User login
How to avoid and manage complications when placing ports and docking



2017 Update on minimally invasive gynecologic surgery
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.
The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
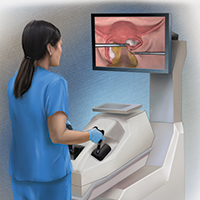
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11
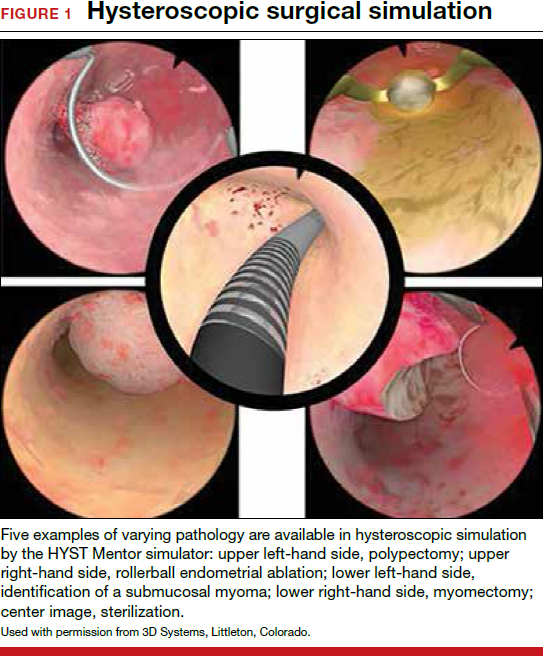
Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18
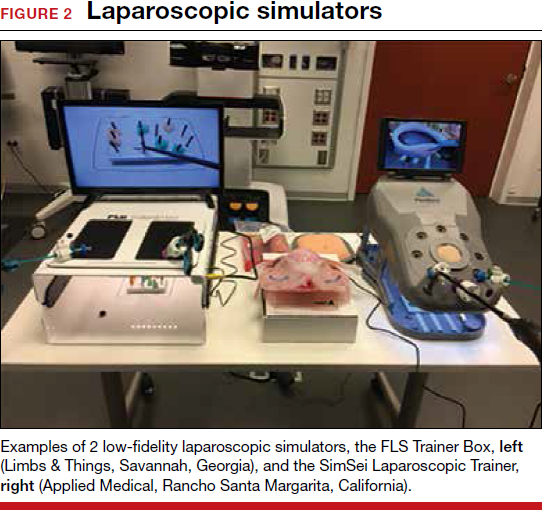
The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
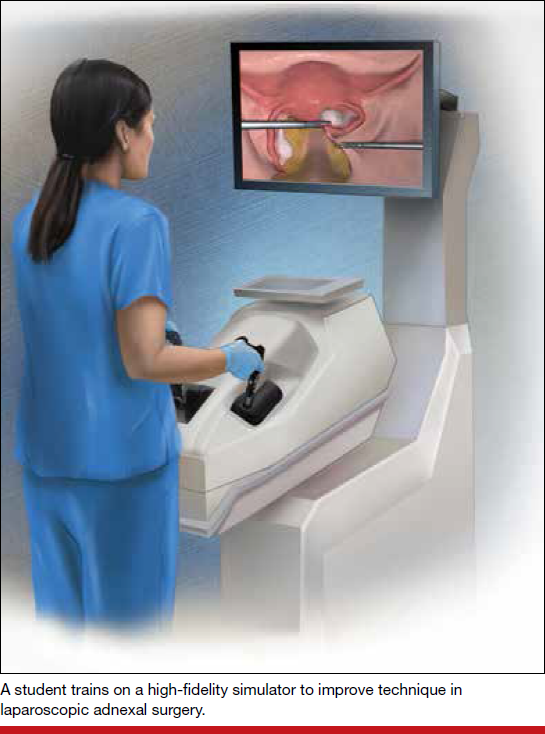
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).
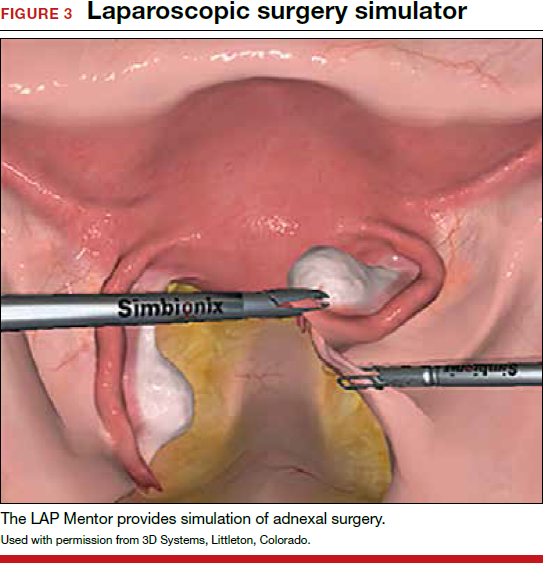
Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.

The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11

Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18

The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).

Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Gynecologic surgeons who trained in the early 1990s may feel that the practice of gynecologic surgery seemed simpler back then. There were really only 2 ways to perform a hysterectomy: vaginally (TVH—total vaginal hysterectomy) and abdominally (TAH—total abdominal hysterectomy). Global endometrial ablation devices were not an established treatment for abnormal uterine bleeding, and therapeutic advancements such as hormonally laden intrauterine devices, vaginal mesh kits, and surgical robots did not exist. The options in the surgical toolbox were limited, and the general expectation in residency was long hours. During that period, consistent exposure to the operating room and case volume allowed one to graduate confidant in one’s surgical skills.

The changing landscape of gynecologic surgery
Fast-forward to 2017. Now, so many variables affect the ability to produce a well-trained gynecologic surgeon. In fact, in 2015 Guntupalli and colleagues studied the preparedness of ObGyn residents for fellowship training in the 4 subspecialties of female pelvic medicine and reconstructive surgery, gynecologic oncology, maternal-fetal medicine, and reproductive endocrinology-infertility.1 Through a validated survey of fellowship program directors, the authors found that only 20% of first-year fellows were able to perform a vaginal hysterectomy independently, and 46%, an abdominal hysterectomy. Barely 50% of first-year fellows in all subspecialties studied could independently set up a retractor for laparotomy and appropriately pack and mobilize the bowel for pelvic surgery.1
Today the hysterectomy procedure has become the proverbial alphabet soup. Trainees are confronted with having to learn not only the TVH and the TAH but also the LAVH (laparoscopic-assisted vaginal hysterectomy), LSH (laparoscopic supracervical hysterectomy), TLH (total laparoscopic hysterectomy), and RALH (robot-assisted laparoscopic hysterectomy).2 With a mandated 80-hour residency workweek restriction and an increasing number of minimally invasive hysterectomies performed nationally, a perfect storm exists for critically evaluating the current paradigm of resident and fellow surgical training.3
One may wonder if current controversies surrounding many of the technologic advancements in gynecologic surgery result from inadequate training and too many treatment options or from flaws in the actual devices. A “see one, do one, teach one” approach to assimilating surgical skills is no longer an accepted approach, and although the “10,000-hour rule” of focused practice to attain expertise makes sense, how can a trainee gain enough exposure to achieve competency?
Related article:
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
Simulation: A creditable training tactic
This is where simulation—whether low or high fidelity—potentially can fill in some of those training gaps. Simulation in medicine is a proven instructional design strategy in which learning is an active and experiential process. Studies clearly have shown that simulation-based medical education (SBME) with deliberate practice is superior to traditional clinical medical education in achieving specific clinical skill acquisition goals.4
This special Update on minimally invasive gynecologic surgery offers a 30,000-foot overview of the current state of simulation in gynecologic surgical training. Equally important to this conversation is the process by which a trained individual can obtain the appropriate credentials and subsequent privileging to perform various surgical procedures. Simulation has begun to play a significant role not only in an individual’s initial credentialing and privileging in surgery but also in maintaining those privileges.
Read about the evolving role of simulation in gyn surgery training.
Simulation's evolving role in gyn surgery training
Recently, the traditional model of gynecologic surgical training has been impacted by the exponential growth of technology (surgical devices), increased surgical options, and the limited work hours of trainees. As a result, simulation-based medical education has been identified as a potential solution to address deficits in surgical training. Fortunately, all modalities of surgery are now amenable to improvements in surgical education via simulation.5
Although basic skill training in the standard areas of hand-eye coordination, tissue handling, and instrument use still is prerequisite, the integration of both low- and high-fidelity simulation technologies--with enhanced functionality--now allows for a more comprehensive approach to understanding surgical anatomy. In addition, simulation training provides the opportunity for independent practice of full surgical procedures and, in many instances, offers objective and instantaneous assessment feedback for the learner. This discussion highlights some of the relevant literature on simulation training and the impact of surgical simulation on hysteroscopy and laparoscopy.
Box trainers and virtual reality simulators in hysteroscopy training
Hysteroscopic surgery allows direct endoscopic visualization of the uterine cavity for both diagnostic and therapeutic purposes. While the majority of these procedures are generally low risk, operative hysteroscopic experience minimizes the possibility of significant procedure-related complications, such as uterine perforation.5 The literature repeatedly shows that significant differences exist in skill and sense of preparedness between the novice or inexperienced surgeon (resident trainee) and the expert in hysteroscopic surgery.6-8
Both low- and high-fidelity hysteroscopic simulators can be used to fine-tune operator skills. Low-fidelity simulators such as box trainers, which focus on skills like endometrial ablation and hysteroscopic resection with energy, have been shown to measurably improve performance, and they are well-received by participants. Low-fidelity simulations that incorporate vegetable/fruit or animal models (for example, porcine bladders and cattle uteri) have also been employed with success.9
On the high-fidelity end, surgical trainees can now experience hysteroscopic surgery simulation through virtual reality simulators, which have evolved with improvements in technology (FIGURE 1). Many high-fidelity simulators have been developed, and technical skill and theoretical knowledge improve with their use. Overall, trainees have provided positive feedback regarding the realism and training capacity afforded by virtual reality simultors.10,11

Various simulators are equipped with complete training curriculums that focus on essential surgical skills. Common troubleshooting techniques taught via simulator include establishing and maintaining clear views, detecting and coagulating bleeding sources, fluid management and handling, and instrument failure. Learners can perform these sessions repeatedly, independent of their respective starting skill level. On completion of simulation training, the trainee is given objective performance assessments on economy of motion, visualization, safety, fluid handling, and other skills.
Related article:
ExCITE: Minimally invasive tissue extraction made simple with simulation
Learning the complexities of laparoscopy through simulation
Laparoscopic surgery (both conventional and robot assisted) allows for a minimally invasive, cost-effective, and rapid-recovery approach to the management of many common gynecologic conditions. In both approaches, the learning curve to reach competency is steep. Conventional laparoscopy requires unique surgical skills, including adapting to a 2-dimensional field with altered depth perception; this creates challenges in spatial reasoning and achieving proficiency in video-eye-hand coordination as a result of the fulcrum effect inherent in laparoscopic instrumentation. This is further complicated by the essential dexterity required to complete dissections and suturing.12,13
Robot-assisted laparoscopic surgery requires significant modifications to adapt to a 3-dimensional view. In addition, this approach incorporates another level of complexity (and challenge to attaining mastery), namely, using remotely controlled multiple instrument arms with no tactile feedback.
Importantly, some residency training programs are structured unevenly, emphasizing one or the other surgical modality.14 As a result, this propagates certain skills--or lack thereof--on graduation, and thus highlights potential areas of laparoscopic training that need to be improved and enhanced.
Increasing the learning potential
The growing integration of low- and high-fidelity simulation training in laparoscopic surgery has led to improved skill acquisition.12,13,15,16 A well-established low-fidelity simulation model is the Fundamentals of Laparoscopic Surgery module, through which trainees are taught vital psychomotor skills via a validated box trainer that is also supported by a cognitive component (FIGURE 2).17,18

The advent of laparoscopic virtual reality training systems has raised the learning potential further, even for experienced surgeons. Some benefits of virtual reality simulation in conventional laparoscopy include education on an interactive 3D pelvis, step-by-step procedural guidance, a comprehensive return of performance metrics on vital laparoscopic skills, and the incorporation of advanced skills such as laparoscopic suturing, complex dissections, and lysis of adhesions.
In the arena of robot-assisted procedures, simulation modules are available for learning fundamental skill development in hand-eye coordination, depth perception, bimanual manipulation, camera navigation, and wrist articulation.
In both conventional and robot-assisted laparoscopy simulation pathways, complete procedural curriculums (for example, hysterectomy with adnexectomy) are available. Thus, learners can start a procedure or technique at a point applicable to them, practice repeatedly until competency, and eventually become proficient (FIGURE 3).

Generally, high-fidelity computerized simulators provide a comprehensive performance report on completion of training, along with a complete recording of the trainee's encounter during accruement of skill. Most importantly, laparoscopic training via simulation has been validated to translate into improved operating room performance by impacting operating times, safety profiles, and surgical skill growth.15,19
Related article:
Complete colpectomy & colpocleisis: Model for simulation
Simulation is a mainstream training tool
The skills gap between expert surgeons and new trainees continues to widen. A comprehensive educational pathway that provides an optimistic safety profile, abides by time constraints, and elevates skill sets will fall to simulation-based surgical training.20,21 Surgical competence is defined not simply by observation and Halstedian technique but by a combination of cognitive and behavioral abilities as well as perceptual and psychomotor skills. It is impractical to expect current learners to become proficient in visuospatial and tactile perception and to demonstrate technical competency without supplementing their training.22-24 Ultimately, as experience with both low- and high-fidelity surgical simulation grows, the predictive validity of this type of training pathway will become more readily apparent. In other words, improved performance in the simulated environment will translate into improved performance in the operating room.
Read about how gyn surgery simulation is being incorporated into credentialing and privileging
Incorporating gyn surgery simulation into credentialing and privileging
Over the last 25 years surgeons have seen unprecedented changes in technology that have revolutionized our surgical approaches to common gynecologic conditions. In the past, granting surgical privileges was pretty straightforward. Surgeons were granted privileges based on successfully completing their training, and subsequent renewal of those privileges was based on not having any significant misadventures or complications. With the advent of laparoscopy, hysteroscopy, and then robot-assisted surgery, training surgeons and verifying their competency has become much more complicated. The variety of surgical approaches now being taught coupled with reduced resident training time and decreasing case volumes have significantly impacted the traditional methodologies of surgical training.25,26
Related article:
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
High-tech surgery demands high-tech training
The development of high-tech surgical approaches has been accompanied by the natural development of simulation models to help with training. Initially, inanimate models, animal labs, and cadavers were used. Over the last 15 years, several innovative companies have developed virtual reality simulation platforms for laparoscopy, hysteroscopy, and even robotics.27 These virtual reality simulators allow students to develop the psychomotor skills necessary to perform minimally invasive procedures and to practice those skills until they can demonstrate proficiency before operating on a live patient.
Most would agree that the key to learning a surgical skill is to "practice, practice, practice."28 Many studies have shown that improvement in surgical outcomes is clearly related to a surgeon's case volume.29,30 But with case volumes decreased, simulation has evolved as the best training alternative. Current surgical simulators enable a student to engage in "deliberate practice"; that is, to have tasks with well-defined goals, to be motivated to improve, and to receive immediate feedback along with opportunities for repetition and refinements of performance.
Simulation allows students to try different surgical techniques and to use "deliberate practice" avoidance of errors in a controlled, safe situation that provides immediate performance feedback.31 Currently, virtual reality simulators are available for hysteroscopy, laparoscopy, and robot-assisted gynecologic applications. Early models focused solely on developing a learner's psychomotor skills necessary to safely perform minimally invasive surgeries. Newer simulators add a cognitive component to help students learn specific procedures, such as adnexectomy and hysterectomy.32
Based on the aviation simulator training model, the AAGL endorsed a Gynecologic Robotic Surgery Credentialing and Privileging Guideline in 2014; this guidance relies heavily on simulation for initial training as well as for subsequent annual recertification.33 Many institutions, including the MultiCare Health System in Tacoma, Washington, require all surgeons--even high-volume surgeons--to demonstrate proficiency annually by passing required robotic simulation exercises at least 2 times consecutively in order to maintain robotic surgery privileges.34
A work-around for a simulation drawback
Using simulation for recertification has been criticized because, although it can confirm that a surgeon is skilled enough to operate the tool, it does not evaluate surgical judgment or technique. In response, crowdsourced review of an individual surgeon's surgical videos has proven to be a useful, dependable way to give a surgeon direct feedback regarding his or her performance on a live patient.35 Many institutions now use this technology not only for initial training but also for helping surgeons improve with direct feedback from master surgeon reviewers. Other institutions have considered replacing annual re-credentialing case volume requirements with this technology, which actually assesses competence in a more accurate way.36
Related article:
Flight plan for robotic surgery credentialing: New AAGL guidelines
A new flight plan
The bottom line is that the training and annual recertification of future surgeons now mimics closely the pathway that all airplane pilots are required to follow.
Initial training will require mastery of surgical techniques using a simulator before taking a "solo flight" on a live patient.
Maintenance of privileges now requires either large case volumes or skills testing on a simulator. Many institutions now also require an annual "check ride," such as a crowdsourced video review of a surgeon's cases, as described above.
Re-credentialing. Just as the "see one, do one, teach one" model is now part of our historical legacy, re-credentialing simply by avoiding misadventures and staying out of trouble will go the way of paper medical records. Our future will certainly require an annual objective evaluation of good surgical judgment and surgical technique proficiency. Surgical simulation will be the norm for all of us.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
- Guntupalli SR, Doo DW, Guy M, et al. Preparedness of obstetrics and gynecology residents for fellowship training. Obstet Gynecol. 2015;126(3):559–568.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395–398.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711.
- Smith ML. Simulation and education in gynecologic surgery. Obstet Gynecol Clin North Am. 2011;38(4):733–740.
- Raymond E, Ternamian A, Leyland N, Tolomiczenko G. Endoscopy teaching in Canada: a survey of obstetrics and gynecology program directors and graduating residents. J Minim Invasive Gynecol. 2006;13(1):10–16.
- Goff BA, VanBlaricom A, Mandel L, Chinn M, Nielsen P. Comparison of objective, structured assessment of technical skills with a virtual reality hysteroscopy trainer and standard latex hysteroscopy model. J Reprod Med. 2007;52(5):407–412.
- Singhi A. Comparison of complications rates in endoscopic surgery performed by a clinical assistant vs an experienced endoscopic surgeon. J Gynecol Endosc Surg. 2009;1(1):40–46.
- Savran MM, Sorensen SM, Konge L, Tolsgaard MG, Bjerrum F. Training and assessment of hysteroscopic skills: a systematic review. J Surg Ed. 2016;73(5):906–918.
- Panel P, Bajka M, Le Tohic A, Ghoneimi AE, Chis C, Cotin S. Hysteroscopic placement of tubal sterilization implants: virtual reality simulator training. Surg Endosc. 2012;26(7):1986–1996.
- Bajka M, Tuchschmid S, Streich M, Fink D, Szekely G, Harders M. Evaluation of a new virtual-reality training simulator for hysteroscopy. Surg Endosc. 2009;23(9):2026–2033.
- Scott DJ, Bergen PC, Rege RV, et al. Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg. 2000;191(3):272–283.
- Scott-Conner CE, Hall TJ, Anglin BL, et al. The integration of laparoscopy into a surgical residency and implications for the training environment. Surg Endosc. 1994;8(9):1054–1057.
- Berkowitz RL, Minkoff H. A call for change in a changing world. Obstet Gynecol. 2016;127(1):153–156.
- Larsen CR, Oestergaard J, Ottesen BS, Soerensen JL. The efficacy of virtual reality simulation training in laparoscopy: a systematic review of randomized trials. Acta Obstet Gynecol Scand. 2012;91(9):1015–1028.
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. AnnSurg. 2007;246(5):771–779.
- Oropesa I, Sanchez-Gonzalez P, Lamata P, et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95.
- Rooney DM, Brissman IC, Finks JF, Gauger PG. Fundamentals of Laparoscopic Surgery manual test: is videotaped performance assessment an option? J Surg Educ. 2015;72(1):90–95.
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463, 63–64.
- Aggarwal R, Tully A, Grantcharov T, et al. Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG. 2006;113(12):1382–1387.
- Darzi A, Smith S, Taffinder N. Assessing operative skill. Needs to become more objective. BMJ. 1999;318(7188):887–888.
- Moorthy K, Munz Y, Sarker SK, Darzi A. Objective assessment of technical skills in surgery. BMJ. 2003;327(7422):1032–1037.
- Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Assessment of technical surgical skills. Eur J Surg. 2002;168(3):139–144.
- Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery. 2003;134(5):750–757.
- Einarsson JI, Young A, Tsien L, Sangi-Haghpeykar H. Perceived proficiency in endoscopic techniques among senior obstetrics and gynecology residents. J Am Assoc Gynecol Laparosc. 2002;9(2):158–164.
- Cohen SL, Hinchcliffe E. Is surgical training in ob-gyn residency adequate? Contemp ObGyn. . Published July 22, 2016. Accessed October 18, 2017.
- Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30(6):2169–2178.
- Gladwell M. Outliers: The Story of Success. New York, New York: Little Brown and Co; 2008.
- Boyd LR, Novetsky AP, Curtain JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119(4):709–716.
- Kotsis SV, Chung KC. Application of the “see one, do one, teach one” concept in surgical training. Plast Reconstr Surg. 2013;131(5):1194–1201.
- Maestro AR Hysterectomy Module. Mimic simulation website. http://www.mimicsimulation.com/hysterectomy/. Accessed October 18, 2017.
- AAGL. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasiv Gynecol, 2014;21(2):157–167.
- Lenihan JP Jr. Navigating credentialing and privileging and learning curves in robotics with an evidence and experienced-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
- Polin MR, Siddiqui NY, Comstock BA, et al. . Am J Obstet Gynecol. 2016;215(5):644.e1–644.e7.
- Continuous People Improvement. C-SATS website. https://www.csats.com/customers-main/. Accessed October 18, 2017.
How the AAGL is trying to improve outcomes for patients undergoing robot-assisted gynecologic surgery
Flight plan for robotic surgery credentialing: New AAGL guidelines
The AAGL, formerly the American Association of Gynecologic Laparoscopists, has approved the first-ever set of privileging and credentialing guidelines for robotic surgery.1
Why has this prestigious minimally invasive surgery organization done that?
Maybe you’ve seen the Internet and TV ads and billboard trucks driving outside of many major medical society meetings recently, advertising “1-800-BAD-Robot.”2 You also are probably aware of recent articles in the headlines of national periodicals like the Wall Street Journal claiming that robotic surgery can be harmful.3
And yet, robotic gynecologic surgery has grown at an unprecedented rate since its approval by the US Food and Drug Administration (FDA) in April 2005. Recent data from the Nationwide Inpatient Sample from the Agency for Healthcare Research and Quality indicate that robot-assisted hysterectomies have increased at a dramatic rate.4 In a recent study of the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, investigators found that more than 30% of injuries during robotic surgery are related to operator error or robot failure, but the majority of problems are not associated with the technology.5
In this article, I use the aviation industry as an example of a sector that has gotten safety right. By emulating many of its standards, our specialty can make great strides toward patient safety and improved outcomes. I also outline the main points of the new AAGL guidelines and the rationale behind them.1 See, for example, the summary box on page 46.
A “shining example”
The robot clearly is an enabling technology. With its high-definition 3D vision and scaled motion with wristed instruments, surgeons are more comfortable performing many complex gynecologic procedures that previously would have required open surgery to safely accomplish … but the da Vinci Robot does not make a poor surgeon a great surgeon.
Hospitals now are being sued for allowing surgeons to perform robotic surgery on patients without documenting adequate surgeon training or providing consistent oversight.6 This new technology has outpaced the ability of hospital medical staffs to establish practice guidelines and rules to ensure patient safety.
The aviation industry is a shining example of a highly reliable industry. Each day, thousands of commercial aircraft fly all over the world with amazing safety. Most of the time, the pilot and copilot have never flown together. However, each crew member knows his or her role precisely and clearly understands what is expected. Crew members must meet standards that transcend all airlines and all aircraft.7 They all practice communication and undergo standardized training, including simulation, prior to taking off with live passengers on board.
In addition, all pilots must demonstrate their proficiency and competence on a regular basis—by exhibiting actual safe flight performance (over multiple takeoffs and landings) and undergoing check rides with flight examiners and practicing routine and emergency procedures on flight simulators. Airline passengers have come to expect that all pilots are equally proficient and safe. Shouldn’t patients be able to expect the same from their surgeons and hospitals? And yet there is no national or local organization that ensures that all surgeons are equally safe in the operating room. That responsibility is too often left up to the courts.
Three requirements of robotic credentialing
In 2008, the MultiCare Health System in the Pacific Northwest adopted a unique system of robotic credentialing that was based on the aviation model.8 This model has three main components, which are identical to the guidelines imposed on pilots:
- Surgeons selected for training should be likely to be successful in performing robotic surgeries safely and efficiently.
- Practice makes perfect. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills. The aviation world calls this concept “currency.”
- Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Adoption of these tried-and-true safety principles would ensure that hospitals exercise their responsibility to protect patients who undergo robotic surgeries in their systems.
The AAGL’s Robotics Special Interest Group, formed in 2010, is now the largest special interest group in the organization. The group was initially tasked to develop evidence-based guidelines for robotic surgery training and credentialing. Using the aviation industry’s model, the group developed a basic template of robotic surgery credentialing and privileging guidelines that can be used anywhere in the world. This proposal is not meant to be a standard-of-care definition; rather, it is intended simply as a starting point.
Key components of new AAGL robotic surgery credentialing and privileging guidelines1
Initial training
- Train only surgeons who have an adequate case volume to get through the learning curve. Recommended: at least 20 major cases per year.
- Current training pathways include computer-based learning, case observations, pig labs, simulation, and proctored cases. More intense validated simulation training could replace pig labs.
- Surgeons should initially perform only simple, basic procedures with surgeon first-assists until they develop the necessary skills to safely operate the robotic console and start performing more complex cases.
Annual currency
- Surgeons should perform at least 20 major cases per year, with at least one case every 8 weeks.
- If surgeons operate less frequently, proficiency should be verified on a simulator before operation on a live patient.
Annual recertification
- All surgeons should demonstrate competency annually on a simulator, regardless of case volume.
Initial training involves a long learning curve
There is a long learning curve for surgeons to become competent in robotic surgery. In initial studies of experienced advanced laparoscopic surgeons, investigators found that learning curves could involve 50 cases or more.9,10 In a recent study of gynecologic oncologists and urogynecologists at the Mayo Clinic, researchers found that it took 91 cases for experienced surgeons to become proficient on the robot.11
ObGyns in the United States are doing fewer hysterectomies than they used to.12 Many surgeons now perform fewer than 10 hysterectomies per year. These surgeons clearly have worse outcomes than surgeons who operate more frequently.13–15 Therefore, these new guidelines suggest that hospitals should choose to train only surgeons who have a case volume that will allow them to get through their learning curve in a short time and continue to have enough surgeries to maintain their skills. These guidelines recommend that surgeons who are candidates for robotic surgery training already perform a minimum of 20 major gynecologic operations per year.
It is important to learn to walk before you run. New student pilots start out with single-engine propeller planes before graduating to multi-engine props, jets, and commercial aircraft. Similarly, new medical students start out with easy surgical tasks before training for more complex procedures. This approach seems like common sense, although many surgeons may feel that, after orienting on the robot, they can start doing complex cases right away, as the robot enables them to do better and more precise surgery. Nothing could be further from the truth.
It is very important that new robotic surgeons start with easy, basic cases to completely familiarize themselves with the operation of the robot console before attempting more complex and difficult cases.
There is no absolute number of cases that ensures competency with the robot; the number depends on the surgeon’s case load, surgical prowess, and psychomotor skills. A surgeon should be restricted to simple cases initially, and should have an experienced robot-credentialed surgeon operating with him or her during this initial learning period.
Practice makes perfect
Musicians will tell you that the more often you practice, the more skilled you become. This is true for anyone whose job requires special training. It would be naïve to assume that surgeons can maintain optimal skills for robotic surgery by performing only a few cases each year.
Psychomotor skill degradation has been explored in relation to various surgical skills. The more complex the skill, the more likely that skill set will deteriorate without use. In recent studies, investigators have shown that robotic surgery skills begin to decline significantly after only 2 weeks of inactivity, and that skills continue to degrade without use.16,17
Based on this information, the currency requirement for surgeons to maintain privileges was set at 20 cases per year—fewer than two cases per month. Although the members of the Robotics Special Interest Group strongly agree that
maintenance of privileges should not be based entirely on an arbitrary currency number, as Tracy and colleagues also argue in a recent publication,18 it is clear that frequent performance of robotic surgery by high-volume surgeons clearly is more efficient and safer, with lower total operative times and complication rates, than robotic surgery performed by lower-volume surgeons.8
Currency is a well-accepted safety standard in aviation, and pilots know the importance of frequent practice and repetition in the cockpit under real-world conditions.
Ensure annual competency
Although a pilot must accomplish a minimum number of flying hours each year to maintain certification, this does not ensure that passengers will be safe. Pilots also must prove their competence by undergoing periodic check rides and demonstrating their skills on flight simulators.
Surgeons also can use these models to verify competency. Proctors who are independently certified by the FDA or another government agency as examiners could observe and evaluate surgeons performing robotic surgery using standardized checklists and grading forms. If done locally, care must be taken to assure standardization, as local hospital politics could interfere.
The only other methods currently available to verify surgeon competency are to demonstrate proficiency on simulation and to review outcomes data, looking for outliers in important areas such as complications, robotic console times, total operative times, length of stay, etc.
Simulation offers a standardized, independent method to monitor competency.19 A passing test score on a robotic simulator exercise could be a way for a surgeon to prove his or her competency. Basic robotic skills such as camera control and clutching, energy use, and sewing and needle control can be practiced on a robotic simulator.
Virtual cases such as hysterectomy and myomectomy are not yet available on the simulator, nor are cases involving typical complications. These are being developed, however, and will be available shortly.
Several gynecologic resident and fellowship training programs are using simulation to train novice surgeons, and some community hospitals are using simulation as an annual requirement for all practicing surgeons to demonstrate proficiency, similar to pilots.8 Some newer validated training protocols require a surgeon to demonstrate mastery of a particular robotic skill by achieving passing scores at least five times, with at least two consecutive passing scores.20,21
As simulators evolve, they will continue to be incorporated into training, used for surgeon warm-up before surgery, as refreshers for surgeons after a period of robotic inactivity, and for annual recertification.
When robotic surgery leads to legal trouble
A recent medical malpractice case highlights the importance of having guidelines in place to protect patients. In Bremerton, Washington, in 2008,1 a urologist performed his first nonproctored robotic prostatectomy. The challenging and difficult procedure took more than 13 hours; he converted to an open procedure after 7 hours. The patient developed significant postoperative complications and died.1
In the litigation that followed, the surgeon was sued for negligence and for failing to disclose that this was his first solo robot-assisted surgery. The surgeon settled, as did the hospital, which was sued for not supervising the surgeon and failing to ensure that he could use the robot safely. The family also sued Intuitive Surgical, the manufacturer of the da Vinci Robot, for failing to provide adequate training to the surgeon.2
The jury ruled in favor of the manufacturer, stating that the verification of adequate surgeon training was the responsibility of the hospital and specialty medical societies, not the industry.
References
- Estate of Fred Taylor v. Intuitive Surgical Inc., 09-2-03136-5, Superior Court, State of Washington, Kitsap County (Port Orchard).
- Ostrom C. Failed robotic surgery focus of Kitsap trial. Seattle Times. http://seattletimes.com/html/localnews/2020918732_robottrialxml.html Published May 3, 2013. Accessed October 10, 2014.
A word to the wise
If hospital departments really want to ensure that they are doing all that they can to make robotic surgeries safe for their patients, they will utilize the recent guidelines approved by AAGL. In order for these guidelines to work, hospital systems need to commit resources for medical staff oversight, including a robotics peer-review committee with a physician chairman and adequate medical staff support to monitor physicians and manage those who cannot meet these goals.
There clearly will be push-back from surgeons who feel that it is unfair to restrict their ability to perform surgery just because their volumes are low or they can’t master the simulation exercises. However, in the final analysis, would we want the airlines to employ pilots who fly only a couple of times a year or who can’t master the required simulation skills to safely operate a commercial passenger jet?
The important question is, what is our focus? Is it to be “fair” to all surgeons, or is it to provide the best and safest outcomes for our patients? As surgeons, we each need to remember the oath we took when we became physicians to “First, do no harm.” By following these new AAGL robotic surgery guidelines, we will reassure our patients that we, as physicians, do take that oath seriously.
INSTANT POLL
For credentialing and privileging of robotic gynecologic surgery, do you agree that the following points are essential components of the process?
1. Surgeons should be selected for training who are most likely to be successful in performing robotic surgeries safely and efficiently.
2. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills.
3. Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Answer:
a. Yes, I agree.
b. No, I believe this approach is too restrictive.
c. No, I believe this approach is not restrictive enough.
To vote, please visit obgmanagement.com and look for “Quick Poll” on the right side of the homepage.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasive Gynecol. 2014;21(2):157–167.
2. Becnel Law Firm LLC. Bad Robot Surgery. http://badrobotsurgery.com. Accessed October 10, 2014.
3. Burton TM. Report raises concern on robotic surgery device. Wall Street Journal. http://online.wsj.com/news/articles/SB10001424052702304672404579186190568061568 Published November 8, 2013. Accessed October 10, 2014.
4. Rosero E, Kho K, Joshi G, Giesecke M, Schaffer J. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122(4):778–786.
5. Fuchs Weizman N, Cohen S, Manoucheri E, Wang K, Einarsson J. Surgical errors associated with robotic surgery in gynecology: a review of the FDA MAUDE database. J Minim Invasive Gynecol. 2013;20(6):S171.
6. Lee YL, Kilic G, Phelps J. Medicolegal review of liability risks for gynecologists stemming from lack of training in robotic assisted surgery. J Minim Invasive Gynecol. 2011;18(4):512–515.
7. Federal Aviation Administration. Pilot Regulations. http://www.faa.gov/pilots/regs/. Updated March 20, 2013. Accessed October 10, 2014.
8. Lenihan JP. Navigating credentialing, privileging, and learning curves in robotics with an evidence- and experience-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
9. Lenihan J, Kovanda C, Kreaden U. What is the learning curve for robotic Gyn surgery? J Minim Invasive Gynecol. 2008;15(5):589–594.
10. Payne T, Dauterive F. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286–291.
11. Woelk J, Casiano E, Weaver A, Gostout B, Trabuco E, Gebhart A. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–96.
12. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
13. Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
14. Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volumes on outcomes for laparoscopic hysterectomy for benign conditions. Obstet Gynecol. 2012;119(4):710–716.
15. Doll K, Milad M, Gossett D. Surgeon volume and outcomes in benign hysterectomy. J Minim Invasive Gynecol. 2013;20(5):554–561.
16. Jenison E, Gil K, Lendvay T, Guy M. Robotic surgical skills: acquisition, maintenance and degradation. JSLS. 2012;16(2):218–228.
17. Guseila L, Jenison E. Maintaining robotic surgical skills during periods of robotic inactivity. J Robotic Surg. 2014;8(3):261–268.
18. Tracy E, Zephyrin L, Rosman D, Berkowitz L. Credentialing based on surgical volume. Physician workforce challenges, and patient access. Obstet Gynecol. 2013;122(5):947–951.
19. Brand T. Madigan Protocol – Si Version. Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=17. Accessed October 10, 2014.
20. Culligan P, Salamon C. Validation of a robotic simulator: transferring simulator skills to the operating room. Validation of a robotic surgery simulator protocol—transfer of simulator skills to the operating room. Fem Pelvic Med Recon Surg. 2014;20(1):48–51.
21. Culligan P. Morristown Protocol (Morristown Memorial Hospital). Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=11. Accessed October 10, 2014.
The AAGL, formerly the American Association of Gynecologic Laparoscopists, has approved the first-ever set of privileging and credentialing guidelines for robotic surgery.1
Why has this prestigious minimally invasive surgery organization done that?
Maybe you’ve seen the Internet and TV ads and billboard trucks driving outside of many major medical society meetings recently, advertising “1-800-BAD-Robot.”2 You also are probably aware of recent articles in the headlines of national periodicals like the Wall Street Journal claiming that robotic surgery can be harmful.3
And yet, robotic gynecologic surgery has grown at an unprecedented rate since its approval by the US Food and Drug Administration (FDA) in April 2005. Recent data from the Nationwide Inpatient Sample from the Agency for Healthcare Research and Quality indicate that robot-assisted hysterectomies have increased at a dramatic rate.4 In a recent study of the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, investigators found that more than 30% of injuries during robotic surgery are related to operator error or robot failure, but the majority of problems are not associated with the technology.5
In this article, I use the aviation industry as an example of a sector that has gotten safety right. By emulating many of its standards, our specialty can make great strides toward patient safety and improved outcomes. I also outline the main points of the new AAGL guidelines and the rationale behind them.1 See, for example, the summary box on page 46.
A “shining example”
The robot clearly is an enabling technology. With its high-definition 3D vision and scaled motion with wristed instruments, surgeons are more comfortable performing many complex gynecologic procedures that previously would have required open surgery to safely accomplish … but the da Vinci Robot does not make a poor surgeon a great surgeon.
Hospitals now are being sued for allowing surgeons to perform robotic surgery on patients without documenting adequate surgeon training or providing consistent oversight.6 This new technology has outpaced the ability of hospital medical staffs to establish practice guidelines and rules to ensure patient safety.
The aviation industry is a shining example of a highly reliable industry. Each day, thousands of commercial aircraft fly all over the world with amazing safety. Most of the time, the pilot and copilot have never flown together. However, each crew member knows his or her role precisely and clearly understands what is expected. Crew members must meet standards that transcend all airlines and all aircraft.7 They all practice communication and undergo standardized training, including simulation, prior to taking off with live passengers on board.
In addition, all pilots must demonstrate their proficiency and competence on a regular basis—by exhibiting actual safe flight performance (over multiple takeoffs and landings) and undergoing check rides with flight examiners and practicing routine and emergency procedures on flight simulators. Airline passengers have come to expect that all pilots are equally proficient and safe. Shouldn’t patients be able to expect the same from their surgeons and hospitals? And yet there is no national or local organization that ensures that all surgeons are equally safe in the operating room. That responsibility is too often left up to the courts.
Three requirements of robotic credentialing
In 2008, the MultiCare Health System in the Pacific Northwest adopted a unique system of robotic credentialing that was based on the aviation model.8 This model has three main components, which are identical to the guidelines imposed on pilots:
- Surgeons selected for training should be likely to be successful in performing robotic surgeries safely and efficiently.
- Practice makes perfect. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills. The aviation world calls this concept “currency.”
- Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Adoption of these tried-and-true safety principles would ensure that hospitals exercise their responsibility to protect patients who undergo robotic surgeries in their systems.
The AAGL’s Robotics Special Interest Group, formed in 2010, is now the largest special interest group in the organization. The group was initially tasked to develop evidence-based guidelines for robotic surgery training and credentialing. Using the aviation industry’s model, the group developed a basic template of robotic surgery credentialing and privileging guidelines that can be used anywhere in the world. This proposal is not meant to be a standard-of-care definition; rather, it is intended simply as a starting point.
Key components of new AAGL robotic surgery credentialing and privileging guidelines1
Initial training
- Train only surgeons who have an adequate case volume to get through the learning curve. Recommended: at least 20 major cases per year.
- Current training pathways include computer-based learning, case observations, pig labs, simulation, and proctored cases. More intense validated simulation training could replace pig labs.
- Surgeons should initially perform only simple, basic procedures with surgeon first-assists until they develop the necessary skills to safely operate the robotic console and start performing more complex cases.
Annual currency
- Surgeons should perform at least 20 major cases per year, with at least one case every 8 weeks.
- If surgeons operate less frequently, proficiency should be verified on a simulator before operation on a live patient.
Annual recertification
- All surgeons should demonstrate competency annually on a simulator, regardless of case volume.
Initial training involves a long learning curve
There is a long learning curve for surgeons to become competent in robotic surgery. In initial studies of experienced advanced laparoscopic surgeons, investigators found that learning curves could involve 50 cases or more.9,10 In a recent study of gynecologic oncologists and urogynecologists at the Mayo Clinic, researchers found that it took 91 cases for experienced surgeons to become proficient on the robot.11
ObGyns in the United States are doing fewer hysterectomies than they used to.12 Many surgeons now perform fewer than 10 hysterectomies per year. These surgeons clearly have worse outcomes than surgeons who operate more frequently.13–15 Therefore, these new guidelines suggest that hospitals should choose to train only surgeons who have a case volume that will allow them to get through their learning curve in a short time and continue to have enough surgeries to maintain their skills. These guidelines recommend that surgeons who are candidates for robotic surgery training already perform a minimum of 20 major gynecologic operations per year.
It is important to learn to walk before you run. New student pilots start out with single-engine propeller planes before graduating to multi-engine props, jets, and commercial aircraft. Similarly, new medical students start out with easy surgical tasks before training for more complex procedures. This approach seems like common sense, although many surgeons may feel that, after orienting on the robot, they can start doing complex cases right away, as the robot enables them to do better and more precise surgery. Nothing could be further from the truth.
It is very important that new robotic surgeons start with easy, basic cases to completely familiarize themselves with the operation of the robot console before attempting more complex and difficult cases.
There is no absolute number of cases that ensures competency with the robot; the number depends on the surgeon’s case load, surgical prowess, and psychomotor skills. A surgeon should be restricted to simple cases initially, and should have an experienced robot-credentialed surgeon operating with him or her during this initial learning period.
Practice makes perfect
Musicians will tell you that the more often you practice, the more skilled you become. This is true for anyone whose job requires special training. It would be naïve to assume that surgeons can maintain optimal skills for robotic surgery by performing only a few cases each year.
Psychomotor skill degradation has been explored in relation to various surgical skills. The more complex the skill, the more likely that skill set will deteriorate without use. In recent studies, investigators have shown that robotic surgery skills begin to decline significantly after only 2 weeks of inactivity, and that skills continue to degrade without use.16,17
Based on this information, the currency requirement for surgeons to maintain privileges was set at 20 cases per year—fewer than two cases per month. Although the members of the Robotics Special Interest Group strongly agree that
maintenance of privileges should not be based entirely on an arbitrary currency number, as Tracy and colleagues also argue in a recent publication,18 it is clear that frequent performance of robotic surgery by high-volume surgeons clearly is more efficient and safer, with lower total operative times and complication rates, than robotic surgery performed by lower-volume surgeons.8
Currency is a well-accepted safety standard in aviation, and pilots know the importance of frequent practice and repetition in the cockpit under real-world conditions.
Ensure annual competency
Although a pilot must accomplish a minimum number of flying hours each year to maintain certification, this does not ensure that passengers will be safe. Pilots also must prove their competence by undergoing periodic check rides and demonstrating their skills on flight simulators.
Surgeons also can use these models to verify competency. Proctors who are independently certified by the FDA or another government agency as examiners could observe and evaluate surgeons performing robotic surgery using standardized checklists and grading forms. If done locally, care must be taken to assure standardization, as local hospital politics could interfere.
The only other methods currently available to verify surgeon competency are to demonstrate proficiency on simulation and to review outcomes data, looking for outliers in important areas such as complications, robotic console times, total operative times, length of stay, etc.
Simulation offers a standardized, independent method to monitor competency.19 A passing test score on a robotic simulator exercise could be a way for a surgeon to prove his or her competency. Basic robotic skills such as camera control and clutching, energy use, and sewing and needle control can be practiced on a robotic simulator.
Virtual cases such as hysterectomy and myomectomy are not yet available on the simulator, nor are cases involving typical complications. These are being developed, however, and will be available shortly.
Several gynecologic resident and fellowship training programs are using simulation to train novice surgeons, and some community hospitals are using simulation as an annual requirement for all practicing surgeons to demonstrate proficiency, similar to pilots.8 Some newer validated training protocols require a surgeon to demonstrate mastery of a particular robotic skill by achieving passing scores at least five times, with at least two consecutive passing scores.20,21
As simulators evolve, they will continue to be incorporated into training, used for surgeon warm-up before surgery, as refreshers for surgeons after a period of robotic inactivity, and for annual recertification.
When robotic surgery leads to legal trouble
A recent medical malpractice case highlights the importance of having guidelines in place to protect patients. In Bremerton, Washington, in 2008,1 a urologist performed his first nonproctored robotic prostatectomy. The challenging and difficult procedure took more than 13 hours; he converted to an open procedure after 7 hours. The patient developed significant postoperative complications and died.1
In the litigation that followed, the surgeon was sued for negligence and for failing to disclose that this was his first solo robot-assisted surgery. The surgeon settled, as did the hospital, which was sued for not supervising the surgeon and failing to ensure that he could use the robot safely. The family also sued Intuitive Surgical, the manufacturer of the da Vinci Robot, for failing to provide adequate training to the surgeon.2
The jury ruled in favor of the manufacturer, stating that the verification of adequate surgeon training was the responsibility of the hospital and specialty medical societies, not the industry.
References
- Estate of Fred Taylor v. Intuitive Surgical Inc., 09-2-03136-5, Superior Court, State of Washington, Kitsap County (Port Orchard).
- Ostrom C. Failed robotic surgery focus of Kitsap trial. Seattle Times. http://seattletimes.com/html/localnews/2020918732_robottrialxml.html Published May 3, 2013. Accessed October 10, 2014.
A word to the wise
If hospital departments really want to ensure that they are doing all that they can to make robotic surgeries safe for their patients, they will utilize the recent guidelines approved by AAGL. In order for these guidelines to work, hospital systems need to commit resources for medical staff oversight, including a robotics peer-review committee with a physician chairman and adequate medical staff support to monitor physicians and manage those who cannot meet these goals.
There clearly will be push-back from surgeons who feel that it is unfair to restrict their ability to perform surgery just because their volumes are low or they can’t master the simulation exercises. However, in the final analysis, would we want the airlines to employ pilots who fly only a couple of times a year or who can’t master the required simulation skills to safely operate a commercial passenger jet?
The important question is, what is our focus? Is it to be “fair” to all surgeons, or is it to provide the best and safest outcomes for our patients? As surgeons, we each need to remember the oath we took when we became physicians to “First, do no harm.” By following these new AAGL robotic surgery guidelines, we will reassure our patients that we, as physicians, do take that oath seriously.
INSTANT POLL
For credentialing and privileging of robotic gynecologic surgery, do you agree that the following points are essential components of the process?
1. Surgeons should be selected for training who are most likely to be successful in performing robotic surgeries safely and efficiently.
2. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills.
3. Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Answer:
a. Yes, I agree.
b. No, I believe this approach is too restrictive.
c. No, I believe this approach is not restrictive enough.
To vote, please visit obgmanagement.com and look for “Quick Poll” on the right side of the homepage.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The AAGL, formerly the American Association of Gynecologic Laparoscopists, has approved the first-ever set of privileging and credentialing guidelines for robotic surgery.1
Why has this prestigious minimally invasive surgery organization done that?
Maybe you’ve seen the Internet and TV ads and billboard trucks driving outside of many major medical society meetings recently, advertising “1-800-BAD-Robot.”2 You also are probably aware of recent articles in the headlines of national periodicals like the Wall Street Journal claiming that robotic surgery can be harmful.3
And yet, robotic gynecologic surgery has grown at an unprecedented rate since its approval by the US Food and Drug Administration (FDA) in April 2005. Recent data from the Nationwide Inpatient Sample from the Agency for Healthcare Research and Quality indicate that robot-assisted hysterectomies have increased at a dramatic rate.4 In a recent study of the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, investigators found that more than 30% of injuries during robotic surgery are related to operator error or robot failure, but the majority of problems are not associated with the technology.5
In this article, I use the aviation industry as an example of a sector that has gotten safety right. By emulating many of its standards, our specialty can make great strides toward patient safety and improved outcomes. I also outline the main points of the new AAGL guidelines and the rationale behind them.1 See, for example, the summary box on page 46.
A “shining example”
The robot clearly is an enabling technology. With its high-definition 3D vision and scaled motion with wristed instruments, surgeons are more comfortable performing many complex gynecologic procedures that previously would have required open surgery to safely accomplish … but the da Vinci Robot does not make a poor surgeon a great surgeon.
Hospitals now are being sued for allowing surgeons to perform robotic surgery on patients without documenting adequate surgeon training or providing consistent oversight.6 This new technology has outpaced the ability of hospital medical staffs to establish practice guidelines and rules to ensure patient safety.
The aviation industry is a shining example of a highly reliable industry. Each day, thousands of commercial aircraft fly all over the world with amazing safety. Most of the time, the pilot and copilot have never flown together. However, each crew member knows his or her role precisely and clearly understands what is expected. Crew members must meet standards that transcend all airlines and all aircraft.7 They all practice communication and undergo standardized training, including simulation, prior to taking off with live passengers on board.
In addition, all pilots must demonstrate their proficiency and competence on a regular basis—by exhibiting actual safe flight performance (over multiple takeoffs and landings) and undergoing check rides with flight examiners and practicing routine and emergency procedures on flight simulators. Airline passengers have come to expect that all pilots are equally proficient and safe. Shouldn’t patients be able to expect the same from their surgeons and hospitals? And yet there is no national or local organization that ensures that all surgeons are equally safe in the operating room. That responsibility is too often left up to the courts.
Three requirements of robotic credentialing
In 2008, the MultiCare Health System in the Pacific Northwest adopted a unique system of robotic credentialing that was based on the aviation model.8 This model has three main components, which are identical to the guidelines imposed on pilots:
- Surgeons selected for training should be likely to be successful in performing robotic surgeries safely and efficiently.
- Practice makes perfect. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills. The aviation world calls this concept “currency.”
- Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Adoption of these tried-and-true safety principles would ensure that hospitals exercise their responsibility to protect patients who undergo robotic surgeries in their systems.
The AAGL’s Robotics Special Interest Group, formed in 2010, is now the largest special interest group in the organization. The group was initially tasked to develop evidence-based guidelines for robotic surgery training and credentialing. Using the aviation industry’s model, the group developed a basic template of robotic surgery credentialing and privileging guidelines that can be used anywhere in the world. This proposal is not meant to be a standard-of-care definition; rather, it is intended simply as a starting point.
Key components of new AAGL robotic surgery credentialing and privileging guidelines1
Initial training
- Train only surgeons who have an adequate case volume to get through the learning curve. Recommended: at least 20 major cases per year.
- Current training pathways include computer-based learning, case observations, pig labs, simulation, and proctored cases. More intense validated simulation training could replace pig labs.
- Surgeons should initially perform only simple, basic procedures with surgeon first-assists until they develop the necessary skills to safely operate the robotic console and start performing more complex cases.
Annual currency
- Surgeons should perform at least 20 major cases per year, with at least one case every 8 weeks.
- If surgeons operate less frequently, proficiency should be verified on a simulator before operation on a live patient.
Annual recertification
- All surgeons should demonstrate competency annually on a simulator, regardless of case volume.
Initial training involves a long learning curve
There is a long learning curve for surgeons to become competent in robotic surgery. In initial studies of experienced advanced laparoscopic surgeons, investigators found that learning curves could involve 50 cases or more.9,10 In a recent study of gynecologic oncologists and urogynecologists at the Mayo Clinic, researchers found that it took 91 cases for experienced surgeons to become proficient on the robot.11
ObGyns in the United States are doing fewer hysterectomies than they used to.12 Many surgeons now perform fewer than 10 hysterectomies per year. These surgeons clearly have worse outcomes than surgeons who operate more frequently.13–15 Therefore, these new guidelines suggest that hospitals should choose to train only surgeons who have a case volume that will allow them to get through their learning curve in a short time and continue to have enough surgeries to maintain their skills. These guidelines recommend that surgeons who are candidates for robotic surgery training already perform a minimum of 20 major gynecologic operations per year.
It is important to learn to walk before you run. New student pilots start out with single-engine propeller planes before graduating to multi-engine props, jets, and commercial aircraft. Similarly, new medical students start out with easy surgical tasks before training for more complex procedures. This approach seems like common sense, although many surgeons may feel that, after orienting on the robot, they can start doing complex cases right away, as the robot enables them to do better and more precise surgery. Nothing could be further from the truth.
It is very important that new robotic surgeons start with easy, basic cases to completely familiarize themselves with the operation of the robot console before attempting more complex and difficult cases.
There is no absolute number of cases that ensures competency with the robot; the number depends on the surgeon’s case load, surgical prowess, and psychomotor skills. A surgeon should be restricted to simple cases initially, and should have an experienced robot-credentialed surgeon operating with him or her during this initial learning period.
Practice makes perfect
Musicians will tell you that the more often you practice, the more skilled you become. This is true for anyone whose job requires special training. It would be naïve to assume that surgeons can maintain optimal skills for robotic surgery by performing only a few cases each year.
Psychomotor skill degradation has been explored in relation to various surgical skills. The more complex the skill, the more likely that skill set will deteriorate without use. In recent studies, investigators have shown that robotic surgery skills begin to decline significantly after only 2 weeks of inactivity, and that skills continue to degrade without use.16,17
Based on this information, the currency requirement for surgeons to maintain privileges was set at 20 cases per year—fewer than two cases per month. Although the members of the Robotics Special Interest Group strongly agree that
maintenance of privileges should not be based entirely on an arbitrary currency number, as Tracy and colleagues also argue in a recent publication,18 it is clear that frequent performance of robotic surgery by high-volume surgeons clearly is more efficient and safer, with lower total operative times and complication rates, than robotic surgery performed by lower-volume surgeons.8
Currency is a well-accepted safety standard in aviation, and pilots know the importance of frequent practice and repetition in the cockpit under real-world conditions.
Ensure annual competency
Although a pilot must accomplish a minimum number of flying hours each year to maintain certification, this does not ensure that passengers will be safe. Pilots also must prove their competence by undergoing periodic check rides and demonstrating their skills on flight simulators.
Surgeons also can use these models to verify competency. Proctors who are independently certified by the FDA or another government agency as examiners could observe and evaluate surgeons performing robotic surgery using standardized checklists and grading forms. If done locally, care must be taken to assure standardization, as local hospital politics could interfere.
The only other methods currently available to verify surgeon competency are to demonstrate proficiency on simulation and to review outcomes data, looking for outliers in important areas such as complications, robotic console times, total operative times, length of stay, etc.
Simulation offers a standardized, independent method to monitor competency.19 A passing test score on a robotic simulator exercise could be a way for a surgeon to prove his or her competency. Basic robotic skills such as camera control and clutching, energy use, and sewing and needle control can be practiced on a robotic simulator.
Virtual cases such as hysterectomy and myomectomy are not yet available on the simulator, nor are cases involving typical complications. These are being developed, however, and will be available shortly.
Several gynecologic resident and fellowship training programs are using simulation to train novice surgeons, and some community hospitals are using simulation as an annual requirement for all practicing surgeons to demonstrate proficiency, similar to pilots.8 Some newer validated training protocols require a surgeon to demonstrate mastery of a particular robotic skill by achieving passing scores at least five times, with at least two consecutive passing scores.20,21
As simulators evolve, they will continue to be incorporated into training, used for surgeon warm-up before surgery, as refreshers for surgeons after a period of robotic inactivity, and for annual recertification.
When robotic surgery leads to legal trouble
A recent medical malpractice case highlights the importance of having guidelines in place to protect patients. In Bremerton, Washington, in 2008,1 a urologist performed his first nonproctored robotic prostatectomy. The challenging and difficult procedure took more than 13 hours; he converted to an open procedure after 7 hours. The patient developed significant postoperative complications and died.1
In the litigation that followed, the surgeon was sued for negligence and for failing to disclose that this was his first solo robot-assisted surgery. The surgeon settled, as did the hospital, which was sued for not supervising the surgeon and failing to ensure that he could use the robot safely. The family also sued Intuitive Surgical, the manufacturer of the da Vinci Robot, for failing to provide adequate training to the surgeon.2
The jury ruled in favor of the manufacturer, stating that the verification of adequate surgeon training was the responsibility of the hospital and specialty medical societies, not the industry.
References
- Estate of Fred Taylor v. Intuitive Surgical Inc., 09-2-03136-5, Superior Court, State of Washington, Kitsap County (Port Orchard).
- Ostrom C. Failed robotic surgery focus of Kitsap trial. Seattle Times. http://seattletimes.com/html/localnews/2020918732_robottrialxml.html Published May 3, 2013. Accessed October 10, 2014.
A word to the wise
If hospital departments really want to ensure that they are doing all that they can to make robotic surgeries safe for their patients, they will utilize the recent guidelines approved by AAGL. In order for these guidelines to work, hospital systems need to commit resources for medical staff oversight, including a robotics peer-review committee with a physician chairman and adequate medical staff support to monitor physicians and manage those who cannot meet these goals.
There clearly will be push-back from surgeons who feel that it is unfair to restrict their ability to perform surgery just because their volumes are low or they can’t master the simulation exercises. However, in the final analysis, would we want the airlines to employ pilots who fly only a couple of times a year or who can’t master the required simulation skills to safely operate a commercial passenger jet?
The important question is, what is our focus? Is it to be “fair” to all surgeons, or is it to provide the best and safest outcomes for our patients? As surgeons, we each need to remember the oath we took when we became physicians to “First, do no harm.” By following these new AAGL robotic surgery guidelines, we will reassure our patients that we, as physicians, do take that oath seriously.
INSTANT POLL
For credentialing and privileging of robotic gynecologic surgery, do you agree that the following points are essential components of the process?
1. Surgeons should be selected for training who are most likely to be successful in performing robotic surgeries safely and efficiently.
2. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills.
3. Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Answer:
a. Yes, I agree.
b. No, I believe this approach is too restrictive.
c. No, I believe this approach is not restrictive enough.
To vote, please visit obgmanagement.com and look for “Quick Poll” on the right side of the homepage.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasive Gynecol. 2014;21(2):157–167.
2. Becnel Law Firm LLC. Bad Robot Surgery. http://badrobotsurgery.com. Accessed October 10, 2014.
3. Burton TM. Report raises concern on robotic surgery device. Wall Street Journal. http://online.wsj.com/news/articles/SB10001424052702304672404579186190568061568 Published November 8, 2013. Accessed October 10, 2014.
4. Rosero E, Kho K, Joshi G, Giesecke M, Schaffer J. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122(4):778–786.
5. Fuchs Weizman N, Cohen S, Manoucheri E, Wang K, Einarsson J. Surgical errors associated with robotic surgery in gynecology: a review of the FDA MAUDE database. J Minim Invasive Gynecol. 2013;20(6):S171.
6. Lee YL, Kilic G, Phelps J. Medicolegal review of liability risks for gynecologists stemming from lack of training in robotic assisted surgery. J Minim Invasive Gynecol. 2011;18(4):512–515.
7. Federal Aviation Administration. Pilot Regulations. http://www.faa.gov/pilots/regs/. Updated March 20, 2013. Accessed October 10, 2014.
8. Lenihan JP. Navigating credentialing, privileging, and learning curves in robotics with an evidence- and experience-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
9. Lenihan J, Kovanda C, Kreaden U. What is the learning curve for robotic Gyn surgery? J Minim Invasive Gynecol. 2008;15(5):589–594.
10. Payne T, Dauterive F. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286–291.
11. Woelk J, Casiano E, Weaver A, Gostout B, Trabuco E, Gebhart A. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–96.
12. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
13. Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
14. Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volumes on outcomes for laparoscopic hysterectomy for benign conditions. Obstet Gynecol. 2012;119(4):710–716.
15. Doll K, Milad M, Gossett D. Surgeon volume and outcomes in benign hysterectomy. J Minim Invasive Gynecol. 2013;20(5):554–561.
16. Jenison E, Gil K, Lendvay T, Guy M. Robotic surgical skills: acquisition, maintenance and degradation. JSLS. 2012;16(2):218–228.
17. Guseila L, Jenison E. Maintaining robotic surgical skills during periods of robotic inactivity. J Robotic Surg. 2014;8(3):261–268.
18. Tracy E, Zephyrin L, Rosman D, Berkowitz L. Credentialing based on surgical volume. Physician workforce challenges, and patient access. Obstet Gynecol. 2013;122(5):947–951.
19. Brand T. Madigan Protocol – Si Version. Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=17. Accessed October 10, 2014.
20. Culligan P, Salamon C. Validation of a robotic simulator: transferring simulator skills to the operating room. Validation of a robotic surgery simulator protocol—transfer of simulator skills to the operating room. Fem Pelvic Med Recon Surg. 2014;20(1):48–51.
21. Culligan P. Morristown Protocol (Morristown Memorial Hospital). Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=11. Accessed October 10, 2014.
1. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasive Gynecol. 2014;21(2):157–167.
2. Becnel Law Firm LLC. Bad Robot Surgery. http://badrobotsurgery.com. Accessed October 10, 2014.
3. Burton TM. Report raises concern on robotic surgery device. Wall Street Journal. http://online.wsj.com/news/articles/SB10001424052702304672404579186190568061568 Published November 8, 2013. Accessed October 10, 2014.
4. Rosero E, Kho K, Joshi G, Giesecke M, Schaffer J. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122(4):778–786.
5. Fuchs Weizman N, Cohen S, Manoucheri E, Wang K, Einarsson J. Surgical errors associated with robotic surgery in gynecology: a review of the FDA MAUDE database. J Minim Invasive Gynecol. 2013;20(6):S171.
6. Lee YL, Kilic G, Phelps J. Medicolegal review of liability risks for gynecologists stemming from lack of training in robotic assisted surgery. J Minim Invasive Gynecol. 2011;18(4):512–515.
7. Federal Aviation Administration. Pilot Regulations. http://www.faa.gov/pilots/regs/. Updated March 20, 2013. Accessed October 10, 2014.
8. Lenihan JP. Navigating credentialing, privileging, and learning curves in robotics with an evidence- and experience-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
9. Lenihan J, Kovanda C, Kreaden U. What is the learning curve for robotic Gyn surgery? J Minim Invasive Gynecol. 2008;15(5):589–594.
10. Payne T, Dauterive F. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286–291.
11. Woelk J, Casiano E, Weaver A, Gostout B, Trabuco E, Gebhart A. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–96.
12. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
13. Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
14. Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volumes on outcomes for laparoscopic hysterectomy for benign conditions. Obstet Gynecol. 2012;119(4):710–716.
15. Doll K, Milad M, Gossett D. Surgeon volume and outcomes in benign hysterectomy. J Minim Invasive Gynecol. 2013;20(5):554–561.
16. Jenison E, Gil K, Lendvay T, Guy M. Robotic surgical skills: acquisition, maintenance and degradation. JSLS. 2012;16(2):218–228.
17. Guseila L, Jenison E. Maintaining robotic surgical skills during periods of robotic inactivity. J Robotic Surg. 2014;8(3):261–268.
18. Tracy E, Zephyrin L, Rosman D, Berkowitz L. Credentialing based on surgical volume. Physician workforce challenges, and patient access. Obstet Gynecol. 2013;122(5):947–951.
19. Brand T. Madigan Protocol – Si Version. Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=17. Accessed October 10, 2014.
20. Culligan P, Salamon C. Validation of a robotic simulator: transferring simulator skills to the operating room. Validation of a robotic surgery simulator protocol—transfer of simulator skills to the operating room. Fem Pelvic Med Recon Surg. 2014;20(1):48–51.
21. Culligan P. Morristown Protocol (Morristown Memorial Hospital). Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=11. Accessed October 10, 2014.