User login
Is it time for health policy M&Ms?
What would happen if hospitalists began to incorporate health policy into morbidity and mortality (M&M) conferences? That was a question Chris Moriates, MD, explored in an entry for SHM’s The Hospital Leader blog1 and an idea that caused a minor stir on Twitter when he proposed it last summer.
In late July 2017, the U.S. Senate was debating a bill to repeal the Affordable Care Act, without a clear vision for replacing it. In response, physicians around the country took to Twitter to share their sentiments about repeal under the hashtag #DoctorsSpeakOut. In one such tweet, Dr. Moriates, assistant dean for health care value and an associate professor of internal medicine at Dell Medical School at the University of Texas, Austin, said this, in 140 characters: “We recently had idea: health policy M&M for residents to discuss adverse outcomes we see as result of lack of access.”
The idea began with a conversation Dr. Moriates had with Beth Miller, MD, program director for the Dell Medical School Internal Medicine Residency Program. “We were meeting and talking about revamping the [resident] M&M conference to have more learning objectives and put in place best practices,” Dr. Moriates said. “Dr. Miller suggested it could be a good forum [for health policy] because it’s an area where we all come together and there’s a natural hook to it, since it is case-based, thus we can use it to recognize the drivers within the system that lead to bad outcomes.”
In his SHM blog post, Dr. Moriates said he has increasingly observed adverse events that result from issues related to health policy. He provided an example: “A patient I admitted for ‘expedited work-up’ for rectal bleeding after he told me he had been trying to get a recommended colonoscopy for many months but could not get it scheduled due to his lack of insurance. He had colon cancer that had spread.”
In another example, he conjured a hypothetical (though not impractical) case where a patient prescribed blood thinners upon hospital discharge returns to the hospital soon after with a blood clot. Unable to afford the medication, or seek primary care follow-up, this kind of patient is readmitted through no direct fault of his physicians. Yet, the patient is worse off and the hospital takes the hit on readmissions penalties.
Dr. Moriates believes that viewing a case like this through a health policy lens is not only moving, but critical to better understanding health care delivery, particularly in an environment where physician performance is measured, in part, by outcomes. He now believes health policy M&Ms would be valuable to all hospital-based physicians, not just residents.
“Hospitalists are being asked to hit these value-based performance metrics, like readmissions and length of stay, and while we deal with the consequences, we are not always the best informed” with respect to policy, he said. “We could use this forum to teach health policy topics and continually update people and contribute, in real time, to all these different discussions and understand how things are changing or could change and impact our patients.”
“It’s important for physicians to know the policies that are aligned with, and the policies that may undermine, what they’re doing in their practice to improve their patients’ health,” Pourat said.
This knowledge can benefit physicians, too, Pourat added, because health policy M&Ms could help providers understand the goals of particular policies and in turn adjust their own behaviors and expectations.
“Physicians could discuss, what are the underlying issues or root causes, like the decision not to expand Medicaid here in Texas,” Dr. Moriates said. “Not all of these things you can fix, but you’re exposing those stories and perhaps we can come up with some actionable steps. How do we ensure in the future that our patients are able to fulfill their prescription so we’re not just sending someone out assuming they will but not knowing they’re unable to afford it?”
Similar to other domains in which physician leaders become champions, such as antibiotic stewardship, Dr. Pourat suggested that hospitalists could champion policy awareness through the kind of M&Ms Dr. Moriates proposed.
While journal clubs and lectures are great ways for hospitalists to learn more about health policy, the emotionally gripping nature of M&Ms could inspire more physicians to act in favor of policies that benefit their patients and themselves, Dr. Moriates said.
For example, physicians may write to or visit legislative offices, or author op-eds in their local newspapers. This collective action carries the potential to effect change. And it need not be partisan.
“I believe that if health policy issues were more explicitly integrated into M&Ms then clinicians would be more inclined and prepared to effectively advocate for specific policy changes,” he wrote in his blog post. “Perhaps entire groups would be moved to engage in the political process.”
On Twitter, even before Dr. Moriates’ first tweet about health policy M&Ms, New Jersey–based Jennifer Chuang, MD, an adolescent medicine physician, wrote: “M&M is heart-wrenching in academic hospitals. I dare @SenateGOP to present their role in M&M’s to come if ACA is repealed.”
While Dr. Moriates believes the chances are quite small that legislators and policymakers would attend health policy M&Ms, he called the notion “provocative and intriguing.”
In his blog post, Dr. Moriates invites state legislators and local members of Congress to join him in reviewing M&M cases where patients have been negatively affected by policy. He also emphasized that, like most modern M&Ms, the point should not be derision or finger-pointing, but an opportunity to learn how policy translates into practice.
Physicians may learn from legislators, too, he said in his blog post. “Just as policymakers could see legislation through the eyes of practitioners and their patients, this is where we as physicians could possibly learn from our legislators,” he wrote. “We may recognize the potential trade-offs, downsides, and barriers to proposals that to us may have seemed like no-brainers.”
What’s clear, said Dr. Pourat, who is also a professor in the UCLA Fielding School of Public Health and the School of Dentistry, is that Dr. Moriates’ blog post and tweet are “touching an important point for a lot of physicians during this whole debate over health reform.”
President Donald Trump campaigned on a promise to fully repeal and replace the Affordable Care Act but Republican efforts have thus far been stymied. In the meantime, some physicians are watching closely, knowing that whatever comes next will continue to affect them and their patients.
Source
1. Moriates C. Is it time for health policy M&Ms? The Hospital Leader. Aug 16, 2017. http://thehospitalleader.org/is-it-time-for-health-policy-mms/. Accessed 2017 Sep 14.
What would happen if hospitalists began to incorporate health policy into morbidity and mortality (M&M) conferences? That was a question Chris Moriates, MD, explored in an entry for SHM’s The Hospital Leader blog1 and an idea that caused a minor stir on Twitter when he proposed it last summer.
In late July 2017, the U.S. Senate was debating a bill to repeal the Affordable Care Act, without a clear vision for replacing it. In response, physicians around the country took to Twitter to share their sentiments about repeal under the hashtag #DoctorsSpeakOut. In one such tweet, Dr. Moriates, assistant dean for health care value and an associate professor of internal medicine at Dell Medical School at the University of Texas, Austin, said this, in 140 characters: “We recently had idea: health policy M&M for residents to discuss adverse outcomes we see as result of lack of access.”
The idea began with a conversation Dr. Moriates had with Beth Miller, MD, program director for the Dell Medical School Internal Medicine Residency Program. “We were meeting and talking about revamping the [resident] M&M conference to have more learning objectives and put in place best practices,” Dr. Moriates said. “Dr. Miller suggested it could be a good forum [for health policy] because it’s an area where we all come together and there’s a natural hook to it, since it is case-based, thus we can use it to recognize the drivers within the system that lead to bad outcomes.”
In his SHM blog post, Dr. Moriates said he has increasingly observed adverse events that result from issues related to health policy. He provided an example: “A patient I admitted for ‘expedited work-up’ for rectal bleeding after he told me he had been trying to get a recommended colonoscopy for many months but could not get it scheduled due to his lack of insurance. He had colon cancer that had spread.”
In another example, he conjured a hypothetical (though not impractical) case where a patient prescribed blood thinners upon hospital discharge returns to the hospital soon after with a blood clot. Unable to afford the medication, or seek primary care follow-up, this kind of patient is readmitted through no direct fault of his physicians. Yet, the patient is worse off and the hospital takes the hit on readmissions penalties.
Dr. Moriates believes that viewing a case like this through a health policy lens is not only moving, but critical to better understanding health care delivery, particularly in an environment where physician performance is measured, in part, by outcomes. He now believes health policy M&Ms would be valuable to all hospital-based physicians, not just residents.
“Hospitalists are being asked to hit these value-based performance metrics, like readmissions and length of stay, and while we deal with the consequences, we are not always the best informed” with respect to policy, he said. “We could use this forum to teach health policy topics and continually update people and contribute, in real time, to all these different discussions and understand how things are changing or could change and impact our patients.”
“It’s important for physicians to know the policies that are aligned with, and the policies that may undermine, what they’re doing in their practice to improve their patients’ health,” Pourat said.
This knowledge can benefit physicians, too, Pourat added, because health policy M&Ms could help providers understand the goals of particular policies and in turn adjust their own behaviors and expectations.
“Physicians could discuss, what are the underlying issues or root causes, like the decision not to expand Medicaid here in Texas,” Dr. Moriates said. “Not all of these things you can fix, but you’re exposing those stories and perhaps we can come up with some actionable steps. How do we ensure in the future that our patients are able to fulfill their prescription so we’re not just sending someone out assuming they will but not knowing they’re unable to afford it?”
Similar to other domains in which physician leaders become champions, such as antibiotic stewardship, Dr. Pourat suggested that hospitalists could champion policy awareness through the kind of M&Ms Dr. Moriates proposed.
While journal clubs and lectures are great ways for hospitalists to learn more about health policy, the emotionally gripping nature of M&Ms could inspire more physicians to act in favor of policies that benefit their patients and themselves, Dr. Moriates said.
For example, physicians may write to or visit legislative offices, or author op-eds in their local newspapers. This collective action carries the potential to effect change. And it need not be partisan.
“I believe that if health policy issues were more explicitly integrated into M&Ms then clinicians would be more inclined and prepared to effectively advocate for specific policy changes,” he wrote in his blog post. “Perhaps entire groups would be moved to engage in the political process.”
On Twitter, even before Dr. Moriates’ first tweet about health policy M&Ms, New Jersey–based Jennifer Chuang, MD, an adolescent medicine physician, wrote: “M&M is heart-wrenching in academic hospitals. I dare @SenateGOP to present their role in M&M’s to come if ACA is repealed.”
While Dr. Moriates believes the chances are quite small that legislators and policymakers would attend health policy M&Ms, he called the notion “provocative and intriguing.”
In his blog post, Dr. Moriates invites state legislators and local members of Congress to join him in reviewing M&M cases where patients have been negatively affected by policy. He also emphasized that, like most modern M&Ms, the point should not be derision or finger-pointing, but an opportunity to learn how policy translates into practice.
Physicians may learn from legislators, too, he said in his blog post. “Just as policymakers could see legislation through the eyes of practitioners and their patients, this is where we as physicians could possibly learn from our legislators,” he wrote. “We may recognize the potential trade-offs, downsides, and barriers to proposals that to us may have seemed like no-brainers.”
What’s clear, said Dr. Pourat, who is also a professor in the UCLA Fielding School of Public Health and the School of Dentistry, is that Dr. Moriates’ blog post and tweet are “touching an important point for a lot of physicians during this whole debate over health reform.”
President Donald Trump campaigned on a promise to fully repeal and replace the Affordable Care Act but Republican efforts have thus far been stymied. In the meantime, some physicians are watching closely, knowing that whatever comes next will continue to affect them and their patients.
Source
1. Moriates C. Is it time for health policy M&Ms? The Hospital Leader. Aug 16, 2017. http://thehospitalleader.org/is-it-time-for-health-policy-mms/. Accessed 2017 Sep 14.
What would happen if hospitalists began to incorporate health policy into morbidity and mortality (M&M) conferences? That was a question Chris Moriates, MD, explored in an entry for SHM’s The Hospital Leader blog1 and an idea that caused a minor stir on Twitter when he proposed it last summer.
In late July 2017, the U.S. Senate was debating a bill to repeal the Affordable Care Act, without a clear vision for replacing it. In response, physicians around the country took to Twitter to share their sentiments about repeal under the hashtag #DoctorsSpeakOut. In one such tweet, Dr. Moriates, assistant dean for health care value and an associate professor of internal medicine at Dell Medical School at the University of Texas, Austin, said this, in 140 characters: “We recently had idea: health policy M&M for residents to discuss adverse outcomes we see as result of lack of access.”
The idea began with a conversation Dr. Moriates had with Beth Miller, MD, program director for the Dell Medical School Internal Medicine Residency Program. “We were meeting and talking about revamping the [resident] M&M conference to have more learning objectives and put in place best practices,” Dr. Moriates said. “Dr. Miller suggested it could be a good forum [for health policy] because it’s an area where we all come together and there’s a natural hook to it, since it is case-based, thus we can use it to recognize the drivers within the system that lead to bad outcomes.”
In his SHM blog post, Dr. Moriates said he has increasingly observed adverse events that result from issues related to health policy. He provided an example: “A patient I admitted for ‘expedited work-up’ for rectal bleeding after he told me he had been trying to get a recommended colonoscopy for many months but could not get it scheduled due to his lack of insurance. He had colon cancer that had spread.”
In another example, he conjured a hypothetical (though not impractical) case where a patient prescribed blood thinners upon hospital discharge returns to the hospital soon after with a blood clot. Unable to afford the medication, or seek primary care follow-up, this kind of patient is readmitted through no direct fault of his physicians. Yet, the patient is worse off and the hospital takes the hit on readmissions penalties.
Dr. Moriates believes that viewing a case like this through a health policy lens is not only moving, but critical to better understanding health care delivery, particularly in an environment where physician performance is measured, in part, by outcomes. He now believes health policy M&Ms would be valuable to all hospital-based physicians, not just residents.
“Hospitalists are being asked to hit these value-based performance metrics, like readmissions and length of stay, and while we deal with the consequences, we are not always the best informed” with respect to policy, he said. “We could use this forum to teach health policy topics and continually update people and contribute, in real time, to all these different discussions and understand how things are changing or could change and impact our patients.”
“It’s important for physicians to know the policies that are aligned with, and the policies that may undermine, what they’re doing in their practice to improve their patients’ health,” Pourat said.
This knowledge can benefit physicians, too, Pourat added, because health policy M&Ms could help providers understand the goals of particular policies and in turn adjust their own behaviors and expectations.
“Physicians could discuss, what are the underlying issues or root causes, like the decision not to expand Medicaid here in Texas,” Dr. Moriates said. “Not all of these things you can fix, but you’re exposing those stories and perhaps we can come up with some actionable steps. How do we ensure in the future that our patients are able to fulfill their prescription so we’re not just sending someone out assuming they will but not knowing they’re unable to afford it?”
Similar to other domains in which physician leaders become champions, such as antibiotic stewardship, Dr. Pourat suggested that hospitalists could champion policy awareness through the kind of M&Ms Dr. Moriates proposed.
While journal clubs and lectures are great ways for hospitalists to learn more about health policy, the emotionally gripping nature of M&Ms could inspire more physicians to act in favor of policies that benefit their patients and themselves, Dr. Moriates said.
For example, physicians may write to or visit legislative offices, or author op-eds in their local newspapers. This collective action carries the potential to effect change. And it need not be partisan.
“I believe that if health policy issues were more explicitly integrated into M&Ms then clinicians would be more inclined and prepared to effectively advocate for specific policy changes,” he wrote in his blog post. “Perhaps entire groups would be moved to engage in the political process.”
On Twitter, even before Dr. Moriates’ first tweet about health policy M&Ms, New Jersey–based Jennifer Chuang, MD, an adolescent medicine physician, wrote: “M&M is heart-wrenching in academic hospitals. I dare @SenateGOP to present their role in M&M’s to come if ACA is repealed.”
While Dr. Moriates believes the chances are quite small that legislators and policymakers would attend health policy M&Ms, he called the notion “provocative and intriguing.”
In his blog post, Dr. Moriates invites state legislators and local members of Congress to join him in reviewing M&M cases where patients have been negatively affected by policy. He also emphasized that, like most modern M&Ms, the point should not be derision or finger-pointing, but an opportunity to learn how policy translates into practice.
Physicians may learn from legislators, too, he said in his blog post. “Just as policymakers could see legislation through the eyes of practitioners and their patients, this is where we as physicians could possibly learn from our legislators,” he wrote. “We may recognize the potential trade-offs, downsides, and barriers to proposals that to us may have seemed like no-brainers.”
What’s clear, said Dr. Pourat, who is also a professor in the UCLA Fielding School of Public Health and the School of Dentistry, is that Dr. Moriates’ blog post and tweet are “touching an important point for a lot of physicians during this whole debate over health reform.”
President Donald Trump campaigned on a promise to fully repeal and replace the Affordable Care Act but Republican efforts have thus far been stymied. In the meantime, some physicians are watching closely, knowing that whatever comes next will continue to affect them and their patients.
Source
1. Moriates C. Is it time for health policy M&Ms? The Hospital Leader. Aug 16, 2017. http://thehospitalleader.org/is-it-time-for-health-policy-mms/. Accessed 2017 Sep 14.
Antibiotic choice for acute otitis media 2018
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 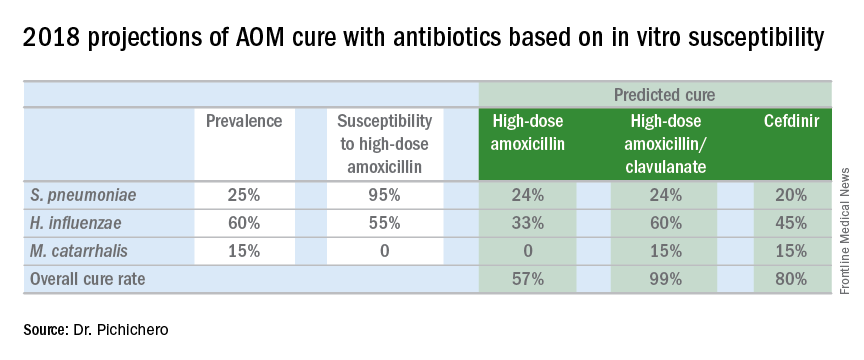
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
Predicting MDR Gram-negative infection mortality risk
Source control, defined as location and elimination of the source of the infection, was critical for patient survival in the case of multidrug resistant bacterial infection, according to the results of a case-control study of 62 critically ill surgical patients who were assessed between 2011 and 2014.
Researchers examined the characteristics of infected patients surviving to hospital discharge compared with those of nonsurvivors to look for predictive factors. Demographically, patients had an overall mean age of 62 years; 30.6% were women; 69.4% were white. The first culture obtained during a surgical ICU admission that grew a carbapenem-resistant Enterobacteriaceae (CRE), MDR Pseudomonas aeruginosa, or MDR Acinetobacter spp. was defined as the index culture.
“In this study, 33.9% [21/62] of critically ill surgical patients with a culture positive for MDR Gram-negative bacteria died prior to hospital discharge,” according to Andrew S. Jarrell, PharmD, of the Johns Hopkins Hospital, Baltimore, and his colleagues.
With multivariate logistic regression, achievement of source control was the only variable associated with decreased in-hospital mortality (odds ratio 0.04, 95% confidence interval, 0.003-0.52); P = .01).
“Source control status was predictive of in-hospital mortality after controlling for other factors. Specifically, the odds of in-hospital mortality were 97% lower when source control was achieved as compared to when source control was not achieved,” the authors stated (J Crit Care. 2018;43:321-6).
Scenarios in which source control was not applicable (pneumonia and urinary tract infection) were also similarly distributed between survivors and nonsurvivors, they reported.
Other than source control, the only significant risk factors for mortality, as seen in univariate analysis, all occurred prior to index culture. They were: vasopressor use (46.3% of survivors, vs. 76.2% of nonsurvivors, P = .03); mechanical ventilation (63.4% vs. 100%, P = .001); and median ICU length of stay (10 days vs. 18 days, P = .001).
“Achievement of source control stands out as a critical factor for patient survival. Clinicians should take this, along with prior ICU LOS, vasopressor use, and mechanical ventilation status, into consideration when evaluating patient prognosis,” Dr. Jarrell and his colleagues concluded.
The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
Source control, defined as location and elimination of the source of the infection, was critical for patient survival in the case of multidrug resistant bacterial infection, according to the results of a case-control study of 62 critically ill surgical patients who were assessed between 2011 and 2014.
Researchers examined the characteristics of infected patients surviving to hospital discharge compared with those of nonsurvivors to look for predictive factors. Demographically, patients had an overall mean age of 62 years; 30.6% were women; 69.4% were white. The first culture obtained during a surgical ICU admission that grew a carbapenem-resistant Enterobacteriaceae (CRE), MDR Pseudomonas aeruginosa, or MDR Acinetobacter spp. was defined as the index culture.
“In this study, 33.9% [21/62] of critically ill surgical patients with a culture positive for MDR Gram-negative bacteria died prior to hospital discharge,” according to Andrew S. Jarrell, PharmD, of the Johns Hopkins Hospital, Baltimore, and his colleagues.
With multivariate logistic regression, achievement of source control was the only variable associated with decreased in-hospital mortality (odds ratio 0.04, 95% confidence interval, 0.003-0.52); P = .01).
“Source control status was predictive of in-hospital mortality after controlling for other factors. Specifically, the odds of in-hospital mortality were 97% lower when source control was achieved as compared to when source control was not achieved,” the authors stated (J Crit Care. 2018;43:321-6).
Scenarios in which source control was not applicable (pneumonia and urinary tract infection) were also similarly distributed between survivors and nonsurvivors, they reported.
Other than source control, the only significant risk factors for mortality, as seen in univariate analysis, all occurred prior to index culture. They were: vasopressor use (46.3% of survivors, vs. 76.2% of nonsurvivors, P = .03); mechanical ventilation (63.4% vs. 100%, P = .001); and median ICU length of stay (10 days vs. 18 days, P = .001).
“Achievement of source control stands out as a critical factor for patient survival. Clinicians should take this, along with prior ICU LOS, vasopressor use, and mechanical ventilation status, into consideration when evaluating patient prognosis,” Dr. Jarrell and his colleagues concluded.
The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
Source control, defined as location and elimination of the source of the infection, was critical for patient survival in the case of multidrug resistant bacterial infection, according to the results of a case-control study of 62 critically ill surgical patients who were assessed between 2011 and 2014.
Researchers examined the characteristics of infected patients surviving to hospital discharge compared with those of nonsurvivors to look for predictive factors. Demographically, patients had an overall mean age of 62 years; 30.6% were women; 69.4% were white. The first culture obtained during a surgical ICU admission that grew a carbapenem-resistant Enterobacteriaceae (CRE), MDR Pseudomonas aeruginosa, or MDR Acinetobacter spp. was defined as the index culture.
“In this study, 33.9% [21/62] of critically ill surgical patients with a culture positive for MDR Gram-negative bacteria died prior to hospital discharge,” according to Andrew S. Jarrell, PharmD, of the Johns Hopkins Hospital, Baltimore, and his colleagues.
With multivariate logistic regression, achievement of source control was the only variable associated with decreased in-hospital mortality (odds ratio 0.04, 95% confidence interval, 0.003-0.52); P = .01).
“Source control status was predictive of in-hospital mortality after controlling for other factors. Specifically, the odds of in-hospital mortality were 97% lower when source control was achieved as compared to when source control was not achieved,” the authors stated (J Crit Care. 2018;43:321-6).
Scenarios in which source control was not applicable (pneumonia and urinary tract infection) were also similarly distributed between survivors and nonsurvivors, they reported.
Other than source control, the only significant risk factors for mortality, as seen in univariate analysis, all occurred prior to index culture. They were: vasopressor use (46.3% of survivors, vs. 76.2% of nonsurvivors, P = .03); mechanical ventilation (63.4% vs. 100%, P = .001); and median ICU length of stay (10 days vs. 18 days, P = .001).
“Achievement of source control stands out as a critical factor for patient survival. Clinicians should take this, along with prior ICU LOS, vasopressor use, and mechanical ventilation status, into consideration when evaluating patient prognosis,” Dr. Jarrell and his colleagues concluded.
The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
FROM THE JOURNAL OF CRITICAL CARE
Key clinical point: Source control was the most important predictor of MDR Gram-negative infection mortality in hospitalized patients.
Major finding: The odds of in-hospital mortality were 97% lower when source control was achieved.
Study details: Case-control study of 62 critically ill surgical patients from 2011 to 2014 who had an MDR infection.
Disclosures: The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
Common food additive makes C. difficile more virulent
, a study showed.
“Out of several carbon sources identified that supported CD2015 growth [epidemic RT027 isolate], we found the disaccharide trehalose increased the growth yield of CD2015 by approximately fivefold, compared with a non-RT027 strain,” according to James Collins, PhD, of Baylor University, Houston, and his colleagues. The increased growth of the epidemic strain of C. difficile observed by Dr. Collins and his team demonstrates that trehalose is a robust carbon source for C. difficile bacterium.
In one experiment, mice with humanized microbiota were infected with two strains of RT027, either R20291 (n = 27) or R20291-delta treA (n = 28), a phosphotrehalase enzyme (TreA) deletion mutant that cannot metabolize trehalose. Mice were then given 5 mM of trehalose ad libitum in their drinking water. Researchers observed that the mice infected with R20291-delta treA had much lower mortality rates than the R20291 group (33.3% vs.78.6%). These findings were then reinforced with a second experiment using mice with humanized microbiota, in which trehalose addition increased mortality in RT027 mice, compared with RT027-infected mice that were not given dietary trehalose.
While Dr. Collins and his team demonstrated the effect of trehalose on C. difficile in mice, they also conducted a limited analysis of ileostomy effluent from three human donors. The researchers found that in two of three samples, treA was strongly induced in CD2015, but not in another ribotype, CD2048. This demonstrates that amounts of trehalose found in food are high enough to be metabolized by certain epidemic strains of C. difficile in humans.
Prior to 2000, trehalose use was limited by a relatively high cost of production, approximately $700 per kilogram. A production innovation that utilized a novel enzymatic method that yielded trehalose from starch brought the price of trehalose to approximately $3 per kilogram, making it a commercially viable food supplement. After being considered “generally recognized as safe” by the U.S. Food and Drug Administration in 2000 and approved for use in Europe, the trehalose concentrations in food skyrocketed from around 2% to 11.25%, and trehalose became widely used in several foods, including ice cream, pasta, and ground beef.
Dr. Collins and his associates said that there is considerable evidence that the widespread use of dietary trehalose has contributed to the spread of epidemic C. difficile ribotypes. First, strains RT027 and RT078 have always had the ability to metabolize trehalose, as evidenced by outbreaks of nonepidemic C. difficile in the 1980s. But no epidemic outbreaks were reported until after 2003, several years after trehalose was approved by the FDA. Second, RT027 and RT078 are phylogenetically distant, but independently evolved the ability to metabolize low levels of trehalose. Third, increased severity of the RT027 strain, which metabolizes trehalose in mice, is consistent with increased virulence of RT078 and RT027 in human patients. Fourth, a competitive advantage is conferred to C. difficile being able to metabolize trehalose in low concentrations in a diverse intestinal setting. Finally, the levels of trehalose in ileostomy fluid from patients who eat a normal diet are high enough to be utilized by RT027 strains.
“On the basis of these observations, we propose that the widespread adoption and use of the disaccharide trehalose in the human diet has played a significant role in the emergence of these epidemic and hypervirulent strains,” Dr. Collins and his colleagues wrote in their article in Nature.
The authors of the study had no relevant financial disclosures to report.
SOURCE: Collins J et al. Nature. 2018 Jan 3. doi: 10.1038/nature25178.
, a study showed.
“Out of several carbon sources identified that supported CD2015 growth [epidemic RT027 isolate], we found the disaccharide trehalose increased the growth yield of CD2015 by approximately fivefold, compared with a non-RT027 strain,” according to James Collins, PhD, of Baylor University, Houston, and his colleagues. The increased growth of the epidemic strain of C. difficile observed by Dr. Collins and his team demonstrates that trehalose is a robust carbon source for C. difficile bacterium.
In one experiment, mice with humanized microbiota were infected with two strains of RT027, either R20291 (n = 27) or R20291-delta treA (n = 28), a phosphotrehalase enzyme (TreA) deletion mutant that cannot metabolize trehalose. Mice were then given 5 mM of trehalose ad libitum in their drinking water. Researchers observed that the mice infected with R20291-delta treA had much lower mortality rates than the R20291 group (33.3% vs.78.6%). These findings were then reinforced with a second experiment using mice with humanized microbiota, in which trehalose addition increased mortality in RT027 mice, compared with RT027-infected mice that were not given dietary trehalose.
While Dr. Collins and his team demonstrated the effect of trehalose on C. difficile in mice, they also conducted a limited analysis of ileostomy effluent from three human donors. The researchers found that in two of three samples, treA was strongly induced in CD2015, but not in another ribotype, CD2048. This demonstrates that amounts of trehalose found in food are high enough to be metabolized by certain epidemic strains of C. difficile in humans.
Prior to 2000, trehalose use was limited by a relatively high cost of production, approximately $700 per kilogram. A production innovation that utilized a novel enzymatic method that yielded trehalose from starch brought the price of trehalose to approximately $3 per kilogram, making it a commercially viable food supplement. After being considered “generally recognized as safe” by the U.S. Food and Drug Administration in 2000 and approved for use in Europe, the trehalose concentrations in food skyrocketed from around 2% to 11.25%, and trehalose became widely used in several foods, including ice cream, pasta, and ground beef.
Dr. Collins and his associates said that there is considerable evidence that the widespread use of dietary trehalose has contributed to the spread of epidemic C. difficile ribotypes. First, strains RT027 and RT078 have always had the ability to metabolize trehalose, as evidenced by outbreaks of nonepidemic C. difficile in the 1980s. But no epidemic outbreaks were reported until after 2003, several years after trehalose was approved by the FDA. Second, RT027 and RT078 are phylogenetically distant, but independently evolved the ability to metabolize low levels of trehalose. Third, increased severity of the RT027 strain, which metabolizes trehalose in mice, is consistent with increased virulence of RT078 and RT027 in human patients. Fourth, a competitive advantage is conferred to C. difficile being able to metabolize trehalose in low concentrations in a diverse intestinal setting. Finally, the levels of trehalose in ileostomy fluid from patients who eat a normal diet are high enough to be utilized by RT027 strains.
“On the basis of these observations, we propose that the widespread adoption and use of the disaccharide trehalose in the human diet has played a significant role in the emergence of these epidemic and hypervirulent strains,” Dr. Collins and his colleagues wrote in their article in Nature.
The authors of the study had no relevant financial disclosures to report.
SOURCE: Collins J et al. Nature. 2018 Jan 3. doi: 10.1038/nature25178.
, a study showed.
“Out of several carbon sources identified that supported CD2015 growth [epidemic RT027 isolate], we found the disaccharide trehalose increased the growth yield of CD2015 by approximately fivefold, compared with a non-RT027 strain,” according to James Collins, PhD, of Baylor University, Houston, and his colleagues. The increased growth of the epidemic strain of C. difficile observed by Dr. Collins and his team demonstrates that trehalose is a robust carbon source for C. difficile bacterium.
In one experiment, mice with humanized microbiota were infected with two strains of RT027, either R20291 (n = 27) or R20291-delta treA (n = 28), a phosphotrehalase enzyme (TreA) deletion mutant that cannot metabolize trehalose. Mice were then given 5 mM of trehalose ad libitum in their drinking water. Researchers observed that the mice infected with R20291-delta treA had much lower mortality rates than the R20291 group (33.3% vs.78.6%). These findings were then reinforced with a second experiment using mice with humanized microbiota, in which trehalose addition increased mortality in RT027 mice, compared with RT027-infected mice that were not given dietary trehalose.
While Dr. Collins and his team demonstrated the effect of trehalose on C. difficile in mice, they also conducted a limited analysis of ileostomy effluent from three human donors. The researchers found that in two of three samples, treA was strongly induced in CD2015, but not in another ribotype, CD2048. This demonstrates that amounts of trehalose found in food are high enough to be metabolized by certain epidemic strains of C. difficile in humans.
Prior to 2000, trehalose use was limited by a relatively high cost of production, approximately $700 per kilogram. A production innovation that utilized a novel enzymatic method that yielded trehalose from starch brought the price of trehalose to approximately $3 per kilogram, making it a commercially viable food supplement. After being considered “generally recognized as safe” by the U.S. Food and Drug Administration in 2000 and approved for use in Europe, the trehalose concentrations in food skyrocketed from around 2% to 11.25%, and trehalose became widely used in several foods, including ice cream, pasta, and ground beef.
Dr. Collins and his associates said that there is considerable evidence that the widespread use of dietary trehalose has contributed to the spread of epidemic C. difficile ribotypes. First, strains RT027 and RT078 have always had the ability to metabolize trehalose, as evidenced by outbreaks of nonepidemic C. difficile in the 1980s. But no epidemic outbreaks were reported until after 2003, several years after trehalose was approved by the FDA. Second, RT027 and RT078 are phylogenetically distant, but independently evolved the ability to metabolize low levels of trehalose. Third, increased severity of the RT027 strain, which metabolizes trehalose in mice, is consistent with increased virulence of RT078 and RT027 in human patients. Fourth, a competitive advantage is conferred to C. difficile being able to metabolize trehalose in low concentrations in a diverse intestinal setting. Finally, the levels of trehalose in ileostomy fluid from patients who eat a normal diet are high enough to be utilized by RT027 strains.
“On the basis of these observations, we propose that the widespread adoption and use of the disaccharide trehalose in the human diet has played a significant role in the emergence of these epidemic and hypervirulent strains,” Dr. Collins and his colleagues wrote in their article in Nature.
The authors of the study had no relevant financial disclosures to report.
SOURCE: Collins J et al. Nature. 2018 Jan 3. doi: 10.1038/nature25178.
FROM NATURE
Key clinical point: Metabolizing trehalose increases the virulence and mortality of C. difficile ribotype 027 (RT027).
Major finding: The ability to metabolize trehalose with the phosphotrehalase enzyme (TreA) increases mortality in RT027.
Study details: Experimental mouse models and an analysis of ileostomy effluent from three anonymous donors.
Disclosures: All authors had no financial disclosures to report.
Source: Collins J et al. Nature. 2018 Jan 3. doi: 10.1038/nature25178.
Clinical Trial: The Checklist to Prevent MRSA Surgical Site Infections
who are undergoing or have undergone cardiac surgery or total joint arthroplasty.
The Department of Veteran Affairs has previously implemented the VA MRSA Prevention Initiative, which successfully reduced patient-to-patient MRSA transmission; however, this initiative does not prevent most MRSA surgical site infections, which are spread differently. The VA has developed a new checklist which will be tested in this study aimed at reducing MRSA surgical site infections (SSIs), and will be implemented at 10 VA medical centers.
The primary outcome measure is superficial and deep/organ space MRSA infections within 90 days of operation. Secondary outcome measures include superficial and deep/organ space MRSA infections within 1 year of operation, presurgical bundle and individual bundle components compliance for 30 days prior to operation, length of postoperative stay, all-cause mortality 1 year after surgery, readmission within 90 days of surgery, and mupirocin and chlorhexidine resistance 30 days presurgery to 90 days postsurgery.
The study will end in April 2019. More than 10,000 people are expected to be included in the final analysis.
Find more information on the study page at Clinicaltrials.gov.
who are undergoing or have undergone cardiac surgery or total joint arthroplasty.
The Department of Veteran Affairs has previously implemented the VA MRSA Prevention Initiative, which successfully reduced patient-to-patient MRSA transmission; however, this initiative does not prevent most MRSA surgical site infections, which are spread differently. The VA has developed a new checklist which will be tested in this study aimed at reducing MRSA surgical site infections (SSIs), and will be implemented at 10 VA medical centers.
The primary outcome measure is superficial and deep/organ space MRSA infections within 90 days of operation. Secondary outcome measures include superficial and deep/organ space MRSA infections within 1 year of operation, presurgical bundle and individual bundle components compliance for 30 days prior to operation, length of postoperative stay, all-cause mortality 1 year after surgery, readmission within 90 days of surgery, and mupirocin and chlorhexidine resistance 30 days presurgery to 90 days postsurgery.
The study will end in April 2019. More than 10,000 people are expected to be included in the final analysis.
Find more information on the study page at Clinicaltrials.gov.
who are undergoing or have undergone cardiac surgery or total joint arthroplasty.
The Department of Veteran Affairs has previously implemented the VA MRSA Prevention Initiative, which successfully reduced patient-to-patient MRSA transmission; however, this initiative does not prevent most MRSA surgical site infections, which are spread differently. The VA has developed a new checklist which will be tested in this study aimed at reducing MRSA surgical site infections (SSIs), and will be implemented at 10 VA medical centers.
The primary outcome measure is superficial and deep/organ space MRSA infections within 90 days of operation. Secondary outcome measures include superficial and deep/organ space MRSA infections within 1 year of operation, presurgical bundle and individual bundle components compliance for 30 days prior to operation, length of postoperative stay, all-cause mortality 1 year after surgery, readmission within 90 days of surgery, and mupirocin and chlorhexidine resistance 30 days presurgery to 90 days postsurgery.
The study will end in April 2019. More than 10,000 people are expected to be included in the final analysis.
Find more information on the study page at Clinicaltrials.gov.
FROM CLINICALTRIALS.GOV
Risks identified for drug-resistant bacteremia in cirrhosis
In patients hospitalized with cirrhosis, biliary cirrhosis, recent health care exposure, nonwhite race, and cultures taken more than 48 hours after admission all independently predicted that bacteremia would be caused by multidrug-resistant organisms (MDROs), according to a medical record review at CHI St. Luke’s Medical Center, an 850-bed tertiary care center in Houston.
“These variables along with severity of infection and liver disease may help clinicians identify patients who will benefit most from broader-spectrum empiric antimicrobial therapy,” wrote the investigators, led by Jennifer Addo Smith, PharmD, of St. Luke’s, in the Journal of Clinical Gastroenterology.
But local epidemiology remains important. “Although a gram-positive agent (e.g., vancomycin) and a carbapenem-sparing gram-negative agent (e.g., ceftriaxone, cefepime) are reasonable empiric agents at our center, other centers with different resistance patterns may warrant different empiric therapy. Given the low prevalence of VRE [vancomycin-resistant Enterococcus] in this study ... and E. faecium in other studies (4%-7%), an empiric agent active against VRE does not seem to be routinely required,” they said.
The team looked into the issue because there hasn’t been much investigation in the United States of the role of multidrug resistant organisms in bacteremia among patients hospitalized with cirrhosis.
Thirty patients in the study had bacteremia caused by MDROs while 60 had bacteremia from non-MDROs, giving a 33% prevalence of MDRO bacteremia, which was consistent with previous, mostly European studies.
Enterobacteriaceae (43%), Staphylococcus aureus (18%), Streptococcus spp. (11%), Enterococcus spp. (10%), and nonfermenting gram-negative bacilli (6%) were the main causes of bacteremia overall.
Among the 30 MDRO cases, methicillin-resistant S. aureus was isolated in seven (23%); methicillin-resistant coagulase-negative Staphylococci in four (13%); fluoroquinolone-resistant Enterobacteriaceae in nine (30%); extended spectrum beta-lactamase–producing Enterobacteriaceae in three (10%), and VRE in two (7%). No carbapenemase-producing gram-negative bacteria were identified.
The predictors of MDRO bacteremia emerged on multivariate analysis and included biliary cirrhosis (adjusted odds ratio, 11.75; 95% confidence interval, 2.08-66.32); recent health care exposure (aOR, 9.81; 95% CI, 2.15-44.88); blood cultures obtained 48 hours after hospital admission (aOR, 6.02; 95% CI, 1.70-21.40) and nonwhite race (aOR , 3.35; 95% CI, 1.19-9.38).
Blood cultures past 48 hours and recent health care exposure – generally hospitalization within the past 90 days – were likely surrogates for nosocomial infection.
The link with biliary cirrhosis is unclear. “Compared with other cirrhotic patients, perhaps patients with PBC [primary biliary cholangitis] have had more cumulative antimicrobial exposure because of [their] higher risk for UTIs [urinary tract infections] and therefore are at increased risk for MDROs,” they wrote.
The median age in the study was 59 years. Half of the patients were white; 46% were women. Hepatitis C was the most common cause of cirrhosis, followed by alcohol.
MDRO was defined in the study as bacteria not susceptible to at least one antibiotic in at least three antimicrobial categories; 90 cirrhosis patients without bacteremia served as controls.
The funding source was not reported. Dr. Addo Smith had no disclosures.
SOURCE: Smith JA et al. J Clin Gastroenterol. 2017 Nov 23. doi: 10.1097/MCG.0000000000000964.
*This story was updated on 1/10/2018.
In patients hospitalized with cirrhosis, biliary cirrhosis, recent health care exposure, nonwhite race, and cultures taken more than 48 hours after admission all independently predicted that bacteremia would be caused by multidrug-resistant organisms (MDROs), according to a medical record review at CHI St. Luke’s Medical Center, an 850-bed tertiary care center in Houston.
“These variables along with severity of infection and liver disease may help clinicians identify patients who will benefit most from broader-spectrum empiric antimicrobial therapy,” wrote the investigators, led by Jennifer Addo Smith, PharmD, of St. Luke’s, in the Journal of Clinical Gastroenterology.
But local epidemiology remains important. “Although a gram-positive agent (e.g., vancomycin) and a carbapenem-sparing gram-negative agent (e.g., ceftriaxone, cefepime) are reasonable empiric agents at our center, other centers with different resistance patterns may warrant different empiric therapy. Given the low prevalence of VRE [vancomycin-resistant Enterococcus] in this study ... and E. faecium in other studies (4%-7%), an empiric agent active against VRE does not seem to be routinely required,” they said.
The team looked into the issue because there hasn’t been much investigation in the United States of the role of multidrug resistant organisms in bacteremia among patients hospitalized with cirrhosis.
Thirty patients in the study had bacteremia caused by MDROs while 60 had bacteremia from non-MDROs, giving a 33% prevalence of MDRO bacteremia, which was consistent with previous, mostly European studies.
Enterobacteriaceae (43%), Staphylococcus aureus (18%), Streptococcus spp. (11%), Enterococcus spp. (10%), and nonfermenting gram-negative bacilli (6%) were the main causes of bacteremia overall.
Among the 30 MDRO cases, methicillin-resistant S. aureus was isolated in seven (23%); methicillin-resistant coagulase-negative Staphylococci in four (13%); fluoroquinolone-resistant Enterobacteriaceae in nine (30%); extended spectrum beta-lactamase–producing Enterobacteriaceae in three (10%), and VRE in two (7%). No carbapenemase-producing gram-negative bacteria were identified.
The predictors of MDRO bacteremia emerged on multivariate analysis and included biliary cirrhosis (adjusted odds ratio, 11.75; 95% confidence interval, 2.08-66.32); recent health care exposure (aOR, 9.81; 95% CI, 2.15-44.88); blood cultures obtained 48 hours after hospital admission (aOR, 6.02; 95% CI, 1.70-21.40) and nonwhite race (aOR , 3.35; 95% CI, 1.19-9.38).
Blood cultures past 48 hours and recent health care exposure – generally hospitalization within the past 90 days – were likely surrogates for nosocomial infection.
The link with biliary cirrhosis is unclear. “Compared with other cirrhotic patients, perhaps patients with PBC [primary biliary cholangitis] have had more cumulative antimicrobial exposure because of [their] higher risk for UTIs [urinary tract infections] and therefore are at increased risk for MDROs,” they wrote.
The median age in the study was 59 years. Half of the patients were white; 46% were women. Hepatitis C was the most common cause of cirrhosis, followed by alcohol.
MDRO was defined in the study as bacteria not susceptible to at least one antibiotic in at least three antimicrobial categories; 90 cirrhosis patients without bacteremia served as controls.
The funding source was not reported. Dr. Addo Smith had no disclosures.
SOURCE: Smith JA et al. J Clin Gastroenterol. 2017 Nov 23. doi: 10.1097/MCG.0000000000000964.
*This story was updated on 1/10/2018.
In patients hospitalized with cirrhosis, biliary cirrhosis, recent health care exposure, nonwhite race, and cultures taken more than 48 hours after admission all independently predicted that bacteremia would be caused by multidrug-resistant organisms (MDROs), according to a medical record review at CHI St. Luke’s Medical Center, an 850-bed tertiary care center in Houston.
“These variables along with severity of infection and liver disease may help clinicians identify patients who will benefit most from broader-spectrum empiric antimicrobial therapy,” wrote the investigators, led by Jennifer Addo Smith, PharmD, of St. Luke’s, in the Journal of Clinical Gastroenterology.
But local epidemiology remains important. “Although a gram-positive agent (e.g., vancomycin) and a carbapenem-sparing gram-negative agent (e.g., ceftriaxone, cefepime) are reasonable empiric agents at our center, other centers with different resistance patterns may warrant different empiric therapy. Given the low prevalence of VRE [vancomycin-resistant Enterococcus] in this study ... and E. faecium in other studies (4%-7%), an empiric agent active against VRE does not seem to be routinely required,” they said.
The team looked into the issue because there hasn’t been much investigation in the United States of the role of multidrug resistant organisms in bacteremia among patients hospitalized with cirrhosis.
Thirty patients in the study had bacteremia caused by MDROs while 60 had bacteremia from non-MDROs, giving a 33% prevalence of MDRO bacteremia, which was consistent with previous, mostly European studies.
Enterobacteriaceae (43%), Staphylococcus aureus (18%), Streptococcus spp. (11%), Enterococcus spp. (10%), and nonfermenting gram-negative bacilli (6%) were the main causes of bacteremia overall.
Among the 30 MDRO cases, methicillin-resistant S. aureus was isolated in seven (23%); methicillin-resistant coagulase-negative Staphylococci in four (13%); fluoroquinolone-resistant Enterobacteriaceae in nine (30%); extended spectrum beta-lactamase–producing Enterobacteriaceae in three (10%), and VRE in two (7%). No carbapenemase-producing gram-negative bacteria were identified.
The predictors of MDRO bacteremia emerged on multivariate analysis and included biliary cirrhosis (adjusted odds ratio, 11.75; 95% confidence interval, 2.08-66.32); recent health care exposure (aOR, 9.81; 95% CI, 2.15-44.88); blood cultures obtained 48 hours after hospital admission (aOR, 6.02; 95% CI, 1.70-21.40) and nonwhite race (aOR , 3.35; 95% CI, 1.19-9.38).
Blood cultures past 48 hours and recent health care exposure – generally hospitalization within the past 90 days – were likely surrogates for nosocomial infection.
The link with biliary cirrhosis is unclear. “Compared with other cirrhotic patients, perhaps patients with PBC [primary biliary cholangitis] have had more cumulative antimicrobial exposure because of [their] higher risk for UTIs [urinary tract infections] and therefore are at increased risk for MDROs,” they wrote.
The median age in the study was 59 years. Half of the patients were white; 46% were women. Hepatitis C was the most common cause of cirrhosis, followed by alcohol.
MDRO was defined in the study as bacteria not susceptible to at least one antibiotic in at least three antimicrobial categories; 90 cirrhosis patients without bacteremia served as controls.
The funding source was not reported. Dr. Addo Smith had no disclosures.
SOURCE: Smith JA et al. J Clin Gastroenterol. 2017 Nov 23. doi: 10.1097/MCG.0000000000000964.
*This story was updated on 1/10/2018.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: In patients hospitalized with cirrhosis, nonwhite race, biliary involvement, recent health care exposure, and cultures taken more than 48 hours after hospital admission all independently predicted that bacteremia would be caused by multidrug-resistant organisms.
Major finding: The predictors of multidrug-resistant organism bacteremia emerged on multivariate analysis and included biliary cirrhosis (aOR 11.75; 95% CI, 2.08-66.32); recent health care exposure (aOR 9.81; 95% CI, 2.15-44.88); and blood cultures obtained 48 hours after hospital admission (aOR 6.02; 95% CI, 1.70-21.40).
Study details: Review of 90 cirrhotic patients with bacteremia, plus 90 controls.
Disclosures: The lead investigator had no disclosures.
Source: Smith JA et al. J Clin Gastroenterol. 2017 Nov 23. doi: 10.1097/MCG.0000000000000964.
FDA bans 24 ingredients from OTC health care antiseptic products
in hospital settings and other health care situations outside the hospital, the U.S. Food and Drug Administration announced in a final rule.
The affected products include health care personnel hand washes and hand rubs, surgical hand scrubs and hand rubs, and patient antiseptic skin preparations. The final rule was published Dec. 20 in the Federal Register and becomes effective in December 2018.
The agency determined that a deferral is warranted for six health care antiseptic active ingredients – benzalkonium chloride, benzethonium chloride, chloroxylenol, alcohol, isopropyl alcohol, and povidone-iodine – to allow more time for interested parties to complete the studies necessary to fill the safety and effectiveness data gaps identified for these ingredients.
“The FDA expects that this information may help better inform us on antiseptic resistance and antibiotic cross-resistance in the health care setting,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Importantly, this doesn’t mean that products containing these six ingredients are ineffective or unsafe. These antiseptic products remain an important resource in health care settings. Personnel should continue to use these products consistent with infection control guidelines while the additional data are gathered.”
No additional data was provided for another 24 products, which were deemed not generally recognized as safe and effective. The minimum data needed to demonstrate safety for all health care antiseptic active ingredients fall into four broad categories: human safety studies, nonclinical safety studies (developmental and reproductive toxicity studies and carcinogenicity studies), data to characterize potential hormonal effects, and data to evaluate the development of antimicrobial resistance, the final rule states.
The FDA noted that manufacturers started to remove nearly all of these 24 active ingredients from their products following a 2015 proposed rule. Triclosan is currently being used in available products.
The active ingredients affected are chlorhexidine gluconate; cloflucarban; fluorosalan; hexachlorophene; hexylresorcinol; iodophors (iodine-containing ingredients including iodine complex [ammonium ether sulfate and polyoxyethylene sorbitan monolaurate], iodine complex [phosphate ester of alkylaryloxy polyethylene glycol], iodine tincture USP, iodine topical solution USP, nonylphenoxypoly [ethyleneoxy] ethanoliodine, poloxamer–iodine complex, undecoylium chloride iodine complex); mercufenol chloride; methylbenzethonium chloride; phenol; secondary amyltricresols; sodium oxychlorosene; tribromsalan; triclocarban; triclosan; triple dye; combination of calomel, oxyquinoline benzoate, triethanolamine, and phenol derivative; and combination of mercufenol chloride and secondary amyltricresols in 50% alcohol.
If manufacturers want to use one or more of these 24 active ingredients in future OTC health care antiseptic drug products, those products will be considered new drugs for which a new drug application approval will be required, the agency said.
The rule does not affect health care antiseptics that are currently marketed under new drug applications and abbreviated new drug applications.
FDA’s action follows a similar final rule published Sept. 6, 2016, which removed triclosan and 18 other active ingredients from consumer antiseptic products.
in hospital settings and other health care situations outside the hospital, the U.S. Food and Drug Administration announced in a final rule.
The affected products include health care personnel hand washes and hand rubs, surgical hand scrubs and hand rubs, and patient antiseptic skin preparations. The final rule was published Dec. 20 in the Federal Register and becomes effective in December 2018.
The agency determined that a deferral is warranted for six health care antiseptic active ingredients – benzalkonium chloride, benzethonium chloride, chloroxylenol, alcohol, isopropyl alcohol, and povidone-iodine – to allow more time for interested parties to complete the studies necessary to fill the safety and effectiveness data gaps identified for these ingredients.
“The FDA expects that this information may help better inform us on antiseptic resistance and antibiotic cross-resistance in the health care setting,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Importantly, this doesn’t mean that products containing these six ingredients are ineffective or unsafe. These antiseptic products remain an important resource in health care settings. Personnel should continue to use these products consistent with infection control guidelines while the additional data are gathered.”
No additional data was provided for another 24 products, which were deemed not generally recognized as safe and effective. The minimum data needed to demonstrate safety for all health care antiseptic active ingredients fall into four broad categories: human safety studies, nonclinical safety studies (developmental and reproductive toxicity studies and carcinogenicity studies), data to characterize potential hormonal effects, and data to evaluate the development of antimicrobial resistance, the final rule states.
The FDA noted that manufacturers started to remove nearly all of these 24 active ingredients from their products following a 2015 proposed rule. Triclosan is currently being used in available products.
The active ingredients affected are chlorhexidine gluconate; cloflucarban; fluorosalan; hexachlorophene; hexylresorcinol; iodophors (iodine-containing ingredients including iodine complex [ammonium ether sulfate and polyoxyethylene sorbitan monolaurate], iodine complex [phosphate ester of alkylaryloxy polyethylene glycol], iodine tincture USP, iodine topical solution USP, nonylphenoxypoly [ethyleneoxy] ethanoliodine, poloxamer–iodine complex, undecoylium chloride iodine complex); mercufenol chloride; methylbenzethonium chloride; phenol; secondary amyltricresols; sodium oxychlorosene; tribromsalan; triclocarban; triclosan; triple dye; combination of calomel, oxyquinoline benzoate, triethanolamine, and phenol derivative; and combination of mercufenol chloride and secondary amyltricresols in 50% alcohol.
If manufacturers want to use one or more of these 24 active ingredients in future OTC health care antiseptic drug products, those products will be considered new drugs for which a new drug application approval will be required, the agency said.
The rule does not affect health care antiseptics that are currently marketed under new drug applications and abbreviated new drug applications.
FDA’s action follows a similar final rule published Sept. 6, 2016, which removed triclosan and 18 other active ingredients from consumer antiseptic products.
in hospital settings and other health care situations outside the hospital, the U.S. Food and Drug Administration announced in a final rule.
The affected products include health care personnel hand washes and hand rubs, surgical hand scrubs and hand rubs, and patient antiseptic skin preparations. The final rule was published Dec. 20 in the Federal Register and becomes effective in December 2018.
The agency determined that a deferral is warranted for six health care antiseptic active ingredients – benzalkonium chloride, benzethonium chloride, chloroxylenol, alcohol, isopropyl alcohol, and povidone-iodine – to allow more time for interested parties to complete the studies necessary to fill the safety and effectiveness data gaps identified for these ingredients.
“The FDA expects that this information may help better inform us on antiseptic resistance and antibiotic cross-resistance in the health care setting,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Importantly, this doesn’t mean that products containing these six ingredients are ineffective or unsafe. These antiseptic products remain an important resource in health care settings. Personnel should continue to use these products consistent with infection control guidelines while the additional data are gathered.”
No additional data was provided for another 24 products, which were deemed not generally recognized as safe and effective. The minimum data needed to demonstrate safety for all health care antiseptic active ingredients fall into four broad categories: human safety studies, nonclinical safety studies (developmental and reproductive toxicity studies and carcinogenicity studies), data to characterize potential hormonal effects, and data to evaluate the development of antimicrobial resistance, the final rule states.
The FDA noted that manufacturers started to remove nearly all of these 24 active ingredients from their products following a 2015 proposed rule. Triclosan is currently being used in available products.
The active ingredients affected are chlorhexidine gluconate; cloflucarban; fluorosalan; hexachlorophene; hexylresorcinol; iodophors (iodine-containing ingredients including iodine complex [ammonium ether sulfate and polyoxyethylene sorbitan monolaurate], iodine complex [phosphate ester of alkylaryloxy polyethylene glycol], iodine tincture USP, iodine topical solution USP, nonylphenoxypoly [ethyleneoxy] ethanoliodine, poloxamer–iodine complex, undecoylium chloride iodine complex); mercufenol chloride; methylbenzethonium chloride; phenol; secondary amyltricresols; sodium oxychlorosene; tribromsalan; triclocarban; triclosan; triple dye; combination of calomel, oxyquinoline benzoate, triethanolamine, and phenol derivative; and combination of mercufenol chloride and secondary amyltricresols in 50% alcohol.
If manufacturers want to use one or more of these 24 active ingredients in future OTC health care antiseptic drug products, those products will be considered new drugs for which a new drug application approval will be required, the agency said.
The rule does not affect health care antiseptics that are currently marketed under new drug applications and abbreviated new drug applications.
FDA’s action follows a similar final rule published Sept. 6, 2016, which removed triclosan and 18 other active ingredients from consumer antiseptic products.
Fecal microbiota transplants by oral capsule noninferior to colonoscopy
Fecal microbiota transplantation (FMT) using oral capsules as the delivery method has been shown to be noninferior to delivery using colonoscopy for the treatment of Clostridium difficile infection, but with a significantly lower price tag.
In an unblended noninferiority trial, published in the Nov. 28 issue of JAMA, 116 adults with at least three documented episodes of C. difficile infection were randomized to either 360 mL of fecal slurry delivered to the cecum via colonoscopy or to 40 capsules of processed fecal microbiota swallowed under direct observation.
Dina Kao, MD, of the department of medicine at the University of Alberta, Edmonton, and coauthors commented that the response rate with the capsules was higher than that seen in other studies of fecal microbiota capsules, which they suggested may partly be due to the larger amount of donor stool used in the study: 80-100 g, compared with 17 g and 25 g used in other studies.
“The higher efficacy observed in this study suggests a dose-dependent response to FMT, and a benefit of bowel lavage prior to FMT, because residual vancomycin was detected up to 8 days despite its discontinuation,” they wrote.
Both treatment modalities achieved similar quality of life improvements. Both groups reported major improvements in domains including physical and emotional health, physical and social functioning, and general health, with no significant differences between the two arms of the study.
The cost per treatment in the colonoscopy group was $874 per patient, compared with $308 per patient in the capsule group.
“Although colonoscopy delivery is more invasive, resource intensive, costly, and inconvenient for patients, it has the advantage of identifying alternative diagnoses,” the authors wrote. “Conversely, when FMT is given by oral capsules, it can be administered in an office setting, which could substantially reduce cost and wait time.”
Both groups also showed significantly improved gut microbiota diversity, which approached that of the donor just 1 week after administration of the treatment.
While 30% of patients characterized FMTs as “unpleasant, gross, or disgusting,” 79% of participants said the unpleasantness was the same or less than anticipated, and 97% said they would undergo the same treatment by the same delivery method again if needed.
However, significantly more patients in the capsule group described their experience as “not at all unpleasant,” compared with the colonoscopy group (66% vs. 44%; 95% CI, 3%-40%; P = .01).
There were no colonic perforations seen in the colonoscopy group, and no infectious complications relating to the treatment in either group. One patient in each group died of underlying cardiopulmonary illness that was unrelated to the treatment, and the rate of minor adverse events was 5.4% in the capsule group and 12.5% in the colonoscopy group.
The authors acknowledged that the lack of a placebo group in the study meant they were not able to measure the size of the effect of fecal microbiota transplantation by either route. One earlier trial had also shown a placebo response rate of 45%.
The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
Clostridium difficile infection costs the U.S. health care system an estimated $1.5 billion each year, with 450,000 cases reported annually, 20% of which involve a recurrence of the infection. Fecal microbiota transplantation is increasingly being used as a treatment, but more widespread adoption is limited partly by the logistical difficulties of delivery.
This study offers encouraging data on delivery of fecal microbiota transplants via capsule, which may reduce barriers to adoption of this treatment; however, there are still some questions to be answered about the treatment’s efficacy, such as the timing of delivery and the relative importance of stool components.
There are also other approaches that should be considered in future research on C. difficile infection, including the use of vancomycin tapers with and without “chasers” of fidaxomicin/rifaximin, the use of defined microbial communities, and the use of sterile, fecal-derived products, which may even supplant standard fecal microbial transplants in the future.
Krishna Rao, MD, Vincent B. Young, MD, PhD, and Preeti N. Malani, MD, are with the division of infectious diseases in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are taken from an accompanying editorial (JAMA. 2017 Nov 28;381:1979-80. doi: 10.1001/jama.2017.17969). Dr. Young reported consulting fees from Vedanta, Merck, and Finch Therapeutics and grants from MedImmune. No other disclosures were reported.
Clostridium difficile infection costs the U.S. health care system an estimated $1.5 billion each year, with 450,000 cases reported annually, 20% of which involve a recurrence of the infection. Fecal microbiota transplantation is increasingly being used as a treatment, but more widespread adoption is limited partly by the logistical difficulties of delivery.
This study offers encouraging data on delivery of fecal microbiota transplants via capsule, which may reduce barriers to adoption of this treatment; however, there are still some questions to be answered about the treatment’s efficacy, such as the timing of delivery and the relative importance of stool components.
There are also other approaches that should be considered in future research on C. difficile infection, including the use of vancomycin tapers with and without “chasers” of fidaxomicin/rifaximin, the use of defined microbial communities, and the use of sterile, fecal-derived products, which may even supplant standard fecal microbial transplants in the future.
Krishna Rao, MD, Vincent B. Young, MD, PhD, and Preeti N. Malani, MD, are with the division of infectious diseases in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are taken from an accompanying editorial (JAMA. 2017 Nov 28;381:1979-80. doi: 10.1001/jama.2017.17969). Dr. Young reported consulting fees from Vedanta, Merck, and Finch Therapeutics and grants from MedImmune. No other disclosures were reported.
Clostridium difficile infection costs the U.S. health care system an estimated $1.5 billion each year, with 450,000 cases reported annually, 20% of which involve a recurrence of the infection. Fecal microbiota transplantation is increasingly being used as a treatment, but more widespread adoption is limited partly by the logistical difficulties of delivery.
This study offers encouraging data on delivery of fecal microbiota transplants via capsule, which may reduce barriers to adoption of this treatment; however, there are still some questions to be answered about the treatment’s efficacy, such as the timing of delivery and the relative importance of stool components.
There are also other approaches that should be considered in future research on C. difficile infection, including the use of vancomycin tapers with and without “chasers” of fidaxomicin/rifaximin, the use of defined microbial communities, and the use of sterile, fecal-derived products, which may even supplant standard fecal microbial transplants in the future.
Krishna Rao, MD, Vincent B. Young, MD, PhD, and Preeti N. Malani, MD, are with the division of infectious diseases in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are taken from an accompanying editorial (JAMA. 2017 Nov 28;381:1979-80. doi: 10.1001/jama.2017.17969). Dr. Young reported consulting fees from Vedanta, Merck, and Finch Therapeutics and grants from MedImmune. No other disclosures were reported.
Fecal microbiota transplantation (FMT) using oral capsules as the delivery method has been shown to be noninferior to delivery using colonoscopy for the treatment of Clostridium difficile infection, but with a significantly lower price tag.
In an unblended noninferiority trial, published in the Nov. 28 issue of JAMA, 116 adults with at least three documented episodes of C. difficile infection were randomized to either 360 mL of fecal slurry delivered to the cecum via colonoscopy or to 40 capsules of processed fecal microbiota swallowed under direct observation.
Dina Kao, MD, of the department of medicine at the University of Alberta, Edmonton, and coauthors commented that the response rate with the capsules was higher than that seen in other studies of fecal microbiota capsules, which they suggested may partly be due to the larger amount of donor stool used in the study: 80-100 g, compared with 17 g and 25 g used in other studies.
“The higher efficacy observed in this study suggests a dose-dependent response to FMT, and a benefit of bowel lavage prior to FMT, because residual vancomycin was detected up to 8 days despite its discontinuation,” they wrote.
Both treatment modalities achieved similar quality of life improvements. Both groups reported major improvements in domains including physical and emotional health, physical and social functioning, and general health, with no significant differences between the two arms of the study.
The cost per treatment in the colonoscopy group was $874 per patient, compared with $308 per patient in the capsule group.
“Although colonoscopy delivery is more invasive, resource intensive, costly, and inconvenient for patients, it has the advantage of identifying alternative diagnoses,” the authors wrote. “Conversely, when FMT is given by oral capsules, it can be administered in an office setting, which could substantially reduce cost and wait time.”
Both groups also showed significantly improved gut microbiota diversity, which approached that of the donor just 1 week after administration of the treatment.
While 30% of patients characterized FMTs as “unpleasant, gross, or disgusting,” 79% of participants said the unpleasantness was the same or less than anticipated, and 97% said they would undergo the same treatment by the same delivery method again if needed.
However, significantly more patients in the capsule group described their experience as “not at all unpleasant,” compared with the colonoscopy group (66% vs. 44%; 95% CI, 3%-40%; P = .01).
There were no colonic perforations seen in the colonoscopy group, and no infectious complications relating to the treatment in either group. One patient in each group died of underlying cardiopulmonary illness that was unrelated to the treatment, and the rate of minor adverse events was 5.4% in the capsule group and 12.5% in the colonoscopy group.
The authors acknowledged that the lack of a placebo group in the study meant they were not able to measure the size of the effect of fecal microbiota transplantation by either route. One earlier trial had also shown a placebo response rate of 45%.
The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
Fecal microbiota transplantation (FMT) using oral capsules as the delivery method has been shown to be noninferior to delivery using colonoscopy for the treatment of Clostridium difficile infection, but with a significantly lower price tag.
In an unblended noninferiority trial, published in the Nov. 28 issue of JAMA, 116 adults with at least three documented episodes of C. difficile infection were randomized to either 360 mL of fecal slurry delivered to the cecum via colonoscopy or to 40 capsules of processed fecal microbiota swallowed under direct observation.
Dina Kao, MD, of the department of medicine at the University of Alberta, Edmonton, and coauthors commented that the response rate with the capsules was higher than that seen in other studies of fecal microbiota capsules, which they suggested may partly be due to the larger amount of donor stool used in the study: 80-100 g, compared with 17 g and 25 g used in other studies.
“The higher efficacy observed in this study suggests a dose-dependent response to FMT, and a benefit of bowel lavage prior to FMT, because residual vancomycin was detected up to 8 days despite its discontinuation,” they wrote.
Both treatment modalities achieved similar quality of life improvements. Both groups reported major improvements in domains including physical and emotional health, physical and social functioning, and general health, with no significant differences between the two arms of the study.
The cost per treatment in the colonoscopy group was $874 per patient, compared with $308 per patient in the capsule group.
“Although colonoscopy delivery is more invasive, resource intensive, costly, and inconvenient for patients, it has the advantage of identifying alternative diagnoses,” the authors wrote. “Conversely, when FMT is given by oral capsules, it can be administered in an office setting, which could substantially reduce cost and wait time.”
Both groups also showed significantly improved gut microbiota diversity, which approached that of the donor just 1 week after administration of the treatment.
While 30% of patients characterized FMTs as “unpleasant, gross, or disgusting,” 79% of participants said the unpleasantness was the same or less than anticipated, and 97% said they would undergo the same treatment by the same delivery method again if needed.
However, significantly more patients in the capsule group described their experience as “not at all unpleasant,” compared with the colonoscopy group (66% vs. 44%; 95% CI, 3%-40%; P = .01).
There were no colonic perforations seen in the colonoscopy group, and no infectious complications relating to the treatment in either group. One patient in each group died of underlying cardiopulmonary illness that was unrelated to the treatment, and the rate of minor adverse events was 5.4% in the capsule group and 12.5% in the colonoscopy group.
The authors acknowledged that the lack of a placebo group in the study meant they were not able to measure the size of the effect of fecal microbiota transplantation by either route. One earlier trial had also shown a placebo response rate of 45%.
The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
FROM JAMA
Key clinical point: Delivering fecal microbiota transplants using oral capsules is noninferior to delivery via colonoscopy in the treatment of Clostridium difficile infection.
Major finding: The rates of resolution of recurrent C. difficile infection with fecal microbiota transplants are similar for delivery via oral capsule or via colonoscopy.
Data source: A randomized, unblended noninferiority trial in 116 adults with recurrent C. difficile infection.
Disclosures: The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
SHORT TAKES
Cardiac testing of Emergency Department patients with chest pain leads to increased revascularization without reduction in admissions for acute MI
Retrospective cohort study of ED patients presenting with chest pain but without evidence of ischemia, shows that non-invasive cardiac testing of these patients lead to more coronary angiograms (92.1 per 1,000 patients) within 30 days, but no significant reduction of admissions for acute MI at 1 year (remained 7.8 per 1,000 patients tested).
Citation: Sandhu AT, Heidenreich PA, Bhattacharya J, Bundorf MK. Cardiovascular Testing and Clinical Outcomes in Emergency Department Patients With Chest Pain. JAMA Intern Med. Published online 2017 June 26. doi: 10.1001/jamainternmed.2017.2432.
Facebook star ratings and “likes” correlate with patient satisfaction scores
In a cross-sectional analysis of 136 New York State hospitals, the study found increased Facebook star ratings correlated (P less than .003) with overall increased HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) score (21/23 HCAHPS). HCAHPS measures also positively correlated (P less than .05) with adjusted number of “likes” on Facebook but to a lesser degree (3/21 HCAHPS). Neither star ratings nor number of “likes” correlate with Medicare spending or 30-day all-cause readmission rate.
Citation: Campbell L, Yue L. Are Facebook user ratings associated with hospital cost, quality, and patient satisfaction? BMJ Qual Saf. 2017 July 19. doi:10.1136/bmjqs-2016-006291.
Candida auris remains an ongoing health care facility transmission risk
Multidrug-resistant fungus Candida auris is an emerging pathogen and a transmission risk across health care facilities. From June 2016 through May 2017, 77 cases of clinical infection have been reported to the Centers for Disease Control and Prevention from seven states, though most (90%) cases are clustered in the New York City metropolitan area. Most patients had multiple medical conditions and extensive health care facility exposure. The CDC recommends contact precautions, private rooming, daily and terminal cleaning with disinfectant active against Clostridium difficile spores, and notification to receiving health care facilities about C. auris colonization or infection on transfer to help reduce the spread of C. auris throughout the United States.
Citation: Tsay S, Welsh RM, Adams EH, et al. Notes from the Field: Ongoing Transmission of Candida auris in Health Care Facilities – United States, June 2016–May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:51415.
Cardiac testing of Emergency Department patients with chest pain leads to increased revascularization without reduction in admissions for acute MI
Retrospective cohort study of ED patients presenting with chest pain but without evidence of ischemia, shows that non-invasive cardiac testing of these patients lead to more coronary angiograms (92.1 per 1,000 patients) within 30 days, but no significant reduction of admissions for acute MI at 1 year (remained 7.8 per 1,000 patients tested).
Citation: Sandhu AT, Heidenreich PA, Bhattacharya J, Bundorf MK. Cardiovascular Testing and Clinical Outcomes in Emergency Department Patients With Chest Pain. JAMA Intern Med. Published online 2017 June 26. doi: 10.1001/jamainternmed.2017.2432.
Facebook star ratings and “likes” correlate with patient satisfaction scores
In a cross-sectional analysis of 136 New York State hospitals, the study found increased Facebook star ratings correlated (P less than .003) with overall increased HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) score (21/23 HCAHPS). HCAHPS measures also positively correlated (P less than .05) with adjusted number of “likes” on Facebook but to a lesser degree (3/21 HCAHPS). Neither star ratings nor number of “likes” correlate with Medicare spending or 30-day all-cause readmission rate.
Citation: Campbell L, Yue L. Are Facebook user ratings associated with hospital cost, quality, and patient satisfaction? BMJ Qual Saf. 2017 July 19. doi:10.1136/bmjqs-2016-006291.
Candida auris remains an ongoing health care facility transmission risk
Multidrug-resistant fungus Candida auris is an emerging pathogen and a transmission risk across health care facilities. From June 2016 through May 2017, 77 cases of clinical infection have been reported to the Centers for Disease Control and Prevention from seven states, though most (90%) cases are clustered in the New York City metropolitan area. Most patients had multiple medical conditions and extensive health care facility exposure. The CDC recommends contact precautions, private rooming, daily and terminal cleaning with disinfectant active against Clostridium difficile spores, and notification to receiving health care facilities about C. auris colonization or infection on transfer to help reduce the spread of C. auris throughout the United States.
Citation: Tsay S, Welsh RM, Adams EH, et al. Notes from the Field: Ongoing Transmission of Candida auris in Health Care Facilities – United States, June 2016–May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:51415.
Cardiac testing of Emergency Department patients with chest pain leads to increased revascularization without reduction in admissions for acute MI
Retrospective cohort study of ED patients presenting with chest pain but without evidence of ischemia, shows that non-invasive cardiac testing of these patients lead to more coronary angiograms (92.1 per 1,000 patients) within 30 days, but no significant reduction of admissions for acute MI at 1 year (remained 7.8 per 1,000 patients tested).
Citation: Sandhu AT, Heidenreich PA, Bhattacharya J, Bundorf MK. Cardiovascular Testing and Clinical Outcomes in Emergency Department Patients With Chest Pain. JAMA Intern Med. Published online 2017 June 26. doi: 10.1001/jamainternmed.2017.2432.
Facebook star ratings and “likes” correlate with patient satisfaction scores
In a cross-sectional analysis of 136 New York State hospitals, the study found increased Facebook star ratings correlated (P less than .003) with overall increased HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) score (21/23 HCAHPS). HCAHPS measures also positively correlated (P less than .05) with adjusted number of “likes” on Facebook but to a lesser degree (3/21 HCAHPS). Neither star ratings nor number of “likes” correlate with Medicare spending or 30-day all-cause readmission rate.
Citation: Campbell L, Yue L. Are Facebook user ratings associated with hospital cost, quality, and patient satisfaction? BMJ Qual Saf. 2017 July 19. doi:10.1136/bmjqs-2016-006291.
Candida auris remains an ongoing health care facility transmission risk
Multidrug-resistant fungus Candida auris is an emerging pathogen and a transmission risk across health care facilities. From June 2016 through May 2017, 77 cases of clinical infection have been reported to the Centers for Disease Control and Prevention from seven states, though most (90%) cases are clustered in the New York City metropolitan area. Most patients had multiple medical conditions and extensive health care facility exposure. The CDC recommends contact precautions, private rooming, daily and terminal cleaning with disinfectant active against Clostridium difficile spores, and notification to receiving health care facilities about C. auris colonization or infection on transfer to help reduce the spread of C. auris throughout the United States.
Citation: Tsay S, Welsh RM, Adams EH, et al. Notes from the Field: Ongoing Transmission of Candida auris in Health Care Facilities – United States, June 2016–May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:51415.
Pediatric hospitalists take on the challenge of antibiotic stewardship
When Carol Glaser, MD, was in training, the philosophy around antibiotic prescribing often went something like this: “Ten days of antibiotics is good, but let’s do a few more days just to be sure,” she said.
Today, however, the new mantra is “less is more.” Dr. Glaser is an experienced pediatric infectious disease physician and the lead physician for pediatric antimicrobial stewardship at The Permanente Medical Group, Kaiser Permanente, at the Oakland (Calif.) Medical Center. While antibiotic stewardship is an issue relevant to nearly all hospitalists, for pediatric patients, the considerations can be unique and particularly serious.
Dr. Shah, a pediatric infectious disease physician at Cincinnati Children’s Hospital, spoke last spring at HM17, the Society of Hospital Medicine’s annual meeting. His talk drew from issues raised on pediatric hospital medicine electronic mailing lists and from audience questions. These centered on decisions regarding the use of intravenous versus oral antibiotics for pediatric patients – or what he refers to as intravenous-to-oral conversion – as well as antibiotic treatment duration.
“For many conditions in pediatrics, we used to treat with intravenous antibiotics initially – and sometimes for the entire course – and now we’re using oral antibiotics for the entire course,” Dr. Shah said. He noted that urinary tract infections were once treated with IV antibiotics in the hospital but are now routinely treated orally in an outpatient setting.
Dr. Shah cited two studies, both of which he coauthored as part of the Pediatric Research in Inpatient Settings Network, which compared intravenous versus oral antibiotics treatments given after discharge: The first, published in JAMA Pediatrics in 2014, examined treatment for osteomyelitis, while the second, which focused on complicated pneumonia, was published in Pediatrics in 2016.1,2
Both were observational, retrospective studies involving more than 2,000 children across more than 30 hospitals. The JAMA Pediatrics study found that roughly half of the patients were discharged with a peripherally inserted central catheter (PICC) line, and half were prescribed oral antibiotics. In some hospitals, 100% of patients were sent home with a PICC line, and in others, all children were sent home on oral antibiotics. Although treatment failure rates were the same for both groups, 15% of the patients sent home with a PICC line had to return to the emergency department because of PICC-related complications. Some were hospitalized.1
The Pediatrics study found less variation in PICC versus oral antibiotic use across hospitals for patients with complicated pneumonia, but the treatment failure rate was slightly higher for PICC patients at 3.2%, compared with 2.6% for those on oral antibiotics. This difference, however, was not statistically significant. PICC-related complications were observed in 7.1% of patients with PICC lines also were more likely to experience adverse drug reactions, compared with patients on oral antibiotics.2
“PICC lines have some advantages, particularly when children are unable or unwilling to take oral antibiotics, but they also have risks” said Dr. Shah. “If outcomes are equivalent, why would you subject patients to the risks of a catheter? And, every time they get a fever at home with a PICC line, they need urgent evaluation for the possibility of a catheter-associated bacterial infection. There is an emotional cost, as well, to taking care of catheters in the home setting.”
Additionally, economic pressures are compelling hospitals to reduce costs and resource utilization while maintaining or improving the quality of care, Dr. Shah pointed out. “Hospitalists do many things well, and quality improvement is one of those areas. That approach really aligns with antimicrobial stewardship, and there is greater incentive with episode-based payment models and financial penalties for excess readmissions. Reducing post-discharge IV antibiotic use aligns with stewardship goals and reduces the likelihood of hospital readmissions.”
The hospital medicine division at Dr. Shah’s hospital helped assemble a multidisciplinary team involving emergency physicians, pharmacists, nursing staff, hospitalists, and infectious disease physicians to encourage the use of appropriate, narrow-spectrum antibiotics and reduce the duration of antibiotic therapies. For example, skin and soft-tissue infections that were once treated for 10-14 days are now sufficiently treated in 5-7days. These efforts to improve outcomes through better adherence to evidence-based practices, including better stewardship, earned the team the SHM Teamwork in Quality Improvement Award in 2014.
“Quality improvement is really about changing the system, and hospitalists, who excel in QI, are poised to help drive antimicrobial stewardship efforts,” Dr. Shah said.
At Oakland Medical Center, Dr. Glaser helped implement handshake rounds, an idea they adopted from a group in Colorado. Every day, with every patient, the antimicrobial stewardship team meets with representatives of the teams – pediatric intensive care, the wards, the NICU, and others – to review antibiotic treatment plans for the choice of antimicrobial drug, for the duration of treatment, and for specific conditions. “We work really closely with hospitalists and our strong pediatric pharmacy team every day to ask: ‘Do we have the right dose? Do we really need to use this antibiotic?’ ” Dr. Glaser said.
Last year, she also worked to incorporate antimicrobial stewardship principles into the hospital’s residency program. “I think the most important thing we’re doing is changing the culture,” she said. “For these young physicians, we’re giving them the knowledge to empower them rather than telling them what to do and giving them a better, fundamental understanding of infectious disease.”
For instance, most pediatric respiratory illnesses are caused by a virus, yet physicians will still prescribe antibiotics for a host of reasons – including the expectations of parents, the guesswork that can go into diagnosing a young patient who cannot describe what is wrong, and the fear that children will get sicker if an antibiotic is not started early.
“A lot of it is figuring out the best approach with the least amount of side effects but covering what we need to cover for a given patient,” she said.
A number of physicians from Dr. Glaser’s team presented stewardship data from their hospital at the July 2017 Pediatric Hospital Medicine meeting in Nashville, demonstrating that, overall, they are using fewer antibiotics and that fewer of those used are broad spectrum. This satisfies the “pillars of stewardship,” Dr. Glaser said. Use antibiotics only when you need them, use them only as long as you need, and then make sure you use the most narrow-spectrum antibiotic you possibly can, she said.
Oakland Medical Center has benefited from a strong commitment to antimicrobial stewardship efforts, Dr. Glaser said, noting that many programs may lack such support, a problem that can be one of the biggest hurdles antimicrobial stewardship efforts face. The support at her hospital “has been an immense help in getting our program to where it is today.”
References
1. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015 Feb:169(2):120-8.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016 Dec;138(6). pii: e20161692.
When Carol Glaser, MD, was in training, the philosophy around antibiotic prescribing often went something like this: “Ten days of antibiotics is good, but let’s do a few more days just to be sure,” she said.
Today, however, the new mantra is “less is more.” Dr. Glaser is an experienced pediatric infectious disease physician and the lead physician for pediatric antimicrobial stewardship at The Permanente Medical Group, Kaiser Permanente, at the Oakland (Calif.) Medical Center. While antibiotic stewardship is an issue relevant to nearly all hospitalists, for pediatric patients, the considerations can be unique and particularly serious.
Dr. Shah, a pediatric infectious disease physician at Cincinnati Children’s Hospital, spoke last spring at HM17, the Society of Hospital Medicine’s annual meeting. His talk drew from issues raised on pediatric hospital medicine electronic mailing lists and from audience questions. These centered on decisions regarding the use of intravenous versus oral antibiotics for pediatric patients – or what he refers to as intravenous-to-oral conversion – as well as antibiotic treatment duration.
“For many conditions in pediatrics, we used to treat with intravenous antibiotics initially – and sometimes for the entire course – and now we’re using oral antibiotics for the entire course,” Dr. Shah said. He noted that urinary tract infections were once treated with IV antibiotics in the hospital but are now routinely treated orally in an outpatient setting.
Dr. Shah cited two studies, both of which he coauthored as part of the Pediatric Research in Inpatient Settings Network, which compared intravenous versus oral antibiotics treatments given after discharge: The first, published in JAMA Pediatrics in 2014, examined treatment for osteomyelitis, while the second, which focused on complicated pneumonia, was published in Pediatrics in 2016.1,2
Both were observational, retrospective studies involving more than 2,000 children across more than 30 hospitals. The JAMA Pediatrics study found that roughly half of the patients were discharged with a peripherally inserted central catheter (PICC) line, and half were prescribed oral antibiotics. In some hospitals, 100% of patients were sent home with a PICC line, and in others, all children were sent home on oral antibiotics. Although treatment failure rates were the same for both groups, 15% of the patients sent home with a PICC line had to return to the emergency department because of PICC-related complications. Some were hospitalized.1
The Pediatrics study found less variation in PICC versus oral antibiotic use across hospitals for patients with complicated pneumonia, but the treatment failure rate was slightly higher for PICC patients at 3.2%, compared with 2.6% for those on oral antibiotics. This difference, however, was not statistically significant. PICC-related complications were observed in 7.1% of patients with PICC lines also were more likely to experience adverse drug reactions, compared with patients on oral antibiotics.2
“PICC lines have some advantages, particularly when children are unable or unwilling to take oral antibiotics, but they also have risks” said Dr. Shah. “If outcomes are equivalent, why would you subject patients to the risks of a catheter? And, every time they get a fever at home with a PICC line, they need urgent evaluation for the possibility of a catheter-associated bacterial infection. There is an emotional cost, as well, to taking care of catheters in the home setting.”
Additionally, economic pressures are compelling hospitals to reduce costs and resource utilization while maintaining or improving the quality of care, Dr. Shah pointed out. “Hospitalists do many things well, and quality improvement is one of those areas. That approach really aligns with antimicrobial stewardship, and there is greater incentive with episode-based payment models and financial penalties for excess readmissions. Reducing post-discharge IV antibiotic use aligns with stewardship goals and reduces the likelihood of hospital readmissions.”
The hospital medicine division at Dr. Shah’s hospital helped assemble a multidisciplinary team involving emergency physicians, pharmacists, nursing staff, hospitalists, and infectious disease physicians to encourage the use of appropriate, narrow-spectrum antibiotics and reduce the duration of antibiotic therapies. For example, skin and soft-tissue infections that were once treated for 10-14 days are now sufficiently treated in 5-7days. These efforts to improve outcomes through better adherence to evidence-based practices, including better stewardship, earned the team the SHM Teamwork in Quality Improvement Award in 2014.
“Quality improvement is really about changing the system, and hospitalists, who excel in QI, are poised to help drive antimicrobial stewardship efforts,” Dr. Shah said.
At Oakland Medical Center, Dr. Glaser helped implement handshake rounds, an idea they adopted from a group in Colorado. Every day, with every patient, the antimicrobial stewardship team meets with representatives of the teams – pediatric intensive care, the wards, the NICU, and others – to review antibiotic treatment plans for the choice of antimicrobial drug, for the duration of treatment, and for specific conditions. “We work really closely with hospitalists and our strong pediatric pharmacy team every day to ask: ‘Do we have the right dose? Do we really need to use this antibiotic?’ ” Dr. Glaser said.
Last year, she also worked to incorporate antimicrobial stewardship principles into the hospital’s residency program. “I think the most important thing we’re doing is changing the culture,” she said. “For these young physicians, we’re giving them the knowledge to empower them rather than telling them what to do and giving them a better, fundamental understanding of infectious disease.”
For instance, most pediatric respiratory illnesses are caused by a virus, yet physicians will still prescribe antibiotics for a host of reasons – including the expectations of parents, the guesswork that can go into diagnosing a young patient who cannot describe what is wrong, and the fear that children will get sicker if an antibiotic is not started early.
“A lot of it is figuring out the best approach with the least amount of side effects but covering what we need to cover for a given patient,” she said.
A number of physicians from Dr. Glaser’s team presented stewardship data from their hospital at the July 2017 Pediatric Hospital Medicine meeting in Nashville, demonstrating that, overall, they are using fewer antibiotics and that fewer of those used are broad spectrum. This satisfies the “pillars of stewardship,” Dr. Glaser said. Use antibiotics only when you need them, use them only as long as you need, and then make sure you use the most narrow-spectrum antibiotic you possibly can, she said.
Oakland Medical Center has benefited from a strong commitment to antimicrobial stewardship efforts, Dr. Glaser said, noting that many programs may lack such support, a problem that can be one of the biggest hurdles antimicrobial stewardship efforts face. The support at her hospital “has been an immense help in getting our program to where it is today.”
References
1. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015 Feb:169(2):120-8.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016 Dec;138(6). pii: e20161692.
When Carol Glaser, MD, was in training, the philosophy around antibiotic prescribing often went something like this: “Ten days of antibiotics is good, but let’s do a few more days just to be sure,” she said.
Today, however, the new mantra is “less is more.” Dr. Glaser is an experienced pediatric infectious disease physician and the lead physician for pediatric antimicrobial stewardship at The Permanente Medical Group, Kaiser Permanente, at the Oakland (Calif.) Medical Center. While antibiotic stewardship is an issue relevant to nearly all hospitalists, for pediatric patients, the considerations can be unique and particularly serious.
Dr. Shah, a pediatric infectious disease physician at Cincinnati Children’s Hospital, spoke last spring at HM17, the Society of Hospital Medicine’s annual meeting. His talk drew from issues raised on pediatric hospital medicine electronic mailing lists and from audience questions. These centered on decisions regarding the use of intravenous versus oral antibiotics for pediatric patients – or what he refers to as intravenous-to-oral conversion – as well as antibiotic treatment duration.
“For many conditions in pediatrics, we used to treat with intravenous antibiotics initially – and sometimes for the entire course – and now we’re using oral antibiotics for the entire course,” Dr. Shah said. He noted that urinary tract infections were once treated with IV antibiotics in the hospital but are now routinely treated orally in an outpatient setting.
Dr. Shah cited two studies, both of which he coauthored as part of the Pediatric Research in Inpatient Settings Network, which compared intravenous versus oral antibiotics treatments given after discharge: The first, published in JAMA Pediatrics in 2014, examined treatment for osteomyelitis, while the second, which focused on complicated pneumonia, was published in Pediatrics in 2016.1,2
Both were observational, retrospective studies involving more than 2,000 children across more than 30 hospitals. The JAMA Pediatrics study found that roughly half of the patients were discharged with a peripherally inserted central catheter (PICC) line, and half were prescribed oral antibiotics. In some hospitals, 100% of patients were sent home with a PICC line, and in others, all children were sent home on oral antibiotics. Although treatment failure rates were the same for both groups, 15% of the patients sent home with a PICC line had to return to the emergency department because of PICC-related complications. Some were hospitalized.1
The Pediatrics study found less variation in PICC versus oral antibiotic use across hospitals for patients with complicated pneumonia, but the treatment failure rate was slightly higher for PICC patients at 3.2%, compared with 2.6% for those on oral antibiotics. This difference, however, was not statistically significant. PICC-related complications were observed in 7.1% of patients with PICC lines also were more likely to experience adverse drug reactions, compared with patients on oral antibiotics.2
“PICC lines have some advantages, particularly when children are unable or unwilling to take oral antibiotics, but they also have risks” said Dr. Shah. “If outcomes are equivalent, why would you subject patients to the risks of a catheter? And, every time they get a fever at home with a PICC line, they need urgent evaluation for the possibility of a catheter-associated bacterial infection. There is an emotional cost, as well, to taking care of catheters in the home setting.”
Additionally, economic pressures are compelling hospitals to reduce costs and resource utilization while maintaining or improving the quality of care, Dr. Shah pointed out. “Hospitalists do many things well, and quality improvement is one of those areas. That approach really aligns with antimicrobial stewardship, and there is greater incentive with episode-based payment models and financial penalties for excess readmissions. Reducing post-discharge IV antibiotic use aligns with stewardship goals and reduces the likelihood of hospital readmissions.”
The hospital medicine division at Dr. Shah’s hospital helped assemble a multidisciplinary team involving emergency physicians, pharmacists, nursing staff, hospitalists, and infectious disease physicians to encourage the use of appropriate, narrow-spectrum antibiotics and reduce the duration of antibiotic therapies. For example, skin and soft-tissue infections that were once treated for 10-14 days are now sufficiently treated in 5-7days. These efforts to improve outcomes through better adherence to evidence-based practices, including better stewardship, earned the team the SHM Teamwork in Quality Improvement Award in 2014.
“Quality improvement is really about changing the system, and hospitalists, who excel in QI, are poised to help drive antimicrobial stewardship efforts,” Dr. Shah said.
At Oakland Medical Center, Dr. Glaser helped implement handshake rounds, an idea they adopted from a group in Colorado. Every day, with every patient, the antimicrobial stewardship team meets with representatives of the teams – pediatric intensive care, the wards, the NICU, and others – to review antibiotic treatment plans for the choice of antimicrobial drug, for the duration of treatment, and for specific conditions. “We work really closely with hospitalists and our strong pediatric pharmacy team every day to ask: ‘Do we have the right dose? Do we really need to use this antibiotic?’ ” Dr. Glaser said.
Last year, she also worked to incorporate antimicrobial stewardship principles into the hospital’s residency program. “I think the most important thing we’re doing is changing the culture,” she said. “For these young physicians, we’re giving them the knowledge to empower them rather than telling them what to do and giving them a better, fundamental understanding of infectious disease.”
For instance, most pediatric respiratory illnesses are caused by a virus, yet physicians will still prescribe antibiotics for a host of reasons – including the expectations of parents, the guesswork that can go into diagnosing a young patient who cannot describe what is wrong, and the fear that children will get sicker if an antibiotic is not started early.
“A lot of it is figuring out the best approach with the least amount of side effects but covering what we need to cover for a given patient,” she said.
A number of physicians from Dr. Glaser’s team presented stewardship data from their hospital at the July 2017 Pediatric Hospital Medicine meeting in Nashville, demonstrating that, overall, they are using fewer antibiotics and that fewer of those used are broad spectrum. This satisfies the “pillars of stewardship,” Dr. Glaser said. Use antibiotics only when you need them, use them only as long as you need, and then make sure you use the most narrow-spectrum antibiotic you possibly can, she said.
Oakland Medical Center has benefited from a strong commitment to antimicrobial stewardship efforts, Dr. Glaser said, noting that many programs may lack such support, a problem that can be one of the biggest hurdles antimicrobial stewardship efforts face. The support at her hospital “has been an immense help in getting our program to where it is today.”
References
1. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015 Feb:169(2):120-8.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016 Dec;138(6). pii: e20161692.