User login
Long-term methimazole therapy improves Graves disease remission rate
VICTORIA, B.C. – In the debate over the optimal duration of methimazole therapy for Graves disease, findings of a new randomized, controlled trial reported at the annual meeting of the American Thyroid Association tip the balance in favor of long-term therapy.
The relapse rate among patients who stayed on the drug long term, for a median of 96 months, was about one-third that among patients who stopped after 18 months, reported lead investigator Fereidoun Azizi, MD, of the Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran. Patients staying on the drug long term did not experience any adverse effects during that time, although only those able to tolerate the drug initially were randomized.
There may be two explanations for this benefit of long-term therapy, according to Dr. Azizi. Long-term therapy may alter immune-related molecular signaling and cell subsets in both the thymus and periphery, ultimately shifting disease course. On the other hand, establishing and maintaining euthyroidism for a prolonged period of time may quell the autoimmune response.
“We are looking at this in depth and also at some of the [molecular factors] in order to elucidate the mechanism behind our striking findings,” he said.
One of the session cochairs, Yaron Tomer, MD, chair of the department of medicine and the Anita and Jack Saltz Chair in Diabetes Research at the Montefiore Medical Center, New York, commented, “There is a move today away from radioactive iodine – many patients do not want radioactive iodine, and we do more surgery now because of that. So this opens up a new option that we didn’t have before.”
The other session cochair, Catherine A. Dinauer, MD, a pediatric endocrinologist and clinician at the Yale Pediatric Thyroid Center, New Haven, Conn., noted that duration of therapy frequently comes up in her practice.
Study details
Relapse of hyperthyroidism after discontinuation of antithyroid drugs remains problematic, Dr. Azizi pointed out when introducing the study.
“Many of the major papers have noted that longer antithyroid drug treatment does not really influence remission rate of Graves, and therefore most of us treat for between 12 and 24 months with antithyroid drugs, and then we stop the medication,” he said. However, recent studies and in particular a meta-analysis (Thyroid. 2017;27:1223-31) suggest there may be an advantage of long-term therapy.
Dr. Azizi and coinvestigators recruited to their trial 302 consecutive patients from a single clinic who had untreated Graves disease and were started on methimazole (Tapazole) therapy.
The 258 patients completing 18 months of therapy were randomized to stop the drug or continue on a maintenance dose long term, for 60-120 months, on a single-blind basis. (The other 44 patients withdrew mainly because of side effects, relapse, and loss to follow-up.)
Patients in the long-term therapy group stayed on the drug for a median of 96 months. The decision about specifically when to stop in this group was guided by thyroid function test results and patients’ clinical status and preferences, according to Dr. Azizi.
The rate of relapse at 48 months after stopping methimazole was 51% among patients in the short-term therapy group but just 16% among patients in the long-term therapy group (P less than or equal to .001). “Definitely, this looks like a cure of the disease if we consider this very low incidence of relapse,” he commented.
Within the group treated long term, patients who did and did not experience relapse were statistically indistinguishable with respect to temporal trends in levels of triiodothyronine (T3), free thyroxine (T4), thyroid-stimulating hormone (TSH), and thyroid-stimulating hormone receptor antibody (TRAb).
Additionally, the daily dose of methimazole therapy required to maintain TSH levels in the normal range fell similarly over time, to about half the initial dose, regardless of whether patients had a relapse or not.
“At the end of treatment, the majority of patients were taking less than 5 mg/day of methimazole,” Dr. Azizi reported. “Some patients needed only two or three pills of 5-mg methimazole per week, and this is very interesting to know, that after you continue, you have definitely more response to methimazole.”
Multivariate analyses showed that in the short-term therapy group, risk factors for relapse were age, sex, and end-of-therapy levels of T3, TSH, and TRAb. In the long-term therapy group, risk factors were end-of-therapy levels of free T4 and TSH.
“We are currently performing more in-depth analysis of genetic markers, including both SNPs [single nucleotide polymorphisms] and HLA [human leukocyte antigen] subtyping on these samples to assess any potential association between relapse rates and genetic background,” Dr. Azizi noted. “However, the problem is the low number of patients who have had a relapse long term.”
During the first 18 months of methimazole therapy, 16 patients had adverse effects in the first 2 months (14 had cutaneous reactions and 2 had elevation of liver enzymes). However, there were no serious complications, such as agranulocytosis.
“It’s very reassuring that after 18 months, in those who had long-term treatment, we did not see any minor or major complications throughout, up to the 120 months of treatment we have had in some of our patients,” Dr. Azizi commented.
Dr. Azizi disclosed that he had no relevant conflicts of interest.
VICTORIA, B.C. – In the debate over the optimal duration of methimazole therapy for Graves disease, findings of a new randomized, controlled trial reported at the annual meeting of the American Thyroid Association tip the balance in favor of long-term therapy.
The relapse rate among patients who stayed on the drug long term, for a median of 96 months, was about one-third that among patients who stopped after 18 months, reported lead investigator Fereidoun Azizi, MD, of the Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran. Patients staying on the drug long term did not experience any adverse effects during that time, although only those able to tolerate the drug initially were randomized.
There may be two explanations for this benefit of long-term therapy, according to Dr. Azizi. Long-term therapy may alter immune-related molecular signaling and cell subsets in both the thymus and periphery, ultimately shifting disease course. On the other hand, establishing and maintaining euthyroidism for a prolonged period of time may quell the autoimmune response.
“We are looking at this in depth and also at some of the [molecular factors] in order to elucidate the mechanism behind our striking findings,” he said.
One of the session cochairs, Yaron Tomer, MD, chair of the department of medicine and the Anita and Jack Saltz Chair in Diabetes Research at the Montefiore Medical Center, New York, commented, “There is a move today away from radioactive iodine – many patients do not want radioactive iodine, and we do more surgery now because of that. So this opens up a new option that we didn’t have before.”
The other session cochair, Catherine A. Dinauer, MD, a pediatric endocrinologist and clinician at the Yale Pediatric Thyroid Center, New Haven, Conn., noted that duration of therapy frequently comes up in her practice.
Study details
Relapse of hyperthyroidism after discontinuation of antithyroid drugs remains problematic, Dr. Azizi pointed out when introducing the study.
“Many of the major papers have noted that longer antithyroid drug treatment does not really influence remission rate of Graves, and therefore most of us treat for between 12 and 24 months with antithyroid drugs, and then we stop the medication,” he said. However, recent studies and in particular a meta-analysis (Thyroid. 2017;27:1223-31) suggest there may be an advantage of long-term therapy.
Dr. Azizi and coinvestigators recruited to their trial 302 consecutive patients from a single clinic who had untreated Graves disease and were started on methimazole (Tapazole) therapy.
The 258 patients completing 18 months of therapy were randomized to stop the drug or continue on a maintenance dose long term, for 60-120 months, on a single-blind basis. (The other 44 patients withdrew mainly because of side effects, relapse, and loss to follow-up.)
Patients in the long-term therapy group stayed on the drug for a median of 96 months. The decision about specifically when to stop in this group was guided by thyroid function test results and patients’ clinical status and preferences, according to Dr. Azizi.
The rate of relapse at 48 months after stopping methimazole was 51% among patients in the short-term therapy group but just 16% among patients in the long-term therapy group (P less than or equal to .001). “Definitely, this looks like a cure of the disease if we consider this very low incidence of relapse,” he commented.
Within the group treated long term, patients who did and did not experience relapse were statistically indistinguishable with respect to temporal trends in levels of triiodothyronine (T3), free thyroxine (T4), thyroid-stimulating hormone (TSH), and thyroid-stimulating hormone receptor antibody (TRAb).
Additionally, the daily dose of methimazole therapy required to maintain TSH levels in the normal range fell similarly over time, to about half the initial dose, regardless of whether patients had a relapse or not.
“At the end of treatment, the majority of patients were taking less than 5 mg/day of methimazole,” Dr. Azizi reported. “Some patients needed only two or three pills of 5-mg methimazole per week, and this is very interesting to know, that after you continue, you have definitely more response to methimazole.”
Multivariate analyses showed that in the short-term therapy group, risk factors for relapse were age, sex, and end-of-therapy levels of T3, TSH, and TRAb. In the long-term therapy group, risk factors were end-of-therapy levels of free T4 and TSH.
“We are currently performing more in-depth analysis of genetic markers, including both SNPs [single nucleotide polymorphisms] and HLA [human leukocyte antigen] subtyping on these samples to assess any potential association between relapse rates and genetic background,” Dr. Azizi noted. “However, the problem is the low number of patients who have had a relapse long term.”
During the first 18 months of methimazole therapy, 16 patients had adverse effects in the first 2 months (14 had cutaneous reactions and 2 had elevation of liver enzymes). However, there were no serious complications, such as agranulocytosis.
“It’s very reassuring that after 18 months, in those who had long-term treatment, we did not see any minor or major complications throughout, up to the 120 months of treatment we have had in some of our patients,” Dr. Azizi commented.
Dr. Azizi disclosed that he had no relevant conflicts of interest.
VICTORIA, B.C. – In the debate over the optimal duration of methimazole therapy for Graves disease, findings of a new randomized, controlled trial reported at the annual meeting of the American Thyroid Association tip the balance in favor of long-term therapy.
The relapse rate among patients who stayed on the drug long term, for a median of 96 months, was about one-third that among patients who stopped after 18 months, reported lead investigator Fereidoun Azizi, MD, of the Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran. Patients staying on the drug long term did not experience any adverse effects during that time, although only those able to tolerate the drug initially were randomized.
There may be two explanations for this benefit of long-term therapy, according to Dr. Azizi. Long-term therapy may alter immune-related molecular signaling and cell subsets in both the thymus and periphery, ultimately shifting disease course. On the other hand, establishing and maintaining euthyroidism for a prolonged period of time may quell the autoimmune response.
“We are looking at this in depth and also at some of the [molecular factors] in order to elucidate the mechanism behind our striking findings,” he said.
One of the session cochairs, Yaron Tomer, MD, chair of the department of medicine and the Anita and Jack Saltz Chair in Diabetes Research at the Montefiore Medical Center, New York, commented, “There is a move today away from radioactive iodine – many patients do not want radioactive iodine, and we do more surgery now because of that. So this opens up a new option that we didn’t have before.”
The other session cochair, Catherine A. Dinauer, MD, a pediatric endocrinologist and clinician at the Yale Pediatric Thyroid Center, New Haven, Conn., noted that duration of therapy frequently comes up in her practice.
Study details
Relapse of hyperthyroidism after discontinuation of antithyroid drugs remains problematic, Dr. Azizi pointed out when introducing the study.
“Many of the major papers have noted that longer antithyroid drug treatment does not really influence remission rate of Graves, and therefore most of us treat for between 12 and 24 months with antithyroid drugs, and then we stop the medication,” he said. However, recent studies and in particular a meta-analysis (Thyroid. 2017;27:1223-31) suggest there may be an advantage of long-term therapy.
Dr. Azizi and coinvestigators recruited to their trial 302 consecutive patients from a single clinic who had untreated Graves disease and were started on methimazole (Tapazole) therapy.
The 258 patients completing 18 months of therapy were randomized to stop the drug or continue on a maintenance dose long term, for 60-120 months, on a single-blind basis. (The other 44 patients withdrew mainly because of side effects, relapse, and loss to follow-up.)
Patients in the long-term therapy group stayed on the drug for a median of 96 months. The decision about specifically when to stop in this group was guided by thyroid function test results and patients’ clinical status and preferences, according to Dr. Azizi.
The rate of relapse at 48 months after stopping methimazole was 51% among patients in the short-term therapy group but just 16% among patients in the long-term therapy group (P less than or equal to .001). “Definitely, this looks like a cure of the disease if we consider this very low incidence of relapse,” he commented.
Within the group treated long term, patients who did and did not experience relapse were statistically indistinguishable with respect to temporal trends in levels of triiodothyronine (T3), free thyroxine (T4), thyroid-stimulating hormone (TSH), and thyroid-stimulating hormone receptor antibody (TRAb).
Additionally, the daily dose of methimazole therapy required to maintain TSH levels in the normal range fell similarly over time, to about half the initial dose, regardless of whether patients had a relapse or not.
“At the end of treatment, the majority of patients were taking less than 5 mg/day of methimazole,” Dr. Azizi reported. “Some patients needed only two or three pills of 5-mg methimazole per week, and this is very interesting to know, that after you continue, you have definitely more response to methimazole.”
Multivariate analyses showed that in the short-term therapy group, risk factors for relapse were age, sex, and end-of-therapy levels of T3, TSH, and TRAb. In the long-term therapy group, risk factors were end-of-therapy levels of free T4 and TSH.
“We are currently performing more in-depth analysis of genetic markers, including both SNPs [single nucleotide polymorphisms] and HLA [human leukocyte antigen] subtyping on these samples to assess any potential association between relapse rates and genetic background,” Dr. Azizi noted. “However, the problem is the low number of patients who have had a relapse long term.”
During the first 18 months of methimazole therapy, 16 patients had adverse effects in the first 2 months (14 had cutaneous reactions and 2 had elevation of liver enzymes). However, there were no serious complications, such as agranulocytosis.
“It’s very reassuring that after 18 months, in those who had long-term treatment, we did not see any minor or major complications throughout, up to the 120 months of treatment we have had in some of our patients,” Dr. Azizi commented.
Dr. Azizi disclosed that he had no relevant conflicts of interest.
AT ATA 2017
Key clinical point:
Major finding: Relative to peers who stopped methimazole after 18 months, patients who continued on the drug for a median of 96 months had a lower rate of relapse after discontinuation (51% vs. 16%; P less than or equal to .001).
Data source: A randomized controlled trial among 258 patients with Graves disease who were relapse free after 18 months on methimazole.
Disclosures: Dr. Azizi disclosed that he had no relevant conflicts of interest.
Rituximab improves salvage in elderly B-cell lymphoma patients
In elderly patients with aggressive B-cell lymphomas who experience treatment failure after CHOP or rituximab-CHOP (R-CHOP), the outcomes of subsequent salvage therapy were improved when rituximab was included, results of a retrospective analysis suggest.
“Survival after rituximab-containing salvage therapy was better in all patient groups, supporting the repeated administration of rituximab to all patients needing salvage therapy,” wrote investigator Bertram Glass, MD, of the department of hematology and stem cell transplantation at Helios Klinikum Berlin-Buch, Berlin, and his coauthors (Ann Oncol. 2017 Oct 6. doi: 10.1093/annonc/mdx556).
Dr. Glass and colleagues reviewed data from the randomized RICOVER-60 trial, which included 1,222 patients aged 61-80 years with aggressive B-cell lymphomas who received CHOP or R-CHOP for six or eight cycles. Based on survival outcomes, six cycles of R-CHOP every 2 weeks should be the preferred regimen, investigators wrote when the study results were published in 2008 (Lancet Oncol. 2008;9[2]:105-16. doi: 10.1016/S1470-2045(08)70002-0).
Of 1,222 patients in the RICOVER-60 trial, 301 (24.6%) had treatment failure, of whom 297 could be included in the present analysis.
Rituximab, included in salvage therapy for 57.4% of those evaluable patients, was found to improve the 2-year survival rate from 20.7% to 46.8% (P less than .001), Dr. Glass and his coinvestigators reported.
The benefit of rituximab in the salvage setting was apparent regardless of whether patients received R-CHOP or CHOP as part of their initial therapy in RICOVER-60, they added.
Among patients who had received CHOP as first-line therapy, 2-year overall survival was 49.6% for those who received rituximab in the salvage setting, compared with 19.1% for those who did not (P less than .001), according to the published data. Likewise, in the initial R-CHOP group, 2-year overall survival was 33.1% for rituximab in salvage and 22.5% for no rituximab in salvage (P = .034).
The investigators also looked for differences in prognosis according to specific patient characteristics, including presence of MYC rearrangements and MYC expression by immunohistochemistry.
In patients with MYC translocation at diagnosis, use of rituximab reduced risk of initial treatment failure from 58.8% to 26.3%, according to the investigators. After treatment failure, patients who initially received CHOP had significantly improved 2-year survival if they had MYC translocations or negative MYC immunohistochemistry, though no such association was found for patients who initially received R-CHOP, they wrote.
Dr. Glass and colleagues concluded that new treatment strategies are needed.
“Overall, the outcome of second-line treatment of elderly patients with refractory and relapsed aggressive B-cell lymphoma is disappointing and worse than in younger patients regardless of the modality chosen,” they wrote. “New drugs and treatment modalities with the potential to change the dismal outlook for elderly patients with aggressive B-cell lymphomas are eagerly awaited.”
Dr. Glass and several coauthors reported honoraria, research funding, and consultancies with Roche.
In elderly patients with aggressive B-cell lymphomas who experience treatment failure after CHOP or rituximab-CHOP (R-CHOP), the outcomes of subsequent salvage therapy were improved when rituximab was included, results of a retrospective analysis suggest.
“Survival after rituximab-containing salvage therapy was better in all patient groups, supporting the repeated administration of rituximab to all patients needing salvage therapy,” wrote investigator Bertram Glass, MD, of the department of hematology and stem cell transplantation at Helios Klinikum Berlin-Buch, Berlin, and his coauthors (Ann Oncol. 2017 Oct 6. doi: 10.1093/annonc/mdx556).
Dr. Glass and colleagues reviewed data from the randomized RICOVER-60 trial, which included 1,222 patients aged 61-80 years with aggressive B-cell lymphomas who received CHOP or R-CHOP for six or eight cycles. Based on survival outcomes, six cycles of R-CHOP every 2 weeks should be the preferred regimen, investigators wrote when the study results were published in 2008 (Lancet Oncol. 2008;9[2]:105-16. doi: 10.1016/S1470-2045(08)70002-0).
Of 1,222 patients in the RICOVER-60 trial, 301 (24.6%) had treatment failure, of whom 297 could be included in the present analysis.
Rituximab, included in salvage therapy for 57.4% of those evaluable patients, was found to improve the 2-year survival rate from 20.7% to 46.8% (P less than .001), Dr. Glass and his coinvestigators reported.
The benefit of rituximab in the salvage setting was apparent regardless of whether patients received R-CHOP or CHOP as part of their initial therapy in RICOVER-60, they added.
Among patients who had received CHOP as first-line therapy, 2-year overall survival was 49.6% for those who received rituximab in the salvage setting, compared with 19.1% for those who did not (P less than .001), according to the published data. Likewise, in the initial R-CHOP group, 2-year overall survival was 33.1% for rituximab in salvage and 22.5% for no rituximab in salvage (P = .034).
The investigators also looked for differences in prognosis according to specific patient characteristics, including presence of MYC rearrangements and MYC expression by immunohistochemistry.
In patients with MYC translocation at diagnosis, use of rituximab reduced risk of initial treatment failure from 58.8% to 26.3%, according to the investigators. After treatment failure, patients who initially received CHOP had significantly improved 2-year survival if they had MYC translocations or negative MYC immunohistochemistry, though no such association was found for patients who initially received R-CHOP, they wrote.
Dr. Glass and colleagues concluded that new treatment strategies are needed.
“Overall, the outcome of second-line treatment of elderly patients with refractory and relapsed aggressive B-cell lymphoma is disappointing and worse than in younger patients regardless of the modality chosen,” they wrote. “New drugs and treatment modalities with the potential to change the dismal outlook for elderly patients with aggressive B-cell lymphomas are eagerly awaited.”
Dr. Glass and several coauthors reported honoraria, research funding, and consultancies with Roche.
In elderly patients with aggressive B-cell lymphomas who experience treatment failure after CHOP or rituximab-CHOP (R-CHOP), the outcomes of subsequent salvage therapy were improved when rituximab was included, results of a retrospective analysis suggest.
“Survival after rituximab-containing salvage therapy was better in all patient groups, supporting the repeated administration of rituximab to all patients needing salvage therapy,” wrote investigator Bertram Glass, MD, of the department of hematology and stem cell transplantation at Helios Klinikum Berlin-Buch, Berlin, and his coauthors (Ann Oncol. 2017 Oct 6. doi: 10.1093/annonc/mdx556).
Dr. Glass and colleagues reviewed data from the randomized RICOVER-60 trial, which included 1,222 patients aged 61-80 years with aggressive B-cell lymphomas who received CHOP or R-CHOP for six or eight cycles. Based on survival outcomes, six cycles of R-CHOP every 2 weeks should be the preferred regimen, investigators wrote when the study results were published in 2008 (Lancet Oncol. 2008;9[2]:105-16. doi: 10.1016/S1470-2045(08)70002-0).
Of 1,222 patients in the RICOVER-60 trial, 301 (24.6%) had treatment failure, of whom 297 could be included in the present analysis.
Rituximab, included in salvage therapy for 57.4% of those evaluable patients, was found to improve the 2-year survival rate from 20.7% to 46.8% (P less than .001), Dr. Glass and his coinvestigators reported.
The benefit of rituximab in the salvage setting was apparent regardless of whether patients received R-CHOP or CHOP as part of their initial therapy in RICOVER-60, they added.
Among patients who had received CHOP as first-line therapy, 2-year overall survival was 49.6% for those who received rituximab in the salvage setting, compared with 19.1% for those who did not (P less than .001), according to the published data. Likewise, in the initial R-CHOP group, 2-year overall survival was 33.1% for rituximab in salvage and 22.5% for no rituximab in salvage (P = .034).
The investigators also looked for differences in prognosis according to specific patient characteristics, including presence of MYC rearrangements and MYC expression by immunohistochemistry.
In patients with MYC translocation at diagnosis, use of rituximab reduced risk of initial treatment failure from 58.8% to 26.3%, according to the investigators. After treatment failure, patients who initially received CHOP had significantly improved 2-year survival if they had MYC translocations or negative MYC immunohistochemistry, though no such association was found for patients who initially received R-CHOP, they wrote.
Dr. Glass and colleagues concluded that new treatment strategies are needed.
“Overall, the outcome of second-line treatment of elderly patients with refractory and relapsed aggressive B-cell lymphoma is disappointing and worse than in younger patients regardless of the modality chosen,” they wrote. “New drugs and treatment modalities with the potential to change the dismal outlook for elderly patients with aggressive B-cell lymphomas are eagerly awaited.”
Dr. Glass and several coauthors reported honoraria, research funding, and consultancies with Roche.
FROM ANNALS OF ONCOLOGY
Key clinical point: Rituximab improved salvage therapy for elderly patients with aggressive-B-cell lymphoma who relapsed after CHOP or R-CHOP.
Major finding: Rituximab as part of a salvage regimen improved the 2-year survival rate from 20.7% to 46.8% (P less than .001).
Data source: Retrospective analysis including 297 elderly patients in the RICOVER-60 trial who had progressive, persistent, or relapsed lymphoma.
Disclosures: Dr. Glass and several coauthors reported honoraria, research funding, and consultancies with Roche.
When do patients with SSTIs require hospital admission and IV antibiotics?
Case
A 54-year old gentleman with a history of type 2 diabetes mellitus presents with several days of progressive left lower extremity redness, pain, swelling, and subjective fevers.
On physical examination the patient is afebrile and hemodynamically stable. The left lower extremity is swollen, warm, and tender to light palpation with an irregular area of erythema extending anteriorly from the ankle to just below the knee. There are no areas of purulence or fluctuance. Labs are notable for a mild leukocytosis of 11,500 cells/mcL. An ultrasound shows no evidence of deep vein thrombosis and the patient is started on vancomycin and ceftazidime and admitted for intravenous antibiotics.
Does the patient require hospital admission and continuation of intravenous antibiotics?
Introduction
The clinical presentation of SSTIs can vary greatly. Consequently, the management of SSTIs can be as simple as a short course of outpatient oral antibiotics or escalate to as complicated as surgical intervention and/or prolonged courses of IV antibiotics. Given the frequency with which these infections result in hospital admission, it is essential for the practicing hospitalist to be able to appropriately triage and treat SSTIs in order to assure adequate therapy, while simultaneously reducing unnecessary hospital days and avoiding indiscriminate exposure to broad spectrum antibiotics.
Pathophysiology and clinical presentation
SSTIs represent a diverse range of presentations and severities from superficial impetigo to life-threatening necrotizing infections, with abscesses and cellulitis being most commonly diagnosed.1
All SSTIs emerge from microbial invasion of the layers of the skin and underlying soft tissues. The accepted minimal criteria for diagnosis of an SSTI are erythema, edema, and warmth and tenderness of the affected area. Comorbid conditions that impair skin integrity, such as lymphedema, chronic inflammation (for example, eczema), intertrigo, or venous insufficiency therefore increase the risk of infection. However, the strongest risk factor for development of an SSTI is disruption of the skin barrier via trauma (foreign body, bite wound), ulceration, laceration, fissures, or surgical wound.2,3
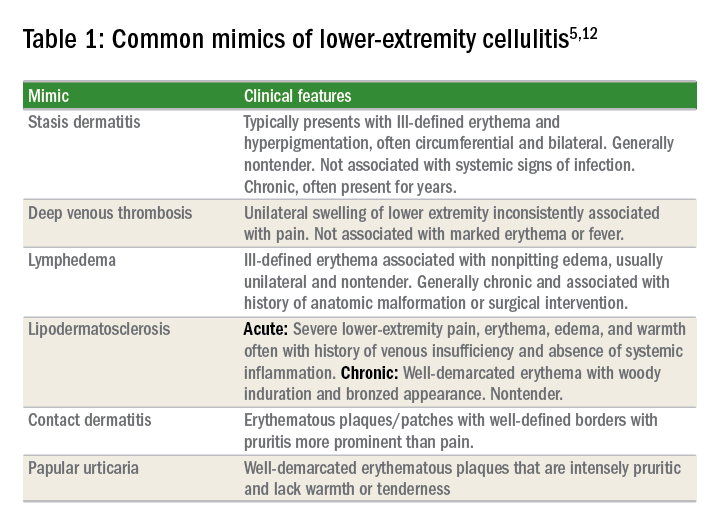
The hallmark features of SSTI are present in other noninfectious skin disorders, thus often yielding misdiagnosis. In a study of 259 patients hospitalized for lower extremity cellulitis, 79 patients (30.5%) were misdiagnosed.4 The most common mimic of SSTI is stasis dermatitis due to chronic venous insufficiency. Other conditions that are often misdiagnosed as SSTI include lymphedema, lipodermatosclerosis, contact dermatitis, papular urticaria and deep venous thrombosis. Differentiating between true SSTI and these “pseudo-cellulitic” conditions is essential to reducing unnecessary hospitalization and exposure to antibiotics, which contribute to nosocomial infection, iatrogenic injury (that is, Clostridium difficile infection, anaphylaxis) and avoidable health care costs.
Microbiology
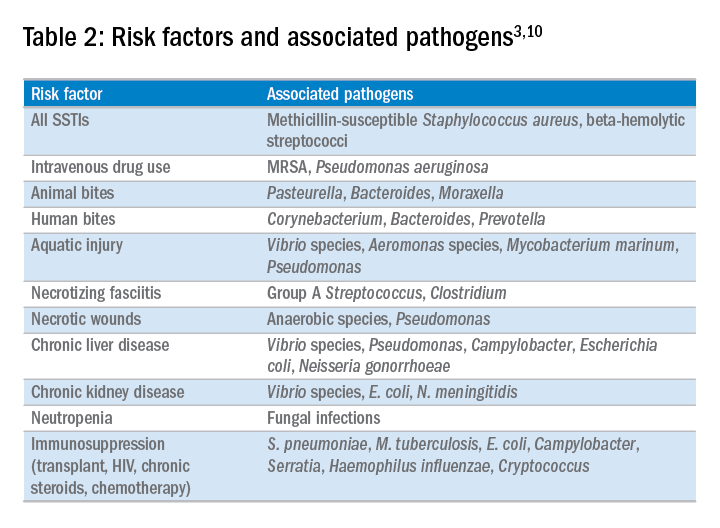
In the majority of cases, the causative pathogen is not identified; superficial culture data is often confounded and positive results do not guarantee pathogenicity of the identified organism. However, the mechanism of bacterial entry, location of infection, and presence of underlying medical conditions also influence the infectious organism(s). For example, infections of the lower extremities may involve enteric organisms such as E. coli and Enterococcus due to fecal runoff. SSTIs due to cat and dog bites commonly involve Pasteurella multocida, while hot tub exposure and intravenous drug use increase the risk of infection with Pseudomonas aeruginosa. Patients with neutropenia are at increased risk for fungal and yeast infections. Consequently, an assessment for potential risk factors is essential in determining appropriate management. Common pathogens associated with various clinical presentations and risk factors are outlined in Table 2.
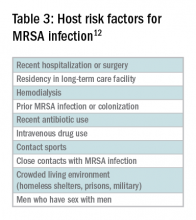
In addition to host risk factors, the type of SSTI may hint at the most likely organisms. Among purulent (“culturable”) SSTIs, up to 76% of infections are due to S. aureus, whereas in diffuse (“nonculturable”) cellulitis, the majority of cases are attributable to B-hemolytic streptococcus.7 The role of S. aureus in SSTIs is further complicated by the rise of methicillin-resistant S. aureus (MRSA), both nosocomial and community-acquired. It is estimated that between 25%-50% of all S. aureus isolates in the United States show methicillin resistance.6,8 Despite the rising prevalence of MRSA, reflexive treatment for MRSA should be avoided in the absence of high-risk presentations (for example, purulent SSTI) or patient risk factors for MRSA (Table 3).
Severity of infection
Given the variety of clinical presentations of SSTIs, an evaluation of the severity of disease is essential to determining appropriate initial management, including the need for hospitalization and intravenous antibiotics. Several grading systems have been proposed to assist in determining severity. High-risk features that are common to these systems include:
• Evidence of systemic infection (fever, tachycardia, altered mental status, tachypnea, hypotension);
• Location of infection with increased risk of local complication (face, brain, hand, perineum);
• Indication of deep tissue infection (for example, crepitus, bullae, or hemorrhage);
• Comorbid conditions predisposing to more severe infection (liver or renal disease, immunocompromised state including neutropenia or active chemotherapy, vascular insufficiency).
In assessing for necrotic infection, the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score can help to distinguish severe cellulitis from necrotizing infections that require immediate surgical evaluation. The LRINEC score uses readily available laboratory markers to stratify patients into tertiles of risk for necrotizing fasciitis. While this objective score can identify patients who may require immediate surgical intervention, any patient with a clinical history or exam concerning for necrotizing infection should be urgently evaluated for possible surgical debridement.11,12
Management
Nonpurulent disease
Nonpurulent SSTIs include cellulitis and necrotizing infections such as necrotizing fasciitis. In the absence of risk factors for particular infectious agents (see above), mild infections can be managed with a trial of oral antibiotics with coverage for streptococcal species such as cephalexin, clindamycin, or amoxicillin-clavulanate.
Empiric coverage for MRSA is not recommended and has been shown to have little benefit. In a trial of 146 patients with mild nonpurulent cellulitis, there was no significant difference in cure rate at 2 weeks between cephalexin monotherapy and dual therapy with cephalexin and trimethoprim-sulfamethoxazole.13
Moderate infections warrant admission for intravenous antibiotics, also with coverage for streptococcal species and MSSA such as penicillin or cefazolin. In most cases, coverage for MRSA is not required but may be considered in patients with risk factors for MRSA. Generally, blood or cutaneous cultures are not recommended given an expected yield of positive culture to be less than 5%,14 but may be considered in patients with immunosuppression or neutropenia or evidence of systemic inflammatory response.
Severe infections should be evaluated for the need for surgical debridement. Empiric antibiotic coverage for Streptococcus pyogenes, MRSA, and gram-negative and anaerobic species is warranted. If necrotizing infection is suspected or diagnosed, immediate surgical debridement is indicated. Culture data from surgical debridement should be obtained and can be useful for tailoring therapy. While blood cultures remain unlikely to yield useful data, it is reasonable to obtain them in severe disease. Empiric antibiotic coverage should be broad and narrowed based on surgical specimens. The general recommendation regarding duration of antibiotics is 5 days; however, longer courses of up to 10-14 days may be required if there is minimal improvement after initial therapy.9
Purulent disease
Purulent SSTIs by definition involve collections of pus and include abscesses, furuncles, and carbuncles. In all purulent SSTIs, incision and drainage is indicated.
For mild disease, incision and drainage is considered definitive management and deep wound cultures and antibiotics are not required. In moderate purulent SSTI, culture of the drained fluid should be obtained, and antibiotics administered with empiric therapy to include coverage for MRSA. Patients at risk for community-acquired MRSA can be given oral agents such as trimethoprim-sulfamethoxazole, clindamycin, or doxycycline (depending upon local antibiogram data) with antibiotics narrowed based on culture data.
In order to minimize treatment failure with oral agents, dosing should be weight based with a minimum of 5 mg/kg per day of bactrim or 10 mg/kg per day of clindamycin.15 Those with risk factors for nosocomial MRSA may warrant intravenous antibiotics, even in moderate disease. Patients with severe purulent disease require intravenous antibiotics with coverage for MRSA with vancomycin, daptomycin or linezolid and subsequently narrowed based on culture data.9
Back to the case
Our patient presented with a case of mild, nonpurulent cellulitis. While he does have a mildly elevated white blood cell count, he has no other signs of systemic infection or underlying conditions predisposing to more severe disease. Hospital admission is not required and de-escalation of antibiotics to an oral agent is appropriate.
If the patient exhibited other signs of systemic infection (that is, fever or tachycardia) hospital admission or admission to observation status for IV antibiotics would be appropriate; however, de-escalation would still be recommended as MRSA coverage is not warranted. We suggest discharge from the emergency department with oral cephalexin, provided no prohibitive allergy is known, with outpatient follow-up to ensure resolution of infection.
Bottom line
SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions. If an infectious process is considered most likely, the need for hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, and not empirically initiated for all presentations.
Dr. Perry is an instructor in the Yale Academic Hospitalist Program at Yale University, New Haven, Conn. Dr. Fogerty is associate professor of medicine in the Yale Academic Hospitalist Program. Dr. Sankey is assistant professor of medicine and interim inpatient medicine clerkship director in the Yale Academic Hospitalist Program.
Key Points
• SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions
• The majority of SSTIs are caused by gram-positive organisms, most notably Staphylococcus aureus and B-hemolytic streptococci
• Evaluation of the severity of disease, using a grading system, is essential to determining appropriate initial management
• Hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, not empirically initiated for all presentations
References
1. Miller L, Eisenberg D, Liu H, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005-2010. BMC Infect Dis. 2015 Aug 21;15:362.
2. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg (cellulitis): case control study. BMJ. 1999;318(7198):1591-4.
3. Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008 Mar;19(2):173-84.
4. Weng QY, Raff AB, Cohen JM, et al. Costs and Consequences Associated with Misdiagnosed Lower Extremity Cellulitis. JAMA Dermatol. 2016 Nov 2.
5. Keller E, Tomecki K, Alraies M. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012 Aug;79(8)547-52.
6. Bassetti, M, Carmelutti A, Righi E. The role of methicillin-resistant Staphylococcus aureus in skin and soft tissue infections. Curr Opin Infect Dis. 2017 Apr.
7. Jeng A, Beheshti M, Li J, Ramesh N. The Role of B-hemolytic Streptococci in Causing Diffuse, Nonculturable Cellulitis. Medicine (Baltimore). 2010 Jul;89(4):217-26.
8. Moran G, Krishnadasan A, Gorwitz R. Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department. N Engl J Med. 2006; 355:666-74.
9. Stevens D, Bisno A, Chambers H, et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft tissue Infections: 2014 Update by the Infectious Disease Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
10. Raff, AB and Kroshinsky D. Cellulitis: A Review. JAMA. 2016 Jul;316(3):325-37.
11. Wong, CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004 Jul;32(7):1535-41.
12. Gunderson CG. Cellulitis: Definition, etiology and clinical features. Am J Med 2011 Dec;124(12):1113-22.
13. Pallin D, et al. Comparative Effectiveness of Cephalexin Plus Trimethoprim-Sulfamethoxazole versus Cephalexin Alone for Treatment of Uncomplicated Cellulitis: A Randomized Controlled Trial. Clin Infect Dis. 2013 Jun;56(12):1754-62.
14. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148-55.
15. Halilovic J, Heintz BH, Brown J.. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect. 2012;65(2):128-34.
Case
A 54-year old gentleman with a history of type 2 diabetes mellitus presents with several days of progressive left lower extremity redness, pain, swelling, and subjective fevers.
On physical examination the patient is afebrile and hemodynamically stable. The left lower extremity is swollen, warm, and tender to light palpation with an irregular area of erythema extending anteriorly from the ankle to just below the knee. There are no areas of purulence or fluctuance. Labs are notable for a mild leukocytosis of 11,500 cells/mcL. An ultrasound shows no evidence of deep vein thrombosis and the patient is started on vancomycin and ceftazidime and admitted for intravenous antibiotics.
Does the patient require hospital admission and continuation of intravenous antibiotics?
Introduction
The clinical presentation of SSTIs can vary greatly. Consequently, the management of SSTIs can be as simple as a short course of outpatient oral antibiotics or escalate to as complicated as surgical intervention and/or prolonged courses of IV antibiotics. Given the frequency with which these infections result in hospital admission, it is essential for the practicing hospitalist to be able to appropriately triage and treat SSTIs in order to assure adequate therapy, while simultaneously reducing unnecessary hospital days and avoiding indiscriminate exposure to broad spectrum antibiotics.
Pathophysiology and clinical presentation
SSTIs represent a diverse range of presentations and severities from superficial impetigo to life-threatening necrotizing infections, with abscesses and cellulitis being most commonly diagnosed.1
All SSTIs emerge from microbial invasion of the layers of the skin and underlying soft tissues. The accepted minimal criteria for diagnosis of an SSTI are erythema, edema, and warmth and tenderness of the affected area. Comorbid conditions that impair skin integrity, such as lymphedema, chronic inflammation (for example, eczema), intertrigo, or venous insufficiency therefore increase the risk of infection. However, the strongest risk factor for development of an SSTI is disruption of the skin barrier via trauma (foreign body, bite wound), ulceration, laceration, fissures, or surgical wound.2,3
The hallmark features of SSTI are present in other noninfectious skin disorders, thus often yielding misdiagnosis. In a study of 259 patients hospitalized for lower extremity cellulitis, 79 patients (30.5%) were misdiagnosed.4 The most common mimic of SSTI is stasis dermatitis due to chronic venous insufficiency. Other conditions that are often misdiagnosed as SSTI include lymphedema, lipodermatosclerosis, contact dermatitis, papular urticaria and deep venous thrombosis. Differentiating between true SSTI and these “pseudo-cellulitic” conditions is essential to reducing unnecessary hospitalization and exposure to antibiotics, which contribute to nosocomial infection, iatrogenic injury (that is, Clostridium difficile infection, anaphylaxis) and avoidable health care costs.
Microbiology
In the majority of cases, the causative pathogen is not identified; superficial culture data is often confounded and positive results do not guarantee pathogenicity of the identified organism. However, the mechanism of bacterial entry, location of infection, and presence of underlying medical conditions also influence the infectious organism(s). For example, infections of the lower extremities may involve enteric organisms such as E. coli and Enterococcus due to fecal runoff. SSTIs due to cat and dog bites commonly involve Pasteurella multocida, while hot tub exposure and intravenous drug use increase the risk of infection with Pseudomonas aeruginosa. Patients with neutropenia are at increased risk for fungal and yeast infections. Consequently, an assessment for potential risk factors is essential in determining appropriate management. Common pathogens associated with various clinical presentations and risk factors are outlined in Table 2.
In addition to host risk factors, the type of SSTI may hint at the most likely organisms. Among purulent (“culturable”) SSTIs, up to 76% of infections are due to S. aureus, whereas in diffuse (“nonculturable”) cellulitis, the majority of cases are attributable to B-hemolytic streptococcus.7 The role of S. aureus in SSTIs is further complicated by the rise of methicillin-resistant S. aureus (MRSA), both nosocomial and community-acquired. It is estimated that between 25%-50% of all S. aureus isolates in the United States show methicillin resistance.6,8 Despite the rising prevalence of MRSA, reflexive treatment for MRSA should be avoided in the absence of high-risk presentations (for example, purulent SSTI) or patient risk factors for MRSA (Table 3).
Severity of infection
Given the variety of clinical presentations of SSTIs, an evaluation of the severity of disease is essential to determining appropriate initial management, including the need for hospitalization and intravenous antibiotics. Several grading systems have been proposed to assist in determining severity. High-risk features that are common to these systems include:
• Evidence of systemic infection (fever, tachycardia, altered mental status, tachypnea, hypotension);
• Location of infection with increased risk of local complication (face, brain, hand, perineum);
• Indication of deep tissue infection (for example, crepitus, bullae, or hemorrhage);
• Comorbid conditions predisposing to more severe infection (liver or renal disease, immunocompromised state including neutropenia or active chemotherapy, vascular insufficiency).
In assessing for necrotic infection, the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score can help to distinguish severe cellulitis from necrotizing infections that require immediate surgical evaluation. The LRINEC score uses readily available laboratory markers to stratify patients into tertiles of risk for necrotizing fasciitis. While this objective score can identify patients who may require immediate surgical intervention, any patient with a clinical history or exam concerning for necrotizing infection should be urgently evaluated for possible surgical debridement.11,12
Management
Nonpurulent disease
Nonpurulent SSTIs include cellulitis and necrotizing infections such as necrotizing fasciitis. In the absence of risk factors for particular infectious agents (see above), mild infections can be managed with a trial of oral antibiotics with coverage for streptococcal species such as cephalexin, clindamycin, or amoxicillin-clavulanate.
Empiric coverage for MRSA is not recommended and has been shown to have little benefit. In a trial of 146 patients with mild nonpurulent cellulitis, there was no significant difference in cure rate at 2 weeks between cephalexin monotherapy and dual therapy with cephalexin and trimethoprim-sulfamethoxazole.13
Moderate infections warrant admission for intravenous antibiotics, also with coverage for streptococcal species and MSSA such as penicillin or cefazolin. In most cases, coverage for MRSA is not required but may be considered in patients with risk factors for MRSA. Generally, blood or cutaneous cultures are not recommended given an expected yield of positive culture to be less than 5%,14 but may be considered in patients with immunosuppression or neutropenia or evidence of systemic inflammatory response.
Severe infections should be evaluated for the need for surgical debridement. Empiric antibiotic coverage for Streptococcus pyogenes, MRSA, and gram-negative and anaerobic species is warranted. If necrotizing infection is suspected or diagnosed, immediate surgical debridement is indicated. Culture data from surgical debridement should be obtained and can be useful for tailoring therapy. While blood cultures remain unlikely to yield useful data, it is reasonable to obtain them in severe disease. Empiric antibiotic coverage should be broad and narrowed based on surgical specimens. The general recommendation regarding duration of antibiotics is 5 days; however, longer courses of up to 10-14 days may be required if there is minimal improvement after initial therapy.9
Purulent disease
Purulent SSTIs by definition involve collections of pus and include abscesses, furuncles, and carbuncles. In all purulent SSTIs, incision and drainage is indicated.
For mild disease, incision and drainage is considered definitive management and deep wound cultures and antibiotics are not required. In moderate purulent SSTI, culture of the drained fluid should be obtained, and antibiotics administered with empiric therapy to include coverage for MRSA. Patients at risk for community-acquired MRSA can be given oral agents such as trimethoprim-sulfamethoxazole, clindamycin, or doxycycline (depending upon local antibiogram data) with antibiotics narrowed based on culture data.
In order to minimize treatment failure with oral agents, dosing should be weight based with a minimum of 5 mg/kg per day of bactrim or 10 mg/kg per day of clindamycin.15 Those with risk factors for nosocomial MRSA may warrant intravenous antibiotics, even in moderate disease. Patients with severe purulent disease require intravenous antibiotics with coverage for MRSA with vancomycin, daptomycin or linezolid and subsequently narrowed based on culture data.9
Back to the case
Our patient presented with a case of mild, nonpurulent cellulitis. While he does have a mildly elevated white blood cell count, he has no other signs of systemic infection or underlying conditions predisposing to more severe disease. Hospital admission is not required and de-escalation of antibiotics to an oral agent is appropriate.
If the patient exhibited other signs of systemic infection (that is, fever or tachycardia) hospital admission or admission to observation status for IV antibiotics would be appropriate; however, de-escalation would still be recommended as MRSA coverage is not warranted. We suggest discharge from the emergency department with oral cephalexin, provided no prohibitive allergy is known, with outpatient follow-up to ensure resolution of infection.
Bottom line
SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions. If an infectious process is considered most likely, the need for hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, and not empirically initiated for all presentations.
Dr. Perry is an instructor in the Yale Academic Hospitalist Program at Yale University, New Haven, Conn. Dr. Fogerty is associate professor of medicine in the Yale Academic Hospitalist Program. Dr. Sankey is assistant professor of medicine and interim inpatient medicine clerkship director in the Yale Academic Hospitalist Program.
Key Points
• SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions
• The majority of SSTIs are caused by gram-positive organisms, most notably Staphylococcus aureus and B-hemolytic streptococci
• Evaluation of the severity of disease, using a grading system, is essential to determining appropriate initial management
• Hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, not empirically initiated for all presentations


References
1. Miller L, Eisenberg D, Liu H, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005-2010. BMC Infect Dis. 2015 Aug 21;15:362.
2. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg (cellulitis): case control study. BMJ. 1999;318(7198):1591-4.
3. Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008 Mar;19(2):173-84.
4. Weng QY, Raff AB, Cohen JM, et al. Costs and Consequences Associated with Misdiagnosed Lower Extremity Cellulitis. JAMA Dermatol. 2016 Nov 2.
5. Keller E, Tomecki K, Alraies M. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012 Aug;79(8)547-52.
6. Bassetti, M, Carmelutti A, Righi E. The role of methicillin-resistant Staphylococcus aureus in skin and soft tissue infections. Curr Opin Infect Dis. 2017 Apr.
7. Jeng A, Beheshti M, Li J, Ramesh N. The Role of B-hemolytic Streptococci in Causing Diffuse, Nonculturable Cellulitis. Medicine (Baltimore). 2010 Jul;89(4):217-26.
8. Moran G, Krishnadasan A, Gorwitz R. Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department. N Engl J Med. 2006; 355:666-74.
9. Stevens D, Bisno A, Chambers H, et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft tissue Infections: 2014 Update by the Infectious Disease Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
10. Raff, AB and Kroshinsky D. Cellulitis: A Review. JAMA. 2016 Jul;316(3):325-37.
11. Wong, CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004 Jul;32(7):1535-41.
12. Gunderson CG. Cellulitis: Definition, etiology and clinical features. Am J Med 2011 Dec;124(12):1113-22.
13. Pallin D, et al. Comparative Effectiveness of Cephalexin Plus Trimethoprim-Sulfamethoxazole versus Cephalexin Alone for Treatment of Uncomplicated Cellulitis: A Randomized Controlled Trial. Clin Infect Dis. 2013 Jun;56(12):1754-62.
14. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148-55.
15. Halilovic J, Heintz BH, Brown J.. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect. 2012;65(2):128-34.
Case
A 54-year old gentleman with a history of type 2 diabetes mellitus presents with several days of progressive left lower extremity redness, pain, swelling, and subjective fevers.
On physical examination the patient is afebrile and hemodynamically stable. The left lower extremity is swollen, warm, and tender to light palpation with an irregular area of erythema extending anteriorly from the ankle to just below the knee. There are no areas of purulence or fluctuance. Labs are notable for a mild leukocytosis of 11,500 cells/mcL. An ultrasound shows no evidence of deep vein thrombosis and the patient is started on vancomycin and ceftazidime and admitted for intravenous antibiotics.
Does the patient require hospital admission and continuation of intravenous antibiotics?
Introduction
The clinical presentation of SSTIs can vary greatly. Consequently, the management of SSTIs can be as simple as a short course of outpatient oral antibiotics or escalate to as complicated as surgical intervention and/or prolonged courses of IV antibiotics. Given the frequency with which these infections result in hospital admission, it is essential for the practicing hospitalist to be able to appropriately triage and treat SSTIs in order to assure adequate therapy, while simultaneously reducing unnecessary hospital days and avoiding indiscriminate exposure to broad spectrum antibiotics.
Pathophysiology and clinical presentation
SSTIs represent a diverse range of presentations and severities from superficial impetigo to life-threatening necrotizing infections, with abscesses and cellulitis being most commonly diagnosed.1
All SSTIs emerge from microbial invasion of the layers of the skin and underlying soft tissues. The accepted minimal criteria for diagnosis of an SSTI are erythema, edema, and warmth and tenderness of the affected area. Comorbid conditions that impair skin integrity, such as lymphedema, chronic inflammation (for example, eczema), intertrigo, or venous insufficiency therefore increase the risk of infection. However, the strongest risk factor for development of an SSTI is disruption of the skin barrier via trauma (foreign body, bite wound), ulceration, laceration, fissures, or surgical wound.2,3
The hallmark features of SSTI are present in other noninfectious skin disorders, thus often yielding misdiagnosis. In a study of 259 patients hospitalized for lower extremity cellulitis, 79 patients (30.5%) were misdiagnosed.4 The most common mimic of SSTI is stasis dermatitis due to chronic venous insufficiency. Other conditions that are often misdiagnosed as SSTI include lymphedema, lipodermatosclerosis, contact dermatitis, papular urticaria and deep venous thrombosis. Differentiating between true SSTI and these “pseudo-cellulitic” conditions is essential to reducing unnecessary hospitalization and exposure to antibiotics, which contribute to nosocomial infection, iatrogenic injury (that is, Clostridium difficile infection, anaphylaxis) and avoidable health care costs.
Microbiology
In the majority of cases, the causative pathogen is not identified; superficial culture data is often confounded and positive results do not guarantee pathogenicity of the identified organism. However, the mechanism of bacterial entry, location of infection, and presence of underlying medical conditions also influence the infectious organism(s). For example, infections of the lower extremities may involve enteric organisms such as E. coli and Enterococcus due to fecal runoff. SSTIs due to cat and dog bites commonly involve Pasteurella multocida, while hot tub exposure and intravenous drug use increase the risk of infection with Pseudomonas aeruginosa. Patients with neutropenia are at increased risk for fungal and yeast infections. Consequently, an assessment for potential risk factors is essential in determining appropriate management. Common pathogens associated with various clinical presentations and risk factors are outlined in Table 2.
In addition to host risk factors, the type of SSTI may hint at the most likely organisms. Among purulent (“culturable”) SSTIs, up to 76% of infections are due to S. aureus, whereas in diffuse (“nonculturable”) cellulitis, the majority of cases are attributable to B-hemolytic streptococcus.7 The role of S. aureus in SSTIs is further complicated by the rise of methicillin-resistant S. aureus (MRSA), both nosocomial and community-acquired. It is estimated that between 25%-50% of all S. aureus isolates in the United States show methicillin resistance.6,8 Despite the rising prevalence of MRSA, reflexive treatment for MRSA should be avoided in the absence of high-risk presentations (for example, purulent SSTI) or patient risk factors for MRSA (Table 3).
Severity of infection
Given the variety of clinical presentations of SSTIs, an evaluation of the severity of disease is essential to determining appropriate initial management, including the need for hospitalization and intravenous antibiotics. Several grading systems have been proposed to assist in determining severity. High-risk features that are common to these systems include:
• Evidence of systemic infection (fever, tachycardia, altered mental status, tachypnea, hypotension);
• Location of infection with increased risk of local complication (face, brain, hand, perineum);
• Indication of deep tissue infection (for example, crepitus, bullae, or hemorrhage);
• Comorbid conditions predisposing to more severe infection (liver or renal disease, immunocompromised state including neutropenia or active chemotherapy, vascular insufficiency).
In assessing for necrotic infection, the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score can help to distinguish severe cellulitis from necrotizing infections that require immediate surgical evaluation. The LRINEC score uses readily available laboratory markers to stratify patients into tertiles of risk for necrotizing fasciitis. While this objective score can identify patients who may require immediate surgical intervention, any patient with a clinical history or exam concerning for necrotizing infection should be urgently evaluated for possible surgical debridement.11,12
Management
Nonpurulent disease
Nonpurulent SSTIs include cellulitis and necrotizing infections such as necrotizing fasciitis. In the absence of risk factors for particular infectious agents (see above), mild infections can be managed with a trial of oral antibiotics with coverage for streptococcal species such as cephalexin, clindamycin, or amoxicillin-clavulanate.
Empiric coverage for MRSA is not recommended and has been shown to have little benefit. In a trial of 146 patients with mild nonpurulent cellulitis, there was no significant difference in cure rate at 2 weeks between cephalexin monotherapy and dual therapy with cephalexin and trimethoprim-sulfamethoxazole.13
Moderate infections warrant admission for intravenous antibiotics, also with coverage for streptococcal species and MSSA such as penicillin or cefazolin. In most cases, coverage for MRSA is not required but may be considered in patients with risk factors for MRSA. Generally, blood or cutaneous cultures are not recommended given an expected yield of positive culture to be less than 5%,14 but may be considered in patients with immunosuppression or neutropenia or evidence of systemic inflammatory response.
Severe infections should be evaluated for the need for surgical debridement. Empiric antibiotic coverage for Streptococcus pyogenes, MRSA, and gram-negative and anaerobic species is warranted. If necrotizing infection is suspected or diagnosed, immediate surgical debridement is indicated. Culture data from surgical debridement should be obtained and can be useful for tailoring therapy. While blood cultures remain unlikely to yield useful data, it is reasonable to obtain them in severe disease. Empiric antibiotic coverage should be broad and narrowed based on surgical specimens. The general recommendation regarding duration of antibiotics is 5 days; however, longer courses of up to 10-14 days may be required if there is minimal improvement after initial therapy.9
Purulent disease
Purulent SSTIs by definition involve collections of pus and include abscesses, furuncles, and carbuncles. In all purulent SSTIs, incision and drainage is indicated.
For mild disease, incision and drainage is considered definitive management and deep wound cultures and antibiotics are not required. In moderate purulent SSTI, culture of the drained fluid should be obtained, and antibiotics administered with empiric therapy to include coverage for MRSA. Patients at risk for community-acquired MRSA can be given oral agents such as trimethoprim-sulfamethoxazole, clindamycin, or doxycycline (depending upon local antibiogram data) with antibiotics narrowed based on culture data.
In order to minimize treatment failure with oral agents, dosing should be weight based with a minimum of 5 mg/kg per day of bactrim or 10 mg/kg per day of clindamycin.15 Those with risk factors for nosocomial MRSA may warrant intravenous antibiotics, even in moderate disease. Patients with severe purulent disease require intravenous antibiotics with coverage for MRSA with vancomycin, daptomycin or linezolid and subsequently narrowed based on culture data.9
Back to the case
Our patient presented with a case of mild, nonpurulent cellulitis. While he does have a mildly elevated white blood cell count, he has no other signs of systemic infection or underlying conditions predisposing to more severe disease. Hospital admission is not required and de-escalation of antibiotics to an oral agent is appropriate.
If the patient exhibited other signs of systemic infection (that is, fever or tachycardia) hospital admission or admission to observation status for IV antibiotics would be appropriate; however, de-escalation would still be recommended as MRSA coverage is not warranted. We suggest discharge from the emergency department with oral cephalexin, provided no prohibitive allergy is known, with outpatient follow-up to ensure resolution of infection.
Bottom line
SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions. If an infectious process is considered most likely, the need for hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, and not empirically initiated for all presentations.
Dr. Perry is an instructor in the Yale Academic Hospitalist Program at Yale University, New Haven, Conn. Dr. Fogerty is associate professor of medicine in the Yale Academic Hospitalist Program. Dr. Sankey is assistant professor of medicine and interim inpatient medicine clerkship director in the Yale Academic Hospitalist Program.
Key Points
• SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions
• The majority of SSTIs are caused by gram-positive organisms, most notably Staphylococcus aureus and B-hemolytic streptococci
• Evaluation of the severity of disease, using a grading system, is essential to determining appropriate initial management
• Hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, not empirically initiated for all presentations


References
1. Miller L, Eisenberg D, Liu H, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005-2010. BMC Infect Dis. 2015 Aug 21;15:362.
2. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg (cellulitis): case control study. BMJ. 1999;318(7198):1591-4.
3. Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008 Mar;19(2):173-84.
4. Weng QY, Raff AB, Cohen JM, et al. Costs and Consequences Associated with Misdiagnosed Lower Extremity Cellulitis. JAMA Dermatol. 2016 Nov 2.
5. Keller E, Tomecki K, Alraies M. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012 Aug;79(8)547-52.
6. Bassetti, M, Carmelutti A, Righi E. The role of methicillin-resistant Staphylococcus aureus in skin and soft tissue infections. Curr Opin Infect Dis. 2017 Apr.
7. Jeng A, Beheshti M, Li J, Ramesh N. The Role of B-hemolytic Streptococci in Causing Diffuse, Nonculturable Cellulitis. Medicine (Baltimore). 2010 Jul;89(4):217-26.
8. Moran G, Krishnadasan A, Gorwitz R. Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department. N Engl J Med. 2006; 355:666-74.
9. Stevens D, Bisno A, Chambers H, et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft tissue Infections: 2014 Update by the Infectious Disease Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
10. Raff, AB and Kroshinsky D. Cellulitis: A Review. JAMA. 2016 Jul;316(3):325-37.
11. Wong, CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004 Jul;32(7):1535-41.
12. Gunderson CG. Cellulitis: Definition, etiology and clinical features. Am J Med 2011 Dec;124(12):1113-22.
13. Pallin D, et al. Comparative Effectiveness of Cephalexin Plus Trimethoprim-Sulfamethoxazole versus Cephalexin Alone for Treatment of Uncomplicated Cellulitis: A Randomized Controlled Trial. Clin Infect Dis. 2013 Jun;56(12):1754-62.
14. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148-55.
15. Halilovic J, Heintz BH, Brown J.. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect. 2012;65(2):128-34.
Nutrition status predicts outcomes in liver transplant
WASHINGTON – Efforts to improve nutritional status prior to transplant may lead to improved patient outcomes and economic benefits after orthotopic liver transplant.
Clinicians at Austin Health, a tertiary health center in Melbourne, reviewed prospectively acquired data on 390 adult patients who underwent orthotopic liver transplant at their institution between January 2009 and June 2016, according to Brooke Chapman, a dietitian on the center’s transplant team.
“Hand-grip strength test is a functional measure of upper-body strength,” Ms. Chapman said at the annual meeting of the American Association for the Study of Liver Diseases. “It’s quick and cheap and reliable but importantly, it does respond quite readily to changes in nutritional intake and nutrition status.”
Assessments were made as patients were wait listed for liver transplant. Hand-grip strength and subjective global assessment were repeated at the time of transplant.
Patients with fulminant liver failure and those requiring retransplantation were excluded from the final analysis, leaving 321 patients in the cohort. More than two-thirds (69%) were men and the median age was 52 years old. About half of patients had a diagnosis of hepatocellular carcinoma or hepatitis C infection. The median MELD (Model for Endstage Liver Disease) score was 18, with a range of 6-40, and the median time on the wait list was 140 days.
We saw a “high prevalence of malnutrition in patients undergoing liver transplant and the deterioration in nutritional status despite our best efforts while they are on the waiting list,” Ms. Chapman said.
At baseline, two-thirds of patients were malnourished – either mildly to moderately or severely; by transplantation, 77% were malnourished.
“At assessment, we are prescribing and educating patients on a high-calorie, high-protein diet initially, and we give oral nutrition support therapies,” she said. “We really try to get them to improve oral intake, but for patients who do require more aggressive intervention, we will feed them via nasogastric tube.”
Just over half (55%) of patients fell below the cutoff for sarcopenia on the hand-grip test at baseline and at transplant. More than a quarter of patients (27%) were not able to complete the 6-minute walk test.
“On univariate analysis, we saw malnutrition to be strongly associated with increased ICU and hospital length of stay,” Ms. Chapman noted. Severely malnourished patients spent significantly more time in the ICU than did well-nourished patients – a mean 147 hours vs. 89 hours (P = .001). Mean length of stay also was significantly longer at 40 days vs. 16 days (P = .003).
There was also an increased incidence of infection in severely malnourished patients as compared with well-nourished patients – 55.2% vs. 33.8%, she said.
“Aggressive strategies to combat malnutrition and deconditioning in the pretransplant period may lead to improved outcomes after transplant,” Ms. Chapman concluded.
The study was funded by Austin Health. Ms. Chapman declared no relevant conflicts of interest.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
WASHINGTON – Efforts to improve nutritional status prior to transplant may lead to improved patient outcomes and economic benefits after orthotopic liver transplant.
Clinicians at Austin Health, a tertiary health center in Melbourne, reviewed prospectively acquired data on 390 adult patients who underwent orthotopic liver transplant at their institution between January 2009 and June 2016, according to Brooke Chapman, a dietitian on the center’s transplant team.
“Hand-grip strength test is a functional measure of upper-body strength,” Ms. Chapman said at the annual meeting of the American Association for the Study of Liver Diseases. “It’s quick and cheap and reliable but importantly, it does respond quite readily to changes in nutritional intake and nutrition status.”
Assessments were made as patients were wait listed for liver transplant. Hand-grip strength and subjective global assessment were repeated at the time of transplant.
Patients with fulminant liver failure and those requiring retransplantation were excluded from the final analysis, leaving 321 patients in the cohort. More than two-thirds (69%) were men and the median age was 52 years old. About half of patients had a diagnosis of hepatocellular carcinoma or hepatitis C infection. The median MELD (Model for Endstage Liver Disease) score was 18, with a range of 6-40, and the median time on the wait list was 140 days.
We saw a “high prevalence of malnutrition in patients undergoing liver transplant and the deterioration in nutritional status despite our best efforts while they are on the waiting list,” Ms. Chapman said.
At baseline, two-thirds of patients were malnourished – either mildly to moderately or severely; by transplantation, 77% were malnourished.
“At assessment, we are prescribing and educating patients on a high-calorie, high-protein diet initially, and we give oral nutrition support therapies,” she said. “We really try to get them to improve oral intake, but for patients who do require more aggressive intervention, we will feed them via nasogastric tube.”
Just over half (55%) of patients fell below the cutoff for sarcopenia on the hand-grip test at baseline and at transplant. More than a quarter of patients (27%) were not able to complete the 6-minute walk test.
“On univariate analysis, we saw malnutrition to be strongly associated with increased ICU and hospital length of stay,” Ms. Chapman noted. Severely malnourished patients spent significantly more time in the ICU than did well-nourished patients – a mean 147 hours vs. 89 hours (P = .001). Mean length of stay also was significantly longer at 40 days vs. 16 days (P = .003).
There was also an increased incidence of infection in severely malnourished patients as compared with well-nourished patients – 55.2% vs. 33.8%, she said.
“Aggressive strategies to combat malnutrition and deconditioning in the pretransplant period may lead to improved outcomes after transplant,” Ms. Chapman concluded.
The study was funded by Austin Health. Ms. Chapman declared no relevant conflicts of interest.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
WASHINGTON – Efforts to improve nutritional status prior to transplant may lead to improved patient outcomes and economic benefits after orthotopic liver transplant.
Clinicians at Austin Health, a tertiary health center in Melbourne, reviewed prospectively acquired data on 390 adult patients who underwent orthotopic liver transplant at their institution between January 2009 and June 2016, according to Brooke Chapman, a dietitian on the center’s transplant team.
“Hand-grip strength test is a functional measure of upper-body strength,” Ms. Chapman said at the annual meeting of the American Association for the Study of Liver Diseases. “It’s quick and cheap and reliable but importantly, it does respond quite readily to changes in nutritional intake and nutrition status.”
Assessments were made as patients were wait listed for liver transplant. Hand-grip strength and subjective global assessment were repeated at the time of transplant.
Patients with fulminant liver failure and those requiring retransplantation were excluded from the final analysis, leaving 321 patients in the cohort. More than two-thirds (69%) were men and the median age was 52 years old. About half of patients had a diagnosis of hepatocellular carcinoma or hepatitis C infection. The median MELD (Model for Endstage Liver Disease) score was 18, with a range of 6-40, and the median time on the wait list was 140 days.
We saw a “high prevalence of malnutrition in patients undergoing liver transplant and the deterioration in nutritional status despite our best efforts while they are on the waiting list,” Ms. Chapman said.
At baseline, two-thirds of patients were malnourished – either mildly to moderately or severely; by transplantation, 77% were malnourished.
“At assessment, we are prescribing and educating patients on a high-calorie, high-protein diet initially, and we give oral nutrition support therapies,” she said. “We really try to get them to improve oral intake, but for patients who do require more aggressive intervention, we will feed them via nasogastric tube.”
Just over half (55%) of patients fell below the cutoff for sarcopenia on the hand-grip test at baseline and at transplant. More than a quarter of patients (27%) were not able to complete the 6-minute walk test.
“On univariate analysis, we saw malnutrition to be strongly associated with increased ICU and hospital length of stay,” Ms. Chapman noted. Severely malnourished patients spent significantly more time in the ICU than did well-nourished patients – a mean 147 hours vs. 89 hours (P = .001). Mean length of stay also was significantly longer at 40 days vs. 16 days (P = .003).
There was also an increased incidence of infection in severely malnourished patients as compared with well-nourished patients – 55.2% vs. 33.8%, she said.
“Aggressive strategies to combat malnutrition and deconditioning in the pretransplant period may lead to improved outcomes after transplant,” Ms. Chapman concluded.
The study was funded by Austin Health. Ms. Chapman declared no relevant conflicts of interest.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: Severely malnourished patients spent a mean of 147 hours in the ICU vs. 89 hours for well-nourished patients. Mean length of stay also was significantly longer at 40 days vs. 16 days (P = .003).
Data source: Retrospective review of data on 390 adults awaiting liver transplantation between Jan. 2009 and June 2016.
Disclosures: The study was funded by the institutions. The authors reported no relevant conflicts of interest.
Irradiation safe, effective as chemotherapy before cell transplant in mantle cell lymphoma
As preparation for autologous stem cell transplantation, total-body irradiation was as safe and effective as chemotherapy conditioning, in a retrospective analysis of mantle cell lymphoma patient records.
“Our data suggest that both TBI [total-body irradiation] and BEAM [carmustine, etoposide, cytarabine, and melphalan]-based conditioning regimens remain viable conditioning options for MCL patients undergoing ASCT [autologous stem cell transplantation],” wrote Yolanda D. Tseng, MD, of the University of Washington, Seattle, and her coinvestigators.
Progression-free survival, the primary outcome, was greater at 5 years’ follow-up in 43 TBI patients (66%) than in the 32 chemotherapy-conditioned patients (52%), but the difference did not reach statistical significance. Overall survival followed a similar pattern: TBI patients’ overall survival at 5 years was 82%, compared with 68% in the TBI patients, but this difference also lacked significance (Biol Blood Marrow Transplant. 2017 Oct 20; doi: 10.1016/j.bbmt.2017.10.029).
Safety outcomes, including early toxicity, nonrelapse mortality, and secondary malignancies, also were similar between groups.
For the analysis, Dr. Tseng and her colleagues reviewed the records of 75 consecutive adult patients treated during 2001-2011 with ASCT at the Fred Hutchinson Cancer Research Center in Seattle. All underwent conditioning with either myeloablative TBI or a BEAM-based regimen.
Most of the patients had chemosensitive disease (97%), and nearly all received rituximab prior to ASCT.
Prior studies have shown that TBI can be at least as effective as chemotherapy conditioning, but none have looked at toxicity. These results indicate that either approach remains viable for patients undergoing ASCT for MCL, Dr. Tseng and her colleagues concluded.
No disclosures were reported.
As preparation for autologous stem cell transplantation, total-body irradiation was as safe and effective as chemotherapy conditioning, in a retrospective analysis of mantle cell lymphoma patient records.
“Our data suggest that both TBI [total-body irradiation] and BEAM [carmustine, etoposide, cytarabine, and melphalan]-based conditioning regimens remain viable conditioning options for MCL patients undergoing ASCT [autologous stem cell transplantation],” wrote Yolanda D. Tseng, MD, of the University of Washington, Seattle, and her coinvestigators.
Progression-free survival, the primary outcome, was greater at 5 years’ follow-up in 43 TBI patients (66%) than in the 32 chemotherapy-conditioned patients (52%), but the difference did not reach statistical significance. Overall survival followed a similar pattern: TBI patients’ overall survival at 5 years was 82%, compared with 68% in the TBI patients, but this difference also lacked significance (Biol Blood Marrow Transplant. 2017 Oct 20; doi: 10.1016/j.bbmt.2017.10.029).
Safety outcomes, including early toxicity, nonrelapse mortality, and secondary malignancies, also were similar between groups.
For the analysis, Dr. Tseng and her colleagues reviewed the records of 75 consecutive adult patients treated during 2001-2011 with ASCT at the Fred Hutchinson Cancer Research Center in Seattle. All underwent conditioning with either myeloablative TBI or a BEAM-based regimen.
Most of the patients had chemosensitive disease (97%), and nearly all received rituximab prior to ASCT.
Prior studies have shown that TBI can be at least as effective as chemotherapy conditioning, but none have looked at toxicity. These results indicate that either approach remains viable for patients undergoing ASCT for MCL, Dr. Tseng and her colleagues concluded.
No disclosures were reported.
As preparation for autologous stem cell transplantation, total-body irradiation was as safe and effective as chemotherapy conditioning, in a retrospective analysis of mantle cell lymphoma patient records.
“Our data suggest that both TBI [total-body irradiation] and BEAM [carmustine, etoposide, cytarabine, and melphalan]-based conditioning regimens remain viable conditioning options for MCL patients undergoing ASCT [autologous stem cell transplantation],” wrote Yolanda D. Tseng, MD, of the University of Washington, Seattle, and her coinvestigators.
Progression-free survival, the primary outcome, was greater at 5 years’ follow-up in 43 TBI patients (66%) than in the 32 chemotherapy-conditioned patients (52%), but the difference did not reach statistical significance. Overall survival followed a similar pattern: TBI patients’ overall survival at 5 years was 82%, compared with 68% in the TBI patients, but this difference also lacked significance (Biol Blood Marrow Transplant. 2017 Oct 20; doi: 10.1016/j.bbmt.2017.10.029).
Safety outcomes, including early toxicity, nonrelapse mortality, and secondary malignancies, also were similar between groups.
For the analysis, Dr. Tseng and her colleagues reviewed the records of 75 consecutive adult patients treated during 2001-2011 with ASCT at the Fred Hutchinson Cancer Research Center in Seattle. All underwent conditioning with either myeloablative TBI or a BEAM-based regimen.
Most of the patients had chemosensitive disease (97%), and nearly all received rituximab prior to ASCT.
Prior studies have shown that TBI can be at least as effective as chemotherapy conditioning, but none have looked at toxicity. These results indicate that either approach remains viable for patients undergoing ASCT for MCL, Dr. Tseng and her colleagues concluded.
No disclosures were reported.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Key clinical point:
Major finding: Five-year progression-free survival was 66% in MCL patients who underwent TBI and 52% in chemotherapy-conditioned patients, a nonsignificant difference.
Data source: An analysis of 75 MCL patients undergoing ASCT in a single institution.
Disclosures: No disclosures were reported.
Post–liver transplant results similar in acute alcoholic hepatitis, stage 1a
WASHINGTON – Patients with acute alcoholic hepatitis (AAH) have similar early post–liver transplant outcomes to those listed with fulminant hepatic failure, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
Patients with severe AAH have high mortality, but many are unable to survive the 6 months of sobriety required to be accepted as liver transplant candidates, said George Cholankeril, MD, of the gastroenterology and hepatology department at Stanford (Calif.) University.
He and his associates studied wait-list mortality and post–liver transplant survival among 1,912 patients listed for either AAH or fulminant hepatic failure on the United Network for Organ Sharing (UNOS) registry between 2011 and 2016.
A total of 193 patients were listed with AAH, 314 were listed with drug-induced liver injury including acetaminophen (DILI-APAP), and 1,405 were listed as non-DILI patients.
One-year post–liver transplant survival among AAH patients was 93.3%, compared with 87.75% for DILI-APAP patients and 88.4% among non-DILI patients (P less than .001). Survival remained the same among AAH patients 3 years following transplantation, but rates dropped for both the DILI-APAP group (80.8%) and the non-DILI group (81.4%), Dr. Cholankeril reported.
Patients were a median age of 45, 33, and 46 years among the AAH, DILI-APAP, and non-DILI, groups respectively. Patients were majority white among all three groups, with a significantly larger female population among the DILI-APAP group (80.6%) than the AAH (34.7%) or non-DILI (59.4%) groups. Patients in the AAH group had a median Model for End-Stage Liver Disease (MELD) score of 32, compared with 34 for DILI-APAP and 21 for non-DILI.
AAH patients could potentially see significant improvement with a liver transplant, according to investigators; however, the current standards for candidacy have created treatment barriers.
“Patients with AAH have comparable early post-transplant outcomes to those with hepatic liver failure,” said Dr. Cholankeril. “However, there is no consensus or national guidelines for liver transplantation within this patient population.”
Wait-list trends have already started to shift toward more AAH patient acceptance. The number of AAH patients added to the transplant wait lists increased from 14 in 2011 to 58 in 2016. Investigators also found that the number of liver transplant centers accepting AAH patients to their transplant lists increased from 3 to 26.
Investigators were limited by the variations in protocols for each transplant center, as well as by the inconsistency of pre–liver transplant psychosocial metrics. The diagnostic criteria of AAH through UNOS was also a limitation for investigators, according to Dr. Cholankeril.
Although liver transplantation may be able to help some patients, it is only a small fix for a much larger problem. “This is only a solution for a minority of patients with the rising epidemic of alcoholic intoxication in the U.S.,” he said. “As the increasing mortality trends show alcohol-related mortality, and alcoholic liver disease is a contributor to it, we must recognize alcoholic liver disease remains an orphan disease and there is still an unmet need.”
Dr. Cholankeril reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
Transplanting patients with alcohol induced liver disease has been controversial since the earliest days of liver transplantation. Initially some programs were reluctant to transplant patients with a disease that was “self-inflicted” based on an ethical concern about using a scarce resource to save the lives of those whose disease was their own fault, while other patients may die waiting. There was also a concern that transplanting alcoholics would be bad publicity for organ donation and reduce the public’s willingness to donate organs. The highly publicized transplantation of baseball legend and known alcoholic Mickey Mantle in 1995 intensified this debate.
Over time it became clear that the concerns regarding transplanting alcoholics were unfounded. The outcomes were equal or better than for other diseases. Liver transplantation was termed “the ultimate eye opening experience” as serious recidivism turned out to be very uncommon. It was realized that a large percentage of all reasons for seeking medical care can be attributed to self-inflicted harm when one considers cigarette induced malignancy and cardiovascular disease and dietary indiscretion leading to obesity and diabetes. It also became clear that from a practical standpoint prohibiting transplantation of alcoholics simply drove patients to programs that would accept such patients, or caused them and their family to withhold disclosure of alcohol use.
While transplantation of patients with chronic liver disease due to alcohol use has become standard of care, transplanting patients with acute alcoholic hepatitis remains controversial and relatively uncommon. Many programs require a period of abstinence, which is impossible in the setting of acute alcoholic hepatitis. The concern is that it is impossible to discern among actively drinking candidates which ones will be able to achieve sobriety after the transplant. The report by Cholankeril and colleagues documents that the tide is changing. There are increasing numbers of patients being transplanted for acute alcoholic hepatitis, and outcomes are acceptable. However, as the authors point out, the numbers are small and represent a highly selected group of patients. Nevertheless, the pressure on programs to modify rigid abstinence criteria is likely to grow as the evidence accumulates showing selected patients with acute alcoholic hepatitis can do well.
Jeffrey Punch, MD, FACS, is transplant specialist at the University of Michigan in Ann Arbor, and on the Editorial Advisory Board of ACS Surgery News.
Transplanting patients with alcohol induced liver disease has been controversial since the earliest days of liver transplantation. Initially some programs were reluctant to transplant patients with a disease that was “self-inflicted” based on an ethical concern about using a scarce resource to save the lives of those whose disease was their own fault, while other patients may die waiting. There was also a concern that transplanting alcoholics would be bad publicity for organ donation and reduce the public’s willingness to donate organs. The highly publicized transplantation of baseball legend and known alcoholic Mickey Mantle in 1995 intensified this debate.
Over time it became clear that the concerns regarding transplanting alcoholics were unfounded. The outcomes were equal or better than for other diseases. Liver transplantation was termed “the ultimate eye opening experience” as serious recidivism turned out to be very uncommon. It was realized that a large percentage of all reasons for seeking medical care can be attributed to self-inflicted harm when one considers cigarette induced malignancy and cardiovascular disease and dietary indiscretion leading to obesity and diabetes. It also became clear that from a practical standpoint prohibiting transplantation of alcoholics simply drove patients to programs that would accept such patients, or caused them and their family to withhold disclosure of alcohol use.
While transplantation of patients with chronic liver disease due to alcohol use has become standard of care, transplanting patients with acute alcoholic hepatitis remains controversial and relatively uncommon. Many programs require a period of abstinence, which is impossible in the setting of acute alcoholic hepatitis. The concern is that it is impossible to discern among actively drinking candidates which ones will be able to achieve sobriety after the transplant. The report by Cholankeril and colleagues documents that the tide is changing. There are increasing numbers of patients being transplanted for acute alcoholic hepatitis, and outcomes are acceptable. However, as the authors point out, the numbers are small and represent a highly selected group of patients. Nevertheless, the pressure on programs to modify rigid abstinence criteria is likely to grow as the evidence accumulates showing selected patients with acute alcoholic hepatitis can do well.
Jeffrey Punch, MD, FACS, is transplant specialist at the University of Michigan in Ann Arbor, and on the Editorial Advisory Board of ACS Surgery News.
Transplanting patients with alcohol induced liver disease has been controversial since the earliest days of liver transplantation. Initially some programs were reluctant to transplant patients with a disease that was “self-inflicted” based on an ethical concern about using a scarce resource to save the lives of those whose disease was their own fault, while other patients may die waiting. There was also a concern that transplanting alcoholics would be bad publicity for organ donation and reduce the public’s willingness to donate organs. The highly publicized transplantation of baseball legend and known alcoholic Mickey Mantle in 1995 intensified this debate.
Over time it became clear that the concerns regarding transplanting alcoholics were unfounded. The outcomes were equal or better than for other diseases. Liver transplantation was termed “the ultimate eye opening experience” as serious recidivism turned out to be very uncommon. It was realized that a large percentage of all reasons for seeking medical care can be attributed to self-inflicted harm when one considers cigarette induced malignancy and cardiovascular disease and dietary indiscretion leading to obesity and diabetes. It also became clear that from a practical standpoint prohibiting transplantation of alcoholics simply drove patients to programs that would accept such patients, or caused them and their family to withhold disclosure of alcohol use.
While transplantation of patients with chronic liver disease due to alcohol use has become standard of care, transplanting patients with acute alcoholic hepatitis remains controversial and relatively uncommon. Many programs require a period of abstinence, which is impossible in the setting of acute alcoholic hepatitis. The concern is that it is impossible to discern among actively drinking candidates which ones will be able to achieve sobriety after the transplant. The report by Cholankeril and colleagues documents that the tide is changing. There are increasing numbers of patients being transplanted for acute alcoholic hepatitis, and outcomes are acceptable. However, as the authors point out, the numbers are small and represent a highly selected group of patients. Nevertheless, the pressure on programs to modify rigid abstinence criteria is likely to grow as the evidence accumulates showing selected patients with acute alcoholic hepatitis can do well.
Jeffrey Punch, MD, FACS, is transplant specialist at the University of Michigan in Ann Arbor, and on the Editorial Advisory Board of ACS Surgery News.
WASHINGTON – Patients with acute alcoholic hepatitis (AAH) have similar early post–liver transplant outcomes to those listed with fulminant hepatic failure, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
Patients with severe AAH have high mortality, but many are unable to survive the 6 months of sobriety required to be accepted as liver transplant candidates, said George Cholankeril, MD, of the gastroenterology and hepatology department at Stanford (Calif.) University.
He and his associates studied wait-list mortality and post–liver transplant survival among 1,912 patients listed for either AAH or fulminant hepatic failure on the United Network for Organ Sharing (UNOS) registry between 2011 and 2016.
A total of 193 patients were listed with AAH, 314 were listed with drug-induced liver injury including acetaminophen (DILI-APAP), and 1,405 were listed as non-DILI patients.
One-year post–liver transplant survival among AAH patients was 93.3%, compared with 87.75% for DILI-APAP patients and 88.4% among non-DILI patients (P less than .001). Survival remained the same among AAH patients 3 years following transplantation, but rates dropped for both the DILI-APAP group (80.8%) and the non-DILI group (81.4%), Dr. Cholankeril reported.
Patients were a median age of 45, 33, and 46 years among the AAH, DILI-APAP, and non-DILI, groups respectively. Patients were majority white among all three groups, with a significantly larger female population among the DILI-APAP group (80.6%) than the AAH (34.7%) or non-DILI (59.4%) groups. Patients in the AAH group had a median Model for End-Stage Liver Disease (MELD) score of 32, compared with 34 for DILI-APAP and 21 for non-DILI.
AAH patients could potentially see significant improvement with a liver transplant, according to investigators; however, the current standards for candidacy have created treatment barriers.
“Patients with AAH have comparable early post-transplant outcomes to those with hepatic liver failure,” said Dr. Cholankeril. “However, there is no consensus or national guidelines for liver transplantation within this patient population.”
Wait-list trends have already started to shift toward more AAH patient acceptance. The number of AAH patients added to the transplant wait lists increased from 14 in 2011 to 58 in 2016. Investigators also found that the number of liver transplant centers accepting AAH patients to their transplant lists increased from 3 to 26.
Investigators were limited by the variations in protocols for each transplant center, as well as by the inconsistency of pre–liver transplant psychosocial metrics. The diagnostic criteria of AAH through UNOS was also a limitation for investigators, according to Dr. Cholankeril.
Although liver transplantation may be able to help some patients, it is only a small fix for a much larger problem. “This is only a solution for a minority of patients with the rising epidemic of alcoholic intoxication in the U.S.,” he said. “As the increasing mortality trends show alcohol-related mortality, and alcoholic liver disease is a contributor to it, we must recognize alcoholic liver disease remains an orphan disease and there is still an unmet need.”
Dr. Cholankeril reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Patients with acute alcoholic hepatitis (AAH) have similar early post–liver transplant outcomes to those listed with fulminant hepatic failure, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
Patients with severe AAH have high mortality, but many are unable to survive the 6 months of sobriety required to be accepted as liver transplant candidates, said George Cholankeril, MD, of the gastroenterology and hepatology department at Stanford (Calif.) University.
He and his associates studied wait-list mortality and post–liver transplant survival among 1,912 patients listed for either AAH or fulminant hepatic failure on the United Network for Organ Sharing (UNOS) registry between 2011 and 2016.
A total of 193 patients were listed with AAH, 314 were listed with drug-induced liver injury including acetaminophen (DILI-APAP), and 1,405 were listed as non-DILI patients.
One-year post–liver transplant survival among AAH patients was 93.3%, compared with 87.75% for DILI-APAP patients and 88.4% among non-DILI patients (P less than .001). Survival remained the same among AAH patients 3 years following transplantation, but rates dropped for both the DILI-APAP group (80.8%) and the non-DILI group (81.4%), Dr. Cholankeril reported.
Patients were a median age of 45, 33, and 46 years among the AAH, DILI-APAP, and non-DILI, groups respectively. Patients were majority white among all three groups, with a significantly larger female population among the DILI-APAP group (80.6%) than the AAH (34.7%) or non-DILI (59.4%) groups. Patients in the AAH group had a median Model for End-Stage Liver Disease (MELD) score of 32, compared with 34 for DILI-APAP and 21 for non-DILI.
AAH patients could potentially see significant improvement with a liver transplant, according to investigators; however, the current standards for candidacy have created treatment barriers.
“Patients with AAH have comparable early post-transplant outcomes to those with hepatic liver failure,” said Dr. Cholankeril. “However, there is no consensus or national guidelines for liver transplantation within this patient population.”
Wait-list trends have already started to shift toward more AAH patient acceptance. The number of AAH patients added to the transplant wait lists increased from 14 in 2011 to 58 in 2016. Investigators also found that the number of liver transplant centers accepting AAH patients to their transplant lists increased from 3 to 26.
Investigators were limited by the variations in protocols for each transplant center, as well as by the inconsistency of pre–liver transplant psychosocial metrics. The diagnostic criteria of AAH through UNOS was also a limitation for investigators, according to Dr. Cholankeril.
Although liver transplantation may be able to help some patients, it is only a small fix for a much larger problem. “This is only a solution for a minority of patients with the rising epidemic of alcoholic intoxication in the U.S.,” he said. “As the increasing mortality trends show alcohol-related mortality, and alcoholic liver disease is a contributor to it, we must recognize alcoholic liver disease remains an orphan disease and there is still an unmet need.”
Dr. Cholankeril reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: Survival 1 and 3 years after liver transplant was comparable in patients with drug-induced liver injury including acetaminophen (P = .10) and significantly higher than other chronic alcoholic liver disease patients (P less that .001).
Data source: Retrospective study of 1,912 liver transplant patients listed for either AAH or status 1A registered on the UNOS registry between 2011 and 2016.
Disclosures: Presenter reported no relevant financial disclosures.
Simplify Your Life; Pay Dues Invoice Online
Don't forget that the end of the year is the time to keep up to date with your SVS membership dues. Invoices were emailed to all members earlier this month and are due by Dec. 31.
It's simple to pay your 2018 dues online -- and there's no need to write out a check or find a stamp! Just log on to vascular.org/payinvoice. (While you're at it, please make sure your record is up to date.) You also can make a donation to the SVS Foundation at the same time. For membership help, e-mail the SVS membership department, or call 312-334-2313
Don't forget that the end of the year is the time to keep up to date with your SVS membership dues. Invoices were emailed to all members earlier this month and are due by Dec. 31.
It's simple to pay your 2018 dues online -- and there's no need to write out a check or find a stamp! Just log on to vascular.org/payinvoice. (While you're at it, please make sure your record is up to date.) You also can make a donation to the SVS Foundation at the same time. For membership help, e-mail the SVS membership department, or call 312-334-2313
Don't forget that the end of the year is the time to keep up to date with your SVS membership dues. Invoices were emailed to all members earlier this month and are due by Dec. 31.
It's simple to pay your 2018 dues online -- and there's no need to write out a check or find a stamp! Just log on to vascular.org/payinvoice. (While you're at it, please make sure your record is up to date.) You also can make a donation to the SVS Foundation at the same time. For membership help, e-mail the SVS membership department, or call 312-334-2313
SVS Establishes Disaster Relief Fund
At its recent meeting, the SVS Board of Directors approved establishing a Disaster Relief Fund in response to disasters in Puerto Rico, Florida, Texas and Mexico. This Fund will support vascular surgeons and their patients who have been impacted by these extraordinary events and ensure that their commitment to vascular health is recognized in times of need.
The Foundation leadership is working to initiate a fundraising campaign and grant application guidelines.
Members are asked to email the SVS Foundation to provide your input on the type of support that would be most helpful to vascular surgeons and their patients in these disaster areas.
At its recent meeting, the SVS Board of Directors approved establishing a Disaster Relief Fund in response to disasters in Puerto Rico, Florida, Texas and Mexico. This Fund will support vascular surgeons and their patients who have been impacted by these extraordinary events and ensure that their commitment to vascular health is recognized in times of need.
The Foundation leadership is working to initiate a fundraising campaign and grant application guidelines.
Members are asked to email the SVS Foundation to provide your input on the type of support that would be most helpful to vascular surgeons and their patients in these disaster areas.
At its recent meeting, the SVS Board of Directors approved establishing a Disaster Relief Fund in response to disasters in Puerto Rico, Florida, Texas and Mexico. This Fund will support vascular surgeons and their patients who have been impacted by these extraordinary events and ensure that their commitment to vascular health is recognized in times of need.
The Foundation leadership is working to initiate a fundraising campaign and grant application guidelines.
Members are asked to email the SVS Foundation to provide your input on the type of support that would be most helpful to vascular surgeons and their patients in these disaster areas.
VIDEO: Fibrosis biomarkers show promise to replace liver biopsy
WASHINGTON – The concentrations of three biomarkers successfully identified the severity of fibrosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
This noninvasive diagnostic method could replace liver biopsy, the current standard used to diagnose patients with NASH.
Liver biopsies are associated with high cost, high rates of complications like infection, and minimal association with morbidity and mortality, Manal Abdelmalek, MD, a hepatologist and liver transplant specialist at Duke University, Durham, N.C., said in a video interview.
Investigators measured serum concentrations of a2-macroglobulin, hyaluronic acid, and metalloproteinase-1 collected from 792 patients with NASH on the same day as patients’ liver biopsies.
Dr. Abdelmalek and her fellow investigators randomly assigned half of the samples to a training group and half the samples to a validation group.
Investigators found that samples in the training group showed a sensitivity of 84.4% (95% confidence interval, 75.5%-91.0%), compared with 81.1% (95% CI, 71.7%-88.4%) in the validation group. Among patients with liver fibrosis, the biomarker test correctly diagnosed 76.5%-100% of patients, with variations depending on placement in the four classifications based on severity.
Investigators feel optimistic that, with more testing, this biomarker test can be used in collaboration with imaging or used independently, minimizing possible patient complications.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – The concentrations of three biomarkers successfully identified the severity of fibrosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
This noninvasive diagnostic method could replace liver biopsy, the current standard used to diagnose patients with NASH.
Liver biopsies are associated with high cost, high rates of complications like infection, and minimal association with morbidity and mortality, Manal Abdelmalek, MD, a hepatologist and liver transplant specialist at Duke University, Durham, N.C., said in a video interview.
Investigators measured serum concentrations of a2-macroglobulin, hyaluronic acid, and metalloproteinase-1 collected from 792 patients with NASH on the same day as patients’ liver biopsies.
Dr. Abdelmalek and her fellow investigators randomly assigned half of the samples to a training group and half the samples to a validation group.
Investigators found that samples in the training group showed a sensitivity of 84.4% (95% confidence interval, 75.5%-91.0%), compared with 81.1% (95% CI, 71.7%-88.4%) in the validation group. Among patients with liver fibrosis, the biomarker test correctly diagnosed 76.5%-100% of patients, with variations depending on placement in the four classifications based on severity.
Investigators feel optimistic that, with more testing, this biomarker test can be used in collaboration with imaging or used independently, minimizing possible patient complications.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – The concentrations of three biomarkers successfully identified the severity of fibrosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
This noninvasive diagnostic method could replace liver biopsy, the current standard used to diagnose patients with NASH.
Liver biopsies are associated with high cost, high rates of complications like infection, and minimal association with morbidity and mortality, Manal Abdelmalek, MD, a hepatologist and liver transplant specialist at Duke University, Durham, N.C., said in a video interview.
Investigators measured serum concentrations of a2-macroglobulin, hyaluronic acid, and metalloproteinase-1 collected from 792 patients with NASH on the same day as patients’ liver biopsies.
Dr. Abdelmalek and her fellow investigators randomly assigned half of the samples to a training group and half the samples to a validation group.
Investigators found that samples in the training group showed a sensitivity of 84.4% (95% confidence interval, 75.5%-91.0%), compared with 81.1% (95% CI, 71.7%-88.4%) in the validation group. Among patients with liver fibrosis, the biomarker test correctly diagnosed 76.5%-100% of patients, with variations depending on placement in the four classifications based on severity.
Investigators feel optimistic that, with more testing, this biomarker test can be used in collaboration with imaging or used independently, minimizing possible patient complications.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
AT THE LIVER MEETING 2017
Do Bedside Visual Tools Improve Patient and Caregiver Satisfaction? A Systematic Review of the Literature
Patient satisfaction with medical care during hospitalization is a common quality metric.1,2 Studies showing higher patient satisfaction have reported lower 30-day hospital readmissions3 and improved overall health.4,5 Conversely, communication failures are associated with dissatisfaction among hospitalized patients and adverse outcomes.6,7 A lack of familiarity with hospital providers weakens collaborative decision making and prevents high-quality patient care.8,9
Bedside visual tools, such as whiteboards and pictures of medical staff, have been widely used to enhance communication between patients, families, and providers.10,11 Results of studies evaluating these tools are varied. For example, 1 study found that 98% of patients were better able to identify physicians when their names were written on whiteboards.12 Yet in another, only 21.1% of patients were more likely to correctly identify ≥1 physicians using pictures.13 Thus, despite widespread use,11 whether visual tools improve patient satisfaction and patient care more broadly remains unclear.14,15
We performed a systematic review to answer the following 3 questions: first, what is the effect of visual tools on outcomes (ie, provider identification, understanding of providers’ roles, patient–provider communication, and satisfaction); second, does impact vary by type of visual tool (eg, whiteboards vs pictures of providers); and third, what factors (eg, study design, patient population) are associated with provider identification, communication, and patient satisfaction?
METHODS
Search Strategy
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis when performing this review.16 A research librarian (WT) conducted serial searches for studies reporting the use of bedside visual tools for hospitalized patients in Medline (via OVID), Embase, SCOPUS, Web of Science, CINAHL, and Cochrane DSR and CENTRAL. Controlled vocabularies (ie, Medical Subject Headings terms) were used to identify synonyms for visual tools of interest. Additional studies were identified manually through bibliographies and meeting abstracts. No study design, publication date, or language restrictions were placed on the search, which was conducted between April 2016 and February 2017 (see supplementary Appendix A).
Study Selection
Two reviewers (AG and KT) independently assessed study eligibility; discrepancies were resolved by a third reviewer (VC). We included all adult or pediatric English language studies in which the effect of visual tool(s) on patient outcomes was reported. Visual tools were defined as the bedside display of information or an instrument given to patients to convey information regarding providers or medical care. Patient-reported outcomes included the following: (a) physician identification, (b) understanding of provider roles, (c) patient–provider communication, and (d) patient satisfaction with care. Providers were defined as physicians, residents, interns, medical students, nurse practitioners, or nurses. We excluded studies that were not original research (eg, conference abstracts, not peer reviewed), reported qualitative data without quantitative outcomes, or did not include a bedside visual tool. Given our interest in hospitalized general medicine patients, studies conducted in emergency departments, surgical units, obstetrics and gynecology wards, and intensive care units were excluded.
Data Extraction and Analysis
Data were extracted independently and in duplicate from all studies by using a template adapted from the Cochrane Collaboration.17 For all studies, we abstracted study design, type of visual tool (eg, whiteboards), unit setting (eg, medical), population studied (eg, adult vs pediatric), and outcomes reported (ie, physician identification, understanding of provider roles, communication, and satisfaction with care). Reviewers independently assessed and categorized the impact of tools on reported outcomes.
To standardize and compare outcomes across studies, the following were used to denote a positive association between visual tools and relevant outcomes: a greater number of physicians correctly identified by name/picture or title/role; the use of terms such as “high,” “agreed,” or “significant” on surveys; or ≥4 Likert scores for domains of identification, understanding of roles, communication, and satisfaction with care. Conversely, the inability to identify providers compared to the control/baseline; poor recall of titles/roles; lower Likert-scale scores (ie, ≤2); or survey terms such as “poor,” “disagreed,” or “insignificant” were considered to connote negative impact. Studies in which Likert scores were rated neither high nor low (ie, 3), or in which patients neither agreed nor disagreed on value were considered neutral.
Owing to clinical heterogeneity within studies, meta-analyses were not performed. Descriptive statistics were used to describe study outcomes. A priori18 studies were evaluated according to the following categories: design (eg, randomized vs observational), outcomes (eg, patient satisfaction), intervention (type of visual tool), and patient population (adult or pediatric). Because pediatric patients have underdeveloped communication skills and include parents and/or guardians, data from pediatric studies were tabulated and reported separately to those from adult studies.
Quality Assessment
As recommended by the Cochrane Collaboration, 2 reviewers (AG, KT) assessed the risk of study bias by using the Downs and Black Scale.17,19 Discrepancies in assessment were resolved by a third reviewer (VC). This instrument uses a point-based system to estimate the quality of a study by rating domains such as internal and external validity, bias, and confounding. In keeping with prior systematic reviews,18,20,21 studies with a score of ≥18 were considered high quality. Interrater agreement for the adjudication of study quality was calculated using the Cohen κ statistic.
RESULTS
STUDIES OF ADULT HOSPITALIZED PATIENTS
Eleven studies were conducted on adult hospitalized patients 12-14,22-24,26,27,29,30,33 and included 3 randomized controlled studies.14,27,33
Results by Outcomes Provider Identification Nine studies measured patients’ ability to identify providers with the use of visual aids, and all 9 reported improvements in this outcome. Visual tools used to measure provider identification included pictures (n = 5),13,14,23,27,33 whiteboards (n = 3),12,22,30 and patient portals (n = 1).26 Within studies that used pictures, individual pictures (n = 2)13,23 and handouts with pictures of multiple providers (n = 3) were used.14,27,33 In 2 studies, care team members such as a dietitian, physiotherapist or pharmacist, were included when measuring identification.14,33
Understanding Providers’ RolesSix studies assessed the effect of visual tools on patients’ understanding of provider roles.13,14,22,26,27,33 Four studies reported a positive effect with the use of pictures,27,33 whiteboards,22 and patient portals.26 However, 2 studies reported either no difference or negative impressions. Appel et al.14 reported no difference in the understanding of physician roles using a handout of providers’ pictures and titles. Arora et al.13 used individual pictures of physicians with descriptions of roles and found a negative association, as demonstrated by fewer patients rating their understanding of physicians’ roles as excellent or very good in the intervention period (45.6%) compared with the baseline (55.3%).
Patient–Provider Communication
Three studies evaluated the influence of visual tools on communication.14,24,29 Using pictures, Appel et al.14 found no difference in the perceived quality of communication. Singh et al.29 used whiteboards and reported improved communication scores for physicians and nurses. With notepads, patients surveyed by Farberg et al.24 stated that the tool improved provider communication.
Patient Satisfaction
Five studies assessed patient satisfaction related to the use of visual tools. 22,23,27,30,33 One study reported satisfaction as positive with the use of individual pictures.23 Two studies that used handouts with pictures of all team members reported either a positive33 or neutral27 impact on satisfaction. Studies that used whiteboards reported a positive association with satisfaction22,30 despite differences in content, such as the inclusion of prewritten prompts for writing goals of care and scheduled tests30 versus the name of the nurse and their education level.22
Results by Type of Visual Tool Pictures
Five studies that used pictures reported a positive effect on provider identification.13,14,23,27,33 Two27,33 of 4 studies13,14,27,33 that assessed patients’ understanding of team member roles reported a positive influence, while 1 reported no difference.14 A fourth study demonstrated a negative association, perhaps due to differences in the description of providers’ roles listed on the tool.13 Only 1 study examined the influence of pictures on patient–provider communication, and this study found no difference.14 Satisfaction with care via the use of pictures varied between positive (2 studies)23,33 and neutral (1 study).27
Whiteboards
Four studies tested the use of whiteboards; of these, 3 reported a positive influence on provider identification.12,22,30 One study reported a positive impact on patient–provider communication.29 Two studies noted a positive effect on patient satisfaction.22,30 Notably, the responsibility for updating whiteboards differed between the studies (ie, nurses only22 vs residents, medical students, and nurses).30
Patient Portal
In 1 study, an electronic portal that included names with pictures of providers, descriptions of their roles, lists of medications, and scheduled tests and/or procedures was used as a visual tool. The portal improved patients’ identification of physicians and patients’ understanding of roles. However, improvements in the knowledge of medication changes and planned tests and/or procedures during hospitalization were not observed.26 This finding would suggest limitations in the hospitalized patient’s knowledge of the plan of care, which could potentially weaken patient–provider communication.
Notepads
Only 1 study assessed the use of formatted notepads on patient–provider communication and noted a positive association. Notepads used prompts for different categories (eg, diagnosis/treatment, medications, etc) to encourage patient questions for providers.24
STUDIES OF PEDIATRIC HOSPITALIZED PATIENTS
Five studies were conducted on hospitalized pediatric units.15,25,28,31,32 All studies surveyed the parents, guardians, or caregivers of pediatric patients. One study excluded patients ≥12 years of age because of legal differences in access to adolescent health information,32 while another interviewed parents and/or guardians of teenagers.15
Results by Outcomes Provider Identification and Understanding of Physicians’ Roles
Four studies that assessed the influence of visual tools on provider identification and understanding of roles reported a positive association.15,25,28,31 Visual tools varied between pictures (n = 2),15,31 patient portal (n = 1),28 and whiteboards and pictures combined (n = 1).25 The measurement of outcomes varied between surveys with free text responses,28 multiple choice questions,25 and 1-5 Likert scales.15,31
Patient–Provider Communication
Two studies assessed the impact of patient portal use on communication and reported a positive association.28,32 The 2 portals autopopulated names, pictures, and roles of providers from electronic medical records. Singh et al.28 used a portal that was also available in Spanish and accommodated for non-English speakers. Kelly et al.32 reported that 90% of parents perceived that portal use was associated with reduced errors in care, with 8% finding errors in their child’s medication list.
Patient Satisfaction
Three studies assessed patient satisfaction via the use of visual tools.15,28,31 Singh et al.28 noted a positive influence on satisfaction via a patient portal. Dudas et al.15 used a single-page handout with names and pictures of each provider, along with information regarding the training and roles of each provider. Distribution of these handouts to patients by investigators led to a positive influence on satisfaction. While Unaka et al.31 used a similar handout, they asked residents to distribute them and found no significant difference in satisfaction scores between the intervention (66%) and control group (62%).
Results by Type of Visual Tool Pictures
Two studies reported a positive impact on provider identification and understanding of roles with the use of pictures.15,31 Dudas et al.15 demonstrated a 4.8-fold increase in the odds of parents identifying a medical student, as compared with the control. Similarly, after adjusting for length of stay and prior hospitalization, Unaka et al.31 reported that a higher percentage of patients correctly identified providers using this approach.
Whiteboard and Picture
One study evaluated the simultaneous use of whiteboards and pictures to improve the identification of providers. The study noted improved identification of supervising doctors and increased recognition of roles for supervising doctors, residents, and medical students.25
Patient Portal
Two studies used patient portals as visual tools. Singh et al.28 assessed the use of a patient portal with names, roles, and pictures of treatment team members. Use of this tool was positively associated with provider identification, understanding of roles, communication, and satisfaction. Kelly et al.32 noted that 60% of parents felt that portal use improved healthcare team communication.
RISK OF STUDY BIAS
The risk of bias was assessed for both adult and pediatric studies in aggregate. The average risk of bias using the Downs and Black Scale was 17.81 (range 14-22, standard deviation [SD] 2.20). Of the 16 included studies, 9 were rated at a low risk of bias (score
- >
18).13-15,26-31 Risk of bias was greatest for measures of external validity (mean 2.88, range 2-3, SD 0.34), internal validity (mean 4.06, range 3-6, SD 1.00), and confounding (mean 2.69, range 1-6, SD 1.35). Two of 3 randomized controlled trials had a low risk of bias.14,27 Interrater reliability for study quality adjudication was 0.90, suggesting excellent agreement (see supplementary Appendix B).
DISCUSSION
In this systematic review, the effects of visual tools on outcomes, such as provider identification, understanding of roles, patient–provider communication, and satisfaction with care, were variable. The majority of included studies were conducted on adult patients (n = 11).12-14,22-24,26,27,29,30,33 Pictures were the most frequently used tool (n = 7)13-15,23,27,31,33 and consequently had the greatest sample size across the review (n = 1297). While pictures had a positive influence on provider identification in all studies, comprehension of provider roles and satisfaction were variable. Although the content of whiteboards varied between studies, they showed favorable effects on provider identification (3 of 4 studies)12,22,30 and satisfaction (2 of 2 studies).22,30 While electronic medical record-based tools had a positive influence on outcomes,26,28 only 1 accounted for language preferences.28 Formatted notepads positively influenced patient–provider communication, but their use was limited by literacy.24 Collectively, these data suggest that visual tools have varying effects on patient-reported outcomes, likely owing to differences in study design, interventions, and evaluation methods.
Theoretically, visual tools should facilitate easier identification of providers and engender collaborative relationships. However, such tools do not replace face-to-face patient–provider and family discussions. Rather, these enhancements best serve as a medium to asynchronously display information to patients and family members. Indeed, within the included studies, we found that the use of visual tools was effective in improving satisfaction (6/8 studies), identification (13/13 studies), and understanding of provider roles (8/10 studies). Thus, it is reasonable to say that, in conjunction with excellent clinical care, these tools have an important role in improving care delivery in the hospital.
Despite this promise, we noted that the effectiveness of individual tools varied, a fact that may relate to differences across studies. First, inconsistencies in the format and/or content of the tools were noted. For example, within studies using pictures, tools varied from individual photographs of each team member13,23 to 1-page handouts with pictures of all team members.14,15,31 Such differences in presentation could affect spatial recognition in identifying providers, as single photos are known to be easier to process than multiple images at the same time.34 Second, no study evaluated patient preference of a visual tool. Thus, personal preferences for pictures versus whiteboards versus electronic modalities or a combination of tools might affect outcomes. Additionally, the utility of visual tools in visually impaired, confused, or non-English-speaking patients may limit effectiveness. Future studies that address these aspects and account for patient preferences may better elucidate the role of visual tools in hospitals.
Our results should be considered in the context of several limitations. First, only 3 studies used randomized trial designs; thus, confounding from unmeasured variables inherent to observational designs is possible. Second, none of the interventions tested were blinded to providers, raising the possibility of a Hawthorne effect (ie, alteration of provider behavior in response to awareness of being observed).35 Third, all studies were conducted at single centers, and only 9 of 16 studies were rated at a low risk of bias; thus, caution in broad extrapolations of this literature is necessary.
However, our study has several strengths, including a thorough search of heterogeneous literature, inclusion of both adult and pediatric populations, and a focus on myriad patient-reported outcomes. Second, by contrasting outcomes and measurement strategies across studies, our review helps explicate differences in results related to variation in outcome measurement or presentation of visual data. Third, because we frame results by outcome and type of visual tool used, we are able to identify strengths and weaknesses of individual tools in novel ways. Finally, our data suggest that the use of picture-based techniques and whiteboards are among the most promising visual interventions. Future studies that pair graphic designers with patients to improve the layout of these tools might prove valuable. Additionally, because the measurement of outcomes is confounded by aspects such as lack of controls, severity of illness, and language barriers, a randomized design would help provide greater clarity regarding effectiveness.
In conclusion, we found that visual tools appear to foster recognition of providers and understanding of their roles. However, variability of format, content, and measurement of outcomes hinders the identification of a single optimal approach. Future work using randomized controlled trial designs and standardized tools and measurements would be welcomed.
Acknowledgments
The authors thank Laura Appel, Kevin O’Leary, and Siddharth Singh for providing unpublished data and clarifications to help these analyses.
Disclosure
Anupama Goyal is the guarantor. Anupama Goyal and Komalpreet Tur performed primary data abstraction and analysis. Anupama Goyal, Scott Flanders, Jason Mann, and Vineet Chopra drafted the manuscript. All authors contributed to the development of the selection criteria, the risk of bias assessment strategy, and the data extraction criteria. Anupama Goyal, Jason Mann, Whitney Townsend, and Vineet Chopra developed the search strategy. Vineet Chopra provided systematic review expertise. All authors read, provided feedback, and approved the final manuscript. The authors declare that they have no conflicts of interest.
1. Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff (Millwood). 2002;21(3):80-90. PubMed
2. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-1931. PubMed
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48. PubMed
4. Little P, Everitt H, Williamson I, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908-911. PubMed
5. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1422-1433. PubMed
6. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
7. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1:i85-i90. PubMed
8. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. PubMed
9. Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201. PubMed
10. Makaryus AN, Friedman EA. Does your patient know your name? An approach to enhancing patients’ awareness of their caretaker’s name. J Healthc Qual. 2005;27(4):53-56. PubMed
11. Sehgal NL, Green A, Vidyarthi AR, Blegen MA, Wachter RM. Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234-239. PubMed
12. Maniaci MJ, Heckman MG, Dawson NL. Increasing a patient’s ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170:1084-1085. PubMed
13. Arora VM, Schaninger C, D’Arcy M, et al. Improving inpatients’ identification of their doctors: Use of FACE™ cards. Jt Comm J Qual Patient Saf. 2009;35(12):613-619. PubMed
14. Appel L, Abrams H, Morra D, Wu RC. Put a face to a name: a randomized controlled trial evaluating the impact of providing clinician photographs on inpatients’ recall. Am J Med. 2015;128(1):82-89. PubMed
15. Dudas RA, Lemerman H, Barone M, Serwint JR. PHACES (Photographs of Academic Clinicians and Their Educational Status): a tool to improve delivery of family-centered care. Acad Pediatr. 2010;10(2):138-145. PubMed
16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
17. Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. West Sussex, UK: The Cochrane Collaboration and Wiley Online Library; 2008.
18. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. PubMed
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139. PubMed
21. Patel R, Chang T, Greysen SR, Chopra V. Social media use in chronic disease: a systematic review and novel taxonomy. Am J Med. 2015;128(12):1335-1350. PubMed
22. Carlin BJ. Using whiteboards: fixed identities. Am J Nurs. 2008;108(11):72A-72B, 72D-72E. PubMed
23. Francis JJ, Pankratz VS, Huddleston JM. Patient satisfaction associated with correct identification of physician’s photographs. Mayo Clin Proc. 2001;76(6):604-608. PubMed
24. Farberg AS, Lin AM, Kuhn L, Flanders SA, Kim CS. Dear Doctor: a tool to facilitate patient-centered communication. J Hosp Med. 2013;8(10):553-558. PubMed
25. Hayes RM, Wickline A, Hensley C, et al. A quality improvement project to improve family recognition of medical team member roles. Hosp Pediatr. 2015;5(9):480-486. PubMed
26. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
27. Simons Y, Caprio T, Furiasse N, Kriss M, Williams MV, O’Leary KJ. The impact of facecards on patients’ knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137-141. PubMed
28. Singh A, Rhee KE, Brennan JJ, Kuelbs C, El-Kareh R, Fisher ES. Who’s my doctor? Using an electronic tool to improve team member identification on an inpatient pediatrics team. Hosp Pediatr. 2016;6(3):157-165. PubMed
29. Singh S, Fletcher KE, Pandl GJ, et al. It’s the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127-131. PubMed
30. Tan M, Hooper Evans K, Braddock CH 3rd, Shieh L. Patient whiteboards to improve patient-centred care in the hospital. Postgrad Med J. 2013;89(1056):604-609. PubMed
31. Unaka NI, White CM, Sucharew HJ, Yau C, Clark SL, Brady PW. Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9(3):186-188. PubMed
32. Kelly MM, Hoonakker PL, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2017;24(1):153-161. PubMed
33. Brener MI, Epstein JA, Cho J, Yeh HC, Dudas RA, Feldman L. Faces of all clinically engaged staff: a quality improvement project that enhances the hospitalised patient experience. Int J Clin Pract. 2016;70(11):923-929. PubMed
34. De Valois RL, De Valois KK. Spatial vision. Annu Rev Psychol. 1980;31:309-341. PubMed
Patient satisfaction with medical care during hospitalization is a common quality metric.1,2 Studies showing higher patient satisfaction have reported lower 30-day hospital readmissions3 and improved overall health.4,5 Conversely, communication failures are associated with dissatisfaction among hospitalized patients and adverse outcomes.6,7 A lack of familiarity with hospital providers weakens collaborative decision making and prevents high-quality patient care.8,9
Bedside visual tools, such as whiteboards and pictures of medical staff, have been widely used to enhance communication between patients, families, and providers.10,11 Results of studies evaluating these tools are varied. For example, 1 study found that 98% of patients were better able to identify physicians when their names were written on whiteboards.12 Yet in another, only 21.1% of patients were more likely to correctly identify ≥1 physicians using pictures.13 Thus, despite widespread use,11 whether visual tools improve patient satisfaction and patient care more broadly remains unclear.14,15
We performed a systematic review to answer the following 3 questions: first, what is the effect of visual tools on outcomes (ie, provider identification, understanding of providers’ roles, patient–provider communication, and satisfaction); second, does impact vary by type of visual tool (eg, whiteboards vs pictures of providers); and third, what factors (eg, study design, patient population) are associated with provider identification, communication, and patient satisfaction?
METHODS
Search Strategy
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis when performing this review.16 A research librarian (WT) conducted serial searches for studies reporting the use of bedside visual tools for hospitalized patients in Medline (via OVID), Embase, SCOPUS, Web of Science, CINAHL, and Cochrane DSR and CENTRAL. Controlled vocabularies (ie, Medical Subject Headings terms) were used to identify synonyms for visual tools of interest. Additional studies were identified manually through bibliographies and meeting abstracts. No study design, publication date, or language restrictions were placed on the search, which was conducted between April 2016 and February 2017 (see supplementary Appendix A).
Study Selection
Two reviewers (AG and KT) independently assessed study eligibility; discrepancies were resolved by a third reviewer (VC). We included all adult or pediatric English language studies in which the effect of visual tool(s) on patient outcomes was reported. Visual tools were defined as the bedside display of information or an instrument given to patients to convey information regarding providers or medical care. Patient-reported outcomes included the following: (a) physician identification, (b) understanding of provider roles, (c) patient–provider communication, and (d) patient satisfaction with care. Providers were defined as physicians, residents, interns, medical students, nurse practitioners, or nurses. We excluded studies that were not original research (eg, conference abstracts, not peer reviewed), reported qualitative data without quantitative outcomes, or did not include a bedside visual tool. Given our interest in hospitalized general medicine patients, studies conducted in emergency departments, surgical units, obstetrics and gynecology wards, and intensive care units were excluded.
Data Extraction and Analysis
Data were extracted independently and in duplicate from all studies by using a template adapted from the Cochrane Collaboration.17 For all studies, we abstracted study design, type of visual tool (eg, whiteboards), unit setting (eg, medical), population studied (eg, adult vs pediatric), and outcomes reported (ie, physician identification, understanding of provider roles, communication, and satisfaction with care). Reviewers independently assessed and categorized the impact of tools on reported outcomes.
To standardize and compare outcomes across studies, the following were used to denote a positive association between visual tools and relevant outcomes: a greater number of physicians correctly identified by name/picture or title/role; the use of terms such as “high,” “agreed,” or “significant” on surveys; or ≥4 Likert scores for domains of identification, understanding of roles, communication, and satisfaction with care. Conversely, the inability to identify providers compared to the control/baseline; poor recall of titles/roles; lower Likert-scale scores (ie, ≤2); or survey terms such as “poor,” “disagreed,” or “insignificant” were considered to connote negative impact. Studies in which Likert scores were rated neither high nor low (ie, 3), or in which patients neither agreed nor disagreed on value were considered neutral.
Owing to clinical heterogeneity within studies, meta-analyses were not performed. Descriptive statistics were used to describe study outcomes. A priori18 studies were evaluated according to the following categories: design (eg, randomized vs observational), outcomes (eg, patient satisfaction), intervention (type of visual tool), and patient population (adult or pediatric). Because pediatric patients have underdeveloped communication skills and include parents and/or guardians, data from pediatric studies were tabulated and reported separately to those from adult studies.
Quality Assessment
As recommended by the Cochrane Collaboration, 2 reviewers (AG, KT) assessed the risk of study bias by using the Downs and Black Scale.17,19 Discrepancies in assessment were resolved by a third reviewer (VC). This instrument uses a point-based system to estimate the quality of a study by rating domains such as internal and external validity, bias, and confounding. In keeping with prior systematic reviews,18,20,21 studies with a score of ≥18 were considered high quality. Interrater agreement for the adjudication of study quality was calculated using the Cohen κ statistic.
RESULTS
STUDIES OF ADULT HOSPITALIZED PATIENTS
Eleven studies were conducted on adult hospitalized patients 12-14,22-24,26,27,29,30,33 and included 3 randomized controlled studies.14,27,33
Results by Outcomes Provider Identification Nine studies measured patients’ ability to identify providers with the use of visual aids, and all 9 reported improvements in this outcome. Visual tools used to measure provider identification included pictures (n = 5),13,14,23,27,33 whiteboards (n = 3),12,22,30 and patient portals (n = 1).26 Within studies that used pictures, individual pictures (n = 2)13,23 and handouts with pictures of multiple providers (n = 3) were used.14,27,33 In 2 studies, care team members such as a dietitian, physiotherapist or pharmacist, were included when measuring identification.14,33
Understanding Providers’ RolesSix studies assessed the effect of visual tools on patients’ understanding of provider roles.13,14,22,26,27,33 Four studies reported a positive effect with the use of pictures,27,33 whiteboards,22 and patient portals.26 However, 2 studies reported either no difference or negative impressions. Appel et al.14 reported no difference in the understanding of physician roles using a handout of providers’ pictures and titles. Arora et al.13 used individual pictures of physicians with descriptions of roles and found a negative association, as demonstrated by fewer patients rating their understanding of physicians’ roles as excellent or very good in the intervention period (45.6%) compared with the baseline (55.3%).
Patient–Provider Communication
Three studies evaluated the influence of visual tools on communication.14,24,29 Using pictures, Appel et al.14 found no difference in the perceived quality of communication. Singh et al.29 used whiteboards and reported improved communication scores for physicians and nurses. With notepads, patients surveyed by Farberg et al.24 stated that the tool improved provider communication.
Patient Satisfaction
Five studies assessed patient satisfaction related to the use of visual tools. 22,23,27,30,33 One study reported satisfaction as positive with the use of individual pictures.23 Two studies that used handouts with pictures of all team members reported either a positive33 or neutral27 impact on satisfaction. Studies that used whiteboards reported a positive association with satisfaction22,30 despite differences in content, such as the inclusion of prewritten prompts for writing goals of care and scheduled tests30 versus the name of the nurse and their education level.22
Results by Type of Visual Tool Pictures
Five studies that used pictures reported a positive effect on provider identification.13,14,23,27,33 Two27,33 of 4 studies13,14,27,33 that assessed patients’ understanding of team member roles reported a positive influence, while 1 reported no difference.14 A fourth study demonstrated a negative association, perhaps due to differences in the description of providers’ roles listed on the tool.13 Only 1 study examined the influence of pictures on patient–provider communication, and this study found no difference.14 Satisfaction with care via the use of pictures varied between positive (2 studies)23,33 and neutral (1 study).27
Whiteboards
Four studies tested the use of whiteboards; of these, 3 reported a positive influence on provider identification.12,22,30 One study reported a positive impact on patient–provider communication.29 Two studies noted a positive effect on patient satisfaction.22,30 Notably, the responsibility for updating whiteboards differed between the studies (ie, nurses only22 vs residents, medical students, and nurses).30
Patient Portal
In 1 study, an electronic portal that included names with pictures of providers, descriptions of their roles, lists of medications, and scheduled tests and/or procedures was used as a visual tool. The portal improved patients’ identification of physicians and patients’ understanding of roles. However, improvements in the knowledge of medication changes and planned tests and/or procedures during hospitalization were not observed.26 This finding would suggest limitations in the hospitalized patient’s knowledge of the plan of care, which could potentially weaken patient–provider communication.
Notepads
Only 1 study assessed the use of formatted notepads on patient–provider communication and noted a positive association. Notepads used prompts for different categories (eg, diagnosis/treatment, medications, etc) to encourage patient questions for providers.24
STUDIES OF PEDIATRIC HOSPITALIZED PATIENTS
Five studies were conducted on hospitalized pediatric units.15,25,28,31,32 All studies surveyed the parents, guardians, or caregivers of pediatric patients. One study excluded patients ≥12 years of age because of legal differences in access to adolescent health information,32 while another interviewed parents and/or guardians of teenagers.15
Results by Outcomes Provider Identification and Understanding of Physicians’ Roles
Four studies that assessed the influence of visual tools on provider identification and understanding of roles reported a positive association.15,25,28,31 Visual tools varied between pictures (n = 2),15,31 patient portal (n = 1),28 and whiteboards and pictures combined (n = 1).25 The measurement of outcomes varied between surveys with free text responses,28 multiple choice questions,25 and 1-5 Likert scales.15,31
Patient–Provider Communication
Two studies assessed the impact of patient portal use on communication and reported a positive association.28,32 The 2 portals autopopulated names, pictures, and roles of providers from electronic medical records. Singh et al.28 used a portal that was also available in Spanish and accommodated for non-English speakers. Kelly et al.32 reported that 90% of parents perceived that portal use was associated with reduced errors in care, with 8% finding errors in their child’s medication list.
Patient Satisfaction
Three studies assessed patient satisfaction via the use of visual tools.15,28,31 Singh et al.28 noted a positive influence on satisfaction via a patient portal. Dudas et al.15 used a single-page handout with names and pictures of each provider, along with information regarding the training and roles of each provider. Distribution of these handouts to patients by investigators led to a positive influence on satisfaction. While Unaka et al.31 used a similar handout, they asked residents to distribute them and found no significant difference in satisfaction scores between the intervention (66%) and control group (62%).
Results by Type of Visual Tool Pictures
Two studies reported a positive impact on provider identification and understanding of roles with the use of pictures.15,31 Dudas et al.15 demonstrated a 4.8-fold increase in the odds of parents identifying a medical student, as compared with the control. Similarly, after adjusting for length of stay and prior hospitalization, Unaka et al.31 reported that a higher percentage of patients correctly identified providers using this approach.
Whiteboard and Picture
One study evaluated the simultaneous use of whiteboards and pictures to improve the identification of providers. The study noted improved identification of supervising doctors and increased recognition of roles for supervising doctors, residents, and medical students.25
Patient Portal
Two studies used patient portals as visual tools. Singh et al.28 assessed the use of a patient portal with names, roles, and pictures of treatment team members. Use of this tool was positively associated with provider identification, understanding of roles, communication, and satisfaction. Kelly et al.32 noted that 60% of parents felt that portal use improved healthcare team communication.
RISK OF STUDY BIAS
The risk of bias was assessed for both adult and pediatric studies in aggregate. The average risk of bias using the Downs and Black Scale was 17.81 (range 14-22, standard deviation [SD] 2.20). Of the 16 included studies, 9 were rated at a low risk of bias (score
- >
18).13-15,26-31 Risk of bias was greatest for measures of external validity (mean 2.88, range 2-3, SD 0.34), internal validity (mean 4.06, range 3-6, SD 1.00), and confounding (mean 2.69, range 1-6, SD 1.35). Two of 3 randomized controlled trials had a low risk of bias.14,27 Interrater reliability for study quality adjudication was 0.90, suggesting excellent agreement (see supplementary Appendix B).
DISCUSSION
In this systematic review, the effects of visual tools on outcomes, such as provider identification, understanding of roles, patient–provider communication, and satisfaction with care, were variable. The majority of included studies were conducted on adult patients (n = 11).12-14,22-24,26,27,29,30,33 Pictures were the most frequently used tool (n = 7)13-15,23,27,31,33 and consequently had the greatest sample size across the review (n = 1297). While pictures had a positive influence on provider identification in all studies, comprehension of provider roles and satisfaction were variable. Although the content of whiteboards varied between studies, they showed favorable effects on provider identification (3 of 4 studies)12,22,30 and satisfaction (2 of 2 studies).22,30 While electronic medical record-based tools had a positive influence on outcomes,26,28 only 1 accounted for language preferences.28 Formatted notepads positively influenced patient–provider communication, but their use was limited by literacy.24 Collectively, these data suggest that visual tools have varying effects on patient-reported outcomes, likely owing to differences in study design, interventions, and evaluation methods.
Theoretically, visual tools should facilitate easier identification of providers and engender collaborative relationships. However, such tools do not replace face-to-face patient–provider and family discussions. Rather, these enhancements best serve as a medium to asynchronously display information to patients and family members. Indeed, within the included studies, we found that the use of visual tools was effective in improving satisfaction (6/8 studies), identification (13/13 studies), and understanding of provider roles (8/10 studies). Thus, it is reasonable to say that, in conjunction with excellent clinical care, these tools have an important role in improving care delivery in the hospital.
Despite this promise, we noted that the effectiveness of individual tools varied, a fact that may relate to differences across studies. First, inconsistencies in the format and/or content of the tools were noted. For example, within studies using pictures, tools varied from individual photographs of each team member13,23 to 1-page handouts with pictures of all team members.14,15,31 Such differences in presentation could affect spatial recognition in identifying providers, as single photos are known to be easier to process than multiple images at the same time.34 Second, no study evaluated patient preference of a visual tool. Thus, personal preferences for pictures versus whiteboards versus electronic modalities or a combination of tools might affect outcomes. Additionally, the utility of visual tools in visually impaired, confused, or non-English-speaking patients may limit effectiveness. Future studies that address these aspects and account for patient preferences may better elucidate the role of visual tools in hospitals.
Our results should be considered in the context of several limitations. First, only 3 studies used randomized trial designs; thus, confounding from unmeasured variables inherent to observational designs is possible. Second, none of the interventions tested were blinded to providers, raising the possibility of a Hawthorne effect (ie, alteration of provider behavior in response to awareness of being observed).35 Third, all studies were conducted at single centers, and only 9 of 16 studies were rated at a low risk of bias; thus, caution in broad extrapolations of this literature is necessary.
However, our study has several strengths, including a thorough search of heterogeneous literature, inclusion of both adult and pediatric populations, and a focus on myriad patient-reported outcomes. Second, by contrasting outcomes and measurement strategies across studies, our review helps explicate differences in results related to variation in outcome measurement or presentation of visual data. Third, because we frame results by outcome and type of visual tool used, we are able to identify strengths and weaknesses of individual tools in novel ways. Finally, our data suggest that the use of picture-based techniques and whiteboards are among the most promising visual interventions. Future studies that pair graphic designers with patients to improve the layout of these tools might prove valuable. Additionally, because the measurement of outcomes is confounded by aspects such as lack of controls, severity of illness, and language barriers, a randomized design would help provide greater clarity regarding effectiveness.
In conclusion, we found that visual tools appear to foster recognition of providers and understanding of their roles. However, variability of format, content, and measurement of outcomes hinders the identification of a single optimal approach. Future work using randomized controlled trial designs and standardized tools and measurements would be welcomed.
Acknowledgments
The authors thank Laura Appel, Kevin O’Leary, and Siddharth Singh for providing unpublished data and clarifications to help these analyses.
Disclosure
Anupama Goyal is the guarantor. Anupama Goyal and Komalpreet Tur performed primary data abstraction and analysis. Anupama Goyal, Scott Flanders, Jason Mann, and Vineet Chopra drafted the manuscript. All authors contributed to the development of the selection criteria, the risk of bias assessment strategy, and the data extraction criteria. Anupama Goyal, Jason Mann, Whitney Townsend, and Vineet Chopra developed the search strategy. Vineet Chopra provided systematic review expertise. All authors read, provided feedback, and approved the final manuscript. The authors declare that they have no conflicts of interest.
Patient satisfaction with medical care during hospitalization is a common quality metric.1,2 Studies showing higher patient satisfaction have reported lower 30-day hospital readmissions3 and improved overall health.4,5 Conversely, communication failures are associated with dissatisfaction among hospitalized patients and adverse outcomes.6,7 A lack of familiarity with hospital providers weakens collaborative decision making and prevents high-quality patient care.8,9
Bedside visual tools, such as whiteboards and pictures of medical staff, have been widely used to enhance communication between patients, families, and providers.10,11 Results of studies evaluating these tools are varied. For example, 1 study found that 98% of patients were better able to identify physicians when their names were written on whiteboards.12 Yet in another, only 21.1% of patients were more likely to correctly identify ≥1 physicians using pictures.13 Thus, despite widespread use,11 whether visual tools improve patient satisfaction and patient care more broadly remains unclear.14,15
We performed a systematic review to answer the following 3 questions: first, what is the effect of visual tools on outcomes (ie, provider identification, understanding of providers’ roles, patient–provider communication, and satisfaction); second, does impact vary by type of visual tool (eg, whiteboards vs pictures of providers); and third, what factors (eg, study design, patient population) are associated with provider identification, communication, and patient satisfaction?
METHODS
Search Strategy
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis when performing this review.16 A research librarian (WT) conducted serial searches for studies reporting the use of bedside visual tools for hospitalized patients in Medline (via OVID), Embase, SCOPUS, Web of Science, CINAHL, and Cochrane DSR and CENTRAL. Controlled vocabularies (ie, Medical Subject Headings terms) were used to identify synonyms for visual tools of interest. Additional studies were identified manually through bibliographies and meeting abstracts. No study design, publication date, or language restrictions were placed on the search, which was conducted between April 2016 and February 2017 (see supplementary Appendix A).
Study Selection
Two reviewers (AG and KT) independently assessed study eligibility; discrepancies were resolved by a third reviewer (VC). We included all adult or pediatric English language studies in which the effect of visual tool(s) on patient outcomes was reported. Visual tools were defined as the bedside display of information or an instrument given to patients to convey information regarding providers or medical care. Patient-reported outcomes included the following: (a) physician identification, (b) understanding of provider roles, (c) patient–provider communication, and (d) patient satisfaction with care. Providers were defined as physicians, residents, interns, medical students, nurse practitioners, or nurses. We excluded studies that were not original research (eg, conference abstracts, not peer reviewed), reported qualitative data without quantitative outcomes, or did not include a bedside visual tool. Given our interest in hospitalized general medicine patients, studies conducted in emergency departments, surgical units, obstetrics and gynecology wards, and intensive care units were excluded.
Data Extraction and Analysis
Data were extracted independently and in duplicate from all studies by using a template adapted from the Cochrane Collaboration.17 For all studies, we abstracted study design, type of visual tool (eg, whiteboards), unit setting (eg, medical), population studied (eg, adult vs pediatric), and outcomes reported (ie, physician identification, understanding of provider roles, communication, and satisfaction with care). Reviewers independently assessed and categorized the impact of tools on reported outcomes.
To standardize and compare outcomes across studies, the following were used to denote a positive association between visual tools and relevant outcomes: a greater number of physicians correctly identified by name/picture or title/role; the use of terms such as “high,” “agreed,” or “significant” on surveys; or ≥4 Likert scores for domains of identification, understanding of roles, communication, and satisfaction with care. Conversely, the inability to identify providers compared to the control/baseline; poor recall of titles/roles; lower Likert-scale scores (ie, ≤2); or survey terms such as “poor,” “disagreed,” or “insignificant” were considered to connote negative impact. Studies in which Likert scores were rated neither high nor low (ie, 3), or in which patients neither agreed nor disagreed on value were considered neutral.
Owing to clinical heterogeneity within studies, meta-analyses were not performed. Descriptive statistics were used to describe study outcomes. A priori18 studies were evaluated according to the following categories: design (eg, randomized vs observational), outcomes (eg, patient satisfaction), intervention (type of visual tool), and patient population (adult or pediatric). Because pediatric patients have underdeveloped communication skills and include parents and/or guardians, data from pediatric studies were tabulated and reported separately to those from adult studies.
Quality Assessment
As recommended by the Cochrane Collaboration, 2 reviewers (AG, KT) assessed the risk of study bias by using the Downs and Black Scale.17,19 Discrepancies in assessment were resolved by a third reviewer (VC). This instrument uses a point-based system to estimate the quality of a study by rating domains such as internal and external validity, bias, and confounding. In keeping with prior systematic reviews,18,20,21 studies with a score of ≥18 were considered high quality. Interrater agreement for the adjudication of study quality was calculated using the Cohen κ statistic.
RESULTS
STUDIES OF ADULT HOSPITALIZED PATIENTS
Eleven studies were conducted on adult hospitalized patients 12-14,22-24,26,27,29,30,33 and included 3 randomized controlled studies.14,27,33
Results by Outcomes Provider Identification Nine studies measured patients’ ability to identify providers with the use of visual aids, and all 9 reported improvements in this outcome. Visual tools used to measure provider identification included pictures (n = 5),13,14,23,27,33 whiteboards (n = 3),12,22,30 and patient portals (n = 1).26 Within studies that used pictures, individual pictures (n = 2)13,23 and handouts with pictures of multiple providers (n = 3) were used.14,27,33 In 2 studies, care team members such as a dietitian, physiotherapist or pharmacist, were included when measuring identification.14,33
Understanding Providers’ RolesSix studies assessed the effect of visual tools on patients’ understanding of provider roles.13,14,22,26,27,33 Four studies reported a positive effect with the use of pictures,27,33 whiteboards,22 and patient portals.26 However, 2 studies reported either no difference or negative impressions. Appel et al.14 reported no difference in the understanding of physician roles using a handout of providers’ pictures and titles. Arora et al.13 used individual pictures of physicians with descriptions of roles and found a negative association, as demonstrated by fewer patients rating their understanding of physicians’ roles as excellent or very good in the intervention period (45.6%) compared with the baseline (55.3%).
Patient–Provider Communication
Three studies evaluated the influence of visual tools on communication.14,24,29 Using pictures, Appel et al.14 found no difference in the perceived quality of communication. Singh et al.29 used whiteboards and reported improved communication scores for physicians and nurses. With notepads, patients surveyed by Farberg et al.24 stated that the tool improved provider communication.
Patient Satisfaction
Five studies assessed patient satisfaction related to the use of visual tools. 22,23,27,30,33 One study reported satisfaction as positive with the use of individual pictures.23 Two studies that used handouts with pictures of all team members reported either a positive33 or neutral27 impact on satisfaction. Studies that used whiteboards reported a positive association with satisfaction22,30 despite differences in content, such as the inclusion of prewritten prompts for writing goals of care and scheduled tests30 versus the name of the nurse and their education level.22
Results by Type of Visual Tool Pictures
Five studies that used pictures reported a positive effect on provider identification.13,14,23,27,33 Two27,33 of 4 studies13,14,27,33 that assessed patients’ understanding of team member roles reported a positive influence, while 1 reported no difference.14 A fourth study demonstrated a negative association, perhaps due to differences in the description of providers’ roles listed on the tool.13 Only 1 study examined the influence of pictures on patient–provider communication, and this study found no difference.14 Satisfaction with care via the use of pictures varied between positive (2 studies)23,33 and neutral (1 study).27
Whiteboards
Four studies tested the use of whiteboards; of these, 3 reported a positive influence on provider identification.12,22,30 One study reported a positive impact on patient–provider communication.29 Two studies noted a positive effect on patient satisfaction.22,30 Notably, the responsibility for updating whiteboards differed between the studies (ie, nurses only22 vs residents, medical students, and nurses).30
Patient Portal
In 1 study, an electronic portal that included names with pictures of providers, descriptions of their roles, lists of medications, and scheduled tests and/or procedures was used as a visual tool. The portal improved patients’ identification of physicians and patients’ understanding of roles. However, improvements in the knowledge of medication changes and planned tests and/or procedures during hospitalization were not observed.26 This finding would suggest limitations in the hospitalized patient’s knowledge of the plan of care, which could potentially weaken patient–provider communication.
Notepads
Only 1 study assessed the use of formatted notepads on patient–provider communication and noted a positive association. Notepads used prompts for different categories (eg, diagnosis/treatment, medications, etc) to encourage patient questions for providers.24
STUDIES OF PEDIATRIC HOSPITALIZED PATIENTS
Five studies were conducted on hospitalized pediatric units.15,25,28,31,32 All studies surveyed the parents, guardians, or caregivers of pediatric patients. One study excluded patients ≥12 years of age because of legal differences in access to adolescent health information,32 while another interviewed parents and/or guardians of teenagers.15
Results by Outcomes Provider Identification and Understanding of Physicians’ Roles
Four studies that assessed the influence of visual tools on provider identification and understanding of roles reported a positive association.15,25,28,31 Visual tools varied between pictures (n = 2),15,31 patient portal (n = 1),28 and whiteboards and pictures combined (n = 1).25 The measurement of outcomes varied between surveys with free text responses,28 multiple choice questions,25 and 1-5 Likert scales.15,31
Patient–Provider Communication
Two studies assessed the impact of patient portal use on communication and reported a positive association.28,32 The 2 portals autopopulated names, pictures, and roles of providers from electronic medical records. Singh et al.28 used a portal that was also available in Spanish and accommodated for non-English speakers. Kelly et al.32 reported that 90% of parents perceived that portal use was associated with reduced errors in care, with 8% finding errors in their child’s medication list.
Patient Satisfaction
Three studies assessed patient satisfaction via the use of visual tools.15,28,31 Singh et al.28 noted a positive influence on satisfaction via a patient portal. Dudas et al.15 used a single-page handout with names and pictures of each provider, along with information regarding the training and roles of each provider. Distribution of these handouts to patients by investigators led to a positive influence on satisfaction. While Unaka et al.31 used a similar handout, they asked residents to distribute them and found no significant difference in satisfaction scores between the intervention (66%) and control group (62%).
Results by Type of Visual Tool Pictures
Two studies reported a positive impact on provider identification and understanding of roles with the use of pictures.15,31 Dudas et al.15 demonstrated a 4.8-fold increase in the odds of parents identifying a medical student, as compared with the control. Similarly, after adjusting for length of stay and prior hospitalization, Unaka et al.31 reported that a higher percentage of patients correctly identified providers using this approach.
Whiteboard and Picture
One study evaluated the simultaneous use of whiteboards and pictures to improve the identification of providers. The study noted improved identification of supervising doctors and increased recognition of roles for supervising doctors, residents, and medical students.25
Patient Portal
Two studies used patient portals as visual tools. Singh et al.28 assessed the use of a patient portal with names, roles, and pictures of treatment team members. Use of this tool was positively associated with provider identification, understanding of roles, communication, and satisfaction. Kelly et al.32 noted that 60% of parents felt that portal use improved healthcare team communication.
RISK OF STUDY BIAS
The risk of bias was assessed for both adult and pediatric studies in aggregate. The average risk of bias using the Downs and Black Scale was 17.81 (range 14-22, standard deviation [SD] 2.20). Of the 16 included studies, 9 were rated at a low risk of bias (score
- >
18).13-15,26-31 Risk of bias was greatest for measures of external validity (mean 2.88, range 2-3, SD 0.34), internal validity (mean 4.06, range 3-6, SD 1.00), and confounding (mean 2.69, range 1-6, SD 1.35). Two of 3 randomized controlled trials had a low risk of bias.14,27 Interrater reliability for study quality adjudication was 0.90, suggesting excellent agreement (see supplementary Appendix B).
DISCUSSION
In this systematic review, the effects of visual tools on outcomes, such as provider identification, understanding of roles, patient–provider communication, and satisfaction with care, were variable. The majority of included studies were conducted on adult patients (n = 11).12-14,22-24,26,27,29,30,33 Pictures were the most frequently used tool (n = 7)13-15,23,27,31,33 and consequently had the greatest sample size across the review (n = 1297). While pictures had a positive influence on provider identification in all studies, comprehension of provider roles and satisfaction were variable. Although the content of whiteboards varied between studies, they showed favorable effects on provider identification (3 of 4 studies)12,22,30 and satisfaction (2 of 2 studies).22,30 While electronic medical record-based tools had a positive influence on outcomes,26,28 only 1 accounted for language preferences.28 Formatted notepads positively influenced patient–provider communication, but their use was limited by literacy.24 Collectively, these data suggest that visual tools have varying effects on patient-reported outcomes, likely owing to differences in study design, interventions, and evaluation methods.
Theoretically, visual tools should facilitate easier identification of providers and engender collaborative relationships. However, such tools do not replace face-to-face patient–provider and family discussions. Rather, these enhancements best serve as a medium to asynchronously display information to patients and family members. Indeed, within the included studies, we found that the use of visual tools was effective in improving satisfaction (6/8 studies), identification (13/13 studies), and understanding of provider roles (8/10 studies). Thus, it is reasonable to say that, in conjunction with excellent clinical care, these tools have an important role in improving care delivery in the hospital.
Despite this promise, we noted that the effectiveness of individual tools varied, a fact that may relate to differences across studies. First, inconsistencies in the format and/or content of the tools were noted. For example, within studies using pictures, tools varied from individual photographs of each team member13,23 to 1-page handouts with pictures of all team members.14,15,31 Such differences in presentation could affect spatial recognition in identifying providers, as single photos are known to be easier to process than multiple images at the same time.34 Second, no study evaluated patient preference of a visual tool. Thus, personal preferences for pictures versus whiteboards versus electronic modalities or a combination of tools might affect outcomes. Additionally, the utility of visual tools in visually impaired, confused, or non-English-speaking patients may limit effectiveness. Future studies that address these aspects and account for patient preferences may better elucidate the role of visual tools in hospitals.
Our results should be considered in the context of several limitations. First, only 3 studies used randomized trial designs; thus, confounding from unmeasured variables inherent to observational designs is possible. Second, none of the interventions tested were blinded to providers, raising the possibility of a Hawthorne effect (ie, alteration of provider behavior in response to awareness of being observed).35 Third, all studies were conducted at single centers, and only 9 of 16 studies were rated at a low risk of bias; thus, caution in broad extrapolations of this literature is necessary.
However, our study has several strengths, including a thorough search of heterogeneous literature, inclusion of both adult and pediatric populations, and a focus on myriad patient-reported outcomes. Second, by contrasting outcomes and measurement strategies across studies, our review helps explicate differences in results related to variation in outcome measurement or presentation of visual data. Third, because we frame results by outcome and type of visual tool used, we are able to identify strengths and weaknesses of individual tools in novel ways. Finally, our data suggest that the use of picture-based techniques and whiteboards are among the most promising visual interventions. Future studies that pair graphic designers with patients to improve the layout of these tools might prove valuable. Additionally, because the measurement of outcomes is confounded by aspects such as lack of controls, severity of illness, and language barriers, a randomized design would help provide greater clarity regarding effectiveness.
In conclusion, we found that visual tools appear to foster recognition of providers and understanding of their roles. However, variability of format, content, and measurement of outcomes hinders the identification of a single optimal approach. Future work using randomized controlled trial designs and standardized tools and measurements would be welcomed.
Acknowledgments
The authors thank Laura Appel, Kevin O’Leary, and Siddharth Singh for providing unpublished data and clarifications to help these analyses.
Disclosure
Anupama Goyal is the guarantor. Anupama Goyal and Komalpreet Tur performed primary data abstraction and analysis. Anupama Goyal, Scott Flanders, Jason Mann, and Vineet Chopra drafted the manuscript. All authors contributed to the development of the selection criteria, the risk of bias assessment strategy, and the data extraction criteria. Anupama Goyal, Jason Mann, Whitney Townsend, and Vineet Chopra developed the search strategy. Vineet Chopra provided systematic review expertise. All authors read, provided feedback, and approved the final manuscript. The authors declare that they have no conflicts of interest.
1. Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff (Millwood). 2002;21(3):80-90. PubMed
2. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-1931. PubMed
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48. PubMed
4. Little P, Everitt H, Williamson I, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908-911. PubMed
5. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1422-1433. PubMed
6. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
7. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1:i85-i90. PubMed
8. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. PubMed
9. Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201. PubMed
10. Makaryus AN, Friedman EA. Does your patient know your name? An approach to enhancing patients’ awareness of their caretaker’s name. J Healthc Qual. 2005;27(4):53-56. PubMed
11. Sehgal NL, Green A, Vidyarthi AR, Blegen MA, Wachter RM. Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234-239. PubMed
12. Maniaci MJ, Heckman MG, Dawson NL. Increasing a patient’s ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170:1084-1085. PubMed
13. Arora VM, Schaninger C, D’Arcy M, et al. Improving inpatients’ identification of their doctors: Use of FACE™ cards. Jt Comm J Qual Patient Saf. 2009;35(12):613-619. PubMed
14. Appel L, Abrams H, Morra D, Wu RC. Put a face to a name: a randomized controlled trial evaluating the impact of providing clinician photographs on inpatients’ recall. Am J Med. 2015;128(1):82-89. PubMed
15. Dudas RA, Lemerman H, Barone M, Serwint JR. PHACES (Photographs of Academic Clinicians and Their Educational Status): a tool to improve delivery of family-centered care. Acad Pediatr. 2010;10(2):138-145. PubMed
16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
17. Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. West Sussex, UK: The Cochrane Collaboration and Wiley Online Library; 2008.
18. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. PubMed
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139. PubMed
21. Patel R, Chang T, Greysen SR, Chopra V. Social media use in chronic disease: a systematic review and novel taxonomy. Am J Med. 2015;128(12):1335-1350. PubMed
22. Carlin BJ. Using whiteboards: fixed identities. Am J Nurs. 2008;108(11):72A-72B, 72D-72E. PubMed
23. Francis JJ, Pankratz VS, Huddleston JM. Patient satisfaction associated with correct identification of physician’s photographs. Mayo Clin Proc. 2001;76(6):604-608. PubMed
24. Farberg AS, Lin AM, Kuhn L, Flanders SA, Kim CS. Dear Doctor: a tool to facilitate patient-centered communication. J Hosp Med. 2013;8(10):553-558. PubMed
25. Hayes RM, Wickline A, Hensley C, et al. A quality improvement project to improve family recognition of medical team member roles. Hosp Pediatr. 2015;5(9):480-486. PubMed
26. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
27. Simons Y, Caprio T, Furiasse N, Kriss M, Williams MV, O’Leary KJ. The impact of facecards on patients’ knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137-141. PubMed
28. Singh A, Rhee KE, Brennan JJ, Kuelbs C, El-Kareh R, Fisher ES. Who’s my doctor? Using an electronic tool to improve team member identification on an inpatient pediatrics team. Hosp Pediatr. 2016;6(3):157-165. PubMed
29. Singh S, Fletcher KE, Pandl GJ, et al. It’s the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127-131. PubMed
30. Tan M, Hooper Evans K, Braddock CH 3rd, Shieh L. Patient whiteboards to improve patient-centred care in the hospital. Postgrad Med J. 2013;89(1056):604-609. PubMed
31. Unaka NI, White CM, Sucharew HJ, Yau C, Clark SL, Brady PW. Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9(3):186-188. PubMed
32. Kelly MM, Hoonakker PL, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2017;24(1):153-161. PubMed
33. Brener MI, Epstein JA, Cho J, Yeh HC, Dudas RA, Feldman L. Faces of all clinically engaged staff: a quality improvement project that enhances the hospitalised patient experience. Int J Clin Pract. 2016;70(11):923-929. PubMed
34. De Valois RL, De Valois KK. Spatial vision. Annu Rev Psychol. 1980;31:309-341. PubMed
1. Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff (Millwood). 2002;21(3):80-90. PubMed
2. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-1931. PubMed
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48. PubMed
4. Little P, Everitt H, Williamson I, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908-911. PubMed
5. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1422-1433. PubMed
6. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
7. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1:i85-i90. PubMed
8. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. PubMed
9. Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201. PubMed
10. Makaryus AN, Friedman EA. Does your patient know your name? An approach to enhancing patients’ awareness of their caretaker’s name. J Healthc Qual. 2005;27(4):53-56. PubMed
11. Sehgal NL, Green A, Vidyarthi AR, Blegen MA, Wachter RM. Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234-239. PubMed
12. Maniaci MJ, Heckman MG, Dawson NL. Increasing a patient’s ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170:1084-1085. PubMed
13. Arora VM, Schaninger C, D’Arcy M, et al. Improving inpatients’ identification of their doctors: Use of FACE™ cards. Jt Comm J Qual Patient Saf. 2009;35(12):613-619. PubMed
14. Appel L, Abrams H, Morra D, Wu RC. Put a face to a name: a randomized controlled trial evaluating the impact of providing clinician photographs on inpatients’ recall. Am J Med. 2015;128(1):82-89. PubMed
15. Dudas RA, Lemerman H, Barone M, Serwint JR. PHACES (Photographs of Academic Clinicians and Their Educational Status): a tool to improve delivery of family-centered care. Acad Pediatr. 2010;10(2):138-145. PubMed
16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
17. Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. West Sussex, UK: The Cochrane Collaboration and Wiley Online Library; 2008.
18. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. PubMed
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139. PubMed
21. Patel R, Chang T, Greysen SR, Chopra V. Social media use in chronic disease: a systematic review and novel taxonomy. Am J Med. 2015;128(12):1335-1350. PubMed
22. Carlin BJ. Using whiteboards: fixed identities. Am J Nurs. 2008;108(11):72A-72B, 72D-72E. PubMed
23. Francis JJ, Pankratz VS, Huddleston JM. Patient satisfaction associated with correct identification of physician’s photographs. Mayo Clin Proc. 2001;76(6):604-608. PubMed
24. Farberg AS, Lin AM, Kuhn L, Flanders SA, Kim CS. Dear Doctor: a tool to facilitate patient-centered communication. J Hosp Med. 2013;8(10):553-558. PubMed
25. Hayes RM, Wickline A, Hensley C, et al. A quality improvement project to improve family recognition of medical team member roles. Hosp Pediatr. 2015;5(9):480-486. PubMed
26. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
27. Simons Y, Caprio T, Furiasse N, Kriss M, Williams MV, O’Leary KJ. The impact of facecards on patients’ knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137-141. PubMed
28. Singh A, Rhee KE, Brennan JJ, Kuelbs C, El-Kareh R, Fisher ES. Who’s my doctor? Using an electronic tool to improve team member identification on an inpatient pediatrics team. Hosp Pediatr. 2016;6(3):157-165. PubMed
29. Singh S, Fletcher KE, Pandl GJ, et al. It’s the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127-131. PubMed
30. Tan M, Hooper Evans K, Braddock CH 3rd, Shieh L. Patient whiteboards to improve patient-centred care in the hospital. Postgrad Med J. 2013;89(1056):604-609. PubMed
31. Unaka NI, White CM, Sucharew HJ, Yau C, Clark SL, Brady PW. Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9(3):186-188. PubMed
32. Kelly MM, Hoonakker PL, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2017;24(1):153-161. PubMed
33. Brener MI, Epstein JA, Cho J, Yeh HC, Dudas RA, Feldman L. Faces of all clinically engaged staff: a quality improvement project that enhances the hospitalised patient experience. Int J Clin Pract. 2016;70(11):923-929. PubMed
34. De Valois RL, De Valois KK. Spatial vision. Annu Rev Psychol. 1980;31:309-341. PubMed
© 2017 Society of Hospital Medicine