User login
Inappropriate antibiotic use is a major public health concern and demonstrates the need for quality improvement initiatives in the delivery of health care.16 Each year nearly 2 million patients in the United States acquire an infection in the hospital, and about 90,000 of them die from these infections.7 More than 70% of the bacteria that cause hospital‐acquired infections are resistant to at least one commonly used drug.7 Persons infected with drug‐resistant organisms have longer hospital stays and higher mortality rates.7
Inappropriate antibiotic use in the inpatient hospital setting can be classified into 5 categories. First, antibiotics may be given for illnesses for which they are not indicated (eg, viral infections). Second, broad‐spectrum antibiotics (such as piperacillin‐tazobactam and quinolones) may be overused in the empiric treatment of common infections.8 Overuse of broad‐spectrum drugs increases selective pressure for antimicrobial resistance and exposes patients to the side effects of some of these drugs, such as Clostridium difficile colitis.8 Third, clinicians occasionally prescribe intravenous (IV) antibiotics when the efficacy of oral agents would be similar. Inappropriate intravenous therapy increases the cost of care and also exposes the patient to the risk of intravenous catheters.8 Fourth, when the correct antibiotic choice is made, inappropriate antibiotic dosage, schedule, and/or duration of treatment can threaten patient safety.8 Fifth, bug‐drug mismatch occurs when susceptibility studies indicate that the drug being used is ineffective or only marginally effective.8 Beyond antimicrobial resistance and safety, these practices also usually increase costs to both the patient and the hospital.7, 910
Influencing providers' prescribing patterns is difficult.11 In this project we assessed the prescribing patterns of hospitalists in an active inpatient environment and then developed an intervention to improve the providers' use of antibiotics. The intervention utilized public health methodologyprior to implementation, we defined the problem, determined its magnitude, identified a behavior change model, and constructed a conceptual framework that identifyied the key determinants. A pilot academic detailing project addressing many determinants was developed, implemented, and evaluated.
Conceptual Model
To change prescribing behaviors is to change learned behaviors. Changing behavior is a complex process affected by several factors including beliefs, expectations, motivations, and the psychosocial environments of the target groups.12 Each of these factors must be considered when attempting to bring about behavior changes. In doing so, a theory that can be depicted in a model often emerges.13 This approach is widely used in understanding and developing public health interventions.
Formulating the Model
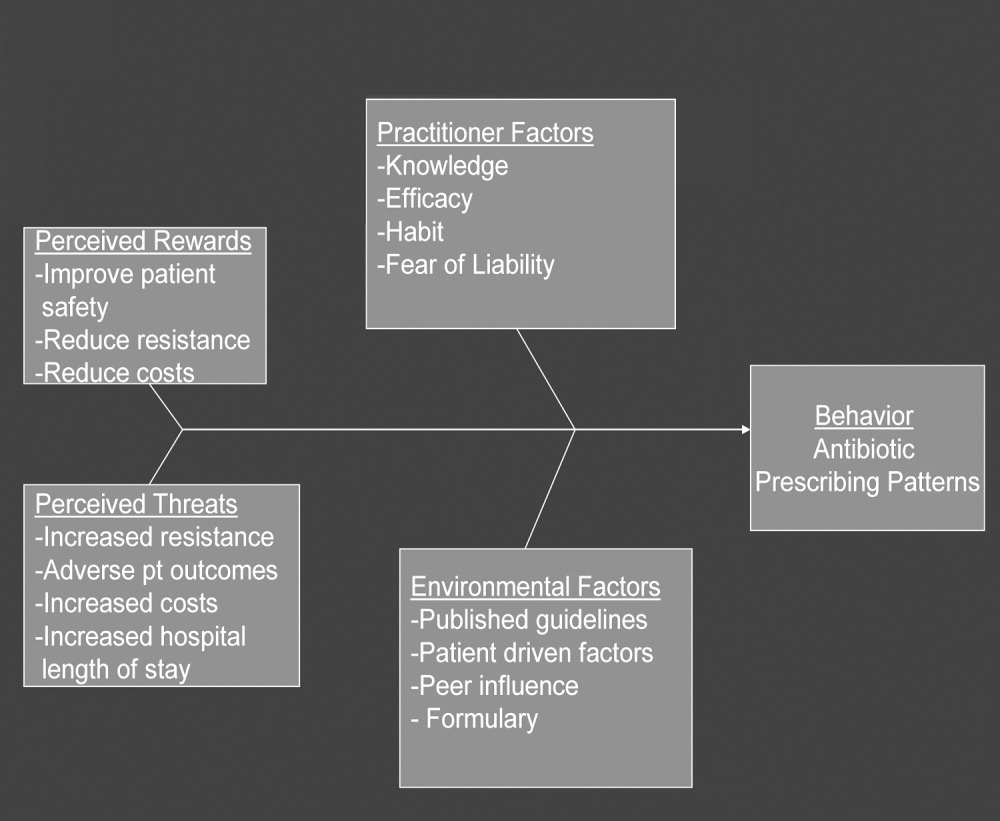
In any public health intervention, recognizing and engaging key stakeholders is a critical step. We identified the following stakeholders: (1) hospitalist practitioners and other prescribing providers including residents and infectious disease specialists; (2) nurses; (3) administrators who are focused on cost effectiveness; (4) patients and their families, who want to get well affordably, without side effects; (5) pharmacists; (6) risk management; and (7) society, which is fearful of the propagation of resistant microbes. In consulting with some of the stakeholders, 4 factors that influence hospitalists' prescribing patterns became apparent. These are practitioner factors, environmental factors, perceived rewards, and perceived threats (Fig. 1).
The practitioner factors shaping prescribing are: (1) knowledge of current best care; (2) self‐efficacy, which determines whether a provider is confident in his or her knowledge to adequately treat a specific infection; (3) habit, which causes providers to pick from a narrow repertoire of antibiotics when treating an infection; and (4) fear of liability, which forces some providers to be cautious. Four environmental factors affecting antibiotic prescriptions are: (1) published guidelines regarding organisms' sensitivity to antibiotics; (2) patient‐driven factors such as affordability, compliance with dosing regimens, side effects, and interactions between the antibiotics and other medications; (3) peer influence, in that providers are reluctant to change a prescription started by another provider (eg, emergency room physician); and (4) the formulary of the hospital, as it forces providers to prescribe within specific parameters. The perceived rewards of specific prescribing practices may include improving patient safety and reducing antibiotic resistance and costs, whereas the perceived threats are increasing antimicrobial resistance, having adverse patient outcomes, and increasing costs and hospital length of stay. We selected a high‐yield, low‐effort intervention in order to have an impact on some of the factors underlying hospitalists' prescribing patterns.
METHODS
Participants
The study participants were 17 hospitalist practitioners including physicians, nurse‐practitioners, and physician assistants who make up the Collaborative Inpatient Medical Service (CIMS) at Johns Hopkins Bayview Medical Center (JHBMC; Table 1). All consented to participate. The study was approved by the institutional review board.
| Age in years, mean (SD) | 36 (6) |
| Female, n (%) | 13 (76%) |
| Physician, n (%) | 9 (53%) |
| Nurse‐practitioner, n (%) | 5 (29%) |
| Physician assistant, n (%) | 3 (18%) |
| Years in practice, mean (SD) | 5.1 (2.8) |
| Number of pharmaceutical representatives exposed to in past year, mean | 1 |
| Number of shifts worked per month, mean (SD) | 14 (4) |
| Primarily works days, n (%) | 13 (76%) |
Data Collection
We collected and assessed prescription patterns over 3 periods: preintervention, interim, and postintervention.
Assessing Appropriateness of Antibiotics
For each order that was assessed in the preintervention, interim, and postintervention periods, the following information was collected: (1) drug ordered, (2) clinical diagnosis, (3) microbiology results available at the time of the order (including relevant results from recent cultures), (4) other medical diagnoses (ICD9 codes), (5) allergies, and (6) exposure to health care facilities (within the past 30 days). The computerized medical record allowed access to the discharge summaries of a patient's hospitalization. These records summarized the patient's hospitalization, allowing the investigators to understand the reasons for a provider's choice of antibiotics. If the rationale was not clear about how to categorize a prescription from reading the data, the investigators performed a chart review. From the information culled from these reviews, the primary investigator and an infectious disease specialist classified each prescription order by consensus as appropriate, effective but inappropriate, or inappropriate therapy.
Prescriptions were classified as appropriate when they were indicated and correlated with sensitivities, if available, or were of a narrow‐enough spectrum and recommended as a first‐line treatment for specific illnesses by either the Johns Hopkins Antibiotic Guide14 or the Stanford Guide to Antimicrobial Therapy.15 For example, cephalexin to treat uncomplicated cellulitis was considered appropriate therapy. Effective but inappropriate prescriptions were broad‐spectrum antibiotics used to treat an infection when a narrower‐spectrum antibiotic would have sufficed. For example, piperacillin‐tazobactam would be effective in treating a simple urinary tract infection but inappropriate to use because of its broad spectrum. Other examples of effective but inappropriate prescriptions were giving an IV when an oral alternative would be equally effective and tolerated or prescribing antibiotic treatment whose duration was too long. Finally, inappropriate prescriptions were those written for conditions for which antibiotics are not indicated or for which the prescribed antibiotic was ineffective for the specified infection (bug‐drug mismatch).
Preintervention
In January 2006 the investigators retrospectively reviewed the prescribing patterns of the 17 providers over the previous year. Using the computerized medical record and physician order entry, consecutive prescriptions of each provider were evaluated, beginning December 31, 2005, going back reverse chronologically until 20 prescriptions had been identified. For 12 of the providers, it was actually possible to review 20 prescriptions. For 2 other providers, both new, part‐time additions to the hospitalist group, only 1 and 7 prescriptions were found for the entire year. The prescribing history of the 3 remaining providers who participated in the study, all physician assistants, could not be evaluated (during any period) because all their orders were linked only to physicians, making it impossible to determine their specific prescriptions using the physician order entry system.
Interim
During the interim period between obtaining informed consent and completing the academic detailing (January 3, 2006, to March 23, 2006), provider prescribing patterns were reviewed to determine if the mere knowledge of the project would produce changes in prescribing behavior.
Postintervention
After the academic detailing was completed (March 23, 2006), the prescribing patterns of the hospitalists were followed through April 23, 2006. Each week after the detailing session, the hospitalists received reminders to prescribe appropriately (including pens with the message Reduce the Overuse).
Detailing Procedures
After the review, a profile was assembled for each of the CIMS providers. The study team detailers (a physician and a pharmacist) met with the individual providers for 30 to 45 minutes. Each hospitalist participant completed a short survey that collected demographic information and was asked about the rationale for his or her antibiotic prescribing pattern. Next, the appraisal of the provider's prescribing pattern was reviewed. This review included looking at the costs of the prescribed antibiotics compared with those of the appropriate alternatives and a reexamination of the guidelines for the selected target drugspiperacillin‐tazobactam, vancomycin, and extended‐spectrum quinolones. These 3 antibiotics were picked because our providers had been particularly vulnerable to inappropriately prescribing them. The hospitalists were provided an antibiotic guide developed specifically for this project and based on the Johns Hopkins Antibiotic Guide14 that summarizes the consensus guidelines.
Data Analysis
The primary outcome variable was the aggregate proportion of inappropriate antibiotic prescribed (as defined earlier) before the intervention, during the interim between obtaining informed consent and intervening on all study subjects, and after the intervention. The percentage of appropriate prescriptions versus total not appropriate prescriptions (combining of the effective but inappropriate and inappropriate categories) were compared across the 3 periods. Ninety‐five percent confidence intervals for comparisons of the proportions were determined using Stata 9.0 (College Station, TX). The difference between the proportions of total not appropriate prescriptions before and after academic detailing was computed in Stata using Fisher's exact test to assess significance.
RESULTS
Demographic information and professional characteristics of the 17 providers are shown in Table 1. Their mean age was 36 years, and 76% were female. The top 4 reasons the providers gave for their prescribing practices were: (1) published guidelines, (2) easier dosing schedule for patient when discharged, (3) continuing an antibiotic course initiated in the emergency room, and (4) broad‐spectrum antibiotics cover all possible microbes.
Comparison of Preintervention, Interim, and Postintervention Periods
Table 2 depicts the results of the prescription appraisals from the retrospective reviews. Of the 14 providers who had ordered antibiotics, 8 (57%) had more prescriptions that were total not appropriate than were appropriate in the preintervention period compared with 3 providers (25%) with this prescribing pattern in the postintervention period (P = .13).
| Provider | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|
| Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | |
| ||||||
| 1 | 20 | 7 (35%) | 13 (65%) | 24 | 17 (70.8%) | 7 (29.2%) |
| 2 | 20 | 10 (50%) | 10 (50%) | 12 | 11 (91.7%) | 1 (8.3%) |
| 3 | 20 | 6 (30%) | 14 (70%) | 8 | 8 (100%) | 0 (0%) |
| 4* | 19 | 10 (52.6%) | 9 (47.4%) | 4 | 3 (75%) | 1 (25%) |
| 5 | 20 | 9 (45%) | 11 (55%) | 10 | 4 (40%) | 6 (60%) |
| 6 | 20 | 5 (25%) | 15 (75%) | 3 | 1 (33.3%) | 2 (66.7%) |
| 7 | 20 | 8 (40%) | 12 (60%) | 8 | 7 (87.5%) | 1 (12.5%) |
| 8* | 1 | 0 (0%) | 1 (100%) | 0 | 0 (0%) | 0 (0%) |
| 9 | 20 | 11 (55%) | 9 (45%) | 5 | 2 (40%) | 3 (60%) |
| 10* | 7 | 3 (42.9%) | 4 (57.1%) | 0 | 0 (0%) | 0 (0%) |
| 11 | 20 | 10 (50%) | 10 (50%) | 17 | 13 (76.5%) | 4 (23.5%) |
| 12 | 20 | 6 (30%) | 14 (70%) | 16 | 14 (87.5%) | 2 (12.5%) |
| 13 | 20 | 12 (60%) | 8 (40%) | 15 | 11 (73.3%) | 4 (26.7%) |
| 14 | 20 | 10 (50%) | 10 (50%) | 7 | 4 (57.1%) | 3 (42.9%) |
| Total | 247 | 107 (43%) | 140 (57%) | 129 | 95 (73.6%) | 34 (26.4%) |
Table 3 shows the proportions of appropriate, effective but inappropriate, and total not appropriate prescriptions in the retrospective, interim, and postintervention periods. Forty‐three percent (95% CI 37%‐49%) of prescriptions were judged to be appropriate, and 57% (95% CI 51%‐63%) to be not appropriate prior to the academic detailing. In the interim period, 59% (95% CI 52%‐65%) of the prescriptions were appropriate, and 41% (95% CI 35%‐48%) were not appropriate; P = .0003. After the intervention, 74% (95% CI 65%‐81%) of the prescriptions were appropriate, and 26% (95% CI 19%‐35%) were not appropriate; P < .0001.
| Period | Appropriate, n (%) | 95% CI | Effective but inappropriate, n (%) | Inappropriate, n (%) | Total not appropriate, n (%) | 95% CI | P value* |
|---|---|---|---|---|---|---|---|
| |||||||
| Retrospective review (pre) | 107 (43%) | 37%‐49% | 75 (30.4%) | 65 (26.6%) | 140 (57%) | 51%‐63% | |
| Interim | 146 (59%) | 52%‐65% | 37 (15%) | 65 (26%) | 102 (41%) | 35%‐48% | .0003 |
| Postintervention | 95 (74%) | 65%‐81% | 8 (6%) | 26 (20%) | 34 (26%) | 19%‐35% | < .0001 |
DISCUSSION
We have demonstrated that academic detailing had a positive impact on the prescribing patterns of hospitalists. The aggregated improvement in antibiotic prescribing patterns can be attributed to improvement in the prescribing patterns of almost every hospitalist practitioner (Table 2). This study focused on aggregate prescriptions as the primary outcome measure because the hospitalists at JHBMC, like at many other institutions, function as a team, with a patient routinely having multiple providers over the course of the hospital stay. The improved prescribing patterns noted during the interim period suggest that the mere knowledge of a project can have an impact on providers. Providers informed the investigators that they were more thoughtful about their choice of antibiotics when they knew that they were being studied. The further statistically significant improvement in prescribing patterns with the intervention shows that the academic detailing itself was successful.
The greatest absolute change in practice was seen in effective but inappropriate prescribing (from 30.4% to 6%), whereas inappropriate prescribing only decreased from 26.6% to 20.6%. Although we aimed to have an impact on all inappropriate antibiotic prescribing patterns, we specifically reviewed the prescribing guidelines for piperacillin‐tazobactam, extended‐spectrum quinolones, and vancomycin. These 3 antibiotics were targeted because our providers had been particularly susceptible to inappropriately prescribing them. The focus on these antibiotics may have resulted in the larger absolute change noted in effective but inappropriate prescribing. We did not collect any data to determine if having an impact on effective but inappropriate prescribing changed the clinical course of the patients, such as shortening their hospital stays. Anecdotal evidence, however, suggests that it does. At our institution it is not uncommon for patients to be kept in the hospital for an extra day to ensure they are stable when transitioned from extended‐spectrum to narrower‐spectrum antibiotics prior to discharge. The effect of reducing effective but inappropriate prescriptions on the clinical course of patients could be an outcome measure assessed by a future, larger study.
Our one‐on‐one appraisal of each provider's prescribing patterns included a review of the cost of the prescribed antibiotics compared with that of the appropriate alternatives. Although decisions on antibiotic choice should be driven by clinical guidelines and appropriateness rather than price, we believed it was relevant to include education about costs and pricing so that providers would be reminded to ascertain whether patients would be able to afford their antibiotics. Antibiotic resistance is influenced by a patient's failure to complete the course of treatment, and noncompliance may be caused by an inability to afford the medication. Often, there are affordable, appropriate alternatives to the newest and most expensive drugs.
A hospitalist‐based academic detailing approach to improving antibiotic prescribing may have far‐reaching benefits and influence. First, it has the potential to affect other practitioners by setting an example and role modeling. In addition to that with their immediate peer group, hospitalists have close and repeated contact with house officers and emergency room physicians and often act as consultants to physicians in other departments such as surgery and psychiatry. Furthermore, some community hospitals have no infectious disease specialists readily available. So this represents an opportunity for hospitalists to promote quality in antibiotic prescribing. Practice‐based learning was very effective because it brought the practitioners face to face with their prescribing patterns. Although intellectually everyone agreed that antibiotics are often misused, this approach forced the providers to stop and reflect on their individual practices. This peer‐delivered intervention allowed for a collaborative approach to solving the problem; the peer (detailer) was approachable, nonjudgmental, and available for further discussion and guidance.
The public health quality improvement approach that we used for our intervention helped us to realize and appreciate the factors underlying prescribing patterns. Only by understanding the motivations for prescribing patterns can we hope to make sustainable changes. This coincides with our previous assertion that hospitalists are engaging in some public health practice.16 In pubic health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole.17 Hospitalist teams aim to make sure that the high‐quality services needed for protecting the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. Antibiotic optimization is a key role that could fall within the mantra of public health practice for the hospitalist.
Several limitations of this pilot should be considered. First, the intervention is labor intensive. However, it is essential to use the problem‐solving paradigm and incorporate behavior change theories in order to identify interventions that can lead to sustainable change. Second, this was not a randomized controlled trial, and it is possible that there might have been some contamination by external forces. However, in reviewing the educational events at our institution, the press, and articles published during the study period, we could not identify any external factors that would have influenced antibiotic prescribing patterns. It would not have been possible to conduct a randomized trial at our institution because the hospitalists work so closely together that we could not ensure complete separation if the subjects were randomized. There would have been contamination from the intervention group to the control group. A trial with randomization at the institution level is the next step. Third, the number of months retrospectively reviewed in order to identify 20 prescriptions of a provider varied. This study assumed there were no other differences during those months that could have affected provider prescribing behavior; this may have introduced some bias. Fourth, the sustainability of this intervention's positive impact is unknown. We assessed outcome soon after the intervention, and it is unknown whether continual booster sessions are required to maintain the positive impact on prescribing patterns.
This pilot was a good starting place to show that behavior change can be realized with a well‐conceived and methodically executed intervention, even among the busiest of physicians. Audit and feedback, or practice‐based learning, appears to be a powerful educational intervention among professionals who take great pride in their work.
- ,.Improving antibiotic use in low‐income countries: an overview of evidence on determinants.Soc Sci Med.2003;57:733–744.
- .Mechanisms of antimicrobial resistance in bacteria.Am J Med.2006;119(6A):S3–S10.
- .Antimicrobial resistance in gram‐positive bacteria.Am J Med.2006;119(6A):S11–S19.
- .Resistance in Gram‐negative bacteria: enterobacteriaceae.Am J Med.2006;119(6A):S20–S28.
- .Pharmacodynamics: relation to antimicrobial resistance.Am J Med.2006;119(6A):S37–S44.
- .Managing methicillin‐resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms.Am J Med.2006;119(6A):S45–S52.
- NIH. The Problem of Antibiotic Resistance. Available at: http://www.niaid.nih.gov.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed.J Hosp Infect.2001;47:198–209.
- ,.The impact of hospital‐acquired bloodstream infections.Emerg Infect Dis.2001;7(2):174–177.
- .Antimicrobial stewardship.Am J Med.2006;119(6A):S53–S61
- ,,, et al.Changing provider behavior: an overview of systemic reviews of interventions.Med Care.2001;39:II–2‐II‐45.
- .A review of current health education theories.Calif J Health Promot.2004;2:74–87
- The Johns Hopkins Hospital Antibiotic Management Program. 2005 Antibiotic Guidelines: Treatment Recommendations for Adult Inpatients. Johns Hopkins Medicine.
- ,,,.The Sanford Guide to Antimicrobial Therapy 2005.35th ed.Hyde Park, VT:Antimicrobial Therapy, Inc.;2005.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
Inappropriate antibiotic use is a major public health concern and demonstrates the need for quality improvement initiatives in the delivery of health care.16 Each year nearly 2 million patients in the United States acquire an infection in the hospital, and about 90,000 of them die from these infections.7 More than 70% of the bacteria that cause hospital‐acquired infections are resistant to at least one commonly used drug.7 Persons infected with drug‐resistant organisms have longer hospital stays and higher mortality rates.7
Inappropriate antibiotic use in the inpatient hospital setting can be classified into 5 categories. First, antibiotics may be given for illnesses for which they are not indicated (eg, viral infections). Second, broad‐spectrum antibiotics (such as piperacillin‐tazobactam and quinolones) may be overused in the empiric treatment of common infections.8 Overuse of broad‐spectrum drugs increases selective pressure for antimicrobial resistance and exposes patients to the side effects of some of these drugs, such as Clostridium difficile colitis.8 Third, clinicians occasionally prescribe intravenous (IV) antibiotics when the efficacy of oral agents would be similar. Inappropriate intravenous therapy increases the cost of care and also exposes the patient to the risk of intravenous catheters.8 Fourth, when the correct antibiotic choice is made, inappropriate antibiotic dosage, schedule, and/or duration of treatment can threaten patient safety.8 Fifth, bug‐drug mismatch occurs when susceptibility studies indicate that the drug being used is ineffective or only marginally effective.8 Beyond antimicrobial resistance and safety, these practices also usually increase costs to both the patient and the hospital.7, 910
Influencing providers' prescribing patterns is difficult.11 In this project we assessed the prescribing patterns of hospitalists in an active inpatient environment and then developed an intervention to improve the providers' use of antibiotics. The intervention utilized public health methodologyprior to implementation, we defined the problem, determined its magnitude, identified a behavior change model, and constructed a conceptual framework that identifyied the key determinants. A pilot academic detailing project addressing many determinants was developed, implemented, and evaluated.
Conceptual Model
To change prescribing behaviors is to change learned behaviors. Changing behavior is a complex process affected by several factors including beliefs, expectations, motivations, and the psychosocial environments of the target groups.12 Each of these factors must be considered when attempting to bring about behavior changes. In doing so, a theory that can be depicted in a model often emerges.13 This approach is widely used in understanding and developing public health interventions.
Formulating the Model
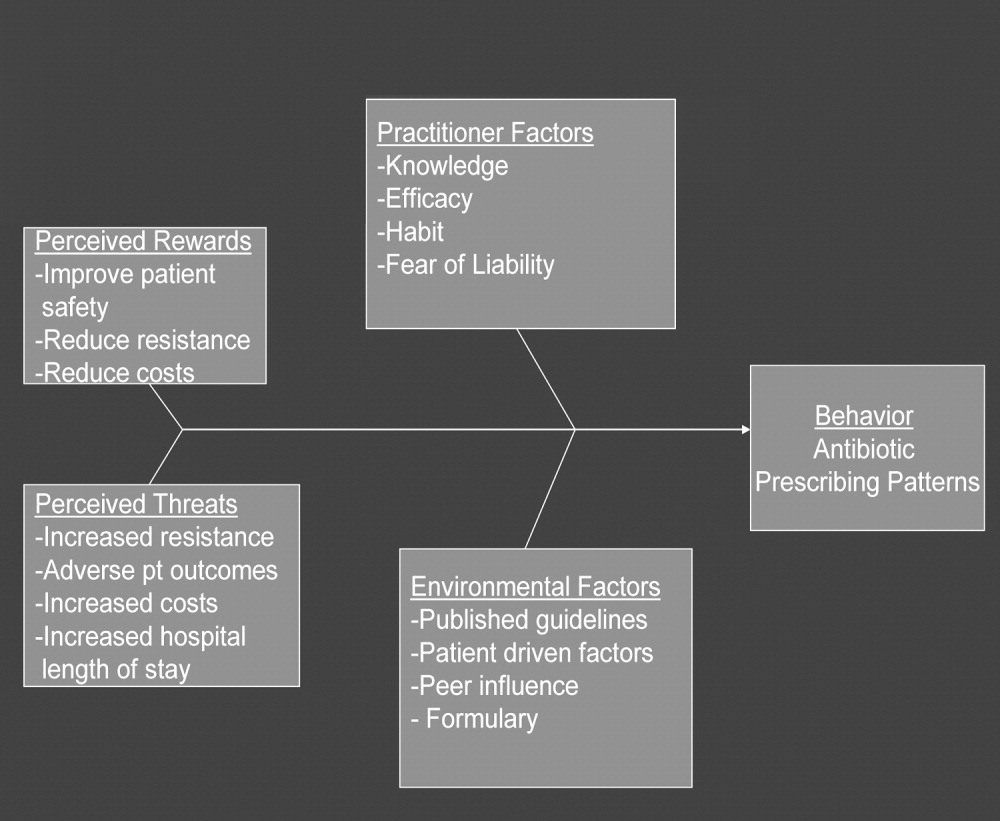
In any public health intervention, recognizing and engaging key stakeholders is a critical step. We identified the following stakeholders: (1) hospitalist practitioners and other prescribing providers including residents and infectious disease specialists; (2) nurses; (3) administrators who are focused on cost effectiveness; (4) patients and their families, who want to get well affordably, without side effects; (5) pharmacists; (6) risk management; and (7) society, which is fearful of the propagation of resistant microbes. In consulting with some of the stakeholders, 4 factors that influence hospitalists' prescribing patterns became apparent. These are practitioner factors, environmental factors, perceived rewards, and perceived threats (Fig. 1).

The practitioner factors shaping prescribing are: (1) knowledge of current best care; (2) self‐efficacy, which determines whether a provider is confident in his or her knowledge to adequately treat a specific infection; (3) habit, which causes providers to pick from a narrow repertoire of antibiotics when treating an infection; and (4) fear of liability, which forces some providers to be cautious. Four environmental factors affecting antibiotic prescriptions are: (1) published guidelines regarding organisms' sensitivity to antibiotics; (2) patient‐driven factors such as affordability, compliance with dosing regimens, side effects, and interactions between the antibiotics and other medications; (3) peer influence, in that providers are reluctant to change a prescription started by another provider (eg, emergency room physician); and (4) the formulary of the hospital, as it forces providers to prescribe within specific parameters. The perceived rewards of specific prescribing practices may include improving patient safety and reducing antibiotic resistance and costs, whereas the perceived threats are increasing antimicrobial resistance, having adverse patient outcomes, and increasing costs and hospital length of stay. We selected a high‐yield, low‐effort intervention in order to have an impact on some of the factors underlying hospitalists' prescribing patterns.
METHODS
Participants
The study participants were 17 hospitalist practitioners including physicians, nurse‐practitioners, and physician assistants who make up the Collaborative Inpatient Medical Service (CIMS) at Johns Hopkins Bayview Medical Center (JHBMC; Table 1). All consented to participate. The study was approved by the institutional review board.
| Age in years, mean (SD) | 36 (6) |
| Female, n (%) | 13 (76%) |
| Physician, n (%) | 9 (53%) |
| Nurse‐practitioner, n (%) | 5 (29%) |
| Physician assistant, n (%) | 3 (18%) |
| Years in practice, mean (SD) | 5.1 (2.8) |
| Number of pharmaceutical representatives exposed to in past year, mean | 1 |
| Number of shifts worked per month, mean (SD) | 14 (4) |
| Primarily works days, n (%) | 13 (76%) |
Data Collection
We collected and assessed prescription patterns over 3 periods: preintervention, interim, and postintervention.
Assessing Appropriateness of Antibiotics
For each order that was assessed in the preintervention, interim, and postintervention periods, the following information was collected: (1) drug ordered, (2) clinical diagnosis, (3) microbiology results available at the time of the order (including relevant results from recent cultures), (4) other medical diagnoses (ICD9 codes), (5) allergies, and (6) exposure to health care facilities (within the past 30 days). The computerized medical record allowed access to the discharge summaries of a patient's hospitalization. These records summarized the patient's hospitalization, allowing the investigators to understand the reasons for a provider's choice of antibiotics. If the rationale was not clear about how to categorize a prescription from reading the data, the investigators performed a chart review. From the information culled from these reviews, the primary investigator and an infectious disease specialist classified each prescription order by consensus as appropriate, effective but inappropriate, or inappropriate therapy.
Prescriptions were classified as appropriate when they were indicated and correlated with sensitivities, if available, or were of a narrow‐enough spectrum and recommended as a first‐line treatment for specific illnesses by either the Johns Hopkins Antibiotic Guide14 or the Stanford Guide to Antimicrobial Therapy.15 For example, cephalexin to treat uncomplicated cellulitis was considered appropriate therapy. Effective but inappropriate prescriptions were broad‐spectrum antibiotics used to treat an infection when a narrower‐spectrum antibiotic would have sufficed. For example, piperacillin‐tazobactam would be effective in treating a simple urinary tract infection but inappropriate to use because of its broad spectrum. Other examples of effective but inappropriate prescriptions were giving an IV when an oral alternative would be equally effective and tolerated or prescribing antibiotic treatment whose duration was too long. Finally, inappropriate prescriptions were those written for conditions for which antibiotics are not indicated or for which the prescribed antibiotic was ineffective for the specified infection (bug‐drug mismatch).
Preintervention
In January 2006 the investigators retrospectively reviewed the prescribing patterns of the 17 providers over the previous year. Using the computerized medical record and physician order entry, consecutive prescriptions of each provider were evaluated, beginning December 31, 2005, going back reverse chronologically until 20 prescriptions had been identified. For 12 of the providers, it was actually possible to review 20 prescriptions. For 2 other providers, both new, part‐time additions to the hospitalist group, only 1 and 7 prescriptions were found for the entire year. The prescribing history of the 3 remaining providers who participated in the study, all physician assistants, could not be evaluated (during any period) because all their orders were linked only to physicians, making it impossible to determine their specific prescriptions using the physician order entry system.
Interim
During the interim period between obtaining informed consent and completing the academic detailing (January 3, 2006, to March 23, 2006), provider prescribing patterns were reviewed to determine if the mere knowledge of the project would produce changes in prescribing behavior.
Postintervention
After the academic detailing was completed (March 23, 2006), the prescribing patterns of the hospitalists were followed through April 23, 2006. Each week after the detailing session, the hospitalists received reminders to prescribe appropriately (including pens with the message Reduce the Overuse).
Detailing Procedures
After the review, a profile was assembled for each of the CIMS providers. The study team detailers (a physician and a pharmacist) met with the individual providers for 30 to 45 minutes. Each hospitalist participant completed a short survey that collected demographic information and was asked about the rationale for his or her antibiotic prescribing pattern. Next, the appraisal of the provider's prescribing pattern was reviewed. This review included looking at the costs of the prescribed antibiotics compared with those of the appropriate alternatives and a reexamination of the guidelines for the selected target drugspiperacillin‐tazobactam, vancomycin, and extended‐spectrum quinolones. These 3 antibiotics were picked because our providers had been particularly vulnerable to inappropriately prescribing them. The hospitalists were provided an antibiotic guide developed specifically for this project and based on the Johns Hopkins Antibiotic Guide14 that summarizes the consensus guidelines.
Data Analysis
The primary outcome variable was the aggregate proportion of inappropriate antibiotic prescribed (as defined earlier) before the intervention, during the interim between obtaining informed consent and intervening on all study subjects, and after the intervention. The percentage of appropriate prescriptions versus total not appropriate prescriptions (combining of the effective but inappropriate and inappropriate categories) were compared across the 3 periods. Ninety‐five percent confidence intervals for comparisons of the proportions were determined using Stata 9.0 (College Station, TX). The difference between the proportions of total not appropriate prescriptions before and after academic detailing was computed in Stata using Fisher's exact test to assess significance.
RESULTS
Demographic information and professional characteristics of the 17 providers are shown in Table 1. Their mean age was 36 years, and 76% were female. The top 4 reasons the providers gave for their prescribing practices were: (1) published guidelines, (2) easier dosing schedule for patient when discharged, (3) continuing an antibiotic course initiated in the emergency room, and (4) broad‐spectrum antibiotics cover all possible microbes.
Comparison of Preintervention, Interim, and Postintervention Periods
Table 2 depicts the results of the prescription appraisals from the retrospective reviews. Of the 14 providers who had ordered antibiotics, 8 (57%) had more prescriptions that were total not appropriate than were appropriate in the preintervention period compared with 3 providers (25%) with this prescribing pattern in the postintervention period (P = .13).
| Provider | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|
| Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | |
| ||||||
| 1 | 20 | 7 (35%) | 13 (65%) | 24 | 17 (70.8%) | 7 (29.2%) |
| 2 | 20 | 10 (50%) | 10 (50%) | 12 | 11 (91.7%) | 1 (8.3%) |
| 3 | 20 | 6 (30%) | 14 (70%) | 8 | 8 (100%) | 0 (0%) |
| 4* | 19 | 10 (52.6%) | 9 (47.4%) | 4 | 3 (75%) | 1 (25%) |
| 5 | 20 | 9 (45%) | 11 (55%) | 10 | 4 (40%) | 6 (60%) |
| 6 | 20 | 5 (25%) | 15 (75%) | 3 | 1 (33.3%) | 2 (66.7%) |
| 7 | 20 | 8 (40%) | 12 (60%) | 8 | 7 (87.5%) | 1 (12.5%) |
| 8* | 1 | 0 (0%) | 1 (100%) | 0 | 0 (0%) | 0 (0%) |
| 9 | 20 | 11 (55%) | 9 (45%) | 5 | 2 (40%) | 3 (60%) |
| 10* | 7 | 3 (42.9%) | 4 (57.1%) | 0 | 0 (0%) | 0 (0%) |
| 11 | 20 | 10 (50%) | 10 (50%) | 17 | 13 (76.5%) | 4 (23.5%) |
| 12 | 20 | 6 (30%) | 14 (70%) | 16 | 14 (87.5%) | 2 (12.5%) |
| 13 | 20 | 12 (60%) | 8 (40%) | 15 | 11 (73.3%) | 4 (26.7%) |
| 14 | 20 | 10 (50%) | 10 (50%) | 7 | 4 (57.1%) | 3 (42.9%) |
| Total | 247 | 107 (43%) | 140 (57%) | 129 | 95 (73.6%) | 34 (26.4%) |
Table 3 shows the proportions of appropriate, effective but inappropriate, and total not appropriate prescriptions in the retrospective, interim, and postintervention periods. Forty‐three percent (95% CI 37%‐49%) of prescriptions were judged to be appropriate, and 57% (95% CI 51%‐63%) to be not appropriate prior to the academic detailing. In the interim period, 59% (95% CI 52%‐65%) of the prescriptions were appropriate, and 41% (95% CI 35%‐48%) were not appropriate; P = .0003. After the intervention, 74% (95% CI 65%‐81%) of the prescriptions were appropriate, and 26% (95% CI 19%‐35%) were not appropriate; P < .0001.
| Period | Appropriate, n (%) | 95% CI | Effective but inappropriate, n (%) | Inappropriate, n (%) | Total not appropriate, n (%) | 95% CI | P value* |
|---|---|---|---|---|---|---|---|
| |||||||
| Retrospective review (pre) | 107 (43%) | 37%‐49% | 75 (30.4%) | 65 (26.6%) | 140 (57%) | 51%‐63% | |
| Interim | 146 (59%) | 52%‐65% | 37 (15%) | 65 (26%) | 102 (41%) | 35%‐48% | .0003 |
| Postintervention | 95 (74%) | 65%‐81% | 8 (6%) | 26 (20%) | 34 (26%) | 19%‐35% | < .0001 |
DISCUSSION
We have demonstrated that academic detailing had a positive impact on the prescribing patterns of hospitalists. The aggregated improvement in antibiotic prescribing patterns can be attributed to improvement in the prescribing patterns of almost every hospitalist practitioner (Table 2). This study focused on aggregate prescriptions as the primary outcome measure because the hospitalists at JHBMC, like at many other institutions, function as a team, with a patient routinely having multiple providers over the course of the hospital stay. The improved prescribing patterns noted during the interim period suggest that the mere knowledge of a project can have an impact on providers. Providers informed the investigators that they were more thoughtful about their choice of antibiotics when they knew that they were being studied. The further statistically significant improvement in prescribing patterns with the intervention shows that the academic detailing itself was successful.
The greatest absolute change in practice was seen in effective but inappropriate prescribing (from 30.4% to 6%), whereas inappropriate prescribing only decreased from 26.6% to 20.6%. Although we aimed to have an impact on all inappropriate antibiotic prescribing patterns, we specifically reviewed the prescribing guidelines for piperacillin‐tazobactam, extended‐spectrum quinolones, and vancomycin. These 3 antibiotics were targeted because our providers had been particularly susceptible to inappropriately prescribing them. The focus on these antibiotics may have resulted in the larger absolute change noted in effective but inappropriate prescribing. We did not collect any data to determine if having an impact on effective but inappropriate prescribing changed the clinical course of the patients, such as shortening their hospital stays. Anecdotal evidence, however, suggests that it does. At our institution it is not uncommon for patients to be kept in the hospital for an extra day to ensure they are stable when transitioned from extended‐spectrum to narrower‐spectrum antibiotics prior to discharge. The effect of reducing effective but inappropriate prescriptions on the clinical course of patients could be an outcome measure assessed by a future, larger study.
Our one‐on‐one appraisal of each provider's prescribing patterns included a review of the cost of the prescribed antibiotics compared with that of the appropriate alternatives. Although decisions on antibiotic choice should be driven by clinical guidelines and appropriateness rather than price, we believed it was relevant to include education about costs and pricing so that providers would be reminded to ascertain whether patients would be able to afford their antibiotics. Antibiotic resistance is influenced by a patient's failure to complete the course of treatment, and noncompliance may be caused by an inability to afford the medication. Often, there are affordable, appropriate alternatives to the newest and most expensive drugs.
A hospitalist‐based academic detailing approach to improving antibiotic prescribing may have far‐reaching benefits and influence. First, it has the potential to affect other practitioners by setting an example and role modeling. In addition to that with their immediate peer group, hospitalists have close and repeated contact with house officers and emergency room physicians and often act as consultants to physicians in other departments such as surgery and psychiatry. Furthermore, some community hospitals have no infectious disease specialists readily available. So this represents an opportunity for hospitalists to promote quality in antibiotic prescribing. Practice‐based learning was very effective because it brought the practitioners face to face with their prescribing patterns. Although intellectually everyone agreed that antibiotics are often misused, this approach forced the providers to stop and reflect on their individual practices. This peer‐delivered intervention allowed for a collaborative approach to solving the problem; the peer (detailer) was approachable, nonjudgmental, and available for further discussion and guidance.
The public health quality improvement approach that we used for our intervention helped us to realize and appreciate the factors underlying prescribing patterns. Only by understanding the motivations for prescribing patterns can we hope to make sustainable changes. This coincides with our previous assertion that hospitalists are engaging in some public health practice.16 In pubic health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole.17 Hospitalist teams aim to make sure that the high‐quality services needed for protecting the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. Antibiotic optimization is a key role that could fall within the mantra of public health practice for the hospitalist.
Several limitations of this pilot should be considered. First, the intervention is labor intensive. However, it is essential to use the problem‐solving paradigm and incorporate behavior change theories in order to identify interventions that can lead to sustainable change. Second, this was not a randomized controlled trial, and it is possible that there might have been some contamination by external forces. However, in reviewing the educational events at our institution, the press, and articles published during the study period, we could not identify any external factors that would have influenced antibiotic prescribing patterns. It would not have been possible to conduct a randomized trial at our institution because the hospitalists work so closely together that we could not ensure complete separation if the subjects were randomized. There would have been contamination from the intervention group to the control group. A trial with randomization at the institution level is the next step. Third, the number of months retrospectively reviewed in order to identify 20 prescriptions of a provider varied. This study assumed there were no other differences during those months that could have affected provider prescribing behavior; this may have introduced some bias. Fourth, the sustainability of this intervention's positive impact is unknown. We assessed outcome soon after the intervention, and it is unknown whether continual booster sessions are required to maintain the positive impact on prescribing patterns.
This pilot was a good starting place to show that behavior change can be realized with a well‐conceived and methodically executed intervention, even among the busiest of physicians. Audit and feedback, or practice‐based learning, appears to be a powerful educational intervention among professionals who take great pride in their work.
Inappropriate antibiotic use is a major public health concern and demonstrates the need for quality improvement initiatives in the delivery of health care.16 Each year nearly 2 million patients in the United States acquire an infection in the hospital, and about 90,000 of them die from these infections.7 More than 70% of the bacteria that cause hospital‐acquired infections are resistant to at least one commonly used drug.7 Persons infected with drug‐resistant organisms have longer hospital stays and higher mortality rates.7
Inappropriate antibiotic use in the inpatient hospital setting can be classified into 5 categories. First, antibiotics may be given for illnesses for which they are not indicated (eg, viral infections). Second, broad‐spectrum antibiotics (such as piperacillin‐tazobactam and quinolones) may be overused in the empiric treatment of common infections.8 Overuse of broad‐spectrum drugs increases selective pressure for antimicrobial resistance and exposes patients to the side effects of some of these drugs, such as Clostridium difficile colitis.8 Third, clinicians occasionally prescribe intravenous (IV) antibiotics when the efficacy of oral agents would be similar. Inappropriate intravenous therapy increases the cost of care and also exposes the patient to the risk of intravenous catheters.8 Fourth, when the correct antibiotic choice is made, inappropriate antibiotic dosage, schedule, and/or duration of treatment can threaten patient safety.8 Fifth, bug‐drug mismatch occurs when susceptibility studies indicate that the drug being used is ineffective or only marginally effective.8 Beyond antimicrobial resistance and safety, these practices also usually increase costs to both the patient and the hospital.7, 910
Influencing providers' prescribing patterns is difficult.11 In this project we assessed the prescribing patterns of hospitalists in an active inpatient environment and then developed an intervention to improve the providers' use of antibiotics. The intervention utilized public health methodologyprior to implementation, we defined the problem, determined its magnitude, identified a behavior change model, and constructed a conceptual framework that identifyied the key determinants. A pilot academic detailing project addressing many determinants was developed, implemented, and evaluated.
Conceptual Model
To change prescribing behaviors is to change learned behaviors. Changing behavior is a complex process affected by several factors including beliefs, expectations, motivations, and the psychosocial environments of the target groups.12 Each of these factors must be considered when attempting to bring about behavior changes. In doing so, a theory that can be depicted in a model often emerges.13 This approach is widely used in understanding and developing public health interventions.
Formulating the Model
In any public health intervention, recognizing and engaging key stakeholders is a critical step. We identified the following stakeholders: (1) hospitalist practitioners and other prescribing providers including residents and infectious disease specialists; (2) nurses; (3) administrators who are focused on cost effectiveness; (4) patients and their families, who want to get well affordably, without side effects; (5) pharmacists; (6) risk management; and (7) society, which is fearful of the propagation of resistant microbes. In consulting with some of the stakeholders, 4 factors that influence hospitalists' prescribing patterns became apparent. These are practitioner factors, environmental factors, perceived rewards, and perceived threats (Fig. 1).

The practitioner factors shaping prescribing are: (1) knowledge of current best care; (2) self‐efficacy, which determines whether a provider is confident in his or her knowledge to adequately treat a specific infection; (3) habit, which causes providers to pick from a narrow repertoire of antibiotics when treating an infection; and (4) fear of liability, which forces some providers to be cautious. Four environmental factors affecting antibiotic prescriptions are: (1) published guidelines regarding organisms' sensitivity to antibiotics; (2) patient‐driven factors such as affordability, compliance with dosing regimens, side effects, and interactions between the antibiotics and other medications; (3) peer influence, in that providers are reluctant to change a prescription started by another provider (eg, emergency room physician); and (4) the formulary of the hospital, as it forces providers to prescribe within specific parameters. The perceived rewards of specific prescribing practices may include improving patient safety and reducing antibiotic resistance and costs, whereas the perceived threats are increasing antimicrobial resistance, having adverse patient outcomes, and increasing costs and hospital length of stay. We selected a high‐yield, low‐effort intervention in order to have an impact on some of the factors underlying hospitalists' prescribing patterns.
METHODS
Participants
The study participants were 17 hospitalist practitioners including physicians, nurse‐practitioners, and physician assistants who make up the Collaborative Inpatient Medical Service (CIMS) at Johns Hopkins Bayview Medical Center (JHBMC; Table 1). All consented to participate. The study was approved by the institutional review board.
| Age in years, mean (SD) | 36 (6) |
| Female, n (%) | 13 (76%) |
| Physician, n (%) | 9 (53%) |
| Nurse‐practitioner, n (%) | 5 (29%) |
| Physician assistant, n (%) | 3 (18%) |
| Years in practice, mean (SD) | 5.1 (2.8) |
| Number of pharmaceutical representatives exposed to in past year, mean | 1 |
| Number of shifts worked per month, mean (SD) | 14 (4) |
| Primarily works days, n (%) | 13 (76%) |
Data Collection
We collected and assessed prescription patterns over 3 periods: preintervention, interim, and postintervention.
Assessing Appropriateness of Antibiotics
For each order that was assessed in the preintervention, interim, and postintervention periods, the following information was collected: (1) drug ordered, (2) clinical diagnosis, (3) microbiology results available at the time of the order (including relevant results from recent cultures), (4) other medical diagnoses (ICD9 codes), (5) allergies, and (6) exposure to health care facilities (within the past 30 days). The computerized medical record allowed access to the discharge summaries of a patient's hospitalization. These records summarized the patient's hospitalization, allowing the investigators to understand the reasons for a provider's choice of antibiotics. If the rationale was not clear about how to categorize a prescription from reading the data, the investigators performed a chart review. From the information culled from these reviews, the primary investigator and an infectious disease specialist classified each prescription order by consensus as appropriate, effective but inappropriate, or inappropriate therapy.
Prescriptions were classified as appropriate when they were indicated and correlated with sensitivities, if available, or were of a narrow‐enough spectrum and recommended as a first‐line treatment for specific illnesses by either the Johns Hopkins Antibiotic Guide14 or the Stanford Guide to Antimicrobial Therapy.15 For example, cephalexin to treat uncomplicated cellulitis was considered appropriate therapy. Effective but inappropriate prescriptions were broad‐spectrum antibiotics used to treat an infection when a narrower‐spectrum antibiotic would have sufficed. For example, piperacillin‐tazobactam would be effective in treating a simple urinary tract infection but inappropriate to use because of its broad spectrum. Other examples of effective but inappropriate prescriptions were giving an IV when an oral alternative would be equally effective and tolerated or prescribing antibiotic treatment whose duration was too long. Finally, inappropriate prescriptions were those written for conditions for which antibiotics are not indicated or for which the prescribed antibiotic was ineffective for the specified infection (bug‐drug mismatch).
Preintervention
In January 2006 the investigators retrospectively reviewed the prescribing patterns of the 17 providers over the previous year. Using the computerized medical record and physician order entry, consecutive prescriptions of each provider were evaluated, beginning December 31, 2005, going back reverse chronologically until 20 prescriptions had been identified. For 12 of the providers, it was actually possible to review 20 prescriptions. For 2 other providers, both new, part‐time additions to the hospitalist group, only 1 and 7 prescriptions were found for the entire year. The prescribing history of the 3 remaining providers who participated in the study, all physician assistants, could not be evaluated (during any period) because all their orders were linked only to physicians, making it impossible to determine their specific prescriptions using the physician order entry system.
Interim
During the interim period between obtaining informed consent and completing the academic detailing (January 3, 2006, to March 23, 2006), provider prescribing patterns were reviewed to determine if the mere knowledge of the project would produce changes in prescribing behavior.
Postintervention
After the academic detailing was completed (March 23, 2006), the prescribing patterns of the hospitalists were followed through April 23, 2006. Each week after the detailing session, the hospitalists received reminders to prescribe appropriately (including pens with the message Reduce the Overuse).
Detailing Procedures
After the review, a profile was assembled for each of the CIMS providers. The study team detailers (a physician and a pharmacist) met with the individual providers for 30 to 45 minutes. Each hospitalist participant completed a short survey that collected demographic information and was asked about the rationale for his or her antibiotic prescribing pattern. Next, the appraisal of the provider's prescribing pattern was reviewed. This review included looking at the costs of the prescribed antibiotics compared with those of the appropriate alternatives and a reexamination of the guidelines for the selected target drugspiperacillin‐tazobactam, vancomycin, and extended‐spectrum quinolones. These 3 antibiotics were picked because our providers had been particularly vulnerable to inappropriately prescribing them. The hospitalists were provided an antibiotic guide developed specifically for this project and based on the Johns Hopkins Antibiotic Guide14 that summarizes the consensus guidelines.
Data Analysis
The primary outcome variable was the aggregate proportion of inappropriate antibiotic prescribed (as defined earlier) before the intervention, during the interim between obtaining informed consent and intervening on all study subjects, and after the intervention. The percentage of appropriate prescriptions versus total not appropriate prescriptions (combining of the effective but inappropriate and inappropriate categories) were compared across the 3 periods. Ninety‐five percent confidence intervals for comparisons of the proportions were determined using Stata 9.0 (College Station, TX). The difference between the proportions of total not appropriate prescriptions before and after academic detailing was computed in Stata using Fisher's exact test to assess significance.
RESULTS
Demographic information and professional characteristics of the 17 providers are shown in Table 1. Their mean age was 36 years, and 76% were female. The top 4 reasons the providers gave for their prescribing practices were: (1) published guidelines, (2) easier dosing schedule for patient when discharged, (3) continuing an antibiotic course initiated in the emergency room, and (4) broad‐spectrum antibiotics cover all possible microbes.
Comparison of Preintervention, Interim, and Postintervention Periods
Table 2 depicts the results of the prescription appraisals from the retrospective reviews. Of the 14 providers who had ordered antibiotics, 8 (57%) had more prescriptions that were total not appropriate than were appropriate in the preintervention period compared with 3 providers (25%) with this prescribing pattern in the postintervention period (P = .13).
| Provider | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|
| Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | |
| ||||||
| 1 | 20 | 7 (35%) | 13 (65%) | 24 | 17 (70.8%) | 7 (29.2%) |
| 2 | 20 | 10 (50%) | 10 (50%) | 12 | 11 (91.7%) | 1 (8.3%) |
| 3 | 20 | 6 (30%) | 14 (70%) | 8 | 8 (100%) | 0 (0%) |
| 4* | 19 | 10 (52.6%) | 9 (47.4%) | 4 | 3 (75%) | 1 (25%) |
| 5 | 20 | 9 (45%) | 11 (55%) | 10 | 4 (40%) | 6 (60%) |
| 6 | 20 | 5 (25%) | 15 (75%) | 3 | 1 (33.3%) | 2 (66.7%) |
| 7 | 20 | 8 (40%) | 12 (60%) | 8 | 7 (87.5%) | 1 (12.5%) |
| 8* | 1 | 0 (0%) | 1 (100%) | 0 | 0 (0%) | 0 (0%) |
| 9 | 20 | 11 (55%) | 9 (45%) | 5 | 2 (40%) | 3 (60%) |
| 10* | 7 | 3 (42.9%) | 4 (57.1%) | 0 | 0 (0%) | 0 (0%) |
| 11 | 20 | 10 (50%) | 10 (50%) | 17 | 13 (76.5%) | 4 (23.5%) |
| 12 | 20 | 6 (30%) | 14 (70%) | 16 | 14 (87.5%) | 2 (12.5%) |
| 13 | 20 | 12 (60%) | 8 (40%) | 15 | 11 (73.3%) | 4 (26.7%) |
| 14 | 20 | 10 (50%) | 10 (50%) | 7 | 4 (57.1%) | 3 (42.9%) |
| Total | 247 | 107 (43%) | 140 (57%) | 129 | 95 (73.6%) | 34 (26.4%) |
Table 3 shows the proportions of appropriate, effective but inappropriate, and total not appropriate prescriptions in the retrospective, interim, and postintervention periods. Forty‐three percent (95% CI 37%‐49%) of prescriptions were judged to be appropriate, and 57% (95% CI 51%‐63%) to be not appropriate prior to the academic detailing. In the interim period, 59% (95% CI 52%‐65%) of the prescriptions were appropriate, and 41% (95% CI 35%‐48%) were not appropriate; P = .0003. After the intervention, 74% (95% CI 65%‐81%) of the prescriptions were appropriate, and 26% (95% CI 19%‐35%) were not appropriate; P < .0001.
| Period | Appropriate, n (%) | 95% CI | Effective but inappropriate, n (%) | Inappropriate, n (%) | Total not appropriate, n (%) | 95% CI | P value* |
|---|---|---|---|---|---|---|---|
| |||||||
| Retrospective review (pre) | 107 (43%) | 37%‐49% | 75 (30.4%) | 65 (26.6%) | 140 (57%) | 51%‐63% | |
| Interim | 146 (59%) | 52%‐65% | 37 (15%) | 65 (26%) | 102 (41%) | 35%‐48% | .0003 |
| Postintervention | 95 (74%) | 65%‐81% | 8 (6%) | 26 (20%) | 34 (26%) | 19%‐35% | < .0001 |
DISCUSSION
We have demonstrated that academic detailing had a positive impact on the prescribing patterns of hospitalists. The aggregated improvement in antibiotic prescribing patterns can be attributed to improvement in the prescribing patterns of almost every hospitalist practitioner (Table 2). This study focused on aggregate prescriptions as the primary outcome measure because the hospitalists at JHBMC, like at many other institutions, function as a team, with a patient routinely having multiple providers over the course of the hospital stay. The improved prescribing patterns noted during the interim period suggest that the mere knowledge of a project can have an impact on providers. Providers informed the investigators that they were more thoughtful about their choice of antibiotics when they knew that they were being studied. The further statistically significant improvement in prescribing patterns with the intervention shows that the academic detailing itself was successful.
The greatest absolute change in practice was seen in effective but inappropriate prescribing (from 30.4% to 6%), whereas inappropriate prescribing only decreased from 26.6% to 20.6%. Although we aimed to have an impact on all inappropriate antibiotic prescribing patterns, we specifically reviewed the prescribing guidelines for piperacillin‐tazobactam, extended‐spectrum quinolones, and vancomycin. These 3 antibiotics were targeted because our providers had been particularly susceptible to inappropriately prescribing them. The focus on these antibiotics may have resulted in the larger absolute change noted in effective but inappropriate prescribing. We did not collect any data to determine if having an impact on effective but inappropriate prescribing changed the clinical course of the patients, such as shortening their hospital stays. Anecdotal evidence, however, suggests that it does. At our institution it is not uncommon for patients to be kept in the hospital for an extra day to ensure they are stable when transitioned from extended‐spectrum to narrower‐spectrum antibiotics prior to discharge. The effect of reducing effective but inappropriate prescriptions on the clinical course of patients could be an outcome measure assessed by a future, larger study.
Our one‐on‐one appraisal of each provider's prescribing patterns included a review of the cost of the prescribed antibiotics compared with that of the appropriate alternatives. Although decisions on antibiotic choice should be driven by clinical guidelines and appropriateness rather than price, we believed it was relevant to include education about costs and pricing so that providers would be reminded to ascertain whether patients would be able to afford their antibiotics. Antibiotic resistance is influenced by a patient's failure to complete the course of treatment, and noncompliance may be caused by an inability to afford the medication. Often, there are affordable, appropriate alternatives to the newest and most expensive drugs.
A hospitalist‐based academic detailing approach to improving antibiotic prescribing may have far‐reaching benefits and influence. First, it has the potential to affect other practitioners by setting an example and role modeling. In addition to that with their immediate peer group, hospitalists have close and repeated contact with house officers and emergency room physicians and often act as consultants to physicians in other departments such as surgery and psychiatry. Furthermore, some community hospitals have no infectious disease specialists readily available. So this represents an opportunity for hospitalists to promote quality in antibiotic prescribing. Practice‐based learning was very effective because it brought the practitioners face to face with their prescribing patterns. Although intellectually everyone agreed that antibiotics are often misused, this approach forced the providers to stop and reflect on their individual practices. This peer‐delivered intervention allowed for a collaborative approach to solving the problem; the peer (detailer) was approachable, nonjudgmental, and available for further discussion and guidance.
The public health quality improvement approach that we used for our intervention helped us to realize and appreciate the factors underlying prescribing patterns. Only by understanding the motivations for prescribing patterns can we hope to make sustainable changes. This coincides with our previous assertion that hospitalists are engaging in some public health practice.16 In pubic health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole.17 Hospitalist teams aim to make sure that the high‐quality services needed for protecting the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. Antibiotic optimization is a key role that could fall within the mantra of public health practice for the hospitalist.
Several limitations of this pilot should be considered. First, the intervention is labor intensive. However, it is essential to use the problem‐solving paradigm and incorporate behavior change theories in order to identify interventions that can lead to sustainable change. Second, this was not a randomized controlled trial, and it is possible that there might have been some contamination by external forces. However, in reviewing the educational events at our institution, the press, and articles published during the study period, we could not identify any external factors that would have influenced antibiotic prescribing patterns. It would not have been possible to conduct a randomized trial at our institution because the hospitalists work so closely together that we could not ensure complete separation if the subjects were randomized. There would have been contamination from the intervention group to the control group. A trial with randomization at the institution level is the next step. Third, the number of months retrospectively reviewed in order to identify 20 prescriptions of a provider varied. This study assumed there were no other differences during those months that could have affected provider prescribing behavior; this may have introduced some bias. Fourth, the sustainability of this intervention's positive impact is unknown. We assessed outcome soon after the intervention, and it is unknown whether continual booster sessions are required to maintain the positive impact on prescribing patterns.
This pilot was a good starting place to show that behavior change can be realized with a well‐conceived and methodically executed intervention, even among the busiest of physicians. Audit and feedback, or practice‐based learning, appears to be a powerful educational intervention among professionals who take great pride in their work.
- ,.Improving antibiotic use in low‐income countries: an overview of evidence on determinants.Soc Sci Med.2003;57:733–744.
- .Mechanisms of antimicrobial resistance in bacteria.Am J Med.2006;119(6A):S3–S10.
- .Antimicrobial resistance in gram‐positive bacteria.Am J Med.2006;119(6A):S11–S19.
- .Resistance in Gram‐negative bacteria: enterobacteriaceae.Am J Med.2006;119(6A):S20–S28.
- .Pharmacodynamics: relation to antimicrobial resistance.Am J Med.2006;119(6A):S37–S44.
- .Managing methicillin‐resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms.Am J Med.2006;119(6A):S45–S52.
- NIH. The Problem of Antibiotic Resistance. Available at: http://www.niaid.nih.gov.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed.J Hosp Infect.2001;47:198–209.
- ,.The impact of hospital‐acquired bloodstream infections.Emerg Infect Dis.2001;7(2):174–177.
- .Antimicrobial stewardship.Am J Med.2006;119(6A):S53–S61
- ,,, et al.Changing provider behavior: an overview of systemic reviews of interventions.Med Care.2001;39:II–2‐II‐45.
- .A review of current health education theories.Calif J Health Promot.2004;2:74–87
- The Johns Hopkins Hospital Antibiotic Management Program. 2005 Antibiotic Guidelines: Treatment Recommendations for Adult Inpatients. Johns Hopkins Medicine.
- ,,,.The Sanford Guide to Antimicrobial Therapy 2005.35th ed.Hyde Park, VT:Antimicrobial Therapy, Inc.;2005.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
- ,.Improving antibiotic use in low‐income countries: an overview of evidence on determinants.Soc Sci Med.2003;57:733–744.
- .Mechanisms of antimicrobial resistance in bacteria.Am J Med.2006;119(6A):S3–S10.
- .Antimicrobial resistance in gram‐positive bacteria.Am J Med.2006;119(6A):S11–S19.
- .Resistance in Gram‐negative bacteria: enterobacteriaceae.Am J Med.2006;119(6A):S20–S28.
- .Pharmacodynamics: relation to antimicrobial resistance.Am J Med.2006;119(6A):S37–S44.
- .Managing methicillin‐resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms.Am J Med.2006;119(6A):S45–S52.
- NIH. The Problem of Antibiotic Resistance. Available at: http://www.niaid.nih.gov.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed.J Hosp Infect.2001;47:198–209.
- ,.The impact of hospital‐acquired bloodstream infections.Emerg Infect Dis.2001;7(2):174–177.
- .Antimicrobial stewardship.Am J Med.2006;119(6A):S53–S61
- ,,, et al.Changing provider behavior: an overview of systemic reviews of interventions.Med Care.2001;39:II–2‐II‐45.
- .A review of current health education theories.Calif J Health Promot.2004;2:74–87
- The Johns Hopkins Hospital Antibiotic Management Program. 2005 Antibiotic Guidelines: Treatment Recommendations for Adult Inpatients. Johns Hopkins Medicine.
- ,,,.The Sanford Guide to Antimicrobial Therapy 2005.35th ed.Hyde Park, VT:Antimicrobial Therapy, Inc.;2005.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
Copyright © 2008 Society of Hospital Medicine