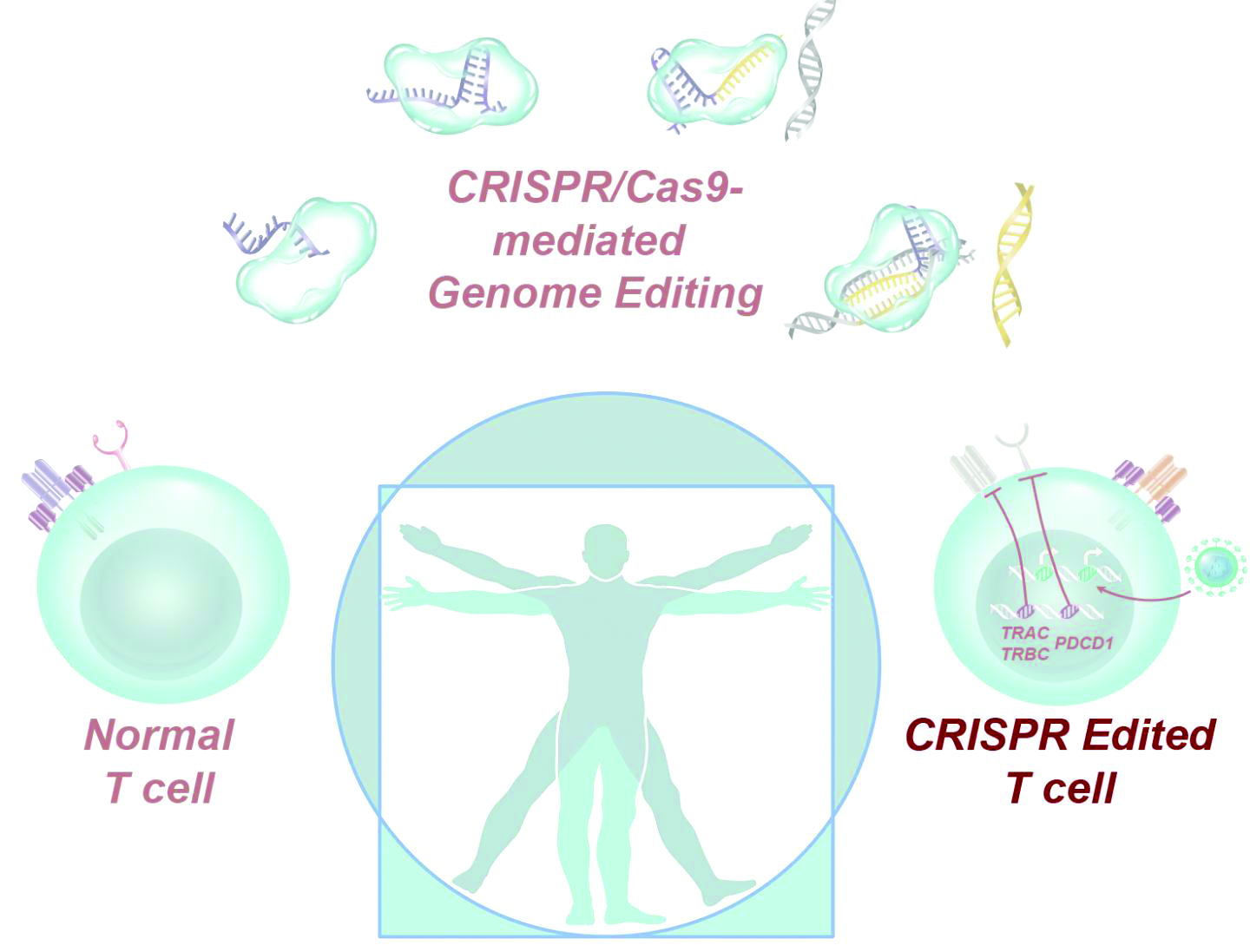
User login
COVID-19: U.S. cardiology groups reaffirm continued use of RAAS-active drugs
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
COVID-19: Older patients with cancer especially vulnerable
For oncologists and other clinicians caring for patients with cancer, the COVID-19 pandemic represents a dynamic clinical challenge that is changing daily and that can feel overwhelming at times, say experts.
“Oncology clinicians are well versed in caring for immunosuppressed patients with cancer, of all ages,” Merry-Jennifer Markham, MD, interim chief of the Division of Hematology and Oncology at the University of Florida Health, Gainesville, told Medscape Medical News.
However, she emphasized that, during this COVID-19 outbreak, “we must be especially diligent about screening for symptoms and exposure, and we must recognize that our older patients with cancer may be especially vulnerable.”
Patients with cancer who are in active treatment are immunosuppressed and are more susceptible to infection and to complications from infection, Markham pointed out. “While we don’t yet have much data on how COVID-19 impacts patients with cancer, I have to suspect that patients undergoing active cancer treatment may be especially vulnerable to the more severe illness associated with COVID-19,” she said.
Indeed, a recent report from China that was published in the Lancet Oncology supports this. The authors suggest that patients with cancer are at higher risk for COVID-19 and have a worse prognosis if they become infected than do those without cancer.
Commonsense rules
Commonsense rules apply for all patients with cancer, regardless of age, said Markham. Measures include thorough handwashing, staying home when sick, and avoiding sick contacts.
Markham, who acts as an expert spokesperson for the American Society of Clinical Oncology, provides information on what patients with cancer need to know about COVID-19 at Cancer.net, the society’s website for patients with cancer.
“Unfortunately, this outbreak of COVID-19 is happening rapidly and in real time,” Markham noted. “The entire medical community is learning as we go, rather than having the luxury of years of evidence-based literature to guide us.”
Another expert agrees. “Unfortunately, there are not a lot of data on how COVID-19 affects cancer patients,” Cardinale Smith, MD, PhD, director of Quality for Cancer Services in the Mount Sinai Health System, New York City, said in an interview.
“We need to minimize the risk for patients and minimize our own exposure by treating this situation like we would a really bad flu season,” Smith told Medscape Medical News. “Some patients have had a bad outcome, but the vast majority do not. The best we can do is stay calm and focused.”
At Mount Sinai, for patients with cancer, routine, nonurgent appointments are being rescheduled for May, Smith said. Those in active treatment are screened by telephone 24 to 48 hours before arrival, after which they undergo a full risk assessment in an isolation room. Those with a respiratory infection are given a mask.
“Patients are very anxious and worried about COVID-19,” said Smith, who has young children and an elderly parent at home. “We don’t have all the answers, and this can heighten anxiety.”
To help allay fears, social workers are asking patients with cancer who express anxiety to discuss their concerns and provide information. A one-page handout on both flu and COVID-10 is available in the waiting room.
The Web portal MyChart gives patients access to updated information on COVID-19 precautions and provides links to the hospital website and to the US Centers for Disease Control and Prevention. Patients who are not feeling well can speak to someone or get answers if they have additional questions.
When counseling patients, Smith advises them to use “an abundance of caution” and to be creative in efforts to minimize risk. “My suggestion is to use FaceTime and Skype to connect and communicate with your community,” she said.
Some churches are conducting services via teleconferencing to minimize risk, and seniors’ centers that offer yoga and other classes are also beginning to provide services virtually, she pointed out.
Data from China
A report published February 14 in the Lancet Oncology appears to be the first analysis in the literature to focus on COVID-19 in patients with cancer.
“Patients with cancer are more susceptible to infection than individuals without cancer because of their systemic immunosuppressive state caused by the malignancy and anticancer treatments, such as chemotherapy or surgery,” write the authors, led by Wenhua Liang, MD, of Guangzhou Medical University. However, in correspondence published in the Lancet Oncology, other experts in China question some of Liang’s and colleagues’ findings.
The report by Liang and colleagues concerns a prospective cohort of 1590 patients with COVID-19.
There were 2007 laboratory-confirmed cases of COVID-19 among patients admitted to 575 hospitals throughout China as of January 31. Of those cases, 417 were excluded from the analysis because of insufficient information regarding disease history.
The team reports that of 18 patients with cancer and COVID-19, 39% were at significantly higher risk for “severe events.” By comparison, of 1572 patients with COVID-19 who did not have cancer, 8% were at significantly higher risk (P = .0003). These events included rapid clinical deterioration that required admission to intensive care; invasive ventilation; or death.
Patients with cancer experienced a much more rapid deterioration in clinical status than did those without cancer. The median time to severe events was 13 days, vs 43 days (hazard ratio [HR] adjusted for age, 3.56; P < .0001).
The analysis also shows that patients who underwent chemotherapy or surgery in the past month had a 75% risk of experiencing clinically severe events, compared with a 43% risk for those who had not received recent treatment.
After adjusting for other risk factors, including age and smoking history, older age was the only risk factor for severe events (odds ratio [OR], 1.43; 95% confidence interval [CI], 0.97 – 2.12; P = .072), the study authors say.
Patients with lung cancer did not have a higher probability of severe events compared with patients with other cancer types (20% vs 62%, respectively; P = .294).
Liang and colleagues conclude that these findings provide “a timely reminder to physicians that more intensive attention should be paid to patients with cancer, in case of rapid deterioration.”
The team also proposes three strategies for managing patients with cancer who are at risk for COVID-19 or any other severe infectious disease. They recommend that intentional postponement of adjuvant chemotherapy or elective surgery be considered for patients with stable cancer who live in areas where disease is endemic. Stronger “personal protection provisions” could also be made for patients with cancer or for cancer survivors. Lastly, for patients with cancer who have COVID-19, especially those who are older or who have comorbidities, more intensive surveillance or treatment should be considered.
However, in comments in the Lancet Oncology, other authors in China say these findings should be interpreted with caution.
One group suggests that the increased susceptibility to COVID-19 in patients with cancer could be the result of higher rates of smoking compared with patients who did not have cancer. “Overall, current evidence remains insufficient to explain a conclusive association between cancer and COVID-19,” say Huahao Shen, PhD, of Zhejiang University School of Medicine, Hangzhou, Zhejiang, and colleagues.
Another group suggests that the significantly higher median age of patients with cancer compared with noncancer patients (63 years vs 49 years) may have contributed to poor prognosis.
These authors, led by Li Zhang, MD, PhD, and Hanping Wang, MD, of Peking Union Medical College and the Chinese Academy of Medical Sciences, Beijing, emphasize that patients with cancer need online medical counseling and that critical cases need to be identified and treated.
“In endemic areas outside Wuhan, decisions on whether or not to postpone cancer treatment need to made on a patient-by-patient basis and according to the risk to the patient and the prevailing situation because delays could lead to tumor progression and ultimately poorer outcomes,” they write.
The study was funded by the China National Science Foundation and the Key Project of Guangzhou Scientific Research Project. Liang and coauthors, Shen and coauthors, Zhang, Wang, and Smith have disclosed no relevant financial relationships. Markham has relationships with Aduro Biotech, Lilly, Tesaro, Novartis, and VBL Therapeutics.
This article first appeared on Medscape.com.
For oncologists and other clinicians caring for patients with cancer, the COVID-19 pandemic represents a dynamic clinical challenge that is changing daily and that can feel overwhelming at times, say experts.
“Oncology clinicians are well versed in caring for immunosuppressed patients with cancer, of all ages,” Merry-Jennifer Markham, MD, interim chief of the Division of Hematology and Oncology at the University of Florida Health, Gainesville, told Medscape Medical News.
However, she emphasized that, during this COVID-19 outbreak, “we must be especially diligent about screening for symptoms and exposure, and we must recognize that our older patients with cancer may be especially vulnerable.”
Patients with cancer who are in active treatment are immunosuppressed and are more susceptible to infection and to complications from infection, Markham pointed out. “While we don’t yet have much data on how COVID-19 impacts patients with cancer, I have to suspect that patients undergoing active cancer treatment may be especially vulnerable to the more severe illness associated with COVID-19,” she said.
Indeed, a recent report from China that was published in the Lancet Oncology supports this. The authors suggest that patients with cancer are at higher risk for COVID-19 and have a worse prognosis if they become infected than do those without cancer.
Commonsense rules
Commonsense rules apply for all patients with cancer, regardless of age, said Markham. Measures include thorough handwashing, staying home when sick, and avoiding sick contacts.
Markham, who acts as an expert spokesperson for the American Society of Clinical Oncology, provides information on what patients with cancer need to know about COVID-19 at Cancer.net, the society’s website for patients with cancer.
“Unfortunately, this outbreak of COVID-19 is happening rapidly and in real time,” Markham noted. “The entire medical community is learning as we go, rather than having the luxury of years of evidence-based literature to guide us.”
Another expert agrees. “Unfortunately, there are not a lot of data on how COVID-19 affects cancer patients,” Cardinale Smith, MD, PhD, director of Quality for Cancer Services in the Mount Sinai Health System, New York City, said in an interview.
“We need to minimize the risk for patients and minimize our own exposure by treating this situation like we would a really bad flu season,” Smith told Medscape Medical News. “Some patients have had a bad outcome, but the vast majority do not. The best we can do is stay calm and focused.”
At Mount Sinai, for patients with cancer, routine, nonurgent appointments are being rescheduled for May, Smith said. Those in active treatment are screened by telephone 24 to 48 hours before arrival, after which they undergo a full risk assessment in an isolation room. Those with a respiratory infection are given a mask.
“Patients are very anxious and worried about COVID-19,” said Smith, who has young children and an elderly parent at home. “We don’t have all the answers, and this can heighten anxiety.”
To help allay fears, social workers are asking patients with cancer who express anxiety to discuss their concerns and provide information. A one-page handout on both flu and COVID-10 is available in the waiting room.
The Web portal MyChart gives patients access to updated information on COVID-19 precautions and provides links to the hospital website and to the US Centers for Disease Control and Prevention. Patients who are not feeling well can speak to someone or get answers if they have additional questions.
When counseling patients, Smith advises them to use “an abundance of caution” and to be creative in efforts to minimize risk. “My suggestion is to use FaceTime and Skype to connect and communicate with your community,” she said.
Some churches are conducting services via teleconferencing to minimize risk, and seniors’ centers that offer yoga and other classes are also beginning to provide services virtually, she pointed out.
Data from China
A report published February 14 in the Lancet Oncology appears to be the first analysis in the literature to focus on COVID-19 in patients with cancer.
“Patients with cancer are more susceptible to infection than individuals without cancer because of their systemic immunosuppressive state caused by the malignancy and anticancer treatments, such as chemotherapy or surgery,” write the authors, led by Wenhua Liang, MD, of Guangzhou Medical University. However, in correspondence published in the Lancet Oncology, other experts in China question some of Liang’s and colleagues’ findings.
The report by Liang and colleagues concerns a prospective cohort of 1590 patients with COVID-19.
There were 2007 laboratory-confirmed cases of COVID-19 among patients admitted to 575 hospitals throughout China as of January 31. Of those cases, 417 were excluded from the analysis because of insufficient information regarding disease history.
The team reports that of 18 patients with cancer and COVID-19, 39% were at significantly higher risk for “severe events.” By comparison, of 1572 patients with COVID-19 who did not have cancer, 8% were at significantly higher risk (P = .0003). These events included rapid clinical deterioration that required admission to intensive care; invasive ventilation; or death.
Patients with cancer experienced a much more rapid deterioration in clinical status than did those without cancer. The median time to severe events was 13 days, vs 43 days (hazard ratio [HR] adjusted for age, 3.56; P < .0001).
The analysis also shows that patients who underwent chemotherapy or surgery in the past month had a 75% risk of experiencing clinically severe events, compared with a 43% risk for those who had not received recent treatment.
After adjusting for other risk factors, including age and smoking history, older age was the only risk factor for severe events (odds ratio [OR], 1.43; 95% confidence interval [CI], 0.97 – 2.12; P = .072), the study authors say.
Patients with lung cancer did not have a higher probability of severe events compared with patients with other cancer types (20% vs 62%, respectively; P = .294).
Liang and colleagues conclude that these findings provide “a timely reminder to physicians that more intensive attention should be paid to patients with cancer, in case of rapid deterioration.”
The team also proposes three strategies for managing patients with cancer who are at risk for COVID-19 or any other severe infectious disease. They recommend that intentional postponement of adjuvant chemotherapy or elective surgery be considered for patients with stable cancer who live in areas where disease is endemic. Stronger “personal protection provisions” could also be made for patients with cancer or for cancer survivors. Lastly, for patients with cancer who have COVID-19, especially those who are older or who have comorbidities, more intensive surveillance or treatment should be considered.
However, in comments in the Lancet Oncology, other authors in China say these findings should be interpreted with caution.
One group suggests that the increased susceptibility to COVID-19 in patients with cancer could be the result of higher rates of smoking compared with patients who did not have cancer. “Overall, current evidence remains insufficient to explain a conclusive association between cancer and COVID-19,” say Huahao Shen, PhD, of Zhejiang University School of Medicine, Hangzhou, Zhejiang, and colleagues.
Another group suggests that the significantly higher median age of patients with cancer compared with noncancer patients (63 years vs 49 years) may have contributed to poor prognosis.
These authors, led by Li Zhang, MD, PhD, and Hanping Wang, MD, of Peking Union Medical College and the Chinese Academy of Medical Sciences, Beijing, emphasize that patients with cancer need online medical counseling and that critical cases need to be identified and treated.
“In endemic areas outside Wuhan, decisions on whether or not to postpone cancer treatment need to made on a patient-by-patient basis and according to the risk to the patient and the prevailing situation because delays could lead to tumor progression and ultimately poorer outcomes,” they write.
The study was funded by the China National Science Foundation and the Key Project of Guangzhou Scientific Research Project. Liang and coauthors, Shen and coauthors, Zhang, Wang, and Smith have disclosed no relevant financial relationships. Markham has relationships with Aduro Biotech, Lilly, Tesaro, Novartis, and VBL Therapeutics.
This article first appeared on Medscape.com.
For oncologists and other clinicians caring for patients with cancer, the COVID-19 pandemic represents a dynamic clinical challenge that is changing daily and that can feel overwhelming at times, say experts.
“Oncology clinicians are well versed in caring for immunosuppressed patients with cancer, of all ages,” Merry-Jennifer Markham, MD, interim chief of the Division of Hematology and Oncology at the University of Florida Health, Gainesville, told Medscape Medical News.
However, she emphasized that, during this COVID-19 outbreak, “we must be especially diligent about screening for symptoms and exposure, and we must recognize that our older patients with cancer may be especially vulnerable.”
Patients with cancer who are in active treatment are immunosuppressed and are more susceptible to infection and to complications from infection, Markham pointed out. “While we don’t yet have much data on how COVID-19 impacts patients with cancer, I have to suspect that patients undergoing active cancer treatment may be especially vulnerable to the more severe illness associated with COVID-19,” she said.
Indeed, a recent report from China that was published in the Lancet Oncology supports this. The authors suggest that patients with cancer are at higher risk for COVID-19 and have a worse prognosis if they become infected than do those without cancer.
Commonsense rules
Commonsense rules apply for all patients with cancer, regardless of age, said Markham. Measures include thorough handwashing, staying home when sick, and avoiding sick contacts.
Markham, who acts as an expert spokesperson for the American Society of Clinical Oncology, provides information on what patients with cancer need to know about COVID-19 at Cancer.net, the society’s website for patients with cancer.
“Unfortunately, this outbreak of COVID-19 is happening rapidly and in real time,” Markham noted. “The entire medical community is learning as we go, rather than having the luxury of years of evidence-based literature to guide us.”
Another expert agrees. “Unfortunately, there are not a lot of data on how COVID-19 affects cancer patients,” Cardinale Smith, MD, PhD, director of Quality for Cancer Services in the Mount Sinai Health System, New York City, said in an interview.
“We need to minimize the risk for patients and minimize our own exposure by treating this situation like we would a really bad flu season,” Smith told Medscape Medical News. “Some patients have had a bad outcome, but the vast majority do not. The best we can do is stay calm and focused.”
At Mount Sinai, for patients with cancer, routine, nonurgent appointments are being rescheduled for May, Smith said. Those in active treatment are screened by telephone 24 to 48 hours before arrival, after which they undergo a full risk assessment in an isolation room. Those with a respiratory infection are given a mask.
“Patients are very anxious and worried about COVID-19,” said Smith, who has young children and an elderly parent at home. “We don’t have all the answers, and this can heighten anxiety.”
To help allay fears, social workers are asking patients with cancer who express anxiety to discuss their concerns and provide information. A one-page handout on both flu and COVID-10 is available in the waiting room.
The Web portal MyChart gives patients access to updated information on COVID-19 precautions and provides links to the hospital website and to the US Centers for Disease Control and Prevention. Patients who are not feeling well can speak to someone or get answers if they have additional questions.
When counseling patients, Smith advises them to use “an abundance of caution” and to be creative in efforts to minimize risk. “My suggestion is to use FaceTime and Skype to connect and communicate with your community,” she said.
Some churches are conducting services via teleconferencing to minimize risk, and seniors’ centers that offer yoga and other classes are also beginning to provide services virtually, she pointed out.
Data from China
A report published February 14 in the Lancet Oncology appears to be the first analysis in the literature to focus on COVID-19 in patients with cancer.
“Patients with cancer are more susceptible to infection than individuals without cancer because of their systemic immunosuppressive state caused by the malignancy and anticancer treatments, such as chemotherapy or surgery,” write the authors, led by Wenhua Liang, MD, of Guangzhou Medical University. However, in correspondence published in the Lancet Oncology, other experts in China question some of Liang’s and colleagues’ findings.
The report by Liang and colleagues concerns a prospective cohort of 1590 patients with COVID-19.
There were 2007 laboratory-confirmed cases of COVID-19 among patients admitted to 575 hospitals throughout China as of January 31. Of those cases, 417 were excluded from the analysis because of insufficient information regarding disease history.
The team reports that of 18 patients with cancer and COVID-19, 39% were at significantly higher risk for “severe events.” By comparison, of 1572 patients with COVID-19 who did not have cancer, 8% were at significantly higher risk (P = .0003). These events included rapid clinical deterioration that required admission to intensive care; invasive ventilation; or death.
Patients with cancer experienced a much more rapid deterioration in clinical status than did those without cancer. The median time to severe events was 13 days, vs 43 days (hazard ratio [HR] adjusted for age, 3.56; P < .0001).
The analysis also shows that patients who underwent chemotherapy or surgery in the past month had a 75% risk of experiencing clinically severe events, compared with a 43% risk for those who had not received recent treatment.
After adjusting for other risk factors, including age and smoking history, older age was the only risk factor for severe events (odds ratio [OR], 1.43; 95% confidence interval [CI], 0.97 – 2.12; P = .072), the study authors say.
Patients with lung cancer did not have a higher probability of severe events compared with patients with other cancer types (20% vs 62%, respectively; P = .294).
Liang and colleagues conclude that these findings provide “a timely reminder to physicians that more intensive attention should be paid to patients with cancer, in case of rapid deterioration.”
The team also proposes three strategies for managing patients with cancer who are at risk for COVID-19 or any other severe infectious disease. They recommend that intentional postponement of adjuvant chemotherapy or elective surgery be considered for patients with stable cancer who live in areas where disease is endemic. Stronger “personal protection provisions” could also be made for patients with cancer or for cancer survivors. Lastly, for patients with cancer who have COVID-19, especially those who are older or who have comorbidities, more intensive surveillance or treatment should be considered.
However, in comments in the Lancet Oncology, other authors in China say these findings should be interpreted with caution.
One group suggests that the increased susceptibility to COVID-19 in patients with cancer could be the result of higher rates of smoking compared with patients who did not have cancer. “Overall, current evidence remains insufficient to explain a conclusive association between cancer and COVID-19,” say Huahao Shen, PhD, of Zhejiang University School of Medicine, Hangzhou, Zhejiang, and colleagues.
Another group suggests that the significantly higher median age of patients with cancer compared with noncancer patients (63 years vs 49 years) may have contributed to poor prognosis.
These authors, led by Li Zhang, MD, PhD, and Hanping Wang, MD, of Peking Union Medical College and the Chinese Academy of Medical Sciences, Beijing, emphasize that patients with cancer need online medical counseling and that critical cases need to be identified and treated.
“In endemic areas outside Wuhan, decisions on whether or not to postpone cancer treatment need to made on a patient-by-patient basis and according to the risk to the patient and the prevailing situation because delays could lead to tumor progression and ultimately poorer outcomes,” they write.
The study was funded by the China National Science Foundation and the Key Project of Guangzhou Scientific Research Project. Liang and coauthors, Shen and coauthors, Zhang, Wang, and Smith have disclosed no relevant financial relationships. Markham has relationships with Aduro Biotech, Lilly, Tesaro, Novartis, and VBL Therapeutics.
This article first appeared on Medscape.com.
FDA approves new drug for relapsed/refractory multiple myeloma
The U.S. Food and Drug Administration today approved isatuximab (Sarclisa, Sanofi) in combination with pomalidomide (Revlimid, Celgene) and dexamethasone for the treatment of adult patients with multiple myeloma who have received two or more prior therapies including lenalidomide and a proteasome inhibitor.
Isatuximab is an anti-CD38 monoclonal antibody administered by intravenous infusion that works by helping the immune system attack multiple myeloma cancer cells.
“While there is no cure for multiple myeloma, Sarclisa is now another CD38-directed treatment option added to the list of FDA-approved treatments of patients with multiple myeloma who have progressive disease after previous therapies,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
“In the clinical trial, there was a 40% reduction in the risk of disease progression or death with this therapy,” he added.
The new approval is based on results from ICARIA-MM, an open-label, randomized phase 3 clinical trial of isatuximab among 307 patients in this setting.
In the trial, at a median follow-up of 11.6 months, median progression-free survival was 11.5 months in the isatuximab-pomalidomide-dexamethasone group versus 6.5 months in the pomalidomide-dexamethasone group (hazard ratio, 0.60; P = .001), as reported last year. Overall response rates were 60.4% for the triplet-treated group versus 35.3% for the doublet-treated group.
The most common side effects for isatuximab included neutropenia, infusion-related reactions, pneumonia, upper respiratory tract infection, diarrhea, anemia, lymphopenia, and thrombocytopenia.
Deaths because of treatment-related adverse events were reported for one patient (less than 1%) in the isatuximab-pomalidomide-dexamethasone group (sepsis) and two patients (1%) in the pomalidomide-dexamethasone group (pneumonia and urinary tract infection).
The drug can also cause serious side effects, including IV infusion-related reactions. In the case of a grade 3 or higher reaction, the drug should be permanently discontinued and health care professionals should institute appropriate medical management.
The FDA notes there have been higher incidences of second primary malignancies observed in a controlled clinical trial of patients with multiple myeloma receiving the drug.
The FDA also highlighted that laboratory test interference may be caused by isatuximab and that blood banks should be informed that patients are receiving the drug. Isatuximab may interfere with, for example, antibody screening for patients who need a blood transfusion. Isatuximab may also interfere with the assays used to monitor M-protein, which may impact the determination of complete response.
This article originally appeared on Medscape.com.
The U.S. Food and Drug Administration today approved isatuximab (Sarclisa, Sanofi) in combination with pomalidomide (Revlimid, Celgene) and dexamethasone for the treatment of adult patients with multiple myeloma who have received two or more prior therapies including lenalidomide and a proteasome inhibitor.
Isatuximab is an anti-CD38 monoclonal antibody administered by intravenous infusion that works by helping the immune system attack multiple myeloma cancer cells.
“While there is no cure for multiple myeloma, Sarclisa is now another CD38-directed treatment option added to the list of FDA-approved treatments of patients with multiple myeloma who have progressive disease after previous therapies,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
“In the clinical trial, there was a 40% reduction in the risk of disease progression or death with this therapy,” he added.
The new approval is based on results from ICARIA-MM, an open-label, randomized phase 3 clinical trial of isatuximab among 307 patients in this setting.
In the trial, at a median follow-up of 11.6 months, median progression-free survival was 11.5 months in the isatuximab-pomalidomide-dexamethasone group versus 6.5 months in the pomalidomide-dexamethasone group (hazard ratio, 0.60; P = .001), as reported last year. Overall response rates were 60.4% for the triplet-treated group versus 35.3% for the doublet-treated group.
The most common side effects for isatuximab included neutropenia, infusion-related reactions, pneumonia, upper respiratory tract infection, diarrhea, anemia, lymphopenia, and thrombocytopenia.
Deaths because of treatment-related adverse events were reported for one patient (less than 1%) in the isatuximab-pomalidomide-dexamethasone group (sepsis) and two patients (1%) in the pomalidomide-dexamethasone group (pneumonia and urinary tract infection).
The drug can also cause serious side effects, including IV infusion-related reactions. In the case of a grade 3 or higher reaction, the drug should be permanently discontinued and health care professionals should institute appropriate medical management.
The FDA notes there have been higher incidences of second primary malignancies observed in a controlled clinical trial of patients with multiple myeloma receiving the drug.
The FDA also highlighted that laboratory test interference may be caused by isatuximab and that blood banks should be informed that patients are receiving the drug. Isatuximab may interfere with, for example, antibody screening for patients who need a blood transfusion. Isatuximab may also interfere with the assays used to monitor M-protein, which may impact the determination of complete response.
This article originally appeared on Medscape.com.
The U.S. Food and Drug Administration today approved isatuximab (Sarclisa, Sanofi) in combination with pomalidomide (Revlimid, Celgene) and dexamethasone for the treatment of adult patients with multiple myeloma who have received two or more prior therapies including lenalidomide and a proteasome inhibitor.
Isatuximab is an anti-CD38 monoclonal antibody administered by intravenous infusion that works by helping the immune system attack multiple myeloma cancer cells.
“While there is no cure for multiple myeloma, Sarclisa is now another CD38-directed treatment option added to the list of FDA-approved treatments of patients with multiple myeloma who have progressive disease after previous therapies,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
“In the clinical trial, there was a 40% reduction in the risk of disease progression or death with this therapy,” he added.
The new approval is based on results from ICARIA-MM, an open-label, randomized phase 3 clinical trial of isatuximab among 307 patients in this setting.
In the trial, at a median follow-up of 11.6 months, median progression-free survival was 11.5 months in the isatuximab-pomalidomide-dexamethasone group versus 6.5 months in the pomalidomide-dexamethasone group (hazard ratio, 0.60; P = .001), as reported last year. Overall response rates were 60.4% for the triplet-treated group versus 35.3% for the doublet-treated group.
The most common side effects for isatuximab included neutropenia, infusion-related reactions, pneumonia, upper respiratory tract infection, diarrhea, anemia, lymphopenia, and thrombocytopenia.
Deaths because of treatment-related adverse events were reported for one patient (less than 1%) in the isatuximab-pomalidomide-dexamethasone group (sepsis) and two patients (1%) in the pomalidomide-dexamethasone group (pneumonia and urinary tract infection).
The drug can also cause serious side effects, including IV infusion-related reactions. In the case of a grade 3 or higher reaction, the drug should be permanently discontinued and health care professionals should institute appropriate medical management.
The FDA notes there have been higher incidences of second primary malignancies observed in a controlled clinical trial of patients with multiple myeloma receiving the drug.
The FDA also highlighted that laboratory test interference may be caused by isatuximab and that blood banks should be informed that patients are receiving the drug. Isatuximab may interfere with, for example, antibody screening for patients who need a blood transfusion. Isatuximab may also interfere with the assays used to monitor M-protein, which may impact the determination of complete response.
This article originally appeared on Medscape.com.
Death from unintentional injury is higher in cancer patients
New research suggests the incidence of death from unintentional injury is higher among patients with cancer than among those in the general U.S. population.
The findings highlight the need for more initiatives to help recognize patients at risk of death from this category of injury, which is the third leading cause of death in the country, according to the study authors.
“The purpose of our study was to present a comprehensive analysis of death from unintentional injury among patients with cancer using a large population-based cohort,” wrote Kunyu Yang, MD, of Huazhong University of Science and Technology in China, and colleagues. Their report is in JAMA Network Open.
The retrospective study included 8,271,020 patients with cancer, 40,599 of whom died from unintentional injury. The researchers identified patients who received a new diagnosis of primary cancer between Jan. 1, 1973, and Dec. 31, 2015, from the Surveillance, Epidemiology, and End Results (SEER) database.
The mean age of study participants was 63.0 years, and most were diagnosed in the 2000-2009 period (41.6%) or the 2010-2015 period (27.6%).
The SEER data was compared with mortality data representative of the general U.S. population obtained from the National Center for Health Statistics. Standardized mortality ratios and rates of death from unintentional injury were measured in both groups.
The rates of death from unintentional injury were 81.90 per 100,000 person-years in cancer patients and 51.21 per 100,000 person-years in the general population. The standardized mortality ratio was 1.60 (95% confidence interval, 1.58-1.61).
Factors associated with higher rates of death from unintentional injury among cancer patients included male sex (relative risk, 1.69; P less than .001), age greater than 80 years at diagnosis (RR, 2.91; P less than .001), and being unmarried (RR, 1.23; P less than .001).
“Rates of death from unintentional injury were the highest in patients with cancers of the liver (200.37 per 100,000 person-years), brain (175.04 per 100,000 person-years), larynx (148.78 per 100,000 person-years), and esophagus (144.98 per 100,000 person-years),” the researchers reported.
They acknowledged that a key limitation of this study was the potential role of reporting bias in death certificate analyses. As a result, death due to unintentional injury and death from suicide and homicide could have been misclassified.
“Cancer-related suicides, like all suicides, are preventable and should be viewed as cancer-related mortality. It is a silent killer with a peak incidence weeks after diagnosis – an undeniably hectic time for most patients and clinicians getting to know their patients,” said Daniel C. McFarland, DO, of Memorial Sloan Kettering Cancer Center in New York. He was not involved in this study.
“Suicide screening with appropriate systems in place to address suicidal thoughts and behavior is crucial for cancer patients throughout the trajectory of their care,” he added.
As with death from unintentional injury, higher rates of suicide have been reported among cancer patients in comparison to the general population (Nat Commun. 2019;10[1]:207).
No funding sources were reported for this study. The authors and Dr. McFarland disclosed no conflicts of interest.
SOURCE: Yang K et al. JAMA Netw Open. 2020 Feb 21. doi: 10.1001/jamanetworkopen.2019.21647.
New research suggests the incidence of death from unintentional injury is higher among patients with cancer than among those in the general U.S. population.
The findings highlight the need for more initiatives to help recognize patients at risk of death from this category of injury, which is the third leading cause of death in the country, according to the study authors.
“The purpose of our study was to present a comprehensive analysis of death from unintentional injury among patients with cancer using a large population-based cohort,” wrote Kunyu Yang, MD, of Huazhong University of Science and Technology in China, and colleagues. Their report is in JAMA Network Open.
The retrospective study included 8,271,020 patients with cancer, 40,599 of whom died from unintentional injury. The researchers identified patients who received a new diagnosis of primary cancer between Jan. 1, 1973, and Dec. 31, 2015, from the Surveillance, Epidemiology, and End Results (SEER) database.
The mean age of study participants was 63.0 years, and most were diagnosed in the 2000-2009 period (41.6%) or the 2010-2015 period (27.6%).
The SEER data was compared with mortality data representative of the general U.S. population obtained from the National Center for Health Statistics. Standardized mortality ratios and rates of death from unintentional injury were measured in both groups.
The rates of death from unintentional injury were 81.90 per 100,000 person-years in cancer patients and 51.21 per 100,000 person-years in the general population. The standardized mortality ratio was 1.60 (95% confidence interval, 1.58-1.61).
Factors associated with higher rates of death from unintentional injury among cancer patients included male sex (relative risk, 1.69; P less than .001), age greater than 80 years at diagnosis (RR, 2.91; P less than .001), and being unmarried (RR, 1.23; P less than .001).
“Rates of death from unintentional injury were the highest in patients with cancers of the liver (200.37 per 100,000 person-years), brain (175.04 per 100,000 person-years), larynx (148.78 per 100,000 person-years), and esophagus (144.98 per 100,000 person-years),” the researchers reported.
They acknowledged that a key limitation of this study was the potential role of reporting bias in death certificate analyses. As a result, death due to unintentional injury and death from suicide and homicide could have been misclassified.
“Cancer-related suicides, like all suicides, are preventable and should be viewed as cancer-related mortality. It is a silent killer with a peak incidence weeks after diagnosis – an undeniably hectic time for most patients and clinicians getting to know their patients,” said Daniel C. McFarland, DO, of Memorial Sloan Kettering Cancer Center in New York. He was not involved in this study.
“Suicide screening with appropriate systems in place to address suicidal thoughts and behavior is crucial for cancer patients throughout the trajectory of their care,” he added.
As with death from unintentional injury, higher rates of suicide have been reported among cancer patients in comparison to the general population (Nat Commun. 2019;10[1]:207).
No funding sources were reported for this study. The authors and Dr. McFarland disclosed no conflicts of interest.
SOURCE: Yang K et al. JAMA Netw Open. 2020 Feb 21. doi: 10.1001/jamanetworkopen.2019.21647.
New research suggests the incidence of death from unintentional injury is higher among patients with cancer than among those in the general U.S. population.
The findings highlight the need for more initiatives to help recognize patients at risk of death from this category of injury, which is the third leading cause of death in the country, according to the study authors.
“The purpose of our study was to present a comprehensive analysis of death from unintentional injury among patients with cancer using a large population-based cohort,” wrote Kunyu Yang, MD, of Huazhong University of Science and Technology in China, and colleagues. Their report is in JAMA Network Open.
The retrospective study included 8,271,020 patients with cancer, 40,599 of whom died from unintentional injury. The researchers identified patients who received a new diagnosis of primary cancer between Jan. 1, 1973, and Dec. 31, 2015, from the Surveillance, Epidemiology, and End Results (SEER) database.
The mean age of study participants was 63.0 years, and most were diagnosed in the 2000-2009 period (41.6%) or the 2010-2015 period (27.6%).
The SEER data was compared with mortality data representative of the general U.S. population obtained from the National Center for Health Statistics. Standardized mortality ratios and rates of death from unintentional injury were measured in both groups.
The rates of death from unintentional injury were 81.90 per 100,000 person-years in cancer patients and 51.21 per 100,000 person-years in the general population. The standardized mortality ratio was 1.60 (95% confidence interval, 1.58-1.61).
Factors associated with higher rates of death from unintentional injury among cancer patients included male sex (relative risk, 1.69; P less than .001), age greater than 80 years at diagnosis (RR, 2.91; P less than .001), and being unmarried (RR, 1.23; P less than .001).
“Rates of death from unintentional injury were the highest in patients with cancers of the liver (200.37 per 100,000 person-years), brain (175.04 per 100,000 person-years), larynx (148.78 per 100,000 person-years), and esophagus (144.98 per 100,000 person-years),” the researchers reported.
They acknowledged that a key limitation of this study was the potential role of reporting bias in death certificate analyses. As a result, death due to unintentional injury and death from suicide and homicide could have been misclassified.
“Cancer-related suicides, like all suicides, are preventable and should be viewed as cancer-related mortality. It is a silent killer with a peak incidence weeks after diagnosis – an undeniably hectic time for most patients and clinicians getting to know their patients,” said Daniel C. McFarland, DO, of Memorial Sloan Kettering Cancer Center in New York. He was not involved in this study.
“Suicide screening with appropriate systems in place to address suicidal thoughts and behavior is crucial for cancer patients throughout the trajectory of their care,” he added.
As with death from unintentional injury, higher rates of suicide have been reported among cancer patients in comparison to the general population (Nat Commun. 2019;10[1]:207).
No funding sources were reported for this study. The authors and Dr. McFarland disclosed no conflicts of interest.
SOURCE: Yang K et al. JAMA Netw Open. 2020 Feb 21. doi: 10.1001/jamanetworkopen.2019.21647.
FROM JAMA NETWORK OPEN
Late effects in young cancer survivors underscore importance of high-risk screening
according to data from the Childhood Cancer Survivor Study.
At a median follow-up of 21 years, the SMR for all-cause mortality was 5.9 among survivors aged 15-20 years and 6.2 among diagnosis-matched children under 15 years, compared with expected rates at the same ages in the general population. For health-related causes – excluding primary cancer recurrence or progression but including late effects of cancer therapy – the SMRs were 4.8 in the older group and 6.8 in the younger group.
Eugene Suh, MD, of Loyola University Chicago Medical Center, Maywood, Ill., and colleagues reported these results in Lancet Oncology.
The difference between the older and younger survivors (n = 5,804 in each group) was most evident at least 20 years after cancer diagnosis, the authors noted.
For both groups, but more so for childhood cancer survivors, the risk of developing any chronic health condition and any grade 3-5 health condition was greater than for siblings of the same age who did not have cancer (hazard ratios, 4.2 for adolescents/young adults and 5.6 for childhood survivors). The same was true for grade 3-5 cardiac conditions (HRs, 4.3 and 5.6, respectively), endocrine conditions (HRs, 3.9 and 6.4, respectively), and musculoskeletal conditions (HRs, 6.5 and 8.0, respectively).
These findings, which confirm those of previous studies suggesting that younger children might be more vulnerable to the adverse effects of cancer treatment, “underscore that focused efforts are needed to ensure early-adolescent and young adult cancer survivors are receiving recommended risk-based care, with a focus on high-risk cancer screening, to reduce morbidity and premature mortality,” the researchers concluded, noting that “studies to date indicate that adherence to such high-risk screening is poor.”
In a related editorial, Päivi Lähteenmäki, MD, PhD, of University of Turku (Finland) and Turku University Hospital, wrote that these findings warrant long-term follow-up of adolescent and young adult cancer survivors. She also argued that the results “might not be fully generalizable to patients treated today who might be on different treatment regimens to those treated in previous decades” and that “[m]ore prospectively collected objective data focusing on survivors ... are needed.”
Accurate characterization of patients at high risk who would benefit from a tailored screening program is most important, and identifying underlying genetic or molecular factors that confer higher risk for late sequelae would be useful for “planning approaches to survivorship,” Dr. Lähteenmäki added.
This study was funded by the National Cancer Institute and American Lebanese-Syrian Associated Charities. Dr. Suh and Dr. Lähteenmäki reported having no competing interests.
SOURCES: Suh E et al. Lancet Oncology. 2020 Feb 14. doi: 10.1016/S1470-2045(19)30800-9;Lähteenmäki P. Lancet Oncol. 2020 Feb 14. doi: 10.106/S1470-2045(19)30858-7.
according to data from the Childhood Cancer Survivor Study.
At a median follow-up of 21 years, the SMR for all-cause mortality was 5.9 among survivors aged 15-20 years and 6.2 among diagnosis-matched children under 15 years, compared with expected rates at the same ages in the general population. For health-related causes – excluding primary cancer recurrence or progression but including late effects of cancer therapy – the SMRs were 4.8 in the older group and 6.8 in the younger group.
Eugene Suh, MD, of Loyola University Chicago Medical Center, Maywood, Ill., and colleagues reported these results in Lancet Oncology.
The difference between the older and younger survivors (n = 5,804 in each group) was most evident at least 20 years after cancer diagnosis, the authors noted.
For both groups, but more so for childhood cancer survivors, the risk of developing any chronic health condition and any grade 3-5 health condition was greater than for siblings of the same age who did not have cancer (hazard ratios, 4.2 for adolescents/young adults and 5.6 for childhood survivors). The same was true for grade 3-5 cardiac conditions (HRs, 4.3 and 5.6, respectively), endocrine conditions (HRs, 3.9 and 6.4, respectively), and musculoskeletal conditions (HRs, 6.5 and 8.0, respectively).
These findings, which confirm those of previous studies suggesting that younger children might be more vulnerable to the adverse effects of cancer treatment, “underscore that focused efforts are needed to ensure early-adolescent and young adult cancer survivors are receiving recommended risk-based care, with a focus on high-risk cancer screening, to reduce morbidity and premature mortality,” the researchers concluded, noting that “studies to date indicate that adherence to such high-risk screening is poor.”
In a related editorial, Päivi Lähteenmäki, MD, PhD, of University of Turku (Finland) and Turku University Hospital, wrote that these findings warrant long-term follow-up of adolescent and young adult cancer survivors. She also argued that the results “might not be fully generalizable to patients treated today who might be on different treatment regimens to those treated in previous decades” and that “[m]ore prospectively collected objective data focusing on survivors ... are needed.”
Accurate characterization of patients at high risk who would benefit from a tailored screening program is most important, and identifying underlying genetic or molecular factors that confer higher risk for late sequelae would be useful for “planning approaches to survivorship,” Dr. Lähteenmäki added.
This study was funded by the National Cancer Institute and American Lebanese-Syrian Associated Charities. Dr. Suh and Dr. Lähteenmäki reported having no competing interests.
SOURCES: Suh E et al. Lancet Oncology. 2020 Feb 14. doi: 10.1016/S1470-2045(19)30800-9;Lähteenmäki P. Lancet Oncol. 2020 Feb 14. doi: 10.106/S1470-2045(19)30858-7.
according to data from the Childhood Cancer Survivor Study.
At a median follow-up of 21 years, the SMR for all-cause mortality was 5.9 among survivors aged 15-20 years and 6.2 among diagnosis-matched children under 15 years, compared with expected rates at the same ages in the general population. For health-related causes – excluding primary cancer recurrence or progression but including late effects of cancer therapy – the SMRs were 4.8 in the older group and 6.8 in the younger group.
Eugene Suh, MD, of Loyola University Chicago Medical Center, Maywood, Ill., and colleagues reported these results in Lancet Oncology.
The difference between the older and younger survivors (n = 5,804 in each group) was most evident at least 20 years after cancer diagnosis, the authors noted.
For both groups, but more so for childhood cancer survivors, the risk of developing any chronic health condition and any grade 3-5 health condition was greater than for siblings of the same age who did not have cancer (hazard ratios, 4.2 for adolescents/young adults and 5.6 for childhood survivors). The same was true for grade 3-5 cardiac conditions (HRs, 4.3 and 5.6, respectively), endocrine conditions (HRs, 3.9 and 6.4, respectively), and musculoskeletal conditions (HRs, 6.5 and 8.0, respectively).
These findings, which confirm those of previous studies suggesting that younger children might be more vulnerable to the adverse effects of cancer treatment, “underscore that focused efforts are needed to ensure early-adolescent and young adult cancer survivors are receiving recommended risk-based care, with a focus on high-risk cancer screening, to reduce morbidity and premature mortality,” the researchers concluded, noting that “studies to date indicate that adherence to such high-risk screening is poor.”
In a related editorial, Päivi Lähteenmäki, MD, PhD, of University of Turku (Finland) and Turku University Hospital, wrote that these findings warrant long-term follow-up of adolescent and young adult cancer survivors. She also argued that the results “might not be fully generalizable to patients treated today who might be on different treatment regimens to those treated in previous decades” and that “[m]ore prospectively collected objective data focusing on survivors ... are needed.”
Accurate characterization of patients at high risk who would benefit from a tailored screening program is most important, and identifying underlying genetic or molecular factors that confer higher risk for late sequelae would be useful for “planning approaches to survivorship,” Dr. Lähteenmäki added.
This study was funded by the National Cancer Institute and American Lebanese-Syrian Associated Charities. Dr. Suh and Dr. Lähteenmäki reported having no competing interests.
SOURCES: Suh E et al. Lancet Oncology. 2020 Feb 14. doi: 10.1016/S1470-2045(19)30800-9;Lähteenmäki P. Lancet Oncol. 2020 Feb 14. doi: 10.106/S1470-2045(19)30858-7.
FROM LANCET ONCOLOGY
Phase 2 data: Inotuzumab, approved in adults with B-ALL, shows promise in kids, too
ORLANDO – Inotuzumab ozogamicin (InO), a CD22-targeted antibody approved for adults with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL), showed promising safety and efficacy in children and young adults with R/R B-ALL in a phase 2 trial.
Of 48 patients aged 1-21 years enrolled in the Children’s Oncology Group (COG) Protocol AALL1621 and evaluable for response and toxicity after treatment with the approved adult InO dose, 19 achieved a complete response (CR) and 9 achieved a complete response with incomplete count recovery (CRi) after the first treatment cycle, for an overall CR/CRi rate of 58.3%, Maureen M. O’Brien, MD, reported at the annual meeting of the American Society of Hematology.
Of those with CR/CRi, 19 (65.5%) achieved minimal residual disease less than 0.01%, said Dr. O’Brien, a pediatric hematologist and medical director of the Leukemia/Lymphoma Program at the Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center.
Three patients had a partial response (PR), nine had stable disease (SD), and eight had progressive disease (PD), and one of each with PR and SD achieved CR/CRi after a second treatment cycle.
“Of note, two patients who were characterized as [having] progressive disease actually had marrow complete response with incomplete count recovery, but had progressive CNS disease,” she said.
Patients included in the single-arm trial had CD22-positive B-ALL, defined as B-ALL with greater than 20% of blasts expressing CD22, and were in at least their second relapse, were refractory to two prior induction regimens, or had a relapse after hematopoietic stem cell transplantation (HSCT). One exception was that patients with Down syndrome were allowed inclusion after a first relapse, she noted.
Median patient age was 9 years, four patients had CNS 3 status, three had Down syndrome, and most were heavily pretreated, with 32 in at least their second relapse.
“Most patients had significant marrow disease burden, with a median marrow blast percentage of 81%,” Dr. O’Brien said. “In terms of prior therapy, 23% had prior transplant, 23% had prior CD19 [chimeric antigen receptor (CAR)] T-cell therapy – including two patients with prior CD22 CAR T, and 29% of patients had received prior blinatumomab.”
All patients received one cycle of InO at a dose of 1.8mg/m2, with .8mg/m2 given on day 1 and 0.5mg/m2 given on days 8 and 15. Intrathecal therapy was determined based on CNS status.
Patients with at least stable disease at day 28 were eligible for a second cycle; those with CR or CRi received InO at a dose of 0.5 mg/m2 on days 1, 8, and 15 in cycle 2, and those without CR/CRi received the same fractionated dose as in cycle 1. Patients with CR/CRi after two cycles were eligible for up to six total cycles at investigator discretion, Dr. O’Brien explained, adding that 26 of 40 patients eligible for cycle 2 proceeded, including 13 of 18 with MRD less than .01%, 6 of 10 with MRD of 0.01% or greater, and 7 of 12 with PR/SD.
After cycle 2, all 13 with MRD less than .01% maintained that MRD level, 3 of 6 with MRD of .01% or greater achieved MRD less than .01%, 2 of 7 with PR/SD achieved CRi with MRD of .01% or greater – and one of those 2 was MRD negative after a third cycle.
Seven patients received three or more cycles.
“Therapy was extremely well tolerated,” Dr. O’Brien said, noting that the most common nonhematological grade 3 or higher adverse events occurring in at least 5% of patients in cycle 1 were febrile neutropenia and infection, occurring in 27.1% and 16.7% of patients, respectively. “But toxicity was quite minimal.”
Hepatic toxicity included four cases of grade 3 alanine transaminase and one grade 3 bilirubin toxicity in cycle 1, and one grade 3 ALT in cycle 2.
“Importantly, there were no inotuzumab dose modifications or delays due to hepatic toxicity,” she said.
Nine patients experienced 11 dose-limiting toxicities in cycle 1, including 7 involving prolonged count recovery beyond day 42, which was not attributable to disease, and 4 nonhematologic events, including drug reaction with eosinophilia and systemic symptoms, bronchopulmonary hemorrhage, respiratory distress, and a postintrathecal methotrexate stroke.
Sinusoidal obstruction syndrome (SOS) developed in 5 of the 48 patients, all in patients who underwent transplant after InO treatment. Only one of the five had undergone a prior transplant. All SOS cases were grade 3 and were treated with defibrotide; four cases resolved quickly, and one was resolving at the time of death from other causes, she said.
“We found no evidence of association with age, conditioning regimen, SOS prophylaxis, cumulative InO exposure, or time from InO to transplant, bearing in mind that it is a small number of cases, so analysis is limited,” she added.
Central CD22 evaluation in 27 patients with pre– and post–cycle 1 samples showed that 11 of those patients had residual disease at the end of cycle 1.
“There is clearly a subset of patients for whom the resistance mechanism does not seem to have any bearing on CD22, as it was still highly expressed at the time of relapse, but there are a significant number of patients who have downregulation of CD22 expression or a subset of blasts that were CD22 negative at the time of relapse,” she said. “Notably, two of three patients with baseline partial CD22 expression – so less than 90% ... did not achieve a morphologic complete response, and both of these patients had KMT2A rearrangements.”
The findings are important, because 10%-20% of children and young adults with B-ALL will relapse, and therapies that can bridge patients to HSCT or CAR T-cell therapy are critical for improving outcomes, Dr. O’Brien said, explaining that InO, a humanized CD22 IgG4 antibody conjugated to calicheamicin, was approved in adults based on “the impressive results from the INNOVATE trial, compared with chemotherapy,” but prospective data on its efficacy and safety in pediatric patients are lacking.
Retrospective data from a compassionate use program in children demonstrated a response rate of 67% in a heavily pretreated population, and phase 1 data from the ITCC-059 trial presented in a poster at the ASH meeting also showed “quite impressive results,” but a major concern has been hepatic toxicity, including SOS, she said.
Given the observed safety and efficacy in the current phase 2 trial, investigation in children will continue, she said, explaining that “COG is now undertaking a phase 3 trial – AALL1732 – which will randomize patients to chemotherapy [with or without] inotuzumab for patients aged 1-25 with newly diagnosed high-risk B-ALL.”
COG AALL1621 was funded by NCTN grants, St. Baldrick’s Foundation, and Pfizer. Dr. O’Brien reported research funding from Pfizer, Celgene, AbbVie, Amgen, Bristol-Myers Squibb, and BTG.
SOURCE: O’Brien M et al. ASH 2019, Abstract 741.
ORLANDO – Inotuzumab ozogamicin (InO), a CD22-targeted antibody approved for adults with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL), showed promising safety and efficacy in children and young adults with R/R B-ALL in a phase 2 trial.
Of 48 patients aged 1-21 years enrolled in the Children’s Oncology Group (COG) Protocol AALL1621 and evaluable for response and toxicity after treatment with the approved adult InO dose, 19 achieved a complete response (CR) and 9 achieved a complete response with incomplete count recovery (CRi) after the first treatment cycle, for an overall CR/CRi rate of 58.3%, Maureen M. O’Brien, MD, reported at the annual meeting of the American Society of Hematology.
Of those with CR/CRi, 19 (65.5%) achieved minimal residual disease less than 0.01%, said Dr. O’Brien, a pediatric hematologist and medical director of the Leukemia/Lymphoma Program at the Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center.
Three patients had a partial response (PR), nine had stable disease (SD), and eight had progressive disease (PD), and one of each with PR and SD achieved CR/CRi after a second treatment cycle.
“Of note, two patients who were characterized as [having] progressive disease actually had marrow complete response with incomplete count recovery, but had progressive CNS disease,” she said.
Patients included in the single-arm trial had CD22-positive B-ALL, defined as B-ALL with greater than 20% of blasts expressing CD22, and were in at least their second relapse, were refractory to two prior induction regimens, or had a relapse after hematopoietic stem cell transplantation (HSCT). One exception was that patients with Down syndrome were allowed inclusion after a first relapse, she noted.
Median patient age was 9 years, four patients had CNS 3 status, three had Down syndrome, and most were heavily pretreated, with 32 in at least their second relapse.
“Most patients had significant marrow disease burden, with a median marrow blast percentage of 81%,” Dr. O’Brien said. “In terms of prior therapy, 23% had prior transplant, 23% had prior CD19 [chimeric antigen receptor (CAR)] T-cell therapy – including two patients with prior CD22 CAR T, and 29% of patients had received prior blinatumomab.”
All patients received one cycle of InO at a dose of 1.8mg/m2, with .8mg/m2 given on day 1 and 0.5mg/m2 given on days 8 and 15. Intrathecal therapy was determined based on CNS status.
Patients with at least stable disease at day 28 were eligible for a second cycle; those with CR or CRi received InO at a dose of 0.5 mg/m2 on days 1, 8, and 15 in cycle 2, and those without CR/CRi received the same fractionated dose as in cycle 1. Patients with CR/CRi after two cycles were eligible for up to six total cycles at investigator discretion, Dr. O’Brien explained, adding that 26 of 40 patients eligible for cycle 2 proceeded, including 13 of 18 with MRD less than .01%, 6 of 10 with MRD of 0.01% or greater, and 7 of 12 with PR/SD.
After cycle 2, all 13 with MRD less than .01% maintained that MRD level, 3 of 6 with MRD of .01% or greater achieved MRD less than .01%, 2 of 7 with PR/SD achieved CRi with MRD of .01% or greater – and one of those 2 was MRD negative after a third cycle.
Seven patients received three or more cycles.
“Therapy was extremely well tolerated,” Dr. O’Brien said, noting that the most common nonhematological grade 3 or higher adverse events occurring in at least 5% of patients in cycle 1 were febrile neutropenia and infection, occurring in 27.1% and 16.7% of patients, respectively. “But toxicity was quite minimal.”
Hepatic toxicity included four cases of grade 3 alanine transaminase and one grade 3 bilirubin toxicity in cycle 1, and one grade 3 ALT in cycle 2.
“Importantly, there were no inotuzumab dose modifications or delays due to hepatic toxicity,” she said.
Nine patients experienced 11 dose-limiting toxicities in cycle 1, including 7 involving prolonged count recovery beyond day 42, which was not attributable to disease, and 4 nonhematologic events, including drug reaction with eosinophilia and systemic symptoms, bronchopulmonary hemorrhage, respiratory distress, and a postintrathecal methotrexate stroke.
Sinusoidal obstruction syndrome (SOS) developed in 5 of the 48 patients, all in patients who underwent transplant after InO treatment. Only one of the five had undergone a prior transplant. All SOS cases were grade 3 and were treated with defibrotide; four cases resolved quickly, and one was resolving at the time of death from other causes, she said.
“We found no evidence of association with age, conditioning regimen, SOS prophylaxis, cumulative InO exposure, or time from InO to transplant, bearing in mind that it is a small number of cases, so analysis is limited,” she added.
Central CD22 evaluation in 27 patients with pre– and post–cycle 1 samples showed that 11 of those patients had residual disease at the end of cycle 1.
“There is clearly a subset of patients for whom the resistance mechanism does not seem to have any bearing on CD22, as it was still highly expressed at the time of relapse, but there are a significant number of patients who have downregulation of CD22 expression or a subset of blasts that were CD22 negative at the time of relapse,” she said. “Notably, two of three patients with baseline partial CD22 expression – so less than 90% ... did not achieve a morphologic complete response, and both of these patients had KMT2A rearrangements.”
The findings are important, because 10%-20% of children and young adults with B-ALL will relapse, and therapies that can bridge patients to HSCT or CAR T-cell therapy are critical for improving outcomes, Dr. O’Brien said, explaining that InO, a humanized CD22 IgG4 antibody conjugated to calicheamicin, was approved in adults based on “the impressive results from the INNOVATE trial, compared with chemotherapy,” but prospective data on its efficacy and safety in pediatric patients are lacking.
Retrospective data from a compassionate use program in children demonstrated a response rate of 67% in a heavily pretreated population, and phase 1 data from the ITCC-059 trial presented in a poster at the ASH meeting also showed “quite impressive results,” but a major concern has been hepatic toxicity, including SOS, she said.
Given the observed safety and efficacy in the current phase 2 trial, investigation in children will continue, she said, explaining that “COG is now undertaking a phase 3 trial – AALL1732 – which will randomize patients to chemotherapy [with or without] inotuzumab for patients aged 1-25 with newly diagnosed high-risk B-ALL.”
COG AALL1621 was funded by NCTN grants, St. Baldrick’s Foundation, and Pfizer. Dr. O’Brien reported research funding from Pfizer, Celgene, AbbVie, Amgen, Bristol-Myers Squibb, and BTG.
SOURCE: O’Brien M et al. ASH 2019, Abstract 741.
ORLANDO – Inotuzumab ozogamicin (InO), a CD22-targeted antibody approved for adults with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL), showed promising safety and efficacy in children and young adults with R/R B-ALL in a phase 2 trial.
Of 48 patients aged 1-21 years enrolled in the Children’s Oncology Group (COG) Protocol AALL1621 and evaluable for response and toxicity after treatment with the approved adult InO dose, 19 achieved a complete response (CR) and 9 achieved a complete response with incomplete count recovery (CRi) after the first treatment cycle, for an overall CR/CRi rate of 58.3%, Maureen M. O’Brien, MD, reported at the annual meeting of the American Society of Hematology.
Of those with CR/CRi, 19 (65.5%) achieved minimal residual disease less than 0.01%, said Dr. O’Brien, a pediatric hematologist and medical director of the Leukemia/Lymphoma Program at the Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center.
Three patients had a partial response (PR), nine had stable disease (SD), and eight had progressive disease (PD), and one of each with PR and SD achieved CR/CRi after a second treatment cycle.
“Of note, two patients who were characterized as [having] progressive disease actually had marrow complete response with incomplete count recovery, but had progressive CNS disease,” she said.
Patients included in the single-arm trial had CD22-positive B-ALL, defined as B-ALL with greater than 20% of blasts expressing CD22, and were in at least their second relapse, were refractory to two prior induction regimens, or had a relapse after hematopoietic stem cell transplantation (HSCT). One exception was that patients with Down syndrome were allowed inclusion after a first relapse, she noted.
Median patient age was 9 years, four patients had CNS 3 status, three had Down syndrome, and most were heavily pretreated, with 32 in at least their second relapse.
“Most patients had significant marrow disease burden, with a median marrow blast percentage of 81%,” Dr. O’Brien said. “In terms of prior therapy, 23% had prior transplant, 23% had prior CD19 [chimeric antigen receptor (CAR)] T-cell therapy – including two patients with prior CD22 CAR T, and 29% of patients had received prior blinatumomab.”
All patients received one cycle of InO at a dose of 1.8mg/m2, with .8mg/m2 given on day 1 and 0.5mg/m2 given on days 8 and 15. Intrathecal therapy was determined based on CNS status.
Patients with at least stable disease at day 28 were eligible for a second cycle; those with CR or CRi received InO at a dose of 0.5 mg/m2 on days 1, 8, and 15 in cycle 2, and those without CR/CRi received the same fractionated dose as in cycle 1. Patients with CR/CRi after two cycles were eligible for up to six total cycles at investigator discretion, Dr. O’Brien explained, adding that 26 of 40 patients eligible for cycle 2 proceeded, including 13 of 18 with MRD less than .01%, 6 of 10 with MRD of 0.01% or greater, and 7 of 12 with PR/SD.
After cycle 2, all 13 with MRD less than .01% maintained that MRD level, 3 of 6 with MRD of .01% or greater achieved MRD less than .01%, 2 of 7 with PR/SD achieved CRi with MRD of .01% or greater – and one of those 2 was MRD negative after a third cycle.
Seven patients received three or more cycles.
“Therapy was extremely well tolerated,” Dr. O’Brien said, noting that the most common nonhematological grade 3 or higher adverse events occurring in at least 5% of patients in cycle 1 were febrile neutropenia and infection, occurring in 27.1% and 16.7% of patients, respectively. “But toxicity was quite minimal.”
Hepatic toxicity included four cases of grade 3 alanine transaminase and one grade 3 bilirubin toxicity in cycle 1, and one grade 3 ALT in cycle 2.
“Importantly, there were no inotuzumab dose modifications or delays due to hepatic toxicity,” she said.
Nine patients experienced 11 dose-limiting toxicities in cycle 1, including 7 involving prolonged count recovery beyond day 42, which was not attributable to disease, and 4 nonhematologic events, including drug reaction with eosinophilia and systemic symptoms, bronchopulmonary hemorrhage, respiratory distress, and a postintrathecal methotrexate stroke.
Sinusoidal obstruction syndrome (SOS) developed in 5 of the 48 patients, all in patients who underwent transplant after InO treatment. Only one of the five had undergone a prior transplant. All SOS cases were grade 3 and were treated with defibrotide; four cases resolved quickly, and one was resolving at the time of death from other causes, she said.
“We found no evidence of association with age, conditioning regimen, SOS prophylaxis, cumulative InO exposure, or time from InO to transplant, bearing in mind that it is a small number of cases, so analysis is limited,” she added.
Central CD22 evaluation in 27 patients with pre– and post–cycle 1 samples showed that 11 of those patients had residual disease at the end of cycle 1.
“There is clearly a subset of patients for whom the resistance mechanism does not seem to have any bearing on CD22, as it was still highly expressed at the time of relapse, but there are a significant number of patients who have downregulation of CD22 expression or a subset of blasts that were CD22 negative at the time of relapse,” she said. “Notably, two of three patients with baseline partial CD22 expression – so less than 90% ... did not achieve a morphologic complete response, and both of these patients had KMT2A rearrangements.”
The findings are important, because 10%-20% of children and young adults with B-ALL will relapse, and therapies that can bridge patients to HSCT or CAR T-cell therapy are critical for improving outcomes, Dr. O’Brien said, explaining that InO, a humanized CD22 IgG4 antibody conjugated to calicheamicin, was approved in adults based on “the impressive results from the INNOVATE trial, compared with chemotherapy,” but prospective data on its efficacy and safety in pediatric patients are lacking.
Retrospective data from a compassionate use program in children demonstrated a response rate of 67% in a heavily pretreated population, and phase 1 data from the ITCC-059 trial presented in a poster at the ASH meeting also showed “quite impressive results,” but a major concern has been hepatic toxicity, including SOS, she said.
Given the observed safety and efficacy in the current phase 2 trial, investigation in children will continue, she said, explaining that “COG is now undertaking a phase 3 trial – AALL1732 – which will randomize patients to chemotherapy [with or without] inotuzumab for patients aged 1-25 with newly diagnosed high-risk B-ALL.”
COG AALL1621 was funded by NCTN grants, St. Baldrick’s Foundation, and Pfizer. Dr. O’Brien reported research funding from Pfizer, Celgene, AbbVie, Amgen, Bristol-Myers Squibb, and BTG.
SOURCE: O’Brien M et al. ASH 2019, Abstract 741.
REPORTING FROM ASH 2019
FDA: Cell phones still look safe
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
Fear drives activity changes in hemophilia patients
Fear of negative events can drive changes in activity levels among patients with hemophilia A, results of the HemACTIVE study suggest.
Patients were more likely to adjust their level of physical activity due to fear of bleeding and joint damage rather than previously experienced bleeding or joint damage.
However, past experience was more likely than fear to make patients stop physical activities altogether.
Mark Skinner, of the Institute for Policy Advancement in Washington, D.C., and colleagues presented these findings in a poster from the annual congress of the European Association for Haemophilia and Allied Disorders.
Mr. Skinner, who is a hemophilia patient himself, said the goal of the HemACTIVE study is to better understand how hemophilia affects patients’ lives.
“We wanted to understand the limitations, challenges, and compromises of individuals living with hemophilia,” Mr. Skinner said. “What has motivated them or prevented them from living more full, active lives doing the kind of work, leisure, and social activities that those without hemophilia do? Is it treatment choice, is it satisfaction with treatment, is it fear?
“We wanted to do a comprehensive study that really looked at the intersection of treatment adherence and satisfaction, the emotional components that relate to those decisions, and the challenges and compromises so that we could better identify what we need to consider as patients think about either changing their therapy or changing their treatment regimen on existing therapy.”
Previous results from the HemACTIVE study showed that, although activity levels differed among hemophilia patients, all patients surveyed wanted greater activity levels, better protection from bleeding, better pain relief, and less-frequent infusions (EAHAD 2019, Abstract P084). In addition, patients who used factor VIII products with an extended half-life were more active and more likely to adhere to their prescribed treatment (ISTH 2019, Abstract PB0210).
The results reported at EAHAD 2020 focus on patients’ reasons for modifying physical activity. Patients and caregivers completed a screening phone interview, followed by a 25-minute, web-based questionnaire on patient activity.
There were 275 respondents – 194 patients with hemophilia A and 81 caregivers – from Canada, France, Germany, Italy, and the United States. Patients had severe (61%) or moderate (39%) hemophilia A, and most (67%) were receiving prophylaxis.
Most patients (70%) were “active” or “extremely/very active,” 77% of patients adjusted their activities because of their hemophilia, and nearly half of patients stopped activities because of their disease.
Fear drives adjustments in activity
Patients were sometimes more likely to adjust their activities based on fear of experiencing an event, as opposed to previously experiencing that event.
Specifically, 44% of patients adjusted their activities due to fear of joint damage, compared with 36% of patients who made adjustments because of past significant joint damage.
Similarly, 41% of patients adjusted activities due to fear of breakthrough bleeds, compared with 36% of patients who made adjustments because of past experience with bleeds and 25% who made adjustments because of significant past bleeds.
On the other hand, a similar percentage of patients adjusted activities because of past experience with pain (43%) and fear of pain (41%). And a similar percentage of patients adjusted activities because of existing joint damage restrictions (35%) and fear of joint deterioration (32%).
Past experience prompts discontinuation of activity
Overall, 47% of patients said anxiety was the most common emotional reason for stopping physical activities. However, patients were consistently more likely to stop activities because of past experience rather than fear or anxiety.
Specifically, 50% of patients stopped activities because of significant past joint damage, 46% stopped because of developing joint problems, and 38% stopped due to fear of joint damage.
More patients stopped activities because of significant past bleeds (41%) rather than fear of breakthrough bleeds (26%). More patients stopped activities because they developed chronic pain (38%) rather than fear of pain (less than 15%). And more patients stopped activities because of existing joint damage restrictions (62%) rather than fear of joint damage (34%).
Applying results to practice: Changing the conversation
Ideally, these findings would be used to promote individualized treatment of hemophilia driven by patients’ goals, Mr. Skinner said. By better understanding patients’ feelings and motivations, clinicians may devise more personalized treatment regimens that align with patients’ goals and improve their quality of life.
Rather than adjusting treatment based only on “hard metrics” such as bleeding events, “we need to take a more holistic approach to looking at outcomes that are more important to patients,” Mr. Skinner said. This type of approach is particularly important to Mr. Skinner as someone who has severe hemophilia A.
“Because hemophilia is a life-long disease, and you’re born with it, you make conscious or unconscious adaptations throughout your life,” he explained. “Your expectations or aspirations adjust to what you’ve been told you can or cannot do because of your hemophilia. The choices I made for my career, where I live, the type of vacations I go on, the type of sports I participate in have all been limited over the course of time, which has meant that I’ve made compromises. There are a lot of individuals with hemophilia who are making decisions that are not what their life goals are.
“What this research helps me understand is that we can change the conversation and build it around an individual patient and understand what their aspirations are. If a clinician understands what I’m wanting to achieve in life … we can build a treatment regime around helping me achieve those goals. That is known to improve adherence.
“The goal, really, is to have hemophilia as a secondary consideration. Instead of saying: ‘You have hemophilia, so these are the options available to you,’ you can say, ‘what is it that you would like to achieve, and then we’ll figure out how your treatment for hemophilia can be adjusted to help you achieve those goals.’ It may sound like a nuance, but it really is reversing the conversation. The goal setting first versus your disease comes first.”
The HemACTIVE study was supported by Bayer. Mr. Skinner disclosed relationships with Bayer and other pharmaceutical companies.
SOURCE: Skinner M et al. EAHAD 2020, Abstract P304.
Fear of negative events can drive changes in activity levels among patients with hemophilia A, results of the HemACTIVE study suggest.
Patients were more likely to adjust their level of physical activity due to fear of bleeding and joint damage rather than previously experienced bleeding or joint damage.
However, past experience was more likely than fear to make patients stop physical activities altogether.
Mark Skinner, of the Institute for Policy Advancement in Washington, D.C., and colleagues presented these findings in a poster from the annual congress of the European Association for Haemophilia and Allied Disorders.
Mr. Skinner, who is a hemophilia patient himself, said the goal of the HemACTIVE study is to better understand how hemophilia affects patients’ lives.
“We wanted to understand the limitations, challenges, and compromises of individuals living with hemophilia,” Mr. Skinner said. “What has motivated them or prevented them from living more full, active lives doing the kind of work, leisure, and social activities that those without hemophilia do? Is it treatment choice, is it satisfaction with treatment, is it fear?
“We wanted to do a comprehensive study that really looked at the intersection of treatment adherence and satisfaction, the emotional components that relate to those decisions, and the challenges and compromises so that we could better identify what we need to consider as patients think about either changing their therapy or changing their treatment regimen on existing therapy.”
Previous results from the HemACTIVE study showed that, although activity levels differed among hemophilia patients, all patients surveyed wanted greater activity levels, better protection from bleeding, better pain relief, and less-frequent infusions (EAHAD 2019, Abstract P084). In addition, patients who used factor VIII products with an extended half-life were more active and more likely to adhere to their prescribed treatment (ISTH 2019, Abstract PB0210).
The results reported at EAHAD 2020 focus on patients’ reasons for modifying physical activity. Patients and caregivers completed a screening phone interview, followed by a 25-minute, web-based questionnaire on patient activity.
There were 275 respondents – 194 patients with hemophilia A and 81 caregivers – from Canada, France, Germany, Italy, and the United States. Patients had severe (61%) or moderate (39%) hemophilia A, and most (67%) were receiving prophylaxis.
Most patients (70%) were “active” or “extremely/very active,” 77% of patients adjusted their activities because of their hemophilia, and nearly half of patients stopped activities because of their disease.
Fear drives adjustments in activity
Patients were sometimes more likely to adjust their activities based on fear of experiencing an event, as opposed to previously experiencing that event.
Specifically, 44% of patients adjusted their activities due to fear of joint damage, compared with 36% of patients who made adjustments because of past significant joint damage.
Similarly, 41% of patients adjusted activities due to fear of breakthrough bleeds, compared with 36% of patients who made adjustments because of past experience with bleeds and 25% who made adjustments because of significant past bleeds.
On the other hand, a similar percentage of patients adjusted activities because of past experience with pain (43%) and fear of pain (41%). And a similar percentage of patients adjusted activities because of existing joint damage restrictions (35%) and fear of joint deterioration (32%).
Past experience prompts discontinuation of activity
Overall, 47% of patients said anxiety was the most common emotional reason for stopping physical activities. However, patients were consistently more likely to stop activities because of past experience rather than fear or anxiety.
Specifically, 50% of patients stopped activities because of significant past joint damage, 46% stopped because of developing joint problems, and 38% stopped due to fear of joint damage.
More patients stopped activities because of significant past bleeds (41%) rather than fear of breakthrough bleeds (26%). More patients stopped activities because they developed chronic pain (38%) rather than fear of pain (less than 15%). And more patients stopped activities because of existing joint damage restrictions (62%) rather than fear of joint damage (34%).
Applying results to practice: Changing the conversation
Ideally, these findings would be used to promote individualized treatment of hemophilia driven by patients’ goals, Mr. Skinner said. By better understanding patients’ feelings and motivations, clinicians may devise more personalized treatment regimens that align with patients’ goals and improve their quality of life.
Rather than adjusting treatment based only on “hard metrics” such as bleeding events, “we need to take a more holistic approach to looking at outcomes that are more important to patients,” Mr. Skinner said. This type of approach is particularly important to Mr. Skinner as someone who has severe hemophilia A.
“Because hemophilia is a life-long disease, and you’re born with it, you make conscious or unconscious adaptations throughout your life,” he explained. “Your expectations or aspirations adjust to what you’ve been told you can or cannot do because of your hemophilia. The choices I made for my career, where I live, the type of vacations I go on, the type of sports I participate in have all been limited over the course of time, which has meant that I’ve made compromises. There are a lot of individuals with hemophilia who are making decisions that are not what their life goals are.
“What this research helps me understand is that we can change the conversation and build it around an individual patient and understand what their aspirations are. If a clinician understands what I’m wanting to achieve in life … we can build a treatment regime around helping me achieve those goals. That is known to improve adherence.
“The goal, really, is to have hemophilia as a secondary consideration. Instead of saying: ‘You have hemophilia, so these are the options available to you,’ you can say, ‘what is it that you would like to achieve, and then we’ll figure out how your treatment for hemophilia can be adjusted to help you achieve those goals.’ It may sound like a nuance, but it really is reversing the conversation. The goal setting first versus your disease comes first.”
The HemACTIVE study was supported by Bayer. Mr. Skinner disclosed relationships with Bayer and other pharmaceutical companies.
SOURCE: Skinner M et al. EAHAD 2020, Abstract P304.
Fear of negative events can drive changes in activity levels among patients with hemophilia A, results of the HemACTIVE study suggest.
Patients were more likely to adjust their level of physical activity due to fear of bleeding and joint damage rather than previously experienced bleeding or joint damage.
However, past experience was more likely than fear to make patients stop physical activities altogether.
Mark Skinner, of the Institute for Policy Advancement in Washington, D.C., and colleagues presented these findings in a poster from the annual congress of the European Association for Haemophilia and Allied Disorders.
Mr. Skinner, who is a hemophilia patient himself, said the goal of the HemACTIVE study is to better understand how hemophilia affects patients’ lives.
“We wanted to understand the limitations, challenges, and compromises of individuals living with hemophilia,” Mr. Skinner said. “What has motivated them or prevented them from living more full, active lives doing the kind of work, leisure, and social activities that those without hemophilia do? Is it treatment choice, is it satisfaction with treatment, is it fear?
“We wanted to do a comprehensive study that really looked at the intersection of treatment adherence and satisfaction, the emotional components that relate to those decisions, and the challenges and compromises so that we could better identify what we need to consider as patients think about either changing their therapy or changing their treatment regimen on existing therapy.”
Previous results from the HemACTIVE study showed that, although activity levels differed among hemophilia patients, all patients surveyed wanted greater activity levels, better protection from bleeding, better pain relief, and less-frequent infusions (EAHAD 2019, Abstract P084). In addition, patients who used factor VIII products with an extended half-life were more active and more likely to adhere to their prescribed treatment (ISTH 2019, Abstract PB0210).
The results reported at EAHAD 2020 focus on patients’ reasons for modifying physical activity. Patients and caregivers completed a screening phone interview, followed by a 25-minute, web-based questionnaire on patient activity.
There were 275 respondents – 194 patients with hemophilia A and 81 caregivers – from Canada, France, Germany, Italy, and the United States. Patients had severe (61%) or moderate (39%) hemophilia A, and most (67%) were receiving prophylaxis.
Most patients (70%) were “active” or “extremely/very active,” 77% of patients adjusted their activities because of their hemophilia, and nearly half of patients stopped activities because of their disease.
Fear drives adjustments in activity
Patients were sometimes more likely to adjust their activities based on fear of experiencing an event, as opposed to previously experiencing that event.
Specifically, 44% of patients adjusted their activities due to fear of joint damage, compared with 36% of patients who made adjustments because of past significant joint damage.
Similarly, 41% of patients adjusted activities due to fear of breakthrough bleeds, compared with 36% of patients who made adjustments because of past experience with bleeds and 25% who made adjustments because of significant past bleeds.
On the other hand, a similar percentage of patients adjusted activities because of past experience with pain (43%) and fear of pain (41%). And a similar percentage of patients adjusted activities because of existing joint damage restrictions (35%) and fear of joint deterioration (32%).
Past experience prompts discontinuation of activity
Overall, 47% of patients said anxiety was the most common emotional reason for stopping physical activities. However, patients were consistently more likely to stop activities because of past experience rather than fear or anxiety.
Specifically, 50% of patients stopped activities because of significant past joint damage, 46% stopped because of developing joint problems, and 38% stopped due to fear of joint damage.
More patients stopped activities because of significant past bleeds (41%) rather than fear of breakthrough bleeds (26%). More patients stopped activities because they developed chronic pain (38%) rather than fear of pain (less than 15%). And more patients stopped activities because of existing joint damage restrictions (62%) rather than fear of joint damage (34%).
Applying results to practice: Changing the conversation
Ideally, these findings would be used to promote individualized treatment of hemophilia driven by patients’ goals, Mr. Skinner said. By better understanding patients’ feelings and motivations, clinicians may devise more personalized treatment regimens that align with patients’ goals and improve their quality of life.
Rather than adjusting treatment based only on “hard metrics” such as bleeding events, “we need to take a more holistic approach to looking at outcomes that are more important to patients,” Mr. Skinner said. This type of approach is particularly important to Mr. Skinner as someone who has severe hemophilia A.
“Because hemophilia is a life-long disease, and you’re born with it, you make conscious or unconscious adaptations throughout your life,” he explained. “Your expectations or aspirations adjust to what you’ve been told you can or cannot do because of your hemophilia. The choices I made for my career, where I live, the type of vacations I go on, the type of sports I participate in have all been limited over the course of time, which has meant that I’ve made compromises. There are a lot of individuals with hemophilia who are making decisions that are not what their life goals are.
“What this research helps me understand is that we can change the conversation and build it around an individual patient and understand what their aspirations are. If a clinician understands what I’m wanting to achieve in life … we can build a treatment regime around helping me achieve those goals. That is known to improve adherence.
“The goal, really, is to have hemophilia as a secondary consideration. Instead of saying: ‘You have hemophilia, so these are the options available to you,’ you can say, ‘what is it that you would like to achieve, and then we’ll figure out how your treatment for hemophilia can be adjusted to help you achieve those goals.’ It may sound like a nuance, but it really is reversing the conversation. The goal setting first versus your disease comes first.”
The HemACTIVE study was supported by Bayer. Mr. Skinner disclosed relationships with Bayer and other pharmaceutical companies.
SOURCE: Skinner M et al. EAHAD 2020, Abstract P304.
REPORTING FROM EAHAD 2020
CRISPR-engineered T cells may be safe for cancer, but do they work?
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
FROM SCIENCE
Global project reveals cancer’s genomic playbook
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
FROM NATURE