User login
- Applying single-incision laparoscopic surgery to gyn practice: What’s involved
Russell P. Atkin, MD; Michael L. Nimaroff, MD; Vrunda Bhavsar, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The uterine leiomyoma is the most common tumor of the female genital tract. Seventy percent of white women and 80% of black women develop one or more of these tumors by the time they reach 50 years, and the myomas are clinically apparent in 25% of patients.1,2 When a fibroid is submucosal, it is often associated with menorrhagia, abnormal uterine bleeding, and infertility.2-4
In this article, I describe three aspects of managing leiomyomata:
- ways of classifying the tumor to better predict the blood loss, operative time and morbidity associated with removal
- the indications for hysteroscopic myomectomy and polypectomy
- new tools for the removal of polyps and myomas.
Preoperative assessment of submucosal myomas is essential
Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: a new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—preliminary report. J Minim Invasive Gynecol. 2005;12(4):308–311.
Wamsteker and colleagues were the first to propose a system for classifying myoma position within the uterine cavity as a means of estimating the degree of difficulty of resectoscopic removal.5 The European Society for Gynaecological Endoscopy (ESGE) later adopted this system, which is now known by its acronym. According to the ESGE system, myomas that lie entirely within the uterine cavity (Type 0) are easier to remove, require less operative time, and involve less fluid deficit and blood loss than myomas that invade the myometrium to varying degrees (FIGURE 1).
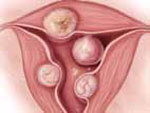
FIGURE 1 ESGE classification
Submucosal myomas are classified as Type 0, Type I, or Type II, according to the degree of myometrial penetration.When more than 50% of a tumor penetrates the myometrium (Type II), the risk of excessive intraoperative fluid absorption is elevated, along with the risk of bleeding and the likelihood of electrolyte abnormalities with the use of non-electrolyte fluid media. Type II tumors also increase operative time and the likelihood that additional procedures will be needed because of incomplete resection—even in the hands of expert hysteroscopic surgeons.5
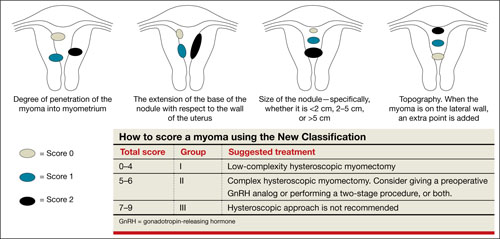
FIGURE 2 New classification
New classification system increases accuracy
Lasmar and colleagues devised a new system for preoperative assessment of submucosal myomas, hoping to estimate more precisely the likelihood of successful removal via resectoscopy. They call their system the New Classification (NC). Besides taking into account the degree of penetration into the myometrium, they consider the percentage of uterine wall encompassed by the myoma and the location of the myoma within the uterus (i.e., fundus, body, or lower segment) (FIGURE 2). The total score is used to categorize the tumor into Group I, II, or III to estimate the likelihood of successful removal.
In devising the system, Lasmar and colleagues used the NC and ESGE systems to analyze 55 myomectomy cases involving 57 myomas. They found that the NC more accurately predicts differences between Groups I and II in regard to completed procedures, fluid deficit, and operative time.
Preoperative hysteroscopic evaluation of submucosal myomas is essential and reliable using the New Classification system.
Hysteroscopic removal of myomas and polyps
yields multiple benefits
Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724–729.
Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity [published online ahead of print January 24, 2011]. Fertil Steril. doi 10.1016/j. fertnstert.2010.12.034.
Afifi K, Anand S. Nallapeta S, Gelbaya TA. Management of endometrial polyps in subfertile women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):117–121.
Studies evaluating the association between infertility and submucosal fibroids have been controversial because the exact mechanism has not been identified. However, new evidence suggests a molecular causal relationship, and Pritts and colleagues demonstrated improved fertility after submucosal myomectomy.3,6
More recently, Shokeir and coworkers conducted a prospective, randomized, age-matched, controlled trial to explore the effects of hysteroscopic myomectomy on otherwise unexplained primary infertility. They enrolled 215 women who had infertility longer than 12 months and who had their fibroids assessed by means of ultrasonography and classified according to the ESGE system.
Women who underwent myomectomy were twice as likely as women in the control group to become pregnant (relative risk = 2.1; 95% confidence interval = 1.5–2.9). Women who had Type 0 and Type I myomas removed had significantly higher pregnancy rates than women in the control group (P < .001). No statistically significant difference in the pregnancy rate between groups was found for Type II myomas.
Polyps may also affect fertility
Rackow and coworkers demonstrated that endometrial polyps affect uterine receptivity on the molecular level, suggesting a relationship between endometrial polyps and infertility. And after a systematic review of endometrial polyps in women who had subfertility, Afifi and colleagues concluded that polypectomy can improve fertility, especially when assisted reproductive technologies are planned.
Myomas, polyps also contribute to bleeding abnormalities
Submucosal myomas have been associated with bleeding abnormalities, such as heavy menstrual bleeding and menopausal bleeding. Although the precise mechanism is unknown by which these bleeding abnormalities arise in the presence of submucosal fibroids, abnormalities within the endometrium or myometrium may play a role at the genetic and molecular level.7,8 There is clear evidence supporting hysteroscopic removal of submucosal fibroids to improve bleeding abnormalities.9,10
Hysteroscopic removal of eSge type 0 and type i submucosal myomas improves the pregnancy rate for patients who have otherwise unexplained primary infertility. Removal of endometrial polyps is also recommended to improve fertility.
Besides improving fertility, hysteroscopic removal of submucosal myomas and endometrial polyps improves menorrhagia and irregular and abnormal uterine bleeding.
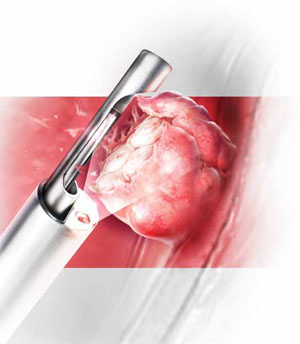
Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. They allow resection using saline, operate without electrical energy, and utilize vacuum suction to remove tissue fragments from the uterine cavity.
Hysteroscopic morcellators ease the task of myomectomy
Hysteroscopic removal of submucosal myomas and polyps is an effective treatment for women who experience bleeding abnormalities or infertility, but the potential for complications deters many gynecologists from performing resectoscopic myomectomy.
Use of a monopolar loop electrode (VIDEO 1) requires an electrolyte-free distention medium, such as 1.5% glycine or 3% sorbitol, and intravasation of these fluids must be limited to minimize the risk of complications such as hyponatremia, cardiovascular compromise, cerebral edema, and, even, death.12 Although the use of normal saline with bipolar resectoscopic instrumentation (VIDEO 2) and automated fluid-management systems reduces the risk of fluid overload, it does not eliminate it entirely, and fluid balance must be carefully scrutinized.13
Intrauterine electrosurgery can burn pelvic organs if an activated electrode perforates the uterine wall and makes contact with bowel or other organs. Burns to the cervix, vagina, and vulva have also been reported when monopolar resectoscopic insulation fails or monopolar electrical current is inadvertently diverted.12
In addition, unless one uses tissue-vaporizing electrodes (VIDEO 3) or is equipped
with newer instrumentation that allows tissue to be removed through the operative sheath of the resectoscope, the myoma must be extracted in pieces, often with repeated removal and reinsertion of the resectoscope and grasping instruments, increasing the risk of cervical injury or uterine perforation with each placement.
Another variable that deters hysteroscopic myomectomy is the lack of training at the residency level. The typical ObGyn resident graduating between 2002 and 2007 had performed a median of only 40 to 51 operative hysteroscopic procedures by the time of graduation.14 This statistic suggests that few residency programs provide adequate training for more demanding hysteroscopic surgeries.
Mechanical morcellators facilitate tissue removal
Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. These morcellators allow resection of a myoma using saline, minimizing the hazards of fluid overload. Because they are mechanical devices that do not require electrical energy, the potential for thermal injury is eliminated.
Mechanical morcellators utilize vacuum suction to remove tissue fragments from the uterine cavity, maintaining a tissue-free operative environment and eliminating the need for repeated manual removal. This feature also reduces the risks of perforation, creation of a false passageway, and gas embolus that have been linked to instrument reinsertion and manual removal of tissue fragments.12
Furthermore, mechanical morcellators are easy to use, reducing operative time and fluid deficit.
Removing Type II myomas with a hysteroscopic morcellator may pose a challenge, however, because of significant myometrial penetration. In addition, bleeding is more likely during removal of a Type II myoma than during removal of other types of tumors, necessitating the use of electric current to address it appropriately. Surgeons who are experienced using the morcellator can overcome these challenges by avoiding the myometrial interface and allowing uterine expulsive contractions to push the myoma into the cavity, making it unnecessary to penetrate the myometrium with the instrument. Thorough preoperative evaluation of Type II myomas is recommended, keeping in mind that removal may be safer and more effective using electrosurgical loop resection.
Option 1: TRUCLEAR morcellator
The TRUCLEAR Hysteroscopic Morcellator (Smith & Nephew) was FDA-approved in 2005 as the first intrauterine mechanical morcellator (VIDEO 4). It requires a dedicated fluid pump and has different instrumentation for myomas and polyps. For myomas, the instrument consists of a rotating tube that reciprocates within an outer 4-mm tube. Both tubes have windows at the end with cutting edges. A vacuum connected to the inner tube provides controlled suction that pulls the tissue into the window on the outer tube and cuts it as the inner tube rotates (VIDEO 5).
For polyps, both inner and outer tubes have oscillating serrated edges on each window (VIDEO 6).
Both instruments are used through a 9-mm offset rod-lens continuous-flow hysteroscope.
In a retrospective analysis, the TRUCLEAR morcellator reduced operative time by about two thirds for polyps and one half for Type 0 and Type I myomas, compared with monopolar loop resection.15 A later study of inexperienced ObGyn residents demonstrated shorter operative times and lower total fluid deficits for the TRUCLEAR morcellator, compared with resectoscopic procedures overall, during polypectomy and myomectomy of Type 0 and Type I myomas.16
Smith & Nephew recently introduced a smaller set of instruments, including a 2.9-mm blade for removal of polyps through a 5.6-mm continuous-flow hysteroscope. However, the new instruments have not yet been approved by the FDA and are unavailable within the United States.
Option 2: MyoSure
The MyoSure Tissue Removal System (Hologic) was FDA-approved in 2009. The hand piece is a rotating and reciprocating 2-mm blade within a 3-mm outer tube. The cutter is connected to a vacuum source that aspirates resected tissue through a side-facing cutting window in the outer tube. The system utilizes standard hysteroscopy set-up for fluid inflow and suction. The instrument is placed through an offset lens continuous-flow hysteroscope with an outer diameter of
6.25 mm. The smaller diameter reduces the amount of cervical dilation required, as well as the risk of uterine perforation.
The smaller size of the instrument renders it ideal for an office setting. Miller and colleagues demonstrated its safety and efficacy for office removal of polyps and myomas (VIDEO 7; VIDEO 8).17
Inadequate reimbursement?
Although both morcellators simplify hysteroscopic myomectomy and polypectomy, insurance reimbursement does not yet differentiate between places of service—unlike other in-office procedures that take into account the cost of the procedural device (see “Reimbursement is limited for hysteroscopic myomectomy in an office setting”). Until the relative value unit (RVU) is modified to reflect this cost, office use of the hysteroscopic morcellator for myomectomy and polypectomy will be financially restrictive to the gynecologist in private practice. Nevertheless, both instruments are easy to use and offer improved safety, increasing access to uterine-preserving surgery.
Thanks to Dr. Andrew I. Brill and Dr. William H. Parker for their thoughtful review of this article.
Since the inception of the resource-based relative value scale, the Centers for Medicare and Medicaid Services (CMS) have provided for different levels of payment to physicians, depending on the place of service and the extent of work involved. The relative value units (RVUs) established for each clinical service are based on three components:
- physician work
- practice expense
- malpractice expense.
The practice expense includes supplies, equipment, clinical and administrative staff, and renting and leasing of space.
When a physician provides a service in a hospital setting or outpatient clinic or surgery unit, the practice expense is lower because the hospital or outpatient facility shoulders those costs. In an office setting, however, the physician practice incurs the full expense of providing the service. In most cases, therefore, the practice is reimbursed at a higher total RVU for office procedures.
The “place of service” code required on your claim form lets the payer know whether the service was rendered in your office (code 11) or a facility such as a hospital or outpatient surgery center (codes 21–24). Physicians who work out of a hospital-owned facility—i.e., physicians who are employed by a hospital—would bill for a facility place of service rather than an office.
The difference in RVUs can be significant. For example, hysteroscopic sterilization (CPT code 58565) has two different RVUs, depending on whether the service is performed in a facility or office (TABLE). However, although hysteroscopic myomectomy can now be safely performed in the office setting for small, less invasive myomas, CMS has not yet assigned a place of service differential for this procedure (CPT code 58561). In other words, CMS has determined that hysteroscopic myomectomy—by definition or practice—is rarely or never performed outside a hospital or outpatient facility.
Medicare reimbursement for hysteroscopic procedures
| Procedure | CPT code | Relative value units | |
|---|---|---|---|
| Facility | Office | ||
| Sterilization | 58565 | 12.90 | 56.66 |
| Endometrial ablation | 58563 | 10.23 | 52.05 |
| Cryoablation | 58356 | 10.34 | 58.92 |
| Myomectomy | 58561 | 16.33 | NA |
| Polypectomy (with dilation and curettage, biopsy) | 58558 | 7.95 | 10.60 |
| To determine reimbursement, multiply the RVU by the Medicare conversion factor, which is $33.9764 | |||
When contracting with a private payer, be sure to ask how the payer reimburses for hysteroscopic myomectomy in an office setting. Payers that do not include a place of service differential may be amenable to negotiation if you can demonstrate that extra compensation can actually save them money and maintain high-quality patient care.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
We want to hear from you! Tell us what you think.
1. Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188(1):100-107.
2. Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: A new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—Preliminary report. J Minim Invasive Gynecol. 2010;12(4):308-311.
3. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215-1223.
4. Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724-729.
5. Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993;82(5):736-740.
6. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027-2034.
7. Stewart EA, Nowak RA. Leiomyoma-related bleeding: a classic hypothesis updated for the molecular era. Human Repro Update. 1996;2(4):295-306.
8. Laughlin SK, Stewart EA. Uterine leiomyomas. Individualizing the approach to a heterogeneous condition. Obstet Gynecol. 2011;117(2 pt 1):396-403.
9. Loffer FD. Improving results of hysteroscopic submucosal myomectomy for menorrhagia by concomitant endometrial ablation. J Minim Invasive Gynecol. 2005;12(3):254-260.
10. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93(5 pt 1):743-748.
11. Nathani F, Clark TJ. Uterine polypectomy in the management of abnormal uterine bleeding: a systematic review. J Minim Invasive Gynecol. 2006;13(4):260-268.
12. Munro MG. Complications of hysteroscopic and uterine resectoscopic surgery. Obstet Gynecol Clin N Am. 2010;37(3):399-425.
13. Kung RC, Vilos GA, Thomas B, Penkin P, Zaltz AP, Stabinsky SA. A new bipolar system for performing operative hysteroscopy in normal saline. J Am Assoc Gynecol Laparosc. 1999;6(3):331-336.
14. Miller CE. Training in minimally iInvasive surgery—you say you want a revolution. J Minim Invasive Gynecol. 2009;16(2):113-120.
15. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic moperating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62-66.
16. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466-471.
17. Miller CE, Glazerman L, Roy K, Lukes A. Clinical evaluation of a new hysteroscopic morcellator—retrospective case review. J Med. 2009;2(3):163-166.
- Applying single-incision laparoscopic surgery to gyn practice: What’s involved
Russell P. Atkin, MD; Michael L. Nimaroff, MD; Vrunda Bhavsar, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The uterine leiomyoma is the most common tumor of the female genital tract. Seventy percent of white women and 80% of black women develop one or more of these tumors by the time they reach 50 years, and the myomas are clinically apparent in 25% of patients.1,2 When a fibroid is submucosal, it is often associated with menorrhagia, abnormal uterine bleeding, and infertility.2-4
In this article, I describe three aspects of managing leiomyomata:
- ways of classifying the tumor to better predict the blood loss, operative time and morbidity associated with removal
- the indications for hysteroscopic myomectomy and polypectomy
- new tools for the removal of polyps and myomas.
Preoperative assessment of submucosal myomas is essential
Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: a new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—preliminary report. J Minim Invasive Gynecol. 2005;12(4):308–311.
Wamsteker and colleagues were the first to propose a system for classifying myoma position within the uterine cavity as a means of estimating the degree of difficulty of resectoscopic removal.5 The European Society for Gynaecological Endoscopy (ESGE) later adopted this system, which is now known by its acronym. According to the ESGE system, myomas that lie entirely within the uterine cavity (Type 0) are easier to remove, require less operative time, and involve less fluid deficit and blood loss than myomas that invade the myometrium to varying degrees (FIGURE 1).

FIGURE 1 ESGE classification
Submucosal myomas are classified as Type 0, Type I, or Type II, according to the degree of myometrial penetration.When more than 50% of a tumor penetrates the myometrium (Type II), the risk of excessive intraoperative fluid absorption is elevated, along with the risk of bleeding and the likelihood of electrolyte abnormalities with the use of non-electrolyte fluid media. Type II tumors also increase operative time and the likelihood that additional procedures will be needed because of incomplete resection—even in the hands of expert hysteroscopic surgeons.5

FIGURE 2 New classification
New classification system increases accuracy
Lasmar and colleagues devised a new system for preoperative assessment of submucosal myomas, hoping to estimate more precisely the likelihood of successful removal via resectoscopy. They call their system the New Classification (NC). Besides taking into account the degree of penetration into the myometrium, they consider the percentage of uterine wall encompassed by the myoma and the location of the myoma within the uterus (i.e., fundus, body, or lower segment) (FIGURE 2). The total score is used to categorize the tumor into Group I, II, or III to estimate the likelihood of successful removal.
In devising the system, Lasmar and colleagues used the NC and ESGE systems to analyze 55 myomectomy cases involving 57 myomas. They found that the NC more accurately predicts differences between Groups I and II in regard to completed procedures, fluid deficit, and operative time.
Preoperative hysteroscopic evaluation of submucosal myomas is essential and reliable using the New Classification system.
Hysteroscopic removal of myomas and polyps
yields multiple benefits
Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724–729.
Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity [published online ahead of print January 24, 2011]. Fertil Steril. doi 10.1016/j. fertnstert.2010.12.034.
Afifi K, Anand S. Nallapeta S, Gelbaya TA. Management of endometrial polyps in subfertile women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):117–121.
Studies evaluating the association between infertility and submucosal fibroids have been controversial because the exact mechanism has not been identified. However, new evidence suggests a molecular causal relationship, and Pritts and colleagues demonstrated improved fertility after submucosal myomectomy.3,6
More recently, Shokeir and coworkers conducted a prospective, randomized, age-matched, controlled trial to explore the effects of hysteroscopic myomectomy on otherwise unexplained primary infertility. They enrolled 215 women who had infertility longer than 12 months and who had their fibroids assessed by means of ultrasonography and classified according to the ESGE system.
Women who underwent myomectomy were twice as likely as women in the control group to become pregnant (relative risk = 2.1; 95% confidence interval = 1.5–2.9). Women who had Type 0 and Type I myomas removed had significantly higher pregnancy rates than women in the control group (P < .001). No statistically significant difference in the pregnancy rate between groups was found for Type II myomas.
Polyps may also affect fertility
Rackow and coworkers demonstrated that endometrial polyps affect uterine receptivity on the molecular level, suggesting a relationship between endometrial polyps and infertility. And after a systematic review of endometrial polyps in women who had subfertility, Afifi and colleagues concluded that polypectomy can improve fertility, especially when assisted reproductive technologies are planned.
Myomas, polyps also contribute to bleeding abnormalities
Submucosal myomas have been associated with bleeding abnormalities, such as heavy menstrual bleeding and menopausal bleeding. Although the precise mechanism is unknown by which these bleeding abnormalities arise in the presence of submucosal fibroids, abnormalities within the endometrium or myometrium may play a role at the genetic and molecular level.7,8 There is clear evidence supporting hysteroscopic removal of submucosal fibroids to improve bleeding abnormalities.9,10
Hysteroscopic removal of eSge type 0 and type i submucosal myomas improves the pregnancy rate for patients who have otherwise unexplained primary infertility. Removal of endometrial polyps is also recommended to improve fertility.
Besides improving fertility, hysteroscopic removal of submucosal myomas and endometrial polyps improves menorrhagia and irregular and abnormal uterine bleeding.

Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. They allow resection using saline, operate without electrical energy, and utilize vacuum suction to remove tissue fragments from the uterine cavity.
Hysteroscopic morcellators ease the task of myomectomy
Hysteroscopic removal of submucosal myomas and polyps is an effective treatment for women who experience bleeding abnormalities or infertility, but the potential for complications deters many gynecologists from performing resectoscopic myomectomy.
Use of a monopolar loop electrode (VIDEO 1) requires an electrolyte-free distention medium, such as 1.5% glycine or 3% sorbitol, and intravasation of these fluids must be limited to minimize the risk of complications such as hyponatremia, cardiovascular compromise, cerebral edema, and, even, death.12 Although the use of normal saline with bipolar resectoscopic instrumentation (VIDEO 2) and automated fluid-management systems reduces the risk of fluid overload, it does not eliminate it entirely, and fluid balance must be carefully scrutinized.13
Intrauterine electrosurgery can burn pelvic organs if an activated electrode perforates the uterine wall and makes contact with bowel or other organs. Burns to the cervix, vagina, and vulva have also been reported when monopolar resectoscopic insulation fails or monopolar electrical current is inadvertently diverted.12
In addition, unless one uses tissue-vaporizing electrodes (VIDEO 3) or is equipped
with newer instrumentation that allows tissue to be removed through the operative sheath of the resectoscope, the myoma must be extracted in pieces, often with repeated removal and reinsertion of the resectoscope and grasping instruments, increasing the risk of cervical injury or uterine perforation with each placement.
Another variable that deters hysteroscopic myomectomy is the lack of training at the residency level. The typical ObGyn resident graduating between 2002 and 2007 had performed a median of only 40 to 51 operative hysteroscopic procedures by the time of graduation.14 This statistic suggests that few residency programs provide adequate training for more demanding hysteroscopic surgeries.
Mechanical morcellators facilitate tissue removal
Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. These morcellators allow resection of a myoma using saline, minimizing the hazards of fluid overload. Because they are mechanical devices that do not require electrical energy, the potential for thermal injury is eliminated.
Mechanical morcellators utilize vacuum suction to remove tissue fragments from the uterine cavity, maintaining a tissue-free operative environment and eliminating the need for repeated manual removal. This feature also reduces the risks of perforation, creation of a false passageway, and gas embolus that have been linked to instrument reinsertion and manual removal of tissue fragments.12
Furthermore, mechanical morcellators are easy to use, reducing operative time and fluid deficit.
Removing Type II myomas with a hysteroscopic morcellator may pose a challenge, however, because of significant myometrial penetration. In addition, bleeding is more likely during removal of a Type II myoma than during removal of other types of tumors, necessitating the use of electric current to address it appropriately. Surgeons who are experienced using the morcellator can overcome these challenges by avoiding the myometrial interface and allowing uterine expulsive contractions to push the myoma into the cavity, making it unnecessary to penetrate the myometrium with the instrument. Thorough preoperative evaluation of Type II myomas is recommended, keeping in mind that removal may be safer and more effective using electrosurgical loop resection.
Option 1: TRUCLEAR morcellator
The TRUCLEAR Hysteroscopic Morcellator (Smith & Nephew) was FDA-approved in 2005 as the first intrauterine mechanical morcellator (VIDEO 4). It requires a dedicated fluid pump and has different instrumentation for myomas and polyps. For myomas, the instrument consists of a rotating tube that reciprocates within an outer 4-mm tube. Both tubes have windows at the end with cutting edges. A vacuum connected to the inner tube provides controlled suction that pulls the tissue into the window on the outer tube and cuts it as the inner tube rotates (VIDEO 5).
For polyps, both inner and outer tubes have oscillating serrated edges on each window (VIDEO 6).
Both instruments are used through a 9-mm offset rod-lens continuous-flow hysteroscope.
In a retrospective analysis, the TRUCLEAR morcellator reduced operative time by about two thirds for polyps and one half for Type 0 and Type I myomas, compared with monopolar loop resection.15 A later study of inexperienced ObGyn residents demonstrated shorter operative times and lower total fluid deficits for the TRUCLEAR morcellator, compared with resectoscopic procedures overall, during polypectomy and myomectomy of Type 0 and Type I myomas.16
Smith & Nephew recently introduced a smaller set of instruments, including a 2.9-mm blade for removal of polyps through a 5.6-mm continuous-flow hysteroscope. However, the new instruments have not yet been approved by the FDA and are unavailable within the United States.
Option 2: MyoSure
The MyoSure Tissue Removal System (Hologic) was FDA-approved in 2009. The hand piece is a rotating and reciprocating 2-mm blade within a 3-mm outer tube. The cutter is connected to a vacuum source that aspirates resected tissue through a side-facing cutting window in the outer tube. The system utilizes standard hysteroscopy set-up for fluid inflow and suction. The instrument is placed through an offset lens continuous-flow hysteroscope with an outer diameter of
6.25 mm. The smaller diameter reduces the amount of cervical dilation required, as well as the risk of uterine perforation.
The smaller size of the instrument renders it ideal for an office setting. Miller and colleagues demonstrated its safety and efficacy for office removal of polyps and myomas (VIDEO 7; VIDEO 8).17
Inadequate reimbursement?
Although both morcellators simplify hysteroscopic myomectomy and polypectomy, insurance reimbursement does not yet differentiate between places of service—unlike other in-office procedures that take into account the cost of the procedural device (see “Reimbursement is limited for hysteroscopic myomectomy in an office setting”). Until the relative value unit (RVU) is modified to reflect this cost, office use of the hysteroscopic morcellator for myomectomy and polypectomy will be financially restrictive to the gynecologist in private practice. Nevertheless, both instruments are easy to use and offer improved safety, increasing access to uterine-preserving surgery.
Thanks to Dr. Andrew I. Brill and Dr. William H. Parker for their thoughtful review of this article.
Since the inception of the resource-based relative value scale, the Centers for Medicare and Medicaid Services (CMS) have provided for different levels of payment to physicians, depending on the place of service and the extent of work involved. The relative value units (RVUs) established for each clinical service are based on three components:
- physician work
- practice expense
- malpractice expense.
The practice expense includes supplies, equipment, clinical and administrative staff, and renting and leasing of space.
When a physician provides a service in a hospital setting or outpatient clinic or surgery unit, the practice expense is lower because the hospital or outpatient facility shoulders those costs. In an office setting, however, the physician practice incurs the full expense of providing the service. In most cases, therefore, the practice is reimbursed at a higher total RVU for office procedures.
The “place of service” code required on your claim form lets the payer know whether the service was rendered in your office (code 11) or a facility such as a hospital or outpatient surgery center (codes 21–24). Physicians who work out of a hospital-owned facility—i.e., physicians who are employed by a hospital—would bill for a facility place of service rather than an office.
The difference in RVUs can be significant. For example, hysteroscopic sterilization (CPT code 58565) has two different RVUs, depending on whether the service is performed in a facility or office (TABLE). However, although hysteroscopic myomectomy can now be safely performed in the office setting for small, less invasive myomas, CMS has not yet assigned a place of service differential for this procedure (CPT code 58561). In other words, CMS has determined that hysteroscopic myomectomy—by definition or practice—is rarely or never performed outside a hospital or outpatient facility.
Medicare reimbursement for hysteroscopic procedures
| Procedure | CPT code | Relative value units | |
|---|---|---|---|
| Facility | Office | ||
| Sterilization | 58565 | 12.90 | 56.66 |
| Endometrial ablation | 58563 | 10.23 | 52.05 |
| Cryoablation | 58356 | 10.34 | 58.92 |
| Myomectomy | 58561 | 16.33 | NA |
| Polypectomy (with dilation and curettage, biopsy) | 58558 | 7.95 | 10.60 |
| To determine reimbursement, multiply the RVU by the Medicare conversion factor, which is $33.9764 | |||
When contracting with a private payer, be sure to ask how the payer reimburses for hysteroscopic myomectomy in an office setting. Payers that do not include a place of service differential may be amenable to negotiation if you can demonstrate that extra compensation can actually save them money and maintain high-quality patient care.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
We want to hear from you! Tell us what you think.
- Applying single-incision laparoscopic surgery to gyn practice: What’s involved
Russell P. Atkin, MD; Michael L. Nimaroff, MD; Vrunda Bhavsar, MD (April 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
The uterine leiomyoma is the most common tumor of the female genital tract. Seventy percent of white women and 80% of black women develop one or more of these tumors by the time they reach 50 years, and the myomas are clinically apparent in 25% of patients.1,2 When a fibroid is submucosal, it is often associated with menorrhagia, abnormal uterine bleeding, and infertility.2-4
In this article, I describe three aspects of managing leiomyomata:
- ways of classifying the tumor to better predict the blood loss, operative time and morbidity associated with removal
- the indications for hysteroscopic myomectomy and polypectomy
- new tools for the removal of polyps and myomas.
Preoperative assessment of submucosal myomas is essential
Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: a new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—preliminary report. J Minim Invasive Gynecol. 2005;12(4):308–311.
Wamsteker and colleagues were the first to propose a system for classifying myoma position within the uterine cavity as a means of estimating the degree of difficulty of resectoscopic removal.5 The European Society for Gynaecological Endoscopy (ESGE) later adopted this system, which is now known by its acronym. According to the ESGE system, myomas that lie entirely within the uterine cavity (Type 0) are easier to remove, require less operative time, and involve less fluid deficit and blood loss than myomas that invade the myometrium to varying degrees (FIGURE 1).

FIGURE 1 ESGE classification
Submucosal myomas are classified as Type 0, Type I, or Type II, according to the degree of myometrial penetration.When more than 50% of a tumor penetrates the myometrium (Type II), the risk of excessive intraoperative fluid absorption is elevated, along with the risk of bleeding and the likelihood of electrolyte abnormalities with the use of non-electrolyte fluid media. Type II tumors also increase operative time and the likelihood that additional procedures will be needed because of incomplete resection—even in the hands of expert hysteroscopic surgeons.5

FIGURE 2 New classification
New classification system increases accuracy
Lasmar and colleagues devised a new system for preoperative assessment of submucosal myomas, hoping to estimate more precisely the likelihood of successful removal via resectoscopy. They call their system the New Classification (NC). Besides taking into account the degree of penetration into the myometrium, they consider the percentage of uterine wall encompassed by the myoma and the location of the myoma within the uterus (i.e., fundus, body, or lower segment) (FIGURE 2). The total score is used to categorize the tumor into Group I, II, or III to estimate the likelihood of successful removal.
In devising the system, Lasmar and colleagues used the NC and ESGE systems to analyze 55 myomectomy cases involving 57 myomas. They found that the NC more accurately predicts differences between Groups I and II in regard to completed procedures, fluid deficit, and operative time.
Preoperative hysteroscopic evaluation of submucosal myomas is essential and reliable using the New Classification system.
Hysteroscopic removal of myomas and polyps
yields multiple benefits
Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724–729.
Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity [published online ahead of print January 24, 2011]. Fertil Steril. doi 10.1016/j. fertnstert.2010.12.034.
Afifi K, Anand S. Nallapeta S, Gelbaya TA. Management of endometrial polyps in subfertile women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):117–121.
Studies evaluating the association between infertility and submucosal fibroids have been controversial because the exact mechanism has not been identified. However, new evidence suggests a molecular causal relationship, and Pritts and colleagues demonstrated improved fertility after submucosal myomectomy.3,6
More recently, Shokeir and coworkers conducted a prospective, randomized, age-matched, controlled trial to explore the effects of hysteroscopic myomectomy on otherwise unexplained primary infertility. They enrolled 215 women who had infertility longer than 12 months and who had their fibroids assessed by means of ultrasonography and classified according to the ESGE system.
Women who underwent myomectomy were twice as likely as women in the control group to become pregnant (relative risk = 2.1; 95% confidence interval = 1.5–2.9). Women who had Type 0 and Type I myomas removed had significantly higher pregnancy rates than women in the control group (P < .001). No statistically significant difference in the pregnancy rate between groups was found for Type II myomas.
Polyps may also affect fertility
Rackow and coworkers demonstrated that endometrial polyps affect uterine receptivity on the molecular level, suggesting a relationship between endometrial polyps and infertility. And after a systematic review of endometrial polyps in women who had subfertility, Afifi and colleagues concluded that polypectomy can improve fertility, especially when assisted reproductive technologies are planned.
Myomas, polyps also contribute to bleeding abnormalities
Submucosal myomas have been associated with bleeding abnormalities, such as heavy menstrual bleeding and menopausal bleeding. Although the precise mechanism is unknown by which these bleeding abnormalities arise in the presence of submucosal fibroids, abnormalities within the endometrium or myometrium may play a role at the genetic and molecular level.7,8 There is clear evidence supporting hysteroscopic removal of submucosal fibroids to improve bleeding abnormalities.9,10
Hysteroscopic removal of eSge type 0 and type i submucosal myomas improves the pregnancy rate for patients who have otherwise unexplained primary infertility. Removal of endometrial polyps is also recommended to improve fertility.
Besides improving fertility, hysteroscopic removal of submucosal myomas and endometrial polyps improves menorrhagia and irregular and abnormal uterine bleeding.

Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. They allow resection using saline, operate without electrical energy, and utilize vacuum suction to remove tissue fragments from the uterine cavity.
Hysteroscopic morcellators ease the task of myomectomy
Hysteroscopic removal of submucosal myomas and polyps is an effective treatment for women who experience bleeding abnormalities or infertility, but the potential for complications deters many gynecologists from performing resectoscopic myomectomy.
Use of a monopolar loop electrode (VIDEO 1) requires an electrolyte-free distention medium, such as 1.5% glycine or 3% sorbitol, and intravasation of these fluids must be limited to minimize the risk of complications such as hyponatremia, cardiovascular compromise, cerebral edema, and, even, death.12 Although the use of normal saline with bipolar resectoscopic instrumentation (VIDEO 2) and automated fluid-management systems reduces the risk of fluid overload, it does not eliminate it entirely, and fluid balance must be carefully scrutinized.13
Intrauterine electrosurgery can burn pelvic organs if an activated electrode perforates the uterine wall and makes contact with bowel or other organs. Burns to the cervix, vagina, and vulva have also been reported when monopolar resectoscopic insulation fails or monopolar electrical current is inadvertently diverted.12
In addition, unless one uses tissue-vaporizing electrodes (VIDEO 3) or is equipped
with newer instrumentation that allows tissue to be removed through the operative sheath of the resectoscope, the myoma must be extracted in pieces, often with repeated removal and reinsertion of the resectoscope and grasping instruments, increasing the risk of cervical injury or uterine perforation with each placement.
Another variable that deters hysteroscopic myomectomy is the lack of training at the residency level. The typical ObGyn resident graduating between 2002 and 2007 had performed a median of only 40 to 51 operative hysteroscopic procedures by the time of graduation.14 This statistic suggests that few residency programs provide adequate training for more demanding hysteroscopic surgeries.
Mechanical morcellators facilitate tissue removal
Hysteroscopic morcellators offer advantages over traditional resectoscopy, making hysteroscopic myomectomy of Type 0 and Type I myomas safer and more feasible for gynecologic surgeons. These morcellators allow resection of a myoma using saline, minimizing the hazards of fluid overload. Because they are mechanical devices that do not require electrical energy, the potential for thermal injury is eliminated.
Mechanical morcellators utilize vacuum suction to remove tissue fragments from the uterine cavity, maintaining a tissue-free operative environment and eliminating the need for repeated manual removal. This feature also reduces the risks of perforation, creation of a false passageway, and gas embolus that have been linked to instrument reinsertion and manual removal of tissue fragments.12
Furthermore, mechanical morcellators are easy to use, reducing operative time and fluid deficit.
Removing Type II myomas with a hysteroscopic morcellator may pose a challenge, however, because of significant myometrial penetration. In addition, bleeding is more likely during removal of a Type II myoma than during removal of other types of tumors, necessitating the use of electric current to address it appropriately. Surgeons who are experienced using the morcellator can overcome these challenges by avoiding the myometrial interface and allowing uterine expulsive contractions to push the myoma into the cavity, making it unnecessary to penetrate the myometrium with the instrument. Thorough preoperative evaluation of Type II myomas is recommended, keeping in mind that removal may be safer and more effective using electrosurgical loop resection.
Option 1: TRUCLEAR morcellator
The TRUCLEAR Hysteroscopic Morcellator (Smith & Nephew) was FDA-approved in 2005 as the first intrauterine mechanical morcellator (VIDEO 4). It requires a dedicated fluid pump and has different instrumentation for myomas and polyps. For myomas, the instrument consists of a rotating tube that reciprocates within an outer 4-mm tube. Both tubes have windows at the end with cutting edges. A vacuum connected to the inner tube provides controlled suction that pulls the tissue into the window on the outer tube and cuts it as the inner tube rotates (VIDEO 5).
For polyps, both inner and outer tubes have oscillating serrated edges on each window (VIDEO 6).
Both instruments are used through a 9-mm offset rod-lens continuous-flow hysteroscope.
In a retrospective analysis, the TRUCLEAR morcellator reduced operative time by about two thirds for polyps and one half for Type 0 and Type I myomas, compared with monopolar loop resection.15 A later study of inexperienced ObGyn residents demonstrated shorter operative times and lower total fluid deficits for the TRUCLEAR morcellator, compared with resectoscopic procedures overall, during polypectomy and myomectomy of Type 0 and Type I myomas.16
Smith & Nephew recently introduced a smaller set of instruments, including a 2.9-mm blade for removal of polyps through a 5.6-mm continuous-flow hysteroscope. However, the new instruments have not yet been approved by the FDA and are unavailable within the United States.
Option 2: MyoSure
The MyoSure Tissue Removal System (Hologic) was FDA-approved in 2009. The hand piece is a rotating and reciprocating 2-mm blade within a 3-mm outer tube. The cutter is connected to a vacuum source that aspirates resected tissue through a side-facing cutting window in the outer tube. The system utilizes standard hysteroscopy set-up for fluid inflow and suction. The instrument is placed through an offset lens continuous-flow hysteroscope with an outer diameter of
6.25 mm. The smaller diameter reduces the amount of cervical dilation required, as well as the risk of uterine perforation.
The smaller size of the instrument renders it ideal for an office setting. Miller and colleagues demonstrated its safety and efficacy for office removal of polyps and myomas (VIDEO 7; VIDEO 8).17
Inadequate reimbursement?
Although both morcellators simplify hysteroscopic myomectomy and polypectomy, insurance reimbursement does not yet differentiate between places of service—unlike other in-office procedures that take into account the cost of the procedural device (see “Reimbursement is limited for hysteroscopic myomectomy in an office setting”). Until the relative value unit (RVU) is modified to reflect this cost, office use of the hysteroscopic morcellator for myomectomy and polypectomy will be financially restrictive to the gynecologist in private practice. Nevertheless, both instruments are easy to use and offer improved safety, increasing access to uterine-preserving surgery.
Thanks to Dr. Andrew I. Brill and Dr. William H. Parker for their thoughtful review of this article.
Since the inception of the resource-based relative value scale, the Centers for Medicare and Medicaid Services (CMS) have provided for different levels of payment to physicians, depending on the place of service and the extent of work involved. The relative value units (RVUs) established for each clinical service are based on three components:
- physician work
- practice expense
- malpractice expense.
The practice expense includes supplies, equipment, clinical and administrative staff, and renting and leasing of space.
When a physician provides a service in a hospital setting or outpatient clinic or surgery unit, the practice expense is lower because the hospital or outpatient facility shoulders those costs. In an office setting, however, the physician practice incurs the full expense of providing the service. In most cases, therefore, the practice is reimbursed at a higher total RVU for office procedures.
The “place of service” code required on your claim form lets the payer know whether the service was rendered in your office (code 11) or a facility such as a hospital or outpatient surgery center (codes 21–24). Physicians who work out of a hospital-owned facility—i.e., physicians who are employed by a hospital—would bill for a facility place of service rather than an office.
The difference in RVUs can be significant. For example, hysteroscopic sterilization (CPT code 58565) has two different RVUs, depending on whether the service is performed in a facility or office (TABLE). However, although hysteroscopic myomectomy can now be safely performed in the office setting for small, less invasive myomas, CMS has not yet assigned a place of service differential for this procedure (CPT code 58561). In other words, CMS has determined that hysteroscopic myomectomy—by definition or practice—is rarely or never performed outside a hospital or outpatient facility.
Medicare reimbursement for hysteroscopic procedures
| Procedure | CPT code | Relative value units | |
|---|---|---|---|
| Facility | Office | ||
| Sterilization | 58565 | 12.90 | 56.66 |
| Endometrial ablation | 58563 | 10.23 | 52.05 |
| Cryoablation | 58356 | 10.34 | 58.92 |
| Myomectomy | 58561 | 16.33 | NA |
| Polypectomy (with dilation and curettage, biopsy) | 58558 | 7.95 | 10.60 |
| To determine reimbursement, multiply the RVU by the Medicare conversion factor, which is $33.9764 | |||
When contracting with a private payer, be sure to ask how the payer reimburses for hysteroscopic myomectomy in an office setting. Payers that do not include a place of service differential may be amenable to negotiation if you can demonstrate that extra compensation can actually save them money and maintain high-quality patient care.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
We want to hear from you! Tell us what you think.
1. Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188(1):100-107.
2. Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: A new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—Preliminary report. J Minim Invasive Gynecol. 2010;12(4):308-311.
3. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215-1223.
4. Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724-729.
5. Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993;82(5):736-740.
6. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027-2034.
7. Stewart EA, Nowak RA. Leiomyoma-related bleeding: a classic hypothesis updated for the molecular era. Human Repro Update. 1996;2(4):295-306.
8. Laughlin SK, Stewart EA. Uterine leiomyomas. Individualizing the approach to a heterogeneous condition. Obstet Gynecol. 2011;117(2 pt 1):396-403.
9. Loffer FD. Improving results of hysteroscopic submucosal myomectomy for menorrhagia by concomitant endometrial ablation. J Minim Invasive Gynecol. 2005;12(3):254-260.
10. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93(5 pt 1):743-748.
11. Nathani F, Clark TJ. Uterine polypectomy in the management of abnormal uterine bleeding: a systematic review. J Minim Invasive Gynecol. 2006;13(4):260-268.
12. Munro MG. Complications of hysteroscopic and uterine resectoscopic surgery. Obstet Gynecol Clin N Am. 2010;37(3):399-425.
13. Kung RC, Vilos GA, Thomas B, Penkin P, Zaltz AP, Stabinsky SA. A new bipolar system for performing operative hysteroscopy in normal saline. J Am Assoc Gynecol Laparosc. 1999;6(3):331-336.
14. Miller CE. Training in minimally iInvasive surgery—you say you want a revolution. J Minim Invasive Gynecol. 2009;16(2):113-120.
15. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic moperating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62-66.
16. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466-471.
17. Miller CE, Glazerman L, Roy K, Lukes A. Clinical evaluation of a new hysteroscopic morcellator—retrospective case review. J Med. 2009;2(3):163-166.
1. Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188(1):100-107.
2. Lasmar RB, Barrozo PR, Dias R, Oliveira MA. Submucous myomas: A new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—Preliminary report. J Minim Invasive Gynecol. 2010;12(4):308-311.
3. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215-1223.
4. Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysteroscopic myomectomy: a randomized matched control study. Fertil Steril. 2010;94(2):724-729.
5. Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993;82(5):736-740.
6. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027-2034.
7. Stewart EA, Nowak RA. Leiomyoma-related bleeding: a classic hypothesis updated for the molecular era. Human Repro Update. 1996;2(4):295-306.
8. Laughlin SK, Stewart EA. Uterine leiomyomas. Individualizing the approach to a heterogeneous condition. Obstet Gynecol. 2011;117(2 pt 1):396-403.
9. Loffer FD. Improving results of hysteroscopic submucosal myomectomy for menorrhagia by concomitant endometrial ablation. J Minim Invasive Gynecol. 2005;12(3):254-260.
10. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93(5 pt 1):743-748.
11. Nathani F, Clark TJ. Uterine polypectomy in the management of abnormal uterine bleeding: a systematic review. J Minim Invasive Gynecol. 2006;13(4):260-268.
12. Munro MG. Complications of hysteroscopic and uterine resectoscopic surgery. Obstet Gynecol Clin N Am. 2010;37(3):399-425.
13. Kung RC, Vilos GA, Thomas B, Penkin P, Zaltz AP, Stabinsky SA. A new bipolar system for performing operative hysteroscopy in normal saline. J Am Assoc Gynecol Laparosc. 1999;6(3):331-336.
14. Miller CE. Training in minimally iInvasive surgery—you say you want a revolution. J Minim Invasive Gynecol. 2009;16(2):113-120.
15. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic moperating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62-66.
16. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466-471.
17. Miller CE, Glazerman L, Roy K, Lukes A. Clinical evaluation of a new hysteroscopic morcellator—retrospective case review. J Med. 2009;2(3):163-166.
