User login
Vaginal hysterectomy with basic instrumentation
In the United States, gynecologic surgeons remove approximately one uterus every minute of the year.1 That rate translates to more than 525,000 hysterectomies annually in this country alone. Yet, despite the widespread availability of information on the benefits of a vaginal approach to hysterectomy, the great majority of these operations—close to 50%—are still performed via an open abdominal approach.2
As I pointed out last month in my “Update on Vaginal Hysterectomy,” the vaginal approach not only is more cosmetically pleasing than laparoscopic and robot-assisted hysterectomy (not to mention open abdominal surgery) but also has a lower complication rate.3
As I also noted, one reason for the low rate of vaginal hysterectomy may be the assumption, on the part of many gynecologic surgeons, that the techniques and tools they learned to use during training are still the only options available today. That assumption is wrong.
In this article, I describe the technique for vaginal hysterectomy using basic instru mentation. This article is based on a master class in vaginal hysterectomy produced by the AAGL and co-sponsored by the Am erican College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. This master class offers continuing medical education credits and is avail able at http://www.aagl.org/vaghystwebinar.
For a look at innovative tools for this procedure, see my “Update on Vaginal Hysterectomy” in the September 2015 issue of this journal at obgmanagement.com.
Vaginal hysterectomy has few contraindications
Many commonly cited contraindications to the vaginal approach are not, in fact, absolute contraindications. An open or laparoscopic approach is preferred when the patient has a known cancer, of course, and when deep infiltrating endometriosis is present at the rectovaginal septum. However, previous pelvic surgery, nulliparity, an enlarged uterus, or lack of a prior vaginal delivery need not exclude the vaginal approach. Nor does a narrow introitus necessarily mandate a laparoscopic or open abdominal approach. In fact, in this article, I describe my basic technique in a patient (a cadaver) with a very narrow pubic arch, and I offer strategies for gaining some needed mobility and avoiding complications (TABLES 1 and 2).
Next month, in the November issue of OBG Management, John B. Gebhart, MD, will describe his vaginal technique for right salpingectomy with ovarian preservation, as well as his technique for right salpingo-oophorectomy.
Proper patient positioning is key
You can simplify the operation by positioning the patient so that her buttocks are over the edge of the table fairly far—at least 1 inch beyond the edge of the table for optimal exposure and greater access. If the patient is thin, it then becomes important to pad the sacrum because, when she is positioned that far off the table, all her weight comes to rest on the sacrum. In overweight patients, this is not an issue, but for thin patients, I place a bit of egg crate or gel beneath the sacrum.
For the procedure, I prefer to place my instruments on a tray that is kept on my lap. This arrangement frees the scrub technician from having to hand tools over my shoulder—and it saves time. I use a narrow, covered Mayo stand, and I place a stepstool beneath my feet to keep my knees at right angles so that things don’t slip during the operation.
Surgical technique
Choose an appropriate retractor
In a woman with a narrow introitus, I find that a posterior weighted speculum takes up too much space. Once I place a clamp on the cervix with that speculum in place, I don’t have much room to work. However, if I substitute a small Deaver retractor, which is narrower, I gain more workspace.
Inject the uterosacral ligaments
Grasp the cervix using a Jacobs vulsellum tenaculum. Use of a single tenaculum allows for much more movement than the use of instruments placed anteriorly and posteriorly. The Jacobs tenaculum obtains a better purchase on the tissue than a single tooth and is considerably less likely to tear through the tissue.
Before beginning the hysterectomy, locate the uterosacral ligaments and inject each one at its junction with the cervix, aspirating slightly before infiltrating the ligament with 0.25% to 0.50% bupivacaine with epinephrine, with dilute vasopressin mixed in. (I place 1 unit in 20 mL of the local solution.) Injection of this solution achieves 2 goals:
- improved intraoperative hemostasis
- postoperative pain relief.
Use a short needle with a needle extender attached to a control syringe rather than a spinal needle for greater control.
Enter the posterior peritoneal cavity
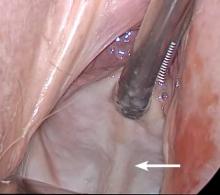
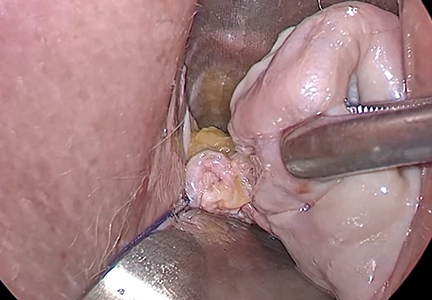
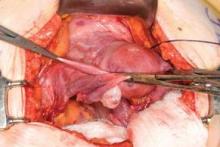
Before entering the peritoneal cavity, create a right angle with the Jacobs tenaculum and Deaver retractor in relation to the surgical field (FIGURE 1). This right angle is difficult to achieve when you are using a weighted speculum in a tight vagina. Once you have a right angle, tent the vaginal tissue in the midline (FIGURE 2).
In a nulliparous patient or a woman with a tight pelvis, you may discover that the peritoneum is pulled up between the uterosacral ligaments. One common pitfall arises when the surgeon, having dissected the vaginal epithelium, continues cutting into the vaginal epithelium instead of reaching into the peritoneal cavity. Palpate the tissue to ensure that there is no bowel in the way and stay at right angles while confidently grasping the peritoneum with a toothed forceps.
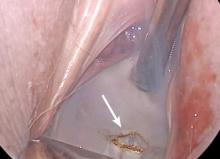
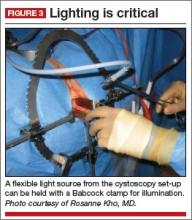
I like to use a bit of electrosurgery to incise the vaginal wall. I don’t begin at the cervix but incise more distally into the vaginal epithelium approximately 2 cm from the cervicovaginal junction. This strategy prevents dissection into the cervix and/or rectovaginal septum rather than the posterior
cul-de-sac (FIGURE 3).
Once the incision is made, it is possible to feel the posterior peritoneum. And as you tent the peritoneum, you can then very confidently extend the incision and enter the cavity posteriorly.
In a patient with significant adhesions such as this one, I feel around posteriorly to determine exactly where I am. One tactic I use is to release the tenaculum and regrasp the cervix with it. This allows for improved visualization and movement of the cervix as the procedure progresses. Depending on the case, it may be necessary to insert a sponge to hold bowel out of the way.
Avoid the bladder
Move the Deaver retractor to the anterior position, switch the Jacobs clamp to the anterior cervix, and pull straight down. Now that you have incised the vaginal epithelium posteriorly, the length of the cervix should be apparent to you, and you can easily determine the location of the bladder reflection.
Keep in mind that, in a postmenopausal patient, there will be fewer vaginal rugae to guide you. Place the Jacobs tenaculum as close to the midline as possible so that you can confidently grab the tissue without fear of grabbing the bladder. If you tilt the Jacobs clamp, you can feel the edge of the bladder reflection. Remember that postmenopausal patients with prolapse (or, occasionally, obese patients with cervical elongation but little actual descensus) may have altered anatomy.
You can create a bit more space in which to dissect by injecting the bupivacaine/ epinephrine solution into the vaginal epithelium. This technique also ensures that the vaginal epithelial incisions won’t bleed.
Now, tilt the Jacobs tenaculum downward and push the junction of the cervix with the bladder reflection toward you so that you have a good sense of how deeply to incise.
Once you’ve made the incision, reclamp the Jacobs tenaculum so that it holds all of that tissue, and repeat the maneuver, tilting the clamp downward and pushing the junction toward you. In this way, you create traction and countertraction, sweeping the tissue out of your way.
Always use sharp dissection. When adhesions are present, surgeons often get into trouble using blunt dissection and may inadvertently enter the bladder if they use a sponge-covered digit for dissection, because adhesions can be much denser than normal tissue. In such cases, the bladder tears open rather than the adhesions being swept away.
Consider this: You don’t need to enter the peritoneal cavity anteriorly in order to continue working on the procedure. You can safely protect the bladder throughout the case, until the very end, if necessary, in patients who have undergone multiple previous surgeries or cesarean deliveries.
Rather than enter the anterior peritoneum, I dissect as much of the vaginal epithelium as possible and place a second Deaver retractor posteriorly.
I massage the uterosacral ligament for about 10 seconds to lengthen it and create more descensus, then place a Ballantine Heaney clamp on the ligament.
Next, I cut the pedicle and suture it, maintaining a clamp on the uterosacral ligament suture so that I can use it later for repair of the vaginal cuff.
I recommend a vessel-sealing device to secure the major blood supply, but I do suture the uterosacral and round ligaments for attachment to the apex at the conclusion of the hysterectomy. I suggest that you place straight clamps to hold the uterosacral ligament sutures and curved clamps on the round ligament ties to help you keep track of what you’re doing.
I generally prefer to use a smaller vessel-sealing device, such as the LigaSure Max (Covidien), because it allows me to take very small bites of tissue. It is also less expensive because it uses a disposable electrode within a reusable Heaney-type clamp.
Many people have argued that we need to teach surgeons to suture vaginally and, for that reason, should avoid vessel sealing. My response: Why wouldn’t we want to use the very best technology available? Randomized trials have demonstrated a 50% reduction in pain relief postoperatively when we use vessel sealing.4 Less foreign material is left in the pelvis, lowering the risk of infection. And it really doesn’t matter which vessel-sealing technology you use, as long as you’re familiar with the specifics of the system you choose. Another advantage: There is no need to pass needles back and forth.
Take small bites of tissue
Because this patient has a very small uterus, a small bite of tissue will get you close to where you want to be. When you take a bite with the vessel sealer, try to protect the vaginal epithelium and vulva from the steam that is emitted. The clamp itself does not heat up, but the steam that is released from the tissue is 100° C, so place a finger between the clamp and the sidewall for protection. It is preferable to burn your own finger than to burn the patient.
Because you haven’t entered the peritoneal cavity anteriorly, it is important to ensure that you don’t take too big of a bite with the vessel sealer. Rather, stay where you know you’ve done your dissection, where things are safe.
One cardinal principle of surgery is that you shouldn’t operate where you can’t feel or see. One of the common errors in vaginal surgery is that surgeons start dissecting higher than they can see. It’s easy to get into trouble when you start pushing tissue or dissecting tissue that you can’t visualize.
At this point, the anterior Deaver retractor is not essential, so I remove it. If you don’t need it, don’t use it. I try to avoid metal when I can.
If I were using suture rather than vessel sealing, I would place a Heaney clamp on the uterosacral ligament and cut. Using a clamp-cut-tie technique, I would pull on the pedicle and cut just beyond the tip of the clamp to ensure that the suture will be secure (FIGURE 4). This approach would not be appropriate during use of a vessel sealer. In that case, you would want to cut to but not beyond the tip of the clamp.
One of the skills helpful in suturing is learning to move your elbow and wrist to achieve the proper angle. Determine where you want the suture to exit the tissue, and then angle your elbow and wrist so that the suture comes out where you want it. It’s easy to lose track of the needle tip, especially when you’re working in a limited space under the pubic symphysis, so use your shoulder, elbow, and wrist to control
suture placement.
Protect the anterior epithelium
Because you have not yet entered the peritoneal cavity anteriorly, it is important to protect the anterior epithelium and bladder. Reinsert a narrow Deaver retractor anteriorly, remove the Jacobs clamp, and replace the clamp laterally so that the cervix can be pulled off to the side (FIGURE 5).
One nice thing about some vessel sealers is that the surgeon can twist them in any direction. It isn’t necessary to move your hand; you simply move the device itself.
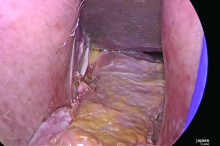
Once you have taken at least the descending branch of the uterine artery, remove the posterior retractor and pull downward on the Jacobs tenaculum. You should have reached just about to the level of the uterine fundus, with the anatomy well visualized (FIGURE 6). Next, open the anterior peritoneum.
Pay attention to the surgical field
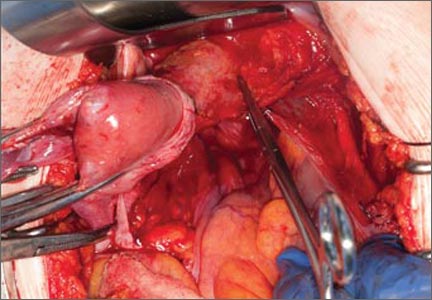
Now that you have entered the peritoneum anteriorly as well as posteriorly, identify the broad ligament, keeping in mind that the ureter is retroperitoneal, not intraperitoneal. If you were to place a clamp from the posterior leaf of the broad ligament across to the anterior leaf of the broad ligament, you would be grasping all the vessels but not the ureter. In fact, the anterior Deaver retractor is lifting both ureters up and out of the way. If you pull the cervix off to the opposite side, you create an additional couple of centimeters—a safe space for the vessel sealer
(FIGURE 7).
In placing the vessel sealer, there is no need to move out laterally, as there is no need for space to place suture. Instead, hug the uterus. At this point, the main concern is the risk of damaging any small bowel behind the uterine fundus that might be coming down into the surgical field, obscured from vision. And because there may be steam emitted at the tip of the vessel-sealing clamp, keep a finger back there to protect anything that might be in the field.
Last steps
Before taking the last bite of tissue on the right-hand side, place the round ligament in a Heaney clamp. Now that the round and utero-ovarian ligaments have been skeletonized, you can grasp the pedicle in a clamp. If the pedicle is especially thick, it may be beneficial to close the clamp, leave it on for a few seconds, and then reapply it. In that way, you obtain a better purchase.
Next, free the rest of the tissue with a vessel sealer, or cut it. I prefer to use a vessel sealer, and I again protect the adjacent tissue with my fingers anteriorly and posteriorly.
With the clamp remaining on the round ligament and utero-ovarian ligament
(FIGURE 8), which will be sutured, push the uterine tissue out of the way, back into the pelvis, to make room for suturing.
Because a postmenopausal vulva may be cut by the suture, it’s important to take pains not to abrade that tissue. Once you have finished suturing the round/utero-ovarian pedicle, leave the needle on the suture so that you can reconnect the round ligament to the anterior pubocervical ring to reconstruct the vaginal apex. For safekeeping, clamp the needle out of the way and tuck it beneath the drape.
Switching to the other side, use a Lahey clamp to flip the uterus, then clamp the pedicle and use the vessel sealer to separate it, again protecting the tissue beneath and ahead of the clamp. Sometimes, with an especially thick pedicle, the vessel sealer will signal that the tissue hasn’t been completely sealed. In that case, get another purchase of the pedicle, protect the adjacent tissue, and seal again.
Once the uterus has been removed completely, suture the utero-ovarian and round ligament on this side.
One tip to aid in the placement of suture is to move your clamped tissue in such a way as to prevent inadvertent suturing of other tissue (FIGURE 9).
An additional strategy for pain relief at this point is to infiltrate the round ligaments with local anesthetic. We know that we’re working with higher-level fibers—T10 to T12—through the round ligaments. By infiltrating them with anesthetic, you achieve denser pain relief for post- operative management.
Uterine reduction strategies facilitate vaginal removal of tissue
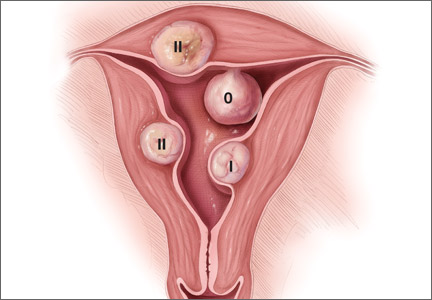
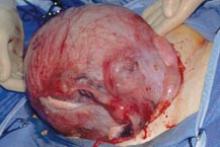
When the uterus is too large to remove intact through the vagina, there are a number of techniques for coring, wedging, and morcellating the tissue. As always, a complete knowledge of anatomy is essential, as well as an understanding that fibroids can frequently distort the uterus, twisting it to the left or right. It is important to anticipate such distortion to avoid the inadvertent destruction of anatomic landmarks or damage to the adnexae.
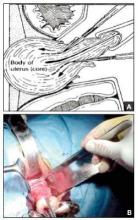
One straight-forward strategy is to debulk the uterus using a knife to core it, removing the central portion. In cases in which you need to keep the entire endometrial cavity intact, you can core the central portion of the uterus while grasping the cervix so that you can remove the endometrium intact for the pathologist (FIGURE).
For this strategy it is important to protect the vaginal sidewalls with metal. You can use another retractor to do that, pulling down on the cervix and beginning the morcellation. I generally prefer to use a short knife handle only because I want to be sure I’m not tempted to cut any higher than I can see.
For more on coring and wedging techniques, see the introductory video for the ACOG/SGS/AAGL master class on vaginal hysterectomy at http://www.aagl.org/vaghystwebinar.
Close the vaginal cuff
The reconstruction of the vaginal cuff is a critical component of any hysterectomy. My approach is to reattach the uterosacral ligaments to the posterior cuff and the round ligaments to the anterior cuff, thereby re- creating an intact pubocervical ring. It is not necessary to include the peritoneum in the cuff closure. In fact, kinking of the ureters is more likely when the peritoneum is closed.
Attach one uterosacral ligament, then place a running, full-thickness stitch across the posterior cuff, and attach the uterosacral ligament on the opposite side. Use the needle you left attached to the round ligament to bring the right pedicle to the anterior cuff at 10 o’clock (be sure you grasp the full thickness of the vaginal epithelium without compromising the bladder). Attach the left round-ligament pedicle at the 2 o’clock position. Then close the cuff side to side down to the uterosacral ligaments. This completely reconstructs the pubocervical ring and provides excellent support at the apex.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www .brighamandwomens.org/Departments_and_Services/obgyn /services/mininvgynsurg/mininvoptions/hysterectomy.aspx. Published October 3, 2014. Accessed September 2, 2015.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009; 114(5):1156–1158.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
In the United States, gynecologic surgeons remove approximately one uterus every minute of the year.1 That rate translates to more than 525,000 hysterectomies annually in this country alone. Yet, despite the widespread availability of information on the benefits of a vaginal approach to hysterectomy, the great majority of these operations—close to 50%—are still performed via an open abdominal approach.2
As I pointed out last month in my “Update on Vaginal Hysterectomy,” the vaginal approach not only is more cosmetically pleasing than laparoscopic and robot-assisted hysterectomy (not to mention open abdominal surgery) but also has a lower complication rate.3
As I also noted, one reason for the low rate of vaginal hysterectomy may be the assumption, on the part of many gynecologic surgeons, that the techniques and tools they learned to use during training are still the only options available today. That assumption is wrong.
In this article, I describe the technique for vaginal hysterectomy using basic instru mentation. This article is based on a master class in vaginal hysterectomy produced by the AAGL and co-sponsored by the Am erican College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. This master class offers continuing medical education credits and is avail able at http://www.aagl.org/vaghystwebinar.
For a look at innovative tools for this procedure, see my “Update on Vaginal Hysterectomy” in the September 2015 issue of this journal at obgmanagement.com.
Vaginal hysterectomy has few contraindications
Many commonly cited contraindications to the vaginal approach are not, in fact, absolute contraindications. An open or laparoscopic approach is preferred when the patient has a known cancer, of course, and when deep infiltrating endometriosis is present at the rectovaginal septum. However, previous pelvic surgery, nulliparity, an enlarged uterus, or lack of a prior vaginal delivery need not exclude the vaginal approach. Nor does a narrow introitus necessarily mandate a laparoscopic or open abdominal approach. In fact, in this article, I describe my basic technique in a patient (a cadaver) with a very narrow pubic arch, and I offer strategies for gaining some needed mobility and avoiding complications (TABLES 1 and 2).
Next month, in the November issue of OBG Management, John B. Gebhart, MD, will describe his vaginal technique for right salpingectomy with ovarian preservation, as well as his technique for right salpingo-oophorectomy.
Proper patient positioning is key
You can simplify the operation by positioning the patient so that her buttocks are over the edge of the table fairly far—at least 1 inch beyond the edge of the table for optimal exposure and greater access. If the patient is thin, it then becomes important to pad the sacrum because, when she is positioned that far off the table, all her weight comes to rest on the sacrum. In overweight patients, this is not an issue, but for thin patients, I place a bit of egg crate or gel beneath the sacrum.
For the procedure, I prefer to place my instruments on a tray that is kept on my lap. This arrangement frees the scrub technician from having to hand tools over my shoulder—and it saves time. I use a narrow, covered Mayo stand, and I place a stepstool beneath my feet to keep my knees at right angles so that things don’t slip during the operation.
Surgical technique
Choose an appropriate retractor
In a woman with a narrow introitus, I find that a posterior weighted speculum takes up too much space. Once I place a clamp on the cervix with that speculum in place, I don’t have much room to work. However, if I substitute a small Deaver retractor, which is narrower, I gain more workspace.
Inject the uterosacral ligaments
Grasp the cervix using a Jacobs vulsellum tenaculum. Use of a single tenaculum allows for much more movement than the use of instruments placed anteriorly and posteriorly. The Jacobs tenaculum obtains a better purchase on the tissue than a single tooth and is considerably less likely to tear through the tissue.
Before beginning the hysterectomy, locate the uterosacral ligaments and inject each one at its junction with the cervix, aspirating slightly before infiltrating the ligament with 0.25% to 0.50% bupivacaine with epinephrine, with dilute vasopressin mixed in. (I place 1 unit in 20 mL of the local solution.) Injection of this solution achieves 2 goals:
- improved intraoperative hemostasis
- postoperative pain relief.
Use a short needle with a needle extender attached to a control syringe rather than a spinal needle for greater control.
Enter the posterior peritoneal cavity
Before entering the peritoneal cavity, create a right angle with the Jacobs tenaculum and Deaver retractor in relation to the surgical field (FIGURE 1). This right angle is difficult to achieve when you are using a weighted speculum in a tight vagina. Once you have a right angle, tent the vaginal tissue in the midline (FIGURE 2).
In a nulliparous patient or a woman with a tight pelvis, you may discover that the peritoneum is pulled up between the uterosacral ligaments. One common pitfall arises when the surgeon, having dissected the vaginal epithelium, continues cutting into the vaginal epithelium instead of reaching into the peritoneal cavity. Palpate the tissue to ensure that there is no bowel in the way and stay at right angles while confidently grasping the peritoneum with a toothed forceps.
I like to use a bit of electrosurgery to incise the vaginal wall. I don’t begin at the cervix but incise more distally into the vaginal epithelium approximately 2 cm from the cervicovaginal junction. This strategy prevents dissection into the cervix and/or rectovaginal septum rather than the posterior
cul-de-sac (FIGURE 3).
Once the incision is made, it is possible to feel the posterior peritoneum. And as you tent the peritoneum, you can then very confidently extend the incision and enter the cavity posteriorly.
In a patient with significant adhesions such as this one, I feel around posteriorly to determine exactly where I am. One tactic I use is to release the tenaculum and regrasp the cervix with it. This allows for improved visualization and movement of the cervix as the procedure progresses. Depending on the case, it may be necessary to insert a sponge to hold bowel out of the way.
Avoid the bladder
Move the Deaver retractor to the anterior position, switch the Jacobs clamp to the anterior cervix, and pull straight down. Now that you have incised the vaginal epithelium posteriorly, the length of the cervix should be apparent to you, and you can easily determine the location of the bladder reflection.
Keep in mind that, in a postmenopausal patient, there will be fewer vaginal rugae to guide you. Place the Jacobs tenaculum as close to the midline as possible so that you can confidently grab the tissue without fear of grabbing the bladder. If you tilt the Jacobs clamp, you can feel the edge of the bladder reflection. Remember that postmenopausal patients with prolapse (or, occasionally, obese patients with cervical elongation but little actual descensus) may have altered anatomy.
You can create a bit more space in which to dissect by injecting the bupivacaine/ epinephrine solution into the vaginal epithelium. This technique also ensures that the vaginal epithelial incisions won’t bleed.
Now, tilt the Jacobs tenaculum downward and push the junction of the cervix with the bladder reflection toward you so that you have a good sense of how deeply to incise.
Once you’ve made the incision, reclamp the Jacobs tenaculum so that it holds all of that tissue, and repeat the maneuver, tilting the clamp downward and pushing the junction toward you. In this way, you create traction and countertraction, sweeping the tissue out of your way.
Always use sharp dissection. When adhesions are present, surgeons often get into trouble using blunt dissection and may inadvertently enter the bladder if they use a sponge-covered digit for dissection, because adhesions can be much denser than normal tissue. In such cases, the bladder tears open rather than the adhesions being swept away.
Consider this: You don’t need to enter the peritoneal cavity anteriorly in order to continue working on the procedure. You can safely protect the bladder throughout the case, until the very end, if necessary, in patients who have undergone multiple previous surgeries or cesarean deliveries.
Rather than enter the anterior peritoneum, I dissect as much of the vaginal epithelium as possible and place a second Deaver retractor posteriorly.
I massage the uterosacral ligament for about 10 seconds to lengthen it and create more descensus, then place a Ballantine Heaney clamp on the ligament.
Next, I cut the pedicle and suture it, maintaining a clamp on the uterosacral ligament suture so that I can use it later for repair of the vaginal cuff.
I recommend a vessel-sealing device to secure the major blood supply, but I do suture the uterosacral and round ligaments for attachment to the apex at the conclusion of the hysterectomy. I suggest that you place straight clamps to hold the uterosacral ligament sutures and curved clamps on the round ligament ties to help you keep track of what you’re doing.
I generally prefer to use a smaller vessel-sealing device, such as the LigaSure Max (Covidien), because it allows me to take very small bites of tissue. It is also less expensive because it uses a disposable electrode within a reusable Heaney-type clamp.
Many people have argued that we need to teach surgeons to suture vaginally and, for that reason, should avoid vessel sealing. My response: Why wouldn’t we want to use the very best technology available? Randomized trials have demonstrated a 50% reduction in pain relief postoperatively when we use vessel sealing.4 Less foreign material is left in the pelvis, lowering the risk of infection. And it really doesn’t matter which vessel-sealing technology you use, as long as you’re familiar with the specifics of the system you choose. Another advantage: There is no need to pass needles back and forth.
Take small bites of tissue
Because this patient has a very small uterus, a small bite of tissue will get you close to where you want to be. When you take a bite with the vessel sealer, try to protect the vaginal epithelium and vulva from the steam that is emitted. The clamp itself does not heat up, but the steam that is released from the tissue is 100° C, so place a finger between the clamp and the sidewall for protection. It is preferable to burn your own finger than to burn the patient.
Because you haven’t entered the peritoneal cavity anteriorly, it is important to ensure that you don’t take too big of a bite with the vessel sealer. Rather, stay where you know you’ve done your dissection, where things are safe.
One cardinal principle of surgery is that you shouldn’t operate where you can’t feel or see. One of the common errors in vaginal surgery is that surgeons start dissecting higher than they can see. It’s easy to get into trouble when you start pushing tissue or dissecting tissue that you can’t visualize.
At this point, the anterior Deaver retractor is not essential, so I remove it. If you don’t need it, don’t use it. I try to avoid metal when I can.
If I were using suture rather than vessel sealing, I would place a Heaney clamp on the uterosacral ligament and cut. Using a clamp-cut-tie technique, I would pull on the pedicle and cut just beyond the tip of the clamp to ensure that the suture will be secure (FIGURE 4). This approach would not be appropriate during use of a vessel sealer. In that case, you would want to cut to but not beyond the tip of the clamp.
One of the skills helpful in suturing is learning to move your elbow and wrist to achieve the proper angle. Determine where you want the suture to exit the tissue, and then angle your elbow and wrist so that the suture comes out where you want it. It’s easy to lose track of the needle tip, especially when you’re working in a limited space under the pubic symphysis, so use your shoulder, elbow, and wrist to control
suture placement.
Protect the anterior epithelium
Because you have not yet entered the peritoneal cavity anteriorly, it is important to protect the anterior epithelium and bladder. Reinsert a narrow Deaver retractor anteriorly, remove the Jacobs clamp, and replace the clamp laterally so that the cervix can be pulled off to the side (FIGURE 5).
One nice thing about some vessel sealers is that the surgeon can twist them in any direction. It isn’t necessary to move your hand; you simply move the device itself.
Once you have taken at least the descending branch of the uterine artery, remove the posterior retractor and pull downward on the Jacobs tenaculum. You should have reached just about to the level of the uterine fundus, with the anatomy well visualized (FIGURE 6). Next, open the anterior peritoneum.
Pay attention to the surgical field
Now that you have entered the peritoneum anteriorly as well as posteriorly, identify the broad ligament, keeping in mind that the ureter is retroperitoneal, not intraperitoneal. If you were to place a clamp from the posterior leaf of the broad ligament across to the anterior leaf of the broad ligament, you would be grasping all the vessels but not the ureter. In fact, the anterior Deaver retractor is lifting both ureters up and out of the way. If you pull the cervix off to the opposite side, you create an additional couple of centimeters—a safe space for the vessel sealer
(FIGURE 7).
In placing the vessel sealer, there is no need to move out laterally, as there is no need for space to place suture. Instead, hug the uterus. At this point, the main concern is the risk of damaging any small bowel behind the uterine fundus that might be coming down into the surgical field, obscured from vision. And because there may be steam emitted at the tip of the vessel-sealing clamp, keep a finger back there to protect anything that might be in the field.
Last steps
Before taking the last bite of tissue on the right-hand side, place the round ligament in a Heaney clamp. Now that the round and utero-ovarian ligaments have been skeletonized, you can grasp the pedicle in a clamp. If the pedicle is especially thick, it may be beneficial to close the clamp, leave it on for a few seconds, and then reapply it. In that way, you obtain a better purchase.
Next, free the rest of the tissue with a vessel sealer, or cut it. I prefer to use a vessel sealer, and I again protect the adjacent tissue with my fingers anteriorly and posteriorly.
With the clamp remaining on the round ligament and utero-ovarian ligament
(FIGURE 8), which will be sutured, push the uterine tissue out of the way, back into the pelvis, to make room for suturing.
Because a postmenopausal vulva may be cut by the suture, it’s important to take pains not to abrade that tissue. Once you have finished suturing the round/utero-ovarian pedicle, leave the needle on the suture so that you can reconnect the round ligament to the anterior pubocervical ring to reconstruct the vaginal apex. For safekeeping, clamp the needle out of the way and tuck it beneath the drape.
Switching to the other side, use a Lahey clamp to flip the uterus, then clamp the pedicle and use the vessel sealer to separate it, again protecting the tissue beneath and ahead of the clamp. Sometimes, with an especially thick pedicle, the vessel sealer will signal that the tissue hasn’t been completely sealed. In that case, get another purchase of the pedicle, protect the adjacent tissue, and seal again.
Once the uterus has been removed completely, suture the utero-ovarian and round ligament on this side.
One tip to aid in the placement of suture is to move your clamped tissue in such a way as to prevent inadvertent suturing of other tissue (FIGURE 9).
An additional strategy for pain relief at this point is to infiltrate the round ligaments with local anesthetic. We know that we’re working with higher-level fibers—T10 to T12—through the round ligaments. By infiltrating them with anesthetic, you achieve denser pain relief for post- operative management.
Uterine reduction strategies facilitate vaginal removal of tissue
When the uterus is too large to remove intact through the vagina, there are a number of techniques for coring, wedging, and morcellating the tissue. As always, a complete knowledge of anatomy is essential, as well as an understanding that fibroids can frequently distort the uterus, twisting it to the left or right. It is important to anticipate such distortion to avoid the inadvertent destruction of anatomic landmarks or damage to the adnexae.
One straight-forward strategy is to debulk the uterus using a knife to core it, removing the central portion. In cases in which you need to keep the entire endometrial cavity intact, you can core the central portion of the uterus while grasping the cervix so that you can remove the endometrium intact for the pathologist (FIGURE).
For this strategy it is important to protect the vaginal sidewalls with metal. You can use another retractor to do that, pulling down on the cervix and beginning the morcellation. I generally prefer to use a short knife handle only because I want to be sure I’m not tempted to cut any higher than I can see.
For more on coring and wedging techniques, see the introductory video for the ACOG/SGS/AAGL master class on vaginal hysterectomy at http://www.aagl.org/vaghystwebinar.
Close the vaginal cuff
The reconstruction of the vaginal cuff is a critical component of any hysterectomy. My approach is to reattach the uterosacral ligaments to the posterior cuff and the round ligaments to the anterior cuff, thereby re- creating an intact pubocervical ring. It is not necessary to include the peritoneum in the cuff closure. In fact, kinking of the ureters is more likely when the peritoneum is closed.
Attach one uterosacral ligament, then place a running, full-thickness stitch across the posterior cuff, and attach the uterosacral ligament on the opposite side. Use the needle you left attached to the round ligament to bring the right pedicle to the anterior cuff at 10 o’clock (be sure you grasp the full thickness of the vaginal epithelium without compromising the bladder). Attach the left round-ligament pedicle at the 2 o’clock position. Then close the cuff side to side down to the uterosacral ligaments. This completely reconstructs the pubocervical ring and provides excellent support at the apex.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In the United States, gynecologic surgeons remove approximately one uterus every minute of the year.1 That rate translates to more than 525,000 hysterectomies annually in this country alone. Yet, despite the widespread availability of information on the benefits of a vaginal approach to hysterectomy, the great majority of these operations—close to 50%—are still performed via an open abdominal approach.2
As I pointed out last month in my “Update on Vaginal Hysterectomy,” the vaginal approach not only is more cosmetically pleasing than laparoscopic and robot-assisted hysterectomy (not to mention open abdominal surgery) but also has a lower complication rate.3
As I also noted, one reason for the low rate of vaginal hysterectomy may be the assumption, on the part of many gynecologic surgeons, that the techniques and tools they learned to use during training are still the only options available today. That assumption is wrong.
In this article, I describe the technique for vaginal hysterectomy using basic instru mentation. This article is based on a master class in vaginal hysterectomy produced by the AAGL and co-sponsored by the Am erican College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. This master class offers continuing medical education credits and is avail able at http://www.aagl.org/vaghystwebinar.
For a look at innovative tools for this procedure, see my “Update on Vaginal Hysterectomy” in the September 2015 issue of this journal at obgmanagement.com.
Vaginal hysterectomy has few contraindications
Many commonly cited contraindications to the vaginal approach are not, in fact, absolute contraindications. An open or laparoscopic approach is preferred when the patient has a known cancer, of course, and when deep infiltrating endometriosis is present at the rectovaginal septum. However, previous pelvic surgery, nulliparity, an enlarged uterus, or lack of a prior vaginal delivery need not exclude the vaginal approach. Nor does a narrow introitus necessarily mandate a laparoscopic or open abdominal approach. In fact, in this article, I describe my basic technique in a patient (a cadaver) with a very narrow pubic arch, and I offer strategies for gaining some needed mobility and avoiding complications (TABLES 1 and 2).
Next month, in the November issue of OBG Management, John B. Gebhart, MD, will describe his vaginal technique for right salpingectomy with ovarian preservation, as well as his technique for right salpingo-oophorectomy.
Proper patient positioning is key
You can simplify the operation by positioning the patient so that her buttocks are over the edge of the table fairly far—at least 1 inch beyond the edge of the table for optimal exposure and greater access. If the patient is thin, it then becomes important to pad the sacrum because, when she is positioned that far off the table, all her weight comes to rest on the sacrum. In overweight patients, this is not an issue, but for thin patients, I place a bit of egg crate or gel beneath the sacrum.
For the procedure, I prefer to place my instruments on a tray that is kept on my lap. This arrangement frees the scrub technician from having to hand tools over my shoulder—and it saves time. I use a narrow, covered Mayo stand, and I place a stepstool beneath my feet to keep my knees at right angles so that things don’t slip during the operation.
Surgical technique
Choose an appropriate retractor
In a woman with a narrow introitus, I find that a posterior weighted speculum takes up too much space. Once I place a clamp on the cervix with that speculum in place, I don’t have much room to work. However, if I substitute a small Deaver retractor, which is narrower, I gain more workspace.
Inject the uterosacral ligaments
Grasp the cervix using a Jacobs vulsellum tenaculum. Use of a single tenaculum allows for much more movement than the use of instruments placed anteriorly and posteriorly. The Jacobs tenaculum obtains a better purchase on the tissue than a single tooth and is considerably less likely to tear through the tissue.
Before beginning the hysterectomy, locate the uterosacral ligaments and inject each one at its junction with the cervix, aspirating slightly before infiltrating the ligament with 0.25% to 0.50% bupivacaine with epinephrine, with dilute vasopressin mixed in. (I place 1 unit in 20 mL of the local solution.) Injection of this solution achieves 2 goals:
- improved intraoperative hemostasis
- postoperative pain relief.
Use a short needle with a needle extender attached to a control syringe rather than a spinal needle for greater control.
Enter the posterior peritoneal cavity
Before entering the peritoneal cavity, create a right angle with the Jacobs tenaculum and Deaver retractor in relation to the surgical field (FIGURE 1). This right angle is difficult to achieve when you are using a weighted speculum in a tight vagina. Once you have a right angle, tent the vaginal tissue in the midline (FIGURE 2).
In a nulliparous patient or a woman with a tight pelvis, you may discover that the peritoneum is pulled up between the uterosacral ligaments. One common pitfall arises when the surgeon, having dissected the vaginal epithelium, continues cutting into the vaginal epithelium instead of reaching into the peritoneal cavity. Palpate the tissue to ensure that there is no bowel in the way and stay at right angles while confidently grasping the peritoneum with a toothed forceps.
I like to use a bit of electrosurgery to incise the vaginal wall. I don’t begin at the cervix but incise more distally into the vaginal epithelium approximately 2 cm from the cervicovaginal junction. This strategy prevents dissection into the cervix and/or rectovaginal septum rather than the posterior
cul-de-sac (FIGURE 3).
Once the incision is made, it is possible to feel the posterior peritoneum. And as you tent the peritoneum, you can then very confidently extend the incision and enter the cavity posteriorly.
In a patient with significant adhesions such as this one, I feel around posteriorly to determine exactly where I am. One tactic I use is to release the tenaculum and regrasp the cervix with it. This allows for improved visualization and movement of the cervix as the procedure progresses. Depending on the case, it may be necessary to insert a sponge to hold bowel out of the way.
Avoid the bladder
Move the Deaver retractor to the anterior position, switch the Jacobs clamp to the anterior cervix, and pull straight down. Now that you have incised the vaginal epithelium posteriorly, the length of the cervix should be apparent to you, and you can easily determine the location of the bladder reflection.
Keep in mind that, in a postmenopausal patient, there will be fewer vaginal rugae to guide you. Place the Jacobs tenaculum as close to the midline as possible so that you can confidently grab the tissue without fear of grabbing the bladder. If you tilt the Jacobs clamp, you can feel the edge of the bladder reflection. Remember that postmenopausal patients with prolapse (or, occasionally, obese patients with cervical elongation but little actual descensus) may have altered anatomy.
You can create a bit more space in which to dissect by injecting the bupivacaine/ epinephrine solution into the vaginal epithelium. This technique also ensures that the vaginal epithelial incisions won’t bleed.
Now, tilt the Jacobs tenaculum downward and push the junction of the cervix with the bladder reflection toward you so that you have a good sense of how deeply to incise.
Once you’ve made the incision, reclamp the Jacobs tenaculum so that it holds all of that tissue, and repeat the maneuver, tilting the clamp downward and pushing the junction toward you. In this way, you create traction and countertraction, sweeping the tissue out of your way.
Always use sharp dissection. When adhesions are present, surgeons often get into trouble using blunt dissection and may inadvertently enter the bladder if they use a sponge-covered digit for dissection, because adhesions can be much denser than normal tissue. In such cases, the bladder tears open rather than the adhesions being swept away.
Consider this: You don’t need to enter the peritoneal cavity anteriorly in order to continue working on the procedure. You can safely protect the bladder throughout the case, until the very end, if necessary, in patients who have undergone multiple previous surgeries or cesarean deliveries.
Rather than enter the anterior peritoneum, I dissect as much of the vaginal epithelium as possible and place a second Deaver retractor posteriorly.
I massage the uterosacral ligament for about 10 seconds to lengthen it and create more descensus, then place a Ballantine Heaney clamp on the ligament.
Next, I cut the pedicle and suture it, maintaining a clamp on the uterosacral ligament suture so that I can use it later for repair of the vaginal cuff.
I recommend a vessel-sealing device to secure the major blood supply, but I do suture the uterosacral and round ligaments for attachment to the apex at the conclusion of the hysterectomy. I suggest that you place straight clamps to hold the uterosacral ligament sutures and curved clamps on the round ligament ties to help you keep track of what you’re doing.
I generally prefer to use a smaller vessel-sealing device, such as the LigaSure Max (Covidien), because it allows me to take very small bites of tissue. It is also less expensive because it uses a disposable electrode within a reusable Heaney-type clamp.
Many people have argued that we need to teach surgeons to suture vaginally and, for that reason, should avoid vessel sealing. My response: Why wouldn’t we want to use the very best technology available? Randomized trials have demonstrated a 50% reduction in pain relief postoperatively when we use vessel sealing.4 Less foreign material is left in the pelvis, lowering the risk of infection. And it really doesn’t matter which vessel-sealing technology you use, as long as you’re familiar with the specifics of the system you choose. Another advantage: There is no need to pass needles back and forth.
Take small bites of tissue
Because this patient has a very small uterus, a small bite of tissue will get you close to where you want to be. When you take a bite with the vessel sealer, try to protect the vaginal epithelium and vulva from the steam that is emitted. The clamp itself does not heat up, but the steam that is released from the tissue is 100° C, so place a finger between the clamp and the sidewall for protection. It is preferable to burn your own finger than to burn the patient.
Because you haven’t entered the peritoneal cavity anteriorly, it is important to ensure that you don’t take too big of a bite with the vessel sealer. Rather, stay where you know you’ve done your dissection, where things are safe.
One cardinal principle of surgery is that you shouldn’t operate where you can’t feel or see. One of the common errors in vaginal surgery is that surgeons start dissecting higher than they can see. It’s easy to get into trouble when you start pushing tissue or dissecting tissue that you can’t visualize.
At this point, the anterior Deaver retractor is not essential, so I remove it. If you don’t need it, don’t use it. I try to avoid metal when I can.
If I were using suture rather than vessel sealing, I would place a Heaney clamp on the uterosacral ligament and cut. Using a clamp-cut-tie technique, I would pull on the pedicle and cut just beyond the tip of the clamp to ensure that the suture will be secure (FIGURE 4). This approach would not be appropriate during use of a vessel sealer. In that case, you would want to cut to but not beyond the tip of the clamp.
One of the skills helpful in suturing is learning to move your elbow and wrist to achieve the proper angle. Determine where you want the suture to exit the tissue, and then angle your elbow and wrist so that the suture comes out where you want it. It’s easy to lose track of the needle tip, especially when you’re working in a limited space under the pubic symphysis, so use your shoulder, elbow, and wrist to control
suture placement.
Protect the anterior epithelium
Because you have not yet entered the peritoneal cavity anteriorly, it is important to protect the anterior epithelium and bladder. Reinsert a narrow Deaver retractor anteriorly, remove the Jacobs clamp, and replace the clamp laterally so that the cervix can be pulled off to the side (FIGURE 5).
One nice thing about some vessel sealers is that the surgeon can twist them in any direction. It isn’t necessary to move your hand; you simply move the device itself.
Once you have taken at least the descending branch of the uterine artery, remove the posterior retractor and pull downward on the Jacobs tenaculum. You should have reached just about to the level of the uterine fundus, with the anatomy well visualized (FIGURE 6). Next, open the anterior peritoneum.
Pay attention to the surgical field
Now that you have entered the peritoneum anteriorly as well as posteriorly, identify the broad ligament, keeping in mind that the ureter is retroperitoneal, not intraperitoneal. If you were to place a clamp from the posterior leaf of the broad ligament across to the anterior leaf of the broad ligament, you would be grasping all the vessels but not the ureter. In fact, the anterior Deaver retractor is lifting both ureters up and out of the way. If you pull the cervix off to the opposite side, you create an additional couple of centimeters—a safe space for the vessel sealer
(FIGURE 7).
In placing the vessel sealer, there is no need to move out laterally, as there is no need for space to place suture. Instead, hug the uterus. At this point, the main concern is the risk of damaging any small bowel behind the uterine fundus that might be coming down into the surgical field, obscured from vision. And because there may be steam emitted at the tip of the vessel-sealing clamp, keep a finger back there to protect anything that might be in the field.
Last steps
Before taking the last bite of tissue on the right-hand side, place the round ligament in a Heaney clamp. Now that the round and utero-ovarian ligaments have been skeletonized, you can grasp the pedicle in a clamp. If the pedicle is especially thick, it may be beneficial to close the clamp, leave it on for a few seconds, and then reapply it. In that way, you obtain a better purchase.
Next, free the rest of the tissue with a vessel sealer, or cut it. I prefer to use a vessel sealer, and I again protect the adjacent tissue with my fingers anteriorly and posteriorly.
With the clamp remaining on the round ligament and utero-ovarian ligament
(FIGURE 8), which will be sutured, push the uterine tissue out of the way, back into the pelvis, to make room for suturing.
Because a postmenopausal vulva may be cut by the suture, it’s important to take pains not to abrade that tissue. Once you have finished suturing the round/utero-ovarian pedicle, leave the needle on the suture so that you can reconnect the round ligament to the anterior pubocervical ring to reconstruct the vaginal apex. For safekeeping, clamp the needle out of the way and tuck it beneath the drape.
Switching to the other side, use a Lahey clamp to flip the uterus, then clamp the pedicle and use the vessel sealer to separate it, again protecting the tissue beneath and ahead of the clamp. Sometimes, with an especially thick pedicle, the vessel sealer will signal that the tissue hasn’t been completely sealed. In that case, get another purchase of the pedicle, protect the adjacent tissue, and seal again.
Once the uterus has been removed completely, suture the utero-ovarian and round ligament on this side.
One tip to aid in the placement of suture is to move your clamped tissue in such a way as to prevent inadvertent suturing of other tissue (FIGURE 9).
An additional strategy for pain relief at this point is to infiltrate the round ligaments with local anesthetic. We know that we’re working with higher-level fibers—T10 to T12—through the round ligaments. By infiltrating them with anesthetic, you achieve denser pain relief for post- operative management.
Uterine reduction strategies facilitate vaginal removal of tissue
When the uterus is too large to remove intact through the vagina, there are a number of techniques for coring, wedging, and morcellating the tissue. As always, a complete knowledge of anatomy is essential, as well as an understanding that fibroids can frequently distort the uterus, twisting it to the left or right. It is important to anticipate such distortion to avoid the inadvertent destruction of anatomic landmarks or damage to the adnexae.
One straight-forward strategy is to debulk the uterus using a knife to core it, removing the central portion. In cases in which you need to keep the entire endometrial cavity intact, you can core the central portion of the uterus while grasping the cervix so that you can remove the endometrium intact for the pathologist (FIGURE).
For this strategy it is important to protect the vaginal sidewalls with metal. You can use another retractor to do that, pulling down on the cervix and beginning the morcellation. I generally prefer to use a short knife handle only because I want to be sure I’m not tempted to cut any higher than I can see.
For more on coring and wedging techniques, see the introductory video for the ACOG/SGS/AAGL master class on vaginal hysterectomy at http://www.aagl.org/vaghystwebinar.
Close the vaginal cuff
The reconstruction of the vaginal cuff is a critical component of any hysterectomy. My approach is to reattach the uterosacral ligaments to the posterior cuff and the round ligaments to the anterior cuff, thereby re- creating an intact pubocervical ring. It is not necessary to include the peritoneum in the cuff closure. In fact, kinking of the ureters is more likely when the peritoneum is closed.
Attach one uterosacral ligament, then place a running, full-thickness stitch across the posterior cuff, and attach the uterosacral ligament on the opposite side. Use the needle you left attached to the round ligament to bring the right pedicle to the anterior cuff at 10 o’clock (be sure you grasp the full thickness of the vaginal epithelium without compromising the bladder). Attach the left round-ligament pedicle at the 2 o’clock position. Then close the cuff side to side down to the uterosacral ligaments. This completely reconstructs the pubocervical ring and provides excellent support at the apex.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www .brighamandwomens.org/Departments_and_Services/obgyn /services/mininvgynsurg/mininvoptions/hysterectomy.aspx. Published October 3, 2014. Accessed September 2, 2015.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009; 114(5):1156–1158.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
- Brigham and Women’s Hospital. Minimally Invasive Gynecologic Surgery: Hysterectomy Options. http://www .brighamandwomens.org/Departments_and_Services/obgyn /services/mininvgynsurg/mininvoptions/hysterectomy.aspx. Published October 3, 2014. Accessed September 2, 2015.
- American Congress of Obstetricians and Gynecologists. 2011 Women’s Health Stats & Facts. Washington, DC: ACOG; 2011. http://www.acog.org/~/media/NewsRoom/MediaKit.pdf. Accessed August 6, 2015.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009; 114(5):1156–1158.
- Silva-Filho AL, Rodrigues AM, Vale de Castro Monteiro M, et al. Randomized study of bipolar vessel sealing system versus conventional suture ligature for vaginal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):200–203.
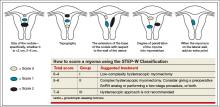
In this Article
- 5 solutions to difficult vaginal access
- The need to take small bites of tissue
- Uterine reduction strategies
Local anesthesia for uterine procedures
A vaginoscopic approach to diagnostic hysteroscopy
In this video, diffuse complex endometrial hyperplasia is identified using a vaginoscopic approach with a 1.9-mm diagnostic rigid hysteroscope. The posterior fornix of the vagina is filled with saline until the cervix is elevated with the fluid and the cervical os is identified. The cervical canal is entered and gentle rotational movement and hydrodistension allows the canal to be traversed into the uterine cavity.
Video provided by Amy L. Garcia, MD

Read Dr. Garcia’s “Update on minimally invasive gynecology” (April 2015)
In this video, diffuse complex endometrial hyperplasia is identified using a vaginoscopic approach with a 1.9-mm diagnostic rigid hysteroscope. The posterior fornix of the vagina is filled with saline until the cervix is elevated with the fluid and the cervical os is identified. The cervical canal is entered and gentle rotational movement and hydrodistension allows the canal to be traversed into the uterine cavity.
Video provided by Amy L. Garcia, MD

Read Dr. Garcia’s “Update on minimally invasive gynecology” (April 2015)
In this video, diffuse complex endometrial hyperplasia is identified using a vaginoscopic approach with a 1.9-mm diagnostic rigid hysteroscope. The posterior fornix of the vagina is filled with saline until the cervix is elevated with the fluid and the cervical os is identified. The cervical canal is entered and gentle rotational movement and hydrodistension allows the canal to be traversed into the uterine cavity.
Video provided by Amy L. Garcia, MD

Read Dr. Garcia’s “Update on minimally invasive gynecology” (April 2015)
Tissue extraction at minimally invasive surgery: Where do we go from here?
The year 2014 marked a sea change in our approach to tissue extraction during minimally invasive surgery. The US Food and Drug Administration (FDA) initiated this transformation in April, when it issued a safety warning on the use of open power morcellation.1 A flurry of statements on the practice followed from professional societies, capped, in late November, with another statement from the FDA.2–4 The new bottom line: The use of open electromechanical (“power”) morcellation is contraindicated in perimenopausal and postmenopausal women, as well as in those who are known or suspected to have a malignancy.4
Most of the concern to date has centered on the risk that an occult leiomyosarcoma could be morcellated inadvertently during uterine surgery, an event that may worsen the prognosis for the patient. To get a gynecologic oncologist’s take on the controversy, OBG Management caught up with Amanda Nickles Fader, MD, director of the Kelly Gynecologic Oncology Service at Johns Hopkins University. Dr. Fader’s perspective is unique in that she treats a relatively high number of patients who have leiomyosarcoma and other uterine cancers.
In this Q&A, we discuss the patient population at Johns Hopkins; why Dr. Fader is especially qualified to speak to the future of electromechanical morcellation in gynecologic surgery; the benefits and risks of minimally invasive surgery, including tissue extraction; her recommendations for preoperative evaluation and counseling of patients undergoing uterine surgery; and guidance on how the specialty of gynecologic surgery should proceed in the future.
OBG Management: Dr. Fader, by way of introduction, could you characterize your patient population?
Amanda Nickles Fader, MD: Like most gynecologic oncologists, I primarily treat women with cancers of the uterus, ovary, cervix, and vulva. Many of us also have the opportunity to treat a number of women each year with complex benign gynecologic conditions that require surgery. As someone who is extremely interested in rare gynecologic tumors, I also treat a relatively high volume of women diagnosed with uterine sarcoma.
Approximately 75% of the women I see in my practice have preinvasive or invasive cancer, and 25% have a benign condition, such as enlarged fibroids or advanced-stage endometriosis.
OBG Management: How many cases of uterine sarcoma do you encounter on an annual basis?
Dr. Fader: Uterine sarcomas are very rare. They represent only 2% of all uterine cancers. Put into perspective, that means that about 0.4 cases of leiomyosarcoma occur in every 100,000 US women—most commonly postmenopausal women. Leiomyosarcoma is a biologically aggressive, high-grade malignancy that often is lethal.5
Endometrial stroma sarcoma is even less common—only 0.3 cases occur in every 100,000 US women. However, this tumor type is more indolent, often diagnosed at an earlier stage, and potentially curable with surgery (with or without hormonal therapy).6
As a referral center for rare uterine tumors, the Kelly Gynecologic Oncology Service and the Sarcoma Center at Johns Hopkins see approximately 35 to 40 new cases of uterine sarcoma annually for treatment of primary disease or recurrence. An additional 15 to 20 consult cases are reviewed from outside hospitals each year by our gynecologic pathology department.
Why a minimally invasive approach is vital
OBG Management: When it comes to uterine surgery for presumed benign conditions, why is a minimally invasive approach important?
Dr. Fader: Minimally invasive surgery clearly benefits women and is one of the greatest advances of the past half-century within our field. Randomized controlled trials and meta-analyses have demonstrated without question that women who undergo minimally invasive surgery for benign conditions or early-stage cancerous gynecologic conditions have superior clinical outcomes, compared with women who undergo surgery via laparotomy.7,8 These outcomes include fewer perioperative complications (including fewer cases of surgical site infection, venous thromboembolism, wound dehiscence, and hospital readmission), shorter hospital stays, less pain, faster recovery, and fewer adhesions. And when women with early-stage cancers undergo minimally invasive surgery, randomized controlled trials show, they have a stage-specific survival rate similar to that observed in women treated with laparotomy.9
Benefits and risks of tissue extraction in minimally invasive surgery
OBG Management: What are the main benefits of tissue extraction, including morcellation?
Dr. Fader: Tissue extraction is a practice that has allowed us to offer minimally invasive surgery to countless more women than we could have in the recent past. It is a technique in which a large specimen (typically a uterus or fibroid) is fragmented into smaller parts in order to remove it through a small laparoscopic incision or orifice (vagina, umbilicus). Without tissue-extraction practices, thousands of women who undergo myomectomy each year to conserve their fertility and hundreds of thousands of women who require hysterectomy potentially would have to undergo a more painful and risky surgery through a larger abdominal incision. That would not be desirable, as we know conclusively that laparotomy is associated with worse outcomes—and even an increased risk of mortality—compared with minimally invasive surgery performed by experienced surgeons.10
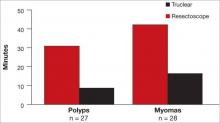
Tissue extraction can be approached in a variety of ways. It can be performed with a scalpel, with a resectoscope, or with an electromechanical morcellator. Tissue extraction can be performed within the uterine cavity, through the vagina, within the abdominal compartment, or within a containment system in any of those compartments.
OBG Management: What are the risks of tissue extraction?
Dr. Fader: The risks of tissue extraction with electromechanical morcellation include potential injury to intra-abdominal organs and vasculature and risk of dissemination of an occult (ie, undiagnosed) uterine cancer. A report by our research group also demonstrated the risks of dissemination of benign uterine tissue requiring subsequent surgery in the setting of open electromechanical morcellation.11 The risk of these events occurring in the hands of a thoughtful and experienced surgeon who conducts comprehensive preoperative patient evaluations is extremely low. However, recent evidence demonstrates that a handful of women worldwide are diagnosed with an occult uterine cancer each year during a morcellation procedure.12–14 Although it is a very rare event (given that most women undergoing hysterectomy and myomectomy procedures are of reproductive age and unlikely to have uterine cancer), this risk is a serious issue. There is an exigent need for the gynecologic surgical community to develop better approaches to tissue extraction that minimize preventable harm in women.
Needed: high-quality data on the risks of morcellation
OBG Management: How does the recent FDA statement urging caution with the use of open power morcellation factor into this equation? The most recent FDA statement noted that open power morcellation is contraindicated in perimenopausal and postmenopausal women.4
Dr. Fader: The FDA’s concern is legitimate. However, the magnitude of the risk of occult uterine sarcoma in women undergoing presumed benign gynecologic surgery has been scrutinized and debated. The FDA panel quoted a risk that roughly 1 in 350 women undergoing presumed benign gynecologic surgery for fibroids will have an occult leiomyosarcoma diagnosed. However, more recent systematic reviews and a review of the prospective published literature demonstrate that the risk is more likely on the order of 1 in 1,700 to 1 in 8,333 women.15 The risk may be even lower in gynecologic surgery practices that see a high volume of hysterectomy/myomectomy cases and utilize meticulous preoperative patient selection criteria to establish a woman’s candidacy for tissue-extraction procedures.
I am concerned with how “occult sarcoma” discovered during surgery for “presumed benign gynecologic disease is being defined in the literature. There is no uniform definition being used. A cancer in this setting is only truly occult or undiagnosed if the physician was thinking about it and made every effort to rule out cancer preoperatively, and the morcellation procedure was performed in a low-risk population (but cancer was still diagnosed on final pathology in this population). However, in the majority of the morcellation studies in the literature, it is not clear that thorough preoperative evaluations occurred in patients to rule out uterine malignancy—in fact, there is a paucity of published information regarding establishing appropriate patient candidacy for morcellation procedures. So we can’t derive any conclusions regarding whether “occult” sarcomas were truly undetectable or not in the published literature.
In addition, the literature is very clear that advancing age and postmenopausal status are risk factors for uterine malignancy.16 The vast majority of uterine sarcoma cases are diagnosed in postmenopausal women. Yet, in one large US population-based hysterectomy study, 20% of the morcellated cases (and the overwhelming majority of the “occult” morcellated uterine cancers) occurred in postmenopausal women!17
Further, in a more recent study by the same authors, again the risks of morcellating a uterine cancer in a population undergoing myomectomy was significantly higher in a postmenopausal population and occurred only rarely in women younger than age 40. But it should come as no surprise that a greater incidence of uterine cancer was identified in these older cohorts—cancer risk increases precipitously with age. That’s not “occult”; that’s basic cancer epidemiology.
In other words, we cannot assume from population-based administrative claims data that morcellation performed in inappropriate populations at higher risk of uterine malignancy (in which we do not know whether patients were properly screened for the procedure preoperatively or whether they had risk factors for uterine cancer but were presumably poor candidates for morcellation due to age alone) helps us define the true incidence of “occult” sarcoma or cancer in a population.
These studies are provocative, however, and do inform us that, as women get older, we are apt to see a greater incidence of uterine cancer. We cannot safely assume that a postmenopausal or elderly woman with symptomatic or enlarging fibroids has “presumed benign disease”—it is cancer until proven otherwise, and we need to be looking for it preoperatively. Therefore, we need to be particularly careful with our surgical practices in this population—ie, the basis for the FDA’s recommendation to avoid morcellation in older women. And I agree with the FDA that open electromechanical morcellation generally is contraindicated in postmenopausal women. However, we need better data from large prospective studies to inform our understanding of the true incidence of undiagnosed or “occult” uterine sarcoma in women undergoing surgery for presumed benign disease. These future studies are likely to demonstrate what we already know—that in young, well-screened, well-selected candidates for minimally invasive hysterectomy or myomectomy, the risk of occult cancer is going to be exceptionally low.
OBG Management: Which is greater—the risks or benefits of tissue extraction?
Dr. Fader: Assessment of risks and benefits in medicine has everything to do with the intervention in question as applied to an individual patient. At the end of the day, there are risks and benefits to every medical or surgical treatment offered to patients in every medical and surgical discipline. But the risk of an occult uterine sarcoma is extremely low in a woman of reproductive age who has been properly selected and comprehensively evaluated for minimally invasive surgery and tissue extraction prior to surgery. And this small—though not negligible—risk must be weighed against the much higher risk of harm that may be incurred with an open abdominal procedure, compared with minimally invasive surgery.
However, in many elderly women, the risks of tissue extraction with an electromechanical morcellator may outweigh the benefits. Even so, there are exceptions in which tissue extraction may be acceptable in postmenopausal women (ie, using alternative tissue-extraction methods in those undergoing minimally invasive supracervical hysterectomy and sacral colpopexy for pelvic organ prolapse).
Few of the data published on the risks of morcellation are of very high quality in terms of scientific rigor or methodology. The best thing we can do as a gynecologic surgical community is conduct sound quality-improvement (QI) programs, disseminate our QI results, publish our data, establish guidelines for best practices in uterine tissue extraction, and collaborate readily to increase the scholarly output on this issue so that national societies and government regulatory agencies have better-quality data to inform future policy on this issue.
A case-based approach
OBG Management: How would you approach tissue extraction in the following case?
CASE: A desire for myomectomy
A 35-year-old woman (G1P1) who delivered by cesarean has an 8-cm symptomatic intramural fibroid. She has regular heavy periods that have led to anemia (hemoglobin level = 10 mg/dL). Her medical history is negative for malignancy, pelvic radiation, or tamoxifen use, and she wants to preserve her fertility. Magnetic resonance imaging (MRI) confirms an 8-cm fibroid. Endometrial biopsy results are negative.
Dr. Fader: At Johns Hopkins, as at many other centers, we use well-defined criteria to determine whether a minimally invasive approach (and tissue extraction) might be appropriate. We also individualize treatment and surgical decision-making on a case-by-case basis. Any candidate for minimally invasive surgery and tissue extraction for uterine fibroids must undergo a thorough preoperative assessment.
Johns Hopkins preoperative assessment criteria include:
- endometrial biopsy
- imaging (often MRI)
- a detailed history and physical, with a comprehensive review of risk factors for malignancy, including family and genetic history or a personal history of malignancy, pelvic radiation, tamoxifen use, or BRCA or hereditary nonpolyposis colorectal cancer (HNPCC) deleterious mutation carrier status, among other things.
- In addition, all cases are discussed at a peer-reviewed, preoperative conference to ensure that a thorough work-up has been conducted and to verify the patient’s candidacy for a minimally invasive procedure with tissue extraction. As the FDA recommends, we conduct an enhanced informed consent process and counsel patients being considered for tissue extraction about the risk of occult sarcoma.
Our top priority is patient safety, so until more data are available, we no longer perform open electromechanical morcellation. We perform all tissue extraction within a containment system and under institutional review board protocol. We primarily perform tissue extraction via scalpel morcellation.
OBG Management: How do the patient’s wishes factor into the decision to perform minimally invasive surgery with morcellation?
Dr. Fader: Our patients make their own decisions regarding surgical approach and procedure after undergoing extensive counseling about the risks and benefits of the proposed procedures. I certainly would offer a patient like the one described in this case the opportunity for a minimally invasive approach (if, after thorough preoperative evaluation, she were deemed to be at very low risk for uterine malignancy). In my experience, most women opt for the minimally invasive approach in this setting; however, if a patient declines minimally invasive surgery, I respect her decision.
Tissue extraction in perimenopause
CASE: A desire for myomectomy at age 48
OBG Management: How would you approach the same case if the patient were a 48-year-old perimenopausal woman?
Dr. Fader: In perimenopausal women, we are more selective about performing morcellation, given the recent FDA safety statement, and because the incidence of occult cancer starts to increase slightly in this patient cohort (although it doesn’t precipitously increase until well into the postmenopausal period).
In addition, myomectomy may have less value in a 48-year-old, given the lower likelihood of achieving successful fertility, although there are exceptions. US cancer statistics and studies on morcellation demonstrate that the vast majority of women in their 30s and 40s have an extremely low risk for sarcomas and other uterine malignancies.2
In select cases in which a woman has undergone a comprehensive preoperative work-up, has a stable-appearing fibroid(s), and is well-educated and counseled about the pros and cons of morcellation, we would consider performing a procedure with contained tissue extraction. As a general matter, however, I would be more inclined to offer a 48-year-old in this situation a uterine artery embolization or minimally invasive hysterectomy than a myomectomy procedure, especially given the recent study by Wright and colleagues demonstrating the significantly increased risks of uterine cancer at myomectomy surgery for a woman in her late 40s or early 50s.18
Preoperative assessment should be comprehensive
OBG Management: What preoperative evaluation do you perform when tissue extraction, including morcellation, is an issue?
Dr. Fader: It is the policy at Johns Hopkins that all women being considered for minimally invasive surgery and tissue extraction must undergo a rigorous preoperative work-up that includes:
- a comprehensive history and physical (to exclude malignancy and risk for occult malignancy)
- an endometrial evaluation (most commonly, an endometrial biopsy)
- uterine imaging (with longitudinal evaluation of imaging findings if performed previously)
- discussion of each patient case at peer-reviewed, preoperative department conferences.
We have separate conferences for the gynecology and gynecologic oncology services and have employed this practice for many years at Hopkins. We are also studying the role of serum lactate dehydrogenase (LDH) isoenzyme levels in stratifying women with uterine fibroids versus cancer/sarcoma.19
OBG Management: Which patients would you exclude from the electromechanical morcellation option?
Dr. Fader: As the American College of Obstetricians and Gynecologists, the AAGL, the Society of Gynecologic Oncology, and the Society of Gynecologic Surgeons appear to agree:
- When utilized in select patients of reproductive age, minimally invasive surgery and morcellation are beneficial.
- Morcellation should categorically not be performed in any woman who has a known or suspected uterine (or other gynecologic) malignancy.2,3
At our institution, we have significantly curtailed the use of electromechanical morcellation at this time and especially do not perform it in women aged 50 or older or in those with confirmed postmenopausal status. We also do not perform electromechanical morcellation in women with a personal history of uterine, cervical, or ovarian preinvasive or invasive cancer, or in women with a strong family history of gynecologic malignancy.
Other populations we exclude are women with:
- a history of mitotically active or atypical fibroids (as determined at previous myomectomy)
- known BRCA or HNPCC deleterious mutation, hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome, hereditary childhood retinoblastoma, or other genetic predisposition to uterine or ovarian cancer
- a history of pelvic radiation
- a history of tamoxifen use.
Counseling the patient
OBG Management: If tissue extraction is an issue, and morcellation will be necessary for a minimally invasive approach, how do you counsel the patient?
Dr. Fader: We have an informed consent protocol we use at Hopkins in this regard. We speak extensively to our patients about the fact that every procedure or intervention performed in medicine carries a benefit/risk ratio. We inform patients of the FDA morcellation safety statement—that their fibroid or fibroids may contain unexpected cancerous tissue and that laparoscopic electromechanical morcellation may spread the cancer and possibly worsen their prognosis. We also explain that, while the FDA quotes a risk of approximately 1 in 350 for occult sarcoma, that this data review was somewhat limited in scope and included postmenopausal women in the estimates. Based on the best available published systematic reviews and internal Hopkins data, we believe the risk of morcellating an occult uterine malignancy in a woman of reproductive age is more likely on the order of approximately 1 in 1,700 to 1 in 8,333.
We discuss our institution’s approach to tissue extraction (ie, that we no longer perform open electromechanical morcellation but instead perform contained tissue extraction on an institutional review board protocol). We tell patients that contained tissue-extraction practices are still experimental, although there is published literature preliminarily supporting the safety of the practice. In addition, we discuss the fact that contained tissue extraction has been used for years to safely remove large intra-abdominal specimens from laparoscopic incisions, from adnexal tissue to gallbladder, spleen, kidney, intestinal, and appendiceal specimens.
We also explain that, as physicians, we do our best to ensure that risks are minimized and reasonable in relation to anticipated benefits but that, even when we use the very best diagnostic measures, no test is 100% sensitive or specific to rule out malignancy in this setting (or in any setting, actually).
Finally, we discuss the fact that minimally invasive surgery and tissue extraction practices performed in appropriate patients by skilled surgeons conclusively benefit hundreds of thousands of women each year around the world. That is a narrative that has been somewhat lacking in the recent dialogue about tissue-extraction practices.
OBG Management: What do we know about outcomes when a leiomyosarcoma is inadvertently morcellated?
Dr. Fader: This is considered a “cut-through” procedure, in that a cancerous tumor that is potentially contained to an organ is not removed intact or with clean margins. The morcellation procedure effectively cuts through the occult cancer, which is not desirable. Intact surgical removal of uterine cancers or sarcomas is the mainstay of therapy for these malignancies, based on NCCN guidelines.22
OBG Management: Does a morcellated leiomyosarcoma carry a worse prognosis than an unmorcellated leiomyosarcoma?
Dr. Fader: When it comes to morcellated versus “intact” uterine leiomyosarcoma, we enter a largely data-free zone. We don’t know with certainty whether the outcome is worsened. If we’re being intellectually honest, we must admit the possibility that a morcellated cancer is more likely to be disseminated, rendering it potentially more difficult to treat. However, sarcomas primarily spread by hematogenous dissemination. It is quite possible that even the act of incising an intact fibroid in an open abdominal procedure or performing a supracervical or total hysterectomy without morcellation may still result in hematogenous cancer dissemination. So there is no indication yet that electromechanical morcellation poses a unique and higher risk of cancer upstaging or worse prognosis compared with techniques such as open myomectomy, supracervical hysterectomy, or hysteroscopic myomectomy.
In addition, the prognosis associated with even early-stage uterine leiomyosarcoma is uniformly poor. A published study from Hopkins that included 108 patients with uterine leiomyosarcoma suggests that the recurrence rate and survival of patients with early-stage, “intact” leiomyosarcoma are very poor and comparable to the survival rate documented in the literature for women with morcellated sarcomas.23
The few retrospective studies that exist suggesting worse outcomes with morcellation have limitations that preclude any definitive conclusions.2 These studies are marred by small numbers, heterogeneity in morcellation practices, poor follow-up times, and a lack of detail regarding how patients with morcellated versus unmorcellated sarcomas were treated. Nevertheless, a couple of these small retrospective reports indicate the possibility of worse outcomes in women with morcellated uterine sarcomas, compared with historical controls with intact sarcoma removal.
Is the morcellator at fault—or the user?
OBG Management: Some would argue that even one case of a morcellated uterine sarcoma is too many. How would you respond?
Dr. Fader: There is no doubt that a handful of women each year have been harmed by morcellation practices. Those women deserve our dedication and best efforts to learn how to better treat morcellated sarcomas—and more importantly—how to mitigate the risks associated with morcellation practices and reduce the risk of preventable harm for all women undergoing minimally invasive fibroid procedures. I think the single best thing we can do to mitigate risk is to be more conscientious about selecting our patients for tissue-extraction procedures (ie, strict selection criteria, appropriate preoperative work-ups). If we did this, we likely would reduce the number of oncologic morcellator mishaps by 50% to 80% without changing anything else.
When we closely scrutinize the literature, and when I reflect on the women with morcellated cancers that we have cared for at Hopkins, we observe that some (but not all) “occult” uterine cancers were not that hidden after all and may have been detected preoperatively if an effort had been made.
We have noted a number of patients in this setting who experienced harm not because a specific device was used to cut up their uterus but because they were never appropriate candidates for the procedure in the first place. For instance, I recently cared for an elderly woman with a morcellated uterine cancer who underwent a laparoscopic supracervical hysterectomy without an appropriate preoperative work-up (ie, no endometrial biopsy or imaging) or informed consent about the possibility that she might have cancer. If an elderly woman presents with concerning symptoms related to her uterus (ie, enlargement, bleeding), she must be evaluated and counseled regarding the considerable risk of potential malignancy. Even in the setting of a normal work-up, I don’t believe it is a good idea to perform electromechanical morcellation in higher-risk women, including elderly women. That does not mean that select, well-screened women cannot be considered for alternative tissue-extraction techniques, but the risks and benefits must be carefully weighed in each patient, and informed consent must be obtained.
By continuing to refine the safety features of the electromechanical morcellator devices and choosing patients more carefully for minimally invasive procedures and tissue extraction, we likely will reduce the risk of preventable harm in women undergoing gynecologic surgery.
Can leiomyosarcoma be detected preoperatively?
OBG Management: How can we improve preoperative detection of uterine malignancy, particularly leiomyosarcoma?
Dr. Fader: For starters, we can improve detection of uterine cancers by simply looking for them. Almost all epithelial uterine cancers will be detectable by endometrial sampling. A comprehensive history and physical and uterine imaging also may be helpful. And although they are more difficult to detect than epithelial uterine cancers, it is a myth that sarcomas cannot be diagnosed preoperatively. Investigators from Columbia University retrospectively evaluated the ability to preoperatively detect epithelial uterine cancers and uterine sarcomas on final pathology. In 72 women who were ultimately diagnosed with a sarcoma, preoperative endometrial sampling suggested an invasive tumor in 86% and predicted the correct histology in 64%. In fact, the rate of detection of invasive cancer by preoperative sampling was not statistically different among sarcomas than it was among epithelial uterine cancers, although there was less of a correlation with appropriate histology seen with endometrial biopsy.20
That being said, sarcomas can be missed, especially in younger women who have extremely large, degenerated, or necrotic-appearing fibroids. Improvements in diagnostic testing are desperately needed to help distinguish benign fibroids from sarcomas, as there are no reliable modalities to exclude a sarcoma at this time. MRI appears to be the most useful imaging modality, although it cannot definitively distinguish a fibroid from a sarcoma. However, a fairly constant finding in leiomyosarcomas is the absence of calcifications. Further, some studies also suggest that ill-defined margins are consistent with a sarcoma. Finally, several centers, including our own, are studying novel biologic markers and revisiting the utility of previously described markers such as LDH in the preoperative detection of uterine sarcoma.21
The way forward
OBG Management: You have said, “Keep patients informed and safe but avoid being too reactionary.” Could you expand on this statement?
Dr. Fader: Certainly. There are many examples throughout the history of medicine in which treatments have brought benefit to thousands or millions of individuals but may cause harm in a select few. We know that when controversial medical issues have arisen in the past, the pendulum has swung widely in terms of societal response.
For example, the landmark Women’s Health Initiative had an immediate and adverse impact on hormone replacement therapy (HRT) administration. The increased risk of cardiovascular disease and breast cancer observed with use of long-term combination therapy with conjugated equine estrogen and medroxyprogesterone acetate prompted many US health-care providers to abandon use of HRT—until more contemporary data demonstrated that, in younger, healthier postmenopausal women, these adverse events were very rare and the benefits of HRT outweighed the risks.
Can we avoid stroke, heart attack, and breast cancer in all younger women taking HRT? Of course not. But we counsel women about the benefit/risk ratio of the therapy and advise them that the likelihood of these events is rare.
Similarly, as with the HRT analogy, younger women have lower risks associated with surgery and morcellation, compared with older women, and are more likely to derive benefit from the procedure (after ensuring appropriate candidacy with a comprehensive preoperative evaluation and informed consent).
However, if the expectation is that no cases of harm will ever occur with a surgical device or procedure in order for it to be deemed acceptable and safe to use in practice, then that is simply an impossible standard to uphold. There is no device, medication, or intervention I know of in medicine that is completely risk-free.
OBG Management: Are there other examples of this type of benefit/risk assessment?
Dr. Fader: Yes. For instance, tamoxifen is a nonsteroidal anti-estrogen agent approved by the FDA for adjuvant treatment of breast cancer, treatment of metastatic breast cancer, and reduction of breast cancer incidence in high-risk women. Tamoxifen has effectively reduced breast cancer rates and significantly improved survival in select breast cancer patients. Yet, it is well known that long-term use of tamoxifen is associated with a twofold increased risk of uterine cancer and uterine sarcoma—a likely far more commonly occurring adverse event than an occult, morcellated uterine sarcoma during a minimally invasive gynecologic procedure.
Should the FDA ban or significantly curtail the use of tamoxifen? No, not likely, because the benefits far outweigh the risks in previvors and hormone-positive breast cancer survivors. “Keep patients informed and safe but avoid being too reactionary” means that we must do our due diligence as physicians by comprehensively counseling and obtaining informed consent from our patients before performing medical interventions. We also must closely scrutinize and improve upon practices that may cause harm.
However, what this also means is that while it may be prudent to restrict or limit a surgical practice in select higher-risk populations or modify it in some way to make it safer, we shouldn’t necessarily completely abandon or ban a practice that has benefited hundreds of thousands of patients at low risk of harm until we have objectively reviewed all of the available science and fully understand the implications of a practice change (ie, would the risks of preventable harm be even greater for women if more of them had to undergo open abdominal surgery?). We need continued cool heads and sound scientific reasoning to decide upon health-care policy changes or treatment paradigm shifts.
At the end of the day, however, it is paramount that we mitigate patient harm. The subject of tissue extraction during minimally invasive surgery is a complex and nuanced issue that merits continued study and open-minded and intelligent dialogue between patient stakeholders, clinicians, scientists, industry, ethicists, regulatory agencies, and the press. I think we can all appreciate how humbling and challenging the morcellation issue has been for many of us, especially our patients—particularly the unfortunate women who have been diagnosed with a uterine sarcoma.
Like many of my colleagues, I have been privileged to care for a number of women with uterine leiomyosarcoma. It is a devastating disease, and the prognosis is very poor, whether it is morcellated or removed intact. We are fortunate that the vast majority of women who undergo a minimally invasive procedure for fibroids or other presumed benign indications will not be at risk for an occult malignancy. But what can we do now to continue to offer the benefits of minimally invasive surgery and tissue extraction to women while simultaneously reducing the risk to the select few who will develop a rare uterine cancer?
We can all make a greater effort to more carefully select our patients for minimally invasive surgery and tissue extraction, to limit the performance of open electromechanical morcellation and collaborate in studying the role of refined tissue-extraction techniques and containment systems, to enhance the informed consent process, to develop improved diagnostic tests for preoperative cancer detection, and to conduct higher-quality studies on minimally invasive tissue-extraction techniques for regulatory agencies to review in the near future. It also goes without saying that we need more federal funding to study rare tumors (and gynecologic cancers in general) and develop better sarcoma treatments.
Although there is no medical treatment or surgical procedure that is completely risk-free, interventions such as HRT, tamoxifen, and uterine morcellation—when used in appropriate patients and for appropriate indications—will allow preventable harm to be minimized and make it possible for countless women to continue to derive tremendous benefit.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed February 3, 2014.
2. AAGL. Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed February 3, 2015.
3. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery: a special report. http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Power-Morcellation-and-Occult-Malignancy-in-Gynecologic-Surgery. Published May 2014. Accessed February 3, 2015.
4. US Food and Drug Administration. Updated Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Published November 24, 2014. Accessed February 2, 2015.
5. American Cancer Society. Uterine sarcoma: What is uterine sarcoma? http://www.cancer.org/cancer/uterinesarcoma/detailedguide/uterine-sarcoma-what-is-uterine-sarcoma. Updated January 12, 2015. Accessed February 3, 2015.
6. D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131–139.
7. Chittawar B, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2014;10:CD004638. doi:10.1002/14651858.CD004638.pub3.
8. Mori KM, Neubauer NL. Minimally invasive surgery in gynecologic oncology. ISRN Obstet Gynecol. 2013; article ID 312982. http://dx.doi.org/10.1155/2013/312982. Accessed February 2, 2015.
9. Li G, Yan X, Shang H, Wang G, Chen L, Han Y. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib IIa cervical cancer. Gynecol Oncol. 2007;105(1):176–180.
10. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study Lap2. J Clin Oncol. 2009;27(32):5331–5336.
11. Ramos A, Fader AN, Long Roche K. Surgical cytoreduction for disseminated benign disease after open power uterine morcellation. Obstet Gynecol. 2014;125(1):99–102.
12. Theben J, Schellong A, Altgassen C, Kelling K, Schneider S, Grobe-Drieling D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): an analysis of 1,584 LASH cases. Arch Gynecol Obstet. 2013;287(3):455–462.
13. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
14. Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One. 2012;7(11):e50058.
15. Pritts EA, Parker WH, Brown J, Olive DL. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22(1):26–33.
16. Wright JD, Tergas AI, Burke WM, et al. Prevalence of uterine pathology in women undergoing minimally invasive hysterectomy employing electric power morcellation. JAMA. 2014;312(12):1253–1255.
17. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA. 2014;312(12):1253–1255.
18. Wright JD, Tergas AI, Cul R, et al. Use of electric power morcellation and prevalence of underlying cancer in women who undergo myomectomy. JAMA Oncol. 2015; published online February 19, 2015. doi:10.1001/jamaoncol.2014.206.
19. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002; 12:354.
20. Ricci S, Giuntoli RL 2nd, Eisenhauer E, et al. Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecol Oncol. 2013;131(3):629–633.
21. Bansal N, Herzog TJ, Burke W, Cohen CJ, Wright JD. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008;110(1):43–48.
22. Koh WJ, Greer BE, Abu-Rustum NR, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw. 2014;12(2):248–280.
23. Schwartz LB, Zawin M, Carcangiu ML, Lange R, McCarthy S. Does pelvic magnetic resonance imaging differentiate among the histologic subtypes of uterine leiomyomata? Fertil Steril. 1998;70(3):580–587.
The year 2014 marked a sea change in our approach to tissue extraction during minimally invasive surgery. The US Food and Drug Administration (FDA) initiated this transformation in April, when it issued a safety warning on the use of open power morcellation.1 A flurry of statements on the practice followed from professional societies, capped, in late November, with another statement from the FDA.2–4 The new bottom line: The use of open electromechanical (“power”) morcellation is contraindicated in perimenopausal and postmenopausal women, as well as in those who are known or suspected to have a malignancy.4
Most of the concern to date has centered on the risk that an occult leiomyosarcoma could be morcellated inadvertently during uterine surgery, an event that may worsen the prognosis for the patient. To get a gynecologic oncologist’s take on the controversy, OBG Management caught up with Amanda Nickles Fader, MD, director of the Kelly Gynecologic Oncology Service at Johns Hopkins University. Dr. Fader’s perspective is unique in that she treats a relatively high number of patients who have leiomyosarcoma and other uterine cancers.
In this Q&A, we discuss the patient population at Johns Hopkins; why Dr. Fader is especially qualified to speak to the future of electromechanical morcellation in gynecologic surgery; the benefits and risks of minimally invasive surgery, including tissue extraction; her recommendations for preoperative evaluation and counseling of patients undergoing uterine surgery; and guidance on how the specialty of gynecologic surgery should proceed in the future.
OBG Management: Dr. Fader, by way of introduction, could you characterize your patient population?
Amanda Nickles Fader, MD: Like most gynecologic oncologists, I primarily treat women with cancers of the uterus, ovary, cervix, and vulva. Many of us also have the opportunity to treat a number of women each year with complex benign gynecologic conditions that require surgery. As someone who is extremely interested in rare gynecologic tumors, I also treat a relatively high volume of women diagnosed with uterine sarcoma.
Approximately 75% of the women I see in my practice have preinvasive or invasive cancer, and 25% have a benign condition, such as enlarged fibroids or advanced-stage endometriosis.
OBG Management: How many cases of uterine sarcoma do you encounter on an annual basis?
Dr. Fader: Uterine sarcomas are very rare. They represent only 2% of all uterine cancers. Put into perspective, that means that about 0.4 cases of leiomyosarcoma occur in every 100,000 US women—most commonly postmenopausal women. Leiomyosarcoma is a biologically aggressive, high-grade malignancy that often is lethal.5
Endometrial stroma sarcoma is even less common—only 0.3 cases occur in every 100,000 US women. However, this tumor type is more indolent, often diagnosed at an earlier stage, and potentially curable with surgery (with or without hormonal therapy).6
As a referral center for rare uterine tumors, the Kelly Gynecologic Oncology Service and the Sarcoma Center at Johns Hopkins see approximately 35 to 40 new cases of uterine sarcoma annually for treatment of primary disease or recurrence. An additional 15 to 20 consult cases are reviewed from outside hospitals each year by our gynecologic pathology department.
Why a minimally invasive approach is vital
OBG Management: When it comes to uterine surgery for presumed benign conditions, why is a minimally invasive approach important?
Dr. Fader: Minimally invasive surgery clearly benefits women and is one of the greatest advances of the past half-century within our field. Randomized controlled trials and meta-analyses have demonstrated without question that women who undergo minimally invasive surgery for benign conditions or early-stage cancerous gynecologic conditions have superior clinical outcomes, compared with women who undergo surgery via laparotomy.7,8 These outcomes include fewer perioperative complications (including fewer cases of surgical site infection, venous thromboembolism, wound dehiscence, and hospital readmission), shorter hospital stays, less pain, faster recovery, and fewer adhesions. And when women with early-stage cancers undergo minimally invasive surgery, randomized controlled trials show, they have a stage-specific survival rate similar to that observed in women treated with laparotomy.9
Benefits and risks of tissue extraction in minimally invasive surgery
OBG Management: What are the main benefits of tissue extraction, including morcellation?
Dr. Fader: Tissue extraction is a practice that has allowed us to offer minimally invasive surgery to countless more women than we could have in the recent past. It is a technique in which a large specimen (typically a uterus or fibroid) is fragmented into smaller parts in order to remove it through a small laparoscopic incision or orifice (vagina, umbilicus). Without tissue-extraction practices, thousands of women who undergo myomectomy each year to conserve their fertility and hundreds of thousands of women who require hysterectomy potentially would have to undergo a more painful and risky surgery through a larger abdominal incision. That would not be desirable, as we know conclusively that laparotomy is associated with worse outcomes—and even an increased risk of mortality—compared with minimally invasive surgery performed by experienced surgeons.10
Tissue extraction can be approached in a variety of ways. It can be performed with a scalpel, with a resectoscope, or with an electromechanical morcellator. Tissue extraction can be performed within the uterine cavity, through the vagina, within the abdominal compartment, or within a containment system in any of those compartments.
OBG Management: What are the risks of tissue extraction?
Dr. Fader: The risks of tissue extraction with electromechanical morcellation include potential injury to intra-abdominal organs and vasculature and risk of dissemination of an occult (ie, undiagnosed) uterine cancer. A report by our research group also demonstrated the risks of dissemination of benign uterine tissue requiring subsequent surgery in the setting of open electromechanical morcellation.11 The risk of these events occurring in the hands of a thoughtful and experienced surgeon who conducts comprehensive preoperative patient evaluations is extremely low. However, recent evidence demonstrates that a handful of women worldwide are diagnosed with an occult uterine cancer each year during a morcellation procedure.12–14 Although it is a very rare event (given that most women undergoing hysterectomy and myomectomy procedures are of reproductive age and unlikely to have uterine cancer), this risk is a serious issue. There is an exigent need for the gynecologic surgical community to develop better approaches to tissue extraction that minimize preventable harm in women.
Needed: high-quality data on the risks of morcellation
OBG Management: How does the recent FDA statement urging caution with the use of open power morcellation factor into this equation? The most recent FDA statement noted that open power morcellation is contraindicated in perimenopausal and postmenopausal women.4
Dr. Fader: The FDA’s concern is legitimate. However, the magnitude of the risk of occult uterine sarcoma in women undergoing presumed benign gynecologic surgery has been scrutinized and debated. The FDA panel quoted a risk that roughly 1 in 350 women undergoing presumed benign gynecologic surgery for fibroids will have an occult leiomyosarcoma diagnosed. However, more recent systematic reviews and a review of the prospective published literature demonstrate that the risk is more likely on the order of 1 in 1,700 to 1 in 8,333 women.15 The risk may be even lower in gynecologic surgery practices that see a high volume of hysterectomy/myomectomy cases and utilize meticulous preoperative patient selection criteria to establish a woman’s candidacy for tissue-extraction procedures.
I am concerned with how “occult sarcoma” discovered during surgery for “presumed benign gynecologic disease is being defined in the literature. There is no uniform definition being used. A cancer in this setting is only truly occult or undiagnosed if the physician was thinking about it and made every effort to rule out cancer preoperatively, and the morcellation procedure was performed in a low-risk population (but cancer was still diagnosed on final pathology in this population). However, in the majority of the morcellation studies in the literature, it is not clear that thorough preoperative evaluations occurred in patients to rule out uterine malignancy—in fact, there is a paucity of published information regarding establishing appropriate patient candidacy for morcellation procedures. So we can’t derive any conclusions regarding whether “occult” sarcomas were truly undetectable or not in the published literature.
In addition, the literature is very clear that advancing age and postmenopausal status are risk factors for uterine malignancy.16 The vast majority of uterine sarcoma cases are diagnosed in postmenopausal women. Yet, in one large US population-based hysterectomy study, 20% of the morcellated cases (and the overwhelming majority of the “occult” morcellated uterine cancers) occurred in postmenopausal women!17
Further, in a more recent study by the same authors, again the risks of morcellating a uterine cancer in a population undergoing myomectomy was significantly higher in a postmenopausal population and occurred only rarely in women younger than age 40. But it should come as no surprise that a greater incidence of uterine cancer was identified in these older cohorts—cancer risk increases precipitously with age. That’s not “occult”; that’s basic cancer epidemiology.
In other words, we cannot assume from population-based administrative claims data that morcellation performed in inappropriate populations at higher risk of uterine malignancy (in which we do not know whether patients were properly screened for the procedure preoperatively or whether they had risk factors for uterine cancer but were presumably poor candidates for morcellation due to age alone) helps us define the true incidence of “occult” sarcoma or cancer in a population.
These studies are provocative, however, and do inform us that, as women get older, we are apt to see a greater incidence of uterine cancer. We cannot safely assume that a postmenopausal or elderly woman with symptomatic or enlarging fibroids has “presumed benign disease”—it is cancer until proven otherwise, and we need to be looking for it preoperatively. Therefore, we need to be particularly careful with our surgical practices in this population—ie, the basis for the FDA’s recommendation to avoid morcellation in older women. And I agree with the FDA that open electromechanical morcellation generally is contraindicated in postmenopausal women. However, we need better data from large prospective studies to inform our understanding of the true incidence of undiagnosed or “occult” uterine sarcoma in women undergoing surgery for presumed benign disease. These future studies are likely to demonstrate what we already know—that in young, well-screened, well-selected candidates for minimally invasive hysterectomy or myomectomy, the risk of occult cancer is going to be exceptionally low.
OBG Management: Which is greater—the risks or benefits of tissue extraction?
Dr. Fader: Assessment of risks and benefits in medicine has everything to do with the intervention in question as applied to an individual patient. At the end of the day, there are risks and benefits to every medical or surgical treatment offered to patients in every medical and surgical discipline. But the risk of an occult uterine sarcoma is extremely low in a woman of reproductive age who has been properly selected and comprehensively evaluated for minimally invasive surgery and tissue extraction prior to surgery. And this small—though not negligible—risk must be weighed against the much higher risk of harm that may be incurred with an open abdominal procedure, compared with minimally invasive surgery.
However, in many elderly women, the risks of tissue extraction with an electromechanical morcellator may outweigh the benefits. Even so, there are exceptions in which tissue extraction may be acceptable in postmenopausal women (ie, using alternative tissue-extraction methods in those undergoing minimally invasive supracervical hysterectomy and sacral colpopexy for pelvic organ prolapse).
Few of the data published on the risks of morcellation are of very high quality in terms of scientific rigor or methodology. The best thing we can do as a gynecologic surgical community is conduct sound quality-improvement (QI) programs, disseminate our QI results, publish our data, establish guidelines for best practices in uterine tissue extraction, and collaborate readily to increase the scholarly output on this issue so that national societies and government regulatory agencies have better-quality data to inform future policy on this issue.
A case-based approach
OBG Management: How would you approach tissue extraction in the following case?
CASE: A desire for myomectomy
A 35-year-old woman (G1P1) who delivered by cesarean has an 8-cm symptomatic intramural fibroid. She has regular heavy periods that have led to anemia (hemoglobin level = 10 mg/dL). Her medical history is negative for malignancy, pelvic radiation, or tamoxifen use, and she wants to preserve her fertility. Magnetic resonance imaging (MRI) confirms an 8-cm fibroid. Endometrial biopsy results are negative.
Dr. Fader: At Johns Hopkins, as at many other centers, we use well-defined criteria to determine whether a minimally invasive approach (and tissue extraction) might be appropriate. We also individualize treatment and surgical decision-making on a case-by-case basis. Any candidate for minimally invasive surgery and tissue extraction for uterine fibroids must undergo a thorough preoperative assessment.
Johns Hopkins preoperative assessment criteria include:
- endometrial biopsy
- imaging (often MRI)
- a detailed history and physical, with a comprehensive review of risk factors for malignancy, including family and genetic history or a personal history of malignancy, pelvic radiation, tamoxifen use, or BRCA or hereditary nonpolyposis colorectal cancer (HNPCC) deleterious mutation carrier status, among other things.
- In addition, all cases are discussed at a peer-reviewed, preoperative conference to ensure that a thorough work-up has been conducted and to verify the patient’s candidacy for a minimally invasive procedure with tissue extraction. As the FDA recommends, we conduct an enhanced informed consent process and counsel patients being considered for tissue extraction about the risk of occult sarcoma.
Our top priority is patient safety, so until more data are available, we no longer perform open electromechanical morcellation. We perform all tissue extraction within a containment system and under institutional review board protocol. We primarily perform tissue extraction via scalpel morcellation.
OBG Management: How do the patient’s wishes factor into the decision to perform minimally invasive surgery with morcellation?
Dr. Fader: Our patients make their own decisions regarding surgical approach and procedure after undergoing extensive counseling about the risks and benefits of the proposed procedures. I certainly would offer a patient like the one described in this case the opportunity for a minimally invasive approach (if, after thorough preoperative evaluation, she were deemed to be at very low risk for uterine malignancy). In my experience, most women opt for the minimally invasive approach in this setting; however, if a patient declines minimally invasive surgery, I respect her decision.
Tissue extraction in perimenopause
CASE: A desire for myomectomy at age 48
OBG Management: How would you approach the same case if the patient were a 48-year-old perimenopausal woman?
Dr. Fader: In perimenopausal women, we are more selective about performing morcellation, given the recent FDA safety statement, and because the incidence of occult cancer starts to increase slightly in this patient cohort (although it doesn’t precipitously increase until well into the postmenopausal period).
In addition, myomectomy may have less value in a 48-year-old, given the lower likelihood of achieving successful fertility, although there are exceptions. US cancer statistics and studies on morcellation demonstrate that the vast majority of women in their 30s and 40s have an extremely low risk for sarcomas and other uterine malignancies.2
In select cases in which a woman has undergone a comprehensive preoperative work-up, has a stable-appearing fibroid(s), and is well-educated and counseled about the pros and cons of morcellation, we would consider performing a procedure with contained tissue extraction. As a general matter, however, I would be more inclined to offer a 48-year-old in this situation a uterine artery embolization or minimally invasive hysterectomy than a myomectomy procedure, especially given the recent study by Wright and colleagues demonstrating the significantly increased risks of uterine cancer at myomectomy surgery for a woman in her late 40s or early 50s.18
Preoperative assessment should be comprehensive
OBG Management: What preoperative evaluation do you perform when tissue extraction, including morcellation, is an issue?
Dr. Fader: It is the policy at Johns Hopkins that all women being considered for minimally invasive surgery and tissue extraction must undergo a rigorous preoperative work-up that includes:
- a comprehensive history and physical (to exclude malignancy and risk for occult malignancy)
- an endometrial evaluation (most commonly, an endometrial biopsy)
- uterine imaging (with longitudinal evaluation of imaging findings if performed previously)
- discussion of each patient case at peer-reviewed, preoperative department conferences.
We have separate conferences for the gynecology and gynecologic oncology services and have employed this practice for many years at Hopkins. We are also studying the role of serum lactate dehydrogenase (LDH) isoenzyme levels in stratifying women with uterine fibroids versus cancer/sarcoma.19
OBG Management: Which patients would you exclude from the electromechanical morcellation option?
Dr. Fader: As the American College of Obstetricians and Gynecologists, the AAGL, the Society of Gynecologic Oncology, and the Society of Gynecologic Surgeons appear to agree:
- When utilized in select patients of reproductive age, minimally invasive surgery and morcellation are beneficial.
- Morcellation should categorically not be performed in any woman who has a known or suspected uterine (or other gynecologic) malignancy.2,3
At our institution, we have significantly curtailed the use of electromechanical morcellation at this time and especially do not perform it in women aged 50 or older or in those with confirmed postmenopausal status. We also do not perform electromechanical morcellation in women with a personal history of uterine, cervical, or ovarian preinvasive or invasive cancer, or in women with a strong family history of gynecologic malignancy.
Other populations we exclude are women with:
- a history of mitotically active or atypical fibroids (as determined at previous myomectomy)
- known BRCA or HNPCC deleterious mutation, hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome, hereditary childhood retinoblastoma, or other genetic predisposition to uterine or ovarian cancer
- a history of pelvic radiation
- a history of tamoxifen use.
Counseling the patient
OBG Management: If tissue extraction is an issue, and morcellation will be necessary for a minimally invasive approach, how do you counsel the patient?
Dr. Fader: We have an informed consent protocol we use at Hopkins in this regard. We speak extensively to our patients about the fact that every procedure or intervention performed in medicine carries a benefit/risk ratio. We inform patients of the FDA morcellation safety statement—that their fibroid or fibroids may contain unexpected cancerous tissue and that laparoscopic electromechanical morcellation may spread the cancer and possibly worsen their prognosis. We also explain that, while the FDA quotes a risk of approximately 1 in 350 for occult sarcoma, that this data review was somewhat limited in scope and included postmenopausal women in the estimates. Based on the best available published systematic reviews and internal Hopkins data, we believe the risk of morcellating an occult uterine malignancy in a woman of reproductive age is more likely on the order of approximately 1 in 1,700 to 1 in 8,333.
We discuss our institution’s approach to tissue extraction (ie, that we no longer perform open electromechanical morcellation but instead perform contained tissue extraction on an institutional review board protocol). We tell patients that contained tissue-extraction practices are still experimental, although there is published literature preliminarily supporting the safety of the practice. In addition, we discuss the fact that contained tissue extraction has been used for years to safely remove large intra-abdominal specimens from laparoscopic incisions, from adnexal tissue to gallbladder, spleen, kidney, intestinal, and appendiceal specimens.
We also explain that, as physicians, we do our best to ensure that risks are minimized and reasonable in relation to anticipated benefits but that, even when we use the very best diagnostic measures, no test is 100% sensitive or specific to rule out malignancy in this setting (or in any setting, actually).
Finally, we discuss the fact that minimally invasive surgery and tissue extraction practices performed in appropriate patients by skilled surgeons conclusively benefit hundreds of thousands of women each year around the world. That is a narrative that has been somewhat lacking in the recent dialogue about tissue-extraction practices.
OBG Management: What do we know about outcomes when a leiomyosarcoma is inadvertently morcellated?
Dr. Fader: This is considered a “cut-through” procedure, in that a cancerous tumor that is potentially contained to an organ is not removed intact or with clean margins. The morcellation procedure effectively cuts through the occult cancer, which is not desirable. Intact surgical removal of uterine cancers or sarcomas is the mainstay of therapy for these malignancies, based on NCCN guidelines.22
OBG Management: Does a morcellated leiomyosarcoma carry a worse prognosis than an unmorcellated leiomyosarcoma?
Dr. Fader: When it comes to morcellated versus “intact” uterine leiomyosarcoma, we enter a largely data-free zone. We don’t know with certainty whether the outcome is worsened. If we’re being intellectually honest, we must admit the possibility that a morcellated cancer is more likely to be disseminated, rendering it potentially more difficult to treat. However, sarcomas primarily spread by hematogenous dissemination. It is quite possible that even the act of incising an intact fibroid in an open abdominal procedure or performing a supracervical or total hysterectomy without morcellation may still result in hematogenous cancer dissemination. So there is no indication yet that electromechanical morcellation poses a unique and higher risk of cancer upstaging or worse prognosis compared with techniques such as open myomectomy, supracervical hysterectomy, or hysteroscopic myomectomy.
In addition, the prognosis associated with even early-stage uterine leiomyosarcoma is uniformly poor. A published study from Hopkins that included 108 patients with uterine leiomyosarcoma suggests that the recurrence rate and survival of patients with early-stage, “intact” leiomyosarcoma are very poor and comparable to the survival rate documented in the literature for women with morcellated sarcomas.23
The few retrospective studies that exist suggesting worse outcomes with morcellation have limitations that preclude any definitive conclusions.2 These studies are marred by small numbers, heterogeneity in morcellation practices, poor follow-up times, and a lack of detail regarding how patients with morcellated versus unmorcellated sarcomas were treated. Nevertheless, a couple of these small retrospective reports indicate the possibility of worse outcomes in women with morcellated uterine sarcomas, compared with historical controls with intact sarcoma removal.
Is the morcellator at fault—or the user?
OBG Management: Some would argue that even one case of a morcellated uterine sarcoma is too many. How would you respond?
Dr. Fader: There is no doubt that a handful of women each year have been harmed by morcellation practices. Those women deserve our dedication and best efforts to learn how to better treat morcellated sarcomas—and more importantly—how to mitigate the risks associated with morcellation practices and reduce the risk of preventable harm for all women undergoing minimally invasive fibroid procedures. I think the single best thing we can do to mitigate risk is to be more conscientious about selecting our patients for tissue-extraction procedures (ie, strict selection criteria, appropriate preoperative work-ups). If we did this, we likely would reduce the number of oncologic morcellator mishaps by 50% to 80% without changing anything else.
When we closely scrutinize the literature, and when I reflect on the women with morcellated cancers that we have cared for at Hopkins, we observe that some (but not all) “occult” uterine cancers were not that hidden after all and may have been detected preoperatively if an effort had been made.
We have noted a number of patients in this setting who experienced harm not because a specific device was used to cut up their uterus but because they were never appropriate candidates for the procedure in the first place. For instance, I recently cared for an elderly woman with a morcellated uterine cancer who underwent a laparoscopic supracervical hysterectomy without an appropriate preoperative work-up (ie, no endometrial biopsy or imaging) or informed consent about the possibility that she might have cancer. If an elderly woman presents with concerning symptoms related to her uterus (ie, enlargement, bleeding), she must be evaluated and counseled regarding the considerable risk of potential malignancy. Even in the setting of a normal work-up, I don’t believe it is a good idea to perform electromechanical morcellation in higher-risk women, including elderly women. That does not mean that select, well-screened women cannot be considered for alternative tissue-extraction techniques, but the risks and benefits must be carefully weighed in each patient, and informed consent must be obtained.
By continuing to refine the safety features of the electromechanical morcellator devices and choosing patients more carefully for minimally invasive procedures and tissue extraction, we likely will reduce the risk of preventable harm in women undergoing gynecologic surgery.
Can leiomyosarcoma be detected preoperatively?
OBG Management: How can we improve preoperative detection of uterine malignancy, particularly leiomyosarcoma?
Dr. Fader: For starters, we can improve detection of uterine cancers by simply looking for them. Almost all epithelial uterine cancers will be detectable by endometrial sampling. A comprehensive history and physical and uterine imaging also may be helpful. And although they are more difficult to detect than epithelial uterine cancers, it is a myth that sarcomas cannot be diagnosed preoperatively. Investigators from Columbia University retrospectively evaluated the ability to preoperatively detect epithelial uterine cancers and uterine sarcomas on final pathology. In 72 women who were ultimately diagnosed with a sarcoma, preoperative endometrial sampling suggested an invasive tumor in 86% and predicted the correct histology in 64%. In fact, the rate of detection of invasive cancer by preoperative sampling was not statistically different among sarcomas than it was among epithelial uterine cancers, although there was less of a correlation with appropriate histology seen with endometrial biopsy.20
That being said, sarcomas can be missed, especially in younger women who have extremely large, degenerated, or necrotic-appearing fibroids. Improvements in diagnostic testing are desperately needed to help distinguish benign fibroids from sarcomas, as there are no reliable modalities to exclude a sarcoma at this time. MRI appears to be the most useful imaging modality, although it cannot definitively distinguish a fibroid from a sarcoma. However, a fairly constant finding in leiomyosarcomas is the absence of calcifications. Further, some studies also suggest that ill-defined margins are consistent with a sarcoma. Finally, several centers, including our own, are studying novel biologic markers and revisiting the utility of previously described markers such as LDH in the preoperative detection of uterine sarcoma.21
The way forward
OBG Management: You have said, “Keep patients informed and safe but avoid being too reactionary.” Could you expand on this statement?
Dr. Fader: Certainly. There are many examples throughout the history of medicine in which treatments have brought benefit to thousands or millions of individuals but may cause harm in a select few. We know that when controversial medical issues have arisen in the past, the pendulum has swung widely in terms of societal response.
For example, the landmark Women’s Health Initiative had an immediate and adverse impact on hormone replacement therapy (HRT) administration. The increased risk of cardiovascular disease and breast cancer observed with use of long-term combination therapy with conjugated equine estrogen and medroxyprogesterone acetate prompted many US health-care providers to abandon use of HRT—until more contemporary data demonstrated that, in younger, healthier postmenopausal women, these adverse events were very rare and the benefits of HRT outweighed the risks.
Can we avoid stroke, heart attack, and breast cancer in all younger women taking HRT? Of course not. But we counsel women about the benefit/risk ratio of the therapy and advise them that the likelihood of these events is rare.
Similarly, as with the HRT analogy, younger women have lower risks associated with surgery and morcellation, compared with older women, and are more likely to derive benefit from the procedure (after ensuring appropriate candidacy with a comprehensive preoperative evaluation and informed consent).
However, if the expectation is that no cases of harm will ever occur with a surgical device or procedure in order for it to be deemed acceptable and safe to use in practice, then that is simply an impossible standard to uphold. There is no device, medication, or intervention I know of in medicine that is completely risk-free.
OBG Management: Are there other examples of this type of benefit/risk assessment?
Dr. Fader: Yes. For instance, tamoxifen is a nonsteroidal anti-estrogen agent approved by the FDA for adjuvant treatment of breast cancer, treatment of metastatic breast cancer, and reduction of breast cancer incidence in high-risk women. Tamoxifen has effectively reduced breast cancer rates and significantly improved survival in select breast cancer patients. Yet, it is well known that long-term use of tamoxifen is associated with a twofold increased risk of uterine cancer and uterine sarcoma—a likely far more commonly occurring adverse event than an occult, morcellated uterine sarcoma during a minimally invasive gynecologic procedure.
Should the FDA ban or significantly curtail the use of tamoxifen? No, not likely, because the benefits far outweigh the risks in previvors and hormone-positive breast cancer survivors. “Keep patients informed and safe but avoid being too reactionary” means that we must do our due diligence as physicians by comprehensively counseling and obtaining informed consent from our patients before performing medical interventions. We also must closely scrutinize and improve upon practices that may cause harm.
However, what this also means is that while it may be prudent to restrict or limit a surgical practice in select higher-risk populations or modify it in some way to make it safer, we shouldn’t necessarily completely abandon or ban a practice that has benefited hundreds of thousands of patients at low risk of harm until we have objectively reviewed all of the available science and fully understand the implications of a practice change (ie, would the risks of preventable harm be even greater for women if more of them had to undergo open abdominal surgery?). We need continued cool heads and sound scientific reasoning to decide upon health-care policy changes or treatment paradigm shifts.
At the end of the day, however, it is paramount that we mitigate patient harm. The subject of tissue extraction during minimally invasive surgery is a complex and nuanced issue that merits continued study and open-minded and intelligent dialogue between patient stakeholders, clinicians, scientists, industry, ethicists, regulatory agencies, and the press. I think we can all appreciate how humbling and challenging the morcellation issue has been for many of us, especially our patients—particularly the unfortunate women who have been diagnosed with a uterine sarcoma.
Like many of my colleagues, I have been privileged to care for a number of women with uterine leiomyosarcoma. It is a devastating disease, and the prognosis is very poor, whether it is morcellated or removed intact. We are fortunate that the vast majority of women who undergo a minimally invasive procedure for fibroids or other presumed benign indications will not be at risk for an occult malignancy. But what can we do now to continue to offer the benefits of minimally invasive surgery and tissue extraction to women while simultaneously reducing the risk to the select few who will develop a rare uterine cancer?
We can all make a greater effort to more carefully select our patients for minimally invasive surgery and tissue extraction, to limit the performance of open electromechanical morcellation and collaborate in studying the role of refined tissue-extraction techniques and containment systems, to enhance the informed consent process, to develop improved diagnostic tests for preoperative cancer detection, and to conduct higher-quality studies on minimally invasive tissue-extraction techniques for regulatory agencies to review in the near future. It also goes without saying that we need more federal funding to study rare tumors (and gynecologic cancers in general) and develop better sarcoma treatments.
Although there is no medical treatment or surgical procedure that is completely risk-free, interventions such as HRT, tamoxifen, and uterine morcellation—when used in appropriate patients and for appropriate indications—will allow preventable harm to be minimized and make it possible for countless women to continue to derive tremendous benefit.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The year 2014 marked a sea change in our approach to tissue extraction during minimally invasive surgery. The US Food and Drug Administration (FDA) initiated this transformation in April, when it issued a safety warning on the use of open power morcellation.1 A flurry of statements on the practice followed from professional societies, capped, in late November, with another statement from the FDA.2–4 The new bottom line: The use of open electromechanical (“power”) morcellation is contraindicated in perimenopausal and postmenopausal women, as well as in those who are known or suspected to have a malignancy.4
Most of the concern to date has centered on the risk that an occult leiomyosarcoma could be morcellated inadvertently during uterine surgery, an event that may worsen the prognosis for the patient. To get a gynecologic oncologist’s take on the controversy, OBG Management caught up with Amanda Nickles Fader, MD, director of the Kelly Gynecologic Oncology Service at Johns Hopkins University. Dr. Fader’s perspective is unique in that she treats a relatively high number of patients who have leiomyosarcoma and other uterine cancers.
In this Q&A, we discuss the patient population at Johns Hopkins; why Dr. Fader is especially qualified to speak to the future of electromechanical morcellation in gynecologic surgery; the benefits and risks of minimally invasive surgery, including tissue extraction; her recommendations for preoperative evaluation and counseling of patients undergoing uterine surgery; and guidance on how the specialty of gynecologic surgery should proceed in the future.
OBG Management: Dr. Fader, by way of introduction, could you characterize your patient population?
Amanda Nickles Fader, MD: Like most gynecologic oncologists, I primarily treat women with cancers of the uterus, ovary, cervix, and vulva. Many of us also have the opportunity to treat a number of women each year with complex benign gynecologic conditions that require surgery. As someone who is extremely interested in rare gynecologic tumors, I also treat a relatively high volume of women diagnosed with uterine sarcoma.
Approximately 75% of the women I see in my practice have preinvasive or invasive cancer, and 25% have a benign condition, such as enlarged fibroids or advanced-stage endometriosis.
OBG Management: How many cases of uterine sarcoma do you encounter on an annual basis?
Dr. Fader: Uterine sarcomas are very rare. They represent only 2% of all uterine cancers. Put into perspective, that means that about 0.4 cases of leiomyosarcoma occur in every 100,000 US women—most commonly postmenopausal women. Leiomyosarcoma is a biologically aggressive, high-grade malignancy that often is lethal.5
Endometrial stroma sarcoma is even less common—only 0.3 cases occur in every 100,000 US women. However, this tumor type is more indolent, often diagnosed at an earlier stage, and potentially curable with surgery (with or without hormonal therapy).6
As a referral center for rare uterine tumors, the Kelly Gynecologic Oncology Service and the Sarcoma Center at Johns Hopkins see approximately 35 to 40 new cases of uterine sarcoma annually for treatment of primary disease or recurrence. An additional 15 to 20 consult cases are reviewed from outside hospitals each year by our gynecologic pathology department.
Why a minimally invasive approach is vital
OBG Management: When it comes to uterine surgery for presumed benign conditions, why is a minimally invasive approach important?
Dr. Fader: Minimally invasive surgery clearly benefits women and is one of the greatest advances of the past half-century within our field. Randomized controlled trials and meta-analyses have demonstrated without question that women who undergo minimally invasive surgery for benign conditions or early-stage cancerous gynecologic conditions have superior clinical outcomes, compared with women who undergo surgery via laparotomy.7,8 These outcomes include fewer perioperative complications (including fewer cases of surgical site infection, venous thromboembolism, wound dehiscence, and hospital readmission), shorter hospital stays, less pain, faster recovery, and fewer adhesions. And when women with early-stage cancers undergo minimally invasive surgery, randomized controlled trials show, they have a stage-specific survival rate similar to that observed in women treated with laparotomy.9
Benefits and risks of tissue extraction in minimally invasive surgery
OBG Management: What are the main benefits of tissue extraction, including morcellation?
Dr. Fader: Tissue extraction is a practice that has allowed us to offer minimally invasive surgery to countless more women than we could have in the recent past. It is a technique in which a large specimen (typically a uterus or fibroid) is fragmented into smaller parts in order to remove it through a small laparoscopic incision or orifice (vagina, umbilicus). Without tissue-extraction practices, thousands of women who undergo myomectomy each year to conserve their fertility and hundreds of thousands of women who require hysterectomy potentially would have to undergo a more painful and risky surgery through a larger abdominal incision. That would not be desirable, as we know conclusively that laparotomy is associated with worse outcomes—and even an increased risk of mortality—compared with minimally invasive surgery performed by experienced surgeons.10
Tissue extraction can be approached in a variety of ways. It can be performed with a scalpel, with a resectoscope, or with an electromechanical morcellator. Tissue extraction can be performed within the uterine cavity, through the vagina, within the abdominal compartment, or within a containment system in any of those compartments.
OBG Management: What are the risks of tissue extraction?
Dr. Fader: The risks of tissue extraction with electromechanical morcellation include potential injury to intra-abdominal organs and vasculature and risk of dissemination of an occult (ie, undiagnosed) uterine cancer. A report by our research group also demonstrated the risks of dissemination of benign uterine tissue requiring subsequent surgery in the setting of open electromechanical morcellation.11 The risk of these events occurring in the hands of a thoughtful and experienced surgeon who conducts comprehensive preoperative patient evaluations is extremely low. However, recent evidence demonstrates that a handful of women worldwide are diagnosed with an occult uterine cancer each year during a morcellation procedure.12–14 Although it is a very rare event (given that most women undergoing hysterectomy and myomectomy procedures are of reproductive age and unlikely to have uterine cancer), this risk is a serious issue. There is an exigent need for the gynecologic surgical community to develop better approaches to tissue extraction that minimize preventable harm in women.
Needed: high-quality data on the risks of morcellation
OBG Management: How does the recent FDA statement urging caution with the use of open power morcellation factor into this equation? The most recent FDA statement noted that open power morcellation is contraindicated in perimenopausal and postmenopausal women.4
Dr. Fader: The FDA’s concern is legitimate. However, the magnitude of the risk of occult uterine sarcoma in women undergoing presumed benign gynecologic surgery has been scrutinized and debated. The FDA panel quoted a risk that roughly 1 in 350 women undergoing presumed benign gynecologic surgery for fibroids will have an occult leiomyosarcoma diagnosed. However, more recent systematic reviews and a review of the prospective published literature demonstrate that the risk is more likely on the order of 1 in 1,700 to 1 in 8,333 women.15 The risk may be even lower in gynecologic surgery practices that see a high volume of hysterectomy/myomectomy cases and utilize meticulous preoperative patient selection criteria to establish a woman’s candidacy for tissue-extraction procedures.
I am concerned with how “occult sarcoma” discovered during surgery for “presumed benign gynecologic disease is being defined in the literature. There is no uniform definition being used. A cancer in this setting is only truly occult or undiagnosed if the physician was thinking about it and made every effort to rule out cancer preoperatively, and the morcellation procedure was performed in a low-risk population (but cancer was still diagnosed on final pathology in this population). However, in the majority of the morcellation studies in the literature, it is not clear that thorough preoperative evaluations occurred in patients to rule out uterine malignancy—in fact, there is a paucity of published information regarding establishing appropriate patient candidacy for morcellation procedures. So we can’t derive any conclusions regarding whether “occult” sarcomas were truly undetectable or not in the published literature.
In addition, the literature is very clear that advancing age and postmenopausal status are risk factors for uterine malignancy.16 The vast majority of uterine sarcoma cases are diagnosed in postmenopausal women. Yet, in one large US population-based hysterectomy study, 20% of the morcellated cases (and the overwhelming majority of the “occult” morcellated uterine cancers) occurred in postmenopausal women!17
Further, in a more recent study by the same authors, again the risks of morcellating a uterine cancer in a population undergoing myomectomy was significantly higher in a postmenopausal population and occurred only rarely in women younger than age 40. But it should come as no surprise that a greater incidence of uterine cancer was identified in these older cohorts—cancer risk increases precipitously with age. That’s not “occult”; that’s basic cancer epidemiology.
In other words, we cannot assume from population-based administrative claims data that morcellation performed in inappropriate populations at higher risk of uterine malignancy (in which we do not know whether patients were properly screened for the procedure preoperatively or whether they had risk factors for uterine cancer but were presumably poor candidates for morcellation due to age alone) helps us define the true incidence of “occult” sarcoma or cancer in a population.
These studies are provocative, however, and do inform us that, as women get older, we are apt to see a greater incidence of uterine cancer. We cannot safely assume that a postmenopausal or elderly woman with symptomatic or enlarging fibroids has “presumed benign disease”—it is cancer until proven otherwise, and we need to be looking for it preoperatively. Therefore, we need to be particularly careful with our surgical practices in this population—ie, the basis for the FDA’s recommendation to avoid morcellation in older women. And I agree with the FDA that open electromechanical morcellation generally is contraindicated in postmenopausal women. However, we need better data from large prospective studies to inform our understanding of the true incidence of undiagnosed or “occult” uterine sarcoma in women undergoing surgery for presumed benign disease. These future studies are likely to demonstrate what we already know—that in young, well-screened, well-selected candidates for minimally invasive hysterectomy or myomectomy, the risk of occult cancer is going to be exceptionally low.
OBG Management: Which is greater—the risks or benefits of tissue extraction?
Dr. Fader: Assessment of risks and benefits in medicine has everything to do with the intervention in question as applied to an individual patient. At the end of the day, there are risks and benefits to every medical or surgical treatment offered to patients in every medical and surgical discipline. But the risk of an occult uterine sarcoma is extremely low in a woman of reproductive age who has been properly selected and comprehensively evaluated for minimally invasive surgery and tissue extraction prior to surgery. And this small—though not negligible—risk must be weighed against the much higher risk of harm that may be incurred with an open abdominal procedure, compared with minimally invasive surgery.
However, in many elderly women, the risks of tissue extraction with an electromechanical morcellator may outweigh the benefits. Even so, there are exceptions in which tissue extraction may be acceptable in postmenopausal women (ie, using alternative tissue-extraction methods in those undergoing minimally invasive supracervical hysterectomy and sacral colpopexy for pelvic organ prolapse).
Few of the data published on the risks of morcellation are of very high quality in terms of scientific rigor or methodology. The best thing we can do as a gynecologic surgical community is conduct sound quality-improvement (QI) programs, disseminate our QI results, publish our data, establish guidelines for best practices in uterine tissue extraction, and collaborate readily to increase the scholarly output on this issue so that national societies and government regulatory agencies have better-quality data to inform future policy on this issue.
A case-based approach
OBG Management: How would you approach tissue extraction in the following case?
CASE: A desire for myomectomy
A 35-year-old woman (G1P1) who delivered by cesarean has an 8-cm symptomatic intramural fibroid. She has regular heavy periods that have led to anemia (hemoglobin level = 10 mg/dL). Her medical history is negative for malignancy, pelvic radiation, or tamoxifen use, and she wants to preserve her fertility. Magnetic resonance imaging (MRI) confirms an 8-cm fibroid. Endometrial biopsy results are negative.
Dr. Fader: At Johns Hopkins, as at many other centers, we use well-defined criteria to determine whether a minimally invasive approach (and tissue extraction) might be appropriate. We also individualize treatment and surgical decision-making on a case-by-case basis. Any candidate for minimally invasive surgery and tissue extraction for uterine fibroids must undergo a thorough preoperative assessment.
Johns Hopkins preoperative assessment criteria include:
- endometrial biopsy
- imaging (often MRI)
- a detailed history and physical, with a comprehensive review of risk factors for malignancy, including family and genetic history or a personal history of malignancy, pelvic radiation, tamoxifen use, or BRCA or hereditary nonpolyposis colorectal cancer (HNPCC) deleterious mutation carrier status, among other things.
- In addition, all cases are discussed at a peer-reviewed, preoperative conference to ensure that a thorough work-up has been conducted and to verify the patient’s candidacy for a minimally invasive procedure with tissue extraction. As the FDA recommends, we conduct an enhanced informed consent process and counsel patients being considered for tissue extraction about the risk of occult sarcoma.
Our top priority is patient safety, so until more data are available, we no longer perform open electromechanical morcellation. We perform all tissue extraction within a containment system and under institutional review board protocol. We primarily perform tissue extraction via scalpel morcellation.
OBG Management: How do the patient’s wishes factor into the decision to perform minimally invasive surgery with morcellation?
Dr. Fader: Our patients make their own decisions regarding surgical approach and procedure after undergoing extensive counseling about the risks and benefits of the proposed procedures. I certainly would offer a patient like the one described in this case the opportunity for a minimally invasive approach (if, after thorough preoperative evaluation, she were deemed to be at very low risk for uterine malignancy). In my experience, most women opt for the minimally invasive approach in this setting; however, if a patient declines minimally invasive surgery, I respect her decision.
Tissue extraction in perimenopause
CASE: A desire for myomectomy at age 48
OBG Management: How would you approach the same case if the patient were a 48-year-old perimenopausal woman?
Dr. Fader: In perimenopausal women, we are more selective about performing morcellation, given the recent FDA safety statement, and because the incidence of occult cancer starts to increase slightly in this patient cohort (although it doesn’t precipitously increase until well into the postmenopausal period).
In addition, myomectomy may have less value in a 48-year-old, given the lower likelihood of achieving successful fertility, although there are exceptions. US cancer statistics and studies on morcellation demonstrate that the vast majority of women in their 30s and 40s have an extremely low risk for sarcomas and other uterine malignancies.2
In select cases in which a woman has undergone a comprehensive preoperative work-up, has a stable-appearing fibroid(s), and is well-educated and counseled about the pros and cons of morcellation, we would consider performing a procedure with contained tissue extraction. As a general matter, however, I would be more inclined to offer a 48-year-old in this situation a uterine artery embolization or minimally invasive hysterectomy than a myomectomy procedure, especially given the recent study by Wright and colleagues demonstrating the significantly increased risks of uterine cancer at myomectomy surgery for a woman in her late 40s or early 50s.18
Preoperative assessment should be comprehensive
OBG Management: What preoperative evaluation do you perform when tissue extraction, including morcellation, is an issue?
Dr. Fader: It is the policy at Johns Hopkins that all women being considered for minimally invasive surgery and tissue extraction must undergo a rigorous preoperative work-up that includes:
- a comprehensive history and physical (to exclude malignancy and risk for occult malignancy)
- an endometrial evaluation (most commonly, an endometrial biopsy)
- uterine imaging (with longitudinal evaluation of imaging findings if performed previously)
- discussion of each patient case at peer-reviewed, preoperative department conferences.
We have separate conferences for the gynecology and gynecologic oncology services and have employed this practice for many years at Hopkins. We are also studying the role of serum lactate dehydrogenase (LDH) isoenzyme levels in stratifying women with uterine fibroids versus cancer/sarcoma.19
OBG Management: Which patients would you exclude from the electromechanical morcellation option?
Dr. Fader: As the American College of Obstetricians and Gynecologists, the AAGL, the Society of Gynecologic Oncology, and the Society of Gynecologic Surgeons appear to agree:
- When utilized in select patients of reproductive age, minimally invasive surgery and morcellation are beneficial.
- Morcellation should categorically not be performed in any woman who has a known or suspected uterine (or other gynecologic) malignancy.2,3
At our institution, we have significantly curtailed the use of electromechanical morcellation at this time and especially do not perform it in women aged 50 or older or in those with confirmed postmenopausal status. We also do not perform electromechanical morcellation in women with a personal history of uterine, cervical, or ovarian preinvasive or invasive cancer, or in women with a strong family history of gynecologic malignancy.
Other populations we exclude are women with:
- a history of mitotically active or atypical fibroids (as determined at previous myomectomy)
- known BRCA or HNPCC deleterious mutation, hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome, hereditary childhood retinoblastoma, or other genetic predisposition to uterine or ovarian cancer
- a history of pelvic radiation
- a history of tamoxifen use.
Counseling the patient
OBG Management: If tissue extraction is an issue, and morcellation will be necessary for a minimally invasive approach, how do you counsel the patient?
Dr. Fader: We have an informed consent protocol we use at Hopkins in this regard. We speak extensively to our patients about the fact that every procedure or intervention performed in medicine carries a benefit/risk ratio. We inform patients of the FDA morcellation safety statement—that their fibroid or fibroids may contain unexpected cancerous tissue and that laparoscopic electromechanical morcellation may spread the cancer and possibly worsen their prognosis. We also explain that, while the FDA quotes a risk of approximately 1 in 350 for occult sarcoma, that this data review was somewhat limited in scope and included postmenopausal women in the estimates. Based on the best available published systematic reviews and internal Hopkins data, we believe the risk of morcellating an occult uterine malignancy in a woman of reproductive age is more likely on the order of approximately 1 in 1,700 to 1 in 8,333.
We discuss our institution’s approach to tissue extraction (ie, that we no longer perform open electromechanical morcellation but instead perform contained tissue extraction on an institutional review board protocol). We tell patients that contained tissue-extraction practices are still experimental, although there is published literature preliminarily supporting the safety of the practice. In addition, we discuss the fact that contained tissue extraction has been used for years to safely remove large intra-abdominal specimens from laparoscopic incisions, from adnexal tissue to gallbladder, spleen, kidney, intestinal, and appendiceal specimens.
We also explain that, as physicians, we do our best to ensure that risks are minimized and reasonable in relation to anticipated benefits but that, even when we use the very best diagnostic measures, no test is 100% sensitive or specific to rule out malignancy in this setting (or in any setting, actually).
Finally, we discuss the fact that minimally invasive surgery and tissue extraction practices performed in appropriate patients by skilled surgeons conclusively benefit hundreds of thousands of women each year around the world. That is a narrative that has been somewhat lacking in the recent dialogue about tissue-extraction practices.
OBG Management: What do we know about outcomes when a leiomyosarcoma is inadvertently morcellated?
Dr. Fader: This is considered a “cut-through” procedure, in that a cancerous tumor that is potentially contained to an organ is not removed intact or with clean margins. The morcellation procedure effectively cuts through the occult cancer, which is not desirable. Intact surgical removal of uterine cancers or sarcomas is the mainstay of therapy for these malignancies, based on NCCN guidelines.22
OBG Management: Does a morcellated leiomyosarcoma carry a worse prognosis than an unmorcellated leiomyosarcoma?
Dr. Fader: When it comes to morcellated versus “intact” uterine leiomyosarcoma, we enter a largely data-free zone. We don’t know with certainty whether the outcome is worsened. If we’re being intellectually honest, we must admit the possibility that a morcellated cancer is more likely to be disseminated, rendering it potentially more difficult to treat. However, sarcomas primarily spread by hematogenous dissemination. It is quite possible that even the act of incising an intact fibroid in an open abdominal procedure or performing a supracervical or total hysterectomy without morcellation may still result in hematogenous cancer dissemination. So there is no indication yet that electromechanical morcellation poses a unique and higher risk of cancer upstaging or worse prognosis compared with techniques such as open myomectomy, supracervical hysterectomy, or hysteroscopic myomectomy.
In addition, the prognosis associated with even early-stage uterine leiomyosarcoma is uniformly poor. A published study from Hopkins that included 108 patients with uterine leiomyosarcoma suggests that the recurrence rate and survival of patients with early-stage, “intact” leiomyosarcoma are very poor and comparable to the survival rate documented in the literature for women with morcellated sarcomas.23
The few retrospective studies that exist suggesting worse outcomes with morcellation have limitations that preclude any definitive conclusions.2 These studies are marred by small numbers, heterogeneity in morcellation practices, poor follow-up times, and a lack of detail regarding how patients with morcellated versus unmorcellated sarcomas were treated. Nevertheless, a couple of these small retrospective reports indicate the possibility of worse outcomes in women with morcellated uterine sarcomas, compared with historical controls with intact sarcoma removal.
Is the morcellator at fault—or the user?
OBG Management: Some would argue that even one case of a morcellated uterine sarcoma is too many. How would you respond?
Dr. Fader: There is no doubt that a handful of women each year have been harmed by morcellation practices. Those women deserve our dedication and best efforts to learn how to better treat morcellated sarcomas—and more importantly—how to mitigate the risks associated with morcellation practices and reduce the risk of preventable harm for all women undergoing minimally invasive fibroid procedures. I think the single best thing we can do to mitigate risk is to be more conscientious about selecting our patients for tissue-extraction procedures (ie, strict selection criteria, appropriate preoperative work-ups). If we did this, we likely would reduce the number of oncologic morcellator mishaps by 50% to 80% without changing anything else.
When we closely scrutinize the literature, and when I reflect on the women with morcellated cancers that we have cared for at Hopkins, we observe that some (but not all) “occult” uterine cancers were not that hidden after all and may have been detected preoperatively if an effort had been made.
We have noted a number of patients in this setting who experienced harm not because a specific device was used to cut up their uterus but because they were never appropriate candidates for the procedure in the first place. For instance, I recently cared for an elderly woman with a morcellated uterine cancer who underwent a laparoscopic supracervical hysterectomy without an appropriate preoperative work-up (ie, no endometrial biopsy or imaging) or informed consent about the possibility that she might have cancer. If an elderly woman presents with concerning symptoms related to her uterus (ie, enlargement, bleeding), she must be evaluated and counseled regarding the considerable risk of potential malignancy. Even in the setting of a normal work-up, I don’t believe it is a good idea to perform electromechanical morcellation in higher-risk women, including elderly women. That does not mean that select, well-screened women cannot be considered for alternative tissue-extraction techniques, but the risks and benefits must be carefully weighed in each patient, and informed consent must be obtained.
By continuing to refine the safety features of the electromechanical morcellator devices and choosing patients more carefully for minimally invasive procedures and tissue extraction, we likely will reduce the risk of preventable harm in women undergoing gynecologic surgery.
Can leiomyosarcoma be detected preoperatively?
OBG Management: How can we improve preoperative detection of uterine malignancy, particularly leiomyosarcoma?
Dr. Fader: For starters, we can improve detection of uterine cancers by simply looking for them. Almost all epithelial uterine cancers will be detectable by endometrial sampling. A comprehensive history and physical and uterine imaging also may be helpful. And although they are more difficult to detect than epithelial uterine cancers, it is a myth that sarcomas cannot be diagnosed preoperatively. Investigators from Columbia University retrospectively evaluated the ability to preoperatively detect epithelial uterine cancers and uterine sarcomas on final pathology. In 72 women who were ultimately diagnosed with a sarcoma, preoperative endometrial sampling suggested an invasive tumor in 86% and predicted the correct histology in 64%. In fact, the rate of detection of invasive cancer by preoperative sampling was not statistically different among sarcomas than it was among epithelial uterine cancers, although there was less of a correlation with appropriate histology seen with endometrial biopsy.20
That being said, sarcomas can be missed, especially in younger women who have extremely large, degenerated, or necrotic-appearing fibroids. Improvements in diagnostic testing are desperately needed to help distinguish benign fibroids from sarcomas, as there are no reliable modalities to exclude a sarcoma at this time. MRI appears to be the most useful imaging modality, although it cannot definitively distinguish a fibroid from a sarcoma. However, a fairly constant finding in leiomyosarcomas is the absence of calcifications. Further, some studies also suggest that ill-defined margins are consistent with a sarcoma. Finally, several centers, including our own, are studying novel biologic markers and revisiting the utility of previously described markers such as LDH in the preoperative detection of uterine sarcoma.21
The way forward
OBG Management: You have said, “Keep patients informed and safe but avoid being too reactionary.” Could you expand on this statement?
Dr. Fader: Certainly. There are many examples throughout the history of medicine in which treatments have brought benefit to thousands or millions of individuals but may cause harm in a select few. We know that when controversial medical issues have arisen in the past, the pendulum has swung widely in terms of societal response.
For example, the landmark Women’s Health Initiative had an immediate and adverse impact on hormone replacement therapy (HRT) administration. The increased risk of cardiovascular disease and breast cancer observed with use of long-term combination therapy with conjugated equine estrogen and medroxyprogesterone acetate prompted many US health-care providers to abandon use of HRT—until more contemporary data demonstrated that, in younger, healthier postmenopausal women, these adverse events were very rare and the benefits of HRT outweighed the risks.
Can we avoid stroke, heart attack, and breast cancer in all younger women taking HRT? Of course not. But we counsel women about the benefit/risk ratio of the therapy and advise them that the likelihood of these events is rare.
Similarly, as with the HRT analogy, younger women have lower risks associated with surgery and morcellation, compared with older women, and are more likely to derive benefit from the procedure (after ensuring appropriate candidacy with a comprehensive preoperative evaluation and informed consent).
However, if the expectation is that no cases of harm will ever occur with a surgical device or procedure in order for it to be deemed acceptable and safe to use in practice, then that is simply an impossible standard to uphold. There is no device, medication, or intervention I know of in medicine that is completely risk-free.
OBG Management: Are there other examples of this type of benefit/risk assessment?
Dr. Fader: Yes. For instance, tamoxifen is a nonsteroidal anti-estrogen agent approved by the FDA for adjuvant treatment of breast cancer, treatment of metastatic breast cancer, and reduction of breast cancer incidence in high-risk women. Tamoxifen has effectively reduced breast cancer rates and significantly improved survival in select breast cancer patients. Yet, it is well known that long-term use of tamoxifen is associated with a twofold increased risk of uterine cancer and uterine sarcoma—a likely far more commonly occurring adverse event than an occult, morcellated uterine sarcoma during a minimally invasive gynecologic procedure.
Should the FDA ban or significantly curtail the use of tamoxifen? No, not likely, because the benefits far outweigh the risks in previvors and hormone-positive breast cancer survivors. “Keep patients informed and safe but avoid being too reactionary” means that we must do our due diligence as physicians by comprehensively counseling and obtaining informed consent from our patients before performing medical interventions. We also must closely scrutinize and improve upon practices that may cause harm.
However, what this also means is that while it may be prudent to restrict or limit a surgical practice in select higher-risk populations or modify it in some way to make it safer, we shouldn’t necessarily completely abandon or ban a practice that has benefited hundreds of thousands of patients at low risk of harm until we have objectively reviewed all of the available science and fully understand the implications of a practice change (ie, would the risks of preventable harm be even greater for women if more of them had to undergo open abdominal surgery?). We need continued cool heads and sound scientific reasoning to decide upon health-care policy changes or treatment paradigm shifts.
At the end of the day, however, it is paramount that we mitigate patient harm. The subject of tissue extraction during minimally invasive surgery is a complex and nuanced issue that merits continued study and open-minded and intelligent dialogue between patient stakeholders, clinicians, scientists, industry, ethicists, regulatory agencies, and the press. I think we can all appreciate how humbling and challenging the morcellation issue has been for many of us, especially our patients—particularly the unfortunate women who have been diagnosed with a uterine sarcoma.
Like many of my colleagues, I have been privileged to care for a number of women with uterine leiomyosarcoma. It is a devastating disease, and the prognosis is very poor, whether it is morcellated or removed intact. We are fortunate that the vast majority of women who undergo a minimally invasive procedure for fibroids or other presumed benign indications will not be at risk for an occult malignancy. But what can we do now to continue to offer the benefits of minimally invasive surgery and tissue extraction to women while simultaneously reducing the risk to the select few who will develop a rare uterine cancer?
We can all make a greater effort to more carefully select our patients for minimally invasive surgery and tissue extraction, to limit the performance of open electromechanical morcellation and collaborate in studying the role of refined tissue-extraction techniques and containment systems, to enhance the informed consent process, to develop improved diagnostic tests for preoperative cancer detection, and to conduct higher-quality studies on minimally invasive tissue-extraction techniques for regulatory agencies to review in the near future. It also goes without saying that we need more federal funding to study rare tumors (and gynecologic cancers in general) and develop better sarcoma treatments.
Although there is no medical treatment or surgical procedure that is completely risk-free, interventions such as HRT, tamoxifen, and uterine morcellation—when used in appropriate patients and for appropriate indications—will allow preventable harm to be minimized and make it possible for countless women to continue to derive tremendous benefit.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed February 3, 2014.
2. AAGL. Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed February 3, 2015.
3. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery: a special report. http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Power-Morcellation-and-Occult-Malignancy-in-Gynecologic-Surgery. Published May 2014. Accessed February 3, 2015.
4. US Food and Drug Administration. Updated Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Published November 24, 2014. Accessed February 2, 2015.
5. American Cancer Society. Uterine sarcoma: What is uterine sarcoma? http://www.cancer.org/cancer/uterinesarcoma/detailedguide/uterine-sarcoma-what-is-uterine-sarcoma. Updated January 12, 2015. Accessed February 3, 2015.
6. D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131–139.
7. Chittawar B, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2014;10:CD004638. doi:10.1002/14651858.CD004638.pub3.
8. Mori KM, Neubauer NL. Minimally invasive surgery in gynecologic oncology. ISRN Obstet Gynecol. 2013; article ID 312982. http://dx.doi.org/10.1155/2013/312982. Accessed February 2, 2015.
9. Li G, Yan X, Shang H, Wang G, Chen L, Han Y. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib IIa cervical cancer. Gynecol Oncol. 2007;105(1):176–180.
10. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study Lap2. J Clin Oncol. 2009;27(32):5331–5336.
11. Ramos A, Fader AN, Long Roche K. Surgical cytoreduction for disseminated benign disease after open power uterine morcellation. Obstet Gynecol. 2014;125(1):99–102.
12. Theben J, Schellong A, Altgassen C, Kelling K, Schneider S, Grobe-Drieling D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): an analysis of 1,584 LASH cases. Arch Gynecol Obstet. 2013;287(3):455–462.
13. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
14. Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One. 2012;7(11):e50058.
15. Pritts EA, Parker WH, Brown J, Olive DL. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22(1):26–33.
16. Wright JD, Tergas AI, Burke WM, et al. Prevalence of uterine pathology in women undergoing minimally invasive hysterectomy employing electric power morcellation. JAMA. 2014;312(12):1253–1255.
17. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA. 2014;312(12):1253–1255.
18. Wright JD, Tergas AI, Cul R, et al. Use of electric power morcellation and prevalence of underlying cancer in women who undergo myomectomy. JAMA Oncol. 2015; published online February 19, 2015. doi:10.1001/jamaoncol.2014.206.
19. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002; 12:354.
20. Ricci S, Giuntoli RL 2nd, Eisenhauer E, et al. Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecol Oncol. 2013;131(3):629–633.
21. Bansal N, Herzog TJ, Burke W, Cohen CJ, Wright JD. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008;110(1):43–48.
22. Koh WJ, Greer BE, Abu-Rustum NR, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw. 2014;12(2):248–280.
23. Schwartz LB, Zawin M, Carcangiu ML, Lange R, McCarthy S. Does pelvic magnetic resonance imaging differentiate among the histologic subtypes of uterine leiomyomata? Fertil Steril. 1998;70(3):580–587.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed February 3, 2014.
2. AAGL. Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed February 3, 2015.
3. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery: a special report. http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Power-Morcellation-and-Occult-Malignancy-in-Gynecologic-Surgery. Published May 2014. Accessed February 3, 2015.
4. US Food and Drug Administration. Updated Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Published November 24, 2014. Accessed February 2, 2015.
5. American Cancer Society. Uterine sarcoma: What is uterine sarcoma? http://www.cancer.org/cancer/uterinesarcoma/detailedguide/uterine-sarcoma-what-is-uterine-sarcoma. Updated January 12, 2015. Accessed February 3, 2015.
6. D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131–139.
7. Chittawar B, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2014;10:CD004638. doi:10.1002/14651858.CD004638.pub3.
8. Mori KM, Neubauer NL. Minimally invasive surgery in gynecologic oncology. ISRN Obstet Gynecol. 2013; article ID 312982. http://dx.doi.org/10.1155/2013/312982. Accessed February 2, 2015.
9. Li G, Yan X, Shang H, Wang G, Chen L, Han Y. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib IIa cervical cancer. Gynecol Oncol. 2007;105(1):176–180.
10. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study Lap2. J Clin Oncol. 2009;27(32):5331–5336.
11. Ramos A, Fader AN, Long Roche K. Surgical cytoreduction for disseminated benign disease after open power uterine morcellation. Obstet Gynecol. 2014;125(1):99–102.
12. Theben J, Schellong A, Altgassen C, Kelling K, Schneider S, Grobe-Drieling D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): an analysis of 1,584 LASH cases. Arch Gynecol Obstet. 2013;287(3):455–462.
13. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
14. Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One. 2012;7(11):e50058.
15. Pritts EA, Parker WH, Brown J, Olive DL. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22(1):26–33.
16. Wright JD, Tergas AI, Burke WM, et al. Prevalence of uterine pathology in women undergoing minimally invasive hysterectomy employing electric power morcellation. JAMA. 2014;312(12):1253–1255.
17. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA. 2014;312(12):1253–1255.
18. Wright JD, Tergas AI, Cul R, et al. Use of electric power morcellation and prevalence of underlying cancer in women who undergo myomectomy. JAMA Oncol. 2015; published online February 19, 2015. doi:10.1001/jamaoncol.2014.206.
19. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002; 12:354.
20. Ricci S, Giuntoli RL 2nd, Eisenhauer E, et al. Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecol Oncol. 2013;131(3):629–633.
21. Bansal N, Herzog TJ, Burke W, Cohen CJ, Wright JD. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008;110(1):43–48.
22. Koh WJ, Greer BE, Abu-Rustum NR, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw. 2014;12(2):248–280.
23. Schwartz LB, Zawin M, Carcangiu ML, Lange R, McCarthy S. Does pelvic magnetic resonance imaging differentiate among the histologic subtypes of uterine leiomyomata? Fertil Steril. 1998;70(3):580–587.
IN THIS ARTICLE
– A case-based approach
– Comprehensive preoperative assessment
– What is the prognosis when a leiomyosarcoma is morcellated?
Hysteroscopic myomectomy using a mechanical approach
Uterine fibroids are a common complaint in gynecology, with an incidence of approximately 30% in women aged 25 to 45 years and a cumulative incidence of 70% to 80% by age 50.1,2 They are more prevalent in women of African descent and are a leading indication for hysterectomy.
Although they can be asymptomatic, submucosal fibroids are frequently associated with:
- abnormal uterine bleeding (AUB)
- dysmenorrhea
- expulsion of an intrauterine device (IUD)
- leukorrhea
- pelvic pain
- urinary frequency
- infertility
- premature labor
- reproductive wastage
- bleeding during hormone replacement therapy.
In postmenopausal women, the risk of malignancy in a leiomyoma ranges from 0.2% to 0.5%.1 The risk is lower in premenopausal women.
In this article, I describe the technique for hysteroscopic myomectomy using a mechanical approach (Truclear Tissue Removal System, Smith & Nephew, Andover, MA), which offers hysteroscopic morcellation as well as quick resection and efficient fluid management. (Note: Unlike open intraperitoneal morcellation, hysteroscopic morcellation carries a low risk of tissue spread.)
Classification of fibroids
Preoperative classification of leiomyomas makes it possible to determine the best route for surgery. The most commonly used classification system was developed by the European Society of Gynaecological Endoscopy (ESGE) (FIGURE 1), which considers the extent of intramural extension. Each fibroid under that system is classified as:
- Type 0 – no intramural extension
- Type I – less than 50% extension
- Type II – more than 50% extension.
A second classification system recently was devised to take into account additional features of the fibroid. The STEP-W classification considers size, topography, extension, penetration, and the lateral wall (FIGURE 2). In general, the lower the score, the less complex the procedure will be, with a lower risk of fluid intravasation, shorter operative time, and a greater likelihood of complete removal of the fibroid.
A multicenter, prospective study of 449 women who underwent hysteroscopic resection of their fibroids correlated the ESGE and STEP-W systems. All 320 fibroids (100%) with a score of 4 or below on the STEP-W classification system were completely removed, compared with 112 of 145 fibroids (77.2%) with a score greater than 4. All 33 cases of incomplete hysteroscopic resection (100%) had a STEP-W score above 4.3
In the same study, 85 of 86 cases (98.9%) with Type 0 fibroids under the ESGE system had complete resection, along with 278 of 298 Type I fibroids (93.3%), and 69 of 81 Type II fibroids (85.2%).3 Complete removal is a goal because it relieves symptoms and averts the need for additional procedures.
Patient selection
Proper patient selection for hysteroscopic myomectomy is extremely important. The most common indications are AUB, pelvic pain or discomfort, recurrent pregnancy loss, and infertility. In addition, the patient should have a strong wish for uterine preservation and desire a minimally invasive transcervical approach.
AAGL guidelines on the diagnosis and management of submucous fibroids note that, in general, submucous leiomyomas as large as 4 or 5 cm in diameter can be removed hysteroscopically by experienced surgeons.4
A hysteroscopic approach is not advised for women in whom hysteroscopic surgery is contraindicated, such as women with intrauterine pregnancy, active pelvic infection, active herpes infection, or cervical or uterine cancer. Women who have medical comorbidities such as coronary heart disease, significant renal disease, or bleeding diathesis may need perioperative clearance from anesthesia or hematology prior to hysteroscopic surgery and close fluid monitoring during the procedure.
Consider the leiomyoma
Penetration into the myometrium. Women who have a fibroid that penetrates more than 50% into the myometrium may benefit from hysteroscopic myomectomy, provided the surgeon is highly experienced. A skilled hysteroscopist can ensure complete enucleation of a penetrating fibroid in these cases.
If you are still in the learning process for hysteroscopy, however, start with easier cases—ie, polyps and Type 0 and Type I fibroids. Type II fibroids require longer operative time, are associated with increased fluid absorption and intravasation, carry an increased risk of perioperative complications, and may not always be completely resected.
Size of the fibroid also is relevant. As size increases, so does the volume of tissue needing to be removed, adding to overall operative time.
Presence of other fibroids. When a woman has an intracavitary fibroid as well as myomas in other locations, the surgeon should consider whether hysteroscopic removal of the intracavitary lesion alone can provide significant relief of all fibroid-related symptoms. In such cases, laparoscopic, robotic, or abdominal myomectomy may be preferable, especially if the volume of the additional myomas is considerable.
To determine the optimal surgical route, the physician must consider the symptoms present—is AUB the only symptom, or are other fibroid-related conditions present as well, such as bulk, pelvic pain, and other quality-of-life issues? If multiple symptoms exist, then other approaches may be better.
How fibroids affect fertility
Fibroids are present in 5% to 10% of women with infertility. In this population, fibroids are the only abnormal finding in 1.0% to 2.4% of cases.4
In a meta-analysis of 23 studies evaluating women with fibroids and infertility, Pritts and colleagues found nine studies involving submucosal fibroids.5 These studies included one randomized controlled trial, two prospective studies, and six retrospective analyses. They found that women who had fibroids with a submucosal component had lower pregnancy and implantation rates, compared with their infertile, myoma-free counterparts. Pritts and colleagues concluded that myomectomy is likely to improve fertility in these cases (TABLE).5
Instrumentation
Among the options are monopolar and bipolar resectoscopy and the mechanical approach using the Truclear System, which includes a morcellator. With conventional resectoscopy all chips must be removed, necessitating multiple insertions of the hysteroscope. Monopolar instrumentation, in particular, carries a risk of energy discharge to healthy tissue. The monopolar resectoscope also has a longer learning curve, compared with the mechanical approach.6
In contrast, the Truclear System requires fewer insertions, has a short learning curve, and omits the need for capture of individual chips, as the mechanical morcellator suctions and captures them throughout the procedure.7 In addition, because resection is performed mechanically, there is no risk of energy discharge to healthy tissue.
The Truclear system also is associated with a significantly shorter operative time, compared with resectoscopy, which may be advantageous for residents, fellows, and other physicians learning the procedure (FIGURE 3).7 Shorter operative time also may result in lower fluid deficits. In addition, saline distension may reduce the risk of fluid absorption and hyponatremia. The tissue-capture feature allows evaluation of the entire pathologic specimen.
Besides hysteroscopic myomectomy, the Truclear System is appropriate for visual dilatation and curettage (D&C), adhesiolysis, polypectomy, and evacuation of retained products of conception.
Preoperative evaluation
A complete history is vital to document which fibroid-related symptoms are present and how they affect quality of life.
Preoperative imaging also is imperative—using either 2D or 3D saline infusion sonography or a combination of diagnostic hysteroscopy and transvaginal ultrasound—to select patients for hysteroscopy, anticipate blood loss, and ensure that the proper instrumentation is available at the time of surgery. Magnetic resonance imaging, computed tomography, and hysterosalpingography are either prohibitively expensive or of limited value in the initial preoperative assessment of uterine fibroids.
Any woman who has AUB and a risk for endometrial hyperplasia or cancer should undergo endometrial assessment as well.
Use of preoperative medications
In most cases, prophylactic administration of antibiotics is not warranted to prevent infection or endocarditis.
Although some clinicians give gonadotropin-releasing hormone (GnRH) agonists to reduce the size of large fibroids, the drug complicates dissection of the fibroid from the surrounding capsule. For this reason, and because we lack data demonstrating that GnRH agonists decrease blood loss and limit absorption of distension media, I do not administer them to patients.8–12 Moreover, this drug can cause vasomotor symptoms, cervical stenosis, and vaginal hemorrhage (related to estrogen flare).
GnRH agonists may be of value to stimulate transient amenorrhea for several months preoperatively in order to correct iron-deficiency anemia. Intravenous iron also can be administered during this interval.
The risk of bleeding in hysteroscopic myomectomy is 2% to 3%.1 When the mechanical approach is used, rather than resectoscopy, continuous flow coupled with suctioning of the chips during the procedure keeps the image clear. Post-procedure contraction of the uterus stops most bleeding. Intrauterine pressure of the pump can be increased to help tamponade any oozing.
Misoprostol. Cervical stenosis is not uncommon in menopausal women. It can also pose a challenge in nulliparous women. Attempting hysteroscopy in the setting of cervical stenosis increases the risk of cervical laceration, creation of a false passage, and uterine perforation. For this reason, I prescribe oral or vaginal misoprostol 200 to 400 µg nightly for 1 to 2 days before the procedure.
Vasopressin can reduce blood loss during hysteroscopic myomectomy when it is injected into the cervical stroma preoperatively. It also reduces absorption of distension fluid and facilitates cervical dilation.
However, vasopressin must be injected with extreme care, with aspiration to confirm the absence of blood prior to each injection, as intravascular injection can lead to bradycardia, profound hypertension, and even death.13 Always notify the anesthesiologist prior to injection when vasopressin will be administered.
I routinely use vasopressin before hysteroscopic myomectomy (0.5 mg in 20 cc of saline or 20 U in 100 cc), injecting 5 cc of the solution at 3, 6, 9, and 12 o’clock positions.
Anesthesia during hysteroscopic myomectomy typically is “monitored anesthesia care,” or MAC, which consists of local anesthesia with sedation and analgesia. The need for regional or general anesthesia is rare. Consider adding a pericervical block or intravenous ketorolac (Toradol) to provide postoperative analgesia.
Surgical technique
Strict attention to fluid management is required throughout the procedure, preferably in accordance with AAGL guidelines on the management of hysteroscopic distending media.14 With the mechanical approach, because the distension fluid is isotonic (normal saline), it does not increase the risk of hyponatremia but can cause pulmonary edema or congestive heart failure. Intravasation usually is the result of excessive operative time, treatment of deeper myometrial fibroids (Type I or II), or high intrauterine pressure. I operate using intrauterine pressure in the range of 75 to 125 mm Hg.
The steps involved in the mechanical hysteroscopy approach are:
- Insert the hysteroscope into the uterus under direct visualization. In general, the greater the number of insertions, the greater the risk of uterine perforation. Preoperative cervical ripening helps facilitate insertion (see “Misoprostol” above).
- Distend the uterus with saline and inspect the uterine cavity, noting again the size and location of the fibroids and whether they are sessile or pedunculated.
- Locate the fibroid or other pathology to be removed, and place the morcellator window against it to begin cutting. Use the tip of the morcellator to elevate the fibroid for easier cutting. Enucleation is accomplished largely by varying the intrauterine pressure, which permits uterine decompression and myometrial contraction and renders the fibroid capsule more visible. If necessary, the hysteroscope can be withdrawn to stimulate myometrial contraction, which also helps to delineate the fibroid capsule.
- Reinspect the uterus to rule out perforation and remove any additional intrauterine pathology with a targeted view.
- Once all designated fibroids have been removed, withdraw the morcellator and hysteroscope from the uterus.
- Inspect the endocervical landscape to rule out injury and other pathology.
- Careful preoperative evaluation is important, preferably using diagnostic hysteroscopy or saline infusion sonography, to choose the optimal route of myomectomy and plan the surgical approach.
- During the myomectomy, pay close attention to fluid management and adhere strictly to predetermined limits.
- Complete removal of the fibroid is essential to relieve symptoms and avert the need for additional procedures.
Postoperative care
A nonsteroidal anti-inflammatory drug or limited use of narcotics usually is sufficient to relieve any postoperative cramping or vaginal discomfort.
Advise the patient to notify you in the event of increasing pain, foul-smelling vaginal discharge, or fever.
Also counsel her that she can return to most normal activities within 24 to 48 hours. Sexual activity is permissible 1 week after surgery. Early and frequent ambulation is important.
Schedule a follow-up visit 4 to 6 weeks after the procedure.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Perez-Medina T, Font EC, eds. Diagnostic and Operative Hysteroscopy. Tunbridge Wells, Kent, UK: Anshan Publishing; 2007:13.
2. Management of uterine fibroids: an update of the evidence. Agency for Healthcare Research and Quality. http://archive.ahrq.gov/clinic/tp/uteruptp.htm. Published July 2007. Accessed January 14, 2015.
3. Lasmar RB, Zinmei Z, Indman PD, Celeste RK, Di Spiezo Sardo A. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95(6):2073–2077.
4. AAGL Practice Report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol. 2012;19(2):152–171.
5. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223.
6. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466–471.
7. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62–66.
8. Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997;68(5):881–886.
9. Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7(3):351.
10. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96(4):517–520.
11. Perino A, Chianchiano N, Petronio M, Cittadini E. Role of leuprolide acetate depot in hysteroscopic surgery: a controlled study. Fertil Steril. 1993;59(3):507–510.
12. Mencaglia L, Tantini C. GnRH agonist analogs and hysteroscopic resection of myomas. Int J Gynaecol Obstet. 1993;43(3):285–288.
13. Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 Pt 2):484–486.
14. Munro MD, Storz K, Abbott JA, et al; AAGL. AAGL Practice Report: practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013;20(2):137–148.
Uterine fibroids are a common complaint in gynecology, with an incidence of approximately 30% in women aged 25 to 45 years and a cumulative incidence of 70% to 80% by age 50.1,2 They are more prevalent in women of African descent and are a leading indication for hysterectomy.
Although they can be asymptomatic, submucosal fibroids are frequently associated with:
- abnormal uterine bleeding (AUB)
- dysmenorrhea
- expulsion of an intrauterine device (IUD)
- leukorrhea
- pelvic pain
- urinary frequency
- infertility
- premature labor
- reproductive wastage
- bleeding during hormone replacement therapy.
In postmenopausal women, the risk of malignancy in a leiomyoma ranges from 0.2% to 0.5%.1 The risk is lower in premenopausal women.
In this article, I describe the technique for hysteroscopic myomectomy using a mechanical approach (Truclear Tissue Removal System, Smith & Nephew, Andover, MA), which offers hysteroscopic morcellation as well as quick resection and efficient fluid management. (Note: Unlike open intraperitoneal morcellation, hysteroscopic morcellation carries a low risk of tissue spread.)
Classification of fibroids
Preoperative classification of leiomyomas makes it possible to determine the best route for surgery. The most commonly used classification system was developed by the European Society of Gynaecological Endoscopy (ESGE) (FIGURE 1), which considers the extent of intramural extension. Each fibroid under that system is classified as:
- Type 0 – no intramural extension
- Type I – less than 50% extension
- Type II – more than 50% extension.
A second classification system recently was devised to take into account additional features of the fibroid. The STEP-W classification considers size, topography, extension, penetration, and the lateral wall (FIGURE 2). In general, the lower the score, the less complex the procedure will be, with a lower risk of fluid intravasation, shorter operative time, and a greater likelihood of complete removal of the fibroid.
A multicenter, prospective study of 449 women who underwent hysteroscopic resection of their fibroids correlated the ESGE and STEP-W systems. All 320 fibroids (100%) with a score of 4 or below on the STEP-W classification system were completely removed, compared with 112 of 145 fibroids (77.2%) with a score greater than 4. All 33 cases of incomplete hysteroscopic resection (100%) had a STEP-W score above 4.3
In the same study, 85 of 86 cases (98.9%) with Type 0 fibroids under the ESGE system had complete resection, along with 278 of 298 Type I fibroids (93.3%), and 69 of 81 Type II fibroids (85.2%).3 Complete removal is a goal because it relieves symptoms and averts the need for additional procedures.
Patient selection
Proper patient selection for hysteroscopic myomectomy is extremely important. The most common indications are AUB, pelvic pain or discomfort, recurrent pregnancy loss, and infertility. In addition, the patient should have a strong wish for uterine preservation and desire a minimally invasive transcervical approach.
AAGL guidelines on the diagnosis and management of submucous fibroids note that, in general, submucous leiomyomas as large as 4 or 5 cm in diameter can be removed hysteroscopically by experienced surgeons.4
A hysteroscopic approach is not advised for women in whom hysteroscopic surgery is contraindicated, such as women with intrauterine pregnancy, active pelvic infection, active herpes infection, or cervical or uterine cancer. Women who have medical comorbidities such as coronary heart disease, significant renal disease, or bleeding diathesis may need perioperative clearance from anesthesia or hematology prior to hysteroscopic surgery and close fluid monitoring during the procedure.
Consider the leiomyoma
Penetration into the myometrium. Women who have a fibroid that penetrates more than 50% into the myometrium may benefit from hysteroscopic myomectomy, provided the surgeon is highly experienced. A skilled hysteroscopist can ensure complete enucleation of a penetrating fibroid in these cases.
If you are still in the learning process for hysteroscopy, however, start with easier cases—ie, polyps and Type 0 and Type I fibroids. Type II fibroids require longer operative time, are associated with increased fluid absorption and intravasation, carry an increased risk of perioperative complications, and may not always be completely resected.
Size of the fibroid also is relevant. As size increases, so does the volume of tissue needing to be removed, adding to overall operative time.
Presence of other fibroids. When a woman has an intracavitary fibroid as well as myomas in other locations, the surgeon should consider whether hysteroscopic removal of the intracavitary lesion alone can provide significant relief of all fibroid-related symptoms. In such cases, laparoscopic, robotic, or abdominal myomectomy may be preferable, especially if the volume of the additional myomas is considerable.
To determine the optimal surgical route, the physician must consider the symptoms present—is AUB the only symptom, or are other fibroid-related conditions present as well, such as bulk, pelvic pain, and other quality-of-life issues? If multiple symptoms exist, then other approaches may be better.
How fibroids affect fertility
Fibroids are present in 5% to 10% of women with infertility. In this population, fibroids are the only abnormal finding in 1.0% to 2.4% of cases.4
In a meta-analysis of 23 studies evaluating women with fibroids and infertility, Pritts and colleagues found nine studies involving submucosal fibroids.5 These studies included one randomized controlled trial, two prospective studies, and six retrospective analyses. They found that women who had fibroids with a submucosal component had lower pregnancy and implantation rates, compared with their infertile, myoma-free counterparts. Pritts and colleagues concluded that myomectomy is likely to improve fertility in these cases (TABLE).5
Instrumentation
Among the options are monopolar and bipolar resectoscopy and the mechanical approach using the Truclear System, which includes a morcellator. With conventional resectoscopy all chips must be removed, necessitating multiple insertions of the hysteroscope. Monopolar instrumentation, in particular, carries a risk of energy discharge to healthy tissue. The monopolar resectoscope also has a longer learning curve, compared with the mechanical approach.6
In contrast, the Truclear System requires fewer insertions, has a short learning curve, and omits the need for capture of individual chips, as the mechanical morcellator suctions and captures them throughout the procedure.7 In addition, because resection is performed mechanically, there is no risk of energy discharge to healthy tissue.
The Truclear system also is associated with a significantly shorter operative time, compared with resectoscopy, which may be advantageous for residents, fellows, and other physicians learning the procedure (FIGURE 3).7 Shorter operative time also may result in lower fluid deficits. In addition, saline distension may reduce the risk of fluid absorption and hyponatremia. The tissue-capture feature allows evaluation of the entire pathologic specimen.
Besides hysteroscopic myomectomy, the Truclear System is appropriate for visual dilatation and curettage (D&C), adhesiolysis, polypectomy, and evacuation of retained products of conception.
Preoperative evaluation
A complete history is vital to document which fibroid-related symptoms are present and how they affect quality of life.
Preoperative imaging also is imperative—using either 2D or 3D saline infusion sonography or a combination of diagnostic hysteroscopy and transvaginal ultrasound—to select patients for hysteroscopy, anticipate blood loss, and ensure that the proper instrumentation is available at the time of surgery. Magnetic resonance imaging, computed tomography, and hysterosalpingography are either prohibitively expensive or of limited value in the initial preoperative assessment of uterine fibroids.
Any woman who has AUB and a risk for endometrial hyperplasia or cancer should undergo endometrial assessment as well.
Use of preoperative medications
In most cases, prophylactic administration of antibiotics is not warranted to prevent infection or endocarditis.
Although some clinicians give gonadotropin-releasing hormone (GnRH) agonists to reduce the size of large fibroids, the drug complicates dissection of the fibroid from the surrounding capsule. For this reason, and because we lack data demonstrating that GnRH agonists decrease blood loss and limit absorption of distension media, I do not administer them to patients.8–12 Moreover, this drug can cause vasomotor symptoms, cervical stenosis, and vaginal hemorrhage (related to estrogen flare).
GnRH agonists may be of value to stimulate transient amenorrhea for several months preoperatively in order to correct iron-deficiency anemia. Intravenous iron also can be administered during this interval.
The risk of bleeding in hysteroscopic myomectomy is 2% to 3%.1 When the mechanical approach is used, rather than resectoscopy, continuous flow coupled with suctioning of the chips during the procedure keeps the image clear. Post-procedure contraction of the uterus stops most bleeding. Intrauterine pressure of the pump can be increased to help tamponade any oozing.
Misoprostol. Cervical stenosis is not uncommon in menopausal women. It can also pose a challenge in nulliparous women. Attempting hysteroscopy in the setting of cervical stenosis increases the risk of cervical laceration, creation of a false passage, and uterine perforation. For this reason, I prescribe oral or vaginal misoprostol 200 to 400 µg nightly for 1 to 2 days before the procedure.
Vasopressin can reduce blood loss during hysteroscopic myomectomy when it is injected into the cervical stroma preoperatively. It also reduces absorption of distension fluid and facilitates cervical dilation.
However, vasopressin must be injected with extreme care, with aspiration to confirm the absence of blood prior to each injection, as intravascular injection can lead to bradycardia, profound hypertension, and even death.13 Always notify the anesthesiologist prior to injection when vasopressin will be administered.
I routinely use vasopressin before hysteroscopic myomectomy (0.5 mg in 20 cc of saline or 20 U in 100 cc), injecting 5 cc of the solution at 3, 6, 9, and 12 o’clock positions.
Anesthesia during hysteroscopic myomectomy typically is “monitored anesthesia care,” or MAC, which consists of local anesthesia with sedation and analgesia. The need for regional or general anesthesia is rare. Consider adding a pericervical block or intravenous ketorolac (Toradol) to provide postoperative analgesia.
Surgical technique
Strict attention to fluid management is required throughout the procedure, preferably in accordance with AAGL guidelines on the management of hysteroscopic distending media.14 With the mechanical approach, because the distension fluid is isotonic (normal saline), it does not increase the risk of hyponatremia but can cause pulmonary edema or congestive heart failure. Intravasation usually is the result of excessive operative time, treatment of deeper myometrial fibroids (Type I or II), or high intrauterine pressure. I operate using intrauterine pressure in the range of 75 to 125 mm Hg.
The steps involved in the mechanical hysteroscopy approach are:
- Insert the hysteroscope into the uterus under direct visualization. In general, the greater the number of insertions, the greater the risk of uterine perforation. Preoperative cervical ripening helps facilitate insertion (see “Misoprostol” above).
- Distend the uterus with saline and inspect the uterine cavity, noting again the size and location of the fibroids and whether they are sessile or pedunculated.
- Locate the fibroid or other pathology to be removed, and place the morcellator window against it to begin cutting. Use the tip of the morcellator to elevate the fibroid for easier cutting. Enucleation is accomplished largely by varying the intrauterine pressure, which permits uterine decompression and myometrial contraction and renders the fibroid capsule more visible. If necessary, the hysteroscope can be withdrawn to stimulate myometrial contraction, which also helps to delineate the fibroid capsule.
- Reinspect the uterus to rule out perforation and remove any additional intrauterine pathology with a targeted view.
- Once all designated fibroids have been removed, withdraw the morcellator and hysteroscope from the uterus.
- Inspect the endocervical landscape to rule out injury and other pathology.
- Careful preoperative evaluation is important, preferably using diagnostic hysteroscopy or saline infusion sonography, to choose the optimal route of myomectomy and plan the surgical approach.
- During the myomectomy, pay close attention to fluid management and adhere strictly to predetermined limits.
- Complete removal of the fibroid is essential to relieve symptoms and avert the need for additional procedures.
Postoperative care
A nonsteroidal anti-inflammatory drug or limited use of narcotics usually is sufficient to relieve any postoperative cramping or vaginal discomfort.
Advise the patient to notify you in the event of increasing pain, foul-smelling vaginal discharge, or fever.
Also counsel her that she can return to most normal activities within 24 to 48 hours. Sexual activity is permissible 1 week after surgery. Early and frequent ambulation is important.
Schedule a follow-up visit 4 to 6 weeks after the procedure.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Uterine fibroids are a common complaint in gynecology, with an incidence of approximately 30% in women aged 25 to 45 years and a cumulative incidence of 70% to 80% by age 50.1,2 They are more prevalent in women of African descent and are a leading indication for hysterectomy.
Although they can be asymptomatic, submucosal fibroids are frequently associated with:
- abnormal uterine bleeding (AUB)
- dysmenorrhea
- expulsion of an intrauterine device (IUD)
- leukorrhea
- pelvic pain
- urinary frequency
- infertility
- premature labor
- reproductive wastage
- bleeding during hormone replacement therapy.
In postmenopausal women, the risk of malignancy in a leiomyoma ranges from 0.2% to 0.5%.1 The risk is lower in premenopausal women.
In this article, I describe the technique for hysteroscopic myomectomy using a mechanical approach (Truclear Tissue Removal System, Smith & Nephew, Andover, MA), which offers hysteroscopic morcellation as well as quick resection and efficient fluid management. (Note: Unlike open intraperitoneal morcellation, hysteroscopic morcellation carries a low risk of tissue spread.)
Classification of fibroids
Preoperative classification of leiomyomas makes it possible to determine the best route for surgery. The most commonly used classification system was developed by the European Society of Gynaecological Endoscopy (ESGE) (FIGURE 1), which considers the extent of intramural extension. Each fibroid under that system is classified as:
- Type 0 – no intramural extension
- Type I – less than 50% extension
- Type II – more than 50% extension.
A second classification system recently was devised to take into account additional features of the fibroid. The STEP-W classification considers size, topography, extension, penetration, and the lateral wall (FIGURE 2). In general, the lower the score, the less complex the procedure will be, with a lower risk of fluid intravasation, shorter operative time, and a greater likelihood of complete removal of the fibroid.
A multicenter, prospective study of 449 women who underwent hysteroscopic resection of their fibroids correlated the ESGE and STEP-W systems. All 320 fibroids (100%) with a score of 4 or below on the STEP-W classification system were completely removed, compared with 112 of 145 fibroids (77.2%) with a score greater than 4. All 33 cases of incomplete hysteroscopic resection (100%) had a STEP-W score above 4.3
In the same study, 85 of 86 cases (98.9%) with Type 0 fibroids under the ESGE system had complete resection, along with 278 of 298 Type I fibroids (93.3%), and 69 of 81 Type II fibroids (85.2%).3 Complete removal is a goal because it relieves symptoms and averts the need for additional procedures.
Patient selection
Proper patient selection for hysteroscopic myomectomy is extremely important. The most common indications are AUB, pelvic pain or discomfort, recurrent pregnancy loss, and infertility. In addition, the patient should have a strong wish for uterine preservation and desire a minimally invasive transcervical approach.
AAGL guidelines on the diagnosis and management of submucous fibroids note that, in general, submucous leiomyomas as large as 4 or 5 cm in diameter can be removed hysteroscopically by experienced surgeons.4
A hysteroscopic approach is not advised for women in whom hysteroscopic surgery is contraindicated, such as women with intrauterine pregnancy, active pelvic infection, active herpes infection, or cervical or uterine cancer. Women who have medical comorbidities such as coronary heart disease, significant renal disease, or bleeding diathesis may need perioperative clearance from anesthesia or hematology prior to hysteroscopic surgery and close fluid monitoring during the procedure.
Consider the leiomyoma
Penetration into the myometrium. Women who have a fibroid that penetrates more than 50% into the myometrium may benefit from hysteroscopic myomectomy, provided the surgeon is highly experienced. A skilled hysteroscopist can ensure complete enucleation of a penetrating fibroid in these cases.
If you are still in the learning process for hysteroscopy, however, start with easier cases—ie, polyps and Type 0 and Type I fibroids. Type II fibroids require longer operative time, are associated with increased fluid absorption and intravasation, carry an increased risk of perioperative complications, and may not always be completely resected.
Size of the fibroid also is relevant. As size increases, so does the volume of tissue needing to be removed, adding to overall operative time.
Presence of other fibroids. When a woman has an intracavitary fibroid as well as myomas in other locations, the surgeon should consider whether hysteroscopic removal of the intracavitary lesion alone can provide significant relief of all fibroid-related symptoms. In such cases, laparoscopic, robotic, or abdominal myomectomy may be preferable, especially if the volume of the additional myomas is considerable.
To determine the optimal surgical route, the physician must consider the symptoms present—is AUB the only symptom, or are other fibroid-related conditions present as well, such as bulk, pelvic pain, and other quality-of-life issues? If multiple symptoms exist, then other approaches may be better.
How fibroids affect fertility
Fibroids are present in 5% to 10% of women with infertility. In this population, fibroids are the only abnormal finding in 1.0% to 2.4% of cases.4
In a meta-analysis of 23 studies evaluating women with fibroids and infertility, Pritts and colleagues found nine studies involving submucosal fibroids.5 These studies included one randomized controlled trial, two prospective studies, and six retrospective analyses. They found that women who had fibroids with a submucosal component had lower pregnancy and implantation rates, compared with their infertile, myoma-free counterparts. Pritts and colleagues concluded that myomectomy is likely to improve fertility in these cases (TABLE).5
Instrumentation
Among the options are monopolar and bipolar resectoscopy and the mechanical approach using the Truclear System, which includes a morcellator. With conventional resectoscopy all chips must be removed, necessitating multiple insertions of the hysteroscope. Monopolar instrumentation, in particular, carries a risk of energy discharge to healthy tissue. The monopolar resectoscope also has a longer learning curve, compared with the mechanical approach.6
In contrast, the Truclear System requires fewer insertions, has a short learning curve, and omits the need for capture of individual chips, as the mechanical morcellator suctions and captures them throughout the procedure.7 In addition, because resection is performed mechanically, there is no risk of energy discharge to healthy tissue.
The Truclear system also is associated with a significantly shorter operative time, compared with resectoscopy, which may be advantageous for residents, fellows, and other physicians learning the procedure (FIGURE 3).7 Shorter operative time also may result in lower fluid deficits. In addition, saline distension may reduce the risk of fluid absorption and hyponatremia. The tissue-capture feature allows evaluation of the entire pathologic specimen.
Besides hysteroscopic myomectomy, the Truclear System is appropriate for visual dilatation and curettage (D&C), adhesiolysis, polypectomy, and evacuation of retained products of conception.
Preoperative evaluation
A complete history is vital to document which fibroid-related symptoms are present and how they affect quality of life.
Preoperative imaging also is imperative—using either 2D or 3D saline infusion sonography or a combination of diagnostic hysteroscopy and transvaginal ultrasound—to select patients for hysteroscopy, anticipate blood loss, and ensure that the proper instrumentation is available at the time of surgery. Magnetic resonance imaging, computed tomography, and hysterosalpingography are either prohibitively expensive or of limited value in the initial preoperative assessment of uterine fibroids.
Any woman who has AUB and a risk for endometrial hyperplasia or cancer should undergo endometrial assessment as well.
Use of preoperative medications
In most cases, prophylactic administration of antibiotics is not warranted to prevent infection or endocarditis.
Although some clinicians give gonadotropin-releasing hormone (GnRH) agonists to reduce the size of large fibroids, the drug complicates dissection of the fibroid from the surrounding capsule. For this reason, and because we lack data demonstrating that GnRH agonists decrease blood loss and limit absorption of distension media, I do not administer them to patients.8–12 Moreover, this drug can cause vasomotor symptoms, cervical stenosis, and vaginal hemorrhage (related to estrogen flare).
GnRH agonists may be of value to stimulate transient amenorrhea for several months preoperatively in order to correct iron-deficiency anemia. Intravenous iron also can be administered during this interval.
The risk of bleeding in hysteroscopic myomectomy is 2% to 3%.1 When the mechanical approach is used, rather than resectoscopy, continuous flow coupled with suctioning of the chips during the procedure keeps the image clear. Post-procedure contraction of the uterus stops most bleeding. Intrauterine pressure of the pump can be increased to help tamponade any oozing.
Misoprostol. Cervical stenosis is not uncommon in menopausal women. It can also pose a challenge in nulliparous women. Attempting hysteroscopy in the setting of cervical stenosis increases the risk of cervical laceration, creation of a false passage, and uterine perforation. For this reason, I prescribe oral or vaginal misoprostol 200 to 400 µg nightly for 1 to 2 days before the procedure.
Vasopressin can reduce blood loss during hysteroscopic myomectomy when it is injected into the cervical stroma preoperatively. It also reduces absorption of distension fluid and facilitates cervical dilation.
However, vasopressin must be injected with extreme care, with aspiration to confirm the absence of blood prior to each injection, as intravascular injection can lead to bradycardia, profound hypertension, and even death.13 Always notify the anesthesiologist prior to injection when vasopressin will be administered.
I routinely use vasopressin before hysteroscopic myomectomy (0.5 mg in 20 cc of saline or 20 U in 100 cc), injecting 5 cc of the solution at 3, 6, 9, and 12 o’clock positions.
Anesthesia during hysteroscopic myomectomy typically is “monitored anesthesia care,” or MAC, which consists of local anesthesia with sedation and analgesia. The need for regional or general anesthesia is rare. Consider adding a pericervical block or intravenous ketorolac (Toradol) to provide postoperative analgesia.
Surgical technique
Strict attention to fluid management is required throughout the procedure, preferably in accordance with AAGL guidelines on the management of hysteroscopic distending media.14 With the mechanical approach, because the distension fluid is isotonic (normal saline), it does not increase the risk of hyponatremia but can cause pulmonary edema or congestive heart failure. Intravasation usually is the result of excessive operative time, treatment of deeper myometrial fibroids (Type I or II), or high intrauterine pressure. I operate using intrauterine pressure in the range of 75 to 125 mm Hg.
The steps involved in the mechanical hysteroscopy approach are:
- Insert the hysteroscope into the uterus under direct visualization. In general, the greater the number of insertions, the greater the risk of uterine perforation. Preoperative cervical ripening helps facilitate insertion (see “Misoprostol” above).
- Distend the uterus with saline and inspect the uterine cavity, noting again the size and location of the fibroids and whether they are sessile or pedunculated.
- Locate the fibroid or other pathology to be removed, and place the morcellator window against it to begin cutting. Use the tip of the morcellator to elevate the fibroid for easier cutting. Enucleation is accomplished largely by varying the intrauterine pressure, which permits uterine decompression and myometrial contraction and renders the fibroid capsule more visible. If necessary, the hysteroscope can be withdrawn to stimulate myometrial contraction, which also helps to delineate the fibroid capsule.
- Reinspect the uterus to rule out perforation and remove any additional intrauterine pathology with a targeted view.
- Once all designated fibroids have been removed, withdraw the morcellator and hysteroscope from the uterus.
- Inspect the endocervical landscape to rule out injury and other pathology.
- Careful preoperative evaluation is important, preferably using diagnostic hysteroscopy or saline infusion sonography, to choose the optimal route of myomectomy and plan the surgical approach.
- During the myomectomy, pay close attention to fluid management and adhere strictly to predetermined limits.
- Complete removal of the fibroid is essential to relieve symptoms and avert the need for additional procedures.
Postoperative care
A nonsteroidal anti-inflammatory drug or limited use of narcotics usually is sufficient to relieve any postoperative cramping or vaginal discomfort.
Advise the patient to notify you in the event of increasing pain, foul-smelling vaginal discharge, or fever.
Also counsel her that she can return to most normal activities within 24 to 48 hours. Sexual activity is permissible 1 week after surgery. Early and frequent ambulation is important.
Schedule a follow-up visit 4 to 6 weeks after the procedure.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Perez-Medina T, Font EC, eds. Diagnostic and Operative Hysteroscopy. Tunbridge Wells, Kent, UK: Anshan Publishing; 2007:13.
2. Management of uterine fibroids: an update of the evidence. Agency for Healthcare Research and Quality. http://archive.ahrq.gov/clinic/tp/uteruptp.htm. Published July 2007. Accessed January 14, 2015.
3. Lasmar RB, Zinmei Z, Indman PD, Celeste RK, Di Spiezo Sardo A. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95(6):2073–2077.
4. AAGL Practice Report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol. 2012;19(2):152–171.
5. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223.
6. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466–471.
7. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62–66.
8. Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997;68(5):881–886.
9. Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7(3):351.
10. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96(4):517–520.
11. Perino A, Chianchiano N, Petronio M, Cittadini E. Role of leuprolide acetate depot in hysteroscopic surgery: a controlled study. Fertil Steril. 1993;59(3):507–510.
12. Mencaglia L, Tantini C. GnRH agonist analogs and hysteroscopic resection of myomas. Int J Gynaecol Obstet. 1993;43(3):285–288.
13. Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 Pt 2):484–486.
14. Munro MD, Storz K, Abbott JA, et al; AAGL. AAGL Practice Report: practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013;20(2):137–148.
1. Perez-Medina T, Font EC, eds. Diagnostic and Operative Hysteroscopy. Tunbridge Wells, Kent, UK: Anshan Publishing; 2007:13.
2. Management of uterine fibroids: an update of the evidence. Agency for Healthcare Research and Quality. http://archive.ahrq.gov/clinic/tp/uteruptp.htm. Published July 2007. Accessed January 14, 2015.
3. Lasmar RB, Zinmei Z, Indman PD, Celeste RK, Di Spiezo Sardo A. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95(6):2073–2077.
4. AAGL Practice Report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol. 2012;19(2):152–171.
5. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223.
6. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466–471.
7. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62–66.
8. Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997;68(5):881–886.
9. Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7(3):351.
10. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96(4):517–520.
11. Perino A, Chianchiano N, Petronio M, Cittadini E. Role of leuprolide acetate depot in hysteroscopic surgery: a controlled study. Fertil Steril. 1993;59(3):507–510.
12. Mencaglia L, Tantini C. GnRH agonist analogs and hysteroscopic resection of myomas. Int J Gynaecol Obstet. 1993;43(3):285–288.
13. Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 Pt 2):484–486.
14. Munro MD, Storz K, Abbott JA, et al; AAGL. AAGL Practice Report: practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013;20(2):137–148.
The posterior colpotomy: An alternative approach to tissue extraction
CASE: Patient opts for myomectomy
A 41-year-old woman, G0, with symptomatic myomas wishes to preserve her reproductive organs rather than undergo hysterectomy. She chooses laparoscopic myomectomy.
Preoperative imaging with transvaginal ultrasound reveals a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma. Preoperative videohysteroscopy reveals external compression of the anterior intramural myoma without intracavitary extension. Both tubal ostia appear normal.
During a multipuncture technique with a 5-mm laparoscope and 5-mm accessory ports,1 the abdomen and pelvis are evaluated. The 4-cm pedunculated myoma is visualized posteriorly and to the left of midline. The 5-cm intramural myoma enlarges the contour of the uterine fundus.
How would you proceed?
With intracorporeal electromechanical “power” morcellation under scrutiny due to the potential dissemination of benign and malignant tissue, many surgeons are seeking alternatives that will allow them to continue offering minimally invasive surgical options.2–4
Intracorporeal power morcellation is used during minimally invasive gynecologic procedures, including total hysterectomy, supracervical hysterectomy, and myomectomy. Two current alternatives—laparoscopic-assisted minilaparotomy and tissue extraction through a posterior colpotomy—show promise in minimizing the risks of tissue dissemination.5–7 Regardless of the route selected for tissue extraction, the use of endoscopic specimen bags and surgical retractors may ease tissue removal and limit dissemination.
In this article, we describe contained transvaginal tissue extraction through a posterior colpotomy in the setting of laparoscopic myomectomy, describing an actual case. A video of our technique is available at obgmanagement.com.
Technique, tips, and tricks
Posterior colpotomy allows the removal of fibroids during laparoscopic myomectomy without the need to enlarge the abdominal incisions and without the use of intracorporeal power morcellation. Instead, tissue is extracted transvaginally. The incision is hidden in a natural orifice, the vagina.
Equipment consists of a:
- 5-mm laparoscope and 5-mm accessory ports
- LapSac specimen-retrieval bag (Cook Medical; various sizes available)
- AirSeal Access Port (SurgiQuest), 12 mm in diameter and 150 mm in length (FIGURE 1).
Figure 1: Equipment   The AirSeal Access Port (Top) and LapSac specimen-retrieval bag (Bottom). |
Preparatory steps Place a manipulator in the uterus and elevate it anteriorly. Position the AirSeal Access Port transvaginally, with the sharp tip below the cervix in the posterior fornix. Take care not to injure the rectum.
Confirm proper placement of the Access Port and visualize the posterior cul-de-sac laparoscopically.
Insert the 12-mm Access Port for pneumoperitoneum and the introduction and removal of suture, curved needles, and the specimen-retrieval bag.
The Access Port also provides excellent smoke evacuation and optimal visualization during the myomectomy. It is a new-concept laparoscopic port without any mechanical seal. The technology assists in maintaining pneumoperitoneum at a constant pressure despite the size of the opening.
Amputating the myomas
Choose a specimen-retrieval bag just slightly larger than the largest myoma. In this case, the larger of the two myomas is approximately 5 cm. Therefore, a 5 × 8 cm LapSac is appropriate. We roll up the LapSac and place it through the Access Port using smooth forceps, situating the bag in the abdomen prior to the start of the myomectomy, with the opening toward the uterus, so that the myomas can be collected as they are removed (FIGURE 2).
We then inject dilute vasopressin (one 20-unit ampule in 60 cc normal saline) near the base of the pedunculated myoma stalk and use monopolar electrosurgery to amputate the myoma. We place the myoma in the specimen-retrieval bag (FIGURE 3).
Next, we inject dilute vasopressin into the serosa overlying the intramural myoma and use electrosurgery to incise the serosa and myometrium. We enucleate the second myoma and place it in the bag. We then close the uterine incision using a combination of interrupted Vicryl and running V-Loc sutures on a curved CT-2 needle introduced through the Access Port (FIGURE 4).
Figure 2: Introduce the bag 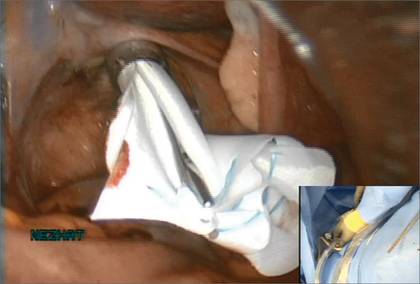 Introduce the LapSac through the Access Port. Figure 3: Contain the specimen 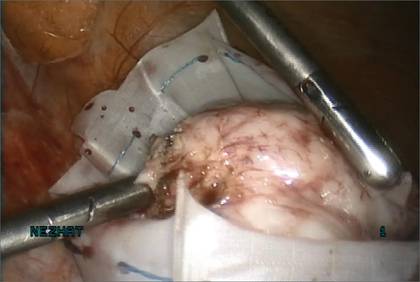 | Figure 4: Close the uterine incision 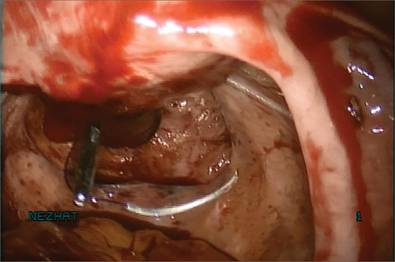 In preparation for closure, insert a curved CT-2 needle and suture material through the Access Port. Figure 5: Cinch the sac 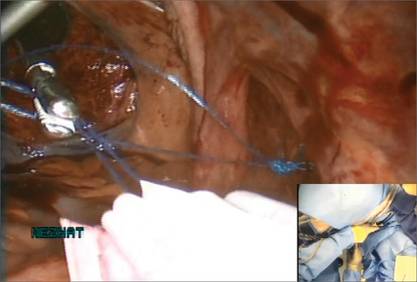 |
Tissue extraction
We place a blunt-tipped grasper transvaginally through the 12-mm Access Port to retrieve the blue polypropylene drawstring of the specimen bag (FIGURE 5). We then deactivate the Access Port and AirSeal system.
The bag containing the myomas is too large to fit through the port and the posterior colpotomy, so it is necessary to remove the Access Port from the vagina without losing the drawstrings of the specimen bag (FIGURE 6).
We vaginally exteriorize the opening of the bag (FIGURE 7), reorient the pedunculated myoma, which is oblong in shape, using forceps, and remove it without morcellation.
Manual morcellation will be necessary for the second, larger myoma. We perform that morcellation sharply using a scalpel within the specimen retrieval bag, taking care not to puncture the bag (FIGURE 8). When the myoma pieces are small enough, we remove them, along with the bag, through the posterior colpotomy. We then close the colpotomy laparoscopically using two interrupted 0 Vicryl sutures, and we copiously irrigate the pelvis (FIGURE 9).
Figure 6: Remove the Access Port 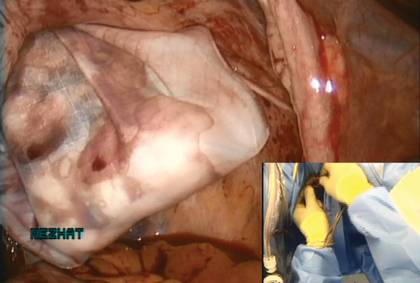 Prior to tissue extraction, remove the Access Port from the vagina. Figure 7: Exteriorize the bag 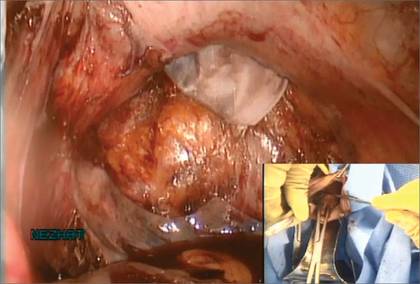 | Figure 8: Contain the morcellation 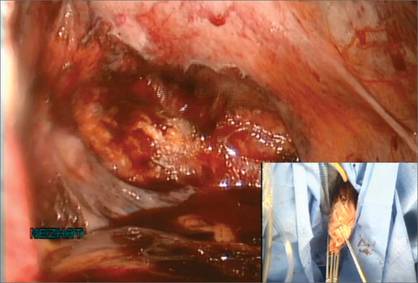 Manually morcellate the specimen within the bag and remove it transvaginally. Figure 9: Close the colpotomy 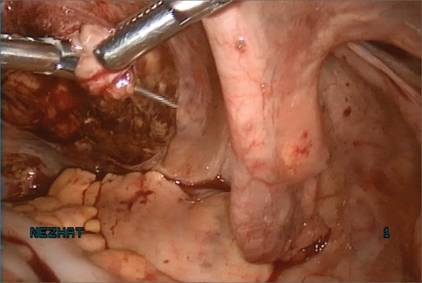 |
Benefits of this approach
The greatest benefit of this technique is the safe removal of specimens when performing fertility-sparing surgery. The 5-mm incisions are cosmetically inconspicuous. Moreover, the risk of port-site hernia is lower with 5-mm incisions, as opposed to extended incisions to remove specimens transabdominally.
The posterior colpotomy is associated with reduced pain and does not increase the rate of dyspareunia or infection; it also helps prevent pelvic adhesions.8–11
In 1993, we reported the results of second-look laparoscopy in 22 women who had undergone laparoscopic posterior colpotomy for tissue extraction. None had obliterative adhesions in the posterior cul-de-sac.11 This advantage is especially important in fertility-sparing surgery.
We have used this approach for specimen removal after several different procedures, including laparoscopic cystectomy and appendectomy.12,13 For laparoscopic cystectomy, once the cyst is drained, we enucleate it and place the cyst capsule into a specimen bag that has been inserted transvaginally through a posterior colpotomy.12 Laparoscopic appendectomy can be performed using a 12-mm stapler introduced via the colpotomy. We simply remove the specimen in its entirety through the posterior colpotomy.13
The bottom line: Gynecologic surgeons need to continue performing minimally invasive surgery for the benefit of patients. Moving forward and innovating to develop alternatives to intracorporeal power morcellation, when possible, should be our aim rather than falling back on surgeries through large abdominal incisions.
CASE: Resolved
At her 1-week postoperative visit, the patient’s 5-mm incisions are healing well and she has minimal pain.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. King LP, Nezhat C, Nezhat F, et al. Laparoscopic access. In: Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:41–53.
2. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311(9):905–906.
3. Kho KA, Anderson TL, Nezhat CH. Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol. 2014;124(4):787–793.
4. Kho K, Nezhat CH. Parasitic myomas. Obstet Gynecol. 2009;114(3):611–615.
5. Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil. 1994;39(1):39–44.
6. Seidman DS, Nezhat CH, Nezhat F, Nezhat C. The role of laparoscopic-assisted myomectomy (LAM). JSLS. 2001;5(4):299–303.
7. Kho KA, Shin JH, Nezhat C. Vaginal extraction of large uteri with the Alexis retractor. JMIG. 2009;16(5):616–617.
8. Ghezzi F, Cromi A, Uccella S, Bogani G, Serati M, Bolis P. Transumbilical versus transvaginal retrieval of surgical specimens at laparoscopy: a randomized trial. Am J Obstet Gynecol. 2012;207(2):112.e1–e6.
9. Ghezzi F, Raio L, Mueller MD, Gyr T, Buttarelli M, Franchi M. Vaginal extraction of pelvic masses following operative laparoscopy. Surg Endosc. 2002;16(12):1691–1696.
10. Guarner-Argente C, Beltrán M, Martínez-Pallí G, et al. Infection during natural orifice transluminal endoscopic surgery peritoneoscopy: a randomized comparative study in a survival porcine model. J Minim Invasive Gynecol. 2011;18(6):741–746.
11. Nezhat F, Brill AI, Nezhat CH, Nezhat C. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993;38(7):534–536.
12. Nezhat CH. Laparoscopic large ovarian cystectomy and removal through a natural orifice in a 16-year-old female. Video presented at: 21st Annual Meeting of the Society of Laparoscopic Surgeons; September 5–8, 2012; Boston, Massachusetts.
13. Nezhat CH, Datta MS, DeFazio A, Nezhat F, Nezhat C. Natural orifice-assisted laparoscopic appendectomy. JSLS. 2009;13(1):14–18.
CASE: Patient opts for myomectomy
A 41-year-old woman, G0, with symptomatic myomas wishes to preserve her reproductive organs rather than undergo hysterectomy. She chooses laparoscopic myomectomy.
Preoperative imaging with transvaginal ultrasound reveals a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma. Preoperative videohysteroscopy reveals external compression of the anterior intramural myoma without intracavitary extension. Both tubal ostia appear normal.
During a multipuncture technique with a 5-mm laparoscope and 5-mm accessory ports,1 the abdomen and pelvis are evaluated. The 4-cm pedunculated myoma is visualized posteriorly and to the left of midline. The 5-cm intramural myoma enlarges the contour of the uterine fundus.
How would you proceed?
With intracorporeal electromechanical “power” morcellation under scrutiny due to the potential dissemination of benign and malignant tissue, many surgeons are seeking alternatives that will allow them to continue offering minimally invasive surgical options.2–4
Intracorporeal power morcellation is used during minimally invasive gynecologic procedures, including total hysterectomy, supracervical hysterectomy, and myomectomy. Two current alternatives—laparoscopic-assisted minilaparotomy and tissue extraction through a posterior colpotomy—show promise in minimizing the risks of tissue dissemination.5–7 Regardless of the route selected for tissue extraction, the use of endoscopic specimen bags and surgical retractors may ease tissue removal and limit dissemination.
In this article, we describe contained transvaginal tissue extraction through a posterior colpotomy in the setting of laparoscopic myomectomy, describing an actual case. A video of our technique is available at obgmanagement.com.
Technique, tips, and tricks
Posterior colpotomy allows the removal of fibroids during laparoscopic myomectomy without the need to enlarge the abdominal incisions and without the use of intracorporeal power morcellation. Instead, tissue is extracted transvaginally. The incision is hidden in a natural orifice, the vagina.
Equipment consists of a:
- 5-mm laparoscope and 5-mm accessory ports
- LapSac specimen-retrieval bag (Cook Medical; various sizes available)
- AirSeal Access Port (SurgiQuest), 12 mm in diameter and 150 mm in length (FIGURE 1).
Figure 1: Equipment   The AirSeal Access Port (Top) and LapSac specimen-retrieval bag (Bottom). |
Preparatory steps Place a manipulator in the uterus and elevate it anteriorly. Position the AirSeal Access Port transvaginally, with the sharp tip below the cervix in the posterior fornix. Take care not to injure the rectum.
Confirm proper placement of the Access Port and visualize the posterior cul-de-sac laparoscopically.
Insert the 12-mm Access Port for pneumoperitoneum and the introduction and removal of suture, curved needles, and the specimen-retrieval bag.
The Access Port also provides excellent smoke evacuation and optimal visualization during the myomectomy. It is a new-concept laparoscopic port without any mechanical seal. The technology assists in maintaining pneumoperitoneum at a constant pressure despite the size of the opening.
Amputating the myomas
Choose a specimen-retrieval bag just slightly larger than the largest myoma. In this case, the larger of the two myomas is approximately 5 cm. Therefore, a 5 × 8 cm LapSac is appropriate. We roll up the LapSac and place it through the Access Port using smooth forceps, situating the bag in the abdomen prior to the start of the myomectomy, with the opening toward the uterus, so that the myomas can be collected as they are removed (FIGURE 2).
We then inject dilute vasopressin (one 20-unit ampule in 60 cc normal saline) near the base of the pedunculated myoma stalk and use monopolar electrosurgery to amputate the myoma. We place the myoma in the specimen-retrieval bag (FIGURE 3).
Next, we inject dilute vasopressin into the serosa overlying the intramural myoma and use electrosurgery to incise the serosa and myometrium. We enucleate the second myoma and place it in the bag. We then close the uterine incision using a combination of interrupted Vicryl and running V-Loc sutures on a curved CT-2 needle introduced through the Access Port (FIGURE 4).
Figure 2: Introduce the bag  Introduce the LapSac through the Access Port. Figure 3: Contain the specimen  | Figure 4: Close the uterine incision  In preparation for closure, insert a curved CT-2 needle and suture material through the Access Port. Figure 5: Cinch the sac  |
Tissue extraction
We place a blunt-tipped grasper transvaginally through the 12-mm Access Port to retrieve the blue polypropylene drawstring of the specimen bag (FIGURE 5). We then deactivate the Access Port and AirSeal system.
The bag containing the myomas is too large to fit through the port and the posterior colpotomy, so it is necessary to remove the Access Port from the vagina without losing the drawstrings of the specimen bag (FIGURE 6).
We vaginally exteriorize the opening of the bag (FIGURE 7), reorient the pedunculated myoma, which is oblong in shape, using forceps, and remove it without morcellation.
Manual morcellation will be necessary for the second, larger myoma. We perform that morcellation sharply using a scalpel within the specimen retrieval bag, taking care not to puncture the bag (FIGURE 8). When the myoma pieces are small enough, we remove them, along with the bag, through the posterior colpotomy. We then close the colpotomy laparoscopically using two interrupted 0 Vicryl sutures, and we copiously irrigate the pelvis (FIGURE 9).
Figure 6: Remove the Access Port  Prior to tissue extraction, remove the Access Port from the vagina. Figure 7: Exteriorize the bag  | Figure 8: Contain the morcellation  Manually morcellate the specimen within the bag and remove it transvaginally. Figure 9: Close the colpotomy  |
Benefits of this approach
The greatest benefit of this technique is the safe removal of specimens when performing fertility-sparing surgery. The 5-mm incisions are cosmetically inconspicuous. Moreover, the risk of port-site hernia is lower with 5-mm incisions, as opposed to extended incisions to remove specimens transabdominally.
The posterior colpotomy is associated with reduced pain and does not increase the rate of dyspareunia or infection; it also helps prevent pelvic adhesions.8–11
In 1993, we reported the results of second-look laparoscopy in 22 women who had undergone laparoscopic posterior colpotomy for tissue extraction. None had obliterative adhesions in the posterior cul-de-sac.11 This advantage is especially important in fertility-sparing surgery.
We have used this approach for specimen removal after several different procedures, including laparoscopic cystectomy and appendectomy.12,13 For laparoscopic cystectomy, once the cyst is drained, we enucleate it and place the cyst capsule into a specimen bag that has been inserted transvaginally through a posterior colpotomy.12 Laparoscopic appendectomy can be performed using a 12-mm stapler introduced via the colpotomy. We simply remove the specimen in its entirety through the posterior colpotomy.13
The bottom line: Gynecologic surgeons need to continue performing minimally invasive surgery for the benefit of patients. Moving forward and innovating to develop alternatives to intracorporeal power morcellation, when possible, should be our aim rather than falling back on surgeries through large abdominal incisions.
CASE: Resolved
At her 1-week postoperative visit, the patient’s 5-mm incisions are healing well and she has minimal pain.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE: Patient opts for myomectomy
A 41-year-old woman, G0, with symptomatic myomas wishes to preserve her reproductive organs rather than undergo hysterectomy. She chooses laparoscopic myomectomy.
Preoperative imaging with transvaginal ultrasound reveals a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma. Preoperative videohysteroscopy reveals external compression of the anterior intramural myoma without intracavitary extension. Both tubal ostia appear normal.
During a multipuncture technique with a 5-mm laparoscope and 5-mm accessory ports,1 the abdomen and pelvis are evaluated. The 4-cm pedunculated myoma is visualized posteriorly and to the left of midline. The 5-cm intramural myoma enlarges the contour of the uterine fundus.
How would you proceed?
With intracorporeal electromechanical “power” morcellation under scrutiny due to the potential dissemination of benign and malignant tissue, many surgeons are seeking alternatives that will allow them to continue offering minimally invasive surgical options.2–4
Intracorporeal power morcellation is used during minimally invasive gynecologic procedures, including total hysterectomy, supracervical hysterectomy, and myomectomy. Two current alternatives—laparoscopic-assisted minilaparotomy and tissue extraction through a posterior colpotomy—show promise in minimizing the risks of tissue dissemination.5–7 Regardless of the route selected for tissue extraction, the use of endoscopic specimen bags and surgical retractors may ease tissue removal and limit dissemination.
In this article, we describe contained transvaginal tissue extraction through a posterior colpotomy in the setting of laparoscopic myomectomy, describing an actual case. A video of our technique is available at obgmanagement.com.
Technique, tips, and tricks
Posterior colpotomy allows the removal of fibroids during laparoscopic myomectomy without the need to enlarge the abdominal incisions and without the use of intracorporeal power morcellation. Instead, tissue is extracted transvaginally. The incision is hidden in a natural orifice, the vagina.
Equipment consists of a:
- 5-mm laparoscope and 5-mm accessory ports
- LapSac specimen-retrieval bag (Cook Medical; various sizes available)
- AirSeal Access Port (SurgiQuest), 12 mm in diameter and 150 mm in length (FIGURE 1).
Figure 1: Equipment   The AirSeal Access Port (Top) and LapSac specimen-retrieval bag (Bottom). |
Preparatory steps Place a manipulator in the uterus and elevate it anteriorly. Position the AirSeal Access Port transvaginally, with the sharp tip below the cervix in the posterior fornix. Take care not to injure the rectum.
Confirm proper placement of the Access Port and visualize the posterior cul-de-sac laparoscopically.
Insert the 12-mm Access Port for pneumoperitoneum and the introduction and removal of suture, curved needles, and the specimen-retrieval bag.
The Access Port also provides excellent smoke evacuation and optimal visualization during the myomectomy. It is a new-concept laparoscopic port without any mechanical seal. The technology assists in maintaining pneumoperitoneum at a constant pressure despite the size of the opening.
Amputating the myomas
Choose a specimen-retrieval bag just slightly larger than the largest myoma. In this case, the larger of the two myomas is approximately 5 cm. Therefore, a 5 × 8 cm LapSac is appropriate. We roll up the LapSac and place it through the Access Port using smooth forceps, situating the bag in the abdomen prior to the start of the myomectomy, with the opening toward the uterus, so that the myomas can be collected as they are removed (FIGURE 2).
We then inject dilute vasopressin (one 20-unit ampule in 60 cc normal saline) near the base of the pedunculated myoma stalk and use monopolar electrosurgery to amputate the myoma. We place the myoma in the specimen-retrieval bag (FIGURE 3).
Next, we inject dilute vasopressin into the serosa overlying the intramural myoma and use electrosurgery to incise the serosa and myometrium. We enucleate the second myoma and place it in the bag. We then close the uterine incision using a combination of interrupted Vicryl and running V-Loc sutures on a curved CT-2 needle introduced through the Access Port (FIGURE 4).
Figure 2: Introduce the bag  Introduce the LapSac through the Access Port. Figure 3: Contain the specimen  | Figure 4: Close the uterine incision  In preparation for closure, insert a curved CT-2 needle and suture material through the Access Port. Figure 5: Cinch the sac  |
Tissue extraction
We place a blunt-tipped grasper transvaginally through the 12-mm Access Port to retrieve the blue polypropylene drawstring of the specimen bag (FIGURE 5). We then deactivate the Access Port and AirSeal system.
The bag containing the myomas is too large to fit through the port and the posterior colpotomy, so it is necessary to remove the Access Port from the vagina without losing the drawstrings of the specimen bag (FIGURE 6).
We vaginally exteriorize the opening of the bag (FIGURE 7), reorient the pedunculated myoma, which is oblong in shape, using forceps, and remove it without morcellation.
Manual morcellation will be necessary for the second, larger myoma. We perform that morcellation sharply using a scalpel within the specimen retrieval bag, taking care not to puncture the bag (FIGURE 8). When the myoma pieces are small enough, we remove them, along with the bag, through the posterior colpotomy. We then close the colpotomy laparoscopically using two interrupted 0 Vicryl sutures, and we copiously irrigate the pelvis (FIGURE 9).
Figure 6: Remove the Access Port  Prior to tissue extraction, remove the Access Port from the vagina. Figure 7: Exteriorize the bag  | Figure 8: Contain the morcellation  Manually morcellate the specimen within the bag and remove it transvaginally. Figure 9: Close the colpotomy  |
Benefits of this approach
The greatest benefit of this technique is the safe removal of specimens when performing fertility-sparing surgery. The 5-mm incisions are cosmetically inconspicuous. Moreover, the risk of port-site hernia is lower with 5-mm incisions, as opposed to extended incisions to remove specimens transabdominally.
The posterior colpotomy is associated with reduced pain and does not increase the rate of dyspareunia or infection; it also helps prevent pelvic adhesions.8–11
In 1993, we reported the results of second-look laparoscopy in 22 women who had undergone laparoscopic posterior colpotomy for tissue extraction. None had obliterative adhesions in the posterior cul-de-sac.11 This advantage is especially important in fertility-sparing surgery.
We have used this approach for specimen removal after several different procedures, including laparoscopic cystectomy and appendectomy.12,13 For laparoscopic cystectomy, once the cyst is drained, we enucleate it and place the cyst capsule into a specimen bag that has been inserted transvaginally through a posterior colpotomy.12 Laparoscopic appendectomy can be performed using a 12-mm stapler introduced via the colpotomy. We simply remove the specimen in its entirety through the posterior colpotomy.13
The bottom line: Gynecologic surgeons need to continue performing minimally invasive surgery for the benefit of patients. Moving forward and innovating to develop alternatives to intracorporeal power morcellation, when possible, should be our aim rather than falling back on surgeries through large abdominal incisions.
CASE: Resolved
At her 1-week postoperative visit, the patient’s 5-mm incisions are healing well and she has minimal pain.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. King LP, Nezhat C, Nezhat F, et al. Laparoscopic access. In: Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:41–53.
2. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311(9):905–906.
3. Kho KA, Anderson TL, Nezhat CH. Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol. 2014;124(4):787–793.
4. Kho K, Nezhat CH. Parasitic myomas. Obstet Gynecol. 2009;114(3):611–615.
5. Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil. 1994;39(1):39–44.
6. Seidman DS, Nezhat CH, Nezhat F, Nezhat C. The role of laparoscopic-assisted myomectomy (LAM). JSLS. 2001;5(4):299–303.
7. Kho KA, Shin JH, Nezhat C. Vaginal extraction of large uteri with the Alexis retractor. JMIG. 2009;16(5):616–617.
8. Ghezzi F, Cromi A, Uccella S, Bogani G, Serati M, Bolis P. Transumbilical versus transvaginal retrieval of surgical specimens at laparoscopy: a randomized trial. Am J Obstet Gynecol. 2012;207(2):112.e1–e6.
9. Ghezzi F, Raio L, Mueller MD, Gyr T, Buttarelli M, Franchi M. Vaginal extraction of pelvic masses following operative laparoscopy. Surg Endosc. 2002;16(12):1691–1696.
10. Guarner-Argente C, Beltrán M, Martínez-Pallí G, et al. Infection during natural orifice transluminal endoscopic surgery peritoneoscopy: a randomized comparative study in a survival porcine model. J Minim Invasive Gynecol. 2011;18(6):741–746.
11. Nezhat F, Brill AI, Nezhat CH, Nezhat C. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993;38(7):534–536.
12. Nezhat CH. Laparoscopic large ovarian cystectomy and removal through a natural orifice in a 16-year-old female. Video presented at: 21st Annual Meeting of the Society of Laparoscopic Surgeons; September 5–8, 2012; Boston, Massachusetts.
13. Nezhat CH, Datta MS, DeFazio A, Nezhat F, Nezhat C. Natural orifice-assisted laparoscopic appendectomy. JSLS. 2009;13(1):14–18.
1. King LP, Nezhat C, Nezhat F, et al. Laparoscopic access. In: Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:41–53.
2. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311(9):905–906.
3. Kho KA, Anderson TL, Nezhat CH. Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol. 2014;124(4):787–793.
4. Kho K, Nezhat CH. Parasitic myomas. Obstet Gynecol. 2009;114(3):611–615.
5. Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil. 1994;39(1):39–44.
6. Seidman DS, Nezhat CH, Nezhat F, Nezhat C. The role of laparoscopic-assisted myomectomy (LAM). JSLS. 2001;5(4):299–303.
7. Kho KA, Shin JH, Nezhat C. Vaginal extraction of large uteri with the Alexis retractor. JMIG. 2009;16(5):616–617.
8. Ghezzi F, Cromi A, Uccella S, Bogani G, Serati M, Bolis P. Transumbilical versus transvaginal retrieval of surgical specimens at laparoscopy: a randomized trial. Am J Obstet Gynecol. 2012;207(2):112.e1–e6.
9. Ghezzi F, Raio L, Mueller MD, Gyr T, Buttarelli M, Franchi M. Vaginal extraction of pelvic masses following operative laparoscopy. Surg Endosc. 2002;16(12):1691–1696.
10. Guarner-Argente C, Beltrán M, Martínez-Pallí G, et al. Infection during natural orifice transluminal endoscopic surgery peritoneoscopy: a randomized comparative study in a survival porcine model. J Minim Invasive Gynecol. 2011;18(6):741–746.
11. Nezhat F, Brill AI, Nezhat CH, Nezhat C. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993;38(7):534–536.
12. Nezhat CH. Laparoscopic large ovarian cystectomy and removal through a natural orifice in a 16-year-old female. Video presented at: 21st Annual Meeting of the Society of Laparoscopic Surgeons; September 5–8, 2012; Boston, Massachusetts.
13. Nezhat CH, Datta MS, DeFazio A, Nezhat F, Nezhat C. Natural orifice-assisted laparoscopic appendectomy. JSLS. 2009;13(1):14–18.
Flight plan for robotic surgery credentialing: New AAGL guidelines
The AAGL, formerly the American Association of Gynecologic Laparoscopists, has approved the first-ever set of privileging and credentialing guidelines for robotic surgery.1
Why has this prestigious minimally invasive surgery organization done that?
Maybe you’ve seen the Internet and TV ads and billboard trucks driving outside of many major medical society meetings recently, advertising “1-800-BAD-Robot.”2 You also are probably aware of recent articles in the headlines of national periodicals like the Wall Street Journal claiming that robotic surgery can be harmful.3
And yet, robotic gynecologic surgery has grown at an unprecedented rate since its approval by the US Food and Drug Administration (FDA) in April 2005. Recent data from the Nationwide Inpatient Sample from the Agency for Healthcare Research and Quality indicate that robot-assisted hysterectomies have increased at a dramatic rate.4 In a recent study of the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, investigators found that more than 30% of injuries during robotic surgery are related to operator error or robot failure, but the majority of problems are not associated with the technology.5
In this article, I use the aviation industry as an example of a sector that has gotten safety right. By emulating many of its standards, our specialty can make great strides toward patient safety and improved outcomes. I also outline the main points of the new AAGL guidelines and the rationale behind them.1 See, for example, the summary box on page 46.
A “shining example”
The robot clearly is an enabling technology. With its high-definition 3D vision and scaled motion with wristed instruments, surgeons are more comfortable performing many complex gynecologic procedures that previously would have required open surgery to safely accomplish … but the da Vinci Robot does not make a poor surgeon a great surgeon.
Hospitals now are being sued for allowing surgeons to perform robotic surgery on patients without documenting adequate surgeon training or providing consistent oversight.6 This new technology has outpaced the ability of hospital medical staffs to establish practice guidelines and rules to ensure patient safety.
The aviation industry is a shining example of a highly reliable industry. Each day, thousands of commercial aircraft fly all over the world with amazing safety. Most of the time, the pilot and copilot have never flown together. However, each crew member knows his or her role precisely and clearly understands what is expected. Crew members must meet standards that transcend all airlines and all aircraft.7 They all practice communication and undergo standardized training, including simulation, prior to taking off with live passengers on board.
In addition, all pilots must demonstrate their proficiency and competence on a regular basis—by exhibiting actual safe flight performance (over multiple takeoffs and landings) and undergoing check rides with flight examiners and practicing routine and emergency procedures on flight simulators. Airline passengers have come to expect that all pilots are equally proficient and safe. Shouldn’t patients be able to expect the same from their surgeons and hospitals? And yet there is no national or local organization that ensures that all surgeons are equally safe in the operating room. That responsibility is too often left up to the courts.
Three requirements of robotic credentialing
In 2008, the MultiCare Health System in the Pacific Northwest adopted a unique system of robotic credentialing that was based on the aviation model.8 This model has three main components, which are identical to the guidelines imposed on pilots:
- Surgeons selected for training should be likely to be successful in performing robotic surgeries safely and efficiently.
- Practice makes perfect. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills. The aviation world calls this concept “currency.”
- Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Adoption of these tried-and-true safety principles would ensure that hospitals exercise their responsibility to protect patients who undergo robotic surgeries in their systems.
The AAGL’s Robotics Special Interest Group, formed in 2010, is now the largest special interest group in the organization. The group was initially tasked to develop evidence-based guidelines for robotic surgery training and credentialing. Using the aviation industry’s model, the group developed a basic template of robotic surgery credentialing and privileging guidelines that can be used anywhere in the world. This proposal is not meant to be a standard-of-care definition; rather, it is intended simply as a starting point.
Key components of new AAGL robotic surgery credentialing and privileging guidelines1
Initial training
- Train only surgeons who have an adequate case volume to get through the learning curve. Recommended: at least 20 major cases per year.
- Current training pathways include computer-based learning, case observations, pig labs, simulation, and proctored cases. More intense validated simulation training could replace pig labs.
- Surgeons should initially perform only simple, basic procedures with surgeon first-assists until they develop the necessary skills to safely operate the robotic console and start performing more complex cases.
Annual currency
- Surgeons should perform at least 20 major cases per year, with at least one case every 8 weeks.
- If surgeons operate less frequently, proficiency should be verified on a simulator before operation on a live patient.
Annual recertification
- All surgeons should demonstrate competency annually on a simulator, regardless of case volume.
Initial training involves a long learning curve
There is a long learning curve for surgeons to become competent in robotic surgery. In initial studies of experienced advanced laparoscopic surgeons, investigators found that learning curves could involve 50 cases or more.9,10 In a recent study of gynecologic oncologists and urogynecologists at the Mayo Clinic, researchers found that it took 91 cases for experienced surgeons to become proficient on the robot.11
ObGyns in the United States are doing fewer hysterectomies than they used to.12 Many surgeons now perform fewer than 10 hysterectomies per year. These surgeons clearly have worse outcomes than surgeons who operate more frequently.13–15 Therefore, these new guidelines suggest that hospitals should choose to train only surgeons who have a case volume that will allow them to get through their learning curve in a short time and continue to have enough surgeries to maintain their skills. These guidelines recommend that surgeons who are candidates for robotic surgery training already perform a minimum of 20 major gynecologic operations per year.
It is important to learn to walk before you run. New student pilots start out with single-engine propeller planes before graduating to multi-engine props, jets, and commercial aircraft. Similarly, new medical students start out with easy surgical tasks before training for more complex procedures. This approach seems like common sense, although many surgeons may feel that, after orienting on the robot, they can start doing complex cases right away, as the robot enables them to do better and more precise surgery. Nothing could be further from the truth.
It is very important that new robotic surgeons start with easy, basic cases to completely familiarize themselves with the operation of the robot console before attempting more complex and difficult cases.
There is no absolute number of cases that ensures competency with the robot; the number depends on the surgeon’s case load, surgical prowess, and psychomotor skills. A surgeon should be restricted to simple cases initially, and should have an experienced robot-credentialed surgeon operating with him or her during this initial learning period.
Practice makes perfect
Musicians will tell you that the more often you practice, the more skilled you become. This is true for anyone whose job requires special training. It would be naïve to assume that surgeons can maintain optimal skills for robotic surgery by performing only a few cases each year.
Psychomotor skill degradation has been explored in relation to various surgical skills. The more complex the skill, the more likely that skill set will deteriorate without use. In recent studies, investigators have shown that robotic surgery skills begin to decline significantly after only 2 weeks of inactivity, and that skills continue to degrade without use.16,17
Based on this information, the currency requirement for surgeons to maintain privileges was set at 20 cases per year—fewer than two cases per month. Although the members of the Robotics Special Interest Group strongly agree that
maintenance of privileges should not be based entirely on an arbitrary currency number, as Tracy and colleagues also argue in a recent publication,18 it is clear that frequent performance of robotic surgery by high-volume surgeons clearly is more efficient and safer, with lower total operative times and complication rates, than robotic surgery performed by lower-volume surgeons.8
Currency is a well-accepted safety standard in aviation, and pilots know the importance of frequent practice and repetition in the cockpit under real-world conditions.
Ensure annual competency
Although a pilot must accomplish a minimum number of flying hours each year to maintain certification, this does not ensure that passengers will be safe. Pilots also must prove their competence by undergoing periodic check rides and demonstrating their skills on flight simulators.
Surgeons also can use these models to verify competency. Proctors who are independently certified by the FDA or another government agency as examiners could observe and evaluate surgeons performing robotic surgery using standardized checklists and grading forms. If done locally, care must be taken to assure standardization, as local hospital politics could interfere.
The only other methods currently available to verify surgeon competency are to demonstrate proficiency on simulation and to review outcomes data, looking for outliers in important areas such as complications, robotic console times, total operative times, length of stay, etc.
Simulation offers a standardized, independent method to monitor competency.19 A passing test score on a robotic simulator exercise could be a way for a surgeon to prove his or her competency. Basic robotic skills such as camera control and clutching, energy use, and sewing and needle control can be practiced on a robotic simulator.
Virtual cases such as hysterectomy and myomectomy are not yet available on the simulator, nor are cases involving typical complications. These are being developed, however, and will be available shortly.
Several gynecologic resident and fellowship training programs are using simulation to train novice surgeons, and some community hospitals are using simulation as an annual requirement for all practicing surgeons to demonstrate proficiency, similar to pilots.8 Some newer validated training protocols require a surgeon to demonstrate mastery of a particular robotic skill by achieving passing scores at least five times, with at least two consecutive passing scores.20,21
As simulators evolve, they will continue to be incorporated into training, used for surgeon warm-up before surgery, as refreshers for surgeons after a period of robotic inactivity, and for annual recertification.
When robotic surgery leads to legal trouble
A recent medical malpractice case highlights the importance of having guidelines in place to protect patients. In Bremerton, Washington, in 2008,1 a urologist performed his first nonproctored robotic prostatectomy. The challenging and difficult procedure took more than 13 hours; he converted to an open procedure after 7 hours. The patient developed significant postoperative complications and died.1
In the litigation that followed, the surgeon was sued for negligence and for failing to disclose that this was his first solo robot-assisted surgery. The surgeon settled, as did the hospital, which was sued for not supervising the surgeon and failing to ensure that he could use the robot safely. The family also sued Intuitive Surgical, the manufacturer of the da Vinci Robot, for failing to provide adequate training to the surgeon.2
The jury ruled in favor of the manufacturer, stating that the verification of adequate surgeon training was the responsibility of the hospital and specialty medical societies, not the industry.
References
- Estate of Fred Taylor v. Intuitive Surgical Inc., 09-2-03136-5, Superior Court, State of Washington, Kitsap County (Port Orchard).
- Ostrom C. Failed robotic surgery focus of Kitsap trial. Seattle Times. http://seattletimes.com/html/localnews/2020918732_robottrialxml.html Published May 3, 2013. Accessed October 10, 2014.
A word to the wise
If hospital departments really want to ensure that they are doing all that they can to make robotic surgeries safe for their patients, they will utilize the recent guidelines approved by AAGL. In order for these guidelines to work, hospital systems need to commit resources for medical staff oversight, including a robotics peer-review committee with a physician chairman and adequate medical staff support to monitor physicians and manage those who cannot meet these goals.
There clearly will be push-back from surgeons who feel that it is unfair to restrict their ability to perform surgery just because their volumes are low or they can’t master the simulation exercises. However, in the final analysis, would we want the airlines to employ pilots who fly only a couple of times a year or who can’t master the required simulation skills to safely operate a commercial passenger jet?
The important question is, what is our focus? Is it to be “fair” to all surgeons, or is it to provide the best and safest outcomes for our patients? As surgeons, we each need to remember the oath we took when we became physicians to “First, do no harm.” By following these new AAGL robotic surgery guidelines, we will reassure our patients that we, as physicians, do take that oath seriously.
INSTANT POLL
For credentialing and privileging of robotic gynecologic surgery, do you agree that the following points are essential components of the process?
1. Surgeons should be selected for training who are most likely to be successful in performing robotic surgeries safely and efficiently.
2. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills.
3. Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Answer:
a. Yes, I agree.
b. No, I believe this approach is too restrictive.
c. No, I believe this approach is not restrictive enough.
To vote, please visit obgmanagement.com and look for “Quick Poll” on the right side of the homepage.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasive Gynecol. 2014;21(2):157–167.
2. Becnel Law Firm LLC. Bad Robot Surgery. http://badrobotsurgery.com. Accessed October 10, 2014.
3. Burton TM. Report raises concern on robotic surgery device. Wall Street Journal. http://online.wsj.com/news/articles/SB10001424052702304672404579186190568061568 Published November 8, 2013. Accessed October 10, 2014.
4. Rosero E, Kho K, Joshi G, Giesecke M, Schaffer J. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122(4):778–786.
5. Fuchs Weizman N, Cohen S, Manoucheri E, Wang K, Einarsson J. Surgical errors associated with robotic surgery in gynecology: a review of the FDA MAUDE database. J Minim Invasive Gynecol. 2013;20(6):S171.
6. Lee YL, Kilic G, Phelps J. Medicolegal review of liability risks for gynecologists stemming from lack of training in robotic assisted surgery. J Minim Invasive Gynecol. 2011;18(4):512–515.
7. Federal Aviation Administration. Pilot Regulations. http://www.faa.gov/pilots/regs/. Updated March 20, 2013. Accessed October 10, 2014.
8. Lenihan JP. Navigating credentialing, privileging, and learning curves in robotics with an evidence- and experience-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
9. Lenihan J, Kovanda C, Kreaden U. What is the learning curve for robotic Gyn surgery? J Minim Invasive Gynecol. 2008;15(5):589–594.
10. Payne T, Dauterive F. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286–291.
11. Woelk J, Casiano E, Weaver A, Gostout B, Trabuco E, Gebhart A. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–96.
12. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
13. Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
14. Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volumes on outcomes for laparoscopic hysterectomy for benign conditions. Obstet Gynecol. 2012;119(4):710–716.
15. Doll K, Milad M, Gossett D. Surgeon volume and outcomes in benign hysterectomy. J Minim Invasive Gynecol. 2013;20(5):554–561.
16. Jenison E, Gil K, Lendvay T, Guy M. Robotic surgical skills: acquisition, maintenance and degradation. JSLS. 2012;16(2):218–228.
17. Guseila L, Jenison E. Maintaining robotic surgical skills during periods of robotic inactivity. J Robotic Surg. 2014;8(3):261–268.
18. Tracy E, Zephyrin L, Rosman D, Berkowitz L. Credentialing based on surgical volume. Physician workforce challenges, and patient access. Obstet Gynecol. 2013;122(5):947–951.
19. Brand T. Madigan Protocol – Si Version. Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=17. Accessed October 10, 2014.
20. Culligan P, Salamon C. Validation of a robotic simulator: transferring simulator skills to the operating room. Validation of a robotic surgery simulator protocol—transfer of simulator skills to the operating room. Fem Pelvic Med Recon Surg. 2014;20(1):48–51.
21. Culligan P. Morristown Protocol (Morristown Memorial Hospital). Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=11. Accessed October 10, 2014.
The AAGL, formerly the American Association of Gynecologic Laparoscopists, has approved the first-ever set of privileging and credentialing guidelines for robotic surgery.1
Why has this prestigious minimally invasive surgery organization done that?
Maybe you’ve seen the Internet and TV ads and billboard trucks driving outside of many major medical society meetings recently, advertising “1-800-BAD-Robot.”2 You also are probably aware of recent articles in the headlines of national periodicals like the Wall Street Journal claiming that robotic surgery can be harmful.3
And yet, robotic gynecologic surgery has grown at an unprecedented rate since its approval by the US Food and Drug Administration (FDA) in April 2005. Recent data from the Nationwide Inpatient Sample from the Agency for Healthcare Research and Quality indicate that robot-assisted hysterectomies have increased at a dramatic rate.4 In a recent study of the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, investigators found that more than 30% of injuries during robotic surgery are related to operator error or robot failure, but the majority of problems are not associated with the technology.5
In this article, I use the aviation industry as an example of a sector that has gotten safety right. By emulating many of its standards, our specialty can make great strides toward patient safety and improved outcomes. I also outline the main points of the new AAGL guidelines and the rationale behind them.1 See, for example, the summary box on page 46.
A “shining example”
The robot clearly is an enabling technology. With its high-definition 3D vision and scaled motion with wristed instruments, surgeons are more comfortable performing many complex gynecologic procedures that previously would have required open surgery to safely accomplish … but the da Vinci Robot does not make a poor surgeon a great surgeon.
Hospitals now are being sued for allowing surgeons to perform robotic surgery on patients without documenting adequate surgeon training or providing consistent oversight.6 This new technology has outpaced the ability of hospital medical staffs to establish practice guidelines and rules to ensure patient safety.
The aviation industry is a shining example of a highly reliable industry. Each day, thousands of commercial aircraft fly all over the world with amazing safety. Most of the time, the pilot and copilot have never flown together. However, each crew member knows his or her role precisely and clearly understands what is expected. Crew members must meet standards that transcend all airlines and all aircraft.7 They all practice communication and undergo standardized training, including simulation, prior to taking off with live passengers on board.
In addition, all pilots must demonstrate their proficiency and competence on a regular basis—by exhibiting actual safe flight performance (over multiple takeoffs and landings) and undergoing check rides with flight examiners and practicing routine and emergency procedures on flight simulators. Airline passengers have come to expect that all pilots are equally proficient and safe. Shouldn’t patients be able to expect the same from their surgeons and hospitals? And yet there is no national or local organization that ensures that all surgeons are equally safe in the operating room. That responsibility is too often left up to the courts.
Three requirements of robotic credentialing
In 2008, the MultiCare Health System in the Pacific Northwest adopted a unique system of robotic credentialing that was based on the aviation model.8 This model has three main components, which are identical to the guidelines imposed on pilots:
- Surgeons selected for training should be likely to be successful in performing robotic surgeries safely and efficiently.
- Practice makes perfect. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills. The aviation world calls this concept “currency.”
- Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Adoption of these tried-and-true safety principles would ensure that hospitals exercise their responsibility to protect patients who undergo robotic surgeries in their systems.
The AAGL’s Robotics Special Interest Group, formed in 2010, is now the largest special interest group in the organization. The group was initially tasked to develop evidence-based guidelines for robotic surgery training and credentialing. Using the aviation industry’s model, the group developed a basic template of robotic surgery credentialing and privileging guidelines that can be used anywhere in the world. This proposal is not meant to be a standard-of-care definition; rather, it is intended simply as a starting point.
Key components of new AAGL robotic surgery credentialing and privileging guidelines1
Initial training
- Train only surgeons who have an adequate case volume to get through the learning curve. Recommended: at least 20 major cases per year.
- Current training pathways include computer-based learning, case observations, pig labs, simulation, and proctored cases. More intense validated simulation training could replace pig labs.
- Surgeons should initially perform only simple, basic procedures with surgeon first-assists until they develop the necessary skills to safely operate the robotic console and start performing more complex cases.
Annual currency
- Surgeons should perform at least 20 major cases per year, with at least one case every 8 weeks.
- If surgeons operate less frequently, proficiency should be verified on a simulator before operation on a live patient.
Annual recertification
- All surgeons should demonstrate competency annually on a simulator, regardless of case volume.
Initial training involves a long learning curve
There is a long learning curve for surgeons to become competent in robotic surgery. In initial studies of experienced advanced laparoscopic surgeons, investigators found that learning curves could involve 50 cases or more.9,10 In a recent study of gynecologic oncologists and urogynecologists at the Mayo Clinic, researchers found that it took 91 cases for experienced surgeons to become proficient on the robot.11
ObGyns in the United States are doing fewer hysterectomies than they used to.12 Many surgeons now perform fewer than 10 hysterectomies per year. These surgeons clearly have worse outcomes than surgeons who operate more frequently.13–15 Therefore, these new guidelines suggest that hospitals should choose to train only surgeons who have a case volume that will allow them to get through their learning curve in a short time and continue to have enough surgeries to maintain their skills. These guidelines recommend that surgeons who are candidates for robotic surgery training already perform a minimum of 20 major gynecologic operations per year.
It is important to learn to walk before you run. New student pilots start out with single-engine propeller planes before graduating to multi-engine props, jets, and commercial aircraft. Similarly, new medical students start out with easy surgical tasks before training for more complex procedures. This approach seems like common sense, although many surgeons may feel that, after orienting on the robot, they can start doing complex cases right away, as the robot enables them to do better and more precise surgery. Nothing could be further from the truth.
It is very important that new robotic surgeons start with easy, basic cases to completely familiarize themselves with the operation of the robot console before attempting more complex and difficult cases.
There is no absolute number of cases that ensures competency with the robot; the number depends on the surgeon’s case load, surgical prowess, and psychomotor skills. A surgeon should be restricted to simple cases initially, and should have an experienced robot-credentialed surgeon operating with him or her during this initial learning period.
Practice makes perfect
Musicians will tell you that the more often you practice, the more skilled you become. This is true for anyone whose job requires special training. It would be naïve to assume that surgeons can maintain optimal skills for robotic surgery by performing only a few cases each year.
Psychomotor skill degradation has been explored in relation to various surgical skills. The more complex the skill, the more likely that skill set will deteriorate without use. In recent studies, investigators have shown that robotic surgery skills begin to decline significantly after only 2 weeks of inactivity, and that skills continue to degrade without use.16,17
Based on this information, the currency requirement for surgeons to maintain privileges was set at 20 cases per year—fewer than two cases per month. Although the members of the Robotics Special Interest Group strongly agree that
maintenance of privileges should not be based entirely on an arbitrary currency number, as Tracy and colleagues also argue in a recent publication,18 it is clear that frequent performance of robotic surgery by high-volume surgeons clearly is more efficient and safer, with lower total operative times and complication rates, than robotic surgery performed by lower-volume surgeons.8
Currency is a well-accepted safety standard in aviation, and pilots know the importance of frequent practice and repetition in the cockpit under real-world conditions.
Ensure annual competency
Although a pilot must accomplish a minimum number of flying hours each year to maintain certification, this does not ensure that passengers will be safe. Pilots also must prove their competence by undergoing periodic check rides and demonstrating their skills on flight simulators.
Surgeons also can use these models to verify competency. Proctors who are independently certified by the FDA or another government agency as examiners could observe and evaluate surgeons performing robotic surgery using standardized checklists and grading forms. If done locally, care must be taken to assure standardization, as local hospital politics could interfere.
The only other methods currently available to verify surgeon competency are to demonstrate proficiency on simulation and to review outcomes data, looking for outliers in important areas such as complications, robotic console times, total operative times, length of stay, etc.
Simulation offers a standardized, independent method to monitor competency.19 A passing test score on a robotic simulator exercise could be a way for a surgeon to prove his or her competency. Basic robotic skills such as camera control and clutching, energy use, and sewing and needle control can be practiced on a robotic simulator.
Virtual cases such as hysterectomy and myomectomy are not yet available on the simulator, nor are cases involving typical complications. These are being developed, however, and will be available shortly.
Several gynecologic resident and fellowship training programs are using simulation to train novice surgeons, and some community hospitals are using simulation as an annual requirement for all practicing surgeons to demonstrate proficiency, similar to pilots.8 Some newer validated training protocols require a surgeon to demonstrate mastery of a particular robotic skill by achieving passing scores at least five times, with at least two consecutive passing scores.20,21
As simulators evolve, they will continue to be incorporated into training, used for surgeon warm-up before surgery, as refreshers for surgeons after a period of robotic inactivity, and for annual recertification.
When robotic surgery leads to legal trouble
A recent medical malpractice case highlights the importance of having guidelines in place to protect patients. In Bremerton, Washington, in 2008,1 a urologist performed his first nonproctored robotic prostatectomy. The challenging and difficult procedure took more than 13 hours; he converted to an open procedure after 7 hours. The patient developed significant postoperative complications and died.1
In the litigation that followed, the surgeon was sued for negligence and for failing to disclose that this was his first solo robot-assisted surgery. The surgeon settled, as did the hospital, which was sued for not supervising the surgeon and failing to ensure that he could use the robot safely. The family also sued Intuitive Surgical, the manufacturer of the da Vinci Robot, for failing to provide adequate training to the surgeon.2
The jury ruled in favor of the manufacturer, stating that the verification of adequate surgeon training was the responsibility of the hospital and specialty medical societies, not the industry.
References
- Estate of Fred Taylor v. Intuitive Surgical Inc., 09-2-03136-5, Superior Court, State of Washington, Kitsap County (Port Orchard).
- Ostrom C. Failed robotic surgery focus of Kitsap trial. Seattle Times. http://seattletimes.com/html/localnews/2020918732_robottrialxml.html Published May 3, 2013. Accessed October 10, 2014.
A word to the wise
If hospital departments really want to ensure that they are doing all that they can to make robotic surgeries safe for their patients, they will utilize the recent guidelines approved by AAGL. In order for these guidelines to work, hospital systems need to commit resources for medical staff oversight, including a robotics peer-review committee with a physician chairman and adequate medical staff support to monitor physicians and manage those who cannot meet these goals.
There clearly will be push-back from surgeons who feel that it is unfair to restrict their ability to perform surgery just because their volumes are low or they can’t master the simulation exercises. However, in the final analysis, would we want the airlines to employ pilots who fly only a couple of times a year or who can’t master the required simulation skills to safely operate a commercial passenger jet?
The important question is, what is our focus? Is it to be “fair” to all surgeons, or is it to provide the best and safest outcomes for our patients? As surgeons, we each need to remember the oath we took when we became physicians to “First, do no harm.” By following these new AAGL robotic surgery guidelines, we will reassure our patients that we, as physicians, do take that oath seriously.
INSTANT POLL
For credentialing and privileging of robotic gynecologic surgery, do you agree that the following points are essential components of the process?
1. Surgeons should be selected for training who are most likely to be successful in performing robotic surgeries safely and efficiently.
2. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills.
3. Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Answer:
a. Yes, I agree.
b. No, I believe this approach is too restrictive.
c. No, I believe this approach is not restrictive enough.
To vote, please visit obgmanagement.com and look for “Quick Poll” on the right side of the homepage.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The AAGL, formerly the American Association of Gynecologic Laparoscopists, has approved the first-ever set of privileging and credentialing guidelines for robotic surgery.1
Why has this prestigious minimally invasive surgery organization done that?
Maybe you’ve seen the Internet and TV ads and billboard trucks driving outside of many major medical society meetings recently, advertising “1-800-BAD-Robot.”2 You also are probably aware of recent articles in the headlines of national periodicals like the Wall Street Journal claiming that robotic surgery can be harmful.3
And yet, robotic gynecologic surgery has grown at an unprecedented rate since its approval by the US Food and Drug Administration (FDA) in April 2005. Recent data from the Nationwide Inpatient Sample from the Agency for Healthcare Research and Quality indicate that robot-assisted hysterectomies have increased at a dramatic rate.4 In a recent study of the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, investigators found that more than 30% of injuries during robotic surgery are related to operator error or robot failure, but the majority of problems are not associated with the technology.5
In this article, I use the aviation industry as an example of a sector that has gotten safety right. By emulating many of its standards, our specialty can make great strides toward patient safety and improved outcomes. I also outline the main points of the new AAGL guidelines and the rationale behind them.1 See, for example, the summary box on page 46.
A “shining example”
The robot clearly is an enabling technology. With its high-definition 3D vision and scaled motion with wristed instruments, surgeons are more comfortable performing many complex gynecologic procedures that previously would have required open surgery to safely accomplish … but the da Vinci Robot does not make a poor surgeon a great surgeon.
Hospitals now are being sued for allowing surgeons to perform robotic surgery on patients without documenting adequate surgeon training or providing consistent oversight.6 This new technology has outpaced the ability of hospital medical staffs to establish practice guidelines and rules to ensure patient safety.
The aviation industry is a shining example of a highly reliable industry. Each day, thousands of commercial aircraft fly all over the world with amazing safety. Most of the time, the pilot and copilot have never flown together. However, each crew member knows his or her role precisely and clearly understands what is expected. Crew members must meet standards that transcend all airlines and all aircraft.7 They all practice communication and undergo standardized training, including simulation, prior to taking off with live passengers on board.
In addition, all pilots must demonstrate their proficiency and competence on a regular basis—by exhibiting actual safe flight performance (over multiple takeoffs and landings) and undergoing check rides with flight examiners and practicing routine and emergency procedures on flight simulators. Airline passengers have come to expect that all pilots are equally proficient and safe. Shouldn’t patients be able to expect the same from their surgeons and hospitals? And yet there is no national or local organization that ensures that all surgeons are equally safe in the operating room. That responsibility is too often left up to the courts.
Three requirements of robotic credentialing
In 2008, the MultiCare Health System in the Pacific Northwest adopted a unique system of robotic credentialing that was based on the aviation model.8 This model has three main components, which are identical to the guidelines imposed on pilots:
- Surgeons selected for training should be likely to be successful in performing robotic surgeries safely and efficiently.
- Practice makes perfect. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills. The aviation world calls this concept “currency.”
- Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Adoption of these tried-and-true safety principles would ensure that hospitals exercise their responsibility to protect patients who undergo robotic surgeries in their systems.
The AAGL’s Robotics Special Interest Group, formed in 2010, is now the largest special interest group in the organization. The group was initially tasked to develop evidence-based guidelines for robotic surgery training and credentialing. Using the aviation industry’s model, the group developed a basic template of robotic surgery credentialing and privileging guidelines that can be used anywhere in the world. This proposal is not meant to be a standard-of-care definition; rather, it is intended simply as a starting point.
Key components of new AAGL robotic surgery credentialing and privileging guidelines1
Initial training
- Train only surgeons who have an adequate case volume to get through the learning curve. Recommended: at least 20 major cases per year.
- Current training pathways include computer-based learning, case observations, pig labs, simulation, and proctored cases. More intense validated simulation training could replace pig labs.
- Surgeons should initially perform only simple, basic procedures with surgeon first-assists until they develop the necessary skills to safely operate the robotic console and start performing more complex cases.
Annual currency
- Surgeons should perform at least 20 major cases per year, with at least one case every 8 weeks.
- If surgeons operate less frequently, proficiency should be verified on a simulator before operation on a live patient.
Annual recertification
- All surgeons should demonstrate competency annually on a simulator, regardless of case volume.
Initial training involves a long learning curve
There is a long learning curve for surgeons to become competent in robotic surgery. In initial studies of experienced advanced laparoscopic surgeons, investigators found that learning curves could involve 50 cases or more.9,10 In a recent study of gynecologic oncologists and urogynecologists at the Mayo Clinic, researchers found that it took 91 cases for experienced surgeons to become proficient on the robot.11
ObGyns in the United States are doing fewer hysterectomies than they used to.12 Many surgeons now perform fewer than 10 hysterectomies per year. These surgeons clearly have worse outcomes than surgeons who operate more frequently.13–15 Therefore, these new guidelines suggest that hospitals should choose to train only surgeons who have a case volume that will allow them to get through their learning curve in a short time and continue to have enough surgeries to maintain their skills. These guidelines recommend that surgeons who are candidates for robotic surgery training already perform a minimum of 20 major gynecologic operations per year.
It is important to learn to walk before you run. New student pilots start out with single-engine propeller planes before graduating to multi-engine props, jets, and commercial aircraft. Similarly, new medical students start out with easy surgical tasks before training for more complex procedures. This approach seems like common sense, although many surgeons may feel that, after orienting on the robot, they can start doing complex cases right away, as the robot enables them to do better and more precise surgery. Nothing could be further from the truth.
It is very important that new robotic surgeons start with easy, basic cases to completely familiarize themselves with the operation of the robot console before attempting more complex and difficult cases.
There is no absolute number of cases that ensures competency with the robot; the number depends on the surgeon’s case load, surgical prowess, and psychomotor skills. A surgeon should be restricted to simple cases initially, and should have an experienced robot-credentialed surgeon operating with him or her during this initial learning period.
Practice makes perfect
Musicians will tell you that the more often you practice, the more skilled you become. This is true for anyone whose job requires special training. It would be naïve to assume that surgeons can maintain optimal skills for robotic surgery by performing only a few cases each year.
Psychomotor skill degradation has been explored in relation to various surgical skills. The more complex the skill, the more likely that skill set will deteriorate without use. In recent studies, investigators have shown that robotic surgery skills begin to decline significantly after only 2 weeks of inactivity, and that skills continue to degrade without use.16,17
Based on this information, the currency requirement for surgeons to maintain privileges was set at 20 cases per year—fewer than two cases per month. Although the members of the Robotics Special Interest Group strongly agree that
maintenance of privileges should not be based entirely on an arbitrary currency number, as Tracy and colleagues also argue in a recent publication,18 it is clear that frequent performance of robotic surgery by high-volume surgeons clearly is more efficient and safer, with lower total operative times and complication rates, than robotic surgery performed by lower-volume surgeons.8
Currency is a well-accepted safety standard in aviation, and pilots know the importance of frequent practice and repetition in the cockpit under real-world conditions.
Ensure annual competency
Although a pilot must accomplish a minimum number of flying hours each year to maintain certification, this does not ensure that passengers will be safe. Pilots also must prove their competence by undergoing periodic check rides and demonstrating their skills on flight simulators.
Surgeons also can use these models to verify competency. Proctors who are independently certified by the FDA or another government agency as examiners could observe and evaluate surgeons performing robotic surgery using standardized checklists and grading forms. If done locally, care must be taken to assure standardization, as local hospital politics could interfere.
The only other methods currently available to verify surgeon competency are to demonstrate proficiency on simulation and to review outcomes data, looking for outliers in important areas such as complications, robotic console times, total operative times, length of stay, etc.
Simulation offers a standardized, independent method to monitor competency.19 A passing test score on a robotic simulator exercise could be a way for a surgeon to prove his or her competency. Basic robotic skills such as camera control and clutching, energy use, and sewing and needle control can be practiced on a robotic simulator.
Virtual cases such as hysterectomy and myomectomy are not yet available on the simulator, nor are cases involving typical complications. These are being developed, however, and will be available shortly.
Several gynecologic resident and fellowship training programs are using simulation to train novice surgeons, and some community hospitals are using simulation as an annual requirement for all practicing surgeons to demonstrate proficiency, similar to pilots.8 Some newer validated training protocols require a surgeon to demonstrate mastery of a particular robotic skill by achieving passing scores at least five times, with at least two consecutive passing scores.20,21
As simulators evolve, they will continue to be incorporated into training, used for surgeon warm-up before surgery, as refreshers for surgeons after a period of robotic inactivity, and for annual recertification.
When robotic surgery leads to legal trouble
A recent medical malpractice case highlights the importance of having guidelines in place to protect patients. In Bremerton, Washington, in 2008,1 a urologist performed his first nonproctored robotic prostatectomy. The challenging and difficult procedure took more than 13 hours; he converted to an open procedure after 7 hours. The patient developed significant postoperative complications and died.1
In the litigation that followed, the surgeon was sued for negligence and for failing to disclose that this was his first solo robot-assisted surgery. The surgeon settled, as did the hospital, which was sued for not supervising the surgeon and failing to ensure that he could use the robot safely. The family also sued Intuitive Surgical, the manufacturer of the da Vinci Robot, for failing to provide adequate training to the surgeon.2
The jury ruled in favor of the manufacturer, stating that the verification of adequate surgeon training was the responsibility of the hospital and specialty medical societies, not the industry.
References
- Estate of Fred Taylor v. Intuitive Surgical Inc., 09-2-03136-5, Superior Court, State of Washington, Kitsap County (Port Orchard).
- Ostrom C. Failed robotic surgery focus of Kitsap trial. Seattle Times. http://seattletimes.com/html/localnews/2020918732_robottrialxml.html Published May 3, 2013. Accessed October 10, 2014.
A word to the wise
If hospital departments really want to ensure that they are doing all that they can to make robotic surgeries safe for their patients, they will utilize the recent guidelines approved by AAGL. In order for these guidelines to work, hospital systems need to commit resources for medical staff oversight, including a robotics peer-review committee with a physician chairman and adequate medical staff support to monitor physicians and manage those who cannot meet these goals.
There clearly will be push-back from surgeons who feel that it is unfair to restrict their ability to perform surgery just because their volumes are low or they can’t master the simulation exercises. However, in the final analysis, would we want the airlines to employ pilots who fly only a couple of times a year or who can’t master the required simulation skills to safely operate a commercial passenger jet?
The important question is, what is our focus? Is it to be “fair” to all surgeons, or is it to provide the best and safest outcomes for our patients? As surgeons, we each need to remember the oath we took when we became physicians to “First, do no harm.” By following these new AAGL robotic surgery guidelines, we will reassure our patients that we, as physicians, do take that oath seriously.
INSTANT POLL
For credentialing and privileging of robotic gynecologic surgery, do you agree that the following points are essential components of the process?
1. Surgeons should be selected for training who are most likely to be successful in performing robotic surgeries safely and efficiently.
2. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills.
3. Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Answer:
a. Yes, I agree.
b. No, I believe this approach is too restrictive.
c. No, I believe this approach is not restrictive enough.
To vote, please visit obgmanagement.com and look for “Quick Poll” on the right side of the homepage.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasive Gynecol. 2014;21(2):157–167.
2. Becnel Law Firm LLC. Bad Robot Surgery. http://badrobotsurgery.com. Accessed October 10, 2014.
3. Burton TM. Report raises concern on robotic surgery device. Wall Street Journal. http://online.wsj.com/news/articles/SB10001424052702304672404579186190568061568 Published November 8, 2013. Accessed October 10, 2014.
4. Rosero E, Kho K, Joshi G, Giesecke M, Schaffer J. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122(4):778–786.
5. Fuchs Weizman N, Cohen S, Manoucheri E, Wang K, Einarsson J. Surgical errors associated with robotic surgery in gynecology: a review of the FDA MAUDE database. J Minim Invasive Gynecol. 2013;20(6):S171.
6. Lee YL, Kilic G, Phelps J. Medicolegal review of liability risks for gynecologists stemming from lack of training in robotic assisted surgery. J Minim Invasive Gynecol. 2011;18(4):512–515.
7. Federal Aviation Administration. Pilot Regulations. http://www.faa.gov/pilots/regs/. Updated March 20, 2013. Accessed October 10, 2014.
8. Lenihan JP. Navigating credentialing, privileging, and learning curves in robotics with an evidence- and experience-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
9. Lenihan J, Kovanda C, Kreaden U. What is the learning curve for robotic Gyn surgery? J Minim Invasive Gynecol. 2008;15(5):589–594.
10. Payne T, Dauterive F. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286–291.
11. Woelk J, Casiano E, Weaver A, Gostout B, Trabuco E, Gebhart A. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–96.
12. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
13. Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
14. Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volumes on outcomes for laparoscopic hysterectomy for benign conditions. Obstet Gynecol. 2012;119(4):710–716.
15. Doll K, Milad M, Gossett D. Surgeon volume and outcomes in benign hysterectomy. J Minim Invasive Gynecol. 2013;20(5):554–561.
16. Jenison E, Gil K, Lendvay T, Guy M. Robotic surgical skills: acquisition, maintenance and degradation. JSLS. 2012;16(2):218–228.
17. Guseila L, Jenison E. Maintaining robotic surgical skills during periods of robotic inactivity. J Robotic Surg. 2014;8(3):261–268.
18. Tracy E, Zephyrin L, Rosman D, Berkowitz L. Credentialing based on surgical volume. Physician workforce challenges, and patient access. Obstet Gynecol. 2013;122(5):947–951.
19. Brand T. Madigan Protocol – Si Version. Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=17. Accessed October 10, 2014.
20. Culligan P, Salamon C. Validation of a robotic simulator: transferring simulator skills to the operating room. Validation of a robotic surgery simulator protocol—transfer of simulator skills to the operating room. Fem Pelvic Med Recon Surg. 2014;20(1):48–51.
21. Culligan P. Morristown Protocol (Morristown Memorial Hospital). Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=11. Accessed October 10, 2014.
1. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasive Gynecol. 2014;21(2):157–167.
2. Becnel Law Firm LLC. Bad Robot Surgery. http://badrobotsurgery.com. Accessed October 10, 2014.
3. Burton TM. Report raises concern on robotic surgery device. Wall Street Journal. http://online.wsj.com/news/articles/SB10001424052702304672404579186190568061568 Published November 8, 2013. Accessed October 10, 2014.
4. Rosero E, Kho K, Joshi G, Giesecke M, Schaffer J. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122(4):778–786.
5. Fuchs Weizman N, Cohen S, Manoucheri E, Wang K, Einarsson J. Surgical errors associated with robotic surgery in gynecology: a review of the FDA MAUDE database. J Minim Invasive Gynecol. 2013;20(6):S171.
6. Lee YL, Kilic G, Phelps J. Medicolegal review of liability risks for gynecologists stemming from lack of training in robotic assisted surgery. J Minim Invasive Gynecol. 2011;18(4):512–515.
7. Federal Aviation Administration. Pilot Regulations. http://www.faa.gov/pilots/regs/. Updated March 20, 2013. Accessed October 10, 2014.
8. Lenihan JP. Navigating credentialing, privileging, and learning curves in robotics with an evidence- and experience-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
9. Lenihan J, Kovanda C, Kreaden U. What is the learning curve for robotic Gyn surgery? J Minim Invasive Gynecol. 2008;15(5):589–594.
10. Payne T, Dauterive F. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286–291.
11. Woelk J, Casiano E, Weaver A, Gostout B, Trabuco E, Gebhart A. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–96.
12. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
13. Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
14. Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volumes on outcomes for laparoscopic hysterectomy for benign conditions. Obstet Gynecol. 2012;119(4):710–716.
15. Doll K, Milad M, Gossett D. Surgeon volume and outcomes in benign hysterectomy. J Minim Invasive Gynecol. 2013;20(5):554–561.
16. Jenison E, Gil K, Lendvay T, Guy M. Robotic surgical skills: acquisition, maintenance and degradation. JSLS. 2012;16(2):218–228.
17. Guseila L, Jenison E. Maintaining robotic surgical skills during periods of robotic inactivity. J Robotic Surg. 2014;8(3):261–268.
18. Tracy E, Zephyrin L, Rosman D, Berkowitz L. Credentialing based on surgical volume. Physician workforce challenges, and patient access. Obstet Gynecol. 2013;122(5):947–951.
19. Brand T. Madigan Protocol – Si Version. Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=17. Accessed October 10, 2014.
20. Culligan P, Salamon C. Validation of a robotic simulator: transferring simulator skills to the operating room. Validation of a robotic surgery simulator protocol—transfer of simulator skills to the operating room. Fem Pelvic Med Recon Surg. 2014;20(1):48–51.
21. Culligan P. Morristown Protocol (Morristown Memorial Hospital). Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=11. Accessed October 10, 2014.
Total abdominal hysterectomy the Mayo Clinic way
The abdominal approach to hysterectomy remains the most common route to hysterectomy in the United States. Its greatest advantage: It allows the uterus to be removed intact.1–3
The recent US Food and Drug Administration (FDA) warning against the use of power morcellation in women with known or suspected uterine malignancy has left many gynecologic surgeons wondering what might be the optimal approach to the removal of a large uterus.4
Although most hysterectomies are performed for benign conditions—namely, uterine fibroids—malignancy should be considered in the differential diagnosis. When hysterectomy is performed laparoscopically, a large uterus must be morcellated intraperitoneally. Since the FDA safety communication was issued, some hospitals have imposed a moratorium on the use of power morcellators for removal of uterine tissue until more definitive evidence is put forth regarding safety and best practices. This chain of events allows us an opportunity to review the basics of abdominal hysterectomy.
For the sake of this discussion, I will assume that the hysterectomy is being performed for a benign indication as I highlight the Mayo Clinic approach to total abdominal hysterectomy (TAH).5
Preoperative considerations
The patient should be medically able to undergo operative intervention. If she has preexisting medical conditions, preoperative clearance should be obtained from her primary care provider, and her medical conditions should be optimized prior to surgical intervention.
Baseline laboratory studies include a complete blood count, electrolyte panel, glucose assessment, and an electrocardiogram (EKG). Bowel prep typically is not required. Provisions should be made to prevent deep venous thrombosis (DVT), usually by utilizing sequential compression devices, based on the individual patient’s risk factors.6,7
A prophylactic antibiotic to prevent surgical site infection (often a first-generation cephalosporin) should be given as a single intravenous (IV) dose prior to the incision.8 If bacterial vaginosis is present, treatment prior to surgery can reduce the frequency of vaginal cuff infection.9
Again, for the sake of this discussion, I will assume that malignancy has been ruled out.
Positioning and preparation
After induction of anesthesia, position the patient either in a dorsal supine (traditional) or lithotomy (yellow-fin stirrups) position and reexamine her to confirm the findings of the pelvic exam. If the patient is positioned in the supine position, use ankle straps to prevent her from moving as the Trendelenburg position advances during the procedure.
Prep the abdominal skin with a bactericidal agent (most often a povidone-iodine solution). Also prep the vagina with a povidone-iodine solution because the vaginal cuff will be opened during the TAH. Place a transurethral catheter to drain urine throughout the case. Use of a three-way catheter allows the bladder to be easily backfilled during the procedure for identification of its borders or assessment of its integrity.
Last, incorporate a surgical pause prior to the incision to confirm that you have the right patient, know the procedure and incision planned, and are aware of any allergies. Also confirm that antibiotics have been given.
Operative technique
Intraoperative principles
A planned approach avoids wasteful time and motion, and an adequate incision allows for sufficient exposure, which is critical but often underappreciated by the novice surgeon. We prefer a midline incision because it allows the most flexibility to adapt to intraoperative findings, but a Pfannenstiel incision also is an option.
Fixed retraction is paramount to “set up” exposure for the remainder of the case. We prefer a Balfour fixed retractor but, with smaller uteri, a self-retaining Alexis retractor (Applied Medical, Rancho Santa Margarita, California) affords decent exposure and may cause less postoperative abdominal wall discomfort; it also avoids the possibility of retractor-related neuropathy.
Moistened abdominal packing allows the bowel to be packed into the upper abdomen for the remainder of the case, which facilitates consistent exposure of the operative field. Adequate lighting is essential, as is one or more knowledgeable assistants.
Use sharp dissection throughout the procedure. Clean, sharp dissection averts injury to adjacent structures, such as the ureter, bladder, and rectum, and promotes recognition of any injuries, permitting immediate repair.
The application of proper traction and counter-traction on tissues allows accurate definition of the correct tissue planes and facilitates identification of important anatomic structures. Vital structures should be identified and, if necessary, mobilized before any clamps are placed or pedicles transected. Adhesions should be sharply lysed to facilitate exposure.
Freeing the bladder anteriorly and the rectum posteriorly prevents their inadvertent inclusion in closure of the vagina and minimizes the risk of fistula formation. The bladder and rectum should be sharply mobilized at least 1 cm beyond the site of planned vaginal transection.
Last, excellent support of the vaginal wall can be provided by securing the uterosacral-cardinal ligaments to the corners of the vaginal vault.
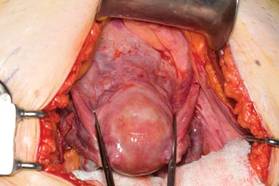 |
| FIGURE 1: Place straight Kocher clamps to facilitate traction during the operation. |
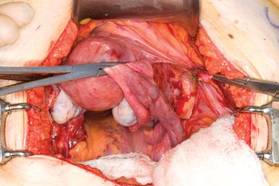 |
| FIGURE 2: Clamp and divide the right round ligament, opening the broad ligament. |
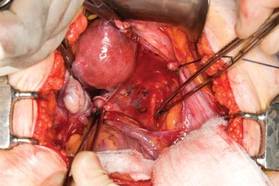 |
| FIGURE 3: Identify the right ureter along the medial leaf of the broad ligament. |
Identifying the ureter
Once good exposure and adequate Trendelenburg position are achieved, place Kocher clamps across the cornual portion of the uterus (incorporating the round ligament, tube, and utero-ovarian pedicle) (FIGURE 1). This facilitates continuous traction and prevents back bleeding throughout the case.
With traction applied to the left, identify the right round ligament, clamp it with a Kocher clamp, and transect it. Incise the peritoneum parallel to the uterus and gonadal vessels (FIGURE 2). This opens the broad ligament and allows identification of the critical underlying structures (ureter, external and internal iliac vessels). Following the medial leaf of the broad ligament downward, identify the ureter by both visualization and palpation (FIGURE 3).
Although I do not discuss salpingo-oophorectomy in this article, be aware that the ureter is at risk when clamping the gonadal vessels near the pelvic brim.
Once the ureter is identified, create a window in the broad ligament above the ureter. In a medial to lateral fashion, place your index finger through that peritoneal window, making certain the ureter is below and out of the way. Place a Kocher clamp across the tube and utero-ovarian pedicle, and transect and suture-ligate the pedicle (preserving the tube and ovary). Repeat this procedure on the patient’s left side, using traction and counter-traction to facilitate exposure (FIGURE 4).
Mobilizing the bladder
With the assistant providing upward traction on the uterus, use Russian forceps to elevate the peritoneum overlying the bladder. Undermine and incise the peritoneum from the patient’s left to the right (FIGURE 5). Begin sharp dissection of the loose areolar tissue. By gently spreading the tissue using the tips of the scissors, and snipping the tissue in the midline, you allow the dissection to proceed down the lower uterine segment (FIGURE 6).
Any bleeding usually means you are too close to the bladder or have ventured too far laterally. If the patient has had a previous cesarean delivery, this area may be densely scarred. Often, it is easiest to dissect laterally around the scar on each side, where there is less dense scarring, and mobilize the tissue until the denser central scarring can be dissected. Note that the bladder attachment curves upward on each side and lateral to the cervix, over the lateral vagina and the uterine vessels.
It is absolutely critical to dissect and expose 1 or 2 cm of the entire anterior vaginal wall below the level of the cervix to be certain that the bladder has been fully mobilized and to prevent later incorporation into the vaginal cuff closure. The exact location of the cervix is best detected by placing a finger behind the uterus and using the thumb to compress the area of the anterior portion of the cervix under the bladder.
Fibroids can cause distortion of the anatomic planes we utilize. Be aware of the distortion and adjust your dissection accordingly. The structures of the urinary tract are most often affected; sharp dissection is necessary to mobilize the ureter and bladder in these cases. (See the case discussions)
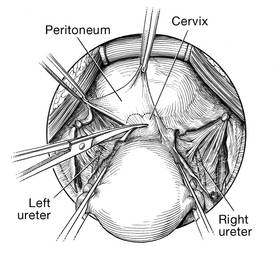 | 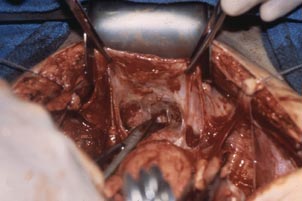 |
FIGURE 5: Upward traction on the peritoneum overlying the bladder facilitates development of the bladder flap off of the lower uterus. | FIGURE 6: Dissect the bladder off the lower uterus |
Large intramural or pedunculated myomas can be difficult surgical challenges. Broad-ligament myomas, however, are unique. Significant anatomic distortion can occur. Always consider the possibility of some degree of ureteral obstruction and be on the lookout for unrecognized bladder injury.
Case 1
This very large myoma essentially filled the pelvis but seems to arise from the left side of the uterus, distorting the anatomy. Note the attenuation of the round ligaments and the normal appearance of the tubes and ovaries (the left tube has a distal paratubal cyst.) Note also the bladder, particularly how sharp dissection will be required to mobilize it off the underlying mass.
To manage removal, at case outset, we placed bilateral external ureteral stents and used a lucite vaginal dilator to aid in respective ureter and vaginal apex identification. The bladder was attenuated over this large mass and was rather easily dissected, given the defined mass around it. The ureters were well lateral and inferior and readily identified with stent palpation. The cervix was certainly elongated and, after the uterine vessels were removed, the hysterectomy was completed without incident.
Surgical pearl: To extract very large masses during total abdominal hysterectomy, sometimes you have more “room” if the fixed retractor is removed. You can then use a series of handheld retractors (Deavor, Harrington, etc) on the side you are operating until the mass has been mobilized enough to place a fixed retractor.
CASE 2
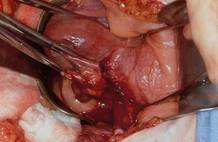 | 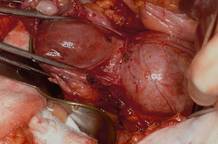 |
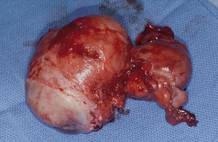 | 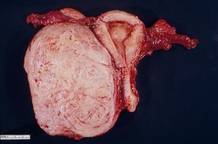 |
This large cervical myoma is creating urinary urgency, frequency, and moderate obstruction of the right ureter. Sharp dissection is critical to mobilize the bladder well free of the myoma. We placed bilateral ureteral stents to start the case to aid in identification of the ureter.
The first illustration at right (top left) shows the operative appearance before the bladder flap was taken down. The second photo (top right) reveals the extent of this large myoma after the bladder has been sharply dissected free of the mass. The third photo (bottom left) displays the specimen sent to pathology (be sure to minimize the amount of vaginal tissue taken with the specimen). Note the distortion of the endocervical canal and cervix. The last photo (bottom right) reveals the sectioned specimen.
Surgical pearl: Use a three-way catheter and backfill the bladder for identification during the procedure and at the conclusion of the case to rule out bladder injury. A few drops of methylene blue added to the solution makes recognition easier.
Ligation of the uterine arteries
Apply cephalad traction to the uterus and place a Harrington retractor anteriorly to retract the bladder away from the cervix on the upper portion of the vagina. With the uterus pulled first to the left, palpate the right ureter between the thumb and index finger at the level of the uterine artery (FIGURE 7). Once you have determined the course of the ureter, place a Kocher clamp well down on the right side of the lower cervix at about a 45° angle, sliding off the side of the cervix (FIGURE 8). The clamp should now include the superior portion of the cardinal ligament with the uterine vessels and paracolpium immediately above the lateral vaginal fornix.
Transect the cardinal pedicle. Repeat the procedure on the left side after adjusting the Harrington retractor slightly to the left and identifying the course of the ureter where the Kocher clamp will be placed. Thus, a single Kocher clamp is placed on each side to control the blood supply.
It is paramount that you know the location of the ureter prior to placement and transection of the uterine vessels to prevent inadvertent injury or obstruction of the ureters.
Divide the uterine vessel–cardinal ligament complex close to the cervix (medial to the Kocher clamp), slightly undercutting the tip (FIGURE 9). This creates a fascial window, the anterior edge of which is the pubocervical fascia. This is subsequently developed and managed as a separate layer during the vaginal vault closure.
Repeat this procedure on the left side.
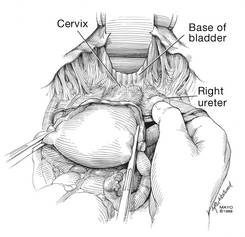 | 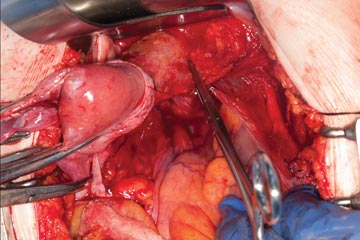 | 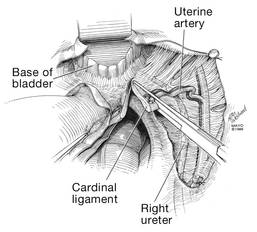 |
| FIGURE 7: After mobilizing the bladder, palpate the right ureter prior to placement of a Kocher clamp on the cardinal vascular pedicle. | FIGURE 8: Place a single Kocher clamp on the right uterine vessels. See the right ureter well lateral of the clamp. | FIGURE 9: The right uterine vessels have been clamped and transected. The clamp is slightly undercut to allow access to the vaginal fornix. |
Preparing and opening the vaginal cuff
Develop the anterior pubocervical fascia by cutting with a scissors horizontally across the anterior vaginal wall at the level of the cervix (FIGURE 10). This layer of tissue will be utilized to close the vaginal cuff in a secondary layer later on. This technique will serve to close the pubocervical fascial ring at the vaginal vault and, with support of the uterosacral ligaments, provide support to the vaginal apex. At this point, the vagina has not yet been entered. The remaining tissue beneath the mobilized layer and the anterior cervix is the anterior vaginal wall.
Pull the uterus up and anterior toward the pubic symphysis to make the uterosacral ligaments prominent. Then transect the uterosacral ligaments close to the uterus (FIGURE 11). You may encounter minor bleeding, but there is no need to ligate the stumps at this point. Cut the tissue between the ligaments horizontally, similarly to the anterior dissection. With gentle finger dissection, as necessary, this should free the rectosigmoid colon from the posterior vaginal wall.
If this is a new technique for you, it may serve you well to place a stitch in each uterosacral ligament, below the spot where you will transect it, prior to cutting. When you have the uterus on tension, the ligaments are most recognizable, and these sutures can then be incorporated into the vaginal angles.
At this point there should be a circular area just beneath the cervix that is the vaginal wall at the apex of the vagina. Retract the uterus anteriorly and to the left, and enter the vagina posteriorly and laterally, just above the stump of the right uterosacral ligament (FIGURE 12). The uterus now can be removed by circumcising the vagina as close to the cervix as possible to avoid vaginal shortening. As the uterus is being removed, place four vulsellum tenacula successively at the 3, 12, 9, and 6 o’clock positions of the vaginal cuff as it is developed (FIGURE 13). Then swab povidone-iodine on the vaginal cuff and canal.
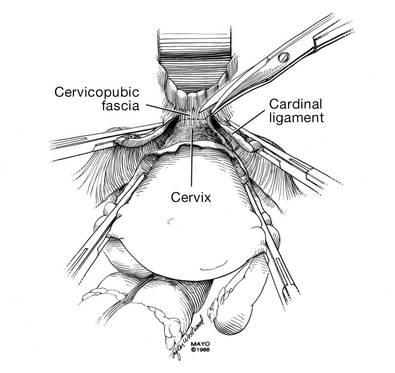 | 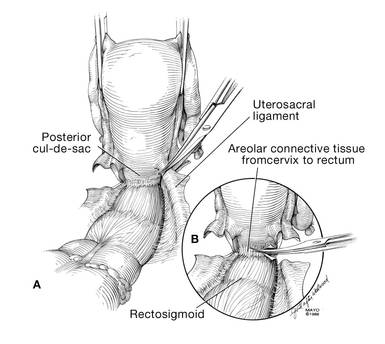 |
| FIGURE 10: After mobilizing the bladder and securing the vascular pedicles, develop the anterior endopelvic fascia. It will be used as a second layer to close the vaginal cuff. | FIGURE 11: A. Transect the uterosacral ligaments. B. Mobilize the rectum posteriorly. |
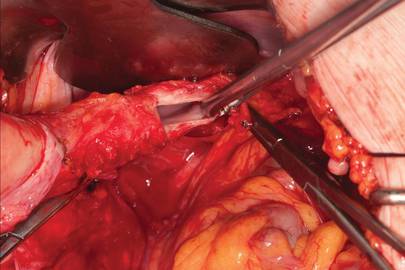 | 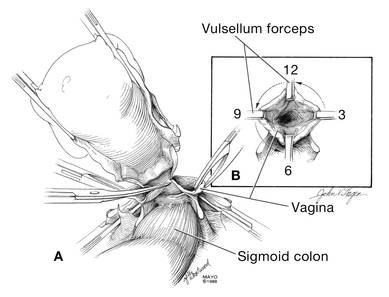 |
| FIGURE 12: Enter the right vaginal fornix and grasp it with a vulsellum tenaculum. | FIGURE 13: A. After entering the right vaginal fornix, extend the incision in a counter-clockwise fashion, preserving vaginal length. B. Placement of the tenaculum as the incision proceeds. |
Cuff closure
Place a lap salt sponge over the Kocher clamps to prevent suture entanglement. Use a 36-inch continuous 0-polyglactin suture to close the vaginal vault and achieve hemostasis. Begin suturing with a right vaginal angle stitch, placed so that the small vessels are ligated and the lateral supporting tissues from the base of the cardinal ligament are attached to the right vaginal angle. This closes the “window” that was created with slight undercutting of the cardinal ligament earlier. Continue suturing toward the left, with each bite placed submucosally so that the epithelial edges are approximated and inverted into the vagina. The suture does not enter the vagina (FIGURE 14). As you reach the left vaginal angle, obtain a healthy purchase of the left uterosacral ligament and then pass the needle laterally to the vaginal fornix angle, through the lateral supporting tissues at the base of the cardinal ligament, as was done on the right vaginal angle. Then lock the suture and return across the vaginal vault. This will plicate together the anterior and posterior pubocervical fascia layers developed earlier, creating a second layer, before closing the fascial ring at the vaginal apex. Incorporate the right uterosacral ligament into the right vaginal angle and tie the suture (FIGURES 15 AND 16).
Close the cuff and verify hemostasis. Use the lap salt sponge covering the Kocher clamps on the cardinal ligament pedicles to wipe the surgical field clean. Then use light cautery along the cuff and the base and back of the bladder. If there is some bleeding at the very corners of the vagina, it can usually be managed during suturing of the cardinal ligament pedicles into the corners of the vault.
Elevate the Kocher clamp containing the cardinal ligament and uterine vessels toward the midline. Then palpate the ureter between your index finger and thumb as it courses through the cardinal ligament toward the bladder. This step provides a second check on the location of the ureter (the first was when the clamp was originally placed) before the cardinal ligament is tied to the corner of the vault (FIGURE 17). The needle should enter the peritoneum, right uterosacral ligament, and full thickness of the right angle of the vagina just lateral to the suture used to close the vaginal apex. Bring the pedicle over the corner of the vagina and tie it close to the lateral aspect of the Kocher clamp, leaving an adequate stump. Then free-tie the stump of the cardinal ligament to add a double ligation of this vascular pedicle. Repeat this procedure on the opposite side (FIGURE 18). Then verify hemostasis throughout the operative field.
Take the patient out of Trendelenburg position and place her flat, and copiously irrigate the pelvis. Once needle and sponge counts are completed, close the abdomen in a layered fashion. Place a wound dressing and a Foley catheter, leaving the latter in place overnight.
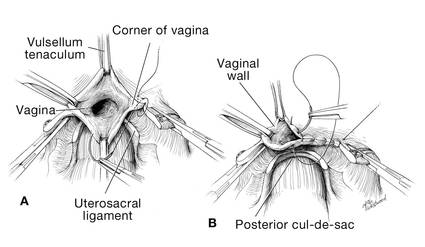 | 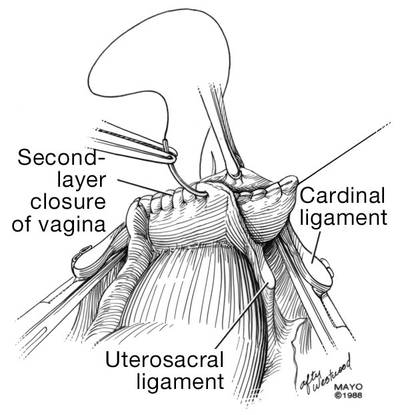 |
| FIGURE 14: A. Begin in the right vaginal corner, closing the area that was “undercut” (see FIGURE 10). B. Close the first layer in in a subcuticular manner. | FIGURE 15: The second layer of cuff closure utilizes the anterior and posterior endopelvic fascia and imbricates the first layer. |
 | |
FIGURE 16: Two-layer closure of the vaginal cuff. | |
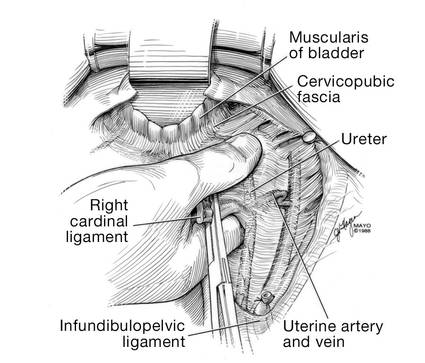 | 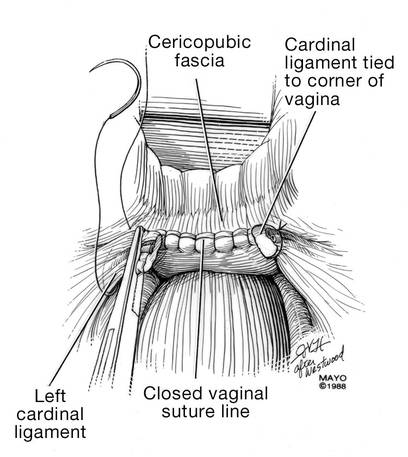 |
| FIGURE 17: Prior to suture ligation of the right uterine vessels, palpate the ureter again to identify its location. | FIGURE 18: Suture-ligate the uterine pedicles into the corners of the vaginal cuff. |
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091–1095.
2. Falcone T, Walters MD. Hysterectomy for benign disease. Obstet Gynecol. 2008;111(3):753–767.
3. Unger JB, Paul R, Caldito G. Hysterectomy for the massive leiomyomatous uterus. Obstet Gynecol. 2002;100(6):1271–1275.
4. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed September 15, 2014.
5. Webb MJ. Mayo Clinic Manual of Pelvic Surgery. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:55–72.
6. Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 84. Prevention of deep vein thrombosis and pulmonary embolism. Obstet Gynecol. 2007;110(2 pt 1):429–440.
7. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis. 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
8. Committee on Practice Bulletins, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 74. Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2006;108(1):225–234.
9. Larsson PG, Carlsson B. Does pre- and postoperative metronidazole treatment lower vaginal cuff infection rate after abdominal hysterectomy among women with bacterial vaginosis? Infect Dis Obstet Gynecol. 2002;10(3):133–140.
The abdominal approach to hysterectomy remains the most common route to hysterectomy in the United States. Its greatest advantage: It allows the uterus to be removed intact.1–3
The recent US Food and Drug Administration (FDA) warning against the use of power morcellation in women with known or suspected uterine malignancy has left many gynecologic surgeons wondering what might be the optimal approach to the removal of a large uterus.4
Although most hysterectomies are performed for benign conditions—namely, uterine fibroids—malignancy should be considered in the differential diagnosis. When hysterectomy is performed laparoscopically, a large uterus must be morcellated intraperitoneally. Since the FDA safety communication was issued, some hospitals have imposed a moratorium on the use of power morcellators for removal of uterine tissue until more definitive evidence is put forth regarding safety and best practices. This chain of events allows us an opportunity to review the basics of abdominal hysterectomy.
For the sake of this discussion, I will assume that the hysterectomy is being performed for a benign indication as I highlight the Mayo Clinic approach to total abdominal hysterectomy (TAH).5
Preoperative considerations
The patient should be medically able to undergo operative intervention. If she has preexisting medical conditions, preoperative clearance should be obtained from her primary care provider, and her medical conditions should be optimized prior to surgical intervention.
Baseline laboratory studies include a complete blood count, electrolyte panel, glucose assessment, and an electrocardiogram (EKG). Bowel prep typically is not required. Provisions should be made to prevent deep venous thrombosis (DVT), usually by utilizing sequential compression devices, based on the individual patient’s risk factors.6,7
A prophylactic antibiotic to prevent surgical site infection (often a first-generation cephalosporin) should be given as a single intravenous (IV) dose prior to the incision.8 If bacterial vaginosis is present, treatment prior to surgery can reduce the frequency of vaginal cuff infection.9
Again, for the sake of this discussion, I will assume that malignancy has been ruled out.
Positioning and preparation
After induction of anesthesia, position the patient either in a dorsal supine (traditional) or lithotomy (yellow-fin stirrups) position and reexamine her to confirm the findings of the pelvic exam. If the patient is positioned in the supine position, use ankle straps to prevent her from moving as the Trendelenburg position advances during the procedure.
Prep the abdominal skin with a bactericidal agent (most often a povidone-iodine solution). Also prep the vagina with a povidone-iodine solution because the vaginal cuff will be opened during the TAH. Place a transurethral catheter to drain urine throughout the case. Use of a three-way catheter allows the bladder to be easily backfilled during the procedure for identification of its borders or assessment of its integrity.
Last, incorporate a surgical pause prior to the incision to confirm that you have the right patient, know the procedure and incision planned, and are aware of any allergies. Also confirm that antibiotics have been given.
Operative technique
Intraoperative principles
A planned approach avoids wasteful time and motion, and an adequate incision allows for sufficient exposure, which is critical but often underappreciated by the novice surgeon. We prefer a midline incision because it allows the most flexibility to adapt to intraoperative findings, but a Pfannenstiel incision also is an option.
Fixed retraction is paramount to “set up” exposure for the remainder of the case. We prefer a Balfour fixed retractor but, with smaller uteri, a self-retaining Alexis retractor (Applied Medical, Rancho Santa Margarita, California) affords decent exposure and may cause less postoperative abdominal wall discomfort; it also avoids the possibility of retractor-related neuropathy.
Moistened abdominal packing allows the bowel to be packed into the upper abdomen for the remainder of the case, which facilitates consistent exposure of the operative field. Adequate lighting is essential, as is one or more knowledgeable assistants.
Use sharp dissection throughout the procedure. Clean, sharp dissection averts injury to adjacent structures, such as the ureter, bladder, and rectum, and promotes recognition of any injuries, permitting immediate repair.
The application of proper traction and counter-traction on tissues allows accurate definition of the correct tissue planes and facilitates identification of important anatomic structures. Vital structures should be identified and, if necessary, mobilized before any clamps are placed or pedicles transected. Adhesions should be sharply lysed to facilitate exposure.
Freeing the bladder anteriorly and the rectum posteriorly prevents their inadvertent inclusion in closure of the vagina and minimizes the risk of fistula formation. The bladder and rectum should be sharply mobilized at least 1 cm beyond the site of planned vaginal transection.
Last, excellent support of the vaginal wall can be provided by securing the uterosacral-cardinal ligaments to the corners of the vaginal vault.
 |
| FIGURE 1: Place straight Kocher clamps to facilitate traction during the operation. |
 |
| FIGURE 2: Clamp and divide the right round ligament, opening the broad ligament. |
 |
| FIGURE 3: Identify the right ureter along the medial leaf of the broad ligament. |
Identifying the ureter
Once good exposure and adequate Trendelenburg position are achieved, place Kocher clamps across the cornual portion of the uterus (incorporating the round ligament, tube, and utero-ovarian pedicle) (FIGURE 1). This facilitates continuous traction and prevents back bleeding throughout the case.
With traction applied to the left, identify the right round ligament, clamp it with a Kocher clamp, and transect it. Incise the peritoneum parallel to the uterus and gonadal vessels (FIGURE 2). This opens the broad ligament and allows identification of the critical underlying structures (ureter, external and internal iliac vessels). Following the medial leaf of the broad ligament downward, identify the ureter by both visualization and palpation (FIGURE 3).
Although I do not discuss salpingo-oophorectomy in this article, be aware that the ureter is at risk when clamping the gonadal vessels near the pelvic brim.
Once the ureter is identified, create a window in the broad ligament above the ureter. In a medial to lateral fashion, place your index finger through that peritoneal window, making certain the ureter is below and out of the way. Place a Kocher clamp across the tube and utero-ovarian pedicle, and transect and suture-ligate the pedicle (preserving the tube and ovary). Repeat this procedure on the patient’s left side, using traction and counter-traction to facilitate exposure (FIGURE 4).
Mobilizing the bladder
With the assistant providing upward traction on the uterus, use Russian forceps to elevate the peritoneum overlying the bladder. Undermine and incise the peritoneum from the patient’s left to the right (FIGURE 5). Begin sharp dissection of the loose areolar tissue. By gently spreading the tissue using the tips of the scissors, and snipping the tissue in the midline, you allow the dissection to proceed down the lower uterine segment (FIGURE 6).
Any bleeding usually means you are too close to the bladder or have ventured too far laterally. If the patient has had a previous cesarean delivery, this area may be densely scarred. Often, it is easiest to dissect laterally around the scar on each side, where there is less dense scarring, and mobilize the tissue until the denser central scarring can be dissected. Note that the bladder attachment curves upward on each side and lateral to the cervix, over the lateral vagina and the uterine vessels.
It is absolutely critical to dissect and expose 1 or 2 cm of the entire anterior vaginal wall below the level of the cervix to be certain that the bladder has been fully mobilized and to prevent later incorporation into the vaginal cuff closure. The exact location of the cervix is best detected by placing a finger behind the uterus and using the thumb to compress the area of the anterior portion of the cervix under the bladder.
Fibroids can cause distortion of the anatomic planes we utilize. Be aware of the distortion and adjust your dissection accordingly. The structures of the urinary tract are most often affected; sharp dissection is necessary to mobilize the ureter and bladder in these cases. (See the case discussions)
 |  |
FIGURE 5: Upward traction on the peritoneum overlying the bladder facilitates development of the bladder flap off of the lower uterus. | FIGURE 6: Dissect the bladder off the lower uterus |
Large intramural or pedunculated myomas can be difficult surgical challenges. Broad-ligament myomas, however, are unique. Significant anatomic distortion can occur. Always consider the possibility of some degree of ureteral obstruction and be on the lookout for unrecognized bladder injury.
Case 1
This very large myoma essentially filled the pelvis but seems to arise from the left side of the uterus, distorting the anatomy. Note the attenuation of the round ligaments and the normal appearance of the tubes and ovaries (the left tube has a distal paratubal cyst.) Note also the bladder, particularly how sharp dissection will be required to mobilize it off the underlying mass.
To manage removal, at case outset, we placed bilateral external ureteral stents and used a lucite vaginal dilator to aid in respective ureter and vaginal apex identification. The bladder was attenuated over this large mass and was rather easily dissected, given the defined mass around it. The ureters were well lateral and inferior and readily identified with stent palpation. The cervix was certainly elongated and, after the uterine vessels were removed, the hysterectomy was completed without incident.
Surgical pearl: To extract very large masses during total abdominal hysterectomy, sometimes you have more “room” if the fixed retractor is removed. You can then use a series of handheld retractors (Deavor, Harrington, etc) on the side you are operating until the mass has been mobilized enough to place a fixed retractor.
CASE 2
 |  |
 |  |
This large cervical myoma is creating urinary urgency, frequency, and moderate obstruction of the right ureter. Sharp dissection is critical to mobilize the bladder well free of the myoma. We placed bilateral ureteral stents to start the case to aid in identification of the ureter.
The first illustration at right (top left) shows the operative appearance before the bladder flap was taken down. The second photo (top right) reveals the extent of this large myoma after the bladder has been sharply dissected free of the mass. The third photo (bottom left) displays the specimen sent to pathology (be sure to minimize the amount of vaginal tissue taken with the specimen). Note the distortion of the endocervical canal and cervix. The last photo (bottom right) reveals the sectioned specimen.
Surgical pearl: Use a three-way catheter and backfill the bladder for identification during the procedure and at the conclusion of the case to rule out bladder injury. A few drops of methylene blue added to the solution makes recognition easier.
Ligation of the uterine arteries
Apply cephalad traction to the uterus and place a Harrington retractor anteriorly to retract the bladder away from the cervix on the upper portion of the vagina. With the uterus pulled first to the left, palpate the right ureter between the thumb and index finger at the level of the uterine artery (FIGURE 7). Once you have determined the course of the ureter, place a Kocher clamp well down on the right side of the lower cervix at about a 45° angle, sliding off the side of the cervix (FIGURE 8). The clamp should now include the superior portion of the cardinal ligament with the uterine vessels and paracolpium immediately above the lateral vaginal fornix.
Transect the cardinal pedicle. Repeat the procedure on the left side after adjusting the Harrington retractor slightly to the left and identifying the course of the ureter where the Kocher clamp will be placed. Thus, a single Kocher clamp is placed on each side to control the blood supply.
It is paramount that you know the location of the ureter prior to placement and transection of the uterine vessels to prevent inadvertent injury or obstruction of the ureters.
Divide the uterine vessel–cardinal ligament complex close to the cervix (medial to the Kocher clamp), slightly undercutting the tip (FIGURE 9). This creates a fascial window, the anterior edge of which is the pubocervical fascia. This is subsequently developed and managed as a separate layer during the vaginal vault closure.
Repeat this procedure on the left side.
 |  |  |
| FIGURE 7: After mobilizing the bladder, palpate the right ureter prior to placement of a Kocher clamp on the cardinal vascular pedicle. | FIGURE 8: Place a single Kocher clamp on the right uterine vessels. See the right ureter well lateral of the clamp. | FIGURE 9: The right uterine vessels have been clamped and transected. The clamp is slightly undercut to allow access to the vaginal fornix. |
Preparing and opening the vaginal cuff
Develop the anterior pubocervical fascia by cutting with a scissors horizontally across the anterior vaginal wall at the level of the cervix (FIGURE 10). This layer of tissue will be utilized to close the vaginal cuff in a secondary layer later on. This technique will serve to close the pubocervical fascial ring at the vaginal vault and, with support of the uterosacral ligaments, provide support to the vaginal apex. At this point, the vagina has not yet been entered. The remaining tissue beneath the mobilized layer and the anterior cervix is the anterior vaginal wall.
Pull the uterus up and anterior toward the pubic symphysis to make the uterosacral ligaments prominent. Then transect the uterosacral ligaments close to the uterus (FIGURE 11). You may encounter minor bleeding, but there is no need to ligate the stumps at this point. Cut the tissue between the ligaments horizontally, similarly to the anterior dissection. With gentle finger dissection, as necessary, this should free the rectosigmoid colon from the posterior vaginal wall.
If this is a new technique for you, it may serve you well to place a stitch in each uterosacral ligament, below the spot where you will transect it, prior to cutting. When you have the uterus on tension, the ligaments are most recognizable, and these sutures can then be incorporated into the vaginal angles.
At this point there should be a circular area just beneath the cervix that is the vaginal wall at the apex of the vagina. Retract the uterus anteriorly and to the left, and enter the vagina posteriorly and laterally, just above the stump of the right uterosacral ligament (FIGURE 12). The uterus now can be removed by circumcising the vagina as close to the cervix as possible to avoid vaginal shortening. As the uterus is being removed, place four vulsellum tenacula successively at the 3, 12, 9, and 6 o’clock positions of the vaginal cuff as it is developed (FIGURE 13). Then swab povidone-iodine on the vaginal cuff and canal.
 |  |
| FIGURE 10: After mobilizing the bladder and securing the vascular pedicles, develop the anterior endopelvic fascia. It will be used as a second layer to close the vaginal cuff. | FIGURE 11: A. Transect the uterosacral ligaments. B. Mobilize the rectum posteriorly. |
 |  |
| FIGURE 12: Enter the right vaginal fornix and grasp it with a vulsellum tenaculum. | FIGURE 13: A. After entering the right vaginal fornix, extend the incision in a counter-clockwise fashion, preserving vaginal length. B. Placement of the tenaculum as the incision proceeds. |
Cuff closure
Place a lap salt sponge over the Kocher clamps to prevent suture entanglement. Use a 36-inch continuous 0-polyglactin suture to close the vaginal vault and achieve hemostasis. Begin suturing with a right vaginal angle stitch, placed so that the small vessels are ligated and the lateral supporting tissues from the base of the cardinal ligament are attached to the right vaginal angle. This closes the “window” that was created with slight undercutting of the cardinal ligament earlier. Continue suturing toward the left, with each bite placed submucosally so that the epithelial edges are approximated and inverted into the vagina. The suture does not enter the vagina (FIGURE 14). As you reach the left vaginal angle, obtain a healthy purchase of the left uterosacral ligament and then pass the needle laterally to the vaginal fornix angle, through the lateral supporting tissues at the base of the cardinal ligament, as was done on the right vaginal angle. Then lock the suture and return across the vaginal vault. This will plicate together the anterior and posterior pubocervical fascia layers developed earlier, creating a second layer, before closing the fascial ring at the vaginal apex. Incorporate the right uterosacral ligament into the right vaginal angle and tie the suture (FIGURES 15 AND 16).
Close the cuff and verify hemostasis. Use the lap salt sponge covering the Kocher clamps on the cardinal ligament pedicles to wipe the surgical field clean. Then use light cautery along the cuff and the base and back of the bladder. If there is some bleeding at the very corners of the vagina, it can usually be managed during suturing of the cardinal ligament pedicles into the corners of the vault.
Elevate the Kocher clamp containing the cardinal ligament and uterine vessels toward the midline. Then palpate the ureter between your index finger and thumb as it courses through the cardinal ligament toward the bladder. This step provides a second check on the location of the ureter (the first was when the clamp was originally placed) before the cardinal ligament is tied to the corner of the vault (FIGURE 17). The needle should enter the peritoneum, right uterosacral ligament, and full thickness of the right angle of the vagina just lateral to the suture used to close the vaginal apex. Bring the pedicle over the corner of the vagina and tie it close to the lateral aspect of the Kocher clamp, leaving an adequate stump. Then free-tie the stump of the cardinal ligament to add a double ligation of this vascular pedicle. Repeat this procedure on the opposite side (FIGURE 18). Then verify hemostasis throughout the operative field.
Take the patient out of Trendelenburg position and place her flat, and copiously irrigate the pelvis. Once needle and sponge counts are completed, close the abdomen in a layered fashion. Place a wound dressing and a Foley catheter, leaving the latter in place overnight.
 |  |
| FIGURE 14: A. Begin in the right vaginal corner, closing the area that was “undercut” (see FIGURE 10). B. Close the first layer in in a subcuticular manner. | FIGURE 15: The second layer of cuff closure utilizes the anterior and posterior endopelvic fascia and imbricates the first layer. |
 | |
FIGURE 16: Two-layer closure of the vaginal cuff. | |
 |  |
| FIGURE 17: Prior to suture ligation of the right uterine vessels, palpate the ureter again to identify its location. | FIGURE 18: Suture-ligate the uterine pedicles into the corners of the vaginal cuff. |
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The abdominal approach to hysterectomy remains the most common route to hysterectomy in the United States. Its greatest advantage: It allows the uterus to be removed intact.1–3
The recent US Food and Drug Administration (FDA) warning against the use of power morcellation in women with known or suspected uterine malignancy has left many gynecologic surgeons wondering what might be the optimal approach to the removal of a large uterus.4
Although most hysterectomies are performed for benign conditions—namely, uterine fibroids—malignancy should be considered in the differential diagnosis. When hysterectomy is performed laparoscopically, a large uterus must be morcellated intraperitoneally. Since the FDA safety communication was issued, some hospitals have imposed a moratorium on the use of power morcellators for removal of uterine tissue until more definitive evidence is put forth regarding safety and best practices. This chain of events allows us an opportunity to review the basics of abdominal hysterectomy.
For the sake of this discussion, I will assume that the hysterectomy is being performed for a benign indication as I highlight the Mayo Clinic approach to total abdominal hysterectomy (TAH).5
Preoperative considerations
The patient should be medically able to undergo operative intervention. If she has preexisting medical conditions, preoperative clearance should be obtained from her primary care provider, and her medical conditions should be optimized prior to surgical intervention.
Baseline laboratory studies include a complete blood count, electrolyte panel, glucose assessment, and an electrocardiogram (EKG). Bowel prep typically is not required. Provisions should be made to prevent deep venous thrombosis (DVT), usually by utilizing sequential compression devices, based on the individual patient’s risk factors.6,7
A prophylactic antibiotic to prevent surgical site infection (often a first-generation cephalosporin) should be given as a single intravenous (IV) dose prior to the incision.8 If bacterial vaginosis is present, treatment prior to surgery can reduce the frequency of vaginal cuff infection.9
Again, for the sake of this discussion, I will assume that malignancy has been ruled out.
Positioning and preparation
After induction of anesthesia, position the patient either in a dorsal supine (traditional) or lithotomy (yellow-fin stirrups) position and reexamine her to confirm the findings of the pelvic exam. If the patient is positioned in the supine position, use ankle straps to prevent her from moving as the Trendelenburg position advances during the procedure.
Prep the abdominal skin with a bactericidal agent (most often a povidone-iodine solution). Also prep the vagina with a povidone-iodine solution because the vaginal cuff will be opened during the TAH. Place a transurethral catheter to drain urine throughout the case. Use of a three-way catheter allows the bladder to be easily backfilled during the procedure for identification of its borders or assessment of its integrity.
Last, incorporate a surgical pause prior to the incision to confirm that you have the right patient, know the procedure and incision planned, and are aware of any allergies. Also confirm that antibiotics have been given.
Operative technique
Intraoperative principles
A planned approach avoids wasteful time and motion, and an adequate incision allows for sufficient exposure, which is critical but often underappreciated by the novice surgeon. We prefer a midline incision because it allows the most flexibility to adapt to intraoperative findings, but a Pfannenstiel incision also is an option.
Fixed retraction is paramount to “set up” exposure for the remainder of the case. We prefer a Balfour fixed retractor but, with smaller uteri, a self-retaining Alexis retractor (Applied Medical, Rancho Santa Margarita, California) affords decent exposure and may cause less postoperative abdominal wall discomfort; it also avoids the possibility of retractor-related neuropathy.
Moistened abdominal packing allows the bowel to be packed into the upper abdomen for the remainder of the case, which facilitates consistent exposure of the operative field. Adequate lighting is essential, as is one or more knowledgeable assistants.
Use sharp dissection throughout the procedure. Clean, sharp dissection averts injury to adjacent structures, such as the ureter, bladder, and rectum, and promotes recognition of any injuries, permitting immediate repair.
The application of proper traction and counter-traction on tissues allows accurate definition of the correct tissue planes and facilitates identification of important anatomic structures. Vital structures should be identified and, if necessary, mobilized before any clamps are placed or pedicles transected. Adhesions should be sharply lysed to facilitate exposure.
Freeing the bladder anteriorly and the rectum posteriorly prevents their inadvertent inclusion in closure of the vagina and minimizes the risk of fistula formation. The bladder and rectum should be sharply mobilized at least 1 cm beyond the site of planned vaginal transection.
Last, excellent support of the vaginal wall can be provided by securing the uterosacral-cardinal ligaments to the corners of the vaginal vault.
 |
| FIGURE 1: Place straight Kocher clamps to facilitate traction during the operation. |
 |
| FIGURE 2: Clamp and divide the right round ligament, opening the broad ligament. |
 |
| FIGURE 3: Identify the right ureter along the medial leaf of the broad ligament. |
Identifying the ureter
Once good exposure and adequate Trendelenburg position are achieved, place Kocher clamps across the cornual portion of the uterus (incorporating the round ligament, tube, and utero-ovarian pedicle) (FIGURE 1). This facilitates continuous traction and prevents back bleeding throughout the case.
With traction applied to the left, identify the right round ligament, clamp it with a Kocher clamp, and transect it. Incise the peritoneum parallel to the uterus and gonadal vessels (FIGURE 2). This opens the broad ligament and allows identification of the critical underlying structures (ureter, external and internal iliac vessels). Following the medial leaf of the broad ligament downward, identify the ureter by both visualization and palpation (FIGURE 3).
Although I do not discuss salpingo-oophorectomy in this article, be aware that the ureter is at risk when clamping the gonadal vessels near the pelvic brim.
Once the ureter is identified, create a window in the broad ligament above the ureter. In a medial to lateral fashion, place your index finger through that peritoneal window, making certain the ureter is below and out of the way. Place a Kocher clamp across the tube and utero-ovarian pedicle, and transect and suture-ligate the pedicle (preserving the tube and ovary). Repeat this procedure on the patient’s left side, using traction and counter-traction to facilitate exposure (FIGURE 4).
Mobilizing the bladder
With the assistant providing upward traction on the uterus, use Russian forceps to elevate the peritoneum overlying the bladder. Undermine and incise the peritoneum from the patient’s left to the right (FIGURE 5). Begin sharp dissection of the loose areolar tissue. By gently spreading the tissue using the tips of the scissors, and snipping the tissue in the midline, you allow the dissection to proceed down the lower uterine segment (FIGURE 6).
Any bleeding usually means you are too close to the bladder or have ventured too far laterally. If the patient has had a previous cesarean delivery, this area may be densely scarred. Often, it is easiest to dissect laterally around the scar on each side, where there is less dense scarring, and mobilize the tissue until the denser central scarring can be dissected. Note that the bladder attachment curves upward on each side and lateral to the cervix, over the lateral vagina and the uterine vessels.
It is absolutely critical to dissect and expose 1 or 2 cm of the entire anterior vaginal wall below the level of the cervix to be certain that the bladder has been fully mobilized and to prevent later incorporation into the vaginal cuff closure. The exact location of the cervix is best detected by placing a finger behind the uterus and using the thumb to compress the area of the anterior portion of the cervix under the bladder.
Fibroids can cause distortion of the anatomic planes we utilize. Be aware of the distortion and adjust your dissection accordingly. The structures of the urinary tract are most often affected; sharp dissection is necessary to mobilize the ureter and bladder in these cases. (See the case discussions)
 |  |
FIGURE 5: Upward traction on the peritoneum overlying the bladder facilitates development of the bladder flap off of the lower uterus. | FIGURE 6: Dissect the bladder off the lower uterus |
Large intramural or pedunculated myomas can be difficult surgical challenges. Broad-ligament myomas, however, are unique. Significant anatomic distortion can occur. Always consider the possibility of some degree of ureteral obstruction and be on the lookout for unrecognized bladder injury.
Case 1
This very large myoma essentially filled the pelvis but seems to arise from the left side of the uterus, distorting the anatomy. Note the attenuation of the round ligaments and the normal appearance of the tubes and ovaries (the left tube has a distal paratubal cyst.) Note also the bladder, particularly how sharp dissection will be required to mobilize it off the underlying mass.
To manage removal, at case outset, we placed bilateral external ureteral stents and used a lucite vaginal dilator to aid in respective ureter and vaginal apex identification. The bladder was attenuated over this large mass and was rather easily dissected, given the defined mass around it. The ureters were well lateral and inferior and readily identified with stent palpation. The cervix was certainly elongated and, after the uterine vessels were removed, the hysterectomy was completed without incident.
Surgical pearl: To extract very large masses during total abdominal hysterectomy, sometimes you have more “room” if the fixed retractor is removed. You can then use a series of handheld retractors (Deavor, Harrington, etc) on the side you are operating until the mass has been mobilized enough to place a fixed retractor.
CASE 2
 |  |
 |  |
This large cervical myoma is creating urinary urgency, frequency, and moderate obstruction of the right ureter. Sharp dissection is critical to mobilize the bladder well free of the myoma. We placed bilateral ureteral stents to start the case to aid in identification of the ureter.
The first illustration at right (top left) shows the operative appearance before the bladder flap was taken down. The second photo (top right) reveals the extent of this large myoma after the bladder has been sharply dissected free of the mass. The third photo (bottom left) displays the specimen sent to pathology (be sure to minimize the amount of vaginal tissue taken with the specimen). Note the distortion of the endocervical canal and cervix. The last photo (bottom right) reveals the sectioned specimen.
Surgical pearl: Use a three-way catheter and backfill the bladder for identification during the procedure and at the conclusion of the case to rule out bladder injury. A few drops of methylene blue added to the solution makes recognition easier.
Ligation of the uterine arteries
Apply cephalad traction to the uterus and place a Harrington retractor anteriorly to retract the bladder away from the cervix on the upper portion of the vagina. With the uterus pulled first to the left, palpate the right ureter between the thumb and index finger at the level of the uterine artery (FIGURE 7). Once you have determined the course of the ureter, place a Kocher clamp well down on the right side of the lower cervix at about a 45° angle, sliding off the side of the cervix (FIGURE 8). The clamp should now include the superior portion of the cardinal ligament with the uterine vessels and paracolpium immediately above the lateral vaginal fornix.
Transect the cardinal pedicle. Repeat the procedure on the left side after adjusting the Harrington retractor slightly to the left and identifying the course of the ureter where the Kocher clamp will be placed. Thus, a single Kocher clamp is placed on each side to control the blood supply.
It is paramount that you know the location of the ureter prior to placement and transection of the uterine vessels to prevent inadvertent injury or obstruction of the ureters.
Divide the uterine vessel–cardinal ligament complex close to the cervix (medial to the Kocher clamp), slightly undercutting the tip (FIGURE 9). This creates a fascial window, the anterior edge of which is the pubocervical fascia. This is subsequently developed and managed as a separate layer during the vaginal vault closure.
Repeat this procedure on the left side.
 |  |  |
| FIGURE 7: After mobilizing the bladder, palpate the right ureter prior to placement of a Kocher clamp on the cardinal vascular pedicle. | FIGURE 8: Place a single Kocher clamp on the right uterine vessels. See the right ureter well lateral of the clamp. | FIGURE 9: The right uterine vessels have been clamped and transected. The clamp is slightly undercut to allow access to the vaginal fornix. |
Preparing and opening the vaginal cuff
Develop the anterior pubocervical fascia by cutting with a scissors horizontally across the anterior vaginal wall at the level of the cervix (FIGURE 10). This layer of tissue will be utilized to close the vaginal cuff in a secondary layer later on. This technique will serve to close the pubocervical fascial ring at the vaginal vault and, with support of the uterosacral ligaments, provide support to the vaginal apex. At this point, the vagina has not yet been entered. The remaining tissue beneath the mobilized layer and the anterior cervix is the anterior vaginal wall.
Pull the uterus up and anterior toward the pubic symphysis to make the uterosacral ligaments prominent. Then transect the uterosacral ligaments close to the uterus (FIGURE 11). You may encounter minor bleeding, but there is no need to ligate the stumps at this point. Cut the tissue between the ligaments horizontally, similarly to the anterior dissection. With gentle finger dissection, as necessary, this should free the rectosigmoid colon from the posterior vaginal wall.
If this is a new technique for you, it may serve you well to place a stitch in each uterosacral ligament, below the spot where you will transect it, prior to cutting. When you have the uterus on tension, the ligaments are most recognizable, and these sutures can then be incorporated into the vaginal angles.
At this point there should be a circular area just beneath the cervix that is the vaginal wall at the apex of the vagina. Retract the uterus anteriorly and to the left, and enter the vagina posteriorly and laterally, just above the stump of the right uterosacral ligament (FIGURE 12). The uterus now can be removed by circumcising the vagina as close to the cervix as possible to avoid vaginal shortening. As the uterus is being removed, place four vulsellum tenacula successively at the 3, 12, 9, and 6 o’clock positions of the vaginal cuff as it is developed (FIGURE 13). Then swab povidone-iodine on the vaginal cuff and canal.
 |  |
| FIGURE 10: After mobilizing the bladder and securing the vascular pedicles, develop the anterior endopelvic fascia. It will be used as a second layer to close the vaginal cuff. | FIGURE 11: A. Transect the uterosacral ligaments. B. Mobilize the rectum posteriorly. |
 |  |
| FIGURE 12: Enter the right vaginal fornix and grasp it with a vulsellum tenaculum. | FIGURE 13: A. After entering the right vaginal fornix, extend the incision in a counter-clockwise fashion, preserving vaginal length. B. Placement of the tenaculum as the incision proceeds. |
Cuff closure
Place a lap salt sponge over the Kocher clamps to prevent suture entanglement. Use a 36-inch continuous 0-polyglactin suture to close the vaginal vault and achieve hemostasis. Begin suturing with a right vaginal angle stitch, placed so that the small vessels are ligated and the lateral supporting tissues from the base of the cardinal ligament are attached to the right vaginal angle. This closes the “window” that was created with slight undercutting of the cardinal ligament earlier. Continue suturing toward the left, with each bite placed submucosally so that the epithelial edges are approximated and inverted into the vagina. The suture does not enter the vagina (FIGURE 14). As you reach the left vaginal angle, obtain a healthy purchase of the left uterosacral ligament and then pass the needle laterally to the vaginal fornix angle, through the lateral supporting tissues at the base of the cardinal ligament, as was done on the right vaginal angle. Then lock the suture and return across the vaginal vault. This will plicate together the anterior and posterior pubocervical fascia layers developed earlier, creating a second layer, before closing the fascial ring at the vaginal apex. Incorporate the right uterosacral ligament into the right vaginal angle and tie the suture (FIGURES 15 AND 16).
Close the cuff and verify hemostasis. Use the lap salt sponge covering the Kocher clamps on the cardinal ligament pedicles to wipe the surgical field clean. Then use light cautery along the cuff and the base and back of the bladder. If there is some bleeding at the very corners of the vagina, it can usually be managed during suturing of the cardinal ligament pedicles into the corners of the vault.
Elevate the Kocher clamp containing the cardinal ligament and uterine vessels toward the midline. Then palpate the ureter between your index finger and thumb as it courses through the cardinal ligament toward the bladder. This step provides a second check on the location of the ureter (the first was when the clamp was originally placed) before the cardinal ligament is tied to the corner of the vault (FIGURE 17). The needle should enter the peritoneum, right uterosacral ligament, and full thickness of the right angle of the vagina just lateral to the suture used to close the vaginal apex. Bring the pedicle over the corner of the vagina and tie it close to the lateral aspect of the Kocher clamp, leaving an adequate stump. Then free-tie the stump of the cardinal ligament to add a double ligation of this vascular pedicle. Repeat this procedure on the opposite side (FIGURE 18). Then verify hemostasis throughout the operative field.
Take the patient out of Trendelenburg position and place her flat, and copiously irrigate the pelvis. Once needle and sponge counts are completed, close the abdomen in a layered fashion. Place a wound dressing and a Foley catheter, leaving the latter in place overnight.
 |  |
| FIGURE 14: A. Begin in the right vaginal corner, closing the area that was “undercut” (see FIGURE 10). B. Close the first layer in in a subcuticular manner. | FIGURE 15: The second layer of cuff closure utilizes the anterior and posterior endopelvic fascia and imbricates the first layer. |
 | |
FIGURE 16: Two-layer closure of the vaginal cuff. | |
 |  |
| FIGURE 17: Prior to suture ligation of the right uterine vessels, palpate the ureter again to identify its location. | FIGURE 18: Suture-ligate the uterine pedicles into the corners of the vaginal cuff. |
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091–1095.
2. Falcone T, Walters MD. Hysterectomy for benign disease. Obstet Gynecol. 2008;111(3):753–767.
3. Unger JB, Paul R, Caldito G. Hysterectomy for the massive leiomyomatous uterus. Obstet Gynecol. 2002;100(6):1271–1275.
4. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed September 15, 2014.
5. Webb MJ. Mayo Clinic Manual of Pelvic Surgery. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:55–72.
6. Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 84. Prevention of deep vein thrombosis and pulmonary embolism. Obstet Gynecol. 2007;110(2 pt 1):429–440.
7. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis. 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
8. Committee on Practice Bulletins, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 74. Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2006;108(1):225–234.
9. Larsson PG, Carlsson B. Does pre- and postoperative metronidazole treatment lower vaginal cuff infection rate after abdominal hysterectomy among women with bacterial vaginosis? Infect Dis Obstet Gynecol. 2002;10(3):133–140.
1. Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091–1095.
2. Falcone T, Walters MD. Hysterectomy for benign disease. Obstet Gynecol. 2008;111(3):753–767.
3. Unger JB, Paul R, Caldito G. Hysterectomy for the massive leiomyomatous uterus. Obstet Gynecol. 2002;100(6):1271–1275.
4. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed September 15, 2014.
5. Webb MJ. Mayo Clinic Manual of Pelvic Surgery. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:55–72.
6. Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 84. Prevention of deep vein thrombosis and pulmonary embolism. Obstet Gynecol. 2007;110(2 pt 1):429–440.
7. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis. 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
8. Committee on Practice Bulletins, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 74. Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2006;108(1):225–234.
9. Larsson PG, Carlsson B. Does pre- and postoperative metronidazole treatment lower vaginal cuff infection rate after abdominal hysterectomy among women with bacterial vaginosis? Infect Dis Obstet Gynecol. 2002;10(3):133–140.
Laparoscopic dual-port contained power morcellation: An offered solution
Minimally invasive surgery utilizing laparoscopy for hysterectomy and myomectomy has become more common in women with gynecologic pathology. The benefits of this approach compared with laparotomy include decreased hospital stay, shorter recovery and, in experienced hands, significantly decreased morbidity.1–3
Approximately 600,000 hysterectomies are performed annually in the United States—30% of which are performed laparoscopically.4 The primary indication for surgical intervention is uterine leiomyoma. This pathology accounts for 40% of procedures.5 During these surgeries, electromechanical morcellation (EMM), or open “power” morcellation, is commonly used to cut large tissue specimens into small pieces for removal and thereby avoid a larger incision. Concerns have been raised regarding the use of open power morcellation because of the risk of spreading an unrecognized malignancy.
Based on case reports and retrospective studies, the FDA issued a statement in April of this year discouraging the use of EMM for hysterectomy and myomectomy in women with uterine fibroids.6 The concern for inadvertent spread of an occult malignancy was the reasoning for the communication. Since that time, the FDA’s Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee held a public meeting in which the panel heard comments from patients, societies, and industry regarding their positions on the safety of laparoscopic power morcellation. The panel made several recommendations to the FDA but, at the time of this writing, the FDA has yet to issue a final decision.
Reaction to FDA’s action/inaction
The FDA’s “safety” communication was in response to the concern of a few who experienced a bad outcome believed to be secondary to open power morcellation of enlarged uteri or fibroid tumors. In its statement, the FDA estimated the risk of an occult sarcoma to be about 1 in 350 and stated that the risk of disseminating a sarcoma with morcellation is substantial. The FDA discouraged the use of the power morcellator during hysterectomy or myomectomy for uterine fibroids.
Many organizations, including the Society of Gynecologic Oncology, The American Association of Gynecologic Laparoscopists (AAGL), and the American College of Obstetricians and Gynecologists, issued less stringent statements regarding this technology.7–9 These organizations stated generally that there were too few data to make a statement at that time, advocated the collection of more data, and encouraged detailed informed consent to be given to patients undergoing these procedures.
However, the FDA’s statement, and lack of a timely follow-up to clarify the role of the laparoscopic power morcellator in gynecologic surgery, has effectively stopped the use of this technology in its current form. In fact, in response to the statement, Ethicon Endosurgery has discontinued the distribution and sales of its power morcellator and many institutions have severely or completely restricted the use of this technology. The reason for these restrictions is that the medicolegal consequences of an adverse outcome would be very difficult to defend given the current, albeit premature, recommendations of the FDA. This statement makes it difficult to defend any adverse outcome that may occur in association with the use of the laparoscopic power morcellator. Furthermore, this statement by the FDA has largely prevented the medical community at large from collecting additional useful information to allow for a data-driven determination.
Power morcellation is not without risks. In fact, we outline them in this article. However, we believe that minimally invasive surgery should be allowed to continue to advance. In that vein, here we describe a technique of dual-port contained EMM. This surgical approach is performed under direct visualization—which solves the problem of poor visualization that hinders other contained EMM techniques.
Risks of power morcellation
The potential for inadvertent spread of occult malignancy is not the only risk of open EMM. Reports of disseminated leiomyomatosis, adenomyosis, and endometriosis also have been described from inadvertent tissue dispersion during open EMM with resulting ectopic reperitonealization.10–12
The procedure itself is not without risks. A recent systematic review documented 55 major and minor complications from EMM.13 Multiple organ systems were injured including bowel, urinary, vascular, and others, resulting in six deaths from these complications. The investigators concluded that “laparoscopic morcellator–related injuries continue to increase and short- and long-term complications are emerging in both the medical literature and device-related databases. Surgeon inexperience is descriptively identified as one of the most common contributing factors.”
All of the above risks must be weighed against the known benefits of laparoscopic surgery and presented to each patient to assist in deciding which route of surgery should be performed.
Tissue extraction options for large specimens
Large specimen extraction options during gynecologic surgery include:
Vaginal coring. Delivery through the vagina or colpotomy during vaginal or laparoscopic hysterectomy uses the technique of coring, which has long been established in our field.
Manual morcellation through a single incision. Mini-laparotomy or laparoendoscopic single-site surgery (LESS) incisions provide another option of removal with manual morcellation after laparoscopic hysterectomy or myomectomy. One study revealed that specimens up to 22 weeks in size can be placed in a large EndoCatch bag and morcellated extracorporeally by circumferentially coring with a scalpel.14
Contained power morcellation through a single port. Finally, the technique of contained EMM was recently described.15 This technique uses a large containment bag placed through a LESS incision with EMM being performed in an artificially created pneumoperitoneum. This technique isolates the specimen so that it can be morcellated without risk of exposing the patient to any malignant cells that might be unrecognized within the specimen.
Each of these techniques allows many patients to consider a minimally invasive option for their surgery. However, the ability to safely morcellate a very large uterus or myoma may be limited by visualization, and the experience of the surgeon is often critical in the successful performance of these procedures.16
Therefore, at Washington Universitywe have developed a technique using dual ports, with isolation of the uterus or myomas to improve visualization and prevent spillage of malignant tumor or dispersion of other benign tissue.
Dual-port EMM: Technique, tips, and tricks
Our technique of dual-port contained EMM allows the removal of large fibroids or uteri much larger than 20 weeks in size safely under direct visualization through a 15-mm incision. The technique uses:
- Karl Storz Rotocut tissue morcellator with spacers (FIGURE 1)
- 15-mm trocar
- 5-mm balloon trocar
- 20320-inch containment bag (FIGURE 2).
Containment bag placement
Once the specimen is free, we place it to the right or left side of the abdomen. The 15-mm trocar is placed through the umbilicus while visualizing from a lateral trocar site. We then fan-fold the containment bag and introduce it through the 15-mm trocar, keeping the bag oriented with the opening anterior (FIGURE 3). The bag is then grasped at the opening along the drawstring with an atraumatic grasper.
Tip: Care must be taken when introducing the bag in order to avoid tearing or making a small hole in it.
The leading edge is then introduced into the deepest part of the pelvis, and the remainder of the bag (left outside of the abdomen) is then fed cephalad into the abdomen.
Once the bag is completely in the abdomen, we orient the bag with the opening as wide as possible. This allows placement of a very large specimen. Once the specimen is within the containment bag, the drawstring is pulled tight and the mouth of the bag is removed through the 15-mm trocar site at the umbilicus.
The abdominal lateral gas port is opened to allow the intra-abdominal pneumoperitoneum to escape. A 5-mm trocar is placed into the bag through the opening at the umbilicus and the containment bag is insufflated with carbon dioxide and the insufflation pressure is set to 30 mm. The laparoscope placed through this trocar allows the artificial pneumoperitoneum being created to be observed (VIDEO).

Tip: The containment bag covers the entire abdominal cavity and should be fully distended. If it does not distend fully, a hole in the bag may be present and the bag must be replaced.
At this point, we place a balloon trocar at the lateral trocar site and into the bag under direct visualization. The balloon tip is inflated and pulled up tightly against the bag and abdominal wall (FIGURE 4). This allows a tight seal so there is no gas leak or spillage of the morcellated specimen. The laparoscope is placed through this trocar and the insufflation tubing is moved to this port.
Morcellator insertion
The morcellator is introduced through the umbilicus under direct visualization using the short morcellator blade in most instances. Spacers are used to set the length of the morcellator within the containment bag. The tip of the morcellator should be approximately 3 cm to 4 cm within the bag but well away from the retroperitoneum. Remember, any bag will be cut easily by the morcellator and should be thought of as peritoneum only and not a tough barrier. Serious injuries could otherwise develop.
At this point, place the patient flat or out of Trendelenburg position. Morcellation may now proceed.
Tip: Morcellation is best performed with the morcellator perpendicular to the abdomen under direct visualization using a 30° laparoscope to optimize the view. Morcellation in this position uses gravity to facilitate “peeling” of the specimen during morcellation and allows for faster removal.
Before removing the morcellator, inspect the containment bag for any large pieces that may have been dispersed during the morcellation process and remove them. Once there are only small fragments remaining, remove the morcellator, allowing the carbon dioxide to escape. Deflate the balloon tip on the trocar.
Now the containment bag with the remaining specimen may be removed through the umbilicus, while simultaneously removing the balloon-tip trocar from the bag.
A safe minimally invasive approach
This technique has allowed us to safely remove specimens larger than 1,500 g while keeping them in a contained environment with no spill of tissue within the abdomen.
Tracking and adaptation needed
The FDA safety communication has severely limited the practice of morcellation in the minimally invasive gynecologic surgical setting. Many hospitals around the country have reacted by placing significant restrictions on the use of EMM or banned it outright. This action may reverse the national trend of increasing rates of laparoscopic hysterectomy and force many practitioners to return to open surgery.
Currently, it is unclear what the true risk of tissue extraction is whether it is performed via EMM or manually. Large national databases including the BOLD database from the Surgical Review Corporation, as well as AAGL, must be utilized to track these cases and their outcomes to guide therapy. In the meantime, in order to continue to offer a minimally invasive approach to gynecologic surgery, new techniques and instrumentation in the operating room will need to be modified to adapt to these new guidelines. This is vital to maintain or even reduce the rates of open hysterectomy and associated morbidity while diminishing the potential risks of inadvertent benign as well as malignant tissue dispersion with tissue extraction.
Share your thoughts on this article! Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677. doi:10.1002/14651858.CD003677.pub4.
2. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
3. Wiser A, Holcroft CA, Tolandi T, Abenhaim HA. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10(2):117–122.
4. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
5. Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34.e1–e7.
6. U S Food and Drug Administration. Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids: Summary and key findings. Silver Spring, Maryland: FDA. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM393589.pdf. Published April 17, 2014. Accessed August 19, 2014.
7. Society of Gynecologic Oncology (SGO). SGO Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation. Published December 2013. Accessed March 1, 2014.
8. AAGL. Member Update: Disseminated leiomyosarcoma with power morcellation (Update #2). https://www.aagl.org/aaglnews/member-update-disseminated-leiomyosarcoma-with-power-morcellation-update-2/. Published July 11, 2014. Accessed August 19, 2014.
9. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery. http://www.acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Power_Morcellation_and_Occult_Malignancy_in_Gynecologic_Surgery. Published May 2014. Accessed August 19, 2014.
10. Sepilian V, Della Badia C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet Gynecol. 2003;102(5 Pt 2):1125–1127.
11. Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14(6):770–775.
12. Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007;14(2):156–160.
13. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21(3):486–491.
14. Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: an original technique that permits the safe and quick removal of a large uterus. Am J. Obstet Gynecol. 2011;204(6):566.e1–e2.
15. Shibley KA. Feasibilty of intra-abdominal tissue isolation and extraction with an artificially created pneumoperitoneum, at laparoscopy for gynecologic procedures. J Min Invasive Gynecol. 2012;19(6):S75.
Minimally invasive surgery utilizing laparoscopy for hysterectomy and myomectomy has become more common in women with gynecologic pathology. The benefits of this approach compared with laparotomy include decreased hospital stay, shorter recovery and, in experienced hands, significantly decreased morbidity.1–3
Approximately 600,000 hysterectomies are performed annually in the United States—30% of which are performed laparoscopically.4 The primary indication for surgical intervention is uterine leiomyoma. This pathology accounts for 40% of procedures.5 During these surgeries, electromechanical morcellation (EMM), or open “power” morcellation, is commonly used to cut large tissue specimens into small pieces for removal and thereby avoid a larger incision. Concerns have been raised regarding the use of open power morcellation because of the risk of spreading an unrecognized malignancy.
Based on case reports and retrospective studies, the FDA issued a statement in April of this year discouraging the use of EMM for hysterectomy and myomectomy in women with uterine fibroids.6 The concern for inadvertent spread of an occult malignancy was the reasoning for the communication. Since that time, the FDA’s Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee held a public meeting in which the panel heard comments from patients, societies, and industry regarding their positions on the safety of laparoscopic power morcellation. The panel made several recommendations to the FDA but, at the time of this writing, the FDA has yet to issue a final decision.
Reaction to FDA’s action/inaction
The FDA’s “safety” communication was in response to the concern of a few who experienced a bad outcome believed to be secondary to open power morcellation of enlarged uteri or fibroid tumors. In its statement, the FDA estimated the risk of an occult sarcoma to be about 1 in 350 and stated that the risk of disseminating a sarcoma with morcellation is substantial. The FDA discouraged the use of the power morcellator during hysterectomy or myomectomy for uterine fibroids.
Many organizations, including the Society of Gynecologic Oncology, The American Association of Gynecologic Laparoscopists (AAGL), and the American College of Obstetricians and Gynecologists, issued less stringent statements regarding this technology.7–9 These organizations stated generally that there were too few data to make a statement at that time, advocated the collection of more data, and encouraged detailed informed consent to be given to patients undergoing these procedures.
However, the FDA’s statement, and lack of a timely follow-up to clarify the role of the laparoscopic power morcellator in gynecologic surgery, has effectively stopped the use of this technology in its current form. In fact, in response to the statement, Ethicon Endosurgery has discontinued the distribution and sales of its power morcellator and many institutions have severely or completely restricted the use of this technology. The reason for these restrictions is that the medicolegal consequences of an adverse outcome would be very difficult to defend given the current, albeit premature, recommendations of the FDA. This statement makes it difficult to defend any adverse outcome that may occur in association with the use of the laparoscopic power morcellator. Furthermore, this statement by the FDA has largely prevented the medical community at large from collecting additional useful information to allow for a data-driven determination.
Power morcellation is not without risks. In fact, we outline them in this article. However, we believe that minimally invasive surgery should be allowed to continue to advance. In that vein, here we describe a technique of dual-port contained EMM. This surgical approach is performed under direct visualization—which solves the problem of poor visualization that hinders other contained EMM techniques.
Risks of power morcellation
The potential for inadvertent spread of occult malignancy is not the only risk of open EMM. Reports of disseminated leiomyomatosis, adenomyosis, and endometriosis also have been described from inadvertent tissue dispersion during open EMM with resulting ectopic reperitonealization.10–12
The procedure itself is not without risks. A recent systematic review documented 55 major and minor complications from EMM.13 Multiple organ systems were injured including bowel, urinary, vascular, and others, resulting in six deaths from these complications. The investigators concluded that “laparoscopic morcellator–related injuries continue to increase and short- and long-term complications are emerging in both the medical literature and device-related databases. Surgeon inexperience is descriptively identified as one of the most common contributing factors.”
All of the above risks must be weighed against the known benefits of laparoscopic surgery and presented to each patient to assist in deciding which route of surgery should be performed.
Tissue extraction options for large specimens
Large specimen extraction options during gynecologic surgery include:
Vaginal coring. Delivery through the vagina or colpotomy during vaginal or laparoscopic hysterectomy uses the technique of coring, which has long been established in our field.
Manual morcellation through a single incision. Mini-laparotomy or laparoendoscopic single-site surgery (LESS) incisions provide another option of removal with manual morcellation after laparoscopic hysterectomy or myomectomy. One study revealed that specimens up to 22 weeks in size can be placed in a large EndoCatch bag and morcellated extracorporeally by circumferentially coring with a scalpel.14
Contained power morcellation through a single port. Finally, the technique of contained EMM was recently described.15 This technique uses a large containment bag placed through a LESS incision with EMM being performed in an artificially created pneumoperitoneum. This technique isolates the specimen so that it can be morcellated without risk of exposing the patient to any malignant cells that might be unrecognized within the specimen.
Each of these techniques allows many patients to consider a minimally invasive option for their surgery. However, the ability to safely morcellate a very large uterus or myoma may be limited by visualization, and the experience of the surgeon is often critical in the successful performance of these procedures.16
Therefore, at Washington Universitywe have developed a technique using dual ports, with isolation of the uterus or myomas to improve visualization and prevent spillage of malignant tumor or dispersion of other benign tissue.
Dual-port EMM: Technique, tips, and tricks
Our technique of dual-port contained EMM allows the removal of large fibroids or uteri much larger than 20 weeks in size safely under direct visualization through a 15-mm incision. The technique uses:
- Karl Storz Rotocut tissue morcellator with spacers (FIGURE 1)
- 15-mm trocar
- 5-mm balloon trocar
- 20320-inch containment bag (FIGURE 2).
Containment bag placement
Once the specimen is free, we place it to the right or left side of the abdomen. The 15-mm trocar is placed through the umbilicus while visualizing from a lateral trocar site. We then fan-fold the containment bag and introduce it through the 15-mm trocar, keeping the bag oriented with the opening anterior (FIGURE 3). The bag is then grasped at the opening along the drawstring with an atraumatic grasper.
Tip: Care must be taken when introducing the bag in order to avoid tearing or making a small hole in it.
The leading edge is then introduced into the deepest part of the pelvis, and the remainder of the bag (left outside of the abdomen) is then fed cephalad into the abdomen.
Once the bag is completely in the abdomen, we orient the bag with the opening as wide as possible. This allows placement of a very large specimen. Once the specimen is within the containment bag, the drawstring is pulled tight and the mouth of the bag is removed through the 15-mm trocar site at the umbilicus.
The abdominal lateral gas port is opened to allow the intra-abdominal pneumoperitoneum to escape. A 5-mm trocar is placed into the bag through the opening at the umbilicus and the containment bag is insufflated with carbon dioxide and the insufflation pressure is set to 30 mm. The laparoscope placed through this trocar allows the artificial pneumoperitoneum being created to be observed (VIDEO).

Tip: The containment bag covers the entire abdominal cavity and should be fully distended. If it does not distend fully, a hole in the bag may be present and the bag must be replaced.
At this point, we place a balloon trocar at the lateral trocar site and into the bag under direct visualization. The balloon tip is inflated and pulled up tightly against the bag and abdominal wall (FIGURE 4). This allows a tight seal so there is no gas leak or spillage of the morcellated specimen. The laparoscope is placed through this trocar and the insufflation tubing is moved to this port.
Morcellator insertion
The morcellator is introduced through the umbilicus under direct visualization using the short morcellator blade in most instances. Spacers are used to set the length of the morcellator within the containment bag. The tip of the morcellator should be approximately 3 cm to 4 cm within the bag but well away from the retroperitoneum. Remember, any bag will be cut easily by the morcellator and should be thought of as peritoneum only and not a tough barrier. Serious injuries could otherwise develop.
At this point, place the patient flat or out of Trendelenburg position. Morcellation may now proceed.
Tip: Morcellation is best performed with the morcellator perpendicular to the abdomen under direct visualization using a 30° laparoscope to optimize the view. Morcellation in this position uses gravity to facilitate “peeling” of the specimen during morcellation and allows for faster removal.
Before removing the morcellator, inspect the containment bag for any large pieces that may have been dispersed during the morcellation process and remove them. Once there are only small fragments remaining, remove the morcellator, allowing the carbon dioxide to escape. Deflate the balloon tip on the trocar.
Now the containment bag with the remaining specimen may be removed through the umbilicus, while simultaneously removing the balloon-tip trocar from the bag.
A safe minimally invasive approach
This technique has allowed us to safely remove specimens larger than 1,500 g while keeping them in a contained environment with no spill of tissue within the abdomen.
Tracking and adaptation needed
The FDA safety communication has severely limited the practice of morcellation in the minimally invasive gynecologic surgical setting. Many hospitals around the country have reacted by placing significant restrictions on the use of EMM or banned it outright. This action may reverse the national trend of increasing rates of laparoscopic hysterectomy and force many practitioners to return to open surgery.
Currently, it is unclear what the true risk of tissue extraction is whether it is performed via EMM or manually. Large national databases including the BOLD database from the Surgical Review Corporation, as well as AAGL, must be utilized to track these cases and their outcomes to guide therapy. In the meantime, in order to continue to offer a minimally invasive approach to gynecologic surgery, new techniques and instrumentation in the operating room will need to be modified to adapt to these new guidelines. This is vital to maintain or even reduce the rates of open hysterectomy and associated morbidity while diminishing the potential risks of inadvertent benign as well as malignant tissue dispersion with tissue extraction.
Share your thoughts on this article! Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Minimally invasive surgery utilizing laparoscopy for hysterectomy and myomectomy has become more common in women with gynecologic pathology. The benefits of this approach compared with laparotomy include decreased hospital stay, shorter recovery and, in experienced hands, significantly decreased morbidity.1–3
Approximately 600,000 hysterectomies are performed annually in the United States—30% of which are performed laparoscopically.4 The primary indication for surgical intervention is uterine leiomyoma. This pathology accounts for 40% of procedures.5 During these surgeries, electromechanical morcellation (EMM), or open “power” morcellation, is commonly used to cut large tissue specimens into small pieces for removal and thereby avoid a larger incision. Concerns have been raised regarding the use of open power morcellation because of the risk of spreading an unrecognized malignancy.
Based on case reports and retrospective studies, the FDA issued a statement in April of this year discouraging the use of EMM for hysterectomy and myomectomy in women with uterine fibroids.6 The concern for inadvertent spread of an occult malignancy was the reasoning for the communication. Since that time, the FDA’s Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee held a public meeting in which the panel heard comments from patients, societies, and industry regarding their positions on the safety of laparoscopic power morcellation. The panel made several recommendations to the FDA but, at the time of this writing, the FDA has yet to issue a final decision.
Reaction to FDA’s action/inaction
The FDA’s “safety” communication was in response to the concern of a few who experienced a bad outcome believed to be secondary to open power morcellation of enlarged uteri or fibroid tumors. In its statement, the FDA estimated the risk of an occult sarcoma to be about 1 in 350 and stated that the risk of disseminating a sarcoma with morcellation is substantial. The FDA discouraged the use of the power morcellator during hysterectomy or myomectomy for uterine fibroids.
Many organizations, including the Society of Gynecologic Oncology, The American Association of Gynecologic Laparoscopists (AAGL), and the American College of Obstetricians and Gynecologists, issued less stringent statements regarding this technology.7–9 These organizations stated generally that there were too few data to make a statement at that time, advocated the collection of more data, and encouraged detailed informed consent to be given to patients undergoing these procedures.
However, the FDA’s statement, and lack of a timely follow-up to clarify the role of the laparoscopic power morcellator in gynecologic surgery, has effectively stopped the use of this technology in its current form. In fact, in response to the statement, Ethicon Endosurgery has discontinued the distribution and sales of its power morcellator and many institutions have severely or completely restricted the use of this technology. The reason for these restrictions is that the medicolegal consequences of an adverse outcome would be very difficult to defend given the current, albeit premature, recommendations of the FDA. This statement makes it difficult to defend any adverse outcome that may occur in association with the use of the laparoscopic power morcellator. Furthermore, this statement by the FDA has largely prevented the medical community at large from collecting additional useful information to allow for a data-driven determination.
Power morcellation is not without risks. In fact, we outline them in this article. However, we believe that minimally invasive surgery should be allowed to continue to advance. In that vein, here we describe a technique of dual-port contained EMM. This surgical approach is performed under direct visualization—which solves the problem of poor visualization that hinders other contained EMM techniques.
Risks of power morcellation
The potential for inadvertent spread of occult malignancy is not the only risk of open EMM. Reports of disseminated leiomyomatosis, adenomyosis, and endometriosis also have been described from inadvertent tissue dispersion during open EMM with resulting ectopic reperitonealization.10–12
The procedure itself is not without risks. A recent systematic review documented 55 major and minor complications from EMM.13 Multiple organ systems were injured including bowel, urinary, vascular, and others, resulting in six deaths from these complications. The investigators concluded that “laparoscopic morcellator–related injuries continue to increase and short- and long-term complications are emerging in both the medical literature and device-related databases. Surgeon inexperience is descriptively identified as one of the most common contributing factors.”
All of the above risks must be weighed against the known benefits of laparoscopic surgery and presented to each patient to assist in deciding which route of surgery should be performed.
Tissue extraction options for large specimens
Large specimen extraction options during gynecologic surgery include:
Vaginal coring. Delivery through the vagina or colpotomy during vaginal or laparoscopic hysterectomy uses the technique of coring, which has long been established in our field.
Manual morcellation through a single incision. Mini-laparotomy or laparoendoscopic single-site surgery (LESS) incisions provide another option of removal with manual morcellation after laparoscopic hysterectomy or myomectomy. One study revealed that specimens up to 22 weeks in size can be placed in a large EndoCatch bag and morcellated extracorporeally by circumferentially coring with a scalpel.14
Contained power morcellation through a single port. Finally, the technique of contained EMM was recently described.15 This technique uses a large containment bag placed through a LESS incision with EMM being performed in an artificially created pneumoperitoneum. This technique isolates the specimen so that it can be morcellated without risk of exposing the patient to any malignant cells that might be unrecognized within the specimen.
Each of these techniques allows many patients to consider a minimally invasive option for their surgery. However, the ability to safely morcellate a very large uterus or myoma may be limited by visualization, and the experience of the surgeon is often critical in the successful performance of these procedures.16
Therefore, at Washington Universitywe have developed a technique using dual ports, with isolation of the uterus or myomas to improve visualization and prevent spillage of malignant tumor or dispersion of other benign tissue.
Dual-port EMM: Technique, tips, and tricks
Our technique of dual-port contained EMM allows the removal of large fibroids or uteri much larger than 20 weeks in size safely under direct visualization through a 15-mm incision. The technique uses:
- Karl Storz Rotocut tissue morcellator with spacers (FIGURE 1)
- 15-mm trocar
- 5-mm balloon trocar
- 20320-inch containment bag (FIGURE 2).
Containment bag placement
Once the specimen is free, we place it to the right or left side of the abdomen. The 15-mm trocar is placed through the umbilicus while visualizing from a lateral trocar site. We then fan-fold the containment bag and introduce it through the 15-mm trocar, keeping the bag oriented with the opening anterior (FIGURE 3). The bag is then grasped at the opening along the drawstring with an atraumatic grasper.
Tip: Care must be taken when introducing the bag in order to avoid tearing or making a small hole in it.
The leading edge is then introduced into the deepest part of the pelvis, and the remainder of the bag (left outside of the abdomen) is then fed cephalad into the abdomen.
Once the bag is completely in the abdomen, we orient the bag with the opening as wide as possible. This allows placement of a very large specimen. Once the specimen is within the containment bag, the drawstring is pulled tight and the mouth of the bag is removed through the 15-mm trocar site at the umbilicus.
The abdominal lateral gas port is opened to allow the intra-abdominal pneumoperitoneum to escape. A 5-mm trocar is placed into the bag through the opening at the umbilicus and the containment bag is insufflated with carbon dioxide and the insufflation pressure is set to 30 mm. The laparoscope placed through this trocar allows the artificial pneumoperitoneum being created to be observed (VIDEO).

Tip: The containment bag covers the entire abdominal cavity and should be fully distended. If it does not distend fully, a hole in the bag may be present and the bag must be replaced.
At this point, we place a balloon trocar at the lateral trocar site and into the bag under direct visualization. The balloon tip is inflated and pulled up tightly against the bag and abdominal wall (FIGURE 4). This allows a tight seal so there is no gas leak or spillage of the morcellated specimen. The laparoscope is placed through this trocar and the insufflation tubing is moved to this port.
Morcellator insertion
The morcellator is introduced through the umbilicus under direct visualization using the short morcellator blade in most instances. Spacers are used to set the length of the morcellator within the containment bag. The tip of the morcellator should be approximately 3 cm to 4 cm within the bag but well away from the retroperitoneum. Remember, any bag will be cut easily by the morcellator and should be thought of as peritoneum only and not a tough barrier. Serious injuries could otherwise develop.
At this point, place the patient flat or out of Trendelenburg position. Morcellation may now proceed.
Tip: Morcellation is best performed with the morcellator perpendicular to the abdomen under direct visualization using a 30° laparoscope to optimize the view. Morcellation in this position uses gravity to facilitate “peeling” of the specimen during morcellation and allows for faster removal.
Before removing the morcellator, inspect the containment bag for any large pieces that may have been dispersed during the morcellation process and remove them. Once there are only small fragments remaining, remove the morcellator, allowing the carbon dioxide to escape. Deflate the balloon tip on the trocar.
Now the containment bag with the remaining specimen may be removed through the umbilicus, while simultaneously removing the balloon-tip trocar from the bag.
A safe minimally invasive approach
This technique has allowed us to safely remove specimens larger than 1,500 g while keeping them in a contained environment with no spill of tissue within the abdomen.
Tracking and adaptation needed
The FDA safety communication has severely limited the practice of morcellation in the minimally invasive gynecologic surgical setting. Many hospitals around the country have reacted by placing significant restrictions on the use of EMM or banned it outright. This action may reverse the national trend of increasing rates of laparoscopic hysterectomy and force many practitioners to return to open surgery.
Currently, it is unclear what the true risk of tissue extraction is whether it is performed via EMM or manually. Large national databases including the BOLD database from the Surgical Review Corporation, as well as AAGL, must be utilized to track these cases and their outcomes to guide therapy. In the meantime, in order to continue to offer a minimally invasive approach to gynecologic surgery, new techniques and instrumentation in the operating room will need to be modified to adapt to these new guidelines. This is vital to maintain or even reduce the rates of open hysterectomy and associated morbidity while diminishing the potential risks of inadvertent benign as well as malignant tissue dispersion with tissue extraction.
Share your thoughts on this article! Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677. doi:10.1002/14651858.CD003677.pub4.
2. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
3. Wiser A, Holcroft CA, Tolandi T, Abenhaim HA. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10(2):117–122.
4. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
5. Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34.e1–e7.
6. U S Food and Drug Administration. Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids: Summary and key findings. Silver Spring, Maryland: FDA. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM393589.pdf. Published April 17, 2014. Accessed August 19, 2014.
7. Society of Gynecologic Oncology (SGO). SGO Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation. Published December 2013. Accessed March 1, 2014.
8. AAGL. Member Update: Disseminated leiomyosarcoma with power morcellation (Update #2). https://www.aagl.org/aaglnews/member-update-disseminated-leiomyosarcoma-with-power-morcellation-update-2/. Published July 11, 2014. Accessed August 19, 2014.
9. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery. http://www.acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Power_Morcellation_and_Occult_Malignancy_in_Gynecologic_Surgery. Published May 2014. Accessed August 19, 2014.
10. Sepilian V, Della Badia C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet Gynecol. 2003;102(5 Pt 2):1125–1127.
11. Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14(6):770–775.
12. Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007;14(2):156–160.
13. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21(3):486–491.
14. Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: an original technique that permits the safe and quick removal of a large uterus. Am J. Obstet Gynecol. 2011;204(6):566.e1–e2.
15. Shibley KA. Feasibilty of intra-abdominal tissue isolation and extraction with an artificially created pneumoperitoneum, at laparoscopy for gynecologic procedures. J Min Invasive Gynecol. 2012;19(6):S75.
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677. doi:10.1002/14651858.CD003677.pub4.
2. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
3. Wiser A, Holcroft CA, Tolandi T, Abenhaim HA. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10(2):117–122.
4. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
5. Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34.e1–e7.
6. U S Food and Drug Administration. Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids: Summary and key findings. Silver Spring, Maryland: FDA. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM393589.pdf. Published April 17, 2014. Accessed August 19, 2014.
7. Society of Gynecologic Oncology (SGO). SGO Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation. Published December 2013. Accessed March 1, 2014.
8. AAGL. Member Update: Disseminated leiomyosarcoma with power morcellation (Update #2). https://www.aagl.org/aaglnews/member-update-disseminated-leiomyosarcoma-with-power-morcellation-update-2/. Published July 11, 2014. Accessed August 19, 2014.
9. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery. http://www.acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Power_Morcellation_and_Occult_Malignancy_in_Gynecologic_Surgery. Published May 2014. Accessed August 19, 2014.
10. Sepilian V, Della Badia C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet Gynecol. 2003;102(5 Pt 2):1125–1127.
11. Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14(6):770–775.
12. Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007;14(2):156–160.
13. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21(3):486–491.
14. Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: an original technique that permits the safe and quick removal of a large uterus. Am J. Obstet Gynecol. 2011;204(6):566.e1–e2.
15. Shibley KA. Feasibilty of intra-abdominal tissue isolation and extraction with an artificially created pneumoperitoneum, at laparoscopy for gynecologic procedures. J Min Invasive Gynecol. 2012;19(6):S75.
Transforming vaginal hysterectomy: 7 solutions to the most daunting challenges
Vaginal hysterectomy is the preferred route to benign hysterectomy because it is associated with better outcomes and fewer complications than the laparoscopic and open abdominal approaches.1,2 Yet, despite superior patient outcomes and cost benefits, the rate of vaginal hysterectomy is declining.
According to the Nationwide Inpatient Sample, the use of vaginal hysterectomy declined from 24.8% in 1998 to 16.7% in 2010.3 In fact, more than 80% of surgeons in the United States now perform fewer than five vaginal procedures in a year.4
The increasing use of other minimally invasive routes, such as laparoscopy and robotics, indicates that most practicing surgeons and recent graduates are choosing these approaches over the vaginal route. In only 3 years, the rate of laparoscopy increased by 6% and robotics increased by almost 10%.3
Many surgeons assume that vaginal hysterectomy exists in a state of suspended animation, with nothing much changed in the way it has been performed over the past few decades. Further, vaginal surgery is difficult to teach and learn, given limitations in exposure and visualization, difficulty in securing hemostasis, and challenges in the removal of the large uterus and adnexae. As a result, vaginal hysterectomy often is thought, erroneously, to be indicated only in procedures involving a small and prolapsing uterus.
To increase the rate of vaginal hysterectomy, we can benefit from experience gained in laparoscopy and robotics—whether we are teachers or learners—while maintaining patient safety and containing costs.
In this article, I describe common challenges in vaginal hysterectomy and offer tools and techniques to overcome them:
- achieving and enhancing ergonomics, exposure, and visualization
- the need to work in a long vaginal vault
- the task of securing vascular and thick tissue pedicles when the introitus and vaginal vault are narrow.
The vaginal approach is less costly
Vaginal hysterectomy costs significantly less to perform than other approaches. At a tertiary referral center, vaginal hysterectomy costs approximately $7,000 to $18,000 per case less than laparoscopic, abdominal, and robotic hysterectomy.5 With declining use of vaginal hysterectomy and increasing use of more costly approaches, we face a health-care crisis.
Residents are inadequately trained to perform vaginal hysterectomy
Data reveal that not only are our recent graduates inadequately prepared to perform vaginal hysterectomy, but national health-care dollars and resources are depleted when surgeons choose to perform more costly approaches. As a result, many eligible patients end up deprived of the benefits of a single, concealed, and minimally invasive procedure.
The increase in laparoscopic and robotic approaches to hysterectomy has affected residency training. National case log reports from the Accreditation Council of Graduate Medical Education show that the number of vaginal hysterectomies performed by residents as “primary surgeons” decreased by 40%, from a mean of 35 cases in 2002 to 19 cases in 2012.6 A recent survey found that only 28% of graduating residents were “completely prepared” to perform a vaginal hysterectomy, compared with 58% for abdominal hysterectomy, 22% for laparoscopic hysterectomy, and 3% for the robotic approach.7
The rate of vaginal hysterectomy will continue to decline if we perform it in the same manner it was done 30 years ago. The current generation of practicing gynecologists and graduates is choosing to perform the procedure laparoscopically or robotically because of the advantages these technologies provide. It is time that we incorporate features from these minimally invasive approaches to streamline vaginal hysterectomy while maintaining patient safety and containing costs.
Challenges: Ergonomics, exposure, and visualization
In conventional vaginal surgery, the surgeon often is the person who has the best and, sometimes, the sole view. Two bedside assistants are required to hold retractors during the entire case, which can lead to fatigue and muscle strain. Poor lighting also can greatly limit visualization into the pelvic cavity.
Both laparoscopy and robotics provide a well-illuminated and magnified view, with three-dimensional images now available in both platforms. This view is projected to overhead monitors for the entire surgical team to see. Magnification of the pelvic anatomic structures and projection to an external monitor facilitate teaching and learning, better anticipation of the surgical and procedural needs, and overall patient safety.
From robotics, where ergonomics is exemplified, we also learn the importance of surgeon comfort during the procedure.
Solution #1: A self-retaining retractor
A self-retaining system such as the Magrina-Bookwalter vaginal retractor (Symmetry Surgical, Nashville, Tennessee) (FIGURE 1)
Solution #2: Seat the surgeon for an optimal view
With the patient in the lithotomy position and her legs in candy cane stirrups, the surgeon can be seated on a high chair so that the operative field is at the approximate level of the assistants’ view (FIGURE 2)
Solution #3: Illuminate the cavity
The deep pelvic cavity can be easily illuminated using a lighted suction tip, a flexible light source (as part of the cystoscopy set) held with a Babcock clamp (FIGURE 3), or a malleable illuminating mat taped to the retractor blades (such as Lightmat surgical illuminator, Lumitex, Inc., Strongsville, Ohio).
Solution #4: Project the image
Cameras attached to an overhead boom or operating room light handles (FIGURE 4) and an external telescope with integrated illumination, such as a standard cystoscope or VITOM Exoscope (Karl Storz, El Segundo, California) (FIGURE 5) provide both magnification and projection of the procedure to an overhead monitor.
Glass technology (Google, Mountain View, California) also has been utilized in surgery and can be a good application of simultaneous projection and recording of the procedure to an external monitor (FIGURE 6). Google Glass is a wearable computer with an optical head-mounted display. The device, similar to eyeglasses, is voice-activated, thereby allowing the surgeon to record the procedure hands-free. Simultaneous projection to an external monitor allows the entire team in the operating room to be aware of the flow of the procedure.
Challenge: Working in a narrow vaginal vault
Without correct instrumentation, this challenge can be especially daunting. Laparoscopy and robotics have changed the way we perform pelvic surgery by providing advanced instrumentation.
Solution #5: Adapt your instruments
Modified vaginal instruments can be used to facilitate a case. Watch the accompanying VIDEO on the use of improved vaginal instruments during morcellation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
| Click to enlarge >>> |
Among the instruments adaptable for vaginal surgery:
- curving, articulating instruments
- long, curved, and rounded knife handles, which allow for better ergonomics during prolonged morcellation
- modified long retractors and use of a single long vaginal pack provide retraction of loops of bowel and easy access to secure pedicles deep in the pelvis.
All of these instruments are available through Marina Medical in Sunrise, Florida.
Challenge: Securing vascular and thick tissue pediclesA narrow introitus and vaginal vault can be difficult to manage during vaginal surgery. Another challenge is a uterus that is large or deformed by multiple fibroids.
Solution #6: Vaginal incision
A simple superficial 2- to 3-cm incision on the distal posterior aspect of the vaginal wall can widen the introitus and vault to facilitate the procedure (FIGURE 7)
Solution #7: Vessel-sealing tools
The use of energy is integral to laparoscopy and robotics for dissection and securing vessels. In a meta-analysis that included seven randomized controlled trials, advanced vessel-sealing devices proved useful in vaginal surgery by decreasing blood loss and operative time.8
In the setting of a difficult vaginal hysterectomy with a narrow introitus and large uterus, the use of vessel-sealing technology allows the surgeon to skeletonize the uterine arteries while allowing progressive descensus to secure the upper pedicles.
In my experience, the use of an advanced vessel-sealing device, compared with traditional clamp-cut-tying technique, facilitated successful completion of vaginal hysterectomy in 650 patients with relative contraindications to the vaginal approach, such as nulliparity, a uterus weighing more than 250 g, and a history of cesarean delivery (Mayo Clinic data; yet unpublished).
We must change with the times
The rate of vaginal hysterectomy will continue to decline unless we modify our technique to incorporate new technology. The current generation of practicing gynecologists and recent graduates are choosing the laparoscopic and robotic approaches because of the advantages these technologies offer. It is time we incorporate relevant features from these minimally invasive approaches while maintaining patient safety and containing costs by performing vaginal hysterectomy whenever possible. A willingness to change and ability to think outside the usual box will help us train new generations of vaginal surgeons who can bring back vaginal hysterectomy as the preferred route to the benign hysterectomy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
3. Wright T, Herzog T, Tsul J, et al. Nationwide trends in inpatient hysterectomy in the United States. Obstet Gynecol. 2013:122(2):233–241.
4. Rogo-Gupta L, Lewyn S, Jum JH, et al. Effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
5. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
6. Washburn EE, Cohen SL, Manoucherie E, Zurawin, RJ, Einarsson JI. Trends in reported residency surgical experience in hysterectomy [published online ahead of print June 4, 2014]. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2014.05.005.
7. Burkett D, Horwitz J, Kennedy V, et al. Assessing current trends in resident hysterectomy training. Female Pelvic Med Reconstr Surg. 2011;17(5):210–214.
8. Kroft J, Selk K. Energy-based vessel sealing in vaginal hysterectomy. A systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
Vaginal hysterectomy is the preferred route to benign hysterectomy because it is associated with better outcomes and fewer complications than the laparoscopic and open abdominal approaches.1,2 Yet, despite superior patient outcomes and cost benefits, the rate of vaginal hysterectomy is declining.
According to the Nationwide Inpatient Sample, the use of vaginal hysterectomy declined from 24.8% in 1998 to 16.7% in 2010.3 In fact, more than 80% of surgeons in the United States now perform fewer than five vaginal procedures in a year.4
The increasing use of other minimally invasive routes, such as laparoscopy and robotics, indicates that most practicing surgeons and recent graduates are choosing these approaches over the vaginal route. In only 3 years, the rate of laparoscopy increased by 6% and robotics increased by almost 10%.3
Many surgeons assume that vaginal hysterectomy exists in a state of suspended animation, with nothing much changed in the way it has been performed over the past few decades. Further, vaginal surgery is difficult to teach and learn, given limitations in exposure and visualization, difficulty in securing hemostasis, and challenges in the removal of the large uterus and adnexae. As a result, vaginal hysterectomy often is thought, erroneously, to be indicated only in procedures involving a small and prolapsing uterus.
To increase the rate of vaginal hysterectomy, we can benefit from experience gained in laparoscopy and robotics—whether we are teachers or learners—while maintaining patient safety and containing costs.
In this article, I describe common challenges in vaginal hysterectomy and offer tools and techniques to overcome them:
- achieving and enhancing ergonomics, exposure, and visualization
- the need to work in a long vaginal vault
- the task of securing vascular and thick tissue pedicles when the introitus and vaginal vault are narrow.
The vaginal approach is less costly
Vaginal hysterectomy costs significantly less to perform than other approaches. At a tertiary referral center, vaginal hysterectomy costs approximately $7,000 to $18,000 per case less than laparoscopic, abdominal, and robotic hysterectomy.5 With declining use of vaginal hysterectomy and increasing use of more costly approaches, we face a health-care crisis.
Residents are inadequately trained to perform vaginal hysterectomy
Data reveal that not only are our recent graduates inadequately prepared to perform vaginal hysterectomy, but national health-care dollars and resources are depleted when surgeons choose to perform more costly approaches. As a result, many eligible patients end up deprived of the benefits of a single, concealed, and minimally invasive procedure.
The increase in laparoscopic and robotic approaches to hysterectomy has affected residency training. National case log reports from the Accreditation Council of Graduate Medical Education show that the number of vaginal hysterectomies performed by residents as “primary surgeons” decreased by 40%, from a mean of 35 cases in 2002 to 19 cases in 2012.6 A recent survey found that only 28% of graduating residents were “completely prepared” to perform a vaginal hysterectomy, compared with 58% for abdominal hysterectomy, 22% for laparoscopic hysterectomy, and 3% for the robotic approach.7
The rate of vaginal hysterectomy will continue to decline if we perform it in the same manner it was done 30 years ago. The current generation of practicing gynecologists and graduates is choosing to perform the procedure laparoscopically or robotically because of the advantages these technologies provide. It is time that we incorporate features from these minimally invasive approaches to streamline vaginal hysterectomy while maintaining patient safety and containing costs.
Challenges: Ergonomics, exposure, and visualization
In conventional vaginal surgery, the surgeon often is the person who has the best and, sometimes, the sole view. Two bedside assistants are required to hold retractors during the entire case, which can lead to fatigue and muscle strain. Poor lighting also can greatly limit visualization into the pelvic cavity.
Both laparoscopy and robotics provide a well-illuminated and magnified view, with three-dimensional images now available in both platforms. This view is projected to overhead monitors for the entire surgical team to see. Magnification of the pelvic anatomic structures and projection to an external monitor facilitate teaching and learning, better anticipation of the surgical and procedural needs, and overall patient safety.
From robotics, where ergonomics is exemplified, we also learn the importance of surgeon comfort during the procedure.
Solution #1: A self-retaining retractor
A self-retaining system such as the Magrina-Bookwalter vaginal retractor (Symmetry Surgical, Nashville, Tennessee) (FIGURE 1)
Solution #2: Seat the surgeon for an optimal view
With the patient in the lithotomy position and her legs in candy cane stirrups, the surgeon can be seated on a high chair so that the operative field is at the approximate level of the assistants’ view (FIGURE 2)
Solution #3: Illuminate the cavity
The deep pelvic cavity can be easily illuminated using a lighted suction tip, a flexible light source (as part of the cystoscopy set) held with a Babcock clamp (FIGURE 3), or a malleable illuminating mat taped to the retractor blades (such as Lightmat surgical illuminator, Lumitex, Inc., Strongsville, Ohio).
Solution #4: Project the image
Cameras attached to an overhead boom or operating room light handles (FIGURE 4) and an external telescope with integrated illumination, such as a standard cystoscope or VITOM Exoscope (Karl Storz, El Segundo, California) (FIGURE 5) provide both magnification and projection of the procedure to an overhead monitor.
Glass technology (Google, Mountain View, California) also has been utilized in surgery and can be a good application of simultaneous projection and recording of the procedure to an external monitor (FIGURE 6). Google Glass is a wearable computer with an optical head-mounted display. The device, similar to eyeglasses, is voice-activated, thereby allowing the surgeon to record the procedure hands-free. Simultaneous projection to an external monitor allows the entire team in the operating room to be aware of the flow of the procedure.
Challenge: Working in a narrow vaginal vault
Without correct instrumentation, this challenge can be especially daunting. Laparoscopy and robotics have changed the way we perform pelvic surgery by providing advanced instrumentation.
Solution #5: Adapt your instruments
Modified vaginal instruments can be used to facilitate a case. Watch the accompanying VIDEO on the use of improved vaginal instruments during morcellation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
| Click to enlarge >>> |
Among the instruments adaptable for vaginal surgery:
- curving, articulating instruments
- long, curved, and rounded knife handles, which allow for better ergonomics during prolonged morcellation
- modified long retractors and use of a single long vaginal pack provide retraction of loops of bowel and easy access to secure pedicles deep in the pelvis.
All of these instruments are available through Marina Medical in Sunrise, Florida.
Challenge: Securing vascular and thick tissue pediclesA narrow introitus and vaginal vault can be difficult to manage during vaginal surgery. Another challenge is a uterus that is large or deformed by multiple fibroids.
Solution #6: Vaginal incision
A simple superficial 2- to 3-cm incision on the distal posterior aspect of the vaginal wall can widen the introitus and vault to facilitate the procedure (FIGURE 7)
Solution #7: Vessel-sealing tools
The use of energy is integral to laparoscopy and robotics for dissection and securing vessels. In a meta-analysis that included seven randomized controlled trials, advanced vessel-sealing devices proved useful in vaginal surgery by decreasing blood loss and operative time.8
In the setting of a difficult vaginal hysterectomy with a narrow introitus and large uterus, the use of vessel-sealing technology allows the surgeon to skeletonize the uterine arteries while allowing progressive descensus to secure the upper pedicles.
In my experience, the use of an advanced vessel-sealing device, compared with traditional clamp-cut-tying technique, facilitated successful completion of vaginal hysterectomy in 650 patients with relative contraindications to the vaginal approach, such as nulliparity, a uterus weighing more than 250 g, and a history of cesarean delivery (Mayo Clinic data; yet unpublished).
We must change with the times
The rate of vaginal hysterectomy will continue to decline unless we modify our technique to incorporate new technology. The current generation of practicing gynecologists and recent graduates are choosing the laparoscopic and robotic approaches because of the advantages these technologies offer. It is time we incorporate relevant features from these minimally invasive approaches while maintaining patient safety and containing costs by performing vaginal hysterectomy whenever possible. A willingness to change and ability to think outside the usual box will help us train new generations of vaginal surgeons who can bring back vaginal hysterectomy as the preferred route to the benign hysterectomy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Vaginal hysterectomy is the preferred route to benign hysterectomy because it is associated with better outcomes and fewer complications than the laparoscopic and open abdominal approaches.1,2 Yet, despite superior patient outcomes and cost benefits, the rate of vaginal hysterectomy is declining.
According to the Nationwide Inpatient Sample, the use of vaginal hysterectomy declined from 24.8% in 1998 to 16.7% in 2010.3 In fact, more than 80% of surgeons in the United States now perform fewer than five vaginal procedures in a year.4
The increasing use of other minimally invasive routes, such as laparoscopy and robotics, indicates that most practicing surgeons and recent graduates are choosing these approaches over the vaginal route. In only 3 years, the rate of laparoscopy increased by 6% and robotics increased by almost 10%.3
Many surgeons assume that vaginal hysterectomy exists in a state of suspended animation, with nothing much changed in the way it has been performed over the past few decades. Further, vaginal surgery is difficult to teach and learn, given limitations in exposure and visualization, difficulty in securing hemostasis, and challenges in the removal of the large uterus and adnexae. As a result, vaginal hysterectomy often is thought, erroneously, to be indicated only in procedures involving a small and prolapsing uterus.
To increase the rate of vaginal hysterectomy, we can benefit from experience gained in laparoscopy and robotics—whether we are teachers or learners—while maintaining patient safety and containing costs.
In this article, I describe common challenges in vaginal hysterectomy and offer tools and techniques to overcome them:
- achieving and enhancing ergonomics, exposure, and visualization
- the need to work in a long vaginal vault
- the task of securing vascular and thick tissue pedicles when the introitus and vaginal vault are narrow.
The vaginal approach is less costly
Vaginal hysterectomy costs significantly less to perform than other approaches. At a tertiary referral center, vaginal hysterectomy costs approximately $7,000 to $18,000 per case less than laparoscopic, abdominal, and robotic hysterectomy.5 With declining use of vaginal hysterectomy and increasing use of more costly approaches, we face a health-care crisis.
Residents are inadequately trained to perform vaginal hysterectomy
Data reveal that not only are our recent graduates inadequately prepared to perform vaginal hysterectomy, but national health-care dollars and resources are depleted when surgeons choose to perform more costly approaches. As a result, many eligible patients end up deprived of the benefits of a single, concealed, and minimally invasive procedure.
The increase in laparoscopic and robotic approaches to hysterectomy has affected residency training. National case log reports from the Accreditation Council of Graduate Medical Education show that the number of vaginal hysterectomies performed by residents as “primary surgeons” decreased by 40%, from a mean of 35 cases in 2002 to 19 cases in 2012.6 A recent survey found that only 28% of graduating residents were “completely prepared” to perform a vaginal hysterectomy, compared with 58% for abdominal hysterectomy, 22% for laparoscopic hysterectomy, and 3% for the robotic approach.7
The rate of vaginal hysterectomy will continue to decline if we perform it in the same manner it was done 30 years ago. The current generation of practicing gynecologists and graduates is choosing to perform the procedure laparoscopically or robotically because of the advantages these technologies provide. It is time that we incorporate features from these minimally invasive approaches to streamline vaginal hysterectomy while maintaining patient safety and containing costs.
Challenges: Ergonomics, exposure, and visualization
In conventional vaginal surgery, the surgeon often is the person who has the best and, sometimes, the sole view. Two bedside assistants are required to hold retractors during the entire case, which can lead to fatigue and muscle strain. Poor lighting also can greatly limit visualization into the pelvic cavity.
Both laparoscopy and robotics provide a well-illuminated and magnified view, with three-dimensional images now available in both platforms. This view is projected to overhead monitors for the entire surgical team to see. Magnification of the pelvic anatomic structures and projection to an external monitor facilitate teaching and learning, better anticipation of the surgical and procedural needs, and overall patient safety.
From robotics, where ergonomics is exemplified, we also learn the importance of surgeon comfort during the procedure.
Solution #1: A self-retaining retractor
A self-retaining system such as the Magrina-Bookwalter vaginal retractor (Symmetry Surgical, Nashville, Tennessee) (FIGURE 1)
Solution #2: Seat the surgeon for an optimal view
With the patient in the lithotomy position and her legs in candy cane stirrups, the surgeon can be seated on a high chair so that the operative field is at the approximate level of the assistants’ view (FIGURE 2)
Solution #3: Illuminate the cavity
The deep pelvic cavity can be easily illuminated using a lighted suction tip, a flexible light source (as part of the cystoscopy set) held with a Babcock clamp (FIGURE 3), or a malleable illuminating mat taped to the retractor blades (such as Lightmat surgical illuminator, Lumitex, Inc., Strongsville, Ohio).
Solution #4: Project the image
Cameras attached to an overhead boom or operating room light handles (FIGURE 4) and an external telescope with integrated illumination, such as a standard cystoscope or VITOM Exoscope (Karl Storz, El Segundo, California) (FIGURE 5) provide both magnification and projection of the procedure to an overhead monitor.
Glass technology (Google, Mountain View, California) also has been utilized in surgery and can be a good application of simultaneous projection and recording of the procedure to an external monitor (FIGURE 6). Google Glass is a wearable computer with an optical head-mounted display. The device, similar to eyeglasses, is voice-activated, thereby allowing the surgeon to record the procedure hands-free. Simultaneous projection to an external monitor allows the entire team in the operating room to be aware of the flow of the procedure.
Challenge: Working in a narrow vaginal vault
Without correct instrumentation, this challenge can be especially daunting. Laparoscopy and robotics have changed the way we perform pelvic surgery by providing advanced instrumentation.
Solution #5: Adapt your instruments
Modified vaginal instruments can be used to facilitate a case. Watch the accompanying VIDEO on the use of improved vaginal instruments during morcellation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
| Click to enlarge >>> |
Among the instruments adaptable for vaginal surgery:
- curving, articulating instruments
- long, curved, and rounded knife handles, which allow for better ergonomics during prolonged morcellation
- modified long retractors and use of a single long vaginal pack provide retraction of loops of bowel and easy access to secure pedicles deep in the pelvis.
All of these instruments are available through Marina Medical in Sunrise, Florida.
Challenge: Securing vascular and thick tissue pediclesA narrow introitus and vaginal vault can be difficult to manage during vaginal surgery. Another challenge is a uterus that is large or deformed by multiple fibroids.
Solution #6: Vaginal incision
A simple superficial 2- to 3-cm incision on the distal posterior aspect of the vaginal wall can widen the introitus and vault to facilitate the procedure (FIGURE 7)
Solution #7: Vessel-sealing tools
The use of energy is integral to laparoscopy and robotics for dissection and securing vessels. In a meta-analysis that included seven randomized controlled trials, advanced vessel-sealing devices proved useful in vaginal surgery by decreasing blood loss and operative time.8
In the setting of a difficult vaginal hysterectomy with a narrow introitus and large uterus, the use of vessel-sealing technology allows the surgeon to skeletonize the uterine arteries while allowing progressive descensus to secure the upper pedicles.
In my experience, the use of an advanced vessel-sealing device, compared with traditional clamp-cut-tying technique, facilitated successful completion of vaginal hysterectomy in 650 patients with relative contraindications to the vaginal approach, such as nulliparity, a uterus weighing more than 250 g, and a history of cesarean delivery (Mayo Clinic data; yet unpublished).
We must change with the times
The rate of vaginal hysterectomy will continue to decline unless we modify our technique to incorporate new technology. The current generation of practicing gynecologists and recent graduates are choosing the laparoscopic and robotic approaches because of the advantages these technologies offer. It is time we incorporate relevant features from these minimally invasive approaches while maintaining patient safety and containing costs by performing vaginal hysterectomy whenever possible. A willingness to change and ability to think outside the usual box will help us train new generations of vaginal surgeons who can bring back vaginal hysterectomy as the preferred route to the benign hysterectomy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
3. Wright T, Herzog T, Tsul J, et al. Nationwide trends in inpatient hysterectomy in the United States. Obstet Gynecol. 2013:122(2):233–241.
4. Rogo-Gupta L, Lewyn S, Jum JH, et al. Effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
5. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
6. Washburn EE, Cohen SL, Manoucherie E, Zurawin, RJ, Einarsson JI. Trends in reported residency surgical experience in hysterectomy [published online ahead of print June 4, 2014]. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2014.05.005.
7. Burkett D, Horwitz J, Kennedy V, et al. Assessing current trends in resident hysterectomy training. Female Pelvic Med Reconstr Surg. 2011;17(5):210–214.
8. Kroft J, Selk K. Energy-based vessel sealing in vaginal hysterectomy. A systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
3. Wright T, Herzog T, Tsul J, et al. Nationwide trends in inpatient hysterectomy in the United States. Obstet Gynecol. 2013:122(2):233–241.
4. Rogo-Gupta L, Lewyn S, Jum JH, et al. Effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
5. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
6. Washburn EE, Cohen SL, Manoucherie E, Zurawin, RJ, Einarsson JI. Trends in reported residency surgical experience in hysterectomy [published online ahead of print June 4, 2014]. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2014.05.005.
7. Burkett D, Horwitz J, Kennedy V, et al. Assessing current trends in resident hysterectomy training. Female Pelvic Med Reconstr Surg. 2011;17(5):210–214.
8. Kroft J, Selk K. Energy-based vessel sealing in vaginal hysterectomy. A systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
![]()
Dr. Kho presents vaginal morcellation by hand, using advanced instrumentation