User login
Mindful mentoring
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Medical professional liability risk and mitigation: An overview for early-career gastroenterologists
Disclaimer: This article is for educational purposes only. All examples are hypothetical and aim to illustrate common clinical scenarios and challenges gastroenterologists may encounter within their scope of practice. The content herein should not be interpreted as legal advice for individual cases nor a substitute for seeking the advice of an attorney.
There are unique potential stressors faced by the gastroenterologist at each career stage, some more so early on. One such stressor, and one particularly important in a procedure-intensive specialty like GI, is medical professional liability (MPL), historically termed “medical malpractice.” Between 2009 and 2018, GI was the second-highest internal medicine subspecialty in both MPL claims made and claims paid,1 yet instruction on MPL risk and mitigation is scarce in fellowship, as is the available GI-related literature on the topic. This scarcity may generate untoward stress and unnecessarily expose gastroenterologists to avoidable MPL pitfalls. Therefore, it is vital for GI trainees, early-career gastroenterologists, and even seasoned gastroenterologists to have a working and updated knowledge of the general principles of MPL and GI-specific considerations. Such understanding can help preserve physician well-being, increase professional satisfaction, strengthen the doctor-patient relationship, and improve health care outcomes.2
To this end, we herein provide a focused review of the following: key MPL concepts, trends in MPL claims, GI-related MPL risk scenarios and considerations, adverse provider defensive mechanisms, documentation tenets, challenges posed by telemedicine, and the concept of “vicarious liability.”
Key MPL concepts
MPL falls under the umbrella of tort law, which itself falls under the umbrella of civil law; that is, civil (as opposed to criminal) justice governs torts – including but not limited to MPL claims – as well as other areas of law concerning noncriminal injury.3 A “tort” is a “civil wrong that unfairly causes another to experience loss or harm resulting in legal liability.”3 MPL claims assert the tort of negligence (similar to the concept of “incompetence”) and endeavor to compensate the harmed patient/individual while simultaneously dissuading suboptimal medical care by the provider in the future.4,5 A successful MPL claim must prove four overlapping elements: that the tortfeasor (here, the gastroenterologist) owed a duty of care to the injured party and breached that duty, which caused damages.6 Given that MPL cases exist within tort law rather than criminal law, the burden of proof for these cases is not “beyond a reasonable doubt”; instead, it’s “to a reasonable medical probability.”7
Trends in MPL claims
According to data compiled by the MPL Association, 278,220 MPL claims were made in the United States from 1985 to 2012.3,8-10 Among these, 1.8% involved gastroenterologists, which puts it at 17th place out of the 20 specialties surveyed.9 While the number of paid claims over this time frame decreased in GI by 34.6% (from 18.5 to 12.1 cases per 1,000 physician-years), there was a concurrent 23.3% increase in average claim compensation; essentially, there were fewer paid GI-related claims but there were higher payouts per paid claim.11,12 From 2009 to 2018, average legal defense costs for paid GI-related claims were $97,392, and average paid amount was $330,876.1
GI-related MPL risk scenarios and considerations
Many MPL claims relate to situations involving medical errors or adverse events (AEs), be they procedural or nonprocedural. However other aspects of GI also carry MPL risk.
Informed consent
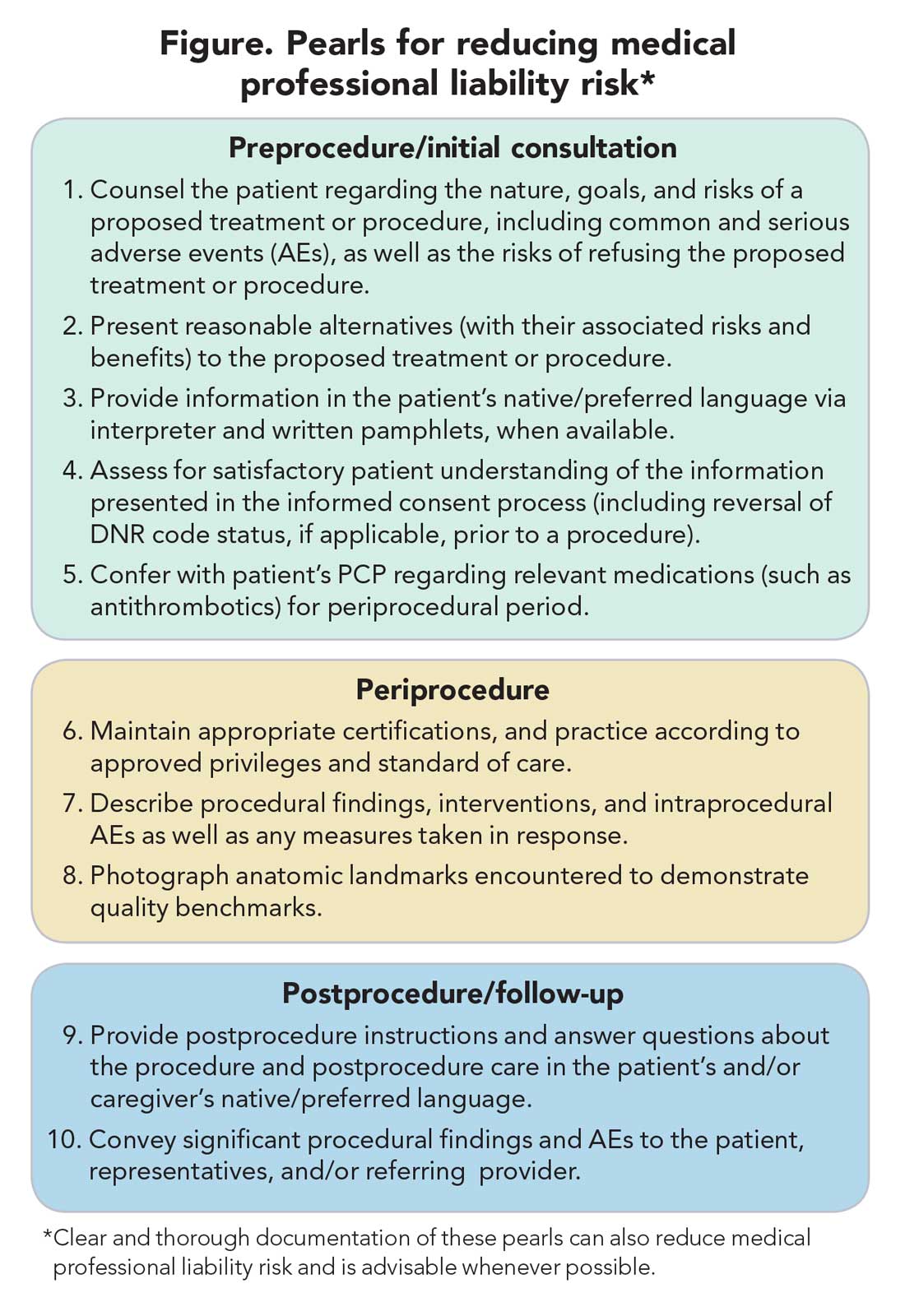
MPL claims may be made not only on the grounds of inadequately informed consent but also inadequately informed refusal.5,13,14 While standards for adequate informed consent vary by state, most states apply the “reasonable patient standard,” i.e., assuming an average patient with enough information to be an active participant in the medical decision-making process. Generally, informed consent should ensure that the patient understands the nature of the procedure/treatment being proposed, there is a discussion of the risks and benefits of undergoing and not undergoing the procedure/treatment, reasonable alternatives are presented, the risks and benefits associated with these alternatives are discussed, and the patient’s comprehension of these things is assessed (Figure).15 Additionally, informed consent should be tailored to each patient and GI procedure/treatment on a case-by-case basis rather than using a one-size-fits-all approach. Moreover, documentation of the patient’s understanding of the (tailored) information provided can concurrently improve quality of the consent and potentially decrease MPL risk (Figure).16
Endoscopic procedures
Procedure-related MPL claims represent approximately 25% of all GI-related claims (8,17). Among these, 52% involve colonoscopy, 16% involve endoscopic retrograde cholangiopancreatography (ERCP), and 11% involve esophagogastroduodenoscopy.8 Albeit generally safe, colonoscopy, as with esophagogastroduodenoscopy, is subject to rare but serious AEs.18,19 Risk of these AEs may be accentuated in certain scenarios (such as severe colonic inflammation or coagulopathy) and, as discussed earlier, may merit tailored informed consent. Regardless of the procedure, in the event of postprocedural development of signs/symptoms (such as tachycardia, fever, chest or abdominal discomfort, or hypotension) indicating a potential AE, stabilizing measures and evaluation (such as blood work and imaging) should be undertaken, and hospital admission (if not already hospitalized) should be considered until discharge is deemed safe.19
ERCP-related MPL claims, for many years, have had the highest average compensation of any GI procedure.11 Though discussion of advanced procedures is beyond the scope of this article, it is worth mentioning the observation that most of such claims involve an allegation that the procedure was not indicated (for example, that it was performed based on inadequate evidence of pancreatobiliary pathology), or was for diagnostic purposes (for example, being done instead of noninvasive imaging) rather than therapeutic.20-23 This emphasizes the importance of appropriate procedure indications.
Percutaneous endoscopic gastrostomy (PEG) placement merits special mention given it can be complicated by ethical challenges (for example, needing a surrogate decision-maker’s consent or representing medical futility) and has a relatively high potential for MPL claims. PEG placement carries a low AE rate (0.1%-1%), but these AEs may result in high morbidity/mortality, in part because of the underlying comorbidities of patients needing PEG placement.24,25 Also, timing of a patient’s demise may coincide with PEG placement, thereby prompting (possibly unfounded) perceptions of causality.24-27 Therefore, such scenarios merit unique additional preprocedure safeguards. For instance, for patients lacking capacity to provide informed consent, especially when family members may differ on whether PEG should be placed, it is advisable to ask the family to select one surrogate decision-maker (if there’s no advance directive) to whom the gastroenterologist should discuss both the risks, benefits, and goals of PEG placement in the context of the patient’s overall clinical trajectory/life expectancy and the need for consent (or refusal) based on what the patient would have wished. In addition, having a medical professional witness this discussion may be useful.27
Antithrombotic agents
Periprocedural management of antithrombotics, including anticoagulants and antiplatelets, can pose challenges for the gastroenterologist. While clinical practice guidelines exist to guide decision-making in this regard, the variables involved may extend beyond the expertise of the gastroenterologist.28 For instance, in addition to the procedural risk for bleeding, the indication for antithrombotic therapy, risk of a thrombotic event, duration of action of the antithrombotic, and available bridging options should all be considered according to recommendations.28,29 While requiring more time on the part of the gastroenterologist, the optimal periprocedural management of antithrombotic agents would usually involve discussion with the provider managing antithrombotic therapy to best conduct a risk-benefit assessment regarding if (and how long) the antithrombotic therapy should be held (Figure). This shared decision-making, which should also include the patient, may help decrease MPL risk and improve outcomes.
Provider defense mechanisms
Physicians may engage in various defensive behaviors in an attempt to mitigate MPL risk; however, these behaviors may, paradoxically, increase risk.30,31
Assurance behaviors
Assurance behaviors refer to the practice of recommending or performing additional services (such as medications, imaging, procedures, and referrals) that are not clearly indicated.2,30,31 Assurance behaviors are driven by fear of MPL risk and/or missing a potential diagnosis. Recent studies have estimated that more than 50% of gastroenterologists worldwide have performed additional invasive procedures without clear indications, and that nearly one-third of endoscopic procedures annually have questionable indications.30,32 While assurance behaviors may seem likely to decrease MPL risk, overall, they may inadvertently increase AE and MPL risk, as well as health care expenditures.3,30,32
Avoidance behaviors
Avoidance behaviors refer to providers avoiding participation in potentially high-risk clinical interventions (for example, the actual procedures), including those for which they are credentialed/certified proficient.30,31 Two clinical scenarios that illustrate this behavior include the following: An advanced endoscopist credentialed to perform ERCP might refer a “high-risk” elderly patient with cholangitis to another provider to perform said ERCP or for percutaneous transhepatic drainage (in the absence of a clear benefit to such), or a gastroenterologist might refer a patient to interventional gastroenterology for resection of a large polyp even though gastroenterologists are usually proficient in this skill and may feel comfortable performing the resection themselves. Avoidance behaviors are driven by a fear of MPL risk and can have several negative consequences.33 For example, patients may not receive indicated interventions. Additionally, patients may have to wait longer for an intervention because they are referred to another provider, which also increases potential for loss to follow-up.2,30,31 This may be viewed as noncompliance with the standard of care, among other hazards, thereby increasing MPL risk.
Documentation tenets
Thorough documentation can decrease MPL risk, especially since it is often used as legal evidence.16 Documenting, for instance, preprocedure discussion of potential risk of AEs (such as bleeding or perforation) or procedural failure (for example, missed lesions)can protect gastroenterologists (Figure).16 While, as discussed previously, these should be covered in the informed consent process (which itself reduces MPL risk), proof of compliance in providing adequate informed consent must come in the form of documentation that indicates that the process took place and specifically what topics were discussed therein. MPL risk may be further decreased by documenting steps taken during a procedure and anatomic landmarks encountered to offer proof of technical competency and compliance with standards of care (Figure).16,34 In this context, it is worth recalling the adage: “If it’s not documented, it did not occur.”
Curbside consults versus consultation
Also germane here is the topic of whether documentation is needed for “curbside consults.” The uncertainty is, in part, semantic; that is, at what point does a “curbside” become a consultation? A curbside is a general question or query (such as anything that could also be answered by searching the Internet or reference materials) in response to which information is provided; once it involves provision of medical advice for a specific patient (for example, when patient identifiers have been shared or their EHR has been accessed), it constitutes a consultation. Based on these definitions, a curbside need not be documented, whereas a consultation – even if seemingly trivial – should be.
Consideration of language and cultural factors
Language barriers should be considered when the gastroenterologist is communicating with the patient, and such efforts, whenever made, should be documented to best protect against MPL.16,35 These considerations arise not only during the consent process but when obtaining a history, providing postprocedure instructions, and during follow-ups. To this end, 24/7 telephone interpreter services may assist the gastroenterologist (when one is communicating with non–English speakers and is not medically certified in the patient’s native/preferred language) and strengthen trust in the provider-patient relationship.36 Additionally, written materials (such as consent forms, procedural information) in patients’ native/preferred languages should be provided, when available, to enhance patient understanding and participation in care (Figure).35
Challenges posed by telemedicine
The COVID-19 pandemic has rapidly led to more virtual encounters. While increased utilization of telemedicine platforms may make health care more accessible, it does not lessen the clinicians’ duty to patients and may actually expose them to greater MPL risk.18,37,38 Therefore, the provider must be cognizant of two key principles to mitigate MPL risk in the context of telemedicine encounters. First, the same standard of care applies to virtual and in-person encounters.18,37,38 Second, patient privacy and HIPAA regulations are not waived during telemedicine encounters, and breaches of such may result in an MPL claim.18,37,38
With regard to the first principle, for patients who have not been physically examined (such as when a telemedicine visit was substituted for an in-person clinic encounter), gastroenterologists should not overlook requesting timely preprocedure anesthesia consultation or obtaining additional laboratory studies as needed to ensure safety and the same standard of care. Moreover, particularly in the context of pandemic-related decreased procedural capacity, triaging procedures can be especially challenging. Standardized institutional criteria which prioritize certain diagnoses/conditions over others, leaving room for justifiable exceptions, are advisable.
Vicarious liability
“Vicarious liability” is defined as that extending to persons who have not committed a wrong but on whose behalf wrongdoers acted.39 Therefore, gastroenterologists may be liable not only for their own actions but also for those of personnel they supervise (such as fellow trainees and non–physician practitioners).39 Vicarious liability aims to ensure that systemic checks and balances are in place so that, if failure occurs, harm can still be mitigated and/or avoided, as illustrated by Reason’s “Swiss Cheese Model.”40
Conclusion
Any gastroenterologist can experience an MPL claim. Such an experience can be especially stressful and confusing to early-career clinicians, especially if they’re unfamiliar with legal proceedings. Although MPL principles are not often taught in medical school or residency, it is important for gastroenterologists to be informed regarding tenets of MPL and cognizant of clinical situations which have relatively higher MPL risk. This can assuage untoward angst regarding MPL and highlight proactive risk-mitigation strategies. In general, gastroenterologist practices that can mitigate MPL risk include effective communication; adequate informed consent/refusal; documentation of preprocedure counseling, periprocedure events, and postprocedure recommendations; and maintenance of proper certification and privileging.
Dr. Azizian and Dr. Dalai are with the University of California, Los Angeles and the department of medicine at Olive View–UCLA Medical Center, Sylmar, Calif. They are co–first authors of this paper. Dr. Dalai is also with the division of gastroenterology at the University of New Mexico, Albuquerque. Dr. Adams is with the Center for Clinical Management Research in Veterans Affairs Ann Arbor Healthcare System, the division of gastroenterology at the University of Michigan Health System, and the Institute for Healthcare Policy and Innovation, all in Ann Arbor, Mich. Dr. Tabibian is with UCLA and the division of gastroenterology at Olive View–UCLA Medical Center. The authors have no conflicts of interest.
References
1. 2020 Data Sharing Project Gastroenterology 2009-2018. Inside Medical Liability: Second Quarter. Accessed 2020 Dec 6.
2. Mello MM et al. Health Aff (Millwood). 2004 Jul-Aug;23(4):42-53.
3. Adams MA et al. JAMA. 2014 Oct;312(13):1348-9.
4. Pegalis SE. American Law of Medical Malpractice 3d, Vol. 2. St. Paul, Minn.: Thomson Reuters, 2005.
5. Feld LD et al. Am J Gastroenterol. 2018 Nov;113(11):1577-9.
6. Sawyer v. Wight, 196 F. Supp. 2d 220, 226 (E.D.N.Y. 2002).
7. Michael A. Sita v. Long Island Jewish-Hillside Medical Center, 22 A.D.3d 743 (N.Y. App. Div. 2005).
8. Conklin LS et al. Clin Gastroenterol Hepatol. 2008 Jun;6(6):677-81.
9. Jena AB et al. N Engl J Med. 2011 Aug 18;365(7):629-36.
10. Kane CK. “Policy Research Perspectives Medical Liability Claim Frequency: A 2007-2008 Snapshot of Physicians.” Chicago: American Medical Association, 2010.
11. Hernandez LV et al. World J Gastrointest Endosc. 2013 Apr 16;5(4):169-73.
12. Schaffer AC et al. JAMA Intern Med. 2017 May 1;177(5):710-8.
13. Natanson v. Kline, 186 Kan. 393, 409, 350 P.2d 1093, 1106, decision clarified on denial of reh’g, 187 Kan. 186, 354 P.2d 670 (1960).
14. Truman v. Thomas, 27 Cal. 3d 285, 292, 611 P.2d 902, 906 (1980).
15. Shah P et al. Informed Consent, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Aug 22.
16. Rex DK. Clin Gastroenterol Hepatol. 2013 Jul;11(7):768-73.
17. Gerstenberger PD, Plumeri PA. Gastrointest Endosc. Mar-Apr 1993;39(2):132-8.
18. Adams MA and Allen JI. Clin Gastroenterol Hepatol. 2019 Nov;17(12):2392-6.e1.
19. Ahlawat R et al. Esophagogastroduodenoscopy, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Dec 9.
20. Cotton PB. Gastrointest Endosc. 2006 Mar;63(3):378-82.
21. Cotton PB. Gastrointest Endosc. 2010 Oct;72(4):904.
22. Adamson TE et al. West J Med. 1989 Mar;150(3):356-60.
23. Trap R et al. Endoscopy. 1999 Feb;31(2):125-30.
24. Funaki B. Semin Intervent Radiol. 2015 Mar;32(1):61-4.
25. Feeding Tube Nursing Home and Hospital Malpractice. Miller & Zois, Attorneys at Law. Accessed 2020 Jun 20.
26. Medical Malpractice Lawsuit Brings $750,000 Settlement: Death of 82-year-old woman from sepsis due to improper placement of feeding tube. Lubin & Meyers PC. Accessed 2020 Jun 20.
27. Brendel RW et al. Med Clin North Am. 2010 Nov;94(6):1229-40, xi-ii.
28. ASGE Standards of Practice Committee; Acosta RD et al. Gastrointest Endosc. 2016 Jan;83(1):3-16.
29. Saleem S and Thomas AL. Cureus. 2018 Jun 25;10(6):e2878.
30. Hiyama T et al. World J Gastroenterol. 2006 Dec 21;12(47):7671-5.
31. Studdert DM et al. JAMA. 2005 Jun 1;293(21):2609-17.
32. Shaheen NJ et al. Gastroenterology. 2018 May;154(7):1993-2003.
33. Oza VM et al. Clin Gastroenterol Hepatol. 2016 Feb;14(2):172-4.
34. Feld AD. Gastrointest Endosc Clin N Am. 2002 Jan;12(1):171-9, viii-ix.
35. Lee JS et al. J Gen Intern Med. 2017 Aug;32(8):863-70.
36. Forrow L and Kontrimas JC. J Gen Intern Med. 2017 Aug;32(8):855-7.
37. Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
38. Tabibian JH. “The Evolution of Telehealth.” Guidepoint: Legal Solutions Blog. Accessed 2020 Aug 12.
39. Feld AD. Am J Gastroenterol. 2004 Sep;99(9):1641-4.
40. Reason J. BMJ. 2000;320(7237):768‐70.
Disclaimer: This article is for educational purposes only. All examples are hypothetical and aim to illustrate common clinical scenarios and challenges gastroenterologists may encounter within their scope of practice. The content herein should not be interpreted as legal advice for individual cases nor a substitute for seeking the advice of an attorney.
There are unique potential stressors faced by the gastroenterologist at each career stage, some more so early on. One such stressor, and one particularly important in a procedure-intensive specialty like GI, is medical professional liability (MPL), historically termed “medical malpractice.” Between 2009 and 2018, GI was the second-highest internal medicine subspecialty in both MPL claims made and claims paid,1 yet instruction on MPL risk and mitigation is scarce in fellowship, as is the available GI-related literature on the topic. This scarcity may generate untoward stress and unnecessarily expose gastroenterologists to avoidable MPL pitfalls. Therefore, it is vital for GI trainees, early-career gastroenterologists, and even seasoned gastroenterologists to have a working and updated knowledge of the general principles of MPL and GI-specific considerations. Such understanding can help preserve physician well-being, increase professional satisfaction, strengthen the doctor-patient relationship, and improve health care outcomes.2
To this end, we herein provide a focused review of the following: key MPL concepts, trends in MPL claims, GI-related MPL risk scenarios and considerations, adverse provider defensive mechanisms, documentation tenets, challenges posed by telemedicine, and the concept of “vicarious liability.”
Key MPL concepts
MPL falls under the umbrella of tort law, which itself falls under the umbrella of civil law; that is, civil (as opposed to criminal) justice governs torts – including but not limited to MPL claims – as well as other areas of law concerning noncriminal injury.3 A “tort” is a “civil wrong that unfairly causes another to experience loss or harm resulting in legal liability.”3 MPL claims assert the tort of negligence (similar to the concept of “incompetence”) and endeavor to compensate the harmed patient/individual while simultaneously dissuading suboptimal medical care by the provider in the future.4,5 A successful MPL claim must prove four overlapping elements: that the tortfeasor (here, the gastroenterologist) owed a duty of care to the injured party and breached that duty, which caused damages.6 Given that MPL cases exist within tort law rather than criminal law, the burden of proof for these cases is not “beyond a reasonable doubt”; instead, it’s “to a reasonable medical probability.”7
Trends in MPL claims
According to data compiled by the MPL Association, 278,220 MPL claims were made in the United States from 1985 to 2012.3,8-10 Among these, 1.8% involved gastroenterologists, which puts it at 17th place out of the 20 specialties surveyed.9 While the number of paid claims over this time frame decreased in GI by 34.6% (from 18.5 to 12.1 cases per 1,000 physician-years), there was a concurrent 23.3% increase in average claim compensation; essentially, there were fewer paid GI-related claims but there were higher payouts per paid claim.11,12 From 2009 to 2018, average legal defense costs for paid GI-related claims were $97,392, and average paid amount was $330,876.1
GI-related MPL risk scenarios and considerations
Many MPL claims relate to situations involving medical errors or adverse events (AEs), be they procedural or nonprocedural. However other aspects of GI also carry MPL risk.

Informed consent
MPL claims may be made not only on the grounds of inadequately informed consent but also inadequately informed refusal.5,13,14 While standards for adequate informed consent vary by state, most states apply the “reasonable patient standard,” i.e., assuming an average patient with enough information to be an active participant in the medical decision-making process. Generally, informed consent should ensure that the patient understands the nature of the procedure/treatment being proposed, there is a discussion of the risks and benefits of undergoing and not undergoing the procedure/treatment, reasonable alternatives are presented, the risks and benefits associated with these alternatives are discussed, and the patient’s comprehension of these things is assessed (Figure).15 Additionally, informed consent should be tailored to each patient and GI procedure/treatment on a case-by-case basis rather than using a one-size-fits-all approach. Moreover, documentation of the patient’s understanding of the (tailored) information provided can concurrently improve quality of the consent and potentially decrease MPL risk (Figure).16
Endoscopic procedures
Procedure-related MPL claims represent approximately 25% of all GI-related claims (8,17). Among these, 52% involve colonoscopy, 16% involve endoscopic retrograde cholangiopancreatography (ERCP), and 11% involve esophagogastroduodenoscopy.8 Albeit generally safe, colonoscopy, as with esophagogastroduodenoscopy, is subject to rare but serious AEs.18,19 Risk of these AEs may be accentuated in certain scenarios (such as severe colonic inflammation or coagulopathy) and, as discussed earlier, may merit tailored informed consent. Regardless of the procedure, in the event of postprocedural development of signs/symptoms (such as tachycardia, fever, chest or abdominal discomfort, or hypotension) indicating a potential AE, stabilizing measures and evaluation (such as blood work and imaging) should be undertaken, and hospital admission (if not already hospitalized) should be considered until discharge is deemed safe.19
ERCP-related MPL claims, for many years, have had the highest average compensation of any GI procedure.11 Though discussion of advanced procedures is beyond the scope of this article, it is worth mentioning the observation that most of such claims involve an allegation that the procedure was not indicated (for example, that it was performed based on inadequate evidence of pancreatobiliary pathology), or was for diagnostic purposes (for example, being done instead of noninvasive imaging) rather than therapeutic.20-23 This emphasizes the importance of appropriate procedure indications.
Percutaneous endoscopic gastrostomy (PEG) placement merits special mention given it can be complicated by ethical challenges (for example, needing a surrogate decision-maker’s consent or representing medical futility) and has a relatively high potential for MPL claims. PEG placement carries a low AE rate (0.1%-1%), but these AEs may result in high morbidity/mortality, in part because of the underlying comorbidities of patients needing PEG placement.24,25 Also, timing of a patient’s demise may coincide with PEG placement, thereby prompting (possibly unfounded) perceptions of causality.24-27 Therefore, such scenarios merit unique additional preprocedure safeguards. For instance, for patients lacking capacity to provide informed consent, especially when family members may differ on whether PEG should be placed, it is advisable to ask the family to select one surrogate decision-maker (if there’s no advance directive) to whom the gastroenterologist should discuss both the risks, benefits, and goals of PEG placement in the context of the patient’s overall clinical trajectory/life expectancy and the need for consent (or refusal) based on what the patient would have wished. In addition, having a medical professional witness this discussion may be useful.27
Antithrombotic agents
Periprocedural management of antithrombotics, including anticoagulants and antiplatelets, can pose challenges for the gastroenterologist. While clinical practice guidelines exist to guide decision-making in this regard, the variables involved may extend beyond the expertise of the gastroenterologist.28 For instance, in addition to the procedural risk for bleeding, the indication for antithrombotic therapy, risk of a thrombotic event, duration of action of the antithrombotic, and available bridging options should all be considered according to recommendations.28,29 While requiring more time on the part of the gastroenterologist, the optimal periprocedural management of antithrombotic agents would usually involve discussion with the provider managing antithrombotic therapy to best conduct a risk-benefit assessment regarding if (and how long) the antithrombotic therapy should be held (Figure). This shared decision-making, which should also include the patient, may help decrease MPL risk and improve outcomes.
Provider defense mechanisms
Physicians may engage in various defensive behaviors in an attempt to mitigate MPL risk; however, these behaviors may, paradoxically, increase risk.30,31
Assurance behaviors
Assurance behaviors refer to the practice of recommending or performing additional services (such as medications, imaging, procedures, and referrals) that are not clearly indicated.2,30,31 Assurance behaviors are driven by fear of MPL risk and/or missing a potential diagnosis. Recent studies have estimated that more than 50% of gastroenterologists worldwide have performed additional invasive procedures without clear indications, and that nearly one-third of endoscopic procedures annually have questionable indications.30,32 While assurance behaviors may seem likely to decrease MPL risk, overall, they may inadvertently increase AE and MPL risk, as well as health care expenditures.3,30,32
Avoidance behaviors
Avoidance behaviors refer to providers avoiding participation in potentially high-risk clinical interventions (for example, the actual procedures), including those for which they are credentialed/certified proficient.30,31 Two clinical scenarios that illustrate this behavior include the following: An advanced endoscopist credentialed to perform ERCP might refer a “high-risk” elderly patient with cholangitis to another provider to perform said ERCP or for percutaneous transhepatic drainage (in the absence of a clear benefit to such), or a gastroenterologist might refer a patient to interventional gastroenterology for resection of a large polyp even though gastroenterologists are usually proficient in this skill and may feel comfortable performing the resection themselves. Avoidance behaviors are driven by a fear of MPL risk and can have several negative consequences.33 For example, patients may not receive indicated interventions. Additionally, patients may have to wait longer for an intervention because they are referred to another provider, which also increases potential for loss to follow-up.2,30,31 This may be viewed as noncompliance with the standard of care, among other hazards, thereby increasing MPL risk.
Documentation tenets
Thorough documentation can decrease MPL risk, especially since it is often used as legal evidence.16 Documenting, for instance, preprocedure discussion of potential risk of AEs (such as bleeding or perforation) or procedural failure (for example, missed lesions)can protect gastroenterologists (Figure).16 While, as discussed previously, these should be covered in the informed consent process (which itself reduces MPL risk), proof of compliance in providing adequate informed consent must come in the form of documentation that indicates that the process took place and specifically what topics were discussed therein. MPL risk may be further decreased by documenting steps taken during a procedure and anatomic landmarks encountered to offer proof of technical competency and compliance with standards of care (Figure).16,34 In this context, it is worth recalling the adage: “If it’s not documented, it did not occur.”
Curbside consults versus consultation
Also germane here is the topic of whether documentation is needed for “curbside consults.” The uncertainty is, in part, semantic; that is, at what point does a “curbside” become a consultation? A curbside is a general question or query (such as anything that could also be answered by searching the Internet or reference materials) in response to which information is provided; once it involves provision of medical advice for a specific patient (for example, when patient identifiers have been shared or their EHR has been accessed), it constitutes a consultation. Based on these definitions, a curbside need not be documented, whereas a consultation – even if seemingly trivial – should be.
Consideration of language and cultural factors
Language barriers should be considered when the gastroenterologist is communicating with the patient, and such efforts, whenever made, should be documented to best protect against MPL.16,35 These considerations arise not only during the consent process but when obtaining a history, providing postprocedure instructions, and during follow-ups. To this end, 24/7 telephone interpreter services may assist the gastroenterologist (when one is communicating with non–English speakers and is not medically certified in the patient’s native/preferred language) and strengthen trust in the provider-patient relationship.36 Additionally, written materials (such as consent forms, procedural information) in patients’ native/preferred languages should be provided, when available, to enhance patient understanding and participation in care (Figure).35
Challenges posed by telemedicine
The COVID-19 pandemic has rapidly led to more virtual encounters. While increased utilization of telemedicine platforms may make health care more accessible, it does not lessen the clinicians’ duty to patients and may actually expose them to greater MPL risk.18,37,38 Therefore, the provider must be cognizant of two key principles to mitigate MPL risk in the context of telemedicine encounters. First, the same standard of care applies to virtual and in-person encounters.18,37,38 Second, patient privacy and HIPAA regulations are not waived during telemedicine encounters, and breaches of such may result in an MPL claim.18,37,38
With regard to the first principle, for patients who have not been physically examined (such as when a telemedicine visit was substituted for an in-person clinic encounter), gastroenterologists should not overlook requesting timely preprocedure anesthesia consultation or obtaining additional laboratory studies as needed to ensure safety and the same standard of care. Moreover, particularly in the context of pandemic-related decreased procedural capacity, triaging procedures can be especially challenging. Standardized institutional criteria which prioritize certain diagnoses/conditions over others, leaving room for justifiable exceptions, are advisable.
Vicarious liability
“Vicarious liability” is defined as that extending to persons who have not committed a wrong but on whose behalf wrongdoers acted.39 Therefore, gastroenterologists may be liable not only for their own actions but also for those of personnel they supervise (such as fellow trainees and non–physician practitioners).39 Vicarious liability aims to ensure that systemic checks and balances are in place so that, if failure occurs, harm can still be mitigated and/or avoided, as illustrated by Reason’s “Swiss Cheese Model.”40
Conclusion
Any gastroenterologist can experience an MPL claim. Such an experience can be especially stressful and confusing to early-career clinicians, especially if they’re unfamiliar with legal proceedings. Although MPL principles are not often taught in medical school or residency, it is important for gastroenterologists to be informed regarding tenets of MPL and cognizant of clinical situations which have relatively higher MPL risk. This can assuage untoward angst regarding MPL and highlight proactive risk-mitigation strategies. In general, gastroenterologist practices that can mitigate MPL risk include effective communication; adequate informed consent/refusal; documentation of preprocedure counseling, periprocedure events, and postprocedure recommendations; and maintenance of proper certification and privileging.
Dr. Azizian and Dr. Dalai are with the University of California, Los Angeles and the department of medicine at Olive View–UCLA Medical Center, Sylmar, Calif. They are co–first authors of this paper. Dr. Dalai is also with the division of gastroenterology at the University of New Mexico, Albuquerque. Dr. Adams is with the Center for Clinical Management Research in Veterans Affairs Ann Arbor Healthcare System, the division of gastroenterology at the University of Michigan Health System, and the Institute for Healthcare Policy and Innovation, all in Ann Arbor, Mich. Dr. Tabibian is with UCLA and the division of gastroenterology at Olive View–UCLA Medical Center. The authors have no conflicts of interest.
References
1. 2020 Data Sharing Project Gastroenterology 2009-2018. Inside Medical Liability: Second Quarter. Accessed 2020 Dec 6.
2. Mello MM et al. Health Aff (Millwood). 2004 Jul-Aug;23(4):42-53.
3. Adams MA et al. JAMA. 2014 Oct;312(13):1348-9.
4. Pegalis SE. American Law of Medical Malpractice 3d, Vol. 2. St. Paul, Minn.: Thomson Reuters, 2005.
5. Feld LD et al. Am J Gastroenterol. 2018 Nov;113(11):1577-9.
6. Sawyer v. Wight, 196 F. Supp. 2d 220, 226 (E.D.N.Y. 2002).
7. Michael A. Sita v. Long Island Jewish-Hillside Medical Center, 22 A.D.3d 743 (N.Y. App. Div. 2005).
8. Conklin LS et al. Clin Gastroenterol Hepatol. 2008 Jun;6(6):677-81.
9. Jena AB et al. N Engl J Med. 2011 Aug 18;365(7):629-36.
10. Kane CK. “Policy Research Perspectives Medical Liability Claim Frequency: A 2007-2008 Snapshot of Physicians.” Chicago: American Medical Association, 2010.
11. Hernandez LV et al. World J Gastrointest Endosc. 2013 Apr 16;5(4):169-73.
12. Schaffer AC et al. JAMA Intern Med. 2017 May 1;177(5):710-8.
13. Natanson v. Kline, 186 Kan. 393, 409, 350 P.2d 1093, 1106, decision clarified on denial of reh’g, 187 Kan. 186, 354 P.2d 670 (1960).
14. Truman v. Thomas, 27 Cal. 3d 285, 292, 611 P.2d 902, 906 (1980).
15. Shah P et al. Informed Consent, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Aug 22.
16. Rex DK. Clin Gastroenterol Hepatol. 2013 Jul;11(7):768-73.
17. Gerstenberger PD, Plumeri PA. Gastrointest Endosc. Mar-Apr 1993;39(2):132-8.
18. Adams MA and Allen JI. Clin Gastroenterol Hepatol. 2019 Nov;17(12):2392-6.e1.
19. Ahlawat R et al. Esophagogastroduodenoscopy, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Dec 9.
20. Cotton PB. Gastrointest Endosc. 2006 Mar;63(3):378-82.
21. Cotton PB. Gastrointest Endosc. 2010 Oct;72(4):904.
22. Adamson TE et al. West J Med. 1989 Mar;150(3):356-60.
23. Trap R et al. Endoscopy. 1999 Feb;31(2):125-30.
24. Funaki B. Semin Intervent Radiol. 2015 Mar;32(1):61-4.
25. Feeding Tube Nursing Home and Hospital Malpractice. Miller & Zois, Attorneys at Law. Accessed 2020 Jun 20.
26. Medical Malpractice Lawsuit Brings $750,000 Settlement: Death of 82-year-old woman from sepsis due to improper placement of feeding tube. Lubin & Meyers PC. Accessed 2020 Jun 20.
27. Brendel RW et al. Med Clin North Am. 2010 Nov;94(6):1229-40, xi-ii.
28. ASGE Standards of Practice Committee; Acosta RD et al. Gastrointest Endosc. 2016 Jan;83(1):3-16.
29. Saleem S and Thomas AL. Cureus. 2018 Jun 25;10(6):e2878.
30. Hiyama T et al. World J Gastroenterol. 2006 Dec 21;12(47):7671-5.
31. Studdert DM et al. JAMA. 2005 Jun 1;293(21):2609-17.
32. Shaheen NJ et al. Gastroenterology. 2018 May;154(7):1993-2003.
33. Oza VM et al. Clin Gastroenterol Hepatol. 2016 Feb;14(2):172-4.
34. Feld AD. Gastrointest Endosc Clin N Am. 2002 Jan;12(1):171-9, viii-ix.
35. Lee JS et al. J Gen Intern Med. 2017 Aug;32(8):863-70.
36. Forrow L and Kontrimas JC. J Gen Intern Med. 2017 Aug;32(8):855-7.
37. Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
38. Tabibian JH. “The Evolution of Telehealth.” Guidepoint: Legal Solutions Blog. Accessed 2020 Aug 12.
39. Feld AD. Am J Gastroenterol. 2004 Sep;99(9):1641-4.
40. Reason J. BMJ. 2000;320(7237):768‐70.
Disclaimer: This article is for educational purposes only. All examples are hypothetical and aim to illustrate common clinical scenarios and challenges gastroenterologists may encounter within their scope of practice. The content herein should not be interpreted as legal advice for individual cases nor a substitute for seeking the advice of an attorney.
There are unique potential stressors faced by the gastroenterologist at each career stage, some more so early on. One such stressor, and one particularly important in a procedure-intensive specialty like GI, is medical professional liability (MPL), historically termed “medical malpractice.” Between 2009 and 2018, GI was the second-highest internal medicine subspecialty in both MPL claims made and claims paid,1 yet instruction on MPL risk and mitigation is scarce in fellowship, as is the available GI-related literature on the topic. This scarcity may generate untoward stress and unnecessarily expose gastroenterologists to avoidable MPL pitfalls. Therefore, it is vital for GI trainees, early-career gastroenterologists, and even seasoned gastroenterologists to have a working and updated knowledge of the general principles of MPL and GI-specific considerations. Such understanding can help preserve physician well-being, increase professional satisfaction, strengthen the doctor-patient relationship, and improve health care outcomes.2
To this end, we herein provide a focused review of the following: key MPL concepts, trends in MPL claims, GI-related MPL risk scenarios and considerations, adverse provider defensive mechanisms, documentation tenets, challenges posed by telemedicine, and the concept of “vicarious liability.”
Key MPL concepts
MPL falls under the umbrella of tort law, which itself falls under the umbrella of civil law; that is, civil (as opposed to criminal) justice governs torts – including but not limited to MPL claims – as well as other areas of law concerning noncriminal injury.3 A “tort” is a “civil wrong that unfairly causes another to experience loss or harm resulting in legal liability.”3 MPL claims assert the tort of negligence (similar to the concept of “incompetence”) and endeavor to compensate the harmed patient/individual while simultaneously dissuading suboptimal medical care by the provider in the future.4,5 A successful MPL claim must prove four overlapping elements: that the tortfeasor (here, the gastroenterologist) owed a duty of care to the injured party and breached that duty, which caused damages.6 Given that MPL cases exist within tort law rather than criminal law, the burden of proof for these cases is not “beyond a reasonable doubt”; instead, it’s “to a reasonable medical probability.”7
Trends in MPL claims
According to data compiled by the MPL Association, 278,220 MPL claims were made in the United States from 1985 to 2012.3,8-10 Among these, 1.8% involved gastroenterologists, which puts it at 17th place out of the 20 specialties surveyed.9 While the number of paid claims over this time frame decreased in GI by 34.6% (from 18.5 to 12.1 cases per 1,000 physician-years), there was a concurrent 23.3% increase in average claim compensation; essentially, there were fewer paid GI-related claims but there were higher payouts per paid claim.11,12 From 2009 to 2018, average legal defense costs for paid GI-related claims were $97,392, and average paid amount was $330,876.1
GI-related MPL risk scenarios and considerations
Many MPL claims relate to situations involving medical errors or adverse events (AEs), be they procedural or nonprocedural. However other aspects of GI also carry MPL risk.

Informed consent
MPL claims may be made not only on the grounds of inadequately informed consent but also inadequately informed refusal.5,13,14 While standards for adequate informed consent vary by state, most states apply the “reasonable patient standard,” i.e., assuming an average patient with enough information to be an active participant in the medical decision-making process. Generally, informed consent should ensure that the patient understands the nature of the procedure/treatment being proposed, there is a discussion of the risks and benefits of undergoing and not undergoing the procedure/treatment, reasonable alternatives are presented, the risks and benefits associated with these alternatives are discussed, and the patient’s comprehension of these things is assessed (Figure).15 Additionally, informed consent should be tailored to each patient and GI procedure/treatment on a case-by-case basis rather than using a one-size-fits-all approach. Moreover, documentation of the patient’s understanding of the (tailored) information provided can concurrently improve quality of the consent and potentially decrease MPL risk (Figure).16
Endoscopic procedures
Procedure-related MPL claims represent approximately 25% of all GI-related claims (8,17). Among these, 52% involve colonoscopy, 16% involve endoscopic retrograde cholangiopancreatography (ERCP), and 11% involve esophagogastroduodenoscopy.8 Albeit generally safe, colonoscopy, as with esophagogastroduodenoscopy, is subject to rare but serious AEs.18,19 Risk of these AEs may be accentuated in certain scenarios (such as severe colonic inflammation or coagulopathy) and, as discussed earlier, may merit tailored informed consent. Regardless of the procedure, in the event of postprocedural development of signs/symptoms (such as tachycardia, fever, chest or abdominal discomfort, or hypotension) indicating a potential AE, stabilizing measures and evaluation (such as blood work and imaging) should be undertaken, and hospital admission (if not already hospitalized) should be considered until discharge is deemed safe.19
ERCP-related MPL claims, for many years, have had the highest average compensation of any GI procedure.11 Though discussion of advanced procedures is beyond the scope of this article, it is worth mentioning the observation that most of such claims involve an allegation that the procedure was not indicated (for example, that it was performed based on inadequate evidence of pancreatobiliary pathology), or was for diagnostic purposes (for example, being done instead of noninvasive imaging) rather than therapeutic.20-23 This emphasizes the importance of appropriate procedure indications.
Percutaneous endoscopic gastrostomy (PEG) placement merits special mention given it can be complicated by ethical challenges (for example, needing a surrogate decision-maker’s consent or representing medical futility) and has a relatively high potential for MPL claims. PEG placement carries a low AE rate (0.1%-1%), but these AEs may result in high morbidity/mortality, in part because of the underlying comorbidities of patients needing PEG placement.24,25 Also, timing of a patient’s demise may coincide with PEG placement, thereby prompting (possibly unfounded) perceptions of causality.24-27 Therefore, such scenarios merit unique additional preprocedure safeguards. For instance, for patients lacking capacity to provide informed consent, especially when family members may differ on whether PEG should be placed, it is advisable to ask the family to select one surrogate decision-maker (if there’s no advance directive) to whom the gastroenterologist should discuss both the risks, benefits, and goals of PEG placement in the context of the patient’s overall clinical trajectory/life expectancy and the need for consent (or refusal) based on what the patient would have wished. In addition, having a medical professional witness this discussion may be useful.27
Antithrombotic agents
Periprocedural management of antithrombotics, including anticoagulants and antiplatelets, can pose challenges for the gastroenterologist. While clinical practice guidelines exist to guide decision-making in this regard, the variables involved may extend beyond the expertise of the gastroenterologist.28 For instance, in addition to the procedural risk for bleeding, the indication for antithrombotic therapy, risk of a thrombotic event, duration of action of the antithrombotic, and available bridging options should all be considered according to recommendations.28,29 While requiring more time on the part of the gastroenterologist, the optimal periprocedural management of antithrombotic agents would usually involve discussion with the provider managing antithrombotic therapy to best conduct a risk-benefit assessment regarding if (and how long) the antithrombotic therapy should be held (Figure). This shared decision-making, which should also include the patient, may help decrease MPL risk and improve outcomes.
Provider defense mechanisms
Physicians may engage in various defensive behaviors in an attempt to mitigate MPL risk; however, these behaviors may, paradoxically, increase risk.30,31
Assurance behaviors
Assurance behaviors refer to the practice of recommending or performing additional services (such as medications, imaging, procedures, and referrals) that are not clearly indicated.2,30,31 Assurance behaviors are driven by fear of MPL risk and/or missing a potential diagnosis. Recent studies have estimated that more than 50% of gastroenterologists worldwide have performed additional invasive procedures without clear indications, and that nearly one-third of endoscopic procedures annually have questionable indications.30,32 While assurance behaviors may seem likely to decrease MPL risk, overall, they may inadvertently increase AE and MPL risk, as well as health care expenditures.3,30,32
Avoidance behaviors
Avoidance behaviors refer to providers avoiding participation in potentially high-risk clinical interventions (for example, the actual procedures), including those for which they are credentialed/certified proficient.30,31 Two clinical scenarios that illustrate this behavior include the following: An advanced endoscopist credentialed to perform ERCP might refer a “high-risk” elderly patient with cholangitis to another provider to perform said ERCP or for percutaneous transhepatic drainage (in the absence of a clear benefit to such), or a gastroenterologist might refer a patient to interventional gastroenterology for resection of a large polyp even though gastroenterologists are usually proficient in this skill and may feel comfortable performing the resection themselves. Avoidance behaviors are driven by a fear of MPL risk and can have several negative consequences.33 For example, patients may not receive indicated interventions. Additionally, patients may have to wait longer for an intervention because they are referred to another provider, which also increases potential for loss to follow-up.2,30,31 This may be viewed as noncompliance with the standard of care, among other hazards, thereby increasing MPL risk.
Documentation tenets
Thorough documentation can decrease MPL risk, especially since it is often used as legal evidence.16 Documenting, for instance, preprocedure discussion of potential risk of AEs (such as bleeding or perforation) or procedural failure (for example, missed lesions)can protect gastroenterologists (Figure).16 While, as discussed previously, these should be covered in the informed consent process (which itself reduces MPL risk), proof of compliance in providing adequate informed consent must come in the form of documentation that indicates that the process took place and specifically what topics were discussed therein. MPL risk may be further decreased by documenting steps taken during a procedure and anatomic landmarks encountered to offer proof of technical competency and compliance with standards of care (Figure).16,34 In this context, it is worth recalling the adage: “If it’s not documented, it did not occur.”
Curbside consults versus consultation
Also germane here is the topic of whether documentation is needed for “curbside consults.” The uncertainty is, in part, semantic; that is, at what point does a “curbside” become a consultation? A curbside is a general question or query (such as anything that could also be answered by searching the Internet or reference materials) in response to which information is provided; once it involves provision of medical advice for a specific patient (for example, when patient identifiers have been shared or their EHR has been accessed), it constitutes a consultation. Based on these definitions, a curbside need not be documented, whereas a consultation – even if seemingly trivial – should be.
Consideration of language and cultural factors
Language barriers should be considered when the gastroenterologist is communicating with the patient, and such efforts, whenever made, should be documented to best protect against MPL.16,35 These considerations arise not only during the consent process but when obtaining a history, providing postprocedure instructions, and during follow-ups. To this end, 24/7 telephone interpreter services may assist the gastroenterologist (when one is communicating with non–English speakers and is not medically certified in the patient’s native/preferred language) and strengthen trust in the provider-patient relationship.36 Additionally, written materials (such as consent forms, procedural information) in patients’ native/preferred languages should be provided, when available, to enhance patient understanding and participation in care (Figure).35
Challenges posed by telemedicine
The COVID-19 pandemic has rapidly led to more virtual encounters. While increased utilization of telemedicine platforms may make health care more accessible, it does not lessen the clinicians’ duty to patients and may actually expose them to greater MPL risk.18,37,38 Therefore, the provider must be cognizant of two key principles to mitigate MPL risk in the context of telemedicine encounters. First, the same standard of care applies to virtual and in-person encounters.18,37,38 Second, patient privacy and HIPAA regulations are not waived during telemedicine encounters, and breaches of such may result in an MPL claim.18,37,38
With regard to the first principle, for patients who have not been physically examined (such as when a telemedicine visit was substituted for an in-person clinic encounter), gastroenterologists should not overlook requesting timely preprocedure anesthesia consultation or obtaining additional laboratory studies as needed to ensure safety and the same standard of care. Moreover, particularly in the context of pandemic-related decreased procedural capacity, triaging procedures can be especially challenging. Standardized institutional criteria which prioritize certain diagnoses/conditions over others, leaving room for justifiable exceptions, are advisable.
Vicarious liability
“Vicarious liability” is defined as that extending to persons who have not committed a wrong but on whose behalf wrongdoers acted.39 Therefore, gastroenterologists may be liable not only for their own actions but also for those of personnel they supervise (such as fellow trainees and non–physician practitioners).39 Vicarious liability aims to ensure that systemic checks and balances are in place so that, if failure occurs, harm can still be mitigated and/or avoided, as illustrated by Reason’s “Swiss Cheese Model.”40
Conclusion
Any gastroenterologist can experience an MPL claim. Such an experience can be especially stressful and confusing to early-career clinicians, especially if they’re unfamiliar with legal proceedings. Although MPL principles are not often taught in medical school or residency, it is important for gastroenterologists to be informed regarding tenets of MPL and cognizant of clinical situations which have relatively higher MPL risk. This can assuage untoward angst regarding MPL and highlight proactive risk-mitigation strategies. In general, gastroenterologist practices that can mitigate MPL risk include effective communication; adequate informed consent/refusal; documentation of preprocedure counseling, periprocedure events, and postprocedure recommendations; and maintenance of proper certification and privileging.
Dr. Azizian and Dr. Dalai are with the University of California, Los Angeles and the department of medicine at Olive View–UCLA Medical Center, Sylmar, Calif. They are co–first authors of this paper. Dr. Dalai is also with the division of gastroenterology at the University of New Mexico, Albuquerque. Dr. Adams is with the Center for Clinical Management Research in Veterans Affairs Ann Arbor Healthcare System, the division of gastroenterology at the University of Michigan Health System, and the Institute for Healthcare Policy and Innovation, all in Ann Arbor, Mich. Dr. Tabibian is with UCLA and the division of gastroenterology at Olive View–UCLA Medical Center. The authors have no conflicts of interest.
References
1. 2020 Data Sharing Project Gastroenterology 2009-2018. Inside Medical Liability: Second Quarter. Accessed 2020 Dec 6.
2. Mello MM et al. Health Aff (Millwood). 2004 Jul-Aug;23(4):42-53.
3. Adams MA et al. JAMA. 2014 Oct;312(13):1348-9.
4. Pegalis SE. American Law of Medical Malpractice 3d, Vol. 2. St. Paul, Minn.: Thomson Reuters, 2005.
5. Feld LD et al. Am J Gastroenterol. 2018 Nov;113(11):1577-9.
6. Sawyer v. Wight, 196 F. Supp. 2d 220, 226 (E.D.N.Y. 2002).
7. Michael A. Sita v. Long Island Jewish-Hillside Medical Center, 22 A.D.3d 743 (N.Y. App. Div. 2005).
8. Conklin LS et al. Clin Gastroenterol Hepatol. 2008 Jun;6(6):677-81.
9. Jena AB et al. N Engl J Med. 2011 Aug 18;365(7):629-36.
10. Kane CK. “Policy Research Perspectives Medical Liability Claim Frequency: A 2007-2008 Snapshot of Physicians.” Chicago: American Medical Association, 2010.
11. Hernandez LV et al. World J Gastrointest Endosc. 2013 Apr 16;5(4):169-73.
12. Schaffer AC et al. JAMA Intern Med. 2017 May 1;177(5):710-8.
13. Natanson v. Kline, 186 Kan. 393, 409, 350 P.2d 1093, 1106, decision clarified on denial of reh’g, 187 Kan. 186, 354 P.2d 670 (1960).
14. Truman v. Thomas, 27 Cal. 3d 285, 292, 611 P.2d 902, 906 (1980).
15. Shah P et al. Informed Consent, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Aug 22.
16. Rex DK. Clin Gastroenterol Hepatol. 2013 Jul;11(7):768-73.
17. Gerstenberger PD, Plumeri PA. Gastrointest Endosc. Mar-Apr 1993;39(2):132-8.
18. Adams MA and Allen JI. Clin Gastroenterol Hepatol. 2019 Nov;17(12):2392-6.e1.
19. Ahlawat R et al. Esophagogastroduodenoscopy, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Dec 9.
20. Cotton PB. Gastrointest Endosc. 2006 Mar;63(3):378-82.
21. Cotton PB. Gastrointest Endosc. 2010 Oct;72(4):904.
22. Adamson TE et al. West J Med. 1989 Mar;150(3):356-60.
23. Trap R et al. Endoscopy. 1999 Feb;31(2):125-30.
24. Funaki B. Semin Intervent Radiol. 2015 Mar;32(1):61-4.
25. Feeding Tube Nursing Home and Hospital Malpractice. Miller & Zois, Attorneys at Law. Accessed 2020 Jun 20.
26. Medical Malpractice Lawsuit Brings $750,000 Settlement: Death of 82-year-old woman from sepsis due to improper placement of feeding tube. Lubin & Meyers PC. Accessed 2020 Jun 20.
27. Brendel RW et al. Med Clin North Am. 2010 Nov;94(6):1229-40, xi-ii.
28. ASGE Standards of Practice Committee; Acosta RD et al. Gastrointest Endosc. 2016 Jan;83(1):3-16.
29. Saleem S and Thomas AL. Cureus. 2018 Jun 25;10(6):e2878.
30. Hiyama T et al. World J Gastroenterol. 2006 Dec 21;12(47):7671-5.
31. Studdert DM et al. JAMA. 2005 Jun 1;293(21):2609-17.
32. Shaheen NJ et al. Gastroenterology. 2018 May;154(7):1993-2003.
33. Oza VM et al. Clin Gastroenterol Hepatol. 2016 Feb;14(2):172-4.
34. Feld AD. Gastrointest Endosc Clin N Am. 2002 Jan;12(1):171-9, viii-ix.
35. Lee JS et al. J Gen Intern Med. 2017 Aug;32(8):863-70.
36. Forrow L and Kontrimas JC. J Gen Intern Med. 2017 Aug;32(8):855-7.
37. Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
38. Tabibian JH. “The Evolution of Telehealth.” Guidepoint: Legal Solutions Blog. Accessed 2020 Aug 12.
39. Feld AD. Am J Gastroenterol. 2004 Sep;99(9):1641-4.
40. Reason J. BMJ. 2000;320(7237):768‐70.
New year, new hopes
Dear colleagues,
I’m pleased to introduce the winter edition of The New Gastroenterologist – the first issue of 2021! The start of the new year has been very much anticipated because many hope that this year will bring some resolution to the challenges we faced in 2020.
With the pandemic came the widespread use of telemedicine, a feature of patient care that is likely here to stay. As physicians, it is imperative that we understand the legal implications of virtual medicine. Experienced medical malpractice lawyers Ashton Hyde and Grace Johnson (Younker Hyde Macfarlane) offer advice on this rapidly evolving realm of medicine.
Early career gastroenterologists often fall victim to self-doubt in a phenomenon referred to as impostor syndrome. Dr. Kimberly Brown (Wayne State University) discusses this important topic: what it is, how to recognize it, and how to mitigate it. One way to temper the effects of impostor syndrome is utilizing the art of coaching. Dr. Ami N. Shah (Rush) takes us through her journey and reviews the personal and professional benefits of implementing coaching in medicine.
Consults about feedings tubes can be daunting because experience with the placement and management of feeding tubes can be limited during training. This quarter’s “In Focus” article, written by Dr. John Fang and Dr. Gregory Toy (University of Utah) reviews the indications for placement, type of tubes available, and common complications and how to troubleshoot them. This is an absolute must-read for any new gastroenterologist.
How do you approach the patient who shows up for an open access endoscopy, but a quick chart review leads you to the realization that the procedure, is in fact, not indicated? There tends to be a lot of inertia which prevents cancellation of cases like this because the patient is already in the endoscopy suite, prepped, and has planned for this procedure in the preceding weeks or months. Dr. Laurel R. Fisher (University of Pennsylvania) unpacks the ethical considerations of this familiar scenario in this fantastic addition to our ethics case series.
In our postfellowship pathways section, Dr. Rena Yadlapati (University of California San Diego) and Dr. Kelli DeLay (University of Colorado) guide us through the path to becoming an esophagologist. In the DHPA Private Practice Perspectives article this quarter, Dr. Nadeem Baig (Allied Digestive Care) and Kevin Harlen (Capital Digestive Care) explain how clinical productivity is measured and how this translates into compensation in practice.
A silver lining of the pandemic is the way in which social media has been used to connect colleagues around the world in fostering medical education. Dr. Sultan Mahmood (State University of New York at Buffalo), Dr. Atoosa Rabiee (Washington DC VA Medical Center), Dr. Sunil Amin (University of Miami), Dr. Allon Kahn (Mayo Clinic Scottsdale), and Dr. Ijlal Akbar Ali (University of Oklahoma) discuss the inception of @GIJournal, a Twitter-based online journal club, and how it has gained popularity in recent months.
The AGA launched a new podcast, “Small Talk, Big Topics,” geared toward trainees and early career gastroenterologists, and through a brief question and answer session, we get to know the hosts: Dr. Matthew Whitson (Zucker School of Medicine at Hofstra-Northwell), Dr. Nina Nandy (Presbyterian Medical Group), and Dr. C.S. Tse (Brown University).
Lastly, I’d like to take a moment to recognize Lora McGlade, who has been instrumental in The New Gastroenterologist as the Medical Communications Editor for our publisher, Frontline. She assumed a new role at the end of last year, and I cannot thank her enough for her contributions in making this publication a success.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu) or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m pleased to introduce the winter edition of The New Gastroenterologist – the first issue of 2021! The start of the new year has been very much anticipated because many hope that this year will bring some resolution to the challenges we faced in 2020.
With the pandemic came the widespread use of telemedicine, a feature of patient care that is likely here to stay. As physicians, it is imperative that we understand the legal implications of virtual medicine. Experienced medical malpractice lawyers Ashton Hyde and Grace Johnson (Younker Hyde Macfarlane) offer advice on this rapidly evolving realm of medicine.
Early career gastroenterologists often fall victim to self-doubt in a phenomenon referred to as impostor syndrome. Dr. Kimberly Brown (Wayne State University) discusses this important topic: what it is, how to recognize it, and how to mitigate it. One way to temper the effects of impostor syndrome is utilizing the art of coaching. Dr. Ami N. Shah (Rush) takes us through her journey and reviews the personal and professional benefits of implementing coaching in medicine.
Consults about feedings tubes can be daunting because experience with the placement and management of feeding tubes can be limited during training. This quarter’s “In Focus” article, written by Dr. John Fang and Dr. Gregory Toy (University of Utah) reviews the indications for placement, type of tubes available, and common complications and how to troubleshoot them. This is an absolute must-read for any new gastroenterologist.
How do you approach the patient who shows up for an open access endoscopy, but a quick chart review leads you to the realization that the procedure, is in fact, not indicated? There tends to be a lot of inertia which prevents cancellation of cases like this because the patient is already in the endoscopy suite, prepped, and has planned for this procedure in the preceding weeks or months. Dr. Laurel R. Fisher (University of Pennsylvania) unpacks the ethical considerations of this familiar scenario in this fantastic addition to our ethics case series.
In our postfellowship pathways section, Dr. Rena Yadlapati (University of California San Diego) and Dr. Kelli DeLay (University of Colorado) guide us through the path to becoming an esophagologist. In the DHPA Private Practice Perspectives article this quarter, Dr. Nadeem Baig (Allied Digestive Care) and Kevin Harlen (Capital Digestive Care) explain how clinical productivity is measured and how this translates into compensation in practice.
A silver lining of the pandemic is the way in which social media has been used to connect colleagues around the world in fostering medical education. Dr. Sultan Mahmood (State University of New York at Buffalo), Dr. Atoosa Rabiee (Washington DC VA Medical Center), Dr. Sunil Amin (University of Miami), Dr. Allon Kahn (Mayo Clinic Scottsdale), and Dr. Ijlal Akbar Ali (University of Oklahoma) discuss the inception of @GIJournal, a Twitter-based online journal club, and how it has gained popularity in recent months.
The AGA launched a new podcast, “Small Talk, Big Topics,” geared toward trainees and early career gastroenterologists, and through a brief question and answer session, we get to know the hosts: Dr. Matthew Whitson (Zucker School of Medicine at Hofstra-Northwell), Dr. Nina Nandy (Presbyterian Medical Group), and Dr. C.S. Tse (Brown University).
Lastly, I’d like to take a moment to recognize Lora McGlade, who has been instrumental in The New Gastroenterologist as the Medical Communications Editor for our publisher, Frontline. She assumed a new role at the end of last year, and I cannot thank her enough for her contributions in making this publication a success.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu) or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m pleased to introduce the winter edition of The New Gastroenterologist – the first issue of 2021! The start of the new year has been very much anticipated because many hope that this year will bring some resolution to the challenges we faced in 2020.
With the pandemic came the widespread use of telemedicine, a feature of patient care that is likely here to stay. As physicians, it is imperative that we understand the legal implications of virtual medicine. Experienced medical malpractice lawyers Ashton Hyde and Grace Johnson (Younker Hyde Macfarlane) offer advice on this rapidly evolving realm of medicine.
Early career gastroenterologists often fall victim to self-doubt in a phenomenon referred to as impostor syndrome. Dr. Kimberly Brown (Wayne State University) discusses this important topic: what it is, how to recognize it, and how to mitigate it. One way to temper the effects of impostor syndrome is utilizing the art of coaching. Dr. Ami N. Shah (Rush) takes us through her journey and reviews the personal and professional benefits of implementing coaching in medicine.
Consults about feedings tubes can be daunting because experience with the placement and management of feeding tubes can be limited during training. This quarter’s “In Focus” article, written by Dr. John Fang and Dr. Gregory Toy (University of Utah) reviews the indications for placement, type of tubes available, and common complications and how to troubleshoot them. This is an absolute must-read for any new gastroenterologist.
How do you approach the patient who shows up for an open access endoscopy, but a quick chart review leads you to the realization that the procedure, is in fact, not indicated? There tends to be a lot of inertia which prevents cancellation of cases like this because the patient is already in the endoscopy suite, prepped, and has planned for this procedure in the preceding weeks or months. Dr. Laurel R. Fisher (University of Pennsylvania) unpacks the ethical considerations of this familiar scenario in this fantastic addition to our ethics case series.
In our postfellowship pathways section, Dr. Rena Yadlapati (University of California San Diego) and Dr. Kelli DeLay (University of Colorado) guide us through the path to becoming an esophagologist. In the DHPA Private Practice Perspectives article this quarter, Dr. Nadeem Baig (Allied Digestive Care) and Kevin Harlen (Capital Digestive Care) explain how clinical productivity is measured and how this translates into compensation in practice.
A silver lining of the pandemic is the way in which social media has been used to connect colleagues around the world in fostering medical education. Dr. Sultan Mahmood (State University of New York at Buffalo), Dr. Atoosa Rabiee (Washington DC VA Medical Center), Dr. Sunil Amin (University of Miami), Dr. Allon Kahn (Mayo Clinic Scottsdale), and Dr. Ijlal Akbar Ali (University of Oklahoma) discuss the inception of @GIJournal, a Twitter-based online journal club, and how it has gained popularity in recent months.
The AGA launched a new podcast, “Small Talk, Big Topics,” geared toward trainees and early career gastroenterologists, and through a brief question and answer session, we get to know the hosts: Dr. Matthew Whitson (Zucker School of Medicine at Hofstra-Northwell), Dr. Nina Nandy (Presbyterian Medical Group), and Dr. C.S. Tse (Brown University).
Lastly, I’d like to take a moment to recognize Lora McGlade, who has been instrumental in The New Gastroenterologist as the Medical Communications Editor for our publisher, Frontline. She assumed a new role at the end of last year, and I cannot thank her enough for her contributions in making this publication a success.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu) or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Update on feeding tubes: Indications and troubleshooting complications
Introduction
Gastroenterologists are in a unique position to manage individuals with feeding tubes as their training underscores principles in digestion, absorption, nutrition support, and enteral tube placement. Adequate management of individuals with feeding tubes and, importantly, the complications that arise from feeding tube use and placement require a basic understanding of intestinal anatomy and physiology. Therefore, gastroenterologists are well suited to both place and manage individuals with feeding tubes in the long term.
Indications for tube feeding
When deciding on the appropriate route for artificial nutrition support, the first decision to be made is enteral access versus parenteral nutrition support. Enteral nutrition confers multiple benefits, including preservation of the mucosal lining, reductions in complicated infections, decreased costs, and improved patient compliance. All attempts at adequate enteral access should be made before deciding on the use of parenteral nutrition. Following the clinical decision to pursue artificial means of nutrition support and enteral access, the next common decision is the anticipated duration of nutrition support. Generally, the oral or nasal tubes are used for short durations (i.e., less than 4 weeks) with percutaneous placement into the stomach or small intestine for longer-term feeding (i.e., percutaneous endoscopic gastrostomy [PEG] or percutaneous endoscopic jejunostomy [PEJ]).
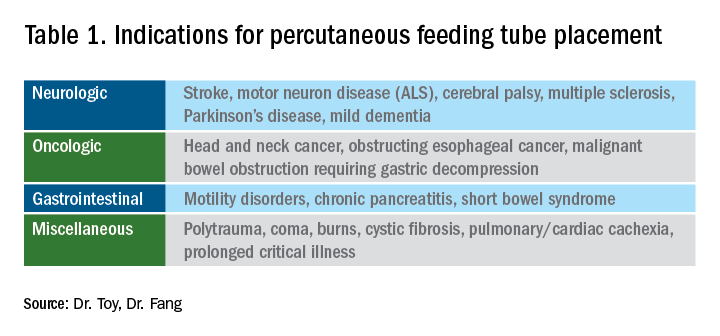
The most general indication for nutrition support is an inability to maintain adequate nutritional needs with oral intake alone. General categories of inadequate oral intake include neurologic disorders, malignancy, and gastrointestinal conditions affecting digestion and absorption (Table 1). Absolute and relative contraindications to PEG placement are listed in Table 2. If an endoscopic placement is not possible, alternative means of placement (i.e., surgery or interventional radiology) can be considered to avoid the consequences of prolonged malnutrition. In-hospital mortality following PEG placement has decreased 40% over the last 10 years, which can be attributed to improved patient selection, enhanced discharge practices, and exclusion of patients with the highest comorbidity and mortality rates, like those with advanced dementia or terminal cancer.1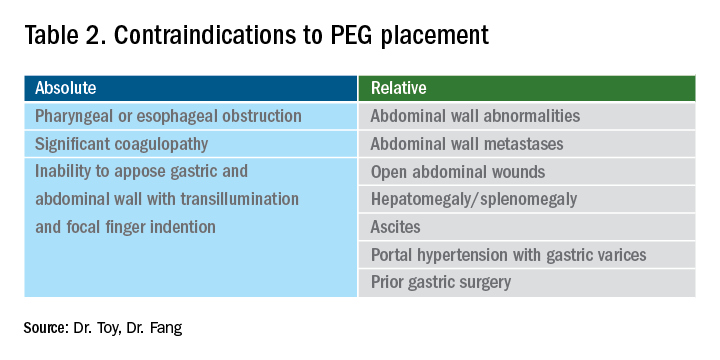
PEG placement in patients with dementia is controversial, with previous studies not demonstrating improved outcomes and association with high mortality rates,2 so the practice is currently not recommended by the American Geriatrics Society in individuals with advanced dementia.3 However, a large Japanese study showed that careful selection of patients with mild dementia to undergo gastrostomy increased independence fourfold; therefore, multidisciplinary involvement is often necessary in the decision to pursue artificial means of nutrition support in this population.4
The recent coronavirus disease 2019 (COVID-19) pandemic has placed additional strains on endoscopic placement and has highlighted the effect of the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) on GI symptoms. A recent meta-analysis showed an overall incidence of GI symptoms of 17.6% in the following conditions in decreasing order of prevalence: anorexia, diarrhea, nausea, vomiting, and abdominal discomfort.5 In addition, the prolonged ventilatory requirements among a subset of individuals with the most severe COVID-19 results in extended periods of nutrition support via enteral tube placements. In individuals with ICU-acquired weakness and discharge to long-term care facilities, the placement of percutaneous endoscopic tubes may be required, although with the additional consideration of the need for an aerosolizing procedure. Delay of placement has been advocated, in addition to appropriate personal protective equipment, in order to ensure safe placement for the endoscopy staff.6
Types of feeding tubes
After deciding to feed a patient enterally and determining the anticipated duration of enteral support, the next decision is to determine the most appropriate location of feeding delivery: into the stomach or the small bowel. Gastric feeding is advantageous most commonly because of its increased capacity, allowing for larger volumes to be delivered over shorter durations. However, in the setting of postsurgical anatomy, gastroparesis, or obstructing tumors/pancreatic inflammation, distal delivery of tube feeds may be required into the jejunum. Additionally, percutaneous tubes placed into the stomach can have extenders into the small bowel (GJ tubes) to allow for feeding into the small bowel and decompression or delivery of medications into the stomach.
In general, gastric feeding is preferred over small bowel feeding as PEG tubes are more stable and have fewer complications than either PEG-J or direct PEJ tubes. Gastrostomy tubes are generally shorter and larger in diameter making them less likely to clog. PEG-J tubes have separate lumens for gastric and small intestinal access, but the smaller-bore jejunal extension tubes are more likely to clog or become dislodged. While direct PEJ is shown to have higher rates of tube patency and decreased rates of endoscopic re-intervention, compared with PEG-J,7 one limitation of a direct PEJ is difficulty in placement and site selection, which can be performed with a pediatric colonoscope or balloon enteroscopy system. Most commonly, this procedure is performed under general anesthesia.
In the case of a critically ill patient in the ICU, it is recommended to start enteral nutrition within 24-48 hours of arrival to avoid complications of prolonged calorie deficits. Nasally inserted feeding tubes (e.g., Cortrak, Avanos Medical Devices, Alpharetta, Ga.) are most commonly used at the bedside and can be placed blindly using electromagnetic image guidance, radiographically, or endoscopy. However, the small caliber of nasoenteric tubes comes with the common complication of clogging, which can be overcome with slightly larger bore gastric feeding tubes. If gastric feeding is not tolerated (e.g., in the case of vomiting, witnessed aspiration), small bowel feeding should be initiated and can be a more durable form of enteral feeding with fewer interruptions as feedings do not need to be held for procedures or symptomatic gastric intolerance. In clinical areas of question, or if there is a concern for intolerance of enteral feeding, a short trial with nasogastric or nasojejunal tube placement should be performed before a more definitive percutaneous placement.
With respect to percutaneous tubes, important characteristics to choose are the size (diameter in French units), type of internal retention device, and external appearance of the tube (standard or low profile). All percutaneous tubes contain an external retention device (i.e., bumper) that fits against the skin and an internal retention device that is either a balloon or plastic dome or funnel that prevents the tube from becoming dislodged. Balloon retention tubes require replacement every 3-6 months, while nonballoon tubes generally require replacement annually in order to prevent the plastic from cracking, which can make removal complicated. Low-profile tubes have an external cap, which, when opened, allows for extension tubing to be securely attached while in use and detached while not in use. Low-profile tubes are often preferred among younger, active patients and those with adequate dexterity to allow for attachment of the external extension tubing. These tubes are most often inserted as a replacement for an initially endoscopically placed tube, although one-step systems for initial placement are available. The size of the low-profile tube is chosen based on the size of the existing PEG tube and by measuring the length of the stoma tract using specialized measuring devices.8 Patients and caregivers can also be trained to replace balloon-type tubes on their own to limit complications of displaced or cracked tubes. Low-profile tubes are commercially available for both gastric placement and gastric placement with extension into the small bowel, which often requires fluoroscopy for secure placement.
All percutaneous enteral tubes are being transitioned to the ENfit connector system, which prevents connections from the enteral system to nonenteral systems (namely intravenous lines, chest tubes) and vice versa. Tubing misconnections have been rarely reported, and the EnFIT system is designed to prevent such misadventures that have resulted in serious complications and even mortality.9 Adapter devices are available that may be required for patients with feeding tubes who have not been transitioned yet. Most commonly with new tube placements and replacements, patients and providers will have to become familiar with the new syringes and feeding bags required with EnFIT connectors.
Gastrostomy placement can be considered a higher-risk endoscopic procedure. One complicating factor is the increased use of antiplatelet and anticoagulant therapies in individuals with a history of neurologic insults. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines recommend that coumadin be held 5 days before the procedure and bridged with heparin if the patient is at high risk of thromboembolic complications. For patients on dual anti-platelet therapy, thienopyridines like clopidogrel are often stopped 5-7 days prior to procedure with continuation of aspirin,10 but there are more recent data that PEG insertion is safe with continued use of DAPT.11 Direct-acting anticoagulants (DOACs) are often stopped 24-48 hours prior to procedure and then restarted 48 hours after tube placement, but this is dependent on the half-life of the specific DOAC and the patient’s renal function. Patients with decreased creatinine clearance may need to hold the DOAC up to 3-4 days prior to the procedure. In this situation, referring to ASGE guidelines and consultation with a hematologist or managing anti-coagulation clinic is advised.10
Troubleshooting complications
Nasoenteric tubes: One of the most common and irritating complications with nasoenteric feeding tubes is clogging. To prevent clogging, the tube should be flushed frequently.12 At least 30 mL of free water should be used to flush the tube every 4-8 hours for continuous feedings or before and after bolus feeding. Additionally, 15-30 mL of water should be given with each separate medication administration, and if possible, medication administration via small-bore small bowel feeding tubes should be avoided.12 Water flushing is especially important with small-caliber tubes and pumps that deliver both feeding and water flushes. It is available for small bowel feeding in order to allow for programmed water delivery.
Warm water flushes can also help unclog the tube,12 and additional pharmacologic and mechanical devices have been promoted for clogged tubes. One common technique is mixing pancreatic enzymes (Viokase) with a crushed 325-mg tablet of nonenteric coated sodium bicarbonate and 5 mL of water to create a solution that has the alkaline properties allowing for both pancreatic enzyme activation and clog dissolution. Additionally, an endoscopic retrograde cholangiopancreatography (ERCP) catheter can be placed into longer feeding tubes to directly infuse the activated agent to the site of the clog.13 If water and enzymes are not successful in unclogging the tube, commercially available brushes can help remove clogs. The TubeClear® system (Actuated Medical, Bellefonte, Penna) has a single-use stem that is connected to AC power to create a jackhammerlike movement to remove clogs in longer nasoenteral and gastrojejunal tubes.
PEG tubes (short-term complications): Procedural and immediate postprocedural complications include bleeding, aspiration, pneumoperitoneum, and perforation. Pneumoperitoneum occurs in approximately 50% of cases and is generally clinically insignificant. The risk of pneumoperitoneum can be reduced by using CO2 insufflation.14 If the patient develops systemic signs of infection or peritoneal signs, CT scan with oral contrast is warranted for further evaluation and to assess for inadvertent perforation of overlying bowel or dislodged tube. Aspiration during or following endoscopy is another common complication of PEG placement and risk factors include over-sedation, supine positioning, advanced age, and neurologic dysfunction. This risk can be mitigated by avoiding over-sedation, immediately aspirating gastric contents when the stomach is reached, and avoiding excessive insufflation.15 In addition, elevating the head of the bed during the procedure and dedicating an assistant to perform oral suctioning during the entire procedure is recommended.
PEG tubes (long-term complications): More delayed complications of PEG insertion include wound infection, buried bumper syndrome, tumor seeding, peristomal leakage, and tube dislodgement. The prevalence of wound infection is 5%- 25%,16 and randomized controlled trials have demonstrated the efficacy of a single dose of an IV antibiotic (i.e., cephalosporin) in those not already receiving a broad spectrum antibiotic and administered prophylactically before tube placement.17 The significance of this reduction is such that antibiotic administration before tube placement should be considered a quality measure for the procedure. A small amount of redness around the tube site (less than 5 mm) is typical, but extension of erythema, warmth, tenderness, purulent drainage, or systemic symptoms is consistent with infection and warrants additional antibiotic administration. Minor infections can be treated with local antiseptics and oral antibiotics, and early intervention is important to prevent need for hospital admission, systemic antibiotics, and even surgical debridement.
Peristomal leakage is reported in approximately 1%-2% of patients.18 Photographs of the site can be very useful in evaluating and managing peristomal leakage and infections. Interventions include reducing gastric secretions with proton pump inhibitors and management of the skin with barrier creams, such as zinc oxide (Calmoseptine®) ointment. Placement of a larger-diameter tube only enlarges the stoma track and worsens the leakage. In such cases, thorough evaluations for delayed gastric emptying (gastroparesis), distal obstruction, or constipation should be performed and managed accordingly. Opiates are common contributors to constipation and delayed gastric emptying and often require reduction in use or directed antagonist therapy to reduce leaking. Continuous feeding over bolus feedings and delivering nutrition distally into the small bowel (PEG-J placement) can improve leaking from gastrostomy tubes. Additional means of management include stabilizing the tube by replacing a traditional tube with a low-profile tube or using right-angle external bumpers. If all measures fail, removing the tube and allowing for stomal closure can be attempted,16 although this option often requires parenteral nutrition support to prevent prolonged periods of inadequate nutrition.
Buried bumper syndrome (BBS) occurs in 1.5%-8.8% of PEG placements and is a common late complication of PEG placement, although early reports have been described.18 The development of BBS occurs when the internal bumper migrates from the gastric lumen through and into the stomach or abdominal wall. It occurs more frequently with solid nonballoon retention tubes and is caused by excessive compression of the external bumper against the skin and abdominal wall. Patients with BBS usually present with an immobile catheter, resistance with feeds (because of a closure of the stomach wall around the internal portion of the gastrostomy tube), abdominal pain, or peristomal leakage. Physicians should be aware of and assess tubes for BBS, in particular when replacing an immobile tube (cannot be pushed into the free stomach lumen) or when there is difficulty in flushing water into the tube. This complication can be easily prevented by allowing a minimum of 0.5-1.0 cm (1 finger breadth) between the external bumper and the abdominal wall. In particular, patients and caregivers should be warned that if the patient gains significant amounts of weight, the outer bumper will need to be loosened. Once BBS is diagnosed, the PEG tube requires removal and replacement as it can cause bleeding, infection, or fasciitis. The general steps to replacement include endoscopic removal of the existing tube and replacement of new PEG in the existing tract as long as the BBS is not severe. In most cases a replacement tube can be pulled into place using the pull-PEG technique at the same gastrostomy site as long as the stoma tract can be cannulated with a wire after the existing tube is removed.
Similar to nasoenteric tubes, PEG tubes can become clogged, although this complication is infrequent. The primary steps for prevention include adequately flushing with water before and after feeds and ensuring that all medications are liquid or well crushed and dissolved before instilling. Timely tube replacement also ensures that the internal portions of the gastrostomy tube remain free of debris. Management is similar to that of unclogging nasoenteral tubes, as discussed above, and specific commercial declogging devices for PEG tubes include the Bionix Declogger® (Bionix Development Corp., Toledo, Ohio) and the Bard® PEG cleaning brush (Bard Peripheral Vascular Inc., Tempe, Ariz.). The Bionix system has a plastic stem with a screw and thread design that will remove clogs in 14-24 French PEG tubes, while the Bard brush has a flexible nylon stem with soft bristles at the end to prevent mucosal injury and can be used for prophylaxis against clogs, as well as removing clogs themselves.12
Lastly, a rare but important complication of PEG placement is tumor seeding of the PEG site in patients with active head and neck or upper gastrointestinal cancer.19 The presumed mechanism is shearing of tumor cells as the PEG is pulled through the upper aerodigestive tract and through the wall of the stomach, as prior studies have demonstrated frequent seeding of tubes and incision sites as shown by brushing the tube for malignant cells after tube placement.20 It is important to recognize this complication and not misdiagnose it as granulation tissue, infection, or bleeding as the spread of the cancer generally portends a poor prognosis. Therefore, it is best to use a PEG insertion technique that does not involve pulling or pushing the PEG through the upper aerodigestive tract in patients with active cancer and instead place tubes via an external approach by colleagues in interventional radiology or via direct surgical placement.
Conclusion
Gastroenterologists occupy a unique role in evaluation, diagnosis, and management of patients requiring enteral feeding. In addition, they are best equipped to place, prevent, and manage complications of tube feeding. For this reason, it is imperative that gastroenterologists familiarize themselves with indications for enteral tubes and types of enteral tubes available, as well as the identification and management of common complications. Comprehensive understanding of these concepts will augment the practicing gastroenterologist’s ability to manage patients requiring enteral nutrition support with confidence.
References
1. Stein DJ et al. Dig Dis Sci. 2020 Jun 19. doi: 10.1007/s10620-020-06396-y.
2. American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. J Am Geriatr Soc. 2014;62(8):1590-3.
3. Dietrich CG, Schoppmeyer K. World J Gastroenterol. 2020;26(20):2464-71.
4. Suzuki Y et al. T Gastroenterology Res.2012 Feb;5(1):10-20.
5. Cheung KS et al. Gastroenterology. 2020 Jul;159(1):81-95.
6. Micic D et al. Am J Gastroenterol. 2020 Sep;115(9):1367-70.
7. Fan AC et al. Gastrointest Endosc. 2002;56(6):890-4.
8. Tang SJ. Video J Encycl GI Endosc. 2014;2(2):70-3.
9. Guenter P, Lyman B. Nutr Clin Pract. 2016;31(6):769-72.
10. Acosta RD et al. Gastrointest Endosc. 2016;83(1):3-16.
11. Richter JA et al. Gastrointest Endosc. 2011;74(1):22-34.
12. Boullata JI et al. JPEN. 2017;41(1):15-103.
13. McClave SA. Tech Gastrointest Endosc. 2021;3(1):62-8.
14. Murphy CJ et al. Endosc Int Open. 2016;4(3):E292. doi: 10.1053/tgie.2001.19915.
15. Lynch CR et al. Pract Gastroenterology. 2004;28:66-77.
16. Hucl T et al. Best Pract Res Clin Gastroenterol. 2016;30(5):769-81. doi: 10.1016/j.bpg.2016.10.002.
17. Jafri NS et al. Aliment Pharmacol & Therapeut. 2007;25(6):647-56. doi: 10.1111/j.1365-2036.2007.03247.x.
18. Blumenstein I et al. World J Gastroenterol. 2014;20(26):8505-24. doi: 10.3748/wjg.v20.i26.8505.
19. Fung E et al. Surgical Endosc. 2017;31(9):3623-7. doi: 10.1007/s00464-016-5394-8.
20. Ellrichmann M et al. Endoscopy. 2013;45(07):526-31. doi: 10.1055/s-0033-1344023.
Dr. Toy is with the department of internal medicine at the University of Utah, Salt Lake City. Dr. Fang is with the division of gastroenterology and hepatology at the University of Utah.
Introduction
Gastroenterologists are in a unique position to manage individuals with feeding tubes as their training underscores principles in digestion, absorption, nutrition support, and enteral tube placement. Adequate management of individuals with feeding tubes and, importantly, the complications that arise from feeding tube use and placement require a basic understanding of intestinal anatomy and physiology. Therefore, gastroenterologists are well suited to both place and manage individuals with feeding tubes in the long term.
Indications for tube feeding
When deciding on the appropriate route for artificial nutrition support, the first decision to be made is enteral access versus parenteral nutrition support. Enteral nutrition confers multiple benefits, including preservation of the mucosal lining, reductions in complicated infections, decreased costs, and improved patient compliance. All attempts at adequate enteral access should be made before deciding on the use of parenteral nutrition. Following the clinical decision to pursue artificial means of nutrition support and enteral access, the next common decision is the anticipated duration of nutrition support. Generally, the oral or nasal tubes are used for short durations (i.e., less than 4 weeks) with percutaneous placement into the stomach or small intestine for longer-term feeding (i.e., percutaneous endoscopic gastrostomy [PEG] or percutaneous endoscopic jejunostomy [PEJ]).

The most general indication for nutrition support is an inability to maintain adequate nutritional needs with oral intake alone. General categories of inadequate oral intake include neurologic disorders, malignancy, and gastrointestinal conditions affecting digestion and absorption (Table 1). Absolute and relative contraindications to PEG placement are listed in Table 2. If an endoscopic placement is not possible, alternative means of placement (i.e., surgery or interventional radiology) can be considered to avoid the consequences of prolonged malnutrition. In-hospital mortality following PEG placement has decreased 40% over the last 10 years, which can be attributed to improved patient selection, enhanced discharge practices, and exclusion of patients with the highest comorbidity and mortality rates, like those with advanced dementia or terminal cancer.1
PEG placement in patients with dementia is controversial, with previous studies not demonstrating improved outcomes and association with high mortality rates,2 so the practice is currently not recommended by the American Geriatrics Society in individuals with advanced dementia.3 However, a large Japanese study showed that careful selection of patients with mild dementia to undergo gastrostomy increased independence fourfold; therefore, multidisciplinary involvement is often necessary in the decision to pursue artificial means of nutrition support in this population.4
The recent coronavirus disease 2019 (COVID-19) pandemic has placed additional strains on endoscopic placement and has highlighted the effect of the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) on GI symptoms. A recent meta-analysis showed an overall incidence of GI symptoms of 17.6% in the following conditions in decreasing order of prevalence: anorexia, diarrhea, nausea, vomiting, and abdominal discomfort.5 In addition, the prolonged ventilatory requirements among a subset of individuals with the most severe COVID-19 results in extended periods of nutrition support via enteral tube placements. In individuals with ICU-acquired weakness and discharge to long-term care facilities, the placement of percutaneous endoscopic tubes may be required, although with the additional consideration of the need for an aerosolizing procedure. Delay of placement has been advocated, in addition to appropriate personal protective equipment, in order to ensure safe placement for the endoscopy staff.6
Types of feeding tubes
After deciding to feed a patient enterally and determining the anticipated duration of enteral support, the next decision is to determine the most appropriate location of feeding delivery: into the stomach or the small bowel. Gastric feeding is advantageous most commonly because of its increased capacity, allowing for larger volumes to be delivered over shorter durations. However, in the setting of postsurgical anatomy, gastroparesis, or obstructing tumors/pancreatic inflammation, distal delivery of tube feeds may be required into the jejunum. Additionally, percutaneous tubes placed into the stomach can have extenders into the small bowel (GJ tubes) to allow for feeding into the small bowel and decompression or delivery of medications into the stomach.
In general, gastric feeding is preferred over small bowel feeding as PEG tubes are more stable and have fewer complications than either PEG-J or direct PEJ tubes. Gastrostomy tubes are generally shorter and larger in diameter making them less likely to clog. PEG-J tubes have separate lumens for gastric and small intestinal access, but the smaller-bore jejunal extension tubes are more likely to clog or become dislodged. While direct PEJ is shown to have higher rates of tube patency and decreased rates of endoscopic re-intervention, compared with PEG-J,7 one limitation of a direct PEJ is difficulty in placement and site selection, which can be performed with a pediatric colonoscope or balloon enteroscopy system. Most commonly, this procedure is performed under general anesthesia.
In the case of a critically ill patient in the ICU, it is recommended to start enteral nutrition within 24-48 hours of arrival to avoid complications of prolonged calorie deficits. Nasally inserted feeding tubes (e.g., Cortrak, Avanos Medical Devices, Alpharetta, Ga.) are most commonly used at the bedside and can be placed blindly using electromagnetic image guidance, radiographically, or endoscopy. However, the small caliber of nasoenteric tubes comes with the common complication of clogging, which can be overcome with slightly larger bore gastric feeding tubes. If gastric feeding is not tolerated (e.g., in the case of vomiting, witnessed aspiration), small bowel feeding should be initiated and can be a more durable form of enteral feeding with fewer interruptions as feedings do not need to be held for procedures or symptomatic gastric intolerance. In clinical areas of question, or if there is a concern for intolerance of enteral feeding, a short trial with nasogastric or nasojejunal tube placement should be performed before a more definitive percutaneous placement.
With respect to percutaneous tubes, important characteristics to choose are the size (diameter in French units), type of internal retention device, and external appearance of the tube (standard or low profile). All percutaneous tubes contain an external retention device (i.e., bumper) that fits against the skin and an internal retention device that is either a balloon or plastic dome or funnel that prevents the tube from becoming dislodged. Balloon retention tubes require replacement every 3-6 months, while nonballoon tubes generally require replacement annually in order to prevent the plastic from cracking, which can make removal complicated. Low-profile tubes have an external cap, which, when opened, allows for extension tubing to be securely attached while in use and detached while not in use. Low-profile tubes are often preferred among younger, active patients and those with adequate dexterity to allow for attachment of the external extension tubing. These tubes are most often inserted as a replacement for an initially endoscopically placed tube, although one-step systems for initial placement are available. The size of the low-profile tube is chosen based on the size of the existing PEG tube and by measuring the length of the stoma tract using specialized measuring devices.8 Patients and caregivers can also be trained to replace balloon-type tubes on their own to limit complications of displaced or cracked tubes. Low-profile tubes are commercially available for both gastric placement and gastric placement with extension into the small bowel, which often requires fluoroscopy for secure placement.
All percutaneous enteral tubes are being transitioned to the ENfit connector system, which prevents connections from the enteral system to nonenteral systems (namely intravenous lines, chest tubes) and vice versa. Tubing misconnections have been rarely reported, and the EnFIT system is designed to prevent such misadventures that have resulted in serious complications and even mortality.9 Adapter devices are available that may be required for patients with feeding tubes who have not been transitioned yet. Most commonly with new tube placements and replacements, patients and providers will have to become familiar with the new syringes and feeding bags required with EnFIT connectors.
Gastrostomy placement can be considered a higher-risk endoscopic procedure. One complicating factor is the increased use of antiplatelet and anticoagulant therapies in individuals with a history of neurologic insults. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines recommend that coumadin be held 5 days before the procedure and bridged with heparin if the patient is at high risk of thromboembolic complications. For patients on dual anti-platelet therapy, thienopyridines like clopidogrel are often stopped 5-7 days prior to procedure with continuation of aspirin,10 but there are more recent data that PEG insertion is safe with continued use of DAPT.11 Direct-acting anticoagulants (DOACs) are often stopped 24-48 hours prior to procedure and then restarted 48 hours after tube placement, but this is dependent on the half-life of the specific DOAC and the patient’s renal function. Patients with decreased creatinine clearance may need to hold the DOAC up to 3-4 days prior to the procedure. In this situation, referring to ASGE guidelines and consultation with a hematologist or managing anti-coagulation clinic is advised.10
Troubleshooting complications
Nasoenteric tubes: One of the most common and irritating complications with nasoenteric feeding tubes is clogging. To prevent clogging, the tube should be flushed frequently.12 At least 30 mL of free water should be used to flush the tube every 4-8 hours for continuous feedings or before and after bolus feeding. Additionally, 15-30 mL of water should be given with each separate medication administration, and if possible, medication administration via small-bore small bowel feeding tubes should be avoided.12 Water flushing is especially important with small-caliber tubes and pumps that deliver both feeding and water flushes. It is available for small bowel feeding in order to allow for programmed water delivery.
Warm water flushes can also help unclog the tube,12 and additional pharmacologic and mechanical devices have been promoted for clogged tubes. One common technique is mixing pancreatic enzymes (Viokase) with a crushed 325-mg tablet of nonenteric coated sodium bicarbonate and 5 mL of water to create a solution that has the alkaline properties allowing for both pancreatic enzyme activation and clog dissolution. Additionally, an endoscopic retrograde cholangiopancreatography (ERCP) catheter can be placed into longer feeding tubes to directly infuse the activated agent to the site of the clog.13 If water and enzymes are not successful in unclogging the tube, commercially available brushes can help remove clogs. The TubeClear® system (Actuated Medical, Bellefonte, Penna) has a single-use stem that is connected to AC power to create a jackhammerlike movement to remove clogs in longer nasoenteral and gastrojejunal tubes.
PEG tubes (short-term complications): Procedural and immediate postprocedural complications include bleeding, aspiration, pneumoperitoneum, and perforation. Pneumoperitoneum occurs in approximately 50% of cases and is generally clinically insignificant. The risk of pneumoperitoneum can be reduced by using CO2 insufflation.14 If the patient develops systemic signs of infection or peritoneal signs, CT scan with oral contrast is warranted for further evaluation and to assess for inadvertent perforation of overlying bowel or dislodged tube. Aspiration during or following endoscopy is another common complication of PEG placement and risk factors include over-sedation, supine positioning, advanced age, and neurologic dysfunction. This risk can be mitigated by avoiding over-sedation, immediately aspirating gastric contents when the stomach is reached, and avoiding excessive insufflation.15 In addition, elevating the head of the bed during the procedure and dedicating an assistant to perform oral suctioning during the entire procedure is recommended.
PEG tubes (long-term complications): More delayed complications of PEG insertion include wound infection, buried bumper syndrome, tumor seeding, peristomal leakage, and tube dislodgement. The prevalence of wound infection is 5%- 25%,16 and randomized controlled trials have demonstrated the efficacy of a single dose of an IV antibiotic (i.e., cephalosporin) in those not already receiving a broad spectrum antibiotic and administered prophylactically before tube placement.17 The significance of this reduction is such that antibiotic administration before tube placement should be considered a quality measure for the procedure. A small amount of redness around the tube site (less than 5 mm) is typical, but extension of erythema, warmth, tenderness, purulent drainage, or systemic symptoms is consistent with infection and warrants additional antibiotic administration. Minor infections can be treated with local antiseptics and oral antibiotics, and early intervention is important to prevent need for hospital admission, systemic antibiotics, and even surgical debridement.
Peristomal leakage is reported in approximately 1%-2% of patients.18 Photographs of the site can be very useful in evaluating and managing peristomal leakage and infections. Interventions include reducing gastric secretions with proton pump inhibitors and management of the skin with barrier creams, such as zinc oxide (Calmoseptine®) ointment. Placement of a larger-diameter tube only enlarges the stoma track and worsens the leakage. In such cases, thorough evaluations for delayed gastric emptying (gastroparesis), distal obstruction, or constipation should be performed and managed accordingly. Opiates are common contributors to constipation and delayed gastric emptying and often require reduction in use or directed antagonist therapy to reduce leaking. Continuous feeding over bolus feedings and delivering nutrition distally into the small bowel (PEG-J placement) can improve leaking from gastrostomy tubes. Additional means of management include stabilizing the tube by replacing a traditional tube with a low-profile tube or using right-angle external bumpers. If all measures fail, removing the tube and allowing for stomal closure can be attempted,16 although this option often requires parenteral nutrition support to prevent prolonged periods of inadequate nutrition.
Buried bumper syndrome (BBS) occurs in 1.5%-8.8% of PEG placements and is a common late complication of PEG placement, although early reports have been described.18 The development of BBS occurs when the internal bumper migrates from the gastric lumen through and into the stomach or abdominal wall. It occurs more frequently with solid nonballoon retention tubes and is caused by excessive compression of the external bumper against the skin and abdominal wall. Patients with BBS usually present with an immobile catheter, resistance with feeds (because of a closure of the stomach wall around the internal portion of the gastrostomy tube), abdominal pain, or peristomal leakage. Physicians should be aware of and assess tubes for BBS, in particular when replacing an immobile tube (cannot be pushed into the free stomach lumen) or when there is difficulty in flushing water into the tube. This complication can be easily prevented by allowing a minimum of 0.5-1.0 cm (1 finger breadth) between the external bumper and the abdominal wall. In particular, patients and caregivers should be warned that if the patient gains significant amounts of weight, the outer bumper will need to be loosened. Once BBS is diagnosed, the PEG tube requires removal and replacement as it can cause bleeding, infection, or fasciitis. The general steps to replacement include endoscopic removal of the existing tube and replacement of new PEG in the existing tract as long as the BBS is not severe. In most cases a replacement tube can be pulled into place using the pull-PEG technique at the same gastrostomy site as long as the stoma tract can be cannulated with a wire after the existing tube is removed.
Similar to nasoenteric tubes, PEG tubes can become clogged, although this complication is infrequent. The primary steps for prevention include adequately flushing with water before and after feeds and ensuring that all medications are liquid or well crushed and dissolved before instilling. Timely tube replacement also ensures that the internal portions of the gastrostomy tube remain free of debris. Management is similar to that of unclogging nasoenteral tubes, as discussed above, and specific commercial declogging devices for PEG tubes include the Bionix Declogger® (Bionix Development Corp., Toledo, Ohio) and the Bard® PEG cleaning brush (Bard Peripheral Vascular Inc., Tempe, Ariz.). The Bionix system has a plastic stem with a screw and thread design that will remove clogs in 14-24 French PEG tubes, while the Bard brush has a flexible nylon stem with soft bristles at the end to prevent mucosal injury and can be used for prophylaxis against clogs, as well as removing clogs themselves.12
Lastly, a rare but important complication of PEG placement is tumor seeding of the PEG site in patients with active head and neck or upper gastrointestinal cancer.19 The presumed mechanism is shearing of tumor cells as the PEG is pulled through the upper aerodigestive tract and through the wall of the stomach, as prior studies have demonstrated frequent seeding of tubes and incision sites as shown by brushing the tube for malignant cells after tube placement.20 It is important to recognize this complication and not misdiagnose it as granulation tissue, infection, or bleeding as the spread of the cancer generally portends a poor prognosis. Therefore, it is best to use a PEG insertion technique that does not involve pulling or pushing the PEG through the upper aerodigestive tract in patients with active cancer and instead place tubes via an external approach by colleagues in interventional radiology or via direct surgical placement.
Conclusion
Gastroenterologists occupy a unique role in evaluation, diagnosis, and management of patients requiring enteral feeding. In addition, they are best equipped to place, prevent, and manage complications of tube feeding. For this reason, it is imperative that gastroenterologists familiarize themselves with indications for enteral tubes and types of enteral tubes available, as well as the identification and management of common complications. Comprehensive understanding of these concepts will augment the practicing gastroenterologist’s ability to manage patients requiring enteral nutrition support with confidence.
References
1. Stein DJ et al. Dig Dis Sci. 2020 Jun 19. doi: 10.1007/s10620-020-06396-y.
2. American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. J Am Geriatr Soc. 2014;62(8):1590-3.
3. Dietrich CG, Schoppmeyer K. World J Gastroenterol. 2020;26(20):2464-71.
4. Suzuki Y et al. T Gastroenterology Res.2012 Feb;5(1):10-20.
5. Cheung KS et al. Gastroenterology. 2020 Jul;159(1):81-95.
6. Micic D et al. Am J Gastroenterol. 2020 Sep;115(9):1367-70.
7. Fan AC et al. Gastrointest Endosc. 2002;56(6):890-4.
8. Tang SJ. Video J Encycl GI Endosc. 2014;2(2):70-3.
9. Guenter P, Lyman B. Nutr Clin Pract. 2016;31(6):769-72.
10. Acosta RD et al. Gastrointest Endosc. 2016;83(1):3-16.
11. Richter JA et al. Gastrointest Endosc. 2011;74(1):22-34.
12. Boullata JI et al. JPEN. 2017;41(1):15-103.
13. McClave SA. Tech Gastrointest Endosc. 2021;3(1):62-8.
14. Murphy CJ et al. Endosc Int Open. 2016;4(3):E292. doi: 10.1053/tgie.2001.19915.
15. Lynch CR et al. Pract Gastroenterology. 2004;28:66-77.
16. Hucl T et al. Best Pract Res Clin Gastroenterol. 2016;30(5):769-81. doi: 10.1016/j.bpg.2016.10.002.
17. Jafri NS et al. Aliment Pharmacol & Therapeut. 2007;25(6):647-56. doi: 10.1111/j.1365-2036.2007.03247.x.
18. Blumenstein I et al. World J Gastroenterol. 2014;20(26):8505-24. doi: 10.3748/wjg.v20.i26.8505.
19. Fung E et al. Surgical Endosc. 2017;31(9):3623-7. doi: 10.1007/s00464-016-5394-8.
20. Ellrichmann M et al. Endoscopy. 2013;45(07):526-31. doi: 10.1055/s-0033-1344023.
Dr. Toy is with the department of internal medicine at the University of Utah, Salt Lake City. Dr. Fang is with the division of gastroenterology and hepatology at the University of Utah.
Introduction
Gastroenterologists are in a unique position to manage individuals with feeding tubes as their training underscores principles in digestion, absorption, nutrition support, and enteral tube placement. Adequate management of individuals with feeding tubes and, importantly, the complications that arise from feeding tube use and placement require a basic understanding of intestinal anatomy and physiology. Therefore, gastroenterologists are well suited to both place and manage individuals with feeding tubes in the long term.
Indications for tube feeding
When deciding on the appropriate route for artificial nutrition support, the first decision to be made is enteral access versus parenteral nutrition support. Enteral nutrition confers multiple benefits, including preservation of the mucosal lining, reductions in complicated infections, decreased costs, and improved patient compliance. All attempts at adequate enteral access should be made before deciding on the use of parenteral nutrition. Following the clinical decision to pursue artificial means of nutrition support and enteral access, the next common decision is the anticipated duration of nutrition support. Generally, the oral or nasal tubes are used for short durations (i.e., less than 4 weeks) with percutaneous placement into the stomach or small intestine for longer-term feeding (i.e., percutaneous endoscopic gastrostomy [PEG] or percutaneous endoscopic jejunostomy [PEJ]).

The most general indication for nutrition support is an inability to maintain adequate nutritional needs with oral intake alone. General categories of inadequate oral intake include neurologic disorders, malignancy, and gastrointestinal conditions affecting digestion and absorption (Table 1). Absolute and relative contraindications to PEG placement are listed in Table 2. If an endoscopic placement is not possible, alternative means of placement (i.e., surgery or interventional radiology) can be considered to avoid the consequences of prolonged malnutrition. In-hospital mortality following PEG placement has decreased 40% over the last 10 years, which can be attributed to improved patient selection, enhanced discharge practices, and exclusion of patients with the highest comorbidity and mortality rates, like those with advanced dementia or terminal cancer.1
PEG placement in patients with dementia is controversial, with previous studies not demonstrating improved outcomes and association with high mortality rates,2 so the practice is currently not recommended by the American Geriatrics Society in individuals with advanced dementia.3 However, a large Japanese study showed that careful selection of patients with mild dementia to undergo gastrostomy increased independence fourfold; therefore, multidisciplinary involvement is often necessary in the decision to pursue artificial means of nutrition support in this population.4
The recent coronavirus disease 2019 (COVID-19) pandemic has placed additional strains on endoscopic placement and has highlighted the effect of the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) on GI symptoms. A recent meta-analysis showed an overall incidence of GI symptoms of 17.6% in the following conditions in decreasing order of prevalence: anorexia, diarrhea, nausea, vomiting, and abdominal discomfort.5 In addition, the prolonged ventilatory requirements among a subset of individuals with the most severe COVID-19 results in extended periods of nutrition support via enteral tube placements. In individuals with ICU-acquired weakness and discharge to long-term care facilities, the placement of percutaneous endoscopic tubes may be required, although with the additional consideration of the need for an aerosolizing procedure. Delay of placement has been advocated, in addition to appropriate personal protective equipment, in order to ensure safe placement for the endoscopy staff.6
Types of feeding tubes
After deciding to feed a patient enterally and determining the anticipated duration of enteral support, the next decision is to determine the most appropriate location of feeding delivery: into the stomach or the small bowel. Gastric feeding is advantageous most commonly because of its increased capacity, allowing for larger volumes to be delivered over shorter durations. However, in the setting of postsurgical anatomy, gastroparesis, or obstructing tumors/pancreatic inflammation, distal delivery of tube feeds may be required into the jejunum. Additionally, percutaneous tubes placed into the stomach can have extenders into the small bowel (GJ tubes) to allow for feeding into the small bowel and decompression or delivery of medications into the stomach.
In general, gastric feeding is preferred over small bowel feeding as PEG tubes are more stable and have fewer complications than either PEG-J or direct PEJ tubes. Gastrostomy tubes are generally shorter and larger in diameter making them less likely to clog. PEG-J tubes have separate lumens for gastric and small intestinal access, but the smaller-bore jejunal extension tubes are more likely to clog or become dislodged. While direct PEJ is shown to have higher rates of tube patency and decreased rates of endoscopic re-intervention, compared with PEG-J,7 one limitation of a direct PEJ is difficulty in placement and site selection, which can be performed with a pediatric colonoscope or balloon enteroscopy system. Most commonly, this procedure is performed under general anesthesia.
In the case of a critically ill patient in the ICU, it is recommended to start enteral nutrition within 24-48 hours of arrival to avoid complications of prolonged calorie deficits. Nasally inserted feeding tubes (e.g., Cortrak, Avanos Medical Devices, Alpharetta, Ga.) are most commonly used at the bedside and can be placed blindly using electromagnetic image guidance, radiographically, or endoscopy. However, the small caliber of nasoenteric tubes comes with the common complication of clogging, which can be overcome with slightly larger bore gastric feeding tubes. If gastric feeding is not tolerated (e.g., in the case of vomiting, witnessed aspiration), small bowel feeding should be initiated and can be a more durable form of enteral feeding with fewer interruptions as feedings do not need to be held for procedures or symptomatic gastric intolerance. In clinical areas of question, or if there is a concern for intolerance of enteral feeding, a short trial with nasogastric or nasojejunal tube placement should be performed before a more definitive percutaneous placement.
With respect to percutaneous tubes, important characteristics to choose are the size (diameter in French units), type of internal retention device, and external appearance of the tube (standard or low profile). All percutaneous tubes contain an external retention device (i.e., bumper) that fits against the skin and an internal retention device that is either a balloon or plastic dome or funnel that prevents the tube from becoming dislodged. Balloon retention tubes require replacement every 3-6 months, while nonballoon tubes generally require replacement annually in order to prevent the plastic from cracking, which can make removal complicated. Low-profile tubes have an external cap, which, when opened, allows for extension tubing to be securely attached while in use and detached while not in use. Low-profile tubes are often preferred among younger, active patients and those with adequate dexterity to allow for attachment of the external extension tubing. These tubes are most often inserted as a replacement for an initially endoscopically placed tube, although one-step systems for initial placement are available. The size of the low-profile tube is chosen based on the size of the existing PEG tube and by measuring the length of the stoma tract using specialized measuring devices.8 Patients and caregivers can also be trained to replace balloon-type tubes on their own to limit complications of displaced or cracked tubes. Low-profile tubes are commercially available for both gastric placement and gastric placement with extension into the small bowel, which often requires fluoroscopy for secure placement.
All percutaneous enteral tubes are being transitioned to the ENfit connector system, which prevents connections from the enteral system to nonenteral systems (namely intravenous lines, chest tubes) and vice versa. Tubing misconnections have been rarely reported, and the EnFIT system is designed to prevent such misadventures that have resulted in serious complications and even mortality.9 Adapter devices are available that may be required for patients with feeding tubes who have not been transitioned yet. Most commonly with new tube placements and replacements, patients and providers will have to become familiar with the new syringes and feeding bags required with EnFIT connectors.
Gastrostomy placement can be considered a higher-risk endoscopic procedure. One complicating factor is the increased use of antiplatelet and anticoagulant therapies in individuals with a history of neurologic insults. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines recommend that coumadin be held 5 days before the procedure and bridged with heparin if the patient is at high risk of thromboembolic complications. For patients on dual anti-platelet therapy, thienopyridines like clopidogrel are often stopped 5-7 days prior to procedure with continuation of aspirin,10 but there are more recent data that PEG insertion is safe with continued use of DAPT.11 Direct-acting anticoagulants (DOACs) are often stopped 24-48 hours prior to procedure and then restarted 48 hours after tube placement, but this is dependent on the half-life of the specific DOAC and the patient’s renal function. Patients with decreased creatinine clearance may need to hold the DOAC up to 3-4 days prior to the procedure. In this situation, referring to ASGE guidelines and consultation with a hematologist or managing anti-coagulation clinic is advised.10
Troubleshooting complications
Nasoenteric tubes: One of the most common and irritating complications with nasoenteric feeding tubes is clogging. To prevent clogging, the tube should be flushed frequently.12 At least 30 mL of free water should be used to flush the tube every 4-8 hours for continuous feedings or before and after bolus feeding. Additionally, 15-30 mL of water should be given with each separate medication administration, and if possible, medication administration via small-bore small bowel feeding tubes should be avoided.12 Water flushing is especially important with small-caliber tubes and pumps that deliver both feeding and water flushes. It is available for small bowel feeding in order to allow for programmed water delivery.
Warm water flushes can also help unclog the tube,12 and additional pharmacologic and mechanical devices have been promoted for clogged tubes. One common technique is mixing pancreatic enzymes (Viokase) with a crushed 325-mg tablet of nonenteric coated sodium bicarbonate and 5 mL of water to create a solution that has the alkaline properties allowing for both pancreatic enzyme activation and clog dissolution. Additionally, an endoscopic retrograde cholangiopancreatography (ERCP) catheter can be placed into longer feeding tubes to directly infuse the activated agent to the site of the clog.13 If water and enzymes are not successful in unclogging the tube, commercially available brushes can help remove clogs. The TubeClear® system (Actuated Medical, Bellefonte, Penna) has a single-use stem that is connected to AC power to create a jackhammerlike movement to remove clogs in longer nasoenteral and gastrojejunal tubes.
PEG tubes (short-term complications): Procedural and immediate postprocedural complications include bleeding, aspiration, pneumoperitoneum, and perforation. Pneumoperitoneum occurs in approximately 50% of cases and is generally clinically insignificant. The risk of pneumoperitoneum can be reduced by using CO2 insufflation.14 If the patient develops systemic signs of infection or peritoneal signs, CT scan with oral contrast is warranted for further evaluation and to assess for inadvertent perforation of overlying bowel or dislodged tube. Aspiration during or following endoscopy is another common complication of PEG placement and risk factors include over-sedation, supine positioning, advanced age, and neurologic dysfunction. This risk can be mitigated by avoiding over-sedation, immediately aspirating gastric contents when the stomach is reached, and avoiding excessive insufflation.15 In addition, elevating the head of the bed during the procedure and dedicating an assistant to perform oral suctioning during the entire procedure is recommended.
PEG tubes (long-term complications): More delayed complications of PEG insertion include wound infection, buried bumper syndrome, tumor seeding, peristomal leakage, and tube dislodgement. The prevalence of wound infection is 5%- 25%,16 and randomized controlled trials have demonstrated the efficacy of a single dose of an IV antibiotic (i.e., cephalosporin) in those not already receiving a broad spectrum antibiotic and administered prophylactically before tube placement.17 The significance of this reduction is such that antibiotic administration before tube placement should be considered a quality measure for the procedure. A small amount of redness around the tube site (less than 5 mm) is typical, but extension of erythema, warmth, tenderness, purulent drainage, or systemic symptoms is consistent with infection and warrants additional antibiotic administration. Minor infections can be treated with local antiseptics and oral antibiotics, and early intervention is important to prevent need for hospital admission, systemic antibiotics, and even surgical debridement.
Peristomal leakage is reported in approximately 1%-2% of patients.18 Photographs of the site can be very useful in evaluating and managing peristomal leakage and infections. Interventions include reducing gastric secretions with proton pump inhibitors and management of the skin with barrier creams, such as zinc oxide (Calmoseptine®) ointment. Placement of a larger-diameter tube only enlarges the stoma track and worsens the leakage. In such cases, thorough evaluations for delayed gastric emptying (gastroparesis), distal obstruction, or constipation should be performed and managed accordingly. Opiates are common contributors to constipation and delayed gastric emptying and often require reduction in use or directed antagonist therapy to reduce leaking. Continuous feeding over bolus feedings and delivering nutrition distally into the small bowel (PEG-J placement) can improve leaking from gastrostomy tubes. Additional means of management include stabilizing the tube by replacing a traditional tube with a low-profile tube or using right-angle external bumpers. If all measures fail, removing the tube and allowing for stomal closure can be attempted,16 although this option often requires parenteral nutrition support to prevent prolonged periods of inadequate nutrition.
Buried bumper syndrome (BBS) occurs in 1.5%-8.8% of PEG placements and is a common late complication of PEG placement, although early reports have been described.18 The development of BBS occurs when the internal bumper migrates from the gastric lumen through and into the stomach or abdominal wall. It occurs more frequently with solid nonballoon retention tubes and is caused by excessive compression of the external bumper against the skin and abdominal wall. Patients with BBS usually present with an immobile catheter, resistance with feeds (because of a closure of the stomach wall around the internal portion of the gastrostomy tube), abdominal pain, or peristomal leakage. Physicians should be aware of and assess tubes for BBS, in particular when replacing an immobile tube (cannot be pushed into the free stomach lumen) or when there is difficulty in flushing water into the tube. This complication can be easily prevented by allowing a minimum of 0.5-1.0 cm (1 finger breadth) between the external bumper and the abdominal wall. In particular, patients and caregivers should be warned that if the patient gains significant amounts of weight, the outer bumper will need to be loosened. Once BBS is diagnosed, the PEG tube requires removal and replacement as it can cause bleeding, infection, or fasciitis. The general steps to replacement include endoscopic removal of the existing tube and replacement of new PEG in the existing tract as long as the BBS is not severe. In most cases a replacement tube can be pulled into place using the pull-PEG technique at the same gastrostomy site as long as the stoma tract can be cannulated with a wire after the existing tube is removed.
Similar to nasoenteric tubes, PEG tubes can become clogged, although this complication is infrequent. The primary steps for prevention include adequately flushing with water before and after feeds and ensuring that all medications are liquid or well crushed and dissolved before instilling. Timely tube replacement also ensures that the internal portions of the gastrostomy tube remain free of debris. Management is similar to that of unclogging nasoenteral tubes, as discussed above, and specific commercial declogging devices for PEG tubes include the Bionix Declogger® (Bionix Development Corp., Toledo, Ohio) and the Bard® PEG cleaning brush (Bard Peripheral Vascular Inc., Tempe, Ariz.). The Bionix system has a plastic stem with a screw and thread design that will remove clogs in 14-24 French PEG tubes, while the Bard brush has a flexible nylon stem with soft bristles at the end to prevent mucosal injury and can be used for prophylaxis against clogs, as well as removing clogs themselves.12
Lastly, a rare but important complication of PEG placement is tumor seeding of the PEG site in patients with active head and neck or upper gastrointestinal cancer.19 The presumed mechanism is shearing of tumor cells as the PEG is pulled through the upper aerodigestive tract and through the wall of the stomach, as prior studies have demonstrated frequent seeding of tubes and incision sites as shown by brushing the tube for malignant cells after tube placement.20 It is important to recognize this complication and not misdiagnose it as granulation tissue, infection, or bleeding as the spread of the cancer generally portends a poor prognosis. Therefore, it is best to use a PEG insertion technique that does not involve pulling or pushing the PEG through the upper aerodigestive tract in patients with active cancer and instead place tubes via an external approach by colleagues in interventional radiology or via direct surgical placement.
Conclusion
Gastroenterologists occupy a unique role in evaluation, diagnosis, and management of patients requiring enteral feeding. In addition, they are best equipped to place, prevent, and manage complications of tube feeding. For this reason, it is imperative that gastroenterologists familiarize themselves with indications for enteral tubes and types of enteral tubes available, as well as the identification and management of common complications. Comprehensive understanding of these concepts will augment the practicing gastroenterologist’s ability to manage patients requiring enteral nutrition support with confidence.
References
1. Stein DJ et al. Dig Dis Sci. 2020 Jun 19. doi: 10.1007/s10620-020-06396-y.
2. American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. J Am Geriatr Soc. 2014;62(8):1590-3.
3. Dietrich CG, Schoppmeyer K. World J Gastroenterol. 2020;26(20):2464-71.
4. Suzuki Y et al. T Gastroenterology Res.2012 Feb;5(1):10-20.
5. Cheung KS et al. Gastroenterology. 2020 Jul;159(1):81-95.
6. Micic D et al. Am J Gastroenterol. 2020 Sep;115(9):1367-70.
7. Fan AC et al. Gastrointest Endosc. 2002;56(6):890-4.
8. Tang SJ. Video J Encycl GI Endosc. 2014;2(2):70-3.
9. Guenter P, Lyman B. Nutr Clin Pract. 2016;31(6):769-72.
10. Acosta RD et al. Gastrointest Endosc. 2016;83(1):3-16.
11. Richter JA et al. Gastrointest Endosc. 2011;74(1):22-34.
12. Boullata JI et al. JPEN. 2017;41(1):15-103.
13. McClave SA. Tech Gastrointest Endosc. 2021;3(1):62-8.
14. Murphy CJ et al. Endosc Int Open. 2016;4(3):E292. doi: 10.1053/tgie.2001.19915.
15. Lynch CR et al. Pract Gastroenterology. 2004;28:66-77.
16. Hucl T et al. Best Pract Res Clin Gastroenterol. 2016;30(5):769-81. doi: 10.1016/j.bpg.2016.10.002.
17. Jafri NS et al. Aliment Pharmacol & Therapeut. 2007;25(6):647-56. doi: 10.1111/j.1365-2036.2007.03247.x.
18. Blumenstein I et al. World J Gastroenterol. 2014;20(26):8505-24. doi: 10.3748/wjg.v20.i26.8505.
19. Fung E et al. Surgical Endosc. 2017;31(9):3623-7. doi: 10.1007/s00464-016-5394-8.
20. Ellrichmann M et al. Endoscopy. 2013;45(07):526-31. doi: 10.1055/s-0033-1344023.
Dr. Toy is with the department of internal medicine at the University of Utah, Salt Lake City. Dr. Fang is with the division of gastroenterology and hepatology at the University of Utah.
How productivity influences compensation in private practice
When starting a career in gastroenterology, physicians tend to work in the hospital, where there is usually high demand for services and productivity goals are easy to meet. This is a little different in private GI groups, where it takes some time to build up your patient base. This might be a significant concern for young physicians considering private practice. But understanding the role that productivity plays in compensation packages can help in choosing the right group to join.
While compensation models may differ from practice to practice, there is usually a base salary provided with a productivity bonus. Some practices may use productivity along with other measures to determine when a physician is eligible to become a partner in the practice. Partnership is often accompanied with the benefits of ancillary services ownership such as ambulatory surgery centers (ASCs) and anesthesia, pathology, and infusion services.
How is productivity measured?
Most practices utilize relative value units (RVUs), a standard used by Medicare to determine the amount to pay physicians according to their productivity. Most public and private payers are utilizing the RVU system first developed for Medicare as a useful, time-saving way to handle physician payments. The RVU defines the volume of work doctors perform for all procedures and services covered under the Medicare Physician Fee Schedule.
The Medicare Physician Payment System has three components:
• The geographic practice cost indices (GPCIs)
• Relative value units (RVUs)
• A conversion factor
It is important to understand the types of RVUs that exist to understand how to calculate them properly – these include the following categories:
• Physician work, which accounts for the time and effort to perform a procedure.
• Practice expense, which is for the costs of nonphysician labor such as rent and supplies.
• Global fees, which includes fees for initial visits, follow-ups, and practice expense, and applies during a predetermined length of time known as the “global periods,” primarily for major surgeries.
• Malpractice expense, such as costs for professional liability insurance.
There is no specific dollar amount attached to an RVU because RVUs are part of a resource-based relative value scale (RBRVS) which uses RVUs to relate medical procedures to each other. Payment for physician work is based on whether the procedure is performed in an ASC or hospital outpatient department or in an office. A separate facility fee payment is made to the ASC or hospital outpatient department for procedures performed there. Other elements include skills and the amount of time needed to perform a procedure. Calculating the reimbursement from an RVU involves several components and a significant amount of complex math.
Meeting goals while building a practice
For many young physicians working in the hospital where patients are plentiful, it might seem daunting to build your practice with productivity goals. Practices should, and many do, design their initial productivity plans to minimum or mean RVUs for young physicians rather than someone 10 years into practice. Younger physicians have fellowship and training, but it takes years to become highly efficient with time and productivity. It’s important for everyone involved to set attainable benchmarks.
The practice should also do its best to support your efforts to grow your patient base. While you should be expected to develop relationships with referring physicians, you’ll benefit from the practice’s marketing efforts. When new patients come in, they usually go to newly hired physicians because more senior physicians are booked weeks or months in advance.
Practice administrators also work hard to time new hires to overlap with expected retirements. Senior partners will always have follow-up colonoscopies and associates will need to take on these cases as their colleagues retire. In some practices, younger physicians are expected to take the hospital on call schedules or respond to emergency department calls, so it shouldn’t be difficult to meet productivity goals.
And once you become a partner and are further along on in your career, your productivity plan will change. Some groups have productivity-based compensation, which allows more senior partners to work when they want to – as long as they are meeting the productivity rates that will cover their portion of the practice expenses.
If a physician is consistently not meeting productivity measures, a practice may exercise the right to terminate the relationship, but this is rare. More often, physicians meet their productivity levels and receive certain bonuses for exceeding their goals. In most practices, the partners you work with will know if you aren’t meeting your goals. In most cases, they will take on a mentorship role to help you succeed.
Ask questions, be engaged
Another thing to be aware of is that all practices worth joining make sure productivity plans do not violate the Stark Law, anti-kickback statutes, or other regulations. A huge red flag to look out for is a productivity plan that is based on the number of procedures – it should never be tied to volume.
It’s also best to consider how often the productivity plan is measured. It might be a red flag if it is measured weekly or monthly or if there are heavy consequences for not meeting RVU goals. Most groups look at productivity on a quarterly basis and integrate those discussions into a standard review process.
The successful early-career GIs we interview in our practices are those who are interested in understanding the ins and outs of our practices and what they can achieve through practicing independently. The practices worth joining will likewise be interested in discussing your level of entrepreneurship, the opportunities for you to grow in your career, and what it takes to be on the track to partner.
Dr. Baig is a practicing gastroenterologist at Allied Digestive Care in New Jersey and is the chair of communications for the Digestive Health Physicians Association (DHPA); Mr. Harlen is the president of PE Practice Solutions and immediate past chief operating officer of Capital Digestive Care in Maryland. He is the executive director of DHPA.
When starting a career in gastroenterology, physicians tend to work in the hospital, where there is usually high demand for services and productivity goals are easy to meet. This is a little different in private GI groups, where it takes some time to build up your patient base. This might be a significant concern for young physicians considering private practice. But understanding the role that productivity plays in compensation packages can help in choosing the right group to join.
While compensation models may differ from practice to practice, there is usually a base salary provided with a productivity bonus. Some practices may use productivity along with other measures to determine when a physician is eligible to become a partner in the practice. Partnership is often accompanied with the benefits of ancillary services ownership such as ambulatory surgery centers (ASCs) and anesthesia, pathology, and infusion services.
How is productivity measured?
Most practices utilize relative value units (RVUs), a standard used by Medicare to determine the amount to pay physicians according to their productivity. Most public and private payers are utilizing the RVU system first developed for Medicare as a useful, time-saving way to handle physician payments. The RVU defines the volume of work doctors perform for all procedures and services covered under the Medicare Physician Fee Schedule.
The Medicare Physician Payment System has three components:
• The geographic practice cost indices (GPCIs)
• Relative value units (RVUs)
• A conversion factor
It is important to understand the types of RVUs that exist to understand how to calculate them properly – these include the following categories:
• Physician work, which accounts for the time and effort to perform a procedure.
• Practice expense, which is for the costs of nonphysician labor such as rent and supplies.
• Global fees, which includes fees for initial visits, follow-ups, and practice expense, and applies during a predetermined length of time known as the “global periods,” primarily for major surgeries.
• Malpractice expense, such as costs for professional liability insurance.
There is no specific dollar amount attached to an RVU because RVUs are part of a resource-based relative value scale (RBRVS) which uses RVUs to relate medical procedures to each other. Payment for physician work is based on whether the procedure is performed in an ASC or hospital outpatient department or in an office. A separate facility fee payment is made to the ASC or hospital outpatient department for procedures performed there. Other elements include skills and the amount of time needed to perform a procedure. Calculating the reimbursement from an RVU involves several components and a significant amount of complex math.
Meeting goals while building a practice
For many young physicians working in the hospital where patients are plentiful, it might seem daunting to build your practice with productivity goals. Practices should, and many do, design their initial productivity plans to minimum or mean RVUs for young physicians rather than someone 10 years into practice. Younger physicians have fellowship and training, but it takes years to become highly efficient with time and productivity. It’s important for everyone involved to set attainable benchmarks.
The practice should also do its best to support your efforts to grow your patient base. While you should be expected to develop relationships with referring physicians, you’ll benefit from the practice’s marketing efforts. When new patients come in, they usually go to newly hired physicians because more senior physicians are booked weeks or months in advance.
Practice administrators also work hard to time new hires to overlap with expected retirements. Senior partners will always have follow-up colonoscopies and associates will need to take on these cases as their colleagues retire. In some practices, younger physicians are expected to take the hospital on call schedules or respond to emergency department calls, so it shouldn’t be difficult to meet productivity goals.
And once you become a partner and are further along on in your career, your productivity plan will change. Some groups have productivity-based compensation, which allows more senior partners to work when they want to – as long as they are meeting the productivity rates that will cover their portion of the practice expenses.
If a physician is consistently not meeting productivity measures, a practice may exercise the right to terminate the relationship, but this is rare. More often, physicians meet their productivity levels and receive certain bonuses for exceeding their goals. In most practices, the partners you work with will know if you aren’t meeting your goals. In most cases, they will take on a mentorship role to help you succeed.
Ask questions, be engaged
Another thing to be aware of is that all practices worth joining make sure productivity plans do not violate the Stark Law, anti-kickback statutes, or other regulations. A huge red flag to look out for is a productivity plan that is based on the number of procedures – it should never be tied to volume.
It’s also best to consider how often the productivity plan is measured. It might be a red flag if it is measured weekly or monthly or if there are heavy consequences for not meeting RVU goals. Most groups look at productivity on a quarterly basis and integrate those discussions into a standard review process.
The successful early-career GIs we interview in our practices are those who are interested in understanding the ins and outs of our practices and what they can achieve through practicing independently. The practices worth joining will likewise be interested in discussing your level of entrepreneurship, the opportunities for you to grow in your career, and what it takes to be on the track to partner.
Dr. Baig is a practicing gastroenterologist at Allied Digestive Care in New Jersey and is the chair of communications for the Digestive Health Physicians Association (DHPA); Mr. Harlen is the president of PE Practice Solutions and immediate past chief operating officer of Capital Digestive Care in Maryland. He is the executive director of DHPA.
When starting a career in gastroenterology, physicians tend to work in the hospital, where there is usually high demand for services and productivity goals are easy to meet. This is a little different in private GI groups, where it takes some time to build up your patient base. This might be a significant concern for young physicians considering private practice. But understanding the role that productivity plays in compensation packages can help in choosing the right group to join.
While compensation models may differ from practice to practice, there is usually a base salary provided with a productivity bonus. Some practices may use productivity along with other measures to determine when a physician is eligible to become a partner in the practice. Partnership is often accompanied with the benefits of ancillary services ownership such as ambulatory surgery centers (ASCs) and anesthesia, pathology, and infusion services.
How is productivity measured?
Most practices utilize relative value units (RVUs), a standard used by Medicare to determine the amount to pay physicians according to their productivity. Most public and private payers are utilizing the RVU system first developed for Medicare as a useful, time-saving way to handle physician payments. The RVU defines the volume of work doctors perform for all procedures and services covered under the Medicare Physician Fee Schedule.
The Medicare Physician Payment System has three components:
• The geographic practice cost indices (GPCIs)
• Relative value units (RVUs)
• A conversion factor
It is important to understand the types of RVUs that exist to understand how to calculate them properly – these include the following categories:
• Physician work, which accounts for the time and effort to perform a procedure.
• Practice expense, which is for the costs of nonphysician labor such as rent and supplies.
• Global fees, which includes fees for initial visits, follow-ups, and practice expense, and applies during a predetermined length of time known as the “global periods,” primarily for major surgeries.
• Malpractice expense, such as costs for professional liability insurance.
There is no specific dollar amount attached to an RVU because RVUs are part of a resource-based relative value scale (RBRVS) which uses RVUs to relate medical procedures to each other. Payment for physician work is based on whether the procedure is performed in an ASC or hospital outpatient department or in an office. A separate facility fee payment is made to the ASC or hospital outpatient department for procedures performed there. Other elements include skills and the amount of time needed to perform a procedure. Calculating the reimbursement from an RVU involves several components and a significant amount of complex math.
Meeting goals while building a practice
For many young physicians working in the hospital where patients are plentiful, it might seem daunting to build your practice with productivity goals. Practices should, and many do, design their initial productivity plans to minimum or mean RVUs for young physicians rather than someone 10 years into practice. Younger physicians have fellowship and training, but it takes years to become highly efficient with time and productivity. It’s important for everyone involved to set attainable benchmarks.
The practice should also do its best to support your efforts to grow your patient base. While you should be expected to develop relationships with referring physicians, you’ll benefit from the practice’s marketing efforts. When new patients come in, they usually go to newly hired physicians because more senior physicians are booked weeks or months in advance.
Practice administrators also work hard to time new hires to overlap with expected retirements. Senior partners will always have follow-up colonoscopies and associates will need to take on these cases as their colleagues retire. In some practices, younger physicians are expected to take the hospital on call schedules or respond to emergency department calls, so it shouldn’t be difficult to meet productivity goals.
And once you become a partner and are further along on in your career, your productivity plan will change. Some groups have productivity-based compensation, which allows more senior partners to work when they want to – as long as they are meeting the productivity rates that will cover their portion of the practice expenses.
If a physician is consistently not meeting productivity measures, a practice may exercise the right to terminate the relationship, but this is rare. More often, physicians meet their productivity levels and receive certain bonuses for exceeding their goals. In most practices, the partners you work with will know if you aren’t meeting your goals. In most cases, they will take on a mentorship role to help you succeed.
Ask questions, be engaged
Another thing to be aware of is that all practices worth joining make sure productivity plans do not violate the Stark Law, anti-kickback statutes, or other regulations. A huge red flag to look out for is a productivity plan that is based on the number of procedures – it should never be tied to volume.
It’s also best to consider how often the productivity plan is measured. It might be a red flag if it is measured weekly or monthly or if there are heavy consequences for not meeting RVU goals. Most groups look at productivity on a quarterly basis and integrate those discussions into a standard review process.
The successful early-career GIs we interview in our practices are those who are interested in understanding the ins and outs of our practices and what they can achieve through practicing independently. The practices worth joining will likewise be interested in discussing your level of entrepreneurship, the opportunities for you to grow in your career, and what it takes to be on the track to partner.
Dr. Baig is a practicing gastroenterologist at Allied Digestive Care in New Jersey and is the chair of communications for the Digestive Health Physicians Association (DHPA); Mr. Harlen is the president of PE Practice Solutions and immediate past chief operating officer of Capital Digestive Care in Maryland. He is the executive director of DHPA.
AGA News
Career Development Workshops Series
The AGA Career Development Workshops equip trainees and early-career GIs with indispensable knowledge and skills to successfully navigate the career path ahead. Over the course of the workshops, you will gain vital insights and advice to advance in your career with education not formally part of the training program curriculum. Workshops take place virtually and include topics like “How to Evaluate a Job in 2021,” “How to Succeed in Academic or Private Practice During COVID-19,” “Life in Industry,” and more. Workshops continue to be added monthly. Register today.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® (DDW) moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, members of the digestive disease community have connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Gastro.org/DDW2021.
We did it!
Thank you for helping us raise $231,357 on AGA Giving Day to fund health disparity research!
The past few months were unlike any we’ve ever experienced, and above all we knew we needed to take action to provide a better future for digestive health patients. That’s why AGA and the AGA Research Foundation launched AGA Giving Day to address health disparities that negatively affect our patients head on. We couldn’t have led the fight to eradicate disparities in GI without our loyal supporters.
AGA Giving Day provided an opportunity to do something about health care differences that lead to poorer outcomes due to race and socioeconomic status. Thanks to the support of all our donors and funders, we raised $231,357 to fund health disparities research.
All donations will go directly into research awards earmarked for GI health disparities research. Health disparities research is the key to understanding how we can improve disease management for every patient.
During these trying times, there is one thing that hasn’t and won’t change: our commitment to our mission of raising funds to support talented researchers in gastroenterology and hepatology. While there is still more work ahead, we know we can move forward with the help of friends like you.
Thank you for being part of our fight to eradicate disparities in GI. Learn more about our other efforts through the AGA Equity Project.
Gastro.org/GivingDay
Career Development Workshops Series
The AGA Career Development Workshops equip trainees and early-career GIs with indispensable knowledge and skills to successfully navigate the career path ahead. Over the course of the workshops, you will gain vital insights and advice to advance in your career with education not formally part of the training program curriculum. Workshops take place virtually and include topics like “How to Evaluate a Job in 2021,” “How to Succeed in Academic or Private Practice During COVID-19,” “Life in Industry,” and more. Workshops continue to be added monthly. Register today.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® (DDW) moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, members of the digestive disease community have connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Gastro.org/DDW2021.
We did it!
Thank you for helping us raise $231,357 on AGA Giving Day to fund health disparity research!
The past few months were unlike any we’ve ever experienced, and above all we knew we needed to take action to provide a better future for digestive health patients. That’s why AGA and the AGA Research Foundation launched AGA Giving Day to address health disparities that negatively affect our patients head on. We couldn’t have led the fight to eradicate disparities in GI without our loyal supporters.
AGA Giving Day provided an opportunity to do something about health care differences that lead to poorer outcomes due to race and socioeconomic status. Thanks to the support of all our donors and funders, we raised $231,357 to fund health disparities research.
All donations will go directly into research awards earmarked for GI health disparities research. Health disparities research is the key to understanding how we can improve disease management for every patient.
During these trying times, there is one thing that hasn’t and won’t change: our commitment to our mission of raising funds to support talented researchers in gastroenterology and hepatology. While there is still more work ahead, we know we can move forward with the help of friends like you.
Thank you for being part of our fight to eradicate disparities in GI. Learn more about our other efforts through the AGA Equity Project.
Gastro.org/GivingDay
Career Development Workshops Series
The AGA Career Development Workshops equip trainees and early-career GIs with indispensable knowledge and skills to successfully navigate the career path ahead. Over the course of the workshops, you will gain vital insights and advice to advance in your career with education not formally part of the training program curriculum. Workshops take place virtually and include topics like “How to Evaluate a Job in 2021,” “How to Succeed in Academic or Private Practice During COVID-19,” “Life in Industry,” and more. Workshops continue to be added monthly. Register today.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® (DDW) moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, members of the digestive disease community have connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Gastro.org/DDW2021.
We did it!
Thank you for helping us raise $231,357 on AGA Giving Day to fund health disparity research!
The past few months were unlike any we’ve ever experienced, and above all we knew we needed to take action to provide a better future for digestive health patients. That’s why AGA and the AGA Research Foundation launched AGA Giving Day to address health disparities that negatively affect our patients head on. We couldn’t have led the fight to eradicate disparities in GI without our loyal supporters.
AGA Giving Day provided an opportunity to do something about health care differences that lead to poorer outcomes due to race and socioeconomic status. Thanks to the support of all our donors and funders, we raised $231,357 to fund health disparities research.
All donations will go directly into research awards earmarked for GI health disparities research. Health disparities research is the key to understanding how we can improve disease management for every patient.
During these trying times, there is one thing that hasn’t and won’t change: our commitment to our mission of raising funds to support talented researchers in gastroenterology and hepatology. While there is still more work ahead, we know we can move forward with the help of friends like you.
Thank you for being part of our fight to eradicate disparities in GI. Learn more about our other efforts through the AGA Equity Project.
Gastro.org/GivingDay
Calendar
For more information about the American Gastroenterological Association’s upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
Upcoming Events
May 1, 2021
2021 AGA Postgraduate Course (Virtual Event)
Discover emerging science, leverage new tools and technologies and build lasting collaborations that will transform GI research and patient care at the AGA Postgraduate Course. Receive updates here.
May 21-23, 2021
Digestive Disease Week® (Virtual Event)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Early bird registration: Jan. 20 to Mar. 31, 2021.
Award Deadlines
AGA Student Abstract Award
This award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Student Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This award provides support to early career (i.e., 35 years of age or younger at the time of Digestive Disease Week® (DDW)) basic, translational or clinical investigators residing outside North America giving abstract-based oral or poster presentations at DDW.
Application Deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Fellow Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Fellowship (SURF)
These fellowships support undergraduate students from groups traditionally underrepresented in biomedical research to perform 10 weeks of research related to digestive diseases under the mentorship of top investigators in the fields of gastroenterology and hepatology. The award provides a stipend, funding to offset travel and meal expenses, and opportunities to learn about future training and career options.
Application Deadline: Feb. 24, 2021
For more information about the American Gastroenterological Association’s upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
Upcoming Events
May 1, 2021
2021 AGA Postgraduate Course (Virtual Event)
Discover emerging science, leverage new tools and technologies and build lasting collaborations that will transform GI research and patient care at the AGA Postgraduate Course. Receive updates here.
May 21-23, 2021
Digestive Disease Week® (Virtual Event)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Early bird registration: Jan. 20 to Mar. 31, 2021.
Award Deadlines
AGA Student Abstract Award
This award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Student Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This award provides support to early career (i.e., 35 years of age or younger at the time of Digestive Disease Week® (DDW)) basic, translational or clinical investigators residing outside North America giving abstract-based oral or poster presentations at DDW.
Application Deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Fellow Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Fellowship (SURF)
These fellowships support undergraduate students from groups traditionally underrepresented in biomedical research to perform 10 weeks of research related to digestive diseases under the mentorship of top investigators in the fields of gastroenterology and hepatology. The award provides a stipend, funding to offset travel and meal expenses, and opportunities to learn about future training and career options.
Application Deadline: Feb. 24, 2021
For more information about the American Gastroenterological Association’s upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
Upcoming Events
May 1, 2021
2021 AGA Postgraduate Course (Virtual Event)
Discover emerging science, leverage new tools and technologies and build lasting collaborations that will transform GI research and patient care at the AGA Postgraduate Course. Receive updates here.
May 21-23, 2021
Digestive Disease Week® (Virtual Event)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Early bird registration: Jan. 20 to Mar. 31, 2021.
Award Deadlines
AGA Student Abstract Award
This award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Student Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This award provides support to early career (i.e., 35 years of age or younger at the time of Digestive Disease Week® (DDW)) basic, translational or clinical investigators residing outside North America giving abstract-based oral or poster presentations at DDW.
Application Deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Fellow Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Fellowship (SURF)
These fellowships support undergraduate students from groups traditionally underrepresented in biomedical research to perform 10 weeks of research related to digestive diseases under the mentorship of top investigators in the fields of gastroenterology and hepatology. The award provides a stipend, funding to offset travel and meal expenses, and opportunities to learn about future training and career options.
Application Deadline: Feb. 24, 2021
February 2021 – ICYMI
GASTROENTEROLOGY
October 2020
How to incorporate a chief fellow into a gastroenterology fellowship program. Mohammad Bilal et al. 2020 Oct;159(4):1227-30. doi: 10.1053/j.gastro.2020.09.001
Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Pu Wang et al. 2020 Oct;159(4):1252-61.e5. doi: 10.1053/j.gastro.2020.06.023
November 2020
Simulation-based mastery learning with virtual coaching: experience in training standardized upper endoscopy to novice endoscopists. Roy Soetikno et al. 2020 Nov;159(5):1632-6. doi: 10.1053/j.gastro.2020.06.096
Risk of small bowel adenocarcinoma, adenomas, and carcinoids in a nationwide cohort of individuals with celiac disease. Louise Emilsson et al. 2020 Nov;159(5):1686-94.e2 doi: 10.1053/j.gastro.2020.07.007
December 2020
Increased intestinal permeability is associated with later development of Crohn’s disease. Williams Turpin et al. 2020 Dec;159(6):2092-100.e5. doi: 10.1053/j.gastro.2020.08.005
January 2021
The role of angiotensin converting enzyme 2 in modulating gut microbiota, intestinal inflammation, and coronavirus infection. Josef M. Penninger et al. 2020 Oct 30:S0016-5085(20)35327-0. doi: 10.1053/j.gastro.2020.07.067
Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. William D. Chey et al. 2020 Oct 19:S0016-5085(20)35281-1. doi: 10.1053/j.gastro.2020.06.099
Efficacy and safety of tradipitant in patients with diabetic and idiopathic gastroparesis in a randomized, placebo-controlled trial. Jesse L. Carlin et al 2020 Jul 18;S0016-5085(20)34958-1. doi: 10.1053/j.gastro.2020.07.029
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
November 2020
The virtual gastroenterology clinic. Toyia James-Stevenson. 2020 Nov;18(12):2679-82. doi: 10.1016/j.cgh.2020.06.012
Risk factors associated with early-onset colorectal cancer. Valerie Gausman et al. 2020 Nov;18(12):2752-9.e2. doi: 10.1016/j.cgh.2019.10.009
Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis c virus infection. Teng-Yu Lee et al. 2020 Nov;18(12):2784-92.e7. doi: 10.1016/j.cgh.2020.04.036
December 2020
Sensitivity of fecal immunochemical test for colorectal cancer detection differs according to stage and location. Tobias Niedermaier et al. 2020 Dec;18(13):2920-2928.e6. doi: 10.1016/j.cgh.2020.01.025
Effects of colesevelam on bowel symptoms, biomarkers, and colonic mucosal gene expression in patients with bile acid diarrhea in a randomized trial. Priya Vijayvargiya et al. 2020 Dec;18(13):2962-70.e6. doi: 10.1016/j.cgh.2020.02.027
Endoscopy for gastric cancer screening is cost effective for Asian Americans in the United States. Shailja C. Shah et al. 2020 Dec;18(13):3026-39. doi: 10.1016/j.cgh.2020.07.031
January 2021
C.O.V.I.D.: A survival guide for GI fellowship training during the COVID-19 pandemic. Tzu-Hao Lee et al. 2021 Jan;19(1):6-9. doi: 10.1016/j.cgh.2020.10.001
Use of proton pump inhibitors increases risk of incident kidney stones. Michael Simonov et al. 2021 Jan;19(1):72-9.e21. doi: 10.1016/j.cgh.2020.02.053
TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Endoscopic extraction of large foreign bodies utilizing a novel push-pull extraction technique. Koushik K. Das and Michael L. Kochman. 2020 Oct;22(4):172-7. doi: 10.1016/j.tige.2020.06.004
GASTROENTEROLOGY
October 2020
How to incorporate a chief fellow into a gastroenterology fellowship program. Mohammad Bilal et al. 2020 Oct;159(4):1227-30. doi: 10.1053/j.gastro.2020.09.001
Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Pu Wang et al. 2020 Oct;159(4):1252-61.e5. doi: 10.1053/j.gastro.2020.06.023
November 2020
Simulation-based mastery learning with virtual coaching: experience in training standardized upper endoscopy to novice endoscopists. Roy Soetikno et al. 2020 Nov;159(5):1632-6. doi: 10.1053/j.gastro.2020.06.096
Risk of small bowel adenocarcinoma, adenomas, and carcinoids in a nationwide cohort of individuals with celiac disease. Louise Emilsson et al. 2020 Nov;159(5):1686-94.e2 doi: 10.1053/j.gastro.2020.07.007
December 2020
Increased intestinal permeability is associated with later development of Crohn’s disease. Williams Turpin et al. 2020 Dec;159(6):2092-100.e5. doi: 10.1053/j.gastro.2020.08.005
January 2021
The role of angiotensin converting enzyme 2 in modulating gut microbiota, intestinal inflammation, and coronavirus infection. Josef M. Penninger et al. 2020 Oct 30:S0016-5085(20)35327-0. doi: 10.1053/j.gastro.2020.07.067
Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. William D. Chey et al. 2020 Oct 19:S0016-5085(20)35281-1. doi: 10.1053/j.gastro.2020.06.099
Efficacy and safety of tradipitant in patients with diabetic and idiopathic gastroparesis in a randomized, placebo-controlled trial. Jesse L. Carlin et al 2020 Jul 18;S0016-5085(20)34958-1. doi: 10.1053/j.gastro.2020.07.029
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
November 2020
The virtual gastroenterology clinic. Toyia James-Stevenson. 2020 Nov;18(12):2679-82. doi: 10.1016/j.cgh.2020.06.012
Risk factors associated with early-onset colorectal cancer. Valerie Gausman et al. 2020 Nov;18(12):2752-9.e2. doi: 10.1016/j.cgh.2019.10.009
Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis c virus infection. Teng-Yu Lee et al. 2020 Nov;18(12):2784-92.e7. doi: 10.1016/j.cgh.2020.04.036
December 2020
Sensitivity of fecal immunochemical test for colorectal cancer detection differs according to stage and location. Tobias Niedermaier et al. 2020 Dec;18(13):2920-2928.e6. doi: 10.1016/j.cgh.2020.01.025
Effects of colesevelam on bowel symptoms, biomarkers, and colonic mucosal gene expression in patients with bile acid diarrhea in a randomized trial. Priya Vijayvargiya et al. 2020 Dec;18(13):2962-70.e6. doi: 10.1016/j.cgh.2020.02.027
Endoscopy for gastric cancer screening is cost effective for Asian Americans in the United States. Shailja C. Shah et al. 2020 Dec;18(13):3026-39. doi: 10.1016/j.cgh.2020.07.031
January 2021
C.O.V.I.D.: A survival guide for GI fellowship training during the COVID-19 pandemic. Tzu-Hao Lee et al. 2021 Jan;19(1):6-9. doi: 10.1016/j.cgh.2020.10.001
Use of proton pump inhibitors increases risk of incident kidney stones. Michael Simonov et al. 2021 Jan;19(1):72-9.e21. doi: 10.1016/j.cgh.2020.02.053
TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Endoscopic extraction of large foreign bodies utilizing a novel push-pull extraction technique. Koushik K. Das and Michael L. Kochman. 2020 Oct;22(4):172-7. doi: 10.1016/j.tige.2020.06.004
GASTROENTEROLOGY
October 2020
How to incorporate a chief fellow into a gastroenterology fellowship program. Mohammad Bilal et al. 2020 Oct;159(4):1227-30. doi: 10.1053/j.gastro.2020.09.001
Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Pu Wang et al. 2020 Oct;159(4):1252-61.e5. doi: 10.1053/j.gastro.2020.06.023
November 2020
Simulation-based mastery learning with virtual coaching: experience in training standardized upper endoscopy to novice endoscopists. Roy Soetikno et al. 2020 Nov;159(5):1632-6. doi: 10.1053/j.gastro.2020.06.096
Risk of small bowel adenocarcinoma, adenomas, and carcinoids in a nationwide cohort of individuals with celiac disease. Louise Emilsson et al. 2020 Nov;159(5):1686-94.e2 doi: 10.1053/j.gastro.2020.07.007
December 2020
Increased intestinal permeability is associated with later development of Crohn’s disease. Williams Turpin et al. 2020 Dec;159(6):2092-100.e5. doi: 10.1053/j.gastro.2020.08.005
January 2021
The role of angiotensin converting enzyme 2 in modulating gut microbiota, intestinal inflammation, and coronavirus infection. Josef M. Penninger et al. 2020 Oct 30:S0016-5085(20)35327-0. doi: 10.1053/j.gastro.2020.07.067
Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. William D. Chey et al. 2020 Oct 19:S0016-5085(20)35281-1. doi: 10.1053/j.gastro.2020.06.099
Efficacy and safety of tradipitant in patients with diabetic and idiopathic gastroparesis in a randomized, placebo-controlled trial. Jesse L. Carlin et al 2020 Jul 18;S0016-5085(20)34958-1. doi: 10.1053/j.gastro.2020.07.029
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
November 2020
The virtual gastroenterology clinic. Toyia James-Stevenson. 2020 Nov;18(12):2679-82. doi: 10.1016/j.cgh.2020.06.012
Risk factors associated with early-onset colorectal cancer. Valerie Gausman et al. 2020 Nov;18(12):2752-9.e2. doi: 10.1016/j.cgh.2019.10.009
Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis c virus infection. Teng-Yu Lee et al. 2020 Nov;18(12):2784-92.e7. doi: 10.1016/j.cgh.2020.04.036
December 2020
Sensitivity of fecal immunochemical test for colorectal cancer detection differs according to stage and location. Tobias Niedermaier et al. 2020 Dec;18(13):2920-2928.e6. doi: 10.1016/j.cgh.2020.01.025
Effects of colesevelam on bowel symptoms, biomarkers, and colonic mucosal gene expression in patients with bile acid diarrhea in a randomized trial. Priya Vijayvargiya et al. 2020 Dec;18(13):2962-70.e6. doi: 10.1016/j.cgh.2020.02.027
Endoscopy for gastric cancer screening is cost effective for Asian Americans in the United States. Shailja C. Shah et al. 2020 Dec;18(13):3026-39. doi: 10.1016/j.cgh.2020.07.031
January 2021
C.O.V.I.D.: A survival guide for GI fellowship training during the COVID-19 pandemic. Tzu-Hao Lee et al. 2021 Jan;19(1):6-9. doi: 10.1016/j.cgh.2020.10.001
Use of proton pump inhibitors increases risk of incident kidney stones. Michael Simonov et al. 2021 Jan;19(1):72-9.e21. doi: 10.1016/j.cgh.2020.02.053
TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Endoscopic extraction of large foreign bodies utilizing a novel push-pull extraction technique. Koushik K. Das and Michael L. Kochman. 2020 Oct;22(4):172-7. doi: 10.1016/j.tige.2020.06.004
Meet the hosts of AGA’s new podcast: Small Talk, Big Topics
Matthew Whitson, MD, MSEd (lead host)
Walk us through your current GI role and your path to getting there:
I am currently the GI fellowship director at Hofstra-Northwell, by way of Mount Sinai in New York City for medical school and residency and the University of Pennsylvania, Philadelphia, for GI fellowship. I’m about 60:40 clinical and scholarship. My clinical focus is in esophageal and swallowing disorders, which came about because of mentorship and clinical exposure while at UPenn. During my fellowship, I also got a master’s in medical education again because of the tremendous sponsorship from the faculty and leadership. I have educational roles in the medical school, the internal medicine residency, and, of course, the GI fellowship.
What is your favorite part about your current role? Least favorite part?
Favorite part: working with students and trainees. When you see a medical concept click for them and then see them apply that concept, or that skill, into practice it is incredibly rewarding. Least favorite part: the amount of written documentation needed to run a fellowship.
What are your interests outside of work?
I love going to see live music in New York and touring the museums of New York, preferably the MOMA, or getting to Storm King (an expansive sculpture garden) outside of the city when we can. Anytime we can get outside to go hiking or play golf is a good day.
What advice would you give to…
- Someone who matches into GI on Dec. 2: Celebrate; you’ve earned it! Those projects you started during residency – finish them now. Otherwise, it’s super hard to get them done during fellowship, especially if you are training at a different institution for GI fellowship.
- Someone who just graduated from GI fellowship: Negotiate that contract, and then negotiate it again. Have a budget, and don’t spend that “attending money” on anything major for at least 6 months.
How do you see the future of GI changing as a new generation of trainees enters the workforce?
The way we access information is changing. Everything is at the tip of your fingers at any time, so much so, it can be overwhelming. I think that learning how to critically appraise and access clinically appropriate data is a skill that everyone will need going forward. I think it will take an even more central role in our medical education. Beyond this, the importance of shared decision-making with your patients will continue to increase in the world of personalized medicine, as will the assortment of noninvasive testing options.
Why did you want to host this podcast?
Reading about mentorship, sponsorship, career development, etc. is important, but it doesn’t do these topics justice. It is such a nuanced thing and talking about it, exploring it, teasing it out is just so fun. Plus, I was a radio DJ when younger and have always dreamed of doing something in the audio medium as a professional.
What’s your favorite episode so far?
I won’t say favorite, but I think the Laurie Keefer episode is up there. It was such a nice conversation about a challenging concept: Building resilience in our trainees and ourselves. I learned a lot from her and have begun integrating some of these skills into my work as a program director.
What’s the best piece of advice you’ve gotten that’s helped you in your career so far?
I’m going to adopt this from a mentor of mine, but it’s the “me or my family rule.” What would you want done if the patient in front of you were your family member? If you keep that as your “True North,” then I think you are off to a good start as a clinician.
Nina Nandy, MD, MS (co-host)
Walk us through your current GI role and your path to getting there:
I think the biggest decision to make in medical school is medicine or surgery, and most things will fall under one of those categories. I liked the problem solving of medicine and the hands-on work of surgery, so I was leaning toward a procedural field then met some wonderful mentors in GI when I was in medical school. I think every field of medicine has a particular personality, and when I met gastroenterologists, it clicked with me, and I thought “I’ve found my people.” So, I went to residency in internal medicine with the goal of GI or bust. I am currently a practicing gastroenterologist, and I do general GI, liver disease, and motility.
What is your favorite part about your current role? Least favorite part?
I really love GI. I feel like I’ve found my calling, and its really exciting to be able to say that. What drew me to GI was the use of technology and minimally invasive endoscopy to see a person inside out and understand their pathology, the mix of chronic and acute conditions, and the educational aspect of talking to folks in clinic. I like putting people at ease, and GI is a great field for jokes. My least favorite part is doing peer-to-peers with insurance companies to get inflammatory bowel disease drugs approved.
What are your interests outside of work?
Outside of work, this podcast, and being division vice chief, I like to learn languages. I speak five and am working on a sixth. I’m writing a secret screenplay. I play piano and guitar, which reminds me of a quote: “All my life I wanted to play guitar badly. And now I play guitar. Badly.” I also love art; I use oil paint, acrylics, pen and ink, mixed media. I love to dance and am just getting into Peloton. But perhaps my most important role is maintaining the Instagram account for my two famous cats who will hopefully enable me to retire early. Are you out there, Purina?
What advice would you give to…
- Someone who matches into GI on Dec. 2: First of all, celebrate! Treat yo’self; you did it! Welcome to the most exciting field of medicine. But seriously, congratulate yourself for your hard work and don’t worry about being terrible at scoping because there’s a learning curve. Don’t worry about what you need to study because you are going to do it. Come in with an inquisitive, open mind. Don’t turn down consults because they seem ridiculous. You can always learn something! I think the best thing to do in fellowship is to do everything. Learn that motility and capsule, cannulate that common bile duct, place that esophageal stent! You won’t have this kind of support in the future, and you should get comfortable with everything possible while you can.
- Someone who just graduated from GI fellowship: As with those matching into GI, celebrate! Treat yo’self; you did it! I think this is the hardest transition; you don’t have that safety net anymore. You are the be-all, end-all last stop on the train. Just kidding. It seems that way, but you can always collaborate with colleagues and look things up on UpToDate. You know more than you think, and it is a continuous learning process, so it’s okay to have questions; it means you care. Yes, there will be more responsibility, and you need to keep up on path and your inbox because it will pile up. You need to think about appropriate follow-up and resources to offer your patients. You can keep up on current guidelines through your GI societies; do continuing medical education and postgraduate courses as well.
How do you see the future of GI changing as a new generation of trainees enters the workforce?
I think the future of GI is innovation, technology, social media, multidisciplinary learning. GI is a technology-centered field, and there will be new developments in medical devices and basic science research, such as the microbiome, which holds the key for numerous pathogenic processes. Physicians will need to be physician-scientists, physician-innovators, physician-business people, and physician-leaders. We must learn things beyond our own field to be successful in this changing world.
Why did you want to host this podcast?
I wanted to host this podcast because I think there is so much in fellowship we learn about GI but also so much we don’t learn about GI careers and the “real world” of practice. I wanted to create content focused on career development for early GIs and trainees and discuss “everything you wanted to know in fellowship but were afraid to ask.” I wanted to interview real successful people in the field, whether it be focusing on a career in medical education, basic science research, transplant hepatology, therapeutic endoscopy, or private practice. There are a lot of podcasts that do a great job focusing on guidelines, case reports, and research, but we wanted to take this one in a different direction. It is a great way to reach a broad audience across many platforms.
What’s your favorite episode so far?
I really like the Janice Jou episode. Not just because I’m on it, but also because she is a great, a dynamic, speaker, and our conversation was so effortless, and because she is a phenomenal program director and educator and has such valuable advice for trainees and early career gastroenterologists, drawing from her own experiences. Her tips – or rather “Janice jewels,” as I am trying to trademark on negotiation – are excellent. Check it out!
What’s the best piece of advice you’ve gotten that’s helped you in your career so far?
Don’t buy a house right out of training. Also, “live your life, not someone else’s.”
C.S. Tse, MD (co-host)
Walk us through your current GI role and your path to getting there:
I grew up in Toronto and moved to the United States for medical school at the Yale University, New Haven, Conn., and internal medicine residency at the Mayo Clinic in Rochester, Minnesota. During my residency, I became interested in gastroenterology with a particular interest in inflammatory bowel disease after studying the postoperative outcomes of IBD patients on biologics and examining the clinical course of IBD patients with coexistent celiac disease. I am a third-year gastroenterology fellow at Brown University. I will spend a year as the advanced IBD fellow at the University of California–San Diego from July 2021 to June 2022. My current research examines IBD patients’ quality of care and the psychosocial impacts on patients’ disease course. I am working with the Crohn’s and Colitis Foundation’s IBD Qorus Learning Health System to improve the quality of care and outcomes of patients with IBD.
What is your favorite part about your current role? Least favorite part?
My favorite part of my current role is to combine patient care with clinical research, particularly for patients with IBD. My least favorite part is encountering “red tape” that may give a false sense of productivity but not actually be beneficial for patient care. Some of this is discussed in this article from the Harvard Business Review.
What are your interests outside of work?
I serve as the National President of the American Medical Women’s Association (AMWA) Residents & Fellows Division. I am a Core Faculty member of the AMWA IGNITE MD program, which is a nation-wide initiative to educate and empower female medical trainees. I currently serve as an abstract reviewer for Digestive Diseases Week® (since 2018). I previously served as an abstract reviewer and judge for the American Medical Association’s Scientific Symposium (2019 & 2020). Outside of work, I enjoy hiking, traveling, and reading.
What advice would you give to someone who matches into GI on Dec. 2:
Identify mentors early. (You can have more than one!) Try to imagine where you want your career to be in 5 years – generalist vs. specialist. Will you have a niche in practice? Is advanced endoscopy (ERCP, EUS, etc.) going to be a part of your practice? Academic, private practice, community practice, or hybrid? Knowing your goals will help tailor the GI fellowship experience to get you to where you want to be in your career. GI fellowship may be like a buffet table where there are many opportunities and options, but one can rarely do it all! Choosing and pursuing experiences that ultimately align with your goals can help you make the most out of your time during GI fellowship training.
How do you see the future of GI changing as a new generation of trainees enters the workforce?
I think that there will be more integration of information technology and artificial intelligence into GI, just as for the rest of society. For example, we can see this clearly illustrated in the rapid uptake of telemedicine (including GI) during COVID-19.
Why did you want to host this podcast?
I am intrigued by the opportunity to connect with GIs broadly through this AGA podcast. It is a portable way to use on-demand technology to engage in conversations relevant to other early GIs who may not be conventionally addressed by other means, such as journal articles, conferences, traditional didactics, and books.
What’s your favorite episode so far?
Janice Jou’s podcast was phenomenal in providing mentorship advice (at a distance) to trainees who are interested in an academic career in clinical medicine.
What’s the best piece of advice you’ve gotten that’s helped you in your career so far?
“We are what we repeatedly do. Excellence, therefore, is not an act, but a habit.” This advice is most commonly credited to Aristotle.
Be sure to subscribe wherever you listen to podcasts or listen on the AGA website: https://gastro.org/podcast.
Dr. Whitson is GI fellowship director, Zucker School of Medicine at Hofstra-Northwell, Great Neck, N.Y. @MJWhitsonMD. Dr. Nandy is a gastroenterologist at Presbyterian Medical Group, Albuquerque, N.M. @NinaNandyMD. Dr. Tse is a GI fellow at Brown University, Providence, R.I. @CSTseMD.
Matthew Whitson, MD, MSEd (lead host)
Walk us through your current GI role and your path to getting there:
I am currently the GI fellowship director at Hofstra-Northwell, by way of Mount Sinai in New York City for medical school and residency and the University of Pennsylvania, Philadelphia, for GI fellowship. I’m about 60:40 clinical and scholarship. My clinical focus is in esophageal and swallowing disorders, which came about because of mentorship and clinical exposure while at UPenn. During my fellowship, I also got a master’s in medical education again because of the tremendous sponsorship from the faculty and leadership. I have educational roles in the medical school, the internal medicine residency, and, of course, the GI fellowship.
What is your favorite part about your current role? Least favorite part?
Favorite part: working with students and trainees. When you see a medical concept click for them and then see them apply that concept, or that skill, into practice it is incredibly rewarding. Least favorite part: the amount of written documentation needed to run a fellowship.
What are your interests outside of work?
I love going to see live music in New York and touring the museums of New York, preferably the MOMA, or getting to Storm King (an expansive sculpture garden) outside of the city when we can. Anytime we can get outside to go hiking or play golf is a good day.
What advice would you give to…
- Someone who matches into GI on Dec. 2: Celebrate; you’ve earned it! Those projects you started during residency – finish them now. Otherwise, it’s super hard to get them done during fellowship, especially if you are training at a different institution for GI fellowship.
- Someone who just graduated from GI fellowship: Negotiate that contract, and then negotiate it again. Have a budget, and don’t spend that “attending money” on anything major for at least 6 months.
How do you see the future of GI changing as a new generation of trainees enters the workforce?
The way we access information is changing. Everything is at the tip of your fingers at any time, so much so, it can be overwhelming. I think that learning how to critically appraise and access clinically appropriate data is a skill that everyone will need going forward. I think it will take an even more central role in our medical education. Beyond this, the importance of shared decision-making with your patients will continue to increase in the world of personalized medicine, as will the assortment of noninvasive testing options.
Why did you want to host this podcast?
Reading about mentorship, sponsorship, career development, etc. is important, but it doesn’t do these topics justice. It is such a nuanced thing and talking about it, exploring it, teasing it out is just so fun. Plus, I was a radio DJ when younger and have always dreamed of doing something in the audio medium as a professional.
What’s your favorite episode so far?
I won’t say favorite, but I think the Laurie Keefer episode is up there. It was such a nice conversation about a challenging concept: Building resilience in our trainees and ourselves. I learned a lot from her and have begun integrating some of these skills into my work as a program director.
What’s the best piece of advice you’ve gotten that’s helped you in your career so far?
I’m going to adopt this from a mentor of mine, but it’s the “me or my family rule.” What would you want done if the patient in front of you were your family member? If you keep that as your “True North,” then I think you are off to a good start as a clinician.
Nina Nandy, MD, MS (co-host)
Walk us through your current GI role and your path to getting there:
I think the biggest decision to make in medical school is medicine or surgery, and most things will fall under one of those categories. I liked the problem solving of medicine and the hands-on work of surgery, so I was leaning toward a procedural field then met some wonderful mentors in GI when I was in medical school. I think every field of medicine has a particular personality, and when I met gastroenterologists, it clicked with me, and I thought “I’ve found my people.” So, I went to residency in internal medicine with the goal of GI or bust. I am currently a practicing gastroenterologist, and I do general GI, liver disease, and motility.
What is your favorite part about your current role? Least favorite part?
I really love GI. I feel like I’ve found my calling, and its really exciting to be able to say that. What drew me to GI was the use of technology and minimally invasive endoscopy to see a person inside out and understand their pathology, the mix of chronic and acute conditions, and the educational aspect of talking to folks in clinic. I like putting people at ease, and GI is a great field for jokes. My least favorite part is doing peer-to-peers with insurance companies to get inflammatory bowel disease drugs approved.
What are your interests outside of work?
Outside of work, this podcast, and being division vice chief, I like to learn languages. I speak five and am working on a sixth. I’m writing a secret screenplay. I play piano and guitar, which reminds me of a quote: “All my life I wanted to play guitar badly. And now I play guitar. Badly.” I also love art; I use oil paint, acrylics, pen and ink, mixed media. I love to dance and am just getting into Peloton. But perhaps my most important role is maintaining the Instagram account for my two famous cats who will hopefully enable me to retire early. Are you out there, Purina?
What advice would you give to…
- Someone who matches into GI on Dec. 2: First of all, celebrate! Treat yo’self; you did it! Welcome to the most exciting field of medicine. But seriously, congratulate yourself for your hard work and don’t worry about being terrible at scoping because there’s a learning curve. Don’t worry about what you need to study because you are going to do it. Come in with an inquisitive, open mind. Don’t turn down consults because they seem ridiculous. You can always learn something! I think the best thing to do in fellowship is to do everything. Learn that motility and capsule, cannulate that common bile duct, place that esophageal stent! You won’t have this kind of support in the future, and you should get comfortable with everything possible while you can.
- Someone who just graduated from GI fellowship: As with those matching into GI, celebrate! Treat yo’self; you did it! I think this is the hardest transition; you don’t have that safety net anymore. You are the be-all, end-all last stop on the train. Just kidding. It seems that way, but you can always collaborate with colleagues and look things up on UpToDate. You know more than you think, and it is a continuous learning process, so it’s okay to have questions; it means you care. Yes, there will be more responsibility, and you need to keep up on path and your inbox because it will pile up. You need to think about appropriate follow-up and resources to offer your patients. You can keep up on current guidelines through your GI societies; do continuing medical education and postgraduate courses as well.
How do you see the future of GI changing as a new generation of trainees enters the workforce?
I think the future of GI is innovation, technology, social media, multidisciplinary learning. GI is a technology-centered field, and there will be new developments in medical devices and basic science research, such as the microbiome, which holds the key for numerous pathogenic processes. Physicians will need to be physician-scientists, physician-innovators, physician-business people, and physician-leaders. We must learn things beyond our own field to be successful in this changing world.
Why did you want to host this podcast?
I wanted to host this podcast because I think there is so much in fellowship we learn about GI but also so much we don’t learn about GI careers and the “real world” of practice. I wanted to create content focused on career development for early GIs and trainees and discuss “everything you wanted to know in fellowship but were afraid to ask.” I wanted to interview real successful people in the field, whether it be focusing on a career in medical education, basic science research, transplant hepatology, therapeutic endoscopy, or private practice. There are a lot of podcasts that do a great job focusing on guidelines, case reports, and research, but we wanted to take this one in a different direction. It is a great way to reach a broad audience across many platforms.
What’s your favorite episode so far?
I really like the Janice Jou episode. Not just because I’m on it, but also because she is a great, a dynamic, speaker, and our conversation was so effortless, and because she is a phenomenal program director and educator and has such valuable advice for trainees and early career gastroenterologists, drawing from her own experiences. Her tips – or rather “Janice jewels,” as I am trying to trademark on negotiation – are excellent. Check it out!
What’s the best piece of advice you’ve gotten that’s helped you in your career so far?
Don’t buy a house right out of training. Also, “live your life, not someone else’s.”
C.S. Tse, MD (co-host)
Walk us through your current GI role and your path to getting there:
I grew up in Toronto and moved to the United States for medical school at the Yale University, New Haven, Conn., and internal medicine residency at the Mayo Clinic in Rochester, Minnesota. During my residency, I became interested in gastroenterology with a particular interest in inflammatory bowel disease after studying the postoperative outcomes of IBD patients on biologics and examining the clinical course of IBD patients with coexistent celiac disease. I am a third-year gastroenterology fellow at Brown University. I will spend a year as the advanced IBD fellow at the University of California–San Diego from July 2021 to June 2022. My current research examines IBD patients’ quality of care and the psychosocial impacts on patients’ disease course. I am working with the Crohn’s and Colitis Foundation’s IBD Qorus Learning Health System to improve the quality of care and outcomes of patients with IBD.
What is your favorite part about your current role? Least favorite part?
My favorite part of my current role is to combine patient care with clinical research, particularly for patients with IBD. My least favorite part is encountering “red tape” that may give a false sense of productivity but not actually be beneficial for patient care. Some of this is discussed in this article from the Harvard Business Review.
What are your interests outside of work?
I serve as the National President of the American Medical Women’s Association (AMWA) Residents & Fellows Division. I am a Core Faculty member of the AMWA IGNITE MD program, which is a nation-wide initiative to educate and empower female medical trainees. I currently serve as an abstract reviewer for Digestive Diseases Week® (since 2018). I previously served as an abstract reviewer and judge for the American Medical Association’s Scientific Symposium (2019 & 2020). Outside of work, I enjoy hiking, traveling, and reading.
What advice would you give to someone who matches into GI on Dec. 2:
Identify mentors early. (You can have more than one!) Try to imagine where you want your career to be in 5 years – generalist vs. specialist. Will you have a niche in practice? Is advanced endoscopy (ERCP, EUS, etc.) going to be a part of your practice? Academic, private practice, community practice, or hybrid? Knowing your goals will help tailor the GI fellowship experience to get you to where you want to be in your career. GI fellowship may be like a buffet table where there are many opportunities and options, but one can rarely do it all! Choosing and pursuing experiences that ultimately align with your goals can help you make the most out of your time during GI fellowship training.
How do you see the future of GI changing as a new generation of trainees enters the workforce?
I think that there will be more integration of information technology and artificial intelligence into GI, just as for the rest of society. For example, we can see this clearly illustrated in the rapid uptake of telemedicine (including GI) during COVID-19.
Why did you want to host this podcast?
I am intrigued by the opportunity to connect with GIs broadly through this AGA podcast. It is a portable way to use on-demand technology to engage in conversations relevant to other early GIs who may not be conventionally addressed by other means, such as journal articles, conferences, traditional didactics, and books.
What’s your favorite episode so far?
Janice Jou’s podcast was phenomenal in providing mentorship advice (at a distance) to trainees who are interested in an academic career in clinical medicine.
What’s the best piece of advice you’ve gotten that’s helped you in your career so far?
“We are what we repeatedly do. Excellence, therefore, is not an act, but a habit.” This advice is most commonly credited to Aristotle.
Be sure to subscribe wherever you listen to podcasts or listen on the AGA website: https://gastro.org/podcast.
Dr. Whitson is GI fellowship director, Zucker School of Medicine at Hofstra-Northwell, Great Neck, N.Y. @MJWhitsonMD. Dr. Nandy is a gastroenterologist at Presbyterian Medical Group, Albuquerque, N.M. @NinaNandyMD. Dr. Tse is a GI fellow at Brown University, Providence, R.I. @CSTseMD.
Matthew Whitson, MD, MSEd (lead host)
Walk us through your current GI role and your path to getting there:
I am currently the GI fellowship director at Hofstra-Northwell, by way of Mount Sinai in New York City for medical school and residency and the University of Pennsylvania, Philadelphia, for GI fellowship. I’m about 60:40 clinical and scholarship. My clinical focus is in esophageal and swallowing disorders, which came about because of mentorship and clinical exposure while at UPenn. During my fellowship, I also got a master’s in medical education again because of the tremendous sponsorship from the faculty and leadership. I have educational roles in the medical school, the internal medicine residency, and, of course, the GI fellowship.
What is your favorite part about your current role? Least favorite part?
Favorite part: working with students and trainees. When you see a medical concept click for them and then see them apply that concept, or that skill, into practice it is incredibly rewarding. Least favorite part: the amount of written documentation needed to run a fellowship.
What are your interests outside of work?
I love going to see live music in New York and touring the museums of New York, preferably the MOMA, or getting to Storm King (an expansive sculpture garden) outside of the city when we can. Anytime we can get outside to go hiking or play golf is a good day.
What advice would you give to…
- Someone who matches into GI on Dec. 2: Celebrate; you’ve earned it! Those projects you started during residency – finish them now. Otherwise, it’s super hard to get them done during fellowship, especially if you are training at a different institution for GI fellowship.
- Someone who just graduated from GI fellowship: Negotiate that contract, and then negotiate it again. Have a budget, and don’t spend that “attending money” on anything major for at least 6 months.
How do you see the future of GI changing as a new generation of trainees enters the workforce?
The way we access information is changing. Everything is at the tip of your fingers at any time, so much so, it can be overwhelming. I think that learning how to critically appraise and access clinically appropriate data is a skill that everyone will need going forward. I think it will take an even more central role in our medical education. Beyond this, the importance of shared decision-making with your patients will continue to increase in the world of personalized medicine, as will the assortment of noninvasive testing options.
Why did you want to host this podcast?
Reading about mentorship, sponsorship, career development, etc. is important, but it doesn’t do these topics justice. It is such a nuanced thing and talking about it, exploring it, teasing it out is just so fun. Plus, I was a radio DJ when younger and have always dreamed of doing something in the audio medium as a professional.
What’s your favorite episode so far?
I won’t say favorite, but I think the Laurie Keefer episode is up there. It was such a nice conversation about a challenging concept: Building resilience in our trainees and ourselves. I learned a lot from her and have begun integrating some of these skills into my work as a program director.
What’s the best piece of advice you’ve gotten that’s helped you in your career so far?
I’m going to adopt this from a mentor of mine, but it’s the “me or my family rule.” What would you want done if the patient in front of you were your family member? If you keep that as your “True North,” then I think you are off to a good start as a clinician.
Nina Nandy, MD, MS (co-host)
Walk us through your current GI role and your path to getting there:
I think the biggest decision to make in medical school is medicine or surgery, and most things will fall under one of those categories. I liked the problem solving of medicine and the hands-on work of surgery, so I was leaning toward a procedural field then met some wonderful mentors in GI when I was in medical school. I think every field of medicine has a particular personality, and when I met gastroenterologists, it clicked with me, and I thought “I’ve found my people.” So, I went to residency in internal medicine with the goal of GI or bust. I am currently a practicing gastroenterologist, and I do general GI, liver disease, and motility.
What is your favorite part about your current role? Least favorite part?
I really love GI. I feel like I’ve found my calling, and its really exciting to be able to say that. What drew me to GI was the use of technology and minimally invasive endoscopy to see a person inside out and understand their pathology, the mix of chronic and acute conditions, and the educational aspect of talking to folks in clinic. I like putting people at ease, and GI is a great field for jokes. My least favorite part is doing peer-to-peers with insurance companies to get inflammatory bowel disease drugs approved.
What are your interests outside of work?
Outside of work, this podcast, and being division vice chief, I like to learn languages. I speak five and am working on a sixth. I’m writing a secret screenplay. I play piano and guitar, which reminds me of a quote: “All my life I wanted to play guitar badly. And now I play guitar. Badly.” I also love art; I use oil paint, acrylics, pen and ink, mixed media. I love to dance and am just getting into Peloton. But perhaps my most important role is maintaining the Instagram account for my two famous cats who will hopefully enable me to retire early. Are you out there, Purina?
What advice would you give to…
- Someone who matches into GI on Dec. 2: First of all, celebrate! Treat yo’self; you did it! Welcome to the most exciting field of medicine. But seriously, congratulate yourself for your hard work and don’t worry about being terrible at scoping because there’s a learning curve. Don’t worry about what you need to study because you are going to do it. Come in with an inquisitive, open mind. Don’t turn down consults because they seem ridiculous. You can always learn something! I think the best thing to do in fellowship is to do everything. Learn that motility and capsule, cannulate that common bile duct, place that esophageal stent! You won’t have this kind of support in the future, and you should get comfortable with everything possible while you can.
- Someone who just graduated from GI fellowship: As with those matching into GI, celebrate! Treat yo’self; you did it! I think this is the hardest transition; you don’t have that safety net anymore. You are the be-all, end-all last stop on the train. Just kidding. It seems that way, but you can always collaborate with colleagues and look things up on UpToDate. You know more than you think, and it is a continuous learning process, so it’s okay to have questions; it means you care. Yes, there will be more responsibility, and you need to keep up on path and your inbox because it will pile up. You need to think about appropriate follow-up and resources to offer your patients. You can keep up on current guidelines through your GI societies; do continuing medical education and postgraduate courses as well.
How do you see the future of GI changing as a new generation of trainees enters the workforce?
I think the future of GI is innovation, technology, social media, multidisciplinary learning. GI is a technology-centered field, and there will be new developments in medical devices and basic science research, such as the microbiome, which holds the key for numerous pathogenic processes. Physicians will need to be physician-scientists, physician-innovators, physician-business people, and physician-leaders. We must learn things beyond our own field to be successful in this changing world.
Why did you want to host this podcast?
I wanted to host this podcast because I think there is so much in fellowship we learn about GI but also so much we don’t learn about GI careers and the “real world” of practice. I wanted to create content focused on career development for early GIs and trainees and discuss “everything you wanted to know in fellowship but were afraid to ask.” I wanted to interview real successful people in the field, whether it be focusing on a career in medical education, basic science research, transplant hepatology, therapeutic endoscopy, or private practice. There are a lot of podcasts that do a great job focusing on guidelines, case reports, and research, but we wanted to take this one in a different direction. It is a great way to reach a broad audience across many platforms.
What’s your favorite episode so far?
I really like the Janice Jou episode. Not just because I’m on it, but also because she is a great, a dynamic, speaker, and our conversation was so effortless, and because she is a phenomenal program director and educator and has such valuable advice for trainees and early career gastroenterologists, drawing from her own experiences. Her tips – or rather “Janice jewels,” as I am trying to trademark on negotiation – are excellent. Check it out!
What’s the best piece of advice you’ve gotten that’s helped you in your career so far?
Don’t buy a house right out of training. Also, “live your life, not someone else’s.”
C.S. Tse, MD (co-host)
Walk us through your current GI role and your path to getting there:
I grew up in Toronto and moved to the United States for medical school at the Yale University, New Haven, Conn., and internal medicine residency at the Mayo Clinic in Rochester, Minnesota. During my residency, I became interested in gastroenterology with a particular interest in inflammatory bowel disease after studying the postoperative outcomes of IBD patients on biologics and examining the clinical course of IBD patients with coexistent celiac disease. I am a third-year gastroenterology fellow at Brown University. I will spend a year as the advanced IBD fellow at the University of California–San Diego from July 2021 to June 2022. My current research examines IBD patients’ quality of care and the psychosocial impacts on patients’ disease course. I am working with the Crohn’s and Colitis Foundation’s IBD Qorus Learning Health System to improve the quality of care and outcomes of patients with IBD.
What is your favorite part about your current role? Least favorite part?
My favorite part of my current role is to combine patient care with clinical research, particularly for patients with IBD. My least favorite part is encountering “red tape” that may give a false sense of productivity but not actually be beneficial for patient care. Some of this is discussed in this article from the Harvard Business Review.
What are your interests outside of work?
I serve as the National President of the American Medical Women’s Association (AMWA) Residents & Fellows Division. I am a Core Faculty member of the AMWA IGNITE MD program, which is a nation-wide initiative to educate and empower female medical trainees. I currently serve as an abstract reviewer for Digestive Diseases Week® (since 2018). I previously served as an abstract reviewer and judge for the American Medical Association’s Scientific Symposium (2019 & 2020). Outside of work, I enjoy hiking, traveling, and reading.
What advice would you give to someone who matches into GI on Dec. 2:
Identify mentors early. (You can have more than one!) Try to imagine where you want your career to be in 5 years – generalist vs. specialist. Will you have a niche in practice? Is advanced endoscopy (ERCP, EUS, etc.) going to be a part of your practice? Academic, private practice, community practice, or hybrid? Knowing your goals will help tailor the GI fellowship experience to get you to where you want to be in your career. GI fellowship may be like a buffet table where there are many opportunities and options, but one can rarely do it all! Choosing and pursuing experiences that ultimately align with your goals can help you make the most out of your time during GI fellowship training.
How do you see the future of GI changing as a new generation of trainees enters the workforce?
I think that there will be more integration of information technology and artificial intelligence into GI, just as for the rest of society. For example, we can see this clearly illustrated in the rapid uptake of telemedicine (including GI) during COVID-19.
Why did you want to host this podcast?
I am intrigued by the opportunity to connect with GIs broadly through this AGA podcast. It is a portable way to use on-demand technology to engage in conversations relevant to other early GIs who may not be conventionally addressed by other means, such as journal articles, conferences, traditional didactics, and books.
What’s your favorite episode so far?
Janice Jou’s podcast was phenomenal in providing mentorship advice (at a distance) to trainees who are interested in an academic career in clinical medicine.
What’s the best piece of advice you’ve gotten that’s helped you in your career so far?
“We are what we repeatedly do. Excellence, therefore, is not an act, but a habit.” This advice is most commonly credited to Aristotle.
Be sure to subscribe wherever you listen to podcasts or listen on the AGA website: https://gastro.org/podcast.
Dr. Whitson is GI fellowship director, Zucker School of Medicine at Hofstra-Northwell, Great Neck, N.Y. @MJWhitsonMD. Dr. Nandy is a gastroenterologist at Presbyterian Medical Group, Albuquerque, N.M. @NinaNandyMD. Dr. Tse is a GI fellow at Brown University, Providence, R.I. @CSTseMD.
Impostor syndrome: Implications for medical professionals
A few years ago, I was asked to give a talk on impostor syndrome at a national conference. My initial thought was “I am not even remotely qualified to give this talk.” Upon reflection, I think that was the first time I acknowledged that I, too, suffer from this syndrome.
There are many definitions and designations (e.g., impostor phenomenon or fraud syndrome), but the one I use most often is high-achieving individuals who are marked by an inability to internalize their accomplishments and a persistent fear of being exposed as a fraud. They live in fear that their incompetence will be exposed and they will be revealed as a fraud both intellectually and within their job or role. First described by Clance and Imes in 1978,1 the original authors observed that many highly respected and accomplished women did not experience an internal sense of success despite their education and evidence of academic achievement. Based in part on previous observations regarding the differential attribution of success in men and women,2 the authors suggested that two general principles were found to be at the heart of this syndrome. The first was that an unexpected performance outcome will be attributed to a temporary cause. The second was that an expected performance outcome will be attributed to a stable cause. As such, the authors originally suggested that women tended to explain failure with lack of ability, whereas men attributed failure to luck or task difficulty. Furthermore, the authors emphasized environmental factors – such as mentorship, competition, and isolation – as the primary influence in the development of these tendencies.
Although originally described in women, this phenomenon can also affect men, as well as a wide variety of people from different occupations and cultures.3-6 Furthermore, although environmental factors were originally linked as the primary driver of these tendencies, further research has suggested that personality factors play a larger role, and that up to 70% of people may experience this phenomenon in their lifetime.7 Personality traits such as perfectionism and neuroticism may be linked to the development of this phenomenon.3,8
There are several online screening questionnaires that can be used to gauge whether individuals experience some or most of these traits. On one such questionnaire, the Clance IP Scale,9 poses such questions as: “I have often succeeded on a test or task even though I was afraid that I would not do well before I undertook the task” and “I am afraid people important to me may find out that I am not as capable as they think I am.” There are 20 questions scored from 1 to 5 and a score of 40 or below suggests few impostor tendencies, while a score of 80 or above suggests the respondent often has intense IP experiences. The higher the score, the more frequently and seriously the impostor syndrome interferes in a person’s life. What is unclear is whether this worsens, improves, or stays the same throughout one’s career. Of interest is that my personal score at this time is 43; however, it would have been 89 had I taken the test during college and medical school. What is unclear to me from the literature is what factors may play a role in a person’s perception of their abilities and their personal confidence over time.
Why is this important? Given that we are all professionals, impostor tendencies appear to have significant impact in the context of our work. This may have impact on us both as employers and as employees.10 Individuals with impostor syndrome tendencies often characterize themselves negatively and perform poorly on self-appraisals.11 In a study of 201 Belgian white-collar workers, Vergauwe and colleagues found that impostor syndrome tendencies were negatively related to job satisfaction and organizational citizenship behavior; both of which could be influenced by a high degree of social support.10 Individuals with impostor syndrome tendencies do less career planning, explore career options less frequently, and are less inclined to lead.12,13 These tendencies can be detrimental as the most qualified people for a position or opportunity may not step forward for consideration. Employers may tend to overlook these individuals for promotions or for pay raises which could negatively influence future earnings. Furthermore, a person may experience increased burnout as they continuously try to overcompensate for what they perceive as their shortcomings. They may feel concerned they are letting others down or not performing to standards. They may derive less enjoyment from life because of the constant focus on feelings of inadequacy.14 Research along these lines suggest impostor syndrome tendencies can have adverse personal and health-related consequences and may increase social anxiety, depression, and overall psychological distress.15,16
What can we do about it? In a very interesting study by Zanchetta and colleagues, the authors studied 103 young employees and randomized them to receive coaching, training, or no intervention.17 Their findings showed that coaching was an effective mindset intervention which resulted in reduced impostor syndrome scores. Furthermore, fear of negative evaluation and the effect of coaching appeared to be significantly associated with a reduction in the impostor syndrome scores. Coaching appeared to improve self-enhancing attributions and self-efficacy with a reduction in the tendency of subjects to fear negative evaluation. The authors concluded that fostering a mindset shift by reducing the fear of negative evaluations through coaching demonstrated measurable and sustained improvements in overall impostor syndrome scores for participants.17
What do I suggest? It is clear this affects a significant percentage of physicians, health care professionals, and professionals in general. Harboring these tendencies can have a negative impact on health, professional achievement, income, and happiness. It is important to self-reflect, identify if you are at risk, and if so, take the opportunity to explore solutions. My recommendations are:
- Name it: Take the test and see how you score.
- Be mindful: Self-reflection will help you identify the behaviors that are interfering with your happiness and success.
- Write it down: Be strategic and document your plan for success to reinforce your accomplishments.
- Create a feedback group: Friends and colleagues can help to mitigate the negative effects of impostor syndrome tendencies.
- Speak up: Ask for help; coaching has been documented to reduce impostor syndrome scores and help lessen the burden of these tendencies.
- Step out of your comfort zone: Develop a mantra, break bigger challenges into smaller pieces, and acknowledge little wins along the way.
In conclusion, impostor syndrome appears to be highly prevalent in professionals including those of us in medicine. The experience can adversely affect our careers and ability to secure key leadership positions. As managers, we also must keep in mind our role in mentoring others and recognizing the potential impact of impostor syndrome on those who report to us. Recognition of this phenomenon – and understanding of the effects on oneself – is the first step in overcoming the negative effects and moving toward realization of one’s potential.
Dr. Brown is a professor of medicine at Wayne State University, division chief of gastroenterology and hepatology at Henry Ford Hospital, and associate medical director at the Henry Ford Hospital Transplant Institute, all in Detroit.
References
1. Clance PR, Imes S. Psychother Theory Res Pract. 1978 Fall;15(3):1-7.
2. Deaux D. In J.H.Harvey, W.J.Ickes and R.F. Kidd (Eds). New directions in attribution research. Vol. 1. New York: Halsted Press Division, Wiley. 1976; p 335-42.
3. Bernard NS et al. J Pers Assess. 2002;78(2):321-33.
4. Topping ME et al. Acad Psychol Bull. 1985;(7):213-26.
5. Langford J et al. Psychotherapy. 1993;30(3):495-501.
6. Chae JH et al. J Pers Assess. 1995;65(3):468-85.
7. Harvey JC et al. If I’m successful, why do I feel like a fake? New York: Random House, 1985.
8. Ross SR et al. Pers Individ Diff. 2001;31:1347-55.
9. Clance PR. The impostor phenomenon: When success makes you feel like a fake. Toronto: Bantam Books, 1985; p 20-2.
10. Vergauwe J et al. J Bus Psychol. 2015;30:565-81.
11. Leary MR et al. J Pers. 2000;68(4):725-56.
12. Neureiter M et al. Front Psychol. 2016;7:48.
13. Neureiter M et al. J Vocat Behav. 2017;98:56-69.
14. Duhigg C. The power of habit: Why we do what we do in life and business. New York: Random House, 2012.
15. Henning K et al. Med Educ. 1998 Sep;32(5):456-64.
16. Oriel K et al. Fam Med. 2004 Apr;36(4):248-52.
17. Zanchetta M et al. Front Psychol. 2020 May 15;11:405.
A few years ago, I was asked to give a talk on impostor syndrome at a national conference. My initial thought was “I am not even remotely qualified to give this talk.” Upon reflection, I think that was the first time I acknowledged that I, too, suffer from this syndrome.
There are many definitions and designations (e.g., impostor phenomenon or fraud syndrome), but the one I use most often is high-achieving individuals who are marked by an inability to internalize their accomplishments and a persistent fear of being exposed as a fraud. They live in fear that their incompetence will be exposed and they will be revealed as a fraud both intellectually and within their job or role. First described by Clance and Imes in 1978,1 the original authors observed that many highly respected and accomplished women did not experience an internal sense of success despite their education and evidence of academic achievement. Based in part on previous observations regarding the differential attribution of success in men and women,2 the authors suggested that two general principles were found to be at the heart of this syndrome. The first was that an unexpected performance outcome will be attributed to a temporary cause. The second was that an expected performance outcome will be attributed to a stable cause. As such, the authors originally suggested that women tended to explain failure with lack of ability, whereas men attributed failure to luck or task difficulty. Furthermore, the authors emphasized environmental factors – such as mentorship, competition, and isolation – as the primary influence in the development of these tendencies.
Although originally described in women, this phenomenon can also affect men, as well as a wide variety of people from different occupations and cultures.3-6 Furthermore, although environmental factors were originally linked as the primary driver of these tendencies, further research has suggested that personality factors play a larger role, and that up to 70% of people may experience this phenomenon in their lifetime.7 Personality traits such as perfectionism and neuroticism may be linked to the development of this phenomenon.3,8
There are several online screening questionnaires that can be used to gauge whether individuals experience some or most of these traits. On one such questionnaire, the Clance IP Scale,9 poses such questions as: “I have often succeeded on a test or task even though I was afraid that I would not do well before I undertook the task” and “I am afraid people important to me may find out that I am not as capable as they think I am.” There are 20 questions scored from 1 to 5 and a score of 40 or below suggests few impostor tendencies, while a score of 80 or above suggests the respondent often has intense IP experiences. The higher the score, the more frequently and seriously the impostor syndrome interferes in a person’s life. What is unclear is whether this worsens, improves, or stays the same throughout one’s career. Of interest is that my personal score at this time is 43; however, it would have been 89 had I taken the test during college and medical school. What is unclear to me from the literature is what factors may play a role in a person’s perception of their abilities and their personal confidence over time.
Why is this important? Given that we are all professionals, impostor tendencies appear to have significant impact in the context of our work. This may have impact on us both as employers and as employees.10 Individuals with impostor syndrome tendencies often characterize themselves negatively and perform poorly on self-appraisals.11 In a study of 201 Belgian white-collar workers, Vergauwe and colleagues found that impostor syndrome tendencies were negatively related to job satisfaction and organizational citizenship behavior; both of which could be influenced by a high degree of social support.10 Individuals with impostor syndrome tendencies do less career planning, explore career options less frequently, and are less inclined to lead.12,13 These tendencies can be detrimental as the most qualified people for a position or opportunity may not step forward for consideration. Employers may tend to overlook these individuals for promotions or for pay raises which could negatively influence future earnings. Furthermore, a person may experience increased burnout as they continuously try to overcompensate for what they perceive as their shortcomings. They may feel concerned they are letting others down or not performing to standards. They may derive less enjoyment from life because of the constant focus on feelings of inadequacy.14 Research along these lines suggest impostor syndrome tendencies can have adverse personal and health-related consequences and may increase social anxiety, depression, and overall psychological distress.15,16
What can we do about it? In a very interesting study by Zanchetta and colleagues, the authors studied 103 young employees and randomized them to receive coaching, training, or no intervention.17 Their findings showed that coaching was an effective mindset intervention which resulted in reduced impostor syndrome scores. Furthermore, fear of negative evaluation and the effect of coaching appeared to be significantly associated with a reduction in the impostor syndrome scores. Coaching appeared to improve self-enhancing attributions and self-efficacy with a reduction in the tendency of subjects to fear negative evaluation. The authors concluded that fostering a mindset shift by reducing the fear of negative evaluations through coaching demonstrated measurable and sustained improvements in overall impostor syndrome scores for participants.17
What do I suggest? It is clear this affects a significant percentage of physicians, health care professionals, and professionals in general. Harboring these tendencies can have a negative impact on health, professional achievement, income, and happiness. It is important to self-reflect, identify if you are at risk, and if so, take the opportunity to explore solutions. My recommendations are:
- Name it: Take the test and see how you score.
- Be mindful: Self-reflection will help you identify the behaviors that are interfering with your happiness and success.
- Write it down: Be strategic and document your plan for success to reinforce your accomplishments.
- Create a feedback group: Friends and colleagues can help to mitigate the negative effects of impostor syndrome tendencies.
- Speak up: Ask for help; coaching has been documented to reduce impostor syndrome scores and help lessen the burden of these tendencies.
- Step out of your comfort zone: Develop a mantra, break bigger challenges into smaller pieces, and acknowledge little wins along the way.
In conclusion, impostor syndrome appears to be highly prevalent in professionals including those of us in medicine. The experience can adversely affect our careers and ability to secure key leadership positions. As managers, we also must keep in mind our role in mentoring others and recognizing the potential impact of impostor syndrome on those who report to us. Recognition of this phenomenon – and understanding of the effects on oneself – is the first step in overcoming the negative effects and moving toward realization of one’s potential.
Dr. Brown is a professor of medicine at Wayne State University, division chief of gastroenterology and hepatology at Henry Ford Hospital, and associate medical director at the Henry Ford Hospital Transplant Institute, all in Detroit.
References
1. Clance PR, Imes S. Psychother Theory Res Pract. 1978 Fall;15(3):1-7.
2. Deaux D. In J.H.Harvey, W.J.Ickes and R.F. Kidd (Eds). New directions in attribution research. Vol. 1. New York: Halsted Press Division, Wiley. 1976; p 335-42.
3. Bernard NS et al. J Pers Assess. 2002;78(2):321-33.
4. Topping ME et al. Acad Psychol Bull. 1985;(7):213-26.
5. Langford J et al. Psychotherapy. 1993;30(3):495-501.
6. Chae JH et al. J Pers Assess. 1995;65(3):468-85.
7. Harvey JC et al. If I’m successful, why do I feel like a fake? New York: Random House, 1985.
8. Ross SR et al. Pers Individ Diff. 2001;31:1347-55.
9. Clance PR. The impostor phenomenon: When success makes you feel like a fake. Toronto: Bantam Books, 1985; p 20-2.
10. Vergauwe J et al. J Bus Psychol. 2015;30:565-81.
11. Leary MR et al. J Pers. 2000;68(4):725-56.
12. Neureiter M et al. Front Psychol. 2016;7:48.
13. Neureiter M et al. J Vocat Behav. 2017;98:56-69.
14. Duhigg C. The power of habit: Why we do what we do in life and business. New York: Random House, 2012.
15. Henning K et al. Med Educ. 1998 Sep;32(5):456-64.
16. Oriel K et al. Fam Med. 2004 Apr;36(4):248-52.
17. Zanchetta M et al. Front Psychol. 2020 May 15;11:405.
A few years ago, I was asked to give a talk on impostor syndrome at a national conference. My initial thought was “I am not even remotely qualified to give this talk.” Upon reflection, I think that was the first time I acknowledged that I, too, suffer from this syndrome.
There are many definitions and designations (e.g., impostor phenomenon or fraud syndrome), but the one I use most often is high-achieving individuals who are marked by an inability to internalize their accomplishments and a persistent fear of being exposed as a fraud. They live in fear that their incompetence will be exposed and they will be revealed as a fraud both intellectually and within their job or role. First described by Clance and Imes in 1978,1 the original authors observed that many highly respected and accomplished women did not experience an internal sense of success despite their education and evidence of academic achievement. Based in part on previous observations regarding the differential attribution of success in men and women,2 the authors suggested that two general principles were found to be at the heart of this syndrome. The first was that an unexpected performance outcome will be attributed to a temporary cause. The second was that an expected performance outcome will be attributed to a stable cause. As such, the authors originally suggested that women tended to explain failure with lack of ability, whereas men attributed failure to luck or task difficulty. Furthermore, the authors emphasized environmental factors – such as mentorship, competition, and isolation – as the primary influence in the development of these tendencies.
Although originally described in women, this phenomenon can also affect men, as well as a wide variety of people from different occupations and cultures.3-6 Furthermore, although environmental factors were originally linked as the primary driver of these tendencies, further research has suggested that personality factors play a larger role, and that up to 70% of people may experience this phenomenon in their lifetime.7 Personality traits such as perfectionism and neuroticism may be linked to the development of this phenomenon.3,8
There are several online screening questionnaires that can be used to gauge whether individuals experience some or most of these traits. On one such questionnaire, the Clance IP Scale,9 poses such questions as: “I have often succeeded on a test or task even though I was afraid that I would not do well before I undertook the task” and “I am afraid people important to me may find out that I am not as capable as they think I am.” There are 20 questions scored from 1 to 5 and a score of 40 or below suggests few impostor tendencies, while a score of 80 or above suggests the respondent often has intense IP experiences. The higher the score, the more frequently and seriously the impostor syndrome interferes in a person’s life. What is unclear is whether this worsens, improves, or stays the same throughout one’s career. Of interest is that my personal score at this time is 43; however, it would have been 89 had I taken the test during college and medical school. What is unclear to me from the literature is what factors may play a role in a person’s perception of their abilities and their personal confidence over time.
Why is this important? Given that we are all professionals, impostor tendencies appear to have significant impact in the context of our work. This may have impact on us both as employers and as employees.10 Individuals with impostor syndrome tendencies often characterize themselves negatively and perform poorly on self-appraisals.11 In a study of 201 Belgian white-collar workers, Vergauwe and colleagues found that impostor syndrome tendencies were negatively related to job satisfaction and organizational citizenship behavior; both of which could be influenced by a high degree of social support.10 Individuals with impostor syndrome tendencies do less career planning, explore career options less frequently, and are less inclined to lead.12,13 These tendencies can be detrimental as the most qualified people for a position or opportunity may not step forward for consideration. Employers may tend to overlook these individuals for promotions or for pay raises which could negatively influence future earnings. Furthermore, a person may experience increased burnout as they continuously try to overcompensate for what they perceive as their shortcomings. They may feel concerned they are letting others down or not performing to standards. They may derive less enjoyment from life because of the constant focus on feelings of inadequacy.14 Research along these lines suggest impostor syndrome tendencies can have adverse personal and health-related consequences and may increase social anxiety, depression, and overall psychological distress.15,16
What can we do about it? In a very interesting study by Zanchetta and colleagues, the authors studied 103 young employees and randomized them to receive coaching, training, or no intervention.17 Their findings showed that coaching was an effective mindset intervention which resulted in reduced impostor syndrome scores. Furthermore, fear of negative evaluation and the effect of coaching appeared to be significantly associated with a reduction in the impostor syndrome scores. Coaching appeared to improve self-enhancing attributions and self-efficacy with a reduction in the tendency of subjects to fear negative evaluation. The authors concluded that fostering a mindset shift by reducing the fear of negative evaluations through coaching demonstrated measurable and sustained improvements in overall impostor syndrome scores for participants.17
What do I suggest? It is clear this affects a significant percentage of physicians, health care professionals, and professionals in general. Harboring these tendencies can have a negative impact on health, professional achievement, income, and happiness. It is important to self-reflect, identify if you are at risk, and if so, take the opportunity to explore solutions. My recommendations are:
- Name it: Take the test and see how you score.
- Be mindful: Self-reflection will help you identify the behaviors that are interfering with your happiness and success.
- Write it down: Be strategic and document your plan for success to reinforce your accomplishments.
- Create a feedback group: Friends and colleagues can help to mitigate the negative effects of impostor syndrome tendencies.
- Speak up: Ask for help; coaching has been documented to reduce impostor syndrome scores and help lessen the burden of these tendencies.
- Step out of your comfort zone: Develop a mantra, break bigger challenges into smaller pieces, and acknowledge little wins along the way.
In conclusion, impostor syndrome appears to be highly prevalent in professionals including those of us in medicine. The experience can adversely affect our careers and ability to secure key leadership positions. As managers, we also must keep in mind our role in mentoring others and recognizing the potential impact of impostor syndrome on those who report to us. Recognition of this phenomenon – and understanding of the effects on oneself – is the first step in overcoming the negative effects and moving toward realization of one’s potential.
Dr. Brown is a professor of medicine at Wayne State University, division chief of gastroenterology and hepatology at Henry Ford Hospital, and associate medical director at the Henry Ford Hospital Transplant Institute, all in Detroit.
References
1. Clance PR, Imes S. Psychother Theory Res Pract. 1978 Fall;15(3):1-7.
2. Deaux D. In J.H.Harvey, W.J.Ickes and R.F. Kidd (Eds). New directions in attribution research. Vol. 1. New York: Halsted Press Division, Wiley. 1976; p 335-42.
3. Bernard NS et al. J Pers Assess. 2002;78(2):321-33.
4. Topping ME et al. Acad Psychol Bull. 1985;(7):213-26.
5. Langford J et al. Psychotherapy. 1993;30(3):495-501.
6. Chae JH et al. J Pers Assess. 1995;65(3):468-85.
7. Harvey JC et al. If I’m successful, why do I feel like a fake? New York: Random House, 1985.
8. Ross SR et al. Pers Individ Diff. 2001;31:1347-55.
9. Clance PR. The impostor phenomenon: When success makes you feel like a fake. Toronto: Bantam Books, 1985; p 20-2.
10. Vergauwe J et al. J Bus Psychol. 2015;30:565-81.
11. Leary MR et al. J Pers. 2000;68(4):725-56.
12. Neureiter M et al. Front Psychol. 2016;7:48.
13. Neureiter M et al. J Vocat Behav. 2017;98:56-69.
14. Duhigg C. The power of habit: Why we do what we do in life and business. New York: Random House, 2012.
15. Henning K et al. Med Educ. 1998 Sep;32(5):456-64.
16. Oriel K et al. Fam Med. 2004 Apr;36(4):248-52.
17. Zanchetta M et al. Front Psychol. 2020 May 15;11:405.