User login
Don’t shorten therapy for older, sicker cellulitis patients
MADRID – An attempt to balance effective treatment with good antibiotic stewardship fell short when patients with cellulitis who got 6 days of flucloxacillin relapsed significantly sooner and more frequently than did those who received the standard 12 days of treatment.
While cellulitis cure rates at 14 and 28 days were similar between the two groups, 90-day relapse rates were significantly higher for those who took the 6-day course (23.5% vs. 6%), Duncan R. Cranendonk, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual conference. The cohort demographics perhaps played into this finding: Most of the group was elderly, hospitalized, and had comorbid conditions.
“However, this is the population clinicians are most likely to see,” said Dr Cranendonk of the University of Amsterdam. “It appears that therapy cannot be safely shortened in this population.”
In light of recent antibiotic trials showing that shorter courses can be as effective as prolonged treatment, Dr. Cranendonk and his colleagues conducted the DANCE (Duration of Antibiotic Therapy for Cellulitis) trial. The study investigated the efficacy of an abbreviated course of intravenous flucloxacillin among 248 patients with cellulitis admitted to 11 Dutch hospitals. At treatment day 6, those who had clinically improved after their initial treatment were randomized to 6 additional days of IV flucloxacillin or to placebo. The primary outcome was cure by day 14 without relapse by day 28.
A 2004 study successfully paved the way for DANCE, Dr. Cranendonk noted. That trial examined 5 versus 10 days of levofloxacin 500 mg for uncomplicated cellulitis in 87 patients. The outcome was positive: There was no significant difference in clinical outcome between the two arms, with a 98% cure rate in both groups.
However, Dr. Cranendonk noted, there were some important differences between the patients in that study and the DANCE cohort. They were, on the whole, younger and generally in better overall health. Also, only 15% of those patients were hospitalized for their infections, while all of the DANCE subjects were treated in the hospital.
Patients enrolled in DANCE were a mean of 62 years old, with a median 28 kg/m2 body mass index. About 40% had experienced cellulitis before, and 25% had diabetes. Most infections were on the leg (84%) and involved the lower leg or the lower leg and the foot. Fever was present in half of the group, lymphadenopathy in a third, and leukocytosis in 70%.
Upon enrollment, all 248 patients received 6 days of 1,000 mg/day IV flucloxacillin, with the option of a step-down to oral treatment (500 mg four times per day) at the treating physician’s discretion. At day 6, patients who were clinically improved (afebrile, no need to an antibiotic switch, no growth in blood culture, and improved symptoms of pain, ulceration, discharge, and fluctuance) were randomized to either another 6 days of flucloxacillin or placebo.
The primary endpoint was cure by day 14, with no relapse and no need for new antibiotics by day 28. The secondary endpoint was relapse by 90 days after initial cure.
After initial treatment, 151 patients entered the randomization phase. At 28 days, relapse-free cure rates were nearly identical: 49% of the 12-day group and 50% of the 6-day group. However, by 90 days, a significant difference became apparent: Patients who had received the 6-day course of flucloxacillin were significantly more likely to have experienced a relapse of cellulitis in the same region (23.5% vs. 6% in the 12-day group). A Kaplan-Meier analysis showed that these patients began to relapse as early as 35 days after the end of therapy. Most relapses occurred during days 60-90. The few relapses in the 12-day group occurred toward the end of the follow-up period, from day 75 onward.
Dr. Cranendonk said the investigation shows that older, less-healthy cellulitis patients can probably benefit from the longer course of antibiotics. “Short-term outcomes aren’t everything,” he noted.
He had no financial disclosures.
A video interview of Dr. Cranendock by ECCMID 2018 is available.
SOURCE: Cranendonk et al. ECCMID 2018, Abstract O1122
MADRID – An attempt to balance effective treatment with good antibiotic stewardship fell short when patients with cellulitis who got 6 days of flucloxacillin relapsed significantly sooner and more frequently than did those who received the standard 12 days of treatment.
While cellulitis cure rates at 14 and 28 days were similar between the two groups, 90-day relapse rates were significantly higher for those who took the 6-day course (23.5% vs. 6%), Duncan R. Cranendonk, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual conference. The cohort demographics perhaps played into this finding: Most of the group was elderly, hospitalized, and had comorbid conditions.
“However, this is the population clinicians are most likely to see,” said Dr Cranendonk of the University of Amsterdam. “It appears that therapy cannot be safely shortened in this population.”
In light of recent antibiotic trials showing that shorter courses can be as effective as prolonged treatment, Dr. Cranendonk and his colleagues conducted the DANCE (Duration of Antibiotic Therapy for Cellulitis) trial. The study investigated the efficacy of an abbreviated course of intravenous flucloxacillin among 248 patients with cellulitis admitted to 11 Dutch hospitals. At treatment day 6, those who had clinically improved after their initial treatment were randomized to 6 additional days of IV flucloxacillin or to placebo. The primary outcome was cure by day 14 without relapse by day 28.
A 2004 study successfully paved the way for DANCE, Dr. Cranendonk noted. That trial examined 5 versus 10 days of levofloxacin 500 mg for uncomplicated cellulitis in 87 patients. The outcome was positive: There was no significant difference in clinical outcome between the two arms, with a 98% cure rate in both groups.
However, Dr. Cranendonk noted, there were some important differences between the patients in that study and the DANCE cohort. They were, on the whole, younger and generally in better overall health. Also, only 15% of those patients were hospitalized for their infections, while all of the DANCE subjects were treated in the hospital.
Patients enrolled in DANCE were a mean of 62 years old, with a median 28 kg/m2 body mass index. About 40% had experienced cellulitis before, and 25% had diabetes. Most infections were on the leg (84%) and involved the lower leg or the lower leg and the foot. Fever was present in half of the group, lymphadenopathy in a third, and leukocytosis in 70%.
Upon enrollment, all 248 patients received 6 days of 1,000 mg/day IV flucloxacillin, with the option of a step-down to oral treatment (500 mg four times per day) at the treating physician’s discretion. At day 6, patients who were clinically improved (afebrile, no need to an antibiotic switch, no growth in blood culture, and improved symptoms of pain, ulceration, discharge, and fluctuance) were randomized to either another 6 days of flucloxacillin or placebo.
The primary endpoint was cure by day 14, with no relapse and no need for new antibiotics by day 28. The secondary endpoint was relapse by 90 days after initial cure.
After initial treatment, 151 patients entered the randomization phase. At 28 days, relapse-free cure rates were nearly identical: 49% of the 12-day group and 50% of the 6-day group. However, by 90 days, a significant difference became apparent: Patients who had received the 6-day course of flucloxacillin were significantly more likely to have experienced a relapse of cellulitis in the same region (23.5% vs. 6% in the 12-day group). A Kaplan-Meier analysis showed that these patients began to relapse as early as 35 days after the end of therapy. Most relapses occurred during days 60-90. The few relapses in the 12-day group occurred toward the end of the follow-up period, from day 75 onward.
Dr. Cranendonk said the investigation shows that older, less-healthy cellulitis patients can probably benefit from the longer course of antibiotics. “Short-term outcomes aren’t everything,” he noted.
He had no financial disclosures.
A video interview of Dr. Cranendock by ECCMID 2018 is available.
SOURCE: Cranendonk et al. ECCMID 2018, Abstract O1122
MADRID – An attempt to balance effective treatment with good antibiotic stewardship fell short when patients with cellulitis who got 6 days of flucloxacillin relapsed significantly sooner and more frequently than did those who received the standard 12 days of treatment.
While cellulitis cure rates at 14 and 28 days were similar between the two groups, 90-day relapse rates were significantly higher for those who took the 6-day course (23.5% vs. 6%), Duncan R. Cranendonk, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual conference. The cohort demographics perhaps played into this finding: Most of the group was elderly, hospitalized, and had comorbid conditions.
“However, this is the population clinicians are most likely to see,” said Dr Cranendonk of the University of Amsterdam. “It appears that therapy cannot be safely shortened in this population.”
In light of recent antibiotic trials showing that shorter courses can be as effective as prolonged treatment, Dr. Cranendonk and his colleagues conducted the DANCE (Duration of Antibiotic Therapy for Cellulitis) trial. The study investigated the efficacy of an abbreviated course of intravenous flucloxacillin among 248 patients with cellulitis admitted to 11 Dutch hospitals. At treatment day 6, those who had clinically improved after their initial treatment were randomized to 6 additional days of IV flucloxacillin or to placebo. The primary outcome was cure by day 14 without relapse by day 28.
A 2004 study successfully paved the way for DANCE, Dr. Cranendonk noted. That trial examined 5 versus 10 days of levofloxacin 500 mg for uncomplicated cellulitis in 87 patients. The outcome was positive: There was no significant difference in clinical outcome between the two arms, with a 98% cure rate in both groups.
However, Dr. Cranendonk noted, there were some important differences between the patients in that study and the DANCE cohort. They were, on the whole, younger and generally in better overall health. Also, only 15% of those patients were hospitalized for their infections, while all of the DANCE subjects were treated in the hospital.
Patients enrolled in DANCE were a mean of 62 years old, with a median 28 kg/m2 body mass index. About 40% had experienced cellulitis before, and 25% had diabetes. Most infections were on the leg (84%) and involved the lower leg or the lower leg and the foot. Fever was present in half of the group, lymphadenopathy in a third, and leukocytosis in 70%.
Upon enrollment, all 248 patients received 6 days of 1,000 mg/day IV flucloxacillin, with the option of a step-down to oral treatment (500 mg four times per day) at the treating physician’s discretion. At day 6, patients who were clinically improved (afebrile, no need to an antibiotic switch, no growth in blood culture, and improved symptoms of pain, ulceration, discharge, and fluctuance) were randomized to either another 6 days of flucloxacillin or placebo.
The primary endpoint was cure by day 14, with no relapse and no need for new antibiotics by day 28. The secondary endpoint was relapse by 90 days after initial cure.
After initial treatment, 151 patients entered the randomization phase. At 28 days, relapse-free cure rates were nearly identical: 49% of the 12-day group and 50% of the 6-day group. However, by 90 days, a significant difference became apparent: Patients who had received the 6-day course of flucloxacillin were significantly more likely to have experienced a relapse of cellulitis in the same region (23.5% vs. 6% in the 12-day group). A Kaplan-Meier analysis showed that these patients began to relapse as early as 35 days after the end of therapy. Most relapses occurred during days 60-90. The few relapses in the 12-day group occurred toward the end of the follow-up period, from day 75 onward.
Dr. Cranendonk said the investigation shows that older, less-healthy cellulitis patients can probably benefit from the longer course of antibiotics. “Short-term outcomes aren’t everything,” he noted.
He had no financial disclosures.
A video interview of Dr. Cranendock by ECCMID 2018 is available.
SOURCE: Cranendonk et al. ECCMID 2018, Abstract O1122
REPORTING FROM ECCMID 2018
Key clinical point: Elderly patients with cellulitis and comorbid conditions probably need a full 12-day course of treatment.
Major finding: Three-month relapse rates were significantly higher in those who received 6 days of flucloxacillin than they were among those who received 12 days (23.5% vs. 6%).
Study details: Patients who improved on 6 days of treatment were randomized to either placebo or another 6 days of therapy.
Disclosures: Dr. Cranendonk had no financial disclosures.
Source: Cranendonk DR et al. ECCMID 2018, Abstract O1122
FDA advisory committee votes to recommend first once-daily aminoglycoside antibiotic
The Antimicrobial Drugs Advisory Committee of the Food and Drug Administration has voted to recommend plazomicin, a new aminoglycoside, for systemic use in the treatment of complicated urinary tract infections (cUTI) but rejected it for treatment of blood stream infections (BSIs) that are caused by multidrug resistant (MDR) Enterobacteriaceae.
Advisers voted unanimously to recommend plazomicin for cUTI, but rejected the drug for BSIs in an 11-4 no vote, based on the results of two phase 3 clinical trials: EPIC and CARE.
“Today’s meeting brought the committee face to face with the crisis of multidrug resistant bacteria,” he said. “Results of the 009 [EPIC] study, in my mind, clearly showed plazomicin met the noninferiority endpoints.”
EPIC study
EPIC was a phase 3 clinical trial to assess the noninferiority of plazomicin to meropenem in patients with cUTI and/or acute pyelonephritis (AP). Many patients with drug resistant infections have limited treatment options, so plazomicin was reviewed under the Limited Population Antibacterial Drug pathway.
Patients in the study were stratified by geographical region and infection type – cUTI or acute pyelonephritis (AP). In total, 609 patients were randomized in the intent-to-treat (ITT) group with 306 and 303 receiving plazomicin or meropenem, respectively. Using the coprimary efficacy endpoints of microbiological eradication and clinical cure, a measure known as composite cure was developed to assess efficacy at Day 5 and the test of cure (TOC) visit in the microbiological modified intent-to-treat (mMITT) population. The mMITT group consisted of all patients who had received any dose of study drug and had at least one qualified baseline pathogen with 105 or more colony-forming units/mL that was susceptible to both meropenem and plazomicin.Plazomicin was noninferior to meropenem with a margin of 15%. At day 5, the composite cure rate was 88% in the plazomicin group, compared with 91.4% in the meropenem group. Similar results were seen at the test of cure visit, with composite cure rates of 81.7% and 70.1% in the plazomicin and meropenem groups, respectively.
In a secondary analysis of microbiologically evaluable populations, or patients who had an appropriately collected urine specimen yielding interpretable culture results, plazomicin again showed noninferiority. Composite cure rates at Day 5 were 89.4% in the plazomicin group, compared with 94.2% in the meropenem group. TOC composite scores also were similar, at 89.4% and 75.1%, respectively.An analysis of composite cure scores at the end of the intravenous therapy visit revealed that Day 5 scores were comparable between the plazomicin and meropenem groups at 93.7% and 94.9%, respectively, demonstrating that high cure rates were achieved with IV treatment, irrespective of the drug used.
In a pooled safety analysis of a phase 2 trial and the EPIC trial, 22.5% experienced a treatment-emergent adverse event (TEAE); of these, 2.9% had a severe TEAE and 2.7% experienced a TEAE that led to discontinuation of the intravenous study drug. All adverse events were related to renal function, diarrhea, headache, nausea, and vomiting.
CARE study
CARE was an open-label trial to assess the safety and efficacy of plazomicin as compared with colistin in patients with hospital-acquired bacterial pneumonia or ventilator-associated bacterial pneumonia (HABP/VABP) or bloodstream infections caused by CRE. The primary endpoint in the study was all-cause mortality at Day 28 or significant disease-related complications in the mMITT population, which included all patients with a confirmed CRE pathogen who had at least one dose of the study drug.
Overall, CARE enrolled 69 patients and split them into two cohorts. In Cohort 1, there were 39 randomized patients; 30 with blood stream infections and 9 with HABP or VABP. Cohort 2 was uncontrolled and consisted of 30 total patients; 27 patients who were in the mMITT population included 14 patients with BSI, 9 with HABP/VABP, and 4 with cUTI, all of whom received plazomicin.
Overall in Cohort 1, all-cause mortality at Day 28 or significant disease-related complications were lower with plazomicin than with colistin (23.5% vs. 50.0%). Because of the small patient HABP/VABP population in Cohort 1, the trial focused on patients with blood stream infections.
The rates of all-cause mortality and significant disease-related complications at Day 28 were much lower with plazomicin therapy than with colistin for patients with blood infections (14.3% vs. 53.3%, respectively). All-cause mortality alone at Day 28 was 7.1% in the plazomicin patients and 40.0% in colistin patients.
In the BSI subgroup, plazomicin reduced the rate of death by 86% and 63% on days 28 and 60, respectively, compared with colistin.
The uncontrolled data from Cohort 2 were supportive of the all-cause mortality rate in Cohort 1, with a rate of 14.3% at Day 28.
Because this was a severely ill patient population, there was a higher rate of adverse events. In fact, nearly all the patients in this study (16/18) experienced one treatment-emergent adverse event; 61.1% experienced severe TEAEs, with 11.1% severe enough to discontinue the study drug use.
Despite the study results, many of the panel members were not comfortable recommending it for approval.
Dr. Green, who supported plazomicin for use in cUTI but rejected it for use in BSIs, shared some of his concerns: “Because of the clear need, I was really tempted to vote yes and I actually came here today, thinking that I was going to vote yes. But this study clearly had a number of limitations that impacted the interpretation of results to support the approval for bloodstream infection,” he said. “The limitation that I could just not overcome was the small numbers [of participants].”
Plazomicin has a Prescription Drug User Fee Act date of June 25 of this year by which time the FDA will decide on its approval. While the FDA does not always follow the recommendations of these committees, they usually accept them and proceed accordingly.
The Antimicrobial Drugs Advisory Committee of the Food and Drug Administration has voted to recommend plazomicin, a new aminoglycoside, for systemic use in the treatment of complicated urinary tract infections (cUTI) but rejected it for treatment of blood stream infections (BSIs) that are caused by multidrug resistant (MDR) Enterobacteriaceae.
Advisers voted unanimously to recommend plazomicin for cUTI, but rejected the drug for BSIs in an 11-4 no vote, based on the results of two phase 3 clinical trials: EPIC and CARE.
“Today’s meeting brought the committee face to face with the crisis of multidrug resistant bacteria,” he said. “Results of the 009 [EPIC] study, in my mind, clearly showed plazomicin met the noninferiority endpoints.”
EPIC study
EPIC was a phase 3 clinical trial to assess the noninferiority of plazomicin to meropenem in patients with cUTI and/or acute pyelonephritis (AP). Many patients with drug resistant infections have limited treatment options, so plazomicin was reviewed under the Limited Population Antibacterial Drug pathway.
Patients in the study were stratified by geographical region and infection type – cUTI or acute pyelonephritis (AP). In total, 609 patients were randomized in the intent-to-treat (ITT) group with 306 and 303 receiving plazomicin or meropenem, respectively. Using the coprimary efficacy endpoints of microbiological eradication and clinical cure, a measure known as composite cure was developed to assess efficacy at Day 5 and the test of cure (TOC) visit in the microbiological modified intent-to-treat (mMITT) population. The mMITT group consisted of all patients who had received any dose of study drug and had at least one qualified baseline pathogen with 105 or more colony-forming units/mL that was susceptible to both meropenem and plazomicin.Plazomicin was noninferior to meropenem with a margin of 15%. At day 5, the composite cure rate was 88% in the plazomicin group, compared with 91.4% in the meropenem group. Similar results were seen at the test of cure visit, with composite cure rates of 81.7% and 70.1% in the plazomicin and meropenem groups, respectively.
In a secondary analysis of microbiologically evaluable populations, or patients who had an appropriately collected urine specimen yielding interpretable culture results, plazomicin again showed noninferiority. Composite cure rates at Day 5 were 89.4% in the plazomicin group, compared with 94.2% in the meropenem group. TOC composite scores also were similar, at 89.4% and 75.1%, respectively.An analysis of composite cure scores at the end of the intravenous therapy visit revealed that Day 5 scores were comparable between the plazomicin and meropenem groups at 93.7% and 94.9%, respectively, demonstrating that high cure rates were achieved with IV treatment, irrespective of the drug used.
In a pooled safety analysis of a phase 2 trial and the EPIC trial, 22.5% experienced a treatment-emergent adverse event (TEAE); of these, 2.9% had a severe TEAE and 2.7% experienced a TEAE that led to discontinuation of the intravenous study drug. All adverse events were related to renal function, diarrhea, headache, nausea, and vomiting.
CARE study
CARE was an open-label trial to assess the safety and efficacy of plazomicin as compared with colistin in patients with hospital-acquired bacterial pneumonia or ventilator-associated bacterial pneumonia (HABP/VABP) or bloodstream infections caused by CRE. The primary endpoint in the study was all-cause mortality at Day 28 or significant disease-related complications in the mMITT population, which included all patients with a confirmed CRE pathogen who had at least one dose of the study drug.
Overall, CARE enrolled 69 patients and split them into two cohorts. In Cohort 1, there were 39 randomized patients; 30 with blood stream infections and 9 with HABP or VABP. Cohort 2 was uncontrolled and consisted of 30 total patients; 27 patients who were in the mMITT population included 14 patients with BSI, 9 with HABP/VABP, and 4 with cUTI, all of whom received plazomicin.
Overall in Cohort 1, all-cause mortality at Day 28 or significant disease-related complications were lower with plazomicin than with colistin (23.5% vs. 50.0%). Because of the small patient HABP/VABP population in Cohort 1, the trial focused on patients with blood stream infections.
The rates of all-cause mortality and significant disease-related complications at Day 28 were much lower with plazomicin therapy than with colistin for patients with blood infections (14.3% vs. 53.3%, respectively). All-cause mortality alone at Day 28 was 7.1% in the plazomicin patients and 40.0% in colistin patients.
In the BSI subgroup, plazomicin reduced the rate of death by 86% and 63% on days 28 and 60, respectively, compared with colistin.
The uncontrolled data from Cohort 2 were supportive of the all-cause mortality rate in Cohort 1, with a rate of 14.3% at Day 28.
Because this was a severely ill patient population, there was a higher rate of adverse events. In fact, nearly all the patients in this study (16/18) experienced one treatment-emergent adverse event; 61.1% experienced severe TEAEs, with 11.1% severe enough to discontinue the study drug use.
Despite the study results, many of the panel members were not comfortable recommending it for approval.
Dr. Green, who supported plazomicin for use in cUTI but rejected it for use in BSIs, shared some of his concerns: “Because of the clear need, I was really tempted to vote yes and I actually came here today, thinking that I was going to vote yes. But this study clearly had a number of limitations that impacted the interpretation of results to support the approval for bloodstream infection,” he said. “The limitation that I could just not overcome was the small numbers [of participants].”
Plazomicin has a Prescription Drug User Fee Act date of June 25 of this year by which time the FDA will decide on its approval. While the FDA does not always follow the recommendations of these committees, they usually accept them and proceed accordingly.
The Antimicrobial Drugs Advisory Committee of the Food and Drug Administration has voted to recommend plazomicin, a new aminoglycoside, for systemic use in the treatment of complicated urinary tract infections (cUTI) but rejected it for treatment of blood stream infections (BSIs) that are caused by multidrug resistant (MDR) Enterobacteriaceae.
Advisers voted unanimously to recommend plazomicin for cUTI, but rejected the drug for BSIs in an 11-4 no vote, based on the results of two phase 3 clinical trials: EPIC and CARE.
“Today’s meeting brought the committee face to face with the crisis of multidrug resistant bacteria,” he said. “Results of the 009 [EPIC] study, in my mind, clearly showed plazomicin met the noninferiority endpoints.”
EPIC study
EPIC was a phase 3 clinical trial to assess the noninferiority of plazomicin to meropenem in patients with cUTI and/or acute pyelonephritis (AP). Many patients with drug resistant infections have limited treatment options, so plazomicin was reviewed under the Limited Population Antibacterial Drug pathway.
Patients in the study were stratified by geographical region and infection type – cUTI or acute pyelonephritis (AP). In total, 609 patients were randomized in the intent-to-treat (ITT) group with 306 and 303 receiving plazomicin or meropenem, respectively. Using the coprimary efficacy endpoints of microbiological eradication and clinical cure, a measure known as composite cure was developed to assess efficacy at Day 5 and the test of cure (TOC) visit in the microbiological modified intent-to-treat (mMITT) population. The mMITT group consisted of all patients who had received any dose of study drug and had at least one qualified baseline pathogen with 105 or more colony-forming units/mL that was susceptible to both meropenem and plazomicin.Plazomicin was noninferior to meropenem with a margin of 15%. At day 5, the composite cure rate was 88% in the plazomicin group, compared with 91.4% in the meropenem group. Similar results were seen at the test of cure visit, with composite cure rates of 81.7% and 70.1% in the plazomicin and meropenem groups, respectively.
In a secondary analysis of microbiologically evaluable populations, or patients who had an appropriately collected urine specimen yielding interpretable culture results, plazomicin again showed noninferiority. Composite cure rates at Day 5 were 89.4% in the plazomicin group, compared with 94.2% in the meropenem group. TOC composite scores also were similar, at 89.4% and 75.1%, respectively.An analysis of composite cure scores at the end of the intravenous therapy visit revealed that Day 5 scores were comparable between the plazomicin and meropenem groups at 93.7% and 94.9%, respectively, demonstrating that high cure rates were achieved with IV treatment, irrespective of the drug used.
In a pooled safety analysis of a phase 2 trial and the EPIC trial, 22.5% experienced a treatment-emergent adverse event (TEAE); of these, 2.9% had a severe TEAE and 2.7% experienced a TEAE that led to discontinuation of the intravenous study drug. All adverse events were related to renal function, diarrhea, headache, nausea, and vomiting.
CARE study
CARE was an open-label trial to assess the safety and efficacy of plazomicin as compared with colistin in patients with hospital-acquired bacterial pneumonia or ventilator-associated bacterial pneumonia (HABP/VABP) or bloodstream infections caused by CRE. The primary endpoint in the study was all-cause mortality at Day 28 or significant disease-related complications in the mMITT population, which included all patients with a confirmed CRE pathogen who had at least one dose of the study drug.
Overall, CARE enrolled 69 patients and split them into two cohorts. In Cohort 1, there were 39 randomized patients; 30 with blood stream infections and 9 with HABP or VABP. Cohort 2 was uncontrolled and consisted of 30 total patients; 27 patients who were in the mMITT population included 14 patients with BSI, 9 with HABP/VABP, and 4 with cUTI, all of whom received plazomicin.
Overall in Cohort 1, all-cause mortality at Day 28 or significant disease-related complications were lower with plazomicin than with colistin (23.5% vs. 50.0%). Because of the small patient HABP/VABP population in Cohort 1, the trial focused on patients with blood stream infections.
The rates of all-cause mortality and significant disease-related complications at Day 28 were much lower with plazomicin therapy than with colistin for patients with blood infections (14.3% vs. 53.3%, respectively). All-cause mortality alone at Day 28 was 7.1% in the plazomicin patients and 40.0% in colistin patients.
In the BSI subgroup, plazomicin reduced the rate of death by 86% and 63% on days 28 and 60, respectively, compared with colistin.
The uncontrolled data from Cohort 2 were supportive of the all-cause mortality rate in Cohort 1, with a rate of 14.3% at Day 28.
Because this was a severely ill patient population, there was a higher rate of adverse events. In fact, nearly all the patients in this study (16/18) experienced one treatment-emergent adverse event; 61.1% experienced severe TEAEs, with 11.1% severe enough to discontinue the study drug use.
Despite the study results, many of the panel members were not comfortable recommending it for approval.
Dr. Green, who supported plazomicin for use in cUTI but rejected it for use in BSIs, shared some of his concerns: “Because of the clear need, I was really tempted to vote yes and I actually came here today, thinking that I was going to vote yes. But this study clearly had a number of limitations that impacted the interpretation of results to support the approval for bloodstream infection,” he said. “The limitation that I could just not overcome was the small numbers [of participants].”
Plazomicin has a Prescription Drug User Fee Act date of June 25 of this year by which time the FDA will decide on its approval. While the FDA does not always follow the recommendations of these committees, they usually accept them and proceed accordingly.
MDedge Daily News: Antibiotic resistance leads to ‘nightmare’ bacteria
PPIs aren’t responsible for cognitive decline. Levothyroxine comes with risks for older patients. And Medicare formulary changes could be on the way.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
PPIs aren’t responsible for cognitive decline. Levothyroxine comes with risks for older patients. And Medicare formulary changes could be on the way.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
PPIs aren’t responsible for cognitive decline. Levothyroxine comes with risks for older patients. And Medicare formulary changes could be on the way.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Unusual antibiotic resistance found in more than 200 bacteria
The Centers for Disease Control and Prevention’s Antibiotic Resistance (AR) Lab Network has detected 221 instances of bacteria with especially rare resistance genes in the United States, according to a Vital Signs report published online and expanded upon in CDC’s MMWR Weekly.
The MMWR Weekly report, “Containment of Novel Multidrug-Resistant Organisms and Resistance Mechanisms,” which goes deeper into the science behind the issue, shows that in 9 months, in all states and Puerto Rico, health department workers in the AR lab network tested 5,776 samples of highly resistant bacteria, according to Anne Schuchat, MD, principal deputy director of the CDC. These bacteria were immediately tested for unusual resistance – “those genes that were highly resistant, or rare, with special resistance that could spread,” she said.
“Of the 5,776, about 1 in 4 of the bacteria had a gene that helped it spread its resistance. And there were 221 instances of an especially rare resistance gene,” she added. This prompted intense screening, revealing “that 1 in 10 tests were also positive. Meaning the unusual resistance may have spread to other patients. And could have continued spreading if left undetected.”
The report looked at carbapenem-resistant Enterobacteriaceae (CRE) and Enterobacteriaceae with extended-spectrum beta-lactamases (ESBL) infection data from the National Healthcare Safety Network from 2006-2015 to calculate changes in the year over year proportions of these infections and how an enhanced detection and control strategy curbs carbapenem resistance.
This strategy includes components such as timely implementation of appropriate infection control measures, conducting a health care and contact investigation with follow-up, and implementing a system to ensure adherence to infection control measures.
“With independent, or single facility approaches to control spread, a dangerous type of unusual resistance in Enterobacteriaceae [ESBL phenotype] decreased by about 2% per year [(risk ratio [RR] = 0.98, P less than .001)].” With a more aggressive approach, using guidance such as CDC’s CRE toolkit, released in 2009, another type of unusual resistance [CRE] in the same bacteria (Enterobacteriaceae) decreased by nearly 15% per year (RR = 0.85, P less than .01).
The difference may be due in part to the more directed response utilized to slow the spread of the “nightmare bacteria,” once it was identified, said Dr. Schuchat.
These results show massive promise even if only partially effective, specifically for CRE.
“CDC estimates show that if only 20% effective, the containment strategy can reduce the number of nightmare bacteria [CRE] cases by 76% over 3 years in one area.”
Due to the nature of antibiotic resistance and its ability to spread, this poses a significant public health threat. Antibiotics are not simply used to treat infections but are a safety net that is used in cancer treatment, surgery, and ICU care, Dr. Schuchat pointed out. The rise of antibiotic resistance is a threat to that safety net and accounts for nearly 2,000,000 antibiotic resistant infections and approximately 23,000 deaths per year.
But aggressive responses to these infections can control their spread. Dr. Schuchat used the analogy of controlling a fire to illustrate the concept.
“Much like a fire, finding and stopping unusual resistance early when it’s just a spark protects people.”
Dr. Schuchat reiterated that simply identifying the issue is only part of the equation.
“Detection is not enough on its own. When there is a fire, somebody needs to put it out. CDC supports more than 500 local staff across the country to combat antibiotic resistance wherever it emerges.”
While the report highlights the strides that have been made in combating antibiotic resistance, Paul Auwaerter, MD, president of the Infectious Diseases Society of America, released a statement highlighting the need to further fund these efforts.
“The report spells out the need to accelerate efforts to curb resistance or face an increasing burden including novel resistance mutations that threaten health,” stated Dr. Auwaerter. “The efforts detailed in the Vital Signs report were made possible through new congressional funding in 2016 to combat antibiotic resistance. We urge Congress to sustain and to grow that investment so that further progress will prepare us to meet the future challenges of antibiotic resistance from a position of strength,” he added.
A fact sheet with a brief summation of the vital signs report is available here.
The Centers for Disease Control and Prevention’s Antibiotic Resistance (AR) Lab Network has detected 221 instances of bacteria with especially rare resistance genes in the United States, according to a Vital Signs report published online and expanded upon in CDC’s MMWR Weekly.
The MMWR Weekly report, “Containment of Novel Multidrug-Resistant Organisms and Resistance Mechanisms,” which goes deeper into the science behind the issue, shows that in 9 months, in all states and Puerto Rico, health department workers in the AR lab network tested 5,776 samples of highly resistant bacteria, according to Anne Schuchat, MD, principal deputy director of the CDC. These bacteria were immediately tested for unusual resistance – “those genes that were highly resistant, or rare, with special resistance that could spread,” she said.
“Of the 5,776, about 1 in 4 of the bacteria had a gene that helped it spread its resistance. And there were 221 instances of an especially rare resistance gene,” she added. This prompted intense screening, revealing “that 1 in 10 tests were also positive. Meaning the unusual resistance may have spread to other patients. And could have continued spreading if left undetected.”
The report looked at carbapenem-resistant Enterobacteriaceae (CRE) and Enterobacteriaceae with extended-spectrum beta-lactamases (ESBL) infection data from the National Healthcare Safety Network from 2006-2015 to calculate changes in the year over year proportions of these infections and how an enhanced detection and control strategy curbs carbapenem resistance.
This strategy includes components such as timely implementation of appropriate infection control measures, conducting a health care and contact investigation with follow-up, and implementing a system to ensure adherence to infection control measures.
“With independent, or single facility approaches to control spread, a dangerous type of unusual resistance in Enterobacteriaceae [ESBL phenotype] decreased by about 2% per year [(risk ratio [RR] = 0.98, P less than .001)].” With a more aggressive approach, using guidance such as CDC’s CRE toolkit, released in 2009, another type of unusual resistance [CRE] in the same bacteria (Enterobacteriaceae) decreased by nearly 15% per year (RR = 0.85, P less than .01).
The difference may be due in part to the more directed response utilized to slow the spread of the “nightmare bacteria,” once it was identified, said Dr. Schuchat.
These results show massive promise even if only partially effective, specifically for CRE.
“CDC estimates show that if only 20% effective, the containment strategy can reduce the number of nightmare bacteria [CRE] cases by 76% over 3 years in one area.”
Due to the nature of antibiotic resistance and its ability to spread, this poses a significant public health threat. Antibiotics are not simply used to treat infections but are a safety net that is used in cancer treatment, surgery, and ICU care, Dr. Schuchat pointed out. The rise of antibiotic resistance is a threat to that safety net and accounts for nearly 2,000,000 antibiotic resistant infections and approximately 23,000 deaths per year.
But aggressive responses to these infections can control their spread. Dr. Schuchat used the analogy of controlling a fire to illustrate the concept.
“Much like a fire, finding and stopping unusual resistance early when it’s just a spark protects people.”
Dr. Schuchat reiterated that simply identifying the issue is only part of the equation.
“Detection is not enough on its own. When there is a fire, somebody needs to put it out. CDC supports more than 500 local staff across the country to combat antibiotic resistance wherever it emerges.”
While the report highlights the strides that have been made in combating antibiotic resistance, Paul Auwaerter, MD, president of the Infectious Diseases Society of America, released a statement highlighting the need to further fund these efforts.
“The report spells out the need to accelerate efforts to curb resistance or face an increasing burden including novel resistance mutations that threaten health,” stated Dr. Auwaerter. “The efforts detailed in the Vital Signs report were made possible through new congressional funding in 2016 to combat antibiotic resistance. We urge Congress to sustain and to grow that investment so that further progress will prepare us to meet the future challenges of antibiotic resistance from a position of strength,” he added.
A fact sheet with a brief summation of the vital signs report is available here.
The Centers for Disease Control and Prevention’s Antibiotic Resistance (AR) Lab Network has detected 221 instances of bacteria with especially rare resistance genes in the United States, according to a Vital Signs report published online and expanded upon in CDC’s MMWR Weekly.
The MMWR Weekly report, “Containment of Novel Multidrug-Resistant Organisms and Resistance Mechanisms,” which goes deeper into the science behind the issue, shows that in 9 months, in all states and Puerto Rico, health department workers in the AR lab network tested 5,776 samples of highly resistant bacteria, according to Anne Schuchat, MD, principal deputy director of the CDC. These bacteria were immediately tested for unusual resistance – “those genes that were highly resistant, or rare, with special resistance that could spread,” she said.
“Of the 5,776, about 1 in 4 of the bacteria had a gene that helped it spread its resistance. And there were 221 instances of an especially rare resistance gene,” she added. This prompted intense screening, revealing “that 1 in 10 tests were also positive. Meaning the unusual resistance may have spread to other patients. And could have continued spreading if left undetected.”
The report looked at carbapenem-resistant Enterobacteriaceae (CRE) and Enterobacteriaceae with extended-spectrum beta-lactamases (ESBL) infection data from the National Healthcare Safety Network from 2006-2015 to calculate changes in the year over year proportions of these infections and how an enhanced detection and control strategy curbs carbapenem resistance.
This strategy includes components such as timely implementation of appropriate infection control measures, conducting a health care and contact investigation with follow-up, and implementing a system to ensure adherence to infection control measures.
“With independent, or single facility approaches to control spread, a dangerous type of unusual resistance in Enterobacteriaceae [ESBL phenotype] decreased by about 2% per year [(risk ratio [RR] = 0.98, P less than .001)].” With a more aggressive approach, using guidance such as CDC’s CRE toolkit, released in 2009, another type of unusual resistance [CRE] in the same bacteria (Enterobacteriaceae) decreased by nearly 15% per year (RR = 0.85, P less than .01).
The difference may be due in part to the more directed response utilized to slow the spread of the “nightmare bacteria,” once it was identified, said Dr. Schuchat.
These results show massive promise even if only partially effective, specifically for CRE.
“CDC estimates show that if only 20% effective, the containment strategy can reduce the number of nightmare bacteria [CRE] cases by 76% over 3 years in one area.”
Due to the nature of antibiotic resistance and its ability to spread, this poses a significant public health threat. Antibiotics are not simply used to treat infections but are a safety net that is used in cancer treatment, surgery, and ICU care, Dr. Schuchat pointed out. The rise of antibiotic resistance is a threat to that safety net and accounts for nearly 2,000,000 antibiotic resistant infections and approximately 23,000 deaths per year.
But aggressive responses to these infections can control their spread. Dr. Schuchat used the analogy of controlling a fire to illustrate the concept.
“Much like a fire, finding and stopping unusual resistance early when it’s just a spark protects people.”
Dr. Schuchat reiterated that simply identifying the issue is only part of the equation.
“Detection is not enough on its own. When there is a fire, somebody needs to put it out. CDC supports more than 500 local staff across the country to combat antibiotic resistance wherever it emerges.”
While the report highlights the strides that have been made in combating antibiotic resistance, Paul Auwaerter, MD, president of the Infectious Diseases Society of America, released a statement highlighting the need to further fund these efforts.
“The report spells out the need to accelerate efforts to curb resistance or face an increasing burden including novel resistance mutations that threaten health,” stated Dr. Auwaerter. “The efforts detailed in the Vital Signs report were made possible through new congressional funding in 2016 to combat antibiotic resistance. We urge Congress to sustain and to grow that investment so that further progress will prepare us to meet the future challenges of antibiotic resistance from a position of strength,” he added.
A fact sheet with a brief summation of the vital signs report is available here.
FROM A CDC TELEBRIEFING
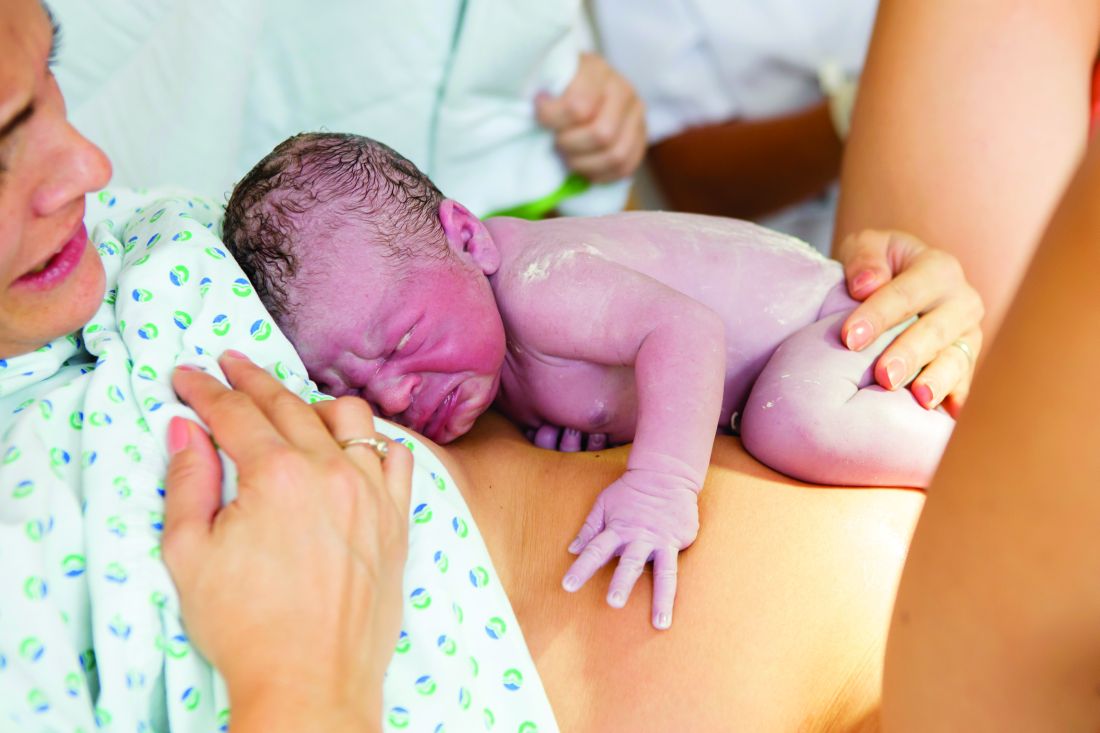
QI initiative reduces antibiotic use in chorioamnionitis-exposed newborns
A hospital quality improvement initiative reduced antibiotic use by more than half when well-appearing newborns exposed to chorioamnionitis were initially monitored for symptoms instead of routinely given antibiotics, found a study in Pediatrics.
“The reduction in both antibiotic use and laboratory testing occurred without clinically relevant delays in care or poor outcomes,” wrote Neha S. Joshi, MD, of Stanford (Calif.) University and her associates.
At Lucile Packard Children’s Hospital Stanford, about half of all antibiotic use for late-preterm or term infants went to newborns exposed to chorioamnionitis. The hospital developed a quality improvement initiative to safely reduce unnecessary antibiotic use in these patients and to decrease unnecessary lab testing given the weak clinical relevance of CBC counts and C-reactive protein labs for determining whether to give a well-appearing child antibiotics, the study authors explained.
Before the initiative began, standard practice included admitting all infants to the neonatal ICU who were at least 34 weeks’ gestation and exposed to chorioamnionitis. They were treated with ampicillin and gentamicin until early-onset sepsis was excluded. Lab evaluations included a CBC count, blood culture, and multiple C-reactive protein labs.
Under the new protocol, symptomatic newborns still had the same labs and received empirical antibiotics. Well-appearing, late-preterm or term infants exposed to chorioamnionitis first spent 2 hours of skin-to-skin contact with their mothers and then were monitored clinically in a level II nursery for at least 24 hours. Unless clinical symptoms developed in that time, the infants then were returned to their mothers until discharge without labs or antibiotics. Those who did develop potentially septic signs/symptoms, as determined by the treating physician, were evaluated and then received antibiotics if deemed appropriate.
During the first 15 months of the quality improvement initiative, 310 infants (5.7% of the 5,425 total births with at least 34 weeks’ gestation) were exposed to chorioamnionitis. Of these, 23 (7.4%) were symptomatic and began antibiotics; another 10 (3.2%) were admitted to the neonatal ICU for a congenital anomaly.
The researchers collected data on antibiotic use, lab tests, cultures, and clinical outcomes from the remaining 277 well-appearing newborns; 88% did not receive antibiotics during their hospital stay, and 83% underwent no laboratory testing. Only 17% of infants had lab testing for sepsis; none had culture result–positive, early-onset sepsis.
Only 12% of infants who initially appeared well developed signs/symptoms of sepsis, underwent laboratory testing, and received antibiotics. Nearly half of these (5% of all infants) received antibiotic treatment for at least 5 days despite negative cultures, while the other 7% received antibiotics for less than 48 hours, Dr. Joshi and her colleagues reported.
Infants with at least 34 weeks’ gestation receiving antibiotics at the hospital dropped from 12.3% before the initiative to 5.5% afterward, a 55% decrease (95% confidence interval, 40%-60%), the researchers said. Study limitations included a lack of postdischarge follow-up, the variability in physician decisions about which infants were symptomatic and which ones needed antibiotics, and an inability to generalize findings to institutions without 24/7 availability of neonatal hospitalists.
Past studies have found that all newborns with positive cultures showed symptoms at birth and needed resuscitation, continuous positive airway pressure, or intubation.
“An infant who is well-appearing at birth likely has an even lower risk of early-onset sepsis even in the setting of chorioamnionitis, and an empirical antibiotic treatment strategy for chorioamnionitis-exposed infants will result in a large number of uninfected infants being treated,” Dr. Joshi and her associates said. “Updated treatment approaches are needed to reduce unnecessary antibiotic exposure and provide higher-value care in this population.”
The study did not use external funding. The authors had no disclosures.
SOURCE: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
A hospital quality improvement initiative reduced antibiotic use by more than half when well-appearing newborns exposed to chorioamnionitis were initially monitored for symptoms instead of routinely given antibiotics, found a study in Pediatrics.
“The reduction in both antibiotic use and laboratory testing occurred without clinically relevant delays in care or poor outcomes,” wrote Neha S. Joshi, MD, of Stanford (Calif.) University and her associates.
At Lucile Packard Children’s Hospital Stanford, about half of all antibiotic use for late-preterm or term infants went to newborns exposed to chorioamnionitis. The hospital developed a quality improvement initiative to safely reduce unnecessary antibiotic use in these patients and to decrease unnecessary lab testing given the weak clinical relevance of CBC counts and C-reactive protein labs for determining whether to give a well-appearing child antibiotics, the study authors explained.
Before the initiative began, standard practice included admitting all infants to the neonatal ICU who were at least 34 weeks’ gestation and exposed to chorioamnionitis. They were treated with ampicillin and gentamicin until early-onset sepsis was excluded. Lab evaluations included a CBC count, blood culture, and multiple C-reactive protein labs.
Under the new protocol, symptomatic newborns still had the same labs and received empirical antibiotics. Well-appearing, late-preterm or term infants exposed to chorioamnionitis first spent 2 hours of skin-to-skin contact with their mothers and then were monitored clinically in a level II nursery for at least 24 hours. Unless clinical symptoms developed in that time, the infants then were returned to their mothers until discharge without labs or antibiotics. Those who did develop potentially septic signs/symptoms, as determined by the treating physician, were evaluated and then received antibiotics if deemed appropriate.
During the first 15 months of the quality improvement initiative, 310 infants (5.7% of the 5,425 total births with at least 34 weeks’ gestation) were exposed to chorioamnionitis. Of these, 23 (7.4%) were symptomatic and began antibiotics; another 10 (3.2%) were admitted to the neonatal ICU for a congenital anomaly.
The researchers collected data on antibiotic use, lab tests, cultures, and clinical outcomes from the remaining 277 well-appearing newborns; 88% did not receive antibiotics during their hospital stay, and 83% underwent no laboratory testing. Only 17% of infants had lab testing for sepsis; none had culture result–positive, early-onset sepsis.
Only 12% of infants who initially appeared well developed signs/symptoms of sepsis, underwent laboratory testing, and received antibiotics. Nearly half of these (5% of all infants) received antibiotic treatment for at least 5 days despite negative cultures, while the other 7% received antibiotics for less than 48 hours, Dr. Joshi and her colleagues reported.
Infants with at least 34 weeks’ gestation receiving antibiotics at the hospital dropped from 12.3% before the initiative to 5.5% afterward, a 55% decrease (95% confidence interval, 40%-60%), the researchers said. Study limitations included a lack of postdischarge follow-up, the variability in physician decisions about which infants were symptomatic and which ones needed antibiotics, and an inability to generalize findings to institutions without 24/7 availability of neonatal hospitalists.
Past studies have found that all newborns with positive cultures showed symptoms at birth and needed resuscitation, continuous positive airway pressure, or intubation.
“An infant who is well-appearing at birth likely has an even lower risk of early-onset sepsis even in the setting of chorioamnionitis, and an empirical antibiotic treatment strategy for chorioamnionitis-exposed infants will result in a large number of uninfected infants being treated,” Dr. Joshi and her associates said. “Updated treatment approaches are needed to reduce unnecessary antibiotic exposure and provide higher-value care in this population.”
The study did not use external funding. The authors had no disclosures.
SOURCE: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
A hospital quality improvement initiative reduced antibiotic use by more than half when well-appearing newborns exposed to chorioamnionitis were initially monitored for symptoms instead of routinely given antibiotics, found a study in Pediatrics.
“The reduction in both antibiotic use and laboratory testing occurred without clinically relevant delays in care or poor outcomes,” wrote Neha S. Joshi, MD, of Stanford (Calif.) University and her associates.
At Lucile Packard Children’s Hospital Stanford, about half of all antibiotic use for late-preterm or term infants went to newborns exposed to chorioamnionitis. The hospital developed a quality improvement initiative to safely reduce unnecessary antibiotic use in these patients and to decrease unnecessary lab testing given the weak clinical relevance of CBC counts and C-reactive protein labs for determining whether to give a well-appearing child antibiotics, the study authors explained.
Before the initiative began, standard practice included admitting all infants to the neonatal ICU who were at least 34 weeks’ gestation and exposed to chorioamnionitis. They were treated with ampicillin and gentamicin until early-onset sepsis was excluded. Lab evaluations included a CBC count, blood culture, and multiple C-reactive protein labs.
Under the new protocol, symptomatic newborns still had the same labs and received empirical antibiotics. Well-appearing, late-preterm or term infants exposed to chorioamnionitis first spent 2 hours of skin-to-skin contact with their mothers and then were monitored clinically in a level II nursery for at least 24 hours. Unless clinical symptoms developed in that time, the infants then were returned to their mothers until discharge without labs or antibiotics. Those who did develop potentially septic signs/symptoms, as determined by the treating physician, were evaluated and then received antibiotics if deemed appropriate.
During the first 15 months of the quality improvement initiative, 310 infants (5.7% of the 5,425 total births with at least 34 weeks’ gestation) were exposed to chorioamnionitis. Of these, 23 (7.4%) were symptomatic and began antibiotics; another 10 (3.2%) were admitted to the neonatal ICU for a congenital anomaly.
The researchers collected data on antibiotic use, lab tests, cultures, and clinical outcomes from the remaining 277 well-appearing newborns; 88% did not receive antibiotics during their hospital stay, and 83% underwent no laboratory testing. Only 17% of infants had lab testing for sepsis; none had culture result–positive, early-onset sepsis.
Only 12% of infants who initially appeared well developed signs/symptoms of sepsis, underwent laboratory testing, and received antibiotics. Nearly half of these (5% of all infants) received antibiotic treatment for at least 5 days despite negative cultures, while the other 7% received antibiotics for less than 48 hours, Dr. Joshi and her colleagues reported.
Infants with at least 34 weeks’ gestation receiving antibiotics at the hospital dropped from 12.3% before the initiative to 5.5% afterward, a 55% decrease (95% confidence interval, 40%-60%), the researchers said. Study limitations included a lack of postdischarge follow-up, the variability in physician decisions about which infants were symptomatic and which ones needed antibiotics, and an inability to generalize findings to institutions without 24/7 availability of neonatal hospitalists.
Past studies have found that all newborns with positive cultures showed symptoms at birth and needed resuscitation, continuous positive airway pressure, or intubation.
“An infant who is well-appearing at birth likely has an even lower risk of early-onset sepsis even in the setting of chorioamnionitis, and an empirical antibiotic treatment strategy for chorioamnionitis-exposed infants will result in a large number of uninfected infants being treated,” Dr. Joshi and her associates said. “Updated treatment approaches are needed to reduce unnecessary antibiotic exposure and provide higher-value care in this population.”
The study did not use external funding. The authors had no disclosures.
SOURCE: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
FROM PEDIATRICS
Key clinical point:
Major finding: After a quality improvement initiative was implemented, 55% fewer late-preterm and term, chorioamnionitis-exposed infants received antibiotics without an increase in negative outcomes.
Data source: A study of 310 chorioamnionitis-exposed newborns who were late preterm or term at a California hospital.
Disclosures: The study did not use external funding. The authors had no relevant financial disclosures.
Source: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
Making structural improvements in health care
Every day, hospitalists devote time and energy to the best practices that can limit the spread of infection and the development of antibiotic resistance. Infection Prevention (IP) and Antimicrobial Stewardship (ASP) are two hospital programs that address that same goal.
But there may be a more effective approach possible, according to Jerome A. Leis, MD, MSc, FRCPC, of the Centre for Quality Improvement and Patient Safety at the University of Toronto.
“Despite the high-quality evidence supporting these IP/ASP interventions, our approach to adding these to our current practice sometimes feels like adding scaffolding to a rickety building,” he said. “It supports the underlying structure, but remove the scaffolding without fixing the building, and it may just come tumbling down.” Sometimes the work seems like an uphill battle, he added, as the same problems continue to recur.
That’s because there’s a systemic element to the problems. “Hospitalists know first hand about how the system that we work in makes it difficult to ensure that all the best IP/ASP practices are adhered to all the time,” Dr. Leis said. “Simply reminding staff to remove a urinary catheter in a timely fashion or clean their hands every single time they touch a patient or the environment can only get us so far.” That’s where improvement science comes in.
The relatively new field of improvement science provides a framework for research focused on health care improvement; its goal is to determine which improvement strategies are most effective. Dr. Leis argued that, “when our approach to IP and ASP incorporate principles of improvement science, we are more likely to be successful in achieving sustainable changes in practice.”
Rather than constantly adding extra steps and reminders for hospitalists about patient safety, he said, we need to recognize that there are systemic factors that lead to specific practices. “Our focus should be to use improvement-science methodology to understand these barriers and redesign the processes of care in a way that makes it easier for hospitalists to adhere to the best IP/ASP practices for our patients.”
These structural changes should come from collaboration among content experts in IP/ASP and those with training in improvement science, he said – many IP and ASP programs are already putting this in practice, using improvement science to create safer systems of care.
Reference
Leis J. Advancing infection prevention and antimicrobial stewardship through improvement science. BMJ Qual Saf. 2017 Jun 14. doi: 10.1136/bmjqs-2017-006793.
Every day, hospitalists devote time and energy to the best practices that can limit the spread of infection and the development of antibiotic resistance. Infection Prevention (IP) and Antimicrobial Stewardship (ASP) are two hospital programs that address that same goal.
But there may be a more effective approach possible, according to Jerome A. Leis, MD, MSc, FRCPC, of the Centre for Quality Improvement and Patient Safety at the University of Toronto.
“Despite the high-quality evidence supporting these IP/ASP interventions, our approach to adding these to our current practice sometimes feels like adding scaffolding to a rickety building,” he said. “It supports the underlying structure, but remove the scaffolding without fixing the building, and it may just come tumbling down.” Sometimes the work seems like an uphill battle, he added, as the same problems continue to recur.
That’s because there’s a systemic element to the problems. “Hospitalists know first hand about how the system that we work in makes it difficult to ensure that all the best IP/ASP practices are adhered to all the time,” Dr. Leis said. “Simply reminding staff to remove a urinary catheter in a timely fashion or clean their hands every single time they touch a patient or the environment can only get us so far.” That’s where improvement science comes in.
The relatively new field of improvement science provides a framework for research focused on health care improvement; its goal is to determine which improvement strategies are most effective. Dr. Leis argued that, “when our approach to IP and ASP incorporate principles of improvement science, we are more likely to be successful in achieving sustainable changes in practice.”
Rather than constantly adding extra steps and reminders for hospitalists about patient safety, he said, we need to recognize that there are systemic factors that lead to specific practices. “Our focus should be to use improvement-science methodology to understand these barriers and redesign the processes of care in a way that makes it easier for hospitalists to adhere to the best IP/ASP practices for our patients.”
These structural changes should come from collaboration among content experts in IP/ASP and those with training in improvement science, he said – many IP and ASP programs are already putting this in practice, using improvement science to create safer systems of care.
Reference
Leis J. Advancing infection prevention and antimicrobial stewardship through improvement science. BMJ Qual Saf. 2017 Jun 14. doi: 10.1136/bmjqs-2017-006793.
Every day, hospitalists devote time and energy to the best practices that can limit the spread of infection and the development of antibiotic resistance. Infection Prevention (IP) and Antimicrobial Stewardship (ASP) are two hospital programs that address that same goal.
But there may be a more effective approach possible, according to Jerome A. Leis, MD, MSc, FRCPC, of the Centre for Quality Improvement and Patient Safety at the University of Toronto.
“Despite the high-quality evidence supporting these IP/ASP interventions, our approach to adding these to our current practice sometimes feels like adding scaffolding to a rickety building,” he said. “It supports the underlying structure, but remove the scaffolding without fixing the building, and it may just come tumbling down.” Sometimes the work seems like an uphill battle, he added, as the same problems continue to recur.
That’s because there’s a systemic element to the problems. “Hospitalists know first hand about how the system that we work in makes it difficult to ensure that all the best IP/ASP practices are adhered to all the time,” Dr. Leis said. “Simply reminding staff to remove a urinary catheter in a timely fashion or clean their hands every single time they touch a patient or the environment can only get us so far.” That’s where improvement science comes in.
The relatively new field of improvement science provides a framework for research focused on health care improvement; its goal is to determine which improvement strategies are most effective. Dr. Leis argued that, “when our approach to IP and ASP incorporate principles of improvement science, we are more likely to be successful in achieving sustainable changes in practice.”
Rather than constantly adding extra steps and reminders for hospitalists about patient safety, he said, we need to recognize that there are systemic factors that lead to specific practices. “Our focus should be to use improvement-science methodology to understand these barriers and redesign the processes of care in a way that makes it easier for hospitalists to adhere to the best IP/ASP practices for our patients.”
These structural changes should come from collaboration among content experts in IP/ASP and those with training in improvement science, he said – many IP and ASP programs are already putting this in practice, using improvement science to create safer systems of care.
Reference
Leis J. Advancing infection prevention and antimicrobial stewardship through improvement science. BMJ Qual Saf. 2017 Jun 14. doi: 10.1136/bmjqs-2017-006793.
FDA warns against clarithromycin use in patients with heart disease
The Food and Drug Administration has added a new warning for an increased risk of death in patients with heart disease who have used clarithromycin (Biaxin), on the basis of results of a 10-year follow-up from the CLARICOR trial.
The CLARICOR trial followed 4,372 randomized patients for at least 2 years after undergoing 14 days of treatment with daily doses of 500 mg clarithromycin. Among these patients, researchers observed an unexpected increase in deaths in patients with coronary heart disease. (The Feb. 22 FDA statement announcing the alert did not provide data from CLARICOR.) As of yet, there is no clear explanation of how clarithromycin would lead to more deaths, compared with a placebo, the agency said.
Regardless, two of the six observational studies published found a link between clarithromycin use and long-term risks; four did not. The CLARICOR trial provides the strongest evidence of increased health risks, the statement said.
The FDA is recommending that health care professionals be aware of the risks associated with clarithromycin use and consider the benefits and risks of use in patients with heart disease. If at all possible, the use of other antibiotics may be a better option. Doctors should advise patients to be aware of signs and symptoms associated with cardiovascular issues.
Patients are also an important piece of the puzzle and should communicate with their health care providers about heart disease, particularly when taking antibiotics to treat for an infection.
The FDA has added the results of the CLARICOR trial to the clarithromycin drug labels. The agency will continue to monitor the safety reports in patients using clarithromycin.
Serious adverse events associated with clarithromycin should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch/.
The Food and Drug Administration has added a new warning for an increased risk of death in patients with heart disease who have used clarithromycin (Biaxin), on the basis of results of a 10-year follow-up from the CLARICOR trial.
The CLARICOR trial followed 4,372 randomized patients for at least 2 years after undergoing 14 days of treatment with daily doses of 500 mg clarithromycin. Among these patients, researchers observed an unexpected increase in deaths in patients with coronary heart disease. (The Feb. 22 FDA statement announcing the alert did not provide data from CLARICOR.) As of yet, there is no clear explanation of how clarithromycin would lead to more deaths, compared with a placebo, the agency said.
Regardless, two of the six observational studies published found a link between clarithromycin use and long-term risks; four did not. The CLARICOR trial provides the strongest evidence of increased health risks, the statement said.
The FDA is recommending that health care professionals be aware of the risks associated with clarithromycin use and consider the benefits and risks of use in patients with heart disease. If at all possible, the use of other antibiotics may be a better option. Doctors should advise patients to be aware of signs and symptoms associated with cardiovascular issues.
Patients are also an important piece of the puzzle and should communicate with their health care providers about heart disease, particularly when taking antibiotics to treat for an infection.
The FDA has added the results of the CLARICOR trial to the clarithromycin drug labels. The agency will continue to monitor the safety reports in patients using clarithromycin.
Serious adverse events associated with clarithromycin should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch/.
The Food and Drug Administration has added a new warning for an increased risk of death in patients with heart disease who have used clarithromycin (Biaxin), on the basis of results of a 10-year follow-up from the CLARICOR trial.
The CLARICOR trial followed 4,372 randomized patients for at least 2 years after undergoing 14 days of treatment with daily doses of 500 mg clarithromycin. Among these patients, researchers observed an unexpected increase in deaths in patients with coronary heart disease. (The Feb. 22 FDA statement announcing the alert did not provide data from CLARICOR.) As of yet, there is no clear explanation of how clarithromycin would lead to more deaths, compared with a placebo, the agency said.
Regardless, two of the six observational studies published found a link between clarithromycin use and long-term risks; four did not. The CLARICOR trial provides the strongest evidence of increased health risks, the statement said.
The FDA is recommending that health care professionals be aware of the risks associated with clarithromycin use and consider the benefits and risks of use in patients with heart disease. If at all possible, the use of other antibiotics may be a better option. Doctors should advise patients to be aware of signs and symptoms associated with cardiovascular issues.
Patients are also an important piece of the puzzle and should communicate with their health care providers about heart disease, particularly when taking antibiotics to treat for an infection.
The FDA has added the results of the CLARICOR trial to the clarithromycin drug labels. The agency will continue to monitor the safety reports in patients using clarithromycin.
Serious adverse events associated with clarithromycin should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch/.
Reported penicillin allergies hike inpatient costs
Total inpatient costs for patients who report being allergic to penicillin are much higher than for those who don’t report an allergy, according to a recent systematic review and meta-analysis.
The review, which eventually included 30 articles, found that total inpatient costs ranged from an average $1,145-$4,254 higher per patient with a reported penicillin allergy compared to nonallergic patients, said T. Joseph Mattingly, PharmD, and his associates. Outpatient prescription costs were also estimated to be steeper, running $14-$93 higher per patient who reported a penicillin allergy.
Although 10%-20% of patients report a penicillin allergy, “[a] majority of patients who report PCN [penicillin] allergy are not truly allergic upon confirmatory testing,” Dr. Mattingly and his colleagues wrote.
This overreporting of penicillin allergies is a problem for the patient and the health care system because “reported antibiotic allergies have been associated with suboptimal antibiotic therapy, increased antimicrobial resistance, increased length of stay, increased antibiotic-related adverse events, increased rates of C. difficile infection, intensive care unit (ICU) admission, death, as well as increased treatment cost,” said Dr. Mattingly and his coauthors.
Health care providers often “tend to take reported allergies at face value,” said coauthor Anne Fulton, suggesting that primary care practices can help by considering skin testing for those patients who carry a label of penicillin allergy, but don’t have a documented confirmatory test. The cost for a commonly used skin test for penicillin allergy runs about $200, said Ms. Fulton, a doctoral candidate at the University of Maryland, Baltimore, in an interview.
When conducting the meta-analysis, Dr. Mattingly and his coauthors converted all figures to 2017 U.S. dollars, using Consumer Price Index figures to adjust for inflation. This yields conservative estimates for cost, as drug and health care prices have far outstripped the general rate of inflation during the period in which the studies occurred, Ms. Fulton acknowledged.
The investigators highlighted the need for ongoing study in this area. “To our knowledge, there are no evaluations of long-term outpatient outcomes related to the effects of PCN allergy and the potential impact of delabeling patients who do not have a true allergy,” they wrote.
Ms. Fulton agreed, noting that the studies covered in the meta-analysis were primarily focused on short-term outcomes, though there are many potential long-term benefits to delabeling patients who are not truly penicillin allergic.
For the patient, this includes the opportunity to receive optimal antimicrobial therapy, as well as potential savings in copays and other out-of-pocket expenses for outpatient medications, she said.
As antimicrobial resistance becomes an ever more pressing problem, there are more opportunities for targeted therapy if inappropriate allergy labeling is addressed, Ms. Fulton added.
Further study should use “cost-effectiveness analysis methods that include societal and health sector perspectives capturing immediate and future outcomes and costs to evaluate the use of skin-testing procedures in either inpatient or outpatient settings,” the investigators wrote.
The study was supported by ALK, the manufacturer of Pre-Pen, a commercially available penicillin allergy skin test.
SOURCE: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
Total inpatient costs for patients who report being allergic to penicillin are much higher than for those who don’t report an allergy, according to a recent systematic review and meta-analysis.
The review, which eventually included 30 articles, found that total inpatient costs ranged from an average $1,145-$4,254 higher per patient with a reported penicillin allergy compared to nonallergic patients, said T. Joseph Mattingly, PharmD, and his associates. Outpatient prescription costs were also estimated to be steeper, running $14-$93 higher per patient who reported a penicillin allergy.
Although 10%-20% of patients report a penicillin allergy, “[a] majority of patients who report PCN [penicillin] allergy are not truly allergic upon confirmatory testing,” Dr. Mattingly and his colleagues wrote.
This overreporting of penicillin allergies is a problem for the patient and the health care system because “reported antibiotic allergies have been associated with suboptimal antibiotic therapy, increased antimicrobial resistance, increased length of stay, increased antibiotic-related adverse events, increased rates of C. difficile infection, intensive care unit (ICU) admission, death, as well as increased treatment cost,” said Dr. Mattingly and his coauthors.
Health care providers often “tend to take reported allergies at face value,” said coauthor Anne Fulton, suggesting that primary care practices can help by considering skin testing for those patients who carry a label of penicillin allergy, but don’t have a documented confirmatory test. The cost for a commonly used skin test for penicillin allergy runs about $200, said Ms. Fulton, a doctoral candidate at the University of Maryland, Baltimore, in an interview.
When conducting the meta-analysis, Dr. Mattingly and his coauthors converted all figures to 2017 U.S. dollars, using Consumer Price Index figures to adjust for inflation. This yields conservative estimates for cost, as drug and health care prices have far outstripped the general rate of inflation during the period in which the studies occurred, Ms. Fulton acknowledged.
The investigators highlighted the need for ongoing study in this area. “To our knowledge, there are no evaluations of long-term outpatient outcomes related to the effects of PCN allergy and the potential impact of delabeling patients who do not have a true allergy,” they wrote.
Ms. Fulton agreed, noting that the studies covered in the meta-analysis were primarily focused on short-term outcomes, though there are many potential long-term benefits to delabeling patients who are not truly penicillin allergic.
For the patient, this includes the opportunity to receive optimal antimicrobial therapy, as well as potential savings in copays and other out-of-pocket expenses for outpatient medications, she said.
As antimicrobial resistance becomes an ever more pressing problem, there are more opportunities for targeted therapy if inappropriate allergy labeling is addressed, Ms. Fulton added.
Further study should use “cost-effectiveness analysis methods that include societal and health sector perspectives capturing immediate and future outcomes and costs to evaluate the use of skin-testing procedures in either inpatient or outpatient settings,” the investigators wrote.
The study was supported by ALK, the manufacturer of Pre-Pen, a commercially available penicillin allergy skin test.
SOURCE: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
Total inpatient costs for patients who report being allergic to penicillin are much higher than for those who don’t report an allergy, according to a recent systematic review and meta-analysis.
The review, which eventually included 30 articles, found that total inpatient costs ranged from an average $1,145-$4,254 higher per patient with a reported penicillin allergy compared to nonallergic patients, said T. Joseph Mattingly, PharmD, and his associates. Outpatient prescription costs were also estimated to be steeper, running $14-$93 higher per patient who reported a penicillin allergy.
Although 10%-20% of patients report a penicillin allergy, “[a] majority of patients who report PCN [penicillin] allergy are not truly allergic upon confirmatory testing,” Dr. Mattingly and his colleagues wrote.
This overreporting of penicillin allergies is a problem for the patient and the health care system because “reported antibiotic allergies have been associated with suboptimal antibiotic therapy, increased antimicrobial resistance, increased length of stay, increased antibiotic-related adverse events, increased rates of C. difficile infection, intensive care unit (ICU) admission, death, as well as increased treatment cost,” said Dr. Mattingly and his coauthors.
Health care providers often “tend to take reported allergies at face value,” said coauthor Anne Fulton, suggesting that primary care practices can help by considering skin testing for those patients who carry a label of penicillin allergy, but don’t have a documented confirmatory test. The cost for a commonly used skin test for penicillin allergy runs about $200, said Ms. Fulton, a doctoral candidate at the University of Maryland, Baltimore, in an interview.
When conducting the meta-analysis, Dr. Mattingly and his coauthors converted all figures to 2017 U.S. dollars, using Consumer Price Index figures to adjust for inflation. This yields conservative estimates for cost, as drug and health care prices have far outstripped the general rate of inflation during the period in which the studies occurred, Ms. Fulton acknowledged.
The investigators highlighted the need for ongoing study in this area. “To our knowledge, there are no evaluations of long-term outpatient outcomes related to the effects of PCN allergy and the potential impact of delabeling patients who do not have a true allergy,” they wrote.
Ms. Fulton agreed, noting that the studies covered in the meta-analysis were primarily focused on short-term outcomes, though there are many potential long-term benefits to delabeling patients who are not truly penicillin allergic.
For the patient, this includes the opportunity to receive optimal antimicrobial therapy, as well as potential savings in copays and other out-of-pocket expenses for outpatient medications, she said.
As antimicrobial resistance becomes an ever more pressing problem, there are more opportunities for targeted therapy if inappropriate allergy labeling is addressed, Ms. Fulton added.
Further study should use “cost-effectiveness analysis methods that include societal and health sector perspectives capturing immediate and future outcomes and costs to evaluate the use of skin-testing procedures in either inpatient or outpatient settings,” the investigators wrote.
The study was supported by ALK, the manufacturer of Pre-Pen, a commercially available penicillin allergy skin test.
SOURCE: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
FROM JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Key clinical point: Inpatient costs were $1,145 – $4,254 higher for those reporting penicillin allergy.
Major finding: Though most studies addressed inpatient admissions, outpatient costs were also significantly higher.
Study details: Systematic review and meta-analysis of 30 articles addressing reported penicillin allergy.
Disclosures: The study was sponsored by ALK.
Source: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
Paring the risk of antibiotic resistance
One unintended consequence of the increased attention to early sepsis identification and intervention can be unnecessary or excessive antibiotic use. Overuse of broad-spectrum antibiotics, in turn, can fuel the emergence of life-threatening infections such as antibiotic-resistant Clostridium difficile, a scourge in many hospitals.
For a sepsis quality improvement (QI) initiative at the University of Utah, Salt Lake City, the hospitalist coleaders took several precautions to lessen the risk of antibiotic overuse. Kencee K. Graves, MD, said she and her colleague Devin J. Horton, MD, designed the hospital’s order sets in collaboration with an infectious disease specialist and pharmacist so they could avoid overly broad antibiotics whenever possible. The project also included an educational effort to get pharmacists in the habit of prompting medical providers to initiate antibiotic de-escalation at 48 hours. The hospital had an antibiotic stewardship program that likely helped as well, she said. As a result of their precautions, the team found no significant difference in the amount of broad-spectrum antibiotics doled out before and after their QI pilot project.
Infection control and antimicrobial specialists also can help; they can monitor an area’s resistance profile, create a antibiogram and reevaluate sepsis pathways and order sets to adjust the recommended antibiotics as the resistance profile changes. “I think we still have a long ways to go,” said Andy Odden, MD, SFHM, patient safety officer in the department of medicine at Washington University in St. Louis. “The initial risk of mortality is so much more dramatic than the long-term risks of developing antimicrobial resistors that unless you have the antimicrobial stewardship people with a seat at the table, that voice can get drowned out very easily.”
The antimicrobial stewardship program at University of Pennsylvania, Philadelphia, has received a boost from technology. The program offers initial guidance on which broad-spectrum antibiotics to consider depending on the suspected source of the sepsis-linked infection. Software by Jackson, Wyo.–based biotech company Teqqa also synthesizes the university hospital’s resistance data based on blood, urine, and sputum cultures. “It can predict the antibiotic sensitivity of a given bug growing out of a given culture on a given unit,” said Craig A. Umscheid, MD, MSCE, of the department of epidemiology and vice chair for quality and safety in the department of medicine at the university.
The bigger issue, Dr. Umscheid said, is when and how to de-escalate antibiotic treatment. “If somebody is feeling better in 48 hours or 72 hours and no cultures have grown back, they have no more fever, and their white counts have normalized, do you start pulling off the antibiotics slowly and, if so, how do you do that?” Several trials are examining such questions, including a multicenter collaboration called DETOURS (De-Escalating Empiric Treatment: Opting-Out of Rx for Selected Patients With Suspected Sepsis). One of the trial’s chief aims is to set up a new opt-out protocol for acute care patients in the wards.
One unintended consequence of the increased attention to early sepsis identification and intervention can be unnecessary or excessive antibiotic use. Overuse of broad-spectrum antibiotics, in turn, can fuel the emergence of life-threatening infections such as antibiotic-resistant Clostridium difficile, a scourge in many hospitals.
For a sepsis quality improvement (QI) initiative at the University of Utah, Salt Lake City, the hospitalist coleaders took several precautions to lessen the risk of antibiotic overuse. Kencee K. Graves, MD, said she and her colleague Devin J. Horton, MD, designed the hospital’s order sets in collaboration with an infectious disease specialist and pharmacist so they could avoid overly broad antibiotics whenever possible. The project also included an educational effort to get pharmacists in the habit of prompting medical providers to initiate antibiotic de-escalation at 48 hours. The hospital had an antibiotic stewardship program that likely helped as well, she said. As a result of their precautions, the team found no significant difference in the amount of broad-spectrum antibiotics doled out before and after their QI pilot project.
Infection control and antimicrobial specialists also can help; they can monitor an area’s resistance profile, create a antibiogram and reevaluate sepsis pathways and order sets to adjust the recommended antibiotics as the resistance profile changes. “I think we still have a long ways to go,” said Andy Odden, MD, SFHM, patient safety officer in the department of medicine at Washington University in St. Louis. “The initial risk of mortality is so much more dramatic than the long-term risks of developing antimicrobial resistors that unless you have the antimicrobial stewardship people with a seat at the table, that voice can get drowned out very easily.”
The antimicrobial stewardship program at University of Pennsylvania, Philadelphia, has received a boost from technology. The program offers initial guidance on which broad-spectrum antibiotics to consider depending on the suspected source of the sepsis-linked infection. Software by Jackson, Wyo.–based biotech company Teqqa also synthesizes the university hospital’s resistance data based on blood, urine, and sputum cultures. “It can predict the antibiotic sensitivity of a given bug growing out of a given culture on a given unit,” said Craig A. Umscheid, MD, MSCE, of the department of epidemiology and vice chair for quality and safety in the department of medicine at the university.
The bigger issue, Dr. Umscheid said, is when and how to de-escalate antibiotic treatment. “If somebody is feeling better in 48 hours or 72 hours and no cultures have grown back, they have no more fever, and their white counts have normalized, do you start pulling off the antibiotics slowly and, if so, how do you do that?” Several trials are examining such questions, including a multicenter collaboration called DETOURS (De-Escalating Empiric Treatment: Opting-Out of Rx for Selected Patients With Suspected Sepsis). One of the trial’s chief aims is to set up a new opt-out protocol for acute care patients in the wards.
One unintended consequence of the increased attention to early sepsis identification and intervention can be unnecessary or excessive antibiotic use. Overuse of broad-spectrum antibiotics, in turn, can fuel the emergence of life-threatening infections such as antibiotic-resistant Clostridium difficile, a scourge in many hospitals.
For a sepsis quality improvement (QI) initiative at the University of Utah, Salt Lake City, the hospitalist coleaders took several precautions to lessen the risk of antibiotic overuse. Kencee K. Graves, MD, said she and her colleague Devin J. Horton, MD, designed the hospital’s order sets in collaboration with an infectious disease specialist and pharmacist so they could avoid overly broad antibiotics whenever possible. The project also included an educational effort to get pharmacists in the habit of prompting medical providers to initiate antibiotic de-escalation at 48 hours. The hospital had an antibiotic stewardship program that likely helped as well, she said. As a result of their precautions, the team found no significant difference in the amount of broad-spectrum antibiotics doled out before and after their QI pilot project.
Infection control and antimicrobial specialists also can help; they can monitor an area’s resistance profile, create a antibiogram and reevaluate sepsis pathways and order sets to adjust the recommended antibiotics as the resistance profile changes. “I think we still have a long ways to go,” said Andy Odden, MD, SFHM, patient safety officer in the department of medicine at Washington University in St. Louis. “The initial risk of mortality is so much more dramatic than the long-term risks of developing antimicrobial resistors that unless you have the antimicrobial stewardship people with a seat at the table, that voice can get drowned out very easily.”
The antimicrobial stewardship program at University of Pennsylvania, Philadelphia, has received a boost from technology. The program offers initial guidance on which broad-spectrum antibiotics to consider depending on the suspected source of the sepsis-linked infection. Software by Jackson, Wyo.–based biotech company Teqqa also synthesizes the university hospital’s resistance data based on blood, urine, and sputum cultures. “It can predict the antibiotic sensitivity of a given bug growing out of a given culture on a given unit,” said Craig A. Umscheid, MD, MSCE, of the department of epidemiology and vice chair for quality and safety in the department of medicine at the university.
The bigger issue, Dr. Umscheid said, is when and how to de-escalate antibiotic treatment. “If somebody is feeling better in 48 hours or 72 hours and no cultures have grown back, they have no more fever, and their white counts have normalized, do you start pulling off the antibiotics slowly and, if so, how do you do that?” Several trials are examining such questions, including a multicenter collaboration called DETOURS (De-Escalating Empiric Treatment: Opting-Out of Rx for Selected Patients With Suspected Sepsis). One of the trial’s chief aims is to set up a new opt-out protocol for acute care patients in the wards.
Drug combo indicated for bacterial pneumonia
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.