User login
Can prophylactic salpingectomies be achieved with the vaginal approach?
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique
Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique
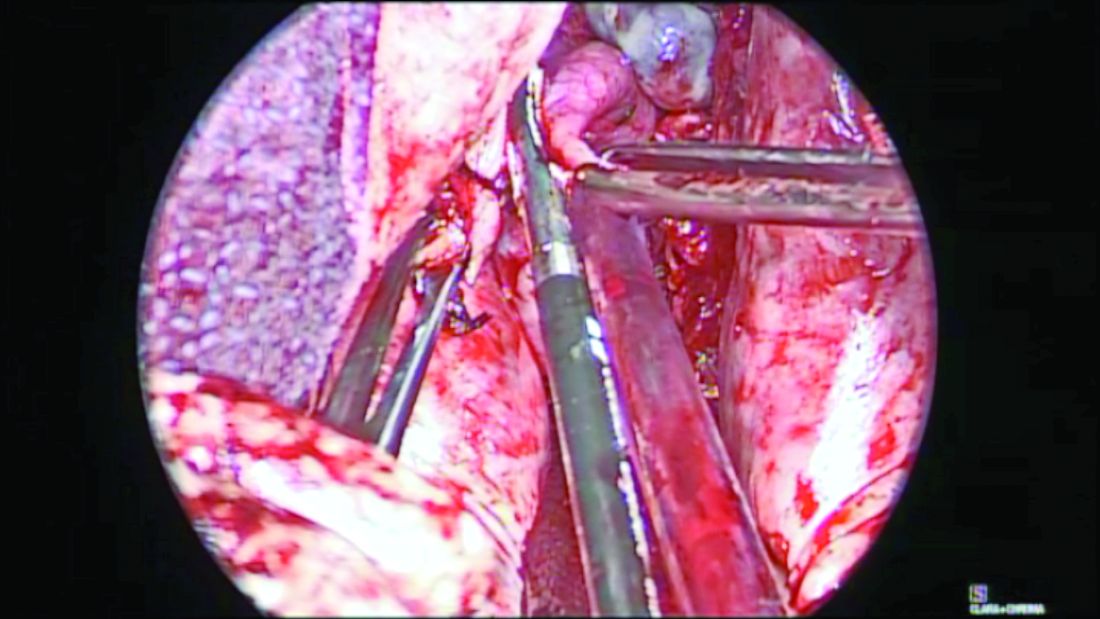
When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

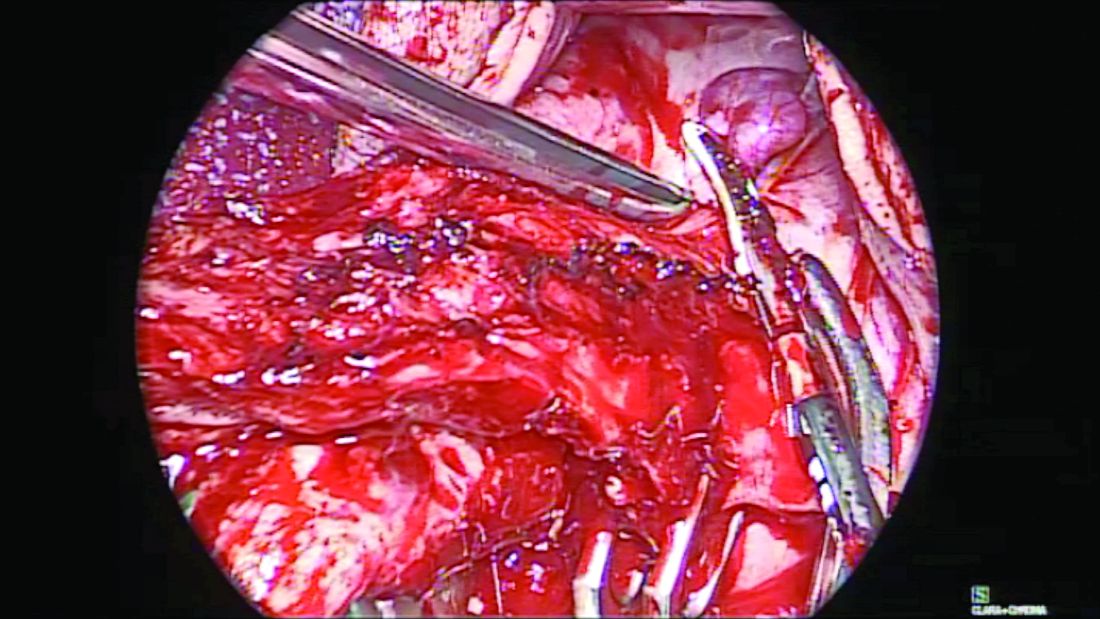
When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique

Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique

When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique

Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique

When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
Highlights from the 2018 Society of Gynecologic Surgeons Scientific Meeting
PART 1
- Leading best gynecologic surgical care into the next decade
- Optimal surgical management of stage 3 and 4 pelvic organ prolapse
- Patient experience: It’s not about satisfaction
Andrew P. Cassidenti, MD
Chief, Female Pelvic Medicine and Reconstructive Surgery
Kern Medical,
Bakersfield, California
Amanda White, MD
Assistant Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Vivian Aguilar, MD
Assistant Professor, Obstetrics and Gynecology
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Rebecca G. Rogers, MD
Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Associate Chair, Clinical Integration and Operations
Dell Medical School, University of Texas
Austin, Texas
Patrick Culligan, MD
Director, Urogynecology and The Center for Female Pelvic Health
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Sarah Huber, MD
Fellow, Female Pelvic Medicine and Reconstructive Surgery
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Vincent R. Lucente, MD, MBA
Chief, Gynecology, St. Luke’s University Health Network
Medical Director, The Institute for Female Pelvic Medicine and Reconstructive Surgery
Allentown, Pennsylvania
Jessica B. Ton, MD
AAGL Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
Bethlehem, Pennsylvania
James I. Merlino, MD
President and Chief Medical Officer of Advisory and Strategic Consulting
Press Ganey Associates
Cleveland, Ohio
Amy A. Merlino, MD
Maternal Fetal Medicine Specialist
Department of Obstetrics and Gynecology
Enterprise Chief Informatics Officer
Cleveland Clinic, Cleveland, Ohio
PART 2
- Deep infiltrating endometriosis: Evaluation and management
- What’s new in simulation training for hysterectomy
Rosanne M. Kho, MD
Head, Section of Benign Gynecology
Women’s Health Institute
Department of Obstetrics and Gynecology
Cleveland Clinic
Cleveland, Ohio
Mauricio S. Abrão, MD
Associate Professor and
Director, Endometriosis Division
Department of Obstetrics and Gynecology
São Paulo University Medical School
São Paulo, Brazil
Alicia Scribner, MD, MPH
Director, Ob/Gyn Simulation Curriculum
Madigan Army Medical Center
Tacoma, Washington
Clinical Instructor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Christine Vaccaro, DO
Medical Director, Andersen Simulation Center
Madigan Army Medical Center
Tacoma, Washington
Clinical Assistant Professor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Uniformed Services University of Health Sciences
Bethesda, Maryland
PART 1
- Leading best gynecologic surgical care into the next decade
- Optimal surgical management of stage 3 and 4 pelvic organ prolapse
- Patient experience: It’s not about satisfaction
Andrew P. Cassidenti, MD
Chief, Female Pelvic Medicine and Reconstructive Surgery
Kern Medical,
Bakersfield, California
Amanda White, MD
Assistant Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Vivian Aguilar, MD
Assistant Professor, Obstetrics and Gynecology
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Rebecca G. Rogers, MD
Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Associate Chair, Clinical Integration and Operations
Dell Medical School, University of Texas
Austin, Texas
Patrick Culligan, MD
Director, Urogynecology and The Center for Female Pelvic Health
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Sarah Huber, MD
Fellow, Female Pelvic Medicine and Reconstructive Surgery
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Vincent R. Lucente, MD, MBA
Chief, Gynecology, St. Luke’s University Health Network
Medical Director, The Institute for Female Pelvic Medicine and Reconstructive Surgery
Allentown, Pennsylvania
Jessica B. Ton, MD
AAGL Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
Bethlehem, Pennsylvania
James I. Merlino, MD
President and Chief Medical Officer of Advisory and Strategic Consulting
Press Ganey Associates
Cleveland, Ohio
Amy A. Merlino, MD
Maternal Fetal Medicine Specialist
Department of Obstetrics and Gynecology
Enterprise Chief Informatics Officer
Cleveland Clinic, Cleveland, Ohio
PART 2
- Deep infiltrating endometriosis: Evaluation and management
- What’s new in simulation training for hysterectomy
Rosanne M. Kho, MD
Head, Section of Benign Gynecology
Women’s Health Institute
Department of Obstetrics and Gynecology
Cleveland Clinic
Cleveland, Ohio
Mauricio S. Abrão, MD
Associate Professor and
Director, Endometriosis Division
Department of Obstetrics and Gynecology
São Paulo University Medical School
São Paulo, Brazil
Alicia Scribner, MD, MPH
Director, Ob/Gyn Simulation Curriculum
Madigan Army Medical Center
Tacoma, Washington
Clinical Instructor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Christine Vaccaro, DO
Medical Director, Andersen Simulation Center
Madigan Army Medical Center
Tacoma, Washington
Clinical Assistant Professor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Uniformed Services University of Health Sciences
Bethesda, Maryland
PART 1
- Leading best gynecologic surgical care into the next decade
- Optimal surgical management of stage 3 and 4 pelvic organ prolapse
- Patient experience: It’s not about satisfaction
Andrew P. Cassidenti, MD
Chief, Female Pelvic Medicine and Reconstructive Surgery
Kern Medical,
Bakersfield, California
Amanda White, MD
Assistant Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Vivian Aguilar, MD
Assistant Professor, Obstetrics and Gynecology
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Rebecca G. Rogers, MD
Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Associate Chair, Clinical Integration and Operations
Dell Medical School, University of Texas
Austin, Texas
Patrick Culligan, MD
Director, Urogynecology and The Center for Female Pelvic Health
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Sarah Huber, MD
Fellow, Female Pelvic Medicine and Reconstructive Surgery
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Vincent R. Lucente, MD, MBA
Chief, Gynecology, St. Luke’s University Health Network
Medical Director, The Institute for Female Pelvic Medicine and Reconstructive Surgery
Allentown, Pennsylvania
Jessica B. Ton, MD
AAGL Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
Bethlehem, Pennsylvania
James I. Merlino, MD
President and Chief Medical Officer of Advisory and Strategic Consulting
Press Ganey Associates
Cleveland, Ohio
Amy A. Merlino, MD
Maternal Fetal Medicine Specialist
Department of Obstetrics and Gynecology
Enterprise Chief Informatics Officer
Cleveland Clinic, Cleveland, Ohio
PART 2
- Deep infiltrating endometriosis: Evaluation and management
- What’s new in simulation training for hysterectomy
Rosanne M. Kho, MD
Head, Section of Benign Gynecology
Women’s Health Institute
Department of Obstetrics and Gynecology
Cleveland Clinic
Cleveland, Ohio
Mauricio S. Abrão, MD
Associate Professor and
Director, Endometriosis Division
Department of Obstetrics and Gynecology
São Paulo University Medical School
São Paulo, Brazil
Alicia Scribner, MD, MPH
Director, Ob/Gyn Simulation Curriculum
Madigan Army Medical Center
Tacoma, Washington
Clinical Instructor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Christine Vaccaro, DO
Medical Director, Andersen Simulation Center
Madigan Army Medical Center
Tacoma, Washington
Clinical Assistant Professor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Uniformed Services University of Health Sciences
Bethesda, Maryland
Deep infiltrating endometriosis: Evaluation and management
Endometriosis affects up to 10% of women of reproductive age or, conservatively, about 6.5 million women in the United States.1,2 There are 3 types of endometriosis—superficial, ovarian, and deep—and in the past each of these was assumed to have a distinct pathogenesis.3 Deep infiltrating endometriosis (DIE) is the presence of one or more endometriotic nodules deeper than 5 mm. In a study at a large tertiary-care center, 40% of patients with endometriosis had deep disease.4 DIE is associated with more severe pain and infertility.5 In patients with endometriosis, diagnosis is commonly made 7 to 9 years after the initial pelvic pain presentation.6 For these reasons, well-directed history taking and proper evaluation and treatment should be pursued to relieve pain and optimize outcomes.
CASE Young woman with intensifying pelvic pain
Mary is a 26-year-old social worker who presents to her ObGyn with symptoms of worsening pain during as well as outside her periods. What additional information would you want to obtain from Mary, given her chief symptom of pain?
Investigate the type of pain
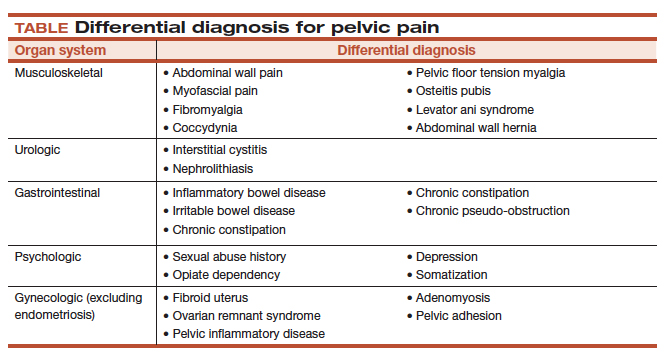
It is important to ask the patient about her menstrual and sexual history, her thoughts regarding near- and long-term fertility, and the type and severity of her pain symptoms. The 5 pain symptoms specific to pelvic pain are dysmenorrhea, dyspareunia, dysuria, dyschezia, and noncyclic pelvic pain. A visual analog scale (VAS) for pain as well as pelvic pain questionnaires can be used to guide evaluation options and monitor treatment outcomes. In addition, it is of paramount importance to understand the differential diagnoses that can present as pelvic pain (TABLE).
CASE Continued: Mary’s history
Mary reports that she always has had painful periods and that she was started on oral contraceptive pills for pain control and regulation of her periods soon after the onset of menses, when she was 12 years old. In college, she was prescribed oral contraceptive pills for contraception. Recently engaged, she is interested in becoming pregnant in 3 years.
A year ago, Mary discontinued the pills because of their adverse effects. Now she has severe pain during (VAS score, 8/10) and outside (VAS score, 7) her monthly periods. Because of this pain, she has taken time off from work twice within the past 6 months. She has pain during intercourse (VAS score, 7) and some pain with bowel movements during her menses (VAS score, 4). Pelvic examination reveals a normal-sized uterus and adnexa as well as a tender nodule in the rectovaginal septum.
What diagnostic tests and imaging would you obtain?
Imaging’s role in diagnosis
At many advanced centers for endometriosis, DIE is successfully diagnosed with specific magnetic resonance imaging (MRI) or transvaginal ultrasound (TVUS) protocols. In a recent review, MRI’s pooled sensitivity and specificity for rectosigmoid endometriosis were 92% and 96%, respectively.7 Choice of imaging for DIE depends on the skills and experience of the clinicians at each center. At a large referral center in São Paulo, Brazil, TVUS with bowel preparation had better sensitivity and specificity for deep retrocervical and rectosigmoid disease compared with MRI and digital pelvic examination.8 In addition, at a center in the United States, we found that proficiency in performing TVUS for DIE was achieved after 70 to 75 cases, and the exam took an average of only 20 minutes.9
Despite recent advances in imaging, most gynecologic societies still hold that endometriosis is to be definitively diagnosed with histologic confirmation from tissue biopsies during surgery. Although surgery remains the diagnostic gold standard, it does not mean that all patients with pelvic pain should undergo diagnostic laparoscopy with tissue biopsies.
The combination of compelling clinical signs, symptoms, and imaging findings (such as absence of findings for ovarian and deep endometriosis) can be used to make a presumptive nonsurgical (that is, clinical) diagnosis of endometriosis. Major societies recommend empiric medical therapy (for example, combination oral contraceptives) for the pain associated with superficial endometriosis.10,11 When there is no response to treatment, or when a patient declines or has contraindications to medical therapy, diagnostic laparoscopy with excision of endometriosis should be considered.
CASE Continued: Diagnosis
Mary undergoes TVUS with bowel preparation, which reveals a normal uterus and adnexa and the presence of 2 lesions, a 2×1.5-cm retrocervical lesion and a 1.8×2-cm rectosigmoid lesion 9 cm above the anal verge. The rectosigmoid lesion involves the external muscularis and compromises 30% of the bowel circumference.
How would you manage the bowel DIE?
Read about management options and individualized care.
Management options: Factor in the variables
DIE can involve the ureters and bladder, the retrocervical and rectovaginal spaces, the appendix, and the bowel. Lesions can be single or multifocal. Although our institutions’ imaging with MRI and TVUS is highly accurate, we additionally recommend the use of colonoscopy (with directed biopsies if appropriate) to evaluate patients who present with rectal bleeding, large endometriotic rectal nodules, or have a family history of bowel cancer.
While many studies have found that surgical resection of DIE improves pain and quality of life, surgery can have significant complications.12 Observation is adequate for asymptomatic patients with DIE. Medical treatment may be offered to patients with mild pain (there is no evidence of a reduction in lesion size with medical therapy). In cases of surgical treatment, we encourage the involvement of a multidisciplinary surgical team to reduce complications and optimize outcomes.
Patients with DIE, significant pain (VAS score, >7), and multiple failed in vitro fertilization treatments are candidates for surgery. When bowel endometriosis is noted on imaging, factors such as size, depth, number of lesions, circumferential involvement, and distance from the anal verge are all used to determine the surgical approach. Rectosigmoid lesions smaller than 3 cm can be treated more conservatively—for example, with shaving or anterior resection with manual repair using disk staplers. Segmental resection generally is indicated for rectosigmoid lesions larger than 3 cm, involvement deeper than the submucosal layer, multiple lesions, circumferential involvement of more than 40%, and the presence of obstructed bowel symptoms.13,14
In patients with DIE who present with both infertility and pain, antimüllerian hormone level and TVUS follicular count are used to evaluate ovarian reserve. As surgical treatment may further reduce ovarian reserve in patients with DIE and infertility, we counsel them regarding assisted reproductive technology options before surgery.
CASE Resolved
After thorough discussion, Mary opts to try a different combination oral contraceptive pill formulation. The pills improve her pain symptoms significantly (VAS score, 4), and she decides to forgo surgery. She will be followed up closely on an outpatient basis with serial TVUS imaging.
Individualize management based on patient parameters
Imaging has been used for the nonsurgical diagnosis of DIE for many years, and this practice increasingly is being accepted and adopted. A presumptive nonsurgical diagnosis of endometriosis can be made based on the clinical signs and symptoms obtained from a thorough history and physical examination, in addition to the absence of imaging findings for ovarian and deep endometriosis.
According to guidelines from major ObGyn societies, such as the American College of Obstetricians and Gynecologists and the European Society of Human Reproduction and Embryology, empiric medical therapy (including combination oral contraceptives, progesterone-containing formulations, and gonadotropin-releasing hormone agonists) can be considered for patients with presumed endometriosis presenting with pain.15
When surgery is chosen, the surgeon must obtain crucial information on the characteristics of the lesion(s) and involve a multidisciplinary team to achieve the best outcomes for the patient.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
- Buck Louis GM, Hediger ML, Peterson CM, et al; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360-365.
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585-596.
- Bellelis P, Dias JA Jr, Podgaec S, Gonzales M, Baracat EC, Abrao MS. Epidemiological and clinical aspects of pelvic endometriosis--a case series. Rev Assoc Med Bras (1992). 2010;56(4):467-471.
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
- Greene R, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32-39.
- Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108(6):886-894.
- Abrão MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092-3097.
- Young SW, Dahiya N, Patel MD, et al. Initial accuracy of and learning curve for transvaginal ultrasound with bowel preparation for deep endometriosis in a US tertiary care center. J Minim Invasive Gynecol. 2017;24(7):1170-1176.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
- de Paula Andres M, Borrelli GM, Kho RM, Abrão MS. The current management of deep endometriosis: a systematic review. Minerva Ginecol. 2017;69(6):587-596.
- Abrão MS, Podgaec S, Dias JA Jr, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15(3):280-285.
- Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21(3):329-339.
- Kho RM, Andres MP, Borrelli GM, Neto JS, Zanluchi A, Abrao MS. Surgical treatment of different types of endometriosis: comparison of major society guidelines and preferred clinical algorithms [published online ahead of print]. Best Pract Res Clin Obstet Gynaecol. 2018. doi:10.1016/j.bpobgyn2018.01.020.
Endometriosis affects up to 10% of women of reproductive age or, conservatively, about 6.5 million women in the United States.1,2 There are 3 types of endometriosis—superficial, ovarian, and deep—and in the past each of these was assumed to have a distinct pathogenesis.3 Deep infiltrating endometriosis (DIE) is the presence of one or more endometriotic nodules deeper than 5 mm. In a study at a large tertiary-care center, 40% of patients with endometriosis had deep disease.4 DIE is associated with more severe pain and infertility.5 In patients with endometriosis, diagnosis is commonly made 7 to 9 years after the initial pelvic pain presentation.6 For these reasons, well-directed history taking and proper evaluation and treatment should be pursued to relieve pain and optimize outcomes.
CASE Young woman with intensifying pelvic pain
Mary is a 26-year-old social worker who presents to her ObGyn with symptoms of worsening pain during as well as outside her periods. What additional information would you want to obtain from Mary, given her chief symptom of pain?
Investigate the type of pain
It is important to ask the patient about her menstrual and sexual history, her thoughts regarding near- and long-term fertility, and the type and severity of her pain symptoms. The 5 pain symptoms specific to pelvic pain are dysmenorrhea, dyspareunia, dysuria, dyschezia, and noncyclic pelvic pain. A visual analog scale (VAS) for pain as well as pelvic pain questionnaires can be used to guide evaluation options and monitor treatment outcomes. In addition, it is of paramount importance to understand the differential diagnoses that can present as pelvic pain (TABLE).

CASE Continued: Mary’s history
Mary reports that she always has had painful periods and that she was started on oral contraceptive pills for pain control and regulation of her periods soon after the onset of menses, when she was 12 years old. In college, she was prescribed oral contraceptive pills for contraception. Recently engaged, she is interested in becoming pregnant in 3 years.
A year ago, Mary discontinued the pills because of their adverse effects. Now she has severe pain during (VAS score, 8/10) and outside (VAS score, 7) her monthly periods. Because of this pain, she has taken time off from work twice within the past 6 months. She has pain during intercourse (VAS score, 7) and some pain with bowel movements during her menses (VAS score, 4). Pelvic examination reveals a normal-sized uterus and adnexa as well as a tender nodule in the rectovaginal septum.
What diagnostic tests and imaging would you obtain?
Imaging’s role in diagnosis
At many advanced centers for endometriosis, DIE is successfully diagnosed with specific magnetic resonance imaging (MRI) or transvaginal ultrasound (TVUS) protocols. In a recent review, MRI’s pooled sensitivity and specificity for rectosigmoid endometriosis were 92% and 96%, respectively.7 Choice of imaging for DIE depends on the skills and experience of the clinicians at each center. At a large referral center in São Paulo, Brazil, TVUS with bowel preparation had better sensitivity and specificity for deep retrocervical and rectosigmoid disease compared with MRI and digital pelvic examination.8 In addition, at a center in the United States, we found that proficiency in performing TVUS for DIE was achieved after 70 to 75 cases, and the exam took an average of only 20 minutes.9
Despite recent advances in imaging, most gynecologic societies still hold that endometriosis is to be definitively diagnosed with histologic confirmation from tissue biopsies during surgery. Although surgery remains the diagnostic gold standard, it does not mean that all patients with pelvic pain should undergo diagnostic laparoscopy with tissue biopsies.
The combination of compelling clinical signs, symptoms, and imaging findings (such as absence of findings for ovarian and deep endometriosis) can be used to make a presumptive nonsurgical (that is, clinical) diagnosis of endometriosis. Major societies recommend empiric medical therapy (for example, combination oral contraceptives) for the pain associated with superficial endometriosis.10,11 When there is no response to treatment, or when a patient declines or has contraindications to medical therapy, diagnostic laparoscopy with excision of endometriosis should be considered.
CASE Continued: Diagnosis
Mary undergoes TVUS with bowel preparation, which reveals a normal uterus and adnexa and the presence of 2 lesions, a 2×1.5-cm retrocervical lesion and a 1.8×2-cm rectosigmoid lesion 9 cm above the anal verge. The rectosigmoid lesion involves the external muscularis and compromises 30% of the bowel circumference.
How would you manage the bowel DIE?
Read about management options and individualized care.
Management options: Factor in the variables
DIE can involve the ureters and bladder, the retrocervical and rectovaginal spaces, the appendix, and the bowel. Lesions can be single or multifocal. Although our institutions’ imaging with MRI and TVUS is highly accurate, we additionally recommend the use of colonoscopy (with directed biopsies if appropriate) to evaluate patients who present with rectal bleeding, large endometriotic rectal nodules, or have a family history of bowel cancer.
While many studies have found that surgical resection of DIE improves pain and quality of life, surgery can have significant complications.12 Observation is adequate for asymptomatic patients with DIE. Medical treatment may be offered to patients with mild pain (there is no evidence of a reduction in lesion size with medical therapy). In cases of surgical treatment, we encourage the involvement of a multidisciplinary surgical team to reduce complications and optimize outcomes.
Patients with DIE, significant pain (VAS score, >7), and multiple failed in vitro fertilization treatments are candidates for surgery. When bowel endometriosis is noted on imaging, factors such as size, depth, number of lesions, circumferential involvement, and distance from the anal verge are all used to determine the surgical approach. Rectosigmoid lesions smaller than 3 cm can be treated more conservatively—for example, with shaving or anterior resection with manual repair using disk staplers. Segmental resection generally is indicated for rectosigmoid lesions larger than 3 cm, involvement deeper than the submucosal layer, multiple lesions, circumferential involvement of more than 40%, and the presence of obstructed bowel symptoms.13,14
In patients with DIE who present with both infertility and pain, antimüllerian hormone level and TVUS follicular count are used to evaluate ovarian reserve. As surgical treatment may further reduce ovarian reserve in patients with DIE and infertility, we counsel them regarding assisted reproductive technology options before surgery.
CASE Resolved
After thorough discussion, Mary opts to try a different combination oral contraceptive pill formulation. The pills improve her pain symptoms significantly (VAS score, 4), and she decides to forgo surgery. She will be followed up closely on an outpatient basis with serial TVUS imaging.
Individualize management based on patient parameters
Imaging has been used for the nonsurgical diagnosis of DIE for many years, and this practice increasingly is being accepted and adopted. A presumptive nonsurgical diagnosis of endometriosis can be made based on the clinical signs and symptoms obtained from a thorough history and physical examination, in addition to the absence of imaging findings for ovarian and deep endometriosis.
According to guidelines from major ObGyn societies, such as the American College of Obstetricians and Gynecologists and the European Society of Human Reproduction and Embryology, empiric medical therapy (including combination oral contraceptives, progesterone-containing formulations, and gonadotropin-releasing hormone agonists) can be considered for patients with presumed endometriosis presenting with pain.15
When surgery is chosen, the surgeon must obtain crucial information on the characteristics of the lesion(s) and involve a multidisciplinary team to achieve the best outcomes for the patient.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Endometriosis affects up to 10% of women of reproductive age or, conservatively, about 6.5 million women in the United States.1,2 There are 3 types of endometriosis—superficial, ovarian, and deep—and in the past each of these was assumed to have a distinct pathogenesis.3 Deep infiltrating endometriosis (DIE) is the presence of one or more endometriotic nodules deeper than 5 mm. In a study at a large tertiary-care center, 40% of patients with endometriosis had deep disease.4 DIE is associated with more severe pain and infertility.5 In patients with endometriosis, diagnosis is commonly made 7 to 9 years after the initial pelvic pain presentation.6 For these reasons, well-directed history taking and proper evaluation and treatment should be pursued to relieve pain and optimize outcomes.
CASE Young woman with intensifying pelvic pain
Mary is a 26-year-old social worker who presents to her ObGyn with symptoms of worsening pain during as well as outside her periods. What additional information would you want to obtain from Mary, given her chief symptom of pain?
Investigate the type of pain
It is important to ask the patient about her menstrual and sexual history, her thoughts regarding near- and long-term fertility, and the type and severity of her pain symptoms. The 5 pain symptoms specific to pelvic pain are dysmenorrhea, dyspareunia, dysuria, dyschezia, and noncyclic pelvic pain. A visual analog scale (VAS) for pain as well as pelvic pain questionnaires can be used to guide evaluation options and monitor treatment outcomes. In addition, it is of paramount importance to understand the differential diagnoses that can present as pelvic pain (TABLE).

CASE Continued: Mary’s history
Mary reports that she always has had painful periods and that she was started on oral contraceptive pills for pain control and regulation of her periods soon after the onset of menses, when she was 12 years old. In college, she was prescribed oral contraceptive pills for contraception. Recently engaged, she is interested in becoming pregnant in 3 years.
A year ago, Mary discontinued the pills because of their adverse effects. Now she has severe pain during (VAS score, 8/10) and outside (VAS score, 7) her monthly periods. Because of this pain, she has taken time off from work twice within the past 6 months. She has pain during intercourse (VAS score, 7) and some pain with bowel movements during her menses (VAS score, 4). Pelvic examination reveals a normal-sized uterus and adnexa as well as a tender nodule in the rectovaginal septum.
What diagnostic tests and imaging would you obtain?
Imaging’s role in diagnosis
At many advanced centers for endometriosis, DIE is successfully diagnosed with specific magnetic resonance imaging (MRI) or transvaginal ultrasound (TVUS) protocols. In a recent review, MRI’s pooled sensitivity and specificity for rectosigmoid endometriosis were 92% and 96%, respectively.7 Choice of imaging for DIE depends on the skills and experience of the clinicians at each center. At a large referral center in São Paulo, Brazil, TVUS with bowel preparation had better sensitivity and specificity for deep retrocervical and rectosigmoid disease compared with MRI and digital pelvic examination.8 In addition, at a center in the United States, we found that proficiency in performing TVUS for DIE was achieved after 70 to 75 cases, and the exam took an average of only 20 minutes.9
Despite recent advances in imaging, most gynecologic societies still hold that endometriosis is to be definitively diagnosed with histologic confirmation from tissue biopsies during surgery. Although surgery remains the diagnostic gold standard, it does not mean that all patients with pelvic pain should undergo diagnostic laparoscopy with tissue biopsies.
The combination of compelling clinical signs, symptoms, and imaging findings (such as absence of findings for ovarian and deep endometriosis) can be used to make a presumptive nonsurgical (that is, clinical) diagnosis of endometriosis. Major societies recommend empiric medical therapy (for example, combination oral contraceptives) for the pain associated with superficial endometriosis.10,11 When there is no response to treatment, or when a patient declines or has contraindications to medical therapy, diagnostic laparoscopy with excision of endometriosis should be considered.
CASE Continued: Diagnosis
Mary undergoes TVUS with bowel preparation, which reveals a normal uterus and adnexa and the presence of 2 lesions, a 2×1.5-cm retrocervical lesion and a 1.8×2-cm rectosigmoid lesion 9 cm above the anal verge. The rectosigmoid lesion involves the external muscularis and compromises 30% of the bowel circumference.
How would you manage the bowel DIE?
Read about management options and individualized care.
Management options: Factor in the variables
DIE can involve the ureters and bladder, the retrocervical and rectovaginal spaces, the appendix, and the bowel. Lesions can be single or multifocal. Although our institutions’ imaging with MRI and TVUS is highly accurate, we additionally recommend the use of colonoscopy (with directed biopsies if appropriate) to evaluate patients who present with rectal bleeding, large endometriotic rectal nodules, or have a family history of bowel cancer.
While many studies have found that surgical resection of DIE improves pain and quality of life, surgery can have significant complications.12 Observation is adequate for asymptomatic patients with DIE. Medical treatment may be offered to patients with mild pain (there is no evidence of a reduction in lesion size with medical therapy). In cases of surgical treatment, we encourage the involvement of a multidisciplinary surgical team to reduce complications and optimize outcomes.
Patients with DIE, significant pain (VAS score, >7), and multiple failed in vitro fertilization treatments are candidates for surgery. When bowel endometriosis is noted on imaging, factors such as size, depth, number of lesions, circumferential involvement, and distance from the anal verge are all used to determine the surgical approach. Rectosigmoid lesions smaller than 3 cm can be treated more conservatively—for example, with shaving or anterior resection with manual repair using disk staplers. Segmental resection generally is indicated for rectosigmoid lesions larger than 3 cm, involvement deeper than the submucosal layer, multiple lesions, circumferential involvement of more than 40%, and the presence of obstructed bowel symptoms.13,14
In patients with DIE who present with both infertility and pain, antimüllerian hormone level and TVUS follicular count are used to evaluate ovarian reserve. As surgical treatment may further reduce ovarian reserve in patients with DIE and infertility, we counsel them regarding assisted reproductive technology options before surgery.
CASE Resolved
After thorough discussion, Mary opts to try a different combination oral contraceptive pill formulation. The pills improve her pain symptoms significantly (VAS score, 4), and she decides to forgo surgery. She will be followed up closely on an outpatient basis with serial TVUS imaging.
Individualize management based on patient parameters
Imaging has been used for the nonsurgical diagnosis of DIE for many years, and this practice increasingly is being accepted and adopted. A presumptive nonsurgical diagnosis of endometriosis can be made based on the clinical signs and symptoms obtained from a thorough history and physical examination, in addition to the absence of imaging findings for ovarian and deep endometriosis.
According to guidelines from major ObGyn societies, such as the American College of Obstetricians and Gynecologists and the European Society of Human Reproduction and Embryology, empiric medical therapy (including combination oral contraceptives, progesterone-containing formulations, and gonadotropin-releasing hormone agonists) can be considered for patients with presumed endometriosis presenting with pain.15
When surgery is chosen, the surgeon must obtain crucial information on the characteristics of the lesion(s) and involve a multidisciplinary team to achieve the best outcomes for the patient.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
- Buck Louis GM, Hediger ML, Peterson CM, et al; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360-365.
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585-596.
- Bellelis P, Dias JA Jr, Podgaec S, Gonzales M, Baracat EC, Abrao MS. Epidemiological and clinical aspects of pelvic endometriosis--a case series. Rev Assoc Med Bras (1992). 2010;56(4):467-471.
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
- Greene R, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32-39.
- Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108(6):886-894.
- Abrão MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092-3097.
- Young SW, Dahiya N, Patel MD, et al. Initial accuracy of and learning curve for transvaginal ultrasound with bowel preparation for deep endometriosis in a US tertiary care center. J Minim Invasive Gynecol. 2017;24(7):1170-1176.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
- de Paula Andres M, Borrelli GM, Kho RM, Abrão MS. The current management of deep endometriosis: a systematic review. Minerva Ginecol. 2017;69(6):587-596.
- Abrão MS, Podgaec S, Dias JA Jr, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15(3):280-285.
- Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21(3):329-339.
- Kho RM, Andres MP, Borrelli GM, Neto JS, Zanluchi A, Abrao MS. Surgical treatment of different types of endometriosis: comparison of major society guidelines and preferred clinical algorithms [published online ahead of print]. Best Pract Res Clin Obstet Gynaecol. 2018. doi:10.1016/j.bpobgyn2018.01.020.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
- Buck Louis GM, Hediger ML, Peterson CM, et al; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360-365.
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585-596.
- Bellelis P, Dias JA Jr, Podgaec S, Gonzales M, Baracat EC, Abrao MS. Epidemiological and clinical aspects of pelvic endometriosis--a case series. Rev Assoc Med Bras (1992). 2010;56(4):467-471.
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
- Greene R, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32-39.
- Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108(6):886-894.
- Abrão MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092-3097.
- Young SW, Dahiya N, Patel MD, et al. Initial accuracy of and learning curve for transvaginal ultrasound with bowel preparation for deep endometriosis in a US tertiary care center. J Minim Invasive Gynecol. 2017;24(7):1170-1176.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
- de Paula Andres M, Borrelli GM, Kho RM, Abrão MS. The current management of deep endometriosis: a systematic review. Minerva Ginecol. 2017;69(6):587-596.
- Abrão MS, Podgaec S, Dias JA Jr, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15(3):280-285.
- Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21(3):329-339.
- Kho RM, Andres MP, Borrelli GM, Neto JS, Zanluchi A, Abrao MS. Surgical treatment of different types of endometriosis: comparison of major society guidelines and preferred clinical algorithms [published online ahead of print]. Best Pract Res Clin Obstet Gynaecol. 2018. doi:10.1016/j.bpobgyn2018.01.020.
Take-home points
- Specific MRI or TVUS protocols are highly accurate in making a nonsurgical diagnosis of deep infiltrating endometriosis (DIE).
- The combination of compelling clinical signs and symptoms and absence of imaging findings for DIE can be used to make a presumptive nonsurgical diagnosis of endometriosis.
- Empiric medical therapy may provide pain relief.
- Conservative treatment, including observation alone, may be considered in asymptomatic patients with DIE and in those with minimal pain.
- Before surgery, it is imperative to know lesion size, depth, circumferential bowel involvement, and location (or distance from the anal verge in cases of rectosigmoid lesion) to optimize surgical outcomes.
Vaginal morcellation by hand using advanced instrumentation

Read Dr. Kho's Surgical Technique article, "Transforming vaginal hysterectomy: 7 solutions to the most daunting challenges" (August 2014)

Read Dr. Kho's Surgical Technique article, "Transforming vaginal hysterectomy: 7 solutions to the most daunting challenges" (August 2014)

Read Dr. Kho's Surgical Technique article, "Transforming vaginal hysterectomy: 7 solutions to the most daunting challenges" (August 2014)
Transforming vaginal hysterectomy: 7 solutions to the most daunting challenges
Vaginal hysterectomy is the preferred route to benign hysterectomy because it is associated with better outcomes and fewer complications than the laparoscopic and open abdominal approaches.1,2 Yet, despite superior patient outcomes and cost benefits, the rate of vaginal hysterectomy is declining.
According to the Nationwide Inpatient Sample, the use of vaginal hysterectomy declined from 24.8% in 1998 to 16.7% in 2010.3 In fact, more than 80% of surgeons in the United States now perform fewer than five vaginal procedures in a year.4
The increasing use of other minimally invasive routes, such as laparoscopy and robotics, indicates that most practicing surgeons and recent graduates are choosing these approaches over the vaginal route. In only 3 years, the rate of laparoscopy increased by 6% and robotics increased by almost 10%.3
Many surgeons assume that vaginal hysterectomy exists in a state of suspended animation, with nothing much changed in the way it has been performed over the past few decades. Further, vaginal surgery is difficult to teach and learn, given limitations in exposure and visualization, difficulty in securing hemostasis, and challenges in the removal of the large uterus and adnexae. As a result, vaginal hysterectomy often is thought, erroneously, to be indicated only in procedures involving a small and prolapsing uterus.
To increase the rate of vaginal hysterectomy, we can benefit from experience gained in laparoscopy and robotics—whether we are teachers or learners—while maintaining patient safety and containing costs.
In this article, I describe common challenges in vaginal hysterectomy and offer tools and techniques to overcome them:
- achieving and enhancing ergonomics, exposure, and visualization
- the need to work in a long vaginal vault
- the task of securing vascular and thick tissue pedicles when the introitus and vaginal vault are narrow.
The vaginal approach is less costly
Vaginal hysterectomy costs significantly less to perform than other approaches. At a tertiary referral center, vaginal hysterectomy costs approximately $7,000 to $18,000 per case less than laparoscopic, abdominal, and robotic hysterectomy.5 With declining use of vaginal hysterectomy and increasing use of more costly approaches, we face a health-care crisis.
Residents are inadequately trained to perform vaginal hysterectomy
Data reveal that not only are our recent graduates inadequately prepared to perform vaginal hysterectomy, but national health-care dollars and resources are depleted when surgeons choose to perform more costly approaches. As a result, many eligible patients end up deprived of the benefits of a single, concealed, and minimally invasive procedure.
The increase in laparoscopic and robotic approaches to hysterectomy has affected residency training. National case log reports from the Accreditation Council of Graduate Medical Education show that the number of vaginal hysterectomies performed by residents as “primary surgeons” decreased by 40%, from a mean of 35 cases in 2002 to 19 cases in 2012.6 A recent survey found that only 28% of graduating residents were “completely prepared” to perform a vaginal hysterectomy, compared with 58% for abdominal hysterectomy, 22% for laparoscopic hysterectomy, and 3% for the robotic approach.7
The rate of vaginal hysterectomy will continue to decline if we perform it in the same manner it was done 30 years ago. The current generation of practicing gynecologists and graduates is choosing to perform the procedure laparoscopically or robotically because of the advantages these technologies provide. It is time that we incorporate features from these minimally invasive approaches to streamline vaginal hysterectomy while maintaining patient safety and containing costs.
Challenges: Ergonomics, exposure, and visualization
In conventional vaginal surgery, the surgeon often is the person who has the best and, sometimes, the sole view. Two bedside assistants are required to hold retractors during the entire case, which can lead to fatigue and muscle strain. Poor lighting also can greatly limit visualization into the pelvic cavity.
Both laparoscopy and robotics provide a well-illuminated and magnified view, with three-dimensional images now available in both platforms. This view is projected to overhead monitors for the entire surgical team to see. Magnification of the pelvic anatomic structures and projection to an external monitor facilitate teaching and learning, better anticipation of the surgical and procedural needs, and overall patient safety.
From robotics, where ergonomics is exemplified, we also learn the importance of surgeon comfort during the procedure.
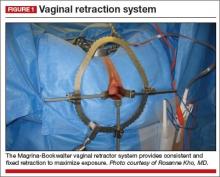
Solution #1: A self-retaining retractor
A self-retaining system such as the Magrina-Bookwalter vaginal retractor (Symmetry Surgical, Nashville, Tennessee) (FIGURE 1)
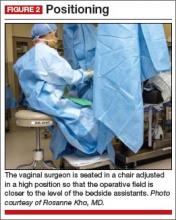
Solution #2: Seat the surgeon for an optimal view
With the patient in the lithotomy position and her legs in candy cane stirrups, the surgeon can be seated on a high chair so that the operative field is at the approximate level of the assistants’ view (FIGURE 2)
Solution #3: Illuminate the cavity
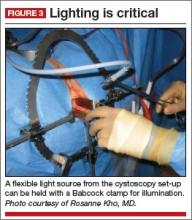
The deep pelvic cavity can be easily illuminated using a lighted suction tip, a flexible light source (as part of the cystoscopy set) held with a Babcock clamp (FIGURE 3), or a malleable illuminating mat taped to the retractor blades (such as Lightmat surgical illuminator, Lumitex, Inc., Strongsville, Ohio).
Solution #4: Project the image
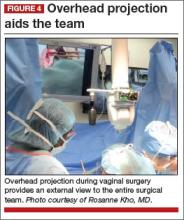
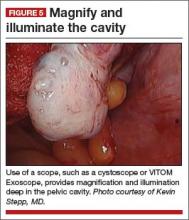
Cameras attached to an overhead boom or operating room light handles (FIGURE 4) and an external telescope with integrated illumination, such as a standard cystoscope or VITOM Exoscope (Karl Storz, El Segundo, California) (FIGURE 5) provide both magnification and projection of the procedure to an overhead monitor.
Glass technology (Google, Mountain View, California) also has been utilized in surgery and can be a good application of simultaneous projection and recording of the procedure to an external monitor (FIGURE 6). Google Glass is a wearable computer with an optical head-mounted display. The device, similar to eyeglasses, is voice-activated, thereby allowing the surgeon to record the procedure hands-free. Simultaneous projection to an external monitor allows the entire team in the operating room to be aware of the flow of the procedure.
Challenge: Working in a narrow vaginal vault
Without correct instrumentation, this challenge can be especially daunting. Laparoscopy and robotics have changed the way we perform pelvic surgery by providing advanced instrumentation.
Solution #5: Adapt your instruments
Modified vaginal instruments can be used to facilitate a case. Watch the accompanying VIDEO on the use of improved vaginal instruments during morcellation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
| Click to enlarge >>> |
Among the instruments adaptable for vaginal surgery:
- curving, articulating instruments
- long, curved, and rounded knife handles, which allow for better ergonomics during prolonged morcellation
- modified long retractors and use of a single long vaginal pack provide retraction of loops of bowel and easy access to secure pedicles deep in the pelvis.
All of these instruments are available through Marina Medical in Sunrise, Florida.
Challenge: Securing vascular and thick tissue pediclesA narrow introitus and vaginal vault can be difficult to manage during vaginal surgery. Another challenge is a uterus that is large or deformed by multiple fibroids.
Solution #6: Vaginal incision
A simple superficial 2- to 3-cm incision on the distal posterior aspect of the vaginal wall can widen the introitus and vault to facilitate the procedure (FIGURE 7)
Solution #7: Vessel-sealing tools
The use of energy is integral to laparoscopy and robotics for dissection and securing vessels. In a meta-analysis that included seven randomized controlled trials, advanced vessel-sealing devices proved useful in vaginal surgery by decreasing blood loss and operative time.8
In the setting of a difficult vaginal hysterectomy with a narrow introitus and large uterus, the use of vessel-sealing technology allows the surgeon to skeletonize the uterine arteries while allowing progressive descensus to secure the upper pedicles.
In my experience, the use of an advanced vessel-sealing device, compared with traditional clamp-cut-tying technique, facilitated successful completion of vaginal hysterectomy in 650 patients with relative contraindications to the vaginal approach, such as nulliparity, a uterus weighing more than 250 g, and a history of cesarean delivery (Mayo Clinic data; yet unpublished).
We must change with the times
The rate of vaginal hysterectomy will continue to decline unless we modify our technique to incorporate new technology. The current generation of practicing gynecologists and recent graduates are choosing the laparoscopic and robotic approaches because of the advantages these technologies offer. It is time we incorporate relevant features from these minimally invasive approaches while maintaining patient safety and containing costs by performing vaginal hysterectomy whenever possible. A willingness to change and ability to think outside the usual box will help us train new generations of vaginal surgeons who can bring back vaginal hysterectomy as the preferred route to the benign hysterectomy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
3. Wright T, Herzog T, Tsul J, et al. Nationwide trends in inpatient hysterectomy in the United States. Obstet Gynecol. 2013:122(2):233–241.
4. Rogo-Gupta L, Lewyn S, Jum JH, et al. Effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
5. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
6. Washburn EE, Cohen SL, Manoucherie E, Zurawin, RJ, Einarsson JI. Trends in reported residency surgical experience in hysterectomy [published online ahead of print June 4, 2014]. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2014.05.005.
7. Burkett D, Horwitz J, Kennedy V, et al. Assessing current trends in resident hysterectomy training. Female Pelvic Med Reconstr Surg. 2011;17(5):210–214.
8. Kroft J, Selk K. Energy-based vessel sealing in vaginal hysterectomy. A systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
Vaginal hysterectomy is the preferred route to benign hysterectomy because it is associated with better outcomes and fewer complications than the laparoscopic and open abdominal approaches.1,2 Yet, despite superior patient outcomes and cost benefits, the rate of vaginal hysterectomy is declining.
According to the Nationwide Inpatient Sample, the use of vaginal hysterectomy declined from 24.8% in 1998 to 16.7% in 2010.3 In fact, more than 80% of surgeons in the United States now perform fewer than five vaginal procedures in a year.4
The increasing use of other minimally invasive routes, such as laparoscopy and robotics, indicates that most practicing surgeons and recent graduates are choosing these approaches over the vaginal route. In only 3 years, the rate of laparoscopy increased by 6% and robotics increased by almost 10%.3
Many surgeons assume that vaginal hysterectomy exists in a state of suspended animation, with nothing much changed in the way it has been performed over the past few decades. Further, vaginal surgery is difficult to teach and learn, given limitations in exposure and visualization, difficulty in securing hemostasis, and challenges in the removal of the large uterus and adnexae. As a result, vaginal hysterectomy often is thought, erroneously, to be indicated only in procedures involving a small and prolapsing uterus.
To increase the rate of vaginal hysterectomy, we can benefit from experience gained in laparoscopy and robotics—whether we are teachers or learners—while maintaining patient safety and containing costs.
In this article, I describe common challenges in vaginal hysterectomy and offer tools and techniques to overcome them:
- achieving and enhancing ergonomics, exposure, and visualization
- the need to work in a long vaginal vault
- the task of securing vascular and thick tissue pedicles when the introitus and vaginal vault are narrow.
The vaginal approach is less costly
Vaginal hysterectomy costs significantly less to perform than other approaches. At a tertiary referral center, vaginal hysterectomy costs approximately $7,000 to $18,000 per case less than laparoscopic, abdominal, and robotic hysterectomy.5 With declining use of vaginal hysterectomy and increasing use of more costly approaches, we face a health-care crisis.
Residents are inadequately trained to perform vaginal hysterectomy
Data reveal that not only are our recent graduates inadequately prepared to perform vaginal hysterectomy, but national health-care dollars and resources are depleted when surgeons choose to perform more costly approaches. As a result, many eligible patients end up deprived of the benefits of a single, concealed, and minimally invasive procedure.
The increase in laparoscopic and robotic approaches to hysterectomy has affected residency training. National case log reports from the Accreditation Council of Graduate Medical Education show that the number of vaginal hysterectomies performed by residents as “primary surgeons” decreased by 40%, from a mean of 35 cases in 2002 to 19 cases in 2012.6 A recent survey found that only 28% of graduating residents were “completely prepared” to perform a vaginal hysterectomy, compared with 58% for abdominal hysterectomy, 22% for laparoscopic hysterectomy, and 3% for the robotic approach.7
The rate of vaginal hysterectomy will continue to decline if we perform it in the same manner it was done 30 years ago. The current generation of practicing gynecologists and graduates is choosing to perform the procedure laparoscopically or robotically because of the advantages these technologies provide. It is time that we incorporate features from these minimally invasive approaches to streamline vaginal hysterectomy while maintaining patient safety and containing costs.
Challenges: Ergonomics, exposure, and visualization
In conventional vaginal surgery, the surgeon often is the person who has the best and, sometimes, the sole view. Two bedside assistants are required to hold retractors during the entire case, which can lead to fatigue and muscle strain. Poor lighting also can greatly limit visualization into the pelvic cavity.
Both laparoscopy and robotics provide a well-illuminated and magnified view, with three-dimensional images now available in both platforms. This view is projected to overhead monitors for the entire surgical team to see. Magnification of the pelvic anatomic structures and projection to an external monitor facilitate teaching and learning, better anticipation of the surgical and procedural needs, and overall patient safety.
From robotics, where ergonomics is exemplified, we also learn the importance of surgeon comfort during the procedure.
Solution #1: A self-retaining retractor
A self-retaining system such as the Magrina-Bookwalter vaginal retractor (Symmetry Surgical, Nashville, Tennessee) (FIGURE 1)
Solution #2: Seat the surgeon for an optimal view
With the patient in the lithotomy position and her legs in candy cane stirrups, the surgeon can be seated on a high chair so that the operative field is at the approximate level of the assistants’ view (FIGURE 2)
Solution #3: Illuminate the cavity
The deep pelvic cavity can be easily illuminated using a lighted suction tip, a flexible light source (as part of the cystoscopy set) held with a Babcock clamp (FIGURE 3), or a malleable illuminating mat taped to the retractor blades (such as Lightmat surgical illuminator, Lumitex, Inc., Strongsville, Ohio).
Solution #4: Project the image
Cameras attached to an overhead boom or operating room light handles (FIGURE 4) and an external telescope with integrated illumination, such as a standard cystoscope or VITOM Exoscope (Karl Storz, El Segundo, California) (FIGURE 5) provide both magnification and projection of the procedure to an overhead monitor.
Glass technology (Google, Mountain View, California) also has been utilized in surgery and can be a good application of simultaneous projection and recording of the procedure to an external monitor (FIGURE 6). Google Glass is a wearable computer with an optical head-mounted display. The device, similar to eyeglasses, is voice-activated, thereby allowing the surgeon to record the procedure hands-free. Simultaneous projection to an external monitor allows the entire team in the operating room to be aware of the flow of the procedure.
Challenge: Working in a narrow vaginal vault
Without correct instrumentation, this challenge can be especially daunting. Laparoscopy and robotics have changed the way we perform pelvic surgery by providing advanced instrumentation.
Solution #5: Adapt your instruments
Modified vaginal instruments can be used to facilitate a case. Watch the accompanying VIDEO on the use of improved vaginal instruments during morcellation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
| Click to enlarge >>> |
Among the instruments adaptable for vaginal surgery:
- curving, articulating instruments
- long, curved, and rounded knife handles, which allow for better ergonomics during prolonged morcellation
- modified long retractors and use of a single long vaginal pack provide retraction of loops of bowel and easy access to secure pedicles deep in the pelvis.
All of these instruments are available through Marina Medical in Sunrise, Florida.
Challenge: Securing vascular and thick tissue pediclesA narrow introitus and vaginal vault can be difficult to manage during vaginal surgery. Another challenge is a uterus that is large or deformed by multiple fibroids.
Solution #6: Vaginal incision
A simple superficial 2- to 3-cm incision on the distal posterior aspect of the vaginal wall can widen the introitus and vault to facilitate the procedure (FIGURE 7)
Solution #7: Vessel-sealing tools
The use of energy is integral to laparoscopy and robotics for dissection and securing vessels. In a meta-analysis that included seven randomized controlled trials, advanced vessel-sealing devices proved useful in vaginal surgery by decreasing blood loss and operative time.8
In the setting of a difficult vaginal hysterectomy with a narrow introitus and large uterus, the use of vessel-sealing technology allows the surgeon to skeletonize the uterine arteries while allowing progressive descensus to secure the upper pedicles.
In my experience, the use of an advanced vessel-sealing device, compared with traditional clamp-cut-tying technique, facilitated successful completion of vaginal hysterectomy in 650 patients with relative contraindications to the vaginal approach, such as nulliparity, a uterus weighing more than 250 g, and a history of cesarean delivery (Mayo Clinic data; yet unpublished).
We must change with the times
The rate of vaginal hysterectomy will continue to decline unless we modify our technique to incorporate new technology. The current generation of practicing gynecologists and recent graduates are choosing the laparoscopic and robotic approaches because of the advantages these technologies offer. It is time we incorporate relevant features from these minimally invasive approaches while maintaining patient safety and containing costs by performing vaginal hysterectomy whenever possible. A willingness to change and ability to think outside the usual box will help us train new generations of vaginal surgeons who can bring back vaginal hysterectomy as the preferred route to the benign hysterectomy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Vaginal hysterectomy is the preferred route to benign hysterectomy because it is associated with better outcomes and fewer complications than the laparoscopic and open abdominal approaches.1,2 Yet, despite superior patient outcomes and cost benefits, the rate of vaginal hysterectomy is declining.
According to the Nationwide Inpatient Sample, the use of vaginal hysterectomy declined from 24.8% in 1998 to 16.7% in 2010.3 In fact, more than 80% of surgeons in the United States now perform fewer than five vaginal procedures in a year.4
The increasing use of other minimally invasive routes, such as laparoscopy and robotics, indicates that most practicing surgeons and recent graduates are choosing these approaches over the vaginal route. In only 3 years, the rate of laparoscopy increased by 6% and robotics increased by almost 10%.3
Many surgeons assume that vaginal hysterectomy exists in a state of suspended animation, with nothing much changed in the way it has been performed over the past few decades. Further, vaginal surgery is difficult to teach and learn, given limitations in exposure and visualization, difficulty in securing hemostasis, and challenges in the removal of the large uterus and adnexae. As a result, vaginal hysterectomy often is thought, erroneously, to be indicated only in procedures involving a small and prolapsing uterus.
To increase the rate of vaginal hysterectomy, we can benefit from experience gained in laparoscopy and robotics—whether we are teachers or learners—while maintaining patient safety and containing costs.
In this article, I describe common challenges in vaginal hysterectomy and offer tools and techniques to overcome them:
- achieving and enhancing ergonomics, exposure, and visualization
- the need to work in a long vaginal vault
- the task of securing vascular and thick tissue pedicles when the introitus and vaginal vault are narrow.
The vaginal approach is less costly
Vaginal hysterectomy costs significantly less to perform than other approaches. At a tertiary referral center, vaginal hysterectomy costs approximately $7,000 to $18,000 per case less than laparoscopic, abdominal, and robotic hysterectomy.5 With declining use of vaginal hysterectomy and increasing use of more costly approaches, we face a health-care crisis.
Residents are inadequately trained to perform vaginal hysterectomy
Data reveal that not only are our recent graduates inadequately prepared to perform vaginal hysterectomy, but national health-care dollars and resources are depleted when surgeons choose to perform more costly approaches. As a result, many eligible patients end up deprived of the benefits of a single, concealed, and minimally invasive procedure.
The increase in laparoscopic and robotic approaches to hysterectomy has affected residency training. National case log reports from the Accreditation Council of Graduate Medical Education show that the number of vaginal hysterectomies performed by residents as “primary surgeons” decreased by 40%, from a mean of 35 cases in 2002 to 19 cases in 2012.6 A recent survey found that only 28% of graduating residents were “completely prepared” to perform a vaginal hysterectomy, compared with 58% for abdominal hysterectomy, 22% for laparoscopic hysterectomy, and 3% for the robotic approach.7
The rate of vaginal hysterectomy will continue to decline if we perform it in the same manner it was done 30 years ago. The current generation of practicing gynecologists and graduates is choosing to perform the procedure laparoscopically or robotically because of the advantages these technologies provide. It is time that we incorporate features from these minimally invasive approaches to streamline vaginal hysterectomy while maintaining patient safety and containing costs.
Challenges: Ergonomics, exposure, and visualization
In conventional vaginal surgery, the surgeon often is the person who has the best and, sometimes, the sole view. Two bedside assistants are required to hold retractors during the entire case, which can lead to fatigue and muscle strain. Poor lighting also can greatly limit visualization into the pelvic cavity.
Both laparoscopy and robotics provide a well-illuminated and magnified view, with three-dimensional images now available in both platforms. This view is projected to overhead monitors for the entire surgical team to see. Magnification of the pelvic anatomic structures and projection to an external monitor facilitate teaching and learning, better anticipation of the surgical and procedural needs, and overall patient safety.
From robotics, where ergonomics is exemplified, we also learn the importance of surgeon comfort during the procedure.
Solution #1: A self-retaining retractor
A self-retaining system such as the Magrina-Bookwalter vaginal retractor (Symmetry Surgical, Nashville, Tennessee) (FIGURE 1)
Solution #2: Seat the surgeon for an optimal view
With the patient in the lithotomy position and her legs in candy cane stirrups, the surgeon can be seated on a high chair so that the operative field is at the approximate level of the assistants’ view (FIGURE 2)
Solution #3: Illuminate the cavity
The deep pelvic cavity can be easily illuminated using a lighted suction tip, a flexible light source (as part of the cystoscopy set) held with a Babcock clamp (FIGURE 3), or a malleable illuminating mat taped to the retractor blades (such as Lightmat surgical illuminator, Lumitex, Inc., Strongsville, Ohio).
Solution #4: Project the image
Cameras attached to an overhead boom or operating room light handles (FIGURE 4) and an external telescope with integrated illumination, such as a standard cystoscope or VITOM Exoscope (Karl Storz, El Segundo, California) (FIGURE 5) provide both magnification and projection of the procedure to an overhead monitor.
Glass technology (Google, Mountain View, California) also has been utilized in surgery and can be a good application of simultaneous projection and recording of the procedure to an external monitor (FIGURE 6). Google Glass is a wearable computer with an optical head-mounted display. The device, similar to eyeglasses, is voice-activated, thereby allowing the surgeon to record the procedure hands-free. Simultaneous projection to an external monitor allows the entire team in the operating room to be aware of the flow of the procedure.
Challenge: Working in a narrow vaginal vault
Without correct instrumentation, this challenge can be especially daunting. Laparoscopy and robotics have changed the way we perform pelvic surgery by providing advanced instrumentation.
Solution #5: Adapt your instruments
Modified vaginal instruments can be used to facilitate a case. Watch the accompanying VIDEO on the use of improved vaginal instruments during morcellation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
| Click to enlarge >>> |
Among the instruments adaptable for vaginal surgery:
- curving, articulating instruments
- long, curved, and rounded knife handles, which allow for better ergonomics during prolonged morcellation
- modified long retractors and use of a single long vaginal pack provide retraction of loops of bowel and easy access to secure pedicles deep in the pelvis.
All of these instruments are available through Marina Medical in Sunrise, Florida.
Challenge: Securing vascular and thick tissue pediclesA narrow introitus and vaginal vault can be difficult to manage during vaginal surgery. Another challenge is a uterus that is large or deformed by multiple fibroids.
Solution #6: Vaginal incision
A simple superficial 2- to 3-cm incision on the distal posterior aspect of the vaginal wall can widen the introitus and vault to facilitate the procedure (FIGURE 7)
Solution #7: Vessel-sealing tools
The use of energy is integral to laparoscopy and robotics for dissection and securing vessels. In a meta-analysis that included seven randomized controlled trials, advanced vessel-sealing devices proved useful in vaginal surgery by decreasing blood loss and operative time.8
In the setting of a difficult vaginal hysterectomy with a narrow introitus and large uterus, the use of vessel-sealing technology allows the surgeon to skeletonize the uterine arteries while allowing progressive descensus to secure the upper pedicles.
In my experience, the use of an advanced vessel-sealing device, compared with traditional clamp-cut-tying technique, facilitated successful completion of vaginal hysterectomy in 650 patients with relative contraindications to the vaginal approach, such as nulliparity, a uterus weighing more than 250 g, and a history of cesarean delivery (Mayo Clinic data; yet unpublished).
We must change with the times
The rate of vaginal hysterectomy will continue to decline unless we modify our technique to incorporate new technology. The current generation of practicing gynecologists and recent graduates are choosing the laparoscopic and robotic approaches because of the advantages these technologies offer. It is time we incorporate relevant features from these minimally invasive approaches while maintaining patient safety and containing costs by performing vaginal hysterectomy whenever possible. A willingness to change and ability to think outside the usual box will help us train new generations of vaginal surgeons who can bring back vaginal hysterectomy as the preferred route to the benign hysterectomy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
3. Wright T, Herzog T, Tsul J, et al. Nationwide trends in inpatient hysterectomy in the United States. Obstet Gynecol. 2013:122(2):233–241.
4. Rogo-Gupta L, Lewyn S, Jum JH, et al. Effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
5. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
6. Washburn EE, Cohen SL, Manoucherie E, Zurawin, RJ, Einarsson JI. Trends in reported residency surgical experience in hysterectomy [published online ahead of print June 4, 2014]. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2014.05.005.
7. Burkett D, Horwitz J, Kennedy V, et al. Assessing current trends in resident hysterectomy training. Female Pelvic Med Reconstr Surg. 2011;17(5):210–214.
8. Kroft J, Selk K. Energy-based vessel sealing in vaginal hysterectomy. A systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
3. Wright T, Herzog T, Tsul J, et al. Nationwide trends in inpatient hysterectomy in the United States. Obstet Gynecol. 2013:122(2):233–241.
4. Rogo-Gupta L, Lewyn S, Jum JH, et al. Effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
5. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
6. Washburn EE, Cohen SL, Manoucherie E, Zurawin, RJ, Einarsson JI. Trends in reported residency surgical experience in hysterectomy [published online ahead of print June 4, 2014]. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2014.05.005.
7. Burkett D, Horwitz J, Kennedy V, et al. Assessing current trends in resident hysterectomy training. Female Pelvic Med Reconstr Surg. 2011;17(5):210–214.
8. Kroft J, Selk K. Energy-based vessel sealing in vaginal hysterectomy. A systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
![]()
Dr. Kho presents vaginal morcellation by hand, using advanced instrumentation
Is same-day discharge feasible and safe for women undergoing vaginal hysterectomy?
Vaginal hysterectomy has a superior profile in terms of morbidity, safety, and cost, compared with other approaches to hysterectomy for benign disease. Despite this standing, vaginal hysterectomy is performed in a minority of cases. As the rates of other minimally invasive approaches—laparoscopic and robotic—have increased in the United States, the vaginal route has declined from 28% in 1998 to 20% in 2010.1,2 In fact, a large majority (85%) of gynecologists in the United States perform fewer than five vaginal hysterectomies a year.3
The concept of same-day discharge after hysterectomy is not new. Previously published studies, including one from an author of this study,4 have shown that discharging patients 12 to 24 hours after laparoscopic or vaginal hysterectomy is feasible. However, outpatient hysterectomy generally has not been adopted to the same extent as outpatient cholecystectomy in the field of general surgery.
At a time of cost-containment and declining medical reimbursements, outpatient hysterectomy has the potential to affect the health-care economic landscape in a significant manner.
Details of the series
Zakaria and Levy describe a consecutive series of 1,071 women who underwent vaginal hysterectomy (performed by a single surgeon) according to a well-outlined outpatient protocol. Participants underwent preoperative counseling and evidence-based interventions (medications, hydration) before, during, and after surgery to preempt postoperative pain and nausea.
Median operative time was 34 minutes (range, 17–210 minutes), and median estimated blood loss was 45 mL (range,5–800 mL). Median uterine weight was 160 g (range, 25–1,380 g).
Following the protocol, same-day discharge (ie, within 12 hours) was accomplished in 96% of patients. A small number (41 women, or approximately 4%) required overnight hospitalization for pain, nausea, or the need to travel a significant distance to return to their home. Five patients required readmission or emergency room evaluation within the first postoperative month due to nausea and vomiting, abdominal pain, fever, pulmonary embolus, or vesicovaginal fistula.
Stengths and limitations
Besides demonstrating that patients can be discharged early, Zakaria and Levy also point out that even traditionally “difficult” vaginal cases—for example, nulliparous women (18% of cases), women with a history of cesarean delivery or pelvic surgery (20% of cases), and patients with uteri larger than 250 g (30% of cases)—can be accomplished vaginally. These cases all were performed using a vessel-sealing device over the 10 years of the series.
Single-surgeon and selection bias may limit the generalizability and conclusions of this study. Future investigations using a comparative cohort (with an inpatient arm) and employing validated measures to evaluate outcomes such as postoperative pain, total narcotic use, return to normal activity, and patient satisfaction, also would be helpful. In addition, it would be beneficial to determine whether the same protocol would be applicable to patients undergoing other hysterectomy approaches.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Most gynecologic practitioners continue to admit patients overnight following hysterectomy. This study demonstrates that same-day discharge is feasible and safe. It also highlights other routinely employed practices, such as the use of an indwelling catheter and liberal administration of intravenous narcotics postoperatively, that may adversely affect a patient’s recovery.
I strongly recommend that readers refer to this study and consider many of the techniques it describes to minimize postoperative pain and nausea in patients undergoing hysterectomy. Even when a patient is admitted overnight, techniques to minimize postoperative discomfort should be considered.
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990–1997. Obstet Gynecol. 2002;99(2):229–234.
2. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
3. Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
4. Levy BS, Luciano DE, Emery LL. Outpatient vaginal hysterectomy is safe for patients and reduces institutional cost. J Minim Invasive Gynecol. 2005;12(6):494–501.
Vaginal hysterectomy has a superior profile in terms of morbidity, safety, and cost, compared with other approaches to hysterectomy for benign disease. Despite this standing, vaginal hysterectomy is performed in a minority of cases. As the rates of other minimally invasive approaches—laparoscopic and robotic—have increased in the United States, the vaginal route has declined from 28% in 1998 to 20% in 2010.1,2 In fact, a large majority (85%) of gynecologists in the United States perform fewer than five vaginal hysterectomies a year.3
The concept of same-day discharge after hysterectomy is not new. Previously published studies, including one from an author of this study,4 have shown that discharging patients 12 to 24 hours after laparoscopic or vaginal hysterectomy is feasible. However, outpatient hysterectomy generally has not been adopted to the same extent as outpatient cholecystectomy in the field of general surgery.
At a time of cost-containment and declining medical reimbursements, outpatient hysterectomy has the potential to affect the health-care economic landscape in a significant manner.
Details of the series
Zakaria and Levy describe a consecutive series of 1,071 women who underwent vaginal hysterectomy (performed by a single surgeon) according to a well-outlined outpatient protocol. Participants underwent preoperative counseling and evidence-based interventions (medications, hydration) before, during, and after surgery to preempt postoperative pain and nausea.
Median operative time was 34 minutes (range, 17–210 minutes), and median estimated blood loss was 45 mL (range,5–800 mL). Median uterine weight was 160 g (range, 25–1,380 g).
Following the protocol, same-day discharge (ie, within 12 hours) was accomplished in 96% of patients. A small number (41 women, or approximately 4%) required overnight hospitalization for pain, nausea, or the need to travel a significant distance to return to their home. Five patients required readmission or emergency room evaluation within the first postoperative month due to nausea and vomiting, abdominal pain, fever, pulmonary embolus, or vesicovaginal fistula.
Stengths and limitations
Besides demonstrating that patients can be discharged early, Zakaria and Levy also point out that even traditionally “difficult” vaginal cases—for example, nulliparous women (18% of cases), women with a history of cesarean delivery or pelvic surgery (20% of cases), and patients with uteri larger than 250 g (30% of cases)—can be accomplished vaginally. These cases all were performed using a vessel-sealing device over the 10 years of the series.
Single-surgeon and selection bias may limit the generalizability and conclusions of this study. Future investigations using a comparative cohort (with an inpatient arm) and employing validated measures to evaluate outcomes such as postoperative pain, total narcotic use, return to normal activity, and patient satisfaction, also would be helpful. In addition, it would be beneficial to determine whether the same protocol would be applicable to patients undergoing other hysterectomy approaches.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Most gynecologic practitioners continue to admit patients overnight following hysterectomy. This study demonstrates that same-day discharge is feasible and safe. It also highlights other routinely employed practices, such as the use of an indwelling catheter and liberal administration of intravenous narcotics postoperatively, that may adversely affect a patient’s recovery.
I strongly recommend that readers refer to this study and consider many of the techniques it describes to minimize postoperative pain and nausea in patients undergoing hysterectomy. Even when a patient is admitted overnight, techniques to minimize postoperative discomfort should be considered.
Vaginal hysterectomy has a superior profile in terms of morbidity, safety, and cost, compared with other approaches to hysterectomy for benign disease. Despite this standing, vaginal hysterectomy is performed in a minority of cases. As the rates of other minimally invasive approaches—laparoscopic and robotic—have increased in the United States, the vaginal route has declined from 28% in 1998 to 20% in 2010.1,2 In fact, a large majority (85%) of gynecologists in the United States perform fewer than five vaginal hysterectomies a year.3
The concept of same-day discharge after hysterectomy is not new. Previously published studies, including one from an author of this study,4 have shown that discharging patients 12 to 24 hours after laparoscopic or vaginal hysterectomy is feasible. However, outpatient hysterectomy generally has not been adopted to the same extent as outpatient cholecystectomy in the field of general surgery.
At a time of cost-containment and declining medical reimbursements, outpatient hysterectomy has the potential to affect the health-care economic landscape in a significant manner.
Details of the series
Zakaria and Levy describe a consecutive series of 1,071 women who underwent vaginal hysterectomy (performed by a single surgeon) according to a well-outlined outpatient protocol. Participants underwent preoperative counseling and evidence-based interventions (medications, hydration) before, during, and after surgery to preempt postoperative pain and nausea.
Median operative time was 34 minutes (range, 17–210 minutes), and median estimated blood loss was 45 mL (range,5–800 mL). Median uterine weight was 160 g (range, 25–1,380 g).
Following the protocol, same-day discharge (ie, within 12 hours) was accomplished in 96% of patients. A small number (41 women, or approximately 4%) required overnight hospitalization for pain, nausea, or the need to travel a significant distance to return to their home. Five patients required readmission or emergency room evaluation within the first postoperative month due to nausea and vomiting, abdominal pain, fever, pulmonary embolus, or vesicovaginal fistula.
Stengths and limitations
Besides demonstrating that patients can be discharged early, Zakaria and Levy also point out that even traditionally “difficult” vaginal cases—for example, nulliparous women (18% of cases), women with a history of cesarean delivery or pelvic surgery (20% of cases), and patients with uteri larger than 250 g (30% of cases)—can be accomplished vaginally. These cases all were performed using a vessel-sealing device over the 10 years of the series.
Single-surgeon and selection bias may limit the generalizability and conclusions of this study. Future investigations using a comparative cohort (with an inpatient arm) and employing validated measures to evaluate outcomes such as postoperative pain, total narcotic use, return to normal activity, and patient satisfaction, also would be helpful. In addition, it would be beneficial to determine whether the same protocol would be applicable to patients undergoing other hysterectomy approaches.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Most gynecologic practitioners continue to admit patients overnight following hysterectomy. This study demonstrates that same-day discharge is feasible and safe. It also highlights other routinely employed practices, such as the use of an indwelling catheter and liberal administration of intravenous narcotics postoperatively, that may adversely affect a patient’s recovery.
I strongly recommend that readers refer to this study and consider many of the techniques it describes to minimize postoperative pain and nausea in patients undergoing hysterectomy. Even when a patient is admitted overnight, techniques to minimize postoperative discomfort should be considered.
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990–1997. Obstet Gynecol. 2002;99(2):229–234.
2. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
3. Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
4. Levy BS, Luciano DE, Emery LL. Outpatient vaginal hysterectomy is safe for patients and reduces institutional cost. J Minim Invasive Gynecol. 2005;12(6):494–501.
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990–1997. Obstet Gynecol. 2002;99(2):229–234.
2. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
3. Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
4. Levy BS, Luciano DE, Emery LL. Outpatient vaginal hysterectomy is safe for patients and reduces institutional cost. J Minim Invasive Gynecol. 2005;12(6):494–501.
The vaginal approach to hysterectomy
The vaginal route is the preferred approach for benign hysterectomy. The most recent Cochrane review of surgical approaches to hysterectomy (abdominal, vaginal, and laparoscopic), which involved more than 3,000 women in 27 randomized controlled trials, shows that vaginal hysterectomy results in fewer complications, shorter hospital stay, and faster recovery and return to normal activity (Cochrane Database Syst. Rev. 2006 (2):CD003677). The vaginal approach also provides the best cosmetic result with its single and concealed incision.
Despite strong evidence for the greater advantage of the vaginal approach, there has not been any increase in the number of hysterectomies performed vaginally. In the United States, the rate appears to have declined in 15 years from 24% in 1990 to 22% in 2005, and this decline may be continuing (Obstet. Gynecol. 2002;99:229-34 and Obstet. Gynecol. 2009;114:1041-8). According to an analysis of a national database from more than 500 acute care hospitals, the majority of gynecologic surgeons in United States (more than 80%) perform fewer than five vaginal surgeries in a year (Obstet. Gynecol. 2010;116:1341-7).
Challenges with exposure, entry into the anterior cul-de-sac, hemostasis, avoidance of ureteral and bladder injury, and removal of the large uterus have been the main stumbling blocks for many surgeons in choosing the vaginal route. I provide, herein, simple techniques, instruments, and devices that can facilitate the performance of the procedure in a safe and efficient manner.
Obtaining exposure
The use of a self-retaining retractor, such as the Magrina-Bookwalter vaginal retractor system (Symmetry Surgical, Nashville, Tenn.) provides consistent and reliable exposure without requiring two surgical assistants at the bedside. Similar to the abdominal self-retractor system, it is attached to the operating table and is designed to fit the contour of the patient’s perineum while in a high lithotomy position. Self-retracting blades of multiple lengths are placed in the four quadrants to maximize room for surgery.
In cases where the introital opening is limited (i.e. = 2.5 cm), such as in nulliparous or menopausal women, a superficial 2- to 3-cm longitudinal incision is performed with bovie cautery in the midline and distal portion of the posterior vaginal wall. This provides additional width to allow placement of the lateral and posterior self-retracting blades.
Additional light from a flexible light source (such as the cystoscopy light) held with a Babcock and a lighted suction irrigator tip (such as Vital Vue, Covidien, Mansfield, Mass.) is extremely helpful in visualization of structures deep within the vagina.
Essential in a vaginal hysterectomy instrument tray are modified deep Deaver retractors that provide additional retraction and visualization particularly in cases of bleeding from pedicles that have retracted to the pelvic sidewall. . A long vaginal pack is also placed to keep loops of bowel out of the operating field. We avoid the use of multiple small sponges that can easily be lost in vaginal cases.
Entry into cul-de-sac
Entry into the anterior cul-de-sac in vaginal hysterectomy can and should be delayed until better descensus of the uterus is obtained. This is achieved with first entering the posterior cul-de-sac, which is often easier to accomplish. To then enter the anterior cul-de-sac, traction is applied posteriorly on the anterior lip of the cervix with the Jacobs tenaculum forceps. The posterior blade is removed to achieve better exposure with a more pronounced angulation of the lower uterine segment.
With ventral traction on the anterior vaginal wall, the bladder is separated from the anterior cervix via sharp dissection with the Mayo curved scissors. The scissor tips are pointed downwards, aimed parallel to the plane of the cervix to reveal the avascular vesicouterine space.
Knowing the anatomy and feel of the tissues is key to mastering entry into the anterior cul-de-sac. Cutting into the cervix will feel tough against the tips of the Metzenbaum scissors, while cutting into the softer beefy-appearing detrusor muscles will manifest with excessive bleeding. The vesicouterine fold is identified as a crescent-shaped peritoneal fold that can be lifted and divided for entry.
In cases where scarring between the bladder and uterus is encountered in patients with multiple previous caesarean sections, dissection is best performed lateral to the midline away from central dense adhesions.
An inability to enter either or both cul-de-sacs should not preclude continuation with the vaginal approach. Securing the uterine arteries can still be accomplished extraperitoneally until better descensus of the uterus is obtained.
Securing vascular pedicles
Achieving hemostasis in vaginal procedures is challenging where there is limited space for placing a suture around the clamp and for securing knots with fingers deep within the vaginal canal. The use of vessel-sealing devices in vaginal hysterectomy overcomes this limitation of tight vaginal access and has proved to be feasible and safe.
Multiple types of energy devices are available, such as PK devices (Gyrus ACMI, Southborough, Mass.), LigaSure instruments (Covidien, Mansfield, Mass.), the Enseal Super Jaw device (Ethicon Endo-Surgery, Nokesville, Va.) and Altrus devices (ConMed Electrosurgery, Centennial, Colo.). These devices can be particularly helpful in cases with narrowed introitus and large uterus. The choice of device is surgeon dependent and requires a learning curve.
Gentle traction is placed on the cervix while the device clamp is pushed up against the pedicle, taking care to avoid leaning against any adjacent tissues or retractor blades to avoid thermal injury. Bringing in a suction device quickly dissipates the hot steam that can be generated from the device.
Avoidance of bladder and ureteral injury
Once the vesicouterine space is entered, bladder pillars are gently pushed superiorly and laterally with the index finger to avoid injury during placement of the vessel-sealing clamp. It is imperative that the surgeon is aware of the location of the ureters, which are easily injured, particularly in cases with pelvic prolapse.
The ureters can be palpated with the index finger at 2 o’clock or at 10 o’clock (for the left and right ureter, respectively) against a curved Deaver retractor placed outside the peritoneal cavity on the lateral vaginal wall. Intraoperative cystoscopy should always be performed at the end of the procedure to diagnose inadvertent bladder and ureteral injury.
Removing the Large Uterus
Morcellation can be initiated after the uterine arteries have been sealed and divided on each side. Orientation of the uterus is maintained by placing two Jacobs tenaculi at the 3 o’clock and at 9 o’clock positions on the cervix. The cervix is bivalved to the level of the lower uterine segment.
If the anterior cul-de-sac has not been entered yet, the cervix should be bivalved to a centimeter below the vesicouterine peritoneal fold and morcellation started within the uterus.
Morcellation is performed with a Schroeder tenaculum (Aesculap Inc., Center Valley, Pa.) placed on the myometrium, and a wedge excision is accomplished with a 10 blade. Serial wedges are performed to decompress the uterus. Entry into the anterior cul-de-sac can now be performed easily with better uterine descensus and visualization of the peritoneal fold.
The surgeon should avoid forceful traction on the cervix during morcellation, which can cause the vascular pedicles to avulse.
Depending on the size of the uterus, morcellation can take over an hour. Use of an articulated long scalpel handle (such as the Precise CMK ergonomic knife by LaparoTools, in Metairie, La., which is rounded) can facilitate morcellation with less fatigue.
The surgeon must persist with morcellation as long as descensus of the uterus is continually achieved. An increase in bleeding is encountered when morcellation is near the fundus. At this point, the utero-ovarian pedicles can be identified and secured to finish the procedure.
Mastering the procedure
Simple techniques and new surgical devices are available to facilitate the difficult vaginal hysterectomy. Cases of narrowed introitus, multiple previous caesarean sections, and the large uteri requiring morcellation can be approached vaginally and achieved safely and efficiently with use of the above techniques.
In recognition of the vaginal approach as a minimally invasive procedure, AAGL has provided postgraduate courses in the recent past that incorporate hands-on workshops with the cadaveric model to master the procedure.
Similarly, AAGL has identified five large-volume vaginal centers throughout the country where observership programs are available to practitioners interested in learning the techniques (www.aagl.org). These opportunities allow the surgeon to advance their skills and reincorporate the vaginal hysterectomy into their armamentarium so that patients can benefit from its most minimally invasive approach.
Dr. Kho is associate professor and director of the minimally invasive gynecologic surgery (MIGS) fellowship program in the department of medical and surgical gynecology at the Mayo Clinic in Phoenix, Ariz. She reported that she has no relevant financial disclosures.
The vaginal route is the preferred approach for benign hysterectomy. The most recent Cochrane review of surgical approaches to hysterectomy (abdominal, vaginal, and laparoscopic), which involved more than 3,000 women in 27 randomized controlled trials, shows that vaginal hysterectomy results in fewer complications, shorter hospital stay, and faster recovery and return to normal activity (Cochrane Database Syst. Rev. 2006 (2):CD003677). The vaginal approach also provides the best cosmetic result with its single and concealed incision.
Despite strong evidence for the greater advantage of the vaginal approach, there has not been any increase in the number of hysterectomies performed vaginally. In the United States, the rate appears to have declined in 15 years from 24% in 1990 to 22% in 2005, and this decline may be continuing (Obstet. Gynecol. 2002;99:229-34 and Obstet. Gynecol. 2009;114:1041-8). According to an analysis of a national database from more than 500 acute care hospitals, the majority of gynecologic surgeons in United States (more than 80%) perform fewer than five vaginal surgeries in a year (Obstet. Gynecol. 2010;116:1341-7).
Challenges with exposure, entry into the anterior cul-de-sac, hemostasis, avoidance of ureteral and bladder injury, and removal of the large uterus have been the main stumbling blocks for many surgeons in choosing the vaginal route. I provide, herein, simple techniques, instruments, and devices that can facilitate the performance of the procedure in a safe and efficient manner.
Obtaining exposure
The use of a self-retaining retractor, such as the Magrina-Bookwalter vaginal retractor system (Symmetry Surgical, Nashville, Tenn.) provides consistent and reliable exposure without requiring two surgical assistants at the bedside. Similar to the abdominal self-retractor system, it is attached to the operating table and is designed to fit the contour of the patient’s perineum while in a high lithotomy position. Self-retracting blades of multiple lengths are placed in the four quadrants to maximize room for surgery.
In cases where the introital opening is limited (i.e. = 2.5 cm), such as in nulliparous or menopausal women, a superficial 2- to 3-cm longitudinal incision is performed with bovie cautery in the midline and distal portion of the posterior vaginal wall. This provides additional width to allow placement of the lateral and posterior self-retracting blades.
Additional light from a flexible light source (such as the cystoscopy light) held with a Babcock and a lighted suction irrigator tip (such as Vital Vue, Covidien, Mansfield, Mass.) is extremely helpful in visualization of structures deep within the vagina.
Essential in a vaginal hysterectomy instrument tray are modified deep Deaver retractors that provide additional retraction and visualization particularly in cases of bleeding from pedicles that have retracted to the pelvic sidewall. . A long vaginal pack is also placed to keep loops of bowel out of the operating field. We avoid the use of multiple small sponges that can easily be lost in vaginal cases.
Entry into cul-de-sac
Entry into the anterior cul-de-sac in vaginal hysterectomy can and should be delayed until better descensus of the uterus is obtained. This is achieved with first entering the posterior cul-de-sac, which is often easier to accomplish. To then enter the anterior cul-de-sac, traction is applied posteriorly on the anterior lip of the cervix with the Jacobs tenaculum forceps. The posterior blade is removed to achieve better exposure with a more pronounced angulation of the lower uterine segment.
With ventral traction on the anterior vaginal wall, the bladder is separated from the anterior cervix via sharp dissection with the Mayo curved scissors. The scissor tips are pointed downwards, aimed parallel to the plane of the cervix to reveal the avascular vesicouterine space.
Knowing the anatomy and feel of the tissues is key to mastering entry into the anterior cul-de-sac. Cutting into the cervix will feel tough against the tips of the Metzenbaum scissors, while cutting into the softer beefy-appearing detrusor muscles will manifest with excessive bleeding. The vesicouterine fold is identified as a crescent-shaped peritoneal fold that can be lifted and divided for entry.
In cases where scarring between the bladder and uterus is encountered in patients with multiple previous caesarean sections, dissection is best performed lateral to the midline away from central dense adhesions.
An inability to enter either or both cul-de-sacs should not preclude continuation with the vaginal approach. Securing the uterine arteries can still be accomplished extraperitoneally until better descensus of the uterus is obtained.
Securing vascular pedicles
Achieving hemostasis in vaginal procedures is challenging where there is limited space for placing a suture around the clamp and for securing knots with fingers deep within the vaginal canal. The use of vessel-sealing devices in vaginal hysterectomy overcomes this limitation of tight vaginal access and has proved to be feasible and safe.
Multiple types of energy devices are available, such as PK devices (Gyrus ACMI, Southborough, Mass.), LigaSure instruments (Covidien, Mansfield, Mass.), the Enseal Super Jaw device (Ethicon Endo-Surgery, Nokesville, Va.) and Altrus devices (ConMed Electrosurgery, Centennial, Colo.). These devices can be particularly helpful in cases with narrowed introitus and large uterus. The choice of device is surgeon dependent and requires a learning curve.
Gentle traction is placed on the cervix while the device clamp is pushed up against the pedicle, taking care to avoid leaning against any adjacent tissues or retractor blades to avoid thermal injury. Bringing in a suction device quickly dissipates the hot steam that can be generated from the device.
Avoidance of bladder and ureteral injury
Once the vesicouterine space is entered, bladder pillars are gently pushed superiorly and laterally with the index finger to avoid injury during placement of the vessel-sealing clamp. It is imperative that the surgeon is aware of the location of the ureters, which are easily injured, particularly in cases with pelvic prolapse.
The ureters can be palpated with the index finger at 2 o’clock or at 10 o’clock (for the left and right ureter, respectively) against a curved Deaver retractor placed outside the peritoneal cavity on the lateral vaginal wall. Intraoperative cystoscopy should always be performed at the end of the procedure to diagnose inadvertent bladder and ureteral injury.
Removing the Large Uterus
Morcellation can be initiated after the uterine arteries have been sealed and divided on each side. Orientation of the uterus is maintained by placing two Jacobs tenaculi at the 3 o’clock and at 9 o’clock positions on the cervix. The cervix is bivalved to the level of the lower uterine segment.
If the anterior cul-de-sac has not been entered yet, the cervix should be bivalved to a centimeter below the vesicouterine peritoneal fold and morcellation started within the uterus.
Morcellation is performed with a Schroeder tenaculum (Aesculap Inc., Center Valley, Pa.) placed on the myometrium, and a wedge excision is accomplished with a 10 blade. Serial wedges are performed to decompress the uterus. Entry into the anterior cul-de-sac can now be performed easily with better uterine descensus and visualization of the peritoneal fold.
The surgeon should avoid forceful traction on the cervix during morcellation, which can cause the vascular pedicles to avulse.
Depending on the size of the uterus, morcellation can take over an hour. Use of an articulated long scalpel handle (such as the Precise CMK ergonomic knife by LaparoTools, in Metairie, La., which is rounded) can facilitate morcellation with less fatigue.
The surgeon must persist with morcellation as long as descensus of the uterus is continually achieved. An increase in bleeding is encountered when morcellation is near the fundus. At this point, the utero-ovarian pedicles can be identified and secured to finish the procedure.
Mastering the procedure
Simple techniques and new surgical devices are available to facilitate the difficult vaginal hysterectomy. Cases of narrowed introitus, multiple previous caesarean sections, and the large uteri requiring morcellation can be approached vaginally and achieved safely and efficiently with use of the above techniques.
In recognition of the vaginal approach as a minimally invasive procedure, AAGL has provided postgraduate courses in the recent past that incorporate hands-on workshops with the cadaveric model to master the procedure.
Similarly, AAGL has identified five large-volume vaginal centers throughout the country where observership programs are available to practitioners interested in learning the techniques (www.aagl.org). These opportunities allow the surgeon to advance their skills and reincorporate the vaginal hysterectomy into their armamentarium so that patients can benefit from its most minimally invasive approach.
Dr. Kho is associate professor and director of the minimally invasive gynecologic surgery (MIGS) fellowship program in the department of medical and surgical gynecology at the Mayo Clinic in Phoenix, Ariz. She reported that she has no relevant financial disclosures.
The vaginal route is the preferred approach for benign hysterectomy. The most recent Cochrane review of surgical approaches to hysterectomy (abdominal, vaginal, and laparoscopic), which involved more than 3,000 women in 27 randomized controlled trials, shows that vaginal hysterectomy results in fewer complications, shorter hospital stay, and faster recovery and return to normal activity (Cochrane Database Syst. Rev. 2006 (2):CD003677). The vaginal approach also provides the best cosmetic result with its single and concealed incision.
Despite strong evidence for the greater advantage of the vaginal approach, there has not been any increase in the number of hysterectomies performed vaginally. In the United States, the rate appears to have declined in 15 years from 24% in 1990 to 22% in 2005, and this decline may be continuing (Obstet. Gynecol. 2002;99:229-34 and Obstet. Gynecol. 2009;114:1041-8). According to an analysis of a national database from more than 500 acute care hospitals, the majority of gynecologic surgeons in United States (more than 80%) perform fewer than five vaginal surgeries in a year (Obstet. Gynecol. 2010;116:1341-7).
Challenges with exposure, entry into the anterior cul-de-sac, hemostasis, avoidance of ureteral and bladder injury, and removal of the large uterus have been the main stumbling blocks for many surgeons in choosing the vaginal route. I provide, herein, simple techniques, instruments, and devices that can facilitate the performance of the procedure in a safe and efficient manner.
Obtaining exposure
The use of a self-retaining retractor, such as the Magrina-Bookwalter vaginal retractor system (Symmetry Surgical, Nashville, Tenn.) provides consistent and reliable exposure without requiring two surgical assistants at the bedside. Similar to the abdominal self-retractor system, it is attached to the operating table and is designed to fit the contour of the patient’s perineum while in a high lithotomy position. Self-retracting blades of multiple lengths are placed in the four quadrants to maximize room for surgery.
In cases where the introital opening is limited (i.e. = 2.5 cm), such as in nulliparous or menopausal women, a superficial 2- to 3-cm longitudinal incision is performed with bovie cautery in the midline and distal portion of the posterior vaginal wall. This provides additional width to allow placement of the lateral and posterior self-retracting blades.
Additional light from a flexible light source (such as the cystoscopy light) held with a Babcock and a lighted suction irrigator tip (such as Vital Vue, Covidien, Mansfield, Mass.) is extremely helpful in visualization of structures deep within the vagina.
Essential in a vaginal hysterectomy instrument tray are modified deep Deaver retractors that provide additional retraction and visualization particularly in cases of bleeding from pedicles that have retracted to the pelvic sidewall. . A long vaginal pack is also placed to keep loops of bowel out of the operating field. We avoid the use of multiple small sponges that can easily be lost in vaginal cases.
Entry into cul-de-sac
Entry into the anterior cul-de-sac in vaginal hysterectomy can and should be delayed until better descensus of the uterus is obtained. This is achieved with first entering the posterior cul-de-sac, which is often easier to accomplish. To then enter the anterior cul-de-sac, traction is applied posteriorly on the anterior lip of the cervix with the Jacobs tenaculum forceps. The posterior blade is removed to achieve better exposure with a more pronounced angulation of the lower uterine segment.
With ventral traction on the anterior vaginal wall, the bladder is separated from the anterior cervix via sharp dissection with the Mayo curved scissors. The scissor tips are pointed downwards, aimed parallel to the plane of the cervix to reveal the avascular vesicouterine space.
Knowing the anatomy and feel of the tissues is key to mastering entry into the anterior cul-de-sac. Cutting into the cervix will feel tough against the tips of the Metzenbaum scissors, while cutting into the softer beefy-appearing detrusor muscles will manifest with excessive bleeding. The vesicouterine fold is identified as a crescent-shaped peritoneal fold that can be lifted and divided for entry.
In cases where scarring between the bladder and uterus is encountered in patients with multiple previous caesarean sections, dissection is best performed lateral to the midline away from central dense adhesions.
An inability to enter either or both cul-de-sacs should not preclude continuation with the vaginal approach. Securing the uterine arteries can still be accomplished extraperitoneally until better descensus of the uterus is obtained.
Securing vascular pedicles
Achieving hemostasis in vaginal procedures is challenging where there is limited space for placing a suture around the clamp and for securing knots with fingers deep within the vaginal canal. The use of vessel-sealing devices in vaginal hysterectomy overcomes this limitation of tight vaginal access and has proved to be feasible and safe.
Multiple types of energy devices are available, such as PK devices (Gyrus ACMI, Southborough, Mass.), LigaSure instruments (Covidien, Mansfield, Mass.), the Enseal Super Jaw device (Ethicon Endo-Surgery, Nokesville, Va.) and Altrus devices (ConMed Electrosurgery, Centennial, Colo.). These devices can be particularly helpful in cases with narrowed introitus and large uterus. The choice of device is surgeon dependent and requires a learning curve.
Gentle traction is placed on the cervix while the device clamp is pushed up against the pedicle, taking care to avoid leaning against any adjacent tissues or retractor blades to avoid thermal injury. Bringing in a suction device quickly dissipates the hot steam that can be generated from the device.
Avoidance of bladder and ureteral injury
Once the vesicouterine space is entered, bladder pillars are gently pushed superiorly and laterally with the index finger to avoid injury during placement of the vessel-sealing clamp. It is imperative that the surgeon is aware of the location of the ureters, which are easily injured, particularly in cases with pelvic prolapse.
The ureters can be palpated with the index finger at 2 o’clock or at 10 o’clock (for the left and right ureter, respectively) against a curved Deaver retractor placed outside the peritoneal cavity on the lateral vaginal wall. Intraoperative cystoscopy should always be performed at the end of the procedure to diagnose inadvertent bladder and ureteral injury.
Removing the Large Uterus
Morcellation can be initiated after the uterine arteries have been sealed and divided on each side. Orientation of the uterus is maintained by placing two Jacobs tenaculi at the 3 o’clock and at 9 o’clock positions on the cervix. The cervix is bivalved to the level of the lower uterine segment.
If the anterior cul-de-sac has not been entered yet, the cervix should be bivalved to a centimeter below the vesicouterine peritoneal fold and morcellation started within the uterus.
Morcellation is performed with a Schroeder tenaculum (Aesculap Inc., Center Valley, Pa.) placed on the myometrium, and a wedge excision is accomplished with a 10 blade. Serial wedges are performed to decompress the uterus. Entry into the anterior cul-de-sac can now be performed easily with better uterine descensus and visualization of the peritoneal fold.
The surgeon should avoid forceful traction on the cervix during morcellation, which can cause the vascular pedicles to avulse.
Depending on the size of the uterus, morcellation can take over an hour. Use of an articulated long scalpel handle (such as the Precise CMK ergonomic knife by LaparoTools, in Metairie, La., which is rounded) can facilitate morcellation with less fatigue.
The surgeon must persist with morcellation as long as descensus of the uterus is continually achieved. An increase in bleeding is encountered when morcellation is near the fundus. At this point, the utero-ovarian pedicles can be identified and secured to finish the procedure.
Mastering the procedure
Simple techniques and new surgical devices are available to facilitate the difficult vaginal hysterectomy. Cases of narrowed introitus, multiple previous caesarean sections, and the large uteri requiring morcellation can be approached vaginally and achieved safely and efficiently with use of the above techniques.
In recognition of the vaginal approach as a minimally invasive procedure, AAGL has provided postgraduate courses in the recent past that incorporate hands-on workshops with the cadaveric model to master the procedure.
Similarly, AAGL has identified five large-volume vaginal centers throughout the country where observership programs are available to practitioners interested in learning the techniques (www.aagl.org). These opportunities allow the surgeon to advance their skills and reincorporate the vaginal hysterectomy into their armamentarium so that patients can benefit from its most minimally invasive approach.
Dr. Kho is associate professor and director of the minimally invasive gynecologic surgery (MIGS) fellowship program in the department of medical and surgical gynecology at the Mayo Clinic in Phoenix, Ariz. She reported that she has no relevant financial disclosures.