User login
Enhanced End‐of‐Life Care and RRTs
In 2007, the Joint Commission for Accreditation of Healthcare Organizations (JCAHO) recommended deployment of rapid response teams (RRTs) in U.S. hospitals to hasten identification and treatment of physiologically unstable hospitalized patients.1 Clinical studies that have focused on whether RRTs improve restorative care outcomes, frequency of cardiac arrest, and critical care utilization have yielded mixed results.2‐11 One study suggested that RRTs might provide an opportunity to enhance palliative care of hospitalized patients.11 In this study, RRT personnel felt that prior do‐not‐resuscitate orders would have been appropriate in nearly a quarter of cases. However, no previous study has examined whether the RRT might be deployed to identify acutely decompensating patients who either do not want or would not benefit from a trial of aggressive restorative treatments. We hypothesized that actuation of an RRT in our hospital would expedite identification of patients not likely to benefit from restorative care and would promote more timely commencement of end‐of‐life comfort care, thereby improving their quality of death (QOD).12‐16
Materials and Methods
Study Design and Settings
This retrospective cohort study was approved by the Institutional Review Board (IRB) of and conducted at Bridgeport Hospital, a 425‐bed community teaching hospital. In October 2006, the hospital deployed its RRT, which includes a critical care nurse, respiratory therapist, and second‐year Medicine resident. Nurses on the hospital wards received educational in‐service training instructing them to request an RRT evaluation for: airway incompetence, oxygen desaturation despite fraction of inspired oxygen (FiO2) 60%, respiratory frequency 8 or >30/minute, heart rate 50 or >110/minute, systolic pressure 90 or >180 mmHg, acute significant bleeding, sudden neurologic changes, or patient changes that troubled the nurse. The critical care nurse and respiratory therapist responded to all calls. If assessment suggested a severe problem that required immediate physician supervision, the resident was summoned immediately. Otherwise, the nurse assessed the patient and suggested to the patient's primary doctor of record a trial of therapies. If ratified, the therapies were provided by the nurse and respiratory therapist until symptoms/signs resolved or failed to improve, in which case the resident‐physician was summoned. The resident‐physician would assess, attempt further relieving therapies, and, if appropriate, arrange for transfer to critical care units (in which case the case was presented to the staff intensivist who supervised care) after discussion with the patient and attending physician. No organizational changes in the administration or education of palliative care were implemented during the study period.
Data Extraction and Analysis
All patients dying in the hospital during the first 8 months of RRT activity (October 1, 2006 to May 31, 2007) and during the same months in the year prior to RRT were eligible for the study. Patients were excluded if they died in areas of the hospital not covered by the RRT, such as intensive care units, operating rooms, emergency department, recovery areas, or pediatric floors, or if they had been admitted or transferred to hospital wards with palliative care/end‐of‐life orders.
Physiologic data, including blood pressures (lowest), heart rate (highest), and respiratory rate (highest), were extracted from records of the 48 hours before and until resolution of the RRT assessment, or prior to death for those without RRT care. Outcomes were defined by World Health Organization (WHO) domains of palliative care (symptoms, social, and spiritual).14 The symptom domain was measured using patients' pain scores, 24 hours prior to death (0‐10). Subjective reports of healthcare providers recorded in hospital records, including the terms suffering, pain, anxiety, or distress were also extracted from notes 24 hours prior to patients' deaths. Administration of opioids in the 24 hours prior to death was also recorded. Social and spiritual domains were measured by documentation of presence of the family and chaplain, respectively, at the bedside in the 24 hours prior to death.
Analysis was performed using SPSS software (SPSS Inc., Chicago, IL). Categorical variables, described as proportions, were compared with chi‐square tests. Continuous variables are reported as means standard errors, or as medians with the interquartile ranges. Means were compared using Student t test if a normal distribution was detected. Nonparametric variables were compared with Wilcoxon rank sum tests. To adjust for confounding and assess possible effect modification, multiple logistic regression, multiple linear regression, and stratified analyses were performed when appropriate. Domains of the QOD were compared between patients who died in the pre‐RRT and post‐RRT epochs. Patients who died on hospital wards without RRT evaluation in the post‐RRT epoch were compared to those who died following RRT care. Unadjusted in‐hospital mortality, frequency of cardiopulmonary resuscitation, frequency of transfer from wards to critical care, and QOD were compiled and compared. A P value of 0.05 was considered statistically significant.
Results
A total of 394 patients died on the hospital wards and were not admitted with palliative, end‐of‐life medical therapies. The combined (pre‐RRT and post‐RRT epochs) cohort had a mean age of 77.2 13.2 years. A total of 48% were male, 79% White, 12% Black, and 8% Hispanic. A total of 128 patients (33%) were admitted to the hospital from a skilled nursing facility and 135 (35%) had written advance directives.
A total of 197 patients met the inclusion criteria during the pre‐RRT (October 1, 2005 to May 31, 2006) and 197 during the post‐RRT epochs (October 1, 2006 to May 31, 2007). There were no differences in age, sex, advance directives, ethnicity, or religion between the groups (Table 1). Primary admission diagnoses were significantly different; pre‐RRT patients were 9% more likely to die with malignancy compared to post‐RRT patients and less likely to come from nursing homes (38% vs. 27%; P = 0.02).
| Total | Pre‐RRT | Post‐RRT | P value | |
|---|---|---|---|---|
| ||||
| Total admissions | 25,943 | 12,926 | 13,017 | |
| Number of deaths | 394 | 197 | 197 | NS |
| Age (years) | 77.5 13.2 | 77.1 13.36 | 77.9 13.13 | 0.5 |
| Male gender | 190 (48%) | 99 (51%) | 91 (46%) | 0.4 |
| From SNF | 128 (32%) | 54 (27%) | 74 (38%) | 0.02 |
| Living will | 135 (34%) | 66 (33%) | 69 (35%) | 0.8 |
| Race | 0.3 | |||
| White | 314 (80%) | 163 (83%) | 151 (77%) | |
| Hispanic | 32 (8%) | 14 (7%) | 18 (9%) | |
| Black | 47 (12%) | 19 (10%) | 28 (14%) | |
| Other | 1 (1%) | 1 (1%) | 0 | |
| Religion (%) | 0.8 | |||
| Christian | 357 (91%) | 177 (90%) | 180 (91%) | |
| Non‐Christian | 37 (9%) | 20 (10%) | 17 (9%) | |
| Admission diagnosis | 0.01 | |||
| Malignancy | 96 (24%) | 56 (28%) | 40 (20%) | * |
| Sepsis | 44 (11%) | 21 (11%) | 23 (12%) | |
| Respiratory | 98 (25%) | 53 (27%) | 45 (23%) | * |
| Stroke | 31 (8%) | 16 (8%) | 15 (8%) | |
| Cardiac | 66 (17%) | 37 (19%) | 29 (15%) | * |
| Hepatic failure | 9 (2%) | 4 (2%) | 5 (2%) | |
| Surgical | 17 (5%) | 6 (3%) | 11 (5%) | |
| Others | 33 (8%) | 4 (2%) | 29 (15%) | * |
| Team | 0.01 | |||
| Medicine | 155 (39%) | 64 (32%) | 94 (47%) | |
| MICU | 44 (11%) | 3 (2%) | 41 (21%) | * |
| Surgery | 12 (3%) | 9 (5%) | 3 (1%) | |
| Restorative outcomes | ||||
| Mortality/1000 | 27/1000 | 30/1000 | 0.9 | |
| Unexpected ICU transfers/1000 | 17/1000 | 19/1000 | 0.8 | |
| CPR/1000 | 3/1000 | 2.5/1000 | 0.9 | |
Restorative Care Outcomes
Crude, unadjusted, in‐hospital mortality (27 vs. 30/1000 admissions), unexpected transfers to intensive care (17 vs. 19/1000 admissions), or cardiac arrests (3 vs. 2.5/1000 admissions) were similar in pre‐RRT and post‐RRT periods (all P > 0.05).
End‐of‐Life Care
At the time of death, 133 patients (68%) who died during the post‐RRT epoch had comfort care only orders whereas 90 (46%) had these orders in the pre‐RRT group (P = 0.0001; Table 2a). Post‐RRT patients were more likely than pre‐RRT patients to receive opioids prior to death (68% vs. 43%, respectively; P = 0.001) and had lower maximum pain scores in their last 24 hours (3.0 3.5 vs. 3.7 3.2; respectively; P = 0.045). Mention of patient distress by nurses in the hospital record following RRT deployment was less than one‐half of that recorded in the pre‐RRT period (26% vs. 62%; P = 0.0001). A chaplain visited post‐RRT patients in the 24 hours prior to death more frequently than in the pre‐RRT period (72% vs. 60%; P = 0.02). The frequency of family at the bedside was similar between epochs (61% post‐RRT vs. 58% pre‐RRT; P = 0.6). These findings were consistent across common primary diagnoses and origins (home vs. nursing home).
| a. Prior to RRT vs. During RRT Deployment | |||
|---|---|---|---|
| Pre‐RRT (n = 197) | Post‐RRT (n = 197) | P Value | |
| Comfort care only | 90 (46%) | 133 (68%) | 0.0001 |
| Pain score (0‐10) | 3.7 3.3 | 3.0 3.5 | 0.045 |
| Opioids administered | 84 (43%) | 134 (68%) | 0.0001 |
| Subjective suffering | 122 (62%) | 52 (26%) | 0.0001 |
| Family present | 115 (58%) | 120 (61%) | 0.6 |
| Chaplain present | 119 (60%) | 142 (72%) | 0.02 |
| b. During RRT Deployment: Those Dying with RRT Assessment vs. Those Dying Without | |||
| Post‐RRT RRT Care (n = 61) | Post‐RRT No RRT Care (n = 136) | P Value | |
| Comfort care only | 46 (75%) | 87 (64%) | 0.1 |
| Pain score (0‐10) | 3.0 3.5 | 3.0 3.5 | 0.9 |
| Opioids administered | 42 (69%) | 92 (67%) | 0.8 |
| Subjective suffering | 18 (29%) | 34 (25%) | 0.9 |
| Family present | 43 (71%) | 77 (57%) | 0.06 |
| Chaplain present | 49 (80%) | 93 (68%) | 0.0001 |
| c. Comparing Before and During RRT Deployment: Those Dying Without RRT Assessment | |||
| Pre‐RRT (n = 197) | Post‐RRT No RRT Care (n = 136) | P Value | |
| Comfort care (only) | 90 (46%) | 87 (64%) | 0.0001 |
| Pain score (0‐10) | 3.7 3.3 | 3.0 3.5 | 0.06 |
| Opioids administered | 84 (43%) | 92 (67%) | 0.0001 |
| Subjective suffering | 122 (62%) | 34 (25%) | 0.0001 |
| Family present | 115 (58%) | 77 (56.6%) | 0.8 |
| Chaplain present | 119 (60) | 74 (54.4%) | 0.2 |
Adjusting for age, gender, and race, the odds ratio (OR) of patients receiving formal end‐of‐life medical orders in post‐RRT was 2.5 that of pre‐RRT (95% confidence interval [CI], 1.7‐3.8), and odds of receiving opioids prior to death were nearly 3 times pre‐RRT (OR, 2.8; 95% CI, 1.9‐4.3). The odds of written mention of post‐RRT patients' suffering in the medical record was less than one‐fourth that of pre‐RRT patients (OR, 0.23; 95% CI, 0.2‐0.4).
To examine whether temporal trends might account for observed differences, patients in the post‐RRT period who received RRT care were compared to those who did not. Sixty‐one patients died with RRT assessments, whereas 136 died without RRT evaluations. End‐of‐life care outcomes were similar for these 2 groups, except more patients with RRT care had chaplain visits proximate to the time of death (80% vs. 68%; P = 0.0001; Table 2b). Outcomes (including comfort care orders, opioid administration, and suffering) of dying patients not cared for by the RRT (after deployment) were superior to those of pre‐RRT dying patients (Table 2c).
Discussion
This pilot study hypothesizes that our RRT impacted patients' QOD. Deployment of the RRT in our hospital was associated with improvement in both symptom and psychospiritual domains of care. Theoretically, RRTs should improve quality‐of‐care via early identification/reversal of physiologic decompensation. By either reversing acute diatheses with an expeditious trial of therapy or failing to reverse with early actuation of palliative therapies, the duration and magnitude of human suffering should be reduced. Attenuation of both duration and magnitude of suffering is the ultimate goal of both restorative and palliative care and is as important an outcome as mortality or length of stay. Previous studies of RRTs have focused on efficacy in reversing the decompensation: preventing cardiopulmonary arrest, avoiding the need for invasive, expensive, labor‐intensive interventions. Our RRT, like others, had no demonstrable impact on restorative outcomes. However, deployment of the RRT was highly associated with improved QOD of our patients. The impact was significant across WHO‐specified domains: pain scores decreased by 19%; (documentation of) patients' distress decreased by 50%; and chaplains' visits were more often documented in the 24 hours prior to death. These relationships held across common disease diagnoses, so the association is unlikely to be spurious.
Outcomes were similarly improved in patients who did not receive RRT care in the post‐RRT epoch. Our hospital did not have a palliative care service in either time period. No new educational efforts among physicians or nurses accounted for this observation. While it is possible that temporal effects accounted for our observation, an equally plausible explanation is that staff observed RRT interventions and applied them to dying patients not seen by the RRT. Our hospital educated caregivers regarding the RRT triggers, and simply making hospital personnel more vigilant for signs of suffering and/or observing the RRT approach may have contributed to enhanced end‐of‐life care for non‐RRT patients.
There are a number of limitations in this study. First, the sample size was relatively small compared to other published studies,2‐11 promoting the possibility that either epoch was not representative of pre‐RRT and post‐RRT parent populations. Another weakness is that QOD was measured using surrogate endpoints. The dead cannot be interviewed to definitively examine QOD; indices of cardiopulmonary distress and psychosocial measures (eg, religious preparations, family involvement) are endpoints suggested by palliative care investigators12, 13 and the World Health Organization.14 While some validated tools17 and consensus measures18 exist for critically ill patients, they do not readily apply to RRT patients. Retrospective records reviews raise the possibility of bias in extracting objective and subjective data. While we attempted to control for this by creating uniform a priori rules for data acquisition (ie, at what intervals and in which parts of the record they could be extracted), we cannot discount the possibility that bias affected the observed results. Finally, improvements in end‐of‐life care could have resulted from temporal trends. This retrospective study cannot prove a causeeffect relationship; a prospective randomized trial would be required to answer the question definitively. Based on the available data suggesting some benefit in restorative outcomes2‐8 and pressure from federal regulators to deploy RRTs regardless,1 a retrospective cohort design may provide the only realistic means of addressing this question.
In conclusion, this is the first (pilot) study to examine end‐of‐life outcomes associated with deployment of an RRT. While the limitations of these observations preclude firm conclusions, the plausibility of the hypothesis, coupled with our observations, suggests that this is a fertile area for future research. While RRTs may enhance restorative outcomes, to the extent that they hasten identification of candidates for palliative end‐of‐life‐care, before administration of invasive modalities that some patients do not want, these teams may simultaneously serve patients and reduce hospital resource utilization.
Addendum
Prior to publication, a contemporaneous study was published that concluded: These findings suggest that rapid response teams may not be decreasing code rates as much as catalyzing a compassionate dialogue of end‐of‐life care among terminally ill patients. This ability to improve end‐of‐life care may be an important benefit of rapid response teams, particularly given the difficulties in prior trials to increase rates of DNR status among seriously ill inpatients and potential decreases in resource use. Chan PS, Khalid A, Longmore LS, Berg RA, Midhail Kosiborod M, Spertus JA. Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA 2008;300: 25062513.
- Joint Commission on the Accreditation of Healthcare Organizations. The Joint Commission 2007 National Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/BD4D59E0‐6D53‐404C‐8507‐883AF3BBC50A/0/audio_conference_091307.pdf. Accessed February2009.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.The effect of a MET team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: a preliminary study.BMJ.2002;324:1–5.
- ,,, et al.Long‐term effect of a medical emergency team on mortality in a teaching hospital.Resuscitation.2007;74:235–241.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Long‐term effect of a rapid response team on cardiac arrests in a teaching hospital.Crit Care.2005;R808–R815.
- ,,, et al.The effect of a rapid response team on major clinical outcome measures in a community teaching hospital.Crit Care Med.2007;35:2076–2082.
- ,,, et al.Introduction of a rapid response team (RRT) system: a cluster‐randomised trail.Lancet.2005;365:2901–2907.
- ,,, et al.Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a children's hospital.JAMA.2007;298:2267–2274.
- ,,,,.The medical emergency team: 12 month analysis of reasons for activation, immediate outcome and not‐for‐resuscitation orders.Resuscitation.2001;50:39–44.
- ,,.Evaluating the quality of dying and death.J Pain Symptom Manage.2001;22:717–726.
- ,.Measuring success of interventions to improve the quality of end‐of‐life care in the intensive care unit.Crit Care Med.2006;34:S341–S347.
- World Health Organization. WHO definition of palliative care. Available at: http://www.who.int/cancer/palliative/definition/en. Accessed February 2009.
- .Does a living will equal a DNR? Are living wills compromising patient safety?J Emerg Med.2007;33:299–305.
- ,,,,,.Quality of dying and death in two medical ICUs.Chest.2005;127:1775–1783.
- ,,,.Using the medical record to evaluate the quality of end‐of‐life care in the intensive care unit.Crit Care Med.2008;36:1138–1146.
- ,,, et al.Proposed quality of measures for palliative care in the critically ill: a consensus from the Robert Wood Johnson Foundation Critical Care Workgroup.Crit Care Med.2006;34:S404–S411.
In 2007, the Joint Commission for Accreditation of Healthcare Organizations (JCAHO) recommended deployment of rapid response teams (RRTs) in U.S. hospitals to hasten identification and treatment of physiologically unstable hospitalized patients.1 Clinical studies that have focused on whether RRTs improve restorative care outcomes, frequency of cardiac arrest, and critical care utilization have yielded mixed results.2‐11 One study suggested that RRTs might provide an opportunity to enhance palliative care of hospitalized patients.11 In this study, RRT personnel felt that prior do‐not‐resuscitate orders would have been appropriate in nearly a quarter of cases. However, no previous study has examined whether the RRT might be deployed to identify acutely decompensating patients who either do not want or would not benefit from a trial of aggressive restorative treatments. We hypothesized that actuation of an RRT in our hospital would expedite identification of patients not likely to benefit from restorative care and would promote more timely commencement of end‐of‐life comfort care, thereby improving their quality of death (QOD).12‐16
Materials and Methods
Study Design and Settings
This retrospective cohort study was approved by the Institutional Review Board (IRB) of and conducted at Bridgeport Hospital, a 425‐bed community teaching hospital. In October 2006, the hospital deployed its RRT, which includes a critical care nurse, respiratory therapist, and second‐year Medicine resident. Nurses on the hospital wards received educational in‐service training instructing them to request an RRT evaluation for: airway incompetence, oxygen desaturation despite fraction of inspired oxygen (FiO2) 60%, respiratory frequency 8 or >30/minute, heart rate 50 or >110/minute, systolic pressure 90 or >180 mmHg, acute significant bleeding, sudden neurologic changes, or patient changes that troubled the nurse. The critical care nurse and respiratory therapist responded to all calls. If assessment suggested a severe problem that required immediate physician supervision, the resident was summoned immediately. Otherwise, the nurse assessed the patient and suggested to the patient's primary doctor of record a trial of therapies. If ratified, the therapies were provided by the nurse and respiratory therapist until symptoms/signs resolved or failed to improve, in which case the resident‐physician was summoned. The resident‐physician would assess, attempt further relieving therapies, and, if appropriate, arrange for transfer to critical care units (in which case the case was presented to the staff intensivist who supervised care) after discussion with the patient and attending physician. No organizational changes in the administration or education of palliative care were implemented during the study period.
Data Extraction and Analysis
All patients dying in the hospital during the first 8 months of RRT activity (October 1, 2006 to May 31, 2007) and during the same months in the year prior to RRT were eligible for the study. Patients were excluded if they died in areas of the hospital not covered by the RRT, such as intensive care units, operating rooms, emergency department, recovery areas, or pediatric floors, or if they had been admitted or transferred to hospital wards with palliative care/end‐of‐life orders.
Physiologic data, including blood pressures (lowest), heart rate (highest), and respiratory rate (highest), were extracted from records of the 48 hours before and until resolution of the RRT assessment, or prior to death for those without RRT care. Outcomes were defined by World Health Organization (WHO) domains of palliative care (symptoms, social, and spiritual).14 The symptom domain was measured using patients' pain scores, 24 hours prior to death (0‐10). Subjective reports of healthcare providers recorded in hospital records, including the terms suffering, pain, anxiety, or distress were also extracted from notes 24 hours prior to patients' deaths. Administration of opioids in the 24 hours prior to death was also recorded. Social and spiritual domains were measured by documentation of presence of the family and chaplain, respectively, at the bedside in the 24 hours prior to death.
Analysis was performed using SPSS software (SPSS Inc., Chicago, IL). Categorical variables, described as proportions, were compared with chi‐square tests. Continuous variables are reported as means standard errors, or as medians with the interquartile ranges. Means were compared using Student t test if a normal distribution was detected. Nonparametric variables were compared with Wilcoxon rank sum tests. To adjust for confounding and assess possible effect modification, multiple logistic regression, multiple linear regression, and stratified analyses were performed when appropriate. Domains of the QOD were compared between patients who died in the pre‐RRT and post‐RRT epochs. Patients who died on hospital wards without RRT evaluation in the post‐RRT epoch were compared to those who died following RRT care. Unadjusted in‐hospital mortality, frequency of cardiopulmonary resuscitation, frequency of transfer from wards to critical care, and QOD were compiled and compared. A P value of 0.05 was considered statistically significant.
Results
A total of 394 patients died on the hospital wards and were not admitted with palliative, end‐of‐life medical therapies. The combined (pre‐RRT and post‐RRT epochs) cohort had a mean age of 77.2 13.2 years. A total of 48% were male, 79% White, 12% Black, and 8% Hispanic. A total of 128 patients (33%) were admitted to the hospital from a skilled nursing facility and 135 (35%) had written advance directives.
A total of 197 patients met the inclusion criteria during the pre‐RRT (October 1, 2005 to May 31, 2006) and 197 during the post‐RRT epochs (October 1, 2006 to May 31, 2007). There were no differences in age, sex, advance directives, ethnicity, or religion between the groups (Table 1). Primary admission diagnoses were significantly different; pre‐RRT patients were 9% more likely to die with malignancy compared to post‐RRT patients and less likely to come from nursing homes (38% vs. 27%; P = 0.02).
| Total | Pre‐RRT | Post‐RRT | P value | |
|---|---|---|---|---|
| ||||
| Total admissions | 25,943 | 12,926 | 13,017 | |
| Number of deaths | 394 | 197 | 197 | NS |
| Age (years) | 77.5 13.2 | 77.1 13.36 | 77.9 13.13 | 0.5 |
| Male gender | 190 (48%) | 99 (51%) | 91 (46%) | 0.4 |
| From SNF | 128 (32%) | 54 (27%) | 74 (38%) | 0.02 |
| Living will | 135 (34%) | 66 (33%) | 69 (35%) | 0.8 |
| Race | 0.3 | |||
| White | 314 (80%) | 163 (83%) | 151 (77%) | |
| Hispanic | 32 (8%) | 14 (7%) | 18 (9%) | |
| Black | 47 (12%) | 19 (10%) | 28 (14%) | |
| Other | 1 (1%) | 1 (1%) | 0 | |
| Religion (%) | 0.8 | |||
| Christian | 357 (91%) | 177 (90%) | 180 (91%) | |
| Non‐Christian | 37 (9%) | 20 (10%) | 17 (9%) | |
| Admission diagnosis | 0.01 | |||
| Malignancy | 96 (24%) | 56 (28%) | 40 (20%) | * |
| Sepsis | 44 (11%) | 21 (11%) | 23 (12%) | |
| Respiratory | 98 (25%) | 53 (27%) | 45 (23%) | * |
| Stroke | 31 (8%) | 16 (8%) | 15 (8%) | |
| Cardiac | 66 (17%) | 37 (19%) | 29 (15%) | * |
| Hepatic failure | 9 (2%) | 4 (2%) | 5 (2%) | |
| Surgical | 17 (5%) | 6 (3%) | 11 (5%) | |
| Others | 33 (8%) | 4 (2%) | 29 (15%) | * |
| Team | 0.01 | |||
| Medicine | 155 (39%) | 64 (32%) | 94 (47%) | |
| MICU | 44 (11%) | 3 (2%) | 41 (21%) | * |
| Surgery | 12 (3%) | 9 (5%) | 3 (1%) | |
| Restorative outcomes | ||||
| Mortality/1000 | 27/1000 | 30/1000 | 0.9 | |
| Unexpected ICU transfers/1000 | 17/1000 | 19/1000 | 0.8 | |
| CPR/1000 | 3/1000 | 2.5/1000 | 0.9 | |
Restorative Care Outcomes
Crude, unadjusted, in‐hospital mortality (27 vs. 30/1000 admissions), unexpected transfers to intensive care (17 vs. 19/1000 admissions), or cardiac arrests (3 vs. 2.5/1000 admissions) were similar in pre‐RRT and post‐RRT periods (all P > 0.05).
End‐of‐Life Care
At the time of death, 133 patients (68%) who died during the post‐RRT epoch had comfort care only orders whereas 90 (46%) had these orders in the pre‐RRT group (P = 0.0001; Table 2a). Post‐RRT patients were more likely than pre‐RRT patients to receive opioids prior to death (68% vs. 43%, respectively; P = 0.001) and had lower maximum pain scores in their last 24 hours (3.0 3.5 vs. 3.7 3.2; respectively; P = 0.045). Mention of patient distress by nurses in the hospital record following RRT deployment was less than one‐half of that recorded in the pre‐RRT period (26% vs. 62%; P = 0.0001). A chaplain visited post‐RRT patients in the 24 hours prior to death more frequently than in the pre‐RRT period (72% vs. 60%; P = 0.02). The frequency of family at the bedside was similar between epochs (61% post‐RRT vs. 58% pre‐RRT; P = 0.6). These findings were consistent across common primary diagnoses and origins (home vs. nursing home).
| a. Prior to RRT vs. During RRT Deployment | |||
|---|---|---|---|
| Pre‐RRT (n = 197) | Post‐RRT (n = 197) | P Value | |
| Comfort care only | 90 (46%) | 133 (68%) | 0.0001 |
| Pain score (0‐10) | 3.7 3.3 | 3.0 3.5 | 0.045 |
| Opioids administered | 84 (43%) | 134 (68%) | 0.0001 |
| Subjective suffering | 122 (62%) | 52 (26%) | 0.0001 |
| Family present | 115 (58%) | 120 (61%) | 0.6 |
| Chaplain present | 119 (60%) | 142 (72%) | 0.02 |
| b. During RRT Deployment: Those Dying with RRT Assessment vs. Those Dying Without | |||
| Post‐RRT RRT Care (n = 61) | Post‐RRT No RRT Care (n = 136) | P Value | |
| Comfort care only | 46 (75%) | 87 (64%) | 0.1 |
| Pain score (0‐10) | 3.0 3.5 | 3.0 3.5 | 0.9 |
| Opioids administered | 42 (69%) | 92 (67%) | 0.8 |
| Subjective suffering | 18 (29%) | 34 (25%) | 0.9 |
| Family present | 43 (71%) | 77 (57%) | 0.06 |
| Chaplain present | 49 (80%) | 93 (68%) | 0.0001 |
| c. Comparing Before and During RRT Deployment: Those Dying Without RRT Assessment | |||
| Pre‐RRT (n = 197) | Post‐RRT No RRT Care (n = 136) | P Value | |
| Comfort care (only) | 90 (46%) | 87 (64%) | 0.0001 |
| Pain score (0‐10) | 3.7 3.3 | 3.0 3.5 | 0.06 |
| Opioids administered | 84 (43%) | 92 (67%) | 0.0001 |
| Subjective suffering | 122 (62%) | 34 (25%) | 0.0001 |
| Family present | 115 (58%) | 77 (56.6%) | 0.8 |
| Chaplain present | 119 (60) | 74 (54.4%) | 0.2 |
Adjusting for age, gender, and race, the odds ratio (OR) of patients receiving formal end‐of‐life medical orders in post‐RRT was 2.5 that of pre‐RRT (95% confidence interval [CI], 1.7‐3.8), and odds of receiving opioids prior to death were nearly 3 times pre‐RRT (OR, 2.8; 95% CI, 1.9‐4.3). The odds of written mention of post‐RRT patients' suffering in the medical record was less than one‐fourth that of pre‐RRT patients (OR, 0.23; 95% CI, 0.2‐0.4).
To examine whether temporal trends might account for observed differences, patients in the post‐RRT period who received RRT care were compared to those who did not. Sixty‐one patients died with RRT assessments, whereas 136 died without RRT evaluations. End‐of‐life care outcomes were similar for these 2 groups, except more patients with RRT care had chaplain visits proximate to the time of death (80% vs. 68%; P = 0.0001; Table 2b). Outcomes (including comfort care orders, opioid administration, and suffering) of dying patients not cared for by the RRT (after deployment) were superior to those of pre‐RRT dying patients (Table 2c).
Discussion
This pilot study hypothesizes that our RRT impacted patients' QOD. Deployment of the RRT in our hospital was associated with improvement in both symptom and psychospiritual domains of care. Theoretically, RRTs should improve quality‐of‐care via early identification/reversal of physiologic decompensation. By either reversing acute diatheses with an expeditious trial of therapy or failing to reverse with early actuation of palliative therapies, the duration and magnitude of human suffering should be reduced. Attenuation of both duration and magnitude of suffering is the ultimate goal of both restorative and palliative care and is as important an outcome as mortality or length of stay. Previous studies of RRTs have focused on efficacy in reversing the decompensation: preventing cardiopulmonary arrest, avoiding the need for invasive, expensive, labor‐intensive interventions. Our RRT, like others, had no demonstrable impact on restorative outcomes. However, deployment of the RRT was highly associated with improved QOD of our patients. The impact was significant across WHO‐specified domains: pain scores decreased by 19%; (documentation of) patients' distress decreased by 50%; and chaplains' visits were more often documented in the 24 hours prior to death. These relationships held across common disease diagnoses, so the association is unlikely to be spurious.
Outcomes were similarly improved in patients who did not receive RRT care in the post‐RRT epoch. Our hospital did not have a palliative care service in either time period. No new educational efforts among physicians or nurses accounted for this observation. While it is possible that temporal effects accounted for our observation, an equally plausible explanation is that staff observed RRT interventions and applied them to dying patients not seen by the RRT. Our hospital educated caregivers regarding the RRT triggers, and simply making hospital personnel more vigilant for signs of suffering and/or observing the RRT approach may have contributed to enhanced end‐of‐life care for non‐RRT patients.
There are a number of limitations in this study. First, the sample size was relatively small compared to other published studies,2‐11 promoting the possibility that either epoch was not representative of pre‐RRT and post‐RRT parent populations. Another weakness is that QOD was measured using surrogate endpoints. The dead cannot be interviewed to definitively examine QOD; indices of cardiopulmonary distress and psychosocial measures (eg, religious preparations, family involvement) are endpoints suggested by palliative care investigators12, 13 and the World Health Organization.14 While some validated tools17 and consensus measures18 exist for critically ill patients, they do not readily apply to RRT patients. Retrospective records reviews raise the possibility of bias in extracting objective and subjective data. While we attempted to control for this by creating uniform a priori rules for data acquisition (ie, at what intervals and in which parts of the record they could be extracted), we cannot discount the possibility that bias affected the observed results. Finally, improvements in end‐of‐life care could have resulted from temporal trends. This retrospective study cannot prove a causeeffect relationship; a prospective randomized trial would be required to answer the question definitively. Based on the available data suggesting some benefit in restorative outcomes2‐8 and pressure from federal regulators to deploy RRTs regardless,1 a retrospective cohort design may provide the only realistic means of addressing this question.
In conclusion, this is the first (pilot) study to examine end‐of‐life outcomes associated with deployment of an RRT. While the limitations of these observations preclude firm conclusions, the plausibility of the hypothesis, coupled with our observations, suggests that this is a fertile area for future research. While RRTs may enhance restorative outcomes, to the extent that they hasten identification of candidates for palliative end‐of‐life‐care, before administration of invasive modalities that some patients do not want, these teams may simultaneously serve patients and reduce hospital resource utilization.
Addendum
Prior to publication, a contemporaneous study was published that concluded: These findings suggest that rapid response teams may not be decreasing code rates as much as catalyzing a compassionate dialogue of end‐of‐life care among terminally ill patients. This ability to improve end‐of‐life care may be an important benefit of rapid response teams, particularly given the difficulties in prior trials to increase rates of DNR status among seriously ill inpatients and potential decreases in resource use. Chan PS, Khalid A, Longmore LS, Berg RA, Midhail Kosiborod M, Spertus JA. Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA 2008;300: 25062513.
In 2007, the Joint Commission for Accreditation of Healthcare Organizations (JCAHO) recommended deployment of rapid response teams (RRTs) in U.S. hospitals to hasten identification and treatment of physiologically unstable hospitalized patients.1 Clinical studies that have focused on whether RRTs improve restorative care outcomes, frequency of cardiac arrest, and critical care utilization have yielded mixed results.2‐11 One study suggested that RRTs might provide an opportunity to enhance palliative care of hospitalized patients.11 In this study, RRT personnel felt that prior do‐not‐resuscitate orders would have been appropriate in nearly a quarter of cases. However, no previous study has examined whether the RRT might be deployed to identify acutely decompensating patients who either do not want or would not benefit from a trial of aggressive restorative treatments. We hypothesized that actuation of an RRT in our hospital would expedite identification of patients not likely to benefit from restorative care and would promote more timely commencement of end‐of‐life comfort care, thereby improving their quality of death (QOD).12‐16
Materials and Methods
Study Design and Settings
This retrospective cohort study was approved by the Institutional Review Board (IRB) of and conducted at Bridgeport Hospital, a 425‐bed community teaching hospital. In October 2006, the hospital deployed its RRT, which includes a critical care nurse, respiratory therapist, and second‐year Medicine resident. Nurses on the hospital wards received educational in‐service training instructing them to request an RRT evaluation for: airway incompetence, oxygen desaturation despite fraction of inspired oxygen (FiO2) 60%, respiratory frequency 8 or >30/minute, heart rate 50 or >110/minute, systolic pressure 90 or >180 mmHg, acute significant bleeding, sudden neurologic changes, or patient changes that troubled the nurse. The critical care nurse and respiratory therapist responded to all calls. If assessment suggested a severe problem that required immediate physician supervision, the resident was summoned immediately. Otherwise, the nurse assessed the patient and suggested to the patient's primary doctor of record a trial of therapies. If ratified, the therapies were provided by the nurse and respiratory therapist until symptoms/signs resolved or failed to improve, in which case the resident‐physician was summoned. The resident‐physician would assess, attempt further relieving therapies, and, if appropriate, arrange for transfer to critical care units (in which case the case was presented to the staff intensivist who supervised care) after discussion with the patient and attending physician. No organizational changes in the administration or education of palliative care were implemented during the study period.
Data Extraction and Analysis
All patients dying in the hospital during the first 8 months of RRT activity (October 1, 2006 to May 31, 2007) and during the same months in the year prior to RRT were eligible for the study. Patients were excluded if they died in areas of the hospital not covered by the RRT, such as intensive care units, operating rooms, emergency department, recovery areas, or pediatric floors, or if they had been admitted or transferred to hospital wards with palliative care/end‐of‐life orders.
Physiologic data, including blood pressures (lowest), heart rate (highest), and respiratory rate (highest), were extracted from records of the 48 hours before and until resolution of the RRT assessment, or prior to death for those without RRT care. Outcomes were defined by World Health Organization (WHO) domains of palliative care (symptoms, social, and spiritual).14 The symptom domain was measured using patients' pain scores, 24 hours prior to death (0‐10). Subjective reports of healthcare providers recorded in hospital records, including the terms suffering, pain, anxiety, or distress were also extracted from notes 24 hours prior to patients' deaths. Administration of opioids in the 24 hours prior to death was also recorded. Social and spiritual domains were measured by documentation of presence of the family and chaplain, respectively, at the bedside in the 24 hours prior to death.
Analysis was performed using SPSS software (SPSS Inc., Chicago, IL). Categorical variables, described as proportions, were compared with chi‐square tests. Continuous variables are reported as means standard errors, or as medians with the interquartile ranges. Means were compared using Student t test if a normal distribution was detected. Nonparametric variables were compared with Wilcoxon rank sum tests. To adjust for confounding and assess possible effect modification, multiple logistic regression, multiple linear regression, and stratified analyses were performed when appropriate. Domains of the QOD were compared between patients who died in the pre‐RRT and post‐RRT epochs. Patients who died on hospital wards without RRT evaluation in the post‐RRT epoch were compared to those who died following RRT care. Unadjusted in‐hospital mortality, frequency of cardiopulmonary resuscitation, frequency of transfer from wards to critical care, and QOD were compiled and compared. A P value of 0.05 was considered statistically significant.
Results
A total of 394 patients died on the hospital wards and were not admitted with palliative, end‐of‐life medical therapies. The combined (pre‐RRT and post‐RRT epochs) cohort had a mean age of 77.2 13.2 years. A total of 48% were male, 79% White, 12% Black, and 8% Hispanic. A total of 128 patients (33%) were admitted to the hospital from a skilled nursing facility and 135 (35%) had written advance directives.
A total of 197 patients met the inclusion criteria during the pre‐RRT (October 1, 2005 to May 31, 2006) and 197 during the post‐RRT epochs (October 1, 2006 to May 31, 2007). There were no differences in age, sex, advance directives, ethnicity, or religion between the groups (Table 1). Primary admission diagnoses were significantly different; pre‐RRT patients were 9% more likely to die with malignancy compared to post‐RRT patients and less likely to come from nursing homes (38% vs. 27%; P = 0.02).
| Total | Pre‐RRT | Post‐RRT | P value | |
|---|---|---|---|---|
| ||||
| Total admissions | 25,943 | 12,926 | 13,017 | |
| Number of deaths | 394 | 197 | 197 | NS |
| Age (years) | 77.5 13.2 | 77.1 13.36 | 77.9 13.13 | 0.5 |
| Male gender | 190 (48%) | 99 (51%) | 91 (46%) | 0.4 |
| From SNF | 128 (32%) | 54 (27%) | 74 (38%) | 0.02 |
| Living will | 135 (34%) | 66 (33%) | 69 (35%) | 0.8 |
| Race | 0.3 | |||
| White | 314 (80%) | 163 (83%) | 151 (77%) | |
| Hispanic | 32 (8%) | 14 (7%) | 18 (9%) | |
| Black | 47 (12%) | 19 (10%) | 28 (14%) | |
| Other | 1 (1%) | 1 (1%) | 0 | |
| Religion (%) | 0.8 | |||
| Christian | 357 (91%) | 177 (90%) | 180 (91%) | |
| Non‐Christian | 37 (9%) | 20 (10%) | 17 (9%) | |
| Admission diagnosis | 0.01 | |||
| Malignancy | 96 (24%) | 56 (28%) | 40 (20%) | * |
| Sepsis | 44 (11%) | 21 (11%) | 23 (12%) | |
| Respiratory | 98 (25%) | 53 (27%) | 45 (23%) | * |
| Stroke | 31 (8%) | 16 (8%) | 15 (8%) | |
| Cardiac | 66 (17%) | 37 (19%) | 29 (15%) | * |
| Hepatic failure | 9 (2%) | 4 (2%) | 5 (2%) | |
| Surgical | 17 (5%) | 6 (3%) | 11 (5%) | |
| Others | 33 (8%) | 4 (2%) | 29 (15%) | * |
| Team | 0.01 | |||
| Medicine | 155 (39%) | 64 (32%) | 94 (47%) | |
| MICU | 44 (11%) | 3 (2%) | 41 (21%) | * |
| Surgery | 12 (3%) | 9 (5%) | 3 (1%) | |
| Restorative outcomes | ||||
| Mortality/1000 | 27/1000 | 30/1000 | 0.9 | |
| Unexpected ICU transfers/1000 | 17/1000 | 19/1000 | 0.8 | |
| CPR/1000 | 3/1000 | 2.5/1000 | 0.9 | |
Restorative Care Outcomes
Crude, unadjusted, in‐hospital mortality (27 vs. 30/1000 admissions), unexpected transfers to intensive care (17 vs. 19/1000 admissions), or cardiac arrests (3 vs. 2.5/1000 admissions) were similar in pre‐RRT and post‐RRT periods (all P > 0.05).
End‐of‐Life Care
At the time of death, 133 patients (68%) who died during the post‐RRT epoch had comfort care only orders whereas 90 (46%) had these orders in the pre‐RRT group (P = 0.0001; Table 2a). Post‐RRT patients were more likely than pre‐RRT patients to receive opioids prior to death (68% vs. 43%, respectively; P = 0.001) and had lower maximum pain scores in their last 24 hours (3.0 3.5 vs. 3.7 3.2; respectively; P = 0.045). Mention of patient distress by nurses in the hospital record following RRT deployment was less than one‐half of that recorded in the pre‐RRT period (26% vs. 62%; P = 0.0001). A chaplain visited post‐RRT patients in the 24 hours prior to death more frequently than in the pre‐RRT period (72% vs. 60%; P = 0.02). The frequency of family at the bedside was similar between epochs (61% post‐RRT vs. 58% pre‐RRT; P = 0.6). These findings were consistent across common primary diagnoses and origins (home vs. nursing home).
| a. Prior to RRT vs. During RRT Deployment | |||
|---|---|---|---|
| Pre‐RRT (n = 197) | Post‐RRT (n = 197) | P Value | |
| Comfort care only | 90 (46%) | 133 (68%) | 0.0001 |
| Pain score (0‐10) | 3.7 3.3 | 3.0 3.5 | 0.045 |
| Opioids administered | 84 (43%) | 134 (68%) | 0.0001 |
| Subjective suffering | 122 (62%) | 52 (26%) | 0.0001 |
| Family present | 115 (58%) | 120 (61%) | 0.6 |
| Chaplain present | 119 (60%) | 142 (72%) | 0.02 |
| b. During RRT Deployment: Those Dying with RRT Assessment vs. Those Dying Without | |||
| Post‐RRT RRT Care (n = 61) | Post‐RRT No RRT Care (n = 136) | P Value | |
| Comfort care only | 46 (75%) | 87 (64%) | 0.1 |
| Pain score (0‐10) | 3.0 3.5 | 3.0 3.5 | 0.9 |
| Opioids administered | 42 (69%) | 92 (67%) | 0.8 |
| Subjective suffering | 18 (29%) | 34 (25%) | 0.9 |
| Family present | 43 (71%) | 77 (57%) | 0.06 |
| Chaplain present | 49 (80%) | 93 (68%) | 0.0001 |
| c. Comparing Before and During RRT Deployment: Those Dying Without RRT Assessment | |||
| Pre‐RRT (n = 197) | Post‐RRT No RRT Care (n = 136) | P Value | |
| Comfort care (only) | 90 (46%) | 87 (64%) | 0.0001 |
| Pain score (0‐10) | 3.7 3.3 | 3.0 3.5 | 0.06 |
| Opioids administered | 84 (43%) | 92 (67%) | 0.0001 |
| Subjective suffering | 122 (62%) | 34 (25%) | 0.0001 |
| Family present | 115 (58%) | 77 (56.6%) | 0.8 |
| Chaplain present | 119 (60) | 74 (54.4%) | 0.2 |
Adjusting for age, gender, and race, the odds ratio (OR) of patients receiving formal end‐of‐life medical orders in post‐RRT was 2.5 that of pre‐RRT (95% confidence interval [CI], 1.7‐3.8), and odds of receiving opioids prior to death were nearly 3 times pre‐RRT (OR, 2.8; 95% CI, 1.9‐4.3). The odds of written mention of post‐RRT patients' suffering in the medical record was less than one‐fourth that of pre‐RRT patients (OR, 0.23; 95% CI, 0.2‐0.4).
To examine whether temporal trends might account for observed differences, patients in the post‐RRT period who received RRT care were compared to those who did not. Sixty‐one patients died with RRT assessments, whereas 136 died without RRT evaluations. End‐of‐life care outcomes were similar for these 2 groups, except more patients with RRT care had chaplain visits proximate to the time of death (80% vs. 68%; P = 0.0001; Table 2b). Outcomes (including comfort care orders, opioid administration, and suffering) of dying patients not cared for by the RRT (after deployment) were superior to those of pre‐RRT dying patients (Table 2c).
Discussion
This pilot study hypothesizes that our RRT impacted patients' QOD. Deployment of the RRT in our hospital was associated with improvement in both symptom and psychospiritual domains of care. Theoretically, RRTs should improve quality‐of‐care via early identification/reversal of physiologic decompensation. By either reversing acute diatheses with an expeditious trial of therapy or failing to reverse with early actuation of palliative therapies, the duration and magnitude of human suffering should be reduced. Attenuation of both duration and magnitude of suffering is the ultimate goal of both restorative and palliative care and is as important an outcome as mortality or length of stay. Previous studies of RRTs have focused on efficacy in reversing the decompensation: preventing cardiopulmonary arrest, avoiding the need for invasive, expensive, labor‐intensive interventions. Our RRT, like others, had no demonstrable impact on restorative outcomes. However, deployment of the RRT was highly associated with improved QOD of our patients. The impact was significant across WHO‐specified domains: pain scores decreased by 19%; (documentation of) patients' distress decreased by 50%; and chaplains' visits were more often documented in the 24 hours prior to death. These relationships held across common disease diagnoses, so the association is unlikely to be spurious.
Outcomes were similarly improved in patients who did not receive RRT care in the post‐RRT epoch. Our hospital did not have a palliative care service in either time period. No new educational efforts among physicians or nurses accounted for this observation. While it is possible that temporal effects accounted for our observation, an equally plausible explanation is that staff observed RRT interventions and applied them to dying patients not seen by the RRT. Our hospital educated caregivers regarding the RRT triggers, and simply making hospital personnel more vigilant for signs of suffering and/or observing the RRT approach may have contributed to enhanced end‐of‐life care for non‐RRT patients.
There are a number of limitations in this study. First, the sample size was relatively small compared to other published studies,2‐11 promoting the possibility that either epoch was not representative of pre‐RRT and post‐RRT parent populations. Another weakness is that QOD was measured using surrogate endpoints. The dead cannot be interviewed to definitively examine QOD; indices of cardiopulmonary distress and psychosocial measures (eg, religious preparations, family involvement) are endpoints suggested by palliative care investigators12, 13 and the World Health Organization.14 While some validated tools17 and consensus measures18 exist for critically ill patients, they do not readily apply to RRT patients. Retrospective records reviews raise the possibility of bias in extracting objective and subjective data. While we attempted to control for this by creating uniform a priori rules for data acquisition (ie, at what intervals and in which parts of the record they could be extracted), we cannot discount the possibility that bias affected the observed results. Finally, improvements in end‐of‐life care could have resulted from temporal trends. This retrospective study cannot prove a causeeffect relationship; a prospective randomized trial would be required to answer the question definitively. Based on the available data suggesting some benefit in restorative outcomes2‐8 and pressure from federal regulators to deploy RRTs regardless,1 a retrospective cohort design may provide the only realistic means of addressing this question.
In conclusion, this is the first (pilot) study to examine end‐of‐life outcomes associated with deployment of an RRT. While the limitations of these observations preclude firm conclusions, the plausibility of the hypothesis, coupled with our observations, suggests that this is a fertile area for future research. While RRTs may enhance restorative outcomes, to the extent that they hasten identification of candidates for palliative end‐of‐life‐care, before administration of invasive modalities that some patients do not want, these teams may simultaneously serve patients and reduce hospital resource utilization.
Addendum
Prior to publication, a contemporaneous study was published that concluded: These findings suggest that rapid response teams may not be decreasing code rates as much as catalyzing a compassionate dialogue of end‐of‐life care among terminally ill patients. This ability to improve end‐of‐life care may be an important benefit of rapid response teams, particularly given the difficulties in prior trials to increase rates of DNR status among seriously ill inpatients and potential decreases in resource use. Chan PS, Khalid A, Longmore LS, Berg RA, Midhail Kosiborod M, Spertus JA. Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA 2008;300: 25062513.
- Joint Commission on the Accreditation of Healthcare Organizations. The Joint Commission 2007 National Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/BD4D59E0‐6D53‐404C‐8507‐883AF3BBC50A/0/audio_conference_091307.pdf. Accessed February2009.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.The effect of a MET team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: a preliminary study.BMJ.2002;324:1–5.
- ,,, et al.Long‐term effect of a medical emergency team on mortality in a teaching hospital.Resuscitation.2007;74:235–241.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Long‐term effect of a rapid response team on cardiac arrests in a teaching hospital.Crit Care.2005;R808–R815.
- ,,, et al.The effect of a rapid response team on major clinical outcome measures in a community teaching hospital.Crit Care Med.2007;35:2076–2082.
- ,,, et al.Introduction of a rapid response team (RRT) system: a cluster‐randomised trail.Lancet.2005;365:2901–2907.
- ,,, et al.Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a children's hospital.JAMA.2007;298:2267–2274.
- ,,,,.The medical emergency team: 12 month analysis of reasons for activation, immediate outcome and not‐for‐resuscitation orders.Resuscitation.2001;50:39–44.
- ,,.Evaluating the quality of dying and death.J Pain Symptom Manage.2001;22:717–726.
- ,.Measuring success of interventions to improve the quality of end‐of‐life care in the intensive care unit.Crit Care Med.2006;34:S341–S347.
- World Health Organization. WHO definition of palliative care. Available at: http://www.who.int/cancer/palliative/definition/en. Accessed February 2009.
- .Does a living will equal a DNR? Are living wills compromising patient safety?J Emerg Med.2007;33:299–305.
- ,,,,,.Quality of dying and death in two medical ICUs.Chest.2005;127:1775–1783.
- ,,,.Using the medical record to evaluate the quality of end‐of‐life care in the intensive care unit.Crit Care Med.2008;36:1138–1146.
- ,,, et al.Proposed quality of measures for palliative care in the critically ill: a consensus from the Robert Wood Johnson Foundation Critical Care Workgroup.Crit Care Med.2006;34:S404–S411.
- Joint Commission on the Accreditation of Healthcare Organizations. The Joint Commission 2007 National Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/BD4D59E0‐6D53‐404C‐8507‐883AF3BBC50A/0/audio_conference_091307.pdf. Accessed February2009.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.The effect of a MET team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: a preliminary study.BMJ.2002;324:1–5.
- ,,, et al.Long‐term effect of a medical emergency team on mortality in a teaching hospital.Resuscitation.2007;74:235–241.
- ,,, et al.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Long‐term effect of a rapid response team on cardiac arrests in a teaching hospital.Crit Care.2005;R808–R815.
- ,,, et al.The effect of a rapid response team on major clinical outcome measures in a community teaching hospital.Crit Care Med.2007;35:2076–2082.
- ,,, et al.Introduction of a rapid response team (RRT) system: a cluster‐randomised trail.Lancet.2005;365:2901–2907.
- ,,, et al.Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a children's hospital.JAMA.2007;298:2267–2274.
- ,,,,.The medical emergency team: 12 month analysis of reasons for activation, immediate outcome and not‐for‐resuscitation orders.Resuscitation.2001;50:39–44.
- ,,.Evaluating the quality of dying and death.J Pain Symptom Manage.2001;22:717–726.
- ,.Measuring success of interventions to improve the quality of end‐of‐life care in the intensive care unit.Crit Care Med.2006;34:S341–S347.
- World Health Organization. WHO definition of palliative care. Available at: http://www.who.int/cancer/palliative/definition/en. Accessed February 2009.
- .Does a living will equal a DNR? Are living wills compromising patient safety?J Emerg Med.2007;33:299–305.
- ,,,,,.Quality of dying and death in two medical ICUs.Chest.2005;127:1775–1783.
- ,,,.Using the medical record to evaluate the quality of end‐of‐life care in the intensive care unit.Crit Care Med.2008;36:1138–1146.
- ,,, et al.Proposed quality of measures for palliative care in the critically ill: a consensus from the Robert Wood Johnson Foundation Critical Care Workgroup.Crit Care Med.2006;34:S404–S411.
Patients' Predilections Regarding Informed Consent
The cornerstones of American medical ethics include respect for patient autonomy and beneficence. Although informed consent is required for surgical procedures and transfusion of blood products, the overwhelming majority of medical treatments administered by physicians to hospitalized patients are given without discussing risks, benefits, and alternatives. Although patients may sign a general permission‐to‐treat form on admission to the hospital, informed consent for medical treatments is generally ad hoc, and there are no national standards or mandates. We hypothesized that given the choice, hospitalized patients would want to participate in informed decision making, especially for therapies associated with substantial risks and benefits.
METHODS
The Institutional Review Board of Bridgeport Hospital approved this study. Each day between June and August 2006, the hospital's admitting department provided investigators with a list that included names and locations of all patients admitted to the Department of Medicine inpatient service. All the patients were eligible for participation in the study. Patients were excluded if they were in a comatose state, were encephalopathic, or were judged to be severely demented. In addition, patients were assessed during the scripted intervention to ascertain whether they had the capacity to make informed decisions based on their ability: (a) to understand the presented information, (b) to consider the information in relation to their personal values, and (c) to communicate their wishes. If personnel doubted an individual's capacity in any of these 3 areas, they were not included in the study.
Study personnel read directly from the script (see Appendix) and recorded answers. Study personnel were permitted to reread questions but did not provide additional guidance beyond the questionnaire. Patients whose primary language was not English were interviewed through in‐house or 3‐way telephone (remote) translators.
Statistical analyses included the chi‐square test to examine responses across the 3 categories of answers (ie, always consent, qualified consent, waive consent) and simple comparisons of percentages. A P value < .05 was considered statistically significant.
RESULTS
A total of 634 patients were admitted to the medicine service during the study period June‐August 2006. Of these, 158 were judged to lack sufficient capacity by study personnel and were excluded from the study. Ninety‐five refused to participate, and 171 were discharged before the questionnaire could be administered. Two hundred and ten patients answered the questionnaire. They ranged in age from 18 to 96 years (mean age standard error, 63.3 1.1 years). One hundred and three (49%) were men, and 107 (51%) were women. A majority (67.5%) were white, 20% (42) were African American, and 11.9% (25) were Hispanic. Most (87.6%) had at least a high school education, and 35% had a college‐/graduate‐level education. Sixty‐seven percent had at least 2 comorbid conditions in addition to their principal reason for hospitalization. Their average acute physiology and chronic health care evaluation (APACHE II) score was 7.5 0.3 (median 7; range 0‐22).
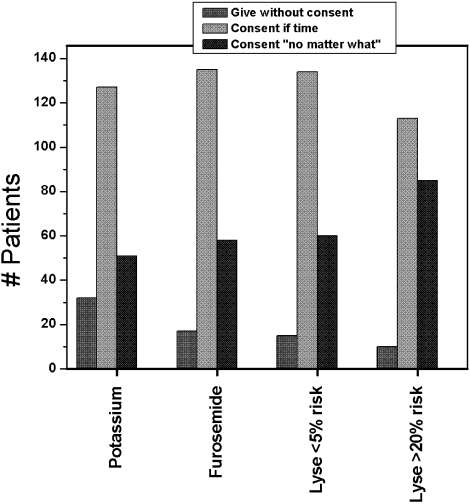
Figure 1 shows the distribution of answers to each of the 4 questions.
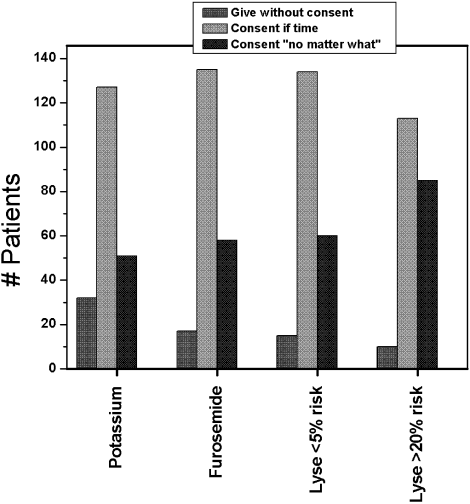
Question 1: Permission for Administration of Diuretics
One hundred and ninety‐three patients (92%) wished to participate in choosing whether to receive diuretics for congestive heart failure (CHF). Of these, 58 (28%) wanted their treating physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and thirty‐five (64%) wanted to be able to give permission if time allowed. Only 8% thought doctors should just give diuretics for CHF without seeking permission.
The pattern of response did not differ by sex, race, number of comorbid conditions, or primary admission diagnosis. Age (>65 vs. <65 years) was significantly associated with predilections to waive permission for administration of diuretics (Pearson chi‐square test P = .01). For example, 36.9% of the younger patients (<65 years) wanted to be consulted under all circumstances compared with only 18.7% of the more elderly patients (P = .004).
Question 2: Permission for Potassium Replacement
Overall, 178 patients (85%) wished to participate in decision making regarding potassium supplementation, and 51 (24%) wanted the managing physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and twenty‐seven patients (61%) responded that they would like to be able to give permission if time allowed. Only 15% thought doctors should just give potassium replacement without seeking their permission. Similar to the responses to diuretic replacement, the pattern of responses differed by age but not by sex, race, level of education, or number of comorbid conditions. Thirty‐one percent of the younger patients wanted to give permission at all times compared with 17.8% of the older patients (P = .03).
Question 3: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Less Than 5%
If the risk of cerebral hemorrhage was less than 5%, only 15 patients (7%) thought it should be given without seeking their permission. A third of the younger patients compared with 24.5% of the elderly patients would want to be consulted for their permission at all times (P = .18). The pattern of responses also did not differ by sex, race or level of education.
Question 4: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Greater Than 20%
Overall, 85 patients (40.8%) would want a discussion and their permission no matter what prior to initiating high‐risk thrombolysis. One hundred and thirteen patients (54%) would want to be able to give permission if time allowed. This pattern of response differed by level of education and by age. Forty‐four percent of those with at least a high school education would want to give permission compared with 19% of those without a high school education (P = .016). Four percent of those with at least a high school education would yield the need for permission at all times compared with 11.5% of those without a high school education (P = .09). Only 1 elderly patient (0.9%) would waive the need for permission at all times compared with 9 younger patients (8.7%; P = .01).
DISCUSSION
The principal finding of this study is that most medical patients prefer to participate in making decisions about their medical care during acute hospitalization, even for relatively low‐risk treatments like potassium supplementation and administration of diuretics. Very few patients were prepared to waive consent and grant their physicians the absolute right to administer therapies such as thrombolysis, even if the risk of bleeding was estimated to be less than 5%. Whereas the elderly patients were less likely to prefer being asked to consent to treatments than were younger patients, most would want to be informed of even trivial therapies if time allowed.
In some situations older patients (65 years old) were more likely than younger patients (<65 years old) to allow their physicians to make unilateral decisions regarding their health care. This could be explained by those age 65 and older having grown up when physician paternalism was more prevalent in American medicine. In the 1970s physician paternalism waned, and respect for patient autonomy emerged as the dominant physicianpatient model. Patients who became adults after 1970 know only this relationship with their physician, and so it makes sense that they would be more inclined to prefer a participatory model.
These data complement and extend a series of studies we conducted with patients admitted to Bridgeport Hospital. Our data suggest that our patients wish to consent for end‐of‐life decisions,1, 2 invasive procedures,3 and, now, to be apprised of medical therapies administered during hospitalization. At the same time, we have found that consent practices at many centers are not consistent with these patient predilections.1, 2, 4 Our study suffered from having a small sample size obtained in one geographic location; so results should be generalized cautiously. Nonetheless, insofar as the expectations of patients for participation are not being met by the health care system in Connecticut (and we suspect elsewhere), clinicians, hospital administrators, and health care policy makers might consider whether more rigorous and explicit consent practices and policies are required. Another important limitation of the study was that patients included may not have entirely understood the implications of their answers (ie, how cumbersome to the system and bothersome to the patient seeking consent for every therapy could become). In fact, we cannot be certain that all patients truly understood the questions, some of which were complex. Nonetheless, these results support that considered in the abstract, most patients prefer to consent for medical therapies. Had the implications for safety and expediency been explained in detail, it is possible that patients would have waived the need to give consent for treatments with minimal risk. The questionnaire also presents an abbreviated list of risks and benefits for each intervention, and although it refers to the formal process of informed consent in its preamble, it uses terminology (ie, permission) that may not reflect the complexity of informed consent. Nonetheless, our goal was to examine the degree to which patients wished to participate in their medical decision making. Notwithstanding these weaknesses of the survey instrument, the data suggest patients want to be in the loop whenever possible.
There are no national standards of consent for medical treatments. The Veterans' Administration, which has led the way in many areas of patients' rights, has a policy:
Treatments and Procedures That Do Not Require Signature Consent. Treatments and procedures that are low risk and are within broadly accepted standards of medical practice (e.g., administration of most drugs or for the performance of minor procedures such as routine X‐rays) do not require signature consent. However, the informed consent process must be documented in the medical record.
Compliance with this standard (ie, consent for every new medication) is not routine in most acute care hospitals. Although some clinicians obtain formal consent for high‐risk therapies (perhaps out of respect for autonomy, perhaps to reduce medical‐legal liability), there are no explicit decision rules to guide clinicians regarding for which treatments they should obtain formal consent. Accordingly, some might obtain formal consent for thrombolysis for massive pulmonary embolus, and others might not. It is not clear that the consent‐to‐treat form signed during hospital admission would legally cover all medical therapies during hospitalization. The legal standard for informed consent is what any reasonable patient would want to consent for. Our data suggest that most reasonable patients wish to at least assent and perhaps consent for much of what they receive during hospitalization. Although we have been unable to find case law predicated entirely on failure to obtain consent prior to administration of a therapy that caused a complication, it is plausible that the reasonable patient standard could be used in this manner. Regardless, it is impractical to require consent for the thousands of medical therapies administered each day in hospitals. Requiring consent for all therapies, if respected rigidly, would threaten the safety and efficiency of American hospitals. Naturally, a balance betweem respect for autonomy, that is, informed consent for the riskiest therapies, and efficiency is necessary. Explicit guidelines issued by accrediting agencies or the federal government would be helpful. The rules for consent (and/or assent) should be more explicit and less arbitrary, that is, determined independently by each clinician.
In conclusion, these data demonstrate that when considered in the abstract, that is, without explaining the logistical hurdles that it would create, inpatients wish to participate in decision making for both low‐ and high‐risk treatments. Clinicians are faced with demands and obligations that preclude full consent for the myriad low‐risk treatments administered daily to hospitalized patients. Some treatments are likely to be covered implicitly under the general consent‐to‐treat process and paperwork. Nonetheless, clinicians should consider explaining the principal risks and benefits of moderate‐risk treatments in order to secure informed assent. Full informed consent may be most appropriate for very high‐risk therapies. Patients expect and deserve frequent communication with caregivers that balances their safety with their right to self‐determination.
APPENDIX
QUESTIONNAIRE
Good morning/afternoon/evening. My name is Dr. _____________, and I am working with Dr. Constantine Manthous in a study to determine what patients want to know about their treatments during hospitalization. The research will not effect your care in any way, and if it is published, your confidential medical information will be protected and will not be mentioned in any publications. In fact, the questions I will ask do not apply to your care plans but are what ifs to find out for what kinds of treatments patients' want to provide permission called informed consent. Informed consent is when a doctor explains a treatment or procedure to the patient, including its risks, benefits, and alternatives, and asks permission before doing it. Are you feeling up to answering 4 questions that should take about 5‐10 minutes? Thank you.
Again, these questions do not apply to your illness or treatments.
If you had fluid on your lungs, a medicine called a diuretic could be given to make you pass more urine to help get the fluid out of the lungs. The benefits are that it can help you breathe easier. The risks are that it will make you have to urinate more often (>50%), and sometimes minerals in the blood get low and can cause the heart to beat abnormally (<1%) if enough replacement minerals aren't given to keep up with losses in the urine. The alternative to receiving this medicine would be not to receive it, which risks continued shortness of breath, and rarely (<5%) untreated patients may need a breathing machine to help breathing. Which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without asking my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there were time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
When a diuretic is given, minerals in the blood can be lost in the urine. If the minerals in the blood get too low, the heart can have abnormal beats that are rarely (<1%) life‐threatening. Doctors can give replacement minerals. The risks of replacement are minimal, and the alternative is not to give the minerals, risking abnormal heartbeats. Which best summarizes your preference?
If I needed replacement minerals, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give me the minerals. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed replacement minerals, I'd want the doctor to talk it over with me first to get my permission no matter what.
During hospitalization, sometimes blood clots can form in the legs and travel to the lungs. Very rarely (<1%), the blood clots can cause shortness of breath and the blood pressure to drop to a dangerous level. In this case there is a medicine called tpa that can dissolve the blood clot. It almost always dissolves the clot, improves breathlessness, and improves heart function. But there is a small risk (<5%) that it can cause serious bleeding into the brain (called a stroke). Which best summarizes your preference?
If I needed tpa for life‐threatening blood clots, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give the tpa. But if there was time and I was able, I'd want the doctor to talk it over with me first to get my permission.
If I needed tpa for life‐threatening blood clots, I'd want the doctor to talk it over with me first to get my permission no matter what.
In the previous example, what if the serious brain bleeding from the clot‐busting drug happened in more than 20% of cases, which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
- ,,,,.Patient, physician and family member understanding of living wills.Am J Respir Crit Care Med.2002;166:1430–1435.
- ,,,,,.Hospitalized patients want to choose whether to receive life‐sustaining therapies.J Hosp Med.2006;1:161–167.
- ,,,,,.Informed consent for invasive medical procedures. From the patient's perspective.Conn Med.2003;67:529–533.
- ,,,.Informed consent for medical procedures: Local and national practices.Chest.2003;124:1978–1984.
- Department of Veterans Affairs. VHA informed consent for clinical treatments and procedures. 2003. Available at: http://www.va.gov/ETHICS/docs/policy/VHA_Handbook_1004‐1_Informed_Consent_Policy_20030129.pdf. Accessed September 5,2006.
The cornerstones of American medical ethics include respect for patient autonomy and beneficence. Although informed consent is required for surgical procedures and transfusion of blood products, the overwhelming majority of medical treatments administered by physicians to hospitalized patients are given without discussing risks, benefits, and alternatives. Although patients may sign a general permission‐to‐treat form on admission to the hospital, informed consent for medical treatments is generally ad hoc, and there are no national standards or mandates. We hypothesized that given the choice, hospitalized patients would want to participate in informed decision making, especially for therapies associated with substantial risks and benefits.
METHODS
The Institutional Review Board of Bridgeport Hospital approved this study. Each day between June and August 2006, the hospital's admitting department provided investigators with a list that included names and locations of all patients admitted to the Department of Medicine inpatient service. All the patients were eligible for participation in the study. Patients were excluded if they were in a comatose state, were encephalopathic, or were judged to be severely demented. In addition, patients were assessed during the scripted intervention to ascertain whether they had the capacity to make informed decisions based on their ability: (a) to understand the presented information, (b) to consider the information in relation to their personal values, and (c) to communicate their wishes. If personnel doubted an individual's capacity in any of these 3 areas, they were not included in the study.
Study personnel read directly from the script (see Appendix) and recorded answers. Study personnel were permitted to reread questions but did not provide additional guidance beyond the questionnaire. Patients whose primary language was not English were interviewed through in‐house or 3‐way telephone (remote) translators.
Statistical analyses included the chi‐square test to examine responses across the 3 categories of answers (ie, always consent, qualified consent, waive consent) and simple comparisons of percentages. A P value < .05 was considered statistically significant.
RESULTS
A total of 634 patients were admitted to the medicine service during the study period June‐August 2006. Of these, 158 were judged to lack sufficient capacity by study personnel and were excluded from the study. Ninety‐five refused to participate, and 171 were discharged before the questionnaire could be administered. Two hundred and ten patients answered the questionnaire. They ranged in age from 18 to 96 years (mean age standard error, 63.3 1.1 years). One hundred and three (49%) were men, and 107 (51%) were women. A majority (67.5%) were white, 20% (42) were African American, and 11.9% (25) were Hispanic. Most (87.6%) had at least a high school education, and 35% had a college‐/graduate‐level education. Sixty‐seven percent had at least 2 comorbid conditions in addition to their principal reason for hospitalization. Their average acute physiology and chronic health care evaluation (APACHE II) score was 7.5 0.3 (median 7; range 0‐22).
Figure 1 shows the distribution of answers to each of the 4 questions.

Question 1: Permission for Administration of Diuretics
One hundred and ninety‐three patients (92%) wished to participate in choosing whether to receive diuretics for congestive heart failure (CHF). Of these, 58 (28%) wanted their treating physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and thirty‐five (64%) wanted to be able to give permission if time allowed. Only 8% thought doctors should just give diuretics for CHF without seeking permission.
The pattern of response did not differ by sex, race, number of comorbid conditions, or primary admission diagnosis. Age (>65 vs. <65 years) was significantly associated with predilections to waive permission for administration of diuretics (Pearson chi‐square test P = .01). For example, 36.9% of the younger patients (<65 years) wanted to be consulted under all circumstances compared with only 18.7% of the more elderly patients (P = .004).
Question 2: Permission for Potassium Replacement
Overall, 178 patients (85%) wished to participate in decision making regarding potassium supplementation, and 51 (24%) wanted the managing physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and twenty‐seven patients (61%) responded that they would like to be able to give permission if time allowed. Only 15% thought doctors should just give potassium replacement without seeking their permission. Similar to the responses to diuretic replacement, the pattern of responses differed by age but not by sex, race, level of education, or number of comorbid conditions. Thirty‐one percent of the younger patients wanted to give permission at all times compared with 17.8% of the older patients (P = .03).
Question 3: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Less Than 5%
If the risk of cerebral hemorrhage was less than 5%, only 15 patients (7%) thought it should be given without seeking their permission. A third of the younger patients compared with 24.5% of the elderly patients would want to be consulted for their permission at all times (P = .18). The pattern of responses also did not differ by sex, race or level of education.
Question 4: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Greater Than 20%
Overall, 85 patients (40.8%) would want a discussion and their permission no matter what prior to initiating high‐risk thrombolysis. One hundred and thirteen patients (54%) would want to be able to give permission if time allowed. This pattern of response differed by level of education and by age. Forty‐four percent of those with at least a high school education would want to give permission compared with 19% of those without a high school education (P = .016). Four percent of those with at least a high school education would yield the need for permission at all times compared with 11.5% of those without a high school education (P = .09). Only 1 elderly patient (0.9%) would waive the need for permission at all times compared with 9 younger patients (8.7%; P = .01).
DISCUSSION
The principal finding of this study is that most medical patients prefer to participate in making decisions about their medical care during acute hospitalization, even for relatively low‐risk treatments like potassium supplementation and administration of diuretics. Very few patients were prepared to waive consent and grant their physicians the absolute right to administer therapies such as thrombolysis, even if the risk of bleeding was estimated to be less than 5%. Whereas the elderly patients were less likely to prefer being asked to consent to treatments than were younger patients, most would want to be informed of even trivial therapies if time allowed.
In some situations older patients (65 years old) were more likely than younger patients (<65 years old) to allow their physicians to make unilateral decisions regarding their health care. This could be explained by those age 65 and older having grown up when physician paternalism was more prevalent in American medicine. In the 1970s physician paternalism waned, and respect for patient autonomy emerged as the dominant physicianpatient model. Patients who became adults after 1970 know only this relationship with their physician, and so it makes sense that they would be more inclined to prefer a participatory model.
These data complement and extend a series of studies we conducted with patients admitted to Bridgeport Hospital. Our data suggest that our patients wish to consent for end‐of‐life decisions,1, 2 invasive procedures,3 and, now, to be apprised of medical therapies administered during hospitalization. At the same time, we have found that consent practices at many centers are not consistent with these patient predilections.1, 2, 4 Our study suffered from having a small sample size obtained in one geographic location; so results should be generalized cautiously. Nonetheless, insofar as the expectations of patients for participation are not being met by the health care system in Connecticut (and we suspect elsewhere), clinicians, hospital administrators, and health care policy makers might consider whether more rigorous and explicit consent practices and policies are required. Another important limitation of the study was that patients included may not have entirely understood the implications of their answers (ie, how cumbersome to the system and bothersome to the patient seeking consent for every therapy could become). In fact, we cannot be certain that all patients truly understood the questions, some of which were complex. Nonetheless, these results support that considered in the abstract, most patients prefer to consent for medical therapies. Had the implications for safety and expediency been explained in detail, it is possible that patients would have waived the need to give consent for treatments with minimal risk. The questionnaire also presents an abbreviated list of risks and benefits for each intervention, and although it refers to the formal process of informed consent in its preamble, it uses terminology (ie, permission) that may not reflect the complexity of informed consent. Nonetheless, our goal was to examine the degree to which patients wished to participate in their medical decision making. Notwithstanding these weaknesses of the survey instrument, the data suggest patients want to be in the loop whenever possible.
There are no national standards of consent for medical treatments. The Veterans' Administration, which has led the way in many areas of patients' rights, has a policy:
Treatments and Procedures That Do Not Require Signature Consent. Treatments and procedures that are low risk and are within broadly accepted standards of medical practice (e.g., administration of most drugs or for the performance of minor procedures such as routine X‐rays) do not require signature consent. However, the informed consent process must be documented in the medical record.
Compliance with this standard (ie, consent for every new medication) is not routine in most acute care hospitals. Although some clinicians obtain formal consent for high‐risk therapies (perhaps out of respect for autonomy, perhaps to reduce medical‐legal liability), there are no explicit decision rules to guide clinicians regarding for which treatments they should obtain formal consent. Accordingly, some might obtain formal consent for thrombolysis for massive pulmonary embolus, and others might not. It is not clear that the consent‐to‐treat form signed during hospital admission would legally cover all medical therapies during hospitalization. The legal standard for informed consent is what any reasonable patient would want to consent for. Our data suggest that most reasonable patients wish to at least assent and perhaps consent for much of what they receive during hospitalization. Although we have been unable to find case law predicated entirely on failure to obtain consent prior to administration of a therapy that caused a complication, it is plausible that the reasonable patient standard could be used in this manner. Regardless, it is impractical to require consent for the thousands of medical therapies administered each day in hospitals. Requiring consent for all therapies, if respected rigidly, would threaten the safety and efficiency of American hospitals. Naturally, a balance betweem respect for autonomy, that is, informed consent for the riskiest therapies, and efficiency is necessary. Explicit guidelines issued by accrediting agencies or the federal government would be helpful. The rules for consent (and/or assent) should be more explicit and less arbitrary, that is, determined independently by each clinician.
In conclusion, these data demonstrate that when considered in the abstract, that is, without explaining the logistical hurdles that it would create, inpatients wish to participate in decision making for both low‐ and high‐risk treatments. Clinicians are faced with demands and obligations that preclude full consent for the myriad low‐risk treatments administered daily to hospitalized patients. Some treatments are likely to be covered implicitly under the general consent‐to‐treat process and paperwork. Nonetheless, clinicians should consider explaining the principal risks and benefits of moderate‐risk treatments in order to secure informed assent. Full informed consent may be most appropriate for very high‐risk therapies. Patients expect and deserve frequent communication with caregivers that balances their safety with their right to self‐determination.
APPENDIX
QUESTIONNAIRE
Good morning/afternoon/evening. My name is Dr. _____________, and I am working with Dr. Constantine Manthous in a study to determine what patients want to know about their treatments during hospitalization. The research will not effect your care in any way, and if it is published, your confidential medical information will be protected and will not be mentioned in any publications. In fact, the questions I will ask do not apply to your care plans but are what ifs to find out for what kinds of treatments patients' want to provide permission called informed consent. Informed consent is when a doctor explains a treatment or procedure to the patient, including its risks, benefits, and alternatives, and asks permission before doing it. Are you feeling up to answering 4 questions that should take about 5‐10 minutes? Thank you.
Again, these questions do not apply to your illness or treatments.
If you had fluid on your lungs, a medicine called a diuretic could be given to make you pass more urine to help get the fluid out of the lungs. The benefits are that it can help you breathe easier. The risks are that it will make you have to urinate more often (>50%), and sometimes minerals in the blood get low and can cause the heart to beat abnormally (<1%) if enough replacement minerals aren't given to keep up with losses in the urine. The alternative to receiving this medicine would be not to receive it, which risks continued shortness of breath, and rarely (<5%) untreated patients may need a breathing machine to help breathing. Which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without asking my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there were time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
When a diuretic is given, minerals in the blood can be lost in the urine. If the minerals in the blood get too low, the heart can have abnormal beats that are rarely (<1%) life‐threatening. Doctors can give replacement minerals. The risks of replacement are minimal, and the alternative is not to give the minerals, risking abnormal heartbeats. Which best summarizes your preference?
If I needed replacement minerals, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give me the minerals. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed replacement minerals, I'd want the doctor to talk it over with me first to get my permission no matter what.
During hospitalization, sometimes blood clots can form in the legs and travel to the lungs. Very rarely (<1%), the blood clots can cause shortness of breath and the blood pressure to drop to a dangerous level. In this case there is a medicine called tpa that can dissolve the blood clot. It almost always dissolves the clot, improves breathlessness, and improves heart function. But there is a small risk (<5%) that it can cause serious bleeding into the brain (called a stroke). Which best summarizes your preference?
If I needed tpa for life‐threatening blood clots, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give the tpa. But if there was time and I was able, I'd want the doctor to talk it over with me first to get my permission.
If I needed tpa for life‐threatening blood clots, I'd want the doctor to talk it over with me first to get my permission no matter what.
In the previous example, what if the serious brain bleeding from the clot‐busting drug happened in more than 20% of cases, which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
The cornerstones of American medical ethics include respect for patient autonomy and beneficence. Although informed consent is required for surgical procedures and transfusion of blood products, the overwhelming majority of medical treatments administered by physicians to hospitalized patients are given without discussing risks, benefits, and alternatives. Although patients may sign a general permission‐to‐treat form on admission to the hospital, informed consent for medical treatments is generally ad hoc, and there are no national standards or mandates. We hypothesized that given the choice, hospitalized patients would want to participate in informed decision making, especially for therapies associated with substantial risks and benefits.
METHODS
The Institutional Review Board of Bridgeport Hospital approved this study. Each day between June and August 2006, the hospital's admitting department provided investigators with a list that included names and locations of all patients admitted to the Department of Medicine inpatient service. All the patients were eligible for participation in the study. Patients were excluded if they were in a comatose state, were encephalopathic, or were judged to be severely demented. In addition, patients were assessed during the scripted intervention to ascertain whether they had the capacity to make informed decisions based on their ability: (a) to understand the presented information, (b) to consider the information in relation to their personal values, and (c) to communicate their wishes. If personnel doubted an individual's capacity in any of these 3 areas, they were not included in the study.
Study personnel read directly from the script (see Appendix) and recorded answers. Study personnel were permitted to reread questions but did not provide additional guidance beyond the questionnaire. Patients whose primary language was not English were interviewed through in‐house or 3‐way telephone (remote) translators.
Statistical analyses included the chi‐square test to examine responses across the 3 categories of answers (ie, always consent, qualified consent, waive consent) and simple comparisons of percentages. A P value < .05 was considered statistically significant.
RESULTS
A total of 634 patients were admitted to the medicine service during the study period June‐August 2006. Of these, 158 were judged to lack sufficient capacity by study personnel and were excluded from the study. Ninety‐five refused to participate, and 171 were discharged before the questionnaire could be administered. Two hundred and ten patients answered the questionnaire. They ranged in age from 18 to 96 years (mean age standard error, 63.3 1.1 years). One hundred and three (49%) were men, and 107 (51%) were women. A majority (67.5%) were white, 20% (42) were African American, and 11.9% (25) were Hispanic. Most (87.6%) had at least a high school education, and 35% had a college‐/graduate‐level education. Sixty‐seven percent had at least 2 comorbid conditions in addition to their principal reason for hospitalization. Their average acute physiology and chronic health care evaluation (APACHE II) score was 7.5 0.3 (median 7; range 0‐22).
Figure 1 shows the distribution of answers to each of the 4 questions.

Question 1: Permission for Administration of Diuretics
One hundred and ninety‐three patients (92%) wished to participate in choosing whether to receive diuretics for congestive heart failure (CHF). Of these, 58 (28%) wanted their treating physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and thirty‐five (64%) wanted to be able to give permission if time allowed. Only 8% thought doctors should just give diuretics for CHF without seeking permission.
The pattern of response did not differ by sex, race, number of comorbid conditions, or primary admission diagnosis. Age (>65 vs. <65 years) was significantly associated with predilections to waive permission for administration of diuretics (Pearson chi‐square test P = .01). For example, 36.9% of the younger patients (<65 years) wanted to be consulted under all circumstances compared with only 18.7% of the more elderly patients (P = .004).
Question 2: Permission for Potassium Replacement
Overall, 178 patients (85%) wished to participate in decision making regarding potassium supplementation, and 51 (24%) wanted the managing physicians to obtain their permission no matter what, even if there was an acute matter of life and death. One hundred and twenty‐seven patients (61%) responded that they would like to be able to give permission if time allowed. Only 15% thought doctors should just give potassium replacement without seeking their permission. Similar to the responses to diuretic replacement, the pattern of responses differed by age but not by sex, race, level of education, or number of comorbid conditions. Thirty‐one percent of the younger patients wanted to give permission at all times compared with 17.8% of the older patients (P = .03).
Question 3: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Less Than 5%
If the risk of cerebral hemorrhage was less than 5%, only 15 patients (7%) thought it should be given without seeking their permission. A third of the younger patients compared with 24.5% of the elderly patients would want to be consulted for their permission at all times (P = .18). The pattern of responses also did not differ by sex, race or level of education.
Question 4: Permission for Thrombolysis of Pulmonary Embolus if Risk of Cerebral Bleed Was Greater Than 20%
Overall, 85 patients (40.8%) would want a discussion and their permission no matter what prior to initiating high‐risk thrombolysis. One hundred and thirteen patients (54%) would want to be able to give permission if time allowed. This pattern of response differed by level of education and by age. Forty‐four percent of those with at least a high school education would want to give permission compared with 19% of those without a high school education (P = .016). Four percent of those with at least a high school education would yield the need for permission at all times compared with 11.5% of those without a high school education (P = .09). Only 1 elderly patient (0.9%) would waive the need for permission at all times compared with 9 younger patients (8.7%; P = .01).
DISCUSSION
The principal finding of this study is that most medical patients prefer to participate in making decisions about their medical care during acute hospitalization, even for relatively low‐risk treatments like potassium supplementation and administration of diuretics. Very few patients were prepared to waive consent and grant their physicians the absolute right to administer therapies such as thrombolysis, even if the risk of bleeding was estimated to be less than 5%. Whereas the elderly patients were less likely to prefer being asked to consent to treatments than were younger patients, most would want to be informed of even trivial therapies if time allowed.
In some situations older patients (65 years old) were more likely than younger patients (<65 years old) to allow their physicians to make unilateral decisions regarding their health care. This could be explained by those age 65 and older having grown up when physician paternalism was more prevalent in American medicine. In the 1970s physician paternalism waned, and respect for patient autonomy emerged as the dominant physicianpatient model. Patients who became adults after 1970 know only this relationship with their physician, and so it makes sense that they would be more inclined to prefer a participatory model.
These data complement and extend a series of studies we conducted with patients admitted to Bridgeport Hospital. Our data suggest that our patients wish to consent for end‐of‐life decisions,1, 2 invasive procedures,3 and, now, to be apprised of medical therapies administered during hospitalization. At the same time, we have found that consent practices at many centers are not consistent with these patient predilections.1, 2, 4 Our study suffered from having a small sample size obtained in one geographic location; so results should be generalized cautiously. Nonetheless, insofar as the expectations of patients for participation are not being met by the health care system in Connecticut (and we suspect elsewhere), clinicians, hospital administrators, and health care policy makers might consider whether more rigorous and explicit consent practices and policies are required. Another important limitation of the study was that patients included may not have entirely understood the implications of their answers (ie, how cumbersome to the system and bothersome to the patient seeking consent for every therapy could become). In fact, we cannot be certain that all patients truly understood the questions, some of which were complex. Nonetheless, these results support that considered in the abstract, most patients prefer to consent for medical therapies. Had the implications for safety and expediency been explained in detail, it is possible that patients would have waived the need to give consent for treatments with minimal risk. The questionnaire also presents an abbreviated list of risks and benefits for each intervention, and although it refers to the formal process of informed consent in its preamble, it uses terminology (ie, permission) that may not reflect the complexity of informed consent. Nonetheless, our goal was to examine the degree to which patients wished to participate in their medical decision making. Notwithstanding these weaknesses of the survey instrument, the data suggest patients want to be in the loop whenever possible.
There are no national standards of consent for medical treatments. The Veterans' Administration, which has led the way in many areas of patients' rights, has a policy:
Treatments and Procedures That Do Not Require Signature Consent. Treatments and procedures that are low risk and are within broadly accepted standards of medical practice (e.g., administration of most drugs or for the performance of minor procedures such as routine X‐rays) do not require signature consent. However, the informed consent process must be documented in the medical record.
Compliance with this standard (ie, consent for every new medication) is not routine in most acute care hospitals. Although some clinicians obtain formal consent for high‐risk therapies (perhaps out of respect for autonomy, perhaps to reduce medical‐legal liability), there are no explicit decision rules to guide clinicians regarding for which treatments they should obtain formal consent. Accordingly, some might obtain formal consent for thrombolysis for massive pulmonary embolus, and others might not. It is not clear that the consent‐to‐treat form signed during hospital admission would legally cover all medical therapies during hospitalization. The legal standard for informed consent is what any reasonable patient would want to consent for. Our data suggest that most reasonable patients wish to at least assent and perhaps consent for much of what they receive during hospitalization. Although we have been unable to find case law predicated entirely on failure to obtain consent prior to administration of a therapy that caused a complication, it is plausible that the reasonable patient standard could be used in this manner. Regardless, it is impractical to require consent for the thousands of medical therapies administered each day in hospitals. Requiring consent for all therapies, if respected rigidly, would threaten the safety and efficiency of American hospitals. Naturally, a balance betweem respect for autonomy, that is, informed consent for the riskiest therapies, and efficiency is necessary. Explicit guidelines issued by accrediting agencies or the federal government would be helpful. The rules for consent (and/or assent) should be more explicit and less arbitrary, that is, determined independently by each clinician.
In conclusion, these data demonstrate that when considered in the abstract, that is, without explaining the logistical hurdles that it would create, inpatients wish to participate in decision making for both low‐ and high‐risk treatments. Clinicians are faced with demands and obligations that preclude full consent for the myriad low‐risk treatments administered daily to hospitalized patients. Some treatments are likely to be covered implicitly under the general consent‐to‐treat process and paperwork. Nonetheless, clinicians should consider explaining the principal risks and benefits of moderate‐risk treatments in order to secure informed assent. Full informed consent may be most appropriate for very high‐risk therapies. Patients expect and deserve frequent communication with caregivers that balances their safety with their right to self‐determination.
APPENDIX
QUESTIONNAIRE
Good morning/afternoon/evening. My name is Dr. _____________, and I am working with Dr. Constantine Manthous in a study to determine what patients want to know about their treatments during hospitalization. The research will not effect your care in any way, and if it is published, your confidential medical information will be protected and will not be mentioned in any publications. In fact, the questions I will ask do not apply to your care plans but are what ifs to find out for what kinds of treatments patients' want to provide permission called informed consent. Informed consent is when a doctor explains a treatment or procedure to the patient, including its risks, benefits, and alternatives, and asks permission before doing it. Are you feeling up to answering 4 questions that should take about 5‐10 minutes? Thank you.
Again, these questions do not apply to your illness or treatments.
If you had fluid on your lungs, a medicine called a diuretic could be given to make you pass more urine to help get the fluid out of the lungs. The benefits are that it can help you breathe easier. The risks are that it will make you have to urinate more often (>50%), and sometimes minerals in the blood get low and can cause the heart to beat abnormally (<1%) if enough replacement minerals aren't given to keep up with losses in the urine. The alternative to receiving this medicine would be not to receive it, which risks continued shortness of breath, and rarely (<5%) untreated patients may need a breathing machine to help breathing. Which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without asking my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there were time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
When a diuretic is given, minerals in the blood can be lost in the urine. If the minerals in the blood get too low, the heart can have abnormal beats that are rarely (<1%) life‐threatening. Doctors can give replacement minerals. The risks of replacement are minimal, and the alternative is not to give the minerals, risking abnormal heartbeats. Which best summarizes your preference?
If I needed replacement minerals, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give me the minerals. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed replacement minerals, I'd want the doctor to talk it over with me first to get my permission no matter what.
During hospitalization, sometimes blood clots can form in the legs and travel to the lungs. Very rarely (<1%), the blood clots can cause shortness of breath and the blood pressure to drop to a dangerous level. In this case there is a medicine called tpa that can dissolve the blood clot. It almost always dissolves the clot, improves breathlessness, and improves heart function. But there is a small risk (<5%) that it can cause serious bleeding into the brain (called a stroke). Which best summarizes your preference?
If I needed tpa for life‐threatening blood clots, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give the tpa. But if there was time and I was able, I'd want the doctor to talk it over with me first to get my permission.
If I needed tpa for life‐threatening blood clots, I'd want the doctor to talk it over with me first to get my permission no matter what.
In the previous example, what if the serious brain bleeding from the clot‐busting drug happened in more than 20% of cases, which best summarizes your preference?
If I needed this treatment, the doctor should give it to me without needing my permission.
If it was a question of life or death and there wasn't enough time to talk it over, I'd want the doctor to just give it. But if there was time, I'd want the doctor to talk it over with me first to get my permission.
If I needed this treatment, I'd want the doctor to talk it over with me first to get my permission no matter what.
- ,,,,.Patient, physician and family member understanding of living wills.Am J Respir Crit Care Med.2002;166:1430–1435.
- ,,,,,.Hospitalized patients want to choose whether to receive life‐sustaining therapies.J Hosp Med.2006;1:161–167.
- ,,,,,.Informed consent for invasive medical procedures. From the patient's perspective.Conn Med.2003;67:529–533.
- ,,,.Informed consent for medical procedures: Local and national practices.Chest.2003;124:1978–1984.
- Department of Veterans Affairs. VHA informed consent for clinical treatments and procedures. 2003. Available at: http://www.va.gov/ETHICS/docs/policy/VHA_Handbook_1004‐1_Informed_Consent_Policy_20030129.pdf. Accessed September 5,2006.
- ,,,,.Patient, physician and family member understanding of living wills.Am J Respir Crit Care Med.2002;166:1430–1435.
- ,,,,,.Hospitalized patients want to choose whether to receive life‐sustaining therapies.J Hosp Med.2006;1:161–167.
- ,,,,,.Informed consent for invasive medical procedures. From the patient's perspective.Conn Med.2003;67:529–533.
- ,,,.Informed consent for medical procedures: Local and national practices.Chest.2003;124:1978–1984.
- Department of Veterans Affairs. VHA informed consent for clinical treatments and procedures. 2003. Available at: http://www.va.gov/ETHICS/docs/policy/VHA_Handbook_1004‐1_Informed_Consent_Policy_20030129.pdf. Accessed September 5,2006.
Copyright © 2008 Society of Hospital Medicine