User login
Delivering Palliative Care in a Community Hospital: Experiences and Lessons Learned from the Front Lines
From the Division of Palliative Care, Butler Health System, Butler, PA (Drs. Stein, Reefer, Selvaggi, Ms. Doverspike); the University of Pittsburgh Medical Center, Pittsburgh, PA (Dr. Rajagopal); and the Duke Cancer Institute and Duke Fuqua School of Business, Durham, NC (Dr. Kamal).
Abstract
- Objective: To describe an approach to develop a community-centric palliative care program in a rural community health system and to review data collected over the program’s first year.
- Methods: We describe the underlying foundations of our program development including the health system’s prioritization of a palliative care program, funding opportunities, collaboration with community supports, and the importance of building a team and program that reflects a community’s needs. Data were collected through a program-maintained spreadsheet and a data monitoring system available through the Global Palliative Care Quality Alliance.
- Results: 516 new inpatient consultations were seen during the first year, for a penetration of 3.7%. The demographics of the patients who received consultation reflect that of the surrounding community. Over 50% of patients seen within the first year died, and hospice utilization at home and within facilities and inpatient hospice units increased. In addition, 79% of the patients seen by the palliative care team had a confirmed code status of do not resuscitate and do not intubate.
- Conclusions: Butler Health System’s approach to development of a palliative care program has resulted in increasing utilization of palliative care services in the hospital. Having hospital administration support, community support, and understanding the individualized needs of a community has been essential for the program’s expansion.
Key words: palliative care; program development; community hospital; rural.
Since its inception, palliative care has been committed to providing specialty-level consultation services to individuals with serious illness and their loved ones. The field has focused heavily on growth and acceptance, consistently moving upstream with regards to illness trajectory, across diseases, and across demographic variables such as age (eg, pediatric quality of life programs) and race (eg, community outreach programs addressing racial disparities in hospice use). An important frontier that remains challenging for much of the field is expansion into the community setting, where resources, implicit acceptance, and patient populations may vary.
As health system leaders appreciate the positive impacts palliative medicine on patient care and care quality, barriers to implementing palliative care programs in community hospitals must be addressed in ways tailored to the unique needs of smaller organizations and their communities. The goal of this paper is to outline the approach taken to develop Butler Health System’s community-centric palliative care program, describe our program’s underlying foundation rooted in community supports, and recount steps we have taken thus far to impact patient care in our hospital, health system, and community through the program’s first year.
Community Hospital Palliative Care—The Necessity and the Challenges
Palliative care has made strides in its growth and acceptance in the last decade; yet, the distribution of that growth has been skewed. Although 67% of hospitals now report access to specialist palliative care programs, most of the 148% growth over the last decade has been actualized in larger hospitals. Ninety percent of hospitals with greater than 300 beds report palliative care service availability whereas only 56% of small hospitals were identified to have this specialty care [1].
The inequity of access is also seen in other countries. A recent Canadian study retrospectively examined access to care of 23,860 deceased patients in Nova Scotia. Although they found 40.9% of study subjects were enrolled in a palliative care program at urban, academic centers, patients in a rural setting were only a third as likely to be enrolled in a palliative care program [2]. This access gap has important effects on patient-level outcomes, as evidence has consistently demonstrated that patients in rural settings who receive palliative care have decreased unnecessary hospitalizations and less in-hospital deaths [3].
While evidence of improved outcomes is strong, important barriers stand in the way. In a 2013 study, 374 health care providers at 236 rural hospitals in 7 states were interviewed to determine barriers to providing palliative care in rural settings. Barriers identified include a lack of administrative support, access to basic palliative care training for primary care physicians, and limited relationships to hospices [4]. Additional challenges include lack of access to tertiary-level specialty clinicians, access to and misconceptions about prescription medications, transportation for patients and providers, and incorporating a patient’s community supports [5–7].
Proposed Solutions
Techniques to improve palliative care access for rural and community centers that have been previously reviewed in the literature include videoconferencing with tertiary care experts in palliative care and education through small community-level lectures [8–10]. Goals of rural and suburban palliative care programs are broadly similar to programs at academic medical centers; however, few studies have identified impact of palliative medicine on patient care in community settings. In one suburban practice, a study found that patients were more likely to die at home if they had multiple caregivers, increased length of time under palliative care, and older age upon referral [11].
The United States has few large-scale pilot programs attempting to address the palliative needs of a more suburban or rural population. Of these, the Minnesota Rural Palliative Care Initiative developed by Stratis Health is perhaps the best publicized. Stratis Health developed and led an 18-month learning collaborative from October 2008 to April 2010 through which community teams developed or improved palliative care services. Through this initiative, a community-based health care practice model was developed that took advantage of the strong interrelationships within rural communities. After 18 months, 6 out of 10 rural Minnesota communities had formal palliative care programs, and 8 to 9 out of 10 had capabilities to at least address advance directives as well as provider and community education [12]. In another initiative, the NIH established a new suburban clinic with tertiary providers specifically for resource intensive, underserved patients [13]. The clinic was established by partnering with a service that was already in place in the community. Twenty-seven patients were seen within 7 months. The most common consults were patients with numerous comorbidities and chronic pain rather than terminal diagnoses. Given the intensive need of these patients, the authors felt that a consultation service and an interdisciplinary team that included psychosocial/spiritual/social work providers offered the most efficient method of delivering advanced palliative care needs.
The research regarding both solutions to challenges and novel methods of addressing the care gap remains sparse as evidenced by the conclusions of multiple systematic reviews and meta-analyses and the inability of the Cochrane review to find papers meeting inclusion criteria regarding techniques of community support in palliative care [14,15]. There remains a need to identify practical techniques of implementing palliative care in rural and suburban settings.
The Butler Health System Experience
In August 2015, we set out to start the first hospital-based palliative care consultation service in the Butler Health System. The health system is a nonprofit, single-hospital system anchored by Butler Memorial Hospital, a 294-bed community hospital located within a rural Pennsylvania county of 186,000 residents, 35 miles north of Pittsburgh. Butler County consists of a predominantly white, non-Hispanic population with over 15% of the residents being older than 65 years of age. The median household income is $61,000 earned primarily through blue collar occupations [16]. Driven by 53 employed primary care physicians, the health system provides services for 75,000 patients at sites covering an area of 4000 square miles. The hospital provides general medical, critical, surgical and subspecialty care and behavior health services as a regional referral center for 4 surrounding rural counties, accepting 12,500 inpatient admissions annually. A hospitalist service admits the majority of oncology patients, and the intensive care unit (ICU) is an open unit, where patients are admitted to the hospitalist, primary care, or surgical service.
While no formal needs assessment was performed prior to program development, perceptions of inadequate pain control, overuse of naloxone, underutilization of hospice services, and lack of consistent quality in end-of-life care were identified. These concerns were voiced at the levels of direct patient care on the floors, and by nursing and physician hospital leadership. Prior to our program, the chief medical officer attended the national Center to Advance Palliative Care conference to better understand the field of palliative care and its impact on improving quality of care. Concurrently, our health system was expanding its inpatient capabilities (eg, advanced neurologic and cardiac services), resulting in admissions with increased disease severity and illness complexity. With the vision of improved patient care, prioritizing quality end-of-life care and symptom management, the hospital board and administration overwhelmingly supported the development of the palliative care program, philosophically and financially.
Laying a Foundation—Funding, Collaboration, and Team Building
Funding and staffing are 2 important factors when building any program. Sources of funding for palliative care programs may include hospital support, billing revenue, grants, and philanthropy. Program development was a priority for the hospital and community. To help offset costs, efforts to raise financial support focused on utilizing the health system’s annual fundraising events. Through the generosity of individuals in the community, the hospital’s annual gala event, and donations from the hospital’s auxiliary, a total of $230,000 was raised prior to program initiation. Funds budgeted through direct hospital support and fundraising were allocated towards hiring palliative care team members and community marketing projects.
The hospital’s surrounding community is fortunate to have 2 local inpatient hospice facilities, and these relationships were imperative to providing quality end-of-life care preceding our palliative care program. A formal partnership was previously established with one while the other remains an important referral facility due to its proximity to the hospital. These hospice services are encouraged to participate in our weekly palliative care interdisciplinary team meetings. Their incorporation has improved coordination, continuity, and translation of care upon patient discharge from the acute hospital setting. Additionally, the relationships have been beneficial in tracking patients’ outcomes and data collection.
The standard structure of a palliative care team described by the Joint Commission and National Consensus Panel for Palliative Care consists of a physician, registered nurse or advanced practice provider, chaplaincy, and social work. Despite this recommendation, less than 40% of surveyed hospitals met the criteria, and less than 25% have dedicated funding to cover these positions [17]. Upon inception of our palliative care program, 2.6 funded full-time equivalents (FTEs) were allocated. These positions included a physician (1.0 FTE), a physician assistant (1.0 FTE), and a part time palliative care social worker (0.6 FTE). The 2015 National Palliative Care Registry found that 3.2 funded FTEs per 10,000 admissions is the average for hospitals with 150 to 299 beds [17]. The uncertainty of the utilization and consult volume, and the limited amount of qualified palliative care trained practitioners, resulted in the palliative program starting below this mean at 2.1 funded FTEs per 10,000 admissions. All the funded positions were located on site at the hospital. The pre-existing volunteer hospital chaplain service was identified as the pastoral care component for the program.
Increased FTEs have been associated with increased palliative care service penetration and ultimately in decreased time to consult [18]. In response to increasing consult volumes, concerns for delays in time to consult, and in preparation for expansion to an outpatient service, the palliative care department acquired an additional funded physician FTE (1.0). Ultimately the service reached a total of 3.6 FTE for inpatient services during its first 12 months; proportionately this resulted in an increase to 2.9 FTE per 10,000 admissions based on the yearly admission rate of 12,500 patients.
Educational Outreach
The success of a palliative care program depends on other clinicians’ acceptance and referral to the clinical program. We took a 2-pronged approach, focusing on both hospital-based and community-based education. The hospital-based nursing education included 30-minute presentations on general overviews of palliative care, differences between palliative care and hospice, and acute symptom management at the end of life. The palliative care team presented to all medical, surgical, and intensive care units and encompassed all shifts of nursing staff. These lectures included pre- and post-tests to assess for impact and feedback. Similar educational presentations, as well as an hour-long presentation on opioids and palliative care, were available for physicians for CME opportunities. We also distributed concise palliative care referral packets to outpatient primary care offices through the health system’s marketing team. The referral packets included examples of diagnoses, clinical scenarios, and symptoms to assist in the physicians’ understanding of palliative care services. The palliative care team also met with clinic office managers to discuss the program and answer questions.
There were also educational opportunities for patients and families in our community. Taking advantage of previously developed partnerships between the hospital system and local media outlets, the palliative care team performed local radio spots to educate the community on topics including an overview of palliative care, how to request palliative care, and the difference between palliative care and hospice care. We partnered with a local hospice agency and developed a well-received bereavement seminar for patients, family members, and employees and included the topic of advanced care planning.
Data Collection
We collect data using 2 different tools: a self-maintained spreadsheet shared between our palliative care clinicians, and a collective data tool (QDACT) included in our membership with and maintained by the Global Palliative Care Quality Alliance. Data collected and tracked in our spreadsheet includes date of consult, patient age, primary and secondary diagnoses, disposition, goals of care discussions, date of death, and 30-day readmissions. Through the QDACT data monitoring program, we are tracking and analyzing quality measures including symptom assessment and management and code status conversion. The QDACT database also provides financial data specific to our institution such as cost savings based on our billing, readmission rates, and length of stay.
Results
Projections, Volumes, and Penetration
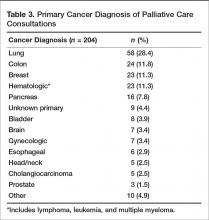
Prior to the start of our program, our chief medical office used Center to Advance Palliative Care tools to project inpatient consultation volumes at our institution. Variables that are recommended by this center to guide projections include number of hospital admissions per year, hospital occupancy, disposition to hospice, as well as generalized estimations of inpatient mortality rates. Based on our data, it was expected that our program would receive 204 new inpatient consults in our first year, and 774 follow-up visits. Our actual new inpatient consults totaled 516, with 919 follow-up visits. Palliative care penetration (percentage of annual hospital admissions seen by the palliative care team) our first year was 3.7% (Table 1).
Consultation Demographics
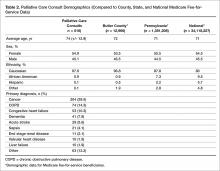
The demographics of the patients seen by the palliative care team reflect that of Butler County’s Medicare fee-for-service (FFS) population (Table 2); however, differences were seen at the state and national level with regard to ethnicity (Table 2).
Almost half of consultations (49%) were placed by the hospitalist service. Since the ICU is an open unit, critical care consults are not adequately reflected by analysis of the ordering physician alone. Analysis of consultation location revealed that 27% of inpatient consults were located within the ICU.
Patient Outcomes and Disposition
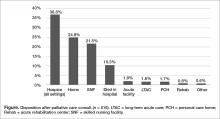
Outcomes and discharge data from the first year were collected and reviewed. Ten percent of the patients seen by palliative care died in the hospital, and 51% of patients that were seen by palliative care died within the program’s first year. Thirty-seven percent of patients discharged from the hospital utilized hospice services at home, in residential nursing facility, or at an inpatient hospice unit. The remaining 53% were discharged without hospice services to home or facility (Figure).
Hospice utilization by the health system increased during our first year. Compared to the 2014 calendar year, there were a total of 263 referrals for hospice services. During the first year of the palliative care program, which started August 2015, there were a total of 293 referrals. Of the 293 total hospice referrals, 190 (64.8%) of these referrals were for patients seen by the palliative care team.
Change of Code Status
Code status and changes in codes status data were collected. Of 462 individual patients prior to or at the time of palliative care consults, 43% were full code, 4% limited code, 8% unknown status, and 45% Do Not Resuscitate. After palliative care consult, 61% of the patients who were previously full/limited/unknown converted to do not resuscitate and do not intubate status. In total, 79% of patients seen by palliative care had a confirmed code status of Do Not Resuscitate and Do Not Intubate status after consult.
Discussion
In our first year, our palliative care program exceeded the expected number of inpatient consults, corresponding with a penetration of 3.7%. With the increase of funded FTEs, preliminary data shows that the department’s penetration continues to rise remaining consistent with the data and expectations [18]. During the second year, it is anticipated that over 600 inpatient palliative care consultations will be performed with an estimated penetration of 4%. This increasing penetration reflects the rising utilization of palliative care within our hospital. Since inception of the program, the service has expanded into an outpatient clinic 2 days per week. The palliative care clinic is staffed by a registered nurse (funded 0.6 FTE) and covered by the same physicians and physician assistant providing the inpatient services. The department acquired an unfunded but designated chaplaincy volunteer to assist with patients’ spiritual needs. We believe that the success of our program during the first year was related to multiple factors: a focus of integration and education by the palliative care department, health system administration buy-in, and identification of surrounding community needs.
In addition to patient care, our palliative care department also prioritizes “tangible” impacts to better establish our contributions to the health system. We have done this through participation on hospital committees, hospital policy revision teams, and by developing innovative solutions such as a terminal extubation protocol and order set for our ICU. The health system and its administration have recognized the importance of educating nursing and physician staff on palliative care services, and have supported these continued efforts alongside our clinical obligations.
Concurrent with administration buy-in, financial supports for our palliative care services were initially supplemented by the health system. Our department understands the importance of recognizing limitations of resources in communities and their hospitals. In efforts to minimize the department’s impact on our own health system’s financial resources, we have strived to offset our costs. We helped the hospital system meet pay-for-performance palliative care metrics set by the large local insurers resulting in financial hospital reimbursement valued at $600,000 in 2016.
The question of how the program may translate into other communities raises a major limitation: the homogeneity our population. The community surrounding the hospital is primarily Caucasian, with minimal representation of minority populations. While the patient population seen by our palliative care team is reflective of our surrounding county, it does not represent Medicare FFS beneficiaries on a national level or many other types of community hospitals across the country. Variations of ethnicity, age, diagnoses, and faith are fundamental, which highlights the importance of understanding the community in which a program is developed.
The rising trajectory of our palliative care service utilization has prompted a discussion of future endeavors for our program. Expectations for a continued shortage of hospice and palliative care physicians [19] and concerns for practitioner burnout [20] underlie our thoughtful approach to expansion of inpatient and outpatient services. At this time, potential projects include a consultation trigger system and incorporation of palliative care providers in ICU rounding, as well as possible expansion of outpatient services through implantation of an advanced practitioner into surrounding nursing homes and primary care offices.
We have found a growing utilization of our program at Butler Health System. Our first year experience has highlighted the importance of identifying community and hospital administrative champions as a foundation. Additionally, understanding the specific characteristics of one’s surrounding community may allow for improved integration and acceptance of palliative care in a community setting. Our program continues to work with the health system, community, and philanthropic organizations to expand the ever-growing need for palliative care services.
1. Dumanovsky T, Augustin R, Rogers M, et al. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med 2016;19:8–15.
2. Lavergne MR, Lethbridge L, Johnston G, et al. Examining palliative care program use and place of death in rural and urban contexts: a Canadian population-based study using linked data. Rural Remote Health 2015;15:3134.
3. Seow H, Brazil K, Sussman J, et al. Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ 2014;348:g3496.
4. Fink RM, Oman KS, Youngwerth J, et al. A palliative care needs assessment of rural hospitals. J Palliat Med 2013;16:638–44.
5. Dumont S, Jacobs P, Turcotte V, et al. Palliative care costs in Canada: A descriptive comparison of studies of urban and rural patients near end of life. J Palliat Med 2015;29:908–17.
6. Kaasalainen S, Brazil K, Williams A, et al. Nurses' experiences providing palliative care to individuals living in rural communities: aspects of the physical residential setting. Rural Remote Health 2014;14:2728.
7. Ahmed N, Bestall JC, Ahmedzai SH, et al. Systematic review of the problems and issues of accessing specialist palliative care by patients, carers and health and social care professionals. J Palliat Med 2004;18:525–42.
8. Ray RA, Fried O, Lindsay D. Palliative care professional education via video conference builds confidence to deliver palliative care in rural and remote locations. BMC Health Serv Res 2014;14:272.
9. Bakitas MA, Elk R, Astin M, et al. Systematic review of palliative care in the rural setting. Cancer Control 2015;22:450–64.
10. Akiyama M, Hirai K, Takebayashi T, et al. The effects of community-wide dissemination of information on perceptions of palliative care, knowledge about opioids, and sense of security among cancer patients, their families, and the general public. Support Care Cancer 2016;24: 347–56.
11. Maida V. Factors that promote success in home palliative care: a study of a large suburban palliative care practice. J Palliat Care 2002;18:282–6.
12. Ceronsky L, Shearer J, Weng K, et al. Minnesota Rural Palliative Care Initiative: building palliative care capacity in rural Minnesota. J Palliat Med 2013;16:310–3.
13. Aggarwal SK, Ghosh A, Cheng MJ, et al. Initiating pain and palliative care outpatient services for the suburban underserved in Montgomery County, Maryland: Lessons learned at the NIH Clinical Center and MobileMed. Palliat Support Care 2015;16:1–6.
14. Rainsford S, MacLeod RD, Glasgow NJ. Place of death in rural palliative care: A systematic review. J Palliat Med 2016;30:745–63.
15. Horey D, Street AF, O'Connor M, et al. Training and supportive programs for palliative care volunteers in community settings. Cochrane Database Syst Rev 2015 Jul 20;(7):CD009500.
16. The United States Census Bureau: QuickFacts: Butler County, Pennsylvania. Accessed 10 Mar 2017 at www.census.gov/quickfacts/table/PST045216/42019,00.
17. Spetz J, Dudley N, Trupin L, et al. Few hospital palliative care programs meet national staffing recommendations. Health Aff 2016;35:1690–7.
18. Dumanovsky T, Rogers M, Spragens LH, et al. Impact of Staffing on Access to Palliative Care in U.S. Hospitals. J Palliat Med. 2015 Dec; 18(12). Pages 998-999.
19. Lupu D, American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage 2010;40:899–911.
20. Kamal AH, Bull JK, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manage 2016;51:690–6.
From the Division of Palliative Care, Butler Health System, Butler, PA (Drs. Stein, Reefer, Selvaggi, Ms. Doverspike); the University of Pittsburgh Medical Center, Pittsburgh, PA (Dr. Rajagopal); and the Duke Cancer Institute and Duke Fuqua School of Business, Durham, NC (Dr. Kamal).
Abstract
- Objective: To describe an approach to develop a community-centric palliative care program in a rural community health system and to review data collected over the program’s first year.
- Methods: We describe the underlying foundations of our program development including the health system’s prioritization of a palliative care program, funding opportunities, collaboration with community supports, and the importance of building a team and program that reflects a community’s needs. Data were collected through a program-maintained spreadsheet and a data monitoring system available through the Global Palliative Care Quality Alliance.
- Results: 516 new inpatient consultations were seen during the first year, for a penetration of 3.7%. The demographics of the patients who received consultation reflect that of the surrounding community. Over 50% of patients seen within the first year died, and hospice utilization at home and within facilities and inpatient hospice units increased. In addition, 79% of the patients seen by the palliative care team had a confirmed code status of do not resuscitate and do not intubate.
- Conclusions: Butler Health System’s approach to development of a palliative care program has resulted in increasing utilization of palliative care services in the hospital. Having hospital administration support, community support, and understanding the individualized needs of a community has been essential for the program’s expansion.
Key words: palliative care; program development; community hospital; rural.
Since its inception, palliative care has been committed to providing specialty-level consultation services to individuals with serious illness and their loved ones. The field has focused heavily on growth and acceptance, consistently moving upstream with regards to illness trajectory, across diseases, and across demographic variables such as age (eg, pediatric quality of life programs) and race (eg, community outreach programs addressing racial disparities in hospice use). An important frontier that remains challenging for much of the field is expansion into the community setting, where resources, implicit acceptance, and patient populations may vary.
As health system leaders appreciate the positive impacts palliative medicine on patient care and care quality, barriers to implementing palliative care programs in community hospitals must be addressed in ways tailored to the unique needs of smaller organizations and their communities. The goal of this paper is to outline the approach taken to develop Butler Health System’s community-centric palliative care program, describe our program’s underlying foundation rooted in community supports, and recount steps we have taken thus far to impact patient care in our hospital, health system, and community through the program’s first year.
Community Hospital Palliative Care—The Necessity and the Challenges
Palliative care has made strides in its growth and acceptance in the last decade; yet, the distribution of that growth has been skewed. Although 67% of hospitals now report access to specialist palliative care programs, most of the 148% growth over the last decade has been actualized in larger hospitals. Ninety percent of hospitals with greater than 300 beds report palliative care service availability whereas only 56% of small hospitals were identified to have this specialty care [1].
The inequity of access is also seen in other countries. A recent Canadian study retrospectively examined access to care of 23,860 deceased patients in Nova Scotia. Although they found 40.9% of study subjects were enrolled in a palliative care program at urban, academic centers, patients in a rural setting were only a third as likely to be enrolled in a palliative care program [2]. This access gap has important effects on patient-level outcomes, as evidence has consistently demonstrated that patients in rural settings who receive palliative care have decreased unnecessary hospitalizations and less in-hospital deaths [3].
While evidence of improved outcomes is strong, important barriers stand in the way. In a 2013 study, 374 health care providers at 236 rural hospitals in 7 states were interviewed to determine barriers to providing palliative care in rural settings. Barriers identified include a lack of administrative support, access to basic palliative care training for primary care physicians, and limited relationships to hospices [4]. Additional challenges include lack of access to tertiary-level specialty clinicians, access to and misconceptions about prescription medications, transportation for patients and providers, and incorporating a patient’s community supports [5–7].
Proposed Solutions
Techniques to improve palliative care access for rural and community centers that have been previously reviewed in the literature include videoconferencing with tertiary care experts in palliative care and education through small community-level lectures [8–10]. Goals of rural and suburban palliative care programs are broadly similar to programs at academic medical centers; however, few studies have identified impact of palliative medicine on patient care in community settings. In one suburban practice, a study found that patients were more likely to die at home if they had multiple caregivers, increased length of time under palliative care, and older age upon referral [11].
The United States has few large-scale pilot programs attempting to address the palliative needs of a more suburban or rural population. Of these, the Minnesota Rural Palliative Care Initiative developed by Stratis Health is perhaps the best publicized. Stratis Health developed and led an 18-month learning collaborative from October 2008 to April 2010 through which community teams developed or improved palliative care services. Through this initiative, a community-based health care practice model was developed that took advantage of the strong interrelationships within rural communities. After 18 months, 6 out of 10 rural Minnesota communities had formal palliative care programs, and 8 to 9 out of 10 had capabilities to at least address advance directives as well as provider and community education [12]. In another initiative, the NIH established a new suburban clinic with tertiary providers specifically for resource intensive, underserved patients [13]. The clinic was established by partnering with a service that was already in place in the community. Twenty-seven patients were seen within 7 months. The most common consults were patients with numerous comorbidities and chronic pain rather than terminal diagnoses. Given the intensive need of these patients, the authors felt that a consultation service and an interdisciplinary team that included psychosocial/spiritual/social work providers offered the most efficient method of delivering advanced palliative care needs.
The research regarding both solutions to challenges and novel methods of addressing the care gap remains sparse as evidenced by the conclusions of multiple systematic reviews and meta-analyses and the inability of the Cochrane review to find papers meeting inclusion criteria regarding techniques of community support in palliative care [14,15]. There remains a need to identify practical techniques of implementing palliative care in rural and suburban settings.
The Butler Health System Experience
In August 2015, we set out to start the first hospital-based palliative care consultation service in the Butler Health System. The health system is a nonprofit, single-hospital system anchored by Butler Memorial Hospital, a 294-bed community hospital located within a rural Pennsylvania county of 186,000 residents, 35 miles north of Pittsburgh. Butler County consists of a predominantly white, non-Hispanic population with over 15% of the residents being older than 65 years of age. The median household income is $61,000 earned primarily through blue collar occupations [16]. Driven by 53 employed primary care physicians, the health system provides services for 75,000 patients at sites covering an area of 4000 square miles. The hospital provides general medical, critical, surgical and subspecialty care and behavior health services as a regional referral center for 4 surrounding rural counties, accepting 12,500 inpatient admissions annually. A hospitalist service admits the majority of oncology patients, and the intensive care unit (ICU) is an open unit, where patients are admitted to the hospitalist, primary care, or surgical service.
While no formal needs assessment was performed prior to program development, perceptions of inadequate pain control, overuse of naloxone, underutilization of hospice services, and lack of consistent quality in end-of-life care were identified. These concerns were voiced at the levels of direct patient care on the floors, and by nursing and physician hospital leadership. Prior to our program, the chief medical officer attended the national Center to Advance Palliative Care conference to better understand the field of palliative care and its impact on improving quality of care. Concurrently, our health system was expanding its inpatient capabilities (eg, advanced neurologic and cardiac services), resulting in admissions with increased disease severity and illness complexity. With the vision of improved patient care, prioritizing quality end-of-life care and symptom management, the hospital board and administration overwhelmingly supported the development of the palliative care program, philosophically and financially.
Laying a Foundation—Funding, Collaboration, and Team Building
Funding and staffing are 2 important factors when building any program. Sources of funding for palliative care programs may include hospital support, billing revenue, grants, and philanthropy. Program development was a priority for the hospital and community. To help offset costs, efforts to raise financial support focused on utilizing the health system’s annual fundraising events. Through the generosity of individuals in the community, the hospital’s annual gala event, and donations from the hospital’s auxiliary, a total of $230,000 was raised prior to program initiation. Funds budgeted through direct hospital support and fundraising were allocated towards hiring palliative care team members and community marketing projects.
The hospital’s surrounding community is fortunate to have 2 local inpatient hospice facilities, and these relationships were imperative to providing quality end-of-life care preceding our palliative care program. A formal partnership was previously established with one while the other remains an important referral facility due to its proximity to the hospital. These hospice services are encouraged to participate in our weekly palliative care interdisciplinary team meetings. Their incorporation has improved coordination, continuity, and translation of care upon patient discharge from the acute hospital setting. Additionally, the relationships have been beneficial in tracking patients’ outcomes and data collection.
The standard structure of a palliative care team described by the Joint Commission and National Consensus Panel for Palliative Care consists of a physician, registered nurse or advanced practice provider, chaplaincy, and social work. Despite this recommendation, less than 40% of surveyed hospitals met the criteria, and less than 25% have dedicated funding to cover these positions [17]. Upon inception of our palliative care program, 2.6 funded full-time equivalents (FTEs) were allocated. These positions included a physician (1.0 FTE), a physician assistant (1.0 FTE), and a part time palliative care social worker (0.6 FTE). The 2015 National Palliative Care Registry found that 3.2 funded FTEs per 10,000 admissions is the average for hospitals with 150 to 299 beds [17]. The uncertainty of the utilization and consult volume, and the limited amount of qualified palliative care trained practitioners, resulted in the palliative program starting below this mean at 2.1 funded FTEs per 10,000 admissions. All the funded positions were located on site at the hospital. The pre-existing volunteer hospital chaplain service was identified as the pastoral care component for the program.
Increased FTEs have been associated with increased palliative care service penetration and ultimately in decreased time to consult [18]. In response to increasing consult volumes, concerns for delays in time to consult, and in preparation for expansion to an outpatient service, the palliative care department acquired an additional funded physician FTE (1.0). Ultimately the service reached a total of 3.6 FTE for inpatient services during its first 12 months; proportionately this resulted in an increase to 2.9 FTE per 10,000 admissions based on the yearly admission rate of 12,500 patients.
Educational Outreach
The success of a palliative care program depends on other clinicians’ acceptance and referral to the clinical program. We took a 2-pronged approach, focusing on both hospital-based and community-based education. The hospital-based nursing education included 30-minute presentations on general overviews of palliative care, differences between palliative care and hospice, and acute symptom management at the end of life. The palliative care team presented to all medical, surgical, and intensive care units and encompassed all shifts of nursing staff. These lectures included pre- and post-tests to assess for impact and feedback. Similar educational presentations, as well as an hour-long presentation on opioids and palliative care, were available for physicians for CME opportunities. We also distributed concise palliative care referral packets to outpatient primary care offices through the health system’s marketing team. The referral packets included examples of diagnoses, clinical scenarios, and symptoms to assist in the physicians’ understanding of palliative care services. The palliative care team also met with clinic office managers to discuss the program and answer questions.
There were also educational opportunities for patients and families in our community. Taking advantage of previously developed partnerships between the hospital system and local media outlets, the palliative care team performed local radio spots to educate the community on topics including an overview of palliative care, how to request palliative care, and the difference between palliative care and hospice care. We partnered with a local hospice agency and developed a well-received bereavement seminar for patients, family members, and employees and included the topic of advanced care planning.
Data Collection
We collect data using 2 different tools: a self-maintained spreadsheet shared between our palliative care clinicians, and a collective data tool (QDACT) included in our membership with and maintained by the Global Palliative Care Quality Alliance. Data collected and tracked in our spreadsheet includes date of consult, patient age, primary and secondary diagnoses, disposition, goals of care discussions, date of death, and 30-day readmissions. Through the QDACT data monitoring program, we are tracking and analyzing quality measures including symptom assessment and management and code status conversion. The QDACT database also provides financial data specific to our institution such as cost savings based on our billing, readmission rates, and length of stay.
Results
Projections, Volumes, and Penetration
Prior to the start of our program, our chief medical office used Center to Advance Palliative Care tools to project inpatient consultation volumes at our institution. Variables that are recommended by this center to guide projections include number of hospital admissions per year, hospital occupancy, disposition to hospice, as well as generalized estimations of inpatient mortality rates. Based on our data, it was expected that our program would receive 204 new inpatient consults in our first year, and 774 follow-up visits. Our actual new inpatient consults totaled 516, with 919 follow-up visits. Palliative care penetration (percentage of annual hospital admissions seen by the palliative care team) our first year was 3.7% (Table 1).
Consultation Demographics
The demographics of the patients seen by the palliative care team reflect that of Butler County’s Medicare fee-for-service (FFS) population (Table 2); however, differences were seen at the state and national level with regard to ethnicity (Table 2).
Almost half of consultations (49%) were placed by the hospitalist service. Since the ICU is an open unit, critical care consults are not adequately reflected by analysis of the ordering physician alone. Analysis of consultation location revealed that 27% of inpatient consults were located within the ICU.
Patient Outcomes and Disposition
Outcomes and discharge data from the first year were collected and reviewed. Ten percent of the patients seen by palliative care died in the hospital, and 51% of patients that were seen by palliative care died within the program’s first year. Thirty-seven percent of patients discharged from the hospital utilized hospice services at home, in residential nursing facility, or at an inpatient hospice unit. The remaining 53% were discharged without hospice services to home or facility (Figure).
Hospice utilization by the health system increased during our first year. Compared to the 2014 calendar year, there were a total of 263 referrals for hospice services. During the first year of the palliative care program, which started August 2015, there were a total of 293 referrals. Of the 293 total hospice referrals, 190 (64.8%) of these referrals were for patients seen by the palliative care team.
Change of Code Status
Code status and changes in codes status data were collected. Of 462 individual patients prior to or at the time of palliative care consults, 43% were full code, 4% limited code, 8% unknown status, and 45% Do Not Resuscitate. After palliative care consult, 61% of the patients who were previously full/limited/unknown converted to do not resuscitate and do not intubate status. In total, 79% of patients seen by palliative care had a confirmed code status of Do Not Resuscitate and Do Not Intubate status after consult.
Discussion
In our first year, our palliative care program exceeded the expected number of inpatient consults, corresponding with a penetration of 3.7%. With the increase of funded FTEs, preliminary data shows that the department’s penetration continues to rise remaining consistent with the data and expectations [18]. During the second year, it is anticipated that over 600 inpatient palliative care consultations will be performed with an estimated penetration of 4%. This increasing penetration reflects the rising utilization of palliative care within our hospital. Since inception of the program, the service has expanded into an outpatient clinic 2 days per week. The palliative care clinic is staffed by a registered nurse (funded 0.6 FTE) and covered by the same physicians and physician assistant providing the inpatient services. The department acquired an unfunded but designated chaplaincy volunteer to assist with patients’ spiritual needs. We believe that the success of our program during the first year was related to multiple factors: a focus of integration and education by the palliative care department, health system administration buy-in, and identification of surrounding community needs.
In addition to patient care, our palliative care department also prioritizes “tangible” impacts to better establish our contributions to the health system. We have done this through participation on hospital committees, hospital policy revision teams, and by developing innovative solutions such as a terminal extubation protocol and order set for our ICU. The health system and its administration have recognized the importance of educating nursing and physician staff on palliative care services, and have supported these continued efforts alongside our clinical obligations.
Concurrent with administration buy-in, financial supports for our palliative care services were initially supplemented by the health system. Our department understands the importance of recognizing limitations of resources in communities and their hospitals. In efforts to minimize the department’s impact on our own health system’s financial resources, we have strived to offset our costs. We helped the hospital system meet pay-for-performance palliative care metrics set by the large local insurers resulting in financial hospital reimbursement valued at $600,000 in 2016.
The question of how the program may translate into other communities raises a major limitation: the homogeneity our population. The community surrounding the hospital is primarily Caucasian, with minimal representation of minority populations. While the patient population seen by our palliative care team is reflective of our surrounding county, it does not represent Medicare FFS beneficiaries on a national level or many other types of community hospitals across the country. Variations of ethnicity, age, diagnoses, and faith are fundamental, which highlights the importance of understanding the community in which a program is developed.
The rising trajectory of our palliative care service utilization has prompted a discussion of future endeavors for our program. Expectations for a continued shortage of hospice and palliative care physicians [19] and concerns for practitioner burnout [20] underlie our thoughtful approach to expansion of inpatient and outpatient services. At this time, potential projects include a consultation trigger system and incorporation of palliative care providers in ICU rounding, as well as possible expansion of outpatient services through implantation of an advanced practitioner into surrounding nursing homes and primary care offices.
We have found a growing utilization of our program at Butler Health System. Our first year experience has highlighted the importance of identifying community and hospital administrative champions as a foundation. Additionally, understanding the specific characteristics of one’s surrounding community may allow for improved integration and acceptance of palliative care in a community setting. Our program continues to work with the health system, community, and philanthropic organizations to expand the ever-growing need for palliative care services.
From the Division of Palliative Care, Butler Health System, Butler, PA (Drs. Stein, Reefer, Selvaggi, Ms. Doverspike); the University of Pittsburgh Medical Center, Pittsburgh, PA (Dr. Rajagopal); and the Duke Cancer Institute and Duke Fuqua School of Business, Durham, NC (Dr. Kamal).
Abstract
- Objective: To describe an approach to develop a community-centric palliative care program in a rural community health system and to review data collected over the program’s first year.
- Methods: We describe the underlying foundations of our program development including the health system’s prioritization of a palliative care program, funding opportunities, collaboration with community supports, and the importance of building a team and program that reflects a community’s needs. Data were collected through a program-maintained spreadsheet and a data monitoring system available through the Global Palliative Care Quality Alliance.
- Results: 516 new inpatient consultations were seen during the first year, for a penetration of 3.7%. The demographics of the patients who received consultation reflect that of the surrounding community. Over 50% of patients seen within the first year died, and hospice utilization at home and within facilities and inpatient hospice units increased. In addition, 79% of the patients seen by the palliative care team had a confirmed code status of do not resuscitate and do not intubate.
- Conclusions: Butler Health System’s approach to development of a palliative care program has resulted in increasing utilization of palliative care services in the hospital. Having hospital administration support, community support, and understanding the individualized needs of a community has been essential for the program’s expansion.
Key words: palliative care; program development; community hospital; rural.
Since its inception, palliative care has been committed to providing specialty-level consultation services to individuals with serious illness and their loved ones. The field has focused heavily on growth and acceptance, consistently moving upstream with regards to illness trajectory, across diseases, and across demographic variables such as age (eg, pediatric quality of life programs) and race (eg, community outreach programs addressing racial disparities in hospice use). An important frontier that remains challenging for much of the field is expansion into the community setting, where resources, implicit acceptance, and patient populations may vary.
As health system leaders appreciate the positive impacts palliative medicine on patient care and care quality, barriers to implementing palliative care programs in community hospitals must be addressed in ways tailored to the unique needs of smaller organizations and their communities. The goal of this paper is to outline the approach taken to develop Butler Health System’s community-centric palliative care program, describe our program’s underlying foundation rooted in community supports, and recount steps we have taken thus far to impact patient care in our hospital, health system, and community through the program’s first year.
Community Hospital Palliative Care—The Necessity and the Challenges
Palliative care has made strides in its growth and acceptance in the last decade; yet, the distribution of that growth has been skewed. Although 67% of hospitals now report access to specialist palliative care programs, most of the 148% growth over the last decade has been actualized in larger hospitals. Ninety percent of hospitals with greater than 300 beds report palliative care service availability whereas only 56% of small hospitals were identified to have this specialty care [1].
The inequity of access is also seen in other countries. A recent Canadian study retrospectively examined access to care of 23,860 deceased patients in Nova Scotia. Although they found 40.9% of study subjects were enrolled in a palliative care program at urban, academic centers, patients in a rural setting were only a third as likely to be enrolled in a palliative care program [2]. This access gap has important effects on patient-level outcomes, as evidence has consistently demonstrated that patients in rural settings who receive palliative care have decreased unnecessary hospitalizations and less in-hospital deaths [3].
While evidence of improved outcomes is strong, important barriers stand in the way. In a 2013 study, 374 health care providers at 236 rural hospitals in 7 states were interviewed to determine barriers to providing palliative care in rural settings. Barriers identified include a lack of administrative support, access to basic palliative care training for primary care physicians, and limited relationships to hospices [4]. Additional challenges include lack of access to tertiary-level specialty clinicians, access to and misconceptions about prescription medications, transportation for patients and providers, and incorporating a patient’s community supports [5–7].
Proposed Solutions
Techniques to improve palliative care access for rural and community centers that have been previously reviewed in the literature include videoconferencing with tertiary care experts in palliative care and education through small community-level lectures [8–10]. Goals of rural and suburban palliative care programs are broadly similar to programs at academic medical centers; however, few studies have identified impact of palliative medicine on patient care in community settings. In one suburban practice, a study found that patients were more likely to die at home if they had multiple caregivers, increased length of time under palliative care, and older age upon referral [11].
The United States has few large-scale pilot programs attempting to address the palliative needs of a more suburban or rural population. Of these, the Minnesota Rural Palliative Care Initiative developed by Stratis Health is perhaps the best publicized. Stratis Health developed and led an 18-month learning collaborative from October 2008 to April 2010 through which community teams developed or improved palliative care services. Through this initiative, a community-based health care practice model was developed that took advantage of the strong interrelationships within rural communities. After 18 months, 6 out of 10 rural Minnesota communities had formal palliative care programs, and 8 to 9 out of 10 had capabilities to at least address advance directives as well as provider and community education [12]. In another initiative, the NIH established a new suburban clinic with tertiary providers specifically for resource intensive, underserved patients [13]. The clinic was established by partnering with a service that was already in place in the community. Twenty-seven patients were seen within 7 months. The most common consults were patients with numerous comorbidities and chronic pain rather than terminal diagnoses. Given the intensive need of these patients, the authors felt that a consultation service and an interdisciplinary team that included psychosocial/spiritual/social work providers offered the most efficient method of delivering advanced palliative care needs.
The research regarding both solutions to challenges and novel methods of addressing the care gap remains sparse as evidenced by the conclusions of multiple systematic reviews and meta-analyses and the inability of the Cochrane review to find papers meeting inclusion criteria regarding techniques of community support in palliative care [14,15]. There remains a need to identify practical techniques of implementing palliative care in rural and suburban settings.
The Butler Health System Experience
In August 2015, we set out to start the first hospital-based palliative care consultation service in the Butler Health System. The health system is a nonprofit, single-hospital system anchored by Butler Memorial Hospital, a 294-bed community hospital located within a rural Pennsylvania county of 186,000 residents, 35 miles north of Pittsburgh. Butler County consists of a predominantly white, non-Hispanic population with over 15% of the residents being older than 65 years of age. The median household income is $61,000 earned primarily through blue collar occupations [16]. Driven by 53 employed primary care physicians, the health system provides services for 75,000 patients at sites covering an area of 4000 square miles. The hospital provides general medical, critical, surgical and subspecialty care and behavior health services as a regional referral center for 4 surrounding rural counties, accepting 12,500 inpatient admissions annually. A hospitalist service admits the majority of oncology patients, and the intensive care unit (ICU) is an open unit, where patients are admitted to the hospitalist, primary care, or surgical service.
While no formal needs assessment was performed prior to program development, perceptions of inadequate pain control, overuse of naloxone, underutilization of hospice services, and lack of consistent quality in end-of-life care were identified. These concerns were voiced at the levels of direct patient care on the floors, and by nursing and physician hospital leadership. Prior to our program, the chief medical officer attended the national Center to Advance Palliative Care conference to better understand the field of palliative care and its impact on improving quality of care. Concurrently, our health system was expanding its inpatient capabilities (eg, advanced neurologic and cardiac services), resulting in admissions with increased disease severity and illness complexity. With the vision of improved patient care, prioritizing quality end-of-life care and symptom management, the hospital board and administration overwhelmingly supported the development of the palliative care program, philosophically and financially.
Laying a Foundation—Funding, Collaboration, and Team Building
Funding and staffing are 2 important factors when building any program. Sources of funding for palliative care programs may include hospital support, billing revenue, grants, and philanthropy. Program development was a priority for the hospital and community. To help offset costs, efforts to raise financial support focused on utilizing the health system’s annual fundraising events. Through the generosity of individuals in the community, the hospital’s annual gala event, and donations from the hospital’s auxiliary, a total of $230,000 was raised prior to program initiation. Funds budgeted through direct hospital support and fundraising were allocated towards hiring palliative care team members and community marketing projects.
The hospital’s surrounding community is fortunate to have 2 local inpatient hospice facilities, and these relationships were imperative to providing quality end-of-life care preceding our palliative care program. A formal partnership was previously established with one while the other remains an important referral facility due to its proximity to the hospital. These hospice services are encouraged to participate in our weekly palliative care interdisciplinary team meetings. Their incorporation has improved coordination, continuity, and translation of care upon patient discharge from the acute hospital setting. Additionally, the relationships have been beneficial in tracking patients’ outcomes and data collection.
The standard structure of a palliative care team described by the Joint Commission and National Consensus Panel for Palliative Care consists of a physician, registered nurse or advanced practice provider, chaplaincy, and social work. Despite this recommendation, less than 40% of surveyed hospitals met the criteria, and less than 25% have dedicated funding to cover these positions [17]. Upon inception of our palliative care program, 2.6 funded full-time equivalents (FTEs) were allocated. These positions included a physician (1.0 FTE), a physician assistant (1.0 FTE), and a part time palliative care social worker (0.6 FTE). The 2015 National Palliative Care Registry found that 3.2 funded FTEs per 10,000 admissions is the average for hospitals with 150 to 299 beds [17]. The uncertainty of the utilization and consult volume, and the limited amount of qualified palliative care trained practitioners, resulted in the palliative program starting below this mean at 2.1 funded FTEs per 10,000 admissions. All the funded positions were located on site at the hospital. The pre-existing volunteer hospital chaplain service was identified as the pastoral care component for the program.
Increased FTEs have been associated with increased palliative care service penetration and ultimately in decreased time to consult [18]. In response to increasing consult volumes, concerns for delays in time to consult, and in preparation for expansion to an outpatient service, the palliative care department acquired an additional funded physician FTE (1.0). Ultimately the service reached a total of 3.6 FTE for inpatient services during its first 12 months; proportionately this resulted in an increase to 2.9 FTE per 10,000 admissions based on the yearly admission rate of 12,500 patients.
Educational Outreach
The success of a palliative care program depends on other clinicians’ acceptance and referral to the clinical program. We took a 2-pronged approach, focusing on both hospital-based and community-based education. The hospital-based nursing education included 30-minute presentations on general overviews of palliative care, differences between palliative care and hospice, and acute symptom management at the end of life. The palliative care team presented to all medical, surgical, and intensive care units and encompassed all shifts of nursing staff. These lectures included pre- and post-tests to assess for impact and feedback. Similar educational presentations, as well as an hour-long presentation on opioids and palliative care, were available for physicians for CME opportunities. We also distributed concise palliative care referral packets to outpatient primary care offices through the health system’s marketing team. The referral packets included examples of diagnoses, clinical scenarios, and symptoms to assist in the physicians’ understanding of palliative care services. The palliative care team also met with clinic office managers to discuss the program and answer questions.
There were also educational opportunities for patients and families in our community. Taking advantage of previously developed partnerships between the hospital system and local media outlets, the palliative care team performed local radio spots to educate the community on topics including an overview of palliative care, how to request palliative care, and the difference between palliative care and hospice care. We partnered with a local hospice agency and developed a well-received bereavement seminar for patients, family members, and employees and included the topic of advanced care planning.
Data Collection
We collect data using 2 different tools: a self-maintained spreadsheet shared between our palliative care clinicians, and a collective data tool (QDACT) included in our membership with and maintained by the Global Palliative Care Quality Alliance. Data collected and tracked in our spreadsheet includes date of consult, patient age, primary and secondary diagnoses, disposition, goals of care discussions, date of death, and 30-day readmissions. Through the QDACT data monitoring program, we are tracking and analyzing quality measures including symptom assessment and management and code status conversion. The QDACT database also provides financial data specific to our institution such as cost savings based on our billing, readmission rates, and length of stay.
Results
Projections, Volumes, and Penetration
Prior to the start of our program, our chief medical office used Center to Advance Palliative Care tools to project inpatient consultation volumes at our institution. Variables that are recommended by this center to guide projections include number of hospital admissions per year, hospital occupancy, disposition to hospice, as well as generalized estimations of inpatient mortality rates. Based on our data, it was expected that our program would receive 204 new inpatient consults in our first year, and 774 follow-up visits. Our actual new inpatient consults totaled 516, with 919 follow-up visits. Palliative care penetration (percentage of annual hospital admissions seen by the palliative care team) our first year was 3.7% (Table 1).
Consultation Demographics
The demographics of the patients seen by the palliative care team reflect that of Butler County’s Medicare fee-for-service (FFS) population (Table 2); however, differences were seen at the state and national level with regard to ethnicity (Table 2).
Almost half of consultations (49%) were placed by the hospitalist service. Since the ICU is an open unit, critical care consults are not adequately reflected by analysis of the ordering physician alone. Analysis of consultation location revealed that 27% of inpatient consults were located within the ICU.
Patient Outcomes and Disposition
Outcomes and discharge data from the first year were collected and reviewed. Ten percent of the patients seen by palliative care died in the hospital, and 51% of patients that were seen by palliative care died within the program’s first year. Thirty-seven percent of patients discharged from the hospital utilized hospice services at home, in residential nursing facility, or at an inpatient hospice unit. The remaining 53% were discharged without hospice services to home or facility (Figure).
Hospice utilization by the health system increased during our first year. Compared to the 2014 calendar year, there were a total of 263 referrals for hospice services. During the first year of the palliative care program, which started August 2015, there were a total of 293 referrals. Of the 293 total hospice referrals, 190 (64.8%) of these referrals were for patients seen by the palliative care team.
Change of Code Status
Code status and changes in codes status data were collected. Of 462 individual patients prior to or at the time of palliative care consults, 43% were full code, 4% limited code, 8% unknown status, and 45% Do Not Resuscitate. After palliative care consult, 61% of the patients who were previously full/limited/unknown converted to do not resuscitate and do not intubate status. In total, 79% of patients seen by palliative care had a confirmed code status of Do Not Resuscitate and Do Not Intubate status after consult.
Discussion
In our first year, our palliative care program exceeded the expected number of inpatient consults, corresponding with a penetration of 3.7%. With the increase of funded FTEs, preliminary data shows that the department’s penetration continues to rise remaining consistent with the data and expectations [18]. During the second year, it is anticipated that over 600 inpatient palliative care consultations will be performed with an estimated penetration of 4%. This increasing penetration reflects the rising utilization of palliative care within our hospital. Since inception of the program, the service has expanded into an outpatient clinic 2 days per week. The palliative care clinic is staffed by a registered nurse (funded 0.6 FTE) and covered by the same physicians and physician assistant providing the inpatient services. The department acquired an unfunded but designated chaplaincy volunteer to assist with patients’ spiritual needs. We believe that the success of our program during the first year was related to multiple factors: a focus of integration and education by the palliative care department, health system administration buy-in, and identification of surrounding community needs.
In addition to patient care, our palliative care department also prioritizes “tangible” impacts to better establish our contributions to the health system. We have done this through participation on hospital committees, hospital policy revision teams, and by developing innovative solutions such as a terminal extubation protocol and order set for our ICU. The health system and its administration have recognized the importance of educating nursing and physician staff on palliative care services, and have supported these continued efforts alongside our clinical obligations.
Concurrent with administration buy-in, financial supports for our palliative care services were initially supplemented by the health system. Our department understands the importance of recognizing limitations of resources in communities and their hospitals. In efforts to minimize the department’s impact on our own health system’s financial resources, we have strived to offset our costs. We helped the hospital system meet pay-for-performance palliative care metrics set by the large local insurers resulting in financial hospital reimbursement valued at $600,000 in 2016.
The question of how the program may translate into other communities raises a major limitation: the homogeneity our population. The community surrounding the hospital is primarily Caucasian, with minimal representation of minority populations. While the patient population seen by our palliative care team is reflective of our surrounding county, it does not represent Medicare FFS beneficiaries on a national level or many other types of community hospitals across the country. Variations of ethnicity, age, diagnoses, and faith are fundamental, which highlights the importance of understanding the community in which a program is developed.
The rising trajectory of our palliative care service utilization has prompted a discussion of future endeavors for our program. Expectations for a continued shortage of hospice and palliative care physicians [19] and concerns for practitioner burnout [20] underlie our thoughtful approach to expansion of inpatient and outpatient services. At this time, potential projects include a consultation trigger system and incorporation of palliative care providers in ICU rounding, as well as possible expansion of outpatient services through implantation of an advanced practitioner into surrounding nursing homes and primary care offices.
We have found a growing utilization of our program at Butler Health System. Our first year experience has highlighted the importance of identifying community and hospital administrative champions as a foundation. Additionally, understanding the specific characteristics of one’s surrounding community may allow for improved integration and acceptance of palliative care in a community setting. Our program continues to work with the health system, community, and philanthropic organizations to expand the ever-growing need for palliative care services.
1. Dumanovsky T, Augustin R, Rogers M, et al. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med 2016;19:8–15.
2. Lavergne MR, Lethbridge L, Johnston G, et al. Examining palliative care program use and place of death in rural and urban contexts: a Canadian population-based study using linked data. Rural Remote Health 2015;15:3134.
3. Seow H, Brazil K, Sussman J, et al. Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ 2014;348:g3496.
4. Fink RM, Oman KS, Youngwerth J, et al. A palliative care needs assessment of rural hospitals. J Palliat Med 2013;16:638–44.
5. Dumont S, Jacobs P, Turcotte V, et al. Palliative care costs in Canada: A descriptive comparison of studies of urban and rural patients near end of life. J Palliat Med 2015;29:908–17.
6. Kaasalainen S, Brazil K, Williams A, et al. Nurses' experiences providing palliative care to individuals living in rural communities: aspects of the physical residential setting. Rural Remote Health 2014;14:2728.
7. Ahmed N, Bestall JC, Ahmedzai SH, et al. Systematic review of the problems and issues of accessing specialist palliative care by patients, carers and health and social care professionals. J Palliat Med 2004;18:525–42.
8. Ray RA, Fried O, Lindsay D. Palliative care professional education via video conference builds confidence to deliver palliative care in rural and remote locations. BMC Health Serv Res 2014;14:272.
9. Bakitas MA, Elk R, Astin M, et al. Systematic review of palliative care in the rural setting. Cancer Control 2015;22:450–64.
10. Akiyama M, Hirai K, Takebayashi T, et al. The effects of community-wide dissemination of information on perceptions of palliative care, knowledge about opioids, and sense of security among cancer patients, their families, and the general public. Support Care Cancer 2016;24: 347–56.
11. Maida V. Factors that promote success in home palliative care: a study of a large suburban palliative care practice. J Palliat Care 2002;18:282–6.
12. Ceronsky L, Shearer J, Weng K, et al. Minnesota Rural Palliative Care Initiative: building palliative care capacity in rural Minnesota. J Palliat Med 2013;16:310–3.
13. Aggarwal SK, Ghosh A, Cheng MJ, et al. Initiating pain and palliative care outpatient services for the suburban underserved in Montgomery County, Maryland: Lessons learned at the NIH Clinical Center and MobileMed. Palliat Support Care 2015;16:1–6.
14. Rainsford S, MacLeod RD, Glasgow NJ. Place of death in rural palliative care: A systematic review. J Palliat Med 2016;30:745–63.
15. Horey D, Street AF, O'Connor M, et al. Training and supportive programs for palliative care volunteers in community settings. Cochrane Database Syst Rev 2015 Jul 20;(7):CD009500.
16. The United States Census Bureau: QuickFacts: Butler County, Pennsylvania. Accessed 10 Mar 2017 at www.census.gov/quickfacts/table/PST045216/42019,00.
17. Spetz J, Dudley N, Trupin L, et al. Few hospital palliative care programs meet national staffing recommendations. Health Aff 2016;35:1690–7.
18. Dumanovsky T, Rogers M, Spragens LH, et al. Impact of Staffing on Access to Palliative Care in U.S. Hospitals. J Palliat Med. 2015 Dec; 18(12). Pages 998-999.
19. Lupu D, American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage 2010;40:899–911.
20. Kamal AH, Bull JK, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manage 2016;51:690–6.
1. Dumanovsky T, Augustin R, Rogers M, et al. The growth of palliative care in U.S. hospitals: a status report. J Palliat Med 2016;19:8–15.
2. Lavergne MR, Lethbridge L, Johnston G, et al. Examining palliative care program use and place of death in rural and urban contexts: a Canadian population-based study using linked data. Rural Remote Health 2015;15:3134.
3. Seow H, Brazil K, Sussman J, et al. Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ 2014;348:g3496.
4. Fink RM, Oman KS, Youngwerth J, et al. A palliative care needs assessment of rural hospitals. J Palliat Med 2013;16:638–44.
5. Dumont S, Jacobs P, Turcotte V, et al. Palliative care costs in Canada: A descriptive comparison of studies of urban and rural patients near end of life. J Palliat Med 2015;29:908–17.
6. Kaasalainen S, Brazil K, Williams A, et al. Nurses' experiences providing palliative care to individuals living in rural communities: aspects of the physical residential setting. Rural Remote Health 2014;14:2728.
7. Ahmed N, Bestall JC, Ahmedzai SH, et al. Systematic review of the problems and issues of accessing specialist palliative care by patients, carers and health and social care professionals. J Palliat Med 2004;18:525–42.
8. Ray RA, Fried O, Lindsay D. Palliative care professional education via video conference builds confidence to deliver palliative care in rural and remote locations. BMC Health Serv Res 2014;14:272.
9. Bakitas MA, Elk R, Astin M, et al. Systematic review of palliative care in the rural setting. Cancer Control 2015;22:450–64.
10. Akiyama M, Hirai K, Takebayashi T, et al. The effects of community-wide dissemination of information on perceptions of palliative care, knowledge about opioids, and sense of security among cancer patients, their families, and the general public. Support Care Cancer 2016;24: 347–56.
11. Maida V. Factors that promote success in home palliative care: a study of a large suburban palliative care practice. J Palliat Care 2002;18:282–6.
12. Ceronsky L, Shearer J, Weng K, et al. Minnesota Rural Palliative Care Initiative: building palliative care capacity in rural Minnesota. J Palliat Med 2013;16:310–3.
13. Aggarwal SK, Ghosh A, Cheng MJ, et al. Initiating pain and palliative care outpatient services for the suburban underserved in Montgomery County, Maryland: Lessons learned at the NIH Clinical Center and MobileMed. Palliat Support Care 2015;16:1–6.
14. Rainsford S, MacLeod RD, Glasgow NJ. Place of death in rural palliative care: A systematic review. J Palliat Med 2016;30:745–63.
15. Horey D, Street AF, O'Connor M, et al. Training and supportive programs for palliative care volunteers in community settings. Cochrane Database Syst Rev 2015 Jul 20;(7):CD009500.
16. The United States Census Bureau: QuickFacts: Butler County, Pennsylvania. Accessed 10 Mar 2017 at www.census.gov/quickfacts/table/PST045216/42019,00.
17. Spetz J, Dudley N, Trupin L, et al. Few hospital palliative care programs meet national staffing recommendations. Health Aff 2016;35:1690–7.
18. Dumanovsky T, Rogers M, Spragens LH, et al. Impact of Staffing on Access to Palliative Care in U.S. Hospitals. J Palliat Med. 2015 Dec; 18(12). Pages 998-999.
19. Lupu D, American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage 2010;40:899–911.
20. Kamal AH, Bull JK, Wolf SP, et al. Prevalence and predictors of burnout among hospice and palliative care clinicians in the U.S. J Pain Symptom Manage 2016;51:690–6.
Does newly discovered vasoactive peptide ELABELA reveal essential mechanisms for preeclampsia development?
EXPERT COMMENTARY
Preeclampsia is a disorder of impaired placentation. ELABELA (ELA) encodes an endogenous ligand for the apelin receptor and is detected in preimplantation human blastocysts and in 2 organs in adults: the placenta and kidney. Recently, an international team of researchers described their study findings on ELA and its role in placental vascular development and preeclampsia in mice.
Details of the study
To delineate the contribution of ELA to mammalian development, Ho and colleagues generated Ela knockout mice. The investigators showed that during development, the ELA protein is first detected in the early placenta and becomes abundant later in placenta formation. They also demonstrated that ELA is a pregnancy hormone that circulates in the blood of pregnant, but not nonpregnant, mice.
Placental structure. The Ela knockout mice had placentas that demonstrated thin labyrinths, with poor vascularization, increased apoptosis, and reduced proliferation. Further, RNA analysis of ELA-lacking placentas revealed a gene expression profile indicative of hypoxia, including the upregulation of certain genes involved in blood vessel building. Placental vessels showed overall stunted architecture characterized by little or no extension and branching of angiogenic sprouts and impaired formation of the adequate labyrinth network required for proper perfusion in the placenta.
Given these gene expression findings and the fact that placental and vascular abnormalities have long been suspected to underlie preeclampsia, the investigators sought to determine if ELA-lacking mice exhibit preeclampsia. Evidence indicated they do.
Indicators of preeclampsia. Pregnant ELA-lacking mice had significantly higher levels of proteinuria and significantly higher blood pressure than either pregnant wild-type mice or nonpregnant ELA-lacking mice. Further, at the end of pregnancy, histology and transmission electron microscopy of kidney glomerular sections from ELA-lacking pregnant mice revealed signs of endotheliosis, a unique renal pathology that is also observed in women with preeclampsia. Pups of ELA-lacking mothers tended to weigh less than those of wild-type mothers, a situation that may be similar to the fetal intrauterine growth restriction commonly seen in women with preeclampsia.
Angiogenic factors. The authors then looked at levels of angiogenic proteins implicated in the pathogenesis of preeclampsia to determine if ELA is upstream. They found that ELA-lacking mice placentas had increased levels of sFlt1, Vegfa, and Plgf mRNA; these transcriptional changes, however, did not translate into significantly elevated plasma levels of the respective proteins. Thus, these findings indicate that ELA acts independently of, and possibly earlier than, angiogenic factors in the pathogenesis of preeclampsia.
Experimental treatment. The authors further showed that infusing recombinant ELA protein could alleviate symptoms of preeclampsia in mice. Injection of ELA protein in ELA-lacking mice led to reduction of blood pressure, reversal of glomerular endotheliosis, and rescue of fetal growth restriction.
Study strengths and weaknesses
This animal study contributes compelling molecular evidence of ELA’s role in mammalian placental development and angiogenesis, revealing that ELA deficiency leads to preeclampsia and placental abnormalities in pregnant mice. How ELA acts in humans and human pregnancy, however, has yet to be explored.
-- Sarosh Rana, MD, MPH
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Preeclampsia is a disorder of impaired placentation. ELABELA (ELA) encodes an endogenous ligand for the apelin receptor and is detected in preimplantation human blastocysts and in 2 organs in adults: the placenta and kidney. Recently, an international team of researchers described their study findings on ELA and its role in placental vascular development and preeclampsia in mice.
Details of the study
To delineate the contribution of ELA to mammalian development, Ho and colleagues generated Ela knockout mice. The investigators showed that during development, the ELA protein is first detected in the early placenta and becomes abundant later in placenta formation. They also demonstrated that ELA is a pregnancy hormone that circulates in the blood of pregnant, but not nonpregnant, mice.
Placental structure. The Ela knockout mice had placentas that demonstrated thin labyrinths, with poor vascularization, increased apoptosis, and reduced proliferation. Further, RNA analysis of ELA-lacking placentas revealed a gene expression profile indicative of hypoxia, including the upregulation of certain genes involved in blood vessel building. Placental vessels showed overall stunted architecture characterized by little or no extension and branching of angiogenic sprouts and impaired formation of the adequate labyrinth network required for proper perfusion in the placenta.
Given these gene expression findings and the fact that placental and vascular abnormalities have long been suspected to underlie preeclampsia, the investigators sought to determine if ELA-lacking mice exhibit preeclampsia. Evidence indicated they do.
Indicators of preeclampsia. Pregnant ELA-lacking mice had significantly higher levels of proteinuria and significantly higher blood pressure than either pregnant wild-type mice or nonpregnant ELA-lacking mice. Further, at the end of pregnancy, histology and transmission electron microscopy of kidney glomerular sections from ELA-lacking pregnant mice revealed signs of endotheliosis, a unique renal pathology that is also observed in women with preeclampsia. Pups of ELA-lacking mothers tended to weigh less than those of wild-type mothers, a situation that may be similar to the fetal intrauterine growth restriction commonly seen in women with preeclampsia.
Angiogenic factors. The authors then looked at levels of angiogenic proteins implicated in the pathogenesis of preeclampsia to determine if ELA is upstream. They found that ELA-lacking mice placentas had increased levels of sFlt1, Vegfa, and Plgf mRNA; these transcriptional changes, however, did not translate into significantly elevated plasma levels of the respective proteins. Thus, these findings indicate that ELA acts independently of, and possibly earlier than, angiogenic factors in the pathogenesis of preeclampsia.
Experimental treatment. The authors further showed that infusing recombinant ELA protein could alleviate symptoms of preeclampsia in mice. Injection of ELA protein in ELA-lacking mice led to reduction of blood pressure, reversal of glomerular endotheliosis, and rescue of fetal growth restriction.
Study strengths and weaknesses
This animal study contributes compelling molecular evidence of ELA’s role in mammalian placental development and angiogenesis, revealing that ELA deficiency leads to preeclampsia and placental abnormalities in pregnant mice. How ELA acts in humans and human pregnancy, however, has yet to be explored.
-- Sarosh Rana, MD, MPH
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Preeclampsia is a disorder of impaired placentation. ELABELA (ELA) encodes an endogenous ligand for the apelin receptor and is detected in preimplantation human blastocysts and in 2 organs in adults: the placenta and kidney. Recently, an international team of researchers described their study findings on ELA and its role in placental vascular development and preeclampsia in mice.
Details of the study
To delineate the contribution of ELA to mammalian development, Ho and colleagues generated Ela knockout mice. The investigators showed that during development, the ELA protein is first detected in the early placenta and becomes abundant later in placenta formation. They also demonstrated that ELA is a pregnancy hormone that circulates in the blood of pregnant, but not nonpregnant, mice.
Placental structure. The Ela knockout mice had placentas that demonstrated thin labyrinths, with poor vascularization, increased apoptosis, and reduced proliferation. Further, RNA analysis of ELA-lacking placentas revealed a gene expression profile indicative of hypoxia, including the upregulation of certain genes involved in blood vessel building. Placental vessels showed overall stunted architecture characterized by little or no extension and branching of angiogenic sprouts and impaired formation of the adequate labyrinth network required for proper perfusion in the placenta.
Given these gene expression findings and the fact that placental and vascular abnormalities have long been suspected to underlie preeclampsia, the investigators sought to determine if ELA-lacking mice exhibit preeclampsia. Evidence indicated they do.
Indicators of preeclampsia. Pregnant ELA-lacking mice had significantly higher levels of proteinuria and significantly higher blood pressure than either pregnant wild-type mice or nonpregnant ELA-lacking mice. Further, at the end of pregnancy, histology and transmission electron microscopy of kidney glomerular sections from ELA-lacking pregnant mice revealed signs of endotheliosis, a unique renal pathology that is also observed in women with preeclampsia. Pups of ELA-lacking mothers tended to weigh less than those of wild-type mothers, a situation that may be similar to the fetal intrauterine growth restriction commonly seen in women with preeclampsia.
Angiogenic factors. The authors then looked at levels of angiogenic proteins implicated in the pathogenesis of preeclampsia to determine if ELA is upstream. They found that ELA-lacking mice placentas had increased levels of sFlt1, Vegfa, and Plgf mRNA; these transcriptional changes, however, did not translate into significantly elevated plasma levels of the respective proteins. Thus, these findings indicate that ELA acts independently of, and possibly earlier than, angiogenic factors in the pathogenesis of preeclampsia.
Experimental treatment. The authors further showed that infusing recombinant ELA protein could alleviate symptoms of preeclampsia in mice. Injection of ELA protein in ELA-lacking mice led to reduction of blood pressure, reversal of glomerular endotheliosis, and rescue of fetal growth restriction.
Study strengths and weaknesses
This animal study contributes compelling molecular evidence of ELA’s role in mammalian placental development and angiogenesis, revealing that ELA deficiency leads to preeclampsia and placental abnormalities in pregnant mice. How ELA acts in humans and human pregnancy, however, has yet to be explored.
-- Sarosh Rana, MD, MPH
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Debunking Actinic Keratosis Myths: Are Patients With Darker Skin At Risk for Actinic Keratoses?
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Maternally derived pneumococcal, meningococcal antibodies may affect vaccine effectiveness
, according to a new study.
That information may be useful in deciding the impact of vaccination programs that use a combination of maternal and infant vaccines and consider schedules with a delayed start, said Merryn Voysey of the University of Oxford (England) and her associates.
In this study, 5,097 children in 16 cohorts from 13 countries had pneumococcal antibody concentrations assessed from blood samples taken before their first dose of vaccine, and 2,925 infants from 5 cohorts in 4 countries had meningococcal antibody concentrations available.
At the time of their first vaccination, the children were ages 5-23 weeks and were from countries in Europe, Africa, Latin America, and South and East Asia. These populations have no routine programs of immunization in pregnancy, the researchers said. So, the maternal antibodies are passively acquired, and the decay rates may differ from those induced by maternal vaccinations.
The seroprevalence of maternal antibodies in infants was 92% for pneumococcal serotype 14 and 80% for serotype 19F; it was 30% for serotype 4 and 34% for serotype 1. Thirteen percent of infants had detectable levels of group C meningococcal antibodies prior to vaccination, and 43% had group A antibodies.
For the pneumococcal antibodies, “there was statistically significant variation in half-life estimates between country cohorts and between serotypes (both P less than .0001),” the researchers said. The half-life estimate was lowest – at 39 days – for serotype 6B, and highest – at 48 days – for serotype 5. The overall estimate across serotypes was 43 days.
“The age of the child was not significantly associated with decay rates (P = .103), confirming the assumption of exponential decay,” they said.
For the meningococcal antibodies, the half-lives were 43 days for group A and 40 days for group C.
“Substantial proportions of infants have antibodies to many vaccine serotypes of pneumococcus at the age when a vaccine program might normally commence,” the investigators noted. “Conversely, antibodies against capsular groups A and C meningococcal polysaccharides were less common, particularly for group C, which was only present in 13% of infants in the four countries contained in this analysis.
“Higher levels of group A meningococcal antibodies than group C have also been seen in unvaccinated adults of childbearing age in the Netherlands, and in mothers in the United Kingdom,” the researchers added. “Passively acquired maternal antibody has been shown to adversely affect the magnitude of the immune response to vaccination with pneumococcal conjugate vaccine, and increase the occurrence of otitis media in infants under 6 months of age.”
The proportion of infants who had maternal antipneumococcal antibodies differed between serotypes, the authors noted. Almost all infants had serotype 14 pneumococcal antibodies, and very high proportions of infants had serotype 19F antibodies.
“We have previously shown that the antibody response to vaccination with pneumococcal conjugate vaccine is adversely affected by the presence of maternal antibody,” the investigators said. “This inhibitory effect is greatest for serotype 14, with children seropositive from maternal antibodies having a response to vaccination that is only three-quarters the magnitude of those with no maternal antibody.”
Read more in Vaccine (2017 Oct 13;35[43]:5850-7).
, according to a new study.
That information may be useful in deciding the impact of vaccination programs that use a combination of maternal and infant vaccines and consider schedules with a delayed start, said Merryn Voysey of the University of Oxford (England) and her associates.
In this study, 5,097 children in 16 cohorts from 13 countries had pneumococcal antibody concentrations assessed from blood samples taken before their first dose of vaccine, and 2,925 infants from 5 cohorts in 4 countries had meningococcal antibody concentrations available.
At the time of their first vaccination, the children were ages 5-23 weeks and were from countries in Europe, Africa, Latin America, and South and East Asia. These populations have no routine programs of immunization in pregnancy, the researchers said. So, the maternal antibodies are passively acquired, and the decay rates may differ from those induced by maternal vaccinations.
The seroprevalence of maternal antibodies in infants was 92% for pneumococcal serotype 14 and 80% for serotype 19F; it was 30% for serotype 4 and 34% for serotype 1. Thirteen percent of infants had detectable levels of group C meningococcal antibodies prior to vaccination, and 43% had group A antibodies.
For the pneumococcal antibodies, “there was statistically significant variation in half-life estimates between country cohorts and between serotypes (both P less than .0001),” the researchers said. The half-life estimate was lowest – at 39 days – for serotype 6B, and highest – at 48 days – for serotype 5. The overall estimate across serotypes was 43 days.
“The age of the child was not significantly associated with decay rates (P = .103), confirming the assumption of exponential decay,” they said.
For the meningococcal antibodies, the half-lives were 43 days for group A and 40 days for group C.
“Substantial proportions of infants have antibodies to many vaccine serotypes of pneumococcus at the age when a vaccine program might normally commence,” the investigators noted. “Conversely, antibodies against capsular groups A and C meningococcal polysaccharides were less common, particularly for group C, which was only present in 13% of infants in the four countries contained in this analysis.
“Higher levels of group A meningococcal antibodies than group C have also been seen in unvaccinated adults of childbearing age in the Netherlands, and in mothers in the United Kingdom,” the researchers added. “Passively acquired maternal antibody has been shown to adversely affect the magnitude of the immune response to vaccination with pneumococcal conjugate vaccine, and increase the occurrence of otitis media in infants under 6 months of age.”
The proportion of infants who had maternal antipneumococcal antibodies differed between serotypes, the authors noted. Almost all infants had serotype 14 pneumococcal antibodies, and very high proportions of infants had serotype 19F antibodies.
“We have previously shown that the antibody response to vaccination with pneumococcal conjugate vaccine is adversely affected by the presence of maternal antibody,” the investigators said. “This inhibitory effect is greatest for serotype 14, with children seropositive from maternal antibodies having a response to vaccination that is only three-quarters the magnitude of those with no maternal antibody.”
Read more in Vaccine (2017 Oct 13;35[43]:5850-7).
, according to a new study.
That information may be useful in deciding the impact of vaccination programs that use a combination of maternal and infant vaccines and consider schedules with a delayed start, said Merryn Voysey of the University of Oxford (England) and her associates.
In this study, 5,097 children in 16 cohorts from 13 countries had pneumococcal antibody concentrations assessed from blood samples taken before their first dose of vaccine, and 2,925 infants from 5 cohorts in 4 countries had meningococcal antibody concentrations available.
At the time of their first vaccination, the children were ages 5-23 weeks and were from countries in Europe, Africa, Latin America, and South and East Asia. These populations have no routine programs of immunization in pregnancy, the researchers said. So, the maternal antibodies are passively acquired, and the decay rates may differ from those induced by maternal vaccinations.
The seroprevalence of maternal antibodies in infants was 92% for pneumococcal serotype 14 and 80% for serotype 19F; it was 30% for serotype 4 and 34% for serotype 1. Thirteen percent of infants had detectable levels of group C meningococcal antibodies prior to vaccination, and 43% had group A antibodies.
For the pneumococcal antibodies, “there was statistically significant variation in half-life estimates between country cohorts and between serotypes (both P less than .0001),” the researchers said. The half-life estimate was lowest – at 39 days – for serotype 6B, and highest – at 48 days – for serotype 5. The overall estimate across serotypes was 43 days.
“The age of the child was not significantly associated with decay rates (P = .103), confirming the assumption of exponential decay,” they said.
For the meningococcal antibodies, the half-lives were 43 days for group A and 40 days for group C.
“Substantial proportions of infants have antibodies to many vaccine serotypes of pneumococcus at the age when a vaccine program might normally commence,” the investigators noted. “Conversely, antibodies against capsular groups A and C meningococcal polysaccharides were less common, particularly for group C, which was only present in 13% of infants in the four countries contained in this analysis.
“Higher levels of group A meningococcal antibodies than group C have also been seen in unvaccinated adults of childbearing age in the Netherlands, and in mothers in the United Kingdom,” the researchers added. “Passively acquired maternal antibody has been shown to adversely affect the magnitude of the immune response to vaccination with pneumococcal conjugate vaccine, and increase the occurrence of otitis media in infants under 6 months of age.”
The proportion of infants who had maternal antipneumococcal antibodies differed between serotypes, the authors noted. Almost all infants had serotype 14 pneumococcal antibodies, and very high proportions of infants had serotype 19F antibodies.
“We have previously shown that the antibody response to vaccination with pneumococcal conjugate vaccine is adversely affected by the presence of maternal antibody,” the investigators said. “This inhibitory effect is greatest for serotype 14, with children seropositive from maternal antibodies having a response to vaccination that is only three-quarters the magnitude of those with no maternal antibody.”
Read more in Vaccine (2017 Oct 13;35[43]:5850-7).
FROM VACCINE
Vitality predicts T2DM major cardiovascular event risk
LISBON – according to the results of a primary care study.
Seldom feeling “full of pep” or not having “a lot of energy” was associated with an increased risk of a major cardiovascular event (MACE) in middle-aged (55-66 years) adults with T2DM, Marta Vergara, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
“It’s well known that patients with type 2 diabetes have a high risk for developing cardiovascular disease, and it is the main cause of death,” said Dr. Vergara of Linköping (Sweden) University.
While several risk factors for cardiovascular disease are known and widely monitored for in clinical practice worldwide, including psychological aspects such as mental stress, ways to identify patients earlier are needed.
“We need more clinically useful and easy-to-manage measurement instruments,” Dr. Vergara said.
Using data from the ongoing Cardiovascular Risk Factors in Patients With Diabetes – a Prospective Study in Primary Care (CARDIPP), Dr. Vergara and colleagues identified two questions used in the 36-Item Short Form (SF-36) that might fit the bill:
- ”How much time during the past 4 weeks did you feel full of pep?”
- “How much time during the past 4 weeks did you have a lot of energy?”
Patients had answered these questions using a 6-point scale ranging from 1, all of the time, to 6 none of the time.
In addition to completing the full SF-36 at recruitment, all patients enrolled in CARDIPP underwent thorough assessment of baseline cardiovascular risk. This included asking about their duration of diabetes and smoking history, taking glycemic and anthropometric readings, and recording their systolic blood pressure. Pulse wave velocity was also used to assess patients’ carotid-femoral artery stiffness.
Over a follow-up period of 7 years, 59 (7.8%) of 761 men and women aged 55-66 years developed ischemic heart disease or had a stroke and needed hospital treatment or died.
“Our data support the use of questions about sense of vitality in order to add prognostic information about subsequent risk of MACE independently of traditional risk markers, such as diabetes duration, age, HbA1c, gender, smoking, systolic blood pressure, and carotid-femoral pulse wave velocity and sagittal abdominal diameter,” Dr. Vergara said.
“We suggest that not feeling full of pep and not having a lot of energy could be used as risk markers for MACE in patients with type 2 diabetes.” These are two easy questions that could be asked as part of the risk assessment process in primary care, she suggested. “Other questions from the SF-36, such as feeling tired or worn out, were not independent markers of MACE,” Dr. Vergara said.
She said she had no relevant financial disclosures.
LISBON – according to the results of a primary care study.
Seldom feeling “full of pep” or not having “a lot of energy” was associated with an increased risk of a major cardiovascular event (MACE) in middle-aged (55-66 years) adults with T2DM, Marta Vergara, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
“It’s well known that patients with type 2 diabetes have a high risk for developing cardiovascular disease, and it is the main cause of death,” said Dr. Vergara of Linköping (Sweden) University.
While several risk factors for cardiovascular disease are known and widely monitored for in clinical practice worldwide, including psychological aspects such as mental stress, ways to identify patients earlier are needed.
“We need more clinically useful and easy-to-manage measurement instruments,” Dr. Vergara said.
Using data from the ongoing Cardiovascular Risk Factors in Patients With Diabetes – a Prospective Study in Primary Care (CARDIPP), Dr. Vergara and colleagues identified two questions used in the 36-Item Short Form (SF-36) that might fit the bill:
- ”How much time during the past 4 weeks did you feel full of pep?”
- “How much time during the past 4 weeks did you have a lot of energy?”
Patients had answered these questions using a 6-point scale ranging from 1, all of the time, to 6 none of the time.
In addition to completing the full SF-36 at recruitment, all patients enrolled in CARDIPP underwent thorough assessment of baseline cardiovascular risk. This included asking about their duration of diabetes and smoking history, taking glycemic and anthropometric readings, and recording their systolic blood pressure. Pulse wave velocity was also used to assess patients’ carotid-femoral artery stiffness.
Over a follow-up period of 7 years, 59 (7.8%) of 761 men and women aged 55-66 years developed ischemic heart disease or had a stroke and needed hospital treatment or died.
“Our data support the use of questions about sense of vitality in order to add prognostic information about subsequent risk of MACE independently of traditional risk markers, such as diabetes duration, age, HbA1c, gender, smoking, systolic blood pressure, and carotid-femoral pulse wave velocity and sagittal abdominal diameter,” Dr. Vergara said.
“We suggest that not feeling full of pep and not having a lot of energy could be used as risk markers for MACE in patients with type 2 diabetes.” These are two easy questions that could be asked as part of the risk assessment process in primary care, she suggested. “Other questions from the SF-36, such as feeling tired or worn out, were not independent markers of MACE,” Dr. Vergara said.
She said she had no relevant financial disclosures.
LISBON – according to the results of a primary care study.
Seldom feeling “full of pep” or not having “a lot of energy” was associated with an increased risk of a major cardiovascular event (MACE) in middle-aged (55-66 years) adults with T2DM, Marta Vergara, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
“It’s well known that patients with type 2 diabetes have a high risk for developing cardiovascular disease, and it is the main cause of death,” said Dr. Vergara of Linköping (Sweden) University.
While several risk factors for cardiovascular disease are known and widely monitored for in clinical practice worldwide, including psychological aspects such as mental stress, ways to identify patients earlier are needed.
“We need more clinically useful and easy-to-manage measurement instruments,” Dr. Vergara said.
Using data from the ongoing Cardiovascular Risk Factors in Patients With Diabetes – a Prospective Study in Primary Care (CARDIPP), Dr. Vergara and colleagues identified two questions used in the 36-Item Short Form (SF-36) that might fit the bill:
- ”How much time during the past 4 weeks did you feel full of pep?”
- “How much time during the past 4 weeks did you have a lot of energy?”
Patients had answered these questions using a 6-point scale ranging from 1, all of the time, to 6 none of the time.
In addition to completing the full SF-36 at recruitment, all patients enrolled in CARDIPP underwent thorough assessment of baseline cardiovascular risk. This included asking about their duration of diabetes and smoking history, taking glycemic and anthropometric readings, and recording their systolic blood pressure. Pulse wave velocity was also used to assess patients’ carotid-femoral artery stiffness.
Over a follow-up period of 7 years, 59 (7.8%) of 761 men and women aged 55-66 years developed ischemic heart disease or had a stroke and needed hospital treatment or died.
“Our data support the use of questions about sense of vitality in order to add prognostic information about subsequent risk of MACE independently of traditional risk markers, such as diabetes duration, age, HbA1c, gender, smoking, systolic blood pressure, and carotid-femoral pulse wave velocity and sagittal abdominal diameter,” Dr. Vergara said.
“We suggest that not feeling full of pep and not having a lot of energy could be used as risk markers for MACE in patients with type 2 diabetes.” These are two easy questions that could be asked as part of the risk assessment process in primary care, she suggested. “Other questions from the SF-36, such as feeling tired or worn out, were not independent markers of MACE,” Dr. Vergara said.
She said she had no relevant financial disclosures.
AT EASD 2017
Key clinical point: Two simple questions about vitality could help assess the risk of major cardiovascular events in patients with type 2 diabetes mellitus.
Major finding: The hazard ratios for seldom feeling “full of pep” and seldom having “lots of energy” and MACE were a respective 1.31 (P = .003) and 1.44 (P less than .0001).
Data source: A prospective, observational primary care study of 761 patients with type 2 diabetes mellitus.
Disclosures: The presenting author had no relevant financial disclosures.
We’ll be there, covering the news
GI & Hepatology News reporters are geared up to cover the Liver Meeting® at the Walter E. Washington Convention Center, in Washington, starting this weekend. The annual meeting of the American Association for the Study of Liver Diseases is a worldwide meeting of liver specialists that will include presentations of new information on every level of knowledge about the liver from the hepatocyte to organ transplantation.
Onsite reporters will cover new biomarkers for nonalcoholic fatty liver disease, surveillance and treatments for hepatocellular carcinoma, and pre- and posttransplant factors that affect morbidity and mortality.
Highly anticipated presentations include:
- Early liver transplant good for patients with severe alcoholic hepatitis.
- Asians have highest rate of herbal dietary supplement DILI liver transplantations.
- Bilirubin levels associated with transplant-free survival in PBS patients.
GI & Hepatology News reporters are geared up to cover the Liver Meeting® at the Walter E. Washington Convention Center, in Washington, starting this weekend. The annual meeting of the American Association for the Study of Liver Diseases is a worldwide meeting of liver specialists that will include presentations of new information on every level of knowledge about the liver from the hepatocyte to organ transplantation.
Onsite reporters will cover new biomarkers for nonalcoholic fatty liver disease, surveillance and treatments for hepatocellular carcinoma, and pre- and posttransplant factors that affect morbidity and mortality.
Highly anticipated presentations include:
- Early liver transplant good for patients with severe alcoholic hepatitis.
- Asians have highest rate of herbal dietary supplement DILI liver transplantations.
- Bilirubin levels associated with transplant-free survival in PBS patients.
GI & Hepatology News reporters are geared up to cover the Liver Meeting® at the Walter E. Washington Convention Center, in Washington, starting this weekend. The annual meeting of the American Association for the Study of Liver Diseases is a worldwide meeting of liver specialists that will include presentations of new information on every level of knowledge about the liver from the hepatocyte to organ transplantation.
Onsite reporters will cover new biomarkers for nonalcoholic fatty liver disease, surveillance and treatments for hepatocellular carcinoma, and pre- and posttransplant factors that affect morbidity and mortality.
Highly anticipated presentations include:
- Early liver transplant good for patients with severe alcoholic hepatitis.
- Asians have highest rate of herbal dietary supplement DILI liver transplantations.
- Bilirubin levels associated with transplant-free survival in PBS patients.
Societies unite to develop cardiology subspecialty MOC products
Four medical societies are banding together to help cardiology subspecialists get through the maintenance of certification process.
The American College of Cardiology, Heart Failure Society of America, Heart Rhythm Society, and Society for Cardiovascular Angiography and Interventions are working together to develop new modules to help subspecialists meet the American Board of Internal Medicine’s current 10-year maintenance of certification examination.
The groups first must reach an agreement with ABIM; they can then collaborate to enhance the existing ACC self-assessment program (SAP) line with CathSAP, EPSAP, and Heart Failure SAP products to help fulfill the MOC needs of interventionalists, electrophysiologists, and heart failure specialists, respectively.
The societies hope to make the SAPs available in time for the ABIM rollout of the 2-year Knowledge Check-in assessment option. The current plan is for the rollout of a general cardiology product in 2019; electrophysiologists, heart failure, and interventionalists in 2020; and adult congenital in 2023.
“It is the shared goal of ACC, HFSA, HRS, and SCAI to help our collective members ensure their patients are receiving the highest-quality, evidence-based care,” ACC President Mary Norine Walsh, MD, said in a statement. “In offering additional pathways for cardiologists who wish to maintain their professional certification, we can more effectively and efficiently help busy clinicians keep up with current knowledge in their specific areas of practice.”
Four medical societies are banding together to help cardiology subspecialists get through the maintenance of certification process.
The American College of Cardiology, Heart Failure Society of America, Heart Rhythm Society, and Society for Cardiovascular Angiography and Interventions are working together to develop new modules to help subspecialists meet the American Board of Internal Medicine’s current 10-year maintenance of certification examination.
The groups first must reach an agreement with ABIM; they can then collaborate to enhance the existing ACC self-assessment program (SAP) line with CathSAP, EPSAP, and Heart Failure SAP products to help fulfill the MOC needs of interventionalists, electrophysiologists, and heart failure specialists, respectively.
The societies hope to make the SAPs available in time for the ABIM rollout of the 2-year Knowledge Check-in assessment option. The current plan is for the rollout of a general cardiology product in 2019; electrophysiologists, heart failure, and interventionalists in 2020; and adult congenital in 2023.
“It is the shared goal of ACC, HFSA, HRS, and SCAI to help our collective members ensure their patients are receiving the highest-quality, evidence-based care,” ACC President Mary Norine Walsh, MD, said in a statement. “In offering additional pathways for cardiologists who wish to maintain their professional certification, we can more effectively and efficiently help busy clinicians keep up with current knowledge in their specific areas of practice.”
Four medical societies are banding together to help cardiology subspecialists get through the maintenance of certification process.
The American College of Cardiology, Heart Failure Society of America, Heart Rhythm Society, and Society for Cardiovascular Angiography and Interventions are working together to develop new modules to help subspecialists meet the American Board of Internal Medicine’s current 10-year maintenance of certification examination.
The groups first must reach an agreement with ABIM; they can then collaborate to enhance the existing ACC self-assessment program (SAP) line with CathSAP, EPSAP, and Heart Failure SAP products to help fulfill the MOC needs of interventionalists, electrophysiologists, and heart failure specialists, respectively.
The societies hope to make the SAPs available in time for the ABIM rollout of the 2-year Knowledge Check-in assessment option. The current plan is for the rollout of a general cardiology product in 2019; electrophysiologists, heart failure, and interventionalists in 2020; and adult congenital in 2023.
“It is the shared goal of ACC, HFSA, HRS, and SCAI to help our collective members ensure their patients are receiving the highest-quality, evidence-based care,” ACC President Mary Norine Walsh, MD, said in a statement. “In offering additional pathways for cardiologists who wish to maintain their professional certification, we can more effectively and efficiently help busy clinicians keep up with current knowledge in their specific areas of practice.”
Guidelines cut acute chest syndrome hospital returns in pediatric sickle cell
Children with sickle cell disease who experience acute chest syndrome benefit from the current guideline-recommended antibiotic regimen, based on data from more than 7,000 patients.
Although acute chest syndrome (ACS) is among the most common complications of sickle cell disease (SCD), data on the effectiveness of the recommended antibiotic therapies (macrolides and cephalosporins) are lacking, wrote David G. Bundy, MD, of the Medical University of South Carolina, Charleston, and colleagues. ACS often leads to intensive hospital care and 1%-2% morbidity, they noted.
The most recent guidelines from the National Heart, Lung, and Blood Institute call for “an intravenous cephalosporin and an oral macrolide antibiotic,” the researchers said.
To determine the impact of antibiotic use as directed on reducing hospital readmissions in young SCD patients, the researchers reviewed data from 14,480 hospitalizations for ACS involving 7,178 children and young adults aged 0-22 years seen at 41 hospitals in the United States (JAMA Pediatr. 2017 Sep 11. doi: 10.1001/jamapediatrics.2017.2526).
“This high level of interhospital variation also suggests possible clinician disagreement regarding the ideal antibiotic treatment for children with ACS,” the researchers wrote.
Rates of all-cause readmission and 30-day ACS-related readmission were significantly lower among patients who received the recommended antibiotics (odds ratio, 0.50 and 0.71, respectively). Children aged 5-9 years were most likely to receive the recommended antibiotics (80%), while young adults aged 19-22 years were the least likely (64%).
The findings were limited by several factors, including coding errors and incomplete clinical information, the researchers noted. But the results suggest that the guideline-recommended antibiotics are effective, “so more robust dissemination and implementation of existing treatment guidelines may reduce readmissions in this high-risk population,” they said.
The researchers had no financial conflicts to disclose. Study coauthor Staci Arnold, MD, was supported in part by the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program.
Children with sickle cell disease who experience acute chest syndrome benefit from the current guideline-recommended antibiotic regimen, based on data from more than 7,000 patients.
Although acute chest syndrome (ACS) is among the most common complications of sickle cell disease (SCD), data on the effectiveness of the recommended antibiotic therapies (macrolides and cephalosporins) are lacking, wrote David G. Bundy, MD, of the Medical University of South Carolina, Charleston, and colleagues. ACS often leads to intensive hospital care and 1%-2% morbidity, they noted.
The most recent guidelines from the National Heart, Lung, and Blood Institute call for “an intravenous cephalosporin and an oral macrolide antibiotic,” the researchers said.
To determine the impact of antibiotic use as directed on reducing hospital readmissions in young SCD patients, the researchers reviewed data from 14,480 hospitalizations for ACS involving 7,178 children and young adults aged 0-22 years seen at 41 hospitals in the United States (JAMA Pediatr. 2017 Sep 11. doi: 10.1001/jamapediatrics.2017.2526).
“This high level of interhospital variation also suggests possible clinician disagreement regarding the ideal antibiotic treatment for children with ACS,” the researchers wrote.
Rates of all-cause readmission and 30-day ACS-related readmission were significantly lower among patients who received the recommended antibiotics (odds ratio, 0.50 and 0.71, respectively). Children aged 5-9 years were most likely to receive the recommended antibiotics (80%), while young adults aged 19-22 years were the least likely (64%).
The findings were limited by several factors, including coding errors and incomplete clinical information, the researchers noted. But the results suggest that the guideline-recommended antibiotics are effective, “so more robust dissemination and implementation of existing treatment guidelines may reduce readmissions in this high-risk population,” they said.
The researchers had no financial conflicts to disclose. Study coauthor Staci Arnold, MD, was supported in part by the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program.
Children with sickle cell disease who experience acute chest syndrome benefit from the current guideline-recommended antibiotic regimen, based on data from more than 7,000 patients.
Although acute chest syndrome (ACS) is among the most common complications of sickle cell disease (SCD), data on the effectiveness of the recommended antibiotic therapies (macrolides and cephalosporins) are lacking, wrote David G. Bundy, MD, of the Medical University of South Carolina, Charleston, and colleagues. ACS often leads to intensive hospital care and 1%-2% morbidity, they noted.
The most recent guidelines from the National Heart, Lung, and Blood Institute call for “an intravenous cephalosporin and an oral macrolide antibiotic,” the researchers said.
To determine the impact of antibiotic use as directed on reducing hospital readmissions in young SCD patients, the researchers reviewed data from 14,480 hospitalizations for ACS involving 7,178 children and young adults aged 0-22 years seen at 41 hospitals in the United States (JAMA Pediatr. 2017 Sep 11. doi: 10.1001/jamapediatrics.2017.2526).
“This high level of interhospital variation also suggests possible clinician disagreement regarding the ideal antibiotic treatment for children with ACS,” the researchers wrote.
Rates of all-cause readmission and 30-day ACS-related readmission were significantly lower among patients who received the recommended antibiotics (odds ratio, 0.50 and 0.71, respectively). Children aged 5-9 years were most likely to receive the recommended antibiotics (80%), while young adults aged 19-22 years were the least likely (64%).
The findings were limited by several factors, including coding errors and incomplete clinical information, the researchers noted. But the results suggest that the guideline-recommended antibiotics are effective, “so more robust dissemination and implementation of existing treatment guidelines may reduce readmissions in this high-risk population,” they said.
The researchers had no financial conflicts to disclose. Study coauthor Staci Arnold, MD, was supported in part by the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program.
FROM JAMA PEDIATRICS
Key clinical point: Treatment with the recommended antibiotics was effective in reducing hospital readmissions for acute chest syndrome in children and young adults up to age 22 years with sickle cell disease.
Major finding: Hospital readmission for 30-day acute chest syndrome and all-cause readmission were significantly lower (odds ratio, 0.71 and 0.50, respectively) among children with sickle cell disease who received antibiotics (macrolides and cephalosporins) according to current guidelines, compared with those who did not.
Data source: A retrospective, multicenter study of 14,480 hospitalizations at 41 locations involving 7,178 children and young adults aged 0-22 years.
Disclosures: The researchers had no financial conflicts to disclose. Study coauthor Staci Arnold, MD, was supported in part by the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program.
Using Contingency Management for the Treatment of Substance Use Disorders in Real-World Settings
From UConn Health, Farmington, CT.
Abstract
- Objective: To discuss the efficacy and generalizability of contingency management (CM) for the treatment of substance use disorders and design considerations for those considering implementing in clinical settings.
- Methods: Review of the literature.
- Results: CM is an efficacious treatment for substance abuse disorders that is widely generalizable across substance use disorders and patient characteristics. CM can be implemented in a number of treatment programs, including residential and outpatient settings, and it can be administered in both individual and group formats. Abstinence and attendance are the most commonly targeted behaviors in substance abuse treatment settings. Design features, including the selection of target behaviors, delivery methods, and reinforcers, are discussed. Schedule parameters, such as frequency, magnitude, immediacy, and escalation of reinforcement, are associated with overall impact of the CM program and are important considerations for those interested in tailoring CM protocols to their needs.
- Conclusion: CM is an efficacious option that is applicable to most substance abuse treatment patients. A number of demonstrations of real-world implementation have been published and suggest CM can be adapted with success to clinic settings. In adopting CM protocols, clinics should aim for those protocols with established efficacy; however, if adaptations are necessary, careful consideration should be given to modifications to minimize risks of undermining CM’s effects.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Contingency management (CM) is a behavioral intervention that is efficacious in the treatment of substance use disorders (SUDs). CM uses a behavior analytic framework and applies principles of learning theory, particularly operant conditioning theory, to change client behavior(s) [1–5]. In basic terms, operant conditioning principles suggests that whether a behavior continues or not is a function of consequences [6]. Reinforced behaviors are more likely to occur in the future. Substance abuse can be viewed as a behavior maintained by the reinforcing effects of the drug itself [5], including the feel-good aspects of intoxication or relaxation and the amelioration of withdrawal symptoms. CM extends these same principles of to a treatment context, such that reinforcers for abstinent behavior are introduced to compete with the reinforcing effects of continued drug use [5].
In CM’s application to substance abuse treatment, drug-negative samples or treatment attendance are reinforced using tangible incentives with the goal of motivating continued abstinence and/or treatment engagement. When clients demonstrate these target behaviors, they earn incentives in the form of goods or services of value to the client, such as small electronics, gift cards, and toiletries. Despite the promising effects observed in research trials, real-world implementation efforts have not kept pace [7–9]. This review briefly discusses CM’s efficacy and highlights key features for professionals considering adopting this intervention. Demonstration efforts that illustrate how CM can be effectively implemented within the constraints and limitations of non-research, clinical settings are also presented.
Efficacy of CM
CM’s efficacy spans a number of SUDs, including cocaine, opioids, alcohol, nicotine, and marijuana [10–13], making it amenable for treatment of most SUD clinic populations. It generates larger effect sizes than other SUD treatments, including cognitive behavioral therapy [14], and it has been evaluated in a wide range of settings. Large-scale evaluations have been conducted in both intensive outpatient [15] and methadone maintenance [16] settings as part of the National Institute on Drug Abuse Clinical Trials Network, demonstrating consistent benefits of CM when added to treatment as usual. In the first of these 2 studies, Petry et al [15] randomized 415 stimulant users from 1 of 8 intensive outpatient clinics to treatment as usual or treatment as usual plus CM for alcohol and stimulant abstinence. CM participants submitted more substance-negative urine and breath samples, achieved continuous abstinence at significantly higher rates, and had longer treatment retention compared to those receiving treatment as usual. The parallel study [16] focused on stimulant use in clients from methadone maintenance clinics and found similar benefits of CM on stimulant abstinence. Beyond these settings, CM has been applied in a number of other contexts, including drop-in centers [17], vocational rehabilitation [18,19], job-skills training [20], and residential programs [21–23]. In addition, several group-based adaptations have been explored [17,24–27].
CM benefits most clients and generalizes across several demographic variables, including gender [28,29], race [30], housing status [31], and income levels [32–34]. Among clinical characteristics, CM is efficacious for those with co-occurring SUDs [35], other substance use [36], psychiatric disorders [37–39], medical problems [40–42], and history of transactional sex [43].
Design Considerations
Design features, including what behavior will be reinforced and how to do so, are among the first decision points for clinicians interested in implementing CM. One of the advantages of CM is that it has a high degree of flexibility in design, which means that it can be readily tailored to client populations and clinic needs. However, this flexibility can lead clinicians astray from the foundational principles of CM and unknowingly weaken the impact of the program. Below, some key considerations for CM protocol design are reviewed. For additional coverage of these topics, readers are referred to additional articles [1,2] or Petry’s comprehensive book on implementing CM [44]. In this review, published examples of CM’s application in real-world settings are presented, highlighting how CM has been adapted in these clinical efforts.
Target Behaviors
The selection of the target behavior will drive many of the subsequent program design decisions. As such, it is important to identify this feature early. Target behaviors must be achievable, objectively verifiable, and well defined. The most common CM targets are drug abstinence or therapy session attendance. CM has also been used to target other behaviors, such as medication adherence [45,46], treatment-related activities [47,48], and exercise [49–51]. Client self-report of behaviors or vaguely defined behaviors (eg, “good participation”) should be avoided. While some of the decisions related to CM protocols are flexible, the use of objectively verifiable target behaviors is a core feature that should not be neglected. If the behavior of interest cannot be objectively verified, an alternate behavior should be chosen.
Selection of the target behavior is often considered in hand with defining which population is eligible to participate in the CM program. Client characteristics are often forefront in this decision, but clinic-driven logistical issues or unmet needs may also play a role. A real-world example of this decision process is evident in the nationwide rollout of CM among the intensive outpatient programs within the Veterans Administration (VA). The VA identified a treatment need for those with stimulant use disorders, as this group did not have efficacious pharmacotherapy options available that targeted stimulant use. As such, the VA applied CM to patients with a focus on stimulant abstinence as the behavioral target [52]. For others, the decision may revolve around addressing underutilization of specific treatment resources (eg, outpatient groups, vocational rehabilitation) [53–56] or treatment needs among certain subgroups of clients, such as adolescents [57–59].
For abstinence targets, clinics would need to select one or more specific substances as the focus of the CM program. In general, targeting a single substance rather than multiple substances is more effective [10,13], is more straightforward for clients to understand, and allows more clients to access the reinforcers. Exposure to the reinforcers is necessary for CM to work; thus, setting a goal that is achievable for most clients should be a priority. Requiring abstinence from multiple substances means that some clients may never experience the reinforcer and thus cannot benefit from its effects at all. Some clinicians or administrators may initially have reservations about reinforcing single drug abstinence in the event that other drug use continues. However, targeting a single substance for reinforcement often results in reduced use of other substances [60]. Clinicians may find that this makes intuitive sense; a client with cocaine use disorder who is trying to maintain cocaine abstinence over a long period is likely to avoid using alcohol or other substances that might lead to relapse. For abstinence, objective verification through urine or breath specimens using tests that include validity checks is relatively straightforward.
Attendance is a popular target for clinics in part because it does not require additional staff time to collect specimen samples and it was the most commonly reported target behavior in samples of SUD providers who use incentives [61,62]. Objective verification of attendance is usually via a staff member, but expectations must be clear to both parties. Clinics should consider potential problems that may arise (eg, arriving late, leaving group early, excused absences) and carefully define and communicate expectations for the CM program. Piloting [19] the CM program with a small group of clients may be valuable in trouble-shooting challenges before wider implementation.
In a recent study [55], clients earned reinforcers for attending clinician-led group counseling sessions and/or the in-clinic patient-led Methadone Anonymous (MA) groups. This non-research, clinical effort addressed historically poor therapy attendance at the clinic, and attendance rates were compared before, during, and after the CM program. CM increased attendance to both groups in the short-term after implementation, but effects were more robust for the MA groups in which increased attendance persisted 3 months following the withdrawal of the contingencies. Overall effects of this program were modest, but they are notable given the use of an ultra-low cost approach.
Delivery Methods
The majority of CM studies used voucher or prize-based methods. Head-to-head comparisons suggest that they are comparable in efficacy [63–65], and each has advantages and disadvantages that may make one option more appealing for a given clinic. Voucher programs are generally straightforward to administer. Clients earn vouchers for each instance of the target behavior. The value of the vouchers typically increases with consecutive performance. The schedule used in the influential Higgins et al studies [66,67] started at $2.50 for the first cocaine-negative sample and increased by $0.50 for each subsequent consecutive cocaine-negative sample. Earned vouchers are exchanged for goods or services selected by the client, increasing the likelihood that the selected items will be highly desirable and allowing for a wide range of client preferences. Clients appear to prefer this approach when given a choice between set schedules or those that introduce an element of chance (ie, prize-based CM, discussed below) [68]. However, voucher programs can be costly (~$1000 per client over 12 weeks) and may require more staff time to fulfill individual requests for specific items. However, staff burden related to shopping can be reduced by limiting these individual requests and using an on-site stocked cabinet of goods similar to prize-CM programs.
Prize-based CM is similar but introduces probabilistic earnings and variability in prize magnitude. Rather than earning vouchers, clients earn draws from a fishbowl for each instance of the target behavior, again typically in an escalating manner. For example, a client may earn one draw from the fishbowl for the first cocaine-negative sample, 2 draws for the second consecutive negative sample, 3 draws for the third, and so on. A typical fishbowl is composed of 500 slips, some noting prizes and some having no prize value. Typically, half the slips in the bowl are non-monetary “good jobs.” The remaining half are small prizes worth about $1 in value (eg, food coupons, bus tokens, small toiletries), large prizes worth about $20 in value (eg, small electronics, gift certificates), and one slip is the jumbo prize worth about $100. When a client draws a winning slip, they select a prize from that category (ie, small, large, jumbo) from an onsite, stocked cabinet. Due to the probabilistic feature of prize-based CM, overall costs of the program can be substantially lower than typical voucher programs, with average maximum expected earnings ranging $250 t $450 per client over a 12-week treatment period [15,16,65,69]. Advantages of this method include potentially lower costs and minimal shopping demands (a once-monthly shopping trip to restock the cabinet will usually suffice) while maintaining comparable efficacy. Relative to voucher programs, prize-based CM involves additional administration time to allow for drawing slips from the fishbowl, which can be compounded when multiple clients want to draw at the same time such as in a group setting. Many of the group-based CM adaptations address this issue by limiting the number of clients who can draw for prizes in a given group or by limiting the number of draws per client [25,27,54].
Reinforcers
Regardless of whether selecting voucher or prize CM, reinforcers are critically important to the success of the program. Reinforcers must be desirable. One of the quickest ways to undermine a CM program is lack of variety or undesirable reinforcers. If stocking a cabinet with prizes onsite, care should be taken to have numerous options within each of the small and large prize categories that are appealing to a wide range of clients. Since a client who is consistently earning draws will choose prizes often, it is imperative to include enough variety so that even these clients find desirable items each time they select a prize. Clients should be asked regularly if they have suggestions for prizes; one program [54] found suggestion boxes useful for encouraging clients to voice their preferences. Donations can be solicited from local businesses to reduce costs [53], and low-cost but high-value options, such as clinic privileges, can also be explored. Petry [1,44] provides some suggestions of the latter, and Amass and Kamien [70] describe their successful strategies to fund and sustain a clinic-based CM program through community donations. Some clinics may already have tangible goods, such as gas or metro cards, that are offered to clients based on need rather than behavior [53]. These existing resources might be redirected to a CM program, in which these goods are contingent on abstinence or attendance, if appropriate.
Schedule Parameters
Once the target behavior, client population, and CM delivery methods are selected, the next step is to design the reinforcement schedule. The following schedule parameters apply to both voucher and prize-based CM systems. The more closely a clinical program adheres to the parameters of effective protocols, the more likely the program is to generate comparable outcomes. If there is a parameter or design feature that is incompatible with clinic needs, modifications can be introduced. However, each deviation away from the ideal has a chance of undermining the success of the CM program. Any changes and their potential impacts should considered carefully, and consultation with a CM expert may aid in the development of successful and efficacious clinic-based protocols. Of note, a meta-analysis [13] of CM studies found that researcher involvement in the planning and design of CM programs is associated with larger treatment effects. CM researchers are especially attuned to the potential impacts and pitfalls associated with modifying CM protocols, and they can be valuable resources for clinics interested in tailoring a CM program to their specific needs. Several examples of clinical demonstration projects that used researcher input are available [19,53,71].
Magnitude
Incentive magnitude was directly related to the size of treatment effects in a meta-analysis [11] of CM studies. Although not all studies find significant differences in outcomes related to magnitude [65,72], the bulk of evidence suggests magnitude is an important parameter and is related to effect size for both voucher [73–75] and prize-based CM [69,76] systems. Thus, although clinics may have restrictive budgets, severely undercutting the magnitude of rewards is not usually the solution as it can undermine treatment effects [76]. Donations can reduce overall costs [53,57,70], and other protocol features discussed below, such as capping the amount of reinforcement available, can reduce the overall magnitude available per patient.
Another approach, used in group-based CM, limits the number of patients who earn prizes per week [25,27]. For example, in a 2011 study by Petry et al, clients added slips with their name to a bowl for attendance and negative samples. Once all names were collected in the bowl, the group leader would pull a specified number of slips (eg, 3 slips per group). These individuals were eligible to draw from the prize bowl for prizes. This approach was associated with longer durations of consecutive abstinence and better treatment attendance relative to treatment as usual. However, clinics can control the overall program costs by limiting the number of patients eligible for prizes.
Frequency
Frequent reinforcement opportunities are ideal, and more frequent assessment is associated with larger treatment effects [10]. However, a number of factors, including which target behavior is selected and logistical issues specific to the clinic such as when groups meet, will play a role in determining the frequency of CM sessions. For abstinence targets, the substance targeted and type of test will largely determine the frequency of CM sessions. The goal would be to test at a frequency that would detect most or nearly all instances of use. For cocaine or opioids, this equates to testing 2 to 3 times weekly. Breath samples for alcohol or cigarette smoking would necessitate testing daily or multiple times per day to detect most instances of use because these tests have short windows of detection. CM protocols based on these breath tests have often had daily or twice daily CM sessions [77,78]; technological adaptations [77,79,80] or residential settings [21,23] may reduce burden to the client for assessment of these substances. Tapering the number of breath tests over time or transitioning from daily breath tests to once or twice weekly urine testing after abstinence is established is another approach [81,82].
Marijuana, on the other hand, poses difficulties because it is detectable in urine samples for up to 2 weeks following use. If relying solely on urine results for reinforcement, clients may not test negative for several days or weeks after last use, resulting in a delay of reinforcement. To address this issue, some CM programs targeting marijuana abstinence initially reinforce attendance in the first 2 weeks and then transition to reinforcing marijuana-negative drug samples for the remainder of the treatment period [48].
In general, more frequent CM sessions can translate to higher costs; however, infrequent reinforcement (ie, less than weekly) is not as effective [45]. In real-world applications, clinics often need to balance feasibility and costs with the ideal CM schedule. In abstinence-based CM, this compromise may result in a testing schedule that may not capture all instances of use. For example, while thrice-weekly testing may be ideal for cocaine or opioids, a twice-weekly schedule may be selected because it lowers costs and is more consistent with clinic schedules.
Immediacy
In general, clinics should aim to deliver reinforcement as immediately as possible, as delays between the target behavior and reinforcement are associated with decreased treatment effects [10,11,83]. For drug abstinence, onsite urine testing systems that provide immediate results are preferred over sending samples for laboratory testing. Clinics that do not have access to or who cannot afford specimen testing that allows onsite collection and immediate results might consider other options for target behaviors, such as attendance.
Immediacy of reinforcement is also important when targeting attendance. One clinic [53] implemented a program that offered a $50 incentive if clients attended 1 month of group therapy sessions. This approach was not effective and no clients earned the incentive for several months. After consultation, the clinic revised the incentive program to a daily drawing for attendance using the fishbowl method, thereby decreasing the delay between the behavior and its consequence. This example illustrates not only problems with delayed reinforcement but also the common mistake of setting expectations for the target behavior too high. Attending a month of group therapy sessions is a high bar that few patients will achieve, resulting in a system that mostly rewards those already doing well [19]. In contrast, attending a single group session in order to earn reinforcers is a reachable goal and increases the likelihood that more clients are exposed to the reinforcers. These small steps (ie, attending a single group or submitting a single drug negative urine) encourage initiation of the behavior(s) targeted. Other features, such as escalation (discussed next), aim to sustain the behavior over time.
Escalation
Escalation involves increasing the amount of reinforcement for each consecutive target behavior. In the voucher programs, the amount earned per negative sample may increase for each consecutive negative sample (eg, $2.50 for the first negative sample, $3.00 for the second, $3.50 for the third, and so on). For prize-based programs, the number of draws escalates with consecutive performance (eg, 1 draw for the first group attended, 2 draws for the second, 3 for the third, and so on). Protocols that include escalation generate larger effects than those that have a set, flat incentive amount even when total costs are the same across comparison conditions [73].
Escalating schedules usually include a reset feature. Following a positive or refused sample or unexcused absence, the amount earned for the next negative sample is reduced to the initial amount and begins escalating anew with consecutive negative samples. Some schedules allow for a rapid reset in which after a specified period of time or consecutive performance, the value jumps to the value achieved when the relapse occurred [66].
Despite its consistent inclusion in CM protocols from randomized clinical trials, our data [61] suggest that more than half of providers using incentives in treatment as part of a clinical effort do not use escalating reinforcers. Escalating schedules require more careful tracking of client progress, possibly contributing to lower uptake of this design feature in clinical practice. Development of simple tracking forms can minimize this challenge.
Another drawback of escalation pertaining to prize-based CM is that escalating schedules can affect the duration of CM sessions when clients are drawing a large number of slips each session and escalation can increase costs of the overall program. Capping the number of draws will help mitigate both issues. For example, once a client reaches 10 draws for group attendance, they continue earning 10 draws for each consecutive session attended with no further escalation.
Putting It All Together
CM sessions can be conducted as stand-alone sessions or incorporated into group or individual therapy sessions. Many clinicians will find the latter approach sets a positive tone for the therapy session given CM's focus on what the client is doing well. Starting the treatment session with the CM component often naturally leads into a discussion of relevant therapeutic issues, such as effective coping, slips, or triggers. The CM session length can be variable, but it is typically under 10 minutes. Thus, the CM component need not dominate the clinical session or content. CM sessions for abstinence are scheduled according to a set schedule (eg, Mondays and Thursdays) and often coincide with other treatment aspects (eg, before or after group therapy on Mondays and Thursdays). CM sessions for attendance also generally follow a set schedule (eg, client expected to attend Monday and Wednesday group therapy sessions). The duration of the CM protocol can also vary, with most clinical trials ranging from 12 to 24 weeks. Very short durations are unlikely to produce lasting behavior change, particularly with complex behaviors such as abstinence. Petry [44] recommends no less than 8 weeks duration and a maximum duration of 24 weeks.
As discussed, CM offers many opportunities for tailoring to optimize its fit within the existing structure of clinics. However, this flexibility must be viewed together with an understanding of the principles that impact CM's efficacy. Specific recommendations for CM protocol development will depend on the behavior targeted, the delivery methods, and format (eg, individual versus group settings). For these reasons, consultation with a CM expert is ideal. However, some general guidelines for developing a CM program that incorporate the principles discussed above include an 8- to 12-week program that (1) provides sufficient magnitude to compete with the behavior you are attempting to change, (2) offers frequent opportunities for reinforcement (eg, 2-3 times/wk for opioids or stimulant abstinence, 1-2 times/week for attendance targets; not less than weekly for most behaviors), (3) delivers the reinforcement immediately or very close in time with the behavior (eg, reinforce attendance at the beginning of the group, use onsite urine testing and reinforce immediately after testing), and (4) incorporates escalating and reset features into the schedule.
Clinician Training and Supervision
Training in CM is an important part of the implementation process. Studies [62,84–87] have identified a number of perceived barriers to and negative beliefs about CM, including philosophical and logistical concerns. Tangible incentives, the core of most CM protocols, are generally viewed less favorably than social or nonspecified incentives [84,86,87]. Philosophical concerns relate to CM’s inability to address the underlying causes of addiction, that it does not address multiple behaviors, and that it may undermine internal motivation for sobriety [62,84]. An additional objection relates to paying someone to do what they should do on their own [86]. Logistical and practical concerns often represent implementation barriers such as costs and access to training and supervision, but they also reflect concern for what happens when contingencies are withdrawn, that clients may sell or trade prizes for drugs, and worries that CM’s evidence does not generalize to clinic populations [62].
Many of these beliefs reflect a limited understanding of CM, and addressing these misperceptions is a first step toward reducing resistance to implementation efforts. For example, a substantial body of literature points to CM’s wide generalizability across a range of characteristics, clients that sell or trade prizes for drugs are likely to disrupt their chain of negative samples or attendance, and most studies do not find negative impacts of CM on intrinsic motivation [88–90]. Fortunately, CM training appears to be an effective way to address negative beliefs. In the VA implementation effort [52], training workshops decreased perceived barriers and increased positive impressions of CM [91]. In other training efforts, brief educational materials were effective in changing perceptions of CM’s efficacy [92].
Beyond initial training, supervision of CM delivery is likely to be necessary [93,94]. Clinician skill in delivering CM is related to client outcomes [93,95] and relatively simple adherence measures are available for monitoring [96,97]. However, the best methods for training and supervision of CM have yet to be established. The VA initiative was developed in consultation with CM experts and employed ongoing phone consultation following initial training workshops [52,91]. This approach represented significant investment by the VA toward staff training and CM protocol development that may not be achievable for individual clinics. As attention to CM’s dissemination and implementation has grown, some free resources have been developed. Promoting Awareness of Motivational Incentives (PAMI; www.bettertxoutcomes.org/bettertxoutcomes/PAMI.html) is a collaborative initiative sponsored by the National Institute of Drug Abuse and the Substance Abuse and Mental Health Services Administration. It offers free resources and training materials.
Conclusion
Overall, CM is a highly efficacious treatment for SUDs that generalizes to most clients. Despite a robust evidence base, CM’s implementation in clinical settings lags behind other empirically supported treatments [92]. At least in part, CM’s costs, which include not only staff training and adherence monitoring (as with other treatments), but also costs of the incentives themselves, may contribute to slow uptake in clinical settings. Clinics often do not have the resources available for CM within their operating budgets. However, a growing number of projects [19,52,53,55–57,70,71] illustrate CM implementation within routine clinical care, and increased revenue from improved attendance to treatment groups may be one mechanism through which to fund a CM program [54,56,57]. These projects are valuable not only for demonstrating that CM can be efficacious outside the research setting, but also for highlighting how implementation barriers can be overcome. Continued efforts of this nature are likely to be particularly valuable for clinicians and administrators considering adopting CM within clinical settings.
1. Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend 2000;58:9–25.
2. Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psychol 2006;2:411–34.
3. Meredith SE, Jarvis BP, Raiff BR, et al. The ABCs of incentive-based treatment in health care: A behavior analytic framework to inform research and practice. Psychol Res Behav Manag 2014;7:103–14.
4. Bigelow GE, Silverman K. Theoretical and empirical foundations of contingency management treatments for drug abuse. In: Higgins ST, Silverman K, editors. Motivating behavior change among illicit drug users. Washington DC: American Psychological Association; 1999: 15–31.
5. Higgins ST, Budney AJ, Bickel WK. Applying behavioral concepts and principles to the treatment of cocaine dependence. Drug Alcohol Depend 1994;34:87–97.
6. Skinner BF. Science and human behavior. New York: Macmillan; 1953.
7. Herbeck DM, Hser YI, Teruya C. Empirically supported substance abuse treatment approaches: A survey of treatment providers’ perspectives and practices. Addict Behav 2008;33:699–712.
8. McGovern MP, Fox TS, Xie H, Drake RE. A survey of clinical practices and readiness to adopt evidence-based practices: Dissemination research in an addiction treatment system. J Subst Abuse Treat 2004;26:305–12.
9. Willenbring ML, Kivlahan D, Kenny M, et al. Beliefs about evidence-based practices in addiction treatment: A survey of Veterans Administration program leaders. J Subst Abuse Treat 2004;26:79–85.
10. Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: A meta-analysis. Drug Alcohol Depend 2000;58:55–66.
11. Lussier JP, Heil SH, Mongeon JA, et al. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192–203.
12. Benishek LA, Dugosh KL, Kirby KC, et al. Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction 2014;109:1426–36.
13. Prendergast M, Podus D, Finney J, et al. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction 2006;101:1546–60.
14. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008;165:179–87.
15. Petry NM, Peirce JM, Stitzer ML, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2005;62:1148–56.
16. Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment - A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2006;63:201–8.
17. Petry NM, Martin B, Finocche C. Contingency management in group treatment: a demonstration project in an HIV drop-in center. J Subst Abuse Treat 2001;21:89–96.
18. Drebing CE, Van Ormer EA, Mueller L, et al. Adding contingency management intervention to vocational rehabilitation: outcomes for dually diagnosed veterans. J Rehabil Res Dev 2007;44:851–65.
19. Kellogg SH, Burns M, Coleman P, et al. Something of value: The introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. J Subst Abuse Treat 2005;28:57–65.
20. Koffarnus MN, Wong CJ, Fingerhood M, et al. Monetary incentives to reinforce engagement and achievement in a job-skills training program for homeless, unemployed adults. J Appl Behav Anal 2013;46:582–91.
21. Rohsenow D, Martin R, Tidey JW, et al. Treating smokers in substance treatment with contingent vouchers, nicotine replacement, and brief advice adapted for sobriety settings. J Subst Abuse Treat 2017.
22. Hunt YM, Rash CJ, Burke RS, Parker JD. Smoking cessation in recovery: Comparing two different cognitive behavioral treatments. Addict Disord Their Treat 2010;9:64–74.
23. Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reductions in residential substance abuse patients. J Appl Behav Anal 2008;41:617–22.
24. Petry NM, Weinstock J, Alessi SM, et al. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol 2010;78:89.
25. Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. J Consult Clin Psychol 2011;79:686–96.
26. Ledgerwood DM, Alessi SM, Hanson T, et al. Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. J Appl Behav Anal 2008;41:517–26.
27. Alessi SM, Hanson T, Wieners M, Petry NM. Low-cost contingency management in community clinics: Delivering incentives partially in group therapy. Exp Clin Psychopharmacol 2007;15:293–300.
28. Burch AE, Rash CJ, Petry NM. Sex effects in cocaine-using methadone patients randomized to contingency management interventions. Exp Clin Psychopharmacol 2015;23:284–90.
29. Rash CJ, Petry NM. Contingency management treatments are equally efficacious for both sexes in intensive outpatient settings. Exp Clin Psychopharmacol 2015;23:369–76.
30. Barry D, Sullivan B, Petry NM. Comparable efficacy of contingency management for cocaine dependence among African American, Hispanic, and White methadone maintenance clients. Psychol Addict Behav 2009;23:168–74.
31. Rash CJ, Alessi SM, Petry NM. Substance abuse treatment patients in housing programs respond to contingency management interventions. J Subst Abuse Treat 2017;72:97–102.
32. Rash CJ, Olmstead TA, Petry NM. Income does not affect response to contingency management treatments among community substance abuse treatment-seekers. Drug Alcohol Depend 2009;104:249–53.
33. Rash CJ, Andrade LF, Petry NM. Income received during treatment does not affect response to contingency management treatments in cocaine-dependent outpatients. Drug Alcohol Depend 2013;132:528–34.
34. Secades-Villa R, García-Fernández G, Peña-Suárez E, et al. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. J Subst Abuse Treat 2013;44:349–54.
35. Rash CJ, Alessi SM, Petry NM. Cocaine abusers with and without alcohol dependence respond equally well to contingency management treatments. Exp Clin Psychopharmacol 2008;16:275–81.
36. Alessi SM, Rash C, Petry NM. Contingency management is efficacious and improves outcomes in cocaine patients with pretreatment marijuana use. Drug Alcohol Depend 2011;118:62–7.
37. Ford JD, Hawke J, Alessi S, et al. Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behav Res Ther 2007;45:2417–31.
38. Weinstock J, Alessi SM, Petry NM. Regardless of psychiatric severity the addition of contingency management to standard treatment improves retention and drug use outcomes. Drug Alcohol Depend 2007;87:288–96.
39. García-Fernández G, Secades-Villa R, García-Rodríguez O, et al. Contingency management improves outcomes in cocaine-dependent outpatients with depressive symptoms. Exp Clin Psychopharmacol 2013;21:482–9.
40. Walter KN, Petry NM. Patients with diabetes respond well to contingency management treatment targeting alcohol and substance use. Psychol Health Med 2015;20:916–26.
41. Burch AE, Morasco BJ, Petry NM. Patients undergoing substance abuse treatment and receiving financial sssistance for a physical disability respond well to contingency management treatment. J Subst Abuse Treat 2015;58:67–71.
42. Burch AE, Rash CJ, Petry NM. Cocaine-using substance abuse treatment patients with and without HIV respond well to contingency management treatment. J Subst Abuse Treat 2017;77:21–5.
43. Rash CJ, Burki M, Montezuma-Rusca JM, Petry NM. A retrospective and prospective analysis of trading sex for drugs or money in women substance abuse treatment patients. Drug Alcohol Depend 2016;162:182–9.
44. Petry NM. Contingency management for substance abuse treatment: A guide to implementing this evidence-based practice. New York: Routledge; 2012.
45. Petry NM, Rash CJ, Byrne S, et al. Financial reinforcers for improving medication adherence: Findings from a meta-analysis. Am J Med 2012;125:888–96.
46. Herrmann ES, Matusiewicz AK, Stitzer ML, et al. Contingency management interventions for HIV, tuberculosis, and hepatitis control among individuals with substance use disorders: a systematized review. J Subst Abuse Treat 2017;72:117–25.
47. Petry NM, Alessi SM, Carroll KM, et al. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. J Consult Clin Psychol 2006;74:592–601.
48. Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: Randomized trial of contingency management and self-efficacy enhancement. Addict Behav 2013;38:1764–75.
49. Kurti AN, Dallery J. A laboratory-based evaluation of exercise plus contingency management for reducing cigarette smoking. Drug Alcohol Depend 2014;144:201–9.
50. Weinstock J, Capizzi J, Weber SM, et al. Exercise as an intervention for sedentary hazardous drinking college students: A pilot study. Ment Health Phys Act 2014;7:55–62.
51. Mitchell MS, Goodman JM, Alter DA, et al. Financial incentives for exercise adherence in adults: Systematic review and meta-analysis. Am J Prev Med 2013;45:658–67.
52. Petry NM, Dephilippis D, Rash CJ, et al. Nationwide dissemination of contingency management: The Veterans Administration initiative. Am J Addict 2014;23:205–10.
53. Walker R, Rosvall T, Field CA, et al. Disseminating contingency management to increase attendance in two community substance abuse treatment centers: Lessons learned. J Subst Abuse Treat 2010;39:202–9.
54. Sigmon SC, Stitzer ML. Use of a low-cost incentive intervention to improve counseling attendance among methadone-maintained patients. J Subst Abuse Treat 2005;29:253–8.
55. Kropp F, Lewis D, Winhusen T. The effectiveness of ultra-low magnitude reinforcers: Findings from a “real-world” application of contingency management. J Subst Abuse Treat 2017;72:111–6.
56. Fitzsimons H, Tuten M, Borsuk C, et al. Clinician-delivered contingency management increases engagement and attendance in drug and alcohol treatment. Drug Alcohol Depend 2015;152:62–7.
57. Lott DC, Jencius S. Effectiveness of very low-cost contingency management in a community adolescent treatment program. Drug Alcohol Depend 2009;102:162–5.
58. Henggeler SW, Chapman JE, Rowland MD, et al. Statewide adoption and initial implementation of contingency management for substance-abusing adolescents. 2008;76:556–67.
59. Henggeler SW, Chapman JE, Rowland MD, et al. If you build it , they will come: Statewide practitioner interest in contingency management for youths. 2007;32:121–31.
60. Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol 2000;68:250–7.
61. Rash CJ, Petry NM, Alessi SM. Examining implementation of contingency management in real-world settings [Abstract]. Alcohol Clin Exp Res 2016;20:103A.
62. Rash CJ, Petry NM, Kirby KC, et al. Identifying provider beliefs related to contingency management adoption using the Contingency Management Beliefs Questionnaire. Drug Alcohol Depend 2012;121:205–12.
63. Petry NM, Alessi SM, Marx J, et al. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. J Consult Clin Psychol 2005;73:1005–14.
64. Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol 2007;75:983–91.
65. Petry NM, Alessi SM, Barry D, Carroll KM. Standard magnitude prize reinforcers can be as efficacious as larger magnitude reinforcers in cocaine-dependent methadone patients. J Consult Clin Psychol 2015;83:464–72.
66. Higgins ST, Budney AJ, Bickel WK, et al. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry 1994;51:568.
67. Higgins ST, Wong CJ, Badger GJ, et al. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol 2000;68:64–72.
68. Hartzler B, Garrett S. Interest and preferences for contingency management design among addiction treatment clientele. Am J Drug Alcohol Abuse 2016;42:287–95.
69. Petry NM, Barry D, Alessi SM, et al. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol 2012;80:276–85.
70. Amass L, Kamien J. A tale of two cities: Financing two voucher programs for substance abusers through community donations. Exp Clin Psychopharmacol 2004;12:147–55.
71. Hartzler B. Building a bonfire that remains stoked: Sustainment of a contingency management intervention developed through collaborative design. Subst Abuse Treat Prev Policy 2015;10:30.
72. Carroll KM, Sinha R, Nich C, et al. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol 2002;10:54–63.
73. Roll JM, Shoptaw S. Contingency management: Schedule effects. Psychiatry Res 2006;144:91–3.
74. Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology (Berl) 1999;146:128–38.
75. Businelle MS, Rash CJ, Burke RS, Parker JD. Using vouchers to increase continuing care participation in veterans: does magnitude matter? Am J Addict 2009;18:122–9.
76. Petry NM, Tedford J, Austin M, et al. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction 2004;99:349–60.
77. Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction 2013;108:900–9.
78. Alessi SM, Petry NM. Smoking reductions and increased self-efficacy in a randomized controlled trial of smoking abstinence--contingent incentives in residential substance abuse treatment patients. Nicotine Tob Res 2014;16:1436–45.
79. Dougherty DM, Hill-Kapturczak N, Liang Y, et al. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend 2014;142:301–6.
80. Dallery J, Meredith S, Jarvis B, Nuzzo PA. Internet-based group contingency management to promote smoking abstinence. Exp Clin Psychopharmacol 2015;23:176–83.
81. Higgins ST, Washio Y, Lopez AA, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med (Baltim) 2014;68:51–7.
82. Higgins ST, Heil SH, Solomon LJ, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 2004;6:1015–20.
83. Packer RR, Howell DN, McPherson S, Roll JM. Investigating reinforcer magnitude and reinforcer delay: A contingency management analog study. Exp Clin Psychopharmacol 2012;20:287–92.
84. Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug Alcohol Depend 2006;85:19–27.
85. Cameron J, Ritter A. Contingency management: Perspectives of Australian service providers. 2007;26:183–9.
86. Hartzler B, Rabun C. Community opioid treatment perspectives on contingency management: Perceived feasibility, effectiveness, and transportability of social and financial incentives. J Subst Abuse Treat 2013;45:242–8.
87. Aletraris L, Shelton JS, Roman PM. Counselor attitudes toward contingency management for substance use disorder: Effectiveness, acceptability, and endorsement of incentives for treatment attendance and abstinence. J Subst Abuse Treat 2015;57:41–8.
88. Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol 2000;68:1051–61.
89. Ledgerwood DM, Petry NM. Does contingency management affect motivation to change substance use? Drug Alcohol Depend 2006;83:65–72.
90. Litt MD, Kadden RM, Kabela-Cormier E, Petry NM. Coping skills training and contingency management treatments for marijuana dependence: Exploring mechanisms of behavior change. Addiction 2008;103:638–48.
91. Rash CJ, DePhilippis D, McKay JR, et al. Training workshops positively impact beliefs about contingency management in a nationwide dissemination effort. J Subst Abuse Treat 2013;45:306–12.
92. Benishek LA, Kirby KC, Dugosh KL, Padovano A. Beliefs about the empirical support of drug abuse treatment interventions: A survey of outpatient treatment providers. Drug Alcohol Depend 2010;107:202–8.
93. Petry NM, Alessi SM, Ledgerwood DM. Contingency management delivered by community therapists in outpatient settings. Drug Alcohol Depend 2012;122:86–92.
94. Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. J Consult Clin Psychol 2012;80:286–98.
95. Hartzler B, Beadnell B, Donovan D. Predictive validity of addiction treatment clinicians’ post-training contingency management skills for subsequent clinical outcomes. J Subst Abuse Treat 2017.
96. Hartzler B. Adapting the Helpful Responses Questionnaire to assess communication skills involved in delivering contingency management: Preliminary psychometrics. J Subst Abuse Treat 2014;55:52–7.
97. Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the Contingency Management Competence Scale. Drug Alcohol Depend 2010;109:167–74.
From UConn Health, Farmington, CT.
Abstract
- Objective: To discuss the efficacy and generalizability of contingency management (CM) for the treatment of substance use disorders and design considerations for those considering implementing in clinical settings.
- Methods: Review of the literature.
- Results: CM is an efficacious treatment for substance abuse disorders that is widely generalizable across substance use disorders and patient characteristics. CM can be implemented in a number of treatment programs, including residential and outpatient settings, and it can be administered in both individual and group formats. Abstinence and attendance are the most commonly targeted behaviors in substance abuse treatment settings. Design features, including the selection of target behaviors, delivery methods, and reinforcers, are discussed. Schedule parameters, such as frequency, magnitude, immediacy, and escalation of reinforcement, are associated with overall impact of the CM program and are important considerations for those interested in tailoring CM protocols to their needs.
- Conclusion: CM is an efficacious option that is applicable to most substance abuse treatment patients. A number of demonstrations of real-world implementation have been published and suggest CM can be adapted with success to clinic settings. In adopting CM protocols, clinics should aim for those protocols with established efficacy; however, if adaptations are necessary, careful consideration should be given to modifications to minimize risks of undermining CM’s effects.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Contingency management (CM) is a behavioral intervention that is efficacious in the treatment of substance use disorders (SUDs). CM uses a behavior analytic framework and applies principles of learning theory, particularly operant conditioning theory, to change client behavior(s) [1–5]. In basic terms, operant conditioning principles suggests that whether a behavior continues or not is a function of consequences [6]. Reinforced behaviors are more likely to occur in the future. Substance abuse can be viewed as a behavior maintained by the reinforcing effects of the drug itself [5], including the feel-good aspects of intoxication or relaxation and the amelioration of withdrawal symptoms. CM extends these same principles of to a treatment context, such that reinforcers for abstinent behavior are introduced to compete with the reinforcing effects of continued drug use [5].
In CM’s application to substance abuse treatment, drug-negative samples or treatment attendance are reinforced using tangible incentives with the goal of motivating continued abstinence and/or treatment engagement. When clients demonstrate these target behaviors, they earn incentives in the form of goods or services of value to the client, such as small electronics, gift cards, and toiletries. Despite the promising effects observed in research trials, real-world implementation efforts have not kept pace [7–9]. This review briefly discusses CM’s efficacy and highlights key features for professionals considering adopting this intervention. Demonstration efforts that illustrate how CM can be effectively implemented within the constraints and limitations of non-research, clinical settings are also presented.
Efficacy of CM
CM’s efficacy spans a number of SUDs, including cocaine, opioids, alcohol, nicotine, and marijuana [10–13], making it amenable for treatment of most SUD clinic populations. It generates larger effect sizes than other SUD treatments, including cognitive behavioral therapy [14], and it has been evaluated in a wide range of settings. Large-scale evaluations have been conducted in both intensive outpatient [15] and methadone maintenance [16] settings as part of the National Institute on Drug Abuse Clinical Trials Network, demonstrating consistent benefits of CM when added to treatment as usual. In the first of these 2 studies, Petry et al [15] randomized 415 stimulant users from 1 of 8 intensive outpatient clinics to treatment as usual or treatment as usual plus CM for alcohol and stimulant abstinence. CM participants submitted more substance-negative urine and breath samples, achieved continuous abstinence at significantly higher rates, and had longer treatment retention compared to those receiving treatment as usual. The parallel study [16] focused on stimulant use in clients from methadone maintenance clinics and found similar benefits of CM on stimulant abstinence. Beyond these settings, CM has been applied in a number of other contexts, including drop-in centers [17], vocational rehabilitation [18,19], job-skills training [20], and residential programs [21–23]. In addition, several group-based adaptations have been explored [17,24–27].
CM benefits most clients and generalizes across several demographic variables, including gender [28,29], race [30], housing status [31], and income levels [32–34]. Among clinical characteristics, CM is efficacious for those with co-occurring SUDs [35], other substance use [36], psychiatric disorders [37–39], medical problems [40–42], and history of transactional sex [43].
Design Considerations
Design features, including what behavior will be reinforced and how to do so, are among the first decision points for clinicians interested in implementing CM. One of the advantages of CM is that it has a high degree of flexibility in design, which means that it can be readily tailored to client populations and clinic needs. However, this flexibility can lead clinicians astray from the foundational principles of CM and unknowingly weaken the impact of the program. Below, some key considerations for CM protocol design are reviewed. For additional coverage of these topics, readers are referred to additional articles [1,2] or Petry’s comprehensive book on implementing CM [44]. In this review, published examples of CM’s application in real-world settings are presented, highlighting how CM has been adapted in these clinical efforts.
Target Behaviors
The selection of the target behavior will drive many of the subsequent program design decisions. As such, it is important to identify this feature early. Target behaviors must be achievable, objectively verifiable, and well defined. The most common CM targets are drug abstinence or therapy session attendance. CM has also been used to target other behaviors, such as medication adherence [45,46], treatment-related activities [47,48], and exercise [49–51]. Client self-report of behaviors or vaguely defined behaviors (eg, “good participation”) should be avoided. While some of the decisions related to CM protocols are flexible, the use of objectively verifiable target behaviors is a core feature that should not be neglected. If the behavior of interest cannot be objectively verified, an alternate behavior should be chosen.
Selection of the target behavior is often considered in hand with defining which population is eligible to participate in the CM program. Client characteristics are often forefront in this decision, but clinic-driven logistical issues or unmet needs may also play a role. A real-world example of this decision process is evident in the nationwide rollout of CM among the intensive outpatient programs within the Veterans Administration (VA). The VA identified a treatment need for those with stimulant use disorders, as this group did not have efficacious pharmacotherapy options available that targeted stimulant use. As such, the VA applied CM to patients with a focus on stimulant abstinence as the behavioral target [52]. For others, the decision may revolve around addressing underutilization of specific treatment resources (eg, outpatient groups, vocational rehabilitation) [53–56] or treatment needs among certain subgroups of clients, such as adolescents [57–59].
For abstinence targets, clinics would need to select one or more specific substances as the focus of the CM program. In general, targeting a single substance rather than multiple substances is more effective [10,13], is more straightforward for clients to understand, and allows more clients to access the reinforcers. Exposure to the reinforcers is necessary for CM to work; thus, setting a goal that is achievable for most clients should be a priority. Requiring abstinence from multiple substances means that some clients may never experience the reinforcer and thus cannot benefit from its effects at all. Some clinicians or administrators may initially have reservations about reinforcing single drug abstinence in the event that other drug use continues. However, targeting a single substance for reinforcement often results in reduced use of other substances [60]. Clinicians may find that this makes intuitive sense; a client with cocaine use disorder who is trying to maintain cocaine abstinence over a long period is likely to avoid using alcohol or other substances that might lead to relapse. For abstinence, objective verification through urine or breath specimens using tests that include validity checks is relatively straightforward.
Attendance is a popular target for clinics in part because it does not require additional staff time to collect specimen samples and it was the most commonly reported target behavior in samples of SUD providers who use incentives [61,62]. Objective verification of attendance is usually via a staff member, but expectations must be clear to both parties. Clinics should consider potential problems that may arise (eg, arriving late, leaving group early, excused absences) and carefully define and communicate expectations for the CM program. Piloting [19] the CM program with a small group of clients may be valuable in trouble-shooting challenges before wider implementation.
In a recent study [55], clients earned reinforcers for attending clinician-led group counseling sessions and/or the in-clinic patient-led Methadone Anonymous (MA) groups. This non-research, clinical effort addressed historically poor therapy attendance at the clinic, and attendance rates were compared before, during, and after the CM program. CM increased attendance to both groups in the short-term after implementation, but effects were more robust for the MA groups in which increased attendance persisted 3 months following the withdrawal of the contingencies. Overall effects of this program were modest, but they are notable given the use of an ultra-low cost approach.
Delivery Methods
The majority of CM studies used voucher or prize-based methods. Head-to-head comparisons suggest that they are comparable in efficacy [63–65], and each has advantages and disadvantages that may make one option more appealing for a given clinic. Voucher programs are generally straightforward to administer. Clients earn vouchers for each instance of the target behavior. The value of the vouchers typically increases with consecutive performance. The schedule used in the influential Higgins et al studies [66,67] started at $2.50 for the first cocaine-negative sample and increased by $0.50 for each subsequent consecutive cocaine-negative sample. Earned vouchers are exchanged for goods or services selected by the client, increasing the likelihood that the selected items will be highly desirable and allowing for a wide range of client preferences. Clients appear to prefer this approach when given a choice between set schedules or those that introduce an element of chance (ie, prize-based CM, discussed below) [68]. However, voucher programs can be costly (~$1000 per client over 12 weeks) and may require more staff time to fulfill individual requests for specific items. However, staff burden related to shopping can be reduced by limiting these individual requests and using an on-site stocked cabinet of goods similar to prize-CM programs.
Prize-based CM is similar but introduces probabilistic earnings and variability in prize magnitude. Rather than earning vouchers, clients earn draws from a fishbowl for each instance of the target behavior, again typically in an escalating manner. For example, a client may earn one draw from the fishbowl for the first cocaine-negative sample, 2 draws for the second consecutive negative sample, 3 draws for the third, and so on. A typical fishbowl is composed of 500 slips, some noting prizes and some having no prize value. Typically, half the slips in the bowl are non-monetary “good jobs.” The remaining half are small prizes worth about $1 in value (eg, food coupons, bus tokens, small toiletries), large prizes worth about $20 in value (eg, small electronics, gift certificates), and one slip is the jumbo prize worth about $100. When a client draws a winning slip, they select a prize from that category (ie, small, large, jumbo) from an onsite, stocked cabinet. Due to the probabilistic feature of prize-based CM, overall costs of the program can be substantially lower than typical voucher programs, with average maximum expected earnings ranging $250 t $450 per client over a 12-week treatment period [15,16,65,69]. Advantages of this method include potentially lower costs and minimal shopping demands (a once-monthly shopping trip to restock the cabinet will usually suffice) while maintaining comparable efficacy. Relative to voucher programs, prize-based CM involves additional administration time to allow for drawing slips from the fishbowl, which can be compounded when multiple clients want to draw at the same time such as in a group setting. Many of the group-based CM adaptations address this issue by limiting the number of clients who can draw for prizes in a given group or by limiting the number of draws per client [25,27,54].
Reinforcers
Regardless of whether selecting voucher or prize CM, reinforcers are critically important to the success of the program. Reinforcers must be desirable. One of the quickest ways to undermine a CM program is lack of variety or undesirable reinforcers. If stocking a cabinet with prizes onsite, care should be taken to have numerous options within each of the small and large prize categories that are appealing to a wide range of clients. Since a client who is consistently earning draws will choose prizes often, it is imperative to include enough variety so that even these clients find desirable items each time they select a prize. Clients should be asked regularly if they have suggestions for prizes; one program [54] found suggestion boxes useful for encouraging clients to voice their preferences. Donations can be solicited from local businesses to reduce costs [53], and low-cost but high-value options, such as clinic privileges, can also be explored. Petry [1,44] provides some suggestions of the latter, and Amass and Kamien [70] describe their successful strategies to fund and sustain a clinic-based CM program through community donations. Some clinics may already have tangible goods, such as gas or metro cards, that are offered to clients based on need rather than behavior [53]. These existing resources might be redirected to a CM program, in which these goods are contingent on abstinence or attendance, if appropriate.
Schedule Parameters
Once the target behavior, client population, and CM delivery methods are selected, the next step is to design the reinforcement schedule. The following schedule parameters apply to both voucher and prize-based CM systems. The more closely a clinical program adheres to the parameters of effective protocols, the more likely the program is to generate comparable outcomes. If there is a parameter or design feature that is incompatible with clinic needs, modifications can be introduced. However, each deviation away from the ideal has a chance of undermining the success of the CM program. Any changes and their potential impacts should considered carefully, and consultation with a CM expert may aid in the development of successful and efficacious clinic-based protocols. Of note, a meta-analysis [13] of CM studies found that researcher involvement in the planning and design of CM programs is associated with larger treatment effects. CM researchers are especially attuned to the potential impacts and pitfalls associated with modifying CM protocols, and they can be valuable resources for clinics interested in tailoring a CM program to their specific needs. Several examples of clinical demonstration projects that used researcher input are available [19,53,71].
Magnitude
Incentive magnitude was directly related to the size of treatment effects in a meta-analysis [11] of CM studies. Although not all studies find significant differences in outcomes related to magnitude [65,72], the bulk of evidence suggests magnitude is an important parameter and is related to effect size for both voucher [73–75] and prize-based CM [69,76] systems. Thus, although clinics may have restrictive budgets, severely undercutting the magnitude of rewards is not usually the solution as it can undermine treatment effects [76]. Donations can reduce overall costs [53,57,70], and other protocol features discussed below, such as capping the amount of reinforcement available, can reduce the overall magnitude available per patient.
Another approach, used in group-based CM, limits the number of patients who earn prizes per week [25,27]. For example, in a 2011 study by Petry et al, clients added slips with their name to a bowl for attendance and negative samples. Once all names were collected in the bowl, the group leader would pull a specified number of slips (eg, 3 slips per group). These individuals were eligible to draw from the prize bowl for prizes. This approach was associated with longer durations of consecutive abstinence and better treatment attendance relative to treatment as usual. However, clinics can control the overall program costs by limiting the number of patients eligible for prizes.
Frequency
Frequent reinforcement opportunities are ideal, and more frequent assessment is associated with larger treatment effects [10]. However, a number of factors, including which target behavior is selected and logistical issues specific to the clinic such as when groups meet, will play a role in determining the frequency of CM sessions. For abstinence targets, the substance targeted and type of test will largely determine the frequency of CM sessions. The goal would be to test at a frequency that would detect most or nearly all instances of use. For cocaine or opioids, this equates to testing 2 to 3 times weekly. Breath samples for alcohol or cigarette smoking would necessitate testing daily or multiple times per day to detect most instances of use because these tests have short windows of detection. CM protocols based on these breath tests have often had daily or twice daily CM sessions [77,78]; technological adaptations [77,79,80] or residential settings [21,23] may reduce burden to the client for assessment of these substances. Tapering the number of breath tests over time or transitioning from daily breath tests to once or twice weekly urine testing after abstinence is established is another approach [81,82].
Marijuana, on the other hand, poses difficulties because it is detectable in urine samples for up to 2 weeks following use. If relying solely on urine results for reinforcement, clients may not test negative for several days or weeks after last use, resulting in a delay of reinforcement. To address this issue, some CM programs targeting marijuana abstinence initially reinforce attendance in the first 2 weeks and then transition to reinforcing marijuana-negative drug samples for the remainder of the treatment period [48].
In general, more frequent CM sessions can translate to higher costs; however, infrequent reinforcement (ie, less than weekly) is not as effective [45]. In real-world applications, clinics often need to balance feasibility and costs with the ideal CM schedule. In abstinence-based CM, this compromise may result in a testing schedule that may not capture all instances of use. For example, while thrice-weekly testing may be ideal for cocaine or opioids, a twice-weekly schedule may be selected because it lowers costs and is more consistent with clinic schedules.
Immediacy
In general, clinics should aim to deliver reinforcement as immediately as possible, as delays between the target behavior and reinforcement are associated with decreased treatment effects [10,11,83]. For drug abstinence, onsite urine testing systems that provide immediate results are preferred over sending samples for laboratory testing. Clinics that do not have access to or who cannot afford specimen testing that allows onsite collection and immediate results might consider other options for target behaviors, such as attendance.
Immediacy of reinforcement is also important when targeting attendance. One clinic [53] implemented a program that offered a $50 incentive if clients attended 1 month of group therapy sessions. This approach was not effective and no clients earned the incentive for several months. After consultation, the clinic revised the incentive program to a daily drawing for attendance using the fishbowl method, thereby decreasing the delay between the behavior and its consequence. This example illustrates not only problems with delayed reinforcement but also the common mistake of setting expectations for the target behavior too high. Attending a month of group therapy sessions is a high bar that few patients will achieve, resulting in a system that mostly rewards those already doing well [19]. In contrast, attending a single group session in order to earn reinforcers is a reachable goal and increases the likelihood that more clients are exposed to the reinforcers. These small steps (ie, attending a single group or submitting a single drug negative urine) encourage initiation of the behavior(s) targeted. Other features, such as escalation (discussed next), aim to sustain the behavior over time.
Escalation
Escalation involves increasing the amount of reinforcement for each consecutive target behavior. In the voucher programs, the amount earned per negative sample may increase for each consecutive negative sample (eg, $2.50 for the first negative sample, $3.00 for the second, $3.50 for the third, and so on). For prize-based programs, the number of draws escalates with consecutive performance (eg, 1 draw for the first group attended, 2 draws for the second, 3 for the third, and so on). Protocols that include escalation generate larger effects than those that have a set, flat incentive amount even when total costs are the same across comparison conditions [73].
Escalating schedules usually include a reset feature. Following a positive or refused sample or unexcused absence, the amount earned for the next negative sample is reduced to the initial amount and begins escalating anew with consecutive negative samples. Some schedules allow for a rapid reset in which after a specified period of time or consecutive performance, the value jumps to the value achieved when the relapse occurred [66].
Despite its consistent inclusion in CM protocols from randomized clinical trials, our data [61] suggest that more than half of providers using incentives in treatment as part of a clinical effort do not use escalating reinforcers. Escalating schedules require more careful tracking of client progress, possibly contributing to lower uptake of this design feature in clinical practice. Development of simple tracking forms can minimize this challenge.
Another drawback of escalation pertaining to prize-based CM is that escalating schedules can affect the duration of CM sessions when clients are drawing a large number of slips each session and escalation can increase costs of the overall program. Capping the number of draws will help mitigate both issues. For example, once a client reaches 10 draws for group attendance, they continue earning 10 draws for each consecutive session attended with no further escalation.
Putting It All Together
CM sessions can be conducted as stand-alone sessions or incorporated into group or individual therapy sessions. Many clinicians will find the latter approach sets a positive tone for the therapy session given CM's focus on what the client is doing well. Starting the treatment session with the CM component often naturally leads into a discussion of relevant therapeutic issues, such as effective coping, slips, or triggers. The CM session length can be variable, but it is typically under 10 minutes. Thus, the CM component need not dominate the clinical session or content. CM sessions for abstinence are scheduled according to a set schedule (eg, Mondays and Thursdays) and often coincide with other treatment aspects (eg, before or after group therapy on Mondays and Thursdays). CM sessions for attendance also generally follow a set schedule (eg, client expected to attend Monday and Wednesday group therapy sessions). The duration of the CM protocol can also vary, with most clinical trials ranging from 12 to 24 weeks. Very short durations are unlikely to produce lasting behavior change, particularly with complex behaviors such as abstinence. Petry [44] recommends no less than 8 weeks duration and a maximum duration of 24 weeks.
As discussed, CM offers many opportunities for tailoring to optimize its fit within the existing structure of clinics. However, this flexibility must be viewed together with an understanding of the principles that impact CM's efficacy. Specific recommendations for CM protocol development will depend on the behavior targeted, the delivery methods, and format (eg, individual versus group settings). For these reasons, consultation with a CM expert is ideal. However, some general guidelines for developing a CM program that incorporate the principles discussed above include an 8- to 12-week program that (1) provides sufficient magnitude to compete with the behavior you are attempting to change, (2) offers frequent opportunities for reinforcement (eg, 2-3 times/wk for opioids or stimulant abstinence, 1-2 times/week for attendance targets; not less than weekly for most behaviors), (3) delivers the reinforcement immediately or very close in time with the behavior (eg, reinforce attendance at the beginning of the group, use onsite urine testing and reinforce immediately after testing), and (4) incorporates escalating and reset features into the schedule.
Clinician Training and Supervision
Training in CM is an important part of the implementation process. Studies [62,84–87] have identified a number of perceived barriers to and negative beliefs about CM, including philosophical and logistical concerns. Tangible incentives, the core of most CM protocols, are generally viewed less favorably than social or nonspecified incentives [84,86,87]. Philosophical concerns relate to CM’s inability to address the underlying causes of addiction, that it does not address multiple behaviors, and that it may undermine internal motivation for sobriety [62,84]. An additional objection relates to paying someone to do what they should do on their own [86]. Logistical and practical concerns often represent implementation barriers such as costs and access to training and supervision, but they also reflect concern for what happens when contingencies are withdrawn, that clients may sell or trade prizes for drugs, and worries that CM’s evidence does not generalize to clinic populations [62].
Many of these beliefs reflect a limited understanding of CM, and addressing these misperceptions is a first step toward reducing resistance to implementation efforts. For example, a substantial body of literature points to CM’s wide generalizability across a range of characteristics, clients that sell or trade prizes for drugs are likely to disrupt their chain of negative samples or attendance, and most studies do not find negative impacts of CM on intrinsic motivation [88–90]. Fortunately, CM training appears to be an effective way to address negative beliefs. In the VA implementation effort [52], training workshops decreased perceived barriers and increased positive impressions of CM [91]. In other training efforts, brief educational materials were effective in changing perceptions of CM’s efficacy [92].
Beyond initial training, supervision of CM delivery is likely to be necessary [93,94]. Clinician skill in delivering CM is related to client outcomes [93,95] and relatively simple adherence measures are available for monitoring [96,97]. However, the best methods for training and supervision of CM have yet to be established. The VA initiative was developed in consultation with CM experts and employed ongoing phone consultation following initial training workshops [52,91]. This approach represented significant investment by the VA toward staff training and CM protocol development that may not be achievable for individual clinics. As attention to CM’s dissemination and implementation has grown, some free resources have been developed. Promoting Awareness of Motivational Incentives (PAMI; www.bettertxoutcomes.org/bettertxoutcomes/PAMI.html) is a collaborative initiative sponsored by the National Institute of Drug Abuse and the Substance Abuse and Mental Health Services Administration. It offers free resources and training materials.
Conclusion
Overall, CM is a highly efficacious treatment for SUDs that generalizes to most clients. Despite a robust evidence base, CM’s implementation in clinical settings lags behind other empirically supported treatments [92]. At least in part, CM’s costs, which include not only staff training and adherence monitoring (as with other treatments), but also costs of the incentives themselves, may contribute to slow uptake in clinical settings. Clinics often do not have the resources available for CM within their operating budgets. However, a growing number of projects [19,52,53,55–57,70,71] illustrate CM implementation within routine clinical care, and increased revenue from improved attendance to treatment groups may be one mechanism through which to fund a CM program [54,56,57]. These projects are valuable not only for demonstrating that CM can be efficacious outside the research setting, but also for highlighting how implementation barriers can be overcome. Continued efforts of this nature are likely to be particularly valuable for clinicians and administrators considering adopting CM within clinical settings.
From UConn Health, Farmington, CT.
Abstract
- Objective: To discuss the efficacy and generalizability of contingency management (CM) for the treatment of substance use disorders and design considerations for those considering implementing in clinical settings.
- Methods: Review of the literature.
- Results: CM is an efficacious treatment for substance abuse disorders that is widely generalizable across substance use disorders and patient characteristics. CM can be implemented in a number of treatment programs, including residential and outpatient settings, and it can be administered in both individual and group formats. Abstinence and attendance are the most commonly targeted behaviors in substance abuse treatment settings. Design features, including the selection of target behaviors, delivery methods, and reinforcers, are discussed. Schedule parameters, such as frequency, magnitude, immediacy, and escalation of reinforcement, are associated with overall impact of the CM program and are important considerations for those interested in tailoring CM protocols to their needs.
- Conclusion: CM is an efficacious option that is applicable to most substance abuse treatment patients. A number of demonstrations of real-world implementation have been published and suggest CM can be adapted with success to clinic settings. In adopting CM protocols, clinics should aim for those protocols with established efficacy; however, if adaptations are necessary, careful consideration should be given to modifications to minimize risks of undermining CM’s effects.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Contingency management (CM) is a behavioral intervention that is efficacious in the treatment of substance use disorders (SUDs). CM uses a behavior analytic framework and applies principles of learning theory, particularly operant conditioning theory, to change client behavior(s) [1–5]. In basic terms, operant conditioning principles suggests that whether a behavior continues or not is a function of consequences [6]. Reinforced behaviors are more likely to occur in the future. Substance abuse can be viewed as a behavior maintained by the reinforcing effects of the drug itself [5], including the feel-good aspects of intoxication or relaxation and the amelioration of withdrawal symptoms. CM extends these same principles of to a treatment context, such that reinforcers for abstinent behavior are introduced to compete with the reinforcing effects of continued drug use [5].
In CM’s application to substance abuse treatment, drug-negative samples or treatment attendance are reinforced using tangible incentives with the goal of motivating continued abstinence and/or treatment engagement. When clients demonstrate these target behaviors, they earn incentives in the form of goods or services of value to the client, such as small electronics, gift cards, and toiletries. Despite the promising effects observed in research trials, real-world implementation efforts have not kept pace [7–9]. This review briefly discusses CM’s efficacy and highlights key features for professionals considering adopting this intervention. Demonstration efforts that illustrate how CM can be effectively implemented within the constraints and limitations of non-research, clinical settings are also presented.
Efficacy of CM
CM’s efficacy spans a number of SUDs, including cocaine, opioids, alcohol, nicotine, and marijuana [10–13], making it amenable for treatment of most SUD clinic populations. It generates larger effect sizes than other SUD treatments, including cognitive behavioral therapy [14], and it has been evaluated in a wide range of settings. Large-scale evaluations have been conducted in both intensive outpatient [15] and methadone maintenance [16] settings as part of the National Institute on Drug Abuse Clinical Trials Network, demonstrating consistent benefits of CM when added to treatment as usual. In the first of these 2 studies, Petry et al [15] randomized 415 stimulant users from 1 of 8 intensive outpatient clinics to treatment as usual or treatment as usual plus CM for alcohol and stimulant abstinence. CM participants submitted more substance-negative urine and breath samples, achieved continuous abstinence at significantly higher rates, and had longer treatment retention compared to those receiving treatment as usual. The parallel study [16] focused on stimulant use in clients from methadone maintenance clinics and found similar benefits of CM on stimulant abstinence. Beyond these settings, CM has been applied in a number of other contexts, including drop-in centers [17], vocational rehabilitation [18,19], job-skills training [20], and residential programs [21–23]. In addition, several group-based adaptations have been explored [17,24–27].
CM benefits most clients and generalizes across several demographic variables, including gender [28,29], race [30], housing status [31], and income levels [32–34]. Among clinical characteristics, CM is efficacious for those with co-occurring SUDs [35], other substance use [36], psychiatric disorders [37–39], medical problems [40–42], and history of transactional sex [43].
Design Considerations
Design features, including what behavior will be reinforced and how to do so, are among the first decision points for clinicians interested in implementing CM. One of the advantages of CM is that it has a high degree of flexibility in design, which means that it can be readily tailored to client populations and clinic needs. However, this flexibility can lead clinicians astray from the foundational principles of CM and unknowingly weaken the impact of the program. Below, some key considerations for CM protocol design are reviewed. For additional coverage of these topics, readers are referred to additional articles [1,2] or Petry’s comprehensive book on implementing CM [44]. In this review, published examples of CM’s application in real-world settings are presented, highlighting how CM has been adapted in these clinical efforts.
Target Behaviors
The selection of the target behavior will drive many of the subsequent program design decisions. As such, it is important to identify this feature early. Target behaviors must be achievable, objectively verifiable, and well defined. The most common CM targets are drug abstinence or therapy session attendance. CM has also been used to target other behaviors, such as medication adherence [45,46], treatment-related activities [47,48], and exercise [49–51]. Client self-report of behaviors or vaguely defined behaviors (eg, “good participation”) should be avoided. While some of the decisions related to CM protocols are flexible, the use of objectively verifiable target behaviors is a core feature that should not be neglected. If the behavior of interest cannot be objectively verified, an alternate behavior should be chosen.
Selection of the target behavior is often considered in hand with defining which population is eligible to participate in the CM program. Client characteristics are often forefront in this decision, but clinic-driven logistical issues or unmet needs may also play a role. A real-world example of this decision process is evident in the nationwide rollout of CM among the intensive outpatient programs within the Veterans Administration (VA). The VA identified a treatment need for those with stimulant use disorders, as this group did not have efficacious pharmacotherapy options available that targeted stimulant use. As such, the VA applied CM to patients with a focus on stimulant abstinence as the behavioral target [52]. For others, the decision may revolve around addressing underutilization of specific treatment resources (eg, outpatient groups, vocational rehabilitation) [53–56] or treatment needs among certain subgroups of clients, such as adolescents [57–59].
For abstinence targets, clinics would need to select one or more specific substances as the focus of the CM program. In general, targeting a single substance rather than multiple substances is more effective [10,13], is more straightforward for clients to understand, and allows more clients to access the reinforcers. Exposure to the reinforcers is necessary for CM to work; thus, setting a goal that is achievable for most clients should be a priority. Requiring abstinence from multiple substances means that some clients may never experience the reinforcer and thus cannot benefit from its effects at all. Some clinicians or administrators may initially have reservations about reinforcing single drug abstinence in the event that other drug use continues. However, targeting a single substance for reinforcement often results in reduced use of other substances [60]. Clinicians may find that this makes intuitive sense; a client with cocaine use disorder who is trying to maintain cocaine abstinence over a long period is likely to avoid using alcohol or other substances that might lead to relapse. For abstinence, objective verification through urine or breath specimens using tests that include validity checks is relatively straightforward.
Attendance is a popular target for clinics in part because it does not require additional staff time to collect specimen samples and it was the most commonly reported target behavior in samples of SUD providers who use incentives [61,62]. Objective verification of attendance is usually via a staff member, but expectations must be clear to both parties. Clinics should consider potential problems that may arise (eg, arriving late, leaving group early, excused absences) and carefully define and communicate expectations for the CM program. Piloting [19] the CM program with a small group of clients may be valuable in trouble-shooting challenges before wider implementation.
In a recent study [55], clients earned reinforcers for attending clinician-led group counseling sessions and/or the in-clinic patient-led Methadone Anonymous (MA) groups. This non-research, clinical effort addressed historically poor therapy attendance at the clinic, and attendance rates were compared before, during, and after the CM program. CM increased attendance to both groups in the short-term after implementation, but effects were more robust for the MA groups in which increased attendance persisted 3 months following the withdrawal of the contingencies. Overall effects of this program were modest, but they are notable given the use of an ultra-low cost approach.
Delivery Methods
The majority of CM studies used voucher or prize-based methods. Head-to-head comparisons suggest that they are comparable in efficacy [63–65], and each has advantages and disadvantages that may make one option more appealing for a given clinic. Voucher programs are generally straightforward to administer. Clients earn vouchers for each instance of the target behavior. The value of the vouchers typically increases with consecutive performance. The schedule used in the influential Higgins et al studies [66,67] started at $2.50 for the first cocaine-negative sample and increased by $0.50 for each subsequent consecutive cocaine-negative sample. Earned vouchers are exchanged for goods or services selected by the client, increasing the likelihood that the selected items will be highly desirable and allowing for a wide range of client preferences. Clients appear to prefer this approach when given a choice between set schedules or those that introduce an element of chance (ie, prize-based CM, discussed below) [68]. However, voucher programs can be costly (~$1000 per client over 12 weeks) and may require more staff time to fulfill individual requests for specific items. However, staff burden related to shopping can be reduced by limiting these individual requests and using an on-site stocked cabinet of goods similar to prize-CM programs.
Prize-based CM is similar but introduces probabilistic earnings and variability in prize magnitude. Rather than earning vouchers, clients earn draws from a fishbowl for each instance of the target behavior, again typically in an escalating manner. For example, a client may earn one draw from the fishbowl for the first cocaine-negative sample, 2 draws for the second consecutive negative sample, 3 draws for the third, and so on. A typical fishbowl is composed of 500 slips, some noting prizes and some having no prize value. Typically, half the slips in the bowl are non-monetary “good jobs.” The remaining half are small prizes worth about $1 in value (eg, food coupons, bus tokens, small toiletries), large prizes worth about $20 in value (eg, small electronics, gift certificates), and one slip is the jumbo prize worth about $100. When a client draws a winning slip, they select a prize from that category (ie, small, large, jumbo) from an onsite, stocked cabinet. Due to the probabilistic feature of prize-based CM, overall costs of the program can be substantially lower than typical voucher programs, with average maximum expected earnings ranging $250 t $450 per client over a 12-week treatment period [15,16,65,69]. Advantages of this method include potentially lower costs and minimal shopping demands (a once-monthly shopping trip to restock the cabinet will usually suffice) while maintaining comparable efficacy. Relative to voucher programs, prize-based CM involves additional administration time to allow for drawing slips from the fishbowl, which can be compounded when multiple clients want to draw at the same time such as in a group setting. Many of the group-based CM adaptations address this issue by limiting the number of clients who can draw for prizes in a given group or by limiting the number of draws per client [25,27,54].
Reinforcers
Regardless of whether selecting voucher or prize CM, reinforcers are critically important to the success of the program. Reinforcers must be desirable. One of the quickest ways to undermine a CM program is lack of variety or undesirable reinforcers. If stocking a cabinet with prizes onsite, care should be taken to have numerous options within each of the small and large prize categories that are appealing to a wide range of clients. Since a client who is consistently earning draws will choose prizes often, it is imperative to include enough variety so that even these clients find desirable items each time they select a prize. Clients should be asked regularly if they have suggestions for prizes; one program [54] found suggestion boxes useful for encouraging clients to voice their preferences. Donations can be solicited from local businesses to reduce costs [53], and low-cost but high-value options, such as clinic privileges, can also be explored. Petry [1,44] provides some suggestions of the latter, and Amass and Kamien [70] describe their successful strategies to fund and sustain a clinic-based CM program through community donations. Some clinics may already have tangible goods, such as gas or metro cards, that are offered to clients based on need rather than behavior [53]. These existing resources might be redirected to a CM program, in which these goods are contingent on abstinence or attendance, if appropriate.
Schedule Parameters
Once the target behavior, client population, and CM delivery methods are selected, the next step is to design the reinforcement schedule. The following schedule parameters apply to both voucher and prize-based CM systems. The more closely a clinical program adheres to the parameters of effective protocols, the more likely the program is to generate comparable outcomes. If there is a parameter or design feature that is incompatible with clinic needs, modifications can be introduced. However, each deviation away from the ideal has a chance of undermining the success of the CM program. Any changes and their potential impacts should considered carefully, and consultation with a CM expert may aid in the development of successful and efficacious clinic-based protocols. Of note, a meta-analysis [13] of CM studies found that researcher involvement in the planning and design of CM programs is associated with larger treatment effects. CM researchers are especially attuned to the potential impacts and pitfalls associated with modifying CM protocols, and they can be valuable resources for clinics interested in tailoring a CM program to their specific needs. Several examples of clinical demonstration projects that used researcher input are available [19,53,71].
Magnitude
Incentive magnitude was directly related to the size of treatment effects in a meta-analysis [11] of CM studies. Although not all studies find significant differences in outcomes related to magnitude [65,72], the bulk of evidence suggests magnitude is an important parameter and is related to effect size for both voucher [73–75] and prize-based CM [69,76] systems. Thus, although clinics may have restrictive budgets, severely undercutting the magnitude of rewards is not usually the solution as it can undermine treatment effects [76]. Donations can reduce overall costs [53,57,70], and other protocol features discussed below, such as capping the amount of reinforcement available, can reduce the overall magnitude available per patient.
Another approach, used in group-based CM, limits the number of patients who earn prizes per week [25,27]. For example, in a 2011 study by Petry et al, clients added slips with their name to a bowl for attendance and negative samples. Once all names were collected in the bowl, the group leader would pull a specified number of slips (eg, 3 slips per group). These individuals were eligible to draw from the prize bowl for prizes. This approach was associated with longer durations of consecutive abstinence and better treatment attendance relative to treatment as usual. However, clinics can control the overall program costs by limiting the number of patients eligible for prizes.
Frequency
Frequent reinforcement opportunities are ideal, and more frequent assessment is associated with larger treatment effects [10]. However, a number of factors, including which target behavior is selected and logistical issues specific to the clinic such as when groups meet, will play a role in determining the frequency of CM sessions. For abstinence targets, the substance targeted and type of test will largely determine the frequency of CM sessions. The goal would be to test at a frequency that would detect most or nearly all instances of use. For cocaine or opioids, this equates to testing 2 to 3 times weekly. Breath samples for alcohol or cigarette smoking would necessitate testing daily or multiple times per day to detect most instances of use because these tests have short windows of detection. CM protocols based on these breath tests have often had daily or twice daily CM sessions [77,78]; technological adaptations [77,79,80] or residential settings [21,23] may reduce burden to the client for assessment of these substances. Tapering the number of breath tests over time or transitioning from daily breath tests to once or twice weekly urine testing after abstinence is established is another approach [81,82].
Marijuana, on the other hand, poses difficulties because it is detectable in urine samples for up to 2 weeks following use. If relying solely on urine results for reinforcement, clients may not test negative for several days or weeks after last use, resulting in a delay of reinforcement. To address this issue, some CM programs targeting marijuana abstinence initially reinforce attendance in the first 2 weeks and then transition to reinforcing marijuana-negative drug samples for the remainder of the treatment period [48].
In general, more frequent CM sessions can translate to higher costs; however, infrequent reinforcement (ie, less than weekly) is not as effective [45]. In real-world applications, clinics often need to balance feasibility and costs with the ideal CM schedule. In abstinence-based CM, this compromise may result in a testing schedule that may not capture all instances of use. For example, while thrice-weekly testing may be ideal for cocaine or opioids, a twice-weekly schedule may be selected because it lowers costs and is more consistent with clinic schedules.
Immediacy
In general, clinics should aim to deliver reinforcement as immediately as possible, as delays between the target behavior and reinforcement are associated with decreased treatment effects [10,11,83]. For drug abstinence, onsite urine testing systems that provide immediate results are preferred over sending samples for laboratory testing. Clinics that do not have access to or who cannot afford specimen testing that allows onsite collection and immediate results might consider other options for target behaviors, such as attendance.
Immediacy of reinforcement is also important when targeting attendance. One clinic [53] implemented a program that offered a $50 incentive if clients attended 1 month of group therapy sessions. This approach was not effective and no clients earned the incentive for several months. After consultation, the clinic revised the incentive program to a daily drawing for attendance using the fishbowl method, thereby decreasing the delay between the behavior and its consequence. This example illustrates not only problems with delayed reinforcement but also the common mistake of setting expectations for the target behavior too high. Attending a month of group therapy sessions is a high bar that few patients will achieve, resulting in a system that mostly rewards those already doing well [19]. In contrast, attending a single group session in order to earn reinforcers is a reachable goal and increases the likelihood that more clients are exposed to the reinforcers. These small steps (ie, attending a single group or submitting a single drug negative urine) encourage initiation of the behavior(s) targeted. Other features, such as escalation (discussed next), aim to sustain the behavior over time.
Escalation
Escalation involves increasing the amount of reinforcement for each consecutive target behavior. In the voucher programs, the amount earned per negative sample may increase for each consecutive negative sample (eg, $2.50 for the first negative sample, $3.00 for the second, $3.50 for the third, and so on). For prize-based programs, the number of draws escalates with consecutive performance (eg, 1 draw for the first group attended, 2 draws for the second, 3 for the third, and so on). Protocols that include escalation generate larger effects than those that have a set, flat incentive amount even when total costs are the same across comparison conditions [73].
Escalating schedules usually include a reset feature. Following a positive or refused sample or unexcused absence, the amount earned for the next negative sample is reduced to the initial amount and begins escalating anew with consecutive negative samples. Some schedules allow for a rapid reset in which after a specified period of time or consecutive performance, the value jumps to the value achieved when the relapse occurred [66].
Despite its consistent inclusion in CM protocols from randomized clinical trials, our data [61] suggest that more than half of providers using incentives in treatment as part of a clinical effort do not use escalating reinforcers. Escalating schedules require more careful tracking of client progress, possibly contributing to lower uptake of this design feature in clinical practice. Development of simple tracking forms can minimize this challenge.
Another drawback of escalation pertaining to prize-based CM is that escalating schedules can affect the duration of CM sessions when clients are drawing a large number of slips each session and escalation can increase costs of the overall program. Capping the number of draws will help mitigate both issues. For example, once a client reaches 10 draws for group attendance, they continue earning 10 draws for each consecutive session attended with no further escalation.
Putting It All Together
CM sessions can be conducted as stand-alone sessions or incorporated into group or individual therapy sessions. Many clinicians will find the latter approach sets a positive tone for the therapy session given CM's focus on what the client is doing well. Starting the treatment session with the CM component often naturally leads into a discussion of relevant therapeutic issues, such as effective coping, slips, or triggers. The CM session length can be variable, but it is typically under 10 minutes. Thus, the CM component need not dominate the clinical session or content. CM sessions for abstinence are scheduled according to a set schedule (eg, Mondays and Thursdays) and often coincide with other treatment aspects (eg, before or after group therapy on Mondays and Thursdays). CM sessions for attendance also generally follow a set schedule (eg, client expected to attend Monday and Wednesday group therapy sessions). The duration of the CM protocol can also vary, with most clinical trials ranging from 12 to 24 weeks. Very short durations are unlikely to produce lasting behavior change, particularly with complex behaviors such as abstinence. Petry [44] recommends no less than 8 weeks duration and a maximum duration of 24 weeks.
As discussed, CM offers many opportunities for tailoring to optimize its fit within the existing structure of clinics. However, this flexibility must be viewed together with an understanding of the principles that impact CM's efficacy. Specific recommendations for CM protocol development will depend on the behavior targeted, the delivery methods, and format (eg, individual versus group settings). For these reasons, consultation with a CM expert is ideal. However, some general guidelines for developing a CM program that incorporate the principles discussed above include an 8- to 12-week program that (1) provides sufficient magnitude to compete with the behavior you are attempting to change, (2) offers frequent opportunities for reinforcement (eg, 2-3 times/wk for opioids or stimulant abstinence, 1-2 times/week for attendance targets; not less than weekly for most behaviors), (3) delivers the reinforcement immediately or very close in time with the behavior (eg, reinforce attendance at the beginning of the group, use onsite urine testing and reinforce immediately after testing), and (4) incorporates escalating and reset features into the schedule.
Clinician Training and Supervision
Training in CM is an important part of the implementation process. Studies [62,84–87] have identified a number of perceived barriers to and negative beliefs about CM, including philosophical and logistical concerns. Tangible incentives, the core of most CM protocols, are generally viewed less favorably than social or nonspecified incentives [84,86,87]. Philosophical concerns relate to CM’s inability to address the underlying causes of addiction, that it does not address multiple behaviors, and that it may undermine internal motivation for sobriety [62,84]. An additional objection relates to paying someone to do what they should do on their own [86]. Logistical and practical concerns often represent implementation barriers such as costs and access to training and supervision, but they also reflect concern for what happens when contingencies are withdrawn, that clients may sell or trade prizes for drugs, and worries that CM’s evidence does not generalize to clinic populations [62].
Many of these beliefs reflect a limited understanding of CM, and addressing these misperceptions is a first step toward reducing resistance to implementation efforts. For example, a substantial body of literature points to CM’s wide generalizability across a range of characteristics, clients that sell or trade prizes for drugs are likely to disrupt their chain of negative samples or attendance, and most studies do not find negative impacts of CM on intrinsic motivation [88–90]. Fortunately, CM training appears to be an effective way to address negative beliefs. In the VA implementation effort [52], training workshops decreased perceived barriers and increased positive impressions of CM [91]. In other training efforts, brief educational materials were effective in changing perceptions of CM’s efficacy [92].
Beyond initial training, supervision of CM delivery is likely to be necessary [93,94]. Clinician skill in delivering CM is related to client outcomes [93,95] and relatively simple adherence measures are available for monitoring [96,97]. However, the best methods for training and supervision of CM have yet to be established. The VA initiative was developed in consultation with CM experts and employed ongoing phone consultation following initial training workshops [52,91]. This approach represented significant investment by the VA toward staff training and CM protocol development that may not be achievable for individual clinics. As attention to CM’s dissemination and implementation has grown, some free resources have been developed. Promoting Awareness of Motivational Incentives (PAMI; www.bettertxoutcomes.org/bettertxoutcomes/PAMI.html) is a collaborative initiative sponsored by the National Institute of Drug Abuse and the Substance Abuse and Mental Health Services Administration. It offers free resources and training materials.
Conclusion
Overall, CM is a highly efficacious treatment for SUDs that generalizes to most clients. Despite a robust evidence base, CM’s implementation in clinical settings lags behind other empirically supported treatments [92]. At least in part, CM’s costs, which include not only staff training and adherence monitoring (as with other treatments), but also costs of the incentives themselves, may contribute to slow uptake in clinical settings. Clinics often do not have the resources available for CM within their operating budgets. However, a growing number of projects [19,52,53,55–57,70,71] illustrate CM implementation within routine clinical care, and increased revenue from improved attendance to treatment groups may be one mechanism through which to fund a CM program [54,56,57]. These projects are valuable not only for demonstrating that CM can be efficacious outside the research setting, but also for highlighting how implementation barriers can be overcome. Continued efforts of this nature are likely to be particularly valuable for clinicians and administrators considering adopting CM within clinical settings.
1. Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend 2000;58:9–25.
2. Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psychol 2006;2:411–34.
3. Meredith SE, Jarvis BP, Raiff BR, et al. The ABCs of incentive-based treatment in health care: A behavior analytic framework to inform research and practice. Psychol Res Behav Manag 2014;7:103–14.
4. Bigelow GE, Silverman K. Theoretical and empirical foundations of contingency management treatments for drug abuse. In: Higgins ST, Silverman K, editors. Motivating behavior change among illicit drug users. Washington DC: American Psychological Association; 1999: 15–31.
5. Higgins ST, Budney AJ, Bickel WK. Applying behavioral concepts and principles to the treatment of cocaine dependence. Drug Alcohol Depend 1994;34:87–97.
6. Skinner BF. Science and human behavior. New York: Macmillan; 1953.
7. Herbeck DM, Hser YI, Teruya C. Empirically supported substance abuse treatment approaches: A survey of treatment providers’ perspectives and practices. Addict Behav 2008;33:699–712.
8. McGovern MP, Fox TS, Xie H, Drake RE. A survey of clinical practices and readiness to adopt evidence-based practices: Dissemination research in an addiction treatment system. J Subst Abuse Treat 2004;26:305–12.
9. Willenbring ML, Kivlahan D, Kenny M, et al. Beliefs about evidence-based practices in addiction treatment: A survey of Veterans Administration program leaders. J Subst Abuse Treat 2004;26:79–85.
10. Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: A meta-analysis. Drug Alcohol Depend 2000;58:55–66.
11. Lussier JP, Heil SH, Mongeon JA, et al. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192–203.
12. Benishek LA, Dugosh KL, Kirby KC, et al. Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction 2014;109:1426–36.
13. Prendergast M, Podus D, Finney J, et al. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction 2006;101:1546–60.
14. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008;165:179–87.
15. Petry NM, Peirce JM, Stitzer ML, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2005;62:1148–56.
16. Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment - A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2006;63:201–8.
17. Petry NM, Martin B, Finocche C. Contingency management in group treatment: a demonstration project in an HIV drop-in center. J Subst Abuse Treat 2001;21:89–96.
18. Drebing CE, Van Ormer EA, Mueller L, et al. Adding contingency management intervention to vocational rehabilitation: outcomes for dually diagnosed veterans. J Rehabil Res Dev 2007;44:851–65.
19. Kellogg SH, Burns M, Coleman P, et al. Something of value: The introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. J Subst Abuse Treat 2005;28:57–65.
20. Koffarnus MN, Wong CJ, Fingerhood M, et al. Monetary incentives to reinforce engagement and achievement in a job-skills training program for homeless, unemployed adults. J Appl Behav Anal 2013;46:582–91.
21. Rohsenow D, Martin R, Tidey JW, et al. Treating smokers in substance treatment with contingent vouchers, nicotine replacement, and brief advice adapted for sobriety settings. J Subst Abuse Treat 2017.
22. Hunt YM, Rash CJ, Burke RS, Parker JD. Smoking cessation in recovery: Comparing two different cognitive behavioral treatments. Addict Disord Their Treat 2010;9:64–74.
23. Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reductions in residential substance abuse patients. J Appl Behav Anal 2008;41:617–22.
24. Petry NM, Weinstock J, Alessi SM, et al. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol 2010;78:89.
25. Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. J Consult Clin Psychol 2011;79:686–96.
26. Ledgerwood DM, Alessi SM, Hanson T, et al. Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. J Appl Behav Anal 2008;41:517–26.
27. Alessi SM, Hanson T, Wieners M, Petry NM. Low-cost contingency management in community clinics: Delivering incentives partially in group therapy. Exp Clin Psychopharmacol 2007;15:293–300.
28. Burch AE, Rash CJ, Petry NM. Sex effects in cocaine-using methadone patients randomized to contingency management interventions. Exp Clin Psychopharmacol 2015;23:284–90.
29. Rash CJ, Petry NM. Contingency management treatments are equally efficacious for both sexes in intensive outpatient settings. Exp Clin Psychopharmacol 2015;23:369–76.
30. Barry D, Sullivan B, Petry NM. Comparable efficacy of contingency management for cocaine dependence among African American, Hispanic, and White methadone maintenance clients. Psychol Addict Behav 2009;23:168–74.
31. Rash CJ, Alessi SM, Petry NM. Substance abuse treatment patients in housing programs respond to contingency management interventions. J Subst Abuse Treat 2017;72:97–102.
32. Rash CJ, Olmstead TA, Petry NM. Income does not affect response to contingency management treatments among community substance abuse treatment-seekers. Drug Alcohol Depend 2009;104:249–53.
33. Rash CJ, Andrade LF, Petry NM. Income received during treatment does not affect response to contingency management treatments in cocaine-dependent outpatients. Drug Alcohol Depend 2013;132:528–34.
34. Secades-Villa R, García-Fernández G, Peña-Suárez E, et al. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. J Subst Abuse Treat 2013;44:349–54.
35. Rash CJ, Alessi SM, Petry NM. Cocaine abusers with and without alcohol dependence respond equally well to contingency management treatments. Exp Clin Psychopharmacol 2008;16:275–81.
36. Alessi SM, Rash C, Petry NM. Contingency management is efficacious and improves outcomes in cocaine patients with pretreatment marijuana use. Drug Alcohol Depend 2011;118:62–7.
37. Ford JD, Hawke J, Alessi S, et al. Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behav Res Ther 2007;45:2417–31.
38. Weinstock J, Alessi SM, Petry NM. Regardless of psychiatric severity the addition of contingency management to standard treatment improves retention and drug use outcomes. Drug Alcohol Depend 2007;87:288–96.
39. García-Fernández G, Secades-Villa R, García-Rodríguez O, et al. Contingency management improves outcomes in cocaine-dependent outpatients with depressive symptoms. Exp Clin Psychopharmacol 2013;21:482–9.
40. Walter KN, Petry NM. Patients with diabetes respond well to contingency management treatment targeting alcohol and substance use. Psychol Health Med 2015;20:916–26.
41. Burch AE, Morasco BJ, Petry NM. Patients undergoing substance abuse treatment and receiving financial sssistance for a physical disability respond well to contingency management treatment. J Subst Abuse Treat 2015;58:67–71.
42. Burch AE, Rash CJ, Petry NM. Cocaine-using substance abuse treatment patients with and without HIV respond well to contingency management treatment. J Subst Abuse Treat 2017;77:21–5.
43. Rash CJ, Burki M, Montezuma-Rusca JM, Petry NM. A retrospective and prospective analysis of trading sex for drugs or money in women substance abuse treatment patients. Drug Alcohol Depend 2016;162:182–9.
44. Petry NM. Contingency management for substance abuse treatment: A guide to implementing this evidence-based practice. New York: Routledge; 2012.
45. Petry NM, Rash CJ, Byrne S, et al. Financial reinforcers for improving medication adherence: Findings from a meta-analysis. Am J Med 2012;125:888–96.
46. Herrmann ES, Matusiewicz AK, Stitzer ML, et al. Contingency management interventions for HIV, tuberculosis, and hepatitis control among individuals with substance use disorders: a systematized review. J Subst Abuse Treat 2017;72:117–25.
47. Petry NM, Alessi SM, Carroll KM, et al. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. J Consult Clin Psychol 2006;74:592–601.
48. Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: Randomized trial of contingency management and self-efficacy enhancement. Addict Behav 2013;38:1764–75.
49. Kurti AN, Dallery J. A laboratory-based evaluation of exercise plus contingency management for reducing cigarette smoking. Drug Alcohol Depend 2014;144:201–9.
50. Weinstock J, Capizzi J, Weber SM, et al. Exercise as an intervention for sedentary hazardous drinking college students: A pilot study. Ment Health Phys Act 2014;7:55–62.
51. Mitchell MS, Goodman JM, Alter DA, et al. Financial incentives for exercise adherence in adults: Systematic review and meta-analysis. Am J Prev Med 2013;45:658–67.
52. Petry NM, Dephilippis D, Rash CJ, et al. Nationwide dissemination of contingency management: The Veterans Administration initiative. Am J Addict 2014;23:205–10.
53. Walker R, Rosvall T, Field CA, et al. Disseminating contingency management to increase attendance in two community substance abuse treatment centers: Lessons learned. J Subst Abuse Treat 2010;39:202–9.
54. Sigmon SC, Stitzer ML. Use of a low-cost incentive intervention to improve counseling attendance among methadone-maintained patients. J Subst Abuse Treat 2005;29:253–8.
55. Kropp F, Lewis D, Winhusen T. The effectiveness of ultra-low magnitude reinforcers: Findings from a “real-world” application of contingency management. J Subst Abuse Treat 2017;72:111–6.
56. Fitzsimons H, Tuten M, Borsuk C, et al. Clinician-delivered contingency management increases engagement and attendance in drug and alcohol treatment. Drug Alcohol Depend 2015;152:62–7.
57. Lott DC, Jencius S. Effectiveness of very low-cost contingency management in a community adolescent treatment program. Drug Alcohol Depend 2009;102:162–5.
58. Henggeler SW, Chapman JE, Rowland MD, et al. Statewide adoption and initial implementation of contingency management for substance-abusing adolescents. 2008;76:556–67.
59. Henggeler SW, Chapman JE, Rowland MD, et al. If you build it , they will come: Statewide practitioner interest in contingency management for youths. 2007;32:121–31.
60. Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol 2000;68:250–7.
61. Rash CJ, Petry NM, Alessi SM. Examining implementation of contingency management in real-world settings [Abstract]. Alcohol Clin Exp Res 2016;20:103A.
62. Rash CJ, Petry NM, Kirby KC, et al. Identifying provider beliefs related to contingency management adoption using the Contingency Management Beliefs Questionnaire. Drug Alcohol Depend 2012;121:205–12.
63. Petry NM, Alessi SM, Marx J, et al. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. J Consult Clin Psychol 2005;73:1005–14.
64. Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol 2007;75:983–91.
65. Petry NM, Alessi SM, Barry D, Carroll KM. Standard magnitude prize reinforcers can be as efficacious as larger magnitude reinforcers in cocaine-dependent methadone patients. J Consult Clin Psychol 2015;83:464–72.
66. Higgins ST, Budney AJ, Bickel WK, et al. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry 1994;51:568.
67. Higgins ST, Wong CJ, Badger GJ, et al. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol 2000;68:64–72.
68. Hartzler B, Garrett S. Interest and preferences for contingency management design among addiction treatment clientele. Am J Drug Alcohol Abuse 2016;42:287–95.
69. Petry NM, Barry D, Alessi SM, et al. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol 2012;80:276–85.
70. Amass L, Kamien J. A tale of two cities: Financing two voucher programs for substance abusers through community donations. Exp Clin Psychopharmacol 2004;12:147–55.
71. Hartzler B. Building a bonfire that remains stoked: Sustainment of a contingency management intervention developed through collaborative design. Subst Abuse Treat Prev Policy 2015;10:30.
72. Carroll KM, Sinha R, Nich C, et al. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol 2002;10:54–63.
73. Roll JM, Shoptaw S. Contingency management: Schedule effects. Psychiatry Res 2006;144:91–3.
74. Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology (Berl) 1999;146:128–38.
75. Businelle MS, Rash CJ, Burke RS, Parker JD. Using vouchers to increase continuing care participation in veterans: does magnitude matter? Am J Addict 2009;18:122–9.
76. Petry NM, Tedford J, Austin M, et al. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction 2004;99:349–60.
77. Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction 2013;108:900–9.
78. Alessi SM, Petry NM. Smoking reductions and increased self-efficacy in a randomized controlled trial of smoking abstinence--contingent incentives in residential substance abuse treatment patients. Nicotine Tob Res 2014;16:1436–45.
79. Dougherty DM, Hill-Kapturczak N, Liang Y, et al. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend 2014;142:301–6.
80. Dallery J, Meredith S, Jarvis B, Nuzzo PA. Internet-based group contingency management to promote smoking abstinence. Exp Clin Psychopharmacol 2015;23:176–83.
81. Higgins ST, Washio Y, Lopez AA, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med (Baltim) 2014;68:51–7.
82. Higgins ST, Heil SH, Solomon LJ, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 2004;6:1015–20.
83. Packer RR, Howell DN, McPherson S, Roll JM. Investigating reinforcer magnitude and reinforcer delay: A contingency management analog study. Exp Clin Psychopharmacol 2012;20:287–92.
84. Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug Alcohol Depend 2006;85:19–27.
85. Cameron J, Ritter A. Contingency management: Perspectives of Australian service providers. 2007;26:183–9.
86. Hartzler B, Rabun C. Community opioid treatment perspectives on contingency management: Perceived feasibility, effectiveness, and transportability of social and financial incentives. J Subst Abuse Treat 2013;45:242–8.
87. Aletraris L, Shelton JS, Roman PM. Counselor attitudes toward contingency management for substance use disorder: Effectiveness, acceptability, and endorsement of incentives for treatment attendance and abstinence. J Subst Abuse Treat 2015;57:41–8.
88. Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol 2000;68:1051–61.
89. Ledgerwood DM, Petry NM. Does contingency management affect motivation to change substance use? Drug Alcohol Depend 2006;83:65–72.
90. Litt MD, Kadden RM, Kabela-Cormier E, Petry NM. Coping skills training and contingency management treatments for marijuana dependence: Exploring mechanisms of behavior change. Addiction 2008;103:638–48.
91. Rash CJ, DePhilippis D, McKay JR, et al. Training workshops positively impact beliefs about contingency management in a nationwide dissemination effort. J Subst Abuse Treat 2013;45:306–12.
92. Benishek LA, Kirby KC, Dugosh KL, Padovano A. Beliefs about the empirical support of drug abuse treatment interventions: A survey of outpatient treatment providers. Drug Alcohol Depend 2010;107:202–8.
93. Petry NM, Alessi SM, Ledgerwood DM. Contingency management delivered by community therapists in outpatient settings. Drug Alcohol Depend 2012;122:86–92.
94. Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. J Consult Clin Psychol 2012;80:286–98.
95. Hartzler B, Beadnell B, Donovan D. Predictive validity of addiction treatment clinicians’ post-training contingency management skills for subsequent clinical outcomes. J Subst Abuse Treat 2017.
96. Hartzler B. Adapting the Helpful Responses Questionnaire to assess communication skills involved in delivering contingency management: Preliminary psychometrics. J Subst Abuse Treat 2014;55:52–7.
97. Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the Contingency Management Competence Scale. Drug Alcohol Depend 2010;109:167–74.
1. Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend 2000;58:9–25.
2. Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psychol 2006;2:411–34.
3. Meredith SE, Jarvis BP, Raiff BR, et al. The ABCs of incentive-based treatment in health care: A behavior analytic framework to inform research and practice. Psychol Res Behav Manag 2014;7:103–14.
4. Bigelow GE, Silverman K. Theoretical and empirical foundations of contingency management treatments for drug abuse. In: Higgins ST, Silverman K, editors. Motivating behavior change among illicit drug users. Washington DC: American Psychological Association; 1999: 15–31.
5. Higgins ST, Budney AJ, Bickel WK. Applying behavioral concepts and principles to the treatment of cocaine dependence. Drug Alcohol Depend 1994;34:87–97.
6. Skinner BF. Science and human behavior. New York: Macmillan; 1953.
7. Herbeck DM, Hser YI, Teruya C. Empirically supported substance abuse treatment approaches: A survey of treatment providers’ perspectives and practices. Addict Behav 2008;33:699–712.
8. McGovern MP, Fox TS, Xie H, Drake RE. A survey of clinical practices and readiness to adopt evidence-based practices: Dissemination research in an addiction treatment system. J Subst Abuse Treat 2004;26:305–12.
9. Willenbring ML, Kivlahan D, Kenny M, et al. Beliefs about evidence-based practices in addiction treatment: A survey of Veterans Administration program leaders. J Subst Abuse Treat 2004;26:79–85.
10. Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: A meta-analysis. Drug Alcohol Depend 2000;58:55–66.
11. Lussier JP, Heil SH, Mongeon JA, et al. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192–203.
12. Benishek LA, Dugosh KL, Kirby KC, et al. Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction 2014;109:1426–36.
13. Prendergast M, Podus D, Finney J, et al. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction 2006;101:1546–60.
14. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008;165:179–87.
15. Petry NM, Peirce JM, Stitzer ML, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2005;62:1148–56.
16. Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment - A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2006;63:201–8.
17. Petry NM, Martin B, Finocche C. Contingency management in group treatment: a demonstration project in an HIV drop-in center. J Subst Abuse Treat 2001;21:89–96.
18. Drebing CE, Van Ormer EA, Mueller L, et al. Adding contingency management intervention to vocational rehabilitation: outcomes for dually diagnosed veterans. J Rehabil Res Dev 2007;44:851–65.
19. Kellogg SH, Burns M, Coleman P, et al. Something of value: The introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. J Subst Abuse Treat 2005;28:57–65.
20. Koffarnus MN, Wong CJ, Fingerhood M, et al. Monetary incentives to reinforce engagement and achievement in a job-skills training program for homeless, unemployed adults. J Appl Behav Anal 2013;46:582–91.
21. Rohsenow D, Martin R, Tidey JW, et al. Treating smokers in substance treatment with contingent vouchers, nicotine replacement, and brief advice adapted for sobriety settings. J Subst Abuse Treat 2017.
22. Hunt YM, Rash CJ, Burke RS, Parker JD. Smoking cessation in recovery: Comparing two different cognitive behavioral treatments. Addict Disord Their Treat 2010;9:64–74.
23. Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reductions in residential substance abuse patients. J Appl Behav Anal 2008;41:617–22.
24. Petry NM, Weinstock J, Alessi SM, et al. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol 2010;78:89.
25. Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. J Consult Clin Psychol 2011;79:686–96.
26. Ledgerwood DM, Alessi SM, Hanson T, et al. Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. J Appl Behav Anal 2008;41:517–26.
27. Alessi SM, Hanson T, Wieners M, Petry NM. Low-cost contingency management in community clinics: Delivering incentives partially in group therapy. Exp Clin Psychopharmacol 2007;15:293–300.
28. Burch AE, Rash CJ, Petry NM. Sex effects in cocaine-using methadone patients randomized to contingency management interventions. Exp Clin Psychopharmacol 2015;23:284–90.
29. Rash CJ, Petry NM. Contingency management treatments are equally efficacious for both sexes in intensive outpatient settings. Exp Clin Psychopharmacol 2015;23:369–76.
30. Barry D, Sullivan B, Petry NM. Comparable efficacy of contingency management for cocaine dependence among African American, Hispanic, and White methadone maintenance clients. Psychol Addict Behav 2009;23:168–74.
31. Rash CJ, Alessi SM, Petry NM. Substance abuse treatment patients in housing programs respond to contingency management interventions. J Subst Abuse Treat 2017;72:97–102.
32. Rash CJ, Olmstead TA, Petry NM. Income does not affect response to contingency management treatments among community substance abuse treatment-seekers. Drug Alcohol Depend 2009;104:249–53.
33. Rash CJ, Andrade LF, Petry NM. Income received during treatment does not affect response to contingency management treatments in cocaine-dependent outpatients. Drug Alcohol Depend 2013;132:528–34.
34. Secades-Villa R, García-Fernández G, Peña-Suárez E, et al. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. J Subst Abuse Treat 2013;44:349–54.
35. Rash CJ, Alessi SM, Petry NM. Cocaine abusers with and without alcohol dependence respond equally well to contingency management treatments. Exp Clin Psychopharmacol 2008;16:275–81.
36. Alessi SM, Rash C, Petry NM. Contingency management is efficacious and improves outcomes in cocaine patients with pretreatment marijuana use. Drug Alcohol Depend 2011;118:62–7.
37. Ford JD, Hawke J, Alessi S, et al. Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behav Res Ther 2007;45:2417–31.
38. Weinstock J, Alessi SM, Petry NM. Regardless of psychiatric severity the addition of contingency management to standard treatment improves retention and drug use outcomes. Drug Alcohol Depend 2007;87:288–96.
39. García-Fernández G, Secades-Villa R, García-Rodríguez O, et al. Contingency management improves outcomes in cocaine-dependent outpatients with depressive symptoms. Exp Clin Psychopharmacol 2013;21:482–9.
40. Walter KN, Petry NM. Patients with diabetes respond well to contingency management treatment targeting alcohol and substance use. Psychol Health Med 2015;20:916–26.
41. Burch AE, Morasco BJ, Petry NM. Patients undergoing substance abuse treatment and receiving financial sssistance for a physical disability respond well to contingency management treatment. J Subst Abuse Treat 2015;58:67–71.
42. Burch AE, Rash CJ, Petry NM. Cocaine-using substance abuse treatment patients with and without HIV respond well to contingency management treatment. J Subst Abuse Treat 2017;77:21–5.
43. Rash CJ, Burki M, Montezuma-Rusca JM, Petry NM. A retrospective and prospective analysis of trading sex for drugs or money in women substance abuse treatment patients. Drug Alcohol Depend 2016;162:182–9.
44. Petry NM. Contingency management for substance abuse treatment: A guide to implementing this evidence-based practice. New York: Routledge; 2012.
45. Petry NM, Rash CJ, Byrne S, et al. Financial reinforcers for improving medication adherence: Findings from a meta-analysis. Am J Med 2012;125:888–96.
46. Herrmann ES, Matusiewicz AK, Stitzer ML, et al. Contingency management interventions for HIV, tuberculosis, and hepatitis control among individuals with substance use disorders: a systematized review. J Subst Abuse Treat 2017;72:117–25.
47. Petry NM, Alessi SM, Carroll KM, et al. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. J Consult Clin Psychol 2006;74:592–601.
48. Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: Randomized trial of contingency management and self-efficacy enhancement. Addict Behav 2013;38:1764–75.
49. Kurti AN, Dallery J. A laboratory-based evaluation of exercise plus contingency management for reducing cigarette smoking. Drug Alcohol Depend 2014;144:201–9.
50. Weinstock J, Capizzi J, Weber SM, et al. Exercise as an intervention for sedentary hazardous drinking college students: A pilot study. Ment Health Phys Act 2014;7:55–62.
51. Mitchell MS, Goodman JM, Alter DA, et al. Financial incentives for exercise adherence in adults: Systematic review and meta-analysis. Am J Prev Med 2013;45:658–67.
52. Petry NM, Dephilippis D, Rash CJ, et al. Nationwide dissemination of contingency management: The Veterans Administration initiative. Am J Addict 2014;23:205–10.
53. Walker R, Rosvall T, Field CA, et al. Disseminating contingency management to increase attendance in two community substance abuse treatment centers: Lessons learned. J Subst Abuse Treat 2010;39:202–9.
54. Sigmon SC, Stitzer ML. Use of a low-cost incentive intervention to improve counseling attendance among methadone-maintained patients. J Subst Abuse Treat 2005;29:253–8.
55. Kropp F, Lewis D, Winhusen T. The effectiveness of ultra-low magnitude reinforcers: Findings from a “real-world” application of contingency management. J Subst Abuse Treat 2017;72:111–6.
56. Fitzsimons H, Tuten M, Borsuk C, et al. Clinician-delivered contingency management increases engagement and attendance in drug and alcohol treatment. Drug Alcohol Depend 2015;152:62–7.
57. Lott DC, Jencius S. Effectiveness of very low-cost contingency management in a community adolescent treatment program. Drug Alcohol Depend 2009;102:162–5.
58. Henggeler SW, Chapman JE, Rowland MD, et al. Statewide adoption and initial implementation of contingency management for substance-abusing adolescents. 2008;76:556–67.
59. Henggeler SW, Chapman JE, Rowland MD, et al. If you build it , they will come: Statewide practitioner interest in contingency management for youths. 2007;32:121–31.
60. Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol 2000;68:250–7.
61. Rash CJ, Petry NM, Alessi SM. Examining implementation of contingency management in real-world settings [Abstract]. Alcohol Clin Exp Res 2016;20:103A.
62. Rash CJ, Petry NM, Kirby KC, et al. Identifying provider beliefs related to contingency management adoption using the Contingency Management Beliefs Questionnaire. Drug Alcohol Depend 2012;121:205–12.
63. Petry NM, Alessi SM, Marx J, et al. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. J Consult Clin Psychol 2005;73:1005–14.
64. Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol 2007;75:983–91.
65. Petry NM, Alessi SM, Barry D, Carroll KM. Standard magnitude prize reinforcers can be as efficacious as larger magnitude reinforcers in cocaine-dependent methadone patients. J Consult Clin Psychol 2015;83:464–72.
66. Higgins ST, Budney AJ, Bickel WK, et al. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry 1994;51:568.
67. Higgins ST, Wong CJ, Badger GJ, et al. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol 2000;68:64–72.
68. Hartzler B, Garrett S. Interest and preferences for contingency management design among addiction treatment clientele. Am J Drug Alcohol Abuse 2016;42:287–95.
69. Petry NM, Barry D, Alessi SM, et al. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol 2012;80:276–85.
70. Amass L, Kamien J. A tale of two cities: Financing two voucher programs for substance abusers through community donations. Exp Clin Psychopharmacol 2004;12:147–55.
71. Hartzler B. Building a bonfire that remains stoked: Sustainment of a contingency management intervention developed through collaborative design. Subst Abuse Treat Prev Policy 2015;10:30.
72. Carroll KM, Sinha R, Nich C, et al. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol 2002;10:54–63.
73. Roll JM, Shoptaw S. Contingency management: Schedule effects. Psychiatry Res 2006;144:91–3.
74. Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology (Berl) 1999;146:128–38.
75. Businelle MS, Rash CJ, Burke RS, Parker JD. Using vouchers to increase continuing care participation in veterans: does magnitude matter? Am J Addict 2009;18:122–9.
76. Petry NM, Tedford J, Austin M, et al. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction 2004;99:349–60.
77. Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction 2013;108:900–9.
78. Alessi SM, Petry NM. Smoking reductions and increased self-efficacy in a randomized controlled trial of smoking abstinence--contingent incentives in residential substance abuse treatment patients. Nicotine Tob Res 2014;16:1436–45.
79. Dougherty DM, Hill-Kapturczak N, Liang Y, et al. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend 2014;142:301–6.
80. Dallery J, Meredith S, Jarvis B, Nuzzo PA. Internet-based group contingency management to promote smoking abstinence. Exp Clin Psychopharmacol 2015;23:176–83.
81. Higgins ST, Washio Y, Lopez AA, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med (Baltim) 2014;68:51–7.
82. Higgins ST, Heil SH, Solomon LJ, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 2004;6:1015–20.
83. Packer RR, Howell DN, McPherson S, Roll JM. Investigating reinforcer magnitude and reinforcer delay: A contingency management analog study. Exp Clin Psychopharmacol 2012;20:287–92.
84. Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug Alcohol Depend 2006;85:19–27.
85. Cameron J, Ritter A. Contingency management: Perspectives of Australian service providers. 2007;26:183–9.
86. Hartzler B, Rabun C. Community opioid treatment perspectives on contingency management: Perceived feasibility, effectiveness, and transportability of social and financial incentives. J Subst Abuse Treat 2013;45:242–8.
87. Aletraris L, Shelton JS, Roman PM. Counselor attitudes toward contingency management for substance use disorder: Effectiveness, acceptability, and endorsement of incentives for treatment attendance and abstinence. J Subst Abuse Treat 2015;57:41–8.
88. Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol 2000;68:1051–61.
89. Ledgerwood DM, Petry NM. Does contingency management affect motivation to change substance use? Drug Alcohol Depend 2006;83:65–72.
90. Litt MD, Kadden RM, Kabela-Cormier E, Petry NM. Coping skills training and contingency management treatments for marijuana dependence: Exploring mechanisms of behavior change. Addiction 2008;103:638–48.
91. Rash CJ, DePhilippis D, McKay JR, et al. Training workshops positively impact beliefs about contingency management in a nationwide dissemination effort. J Subst Abuse Treat 2013;45:306–12.
92. Benishek LA, Kirby KC, Dugosh KL, Padovano A. Beliefs about the empirical support of drug abuse treatment interventions: A survey of outpatient treatment providers. Drug Alcohol Depend 2010;107:202–8.
93. Petry NM, Alessi SM, Ledgerwood DM. Contingency management delivered by community therapists in outpatient settings. Drug Alcohol Depend 2012;122:86–92.
94. Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. J Consult Clin Psychol 2012;80:286–98.
95. Hartzler B, Beadnell B, Donovan D. Predictive validity of addiction treatment clinicians’ post-training contingency management skills for subsequent clinical outcomes. J Subst Abuse Treat 2017.
96. Hartzler B. Adapting the Helpful Responses Questionnaire to assess communication skills involved in delivering contingency management: Preliminary psychometrics. J Subst Abuse Treat 2014;55:52–7.
97. Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the Contingency Management Competence Scale. Drug Alcohol Depend 2010;109:167–74.
Flashback to 2016
For the final installment of this series, we “flashback” to our April 2016 issue, which featured a study examining 30-day complications among commercially insured adults undergoing colonoscopy with and without anesthesia-assisted sedation using Marketscan data (2008-2011).
Several letters to the editor challenged the methods used in this systematic review/meta-analysis, such that this question remains largely unresolved. What is clear is that we continue to lack an adequate understanding of which patients are most likely to benefit from anesthesia-assisted sedation, whether due to increased risk of failing standard sedation or increased risk of complications with standard sedation. This lack of clarity, as manifested in poorly specified guidelines, has fueled likely inappropriate allocation of monitored anesthesia care to low risk-patients (driven by a complex interplay of patient, provider, organizational, and economic factors), which has contributed to ballooning health care costs
Megan A. Adams, MS, JD, MSc, is a clinical lecturer in the division of gastroenterology at the University of Michigan, a gastroenterologist at the Ann Arbor Mich, VA, and an investigator in the VA Ann Arbor Center for Clinical Management Research. She is an associate editor of GI & Hepatology News.
For the final installment of this series, we “flashback” to our April 2016 issue, which featured a study examining 30-day complications among commercially insured adults undergoing colonoscopy with and without anesthesia-assisted sedation using Marketscan data (2008-2011).
Several letters to the editor challenged the methods used in this systematic review/meta-analysis, such that this question remains largely unresolved. What is clear is that we continue to lack an adequate understanding of which patients are most likely to benefit from anesthesia-assisted sedation, whether due to increased risk of failing standard sedation or increased risk of complications with standard sedation. This lack of clarity, as manifested in poorly specified guidelines, has fueled likely inappropriate allocation of monitored anesthesia care to low risk-patients (driven by a complex interplay of patient, provider, organizational, and economic factors), which has contributed to ballooning health care costs
Megan A. Adams, MS, JD, MSc, is a clinical lecturer in the division of gastroenterology at the University of Michigan, a gastroenterologist at the Ann Arbor Mich, VA, and an investigator in the VA Ann Arbor Center for Clinical Management Research. She is an associate editor of GI & Hepatology News.
For the final installment of this series, we “flashback” to our April 2016 issue, which featured a study examining 30-day complications among commercially insured adults undergoing colonoscopy with and without anesthesia-assisted sedation using Marketscan data (2008-2011).
Several letters to the editor challenged the methods used in this systematic review/meta-analysis, such that this question remains largely unresolved. What is clear is that we continue to lack an adequate understanding of which patients are most likely to benefit from anesthesia-assisted sedation, whether due to increased risk of failing standard sedation or increased risk of complications with standard sedation. This lack of clarity, as manifested in poorly specified guidelines, has fueled likely inappropriate allocation of monitored anesthesia care to low risk-patients (driven by a complex interplay of patient, provider, organizational, and economic factors), which has contributed to ballooning health care costs
Megan A. Adams, MS, JD, MSc, is a clinical lecturer in the division of gastroenterology at the University of Michigan, a gastroenterologist at the Ann Arbor Mich, VA, and an investigator in the VA Ann Arbor Center for Clinical Management Research. She is an associate editor of GI & Hepatology News.