User login
Utilizing a Sleep Mask to Reduce Patient Anxiety During Nail Surgery
Practice Gap
Perioperative anxiety is common in patients undergoing nail surgery. Patients might worry about seeing blood; about the procedure itself, including nail avulsion; and about associated pain and disfigurement. Nail surgery causes a high level of anxiety that correlates positively with postoperative pain1 and overall patient dissatisfaction. Furthermore, surgery-related anxiety is a predictor of increased postoperative analgesic use2 and delayed recovery.3
Therefore, implementing strategies that reduce perioperative anxiety may help minimize postoperative pain. Squeezing a stress ball, hand-holding, virtual reality, and music are tools that have been studied to reduce anxiety in the context of Mohs micrographic surgery; these strategies have not been studied for nail surgery.
The Technique
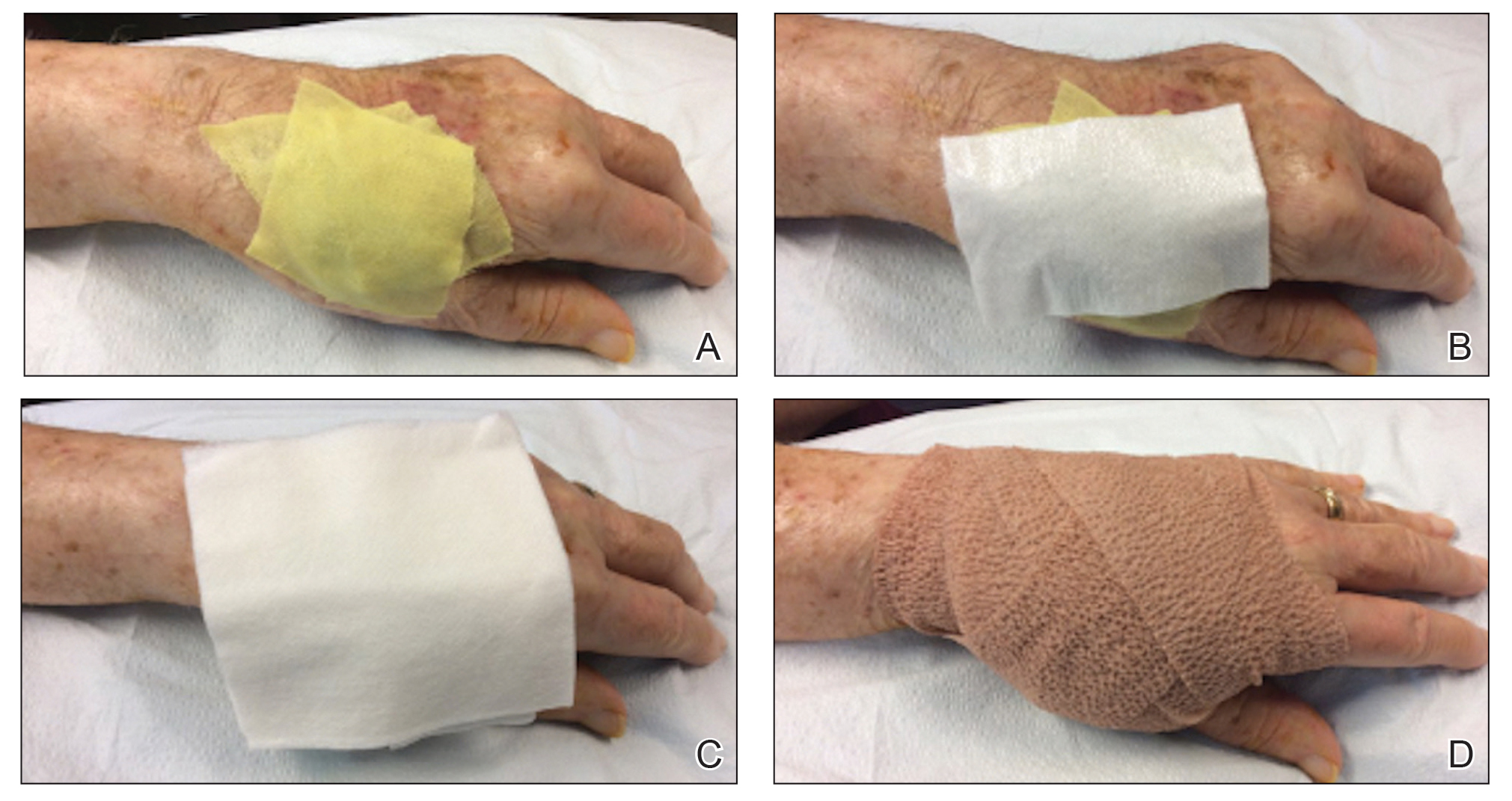
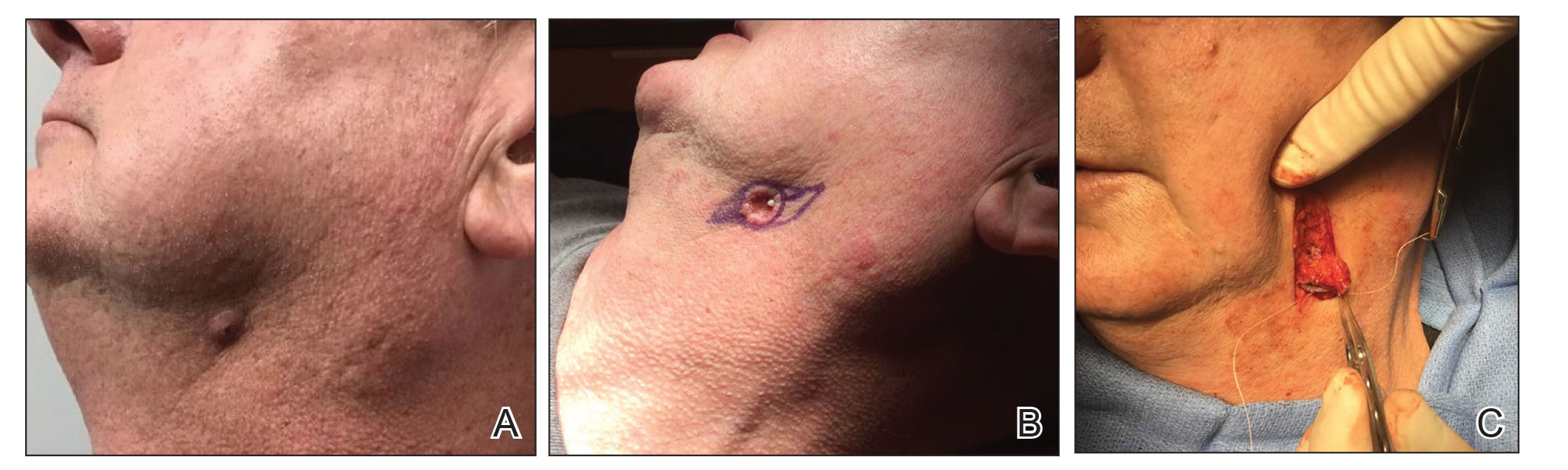
Using a sleep mask is a practical solution to reduce patient anxiety during nail surgery. A minority of patients will choose to watch their surgical procedure; most become unnerved observing their nail surgery. Using a sleep mask diverts visual attention from the surgical field without physically interfering with the nail surgeon. Utilizing a sleep mask is cost-effective, with disposable sleep masks available online for less than $0.30 each. Patients can bring their own mask, or a mask can be offered prior to surgery.
If desired, patients are instructed to wear the sleep mask during the entirety of the procedure, starting from anesthetic infiltration until wound closure and dressing application. Any adjustments can be made with the patient’s free hand. The sleep mask can be offered to patients of all ages undergoing nail surgery under local anesthesia, except babies and young children, who require general anesthesia.
Practical Implications
Distraction is an important strategy to reduce anxiety and pain in patients undergoing surgical procedures. In an observational study of 3087 surgical patients, 36% reported that self-distraction was the most helpful strategy for coping with preoperative anxiety.4 In a randomized, open-label clinical trial of 72 patients undergoing peripheral venous catheterization, asking the patients simple questions during the procedure was more effective than local anesthesia in reducing the perception of pain.5
It is crucial to implement strategies to reduce anxiety in patients undergoing nail surgery. Using a sleep mask impedes direct visualization of the surgical field, thus distracting the patient’s sight and attention from the procedure. Furthermore, this technique is safe and cost-effective.
Controlled clinical trials are necessary to assess the efficacy of this method in reducing nail surgery–related anxiety in comparison to other techniques.
- Navarro-Gastón D, Munuera-Martínez PV. Prevalence of preoperative anxiety and its relationship with postoperative pain in foot nail surgery: a cross-sectional study. Int J Environ Res Public Health. 2020;17:4481. doi:10.3390/ijerph17124481
- Ip HYV, Abrishami A, Peng PWH, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Aust H, Rüsch D, Schuster M, et al. Coping strategies in anxious surgical patients. BMC Health Serv Res. 2016;16:250. doi:10.1186/s12913-016-1492-5
- Balanyuk I, Ledonne G, Provenzano M, et al. Distraction technique for pain reduction in peripheral venous catheterization: randomized, controlled trial. Acta Biomed. 2018;89(suppl 4):55-63. doi:10.23750/abmv89i4-S.7115
Practice Gap
Perioperative anxiety is common in patients undergoing nail surgery. Patients might worry about seeing blood; about the procedure itself, including nail avulsion; and about associated pain and disfigurement. Nail surgery causes a high level of anxiety that correlates positively with postoperative pain1 and overall patient dissatisfaction. Furthermore, surgery-related anxiety is a predictor of increased postoperative analgesic use2 and delayed recovery.3
Therefore, implementing strategies that reduce perioperative anxiety may help minimize postoperative pain. Squeezing a stress ball, hand-holding, virtual reality, and music are tools that have been studied to reduce anxiety in the context of Mohs micrographic surgery; these strategies have not been studied for nail surgery.
The Technique
Using a sleep mask is a practical solution to reduce patient anxiety during nail surgery. A minority of patients will choose to watch their surgical procedure; most become unnerved observing their nail surgery. Using a sleep mask diverts visual attention from the surgical field without physically interfering with the nail surgeon. Utilizing a sleep mask is cost-effective, with disposable sleep masks available online for less than $0.30 each. Patients can bring their own mask, or a mask can be offered prior to surgery.
If desired, patients are instructed to wear the sleep mask during the entirety of the procedure, starting from anesthetic infiltration until wound closure and dressing application. Any adjustments can be made with the patient’s free hand. The sleep mask can be offered to patients of all ages undergoing nail surgery under local anesthesia, except babies and young children, who require general anesthesia.
Practical Implications
Distraction is an important strategy to reduce anxiety and pain in patients undergoing surgical procedures. In an observational study of 3087 surgical patients, 36% reported that self-distraction was the most helpful strategy for coping with preoperative anxiety.4 In a randomized, open-label clinical trial of 72 patients undergoing peripheral venous catheterization, asking the patients simple questions during the procedure was more effective than local anesthesia in reducing the perception of pain.5
It is crucial to implement strategies to reduce anxiety in patients undergoing nail surgery. Using a sleep mask impedes direct visualization of the surgical field, thus distracting the patient’s sight and attention from the procedure. Furthermore, this technique is safe and cost-effective.
Controlled clinical trials are necessary to assess the efficacy of this method in reducing nail surgery–related anxiety in comparison to other techniques.
Practice Gap
Perioperative anxiety is common in patients undergoing nail surgery. Patients might worry about seeing blood; about the procedure itself, including nail avulsion; and about associated pain and disfigurement. Nail surgery causes a high level of anxiety that correlates positively with postoperative pain1 and overall patient dissatisfaction. Furthermore, surgery-related anxiety is a predictor of increased postoperative analgesic use2 and delayed recovery.3
Therefore, implementing strategies that reduce perioperative anxiety may help minimize postoperative pain. Squeezing a stress ball, hand-holding, virtual reality, and music are tools that have been studied to reduce anxiety in the context of Mohs micrographic surgery; these strategies have not been studied for nail surgery.
The Technique
Using a sleep mask is a practical solution to reduce patient anxiety during nail surgery. A minority of patients will choose to watch their surgical procedure; most become unnerved observing their nail surgery. Using a sleep mask diverts visual attention from the surgical field without physically interfering with the nail surgeon. Utilizing a sleep mask is cost-effective, with disposable sleep masks available online for less than $0.30 each. Patients can bring their own mask, or a mask can be offered prior to surgery.
If desired, patients are instructed to wear the sleep mask during the entirety of the procedure, starting from anesthetic infiltration until wound closure and dressing application. Any adjustments can be made with the patient’s free hand. The sleep mask can be offered to patients of all ages undergoing nail surgery under local anesthesia, except babies and young children, who require general anesthesia.
Practical Implications
Distraction is an important strategy to reduce anxiety and pain in patients undergoing surgical procedures. In an observational study of 3087 surgical patients, 36% reported that self-distraction was the most helpful strategy for coping with preoperative anxiety.4 In a randomized, open-label clinical trial of 72 patients undergoing peripheral venous catheterization, asking the patients simple questions during the procedure was more effective than local anesthesia in reducing the perception of pain.5
It is crucial to implement strategies to reduce anxiety in patients undergoing nail surgery. Using a sleep mask impedes direct visualization of the surgical field, thus distracting the patient’s sight and attention from the procedure. Furthermore, this technique is safe and cost-effective.
Controlled clinical trials are necessary to assess the efficacy of this method in reducing nail surgery–related anxiety in comparison to other techniques.
- Navarro-Gastón D, Munuera-Martínez PV. Prevalence of preoperative anxiety and its relationship with postoperative pain in foot nail surgery: a cross-sectional study. Int J Environ Res Public Health. 2020;17:4481. doi:10.3390/ijerph17124481
- Ip HYV, Abrishami A, Peng PWH, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Aust H, Rüsch D, Schuster M, et al. Coping strategies in anxious surgical patients. BMC Health Serv Res. 2016;16:250. doi:10.1186/s12913-016-1492-5
- Balanyuk I, Ledonne G, Provenzano M, et al. Distraction technique for pain reduction in peripheral venous catheterization: randomized, controlled trial. Acta Biomed. 2018;89(suppl 4):55-63. doi:10.23750/abmv89i4-S.7115
- Navarro-Gastón D, Munuera-Martínez PV. Prevalence of preoperative anxiety and its relationship with postoperative pain in foot nail surgery: a cross-sectional study. Int J Environ Res Public Health. 2020;17:4481. doi:10.3390/ijerph17124481
- Ip HYV, Abrishami A, Peng PWH, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Aust H, Rüsch D, Schuster M, et al. Coping strategies in anxious surgical patients. BMC Health Serv Res. 2016;16:250. doi:10.1186/s12913-016-1492-5
- Balanyuk I, Ledonne G, Provenzano M, et al. Distraction technique for pain reduction in peripheral venous catheterization: randomized, controlled trial. Acta Biomed. 2018;89(suppl 4):55-63. doi:10.23750/abmv89i4-S.7115
24-7 Dressing Technique to Optimize Wound Healing After Mohs Micrographic Surgery
Practice Gap
Management of surgical wounds is a critical component of postsurgical care for patients during recovery at home.1 However, postoperative wound care can be troublesome, time consuming, and expensive. Common problems with current standard dressings include an increased risk for infection, pain, and wound damage with frequent dressing changes.2-4
Patients often are unable to take proper care of wounds themselves and may not have the financial means or social support to have others assist them.4-6 For these patients, the option of a hassle-free dressing that they can leave on until their follow-up appointment is preferred. In our experience, what we call a 24-7 bandage has been remarkably successful in patients who are vulnerable to wound complications.
We report a comfortable, effective, and simple technique for wound dressings after dermatologic surgery.
The Technique
In Figure 1, we demonstrate a simple dressing technique that can be used to optimize wound healing in patients unable to provide adequate wound care for themselves:
1. The surgical site is covered with mupirocin ointment, followed by bismuth tribromophenate gauze (Figure 1A). The bismuth-impregnated gauze helps make the dressing nonadherent and moderately occlusive. It also adds moisture to the wound bed.
2. The gauze is then covered with excess mupirocin. A nonadherent dressing is applied (Figure 1B).
3. The entire area is covered with gauze and cover-roll nonlatex bandaging tape to ensure maximum adhesion (Figures 1C and 1D).
4. When the surgical site is on an extremity, it is wrapped in a self-adherent wrap or bandage roll to prevent clothing from pulling the tape loose.
Once this dressing technique is performed in the office, the bandage requires no wound care at home other than ensuring that the bandage is kept dry. The 24-7 dressing can be left on the surgical site for 7 days until the follow-up appointment. If necessary, it also can be applied for a second week after bolster removal or for multiple weeks following advanced flap repair.
Our patients find this dressing comfortable and unobtrusive. It is easy for the staff to apply and inexpensive.
Practical Implications
We have treated approximately 200 patients with the 24-7 dressing technique. Our experience is that these patients demonstrated an excellent aesthetic outcome without complications (Figure 2). We have successfully utilized the dressing in several anatomic locations, including the arms, legs, neck, face, and scalp. We use mupirocin for its antimicrobial activity, but we have not performed a study at our clinic looking at the difference between rate of infection and wound healing using mupirocin vs petrolatum. We prefer adding bulk gauze under the tape and leaving the dressing on for 7 days. We seldom have issues with bleeding, and if there is an issue, the patient is told to come back to our clinic so we can change the bandage for them.
This dressing technique is cost-effective to the patient and clinical staff, provides protection from potential injury to the sutures, decreases the risk for infection, and removes the stress and burden on the patient and family of frequent dressing changes. Furthermore, by preventing patient manipulation and frequent removal of the dressing, the wound retains adequate moisture during healing. This technique also can be applied to a variety of outpatient procedures other than Mohs micrographic surgery.
We hope that our colleagues find this 24-7 dressing technique for dressing wounds after dermatologic surgery useful in patient populations vulnerable to wound complications.
- Winton GB, Salasche SJ. Wound dressings for dermatologic surgery. J Am Acad Dermatol. 1995;13:1026-1044.
- Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14:449-459.
- Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. a clinical review. Dermatol Surg. 1995;21:583-590.
- Jones AM, San Miguel L. Are modern wound dressings a clinical and cost-effective alternative to the use of gauze? J Wound Care. 2006;15:65-66.
- Ubbink DT, Vermeulen H, Goossens A. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg. 2008;143:950-955.
- Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014;3;511-529.
Practice Gap
Management of surgical wounds is a critical component of postsurgical care for patients during recovery at home.1 However, postoperative wound care can be troublesome, time consuming, and expensive. Common problems with current standard dressings include an increased risk for infection, pain, and wound damage with frequent dressing changes.2-4
Patients often are unable to take proper care of wounds themselves and may not have the financial means or social support to have others assist them.4-6 For these patients, the option of a hassle-free dressing that they can leave on until their follow-up appointment is preferred. In our experience, what we call a 24-7 bandage has been remarkably successful in patients who are vulnerable to wound complications.
We report a comfortable, effective, and simple technique for wound dressings after dermatologic surgery.
The Technique
In Figure 1, we demonstrate a simple dressing technique that can be used to optimize wound healing in patients unable to provide adequate wound care for themselves:
1. The surgical site is covered with mupirocin ointment, followed by bismuth tribromophenate gauze (Figure 1A). The bismuth-impregnated gauze helps make the dressing nonadherent and moderately occlusive. It also adds moisture to the wound bed.
2. The gauze is then covered with excess mupirocin. A nonadherent dressing is applied (Figure 1B).
3. The entire area is covered with gauze and cover-roll nonlatex bandaging tape to ensure maximum adhesion (Figures 1C and 1D).
4. When the surgical site is on an extremity, it is wrapped in a self-adherent wrap or bandage roll to prevent clothing from pulling the tape loose.
Once this dressing technique is performed in the office, the bandage requires no wound care at home other than ensuring that the bandage is kept dry. The 24-7 dressing can be left on the surgical site for 7 days until the follow-up appointment. If necessary, it also can be applied for a second week after bolster removal or for multiple weeks following advanced flap repair.
Our patients find this dressing comfortable and unobtrusive. It is easy for the staff to apply and inexpensive.
Practical Implications
We have treated approximately 200 patients with the 24-7 dressing technique. Our experience is that these patients demonstrated an excellent aesthetic outcome without complications (Figure 2). We have successfully utilized the dressing in several anatomic locations, including the arms, legs, neck, face, and scalp. We use mupirocin for its antimicrobial activity, but we have not performed a study at our clinic looking at the difference between rate of infection and wound healing using mupirocin vs petrolatum. We prefer adding bulk gauze under the tape and leaving the dressing on for 7 days. We seldom have issues with bleeding, and if there is an issue, the patient is told to come back to our clinic so we can change the bandage for them.
This dressing technique is cost-effective to the patient and clinical staff, provides protection from potential injury to the sutures, decreases the risk for infection, and removes the stress and burden on the patient and family of frequent dressing changes. Furthermore, by preventing patient manipulation and frequent removal of the dressing, the wound retains adequate moisture during healing. This technique also can be applied to a variety of outpatient procedures other than Mohs micrographic surgery.
We hope that our colleagues find this 24-7 dressing technique for dressing wounds after dermatologic surgery useful in patient populations vulnerable to wound complications.
Practice Gap
Management of surgical wounds is a critical component of postsurgical care for patients during recovery at home.1 However, postoperative wound care can be troublesome, time consuming, and expensive. Common problems with current standard dressings include an increased risk for infection, pain, and wound damage with frequent dressing changes.2-4
Patients often are unable to take proper care of wounds themselves and may not have the financial means or social support to have others assist them.4-6 For these patients, the option of a hassle-free dressing that they can leave on until their follow-up appointment is preferred. In our experience, what we call a 24-7 bandage has been remarkably successful in patients who are vulnerable to wound complications.
We report a comfortable, effective, and simple technique for wound dressings after dermatologic surgery.
The Technique
In Figure 1, we demonstrate a simple dressing technique that can be used to optimize wound healing in patients unable to provide adequate wound care for themselves:
1. The surgical site is covered with mupirocin ointment, followed by bismuth tribromophenate gauze (Figure 1A). The bismuth-impregnated gauze helps make the dressing nonadherent and moderately occlusive. It also adds moisture to the wound bed.
2. The gauze is then covered with excess mupirocin. A nonadherent dressing is applied (Figure 1B).
3. The entire area is covered with gauze and cover-roll nonlatex bandaging tape to ensure maximum adhesion (Figures 1C and 1D).
4. When the surgical site is on an extremity, it is wrapped in a self-adherent wrap or bandage roll to prevent clothing from pulling the tape loose.
Once this dressing technique is performed in the office, the bandage requires no wound care at home other than ensuring that the bandage is kept dry. The 24-7 dressing can be left on the surgical site for 7 days until the follow-up appointment. If necessary, it also can be applied for a second week after bolster removal or for multiple weeks following advanced flap repair.
Our patients find this dressing comfortable and unobtrusive. It is easy for the staff to apply and inexpensive.
Practical Implications
We have treated approximately 200 patients with the 24-7 dressing technique. Our experience is that these patients demonstrated an excellent aesthetic outcome without complications (Figure 2). We have successfully utilized the dressing in several anatomic locations, including the arms, legs, neck, face, and scalp. We use mupirocin for its antimicrobial activity, but we have not performed a study at our clinic looking at the difference between rate of infection and wound healing using mupirocin vs petrolatum. We prefer adding bulk gauze under the tape and leaving the dressing on for 7 days. We seldom have issues with bleeding, and if there is an issue, the patient is told to come back to our clinic so we can change the bandage for them.
This dressing technique is cost-effective to the patient and clinical staff, provides protection from potential injury to the sutures, decreases the risk for infection, and removes the stress and burden on the patient and family of frequent dressing changes. Furthermore, by preventing patient manipulation and frequent removal of the dressing, the wound retains adequate moisture during healing. This technique also can be applied to a variety of outpatient procedures other than Mohs micrographic surgery.
We hope that our colleagues find this 24-7 dressing technique for dressing wounds after dermatologic surgery useful in patient populations vulnerable to wound complications.
- Winton GB, Salasche SJ. Wound dressings for dermatologic surgery. J Am Acad Dermatol. 1995;13:1026-1044.
- Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14:449-459.
- Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. a clinical review. Dermatol Surg. 1995;21:583-590.
- Jones AM, San Miguel L. Are modern wound dressings a clinical and cost-effective alternative to the use of gauze? J Wound Care. 2006;15:65-66.
- Ubbink DT, Vermeulen H, Goossens A. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg. 2008;143:950-955.
- Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014;3;511-529.
- Winton GB, Salasche SJ. Wound dressings for dermatologic surgery. J Am Acad Dermatol. 1995;13:1026-1044.
- Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14:449-459.
- Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. a clinical review. Dermatol Surg. 1995;21:583-590.
- Jones AM, San Miguel L. Are modern wound dressings a clinical and cost-effective alternative to the use of gauze? J Wound Care. 2006;15:65-66.
- Ubbink DT, Vermeulen H, Goossens A. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg. 2008;143:950-955.
- Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle). 2014;3;511-529.
Combined Treatment of Disfiguring Facial Angiofibromas in Tuberous Sclerosis Complex With Surgical Debulking and Topical Sirolimus
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
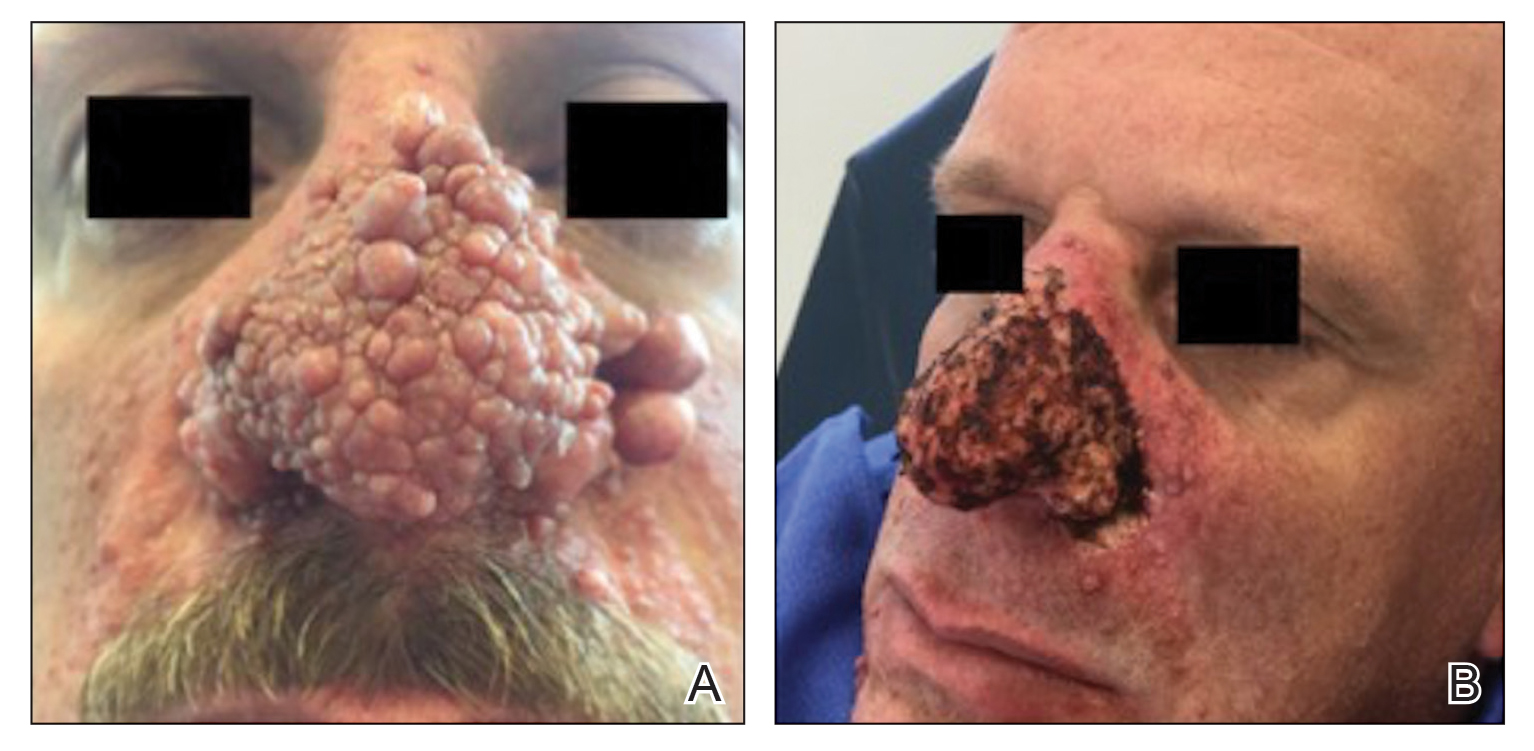
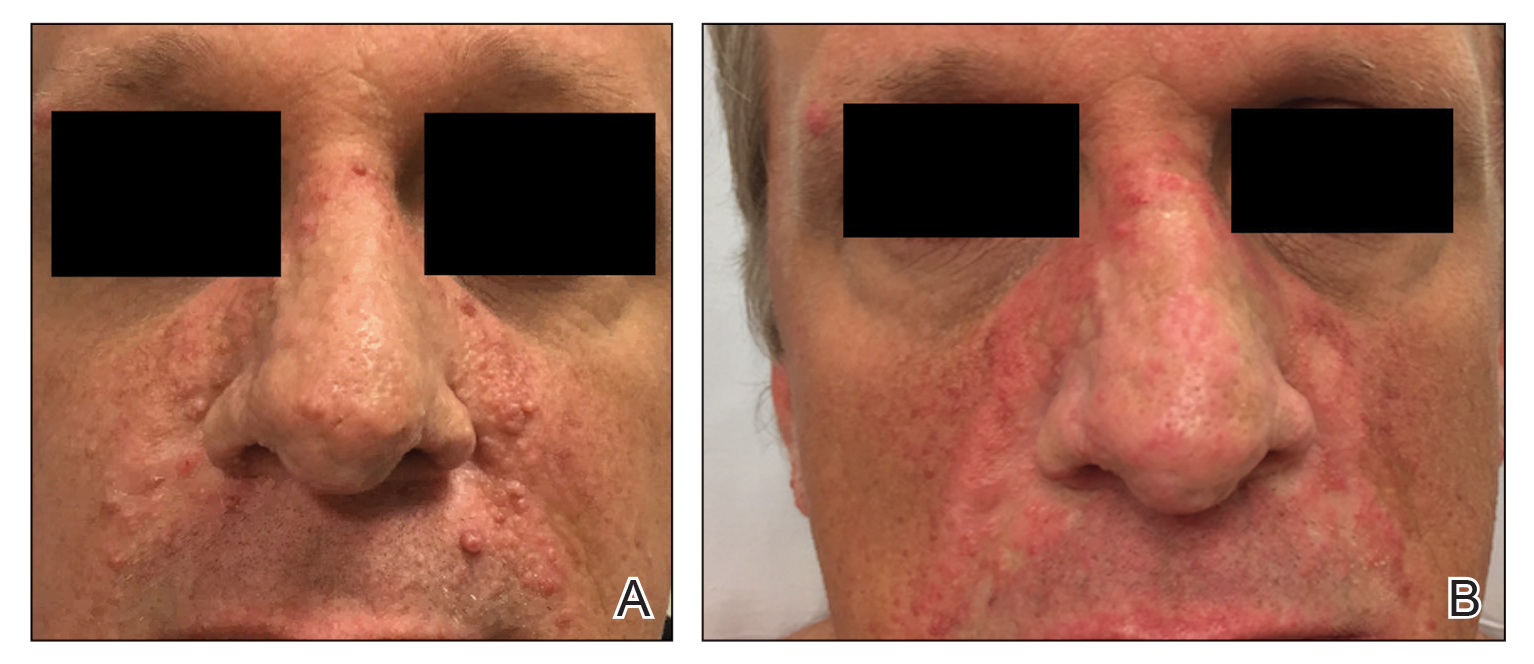
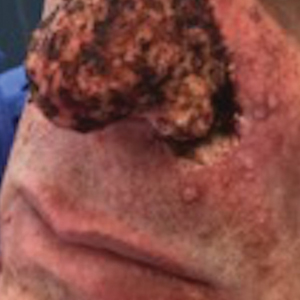
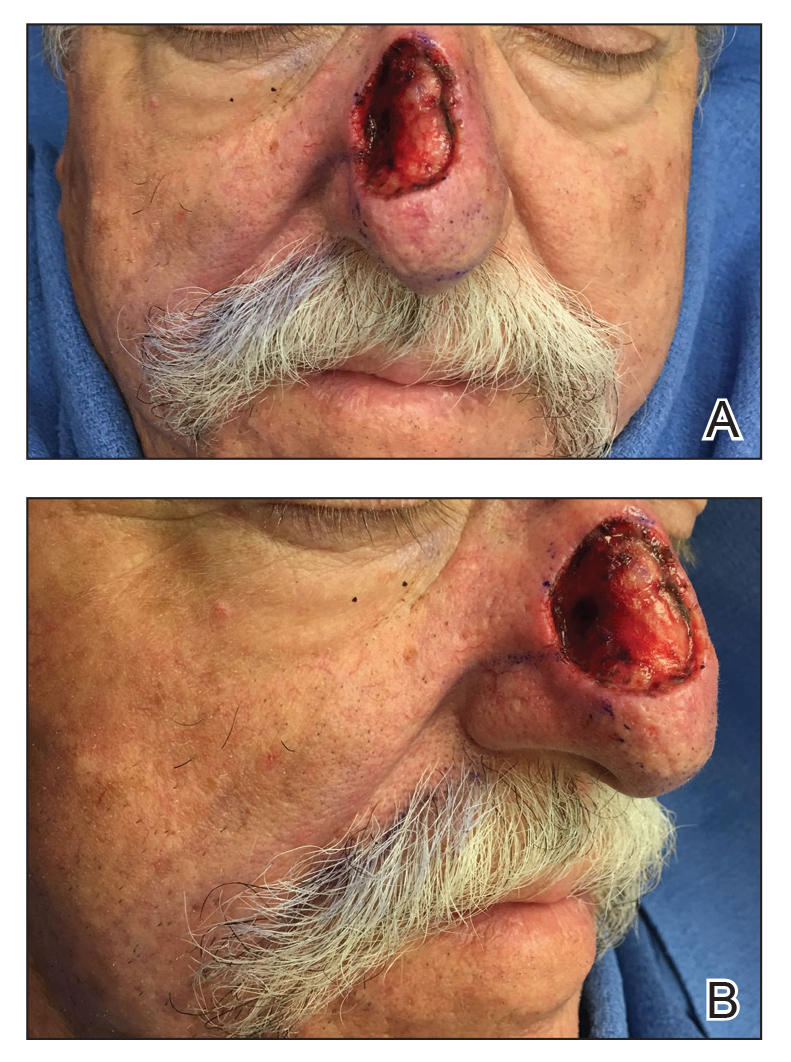
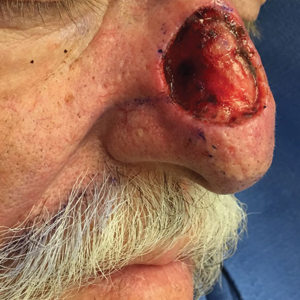
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.
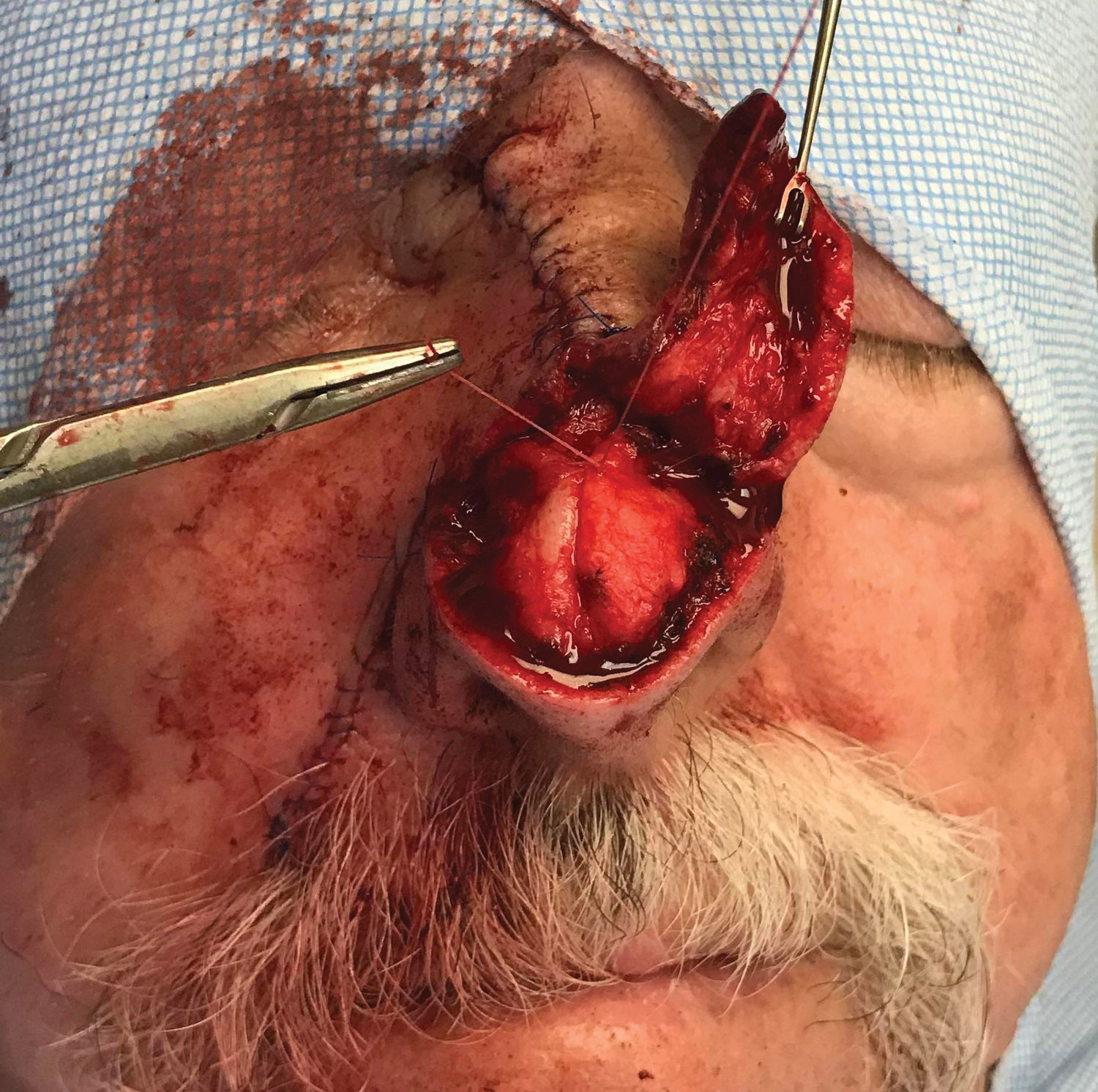
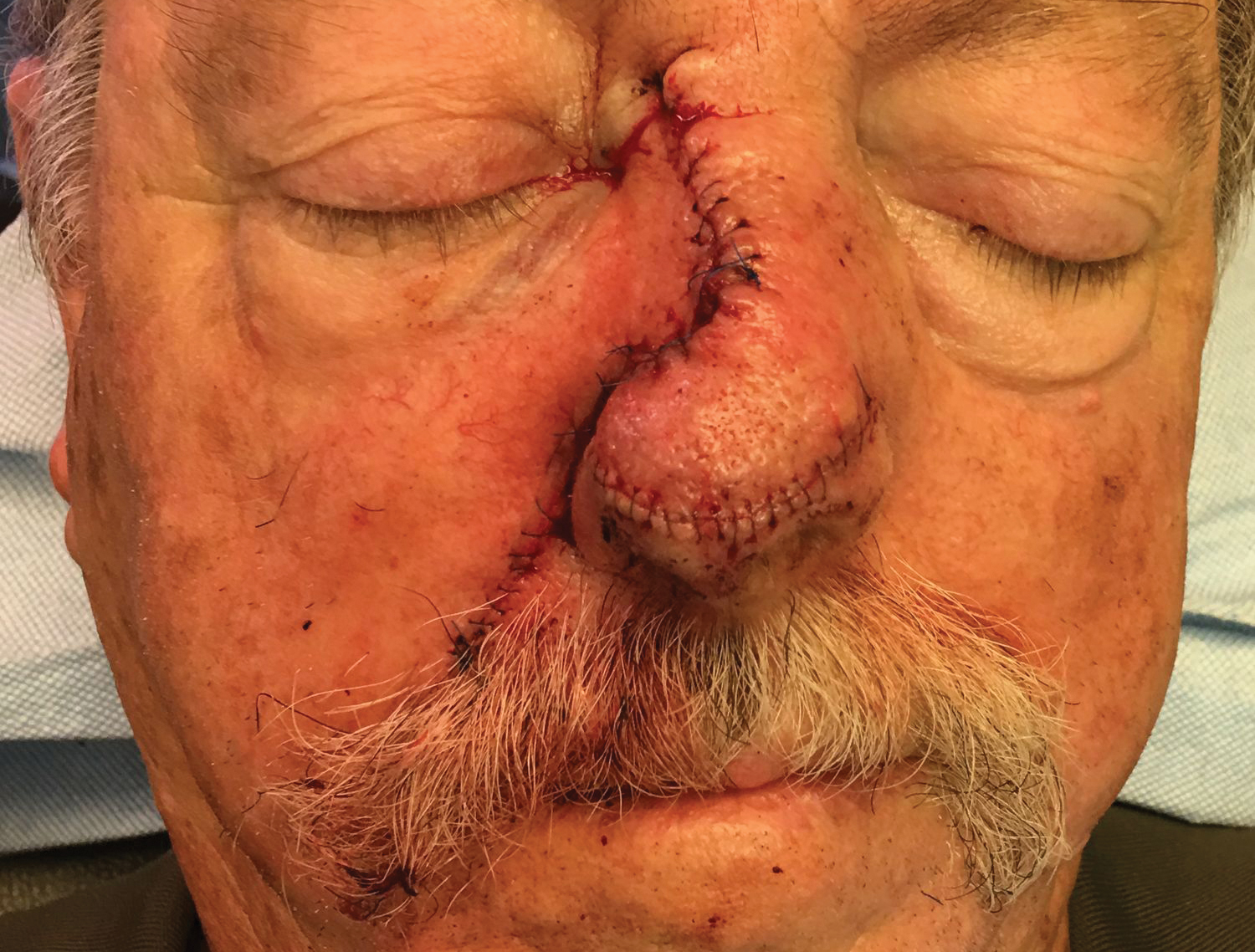
Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
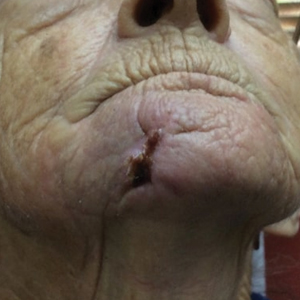
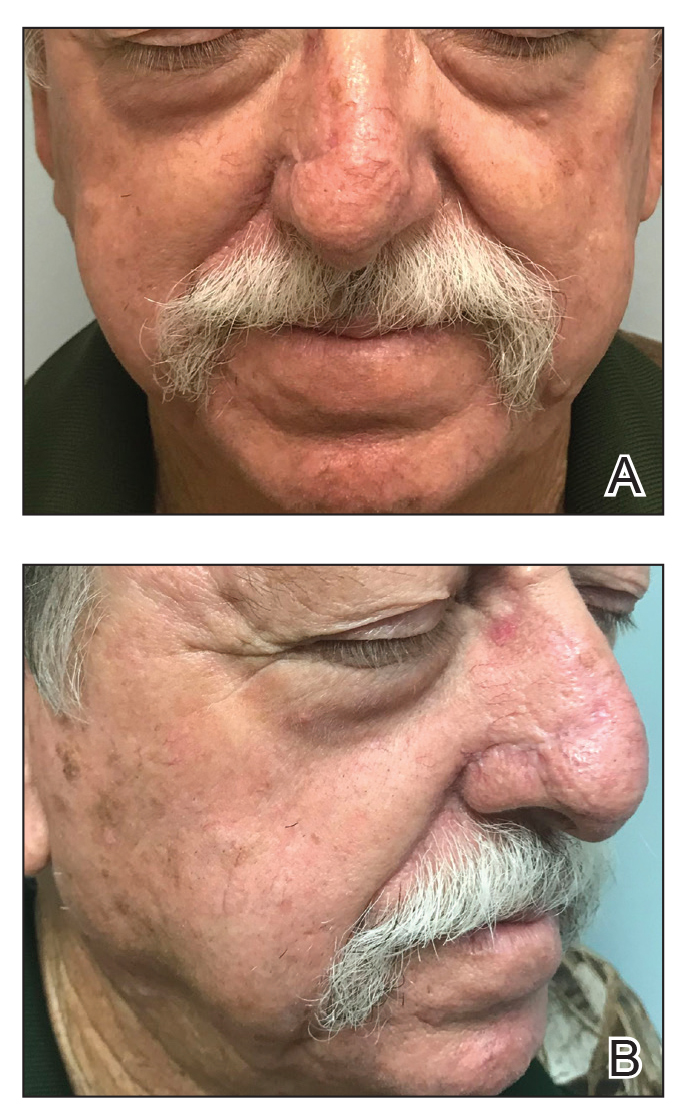
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).
Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.
Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).
Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.
Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).
Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
Cutaneous Odontogenic Sinus: An Inflammatory Mimicker of Squamous Cell Carcinoma and Epidermal Cysts
Clinical Challenge
An
Practice Gap
It is estimated that half of patients with an extraoral fistula are treated with multiple dermatologic surgical operations, radiotherapy, antibiotic therapy, and chemotherapy before the correct diagnosis is made.1 Thus, proper identification of these lesions is crucial for prognosis and treatment. The most common locations for OCSTs are the mandibular, submandibular, and cervical skin.1,2 Given these locations, patients with OCSTs commonly present to the dermatology office for evaluation. Education regarding the clinical presentation, histopathology, and proper evaluation and further referral for treatment is essential for dermatologists.
Tools and Technique for Diagnosis
We present 2 patients with OCSTs who were referred for cutaneous surgery for an SCC and epidermal cyst, but the proper diagnosis was rendered after an index of suspicion and clinicopathologic correlation led to additional testing and eventual referral for imaging.
Patient 1
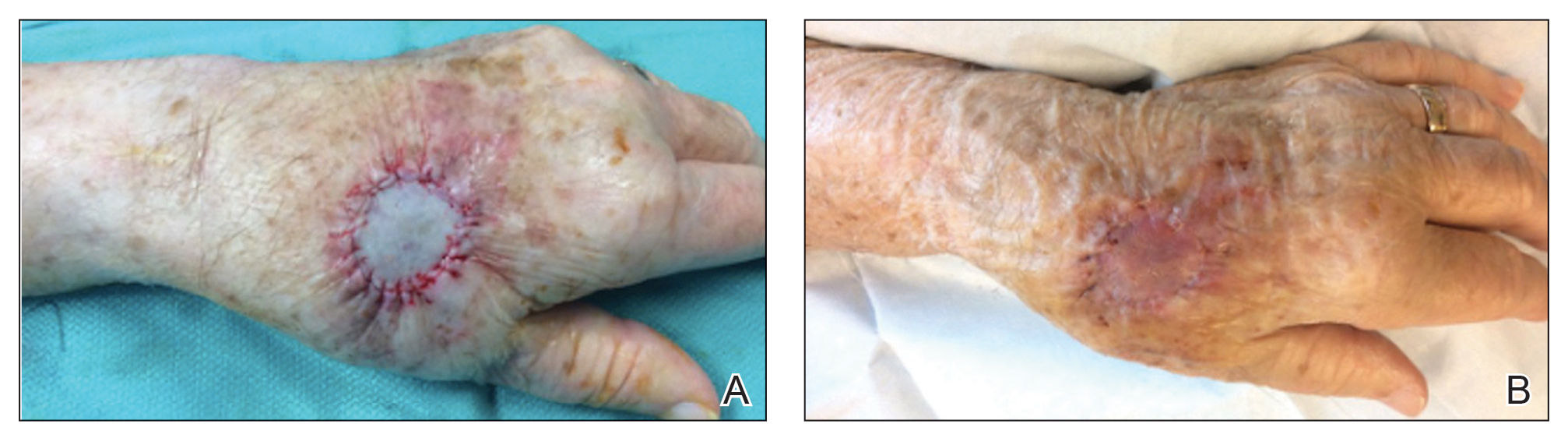
A 68-year-old woman presented for Mohs micrographic surgery (MMS) of a biopsy-proven SCC on the chin. The tumor cleared after 2 MMS stages (Figure 1A). Due to notable inflammation in each stage, the slides were sent to a pathologist who confirmed clear margins. Within 2 weeks of MMS, the wound began to dehisce (Figure 1B). The patient presented 4 months later with a crusted ulcerated nodule at the MMS site (Figure 1C). A biopsy showed likely recurrence of SCC. Upon presentation to the Mohs surgeon, the nodule felt fixed to the underlying jaw, and the patient was noted to have poor dentition. The patient was sent for computed tomography (CT), which showed focal thinning of the mandible, likely postsurgical, and clear maxillary sinuses. Due to the clinical appearance and anatomic location of the lesion, a request was made for a second read of the CT, specifically looking for an OCST at the prior surgical site. With this information, the radiologist noted an OCST extending from the mandible to the lesion, reported as a periapical lucency (representing a periapical abscess) at a mandibular tooth with a dental sinus draining into the soft tissues. The patient was started on antibiotics and referred to an oral surgeon for OCST excision.

Patient 2
A 62-year-old man presented with an inflamed subcutaneous nodule on the left anterior neck. A biopsy showed a ruptured cyst, and the patient was referred for excision. Clinical examination revealed a subcutaneous nodule fixed to the lower portion of the mandible (Figure 2A) that exhibited a rubbery retraction when pulled (Figure 2B). After a discussion about the atypical feel and appearance of this cyst, the patient preferred to undergo excision. During excision, the lesion felt deep and fixed with retraction (Figure 2C). With intraoperative re-evaluation of the clinical scenario and location, the patient was sent for CT. The initial read noted clear maxillary and ethmoid sinuses, with no mention of an OCST. After discussing the clinical history and suspicion specifically for an OCST with the radiologist, the re-read showed notable inflammation and decay of the tooth adjacent to the area of interest. An OCST was diagnosed, and the patient was sent to an oral surgeon for excision after antibiotics were prescribed.
Practice Implications
Odontogenic cutaneous sinus tracts commonly are misdiagnosed due to variations in clinical presentations resembling more common cutaneous diagnoses, nonspecific histopathologic findings, and lack of dental symptoms or concerns about dentition. Clinically, an OCST presents as a fixed, red, crusty, nontender nodule with intermittent draining. With palpation of the involved area, the clinician may feel a cord of tissue connecting the skin lesion intraorally.2,4 A clinician should have a high index of suspicion for an OCST when evaluating fixed lesions of the lower face, jawline, and neck due to the possibility of a dental origin,1 which is important because an OCST can have similar clinical findings to lesions such as congenital fistulas, pustules, cysts, osteomyelitis, foreign-body granulomas, pyogenic granulomas, syphilis, metastatic carcinomas, basal cell carcinomas, and SCCs.2,4 A PubMed search of articles indexed for MEDLINE using the terms Mohs, MMS, chemosurgery, odontogenic sinus, odontogenic cutaneous sinus tract, and dental sinus yielded only 2 OCSTs that were referred for MMS in the last 30 years, both of which were in the nasolabial fold/medial malar cheek.2,4 Histopathologic findings of an OCST are nonspecific; a mixed or granulomatous inflammatory infiltrate, granulation tissue, and scarring can be seen.1 Pseudocarcinomatous/pseudoepitheliomatous hyperplasia of the epidermis can be seen and cause histologic misinterpretation for an SCC.2 Given that these findings are nonspecific without a clinical context, even with a histopathologic diagnosis of SCC or cyst, a clinical suspicion for an OCST should lead to an intraoral examination. Imaging can be ordered to look for an OCST in the area of interest. Although panoramic or periapical radiography with or without dental probes/radiopaque markers commonly have been used, more recent literature has suggested that CT may be superior to radiographs for making an OCST diagnosis.1,3 If imaging is not consistent with the clinically suspected OCST, we recommend directly contacting the radiologist to explain the clinical history and even refresh his/her suspicion for this diagnosis.
If a diagnosis of an OCST is made, oral antibiotics can be prescribed, though the use of antibiotics has been controversial. For severe odontogenic infections, typically beta-lactam antibiotics, cephalosporins, metronidazole, clindamycin, moxifloxacin, or erythromycin can be given for 7 days or until 3 days after symptoms have resolved.5 Although antibiotics can bring temporary resolution, it is imperative to treat the source of infection to prevent recurrence. It is crucial for these patients to be referred to an oral surgeon for evaluation and treatment of OCST by either a root canal or tooth extraction.
Final Thoughts
We present this pearl on the diagnosis and management of an OCST, also known as a dental sinus, to better assist clinicians in making this diagnosis. With an index of suspicion as well as intraoral and radiologic evaluations, a proper diagnosis may be rendered, potentially avoiding unnecessary cutaneous surgery. In addition, we highlight the importance of communication between the clinician and the radiologist to directly look for OCST in the area of concern and consider a re-read of the images when clinical suspicion does not correlate with the radiology report.
- Bai J, Ji AP, Huang MW. Submental cutaneous sinus tract of mandibular second molar origin. Int Endod J. 2014;47:1185-1191.
- Plast Reconstr Surg.
- Gregoire C. How are odontogenic infections best managed? J Can Dent Assoc. 2010;76:a37.
- Bodner L, Bar-Ziv J. Cutaneous sinus tract of dental origin—imaging with a dental CT software programme. Br J Oral Maxillofac Surg. 1998;36:311-313.
- Peermohamed S, Barber D, Kurwa H. Diagnostic challenges of cutaneous draining sinus tracts of odontogenic origin: a case report. Dermatol Surg. 2011;37:1525-1527.
Clinical Challenge
An
Practice Gap
It is estimated that half of patients with an extraoral fistula are treated with multiple dermatologic surgical operations, radiotherapy, antibiotic therapy, and chemotherapy before the correct diagnosis is made.1 Thus, proper identification of these lesions is crucial for prognosis and treatment. The most common locations for OCSTs are the mandibular, submandibular, and cervical skin.1,2 Given these locations, patients with OCSTs commonly present to the dermatology office for evaluation. Education regarding the clinical presentation, histopathology, and proper evaluation and further referral for treatment is essential for dermatologists.
Tools and Technique for Diagnosis
We present 2 patients with OCSTs who were referred for cutaneous surgery for an SCC and epidermal cyst, but the proper diagnosis was rendered after an index of suspicion and clinicopathologic correlation led to additional testing and eventual referral for imaging.
Patient 1
A 68-year-old woman presented for Mohs micrographic surgery (MMS) of a biopsy-proven SCC on the chin. The tumor cleared after 2 MMS stages (Figure 1A). Due to notable inflammation in each stage, the slides were sent to a pathologist who confirmed clear margins. Within 2 weeks of MMS, the wound began to dehisce (Figure 1B). The patient presented 4 months later with a crusted ulcerated nodule at the MMS site (Figure 1C). A biopsy showed likely recurrence of SCC. Upon presentation to the Mohs surgeon, the nodule felt fixed to the underlying jaw, and the patient was noted to have poor dentition. The patient was sent for computed tomography (CT), which showed focal thinning of the mandible, likely postsurgical, and clear maxillary sinuses. Due to the clinical appearance and anatomic location of the lesion, a request was made for a second read of the CT, specifically looking for an OCST at the prior surgical site. With this information, the radiologist noted an OCST extending from the mandible to the lesion, reported as a periapical lucency (representing a periapical abscess) at a mandibular tooth with a dental sinus draining into the soft tissues. The patient was started on antibiotics and referred to an oral surgeon for OCST excision.

Patient 2
A 62-year-old man presented with an inflamed subcutaneous nodule on the left anterior neck. A biopsy showed a ruptured cyst, and the patient was referred for excision. Clinical examination revealed a subcutaneous nodule fixed to the lower portion of the mandible (Figure 2A) that exhibited a rubbery retraction when pulled (Figure 2B). After a discussion about the atypical feel and appearance of this cyst, the patient preferred to undergo excision. During excision, the lesion felt deep and fixed with retraction (Figure 2C). With intraoperative re-evaluation of the clinical scenario and location, the patient was sent for CT. The initial read noted clear maxillary and ethmoid sinuses, with no mention of an OCST. After discussing the clinical history and suspicion specifically for an OCST with the radiologist, the re-read showed notable inflammation and decay of the tooth adjacent to the area of interest. An OCST was diagnosed, and the patient was sent to an oral surgeon for excision after antibiotics were prescribed.

Practice Implications
Odontogenic cutaneous sinus tracts commonly are misdiagnosed due to variations in clinical presentations resembling more common cutaneous diagnoses, nonspecific histopathologic findings, and lack of dental symptoms or concerns about dentition. Clinically, an OCST presents as a fixed, red, crusty, nontender nodule with intermittent draining. With palpation of the involved area, the clinician may feel a cord of tissue connecting the skin lesion intraorally.2,4 A clinician should have a high index of suspicion for an OCST when evaluating fixed lesions of the lower face, jawline, and neck due to the possibility of a dental origin,1 which is important because an OCST can have similar clinical findings to lesions such as congenital fistulas, pustules, cysts, osteomyelitis, foreign-body granulomas, pyogenic granulomas, syphilis, metastatic carcinomas, basal cell carcinomas, and SCCs.2,4 A PubMed search of articles indexed for MEDLINE using the terms Mohs, MMS, chemosurgery, odontogenic sinus, odontogenic cutaneous sinus tract, and dental sinus yielded only 2 OCSTs that were referred for MMS in the last 30 years, both of which were in the nasolabial fold/medial malar cheek.2,4 Histopathologic findings of an OCST are nonspecific; a mixed or granulomatous inflammatory infiltrate, granulation tissue, and scarring can be seen.1 Pseudocarcinomatous/pseudoepitheliomatous hyperplasia of the epidermis can be seen and cause histologic misinterpretation for an SCC.2 Given that these findings are nonspecific without a clinical context, even with a histopathologic diagnosis of SCC or cyst, a clinical suspicion for an OCST should lead to an intraoral examination. Imaging can be ordered to look for an OCST in the area of interest. Although panoramic or periapical radiography with or without dental probes/radiopaque markers commonly have been used, more recent literature has suggested that CT may be superior to radiographs for making an OCST diagnosis.1,3 If imaging is not consistent with the clinically suspected OCST, we recommend directly contacting the radiologist to explain the clinical history and even refresh his/her suspicion for this diagnosis.
If a diagnosis of an OCST is made, oral antibiotics can be prescribed, though the use of antibiotics has been controversial. For severe odontogenic infections, typically beta-lactam antibiotics, cephalosporins, metronidazole, clindamycin, moxifloxacin, or erythromycin can be given for 7 days or until 3 days after symptoms have resolved.5 Although antibiotics can bring temporary resolution, it is imperative to treat the source of infection to prevent recurrence. It is crucial for these patients to be referred to an oral surgeon for evaluation and treatment of OCST by either a root canal or tooth extraction.
Final Thoughts
We present this pearl on the diagnosis and management of an OCST, also known as a dental sinus, to better assist clinicians in making this diagnosis. With an index of suspicion as well as intraoral and radiologic evaluations, a proper diagnosis may be rendered, potentially avoiding unnecessary cutaneous surgery. In addition, we highlight the importance of communication between the clinician and the radiologist to directly look for OCST in the area of concern and consider a re-read of the images when clinical suspicion does not correlate with the radiology report.
Clinical Challenge
An
Practice Gap
It is estimated that half of patients with an extraoral fistula are treated with multiple dermatologic surgical operations, radiotherapy, antibiotic therapy, and chemotherapy before the correct diagnosis is made.1 Thus, proper identification of these lesions is crucial for prognosis and treatment. The most common locations for OCSTs are the mandibular, submandibular, and cervical skin.1,2 Given these locations, patients with OCSTs commonly present to the dermatology office for evaluation. Education regarding the clinical presentation, histopathology, and proper evaluation and further referral for treatment is essential for dermatologists.
Tools and Technique for Diagnosis
We present 2 patients with OCSTs who were referred for cutaneous surgery for an SCC and epidermal cyst, but the proper diagnosis was rendered after an index of suspicion and clinicopathologic correlation led to additional testing and eventual referral for imaging.
Patient 1
A 68-year-old woman presented for Mohs micrographic surgery (MMS) of a biopsy-proven SCC on the chin. The tumor cleared after 2 MMS stages (Figure 1A). Due to notable inflammation in each stage, the slides were sent to a pathologist who confirmed clear margins. Within 2 weeks of MMS, the wound began to dehisce (Figure 1B). The patient presented 4 months later with a crusted ulcerated nodule at the MMS site (Figure 1C). A biopsy showed likely recurrence of SCC. Upon presentation to the Mohs surgeon, the nodule felt fixed to the underlying jaw, and the patient was noted to have poor dentition. The patient was sent for computed tomography (CT), which showed focal thinning of the mandible, likely postsurgical, and clear maxillary sinuses. Due to the clinical appearance and anatomic location of the lesion, a request was made for a second read of the CT, specifically looking for an OCST at the prior surgical site. With this information, the radiologist noted an OCST extending from the mandible to the lesion, reported as a periapical lucency (representing a periapical abscess) at a mandibular tooth with a dental sinus draining into the soft tissues. The patient was started on antibiotics and referred to an oral surgeon for OCST excision.

Patient 2
A 62-year-old man presented with an inflamed subcutaneous nodule on the left anterior neck. A biopsy showed a ruptured cyst, and the patient was referred for excision. Clinical examination revealed a subcutaneous nodule fixed to the lower portion of the mandible (Figure 2A) that exhibited a rubbery retraction when pulled (Figure 2B). After a discussion about the atypical feel and appearance of this cyst, the patient preferred to undergo excision. During excision, the lesion felt deep and fixed with retraction (Figure 2C). With intraoperative re-evaluation of the clinical scenario and location, the patient was sent for CT. The initial read noted clear maxillary and ethmoid sinuses, with no mention of an OCST. After discussing the clinical history and suspicion specifically for an OCST with the radiologist, the re-read showed notable inflammation and decay of the tooth adjacent to the area of interest. An OCST was diagnosed, and the patient was sent to an oral surgeon for excision after antibiotics were prescribed.

Practice Implications
Odontogenic cutaneous sinus tracts commonly are misdiagnosed due to variations in clinical presentations resembling more common cutaneous diagnoses, nonspecific histopathologic findings, and lack of dental symptoms or concerns about dentition. Clinically, an OCST presents as a fixed, red, crusty, nontender nodule with intermittent draining. With palpation of the involved area, the clinician may feel a cord of tissue connecting the skin lesion intraorally.2,4 A clinician should have a high index of suspicion for an OCST when evaluating fixed lesions of the lower face, jawline, and neck due to the possibility of a dental origin,1 which is important because an OCST can have similar clinical findings to lesions such as congenital fistulas, pustules, cysts, osteomyelitis, foreign-body granulomas, pyogenic granulomas, syphilis, metastatic carcinomas, basal cell carcinomas, and SCCs.2,4 A PubMed search of articles indexed for MEDLINE using the terms Mohs, MMS, chemosurgery, odontogenic sinus, odontogenic cutaneous sinus tract, and dental sinus yielded only 2 OCSTs that were referred for MMS in the last 30 years, both of which were in the nasolabial fold/medial malar cheek.2,4 Histopathologic findings of an OCST are nonspecific; a mixed or granulomatous inflammatory infiltrate, granulation tissue, and scarring can be seen.1 Pseudocarcinomatous/pseudoepitheliomatous hyperplasia of the epidermis can be seen and cause histologic misinterpretation for an SCC.2 Given that these findings are nonspecific without a clinical context, even with a histopathologic diagnosis of SCC or cyst, a clinical suspicion for an OCST should lead to an intraoral examination. Imaging can be ordered to look for an OCST in the area of interest. Although panoramic or periapical radiography with or without dental probes/radiopaque markers commonly have been used, more recent literature has suggested that CT may be superior to radiographs for making an OCST diagnosis.1,3 If imaging is not consistent with the clinically suspected OCST, we recommend directly contacting the radiologist to explain the clinical history and even refresh his/her suspicion for this diagnosis.
If a diagnosis of an OCST is made, oral antibiotics can be prescribed, though the use of antibiotics has been controversial. For severe odontogenic infections, typically beta-lactam antibiotics, cephalosporins, metronidazole, clindamycin, moxifloxacin, or erythromycin can be given for 7 days or until 3 days after symptoms have resolved.5 Although antibiotics can bring temporary resolution, it is imperative to treat the source of infection to prevent recurrence. It is crucial for these patients to be referred to an oral surgeon for evaluation and treatment of OCST by either a root canal or tooth extraction.
Final Thoughts
We present this pearl on the diagnosis and management of an OCST, also known as a dental sinus, to better assist clinicians in making this diagnosis. With an index of suspicion as well as intraoral and radiologic evaluations, a proper diagnosis may be rendered, potentially avoiding unnecessary cutaneous surgery. In addition, we highlight the importance of communication between the clinician and the radiologist to directly look for OCST in the area of concern and consider a re-read of the images when clinical suspicion does not correlate with the radiology report.
- Bai J, Ji AP, Huang MW. Submental cutaneous sinus tract of mandibular second molar origin. Int Endod J. 2014;47:1185-1191.
- Plast Reconstr Surg.
- Gregoire C. How are odontogenic infections best managed? J Can Dent Assoc. 2010;76:a37.
- Bodner L, Bar-Ziv J. Cutaneous sinus tract of dental origin—imaging with a dental CT software programme. Br J Oral Maxillofac Surg. 1998;36:311-313.
- Peermohamed S, Barber D, Kurwa H. Diagnostic challenges of cutaneous draining sinus tracts of odontogenic origin: a case report. Dermatol Surg. 2011;37:1525-1527.
- Bai J, Ji AP, Huang MW. Submental cutaneous sinus tract of mandibular second molar origin. Int Endod J. 2014;47:1185-1191.
- Plast Reconstr Surg.
- Gregoire C. How are odontogenic infections best managed? J Can Dent Assoc. 2010;76:a37.
- Bodner L, Bar-Ziv J. Cutaneous sinus tract of dental origin—imaging with a dental CT software programme. Br J Oral Maxillofac Surg. 1998;36:311-313.
- Peermohamed S, Barber D, Kurwa H. Diagnostic challenges of cutaneous draining sinus tracts of odontogenic origin: a case report. Dermatol Surg. 2011;37:1525-1527.
Rapid Screening of Invasive Fungal Infections in the Hospital Setting Using the (1,3)-β-D-glucan Assay
Practice Gap
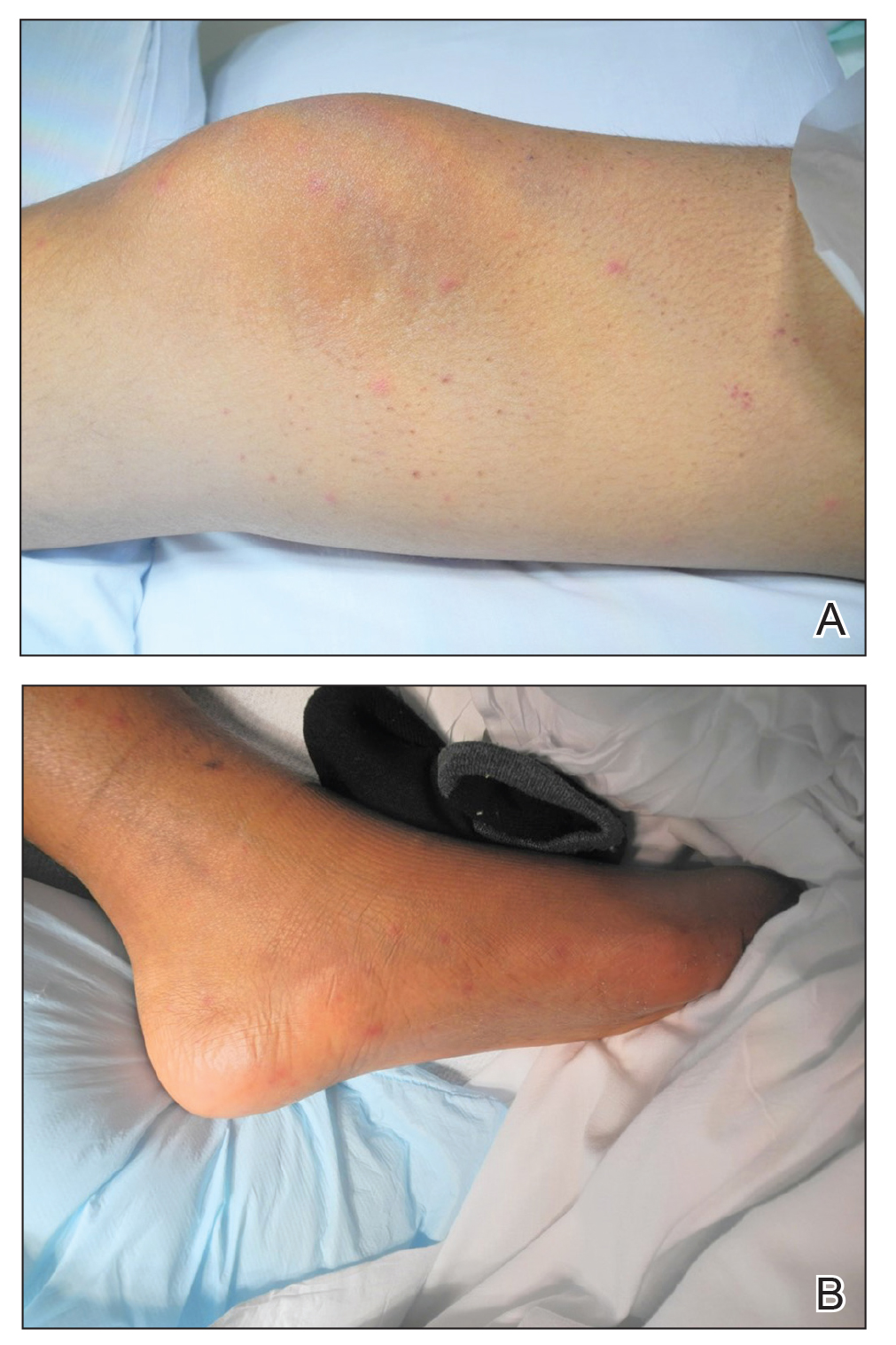
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.
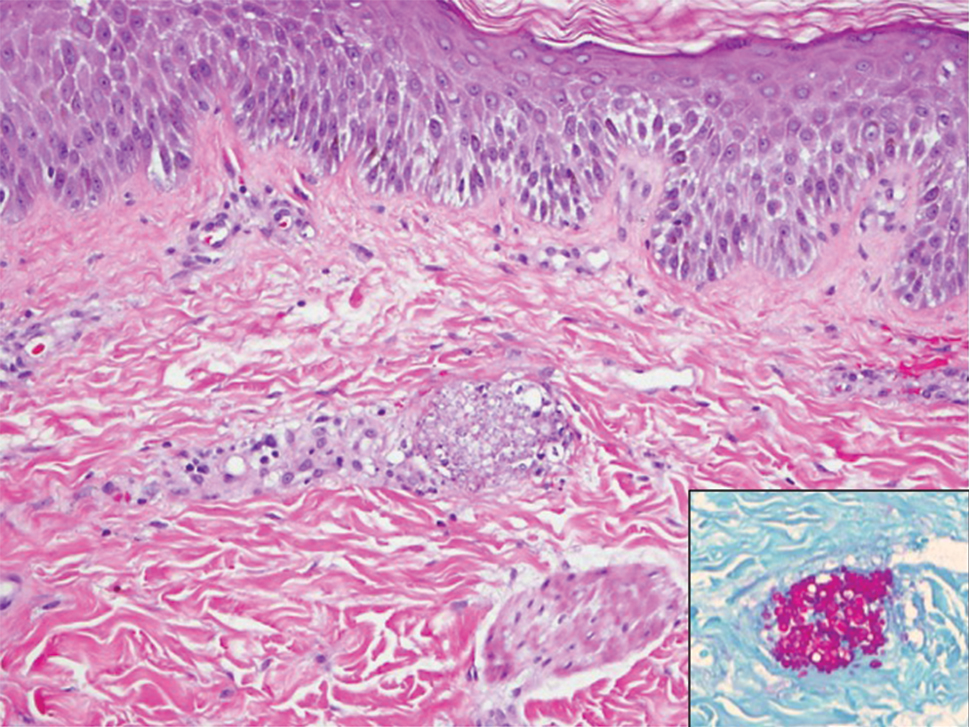
Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.



Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
Practice Gap
Invasive fungal infections are a leading cause of morbidity and mortality among neutropenic, immunocompromised, and critically ill patients. Candida species are the most common cause of fungemia, with portals of entry into the bloodstream including the gastrointestinal tract, contaminated intravascular catheters, and localized foci of infection.1 Diagnosis of invasive candidiasis remains challenging due to an absence of specific clinical signs and symptoms, varying from a mild fever that is unresponsive to antibiotics to florid sepsis. When present, clinical clues may include chorioretinitis; muscle abscesses; and skin eruptions, characteristically with Candida tropicalis. Cutaneous manifestations of disseminated Candida infections appear in only 13% of affected patients.1 The lesions typically present as 5- to 10-mm pink dermal papules or painless pustules on an erythematous base and may be singular, localized, or diffuse in distribution. Body regions normally involved are the trunk, arms, and legs, rarely the head and neck.1 Cutaneous lesions often develop at a time when patients are febrile, are not responding to antibiotics, and are clinically deteriorating.
A 15-year-old adolescent boy with pre–B-cell acute lymphoblastic leukemia was admitted with febrile neutropenia for presumed septic shock secondary to an unknown infectious etiology. The patient was started on broad-spectrum intravenous antibiotics, and blood cultures were obtained. On the second day of hospitalization, he developed approximately 10 to 15 discrete, 3- to 6-mm, pink to violaceous papules scattered on the chest and arms (Figure 1). Over several hours, the number of lesions increased to more than 50 with involvement of the legs (Figure 2). A punch biopsy of lesional skin from the left dorsal wrist demonstrated a circumscribed abscess of yeast in the papillary dermis, which was highlighted by periodic acid–Schiff staining with minimal associated inflammation (Figure 3). Blood and tissue cultures persistently grew C tropicalis. The patient was started on intravenous liposomal amphotericin B but died on day 5 of hospitalization after developing endocarditis.



Early and reliable diagnosis of Candida species fungemia is of critical importance to successful treatment, particularly with the emergence of multidrug-resistant strains such as Candida auris.2 In patients with apparent cutaneous manifestations, a lesional punch biopsy for culture and histopathologic evaluation is recommended in addition to blood culture; however, organisms may or may not be present in large numbers, and they may be difficult to identify on routine hematoxylin and eosin–stained tissue sections. To enhance the likelihood of highlighting the fungus within the sample, the pathologist must be made aware of the presumptive diagnosis of disseminated candidiasis so that special techniques can be utilized, such as periodic acid–Schiff stain.
Although positive blood culture is the gold standard for candidemia diagnosis, only 30% to 50% of patients with disseminated candidiasis had positive blood cultures at autopsy.1 Another study showed the sensitivity of blood culture for the detection of invasive fungal infection to be as low as 8.3%.3 In cases with positive blood cultures, the median time to positivity is 2 to 3 days, but it can take as long as 8 days, thus limiting its clinical utility in acutely ill patients.4 Given the low sensitivity and prolonged time required for culture growth of most fungal organisms, novel assays for rapid, non–culture-based diagnosis of systemic fungal infections hold substantial clinical promise moving forward.
The Technique
One of the more promising non–culture-based fungal diagnostic methodologies is an antigen assay based on the detection of serum (1,3)-β-D-glucan (BDG), a major cell wall constituent of most pathogenic fungi. This assay is not specific for Candida species and can be positive for Aspergillosis species, Fusarium species, Coccidioides immitis, Histoplasma capsulatum, and Pneumocystis jirovecii pneumonia, among others; therefore, it functions as a general biomarker for fungi in the bloodstream.4,5 (1,3)-β-D-glucan assay can be useful as an adjunct for blood cultures and punch biopsy, especially when cultures are negative or the results remain outstanding. The results of the BDG assay are available in less than 24 hours at minimal cost, and the test is approved by the US Food and Drug Administration for use as an aid in invasive fungal disease diagnosis. In a meta-analysis of 11 studies, BDG sensitivity was 75%.4 In a study based on autopsy cases from 6 years, BDG specificity was 98.4% with positive and negative predictive values of 86.7% and 97.1%, respectively.3 Optimal results were achieved when 2 consecutive tests were positive.4 The serum assay output is based on spectrophotometer readings, which are converted to BDG concentrations (negative, <60 pg/mL; indeterminate, 60–79 pg/mL; positive ≥80 pg/mL).5 Although we cannot be certain, utilizing the BDG assay in our patient may have led to earlier treatment and a better outcome.
A disadvantage of the BDG assay is the potential for false-positive results, which have been reported in lung transplant recipients with respiratory mold colonization and patients with other systemic bacterial infections.4 False-positive results also have been associated with use of ampicillin-clavulanate and piperacillin-tazobactam antibiotics and human blood products, hemodialysis, and severe mucositis, thus reaffirming the importance of judicious interpretation of BDG assay results by the clinician.4,6 There also is a potential for false-negative results, as the BDG assay does not detect certain fungal species such as Cryptococcus species and Blastomyces dermatitidis, which produce very low levels of BDG, or zygomycetes (Absidia, Mucor, and Rizopus species), which are not known to produce BDG.6
Practice Implications
In the setting of invasive fungal infections, a high degree of clinical suspicion is paramount due to the often subtle nature of cutaneous manifestations. A positive BDG assay can be used to identify high-risk patients for empiric antifungal therapy, prompting early intervention and improved outcomes in these acutely ill patients. The BDG assay’s excellent negative predictive value is useful in ruling out invasive Candida infections and may justify stopping unnecessary empiric antifungal therapy.4 For the dermatology hospitalist, incorporation of the BDG assay as a noninvasive screening tool may allow for more rapid initiation of appropriate antifungal therapy while awaiting confirmatory skin biopsy or culture results in disseminated candidemia and other invasive fungal infections.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
- Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31-43.
- Candida auris. Centers for Disease Control and Prevention website. https://www.cdc.gov/fungal/candida-auris/. Updated May 15, 2020. Accessed July 10, 2020.
- Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1,3)-β-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864-1870.
- Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284-1292.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational development and application of (1→3)-β-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses [published online May 24, 2017]. Int J Mol Sci. doi:10.3390/ijms18061124.
- Beta-D glucan assay. MiraVista Diagnostics website. https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/beta-d-glucan-test/. Accessed June 5, 2020.
Utilization of a Stress Ball to Diminish Anxiety During Nail Surgery
Practice Gap
Anxiety is common in patients undergoing surgery with general anesthesia and may be exacerbated in patients undergoing dermatologic surgery with local anesthesia. Apprehension might be worse for nail surgery patients because the nail unit is highly innervated and vascular. Many patients fear the anesthetic injections, and there often is pain postoperatively. Perioperative anxiety correlates with increased postoperative pain,1 analgesic use,2 and delayed recovery.3 Several alternatives have been proposed to decrease perioperative anxiety, including nonpharmacologic interventions such as using educational videos, personalized music, hand holding, art activities, and virtual reality, as well as pharmacologic interventions such as benzodiazepines. However, these techniques have not been well studied for nail surgery.
The Technique
Patients generally are anxious about nail surgery secondary to the pain associated with the local anesthetic infiltration; hence, it is crucial to decrease anxiety during this initial step. In our practice, we provide patients with a palm-sized stress ball made of closed-cell polyurethane foam rubber before surgery. Patients are then instructed to hold the stress ball with the free hand and squeeze it whenever they feel anxious or when they feel any discomfort related to the procedure (Figure). A variety of balls can be bought for less than $1 each, thus making it a cost-effective option.
Practice Implications
Holding a stress ball has been found to reduce both pain and anxiety in patients undergoing conscious surgery.4 Furthermore, squeezing a stress ball perioperatively may increase feelings of empowerment, given that patients have direct control over the object, which in turn may have a positive effect on anxiety and patient satisfaction without interfering with the surgical procedure.5 Holding a stress ball is a safe, widely accessible, and inexpensive technique that may aid in decreasing patients’ anxiety related to nail surgery. Nonetheless, controlled clinical trials assessing the efficacy of this method in reducing anxiety related to nail surgery are needed to determine its benefit compared to other methods.
- Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42:521-530.
- Powell R, Johnston M, Smith WC, et al. Psychological risk factors for chronic post-surgical pain after inguinal hernia repair surgery: a prospective cohort study. Eur J Pain. 2012;16:600-610.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:e20306.
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455.
- Foy CR, Timmins F. Improving communication in day surgery settings. Nurs Stand. 2004;19:37-42.
Practice Gap
Anxiety is common in patients undergoing surgery with general anesthesia and may be exacerbated in patients undergoing dermatologic surgery with local anesthesia. Apprehension might be worse for nail surgery patients because the nail unit is highly innervated and vascular. Many patients fear the anesthetic injections, and there often is pain postoperatively. Perioperative anxiety correlates with increased postoperative pain,1 analgesic use,2 and delayed recovery.3 Several alternatives have been proposed to decrease perioperative anxiety, including nonpharmacologic interventions such as using educational videos, personalized music, hand holding, art activities, and virtual reality, as well as pharmacologic interventions such as benzodiazepines. However, these techniques have not been well studied for nail surgery.
The Technique
Patients generally are anxious about nail surgery secondary to the pain associated with the local anesthetic infiltration; hence, it is crucial to decrease anxiety during this initial step. In our practice, we provide patients with a palm-sized stress ball made of closed-cell polyurethane foam rubber before surgery. Patients are then instructed to hold the stress ball with the free hand and squeeze it whenever they feel anxious or when they feel any discomfort related to the procedure (Figure). A variety of balls can be bought for less than $1 each, thus making it a cost-effective option.

Practice Implications
Holding a stress ball has been found to reduce both pain and anxiety in patients undergoing conscious surgery.4 Furthermore, squeezing a stress ball perioperatively may increase feelings of empowerment, given that patients have direct control over the object, which in turn may have a positive effect on anxiety and patient satisfaction without interfering with the surgical procedure.5 Holding a stress ball is a safe, widely accessible, and inexpensive technique that may aid in decreasing patients’ anxiety related to nail surgery. Nonetheless, controlled clinical trials assessing the efficacy of this method in reducing anxiety related to nail surgery are needed to determine its benefit compared to other methods.
Practice Gap
Anxiety is common in patients undergoing surgery with general anesthesia and may be exacerbated in patients undergoing dermatologic surgery with local anesthesia. Apprehension might be worse for nail surgery patients because the nail unit is highly innervated and vascular. Many patients fear the anesthetic injections, and there often is pain postoperatively. Perioperative anxiety correlates with increased postoperative pain,1 analgesic use,2 and delayed recovery.3 Several alternatives have been proposed to decrease perioperative anxiety, including nonpharmacologic interventions such as using educational videos, personalized music, hand holding, art activities, and virtual reality, as well as pharmacologic interventions such as benzodiazepines. However, these techniques have not been well studied for nail surgery.
The Technique
Patients generally are anxious about nail surgery secondary to the pain associated with the local anesthetic infiltration; hence, it is crucial to decrease anxiety during this initial step. In our practice, we provide patients with a palm-sized stress ball made of closed-cell polyurethane foam rubber before surgery. Patients are then instructed to hold the stress ball with the free hand and squeeze it whenever they feel anxious or when they feel any discomfort related to the procedure (Figure). A variety of balls can be bought for less than $1 each, thus making it a cost-effective option.

Practice Implications
Holding a stress ball has been found to reduce both pain and anxiety in patients undergoing conscious surgery.4 Furthermore, squeezing a stress ball perioperatively may increase feelings of empowerment, given that patients have direct control over the object, which in turn may have a positive effect on anxiety and patient satisfaction without interfering with the surgical procedure.5 Holding a stress ball is a safe, widely accessible, and inexpensive technique that may aid in decreasing patients’ anxiety related to nail surgery. Nonetheless, controlled clinical trials assessing the efficacy of this method in reducing anxiety related to nail surgery are needed to determine its benefit compared to other methods.
- Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42:521-530.
- Powell R, Johnston M, Smith WC, et al. Psychological risk factors for chronic post-surgical pain after inguinal hernia repair surgery: a prospective cohort study. Eur J Pain. 2012;16:600-610.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:e20306.
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455.
- Foy CR, Timmins F. Improving communication in day surgery settings. Nurs Stand. 2004;19:37-42.
- Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42:521-530.
- Powell R, Johnston M, Smith WC, et al. Psychological risk factors for chronic post-surgical pain after inguinal hernia repair surgery: a prospective cohort study. Eur J Pain. 2012;16:600-610.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:e20306.
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455.
- Foy CR, Timmins F. Improving communication in day surgery settings. Nurs Stand. 2004;19:37-42.
Cartilage Sutures for a Large Nasal Defect
Practice Gap
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.
Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.
At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).
Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
Practice Gap
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.
Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.
At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).
Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
Practice Gap
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.
Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.
At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).
Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
Hydrogen Peroxide as a Hemostatic Agent During Dermatologic Surgery
The number of skin cancer surgeries continues to rise, especially in the older population, many of whom are on blood thinners. The sequela of bleeding, even in minor cases, is one of the most frequently encountered complications of cutaneous surgery. Surgical site bleeding can increase the risk for infection, skin graft failure, wound dehiscence, and hematoma formation, which may lead to disrupted wound healing and eventual poor scar outcome. Although achieving hemostasis is important, it is recommended to limit certain alternative modalities such as electrosurgery due to the accompanied thermal tissue damage that in turn can prolong healing time, worsen scarring, and increase the risk for infection.1
Practice Gap
Hydrogen peroxide (H2O2) is a common topical antiseptic used to clean wounds by killing pathogens through oxidation burst and local oxygen production.2 It is generally affordable, nonallergenic, and easy to obtain. We describe our positive experience using H2O2 as a hemostatic agent during dermatologic surgery, highlighting the agent’s underutilization as well as the recent literature negating traditional viewpoints that it probably causes tissue necrosis and impaired wound healing through its high oxidative property.
The Technique
Before surgery, the site is prepared with chlorhexidine gluconate. A stack of 4×4-in gauze on the surgical tray is saturated with 3% H2O2 and used by the surgeon and surgical assistant throughout the procedure. We currently use this technique during standard excisions, Mohs micrographic surgery stages, repairs, and dermabrasion. Additionally, as a first measure of hemostasis, we recommend H2O2 soaks immediately postoperatively in patients with active bleeding.
We have been utilizing this technique since H2O2 was described as an intraprocedural hemostatic agent during manual dermabrasion.3 Hydrogen peroxide is known to facilitate hemostasis with several accepted mechanisms that include regulating the contractility and barrier function of endothelial cells, activating latent cell surface tissue factor and platelet aggregation, and stimulating platelet-derived growth factor activation.4 It has been reported that increasing H2O2 levels leads to a dose-response increase in aggregation in the presence of subaggregating amounts of collagen.5 This concept was described in an article that utilizes H2O2 as a way to obtain hemostasis before skin grafting burn patients.6 A PubMed search of articles indexed for MEDLINE using the terms h202, hydrogen peroxide, hemostasis, wound healing, surgery, and wound produced several surgical specialties—neurosurgery, orthopedics, gastroenterology, and maxillofacial surgery—that also utilize H2O2 as a hemostatic agent.7,8 One article described a dual-enzyme H2O2 generation machinery in hydrogels as a novel antimicrobial wound treatment.9
Practice Implications
The use of H2O2 as a topical hemostatic agent during surgery was described in 1984.2 The use of H2O2 is not suggested as a substitute for other strong and well-known hemostatic agents, such as aluminum chloride and ferric subsulfate, but rather as a technique that can be used in conjunction with standard methods of hemostasis and antisepsis. For surgical sites that are intended to be closed, we do not suggest these hemostatic agents, as they are known to be caustic, irritating, and pigmenting. In addition to H2O2’s known hemostatic and antiseptic properties, more recent literature invalidates wound impairment concerns and describes its possible role in signaling effector cells to respond downstream, contributing to tissue formation and remodeling.4 The use of H2O2 in wound and incision care has been controversial and avoided due to described skin irritation and possible premature removal of suture10; however, positive biochemical effects of H2O2 on acute wounds have been reported and dispel arguments that this agent causes tissue damage.4 Contrary to the traditional viewpoint that H2O2 probably impairs tissue through its high oxidative property, a proper level of H2O2 is considered an important requirement for normal wound healing. The report published in 1985 that raised concerns of H2O2 causing impaired wound healing through its effect on fibroblasts has been challenged given that the killed cultured fibroblasts were in an in vitro model and not likely representative of the complexities of a healing wound.10 In our experience, the use of H2O2 has not demonstrated any impairments or delays in wound healing, and we postulate that the exposure to H2O2 as described in our technique is not sufficient to cause notable impairment in fibroblast function in vivo. In addition, the role of H2O2 promoting oxidative stress as well as resolving inflammation may suggest it serves as a bidirectional regulator.
Future Directions
Additional studies are needed to assess this precise balance of H2O2 forming a favorable microenvironment in wounds. Similarly, although we discuss minimal and brief use of H2O2 during a procedure, the lack of data on the role of H2O2 as a prophylactic anti-infective agent for postoperative wound care also may be an area of future exploration.
- Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract. 2013;2013:279289.
- Hankin FM, Campbell SE, Goldstein SA, et al. Hydrogen peroxide as a topical hemostatic agent. Clin Orthop Relat Res. 1984;186:244-247.
- Weiss J, Winkleman FJ, Titone A, et al. Evaluation of hydrogen peroxide as an intraprocedural hemostatic agent in manual dermabrasion. Dermatol Surg. 2010;36:1601-1603.
- Zhu G, Wang Q, Lu S, et al. Hydrogen peroxide: a potential wound therapeutic target? Med Princ Pract. 2017;26:301-308.
- Practicò D, Iuliano L, Ghiselli A, et al. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis. 1991;21:169-174.
- Potyondy L, Lottenberg L, Anderson J, et al. The use of hydrogen peroxide for achieving dermal hemostasis after burn excision in a patient with platelet dysfunction. J Burn Care Res. 2006;27:99-101.
- Mawk JR. Hydrogen peroxide for hemostasis. Neurosurgery. 1986;18:827.
- Arakeri G, Brennan PA. Povidone-iodine and hydrogen peroxide mixture soaked gauze pack: a novel hemostatic technique. J Oral Maxillofac Surg. 2013;71:1833.e1-1833.e3.
- Huber D, Tegl G, Mensah A, et al. A dual-enzyme hydrogen peroxide generation machinery in hydrogels supports antimicrobial wound treatment. ACS Appl Mater Interfaces. 2017;9:15307-15316.
- Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
The number of skin cancer surgeries continues to rise, especially in the older population, many of whom are on blood thinners. The sequela of bleeding, even in minor cases, is one of the most frequently encountered complications of cutaneous surgery. Surgical site bleeding can increase the risk for infection, skin graft failure, wound dehiscence, and hematoma formation, which may lead to disrupted wound healing and eventual poor scar outcome. Although achieving hemostasis is important, it is recommended to limit certain alternative modalities such as electrosurgery due to the accompanied thermal tissue damage that in turn can prolong healing time, worsen scarring, and increase the risk for infection.1
Practice Gap
Hydrogen peroxide (H2O2) is a common topical antiseptic used to clean wounds by killing pathogens through oxidation burst and local oxygen production.2 It is generally affordable, nonallergenic, and easy to obtain. We describe our positive experience using H2O2 as a hemostatic agent during dermatologic surgery, highlighting the agent’s underutilization as well as the recent literature negating traditional viewpoints that it probably causes tissue necrosis and impaired wound healing through its high oxidative property.
The Technique
Before surgery, the site is prepared with chlorhexidine gluconate. A stack of 4×4-in gauze on the surgical tray is saturated with 3% H2O2 and used by the surgeon and surgical assistant throughout the procedure. We currently use this technique during standard excisions, Mohs micrographic surgery stages, repairs, and dermabrasion. Additionally, as a first measure of hemostasis, we recommend H2O2 soaks immediately postoperatively in patients with active bleeding.
We have been utilizing this technique since H2O2 was described as an intraprocedural hemostatic agent during manual dermabrasion.3 Hydrogen peroxide is known to facilitate hemostasis with several accepted mechanisms that include regulating the contractility and barrier function of endothelial cells, activating latent cell surface tissue factor and platelet aggregation, and stimulating platelet-derived growth factor activation.4 It has been reported that increasing H2O2 levels leads to a dose-response increase in aggregation in the presence of subaggregating amounts of collagen.5 This concept was described in an article that utilizes H2O2 as a way to obtain hemostasis before skin grafting burn patients.6 A PubMed search of articles indexed for MEDLINE using the terms h202, hydrogen peroxide, hemostasis, wound healing, surgery, and wound produced several surgical specialties—neurosurgery, orthopedics, gastroenterology, and maxillofacial surgery—that also utilize H2O2 as a hemostatic agent.7,8 One article described a dual-enzyme H2O2 generation machinery in hydrogels as a novel antimicrobial wound treatment.9
Practice Implications
The use of H2O2 as a topical hemostatic agent during surgery was described in 1984.2 The use of H2O2 is not suggested as a substitute for other strong and well-known hemostatic agents, such as aluminum chloride and ferric subsulfate, but rather as a technique that can be used in conjunction with standard methods of hemostasis and antisepsis. For surgical sites that are intended to be closed, we do not suggest these hemostatic agents, as they are known to be caustic, irritating, and pigmenting. In addition to H2O2’s known hemostatic and antiseptic properties, more recent literature invalidates wound impairment concerns and describes its possible role in signaling effector cells to respond downstream, contributing to tissue formation and remodeling.4 The use of H2O2 in wound and incision care has been controversial and avoided due to described skin irritation and possible premature removal of suture10; however, positive biochemical effects of H2O2 on acute wounds have been reported and dispel arguments that this agent causes tissue damage.4 Contrary to the traditional viewpoint that H2O2 probably impairs tissue through its high oxidative property, a proper level of H2O2 is considered an important requirement for normal wound healing. The report published in 1985 that raised concerns of H2O2 causing impaired wound healing through its effect on fibroblasts has been challenged given that the killed cultured fibroblasts were in an in vitro model and not likely representative of the complexities of a healing wound.10 In our experience, the use of H2O2 has not demonstrated any impairments or delays in wound healing, and we postulate that the exposure to H2O2 as described in our technique is not sufficient to cause notable impairment in fibroblast function in vivo. In addition, the role of H2O2 promoting oxidative stress as well as resolving inflammation may suggest it serves as a bidirectional regulator.
Future Directions
Additional studies are needed to assess this precise balance of H2O2 forming a favorable microenvironment in wounds. Similarly, although we discuss minimal and brief use of H2O2 during a procedure, the lack of data on the role of H2O2 as a prophylactic anti-infective agent for postoperative wound care also may be an area of future exploration.
The number of skin cancer surgeries continues to rise, especially in the older population, many of whom are on blood thinners. The sequela of bleeding, even in minor cases, is one of the most frequently encountered complications of cutaneous surgery. Surgical site bleeding can increase the risk for infection, skin graft failure, wound dehiscence, and hematoma formation, which may lead to disrupted wound healing and eventual poor scar outcome. Although achieving hemostasis is important, it is recommended to limit certain alternative modalities such as electrosurgery due to the accompanied thermal tissue damage that in turn can prolong healing time, worsen scarring, and increase the risk for infection.1
Practice Gap
Hydrogen peroxide (H2O2) is a common topical antiseptic used to clean wounds by killing pathogens through oxidation burst and local oxygen production.2 It is generally affordable, nonallergenic, and easy to obtain. We describe our positive experience using H2O2 as a hemostatic agent during dermatologic surgery, highlighting the agent’s underutilization as well as the recent literature negating traditional viewpoints that it probably causes tissue necrosis and impaired wound healing through its high oxidative property.
The Technique
Before surgery, the site is prepared with chlorhexidine gluconate. A stack of 4×4-in gauze on the surgical tray is saturated with 3% H2O2 and used by the surgeon and surgical assistant throughout the procedure. We currently use this technique during standard excisions, Mohs micrographic surgery stages, repairs, and dermabrasion. Additionally, as a first measure of hemostasis, we recommend H2O2 soaks immediately postoperatively in patients with active bleeding.
We have been utilizing this technique since H2O2 was described as an intraprocedural hemostatic agent during manual dermabrasion.3 Hydrogen peroxide is known to facilitate hemostasis with several accepted mechanisms that include regulating the contractility and barrier function of endothelial cells, activating latent cell surface tissue factor and platelet aggregation, and stimulating platelet-derived growth factor activation.4 It has been reported that increasing H2O2 levels leads to a dose-response increase in aggregation in the presence of subaggregating amounts of collagen.5 This concept was described in an article that utilizes H2O2 as a way to obtain hemostasis before skin grafting burn patients.6 A PubMed search of articles indexed for MEDLINE using the terms h202, hydrogen peroxide, hemostasis, wound healing, surgery, and wound produced several surgical specialties—neurosurgery, orthopedics, gastroenterology, and maxillofacial surgery—that also utilize H2O2 as a hemostatic agent.7,8 One article described a dual-enzyme H2O2 generation machinery in hydrogels as a novel antimicrobial wound treatment.9
Practice Implications
The use of H2O2 as a topical hemostatic agent during surgery was described in 1984.2 The use of H2O2 is not suggested as a substitute for other strong and well-known hemostatic agents, such as aluminum chloride and ferric subsulfate, but rather as a technique that can be used in conjunction with standard methods of hemostasis and antisepsis. For surgical sites that are intended to be closed, we do not suggest these hemostatic agents, as they are known to be caustic, irritating, and pigmenting. In addition to H2O2’s known hemostatic and antiseptic properties, more recent literature invalidates wound impairment concerns and describes its possible role in signaling effector cells to respond downstream, contributing to tissue formation and remodeling.4 The use of H2O2 in wound and incision care has been controversial and avoided due to described skin irritation and possible premature removal of suture10; however, positive biochemical effects of H2O2 on acute wounds have been reported and dispel arguments that this agent causes tissue damage.4 Contrary to the traditional viewpoint that H2O2 probably impairs tissue through its high oxidative property, a proper level of H2O2 is considered an important requirement for normal wound healing. The report published in 1985 that raised concerns of H2O2 causing impaired wound healing through its effect on fibroblasts has been challenged given that the killed cultured fibroblasts were in an in vitro model and not likely representative of the complexities of a healing wound.10 In our experience, the use of H2O2 has not demonstrated any impairments or delays in wound healing, and we postulate that the exposure to H2O2 as described in our technique is not sufficient to cause notable impairment in fibroblast function in vivo. In addition, the role of H2O2 promoting oxidative stress as well as resolving inflammation may suggest it serves as a bidirectional regulator.
Future Directions
Additional studies are needed to assess this precise balance of H2O2 forming a favorable microenvironment in wounds. Similarly, although we discuss minimal and brief use of H2O2 during a procedure, the lack of data on the role of H2O2 as a prophylactic anti-infective agent for postoperative wound care also may be an area of future exploration.
- Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract. 2013;2013:279289.
- Hankin FM, Campbell SE, Goldstein SA, et al. Hydrogen peroxide as a topical hemostatic agent. Clin Orthop Relat Res. 1984;186:244-247.
- Weiss J, Winkleman FJ, Titone A, et al. Evaluation of hydrogen peroxide as an intraprocedural hemostatic agent in manual dermabrasion. Dermatol Surg. 2010;36:1601-1603.
- Zhu G, Wang Q, Lu S, et al. Hydrogen peroxide: a potential wound therapeutic target? Med Princ Pract. 2017;26:301-308.
- Practicò D, Iuliano L, Ghiselli A, et al. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis. 1991;21:169-174.
- Potyondy L, Lottenberg L, Anderson J, et al. The use of hydrogen peroxide for achieving dermal hemostasis after burn excision in a patient with platelet dysfunction. J Burn Care Res. 2006;27:99-101.
- Mawk JR. Hydrogen peroxide for hemostasis. Neurosurgery. 1986;18:827.
- Arakeri G, Brennan PA. Povidone-iodine and hydrogen peroxide mixture soaked gauze pack: a novel hemostatic technique. J Oral Maxillofac Surg. 2013;71:1833.e1-1833.e3.
- Huber D, Tegl G, Mensah A, et al. A dual-enzyme hydrogen peroxide generation machinery in hydrogels supports antimicrobial wound treatment. ACS Appl Mater Interfaces. 2017;9:15307-15316.
- Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
- Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract. 2013;2013:279289.
- Hankin FM, Campbell SE, Goldstein SA, et al. Hydrogen peroxide as a topical hemostatic agent. Clin Orthop Relat Res. 1984;186:244-247.
- Weiss J, Winkleman FJ, Titone A, et al. Evaluation of hydrogen peroxide as an intraprocedural hemostatic agent in manual dermabrasion. Dermatol Surg. 2010;36:1601-1603.
- Zhu G, Wang Q, Lu S, et al. Hydrogen peroxide: a potential wound therapeutic target? Med Princ Pract. 2017;26:301-308.
- Practicò D, Iuliano L, Ghiselli A, et al. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis. 1991;21:169-174.
- Potyondy L, Lottenberg L, Anderson J, et al. The use of hydrogen peroxide for achieving dermal hemostasis after burn excision in a patient with platelet dysfunction. J Burn Care Res. 2006;27:99-101.
- Mawk JR. Hydrogen peroxide for hemostasis. Neurosurgery. 1986;18:827.
- Arakeri G, Brennan PA. Povidone-iodine and hydrogen peroxide mixture soaked gauze pack: a novel hemostatic technique. J Oral Maxillofac Surg. 2013;71:1833.e1-1833.e3.
- Huber D, Tegl G, Mensah A, et al. A dual-enzyme hydrogen peroxide generation machinery in hydrogels supports antimicrobial wound treatment. ACS Appl Mater Interfaces. 2017;9:15307-15316.
- Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
Reflectance Confocal Microscopy to Facilitate Knifeless Skin Cancer Management
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.