User login
The path to becoming an esophagologist
Esophagology was a term coined in 1948 to describe a medical specialty devoted to the study of the anatomy, physiology, and pathology of the esophagus. The term was born out of increased interest and evolution in esophagology and supported by development in esophagoscopy.1 While still rooted in these basic tenets, the landscape of esophagology is dramatically different in 2020. The last decade alone has seen unprecedented technological advances in esophagology, from the transformation of line tracings to high-resolution esophageal pressure topography to more recent innovations such as the functional lumen imaging probe. Successful therapeutic developments have increased opportunities for effective and less invasive treatment approaches for achalasia and gastroesophageal reflux disease (GERD). With changing concepts in esophageal diseases such as eosinophilic esophagitis, successful management now incorporates findings from recent discoveries that have revolutionized care pathways. (see Figure 1).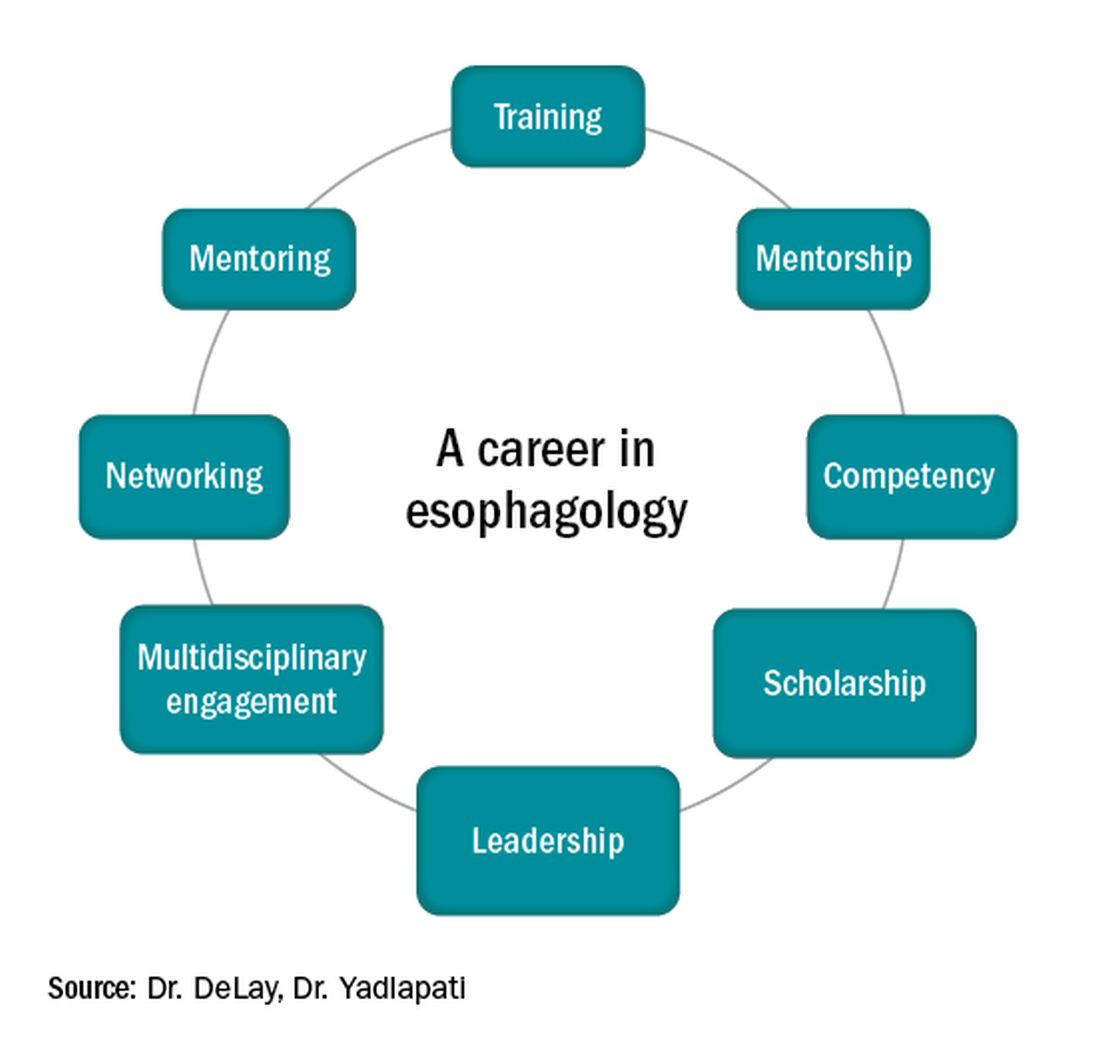
Figure 1
Optimizing esophagology training during fellowship
First, and most importantly, an esophagologist must have a foundation in the basic principles of esophageal anatomy, physiology, and pathology (see Figure 2). While newer digital learning resources exist, tried and true book-based resources – text books, chapters, and reviews – related to esophageal mechanics, the interplay between muscle function and neurogenics, and factors associated with nociception, remain the optimal learning strategy.
Once equipped with a foundation in esophageal physiology, one can readily engage with esophageal technologies, as there exists a vast array of testing to assess esophageal function. A comprehensive understanding of each, including device configuration, clinical protocol, and data storage, promotes a depth of knowledge every esophagologist should develop. Aspiring esophagologists should take time to observe and perform procedures in their motility labs, particularly esophageal high-resolution manometry and ambulatory reflux monitoring studies. If afforded the opportunity through a research study or a clinical indication, esophagologists should also undergo the tests themselves. Empathy regarding the discomfort and tolerability of motility tests, which are notoriously challenging for patients, can promote rapport and trust with patients, increase patient satisfaction, and enhance one’s own understanding of resource utilization and safety.
Perhaps most critical to becoming an esophagologist, is acquiring sufficient competency in interpretation of esophageal studies. Prior research highlights the limitations in achieving competency when trainees adhere to the minimum case volume of studies recommended by the GI core curriculum.2,3 With the bar set higher for the burgeoning esophagologist, one must not only practice with a higher case volume, but also engage in competency-based assessments and performance feedback.4 Trainees should start by reviewing tracings for their own patients. Preliminary interpretation of pending studies and review with a mentor before the final sign-off, participation in research that requires study, or even teaching co-trainees basic tenets of motility are other creative approaches to learning. Esophagologists will be expected to know how to navigate the software to access studies, manually review tracings, and generate reports. Trainees should refer to the multitude of societal guidelines and classification scheme recommendations available when developing competency in diagnostic impression.5
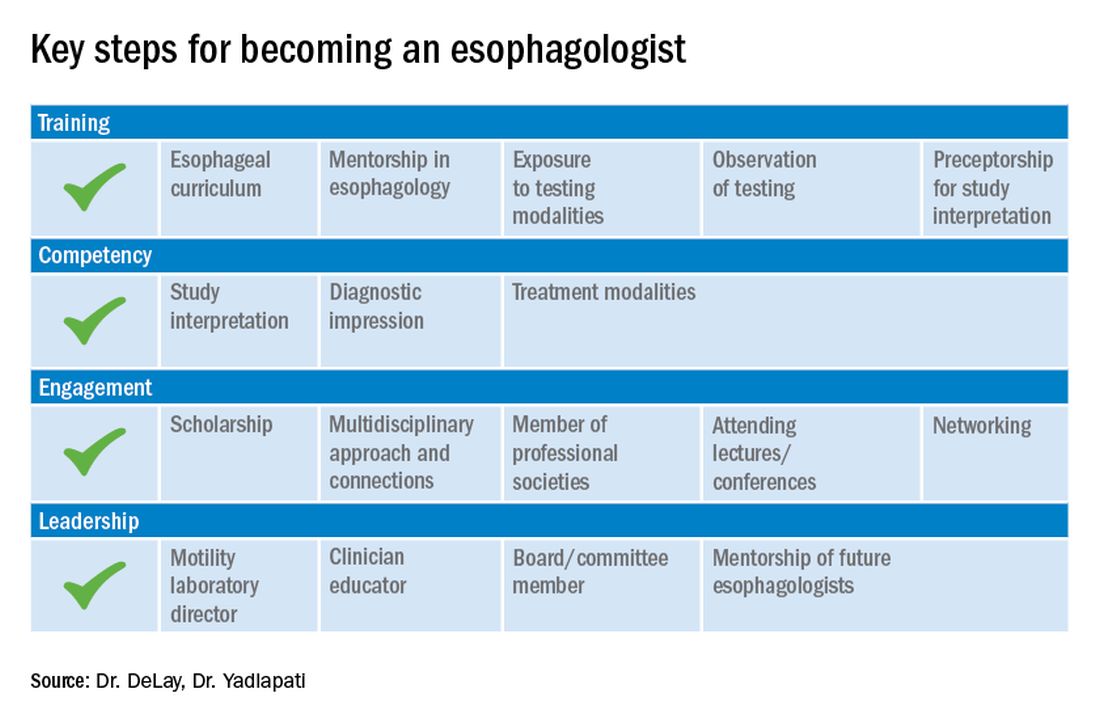
Figure 2
While esophagology is a medical specialty, it is imperative that the esophagologist has a robust understanding of therapeutic options and surgical interventions for esophageal pathology. Scrubbing into the operating room during foregut surgeries is an eye-opening experience. This includes thoracic and abdominal approaches, robotic, laparoscopic, and open techniques, and interventions for GERD, achalasia, diverticular disease, and bariatric management. Equally important is working alongside advanced endoscopy faculty to understand utilities of endoscopic ultrasound, ablative methods for Barrett’s esophagus, and advanced techniques such as peroral endoscopic myotomy and transoral incisionless fundoplication. This exposure is critical as the role of the esophagologist is to speak knowledgably of therapeutic options and the risks and benefits of alternative approaches. Further, the patient’s journey rarely ends with the intervention, and an esophagologist must understand how to evaluate symptoms and manage complications following therapy.
As with broader digestive health, the management of esophageal disorders is becoming increasingly integrated with psychological, lifestyle, and dietary interventions. Observing and understanding how other health care members interact with the patient and relay concepts of brain-gut interaction is helpful in one’s own practice and ability to speak to the value of focused interventions.
These key training aspects in esophagology can be acquired through different avenues (see Figure 3). Formal 1-year advanced esophageal or motility focused fellowships are available at leading esophageal centers. The American Neurogastroenterology and Motility Society (ANMS) offers a clinical training program for selected fellows to pursue apprenticeship-based training in gastrointestinal motility. A review of the benefits of additional training, available programs, and how to apply, can be found at The New Gastroenterologist. It may be possible to customize parts of the general clinical fellowship with a strong focus on esophagology. All budding esophagologists are strongly encouraged to attend and participate in subspecialty national meetings such as through the ANMS or the American Foregut Society.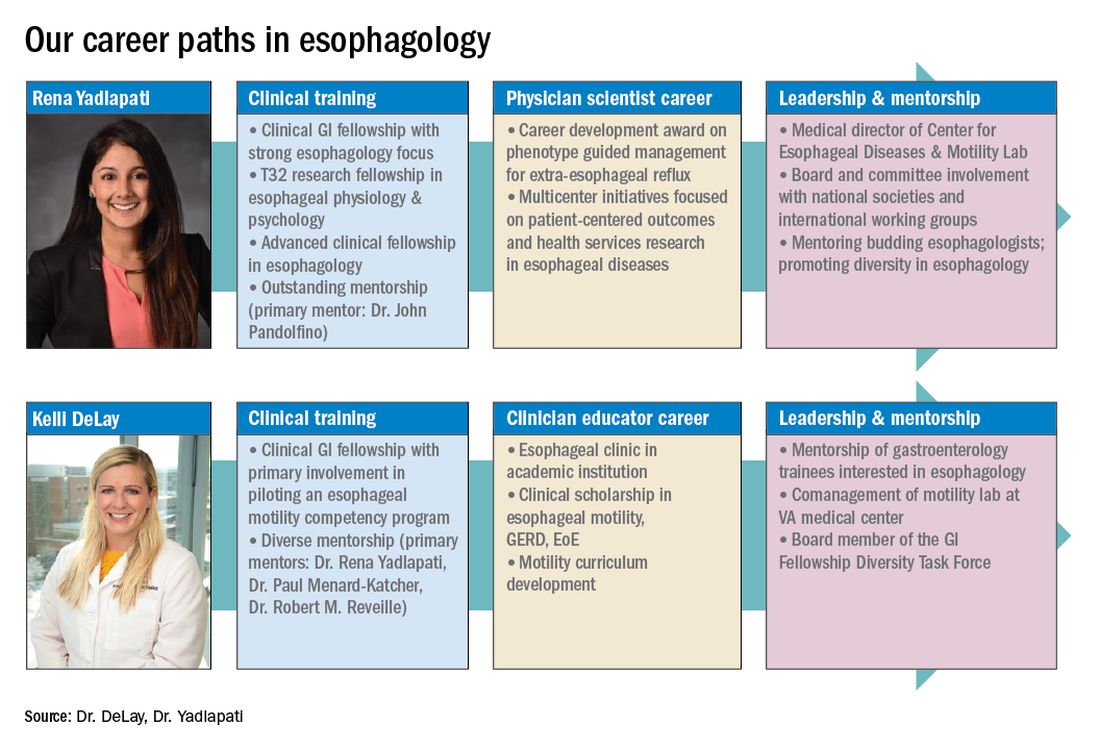
Figure 3
Steep learning curve post fellowship
Regardless of the robust nature of clinical esophagology training, early career esophagologists will face challenges and learn on the job.
Many esophagologists are directors of a motility lab early in their careers. This is often uncharted territory in terms of managing a team of nurses, technicians, and other providers. The director of a motility lab will be called upon to troubleshoot various arenas of diagnostic workup, from study acquisition and interpretation to technical barriers with equipment or software. Keys to maintaining a successful motility lab further include optimizing schedules and protocols, delineating roles and responsibilities of team members, ensuring adequate training across staff and providers, communicating expectations, and cultivating an open relationship with the motility lab supervisor. Crucial, yet often neglected during fellowship training, are the economic considerations of operating and expanding the motility lab, and the financial implications for one’s own practice.6 Participating in professional development workshops can be especially valuable in cultivating leadership skills.
The care an esophagologist provides relies heavily on collaborative relationships within the organization and peer mentorship, cooperation, and feedback. It is essential to cultivate multidisciplinary relationships with surgical (e.g., foregut surgery, laryngology), medical (e.g., pulmonology, allergy), radiology, and pathology colleagues, as well as with integrated health specialists including psychologists, dietitians, and speech language pathologists. It is also important to have open industry partnerships to ensure appropriate technical support and access to advancements.
Often organizations will have only one esophageal specialist within the group. Fortunately, the national and global community of esophagologists is highly collaborative and collegial. All esophagologists should have a network of mentors and colleagues within and outside of their organization to review complex cases, discuss challenges in the workplace, and foster research and innovation. Along these lines, both aspiring and practicing esophagologists should engage with professional societies as opportunities are abundant. Esophageal-focused societies include the ANMS, American Foregut Society, and International Society of Diseases of Esophagus, and the overarching GI societies also have a strong esophageal focus.
The path to becoming an esophagologist does not mirror the structure of the organ itself. Development is neither confined, unidirectional, nor set in length, but gradual, each step thoughtfully built on the last. Esophageal pathology is diverse, complex, and fascinating. With the appropriate training, mentorship, engagement, and leadership, esophagologists have the privilege of making a great impact on the lives of patients we meet, a fulfilling journey worth the time and effort it takes.
Dr. Delay is in the division of gastroenterology & hepatology, University of Colorado Anschutz Medical Campus, Aurora. Dr. Yadlapati is at the Center for Esophageal Diseases, division of gastroenterology, University of California San Diego, La Jolla. She is a consultant through institutional agreement to Medtronic, Ironwood Pharmaceuticals, and Diversatek; she has received research support from Ironwood Pharmaceuticals; and is on the advisory board of Phathom Pharmaceuticals.
References
1. Holinger PH. Arch Otolaryngol. 1948;47:119-26.
2. Yadlapati R et al. Clin Gastroenterol Hepatol. 2017;15:1708-14.e3.
3. Oversight Working Network et al. Gastrointest Endosc. 2014;80:16-27.
4. DeLay K et al. Am J Gastroenterol. 2020;115:1453-9.
5. Gyawali CP et al. Neurogastroenterol Motil. 2018;30(9):e13341.
6. Yadlapati R et al. Gastroenterology. 2020;158:1202-10.
Esophagology was a term coined in 1948 to describe a medical specialty devoted to the study of the anatomy, physiology, and pathology of the esophagus. The term was born out of increased interest and evolution in esophagology and supported by development in esophagoscopy.1 While still rooted in these basic tenets, the landscape of esophagology is dramatically different in 2020. The last decade alone has seen unprecedented technological advances in esophagology, from the transformation of line tracings to high-resolution esophageal pressure topography to more recent innovations such as the functional lumen imaging probe. Successful therapeutic developments have increased opportunities for effective and less invasive treatment approaches for achalasia and gastroesophageal reflux disease (GERD). With changing concepts in esophageal diseases such as eosinophilic esophagitis, successful management now incorporates findings from recent discoveries that have revolutionized care pathways. (see Figure 1).
Figure 1
Optimizing esophagology training during fellowship
First, and most importantly, an esophagologist must have a foundation in the basic principles of esophageal anatomy, physiology, and pathology (see Figure 2). While newer digital learning resources exist, tried and true book-based resources – text books, chapters, and reviews – related to esophageal mechanics, the interplay between muscle function and neurogenics, and factors associated with nociception, remain the optimal learning strategy.
Once equipped with a foundation in esophageal physiology, one can readily engage with esophageal technologies, as there exists a vast array of testing to assess esophageal function. A comprehensive understanding of each, including device configuration, clinical protocol, and data storage, promotes a depth of knowledge every esophagologist should develop. Aspiring esophagologists should take time to observe and perform procedures in their motility labs, particularly esophageal high-resolution manometry and ambulatory reflux monitoring studies. If afforded the opportunity through a research study or a clinical indication, esophagologists should also undergo the tests themselves. Empathy regarding the discomfort and tolerability of motility tests, which are notoriously challenging for patients, can promote rapport and trust with patients, increase patient satisfaction, and enhance one’s own understanding of resource utilization and safety.
Perhaps most critical to becoming an esophagologist, is acquiring sufficient competency in interpretation of esophageal studies. Prior research highlights the limitations in achieving competency when trainees adhere to the minimum case volume of studies recommended by the GI core curriculum.2,3 With the bar set higher for the burgeoning esophagologist, one must not only practice with a higher case volume, but also engage in competency-based assessments and performance feedback.4 Trainees should start by reviewing tracings for their own patients. Preliminary interpretation of pending studies and review with a mentor before the final sign-off, participation in research that requires study, or even teaching co-trainees basic tenets of motility are other creative approaches to learning. Esophagologists will be expected to know how to navigate the software to access studies, manually review tracings, and generate reports. Trainees should refer to the multitude of societal guidelines and classification scheme recommendations available when developing competency in diagnostic impression.5

Figure 2
While esophagology is a medical specialty, it is imperative that the esophagologist has a robust understanding of therapeutic options and surgical interventions for esophageal pathology. Scrubbing into the operating room during foregut surgeries is an eye-opening experience. This includes thoracic and abdominal approaches, robotic, laparoscopic, and open techniques, and interventions for GERD, achalasia, diverticular disease, and bariatric management. Equally important is working alongside advanced endoscopy faculty to understand utilities of endoscopic ultrasound, ablative methods for Barrett’s esophagus, and advanced techniques such as peroral endoscopic myotomy and transoral incisionless fundoplication. This exposure is critical as the role of the esophagologist is to speak knowledgably of therapeutic options and the risks and benefits of alternative approaches. Further, the patient’s journey rarely ends with the intervention, and an esophagologist must understand how to evaluate symptoms and manage complications following therapy.
As with broader digestive health, the management of esophageal disorders is becoming increasingly integrated with psychological, lifestyle, and dietary interventions. Observing and understanding how other health care members interact with the patient and relay concepts of brain-gut interaction is helpful in one’s own practice and ability to speak to the value of focused interventions.
These key training aspects in esophagology can be acquired through different avenues (see Figure 3). Formal 1-year advanced esophageal or motility focused fellowships are available at leading esophageal centers. The American Neurogastroenterology and Motility Society (ANMS) offers a clinical training program for selected fellows to pursue apprenticeship-based training in gastrointestinal motility. A review of the benefits of additional training, available programs, and how to apply, can be found at The New Gastroenterologist. It may be possible to customize parts of the general clinical fellowship with a strong focus on esophagology. All budding esophagologists are strongly encouraged to attend and participate in subspecialty national meetings such as through the ANMS or the American Foregut Society.
Figure 3
Steep learning curve post fellowship
Regardless of the robust nature of clinical esophagology training, early career esophagologists will face challenges and learn on the job.
Many esophagologists are directors of a motility lab early in their careers. This is often uncharted territory in terms of managing a team of nurses, technicians, and other providers. The director of a motility lab will be called upon to troubleshoot various arenas of diagnostic workup, from study acquisition and interpretation to technical barriers with equipment or software. Keys to maintaining a successful motility lab further include optimizing schedules and protocols, delineating roles and responsibilities of team members, ensuring adequate training across staff and providers, communicating expectations, and cultivating an open relationship with the motility lab supervisor. Crucial, yet often neglected during fellowship training, are the economic considerations of operating and expanding the motility lab, and the financial implications for one’s own practice.6 Participating in professional development workshops can be especially valuable in cultivating leadership skills.
The care an esophagologist provides relies heavily on collaborative relationships within the organization and peer mentorship, cooperation, and feedback. It is essential to cultivate multidisciplinary relationships with surgical (e.g., foregut surgery, laryngology), medical (e.g., pulmonology, allergy), radiology, and pathology colleagues, as well as with integrated health specialists including psychologists, dietitians, and speech language pathologists. It is also important to have open industry partnerships to ensure appropriate technical support and access to advancements.
Often organizations will have only one esophageal specialist within the group. Fortunately, the national and global community of esophagologists is highly collaborative and collegial. All esophagologists should have a network of mentors and colleagues within and outside of their organization to review complex cases, discuss challenges in the workplace, and foster research and innovation. Along these lines, both aspiring and practicing esophagologists should engage with professional societies as opportunities are abundant. Esophageal-focused societies include the ANMS, American Foregut Society, and International Society of Diseases of Esophagus, and the overarching GI societies also have a strong esophageal focus.
The path to becoming an esophagologist does not mirror the structure of the organ itself. Development is neither confined, unidirectional, nor set in length, but gradual, each step thoughtfully built on the last. Esophageal pathology is diverse, complex, and fascinating. With the appropriate training, mentorship, engagement, and leadership, esophagologists have the privilege of making a great impact on the lives of patients we meet, a fulfilling journey worth the time and effort it takes.
Dr. Delay is in the division of gastroenterology & hepatology, University of Colorado Anschutz Medical Campus, Aurora. Dr. Yadlapati is at the Center for Esophageal Diseases, division of gastroenterology, University of California San Diego, La Jolla. She is a consultant through institutional agreement to Medtronic, Ironwood Pharmaceuticals, and Diversatek; she has received research support from Ironwood Pharmaceuticals; and is on the advisory board of Phathom Pharmaceuticals.
References
1. Holinger PH. Arch Otolaryngol. 1948;47:119-26.
2. Yadlapati R et al. Clin Gastroenterol Hepatol. 2017;15:1708-14.e3.
3. Oversight Working Network et al. Gastrointest Endosc. 2014;80:16-27.
4. DeLay K et al. Am J Gastroenterol. 2020;115:1453-9.
5. Gyawali CP et al. Neurogastroenterol Motil. 2018;30(9):e13341.
6. Yadlapati R et al. Gastroenterology. 2020;158:1202-10.
Esophagology was a term coined in 1948 to describe a medical specialty devoted to the study of the anatomy, physiology, and pathology of the esophagus. The term was born out of increased interest and evolution in esophagology and supported by development in esophagoscopy.1 While still rooted in these basic tenets, the landscape of esophagology is dramatically different in 2020. The last decade alone has seen unprecedented technological advances in esophagology, from the transformation of line tracings to high-resolution esophageal pressure topography to more recent innovations such as the functional lumen imaging probe. Successful therapeutic developments have increased opportunities for effective and less invasive treatment approaches for achalasia and gastroesophageal reflux disease (GERD). With changing concepts in esophageal diseases such as eosinophilic esophagitis, successful management now incorporates findings from recent discoveries that have revolutionized care pathways. (see Figure 1).
Figure 1
Optimizing esophagology training during fellowship
First, and most importantly, an esophagologist must have a foundation in the basic principles of esophageal anatomy, physiology, and pathology (see Figure 2). While newer digital learning resources exist, tried and true book-based resources – text books, chapters, and reviews – related to esophageal mechanics, the interplay between muscle function and neurogenics, and factors associated with nociception, remain the optimal learning strategy.
Once equipped with a foundation in esophageal physiology, one can readily engage with esophageal technologies, as there exists a vast array of testing to assess esophageal function. A comprehensive understanding of each, including device configuration, clinical protocol, and data storage, promotes a depth of knowledge every esophagologist should develop. Aspiring esophagologists should take time to observe and perform procedures in their motility labs, particularly esophageal high-resolution manometry and ambulatory reflux monitoring studies. If afforded the opportunity through a research study or a clinical indication, esophagologists should also undergo the tests themselves. Empathy regarding the discomfort and tolerability of motility tests, which are notoriously challenging for patients, can promote rapport and trust with patients, increase patient satisfaction, and enhance one’s own understanding of resource utilization and safety.
Perhaps most critical to becoming an esophagologist, is acquiring sufficient competency in interpretation of esophageal studies. Prior research highlights the limitations in achieving competency when trainees adhere to the minimum case volume of studies recommended by the GI core curriculum.2,3 With the bar set higher for the burgeoning esophagologist, one must not only practice with a higher case volume, but also engage in competency-based assessments and performance feedback.4 Trainees should start by reviewing tracings for their own patients. Preliminary interpretation of pending studies and review with a mentor before the final sign-off, participation in research that requires study, or even teaching co-trainees basic tenets of motility are other creative approaches to learning. Esophagologists will be expected to know how to navigate the software to access studies, manually review tracings, and generate reports. Trainees should refer to the multitude of societal guidelines and classification scheme recommendations available when developing competency in diagnostic impression.5

Figure 2
While esophagology is a medical specialty, it is imperative that the esophagologist has a robust understanding of therapeutic options and surgical interventions for esophageal pathology. Scrubbing into the operating room during foregut surgeries is an eye-opening experience. This includes thoracic and abdominal approaches, robotic, laparoscopic, and open techniques, and interventions for GERD, achalasia, diverticular disease, and bariatric management. Equally important is working alongside advanced endoscopy faculty to understand utilities of endoscopic ultrasound, ablative methods for Barrett’s esophagus, and advanced techniques such as peroral endoscopic myotomy and transoral incisionless fundoplication. This exposure is critical as the role of the esophagologist is to speak knowledgably of therapeutic options and the risks and benefits of alternative approaches. Further, the patient’s journey rarely ends with the intervention, and an esophagologist must understand how to evaluate symptoms and manage complications following therapy.
As with broader digestive health, the management of esophageal disorders is becoming increasingly integrated with psychological, lifestyle, and dietary interventions. Observing and understanding how other health care members interact with the patient and relay concepts of brain-gut interaction is helpful in one’s own practice and ability to speak to the value of focused interventions.
These key training aspects in esophagology can be acquired through different avenues (see Figure 3). Formal 1-year advanced esophageal or motility focused fellowships are available at leading esophageal centers. The American Neurogastroenterology and Motility Society (ANMS) offers a clinical training program for selected fellows to pursue apprenticeship-based training in gastrointestinal motility. A review of the benefits of additional training, available programs, and how to apply, can be found at The New Gastroenterologist. It may be possible to customize parts of the general clinical fellowship with a strong focus on esophagology. All budding esophagologists are strongly encouraged to attend and participate in subspecialty national meetings such as through the ANMS or the American Foregut Society.
Figure 3
Steep learning curve post fellowship
Regardless of the robust nature of clinical esophagology training, early career esophagologists will face challenges and learn on the job.
Many esophagologists are directors of a motility lab early in their careers. This is often uncharted territory in terms of managing a team of nurses, technicians, and other providers. The director of a motility lab will be called upon to troubleshoot various arenas of diagnostic workup, from study acquisition and interpretation to technical barriers with equipment or software. Keys to maintaining a successful motility lab further include optimizing schedules and protocols, delineating roles and responsibilities of team members, ensuring adequate training across staff and providers, communicating expectations, and cultivating an open relationship with the motility lab supervisor. Crucial, yet often neglected during fellowship training, are the economic considerations of operating and expanding the motility lab, and the financial implications for one’s own practice.6 Participating in professional development workshops can be especially valuable in cultivating leadership skills.
The care an esophagologist provides relies heavily on collaborative relationships within the organization and peer mentorship, cooperation, and feedback. It is essential to cultivate multidisciplinary relationships with surgical (e.g., foregut surgery, laryngology), medical (e.g., pulmonology, allergy), radiology, and pathology colleagues, as well as with integrated health specialists including psychologists, dietitians, and speech language pathologists. It is also important to have open industry partnerships to ensure appropriate technical support and access to advancements.
Often organizations will have only one esophageal specialist within the group. Fortunately, the national and global community of esophagologists is highly collaborative and collegial. All esophagologists should have a network of mentors and colleagues within and outside of their organization to review complex cases, discuss challenges in the workplace, and foster research and innovation. Along these lines, both aspiring and practicing esophagologists should engage with professional societies as opportunities are abundant. Esophageal-focused societies include the ANMS, American Foregut Society, and International Society of Diseases of Esophagus, and the overarching GI societies also have a strong esophageal focus.
The path to becoming an esophagologist does not mirror the structure of the organ itself. Development is neither confined, unidirectional, nor set in length, but gradual, each step thoughtfully built on the last. Esophageal pathology is diverse, complex, and fascinating. With the appropriate training, mentorship, engagement, and leadership, esophagologists have the privilege of making a great impact on the lives of patients we meet, a fulfilling journey worth the time and effort it takes.
Dr. Delay is in the division of gastroenterology & hepatology, University of Colorado Anschutz Medical Campus, Aurora. Dr. Yadlapati is at the Center for Esophageal Diseases, division of gastroenterology, University of California San Diego, La Jolla. She is a consultant through institutional agreement to Medtronic, Ironwood Pharmaceuticals, and Diversatek; she has received research support from Ironwood Pharmaceuticals; and is on the advisory board of Phathom Pharmaceuticals.
References
1. Holinger PH. Arch Otolaryngol. 1948;47:119-26.
2. Yadlapati R et al. Clin Gastroenterol Hepatol. 2017;15:1708-14.e3.
3. Oversight Working Network et al. Gastrointest Endosc. 2014;80:16-27.
4. DeLay K et al. Am J Gastroenterol. 2020;115:1453-9.
5. Gyawali CP et al. Neurogastroenterol Motil. 2018;30(9):e13341.
6. Yadlapati R et al. Gastroenterology. 2020;158:1202-10.
@GiJournal: An online platform to discuss the latest gastroenterology and hepatology publications
The last decade has seen an increased focus on the use of social media for medical education. Twitter, with over 330 million active users, is the most popular social media platform for medical education. We describe here our recent initiative to establish a weekly online gastroenterology-focused journal club on Twitter.
How was the idea conceived?
Sultan Mahmood, MD (@SultanMahmoodMD)
I joined #GITwitter at the end of 2019 and started following some of the leading experts in the field of gastroenterology and hepatology. It was a pleasant surprise to see how easy it was to engage with them and get expert opinions from across the world in real time. #MondayNightIBD, led by Aline Charabaty, MD, had become a phenomenon in the GI community and changed the perception of medical education in the digital world. There were online journal clubs for different medical subspecialties, including #NephroJC, #HOJournalClub, and #DermJC, but none for gastroenterology. Realizing this opportunity, and with guidance from Dr. Charabaty, we started @GiJournal in December of 2019 with weekly discussions.
@GiJournal started off as an informal discussion in which we would post a summary of the article and invite an expert in the field to comment. However, the interest in the journal club quickly took off as we gained more followers and a worldwide audience joined our journal club discussions on a weekly basis. As the COVID-19 pandemic took hold and endoscopy suites around the word closed, interest in online medical education grew. @GIJournal provided a platform for trainees and practicing physicians alike to stay up to date with the latest publications from the comfort of their homes. Needless to say, the journal club has evolved since its inception in that we now work with a team of experts and trainees who run the journal club on a rotating basis.
How does @GiJournal work?
Ijlal Akbar Ali, MD (@IjlalAkbar)
We have a large editorial board with volunteer faculty and trainees, all divided into four special interest groups (general GI/inflammatory bowel disease, interventional endoscopy/bariatric endoscopy, hepatology, and esophageal/motility disorders). Each week, a faculty member and a trainee pick a recently published article from a high-impact GI-focused journal. We also try to invite an expert of international repute (often the authors of the article themselves!) to engage as well. The faculty moderator and invited expert then work with the trainee to plan the session content. We post the topic and article on Monday. At 8 p.m. EST on Wednesday, the trainee posts a series of six to eight tweets summarizing the article. The faculty then asks the invited expert (and audience at large) a series of predetermined questions. Anyone can respond, share their opinion, and direct their own questions toward the moderator and expert who continually check their notifications and respond in real time. This brews into an hour-long discussion which covers not only the methodologic aspects of the article, but clinical practice in general. Discussions often trickle into the next day as people from different time zones participate. Everyone uses #GIJC at the end of their tweets which assists those following the article and facilitates indexing for future review. For those who miss or want to review sessions, we conveniently summarize all articles and corresponding discussions in a monthly publication, @GiJournal Digest, that is posted on Twitter for anyone to download, read and enjoy (Figure 1).
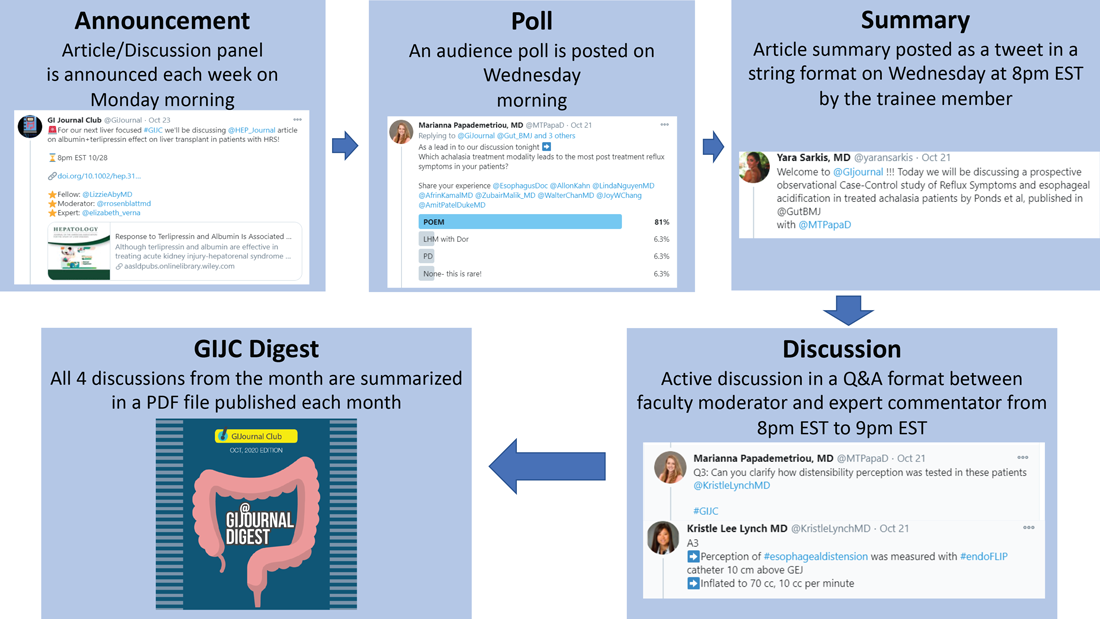
How is this different from any other journal club?
Atoosa Rabiee, MD (@AtoosaRabiee)
@GiJournal is unique in that it provides trainees and practicing gastroenterologists access to interactive discussions with both authors and world-renowned experts in the field. Online journal clubs operate with a flattened hierarchy; as such, they inherently break down access barriers to both the researchers who performed the study and key opinion leaders who commonly participate. There is no boundary as far as institutions or even countries. As a result, our platform has uncovered an unexpected degree of interest in live online discussion, and we have enjoyed collaborating and learning from experts from all over the world. @GiJournal also differs from conventional journal clubs by allowing trainees the opportunity to collaborate and engage with mentors from other institutions. As such, trainees develop relationships with experts in the field outside their home institutions, experts with whom they may not have had contact otherwise.
Although worldwide participation is a key strength of the online @GiJournal platform, it may be challenging for some members to attend the live discussion based on time difference. We account for this in two ways. First, participants are encouraged to continue with comments and questions afterward at their convenience, which allows experts and moderators to continue the conversation, often for several days. Second, to promote inclusivity, we have created a unique, customized publication to summarize and present the key points of conversation for each session. This asynchronous access is a quality not found in more traditional journal club formats. Finally, studies have shown that articles shared on social media tend to have increased citations and higher Altmetric scores.
What are the opportunities for trainees and recent graduates?
Sunil Amin, MD, MPH (@SunilAminMD)
Our surveys have shown that 30%-45% of the @GiJournal discussion participants are trainees. Both gastroenterology fellows and internal medicine residents from around the world are an integral part of each specialty panel for the weekly @GIjournal discussions. Trainees are paired up with a specific faculty mentor and together they choose an article for discussion, create a summary, informal twitter poll, and questions for the discussion. This direct access provides an opportunity for trainees to interact, ask questions, and learn from faculty in an informal atmosphere.
We have heard from multiple trainees who have developed long-term relationships with the experts and faculty mentors they worked with and are now also working on research projects. Additionally, trainees can bring the expertise they have now acquired back to their home institutions to pick articles, add specific teaching points, and enrich their local journal club discussions. Finally, trainees who present on the @GiJournal platform are given unique visibility to the many faculty members and opinion leaders participating in each discussion. This may facilitate future networking opportunities and enhance their CVs for future fellowship or employment applications.
Plans for the future?
Allon Kahn, MD (@AllonKahn)
Despite significant evolution and growth in @GiJournal over the past year, we are still actively working to expand our platform. Modes of online medical education, specifically Twitter-based GI journal club discussions, remain in their infancy. We see this @GiJournal as an opportunity for innovation as we plan for the year ahead. Our top priority for the upcoming year includes obtaining CME approval, which we are currently developing with Integrity CE (an Accreditation Council for Continuing Medical Education–accredited provider of CME for health care professionals). This will give an opportunity for the participants to be awarded CME credit when they participate in our weekly discussions. Other options being explored include starting a podcast and translation of @GiJournal Digest in different languages to reach a wider international audience. Furthermore, with the continued expansion of GI leaders and experts joining and engaging in Twitter, our options for unique and multidisciplinary discussion topics will continue to grow.
How can you join the @GiJournal discussions?
@SultanMahmoodMD
Joining the journal club discussion is easy. Just follow the @GiJournal handle on Twitter and turn on the notifications icon. Although we encourage everyone to “actively” participate in the discussion by asking questions or sharing your personal experience, joining the discussion as an “observer” is also a great way to learn. The discussion starts at 8 p.m. EST every Wednesday. Follow the #GIJC and the @GiJournal handle as questions are posted by the faculty moderator and answered by the experts. Even if you miss the discussion, the @GiJournal Digest is a great way to recap the discussions in an easy-to-read PDF format. The @GiJournal Digest is a monthly publication that archives the four @GiJournal club discussions in the previous month. Follow the link below to access the recent publications: http://ow.ly/uu2550C3RXX
Conclusion
In summary, we believe Twitter-based journal clubs offer an engaging way of virtual learning from the comfort of one’s home and a convenient way to directly interact with the experts. The success of @GiJournal highlights the importance of social media for medical education in the field of gastroenterology and hepatology and we look forward to developing this endeavor further.
Dr. Mahmood is clinical assistant professor of medicine, co–program director of the GI fellowship program, UB division of gastroenterology, hepatology & nutrition, State University of New York at Buffalo; Dr. Rabiee is assistant professor of medicine, director of hepatology, division of gastroenterology and hepatology, Washington DC VA Medical Center, Washington; Dr. Amin is assistant professor of medicine, director of endoscopy, The Lennar Foundation Medical Center, division of digestive health and liver disease, department of medicine, University of Miami; Dr. Kahn is assistant professor of medicine, division of gastroenterology & hepatology, Mayo Clinic, Scottsdale, Ariz.; and Dr. Akbar Ali is a gastroenterology fellow in the division of digestive diseases and nutrition, University of Oklahoma Health Sciences Center, Oklahoma City.
The last decade has seen an increased focus on the use of social media for medical education. Twitter, with over 330 million active users, is the most popular social media platform for medical education. We describe here our recent initiative to establish a weekly online gastroenterology-focused journal club on Twitter.
How was the idea conceived?
Sultan Mahmood, MD (@SultanMahmoodMD)
I joined #GITwitter at the end of 2019 and started following some of the leading experts in the field of gastroenterology and hepatology. It was a pleasant surprise to see how easy it was to engage with them and get expert opinions from across the world in real time. #MondayNightIBD, led by Aline Charabaty, MD, had become a phenomenon in the GI community and changed the perception of medical education in the digital world. There were online journal clubs for different medical subspecialties, including #NephroJC, #HOJournalClub, and #DermJC, but none for gastroenterology. Realizing this opportunity, and with guidance from Dr. Charabaty, we started @GiJournal in December of 2019 with weekly discussions.
@GiJournal started off as an informal discussion in which we would post a summary of the article and invite an expert in the field to comment. However, the interest in the journal club quickly took off as we gained more followers and a worldwide audience joined our journal club discussions on a weekly basis. As the COVID-19 pandemic took hold and endoscopy suites around the word closed, interest in online medical education grew. @GIJournal provided a platform for trainees and practicing physicians alike to stay up to date with the latest publications from the comfort of their homes. Needless to say, the journal club has evolved since its inception in that we now work with a team of experts and trainees who run the journal club on a rotating basis.
How does @GiJournal work?
Ijlal Akbar Ali, MD (@IjlalAkbar)
We have a large editorial board with volunteer faculty and trainees, all divided into four special interest groups (general GI/inflammatory bowel disease, interventional endoscopy/bariatric endoscopy, hepatology, and esophageal/motility disorders). Each week, a faculty member and a trainee pick a recently published article from a high-impact GI-focused journal. We also try to invite an expert of international repute (often the authors of the article themselves!) to engage as well. The faculty moderator and invited expert then work with the trainee to plan the session content. We post the topic and article on Monday. At 8 p.m. EST on Wednesday, the trainee posts a series of six to eight tweets summarizing the article. The faculty then asks the invited expert (and audience at large) a series of predetermined questions. Anyone can respond, share their opinion, and direct their own questions toward the moderator and expert who continually check their notifications and respond in real time. This brews into an hour-long discussion which covers not only the methodologic aspects of the article, but clinical practice in general. Discussions often trickle into the next day as people from different time zones participate. Everyone uses #GIJC at the end of their tweets which assists those following the article and facilitates indexing for future review. For those who miss or want to review sessions, we conveniently summarize all articles and corresponding discussions in a monthly publication, @GiJournal Digest, that is posted on Twitter for anyone to download, read and enjoy (Figure 1).

How is this different from any other journal club?
Atoosa Rabiee, MD (@AtoosaRabiee)
@GiJournal is unique in that it provides trainees and practicing gastroenterologists access to interactive discussions with both authors and world-renowned experts in the field. Online journal clubs operate with a flattened hierarchy; as such, they inherently break down access barriers to both the researchers who performed the study and key opinion leaders who commonly participate. There is no boundary as far as institutions or even countries. As a result, our platform has uncovered an unexpected degree of interest in live online discussion, and we have enjoyed collaborating and learning from experts from all over the world. @GiJournal also differs from conventional journal clubs by allowing trainees the opportunity to collaborate and engage with mentors from other institutions. As such, trainees develop relationships with experts in the field outside their home institutions, experts with whom they may not have had contact otherwise.
Although worldwide participation is a key strength of the online @GiJournal platform, it may be challenging for some members to attend the live discussion based on time difference. We account for this in two ways. First, participants are encouraged to continue with comments and questions afterward at their convenience, which allows experts and moderators to continue the conversation, often for several days. Second, to promote inclusivity, we have created a unique, customized publication to summarize and present the key points of conversation for each session. This asynchronous access is a quality not found in more traditional journal club formats. Finally, studies have shown that articles shared on social media tend to have increased citations and higher Altmetric scores.
What are the opportunities for trainees and recent graduates?
Sunil Amin, MD, MPH (@SunilAminMD)
Our surveys have shown that 30%-45% of the @GiJournal discussion participants are trainees. Both gastroenterology fellows and internal medicine residents from around the world are an integral part of each specialty panel for the weekly @GIjournal discussions. Trainees are paired up with a specific faculty mentor and together they choose an article for discussion, create a summary, informal twitter poll, and questions for the discussion. This direct access provides an opportunity for trainees to interact, ask questions, and learn from faculty in an informal atmosphere.
We have heard from multiple trainees who have developed long-term relationships with the experts and faculty mentors they worked with and are now also working on research projects. Additionally, trainees can bring the expertise they have now acquired back to their home institutions to pick articles, add specific teaching points, and enrich their local journal club discussions. Finally, trainees who present on the @GiJournal platform are given unique visibility to the many faculty members and opinion leaders participating in each discussion. This may facilitate future networking opportunities and enhance their CVs for future fellowship or employment applications.
Plans for the future?
Allon Kahn, MD (@AllonKahn)
Despite significant evolution and growth in @GiJournal over the past year, we are still actively working to expand our platform. Modes of online medical education, specifically Twitter-based GI journal club discussions, remain in their infancy. We see this @GiJournal as an opportunity for innovation as we plan for the year ahead. Our top priority for the upcoming year includes obtaining CME approval, which we are currently developing with Integrity CE (an Accreditation Council for Continuing Medical Education–accredited provider of CME for health care professionals). This will give an opportunity for the participants to be awarded CME credit when they participate in our weekly discussions. Other options being explored include starting a podcast and translation of @GiJournal Digest in different languages to reach a wider international audience. Furthermore, with the continued expansion of GI leaders and experts joining and engaging in Twitter, our options for unique and multidisciplinary discussion topics will continue to grow.
How can you join the @GiJournal discussions?
@SultanMahmoodMD
Joining the journal club discussion is easy. Just follow the @GiJournal handle on Twitter and turn on the notifications icon. Although we encourage everyone to “actively” participate in the discussion by asking questions or sharing your personal experience, joining the discussion as an “observer” is also a great way to learn. The discussion starts at 8 p.m. EST every Wednesday. Follow the #GIJC and the @GiJournal handle as questions are posted by the faculty moderator and answered by the experts. Even if you miss the discussion, the @GiJournal Digest is a great way to recap the discussions in an easy-to-read PDF format. The @GiJournal Digest is a monthly publication that archives the four @GiJournal club discussions in the previous month. Follow the link below to access the recent publications: http://ow.ly/uu2550C3RXX
Conclusion
In summary, we believe Twitter-based journal clubs offer an engaging way of virtual learning from the comfort of one’s home and a convenient way to directly interact with the experts. The success of @GiJournal highlights the importance of social media for medical education in the field of gastroenterology and hepatology and we look forward to developing this endeavor further.
Dr. Mahmood is clinical assistant professor of medicine, co–program director of the GI fellowship program, UB division of gastroenterology, hepatology & nutrition, State University of New York at Buffalo; Dr. Rabiee is assistant professor of medicine, director of hepatology, division of gastroenterology and hepatology, Washington DC VA Medical Center, Washington; Dr. Amin is assistant professor of medicine, director of endoscopy, The Lennar Foundation Medical Center, division of digestive health and liver disease, department of medicine, University of Miami; Dr. Kahn is assistant professor of medicine, division of gastroenterology & hepatology, Mayo Clinic, Scottsdale, Ariz.; and Dr. Akbar Ali is a gastroenterology fellow in the division of digestive diseases and nutrition, University of Oklahoma Health Sciences Center, Oklahoma City.
The last decade has seen an increased focus on the use of social media for medical education. Twitter, with over 330 million active users, is the most popular social media platform for medical education. We describe here our recent initiative to establish a weekly online gastroenterology-focused journal club on Twitter.
How was the idea conceived?
Sultan Mahmood, MD (@SultanMahmoodMD)
I joined #GITwitter at the end of 2019 and started following some of the leading experts in the field of gastroenterology and hepatology. It was a pleasant surprise to see how easy it was to engage with them and get expert opinions from across the world in real time. #MondayNightIBD, led by Aline Charabaty, MD, had become a phenomenon in the GI community and changed the perception of medical education in the digital world. There were online journal clubs for different medical subspecialties, including #NephroJC, #HOJournalClub, and #DermJC, but none for gastroenterology. Realizing this opportunity, and with guidance from Dr. Charabaty, we started @GiJournal in December of 2019 with weekly discussions.
@GiJournal started off as an informal discussion in which we would post a summary of the article and invite an expert in the field to comment. However, the interest in the journal club quickly took off as we gained more followers and a worldwide audience joined our journal club discussions on a weekly basis. As the COVID-19 pandemic took hold and endoscopy suites around the word closed, interest in online medical education grew. @GIJournal provided a platform for trainees and practicing physicians alike to stay up to date with the latest publications from the comfort of their homes. Needless to say, the journal club has evolved since its inception in that we now work with a team of experts and trainees who run the journal club on a rotating basis.
How does @GiJournal work?
Ijlal Akbar Ali, MD (@IjlalAkbar)
We have a large editorial board with volunteer faculty and trainees, all divided into four special interest groups (general GI/inflammatory bowel disease, interventional endoscopy/bariatric endoscopy, hepatology, and esophageal/motility disorders). Each week, a faculty member and a trainee pick a recently published article from a high-impact GI-focused journal. We also try to invite an expert of international repute (often the authors of the article themselves!) to engage as well. The faculty moderator and invited expert then work with the trainee to plan the session content. We post the topic and article on Monday. At 8 p.m. EST on Wednesday, the trainee posts a series of six to eight tweets summarizing the article. The faculty then asks the invited expert (and audience at large) a series of predetermined questions. Anyone can respond, share their opinion, and direct their own questions toward the moderator and expert who continually check their notifications and respond in real time. This brews into an hour-long discussion which covers not only the methodologic aspects of the article, but clinical practice in general. Discussions often trickle into the next day as people from different time zones participate. Everyone uses #GIJC at the end of their tweets which assists those following the article and facilitates indexing for future review. For those who miss or want to review sessions, we conveniently summarize all articles and corresponding discussions in a monthly publication, @GiJournal Digest, that is posted on Twitter for anyone to download, read and enjoy (Figure 1).

How is this different from any other journal club?
Atoosa Rabiee, MD (@AtoosaRabiee)
@GiJournal is unique in that it provides trainees and practicing gastroenterologists access to interactive discussions with both authors and world-renowned experts in the field. Online journal clubs operate with a flattened hierarchy; as such, they inherently break down access barriers to both the researchers who performed the study and key opinion leaders who commonly participate. There is no boundary as far as institutions or even countries. As a result, our platform has uncovered an unexpected degree of interest in live online discussion, and we have enjoyed collaborating and learning from experts from all over the world. @GiJournal also differs from conventional journal clubs by allowing trainees the opportunity to collaborate and engage with mentors from other institutions. As such, trainees develop relationships with experts in the field outside their home institutions, experts with whom they may not have had contact otherwise.
Although worldwide participation is a key strength of the online @GiJournal platform, it may be challenging for some members to attend the live discussion based on time difference. We account for this in two ways. First, participants are encouraged to continue with comments and questions afterward at their convenience, which allows experts and moderators to continue the conversation, often for several days. Second, to promote inclusivity, we have created a unique, customized publication to summarize and present the key points of conversation for each session. This asynchronous access is a quality not found in more traditional journal club formats. Finally, studies have shown that articles shared on social media tend to have increased citations and higher Altmetric scores.
What are the opportunities for trainees and recent graduates?
Sunil Amin, MD, MPH (@SunilAminMD)
Our surveys have shown that 30%-45% of the @GiJournal discussion participants are trainees. Both gastroenterology fellows and internal medicine residents from around the world are an integral part of each specialty panel for the weekly @GIjournal discussions. Trainees are paired up with a specific faculty mentor and together they choose an article for discussion, create a summary, informal twitter poll, and questions for the discussion. This direct access provides an opportunity for trainees to interact, ask questions, and learn from faculty in an informal atmosphere.
We have heard from multiple trainees who have developed long-term relationships with the experts and faculty mentors they worked with and are now also working on research projects. Additionally, trainees can bring the expertise they have now acquired back to their home institutions to pick articles, add specific teaching points, and enrich their local journal club discussions. Finally, trainees who present on the @GiJournal platform are given unique visibility to the many faculty members and opinion leaders participating in each discussion. This may facilitate future networking opportunities and enhance their CVs for future fellowship or employment applications.
Plans for the future?
Allon Kahn, MD (@AllonKahn)
Despite significant evolution and growth in @GiJournal over the past year, we are still actively working to expand our platform. Modes of online medical education, specifically Twitter-based GI journal club discussions, remain in their infancy. We see this @GiJournal as an opportunity for innovation as we plan for the year ahead. Our top priority for the upcoming year includes obtaining CME approval, which we are currently developing with Integrity CE (an Accreditation Council for Continuing Medical Education–accredited provider of CME for health care professionals). This will give an opportunity for the participants to be awarded CME credit when they participate in our weekly discussions. Other options being explored include starting a podcast and translation of @GiJournal Digest in different languages to reach a wider international audience. Furthermore, with the continued expansion of GI leaders and experts joining and engaging in Twitter, our options for unique and multidisciplinary discussion topics will continue to grow.
How can you join the @GiJournal discussions?
@SultanMahmoodMD
Joining the journal club discussion is easy. Just follow the @GiJournal handle on Twitter and turn on the notifications icon. Although we encourage everyone to “actively” participate in the discussion by asking questions or sharing your personal experience, joining the discussion as an “observer” is also a great way to learn. The discussion starts at 8 p.m. EST every Wednesday. Follow the #GIJC and the @GiJournal handle as questions are posted by the faculty moderator and answered by the experts. Even if you miss the discussion, the @GiJournal Digest is a great way to recap the discussions in an easy-to-read PDF format. The @GiJournal Digest is a monthly publication that archives the four @GiJournal club discussions in the previous month. Follow the link below to access the recent publications: http://ow.ly/uu2550C3RXX
Conclusion
In summary, we believe Twitter-based journal clubs offer an engaging way of virtual learning from the comfort of one’s home and a convenient way to directly interact with the experts. The success of @GiJournal highlights the importance of social media for medical education in the field of gastroenterology and hepatology and we look forward to developing this endeavor further.
Dr. Mahmood is clinical assistant professor of medicine, co–program director of the GI fellowship program, UB division of gastroenterology, hepatology & nutrition, State University of New York at Buffalo; Dr. Rabiee is assistant professor of medicine, director of hepatology, division of gastroenterology and hepatology, Washington DC VA Medical Center, Washington; Dr. Amin is assistant professor of medicine, director of endoscopy, The Lennar Foundation Medical Center, division of digestive health and liver disease, department of medicine, University of Miami; Dr. Kahn is assistant professor of medicine, division of gastroenterology & hepatology, Mayo Clinic, Scottsdale, Ariz.; and Dr. Akbar Ali is a gastroenterology fellow in the division of digestive diseases and nutrition, University of Oklahoma Health Sciences Center, Oklahoma City.
Case of the inappropriate endoscopy referral
A 53-year-old woman was referred for surveillance colonoscopy. She is a current smoker with a history of chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation, and two diminutive hyperplastic polyps found on average-risk screening colonoscopy 3 years previously. Her prep at the time was excellent and she was advised to return in 10 years for follow-up. She has taken the day off work, arranged for a driver, is prepped, and is on your schedule for a colonoscopy for a “history of polyps.” Is this an appropriate referral and how should you handle it?
Most of us have had questionable referrals on our endoscopy schedules. While judgments can vary among providers about when a patient should undergo a procedure or what intervention is most needed, some direct-access referrals for endoscopy are considered inappropriate by most standards. In examining referrals for colonoscopy, studies have shown that as many as 23% of screening colonoscopies among Medicare beneficiaries and 14.2% of Veterans Affairs patients in a large colorectal cancer screening study are inappropriate.1,2 A prospective multicenter study found 29% of colonoscopies to be inappropriate, and surveillance studies were confirmed as the most frequent source of inappropriate procedures.3,4 Endoscopies are performed so frequently, effectively, and safely that they can be readily scheduled by gastroenterologists and nongastroenterologists alike. Open access has facilitated and expedited needed procedures, providing benefit to patient and provider and freeing clinic visit time for more complex consults. But while endoscopy is very safe, it is not without risk or cost. What should be the response when a patient in the endoscopy unit appears to be inappropriately referred?
The first step is to determine what is inappropriate. There are several situations when a procedure might be considered inappropriate, particularly when we try to apply ethical principles.
1. The performance of the procedure is contrary to society guidelines. The American Gastroenterological Association, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology publish clinical guidelines. These documents are drafted after rigorous research and literature review, and the strength of the recommendations is confirmed by incorporation of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology. Such guidelines allow gastroenterologists across the country to practice confidently in a manner consistent with the current available data and the standards of care for the GI community. A patient who is referred for a procedure for an indication that does not adhere to – or contradicts – guidelines, may be at risk for substandard care and possibly at risk for harm. It is the physician’s ethical responsibility to provide the most “good” and the least harm for patients, consistent with the ethical principle of beneficence.
Guidelines, however, are not mandates, and an argument may be made that in order to provide the best care, alternatives may be offered to a patient. Some circumstances require clinical judgments based on unique patient characteristics and the need for individualized care. As a rule, however, the goal of guidelines is to assist doctors in providing the best care.
2. The procedure is not the correct test for the clinical question. While endoscopy can address many clinical queries, endoscopy is not always the right procedure for a specific medical question. A patient referred for an esophagogastroduodenoscopy (EGD) to rule out gastroparesis is being subjected to the wrong test to answer the clinical question. Some information may be obtained from an EGD (e.g., retained food may suggest dysmotility or the patient could have gastric outlet obstruction) but this is not the recommended initial management step. Is it reasonable to proceed with a test that cannot answer the question asked? Continuing with the endoscopy would not enhance beneficence and might be a futile service for the patient. Is this doing the best for the patient?
3. The risks of the procedure outweigh the benefits. Some procedures may be consistent with guidelines and able to answer the questions asked, but may present more risk than benefit. Should an elderly patient with multiple significant comorbidities and a likely limited life span undergo a follow-up colonoscopy even at an appropriate interval? The principle of nonmaleficence is the clear standard here.
4. The intent for doing the procedure has questionable merit. Some patients may request an EGD at the time of the screening colonoscopy just to “check,” regardless of symptoms or risk category. A patient has a right to make her/his own decisions but patient autonomy should not be an excuse for a nonindicated procedure.
In the case of the 53-year-old woman referred for surveillance colonoscopy, the physician needs to consider whether performing the test is inappropriate for any of the above reasons. First and foremost, is it doing the most good for the patient?
On the one hand, performing an inappropriately referred procedure contradicts guidelines and may present undue risk of complication from anesthesia or endoscopy. Would the physician be ethically compromised in this situation, or even legally liable should a complication arise during a procedure done for a questionable indication?
On the other hand, canceling such a procedure creates multiple dilemmas. The autonomy and the convenience of the patient need to be respected. The patient who has followed all the instructions, is prepped, has taken off work, arranged for transportation, and wants to have the procedure done may have difficulty accepting a cancellation. Colonoscopy is a safe test. Is it the right thing to cancel her procedure because of an imprudent referral? Would this undermine the patient’s confidence in her referring provider? Physicians may face other pressures to proceed, such as practice or institutional restraints that discourage same-day cancellations. Maintenance of robust financial practices, stable referral sources, and excellent patient satisfaction measures are critical to running an efficient endoscopy unit and maximizing patient service and care.
Is there a sensible way to address the dilemma? One approach is simply to move ahead with the procedure if the physician feels that the benefits outweigh the medical and ethical risks. Besides patient convenience, other “benefits” could be relevant: clinical value from an unexpected finding, affirmation of the patient’s invested time and effort, and avoidance of the apparent undermining of the authority of a referring colleague. Finally, maintaining productive and efficient practices or institutions ultimately allows for better patient care. The physician can explain the enhanced risks, present the alternatives, and – perhaps in less time than the ethical deliberations might take – complete the procedure and have the patient resting comfortably in the recovery unit.
An alternative approach is to cancel the procedure if the physician feels that the indication is not legitimate, or that the risks to the patient and the physician are significant. Explaining the cancellation can be difficult but may be the right decision if ethical principles of beneficence are upheld. It is understood that procedures consume health care resources and can present an undue expense to society if done for improper reasons. Unnecessary procedures clutter schedules for patients who truly need an endoscopy.
Neither approach is completely satisfying, although moving forward with a likely very safe procedure is often the easiest step and probably what many physicians do in this setting.
Is there a better way to approach this problem? Preventing the ethical dilemma is the ideal scenario, although not always feasible. Here are some suggestions to consider.
Reviewing referrals prior to the procedure day allows endoscopists to contact and cancel patients if needed, before the prep and travel begin. This addresses the convenience aspects but not the issue regarding the underlying indication.
The most important step toward avoiding inappropriate referrals is better education for referring providers. Even gastroenterologists, let alone primary care physicians, may struggle to stay current on changing clinical GI guidelines. Colorectal cancer screening, for example, is an area that gives gastroenterologists an opportunity to communicate with and educate colleagues about appropriate management. Keeping our referral base up to date about guidelines and prep and safety recommendations will likely reduce the number of inappropriate colonoscopy referrals and provide many of the benefits described above.
Providing the best care for patients by adhering to medical ethical principles is the goal of our work as physicians. Implementing this goal may demand tough decisions.
Dr. Fisher is professor of clinical medicine and director of small-bowel imaging, division of gastroenterology, University of Pennsylvania, Philadelphia.
References
1. Sheffield KM et al. JAMA Intern Med. 2013 Apr 8;173(7):542-50.
2. Powell AA et al. J Gen Intern Med. 2015 Jun;30(6):732-41.
3. Petruzziello L et al. J Clin Gastroenterol. 2012;46(7):590-4.
4. Kapila N et al. Dig Dis Sci. 2019;64(10):2798-805.
A 53-year-old woman was referred for surveillance colonoscopy. She is a current smoker with a history of chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation, and two diminutive hyperplastic polyps found on average-risk screening colonoscopy 3 years previously. Her prep at the time was excellent and she was advised to return in 10 years for follow-up. She has taken the day off work, arranged for a driver, is prepped, and is on your schedule for a colonoscopy for a “history of polyps.” Is this an appropriate referral and how should you handle it?
Most of us have had questionable referrals on our endoscopy schedules. While judgments can vary among providers about when a patient should undergo a procedure or what intervention is most needed, some direct-access referrals for endoscopy are considered inappropriate by most standards. In examining referrals for colonoscopy, studies have shown that as many as 23% of screening colonoscopies among Medicare beneficiaries and 14.2% of Veterans Affairs patients in a large colorectal cancer screening study are inappropriate.1,2 A prospective multicenter study found 29% of colonoscopies to be inappropriate, and surveillance studies were confirmed as the most frequent source of inappropriate procedures.3,4 Endoscopies are performed so frequently, effectively, and safely that they can be readily scheduled by gastroenterologists and nongastroenterologists alike. Open access has facilitated and expedited needed procedures, providing benefit to patient and provider and freeing clinic visit time for more complex consults. But while endoscopy is very safe, it is not without risk or cost. What should be the response when a patient in the endoscopy unit appears to be inappropriately referred?
The first step is to determine what is inappropriate. There are several situations when a procedure might be considered inappropriate, particularly when we try to apply ethical principles.
1. The performance of the procedure is contrary to society guidelines. The American Gastroenterological Association, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology publish clinical guidelines. These documents are drafted after rigorous research and literature review, and the strength of the recommendations is confirmed by incorporation of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology. Such guidelines allow gastroenterologists across the country to practice confidently in a manner consistent with the current available data and the standards of care for the GI community. A patient who is referred for a procedure for an indication that does not adhere to – or contradicts – guidelines, may be at risk for substandard care and possibly at risk for harm. It is the physician’s ethical responsibility to provide the most “good” and the least harm for patients, consistent with the ethical principle of beneficence.
Guidelines, however, are not mandates, and an argument may be made that in order to provide the best care, alternatives may be offered to a patient. Some circumstances require clinical judgments based on unique patient characteristics and the need for individualized care. As a rule, however, the goal of guidelines is to assist doctors in providing the best care.
2. The procedure is not the correct test for the clinical question. While endoscopy can address many clinical queries, endoscopy is not always the right procedure for a specific medical question. A patient referred for an esophagogastroduodenoscopy (EGD) to rule out gastroparesis is being subjected to the wrong test to answer the clinical question. Some information may be obtained from an EGD (e.g., retained food may suggest dysmotility or the patient could have gastric outlet obstruction) but this is not the recommended initial management step. Is it reasonable to proceed with a test that cannot answer the question asked? Continuing with the endoscopy would not enhance beneficence and might be a futile service for the patient. Is this doing the best for the patient?
3. The risks of the procedure outweigh the benefits. Some procedures may be consistent with guidelines and able to answer the questions asked, but may present more risk than benefit. Should an elderly patient with multiple significant comorbidities and a likely limited life span undergo a follow-up colonoscopy even at an appropriate interval? The principle of nonmaleficence is the clear standard here.
4. The intent for doing the procedure has questionable merit. Some patients may request an EGD at the time of the screening colonoscopy just to “check,” regardless of symptoms or risk category. A patient has a right to make her/his own decisions but patient autonomy should not be an excuse for a nonindicated procedure.
In the case of the 53-year-old woman referred for surveillance colonoscopy, the physician needs to consider whether performing the test is inappropriate for any of the above reasons. First and foremost, is it doing the most good for the patient?
On the one hand, performing an inappropriately referred procedure contradicts guidelines and may present undue risk of complication from anesthesia or endoscopy. Would the physician be ethically compromised in this situation, or even legally liable should a complication arise during a procedure done for a questionable indication?
On the other hand, canceling such a procedure creates multiple dilemmas. The autonomy and the convenience of the patient need to be respected. The patient who has followed all the instructions, is prepped, has taken off work, arranged for transportation, and wants to have the procedure done may have difficulty accepting a cancellation. Colonoscopy is a safe test. Is it the right thing to cancel her procedure because of an imprudent referral? Would this undermine the patient’s confidence in her referring provider? Physicians may face other pressures to proceed, such as practice or institutional restraints that discourage same-day cancellations. Maintenance of robust financial practices, stable referral sources, and excellent patient satisfaction measures are critical to running an efficient endoscopy unit and maximizing patient service and care.
Is there a sensible way to address the dilemma? One approach is simply to move ahead with the procedure if the physician feels that the benefits outweigh the medical and ethical risks. Besides patient convenience, other “benefits” could be relevant: clinical value from an unexpected finding, affirmation of the patient’s invested time and effort, and avoidance of the apparent undermining of the authority of a referring colleague. Finally, maintaining productive and efficient practices or institutions ultimately allows for better patient care. The physician can explain the enhanced risks, present the alternatives, and – perhaps in less time than the ethical deliberations might take – complete the procedure and have the patient resting comfortably in the recovery unit.
An alternative approach is to cancel the procedure if the physician feels that the indication is not legitimate, or that the risks to the patient and the physician are significant. Explaining the cancellation can be difficult but may be the right decision if ethical principles of beneficence are upheld. It is understood that procedures consume health care resources and can present an undue expense to society if done for improper reasons. Unnecessary procedures clutter schedules for patients who truly need an endoscopy.
Neither approach is completely satisfying, although moving forward with a likely very safe procedure is often the easiest step and probably what many physicians do in this setting.
Is there a better way to approach this problem? Preventing the ethical dilemma is the ideal scenario, although not always feasible. Here are some suggestions to consider.
Reviewing referrals prior to the procedure day allows endoscopists to contact and cancel patients if needed, before the prep and travel begin. This addresses the convenience aspects but not the issue regarding the underlying indication.
The most important step toward avoiding inappropriate referrals is better education for referring providers. Even gastroenterologists, let alone primary care physicians, may struggle to stay current on changing clinical GI guidelines. Colorectal cancer screening, for example, is an area that gives gastroenterologists an opportunity to communicate with and educate colleagues about appropriate management. Keeping our referral base up to date about guidelines and prep and safety recommendations will likely reduce the number of inappropriate colonoscopy referrals and provide many of the benefits described above.
Providing the best care for patients by adhering to medical ethical principles is the goal of our work as physicians. Implementing this goal may demand tough decisions.
Dr. Fisher is professor of clinical medicine and director of small-bowel imaging, division of gastroenterology, University of Pennsylvania, Philadelphia.
References
1. Sheffield KM et al. JAMA Intern Med. 2013 Apr 8;173(7):542-50.
2. Powell AA et al. J Gen Intern Med. 2015 Jun;30(6):732-41.
3. Petruzziello L et al. J Clin Gastroenterol. 2012;46(7):590-4.
4. Kapila N et al. Dig Dis Sci. 2019;64(10):2798-805.
A 53-year-old woman was referred for surveillance colonoscopy. She is a current smoker with a history of chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation, and two diminutive hyperplastic polyps found on average-risk screening colonoscopy 3 years previously. Her prep at the time was excellent and she was advised to return in 10 years for follow-up. She has taken the day off work, arranged for a driver, is prepped, and is on your schedule for a colonoscopy for a “history of polyps.” Is this an appropriate referral and how should you handle it?
Most of us have had questionable referrals on our endoscopy schedules. While judgments can vary among providers about when a patient should undergo a procedure or what intervention is most needed, some direct-access referrals for endoscopy are considered inappropriate by most standards. In examining referrals for colonoscopy, studies have shown that as many as 23% of screening colonoscopies among Medicare beneficiaries and 14.2% of Veterans Affairs patients in a large colorectal cancer screening study are inappropriate.1,2 A prospective multicenter study found 29% of colonoscopies to be inappropriate, and surveillance studies were confirmed as the most frequent source of inappropriate procedures.3,4 Endoscopies are performed so frequently, effectively, and safely that they can be readily scheduled by gastroenterologists and nongastroenterologists alike. Open access has facilitated and expedited needed procedures, providing benefit to patient and provider and freeing clinic visit time for more complex consults. But while endoscopy is very safe, it is not without risk or cost. What should be the response when a patient in the endoscopy unit appears to be inappropriately referred?
The first step is to determine what is inappropriate. There are several situations when a procedure might be considered inappropriate, particularly when we try to apply ethical principles.
1. The performance of the procedure is contrary to society guidelines. The American Gastroenterological Association, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology publish clinical guidelines. These documents are drafted after rigorous research and literature review, and the strength of the recommendations is confirmed by incorporation of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology. Such guidelines allow gastroenterologists across the country to practice confidently in a manner consistent with the current available data and the standards of care for the GI community. A patient who is referred for a procedure for an indication that does not adhere to – or contradicts – guidelines, may be at risk for substandard care and possibly at risk for harm. It is the physician’s ethical responsibility to provide the most “good” and the least harm for patients, consistent with the ethical principle of beneficence.
Guidelines, however, are not mandates, and an argument may be made that in order to provide the best care, alternatives may be offered to a patient. Some circumstances require clinical judgments based on unique patient characteristics and the need for individualized care. As a rule, however, the goal of guidelines is to assist doctors in providing the best care.
2. The procedure is not the correct test for the clinical question. While endoscopy can address many clinical queries, endoscopy is not always the right procedure for a specific medical question. A patient referred for an esophagogastroduodenoscopy (EGD) to rule out gastroparesis is being subjected to the wrong test to answer the clinical question. Some information may be obtained from an EGD (e.g., retained food may suggest dysmotility or the patient could have gastric outlet obstruction) but this is not the recommended initial management step. Is it reasonable to proceed with a test that cannot answer the question asked? Continuing with the endoscopy would not enhance beneficence and might be a futile service for the patient. Is this doing the best for the patient?
3. The risks of the procedure outweigh the benefits. Some procedures may be consistent with guidelines and able to answer the questions asked, but may present more risk than benefit. Should an elderly patient with multiple significant comorbidities and a likely limited life span undergo a follow-up colonoscopy even at an appropriate interval? The principle of nonmaleficence is the clear standard here.
4. The intent for doing the procedure has questionable merit. Some patients may request an EGD at the time of the screening colonoscopy just to “check,” regardless of symptoms or risk category. A patient has a right to make her/his own decisions but patient autonomy should not be an excuse for a nonindicated procedure.
In the case of the 53-year-old woman referred for surveillance colonoscopy, the physician needs to consider whether performing the test is inappropriate for any of the above reasons. First and foremost, is it doing the most good for the patient?
On the one hand, performing an inappropriately referred procedure contradicts guidelines and may present undue risk of complication from anesthesia or endoscopy. Would the physician be ethically compromised in this situation, or even legally liable should a complication arise during a procedure done for a questionable indication?
On the other hand, canceling such a procedure creates multiple dilemmas. The autonomy and the convenience of the patient need to be respected. The patient who has followed all the instructions, is prepped, has taken off work, arranged for transportation, and wants to have the procedure done may have difficulty accepting a cancellation. Colonoscopy is a safe test. Is it the right thing to cancel her procedure because of an imprudent referral? Would this undermine the patient’s confidence in her referring provider? Physicians may face other pressures to proceed, such as practice or institutional restraints that discourage same-day cancellations. Maintenance of robust financial practices, stable referral sources, and excellent patient satisfaction measures are critical to running an efficient endoscopy unit and maximizing patient service and care.
Is there a sensible way to address the dilemma? One approach is simply to move ahead with the procedure if the physician feels that the benefits outweigh the medical and ethical risks. Besides patient convenience, other “benefits” could be relevant: clinical value from an unexpected finding, affirmation of the patient’s invested time and effort, and avoidance of the apparent undermining of the authority of a referring colleague. Finally, maintaining productive and efficient practices or institutions ultimately allows for better patient care. The physician can explain the enhanced risks, present the alternatives, and – perhaps in less time than the ethical deliberations might take – complete the procedure and have the patient resting comfortably in the recovery unit.
An alternative approach is to cancel the procedure if the physician feels that the indication is not legitimate, or that the risks to the patient and the physician are significant. Explaining the cancellation can be difficult but may be the right decision if ethical principles of beneficence are upheld. It is understood that procedures consume health care resources and can present an undue expense to society if done for improper reasons. Unnecessary procedures clutter schedules for patients who truly need an endoscopy.
Neither approach is completely satisfying, although moving forward with a likely very safe procedure is often the easiest step and probably what many physicians do in this setting.
Is there a better way to approach this problem? Preventing the ethical dilemma is the ideal scenario, although not always feasible. Here are some suggestions to consider.
Reviewing referrals prior to the procedure day allows endoscopists to contact and cancel patients if needed, before the prep and travel begin. This addresses the convenience aspects but not the issue regarding the underlying indication.
The most important step toward avoiding inappropriate referrals is better education for referring providers. Even gastroenterologists, let alone primary care physicians, may struggle to stay current on changing clinical GI guidelines. Colorectal cancer screening, for example, is an area that gives gastroenterologists an opportunity to communicate with and educate colleagues about appropriate management. Keeping our referral base up to date about guidelines and prep and safety recommendations will likely reduce the number of inappropriate colonoscopy referrals and provide many of the benefits described above.
Providing the best care for patients by adhering to medical ethical principles is the goal of our work as physicians. Implementing this goal may demand tough decisions.
Dr. Fisher is professor of clinical medicine and director of small-bowel imaging, division of gastroenterology, University of Pennsylvania, Philadelphia.
References
1. Sheffield KM et al. JAMA Intern Med. 2013 Apr 8;173(7):542-50.
2. Powell AA et al. J Gen Intern Med. 2015 Jun;30(6):732-41.
3. Petruzziello L et al. J Clin Gastroenterol. 2012;46(7):590-4.
4. Kapila N et al. Dig Dis Sci. 2019;64(10):2798-805.
Coaching in medicine: A perspective
Coaching is a new topic in medicine. I first heard about coaching several years ago and met the term with skepticism. I was unsure how coaching was different than mentoring or advising and I wondered about its usefulness. However, the reason that I even started to learn about coaching was because I was struggling. I had finally arrived in my career, I had my dream job with two healthy kids, a perfect house, and good marriage. I kept hearing the refrain in my head: “Is this all there is?” I had this arrival fallacy that after all this striving and straining that I would finally be content. I felt unfulfilled and was dissatisfied with where I was that was affecting all parts of my life.
As I was wrestling with these thoughts, I had an opportunity to become a coach to residents around the country through the Association of Women Surgeons. I discussed with them what fills them up, what gets them down, how to set goals, and what their goals were for the year, as well as imposter syndrome. Impostor syndrome is defined as a pattern in which an individual doubts their accomplishments or talents and has a persistent internalized fear of being exposed as a “fraud.” Despite external evidence of their competence, those experiencing this phenomenon remain convinced that they are fooling everyone around them and do not deserve all they have achieved. Individuals incorrectly attribute their success to luck or interpret it as a result of deceiving others into thinking they are more intelligent than they perceive themselves to be. Imposter syndrome is prevalent and deep in medicine. As perfectionists, we are especially vulnerable to imposter syndrome as we set unrealistic ideals for ourselves. When we fail to reach these ideals, we feel like frauds, setting up this cycle of self-doubt that is toxic. When we feel that we can’t achieve the goals that we are striving for we will always find ourselves lacking. There is a slow, insidious erosion of self over the years. Imposter syndrome is well documented in medicine and is even felt as early as medical school.1,2
When I began coaching these residents the most profound thing that came out of these sessions was that my life was getting better – I knew what filled me up, what got me down, what my goals were for the year, and how I still deal with imposter syndrome. Coaching gave me a framework for helping determine what I wanted for the rest of my life. As I began coaching, I started learning all the ways in which I could figure out my values, my personal and professional goals, and perhaps most importantly, my relationships with myself and others.
Another perspective on coaching is to look at a professional athlete such as Tom Brady, one of the greatest quarterbacks of all time. He has a quarterback coach. No coach is going to be a better quarterback than Tom Brady. A coach for him is to be there as an advocate, break his fundamentals down technically, and help him improve upon what he already knows. A coach also identifies strengths and weaknesses, and helps him capitalize on both by bringing awareness, reflection, accountability, and support. If world-class athletes still want and benefit from coaching in a sport they have already mastered, coaching for physicians is just another tool to help us improve our abilities in and out of medicine.
Coaching is more encompassing than advising or mentoring. It is about examining deeply held beliefs to see if they are really serving us, if they are in line with our values and how we want to live our lives.
Coaching has also been validated in medicine in several papers. In an article by Dyrbye et al. in JAMA Internal Medicine, measures of emotional exhaustion and burnout decreased in physicians who were coached and increased in those who were not.3 In another study from this year by McGonagle et al., a randomized, controlled trial showed that primary care physicians who had sessions (as short as 6 weeks) to address burnout, psychological capital, and job satisfaction experienced an improvement in measures which persisted for 6 months after intervention.4 Numerous other articles in medicine also exist to demonstrate the effect of coaching on mitigating burnout at an institutional level.
Physicians are inherently driven by their love of learning. As physicians, we love getting to the root cause of any problem and coming up with creative solutions. Any challenge we have, or just wanting to improve the quality of our lives, can be addressed with coaching. As perpetual students we can use coaching to truly master ourselves.
Dr. Shah is associate professor of surgery, Rush University Medical Center, Chicago. Instagram: ami.shahmdcoaching.
References
1. Gottlieb M et al. Med Educ. 2020 Feb;54(2):116-24.
2. Villwock JA et al. Int J Med Educ. 2016 Oct 31;7:364-9.
3. Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5;179(10):1406-14.
4. McGonagle AK et al. J Occup Health Psychol. 2020 Apr 16. doi: 10.1037/ocp0000180.
Coaching is a new topic in medicine. I first heard about coaching several years ago and met the term with skepticism. I was unsure how coaching was different than mentoring or advising and I wondered about its usefulness. However, the reason that I even started to learn about coaching was because I was struggling. I had finally arrived in my career, I had my dream job with two healthy kids, a perfect house, and good marriage. I kept hearing the refrain in my head: “Is this all there is?” I had this arrival fallacy that after all this striving and straining that I would finally be content. I felt unfulfilled and was dissatisfied with where I was that was affecting all parts of my life.
As I was wrestling with these thoughts, I had an opportunity to become a coach to residents around the country through the Association of Women Surgeons. I discussed with them what fills them up, what gets them down, how to set goals, and what their goals were for the year, as well as imposter syndrome. Impostor syndrome is defined as a pattern in which an individual doubts their accomplishments or talents and has a persistent internalized fear of being exposed as a “fraud.” Despite external evidence of their competence, those experiencing this phenomenon remain convinced that they are fooling everyone around them and do not deserve all they have achieved. Individuals incorrectly attribute their success to luck or interpret it as a result of deceiving others into thinking they are more intelligent than they perceive themselves to be. Imposter syndrome is prevalent and deep in medicine. As perfectionists, we are especially vulnerable to imposter syndrome as we set unrealistic ideals for ourselves. When we fail to reach these ideals, we feel like frauds, setting up this cycle of self-doubt that is toxic. When we feel that we can’t achieve the goals that we are striving for we will always find ourselves lacking. There is a slow, insidious erosion of self over the years. Imposter syndrome is well documented in medicine and is even felt as early as medical school.1,2
When I began coaching these residents the most profound thing that came out of these sessions was that my life was getting better – I knew what filled me up, what got me down, what my goals were for the year, and how I still deal with imposter syndrome. Coaching gave me a framework for helping determine what I wanted for the rest of my life. As I began coaching, I started learning all the ways in which I could figure out my values, my personal and professional goals, and perhaps most importantly, my relationships with myself and others.
Another perspective on coaching is to look at a professional athlete such as Tom Brady, one of the greatest quarterbacks of all time. He has a quarterback coach. No coach is going to be a better quarterback than Tom Brady. A coach for him is to be there as an advocate, break his fundamentals down technically, and help him improve upon what he already knows. A coach also identifies strengths and weaknesses, and helps him capitalize on both by bringing awareness, reflection, accountability, and support. If world-class athletes still want and benefit from coaching in a sport they have already mastered, coaching for physicians is just another tool to help us improve our abilities in and out of medicine.
Coaching is more encompassing than advising or mentoring. It is about examining deeply held beliefs to see if they are really serving us, if they are in line with our values and how we want to live our lives.
Coaching has also been validated in medicine in several papers. In an article by Dyrbye et al. in JAMA Internal Medicine, measures of emotional exhaustion and burnout decreased in physicians who were coached and increased in those who were not.3 In another study from this year by McGonagle et al., a randomized, controlled trial showed that primary care physicians who had sessions (as short as 6 weeks) to address burnout, psychological capital, and job satisfaction experienced an improvement in measures which persisted for 6 months after intervention.4 Numerous other articles in medicine also exist to demonstrate the effect of coaching on mitigating burnout at an institutional level.
Physicians are inherently driven by their love of learning. As physicians, we love getting to the root cause of any problem and coming up with creative solutions. Any challenge we have, or just wanting to improve the quality of our lives, can be addressed with coaching. As perpetual students we can use coaching to truly master ourselves.
Dr. Shah is associate professor of surgery, Rush University Medical Center, Chicago. Instagram: ami.shahmdcoaching.
References
1. Gottlieb M et al. Med Educ. 2020 Feb;54(2):116-24.
2. Villwock JA et al. Int J Med Educ. 2016 Oct 31;7:364-9.
3. Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5;179(10):1406-14.
4. McGonagle AK et al. J Occup Health Psychol. 2020 Apr 16. doi: 10.1037/ocp0000180.
Coaching is a new topic in medicine. I first heard about coaching several years ago and met the term with skepticism. I was unsure how coaching was different than mentoring or advising and I wondered about its usefulness. However, the reason that I even started to learn about coaching was because I was struggling. I had finally arrived in my career, I had my dream job with two healthy kids, a perfect house, and good marriage. I kept hearing the refrain in my head: “Is this all there is?” I had this arrival fallacy that after all this striving and straining that I would finally be content. I felt unfulfilled and was dissatisfied with where I was that was affecting all parts of my life.
As I was wrestling with these thoughts, I had an opportunity to become a coach to residents around the country through the Association of Women Surgeons. I discussed with them what fills them up, what gets them down, how to set goals, and what their goals were for the year, as well as imposter syndrome. Impostor syndrome is defined as a pattern in which an individual doubts their accomplishments or talents and has a persistent internalized fear of being exposed as a “fraud.” Despite external evidence of their competence, those experiencing this phenomenon remain convinced that they are fooling everyone around them and do not deserve all they have achieved. Individuals incorrectly attribute their success to luck or interpret it as a result of deceiving others into thinking they are more intelligent than they perceive themselves to be. Imposter syndrome is prevalent and deep in medicine. As perfectionists, we are especially vulnerable to imposter syndrome as we set unrealistic ideals for ourselves. When we fail to reach these ideals, we feel like frauds, setting up this cycle of self-doubt that is toxic. When we feel that we can’t achieve the goals that we are striving for we will always find ourselves lacking. There is a slow, insidious erosion of self over the years. Imposter syndrome is well documented in medicine and is even felt as early as medical school.1,2
When I began coaching these residents the most profound thing that came out of these sessions was that my life was getting better – I knew what filled me up, what got me down, what my goals were for the year, and how I still deal with imposter syndrome. Coaching gave me a framework for helping determine what I wanted for the rest of my life. As I began coaching, I started learning all the ways in which I could figure out my values, my personal and professional goals, and perhaps most importantly, my relationships with myself and others.
Another perspective on coaching is to look at a professional athlete such as Tom Brady, one of the greatest quarterbacks of all time. He has a quarterback coach. No coach is going to be a better quarterback than Tom Brady. A coach for him is to be there as an advocate, break his fundamentals down technically, and help him improve upon what he already knows. A coach also identifies strengths and weaknesses, and helps him capitalize on both by bringing awareness, reflection, accountability, and support. If world-class athletes still want and benefit from coaching in a sport they have already mastered, coaching for physicians is just another tool to help us improve our abilities in and out of medicine.
Coaching is more encompassing than advising or mentoring. It is about examining deeply held beliefs to see if they are really serving us, if they are in line with our values and how we want to live our lives.
Coaching has also been validated in medicine in several papers. In an article by Dyrbye et al. in JAMA Internal Medicine, measures of emotional exhaustion and burnout decreased in physicians who were coached and increased in those who were not.3 In another study from this year by McGonagle et al., a randomized, controlled trial showed that primary care physicians who had sessions (as short as 6 weeks) to address burnout, psychological capital, and job satisfaction experienced an improvement in measures which persisted for 6 months after intervention.4 Numerous other articles in medicine also exist to demonstrate the effect of coaching on mitigating burnout at an institutional level.
Physicians are inherently driven by their love of learning. As physicians, we love getting to the root cause of any problem and coming up with creative solutions. Any challenge we have, or just wanting to improve the quality of our lives, can be addressed with coaching. As perpetual students we can use coaching to truly master ourselves.
Dr. Shah is associate professor of surgery, Rush University Medical Center, Chicago. Instagram: ami.shahmdcoaching.
References
1. Gottlieb M et al. Med Educ. 2020 Feb;54(2):116-24.
2. Villwock JA et al. Int J Med Educ. 2016 Oct 31;7:364-9.
3. Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5;179(10):1406-14.
4. McGonagle AK et al. J Occup Health Psychol. 2020 Apr 16. doi: 10.1037/ocp0000180.
Understand the legal implications of telehealth medicine
Telehealth has been steadily gaining mainstream use throughout the last decade, but the practice was recently shoved, almost overnight, into the forefront of the health care profession. Telehealth is now used more frequently by medical groups and physicians than ever before. General reports before the COVID-19 pandemic approximated 90% of health care organizations used or planned to use telehealth in the future. This future may already be a reality, with a McKinsey & Company report estimating that physicians saw 50-175 times more patients over telehealth platforms since the pandemic’s start.1
In general, telehealth includes use of electronic communication and information technologies to deliver long-distance or remote health care. A physician’s use of telemedicine (clinical services) is one of the most common uses, but the industry also includes other professionals, such as pharmacists and nurses.
Telehealth platforms can be used to monitor, diagnose, treat, and counsel patients successfully. It works best for reading images, follow-up care, outpatient care, and long-term care. However, telemedicine is inappropriate for urgent issues, diagnosing underlying health conditions, or any practice where the standard of care would require a physical exam. There is potential liability for decision making without a proper physical exam.
There are many advantages to telehealth over more traditional health care options. Some of these advantages include:
- Increased access to health care.
- Increased access to medical specialists in small and rural communities.
- Improved long-term care from the comfort of patients’ homes.
- Improved platforms to document patient care outside regular business hours.
But along with these benefits, telehealth carries the disadvantage of potential increased liability. This increased liability could stem from:
- Breached standards of care.
- Inadequate or improper licensing.
- Limited care options.
- Decision making without a proper physical exam.
- Increased informed consent requirements.
- Restricted prescription access.
Before expanding any practice into telemedicine, awareness of potential legal issues is crucial.
Standard of care
Currently, telehealth laws and regulations vary significantly from state to state. But one rule is consistent across the board – that the standard of care for practicing medicine through telemedicine is identical to the standard of care required for practicing medicine during physical practice. It still requires the appropriate examination, testing, labs, imaging, and consultations that any in-person diagnosis needs. For physicians, it also includes supervising nonphysician clinicians, where state law requires supervision.
The American Telemedicine Association currently determines the primary governing standards and guidelines for telemedicine. These can help physicians understand best practices in meeting the standard of care through telemedicine. The American Gastroenterological Association provides coding guidelines and other resources to help physicians with telehealth and e-visits. Other professional societies, such as the American College of Radiology and the American Academy of Dermatology, offer guidelines specific to their medical specialties’ standards of care. These standards still vary from state to state, so medical professionals must be aware of any differences before treating patients in multiple states.
Licensing
Licensing is one of telemedicine’s most confusing legal issues. All states require a license to practice medicine (traditional or telehealth) within their borders. Without that license, practicing medicine in the state is a crime. On top of being criminal, unlicensed practice can affect insurance, liability, billing, and malpractice coverage. When in a brick-and-mortar clinic, a physician’s confidence in practicing within the licensed jurisdiction is easy. Now, the distinction is not so clear. Patients and physicians no longer have to be in the same room, city, or even state, meaning there could be unknown conflicting laws between the two locations. With rare exceptions, standards of care are based on the patient’s location, not the physician’s location. This increases the risk of practicing without being correctly licensed to higher than ever.
Because licensing is a significant roadblock in providing telemedicine, efforts are underway to make the process simpler and more streamlined. The Federation of State Medical Boards developed the Interstate Medical Licensure Compact (IMLC).2 This can qualify physicians to practice medicine across state lines within the compact so long as they meet specific eligibility requirements. The IMLC creates a fast-track option for physicians to fill out one application and receive licenses from multiple states at once. Currently, the compact includes 32 states, the District of Columbia, and Guam.3
Informed consent
Telemedicine health care still requires informed consent from patients. In fact, in some states, the requirements for care provided through telehealth are actually stricter than requirements for informed consent obtained in person.
Most informed consent laws require physicians to cover the risks and benefits of a recommended course of treatment and all feasible and reasonable material alternatives. On top of this traditional informed consent, physicians must get additional consent to receive care over a telehealth platform. This unique requirement explains what telehealth is, possible risks and expected benefits, and security measures used to protect patient information. States vary regarding when verbal consent is sufficient, and when written consent is required.
Prescriptions
Telemedicine is still a relatively new industry, and few legal opinions specifically address telemedicine malpractice. However, prescribing medication based on telemedicine information is among the few issues the courts have addressed. A 2008 decision found that a physician review of patient questionnaires submitted over the Internet was insufficient to prescribe medication without a physical examination determining patient health.4 This cautious approach stemmed from telehealth’s early concern about the absence of patient-physician relationships and potential online pharmacy abuse. Since this decision, many states require an “in-person” visit with a patient before prescribing medication. The definition of what qualifies as an in-person visit varies from state to state – some still consider the use of real-time, audiovisual conferencing sufficient.
The law is still evolving for prescriptions. Some states don’t allow any prescriptions, while others allow physicians to prescribe their patients’ medications as part of an appropriate treatment plan according to their professional discretion. Almost every state prohibits the prescription of controlled substances based on telemedicine.
Conclusion
Telemedicine is becoming an increasingly significant part of both physician-patient relationships and the broader health care industry. Used appropriately, it can be an incredibly effective method of care for physicians and patients. Physicians should learn the laws governing telemedicine in every state they want to practice and continue to stay current on any changes. The Center for Connected Health Policy offers a report, updated semiannually, to help physicians stay up to date on their state laws. These efforts will help prevent physicians from exposure to liability and medical malpractice claims.
Mr. Hyde is a partner at Younker Hyde Macfarlane, a law firm that focuses on prosecuting medical malpractice claims on behalf of injured patients. Ms. Johnson is an associate attorney with the firm. You can find them at YHMLaw.com.
References
1. Bestsennyy O, Harris A, Rost J. Telehealth: A quarter-trillion-dollar post-COVID-19 reality? Mckinsey & Company, May 29, 2020.
2. FSMB: Draft Interstate Compact for Physician Licensure Nears Completion, 2014.
3. Interstate Medical Licensure Compact: U.S. State Participation in the Compact.
4. See, Low Cost Pharm., Inc. v. Ariz. State Bd. Of Pharm, 2008 Ariz. App. Unpub. LEXIS 790, referencing conclusion of Arizona Medical Board.
Telehealth has been steadily gaining mainstream use throughout the last decade, but the practice was recently shoved, almost overnight, into the forefront of the health care profession. Telehealth is now used more frequently by medical groups and physicians than ever before. General reports before the COVID-19 pandemic approximated 90% of health care organizations used or planned to use telehealth in the future. This future may already be a reality, with a McKinsey & Company report estimating that physicians saw 50-175 times more patients over telehealth platforms since the pandemic’s start.1
In general, telehealth includes use of electronic communication and information technologies to deliver long-distance or remote health care. A physician’s use of telemedicine (clinical services) is one of the most common uses, but the industry also includes other professionals, such as pharmacists and nurses.
Telehealth platforms can be used to monitor, diagnose, treat, and counsel patients successfully. It works best for reading images, follow-up care, outpatient care, and long-term care. However, telemedicine is inappropriate for urgent issues, diagnosing underlying health conditions, or any practice where the standard of care would require a physical exam. There is potential liability for decision making without a proper physical exam.
There are many advantages to telehealth over more traditional health care options. Some of these advantages include:
- Increased access to health care.
- Increased access to medical specialists in small and rural communities.
- Improved long-term care from the comfort of patients’ homes.
- Improved platforms to document patient care outside regular business hours.
But along with these benefits, telehealth carries the disadvantage of potential increased liability. This increased liability could stem from:
- Breached standards of care.
- Inadequate or improper licensing.
- Limited care options.
- Decision making without a proper physical exam.
- Increased informed consent requirements.
- Restricted prescription access.
Before expanding any practice into telemedicine, awareness of potential legal issues is crucial.
Standard of care
Currently, telehealth laws and regulations vary significantly from state to state. But one rule is consistent across the board – that the standard of care for practicing medicine through telemedicine is identical to the standard of care required for practicing medicine during physical practice. It still requires the appropriate examination, testing, labs, imaging, and consultations that any in-person diagnosis needs. For physicians, it also includes supervising nonphysician clinicians, where state law requires supervision.
The American Telemedicine Association currently determines the primary governing standards and guidelines for telemedicine. These can help physicians understand best practices in meeting the standard of care through telemedicine. The American Gastroenterological Association provides coding guidelines and other resources to help physicians with telehealth and e-visits. Other professional societies, such as the American College of Radiology and the American Academy of Dermatology, offer guidelines specific to their medical specialties’ standards of care. These standards still vary from state to state, so medical professionals must be aware of any differences before treating patients in multiple states.
Licensing
Licensing is one of telemedicine’s most confusing legal issues. All states require a license to practice medicine (traditional or telehealth) within their borders. Without that license, practicing medicine in the state is a crime. On top of being criminal, unlicensed practice can affect insurance, liability, billing, and malpractice coverage. When in a brick-and-mortar clinic, a physician’s confidence in practicing within the licensed jurisdiction is easy. Now, the distinction is not so clear. Patients and physicians no longer have to be in the same room, city, or even state, meaning there could be unknown conflicting laws between the two locations. With rare exceptions, standards of care are based on the patient’s location, not the physician’s location. This increases the risk of practicing without being correctly licensed to higher than ever.
Because licensing is a significant roadblock in providing telemedicine, efforts are underway to make the process simpler and more streamlined. The Federation of State Medical Boards developed the Interstate Medical Licensure Compact (IMLC).2 This can qualify physicians to practice medicine across state lines within the compact so long as they meet specific eligibility requirements. The IMLC creates a fast-track option for physicians to fill out one application and receive licenses from multiple states at once. Currently, the compact includes 32 states, the District of Columbia, and Guam.3
Informed consent
Telemedicine health care still requires informed consent from patients. In fact, in some states, the requirements for care provided through telehealth are actually stricter than requirements for informed consent obtained in person.
Most informed consent laws require physicians to cover the risks and benefits of a recommended course of treatment and all feasible and reasonable material alternatives. On top of this traditional informed consent, physicians must get additional consent to receive care over a telehealth platform. This unique requirement explains what telehealth is, possible risks and expected benefits, and security measures used to protect patient information. States vary regarding when verbal consent is sufficient, and when written consent is required.
Prescriptions
Telemedicine is still a relatively new industry, and few legal opinions specifically address telemedicine malpractice. However, prescribing medication based on telemedicine information is among the few issues the courts have addressed. A 2008 decision found that a physician review of patient questionnaires submitted over the Internet was insufficient to prescribe medication without a physical examination determining patient health.4 This cautious approach stemmed from telehealth’s early concern about the absence of patient-physician relationships and potential online pharmacy abuse. Since this decision, many states require an “in-person” visit with a patient before prescribing medication. The definition of what qualifies as an in-person visit varies from state to state – some still consider the use of real-time, audiovisual conferencing sufficient.
The law is still evolving for prescriptions. Some states don’t allow any prescriptions, while others allow physicians to prescribe their patients’ medications as part of an appropriate treatment plan according to their professional discretion. Almost every state prohibits the prescription of controlled substances based on telemedicine.
Conclusion
Telemedicine is becoming an increasingly significant part of both physician-patient relationships and the broader health care industry. Used appropriately, it can be an incredibly effective method of care for physicians and patients. Physicians should learn the laws governing telemedicine in every state they want to practice and continue to stay current on any changes. The Center for Connected Health Policy offers a report, updated semiannually, to help physicians stay up to date on their state laws. These efforts will help prevent physicians from exposure to liability and medical malpractice claims.
Mr. Hyde is a partner at Younker Hyde Macfarlane, a law firm that focuses on prosecuting medical malpractice claims on behalf of injured patients. Ms. Johnson is an associate attorney with the firm. You can find them at YHMLaw.com.
References
1. Bestsennyy O, Harris A, Rost J. Telehealth: A quarter-trillion-dollar post-COVID-19 reality? Mckinsey & Company, May 29, 2020.
2. FSMB: Draft Interstate Compact for Physician Licensure Nears Completion, 2014.
3. Interstate Medical Licensure Compact: U.S. State Participation in the Compact.
4. See, Low Cost Pharm., Inc. v. Ariz. State Bd. Of Pharm, 2008 Ariz. App. Unpub. LEXIS 790, referencing conclusion of Arizona Medical Board.
Telehealth has been steadily gaining mainstream use throughout the last decade, but the practice was recently shoved, almost overnight, into the forefront of the health care profession. Telehealth is now used more frequently by medical groups and physicians than ever before. General reports before the COVID-19 pandemic approximated 90% of health care organizations used or planned to use telehealth in the future. This future may already be a reality, with a McKinsey & Company report estimating that physicians saw 50-175 times more patients over telehealth platforms since the pandemic’s start.1
In general, telehealth includes use of electronic communication and information technologies to deliver long-distance or remote health care. A physician’s use of telemedicine (clinical services) is one of the most common uses, but the industry also includes other professionals, such as pharmacists and nurses.
Telehealth platforms can be used to monitor, diagnose, treat, and counsel patients successfully. It works best for reading images, follow-up care, outpatient care, and long-term care. However, telemedicine is inappropriate for urgent issues, diagnosing underlying health conditions, or any practice where the standard of care would require a physical exam. There is potential liability for decision making without a proper physical exam.
There are many advantages to telehealth over more traditional health care options. Some of these advantages include:
- Increased access to health care.
- Increased access to medical specialists in small and rural communities.
- Improved long-term care from the comfort of patients’ homes.
- Improved platforms to document patient care outside regular business hours.
But along with these benefits, telehealth carries the disadvantage of potential increased liability. This increased liability could stem from:
- Breached standards of care.
- Inadequate or improper licensing.
- Limited care options.
- Decision making without a proper physical exam.
- Increased informed consent requirements.
- Restricted prescription access.
Before expanding any practice into telemedicine, awareness of potential legal issues is crucial.
Standard of care
Currently, telehealth laws and regulations vary significantly from state to state. But one rule is consistent across the board – that the standard of care for practicing medicine through telemedicine is identical to the standard of care required for practicing medicine during physical practice. It still requires the appropriate examination, testing, labs, imaging, and consultations that any in-person diagnosis needs. For physicians, it also includes supervising nonphysician clinicians, where state law requires supervision.
The American Telemedicine Association currently determines the primary governing standards and guidelines for telemedicine. These can help physicians understand best practices in meeting the standard of care through telemedicine. The American Gastroenterological Association provides coding guidelines and other resources to help physicians with telehealth and e-visits. Other professional societies, such as the American College of Radiology and the American Academy of Dermatology, offer guidelines specific to their medical specialties’ standards of care. These standards still vary from state to state, so medical professionals must be aware of any differences before treating patients in multiple states.
Licensing
Licensing is one of telemedicine’s most confusing legal issues. All states require a license to practice medicine (traditional or telehealth) within their borders. Without that license, practicing medicine in the state is a crime. On top of being criminal, unlicensed practice can affect insurance, liability, billing, and malpractice coverage. When in a brick-and-mortar clinic, a physician’s confidence in practicing within the licensed jurisdiction is easy. Now, the distinction is not so clear. Patients and physicians no longer have to be in the same room, city, or even state, meaning there could be unknown conflicting laws between the two locations. With rare exceptions, standards of care are based on the patient’s location, not the physician’s location. This increases the risk of practicing without being correctly licensed to higher than ever.
Because licensing is a significant roadblock in providing telemedicine, efforts are underway to make the process simpler and more streamlined. The Federation of State Medical Boards developed the Interstate Medical Licensure Compact (IMLC).2 This can qualify physicians to practice medicine across state lines within the compact so long as they meet specific eligibility requirements. The IMLC creates a fast-track option for physicians to fill out one application and receive licenses from multiple states at once. Currently, the compact includes 32 states, the District of Columbia, and Guam.3
Informed consent
Telemedicine health care still requires informed consent from patients. In fact, in some states, the requirements for care provided through telehealth are actually stricter than requirements for informed consent obtained in person.
Most informed consent laws require physicians to cover the risks and benefits of a recommended course of treatment and all feasible and reasonable material alternatives. On top of this traditional informed consent, physicians must get additional consent to receive care over a telehealth platform. This unique requirement explains what telehealth is, possible risks and expected benefits, and security measures used to protect patient information. States vary regarding when verbal consent is sufficient, and when written consent is required.
Prescriptions
Telemedicine is still a relatively new industry, and few legal opinions specifically address telemedicine malpractice. However, prescribing medication based on telemedicine information is among the few issues the courts have addressed. A 2008 decision found that a physician review of patient questionnaires submitted over the Internet was insufficient to prescribe medication without a physical examination determining patient health.4 This cautious approach stemmed from telehealth’s early concern about the absence of patient-physician relationships and potential online pharmacy abuse. Since this decision, many states require an “in-person” visit with a patient before prescribing medication. The definition of what qualifies as an in-person visit varies from state to state – some still consider the use of real-time, audiovisual conferencing sufficient.
The law is still evolving for prescriptions. Some states don’t allow any prescriptions, while others allow physicians to prescribe their patients’ medications as part of an appropriate treatment plan according to their professional discretion. Almost every state prohibits the prescription of controlled substances based on telemedicine.
Conclusion
Telemedicine is becoming an increasingly significant part of both physician-patient relationships and the broader health care industry. Used appropriately, it can be an incredibly effective method of care for physicians and patients. Physicians should learn the laws governing telemedicine in every state they want to practice and continue to stay current on any changes. The Center for Connected Health Policy offers a report, updated semiannually, to help physicians stay up to date on their state laws. These efforts will help prevent physicians from exposure to liability and medical malpractice claims.
Mr. Hyde is a partner at Younker Hyde Macfarlane, a law firm that focuses on prosecuting medical malpractice claims on behalf of injured patients. Ms. Johnson is an associate attorney with the firm. You can find them at YHMLaw.com.
References
1. Bestsennyy O, Harris A, Rost J. Telehealth: A quarter-trillion-dollar post-COVID-19 reality? Mckinsey & Company, May 29, 2020.
2. FSMB: Draft Interstate Compact for Physician Licensure Nears Completion, 2014.
3. Interstate Medical Licensure Compact: U.S. State Participation in the Compact.
4. See, Low Cost Pharm., Inc. v. Ariz. State Bd. Of Pharm, 2008 Ariz. App. Unpub. LEXIS 790, referencing conclusion of Arizona Medical Board.
Web-based interviews, financial planning in a pandemic, and more
Dear colleagues,
I’m excited to introduce the November issue of The New Gastroenterologist – the last edition of 2020 features a fantastic line-up of articles! As the year comes to a close, we reflect on what has certainly been an interesting year, defined by a set of unique challenges we have faced as a nation and as a specialty.
The fellowship recruitment season is one that has looked starkly different as interviews have converted to a virtual format. Dr. Wissam Khan, Dr. Nada Al Masalmeh, Dr. Stephanie Judd, and Dr. Diane Levine (Wayne State University) compile a helpful list of tips and tricks on proper interview etiquette in the new era of web-based interviews.
Financial planning in the face of a pandemic is a formidable task – Jonathan Tudor (Fidelity Investments) offers valuable advice for gastroenterologists on how to remain secure in your finances even in uncertain circumstances.
This quarter’s “In Focus” feature, written by Dr. Yutaka Tomizawa (University of Washington), is a comprehensive piece elucidating the role of gastroenterologists in the management of gastric cancer. The article reviews the individual risk factors that exist for gastric cancer and provides guidance on how to stratify patients accordingly, which is critical in the ethnically diverse population of the United States.
Keeping a procedure log during fellowship can seem daunting and cumbersome, but it is important. Dr. Houman Rezaizadeh (University of Connecticut) shares his program’s experience with the AGA Procedure Log, a convenient online tracking tool, which can provide accurate and secure documentation of endoscopic procedures performed throughout fellowship.
Dr. Nazia Hasan (North Bay Health Care) and Dr. Allison Schulman (University of Michigan) broach an incredibly important topic: the paucity of women in interventional endoscopy. Dr. Hasan and Dr. Shulman candidly discuss the barriers women face in pursuing this subspecialty and offer practical solutions on how to approach these challenges – a piece that will surely resonate with many young gastroenterologists.
We wrap up our first year of TNG’s ethics series with two cases discussing the utilization of cannabis therapy in inflammatory bowel disease (IBD). Dr. Jami Kinnucan (University of Michigan) and Dr. Arun Swaminath (Lenox Hill Hospital) systematically review existing data on the efficacy of cannabis use in IBD, the risks associated with therapy, and legal implications for both physicians and patients.
Also in this issue is a high-yield clinical review on the endoscopic drainage of pancreatic fluid collections by Dr. Robert Moran and Dr. Joseph Elmunzer (Medical University of South Carolina). Dr. Manol Jovani (Johns Hopkins) teaches us about confounding – a critical concept to keep in mind when evaluating any manuscript. Lastly, our DHPA Private Practice Perspectives article, written by Dr. Mehul Lalani (US Digestive), reviews how quality measures and initiatives are tracked and implemented in private practice.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m excited to introduce the November issue of The New Gastroenterologist – the last edition of 2020 features a fantastic line-up of articles! As the year comes to a close, we reflect on what has certainly been an interesting year, defined by a set of unique challenges we have faced as a nation and as a specialty.
The fellowship recruitment season is one that has looked starkly different as interviews have converted to a virtual format. Dr. Wissam Khan, Dr. Nada Al Masalmeh, Dr. Stephanie Judd, and Dr. Diane Levine (Wayne State University) compile a helpful list of tips and tricks on proper interview etiquette in the new era of web-based interviews.
Financial planning in the face of a pandemic is a formidable task – Jonathan Tudor (Fidelity Investments) offers valuable advice for gastroenterologists on how to remain secure in your finances even in uncertain circumstances.
This quarter’s “In Focus” feature, written by Dr. Yutaka Tomizawa (University of Washington), is a comprehensive piece elucidating the role of gastroenterologists in the management of gastric cancer. The article reviews the individual risk factors that exist for gastric cancer and provides guidance on how to stratify patients accordingly, which is critical in the ethnically diverse population of the United States.
Keeping a procedure log during fellowship can seem daunting and cumbersome, but it is important. Dr. Houman Rezaizadeh (University of Connecticut) shares his program’s experience with the AGA Procedure Log, a convenient online tracking tool, which can provide accurate and secure documentation of endoscopic procedures performed throughout fellowship.
Dr. Nazia Hasan (North Bay Health Care) and Dr. Allison Schulman (University of Michigan) broach an incredibly important topic: the paucity of women in interventional endoscopy. Dr. Hasan and Dr. Shulman candidly discuss the barriers women face in pursuing this subspecialty and offer practical solutions on how to approach these challenges – a piece that will surely resonate with many young gastroenterologists.
We wrap up our first year of TNG’s ethics series with two cases discussing the utilization of cannabis therapy in inflammatory bowel disease (IBD). Dr. Jami Kinnucan (University of Michigan) and Dr. Arun Swaminath (Lenox Hill Hospital) systematically review existing data on the efficacy of cannabis use in IBD, the risks associated with therapy, and legal implications for both physicians and patients.
Also in this issue is a high-yield clinical review on the endoscopic drainage of pancreatic fluid collections by Dr. Robert Moran and Dr. Joseph Elmunzer (Medical University of South Carolina). Dr. Manol Jovani (Johns Hopkins) teaches us about confounding – a critical concept to keep in mind when evaluating any manuscript. Lastly, our DHPA Private Practice Perspectives article, written by Dr. Mehul Lalani (US Digestive), reviews how quality measures and initiatives are tracked and implemented in private practice.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m excited to introduce the November issue of The New Gastroenterologist – the last edition of 2020 features a fantastic line-up of articles! As the year comes to a close, we reflect on what has certainly been an interesting year, defined by a set of unique challenges we have faced as a nation and as a specialty.
The fellowship recruitment season is one that has looked starkly different as interviews have converted to a virtual format. Dr. Wissam Khan, Dr. Nada Al Masalmeh, Dr. Stephanie Judd, and Dr. Diane Levine (Wayne State University) compile a helpful list of tips and tricks on proper interview etiquette in the new era of web-based interviews.
Financial planning in the face of a pandemic is a formidable task – Jonathan Tudor (Fidelity Investments) offers valuable advice for gastroenterologists on how to remain secure in your finances even in uncertain circumstances.
This quarter’s “In Focus” feature, written by Dr. Yutaka Tomizawa (University of Washington), is a comprehensive piece elucidating the role of gastroenterologists in the management of gastric cancer. The article reviews the individual risk factors that exist for gastric cancer and provides guidance on how to stratify patients accordingly, which is critical in the ethnically diverse population of the United States.
Keeping a procedure log during fellowship can seem daunting and cumbersome, but it is important. Dr. Houman Rezaizadeh (University of Connecticut) shares his program’s experience with the AGA Procedure Log, a convenient online tracking tool, which can provide accurate and secure documentation of endoscopic procedures performed throughout fellowship.
Dr. Nazia Hasan (North Bay Health Care) and Dr. Allison Schulman (University of Michigan) broach an incredibly important topic: the paucity of women in interventional endoscopy. Dr. Hasan and Dr. Shulman candidly discuss the barriers women face in pursuing this subspecialty and offer practical solutions on how to approach these challenges – a piece that will surely resonate with many young gastroenterologists.
We wrap up our first year of TNG’s ethics series with two cases discussing the utilization of cannabis therapy in inflammatory bowel disease (IBD). Dr. Jami Kinnucan (University of Michigan) and Dr. Arun Swaminath (Lenox Hill Hospital) systematically review existing data on the efficacy of cannabis use in IBD, the risks associated with therapy, and legal implications for both physicians and patients.
Also in this issue is a high-yield clinical review on the endoscopic drainage of pancreatic fluid collections by Dr. Robert Moran and Dr. Joseph Elmunzer (Medical University of South Carolina). Dr. Manol Jovani (Johns Hopkins) teaches us about confounding – a critical concept to keep in mind when evaluating any manuscript. Lastly, our DHPA Private Practice Perspectives article, written by Dr. Mehul Lalani (US Digestive), reviews how quality measures and initiatives are tracked and implemented in private practice.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Role of gastroenterologists in the U.S. in the management of gastric cancer
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.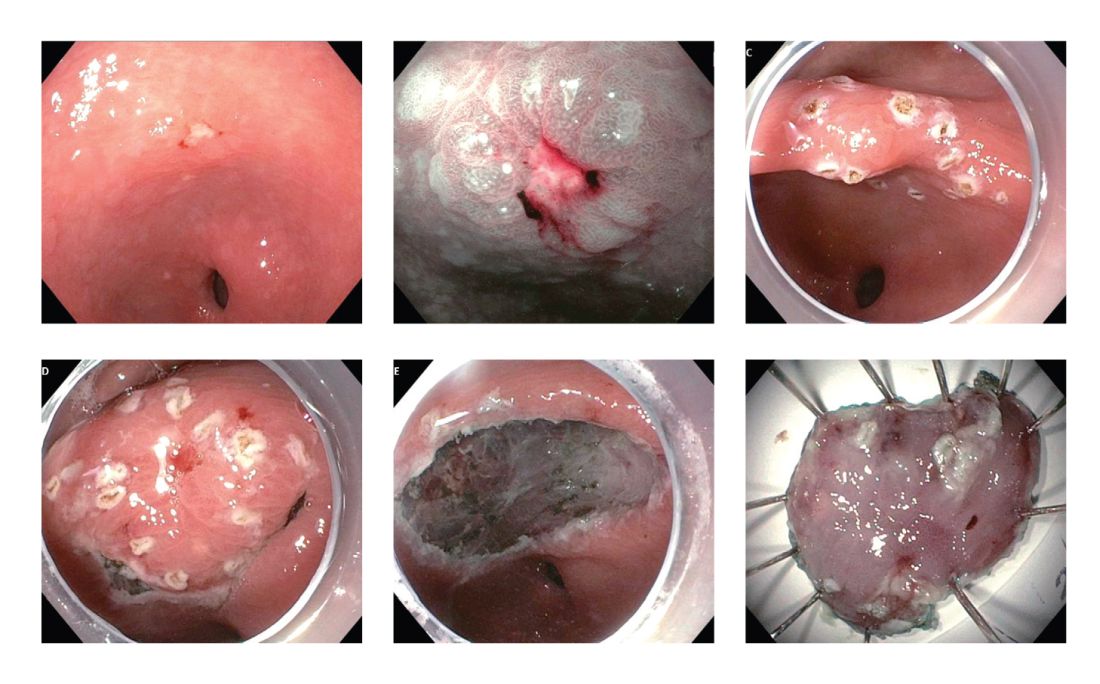
These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.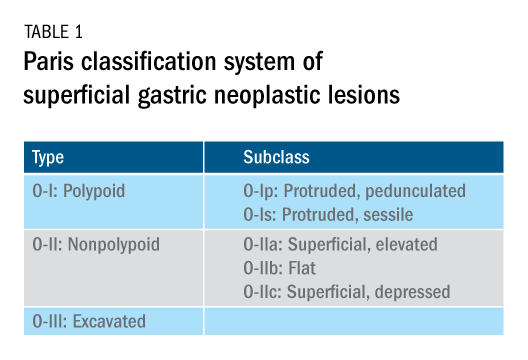
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.
These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.
These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Jan. 15-17, 2021
Gastrointestinal Cancers Symposium
Through an engaging lineup of novel science, education, and exhibits, the virtual 2021 Gastrointestinal (GI) Cancers Symposium offers new, innovative findings in GI cancer treatment, research, and care.
Early-bird deadline: Dec. 16, 2020.
Jan. 21-24, 2021
Crohn’s & Colitis Congress®Join health care professionals and researchers virtually at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, practical information you can immediately implement, and potential treatments on the horizon.
Early-bird deadline: Friday, Nov. 6, 2020.
May 21-23, 2021
Digestive Disease Week® (DDW)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Abstract submission window Oct. 15 to Dec. 3, 2020.
AWARD DEADLINES
American Gastroenterological Association (AGA) Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students,or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early-career (that is, 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations DDW. The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Jan. 15-17, 2021
Gastrointestinal Cancers Symposium
Through an engaging lineup of novel science, education, and exhibits, the virtual 2021 Gastrointestinal (GI) Cancers Symposium offers new, innovative findings in GI cancer treatment, research, and care.
Early-bird deadline: Dec. 16, 2020.
Jan. 21-24, 2021
Crohn’s & Colitis Congress®Join health care professionals and researchers virtually at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, practical information you can immediately implement, and potential treatments on the horizon.
Early-bird deadline: Friday, Nov. 6, 2020.
May 21-23, 2021
Digestive Disease Week® (DDW)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Abstract submission window Oct. 15 to Dec. 3, 2020.
AWARD DEADLINES
American Gastroenterological Association (AGA) Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students,or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early-career (that is, 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations DDW. The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Jan. 15-17, 2021
Gastrointestinal Cancers Symposium
Through an engaging lineup of novel science, education, and exhibits, the virtual 2021 Gastrointestinal (GI) Cancers Symposium offers new, innovative findings in GI cancer treatment, research, and care.
Early-bird deadline: Dec. 16, 2020.
Jan. 21-24, 2021
Crohn’s & Colitis Congress®Join health care professionals and researchers virtually at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, practical information you can immediately implement, and potential treatments on the horizon.
Early-bird deadline: Friday, Nov. 6, 2020.
May 21-23, 2021
Digestive Disease Week® (DDW)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Abstract submission window Oct. 15 to Dec. 3, 2020.
AWARD DEADLINES
American Gastroenterological Association (AGA) Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students,or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early-career (that is, 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations DDW. The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
Quality measures and initiatives in private practices
It has been almost 15 years since the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy established the Task Force on Quality Endoscopy and published the first set of quality indicators for GI endoscopic procedures.
This work was motivated by two seminal reports on patient safety that fostered a demand by the public, policy makers, and payers to accurately define and measure the quality of health care services.
While the Centers for Medicare & Medicaid Services initially designated and required reporting on several basic outcome measures, leaders within the field of gastroenterology recognized the importance of developing evidence-based quality measures for our field, and specifically for endoscopic procedures.
Integrating safety measures into our daily operations has always been important, and over the years, policies have been implemented to incentivize health care providers to meet standards in everything from patient safety to patient satisfaction.
Defining quality and how to measure it
The goals of implementing quality measures within private practices include effective patient care and safety, but they also include issues like access and affordability, as well as the professionalism of your physicians and advanced practice providers.
As a larger practice, we have the resources to support a quality coordinator who spends half their time focused on quality measures. Every provider is required to complete annual education on quality parameters.
We have two committees that propose and track quality initiatives in our practice. We have one on the practice side and one for our ambulatory surgery centers (ASCs). The committees are made of physicians who have a particular interest in quality measures. On the ASC side, our ASC center director from our management partner AmSurg is also a member of the committee.
The road to improving quality within a private practice starts by defining the aspects of care that affect the quality of the patient experience.
Tracking quality in the office and in the surgery center
In our practices we have about 60 physicians. Start times and coding accuracy are good examples of what we have tracked in the past as areas of quality improvement. For instance, if only one or two providers get started late, it can cause a domino effect. Schedules get cramped, which can increase stress and possibly cause our team members to rush. Even things that seem like patient satisfaction issues can affect patient care, so it is important to make sure they are being measured.
On the ASC side, we track adenoma detection rates, colonoscopy intervals, complication rates, and many other additional criteria. As an example, when a pathology report is issued, we require our physicians to provide results to our patients within 72 hours.
Data on all providers are tabulated quarterly and then distributed to the providers in the form of a scorecard. The scorecard is then used for constructive feedback on improvements that can be made. A cumulative annual report is given to the providers, which is also incorporated into reviews. Not paying attention to quality measures can potentially have financial ramifications for providers in our group.
Find the right fit from a quality standpoint
In terms of what we are tracking, we are probably not that different from most groups of our size. Standardization will continue to increase, and it is important as an early career physician to familiarize yourself with quality measures in gastroenterology.
I often interview early career physicians who would like to join Regional GI, and the most impressive are the young men and women who ask about our processes for tracking quality measures and implementing programs geared toward improvement. If you are thinking of joining a practice, bring it up. You will be glad you did.
The interest in quality shows that you are invested in providing the best evidence-based patient care. As an independent group, this is critical because so much of what we do depends on having a track record of measurement. For instance, an ASC might not be credentialed if the quality metrics do not meet a certain threshold.
We are looking for potential partners who are seriously interested in joining us on our mission to provide the highest-quality care to our patients. After all, that is why became gastroenterologists in the first place.
Dr. Lalani serves as treasurer on the executive committee of the Digestive Health Physicians Association and is a practicing gastroenterologist at U.S. Digestive Health.
It has been almost 15 years since the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy established the Task Force on Quality Endoscopy and published the first set of quality indicators for GI endoscopic procedures.
This work was motivated by two seminal reports on patient safety that fostered a demand by the public, policy makers, and payers to accurately define and measure the quality of health care services.
While the Centers for Medicare & Medicaid Services initially designated and required reporting on several basic outcome measures, leaders within the field of gastroenterology recognized the importance of developing evidence-based quality measures for our field, and specifically for endoscopic procedures.
Integrating safety measures into our daily operations has always been important, and over the years, policies have been implemented to incentivize health care providers to meet standards in everything from patient safety to patient satisfaction.
Defining quality and how to measure it
The goals of implementing quality measures within private practices include effective patient care and safety, but they also include issues like access and affordability, as well as the professionalism of your physicians and advanced practice providers.
As a larger practice, we have the resources to support a quality coordinator who spends half their time focused on quality measures. Every provider is required to complete annual education on quality parameters.
We have two committees that propose and track quality initiatives in our practice. We have one on the practice side and one for our ambulatory surgery centers (ASCs). The committees are made of physicians who have a particular interest in quality measures. On the ASC side, our ASC center director from our management partner AmSurg is also a member of the committee.
The road to improving quality within a private practice starts by defining the aspects of care that affect the quality of the patient experience.
Tracking quality in the office and in the surgery center
In our practices we have about 60 physicians. Start times and coding accuracy are good examples of what we have tracked in the past as areas of quality improvement. For instance, if only one or two providers get started late, it can cause a domino effect. Schedules get cramped, which can increase stress and possibly cause our team members to rush. Even things that seem like patient satisfaction issues can affect patient care, so it is important to make sure they are being measured.
On the ASC side, we track adenoma detection rates, colonoscopy intervals, complication rates, and many other additional criteria. As an example, when a pathology report is issued, we require our physicians to provide results to our patients within 72 hours.
Data on all providers are tabulated quarterly and then distributed to the providers in the form of a scorecard. The scorecard is then used for constructive feedback on improvements that can be made. A cumulative annual report is given to the providers, which is also incorporated into reviews. Not paying attention to quality measures can potentially have financial ramifications for providers in our group.
Find the right fit from a quality standpoint
In terms of what we are tracking, we are probably not that different from most groups of our size. Standardization will continue to increase, and it is important as an early career physician to familiarize yourself with quality measures in gastroenterology.
I often interview early career physicians who would like to join Regional GI, and the most impressive are the young men and women who ask about our processes for tracking quality measures and implementing programs geared toward improvement. If you are thinking of joining a practice, bring it up. You will be glad you did.
The interest in quality shows that you are invested in providing the best evidence-based patient care. As an independent group, this is critical because so much of what we do depends on having a track record of measurement. For instance, an ASC might not be credentialed if the quality metrics do not meet a certain threshold.
We are looking for potential partners who are seriously interested in joining us on our mission to provide the highest-quality care to our patients. After all, that is why became gastroenterologists in the first place.
Dr. Lalani serves as treasurer on the executive committee of the Digestive Health Physicians Association and is a practicing gastroenterologist at U.S. Digestive Health.
It has been almost 15 years since the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy established the Task Force on Quality Endoscopy and published the first set of quality indicators for GI endoscopic procedures.
This work was motivated by two seminal reports on patient safety that fostered a demand by the public, policy makers, and payers to accurately define and measure the quality of health care services.
While the Centers for Medicare & Medicaid Services initially designated and required reporting on several basic outcome measures, leaders within the field of gastroenterology recognized the importance of developing evidence-based quality measures for our field, and specifically for endoscopic procedures.
Integrating safety measures into our daily operations has always been important, and over the years, policies have been implemented to incentivize health care providers to meet standards in everything from patient safety to patient satisfaction.
Defining quality and how to measure it
The goals of implementing quality measures within private practices include effective patient care and safety, but they also include issues like access and affordability, as well as the professionalism of your physicians and advanced practice providers.
As a larger practice, we have the resources to support a quality coordinator who spends half their time focused on quality measures. Every provider is required to complete annual education on quality parameters.
We have two committees that propose and track quality initiatives in our practice. We have one on the practice side and one for our ambulatory surgery centers (ASCs). The committees are made of physicians who have a particular interest in quality measures. On the ASC side, our ASC center director from our management partner AmSurg is also a member of the committee.
The road to improving quality within a private practice starts by defining the aspects of care that affect the quality of the patient experience.
Tracking quality in the office and in the surgery center
In our practices we have about 60 physicians. Start times and coding accuracy are good examples of what we have tracked in the past as areas of quality improvement. For instance, if only one or two providers get started late, it can cause a domino effect. Schedules get cramped, which can increase stress and possibly cause our team members to rush. Even things that seem like patient satisfaction issues can affect patient care, so it is important to make sure they are being measured.
On the ASC side, we track adenoma detection rates, colonoscopy intervals, complication rates, and many other additional criteria. As an example, when a pathology report is issued, we require our physicians to provide results to our patients within 72 hours.
Data on all providers are tabulated quarterly and then distributed to the providers in the form of a scorecard. The scorecard is then used for constructive feedback on improvements that can be made. A cumulative annual report is given to the providers, which is also incorporated into reviews. Not paying attention to quality measures can potentially have financial ramifications for providers in our group.
Find the right fit from a quality standpoint
In terms of what we are tracking, we are probably not that different from most groups of our size. Standardization will continue to increase, and it is important as an early career physician to familiarize yourself with quality measures in gastroenterology.
I often interview early career physicians who would like to join Regional GI, and the most impressive are the young men and women who ask about our processes for tracking quality measures and implementing programs geared toward improvement. If you are thinking of joining a practice, bring it up. You will be glad you did.
The interest in quality shows that you are invested in providing the best evidence-based patient care. As an independent group, this is critical because so much of what we do depends on having a track record of measurement. For instance, an ASC might not be credentialed if the quality metrics do not meet a certain threshold.
We are looking for potential partners who are seriously interested in joining us on our mission to provide the highest-quality care to our patients. After all, that is why became gastroenterologists in the first place.
Dr. Lalani serves as treasurer on the executive committee of the Digestive Health Physicians Association and is a practicing gastroenterologist at U.S. Digestive Health.
November 2020 – ICYMI
Gastroenterology
July 2020
Role of cannabis and its derivatives in gastrointestinal and hepatic disease. Jonathan Gotfried et al. 2020 July;159(1):62-80. doi: 10.1053/j.gastro.2020.03.087
Effects of blended (yellow) vs forced coagulation (blue) currents on adverse events, complete resection, or polyp recurrence after polypectomy in a large randomized trial. Heiko Pohl et al. 2020 July;159(1):119-28.e2. doi: 10.1053/j.gastro.2020.03.014
Calculating the starting age for screening in relatives of patients with colorectal cancer based on data from large nationwide data sets.
Yu Tian et al. July 2020;159(1):159-168.e3. doi: 10.1053/j.gastro.2020.03.063
August 2020
Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Erica J. Brenner et al. 2020 Aug 159;(2):481-91.e3. doi: 10.1053/j.gastro.2020.05.032
Collagenous colitis is associated with HLA signature and shares genetic risks with other immune-mediated diseases. Eli Stahl et al. 2020 Aug;159(2):549-61.e8. doi: 10.1053/j.gastro.2020.04.063
Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Alessandro Repici et al. 2020 Aug;159(2):512-20.e7. doi: 10.1053/j.gastro.2020.04.062
September 2020
Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Chun-Han Lo et al. 2020 Sept;159(3):p873-83.e1. doi: 10.1053/j.gastro.2020.05.011
Rates of incomplete resection of 1- to 20-mm colorectal polyps: A systematic review and meta-analysis. Roupen Djinbachian et al. 2020 Sept;159(3):904-14.e12. doi: 10.1053/j.gastro.2020.05.018
Clinical Gastroenterology and Hepatology
August 2020
Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Helen Burton Murray et al. 2020 Aug;18(9):1995-2002.e1. doi: 10.1016/j.cgh.2019.10.030
Ten things every gastroenterologist should know about antireflux surgery. Steven Park et al. 2020 Aug;18(9):1923-9. doi: 10.1016/j.cgh.2020.02.041
Biopsies from ascending and descending colon are sufficient for diagnosis of microscopic colitis. Boris Virine et al. 2020 Aug;18(9):2003-9. doi: 10.1016/j.cgh.2020.02.036
September 2020
Association between endoscopist annual procedure volume and colonoscopy quality: Systematic review and meta-analysis. Nauzer Forbes et al. 2020 Sept:18(10):2192-208.e12. doi: 10.1016/j.cgh.2020.03.046
Plans to reactivate gastroenterology practices following the COVID-19 pandemic: A survey of North American centers. Vladimir M. Kushnir et al on Behalf of the North American Alliance for the Study of Digestive Manifestations of COVID-19. 2020 Sept;18(10):2287-94.e1. doi: 10.1016/j.cgh.2020.05.030
Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Eduardo Vilar-Gomez et al. 2020 Sept;18(10):2305-14.e12. doi: 10.1016/j.cgh.2020.04.017
October 2020
AGA Clinical Practice Update on young adult–onset colorectal cancer diagnosis and management: Expert review. Lisa A. Boardman et al. 2020 Oct:18(11):2415-24. doi: 10.1016/j.cgh.2020.05.058
Frequency of eating disorder pathology among patients with chronic constipation and contribution of gastrointestinal-specific anxiety. Helen Burton Murray et al. 2020 Oct;18(11):2471-8. doi: 10.1016/j.cgh.2019.12.030
Correction of dyssynergic defecation, but not fiber supplementation, reduces symptoms of functional dyspepsia in patients with constipation in a randomized trial. Jose-Walter Huaman et al. 2020 Oct;18(11):2463-70.e1. doi: 10.1016/j.cgh.2019.11.048
Cellular and Molecular Gastroenterology and Hepatology
A new treatment for chronic hepatitis B and D offers novel insights into obesity and hepatic steatosis. Robert Schierwagen et al. 2020;10(3):649-51. doi: 10.1016/j.jcmgh.2020.05.011
Techniques and Innovations in Gastrointestinal Endoscopy
The impact of endoscopic submucosal dissection for gastric adenocarcinomas in the United States. Shria Kumar et al. 2020 July:22(3):93-8. doi: 10.1016/j.tige.2020.03.009
Gastroenterology
July 2020
Role of cannabis and its derivatives in gastrointestinal and hepatic disease. Jonathan Gotfried et al. 2020 July;159(1):62-80. doi: 10.1053/j.gastro.2020.03.087
Effects of blended (yellow) vs forced coagulation (blue) currents on adverse events, complete resection, or polyp recurrence after polypectomy in a large randomized trial. Heiko Pohl et al. 2020 July;159(1):119-28.e2. doi: 10.1053/j.gastro.2020.03.014
Calculating the starting age for screening in relatives of patients with colorectal cancer based on data from large nationwide data sets.
Yu Tian et al. July 2020;159(1):159-168.e3. doi: 10.1053/j.gastro.2020.03.063
August 2020
Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Erica J. Brenner et al. 2020 Aug 159;(2):481-91.e3. doi: 10.1053/j.gastro.2020.05.032
Collagenous colitis is associated with HLA signature and shares genetic risks with other immune-mediated diseases. Eli Stahl et al. 2020 Aug;159(2):549-61.e8. doi: 10.1053/j.gastro.2020.04.063
Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Alessandro Repici et al. 2020 Aug;159(2):512-20.e7. doi: 10.1053/j.gastro.2020.04.062
September 2020
Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Chun-Han Lo et al. 2020 Sept;159(3):p873-83.e1. doi: 10.1053/j.gastro.2020.05.011
Rates of incomplete resection of 1- to 20-mm colorectal polyps: A systematic review and meta-analysis. Roupen Djinbachian et al. 2020 Sept;159(3):904-14.e12. doi: 10.1053/j.gastro.2020.05.018
Clinical Gastroenterology and Hepatology
August 2020
Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Helen Burton Murray et al. 2020 Aug;18(9):1995-2002.e1. doi: 10.1016/j.cgh.2019.10.030
Ten things every gastroenterologist should know about antireflux surgery. Steven Park et al. 2020 Aug;18(9):1923-9. doi: 10.1016/j.cgh.2020.02.041
Biopsies from ascending and descending colon are sufficient for diagnosis of microscopic colitis. Boris Virine et al. 2020 Aug;18(9):2003-9. doi: 10.1016/j.cgh.2020.02.036
September 2020
Association between endoscopist annual procedure volume and colonoscopy quality: Systematic review and meta-analysis. Nauzer Forbes et al. 2020 Sept:18(10):2192-208.e12. doi: 10.1016/j.cgh.2020.03.046
Plans to reactivate gastroenterology practices following the COVID-19 pandemic: A survey of North American centers. Vladimir M. Kushnir et al on Behalf of the North American Alliance for the Study of Digestive Manifestations of COVID-19. 2020 Sept;18(10):2287-94.e1. doi: 10.1016/j.cgh.2020.05.030
Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Eduardo Vilar-Gomez et al. 2020 Sept;18(10):2305-14.e12. doi: 10.1016/j.cgh.2020.04.017
October 2020
AGA Clinical Practice Update on young adult–onset colorectal cancer diagnosis and management: Expert review. Lisa A. Boardman et al. 2020 Oct:18(11):2415-24. doi: 10.1016/j.cgh.2020.05.058
Frequency of eating disorder pathology among patients with chronic constipation and contribution of gastrointestinal-specific anxiety. Helen Burton Murray et al. 2020 Oct;18(11):2471-8. doi: 10.1016/j.cgh.2019.12.030
Correction of dyssynergic defecation, but not fiber supplementation, reduces symptoms of functional dyspepsia in patients with constipation in a randomized trial. Jose-Walter Huaman et al. 2020 Oct;18(11):2463-70.e1. doi: 10.1016/j.cgh.2019.11.048
Cellular and Molecular Gastroenterology and Hepatology
A new treatment for chronic hepatitis B and D offers novel insights into obesity and hepatic steatosis. Robert Schierwagen et al. 2020;10(3):649-51. doi: 10.1016/j.jcmgh.2020.05.011
Techniques and Innovations in Gastrointestinal Endoscopy
The impact of endoscopic submucosal dissection for gastric adenocarcinomas in the United States. Shria Kumar et al. 2020 July:22(3):93-8. doi: 10.1016/j.tige.2020.03.009
Gastroenterology
July 2020
Role of cannabis and its derivatives in gastrointestinal and hepatic disease. Jonathan Gotfried et al. 2020 July;159(1):62-80. doi: 10.1053/j.gastro.2020.03.087
Effects of blended (yellow) vs forced coagulation (blue) currents on adverse events, complete resection, or polyp recurrence after polypectomy in a large randomized trial. Heiko Pohl et al. 2020 July;159(1):119-28.e2. doi: 10.1053/j.gastro.2020.03.014
Calculating the starting age for screening in relatives of patients with colorectal cancer based on data from large nationwide data sets.
Yu Tian et al. July 2020;159(1):159-168.e3. doi: 10.1053/j.gastro.2020.03.063
August 2020
Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Erica J. Brenner et al. 2020 Aug 159;(2):481-91.e3. doi: 10.1053/j.gastro.2020.05.032
Collagenous colitis is associated with HLA signature and shares genetic risks with other immune-mediated diseases. Eli Stahl et al. 2020 Aug;159(2):549-61.e8. doi: 10.1053/j.gastro.2020.04.063
Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Alessandro Repici et al. 2020 Aug;159(2):512-20.e7. doi: 10.1053/j.gastro.2020.04.062
September 2020
Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Chun-Han Lo et al. 2020 Sept;159(3):p873-83.e1. doi: 10.1053/j.gastro.2020.05.011
Rates of incomplete resection of 1- to 20-mm colorectal polyps: A systematic review and meta-analysis. Roupen Djinbachian et al. 2020 Sept;159(3):904-14.e12. doi: 10.1053/j.gastro.2020.05.018
Clinical Gastroenterology and Hepatology
August 2020
Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Helen Burton Murray et al. 2020 Aug;18(9):1995-2002.e1. doi: 10.1016/j.cgh.2019.10.030
Ten things every gastroenterologist should know about antireflux surgery. Steven Park et al. 2020 Aug;18(9):1923-9. doi: 10.1016/j.cgh.2020.02.041
Biopsies from ascending and descending colon are sufficient for diagnosis of microscopic colitis. Boris Virine et al. 2020 Aug;18(9):2003-9. doi: 10.1016/j.cgh.2020.02.036
September 2020
Association between endoscopist annual procedure volume and colonoscopy quality: Systematic review and meta-analysis. Nauzer Forbes et al. 2020 Sept:18(10):2192-208.e12. doi: 10.1016/j.cgh.2020.03.046
Plans to reactivate gastroenterology practices following the COVID-19 pandemic: A survey of North American centers. Vladimir M. Kushnir et al on Behalf of the North American Alliance for the Study of Digestive Manifestations of COVID-19. 2020 Sept;18(10):2287-94.e1. doi: 10.1016/j.cgh.2020.05.030
Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Eduardo Vilar-Gomez et al. 2020 Sept;18(10):2305-14.e12. doi: 10.1016/j.cgh.2020.04.017
October 2020
AGA Clinical Practice Update on young adult–onset colorectal cancer diagnosis and management: Expert review. Lisa A. Boardman et al. 2020 Oct:18(11):2415-24. doi: 10.1016/j.cgh.2020.05.058
Frequency of eating disorder pathology among patients with chronic constipation and contribution of gastrointestinal-specific anxiety. Helen Burton Murray et al. 2020 Oct;18(11):2471-8. doi: 10.1016/j.cgh.2019.12.030
Correction of dyssynergic defecation, but not fiber supplementation, reduces symptoms of functional dyspepsia in patients with constipation in a randomized trial. Jose-Walter Huaman et al. 2020 Oct;18(11):2463-70.e1. doi: 10.1016/j.cgh.2019.11.048
Cellular and Molecular Gastroenterology and Hepatology
A new treatment for chronic hepatitis B and D offers novel insights into obesity and hepatic steatosis. Robert Schierwagen et al. 2020;10(3):649-51. doi: 10.1016/j.jcmgh.2020.05.011
Techniques and Innovations in Gastrointestinal Endoscopy
The impact of endoscopic submucosal dissection for gastric adenocarcinomas in the United States. Shria Kumar et al. 2020 July:22(3):93-8. doi: 10.1016/j.tige.2020.03.009