;27(9):1696-1700.
3. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient [published correction appears in Endocr Pract. 2009;15(7):768]. Endocr Pract. 2008;14(suppl 1):1-83.
4. Hocking MP, Duerson MC, O’Leary JP, Woodward ER. Jejunoilial bypass for morbid obesity. Late follow-up in 100 cases. N Engl J Med. 1983;308(17):995-999.
5. DeMaria EJ. Morbid obesity. In: Mulholland MW, Lillemoe KD, Doherty GM, et al, eds. Greenfield’s Surgery: Scientific Principles & Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:736-743.
6. O’Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol. 2010;25(8):1358-1365.
7. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737.
8. DeMaria EJ, Sugerman HJ, Meador JG, et al. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233(6):809-818.
9. Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8(1):48-55.
10. Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004;7(5):569-575.
11. Schweiger C, Weiss R, Berry E, Keidar A. Nutritional deficiencies in bariatric surgery candidates. Obes Surg. 2010;20(2):193-197.
12. Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33(1):13-24.
13. Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)—a prospective study. Obes Surg. 2010;20(4):447-453.
14. Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12(1):40-47.
15. Ducloux R, Nobécourt E, Chevallier JM, Ducloux H, Elian N, Altman JJ. Vitamin D deficiency before bariatric surgery: should supplement intake be routinely prescribed? Obes Surg. 2011;21(5):556-560.
16. Wang A, Powell A. The effects of obesity surgery on bone metabolism: what orthopedic surgeons need to know. Am J Orthop. 2009;38(2):77-79.
17. Baghdasarian KL. Gastric bypass and megaloblastic anemia. J Am Diet Assoc. 1982;80(4):368-371.
18. Crowley LV, Olson RW. Megaloblastic anemia after gastric bypass for obesity. Am J Gastroenterol. 1983;78(7):406-410.
19. Sorg H, Schulz T, Krueger C, Vollmar B. Consequences of surgical stress on the kinetics of skin wound healing: partial hepatectomy delays and functionally alters dermal repair. Wound Repair Regen. 2009;17(3):367-377.
20. D’Ettorre M, Gniuli D, Iaconelli A, Massi G, Mingrone G, Bracaglia R. Wound healing process in post-bariatric patients: an experimental evaluation. Obes Surg. 2010;20(11):1552-1558.
21. Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19(1);41-46.
22. Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735-3740.
23. von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53(7):918-921.
24. Vilarrasa N, Gómez JM, Elio I, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009;19(7):860-866.
25. Hey H, Lund B, Sørensen OH, Lund B. Delayed fracture healing following jejunoileal bypass surgery for obesity. Calcif Tissue Int. 1982;34(1):13-15.
26. Borrelli J Jr, Pape C, Hak D, et al. Physiological challenges of bone repair. J Orthop Trauma. 2012;26(12):708-711.
27. Sardo P, Walker JH. Bariatric surgery: impact on medication management. Hosp Pharm. 2008;43(2):113-120.
28. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
29. Chopra T, Zhao JJ, Alangaden G, Wood MH, Kaye KS. Preventing surgical site infections after bariatric surgery: value of perioperative antibiotic regimens. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):317-328.
30. Payne KD, Hall RG 2nd. Dosing of antibacterial agents in obese adults: does one size fit all? Expert Rev Anti Infect Ther. 2014;12(7):829-854.
31. Sasse KC, Ganser J, Kozar M, et al. Seven cases of gastric perforation in Roux-en-Y gastric bypass patients: what lessons can we learn? Obes Surg. 2008;18(5):530-534.
32. Miller AD, Smith KM. Medication use in bariatric surgery patients: what orthopedists need to know. Orthopedics. 2006;29(2):121-123.
33. Huang R, Greenky M, Kerr GJ, Austin MS, Parvizi J. The effect of malnutrition on patients undergoing elective joint arthroplasty. J Arthroplasty. 2013;28(8 suppl):21-24.
34. Naghshineh N, O’Brien Coon D, McTigue K, Courcoulas AP, Fernstrom M, Rubin JP. Nutritional assessment of bariatric surgery patients presenting for plastic surgery: a prospective analysis. Plast Reconstr Surg. 2010;126(2):602-610.
35. Zorrilla P, Gómez LA, Salido JA, Silva A, López-Alonso A. Low serum zinc level as a predictive factor of delayed wound healing in total hip replacement. Wound Repair Regen. 2006;14(2):119-122.
36. Zorrilla P, Salido JA, López-Alonso A, Silva A. Serum zinc as a prognostic tool for wound healing in hip hemiarthroplasty. Clin Orthop Relat Res. 2004;(420):304-308.
37. Williams SE, Cooper K, Richmond B, Schauer P. Perioperative management of bariatric surgery patients: focus on metabolic bone disease. Cleve Clin J Med. 2008;75(5):333-349.
38. Kini S, Kannan U. Effect of bariatric surgery on future general surgical procedures. J Minim Access Surg. 2011;7(2):126-131.
39. Hidalgo JE, Roy M, Ramirez A, Szomstein S, Rosenthal RJ. Laparoscopic sleeve gastrectomy: a first step for rapid weight loss in morbidly obese patients requiring a second non-bariatric procedure. Obes Surg. 2012;22(4):555-559.
40. O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032-1040.
41. Inacio MC, Paxton EW, Fisher D, Li RA, Barber TC, Singh JA. Bariatric surgery prior to total joint arthroplasty may not provide dramatic improvements in post-arthroplasty surgical outcomes. J Arthroplasty. 2014;29(7):1359-1364.
42. Gill RS, Al‐Adra DP, Shi X, Sharma AM, Birch DW, Karmali S. The benefits of bariatric surgery in obese patients with hip and knee osteoarthritis: a systematic review. Obes Rev. 2011;12(12):1083-1089.
43. Vartiainen P, Bragge T, Lyytinen T, Hakkarainen M, Karjalainen PA, Arokoski JP. Kinematic and kinetic changes in obese gait in bariatric surgery–induced weight loss. J Biomech. 2012;45(10):1769-1774.
44. Vincent HK, Ben-David K, Conrad BP, Lamb KM, Seay AN, Vincent KR. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346-354.
45. Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes. 2007;31(1):114-120.
46. Booth RE Jr. Total knee arthroplasty in the obese patient: tips and quips. J Arthroplasty. 2002;17(4 suppl 1):69-70.
47. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
48. Inacio MC, Silverstein DK, Raman R, et al. Weight patterns before and after total joint arthroplasty and characteristics associated with weight change. Perm J. 2014;18(1):25-31.
49. Trofa D, Smith EL, Shah V, Shikora S. Total weight loss associated with increased physical activity after bariatric surgery may increase the need for total joint arthroplasty. Surg Obes Relat Dis. 2014;10(2):335-339.
50. Parvizi J, Trousdale RT, Sarr MG. Total joint arthroplasty in patients surgically treated for morbid obesity. J Arthroplasty. 2000;15(8):1003-1008.
An estimated 220,000 bariatric surgeries are performed annually in the United States and Canada, and 344,221 procedures worldwide.1 Not only are orthopedic surgeons seeing more patients who have had bariatric surgery, they are also referring morbidly obese patients to bariatric surgeons before elective procedures.2 Patients with body mass index (BMI) over 40 kg/m2 are candidates for surgical treatment of obesity. Comorbid conditions directly related to obesity, including diabetes, respiratory insufficiency, and pseudotumor cerebri, decrease the BMI of eligibility to 35 kg/m2. Other considerations are failure of nonsurgical weight-loss methods, such as dietary programs for weight reduction, behavioral modification programs, and pharmacotherapy. Patients’ psychological stability is extremely important given the rigorous dietary changes required after surgery.3 Although weight-loss surgery can eliminate many of the complications of obesity, bariatric patients even with weight loss have increased operative and postoperative risks, likely because of alterations in nutrient absorption. Knowledge of the pathophysiology associated with bariatric surgery can assist orthopedic surgeons in optimizing medical and surgical management of patients’ musculoskeletal issues.
Bariatric Surgery
Surgically induced weight loss works by reducing quantity of food consumed and absorption of calories. Jejunoileal bypass, one of the first procedures used, significantly decreased the absorptive area for nutrients, which led to complications such as diarrhea, cirrhosis, and nephrolithiasis.4 This surgery is no longer performed, and current procedures try to minimize the risks of malabsorption.5
The 2 types of bariatric surgeries now available in the United States are gastroplasty and gastric bypass, both of which are performed laparoscopically.6 Gastroplasties are purely restrictive procedures, which reduce stomach volume. In gastric banding, the most common gastroplasty, a silicone band is placed around the proximal stomach to create a 15-mL pouch in the cardia. Sleeve gastrectomy also reduces stomach volume, to about 25%, by stapling along the greater curvature. In both procedures, consumed calories are restricted, but the gastrointestinal tract is left in continuity, and essential nutrients are properly absorbed.7 However, failure rates are higher, and weight loss more variable, than with gastric bypass procedures.8
Gastric bypass uses both restriction and malabsorption to increase weight loss.7 A gastric pouch (15-30 mL) is created by stapling across the cardia of the stomach. The jejunum is then divided, and the distal portion of the divided jejunum anastomosed to the small proximal stomach pouch. This creates the roux limb where food passes. The duodenum is excluded, and the proximal portion of the jejunum is attached to the roux limb to provide a conduit for biliary and pancreatic digestive secretions. Weight loss is caused by both reduction in stomach size and malabsorption of calories owing to the diversion of digestive enzymes and the decrease in absorptive surface area. Only 28% of ingested fat and 57% of ingested protein are absorbed9 (Table 1).
Metabolic Consequences
Nutrient deficiencies are seen more often in the malabsorptive procedures; however, patients with restrictive procedures may have poor eating habits and are therefore also at risk.10 In fact, many patients have nutritional deficiencies predating their bariatric surgery, as obesity creates a chronic inflammatory state that leads to anemia of chronic disease. Schweiger and colleagues11 assessed the nutritional status of bariatric surgery candidates and noted a high incidence of iron and folic acid deficiencies with corresponding anemia. They concluded these deficiencies stemmed from calorie-dense diets high in carbohydrates and fats. Although patients may improve their diet after surgery with concomitant nutritional counseling, deficiencies in iron, calcium, vitamin B12, folate, and vitamin D are common12 (Table 2).
Iron deficiency continues after bariatric surgery because dietary iron must be converted to its absorbable form by hydrochloric acid secreted from the stomach. As stomach volume is reduced, there is a corresponding decrease in acid secretion. The result is that iron deficiency occurs in both restrictive and malabsorptive procedures.13 Moreover, with the diversion from the duodenum and the proximal jejunum in bypass surgery, the main areas of absorption are excluded.10 Patients may require intravenous therapy for iron-deficiency anemia—or oral supplementation combined with ascorbic acid to increase stomach acidity.
As calcium is absorbed mainly in the duodenum and the jejunum, patients who undergo malabsorptive procedures can absorb only 20% of the amount ingested.14 Restrictive procedures do not have the same effect on calcium absorption; however, patients may have reduced dietary lactose intake and be at risk for deficiency.
A study by Ducloux and colleagues15 found that 96% of bariatric surgery patients had vitamin D deficiency before the procedure. After malabsorptive procedures, the decrease in bile salts leads to an inability to break down fat-soluble vitamins and to uncoordinated mixing of food and bile secretions.16 Restrictive procedures do not carry this risk, though many patients still require supplementation because of their underlying deficiency.
The decrease in stomach size causes a decrease in intrinsic factor from parietal cells, with subsequent inability to appropriately transport vitamin B12. Exclusion of the duodenum also eliminates the site of absorption; therefore, B12 should be replaced orally.11 Megaloblastic anemia is a rarely reported sequela.17,18 Folate deficiency is less common because it can take place in the entire intestine after surgery, even though absorption occurs primarily in the proximal portion of the small intestine.10
Protein deficiency can result in loss of muscle mass and subsequent muscle weakness, edema, and anomalies of the skin, mucosa, and nails.12 It is seen after both types of procedures because of decreased dietary intake from intolerance. Malabsorptive procedures also decrease pepsinogen secretion and reduce the intestinal absorption surface.
Considerations for Orthopedic Surgeons
Wound Healing
Much of our knowledge of the effects of bariatric surgery on skin and wound healing has been gleaned from samples obtained from patients during abdominoplasty or other body-contouring procedures. These samples have all shown a decrease in hydroxyproline, the major constituent of collagen and the main factor in determining the tensile strength of a wound.19 D’Ettorre and colleagues20 performed biopsies of abdominal skin before and after biliopancreatic diversion and noted that tissue proteins, including hydroxyproline, were significantly reduced. Histologic examination revealed disorganized dermal elastic and collagen fibers. In addition, all patients involved in the study had wound-healing problems, with delayed healing of 25 days, compared with 12 days in nonbariatric patients. Deficiencies in vitamins B12, D, and E, as well as folate and total tissue protein, were implicated as causative factors.
Effects on Bone
Malabsorptive procedures decrease bone mineral density (BMD) through their effects on calcium and vitamin D. BMD is also decreased because these procedures lower levels of plasma leptin and ghrelin, increase adiponectin, and reduce estrogen in women.21 The BMD decline correlates with amount of weight lost.22 This complication is not seen in restrictive procedures, even though patients may have decreased calcium and vitamin D levels.23 The exact effect on BMD and on subsequent risk for osteopenia and osteoporosis is difficult to quantify, as obese patients have higher BMD than age-matched controls do, because of increased mechanical loading. In a prospective study, Vilarrasa and colleagues24 found a 10.9% decrease in femoral neck BMD in women 1 year after Roux-en-Y with 34% weight loss, despite supplementation with 800 IU of vitamin D and 1200 mg of calcium daily.
Fracture Healing
Although BMD is decreased in patients after gastric bypass surgery, there has been only 1 recorded case of fracture nonunion after bariatric surgery—involving a distal radius fracture in a patient who had undergone jejunoileal bypass surgery.25 Hypovitaminosis has a detrimental effect on bone repair and BMD, increasing the risk for fracture from minor trauma; however, delayed union and nonunion have not been reported as consequences.26
Pharmacology
Both restrictive and malabsorptive procedures decrease drug bioavailabilty from tablet preparations by shortening the surface area available for absorption and diminishing stomach acidity.27 These consequences pose a problem particularly for extended-release formulations, as these formulations are not given enough time to dissolve and reach therapeutic concentrations.28 Also affected is warfarin, which requires a larger dose to maintain therapeutic international normalized ratio. Antibiotics may have reduced bioavailability because of decreased transit time. Therefore, liquid preparations are preferred, as they need not be dissolved.
As there is no reported change in intravenous bioavailability with preoperative and postoperative antimicrobial prophylaxis, this is the preferred administration method.29 However, obese patients in general may have altered pharmacokinetics, including increased glomerular filtration rate, and in most cases they should be treated with higher levels of antibiotics.30
Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided in all patients. The acidic composition of NSAIDs causes direct injury to the gastric pouch. NSAIDs also injure the gastrointestinal lining by inhibiting prostaglandin synthesis, which thins the mucosa. In turn, erosions and ulcers may form in the epithelial layer.31 Acetaminophen or a centrally acting agent (eg, tramadol) is recommended instead. Aspirin has a chemical structure similar to that of NSAIDs and should not be used either. Alendronate causes esophageal ulceration; however, no such complication has been reported with teriparatide32 (Table 3).
Preoperative Evaluation
As already discussed, patients who undergo weight-loss surgery are at higher risk for wound-healing complications because of nutritional deficiencies. Total protein, albumin, and prealbumin levels and total lymphocyte count should be used to identify protein deficiency, which can decrease the likelihood of organized collagen formation. Huang and colleagues33 noted a statistically significant increase in complications after total knee arthroplasty (TKA) in patients with a prealbumin level under 3.5 mg/dL or a transferrin level under 200 mg/dL. Rates of prosthetic joint infection and development of hematoma or seroma requiring operative management were much higher, as were rates of postoperative neurovascular, renal, and cardiovascular complications.
Serum levels of vitamin A, folate, vitamin B12, and vitamin C should also be ordered, as many patients are deficient. Transferrin levels should be checked before surgery, as iron-deficiency anemia is common. Naghshineh and colleagues34 noted an anecdotal decrease in wound-healing complications in body-contouring surgery after correction of subclinical and clinical deficiencies in protein, arginine, glutamine, vitamin A, vitamin B12, vitamin C, folate, thiamine, iron, zinc, and selenium. Zinc deficiency was similarly implicated in wound-healing complications by Zorrilla and colleagues,35,36 who found a statistically significant delay in wound healing in patients with serum zinc levels under 95 mg/dL after total hip arthroplasty (THA)35 and hip hemiarthroplasty.36 To facilitate bone healing, physicians should give patients a thorough workup of levels of serum and urine calcium, 24-hydroxyvitamin D, and alkaline phosphatase. Osteomalacia typically presents with high alkaline phosphatase levels37 and secondary hyperparathyroidism. Therefore, physicians should monitor for these conditions. Although nonunion and aseptic loosening have not been reported as consequences of bariatric surgery, bone health should nevertheless be optimized when possible (Table 4).
Elective Orthopedic Surgery
Little is known about the true effect of weight-loss surgery on subsequent orthopedic procedures. Few investigators have explored the effect of surgery on arthrodesis, and the only recommendation for orthopedic surgeons is to be prepared for poor bone healing and the possibility of nonunion.38 Hidalgo and colleagues39 studied laparoscopic sleeve gastrectomy performed a minimum of 6 months before another elective surgery. Two patients had lumbar laminectomies, 2 had lumbar discectomies, 1 had a cervical discectomy, and 1 had a rotator cuff repair. By most recent follow-up, there were no complications of any of the orthopedic procedures, and all patients had healed.
There is no recommended amount of time between bariatric surgery and elective orthopedic surgery. Maximum weight loss and stabilization are typically achieved 2 years after surgery.40 However, elective orthopedic surgery has been performed as early as 6 months after bariatric surgery. Inacio and colleagues41 studied 3 groups of patients who underwent total joint arthroplasty (TJA): those who had it less than 2 years after bariatric surgery, those who had it more than 2 years after bariatric surgery, and those who were obese but did not have bariatric surgery. Complications of TJA occurred within the first year in 2.9% of the patients who had it more than 2 years after bariatric surgery, in 5.9% of the patients who had it less than 2 years after bariatric surgery, and in 4.1% of the patients who did not undergo bariatric surgery. Similarly, Severson and colleagues2 evaluated intraoperative and postoperative complications of TKA in 3 groups of obese patients: those who had TKA before bariatric surgery, those who had TKA less than 2 years after bariatric surgery, and those who had TKA more than 2 years after bariatric surgery. Gastroplasty and bypass patients were included. BMI was statistically significantly higher in the preoperative group than in the other 2 groups, though mean BMI for all groups was above 35 kg/m2. Operative time and tourniquet time were reduced in patients who had TKA more than 2 years after bariatric surgery, but there was no significant difference in anesthesia time. There was also no difference in 90-day complication rates between patients who had TKA before bariatric surgery and those who had it afterward. Severson and colleagues2 recommended communicating the risks to all obese patients, whether they undergo weight-loss surgery or not.
Arthroplasty
Obese patients have a higher rate of complications after arthroplasty—hence the referrals to bariatric surgeons. Bariatric surgery and its associated weight loss might improve joint pain and delay the need for arthroplasty in some cases.42 Obese people are prone to joint degeneration from the excess weight, and from altered gait patterns (eg, exaggerated step width, slower walking speed, increased time in double-limb stance).43 Gait changes are reversible after weight loss.44 Hooper and colleagues45 found a 37% decrease in lower extremity complaints after surgical weight loss, even though mean BMI at final follow-up was still in the obese range.
Obesity itself is a risk factor for ligamentous instability, but it is unclear whether the risk is mitigated by bariatric surgery. Disruption of the anterior fibers of the medial collateral ligament is more common in obese patients, as are complications involving the extensor mechanism (eg, patellofemoral dislocation). As a result, slower postoperative rehabilitation is recommended.46 Although there is no recorded link between bariatric surgery and the development of ligamentous laxity, surgeons should be aware of the potential for medial collateral ligament avulsion in obese and formerly obese patients and have appropriate implants available.
Kulkarni and colleagues47 compared the rates of hip and knee arthroplasty complications in patients who were obese before bariatric surgery and patients who were still obese after bariatric surgery. Gastroplasty and bypass patients were included. Data on superficial wound infections were excluded; however, the bariatric surgery group’s deep wound infection rate was 3.5 times lower, and its 30-day readmission rate was 7 times lower. There was no difference in dislocation and hip revision rates at 1 year. Although 1 patient in the bariatric surgery group died of an unknown cause 9 days after surgery, Kulkarni and colleagues47 concluded it is safer to operate on obese patients after versus before bariatric surgery. However, their study did not include mean BMI, so no conclusion can be drawn about the risk of operating on patients who were still obese after bariatric surgery.
Studies of weight loss in primary TJA patients have had conflicting findings.48 Trofa and colleagues49 reported that 15 patients who underwent arthroplasty a mean of 42.4 months after bariatric surgery lost 27.9% more of their original BMI compared with patients who underwent bariatric surgery but not arthroplasty. This relationship between arthroplasty and weight loss was strongest in patients who underwent knee arthroplasty, with an average of 43.9% more BMI lost compared to patients who did not undergo TKA. There was no significant change in BMI in patients who underwent THA and bariatric surgery compared with patients who underwent bariatric surgery but not THA.
Parvizi and colleagues50 assessed the results of 20 arthroplasties (8 THAs, 12 TKAs) performed in 14 patients a mean of 23 months after bariatric surgery (2 gastroplasties, 12 bypass surgeries). Mean BMI was 29 kg/m2. At final follow-up, 1 patient required revision THA for aseptic loosening, but all the others showed no evidence of radiographic loosening or wear. One patient had a superficial wound infection, and 1 had a deep wound infection. Parvizi and colleagues50 reported that arthroplasty after bariatric surgery is a viable option and is preferable to operating on morbidly obese patients.
Summary
Orthopedic surgeons are increasingly performing elective hip and knee arthroplasties on patients who have undergone bariatric surgery. Although bariatric surgery may alleviate some of the complications associated with surgery on morbidly obese patients, it should be approached with caution. Studies have shown that bariatric surgery patients are at increased risk for wound-healing and other complications, often caused by unrecognized preoperative nutrient deficiencies. In addition, patients are often unable to tolerate commonly used medications. The exact timing of bariatric surgery relative to elective orthopedic procedures is unclear. Surgeons should perform a preoperative evaluation based on type of bariatric surgery in order to reduce the likelihood of adverse events. Such preemptive therapy may improve the short- and long-term results of major reconstructive surgery. Further research is needed to determine the true effect of bariatric surgery on orthopedic procedures.
An estimated 220,000 bariatric surgeries are performed annually in the United States and Canada, and 344,221 procedures worldwide.1 Not only are orthopedic surgeons seeing more patients who have had bariatric surgery, they are also referring morbidly obese patients to bariatric surgeons before elective procedures.2 Patients with body mass index (BMI) over 40 kg/m2 are candidates for surgical treatment of obesity. Comorbid conditions directly related to obesity, including diabetes, respiratory insufficiency, and pseudotumor cerebri, decrease the BMI of eligibility to 35 kg/m2. Other considerations are failure of nonsurgical weight-loss methods, such as dietary programs for weight reduction, behavioral modification programs, and pharmacotherapy. Patients’ psychological stability is extremely important given the rigorous dietary changes required after surgery.3 Although weight-loss surgery can eliminate many of the complications of obesity, bariatric patients even with weight loss have increased operative and postoperative risks, likely because of alterations in nutrient absorption. Knowledge of the pathophysiology associated with bariatric surgery can assist orthopedic surgeons in optimizing medical and surgical management of patients’ musculoskeletal issues.
Bariatric Surgery
Surgically induced weight loss works by reducing quantity of food consumed and absorption of calories. Jejunoileal bypass, one of the first procedures used, significantly decreased the absorptive area for nutrients, which led to complications such as diarrhea, cirrhosis, and nephrolithiasis.4 This surgery is no longer performed, and current procedures try to minimize the risks of malabsorption.5
The 2 types of bariatric surgeries now available in the United States are gastroplasty and gastric bypass, both of which are performed laparoscopically.6 Gastroplasties are purely restrictive procedures, which reduce stomach volume. In gastric banding, the most common gastroplasty, a silicone band is placed around the proximal stomach to create a 15-mL pouch in the cardia. Sleeve gastrectomy also reduces stomach volume, to about 25%, by stapling along the greater curvature. In both procedures, consumed calories are restricted, but the gastrointestinal tract is left in continuity, and essential nutrients are properly absorbed.7 However, failure rates are higher, and weight loss more variable, than with gastric bypass procedures.8
Gastric bypass uses both restriction and malabsorption to increase weight loss.7 A gastric pouch (15-30 mL) is created by stapling across the cardia of the stomach. The jejunum is then divided, and the distal portion of the divided jejunum anastomosed to the small proximal stomach pouch. This creates the roux limb where food passes. The duodenum is excluded, and the proximal portion of the jejunum is attached to the roux limb to provide a conduit for biliary and pancreatic digestive secretions. Weight loss is caused by both reduction in stomach size and malabsorption of calories owing to the diversion of digestive enzymes and the decrease in absorptive surface area. Only 28% of ingested fat and 57% of ingested protein are absorbed9 (Table 1).
Metabolic Consequences
Nutrient deficiencies are seen more often in the malabsorptive procedures; however, patients with restrictive procedures may have poor eating habits and are therefore also at risk.10 In fact, many patients have nutritional deficiencies predating their bariatric surgery, as obesity creates a chronic inflammatory state that leads to anemia of chronic disease. Schweiger and colleagues11 assessed the nutritional status of bariatric surgery candidates and noted a high incidence of iron and folic acid deficiencies with corresponding anemia. They concluded these deficiencies stemmed from calorie-dense diets high in carbohydrates and fats. Although patients may improve their diet after surgery with concomitant nutritional counseling, deficiencies in iron, calcium, vitamin B12, folate, and vitamin D are common12 (Table 2).
Iron deficiency continues after bariatric surgery because dietary iron must be converted to its absorbable form by hydrochloric acid secreted from the stomach. As stomach volume is reduced, there is a corresponding decrease in acid secretion. The result is that iron deficiency occurs in both restrictive and malabsorptive procedures.13 Moreover, with the diversion from the duodenum and the proximal jejunum in bypass surgery, the main areas of absorption are excluded.10 Patients may require intravenous therapy for iron-deficiency anemia—or oral supplementation combined with ascorbic acid to increase stomach acidity.
As calcium is absorbed mainly in the duodenum and the jejunum, patients who undergo malabsorptive procedures can absorb only 20% of the amount ingested.14 Restrictive procedures do not have the same effect on calcium absorption; however, patients may have reduced dietary lactose intake and be at risk for deficiency.
A study by Ducloux and colleagues15 found that 96% of bariatric surgery patients had vitamin D deficiency before the procedure. After malabsorptive procedures, the decrease in bile salts leads to an inability to break down fat-soluble vitamins and to uncoordinated mixing of food and bile secretions.16 Restrictive procedures do not carry this risk, though many patients still require supplementation because of their underlying deficiency.
The decrease in stomach size causes a decrease in intrinsic factor from parietal cells, with subsequent inability to appropriately transport vitamin B12. Exclusion of the duodenum also eliminates the site of absorption; therefore, B12 should be replaced orally.11 Megaloblastic anemia is a rarely reported sequela.17,18 Folate deficiency is less common because it can take place in the entire intestine after surgery, even though absorption occurs primarily in the proximal portion of the small intestine.10
Protein deficiency can result in loss of muscle mass and subsequent muscle weakness, edema, and anomalies of the skin, mucosa, and nails.12 It is seen after both types of procedures because of decreased dietary intake from intolerance. Malabsorptive procedures also decrease pepsinogen secretion and reduce the intestinal absorption surface.
Considerations for Orthopedic Surgeons
Wound Healing
Much of our knowledge of the effects of bariatric surgery on skin and wound healing has been gleaned from samples obtained from patients during abdominoplasty or other body-contouring procedures. These samples have all shown a decrease in hydroxyproline, the major constituent of collagen and the main factor in determining the tensile strength of a wound.19 D’Ettorre and colleagues20 performed biopsies of abdominal skin before and after biliopancreatic diversion and noted that tissue proteins, including hydroxyproline, were significantly reduced. Histologic examination revealed disorganized dermal elastic and collagen fibers. In addition, all patients involved in the study had wound-healing problems, with delayed healing of 25 days, compared with 12 days in nonbariatric patients. Deficiencies in vitamins B12, D, and E, as well as folate and total tissue protein, were implicated as causative factors.
Effects on Bone
Malabsorptive procedures decrease bone mineral density (BMD) through their effects on calcium and vitamin D. BMD is also decreased because these procedures lower levels of plasma leptin and ghrelin, increase adiponectin, and reduce estrogen in women.21 The BMD decline correlates with amount of weight lost.22 This complication is not seen in restrictive procedures, even though patients may have decreased calcium and vitamin D levels.23 The exact effect on BMD and on subsequent risk for osteopenia and osteoporosis is difficult to quantify, as obese patients have higher BMD than age-matched controls do, because of increased mechanical loading. In a prospective study, Vilarrasa and colleagues24 found a 10.9% decrease in femoral neck BMD in women 1 year after Roux-en-Y with 34% weight loss, despite supplementation with 800 IU of vitamin D and 1200 mg of calcium daily.
Fracture Healing
Although BMD is decreased in patients after gastric bypass surgery, there has been only 1 recorded case of fracture nonunion after bariatric surgery—involving a distal radius fracture in a patient who had undergone jejunoileal bypass surgery.25 Hypovitaminosis has a detrimental effect on bone repair and BMD, increasing the risk for fracture from minor trauma; however, delayed union and nonunion have not been reported as consequences.26
Pharmacology
Both restrictive and malabsorptive procedures decrease drug bioavailabilty from tablet preparations by shortening the surface area available for absorption and diminishing stomach acidity.27 These consequences pose a problem particularly for extended-release formulations, as these formulations are not given enough time to dissolve and reach therapeutic concentrations.28 Also affected is warfarin, which requires a larger dose to maintain therapeutic international normalized ratio. Antibiotics may have reduced bioavailability because of decreased transit time. Therefore, liquid preparations are preferred, as they need not be dissolved.
As there is no reported change in intravenous bioavailability with preoperative and postoperative antimicrobial prophylaxis, this is the preferred administration method.29 However, obese patients in general may have altered pharmacokinetics, including increased glomerular filtration rate, and in most cases they should be treated with higher levels of antibiotics.30
Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided in all patients. The acidic composition of NSAIDs causes direct injury to the gastric pouch. NSAIDs also injure the gastrointestinal lining by inhibiting prostaglandin synthesis, which thins the mucosa. In turn, erosions and ulcers may form in the epithelial layer.31 Acetaminophen or a centrally acting agent (eg, tramadol) is recommended instead. Aspirin has a chemical structure similar to that of NSAIDs and should not be used either. Alendronate causes esophageal ulceration; however, no such complication has been reported with teriparatide32 (Table 3).
Preoperative Evaluation
As already discussed, patients who undergo weight-loss surgery are at higher risk for wound-healing complications because of nutritional deficiencies. Total protein, albumin, and prealbumin levels and total lymphocyte count should be used to identify protein deficiency, which can decrease the likelihood of organized collagen formation. Huang and colleagues33 noted a statistically significant increase in complications after total knee arthroplasty (TKA) in patients with a prealbumin level under 3.5 mg/dL or a transferrin level under 200 mg/dL. Rates of prosthetic joint infection and development of hematoma or seroma requiring operative management were much higher, as were rates of postoperative neurovascular, renal, and cardiovascular complications.
Serum levels of vitamin A, folate, vitamin B12, and vitamin C should also be ordered, as many patients are deficient. Transferrin levels should be checked before surgery, as iron-deficiency anemia is common. Naghshineh and colleagues34 noted an anecdotal decrease in wound-healing complications in body-contouring surgery after correction of subclinical and clinical deficiencies in protein, arginine, glutamine, vitamin A, vitamin B12, vitamin C, folate, thiamine, iron, zinc, and selenium. Zinc deficiency was similarly implicated in wound-healing complications by Zorrilla and colleagues,35,36 who found a statistically significant delay in wound healing in patients with serum zinc levels under 95 mg/dL after total hip arthroplasty (THA)35 and hip hemiarthroplasty.36 To facilitate bone healing, physicians should give patients a thorough workup of levels of serum and urine calcium, 24-hydroxyvitamin D, and alkaline phosphatase. Osteomalacia typically presents with high alkaline phosphatase levels37 and secondary hyperparathyroidism. Therefore, physicians should monitor for these conditions. Although nonunion and aseptic loosening have not been reported as consequences of bariatric surgery, bone health should nevertheless be optimized when possible (Table 4).
Elective Orthopedic Surgery
Little is known about the true effect of weight-loss surgery on subsequent orthopedic procedures. Few investigators have explored the effect of surgery on arthrodesis, and the only recommendation for orthopedic surgeons is to be prepared for poor bone healing and the possibility of nonunion.38 Hidalgo and colleagues39 studied laparoscopic sleeve gastrectomy performed a minimum of 6 months before another elective surgery. Two patients had lumbar laminectomies, 2 had lumbar discectomies, 1 had a cervical discectomy, and 1 had a rotator cuff repair. By most recent follow-up, there were no complications of any of the orthopedic procedures, and all patients had healed.
There is no recommended amount of time between bariatric surgery and elective orthopedic surgery. Maximum weight loss and stabilization are typically achieved 2 years after surgery.40 However, elective orthopedic surgery has been performed as early as 6 months after bariatric surgery. Inacio and colleagues41 studied 3 groups of patients who underwent total joint arthroplasty (TJA): those who had it less than 2 years after bariatric surgery, those who had it more than 2 years after bariatric surgery, and those who were obese but did not have bariatric surgery. Complications of TJA occurred within the first year in 2.9% of the patients who had it more than 2 years after bariatric surgery, in 5.9% of the patients who had it less than 2 years after bariatric surgery, and in 4.1% of the patients who did not undergo bariatric surgery. Similarly, Severson and colleagues2 evaluated intraoperative and postoperative complications of TKA in 3 groups of obese patients: those who had TKA before bariatric surgery, those who had TKA less than 2 years after bariatric surgery, and those who had TKA more than 2 years after bariatric surgery. Gastroplasty and bypass patients were included. BMI was statistically significantly higher in the preoperative group than in the other 2 groups, though mean BMI for all groups was above 35 kg/m2. Operative time and tourniquet time were reduced in patients who had TKA more than 2 years after bariatric surgery, but there was no significant difference in anesthesia time. There was also no difference in 90-day complication rates between patients who had TKA before bariatric surgery and those who had it afterward. Severson and colleagues2 recommended communicating the risks to all obese patients, whether they undergo weight-loss surgery or not.
Arthroplasty
Obese patients have a higher rate of complications after arthroplasty—hence the referrals to bariatric surgeons. Bariatric surgery and its associated weight loss might improve joint pain and delay the need for arthroplasty in some cases.42 Obese people are prone to joint degeneration from the excess weight, and from altered gait patterns (eg, exaggerated step width, slower walking speed, increased time in double-limb stance).43 Gait changes are reversible after weight loss.44 Hooper and colleagues45 found a 37% decrease in lower extremity complaints after surgical weight loss, even though mean BMI at final follow-up was still in the obese range.
Obesity itself is a risk factor for ligamentous instability, but it is unclear whether the risk is mitigated by bariatric surgery. Disruption of the anterior fibers of the medial collateral ligament is more common in obese patients, as are complications involving the extensor mechanism (eg, patellofemoral dislocation). As a result, slower postoperative rehabilitation is recommended.46 Although there is no recorded link between bariatric surgery and the development of ligamentous laxity, surgeons should be aware of the potential for medial collateral ligament avulsion in obese and formerly obese patients and have appropriate implants available.
Kulkarni and colleagues47 compared the rates of hip and knee arthroplasty complications in patients who were obese before bariatric surgery and patients who were still obese after bariatric surgery. Gastroplasty and bypass patients were included. Data on superficial wound infections were excluded; however, the bariatric surgery group’s deep wound infection rate was 3.5 times lower, and its 30-day readmission rate was 7 times lower. There was no difference in dislocation and hip revision rates at 1 year. Although 1 patient in the bariatric surgery group died of an unknown cause 9 days after surgery, Kulkarni and colleagues47 concluded it is safer to operate on obese patients after versus before bariatric surgery. However, their study did not include mean BMI, so no conclusion can be drawn about the risk of operating on patients who were still obese after bariatric surgery.
Studies of weight loss in primary TJA patients have had conflicting findings.48 Trofa and colleagues49 reported that 15 patients who underwent arthroplasty a mean of 42.4 months after bariatric surgery lost 27.9% more of their original BMI compared with patients who underwent bariatric surgery but not arthroplasty. This relationship between arthroplasty and weight loss was strongest in patients who underwent knee arthroplasty, with an average of 43.9% more BMI lost compared to patients who did not undergo TKA. There was no significant change in BMI in patients who underwent THA and bariatric surgery compared with patients who underwent bariatric surgery but not THA.
Parvizi and colleagues50 assessed the results of 20 arthroplasties (8 THAs, 12 TKAs) performed in 14 patients a mean of 23 months after bariatric surgery (2 gastroplasties, 12 bypass surgeries). Mean BMI was 29 kg/m2. At final follow-up, 1 patient required revision THA for aseptic loosening, but all the others showed no evidence of radiographic loosening or wear. One patient had a superficial wound infection, and 1 had a deep wound infection. Parvizi and colleagues50 reported that arthroplasty after bariatric surgery is a viable option and is preferable to operating on morbidly obese patients.
Summary
Orthopedic surgeons are increasingly performing elective hip and knee arthroplasties on patients who have undergone bariatric surgery. Although bariatric surgery may alleviate some of the complications associated with surgery on morbidly obese patients, it should be approached with caution. Studies have shown that bariatric surgery patients are at increased risk for wound-healing and other complications, often caused by unrecognized preoperative nutrient deficiencies. In addition, patients are often unable to tolerate commonly used medications. The exact timing of bariatric surgery relative to elective orthopedic procedures is unclear. Surgeons should perform a preoperative evaluation based on type of bariatric surgery in order to reduce the likelihood of adverse events. Such preemptive therapy may improve the short- and long-term results of major reconstructive surgery. Further research is needed to determine the true effect of bariatric surgery on orthopedic procedures.
1. Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19(12):1605-1611.
2. Severson EP, Singh JA, Browne JA, Trousdale RT, Sarr MG, Lewallen DG. Total knee arthroplasty in morbidly obese patients treated with bariatric surgery. J Arthroplasty. 2012;27(9):1696-1700.
3. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient [published correction appears in Endocr Pract. 2009;15(7):768]. Endocr Pract. 2008;14(suppl 1):1-83.
4. Hocking MP, Duerson MC, O’Leary JP, Woodward ER. Jejunoilial bypass for morbid obesity. Late follow-up in 100 cases. N Engl J Med. 1983;308(17):995-999.
5. DeMaria EJ. Morbid obesity. In: Mulholland MW, Lillemoe KD, Doherty GM, et al, eds. Greenfield’s Surgery: Scientific Principles & Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:736-743.
6. O’Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol. 2010;25(8):1358-1365.
7. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737.
8. DeMaria EJ, Sugerman HJ, Meador JG, et al. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233(6):809-818.
9. Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8(1):48-55.
10. Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004;7(5):569-575.
11. Schweiger C, Weiss R, Berry E, Keidar A. Nutritional deficiencies in bariatric surgery candidates. Obes Surg. 2010;20(2):193-197.
12. Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33(1):13-24.
13. Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)—a prospective study. Obes Surg. 2010;20(4):447-453.
14. Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12(1):40-47.
15. Ducloux R, Nobécourt E, Chevallier JM, Ducloux H, Elian N, Altman JJ. Vitamin D deficiency before bariatric surgery: should supplement intake be routinely prescribed? Obes Surg. 2011;21(5):556-560.
16. Wang A, Powell A. The effects of obesity surgery on bone metabolism: what orthopedic surgeons need to know. Am J Orthop. 2009;38(2):77-79.
17. Baghdasarian KL. Gastric bypass and megaloblastic anemia. J Am Diet Assoc. 1982;80(4):368-371.
18. Crowley LV, Olson RW. Megaloblastic anemia after gastric bypass for obesity. Am J Gastroenterol. 1983;78(7):406-410.
19. Sorg H, Schulz T, Krueger C, Vollmar B. Consequences of surgical stress on the kinetics of skin wound healing: partial hepatectomy delays and functionally alters dermal repair. Wound Repair Regen. 2009;17(3):367-377.
20. D’Ettorre M, Gniuli D, Iaconelli A, Massi G, Mingrone G, Bracaglia R. Wound healing process in post-bariatric patients: an experimental evaluation. Obes Surg. 2010;20(11):1552-1558.
21. Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19(1);41-46.
22. Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735-3740.
23. von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53(7):918-921.
24. Vilarrasa N, Gómez JM, Elio I, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009;19(7):860-866.
25. Hey H, Lund B, Sørensen OH, Lund B. Delayed fracture healing following jejunoileal bypass surgery for obesity. Calcif Tissue Int. 1982;34(1):13-15.
26. Borrelli J Jr, Pape C, Hak D, et al. Physiological challenges of bone repair. J Orthop Trauma. 2012;26(12):708-711.
27. Sardo P, Walker JH. Bariatric surgery: impact on medication management. Hosp Pharm. 2008;43(2):113-120.
28. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
29. Chopra T, Zhao JJ, Alangaden G, Wood MH, Kaye KS. Preventing surgical site infections after bariatric surgery: value of perioperative antibiotic regimens. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):317-328.
30. Payne KD, Hall RG 2nd. Dosing of antibacterial agents in obese adults: does one size fit all? Expert Rev Anti Infect Ther. 2014;12(7):829-854.
31. Sasse KC, Ganser J, Kozar M, et al. Seven cases of gastric perforation in Roux-en-Y gastric bypass patients: what lessons can we learn? Obes Surg. 2008;18(5):530-534.
32. Miller AD, Smith KM. Medication use in bariatric surgery patients: what orthopedists need to know. Orthopedics. 2006;29(2):121-123.
33. Huang R, Greenky M, Kerr GJ, Austin MS, Parvizi J. The effect of malnutrition on patients undergoing elective joint arthroplasty. J Arthroplasty. 2013;28(8 suppl):21-24.
34. Naghshineh N, O’Brien Coon D, McTigue K, Courcoulas AP, Fernstrom M, Rubin JP. Nutritional assessment of bariatric surgery patients presenting for plastic surgery: a prospective analysis. Plast Reconstr Surg. 2010;126(2):602-610.
35. Zorrilla P, Gómez LA, Salido JA, Silva A, López-Alonso A. Low serum zinc level as a predictive factor of delayed wound healing in total hip replacement. Wound Repair Regen. 2006;14(2):119-122.
36. Zorrilla P, Salido JA, López-Alonso A, Silva A. Serum zinc as a prognostic tool for wound healing in hip hemiarthroplasty. Clin Orthop Relat Res. 2004;(420):304-308.
37. Williams SE, Cooper K, Richmond B, Schauer P. Perioperative management of bariatric surgery patients: focus on metabolic bone disease. Cleve Clin J Med. 2008;75(5):333-349.
38. Kini S, Kannan U. Effect of bariatric surgery on future general surgical procedures. J Minim Access Surg. 2011;7(2):126-131.
39. Hidalgo JE, Roy M, Ramirez A, Szomstein S, Rosenthal RJ. Laparoscopic sleeve gastrectomy: a first step for rapid weight loss in morbidly obese patients requiring a second non-bariatric procedure. Obes Surg. 2012;22(4):555-559.
40. O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032-1040.
41. Inacio MC, Paxton EW, Fisher D, Li RA, Barber TC, Singh JA. Bariatric surgery prior to total joint arthroplasty may not provide dramatic improvements in post-arthroplasty surgical outcomes. J Arthroplasty. 2014;29(7):1359-1364.
42. Gill RS, Al‐Adra DP, Shi X, Sharma AM, Birch DW, Karmali S. The benefits of bariatric surgery in obese patients with hip and knee osteoarthritis: a systematic review. Obes Rev. 2011;12(12):1083-1089.
43. Vartiainen P, Bragge T, Lyytinen T, Hakkarainen M, Karjalainen PA, Arokoski JP. Kinematic and kinetic changes in obese gait in bariatric surgery–induced weight loss. J Biomech. 2012;45(10):1769-1774.
44. Vincent HK, Ben-David K, Conrad BP, Lamb KM, Seay AN, Vincent KR. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346-354.
45. Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes. 2007;31(1):114-120.
46. Booth RE Jr. Total knee arthroplasty in the obese patient: tips and quips. J Arthroplasty. 2002;17(4 suppl 1):69-70.
47. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
48. Inacio MC, Silverstein DK, Raman R, et al. Weight patterns before and after total joint arthroplasty and characteristics associated with weight change. Perm J. 2014;18(1):25-31.
49. Trofa D, Smith EL, Shah V, Shikora S. Total weight loss associated with increased physical activity after bariatric surgery may increase the need for total joint arthroplasty. Surg Obes Relat Dis. 2014;10(2):335-339.
50. Parvizi J, Trousdale RT, Sarr MG. Total joint arthroplasty in patients surgically treated for morbid obesity. J Arthroplasty. 2000;15(8):1003-1008.
1. Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19(12):1605-1611.
2. Severson EP, Singh JA, Browne JA, Trousdale RT, Sarr MG, Lewallen DG. Total knee arthroplasty in morbidly obese patients treated with bariatric surgery. J Arthroplasty. 2012;27(9):1696-1700.
3. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient [published correction appears in Endocr Pract. 2009;15(7):768]. Endocr Pract. 2008;14(suppl 1):1-83.
4. Hocking MP, Duerson MC, O’Leary JP, Woodward ER. Jejunoilial bypass for morbid obesity. Late follow-up in 100 cases. N Engl J Med. 1983;308(17):995-999.
5. DeMaria EJ. Morbid obesity. In: Mulholland MW, Lillemoe KD, Doherty GM, et al, eds. Greenfield’s Surgery: Scientific Principles & Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:736-743.
6. O’Brien PE. Bariatric surgery: mechanisms, indications and outcomes. J Gastroenterol Hepatol. 2010;25(8):1358-1365.
7. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737.
8. DeMaria EJ, Sugerman HJ, Meador JG, et al. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233(6):809-818.
9. Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8(1):48-55.
10. Alvarez-Leite JI. Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2004;7(5):569-575.
11. Schweiger C, Weiss R, Berry E, Keidar A. Nutritional deficiencies in bariatric surgery candidates. Obes Surg. 2010;20(2):193-197.
12. Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33(1):13-24.
13. Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)—a prospective study. Obes Surg. 2010;20(4):447-453.
14. Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12(1):40-47.
15. Ducloux R, Nobécourt E, Chevallier JM, Ducloux H, Elian N, Altman JJ. Vitamin D deficiency before bariatric surgery: should supplement intake be routinely prescribed? Obes Surg. 2011;21(5):556-560.
16. Wang A, Powell A. The effects of obesity surgery on bone metabolism: what orthopedic surgeons need to know. Am J Orthop. 2009;38(2):77-79.
17. Baghdasarian KL. Gastric bypass and megaloblastic anemia. J Am Diet Assoc. 1982;80(4):368-371.
18. Crowley LV, Olson RW. Megaloblastic anemia after gastric bypass for obesity. Am J Gastroenterol. 1983;78(7):406-410.
19. Sorg H, Schulz T, Krueger C, Vollmar B. Consequences of surgical stress on the kinetics of skin wound healing: partial hepatectomy delays and functionally alters dermal repair. Wound Repair Regen. 2009;17(3):367-377.
20. D’Ettorre M, Gniuli D, Iaconelli A, Massi G, Mingrone G, Bracaglia R. Wound healing process in post-bariatric patients: an experimental evaluation. Obes Surg. 2010;20(11):1552-1558.
21. Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19(1);41-46.
22. Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735-3740.
23. von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53(7):918-921.
24. Vilarrasa N, Gómez JM, Elio I, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009;19(7):860-866.
25. Hey H, Lund B, Sørensen OH, Lund B. Delayed fracture healing following jejunoileal bypass surgery for obesity. Calcif Tissue Int. 1982;34(1):13-15.
26. Borrelli J Jr, Pape C, Hak D, et al. Physiological challenges of bone repair. J Orthop Trauma. 2012;26(12):708-711.
27. Sardo P, Walker JH. Bariatric surgery: impact on medication management. Hosp Pharm. 2008;43(2):113-120.
28. Lizer MH, Papageorgeon H, Glembot TM. Nutritional and pharmacologic challenges in the bariatric surgery patient. Obes Surg. 2010;20(12):1654-1659.
29. Chopra T, Zhao JJ, Alangaden G, Wood MH, Kaye KS. Preventing surgical site infections after bariatric surgery: value of perioperative antibiotic regimens. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):317-328.
30. Payne KD, Hall RG 2nd. Dosing of antibacterial agents in obese adults: does one size fit all? Expert Rev Anti Infect Ther. 2014;12(7):829-854.
31. Sasse KC, Ganser J, Kozar M, et al. Seven cases of gastric perforation in Roux-en-Y gastric bypass patients: what lessons can we learn? Obes Surg. 2008;18(5):530-534.
32. Miller AD, Smith KM. Medication use in bariatric surgery patients: what orthopedists need to know. Orthopedics. 2006;29(2):121-123.
33. Huang R, Greenky M, Kerr GJ, Austin MS, Parvizi J. The effect of malnutrition on patients undergoing elective joint arthroplasty. J Arthroplasty. 2013;28(8 suppl):21-24.
34. Naghshineh N, O’Brien Coon D, McTigue K, Courcoulas AP, Fernstrom M, Rubin JP. Nutritional assessment of bariatric surgery patients presenting for plastic surgery: a prospective analysis. Plast Reconstr Surg. 2010;126(2):602-610.
35. Zorrilla P, Gómez LA, Salido JA, Silva A, López-Alonso A. Low serum zinc level as a predictive factor of delayed wound healing in total hip replacement. Wound Repair Regen. 2006;14(2):119-122.
36. Zorrilla P, Salido JA, López-Alonso A, Silva A. Serum zinc as a prognostic tool for wound healing in hip hemiarthroplasty. Clin Orthop Relat Res. 2004;(420):304-308.
37. Williams SE, Cooper K, Richmond B, Schauer P. Perioperative management of bariatric surgery patients: focus on metabolic bone disease. Cleve Clin J Med. 2008;75(5):333-349.
38. Kini S, Kannan U. Effect of bariatric surgery on future general surgical procedures. J Minim Access Surg. 2011;7(2):126-131.
39. Hidalgo JE, Roy M, Ramirez A, Szomstein S, Rosenthal RJ. Laparoscopic sleeve gastrectomy: a first step for rapid weight loss in morbidly obese patients requiring a second non-bariatric procedure. Obes Surg. 2012;22(4):555-559.
40. O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032-1040.
41. Inacio MC, Paxton EW, Fisher D, Li RA, Barber TC, Singh JA. Bariatric surgery prior to total joint arthroplasty may not provide dramatic improvements in post-arthroplasty surgical outcomes. J Arthroplasty. 2014;29(7):1359-1364.
42. Gill RS, Al‐Adra DP, Shi X, Sharma AM, Birch DW, Karmali S. The benefits of bariatric surgery in obese patients with hip and knee osteoarthritis: a systematic review. Obes Rev. 2011;12(12):1083-1089.
43. Vartiainen P, Bragge T, Lyytinen T, Hakkarainen M, Karjalainen PA, Arokoski JP. Kinematic and kinetic changes in obese gait in bariatric surgery–induced weight loss. J Biomech. 2012;45(10):1769-1774.
44. Vincent HK, Ben-David K, Conrad BP, Lamb KM, Seay AN, Vincent KR. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346-354.
45. Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes. 2007;31(1):114-120.
46. Booth RE Jr. Total knee arthroplasty in the obese patient: tips and quips. J Arthroplasty. 2002;17(4 suppl 1):69-70.
47. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
48. Inacio MC, Silverstein DK, Raman R, et al. Weight patterns before and after total joint arthroplasty and characteristics associated with weight change. Perm J. 2014;18(1):25-31.
49. Trofa D, Smith EL, Shah V, Shikora S. Total weight loss associated with increased physical activity after bariatric surgery may increase the need for total joint arthroplasty. Surg Obes Relat Dis. 2014;10(2):335-339.
50. Parvizi J, Trousdale RT, Sarr MG. Total joint arthroplasty in patients surgically treated for morbid obesity. J Arthroplasty. 2000;15(8):1003-1008.
Patient-Directed Valgus Stress Radiograph of the Knee: A New and Novel Technique
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
Concomitant Ulnar Styloid Fracture and Distal Radius Fracture Portend Poorer Outcome
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
Distal radius fracture is a common injury treated by orthopedic surgeons. Fifty percent or more of distal radius fractures (DRFs) occur with concomitant ulnar styloid fractures (USFs)1-3 (Figure). The base of the ulnar styloid is the insertion site for portions of the triangular fibrocartilaginous complex (TFCC), which is a primary stabilizer of the distal radioulnar joint (DRUJ).4,5
Although the topic has received significant attention in the literature, there remains a lack of consensus on the prognostic and clinical significance of USF occurring with DRF. In a series reported by May and colleagues,6 all patients with DRUJ instability after DRF also had an USF. Some authors have reported USF as a poor prognostic indicator for DRF, as the occurrence of USF was taken as a proxy for DRUJ instability.7,8 Conversely, other authors have reported that USF nonunion has no effect on the outcome of volar plating of DRF.9-11 In a retrospective cohort study of 182 patients, Li and colleagues12 found no clinically significant difference in outcome between presence or absence of USF with DRF. They also reported that the quality of the DRF reduction was the main determinant of clinical outcome in patients with USF.
We examined a large cohort of patients treated for DRF to identify any possible effect of an associated USF on clinical outcome. All patients provided written informed consent for study inclusion.
Materials and Methods
We retrospectively evaluated 315 cases of DRFs treated (184 operatively, 131 nonoperatively) by members of the Trauma and Hand divisions at our institution over a 7-year period. All cases had sufficient follow-up. In each group, patients with concomitant USF were identified.
At presentation, all displaced fractures underwent closed reduction and immobilization with a sugar-tong splint. Baseline demographic data, injury information, and baseline functional scores on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the 36-Item Short Form Health Survey (SF-36) were recorded. Complete histories were taken and physical examinations performed. Standard radiographs of the injured and contralateral wrists were obtained at time of initial injury.13
Surgery was indicated in patients with an open fracture and in patients with an inherently unstable fracture pattern, using the instability criteria of Cooney and colleagues.14 According to these criteria, unstable fractures have lost alignment after closed reduction or have more than 20° of dorsal angulation, more than 10 mm of longitudinal shortening, or more than 2 mm of articular displacement.14 Patients were treated with either a volar locked plate or bridging external fixation with supplemental Kirschner-wire fixation (usually 2 or 3 wires). Patients in both groups (operative, nonoperative) participated in a formal outpatient therapy program that emphasized active and passive range of motion (ROM) of the finger, wrist motion (if clinically appropriate), and forearm motion. Mean clinical follow-up was 12 months (range, 8-18 months). At each clinic visit, we used a handheld dynamometer to measure ROM, grip strength, and other parameters and compared them with the same parameters on the uninjured side, along with functional outcome.
Differences in demographic characteristics were evaluated with 2 tests—the χ2 test for categorical variables (eg, USF incidence, sex, hand dominance, fracture pattern) and the Student t test for continuous variables. Mann-Whitney U tests were used to assess differences between groups in DASH and SF-36 scores at long-term follow-up, as well as differences in ROM and radiographic measurements. Statistical significance was set at P < .05.
Results
DRFs occurred in the dominant-side wrist more commonly (P < .05) in the nonoperative group than in the operative group, though there was no difference in hand dominance and presence or absence of USF. There was a significant correlation of intra-articular fractures in the operative group (70%) compared with the nonoperative group (34%), though no association was found between presence of USF and intra-articular fracture location.
The percentage of concomitant USF was higher (P< .0002) in patients treated operatively (64.1%) than in those treated nonoperatively (38.9%). Mean (SD) pain score was higher (P = .0001) for patients with USF, 1.80 (2.43), than for patients without USF, 0.80 (1.55). This relationship held in both the operative group, 1.95 (2.48) versus 1.04 (1.58) (P = .027), and the nonoperative group, 1.29 (2.09) versus 0.66 (1.53) (P = .048). Similarly, at long-term follow-up for the entire patient cohort, mean (SD) DASH score was negatively affected by presence of USF, 17.03 (18.94) versus 9.21 (14.06) (P = .001), as was mean (SD) SF-36 score, 77.16 (17.69) versus 82.68 (16.10) (P = .022). This relationship also held in the operative and nonoperative groups with respect to pain and DASH scores, though there were only trends in this direction with respect to SF-36 scores. At final follow-up, there was no significant correlation of pain, SF-36, or DASH scores with presence of an intra-articular fracture as compared with an extra-articular fracture.
Time to radiographic healing was not influenced by presence of USF compared with absence of USF (11 vs 10.06 weeks; P > .05). Similarly, healing was no different in intra-articular fractures compared with extra-articular fractures (11 vs 10 weeks; P > .05).
Wrist ROM at final follow-up was not affected by presence of USF; there was no significant difference in wrist flexion, extension, or forearm rotation. In addition, mean (SD) grip strength was unaffected (P = .132) by presence or absence of USF with DRF overall, 45.45% (31.92) of contralateral versus 52.88% (30.03). However, grip strength was negatively affected (P = .035) by presence of USF in the nonoperative group, 37.79% (20.58) versus 54.52% (31.89) (Table).
Discussion
In this study, we determined that presence of USF was a negative predictor for clinical outcomes after DRF. Given the higher incidence of USF in operatively treated DRFs, USF likely represents a higher-energy mechanism of injury. We think these inferior clinical results are attributable to other wrist pathologies that commonly occur with these injuries. These pathologies, identified in the past, include stylocarpal impaction, extensor carpi ulnaris tendinitis, and pain at USF site.6,10,15 In addition, intracarpal ligamentous injuries, including damage to scapholunate and lunotriquetral ligaments, have been shown to occur in roughly 80% of patients who sustain DRFs, with TFCC injuries occurring at a rate of 60%.16
Patient outcome is multifactorial and depends on initial injury characteristics, reduction quality, associated injuries, and patient demographics and lifestyle factors. Li and colleagues12 showed that the quality of the DRF reduction influenced outcomes in these injuries, as the ulnar styloid and its associated TFCC are in turn reduced more anatomically with a restored DRF reduction. This concept applies to injuries treated both operatively and nonoperatively. Similarly, Xarchas and colleagues17 identified malunion of the ulnar styloid as causing chronic wrist pain because of triquetral impingement, which was treated successfully with ulnar styloidectomy. The poor results at final follow-up in their study may reflect severity of the initial injury, as reported by Frykman.18
Additional factors may compromise clinical outcomes after such injuries. For example, the effect of USF fragment size on outcome has been suggested and debated. In a retrospective series, May and colleagues6 identified fractures involving the base of the ulnar styloid or fovea as potentially destabilizing the DRUJ and in turn leading to chronic instability. This mechanism should be considered a potential contributor to protracted clinical recovery. Other studies have shown that, irrespective of USF fragment size, presence of USF with DRF is not a reliable predictor of DRUJ instability.2,10,19 In the present study, we simply identified presence or absence of USF, irrespective of either stability or fragment size. In cases in which there was an USF without instability, we fixed the DRF in isolation, without surgically addressing the USF. Our data demonstrated that, even in the absence of DRUJ instability, presence of USF was a negative prognostic indicator for patient outcome.
This study had several limitations. First, its design was retrospective. A prospective study would have been ideal for eliminating certain inherent bias. Second, USF represents a higher association with DRUJ instability.6 As there are no validated tests for this clinical entity, identification is somewhat subjective. We did not separate patients by presence or absence of DRUJ instability and thus were not able to directly correlate the connection between USF, DRUJ instability, and poor outcomes in association with DRF. In addition, management of an unstable DRUJ after operative fixation of DRF is controversial, with techniques ranging from splinting in supination to pinning the DRUJ. This inconsistency likely contributed to some error between groups of patients in this study. Last, we did not stratify patients by USF fragment size, as previously discussed, which may have affected outcomes within patient groups.
Our data add to the evidence showing that USF in association with DRF portends poorer clinical outcomes. Concomitant USF should alert the treating physician to a higher-energy mechanism of injury and raise the index of suspicion for other associated injuries in the carpus.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
1. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
2. Sammer DM, Shah HM, Shauver MJ, Chung KC. The effect of ulnar styloid fractures on patient-rated outcomes after volar locking plating of distal radius fractures. J Hand Surg Am. 2009;34(9):1595-1602.
3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture. A prospective review. J Bone Joint Surg Br. 1987;69(4):635-638.
4. Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist—anatomy and function. J Hand Surg Am. 1981;6(2):153-162.
5. Stuart PR, Berger RA, Linscheid RL, An KN. The dorsopalmar stability of the distal radioulnar joint. J Hand Surg Am. 2000;25(4):689-699.
6. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
7. Oskarsson GV, Aaser P, Hjall A. Do we underestimate the predictive value of the ulnar styloid affection in Colles fractures? Arch Orthop Trauma Surg. 1997;116(6-7):341-344.
8. Stoffelen D, De Smet L, Broos P. The importance of the distal radioulnar joint in distal radial fractures. J Hand Surg Br. 1998;23(4):507-511.
9. Buijze GA, Ring D. Clinical impact of united versus nonunited fractures of the proximal half of the ulnar styloid following volar plate fixation of the distal radius. J Hand Surg Am. 2010;35(2):223-227.
10. Kim JK, Yun YH, Kim DJ, Yun GU. Comparison of united and nonunited fractures of the ulnar styloid following volar-plate fixation of distal radius fractures. Injury. 2011;42(4):371-375.
11. Wijffels M, Ring D. The influence of non-union of the ulnar styloid on pain, wrist function and instability after distal radius fracture. J Hand Microsurg. 2011;3(1):11-14.
12. Li S, Chen Y, Lin Z, Fan Q, Cui W, Feng Z. Effect of associated ulnar styloid fracture on wrist function after distal radius [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26(6):666-670.
13. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
14. Cooney WP 3rd, Linscheid RL, Dobyns JH. External pin fixation for unstable Colles’ fractures. J Bone Joint Surg Am. 1979;61(6):840-845.
15. Cerezal L, del Piñal F, Abascal F, García-Valtuille R, Pereda T, Canga A. Imaging findings in ulnar-sided wrist impaction syndromes. Radiographics. 2002;22(1):105-121.
16. Ogawa T, Tanaka T, Yanai T, Kumagai H, Ochiai N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil. 2013;5(1):19.
17. Xarchas KC, Yfandithis P, Kazakos K. Malunion of the ulnar styloid as a cause of ulnar wrist pain. Clin Anat. 2004;17(5):418-422.
18. Frykman G. Fracture of the distal radius including sequelae—shoulder–hand–finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967:(suppl 108):3+.
19. Fujitani R, Omokawa S, Akahane M, Iida A, Ono H, Tanaka Y. Predictors of distal radioulnar joint instability in distal radius fractures. J Hand Surg Am. 2011;36(12):1919-1925.
Pigmented Villonodular Synovitis of the Hip: A Systematic Review
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
Total Knee Arthroplasty in Hemophilic Arthropathy
Chronic hemophilic arthropathy, a well-known complication of hemophilia, develops as a long-term consequence of recurrent joint bleeds resulting in synovial hypertrophy (chronic proliferative synovitis) and joint cartilage destruction. Hemophilic arthropathy mostly affects the knees, ankles, and elbows and causes chronic joint pain and functional impairment in relatively young patients who have not received adequate primary prophylactic replacement therapy with factor concentrates from early childhood.1-3
In the late stages of hemophilic arthropathy of the knee, total knee arthroplasty (TKA) provides dramatic joint pain relief, improves knee functional status, and reduces rebleeding into the joint.4-8 TKA performed on a patient with hemophilia was first reported in the mid-1970s.9,10 In these cases, the surgical procedure itself is often complicated by severe fibrosis developing in the joint soft tissues, flexion joint contracture, and poor quality of the joint bone structures. Even though TKA significantly reduces joint pain in patients with chronic hemophilic arthropathy, some authors have achieved only modest functional outcomes and experienced a high rate of complications (infection, prosthetic loosening).11-13 Data on TKA outcomes are still scarce, and most studies have enrolled a limited number of patients.
We retrospectively evaluated the outcomes of 88 primary TKAs performed on patients with severe hemophilia at a single institution. Clinical outcomes and complications were assessed with a special focus on prosthetic survival and infection.
Patients and Methods
Ninety-one primary TKAs were performed in 77 patients with severe hemophilia A and B (factor VIII [FVIII] and factor IX plasma concentration, <1% each) between January 1, 1999, and December 31, 2011, and the medical records of all these patients were thoroughly reviewed in 2013. The cases of 3 patients who died shortly after surgery were excluded from analysis. Thus, 88 TKAs and 74 patients (74 males) were finally available for evaluation. Fourteen patients underwent bilateral TKAs but none concurrently. The patients provided written informed consent for print and electronic publication of their outcomes.
We recorded demographic data, type and severity of hemophilia, human immunodeficiency virus (HIV) status, hepatitis C virus (HCV) status, and Knee Society Scale (KSS) scores.14 KSS scores include Knee score (pain, range of motion [ROM], stability) and Function score (walking, stairs), both of which range from 0 (normal knee) to 100 (most affected knee). Prosthetic infection was classified (Segawa and colleagues15) as early or late, depending on timing of symptom onset (4 weeks after replacement surgery was the threshold used).
Patients received an intravenous bolus infusion of the deficient factor concentrate followed by continuous infusion to reach a plasma factor level of 100% just before surgery and during the first 7 postoperative days and 50% over the next 7 days (Table 1). Patients with a circulating inhibitor (3 overall) received bypassing agents FEIBA (FVIII inhibitor bypassing agent) or rFVIIa (recombinant factor VII activated) (Table 2). Patients were not given any antifibrinolytic treatment or thromboprophylaxis.
Surgery was performed in a standard surgical room. Patients were placed on the operating table in decubitus supinus position. A parapatellar medial incision was made on a bloodless surgical field (achieved with tourniquet ischemia). The prosthesis model used was always the cemented (gentamicin bone cement) NexGen (Zimmer). Patellar resurfacing was done in all cases (Figures 1A–1D). All TKAs were performed by Dr. Rodríguez-Merchán. Intravenous antibiotic prophylaxis was administered at anesthetic induction and during the first 48 hours after surgery (3 further doses). Active exercises were started on postoperative day 1. Joint load aided with 2 crutches was allowed starting on postoperative day 2.
Mean patient age was 38.2 years (range, 24-73 years). Of the 74 patients, 55 had a diagnosis of severe hemophilia A, and 19 had a diagnosis of severe hemophilia B. During the follow-up period, 23 patients died (mean time, 6.4 years; range, 4-9 years). Causes of death were acquired immune deficiency syndrome (AIDS), liver cirrhosis, and intracranial bleeding. Mean follow-up for the full series of patients was 8 years (range, 1-13 years).
Descriptive statistical analysis was performed with SPSS Windows Version 18.0. Prosthetic failure was regarded as implant removal for any reason. Student t test was used to compare continuous variables, and either χ2 test or Fisher exact test was used to compare categorical variables. P < .05 (2-sided) was considered significant.
Results
Prosthetic survival rates with implant removal for any reason regarded as final endpoint was 92%. Causes of failure were prosthetic infection (6 cases, 6.8%) and loosening (2 cases, 2.2%). Of the 6 prosthetic infections, 5 were regarded as late and 1 as early. Late infections were successfully sorted by performing 2-stage revision TKA with the Constrained Condylar Knee (Zimmer). Acute infections were managed by open joint débridement and polyethylene exchange. Both cases of aseptic loosening of the TKA were successfully managed with 1-stage revision TKA using the same implant model (Figures 2A–2D).
Mean KSS Knee score improved from 79 before surgery to 36 after surgery, and mean KSS Function score improved from 63 to 33. KSS Pain score, which is included in the Knee score, 0 (no pain) to 50 (most severe pain), improved from 47 to 8. Patients receiving inhibitors and patients who were HIV- or HCV-positive did not have poorer outcomes relative to those of patients not receiving inhibitors and patients who were HIV- or HCV-negative. Patients with liver cirrhosis had a lower prosthetic survival rate and lower Knee scores.
Discussion
The prosthetic survival rate found in this study compares well with other reported rates for patients with hemophilia and other bleeding disorders. However, evidence regarding long-term prosthesis survival in TKAs performed for patients with hemophilia is limited. Table 3 summarizes the main reported series of patients with hemophilia with 10-year prosthetic survival rates, number of TKAs performed, and mean follow-up period; in all these series, implant removal for any reason was regarded as the final endpoint.5-8,16,17 Mean follow-up in our study was 8 years. Clinical outcomes of TKA in patients with severe hemophilia and related disorders are expected to be inferior to those achieved in patients without a bleeding condition. The overall 10-year prosthetic survival rate for cemented TKA implants, as reported by the Norwegian Arthroplasty Register, was on the order of 93%.18 Mean age of our patients at time of surgery was only 38.2 years. TKAs performed in younger patients without a bleeding disorder have been associated with shorter implant survival times relative to those of elderly patients.19 Thus, Diduch and colleagues20 reported a prosthetic survival rate of 87% at 18 years in 108 TKAs performed on patients under age 55 years. Lonner and colleagues21 reported a better implant survival rate (90% at 8 years) in a series of patients under age 40 years (32 TKAs). In a study by Duffy and colleagues,22 the implant survival rate was 85% at 15 years in patients under age 55 years (74 TKAs). The results from our retrospective case assessment are quite similar to the overall prosthetic survival rates reported for TKAs performed on patients without hemophilia.
Rates of periprosthetic infection after primary TKA in patients with hemophilia and other bleeding conditions are much higher (up to 11%), with a mean infection rate of 6.2% (range, 1% to 11%), consistent with the rate found in our series of patients (6.8%)7,16,17,23,24 (Table 4). This rate is much higher than that reported after primary TKA in patients without hemophilia but is similar to some rates reported for patients with hemophilia. In our experience, most periprosthetic infections (5/6) were sorted as late.
Late infection is a major concern after TKA in patients with hemophilia, and various factors have been hypothesized as contributing to the high prevalence. An important factor is the high rate of HIV-positive patients among patients with hemophilia—which acts as a strong predisposing factor because of the often low CD4 counts and associated immune deficiency,25 but different reports have provided conflicting results in this respect.5,6,12 We found no relationship between HIV status and risk for periprosthetic infection, but conclusions are limited by the low number of HIV-positive patients in our series (14/74, 18.9%). Our patients’ late periprosthetic infections were diagnosed several years after TKA, suggesting hematogenous spread of infection. Most of these patients either were on regular prophylactic factor infusions or were being treated on demand, which might entail a risk for contamination of infusions by skin bacteria from the puncture site. Therefore, having an aseptic technique for administering coagulation factor concentrates is of paramount importance for patients with hemophilia and a knee implant.
Another important complication of TKA surgery is aseptic loosening of the prosthesis. Aseptic loosening occurred in 2.2% of our patients, but higher rates have been reported elsewhere.11,26 Rates of this complication increase over follow-up, and some authors have linked this complication to TKA polyethylene wear.27 Development of a reactive and destructive bone–cement interface and microhemorrhages into such interface might be implicated in the higher rate of loosening observed among patients with hemophilia.28
In the present study, preoperative and postoperative functional outcomes differed significantly. A modest postoperative total ROM of 69º to 79º has been reported by several authors.5,6 Postoperative ROM may vary—may be slightly increased, remain unchanged, or may even be reduced.4,23,26 Even though little improvement in total ROM is achieved after TKA, many authors have reported reduced flexion contracture and hence an easier gait. However, along with functional improvement, dramatic pain relief after TKA is perhaps the most remarkable aspect, and it has a strong effect on patient satisfaction after surgery.5,7,8,18,23
Our study had 2 main limitations. First, it was a retrospective case series evaluation with the usual issues of potential inaccuracy of medical records and information bias. Second, the study did not include a control group.
Conclusion
The primary TKAs performed in our patients with hemophilia have had a good prosthetic survival rate. Even though such a result is slightly inferior to results in patients without hemophilia, our prosthetic survival rate is not significantly different from the rates reported in other, younger patient subsets. Late periprosthetic infections are a major concern, and taking precautions to avoid hematogenous spread of infections during factor concentrate infusions is strongly encouraged.
1. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59(3):287-305.
2. Rodriguez-Merchan EC. Common orthopaedic problems in haemophilia. Haemophilia. 1999;5(suppl 1):53-60.
3. Steen Carlsson K, Höjgård S, Glomstein A, et al. On-demand vs. prophylactic treatment for severe haemophilia in Norway and Sweden: differences in treatment characteristics and outcome. Haemophilia. 2003;9(5):555-566.
4. Teigland JC, Tjønnfjord GE, Evensen SA, Charania B. Knee arthroplasty in hemophilia. 5-12 year follow-up of 15 patients. Acta Orthop Scand. 1993;64(2):153-156.
5. Silva M, Luck JV Jr. Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87(1):85-91.
6. Wang K, Street A, Dowrick A, Liew S. Clinical outcomes and patient satisfaction following total joint replacement in haemophilia—23-year experience in knees, hips and elbows. Haemophilia. 2012;18(1):86-93.
7. Chevalier Y, Dargaud Y, Lienhart A, Chamouard V, Negrier C. Seventy-two total knee arthroplasties performed in patients with haemophilia using continuous infusion. Vox Sang. 2013;104(2):135-143.
8. Zingg PO, Fucentese SF, Lutz W, Brand B, Mamisch N, Koch PP. Haemophilic knee arthropathy: long-term outcome after total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2465-2470.
9. Kjaersgaard-Andersen P, Christiansen SE, Ingerslev J, Sneppen O. Total knee arthroplasty in classic hemophilia. Clin Orthop Relat Res. 1990;(256):137-146.
10. Cohen I, Heim M, Martinowitz U, Chechick A. Orthopaedic outcome of total knee replacement in haemophilia A. Haemophilia. 2000;6(2):104-109.
11. Fehily M, Fleming P, O’Shea E, Smith O, Smyth H. Total knee arthroplasty in patients with severe haemophilia. Int Orthop. 2002;26(2):89-91.
12. Legroux-Gérot I, Strouk G, Parquet A, Goodemand J, Gougeon F, Duquesnoy B. Total knee arthroplasty in hemophilic arthropathy. Joint Bone Spine. 2003;70(1):22-32.
13. Sheth DS, Oldfield D, Ambrose C, Clyburn T. Total knee arthroplasty in hemophilic arthropathy. J Arthroplasty. 2004;19(1):56-60.
14. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
15. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
16. Goddard NJ, Mann HA, Lee CA. Total knee replacement in patients with end-stage haemophilic arthropathy. 25-year results. J Bone Joint Surg Br. 2010;92(8):1085-1089.
17. Westberg M, Paus AC, Holme PA, Tjønnfjord GE. Haemophilic arthropathy: long-term outcomes in 107 primary total knee arthroplasties. Knee. 2014;21(1):147-150.
18. Lygre SH, Espehaug B, Havelin LI, Vollset SE, Furnes O. Failure of total knee arthroplasty with or without patella resurfacing. A study from the Norwegian Arthroplasty Register with 0-15 years of follow-up. Acta Orthop. 2011;82(3):282-292.
19. Post M, Telfer MC. Surgery in hemophilic patients. J Bone Joint Surg Am. 1975;57(8):1136-1145.
20. Diduch DR, Insall JN, Scott WN, Scuderi GR, Font-Rodriguez D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79(4):575-582.
21. Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;(380):85-90.
22. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 suppl 2):67-70.
23. Chiang CC, Chen PQ, Shen MC, Tsai W. Total knee arthroplasty for severe haemophilic arthropathy: long-term experience in Taiwan. Haemophilia. 2008;14(4):828-834.
24. Solimeno LP, Mancuso ME, Pasta G, Santagostino E, Perfetto S, Mannucci PM. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institution. Br J Haematol. 2009;145(2):227-234.
25. Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25(1):87-92.
26. Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopaedic surgery in human immunodeficiency virus–infected hemophiliacs with CD4 counts < or = 200/mm3. J Arthroplasty. 1995;10(6):716-721.
27. Hicks JL, Ribbans WJ, Buzzard B, et al. Infected joint replacements in HIV-positive patients with haemophilia. J Bone Joint Surg Br. 2001;83(7):1050-1054.
28. Figgie MP, Goldberg VM, Figgie HE 3rd, Heiple KG, Sobel M. Total knee arthroplasty for the treatment of chronic hemophilic arthropathy. Clin Orthop Relat Res. 1989;(248):98-107.
Chronic hemophilic arthropathy, a well-known complication of hemophilia, develops as a long-term consequence of recurrent joint bleeds resulting in synovial hypertrophy (chronic proliferative synovitis) and joint cartilage destruction. Hemophilic arthropathy mostly affects the knees, ankles, and elbows and causes chronic joint pain and functional impairment in relatively young patients who have not received adequate primary prophylactic replacement therapy with factor concentrates from early childhood.1-3
In the late stages of hemophilic arthropathy of the knee, total knee arthroplasty (TKA) provides dramatic joint pain relief, improves knee functional status, and reduces rebleeding into the joint.4-8 TKA performed on a patient with hemophilia was first reported in the mid-1970s.9,10 In these cases, the surgical procedure itself is often complicated by severe fibrosis developing in the joint soft tissues, flexion joint contracture, and poor quality of the joint bone structures. Even though TKA significantly reduces joint pain in patients with chronic hemophilic arthropathy, some authors have achieved only modest functional outcomes and experienced a high rate of complications (infection, prosthetic loosening).11-13 Data on TKA outcomes are still scarce, and most studies have enrolled a limited number of patients.
We retrospectively evaluated the outcomes of 88 primary TKAs performed on patients with severe hemophilia at a single institution. Clinical outcomes and complications were assessed with a special focus on prosthetic survival and infection.
Patients and Methods
Ninety-one primary TKAs were performed in 77 patients with severe hemophilia A and B (factor VIII [FVIII] and factor IX plasma concentration, <1% each) between January 1, 1999, and December 31, 2011, and the medical records of all these patients were thoroughly reviewed in 2013. The cases of 3 patients who died shortly after surgery were excluded from analysis. Thus, 88 TKAs and 74 patients (74 males) were finally available for evaluation. Fourteen patients underwent bilateral TKAs but none concurrently. The patients provided written informed consent for print and electronic publication of their outcomes.
We recorded demographic data, type and severity of hemophilia, human immunodeficiency virus (HIV) status, hepatitis C virus (HCV) status, and Knee Society Scale (KSS) scores.14 KSS scores include Knee score (pain, range of motion [ROM], stability) and Function score (walking, stairs), both of which range from 0 (normal knee) to 100 (most affected knee). Prosthetic infection was classified (Segawa and colleagues15) as early or late, depending on timing of symptom onset (4 weeks after replacement surgery was the threshold used).
Patients received an intravenous bolus infusion of the deficient factor concentrate followed by continuous infusion to reach a plasma factor level of 100% just before surgery and during the first 7 postoperative days and 50% over the next 7 days (Table 1). Patients with a circulating inhibitor (3 overall) received bypassing agents FEIBA (FVIII inhibitor bypassing agent) or rFVIIa (recombinant factor VII activated) (Table 2). Patients were not given any antifibrinolytic treatment or thromboprophylaxis.
Surgery was performed in a standard surgical room. Patients were placed on the operating table in decubitus supinus position. A parapatellar medial incision was made on a bloodless surgical field (achieved with tourniquet ischemia). The prosthesis model used was always the cemented (gentamicin bone cement) NexGen (Zimmer). Patellar resurfacing was done in all cases (Figures 1A–1D). All TKAs were performed by Dr. Rodríguez-Merchán. Intravenous antibiotic prophylaxis was administered at anesthetic induction and during the first 48 hours after surgery (3 further doses). Active exercises were started on postoperative day 1. Joint load aided with 2 crutches was allowed starting on postoperative day 2.
Mean patient age was 38.2 years (range, 24-73 years). Of the 74 patients, 55 had a diagnosis of severe hemophilia A, and 19 had a diagnosis of severe hemophilia B. During the follow-up period, 23 patients died (mean time, 6.4 years; range, 4-9 years). Causes of death were acquired immune deficiency syndrome (AIDS), liver cirrhosis, and intracranial bleeding. Mean follow-up for the full series of patients was 8 years (range, 1-13 years).
Descriptive statistical analysis was performed with SPSS Windows Version 18.0. Prosthetic failure was regarded as implant removal for any reason. Student t test was used to compare continuous variables, and either χ2 test or Fisher exact test was used to compare categorical variables. P < .05 (2-sided) was considered significant.
Results
Prosthetic survival rates with implant removal for any reason regarded as final endpoint was 92%. Causes of failure were prosthetic infection (6 cases, 6.8%) and loosening (2 cases, 2.2%). Of the 6 prosthetic infections, 5 were regarded as late and 1 as early. Late infections were successfully sorted by performing 2-stage revision TKA with the Constrained Condylar Knee (Zimmer). Acute infections were managed by open joint débridement and polyethylene exchange. Both cases of aseptic loosening of the TKA were successfully managed with 1-stage revision TKA using the same implant model (Figures 2A–2D).
Mean KSS Knee score improved from 79 before surgery to 36 after surgery, and mean KSS Function score improved from 63 to 33. KSS Pain score, which is included in the Knee score, 0 (no pain) to 50 (most severe pain), improved from 47 to 8. Patients receiving inhibitors and patients who were HIV- or HCV-positive did not have poorer outcomes relative to those of patients not receiving inhibitors and patients who were HIV- or HCV-negative. Patients with liver cirrhosis had a lower prosthetic survival rate and lower Knee scores.
Discussion
The prosthetic survival rate found in this study compares well with other reported rates for patients with hemophilia and other bleeding disorders. However, evidence regarding long-term prosthesis survival in TKAs performed for patients with hemophilia is limited. Table 3 summarizes the main reported series of patients with hemophilia with 10-year prosthetic survival rates, number of TKAs performed, and mean follow-up period; in all these series, implant removal for any reason was regarded as the final endpoint.5-8,16,17 Mean follow-up in our study was 8 years. Clinical outcomes of TKA in patients with severe hemophilia and related disorders are expected to be inferior to those achieved in patients without a bleeding condition. The overall 10-year prosthetic survival rate for cemented TKA implants, as reported by the Norwegian Arthroplasty Register, was on the order of 93%.18 Mean age of our patients at time of surgery was only 38.2 years. TKAs performed in younger patients without a bleeding disorder have been associated with shorter implant survival times relative to those of elderly patients.19 Thus, Diduch and colleagues20 reported a prosthetic survival rate of 87% at 18 years in 108 TKAs performed on patients under age 55 years. Lonner and colleagues21 reported a better implant survival rate (90% at 8 years) in a series of patients under age 40 years (32 TKAs). In a study by Duffy and colleagues,22 the implant survival rate was 85% at 15 years in patients under age 55 years (74 TKAs). The results from our retrospective case assessment are quite similar to the overall prosthetic survival rates reported for TKAs performed on patients without hemophilia.
Rates of periprosthetic infection after primary TKA in patients with hemophilia and other bleeding conditions are much higher (up to 11%), with a mean infection rate of 6.2% (range, 1% to 11%), consistent with the rate found in our series of patients (6.8%)7,16,17,23,24 (Table 4). This rate is much higher than that reported after primary TKA in patients without hemophilia but is similar to some rates reported for patients with hemophilia. In our experience, most periprosthetic infections (5/6) were sorted as late.
Late infection is a major concern after TKA in patients with hemophilia, and various factors have been hypothesized as contributing to the high prevalence. An important factor is the high rate of HIV-positive patients among patients with hemophilia—which acts as a strong predisposing factor because of the often low CD4 counts and associated immune deficiency,25 but different reports have provided conflicting results in this respect.5,6,12 We found no relationship between HIV status and risk for periprosthetic infection, but conclusions are limited by the low number of HIV-positive patients in our series (14/74, 18.9%). Our patients’ late periprosthetic infections were diagnosed several years after TKA, suggesting hematogenous spread of infection. Most of these patients either were on regular prophylactic factor infusions or were being treated on demand, which might entail a risk for contamination of infusions by skin bacteria from the puncture site. Therefore, having an aseptic technique for administering coagulation factor concentrates is of paramount importance for patients with hemophilia and a knee implant.
Another important complication of TKA surgery is aseptic loosening of the prosthesis. Aseptic loosening occurred in 2.2% of our patients, but higher rates have been reported elsewhere.11,26 Rates of this complication increase over follow-up, and some authors have linked this complication to TKA polyethylene wear.27 Development of a reactive and destructive bone–cement interface and microhemorrhages into such interface might be implicated in the higher rate of loosening observed among patients with hemophilia.28
In the present study, preoperative and postoperative functional outcomes differed significantly. A modest postoperative total ROM of 69º to 79º has been reported by several authors.5,6 Postoperative ROM may vary—may be slightly increased, remain unchanged, or may even be reduced.4,23,26 Even though little improvement in total ROM is achieved after TKA, many authors have reported reduced flexion contracture and hence an easier gait. However, along with functional improvement, dramatic pain relief after TKA is perhaps the most remarkable aspect, and it has a strong effect on patient satisfaction after surgery.5,7,8,18,23
Our study had 2 main limitations. First, it was a retrospective case series evaluation with the usual issues of potential inaccuracy of medical records and information bias. Second, the study did not include a control group.
Conclusion
The primary TKAs performed in our patients with hemophilia have had a good prosthetic survival rate. Even though such a result is slightly inferior to results in patients without hemophilia, our prosthetic survival rate is not significantly different from the rates reported in other, younger patient subsets. Late periprosthetic infections are a major concern, and taking precautions to avoid hematogenous spread of infections during factor concentrate infusions is strongly encouraged.
Chronic hemophilic arthropathy, a well-known complication of hemophilia, develops as a long-term consequence of recurrent joint bleeds resulting in synovial hypertrophy (chronic proliferative synovitis) and joint cartilage destruction. Hemophilic arthropathy mostly affects the knees, ankles, and elbows and causes chronic joint pain and functional impairment in relatively young patients who have not received adequate primary prophylactic replacement therapy with factor concentrates from early childhood.1-3
In the late stages of hemophilic arthropathy of the knee, total knee arthroplasty (TKA) provides dramatic joint pain relief, improves knee functional status, and reduces rebleeding into the joint.4-8 TKA performed on a patient with hemophilia was first reported in the mid-1970s.9,10 In these cases, the surgical procedure itself is often complicated by severe fibrosis developing in the joint soft tissues, flexion joint contracture, and poor quality of the joint bone structures. Even though TKA significantly reduces joint pain in patients with chronic hemophilic arthropathy, some authors have achieved only modest functional outcomes and experienced a high rate of complications (infection, prosthetic loosening).11-13 Data on TKA outcomes are still scarce, and most studies have enrolled a limited number of patients.
We retrospectively evaluated the outcomes of 88 primary TKAs performed on patients with severe hemophilia at a single institution. Clinical outcomes and complications were assessed with a special focus on prosthetic survival and infection.
Patients and Methods
Ninety-one primary TKAs were performed in 77 patients with severe hemophilia A and B (factor VIII [FVIII] and factor IX plasma concentration, <1% each) between January 1, 1999, and December 31, 2011, and the medical records of all these patients were thoroughly reviewed in 2013. The cases of 3 patients who died shortly after surgery were excluded from analysis. Thus, 88 TKAs and 74 patients (74 males) were finally available for evaluation. Fourteen patients underwent bilateral TKAs but none concurrently. The patients provided written informed consent for print and electronic publication of their outcomes.
We recorded demographic data, type and severity of hemophilia, human immunodeficiency virus (HIV) status, hepatitis C virus (HCV) status, and Knee Society Scale (KSS) scores.14 KSS scores include Knee score (pain, range of motion [ROM], stability) and Function score (walking, stairs), both of which range from 0 (normal knee) to 100 (most affected knee). Prosthetic infection was classified (Segawa and colleagues15) as early or late, depending on timing of symptom onset (4 weeks after replacement surgery was the threshold used).
Patients received an intravenous bolus infusion of the deficient factor concentrate followed by continuous infusion to reach a plasma factor level of 100% just before surgery and during the first 7 postoperative days and 50% over the next 7 days (Table 1). Patients with a circulating inhibitor (3 overall) received bypassing agents FEIBA (FVIII inhibitor bypassing agent) or rFVIIa (recombinant factor VII activated) (Table 2). Patients were not given any antifibrinolytic treatment or thromboprophylaxis.
Surgery was performed in a standard surgical room. Patients were placed on the operating table in decubitus supinus position. A parapatellar medial incision was made on a bloodless surgical field (achieved with tourniquet ischemia). The prosthesis model used was always the cemented (gentamicin bone cement) NexGen (Zimmer). Patellar resurfacing was done in all cases (Figures 1A–1D). All TKAs were performed by Dr. Rodríguez-Merchán. Intravenous antibiotic prophylaxis was administered at anesthetic induction and during the first 48 hours after surgery (3 further doses). Active exercises were started on postoperative day 1. Joint load aided with 2 crutches was allowed starting on postoperative day 2.
Mean patient age was 38.2 years (range, 24-73 years). Of the 74 patients, 55 had a diagnosis of severe hemophilia A, and 19 had a diagnosis of severe hemophilia B. During the follow-up period, 23 patients died (mean time, 6.4 years; range, 4-9 years). Causes of death were acquired immune deficiency syndrome (AIDS), liver cirrhosis, and intracranial bleeding. Mean follow-up for the full series of patients was 8 years (range, 1-13 years).
Descriptive statistical analysis was performed with SPSS Windows Version 18.0. Prosthetic failure was regarded as implant removal for any reason. Student t test was used to compare continuous variables, and either χ2 test or Fisher exact test was used to compare categorical variables. P < .05 (2-sided) was considered significant.
Results
Prosthetic survival rates with implant removal for any reason regarded as final endpoint was 92%. Causes of failure were prosthetic infection (6 cases, 6.8%) and loosening (2 cases, 2.2%). Of the 6 prosthetic infections, 5 were regarded as late and 1 as early. Late infections were successfully sorted by performing 2-stage revision TKA with the Constrained Condylar Knee (Zimmer). Acute infections were managed by open joint débridement and polyethylene exchange. Both cases of aseptic loosening of the TKA were successfully managed with 1-stage revision TKA using the same implant model (Figures 2A–2D).
Mean KSS Knee score improved from 79 before surgery to 36 after surgery, and mean KSS Function score improved from 63 to 33. KSS Pain score, which is included in the Knee score, 0 (no pain) to 50 (most severe pain), improved from 47 to 8. Patients receiving inhibitors and patients who were HIV- or HCV-positive did not have poorer outcomes relative to those of patients not receiving inhibitors and patients who were HIV- or HCV-negative. Patients with liver cirrhosis had a lower prosthetic survival rate and lower Knee scores.
Discussion
The prosthetic survival rate found in this study compares well with other reported rates for patients with hemophilia and other bleeding disorders. However, evidence regarding long-term prosthesis survival in TKAs performed for patients with hemophilia is limited. Table 3 summarizes the main reported series of patients with hemophilia with 10-year prosthetic survival rates, number of TKAs performed, and mean follow-up period; in all these series, implant removal for any reason was regarded as the final endpoint.5-8,16,17 Mean follow-up in our study was 8 years. Clinical outcomes of TKA in patients with severe hemophilia and related disorders are expected to be inferior to those achieved in patients without a bleeding condition. The overall 10-year prosthetic survival rate for cemented TKA implants, as reported by the Norwegian Arthroplasty Register, was on the order of 93%.18 Mean age of our patients at time of surgery was only 38.2 years. TKAs performed in younger patients without a bleeding disorder have been associated with shorter implant survival times relative to those of elderly patients.19 Thus, Diduch and colleagues20 reported a prosthetic survival rate of 87% at 18 years in 108 TKAs performed on patients under age 55 years. Lonner and colleagues21 reported a better implant survival rate (90% at 8 years) in a series of patients under age 40 years (32 TKAs). In a study by Duffy and colleagues,22 the implant survival rate was 85% at 15 years in patients under age 55 years (74 TKAs). The results from our retrospective case assessment are quite similar to the overall prosthetic survival rates reported for TKAs performed on patients without hemophilia.
Rates of periprosthetic infection after primary TKA in patients with hemophilia and other bleeding conditions are much higher (up to 11%), with a mean infection rate of 6.2% (range, 1% to 11%), consistent with the rate found in our series of patients (6.8%)7,16,17,23,24 (Table 4). This rate is much higher than that reported after primary TKA in patients without hemophilia but is similar to some rates reported for patients with hemophilia. In our experience, most periprosthetic infections (5/6) were sorted as late.
Late infection is a major concern after TKA in patients with hemophilia, and various factors have been hypothesized as contributing to the high prevalence. An important factor is the high rate of HIV-positive patients among patients with hemophilia—which acts as a strong predisposing factor because of the often low CD4 counts and associated immune deficiency,25 but different reports have provided conflicting results in this respect.5,6,12 We found no relationship between HIV status and risk for periprosthetic infection, but conclusions are limited by the low number of HIV-positive patients in our series (14/74, 18.9%). Our patients’ late periprosthetic infections were diagnosed several years after TKA, suggesting hematogenous spread of infection. Most of these patients either were on regular prophylactic factor infusions or were being treated on demand, which might entail a risk for contamination of infusions by skin bacteria from the puncture site. Therefore, having an aseptic technique for administering coagulation factor concentrates is of paramount importance for patients with hemophilia and a knee implant.
Another important complication of TKA surgery is aseptic loosening of the prosthesis. Aseptic loosening occurred in 2.2% of our patients, but higher rates have been reported elsewhere.11,26 Rates of this complication increase over follow-up, and some authors have linked this complication to TKA polyethylene wear.27 Development of a reactive and destructive bone–cement interface and microhemorrhages into such interface might be implicated in the higher rate of loosening observed among patients with hemophilia.28
In the present study, preoperative and postoperative functional outcomes differed significantly. A modest postoperative total ROM of 69º to 79º has been reported by several authors.5,6 Postoperative ROM may vary—may be slightly increased, remain unchanged, or may even be reduced.4,23,26 Even though little improvement in total ROM is achieved after TKA, many authors have reported reduced flexion contracture and hence an easier gait. However, along with functional improvement, dramatic pain relief after TKA is perhaps the most remarkable aspect, and it has a strong effect on patient satisfaction after surgery.5,7,8,18,23
Our study had 2 main limitations. First, it was a retrospective case series evaluation with the usual issues of potential inaccuracy of medical records and information bias. Second, the study did not include a control group.
Conclusion
The primary TKAs performed in our patients with hemophilia have had a good prosthetic survival rate. Even though such a result is slightly inferior to results in patients without hemophilia, our prosthetic survival rate is not significantly different from the rates reported in other, younger patient subsets. Late periprosthetic infections are a major concern, and taking precautions to avoid hematogenous spread of infections during factor concentrate infusions is strongly encouraged.
1. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59(3):287-305.
2. Rodriguez-Merchan EC. Common orthopaedic problems in haemophilia. Haemophilia. 1999;5(suppl 1):53-60.
3. Steen Carlsson K, Höjgård S, Glomstein A, et al. On-demand vs. prophylactic treatment for severe haemophilia in Norway and Sweden: differences in treatment characteristics and outcome. Haemophilia. 2003;9(5):555-566.
4. Teigland JC, Tjønnfjord GE, Evensen SA, Charania B. Knee arthroplasty in hemophilia. 5-12 year follow-up of 15 patients. Acta Orthop Scand. 1993;64(2):153-156.
5. Silva M, Luck JV Jr. Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87(1):85-91.
6. Wang K, Street A, Dowrick A, Liew S. Clinical outcomes and patient satisfaction following total joint replacement in haemophilia—23-year experience in knees, hips and elbows. Haemophilia. 2012;18(1):86-93.
7. Chevalier Y, Dargaud Y, Lienhart A, Chamouard V, Negrier C. Seventy-two total knee arthroplasties performed in patients with haemophilia using continuous infusion. Vox Sang. 2013;104(2):135-143.
8. Zingg PO, Fucentese SF, Lutz W, Brand B, Mamisch N, Koch PP. Haemophilic knee arthropathy: long-term outcome after total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2465-2470.
9. Kjaersgaard-Andersen P, Christiansen SE, Ingerslev J, Sneppen O. Total knee arthroplasty in classic hemophilia. Clin Orthop Relat Res. 1990;(256):137-146.
10. Cohen I, Heim M, Martinowitz U, Chechick A. Orthopaedic outcome of total knee replacement in haemophilia A. Haemophilia. 2000;6(2):104-109.
11. Fehily M, Fleming P, O’Shea E, Smith O, Smyth H. Total knee arthroplasty in patients with severe haemophilia. Int Orthop. 2002;26(2):89-91.
12. Legroux-Gérot I, Strouk G, Parquet A, Goodemand J, Gougeon F, Duquesnoy B. Total knee arthroplasty in hemophilic arthropathy. Joint Bone Spine. 2003;70(1):22-32.
13. Sheth DS, Oldfield D, Ambrose C, Clyburn T. Total knee arthroplasty in hemophilic arthropathy. J Arthroplasty. 2004;19(1):56-60.
14. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
15. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
16. Goddard NJ, Mann HA, Lee CA. Total knee replacement in patients with end-stage haemophilic arthropathy. 25-year results. J Bone Joint Surg Br. 2010;92(8):1085-1089.
17. Westberg M, Paus AC, Holme PA, Tjønnfjord GE. Haemophilic arthropathy: long-term outcomes in 107 primary total knee arthroplasties. Knee. 2014;21(1):147-150.
18. Lygre SH, Espehaug B, Havelin LI, Vollset SE, Furnes O. Failure of total knee arthroplasty with or without patella resurfacing. A study from the Norwegian Arthroplasty Register with 0-15 years of follow-up. Acta Orthop. 2011;82(3):282-292.
19. Post M, Telfer MC. Surgery in hemophilic patients. J Bone Joint Surg Am. 1975;57(8):1136-1145.
20. Diduch DR, Insall JN, Scott WN, Scuderi GR, Font-Rodriguez D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79(4):575-582.
21. Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;(380):85-90.
22. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 suppl 2):67-70.
23. Chiang CC, Chen PQ, Shen MC, Tsai W. Total knee arthroplasty for severe haemophilic arthropathy: long-term experience in Taiwan. Haemophilia. 2008;14(4):828-834.
24. Solimeno LP, Mancuso ME, Pasta G, Santagostino E, Perfetto S, Mannucci PM. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institution. Br J Haematol. 2009;145(2):227-234.
25. Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25(1):87-92.
26. Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopaedic surgery in human immunodeficiency virus–infected hemophiliacs with CD4 counts < or = 200/mm3. J Arthroplasty. 1995;10(6):716-721.
27. Hicks JL, Ribbans WJ, Buzzard B, et al. Infected joint replacements in HIV-positive patients with haemophilia. J Bone Joint Surg Br. 2001;83(7):1050-1054.
28. Figgie MP, Goldberg VM, Figgie HE 3rd, Heiple KG, Sobel M. Total knee arthroplasty for the treatment of chronic hemophilic arthropathy. Clin Orthop Relat Res. 1989;(248):98-107.
1. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59(3):287-305.
2. Rodriguez-Merchan EC. Common orthopaedic problems in haemophilia. Haemophilia. 1999;5(suppl 1):53-60.
3. Steen Carlsson K, Höjgård S, Glomstein A, et al. On-demand vs. prophylactic treatment for severe haemophilia in Norway and Sweden: differences in treatment characteristics and outcome. Haemophilia. 2003;9(5):555-566.
4. Teigland JC, Tjønnfjord GE, Evensen SA, Charania B. Knee arthroplasty in hemophilia. 5-12 year follow-up of 15 patients. Acta Orthop Scand. 1993;64(2):153-156.
5. Silva M, Luck JV Jr. Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87(1):85-91.
6. Wang K, Street A, Dowrick A, Liew S. Clinical outcomes and patient satisfaction following total joint replacement in haemophilia—23-year experience in knees, hips and elbows. Haemophilia. 2012;18(1):86-93.
7. Chevalier Y, Dargaud Y, Lienhart A, Chamouard V, Negrier C. Seventy-two total knee arthroplasties performed in patients with haemophilia using continuous infusion. Vox Sang. 2013;104(2):135-143.
8. Zingg PO, Fucentese SF, Lutz W, Brand B, Mamisch N, Koch PP. Haemophilic knee arthropathy: long-term outcome after total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2465-2470.
9. Kjaersgaard-Andersen P, Christiansen SE, Ingerslev J, Sneppen O. Total knee arthroplasty in classic hemophilia. Clin Orthop Relat Res. 1990;(256):137-146.
10. Cohen I, Heim M, Martinowitz U, Chechick A. Orthopaedic outcome of total knee replacement in haemophilia A. Haemophilia. 2000;6(2):104-109.
11. Fehily M, Fleming P, O’Shea E, Smith O, Smyth H. Total knee arthroplasty in patients with severe haemophilia. Int Orthop. 2002;26(2):89-91.
12. Legroux-Gérot I, Strouk G, Parquet A, Goodemand J, Gougeon F, Duquesnoy B. Total knee arthroplasty in hemophilic arthropathy. Joint Bone Spine. 2003;70(1):22-32.
13. Sheth DS, Oldfield D, Ambrose C, Clyburn T. Total knee arthroplasty in hemophilic arthropathy. J Arthroplasty. 2004;19(1):56-60.
14. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
15. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
16. Goddard NJ, Mann HA, Lee CA. Total knee replacement in patients with end-stage haemophilic arthropathy. 25-year results. J Bone Joint Surg Br. 2010;92(8):1085-1089.
17. Westberg M, Paus AC, Holme PA, Tjønnfjord GE. Haemophilic arthropathy: long-term outcomes in 107 primary total knee arthroplasties. Knee. 2014;21(1):147-150.
18. Lygre SH, Espehaug B, Havelin LI, Vollset SE, Furnes O. Failure of total knee arthroplasty with or without patella resurfacing. A study from the Norwegian Arthroplasty Register with 0-15 years of follow-up. Acta Orthop. 2011;82(3):282-292.
19. Post M, Telfer MC. Surgery in hemophilic patients. J Bone Joint Surg Am. 1975;57(8):1136-1145.
20. Diduch DR, Insall JN, Scott WN, Scuderi GR, Font-Rodriguez D. Total knee replacement in young, active patients. Long-term follow-up and functional outcome. J Bone Joint Surg Am. 1997;79(4):575-582.
21. Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;(380):85-90.
22. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 suppl 2):67-70.
23. Chiang CC, Chen PQ, Shen MC, Tsai W. Total knee arthroplasty for severe haemophilic arthropathy: long-term experience in Taiwan. Haemophilia. 2008;14(4):828-834.
24. Solimeno LP, Mancuso ME, Pasta G, Santagostino E, Perfetto S, Mannucci PM. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institution. Br J Haematol. 2009;145(2):227-234.
25. Jämsen E, Varonen M, Huhtala H, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty. 2010;25(1):87-92.
26. Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopaedic surgery in human immunodeficiency virus–infected hemophiliacs with CD4 counts < or = 200/mm3. J Arthroplasty. 1995;10(6):716-721.
27. Hicks JL, Ribbans WJ, Buzzard B, et al. Infected joint replacements in HIV-positive patients with haemophilia. J Bone Joint Surg Br. 2001;83(7):1050-1054.
28. Figgie MP, Goldberg VM, Figgie HE 3rd, Heiple KG, Sobel M. Total knee arthroplasty for the treatment of chronic hemophilic arthropathy. Clin Orthop Relat Res. 1989;(248):98-107.
Effects of Tranexamic Acid Cytotoxicity on In Vitro Chondrocytes
For decades, tranexamic acid (TXA) has been used off-label to reduce perioperative blood loss in various surgical procedures, including orthopedic surgery, neurosurgery, urologic surgery, obstetrics and gynecology, and trauma surgery.1 TXA, a synthetic derivative of the amino acid lysine, produces antifibrinolytic activity by competitively inhibiting lysine-binding sites on plasminogen molecules—inhibiting the activation of plasmin and thus preserving the function of fibrin in clot formation. It is believed that, through this method, TXA retains blood clots more effectively, thereby reducing bleeding. Although intravenous delivery of TXA is generally accepted as safe, some studies have indicated that it may contribute to postoperative seizure activity as well as increased thromboembolic events.2,3 For these and other reasons, interest in topical (intra-articular) administration of TXA has increased.
Use of topical TXA in surgery has been expanding over the past several years, with reports of significant reductions in perioperative blood loss and transfusion requirements.4 Orthopedic surgeons specifically have explored the topical use of TXA, especially in total joint arthroplasty (TJA).5 The benefits are increased concentration at the operative site with reduced systemic exposure; cost reduction; and surgeon control.6,7 Several recent studies have yielded significant reductions in perioperative blood loss and transfusions with use of topical TXA.8-10 In the literature, effective dosing for topical TXA in TJA ranges from 250 mg to 3 g.11 The concentration of topical TXA is not consistently described but appears to fall between 15 and 100 mg/mL.12,13 Our institutions14 and several investigators15,16 have used topical TXA in TJA at a concentration of 100 mg/mL, so this was the initial TXA concentration we decided to study. We selected certain time points to allow for relatively early detection of cartilage damage and then followed it to 48 hours of exposure. In cases in which TXA is injected after capsular closure, it is unclear how rapidly the TXA diffuses out of the joint or to what degree it becomes diluted by bleeding or synovial fluid. Certainly, this varies from patient to patient. Clearly, TXA generally passes through the body unmodified when injected intravenously1 and therefore is unlikely to be chemically modified while in the joint.
Very little has been published on use of topical TXA in other orthopedic surgeries, such as intra-articular fracture fixation, ligament reconstruction, hemiarthroplasty, and unicompartmental arthroplasty. Unlike TJA, which removes all native cartilage, these procedures retain and depend on the viability of the native cartilage. Sitek and colleagues17 noted the effect of TXA on chondrocytes within the context of creating an extracellular fibrin matrix for chondrocyte transplant. There was no decrease in chondrocyte viability with TXA 10 mg/mL or 20 mg/mL. Use of fresh bovine cartilage explants as a model for the in vitro study of cartilage damage is well established, including chondrocyte viability and glycosaminoglycan (GAG) release as outcome measures.18,19 Human cartilage has also been studied in vitro using this model.20
In the present study, the primary goal was to test the hypothesis that TXA could be safely used in the presence of native cartilage. The secondary goal was to identify a safe concentration for intra-articular use if toxic characteristics were noted.
Materials and Methods
Young bovine stifle joints were obtained within 3 hours of slaughter at a local abattoir. The joint was disarticulated under sterile conditions, and the distal femoral articular surface was evaluated for any signs of damage or arthritis. All specimens contained healthy, undamaged articular surfaces. Full-thickness cartilage explants (excluding subchondral bone) were then immediately harvested—with use of a scalpel blade and a dermatologic biopsy punch 4 mm in diameter—from the distal, weight-bearing femur. The explants were placed in 24-well tissue-culture plates (USA Scientific), incubated in culture media (high-glucose Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, 1% penicillin/streptomycin, 1% fungizone; Life Technologies), and kept at 37°C and 5% CO2. Explants were allowed to rest in culture media for a minimum of 24 hours after harvest. The pH of the medium was not altered by the addition of TXA.
Bovine explants were randomly assigned to either TXA-exposure or control groups at several time points in replicates of 6. Culture medium was aspirated, and each explant was washed twice with sterile phosphate-buffered saline (PBS). Explants were then incubated at 37°C in culture medium as previously described, or in the same culture medium containing dissolved TXA at a concentration of 100 mg/mL. The explants were incubated at 37°C until harvest at 8, 24, or 48 hours after media addition. For harvest, the media were aspirated and stored at –20°C for GAG content analysis, and the explants were then harvested for LIVE/DEAD assay (Life Technologies) and GAG content analysis.
Explants were washed once in 75% ethanol and then digested in 0.5 mL papain digestion buffer (100 mM sodium phosphate, 10 mM EDTA, 10 mM L-cysteine, 0.125 mg/mL papain; Sigma-Aldrich) at 60°C for 24 hours. Digested samples were then diluted and subjected to GAG analysis.
Murine chondrocytes were harvested from the freshly harvested rib cages and sternums of mice (1-4 days old) as previously described.21 In brief, rib cages were washed twice in D-PBS and then incubated at 37°C for 60 min in 5 mL of 0.25% Trypsin-EDTA (Life Technologies). They were then washed in DMEM with 10% FBS, centrifuged at 1500 rpm for 5 min to remove the supernatant, and washed in sterile PBS. After removal of the PBS wash, the ribs were incubated in 2 mg/mL hyaluronidase in plain DMEM on a shaker at 37°C for 2 hours. Once soft tissue was removed, the rib cages were discarded, and the remaining soft tissue was incubated in a collagenase D/hyaluronidase digestion solution (collagenase D, 1 mg/mL; hyaluronidase, 1 mg/mL; BSA, 40 mg/mL in plain DMEM; Life Technologies) for 8 hours. The resultant cell suspension was filtered through a 40-µm cell strainer (BD Falcon). Isolated chondrocytes were then plated on culture slides (0.5×x106 cells; BD Falcon) and incubated in DMEM/F12 (1:1) complete media at 37°C and 5% CO2. Before experimental treatment, all cultures were visualized under phase microscopy to verify viability and morphology.
Murine chondrocytes were incubated in media (described above) containing TXA 0, 25, 50, or 100 mg/mL and were harvested 8, 24, or 48 hours after initial exposure. Cultures were maintained at 37°C and 5% CO2 until harvest. Culture medium was aspirated, and each sample was washed twice in sterile PBS before analysis with the LIVE/DEAD assay.
The amount of GAG released into the culture media was measured with a 1,9-dimethyl-methylene blue colorimetric assay (DMMB; 38.5 µM 1,9-dimethylmethylene blue, 40 mM glycine, 40.5 mM sodium chloride, 9.5 mM hydrochloric acid; Sigma-Aldrich) based on the method of Farndale and colleagues.22 In brief, 20 µL of media was mixed with the DMMB assay solution in a 96-well plate, and absorbance was read immediately at 530 nm on a microplate reader. Chondroitin 4-sulfate was used to produce a standard curve. Total GAG released into the media was then calculated based on the standard curve and calculated as a percentage of the total GAG content of each explant. Each sample time point and concentration had a replicate of 3.
Chondrocyte viability was assessed with use of the LIVE/DEAD Viability/Cytotoxicity Kit (Life Technologies) following the protocol. Cartilage explants were sectioned orthogonally to the articular surface at 100 µm per section. Four sections were obtained from each explant. Sections were then incubated in 60 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at room temperature in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 3 digital photographs (magnification ×4) were taken per sample with use of a fluorescein filter and a Texas red filter. The live and dead cells in an area were quantified with use of ImageJ, freely available image analysis software.23 This software was verified initially by blinded, manual count for accuracy. Each sample time point and concentration had a replicate of 3 or 4 explants.
Chondrocyte viability was assessed with the LIVE/DEAD Viability/Cytotoxicity Kit following the protocol. Slides were incubated in 200 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at 37°C in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 4 digital photographs (magnification ×4) were taken with use of a fluorescein filter and a Texas red filter. Live and dead cells in an area were quantified with use of ImageJ. Each sample time point and concentration had a replicate of 4 plates.
Statistical analyses were performed in the statistical environment R.24 Data were analyzed with a 2-tailed Student t test with Holm-Bonferroni correction made for multiple comparisons, and a family-wise error rate was set at α = 0.05.
Results
GAG release was notably higher in the explants exposed to TXA 100 mg/mL at all time points (Figure 1). Beginning 8 hours after initial incubation, there was a small but significant (P = .01) loss of GAG in TXA-treated explants (mean, 1.86%; SD, 0.44%) versus control explants (mean, 0.31%; SD, 0.24%). There was a trend of increasing loss with increasing time after initial incubation through 24 hours, GAG (mean, 3.92%; SD, 0.83%) versus control (mean, 1.63%; SD, 0.65%) (P = .02), reaching a peak at 48 hours, GAG (mean, 8.29%; SD, 1.82%) versus control (mean, 3.19%; SD, 0.53%) (P = .03).
Cell viability was notably higher in the control groups 24 and 48 hours after initial incubation (Figure 2), with a visually observable (Figure 3) but variable and nonsignificant (P = .33) difference in viability at 8 hours, control (mean, 63.87%; SD, 13.63%) versus TXA (mean, 46.08%; SD, 22.51%). As incubation time increased from 8 hours, there were significant decreases in cell viability at 24 hours (mean, 39.28%; SD, 4.12%; P = .024) and 48 hours (mean, 21.98%; SD, 2.15%; P = .0005) relative to controls.
After results of exposing murine cells to TXA at different concentrations were obtained, bovine explants were exposed to TXA 25 mg/mL, and viability was recorded 24 and 48 hours after exposure (Figure 4). There was no significant difference in viability between samples.
Cell viability was similar between the TXA 25 mg/mL and control samples at all time points (Figure 5). The TXA 50 mg/mL sample dropped from 66.51% viability at 8 hours to 6.81% viability at 24 hours and complete cell death by 48 hours. The TXA 100 mg/mL samples had no observable viable cells at 8, 24, and 48 hours (Figure 6, confirmed with light microscopy). The TXA 0 mg/mL and 25 mg/mL samples remained largely unchanged: 78.28% and 92.99% viable at 8 hours, 97.29% and 90.22% viable at 24 hours, and 91.62% and 91.35% viable at 48 hours, respectively. See Figures 4 and 5 for viability at all time points and concentrations. Statistical analyses were not performed on these data because the zero values obtained for all samples incubated in TXA 100 mg/mL and the 48-hour TXA 50 mg/mL samples prevented accurate estimation of P values and thus meaningful comparisons of the treatment groups.
Discussion
The results of this study showed that TXA is cytotoxic to both bovine and murine chondrocytes at a concentration of 100 mg/mL. There is a time-dependent increase in GAG release as well as a decrease in chondrocyte viability in intact bovine cartilage. These data suggest that topical or intra-articular administration of TXA at this concentration in the setting of native cartilage may have unintended, detrimental effects.
Murine chondrocyte monolayer cultures exposed to TXA at lower concentrations did not exhibit a concentration-dependent curve with respect to viability. Chondrocytes exposed to TXA 25 mg/mL had no reduction in viability relative to control samples. When the concentration was doubled to 50 mg/mL, however, viability was reduced to 6.81% by 24 hours (Figure 5). These data suggest that, between 25 mg/mL and 50 mg/mL, there is a concentration at which TXA becomes cytotoxic to murine chondrocytes. It should be cautioned that, though TXA was cytotoxic to chondrocytes in this study, the effects are still unknown and indeed may be similar to effects on other types of cells that are present in a replaced joint—such as synovial cells, inflammatory cells, and osteoblasts.
The unaffected viability of murine chondrocytes with TXA 25 mg/mL indicated that this may be a cutoff concentration for safety in the presence of cartilage. To confirm these results, we exposed the bovine explants to TXA 25 mg/mL as well. Consistent with the prior study, chondrocyte viability was unaffected at 48 hours. Some clinical studies have effectively used topical TXA at this concentration, or at a lower concentration, to reduce blood loss in TJA,25 which suggests that 25 mg/mL may be a safe yet effective dose for clinical use of topical TXA.
As the methods used in this study did not distinguish between late-apoptotic and necrotic cell death, we could not determine which mechanism of death led to the viability loss observed. If apoptosis is occurring, how TXA initiates this sequence is unclear. There have been no studies directly linking TXA to apoptotic events, though some studies have indicated that TXA interacts with several molecules other than plasminogen, including GABA (γ-aminobutyric acid) receptors, glycine receptors, and tachykinin neurokinin 1 receptors.26-28 According to these studies, these interactions may be responsible for seizure activity and increased emesis caused by TXA use. In addition, TXA-containing compounds, such as trans-aminomethylcyclohexanecarbonyl-l-(O-picolyl)tyrosine-octylamide, have been shown to induce apoptosis.29
It appears that the extracellular matrix (ECM) of native cartilage explants has a protective effect on chondrocytes. With exposure to TXA 100 mg/mL, the explants retained 52% viability at 24 hours, whereas the monolayer cultures were nonviable at that point. The weak negative charge of the molecule may retard its penetration into the ECM, though there was an inconsistent presentation of cell death at explant superficial zones in treated samples (Figure 3). Consistent surface layer cell death would be expected if slowed penetration were the only protective mechanism. It is possible that the ECM acts as a buffer or solvent, effectively reducing the concentration of TXA directly interacting with the chondrocytes. Further exploration is needed to elucidate the significance of the ECM in protecting chondrocytes from TXA.
Although its findings were highly reproducible, the present study had several limitations, including its in vitro nature and its use of a bovine and murine model rather than a human cell and tissue platform. It may be prudent to expose chondrocytes to TXA for a shorter time to try to mimic what theoretically occurs in vivo. In vivo studies may be a reasonable direction for experimentation. Clarifying the mechanism of cell death is of experimental interest as well. As the first of its kind, the present study provides an important initial database for exploration.
This study is the first to show that TXA has a cytotoxic effect on chondrocytes and that it damages cartilage at clinically used concentrations. Although more studies are needed to verify a safe concentration of TXA for topical use with human cartilage, our data indicate that TXA 25 mg/mL may be an effective yet safe dose for intra-articular use in native joints.
1. McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72(5):585-617.
2. Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350-353.
3. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119.
4. Ker K, Prieto‐Merino D, Roberts I. Systematic review, meta‐analysis and meta‐regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100(10):1271-1279.
5. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309.
6. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968.
7. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
8. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
9. Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2611-2617.
10. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
11. Aguilera-Roig X, Jordán-Sales M, Natera-Cisneros L, Monllau-García JC, Martínez-Zapata MJ. Tranexamic acid in orthopedic surgery [in Spanish]. Rev Esp Cir Ortop Traumatol. 2014;58(1):52-56.
12. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
13. Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):78-82.
14. Tuttle JR, Ritterman SA, Cassidy DB, Anazonwu WA, Froehlich JA, Rubin LE. Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty. 2014;29(8):1512-1515.
15. Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2494-2501.
16. Ishida K, Tsumura N, Kitagawa A, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35(11):1639-1645.
17. Sitek P, Wysocka-Wycisk A, Kępski F, Król D, Bursig H, Dyląg S. PRP-fibrinogen gel-like chondrocyte carrier stabilized by TXA-preliminary study. Cell Tissue Bank. 2013;14(1):133-140.
18. Lo IK, Sciore P, Chung M, et al. Local anesthetics induce chondrocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy. 2009;25(7):707-715.
19. Blumberg TJ, Natoli RM, Athanasiou KA. Effects of doxycycline on articular cartilage GAG release and mechanical properties following impact. Biotechnol Bioeng. 2008;100(3):506-515.
20. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90(5):986-991.
21. Lefebvre V, Garofalo S, Zhou G, Metsäranta M, Vuorio E, De Crombrugghe B. Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. Matrix Biol. 1994;14(4):329-335.
22. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;833(2):173-177.
23. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-675.
24. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008.
25. Sa-Ngasoongsong P, Channoom T, Kawinwonggowit V, et al. Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-hour clamp drain: a prospective triple-blinded randomized controlled trial. Orthop Rev. 2011;3(2):e12.
26. Lecker I, Wang DS, Romaschin AD, Peterson M, Mazer CD, Orser BA. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest. 2012;122(12):4654-4666.
27. Kakiuchi H, Kawarai-Shimamura A, Kuwagata M, Orito K. Tranexamic acid induces kaolin intake stimulating a pathway involving tachykinin neurokinin 1 receptors in rats. Eur J Pharmacol. 2014;723:1-6.
28. Kratzer S, Irl H, Mattusch C, et al. Tranexamic acid impairs γ-aminobutyric acid receptor type A–mediated synaptic transmission in the murine amygdala: a potential mechanism for drug-induced seizures? Anesthesiology. 2014;120(3):639-649.
29. Lee E, Enomoto R, Takemura K, Tsuda Y, Okada Y. A selective plasmin inhibitor, trans-aminomethylcyclohexanecarbonyl-L-(O-picolyl)tyrosine-octylamide (YO-2), induces thymocyte apoptosis. Biochem Pharmacol. 2002;63(7):1315-1323.
For decades, tranexamic acid (TXA) has been used off-label to reduce perioperative blood loss in various surgical procedures, including orthopedic surgery, neurosurgery, urologic surgery, obstetrics and gynecology, and trauma surgery.1 TXA, a synthetic derivative of the amino acid lysine, produces antifibrinolytic activity by competitively inhibiting lysine-binding sites on plasminogen molecules—inhibiting the activation of plasmin and thus preserving the function of fibrin in clot formation. It is believed that, through this method, TXA retains blood clots more effectively, thereby reducing bleeding. Although intravenous delivery of TXA is generally accepted as safe, some studies have indicated that it may contribute to postoperative seizure activity as well as increased thromboembolic events.2,3 For these and other reasons, interest in topical (intra-articular) administration of TXA has increased.
Use of topical TXA in surgery has been expanding over the past several years, with reports of significant reductions in perioperative blood loss and transfusion requirements.4 Orthopedic surgeons specifically have explored the topical use of TXA, especially in total joint arthroplasty (TJA).5 The benefits are increased concentration at the operative site with reduced systemic exposure; cost reduction; and surgeon control.6,7 Several recent studies have yielded significant reductions in perioperative blood loss and transfusions with use of topical TXA.8-10 In the literature, effective dosing for topical TXA in TJA ranges from 250 mg to 3 g.11 The concentration of topical TXA is not consistently described but appears to fall between 15 and 100 mg/mL.12,13 Our institutions14 and several investigators15,16 have used topical TXA in TJA at a concentration of 100 mg/mL, so this was the initial TXA concentration we decided to study. We selected certain time points to allow for relatively early detection of cartilage damage and then followed it to 48 hours of exposure. In cases in which TXA is injected after capsular closure, it is unclear how rapidly the TXA diffuses out of the joint or to what degree it becomes diluted by bleeding or synovial fluid. Certainly, this varies from patient to patient. Clearly, TXA generally passes through the body unmodified when injected intravenously1 and therefore is unlikely to be chemically modified while in the joint.
Very little has been published on use of topical TXA in other orthopedic surgeries, such as intra-articular fracture fixation, ligament reconstruction, hemiarthroplasty, and unicompartmental arthroplasty. Unlike TJA, which removes all native cartilage, these procedures retain and depend on the viability of the native cartilage. Sitek and colleagues17 noted the effect of TXA on chondrocytes within the context of creating an extracellular fibrin matrix for chondrocyte transplant. There was no decrease in chondrocyte viability with TXA 10 mg/mL or 20 mg/mL. Use of fresh bovine cartilage explants as a model for the in vitro study of cartilage damage is well established, including chondrocyte viability and glycosaminoglycan (GAG) release as outcome measures.18,19 Human cartilage has also been studied in vitro using this model.20
In the present study, the primary goal was to test the hypothesis that TXA could be safely used in the presence of native cartilage. The secondary goal was to identify a safe concentration for intra-articular use if toxic characteristics were noted.
Materials and Methods
Young bovine stifle joints were obtained within 3 hours of slaughter at a local abattoir. The joint was disarticulated under sterile conditions, and the distal femoral articular surface was evaluated for any signs of damage or arthritis. All specimens contained healthy, undamaged articular surfaces. Full-thickness cartilage explants (excluding subchondral bone) were then immediately harvested—with use of a scalpel blade and a dermatologic biopsy punch 4 mm in diameter—from the distal, weight-bearing femur. The explants were placed in 24-well tissue-culture plates (USA Scientific), incubated in culture media (high-glucose Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, 1% penicillin/streptomycin, 1% fungizone; Life Technologies), and kept at 37°C and 5% CO2. Explants were allowed to rest in culture media for a minimum of 24 hours after harvest. The pH of the medium was not altered by the addition of TXA.
Bovine explants were randomly assigned to either TXA-exposure or control groups at several time points in replicates of 6. Culture medium was aspirated, and each explant was washed twice with sterile phosphate-buffered saline (PBS). Explants were then incubated at 37°C in culture medium as previously described, or in the same culture medium containing dissolved TXA at a concentration of 100 mg/mL. The explants were incubated at 37°C until harvest at 8, 24, or 48 hours after media addition. For harvest, the media were aspirated and stored at –20°C for GAG content analysis, and the explants were then harvested for LIVE/DEAD assay (Life Technologies) and GAG content analysis.
Explants were washed once in 75% ethanol and then digested in 0.5 mL papain digestion buffer (100 mM sodium phosphate, 10 mM EDTA, 10 mM L-cysteine, 0.125 mg/mL papain; Sigma-Aldrich) at 60°C for 24 hours. Digested samples were then diluted and subjected to GAG analysis.
Murine chondrocytes were harvested from the freshly harvested rib cages and sternums of mice (1-4 days old) as previously described.21 In brief, rib cages were washed twice in D-PBS and then incubated at 37°C for 60 min in 5 mL of 0.25% Trypsin-EDTA (Life Technologies). They were then washed in DMEM with 10% FBS, centrifuged at 1500 rpm for 5 min to remove the supernatant, and washed in sterile PBS. After removal of the PBS wash, the ribs were incubated in 2 mg/mL hyaluronidase in plain DMEM on a shaker at 37°C for 2 hours. Once soft tissue was removed, the rib cages were discarded, and the remaining soft tissue was incubated in a collagenase D/hyaluronidase digestion solution (collagenase D, 1 mg/mL; hyaluronidase, 1 mg/mL; BSA, 40 mg/mL in plain DMEM; Life Technologies) for 8 hours. The resultant cell suspension was filtered through a 40-µm cell strainer (BD Falcon). Isolated chondrocytes were then plated on culture slides (0.5×x106 cells; BD Falcon) and incubated in DMEM/F12 (1:1) complete media at 37°C and 5% CO2. Before experimental treatment, all cultures were visualized under phase microscopy to verify viability and morphology.
Murine chondrocytes were incubated in media (described above) containing TXA 0, 25, 50, or 100 mg/mL and were harvested 8, 24, or 48 hours after initial exposure. Cultures were maintained at 37°C and 5% CO2 until harvest. Culture medium was aspirated, and each sample was washed twice in sterile PBS before analysis with the LIVE/DEAD assay.
The amount of GAG released into the culture media was measured with a 1,9-dimethyl-methylene blue colorimetric assay (DMMB; 38.5 µM 1,9-dimethylmethylene blue, 40 mM glycine, 40.5 mM sodium chloride, 9.5 mM hydrochloric acid; Sigma-Aldrich) based on the method of Farndale and colleagues.22 In brief, 20 µL of media was mixed with the DMMB assay solution in a 96-well plate, and absorbance was read immediately at 530 nm on a microplate reader. Chondroitin 4-sulfate was used to produce a standard curve. Total GAG released into the media was then calculated based on the standard curve and calculated as a percentage of the total GAG content of each explant. Each sample time point and concentration had a replicate of 3.
Chondrocyte viability was assessed with use of the LIVE/DEAD Viability/Cytotoxicity Kit (Life Technologies) following the protocol. Cartilage explants were sectioned orthogonally to the articular surface at 100 µm per section. Four sections were obtained from each explant. Sections were then incubated in 60 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at room temperature in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 3 digital photographs (magnification ×4) were taken per sample with use of a fluorescein filter and a Texas red filter. The live and dead cells in an area were quantified with use of ImageJ, freely available image analysis software.23 This software was verified initially by blinded, manual count for accuracy. Each sample time point and concentration had a replicate of 3 or 4 explants.
Chondrocyte viability was assessed with the LIVE/DEAD Viability/Cytotoxicity Kit following the protocol. Slides were incubated in 200 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at 37°C in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 4 digital photographs (magnification ×4) were taken with use of a fluorescein filter and a Texas red filter. Live and dead cells in an area were quantified with use of ImageJ. Each sample time point and concentration had a replicate of 4 plates.
Statistical analyses were performed in the statistical environment R.24 Data were analyzed with a 2-tailed Student t test with Holm-Bonferroni correction made for multiple comparisons, and a family-wise error rate was set at α = 0.05.
Results
GAG release was notably higher in the explants exposed to TXA 100 mg/mL at all time points (Figure 1). Beginning 8 hours after initial incubation, there was a small but significant (P = .01) loss of GAG in TXA-treated explants (mean, 1.86%; SD, 0.44%) versus control explants (mean, 0.31%; SD, 0.24%). There was a trend of increasing loss with increasing time after initial incubation through 24 hours, GAG (mean, 3.92%; SD, 0.83%) versus control (mean, 1.63%; SD, 0.65%) (P = .02), reaching a peak at 48 hours, GAG (mean, 8.29%; SD, 1.82%) versus control (mean, 3.19%; SD, 0.53%) (P = .03).
Cell viability was notably higher in the control groups 24 and 48 hours after initial incubation (Figure 2), with a visually observable (Figure 3) but variable and nonsignificant (P = .33) difference in viability at 8 hours, control (mean, 63.87%; SD, 13.63%) versus TXA (mean, 46.08%; SD, 22.51%). As incubation time increased from 8 hours, there were significant decreases in cell viability at 24 hours (mean, 39.28%; SD, 4.12%; P = .024) and 48 hours (mean, 21.98%; SD, 2.15%; P = .0005) relative to controls.
After results of exposing murine cells to TXA at different concentrations were obtained, bovine explants were exposed to TXA 25 mg/mL, and viability was recorded 24 and 48 hours after exposure (Figure 4). There was no significant difference in viability between samples.
Cell viability was similar between the TXA 25 mg/mL and control samples at all time points (Figure 5). The TXA 50 mg/mL sample dropped from 66.51% viability at 8 hours to 6.81% viability at 24 hours and complete cell death by 48 hours. The TXA 100 mg/mL samples had no observable viable cells at 8, 24, and 48 hours (Figure 6, confirmed with light microscopy). The TXA 0 mg/mL and 25 mg/mL samples remained largely unchanged: 78.28% and 92.99% viable at 8 hours, 97.29% and 90.22% viable at 24 hours, and 91.62% and 91.35% viable at 48 hours, respectively. See Figures 4 and 5 for viability at all time points and concentrations. Statistical analyses were not performed on these data because the zero values obtained for all samples incubated in TXA 100 mg/mL and the 48-hour TXA 50 mg/mL samples prevented accurate estimation of P values and thus meaningful comparisons of the treatment groups.
Discussion
The results of this study showed that TXA is cytotoxic to both bovine and murine chondrocytes at a concentration of 100 mg/mL. There is a time-dependent increase in GAG release as well as a decrease in chondrocyte viability in intact bovine cartilage. These data suggest that topical or intra-articular administration of TXA at this concentration in the setting of native cartilage may have unintended, detrimental effects.
Murine chondrocyte monolayer cultures exposed to TXA at lower concentrations did not exhibit a concentration-dependent curve with respect to viability. Chondrocytes exposed to TXA 25 mg/mL had no reduction in viability relative to control samples. When the concentration was doubled to 50 mg/mL, however, viability was reduced to 6.81% by 24 hours (Figure 5). These data suggest that, between 25 mg/mL and 50 mg/mL, there is a concentration at which TXA becomes cytotoxic to murine chondrocytes. It should be cautioned that, though TXA was cytotoxic to chondrocytes in this study, the effects are still unknown and indeed may be similar to effects on other types of cells that are present in a replaced joint—such as synovial cells, inflammatory cells, and osteoblasts.
The unaffected viability of murine chondrocytes with TXA 25 mg/mL indicated that this may be a cutoff concentration for safety in the presence of cartilage. To confirm these results, we exposed the bovine explants to TXA 25 mg/mL as well. Consistent with the prior study, chondrocyte viability was unaffected at 48 hours. Some clinical studies have effectively used topical TXA at this concentration, or at a lower concentration, to reduce blood loss in TJA,25 which suggests that 25 mg/mL may be a safe yet effective dose for clinical use of topical TXA.
As the methods used in this study did not distinguish between late-apoptotic and necrotic cell death, we could not determine which mechanism of death led to the viability loss observed. If apoptosis is occurring, how TXA initiates this sequence is unclear. There have been no studies directly linking TXA to apoptotic events, though some studies have indicated that TXA interacts with several molecules other than plasminogen, including GABA (γ-aminobutyric acid) receptors, glycine receptors, and tachykinin neurokinin 1 receptors.26-28 According to these studies, these interactions may be responsible for seizure activity and increased emesis caused by TXA use. In addition, TXA-containing compounds, such as trans-aminomethylcyclohexanecarbonyl-l-(O-picolyl)tyrosine-octylamide, have been shown to induce apoptosis.29
It appears that the extracellular matrix (ECM) of native cartilage explants has a protective effect on chondrocytes. With exposure to TXA 100 mg/mL, the explants retained 52% viability at 24 hours, whereas the monolayer cultures were nonviable at that point. The weak negative charge of the molecule may retard its penetration into the ECM, though there was an inconsistent presentation of cell death at explant superficial zones in treated samples (Figure 3). Consistent surface layer cell death would be expected if slowed penetration were the only protective mechanism. It is possible that the ECM acts as a buffer or solvent, effectively reducing the concentration of TXA directly interacting with the chondrocytes. Further exploration is needed to elucidate the significance of the ECM in protecting chondrocytes from TXA.
Although its findings were highly reproducible, the present study had several limitations, including its in vitro nature and its use of a bovine and murine model rather than a human cell and tissue platform. It may be prudent to expose chondrocytes to TXA for a shorter time to try to mimic what theoretically occurs in vivo. In vivo studies may be a reasonable direction for experimentation. Clarifying the mechanism of cell death is of experimental interest as well. As the first of its kind, the present study provides an important initial database for exploration.
This study is the first to show that TXA has a cytotoxic effect on chondrocytes and that it damages cartilage at clinically used concentrations. Although more studies are needed to verify a safe concentration of TXA for topical use with human cartilage, our data indicate that TXA 25 mg/mL may be an effective yet safe dose for intra-articular use in native joints.
For decades, tranexamic acid (TXA) has been used off-label to reduce perioperative blood loss in various surgical procedures, including orthopedic surgery, neurosurgery, urologic surgery, obstetrics and gynecology, and trauma surgery.1 TXA, a synthetic derivative of the amino acid lysine, produces antifibrinolytic activity by competitively inhibiting lysine-binding sites on plasminogen molecules—inhibiting the activation of plasmin and thus preserving the function of fibrin in clot formation. It is believed that, through this method, TXA retains blood clots more effectively, thereby reducing bleeding. Although intravenous delivery of TXA is generally accepted as safe, some studies have indicated that it may contribute to postoperative seizure activity as well as increased thromboembolic events.2,3 For these and other reasons, interest in topical (intra-articular) administration of TXA has increased.
Use of topical TXA in surgery has been expanding over the past several years, with reports of significant reductions in perioperative blood loss and transfusion requirements.4 Orthopedic surgeons specifically have explored the topical use of TXA, especially in total joint arthroplasty (TJA).5 The benefits are increased concentration at the operative site with reduced systemic exposure; cost reduction; and surgeon control.6,7 Several recent studies have yielded significant reductions in perioperative blood loss and transfusions with use of topical TXA.8-10 In the literature, effective dosing for topical TXA in TJA ranges from 250 mg to 3 g.11 The concentration of topical TXA is not consistently described but appears to fall between 15 and 100 mg/mL.12,13 Our institutions14 and several investigators15,16 have used topical TXA in TJA at a concentration of 100 mg/mL, so this was the initial TXA concentration we decided to study. We selected certain time points to allow for relatively early detection of cartilage damage and then followed it to 48 hours of exposure. In cases in which TXA is injected after capsular closure, it is unclear how rapidly the TXA diffuses out of the joint or to what degree it becomes diluted by bleeding or synovial fluid. Certainly, this varies from patient to patient. Clearly, TXA generally passes through the body unmodified when injected intravenously1 and therefore is unlikely to be chemically modified while in the joint.
Very little has been published on use of topical TXA in other orthopedic surgeries, such as intra-articular fracture fixation, ligament reconstruction, hemiarthroplasty, and unicompartmental arthroplasty. Unlike TJA, which removes all native cartilage, these procedures retain and depend on the viability of the native cartilage. Sitek and colleagues17 noted the effect of TXA on chondrocytes within the context of creating an extracellular fibrin matrix for chondrocyte transplant. There was no decrease in chondrocyte viability with TXA 10 mg/mL or 20 mg/mL. Use of fresh bovine cartilage explants as a model for the in vitro study of cartilage damage is well established, including chondrocyte viability and glycosaminoglycan (GAG) release as outcome measures.18,19 Human cartilage has also been studied in vitro using this model.20
In the present study, the primary goal was to test the hypothesis that TXA could be safely used in the presence of native cartilage. The secondary goal was to identify a safe concentration for intra-articular use if toxic characteristics were noted.
Materials and Methods
Young bovine stifle joints were obtained within 3 hours of slaughter at a local abattoir. The joint was disarticulated under sterile conditions, and the distal femoral articular surface was evaluated for any signs of damage or arthritis. All specimens contained healthy, undamaged articular surfaces. Full-thickness cartilage explants (excluding subchondral bone) were then immediately harvested—with use of a scalpel blade and a dermatologic biopsy punch 4 mm in diameter—from the distal, weight-bearing femur. The explants were placed in 24-well tissue-culture plates (USA Scientific), incubated in culture media (high-glucose Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, 1% penicillin/streptomycin, 1% fungizone; Life Technologies), and kept at 37°C and 5% CO2. Explants were allowed to rest in culture media for a minimum of 24 hours after harvest. The pH of the medium was not altered by the addition of TXA.
Bovine explants were randomly assigned to either TXA-exposure or control groups at several time points in replicates of 6. Culture medium was aspirated, and each explant was washed twice with sterile phosphate-buffered saline (PBS). Explants were then incubated at 37°C in culture medium as previously described, or in the same culture medium containing dissolved TXA at a concentration of 100 mg/mL. The explants were incubated at 37°C until harvest at 8, 24, or 48 hours after media addition. For harvest, the media were aspirated and stored at –20°C for GAG content analysis, and the explants were then harvested for LIVE/DEAD assay (Life Technologies) and GAG content analysis.
Explants were washed once in 75% ethanol and then digested in 0.5 mL papain digestion buffer (100 mM sodium phosphate, 10 mM EDTA, 10 mM L-cysteine, 0.125 mg/mL papain; Sigma-Aldrich) at 60°C for 24 hours. Digested samples were then diluted and subjected to GAG analysis.
Murine chondrocytes were harvested from the freshly harvested rib cages and sternums of mice (1-4 days old) as previously described.21 In brief, rib cages were washed twice in D-PBS and then incubated at 37°C for 60 min in 5 mL of 0.25% Trypsin-EDTA (Life Technologies). They were then washed in DMEM with 10% FBS, centrifuged at 1500 rpm for 5 min to remove the supernatant, and washed in sterile PBS. After removal of the PBS wash, the ribs were incubated in 2 mg/mL hyaluronidase in plain DMEM on a shaker at 37°C for 2 hours. Once soft tissue was removed, the rib cages were discarded, and the remaining soft tissue was incubated in a collagenase D/hyaluronidase digestion solution (collagenase D, 1 mg/mL; hyaluronidase, 1 mg/mL; BSA, 40 mg/mL in plain DMEM; Life Technologies) for 8 hours. The resultant cell suspension was filtered through a 40-µm cell strainer (BD Falcon). Isolated chondrocytes were then plated on culture slides (0.5×x106 cells; BD Falcon) and incubated in DMEM/F12 (1:1) complete media at 37°C and 5% CO2. Before experimental treatment, all cultures were visualized under phase microscopy to verify viability and morphology.
Murine chondrocytes were incubated in media (described above) containing TXA 0, 25, 50, or 100 mg/mL and were harvested 8, 24, or 48 hours after initial exposure. Cultures were maintained at 37°C and 5% CO2 until harvest. Culture medium was aspirated, and each sample was washed twice in sterile PBS before analysis with the LIVE/DEAD assay.
The amount of GAG released into the culture media was measured with a 1,9-dimethyl-methylene blue colorimetric assay (DMMB; 38.5 µM 1,9-dimethylmethylene blue, 40 mM glycine, 40.5 mM sodium chloride, 9.5 mM hydrochloric acid; Sigma-Aldrich) based on the method of Farndale and colleagues.22 In brief, 20 µL of media was mixed with the DMMB assay solution in a 96-well plate, and absorbance was read immediately at 530 nm on a microplate reader. Chondroitin 4-sulfate was used to produce a standard curve. Total GAG released into the media was then calculated based on the standard curve and calculated as a percentage of the total GAG content of each explant. Each sample time point and concentration had a replicate of 3.
Chondrocyte viability was assessed with use of the LIVE/DEAD Viability/Cytotoxicity Kit (Life Technologies) following the protocol. Cartilage explants were sectioned orthogonally to the articular surface at 100 µm per section. Four sections were obtained from each explant. Sections were then incubated in 60 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at room temperature in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 3 digital photographs (magnification ×4) were taken per sample with use of a fluorescein filter and a Texas red filter. The live and dead cells in an area were quantified with use of ImageJ, freely available image analysis software.23 This software was verified initially by blinded, manual count for accuracy. Each sample time point and concentration had a replicate of 3 or 4 explants.
Chondrocyte viability was assessed with the LIVE/DEAD Viability/Cytotoxicity Kit following the protocol. Slides were incubated in 200 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at 37°C in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 4 digital photographs (magnification ×4) were taken with use of a fluorescein filter and a Texas red filter. Live and dead cells in an area were quantified with use of ImageJ. Each sample time point and concentration had a replicate of 4 plates.
Statistical analyses were performed in the statistical environment R.24 Data were analyzed with a 2-tailed Student t test with Holm-Bonferroni correction made for multiple comparisons, and a family-wise error rate was set at α = 0.05.
Results
GAG release was notably higher in the explants exposed to TXA 100 mg/mL at all time points (Figure 1). Beginning 8 hours after initial incubation, there was a small but significant (P = .01) loss of GAG in TXA-treated explants (mean, 1.86%; SD, 0.44%) versus control explants (mean, 0.31%; SD, 0.24%). There was a trend of increasing loss with increasing time after initial incubation through 24 hours, GAG (mean, 3.92%; SD, 0.83%) versus control (mean, 1.63%; SD, 0.65%) (P = .02), reaching a peak at 48 hours, GAG (mean, 8.29%; SD, 1.82%) versus control (mean, 3.19%; SD, 0.53%) (P = .03).
Cell viability was notably higher in the control groups 24 and 48 hours after initial incubation (Figure 2), with a visually observable (Figure 3) but variable and nonsignificant (P = .33) difference in viability at 8 hours, control (mean, 63.87%; SD, 13.63%) versus TXA (mean, 46.08%; SD, 22.51%). As incubation time increased from 8 hours, there were significant decreases in cell viability at 24 hours (mean, 39.28%; SD, 4.12%; P = .024) and 48 hours (mean, 21.98%; SD, 2.15%; P = .0005) relative to controls.
After results of exposing murine cells to TXA at different concentrations were obtained, bovine explants were exposed to TXA 25 mg/mL, and viability was recorded 24 and 48 hours after exposure (Figure 4). There was no significant difference in viability between samples.
Cell viability was similar between the TXA 25 mg/mL and control samples at all time points (Figure 5). The TXA 50 mg/mL sample dropped from 66.51% viability at 8 hours to 6.81% viability at 24 hours and complete cell death by 48 hours. The TXA 100 mg/mL samples had no observable viable cells at 8, 24, and 48 hours (Figure 6, confirmed with light microscopy). The TXA 0 mg/mL and 25 mg/mL samples remained largely unchanged: 78.28% and 92.99% viable at 8 hours, 97.29% and 90.22% viable at 24 hours, and 91.62% and 91.35% viable at 48 hours, respectively. See Figures 4 and 5 for viability at all time points and concentrations. Statistical analyses were not performed on these data because the zero values obtained for all samples incubated in TXA 100 mg/mL and the 48-hour TXA 50 mg/mL samples prevented accurate estimation of P values and thus meaningful comparisons of the treatment groups.
Discussion
The results of this study showed that TXA is cytotoxic to both bovine and murine chondrocytes at a concentration of 100 mg/mL. There is a time-dependent increase in GAG release as well as a decrease in chondrocyte viability in intact bovine cartilage. These data suggest that topical or intra-articular administration of TXA at this concentration in the setting of native cartilage may have unintended, detrimental effects.
Murine chondrocyte monolayer cultures exposed to TXA at lower concentrations did not exhibit a concentration-dependent curve with respect to viability. Chondrocytes exposed to TXA 25 mg/mL had no reduction in viability relative to control samples. When the concentration was doubled to 50 mg/mL, however, viability was reduced to 6.81% by 24 hours (Figure 5). These data suggest that, between 25 mg/mL and 50 mg/mL, there is a concentration at which TXA becomes cytotoxic to murine chondrocytes. It should be cautioned that, though TXA was cytotoxic to chondrocytes in this study, the effects are still unknown and indeed may be similar to effects on other types of cells that are present in a replaced joint—such as synovial cells, inflammatory cells, and osteoblasts.
The unaffected viability of murine chondrocytes with TXA 25 mg/mL indicated that this may be a cutoff concentration for safety in the presence of cartilage. To confirm these results, we exposed the bovine explants to TXA 25 mg/mL as well. Consistent with the prior study, chondrocyte viability was unaffected at 48 hours. Some clinical studies have effectively used topical TXA at this concentration, or at a lower concentration, to reduce blood loss in TJA,25 which suggests that 25 mg/mL may be a safe yet effective dose for clinical use of topical TXA.
As the methods used in this study did not distinguish between late-apoptotic and necrotic cell death, we could not determine which mechanism of death led to the viability loss observed. If apoptosis is occurring, how TXA initiates this sequence is unclear. There have been no studies directly linking TXA to apoptotic events, though some studies have indicated that TXA interacts with several molecules other than plasminogen, including GABA (γ-aminobutyric acid) receptors, glycine receptors, and tachykinin neurokinin 1 receptors.26-28 According to these studies, these interactions may be responsible for seizure activity and increased emesis caused by TXA use. In addition, TXA-containing compounds, such as trans-aminomethylcyclohexanecarbonyl-l-(O-picolyl)tyrosine-octylamide, have been shown to induce apoptosis.29
It appears that the extracellular matrix (ECM) of native cartilage explants has a protective effect on chondrocytes. With exposure to TXA 100 mg/mL, the explants retained 52% viability at 24 hours, whereas the monolayer cultures were nonviable at that point. The weak negative charge of the molecule may retard its penetration into the ECM, though there was an inconsistent presentation of cell death at explant superficial zones in treated samples (Figure 3). Consistent surface layer cell death would be expected if slowed penetration were the only protective mechanism. It is possible that the ECM acts as a buffer or solvent, effectively reducing the concentration of TXA directly interacting with the chondrocytes. Further exploration is needed to elucidate the significance of the ECM in protecting chondrocytes from TXA.
Although its findings were highly reproducible, the present study had several limitations, including its in vitro nature and its use of a bovine and murine model rather than a human cell and tissue platform. It may be prudent to expose chondrocytes to TXA for a shorter time to try to mimic what theoretically occurs in vivo. In vivo studies may be a reasonable direction for experimentation. Clarifying the mechanism of cell death is of experimental interest as well. As the first of its kind, the present study provides an important initial database for exploration.
This study is the first to show that TXA has a cytotoxic effect on chondrocytes and that it damages cartilage at clinically used concentrations. Although more studies are needed to verify a safe concentration of TXA for topical use with human cartilage, our data indicate that TXA 25 mg/mL may be an effective yet safe dose for intra-articular use in native joints.
1. McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72(5):585-617.
2. Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350-353.
3. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119.
4. Ker K, Prieto‐Merino D, Roberts I. Systematic review, meta‐analysis and meta‐regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100(10):1271-1279.
5. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309.
6. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968.
7. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
8. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
9. Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2611-2617.
10. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
11. Aguilera-Roig X, Jordán-Sales M, Natera-Cisneros L, Monllau-García JC, Martínez-Zapata MJ. Tranexamic acid in orthopedic surgery [in Spanish]. Rev Esp Cir Ortop Traumatol. 2014;58(1):52-56.
12. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
13. Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):78-82.
14. Tuttle JR, Ritterman SA, Cassidy DB, Anazonwu WA, Froehlich JA, Rubin LE. Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty. 2014;29(8):1512-1515.
15. Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2494-2501.
16. Ishida K, Tsumura N, Kitagawa A, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35(11):1639-1645.
17. Sitek P, Wysocka-Wycisk A, Kępski F, Król D, Bursig H, Dyląg S. PRP-fibrinogen gel-like chondrocyte carrier stabilized by TXA-preliminary study. Cell Tissue Bank. 2013;14(1):133-140.
18. Lo IK, Sciore P, Chung M, et al. Local anesthetics induce chondrocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy. 2009;25(7):707-715.
19. Blumberg TJ, Natoli RM, Athanasiou KA. Effects of doxycycline on articular cartilage GAG release and mechanical properties following impact. Biotechnol Bioeng. 2008;100(3):506-515.
20. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90(5):986-991.
21. Lefebvre V, Garofalo S, Zhou G, Metsäranta M, Vuorio E, De Crombrugghe B. Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. Matrix Biol. 1994;14(4):329-335.
22. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;833(2):173-177.
23. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-675.
24. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008.
25. Sa-Ngasoongsong P, Channoom T, Kawinwonggowit V, et al. Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-hour clamp drain: a prospective triple-blinded randomized controlled trial. Orthop Rev. 2011;3(2):e12.
26. Lecker I, Wang DS, Romaschin AD, Peterson M, Mazer CD, Orser BA. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest. 2012;122(12):4654-4666.
27. Kakiuchi H, Kawarai-Shimamura A, Kuwagata M, Orito K. Tranexamic acid induces kaolin intake stimulating a pathway involving tachykinin neurokinin 1 receptors in rats. Eur J Pharmacol. 2014;723:1-6.
28. Kratzer S, Irl H, Mattusch C, et al. Tranexamic acid impairs γ-aminobutyric acid receptor type A–mediated synaptic transmission in the murine amygdala: a potential mechanism for drug-induced seizures? Anesthesiology. 2014;120(3):639-649.
29. Lee E, Enomoto R, Takemura K, Tsuda Y, Okada Y. A selective plasmin inhibitor, trans-aminomethylcyclohexanecarbonyl-L-(O-picolyl)tyrosine-octylamide (YO-2), induces thymocyte apoptosis. Biochem Pharmacol. 2002;63(7):1315-1323.
1. McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72(5):585-617.
2. Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350-353.
3. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119.
4. Ker K, Prieto‐Merino D, Roberts I. Systematic review, meta‐analysis and meta‐regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100(10):1271-1279.
5. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309.
6. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968.
7. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
8. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
9. Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2611-2617.
10. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
11. Aguilera-Roig X, Jordán-Sales M, Natera-Cisneros L, Monllau-García JC, Martínez-Zapata MJ. Tranexamic acid in orthopedic surgery [in Spanish]. Rev Esp Cir Ortop Traumatol. 2014;58(1):52-56.
12. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
13. Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):78-82.
14. Tuttle JR, Ritterman SA, Cassidy DB, Anazonwu WA, Froehlich JA, Rubin LE. Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty. 2014;29(8):1512-1515.
15. Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2494-2501.
16. Ishida K, Tsumura N, Kitagawa A, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35(11):1639-1645.
17. Sitek P, Wysocka-Wycisk A, Kępski F, Król D, Bursig H, Dyląg S. PRP-fibrinogen gel-like chondrocyte carrier stabilized by TXA-preliminary study. Cell Tissue Bank. 2013;14(1):133-140.
18. Lo IK, Sciore P, Chung M, et al. Local anesthetics induce chondrocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy. 2009;25(7):707-715.
19. Blumberg TJ, Natoli RM, Athanasiou KA. Effects of doxycycline on articular cartilage GAG release and mechanical properties following impact. Biotechnol Bioeng. 2008;100(3):506-515.
20. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90(5):986-991.
21. Lefebvre V, Garofalo S, Zhou G, Metsäranta M, Vuorio E, De Crombrugghe B. Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. Matrix Biol. 1994;14(4):329-335.
22. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;833(2):173-177.
23. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-675.
24. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008.
25. Sa-Ngasoongsong P, Channoom T, Kawinwonggowit V, et al. Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-hour clamp drain: a prospective triple-blinded randomized controlled trial. Orthop Rev. 2011;3(2):e12.
26. Lecker I, Wang DS, Romaschin AD, Peterson M, Mazer CD, Orser BA. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest. 2012;122(12):4654-4666.
27. Kakiuchi H, Kawarai-Shimamura A, Kuwagata M, Orito K. Tranexamic acid induces kaolin intake stimulating a pathway involving tachykinin neurokinin 1 receptors in rats. Eur J Pharmacol. 2014;723:1-6.
28. Kratzer S, Irl H, Mattusch C, et al. Tranexamic acid impairs γ-aminobutyric acid receptor type A–mediated synaptic transmission in the murine amygdala: a potential mechanism for drug-induced seizures? Anesthesiology. 2014;120(3):639-649.
29. Lee E, Enomoto R, Takemura K, Tsuda Y, Okada Y. A selective plasmin inhibitor, trans-aminomethylcyclohexanecarbonyl-L-(O-picolyl)tyrosine-octylamide (YO-2), induces thymocyte apoptosis. Biochem Pharmacol. 2002;63(7):1315-1323.
Prevalence of Low Bone Mineral Density in Younger Versus Older Women With Distal Radius Fractures
Many organizations and work groups have issued recommendations regarding which patients should undergo bone densitometry. In 2004, the US Surgeon General recommended bone mineral density (BMD) evaluation for all women over age 65 years and for women and men with fragility fractures.1 The Centers for Medicare & Medicaid Services recommended BMD assessment for estrogen-deficient patients, for patients with vertebral abnormalities or hyperparathyroidism, and for patients receiving either steroid therapy or osteoporosis medications approved by the US Food and Drug Administration.2 The US Preventive Services Task Force and the National Osteoporosis Foundation each recommended screening for all women age 65 years or older and for postmenopausal women (age, 60-64 years) at high risk.3,4 The International Society for Clinical Densitometry (ISCD) recommended screening for all women age 65 years or older, all men age 70 years or older, and high-risk women under age 65 years.5
These current recommendations for BMD evaluation focus on women over age 65 years. More recent studies of postmenopausal women with distal radius fractures (DRFs) have found that both younger women (age, 45-65 years) and older women (age, ≥65 years) can have lower BMD and increased risk for hip and spine fracture.6,7 The authors of those studies recommended that all postmenopausal women with DRFs be evaluated for low BMD and that fracture prevention treatment be initiated. Earnshaw and colleagues8 and Oyen and colleagues9 found that men and women (age, ≥50 years) with DRFs had low BMD and elevated 10-year fracture rates. They concluded that BMD should be evaluated and treated in all DRF patients age 50 years or older. Other studies have shown low BMD in the contralateral distal radius of patients of all ages who presented with Colles fractures.10,11 These 2 studies did not measure spine or hip BMD.
The literature on BMD of younger women with DRFs is limited, relying solely on data collected for the contralateral distal radius.10,11 The ISCD recommended measuring both hip and spine BMD in premenopausal women. They also stated that z scores, not t scores, should be used for premenopausal women.5 The causes of low BMD in women over age 55 years are primarily nutritional deficiency and normal aging.1 In younger females, low BMD results from secondary causes, such as diet, medications, medical conditions, and endocrine disorders. When the secondary cause of low BMD can be identified and treated, osteoporosis can be stopped and even reversed in younger patients.12-14 Low BMD is more amenable to treatment in younger patients than in postmenopausal women. Younger patients with low BMD carry a higher lifetime fracture risk because they have more years of life with low BMD; therefore, early identification and treatment have a more significant impact on fracture prevention in these patients.
In the present study, we determined the prevalence of osteoporosis and osteopenia in younger women (age, 35-50 years) with DRFs and compared BMD measurements from younger women (age, 35-50 years) and older women (age, >50 years) with DRFs. The main goal was to determine which patients should be referred for bone densitometry and subsequent treatment.
Patients and Methods
This study received institutional review board approval. During a 5-year period (January 2005–August 2010), we prospectively collected dual-energy x-ray absorptiometry (DXA) scans for 128 women (age, >35 years) who presented with DRFs to our level I trauma center. Age ranged from 35 to 86 years. Data on mechanism of injury, treatment, and body mass index (BMI) were collected. The 128 patients were divided into a younger group (47 women; age range, 35-50 years; mean age, 44 years) and an older group (81 women; age, ≥51 years; mean age, 61 years). Mean BMI was 29.3 in the younger group and 28.8 in the older group (P = .88) (Table).
BMD was measured with a General Electric Lunar Prodigy Advance scanner that was tested annually for accuracy and precision. BMD of hips and lumbar spines was measured with a 76-Kv x-ray source. All DXA scans were analyzed by the same physician. BMD was omitted in cases of patients with a history of lumbar spine or hip fracture.
Two-sample Student t test was used to compare the 2 groups’ data. When multiple groups were being compared, analysis of variance was used. Spearman rank-order test was used to calculate a correlation coefficient for evaluation of the relationships between age and BMD.
Results
Mean lumbar spine (L1–L4) BMD was 1.12 in the younger group and 1.063 in the older group (P = .02); t scores were –0.63 and –1.132, respectively (P = .02); and mean z scores were –0.69 and –0.61, respectively (P = .81). Mean femoral neck BMD was 0.91 in the younger group and 0.80 in the older group (P < .05); t scores were –0.87 and –1.65, respectively (P < .01), and mean femoral neck z scores were –0.69 and –0.67, respectively (P = .92).
To further analyze BMD of specific age groups, we divided patients by decade: 35-39, 40-49, 50-59, 60-69, 70-79, 80-89 years. Among all 6 decades, there were no statistically significant differences between hip z scores (P = .83) (Figure 1). Spearman rank-order correlation test showed a moderate inverse correlation between age and femoral neck BMD (R = –0.42) and t score (R = –0.43). There was a weak correlation between increasing age and decreasing spine BMD, t score, and z score (Rs = –0.27, –0.31, 0.03). There was no correlation between age and femoral neck z score (R = –0.04).
According to the WHO classification system, 11 (23%) of the 47 women in the younger group were osteopenic, and 8 (17%) were osteoporotic, based on spine BMD. Hip BMD values indicated that 20 patients (43%) were osteopenic, and 3 (6%) were osteoporotic. One patient in the younger group had a hip z score of less than –2, and 14 patients (39%) had a hip z score between –2 and –1. Six patients (18%) had a spine z score of less than –2, and 6 patients (18%) had a spine z score between –2 and –1. Of the 81 older patients, 22 (27%) were osteopenic, and 21 (26%) were osteoporotic, according to spine measurements. The femoral neck data indicated that 39 (48%) of the older patients were osteopenic, and 22 (27%) were osteoporotic.
In both groups, mechanisms of injury were identified. Of the 47 younger patients, 26 fell from standing, 7 fell from a height of more than 6 feet, and 14 were injured in motor vehicle collisions (MVCs). Of the 81 older patients, 2 sustained a direct blow, 64 fell from standing, 4 fell from a height of more than 6 feet, and 11 were injured in MVCs. The differences in z scores based on mechanism of injury were not statistically significant (P = .22) (Figure 2).
Discussion
Several studies have shown that older women with DRFs have low BMD in the spine and femoral neck.8,9 These studies focused on older women who sustained low-energy fractures caused by a fall from a standing height. Studies of younger women with DRFs focused on BMD of the contralateral distal radius, not the spine or femoral neck.10,11 Those study groups also had low BMD. Findings from a multitude of studies have established that patients who are older than 50 years when they sustain distal radius fragility fractures should be referred for bone densitometry studies, and there is increasing evidence that younger patients with fragility fractures should undergo this evaluation as well.
The present study was designed to expand the range of patients and mechanisms of injury. Women in this study were 35 years or older. In addition to collecting data from patients injured in a fall from standing, we examined the medical records of women injured in MVCs, in falls from heights of more than 6 feet, and from direct trauma to the wrist. We measured the BMD of the spine and femoral neck and of the contralateral distal radius.
For this discussion, several key points should be made about BMD evaluation in younger versus older women. Most organizations caution against using spine BMD in older women. The ISCD, however, recommended measuring both hip and spine BMD; whereas BMD can be falsely elevated by spine osteoarthritis in older patients, spine BMD measurements are accurate in younger patients not affected by osteoarthritis. The ISCD also stipulated that z scores should be used in examining BMD in younger patients. The z score is a value of how many standard deviations BMD differs from a matched population of the same age, sex, ethnicity, and weight. The t score, which is useful in evaluating older patients, compares a patient’s BMD with that of an average 30-year-old.12
According to the WHO classification system (intended for older women), osteopenia is indicated by a t score between –1.0 and –2.5, and osteoporosis is indicated by a t score of less than –2.5. In the present study, about 43% of the younger patients (age, 35-50 years) with DRFs were osteopenic, and 6% of these patients were osteoporotic. In concert with previous studies,9 48% of our older women (age, >50 years) with DRFs were osteopenic, and 27% were osteoporotic. The difference in mean spinal z scores between the younger and older groups was not statistically significant (P = .81).
As mentioned, when examining BMD of younger patients, it is imperative to use spine z scores. About 18% of our younger patients had a z score of less than –2, and 18% had a z score between –2 and –1. In our comparison of patients from 5 different age decades (range, 35-79 years), there was no statistically significant difference in z scores (P = .83). In addition, there was no correlation between increasing age and decreasing z score (R = –0.04).
Secondary causes of osteoporosis have been documented in 30% of premenopausal women and 55% of men with vertebral fractures.13-15 Primary osteoporosis results from the normal aging process; secondary osteoporosis results from reversible causes, including medications, gastrointestinal disorders, renal disease, endocrine disorders, and sedentary lifestyle.15,16 When a secondary cause of osteoporosis is identified, treatment can be initiated to increase BMD. As younger patients can reverse bone loss and even increase BMD, it is important to identify reversible causes of osteopenia and osteoporosis in this age group. It is well documented that both younger and older patients with DRFs are at increased risk for subsequent fractures.6 Preventing further bone loss at a younger age may drastically decrease lifetime fracture risk.12,17
Most previous studies of BMD in women were limited to patients with DRFs caused by a low-energy mechanism or by a fall from standing. Current recommendations for BMD testing focus on postmenopausal women who have sustained a fragility or low-energy DRF. When an osteoporotic or osteopenic patient’s distal radius is subjected to a high-energy force, a fracture is likely. Therefore, we expanded our study to include high-energy mechanisms of injury. Our analysis of BMD in patients with DRFs sustained in MVCs indicated that 12% of this group were osteoporotic, and 44% were osteopenic. Forty-three percent of our younger patients with a DRF fractured in a MVC were osteopenic, and 6% were osteoporotic. Among 4 mechanisms of injury for DRFs, there was no statistically significant difference in z scores (P = .22) (Figure 2). This provides evidence that a significant portion of patients with DRFs from both high- and low-energy mechanisms are osteoporotic or osteopenic. Patients with DRFs sustained in MVCs or in falls from heights of more than 6 feet should be referred for BMD evaluation.
Conclusion
A significant proportion of younger patients with DRFs are osteopenic or osteoporotic (43% and 6%, respectively), and their z scores are comparable to those of older patients with DRFs. There was no statistically significant difference in BMD z scores between younger and older patients and no difference in mechanisms of injury. This is evidence that younger patients with DRFs caused by a high- or low-energy mechanism of injury should undergo both DXA scan and BMD evaluation. If osteoporosis or osteopenia can be diagnosed at an earlier age, and if these patients can be properly treated, subsequent fractures could be prevented. The present study provides evidence supporting a simplification of the current recommendations for BMD evaluation: All women with DRFs should undergo bone densitometry.
1. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. http://www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf. Accessed November 3, 2015.
2. Bone mass measurement (bone density). Medicare website. https://www.medicare.gov/coverage/bone-density.html. Accessed November 3, 2015.
3. Final update summary: osteoporosis: screening. US Preventive Services Task Force website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/osteoporosis-screening. Updated July 2015. Accessed November 3, 2015.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. http://nof.org/files/nof/public/content/file/344/upload/159.pdf. Accessed November 3, 2015.
5. Khan AA, Bachrach L, Brown JP, et al. Canadian Panel of International Society of Clinical Densitometry. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7(1):51-64.
6. Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2008;19(5):607-613.
7. Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3(3):127-132.
8. Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8(1):53-60.
9. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radius fractures: a case–control study. J Bone Joint Surg Am. 2011;93(4):348-356.
10. Wigderowitz CA, Cunningham T, Rowley DI, Mole PA, Paterson CR. Peripheral bone mineral density in patients with distal radial fractures. J Bone Joint Surg Br. 2003;85(3):423-425.
11. Wigderowitz CA, Rowley DI, Mole PA, Paterson CR, Abel EW. Bone mineral density of the radius in patients with Colles’ fracture. J Bone Joint Surg Br. 2000;82(1):87-89.
12. Khan A, Syed Z. Bone mineral density assessment in premenopausal women. Womens Health. 2006;2(4):639-645.
13. Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77(5):453-468.
14. Hudec SM, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19(1):120-128.
15. Scane AC, Sutcliffe AM, Francis RM. Osteoporosis in men. Baillieres Clin Rheumatol. 1993;7(3):589-601.
16. Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643-645.
17. Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med. 1995;123(6):452-460.
Many organizations and work groups have issued recommendations regarding which patients should undergo bone densitometry. In 2004, the US Surgeon General recommended bone mineral density (BMD) evaluation for all women over age 65 years and for women and men with fragility fractures.1 The Centers for Medicare & Medicaid Services recommended BMD assessment for estrogen-deficient patients, for patients with vertebral abnormalities or hyperparathyroidism, and for patients receiving either steroid therapy or osteoporosis medications approved by the US Food and Drug Administration.2 The US Preventive Services Task Force and the National Osteoporosis Foundation each recommended screening for all women age 65 years or older and for postmenopausal women (age, 60-64 years) at high risk.3,4 The International Society for Clinical Densitometry (ISCD) recommended screening for all women age 65 years or older, all men age 70 years or older, and high-risk women under age 65 years.5
These current recommendations for BMD evaluation focus on women over age 65 years. More recent studies of postmenopausal women with distal radius fractures (DRFs) have found that both younger women (age, 45-65 years) and older women (age, ≥65 years) can have lower BMD and increased risk for hip and spine fracture.6,7 The authors of those studies recommended that all postmenopausal women with DRFs be evaluated for low BMD and that fracture prevention treatment be initiated. Earnshaw and colleagues8 and Oyen and colleagues9 found that men and women (age, ≥50 years) with DRFs had low BMD and elevated 10-year fracture rates. They concluded that BMD should be evaluated and treated in all DRF patients age 50 years or older. Other studies have shown low BMD in the contralateral distal radius of patients of all ages who presented with Colles fractures.10,11 These 2 studies did not measure spine or hip BMD.
The literature on BMD of younger women with DRFs is limited, relying solely on data collected for the contralateral distal radius.10,11 The ISCD recommended measuring both hip and spine BMD in premenopausal women. They also stated that z scores, not t scores, should be used for premenopausal women.5 The causes of low BMD in women over age 55 years are primarily nutritional deficiency and normal aging.1 In younger females, low BMD results from secondary causes, such as diet, medications, medical conditions, and endocrine disorders. When the secondary cause of low BMD can be identified and treated, osteoporosis can be stopped and even reversed in younger patients.12-14 Low BMD is more amenable to treatment in younger patients than in postmenopausal women. Younger patients with low BMD carry a higher lifetime fracture risk because they have more years of life with low BMD; therefore, early identification and treatment have a more significant impact on fracture prevention in these patients.
In the present study, we determined the prevalence of osteoporosis and osteopenia in younger women (age, 35-50 years) with DRFs and compared BMD measurements from younger women (age, 35-50 years) and older women (age, >50 years) with DRFs. The main goal was to determine which patients should be referred for bone densitometry and subsequent treatment.
Patients and Methods
This study received institutional review board approval. During a 5-year period (January 2005–August 2010), we prospectively collected dual-energy x-ray absorptiometry (DXA) scans for 128 women (age, >35 years) who presented with DRFs to our level I trauma center. Age ranged from 35 to 86 years. Data on mechanism of injury, treatment, and body mass index (BMI) were collected. The 128 patients were divided into a younger group (47 women; age range, 35-50 years; mean age, 44 years) and an older group (81 women; age, ≥51 years; mean age, 61 years). Mean BMI was 29.3 in the younger group and 28.8 in the older group (P = .88) (Table).
BMD was measured with a General Electric Lunar Prodigy Advance scanner that was tested annually for accuracy and precision. BMD of hips and lumbar spines was measured with a 76-Kv x-ray source. All DXA scans were analyzed by the same physician. BMD was omitted in cases of patients with a history of lumbar spine or hip fracture.
Two-sample Student t test was used to compare the 2 groups’ data. When multiple groups were being compared, analysis of variance was used. Spearman rank-order test was used to calculate a correlation coefficient for evaluation of the relationships between age and BMD.
Results
Mean lumbar spine (L1–L4) BMD was 1.12 in the younger group and 1.063 in the older group (P = .02); t scores were –0.63 and –1.132, respectively (P = .02); and mean z scores were –0.69 and –0.61, respectively (P = .81). Mean femoral neck BMD was 0.91 in the younger group and 0.80 in the older group (P < .05); t scores were –0.87 and –1.65, respectively (P < .01), and mean femoral neck z scores were –0.69 and –0.67, respectively (P = .92).
To further analyze BMD of specific age groups, we divided patients by decade: 35-39, 40-49, 50-59, 60-69, 70-79, 80-89 years. Among all 6 decades, there were no statistically significant differences between hip z scores (P = .83) (Figure 1). Spearman rank-order correlation test showed a moderate inverse correlation between age and femoral neck BMD (R = –0.42) and t score (R = –0.43). There was a weak correlation between increasing age and decreasing spine BMD, t score, and z score (Rs = –0.27, –0.31, 0.03). There was no correlation between age and femoral neck z score (R = –0.04).
According to the WHO classification system, 11 (23%) of the 47 women in the younger group were osteopenic, and 8 (17%) were osteoporotic, based on spine BMD. Hip BMD values indicated that 20 patients (43%) were osteopenic, and 3 (6%) were osteoporotic. One patient in the younger group had a hip z score of less than –2, and 14 patients (39%) had a hip z score between –2 and –1. Six patients (18%) had a spine z score of less than –2, and 6 patients (18%) had a spine z score between –2 and –1. Of the 81 older patients, 22 (27%) were osteopenic, and 21 (26%) were osteoporotic, according to spine measurements. The femoral neck data indicated that 39 (48%) of the older patients were osteopenic, and 22 (27%) were osteoporotic.
In both groups, mechanisms of injury were identified. Of the 47 younger patients, 26 fell from standing, 7 fell from a height of more than 6 feet, and 14 were injured in motor vehicle collisions (MVCs). Of the 81 older patients, 2 sustained a direct blow, 64 fell from standing, 4 fell from a height of more than 6 feet, and 11 were injured in MVCs. The differences in z scores based on mechanism of injury were not statistically significant (P = .22) (Figure 2).
Discussion
Several studies have shown that older women with DRFs have low BMD in the spine and femoral neck.8,9 These studies focused on older women who sustained low-energy fractures caused by a fall from a standing height. Studies of younger women with DRFs focused on BMD of the contralateral distal radius, not the spine or femoral neck.10,11 Those study groups also had low BMD. Findings from a multitude of studies have established that patients who are older than 50 years when they sustain distal radius fragility fractures should be referred for bone densitometry studies, and there is increasing evidence that younger patients with fragility fractures should undergo this evaluation as well.
The present study was designed to expand the range of patients and mechanisms of injury. Women in this study were 35 years or older. In addition to collecting data from patients injured in a fall from standing, we examined the medical records of women injured in MVCs, in falls from heights of more than 6 feet, and from direct trauma to the wrist. We measured the BMD of the spine and femoral neck and of the contralateral distal radius.
For this discussion, several key points should be made about BMD evaluation in younger versus older women. Most organizations caution against using spine BMD in older women. The ISCD, however, recommended measuring both hip and spine BMD; whereas BMD can be falsely elevated by spine osteoarthritis in older patients, spine BMD measurements are accurate in younger patients not affected by osteoarthritis. The ISCD also stipulated that z scores should be used in examining BMD in younger patients. The z score is a value of how many standard deviations BMD differs from a matched population of the same age, sex, ethnicity, and weight. The t score, which is useful in evaluating older patients, compares a patient’s BMD with that of an average 30-year-old.12
According to the WHO classification system (intended for older women), osteopenia is indicated by a t score between –1.0 and –2.5, and osteoporosis is indicated by a t score of less than –2.5. In the present study, about 43% of the younger patients (age, 35-50 years) with DRFs were osteopenic, and 6% of these patients were osteoporotic. In concert with previous studies,9 48% of our older women (age, >50 years) with DRFs were osteopenic, and 27% were osteoporotic. The difference in mean spinal z scores between the younger and older groups was not statistically significant (P = .81).
As mentioned, when examining BMD of younger patients, it is imperative to use spine z scores. About 18% of our younger patients had a z score of less than –2, and 18% had a z score between –2 and –1. In our comparison of patients from 5 different age decades (range, 35-79 years), there was no statistically significant difference in z scores (P = .83). In addition, there was no correlation between increasing age and decreasing z score (R = –0.04).
Secondary causes of osteoporosis have been documented in 30% of premenopausal women and 55% of men with vertebral fractures.13-15 Primary osteoporosis results from the normal aging process; secondary osteoporosis results from reversible causes, including medications, gastrointestinal disorders, renal disease, endocrine disorders, and sedentary lifestyle.15,16 When a secondary cause of osteoporosis is identified, treatment can be initiated to increase BMD. As younger patients can reverse bone loss and even increase BMD, it is important to identify reversible causes of osteopenia and osteoporosis in this age group. It is well documented that both younger and older patients with DRFs are at increased risk for subsequent fractures.6 Preventing further bone loss at a younger age may drastically decrease lifetime fracture risk.12,17
Most previous studies of BMD in women were limited to patients with DRFs caused by a low-energy mechanism or by a fall from standing. Current recommendations for BMD testing focus on postmenopausal women who have sustained a fragility or low-energy DRF. When an osteoporotic or osteopenic patient’s distal radius is subjected to a high-energy force, a fracture is likely. Therefore, we expanded our study to include high-energy mechanisms of injury. Our analysis of BMD in patients with DRFs sustained in MVCs indicated that 12% of this group were osteoporotic, and 44% were osteopenic. Forty-three percent of our younger patients with a DRF fractured in a MVC were osteopenic, and 6% were osteoporotic. Among 4 mechanisms of injury for DRFs, there was no statistically significant difference in z scores (P = .22) (Figure 2). This provides evidence that a significant portion of patients with DRFs from both high- and low-energy mechanisms are osteoporotic or osteopenic. Patients with DRFs sustained in MVCs or in falls from heights of more than 6 feet should be referred for BMD evaluation.
Conclusion
A significant proportion of younger patients with DRFs are osteopenic or osteoporotic (43% and 6%, respectively), and their z scores are comparable to those of older patients with DRFs. There was no statistically significant difference in BMD z scores between younger and older patients and no difference in mechanisms of injury. This is evidence that younger patients with DRFs caused by a high- or low-energy mechanism of injury should undergo both DXA scan and BMD evaluation. If osteoporosis or osteopenia can be diagnosed at an earlier age, and if these patients can be properly treated, subsequent fractures could be prevented. The present study provides evidence supporting a simplification of the current recommendations for BMD evaluation: All women with DRFs should undergo bone densitometry.
Many organizations and work groups have issued recommendations regarding which patients should undergo bone densitometry. In 2004, the US Surgeon General recommended bone mineral density (BMD) evaluation for all women over age 65 years and for women and men with fragility fractures.1 The Centers for Medicare & Medicaid Services recommended BMD assessment for estrogen-deficient patients, for patients with vertebral abnormalities or hyperparathyroidism, and for patients receiving either steroid therapy or osteoporosis medications approved by the US Food and Drug Administration.2 The US Preventive Services Task Force and the National Osteoporosis Foundation each recommended screening for all women age 65 years or older and for postmenopausal women (age, 60-64 years) at high risk.3,4 The International Society for Clinical Densitometry (ISCD) recommended screening for all women age 65 years or older, all men age 70 years or older, and high-risk women under age 65 years.5
These current recommendations for BMD evaluation focus on women over age 65 years. More recent studies of postmenopausal women with distal radius fractures (DRFs) have found that both younger women (age, 45-65 years) and older women (age, ≥65 years) can have lower BMD and increased risk for hip and spine fracture.6,7 The authors of those studies recommended that all postmenopausal women with DRFs be evaluated for low BMD and that fracture prevention treatment be initiated. Earnshaw and colleagues8 and Oyen and colleagues9 found that men and women (age, ≥50 years) with DRFs had low BMD and elevated 10-year fracture rates. They concluded that BMD should be evaluated and treated in all DRF patients age 50 years or older. Other studies have shown low BMD in the contralateral distal radius of patients of all ages who presented with Colles fractures.10,11 These 2 studies did not measure spine or hip BMD.
The literature on BMD of younger women with DRFs is limited, relying solely on data collected for the contralateral distal radius.10,11 The ISCD recommended measuring both hip and spine BMD in premenopausal women. They also stated that z scores, not t scores, should be used for premenopausal women.5 The causes of low BMD in women over age 55 years are primarily nutritional deficiency and normal aging.1 In younger females, low BMD results from secondary causes, such as diet, medications, medical conditions, and endocrine disorders. When the secondary cause of low BMD can be identified and treated, osteoporosis can be stopped and even reversed in younger patients.12-14 Low BMD is more amenable to treatment in younger patients than in postmenopausal women. Younger patients with low BMD carry a higher lifetime fracture risk because they have more years of life with low BMD; therefore, early identification and treatment have a more significant impact on fracture prevention in these patients.
In the present study, we determined the prevalence of osteoporosis and osteopenia in younger women (age, 35-50 years) with DRFs and compared BMD measurements from younger women (age, 35-50 years) and older women (age, >50 years) with DRFs. The main goal was to determine which patients should be referred for bone densitometry and subsequent treatment.
Patients and Methods
This study received institutional review board approval. During a 5-year period (January 2005–August 2010), we prospectively collected dual-energy x-ray absorptiometry (DXA) scans for 128 women (age, >35 years) who presented with DRFs to our level I trauma center. Age ranged from 35 to 86 years. Data on mechanism of injury, treatment, and body mass index (BMI) were collected. The 128 patients were divided into a younger group (47 women; age range, 35-50 years; mean age, 44 years) and an older group (81 women; age, ≥51 years; mean age, 61 years). Mean BMI was 29.3 in the younger group and 28.8 in the older group (P = .88) (Table).
BMD was measured with a General Electric Lunar Prodigy Advance scanner that was tested annually for accuracy and precision. BMD of hips and lumbar spines was measured with a 76-Kv x-ray source. All DXA scans were analyzed by the same physician. BMD was omitted in cases of patients with a history of lumbar spine or hip fracture.
Two-sample Student t test was used to compare the 2 groups’ data. When multiple groups were being compared, analysis of variance was used. Spearman rank-order test was used to calculate a correlation coefficient for evaluation of the relationships between age and BMD.
Results
Mean lumbar spine (L1–L4) BMD was 1.12 in the younger group and 1.063 in the older group (P = .02); t scores were –0.63 and –1.132, respectively (P = .02); and mean z scores were –0.69 and –0.61, respectively (P = .81). Mean femoral neck BMD was 0.91 in the younger group and 0.80 in the older group (P < .05); t scores were –0.87 and –1.65, respectively (P < .01), and mean femoral neck z scores were –0.69 and –0.67, respectively (P = .92).
To further analyze BMD of specific age groups, we divided patients by decade: 35-39, 40-49, 50-59, 60-69, 70-79, 80-89 years. Among all 6 decades, there were no statistically significant differences between hip z scores (P = .83) (Figure 1). Spearman rank-order correlation test showed a moderate inverse correlation between age and femoral neck BMD (R = –0.42) and t score (R = –0.43). There was a weak correlation between increasing age and decreasing spine BMD, t score, and z score (Rs = –0.27, –0.31, 0.03). There was no correlation between age and femoral neck z score (R = –0.04).
According to the WHO classification system, 11 (23%) of the 47 women in the younger group were osteopenic, and 8 (17%) were osteoporotic, based on spine BMD. Hip BMD values indicated that 20 patients (43%) were osteopenic, and 3 (6%) were osteoporotic. One patient in the younger group had a hip z score of less than –2, and 14 patients (39%) had a hip z score between –2 and –1. Six patients (18%) had a spine z score of less than –2, and 6 patients (18%) had a spine z score between –2 and –1. Of the 81 older patients, 22 (27%) were osteopenic, and 21 (26%) were osteoporotic, according to spine measurements. The femoral neck data indicated that 39 (48%) of the older patients were osteopenic, and 22 (27%) were osteoporotic.
In both groups, mechanisms of injury were identified. Of the 47 younger patients, 26 fell from standing, 7 fell from a height of more than 6 feet, and 14 were injured in motor vehicle collisions (MVCs). Of the 81 older patients, 2 sustained a direct blow, 64 fell from standing, 4 fell from a height of more than 6 feet, and 11 were injured in MVCs. The differences in z scores based on mechanism of injury were not statistically significant (P = .22) (Figure 2).
Discussion
Several studies have shown that older women with DRFs have low BMD in the spine and femoral neck.8,9 These studies focused on older women who sustained low-energy fractures caused by a fall from a standing height. Studies of younger women with DRFs focused on BMD of the contralateral distal radius, not the spine or femoral neck.10,11 Those study groups also had low BMD. Findings from a multitude of studies have established that patients who are older than 50 years when they sustain distal radius fragility fractures should be referred for bone densitometry studies, and there is increasing evidence that younger patients with fragility fractures should undergo this evaluation as well.
The present study was designed to expand the range of patients and mechanisms of injury. Women in this study were 35 years or older. In addition to collecting data from patients injured in a fall from standing, we examined the medical records of women injured in MVCs, in falls from heights of more than 6 feet, and from direct trauma to the wrist. We measured the BMD of the spine and femoral neck and of the contralateral distal radius.
For this discussion, several key points should be made about BMD evaluation in younger versus older women. Most organizations caution against using spine BMD in older women. The ISCD, however, recommended measuring both hip and spine BMD; whereas BMD can be falsely elevated by spine osteoarthritis in older patients, spine BMD measurements are accurate in younger patients not affected by osteoarthritis. The ISCD also stipulated that z scores should be used in examining BMD in younger patients. The z score is a value of how many standard deviations BMD differs from a matched population of the same age, sex, ethnicity, and weight. The t score, which is useful in evaluating older patients, compares a patient’s BMD with that of an average 30-year-old.12
According to the WHO classification system (intended for older women), osteopenia is indicated by a t score between –1.0 and –2.5, and osteoporosis is indicated by a t score of less than –2.5. In the present study, about 43% of the younger patients (age, 35-50 years) with DRFs were osteopenic, and 6% of these patients were osteoporotic. In concert with previous studies,9 48% of our older women (age, >50 years) with DRFs were osteopenic, and 27% were osteoporotic. The difference in mean spinal z scores between the younger and older groups was not statistically significant (P = .81).
As mentioned, when examining BMD of younger patients, it is imperative to use spine z scores. About 18% of our younger patients had a z score of less than –2, and 18% had a z score between –2 and –1. In our comparison of patients from 5 different age decades (range, 35-79 years), there was no statistically significant difference in z scores (P = .83). In addition, there was no correlation between increasing age and decreasing z score (R = –0.04).
Secondary causes of osteoporosis have been documented in 30% of premenopausal women and 55% of men with vertebral fractures.13-15 Primary osteoporosis results from the normal aging process; secondary osteoporosis results from reversible causes, including medications, gastrointestinal disorders, renal disease, endocrine disorders, and sedentary lifestyle.15,16 When a secondary cause of osteoporosis is identified, treatment can be initiated to increase BMD. As younger patients can reverse bone loss and even increase BMD, it is important to identify reversible causes of osteopenia and osteoporosis in this age group. It is well documented that both younger and older patients with DRFs are at increased risk for subsequent fractures.6 Preventing further bone loss at a younger age may drastically decrease lifetime fracture risk.12,17
Most previous studies of BMD in women were limited to patients with DRFs caused by a low-energy mechanism or by a fall from standing. Current recommendations for BMD testing focus on postmenopausal women who have sustained a fragility or low-energy DRF. When an osteoporotic or osteopenic patient’s distal radius is subjected to a high-energy force, a fracture is likely. Therefore, we expanded our study to include high-energy mechanisms of injury. Our analysis of BMD in patients with DRFs sustained in MVCs indicated that 12% of this group were osteoporotic, and 44% were osteopenic. Forty-three percent of our younger patients with a DRF fractured in a MVC were osteopenic, and 6% were osteoporotic. Among 4 mechanisms of injury for DRFs, there was no statistically significant difference in z scores (P = .22) (Figure 2). This provides evidence that a significant portion of patients with DRFs from both high- and low-energy mechanisms are osteoporotic or osteopenic. Patients with DRFs sustained in MVCs or in falls from heights of more than 6 feet should be referred for BMD evaluation.
Conclusion
A significant proportion of younger patients with DRFs are osteopenic or osteoporotic (43% and 6%, respectively), and their z scores are comparable to those of older patients with DRFs. There was no statistically significant difference in BMD z scores between younger and older patients and no difference in mechanisms of injury. This is evidence that younger patients with DRFs caused by a high- or low-energy mechanism of injury should undergo both DXA scan and BMD evaluation. If osteoporosis or osteopenia can be diagnosed at an earlier age, and if these patients can be properly treated, subsequent fractures could be prevented. The present study provides evidence supporting a simplification of the current recommendations for BMD evaluation: All women with DRFs should undergo bone densitometry.
1. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. http://www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf. Accessed November 3, 2015.
2. Bone mass measurement (bone density). Medicare website. https://www.medicare.gov/coverage/bone-density.html. Accessed November 3, 2015.
3. Final update summary: osteoporosis: screening. US Preventive Services Task Force website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/osteoporosis-screening. Updated July 2015. Accessed November 3, 2015.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. http://nof.org/files/nof/public/content/file/344/upload/159.pdf. Accessed November 3, 2015.
5. Khan AA, Bachrach L, Brown JP, et al. Canadian Panel of International Society of Clinical Densitometry. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7(1):51-64.
6. Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2008;19(5):607-613.
7. Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3(3):127-132.
8. Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8(1):53-60.
9. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radius fractures: a case–control study. J Bone Joint Surg Am. 2011;93(4):348-356.
10. Wigderowitz CA, Cunningham T, Rowley DI, Mole PA, Paterson CR. Peripheral bone mineral density in patients with distal radial fractures. J Bone Joint Surg Br. 2003;85(3):423-425.
11. Wigderowitz CA, Rowley DI, Mole PA, Paterson CR, Abel EW. Bone mineral density of the radius in patients with Colles’ fracture. J Bone Joint Surg Br. 2000;82(1):87-89.
12. Khan A, Syed Z. Bone mineral density assessment in premenopausal women. Womens Health. 2006;2(4):639-645.
13. Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77(5):453-468.
14. Hudec SM, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19(1):120-128.
15. Scane AC, Sutcliffe AM, Francis RM. Osteoporosis in men. Baillieres Clin Rheumatol. 1993;7(3):589-601.
16. Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643-645.
17. Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med. 1995;123(6):452-460.
1. US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004. http://www.ncbi.nlm.nih.gov/books/NBK45513/pdf/Bookshelf_NBK45513.pdf. Accessed November 3, 2015.
2. Bone mass measurement (bone density). Medicare website. https://www.medicare.gov/coverage/bone-density.html. Accessed November 3, 2015.
3. Final update summary: osteoporosis: screening. US Preventive Services Task Force website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/osteoporosis-screening. Updated July 2015. Accessed November 3, 2015.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. http://nof.org/files/nof/public/content/file/344/upload/159.pdf. Accessed November 3, 2015.
5. Khan AA, Bachrach L, Brown JP, et al. Canadian Panel of International Society of Clinical Densitometry. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7(1):51-64.
6. Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2008;19(5):607-613.
7. Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int. 1993;3(3):127-132.
8. Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8(1):53-60.
9. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radius fractures: a case–control study. J Bone Joint Surg Am. 2011;93(4):348-356.
10. Wigderowitz CA, Cunningham T, Rowley DI, Mole PA, Paterson CR. Peripheral bone mineral density in patients with distal radial fractures. J Bone Joint Surg Br. 2003;85(3):423-425.
11. Wigderowitz CA, Rowley DI, Mole PA, Paterson CR, Abel EW. Bone mineral density of the radius in patients with Colles’ fracture. J Bone Joint Surg Br. 2000;82(1):87-89.
12. Khan A, Syed Z. Bone mineral density assessment in premenopausal women. Womens Health. 2006;2(4):639-645.
13. Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;77(5):453-468.
14. Hudec SM, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19(1):120-128.
15. Scane AC, Sutcliffe AM, Francis RM. Osteoporosis in men. Baillieres Clin Rheumatol. 1993;7(3):589-601.
16. Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643-645.
17. Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med. 1995;123(6):452-460.