User login
Erythema multiforme secondary to HSV labialis precipitating sickle cell pain crisis
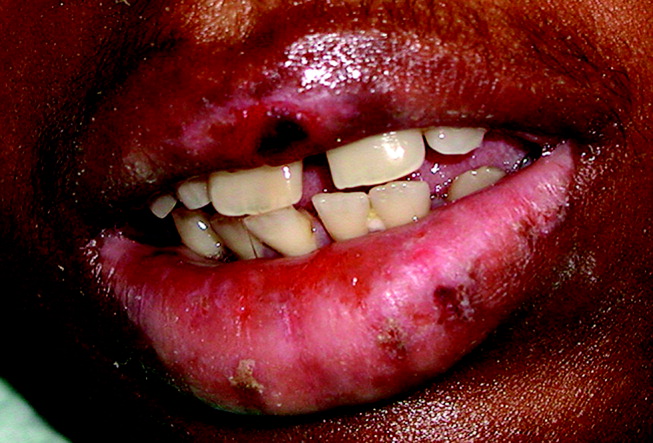
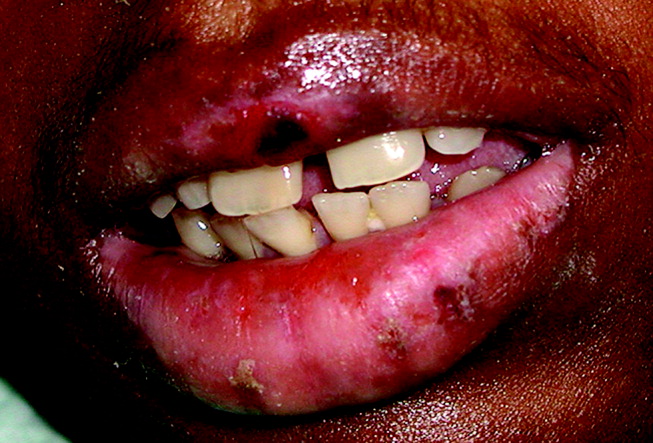
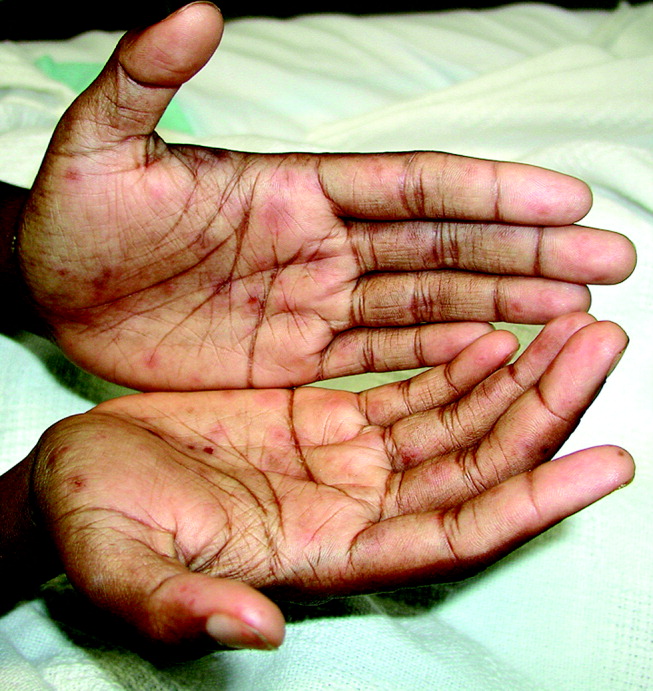
A 28‐year‐old man with sickle cell anemia was admitted with generalized pain. He noted an upper lip lesion 2 weeks prior to admission. He subsequently developed generalized pain in his legs, chest, and back typical of his pain crises. At admission he noted subjective fevers without chills for a week. Vital signs revealed a blood pressure of 135/80, a pulse of 81, a respiratory rate of 16, and an initial temperature of 37.7C. On examination he had scleral icterus and a large upper lip ulcer (Fig. 1). His hospital course was complicated by persistent fevers, a hepatic sequestration crisis, persistent hemolytic anemia requiring blood transfusion, and ultimately the identification of iris‐shaped targetoid lesions on the palms (Fig. 2).These lesions were believed to be consistent with erythema multiforme (EM) secondary to his recent HSV labialis, confirmed by a herpes culture. The patient recovered uneventfully after a 10‐day hospitalization. Erythema multiforme is an acute, self‐limited, but sometimes recurrent dermatologic condition considered to be a distinct hypersensitivity reaction.1 It is associated with certain infections such as herpes simplex 1 and 2, Mycoplasma pneumoniae and fungal infections, and a number of medications in the classes barbiturates, nonsteroidal anti‐inflammatory drugs, penicillins, hydantoins, phenothiazines, and sulfonamides.2 EM is diagnosed clinically by the characteristic rash on the hands and feet, with some cases involving the oral cavity. Treatment is typically focused on resolving the underlying infection or removing the offending drug. Dermatologic manifestations usually improve over 3‐5 weeks without residual sequelae.
- ,,.Understanding the pathogenesis of HSV‐associated erythema multiforme.Dermatology.1998;197:219–222.
- ,,.Erythema multiforme.Am Fam Physician.2006;74:1883–1888.
A 28‐year‐old man with sickle cell anemia was admitted with generalized pain. He noted an upper lip lesion 2 weeks prior to admission. He subsequently developed generalized pain in his legs, chest, and back typical of his pain crises. At admission he noted subjective fevers without chills for a week. Vital signs revealed a blood pressure of 135/80, a pulse of 81, a respiratory rate of 16, and an initial temperature of 37.7C. On examination he had scleral icterus and a large upper lip ulcer (Fig. 1). His hospital course was complicated by persistent fevers, a hepatic sequestration crisis, persistent hemolytic anemia requiring blood transfusion, and ultimately the identification of iris‐shaped targetoid lesions on the palms (Fig. 2).These lesions were believed to be consistent with erythema multiforme (EM) secondary to his recent HSV labialis, confirmed by a herpes culture. The patient recovered uneventfully after a 10‐day hospitalization. Erythema multiforme is an acute, self‐limited, but sometimes recurrent dermatologic condition considered to be a distinct hypersensitivity reaction.1 It is associated with certain infections such as herpes simplex 1 and 2, Mycoplasma pneumoniae and fungal infections, and a number of medications in the classes barbiturates, nonsteroidal anti‐inflammatory drugs, penicillins, hydantoins, phenothiazines, and sulfonamides.2 EM is diagnosed clinically by the characteristic rash on the hands and feet, with some cases involving the oral cavity. Treatment is typically focused on resolving the underlying infection or removing the offending drug. Dermatologic manifestations usually improve over 3‐5 weeks without residual sequelae.


A 28‐year‐old man with sickle cell anemia was admitted with generalized pain. He noted an upper lip lesion 2 weeks prior to admission. He subsequently developed generalized pain in his legs, chest, and back typical of his pain crises. At admission he noted subjective fevers without chills for a week. Vital signs revealed a blood pressure of 135/80, a pulse of 81, a respiratory rate of 16, and an initial temperature of 37.7C. On examination he had scleral icterus and a large upper lip ulcer (Fig. 1). His hospital course was complicated by persistent fevers, a hepatic sequestration crisis, persistent hemolytic anemia requiring blood transfusion, and ultimately the identification of iris‐shaped targetoid lesions on the palms (Fig. 2).These lesions were believed to be consistent with erythema multiforme (EM) secondary to his recent HSV labialis, confirmed by a herpes culture. The patient recovered uneventfully after a 10‐day hospitalization. Erythema multiforme is an acute, self‐limited, but sometimes recurrent dermatologic condition considered to be a distinct hypersensitivity reaction.1 It is associated with certain infections such as herpes simplex 1 and 2, Mycoplasma pneumoniae and fungal infections, and a number of medications in the classes barbiturates, nonsteroidal anti‐inflammatory drugs, penicillins, hydantoins, phenothiazines, and sulfonamides.2 EM is diagnosed clinically by the characteristic rash on the hands and feet, with some cases involving the oral cavity. Treatment is typically focused on resolving the underlying infection or removing the offending drug. Dermatologic manifestations usually improve over 3‐5 weeks without residual sequelae.


- ,,.Understanding the pathogenesis of HSV‐associated erythema multiforme.Dermatology.1998;197:219–222.
- ,,.Erythema multiforme.Am Fam Physician.2006;74:1883–1888.
- ,,.Understanding the pathogenesis of HSV‐associated erythema multiforme.Dermatology.1998;197:219–222.
- ,,.Erythema multiforme.Am Fam Physician.2006;74:1883–1888.
Patients' Predilections Regarding Informed Consent
The cornerstones of American medical ethics include respect for patient autonomy and beneficence. Although informed consent is required for surgical procedures and transfusion of blood products, the overwhelming majority of medical treatments administered by physicians to hospitalized patients are given without discussing risks, benefits, and alternatives. Although patients may sign a general permission‐to‐treat form on admission to the hospital, informed consent for medical treatments is generally ad hoc, and there are no national standards or mandates. We hypothesized that given the choice, hospitalized patients would want to participate in informed decision making, especially for therapies associated with substantial risks and benefits.
METHODS
The Institutional Review Board of Bridgeport Hospital approved this study. Each day between June and August 2006, the hospital's admitting department provided investigators with a list that included names and locations of all patients admitted to the Department of Medicine inpatient service. All the patients were eligible for participation in the study. Patients were excluded if they were in a comatose state, were encephalopathic, or were judged to be severely demented. In addition, patients were assessed during the scripted intervention to ascertain whether they had the capacity to make informed decisions based on their ability: (a) to understand the presented information, (b) to consider the information in relation to their personal values, and (c) to communicate their wishes. If personnel doubted an individual's capacity in any of these 3 areas, they were not included in the study.
Study personnel read directly from the script (see Appendix) and recorded answers. Study personnel were permitted to reread questions but did not provide additional guidance beyond the questionnaire. Patients whose primary language was not English were interviewed through in‐house or 3‐way telephone (remote) translators.
Statistical analyses included the chi‐square test to examine responses across the 3 categories of answers (ie, always consent, qualified consent, waive consent) and simple comparisons of percentages. A P value < .05 was considered statistically significant.
RESULTS
A total of 634 patients were admitted to the medicine service during the study period June‐August 2006. Of these, 158 were judged to lack sufficient capacity by study personnel and were excluded from the study. Ninety‐five refused to participate, and 171 were discharged before the questionnaire could be administered. Two hundred and ten patients answered the questionnaire. They ranged in age from 18 to 96 years (mean age standard error, 63.3 1.1 years). One hundred and three (49%) were men, and 107 (51%) were women. A majority (67.5%) were white, 20% (42) were African American, and 11.9% (25) were Hispanic. Most (87.6%) had at least a high school education, and 35% had a college‐/graduate‐level education. Sixty‐seven percent had at least 2 comorbid conditions in addition to their principal reason for hospitalization. Their average acute physiology and chronic health care evaluation (APACHE II) score was 7.5 0.3 (median 7; range 0‐22).
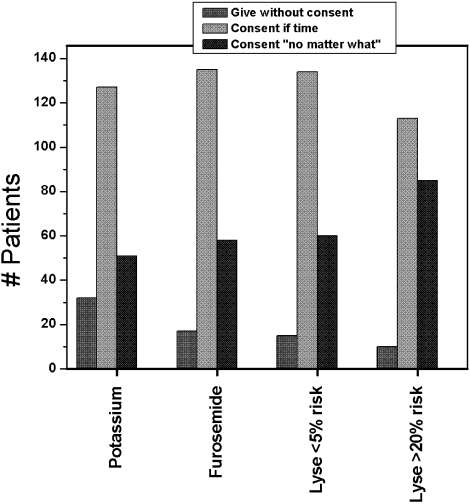
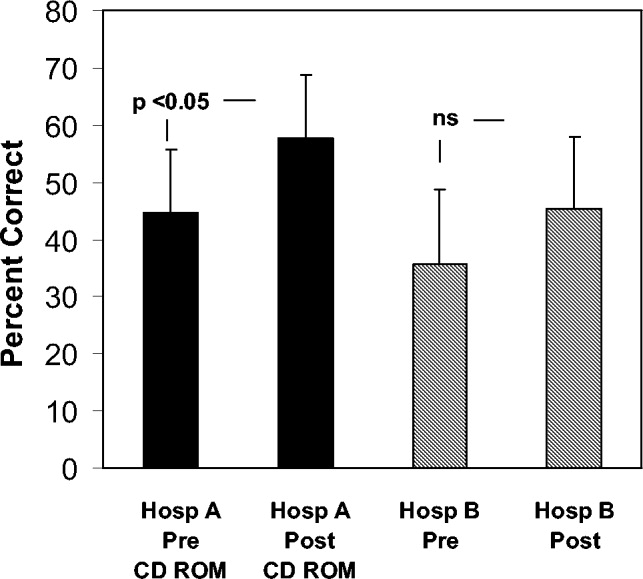
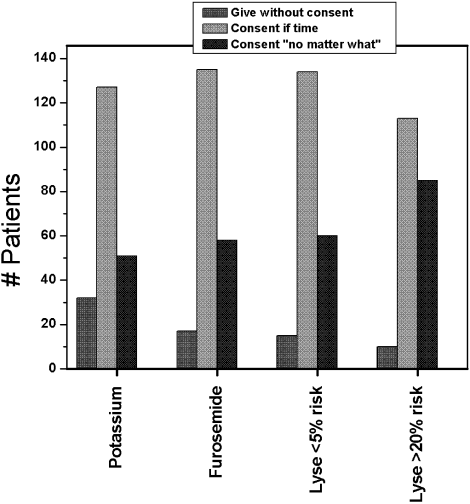
Figure 1 shows the distribution of answers to each of the 4 questions.
Question 1: Permission for Administration of Diuretics
One hundred and ninety‐three patients (92%) wished to participate in choosing whether to receive diuretics for congestive heart failure (CHF). Of these, 58 (28%) wanted their treating physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and thirty‐five (64%) wanted to be able to give permission if time allowed. Only 8% thought doctors should just give diuretics for CHF without seeking permission.
The pattern of response did not differ by sex, race, number of comorbid conditions, or primary admission diagnosis. Age (>65 vs. <65 years) was significantly associated with predilections to waive permission for administration of diuretics (Pearson chi‐square test P = .01). For example, 36.9% of the younger patients (<65 years) wanted to be consulted under all circumstances compared with only 18.7% of the more elderly patients (P = .004).
Question 2: Permission for Potassium Replacement
Overall, 178 patients (85%) wished to participate in decision making regarding potassium supplementation, and 51 (24%) wanted the managing physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and twenty‐seven patients (61%) responded that they would like to be able to give permission if time allowed. Only 15% thought doctors should just give potassium replacement without seeking their permission. Similar to the responses to diuretic replacement, the pattern of responses differed by age but not by sex, race, level of education, or number of comorbid conditions. Thirty‐one percent of the younger patients wanted to give permission at all times compared with 17.8% of the older patients (P = .03).
Question 3: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Less Than 5%
If the risk of cerebral hemorrhage was less than 5%, only 15 patients (7%) thought it should be given without seeking their permission. A third of the younger patients compared with 24.5% of the elderly patients would want to be consulted for their permission at all times (P = .18). The pattern of responses also did not differ by sex, race or level of education.
Question 4: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Greater Than 20%
Overall, 85 patients (40.8%) would want a discussion and their permission no matter what prior to initiating high‐risk thrombolysis. One hundred and thirteen patients (54%) would want to be able to give permission if time allowed. This pattern of response differed by level of education and by age. Forty‐four percent of those with at least a high school education would want to give permission compared with 19% of those without a high school education (P = .016). Four percent of those with at least a high school education would yield the need for permission at all times compared with 11.5% of those without a high school education (P = .09). Only 1 elderly patient (0.9%) would waive the need for permission at all times compared with 9 younger patients (8.7%; P = .01).
DISCUSSION
The principal finding of this study is that most medical patients prefer to participate in making decisions about their medical care during acute hospitalization, even for relatively low‐risk treatments like potassium supplementation and administration of diuretics. Very few patients were prepared to waive consent and grant their physicians the absolute right to administer therapies such as thrombolysis, even if the risk of bleeding was estimated to be less than 5%. Whereas the elderly patients were less likely to prefer being asked to consent to treatments than were younger patients, most would want to be informed of even trivial therapies if time allowed.
In some situations older patients (65 years old) were more likely than younger patients (<65 years old) to allow their physicians to make unilateral decisions regarding their health care. This could be explained by those age 65 and older having grown up when physician paternalism was more prevalent in American medicine. In the 1970s physician paternalism waned, and respect for patient autonomy emerged as the dominant physicianpatient model. Patients who became adults after 1970 know only this relationship with their physician, and so it makes sense that they would be more inclined to prefer a participatory model.
These data complement and extend a series of studies we conducted with patients admitted to Bridgeport Hospital. Our data suggest that our patients wish to consent for end‐of‐life decisions,1, 2 invasive procedures,3 and, now, to be apprised of medical therapies administered during hospitalization. At the same time, we have found that consent practices at many centers are not consistent with these patient predilections.1, 2, 4 Our study suffered from having a small sample size obtained in one geographic location; so results should be generalized cautiously. Nonetheless, insofar as the expectations of patients for participation are not being met by the health care system in Connecticut (and we suspect elsewhere), clinicians, hospital administrators, and health care policy makers might consider whether more rigorous and explicit consent practices and policies are required. Another important limitation of the study was that patients included may not have entirely understood the implications of their answers (ie, how cumbersome to the system and bothersome to the patient seeking consent for every therapy could become). In fact, we cannot be certain that all patients truly understood the questions, some of which were complex. Nonetheless, these results support that considered in the abstract, most patients prefer to consent for medical therapies. Had the implications for safety and expediency been explained in detail, it is possible that patients would have waived the need to give consent for treatments with minimal risk. The questionnaire also presents an abbreviated list of risks and benefits for each intervention, and although it refers to the formal process of informed consent in its preamble, it uses terminology (ie, permission) that may not reflect the complexity of informed consent. Nonetheless, our goal was to examine the degree to which patients wished to participate in their medical decision making. Notwithstanding these weaknesses of the survey instrument, the data suggest patients want to be in the loop whenever possible.
There are no national standards of consent for medical treatments. The Veterans' Administration, which has led the way in many areas of patients' rights, has a policy:
Treatments and Procedures That Do Not Require Signature Consent. Treatments and procedures that are low risk and are within broadly accepted standards of medical practice (e.g., administration of most drugs or for the performance of minor procedures such as routine X‐rays) do not require signature consent. However, the informed consent process must be documented in the medical record.
Compliance with this standard (ie, consent for every new medication) is not routine in most acute care hospitals. Although some clinicians obtain formal consent for high‐risk therapies (perhaps out of respect for autonomy, perhaps to reduce medical‐legal liability), there are no explicit decision rules to guide clinicians regarding for which treatments they should obtain formal consent. Accordingly, some might obtain formal consent for thrombolysis for massive pulmonary embolus, and others might not. It is not clear that the consent‐to‐treat form signed during hospital admission would legally cover all medical therapies during hospitalization. The legal standard for informed consent is what any reasonable patient would want to consent for. Our data suggest that most reasonable patients wish to at least assent and perhaps consent for much of what they receive during hospitalization. Although we have been unable to find case law predicated entirely on failure to obtain consent prior to administration of a therapy that caused a complication, it is plausible that the reasonable patient standard could be used in this manner. Regardless, it is impractical to require consent for the thousands of medical therapies administered each day in hospitals. Requiring consent for all therapies, if respected rigidly, would threaten the safety and efficiency of American hospitals. Naturally, a balance betweem respect for autonomy, that is, informed consent for the riskiest therapies, and efficiency is necessary. Explicit guidelines issued by accrediting agencies or the federal government would be helpful. The rules for consent (and/or assent) should be more explicit and less arbitrary, that is, determined independently by each clinician.
In conclusion, these data demonstrate that when considered in the abstract, that is, without explaining the logistical hurdles that it would create, inpatients wish to participate in decision making for both low‐ and high‐risk treatments. Clinicians are faced with demands and obligations that preclude full consent for the myriad low‐risk treatments administered daily to hospitalized patients. Some treatments are likely to be covered implicitly under the general consent‐to‐treat process and paperwork. Nonetheless, clinicians should consider explaining the principal risks and benefits of moderate‐risk treatments in order to secure informed assent. Full informed consent may be most appropriate for very high‐risk therapies. Patients expect and deserve frequent communication with caregivers that balances their safety with their right to self‐determination.
APPENDIX
QUESTIONNAIRE
Good morning/afternoon/evening. My name is Dr. _____________, and I am working with Dr. Constantine Manthous in a study to determine what patients want to know about their treatments during hospitalization. The research will not effect your care in any way, and if it is published, your confidential medical information will be protected and will not be mentioned in any publications. In fact, the questions I will ask do not apply to your care plans but are what ifs to find out for what kinds of treatments patients' want to provide permission called informed consent. Informed consent is when a doctor explains a treatment or procedure to the patient, including its risks, benefits, and alternatives, and asks permission before doing it. Are you feeling up to answering 4 questions that should take about 5‐10 minutes? Thank you.
Again, these questions do not apply to your illness or treatments.
If you had fluid on your lungs, a medicine called a diuretic could be given to make you pass more urine to help get the fluid out of the lungs. The benefits are that it can help you breathe easier. The risks are that it will make you have to urinate more often (>50%), and sometimes minerals in the blood get low and can cause the heart to beat abnormally (<1%) if enough replacement minerals aren't given to keep up with losses in the urine. The alternative to receiving this medicine would be not to receive it, which risks continued shortness of breath, and rarely (<5%) untreated patients may need a breathing machine to help breathing. Which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without asking my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there were time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
When a diuretic is given, minerals in the blood can be lost in the urine. If the minerals in the blood get too low, the heart can have abnormal beats that are rarely (<1%) life‐threatening. Doctors can give replacement minerals. The risks of replacement are minimal, and the alternative is not to give the minerals, risking abnormal heartbeats. Which best summarizes your preference?
If I needed replacement minerals, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give me the minerals. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed replacement minerals, I'd want the doctor to talk it over with me first to get my permission no matter what.
During hospitalization, sometimes blood clots can form in the legs and travel to the lungs. Very rarely (<1%), the blood clots can cause shortness of breath and the blood pressure to drop to a dangerous level. In this case there is a medicine called tpa that can dissolve the blood clot. It almost always dissolves the clot, improves breathlessness, and improves heart function. But there is a small risk (<5%) that it can cause serious bleeding into the brain (called a stroke). Which best summarizes your preference?
If I needed tpa for life‐threatening blood clots, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give the tpa. But if there was time and I was able, I'd want the doctor to talk it over with me first to get my permission.
If I needed tpa for life‐threatening blood clots, I'd want the doctor to talk it over with me first to get my permission no matter what.
In the previous example, what if the serious brain bleeding from the clot‐busting drug happened in more than 20% of cases, which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
- ,,,,.Patient, physician and family member understanding of living wills.Am J Respir Crit Care Med.2002;166:1430–1435.
- ,,,,,.Hospitalized patients want to choose whether to receive life‐sustaining therapies.J Hosp Med.2006;1:161–167.
- ,,,,,.Informed consent for invasive medical procedures. From the patient's perspective.Conn Med.2003;67:529–533.
- ,,,.Informed consent for medical procedures: Local and national practices.Chest.2003;124:1978–1984.
- Department of Veterans Affairs. VHA informed consent for clinical treatments and procedures. 2003. Available at: http://www.va.gov/ETHICS/docs/policy/VHA_Handbook_1004‐1_Informed_Consent_Policy_20030129.pdf. Accessed September 5,2006.
The cornerstones of American medical ethics include respect for patient autonomy and beneficence. Although informed consent is required for surgical procedures and transfusion of blood products, the overwhelming majority of medical treatments administered by physicians to hospitalized patients are given without discussing risks, benefits, and alternatives. Although patients may sign a general permission‐to‐treat form on admission to the hospital, informed consent for medical treatments is generally ad hoc, and there are no national standards or mandates. We hypothesized that given the choice, hospitalized patients would want to participate in informed decision making, especially for therapies associated with substantial risks and benefits.
METHODS
The Institutional Review Board of Bridgeport Hospital approved this study. Each day between June and August 2006, the hospital's admitting department provided investigators with a list that included names and locations of all patients admitted to the Department of Medicine inpatient service. All the patients were eligible for participation in the study. Patients were excluded if they were in a comatose state, were encephalopathic, or were judged to be severely demented. In addition, patients were assessed during the scripted intervention to ascertain whether they had the capacity to make informed decisions based on their ability: (a) to understand the presented information, (b) to consider the information in relation to their personal values, and (c) to communicate their wishes. If personnel doubted an individual's capacity in any of these 3 areas, they were not included in the study.
Study personnel read directly from the script (see Appendix) and recorded answers. Study personnel were permitted to reread questions but did not provide additional guidance beyond the questionnaire. Patients whose primary language was not English were interviewed through in‐house or 3‐way telephone (remote) translators.
Statistical analyses included the chi‐square test to examine responses across the 3 categories of answers (ie, always consent, qualified consent, waive consent) and simple comparisons of percentages. A P value < .05 was considered statistically significant.
RESULTS
A total of 634 patients were admitted to the medicine service during the study period June‐August 2006. Of these, 158 were judged to lack sufficient capacity by study personnel and were excluded from the study. Ninety‐five refused to participate, and 171 were discharged before the questionnaire could be administered. Two hundred and ten patients answered the questionnaire. They ranged in age from 18 to 96 years (mean age standard error, 63.3 1.1 years). One hundred and three (49%) were men, and 107 (51%) were women. A majority (67.5%) were white, 20% (42) were African American, and 11.9% (25) were Hispanic. Most (87.6%) had at least a high school education, and 35% had a college‐/graduate‐level education. Sixty‐seven percent had at least 2 comorbid conditions in addition to their principal reason for hospitalization. Their average acute physiology and chronic health care evaluation (APACHE II) score was 7.5 0.3 (median 7; range 0‐22).
Figure 1 shows the distribution of answers to each of the 4 questions.

Question 1: Permission for Administration of Diuretics
One hundred and ninety‐three patients (92%) wished to participate in choosing whether to receive diuretics for congestive heart failure (CHF). Of these, 58 (28%) wanted their treating physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and thirty‐five (64%) wanted to be able to give permission if time allowed. Only 8% thought doctors should just give diuretics for CHF without seeking permission.
The pattern of response did not differ by sex, race, number of comorbid conditions, or primary admission diagnosis. Age (>65 vs. <65 years) was significantly associated with predilections to waive permission for administration of diuretics (Pearson chi‐square test P = .01). For example, 36.9% of the younger patients (<65 years) wanted to be consulted under all circumstances compared with only 18.7% of the more elderly patients (P = .004).
Question 2: Permission for Potassium Replacement
Overall, 178 patients (85%) wished to participate in decision making regarding potassium supplementation, and 51 (24%) wanted the managing physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and twenty‐seven patients (61%) responded that they would like to be able to give permission if time allowed. Only 15% thought doctors should just give potassium replacement without seeking their permission. Similar to the responses to diuretic replacement, the pattern of responses differed by age but not by sex, race, level of education, or number of comorbid conditions. Thirty‐one percent of the younger patients wanted to give permission at all times compared with 17.8% of the older patients (P = .03).
Question 3: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Less Than 5%
If the risk of cerebral hemorrhage was less than 5%, only 15 patients (7%) thought it should be given without seeking their permission. A third of the younger patients compared with 24.5% of the elderly patients would want to be consulted for their permission at all times (P = .18). The pattern of responses also did not differ by sex, race or level of education.
Question 4: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Greater Than 20%
Overall, 85 patients (40.8%) would want a discussion and their permission no matter what prior to initiating high‐risk thrombolysis. One hundred and thirteen patients (54%) would want to be able to give permission if time allowed. This pattern of response differed by level of education and by age. Forty‐four percent of those with at least a high school education would want to give permission compared with 19% of those without a high school education (P = .016). Four percent of those with at least a high school education would yield the need for permission at all times compared with 11.5% of those without a high school education (P = .09). Only 1 elderly patient (0.9%) would waive the need for permission at all times compared with 9 younger patients (8.7%; P = .01).
DISCUSSION
The principal finding of this study is that most medical patients prefer to participate in making decisions about their medical care during acute hospitalization, even for relatively low‐risk treatments like potassium supplementation and administration of diuretics. Very few patients were prepared to waive consent and grant their physicians the absolute right to administer therapies such as thrombolysis, even if the risk of bleeding was estimated to be less than 5%. Whereas the elderly patients were less likely to prefer being asked to consent to treatments than were younger patients, most would want to be informed of even trivial therapies if time allowed.
In some situations older patients (65 years old) were more likely than younger patients (<65 years old) to allow their physicians to make unilateral decisions regarding their health care. This could be explained by those age 65 and older having grown up when physician paternalism was more prevalent in American medicine. In the 1970s physician paternalism waned, and respect for patient autonomy emerged as the dominant physicianpatient model. Patients who became adults after 1970 know only this relationship with their physician, and so it makes sense that they would be more inclined to prefer a participatory model.
These data complement and extend a series of studies we conducted with patients admitted to Bridgeport Hospital. Our data suggest that our patients wish to consent for end‐of‐life decisions,1, 2 invasive procedures,3 and, now, to be apprised of medical therapies administered during hospitalization. At the same time, we have found that consent practices at many centers are not consistent with these patient predilections.1, 2, 4 Our study suffered from having a small sample size obtained in one geographic location; so results should be generalized cautiously. Nonetheless, insofar as the expectations of patients for participation are not being met by the health care system in Connecticut (and we suspect elsewhere), clinicians, hospital administrators, and health care policy makers might consider whether more rigorous and explicit consent practices and policies are required. Another important limitation of the study was that patients included may not have entirely understood the implications of their answers (ie, how cumbersome to the system and bothersome to the patient seeking consent for every therapy could become). In fact, we cannot be certain that all patients truly understood the questions, some of which were complex. Nonetheless, these results support that considered in the abstract, most patients prefer to consent for medical therapies. Had the implications for safety and expediency been explained in detail, it is possible that patients would have waived the need to give consent for treatments with minimal risk. The questionnaire also presents an abbreviated list of risks and benefits for each intervention, and although it refers to the formal process of informed consent in its preamble, it uses terminology (ie, permission) that may not reflect the complexity of informed consent. Nonetheless, our goal was to examine the degree to which patients wished to participate in their medical decision making. Notwithstanding these weaknesses of the survey instrument, the data suggest patients want to be in the loop whenever possible.
There are no national standards of consent for medical treatments. The Veterans' Administration, which has led the way in many areas of patients' rights, has a policy:
Treatments and Procedures That Do Not Require Signature Consent. Treatments and procedures that are low risk and are within broadly accepted standards of medical practice (e.g., administration of most drugs or for the performance of minor procedures such as routine X‐rays) do not require signature consent. However, the informed consent process must be documented in the medical record.
Compliance with this standard (ie, consent for every new medication) is not routine in most acute care hospitals. Although some clinicians obtain formal consent for high‐risk therapies (perhaps out of respect for autonomy, perhaps to reduce medical‐legal liability), there are no explicit decision rules to guide clinicians regarding for which treatments they should obtain formal consent. Accordingly, some might obtain formal consent for thrombolysis for massive pulmonary embolus, and others might not. It is not clear that the consent‐to‐treat form signed during hospital admission would legally cover all medical therapies during hospitalization. The legal standard for informed consent is what any reasonable patient would want to consent for. Our data suggest that most reasonable patients wish to at least assent and perhaps consent for much of what they receive during hospitalization. Although we have been unable to find case law predicated entirely on failure to obtain consent prior to administration of a therapy that caused a complication, it is plausible that the reasonable patient standard could be used in this manner. Regardless, it is impractical to require consent for the thousands of medical therapies administered each day in hospitals. Requiring consent for all therapies, if respected rigidly, would threaten the safety and efficiency of American hospitals. Naturally, a balance betweem respect for autonomy, that is, informed consent for the riskiest therapies, and efficiency is necessary. Explicit guidelines issued by accrediting agencies or the federal government would be helpful. The rules for consent (and/or assent) should be more explicit and less arbitrary, that is, determined independently by each clinician.
In conclusion, these data demonstrate that when considered in the abstract, that is, without explaining the logistical hurdles that it would create, inpatients wish to participate in decision making for both low‐ and high‐risk treatments. Clinicians are faced with demands and obligations that preclude full consent for the myriad low‐risk treatments administered daily to hospitalized patients. Some treatments are likely to be covered implicitly under the general consent‐to‐treat process and paperwork. Nonetheless, clinicians should consider explaining the principal risks and benefits of moderate‐risk treatments in order to secure informed assent. Full informed consent may be most appropriate for very high‐risk therapies. Patients expect and deserve frequent communication with caregivers that balances their safety with their right to self‐determination.
APPENDIX
QUESTIONNAIRE
Good morning/afternoon/evening. My name is Dr. _____________, and I am working with Dr. Constantine Manthous in a study to determine what patients want to know about their treatments during hospitalization. The research will not effect your care in any way, and if it is published, your confidential medical information will be protected and will not be mentioned in any publications. In fact, the questions I will ask do not apply to your care plans but are what ifs to find out for what kinds of treatments patients' want to provide permission called informed consent. Informed consent is when a doctor explains a treatment or procedure to the patient, including its risks, benefits, and alternatives, and asks permission before doing it. Are you feeling up to answering 4 questions that should take about 5‐10 minutes? Thank you.
Again, these questions do not apply to your illness or treatments.
If you had fluid on your lungs, a medicine called a diuretic could be given to make you pass more urine to help get the fluid out of the lungs. The benefits are that it can help you breathe easier. The risks are that it will make you have to urinate more often (>50%), and sometimes minerals in the blood get low and can cause the heart to beat abnormally (<1%) if enough replacement minerals aren't given to keep up with losses in the urine. The alternative to receiving this medicine would be not to receive it, which risks continued shortness of breath, and rarely (<5%) untreated patients may need a breathing machine to help breathing. Which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without asking my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there were time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
When a diuretic is given, minerals in the blood can be lost in the urine. If the minerals in the blood get too low, the heart can have abnormal beats that are rarely (<1%) life‐threatening. Doctors can give replacement minerals. The risks of replacement are minimal, and the alternative is not to give the minerals, risking abnormal heartbeats. Which best summarizes your preference?
If I needed replacement minerals, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give me the minerals. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed replacement minerals, I'd want the doctor to talk it over with me first to get my permission no matter what.
During hospitalization, sometimes blood clots can form in the legs and travel to the lungs. Very rarely (<1%), the blood clots can cause shortness of breath and the blood pressure to drop to a dangerous level. In this case there is a medicine called tpa that can dissolve the blood clot. It almost always dissolves the clot, improves breathlessness, and improves heart function. But there is a small risk (<5%) that it can cause serious bleeding into the brain (called a stroke). Which best summarizes your preference?
If I needed tpa for life‐threatening blood clots, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give the tpa. But if there was time and I was able, I'd want the doctor to talk it over with me first to get my permission.
If I needed tpa for life‐threatening blood clots, I'd want the doctor to talk it over with me first to get my permission no matter what.
In the previous example, what if the serious brain bleeding from the clot‐busting drug happened in more than 20% of cases, which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
The cornerstones of American medical ethics include respect for patient autonomy and beneficence. Although informed consent is required for surgical procedures and transfusion of blood products, the overwhelming majority of medical treatments administered by physicians to hospitalized patients are given without discussing risks, benefits, and alternatives. Although patients may sign a general permission‐to‐treat form on admission to the hospital, informed consent for medical treatments is generally ad hoc, and there are no national standards or mandates. We hypothesized that given the choice, hospitalized patients would want to participate in informed decision making, especially for therapies associated with substantial risks and benefits.
METHODS
The Institutional Review Board of Bridgeport Hospital approved this study. Each day between June and August 2006, the hospital's admitting department provided investigators with a list that included names and locations of all patients admitted to the Department of Medicine inpatient service. All the patients were eligible for participation in the study. Patients were excluded if they were in a comatose state, were encephalopathic, or were judged to be severely demented. In addition, patients were assessed during the scripted intervention to ascertain whether they had the capacity to make informed decisions based on their ability: (a) to understand the presented information, (b) to consider the information in relation to their personal values, and (c) to communicate their wishes. If personnel doubted an individual's capacity in any of these 3 areas, they were not included in the study.
Study personnel read directly from the script (see Appendix) and recorded answers. Study personnel were permitted to reread questions but did not provide additional guidance beyond the questionnaire. Patients whose primary language was not English were interviewed through in‐house or 3‐way telephone (remote) translators.
Statistical analyses included the chi‐square test to examine responses across the 3 categories of answers (ie, always consent, qualified consent, waive consent) and simple comparisons of percentages. A P value < .05 was considered statistically significant.
RESULTS
A total of 634 patients were admitted to the medicine service during the study period June‐August 2006. Of these, 158 were judged to lack sufficient capacity by study personnel and were excluded from the study. Ninety‐five refused to participate, and 171 were discharged before the questionnaire could be administered. Two hundred and ten patients answered the questionnaire. They ranged in age from 18 to 96 years (mean age standard error, 63.3 1.1 years). One hundred and three (49%) were men, and 107 (51%) were women. A majority (67.5%) were white, 20% (42) were African American, and 11.9% (25) were Hispanic. Most (87.6%) had at least a high school education, and 35% had a college‐/graduate‐level education. Sixty‐seven percent had at least 2 comorbid conditions in addition to their principal reason for hospitalization. Their average acute physiology and chronic health care evaluation (APACHE II) score was 7.5 0.3 (median 7; range 0‐22).
Figure 1 shows the distribution of answers to each of the 4 questions.

Question 1: Permission for Administration of Diuretics
One hundred and ninety‐three patients (92%) wished to participate in choosing whether to receive diuretics for congestive heart failure (CHF). Of these, 58 (28%) wanted their treating physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and thirty‐five (64%) wanted to be able to give permission if time allowed. Only 8% thought doctors should just give diuretics for CHF without seeking permission.
The pattern of response did not differ by sex, race, number of comorbid conditions, or primary admission diagnosis. Age (>65 vs. <65 years) was significantly associated with predilections to waive permission for administration of diuretics (Pearson chi‐square test P = .01). For example, 36.9% of the younger patients (<65 years) wanted to be consulted under all circumstances compared with only 18.7% of the more elderly patients (P = .004).
Question 2: Permission for Potassium Replacement
Overall, 178 patients (85%) wished to participate in decision making regarding potassium supplementation, and 51 (24%) wanted the managing physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and twenty‐seven patients (61%) responded that they would like to be able to give permission if time allowed. Only 15% thought doctors should just give potassium replacement without seeking their permission. Similar to the responses to diuretic replacement, the pattern of responses differed by age but not by sex, race, level of education, or number of comorbid conditions. Thirty‐one percent of the younger patients wanted to give permission at all times compared with 17.8% of the older patients (P = .03).
Question 3: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Less Than 5%
If the risk of cerebral hemorrhage was less than 5%, only 15 patients (7%) thought it should be given without seeking their permission. A third of the younger patients compared with 24.5% of the elderly patients would want to be consulted for their permission at all times (P = .18). The pattern of responses also did not differ by sex, race or level of education.
Question 4: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Greater Than 20%
Overall, 85 patients (40.8%) would want a discussion and their permission no matter what prior to initiating high‐risk thrombolysis. One hundred and thirteen patients (54%) would want to be able to give permission if time allowed. This pattern of response differed by level of education and by age. Forty‐four percent of those with at least a high school education would want to give permission compared with 19% of those without a high school education (P = .016). Four percent of those with at least a high school education would yield the need for permission at all times compared with 11.5% of those without a high school education (P = .09). Only 1 elderly patient (0.9%) would waive the need for permission at all times compared with 9 younger patients (8.7%; P = .01).
DISCUSSION
The principal finding of this study is that most medical patients prefer to participate in making decisions about their medical care during acute hospitalization, even for relatively low‐risk treatments like potassium supplementation and administration of diuretics. Very few patients were prepared to waive consent and grant their physicians the absolute right to administer therapies such as thrombolysis, even if the risk of bleeding was estimated to be less than 5%. Whereas the elderly patients were less likely to prefer being asked to consent to treatments than were younger patients, most would want to be informed of even trivial therapies if time allowed.
In some situations older patients (65 years old) were more likely than younger patients (<65 years old) to allow their physicians to make unilateral decisions regarding their health care. This could be explained by those age 65 and older having grown up when physician paternalism was more prevalent in American medicine. In the 1970s physician paternalism waned, and respect for patient autonomy emerged as the dominant physicianpatient model. Patients who became adults after 1970 know only this relationship with their physician, and so it makes sense that they would be more inclined to prefer a participatory model.
These data complement and extend a series of studies we conducted with patients admitted to Bridgeport Hospital. Our data suggest that our patients wish to consent for end‐of‐life decisions,1, 2 invasive procedures,3 and, now, to be apprised of medical therapies administered during hospitalization. At the same time, we have found that consent practices at many centers are not consistent with these patient predilections.1, 2, 4 Our study suffered from having a small sample size obtained in one geographic location; so results should be generalized cautiously. Nonetheless, insofar as the expectations of patients for participation are not being met by the health care system in Connecticut (and we suspect elsewhere), clinicians, hospital administrators, and health care policy makers might consider whether more rigorous and explicit consent practices and policies are required. Another important limitation of the study was that patients included may not have entirely understood the implications of their answers (ie, how cumbersome to the system and bothersome to the patient seeking consent for every therapy could become). In fact, we cannot be certain that all patients truly understood the questions, some of which were complex. Nonetheless, these results support that considered in the abstract, most patients prefer to consent for medical therapies. Had the implications for safety and expediency been explained in detail, it is possible that patients would have waived the need to give consent for treatments with minimal risk. The questionnaire also presents an abbreviated list of risks and benefits for each intervention, and although it refers to the formal process of informed consent in its preamble, it uses terminology (ie, permission) that may not reflect the complexity of informed consent. Nonetheless, our goal was to examine the degree to which patients wished to participate in their medical decision making. Notwithstanding these weaknesses of the survey instrument, the data suggest patients want to be in the loop whenever possible.
There are no national standards of consent for medical treatments. The Veterans' Administration, which has led the way in many areas of patients' rights, has a policy:
Treatments and Procedures That Do Not Require Signature Consent. Treatments and procedures that are low risk and are within broadly accepted standards of medical practice (e.g., administration of most drugs or for the performance of minor procedures such as routine X‐rays) do not require signature consent. However, the informed consent process must be documented in the medical record.
Compliance with this standard (ie, consent for every new medication) is not routine in most acute care hospitals. Although some clinicians obtain formal consent for high‐risk therapies (perhaps out of respect for autonomy, perhaps to reduce medical‐legal liability), there are no explicit decision rules to guide clinicians regarding for which treatments they should obtain formal consent. Accordingly, some might obtain formal consent for thrombolysis for massive pulmonary embolus, and others might not. It is not clear that the consent‐to‐treat form signed during hospital admission would legally cover all medical therapies during hospitalization. The legal standard for informed consent is what any reasonable patient would want to consent for. Our data suggest that most reasonable patients wish to at least assent and perhaps consent for much of what they receive during hospitalization. Although we have been unable to find case law predicated entirely on failure to obtain consent prior to administration of a therapy that caused a complication, it is plausible that the reasonable patient standard could be used in this manner. Regardless, it is impractical to require consent for the thousands of medical therapies administered each day in hospitals. Requiring consent for all therapies, if respected rigidly, would threaten the safety and efficiency of American hospitals. Naturally, a balance betweem respect for autonomy, that is, informed consent for the riskiest therapies, and efficiency is necessary. Explicit guidelines issued by accrediting agencies or the federal government would be helpful. The rules for consent (and/or assent) should be more explicit and less arbitrary, that is, determined independently by each clinician.
In conclusion, these data demonstrate that when considered in the abstract, that is, without explaining the logistical hurdles that it would create, inpatients wish to participate in decision making for both low‐ and high‐risk treatments. Clinicians are faced with demands and obligations that preclude full consent for the myriad low‐risk treatments administered daily to hospitalized patients. Some treatments are likely to be covered implicitly under the general consent‐to‐treat process and paperwork. Nonetheless, clinicians should consider explaining the principal risks and benefits of moderate‐risk treatments in order to secure informed assent. Full informed consent may be most appropriate for very high‐risk therapies. Patients expect and deserve frequent communication with caregivers that balances their safety with their right to self‐determination.
APPENDIX
QUESTIONNAIRE
Good morning/afternoon/evening. My name is Dr. _____________, and I am working with Dr. Constantine Manthous in a study to determine what patients want to know about their treatments during hospitalization. The research will not effect your care in any way, and if it is published, your confidential medical information will be protected and will not be mentioned in any publications. In fact, the questions I will ask do not apply to your care plans but are what ifs to find out for what kinds of treatments patients' want to provide permission called informed consent. Informed consent is when a doctor explains a treatment or procedure to the patient, including its risks, benefits, and alternatives, and asks permission before doing it. Are you feeling up to answering 4 questions that should take about 5‐10 minutes? Thank you.
Again, these questions do not apply to your illness or treatments.
If you had fluid on your lungs, a medicine called a diuretic could be given to make you pass more urine to help get the fluid out of the lungs. The benefits are that it can help you breathe easier. The risks are that it will make you have to urinate more often (>50%), and sometimes minerals in the blood get low and can cause the heart to beat abnormally (<1%) if enough replacement minerals aren't given to keep up with losses in the urine. The alternative to receiving this medicine would be not to receive it, which risks continued shortness of breath, and rarely (<5%) untreated patients may need a breathing machine to help breathing. Which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without asking my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there were time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
When a diuretic is given, minerals in the blood can be lost in the urine. If the minerals in the blood get too low, the heart can have abnormal beats that are rarely (<1%) life‐threatening. Doctors can give replacement minerals. The risks of replacement are minimal, and the alternative is not to give the minerals, risking abnormal heartbeats. Which best summarizes your preference?
If I needed replacement minerals, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give me the minerals. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed replacement minerals, I'd want the doctor to talk it over with me first to get my permission no matter what.
During hospitalization, sometimes blood clots can form in the legs and travel to the lungs. Very rarely (<1%), the blood clots can cause shortness of breath and the blood pressure to drop to a dangerous level. In this case there is a medicine called tpa that can dissolve the blood clot. It almost always dissolves the clot, improves breathlessness, and improves heart function. But there is a small risk (<5%) that it can cause serious bleeding into the brain (called a stroke). Which best summarizes your preference?
If I needed tpa for life‐threatening blood clots, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give the tpa. But if there was time and I was able, I'd want the doctor to talk it over with me first to get my permission.
If I needed tpa for life‐threatening blood clots, I'd want the doctor to talk it over with me first to get my permission no matter what.
In the previous example, what if the serious brain bleeding from the clot‐busting drug happened in more than 20% of cases, which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
- ,,,,.Patient, physician and family member understanding of living wills.Am J Respir Crit Care Med.2002;166:1430–1435.
- ,,,,,.Hospitalized patients want to choose whether to receive life‐sustaining therapies.J Hosp Med.2006;1:161–167.
- ,,,,,.Informed consent for invasive medical procedures. From the patient's perspective.Conn Med.2003;67:529–533.
- ,,,.Informed consent for medical procedures: Local and national practices.Chest.2003;124:1978–1984.
- Department of Veterans Affairs. VHA informed consent for clinical treatments and procedures. 2003. Available at: http://www.va.gov/ETHICS/docs/policy/VHA_Handbook_1004‐1_Informed_Consent_Policy_20030129.pdf. Accessed September 5,2006.
- ,,,,.Patient, physician and family member understanding of living wills.Am J Respir Crit Care Med.2002;166:1430–1435.
- ,,,,,.Hospitalized patients want to choose whether to receive life‐sustaining therapies.J Hosp Med.2006;1:161–167.
- ,,,,,.Informed consent for invasive medical procedures. From the patient's perspective.Conn Med.2003;67:529–533.
- ,,,.Informed consent for medical procedures: Local and national practices.Chest.2003;124:1978–1984.
- Department of Veterans Affairs. VHA informed consent for clinical treatments and procedures. 2003. Available at: http://www.va.gov/ETHICS/docs/policy/VHA_Handbook_1004‐1_Informed_Consent_Policy_20030129.pdf. Accessed September 5,2006.
Copyright © 2008 Society of Hospital Medicine
Editorial
We live in a moment of history where change is so speeded up that we begin to see the present only when it is already disappearing.
Two years ago we published the first issue of the Journal of Hospital Medicine and declared, Our goal is that JHM become the premier forum for peer‐reviewed research articles and evidence‐based reviews in the specialty of hospital medicine.1 That first issue was just one of many steps toward this ambition. At the completion of its first year, JHM was selected for indexing and inclusion in the National Library of Medicine's Medical Literature Analysis and Retrieval System Online (MEDLINE), the primary component of PubMed. Following this huge step, we welcomed a remarkable increase in submissions and will have exceeded 300 in our second year, an approximately 50% increase from our first year!
As important, JHM quickly became a valuable benefit of membership in the Society of Hospital Medicine, and the innumerable compliments received by the staff reflect the diligent efforts of a remarkable editorial staff and work by our reviewers. With profound gratitude we list on page 86 these 325 reviewers who donated their priceless time and expertise to enhancing the quality of the manuscripts. To handle the marked increase in submissions, we are expanding and modifying our editorial staff. Please welcome Sunil Kripalani (Vanderbilt) and Daniel Brotman (Johns Hopkins), who join our previous six associate editors and all eight will now serve as JHM's deputy editors. Seven new associate editors also join our team. Among them, Tom Baudendistel (California Pacific Medical Center, San Francisco), Eric Alper (UMass Memorial Health Care, Worcester), Brian Harte (Cleveland Clinic), and Rehan Qayyum (Johns Hopkins) will all focus on optimizing content for practicing hospitalists. Paul Aronowitz will continue to develop our Images section as an associate editor. Recognizing the growing number of pediatric hospitalists, Lisa Zauotis (Childrens Hospital of Philadelphia) and Erin Stucky (Children's Hospital San Diego) join JHM as the other 2 new associate editors. Finally, we welcome new Editorial Board members Mary C. Ottolini (Children's National Medical Center), Douglas Carlson (St. Louis Children's Hospital), and Daniel Rauch (NYU Children's Hospital). The welcome addition of these nationally recognized academicians prepares us for continued growth in manuscript submissions to JHM.
Although we could not excel without the editors, reviewers and our terrific new managing editor, Phaedra McGuinness, we would not survive without the authors who submit their manuscripts to JHMthey are responsible for the caliber of the journal, and we are immensely indebted to them. Originally, we hoped to include individuals involved in all aspects of hospital care,1 and fortunately this is now happening. Complementing hospitalists are nurses and pharmacists2 who recognize the importance of teamwork in the care of hospitalized patients. I encourage all members of the hospital care team to send us the results of their research, teaching, and quality improvement efforts.
As the specialty of hospital medicine continues to evolve, now with more than 20,000 hospitalists, JHM will develop with it. I am honored and grateful to collaborate with such a remarkable group of colleagues as we build the premier journal for the fastest growing specialty in the history of medicine in the United States. On to year 3!
P.S. Our tenuous hold on life confronted me this past Thanksgiving holiday. A fellow hospitalist and dear friend died unexpectedly. Two years before, he posted on the wall of the office shared with his colleagues the following quote:
What we do for ourselves fades, but what we do for another may be etched into eternity.
The smile and humanity of John Allen Garner (19632007) is etched into the lives of his family, many friends and colleagues, and innumerable grateful patients.
- .Hospital medicine's evolution—the next steps.J Hosp Med.2006;1:1–2.
- ,,, et al.ASHP–SHM joint statement on hospitalist–pharmacist collaboration.Am J Health‐Syst Pharm.2008;65:260–263.
We live in a moment of history where change is so speeded up that we begin to see the present only when it is already disappearing.
Two years ago we published the first issue of the Journal of Hospital Medicine and declared, Our goal is that JHM become the premier forum for peer‐reviewed research articles and evidence‐based reviews in the specialty of hospital medicine.1 That first issue was just one of many steps toward this ambition. At the completion of its first year, JHM was selected for indexing and inclusion in the National Library of Medicine's Medical Literature Analysis and Retrieval System Online (MEDLINE), the primary component of PubMed. Following this huge step, we welcomed a remarkable increase in submissions and will have exceeded 300 in our second year, an approximately 50% increase from our first year!
As important, JHM quickly became a valuable benefit of membership in the Society of Hospital Medicine, and the innumerable compliments received by the staff reflect the diligent efforts of a remarkable editorial staff and work by our reviewers. With profound gratitude we list on page 86 these 325 reviewers who donated their priceless time and expertise to enhancing the quality of the manuscripts. To handle the marked increase in submissions, we are expanding and modifying our editorial staff. Please welcome Sunil Kripalani (Vanderbilt) and Daniel Brotman (Johns Hopkins), who join our previous six associate editors and all eight will now serve as JHM's deputy editors. Seven new associate editors also join our team. Among them, Tom Baudendistel (California Pacific Medical Center, San Francisco), Eric Alper (UMass Memorial Health Care, Worcester), Brian Harte (Cleveland Clinic), and Rehan Qayyum (Johns Hopkins) will all focus on optimizing content for practicing hospitalists. Paul Aronowitz will continue to develop our Images section as an associate editor. Recognizing the growing number of pediatric hospitalists, Lisa Zauotis (Childrens Hospital of Philadelphia) and Erin Stucky (Children's Hospital San Diego) join JHM as the other 2 new associate editors. Finally, we welcome new Editorial Board members Mary C. Ottolini (Children's National Medical Center), Douglas Carlson (St. Louis Children's Hospital), and Daniel Rauch (NYU Children's Hospital). The welcome addition of these nationally recognized academicians prepares us for continued growth in manuscript submissions to JHM.
Although we could not excel without the editors, reviewers and our terrific new managing editor, Phaedra McGuinness, we would not survive without the authors who submit their manuscripts to JHMthey are responsible for the caliber of the journal, and we are immensely indebted to them. Originally, we hoped to include individuals involved in all aspects of hospital care,1 and fortunately this is now happening. Complementing hospitalists are nurses and pharmacists2 who recognize the importance of teamwork in the care of hospitalized patients. I encourage all members of the hospital care team to send us the results of their research, teaching, and quality improvement efforts.
As the specialty of hospital medicine continues to evolve, now with more than 20,000 hospitalists, JHM will develop with it. I am honored and grateful to collaborate with such a remarkable group of colleagues as we build the premier journal for the fastest growing specialty in the history of medicine in the United States. On to year 3!
P.S. Our tenuous hold on life confronted me this past Thanksgiving holiday. A fellow hospitalist and dear friend died unexpectedly. Two years before, he posted on the wall of the office shared with his colleagues the following quote:
What we do for ourselves fades, but what we do for another may be etched into eternity.
The smile and humanity of John Allen Garner (19632007) is etched into the lives of his family, many friends and colleagues, and innumerable grateful patients.
We live in a moment of history where change is so speeded up that we begin to see the present only when it is already disappearing.
Two years ago we published the first issue of the Journal of Hospital Medicine and declared, Our goal is that JHM become the premier forum for peer‐reviewed research articles and evidence‐based reviews in the specialty of hospital medicine.1 That first issue was just one of many steps toward this ambition. At the completion of its first year, JHM was selected for indexing and inclusion in the National Library of Medicine's Medical Literature Analysis and Retrieval System Online (MEDLINE), the primary component of PubMed. Following this huge step, we welcomed a remarkable increase in submissions and will have exceeded 300 in our second year, an approximately 50% increase from our first year!
As important, JHM quickly became a valuable benefit of membership in the Society of Hospital Medicine, and the innumerable compliments received by the staff reflect the diligent efforts of a remarkable editorial staff and work by our reviewers. With profound gratitude we list on page 86 these 325 reviewers who donated their priceless time and expertise to enhancing the quality of the manuscripts. To handle the marked increase in submissions, we are expanding and modifying our editorial staff. Please welcome Sunil Kripalani (Vanderbilt) and Daniel Brotman (Johns Hopkins), who join our previous six associate editors and all eight will now serve as JHM's deputy editors. Seven new associate editors also join our team. Among them, Tom Baudendistel (California Pacific Medical Center, San Francisco), Eric Alper (UMass Memorial Health Care, Worcester), Brian Harte (Cleveland Clinic), and Rehan Qayyum (Johns Hopkins) will all focus on optimizing content for practicing hospitalists. Paul Aronowitz will continue to develop our Images section as an associate editor. Recognizing the growing number of pediatric hospitalists, Lisa Zauotis (Childrens Hospital of Philadelphia) and Erin Stucky (Children's Hospital San Diego) join JHM as the other 2 new associate editors. Finally, we welcome new Editorial Board members Mary C. Ottolini (Children's National Medical Center), Douglas Carlson (St. Louis Children's Hospital), and Daniel Rauch (NYU Children's Hospital). The welcome addition of these nationally recognized academicians prepares us for continued growth in manuscript submissions to JHM.
Although we could not excel without the editors, reviewers and our terrific new managing editor, Phaedra McGuinness, we would not survive without the authors who submit their manuscripts to JHMthey are responsible for the caliber of the journal, and we are immensely indebted to them. Originally, we hoped to include individuals involved in all aspects of hospital care,1 and fortunately this is now happening. Complementing hospitalists are nurses and pharmacists2 who recognize the importance of teamwork in the care of hospitalized patients. I encourage all members of the hospital care team to send us the results of their research, teaching, and quality improvement efforts.
As the specialty of hospital medicine continues to evolve, now with more than 20,000 hospitalists, JHM will develop with it. I am honored and grateful to collaborate with such a remarkable group of colleagues as we build the premier journal for the fastest growing specialty in the history of medicine in the United States. On to year 3!
P.S. Our tenuous hold on life confronted me this past Thanksgiving holiday. A fellow hospitalist and dear friend died unexpectedly. Two years before, he posted on the wall of the office shared with his colleagues the following quote:
What we do for ourselves fades, but what we do for another may be etched into eternity.
The smile and humanity of John Allen Garner (19632007) is etched into the lives of his family, many friends and colleagues, and innumerable grateful patients.
- .Hospital medicine's evolution—the next steps.J Hosp Med.2006;1:1–2.
- ,,, et al.ASHP–SHM joint statement on hospitalist–pharmacist collaboration.Am J Health‐Syst Pharm.2008;65:260–263.
- .Hospital medicine's evolution—the next steps.J Hosp Med.2006;1:1–2.
- ,,, et al.ASHP–SHM joint statement on hospitalist–pharmacist collaboration.Am J Health‐Syst Pharm.2008;65:260–263.
Thinking Inside the Box
A 65‐year‐old man was referred for evaluation of worsening ascites and end‐stage liver disease. The patient had been well until 1 year ago, when he developed lower extremity edema and abdominal distention. After evaluation by his primary care physician, he was given a diagnosis of cryptogenic cirrhosis. He underwent several paracenteses and was placed on furosemide and spironolactone. The patient had been stable on his diuretic regimen until 2 weeks previously, when he suddenly developed worsening edema and ascites, along with dizziness, nausea, and hypotension. His physician stopped the diuretics and referred him to the hospital.
Before diagnosing a patient with cryptogenic cirrhosis, it is necessary to exclude common etiologies of cirrhosis such as alcohol, viral hepatitis, and non‐alcoholic fatty liver disease and numerous uncommon causes, including Wilson's disease, hemochromatosis, Budd‐Chiari, and biliary cirrhosis. It is also important to remember that patients with liver disease are not immune to extrahepatic causes of ascites, such as peritoneal carcinomatosis and tuberculous ascites. Simultaneously, reasons for chronic liver disease decompensating acutely must be considered: medication nonadherence, excess salt intake, hepatotoxicity from acetaminophen or alcohol, and other acute insults, such as hepatocellular carcinoma, an intervening infection (especially spontaneous bacterial peritonitis), ascending cholangitis, or a flare of chronic viral hepatitis.
Past medical and surgical history included diabetes mellitus (diagnosed 10 years previously), obstructive sleep apnea, hypertension, hypothyroidism, and mild chronic kidney disease. Medications included levothyroxine, lactulose, sulfamethoxazole, pioglitazone (started 4 months prior), and ibuprofen. Furosemide and spironolactone had been discontinued 2 weeks previously. He currently resided in the Central Valley of California. He had lived in Thailand from age 7 to 17 and traveled to India more than 1 year ago. He did not smoke and had never used intravenous drugs or received a blood transfusion. He rarely drank alcohol. He worked as a chemist. There was no family history of liver disease.
There is no obvious explanation for the underlying liver disease or the acute decompensation. Sulfamethoxazole is a rare cause of allergic or granulomatous hepatitis. Pioglitazone is a thiazolinedione which in earlier formulations was linked to hepatitis but can be excluded as a cause of this patient's cirrhosis because it was started after liver disease was detected. As a chemist, he might have been exposed to carbon tetrachloride, a known hepatotoxin. Obstructive sleep apnea causes pulmonary hypertension, but severe ascites and acute hepatic decompensation would be unusual. Ibuprofen might precipitate worsening renal function and fluid accumulation. Time in Thailand and India raises the possibility of tuberculous ascites.
The patient had no headache, vision changes, abdominal pain, emesis, melena, hematochezia, chest pain, palpitations, dysuria, polyuria, pruritus, dark urine, or rashes. He reported difficulty with concentration when lactulose was decreased. He noted worsening exercise tolerance with dyspnea after 10 steps and reported a weight gain of 12 pounds in the past 2 weeks.
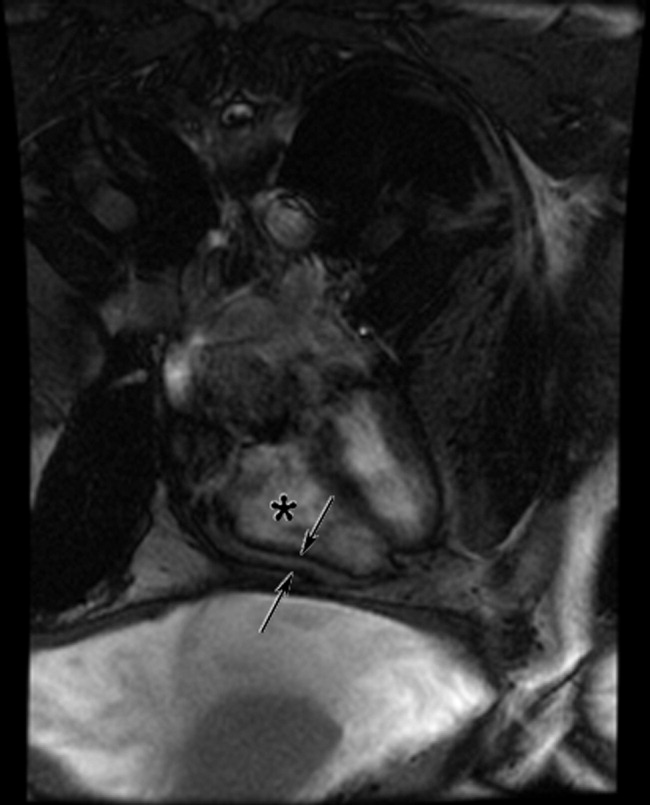
On examination, temperature was 36.8C; blood pressure, 129/87 mm Hg; heart rate, 85 beats per minute; respirations, 20 per minute; and oxygen saturation, 94% on room air. He was uncomfortable but alert. There was no scleral icterus or conjunctival pallor. Jugular venous pressure was elevated. The lungs were clear, and the heart was regular, with no murmur, rub, or gallops. The abdomen was massively distended with a fluid wave; the liver and spleen could not be palpated. There was pitting edema of the sacrum and lower extremities. There was no asterixis, palmar erythema, spider angiomata, or skin discoloration.
The additional history and physical exam suggest that the primary problem may lie outside the liver, especially as signs of advanced liver disease (other than ascites) are absent. Dyspnea on exertion is consistent with the physical stress of a large volume of ascites or could be secondary to several pulmonary complications associated with liver disease, including portopulmonary hypertension, hepatopulmonary syndrome, or hepatic hydrothorax. Alternatively, the dyspnea raises the possibility that the ascites is not related to a primary liver disorder but rather to anemia or to a cardiac disorder, such as chronic left ventricular failure, isolated right‐sided heart failure, or constrictive pericarditis. These diagnoses are suggested by the elevated jugular venous pressure, which is atypical in cirrhosis.
Although portal hypertension accounts for most cases of ascites, peritoneal fluid should be examined to exclude peritoneal carcinomatosis and tuberculous ascites. I am interested in the results of an echocardiogram.
Initial laboratory studies demonstrated a sodium concentration of 136 mEq/dL; potassium, 4.7 mEq/dL; chloride, 99 mEq/dL; bicarbonate, 24 mEq/dL; blood urea nitrogen, 54 mg/dL; creatinine, 3.3 mg/dL (increased from baseline of 1.6 mg/dL 4 months previously); white cell count, 7000/mm3; hemoglobin, 10.5 g/dL; MCV, 89 fL; platelet count, 205,000/mm3; bilirubin, 0.6 mg/dL; aspartate aminotransferase, 15 U/L; alanine aminotransferase, 8 U/L; alkaline phosphatase, 102 U/L; albumin, 4.2 g/dL; total protein, 8.2 g/dL; international normalized ratio, 1.2; and partial thromboplastin time, 31.8 seconds. A urine dipstick demonstrated 1+ protein. The chest radiograph was normal. Electrocardiogram had borderline low voltage with nonspecific T‐wave abnormalities. Additional studies showed a serum iron concentration of 49 mg/dL, transferrin saturation of 16%, total iron binding capacity of 310 mg/dL, and ferritin of 247 mg/mL. Hemoglobin A1c was 7.0%. Acute and chronic antibodies to hepatitis A, B, and C viruses were negative. The following study results were normal or negative: antinuclear antibody, alpha‐1‐antitrypsin, ceruloplasmin, alpha‐fetoprotein, carcinoembryonic antigen, and 24‐hour urinary copper. The thyroid function studies were normal. A purified protein derivative (PPD) skin test was nonreactive.
There continues to be a paucity of evidence of a primary liver disorder. The hepatic enzymes and tests of liver synthetic function are normal, and there is no pancytopenia, as might result from hypersplenism. I remain most suspicious of either a primary cardiac or pericardial disorder with secondary hepatic congestion or a disease that simultaneously affects the heart and liver.
The reasons for the low voltage on the electrocardiogram include processes that infiltrate the myocardium (amyloidosis, sarcoidosis, hemochromatosis, and myxedema fluid) and processes that increase the distance between the myocardium and surface electrodes, such as adipose tissue, air (from emphysema or pneumothorax), or pericardial effusion. Pericardial effusion may present subacutely with predominant features of right ventricular failure. Low voltage, liver disease, and possible heart failure raise the possibility of amyloidosis or hemochromatosis. The low transferrin saturation renders hemochromatosis unlikely. Although normal alkaline phosphatase and serum albumin are not characteristic when AL amyloid affects the liver and kidneys, serum and urine protein electrophoresis and immunofixation should be considered.
With paracentesis 3.5 L of ascitic fluid was removed. The red cell count was 4000/mm3, and white blood cell count was 505/mm3, of which 25% were polymorphonuclear cells, 22% were lymphocytes, and 53% were monocytes. Additional peritoneal fluid chemistries included albumin of 3.0 g/dL and total protein of 5.3 g/dL. Abdominal ultrasound with Doppler demonstrated a liver of normal size and echogenicity with patent hepatic arteries, hepatic veins, and portal vein. There was mild splenomegaly with normal kidneys. Evaluation for a possible liver transplant was initiated. Blood, urine, and peritoneal fluid cultures demonstrated no growth. Echocardiography demonstrated borderline concentric left ventricular hypertrophy, normal right and left ventricular function, dilated superior and inferior vena cavae, and no pericardial effusion or thickening.
The serum‐ascites albumin gradient (SAAG) of 1.2 is consistent with portal hypertension as the cause of the ascites. The Doppler findings exclude postsinusoidal causes of portal hypertension from hepatic vein obstruction or thrombosis. The combination of the elevated SAAG, elevated jugular venous pressure, borderline low voltage on ECG, and elevated peritoneal total protein make cardiac and pericardial disease the leading considerations. Given the normal ventricular function, I am concerned about elevated intracardiac pressures resulting from pericardial disease or restrictive cardiomyopathy. At this point, right heart catheterization would be useful for assessing intracardiac pressures.
On the fourth hospital day, paracentesis was repeated, and 15 L of fluid was removed. A transjugular liver biopsy demonstrated diffuse patchy fibrosis consistent with early cirrhosis and minor intralobular changes with minimal ballooning. There was no steatosis, active inflammation, granulomata, iron deposition, or evidence of viral hepatitis. Right heart catheterization revealed a right atrial pressure of 18 cm H20, right ventricular pressure of 34/20 cm H20, pulmonary artery pressure of 34/18 cm H20 (mean 25), pulmonary capillary wedge pressure of 20 cm H20, cardiac output of 5.8 L/min, and cardiac index of 2.5 L/min/m2.
The mild hepatic histologic abnormalities do not support an intrinsic liver disease as the cause of his massive ascites and end‐stage liver disease physiology. Cardiac catheterization demonstrates equalization of diastolic pressures, which suggests constrictive pericarditis or restrictive cardiomyopathy. Despite the normal chest radiograph and nonreactive PPD, tuberculosis would be my leading explanation for constrictive pericarditis given the time spent in areas endemic with TB. Although lateral chest radiography may demonstrate pericardial calcifications, magnetic resonance imaging (MRI) is the best imaging modality to detect constrictive pericarditis. Alternately, cardiac amyloidosis could cause restrictive cardiomyopathy and has not been definitively excluded. A cardiac MRI to assess the pericardium would be my next test, and I would request Congo red stains of the liver biopsy. If these tests are unrevealing, endomyocardial biopsy may be necessary.
The cardiac MRI revealed a severely thickened 7‐mm pericardium (normal < 3 mm) most prominent over the right atrium and ventricle. The right ventricle was described as bullet‐shaped, suggesting constrictive pericardial disease (Fig. 1). Left heart catheterization to evaluate coronary anatomy and left ventricular pressures revealed no significant coronary arterial disease and demonstrated an elevated left ventricular end‐diastolic pressure consistent with constrictive pericarditis. Endomyocardial biopsy showed no evidence of infiltrative disease, granulomata, or other significant abnormality. The following day the patient underwent pericardiectomy. Postoperatively, his ascites was easily managed with low doses of diuretics. The pericardial tissue revealed chronic inflammatory cells and dense collagenous fibrosis characteristic of constrictive pericarditis without evidence of malignancy or granulomatous disease. Pericardial cultures were negative for bacteria, viruses, fungi, and mycobacteria.
DISCUSSION
Constrictive pericarditis is characterized by chronic fibrous thickening of the once‐elastic pericardial sac and can occur following any disease process that affects the pericardium (Table 1).1, 2 The challenge in the diagnosis of constrictive pericarditis lies in the recognition of this slowly progressive and uncommon disease. In many cases, nonspecific symptoms of reduced cardiac output and insidious right‐sided heart failure are present for 12 months or longer before a diagnosis is established.1, 3 A typical presentation of constrictive pericarditis is peripheral edema, ascites, and hepatomegaly, a combination that may understandably lead to a misdiagnosis of chronic liver disease and even subject a patient to the unnecessary risk of a liver biopsy, as in this case.
|
Cryptogenic cirrhosis, the initial diagnosis of this patient, is a term used only after excluding the common and uncommon causes of cirrhosis (Table 2).46 With expanded knowledge of the causes of cirrhosis, especially nonalcoholic fatty liver disease, the number of cases of cirrhosis considered to be cryptogenic has decreased from nearly one‐third of all cases in 1960 to approximately 5% in a modern series.7, 8 Chronic or repetitive heart failure can lead to progressive hepatic fibrosis and cirrhosis. Distinguishing features compared to other causes of cirrhosis include an ascitic protein concentration greater than 2.5 g/dL, relatively preserved synthetic function, and infrequent stigmata of end‐stage liver disease such as spider angiomata or pronounced jaundice.9, 10
|
Most common
|
Less common
|
A key exam feature that distinguishes cardiac cirrhosis from other causes of liver failure is an elevated jugular venous pressure. Hepatic causes of cirrhosis induce increased nitric oxide production, which leads to splanchnic and peripheral arterial vasodilatation with a reduced effective circulating volume and normal or low jugular venous pressure.11, 12 Therefore, a patient with cirrhosis and ascites having an elevated jugular venous pressure should prompt echocardiographic evaluation.13 When echocardiography excludes ventricular dysfunction, valvular abnormalities, and pulmonary hypertension, constrictive pericarditis and restrictive cardiomyopathy remain important diagnostic considerations.
In both constrictive pericarditis and restrictive cardiomyopathy, ventricular filling is limited. Pressures in the chambers rise abruptly and rapidly during ventricular filling until equilibrium is reached in early diastole. This can be conceptualized as the cardiac chambers being constrained by the limitations of a rigid external box. In constrictive pericarditis, the rigid external box is the fibrosed and thickened pericardial sac, which loses its elasticity and impairs filling of the ventricles. In restrictive cardiomyopathy, the stiff myocardium limits ventricular filling.
There is considerable overlap in the clinical, echocardiographic, and hemodynamic findings of constrictive pericarditis and restrictive cardiomyopathy.14 Both may present insidiously with progressive heart failure. Echocardiography demonstrates impaired diastolic function. Cardiac hemodynamics demonstrate abrupt and rapid early diastolic filling, elevated and equal ventricular end‐diastolic pressures, and reduced stroke volume and cardiac output. A diagnosis of constrictive pericarditis is favored when a marked inspiratory increase in right ventricular pressures and decrease in left ventricular pressures are seen on heart catheterization or a similar inspiratory increase in transvalvular flow velocities across the tricuspid valve compared with the mitral valve is shown by echocardiography. This finding results from normal inspiratory increases in intrathoracic pressures, which are unable to be transmitted through the rigid pericardium but continue to augment venous return to the right side of the heart. As many as one‐third of patients with pericardial constriction lack these characteristic findings on echocardiogram.14
The results of pericardial imaging may suggest a diagnosis of constrictive pericarditis. Lateral chest radiography demonstrates pericardial calcifications in less than 30% of cases.15 Cardiac computed tomography (CT) and MRI are the best imaging modalities for detecting an increase in pericardial thickness (3 mm or greater).16 However, in as many as 20% of patients with surgically confirmed constrictive pericarditis, CT and MRI will demonstrate a pericardium of normal thickness.17
When faced with the diagnostic conundrum of constrictive pericarditis versus restrictive cardiomyopathy, strong clinical suspicion, thorough echocardiography, careful hemodynamic assessment with right and left heart catheterization,14, 18 pericardial imaging, and sometimes endomyocardial biopsy to exclude restrictive cardiomyopathy are often needed before proceeding to pericardiectomy, which carries a significant surgical risk but can also be curative.
This case highlights many of the features of constrictive pericarditis, the challenges and delay in its diagnosis, and its occasional misdiagnosis as chronic liver disease. Clinicians may recognize the typical combination of cirrhosis (or suspected cirrhosis), high SAAG ascites, and edema as characteristic of advanced intrinsic liver disease. However, they must not be seduced into immediate pattern recognition when contrary evidencesuch as elevated neck veins, elevated ascitic total protein, or relatively preserved hepatic synthetic functionaccompanies that picture. Under such circumstances, they must remember to think outside the box and bear in mind that the heart may be trapped inside a box.
Take‐Home Points
-
Constrictive pericarditis is often unrecognized initially, resulting in delayed diagnosis. Patients typically present with nonspecific signs and symptoms of low cardiac output and progressive right‐sided heart failure. Clinical suspicion is key to prompt diagnosis and pericardiectomy, which may be curative.
-
Distinguishing features in the presentation of cardiac or pericardial etiologies of ascites and cirrhosis include elevated neck veins, elevated ascitic protein content, relatively preserved hepatic synthetic function, and absence of the stigmata of end‐stage liver disease.
-
Constrictive pericarditis and restrictive cardiomyopathy can present with a similar clinical picture and hemodynamics showing impaired ventricular filling. Right and left heart catheterization, pericardial imaging, and endomyocardial biopsy may differentiate the 2 conditions. For constrictive pericarditis, surgical and pathological confirmation is the gold standard for diagnosis and the only definitive treatment.
- ,,, et al.Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy.Circulation.1999;100:1380–1386.
- ,,, et al.Constrictive pericarditis: etiology and cause‐specific survival after pericardiectomy.J Am Coll Cardiol.2004;43:1445–1452.
- .Chronic constrictive pericarditis.Am J Cardiol.1961;7:48–61.
- American Gastroenterological Association.AGA technical review on the evaluation of liver chemistry tests.Gastroenterology.2002;123:1367–1384.
- ,.AASLD practice guidelines: evaluation of the patient for liver transplantation.Hepatology.2005;41:1–26.
- Feldman M,Friedman LS,Brandt LJ, eds.Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management.Philadelphia:Saunders Elsevier;2006.
- ,,, et al.Cirrhosis of the liver: a study of alcoholic and nonalcoholic patients in Boston and London.N Engl J Med.1960;261:1–9.
- ,,, et al.Liver transplantation for cryptogenic cirrhosis.Liver Transpl Surg.1997;3:359–364.
- ,,, et al.Heart diseases affecting the liver and liver disease affecting the heart.Am Heart J.2000;140:111–120.
- ,,.The liver in heart failure.Clin Liver Dis.2002;6:947–967.
- ,,, et al.Portal hypertension: from pathophysiology to clinical practice.Liver Int.2005;25:1079–1090.
- .Portal hypertension.Curr Opin Gastroenterol.2006;22:254–262.
- ,,, et al.Negative influences of ascites on the cardiac function of cirrhotic patients.Am J Med.1975;59:165–170.
- .Constrictive pericarditis in the modern era: a diagnostic dilemma.Heart.2001;86:619–623.
- ,,, et al.Calcific constrictive pericarditis: is it still with us?Ann Intern Med.2000;132:444–450.
- ,,,,,.CT and MR imaging of pericardial disease.Radiographics.2003;23:S167–S180.
- ,,, et al.Constrictive pericarditis in 26 patients with histologically normal pericardial thickness.Circulation.2003;108:1852–1857.
- ,,, et al.Value of dynamic respiratory changes in left and right ventricular pressures for the diagnosis of constrictive pericarditis.Circulation.1996;93:2007–2013.
A 65‐year‐old man was referred for evaluation of worsening ascites and end‐stage liver disease. The patient had been well until 1 year ago, when he developed lower extremity edema and abdominal distention. After evaluation by his primary care physician, he was given a diagnosis of cryptogenic cirrhosis. He underwent several paracenteses and was placed on furosemide and spironolactone. The patient had been stable on his diuretic regimen until 2 weeks previously, when he suddenly developed worsening edema and ascites, along with dizziness, nausea, and hypotension. His physician stopped the diuretics and referred him to the hospital.
Before diagnosing a patient with cryptogenic cirrhosis, it is necessary to exclude common etiologies of cirrhosis such as alcohol, viral hepatitis, and non‐alcoholic fatty liver disease and numerous uncommon causes, including Wilson's disease, hemochromatosis, Budd‐Chiari, and biliary cirrhosis. It is also important to remember that patients with liver disease are not immune to extrahepatic causes of ascites, such as peritoneal carcinomatosis and tuberculous ascites. Simultaneously, reasons for chronic liver disease decompensating acutely must be considered: medication nonadherence, excess salt intake, hepatotoxicity from acetaminophen or alcohol, and other acute insults, such as hepatocellular carcinoma, an intervening infection (especially spontaneous bacterial peritonitis), ascending cholangitis, or a flare of chronic viral hepatitis.
Past medical and surgical history included diabetes mellitus (diagnosed 10 years previously), obstructive sleep apnea, hypertension, hypothyroidism, and mild chronic kidney disease. Medications included levothyroxine, lactulose, sulfamethoxazole, pioglitazone (started 4 months prior), and ibuprofen. Furosemide and spironolactone had been discontinued 2 weeks previously. He currently resided in the Central Valley of California. He had lived in Thailand from age 7 to 17 and traveled to India more than 1 year ago. He did not smoke and had never used intravenous drugs or received a blood transfusion. He rarely drank alcohol. He worked as a chemist. There was no family history of liver disease.
There is no obvious explanation for the underlying liver disease or the acute decompensation. Sulfamethoxazole is a rare cause of allergic or granulomatous hepatitis. Pioglitazone is a thiazolinedione which in earlier formulations was linked to hepatitis but can be excluded as a cause of this patient's cirrhosis because it was started after liver disease was detected. As a chemist, he might have been exposed to carbon tetrachloride, a known hepatotoxin. Obstructive sleep apnea causes pulmonary hypertension, but severe ascites and acute hepatic decompensation would be unusual. Ibuprofen might precipitate worsening renal function and fluid accumulation. Time in Thailand and India raises the possibility of tuberculous ascites.
The patient had no headache, vision changes, abdominal pain, emesis, melena, hematochezia, chest pain, palpitations, dysuria, polyuria, pruritus, dark urine, or rashes. He reported difficulty with concentration when lactulose was decreased. He noted worsening exercise tolerance with dyspnea after 10 steps and reported a weight gain of 12 pounds in the past 2 weeks.
On examination, temperature was 36.8C; blood pressure, 129/87 mm Hg; heart rate, 85 beats per minute; respirations, 20 per minute; and oxygen saturation, 94% on room air. He was uncomfortable but alert. There was no scleral icterus or conjunctival pallor. Jugular venous pressure was elevated. The lungs were clear, and the heart was regular, with no murmur, rub, or gallops. The abdomen was massively distended with a fluid wave; the liver and spleen could not be palpated. There was pitting edema of the sacrum and lower extremities. There was no asterixis, palmar erythema, spider angiomata, or skin discoloration.
The additional history and physical exam suggest that the primary problem may lie outside the liver, especially as signs of advanced liver disease (other than ascites) are absent. Dyspnea on exertion is consistent with the physical stress of a large volume of ascites or could be secondary to several pulmonary complications associated with liver disease, including portopulmonary hypertension, hepatopulmonary syndrome, or hepatic hydrothorax. Alternatively, the dyspnea raises the possibility that the ascites is not related to a primary liver disorder but rather to anemia or to a cardiac disorder, such as chronic left ventricular failure, isolated right‐sided heart failure, or constrictive pericarditis. These diagnoses are suggested by the elevated jugular venous pressure, which is atypical in cirrhosis.
Although portal hypertension accounts for most cases of ascites, peritoneal fluid should be examined to exclude peritoneal carcinomatosis and tuberculous ascites. I am interested in the results of an echocardiogram.
Initial laboratory studies demonstrated a sodium concentration of 136 mEq/dL; potassium, 4.7 mEq/dL; chloride, 99 mEq/dL; bicarbonate, 24 mEq/dL; blood urea nitrogen, 54 mg/dL; creatinine, 3.3 mg/dL (increased from baseline of 1.6 mg/dL 4 months previously); white cell count, 7000/mm3; hemoglobin, 10.5 g/dL; MCV, 89 fL; platelet count, 205,000/mm3; bilirubin, 0.6 mg/dL; aspartate aminotransferase, 15 U/L; alanine aminotransferase, 8 U/L; alkaline phosphatase, 102 U/L; albumin, 4.2 g/dL; total protein, 8.2 g/dL; international normalized ratio, 1.2; and partial thromboplastin time, 31.8 seconds. A urine dipstick demonstrated 1+ protein. The chest radiograph was normal. Electrocardiogram had borderline low voltage with nonspecific T‐wave abnormalities. Additional studies showed a serum iron concentration of 49 mg/dL, transferrin saturation of 16%, total iron binding capacity of 310 mg/dL, and ferritin of 247 mg/mL. Hemoglobin A1c was 7.0%. Acute and chronic antibodies to hepatitis A, B, and C viruses were negative. The following study results were normal or negative: antinuclear antibody, alpha‐1‐antitrypsin, ceruloplasmin, alpha‐fetoprotein, carcinoembryonic antigen, and 24‐hour urinary copper. The thyroid function studies were normal. A purified protein derivative (PPD) skin test was nonreactive.
There continues to be a paucity of evidence of a primary liver disorder. The hepatic enzymes and tests of liver synthetic function are normal, and there is no pancytopenia, as might result from hypersplenism. I remain most suspicious of either a primary cardiac or pericardial disorder with secondary hepatic congestion or a disease that simultaneously affects the heart and liver.
The reasons for the low voltage on the electrocardiogram include processes that infiltrate the myocardium (amyloidosis, sarcoidosis, hemochromatosis, and myxedema fluid) and processes that increase the distance between the myocardium and surface electrodes, such as adipose tissue, air (from emphysema or pneumothorax), or pericardial effusion. Pericardial effusion may present subacutely with predominant features of right ventricular failure. Low voltage, liver disease, and possible heart failure raise the possibility of amyloidosis or hemochromatosis. The low transferrin saturation renders hemochromatosis unlikely. Although normal alkaline phosphatase and serum albumin are not characteristic when AL amyloid affects the liver and kidneys, serum and urine protein electrophoresis and immunofixation should be considered.
With paracentesis 3.5 L of ascitic fluid was removed. The red cell count was 4000/mm3, and white blood cell count was 505/mm3, of which 25% were polymorphonuclear cells, 22% were lymphocytes, and 53% were monocytes. Additional peritoneal fluid chemistries included albumin of 3.0 g/dL and total protein of 5.3 g/dL. Abdominal ultrasound with Doppler demonstrated a liver of normal size and echogenicity with patent hepatic arteries, hepatic veins, and portal vein. There was mild splenomegaly with normal kidneys. Evaluation for a possible liver transplant was initiated. Blood, urine, and peritoneal fluid cultures demonstrated no growth. Echocardiography demonstrated borderline concentric left ventricular hypertrophy, normal right and left ventricular function, dilated superior and inferior vena cavae, and no pericardial effusion or thickening.
The serum‐ascites albumin gradient (SAAG) of 1.2 is consistent with portal hypertension as the cause of the ascites. The Doppler findings exclude postsinusoidal causes of portal hypertension from hepatic vein obstruction or thrombosis. The combination of the elevated SAAG, elevated jugular venous pressure, borderline low voltage on ECG, and elevated peritoneal total protein make cardiac and pericardial disease the leading considerations. Given the normal ventricular function, I am concerned about elevated intracardiac pressures resulting from pericardial disease or restrictive cardiomyopathy. At this point, right heart catheterization would be useful for assessing intracardiac pressures.
On the fourth hospital day, paracentesis was repeated, and 15 L of fluid was removed. A transjugular liver biopsy demonstrated diffuse patchy fibrosis consistent with early cirrhosis and minor intralobular changes with minimal ballooning. There was no steatosis, active inflammation, granulomata, iron deposition, or evidence of viral hepatitis. Right heart catheterization revealed a right atrial pressure of 18 cm H20, right ventricular pressure of 34/20 cm H20, pulmonary artery pressure of 34/18 cm H20 (mean 25), pulmonary capillary wedge pressure of 20 cm H20, cardiac output of 5.8 L/min, and cardiac index of 2.5 L/min/m2.
The mild hepatic histologic abnormalities do not support an intrinsic liver disease as the cause of his massive ascites and end‐stage liver disease physiology. Cardiac catheterization demonstrates equalization of diastolic pressures, which suggests constrictive pericarditis or restrictive cardiomyopathy. Despite the normal chest radiograph and nonreactive PPD, tuberculosis would be my leading explanation for constrictive pericarditis given the time spent in areas endemic with TB. Although lateral chest radiography may demonstrate pericardial calcifications, magnetic resonance imaging (MRI) is the best imaging modality to detect constrictive pericarditis. Alternately, cardiac amyloidosis could cause restrictive cardiomyopathy and has not been definitively excluded. A cardiac MRI to assess the pericardium would be my next test, and I would request Congo red stains of the liver biopsy. If these tests are unrevealing, endomyocardial biopsy may be necessary.
The cardiac MRI revealed a severely thickened 7‐mm pericardium (normal < 3 mm) most prominent over the right atrium and ventricle. The right ventricle was described as bullet‐shaped, suggesting constrictive pericardial disease (Fig. 1). Left heart catheterization to evaluate coronary anatomy and left ventricular pressures revealed no significant coronary arterial disease and demonstrated an elevated left ventricular end‐diastolic pressure consistent with constrictive pericarditis. Endomyocardial biopsy showed no evidence of infiltrative disease, granulomata, or other significant abnormality. The following day the patient underwent pericardiectomy. Postoperatively, his ascites was easily managed with low doses of diuretics. The pericardial tissue revealed chronic inflammatory cells and dense collagenous fibrosis characteristic of constrictive pericarditis without evidence of malignancy or granulomatous disease. Pericardial cultures were negative for bacteria, viruses, fungi, and mycobacteria.

DISCUSSION
Constrictive pericarditis is characterized by chronic fibrous thickening of the once‐elastic pericardial sac and can occur following any disease process that affects the pericardium (Table 1).1, 2 The challenge in the diagnosis of constrictive pericarditis lies in the recognition of this slowly progressive and uncommon disease. In many cases, nonspecific symptoms of reduced cardiac output and insidious right‐sided heart failure are present for 12 months or longer before a diagnosis is established.1, 3 A typical presentation of constrictive pericarditis is peripheral edema, ascites, and hepatomegaly, a combination that may understandably lead to a misdiagnosis of chronic liver disease and even subject a patient to the unnecessary risk of a liver biopsy, as in this case.
|
Cryptogenic cirrhosis, the initial diagnosis of this patient, is a term used only after excluding the common and uncommon causes of cirrhosis (Table 2).46 With expanded knowledge of the causes of cirrhosis, especially nonalcoholic fatty liver disease, the number of cases of cirrhosis considered to be cryptogenic has decreased from nearly one‐third of all cases in 1960 to approximately 5% in a modern series.7, 8 Chronic or repetitive heart failure can lead to progressive hepatic fibrosis and cirrhosis. Distinguishing features compared to other causes of cirrhosis include an ascitic protein concentration greater than 2.5 g/dL, relatively preserved synthetic function, and infrequent stigmata of end‐stage liver disease such as spider angiomata or pronounced jaundice.9, 10
|
Most common
|
Less common
|
A key exam feature that distinguishes cardiac cirrhosis from other causes of liver failure is an elevated jugular venous pressure. Hepatic causes of cirrhosis induce increased nitric oxide production, which leads to splanchnic and peripheral arterial vasodilatation with a reduced effective circulating volume and normal or low jugular venous pressure.11, 12 Therefore, a patient with cirrhosis and ascites having an elevated jugular venous pressure should prompt echocardiographic evaluation.13 When echocardiography excludes ventricular dysfunction, valvular abnormalities, and pulmonary hypertension, constrictive pericarditis and restrictive cardiomyopathy remain important diagnostic considerations.
In both constrictive pericarditis and restrictive cardiomyopathy, ventricular filling is limited. Pressures in the chambers rise abruptly and rapidly during ventricular filling until equilibrium is reached in early diastole. This can be conceptualized as the cardiac chambers being constrained by the limitations of a rigid external box. In constrictive pericarditis, the rigid external box is the fibrosed and thickened pericardial sac, which loses its elasticity and impairs filling of the ventricles. In restrictive cardiomyopathy, the stiff myocardium limits ventricular filling.
There is considerable overlap in the clinical, echocardiographic, and hemodynamic findings of constrictive pericarditis and restrictive cardiomyopathy.14 Both may present insidiously with progressive heart failure. Echocardiography demonstrates impaired diastolic function. Cardiac hemodynamics demonstrate abrupt and rapid early diastolic filling, elevated and equal ventricular end‐diastolic pressures, and reduced stroke volume and cardiac output. A diagnosis of constrictive pericarditis is favored when a marked inspiratory increase in right ventricular pressures and decrease in left ventricular pressures are seen on heart catheterization or a similar inspiratory increase in transvalvular flow velocities across the tricuspid valve compared with the mitral valve is shown by echocardiography. This finding results from normal inspiratory increases in intrathoracic pressures, which are unable to be transmitted through the rigid pericardium but continue to augment venous return to the right side of the heart. As many as one‐third of patients with pericardial constriction lack these characteristic findings on echocardiogram.14
The results of pericardial imaging may suggest a diagnosis of constrictive pericarditis. Lateral chest radiography demonstrates pericardial calcifications in less than 30% of cases.15 Cardiac computed tomography (CT) and MRI are the best imaging modalities for detecting an increase in pericardial thickness (3 mm or greater).16 However, in as many as 20% of patients with surgically confirmed constrictive pericarditis, CT and MRI will demonstrate a pericardium of normal thickness.17
When faced with the diagnostic conundrum of constrictive pericarditis versus restrictive cardiomyopathy, strong clinical suspicion, thorough echocardiography, careful hemodynamic assessment with right and left heart catheterization,14, 18 pericardial imaging, and sometimes endomyocardial biopsy to exclude restrictive cardiomyopathy are often needed before proceeding to pericardiectomy, which carries a significant surgical risk but can also be curative.
This case highlights many of the features of constrictive pericarditis, the challenges and delay in its diagnosis, and its occasional misdiagnosis as chronic liver disease. Clinicians may recognize the typical combination of cirrhosis (or suspected cirrhosis), high SAAG ascites, and edema as characteristic of advanced intrinsic liver disease. However, they must not be seduced into immediate pattern recognition when contrary evidencesuch as elevated neck veins, elevated ascitic total protein, or relatively preserved hepatic synthetic functionaccompanies that picture. Under such circumstances, they must remember to think outside the box and bear in mind that the heart may be trapped inside a box.
Take‐Home Points
-
Constrictive pericarditis is often unrecognized initially, resulting in delayed diagnosis. Patients typically present with nonspecific signs and symptoms of low cardiac output and progressive right‐sided heart failure. Clinical suspicion is key to prompt diagnosis and pericardiectomy, which may be curative.
-
Distinguishing features in the presentation of cardiac or pericardial etiologies of ascites and cirrhosis include elevated neck veins, elevated ascitic protein content, relatively preserved hepatic synthetic function, and absence of the stigmata of end‐stage liver disease.
-
Constrictive pericarditis and restrictive cardiomyopathy can present with a similar clinical picture and hemodynamics showing impaired ventricular filling. Right and left heart catheterization, pericardial imaging, and endomyocardial biopsy may differentiate the 2 conditions. For constrictive pericarditis, surgical and pathological confirmation is the gold standard for diagnosis and the only definitive treatment.
A 65‐year‐old man was referred for evaluation of worsening ascites and end‐stage liver disease. The patient had been well until 1 year ago, when he developed lower extremity edema and abdominal distention. After evaluation by his primary care physician, he was given a diagnosis of cryptogenic cirrhosis. He underwent several paracenteses and was placed on furosemide and spironolactone. The patient had been stable on his diuretic regimen until 2 weeks previously, when he suddenly developed worsening edema and ascites, along with dizziness, nausea, and hypotension. His physician stopped the diuretics and referred him to the hospital.
Before diagnosing a patient with cryptogenic cirrhosis, it is necessary to exclude common etiologies of cirrhosis such as alcohol, viral hepatitis, and non‐alcoholic fatty liver disease and numerous uncommon causes, including Wilson's disease, hemochromatosis, Budd‐Chiari, and biliary cirrhosis. It is also important to remember that patients with liver disease are not immune to extrahepatic causes of ascites, such as peritoneal carcinomatosis and tuberculous ascites. Simultaneously, reasons for chronic liver disease decompensating acutely must be considered: medication nonadherence, excess salt intake, hepatotoxicity from acetaminophen or alcohol, and other acute insults, such as hepatocellular carcinoma, an intervening infection (especially spontaneous bacterial peritonitis), ascending cholangitis, or a flare of chronic viral hepatitis.
Past medical and surgical history included diabetes mellitus (diagnosed 10 years previously), obstructive sleep apnea, hypertension, hypothyroidism, and mild chronic kidney disease. Medications included levothyroxine, lactulose, sulfamethoxazole, pioglitazone (started 4 months prior), and ibuprofen. Furosemide and spironolactone had been discontinued 2 weeks previously. He currently resided in the Central Valley of California. He had lived in Thailand from age 7 to 17 and traveled to India more than 1 year ago. He did not smoke and had never used intravenous drugs or received a blood transfusion. He rarely drank alcohol. He worked as a chemist. There was no family history of liver disease.
There is no obvious explanation for the underlying liver disease or the acute decompensation. Sulfamethoxazole is a rare cause of allergic or granulomatous hepatitis. Pioglitazone is a thiazolinedione which in earlier formulations was linked to hepatitis but can be excluded as a cause of this patient's cirrhosis because it was started after liver disease was detected. As a chemist, he might have been exposed to carbon tetrachloride, a known hepatotoxin. Obstructive sleep apnea causes pulmonary hypertension, but severe ascites and acute hepatic decompensation would be unusual. Ibuprofen might precipitate worsening renal function and fluid accumulation. Time in Thailand and India raises the possibility of tuberculous ascites.
The patient had no headache, vision changes, abdominal pain, emesis, melena, hematochezia, chest pain, palpitations, dysuria, polyuria, pruritus, dark urine, or rashes. He reported difficulty with concentration when lactulose was decreased. He noted worsening exercise tolerance with dyspnea after 10 steps and reported a weight gain of 12 pounds in the past 2 weeks.
On examination, temperature was 36.8C; blood pressure, 129/87 mm Hg; heart rate, 85 beats per minute; respirations, 20 per minute; and oxygen saturation, 94% on room air. He was uncomfortable but alert. There was no scleral icterus or conjunctival pallor. Jugular venous pressure was elevated. The lungs were clear, and the heart was regular, with no murmur, rub, or gallops. The abdomen was massively distended with a fluid wave; the liver and spleen could not be palpated. There was pitting edema of the sacrum and lower extremities. There was no asterixis, palmar erythema, spider angiomata, or skin discoloration.
The additional history and physical exam suggest that the primary problem may lie outside the liver, especially as signs of advanced liver disease (other than ascites) are absent. Dyspnea on exertion is consistent with the physical stress of a large volume of ascites or could be secondary to several pulmonary complications associated with liver disease, including portopulmonary hypertension, hepatopulmonary syndrome, or hepatic hydrothorax. Alternatively, the dyspnea raises the possibility that the ascites is not related to a primary liver disorder but rather to anemia or to a cardiac disorder, such as chronic left ventricular failure, isolated right‐sided heart failure, or constrictive pericarditis. These diagnoses are suggested by the elevated jugular venous pressure, which is atypical in cirrhosis.
Although portal hypertension accounts for most cases of ascites, peritoneal fluid should be examined to exclude peritoneal carcinomatosis and tuberculous ascites. I am interested in the results of an echocardiogram.
Initial laboratory studies demonstrated a sodium concentration of 136 mEq/dL; potassium, 4.7 mEq/dL; chloride, 99 mEq/dL; bicarbonate, 24 mEq/dL; blood urea nitrogen, 54 mg/dL; creatinine, 3.3 mg/dL (increased from baseline of 1.6 mg/dL 4 months previously); white cell count, 7000/mm3; hemoglobin, 10.5 g/dL; MCV, 89 fL; platelet count, 205,000/mm3; bilirubin, 0.6 mg/dL; aspartate aminotransferase, 15 U/L; alanine aminotransferase, 8 U/L; alkaline phosphatase, 102 U/L; albumin, 4.2 g/dL; total protein, 8.2 g/dL; international normalized ratio, 1.2; and partial thromboplastin time, 31.8 seconds. A urine dipstick demonstrated 1+ protein. The chest radiograph was normal. Electrocardiogram had borderline low voltage with nonspecific T‐wave abnormalities. Additional studies showed a serum iron concentration of 49 mg/dL, transferrin saturation of 16%, total iron binding capacity of 310 mg/dL, and ferritin of 247 mg/mL. Hemoglobin A1c was 7.0%. Acute and chronic antibodies to hepatitis A, B, and C viruses were negative. The following study results were normal or negative: antinuclear antibody, alpha‐1‐antitrypsin, ceruloplasmin, alpha‐fetoprotein, carcinoembryonic antigen, and 24‐hour urinary copper. The thyroid function studies were normal. A purified protein derivative (PPD) skin test was nonreactive.
There continues to be a paucity of evidence of a primary liver disorder. The hepatic enzymes and tests of liver synthetic function are normal, and there is no pancytopenia, as might result from hypersplenism. I remain most suspicious of either a primary cardiac or pericardial disorder with secondary hepatic congestion or a disease that simultaneously affects the heart and liver.
The reasons for the low voltage on the electrocardiogram include processes that infiltrate the myocardium (amyloidosis, sarcoidosis, hemochromatosis, and myxedema fluid) and processes that increase the distance between the myocardium and surface electrodes, such as adipose tissue, air (from emphysema or pneumothorax), or pericardial effusion. Pericardial effusion may present subacutely with predominant features of right ventricular failure. Low voltage, liver disease, and possible heart failure raise the possibility of amyloidosis or hemochromatosis. The low transferrin saturation renders hemochromatosis unlikely. Although normal alkaline phosphatase and serum albumin are not characteristic when AL amyloid affects the liver and kidneys, serum and urine protein electrophoresis and immunofixation should be considered.
With paracentesis 3.5 L of ascitic fluid was removed. The red cell count was 4000/mm3, and white blood cell count was 505/mm3, of which 25% were polymorphonuclear cells, 22% were lymphocytes, and 53% were monocytes. Additional peritoneal fluid chemistries included albumin of 3.0 g/dL and total protein of 5.3 g/dL. Abdominal ultrasound with Doppler demonstrated a liver of normal size and echogenicity with patent hepatic arteries, hepatic veins, and portal vein. There was mild splenomegaly with normal kidneys. Evaluation for a possible liver transplant was initiated. Blood, urine, and peritoneal fluid cultures demonstrated no growth. Echocardiography demonstrated borderline concentric left ventricular hypertrophy, normal right and left ventricular function, dilated superior and inferior vena cavae, and no pericardial effusion or thickening.
The serum‐ascites albumin gradient (SAAG) of 1.2 is consistent with portal hypertension as the cause of the ascites. The Doppler findings exclude postsinusoidal causes of portal hypertension from hepatic vein obstruction or thrombosis. The combination of the elevated SAAG, elevated jugular venous pressure, borderline low voltage on ECG, and elevated peritoneal total protein make cardiac and pericardial disease the leading considerations. Given the normal ventricular function, I am concerned about elevated intracardiac pressures resulting from pericardial disease or restrictive cardiomyopathy. At this point, right heart catheterization would be useful for assessing intracardiac pressures.
On the fourth hospital day, paracentesis was repeated, and 15 L of fluid was removed. A transjugular liver biopsy demonstrated diffuse patchy fibrosis consistent with early cirrhosis and minor intralobular changes with minimal ballooning. There was no steatosis, active inflammation, granulomata, iron deposition, or evidence of viral hepatitis. Right heart catheterization revealed a right atrial pressure of 18 cm H20, right ventricular pressure of 34/20 cm H20, pulmonary artery pressure of 34/18 cm H20 (mean 25), pulmonary capillary wedge pressure of 20 cm H20, cardiac output of 5.8 L/min, and cardiac index of 2.5 L/min/m2.
The mild hepatic histologic abnormalities do not support an intrinsic liver disease as the cause of his massive ascites and end‐stage liver disease physiology. Cardiac catheterization demonstrates equalization of diastolic pressures, which suggests constrictive pericarditis or restrictive cardiomyopathy. Despite the normal chest radiograph and nonreactive PPD, tuberculosis would be my leading explanation for constrictive pericarditis given the time spent in areas endemic with TB. Although lateral chest radiography may demonstrate pericardial calcifications, magnetic resonance imaging (MRI) is the best imaging modality to detect constrictive pericarditis. Alternately, cardiac amyloidosis could cause restrictive cardiomyopathy and has not been definitively excluded. A cardiac MRI to assess the pericardium would be my next test, and I would request Congo red stains of the liver biopsy. If these tests are unrevealing, endomyocardial biopsy may be necessary.
The cardiac MRI revealed a severely thickened 7‐mm pericardium (normal < 3 mm) most prominent over the right atrium and ventricle. The right ventricle was described as bullet‐shaped, suggesting constrictive pericardial disease (Fig. 1). Left heart catheterization to evaluate coronary anatomy and left ventricular pressures revealed no significant coronary arterial disease and demonstrated an elevated left ventricular end‐diastolic pressure consistent with constrictive pericarditis. Endomyocardial biopsy showed no evidence of infiltrative disease, granulomata, or other significant abnormality. The following day the patient underwent pericardiectomy. Postoperatively, his ascites was easily managed with low doses of diuretics. The pericardial tissue revealed chronic inflammatory cells and dense collagenous fibrosis characteristic of constrictive pericarditis without evidence of malignancy or granulomatous disease. Pericardial cultures were negative for bacteria, viruses, fungi, and mycobacteria.

DISCUSSION
Constrictive pericarditis is characterized by chronic fibrous thickening of the once‐elastic pericardial sac and can occur following any disease process that affects the pericardium (Table 1).1, 2 The challenge in the diagnosis of constrictive pericarditis lies in the recognition of this slowly progressive and uncommon disease. In many cases, nonspecific symptoms of reduced cardiac output and insidious right‐sided heart failure are present for 12 months or longer before a diagnosis is established.1, 3 A typical presentation of constrictive pericarditis is peripheral edema, ascites, and hepatomegaly, a combination that may understandably lead to a misdiagnosis of chronic liver disease and even subject a patient to the unnecessary risk of a liver biopsy, as in this case.
|
Cryptogenic cirrhosis, the initial diagnosis of this patient, is a term used only after excluding the common and uncommon causes of cirrhosis (Table 2).46 With expanded knowledge of the causes of cirrhosis, especially nonalcoholic fatty liver disease, the number of cases of cirrhosis considered to be cryptogenic has decreased from nearly one‐third of all cases in 1960 to approximately 5% in a modern series.7, 8 Chronic or repetitive heart failure can lead to progressive hepatic fibrosis and cirrhosis. Distinguishing features compared to other causes of cirrhosis include an ascitic protein concentration greater than 2.5 g/dL, relatively preserved synthetic function, and infrequent stigmata of end‐stage liver disease such as spider angiomata or pronounced jaundice.9, 10
|
Most common
|
Less common
|
A key exam feature that distinguishes cardiac cirrhosis from other causes of liver failure is an elevated jugular venous pressure. Hepatic causes of cirrhosis induce increased nitric oxide production, which leads to splanchnic and peripheral arterial vasodilatation with a reduced effective circulating volume and normal or low jugular venous pressure.11, 12 Therefore, a patient with cirrhosis and ascites having an elevated jugular venous pressure should prompt echocardiographic evaluation.13 When echocardiography excludes ventricular dysfunction, valvular abnormalities, and pulmonary hypertension, constrictive pericarditis and restrictive cardiomyopathy remain important diagnostic considerations.
In both constrictive pericarditis and restrictive cardiomyopathy, ventricular filling is limited. Pressures in the chambers rise abruptly and rapidly during ventricular filling until equilibrium is reached in early diastole. This can be conceptualized as the cardiac chambers being constrained by the limitations of a rigid external box. In constrictive pericarditis, the rigid external box is the fibrosed and thickened pericardial sac, which loses its elasticity and impairs filling of the ventricles. In restrictive cardiomyopathy, the stiff myocardium limits ventricular filling.
There is considerable overlap in the clinical, echocardiographic, and hemodynamic findings of constrictive pericarditis and restrictive cardiomyopathy.14 Both may present insidiously with progressive heart failure. Echocardiography demonstrates impaired diastolic function. Cardiac hemodynamics demonstrate abrupt and rapid early diastolic filling, elevated and equal ventricular end‐diastolic pressures, and reduced stroke volume and cardiac output. A diagnosis of constrictive pericarditis is favored when a marked inspiratory increase in right ventricular pressures and decrease in left ventricular pressures are seen on heart catheterization or a similar inspiratory increase in transvalvular flow velocities across the tricuspid valve compared with the mitral valve is shown by echocardiography. This finding results from normal inspiratory increases in intrathoracic pressures, which are unable to be transmitted through the rigid pericardium but continue to augment venous return to the right side of the heart. As many as one‐third of patients with pericardial constriction lack these characteristic findings on echocardiogram.14
The results of pericardial imaging may suggest a diagnosis of constrictive pericarditis. Lateral chest radiography demonstrates pericardial calcifications in less than 30% of cases.15 Cardiac computed tomography (CT) and MRI are the best imaging modalities for detecting an increase in pericardial thickness (3 mm or greater).16 However, in as many as 20% of patients with surgically confirmed constrictive pericarditis, CT and MRI will demonstrate a pericardium of normal thickness.17
When faced with the diagnostic conundrum of constrictive pericarditis versus restrictive cardiomyopathy, strong clinical suspicion, thorough echocardiography, careful hemodynamic assessment with right and left heart catheterization,14, 18 pericardial imaging, and sometimes endomyocardial biopsy to exclude restrictive cardiomyopathy are often needed before proceeding to pericardiectomy, which carries a significant surgical risk but can also be curative.
This case highlights many of the features of constrictive pericarditis, the challenges and delay in its diagnosis, and its occasional misdiagnosis as chronic liver disease. Clinicians may recognize the typical combination of cirrhosis (or suspected cirrhosis), high SAAG ascites, and edema as characteristic of advanced intrinsic liver disease. However, they must not be seduced into immediate pattern recognition when contrary evidencesuch as elevated neck veins, elevated ascitic total protein, or relatively preserved hepatic synthetic functionaccompanies that picture. Under such circumstances, they must remember to think outside the box and bear in mind that the heart may be trapped inside a box.
Take‐Home Points
-
Constrictive pericarditis is often unrecognized initially, resulting in delayed diagnosis. Patients typically present with nonspecific signs and symptoms of low cardiac output and progressive right‐sided heart failure. Clinical suspicion is key to prompt diagnosis and pericardiectomy, which may be curative.
-
Distinguishing features in the presentation of cardiac or pericardial etiologies of ascites and cirrhosis include elevated neck veins, elevated ascitic protein content, relatively preserved hepatic synthetic function, and absence of the stigmata of end‐stage liver disease.
-
Constrictive pericarditis and restrictive cardiomyopathy can present with a similar clinical picture and hemodynamics showing impaired ventricular filling. Right and left heart catheterization, pericardial imaging, and endomyocardial biopsy may differentiate the 2 conditions. For constrictive pericarditis, surgical and pathological confirmation is the gold standard for diagnosis and the only definitive treatment.
- ,,, et al.Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy.Circulation.1999;100:1380–1386.
- ,,, et al.Constrictive pericarditis: etiology and cause‐specific survival after pericardiectomy.J Am Coll Cardiol.2004;43:1445–1452.
- .Chronic constrictive pericarditis.Am J Cardiol.1961;7:48–61.
- American Gastroenterological Association.AGA technical review on the evaluation of liver chemistry tests.Gastroenterology.2002;123:1367–1384.
- ,.AASLD practice guidelines: evaluation of the patient for liver transplantation.Hepatology.2005;41:1–26.
- Feldman M,Friedman LS,Brandt LJ, eds.Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management.Philadelphia:Saunders Elsevier;2006.
- ,,, et al.Cirrhosis of the liver: a study of alcoholic and nonalcoholic patients in Boston and London.N Engl J Med.1960;261:1–9.
- ,,, et al.Liver transplantation for cryptogenic cirrhosis.Liver Transpl Surg.1997;3:359–364.
- ,,, et al.Heart diseases affecting the liver and liver disease affecting the heart.Am Heart J.2000;140:111–120.
- ,,.The liver in heart failure.Clin Liver Dis.2002;6:947–967.
- ,,, et al.Portal hypertension: from pathophysiology to clinical practice.Liver Int.2005;25:1079–1090.
- .Portal hypertension.Curr Opin Gastroenterol.2006;22:254–262.
- ,,, et al.Negative influences of ascites on the cardiac function of cirrhotic patients.Am J Med.1975;59:165–170.
- .Constrictive pericarditis in the modern era: a diagnostic dilemma.Heart.2001;86:619–623.
- ,,, et al.Calcific constrictive pericarditis: is it still with us?Ann Intern Med.2000;132:444–450.
- ,,,,,.CT and MR imaging of pericardial disease.Radiographics.2003;23:S167–S180.
- ,,, et al.Constrictive pericarditis in 26 patients with histologically normal pericardial thickness.Circulation.2003;108:1852–1857.
- ,,, et al.Value of dynamic respiratory changes in left and right ventricular pressures for the diagnosis of constrictive pericarditis.Circulation.1996;93:2007–2013.
- ,,, et al.Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy.Circulation.1999;100:1380–1386.
- ,,, et al.Constrictive pericarditis: etiology and cause‐specific survival after pericardiectomy.J Am Coll Cardiol.2004;43:1445–1452.
- .Chronic constrictive pericarditis.Am J Cardiol.1961;7:48–61.
- American Gastroenterological Association.AGA technical review on the evaluation of liver chemistry tests.Gastroenterology.2002;123:1367–1384.
- ,.AASLD practice guidelines: evaluation of the patient for liver transplantation.Hepatology.2005;41:1–26.
- Feldman M,Friedman LS,Brandt LJ, eds.Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management.Philadelphia:Saunders Elsevier;2006.
- ,,, et al.Cirrhosis of the liver: a study of alcoholic and nonalcoholic patients in Boston and London.N Engl J Med.1960;261:1–9.
- ,,, et al.Liver transplantation for cryptogenic cirrhosis.Liver Transpl Surg.1997;3:359–364.
- ,,, et al.Heart diseases affecting the liver and liver disease affecting the heart.Am Heart J.2000;140:111–120.
- ,,.The liver in heart failure.Clin Liver Dis.2002;6:947–967.
- ,,, et al.Portal hypertension: from pathophysiology to clinical practice.Liver Int.2005;25:1079–1090.
- .Portal hypertension.Curr Opin Gastroenterol.2006;22:254–262.
- ,,, et al.Negative influences of ascites on the cardiac function of cirrhotic patients.Am J Med.1975;59:165–170.
- .Constrictive pericarditis in the modern era: a diagnostic dilemma.Heart.2001;86:619–623.
- ,,, et al.Calcific constrictive pericarditis: is it still with us?Ann Intern Med.2000;132:444–450.
- ,,,,,.CT and MR imaging of pericardial disease.Radiographics.2003;23:S167–S180.
- ,,, et al.Constrictive pericarditis in 26 patients with histologically normal pericardial thickness.Circulation.2003;108:1852–1857.
- ,,, et al.Value of dynamic respiratory changes in left and right ventricular pressures for the diagnosis of constrictive pericarditis.Circulation.1996;93:2007–2013.
Annual reviewers list
We deeply appreciate the involvement of our reviewers who made the Journal of Hospital Medicine so successful in its second year. Listed below are the many reviewers and volume of their contributions. They have our sincere gratitude.
Reviewed 4 or More Articles
Thomas E. Baudendistel (7)
Renee Patrice Bullock‐Palmer (4)
Vincent W. Chiang (4)
Jasminka M. Criley (4)
Gurpreet Dhaliwal (6)
Lorenzo Di Francesco (4)
Dana Edelson, MD (7)
Kellie L. Flood (5)
Jeffrey Glasheen (5)
Jeffrey L. Greenwald (4)
Brian Harte (11)
Sunil Kripalani (4)
Matthew Landler (4)
Greg Maynard (5)
Kevin J. O'Leary (4)
Sameer Parikh (6)
James C. Pile (6)
Jason S. Schneider (4)
Doug Wright (4)
Reviewed 3 Articles
Eric Alper
Alpesh Amin
Robert Neal Axon
David L. Blazes
Douglas Carlson
Param Dedhia
Margaret Fang
Jonathan M. Flacker
Rajesh Garg
Stephanie Grossman
Daniel Payson Hunt
Christopher Seoung Kim
Christopher P. Landrigan
Kristine H. Lethert
Marcia Levetown
David Liebovitz
David Likosky
Navneet Majhail
Jennifer Myers
Janet Nagamine
Daniel A. Rauch
Steve Ross
Danielle Bowen Scheurer
Jeffrey Lawrence Schnipper
Hiren Shah
Jason Stein
Reviewed 2 Articles
Nasim Afsarmanesh
Mel L. Anderson III
Vineet Arora
John Banja
Susan S. Braithwaite
Cynthia Jean Brown
Daniel Seth Budnitz
Beril Cakir
Rachel N. Caskey
Murtaza Cassoobhoy
Eva Chittenden
Eugene Shu‐Sen Chu
Steven L. Cohn
Randolph Cole
Nathan T. Connell
Richard I. Cook
Aashish Didwania
Daniel David Dressler
Matthew Eisen
Terry England
Leonard Samuel Feldman
Regina Fink
Raminder Singh Gill
Philip H. Goodman
Mohan Gounder
Susan Grant
Paul Grant
Sajeev Handa
Jeanne M. Huddleston
Carlos Manuel Isada
Amir K. Jaffer
Peter John Kaboli
Jennifer Kapo
Ariel R. Katz
Ujjaini Khanderia
Lisa Kirkland
Cindy J. Lai
Cecilia Lansang
Michael Edwin Lazarus
Bennett Leslie
Blake J. Lesselroth
Lee Lindquist
Michael Lubin
Michelle Magee
David Malkenson
Michael Matheny
Laurence McMahon
Donna Leco Mercado
Melissa Munsell
Brahmajee Nallamothu
James Newman
Vikas Parekh
Mohammed A. Qadeer
Kara Quan
Cate E. Ranheim
Sumant Ranji
Gustavo Rivero
John James Ross
Richard Saitz
Elias G. Sakalis
Maryam Sattari
Bradley Allen Sharpe
Tamara D. Simon
Jeff Sperring
Erin Stucky
Rebecca Sudore
Patrick John Torcson
Arpana Vidyarthi
Diane B. Wayne
Chad Whelan
Stephen Wilson
Jeanie Youngwerth
Iris Yung
Lisa B. Zaoutis
Thomas Zipp
Reviewed 1 Article
Adebola Adesanya
Deborah Adey
Bianca Borges Afonso
Dewesh Agrawal
Meenakshy Aiyer
Richard Keith Albert
Shafic S. Al‐Nammari
Wendy Anderson
Ashish Aneja
Armand H. M. Antommaria
Paul Aronowitz
Deepak Asudani
Andrew Auerbach
Ann Avery
Ahmed BaHammam
Thomas W. Barrett
Jeffrey Barsuk
John Alexander Batsis
Brent Beasley
Deepti Behl
Chaim Bell
Rachelle Bernacki
Jennifer Best
Nisha L. Bhatia
Richard D. Blondell
Bema Bonsu
Thomas Bookwalter
Jeffrey Boord
Debra Boyer
Dawn Brezina
Mandy Brown Belfort
Lucinda Bryant
Alfred Paul Burger
David Busch
Bill Carruth
Brian Carter
Peter J. Cawley
Shiven B. Chabria
Kevin Chan
Carol Chenoweth
Barbara Cleary
Jennifer Cohen
Curtiss B. Cook
Otto Costantini
Kenneth Covinsky
Donald Craven
Timothy J. Crone
Yvette Marie Cua
Ethan Ulysses Cumbler
A. Mark Dalzell
Jennifer Daru
Mellar Davis
William DeMarco
Thomas Donner
Abhijit Duggal
Beatrice Edwards
David Efron
Erin Egan
Kristin Englund
Edward Etchells
Mark Fagan
Tonya Fancher
Randy Joe Ferrance
Chris Feudtner
Christopher K. Finch
Kathleen M. Finn
Alan John Forster
Michael Sebastian Galindo
Joseph Michael Geskey
Roma Y. Gianchandani
Adit A. Ginde
Alan Go
Sherita Hill Golden
Jill Deborah Goldenberg
Adrienne Green
Brian Greffe
Douglas Gregory
Merik Gross
Munish Gupta
Lakshmi Halasyamani
Leslie W. Hall
Jennifer Hanrahan
Lee Hargraves
Gregory Adam Harlan
Michael Harper
Julie Hauer
Nicola Helm
Timothy Hoff
Eric Edwin Howell
Michael Howell
Stephen Hwang
Robert Hyzy
Brian Jack
Joshua Levi Jacobs
Jay Jahanmir
Neeta Jain
William Janssen
Michael Jibson
Karnjit Johl
Robert Kalayjian
Andrew Karson
Dan Kaul
Abel Ngo Kho
Flora Kisuule
Antonios E. Kopanakis
Collin Kroen
Damon Kwan
Alan Labonte
Robert Lash
Usman Latif
Joshua Lee
Sei Lee
Joseph Li
Peter K. Lindenauer
David Ling
Ian Logan
Vanessa London
Jennifer Lukela
Eusni Rahayu M. Tohit
Alejandro E. Macias
Jennifer Mack
Brian Mandell
Efren C. Manjarrez
Brian Markoff
George Mathew
Sarah McBride
Michael McFarlane
Sylvia Cheney McKean
David Meltzer
Franklin Michota
Sherif Mossad
Joseph Munsayac
Paul Murphree
Thomas Aquinas Murphy
Nina Naeger Murphy
Eric Neurmberger
Kathrin Nicolacakis
Craig Nielsen
Lise Nigrovic
Timothy O'Brien
Mary Ottolini
Thomas Andrew Owens
Robert Pascucci
Raffaele Pesavento
Diana Pi
Ann Poncelet
Maryjo Prince‐Paul
Rehan Qayyum
Hossam A. Rahman
Vijay Rajput
Shawn Ralston
Sadat Rashid
Kimberly Rask
Mathew J. Reeves
Robert Reilly
Larry Rhein
Erinn Rhodes
Willaim David Rifkin
Hilary F. Ryder
Camille Sabella
Sandeep Sachdeva
Sanjay Saint
David Schulman
Thomas Schwenk
Gregory B. Seymann
Hasan Shabbir
Samir S. Shah
Lisa Shah
Kaveh G. Shojania
Rebecca Shunk
Eric M. Siegal
Jeffrey Simmons
Siddhartha Singh
G. Randall Smith Jr.
Lailey Sooriash
Ted Speroff
Diane Stafford
Brett Stauffer
Andrea Stracciolini
Hemali Sudhalkar
Alan Taege
Benjamin Taylor
Jay Thomas
Rachel E. Thompson
Sharlene Toney
Joan Trey
Jennifer Michelle Trujillo
Alexander Turchin
Guillermo E. Umpierrez
Bobbak Vahid
Jay Vaidya
Tamara Vesel
Sally Vitali
Polychronopoulos Vlasis
Natalia Borisovna Volkova
Heidi Wald
H. Kenneth Walker
Jeff Wiese
Jonathan Winickoff
Bradford Winters
Scott Wright
David Zipes
We deeply appreciate the involvement of our reviewers who made the Journal of Hospital Medicine so successful in its second year. Listed below are the many reviewers and volume of their contributions. They have our sincere gratitude.
Reviewed 4 or More Articles
Thomas E. Baudendistel (7)
Renee Patrice Bullock‐Palmer (4)
Vincent W. Chiang (4)
Jasminka M. Criley (4)
Gurpreet Dhaliwal (6)
Lorenzo Di Francesco (4)
Dana Edelson, MD (7)
Kellie L. Flood (5)
Jeffrey Glasheen (5)
Jeffrey L. Greenwald (4)
Brian Harte (11)
Sunil Kripalani (4)
Matthew Landler (4)
Greg Maynard (5)
Kevin J. O'Leary (4)
Sameer Parikh (6)
James C. Pile (6)
Jason S. Schneider (4)
Doug Wright (4)
Reviewed 3 Articles
Eric Alper
Alpesh Amin
Robert Neal Axon
David L. Blazes
Douglas Carlson
Param Dedhia
Margaret Fang
Jonathan M. Flacker
Rajesh Garg
Stephanie Grossman
Daniel Payson Hunt
Christopher Seoung Kim
Christopher P. Landrigan
Kristine H. Lethert
Marcia Levetown
David Liebovitz
David Likosky
Navneet Majhail
Jennifer Myers
Janet Nagamine
Daniel A. Rauch
Steve Ross
Danielle Bowen Scheurer
Jeffrey Lawrence Schnipper
Hiren Shah
Jason Stein
Reviewed 2 Articles
Nasim Afsarmanesh
Mel L. Anderson III
Vineet Arora
John Banja
Susan S. Braithwaite
Cynthia Jean Brown
Daniel Seth Budnitz
Beril Cakir
Rachel N. Caskey
Murtaza Cassoobhoy
Eva Chittenden
Eugene Shu‐Sen Chu
Steven L. Cohn
Randolph Cole
Nathan T. Connell
Richard I. Cook
Aashish Didwania
Daniel David Dressler
Matthew Eisen
Terry England
Leonard Samuel Feldman
Regina Fink
Raminder Singh Gill
Philip H. Goodman
Mohan Gounder
Susan Grant
Paul Grant
Sajeev Handa
Jeanne M. Huddleston
Carlos Manuel Isada
Amir K. Jaffer
Peter John Kaboli
Jennifer Kapo
Ariel R. Katz
Ujjaini Khanderia
Lisa Kirkland
Cindy J. Lai
Cecilia Lansang
Michael Edwin Lazarus
Bennett Leslie
Blake J. Lesselroth
Lee Lindquist
Michael Lubin
Michelle Magee
David Malkenson
Michael Matheny
Laurence McMahon
Donna Leco Mercado
Melissa Munsell
Brahmajee Nallamothu
James Newman
Vikas Parekh
Mohammed A. Qadeer
Kara Quan
Cate E. Ranheim
Sumant Ranji
Gustavo Rivero
John James Ross
Richard Saitz
Elias G. Sakalis
Maryam Sattari
Bradley Allen Sharpe
Tamara D. Simon
Jeff Sperring
Erin Stucky
Rebecca Sudore
Patrick John Torcson
Arpana Vidyarthi
Diane B. Wayne
Chad Whelan
Stephen Wilson
Jeanie Youngwerth
Iris Yung
Lisa B. Zaoutis
Thomas Zipp
Reviewed 1 Article
Adebola Adesanya
Deborah Adey
Bianca Borges Afonso
Dewesh Agrawal
Meenakshy Aiyer
Richard Keith Albert
Shafic S. Al‐Nammari
Wendy Anderson
Ashish Aneja
Armand H. M. Antommaria
Paul Aronowitz
Deepak Asudani
Andrew Auerbach
Ann Avery
Ahmed BaHammam
Thomas W. Barrett
Jeffrey Barsuk
John Alexander Batsis
Brent Beasley
Deepti Behl
Chaim Bell
Rachelle Bernacki
Jennifer Best
Nisha L. Bhatia
Richard D. Blondell
Bema Bonsu
Thomas Bookwalter
Jeffrey Boord
Debra Boyer
Dawn Brezina
Mandy Brown Belfort
Lucinda Bryant
Alfred Paul Burger
David Busch
Bill Carruth
Brian Carter
Peter J. Cawley
Shiven B. Chabria
Kevin Chan
Carol Chenoweth
Barbara Cleary
Jennifer Cohen
Curtiss B. Cook
Otto Costantini
Kenneth Covinsky
Donald Craven
Timothy J. Crone
Yvette Marie Cua
Ethan Ulysses Cumbler
A. Mark Dalzell
Jennifer Daru
Mellar Davis
William DeMarco
Thomas Donner
Abhijit Duggal
Beatrice Edwards
David Efron
Erin Egan
Kristin Englund
Edward Etchells
Mark Fagan
Tonya Fancher
Randy Joe Ferrance
Chris Feudtner
Christopher K. Finch
Kathleen M. Finn
Alan John Forster
Michael Sebastian Galindo
Joseph Michael Geskey
Roma Y. Gianchandani
Adit A. Ginde
Alan Go
Sherita Hill Golden
Jill Deborah Goldenberg
Adrienne Green
Brian Greffe
Douglas Gregory
Merik Gross
Munish Gupta
Lakshmi Halasyamani
Leslie W. Hall
Jennifer Hanrahan
Lee Hargraves
Gregory Adam Harlan
Michael Harper
Julie Hauer
Nicola Helm
Timothy Hoff
Eric Edwin Howell
Michael Howell
Stephen Hwang
Robert Hyzy
Brian Jack
Joshua Levi Jacobs
Jay Jahanmir
Neeta Jain
William Janssen
Michael Jibson
Karnjit Johl
Robert Kalayjian
Andrew Karson
Dan Kaul
Abel Ngo Kho
Flora Kisuule
Antonios E. Kopanakis
Collin Kroen
Damon Kwan
Alan Labonte
Robert Lash
Usman Latif
Joshua Lee
Sei Lee
Joseph Li
Peter K. Lindenauer
David Ling
Ian Logan
Vanessa London
Jennifer Lukela
Eusni Rahayu M. Tohit
Alejandro E. Macias
Jennifer Mack
Brian Mandell
Efren C. Manjarrez
Brian Markoff
George Mathew
Sarah McBride
Michael McFarlane
Sylvia Cheney McKean
David Meltzer
Franklin Michota
Sherif Mossad
Joseph Munsayac
Paul Murphree
Thomas Aquinas Murphy
Nina Naeger Murphy
Eric Neurmberger
Kathrin Nicolacakis
Craig Nielsen
Lise Nigrovic
Timothy O'Brien
Mary Ottolini
Thomas Andrew Owens
Robert Pascucci
Raffaele Pesavento
Diana Pi
Ann Poncelet
Maryjo Prince‐Paul
Rehan Qayyum
Hossam A. Rahman
Vijay Rajput
Shawn Ralston
Sadat Rashid
Kimberly Rask
Mathew J. Reeves
Robert Reilly
Larry Rhein
Erinn Rhodes
Willaim David Rifkin
Hilary F. Ryder
Camille Sabella
Sandeep Sachdeva
Sanjay Saint
David Schulman
Thomas Schwenk
Gregory B. Seymann
Hasan Shabbir
Samir S. Shah
Lisa Shah
Kaveh G. Shojania
Rebecca Shunk
Eric M. Siegal
Jeffrey Simmons
Siddhartha Singh
G. Randall Smith Jr.
Lailey Sooriash
Ted Speroff
Diane Stafford
Brett Stauffer
Andrea Stracciolini
Hemali Sudhalkar
Alan Taege
Benjamin Taylor
Jay Thomas
Rachel E. Thompson
Sharlene Toney
Joan Trey
Jennifer Michelle Trujillo
Alexander Turchin
Guillermo E. Umpierrez
Bobbak Vahid
Jay Vaidya
Tamara Vesel
Sally Vitali
Polychronopoulos Vlasis
Natalia Borisovna Volkova
Heidi Wald
H. Kenneth Walker
Jeff Wiese
Jonathan Winickoff
Bradford Winters
Scott Wright
David Zipes
We deeply appreciate the involvement of our reviewers who made the Journal of Hospital Medicine so successful in its second year. Listed below are the many reviewers and volume of their contributions. They have our sincere gratitude.
Reviewed 4 or More Articles
Thomas E. Baudendistel (7)
Renee Patrice Bullock‐Palmer (4)
Vincent W. Chiang (4)
Jasminka M. Criley (4)
Gurpreet Dhaliwal (6)
Lorenzo Di Francesco (4)
Dana Edelson, MD (7)
Kellie L. Flood (5)
Jeffrey Glasheen (5)
Jeffrey L. Greenwald (4)
Brian Harte (11)
Sunil Kripalani (4)
Matthew Landler (4)
Greg Maynard (5)
Kevin J. O'Leary (4)
Sameer Parikh (6)
James C. Pile (6)
Jason S. Schneider (4)
Doug Wright (4)
Reviewed 3 Articles
Eric Alper
Alpesh Amin
Robert Neal Axon
David L. Blazes
Douglas Carlson
Param Dedhia
Margaret Fang
Jonathan M. Flacker
Rajesh Garg
Stephanie Grossman
Daniel Payson Hunt
Christopher Seoung Kim
Christopher P. Landrigan
Kristine H. Lethert
Marcia Levetown
David Liebovitz
David Likosky
Navneet Majhail
Jennifer Myers
Janet Nagamine
Daniel A. Rauch
Steve Ross
Danielle Bowen Scheurer
Jeffrey Lawrence Schnipper
Hiren Shah
Jason Stein
Reviewed 2 Articles
Nasim Afsarmanesh
Mel L. Anderson III
Vineet Arora
John Banja
Susan S. Braithwaite
Cynthia Jean Brown
Daniel Seth Budnitz
Beril Cakir
Rachel N. Caskey
Murtaza Cassoobhoy
Eva Chittenden
Eugene Shu‐Sen Chu
Steven L. Cohn
Randolph Cole
Nathan T. Connell
Richard I. Cook
Aashish Didwania
Daniel David Dressler
Matthew Eisen
Terry England
Leonard Samuel Feldman
Regina Fink
Raminder Singh Gill
Philip H. Goodman
Mohan Gounder
Susan Grant
Paul Grant
Sajeev Handa
Jeanne M. Huddleston
Carlos Manuel Isada
Amir K. Jaffer
Peter John Kaboli
Jennifer Kapo
Ariel R. Katz
Ujjaini Khanderia
Lisa Kirkland
Cindy J. Lai
Cecilia Lansang
Michael Edwin Lazarus
Bennett Leslie
Blake J. Lesselroth
Lee Lindquist
Michael Lubin
Michelle Magee
David Malkenson
Michael Matheny
Laurence McMahon
Donna Leco Mercado
Melissa Munsell
Brahmajee Nallamothu
James Newman
Vikas Parekh
Mohammed A. Qadeer
Kara Quan
Cate E. Ranheim
Sumant Ranji
Gustavo Rivero
John James Ross
Richard Saitz
Elias G. Sakalis
Maryam Sattari
Bradley Allen Sharpe
Tamara D. Simon
Jeff Sperring
Erin Stucky
Rebecca Sudore
Patrick John Torcson
Arpana Vidyarthi
Diane B. Wayne
Chad Whelan
Stephen Wilson
Jeanie Youngwerth
Iris Yung
Lisa B. Zaoutis
Thomas Zipp
Reviewed 1 Article
Adebola Adesanya
Deborah Adey
Bianca Borges Afonso
Dewesh Agrawal
Meenakshy Aiyer
Richard Keith Albert
Shafic S. Al‐Nammari
Wendy Anderson
Ashish Aneja
Armand H. M. Antommaria
Paul Aronowitz
Deepak Asudani
Andrew Auerbach
Ann Avery
Ahmed BaHammam
Thomas W. Barrett
Jeffrey Barsuk
John Alexander Batsis
Brent Beasley
Deepti Behl
Chaim Bell
Rachelle Bernacki
Jennifer Best
Nisha L. Bhatia
Richard D. Blondell
Bema Bonsu
Thomas Bookwalter
Jeffrey Boord
Debra Boyer
Dawn Brezina
Mandy Brown Belfort
Lucinda Bryant
Alfred Paul Burger
David Busch
Bill Carruth
Brian Carter
Peter J. Cawley
Shiven B. Chabria
Kevin Chan
Carol Chenoweth
Barbara Cleary
Jennifer Cohen
Curtiss B. Cook
Otto Costantini
Kenneth Covinsky
Donald Craven
Timothy J. Crone
Yvette Marie Cua
Ethan Ulysses Cumbler
A. Mark Dalzell
Jennifer Daru
Mellar Davis
William DeMarco
Thomas Donner
Abhijit Duggal
Beatrice Edwards
David Efron
Erin Egan
Kristin Englund
Edward Etchells
Mark Fagan
Tonya Fancher
Randy Joe Ferrance
Chris Feudtner
Christopher K. Finch
Kathleen M. Finn
Alan John Forster
Michael Sebastian Galindo
Joseph Michael Geskey
Roma Y. Gianchandani
Adit A. Ginde
Alan Go
Sherita Hill Golden
Jill Deborah Goldenberg
Adrienne Green
Brian Greffe
Douglas Gregory
Merik Gross
Munish Gupta
Lakshmi Halasyamani
Leslie W. Hall
Jennifer Hanrahan
Lee Hargraves
Gregory Adam Harlan
Michael Harper
Julie Hauer
Nicola Helm
Timothy Hoff
Eric Edwin Howell
Michael Howell
Stephen Hwang
Robert Hyzy
Brian Jack
Joshua Levi Jacobs
Jay Jahanmir
Neeta Jain
William Janssen
Michael Jibson
Karnjit Johl
Robert Kalayjian
Andrew Karson
Dan Kaul
Abel Ngo Kho
Flora Kisuule
Antonios E. Kopanakis
Collin Kroen
Damon Kwan
Alan Labonte
Robert Lash
Usman Latif
Joshua Lee
Sei Lee
Joseph Li
Peter K. Lindenauer
David Ling
Ian Logan
Vanessa London
Jennifer Lukela
Eusni Rahayu M. Tohit
Alejandro E. Macias
Jennifer Mack
Brian Mandell
Efren C. Manjarrez
Brian Markoff
George Mathew
Sarah McBride
Michael McFarlane
Sylvia Cheney McKean
David Meltzer
Franklin Michota
Sherif Mossad
Joseph Munsayac
Paul Murphree
Thomas Aquinas Murphy
Nina Naeger Murphy
Eric Neurmberger
Kathrin Nicolacakis
Craig Nielsen
Lise Nigrovic
Timothy O'Brien
Mary Ottolini
Thomas Andrew Owens
Robert Pascucci
Raffaele Pesavento
Diana Pi
Ann Poncelet
Maryjo Prince‐Paul
Rehan Qayyum
Hossam A. Rahman
Vijay Rajput
Shawn Ralston
Sadat Rashid
Kimberly Rask
Mathew J. Reeves
Robert Reilly
Larry Rhein
Erinn Rhodes
Willaim David Rifkin
Hilary F. Ryder
Camille Sabella
Sandeep Sachdeva
Sanjay Saint
David Schulman
Thomas Schwenk
Gregory B. Seymann
Hasan Shabbir
Samir S. Shah
Lisa Shah
Kaveh G. Shojania
Rebecca Shunk
Eric M. Siegal
Jeffrey Simmons
Siddhartha Singh
G. Randall Smith Jr.
Lailey Sooriash
Ted Speroff
Diane Stafford
Brett Stauffer
Andrea Stracciolini
Hemali Sudhalkar
Alan Taege
Benjamin Taylor
Jay Thomas
Rachel E. Thompson
Sharlene Toney
Joan Trey
Jennifer Michelle Trujillo
Alexander Turchin
Guillermo E. Umpierrez
Bobbak Vahid
Jay Vaidya
Tamara Vesel
Sally Vitali
Polychronopoulos Vlasis
Natalia Borisovna Volkova
Heidi Wald
H. Kenneth Walker
Jeff Wiese
Jonathan Winickoff
Bradford Winters
Scott Wright
David Zipes
Improving Antibiotic Utilization among Hospitalists
Inappropriate antibiotic use is a major public health concern and demonstrates the need for quality improvement initiatives in the delivery of health care.16 Each year nearly 2 million patients in the United States acquire an infection in the hospital, and about 90,000 of them die from these infections.7 More than 70% of the bacteria that cause hospital‐acquired infections are resistant to at least one commonly used drug.7 Persons infected with drug‐resistant organisms have longer hospital stays and higher mortality rates.7
Inappropriate antibiotic use in the inpatient hospital setting can be classified into 5 categories. First, antibiotics may be given for illnesses for which they are not indicated (eg, viral infections). Second, broad‐spectrum antibiotics (such as piperacillin‐tazobactam and quinolones) may be overused in the empiric treatment of common infections.8 Overuse of broad‐spectrum drugs increases selective pressure for antimicrobial resistance and exposes patients to the side effects of some of these drugs, such as Clostridium difficile colitis.8 Third, clinicians occasionally prescribe intravenous (IV) antibiotics when the efficacy of oral agents would be similar. Inappropriate intravenous therapy increases the cost of care and also exposes the patient to the risk of intravenous catheters.8 Fourth, when the correct antibiotic choice is made, inappropriate antibiotic dosage, schedule, and/or duration of treatment can threaten patient safety.8 Fifth, bug‐drug mismatch occurs when susceptibility studies indicate that the drug being used is ineffective or only marginally effective.8 Beyond antimicrobial resistance and safety, these practices also usually increase costs to both the patient and the hospital.7, 910
Influencing providers' prescribing patterns is difficult.11 In this project we assessed the prescribing patterns of hospitalists in an active inpatient environment and then developed an intervention to improve the providers' use of antibiotics. The intervention utilized public health methodologyprior to implementation, we defined the problem, determined its magnitude, identified a behavior change model, and constructed a conceptual framework that identifyied the key determinants. A pilot academic detailing project addressing many determinants was developed, implemented, and evaluated.
Conceptual Model
To change prescribing behaviors is to change learned behaviors. Changing behavior is a complex process affected by several factors including beliefs, expectations, motivations, and the psychosocial environments of the target groups.12 Each of these factors must be considered when attempting to bring about behavior changes. In doing so, a theory that can be depicted in a model often emerges.13 This approach is widely used in understanding and developing public health interventions.
Formulating the Model
In any public health intervention, recognizing and engaging key stakeholders is a critical step. We identified the following stakeholders: (1) hospitalist practitioners and other prescribing providers including residents and infectious disease specialists; (2) nurses; (3) administrators who are focused on cost effectiveness; (4) patients and their families, who want to get well affordably, without side effects; (5) pharmacists; (6) risk management; and (7) society, which is fearful of the propagation of resistant microbes. In consulting with some of the stakeholders, 4 factors that influence hospitalists' prescribing patterns became apparent. These are practitioner factors, environmental factors, perceived rewards, and perceived threats (Fig. 1).
The practitioner factors shaping prescribing are: (1) knowledge of current best care; (2) self‐efficacy, which determines whether a provider is confident in his or her knowledge to adequately treat a specific infection; (3) habit, which causes providers to pick from a narrow repertoire of antibiotics when treating an infection; and (4) fear of liability, which forces some providers to be cautious. Four environmental factors affecting antibiotic prescriptions are: (1) published guidelines regarding organisms' sensitivity to antibiotics; (2) patient‐driven factors such as affordability, compliance with dosing regimens, side effects, and interactions between the antibiotics and other medications; (3) peer influence, in that providers are reluctant to change a prescription started by another provider (eg, emergency room physician); and (4) the formulary of the hospital, as it forces providers to prescribe within specific parameters. The perceived rewards of specific prescribing practices may include improving patient safety and reducing antibiotic resistance and costs, whereas the perceived threats are increasing antimicrobial resistance, having adverse patient outcomes, and increasing costs and hospital length of stay. We selected a high‐yield, low‐effort intervention in order to have an impact on some of the factors underlying hospitalists' prescribing patterns.
METHODS
Participants
The study participants were 17 hospitalist practitioners including physicians, nurse‐practitioners, and physician assistants who make up the Collaborative Inpatient Medical Service (CIMS) at Johns Hopkins Bayview Medical Center (JHBMC; Table 1). All consented to participate. The study was approved by the institutional review board.
| Age in years, mean (SD) | 36 (6) |
| Female, n (%) | 13 (76%) |
| Physician, n (%) | 9 (53%) |
| Nurse‐practitioner, n (%) | 5 (29%) |
| Physician assistant, n (%) | 3 (18%) |
| Years in practice, mean (SD) | 5.1 (2.8) |
| Number of pharmaceutical representatives exposed to in past year, mean | 1 |
| Number of shifts worked per month, mean (SD) | 14 (4) |
| Primarily works days, n (%) | 13 (76%) |
Data Collection
We collected and assessed prescription patterns over 3 periods: preintervention, interim, and postintervention.
Assessing Appropriateness of Antibiotics
For each order that was assessed in the preintervention, interim, and postintervention periods, the following information was collected: (1) drug ordered, (2) clinical diagnosis, (3) microbiology results available at the time of the order (including relevant results from recent cultures), (4) other medical diagnoses (ICD9 codes), (5) allergies, and (6) exposure to health care facilities (within the past 30 days). The computerized medical record allowed access to the discharge summaries of a patient's hospitalization. These records summarized the patient's hospitalization, allowing the investigators to understand the reasons for a provider's choice of antibiotics. If the rationale was not clear about how to categorize a prescription from reading the data, the investigators performed a chart review. From the information culled from these reviews, the primary investigator and an infectious disease specialist classified each prescription order by consensus as appropriate, effective but inappropriate, or inappropriate therapy.
Prescriptions were classified as appropriate when they were indicated and correlated with sensitivities, if available, or were of a narrow‐enough spectrum and recommended as a first‐line treatment for specific illnesses by either the Johns Hopkins Antibiotic Guide14 or the Stanford Guide to Antimicrobial Therapy.15 For example, cephalexin to treat uncomplicated cellulitis was considered appropriate therapy. Effective but inappropriate prescriptions were broad‐spectrum antibiotics used to treat an infection when a narrower‐spectrum antibiotic would have sufficed. For example, piperacillin‐tazobactam would be effective in treating a simple urinary tract infection but inappropriate to use because of its broad spectrum. Other examples of effective but inappropriate prescriptions were giving an IV when an oral alternative would be equally effective and tolerated or prescribing antibiotic treatment whose duration was too long. Finally, inappropriate prescriptions were those written for conditions for which antibiotics are not indicated or for which the prescribed antibiotic was ineffective for the specified infection (bug‐drug mismatch).
Preintervention
In January 2006 the investigators retrospectively reviewed the prescribing patterns of the 17 providers over the previous year. Using the computerized medical record and physician order entry, consecutive prescriptions of each provider were evaluated, beginning December 31, 2005, going back reverse chronologically until 20 prescriptions had been identified. For 12 of the providers, it was actually possible to review 20 prescriptions. For 2 other providers, both new, part‐time additions to the hospitalist group, only 1 and 7 prescriptions were found for the entire year. The prescribing history of the 3 remaining providers who participated in the study, all physician assistants, could not be evaluated (during any period) because all their orders were linked only to physicians, making it impossible to determine their specific prescriptions using the physician order entry system.
Interim
During the interim period between obtaining informed consent and completing the academic detailing (January 3, 2006, to March 23, 2006), provider prescribing patterns were reviewed to determine if the mere knowledge of the project would produce changes in prescribing behavior.
Postintervention
After the academic detailing was completed (March 23, 2006), the prescribing patterns of the hospitalists were followed through April 23, 2006. Each week after the detailing session, the hospitalists received reminders to prescribe appropriately (including pens with the message Reduce the Overuse).
Detailing Procedures
After the review, a profile was assembled for each of the CIMS providers. The study team detailers (a physician and a pharmacist) met with the individual providers for 30 to 45 minutes. Each hospitalist participant completed a short survey that collected demographic information and was asked about the rationale for his or her antibiotic prescribing pattern. Next, the appraisal of the provider's prescribing pattern was reviewed. This review included looking at the costs of the prescribed antibiotics compared with those of the appropriate alternatives and a reexamination of the guidelines for the selected target drugspiperacillin‐tazobactam, vancomycin, and extended‐spectrum quinolones. These 3 antibiotics were picked because our providers had been particularly vulnerable to inappropriately prescribing them. The hospitalists were provided an antibiotic guide developed specifically for this project and based on the Johns Hopkins Antibiotic Guide14 that summarizes the consensus guidelines.
Data Analysis
The primary outcome variable was the aggregate proportion of inappropriate antibiotic prescribed (as defined earlier) before the intervention, during the interim between obtaining informed consent and intervening on all study subjects, and after the intervention. The percentage of appropriate prescriptions versus total not appropriate prescriptions (combining of the effective but inappropriate and inappropriate categories) were compared across the 3 periods. Ninety‐five percent confidence intervals for comparisons of the proportions were determined using Stata 9.0 (College Station, TX). The difference between the proportions of total not appropriate prescriptions before and after academic detailing was computed in Stata using Fisher's exact test to assess significance.
RESULTS
Demographic information and professional characteristics of the 17 providers are shown in Table 1. Their mean age was 36 years, and 76% were female. The top 4 reasons the providers gave for their prescribing practices were: (1) published guidelines, (2) easier dosing schedule for patient when discharged, (3) continuing an antibiotic course initiated in the emergency room, and (4) broad‐spectrum antibiotics cover all possible microbes.
Comparison of Preintervention, Interim, and Postintervention Periods
Table 2 depicts the results of the prescription appraisals from the retrospective reviews. Of the 14 providers who had ordered antibiotics, 8 (57%) had more prescriptions that were total not appropriate than were appropriate in the preintervention period compared with 3 providers (25%) with this prescribing pattern in the postintervention period (P = .13).
| Provider | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|
| Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | |
| ||||||
| 1 | 20 | 7 (35%) | 13 (65%) | 24 | 17 (70.8%) | 7 (29.2%) |
| 2 | 20 | 10 (50%) | 10 (50%) | 12 | 11 (91.7%) | 1 (8.3%) |
| 3 | 20 | 6 (30%) | 14 (70%) | 8 | 8 (100%) | 0 (0%) |
| 4* | 19 | 10 (52.6%) | 9 (47.4%) | 4 | 3 (75%) | 1 (25%) |
| 5 | 20 | 9 (45%) | 11 (55%) | 10 | 4 (40%) | 6 (60%) |
| 6 | 20 | 5 (25%) | 15 (75%) | 3 | 1 (33.3%) | 2 (66.7%) |
| 7 | 20 | 8 (40%) | 12 (60%) | 8 | 7 (87.5%) | 1 (12.5%) |
| 8* | 1 | 0 (0%) | 1 (100%) | 0 | 0 (0%) | 0 (0%) |
| 9 | 20 | 11 (55%) | 9 (45%) | 5 | 2 (40%) | 3 (60%) |
| 10* | 7 | 3 (42.9%) | 4 (57.1%) | 0 | 0 (0%) | 0 (0%) |
| 11 | 20 | 10 (50%) | 10 (50%) | 17 | 13 (76.5%) | 4 (23.5%) |
| 12 | 20 | 6 (30%) | 14 (70%) | 16 | 14 (87.5%) | 2 (12.5%) |
| 13 | 20 | 12 (60%) | 8 (40%) | 15 | 11 (73.3%) | 4 (26.7%) |
| 14 | 20 | 10 (50%) | 10 (50%) | 7 | 4 (57.1%) | 3 (42.9%) |
| Total | 247 | 107 (43%) | 140 (57%) | 129 | 95 (73.6%) | 34 (26.4%) |
Table 3 shows the proportions of appropriate, effective but inappropriate, and total not appropriate prescriptions in the retrospective, interim, and postintervention periods. Forty‐three percent (95% CI 37%‐49%) of prescriptions were judged to be appropriate, and 57% (95% CI 51%‐63%) to be not appropriate prior to the academic detailing. In the interim period, 59% (95% CI 52%‐65%) of the prescriptions were appropriate, and 41% (95% CI 35%‐48%) were not appropriate; P = .0003. After the intervention, 74% (95% CI 65%‐81%) of the prescriptions were appropriate, and 26% (95% CI 19%‐35%) were not appropriate; P < .0001.
| Period | Appropriate, n (%) | 95% CI | Effective but inappropriate, n (%) | Inappropriate, n (%) | Total not appropriate, n (%) | 95% CI | P value* |
|---|---|---|---|---|---|---|---|
| |||||||
| Retrospective review (pre) | 107 (43%) | 37%‐49% | 75 (30.4%) | 65 (26.6%) | 140 (57%) | 51%‐63% | |
| Interim | 146 (59%) | 52%‐65% | 37 (15%) | 65 (26%) | 102 (41%) | 35%‐48% | .0003 |
| Postintervention | 95 (74%) | 65%‐81% | 8 (6%) | 26 (20%) | 34 (26%) | 19%‐35% | < .0001 |
DISCUSSION
We have demonstrated that academic detailing had a positive impact on the prescribing patterns of hospitalists. The aggregated improvement in antibiotic prescribing patterns can be attributed to improvement in the prescribing patterns of almost every hospitalist practitioner (Table 2). This study focused on aggregate prescriptions as the primary outcome measure because the hospitalists at JHBMC, like at many other institutions, function as a team, with a patient routinely having multiple providers over the course of the hospital stay. The improved prescribing patterns noted during the interim period suggest that the mere knowledge of a project can have an impact on providers. Providers informed the investigators that they were more thoughtful about their choice of antibiotics when they knew that they were being studied. The further statistically significant improvement in prescribing patterns with the intervention shows that the academic detailing itself was successful.
The greatest absolute change in practice was seen in effective but inappropriate prescribing (from 30.4% to 6%), whereas inappropriate prescribing only decreased from 26.6% to 20.6%. Although we aimed to have an impact on all inappropriate antibiotic prescribing patterns, we specifically reviewed the prescribing guidelines for piperacillin‐tazobactam, extended‐spectrum quinolones, and vancomycin. These 3 antibiotics were targeted because our providers had been particularly susceptible to inappropriately prescribing them. The focus on these antibiotics may have resulted in the larger absolute change noted in effective but inappropriate prescribing. We did not collect any data to determine if having an impact on effective but inappropriate prescribing changed the clinical course of the patients, such as shortening their hospital stays. Anecdotal evidence, however, suggests that it does. At our institution it is not uncommon for patients to be kept in the hospital for an extra day to ensure they are stable when transitioned from extended‐spectrum to narrower‐spectrum antibiotics prior to discharge. The effect of reducing effective but inappropriate prescriptions on the clinical course of patients could be an outcome measure assessed by a future, larger study.
Our one‐on‐one appraisal of each provider's prescribing patterns included a review of the cost of the prescribed antibiotics compared with that of the appropriate alternatives. Although decisions on antibiotic choice should be driven by clinical guidelines and appropriateness rather than price, we believed it was relevant to include education about costs and pricing so that providers would be reminded to ascertain whether patients would be able to afford their antibiotics. Antibiotic resistance is influenced by a patient's failure to complete the course of treatment, and noncompliance may be caused by an inability to afford the medication. Often, there are affordable, appropriate alternatives to the newest and most expensive drugs.
A hospitalist‐based academic detailing approach to improving antibiotic prescribing may have far‐reaching benefits and influence. First, it has the potential to affect other practitioners by setting an example and role modeling. In addition to that with their immediate peer group, hospitalists have close and repeated contact with house officers and emergency room physicians and often act as consultants to physicians in other departments such as surgery and psychiatry. Furthermore, some community hospitals have no infectious disease specialists readily available. So this represents an opportunity for hospitalists to promote quality in antibiotic prescribing. Practice‐based learning was very effective because it brought the practitioners face to face with their prescribing patterns. Although intellectually everyone agreed that antibiotics are often misused, this approach forced the providers to stop and reflect on their individual practices. This peer‐delivered intervention allowed for a collaborative approach to solving the problem; the peer (detailer) was approachable, nonjudgmental, and available for further discussion and guidance.
The public health quality improvement approach that we used for our intervention helped us to realize and appreciate the factors underlying prescribing patterns. Only by understanding the motivations for prescribing patterns can we hope to make sustainable changes. This coincides with our previous assertion that hospitalists are engaging in some public health practice.16 In pubic health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole.17 Hospitalist teams aim to make sure that the high‐quality services needed for protecting the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. Antibiotic optimization is a key role that could fall within the mantra of public health practice for the hospitalist.
Several limitations of this pilot should be considered. First, the intervention is labor intensive. However, it is essential to use the problem‐solving paradigm and incorporate behavior change theories in order to identify interventions that can lead to sustainable change. Second, this was not a randomized controlled trial, and it is possible that there might have been some contamination by external forces. However, in reviewing the educational events at our institution, the press, and articles published during the study period, we could not identify any external factors that would have influenced antibiotic prescribing patterns. It would not have been possible to conduct a randomized trial at our institution because the hospitalists work so closely together that we could not ensure complete separation if the subjects were randomized. There would have been contamination from the intervention group to the control group. A trial with randomization at the institution level is the next step. Third, the number of months retrospectively reviewed in order to identify 20 prescriptions of a provider varied. This study assumed there were no other differences during those months that could have affected provider prescribing behavior; this may have introduced some bias. Fourth, the sustainability of this intervention's positive impact is unknown. We assessed outcome soon after the intervention, and it is unknown whether continual booster sessions are required to maintain the positive impact on prescribing patterns.
This pilot was a good starting place to show that behavior change can be realized with a well‐conceived and methodically executed intervention, even among the busiest of physicians. Audit and feedback, or practice‐based learning, appears to be a powerful educational intervention among professionals who take great pride in their work.
- ,.Improving antibiotic use in low‐income countries: an overview of evidence on determinants.Soc Sci Med.2003;57:733–744.
- .Mechanisms of antimicrobial resistance in bacteria.Am J Med.2006;119(6A):S3–S10.
- .Antimicrobial resistance in gram‐positive bacteria.Am J Med.2006;119(6A):S11–S19.
- .Resistance in Gram‐negative bacteria: enterobacteriaceae.Am J Med.2006;119(6A):S20–S28.
- .Pharmacodynamics: relation to antimicrobial resistance.Am J Med.2006;119(6A):S37–S44.
- .Managing methicillin‐resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms.Am J Med.2006;119(6A):S45–S52.
- NIH. The Problem of Antibiotic Resistance. Available at: http://www.niaid.nih.gov.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed.J Hosp Infect.2001;47:198–209.
- ,.The impact of hospital‐acquired bloodstream infections.Emerg Infect Dis.2001;7(2):174–177.
- .Antimicrobial stewardship.Am J Med.2006;119(6A):S53–S61
- ,,, et al.Changing provider behavior: an overview of systemic reviews of interventions.Med Care.2001;39:II–2‐II‐45.
- .A review of current health education theories.Calif J Health Promot.2004;2:74–87
- The Johns Hopkins Hospital Antibiotic Management Program. 2005 Antibiotic Guidelines: Treatment Recommendations for Adult Inpatients. Johns Hopkins Medicine.
- ,,,.The Sanford Guide to Antimicrobial Therapy 2005.35th ed.Hyde Park, VT:Antimicrobial Therapy, Inc.;2005.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
Inappropriate antibiotic use is a major public health concern and demonstrates the need for quality improvement initiatives in the delivery of health care.16 Each year nearly 2 million patients in the United States acquire an infection in the hospital, and about 90,000 of them die from these infections.7 More than 70% of the bacteria that cause hospital‐acquired infections are resistant to at least one commonly used drug.7 Persons infected with drug‐resistant organisms have longer hospital stays and higher mortality rates.7
Inappropriate antibiotic use in the inpatient hospital setting can be classified into 5 categories. First, antibiotics may be given for illnesses for which they are not indicated (eg, viral infections). Second, broad‐spectrum antibiotics (such as piperacillin‐tazobactam and quinolones) may be overused in the empiric treatment of common infections.8 Overuse of broad‐spectrum drugs increases selective pressure for antimicrobial resistance and exposes patients to the side effects of some of these drugs, such as Clostridium difficile colitis.8 Third, clinicians occasionally prescribe intravenous (IV) antibiotics when the efficacy of oral agents would be similar. Inappropriate intravenous therapy increases the cost of care and also exposes the patient to the risk of intravenous catheters.8 Fourth, when the correct antibiotic choice is made, inappropriate antibiotic dosage, schedule, and/or duration of treatment can threaten patient safety.8 Fifth, bug‐drug mismatch occurs when susceptibility studies indicate that the drug being used is ineffective or only marginally effective.8 Beyond antimicrobial resistance and safety, these practices also usually increase costs to both the patient and the hospital.7, 910
Influencing providers' prescribing patterns is difficult.11 In this project we assessed the prescribing patterns of hospitalists in an active inpatient environment and then developed an intervention to improve the providers' use of antibiotics. The intervention utilized public health methodologyprior to implementation, we defined the problem, determined its magnitude, identified a behavior change model, and constructed a conceptual framework that identifyied the key determinants. A pilot academic detailing project addressing many determinants was developed, implemented, and evaluated.
Conceptual Model
To change prescribing behaviors is to change learned behaviors. Changing behavior is a complex process affected by several factors including beliefs, expectations, motivations, and the psychosocial environments of the target groups.12 Each of these factors must be considered when attempting to bring about behavior changes. In doing so, a theory that can be depicted in a model often emerges.13 This approach is widely used in understanding and developing public health interventions.
Formulating the Model
In any public health intervention, recognizing and engaging key stakeholders is a critical step. We identified the following stakeholders: (1) hospitalist practitioners and other prescribing providers including residents and infectious disease specialists; (2) nurses; (3) administrators who are focused on cost effectiveness; (4) patients and their families, who want to get well affordably, without side effects; (5) pharmacists; (6) risk management; and (7) society, which is fearful of the propagation of resistant microbes. In consulting with some of the stakeholders, 4 factors that influence hospitalists' prescribing patterns became apparent. These are practitioner factors, environmental factors, perceived rewards, and perceived threats (Fig. 1).

The practitioner factors shaping prescribing are: (1) knowledge of current best care; (2) self‐efficacy, which determines whether a provider is confident in his or her knowledge to adequately treat a specific infection; (3) habit, which causes providers to pick from a narrow repertoire of antibiotics when treating an infection; and (4) fear of liability, which forces some providers to be cautious. Four environmental factors affecting antibiotic prescriptions are: (1) published guidelines regarding organisms' sensitivity to antibiotics; (2) patient‐driven factors such as affordability, compliance with dosing regimens, side effects, and interactions between the antibiotics and other medications; (3) peer influence, in that providers are reluctant to change a prescription started by another provider (eg, emergency room physician); and (4) the formulary of the hospital, as it forces providers to prescribe within specific parameters. The perceived rewards of specific prescribing practices may include improving patient safety and reducing antibiotic resistance and costs, whereas the perceived threats are increasing antimicrobial resistance, having adverse patient outcomes, and increasing costs and hospital length of stay. We selected a high‐yield, low‐effort intervention in order to have an impact on some of the factors underlying hospitalists' prescribing patterns.
METHODS
Participants
The study participants were 17 hospitalist practitioners including physicians, nurse‐practitioners, and physician assistants who make up the Collaborative Inpatient Medical Service (CIMS) at Johns Hopkins Bayview Medical Center (JHBMC; Table 1). All consented to participate. The study was approved by the institutional review board.
| Age in years, mean (SD) | 36 (6) |
| Female, n (%) | 13 (76%) |
| Physician, n (%) | 9 (53%) |
| Nurse‐practitioner, n (%) | 5 (29%) |
| Physician assistant, n (%) | 3 (18%) |
| Years in practice, mean (SD) | 5.1 (2.8) |
| Number of pharmaceutical representatives exposed to in past year, mean | 1 |
| Number of shifts worked per month, mean (SD) | 14 (4) |
| Primarily works days, n (%) | 13 (76%) |
Data Collection
We collected and assessed prescription patterns over 3 periods: preintervention, interim, and postintervention.
Assessing Appropriateness of Antibiotics
For each order that was assessed in the preintervention, interim, and postintervention periods, the following information was collected: (1) drug ordered, (2) clinical diagnosis, (3) microbiology results available at the time of the order (including relevant results from recent cultures), (4) other medical diagnoses (ICD9 codes), (5) allergies, and (6) exposure to health care facilities (within the past 30 days). The computerized medical record allowed access to the discharge summaries of a patient's hospitalization. These records summarized the patient's hospitalization, allowing the investigators to understand the reasons for a provider's choice of antibiotics. If the rationale was not clear about how to categorize a prescription from reading the data, the investigators performed a chart review. From the information culled from these reviews, the primary investigator and an infectious disease specialist classified each prescription order by consensus as appropriate, effective but inappropriate, or inappropriate therapy.
Prescriptions were classified as appropriate when they were indicated and correlated with sensitivities, if available, or were of a narrow‐enough spectrum and recommended as a first‐line treatment for specific illnesses by either the Johns Hopkins Antibiotic Guide14 or the Stanford Guide to Antimicrobial Therapy.15 For example, cephalexin to treat uncomplicated cellulitis was considered appropriate therapy. Effective but inappropriate prescriptions were broad‐spectrum antibiotics used to treat an infection when a narrower‐spectrum antibiotic would have sufficed. For example, piperacillin‐tazobactam would be effective in treating a simple urinary tract infection but inappropriate to use because of its broad spectrum. Other examples of effective but inappropriate prescriptions were giving an IV when an oral alternative would be equally effective and tolerated or prescribing antibiotic treatment whose duration was too long. Finally, inappropriate prescriptions were those written for conditions for which antibiotics are not indicated or for which the prescribed antibiotic was ineffective for the specified infection (bug‐drug mismatch).
Preintervention
In January 2006 the investigators retrospectively reviewed the prescribing patterns of the 17 providers over the previous year. Using the computerized medical record and physician order entry, consecutive prescriptions of each provider were evaluated, beginning December 31, 2005, going back reverse chronologically until 20 prescriptions had been identified. For 12 of the providers, it was actually possible to review 20 prescriptions. For 2 other providers, both new, part‐time additions to the hospitalist group, only 1 and 7 prescriptions were found for the entire year. The prescribing history of the 3 remaining providers who participated in the study, all physician assistants, could not be evaluated (during any period) because all their orders were linked only to physicians, making it impossible to determine their specific prescriptions using the physician order entry system.
Interim
During the interim period between obtaining informed consent and completing the academic detailing (January 3, 2006, to March 23, 2006), provider prescribing patterns were reviewed to determine if the mere knowledge of the project would produce changes in prescribing behavior.
Postintervention
After the academic detailing was completed (March 23, 2006), the prescribing patterns of the hospitalists were followed through April 23, 2006. Each week after the detailing session, the hospitalists received reminders to prescribe appropriately (including pens with the message Reduce the Overuse).
Detailing Procedures
After the review, a profile was assembled for each of the CIMS providers. The study team detailers (a physician and a pharmacist) met with the individual providers for 30 to 45 minutes. Each hospitalist participant completed a short survey that collected demographic information and was asked about the rationale for his or her antibiotic prescribing pattern. Next, the appraisal of the provider's prescribing pattern was reviewed. This review included looking at the costs of the prescribed antibiotics compared with those of the appropriate alternatives and a reexamination of the guidelines for the selected target drugspiperacillin‐tazobactam, vancomycin, and extended‐spectrum quinolones. These 3 antibiotics were picked because our providers had been particularly vulnerable to inappropriately prescribing them. The hospitalists were provided an antibiotic guide developed specifically for this project and based on the Johns Hopkins Antibiotic Guide14 that summarizes the consensus guidelines.
Data Analysis
The primary outcome variable was the aggregate proportion of inappropriate antibiotic prescribed (as defined earlier) before the intervention, during the interim between obtaining informed consent and intervening on all study subjects, and after the intervention. The percentage of appropriate prescriptions versus total not appropriate prescriptions (combining of the effective but inappropriate and inappropriate categories) were compared across the 3 periods. Ninety‐five percent confidence intervals for comparisons of the proportions were determined using Stata 9.0 (College Station, TX). The difference between the proportions of total not appropriate prescriptions before and after academic detailing was computed in Stata using Fisher's exact test to assess significance.
RESULTS
Demographic information and professional characteristics of the 17 providers are shown in Table 1. Their mean age was 36 years, and 76% were female. The top 4 reasons the providers gave for their prescribing practices were: (1) published guidelines, (2) easier dosing schedule for patient when discharged, (3) continuing an antibiotic course initiated in the emergency room, and (4) broad‐spectrum antibiotics cover all possible microbes.
Comparison of Preintervention, Interim, and Postintervention Periods
Table 2 depicts the results of the prescription appraisals from the retrospective reviews. Of the 14 providers who had ordered antibiotics, 8 (57%) had more prescriptions that were total not appropriate than were appropriate in the preintervention period compared with 3 providers (25%) with this prescribing pattern in the postintervention period (P = .13).
| Provider | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|
| Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | |
| ||||||
| 1 | 20 | 7 (35%) | 13 (65%) | 24 | 17 (70.8%) | 7 (29.2%) |
| 2 | 20 | 10 (50%) | 10 (50%) | 12 | 11 (91.7%) | 1 (8.3%) |
| 3 | 20 | 6 (30%) | 14 (70%) | 8 | 8 (100%) | 0 (0%) |
| 4* | 19 | 10 (52.6%) | 9 (47.4%) | 4 | 3 (75%) | 1 (25%) |
| 5 | 20 | 9 (45%) | 11 (55%) | 10 | 4 (40%) | 6 (60%) |
| 6 | 20 | 5 (25%) | 15 (75%) | 3 | 1 (33.3%) | 2 (66.7%) |
| 7 | 20 | 8 (40%) | 12 (60%) | 8 | 7 (87.5%) | 1 (12.5%) |
| 8* | 1 | 0 (0%) | 1 (100%) | 0 | 0 (0%) | 0 (0%) |
| 9 | 20 | 11 (55%) | 9 (45%) | 5 | 2 (40%) | 3 (60%) |
| 10* | 7 | 3 (42.9%) | 4 (57.1%) | 0 | 0 (0%) | 0 (0%) |
| 11 | 20 | 10 (50%) | 10 (50%) | 17 | 13 (76.5%) | 4 (23.5%) |
| 12 | 20 | 6 (30%) | 14 (70%) | 16 | 14 (87.5%) | 2 (12.5%) |
| 13 | 20 | 12 (60%) | 8 (40%) | 15 | 11 (73.3%) | 4 (26.7%) |
| 14 | 20 | 10 (50%) | 10 (50%) | 7 | 4 (57.1%) | 3 (42.9%) |
| Total | 247 | 107 (43%) | 140 (57%) | 129 | 95 (73.6%) | 34 (26.4%) |
Table 3 shows the proportions of appropriate, effective but inappropriate, and total not appropriate prescriptions in the retrospective, interim, and postintervention periods. Forty‐three percent (95% CI 37%‐49%) of prescriptions were judged to be appropriate, and 57% (95% CI 51%‐63%) to be not appropriate prior to the academic detailing. In the interim period, 59% (95% CI 52%‐65%) of the prescriptions were appropriate, and 41% (95% CI 35%‐48%) were not appropriate; P = .0003. After the intervention, 74% (95% CI 65%‐81%) of the prescriptions were appropriate, and 26% (95% CI 19%‐35%) were not appropriate; P < .0001.
| Period | Appropriate, n (%) | 95% CI | Effective but inappropriate, n (%) | Inappropriate, n (%) | Total not appropriate, n (%) | 95% CI | P value* |
|---|---|---|---|---|---|---|---|
| |||||||
| Retrospective review (pre) | 107 (43%) | 37%‐49% | 75 (30.4%) | 65 (26.6%) | 140 (57%) | 51%‐63% | |
| Interim | 146 (59%) | 52%‐65% | 37 (15%) | 65 (26%) | 102 (41%) | 35%‐48% | .0003 |
| Postintervention | 95 (74%) | 65%‐81% | 8 (6%) | 26 (20%) | 34 (26%) | 19%‐35% | < .0001 |
DISCUSSION
We have demonstrated that academic detailing had a positive impact on the prescribing patterns of hospitalists. The aggregated improvement in antibiotic prescribing patterns can be attributed to improvement in the prescribing patterns of almost every hospitalist practitioner (Table 2). This study focused on aggregate prescriptions as the primary outcome measure because the hospitalists at JHBMC, like at many other institutions, function as a team, with a patient routinely having multiple providers over the course of the hospital stay. The improved prescribing patterns noted during the interim period suggest that the mere knowledge of a project can have an impact on providers. Providers informed the investigators that they were more thoughtful about their choice of antibiotics when they knew that they were being studied. The further statistically significant improvement in prescribing patterns with the intervention shows that the academic detailing itself was successful.
The greatest absolute change in practice was seen in effective but inappropriate prescribing (from 30.4% to 6%), whereas inappropriate prescribing only decreased from 26.6% to 20.6%. Although we aimed to have an impact on all inappropriate antibiotic prescribing patterns, we specifically reviewed the prescribing guidelines for piperacillin‐tazobactam, extended‐spectrum quinolones, and vancomycin. These 3 antibiotics were targeted because our providers had been particularly susceptible to inappropriately prescribing them. The focus on these antibiotics may have resulted in the larger absolute change noted in effective but inappropriate prescribing. We did not collect any data to determine if having an impact on effective but inappropriate prescribing changed the clinical course of the patients, such as shortening their hospital stays. Anecdotal evidence, however, suggests that it does. At our institution it is not uncommon for patients to be kept in the hospital for an extra day to ensure they are stable when transitioned from extended‐spectrum to narrower‐spectrum antibiotics prior to discharge. The effect of reducing effective but inappropriate prescriptions on the clinical course of patients could be an outcome measure assessed by a future, larger study.
Our one‐on‐one appraisal of each provider's prescribing patterns included a review of the cost of the prescribed antibiotics compared with that of the appropriate alternatives. Although decisions on antibiotic choice should be driven by clinical guidelines and appropriateness rather than price, we believed it was relevant to include education about costs and pricing so that providers would be reminded to ascertain whether patients would be able to afford their antibiotics. Antibiotic resistance is influenced by a patient's failure to complete the course of treatment, and noncompliance may be caused by an inability to afford the medication. Often, there are affordable, appropriate alternatives to the newest and most expensive drugs.
A hospitalist‐based academic detailing approach to improving antibiotic prescribing may have far‐reaching benefits and influence. First, it has the potential to affect other practitioners by setting an example and role modeling. In addition to that with their immediate peer group, hospitalists have close and repeated contact with house officers and emergency room physicians and often act as consultants to physicians in other departments such as surgery and psychiatry. Furthermore, some community hospitals have no infectious disease specialists readily available. So this represents an opportunity for hospitalists to promote quality in antibiotic prescribing. Practice‐based learning was very effective because it brought the practitioners face to face with their prescribing patterns. Although intellectually everyone agreed that antibiotics are often misused, this approach forced the providers to stop and reflect on their individual practices. This peer‐delivered intervention allowed for a collaborative approach to solving the problem; the peer (detailer) was approachable, nonjudgmental, and available for further discussion and guidance.
The public health quality improvement approach that we used for our intervention helped us to realize and appreciate the factors underlying prescribing patterns. Only by understanding the motivations for prescribing patterns can we hope to make sustainable changes. This coincides with our previous assertion that hospitalists are engaging in some public health practice.16 In pubic health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole.17 Hospitalist teams aim to make sure that the high‐quality services needed for protecting the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. Antibiotic optimization is a key role that could fall within the mantra of public health practice for the hospitalist.
Several limitations of this pilot should be considered. First, the intervention is labor intensive. However, it is essential to use the problem‐solving paradigm and incorporate behavior change theories in order to identify interventions that can lead to sustainable change. Second, this was not a randomized controlled trial, and it is possible that there might have been some contamination by external forces. However, in reviewing the educational events at our institution, the press, and articles published during the study period, we could not identify any external factors that would have influenced antibiotic prescribing patterns. It would not have been possible to conduct a randomized trial at our institution because the hospitalists work so closely together that we could not ensure complete separation if the subjects were randomized. There would have been contamination from the intervention group to the control group. A trial with randomization at the institution level is the next step. Third, the number of months retrospectively reviewed in order to identify 20 prescriptions of a provider varied. This study assumed there were no other differences during those months that could have affected provider prescribing behavior; this may have introduced some bias. Fourth, the sustainability of this intervention's positive impact is unknown. We assessed outcome soon after the intervention, and it is unknown whether continual booster sessions are required to maintain the positive impact on prescribing patterns.
This pilot was a good starting place to show that behavior change can be realized with a well‐conceived and methodically executed intervention, even among the busiest of physicians. Audit and feedback, or practice‐based learning, appears to be a powerful educational intervention among professionals who take great pride in their work.
Inappropriate antibiotic use is a major public health concern and demonstrates the need for quality improvement initiatives in the delivery of health care.16 Each year nearly 2 million patients in the United States acquire an infection in the hospital, and about 90,000 of them die from these infections.7 More than 70% of the bacteria that cause hospital‐acquired infections are resistant to at least one commonly used drug.7 Persons infected with drug‐resistant organisms have longer hospital stays and higher mortality rates.7
Inappropriate antibiotic use in the inpatient hospital setting can be classified into 5 categories. First, antibiotics may be given for illnesses for which they are not indicated (eg, viral infections). Second, broad‐spectrum antibiotics (such as piperacillin‐tazobactam and quinolones) may be overused in the empiric treatment of common infections.8 Overuse of broad‐spectrum drugs increases selective pressure for antimicrobial resistance and exposes patients to the side effects of some of these drugs, such as Clostridium difficile colitis.8 Third, clinicians occasionally prescribe intravenous (IV) antibiotics when the efficacy of oral agents would be similar. Inappropriate intravenous therapy increases the cost of care and also exposes the patient to the risk of intravenous catheters.8 Fourth, when the correct antibiotic choice is made, inappropriate antibiotic dosage, schedule, and/or duration of treatment can threaten patient safety.8 Fifth, bug‐drug mismatch occurs when susceptibility studies indicate that the drug being used is ineffective or only marginally effective.8 Beyond antimicrobial resistance and safety, these practices also usually increase costs to both the patient and the hospital.7, 910
Influencing providers' prescribing patterns is difficult.11 In this project we assessed the prescribing patterns of hospitalists in an active inpatient environment and then developed an intervention to improve the providers' use of antibiotics. The intervention utilized public health methodologyprior to implementation, we defined the problem, determined its magnitude, identified a behavior change model, and constructed a conceptual framework that identifyied the key determinants. A pilot academic detailing project addressing many determinants was developed, implemented, and evaluated.
Conceptual Model
To change prescribing behaviors is to change learned behaviors. Changing behavior is a complex process affected by several factors including beliefs, expectations, motivations, and the psychosocial environments of the target groups.12 Each of these factors must be considered when attempting to bring about behavior changes. In doing so, a theory that can be depicted in a model often emerges.13 This approach is widely used in understanding and developing public health interventions.
Formulating the Model
In any public health intervention, recognizing and engaging key stakeholders is a critical step. We identified the following stakeholders: (1) hospitalist practitioners and other prescribing providers including residents and infectious disease specialists; (2) nurses; (3) administrators who are focused on cost effectiveness; (4) patients and their families, who want to get well affordably, without side effects; (5) pharmacists; (6) risk management; and (7) society, which is fearful of the propagation of resistant microbes. In consulting with some of the stakeholders, 4 factors that influence hospitalists' prescribing patterns became apparent. These are practitioner factors, environmental factors, perceived rewards, and perceived threats (Fig. 1).

The practitioner factors shaping prescribing are: (1) knowledge of current best care; (2) self‐efficacy, which determines whether a provider is confident in his or her knowledge to adequately treat a specific infection; (3) habit, which causes providers to pick from a narrow repertoire of antibiotics when treating an infection; and (4) fear of liability, which forces some providers to be cautious. Four environmental factors affecting antibiotic prescriptions are: (1) published guidelines regarding organisms' sensitivity to antibiotics; (2) patient‐driven factors such as affordability, compliance with dosing regimens, side effects, and interactions between the antibiotics and other medications; (3) peer influence, in that providers are reluctant to change a prescription started by another provider (eg, emergency room physician); and (4) the formulary of the hospital, as it forces providers to prescribe within specific parameters. The perceived rewards of specific prescribing practices may include improving patient safety and reducing antibiotic resistance and costs, whereas the perceived threats are increasing antimicrobial resistance, having adverse patient outcomes, and increasing costs and hospital length of stay. We selected a high‐yield, low‐effort intervention in order to have an impact on some of the factors underlying hospitalists' prescribing patterns.
METHODS
Participants
The study participants were 17 hospitalist practitioners including physicians, nurse‐practitioners, and physician assistants who make up the Collaborative Inpatient Medical Service (CIMS) at Johns Hopkins Bayview Medical Center (JHBMC; Table 1). All consented to participate. The study was approved by the institutional review board.
| Age in years, mean (SD) | 36 (6) |
| Female, n (%) | 13 (76%) |
| Physician, n (%) | 9 (53%) |
| Nurse‐practitioner, n (%) | 5 (29%) |
| Physician assistant, n (%) | 3 (18%) |
| Years in practice, mean (SD) | 5.1 (2.8) |
| Number of pharmaceutical representatives exposed to in past year, mean | 1 |
| Number of shifts worked per month, mean (SD) | 14 (4) |
| Primarily works days, n (%) | 13 (76%) |
Data Collection
We collected and assessed prescription patterns over 3 periods: preintervention, interim, and postintervention.
Assessing Appropriateness of Antibiotics
For each order that was assessed in the preintervention, interim, and postintervention periods, the following information was collected: (1) drug ordered, (2) clinical diagnosis, (3) microbiology results available at the time of the order (including relevant results from recent cultures), (4) other medical diagnoses (ICD9 codes), (5) allergies, and (6) exposure to health care facilities (within the past 30 days). The computerized medical record allowed access to the discharge summaries of a patient's hospitalization. These records summarized the patient's hospitalization, allowing the investigators to understand the reasons for a provider's choice of antibiotics. If the rationale was not clear about how to categorize a prescription from reading the data, the investigators performed a chart review. From the information culled from these reviews, the primary investigator and an infectious disease specialist classified each prescription order by consensus as appropriate, effective but inappropriate, or inappropriate therapy.
Prescriptions were classified as appropriate when they were indicated and correlated with sensitivities, if available, or were of a narrow‐enough spectrum and recommended as a first‐line treatment for specific illnesses by either the Johns Hopkins Antibiotic Guide14 or the Stanford Guide to Antimicrobial Therapy.15 For example, cephalexin to treat uncomplicated cellulitis was considered appropriate therapy. Effective but inappropriate prescriptions were broad‐spectrum antibiotics used to treat an infection when a narrower‐spectrum antibiotic would have sufficed. For example, piperacillin‐tazobactam would be effective in treating a simple urinary tract infection but inappropriate to use because of its broad spectrum. Other examples of effective but inappropriate prescriptions were giving an IV when an oral alternative would be equally effective and tolerated or prescribing antibiotic treatment whose duration was too long. Finally, inappropriate prescriptions were those written for conditions for which antibiotics are not indicated or for which the prescribed antibiotic was ineffective for the specified infection (bug‐drug mismatch).
Preintervention
In January 2006 the investigators retrospectively reviewed the prescribing patterns of the 17 providers over the previous year. Using the computerized medical record and physician order entry, consecutive prescriptions of each provider were evaluated, beginning December 31, 2005, going back reverse chronologically until 20 prescriptions had been identified. For 12 of the providers, it was actually possible to review 20 prescriptions. For 2 other providers, both new, part‐time additions to the hospitalist group, only 1 and 7 prescriptions were found for the entire year. The prescribing history of the 3 remaining providers who participated in the study, all physician assistants, could not be evaluated (during any period) because all their orders were linked only to physicians, making it impossible to determine their specific prescriptions using the physician order entry system.
Interim
During the interim period between obtaining informed consent and completing the academic detailing (January 3, 2006, to March 23, 2006), provider prescribing patterns were reviewed to determine if the mere knowledge of the project would produce changes in prescribing behavior.
Postintervention
After the academic detailing was completed (March 23, 2006), the prescribing patterns of the hospitalists were followed through April 23, 2006. Each week after the detailing session, the hospitalists received reminders to prescribe appropriately (including pens with the message Reduce the Overuse).
Detailing Procedures
After the review, a profile was assembled for each of the CIMS providers. The study team detailers (a physician and a pharmacist) met with the individual providers for 30 to 45 minutes. Each hospitalist participant completed a short survey that collected demographic information and was asked about the rationale for his or her antibiotic prescribing pattern. Next, the appraisal of the provider's prescribing pattern was reviewed. This review included looking at the costs of the prescribed antibiotics compared with those of the appropriate alternatives and a reexamination of the guidelines for the selected target drugspiperacillin‐tazobactam, vancomycin, and extended‐spectrum quinolones. These 3 antibiotics were picked because our providers had been particularly vulnerable to inappropriately prescribing them. The hospitalists were provided an antibiotic guide developed specifically for this project and based on the Johns Hopkins Antibiotic Guide14 that summarizes the consensus guidelines.
Data Analysis
The primary outcome variable was the aggregate proportion of inappropriate antibiotic prescribed (as defined earlier) before the intervention, during the interim between obtaining informed consent and intervening on all study subjects, and after the intervention. The percentage of appropriate prescriptions versus total not appropriate prescriptions (combining of the effective but inappropriate and inappropriate categories) were compared across the 3 periods. Ninety‐five percent confidence intervals for comparisons of the proportions were determined using Stata 9.0 (College Station, TX). The difference between the proportions of total not appropriate prescriptions before and after academic detailing was computed in Stata using Fisher's exact test to assess significance.
RESULTS
Demographic information and professional characteristics of the 17 providers are shown in Table 1. Their mean age was 36 years, and 76% were female. The top 4 reasons the providers gave for their prescribing practices were: (1) published guidelines, (2) easier dosing schedule for patient when discharged, (3) continuing an antibiotic course initiated in the emergency room, and (4) broad‐spectrum antibiotics cover all possible microbes.
Comparison of Preintervention, Interim, and Postintervention Periods
Table 2 depicts the results of the prescription appraisals from the retrospective reviews. Of the 14 providers who had ordered antibiotics, 8 (57%) had more prescriptions that were total not appropriate than were appropriate in the preintervention period compared with 3 providers (25%) with this prescribing pattern in the postintervention period (P = .13).
| Provider | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|
| Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | |
| ||||||
| 1 | 20 | 7 (35%) | 13 (65%) | 24 | 17 (70.8%) | 7 (29.2%) |
| 2 | 20 | 10 (50%) | 10 (50%) | 12 | 11 (91.7%) | 1 (8.3%) |
| 3 | 20 | 6 (30%) | 14 (70%) | 8 | 8 (100%) | 0 (0%) |
| 4* | 19 | 10 (52.6%) | 9 (47.4%) | 4 | 3 (75%) | 1 (25%) |
| 5 | 20 | 9 (45%) | 11 (55%) | 10 | 4 (40%) | 6 (60%) |
| 6 | 20 | 5 (25%) | 15 (75%) | 3 | 1 (33.3%) | 2 (66.7%) |
| 7 | 20 | 8 (40%) | 12 (60%) | 8 | 7 (87.5%) | 1 (12.5%) |
| 8* | 1 | 0 (0%) | 1 (100%) | 0 | 0 (0%) | 0 (0%) |
| 9 | 20 | 11 (55%) | 9 (45%) | 5 | 2 (40%) | 3 (60%) |
| 10* | 7 | 3 (42.9%) | 4 (57.1%) | 0 | 0 (0%) | 0 (0%) |
| 11 | 20 | 10 (50%) | 10 (50%) | 17 | 13 (76.5%) | 4 (23.5%) |
| 12 | 20 | 6 (30%) | 14 (70%) | 16 | 14 (87.5%) | 2 (12.5%) |
| 13 | 20 | 12 (60%) | 8 (40%) | 15 | 11 (73.3%) | 4 (26.7%) |
| 14 | 20 | 10 (50%) | 10 (50%) | 7 | 4 (57.1%) | 3 (42.9%) |
| Total | 247 | 107 (43%) | 140 (57%) | 129 | 95 (73.6%) | 34 (26.4%) |
Table 3 shows the proportions of appropriate, effective but inappropriate, and total not appropriate prescriptions in the retrospective, interim, and postintervention periods. Forty‐three percent (95% CI 37%‐49%) of prescriptions were judged to be appropriate, and 57% (95% CI 51%‐63%) to be not appropriate prior to the academic detailing. In the interim period, 59% (95% CI 52%‐65%) of the prescriptions were appropriate, and 41% (95% CI 35%‐48%) were not appropriate; P = .0003. After the intervention, 74% (95% CI 65%‐81%) of the prescriptions were appropriate, and 26% (95% CI 19%‐35%) were not appropriate; P < .0001.
| Period | Appropriate, n (%) | 95% CI | Effective but inappropriate, n (%) | Inappropriate, n (%) | Total not appropriate, n (%) | 95% CI | P value* |
|---|---|---|---|---|---|---|---|
| |||||||
| Retrospective review (pre) | 107 (43%) | 37%‐49% | 75 (30.4%) | 65 (26.6%) | 140 (57%) | 51%‐63% | |
| Interim | 146 (59%) | 52%‐65% | 37 (15%) | 65 (26%) | 102 (41%) | 35%‐48% | .0003 |
| Postintervention | 95 (74%) | 65%‐81% | 8 (6%) | 26 (20%) | 34 (26%) | 19%‐35% | < .0001 |
DISCUSSION
We have demonstrated that academic detailing had a positive impact on the prescribing patterns of hospitalists. The aggregated improvement in antibiotic prescribing patterns can be attributed to improvement in the prescribing patterns of almost every hospitalist practitioner (Table 2). This study focused on aggregate prescriptions as the primary outcome measure because the hospitalists at JHBMC, like at many other institutions, function as a team, with a patient routinely having multiple providers over the course of the hospital stay. The improved prescribing patterns noted during the interim period suggest that the mere knowledge of a project can have an impact on providers. Providers informed the investigators that they were more thoughtful about their choice of antibiotics when they knew that they were being studied. The further statistically significant improvement in prescribing patterns with the intervention shows that the academic detailing itself was successful.
The greatest absolute change in practice was seen in effective but inappropriate prescribing (from 30.4% to 6%), whereas inappropriate prescribing only decreased from 26.6% to 20.6%. Although we aimed to have an impact on all inappropriate antibiotic prescribing patterns, we specifically reviewed the prescribing guidelines for piperacillin‐tazobactam, extended‐spectrum quinolones, and vancomycin. These 3 antibiotics were targeted because our providers had been particularly susceptible to inappropriately prescribing them. The focus on these antibiotics may have resulted in the larger absolute change noted in effective but inappropriate prescribing. We did not collect any data to determine if having an impact on effective but inappropriate prescribing changed the clinical course of the patients, such as shortening their hospital stays. Anecdotal evidence, however, suggests that it does. At our institution it is not uncommon for patients to be kept in the hospital for an extra day to ensure they are stable when transitioned from extended‐spectrum to narrower‐spectrum antibiotics prior to discharge. The effect of reducing effective but inappropriate prescriptions on the clinical course of patients could be an outcome measure assessed by a future, larger study.
Our one‐on‐one appraisal of each provider's prescribing patterns included a review of the cost of the prescribed antibiotics compared with that of the appropriate alternatives. Although decisions on antibiotic choice should be driven by clinical guidelines and appropriateness rather than price, we believed it was relevant to include education about costs and pricing so that providers would be reminded to ascertain whether patients would be able to afford their antibiotics. Antibiotic resistance is influenced by a patient's failure to complete the course of treatment, and noncompliance may be caused by an inability to afford the medication. Often, there are affordable, appropriate alternatives to the newest and most expensive drugs.
A hospitalist‐based academic detailing approach to improving antibiotic prescribing may have far‐reaching benefits and influence. First, it has the potential to affect other practitioners by setting an example and role modeling. In addition to that with their immediate peer group, hospitalists have close and repeated contact with house officers and emergency room physicians and often act as consultants to physicians in other departments such as surgery and psychiatry. Furthermore, some community hospitals have no infectious disease specialists readily available. So this represents an opportunity for hospitalists to promote quality in antibiotic prescribing. Practice‐based learning was very effective because it brought the practitioners face to face with their prescribing patterns. Although intellectually everyone agreed that antibiotics are often misused, this approach forced the providers to stop and reflect on their individual practices. This peer‐delivered intervention allowed for a collaborative approach to solving the problem; the peer (detailer) was approachable, nonjudgmental, and available for further discussion and guidance.
The public health quality improvement approach that we used for our intervention helped us to realize and appreciate the factors underlying prescribing patterns. Only by understanding the motivations for prescribing patterns can we hope to make sustainable changes. This coincides with our previous assertion that hospitalists are engaging in some public health practice.16 In pubic health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole.17 Hospitalist teams aim to make sure that the high‐quality services needed for protecting the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. Antibiotic optimization is a key role that could fall within the mantra of public health practice for the hospitalist.
Several limitations of this pilot should be considered. First, the intervention is labor intensive. However, it is essential to use the problem‐solving paradigm and incorporate behavior change theories in order to identify interventions that can lead to sustainable change. Second, this was not a randomized controlled trial, and it is possible that there might have been some contamination by external forces. However, in reviewing the educational events at our institution, the press, and articles published during the study period, we could not identify any external factors that would have influenced antibiotic prescribing patterns. It would not have been possible to conduct a randomized trial at our institution because the hospitalists work so closely together that we could not ensure complete separation if the subjects were randomized. There would have been contamination from the intervention group to the control group. A trial with randomization at the institution level is the next step. Third, the number of months retrospectively reviewed in order to identify 20 prescriptions of a provider varied. This study assumed there were no other differences during those months that could have affected provider prescribing behavior; this may have introduced some bias. Fourth, the sustainability of this intervention's positive impact is unknown. We assessed outcome soon after the intervention, and it is unknown whether continual booster sessions are required to maintain the positive impact on prescribing patterns.
This pilot was a good starting place to show that behavior change can be realized with a well‐conceived and methodically executed intervention, even among the busiest of physicians. Audit and feedback, or practice‐based learning, appears to be a powerful educational intervention among professionals who take great pride in their work.
- ,.Improving antibiotic use in low‐income countries: an overview of evidence on determinants.Soc Sci Med.2003;57:733–744.
- .Mechanisms of antimicrobial resistance in bacteria.Am J Med.2006;119(6A):S3–S10.
- .Antimicrobial resistance in gram‐positive bacteria.Am J Med.2006;119(6A):S11–S19.
- .Resistance in Gram‐negative bacteria: enterobacteriaceae.Am J Med.2006;119(6A):S20–S28.
- .Pharmacodynamics: relation to antimicrobial resistance.Am J Med.2006;119(6A):S37–S44.
- .Managing methicillin‐resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms.Am J Med.2006;119(6A):S45–S52.
- NIH. The Problem of Antibiotic Resistance. Available at: http://www.niaid.nih.gov.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed.J Hosp Infect.2001;47:198–209.
- ,.The impact of hospital‐acquired bloodstream infections.Emerg Infect Dis.2001;7(2):174–177.
- .Antimicrobial stewardship.Am J Med.2006;119(6A):S53–S61
- ,,, et al.Changing provider behavior: an overview of systemic reviews of interventions.Med Care.2001;39:II–2‐II‐45.
- .A review of current health education theories.Calif J Health Promot.2004;2:74–87
- The Johns Hopkins Hospital Antibiotic Management Program. 2005 Antibiotic Guidelines: Treatment Recommendations for Adult Inpatients. Johns Hopkins Medicine.
- ,,,.The Sanford Guide to Antimicrobial Therapy 2005.35th ed.Hyde Park, VT:Antimicrobial Therapy, Inc.;2005.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
- ,.Improving antibiotic use in low‐income countries: an overview of evidence on determinants.Soc Sci Med.2003;57:733–744.
- .Mechanisms of antimicrobial resistance in bacteria.Am J Med.2006;119(6A):S3–S10.
- .Antimicrobial resistance in gram‐positive bacteria.Am J Med.2006;119(6A):S11–S19.
- .Resistance in Gram‐negative bacteria: enterobacteriaceae.Am J Med.2006;119(6A):S20–S28.
- .Pharmacodynamics: relation to antimicrobial resistance.Am J Med.2006;119(6A):S37–S44.
- .Managing methicillin‐resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms.Am J Med.2006;119(6A):S45–S52.
- NIH. The Problem of Antibiotic Resistance. Available at: http://www.niaid.nih.gov.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed.J Hosp Infect.2001;47:198–209.
- ,.The impact of hospital‐acquired bloodstream infections.Emerg Infect Dis.2001;7(2):174–177.
- .Antimicrobial stewardship.Am J Med.2006;119(6A):S53–S61
- ,,, et al.Changing provider behavior: an overview of systemic reviews of interventions.Med Care.2001;39:II–2‐II‐45.
- .A review of current health education theories.Calif J Health Promot.2004;2:74–87
- The Johns Hopkins Hospital Antibiotic Management Program. 2005 Antibiotic Guidelines: Treatment Recommendations for Adult Inpatients. Johns Hopkins Medicine.
- ,,,.The Sanford Guide to Antimicrobial Therapy 2005.35th ed.Hyde Park, VT:Antimicrobial Therapy, Inc.;2005.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
Copyright © 2008 Society of Hospital Medicine
CD‐ROM‐Based Education on Anticoagulation
Given recent changes in the goals and objectives of residency training as well as changes in the functioning of teaching hospitals, traditional educational formats may need to be supplemented or replaced.1 The Accreditation Council for Graduate Medical Education (ACGME) is promoting changes in resident education with the goal of not only enhancing trainee competency using innovative methods but also of demonstrating that these educational innovations result in enhanced quality of patient care and improved patient safety.1 A challenging aspect of these initiatives is that programs are working to implement them at a time when there are greater nonteaching demands on faculty time, mandated resident work‐hour limitations have been instituted, in some states by law, and resident patient care and educational activities are prone to disruptions inherent in caring for patients in a complex health system. Various solutions have been proposed including increased incorporation of self‐directed learning as a means of meeting modern resident educational challenges, yet the ideal tools with which to accomplish this are unknown.
Computer‐based instruction in medicine has been available since the 1960s, and although its use had initially been more widespread in medical student, nursing, allied health professional, and patient education,2, 3 it is being increasingly incorporated into resident education as well. Some studies have shown that for medical students, computer‐based teaching is at least as effective in improving knowledge as conventional lectures4 and that learners' satisfaction with computer‐based formats appears comparable with that of traditional didactic lectures.5 In recent years computer‐based teaching has been applied to resident education in various fields including surgery and surgical subspecialties, pediatrics, and obstetrics and gynecology.612 Little is known, however, about how computer‐based educational methods affect resident knowledge and especially how these methods might affect clinical practice.
Venous thromboembolism (VTE) is a common and hazardous complication of acute inpatient hospitalization.13 Recognizing that errors in proper prescribing and monitoring of anticoagulants are a major cause of acute inpatient morbidity and mortality,14 we began an initiative to educate our residents and improve their patient care practices regarding the proper use of anticoagulants. To accomplish this, we developed a CD‐ROM‐based learning module with the aim of increasing resident knowledge of anticoagulation as well as compliance with national standards for VTE prevention. In this study we assessed the impact of the CD‐ROM intervention on resident knowledge and their appropriate use of VTE prophylaxis.
METHODS
The study was approved by the institutional review board. With the participation of faculty educators in the departments of medicine, surgery, and neurology, one of the authors (H.K.) coordinated the development of a CD‐ROM containing concise modules on core topics in anticoagulation (Table 1). The presenters for these topics included the director of clinical hematology, 2 cardiologists including the director of the coronary care unit, the director of the medical intensive care unit, the director of cerebrovascular diseases, and 2 vascular surgeons, one of whom serves as vice chair of surgery. These modules, each lasting about 1 hour, had audio and slide components detailing the proper indications, monitoring, and efficacy of anticoagulants in atrial fibrillation, acute ischemic stroke, acute coronary syndromes, and VTE prevention in acutely ill hospitalized patients. The guidelines presented were based on the sixth (2000) ACCP guidelines for antithrombotic therapy for the prevention and treatment of thrombosis.15 The content of the CD‐ROM was reviewed for accuracy by the authors, though none of them were speakers. We asked that before all current residents in the departments of cardiothoracic surgery, emergency medicine, otolaryngology, internal medicine, neurosurgery, dental medicine, neurology, obstetrics and gynecology, orthopedics, surgery, and urology viewed the CD‐ROM, they complete a pretest to determine their baseline knowledge of this subject. After completing the pretest, the residents were required to view the CD‐ROM and retake the same test. We then compared pre‐ and posttest scores.
| Overview of anticoagulation |
| Venous thromboembolism |
| Atrial fibrillation |
| Unfractionated heparin in acute coronary syndrome |
| Treatment of thromboembolic events with intravenous heparin |
| Anticoagulants in the management of patients with acute ischemic stroke |
| Deep venous thrombosis prophylaxis |
To determine whether an increase in knowledge was secondary to the CD‐ROM intervention or simply a consequence of acquired clinical experience during training, we compared test scores of residents who did and did not receive the CD‐ROM intervention. In the academic year following our initial testing, we asked the incoming categorical medical PGY‐1 classes at our hospital and at a comparable local tertiary‐care hospital in our health system 2 miles away to take an anticoagulation pretest (different from the examination given for the initial testing) during their PGY‐1 orientations. The 2 institutions are comparable in many ways including in patient demographics and size and most residents come from the same medical schools, have a similar rotation structure, and use a comparable curriculum under a unified graduate medical education office. The CD‐ROMs were only given to categorical PGY‐1 residents at our institution. Both groups then retook the same test (posttest) 3 months into their clinical training, a time chosen because it is when all PGY‐1s would be expected to have gained significant clinical experience on the medical wards and or in the intensive care units. The exam questions were generated by one of the authors (B.M.) and covered all the topics in the CD‐ROM.
An Anticoagulation Steering Committee was formed to assess whether the CD‐ROM intervention affected our residents' patient care practices. None of the members of this committee were authors of this work. Members of this committee reviewed inpatient charts and documented resident compliance with VTE prevention standards during periods before and after they had viewed the CD‐ROMs. We chose this particular portion of the CD‐ROM because at both the test and control hospitals, initiatives were underway using order sets to improve anticoagulation in cardiac, neurological, and surgical patients but not in VTE prophylaxis. Charts from the same 2 nursing units on the medical service were reviewed in each period and included patients with a discharge diagnosis of congestive heart failure, any oncologic diagnosis, or sepsis. The chart review tool was developed by the anticoagulation committee and included a thrombosis risk factor assessment section as well as a list of contraindications to anticoagulation to determine if anticoagulation was appropriately implemented. Charts were reviewed for compliance with VTE prophylaxis after the CD‐ROM intervention (given in July 2004) in August 2004. To have a comparable pre‐CD‐ROM comparison, charts of patients with the diagnoses stated above were reviewed from August of the preceding year. The same month was chosen in the previous year to minimize any impact of resident experience, which would likely be a confounding factor if charts from May or June of the academic year were used as a control, for example. To determine whether an improvement in adherence to VTE prophylaxis standards was sustained, an additional chart review was carried out 7 months after the initial CD‐ROM viewing. The same group of observers, none of whom were authors, did all the chart assessments.
Statistics
Continuous variables are reported as means SDs. Comparisons of test scores before and after the CD‐ROM intervention were carried out using paired t testing. Comparisons of pre‐ and posttest scores between both institutions were carried out using analysis of variance with Tukey‐Kramer multiple‐comparisons testing (GraphPad InStat Statistical Software, version 3.01, GraphPad Software, Inc.). We calculated that 13 residents would need to be tested in order to have a statistical power of 80% to detect a 25% increase in test scores with a type I error of 0.05. Comparisons of the proportions of patients who received appropriate VTE prophylaxis were carried out using chi‐square testing. Statistical significance was defined as a 2‐tailed P value less than 0.05.
RESULTS
Overall and Departmental Resident Test Results
One hundred and seventeen residents from all departments participated in the project including taking the pre‐ and posttests. The response rate was 44% overall and ranged from 10% to 100% for individual departments. For all residents combined, there was a statistically significant increase in scores (pretest 46.7% 15.1%, posttest 77.8% 15.1%, P < .005). Overall scores and those for individual departments are summarized in Table 2. As can be seen, there was a significant increase in test results for each department. The only exception was a department that already had a high baseline score and that had only 4 residents, limiting the power of statistical analysis. These findings suggest that the CD‐ROM intervention favorably affected resident knowledge of anticoagulation across all medical specialties tested.
| Department | n | Prescore | Postscore | P value* |
|---|---|---|---|---|
| ||||
| Cardiothoracic surgery | 1 | 72 | 83 | NA |
| Dentistry | 22 | 34.9 10.3 | 72.3 12.4 | < .0001 |
| Surgery | 19 | 52.6 14.5 | 77.1 14.5 | < .0001 |
| Medicine | 21 | 54.3 11.6 | 84.0 8.9 | < .0001 |
| Emergency medicine | 4 | 61.3 4.5 | 94.3 8.0 | < .05 |
| Otolaryngology | 5 | 48.8 5.0 | 80.0 11.6 | < .01 |
| Urology | 4 | 66.5 23.6 | 84.5 15.8 | 0.15 |
| Neurology | 10 | 42.1 11.5 | 68.8 18.9 | < .01 |
| Orthopedics | 12 | 43.4 16.0 | 70.4 24.1 | < .01 |
| Obstetrics/gynecology | 19 | 41.4 13.1 | 81.8 11.0 | < .0001 |
| ALL | 117 | 46.7 15.1 | 77.8 15.1 | < .005 |
Assessment of Independent Effect of CD‐ROM Intervention
To determine what independent effect the CD‐ROM intervention might have, given that scores may improve with the acquisition of clinical experience alone, in July 2004 we tested internal medicine categorical PGY‐1s at our institution and at another tertiary‐care hospital, as described in the Methods section. The results of testing both groups are shown in Figure 1. Nineteen medical PGY‐1s at our hospital (hospital A) completed the anticoagulation pretest, and 16 completed the posttest. Twenty‐two medical PGY‐1s completed the pretest, and 17 completed the posttest at our neighboring hospital (hospital B). Although posttest scores were higher at both institutions, the increase in scores at our institution, which received the CD‐ROM intervention, was statistically significant, whereas the increase for the group not receiving the intervention was not significant. These findings suggest that the CD‐ROM intervention may have had an independent effect on resident knowledge of anticoagulation.
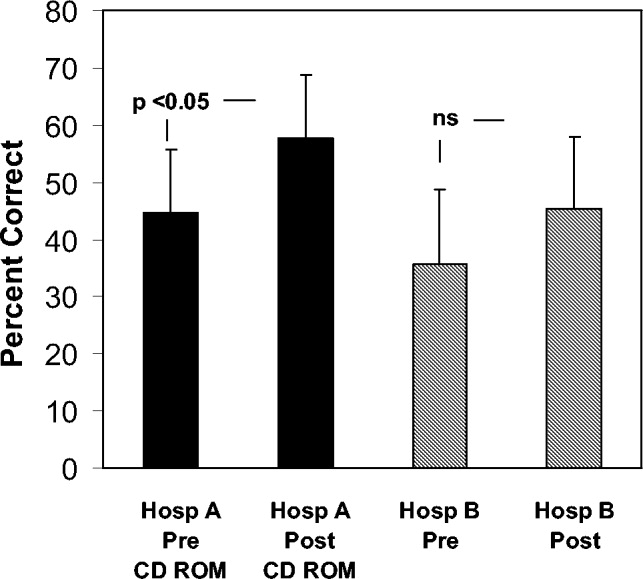
Effect of CD‐ROM Intervention on Resident Use of VTE Prophylaxis
Appropriate use of VTE prophylaxis by residents was assessed at 3 points, as detailed in the Methods section: 1 year before the CD‐ROM intervention (baseline), immediately after the CD‐ROM intervention, and 7 months after the CD‐ROM intervention. VTE prophylaxis, one element of the CD‐ROM, was chosen as a surrogate marker for the impact of the CD‐ROM initiative. A review of 40 charts of patients with the specified diagnoses (100% of the patients with the specified diagnoses, which represented about one third of admissions to the unit) before the CD‐ROM intervention revealed that 30 patients (75%) received appropriate VTE prophylaxis. A review of 38 charts after the CD‐ROM intervention showed that 36 patients (95%) received appropriate prophylaxis; similar findings were obtained 7 months after the CD‐ROM intervention (33 of 35 patients, 94%, P = .0107). These findings, which are shown in Figure 2, suggest that the CD‐ROM intervention enhanced resident compliance with VTE prophylaxis guidelines and that this effect was sustained for at least 7 months.
DISCUSSION
Residency training is facing challenges on several fronts. In addition to substantially changed educational requirements, strict limits on the amount of time that trainees can spend in the hospital have resulted from ACGME requirements and several state laws. Residents who are on night rotations or were on call the night before often miss educational conferences or must choose between attending patient carerelated activities and educational sessions. Time constraints on faculty have compounded this problem, and for residents to effectively learn, the focus of graduate medical education may need to shift somewhat from teaching medical information toward teaching the practice of self‐directed learning, with CD‐ROMs one such mode by which this can take place. Accomplishing this will require novel teaching approaches, and residency programs will need to document their effectiveness.
In this study we demonstrated that our residents increased their knowledge and improved their patient care practices using a CD‐ROM‐based educational tool. Residents frequently make use of computer‐based educational resources in the form of journals, textbooks, informational databases such as comprehensive drug listings, and personal digital assistantbased tools. Advantages of the computer‐based learning format include increased accessibility and flexibility in viewing the material. Residents have the option of repeated screening as desired and of viewing the CD‐ROM in segments if necessary. Although residents often must choose to attend a scheduled traditional lecture or engage in a patient carerelated activity, the CD‐ROM format allows the resident to choose the ideal time and setting to engage in structured educational activities. Other advantages of the CD‐ROM format would be ease of monitoring for accuracy, applicability, and comprehensiveness as well as more flexibility in faculty time commitments. It should be noted that we have no information about how much time residents devoted to the CD‐ROM program and how often they may have returned to the module for review. It should also be noted that although there have been some reports suggesting that CD‐ROM‐based education may play a useful role in student and perhaps resident education,1618 there is no evidence to date demonstrating that widespread use of CD‐ROMs in residency training can differentially affect resident behavior compared with the use of traditional methods.
A number of variables could have affected our results. For overall test scores, the response rate was less than 50%, with variability between departments suggesting that perhaps it was more motivated residents who participated and were therefore more likely to demonstrate improvement. Although our data comparing institutions with and without the CD‐ROM intervention suggested that the CD‐ROM intervention had a discernable effect on resident knowledge, we must also consider the possibility that the 2 groups might not have been comparable, as attitudes, expectations, and other variables might have differed. All the residents were categorical trainees, and given the similarities in many aspects of the training programs in these 2 tertiary‐care hospitals, as described in the Methods section, it is hoped that any such differences were minor. Nevertheless, this must be considered a limitation of our study. Also of note, the number of trainees was small, as was the patient population studied with VTE prophylaxis; hence, we recognize that our work can best be regarded as a pilot study using an alternative learning method. We also realize that giving a group of residents a test followed by distribution of a CD‐ROM might have suggested that we were directing them toward a goal, and this may have affected the results. Heightened awareness of the importance of anticoagulation from the introduction of new guidelines and other variables also could have affected our findings. The taking of an examination itself might also have had an impact on knowledge that could affect subsequent test scores. An additional point to consider is that if knowledge and patient care did improve, we do not know whether this affects residents acquiring other knowledge or whether this will translate into improved patient care in other areas.
Although CD‐ROM‐based learning could serve a useful function in the increasingly complex environment of residency training, this learning method also has disadvantages, including not providing personal contact or having the capability of question‐and‐answer sessions between teacher and resident. This could be overcome by providing time for faculty‐precepted question‐and‐answer sessions or perhaps creating a Web‐based venue for questions to be submitted and answered. In addition, the CD‐ROMs themselves can be designed in an interactive format in which residents can provide answers to clinical questions with feedback based on their selections provided as part of the CD‐ROM program.
In summary, the CD‐ROM‐based program in this study appears to have had an effect on not only knowledge but also patient care practice and suggests that this type of format could serve a useful role in residency training. Studies of additional interventions such as this one might allow for more extensive evaluation of the utility of CD‐ROM‐based learning as a residency training tool.
- ,,, et al., for theResidency Review Committee for Internal Medicine.A new model for accreditation of residency programs in internal medicine.Ann Intern Med.2004;140:902–909.
- ,,.Technology‐based vs. traditional instruction. A comparison of two methods for teaching the skill of performing a 12‐lead ECG.Nurs Educ Perspect.2003;24:70–74.
- ,,.Canadian physical therapists' interest in web‐based and computer‐assisted continuing education.Phys Ther.2005;85:226–237.
- ,,,,,.Computer Assisted Learning is an effective way of teaching endocrinology.Clin Endocrinol.2001;55:537–542.
- ,.Web‐based minimally invasive surgery training: competency assessment in PGY 1‐2 surgical residents.Curr Surg.2004;61:120–124.
- ,.Use of computer‐assisted learning module to achieve ACGME competencies in orthopaedic foot and ankle surgery.Foot Ankle Int.2003;24:938–941.
- ,,,.Tutor versus computer: a prospective comparison of interactive tutorial and computer‐assisted instruction in radiology education.Acad Radiol.2002;9:40–49.
- ,,, et al.Using simulation to instruct emergency medicine residents in cognitive forcing strategies.Acad Med.2004;79:438–446
- ,,, et al.Successful implementation of a novel internet hybrid surgery curriculum: the early phase outcome of thoracic surgery prerequisite curriculum e‐learning project.Ann Surg.2004;240:499–507.
- ,.Development and evaluation of a CD‐ROM computer program to teach residents telephone management.Pediatrics.1998;101:E2.
- ,,,,.The Pediatric Residency Training on Tobacco Project: baseline findings from the resident tobacco survey and observed structured clinical examinations.Prev Med.2004;39:507–516.
- ,,,.Development of a CD‐ROM Internet hybrid: a new thoracic surgery curriculum.Ann Thorac Surg.2002;74:1741–1746
- ,,, et al.A population‐based perspective of the hospital incidence and case fatality rates of deep venous thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,.Relationship between time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis.Arch Intern Med.1997;22:2562–2568.
- ,,;American College of Chest Physicians.The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis.American College of Chest Physicians.Chest.2001;119(1 Suppl):1S–2S.
- ,,.Student assessment of the educational benefits of using a CD‐ROM for instruction of basic surgical skills.J Vet Med Educ.2005;32:138–143.
- .A multimedia CD‐ROM tool to improve student understanding of bile salts and bilirubin metabolism: evaluation of its use in a medical hybrid PBL course.Adv Physiol Educ.2005;29:40–50.
- ,,,.Testing a multimedia module in cancer pain management.J Cancer Educ.1999;14:161–163.
Given recent changes in the goals and objectives of residency training as well as changes in the functioning of teaching hospitals, traditional educational formats may need to be supplemented or replaced.1 The Accreditation Council for Graduate Medical Education (ACGME) is promoting changes in resident education with the goal of not only enhancing trainee competency using innovative methods but also of demonstrating that these educational innovations result in enhanced quality of patient care and improved patient safety.1 A challenging aspect of these initiatives is that programs are working to implement them at a time when there are greater nonteaching demands on faculty time, mandated resident work‐hour limitations have been instituted, in some states by law, and resident patient care and educational activities are prone to disruptions inherent in caring for patients in a complex health system. Various solutions have been proposed including increased incorporation of self‐directed learning as a means of meeting modern resident educational challenges, yet the ideal tools with which to accomplish this are unknown.
Computer‐based instruction in medicine has been available since the 1960s, and although its use had initially been more widespread in medical student, nursing, allied health professional, and patient education,2, 3 it is being increasingly incorporated into resident education as well. Some studies have shown that for medical students, computer‐based teaching is at least as effective in improving knowledge as conventional lectures4 and that learners' satisfaction with computer‐based formats appears comparable with that of traditional didactic lectures.5 In recent years computer‐based teaching has been applied to resident education in various fields including surgery and surgical subspecialties, pediatrics, and obstetrics and gynecology.612 Little is known, however, about how computer‐based educational methods affect resident knowledge and especially how these methods might affect clinical practice.
Venous thromboembolism (VTE) is a common and hazardous complication of acute inpatient hospitalization.13 Recognizing that errors in proper prescribing and monitoring of anticoagulants are a major cause of acute inpatient morbidity and mortality,14 we began an initiative to educate our residents and improve their patient care practices regarding the proper use of anticoagulants. To accomplish this, we developed a CD‐ROM‐based learning module with the aim of increasing resident knowledge of anticoagulation as well as compliance with national standards for VTE prevention. In this study we assessed the impact of the CD‐ROM intervention on resident knowledge and their appropriate use of VTE prophylaxis.
METHODS
The study was approved by the institutional review board. With the participation of faculty educators in the departments of medicine, surgery, and neurology, one of the authors (H.K.) coordinated the development of a CD‐ROM containing concise modules on core topics in anticoagulation (Table 1). The presenters for these topics included the director of clinical hematology, 2 cardiologists including the director of the coronary care unit, the director of the medical intensive care unit, the director of cerebrovascular diseases, and 2 vascular surgeons, one of whom serves as vice chair of surgery. These modules, each lasting about 1 hour, had audio and slide components detailing the proper indications, monitoring, and efficacy of anticoagulants in atrial fibrillation, acute ischemic stroke, acute coronary syndromes, and VTE prevention in acutely ill hospitalized patients. The guidelines presented were based on the sixth (2000) ACCP guidelines for antithrombotic therapy for the prevention and treatment of thrombosis.15 The content of the CD‐ROM was reviewed for accuracy by the authors, though none of them were speakers. We asked that before all current residents in the departments of cardiothoracic surgery, emergency medicine, otolaryngology, internal medicine, neurosurgery, dental medicine, neurology, obstetrics and gynecology, orthopedics, surgery, and urology viewed the CD‐ROM, they complete a pretest to determine their baseline knowledge of this subject. After completing the pretest, the residents were required to view the CD‐ROM and retake the same test. We then compared pre‐ and posttest scores.
| Overview of anticoagulation |
| Venous thromboembolism |
| Atrial fibrillation |
| Unfractionated heparin in acute coronary syndrome |
| Treatment of thromboembolic events with intravenous heparin |
| Anticoagulants in the management of patients with acute ischemic stroke |
| Deep venous thrombosis prophylaxis |
To determine whether an increase in knowledge was secondary to the CD‐ROM intervention or simply a consequence of acquired clinical experience during training, we compared test scores of residents who did and did not receive the CD‐ROM intervention. In the academic year following our initial testing, we asked the incoming categorical medical PGY‐1 classes at our hospital and at a comparable local tertiary‐care hospital in our health system 2 miles away to take an anticoagulation pretest (different from the examination given for the initial testing) during their PGY‐1 orientations. The 2 institutions are comparable in many ways including in patient demographics and size and most residents come from the same medical schools, have a similar rotation structure, and use a comparable curriculum under a unified graduate medical education office. The CD‐ROMs were only given to categorical PGY‐1 residents at our institution. Both groups then retook the same test (posttest) 3 months into their clinical training, a time chosen because it is when all PGY‐1s would be expected to have gained significant clinical experience on the medical wards and or in the intensive care units. The exam questions were generated by one of the authors (B.M.) and covered all the topics in the CD‐ROM.
An Anticoagulation Steering Committee was formed to assess whether the CD‐ROM intervention affected our residents' patient care practices. None of the members of this committee were authors of this work. Members of this committee reviewed inpatient charts and documented resident compliance with VTE prevention standards during periods before and after they had viewed the CD‐ROMs. We chose this particular portion of the CD‐ROM because at both the test and control hospitals, initiatives were underway using order sets to improve anticoagulation in cardiac, neurological, and surgical patients but not in VTE prophylaxis. Charts from the same 2 nursing units on the medical service were reviewed in each period and included patients with a discharge diagnosis of congestive heart failure, any oncologic diagnosis, or sepsis. The chart review tool was developed by the anticoagulation committee and included a thrombosis risk factor assessment section as well as a list of contraindications to anticoagulation to determine if anticoagulation was appropriately implemented. Charts were reviewed for compliance with VTE prophylaxis after the CD‐ROM intervention (given in July 2004) in August 2004. To have a comparable pre‐CD‐ROM comparison, charts of patients with the diagnoses stated above were reviewed from August of the preceding year. The same month was chosen in the previous year to minimize any impact of resident experience, which would likely be a confounding factor if charts from May or June of the academic year were used as a control, for example. To determine whether an improvement in adherence to VTE prophylaxis standards was sustained, an additional chart review was carried out 7 months after the initial CD‐ROM viewing. The same group of observers, none of whom were authors, did all the chart assessments.
Statistics
Continuous variables are reported as means SDs. Comparisons of test scores before and after the CD‐ROM intervention were carried out using paired t testing. Comparisons of pre‐ and posttest scores between both institutions were carried out using analysis of variance with Tukey‐Kramer multiple‐comparisons testing (GraphPad InStat Statistical Software, version 3.01, GraphPad Software, Inc.). We calculated that 13 residents would need to be tested in order to have a statistical power of 80% to detect a 25% increase in test scores with a type I error of 0.05. Comparisons of the proportions of patients who received appropriate VTE prophylaxis were carried out using chi‐square testing. Statistical significance was defined as a 2‐tailed P value less than 0.05.
RESULTS
Overall and Departmental Resident Test Results
One hundred and seventeen residents from all departments participated in the project including taking the pre‐ and posttests. The response rate was 44% overall and ranged from 10% to 100% for individual departments. For all residents combined, there was a statistically significant increase in scores (pretest 46.7% 15.1%, posttest 77.8% 15.1%, P < .005). Overall scores and those for individual departments are summarized in Table 2. As can be seen, there was a significant increase in test results for each department. The only exception was a department that already had a high baseline score and that had only 4 residents, limiting the power of statistical analysis. These findings suggest that the CD‐ROM intervention favorably affected resident knowledge of anticoagulation across all medical specialties tested.
| Department | n | Prescore | Postscore | P value* |
|---|---|---|---|---|
| ||||
| Cardiothoracic surgery | 1 | 72 | 83 | NA |
| Dentistry | 22 | 34.9 10.3 | 72.3 12.4 | < .0001 |
| Surgery | 19 | 52.6 14.5 | 77.1 14.5 | < .0001 |
| Medicine | 21 | 54.3 11.6 | 84.0 8.9 | < .0001 |
| Emergency medicine | 4 | 61.3 4.5 | 94.3 8.0 | < .05 |
| Otolaryngology | 5 | 48.8 5.0 | 80.0 11.6 | < .01 |
| Urology | 4 | 66.5 23.6 | 84.5 15.8 | 0.15 |
| Neurology | 10 | 42.1 11.5 | 68.8 18.9 | < .01 |
| Orthopedics | 12 | 43.4 16.0 | 70.4 24.1 | < .01 |
| Obstetrics/gynecology | 19 | 41.4 13.1 | 81.8 11.0 | < .0001 |
| ALL | 117 | 46.7 15.1 | 77.8 15.1 | < .005 |
Assessment of Independent Effect of CD‐ROM Intervention
To determine what independent effect the CD‐ROM intervention might have, given that scores may improve with the acquisition of clinical experience alone, in July 2004 we tested internal medicine categorical PGY‐1s at our institution and at another tertiary‐care hospital, as described in the Methods section. The results of testing both groups are shown in Figure 1. Nineteen medical PGY‐1s at our hospital (hospital A) completed the anticoagulation pretest, and 16 completed the posttest. Twenty‐two medical PGY‐1s completed the pretest, and 17 completed the posttest at our neighboring hospital (hospital B). Although posttest scores were higher at both institutions, the increase in scores at our institution, which received the CD‐ROM intervention, was statistically significant, whereas the increase for the group not receiving the intervention was not significant. These findings suggest that the CD‐ROM intervention may have had an independent effect on resident knowledge of anticoagulation.

Effect of CD‐ROM Intervention on Resident Use of VTE Prophylaxis
Appropriate use of VTE prophylaxis by residents was assessed at 3 points, as detailed in the Methods section: 1 year before the CD‐ROM intervention (baseline), immediately after the CD‐ROM intervention, and 7 months after the CD‐ROM intervention. VTE prophylaxis, one element of the CD‐ROM, was chosen as a surrogate marker for the impact of the CD‐ROM initiative. A review of 40 charts of patients with the specified diagnoses (100% of the patients with the specified diagnoses, which represented about one third of admissions to the unit) before the CD‐ROM intervention revealed that 30 patients (75%) received appropriate VTE prophylaxis. A review of 38 charts after the CD‐ROM intervention showed that 36 patients (95%) received appropriate prophylaxis; similar findings were obtained 7 months after the CD‐ROM intervention (33 of 35 patients, 94%, P = .0107). These findings, which are shown in Figure 2, suggest that the CD‐ROM intervention enhanced resident compliance with VTE prophylaxis guidelines and that this effect was sustained for at least 7 months.

DISCUSSION
Residency training is facing challenges on several fronts. In addition to substantially changed educational requirements, strict limits on the amount of time that trainees can spend in the hospital have resulted from ACGME requirements and several state laws. Residents who are on night rotations or were on call the night before often miss educational conferences or must choose between attending patient carerelated activities and educational sessions. Time constraints on faculty have compounded this problem, and for residents to effectively learn, the focus of graduate medical education may need to shift somewhat from teaching medical information toward teaching the practice of self‐directed learning, with CD‐ROMs one such mode by which this can take place. Accomplishing this will require novel teaching approaches, and residency programs will need to document their effectiveness.
In this study we demonstrated that our residents increased their knowledge and improved their patient care practices using a CD‐ROM‐based educational tool. Residents frequently make use of computer‐based educational resources in the form of journals, textbooks, informational databases such as comprehensive drug listings, and personal digital assistantbased tools. Advantages of the computer‐based learning format include increased accessibility and flexibility in viewing the material. Residents have the option of repeated screening as desired and of viewing the CD‐ROM in segments if necessary. Although residents often must choose to attend a scheduled traditional lecture or engage in a patient carerelated activity, the CD‐ROM format allows the resident to choose the ideal time and setting to engage in structured educational activities. Other advantages of the CD‐ROM format would be ease of monitoring for accuracy, applicability, and comprehensiveness as well as more flexibility in faculty time commitments. It should be noted that we have no information about how much time residents devoted to the CD‐ROM program and how often they may have returned to the module for review. It should also be noted that although there have been some reports suggesting that CD‐ROM‐based education may play a useful role in student and perhaps resident education,1618 there is no evidence to date demonstrating that widespread use of CD‐ROMs in residency training can differentially affect resident behavior compared with the use of traditional methods.
A number of variables could have affected our results. For overall test scores, the response rate was less than 50%, with variability between departments suggesting that perhaps it was more motivated residents who participated and were therefore more likely to demonstrate improvement. Although our data comparing institutions with and without the CD‐ROM intervention suggested that the CD‐ROM intervention had a discernable effect on resident knowledge, we must also consider the possibility that the 2 groups might not have been comparable, as attitudes, expectations, and other variables might have differed. All the residents were categorical trainees, and given the similarities in many aspects of the training programs in these 2 tertiary‐care hospitals, as described in the Methods section, it is hoped that any such differences were minor. Nevertheless, this must be considered a limitation of our study. Also of note, the number of trainees was small, as was the patient population studied with VTE prophylaxis; hence, we recognize that our work can best be regarded as a pilot study using an alternative learning method. We also realize that giving a group of residents a test followed by distribution of a CD‐ROM might have suggested that we were directing them toward a goal, and this may have affected the results. Heightened awareness of the importance of anticoagulation from the introduction of new guidelines and other variables also could have affected our findings. The taking of an examination itself might also have had an impact on knowledge that could affect subsequent test scores. An additional point to consider is that if knowledge and patient care did improve, we do not know whether this affects residents acquiring other knowledge or whether this will translate into improved patient care in other areas.
Although CD‐ROM‐based learning could serve a useful function in the increasingly complex environment of residency training, this learning method also has disadvantages, including not providing personal contact or having the capability of question‐and‐answer sessions between teacher and resident. This could be overcome by providing time for faculty‐precepted question‐and‐answer sessions or perhaps creating a Web‐based venue for questions to be submitted and answered. In addition, the CD‐ROMs themselves can be designed in an interactive format in which residents can provide answers to clinical questions with feedback based on their selections provided as part of the CD‐ROM program.
In summary, the CD‐ROM‐based program in this study appears to have had an effect on not only knowledge but also patient care practice and suggests that this type of format could serve a useful role in residency training. Studies of additional interventions such as this one might allow for more extensive evaluation of the utility of CD‐ROM‐based learning as a residency training tool.
Given recent changes in the goals and objectives of residency training as well as changes in the functioning of teaching hospitals, traditional educational formats may need to be supplemented or replaced.1 The Accreditation Council for Graduate Medical Education (ACGME) is promoting changes in resident education with the goal of not only enhancing trainee competency using innovative methods but also of demonstrating that these educational innovations result in enhanced quality of patient care and improved patient safety.1 A challenging aspect of these initiatives is that programs are working to implement them at a time when there are greater nonteaching demands on faculty time, mandated resident work‐hour limitations have been instituted, in some states by law, and resident patient care and educational activities are prone to disruptions inherent in caring for patients in a complex health system. Various solutions have been proposed including increased incorporation of self‐directed learning as a means of meeting modern resident educational challenges, yet the ideal tools with which to accomplish this are unknown.
Computer‐based instruction in medicine has been available since the 1960s, and although its use had initially been more widespread in medical student, nursing, allied health professional, and patient education,2, 3 it is being increasingly incorporated into resident education as well. Some studies have shown that for medical students, computer‐based teaching is at least as effective in improving knowledge as conventional lectures4 and that learners' satisfaction with computer‐based formats appears comparable with that of traditional didactic lectures.5 In recent years computer‐based teaching has been applied to resident education in various fields including surgery and surgical subspecialties, pediatrics, and obstetrics and gynecology.612 Little is known, however, about how computer‐based educational methods affect resident knowledge and especially how these methods might affect clinical practice.
Venous thromboembolism (VTE) is a common and hazardous complication of acute inpatient hospitalization.13 Recognizing that errors in proper prescribing and monitoring of anticoagulants are a major cause of acute inpatient morbidity and mortality,14 we began an initiative to educate our residents and improve their patient care practices regarding the proper use of anticoagulants. To accomplish this, we developed a CD‐ROM‐based learning module with the aim of increasing resident knowledge of anticoagulation as well as compliance with national standards for VTE prevention. In this study we assessed the impact of the CD‐ROM intervention on resident knowledge and their appropriate use of VTE prophylaxis.
METHODS
The study was approved by the institutional review board. With the participation of faculty educators in the departments of medicine, surgery, and neurology, one of the authors (H.K.) coordinated the development of a CD‐ROM containing concise modules on core topics in anticoagulation (Table 1). The presenters for these topics included the director of clinical hematology, 2 cardiologists including the director of the coronary care unit, the director of the medical intensive care unit, the director of cerebrovascular diseases, and 2 vascular surgeons, one of whom serves as vice chair of surgery. These modules, each lasting about 1 hour, had audio and slide components detailing the proper indications, monitoring, and efficacy of anticoagulants in atrial fibrillation, acute ischemic stroke, acute coronary syndromes, and VTE prevention in acutely ill hospitalized patients. The guidelines presented were based on the sixth (2000) ACCP guidelines for antithrombotic therapy for the prevention and treatment of thrombosis.15 The content of the CD‐ROM was reviewed for accuracy by the authors, though none of them were speakers. We asked that before all current residents in the departments of cardiothoracic surgery, emergency medicine, otolaryngology, internal medicine, neurosurgery, dental medicine, neurology, obstetrics and gynecology, orthopedics, surgery, and urology viewed the CD‐ROM, they complete a pretest to determine their baseline knowledge of this subject. After completing the pretest, the residents were required to view the CD‐ROM and retake the same test. We then compared pre‐ and posttest scores.
| Overview of anticoagulation |
| Venous thromboembolism |
| Atrial fibrillation |
| Unfractionated heparin in acute coronary syndrome |
| Treatment of thromboembolic events with intravenous heparin |
| Anticoagulants in the management of patients with acute ischemic stroke |
| Deep venous thrombosis prophylaxis |
To determine whether an increase in knowledge was secondary to the CD‐ROM intervention or simply a consequence of acquired clinical experience during training, we compared test scores of residents who did and did not receive the CD‐ROM intervention. In the academic year following our initial testing, we asked the incoming categorical medical PGY‐1 classes at our hospital and at a comparable local tertiary‐care hospital in our health system 2 miles away to take an anticoagulation pretest (different from the examination given for the initial testing) during their PGY‐1 orientations. The 2 institutions are comparable in many ways including in patient demographics and size and most residents come from the same medical schools, have a similar rotation structure, and use a comparable curriculum under a unified graduate medical education office. The CD‐ROMs were only given to categorical PGY‐1 residents at our institution. Both groups then retook the same test (posttest) 3 months into their clinical training, a time chosen because it is when all PGY‐1s would be expected to have gained significant clinical experience on the medical wards and or in the intensive care units. The exam questions were generated by one of the authors (B.M.) and covered all the topics in the CD‐ROM.
An Anticoagulation Steering Committee was formed to assess whether the CD‐ROM intervention affected our residents' patient care practices. None of the members of this committee were authors of this work. Members of this committee reviewed inpatient charts and documented resident compliance with VTE prevention standards during periods before and after they had viewed the CD‐ROMs. We chose this particular portion of the CD‐ROM because at both the test and control hospitals, initiatives were underway using order sets to improve anticoagulation in cardiac, neurological, and surgical patients but not in VTE prophylaxis. Charts from the same 2 nursing units on the medical service were reviewed in each period and included patients with a discharge diagnosis of congestive heart failure, any oncologic diagnosis, or sepsis. The chart review tool was developed by the anticoagulation committee and included a thrombosis risk factor assessment section as well as a list of contraindications to anticoagulation to determine if anticoagulation was appropriately implemented. Charts were reviewed for compliance with VTE prophylaxis after the CD‐ROM intervention (given in July 2004) in August 2004. To have a comparable pre‐CD‐ROM comparison, charts of patients with the diagnoses stated above were reviewed from August of the preceding year. The same month was chosen in the previous year to minimize any impact of resident experience, which would likely be a confounding factor if charts from May or June of the academic year were used as a control, for example. To determine whether an improvement in adherence to VTE prophylaxis standards was sustained, an additional chart review was carried out 7 months after the initial CD‐ROM viewing. The same group of observers, none of whom were authors, did all the chart assessments.
Statistics
Continuous variables are reported as means SDs. Comparisons of test scores before and after the CD‐ROM intervention were carried out using paired t testing. Comparisons of pre‐ and posttest scores between both institutions were carried out using analysis of variance with Tukey‐Kramer multiple‐comparisons testing (GraphPad InStat Statistical Software, version 3.01, GraphPad Software, Inc.). We calculated that 13 residents would need to be tested in order to have a statistical power of 80% to detect a 25% increase in test scores with a type I error of 0.05. Comparisons of the proportions of patients who received appropriate VTE prophylaxis were carried out using chi‐square testing. Statistical significance was defined as a 2‐tailed P value less than 0.05.
RESULTS
Overall and Departmental Resident Test Results
One hundred and seventeen residents from all departments participated in the project including taking the pre‐ and posttests. The response rate was 44% overall and ranged from 10% to 100% for individual departments. For all residents combined, there was a statistically significant increase in scores (pretest 46.7% 15.1%, posttest 77.8% 15.1%, P < .005). Overall scores and those for individual departments are summarized in Table 2. As can be seen, there was a significant increase in test results for each department. The only exception was a department that already had a high baseline score and that had only 4 residents, limiting the power of statistical analysis. These findings suggest that the CD‐ROM intervention favorably affected resident knowledge of anticoagulation across all medical specialties tested.
| Department | n | Prescore | Postscore | P value* |
|---|---|---|---|---|
| ||||
| Cardiothoracic surgery | 1 | 72 | 83 | NA |
| Dentistry | 22 | 34.9 10.3 | 72.3 12.4 | < .0001 |
| Surgery | 19 | 52.6 14.5 | 77.1 14.5 | < .0001 |
| Medicine | 21 | 54.3 11.6 | 84.0 8.9 | < .0001 |
| Emergency medicine | 4 | 61.3 4.5 | 94.3 8.0 | < .05 |
| Otolaryngology | 5 | 48.8 5.0 | 80.0 11.6 | < .01 |
| Urology | 4 | 66.5 23.6 | 84.5 15.8 | 0.15 |
| Neurology | 10 | 42.1 11.5 | 68.8 18.9 | < .01 |
| Orthopedics | 12 | 43.4 16.0 | 70.4 24.1 | < .01 |
| Obstetrics/gynecology | 19 | 41.4 13.1 | 81.8 11.0 | < .0001 |
| ALL | 117 | 46.7 15.1 | 77.8 15.1 | < .005 |
Assessment of Independent Effect of CD‐ROM Intervention
To determine what independent effect the CD‐ROM intervention might have, given that scores may improve with the acquisition of clinical experience alone, in July 2004 we tested internal medicine categorical PGY‐1s at our institution and at another tertiary‐care hospital, as described in the Methods section. The results of testing both groups are shown in Figure 1. Nineteen medical PGY‐1s at our hospital (hospital A) completed the anticoagulation pretest, and 16 completed the posttest. Twenty‐two medical PGY‐1s completed the pretest, and 17 completed the posttest at our neighboring hospital (hospital B). Although posttest scores were higher at both institutions, the increase in scores at our institution, which received the CD‐ROM intervention, was statistically significant, whereas the increase for the group not receiving the intervention was not significant. These findings suggest that the CD‐ROM intervention may have had an independent effect on resident knowledge of anticoagulation.

Effect of CD‐ROM Intervention on Resident Use of VTE Prophylaxis
Appropriate use of VTE prophylaxis by residents was assessed at 3 points, as detailed in the Methods section: 1 year before the CD‐ROM intervention (baseline), immediately after the CD‐ROM intervention, and 7 months after the CD‐ROM intervention. VTE prophylaxis, one element of the CD‐ROM, was chosen as a surrogate marker for the impact of the CD‐ROM initiative. A review of 40 charts of patients with the specified diagnoses (100% of the patients with the specified diagnoses, which represented about one third of admissions to the unit) before the CD‐ROM intervention revealed that 30 patients (75%) received appropriate VTE prophylaxis. A review of 38 charts after the CD‐ROM intervention showed that 36 patients (95%) received appropriate prophylaxis; similar findings were obtained 7 months after the CD‐ROM intervention (33 of 35 patients, 94%, P = .0107). These findings, which are shown in Figure 2, suggest that the CD‐ROM intervention enhanced resident compliance with VTE prophylaxis guidelines and that this effect was sustained for at least 7 months.

DISCUSSION
Residency training is facing challenges on several fronts. In addition to substantially changed educational requirements, strict limits on the amount of time that trainees can spend in the hospital have resulted from ACGME requirements and several state laws. Residents who are on night rotations or were on call the night before often miss educational conferences or must choose between attending patient carerelated activities and educational sessions. Time constraints on faculty have compounded this problem, and for residents to effectively learn, the focus of graduate medical education may need to shift somewhat from teaching medical information toward teaching the practice of self‐directed learning, with CD‐ROMs one such mode by which this can take place. Accomplishing this will require novel teaching approaches, and residency programs will need to document their effectiveness.
In this study we demonstrated that our residents increased their knowledge and improved their patient care practices using a CD‐ROM‐based educational tool. Residents frequently make use of computer‐based educational resources in the form of journals, textbooks, informational databases such as comprehensive drug listings, and personal digital assistantbased tools. Advantages of the computer‐based learning format include increased accessibility and flexibility in viewing the material. Residents have the option of repeated screening as desired and of viewing the CD‐ROM in segments if necessary. Although residents often must choose to attend a scheduled traditional lecture or engage in a patient carerelated activity, the CD‐ROM format allows the resident to choose the ideal time and setting to engage in structured educational activities. Other advantages of the CD‐ROM format would be ease of monitoring for accuracy, applicability, and comprehensiveness as well as more flexibility in faculty time commitments. It should be noted that we have no information about how much time residents devoted to the CD‐ROM program and how often they may have returned to the module for review. It should also be noted that although there have been some reports suggesting that CD‐ROM‐based education may play a useful role in student and perhaps resident education,1618 there is no evidence to date demonstrating that widespread use of CD‐ROMs in residency training can differentially affect resident behavior compared with the use of traditional methods.
A number of variables could have affected our results. For overall test scores, the response rate was less than 50%, with variability between departments suggesting that perhaps it was more motivated residents who participated and were therefore more likely to demonstrate improvement. Although our data comparing institutions with and without the CD‐ROM intervention suggested that the CD‐ROM intervention had a discernable effect on resident knowledge, we must also consider the possibility that the 2 groups might not have been comparable, as attitudes, expectations, and other variables might have differed. All the residents were categorical trainees, and given the similarities in many aspects of the training programs in these 2 tertiary‐care hospitals, as described in the Methods section, it is hoped that any such differences were minor. Nevertheless, this must be considered a limitation of our study. Also of note, the number of trainees was small, as was the patient population studied with VTE prophylaxis; hence, we recognize that our work can best be regarded as a pilot study using an alternative learning method. We also realize that giving a group of residents a test followed by distribution of a CD‐ROM might have suggested that we were directing them toward a goal, and this may have affected the results. Heightened awareness of the importance of anticoagulation from the introduction of new guidelines and other variables also could have affected our findings. The taking of an examination itself might also have had an impact on knowledge that could affect subsequent test scores. An additional point to consider is that if knowledge and patient care did improve, we do not know whether this affects residents acquiring other knowledge or whether this will translate into improved patient care in other areas.
Although CD‐ROM‐based learning could serve a useful function in the increasingly complex environment of residency training, this learning method also has disadvantages, including not providing personal contact or having the capability of question‐and‐answer sessions between teacher and resident. This could be overcome by providing time for faculty‐precepted question‐and‐answer sessions or perhaps creating a Web‐based venue for questions to be submitted and answered. In addition, the CD‐ROMs themselves can be designed in an interactive format in which residents can provide answers to clinical questions with feedback based on their selections provided as part of the CD‐ROM program.
In summary, the CD‐ROM‐based program in this study appears to have had an effect on not only knowledge but also patient care practice and suggests that this type of format could serve a useful role in residency training. Studies of additional interventions such as this one might allow for more extensive evaluation of the utility of CD‐ROM‐based learning as a residency training tool.
- ,,, et al., for theResidency Review Committee for Internal Medicine.A new model for accreditation of residency programs in internal medicine.Ann Intern Med.2004;140:902–909.
- ,,.Technology‐based vs. traditional instruction. A comparison of two methods for teaching the skill of performing a 12‐lead ECG.Nurs Educ Perspect.2003;24:70–74.
- ,,.Canadian physical therapists' interest in web‐based and computer‐assisted continuing education.Phys Ther.2005;85:226–237.
- ,,,,,.Computer Assisted Learning is an effective way of teaching endocrinology.Clin Endocrinol.2001;55:537–542.
- ,.Web‐based minimally invasive surgery training: competency assessment in PGY 1‐2 surgical residents.Curr Surg.2004;61:120–124.
- ,.Use of computer‐assisted learning module to achieve ACGME competencies in orthopaedic foot and ankle surgery.Foot Ankle Int.2003;24:938–941.
- ,,,.Tutor versus computer: a prospective comparison of interactive tutorial and computer‐assisted instruction in radiology education.Acad Radiol.2002;9:40–49.
- ,,, et al.Using simulation to instruct emergency medicine residents in cognitive forcing strategies.Acad Med.2004;79:438–446
- ,,, et al.Successful implementation of a novel internet hybrid surgery curriculum: the early phase outcome of thoracic surgery prerequisite curriculum e‐learning project.Ann Surg.2004;240:499–507.
- ,.Development and evaluation of a CD‐ROM computer program to teach residents telephone management.Pediatrics.1998;101:E2.
- ,,,,.The Pediatric Residency Training on Tobacco Project: baseline findings from the resident tobacco survey and observed structured clinical examinations.Prev Med.2004;39:507–516.
- ,,,.Development of a CD‐ROM Internet hybrid: a new thoracic surgery curriculum.Ann Thorac Surg.2002;74:1741–1746
- ,,, et al.A population‐based perspective of the hospital incidence and case fatality rates of deep venous thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,.Relationship between time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis.Arch Intern Med.1997;22:2562–2568.
- ,,;American College of Chest Physicians.The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis.American College of Chest Physicians.Chest.2001;119(1 Suppl):1S–2S.
- ,,.Student assessment of the educational benefits of using a CD‐ROM for instruction of basic surgical skills.J Vet Med Educ.2005;32:138–143.
- .A multimedia CD‐ROM tool to improve student understanding of bile salts and bilirubin metabolism: evaluation of its use in a medical hybrid PBL course.Adv Physiol Educ.2005;29:40–50.
- ,,,.Testing a multimedia module in cancer pain management.J Cancer Educ.1999;14:161–163.
- ,,, et al., for theResidency Review Committee for Internal Medicine.A new model for accreditation of residency programs in internal medicine.Ann Intern Med.2004;140:902–909.
- ,,.Technology‐based vs. traditional instruction. A comparison of two methods for teaching the skill of performing a 12‐lead ECG.Nurs Educ Perspect.2003;24:70–74.
- ,,.Canadian physical therapists' interest in web‐based and computer‐assisted continuing education.Phys Ther.2005;85:226–237.
- ,,,,,.Computer Assisted Learning is an effective way of teaching endocrinology.Clin Endocrinol.2001;55:537–542.
- ,.Web‐based minimally invasive surgery training: competency assessment in PGY 1‐2 surgical residents.Curr Surg.2004;61:120–124.
- ,.Use of computer‐assisted learning module to achieve ACGME competencies in orthopaedic foot and ankle surgery.Foot Ankle Int.2003;24:938–941.
- ,,,.Tutor versus computer: a prospective comparison of interactive tutorial and computer‐assisted instruction in radiology education.Acad Radiol.2002;9:40–49.
- ,,, et al.Using simulation to instruct emergency medicine residents in cognitive forcing strategies.Acad Med.2004;79:438–446
- ,,, et al.Successful implementation of a novel internet hybrid surgery curriculum: the early phase outcome of thoracic surgery prerequisite curriculum e‐learning project.Ann Surg.2004;240:499–507.
- ,.Development and evaluation of a CD‐ROM computer program to teach residents telephone management.Pediatrics.1998;101:E2.
- ,,,,.The Pediatric Residency Training on Tobacco Project: baseline findings from the resident tobacco survey and observed structured clinical examinations.Prev Med.2004;39:507–516.
- ,,,.Development of a CD‐ROM Internet hybrid: a new thoracic surgery curriculum.Ann Thorac Surg.2002;74:1741–1746
- ,,, et al.A population‐based perspective of the hospital incidence and case fatality rates of deep venous thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,.Relationship between time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis.Arch Intern Med.1997;22:2562–2568.
- ,,;American College of Chest Physicians.The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis.American College of Chest Physicians.Chest.2001;119(1 Suppl):1S–2S.
- ,,.Student assessment of the educational benefits of using a CD‐ROM for instruction of basic surgical skills.J Vet Med Educ.2005;32:138–143.
- .A multimedia CD‐ROM tool to improve student understanding of bile salts and bilirubin metabolism: evaluation of its use in a medical hybrid PBL course.Adv Physiol Educ.2005;29:40–50.
- ,,,.Testing a multimedia module in cancer pain management.J Cancer Educ.1999;14:161–163.
Copyright © 2008 Society of Hospital Medicine
Case Report
Hyperhemolysis syndrome is a form of atypical hemolytic transfusion reaction (HTR). It is characterized by a significant drop in hemoglobin (Hb) after seemingly compatible red blood cell transfusions, suggesting destruction of both transfused and autologous red blood cells. Its pathophysiology is not well understood, and a serologic cause is often not identified.14 In contrast, delayed HTRs are typically characterized by a positive direct antiglobulin test (DAT), suggesting that the patient's red blood cells are coated by immunoglobulin G and/or complement components and by the appearance of previously undetected red blood cell alloantibody or antibodies that developed from a secondary anamnestic response; however, autologous red cells are not destroyed.
CASE
A 48‐year‐old African American woman with sickle cell disease (SCD) was readmitted for pain crisis. Her medical history included stroke, pulmonary hypertension, and congestive heart failure. She had received several transfusions and consequently had developed antibodies to seven clinically significant red blood cell antigens. A week prior to readmission, she was discharged from the hospital with an Hb of 6.9 g/dL after a sickle cell crisis precipitated by pneumonia. She was treated with hydration, pain medications, antibiotics, and a unit of cross‐match‐compatible red blood cells (RBCs) that was antigen negative for her antibodies.
On readmission, she had an Hb of 5.6 g/dL and an uncorrected reticulocyte count of 17.6%. Her reticulocyte production index, a reticulocyte count corrected for the degree of anemia and reticulocyte maturation time, was elevated at 2.6. She was transfused with 1 unit of phenotypically matched and cross‐match‐compatible RBCs. Three hours after transfusion, she developed dark‐colored urine. The transfusion reaction investigation revealed no clerical error or incompatibility, a negative DAT, and an antibody panel identical to that from pretransfusion testing. During hospitalization, the hemolytic anemia worsened (Fig. 1). On the 10th hospital day, she became severely dyspneic as her Hb reached its nadir of 3.6 g/dL despite ongoing erythropoiesis. She developed decompensated heart failure and renal insufficiency, precipitated by the acutely worsening anemia. Along with diuretic and vasodilator therapies, she was treated with methylprednisolone at 125 mg twice daily for 3 days followed by tapering doses of prednisone for 2 weeks, intravenous immunoglobulin (IVIG) at 400 mg/kg a day for 5 days, and 4 cross‐match‐compatible RBC transfusions that were antigen negative for her antibodies. The hemolysis resolved and the patient improved. Throughout hospitalization, her DAT remained negative. The Hb remained stable at 7 g/dL until she was discharged. Ten months of follow‐up showed no new red blood cell antibody in her serum or recurrence of hyperhemolysis syndrome despite receiving subsequent transfusions.
DISCUSSION
Hyperhemolysis syndrome has been described in patients with SCD,14, 6, 7 suggesting that an underlying hemoglobinopathy may be a risk factor; however, a patient with anemia of chronic disease was recently described in the literature to have developed hyperhemolysis syndrome.5 Possible mechanisms include innocent bystander hemolysis through complement‐mediated lysis and/or formation of red blood cell alloantibody or autoantibody;1, 2 and hyperactive macrophages of the reticuloendothelial system that recognize Hb S RBCs of patients with SCD more avidly than normal RBCs because of the exposure of aminophosphatides in the outer layer of the sickled RBC membrane.3 In effect, red blood cells may be destroyed regardless of whether they are autologous or transfused. Additionally, transfusion‐related suppression of erythropoiesis may worsen the severity of anemia.2 Recent studies of patients with SCD suggest that the presence of free plasma Hb, as a consequence of hemolysis, reduces nitric oxide bioavailability, promotes endothelial dysfunction, and contributes to the development of pulmonary hypertension and the varying presentations of vasoocclusion.6 A common observation among patients who experience hyperhemolysis syndrome is that withholding transfusion seems beneficial, probably because immunologic reactions are not exacerbated, whereas treatment with steroids1, 2, 4 and/or IVIG3, 7 resolves hemolysis because of their immunomodulatory effects.
CONCLUSIONS
Hyperhemolysis syndrome is a potentially life‐threatening complication of RBC transfusion. It is important to recognize this syndrome when managing patients with SCD who present with worsening anemia after RBC transfusions. Although further transfusions can exacerbate hemolysis4, 7 and may be relatively contraindicated, in severe and desperate situations, simultaneous treatment with steroids and IVIG, together with RBC transfusions, may be lifesaving.
- ,,,,.Delayed hemolytic transfusion reactions in sickle cell disease: simultaneous destruction of recipients' red cells.Transfusion.1997;37:376–381.
- ,,,,.The sickle cell hemolytic transfusion reaction syndrome.Transfusion.1997;37:382–392.
- ,,,,.Hyperhemolytic transfusion reaction in sickle cell disease.Transfusion.2001;41:323–328.
- ,,,,.Delayed hemolytic transfusion reaction/hyperhemolysis syndrome in children with sickle cell disease.Pediatrics.2003;111(6 Pt 1):e661–e665.
- ,.Hyperhemolysis syndrome in anemia of chronic disease.Transfusion.2005;45:1930–1933.
- and.Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia.Transfusion.2006;46:105–110.
- ,,,.Post‐transfusion hyperhemolysis in a patient with sickle cell disease: use of steroids and intravenous immunoglobulin to prevent further red cell destruction.Vox Sang.1995;69:355–357.
Hyperhemolysis syndrome is a form of atypical hemolytic transfusion reaction (HTR). It is characterized by a significant drop in hemoglobin (Hb) after seemingly compatible red blood cell transfusions, suggesting destruction of both transfused and autologous red blood cells. Its pathophysiology is not well understood, and a serologic cause is often not identified.14 In contrast, delayed HTRs are typically characterized by a positive direct antiglobulin test (DAT), suggesting that the patient's red blood cells are coated by immunoglobulin G and/or complement components and by the appearance of previously undetected red blood cell alloantibody or antibodies that developed from a secondary anamnestic response; however, autologous red cells are not destroyed.
CASE
A 48‐year‐old African American woman with sickle cell disease (SCD) was readmitted for pain crisis. Her medical history included stroke, pulmonary hypertension, and congestive heart failure. She had received several transfusions and consequently had developed antibodies to seven clinically significant red blood cell antigens. A week prior to readmission, she was discharged from the hospital with an Hb of 6.9 g/dL after a sickle cell crisis precipitated by pneumonia. She was treated with hydration, pain medications, antibiotics, and a unit of cross‐match‐compatible red blood cells (RBCs) that was antigen negative for her antibodies.
On readmission, she had an Hb of 5.6 g/dL and an uncorrected reticulocyte count of 17.6%. Her reticulocyte production index, a reticulocyte count corrected for the degree of anemia and reticulocyte maturation time, was elevated at 2.6. She was transfused with 1 unit of phenotypically matched and cross‐match‐compatible RBCs. Three hours after transfusion, she developed dark‐colored urine. The transfusion reaction investigation revealed no clerical error or incompatibility, a negative DAT, and an antibody panel identical to that from pretransfusion testing. During hospitalization, the hemolytic anemia worsened (Fig. 1). On the 10th hospital day, she became severely dyspneic as her Hb reached its nadir of 3.6 g/dL despite ongoing erythropoiesis. She developed decompensated heart failure and renal insufficiency, precipitated by the acutely worsening anemia. Along with diuretic and vasodilator therapies, she was treated with methylprednisolone at 125 mg twice daily for 3 days followed by tapering doses of prednisone for 2 weeks, intravenous immunoglobulin (IVIG) at 400 mg/kg a day for 5 days, and 4 cross‐match‐compatible RBC transfusions that were antigen negative for her antibodies. The hemolysis resolved and the patient improved. Throughout hospitalization, her DAT remained negative. The Hb remained stable at 7 g/dL until she was discharged. Ten months of follow‐up showed no new red blood cell antibody in her serum or recurrence of hyperhemolysis syndrome despite receiving subsequent transfusions.

DISCUSSION
Hyperhemolysis syndrome has been described in patients with SCD,14, 6, 7 suggesting that an underlying hemoglobinopathy may be a risk factor; however, a patient with anemia of chronic disease was recently described in the literature to have developed hyperhemolysis syndrome.5 Possible mechanisms include innocent bystander hemolysis through complement‐mediated lysis and/or formation of red blood cell alloantibody or autoantibody;1, 2 and hyperactive macrophages of the reticuloendothelial system that recognize Hb S RBCs of patients with SCD more avidly than normal RBCs because of the exposure of aminophosphatides in the outer layer of the sickled RBC membrane.3 In effect, red blood cells may be destroyed regardless of whether they are autologous or transfused. Additionally, transfusion‐related suppression of erythropoiesis may worsen the severity of anemia.2 Recent studies of patients with SCD suggest that the presence of free plasma Hb, as a consequence of hemolysis, reduces nitric oxide bioavailability, promotes endothelial dysfunction, and contributes to the development of pulmonary hypertension and the varying presentations of vasoocclusion.6 A common observation among patients who experience hyperhemolysis syndrome is that withholding transfusion seems beneficial, probably because immunologic reactions are not exacerbated, whereas treatment with steroids1, 2, 4 and/or IVIG3, 7 resolves hemolysis because of their immunomodulatory effects.
CONCLUSIONS
Hyperhemolysis syndrome is a potentially life‐threatening complication of RBC transfusion. It is important to recognize this syndrome when managing patients with SCD who present with worsening anemia after RBC transfusions. Although further transfusions can exacerbate hemolysis4, 7 and may be relatively contraindicated, in severe and desperate situations, simultaneous treatment with steroids and IVIG, together with RBC transfusions, may be lifesaving.
Hyperhemolysis syndrome is a form of atypical hemolytic transfusion reaction (HTR). It is characterized by a significant drop in hemoglobin (Hb) after seemingly compatible red blood cell transfusions, suggesting destruction of both transfused and autologous red blood cells. Its pathophysiology is not well understood, and a serologic cause is often not identified.14 In contrast, delayed HTRs are typically characterized by a positive direct antiglobulin test (DAT), suggesting that the patient's red blood cells are coated by immunoglobulin G and/or complement components and by the appearance of previously undetected red blood cell alloantibody or antibodies that developed from a secondary anamnestic response; however, autologous red cells are not destroyed.
CASE
A 48‐year‐old African American woman with sickle cell disease (SCD) was readmitted for pain crisis. Her medical history included stroke, pulmonary hypertension, and congestive heart failure. She had received several transfusions and consequently had developed antibodies to seven clinically significant red blood cell antigens. A week prior to readmission, she was discharged from the hospital with an Hb of 6.9 g/dL after a sickle cell crisis precipitated by pneumonia. She was treated with hydration, pain medications, antibiotics, and a unit of cross‐match‐compatible red blood cells (RBCs) that was antigen negative for her antibodies.
On readmission, she had an Hb of 5.6 g/dL and an uncorrected reticulocyte count of 17.6%. Her reticulocyte production index, a reticulocyte count corrected for the degree of anemia and reticulocyte maturation time, was elevated at 2.6. She was transfused with 1 unit of phenotypically matched and cross‐match‐compatible RBCs. Three hours after transfusion, she developed dark‐colored urine. The transfusion reaction investigation revealed no clerical error or incompatibility, a negative DAT, and an antibody panel identical to that from pretransfusion testing. During hospitalization, the hemolytic anemia worsened (Fig. 1). On the 10th hospital day, she became severely dyspneic as her Hb reached its nadir of 3.6 g/dL despite ongoing erythropoiesis. She developed decompensated heart failure and renal insufficiency, precipitated by the acutely worsening anemia. Along with diuretic and vasodilator therapies, she was treated with methylprednisolone at 125 mg twice daily for 3 days followed by tapering doses of prednisone for 2 weeks, intravenous immunoglobulin (IVIG) at 400 mg/kg a day for 5 days, and 4 cross‐match‐compatible RBC transfusions that were antigen negative for her antibodies. The hemolysis resolved and the patient improved. Throughout hospitalization, her DAT remained negative. The Hb remained stable at 7 g/dL until she was discharged. Ten months of follow‐up showed no new red blood cell antibody in her serum or recurrence of hyperhemolysis syndrome despite receiving subsequent transfusions.

DISCUSSION
Hyperhemolysis syndrome has been described in patients with SCD,14, 6, 7 suggesting that an underlying hemoglobinopathy may be a risk factor; however, a patient with anemia of chronic disease was recently described in the literature to have developed hyperhemolysis syndrome.5 Possible mechanisms include innocent bystander hemolysis through complement‐mediated lysis and/or formation of red blood cell alloantibody or autoantibody;1, 2 and hyperactive macrophages of the reticuloendothelial system that recognize Hb S RBCs of patients with SCD more avidly than normal RBCs because of the exposure of aminophosphatides in the outer layer of the sickled RBC membrane.3 In effect, red blood cells may be destroyed regardless of whether they are autologous or transfused. Additionally, transfusion‐related suppression of erythropoiesis may worsen the severity of anemia.2 Recent studies of patients with SCD suggest that the presence of free plasma Hb, as a consequence of hemolysis, reduces nitric oxide bioavailability, promotes endothelial dysfunction, and contributes to the development of pulmonary hypertension and the varying presentations of vasoocclusion.6 A common observation among patients who experience hyperhemolysis syndrome is that withholding transfusion seems beneficial, probably because immunologic reactions are not exacerbated, whereas treatment with steroids1, 2, 4 and/or IVIG3, 7 resolves hemolysis because of their immunomodulatory effects.
CONCLUSIONS
Hyperhemolysis syndrome is a potentially life‐threatening complication of RBC transfusion. It is important to recognize this syndrome when managing patients with SCD who present with worsening anemia after RBC transfusions. Although further transfusions can exacerbate hemolysis4, 7 and may be relatively contraindicated, in severe and desperate situations, simultaneous treatment with steroids and IVIG, together with RBC transfusions, may be lifesaving.
- ,,,,.Delayed hemolytic transfusion reactions in sickle cell disease: simultaneous destruction of recipients' red cells.Transfusion.1997;37:376–381.
- ,,,,.The sickle cell hemolytic transfusion reaction syndrome.Transfusion.1997;37:382–392.
- ,,,,.Hyperhemolytic transfusion reaction in sickle cell disease.Transfusion.2001;41:323–328.
- ,,,,.Delayed hemolytic transfusion reaction/hyperhemolysis syndrome in children with sickle cell disease.Pediatrics.2003;111(6 Pt 1):e661–e665.
- ,.Hyperhemolysis syndrome in anemia of chronic disease.Transfusion.2005;45:1930–1933.
- and.Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia.Transfusion.2006;46:105–110.
- ,,,.Post‐transfusion hyperhemolysis in a patient with sickle cell disease: use of steroids and intravenous immunoglobulin to prevent further red cell destruction.Vox Sang.1995;69:355–357.
- ,,,,.Delayed hemolytic transfusion reactions in sickle cell disease: simultaneous destruction of recipients' red cells.Transfusion.1997;37:376–381.
- ,,,,.The sickle cell hemolytic transfusion reaction syndrome.Transfusion.1997;37:382–392.
- ,,,,.Hyperhemolytic transfusion reaction in sickle cell disease.Transfusion.2001;41:323–328.
- ,,,,.Delayed hemolytic transfusion reaction/hyperhemolysis syndrome in children with sickle cell disease.Pediatrics.2003;111(6 Pt 1):e661–e665.
- ,.Hyperhemolysis syndrome in anemia of chronic disease.Transfusion.2005;45:1930–1933.
- and.Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia.Transfusion.2006;46:105–110.
- ,,,.Post‐transfusion hyperhemolysis in a patient with sickle cell disease: use of steroids and intravenous immunoglobulin to prevent further red cell destruction.Vox Sang.1995;69:355–357.
Practice Patterns of Hospitalists and Community Physicians
The use of hospitalists, physicians who specialize in inpatient care, has seen a rapid expansion over the last decade.1 Several studies have shown that with hospitalists there is a shorter length of stay (LOS) and decreased utilization of resources and that hospitalists play a positive role in medical education.24 However, only a few studies have examined the specific strategies employed by hospitalists to achieve improved efficiency and outcomes.
Congestive heart failure (CHF) is the most common diagnosis of hospitalized patients older than age 65, with more Medicare spending devoted to patients with CHF than to any other diagnosis‐related group (DRG).5, 6 Over the last 2 decades hospital discharges for congestive heart failure increased by 165%.7 In addition, the rate of hospital readmission of patients with CHF remains high: 2%, 20%, and 50% within 2 days, 1 month, and 6 months, respectively.8
Several previous studies have shown that patients cared for by hospitalists had improved clinical outcomes. Meltzer et al. found that 30‐day mortality of hospitalists' patients was lower than that of non‐hospitalists' patients, 4.2% versus 6.0%, respectively, in the second year of implementation of a hospitalist program.3 A study by Huddleston et al. showed a reduction of 11.8% in the rate of complications experienced by postsurgical orthopedic patients with the involvement of hospitalists in their care in conjunction with the surgeons.4
Many previous studies have pointed to improvements in economic outcomes such as LOS and costs for patients followed by hospitalists. Kulaga et al. showed that patients cared for by hospitalists had reductions of approximately 20% in LOS and 18% in total costs per case compared with those cared for by community‐based physicians.2 Meltzer et al. found a decrease in the average adjusted LOS of 0.49 days in the second year of implementation of a hospitalist program.3 Rifkin et al. found that patients with pneumonia cared for by hospitalists had a mean adjusted LOS of 5.6 days versus 6.5 days for those cared for by non‐hospitalists.9
Few previous studies have looked at specific practice patterns of hospitalists that result in improved efficiency and better outcomes. Rifkin et al., who found that patients with pneumonia cared for by hospitalists had a shorter LOS, suggested this finding was a result of the earlier recognition by hospitalists that patients were stable and more rapid conversion to oral antibiotics.9 Likewise, Stein et al. found that community‐acquired pneumonia patients treated by hospitalists had a shorter LOS than those treated by non‐hospitalists. However, they were unable to assess the differences in patient management that led to this result because of the design of the study.10
Lindenauer et al. compared quality‐of‐care indicators and resource utilization for patients with congestive heart failure treated by hospitalists and non‐hospitalist general internists. They found that patients under the care of hospitalists had a shorter LOS than those cared for by general internists but that the overall costs of care were similar between the groups.11 They compared the quality indicators developed by the Joint Commission on Accreditation of Healthcare Organizations in the Core Measures Initiative, but did not focus on patterns of practices of hospitalists and nonhospitalists. Moreover, they did not look at full‐time hospitalists but focused on physicians who spent at least 25% of their practice caring for inpatients.
We sought to identify distinct, quantifiable practices of full‐time hospitalists in the management of their patients with CHF. We hypothesized that hospitalists would adhere more closely to the current congestive heart failure guidelines and would utilize available resources more judiciously, leading to improved clinical and economic outcomes. To identify these practices, we compared utilization of well‐established therapeutic and diagnostic modalities such as use of ACE‐I, ARB, and beta‐blockers; ordering of chest x‐rays; measurement of brain natriuretic peptide (BNP); and use of medical subspecialty consultants. We also compared standard clinical and economic outcomes such as in‐hospital mortality, readmission rate, LOS, and costs per case between hospitalists and community‐based physicians.
METHODS
Design and Setting
The study was a retrospective chart review of 447 patients treated for CHF from July 1, 2003, through June 30, 2004, at the Queen's Medical Center, a 505‐bed community‐based teaching hospital in Honolulu, Hawaii, and the leading medical referral center in the Pacific Basin. All patients had been cared for by either a community‐based physician or a hospitalist. The community‐based physicians (referred to as non‐hospitalists from here on) were a diverse group of internists and subspecialists, in solo or group practice, who provided inpatient and ambulatory care. The non‐hospitalist group included 119 cardiologists (55%), 83 general internists (38%), and 3 family practitioners (1%), with the other 6% made up of clinicians in the medical oncology, pediatrics, pulmonary, radiation oncology, and thoracic/cardiovascular surgery subspecialties.
The hospitalist group comprised 10 full‐time internists employed by the hospital who provided care for patients only in the inpatient setting and 3 part‐time hospitalists who practiced in the ambulatory setting in addition to providing inpatient night coverage for the group. During the study period, 2 hospitalists left the group, and 2 hospitalists were hired. On average the length of involvement of a full‐time hospitalist in the study was 9 months. Permission to conduct this study was granted by the Queen's Medical Center Institutional Review Board.
Patient Population
Patients were included in the study if they were admitted to Queen's Medical Center during the 18‐month study period, were at least 18 years old, and were coded on discharge by the medical records department with a principal diagnosis of congestive heart failure (International Classification of Diseases, 9th Revision, codes 428, 428.1, 428.9, 402.01, 402.11, 402.91, 404.01, 404.11, and 404.91). Baseline characteristics of patients collected were age, sex, insurance status, comorbidities, and code status on admission. Comorbidities included coronary artery disease, diabetes mellitus (type 1 or 2), hypertension, chronic renal insufficiency (creatinine > 2 mg/dL), and chronic obstructive pulmonary disease (COPD). Patients were excluded if they had initially been admitted to the medical intensive care unit, required ventilatory support, had end‐stage renal disease requiring hemodialysis, or had an LOS greater than 14 days.
Data Collection
Medical records were reviewed by research nurses not directly involved with the hospitalist group. Training to ensure high‐level reliability of data collection was provided, and reliability was verified by the primary author (M.M.R.). The following data were collected: use of ACE‐I, ARB, and beta‐blockers on admission and discharge; use of intravenous and oral diuretics; time to switch to oral diuretic; rates of utilization of medical consultants, physical therapy, dietary consults, social work, and sodium and fluid restriction; and number of repeat chest radiographs, echocardiograms, and BNP measurements. These criteria were developed based on ACC/AHA 2005 guidelines for diagnosis and management of congestive heart failure in adults,11 several studies delineating the importance of initiating therapy in the inpatient setting, and the experience of the Cardiovascular Hospital Atherosclerosis Management Program (CHAMP) for patients with established coronary artery disease.1315 Data on medical resident involvement in patient care were collected for hospitalists and non‐hospitalists.
Additional outcomes included in‐hospital mortality, rate of acute renal failure, readmission rate, LOS, expense, revenue, and margin per case. Acute renal failure was defined as a doubling of the admission creatinine value. The rate of readmissiondefined as readmission to Queen's Medical Center for any reasonwas evaluated after 7, 14, and 30 days and was stratified further for readmissions for CHF. Expense was defined as costs directly related to patient care plus costs related to operating a hospital facility. Revenue was defined as the compensation the hospital expected to collect for service rendered adjusted for bad debt/charity care. Margin was defined as revenue minus expense.
Data Analysis
Descriptive statistics are reported for baseline patient characteristics (age, sex, insurance status, etc.), quality‐of‐care measures (ACE‐I, ARB, diuretic, and beta‐blocker use, time to oral diuretic, etc.), and outcome measures (readmission rate, in‐hospital mortality, LOS, cost data) using frequencies and proportions for categorical variables (eg, sex, ethnicity, insurance status), means and standard deviations (SDs) for continuous variables (age), and medians and interquartile ranges (Q1‐Q3) for skewed variables (eg, LOS, cost data). The patients cared for by hospitalists were compared with those cared for by non‐hospitalists using the chi‐square test or Fisher's exact test for categorical data and the Student t test for continuous data. All‐Payer Severity‐adjusted Diagnosis Related Groups (APS‐DRGs) were used to control for severity of patient illness. The severity of illness codes were taken from 3M APR Benchmarking software for DRGs adjusted for severity of illness and risk of mortality. 3M defined severity of illness as the extent of physiologic decompensation or organ system loss of function. Each diagnosis was assigned 1 of 4 severity levels: minor, moderate, major, or extreme. Kruskal‐Wallis analysis of covariance was used for LOS and cost outcomes, adjusting for age, insurance status, comorbidities, and severity of illness. Multivariate logistic regression was performed for binary outcomes (eg, ACE‐I, ARB, beta‐blocker use) to adjust for confounding variables. Statistical analysis was performed using SAS version 9 (SAS Institute Inc., Cary, NC). All tests were 2‐sided, and differences with a P value < .05 were considered significant.
RESULTS
Patient Characteristics
Table 1 shows the patient characteristic data. There were 447 admissions for congestive heart failure during the study period, 342 of which met study inclusion criteria. Hospitalists provided care for 126 of these patients and non‐hospitalists for 216 patients. Mean age of patients in the hospitalist and nonhospitalist groups was 63 and 73 years, respectively. There were significant differences in insurance status, with hospitalists more frequently caring for patients covered by Medicaid (26% vs. 7%; P < .001) and patients who were uninsured (6% vs. 1%; P = .04). Patients cared for by hospitalists had a lower incidence of coronary artery disease (42% vs. 59%; P = .003) and prior CHF (44% vs. 56%; P = .05). The hospitalists' patients were more likely to have a full resuscitation code status on admission; however, this difference did not reach statistical significance (90% vs. 81%; P = .07). There were no significant differences between patients cared for by hospitalists and non‐hospitalists in sex, ethnic background, other comorbidities, or house staff involvement.
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Age (years, mean SD) | 73 15 | 63 16 | < .001 |
| Male sex | 124 (57) | 78 (62) | .41 |
| Caucasian ethnicity | 41 (19) | 30 (24) | .29 |
| Insurance status | |||
| Medicare | 119 (55) | 58 (46) | .11 |
| Medicaid/Quest | 16 (7) | 33 (26) | < .001 |
| HMSA | 68 (31) | 19 (15) | < .001 |
| Self‐pay | 3 (1) | 7 (6) | .04 |
| Other | 10(5) | 9(7) | .33 |
| Comorbidy | |||
| CAD | 127 (59) | 53 (42) | .003 |
| DM | 78 (36) | 53 (4) | .27 |
| HTN | 139 (64) | 80 (63) | .87 |
| CRI | 43 (20) | 28 (22) | .61 |
| COPD | 30 (14) | 26 (21) | .10 |
| Prior CHF | 120 (56) | 56 (44) | .05 |
| Full code | 174 (81) | 113 (90) | .07 |
| House staff involvement | 42 (19) | 20 (16) | .41 |
Practice Patterns and Resource Utilization
Practice patterns and resource utilization are shown in Table 2. Hospitalists used more ACE‐I/ARBs, with 86% of patients receiving these interventions within 24 hours of admission versus 72% of the patients of non‐hospitalists (adjusted P = .001). Hospitalists treated fewer patients with beta‐blockers on admission and on discharge and more patients with intravenous diuretics (90% vs. 73%; adjusted P = .001). The rate of beta‐blocker use did not change significantly after controlling for patients with COPD (data not shown).
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value* | |
|---|---|---|---|
| |||
| ACE‐I/ARB within 24 hours | 155 (72) | 108 (86) | .001 |
| Beta‐blocker within 24 hours | 119 (55) | 50 (40) | .004 |
| ACE‐I/ARB at discharge | 147 (69) | 95 (75) | .24 |
| Beta‐blocker at discharge | 116 (54) | 52 (41) | .03 |
| Echocardiogram 1 | 125 (58) | 81 (64) | .50 |
| MD Consultants 2 | 35 (16) | 10 (8) | .01 |
| Chest x‐ray 2 | 27 (13) | 5 (4) | .02 |
| BNP 1 | 128 (59) | 95 (75) | .005 |
| BNP > 1 | 22 (10) | 7 (6) | .14 |
| Physical therapy | 35 (16) | 17 (13) | .48 |
| Dietary consult | 29 (13) | 19 (15) | .67 |
| Social work | 62 (29) | 60 (48) | .003 |
| Sodium restriction | 184 (85) | 102 (81) | .31 |
| Fluid restriction | 47 (22) | 35 (28) | .21 |
| IV diuretic | 158 (73) | 114 (90) | .001 |
| Time to oral diuretic (days), median (Q1,Q3) | 1 (1, 3) | 1 (0, 2) | .30 |
Hospitalists were less likely to obtain 2 or more chest x‐rays (4% vs. 13%; adjusted P = .02) or to obtain 2 or more medical consultations (8% vs. 16%; adjusted P = .01). In addition, they obtained more initial measurements of BNP; however, there was a trend toward fewer repeat BNP measurements (6% vs. 10%; P = .14). There was a significantly higher rate of social work utilization by hospitalists than by nonhospitalists (48% vs. 29%; adjusted P = .003). There were no differences between the groups in the rates of obtaining echocardiograms, physical therapy, and dietary consults or in sodium and fluid restrictions.
Outcomes
Significant differences were noted in LOS and cost outcomes between hospitalists and non‐hospitalists after adjusting for age, insurance status, comorbidities, and severity of illness (Tables 3 and 4). Patients cared for by hospitalists had a shorter overall LOS than did patients cared for by non‐hospitalists (adjusted P = .002). A shorter LOS was noted for patients in the minor (median 3 vs. 5 days), moderate (median 4 vs. 5 days), and extreme (7 vs. 8 days) severity categories. Overall adjusted expense was significantly lower for the care of hospitalists' patients across all severity categories (P < .001). There was a trend toward lower adjusted revenue for patients of hospitalists than those of non‐hospitalist (P = .06). The adjusted profit margin did not significantly differ between the groups (P =.14).
| Severity | Nonhospitalist cases (n = 216) | Hospitalist cases (n = 126) | P value | |
|---|---|---|---|---|
| ||||
| Severity (%) | Minor | 40 (19) | 30 (24) | .13 |
| Moderate | 99 (46) | 64 (51) | ||
| Major | 72 (33) | 27 (21) | ||
| Extreme | 5 (2) | 4 (3) | ||
| LOS (days) | Minor | 5 (3, 6) | 3 (2, 4) | .002 |
| Moderate | 5 (3, 7) | 4 (3, 6) | ||
| Major | 6 (4,10) | 6 (4, 10) | ||
| Extreme | 8 (2, 8) | 7 (6, 8) | ||
| Expense ($) | Minor | 5792 (4414, 6715) | 4164 (2401, 5499) | < .001 |
| Moderate | 6953 (4273, 10,224) | 5951 (4301, 8621) | ||
| Major | 13,622 (8219, 28,553) | 10,519 (5249, 15,581) | ||
| Extreme | 18,908 (12913, 24,688) | 16,192 (6135, 26,147) | ||
| Revenue ($) | Minor | 7095 (6611, 7212) | 7116 (4160, 7218) | .06 |
| Moderate | 7118 (7025, 7215) | 6893 (3755, 7164) | ||
| Major | 9601 (6972, 16,668) | 6743 (4612, 7116) | ||
| Extreme | 11,019 (10,009, 24,897) | 9184 (5783, 13,931) | ||
| Margin ($) | Minor | 786 (162, 2997) | 2290 (409, 4768) | .14 |
| Moderate | 256 (1999, 3366) | 796 (2741, 1565) | ||
| Major | 2314 (7870, 1448) | 3499 (8818, 1008) | ||
| Extreme | 1263 (2904, 4012) | 6537 (15,617, 3050) | ||
| Nonhospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Acute renal failure | 2 (1) | 0 (0) | 0.53 |
| In‐hospital mortality | 9 (4) | 0 (0) | 0.03 |
| Readmission for any reason | 53 (25) | 35 (28) | 0.52* |
| Readmission for CHF | 19 (9) | 18 (14) | 0.16* |
In‐hospital mortality of patients treated by hospitalists was lower than that of non‐hospitalist‐treated patients (0% vs. 4%; P =.03). Rates of acute renal failure, overall readmissions and readmissions specifically for congestive heart failure did not differ significantly. Notably, severity of illness assessed by APS‐DRG did not differ between hospitalists' and nonhospitalists' patients (P = .13).
DISCUSSION
Practice Patterns
Our study identified specific practices that hospitalists use more than non‐hospitalists in the management of patients with CHF. These practices, which may have resulted in decreased LOS and lower costs, included higher use of ACE‐I/ARB within 24 hours of admission and of intravenous diuretics. We hypothesized that earlier and more aggressive use of ACE‐I/ARB contributed to after‐load reduction and alteration of cardiac remodeling5 and may have led to faster recovery and improved outcomes. Greater use of intravenous diuretics may signify that hospitalists have a more aggressive approach to managing exacerbations of acute congestive heart failure, which may also lead to faster recovery.
Hospitalists used fewer beta‐blockers on admission and at discharge. Reasons for this finding remain unclear; however, it may have been a result of the practice of avoiding beta‐blockers during exacerbations of acute CHF and the subsequent reliance on primary care providers to restart beta‐blockers after discharge. Lower use of beta‐blockers did not appear to have a negative impact on mortality or readmission rates.
Resource Utilization
Hospitalists used fewer serial chest x‐rays, more initial BNP measurements, and more social work consults, and there was a trend toward their using fewer repeat BNP measurements. The less frequent use of serial chest x‐rays may be a result of hospitalists being able to assess patients more frequently and to rely less on imaging. Higher rates of initial BNP measurement by hospitalists may reflect the ordering patterns of the emergency room physicians because most patients are admitted to the hospitalists via the emergency room. The trend toward fewer repeat BNP measurements by hospitalists may again reflect their ability to perform more frequent clinical assessments and to rely less on laboratory data. The higher rate of utilization of social workers by hospitalists is likely a reflection of a population in need of such interventions rather than the hospitalists having a lower threshold before requesting a social work consultation. There were no differences in the rates of obtaining echocardiograms, physical therapy, and dietary consults and of sodium and fluid restrictions.
Clinical Outcomes
Severity of illness assessed by APS‐DRG did not differ between the patients cared for by hospitalists and those care for by non‐hospitalists (P = .13) despite the hospitalists caring for a younger population. In‐hospital mortality of hospitalist‐treated patients was lower (0% vs. 4%), whereas the rates of readmission and renal failure did not differ between the 2 groups. A slight advantage in the mortality rate appears to be in agreement with prior findings3, 4; however, this may have been a result of the non‐hospitalists caring for an older patient population.
Economic Outcomes
The shorter LOS and lower overall costs of patients followed by hospitalists supports previous findings.2, 3, 10 The LOS in our study was found to be shorter for hospitalist‐treated patients whose illnesses were in the minor, moderate, and extreme severity categories by 40%, 20%, and 13%, respectively. The median expense per case was less across all severity categories, ranging from $1000 to $3100 for the patients followed by hospitalists compared with those followed by non‐hospitalists. There was a trend toward lower adjusted median revenue in all categories except for minor severity for hospitalists' patients (P = .06). The profit margin per case did not differ significantly between patients cared for by hospitalists and non‐hospitalists. The shorter LOS and lower expenses per case of patients under the care of hospitalists should have led to higher revenue and profit margin. However, our study showed lower revenue and no significant differences in profit margin, which may be explained by the fact that the hospitalists' patients had a worse insurance mix with a higher proportion of uninsured and Medicaid patients. It is also possible that non‐hospitalists, in particular, cardiologists, generate higher revenue by performing more procedures such as cardiac catheterizations, thus offsetting the costs.
As noted above, the analysis of LOS, expenses, revenue, and margin controlled for age, comorbidities, severity of illness, and insurance status (Table 3). The results were not significantly affected by adjusting for age, insurance status, and comorbidities after controlling for severity. The difference in age may in part be a result of older patients having established relationships with primary care physicians and being less likely to be admitted by hospitalists. It may also reflect the high prevalence of methamphetamine abuse, which has reached epidemic proportions in Hawaii, and methamphetamine‐induced cardiomyopathy in a younger population of patients followed by hospitalists. Further studies would be necessary to estimate the impact of drug‐induced congestive heart failure in these populations.
Although our study provided a detailed look at practice patterns of a coherent hospitalist group, it had several important limitations. It was a retrospective study conducted at a single institution, making the findings difficult to generalize to hospitalist practices nationwide. It included an unusually large number of non‐Caucasian patients, reflecting the demographics of the state of Hawaii. Data on contraindications to ACE‐I/ARB were not collected because the degree of renal dysfunction that would serve as a contraindication was difficult to define. The primary mode of adjustment was APS, which may have been a limiting factor in assessing severity of illness. The inability to follow patients' course after discharge limited collection of long‐term outcomes data.
In agreement with previous studies, we showed a decreased LOS and lower expenses per case of patients cared for by full‐time hospitalists while preserving quality of care and improving clinical outcomes. We identified specific practices of hospitalists in the management of patients with CHF that differ from those of non‐hospitalists. These practices include early use of ACE‐I/ARB, aggressive approach to diuresis, higher utilization of social work services, and decreased utilization of serial chest x‐rays, medical consultants, and serial BNP measurements. Our study was not designed to identify a direct causal relationship between hospitalist practices and improved outcomes; however, we believe it to be the first step in understanding practice patterns and the impact of the hospitalist movement.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin North Am.2002;86:797–823.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,.Advances in the management of acute and chronic decompensated heart failure.Lippincotts Case Manag.2004;9:S1–S15.
- ,,,, et al.ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult.Circulation.2001;104:2996–3007.
- American Heart Association.Heart disease and stroke statistics—2003 update.2003.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl):S3–S9.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,,.Economic effects of community versus hospital‐based faculty pneumonia care.J Gen Intern Med.1998;13:774–777.
- ,,,,.Quality of care for patients hospitalized with heart failure. Assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in adult.ACC/AHA Pract Guidel.2005:1–82
- ,,.Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure—a review.Am J Cardiol.2004;94:1155–1160.
- .Role of in‐hospital initiation of carvedilol to improve treatment rates and clinical outcomes.Am J Cardiol.2004;93(suppl):77B–81B.
- ,.Rationale and design of the Cardiac Hospitalization Atherosclerosis Management Program at the University of California Los Angeles.Am J Cardiol.2000;85:10A–17A.
The use of hospitalists, physicians who specialize in inpatient care, has seen a rapid expansion over the last decade.1 Several studies have shown that with hospitalists there is a shorter length of stay (LOS) and decreased utilization of resources and that hospitalists play a positive role in medical education.24 However, only a few studies have examined the specific strategies employed by hospitalists to achieve improved efficiency and outcomes.
Congestive heart failure (CHF) is the most common diagnosis of hospitalized patients older than age 65, with more Medicare spending devoted to patients with CHF than to any other diagnosis‐related group (DRG).5, 6 Over the last 2 decades hospital discharges for congestive heart failure increased by 165%.7 In addition, the rate of hospital readmission of patients with CHF remains high: 2%, 20%, and 50% within 2 days, 1 month, and 6 months, respectively.8
Several previous studies have shown that patients cared for by hospitalists had improved clinical outcomes. Meltzer et al. found that 30‐day mortality of hospitalists' patients was lower than that of non‐hospitalists' patients, 4.2% versus 6.0%, respectively, in the second year of implementation of a hospitalist program.3 A study by Huddleston et al. showed a reduction of 11.8% in the rate of complications experienced by postsurgical orthopedic patients with the involvement of hospitalists in their care in conjunction with the surgeons.4
Many previous studies have pointed to improvements in economic outcomes such as LOS and costs for patients followed by hospitalists. Kulaga et al. showed that patients cared for by hospitalists had reductions of approximately 20% in LOS and 18% in total costs per case compared with those cared for by community‐based physicians.2 Meltzer et al. found a decrease in the average adjusted LOS of 0.49 days in the second year of implementation of a hospitalist program.3 Rifkin et al. found that patients with pneumonia cared for by hospitalists had a mean adjusted LOS of 5.6 days versus 6.5 days for those cared for by non‐hospitalists.9
Few previous studies have looked at specific practice patterns of hospitalists that result in improved efficiency and better outcomes. Rifkin et al., who found that patients with pneumonia cared for by hospitalists had a shorter LOS, suggested this finding was a result of the earlier recognition by hospitalists that patients were stable and more rapid conversion to oral antibiotics.9 Likewise, Stein et al. found that community‐acquired pneumonia patients treated by hospitalists had a shorter LOS than those treated by non‐hospitalists. However, they were unable to assess the differences in patient management that led to this result because of the design of the study.10
Lindenauer et al. compared quality‐of‐care indicators and resource utilization for patients with congestive heart failure treated by hospitalists and non‐hospitalist general internists. They found that patients under the care of hospitalists had a shorter LOS than those cared for by general internists but that the overall costs of care were similar between the groups.11 They compared the quality indicators developed by the Joint Commission on Accreditation of Healthcare Organizations in the Core Measures Initiative, but did not focus on patterns of practices of hospitalists and nonhospitalists. Moreover, they did not look at full‐time hospitalists but focused on physicians who spent at least 25% of their practice caring for inpatients.
We sought to identify distinct, quantifiable practices of full‐time hospitalists in the management of their patients with CHF. We hypothesized that hospitalists would adhere more closely to the current congestive heart failure guidelines and would utilize available resources more judiciously, leading to improved clinical and economic outcomes. To identify these practices, we compared utilization of well‐established therapeutic and diagnostic modalities such as use of ACE‐I, ARB, and beta‐blockers; ordering of chest x‐rays; measurement of brain natriuretic peptide (BNP); and use of medical subspecialty consultants. We also compared standard clinical and economic outcomes such as in‐hospital mortality, readmission rate, LOS, and costs per case between hospitalists and community‐based physicians.
METHODS
Design and Setting
The study was a retrospective chart review of 447 patients treated for CHF from July 1, 2003, through June 30, 2004, at the Queen's Medical Center, a 505‐bed community‐based teaching hospital in Honolulu, Hawaii, and the leading medical referral center in the Pacific Basin. All patients had been cared for by either a community‐based physician or a hospitalist. The community‐based physicians (referred to as non‐hospitalists from here on) were a diverse group of internists and subspecialists, in solo or group practice, who provided inpatient and ambulatory care. The non‐hospitalist group included 119 cardiologists (55%), 83 general internists (38%), and 3 family practitioners (1%), with the other 6% made up of clinicians in the medical oncology, pediatrics, pulmonary, radiation oncology, and thoracic/cardiovascular surgery subspecialties.
The hospitalist group comprised 10 full‐time internists employed by the hospital who provided care for patients only in the inpatient setting and 3 part‐time hospitalists who practiced in the ambulatory setting in addition to providing inpatient night coverage for the group. During the study period, 2 hospitalists left the group, and 2 hospitalists were hired. On average the length of involvement of a full‐time hospitalist in the study was 9 months. Permission to conduct this study was granted by the Queen's Medical Center Institutional Review Board.
Patient Population
Patients were included in the study if they were admitted to Queen's Medical Center during the 18‐month study period, were at least 18 years old, and were coded on discharge by the medical records department with a principal diagnosis of congestive heart failure (International Classification of Diseases, 9th Revision, codes 428, 428.1, 428.9, 402.01, 402.11, 402.91, 404.01, 404.11, and 404.91). Baseline characteristics of patients collected were age, sex, insurance status, comorbidities, and code status on admission. Comorbidities included coronary artery disease, diabetes mellitus (type 1 or 2), hypertension, chronic renal insufficiency (creatinine > 2 mg/dL), and chronic obstructive pulmonary disease (COPD). Patients were excluded if they had initially been admitted to the medical intensive care unit, required ventilatory support, had end‐stage renal disease requiring hemodialysis, or had an LOS greater than 14 days.
Data Collection
Medical records were reviewed by research nurses not directly involved with the hospitalist group. Training to ensure high‐level reliability of data collection was provided, and reliability was verified by the primary author (M.M.R.). The following data were collected: use of ACE‐I, ARB, and beta‐blockers on admission and discharge; use of intravenous and oral diuretics; time to switch to oral diuretic; rates of utilization of medical consultants, physical therapy, dietary consults, social work, and sodium and fluid restriction; and number of repeat chest radiographs, echocardiograms, and BNP measurements. These criteria were developed based on ACC/AHA 2005 guidelines for diagnosis and management of congestive heart failure in adults,11 several studies delineating the importance of initiating therapy in the inpatient setting, and the experience of the Cardiovascular Hospital Atherosclerosis Management Program (CHAMP) for patients with established coronary artery disease.1315 Data on medical resident involvement in patient care were collected for hospitalists and non‐hospitalists.
Additional outcomes included in‐hospital mortality, rate of acute renal failure, readmission rate, LOS, expense, revenue, and margin per case. Acute renal failure was defined as a doubling of the admission creatinine value. The rate of readmissiondefined as readmission to Queen's Medical Center for any reasonwas evaluated after 7, 14, and 30 days and was stratified further for readmissions for CHF. Expense was defined as costs directly related to patient care plus costs related to operating a hospital facility. Revenue was defined as the compensation the hospital expected to collect for service rendered adjusted for bad debt/charity care. Margin was defined as revenue minus expense.
Data Analysis
Descriptive statistics are reported for baseline patient characteristics (age, sex, insurance status, etc.), quality‐of‐care measures (ACE‐I, ARB, diuretic, and beta‐blocker use, time to oral diuretic, etc.), and outcome measures (readmission rate, in‐hospital mortality, LOS, cost data) using frequencies and proportions for categorical variables (eg, sex, ethnicity, insurance status), means and standard deviations (SDs) for continuous variables (age), and medians and interquartile ranges (Q1‐Q3) for skewed variables (eg, LOS, cost data). The patients cared for by hospitalists were compared with those cared for by non‐hospitalists using the chi‐square test or Fisher's exact test for categorical data and the Student t test for continuous data. All‐Payer Severity‐adjusted Diagnosis Related Groups (APS‐DRGs) were used to control for severity of patient illness. The severity of illness codes were taken from 3M APR Benchmarking software for DRGs adjusted for severity of illness and risk of mortality. 3M defined severity of illness as the extent of physiologic decompensation or organ system loss of function. Each diagnosis was assigned 1 of 4 severity levels: minor, moderate, major, or extreme. Kruskal‐Wallis analysis of covariance was used for LOS and cost outcomes, adjusting for age, insurance status, comorbidities, and severity of illness. Multivariate logistic regression was performed for binary outcomes (eg, ACE‐I, ARB, beta‐blocker use) to adjust for confounding variables. Statistical analysis was performed using SAS version 9 (SAS Institute Inc., Cary, NC). All tests were 2‐sided, and differences with a P value < .05 were considered significant.
RESULTS
Patient Characteristics
Table 1 shows the patient characteristic data. There were 447 admissions for congestive heart failure during the study period, 342 of which met study inclusion criteria. Hospitalists provided care for 126 of these patients and non‐hospitalists for 216 patients. Mean age of patients in the hospitalist and nonhospitalist groups was 63 and 73 years, respectively. There were significant differences in insurance status, with hospitalists more frequently caring for patients covered by Medicaid (26% vs. 7%; P < .001) and patients who were uninsured (6% vs. 1%; P = .04). Patients cared for by hospitalists had a lower incidence of coronary artery disease (42% vs. 59%; P = .003) and prior CHF (44% vs. 56%; P = .05). The hospitalists' patients were more likely to have a full resuscitation code status on admission; however, this difference did not reach statistical significance (90% vs. 81%; P = .07). There were no significant differences between patients cared for by hospitalists and non‐hospitalists in sex, ethnic background, other comorbidities, or house staff involvement.
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Age (years, mean SD) | 73 15 | 63 16 | < .001 |
| Male sex | 124 (57) | 78 (62) | .41 |
| Caucasian ethnicity | 41 (19) | 30 (24) | .29 |
| Insurance status | |||
| Medicare | 119 (55) | 58 (46) | .11 |
| Medicaid/Quest | 16 (7) | 33 (26) | < .001 |
| HMSA | 68 (31) | 19 (15) | < .001 |
| Self‐pay | 3 (1) | 7 (6) | .04 |
| Other | 10(5) | 9(7) | .33 |
| Comorbidy | |||
| CAD | 127 (59) | 53 (42) | .003 |
| DM | 78 (36) | 53 (4) | .27 |
| HTN | 139 (64) | 80 (63) | .87 |
| CRI | 43 (20) | 28 (22) | .61 |
| COPD | 30 (14) | 26 (21) | .10 |
| Prior CHF | 120 (56) | 56 (44) | .05 |
| Full code | 174 (81) | 113 (90) | .07 |
| House staff involvement | 42 (19) | 20 (16) | .41 |
Practice Patterns and Resource Utilization
Practice patterns and resource utilization are shown in Table 2. Hospitalists used more ACE‐I/ARBs, with 86% of patients receiving these interventions within 24 hours of admission versus 72% of the patients of non‐hospitalists (adjusted P = .001). Hospitalists treated fewer patients with beta‐blockers on admission and on discharge and more patients with intravenous diuretics (90% vs. 73%; adjusted P = .001). The rate of beta‐blocker use did not change significantly after controlling for patients with COPD (data not shown).
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value* | |
|---|---|---|---|
| |||
| ACE‐I/ARB within 24 hours | 155 (72) | 108 (86) | .001 |
| Beta‐blocker within 24 hours | 119 (55) | 50 (40) | .004 |
| ACE‐I/ARB at discharge | 147 (69) | 95 (75) | .24 |
| Beta‐blocker at discharge | 116 (54) | 52 (41) | .03 |
| Echocardiogram 1 | 125 (58) | 81 (64) | .50 |
| MD Consultants 2 | 35 (16) | 10 (8) | .01 |
| Chest x‐ray 2 | 27 (13) | 5 (4) | .02 |
| BNP 1 | 128 (59) | 95 (75) | .005 |
| BNP > 1 | 22 (10) | 7 (6) | .14 |
| Physical therapy | 35 (16) | 17 (13) | .48 |
| Dietary consult | 29 (13) | 19 (15) | .67 |
| Social work | 62 (29) | 60 (48) | .003 |
| Sodium restriction | 184 (85) | 102 (81) | .31 |
| Fluid restriction | 47 (22) | 35 (28) | .21 |
| IV diuretic | 158 (73) | 114 (90) | .001 |
| Time to oral diuretic (days), median (Q1,Q3) | 1 (1, 3) | 1 (0, 2) | .30 |
Hospitalists were less likely to obtain 2 or more chest x‐rays (4% vs. 13%; adjusted P = .02) or to obtain 2 or more medical consultations (8% vs. 16%; adjusted P = .01). In addition, they obtained more initial measurements of BNP; however, there was a trend toward fewer repeat BNP measurements (6% vs. 10%; P = .14). There was a significantly higher rate of social work utilization by hospitalists than by nonhospitalists (48% vs. 29%; adjusted P = .003). There were no differences between the groups in the rates of obtaining echocardiograms, physical therapy, and dietary consults or in sodium and fluid restrictions.
Outcomes
Significant differences were noted in LOS and cost outcomes between hospitalists and non‐hospitalists after adjusting for age, insurance status, comorbidities, and severity of illness (Tables 3 and 4). Patients cared for by hospitalists had a shorter overall LOS than did patients cared for by non‐hospitalists (adjusted P = .002). A shorter LOS was noted for patients in the minor (median 3 vs. 5 days), moderate (median 4 vs. 5 days), and extreme (7 vs. 8 days) severity categories. Overall adjusted expense was significantly lower for the care of hospitalists' patients across all severity categories (P < .001). There was a trend toward lower adjusted revenue for patients of hospitalists than those of non‐hospitalist (P = .06). The adjusted profit margin did not significantly differ between the groups (P =.14).
| Severity | Nonhospitalist cases (n = 216) | Hospitalist cases (n = 126) | P value | |
|---|---|---|---|---|
| ||||
| Severity (%) | Minor | 40 (19) | 30 (24) | .13 |
| Moderate | 99 (46) | 64 (51) | ||
| Major | 72 (33) | 27 (21) | ||
| Extreme | 5 (2) | 4 (3) | ||
| LOS (days) | Minor | 5 (3, 6) | 3 (2, 4) | .002 |
| Moderate | 5 (3, 7) | 4 (3, 6) | ||
| Major | 6 (4,10) | 6 (4, 10) | ||
| Extreme | 8 (2, 8) | 7 (6, 8) | ||
| Expense ($) | Minor | 5792 (4414, 6715) | 4164 (2401, 5499) | < .001 |
| Moderate | 6953 (4273, 10,224) | 5951 (4301, 8621) | ||
| Major | 13,622 (8219, 28,553) | 10,519 (5249, 15,581) | ||
| Extreme | 18,908 (12913, 24,688) | 16,192 (6135, 26,147) | ||
| Revenue ($) | Minor | 7095 (6611, 7212) | 7116 (4160, 7218) | .06 |
| Moderate | 7118 (7025, 7215) | 6893 (3755, 7164) | ||
| Major | 9601 (6972, 16,668) | 6743 (4612, 7116) | ||
| Extreme | 11,019 (10,009, 24,897) | 9184 (5783, 13,931) | ||
| Margin ($) | Minor | 786 (162, 2997) | 2290 (409, 4768) | .14 |
| Moderate | 256 (1999, 3366) | 796 (2741, 1565) | ||
| Major | 2314 (7870, 1448) | 3499 (8818, 1008) | ||
| Extreme | 1263 (2904, 4012) | 6537 (15,617, 3050) | ||
| Nonhospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Acute renal failure | 2 (1) | 0 (0) | 0.53 |
| In‐hospital mortality | 9 (4) | 0 (0) | 0.03 |
| Readmission for any reason | 53 (25) | 35 (28) | 0.52* |
| Readmission for CHF | 19 (9) | 18 (14) | 0.16* |
In‐hospital mortality of patients treated by hospitalists was lower than that of non‐hospitalist‐treated patients (0% vs. 4%; P =.03). Rates of acute renal failure, overall readmissions and readmissions specifically for congestive heart failure did not differ significantly. Notably, severity of illness assessed by APS‐DRG did not differ between hospitalists' and nonhospitalists' patients (P = .13).
DISCUSSION
Practice Patterns
Our study identified specific practices that hospitalists use more than non‐hospitalists in the management of patients with CHF. These practices, which may have resulted in decreased LOS and lower costs, included higher use of ACE‐I/ARB within 24 hours of admission and of intravenous diuretics. We hypothesized that earlier and more aggressive use of ACE‐I/ARB contributed to after‐load reduction and alteration of cardiac remodeling5 and may have led to faster recovery and improved outcomes. Greater use of intravenous diuretics may signify that hospitalists have a more aggressive approach to managing exacerbations of acute congestive heart failure, which may also lead to faster recovery.
Hospitalists used fewer beta‐blockers on admission and at discharge. Reasons for this finding remain unclear; however, it may have been a result of the practice of avoiding beta‐blockers during exacerbations of acute CHF and the subsequent reliance on primary care providers to restart beta‐blockers after discharge. Lower use of beta‐blockers did not appear to have a negative impact on mortality or readmission rates.
Resource Utilization
Hospitalists used fewer serial chest x‐rays, more initial BNP measurements, and more social work consults, and there was a trend toward their using fewer repeat BNP measurements. The less frequent use of serial chest x‐rays may be a result of hospitalists being able to assess patients more frequently and to rely less on imaging. Higher rates of initial BNP measurement by hospitalists may reflect the ordering patterns of the emergency room physicians because most patients are admitted to the hospitalists via the emergency room. The trend toward fewer repeat BNP measurements by hospitalists may again reflect their ability to perform more frequent clinical assessments and to rely less on laboratory data. The higher rate of utilization of social workers by hospitalists is likely a reflection of a population in need of such interventions rather than the hospitalists having a lower threshold before requesting a social work consultation. There were no differences in the rates of obtaining echocardiograms, physical therapy, and dietary consults and of sodium and fluid restrictions.
Clinical Outcomes
Severity of illness assessed by APS‐DRG did not differ between the patients cared for by hospitalists and those care for by non‐hospitalists (P = .13) despite the hospitalists caring for a younger population. In‐hospital mortality of hospitalist‐treated patients was lower (0% vs. 4%), whereas the rates of readmission and renal failure did not differ between the 2 groups. A slight advantage in the mortality rate appears to be in agreement with prior findings3, 4; however, this may have been a result of the non‐hospitalists caring for an older patient population.
Economic Outcomes
The shorter LOS and lower overall costs of patients followed by hospitalists supports previous findings.2, 3, 10 The LOS in our study was found to be shorter for hospitalist‐treated patients whose illnesses were in the minor, moderate, and extreme severity categories by 40%, 20%, and 13%, respectively. The median expense per case was less across all severity categories, ranging from $1000 to $3100 for the patients followed by hospitalists compared with those followed by non‐hospitalists. There was a trend toward lower adjusted median revenue in all categories except for minor severity for hospitalists' patients (P = .06). The profit margin per case did not differ significantly between patients cared for by hospitalists and non‐hospitalists. The shorter LOS and lower expenses per case of patients under the care of hospitalists should have led to higher revenue and profit margin. However, our study showed lower revenue and no significant differences in profit margin, which may be explained by the fact that the hospitalists' patients had a worse insurance mix with a higher proportion of uninsured and Medicaid patients. It is also possible that non‐hospitalists, in particular, cardiologists, generate higher revenue by performing more procedures such as cardiac catheterizations, thus offsetting the costs.
As noted above, the analysis of LOS, expenses, revenue, and margin controlled for age, comorbidities, severity of illness, and insurance status (Table 3). The results were not significantly affected by adjusting for age, insurance status, and comorbidities after controlling for severity. The difference in age may in part be a result of older patients having established relationships with primary care physicians and being less likely to be admitted by hospitalists. It may also reflect the high prevalence of methamphetamine abuse, which has reached epidemic proportions in Hawaii, and methamphetamine‐induced cardiomyopathy in a younger population of patients followed by hospitalists. Further studies would be necessary to estimate the impact of drug‐induced congestive heart failure in these populations.
Although our study provided a detailed look at practice patterns of a coherent hospitalist group, it had several important limitations. It was a retrospective study conducted at a single institution, making the findings difficult to generalize to hospitalist practices nationwide. It included an unusually large number of non‐Caucasian patients, reflecting the demographics of the state of Hawaii. Data on contraindications to ACE‐I/ARB were not collected because the degree of renal dysfunction that would serve as a contraindication was difficult to define. The primary mode of adjustment was APS, which may have been a limiting factor in assessing severity of illness. The inability to follow patients' course after discharge limited collection of long‐term outcomes data.
In agreement with previous studies, we showed a decreased LOS and lower expenses per case of patients cared for by full‐time hospitalists while preserving quality of care and improving clinical outcomes. We identified specific practices of hospitalists in the management of patients with CHF that differ from those of non‐hospitalists. These practices include early use of ACE‐I/ARB, aggressive approach to diuresis, higher utilization of social work services, and decreased utilization of serial chest x‐rays, medical consultants, and serial BNP measurements. Our study was not designed to identify a direct causal relationship between hospitalist practices and improved outcomes; however, we believe it to be the first step in understanding practice patterns and the impact of the hospitalist movement.
The use of hospitalists, physicians who specialize in inpatient care, has seen a rapid expansion over the last decade.1 Several studies have shown that with hospitalists there is a shorter length of stay (LOS) and decreased utilization of resources and that hospitalists play a positive role in medical education.24 However, only a few studies have examined the specific strategies employed by hospitalists to achieve improved efficiency and outcomes.
Congestive heart failure (CHF) is the most common diagnosis of hospitalized patients older than age 65, with more Medicare spending devoted to patients with CHF than to any other diagnosis‐related group (DRG).5, 6 Over the last 2 decades hospital discharges for congestive heart failure increased by 165%.7 In addition, the rate of hospital readmission of patients with CHF remains high: 2%, 20%, and 50% within 2 days, 1 month, and 6 months, respectively.8
Several previous studies have shown that patients cared for by hospitalists had improved clinical outcomes. Meltzer et al. found that 30‐day mortality of hospitalists' patients was lower than that of non‐hospitalists' patients, 4.2% versus 6.0%, respectively, in the second year of implementation of a hospitalist program.3 A study by Huddleston et al. showed a reduction of 11.8% in the rate of complications experienced by postsurgical orthopedic patients with the involvement of hospitalists in their care in conjunction with the surgeons.4
Many previous studies have pointed to improvements in economic outcomes such as LOS and costs for patients followed by hospitalists. Kulaga et al. showed that patients cared for by hospitalists had reductions of approximately 20% in LOS and 18% in total costs per case compared with those cared for by community‐based physicians.2 Meltzer et al. found a decrease in the average adjusted LOS of 0.49 days in the second year of implementation of a hospitalist program.3 Rifkin et al. found that patients with pneumonia cared for by hospitalists had a mean adjusted LOS of 5.6 days versus 6.5 days for those cared for by non‐hospitalists.9
Few previous studies have looked at specific practice patterns of hospitalists that result in improved efficiency and better outcomes. Rifkin et al., who found that patients with pneumonia cared for by hospitalists had a shorter LOS, suggested this finding was a result of the earlier recognition by hospitalists that patients were stable and more rapid conversion to oral antibiotics.9 Likewise, Stein et al. found that community‐acquired pneumonia patients treated by hospitalists had a shorter LOS than those treated by non‐hospitalists. However, they were unable to assess the differences in patient management that led to this result because of the design of the study.10
Lindenauer et al. compared quality‐of‐care indicators and resource utilization for patients with congestive heart failure treated by hospitalists and non‐hospitalist general internists. They found that patients under the care of hospitalists had a shorter LOS than those cared for by general internists but that the overall costs of care were similar between the groups.11 They compared the quality indicators developed by the Joint Commission on Accreditation of Healthcare Organizations in the Core Measures Initiative, but did not focus on patterns of practices of hospitalists and nonhospitalists. Moreover, they did not look at full‐time hospitalists but focused on physicians who spent at least 25% of their practice caring for inpatients.
We sought to identify distinct, quantifiable practices of full‐time hospitalists in the management of their patients with CHF. We hypothesized that hospitalists would adhere more closely to the current congestive heart failure guidelines and would utilize available resources more judiciously, leading to improved clinical and economic outcomes. To identify these practices, we compared utilization of well‐established therapeutic and diagnostic modalities such as use of ACE‐I, ARB, and beta‐blockers; ordering of chest x‐rays; measurement of brain natriuretic peptide (BNP); and use of medical subspecialty consultants. We also compared standard clinical and economic outcomes such as in‐hospital mortality, readmission rate, LOS, and costs per case between hospitalists and community‐based physicians.
METHODS
Design and Setting
The study was a retrospective chart review of 447 patients treated for CHF from July 1, 2003, through June 30, 2004, at the Queen's Medical Center, a 505‐bed community‐based teaching hospital in Honolulu, Hawaii, and the leading medical referral center in the Pacific Basin. All patients had been cared for by either a community‐based physician or a hospitalist. The community‐based physicians (referred to as non‐hospitalists from here on) were a diverse group of internists and subspecialists, in solo or group practice, who provided inpatient and ambulatory care. The non‐hospitalist group included 119 cardiologists (55%), 83 general internists (38%), and 3 family practitioners (1%), with the other 6% made up of clinicians in the medical oncology, pediatrics, pulmonary, radiation oncology, and thoracic/cardiovascular surgery subspecialties.
The hospitalist group comprised 10 full‐time internists employed by the hospital who provided care for patients only in the inpatient setting and 3 part‐time hospitalists who practiced in the ambulatory setting in addition to providing inpatient night coverage for the group. During the study period, 2 hospitalists left the group, and 2 hospitalists were hired. On average the length of involvement of a full‐time hospitalist in the study was 9 months. Permission to conduct this study was granted by the Queen's Medical Center Institutional Review Board.
Patient Population
Patients were included in the study if they were admitted to Queen's Medical Center during the 18‐month study period, were at least 18 years old, and were coded on discharge by the medical records department with a principal diagnosis of congestive heart failure (International Classification of Diseases, 9th Revision, codes 428, 428.1, 428.9, 402.01, 402.11, 402.91, 404.01, 404.11, and 404.91). Baseline characteristics of patients collected were age, sex, insurance status, comorbidities, and code status on admission. Comorbidities included coronary artery disease, diabetes mellitus (type 1 or 2), hypertension, chronic renal insufficiency (creatinine > 2 mg/dL), and chronic obstructive pulmonary disease (COPD). Patients were excluded if they had initially been admitted to the medical intensive care unit, required ventilatory support, had end‐stage renal disease requiring hemodialysis, or had an LOS greater than 14 days.
Data Collection
Medical records were reviewed by research nurses not directly involved with the hospitalist group. Training to ensure high‐level reliability of data collection was provided, and reliability was verified by the primary author (M.M.R.). The following data were collected: use of ACE‐I, ARB, and beta‐blockers on admission and discharge; use of intravenous and oral diuretics; time to switch to oral diuretic; rates of utilization of medical consultants, physical therapy, dietary consults, social work, and sodium and fluid restriction; and number of repeat chest radiographs, echocardiograms, and BNP measurements. These criteria were developed based on ACC/AHA 2005 guidelines for diagnosis and management of congestive heart failure in adults,11 several studies delineating the importance of initiating therapy in the inpatient setting, and the experience of the Cardiovascular Hospital Atherosclerosis Management Program (CHAMP) for patients with established coronary artery disease.1315 Data on medical resident involvement in patient care were collected for hospitalists and non‐hospitalists.
Additional outcomes included in‐hospital mortality, rate of acute renal failure, readmission rate, LOS, expense, revenue, and margin per case. Acute renal failure was defined as a doubling of the admission creatinine value. The rate of readmissiondefined as readmission to Queen's Medical Center for any reasonwas evaluated after 7, 14, and 30 days and was stratified further for readmissions for CHF. Expense was defined as costs directly related to patient care plus costs related to operating a hospital facility. Revenue was defined as the compensation the hospital expected to collect for service rendered adjusted for bad debt/charity care. Margin was defined as revenue minus expense.
Data Analysis
Descriptive statistics are reported for baseline patient characteristics (age, sex, insurance status, etc.), quality‐of‐care measures (ACE‐I, ARB, diuretic, and beta‐blocker use, time to oral diuretic, etc.), and outcome measures (readmission rate, in‐hospital mortality, LOS, cost data) using frequencies and proportions for categorical variables (eg, sex, ethnicity, insurance status), means and standard deviations (SDs) for continuous variables (age), and medians and interquartile ranges (Q1‐Q3) for skewed variables (eg, LOS, cost data). The patients cared for by hospitalists were compared with those cared for by non‐hospitalists using the chi‐square test or Fisher's exact test for categorical data and the Student t test for continuous data. All‐Payer Severity‐adjusted Diagnosis Related Groups (APS‐DRGs) were used to control for severity of patient illness. The severity of illness codes were taken from 3M APR Benchmarking software for DRGs adjusted for severity of illness and risk of mortality. 3M defined severity of illness as the extent of physiologic decompensation or organ system loss of function. Each diagnosis was assigned 1 of 4 severity levels: minor, moderate, major, or extreme. Kruskal‐Wallis analysis of covariance was used for LOS and cost outcomes, adjusting for age, insurance status, comorbidities, and severity of illness. Multivariate logistic regression was performed for binary outcomes (eg, ACE‐I, ARB, beta‐blocker use) to adjust for confounding variables. Statistical analysis was performed using SAS version 9 (SAS Institute Inc., Cary, NC). All tests were 2‐sided, and differences with a P value < .05 were considered significant.
RESULTS
Patient Characteristics
Table 1 shows the patient characteristic data. There were 447 admissions for congestive heart failure during the study period, 342 of which met study inclusion criteria. Hospitalists provided care for 126 of these patients and non‐hospitalists for 216 patients. Mean age of patients in the hospitalist and nonhospitalist groups was 63 and 73 years, respectively. There were significant differences in insurance status, with hospitalists more frequently caring for patients covered by Medicaid (26% vs. 7%; P < .001) and patients who were uninsured (6% vs. 1%; P = .04). Patients cared for by hospitalists had a lower incidence of coronary artery disease (42% vs. 59%; P = .003) and prior CHF (44% vs. 56%; P = .05). The hospitalists' patients were more likely to have a full resuscitation code status on admission; however, this difference did not reach statistical significance (90% vs. 81%; P = .07). There were no significant differences between patients cared for by hospitalists and non‐hospitalists in sex, ethnic background, other comorbidities, or house staff involvement.
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Age (years, mean SD) | 73 15 | 63 16 | < .001 |
| Male sex | 124 (57) | 78 (62) | .41 |
| Caucasian ethnicity | 41 (19) | 30 (24) | .29 |
| Insurance status | |||
| Medicare | 119 (55) | 58 (46) | .11 |
| Medicaid/Quest | 16 (7) | 33 (26) | < .001 |
| HMSA | 68 (31) | 19 (15) | < .001 |
| Self‐pay | 3 (1) | 7 (6) | .04 |
| Other | 10(5) | 9(7) | .33 |
| Comorbidy | |||
| CAD | 127 (59) | 53 (42) | .003 |
| DM | 78 (36) | 53 (4) | .27 |
| HTN | 139 (64) | 80 (63) | .87 |
| CRI | 43 (20) | 28 (22) | .61 |
| COPD | 30 (14) | 26 (21) | .10 |
| Prior CHF | 120 (56) | 56 (44) | .05 |
| Full code | 174 (81) | 113 (90) | .07 |
| House staff involvement | 42 (19) | 20 (16) | .41 |
Practice Patterns and Resource Utilization
Practice patterns and resource utilization are shown in Table 2. Hospitalists used more ACE‐I/ARBs, with 86% of patients receiving these interventions within 24 hours of admission versus 72% of the patients of non‐hospitalists (adjusted P = .001). Hospitalists treated fewer patients with beta‐blockers on admission and on discharge and more patients with intravenous diuretics (90% vs. 73%; adjusted P = .001). The rate of beta‐blocker use did not change significantly after controlling for patients with COPD (data not shown).
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value* | |
|---|---|---|---|
| |||
| ACE‐I/ARB within 24 hours | 155 (72) | 108 (86) | .001 |
| Beta‐blocker within 24 hours | 119 (55) | 50 (40) | .004 |
| ACE‐I/ARB at discharge | 147 (69) | 95 (75) | .24 |
| Beta‐blocker at discharge | 116 (54) | 52 (41) | .03 |
| Echocardiogram 1 | 125 (58) | 81 (64) | .50 |
| MD Consultants 2 | 35 (16) | 10 (8) | .01 |
| Chest x‐ray 2 | 27 (13) | 5 (4) | .02 |
| BNP 1 | 128 (59) | 95 (75) | .005 |
| BNP > 1 | 22 (10) | 7 (6) | .14 |
| Physical therapy | 35 (16) | 17 (13) | .48 |
| Dietary consult | 29 (13) | 19 (15) | .67 |
| Social work | 62 (29) | 60 (48) | .003 |
| Sodium restriction | 184 (85) | 102 (81) | .31 |
| Fluid restriction | 47 (22) | 35 (28) | .21 |
| IV diuretic | 158 (73) | 114 (90) | .001 |
| Time to oral diuretic (days), median (Q1,Q3) | 1 (1, 3) | 1 (0, 2) | .30 |
Hospitalists were less likely to obtain 2 or more chest x‐rays (4% vs. 13%; adjusted P = .02) or to obtain 2 or more medical consultations (8% vs. 16%; adjusted P = .01). In addition, they obtained more initial measurements of BNP; however, there was a trend toward fewer repeat BNP measurements (6% vs. 10%; P = .14). There was a significantly higher rate of social work utilization by hospitalists than by nonhospitalists (48% vs. 29%; adjusted P = .003). There were no differences between the groups in the rates of obtaining echocardiograms, physical therapy, and dietary consults or in sodium and fluid restrictions.
Outcomes
Significant differences were noted in LOS and cost outcomes between hospitalists and non‐hospitalists after adjusting for age, insurance status, comorbidities, and severity of illness (Tables 3 and 4). Patients cared for by hospitalists had a shorter overall LOS than did patients cared for by non‐hospitalists (adjusted P = .002). A shorter LOS was noted for patients in the minor (median 3 vs. 5 days), moderate (median 4 vs. 5 days), and extreme (7 vs. 8 days) severity categories. Overall adjusted expense was significantly lower for the care of hospitalists' patients across all severity categories (P < .001). There was a trend toward lower adjusted revenue for patients of hospitalists than those of non‐hospitalist (P = .06). The adjusted profit margin did not significantly differ between the groups (P =.14).
| Severity | Nonhospitalist cases (n = 216) | Hospitalist cases (n = 126) | P value | |
|---|---|---|---|---|
| ||||
| Severity (%) | Minor | 40 (19) | 30 (24) | .13 |
| Moderate | 99 (46) | 64 (51) | ||
| Major | 72 (33) | 27 (21) | ||
| Extreme | 5 (2) | 4 (3) | ||
| LOS (days) | Minor | 5 (3, 6) | 3 (2, 4) | .002 |
| Moderate | 5 (3, 7) | 4 (3, 6) | ||
| Major | 6 (4,10) | 6 (4, 10) | ||
| Extreme | 8 (2, 8) | 7 (6, 8) | ||
| Expense ($) | Minor | 5792 (4414, 6715) | 4164 (2401, 5499) | < .001 |
| Moderate | 6953 (4273, 10,224) | 5951 (4301, 8621) | ||
| Major | 13,622 (8219, 28,553) | 10,519 (5249, 15,581) | ||
| Extreme | 18,908 (12913, 24,688) | 16,192 (6135, 26,147) | ||
| Revenue ($) | Minor | 7095 (6611, 7212) | 7116 (4160, 7218) | .06 |
| Moderate | 7118 (7025, 7215) | 6893 (3755, 7164) | ||
| Major | 9601 (6972, 16,668) | 6743 (4612, 7116) | ||
| Extreme | 11,019 (10,009, 24,897) | 9184 (5783, 13,931) | ||
| Margin ($) | Minor | 786 (162, 2997) | 2290 (409, 4768) | .14 |
| Moderate | 256 (1999, 3366) | 796 (2741, 1565) | ||
| Major | 2314 (7870, 1448) | 3499 (8818, 1008) | ||
| Extreme | 1263 (2904, 4012) | 6537 (15,617, 3050) | ||
| Nonhospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Acute renal failure | 2 (1) | 0 (0) | 0.53 |
| In‐hospital mortality | 9 (4) | 0 (0) | 0.03 |
| Readmission for any reason | 53 (25) | 35 (28) | 0.52* |
| Readmission for CHF | 19 (9) | 18 (14) | 0.16* |
In‐hospital mortality of patients treated by hospitalists was lower than that of non‐hospitalist‐treated patients (0% vs. 4%; P =.03). Rates of acute renal failure, overall readmissions and readmissions specifically for congestive heart failure did not differ significantly. Notably, severity of illness assessed by APS‐DRG did not differ between hospitalists' and nonhospitalists' patients (P = .13).
DISCUSSION
Practice Patterns
Our study identified specific practices that hospitalists use more than non‐hospitalists in the management of patients with CHF. These practices, which may have resulted in decreased LOS and lower costs, included higher use of ACE‐I/ARB within 24 hours of admission and of intravenous diuretics. We hypothesized that earlier and more aggressive use of ACE‐I/ARB contributed to after‐load reduction and alteration of cardiac remodeling5 and may have led to faster recovery and improved outcomes. Greater use of intravenous diuretics may signify that hospitalists have a more aggressive approach to managing exacerbations of acute congestive heart failure, which may also lead to faster recovery.
Hospitalists used fewer beta‐blockers on admission and at discharge. Reasons for this finding remain unclear; however, it may have been a result of the practice of avoiding beta‐blockers during exacerbations of acute CHF and the subsequent reliance on primary care providers to restart beta‐blockers after discharge. Lower use of beta‐blockers did not appear to have a negative impact on mortality or readmission rates.
Resource Utilization
Hospitalists used fewer serial chest x‐rays, more initial BNP measurements, and more social work consults, and there was a trend toward their using fewer repeat BNP measurements. The less frequent use of serial chest x‐rays may be a result of hospitalists being able to assess patients more frequently and to rely less on imaging. Higher rates of initial BNP measurement by hospitalists may reflect the ordering patterns of the emergency room physicians because most patients are admitted to the hospitalists via the emergency room. The trend toward fewer repeat BNP measurements by hospitalists may again reflect their ability to perform more frequent clinical assessments and to rely less on laboratory data. The higher rate of utilization of social workers by hospitalists is likely a reflection of a population in need of such interventions rather than the hospitalists having a lower threshold before requesting a social work consultation. There were no differences in the rates of obtaining echocardiograms, physical therapy, and dietary consults and of sodium and fluid restrictions.
Clinical Outcomes
Severity of illness assessed by APS‐DRG did not differ between the patients cared for by hospitalists and those care for by non‐hospitalists (P = .13) despite the hospitalists caring for a younger population. In‐hospital mortality of hospitalist‐treated patients was lower (0% vs. 4%), whereas the rates of readmission and renal failure did not differ between the 2 groups. A slight advantage in the mortality rate appears to be in agreement with prior findings3, 4; however, this may have been a result of the non‐hospitalists caring for an older patient population.
Economic Outcomes
The shorter LOS and lower overall costs of patients followed by hospitalists supports previous findings.2, 3, 10 The LOS in our study was found to be shorter for hospitalist‐treated patients whose illnesses were in the minor, moderate, and extreme severity categories by 40%, 20%, and 13%, respectively. The median expense per case was less across all severity categories, ranging from $1000 to $3100 for the patients followed by hospitalists compared with those followed by non‐hospitalists. There was a trend toward lower adjusted median revenue in all categories except for minor severity for hospitalists' patients (P = .06). The profit margin per case did not differ significantly between patients cared for by hospitalists and non‐hospitalists. The shorter LOS and lower expenses per case of patients under the care of hospitalists should have led to higher revenue and profit margin. However, our study showed lower revenue and no significant differences in profit margin, which may be explained by the fact that the hospitalists' patients had a worse insurance mix with a higher proportion of uninsured and Medicaid patients. It is also possible that non‐hospitalists, in particular, cardiologists, generate higher revenue by performing more procedures such as cardiac catheterizations, thus offsetting the costs.
As noted above, the analysis of LOS, expenses, revenue, and margin controlled for age, comorbidities, severity of illness, and insurance status (Table 3). The results were not significantly affected by adjusting for age, insurance status, and comorbidities after controlling for severity. The difference in age may in part be a result of older patients having established relationships with primary care physicians and being less likely to be admitted by hospitalists. It may also reflect the high prevalence of methamphetamine abuse, which has reached epidemic proportions in Hawaii, and methamphetamine‐induced cardiomyopathy in a younger population of patients followed by hospitalists. Further studies would be necessary to estimate the impact of drug‐induced congestive heart failure in these populations.
Although our study provided a detailed look at practice patterns of a coherent hospitalist group, it had several important limitations. It was a retrospective study conducted at a single institution, making the findings difficult to generalize to hospitalist practices nationwide. It included an unusually large number of non‐Caucasian patients, reflecting the demographics of the state of Hawaii. Data on contraindications to ACE‐I/ARB were not collected because the degree of renal dysfunction that would serve as a contraindication was difficult to define. The primary mode of adjustment was APS, which may have been a limiting factor in assessing severity of illness. The inability to follow patients' course after discharge limited collection of long‐term outcomes data.
In agreement with previous studies, we showed a decreased LOS and lower expenses per case of patients cared for by full‐time hospitalists while preserving quality of care and improving clinical outcomes. We identified specific practices of hospitalists in the management of patients with CHF that differ from those of non‐hospitalists. These practices include early use of ACE‐I/ARB, aggressive approach to diuresis, higher utilization of social work services, and decreased utilization of serial chest x‐rays, medical consultants, and serial BNP measurements. Our study was not designed to identify a direct causal relationship between hospitalist practices and improved outcomes; however, we believe it to be the first step in understanding practice patterns and the impact of the hospitalist movement.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin North Am.2002;86:797–823.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,.Advances in the management of acute and chronic decompensated heart failure.Lippincotts Case Manag.2004;9:S1–S15.
- ,,,, et al.ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult.Circulation.2001;104:2996–3007.
- American Heart Association.Heart disease and stroke statistics—2003 update.2003.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl):S3–S9.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,,.Economic effects of community versus hospital‐based faculty pneumonia care.J Gen Intern Med.1998;13:774–777.
- ,,,,.Quality of care for patients hospitalized with heart failure. Assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in adult.ACC/AHA Pract Guidel.2005:1–82
- ,,.Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure—a review.Am J Cardiol.2004;94:1155–1160.
- .Role of in‐hospital initiation of carvedilol to improve treatment rates and clinical outcomes.Am J Cardiol.2004;93(suppl):77B–81B.
- ,.Rationale and design of the Cardiac Hospitalization Atherosclerosis Management Program at the University of California Los Angeles.Am J Cardiol.2000;85:10A–17A.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin North Am.2002;86:797–823.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,.Advances in the management of acute and chronic decompensated heart failure.Lippincotts Case Manag.2004;9:S1–S15.
- ,,,, et al.ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult.Circulation.2001;104:2996–3007.
- American Heart Association.Heart disease and stroke statistics—2003 update.2003.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl):S3–S9.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,,.Economic effects of community versus hospital‐based faculty pneumonia care.J Gen Intern Med.1998;13:774–777.
- ,,,,.Quality of care for patients hospitalized with heart failure. Assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in adult.ACC/AHA Pract Guidel.2005:1–82
- ,,.Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure—a review.Am J Cardiol.2004;94:1155–1160.
- .Role of in‐hospital initiation of carvedilol to improve treatment rates and clinical outcomes.Am J Cardiol.2004;93(suppl):77B–81B.
- ,.Rationale and design of the Cardiac Hospitalization Atherosclerosis Management Program at the University of California Los Angeles.Am J Cardiol.2000;85:10A–17A.
Copyright © 2008 Society of Hospital Medicine
Glycemic Control in Medical Inpatients
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).
Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).

Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).

Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.