User login
Clinical Pearl: Topical Timolol for Refractory Hypergranulation
Practice Gap
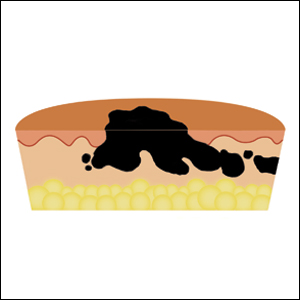
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
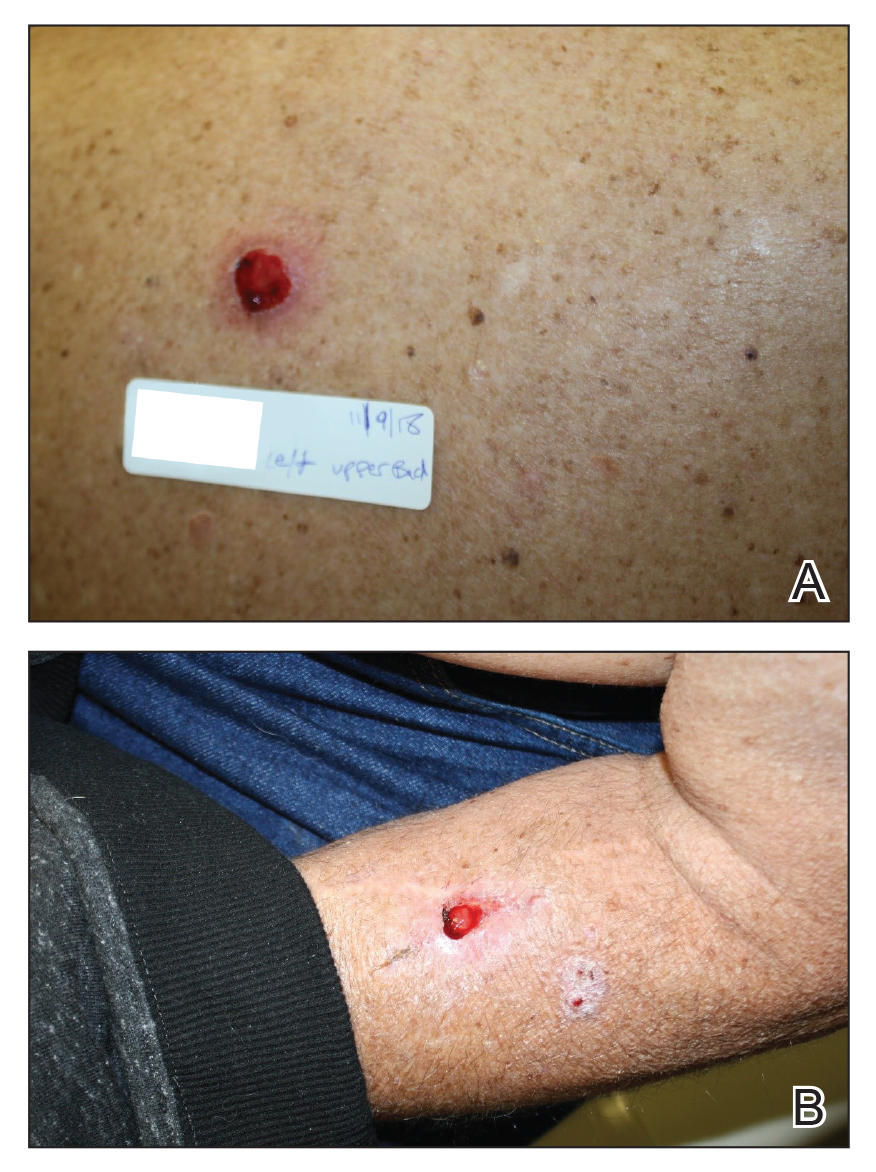
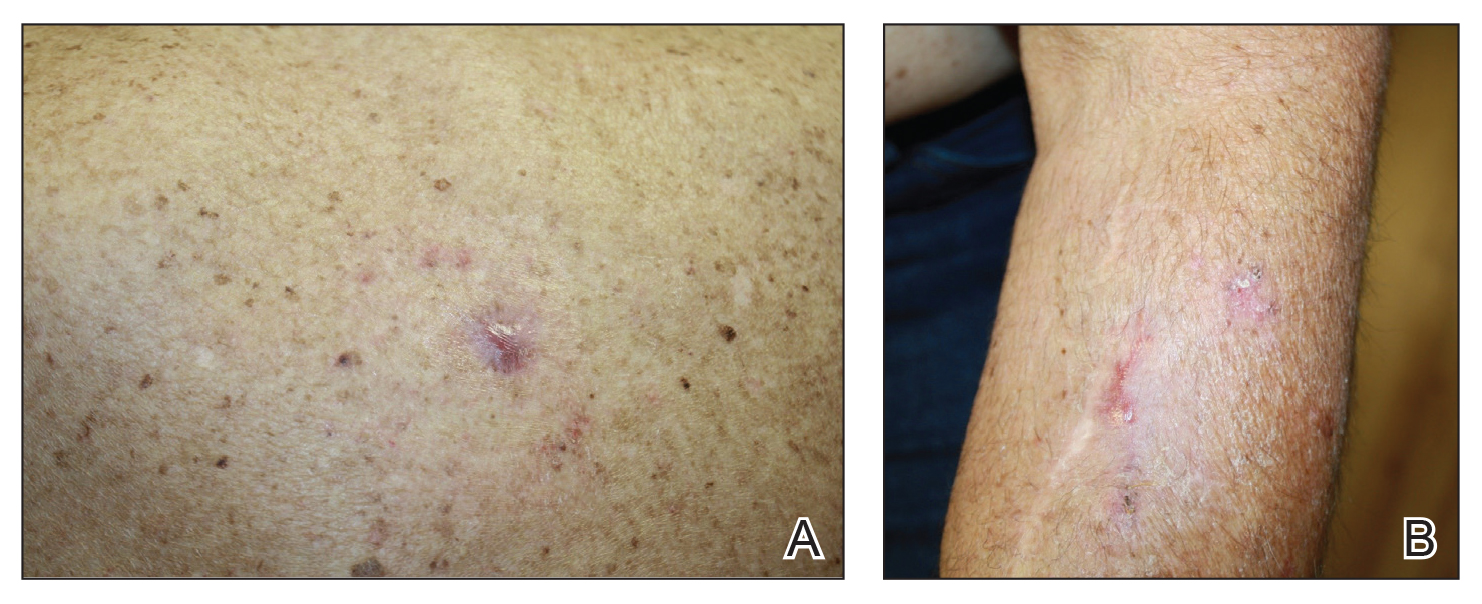
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
Clinical Pearl: Benzethonium Chloride for Habit-Tic Nail Deformity
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
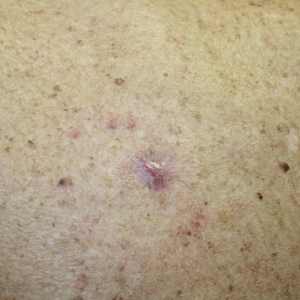
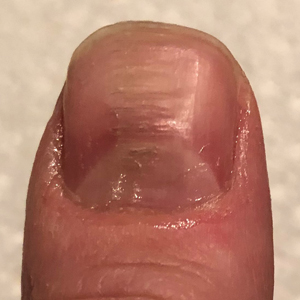
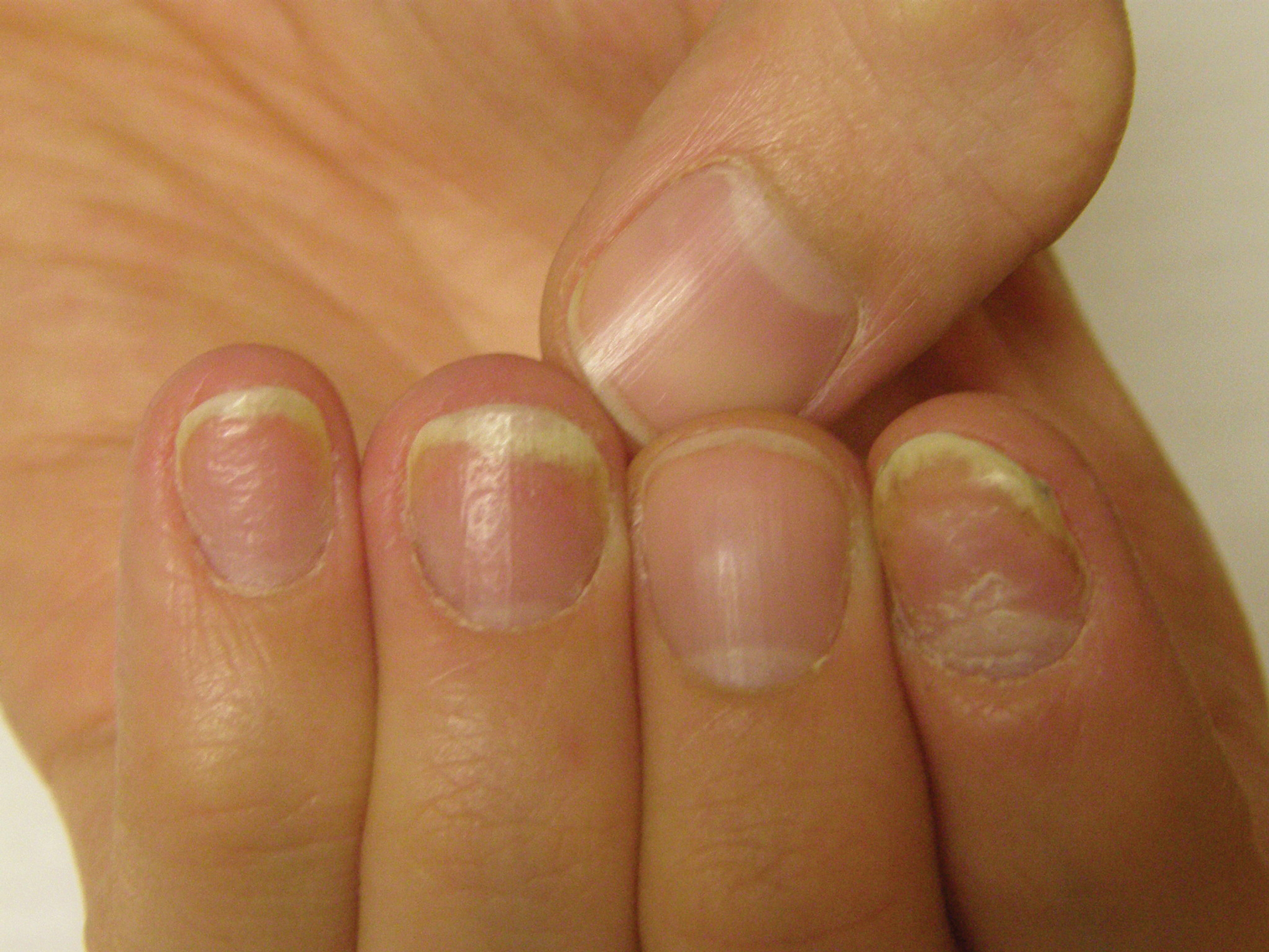
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).
Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).
Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).
Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
Clinical Pearl: Advantages of the Scalp as a Split-Thickness Skin Graft Donor Site
Practice Gap
Common donor sites for split-thickness skin grafts (STSGs) include the abdomen, buttocks, inner upper arms and forearms, and thighs. Challenges associated with donor site wounds in these areas include slow healing times and poor scar cosmesis. Although the scalp is not commonly considered when selecting a STSG donor site, harvesting from this area yields optimal results to improve these shortcomings.
Tools
A Weck knife facilitates STSG harvesting in an operationally timely, convenient fashion from larger donor sites up to 5.5 cm in width, such as the scalp, using adjustable thickness control guards.
The Technique
The donor site is lubricated with a sterile mineral oil. An assistant provides tension, leading the trajectory of the Weck knife with a guard. Small, gentle, back-and-forth strokes are made with the Weck knife to harvest the graft, which is then meshed with a No. 15 blade by placing the belly of the blade on the tissue and rolling it to-and-fro. The recipient site cartilage is fenestrated with a 2-mm punch biopsy.
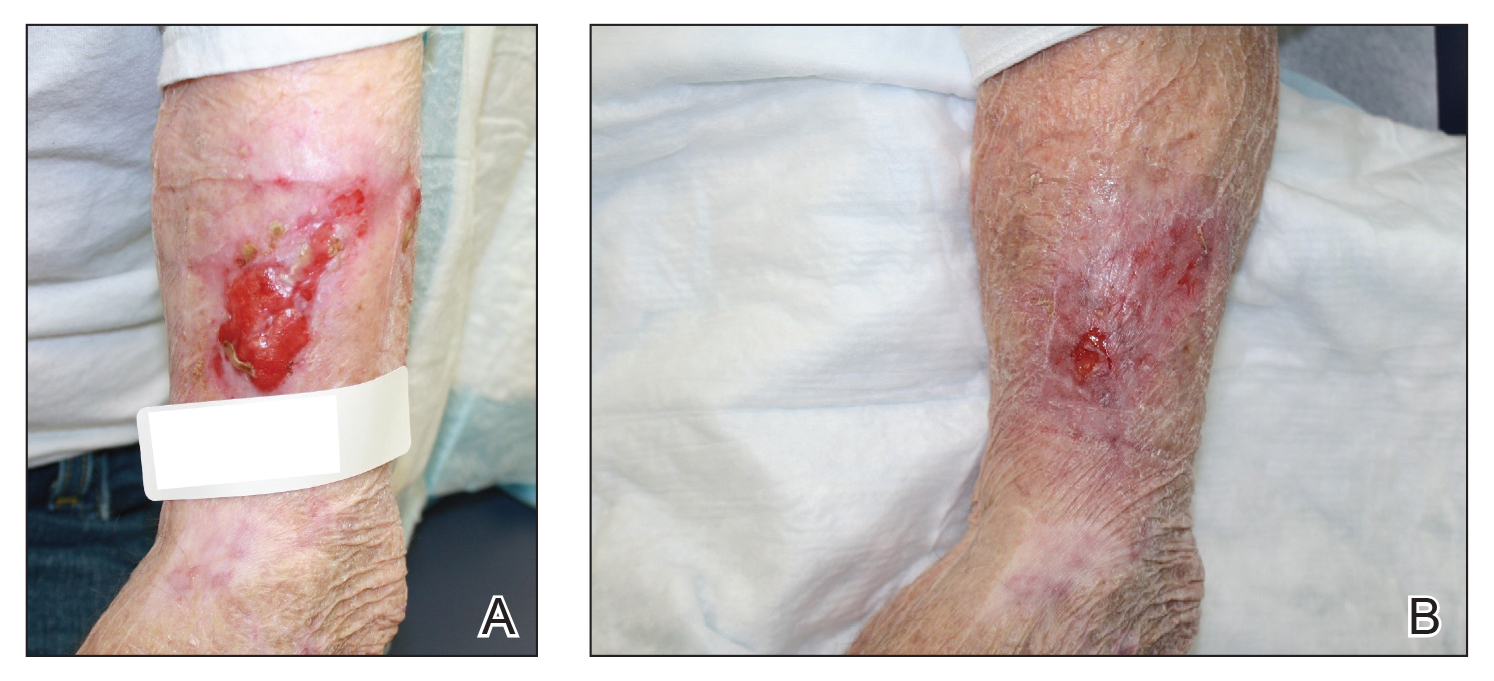
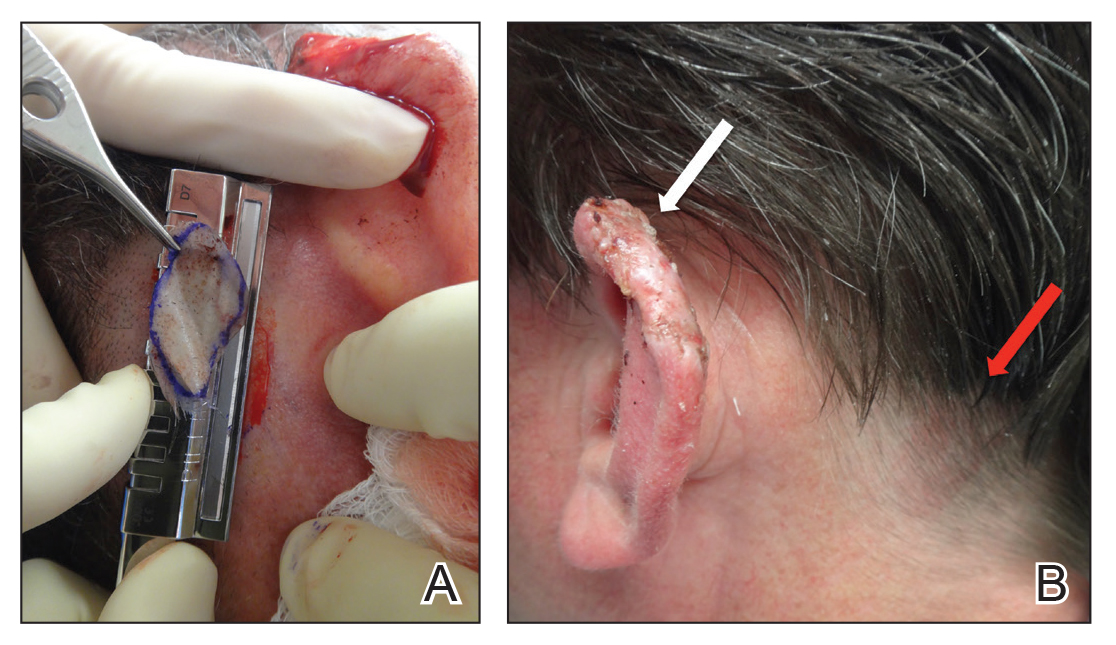
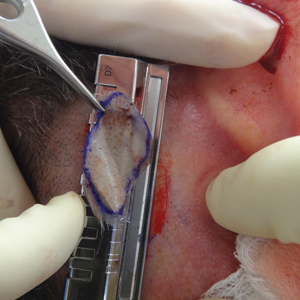
A 48-year-old man underwent Mohs micrographic surgery for treatment of a primary basal cell carcinoma of the left helix, resulting in a 2.5×1.3-cm defect after 2 stages. A Weck knife with a 0.012-in guard was used to harvest an STSG from the postauricular scalp (Figure, A), and the graft was inset to the recipient wound bed. Hemostasis at the scalp donor site was achieved through application of pressure and sterile gauze that was saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine. Both recipient and donor sites were dressed with tie-over bolsters that were sutured into place. At 2-week follow-up, the donor site was fully reepithelialized and hair regrowth obscured the defect (Figure, B).
Practice Implications
Our case demonstrates the advantages of the scalp as an STSG donor site with prompt healing time and excellent cosmesis. Because grafts are harvested at a depth superficial to the hair follicle, the hair regrows to conceal the donor site scar. Additionally, the robust blood supply of the scalp and hair follicle density optimize healing time. The location of the donor site at the postauricular scalp facilitates accessibility for wound care by the patient. Electrocautery or chemical styptics used for hemostasis may traumatize the hair follicles and risk causing alopecia; therefore, as demonstrated in our case, the preferred method to achieve hemostasis is the use of pressure or application of sterile gauze that has been saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine, followed by a pressure dressing provided by a sutured bolster.
Our case also demonstrates the utility of the Weck knife, which was introduced in 1968 as a modification of existing instruments to improve the ease of harvesting STSGs by appending a fixed handle and interchangeable depth gauges to a straight razor.1,2 The Weck knife can obtain grafts up to 5.5 cm in width (length may be as long as anatomically available), often circumventing the need to overlap grafts of smaller widths for repair of larger defects. Furthermore, grafts are harvested at a depth superficial to the hair follicle, averting donor site alopecia. These characteristics make the technique an ideal option for harvesting grafts from the scalp and other large donor sites.
Limitations of the Weck knife technique include the inability to harvest grafts from small donor sites in difficult-to-access anatomic regions or from areas with notable 3-dimensional structure. For harvesting such grafts, we prefer the DermaBlade (AccuTec Blades). Furthermore, assistance for providing tension along the trajectory of the Weck blade with a guard is optimal when performing the procedure. For practices not already utilizing a Weck knife, the technique necessitates additional training and cost. Nonetheless, for STSGs in which large donor site surface area, adjustable thickness, and convenient and timely operational technique are desired, the Weck knife should be considered as part of the dermatologic surgeon’s armamentarium.
- Aneer F, Singh AK, Kumar S. Evolution of instruments for harvest of the skin grafts. Indian J Plast Surg. 2013;46:28-35.
- Goulian D. A new economical dermatome. Plast Reconstr Surg. 1968;42:85-86.
Practice Gap
Common donor sites for split-thickness skin grafts (STSGs) include the abdomen, buttocks, inner upper arms and forearms, and thighs. Challenges associated with donor site wounds in these areas include slow healing times and poor scar cosmesis. Although the scalp is not commonly considered when selecting a STSG donor site, harvesting from this area yields optimal results to improve these shortcomings.
Tools
A Weck knife facilitates STSG harvesting in an operationally timely, convenient fashion from larger donor sites up to 5.5 cm in width, such as the scalp, using adjustable thickness control guards.
The Technique
The donor site is lubricated with a sterile mineral oil. An assistant provides tension, leading the trajectory of the Weck knife with a guard. Small, gentle, back-and-forth strokes are made with the Weck knife to harvest the graft, which is then meshed with a No. 15 blade by placing the belly of the blade on the tissue and rolling it to-and-fro. The recipient site cartilage is fenestrated with a 2-mm punch biopsy.
A 48-year-old man underwent Mohs micrographic surgery for treatment of a primary basal cell carcinoma of the left helix, resulting in a 2.5×1.3-cm defect after 2 stages. A Weck knife with a 0.012-in guard was used to harvest an STSG from the postauricular scalp (Figure, A), and the graft was inset to the recipient wound bed. Hemostasis at the scalp donor site was achieved through application of pressure and sterile gauze that was saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine. Both recipient and donor sites were dressed with tie-over bolsters that were sutured into place. At 2-week follow-up, the donor site was fully reepithelialized and hair regrowth obscured the defect (Figure, B).
Practice Implications
Our case demonstrates the advantages of the scalp as an STSG donor site with prompt healing time and excellent cosmesis. Because grafts are harvested at a depth superficial to the hair follicle, the hair regrows to conceal the donor site scar. Additionally, the robust blood supply of the scalp and hair follicle density optimize healing time. The location of the donor site at the postauricular scalp facilitates accessibility for wound care by the patient. Electrocautery or chemical styptics used for hemostasis may traumatize the hair follicles and risk causing alopecia; therefore, as demonstrated in our case, the preferred method to achieve hemostasis is the use of pressure or application of sterile gauze that has been saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine, followed by a pressure dressing provided by a sutured bolster.
Our case also demonstrates the utility of the Weck knife, which was introduced in 1968 as a modification of existing instruments to improve the ease of harvesting STSGs by appending a fixed handle and interchangeable depth gauges to a straight razor.1,2 The Weck knife can obtain grafts up to 5.5 cm in width (length may be as long as anatomically available), often circumventing the need to overlap grafts of smaller widths for repair of larger defects. Furthermore, grafts are harvested at a depth superficial to the hair follicle, averting donor site alopecia. These characteristics make the technique an ideal option for harvesting grafts from the scalp and other large donor sites.
Limitations of the Weck knife technique include the inability to harvest grafts from small donor sites in difficult-to-access anatomic regions or from areas with notable 3-dimensional structure. For harvesting such grafts, we prefer the DermaBlade (AccuTec Blades). Furthermore, assistance for providing tension along the trajectory of the Weck blade with a guard is optimal when performing the procedure. For practices not already utilizing a Weck knife, the technique necessitates additional training and cost. Nonetheless, for STSGs in which large donor site surface area, adjustable thickness, and convenient and timely operational technique are desired, the Weck knife should be considered as part of the dermatologic surgeon’s armamentarium.
Practice Gap
Common donor sites for split-thickness skin grafts (STSGs) include the abdomen, buttocks, inner upper arms and forearms, and thighs. Challenges associated with donor site wounds in these areas include slow healing times and poor scar cosmesis. Although the scalp is not commonly considered when selecting a STSG donor site, harvesting from this area yields optimal results to improve these shortcomings.
Tools
A Weck knife facilitates STSG harvesting in an operationally timely, convenient fashion from larger donor sites up to 5.5 cm in width, such as the scalp, using adjustable thickness control guards.
The Technique
The donor site is lubricated with a sterile mineral oil. An assistant provides tension, leading the trajectory of the Weck knife with a guard. Small, gentle, back-and-forth strokes are made with the Weck knife to harvest the graft, which is then meshed with a No. 15 blade by placing the belly of the blade on the tissue and rolling it to-and-fro. The recipient site cartilage is fenestrated with a 2-mm punch biopsy.
A 48-year-old man underwent Mohs micrographic surgery for treatment of a primary basal cell carcinoma of the left helix, resulting in a 2.5×1.3-cm defect after 2 stages. A Weck knife with a 0.012-in guard was used to harvest an STSG from the postauricular scalp (Figure, A), and the graft was inset to the recipient wound bed. Hemostasis at the scalp donor site was achieved through application of pressure and sterile gauze that was saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine. Both recipient and donor sites were dressed with tie-over bolsters that were sutured into place. At 2-week follow-up, the donor site was fully reepithelialized and hair regrowth obscured the defect (Figure, B).
Practice Implications
Our case demonstrates the advantages of the scalp as an STSG donor site with prompt healing time and excellent cosmesis. Because grafts are harvested at a depth superficial to the hair follicle, the hair regrows to conceal the donor site scar. Additionally, the robust blood supply of the scalp and hair follicle density optimize healing time. The location of the donor site at the postauricular scalp facilitates accessibility for wound care by the patient. Electrocautery or chemical styptics used for hemostasis may traumatize the hair follicles and risk causing alopecia; therefore, as demonstrated in our case, the preferred method to achieve hemostasis is the use of pressure or application of sterile gauze that has been saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine, followed by a pressure dressing provided by a sutured bolster.
Our case also demonstrates the utility of the Weck knife, which was introduced in 1968 as a modification of existing instruments to improve the ease of harvesting STSGs by appending a fixed handle and interchangeable depth gauges to a straight razor.1,2 The Weck knife can obtain grafts up to 5.5 cm in width (length may be as long as anatomically available), often circumventing the need to overlap grafts of smaller widths for repair of larger defects. Furthermore, grafts are harvested at a depth superficial to the hair follicle, averting donor site alopecia. These characteristics make the technique an ideal option for harvesting grafts from the scalp and other large donor sites.
Limitations of the Weck knife technique include the inability to harvest grafts from small donor sites in difficult-to-access anatomic regions or from areas with notable 3-dimensional structure. For harvesting such grafts, we prefer the DermaBlade (AccuTec Blades). Furthermore, assistance for providing tension along the trajectory of the Weck blade with a guard is optimal when performing the procedure. For practices not already utilizing a Weck knife, the technique necessitates additional training and cost. Nonetheless, for STSGs in which large donor site surface area, adjustable thickness, and convenient and timely operational technique are desired, the Weck knife should be considered as part of the dermatologic surgeon’s armamentarium.
- Aneer F, Singh AK, Kumar S. Evolution of instruments for harvest of the skin grafts. Indian J Plast Surg. 2013;46:28-35.
- Goulian D. A new economical dermatome. Plast Reconstr Surg. 1968;42:85-86.
- Aneer F, Singh AK, Kumar S. Evolution of instruments for harvest of the skin grafts. Indian J Plast Surg. 2013;46:28-35.
- Goulian D. A new economical dermatome. Plast Reconstr Surg. 1968;42:85-86.
"Doctor, Do I Need a Skin Check?"
What does your patient need to know at the first visit?
A patient may be scheduled for a total-body skin examination (TBSE) through several routes: primary care referral, continued cancer screening for an at-risk patient or patient transfer, or patient-directed scheduling for general screening regardless of risk factors. At the patient's first visit, it is imperative that the course of the appointment is smooth and predictable for patient comfort and for a thorough and effective examination. The nurse initially solicits salient medical history, particularly personal and family history of skin cancer, current medications, and any acute concerns. The nurse then prepares the patient for the logistics of the TBSE, namely to undress, don a gown that ties and opens in the back, and be seated on the examination table. When I enter the room, the conversation commences with me seated across from the patient, reviewing specifics about his/her history and risk factors. Then the TBSE is executed from head to toe.
Do you broadly recommend TBSE?
Firstly, TBSE is a safe clinical tool, supported by data outlining a lack of notable patient morbidity during the examination, including psychosocial factors, and it is generally well-received by patients (Risica et al). In 2016, the US Preventative Services Task Force (USPSTF) outlined its recommendations regarding screening for skin cancer, concluding that there is insufficient evidence to broadly recommend TBSE. Unfortunately, USPSTF findings amassed data from all types of screenings, including those by nondermatologists, and did not extract specialty-specific benefits and risks to patients. The recommendation also did not outline the influence of TBSE on morbidity and mortality for at-risk groups. The guidelines target primary care practice trends; therefore, specialty societies such as the American Academy of Dermatology issued statements following the USPSTF recommendation outlining these salient clarifications, namely that TBSE detects melanoma and keratinocyte carcinomas earlier than in patients who are not screened. Randomized controlled trials to prove this observation are lacking, particularly because of the ethics of withholding screening from a prospective study group. However, in 2017, Johnson et al outlined the best available survival data in concert with the USPSTF statement to arrive at the most beneficial screening recommendations for patients, specifically targeting risk groups--those with a history of skin cancer, immunosuppression, indoor tanning and/or many blistering sunburns, and several other genetic parameters--for at least annual TBSE.
The technique and reproducibility of TBSE also are not standardized, though they seem to have been endearingly apprenticed but variably implemented through generations of dermatology residents going forward into practice. As it is, depending on patient body surface area, mobility, willingness to disrobe, and adornments (eg, tattoos, hair appliances), multiple factors can restrict full view of a patient's skin. Recently, Helm et al proposed standardizing the TBSE sequence to minimize omitted areas of the body, which may become an imperative tool for streamlined resident teaching and optimal screening encounters.
How do you keep patients compliant with TBSE?
During and following TBSE, I typically outline any lesions of concern and plan for further testing, screening, and behavioral prevention strategies. Frequency of TBSE and importance of compliance are discussed during the visit and reinforced at checkout where the appointment templates are established a year in advance for those with skin cancer. Further, for those with melanoma, their appointment slots are given priority status so that any cancellations or delays are rescheduled preferentially. Particularly during the discussion about TBSE frequency, I emphasize the comparison and importance of this visit akin to other recommended screenings, such as mammograms and colonoscopies, and that we, as dermatologists, are part of their cancer surveillance team.
What do you do if patients refuse your recommendations?
Some patients refuse a gown or removal of certain clothing items (eg, undergarments, socks, wigs). Some patients defer a yearly TBSE upon checkout and schedule an appointment only when a lesion of concern arises. My advice is not to shame patients and to take advantage of as much as the patient is able and comfortable to show us and be present for, welcoming that we have the opportunity to take care of them and screen for cancer in any capacity. In underserved or limited budget practice regions, lesion-directed examination vs TBSE may be the only screening method utilized and may even attract more patients to a screening facility (Hoorens et al).
In the opposite corner are those patients who deem the recommended TBSE interval as too infrequent, which poses a delicate dilemma. In my opinion, these situations present another cohort of risks. Namely, the patient may become (or continue to be) overly fixated on the small details of every skin lesion, and in my experience, they tend to develop the habit of expecting at least 1 biopsy at each visit, typically of a lesion of their choosing. Depending on the validity of this expectation vs my clinical examination, it can lead to a difficult discussion with the patient about oversampling lesions and the potential for many scars, copious reexcisions for ambiguous lesion pathology, and a trend away from prudent clinical care. In addition, multiple visits incur more patient co-pays and time away from school, work, or home. To ease the patient's mind, I advise to call our office for a more acute visit if there is a lesion of concern; I additionally recommend taking a smartphone photograph of a concerning lesion and monitoring it for changes or sending the photograph to our patient portal messaging system so we can evaluate its acuity.
What take-home advice do you give to patients?
As the visit ends, I further explain that home self-examination or examination by a partner between visits is intuitively a valuable screening adjunct for skin cancer. In 2018, the USPSTF recommended behavioral skin cancer prevention counseling and self-examination only for younger-age cohorts with fair skin (6 months to 24 years), but its utility in specialty practice must be qualified. The American Academy of Dermatology Association subsequently issued a statement to support safe sun-protective practices and diligent self-screening for changing lesions, as earlier detection and management of skin cancer can lead to decreased morbidity and mortality from these neoplasms.
Resources for Patients
American Academy of Dermatology's SPOT Skin Cancer
Centers for Disease Control and Prevention: What Screening Tests Are There?
Suggested Readings
AAD statement on USPSTF recommendation on skin cancer screening. Schaumburg, IL: American Academy of Dermatology; July 26, 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf. Accessed April 26, 2019.
AADA responds to USPSTF recommendation on skin cancer prevention counseling. Rosemont, IL: American Academy of Dermatology Association; March 20, 2018. https://www.aad.org/media/news-releases/skin-cancer-prevention-counseling. Accessed April 26, 2019.
Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total body skin exam: an observational cohort study [published online February 15, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.02.028.
Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
What does your patient need to know at the first visit?
A patient may be scheduled for a total-body skin examination (TBSE) through several routes: primary care referral, continued cancer screening for an at-risk patient or patient transfer, or patient-directed scheduling for general screening regardless of risk factors. At the patient's first visit, it is imperative that the course of the appointment is smooth and predictable for patient comfort and for a thorough and effective examination. The nurse initially solicits salient medical history, particularly personal and family history of skin cancer, current medications, and any acute concerns. The nurse then prepares the patient for the logistics of the TBSE, namely to undress, don a gown that ties and opens in the back, and be seated on the examination table. When I enter the room, the conversation commences with me seated across from the patient, reviewing specifics about his/her history and risk factors. Then the TBSE is executed from head to toe.
Do you broadly recommend TBSE?
Firstly, TBSE is a safe clinical tool, supported by data outlining a lack of notable patient morbidity during the examination, including psychosocial factors, and it is generally well-received by patients (Risica et al). In 2016, the US Preventative Services Task Force (USPSTF) outlined its recommendations regarding screening for skin cancer, concluding that there is insufficient evidence to broadly recommend TBSE. Unfortunately, USPSTF findings amassed data from all types of screenings, including those by nondermatologists, and did not extract specialty-specific benefits and risks to patients. The recommendation also did not outline the influence of TBSE on morbidity and mortality for at-risk groups. The guidelines target primary care practice trends; therefore, specialty societies such as the American Academy of Dermatology issued statements following the USPSTF recommendation outlining these salient clarifications, namely that TBSE detects melanoma and keratinocyte carcinomas earlier than in patients who are not screened. Randomized controlled trials to prove this observation are lacking, particularly because of the ethics of withholding screening from a prospective study group. However, in 2017, Johnson et al outlined the best available survival data in concert with the USPSTF statement to arrive at the most beneficial screening recommendations for patients, specifically targeting risk groups--those with a history of skin cancer, immunosuppression, indoor tanning and/or many blistering sunburns, and several other genetic parameters--for at least annual TBSE.
The technique and reproducibility of TBSE also are not standardized, though they seem to have been endearingly apprenticed but variably implemented through generations of dermatology residents going forward into practice. As it is, depending on patient body surface area, mobility, willingness to disrobe, and adornments (eg, tattoos, hair appliances), multiple factors can restrict full view of a patient's skin. Recently, Helm et al proposed standardizing the TBSE sequence to minimize omitted areas of the body, which may become an imperative tool for streamlined resident teaching and optimal screening encounters.
How do you keep patients compliant with TBSE?
During and following TBSE, I typically outline any lesions of concern and plan for further testing, screening, and behavioral prevention strategies. Frequency of TBSE and importance of compliance are discussed during the visit and reinforced at checkout where the appointment templates are established a year in advance for those with skin cancer. Further, for those with melanoma, their appointment slots are given priority status so that any cancellations or delays are rescheduled preferentially. Particularly during the discussion about TBSE frequency, I emphasize the comparison and importance of this visit akin to other recommended screenings, such as mammograms and colonoscopies, and that we, as dermatologists, are part of their cancer surveillance team.
What do you do if patients refuse your recommendations?
Some patients refuse a gown or removal of certain clothing items (eg, undergarments, socks, wigs). Some patients defer a yearly TBSE upon checkout and schedule an appointment only when a lesion of concern arises. My advice is not to shame patients and to take advantage of as much as the patient is able and comfortable to show us and be present for, welcoming that we have the opportunity to take care of them and screen for cancer in any capacity. In underserved or limited budget practice regions, lesion-directed examination vs TBSE may be the only screening method utilized and may even attract more patients to a screening facility (Hoorens et al).
In the opposite corner are those patients who deem the recommended TBSE interval as too infrequent, which poses a delicate dilemma. In my opinion, these situations present another cohort of risks. Namely, the patient may become (or continue to be) overly fixated on the small details of every skin lesion, and in my experience, they tend to develop the habit of expecting at least 1 biopsy at each visit, typically of a lesion of their choosing. Depending on the validity of this expectation vs my clinical examination, it can lead to a difficult discussion with the patient about oversampling lesions and the potential for many scars, copious reexcisions for ambiguous lesion pathology, and a trend away from prudent clinical care. In addition, multiple visits incur more patient co-pays and time away from school, work, or home. To ease the patient's mind, I advise to call our office for a more acute visit if there is a lesion of concern; I additionally recommend taking a smartphone photograph of a concerning lesion and monitoring it for changes or sending the photograph to our patient portal messaging system so we can evaluate its acuity.
What take-home advice do you give to patients?
As the visit ends, I further explain that home self-examination or examination by a partner between visits is intuitively a valuable screening adjunct for skin cancer. In 2018, the USPSTF recommended behavioral skin cancer prevention counseling and self-examination only for younger-age cohorts with fair skin (6 months to 24 years), but its utility in specialty practice must be qualified. The American Academy of Dermatology Association subsequently issued a statement to support safe sun-protective practices and diligent self-screening for changing lesions, as earlier detection and management of skin cancer can lead to decreased morbidity and mortality from these neoplasms.
Resources for Patients
American Academy of Dermatology's SPOT Skin Cancer
Centers for Disease Control and Prevention: What Screening Tests Are There?
What does your patient need to know at the first visit?
A patient may be scheduled for a total-body skin examination (TBSE) through several routes: primary care referral, continued cancer screening for an at-risk patient or patient transfer, or patient-directed scheduling for general screening regardless of risk factors. At the patient's first visit, it is imperative that the course of the appointment is smooth and predictable for patient comfort and for a thorough and effective examination. The nurse initially solicits salient medical history, particularly personal and family history of skin cancer, current medications, and any acute concerns. The nurse then prepares the patient for the logistics of the TBSE, namely to undress, don a gown that ties and opens in the back, and be seated on the examination table. When I enter the room, the conversation commences with me seated across from the patient, reviewing specifics about his/her history and risk factors. Then the TBSE is executed from head to toe.
Do you broadly recommend TBSE?
Firstly, TBSE is a safe clinical tool, supported by data outlining a lack of notable patient morbidity during the examination, including psychosocial factors, and it is generally well-received by patients (Risica et al). In 2016, the US Preventative Services Task Force (USPSTF) outlined its recommendations regarding screening for skin cancer, concluding that there is insufficient evidence to broadly recommend TBSE. Unfortunately, USPSTF findings amassed data from all types of screenings, including those by nondermatologists, and did not extract specialty-specific benefits and risks to patients. The recommendation also did not outline the influence of TBSE on morbidity and mortality for at-risk groups. The guidelines target primary care practice trends; therefore, specialty societies such as the American Academy of Dermatology issued statements following the USPSTF recommendation outlining these salient clarifications, namely that TBSE detects melanoma and keratinocyte carcinomas earlier than in patients who are not screened. Randomized controlled trials to prove this observation are lacking, particularly because of the ethics of withholding screening from a prospective study group. However, in 2017, Johnson et al outlined the best available survival data in concert with the USPSTF statement to arrive at the most beneficial screening recommendations for patients, specifically targeting risk groups--those with a history of skin cancer, immunosuppression, indoor tanning and/or many blistering sunburns, and several other genetic parameters--for at least annual TBSE.
The technique and reproducibility of TBSE also are not standardized, though they seem to have been endearingly apprenticed but variably implemented through generations of dermatology residents going forward into practice. As it is, depending on patient body surface area, mobility, willingness to disrobe, and adornments (eg, tattoos, hair appliances), multiple factors can restrict full view of a patient's skin. Recently, Helm et al proposed standardizing the TBSE sequence to minimize omitted areas of the body, which may become an imperative tool for streamlined resident teaching and optimal screening encounters.
How do you keep patients compliant with TBSE?
During and following TBSE, I typically outline any lesions of concern and plan for further testing, screening, and behavioral prevention strategies. Frequency of TBSE and importance of compliance are discussed during the visit and reinforced at checkout where the appointment templates are established a year in advance for those with skin cancer. Further, for those with melanoma, their appointment slots are given priority status so that any cancellations or delays are rescheduled preferentially. Particularly during the discussion about TBSE frequency, I emphasize the comparison and importance of this visit akin to other recommended screenings, such as mammograms and colonoscopies, and that we, as dermatologists, are part of their cancer surveillance team.
What do you do if patients refuse your recommendations?
Some patients refuse a gown or removal of certain clothing items (eg, undergarments, socks, wigs). Some patients defer a yearly TBSE upon checkout and schedule an appointment only when a lesion of concern arises. My advice is not to shame patients and to take advantage of as much as the patient is able and comfortable to show us and be present for, welcoming that we have the opportunity to take care of them and screen for cancer in any capacity. In underserved or limited budget practice regions, lesion-directed examination vs TBSE may be the only screening method utilized and may even attract more patients to a screening facility (Hoorens et al).
In the opposite corner are those patients who deem the recommended TBSE interval as too infrequent, which poses a delicate dilemma. In my opinion, these situations present another cohort of risks. Namely, the patient may become (or continue to be) overly fixated on the small details of every skin lesion, and in my experience, they tend to develop the habit of expecting at least 1 biopsy at each visit, typically of a lesion of their choosing. Depending on the validity of this expectation vs my clinical examination, it can lead to a difficult discussion with the patient about oversampling lesions and the potential for many scars, copious reexcisions for ambiguous lesion pathology, and a trend away from prudent clinical care. In addition, multiple visits incur more patient co-pays and time away from school, work, or home. To ease the patient's mind, I advise to call our office for a more acute visit if there is a lesion of concern; I additionally recommend taking a smartphone photograph of a concerning lesion and monitoring it for changes or sending the photograph to our patient portal messaging system so we can evaluate its acuity.
What take-home advice do you give to patients?
As the visit ends, I further explain that home self-examination or examination by a partner between visits is intuitively a valuable screening adjunct for skin cancer. In 2018, the USPSTF recommended behavioral skin cancer prevention counseling and self-examination only for younger-age cohorts with fair skin (6 months to 24 years), but its utility in specialty practice must be qualified. The American Academy of Dermatology Association subsequently issued a statement to support safe sun-protective practices and diligent self-screening for changing lesions, as earlier detection and management of skin cancer can lead to decreased morbidity and mortality from these neoplasms.
Resources for Patients
American Academy of Dermatology's SPOT Skin Cancer
Centers for Disease Control and Prevention: What Screening Tests Are There?
Suggested Readings
AAD statement on USPSTF recommendation on skin cancer screening. Schaumburg, IL: American Academy of Dermatology; July 26, 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf. Accessed April 26, 2019.
AADA responds to USPSTF recommendation on skin cancer prevention counseling. Rosemont, IL: American Academy of Dermatology Association; March 20, 2018. https://www.aad.org/media/news-releases/skin-cancer-prevention-counseling. Accessed April 26, 2019.
Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total body skin exam: an observational cohort study [published online February 15, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.02.028.
Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
Suggested Readings
AAD statement on USPSTF recommendation on skin cancer screening. Schaumburg, IL: American Academy of Dermatology; July 26, 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf. Accessed April 26, 2019.
AADA responds to USPSTF recommendation on skin cancer prevention counseling. Rosemont, IL: American Academy of Dermatology Association; March 20, 2018. https://www.aad.org/media/news-releases/skin-cancer-prevention-counseling. Accessed April 26, 2019.
Helm MF, Hallock KK, Bisbee E, et al. Optimizing the total body skin exam: an observational cohort study [published online February 15, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.02.028.
Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
Hair Loss in Skin of Color Patients
What does your patient need to know at the first visit?
All patients, regardless of race, gender, or age, are afraid of an alopecia diagnosis. Often, the first thing a patient may say when I enter the examination room is, "Please don't tell me I have alopecia."
The first step to a successful initial visit for hair loss is addressing the angst around the word alopecia, which helps to manage the patient's hair-induced anxiety. The next priority is setting expectations for the journey including what to expect during the diagnosis process, treatment, and beyond.
Next is data collection. An extensive hair care practice investigation can begin with a survey that the patient fills out before the visit. Dive into and expand on hair loss history questions, including medical history as well as hair care practices (eg, history of use, frequency, number of years, maintenance for that particular hairstyle) such as braids (eg, individual braids, cornrow braids, with or without added synthetic or human hair), locs (eg, length of locs), chemical relaxers (eg, number of years, frequency, professionally applied or applied at home), hair color, weaves (eg, glued in, sewn in, combination), and more.1 Include a family history of hair loss, both maternal and paternal.
The hair loss investigation almost always includes a scalp biopsy, hair-pull test, dermoscopy, photographs, and even blood work, if applicable. Scalp biopsies may reveal more than one type of alopecia diagnosis, which may impact the treatment plan.2 Sending the scalp biopsy specimen to a dermatopathologist specializing in alopecia along with clinical information about the patient is preferred.
What are your go-to treatments?
My go-to treatments for patients with skin of color (SOC) and hair loss really depend on the specific diagnosis. Randomized, placebo-controlled clinical trials focusing on treatment are lacking in central centrifugal cicatricial alopecia and traction alopecia, which holds true for many other types of alopecia.
For black patients with central centrifugal cicatricial alopecia, I often address the inflammatory component of the disease with oral doxycycline and either a topical corticosteroid, such as clobetasol, or intralesional triamcinolone. Adding minoxidil-containing products later in the treatment process can be helpful. Various treatment protocols exist but are mainly based on anecdotal evidence.1
For those with traction alopecia, modification of offending hairstyle practices is a must.3 Also, treatment of inflammation is key. Typically, I gravitate to topical or intralesional corticosteroids, followed by minoxidil-containing products. However, a challenge of treating traction alopecia is changing the hair care practices that cause tight pulling, friction, or pressure on the scalp, such as from the band of a tightly fitted wig.
It is important to discuss potential side effects of any treatment with the patient. For the most common side effects, discuss how to best prevent them. For example, because of the photosensitivity potential of doxycycline, I ask patients to wear sunscreen daily. To prevent nausea, I recommend that they avoid taking doxycycline on an empty stomach, drink plenty of fluids, and avoid laying down within a few hours after taking the medication.
How do you keep patients compliant with treatment?
Dermatologists should try to understand their patients' hair. A study of 200 black women demonstrated that 68% of the patients did not think their physician understood their hair,4 which likely impacts patients' perceptions of their physician, confidence in the treatment plan, and even compliance with the plan. Attempting to understand the nuances of tightly coiled hair in those of African descent is the first step in the journey of diagnosing and treating hair loss in partnership with the patient.
Setting the goal is a crucial step toward patient compliance. It may be going out in public without a wig or weave and feeling confident, providing more coverage so affected areas do not show as much, improving scalp tenderness, and/or preventing further progression of the condition. These are all reasonable outcomes and each goal is uniquely tailored to each patient.
Familiarize yourself with various hair types, hairstyles, and preferred medication vehicles by attending continuing medical education lectures on alopecia in patients with SOC and on nuances to diagnosis and treatment, reading textbooks focusing on SOC, or seeking out mentorship from a dermatologist who is a hair expert in the types of alopecia most commonly affecting patients with SOC.
What resources do you recommend to patients for more information
For patients with scarring alopecia, the Cicatricial Alopecia Research Foundation (http://www.carfintl.org/) is a great resource for medical information and support groups. Also, the Skin of Color Society has dermatology patient education information (http://skinofcolorsociety.org/).
For patients who are extremely distressed by hair loss, I encourage them to see a mental health professional. The mental health impact of alopecia, despite the extent of disease, is likely underestimated. Patients sometimes need our permission to seek help, especially in many SOC communities where even seeking mental health care often is frowned upon.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric skin of color patients: bootcamp discussion. Cutis. 2017;100:31-35.
- Wohltmann WE, Sperling L. Histopathologic diagnosis of multifactorial alopecia. J Cutan Pathol. 2016;43:483-491.
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia. J Am Acad Dermatol. 2016;75:606-611.
- Gathers RC, Mahan MG. African American women, hair care and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
What does your patient need to know at the first visit?
All patients, regardless of race, gender, or age, are afraid of an alopecia diagnosis. Often, the first thing a patient may say when I enter the examination room is, "Please don't tell me I have alopecia."
The first step to a successful initial visit for hair loss is addressing the angst around the word alopecia, which helps to manage the patient's hair-induced anxiety. The next priority is setting expectations for the journey including what to expect during the diagnosis process, treatment, and beyond.
Next is data collection. An extensive hair care practice investigation can begin with a survey that the patient fills out before the visit. Dive into and expand on hair loss history questions, including medical history as well as hair care practices (eg, history of use, frequency, number of years, maintenance for that particular hairstyle) such as braids (eg, individual braids, cornrow braids, with or without added synthetic or human hair), locs (eg, length of locs), chemical relaxers (eg, number of years, frequency, professionally applied or applied at home), hair color, weaves (eg, glued in, sewn in, combination), and more.1 Include a family history of hair loss, both maternal and paternal.
The hair loss investigation almost always includes a scalp biopsy, hair-pull test, dermoscopy, photographs, and even blood work, if applicable. Scalp biopsies may reveal more than one type of alopecia diagnosis, which may impact the treatment plan.2 Sending the scalp biopsy specimen to a dermatopathologist specializing in alopecia along with clinical information about the patient is preferred.
What are your go-to treatments?
My go-to treatments for patients with skin of color (SOC) and hair loss really depend on the specific diagnosis. Randomized, placebo-controlled clinical trials focusing on treatment are lacking in central centrifugal cicatricial alopecia and traction alopecia, which holds true for many other types of alopecia.
For black patients with central centrifugal cicatricial alopecia, I often address the inflammatory component of the disease with oral doxycycline and either a topical corticosteroid, such as clobetasol, or intralesional triamcinolone. Adding minoxidil-containing products later in the treatment process can be helpful. Various treatment protocols exist but are mainly based on anecdotal evidence.1
For those with traction alopecia, modification of offending hairstyle practices is a must.3 Also, treatment of inflammation is key. Typically, I gravitate to topical or intralesional corticosteroids, followed by minoxidil-containing products. However, a challenge of treating traction alopecia is changing the hair care practices that cause tight pulling, friction, or pressure on the scalp, such as from the band of a tightly fitted wig.
It is important to discuss potential side effects of any treatment with the patient. For the most common side effects, discuss how to best prevent them. For example, because of the photosensitivity potential of doxycycline, I ask patients to wear sunscreen daily. To prevent nausea, I recommend that they avoid taking doxycycline on an empty stomach, drink plenty of fluids, and avoid laying down within a few hours after taking the medication.
How do you keep patients compliant with treatment?
Dermatologists should try to understand their patients' hair. A study of 200 black women demonstrated that 68% of the patients did not think their physician understood their hair,4 which likely impacts patients' perceptions of their physician, confidence in the treatment plan, and even compliance with the plan. Attempting to understand the nuances of tightly coiled hair in those of African descent is the first step in the journey of diagnosing and treating hair loss in partnership with the patient.
Setting the goal is a crucial step toward patient compliance. It may be going out in public without a wig or weave and feeling confident, providing more coverage so affected areas do not show as much, improving scalp tenderness, and/or preventing further progression of the condition. These are all reasonable outcomes and each goal is uniquely tailored to each patient.
Familiarize yourself with various hair types, hairstyles, and preferred medication vehicles by attending continuing medical education lectures on alopecia in patients with SOC and on nuances to diagnosis and treatment, reading textbooks focusing on SOC, or seeking out mentorship from a dermatologist who is a hair expert in the types of alopecia most commonly affecting patients with SOC.
What resources do you recommend to patients for more information
For patients with scarring alopecia, the Cicatricial Alopecia Research Foundation (http://www.carfintl.org/) is a great resource for medical information and support groups. Also, the Skin of Color Society has dermatology patient education information (http://skinofcolorsociety.org/).
For patients who are extremely distressed by hair loss, I encourage them to see a mental health professional. The mental health impact of alopecia, despite the extent of disease, is likely underestimated. Patients sometimes need our permission to seek help, especially in many SOC communities where even seeking mental health care often is frowned upon.
What does your patient need to know at the first visit?
All patients, regardless of race, gender, or age, are afraid of an alopecia diagnosis. Often, the first thing a patient may say when I enter the examination room is, "Please don't tell me I have alopecia."
The first step to a successful initial visit for hair loss is addressing the angst around the word alopecia, which helps to manage the patient's hair-induced anxiety. The next priority is setting expectations for the journey including what to expect during the diagnosis process, treatment, and beyond.
Next is data collection. An extensive hair care practice investigation can begin with a survey that the patient fills out before the visit. Dive into and expand on hair loss history questions, including medical history as well as hair care practices (eg, history of use, frequency, number of years, maintenance for that particular hairstyle) such as braids (eg, individual braids, cornrow braids, with or without added synthetic or human hair), locs (eg, length of locs), chemical relaxers (eg, number of years, frequency, professionally applied or applied at home), hair color, weaves (eg, glued in, sewn in, combination), and more.1 Include a family history of hair loss, both maternal and paternal.
The hair loss investigation almost always includes a scalp biopsy, hair-pull test, dermoscopy, photographs, and even blood work, if applicable. Scalp biopsies may reveal more than one type of alopecia diagnosis, which may impact the treatment plan.2 Sending the scalp biopsy specimen to a dermatopathologist specializing in alopecia along with clinical information about the patient is preferred.
What are your go-to treatments?
My go-to treatments for patients with skin of color (SOC) and hair loss really depend on the specific diagnosis. Randomized, placebo-controlled clinical trials focusing on treatment are lacking in central centrifugal cicatricial alopecia and traction alopecia, which holds true for many other types of alopecia.
For black patients with central centrifugal cicatricial alopecia, I often address the inflammatory component of the disease with oral doxycycline and either a topical corticosteroid, such as clobetasol, or intralesional triamcinolone. Adding minoxidil-containing products later in the treatment process can be helpful. Various treatment protocols exist but are mainly based on anecdotal evidence.1
For those with traction alopecia, modification of offending hairstyle practices is a must.3 Also, treatment of inflammation is key. Typically, I gravitate to topical or intralesional corticosteroids, followed by minoxidil-containing products. However, a challenge of treating traction alopecia is changing the hair care practices that cause tight pulling, friction, or pressure on the scalp, such as from the band of a tightly fitted wig.
It is important to discuss potential side effects of any treatment with the patient. For the most common side effects, discuss how to best prevent them. For example, because of the photosensitivity potential of doxycycline, I ask patients to wear sunscreen daily. To prevent nausea, I recommend that they avoid taking doxycycline on an empty stomach, drink plenty of fluids, and avoid laying down within a few hours after taking the medication.
How do you keep patients compliant with treatment?
Dermatologists should try to understand their patients' hair. A study of 200 black women demonstrated that 68% of the patients did not think their physician understood their hair,4 which likely impacts patients' perceptions of their physician, confidence in the treatment plan, and even compliance with the plan. Attempting to understand the nuances of tightly coiled hair in those of African descent is the first step in the journey of diagnosing and treating hair loss in partnership with the patient.
Setting the goal is a crucial step toward patient compliance. It may be going out in public without a wig or weave and feeling confident, providing more coverage so affected areas do not show as much, improving scalp tenderness, and/or preventing further progression of the condition. These are all reasonable outcomes and each goal is uniquely tailored to each patient.
Familiarize yourself with various hair types, hairstyles, and preferred medication vehicles by attending continuing medical education lectures on alopecia in patients with SOC and on nuances to diagnosis and treatment, reading textbooks focusing on SOC, or seeking out mentorship from a dermatologist who is a hair expert in the types of alopecia most commonly affecting patients with SOC.
What resources do you recommend to patients for more information
For patients with scarring alopecia, the Cicatricial Alopecia Research Foundation (http://www.carfintl.org/) is a great resource for medical information and support groups. Also, the Skin of Color Society has dermatology patient education information (http://skinofcolorsociety.org/).
For patients who are extremely distressed by hair loss, I encourage them to see a mental health professional. The mental health impact of alopecia, despite the extent of disease, is likely underestimated. Patients sometimes need our permission to seek help, especially in many SOC communities where even seeking mental health care often is frowned upon.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric skin of color patients: bootcamp discussion. Cutis. 2017;100:31-35.
- Wohltmann WE, Sperling L. Histopathologic diagnosis of multifactorial alopecia. J Cutan Pathol. 2016;43:483-491.
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia. J Am Acad Dermatol. 2016;75:606-611.
- Gathers RC, Mahan MG. African American women, hair care and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric skin of color patients: bootcamp discussion. Cutis. 2017;100:31-35.
- Wohltmann WE, Sperling L. Histopathologic diagnosis of multifactorial alopecia. J Cutan Pathol. 2016;43:483-491.
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia. J Am Acad Dermatol. 2016;75:606-611.
- Gathers RC, Mahan MG. African American women, hair care and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
Clinical Pearl: Kinesiology Tape for Onychocryptosis
Practice Gap
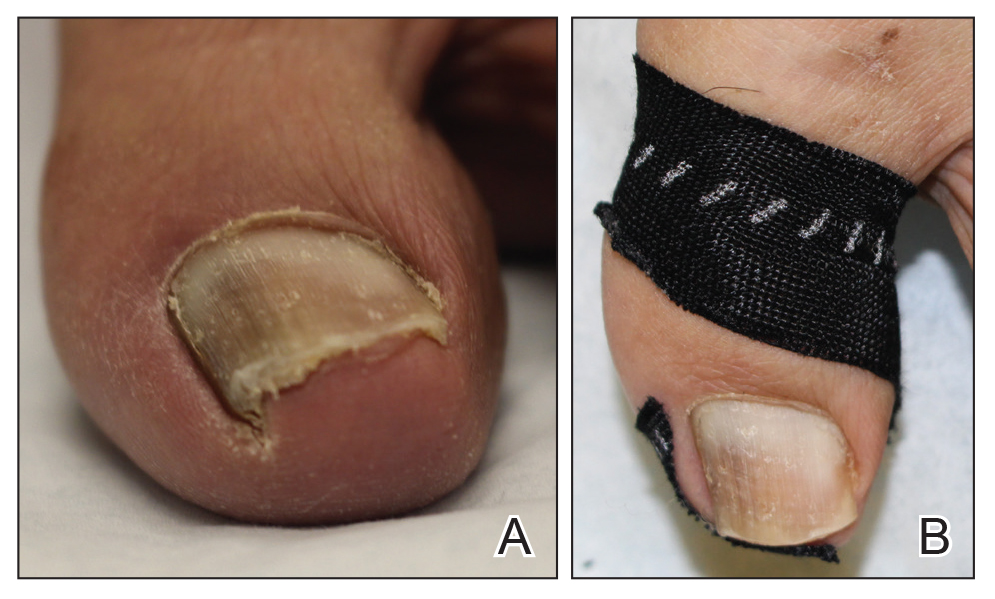
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.
Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
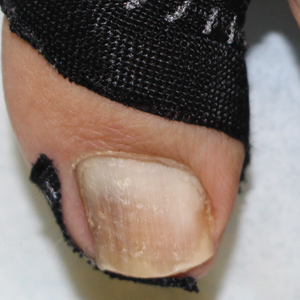
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
Practice Gap
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.
Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
Practice Gap
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.
Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
Nail Psoriasis Tips
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.
The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.
The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.
The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
Wound Closure Tips
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
Clinical Pearl: Mohs Cantaloupe Analogy for the Dermatology Resident
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).

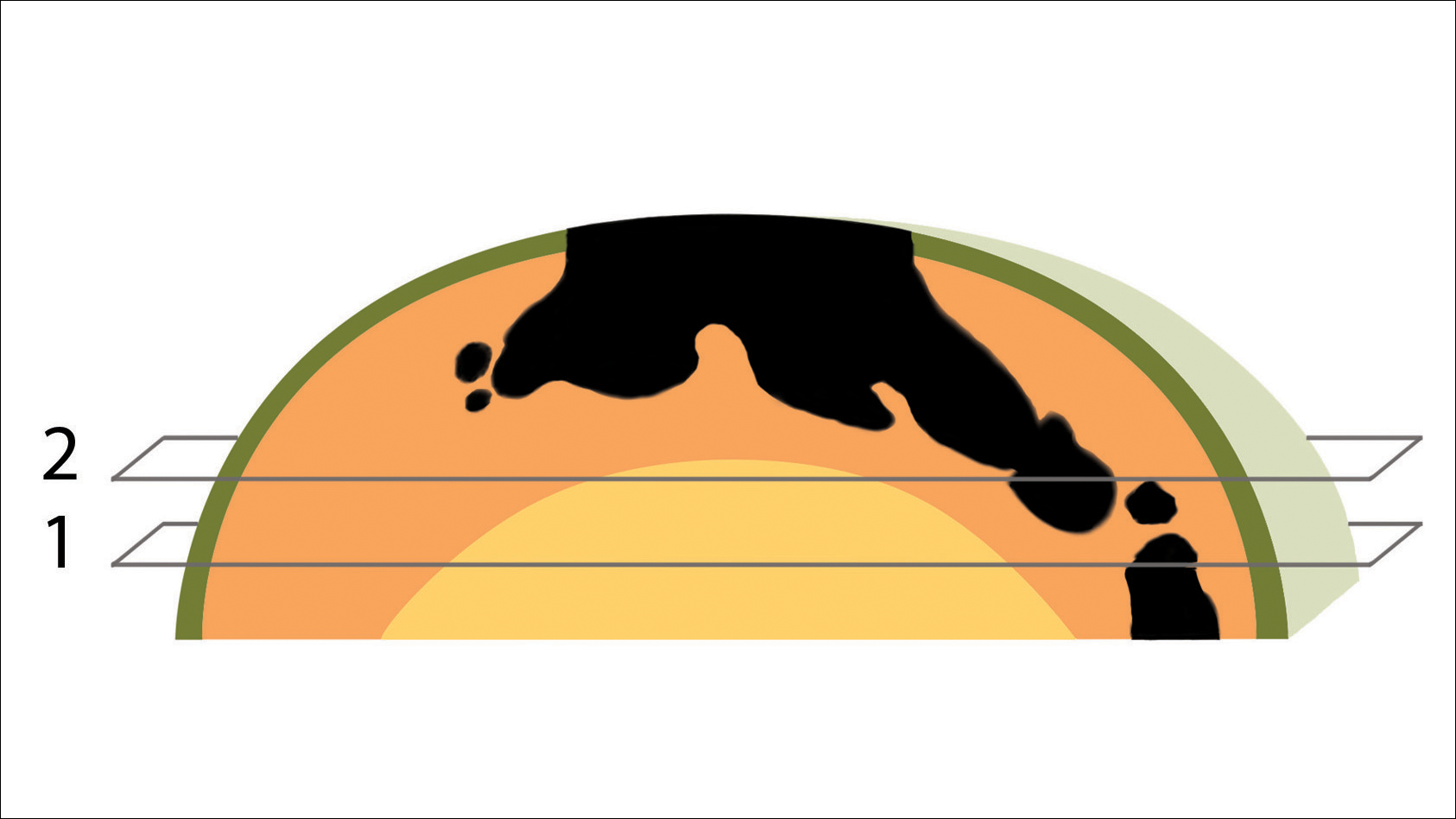
In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.
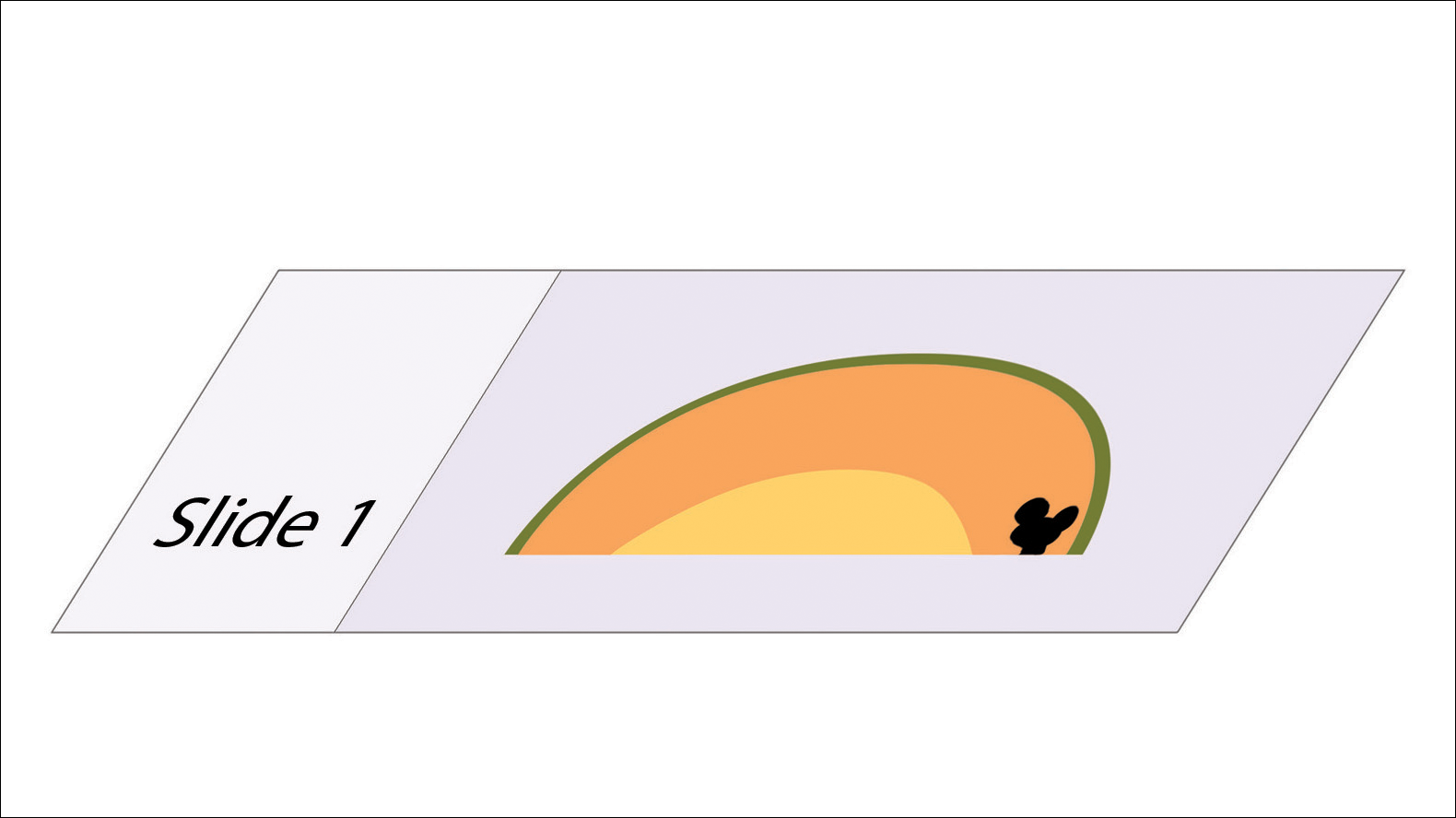
Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
Biologics and Systemic Therapies for Psoriasis: Treat the Patient, Not the Disease
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.