User login
Going into solo practice? An expert shares tips
SAN DIEGO – When the Boston-based cosmetic dermatology practice that employed Catherine M. DiGiorgio, MD, MS, was sold to a private equity firm a few years ago, she found herself at a crossroads: Stay and work for a large corporation, or open a solo practice?
She opted to start her own practice in Boston, “because I didn’t want to work for a large corporation, and I want to provide the best care for my patients in a more intimate manner,” Dr. DiGiorgio, a board-certified laser and cosmetic dermatologist, said at the annual Masters of Aesthetics Symposium.
The decision also tested her mettle. “I spoke to several colleagues and friends, and I was terrified,” she said. “I was like: ‘I don’t even know where to start.’ ”
On the heels of opening a new office in a matter of weeks, she offered the following tips and questions to consider when launching a solo dermatology practice:
Select a location. “That’s your first decision,” she said. “Where in the city are you going to open? Are you going to a new city, or are you moving back home? Don’t be afraid to start from scratch, and don’t be afraid to start a [solo] practice if you already have a patient base.”
Will you lease or purchase your space? After she secured a bank loan, Dr. DiGiorgio chose to lease the space for her new practice, “because you can kind of see where things go, get all the kinks out and figure out how to build things in a space that you don’t own. Then, when you’re ready and you have grown, you can invest more into your practice.”
Will you accept insurance? She built her practice around the direct specialty care model, which emphasizes the patient-physician relationship and removes third-party payors. “It’s not a concierge practice, but it’s a transparent, reasonable fee schedule for medical dermatology,” she explained. “I’ve done 100% cosmetics for about 5 years now, [but] I do medical dermatology for a fee. On my website I have a full price list on how much a full skin check is, [and] how much biopsies are. It’s completely transparent. Patients can submit to their insurance for reimbursement, but we don’t guarantee that they’re going to be reimbursed.”
Where will your patients come from? Will you advertise? Do you have physicians in the area who will refer to you if you’re a board-certified dermatologist? She emphasized the importance of “learning how to present yourself” on a website dedicated to your own practice. “Instagram, Facebook, and social media are great, but you don’t own those pages,” noted Dr. DiGiorgio, who served as the program cochair of the 2023 annual meeting of the American Society for Laser Medicine and Surgery and was recently elected to serve on the board of directors for the American Society for Dermatology Surgery. “You don’t own one of those pictures that are posted on your social media page. They can disappear in a second. If that happens, how are people going to find you?”
Are you going to hire more physicians in the future? That will influence the size of the new office and the floor plan.
Lawyer up. Hiring a health care attorney can “help you navigate transitioning from whatever position you’re in to opening up your own practice, as well as setting up the regulatory paperwork necessary for your new practice. You’ll also need a real estate attorney to help once you have selected a place, to help you navigate through that process,” she said, such as figuring out if the elevator in the building meets the Americans With Disabilities Act (ADA) requirements.
Create a mission statement. That way, “you know why you’re doing this, and it stays with you as you’re getting through the hard roadblocks.”
Find an architect, contractor, or designer. “If you’re building out a space from scratch, you’re going to need an architect,” she said. “Along with that architect will come a full-on contracting firm. I ended up hiring everyone individually, because I’m trying to spend as little money as possible.” She also hired a designer to help select furnishings and create the office atmosphere.
Secure a building permit ASAP. “It’s almost better to have the city permit before you sign the lease, because the permits can take a year, and you don’t want to pay rent on an empty space for a year if you don’t have a permit or if there are other hoops to go through,” Dr. DiGiorgio said.
Find an agent to help you set up medical malpractice insurance, liability insurance, and worker’s compensation insurance. “Make sure you read all the paperwork, because it can be very intricate,” she said.
Find an accountant. That person can help set up a bookkeeping process.
What equipment and devices will you need? That depends largely on the patient population a physician serves. Dr. DiGiorgio noted that eligible small businesses may take a tax credit of up to $5,000 per year for accommodations made to comply with the ADA. “That’s a nice feature, so that you can purchase ADA compliant items like a larger exam chair and custom reception areas.”
Dr. DiGiorgio reported having no relevant disclosures.
SAN DIEGO – When the Boston-based cosmetic dermatology practice that employed Catherine M. DiGiorgio, MD, MS, was sold to a private equity firm a few years ago, she found herself at a crossroads: Stay and work for a large corporation, or open a solo practice?
She opted to start her own practice in Boston, “because I didn’t want to work for a large corporation, and I want to provide the best care for my patients in a more intimate manner,” Dr. DiGiorgio, a board-certified laser and cosmetic dermatologist, said at the annual Masters of Aesthetics Symposium.
The decision also tested her mettle. “I spoke to several colleagues and friends, and I was terrified,” she said. “I was like: ‘I don’t even know where to start.’ ”
On the heels of opening a new office in a matter of weeks, she offered the following tips and questions to consider when launching a solo dermatology practice:
Select a location. “That’s your first decision,” she said. “Where in the city are you going to open? Are you going to a new city, or are you moving back home? Don’t be afraid to start from scratch, and don’t be afraid to start a [solo] practice if you already have a patient base.”
Will you lease or purchase your space? After she secured a bank loan, Dr. DiGiorgio chose to lease the space for her new practice, “because you can kind of see where things go, get all the kinks out and figure out how to build things in a space that you don’t own. Then, when you’re ready and you have grown, you can invest more into your practice.”
Will you accept insurance? She built her practice around the direct specialty care model, which emphasizes the patient-physician relationship and removes third-party payors. “It’s not a concierge practice, but it’s a transparent, reasonable fee schedule for medical dermatology,” she explained. “I’ve done 100% cosmetics for about 5 years now, [but] I do medical dermatology for a fee. On my website I have a full price list on how much a full skin check is, [and] how much biopsies are. It’s completely transparent. Patients can submit to their insurance for reimbursement, but we don’t guarantee that they’re going to be reimbursed.”
Where will your patients come from? Will you advertise? Do you have physicians in the area who will refer to you if you’re a board-certified dermatologist? She emphasized the importance of “learning how to present yourself” on a website dedicated to your own practice. “Instagram, Facebook, and social media are great, but you don’t own those pages,” noted Dr. DiGiorgio, who served as the program cochair of the 2023 annual meeting of the American Society for Laser Medicine and Surgery and was recently elected to serve on the board of directors for the American Society for Dermatology Surgery. “You don’t own one of those pictures that are posted on your social media page. They can disappear in a second. If that happens, how are people going to find you?”
Are you going to hire more physicians in the future? That will influence the size of the new office and the floor plan.
Lawyer up. Hiring a health care attorney can “help you navigate transitioning from whatever position you’re in to opening up your own practice, as well as setting up the regulatory paperwork necessary for your new practice. You’ll also need a real estate attorney to help once you have selected a place, to help you navigate through that process,” she said, such as figuring out if the elevator in the building meets the Americans With Disabilities Act (ADA) requirements.
Create a mission statement. That way, “you know why you’re doing this, and it stays with you as you’re getting through the hard roadblocks.”
Find an architect, contractor, or designer. “If you’re building out a space from scratch, you’re going to need an architect,” she said. “Along with that architect will come a full-on contracting firm. I ended up hiring everyone individually, because I’m trying to spend as little money as possible.” She also hired a designer to help select furnishings and create the office atmosphere.
Secure a building permit ASAP. “It’s almost better to have the city permit before you sign the lease, because the permits can take a year, and you don’t want to pay rent on an empty space for a year if you don’t have a permit or if there are other hoops to go through,” Dr. DiGiorgio said.
Find an agent to help you set up medical malpractice insurance, liability insurance, and worker’s compensation insurance. “Make sure you read all the paperwork, because it can be very intricate,” she said.
Find an accountant. That person can help set up a bookkeeping process.
What equipment and devices will you need? That depends largely on the patient population a physician serves. Dr. DiGiorgio noted that eligible small businesses may take a tax credit of up to $5,000 per year for accommodations made to comply with the ADA. “That’s a nice feature, so that you can purchase ADA compliant items like a larger exam chair and custom reception areas.”
Dr. DiGiorgio reported having no relevant disclosures.
SAN DIEGO – When the Boston-based cosmetic dermatology practice that employed Catherine M. DiGiorgio, MD, MS, was sold to a private equity firm a few years ago, she found herself at a crossroads: Stay and work for a large corporation, or open a solo practice?
She opted to start her own practice in Boston, “because I didn’t want to work for a large corporation, and I want to provide the best care for my patients in a more intimate manner,” Dr. DiGiorgio, a board-certified laser and cosmetic dermatologist, said at the annual Masters of Aesthetics Symposium.
The decision also tested her mettle. “I spoke to several colleagues and friends, and I was terrified,” she said. “I was like: ‘I don’t even know where to start.’ ”
On the heels of opening a new office in a matter of weeks, she offered the following tips and questions to consider when launching a solo dermatology practice:
Select a location. “That’s your first decision,” she said. “Where in the city are you going to open? Are you going to a new city, or are you moving back home? Don’t be afraid to start from scratch, and don’t be afraid to start a [solo] practice if you already have a patient base.”
Will you lease or purchase your space? After she secured a bank loan, Dr. DiGiorgio chose to lease the space for her new practice, “because you can kind of see where things go, get all the kinks out and figure out how to build things in a space that you don’t own. Then, when you’re ready and you have grown, you can invest more into your practice.”
Will you accept insurance? She built her practice around the direct specialty care model, which emphasizes the patient-physician relationship and removes third-party payors. “It’s not a concierge practice, but it’s a transparent, reasonable fee schedule for medical dermatology,” she explained. “I’ve done 100% cosmetics for about 5 years now, [but] I do medical dermatology for a fee. On my website I have a full price list on how much a full skin check is, [and] how much biopsies are. It’s completely transparent. Patients can submit to their insurance for reimbursement, but we don’t guarantee that they’re going to be reimbursed.”
Where will your patients come from? Will you advertise? Do you have physicians in the area who will refer to you if you’re a board-certified dermatologist? She emphasized the importance of “learning how to present yourself” on a website dedicated to your own practice. “Instagram, Facebook, and social media are great, but you don’t own those pages,” noted Dr. DiGiorgio, who served as the program cochair of the 2023 annual meeting of the American Society for Laser Medicine and Surgery and was recently elected to serve on the board of directors for the American Society for Dermatology Surgery. “You don’t own one of those pictures that are posted on your social media page. They can disappear in a second. If that happens, how are people going to find you?”
Are you going to hire more physicians in the future? That will influence the size of the new office and the floor plan.
Lawyer up. Hiring a health care attorney can “help you navigate transitioning from whatever position you’re in to opening up your own practice, as well as setting up the regulatory paperwork necessary for your new practice. You’ll also need a real estate attorney to help once you have selected a place, to help you navigate through that process,” she said, such as figuring out if the elevator in the building meets the Americans With Disabilities Act (ADA) requirements.
Create a mission statement. That way, “you know why you’re doing this, and it stays with you as you’re getting through the hard roadblocks.”
Find an architect, contractor, or designer. “If you’re building out a space from scratch, you’re going to need an architect,” she said. “Along with that architect will come a full-on contracting firm. I ended up hiring everyone individually, because I’m trying to spend as little money as possible.” She also hired a designer to help select furnishings and create the office atmosphere.
Secure a building permit ASAP. “It’s almost better to have the city permit before you sign the lease, because the permits can take a year, and you don’t want to pay rent on an empty space for a year if you don’t have a permit or if there are other hoops to go through,” Dr. DiGiorgio said.
Find an agent to help you set up medical malpractice insurance, liability insurance, and worker’s compensation insurance. “Make sure you read all the paperwork, because it can be very intricate,” she said.
Find an accountant. That person can help set up a bookkeeping process.
What equipment and devices will you need? That depends largely on the patient population a physician serves. Dr. DiGiorgio noted that eligible small businesses may take a tax credit of up to $5,000 per year for accommodations made to comply with the ADA. “That’s a nice feature, so that you can purchase ADA compliant items like a larger exam chair and custom reception areas.”
Dr. DiGiorgio reported having no relevant disclosures.
AT MOAS 2023
FDA to step up oversight of cosmetics, assess ‘forever chemicals’
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
Low-dose oral minoxidil for female pattern hair loss: Benefits, impact on BP, heart rate evaluated
results from a small retrospective analysis showed.
“Additionally, few patients experienced hair loss progression while slightly over a third experienced hair regrowth,” the study’s first author, Reese Imhof, MD, a third-year resident in the department of dermatology at Mayo Clinic, Rochester, Minn., said in an interview. The results were published online in JAAD International.
At low doses, oral minoxidil, approved as an antihypertensive over 40 years ago, has become an increasingly popular treatment for hair loss, particularly since an article about its use for hair loss was published in the New York Times in August 2022. (Oral minoxidil is not approved for treating alopecia, and is used off label for this purpose.)
To evaluate the effects of LDOM in female patients with female pattern hair loss, Dr. Imhof, along with colleagues Beija Villalpando, MD, of the department of medicine and Rochelle R. Torgerson, MD, PhD, of the department of dermatology at the Mayo Clinic, reviewed the records of 25 adult women who were evaluated for female pattern hair loss at the Mayo Clinic over a 5-year period that ended on Nov. 27, 2022. Previous studies have looked at the cardiovascular effects of treatment with oral minoxidil and impact on BP in men, but “few studies have reported on female patients receiving LDOM as monotherapy for female pattern hair loss,” the authors noted.
The mean age of the women in their study was 61 years, and they took LDOM for a mean of 6.2 months. Slightly more than half (52%) took a dose of 1.25 mg daily, while 40% took 2.5 mg daily and 8% took 0.625 mg daily.
Of the 25 patients, 10 (40%) had previously tried topical minoxidil but had discontinued it because of local side effects or challenges with adherence. Also, three patients (12%) had previously tried finasteride and spironolactone but discontinued those medications because of adverse side effects.
The researchers noted disease improvement and hair regrowth was observed in nine patients who were treated with LDOM (36%), while three patients (12%) had “unaltered disease progression.” Adverse side effects observed in the cohort included four patients with facial hypertrichosis (16%) and one patient with fluid retention/lower limb edema (4%).
The patients who developed hypertrichosis did not discontinue LDOM, but the patient who developed edema did stop treatment.
At baseline, systolic BP (SBP) ranged from 107-161 mm Hg, diastolic BP (DBP) ranged from 58-88 mm Hg, and heart rate ranged from 54-114 beats per minute. Post treatment, SBP ranged from 102-152 mm Hg, DBP ranged from 63-90 mm Hg, and heart rate ranged from 56 to 105 bpm. “It was surprising how little ambulatory blood pressure and heart rate changed after an average of 6 months of treatment,” Dr. Imhof said in an interview. “On average, SBP decreased by 2.8 mm HG while DBP decreased by 1.4 mm Hg. Heart rate increased an average of 4.4 beats per minute.”
He acknowledged certain limitations of the study, including its small sample size and lack of inclusion of patients who were being treated for hypertension with concomitant antihypertensive medications. “Some unique aspects of our study are that we focused on women, and we had a slightly older cohort than prior studies (61 years old on average) as well as exposure to higher doses of LDOM, with most patients on either 1.25 mg daily or 2.5 mg daily,” Dr. Imhof said.
The researchers reported having no relevant disclosures, and there was no funding source for the study.
results from a small retrospective analysis showed.
“Additionally, few patients experienced hair loss progression while slightly over a third experienced hair regrowth,” the study’s first author, Reese Imhof, MD, a third-year resident in the department of dermatology at Mayo Clinic, Rochester, Minn., said in an interview. The results were published online in JAAD International.
At low doses, oral minoxidil, approved as an antihypertensive over 40 years ago, has become an increasingly popular treatment for hair loss, particularly since an article about its use for hair loss was published in the New York Times in August 2022. (Oral minoxidil is not approved for treating alopecia, and is used off label for this purpose.)
To evaluate the effects of LDOM in female patients with female pattern hair loss, Dr. Imhof, along with colleagues Beija Villalpando, MD, of the department of medicine and Rochelle R. Torgerson, MD, PhD, of the department of dermatology at the Mayo Clinic, reviewed the records of 25 adult women who were evaluated for female pattern hair loss at the Mayo Clinic over a 5-year period that ended on Nov. 27, 2022. Previous studies have looked at the cardiovascular effects of treatment with oral minoxidil and impact on BP in men, but “few studies have reported on female patients receiving LDOM as monotherapy for female pattern hair loss,” the authors noted.
The mean age of the women in their study was 61 years, and they took LDOM for a mean of 6.2 months. Slightly more than half (52%) took a dose of 1.25 mg daily, while 40% took 2.5 mg daily and 8% took 0.625 mg daily.
Of the 25 patients, 10 (40%) had previously tried topical minoxidil but had discontinued it because of local side effects or challenges with adherence. Also, three patients (12%) had previously tried finasteride and spironolactone but discontinued those medications because of adverse side effects.
The researchers noted disease improvement and hair regrowth was observed in nine patients who were treated with LDOM (36%), while three patients (12%) had “unaltered disease progression.” Adverse side effects observed in the cohort included four patients with facial hypertrichosis (16%) and one patient with fluid retention/lower limb edema (4%).
The patients who developed hypertrichosis did not discontinue LDOM, but the patient who developed edema did stop treatment.
At baseline, systolic BP (SBP) ranged from 107-161 mm Hg, diastolic BP (DBP) ranged from 58-88 mm Hg, and heart rate ranged from 54-114 beats per minute. Post treatment, SBP ranged from 102-152 mm Hg, DBP ranged from 63-90 mm Hg, and heart rate ranged from 56 to 105 bpm. “It was surprising how little ambulatory blood pressure and heart rate changed after an average of 6 months of treatment,” Dr. Imhof said in an interview. “On average, SBP decreased by 2.8 mm HG while DBP decreased by 1.4 mm Hg. Heart rate increased an average of 4.4 beats per minute.”
He acknowledged certain limitations of the study, including its small sample size and lack of inclusion of patients who were being treated for hypertension with concomitant antihypertensive medications. “Some unique aspects of our study are that we focused on women, and we had a slightly older cohort than prior studies (61 years old on average) as well as exposure to higher doses of LDOM, with most patients on either 1.25 mg daily or 2.5 mg daily,” Dr. Imhof said.
The researchers reported having no relevant disclosures, and there was no funding source for the study.
results from a small retrospective analysis showed.
“Additionally, few patients experienced hair loss progression while slightly over a third experienced hair regrowth,” the study’s first author, Reese Imhof, MD, a third-year resident in the department of dermatology at Mayo Clinic, Rochester, Minn., said in an interview. The results were published online in JAAD International.
At low doses, oral minoxidil, approved as an antihypertensive over 40 years ago, has become an increasingly popular treatment for hair loss, particularly since an article about its use for hair loss was published in the New York Times in August 2022. (Oral minoxidil is not approved for treating alopecia, and is used off label for this purpose.)
To evaluate the effects of LDOM in female patients with female pattern hair loss, Dr. Imhof, along with colleagues Beija Villalpando, MD, of the department of medicine and Rochelle R. Torgerson, MD, PhD, of the department of dermatology at the Mayo Clinic, reviewed the records of 25 adult women who were evaluated for female pattern hair loss at the Mayo Clinic over a 5-year period that ended on Nov. 27, 2022. Previous studies have looked at the cardiovascular effects of treatment with oral minoxidil and impact on BP in men, but “few studies have reported on female patients receiving LDOM as monotherapy for female pattern hair loss,” the authors noted.
The mean age of the women in their study was 61 years, and they took LDOM for a mean of 6.2 months. Slightly more than half (52%) took a dose of 1.25 mg daily, while 40% took 2.5 mg daily and 8% took 0.625 mg daily.
Of the 25 patients, 10 (40%) had previously tried topical minoxidil but had discontinued it because of local side effects or challenges with adherence. Also, three patients (12%) had previously tried finasteride and spironolactone but discontinued those medications because of adverse side effects.
The researchers noted disease improvement and hair regrowth was observed in nine patients who were treated with LDOM (36%), while three patients (12%) had “unaltered disease progression.” Adverse side effects observed in the cohort included four patients with facial hypertrichosis (16%) and one patient with fluid retention/lower limb edema (4%).
The patients who developed hypertrichosis did not discontinue LDOM, but the patient who developed edema did stop treatment.
At baseline, systolic BP (SBP) ranged from 107-161 mm Hg, diastolic BP (DBP) ranged from 58-88 mm Hg, and heart rate ranged from 54-114 beats per minute. Post treatment, SBP ranged from 102-152 mm Hg, DBP ranged from 63-90 mm Hg, and heart rate ranged from 56 to 105 bpm. “It was surprising how little ambulatory blood pressure and heart rate changed after an average of 6 months of treatment,” Dr. Imhof said in an interview. “On average, SBP decreased by 2.8 mm HG while DBP decreased by 1.4 mm Hg. Heart rate increased an average of 4.4 beats per minute.”
He acknowledged certain limitations of the study, including its small sample size and lack of inclusion of patients who were being treated for hypertension with concomitant antihypertensive medications. “Some unique aspects of our study are that we focused on women, and we had a slightly older cohort than prior studies (61 years old on average) as well as exposure to higher doses of LDOM, with most patients on either 1.25 mg daily or 2.5 mg daily,” Dr. Imhof said.
The researchers reported having no relevant disclosures, and there was no funding source for the study.
FROM JAAD INTERNATIONAL
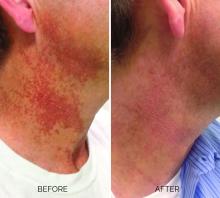
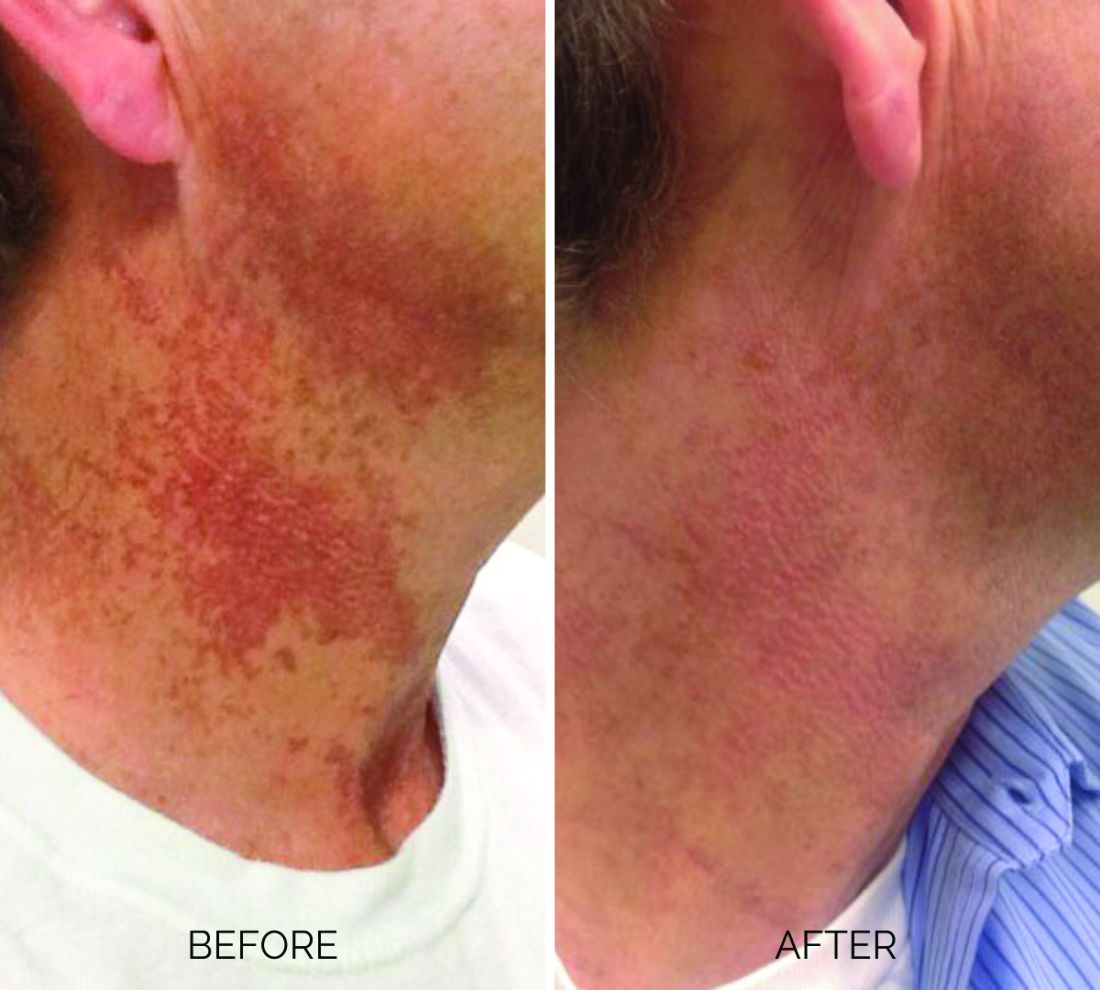
Treating poikiloderma
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
Black women weigh emerging risks of ‘creamy crack’ hair straighteners
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Weekend Botox training: Shortcut to cash or risky business?
This transcript has been edited for clarity.
Dr. Patel: A friend recently joked with me and said, “I wish you were a dermatologist so you could hook me up with Botox and fillers.” Well, little does this friend know that I could be a certified cosmetic injector just after a weekend course. Botox parties, here I come?
I can’t blame any health care professional for having a side hustle. People are burned out, want to supplement their income, or scale back clinical hours. According to one Medscape survey, almost 40% of physicians do have some form of a side hustle, whether it is consulting, speaking engagements, being an expert witness, or moonlighting. I know plenty of doctors and nurses who have taken on Botox injecting as a way to make some extra cash.
Now, going back to me and smoothing out wrinkles. I’m a pediatric hospitalist. I’ve never injected an aesthetic product in anyone’s face. When it comes to sharp objects and faces, I’ve sewn lacerations and drained abscesses. In my world, when we talk about botulinum toxin, we’re usually talking about botulism or the therapeutic treatment of migraines and muscle spasms – pathology.
The National Laser Institute has a 2-day Botox and dermal filler training. “Our 2-day Botox and filler course will also teach you how to build a practice and capitalize on the enormous Botox and dermal filler market that exists in the United States.” That’s a lot to cover in 2 days. They even have lunch breaks.
Just from a quick search, I even found an online video course for $1,500. For an additional fee, you can have a live, hands-on component. There are so many trainings out there, including one that’s only 8 hours long, offered by Empire Medical. I also went and spoke with an employee at Empire Medical who told me that because I’m an MD, if I do the course, I can use my certificate and go directly to a manufacturer, buy Botox, and start injecting right away.
Now, is this training actually sufficient for me to go and get good results while minimizing adverse effects like brow ptosis, dry eyes, and asymmetry? I have no idea. According to a review from the Journal of Cosmetic Dermatology, it’s crucial to understand anatomic landmarks, muscle function, baseline asymmetry, potential migration of the toxin, and site-specific precautions.
Okay, that sounds really intimidating, but people still do it. I saw a Business Insider article about a hospitalist who took a 2-day Botox course and then, to her credit, she trained under supervision for an additional 6 months. She then started hosting Botox parties and each time was making $3,500 to upwards of $20,000.
Let’s do some quick mental math. If I were to go online and buy Botox for $3-$6 a unit and then charge patients $15 a unit, and then I consider that in areas like the forehead or in between the eyes – I read that could take 25-50 units – and I repeat this for multiple patients, I can make a few thousand dollars. Well, I may have to adjust my prices according to the market, obviously, because I did see some Groupons advertising $10 per unit.
Who can get in on some Botox cosmetic cash action? Well, physicians can right away. For other health care professionals, it depends on the state. For example, in California, dentists cannot get Botox solely for cosmetic purposes, whereas in Arizona, they can. Generally speaking, NPs and PAs require some type of physician oversight or supervision, but again, it depends on the state.
Oh, and fun fact: Connecticut outright banned Botox parties and said that Botox must be performed “in a medical spa or licensed health care facility and by a Connecticut-licensed health care provider within his or her scope of practice.”
It definitely worries me that someone could go online or go overseas, buy Botox, claim to be a health care professional, and literally commit fraud. I found stories out there such as a couple in San Jose who are giving out Botox from their home without a license. They got arrested. Also, a woman in Alabama who lied about being a licensed dermatologist and did the same, or another woman in Los Angeles who got arrested after selling counterfeit Botox to undercover law enforcement. Surely, there are plenty more cases out there like this.
I asked Dr. Jacqueline Watchmaker, a board-certified dermatologist at U.S. Dermatology Partners in Arizona who has an expertise in cosmetic procedures, what she thought about the booming med spa industry and what, if any, regulatory changes she wanted to see.
Jacqueline Watchmaker, MD: I do think the fact that people can just go to a 1- or 2-day injection course and inject filler and Botox is concerning. I think the lack of regulation surrounding this topic is also very concerning.
There’s so much that goes into being a skilled injector. It’s an intricate knowledge of facial anatomy, which takes weeks, if not months, to really master. There’s actually injection technique, which can be very complex depending on the part of the face that you’re injecting. Even more important, it’s how to prevent complications, but also how to deal with complications if they do occur. There’s no way that these weekend injection courses are able to cover those topics in a thorough and satisfactory manner.
I see complications from med spas all the time, and I think it’s people going to injectors who are not skilled. They don’t know their anatomy, they don’t know the appropriate filler to use, and then heaven forbid there is a complication, they don’t know how to manage the complication – and then those patients get sent to me.
I think patients sometimes forget that these cosmetic procedures are true medical procedures. You need sterile technique. Again, you need to know the anatomy. It can look easy on social media, but there’s a large amount of thought behind it. I think there needs to be more regulation around this topic.
Dr. Patel: In one study, out of 400 people who received a cosmetic procedure, 50 reported an adverse event, such as discoloration or burns, and these adverse events were more likely to occur if a nonphysician was doing the procedure. Granted, this was a small study. You can’t make a generalization out of it, but this does add to the argument that there needs to be more regulation and oversight.
Let’s be real. The cosmetic injection side hustle is alive and well, but I’m good. I’m not going there. Maybe there should be some more quality control. At Botox parties, do people even ask if their injectors are certified or where they bought their vials?
You might be thinking that this isn’t a big deal because it’s just Botox. Let me ask you all a question: If you or your family member were going to go get Botox or another cosmetic injection, would it still not be a big deal?
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He reported conflicts of interest with Medumo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dr. Patel: A friend recently joked with me and said, “I wish you were a dermatologist so you could hook me up with Botox and fillers.” Well, little does this friend know that I could be a certified cosmetic injector just after a weekend course. Botox parties, here I come?
I can’t blame any health care professional for having a side hustle. People are burned out, want to supplement their income, or scale back clinical hours. According to one Medscape survey, almost 40% of physicians do have some form of a side hustle, whether it is consulting, speaking engagements, being an expert witness, or moonlighting. I know plenty of doctors and nurses who have taken on Botox injecting as a way to make some extra cash.
Now, going back to me and smoothing out wrinkles. I’m a pediatric hospitalist. I’ve never injected an aesthetic product in anyone’s face. When it comes to sharp objects and faces, I’ve sewn lacerations and drained abscesses. In my world, when we talk about botulinum toxin, we’re usually talking about botulism or the therapeutic treatment of migraines and muscle spasms – pathology.
The National Laser Institute has a 2-day Botox and dermal filler training. “Our 2-day Botox and filler course will also teach you how to build a practice and capitalize on the enormous Botox and dermal filler market that exists in the United States.” That’s a lot to cover in 2 days. They even have lunch breaks.
Just from a quick search, I even found an online video course for $1,500. For an additional fee, you can have a live, hands-on component. There are so many trainings out there, including one that’s only 8 hours long, offered by Empire Medical. I also went and spoke with an employee at Empire Medical who told me that because I’m an MD, if I do the course, I can use my certificate and go directly to a manufacturer, buy Botox, and start injecting right away.
Now, is this training actually sufficient for me to go and get good results while minimizing adverse effects like brow ptosis, dry eyes, and asymmetry? I have no idea. According to a review from the Journal of Cosmetic Dermatology, it’s crucial to understand anatomic landmarks, muscle function, baseline asymmetry, potential migration of the toxin, and site-specific precautions.
Okay, that sounds really intimidating, but people still do it. I saw a Business Insider article about a hospitalist who took a 2-day Botox course and then, to her credit, she trained under supervision for an additional 6 months. She then started hosting Botox parties and each time was making $3,500 to upwards of $20,000.
Let’s do some quick mental math. If I were to go online and buy Botox for $3-$6 a unit and then charge patients $15 a unit, and then I consider that in areas like the forehead or in between the eyes – I read that could take 25-50 units – and I repeat this for multiple patients, I can make a few thousand dollars. Well, I may have to adjust my prices according to the market, obviously, because I did see some Groupons advertising $10 per unit.
Who can get in on some Botox cosmetic cash action? Well, physicians can right away. For other health care professionals, it depends on the state. For example, in California, dentists cannot get Botox solely for cosmetic purposes, whereas in Arizona, they can. Generally speaking, NPs and PAs require some type of physician oversight or supervision, but again, it depends on the state.
Oh, and fun fact: Connecticut outright banned Botox parties and said that Botox must be performed “in a medical spa or licensed health care facility and by a Connecticut-licensed health care provider within his or her scope of practice.”
It definitely worries me that someone could go online or go overseas, buy Botox, claim to be a health care professional, and literally commit fraud. I found stories out there such as a couple in San Jose who are giving out Botox from their home without a license. They got arrested. Also, a woman in Alabama who lied about being a licensed dermatologist and did the same, or another woman in Los Angeles who got arrested after selling counterfeit Botox to undercover law enforcement. Surely, there are plenty more cases out there like this.
I asked Dr. Jacqueline Watchmaker, a board-certified dermatologist at U.S. Dermatology Partners in Arizona who has an expertise in cosmetic procedures, what she thought about the booming med spa industry and what, if any, regulatory changes she wanted to see.
Jacqueline Watchmaker, MD: I do think the fact that people can just go to a 1- or 2-day injection course and inject filler and Botox is concerning. I think the lack of regulation surrounding this topic is also very concerning.
There’s so much that goes into being a skilled injector. It’s an intricate knowledge of facial anatomy, which takes weeks, if not months, to really master. There’s actually injection technique, which can be very complex depending on the part of the face that you’re injecting. Even more important, it’s how to prevent complications, but also how to deal with complications if they do occur. There’s no way that these weekend injection courses are able to cover those topics in a thorough and satisfactory manner.
I see complications from med spas all the time, and I think it’s people going to injectors who are not skilled. They don’t know their anatomy, they don’t know the appropriate filler to use, and then heaven forbid there is a complication, they don’t know how to manage the complication – and then those patients get sent to me.
I think patients sometimes forget that these cosmetic procedures are true medical procedures. You need sterile technique. Again, you need to know the anatomy. It can look easy on social media, but there’s a large amount of thought behind it. I think there needs to be more regulation around this topic.
Dr. Patel: In one study, out of 400 people who received a cosmetic procedure, 50 reported an adverse event, such as discoloration or burns, and these adverse events were more likely to occur if a nonphysician was doing the procedure. Granted, this was a small study. You can’t make a generalization out of it, but this does add to the argument that there needs to be more regulation and oversight.
Let’s be real. The cosmetic injection side hustle is alive and well, but I’m good. I’m not going there. Maybe there should be some more quality control. At Botox parties, do people even ask if their injectors are certified or where they bought their vials?
You might be thinking that this isn’t a big deal because it’s just Botox. Let me ask you all a question: If you or your family member were going to go get Botox or another cosmetic injection, would it still not be a big deal?
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He reported conflicts of interest with Medumo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dr. Patel: A friend recently joked with me and said, “I wish you were a dermatologist so you could hook me up with Botox and fillers.” Well, little does this friend know that I could be a certified cosmetic injector just after a weekend course. Botox parties, here I come?
I can’t blame any health care professional for having a side hustle. People are burned out, want to supplement their income, or scale back clinical hours. According to one Medscape survey, almost 40% of physicians do have some form of a side hustle, whether it is consulting, speaking engagements, being an expert witness, or moonlighting. I know plenty of doctors and nurses who have taken on Botox injecting as a way to make some extra cash.
Now, going back to me and smoothing out wrinkles. I’m a pediatric hospitalist. I’ve never injected an aesthetic product in anyone’s face. When it comes to sharp objects and faces, I’ve sewn lacerations and drained abscesses. In my world, when we talk about botulinum toxin, we’re usually talking about botulism or the therapeutic treatment of migraines and muscle spasms – pathology.
The National Laser Institute has a 2-day Botox and dermal filler training. “Our 2-day Botox and filler course will also teach you how to build a practice and capitalize on the enormous Botox and dermal filler market that exists in the United States.” That’s a lot to cover in 2 days. They even have lunch breaks.
Just from a quick search, I even found an online video course for $1,500. For an additional fee, you can have a live, hands-on component. There are so many trainings out there, including one that’s only 8 hours long, offered by Empire Medical. I also went and spoke with an employee at Empire Medical who told me that because I’m an MD, if I do the course, I can use my certificate and go directly to a manufacturer, buy Botox, and start injecting right away.
Now, is this training actually sufficient for me to go and get good results while minimizing adverse effects like brow ptosis, dry eyes, and asymmetry? I have no idea. According to a review from the Journal of Cosmetic Dermatology, it’s crucial to understand anatomic landmarks, muscle function, baseline asymmetry, potential migration of the toxin, and site-specific precautions.
Okay, that sounds really intimidating, but people still do it. I saw a Business Insider article about a hospitalist who took a 2-day Botox course and then, to her credit, she trained under supervision for an additional 6 months. She then started hosting Botox parties and each time was making $3,500 to upwards of $20,000.
Let’s do some quick mental math. If I were to go online and buy Botox for $3-$6 a unit and then charge patients $15 a unit, and then I consider that in areas like the forehead or in between the eyes – I read that could take 25-50 units – and I repeat this for multiple patients, I can make a few thousand dollars. Well, I may have to adjust my prices according to the market, obviously, because I did see some Groupons advertising $10 per unit.
Who can get in on some Botox cosmetic cash action? Well, physicians can right away. For other health care professionals, it depends on the state. For example, in California, dentists cannot get Botox solely for cosmetic purposes, whereas in Arizona, they can. Generally speaking, NPs and PAs require some type of physician oversight or supervision, but again, it depends on the state.
Oh, and fun fact: Connecticut outright banned Botox parties and said that Botox must be performed “in a medical spa or licensed health care facility and by a Connecticut-licensed health care provider within his or her scope of practice.”
It definitely worries me that someone could go online or go overseas, buy Botox, claim to be a health care professional, and literally commit fraud. I found stories out there such as a couple in San Jose who are giving out Botox from their home without a license. They got arrested. Also, a woman in Alabama who lied about being a licensed dermatologist and did the same, or another woman in Los Angeles who got arrested after selling counterfeit Botox to undercover law enforcement. Surely, there are plenty more cases out there like this.
I asked Dr. Jacqueline Watchmaker, a board-certified dermatologist at U.S. Dermatology Partners in Arizona who has an expertise in cosmetic procedures, what she thought about the booming med spa industry and what, if any, regulatory changes she wanted to see.
Jacqueline Watchmaker, MD: I do think the fact that people can just go to a 1- or 2-day injection course and inject filler and Botox is concerning. I think the lack of regulation surrounding this topic is also very concerning.
There’s so much that goes into being a skilled injector. It’s an intricate knowledge of facial anatomy, which takes weeks, if not months, to really master. There’s actually injection technique, which can be very complex depending on the part of the face that you’re injecting. Even more important, it’s how to prevent complications, but also how to deal with complications if they do occur. There’s no way that these weekend injection courses are able to cover those topics in a thorough and satisfactory manner.
I see complications from med spas all the time, and I think it’s people going to injectors who are not skilled. They don’t know their anatomy, they don’t know the appropriate filler to use, and then heaven forbid there is a complication, they don’t know how to manage the complication – and then those patients get sent to me.
I think patients sometimes forget that these cosmetic procedures are true medical procedures. You need sterile technique. Again, you need to know the anatomy. It can look easy on social media, but there’s a large amount of thought behind it. I think there needs to be more regulation around this topic.
Dr. Patel: In one study, out of 400 people who received a cosmetic procedure, 50 reported an adverse event, such as discoloration or burns, and these adverse events were more likely to occur if a nonphysician was doing the procedure. Granted, this was a small study. You can’t make a generalization out of it, but this does add to the argument that there needs to be more regulation and oversight.
Let’s be real. The cosmetic injection side hustle is alive and well, but I’m good. I’m not going there. Maybe there should be some more quality control. At Botox parties, do people even ask if their injectors are certified or where they bought their vials?
You might be thinking that this isn’t a big deal because it’s just Botox. Let me ask you all a question: If you or your family member were going to go get Botox or another cosmetic injection, would it still not be a big deal?
Dr. Patel is a pediatric hospitalist, television producer, media contributor, and digital health enthusiast. He splits his time between New York City and San Francisco, as he is on faculty at Columbia University/Morgan Stanley Children’s Hospital and UCSF Benioff Children’s Hospital. He reported conflicts of interest with Medumo.
A version of this article first appeared on Medscape.com.
Methemoglobinemia Induced by Application of an Anesthetic Cream
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.
After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.

After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.

After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
Practice Points
- Consideration should be given to patients applying anesthetic creams to areas with high absorption capacity.
- Dermatologists should be aware of methemoglobinemia as a serious adverse effect of local anesthetics and guide patients accordingly, as patients do not tend to consider these products to be drugs.
FDA adds safety-related information to its dermal filler webpage
On July 6, the .
Along with a list of common reactions such as bruising, redness, swelling, and pain, the webpage now includes language to inform the public and health care providers about reports of delayed-onset inflammation that have been reported to occur near the dermal filler treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. According to an FDA spokesperson, the update is based on several sources of information, including postmarketing data from adverse event–reporting databases, such as the Manufacturer and User Facility Device Experience (MAUDE) for devices and the Vaccine Adverse Event Reporting System (VAERS) for vaccines, published literature, and recommendations from federal agencies and professional societies.
“More specifically, the site was updated to include certain risks of using dermal fillers such as swelling and bruising as well as some less common risks such as inflammation – swelling or redness near the dermal filler injection site – following viral or bacterial illnesses or infections, vaccinations, or dental procedures,” the spokesperson said.
The announcement about the update also states that “typically, the reported inflammation is responsive to treatment or resolves on its own.”
Other less common risks from dermal filler use listed on the website include bumps in or under the skin (nodules or granulomas) that may need to be treated with injections, oral antibiotics, or surgical removal; infection; open or draining wounds; a sore at the injection site; allergic reactions; or necrosis.
Meanwhile, rare risks from dermal filler use that have been reported to the FDA include severe allergic reactions (anaphylactic shock) that require immediate emergency medical assistance; migration (movement of filler material from the site of injection); leakage or rupture of the filler material at the injection site or through the skin (which may result from a tissue reaction or an infection); the formation of permanent hard nodules; and injury to the blood supply after an unintentional injection into a blood vessel, resulting in necrosis, vision abnormalities (including blindness), or stroke.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment about the FDA update on dermal fillers, said that the agency “is doing its job by making consumers aware of all possible complications [common and uncommon], as it does when it creates a package insert for a medication. Fortunately, however, comprehensive reviews published in the peer-reviewed dermatology literature show delayed inflammation to be a very rare event. So, while it is important for dermatologists during informed consent – prior to filler – to discuss that redness and/or nodules after infection/vaccinations, etc. are possible, it is important to add that based on the data, they are also highly unlikely.”
Sue Ellen Cox, MD, a dermatologist who practices in Chapel Hill, N.C., said that she was glad to see separate sections of recommendations geared to patients and health care providers. For example, the website recommends that patients seek a physician in the field of dermatology or plastic surgery to perform procedures that use dermal fillers. “These are not procedures to be done in an unsupervised spa setting,” said Dr. Cox, a past president of the American Society for Dermatologic Surgery and one of the task force authors of recommendations on preventing and treating adverse events of injectable fillers.
“It also makes the point of using products that are acquired from FDA-approved manufacturers, not products sold online or bootlegged from other countries. Finally, it goes into detail about the importance of in-depth knowledge of anatomy, which is crucial for safe injections and reviews potential complications such as intravascular events and hypersensitivity reactions. The administering physician should have extensive knowledge regarding how to treat any potential problems that arise.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies. Dr. Cox disclosed that she has been a clinical investigator for many injectable companies including AbbVie, Galderma, Revance, and Chroma.
Health care professionals, patients, and others can report adverse events related to dermal fillers and other medical devices to the FDA at 800-FDA-1088 or on the MAUDE website.
On July 6, the .
Along with a list of common reactions such as bruising, redness, swelling, and pain, the webpage now includes language to inform the public and health care providers about reports of delayed-onset inflammation that have been reported to occur near the dermal filler treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. According to an FDA spokesperson, the update is based on several sources of information, including postmarketing data from adverse event–reporting databases, such as the Manufacturer and User Facility Device Experience (MAUDE) for devices and the Vaccine Adverse Event Reporting System (VAERS) for vaccines, published literature, and recommendations from federal agencies and professional societies.
“More specifically, the site was updated to include certain risks of using dermal fillers such as swelling and bruising as well as some less common risks such as inflammation – swelling or redness near the dermal filler injection site – following viral or bacterial illnesses or infections, vaccinations, or dental procedures,” the spokesperson said.
The announcement about the update also states that “typically, the reported inflammation is responsive to treatment or resolves on its own.”
Other less common risks from dermal filler use listed on the website include bumps in or under the skin (nodules or granulomas) that may need to be treated with injections, oral antibiotics, or surgical removal; infection; open or draining wounds; a sore at the injection site; allergic reactions; or necrosis.
Meanwhile, rare risks from dermal filler use that have been reported to the FDA include severe allergic reactions (anaphylactic shock) that require immediate emergency medical assistance; migration (movement of filler material from the site of injection); leakage or rupture of the filler material at the injection site or through the skin (which may result from a tissue reaction or an infection); the formation of permanent hard nodules; and injury to the blood supply after an unintentional injection into a blood vessel, resulting in necrosis, vision abnormalities (including blindness), or stroke.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment about the FDA update on dermal fillers, said that the agency “is doing its job by making consumers aware of all possible complications [common and uncommon], as it does when it creates a package insert for a medication. Fortunately, however, comprehensive reviews published in the peer-reviewed dermatology literature show delayed inflammation to be a very rare event. So, while it is important for dermatologists during informed consent – prior to filler – to discuss that redness and/or nodules after infection/vaccinations, etc. are possible, it is important to add that based on the data, they are also highly unlikely.”
Sue Ellen Cox, MD, a dermatologist who practices in Chapel Hill, N.C., said that she was glad to see separate sections of recommendations geared to patients and health care providers. For example, the website recommends that patients seek a physician in the field of dermatology or plastic surgery to perform procedures that use dermal fillers. “These are not procedures to be done in an unsupervised spa setting,” said Dr. Cox, a past president of the American Society for Dermatologic Surgery and one of the task force authors of recommendations on preventing and treating adverse events of injectable fillers.
“It also makes the point of using products that are acquired from FDA-approved manufacturers, not products sold online or bootlegged from other countries. Finally, it goes into detail about the importance of in-depth knowledge of anatomy, which is crucial for safe injections and reviews potential complications such as intravascular events and hypersensitivity reactions. The administering physician should have extensive knowledge regarding how to treat any potential problems that arise.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies. Dr. Cox disclosed that she has been a clinical investigator for many injectable companies including AbbVie, Galderma, Revance, and Chroma.
Health care professionals, patients, and others can report adverse events related to dermal fillers and other medical devices to the FDA at 800-FDA-1088 or on the MAUDE website.
On July 6, the .
Along with a list of common reactions such as bruising, redness, swelling, and pain, the webpage now includes language to inform the public and health care providers about reports of delayed-onset inflammation that have been reported to occur near the dermal filler treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. According to an FDA spokesperson, the update is based on several sources of information, including postmarketing data from adverse event–reporting databases, such as the Manufacturer and User Facility Device Experience (MAUDE) for devices and the Vaccine Adverse Event Reporting System (VAERS) for vaccines, published literature, and recommendations from federal agencies and professional societies.
“More specifically, the site was updated to include certain risks of using dermal fillers such as swelling and bruising as well as some less common risks such as inflammation – swelling or redness near the dermal filler injection site – following viral or bacterial illnesses or infections, vaccinations, or dental procedures,” the spokesperson said.
The announcement about the update also states that “typically, the reported inflammation is responsive to treatment or resolves on its own.”
Other less common risks from dermal filler use listed on the website include bumps in or under the skin (nodules or granulomas) that may need to be treated with injections, oral antibiotics, or surgical removal; infection; open or draining wounds; a sore at the injection site; allergic reactions; or necrosis.
Meanwhile, rare risks from dermal filler use that have been reported to the FDA include severe allergic reactions (anaphylactic shock) that require immediate emergency medical assistance; migration (movement of filler material from the site of injection); leakage or rupture of the filler material at the injection site or through the skin (which may result from a tissue reaction or an infection); the formation of permanent hard nodules; and injury to the blood supply after an unintentional injection into a blood vessel, resulting in necrosis, vision abnormalities (including blindness), or stroke.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment about the FDA update on dermal fillers, said that the agency “is doing its job by making consumers aware of all possible complications [common and uncommon], as it does when it creates a package insert for a medication. Fortunately, however, comprehensive reviews published in the peer-reviewed dermatology literature show delayed inflammation to be a very rare event. So, while it is important for dermatologists during informed consent – prior to filler – to discuss that redness and/or nodules after infection/vaccinations, etc. are possible, it is important to add that based on the data, they are also highly unlikely.”
Sue Ellen Cox, MD, a dermatologist who practices in Chapel Hill, N.C., said that she was glad to see separate sections of recommendations geared to patients and health care providers. For example, the website recommends that patients seek a physician in the field of dermatology or plastic surgery to perform procedures that use dermal fillers. “These are not procedures to be done in an unsupervised spa setting,” said Dr. Cox, a past president of the American Society for Dermatologic Surgery and one of the task force authors of recommendations on preventing and treating adverse events of injectable fillers.
“It also makes the point of using products that are acquired from FDA-approved manufacturers, not products sold online or bootlegged from other countries. Finally, it goes into detail about the importance of in-depth knowledge of anatomy, which is crucial for safe injections and reviews potential complications such as intravascular events and hypersensitivity reactions. The administering physician should have extensive knowledge regarding how to treat any potential problems that arise.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies. Dr. Cox disclosed that she has been a clinical investigator for many injectable companies including AbbVie, Galderma, Revance, and Chroma.
Health care professionals, patients, and others can report adverse events related to dermal fillers and other medical devices to the FDA at 800-FDA-1088 or on the MAUDE website.
Hairy moles may contain the cure for baldness: Study
.
The researchers found that a specific molecule in those hairy moles “causes normally dormant and diminutive hair follicles to activate their stem cells for robust growth of long and thick hairs,” lead researcher Maksim Plikus, PhD, professor of developmental and cell biology at the University of California, Irvine, said in a statement.
The findings could lead to new treatments for the hair loss condition known as androgenetic alopecia, which researchers said occurs in both men and women. It is also known as male-pattern baldness in men.
The global team led by researchers at the university analyzed hair follicle stem cells and discovered that a molecule called osteopontin drives accelerated hair growth. Stem cells can develop into different kinds of cells, whether they are in the body or in a laboratory, and are often involved in regenerative or repair processes, according to the Mayo Clinic.
This latest study, published in the journal Nature, was done on mice. A drug company cofounded by Dr. Plikus said in a news release that it had further tested the hair growth technique on human hair follicles, and “the researchers were able to induce new growth by human hair follicles in a robust preclinical model.” The company, Amplifica, said in the release that it has an exclusive licensing agreement with the university for the new hair growth “inventions” described in the newly published findings.
Hair loss from androgenetic alopecia occurs in two out of every three men, according to the Cleveland Clinic. Amplifica said the condition affects an estimated 50 million men and 30 million women in the United States.
The hair loss and thinning can begin as early as the late teens, the Cleveland Clinic says. The condition is progressive and can follow a specific pattern, such as the hairline creating an “M” or “U” shape midway through the process toward complete baldness on the top of the head, with a remaining thin band of hair around the sides of the head.
A version of this article first appeared on WebMD.com.
.
The researchers found that a specific molecule in those hairy moles “causes normally dormant and diminutive hair follicles to activate their stem cells for robust growth of long and thick hairs,” lead researcher Maksim Plikus, PhD, professor of developmental and cell biology at the University of California, Irvine, said in a statement.
The findings could lead to new treatments for the hair loss condition known as androgenetic alopecia, which researchers said occurs in both men and women. It is also known as male-pattern baldness in men.
The global team led by researchers at the university analyzed hair follicle stem cells and discovered that a molecule called osteopontin drives accelerated hair growth. Stem cells can develop into different kinds of cells, whether they are in the body or in a laboratory, and are often involved in regenerative or repair processes, according to the Mayo Clinic.
This latest study, published in the journal Nature, was done on mice. A drug company cofounded by Dr. Plikus said in a news release that it had further tested the hair growth technique on human hair follicles, and “the researchers were able to induce new growth by human hair follicles in a robust preclinical model.” The company, Amplifica, said in the release that it has an exclusive licensing agreement with the university for the new hair growth “inventions” described in the newly published findings.
Hair loss from androgenetic alopecia occurs in two out of every three men, according to the Cleveland Clinic. Amplifica said the condition affects an estimated 50 million men and 30 million women in the United States.
The hair loss and thinning can begin as early as the late teens, the Cleveland Clinic says. The condition is progressive and can follow a specific pattern, such as the hairline creating an “M” or “U” shape midway through the process toward complete baldness on the top of the head, with a remaining thin band of hair around the sides of the head.
A version of this article first appeared on WebMD.com.
.
The researchers found that a specific molecule in those hairy moles “causes normally dormant and diminutive hair follicles to activate their stem cells for robust growth of long and thick hairs,” lead researcher Maksim Plikus, PhD, professor of developmental and cell biology at the University of California, Irvine, said in a statement.
The findings could lead to new treatments for the hair loss condition known as androgenetic alopecia, which researchers said occurs in both men and women. It is also known as male-pattern baldness in men.
The global team led by researchers at the university analyzed hair follicle stem cells and discovered that a molecule called osteopontin drives accelerated hair growth. Stem cells can develop into different kinds of cells, whether they are in the body or in a laboratory, and are often involved in regenerative or repair processes, according to the Mayo Clinic.
This latest study, published in the journal Nature, was done on mice. A drug company cofounded by Dr. Plikus said in a news release that it had further tested the hair growth technique on human hair follicles, and “the researchers were able to induce new growth by human hair follicles in a robust preclinical model.” The company, Amplifica, said in the release that it has an exclusive licensing agreement with the university for the new hair growth “inventions” described in the newly published findings.
Hair loss from androgenetic alopecia occurs in two out of every three men, according to the Cleveland Clinic. Amplifica said the condition affects an estimated 50 million men and 30 million women in the United States.
The hair loss and thinning can begin as early as the late teens, the Cleveland Clinic says. The condition is progressive and can follow a specific pattern, such as the hairline creating an “M” or “U” shape midway through the process toward complete baldness on the top of the head, with a remaining thin band of hair around the sides of the head.
A version of this article first appeared on WebMD.com.
FROM NATURE
Macular dermal hyperpigmentation: Treatment tips from an expert
CHICAGO – based on cases she has treated in her practice.
Heather Woolery-Lloyd, MD, director of the skin of color division in the dermatology department at University of Miami, provided three general pointers.
- When in doubt, biopsy.
- For inflammatory disorders, always treat the inflammation in addition to the hyperpigmentation.
- Avoid long-term hydroquinone use in these patients.
Dr. Woolery-Lloyd also reviewed examples of what she has found successful in treating her patients with these conditions.
Lichen planus pigmentosus (LPP)
“It’s one of the hardest things that we treat,” said Dr. Woolery-Lloyd, who often sees cases of LPP in patients in their 30s, 40s, and 50s.
Lesions first appear as small, ill-defined oval-to-round macules, which later become confluent and form large areas of pigmentation. In different patients, the pigment on the face and neck, and sometimes on the forearms can be slate gray or brownish black.
In 2013, dermatologist N.C. Dlova, MD, at the University of KwaZulu‐Natal, Durban, South Africa, reported a link between frontal fibrosing alopecia and LPP in the British Journal of Dermatology. “I definitely see this connection in my practice,” said Dr. Woolery-Lloyd, noting that “both conditions often result in the loss of both eyebrows.”
She recommends always using a topical anti-inflammatory that is safe for the face. One combination she uses is azelaic acid 20% plus hydrocortisone 2.5%.
“We do use a lot of azelaic acid in my practice because it’s affordable,” she said, at the meeting, provided by MedscapeLive! She added that the hardest area to treat in women is around the chin.
Two other conditions, ashy dermatosis and erythema dyschromicum perstans (EDP), are similar. Ashy dermatosis mimics LPP but occurs more prominently on the trunk and extremities. EDP often has a preceding ring of erythema.
Dr. Woolery-Lloyd said the term EDP is often used to cover both EDP and ashy dermatosis in North America because “ashy” can have a negative connotation.
She noted there is no consensus on effective therapy for LPP, ashy dermatosis, or EDP.
A review of the literature on EDP, which included 16 studies on treatment outcomes, found the following:
- Narrow-band ultraviolet B and tacrolimus were effective treatments with minimal side effects.
- Clofazimine was effective, but had side effects, which, ironically, included pigmentary changes.
- Griseofulvin, isotretinoin, and dapsone were comparatively ineffective as lesions recurred after discontinuation.
- Lasers were largely ineffective and can also result in postinflammatory hyperpigmentation and fibrosis.
Ochronosis
Dr. Woolery-Lloyd said she may see one to two patients a year with ochronosis, which is characterized by paradoxical darkening of the skin with long-term hydroquinone use. It usually starts with redness followed by blue-black patches on the face where hydroquinone is applied. In severe cases, blue-black papules and nodules can occur.
“When I give a patient hydroquinone, I always say: ‘I don’t want to see any redness,’” Dr. Woolery-Lloyd said. “If you have any redness, please stop because ochronosis is typically preceded by this redness.”
But, she noted, “people will come in actively using hydroquinone, will have the dark brown or deep black papules or macules on their face, and then this background of redness because they are so inflamed.”
She said that ochronosis can occur in any skin type, not just in patients with darker skin tones. Dr. Woolery-Lloyd advised: “Do not hesitate to biopsy the face if ochronosis is suspected. I always biopsy ochronosis.”
There are two reasons for doing so, she explained. It can help with the diagnosis but it will also provide the patient with an incentive to stop using hydroquinone. “People who are using hydroquinone are addicted to it. They love it. They don’t want to stop. They keep using it despite the fact that their face is getting darker.” When they see a biopsy report, they may be convinced to stop.
Dr. Woolery-Lloyd said she does a 2-mm punch biopsy in the crow’s feet area because there’s almost always ochronosis in that area and it does not leave an obvious scar.
Eventually, she said, if the person stops using hydroquinone, it will clear up, “but it will take years.” Again, here she has had success with her “special formula” of azelaic acid 20% plus hydrocortisone 2.5%
“Don’t tell patients there’s no treatment. That’s the take-home,” she said.
Drug-induced facial hyperpigmentation
“I see this all the time in my African American patients,” Dr. Woolery-Lloyd said. The condition usually is characterized by dark brown hyperpigmentation on the face.
In this situation, the first question to ask is whether the patient is taking medication for hypertension, and the second question is whether it is “HCTZ.” It’s important to use the abbreviation for hydrochlorothiazide – the most common cause of drug-induced facial hyperpigmentation – because that’s what a patient sees on the bottle.
If they are taking HCTZ or another blood pressure medication associated with photosensitivity, they need to switch to a nonphotosensitizing antihypertensive agent (there are several options) and they should start treatment with a topical anti-inflammatory, Dr. Woolery-Lloyd said. Then, she suggests introducing hydrocortisone 2.5% cream and a hydroquinone-free skin brightener (azelaic acid, for example).
Importantly, with any of these conditions, Dr Woolery-Lloyd said, dermatologists should talk with patients about realistic expectations. “It takes a long time for dermal pigment to clear,” she emphasized.
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, L’Oreal, and EPI; has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion; and has been on advisory boards for L’Oreal, Allergan, Ortho Dermatologics, Pfizer, and Merz.
CHICAGO – based on cases she has treated in her practice.
Heather Woolery-Lloyd, MD, director of the skin of color division in the dermatology department at University of Miami, provided three general pointers.
- When in doubt, biopsy.
- For inflammatory disorders, always treat the inflammation in addition to the hyperpigmentation.
- Avoid long-term hydroquinone use in these patients.
Dr. Woolery-Lloyd also reviewed examples of what she has found successful in treating her patients with these conditions.
Lichen planus pigmentosus (LPP)
“It’s one of the hardest things that we treat,” said Dr. Woolery-Lloyd, who often sees cases of LPP in patients in their 30s, 40s, and 50s.
Lesions first appear as small, ill-defined oval-to-round macules, which later become confluent and form large areas of pigmentation. In different patients, the pigment on the face and neck, and sometimes on the forearms can be slate gray or brownish black.
In 2013, dermatologist N.C. Dlova, MD, at the University of KwaZulu‐Natal, Durban, South Africa, reported a link between frontal fibrosing alopecia and LPP in the British Journal of Dermatology. “I definitely see this connection in my practice,” said Dr. Woolery-Lloyd, noting that “both conditions often result in the loss of both eyebrows.”
She recommends always using a topical anti-inflammatory that is safe for the face. One combination she uses is azelaic acid 20% plus hydrocortisone 2.5%.
“We do use a lot of azelaic acid in my practice because it’s affordable,” she said, at the meeting, provided by MedscapeLive! She added that the hardest area to treat in women is around the chin.
Two other conditions, ashy dermatosis and erythema dyschromicum perstans (EDP), are similar. Ashy dermatosis mimics LPP but occurs more prominently on the trunk and extremities. EDP often has a preceding ring of erythema.
Dr. Woolery-Lloyd said the term EDP is often used to cover both EDP and ashy dermatosis in North America because “ashy” can have a negative connotation.
She noted there is no consensus on effective therapy for LPP, ashy dermatosis, or EDP.
A review of the literature on EDP, which included 16 studies on treatment outcomes, found the following:
- Narrow-band ultraviolet B and tacrolimus were effective treatments with minimal side effects.
- Clofazimine was effective, but had side effects, which, ironically, included pigmentary changes.
- Griseofulvin, isotretinoin, and dapsone were comparatively ineffective as lesions recurred after discontinuation.
- Lasers were largely ineffective and can also result in postinflammatory hyperpigmentation and fibrosis.
Ochronosis
Dr. Woolery-Lloyd said she may see one to two patients a year with ochronosis, which is characterized by paradoxical darkening of the skin with long-term hydroquinone use. It usually starts with redness followed by blue-black patches on the face where hydroquinone is applied. In severe cases, blue-black papules and nodules can occur.
“When I give a patient hydroquinone, I always say: ‘I don’t want to see any redness,’” Dr. Woolery-Lloyd said. “If you have any redness, please stop because ochronosis is typically preceded by this redness.”
But, she noted, “people will come in actively using hydroquinone, will have the dark brown or deep black papules or macules on their face, and then this background of redness because they are so inflamed.”
She said that ochronosis can occur in any skin type, not just in patients with darker skin tones. Dr. Woolery-Lloyd advised: “Do not hesitate to biopsy the face if ochronosis is suspected. I always biopsy ochronosis.”
There are two reasons for doing so, she explained. It can help with the diagnosis but it will also provide the patient with an incentive to stop using hydroquinone. “People who are using hydroquinone are addicted to it. They love it. They don’t want to stop. They keep using it despite the fact that their face is getting darker.” When they see a biopsy report, they may be convinced to stop.
Dr. Woolery-Lloyd said she does a 2-mm punch biopsy in the crow’s feet area because there’s almost always ochronosis in that area and it does not leave an obvious scar.
Eventually, she said, if the person stops using hydroquinone, it will clear up, “but it will take years.” Again, here she has had success with her “special formula” of azelaic acid 20% plus hydrocortisone 2.5%
“Don’t tell patients there’s no treatment. That’s the take-home,” she said.
Drug-induced facial hyperpigmentation
“I see this all the time in my African American patients,” Dr. Woolery-Lloyd said. The condition usually is characterized by dark brown hyperpigmentation on the face.
In this situation, the first question to ask is whether the patient is taking medication for hypertension, and the second question is whether it is “HCTZ.” It’s important to use the abbreviation for hydrochlorothiazide – the most common cause of drug-induced facial hyperpigmentation – because that’s what a patient sees on the bottle.
If they are taking HCTZ or another blood pressure medication associated with photosensitivity, they need to switch to a nonphotosensitizing antihypertensive agent (there are several options) and they should start treatment with a topical anti-inflammatory, Dr. Woolery-Lloyd said. Then, she suggests introducing hydrocortisone 2.5% cream and a hydroquinone-free skin brightener (azelaic acid, for example).
Importantly, with any of these conditions, Dr Woolery-Lloyd said, dermatologists should talk with patients about realistic expectations. “It takes a long time for dermal pigment to clear,” she emphasized.
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, L’Oreal, and EPI; has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion; and has been on advisory boards for L’Oreal, Allergan, Ortho Dermatologics, Pfizer, and Merz.
CHICAGO – based on cases she has treated in her practice.
Heather Woolery-Lloyd, MD, director of the skin of color division in the dermatology department at University of Miami, provided three general pointers.
- When in doubt, biopsy.
- For inflammatory disorders, always treat the inflammation in addition to the hyperpigmentation.
- Avoid long-term hydroquinone use in these patients.
Dr. Woolery-Lloyd also reviewed examples of what she has found successful in treating her patients with these conditions.
Lichen planus pigmentosus (LPP)
“It’s one of the hardest things that we treat,” said Dr. Woolery-Lloyd, who often sees cases of LPP in patients in their 30s, 40s, and 50s.
Lesions first appear as small, ill-defined oval-to-round macules, which later become confluent and form large areas of pigmentation. In different patients, the pigment on the face and neck, and sometimes on the forearms can be slate gray or brownish black.
In 2013, dermatologist N.C. Dlova, MD, at the University of KwaZulu‐Natal, Durban, South Africa, reported a link between frontal fibrosing alopecia and LPP in the British Journal of Dermatology. “I definitely see this connection in my practice,” said Dr. Woolery-Lloyd, noting that “both conditions often result in the loss of both eyebrows.”
She recommends always using a topical anti-inflammatory that is safe for the face. One combination she uses is azelaic acid 20% plus hydrocortisone 2.5%.
“We do use a lot of azelaic acid in my practice because it’s affordable,” she said, at the meeting, provided by MedscapeLive! She added that the hardest area to treat in women is around the chin.
Two other conditions, ashy dermatosis and erythema dyschromicum perstans (EDP), are similar. Ashy dermatosis mimics LPP but occurs more prominently on the trunk and extremities. EDP often has a preceding ring of erythema.
Dr. Woolery-Lloyd said the term EDP is often used to cover both EDP and ashy dermatosis in North America because “ashy” can have a negative connotation.
She noted there is no consensus on effective therapy for LPP, ashy dermatosis, or EDP.
A review of the literature on EDP, which included 16 studies on treatment outcomes, found the following:
- Narrow-band ultraviolet B and tacrolimus were effective treatments with minimal side effects.
- Clofazimine was effective, but had side effects, which, ironically, included pigmentary changes.
- Griseofulvin, isotretinoin, and dapsone were comparatively ineffective as lesions recurred after discontinuation.
- Lasers were largely ineffective and can also result in postinflammatory hyperpigmentation and fibrosis.
Ochronosis
Dr. Woolery-Lloyd said she may see one to two patients a year with ochronosis, which is characterized by paradoxical darkening of the skin with long-term hydroquinone use. It usually starts with redness followed by blue-black patches on the face where hydroquinone is applied. In severe cases, blue-black papules and nodules can occur.
“When I give a patient hydroquinone, I always say: ‘I don’t want to see any redness,’” Dr. Woolery-Lloyd said. “If you have any redness, please stop because ochronosis is typically preceded by this redness.”
But, she noted, “people will come in actively using hydroquinone, will have the dark brown or deep black papules or macules on their face, and then this background of redness because they are so inflamed.”
She said that ochronosis can occur in any skin type, not just in patients with darker skin tones. Dr. Woolery-Lloyd advised: “Do not hesitate to biopsy the face if ochronosis is suspected. I always biopsy ochronosis.”
There are two reasons for doing so, she explained. It can help with the diagnosis but it will also provide the patient with an incentive to stop using hydroquinone. “People who are using hydroquinone are addicted to it. They love it. They don’t want to stop. They keep using it despite the fact that their face is getting darker.” When they see a biopsy report, they may be convinced to stop.
Dr. Woolery-Lloyd said she does a 2-mm punch biopsy in the crow’s feet area because there’s almost always ochronosis in that area and it does not leave an obvious scar.
Eventually, she said, if the person stops using hydroquinone, it will clear up, “but it will take years.” Again, here she has had success with her “special formula” of azelaic acid 20% plus hydrocortisone 2.5%
“Don’t tell patients there’s no treatment. That’s the take-home,” she said.
Drug-induced facial hyperpigmentation
“I see this all the time in my African American patients,” Dr. Woolery-Lloyd said. The condition usually is characterized by dark brown hyperpigmentation on the face.
In this situation, the first question to ask is whether the patient is taking medication for hypertension, and the second question is whether it is “HCTZ.” It’s important to use the abbreviation for hydrochlorothiazide – the most common cause of drug-induced facial hyperpigmentation – because that’s what a patient sees on the bottle.
If they are taking HCTZ or another blood pressure medication associated with photosensitivity, they need to switch to a nonphotosensitizing antihypertensive agent (there are several options) and they should start treatment with a topical anti-inflammatory, Dr. Woolery-Lloyd said. Then, she suggests introducing hydrocortisone 2.5% cream and a hydroquinone-free skin brightener (azelaic acid, for example).
Importantly, with any of these conditions, Dr Woolery-Lloyd said, dermatologists should talk with patients about realistic expectations. “It takes a long time for dermal pigment to clear,” she emphasized.
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, L’Oreal, and EPI; has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion; and has been on advisory boards for L’Oreal, Allergan, Ortho Dermatologics, Pfizer, and Merz.
AT THE MEDSCAPELIVE! PIGMENTARY DISORDERS SYMPOSIUM