User login
Some data support botulinum toxin for psoriasis and rosacea
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
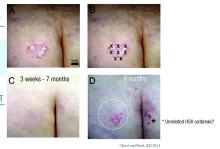
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17
Strontium, ketamine target troublesome itch
ORLANDO – Two drugs that target ion channels in nerves are being used to quiet neurogenic itch.
The powerful anesthetic ketamine and the element strontium have both been formulated into topical compounds that do very well in quelling itches that have been stubbornly resistant to other therapies, Gil Yosipovitch, MD, said at the annual meeting of the American Academy of Dermatology.
Both studies employed a 4% strontium hydrogel that is available over the counter (TriCalm). The product is designed to alleviate skin irritation (itching, burning, or stinging sensations), according to the manufacturer’s website.
The first study, published in 2013, comprised 32 healthy subjects in whom itch was induced with cowhage before and after skin treatment with the strontium gel, a control vehicle, topical 1% hydrocortisone, and topical 2% diphenhydramine (Acta Derm Venereol. 2013 Sep 4;93[5]:520-6).
Strontium significantly reduced the peak intensity and duration of itch relative to all three of the comparators.
A confirmatory study was published in 2015. The vehicle-controlled, randomized, crossover study recorded cowhage-induced itch intensity and duration in 48 healthy subjects before and after skin treatment with TriCalm, 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle (Clin Cosmet Investig Dermatol. 2015 Apr 24;8:223-9). The results were similar, Dr. Yosipovitch said.
TriCalm effectively reduced peak itch intensity by about 3 points on a visual analog scale – a 41% reduction. Itch duration was reduced by 40%. These results were both clinically and statistically significantly better than those achieved by the other active comparators and the vehicle control.
Dr. Yosipovitch said the gel is most effective on nonhistaminergic itches, including those with a neurogenic component, nummular eczema, and facial itch.
“The most powerful antipruritic we have seen in the last 3 years, however, is topical ketamine,” Dr. Yosipovitch said. Typically formulated in 2%-10% creams, the anesthetic is usually combined with amitriptyline and lidocaine. “I see this as the most effective topical antipruritic and antinociceptive we have been using.”
Ketamine is an antagonist of the n-methyl-D-aspartate (NMDA) glutamate receptor and an ion channel protein. Amitriptyline serves primarily as a voltage-gated sodium channel antagonist, and lidocaine as a local anesthetic.
Mark Davis, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., and associates initially investigated 0.5% ketamine in a topical combination with amitriptyline 1% in a cream. The compound was remarkably effective for a 41-year-old man with a recalcitrant case of brachioradial pruritus – a neuropathic condition characterized by upper-extremity itching (JAMA Dermatol. 2013;149[2]:148-50). The patient had already failed treatment with halobetasol propionate, pimecrolimus, capsaicin, doxepin hydrochloride creams, and oral hydroxyzine hydrochloride and desloratadine. However, he had complete resolution of the itch soon after using the combination cream two to three times daily. At last follow-up, 4 years later, he was still using it at least once daily and continued to obtain complete relief.
Dr. Yosipovitch said this case was followed by a retrospective study of 16 patients who had used the 0.5% ketamine cream with either 1% or 2% amitriptyline for recalcitrant pruritus. The etiologies included neurodermatitis, pruritus caused by postherpetic neuralgia, nostalgia paresthetica, anesthesia dolorosa, nasal pruritus, and diabetic neuropathy (J Am Acad Dermatol. 2013 Aug;69[2]:320-1).
They used the medication one to five times per day for a mean duration of 10 months. Of the 16 patients, two had complete relief; two had substantial relief; six had some relief; five had no relief; and one reported increased itching.
Most recently, Dr. Yosipovitch and associates reported the results of a retrospective case review of 96 patients with a variety of pruritic conditions. The most frequent indications were neuropathic conditions (29%) and prurigo nodularis (19%). Most patients got a compounded cream of 10% ketamine, 5% amitriptyline, and 2% lidocaine;16 patients got a compound with 5% ketamine. The medication worked quickly, providing itch relief within a median of about 4 minutes, with an average of about a 50% decrease in itch rating (J Am Acad Dermatol. 2017 Apr;76[4]:760-1).
Forty patients participated in a pharmacy-administered telephone survey that assessed medication tolerability and efficacy. Of these, 23 patients (58%) had relief “to a great extent” and 14 (35%) “to a moderate extent.”
There were mild side effects (burning and redness at the application site) in 16 patients. “We attributed this mainly to the lidocaine component,” Dr. Yosipovitch said. “Itch reduction lasted from 30 minutes to 7 hours, so we think this is quite a powerful tool. I now often use this topical for patients with severe intractable itch.”
He added that a case report of encephalopathy associated with the cream has recently surfaced. The patient was an elderly man with Parkinson’s disease who had been using 10% ketamine compounded with amitriptyline and lidocaine for 4 days. He gradually increased the use until he was applying it onto almost all of his upper body. The day after this extensive application, the patient presented to an emergency department with slurred speech, ataxia, and altered mental status (JAMA Dermatol. 2016;152[12]:1390-1).
“So a word of warning here: I don’t recommend using it all over the body,” Dr. Yosipovitch said.
Dr. Yosipovitch has financial relationships with numerous companies that are investigating antipruritic compounds, including strontium.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – Two drugs that target ion channels in nerves are being used to quiet neurogenic itch.
The powerful anesthetic ketamine and the element strontium have both been formulated into topical compounds that do very well in quelling itches that have been stubbornly resistant to other therapies, Gil Yosipovitch, MD, said at the annual meeting of the American Academy of Dermatology.
Both studies employed a 4% strontium hydrogel that is available over the counter (TriCalm). The product is designed to alleviate skin irritation (itching, burning, or stinging sensations), according to the manufacturer’s website.
The first study, published in 2013, comprised 32 healthy subjects in whom itch was induced with cowhage before and after skin treatment with the strontium gel, a control vehicle, topical 1% hydrocortisone, and topical 2% diphenhydramine (Acta Derm Venereol. 2013 Sep 4;93[5]:520-6).
Strontium significantly reduced the peak intensity and duration of itch relative to all three of the comparators.
A confirmatory study was published in 2015. The vehicle-controlled, randomized, crossover study recorded cowhage-induced itch intensity and duration in 48 healthy subjects before and after skin treatment with TriCalm, 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle (Clin Cosmet Investig Dermatol. 2015 Apr 24;8:223-9). The results were similar, Dr. Yosipovitch said.
TriCalm effectively reduced peak itch intensity by about 3 points on a visual analog scale – a 41% reduction. Itch duration was reduced by 40%. These results were both clinically and statistically significantly better than those achieved by the other active comparators and the vehicle control.
Dr. Yosipovitch said the gel is most effective on nonhistaminergic itches, including those with a neurogenic component, nummular eczema, and facial itch.
“The most powerful antipruritic we have seen in the last 3 years, however, is topical ketamine,” Dr. Yosipovitch said. Typically formulated in 2%-10% creams, the anesthetic is usually combined with amitriptyline and lidocaine. “I see this as the most effective topical antipruritic and antinociceptive we have been using.”
Ketamine is an antagonist of the n-methyl-D-aspartate (NMDA) glutamate receptor and an ion channel protein. Amitriptyline serves primarily as a voltage-gated sodium channel antagonist, and lidocaine as a local anesthetic.
Mark Davis, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., and associates initially investigated 0.5% ketamine in a topical combination with amitriptyline 1% in a cream. The compound was remarkably effective for a 41-year-old man with a recalcitrant case of brachioradial pruritus – a neuropathic condition characterized by upper-extremity itching (JAMA Dermatol. 2013;149[2]:148-50). The patient had already failed treatment with halobetasol propionate, pimecrolimus, capsaicin, doxepin hydrochloride creams, and oral hydroxyzine hydrochloride and desloratadine. However, he had complete resolution of the itch soon after using the combination cream two to three times daily. At last follow-up, 4 years later, he was still using it at least once daily and continued to obtain complete relief.
Dr. Yosipovitch said this case was followed by a retrospective study of 16 patients who had used the 0.5% ketamine cream with either 1% or 2% amitriptyline for recalcitrant pruritus. The etiologies included neurodermatitis, pruritus caused by postherpetic neuralgia, nostalgia paresthetica, anesthesia dolorosa, nasal pruritus, and diabetic neuropathy (J Am Acad Dermatol. 2013 Aug;69[2]:320-1).
They used the medication one to five times per day for a mean duration of 10 months. Of the 16 patients, two had complete relief; two had substantial relief; six had some relief; five had no relief; and one reported increased itching.
Most recently, Dr. Yosipovitch and associates reported the results of a retrospective case review of 96 patients with a variety of pruritic conditions. The most frequent indications were neuropathic conditions (29%) and prurigo nodularis (19%). Most patients got a compounded cream of 10% ketamine, 5% amitriptyline, and 2% lidocaine;16 patients got a compound with 5% ketamine. The medication worked quickly, providing itch relief within a median of about 4 minutes, with an average of about a 50% decrease in itch rating (J Am Acad Dermatol. 2017 Apr;76[4]:760-1).
Forty patients participated in a pharmacy-administered telephone survey that assessed medication tolerability and efficacy. Of these, 23 patients (58%) had relief “to a great extent” and 14 (35%) “to a moderate extent.”
There were mild side effects (burning and redness at the application site) in 16 patients. “We attributed this mainly to the lidocaine component,” Dr. Yosipovitch said. “Itch reduction lasted from 30 minutes to 7 hours, so we think this is quite a powerful tool. I now often use this topical for patients with severe intractable itch.”
He added that a case report of encephalopathy associated with the cream has recently surfaced. The patient was an elderly man with Parkinson’s disease who had been using 10% ketamine compounded with amitriptyline and lidocaine for 4 days. He gradually increased the use until he was applying it onto almost all of his upper body. The day after this extensive application, the patient presented to an emergency department with slurred speech, ataxia, and altered mental status (JAMA Dermatol. 2016;152[12]:1390-1).
“So a word of warning here: I don’t recommend using it all over the body,” Dr. Yosipovitch said.
Dr. Yosipovitch has financial relationships with numerous companies that are investigating antipruritic compounds, including strontium.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – Two drugs that target ion channels in nerves are being used to quiet neurogenic itch.
The powerful anesthetic ketamine and the element strontium have both been formulated into topical compounds that do very well in quelling itches that have been stubbornly resistant to other therapies, Gil Yosipovitch, MD, said at the annual meeting of the American Academy of Dermatology.
Both studies employed a 4% strontium hydrogel that is available over the counter (TriCalm). The product is designed to alleviate skin irritation (itching, burning, or stinging sensations), according to the manufacturer’s website.
The first study, published in 2013, comprised 32 healthy subjects in whom itch was induced with cowhage before and after skin treatment with the strontium gel, a control vehicle, topical 1% hydrocortisone, and topical 2% diphenhydramine (Acta Derm Venereol. 2013 Sep 4;93[5]:520-6).
Strontium significantly reduced the peak intensity and duration of itch relative to all three of the comparators.
A confirmatory study was published in 2015. The vehicle-controlled, randomized, crossover study recorded cowhage-induced itch intensity and duration in 48 healthy subjects before and after skin treatment with TriCalm, 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle (Clin Cosmet Investig Dermatol. 2015 Apr 24;8:223-9). The results were similar, Dr. Yosipovitch said.
TriCalm effectively reduced peak itch intensity by about 3 points on a visual analog scale – a 41% reduction. Itch duration was reduced by 40%. These results were both clinically and statistically significantly better than those achieved by the other active comparators and the vehicle control.
Dr. Yosipovitch said the gel is most effective on nonhistaminergic itches, including those with a neurogenic component, nummular eczema, and facial itch.
“The most powerful antipruritic we have seen in the last 3 years, however, is topical ketamine,” Dr. Yosipovitch said. Typically formulated in 2%-10% creams, the anesthetic is usually combined with amitriptyline and lidocaine. “I see this as the most effective topical antipruritic and antinociceptive we have been using.”
Ketamine is an antagonist of the n-methyl-D-aspartate (NMDA) glutamate receptor and an ion channel protein. Amitriptyline serves primarily as a voltage-gated sodium channel antagonist, and lidocaine as a local anesthetic.
Mark Davis, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., and associates initially investigated 0.5% ketamine in a topical combination with amitriptyline 1% in a cream. The compound was remarkably effective for a 41-year-old man with a recalcitrant case of brachioradial pruritus – a neuropathic condition characterized by upper-extremity itching (JAMA Dermatol. 2013;149[2]:148-50). The patient had already failed treatment with halobetasol propionate, pimecrolimus, capsaicin, doxepin hydrochloride creams, and oral hydroxyzine hydrochloride and desloratadine. However, he had complete resolution of the itch soon after using the combination cream two to three times daily. At last follow-up, 4 years later, he was still using it at least once daily and continued to obtain complete relief.
Dr. Yosipovitch said this case was followed by a retrospective study of 16 patients who had used the 0.5% ketamine cream with either 1% or 2% amitriptyline for recalcitrant pruritus. The etiologies included neurodermatitis, pruritus caused by postherpetic neuralgia, nostalgia paresthetica, anesthesia dolorosa, nasal pruritus, and diabetic neuropathy (J Am Acad Dermatol. 2013 Aug;69[2]:320-1).
They used the medication one to five times per day for a mean duration of 10 months. Of the 16 patients, two had complete relief; two had substantial relief; six had some relief; five had no relief; and one reported increased itching.
Most recently, Dr. Yosipovitch and associates reported the results of a retrospective case review of 96 patients with a variety of pruritic conditions. The most frequent indications were neuropathic conditions (29%) and prurigo nodularis (19%). Most patients got a compounded cream of 10% ketamine, 5% amitriptyline, and 2% lidocaine;16 patients got a compound with 5% ketamine. The medication worked quickly, providing itch relief within a median of about 4 minutes, with an average of about a 50% decrease in itch rating (J Am Acad Dermatol. 2017 Apr;76[4]:760-1).
Forty patients participated in a pharmacy-administered telephone survey that assessed medication tolerability and efficacy. Of these, 23 patients (58%) had relief “to a great extent” and 14 (35%) “to a moderate extent.”
There were mild side effects (burning and redness at the application site) in 16 patients. “We attributed this mainly to the lidocaine component,” Dr. Yosipovitch said. “Itch reduction lasted from 30 minutes to 7 hours, so we think this is quite a powerful tool. I now often use this topical for patients with severe intractable itch.”
He added that a case report of encephalopathy associated with the cream has recently surfaced. The patient was an elderly man with Parkinson’s disease who had been using 10% ketamine compounded with amitriptyline and lidocaine for 4 days. He gradually increased the use until he was applying it onto almost all of his upper body. The day after this extensive application, the patient presented to an emergency department with slurred speech, ataxia, and altered mental status (JAMA Dermatol. 2016;152[12]:1390-1).
“So a word of warning here: I don’t recommend using it all over the body,” Dr. Yosipovitch said.
Dr. Yosipovitch has financial relationships with numerous companies that are investigating antipruritic compounds, including strontium.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17
Omalizumab effects rapid, often complete, clearance of refractory bullous pemphigoid
ORLANDO – Omalizumab, a monoclonal anti-IgE antibody, may be a good option for patients with treatment-refractory bullous pemphigoid.
Patients who received omalizumab (Xolair) experienced rapid improvements, with 30%-50% lesion clearance within a week and complete clearance by 3 weeks, Kenneth Yu, MD, said at the annual meeting of the American Academy of Dermatology. With regular injections, they were kept symptom free for months. Some patients did flare, but were then easily controlled on standard treatment. Omalizumab, approved by the Food and Drug Administration in 2003, is indicated for moderate to severe persistent asthma and chronic idiopathic urticaria.
“We have now treated six patients with omalizumab with very good results with five of them. These are not your garden-variety BP patients, but people with very treatment-resistant disease who have failed treatment with corticosteroids alone, and in combination with other immunosuppressants.”
The rapid clinical improvements, along with observations that eosinophilia decreased with treatment, “strengthen the evidence that BP is an IgE-mediated, organ-specific autoimmune disease,” said Dr. Yu, senior resident in dermatology at the University of Michigan, Ann Arbor.
“Would I use this as a first-line treatment for BP? Probably not. But if you are seeing someone who’s nonresponsive to therapy, you might want to check IgE and eosinophil levels and if those are elevated, you might consider omalizumab as an adjunct treatment – and you might observe a fairly dramatic response.”
Three of Dr. Yu’s patients received omalizumab as monotherapy, and three received it in conjunction with other immunosuppressants. He described their disease presentation, treatment, and progression.
In general, Dr. Yu reserves omalizumab for patients with refractory disease and two particular clinical characteristics: high eosinophil count and elevated serum IgE. The initial dosing is based on the asthma treatment nomogram for the drug; he titrates it according to clinical response. “We don’t alter the total dose given, but we do adjust the frequency with which we give it.”
His first patient was a 70-year-old woman with a 1-year history of poorly controlled BP; she had failed prednisone, azathioprine, and minocycline. She also had a history of steroid-related vertebral compression fractures. She presented with an eosinophil count of over 400 cells/microL.
“We treated her with subcutaneous injections of 300 mg every 2 weeks for 16 weeks,” Dr. Yu said. Within 1 week, she had a 44% reduction in blisters; within 4 weeks, she had gone from 50% body surface area involvement to 5%.
After eight injections, the patient was disease free and Dr. Yu discontinued treatment. She remained clear until week 32 after treatment initiation; she had a flare manifested by increased pruritus and recurrence of lesions. Dr. Yu restarted omalizumab and the lesions cleared within 2 weeks. From weeks 35-72, the patient received five more injections and remained disease free.
“After that, she did have another flare, so we used omalizumab again,” but without the same excellent results. “She had an initial decrease in pruritus, and symptom improvement, but her disease subsequently worsened. We restarted her on prednisone and azathioprine and she has done well.”
Dr. Yu said he made “a couple of interesting observations on this case.”
“We saw no real correlation between disease activity score, and the levels of serum IgG antibodies. But we did notice a parallel correlation with the level of eosinophil and disease severity and also treatment response,” he said. “It was quite clear that immediately after injection, she had a dramatic drop in eosinophils” from 1,600 to 60 cells/microL within 24 hours.
His next case was a 72-year-old woman with a history of somewhat controlled essential tremor, and 6 months of highly pruritic BP blistering. She had been treated with 60 mg/day prednisone, but didn’t tolerate it well, developing steroid-induced psychosis with agitation and violence, and a worsening of her tremor. The steroid was tapered to 40 mg/day and azathioprine was added, but she was did not respond to this change and continued to develop new blisters each day. She was admitted to the hospital for plasmapheresis, which was not helpful. Nor did she respond to six cycles of cyclophosphamide.
At that point, Dr. Yu drew IgE and eosinophil levels: Her absolute eosinophil count was 1,600 cells/microL and IgE was 287 units/mL. He then gave the patient 300 mg omalizumab subcutaneously.
“Ten days after a single injection, her blisters had almost completely resolved,” he said. “To briefly describe her disease course, the blisters went away, and she had resolution of her pruritus. She was discharged with 1 month of prednisone, but we tapered that and have been able to maintain her on omalizumab alone. She had one mild flare, which was readily controlled with prednisone. The last time we saw her, she was disease free.”
He also described four other steroid-refractory BP patients treated with omalizumab.“Their commonalities were that they all had steroid-refractory disease that was resistant to immunosuppressants, had a high level of IgE, and most of them also had eosinophilia.”
Dr. Yu’s descriptions:
• A 78-year-old woman with refractory BP of 1.5 years responded well to three initial injections spaced 6 and 4 weeks apart, and has been well maintained for 20 months with 300-mL injections administered once a month. One relapse was easily controlled.
• A 72-year-old woman with 3.5 years of refractory BP responded well to 375 mg injections every 4 weeks and has been symptom free for a year on that maintenance dose.
• A 55-year-old woman with a 7-month history of refractory BP experienced a 30% reduction in body surface area blistering within 1 week of her first 375-mg injection. By 3 weeks, she was clear. She had three injections, 2 weeks apart, and was disease free for 3 months.
• An 86-year-old woman with longstanding refractory BP experienced a 22% reduction in blister count within a week of her first 375-mL injection. After a series of injections every 2 weeks, however, she developed an exacerbation of her preexisting chronic obstructive pulmonary disease, which was due primarily to tapering her prednisone. However, she no longer uses omalizumab.
“It is difficult to make recommendations because of the limitations of our data,” Dr. Yu said. “But based on the small number of patients we have treated, I would consider using omalizumab in patients with resistant disease who have an elevated IgE and eosinophil count. The optimal dosing regimen is not yet determined. Our approach is to start out with the asthma dosing and titrate until we see improvement. We use the highest dose indicated for the patient’s weight and IgE levels, typically 300-375 mg subcutaneously every 2-8 weeks, and start tapering when the patient gets control.”
He had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – Omalizumab, a monoclonal anti-IgE antibody, may be a good option for patients with treatment-refractory bullous pemphigoid.
Patients who received omalizumab (Xolair) experienced rapid improvements, with 30%-50% lesion clearance within a week and complete clearance by 3 weeks, Kenneth Yu, MD, said at the annual meeting of the American Academy of Dermatology. With regular injections, they were kept symptom free for months. Some patients did flare, but were then easily controlled on standard treatment. Omalizumab, approved by the Food and Drug Administration in 2003, is indicated for moderate to severe persistent asthma and chronic idiopathic urticaria.
“We have now treated six patients with omalizumab with very good results with five of them. These are not your garden-variety BP patients, but people with very treatment-resistant disease who have failed treatment with corticosteroids alone, and in combination with other immunosuppressants.”
The rapid clinical improvements, along with observations that eosinophilia decreased with treatment, “strengthen the evidence that BP is an IgE-mediated, organ-specific autoimmune disease,” said Dr. Yu, senior resident in dermatology at the University of Michigan, Ann Arbor.
“Would I use this as a first-line treatment for BP? Probably not. But if you are seeing someone who’s nonresponsive to therapy, you might want to check IgE and eosinophil levels and if those are elevated, you might consider omalizumab as an adjunct treatment – and you might observe a fairly dramatic response.”
Three of Dr. Yu’s patients received omalizumab as monotherapy, and three received it in conjunction with other immunosuppressants. He described their disease presentation, treatment, and progression.
In general, Dr. Yu reserves omalizumab for patients with refractory disease and two particular clinical characteristics: high eosinophil count and elevated serum IgE. The initial dosing is based on the asthma treatment nomogram for the drug; he titrates it according to clinical response. “We don’t alter the total dose given, but we do adjust the frequency with which we give it.”
His first patient was a 70-year-old woman with a 1-year history of poorly controlled BP; she had failed prednisone, azathioprine, and minocycline. She also had a history of steroid-related vertebral compression fractures. She presented with an eosinophil count of over 400 cells/microL.
“We treated her with subcutaneous injections of 300 mg every 2 weeks for 16 weeks,” Dr. Yu said. Within 1 week, she had a 44% reduction in blisters; within 4 weeks, she had gone from 50% body surface area involvement to 5%.
After eight injections, the patient was disease free and Dr. Yu discontinued treatment. She remained clear until week 32 after treatment initiation; she had a flare manifested by increased pruritus and recurrence of lesions. Dr. Yu restarted omalizumab and the lesions cleared within 2 weeks. From weeks 35-72, the patient received five more injections and remained disease free.
“After that, she did have another flare, so we used omalizumab again,” but without the same excellent results. “She had an initial decrease in pruritus, and symptom improvement, but her disease subsequently worsened. We restarted her on prednisone and azathioprine and she has done well.”
Dr. Yu said he made “a couple of interesting observations on this case.”
“We saw no real correlation between disease activity score, and the levels of serum IgG antibodies. But we did notice a parallel correlation with the level of eosinophil and disease severity and also treatment response,” he said. “It was quite clear that immediately after injection, she had a dramatic drop in eosinophils” from 1,600 to 60 cells/microL within 24 hours.
His next case was a 72-year-old woman with a history of somewhat controlled essential tremor, and 6 months of highly pruritic BP blistering. She had been treated with 60 mg/day prednisone, but didn’t tolerate it well, developing steroid-induced psychosis with agitation and violence, and a worsening of her tremor. The steroid was tapered to 40 mg/day and azathioprine was added, but she was did not respond to this change and continued to develop new blisters each day. She was admitted to the hospital for plasmapheresis, which was not helpful. Nor did she respond to six cycles of cyclophosphamide.
At that point, Dr. Yu drew IgE and eosinophil levels: Her absolute eosinophil count was 1,600 cells/microL and IgE was 287 units/mL. He then gave the patient 300 mg omalizumab subcutaneously.
“Ten days after a single injection, her blisters had almost completely resolved,” he said. “To briefly describe her disease course, the blisters went away, and she had resolution of her pruritus. She was discharged with 1 month of prednisone, but we tapered that and have been able to maintain her on omalizumab alone. She had one mild flare, which was readily controlled with prednisone. The last time we saw her, she was disease free.”
He also described four other steroid-refractory BP patients treated with omalizumab.“Their commonalities were that they all had steroid-refractory disease that was resistant to immunosuppressants, had a high level of IgE, and most of them also had eosinophilia.”
Dr. Yu’s descriptions:
• A 78-year-old woman with refractory BP of 1.5 years responded well to three initial injections spaced 6 and 4 weeks apart, and has been well maintained for 20 months with 300-mL injections administered once a month. One relapse was easily controlled.
• A 72-year-old woman with 3.5 years of refractory BP responded well to 375 mg injections every 4 weeks and has been symptom free for a year on that maintenance dose.
• A 55-year-old woman with a 7-month history of refractory BP experienced a 30% reduction in body surface area blistering within 1 week of her first 375-mg injection. By 3 weeks, she was clear. She had three injections, 2 weeks apart, and was disease free for 3 months.
• An 86-year-old woman with longstanding refractory BP experienced a 22% reduction in blister count within a week of her first 375-mL injection. After a series of injections every 2 weeks, however, she developed an exacerbation of her preexisting chronic obstructive pulmonary disease, which was due primarily to tapering her prednisone. However, she no longer uses omalizumab.
“It is difficult to make recommendations because of the limitations of our data,” Dr. Yu said. “But based on the small number of patients we have treated, I would consider using omalizumab in patients with resistant disease who have an elevated IgE and eosinophil count. The optimal dosing regimen is not yet determined. Our approach is to start out with the asthma dosing and titrate until we see improvement. We use the highest dose indicated for the patient’s weight and IgE levels, typically 300-375 mg subcutaneously every 2-8 weeks, and start tapering when the patient gets control.”
He had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – Omalizumab, a monoclonal anti-IgE antibody, may be a good option for patients with treatment-refractory bullous pemphigoid.
Patients who received omalizumab (Xolair) experienced rapid improvements, with 30%-50% lesion clearance within a week and complete clearance by 3 weeks, Kenneth Yu, MD, said at the annual meeting of the American Academy of Dermatology. With regular injections, they were kept symptom free for months. Some patients did flare, but were then easily controlled on standard treatment. Omalizumab, approved by the Food and Drug Administration in 2003, is indicated for moderate to severe persistent asthma and chronic idiopathic urticaria.
“We have now treated six patients with omalizumab with very good results with five of them. These are not your garden-variety BP patients, but people with very treatment-resistant disease who have failed treatment with corticosteroids alone, and in combination with other immunosuppressants.”
The rapid clinical improvements, along with observations that eosinophilia decreased with treatment, “strengthen the evidence that BP is an IgE-mediated, organ-specific autoimmune disease,” said Dr. Yu, senior resident in dermatology at the University of Michigan, Ann Arbor.
“Would I use this as a first-line treatment for BP? Probably not. But if you are seeing someone who’s nonresponsive to therapy, you might want to check IgE and eosinophil levels and if those are elevated, you might consider omalizumab as an adjunct treatment – and you might observe a fairly dramatic response.”
Three of Dr. Yu’s patients received omalizumab as monotherapy, and three received it in conjunction with other immunosuppressants. He described their disease presentation, treatment, and progression.
In general, Dr. Yu reserves omalizumab for patients with refractory disease and two particular clinical characteristics: high eosinophil count and elevated serum IgE. The initial dosing is based on the asthma treatment nomogram for the drug; he titrates it according to clinical response. “We don’t alter the total dose given, but we do adjust the frequency with which we give it.”
His first patient was a 70-year-old woman with a 1-year history of poorly controlled BP; she had failed prednisone, azathioprine, and minocycline. She also had a history of steroid-related vertebral compression fractures. She presented with an eosinophil count of over 400 cells/microL.
“We treated her with subcutaneous injections of 300 mg every 2 weeks for 16 weeks,” Dr. Yu said. Within 1 week, she had a 44% reduction in blisters; within 4 weeks, she had gone from 50% body surface area involvement to 5%.
After eight injections, the patient was disease free and Dr. Yu discontinued treatment. She remained clear until week 32 after treatment initiation; she had a flare manifested by increased pruritus and recurrence of lesions. Dr. Yu restarted omalizumab and the lesions cleared within 2 weeks. From weeks 35-72, the patient received five more injections and remained disease free.
“After that, she did have another flare, so we used omalizumab again,” but without the same excellent results. “She had an initial decrease in pruritus, and symptom improvement, but her disease subsequently worsened. We restarted her on prednisone and azathioprine and she has done well.”
Dr. Yu said he made “a couple of interesting observations on this case.”
“We saw no real correlation between disease activity score, and the levels of serum IgG antibodies. But we did notice a parallel correlation with the level of eosinophil and disease severity and also treatment response,” he said. “It was quite clear that immediately after injection, she had a dramatic drop in eosinophils” from 1,600 to 60 cells/microL within 24 hours.
His next case was a 72-year-old woman with a history of somewhat controlled essential tremor, and 6 months of highly pruritic BP blistering. She had been treated with 60 mg/day prednisone, but didn’t tolerate it well, developing steroid-induced psychosis with agitation and violence, and a worsening of her tremor. The steroid was tapered to 40 mg/day and azathioprine was added, but she was did not respond to this change and continued to develop new blisters each day. She was admitted to the hospital for plasmapheresis, which was not helpful. Nor did she respond to six cycles of cyclophosphamide.
At that point, Dr. Yu drew IgE and eosinophil levels: Her absolute eosinophil count was 1,600 cells/microL and IgE was 287 units/mL. He then gave the patient 300 mg omalizumab subcutaneously.
“Ten days after a single injection, her blisters had almost completely resolved,” he said. “To briefly describe her disease course, the blisters went away, and she had resolution of her pruritus. She was discharged with 1 month of prednisone, but we tapered that and have been able to maintain her on omalizumab alone. She had one mild flare, which was readily controlled with prednisone. The last time we saw her, she was disease free.”
He also described four other steroid-refractory BP patients treated with omalizumab.“Their commonalities were that they all had steroid-refractory disease that was resistant to immunosuppressants, had a high level of IgE, and most of them also had eosinophilia.”
Dr. Yu’s descriptions:
• A 78-year-old woman with refractory BP of 1.5 years responded well to three initial injections spaced 6 and 4 weeks apart, and has been well maintained for 20 months with 300-mL injections administered once a month. One relapse was easily controlled.
• A 72-year-old woman with 3.5 years of refractory BP responded well to 375 mg injections every 4 weeks and has been symptom free for a year on that maintenance dose.
• A 55-year-old woman with a 7-month history of refractory BP experienced a 30% reduction in body surface area blistering within 1 week of her first 375-mg injection. By 3 weeks, she was clear. She had three injections, 2 weeks apart, and was disease free for 3 months.
• An 86-year-old woman with longstanding refractory BP experienced a 22% reduction in blister count within a week of her first 375-mL injection. After a series of injections every 2 weeks, however, she developed an exacerbation of her preexisting chronic obstructive pulmonary disease, which was due primarily to tapering her prednisone. However, she no longer uses omalizumab.
“It is difficult to make recommendations because of the limitations of our data,” Dr. Yu said. “But based on the small number of patients we have treated, I would consider using omalizumab in patients with resistant disease who have an elevated IgE and eosinophil count. The optimal dosing regimen is not yet determined. Our approach is to start out with the asthma dosing and titrate until we see improvement. We use the highest dose indicated for the patient’s weight and IgE levels, typically 300-375 mg subcutaneously every 2-8 weeks, and start tapering when the patient gets control.”
He had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT AAD 17
Milk: Friend to bones, foe to faces?
ORLANDO – A greasy hamburger and fries and a chocolate milkshake may all earn the finger of blame when teens fret over acne. But which of these foods is the real culprit?
A growing body of data suggests it may be the milk – especially if it’s fat-free milk, according to Andrea Zaenglein, MD, who spoke at the annual meeting of the American Academy of Dermatology. Skim milk has been at the center of a long-simmering acne controversy, said Dr. Zaenglein, professor of dermatology and pediatric dermatology at Pennsylvania State University, Hershey.
The same association was seen in a subsequent study of 4,273 teen boys, published in 2008. There was a 10% increased risk for acne associated with intake of whole or 2% milk, a 17% increased risk for 1% milk, and a 19% increased risk for skim milk (J Am Acad Dermatol. 2008 May;58[5]: 787-93).
A prospective study of about 6,000 girls found similar risks associated with all types of milk: a 19% increased risk for whole milk, 17% for low-fat milk, and 19% for skim milk (Dermatol Online J. 2006 May 30;12[4]:1).
They found positive associations with total dairy and with nonfat dairy, but not with whole-fat or low-fat dairy. “The association was driven by the nonfat dairy,” Dr. Zaenglein said. “When we took nonfat [dairy] out of the total dairy, the association there was no longer significant.” They also found no significant association with body mass index, glycemic index, or glycemic load, she added.
“You have to wonder, what could this association between dairy – and skim milk in particular – be? Could dairy actually be involved in the pathogenesis of acne?” There are a number of proposed mechanisms, none of which have ever been confirmed, she said. “Could it be related to steroids? Milk is a very bioactive substance with estrogens and other hormones, but these are fat soluble and would be removed in skim milk.”
Another theory suggests that insulinlike growth factor-1, either in milk or endogenously stimulated by its consumption, may make a contribution. “People who are passionate about this have published prolifically about the activation of this pathway,” Dr. Zaenglein said, “but it remains speculative.”
She added, there are a plethora of studies showing milk’s benefits in many other areas, including the benefits exerted by milk’s medium-chain fatty acids on cardiovascular health, glycemic control, insulin regulation, and even obesity.
Finally, dairy’s importance to bone health in the United States can’t be ignored. In fact, dairy products are the most commonly recommended foods for ensuring adequate calcium intake in children, teens, and young adults.
“It’s really hard to make a firm recommendation to eliminate dairy, because, in this country, it makes up a good portion of the calcium teens need during their bone-building years, and kids are already at high risk for not meeting these requirements.”
National nutritional guidelines recommend 1,300 mg of calcium every day, which can be accomplished in three to five servings of dairy. “An 8-ounce glass of milk has 300 mg. Yogurt, cheese, and calcium-fortified juice are all highly accepted by teens. But, to get that same amount from vegetables, for example, you’d have to eat 3 cups of cooked kale. That’s a lot of kale,” Dr. Zaenglein said.
She had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – A greasy hamburger and fries and a chocolate milkshake may all earn the finger of blame when teens fret over acne. But which of these foods is the real culprit?
A growing body of data suggests it may be the milk – especially if it’s fat-free milk, according to Andrea Zaenglein, MD, who spoke at the annual meeting of the American Academy of Dermatology. Skim milk has been at the center of a long-simmering acne controversy, said Dr. Zaenglein, professor of dermatology and pediatric dermatology at Pennsylvania State University, Hershey.
The same association was seen in a subsequent study of 4,273 teen boys, published in 2008. There was a 10% increased risk for acne associated with intake of whole or 2% milk, a 17% increased risk for 1% milk, and a 19% increased risk for skim milk (J Am Acad Dermatol. 2008 May;58[5]: 787-93).
A prospective study of about 6,000 girls found similar risks associated with all types of milk: a 19% increased risk for whole milk, 17% for low-fat milk, and 19% for skim milk (Dermatol Online J. 2006 May 30;12[4]:1).
They found positive associations with total dairy and with nonfat dairy, but not with whole-fat or low-fat dairy. “The association was driven by the nonfat dairy,” Dr. Zaenglein said. “When we took nonfat [dairy] out of the total dairy, the association there was no longer significant.” They also found no significant association with body mass index, glycemic index, or glycemic load, she added.
“You have to wonder, what could this association between dairy – and skim milk in particular – be? Could dairy actually be involved in the pathogenesis of acne?” There are a number of proposed mechanisms, none of which have ever been confirmed, she said. “Could it be related to steroids? Milk is a very bioactive substance with estrogens and other hormones, but these are fat soluble and would be removed in skim milk.”
Another theory suggests that insulinlike growth factor-1, either in milk or endogenously stimulated by its consumption, may make a contribution. “People who are passionate about this have published prolifically about the activation of this pathway,” Dr. Zaenglein said, “but it remains speculative.”
She added, there are a plethora of studies showing milk’s benefits in many other areas, including the benefits exerted by milk’s medium-chain fatty acids on cardiovascular health, glycemic control, insulin regulation, and even obesity.
Finally, dairy’s importance to bone health in the United States can’t be ignored. In fact, dairy products are the most commonly recommended foods for ensuring adequate calcium intake in children, teens, and young adults.
“It’s really hard to make a firm recommendation to eliminate dairy, because, in this country, it makes up a good portion of the calcium teens need during their bone-building years, and kids are already at high risk for not meeting these requirements.”
National nutritional guidelines recommend 1,300 mg of calcium every day, which can be accomplished in three to five servings of dairy. “An 8-ounce glass of milk has 300 mg. Yogurt, cheese, and calcium-fortified juice are all highly accepted by teens. But, to get that same amount from vegetables, for example, you’d have to eat 3 cups of cooked kale. That’s a lot of kale,” Dr. Zaenglein said.
She had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – A greasy hamburger and fries and a chocolate milkshake may all earn the finger of blame when teens fret over acne. But which of these foods is the real culprit?
A growing body of data suggests it may be the milk – especially if it’s fat-free milk, according to Andrea Zaenglein, MD, who spoke at the annual meeting of the American Academy of Dermatology. Skim milk has been at the center of a long-simmering acne controversy, said Dr. Zaenglein, professor of dermatology and pediatric dermatology at Pennsylvania State University, Hershey.
The same association was seen in a subsequent study of 4,273 teen boys, published in 2008. There was a 10% increased risk for acne associated with intake of whole or 2% milk, a 17% increased risk for 1% milk, and a 19% increased risk for skim milk (J Am Acad Dermatol. 2008 May;58[5]: 787-93).
A prospective study of about 6,000 girls found similar risks associated with all types of milk: a 19% increased risk for whole milk, 17% for low-fat milk, and 19% for skim milk (Dermatol Online J. 2006 May 30;12[4]:1).
They found positive associations with total dairy and with nonfat dairy, but not with whole-fat or low-fat dairy. “The association was driven by the nonfat dairy,” Dr. Zaenglein said. “When we took nonfat [dairy] out of the total dairy, the association there was no longer significant.” They also found no significant association with body mass index, glycemic index, or glycemic load, she added.
“You have to wonder, what could this association between dairy – and skim milk in particular – be? Could dairy actually be involved in the pathogenesis of acne?” There are a number of proposed mechanisms, none of which have ever been confirmed, she said. “Could it be related to steroids? Milk is a very bioactive substance with estrogens and other hormones, but these are fat soluble and would be removed in skim milk.”
Another theory suggests that insulinlike growth factor-1, either in milk or endogenously stimulated by its consumption, may make a contribution. “People who are passionate about this have published prolifically about the activation of this pathway,” Dr. Zaenglein said, “but it remains speculative.”
She added, there are a plethora of studies showing milk’s benefits in many other areas, including the benefits exerted by milk’s medium-chain fatty acids on cardiovascular health, glycemic control, insulin regulation, and even obesity.
Finally, dairy’s importance to bone health in the United States can’t be ignored. In fact, dairy products are the most commonly recommended foods for ensuring adequate calcium intake in children, teens, and young adults.
“It’s really hard to make a firm recommendation to eliminate dairy, because, in this country, it makes up a good portion of the calcium teens need during their bone-building years, and kids are already at high risk for not meeting these requirements.”
National nutritional guidelines recommend 1,300 mg of calcium every day, which can be accomplished in three to five servings of dairy. “An 8-ounce glass of milk has 300 mg. Yogurt, cheese, and calcium-fortified juice are all highly accepted by teens. But, to get that same amount from vegetables, for example, you’d have to eat 3 cups of cooked kale. That’s a lot of kale,” Dr. Zaenglein said.
She had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17
VIDEO: Picowave laser uses are expanding beyond tattoo removal
ORLANDO – The applications for picowavelength lasers are expanding, with emerging data on their uses for cosmetic indications other than tattoo removal, according to Anne Chapas, MD, who is in private practice in New York.
First introduced and cleared by the Food and Drug Administration for tattoo removal, “picowave devices ... are now being studied in multiple different cosmetic conditions, including their use in acne scars, fine lines and wrinkles, and melasma,” Dr. Chapas said in a video interview at the annual meeting of the American Academy of Dermatology.
The No. 1 thing dermatologists need to know is that these types of lasers are delivering energy extremely quickly,” at 1,000 times faster than nanosecond lasers, she said. Another difference between the two is that “the laser tissue interaction between the two types of devices is completely different.”
In the interview, she highlighted other important points about picowave lasers, including less downtime after treatment.
At the meeting, Dr. Chapas spoke during a session entitled “the Science Behind New Devices in Dermatology.”
Her disclosures include serving as a consultant and investigator for Syneron and Candela.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – The applications for picowavelength lasers are expanding, with emerging data on their uses for cosmetic indications other than tattoo removal, according to Anne Chapas, MD, who is in private practice in New York.
First introduced and cleared by the Food and Drug Administration for tattoo removal, “picowave devices ... are now being studied in multiple different cosmetic conditions, including their use in acne scars, fine lines and wrinkles, and melasma,” Dr. Chapas said in a video interview at the annual meeting of the American Academy of Dermatology.
The No. 1 thing dermatologists need to know is that these types of lasers are delivering energy extremely quickly,” at 1,000 times faster than nanosecond lasers, she said. Another difference between the two is that “the laser tissue interaction between the two types of devices is completely different.”
In the interview, she highlighted other important points about picowave lasers, including less downtime after treatment.
At the meeting, Dr. Chapas spoke during a session entitled “the Science Behind New Devices in Dermatology.”
Her disclosures include serving as a consultant and investigator for Syneron and Candela.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – The applications for picowavelength lasers are expanding, with emerging data on their uses for cosmetic indications other than tattoo removal, according to Anne Chapas, MD, who is in private practice in New York.
First introduced and cleared by the Food and Drug Administration for tattoo removal, “picowave devices ... are now being studied in multiple different cosmetic conditions, including their use in acne scars, fine lines and wrinkles, and melasma,” Dr. Chapas said in a video interview at the annual meeting of the American Academy of Dermatology.
The No. 1 thing dermatologists need to know is that these types of lasers are delivering energy extremely quickly,” at 1,000 times faster than nanosecond lasers, she said. Another difference between the two is that “the laser tissue interaction between the two types of devices is completely different.”
In the interview, she highlighted other important points about picowave lasers, including less downtime after treatment.
At the meeting, Dr. Chapas spoke during a session entitled “the Science Behind New Devices in Dermatology.”
Her disclosures include serving as a consultant and investigator for Syneron and Candela.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AAD 17
VIDEO: Looking at keloids from a different perspective
ORLANDO – It may be time to start considering new options for treating keloids, according to Amy McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
At the annual meeting of the American Academy of Dermatology, Dr. McMichael discussed one of the highlights of the annual symposium of the Skin of Color Society, held right before the annual meeting, a presentation by Michael Tirgan, MD, who has treated patients with keloids for about 10 years.
Dr. Tirgan, an oncologist based in New York, has a large database and registry of patients and shared some interesting data at the symposium. “What he’s found is that those who come with the supermassive and massive keloids are those who have had the most surgery on their keloid,” Dr. McMichael said in a video interview at the meeting.
His findings shed light on what she described as “a new perspective in the way that we think about keloids” and a new approach to treatment – considering keloids as tumors – not just a scar.
Dr. McMichael had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – It may be time to start considering new options for treating keloids, according to Amy McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
At the annual meeting of the American Academy of Dermatology, Dr. McMichael discussed one of the highlights of the annual symposium of the Skin of Color Society, held right before the annual meeting, a presentation by Michael Tirgan, MD, who has treated patients with keloids for about 10 years.
Dr. Tirgan, an oncologist based in New York, has a large database and registry of patients and shared some interesting data at the symposium. “What he’s found is that those who come with the supermassive and massive keloids are those who have had the most surgery on their keloid,” Dr. McMichael said in a video interview at the meeting.
His findings shed light on what she described as “a new perspective in the way that we think about keloids” and a new approach to treatment – considering keloids as tumors – not just a scar.
Dr. McMichael had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – It may be time to start considering new options for treating keloids, according to Amy McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
At the annual meeting of the American Academy of Dermatology, Dr. McMichael discussed one of the highlights of the annual symposium of the Skin of Color Society, held right before the annual meeting, a presentation by Michael Tirgan, MD, who has treated patients with keloids for about 10 years.
Dr. Tirgan, an oncologist based in New York, has a large database and registry of patients and shared some interesting data at the symposium. “What he’s found is that those who come with the supermassive and massive keloids are those who have had the most surgery on their keloid,” Dr. McMichael said in a video interview at the meeting.
His findings shed light on what she described as “a new perspective in the way that we think about keloids” and a new approach to treatment – considering keloids as tumors – not just a scar.
Dr. McMichael had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AAD 17
Novel antifungal had favorable safety, efficacy profile for onychomycosis in phase IIB study
ORLANDO – A novel orally administered antifungal showed a favorable safety and efficacy profile in the treatment of distal lateral subungual onychomycosis, in a phase IIB study presented at the annual meeting of the American Academy of Dermatology.
In the RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study, a randomized, double-blind, placebo-controlled, dose-ranging trial, 259 adults with moderate to severe distal lateral subungual onychomycosis of the large toenail were assigned to either one of four treatment arms. They were given the antifungal, currently named VT-1161, a selective CYP51 inhibitor, at doses of 300 mg or 600 mg once weekly for 10 or 22 weeks, after receiving daily loading doses for the initial 2 weeks. The trial evaluated two dose levels of VT-1161 (300 mg and 600 mg) administered once weekly for either 10 or 22 weeks following an initial 2-week, once-daily loading dose period.
At baseline, the average involvement of the large toenail was 46%, with an average of 4.6 toenails affected. In the intent-to-treat analysis, at 48 weeks, complete cure rates in the four study drug arms ranged from 32% to 42%, compared with 0% in the placebo arm.
Amir Tavakkol, PhD, chief development officer at Viamet Pharmaceuticals, which is developing VT01161, presented the study findings during a late breaking clinical session at the meeting.
Adverse event rates and discontinuation rates were comparable to placebo through week 60, with no patients discontinuing due to any laboratory abnormalities. Nausea and muscle spasms were the most commonly reported adverse events, which Dr. Tavakkol said seemed to occur in patients given the higher doses. VT-1161 is also being studied for treatment of vulvovaginal candidiasis. In October 2016, the FDA granted the drug Qualified Infectious Disease Product and Fast Track designations for the treatment of recurrent vulvovaginal candidiasis, according to the company.
Viamet sponsored the study and Dr. Tavakkol is an employee of the company.
ORLANDO – A novel orally administered antifungal showed a favorable safety and efficacy profile in the treatment of distal lateral subungual onychomycosis, in a phase IIB study presented at the annual meeting of the American Academy of Dermatology.
In the RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study, a randomized, double-blind, placebo-controlled, dose-ranging trial, 259 adults with moderate to severe distal lateral subungual onychomycosis of the large toenail were assigned to either one of four treatment arms. They were given the antifungal, currently named VT-1161, a selective CYP51 inhibitor, at doses of 300 mg or 600 mg once weekly for 10 or 22 weeks, after receiving daily loading doses for the initial 2 weeks. The trial evaluated two dose levels of VT-1161 (300 mg and 600 mg) administered once weekly for either 10 or 22 weeks following an initial 2-week, once-daily loading dose period.
At baseline, the average involvement of the large toenail was 46%, with an average of 4.6 toenails affected. In the intent-to-treat analysis, at 48 weeks, complete cure rates in the four study drug arms ranged from 32% to 42%, compared with 0% in the placebo arm.
Amir Tavakkol, PhD, chief development officer at Viamet Pharmaceuticals, which is developing VT01161, presented the study findings during a late breaking clinical session at the meeting.
Adverse event rates and discontinuation rates were comparable to placebo through week 60, with no patients discontinuing due to any laboratory abnormalities. Nausea and muscle spasms were the most commonly reported adverse events, which Dr. Tavakkol said seemed to occur in patients given the higher doses. VT-1161 is also being studied for treatment of vulvovaginal candidiasis. In October 2016, the FDA granted the drug Qualified Infectious Disease Product and Fast Track designations for the treatment of recurrent vulvovaginal candidiasis, according to the company.
Viamet sponsored the study and Dr. Tavakkol is an employee of the company.
ORLANDO – A novel orally administered antifungal showed a favorable safety and efficacy profile in the treatment of distal lateral subungual onychomycosis, in a phase IIB study presented at the annual meeting of the American Academy of Dermatology.
In the RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study, a randomized, double-blind, placebo-controlled, dose-ranging trial, 259 adults with moderate to severe distal lateral subungual onychomycosis of the large toenail were assigned to either one of four treatment arms. They were given the antifungal, currently named VT-1161, a selective CYP51 inhibitor, at doses of 300 mg or 600 mg once weekly for 10 or 22 weeks, after receiving daily loading doses for the initial 2 weeks. The trial evaluated two dose levels of VT-1161 (300 mg and 600 mg) administered once weekly for either 10 or 22 weeks following an initial 2-week, once-daily loading dose period.
At baseline, the average involvement of the large toenail was 46%, with an average of 4.6 toenails affected. In the intent-to-treat analysis, at 48 weeks, complete cure rates in the four study drug arms ranged from 32% to 42%, compared with 0% in the placebo arm.
Amir Tavakkol, PhD, chief development officer at Viamet Pharmaceuticals, which is developing VT01161, presented the study findings during a late breaking clinical session at the meeting.
Adverse event rates and discontinuation rates were comparable to placebo through week 60, with no patients discontinuing due to any laboratory abnormalities. Nausea and muscle spasms were the most commonly reported adverse events, which Dr. Tavakkol said seemed to occur in patients given the higher doses. VT-1161 is also being studied for treatment of vulvovaginal candidiasis. In October 2016, the FDA granted the drug Qualified Infectious Disease Product and Fast Track designations for the treatment of recurrent vulvovaginal candidiasis, according to the company.
Viamet sponsored the study and Dr. Tavakkol is an employee of the company.
AT AAD 17
Key clinical point:
Major finding: A new selective CYP51 inhibitor, administered orally, met the primary endpoint of complete cure rates at 48 weeks.
Data source: A phase IIB, randomized, double-blind, placebo-controlled, dose-ranging study of 259 adults with moderate-to-severe distal lateral subungual onychomycosis of the large toenail.
Disclosures: Dr. Tavakkol is the chief development officer of Viamet Pharmaceuticals, the sponsor of this trial.
Ixekizumab found superior to ustekinumab in psoriasis at 24 weeks
ORLANDO – The interleukin-17A inhibitor ixekizumab was associated with superior efficacy and safety when compared with ustekinumab at 24 weeks, a head-to-head trial of the two monoclonal antibodies in plaque psoriasis has shown.
The 24-week data from the IXORA-S trial were presented during a late-breaking clinical trial session at the annual meeting of the American Academy of Dermatology by Kristian Reich, MD, PhD, a founder of SCIderm in Hamburg, Germany.
At 24 weeks, 49% in the ixekizumab arm achieved a Psoriasis Area and Severity Index (PASI) 100 level of skin clearance, compared with 24% in the ustekinumab arm (P = .001). Ixekizumab treatment also reached significantly higher skin clearances than ustekinumab at 24 weeks at the level of PASI 90 (83% vs. 59%; P less than .001) and PASI 75 (91% vs. 82%; P = .015).
Treatment with ixekizumab produced a Static Physician’s Global Assessment (sPGA) score of 0 in 54% at 24 weeks, compared with 24% with ustekinumab (P less than .001). A sPGA score of 0 or 1 occurred in 87% of patients who took ixekizumab and in 59% of the ustekinumab group.
An improvement of 4 or more points in the pruritus Numeric Rating Scale was reported by 86% of ixekizumab patients at 24 weeks, compared with 72% of those who took ustekinumab (P = .018).
Dr. Reich reported that there were no deaths or any significant differences in overall treatment-related adverse events across both arms, although he cautioned against putting too much stock in 24-week safety data. “I hesitate to show safety data for only 300 patients at 24 weeks, but it’s good to see here that there doesn’t seem to be a difference. I still would want to see larger patient numbers and more long-term data,” he said.
Dr. Reich had numerous disclosures, including honoraria for serving as a speaker for Eli Lilly, the sponsor of this trial.
ORLANDO – The interleukin-17A inhibitor ixekizumab was associated with superior efficacy and safety when compared with ustekinumab at 24 weeks, a head-to-head trial of the two monoclonal antibodies in plaque psoriasis has shown.
The 24-week data from the IXORA-S trial were presented during a late-breaking clinical trial session at the annual meeting of the American Academy of Dermatology by Kristian Reich, MD, PhD, a founder of SCIderm in Hamburg, Germany.
At 24 weeks, 49% in the ixekizumab arm achieved a Psoriasis Area and Severity Index (PASI) 100 level of skin clearance, compared with 24% in the ustekinumab arm (P = .001). Ixekizumab treatment also reached significantly higher skin clearances than ustekinumab at 24 weeks at the level of PASI 90 (83% vs. 59%; P less than .001) and PASI 75 (91% vs. 82%; P = .015).
Treatment with ixekizumab produced a Static Physician’s Global Assessment (sPGA) score of 0 in 54% at 24 weeks, compared with 24% with ustekinumab (P less than .001). A sPGA score of 0 or 1 occurred in 87% of patients who took ixekizumab and in 59% of the ustekinumab group.
An improvement of 4 or more points in the pruritus Numeric Rating Scale was reported by 86% of ixekizumab patients at 24 weeks, compared with 72% of those who took ustekinumab (P = .018).
Dr. Reich reported that there were no deaths or any significant differences in overall treatment-related adverse events across both arms, although he cautioned against putting too much stock in 24-week safety data. “I hesitate to show safety data for only 300 patients at 24 weeks, but it’s good to see here that there doesn’t seem to be a difference. I still would want to see larger patient numbers and more long-term data,” he said.
Dr. Reich had numerous disclosures, including honoraria for serving as a speaker for Eli Lilly, the sponsor of this trial.
ORLANDO – The interleukin-17A inhibitor ixekizumab was associated with superior efficacy and safety when compared with ustekinumab at 24 weeks, a head-to-head trial of the two monoclonal antibodies in plaque psoriasis has shown.
The 24-week data from the IXORA-S trial were presented during a late-breaking clinical trial session at the annual meeting of the American Academy of Dermatology by Kristian Reich, MD, PhD, a founder of SCIderm in Hamburg, Germany.
At 24 weeks, 49% in the ixekizumab arm achieved a Psoriasis Area and Severity Index (PASI) 100 level of skin clearance, compared with 24% in the ustekinumab arm (P = .001). Ixekizumab treatment also reached significantly higher skin clearances than ustekinumab at 24 weeks at the level of PASI 90 (83% vs. 59%; P less than .001) and PASI 75 (91% vs. 82%; P = .015).
Treatment with ixekizumab produced a Static Physician’s Global Assessment (sPGA) score of 0 in 54% at 24 weeks, compared with 24% with ustekinumab (P less than .001). A sPGA score of 0 or 1 occurred in 87% of patients who took ixekizumab and in 59% of the ustekinumab group.
An improvement of 4 or more points in the pruritus Numeric Rating Scale was reported by 86% of ixekizumab patients at 24 weeks, compared with 72% of those who took ustekinumab (P = .018).
Dr. Reich reported that there were no deaths or any significant differences in overall treatment-related adverse events across both arms, although he cautioned against putting too much stock in 24-week safety data. “I hesitate to show safety data for only 300 patients at 24 weeks, but it’s good to see here that there doesn’t seem to be a difference. I still would want to see larger patient numbers and more long-term data,” he said.
Dr. Reich had numerous disclosures, including honoraria for serving as a speaker for Eli Lilly, the sponsor of this trial.
AT AAD 2017
Key clinical point:
Major finding: At 24 weeks, ixekizumab was associated with significantly better PASI response rates and reduction in itch than ustekinumab in patients with moderate to severe plaque psoriasis.
Data source: Head-to-head trial of 302 adults with moderate to severe plaque psoriasis treated with either ixekizumab or ustekinumab.
Disclosures: Dr. Reich had numerous disclosures, including honoraria for serving as a speaker for Eli Lilly, the sponsor of this trial.
Lasers for Latino skin – A balance of gentleness and strength
ORLANDO – With its unique tendency to develop postinflammatory hyperpigmentation (PIH), Latino skin needs a gentle touch from powerful lasers, according to Eduardo Weiss, MD.
“It’s often best to use a lower power, even though you trade off some efficacy for safety,” said Dr. Weiss, a dermatologist in Miami. “In general, it’s better to go with less aggressive treatment and more sessions, than to risk getting too aggressive and having a bad outcome.”
Dr. Weiss stressed that there are no “one size fits all” recommendations about laser treatment in typical Latino skin, because there’s no such thing as typical Latino skin. The group comprises all Fitzpatrick phototypes. But, in general, he said, the darker the skin, the greater the chance of an acute laser-induced burn, postinflammatory hyperpigmentation, and scarring.
Although general skin tone can provide a good first guess about the potential for hyperreaction to lasers, Dr. Weiss bolsters his judgments with a very simple – but effective – screen: palmar and digital crease pigmentation. First suggested by Hector G. Leal-Silva, MD, of the Institute of Dermatology and Cosmetic Surgery, Monterrey, Mexico, the screen divides patients into four groups, depending on the concentration of pigment present in palmar creases. A score of 0 means no pigment is visible, and the risk of PIH is negligible; a score of 3 means the creases are highly pigmented, and that the risk of PIH is very high.
But, with some adjustments in delivery – including using longer wavelengths and pulse duration, lower fluence and density, and smaller spot sizes – lasers can be used safely and effectively in these at-risk patients, Dr. Weiss said.
Safe treatment starts with pretreatment. It’s best to avoid laser procedures during the summer, when skin is at its darkest. Dr. Weiss also recommends a 6-week regimen aimed at lightening the area to be treated. This can include:
- Sun avoidance and the regular use of a high SPF sunscreen.
- Hydroquinone 4%-8%.
- A “Miami peel,” which is a modified Jessner’s peel with kojic acid and hydroquinone.
- Kligman’s formula of hydroquinone, tretinoin, and a corticosteroid.
- Heliocare, an oral extract of the Polypodium leucotomos fern.
About a month before the procedure, he performs a test spot with the intended laser and its planned settings, in the preauricular area. Any PIH will be obvious within 2-4 weeks. He also carefully screens patients or any photosensitizing condition, like lupus or herpes simplex, or a history of any photosensitizing drugs, such as tetracycline.
Dr. Weiss made specific suggestions for laser treatment of some common Latino skin issues:
Pigmented lesions
The high density of epidermal melanin in Fitzpatrick types IV-VI acts as a competitive chromophore against hemoglobin and oxyhemoglobin. This makes it quite challenging to treat vascular lesions such as port wine stains and telangiectasias, he said. The pulsed dye laser is a good choice, with wavelengths of 585-590 nm especially effective. “The longer 595-nm wavelength allows for a slightly deeper penetration,” Dr. Weiss said. “However, the absorption coefficient of oxyhemoglobin is three times higher at 585 nm than [at] 590 nm.”
Longer pulses are generally safer in dark-skinned patients, he noted. “It will be much less effective than the 585, but for darker-skinned patients, we must sacrifice a little efficacy for safety.”
For rosacea and telangiectasias, Dr. Weiss suggests a 515-nm intense pulsed light with pulse duration of 12-15/ms for these lesions, or pigmentation, he also uses 540 nm or 500-600 nm with pulse duration of 12-15/ms.
Melasma, a very common condition in dark-skinned Latinos, is also “one of the most difficult and frustrating conditions to manage,” he pointed out. He turns to a laser only when the case is resistant to more conservative treatment, which typically includes Kligman’s formula, sunscreen, light peels, and azelaic or kojic acid.
“Lasers are still controversial and, in my opinion, a last resort for melasma. I wouldn’t start unless everything else fails. I reserve them for the deep nonresponding melasmas.”
The Q-switched Nd:YAG is the most widely used for melasma. The fluence used is less than 5 J/cm2, with a 6-mm spot size and a frequency of 10 Hz. The number of treatment sessions varies from 5 to 10 at 1-week intervals, Dr. Weiss said. “Keep in mind that rebound hyperpigmentation could be due to the multiple subthreshold exposures that can stimulate melanogenesis in some areas.”
Skin rejuvenation
Ablative lasers – long the gold standard for skin rejuvenation in those with light skin – can be problematic for darker-skinned patients, Dr. Weiss said.
“These lasers, like the CO2 and Erbium:YAG, can cause several unwanted side effects in Latino skin.” These can include hyperpigmentation, which occurs in 50% of Fitzpatrick III or higher phototypes; erythema that can last for months; and delayed-onset hypopigmentation.
“I think better options for our darker-skinned patients are nonablative infrared, microneedling, and radiofrequency devices,” Dr. Weiss said. “There are, however, newer microablative resurfacing lasers. Fractional CO2, fractional Erbium, and the 2,790-nm yttrium scandium gallium garnet, offer a safer modality with which to treat skin types IV and above. Compared with the older-generation resurfacing lasers, the microablative lasers minimize the amount and duration of erythema and edema, which can last just 3-4 days.”
Dr. Weiss had no relevant financial disclosures.
ORLANDO – With its unique tendency to develop postinflammatory hyperpigmentation (PIH), Latino skin needs a gentle touch from powerful lasers, according to Eduardo Weiss, MD.
“It’s often best to use a lower power, even though you trade off some efficacy for safety,” said Dr. Weiss, a dermatologist in Miami. “In general, it’s better to go with less aggressive treatment and more sessions, than to risk getting too aggressive and having a bad outcome.”
Dr. Weiss stressed that there are no “one size fits all” recommendations about laser treatment in typical Latino skin, because there’s no such thing as typical Latino skin. The group comprises all Fitzpatrick phototypes. But, in general, he said, the darker the skin, the greater the chance of an acute laser-induced burn, postinflammatory hyperpigmentation, and scarring.
Although general skin tone can provide a good first guess about the potential for hyperreaction to lasers, Dr. Weiss bolsters his judgments with a very simple – but effective – screen: palmar and digital crease pigmentation. First suggested by Hector G. Leal-Silva, MD, of the Institute of Dermatology and Cosmetic Surgery, Monterrey, Mexico, the screen divides patients into four groups, depending on the concentration of pigment present in palmar creases. A score of 0 means no pigment is visible, and the risk of PIH is negligible; a score of 3 means the creases are highly pigmented, and that the risk of PIH is very high.
But, with some adjustments in delivery – including using longer wavelengths and pulse duration, lower fluence and density, and smaller spot sizes – lasers can be used safely and effectively in these at-risk patients, Dr. Weiss said.
Safe treatment starts with pretreatment. It’s best to avoid laser procedures during the summer, when skin is at its darkest. Dr. Weiss also recommends a 6-week regimen aimed at lightening the area to be treated. This can include:
- Sun avoidance and the regular use of a high SPF sunscreen.
- Hydroquinone 4%-8%.
- A “Miami peel,” which is a modified Jessner’s peel with kojic acid and hydroquinone.
- Kligman’s formula of hydroquinone, tretinoin, and a corticosteroid.
- Heliocare, an oral extract of the Polypodium leucotomos fern.
About a month before the procedure, he performs a test spot with the intended laser and its planned settings, in the preauricular area. Any PIH will be obvious within 2-4 weeks. He also carefully screens patients or any photosensitizing condition, like lupus or herpes simplex, or a history of any photosensitizing drugs, such as tetracycline.
Dr. Weiss made specific suggestions for laser treatment of some common Latino skin issues:
Pigmented lesions
The high density of epidermal melanin in Fitzpatrick types IV-VI acts as a competitive chromophore against hemoglobin and oxyhemoglobin. This makes it quite challenging to treat vascular lesions such as port wine stains and telangiectasias, he said. The pulsed dye laser is a good choice, with wavelengths of 585-590 nm especially effective. “The longer 595-nm wavelength allows for a slightly deeper penetration,” Dr. Weiss said. “However, the absorption coefficient of oxyhemoglobin is three times higher at 585 nm than [at] 590 nm.”
Longer pulses are generally safer in dark-skinned patients, he noted. “It will be much less effective than the 585, but for darker-skinned patients, we must sacrifice a little efficacy for safety.”
For rosacea and telangiectasias, Dr. Weiss suggests a 515-nm intense pulsed light with pulse duration of 12-15/ms for these lesions, or pigmentation, he also uses 540 nm or 500-600 nm with pulse duration of 12-15/ms.
Melasma, a very common condition in dark-skinned Latinos, is also “one of the most difficult and frustrating conditions to manage,” he pointed out. He turns to a laser only when the case is resistant to more conservative treatment, which typically includes Kligman’s formula, sunscreen, light peels, and azelaic or kojic acid.
“Lasers are still controversial and, in my opinion, a last resort for melasma. I wouldn’t start unless everything else fails. I reserve them for the deep nonresponding melasmas.”
The Q-switched Nd:YAG is the most widely used for melasma. The fluence used is less than 5 J/cm2, with a 6-mm spot size and a frequency of 10 Hz. The number of treatment sessions varies from 5 to 10 at 1-week intervals, Dr. Weiss said. “Keep in mind that rebound hyperpigmentation could be due to the multiple subthreshold exposures that can stimulate melanogenesis in some areas.”
Skin rejuvenation
Ablative lasers – long the gold standard for skin rejuvenation in those with light skin – can be problematic for darker-skinned patients, Dr. Weiss said.
“These lasers, like the CO2 and Erbium:YAG, can cause several unwanted side effects in Latino skin.” These can include hyperpigmentation, which occurs in 50% of Fitzpatrick III or higher phototypes; erythema that can last for months; and delayed-onset hypopigmentation.
“I think better options for our darker-skinned patients are nonablative infrared, microneedling, and radiofrequency devices,” Dr. Weiss said. “There are, however, newer microablative resurfacing lasers. Fractional CO2, fractional Erbium, and the 2,790-nm yttrium scandium gallium garnet, offer a safer modality with which to treat skin types IV and above. Compared with the older-generation resurfacing lasers, the microablative lasers minimize the amount and duration of erythema and edema, which can last just 3-4 days.”
Dr. Weiss had no relevant financial disclosures.
ORLANDO – With its unique tendency to develop postinflammatory hyperpigmentation (PIH), Latino skin needs a gentle touch from powerful lasers, according to Eduardo Weiss, MD.
“It’s often best to use a lower power, even though you trade off some efficacy for safety,” said Dr. Weiss, a dermatologist in Miami. “In general, it’s better to go with less aggressive treatment and more sessions, than to risk getting too aggressive and having a bad outcome.”
Dr. Weiss stressed that there are no “one size fits all” recommendations about laser treatment in typical Latino skin, because there’s no such thing as typical Latino skin. The group comprises all Fitzpatrick phototypes. But, in general, he said, the darker the skin, the greater the chance of an acute laser-induced burn, postinflammatory hyperpigmentation, and scarring.
Although general skin tone can provide a good first guess about the potential for hyperreaction to lasers, Dr. Weiss bolsters his judgments with a very simple – but effective – screen: palmar and digital crease pigmentation. First suggested by Hector G. Leal-Silva, MD, of the Institute of Dermatology and Cosmetic Surgery, Monterrey, Mexico, the screen divides patients into four groups, depending on the concentration of pigment present in palmar creases. A score of 0 means no pigment is visible, and the risk of PIH is negligible; a score of 3 means the creases are highly pigmented, and that the risk of PIH is very high.
But, with some adjustments in delivery – including using longer wavelengths and pulse duration, lower fluence and density, and smaller spot sizes – lasers can be used safely and effectively in these at-risk patients, Dr. Weiss said.
Safe treatment starts with pretreatment. It’s best to avoid laser procedures during the summer, when skin is at its darkest. Dr. Weiss also recommends a 6-week regimen aimed at lightening the area to be treated. This can include:
- Sun avoidance and the regular use of a high SPF sunscreen.
- Hydroquinone 4%-8%.
- A “Miami peel,” which is a modified Jessner’s peel with kojic acid and hydroquinone.
- Kligman’s formula of hydroquinone, tretinoin, and a corticosteroid.
- Heliocare, an oral extract of the Polypodium leucotomos fern.
About a month before the procedure, he performs a test spot with the intended laser and its planned settings, in the preauricular area. Any PIH will be obvious within 2-4 weeks. He also carefully screens patients or any photosensitizing condition, like lupus or herpes simplex, or a history of any photosensitizing drugs, such as tetracycline.
Dr. Weiss made specific suggestions for laser treatment of some common Latino skin issues:
Pigmented lesions
The high density of epidermal melanin in Fitzpatrick types IV-VI acts as a competitive chromophore against hemoglobin and oxyhemoglobin. This makes it quite challenging to treat vascular lesions such as port wine stains and telangiectasias, he said. The pulsed dye laser is a good choice, with wavelengths of 585-590 nm especially effective. “The longer 595-nm wavelength allows for a slightly deeper penetration,” Dr. Weiss said. “However, the absorption coefficient of oxyhemoglobin is three times higher at 585 nm than [at] 590 nm.”
Longer pulses are generally safer in dark-skinned patients, he noted. “It will be much less effective than the 585, but for darker-skinned patients, we must sacrifice a little efficacy for safety.”
For rosacea and telangiectasias, Dr. Weiss suggests a 515-nm intense pulsed light with pulse duration of 12-15/ms for these lesions, or pigmentation, he also uses 540 nm or 500-600 nm with pulse duration of 12-15/ms.
Melasma, a very common condition in dark-skinned Latinos, is also “one of the most difficult and frustrating conditions to manage,” he pointed out. He turns to a laser only when the case is resistant to more conservative treatment, which typically includes Kligman’s formula, sunscreen, light peels, and azelaic or kojic acid.
“Lasers are still controversial and, in my opinion, a last resort for melasma. I wouldn’t start unless everything else fails. I reserve them for the deep nonresponding melasmas.”
The Q-switched Nd:YAG is the most widely used for melasma. The fluence used is less than 5 J/cm2, with a 6-mm spot size and a frequency of 10 Hz. The number of treatment sessions varies from 5 to 10 at 1-week intervals, Dr. Weiss said. “Keep in mind that rebound hyperpigmentation could be due to the multiple subthreshold exposures that can stimulate melanogenesis in some areas.”
Skin rejuvenation
Ablative lasers – long the gold standard for skin rejuvenation in those with light skin – can be problematic for darker-skinned patients, Dr. Weiss said.
“These lasers, like the CO2 and Erbium:YAG, can cause several unwanted side effects in Latino skin.” These can include hyperpigmentation, which occurs in 50% of Fitzpatrick III or higher phototypes; erythema that can last for months; and delayed-onset hypopigmentation.
“I think better options for our darker-skinned patients are nonablative infrared, microneedling, and radiofrequency devices,” Dr. Weiss said. “There are, however, newer microablative resurfacing lasers. Fractional CO2, fractional Erbium, and the 2,790-nm yttrium scandium gallium garnet, offer a safer modality with which to treat skin types IV and above. Compared with the older-generation resurfacing lasers, the microablative lasers minimize the amount and duration of erythema and edema, which can last just 3-4 days.”
Dr. Weiss had no relevant financial disclosures.
EXPERT ANALYSIS FROM AAD 17
For Latinos, misperceptions and lack of medical care make preventing melanoma risky business
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM AAD 17