User login
Visual Tools to Increase Patient Satisfaction: Just Decorative or Actually Effective?
Patient satisfaction and the ability to effectively communicate with hospitalized patients has become a core tenet to providing high-quality healthcare. Over the past few decades, medicine has gradually moved away from many paternalistic practices, and the profession has sought to engage patients as true partners in their own care. It is in this setting that effective communication has risen to be a key factor in the patient and provider relationship. It has also become a closely monitored quality metric tied to financial incentives and penalties. Most importantly, it has been well documented that failures in communication are a frequent cause of adverse events that compromise the ability of healthcare providers to provide safe and effective care.1 It is in this climate that healthcare systems have worked to implement solutions designed to engage patients and their families to improve their healthcare experience. These solutions vary from low to high tech and include patient whiteboards, provider face cards, and web-based patient portals. Despite the numerous innovative solutions being implemented by hospitalists, studies supporting their effectiveness are few. There continues to be limited evidence on the value of these practices and whether they positively impact the desired outcomes of patient satisfaction and engagement.
In this issue of the Journal of Hospital Medicine, Goyal et al.2 performed a systematic review to evaluate whether the use of bedside visual tools for hospitalized medical patients impacts patient satisfaction, patient–provider communication, and provider identification and understanding of roles. The authors were able to identify 16 studies that evaluated the use of these tools, which included provider face cards and whiteboards. The majority of the studies reviewed showed a positive effect on provider identification, understanding providers’ role, and patient satisfaction. The authors found that of the tools evaluated, whiteboards and picture-based techniques were the most effective visually based interventions. However, the authors also highlighted the difficulty in identifying 1 optimal approach to the use of these tools as a result of variations in content, format, and outcome measurement.
Variation in the use of visual tools to improve communication and patient satisfaction limits the ability to identify and evaluate the most effective approaches to their use. Without a streamlined approach, these tools may not produce the desired effect of improving patient and provider communication, which is essential in providing high-quality inpatient care and ensuring patient satisfaction. It has been documented that many patients cannot even identify their providers in the hospital setting, which limits the ability of the patient to be fully engaged in decisions made about their care.3 In addition, substantial portions of hospitalized patients do not understand their plan of care.4 Patients’ understanding of their plan of care is essential for patients to provide informed consent for hospital treatments and better prepare them to assume their own care after discharge, with a full understanding of their diagnosis.5 It has become increasingly clear that healthcare providers must incorporate effective approaches in their daily workflow to address these findings.
Aside from patient satisfaction and engagement, the effect communications failures have on patient safety have been evaluated and recognized. From the National Academy of Medicine’s report emphasizing patient-centered care to the addition of patients’ active engagement in their care as a National Patient Safety Goal by The Joint Commission, the medical field has committed to a continued focus in this area.5,6
The business case can also be made for identifying effective tools that improve patient satisfaction and patient–provider communication. Private and public health insurance providers have incentivized high performance in these areas and have now begun to levy penalties for underperformers. As patients’ level of satisfaction and engagement continue to be assessed via patient surveys, healthcare systems continue to search for effective practices to improve performance in patient-perceived provider communication. Patients’ reporting of their assessment of nurse and physician communication through questions such as “How often did nurses/doctors explain things in a way you could understand?” will continue to be a moving target requiring future studies of effective interventions
Are visual aids the effective tools that hospitals need to improve communication and patient satisfaction, or are they merely decorations? The whiteboard provides an excellent example of the effectiveness that can be seen with the use of these tools. Used to improve patient-provider communication in medicine, the whiteboard has become almost ubiquitous in patient hospital rooms.7 It is now an expected aspect of hospital design and has inspired the development of higher tech solutions, including patient tablets and media walls. It is known to enhance the interaction for both the provider and patient and facilitate the exchange of complicated medical information within an anxiety prone environment in a simple manner by using short phrases or drawings.6 Yet, there is a scarcity of strong evidence to support the most effective approach to the use of whiteboards in improving patient satisfaction and communication. Standardizing how the whiteboard is used during the patient interaction will allow for the effectiveness of this tool to be realized and evaluated and prevent it from becoming another ornamental fixture on our hospital walls.
The systematic review by Goyal et al.2 is a necessary step in the evaluation of common communication tools for their effectiveness and ability to improve patient satisfaction. This exhaustive review of key studies in this area is an excellent addition to the current literature, which has a paucity of extensive evaluations of these approaches. It provides an important signal that visual tools are more than decorative and can be effective when a streamlined approach is utilized. It highlights the importance of identifying effective best practices for the use of these tools that can be studied empirically and subsequently disseminated for widespread use. Continued work is necessary to fill this void and to enable healthcare professionals to provide the highest level of safe, effective, and engaging care that our patients deserve.
Disclosure
The authors have no conflicts of interest.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. PubMed
2. Goyal AA, Komalpreet T, Mann J, Townsend W, Flanders SA, Chopra V. Do bedside visual tools improve patient and caregiver satisfaction? A systematic review of the literature. J Hosp Med. 2017. In press. PubMed
3. Makaryus AN, Friedman EA. Does your patient know your name? An approach to enhancing patients’ awareness of their caretaker’s name. J Healthc Qual. 2005;27(4):53-56. PubMed
4. O’Leary KJ, Kulkarni N, Landler MP, et al. Hospitalized patients’ understanding of their plan of care. Mayo Clin Proc.2010;85(1):47-52. PubMed
5. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. [Internet] Washington, DC: National Academy Press; 2001. 8 p. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed on
6. The Joint Commission’s National Patient Safety Goals 2007 for Hospital/Critical Access Hospital. http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/07_hap_cah_npsgs.htm. Accessed on July 2017.
7. Singh S, Fletcher KE, Pandl GJ, et al. It’s the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual.2011;26(2):127-131. PubMed
Patient satisfaction and the ability to effectively communicate with hospitalized patients has become a core tenet to providing high-quality healthcare. Over the past few decades, medicine has gradually moved away from many paternalistic practices, and the profession has sought to engage patients as true partners in their own care. It is in this setting that effective communication has risen to be a key factor in the patient and provider relationship. It has also become a closely monitored quality metric tied to financial incentives and penalties. Most importantly, it has been well documented that failures in communication are a frequent cause of adverse events that compromise the ability of healthcare providers to provide safe and effective care.1 It is in this climate that healthcare systems have worked to implement solutions designed to engage patients and their families to improve their healthcare experience. These solutions vary from low to high tech and include patient whiteboards, provider face cards, and web-based patient portals. Despite the numerous innovative solutions being implemented by hospitalists, studies supporting their effectiveness are few. There continues to be limited evidence on the value of these practices and whether they positively impact the desired outcomes of patient satisfaction and engagement.
In this issue of the Journal of Hospital Medicine, Goyal et al.2 performed a systematic review to evaluate whether the use of bedside visual tools for hospitalized medical patients impacts patient satisfaction, patient–provider communication, and provider identification and understanding of roles. The authors were able to identify 16 studies that evaluated the use of these tools, which included provider face cards and whiteboards. The majority of the studies reviewed showed a positive effect on provider identification, understanding providers’ role, and patient satisfaction. The authors found that of the tools evaluated, whiteboards and picture-based techniques were the most effective visually based interventions. However, the authors also highlighted the difficulty in identifying 1 optimal approach to the use of these tools as a result of variations in content, format, and outcome measurement.
Variation in the use of visual tools to improve communication and patient satisfaction limits the ability to identify and evaluate the most effective approaches to their use. Without a streamlined approach, these tools may not produce the desired effect of improving patient and provider communication, which is essential in providing high-quality inpatient care and ensuring patient satisfaction. It has been documented that many patients cannot even identify their providers in the hospital setting, which limits the ability of the patient to be fully engaged in decisions made about their care.3 In addition, substantial portions of hospitalized patients do not understand their plan of care.4 Patients’ understanding of their plan of care is essential for patients to provide informed consent for hospital treatments and better prepare them to assume their own care after discharge, with a full understanding of their diagnosis.5 It has become increasingly clear that healthcare providers must incorporate effective approaches in their daily workflow to address these findings.
Aside from patient satisfaction and engagement, the effect communications failures have on patient safety have been evaluated and recognized. From the National Academy of Medicine’s report emphasizing patient-centered care to the addition of patients’ active engagement in their care as a National Patient Safety Goal by The Joint Commission, the medical field has committed to a continued focus in this area.5,6
The business case can also be made for identifying effective tools that improve patient satisfaction and patient–provider communication. Private and public health insurance providers have incentivized high performance in these areas and have now begun to levy penalties for underperformers. As patients’ level of satisfaction and engagement continue to be assessed via patient surveys, healthcare systems continue to search for effective practices to improve performance in patient-perceived provider communication. Patients’ reporting of their assessment of nurse and physician communication through questions such as “How often did nurses/doctors explain things in a way you could understand?” will continue to be a moving target requiring future studies of effective interventions
Are visual aids the effective tools that hospitals need to improve communication and patient satisfaction, or are they merely decorations? The whiteboard provides an excellent example of the effectiveness that can be seen with the use of these tools. Used to improve patient-provider communication in medicine, the whiteboard has become almost ubiquitous in patient hospital rooms.7 It is now an expected aspect of hospital design and has inspired the development of higher tech solutions, including patient tablets and media walls. It is known to enhance the interaction for both the provider and patient and facilitate the exchange of complicated medical information within an anxiety prone environment in a simple manner by using short phrases or drawings.6 Yet, there is a scarcity of strong evidence to support the most effective approach to the use of whiteboards in improving patient satisfaction and communication. Standardizing how the whiteboard is used during the patient interaction will allow for the effectiveness of this tool to be realized and evaluated and prevent it from becoming another ornamental fixture on our hospital walls.
The systematic review by Goyal et al.2 is a necessary step in the evaluation of common communication tools for their effectiveness and ability to improve patient satisfaction. This exhaustive review of key studies in this area is an excellent addition to the current literature, which has a paucity of extensive evaluations of these approaches. It provides an important signal that visual tools are more than decorative and can be effective when a streamlined approach is utilized. It highlights the importance of identifying effective best practices for the use of these tools that can be studied empirically and subsequently disseminated for widespread use. Continued work is necessary to fill this void and to enable healthcare professionals to provide the highest level of safe, effective, and engaging care that our patients deserve.
Disclosure
The authors have no conflicts of interest.
Patient satisfaction and the ability to effectively communicate with hospitalized patients has become a core tenet to providing high-quality healthcare. Over the past few decades, medicine has gradually moved away from many paternalistic practices, and the profession has sought to engage patients as true partners in their own care. It is in this setting that effective communication has risen to be a key factor in the patient and provider relationship. It has also become a closely monitored quality metric tied to financial incentives and penalties. Most importantly, it has been well documented that failures in communication are a frequent cause of adverse events that compromise the ability of healthcare providers to provide safe and effective care.1 It is in this climate that healthcare systems have worked to implement solutions designed to engage patients and their families to improve their healthcare experience. These solutions vary from low to high tech and include patient whiteboards, provider face cards, and web-based patient portals. Despite the numerous innovative solutions being implemented by hospitalists, studies supporting their effectiveness are few. There continues to be limited evidence on the value of these practices and whether they positively impact the desired outcomes of patient satisfaction and engagement.
In this issue of the Journal of Hospital Medicine, Goyal et al.2 performed a systematic review to evaluate whether the use of bedside visual tools for hospitalized medical patients impacts patient satisfaction, patient–provider communication, and provider identification and understanding of roles. The authors were able to identify 16 studies that evaluated the use of these tools, which included provider face cards and whiteboards. The majority of the studies reviewed showed a positive effect on provider identification, understanding providers’ role, and patient satisfaction. The authors found that of the tools evaluated, whiteboards and picture-based techniques were the most effective visually based interventions. However, the authors also highlighted the difficulty in identifying 1 optimal approach to the use of these tools as a result of variations in content, format, and outcome measurement.
Variation in the use of visual tools to improve communication and patient satisfaction limits the ability to identify and evaluate the most effective approaches to their use. Without a streamlined approach, these tools may not produce the desired effect of improving patient and provider communication, which is essential in providing high-quality inpatient care and ensuring patient satisfaction. It has been documented that many patients cannot even identify their providers in the hospital setting, which limits the ability of the patient to be fully engaged in decisions made about their care.3 In addition, substantial portions of hospitalized patients do not understand their plan of care.4 Patients’ understanding of their plan of care is essential for patients to provide informed consent for hospital treatments and better prepare them to assume their own care after discharge, with a full understanding of their diagnosis.5 It has become increasingly clear that healthcare providers must incorporate effective approaches in their daily workflow to address these findings.
Aside from patient satisfaction and engagement, the effect communications failures have on patient safety have been evaluated and recognized. From the National Academy of Medicine’s report emphasizing patient-centered care to the addition of patients’ active engagement in their care as a National Patient Safety Goal by The Joint Commission, the medical field has committed to a continued focus in this area.5,6
The business case can also be made for identifying effective tools that improve patient satisfaction and patient–provider communication. Private and public health insurance providers have incentivized high performance in these areas and have now begun to levy penalties for underperformers. As patients’ level of satisfaction and engagement continue to be assessed via patient surveys, healthcare systems continue to search for effective practices to improve performance in patient-perceived provider communication. Patients’ reporting of their assessment of nurse and physician communication through questions such as “How often did nurses/doctors explain things in a way you could understand?” will continue to be a moving target requiring future studies of effective interventions
Are visual aids the effective tools that hospitals need to improve communication and patient satisfaction, or are they merely decorations? The whiteboard provides an excellent example of the effectiveness that can be seen with the use of these tools. Used to improve patient-provider communication in medicine, the whiteboard has become almost ubiquitous in patient hospital rooms.7 It is now an expected aspect of hospital design and has inspired the development of higher tech solutions, including patient tablets and media walls. It is known to enhance the interaction for both the provider and patient and facilitate the exchange of complicated medical information within an anxiety prone environment in a simple manner by using short phrases or drawings.6 Yet, there is a scarcity of strong evidence to support the most effective approach to the use of whiteboards in improving patient satisfaction and communication. Standardizing how the whiteboard is used during the patient interaction will allow for the effectiveness of this tool to be realized and evaluated and prevent it from becoming another ornamental fixture on our hospital walls.
The systematic review by Goyal et al.2 is a necessary step in the evaluation of common communication tools for their effectiveness and ability to improve patient satisfaction. This exhaustive review of key studies in this area is an excellent addition to the current literature, which has a paucity of extensive evaluations of these approaches. It provides an important signal that visual tools are more than decorative and can be effective when a streamlined approach is utilized. It highlights the importance of identifying effective best practices for the use of these tools that can be studied empirically and subsequently disseminated for widespread use. Continued work is necessary to fill this void and to enable healthcare professionals to provide the highest level of safe, effective, and engaging care that our patients deserve.
Disclosure
The authors have no conflicts of interest.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. PubMed
2. Goyal AA, Komalpreet T, Mann J, Townsend W, Flanders SA, Chopra V. Do bedside visual tools improve patient and caregiver satisfaction? A systematic review of the literature. J Hosp Med. 2017. In press. PubMed
3. Makaryus AN, Friedman EA. Does your patient know your name? An approach to enhancing patients’ awareness of their caretaker’s name. J Healthc Qual. 2005;27(4):53-56. PubMed
4. O’Leary KJ, Kulkarni N, Landler MP, et al. Hospitalized patients’ understanding of their plan of care. Mayo Clin Proc.2010;85(1):47-52. PubMed
5. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. [Internet] Washington, DC: National Academy Press; 2001. 8 p. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed on
6. The Joint Commission’s National Patient Safety Goals 2007 for Hospital/Critical Access Hospital. http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/07_hap_cah_npsgs.htm. Accessed on July 2017.
7. Singh S, Fletcher KE, Pandl GJ, et al. It’s the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual.2011;26(2):127-131. PubMed
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. PubMed
2. Goyal AA, Komalpreet T, Mann J, Townsend W, Flanders SA, Chopra V. Do bedside visual tools improve patient and caregiver satisfaction? A systematic review of the literature. J Hosp Med. 2017. In press. PubMed
3. Makaryus AN, Friedman EA. Does your patient know your name? An approach to enhancing patients’ awareness of their caretaker’s name. J Healthc Qual. 2005;27(4):53-56. PubMed
4. O’Leary KJ, Kulkarni N, Landler MP, et al. Hospitalized patients’ understanding of their plan of care. Mayo Clin Proc.2010;85(1):47-52. PubMed
5. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. [Internet] Washington, DC: National Academy Press; 2001. 8 p. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed on
6. The Joint Commission’s National Patient Safety Goals 2007 for Hospital/Critical Access Hospital. http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/07_hap_cah_npsgs.htm. Accessed on July 2017.
7. Singh S, Fletcher KE, Pandl GJ, et al. It’s the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual.2011;26(2):127-131. PubMed
© 2017 Society of Hospital Medicine
MACRA Monday: Colorectal cancer screening
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #113: Colorectal Cancer Screening
This measure is aimed at capturing the percentage of patients aged 50-75 years who were screened for colorectal cancer.
What you need to do: The patient should be screened for colorectal cancer during calendar 2017 (or specified alternative time frame) using an appropriate test. Document the screening and results in the medical record.
Appropriate tests include:
- Fecal occult blood test (FOBT) during the performance period (calendar 2017).
- Flexible sigmoidoscopy during the performance period or the 4 years prior.
- Colonoscopy during the performance period or the 9 years prior.
- Computed tomography (CT) colonography during the performance period or the 4 years prior.
- Fecal immunochemical DNA test (FIT-DNA) during the measurement period or the 2 years prior.
Eligible cases include patients aged 50-75 years of age on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3017F indicates that colorectal cancer screening results were documented and reviewed. The exclusion code G9711 should be used for patients with a diagnosis or past history of total colectomy or colorectal cancer.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #113: Colorectal Cancer Screening
This measure is aimed at capturing the percentage of patients aged 50-75 years who were screened for colorectal cancer.
What you need to do: The patient should be screened for colorectal cancer during calendar 2017 (or specified alternative time frame) using an appropriate test. Document the screening and results in the medical record.
Appropriate tests include:
- Fecal occult blood test (FOBT) during the performance period (calendar 2017).
- Flexible sigmoidoscopy during the performance period or the 4 years prior.
- Colonoscopy during the performance period or the 9 years prior.
- Computed tomography (CT) colonography during the performance period or the 4 years prior.
- Fecal immunochemical DNA test (FIT-DNA) during the measurement period or the 2 years prior.
Eligible cases include patients aged 50-75 years of age on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3017F indicates that colorectal cancer screening results were documented and reviewed. The exclusion code G9711 should be used for patients with a diagnosis or past history of total colectomy or colorectal cancer.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #113: Colorectal Cancer Screening
This measure is aimed at capturing the percentage of patients aged 50-75 years who were screened for colorectal cancer.
What you need to do: The patient should be screened for colorectal cancer during calendar 2017 (or specified alternative time frame) using an appropriate test. Document the screening and results in the medical record.
Appropriate tests include:
- Fecal occult blood test (FOBT) during the performance period (calendar 2017).
- Flexible sigmoidoscopy during the performance period or the 4 years prior.
- Colonoscopy during the performance period or the 9 years prior.
- Computed tomography (CT) colonography during the performance period or the 4 years prior.
- Fecal immunochemical DNA test (FIT-DNA) during the measurement period or the 2 years prior.
Eligible cases include patients aged 50-75 years of age on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3017F indicates that colorectal cancer screening results were documented and reviewed. The exclusion code G9711 should be used for patients with a diagnosis or past history of total colectomy or colorectal cancer.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
FDA Boxed Warning Updates
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
PRANDIMET (METFORMIN HYDROCHLORIDE; REPAGLINIDE):
- Edited boxed warning April 7, 2017
Post-marketing cases of metforminassociated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, carbonic anyhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue PrandiMet and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
AVANDAMET (METFORMIN HYDROCHLORIDE; ROSIGLITAZONE MALEATE):
- Edited and updated boxed warning April 7, 2017
WARNING: CONGESTIVE HEART FAILURE and LACTIC ACIDOSIS
Rosiglitazone maleate: CONGESTIVE HEART FAILURE
- Thiazolidinediones, including rosiglitazone, cause or exacerbate congestive heart failure in some patients. After initiation of Avandamet, and after dose increases, observe patients carefully for signs and symptoms of heart failure (including excessive, rapid weight gain, dyspnea, and/or edema). If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of Avandamet must be considered.
- Avandamet is not recommended in patients with symptomatic heart failure. Initiation of Avandamet in patients with established NYHA Class III or IV heart failure is contraindicated.
Metformin hydrochloride: LACTIC ACIDOSIS
- Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin- associated lactic acidosis often subtle, accompanied only by nonspeci c symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin- associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), and increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
- Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, carbonic anhydrase
inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment. Steps to reduce the risk of and manage metformin-associated lactic acidosis in these highrisk groups are provided in the Full Prescribing Information. - If metformin-associated lactic acidosis is suspected, immediately discontinue Avandamet and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
OPTIRAY (160, 240, 300, 320, AND 350):
- Edited boxed warning April 7, 2017
PLR conversion, addition of the following:
WARNING: NOT FOR INTRATHECAL USE
Inadvertent intrathecal administration may cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema.
GLUMETZA (METFORMIN HYDROCHLORIDE):
- Edited boxed warning April 7, 2017
LACTIC ACIDOSIS
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metforminassociated lactic acidosis is often subtle, accompanied only by nonspeci c symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio, and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue Glumetza and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
SABRIL (VIGABATRIN):
- Edited boxed warning April 7, 2017
WARNING: PERMANENT VISION LOSS
Because of the risk of permanent vision loss, Sabril is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Vigabatrin REMS Program. Further information is available at www.vigabatrinREMS.com or 1-866-244-8175.
VALCYTE (VALGANCICLOVIR HYDROCHLORIDE):
- Edited boxed warning April 7, 2017
WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY, MUTAGENESIS AND CARCINOGENESIS
- Hematologic Toxicity: Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, and bone marrow failure including aplastic anemia have been reported in patients treated with Valcyte.
- Impairment of Fertility: Based on animal data, Valcyte may cause temporary or permanent inhibition of spermatogenesis in males and suppression of fertility in females.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
PRANDIMET (METFORMIN HYDROCHLORIDE; REPAGLINIDE):
- Edited boxed warning April 7, 2017
Post-marketing cases of metforminassociated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, carbonic anyhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue PrandiMet and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
AVANDAMET (METFORMIN HYDROCHLORIDE; ROSIGLITAZONE MALEATE):
- Edited and updated boxed warning April 7, 2017
WARNING: CONGESTIVE HEART FAILURE and LACTIC ACIDOSIS
Rosiglitazone maleate: CONGESTIVE HEART FAILURE
- Thiazolidinediones, including rosiglitazone, cause or exacerbate congestive heart failure in some patients. After initiation of Avandamet, and after dose increases, observe patients carefully for signs and symptoms of heart failure (including excessive, rapid weight gain, dyspnea, and/or edema). If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of Avandamet must be considered.
- Avandamet is not recommended in patients with symptomatic heart failure. Initiation of Avandamet in patients with established NYHA Class III or IV heart failure is contraindicated.
Metformin hydrochloride: LACTIC ACIDOSIS
- Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin- associated lactic acidosis often subtle, accompanied only by nonspeci c symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin- associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), and increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
- Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, carbonic anhydrase
inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment. Steps to reduce the risk of and manage metformin-associated lactic acidosis in these highrisk groups are provided in the Full Prescribing Information. - If metformin-associated lactic acidosis is suspected, immediately discontinue Avandamet and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
OPTIRAY (160, 240, 300, 320, AND 350):
- Edited boxed warning April 7, 2017
PLR conversion, addition of the following:
WARNING: NOT FOR INTRATHECAL USE
Inadvertent intrathecal administration may cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema.
GLUMETZA (METFORMIN HYDROCHLORIDE):
- Edited boxed warning April 7, 2017
LACTIC ACIDOSIS
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metforminassociated lactic acidosis is often subtle, accompanied only by nonspeci c symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio, and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue Glumetza and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
SABRIL (VIGABATRIN):
- Edited boxed warning April 7, 2017
WARNING: PERMANENT VISION LOSS
Because of the risk of permanent vision loss, Sabril is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Vigabatrin REMS Program. Further information is available at www.vigabatrinREMS.com or 1-866-244-8175.
VALCYTE (VALGANCICLOVIR HYDROCHLORIDE):
- Edited boxed warning April 7, 2017
WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY, MUTAGENESIS AND CARCINOGENESIS
- Hematologic Toxicity: Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, and bone marrow failure including aplastic anemia have been reported in patients treated with Valcyte.
- Impairment of Fertility: Based on animal data, Valcyte may cause temporary or permanent inhibition of spermatogenesis in males and suppression of fertility in females.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
PRANDIMET (METFORMIN HYDROCHLORIDE; REPAGLINIDE):
- Edited boxed warning April 7, 2017
Post-marketing cases of metforminassociated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, carbonic anyhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue PrandiMet and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
AVANDAMET (METFORMIN HYDROCHLORIDE; ROSIGLITAZONE MALEATE):
- Edited and updated boxed warning April 7, 2017
WARNING: CONGESTIVE HEART FAILURE and LACTIC ACIDOSIS
Rosiglitazone maleate: CONGESTIVE HEART FAILURE
- Thiazolidinediones, including rosiglitazone, cause or exacerbate congestive heart failure in some patients. After initiation of Avandamet, and after dose increases, observe patients carefully for signs and symptoms of heart failure (including excessive, rapid weight gain, dyspnea, and/or edema). If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of Avandamet must be considered.
- Avandamet is not recommended in patients with symptomatic heart failure. Initiation of Avandamet in patients with established NYHA Class III or IV heart failure is contraindicated.
Metformin hydrochloride: LACTIC ACIDOSIS
- Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin- associated lactic acidosis often subtle, accompanied only by nonspeci c symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin- associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), and increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
- Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, carbonic anhydrase
inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment. Steps to reduce the risk of and manage metformin-associated lactic acidosis in these highrisk groups are provided in the Full Prescribing Information. - If metformin-associated lactic acidosis is suspected, immediately discontinue Avandamet and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
OPTIRAY (160, 240, 300, 320, AND 350):
- Edited boxed warning April 7, 2017
PLR conversion, addition of the following:
WARNING: NOT FOR INTRATHECAL USE
Inadvertent intrathecal administration may cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema.
GLUMETZA (METFORMIN HYDROCHLORIDE):
- Edited boxed warning April 7, 2017
LACTIC ACIDOSIS
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metforminassociated lactic acidosis is often subtle, accompanied only by nonspeci c symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio, and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (eg, carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (eg, acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue Glumetza and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
SABRIL (VIGABATRIN):
- Edited boxed warning April 7, 2017
WARNING: PERMANENT VISION LOSS
Because of the risk of permanent vision loss, Sabril is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Vigabatrin REMS Program. Further information is available at www.vigabatrinREMS.com or 1-866-244-8175.
VALCYTE (VALGANCICLOVIR HYDROCHLORIDE):
- Edited boxed warning April 7, 2017
WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY, MUTAGENESIS AND CARCINOGENESIS
- Hematologic Toxicity: Severe leukopenia, neutropenia, anemia, thrombocytopenia, pancytopenia, and bone marrow failure including aplastic anemia have been reported in patients treated with Valcyte.
- Impairment of Fertility: Based on animal data, Valcyte may cause temporary or permanent inhibition of spermatogenesis in males and suppression of fertility in females.
JZP-110 improves excessive sleepiness in OSA patients
The selective dopamine norepinephrine reuptake inhibitor JZP-110 was effective at treating excessive sleepiness in obstructive sleep apnea patients, according to an abstract on a study to be presented at the CHEST annual meeting.
In a 12-week, phase 3 trial, adult patients with obstructive sleep apnea were randomized to receive placebo or once-daily JZP-110 at dosages of 37.5 mg, 75 mg, 150 mg, or 300 mg. A total of 459 patients were included in the final analysis.
At the end of the trial, the mean reduction from baseline on a maintenance of wakefulness test was 0.2 minutes for patients in the placebo group, 4.7 minutes in the 37.5-mg group, 9.1 minutes in the 75-mg group, 11 minutes in the 150-mg group, and 13 minutes in the 300-mg group. Mean changes in Epworth Sleepiness Scale scores were –3.3 for patients in the placebo group, –5.1 in the 37.5-mg group, –5 in the 75-mg group, –7.7 in the 150-mg group, and –7.9 in the 300-mg group.
A significantly higher rate of patients in the 75-mg (72.4%), 150-mg (89.7%), and 300-mg (88.7%) groups improved on the Patient Global Impression of Change scale, compared with patients in the placebo (49.1%) and 37.5-mg (55.4%) groups.
Just under 68% of patients who received JZP-110 experienced at least one adverse event, compared with 47.9% of patients who received placebo. The most common adverse events were headache, nausea, decreased appetite, and anxiety. Six serious adverse events were reported over the study period, but none was related to JZP-110.
The study is scheduled to be presented on Sunday, Oct. 29, from 1:30 p.m. to 1:45 p.m. in Room 601A of the Toronto Convention Centre South Building as part of the “Obstructive Sleep Apnea: Insights & Management” session, which will run from 1:30 p.m. to 3 p.m.
The selective dopamine norepinephrine reuptake inhibitor JZP-110 was effective at treating excessive sleepiness in obstructive sleep apnea patients, according to an abstract on a study to be presented at the CHEST annual meeting.
In a 12-week, phase 3 trial, adult patients with obstructive sleep apnea were randomized to receive placebo or once-daily JZP-110 at dosages of 37.5 mg, 75 mg, 150 mg, or 300 mg. A total of 459 patients were included in the final analysis.
At the end of the trial, the mean reduction from baseline on a maintenance of wakefulness test was 0.2 minutes for patients in the placebo group, 4.7 minutes in the 37.5-mg group, 9.1 minutes in the 75-mg group, 11 minutes in the 150-mg group, and 13 minutes in the 300-mg group. Mean changes in Epworth Sleepiness Scale scores were –3.3 for patients in the placebo group, –5.1 in the 37.5-mg group, –5 in the 75-mg group, –7.7 in the 150-mg group, and –7.9 in the 300-mg group.
A significantly higher rate of patients in the 75-mg (72.4%), 150-mg (89.7%), and 300-mg (88.7%) groups improved on the Patient Global Impression of Change scale, compared with patients in the placebo (49.1%) and 37.5-mg (55.4%) groups.
Just under 68% of patients who received JZP-110 experienced at least one adverse event, compared with 47.9% of patients who received placebo. The most common adverse events were headache, nausea, decreased appetite, and anxiety. Six serious adverse events were reported over the study period, but none was related to JZP-110.
The study is scheduled to be presented on Sunday, Oct. 29, from 1:30 p.m. to 1:45 p.m. in Room 601A of the Toronto Convention Centre South Building as part of the “Obstructive Sleep Apnea: Insights & Management” session, which will run from 1:30 p.m. to 3 p.m.
The selective dopamine norepinephrine reuptake inhibitor JZP-110 was effective at treating excessive sleepiness in obstructive sleep apnea patients, according to an abstract on a study to be presented at the CHEST annual meeting.
In a 12-week, phase 3 trial, adult patients with obstructive sleep apnea were randomized to receive placebo or once-daily JZP-110 at dosages of 37.5 mg, 75 mg, 150 mg, or 300 mg. A total of 459 patients were included in the final analysis.
At the end of the trial, the mean reduction from baseline on a maintenance of wakefulness test was 0.2 minutes for patients in the placebo group, 4.7 minutes in the 37.5-mg group, 9.1 minutes in the 75-mg group, 11 minutes in the 150-mg group, and 13 minutes in the 300-mg group. Mean changes in Epworth Sleepiness Scale scores were –3.3 for patients in the placebo group, –5.1 in the 37.5-mg group, –5 in the 75-mg group, –7.7 in the 150-mg group, and –7.9 in the 300-mg group.
A significantly higher rate of patients in the 75-mg (72.4%), 150-mg (89.7%), and 300-mg (88.7%) groups improved on the Patient Global Impression of Change scale, compared with patients in the placebo (49.1%) and 37.5-mg (55.4%) groups.
Just under 68% of patients who received JZP-110 experienced at least one adverse event, compared with 47.9% of patients who received placebo. The most common adverse events were headache, nausea, decreased appetite, and anxiety. Six serious adverse events were reported over the study period, but none was related to JZP-110.
The study is scheduled to be presented on Sunday, Oct. 29, from 1:30 p.m. to 1:45 p.m. in Room 601A of the Toronto Convention Centre South Building as part of the “Obstructive Sleep Apnea: Insights & Management” session, which will run from 1:30 p.m. to 3 p.m.
FROM CHEST 2017
Sepsis response team does not improve mortality/organ dysfunction
A sepsis response team did not have a positive effect on mortality or organ dysfunction in septic patients, compared with standard treatment by a primary care team, according to a study abstract scheduled to be presented at CHEST 2017.
Compared with the primary care team, the sepsis team was more likely to intervene on patients with a quick Sepsis-Related Organ Failure Assessment score greater than 1 (33.8% vs. 22.8%), change or initiate antibiotics within 3 hours (64.6% vs. 37.2%), and obtain blood cultures on time (66.4% vs 45.2%). An additional difference between the two groups was that the sepsis team had better compliance with the 3-hour bundle (15.2% vs 8.4%).
Despite the sepsis team’s higher level of compliance with certain protocols, the combined outcome measure of mortality and organ dysfunction within 28 days was not significantly higher for patients treated by the sepsis team (11.3% vs. 9.8%; P = .6). In fact, there was at least one downside to being treated by the sepsis team, which was having a 14% longer hospital stay.
Chhaya Patel, MD, is scheduled to present the abstract on Sun., Oct. 29th, at 2:30-2:45 p.m. in Convention Center – 602B. This research is part of the Sepsis & Septic Shock session at the CHEST annual meeting, which will run from 1:30 to 3:00 p.m.
A sepsis response team did not have a positive effect on mortality or organ dysfunction in septic patients, compared with standard treatment by a primary care team, according to a study abstract scheduled to be presented at CHEST 2017.
Compared with the primary care team, the sepsis team was more likely to intervene on patients with a quick Sepsis-Related Organ Failure Assessment score greater than 1 (33.8% vs. 22.8%), change or initiate antibiotics within 3 hours (64.6% vs. 37.2%), and obtain blood cultures on time (66.4% vs 45.2%). An additional difference between the two groups was that the sepsis team had better compliance with the 3-hour bundle (15.2% vs 8.4%).
Despite the sepsis team’s higher level of compliance with certain protocols, the combined outcome measure of mortality and organ dysfunction within 28 days was not significantly higher for patients treated by the sepsis team (11.3% vs. 9.8%; P = .6). In fact, there was at least one downside to being treated by the sepsis team, which was having a 14% longer hospital stay.
Chhaya Patel, MD, is scheduled to present the abstract on Sun., Oct. 29th, at 2:30-2:45 p.m. in Convention Center – 602B. This research is part of the Sepsis & Septic Shock session at the CHEST annual meeting, which will run from 1:30 to 3:00 p.m.
A sepsis response team did not have a positive effect on mortality or organ dysfunction in septic patients, compared with standard treatment by a primary care team, according to a study abstract scheduled to be presented at CHEST 2017.
Compared with the primary care team, the sepsis team was more likely to intervene on patients with a quick Sepsis-Related Organ Failure Assessment score greater than 1 (33.8% vs. 22.8%), change or initiate antibiotics within 3 hours (64.6% vs. 37.2%), and obtain blood cultures on time (66.4% vs 45.2%). An additional difference between the two groups was that the sepsis team had better compliance with the 3-hour bundle (15.2% vs 8.4%).
Despite the sepsis team’s higher level of compliance with certain protocols, the combined outcome measure of mortality and organ dysfunction within 28 days was not significantly higher for patients treated by the sepsis team (11.3% vs. 9.8%; P = .6). In fact, there was at least one downside to being treated by the sepsis team, which was having a 14% longer hospital stay.
Chhaya Patel, MD, is scheduled to present the abstract on Sun., Oct. 29th, at 2:30-2:45 p.m. in Convention Center – 602B. This research is part of the Sepsis & Septic Shock session at the CHEST annual meeting, which will run from 1:30 to 3:00 p.m.
FROM CHEST 2017
Robotic-assisted pulmonary lobectomy effective for large tumors
Robotic-assisted pulmonary lobectomy is a safe and effective way to remove large tumors in patients with non–small cell lung cancer (NSCLC), according to the abstract of a study scheduled to be presented at the CHEST annual meeting.
The study covers a retrospective analysis of 345 NSCLC patients with tumors who underwent robotic-assisted pulmonary lobectomy performed by one surgeon from September 2010 through August 2016. The participants were grouped into the following three cohorts: patients with tumors less than 5 cm in diameter, patients with tumors from 5 to 7 cm, and patients with tumors larger than 7 cm. The researchers excluded patients with pulmonary metastases or benign lesions from the study.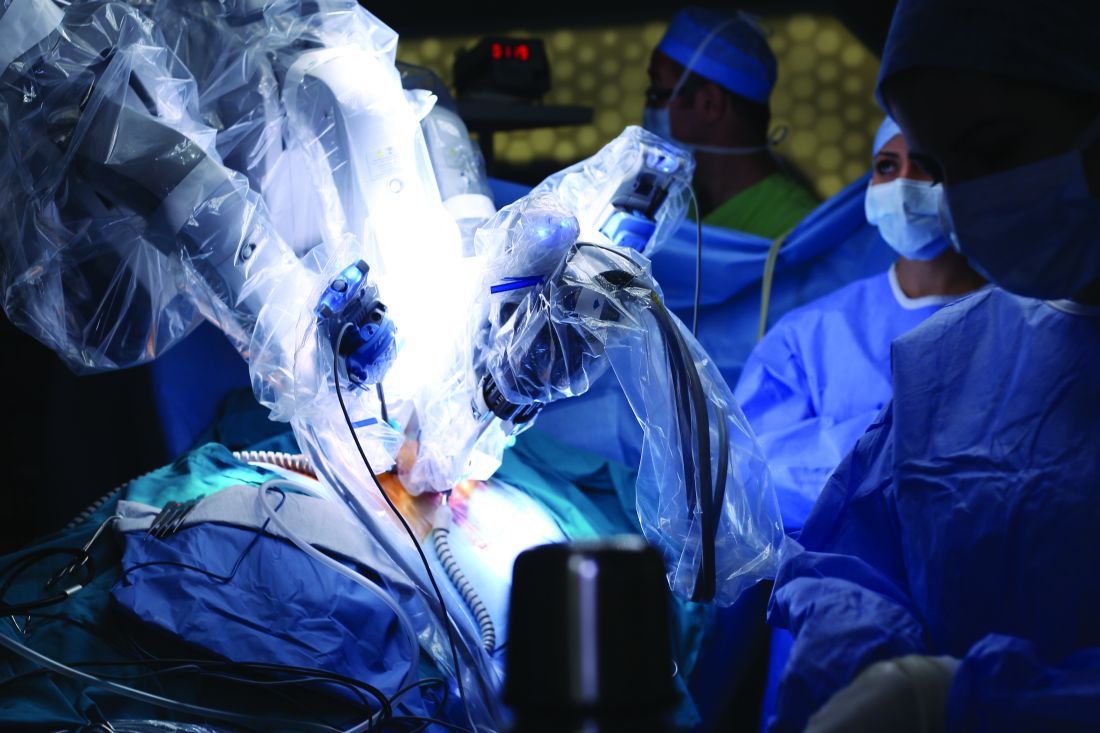
Patients with smaller tumors were more likely to have simple lobectomy or lobectomy plus wedge, while patients with larger tumors were more likely to require lobectomy with chest wall resection. Increased tumor size was also associated with increased intraoperative estimated blood loss, skin-to-skin operative time, hospital length of stay, and overall conversion to open lobectomy.
There was no association found between tumor size and increased overall intraoperative or postoperative complications, or in-hospital mortality.
Nirav Patel, MD, FCCP, of the Tampa Bay Sleep Center is scheduled to present his abstract on Sunday Oct. 29th, between 2:15 and 2:30 p.m. in Convention Center – 606. Dr. Patel’s research is part of the Cardiothoracic Surgery session, running from 1:30 p.m. to 3:00 p.m. at the CHEST annual meeting.
Robotic-assisted pulmonary lobectomy is a safe and effective way to remove large tumors in patients with non–small cell lung cancer (NSCLC), according to the abstract of a study scheduled to be presented at the CHEST annual meeting.
The study covers a retrospective analysis of 345 NSCLC patients with tumors who underwent robotic-assisted pulmonary lobectomy performed by one surgeon from September 2010 through August 2016. The participants were grouped into the following three cohorts: patients with tumors less than 5 cm in diameter, patients with tumors from 5 to 7 cm, and patients with tumors larger than 7 cm. The researchers excluded patients with pulmonary metastases or benign lesions from the study.
Patients with smaller tumors were more likely to have simple lobectomy or lobectomy plus wedge, while patients with larger tumors were more likely to require lobectomy with chest wall resection. Increased tumor size was also associated with increased intraoperative estimated blood loss, skin-to-skin operative time, hospital length of stay, and overall conversion to open lobectomy.
There was no association found between tumor size and increased overall intraoperative or postoperative complications, or in-hospital mortality.
Nirav Patel, MD, FCCP, of the Tampa Bay Sleep Center is scheduled to present his abstract on Sunday Oct. 29th, between 2:15 and 2:30 p.m. in Convention Center – 606. Dr. Patel’s research is part of the Cardiothoracic Surgery session, running from 1:30 p.m. to 3:00 p.m. at the CHEST annual meeting.
Robotic-assisted pulmonary lobectomy is a safe and effective way to remove large tumors in patients with non–small cell lung cancer (NSCLC), according to the abstract of a study scheduled to be presented at the CHEST annual meeting.
The study covers a retrospective analysis of 345 NSCLC patients with tumors who underwent robotic-assisted pulmonary lobectomy performed by one surgeon from September 2010 through August 2016. The participants were grouped into the following three cohorts: patients with tumors less than 5 cm in diameter, patients with tumors from 5 to 7 cm, and patients with tumors larger than 7 cm. The researchers excluded patients with pulmonary metastases or benign lesions from the study.
Patients with smaller tumors were more likely to have simple lobectomy or lobectomy plus wedge, while patients with larger tumors were more likely to require lobectomy with chest wall resection. Increased tumor size was also associated with increased intraoperative estimated blood loss, skin-to-skin operative time, hospital length of stay, and overall conversion to open lobectomy.
There was no association found between tumor size and increased overall intraoperative or postoperative complications, or in-hospital mortality.
Nirav Patel, MD, FCCP, of the Tampa Bay Sleep Center is scheduled to present his abstract on Sunday Oct. 29th, between 2:15 and 2:30 p.m. in Convention Center – 606. Dr. Patel’s research is part of the Cardiothoracic Surgery session, running from 1:30 p.m. to 3:00 p.m. at the CHEST annual meeting.
FROM CHEST 2017
Near-fatal asthma treated effectively by ECMO
Extracorporeal membrane oxygenation (ECMO) is an effective way to treat near fatal asthma, but physicians must remember the risk of complications, according to an abstract on a study scheduled to be presented at CHEST 2017.
The study covers a retrospective analysis of 371 children with asthma who were treated with ECMO; it used data collected by the Extracorporeal Life Support Organization registry from 1988 to 2016. The median age of the children in the study was 7.5 years; the participant group was 43% white and 39% black, as well as 56% male, according to the abstract, which is mentioned in the program for the CHEST annual meeting.
About 80% of children experienced at least one complication, with 20% experiencing three or more. Children who had three or more complications were significantly less likely to experience lung recovery.
Of the children who received VV cannulation, 90% experienced lung recovery, whereas only 69% of children who received VA cannulation recovered. (P less than .0001). VA cannulation was also associated with a higher risk of neurological complications, while those who received VV cannulation were significantly more likely to survive.
The abstract is scheduled to be presented on Sunday Oct. 29 from 2:30 p.m. to 2:45 p.m. in Room 603 of Toronto Convention Centre South Building as part of the Acute Lung Injury & Respiratory Failure session, which will run from 1:30 p.m. to 3 p.m.
Extracorporeal membrane oxygenation (ECMO) is an effective way to treat near fatal asthma, but physicians must remember the risk of complications, according to an abstract on a study scheduled to be presented at CHEST 2017.
The study covers a retrospective analysis of 371 children with asthma who were treated with ECMO; it used data collected by the Extracorporeal Life Support Organization registry from 1988 to 2016. The median age of the children in the study was 7.5 years; the participant group was 43% white and 39% black, as well as 56% male, according to the abstract, which is mentioned in the program for the CHEST annual meeting.
About 80% of children experienced at least one complication, with 20% experiencing three or more. Children who had three or more complications were significantly less likely to experience lung recovery.
Of the children who received VV cannulation, 90% experienced lung recovery, whereas only 69% of children who received VA cannulation recovered. (P less than .0001). VA cannulation was also associated with a higher risk of neurological complications, while those who received VV cannulation were significantly more likely to survive.
The abstract is scheduled to be presented on Sunday Oct. 29 from 2:30 p.m. to 2:45 p.m. in Room 603 of Toronto Convention Centre South Building as part of the Acute Lung Injury & Respiratory Failure session, which will run from 1:30 p.m. to 3 p.m.
Extracorporeal membrane oxygenation (ECMO) is an effective way to treat near fatal asthma, but physicians must remember the risk of complications, according to an abstract on a study scheduled to be presented at CHEST 2017.
The study covers a retrospective analysis of 371 children with asthma who were treated with ECMO; it used data collected by the Extracorporeal Life Support Organization registry from 1988 to 2016. The median age of the children in the study was 7.5 years; the participant group was 43% white and 39% black, as well as 56% male, according to the abstract, which is mentioned in the program for the CHEST annual meeting.
About 80% of children experienced at least one complication, with 20% experiencing three or more. Children who had three or more complications were significantly less likely to experience lung recovery.
Of the children who received VV cannulation, 90% experienced lung recovery, whereas only 69% of children who received VA cannulation recovered. (P less than .0001). VA cannulation was also associated with a higher risk of neurological complications, while those who received VV cannulation were significantly more likely to survive.
The abstract is scheduled to be presented on Sunday Oct. 29 from 2:30 p.m. to 2:45 p.m. in Room 603 of Toronto Convention Centre South Building as part of the Acute Lung Injury & Respiratory Failure session, which will run from 1:30 p.m. to 3 p.m.
New assay may aid diagnosis, treatment of DLBCL
A new assay may help improve the diagnosis and treatment of diffuse large B-cell lymphoma (DLBCL), according to researchers.
The gene expression signature assay can be used to classify subtypes of DLBCL and may enhance disease management by helping to match tumors with the appropriate targeted therapy.
Researchers described the assay in the Journal of Molecular Diagnostics.
The assay is a novel gene expression profiling DLBCL classifier based on reverse transcriptase multiplex ligation-dependent probe amplification (RT-MLPA).
It can simultaneously evaluate the expression of 21 markers, allowing differentiation of the 3 subtypes of DLBCL—germinal center B-cell-like (GCB), activated B-cell-like (ABC), and primary mediastinal B-cell lymphoma (PMBL)—as well as other individualized disease characteristics, such as Epstein-Barr infection status.
Researchers used the RT-MLPA assay to test 150 samples from DLBCL patients. Forty-two percent of the samples were the ABC subtype, 37% the GCB subtype, and 10% molecular PMBL. Eleven percent of the samples could not be classified.
Overall, the RT-MLPA assay correctly assigned 85.0% of the cases into the expected subtypes, compared to 78.8% of samples assigned via immunohistochemistry.
The RT-MLPA assay was also able to detect the MYD88 L265P mutation, one of the most common genetic abnormalities found in ABC DLBCLs. This information can influence treatment, since the presence of the mutation is thought to be predictive of ibrutinib sensitivity.
The researchers said RT-MLPA is a robust, efficient, rapid, and cost-effective alternative to current methods used in the clinic to establish the cell-of-origin classification of DLBCLs.
RT-MLPA requires only common laboratory equipment and can be applied to formalin-fixed, paraffin-embedded samples. Other types of diagnostic methods may not provide the level of detail needed and may also be limited by poor reproducibility and lack of adaptability to routine use in standard laboratories.
“Because we have provided the classification algorithms, other laboratories will be able to verify our results and adjust the procedures to suit their environment,” said study author Philippe Ruminy, PhD, of the Henri Becquerel Cancer Treatment Center, INSERM U1245 in Rouen, France.
“It is our hope that the assay we have developed, which addresses an important recommendation of the recent WHO classifications, will contribute to better management of these tumors and improved patient outcomes.” ![]()
A new assay may help improve the diagnosis and treatment of diffuse large B-cell lymphoma (DLBCL), according to researchers.
The gene expression signature assay can be used to classify subtypes of DLBCL and may enhance disease management by helping to match tumors with the appropriate targeted therapy.
Researchers described the assay in the Journal of Molecular Diagnostics.
The assay is a novel gene expression profiling DLBCL classifier based on reverse transcriptase multiplex ligation-dependent probe amplification (RT-MLPA).
It can simultaneously evaluate the expression of 21 markers, allowing differentiation of the 3 subtypes of DLBCL—germinal center B-cell-like (GCB), activated B-cell-like (ABC), and primary mediastinal B-cell lymphoma (PMBL)—as well as other individualized disease characteristics, such as Epstein-Barr infection status.
Researchers used the RT-MLPA assay to test 150 samples from DLBCL patients. Forty-two percent of the samples were the ABC subtype, 37% the GCB subtype, and 10% molecular PMBL. Eleven percent of the samples could not be classified.
Overall, the RT-MLPA assay correctly assigned 85.0% of the cases into the expected subtypes, compared to 78.8% of samples assigned via immunohistochemistry.
The RT-MLPA assay was also able to detect the MYD88 L265P mutation, one of the most common genetic abnormalities found in ABC DLBCLs. This information can influence treatment, since the presence of the mutation is thought to be predictive of ibrutinib sensitivity.
The researchers said RT-MLPA is a robust, efficient, rapid, and cost-effective alternative to current methods used in the clinic to establish the cell-of-origin classification of DLBCLs.
RT-MLPA requires only common laboratory equipment and can be applied to formalin-fixed, paraffin-embedded samples. Other types of diagnostic methods may not provide the level of detail needed and may also be limited by poor reproducibility and lack of adaptability to routine use in standard laboratories.
“Because we have provided the classification algorithms, other laboratories will be able to verify our results and adjust the procedures to suit their environment,” said study author Philippe Ruminy, PhD, of the Henri Becquerel Cancer Treatment Center, INSERM U1245 in Rouen, France.
“It is our hope that the assay we have developed, which addresses an important recommendation of the recent WHO classifications, will contribute to better management of these tumors and improved patient outcomes.” ![]()
A new assay may help improve the diagnosis and treatment of diffuse large B-cell lymphoma (DLBCL), according to researchers.
The gene expression signature assay can be used to classify subtypes of DLBCL and may enhance disease management by helping to match tumors with the appropriate targeted therapy.
Researchers described the assay in the Journal of Molecular Diagnostics.
The assay is a novel gene expression profiling DLBCL classifier based on reverse transcriptase multiplex ligation-dependent probe amplification (RT-MLPA).
It can simultaneously evaluate the expression of 21 markers, allowing differentiation of the 3 subtypes of DLBCL—germinal center B-cell-like (GCB), activated B-cell-like (ABC), and primary mediastinal B-cell lymphoma (PMBL)—as well as other individualized disease characteristics, such as Epstein-Barr infection status.
Researchers used the RT-MLPA assay to test 150 samples from DLBCL patients. Forty-two percent of the samples were the ABC subtype, 37% the GCB subtype, and 10% molecular PMBL. Eleven percent of the samples could not be classified.
Overall, the RT-MLPA assay correctly assigned 85.0% of the cases into the expected subtypes, compared to 78.8% of samples assigned via immunohistochemistry.
The RT-MLPA assay was also able to detect the MYD88 L265P mutation, one of the most common genetic abnormalities found in ABC DLBCLs. This information can influence treatment, since the presence of the mutation is thought to be predictive of ibrutinib sensitivity.
The researchers said RT-MLPA is a robust, efficient, rapid, and cost-effective alternative to current methods used in the clinic to establish the cell-of-origin classification of DLBCLs.
RT-MLPA requires only common laboratory equipment and can be applied to formalin-fixed, paraffin-embedded samples. Other types of diagnostic methods may not provide the level of detail needed and may also be limited by poor reproducibility and lack of adaptability to routine use in standard laboratories.
“Because we have provided the classification algorithms, other laboratories will be able to verify our results and adjust the procedures to suit their environment,” said study author Philippe Ruminy, PhD, of the Henri Becquerel Cancer Treatment Center, INSERM U1245 in Rouen, France.
“It is our hope that the assay we have developed, which addresses an important recommendation of the recent WHO classifications, will contribute to better management of these tumors and improved patient outcomes.” ![]()
Heart Failure Practical Approaches
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Psoriasis: Biologics bring potential for long-term remission off treatment
GENEVA – Can potent, highly efficacious biologic therapy induce longstanding remission of moderate to severe plaque psoriasis following discontinuation of all treatment?
The answer appears to be yes, in a minority of patients, based on results of a long-term extension study of two pivotal phase 3 clinical trials of secukinumab (Cosentyx), Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.

This 2-year extension study provides the first double-blind, long-term evidence regarding the natural history of psoriasis following discontinuation of a contemporary potent targeted therapy.
The results are exciting because spontaneous remission of moderate to severe psoriasis is rare; psoriasis typically reverts to baseline severity following discontinuation of treatment. In the extension study, though, 21% of patients who achieved a 75% reduction in the Psoriasis Area Severity Index (PASI 75) response on secukinumab at the 300-mg dose for 52 weeks and were then crossed over double-blind to placebo remained continuously relapse-free after 12 months on placebo, and 10% remained relapse-free at 2 years, at which point they were unblinded and put back on secukinumab. No psoriasis therapies were permitted during the placebo phase.
Those findings are particularly impressive because participants in the two pivotal trials of secukinumab combined for the treatment withdrawal extension study – ERASURE and FIXTURE – had a mean 14- and 16-year history of psoriasis at baseline.
“Secukinumab appears to have modified the course of chronic moderate to severe psoriasis, despite intervening late in its course. Treating patients early is expected to have an even greater potential for disease modification,” commented Dr. Lebwohl, the Sol and Clara Kest Professor and chair of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
That hypothesis is under investigation in the ongoing multinational STEPin trial (Study of the Efficacy of Early Intervention With Secukinumab 300 mg s.c. [subcutaneous] Compared to Narrow-band UVB in Patients With New-onset Moderate to Severe Plaque Psoriasis). STEPin involves about 200 patients with newly diagnosed disease not previously treated with systemic agents or phototherapy.
Dr. Lebwohl focused on the 120 patients in the extension study who had a PASI 75 response to secukinumab at the 300-mg dose and the 100 patients with a PASI 75 response to the 150-mg dose who, after 52 weeks on the fully human anti-interleukin-17A monoclonal antibody, were crossed over double-blind to placebo for 2 years or until relapse occurred. Patients were assessed monthly during the extension study.
Relapse was defined as loss of 50% of the maximum PASI improvement achieved while on secukinumab. The relapse-free rate was better in patients who had been on secukinumab 300 mg than in those on 150 mg. At 1 year on placebo, 21% of patients formerly on secukinumab at the higher dose remained free of relapse, as did 10% at 2 years. In contrast, the 1- and 2-year relapse-free rates in the group formerly on secukinumab 150 mg were lower at 14% and 6%, respectively, even though everyone in the extension study had a PASI 75 response when they went off treatment.
The mean baseline PASI score in the ERASURE and FIXTURE trials was 23. Among patients who remained relapse-free for 1 year, the mean PASI score was dramatically lower, at 0.7; among those in remission for 2 years, it was 0.4.
Importantly, the longer a patient’s history of psoriasis, the less likely a long-term relapse-free experience, the dermatologist observed.
A separate mechanistic study in a different group of psoriasis patients provided a likely explanation for the long relapse-free period seen off therapy in some patients in the extension study. The mechanistic study entailed transcriptional analysis of psoriasis-related genes in lesional and nonlesional skin before and after 1 year of treatment with secukinumab 300 mg. The major finding: 85% of the upregulated genes and 75% of downregulated genes in lesional skin pretreatment reverted to normal levels in healed lesional skin post treatment.
Moreover, global mRNA expression for key genes involved in the interleukin-23/interleukin-17 inflammatory cytokine pathway closely resembled healthy skin following treatment with secukinumab. This is evidence of profound molecular normalization, Dr. Lebwohl said.
These secukinumab studies were sponsored by Novartis. Dr. Lebwohl reported having no personal financial conflicts of interest, although his department receives research funding from Novartis and numerous other pharmaceutical companies.
GENEVA – Can potent, highly efficacious biologic therapy induce longstanding remission of moderate to severe plaque psoriasis following discontinuation of all treatment?
The answer appears to be yes, in a minority of patients, based on results of a long-term extension study of two pivotal phase 3 clinical trials of secukinumab (Cosentyx), Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.

This 2-year extension study provides the first double-blind, long-term evidence regarding the natural history of psoriasis following discontinuation of a contemporary potent targeted therapy.
The results are exciting because spontaneous remission of moderate to severe psoriasis is rare; psoriasis typically reverts to baseline severity following discontinuation of treatment. In the extension study, though, 21% of patients who achieved a 75% reduction in the Psoriasis Area Severity Index (PASI 75) response on secukinumab at the 300-mg dose for 52 weeks and were then crossed over double-blind to placebo remained continuously relapse-free after 12 months on placebo, and 10% remained relapse-free at 2 years, at which point they were unblinded and put back on secukinumab. No psoriasis therapies were permitted during the placebo phase.
Those findings are particularly impressive because participants in the two pivotal trials of secukinumab combined for the treatment withdrawal extension study – ERASURE and FIXTURE – had a mean 14- and 16-year history of psoriasis at baseline.
“Secukinumab appears to have modified the course of chronic moderate to severe psoriasis, despite intervening late in its course. Treating patients early is expected to have an even greater potential for disease modification,” commented Dr. Lebwohl, the Sol and Clara Kest Professor and chair of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
That hypothesis is under investigation in the ongoing multinational STEPin trial (Study of the Efficacy of Early Intervention With Secukinumab 300 mg s.c. [subcutaneous] Compared to Narrow-band UVB in Patients With New-onset Moderate to Severe Plaque Psoriasis). STEPin involves about 200 patients with newly diagnosed disease not previously treated with systemic agents or phototherapy.
Dr. Lebwohl focused on the 120 patients in the extension study who had a PASI 75 response to secukinumab at the 300-mg dose and the 100 patients with a PASI 75 response to the 150-mg dose who, after 52 weeks on the fully human anti-interleukin-17A monoclonal antibody, were crossed over double-blind to placebo for 2 years or until relapse occurred. Patients were assessed monthly during the extension study.
Relapse was defined as loss of 50% of the maximum PASI improvement achieved while on secukinumab. The relapse-free rate was better in patients who had been on secukinumab 300 mg than in those on 150 mg. At 1 year on placebo, 21% of patients formerly on secukinumab at the higher dose remained free of relapse, as did 10% at 2 years. In contrast, the 1- and 2-year relapse-free rates in the group formerly on secukinumab 150 mg were lower at 14% and 6%, respectively, even though everyone in the extension study had a PASI 75 response when they went off treatment.
The mean baseline PASI score in the ERASURE and FIXTURE trials was 23. Among patients who remained relapse-free for 1 year, the mean PASI score was dramatically lower, at 0.7; among those in remission for 2 years, it was 0.4.
Importantly, the longer a patient’s history of psoriasis, the less likely a long-term relapse-free experience, the dermatologist observed.
A separate mechanistic study in a different group of psoriasis patients provided a likely explanation for the long relapse-free period seen off therapy in some patients in the extension study. The mechanistic study entailed transcriptional analysis of psoriasis-related genes in lesional and nonlesional skin before and after 1 year of treatment with secukinumab 300 mg. The major finding: 85% of the upregulated genes and 75% of downregulated genes in lesional skin pretreatment reverted to normal levels in healed lesional skin post treatment.
Moreover, global mRNA expression for key genes involved in the interleukin-23/interleukin-17 inflammatory cytokine pathway closely resembled healthy skin following treatment with secukinumab. This is evidence of profound molecular normalization, Dr. Lebwohl said.
These secukinumab studies were sponsored by Novartis. Dr. Lebwohl reported having no personal financial conflicts of interest, although his department receives research funding from Novartis and numerous other pharmaceutical companies.
GENEVA – Can potent, highly efficacious biologic therapy induce longstanding remission of moderate to severe plaque psoriasis following discontinuation of all treatment?
The answer appears to be yes, in a minority of patients, based on results of a long-term extension study of two pivotal phase 3 clinical trials of secukinumab (Cosentyx), Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.

This 2-year extension study provides the first double-blind, long-term evidence regarding the natural history of psoriasis following discontinuation of a contemporary potent targeted therapy.
The results are exciting because spontaneous remission of moderate to severe psoriasis is rare; psoriasis typically reverts to baseline severity following discontinuation of treatment. In the extension study, though, 21% of patients who achieved a 75% reduction in the Psoriasis Area Severity Index (PASI 75) response on secukinumab at the 300-mg dose for 52 weeks and were then crossed over double-blind to placebo remained continuously relapse-free after 12 months on placebo, and 10% remained relapse-free at 2 years, at which point they were unblinded and put back on secukinumab. No psoriasis therapies were permitted during the placebo phase.
Those findings are particularly impressive because participants in the two pivotal trials of secukinumab combined for the treatment withdrawal extension study – ERASURE and FIXTURE – had a mean 14- and 16-year history of psoriasis at baseline.
“Secukinumab appears to have modified the course of chronic moderate to severe psoriasis, despite intervening late in its course. Treating patients early is expected to have an even greater potential for disease modification,” commented Dr. Lebwohl, the Sol and Clara Kest Professor and chair of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
That hypothesis is under investigation in the ongoing multinational STEPin trial (Study of the Efficacy of Early Intervention With Secukinumab 300 mg s.c. [subcutaneous] Compared to Narrow-band UVB in Patients With New-onset Moderate to Severe Plaque Psoriasis). STEPin involves about 200 patients with newly diagnosed disease not previously treated with systemic agents or phototherapy.
Dr. Lebwohl focused on the 120 patients in the extension study who had a PASI 75 response to secukinumab at the 300-mg dose and the 100 patients with a PASI 75 response to the 150-mg dose who, after 52 weeks on the fully human anti-interleukin-17A monoclonal antibody, were crossed over double-blind to placebo for 2 years or until relapse occurred. Patients were assessed monthly during the extension study.
Relapse was defined as loss of 50% of the maximum PASI improvement achieved while on secukinumab. The relapse-free rate was better in patients who had been on secukinumab 300 mg than in those on 150 mg. At 1 year on placebo, 21% of patients formerly on secukinumab at the higher dose remained free of relapse, as did 10% at 2 years. In contrast, the 1- and 2-year relapse-free rates in the group formerly on secukinumab 150 mg were lower at 14% and 6%, respectively, even though everyone in the extension study had a PASI 75 response when they went off treatment.
The mean baseline PASI score in the ERASURE and FIXTURE trials was 23. Among patients who remained relapse-free for 1 year, the mean PASI score was dramatically lower, at 0.7; among those in remission for 2 years, it was 0.4.
Importantly, the longer a patient’s history of psoriasis, the less likely a long-term relapse-free experience, the dermatologist observed.
A separate mechanistic study in a different group of psoriasis patients provided a likely explanation for the long relapse-free period seen off therapy in some patients in the extension study. The mechanistic study entailed transcriptional analysis of psoriasis-related genes in lesional and nonlesional skin before and after 1 year of treatment with secukinumab 300 mg. The major finding: 85% of the upregulated genes and 75% of downregulated genes in lesional skin pretreatment reverted to normal levels in healed lesional skin post treatment.
Moreover, global mRNA expression for key genes involved in the interleukin-23/interleukin-17 inflammatory cytokine pathway closely resembled healthy skin following treatment with secukinumab. This is evidence of profound molecular normalization, Dr. Lebwohl said.
These secukinumab studies were sponsored by Novartis. Dr. Lebwohl reported having no personal financial conflicts of interest, although his department receives research funding from Novartis and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: , enabling some patients to experience long-term remission off therapy.
Major finding: After 1 and 2 years without any psoriasis therapy following successful treatment with secukinumab for 52 weeks, 21% and 10% of patients, respectively, remained relapse-free.
Data source: This prospective extension of two phase 3 pivotal trials of secukinumab included 220 psoriasis patients who were taken off the biologic after 52 weeks and crossed over double blind to placebo for up to 2 years.
Disclosures: The study was sponsored by Novartis. Dr. Lebwohl reported having no financial conflicts.