User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
High Rate of Rehospitalization After First Ischemic Stroke
TOPLINE:
Among patients hospitalized with a first ischemic stroke, 80% were rehospitalized, primarily because of subsequent primary cardiovascular and cerebrovascular diagnoses.
METHODOLOGY:
- To gather information on post-stroke hospital admission, investigators followed 1412 participants (mean age, 72.4 years; 52.1% women, 35.3% Black individuals) from the Atherosclerosis Risk in Communities (ARIC) study who were living in Maryland, Minnesota, North Carolina, and Mississippi.
- Participants were recruited between 1987 and 1989 when they were 45-64 years old and were followed on an annual and then semiannual basis from the index discharge until discharge after their second hospitalization, death, or end of the study in December 2019.
- Specific diagnoses for each hospitalization were based on hospital records, discharge diagnoses, and annual and semiannual phone interviews.
TAKEAWAY:
- During the study period, 1143 hospitalizations occurred over 41,849 person-months.
- 81% of participants were hospitalized over a maximum of 26.6 years of follow-up. Primary cardiovascular and cerebrovascular diagnoses were reported for half of readmissions.
- Over the follow-up period, compared with cardioembolic stroke, readmission risk was lower for thrombotic/lacunar stroke (adjusted hazard ratio [aHR], 0.82; 95% CI, 0.71-0.95) and hemorrhagic stroke (aHR, 0.74; 95% CI, 0.58-0.93). However, when adjusting for atrial fibrillation and competing risk for death, there were no significant differences between stroke subtypes.
- Compared with cardioembolic stroke, thrombotic/lacunar stroke was associated with lower readmission risk within 1 month (aHR, 0.66; 95% CI, 0.46-0.93) and from 1 month to 1 year (aHR, 0.78; 95% CI, 0.62-0.97), and hemorrhagic stroke was associated with lower risk from 1 month to 1 year (aHR, 0.60; 95% CI, 0.41-0.87).
IN PRACTICE:
“These results suggest that prevention strategies focused on cardiovascular and cerebrovascular health warrant further investigation, especially within the first year after incident stroke and perhaps particularly among individuals with an incident cardioembolic stroke,” the authors wrote.
SOURCE:
Kelly Sloane, MD, of the University of Pennsylvania Perelman School of Medicine in Philadelphia, led the study along with colleagues at the National Institute of Neurological Disorders and Stroke, Johns Hopkins University in Baltimore, and the University of North Carolina, Chapel Hill. The article was published online on January 5 in Neurology.
LIMITATIONS:
The ARIC study classification of stroke subtype grouped embolic strokes of undetermined source as thrombotic strokes, and investigators were unable to distinguish between the groups. In addition, there was no way to measure stroke severity, which could have played a role in readmission risk.
DISCLOSURES:
The study was funded by the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institutes of Health.
A version of this article appeared on Medscape.com.
TOPLINE:
Among patients hospitalized with a first ischemic stroke, 80% were rehospitalized, primarily because of subsequent primary cardiovascular and cerebrovascular diagnoses.
METHODOLOGY:
- To gather information on post-stroke hospital admission, investigators followed 1412 participants (mean age, 72.4 years; 52.1% women, 35.3% Black individuals) from the Atherosclerosis Risk in Communities (ARIC) study who were living in Maryland, Minnesota, North Carolina, and Mississippi.
- Participants were recruited between 1987 and 1989 when they were 45-64 years old and were followed on an annual and then semiannual basis from the index discharge until discharge after their second hospitalization, death, or end of the study in December 2019.
- Specific diagnoses for each hospitalization were based on hospital records, discharge diagnoses, and annual and semiannual phone interviews.
TAKEAWAY:
- During the study period, 1143 hospitalizations occurred over 41,849 person-months.
- 81% of participants were hospitalized over a maximum of 26.6 years of follow-up. Primary cardiovascular and cerebrovascular diagnoses were reported for half of readmissions.
- Over the follow-up period, compared with cardioembolic stroke, readmission risk was lower for thrombotic/lacunar stroke (adjusted hazard ratio [aHR], 0.82; 95% CI, 0.71-0.95) and hemorrhagic stroke (aHR, 0.74; 95% CI, 0.58-0.93). However, when adjusting for atrial fibrillation and competing risk for death, there were no significant differences between stroke subtypes.
- Compared with cardioembolic stroke, thrombotic/lacunar stroke was associated with lower readmission risk within 1 month (aHR, 0.66; 95% CI, 0.46-0.93) and from 1 month to 1 year (aHR, 0.78; 95% CI, 0.62-0.97), and hemorrhagic stroke was associated with lower risk from 1 month to 1 year (aHR, 0.60; 95% CI, 0.41-0.87).
IN PRACTICE:
“These results suggest that prevention strategies focused on cardiovascular and cerebrovascular health warrant further investigation, especially within the first year after incident stroke and perhaps particularly among individuals with an incident cardioembolic stroke,” the authors wrote.
SOURCE:
Kelly Sloane, MD, of the University of Pennsylvania Perelman School of Medicine in Philadelphia, led the study along with colleagues at the National Institute of Neurological Disorders and Stroke, Johns Hopkins University in Baltimore, and the University of North Carolina, Chapel Hill. The article was published online on January 5 in Neurology.
LIMITATIONS:
The ARIC study classification of stroke subtype grouped embolic strokes of undetermined source as thrombotic strokes, and investigators were unable to distinguish between the groups. In addition, there was no way to measure stroke severity, which could have played a role in readmission risk.
DISCLOSURES:
The study was funded by the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institutes of Health.
A version of this article appeared on Medscape.com.
TOPLINE:
Among patients hospitalized with a first ischemic stroke, 80% were rehospitalized, primarily because of subsequent primary cardiovascular and cerebrovascular diagnoses.
METHODOLOGY:
- To gather information on post-stroke hospital admission, investigators followed 1412 participants (mean age, 72.4 years; 52.1% women, 35.3% Black individuals) from the Atherosclerosis Risk in Communities (ARIC) study who were living in Maryland, Minnesota, North Carolina, and Mississippi.
- Participants were recruited between 1987 and 1989 when they were 45-64 years old and were followed on an annual and then semiannual basis from the index discharge until discharge after their second hospitalization, death, or end of the study in December 2019.
- Specific diagnoses for each hospitalization were based on hospital records, discharge diagnoses, and annual and semiannual phone interviews.
TAKEAWAY:
- During the study period, 1143 hospitalizations occurred over 41,849 person-months.
- 81% of participants were hospitalized over a maximum of 26.6 years of follow-up. Primary cardiovascular and cerebrovascular diagnoses were reported for half of readmissions.
- Over the follow-up period, compared with cardioembolic stroke, readmission risk was lower for thrombotic/lacunar stroke (adjusted hazard ratio [aHR], 0.82; 95% CI, 0.71-0.95) and hemorrhagic stroke (aHR, 0.74; 95% CI, 0.58-0.93). However, when adjusting for atrial fibrillation and competing risk for death, there were no significant differences between stroke subtypes.
- Compared with cardioembolic stroke, thrombotic/lacunar stroke was associated with lower readmission risk within 1 month (aHR, 0.66; 95% CI, 0.46-0.93) and from 1 month to 1 year (aHR, 0.78; 95% CI, 0.62-0.97), and hemorrhagic stroke was associated with lower risk from 1 month to 1 year (aHR, 0.60; 95% CI, 0.41-0.87).
IN PRACTICE:
“These results suggest that prevention strategies focused on cardiovascular and cerebrovascular health warrant further investigation, especially within the first year after incident stroke and perhaps particularly among individuals with an incident cardioembolic stroke,” the authors wrote.
SOURCE:
Kelly Sloane, MD, of the University of Pennsylvania Perelman School of Medicine in Philadelphia, led the study along with colleagues at the National Institute of Neurological Disorders and Stroke, Johns Hopkins University in Baltimore, and the University of North Carolina, Chapel Hill. The article was published online on January 5 in Neurology.
LIMITATIONS:
The ARIC study classification of stroke subtype grouped embolic strokes of undetermined source as thrombotic strokes, and investigators were unable to distinguish between the groups. In addition, there was no way to measure stroke severity, which could have played a role in readmission risk.
DISCLOSURES:
The study was funded by the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institutes of Health.
A version of this article appeared on Medscape.com.
Researchers Uncover Nanoplastics in Water Bottles
Using an advanced microscopic technique, American researchers have detected 100,000 nanoplastic molecules per liter of water in plastic bottles. Because of their small size, these particles can enter the bloodstream, cells, and the brain, thus posing potential health risks. The study, recently published in the Proceedings of the National Academy of Sciences, raises concerns about the impact of these nanoparticles.
An Unknown Realm
Formed as plastics break down into increasingly small pieces, these particles are consumed by humans and other organisms, with unknown effects on health and ecosystems. Whereas macroplastics have been found in various organs, including the lungs and liver, the study marks a unique exploration into the world of nanoplastics.
Concerns about nanoplastic presence in humans intensified when a 2018 study revealed contamination signs in 93% of 259 examined bottles from nine countries.
The novelty of this research lies in its focus, using a refined spectrometry method, on the poorly understood world of nanoplastics, which derive from the decomposition of microplastics. For the first time, American researchers, including biophysicists and chemists, counted and identified these tiny particles in bottled water. On average, they found around 240,000 detectable plastic fragments per liter, which is 10-100 times more than previous estimates based on larger sizes.
Microplastics are defined as fragments ranging from 5 mm to 1 µm, whereas nanoplastics, particles < 1 µm, are measured in billionths of a meter.
In contrast to microplastics, nanoplastics are so small that they can traverse the intestines and lungs and move directly into the bloodstream, reaching organs such as the heart or brain or even the fetus via the placenta.
“This was previously an obscure, unexplored area. Toxicity studies could only speculate about what was in there,” said Beizhan Yan, PhD, coauthor of the study and environmental chemist at the Lamont–Doherty Earth Observatory of Columbia University, New York. “This study opens a window for us to observe a world we were not exposed to before.”
90% Nanoplastics Found
The new study employed a technique called stimulated Raman scattering microscopy, which was invented by study coauthor Wei Min, a biophysicist at Columbia. This method involves probing samples simultaneously with two lasers tuned to resonate specific molecules.
Researchers tested three bottled water brands that are popular in the United States, analyzing plastic particles up to 100 nm in size. They identified 110,000-370,000 plastic particles per liter. About 90% were nanoplastics — which are invisible by standard imaging techniques — and the rest were microplastics. The study also identified the seven plastics involved.
The most common is polyamide, a type of nylon, likely from plastic filters purportedly used to purify water before bottling. Next is polyethylene terephthalate, which is commonly used for water bottles and other food containers. Researchers also found other common plastics, including polystyrene, polyvinyl chloride, and methyl methacrylate, used in various industrial processes.
Not Size But Quantity
What’s more concerning is that the seven types of plastics accounted for only about 10% of all nanoparticles found in the samples. Researchers have no idea about the composition of the remaining 90%. If these are all nanoparticles, their number could reach tens of millions per liter, representing the complex composition of seemingly simple water samples, as noted by the authors.
Researchers now plan to expand beyond bottled water, exploring the vast realm of nanoplastics. They emphasize that, in terms of mass, nanoplastics are far smaller than microplastics, but “it’s not about size. It’s about the numbers as smaller things can easily penetrate us.”
The team aims to study tap water, which also contains microplastics but in much smaller proportions than bottled water.
This article was translated from the Medscape French edition.
Using an advanced microscopic technique, American researchers have detected 100,000 nanoplastic molecules per liter of water in plastic bottles. Because of their small size, these particles can enter the bloodstream, cells, and the brain, thus posing potential health risks. The study, recently published in the Proceedings of the National Academy of Sciences, raises concerns about the impact of these nanoparticles.
An Unknown Realm
Formed as plastics break down into increasingly small pieces, these particles are consumed by humans and other organisms, with unknown effects on health and ecosystems. Whereas macroplastics have been found in various organs, including the lungs and liver, the study marks a unique exploration into the world of nanoplastics.
Concerns about nanoplastic presence in humans intensified when a 2018 study revealed contamination signs in 93% of 259 examined bottles from nine countries.
The novelty of this research lies in its focus, using a refined spectrometry method, on the poorly understood world of nanoplastics, which derive from the decomposition of microplastics. For the first time, American researchers, including biophysicists and chemists, counted and identified these tiny particles in bottled water. On average, they found around 240,000 detectable plastic fragments per liter, which is 10-100 times more than previous estimates based on larger sizes.
Microplastics are defined as fragments ranging from 5 mm to 1 µm, whereas nanoplastics, particles < 1 µm, are measured in billionths of a meter.
In contrast to microplastics, nanoplastics are so small that they can traverse the intestines and lungs and move directly into the bloodstream, reaching organs such as the heart or brain or even the fetus via the placenta.
“This was previously an obscure, unexplored area. Toxicity studies could only speculate about what was in there,” said Beizhan Yan, PhD, coauthor of the study and environmental chemist at the Lamont–Doherty Earth Observatory of Columbia University, New York. “This study opens a window for us to observe a world we were not exposed to before.”
90% Nanoplastics Found
The new study employed a technique called stimulated Raman scattering microscopy, which was invented by study coauthor Wei Min, a biophysicist at Columbia. This method involves probing samples simultaneously with two lasers tuned to resonate specific molecules.
Researchers tested three bottled water brands that are popular in the United States, analyzing plastic particles up to 100 nm in size. They identified 110,000-370,000 plastic particles per liter. About 90% were nanoplastics — which are invisible by standard imaging techniques — and the rest were microplastics. The study also identified the seven plastics involved.
The most common is polyamide, a type of nylon, likely from plastic filters purportedly used to purify water before bottling. Next is polyethylene terephthalate, which is commonly used for water bottles and other food containers. Researchers also found other common plastics, including polystyrene, polyvinyl chloride, and methyl methacrylate, used in various industrial processes.
Not Size But Quantity
What’s more concerning is that the seven types of plastics accounted for only about 10% of all nanoparticles found in the samples. Researchers have no idea about the composition of the remaining 90%. If these are all nanoparticles, their number could reach tens of millions per liter, representing the complex composition of seemingly simple water samples, as noted by the authors.
Researchers now plan to expand beyond bottled water, exploring the vast realm of nanoplastics. They emphasize that, in terms of mass, nanoplastics are far smaller than microplastics, but “it’s not about size. It’s about the numbers as smaller things can easily penetrate us.”
The team aims to study tap water, which also contains microplastics but in much smaller proportions than bottled water.
This article was translated from the Medscape French edition.
Using an advanced microscopic technique, American researchers have detected 100,000 nanoplastic molecules per liter of water in plastic bottles. Because of their small size, these particles can enter the bloodstream, cells, and the brain, thus posing potential health risks. The study, recently published in the Proceedings of the National Academy of Sciences, raises concerns about the impact of these nanoparticles.
An Unknown Realm
Formed as plastics break down into increasingly small pieces, these particles are consumed by humans and other organisms, with unknown effects on health and ecosystems. Whereas macroplastics have been found in various organs, including the lungs and liver, the study marks a unique exploration into the world of nanoplastics.
Concerns about nanoplastic presence in humans intensified when a 2018 study revealed contamination signs in 93% of 259 examined bottles from nine countries.
The novelty of this research lies in its focus, using a refined spectrometry method, on the poorly understood world of nanoplastics, which derive from the decomposition of microplastics. For the first time, American researchers, including biophysicists and chemists, counted and identified these tiny particles in bottled water. On average, they found around 240,000 detectable plastic fragments per liter, which is 10-100 times more than previous estimates based on larger sizes.
Microplastics are defined as fragments ranging from 5 mm to 1 µm, whereas nanoplastics, particles < 1 µm, are measured in billionths of a meter.
In contrast to microplastics, nanoplastics are so small that they can traverse the intestines and lungs and move directly into the bloodstream, reaching organs such as the heart or brain or even the fetus via the placenta.
“This was previously an obscure, unexplored area. Toxicity studies could only speculate about what was in there,” said Beizhan Yan, PhD, coauthor of the study and environmental chemist at the Lamont–Doherty Earth Observatory of Columbia University, New York. “This study opens a window for us to observe a world we were not exposed to before.”
90% Nanoplastics Found
The new study employed a technique called stimulated Raman scattering microscopy, which was invented by study coauthor Wei Min, a biophysicist at Columbia. This method involves probing samples simultaneously with two lasers tuned to resonate specific molecules.
Researchers tested three bottled water brands that are popular in the United States, analyzing plastic particles up to 100 nm in size. They identified 110,000-370,000 plastic particles per liter. About 90% were nanoplastics — which are invisible by standard imaging techniques — and the rest were microplastics. The study also identified the seven plastics involved.
The most common is polyamide, a type of nylon, likely from plastic filters purportedly used to purify water before bottling. Next is polyethylene terephthalate, which is commonly used for water bottles and other food containers. Researchers also found other common plastics, including polystyrene, polyvinyl chloride, and methyl methacrylate, used in various industrial processes.
Not Size But Quantity
What’s more concerning is that the seven types of plastics accounted for only about 10% of all nanoparticles found in the samples. Researchers have no idea about the composition of the remaining 90%. If these are all nanoparticles, their number could reach tens of millions per liter, representing the complex composition of seemingly simple water samples, as noted by the authors.
Researchers now plan to expand beyond bottled water, exploring the vast realm of nanoplastics. They emphasize that, in terms of mass, nanoplastics are far smaller than microplastics, but “it’s not about size. It’s about the numbers as smaller things can easily penetrate us.”
The team aims to study tap water, which also contains microplastics but in much smaller proportions than bottled water.
This article was translated from the Medscape French edition.
FROM THE PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Why Don’t Physicians Call In Sick?
I began practicing medicine on July 1, 1981. In the 43-plus years since then,
There are several reasons, both good and bad, why this is so: (1) like most physicians, I am a terrible patient; (2) as a solo practitioner, there was (until recently — I’ll get to that in a minute) no one else to see an office full of patients who had waited significant amounts of time for their appointments and in many cases had taken off work themselves to keep them; and (3) there is an unspoken rule against it. Taking sick days is highly frowned upon in the medical world. As a medical student, intern, and resident I was told in so many words not to call in sick, no matter how serious the illness might be.
Apparently, I was not the only doctor-in-training to receive that message. In a survey reported in JAMA Pediatrics several years ago, 95% of the physicians and advanced practice clinicians (APCs) surveyed believed that working while sick put patients at risk — yet 83% reported working sick at least one time over the prior year. They understood the risks, but did it anyway.
There is no question that this practice does put patients’ health at risk. The JAMA study linked numerous reports of outbreaks traceable to symptomatic healthcare workers. Some outbreaks of flu, staph infections, norovirus, and pertussis were shown to originate from a sick physician or supporting staff member. These associations have led to increased morbidity and mortality, as well as excess costs. Those of us who treat immunocompromised patients on a regular basis risk inducing a life-threatening illness by unnecessarily exposing them to pathogens.
The JAMA survey results also confirmed my own observation that many physicians feel boxed in by their institutions or practice situations. “The study illustrates the complex social and logistic factors that cause this behavior,” the authors wrote. “These results may inform efforts to design systems at our hospital to provide support for attending physicians and APCs and help them make the right choice to keep their patients and colleagues safe while caring for themselves.”
What might those efforts look like? For one thing, we can take the obvious and necessary steps to avoid getting sick in the first place, such as staying fit and hydrated, and eating well. We can keep up with routine health visits and measures such as colorectal screening, pap smears, and mammograms, and stay up to date with flu shots and all other essential immunizations.
Next, we can minimize the risk of spreading any illnesses we encounter in the course of our work by practicing the basic infectious disease prevention measures driven home so forcefully by the recent COVID-19 pandemic — washing our hands, using hand sanitizers, and, when appropriate, wearing gloves and masks.
Finally, we can work to overcome this institutional taboo against staying home when we do get sick. Work out a system of mutual coverage for such situations. Two years ago, I merged my solo practice with a local, larger group. I did it for a variety of reasons, but a principal one was to assure that a partner could cover for me if I became ill. Practitioners who choose to remain solo or in small groups should contact colleagues and work out a coverage agreement.
Now, during flu season, it is especially important to resist the temptation to work while sick. The CDC has guidelines for employees specific for the flu, which notes that “persons with the flu are most contagious during the first 3 days of their illness,” and should remain at home until at least 24 hours after their fever subsides (without the use of fever-reducing medications) or after symptoms have improved (at least 4-5 days after they started) — or, if they do not have a fever, after symptoms improve “for at least 4-5 days after the onset of symptoms.”
Of course, we need to remember that COVID-19 is still with us. With the constant evolution of new strains, it is especially important to avoid exposing patients and colleagues to the disease should you become infected. The most recent advice from the CDC includes the recommendation that those who are mildly ill and not moderately or severely immunocompromised should isolate after SARS-CoV-2 infection for at least 5 days after symptom onset (day 0 is the day symptoms appeared, and day 1 is the next full day thereafter) if fever has resolved for at least 24 hours (without taking fever-reducing medications) and other symptoms are improving. In addition, “a high-quality mask should be worn around others at home and in public through day 10.”
We should also extend these rules to our support staff, starting with providing them with adequate sick leave and encouraging them to use it when necessary. Research has found a direct correlation between preventative health care and the number of paid sick leave days a worker gets. In a study of over 3000 US workers, those with 10 paid sick days or more annually accessed preventative care more frequently than those without paid sick days.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
I began practicing medicine on July 1, 1981. In the 43-plus years since then,
There are several reasons, both good and bad, why this is so: (1) like most physicians, I am a terrible patient; (2) as a solo practitioner, there was (until recently — I’ll get to that in a minute) no one else to see an office full of patients who had waited significant amounts of time for their appointments and in many cases had taken off work themselves to keep them; and (3) there is an unspoken rule against it. Taking sick days is highly frowned upon in the medical world. As a medical student, intern, and resident I was told in so many words not to call in sick, no matter how serious the illness might be.
Apparently, I was not the only doctor-in-training to receive that message. In a survey reported in JAMA Pediatrics several years ago, 95% of the physicians and advanced practice clinicians (APCs) surveyed believed that working while sick put patients at risk — yet 83% reported working sick at least one time over the prior year. They understood the risks, but did it anyway.
There is no question that this practice does put patients’ health at risk. The JAMA study linked numerous reports of outbreaks traceable to symptomatic healthcare workers. Some outbreaks of flu, staph infections, norovirus, and pertussis were shown to originate from a sick physician or supporting staff member. These associations have led to increased morbidity and mortality, as well as excess costs. Those of us who treat immunocompromised patients on a regular basis risk inducing a life-threatening illness by unnecessarily exposing them to pathogens.
The JAMA survey results also confirmed my own observation that many physicians feel boxed in by their institutions or practice situations. “The study illustrates the complex social and logistic factors that cause this behavior,” the authors wrote. “These results may inform efforts to design systems at our hospital to provide support for attending physicians and APCs and help them make the right choice to keep their patients and colleagues safe while caring for themselves.”
What might those efforts look like? For one thing, we can take the obvious and necessary steps to avoid getting sick in the first place, such as staying fit and hydrated, and eating well. We can keep up with routine health visits and measures such as colorectal screening, pap smears, and mammograms, and stay up to date with flu shots and all other essential immunizations.
Next, we can minimize the risk of spreading any illnesses we encounter in the course of our work by practicing the basic infectious disease prevention measures driven home so forcefully by the recent COVID-19 pandemic — washing our hands, using hand sanitizers, and, when appropriate, wearing gloves and masks.
Finally, we can work to overcome this institutional taboo against staying home when we do get sick. Work out a system of mutual coverage for such situations. Two years ago, I merged my solo practice with a local, larger group. I did it for a variety of reasons, but a principal one was to assure that a partner could cover for me if I became ill. Practitioners who choose to remain solo or in small groups should contact colleagues and work out a coverage agreement.
Now, during flu season, it is especially important to resist the temptation to work while sick. The CDC has guidelines for employees specific for the flu, which notes that “persons with the flu are most contagious during the first 3 days of their illness,” and should remain at home until at least 24 hours after their fever subsides (without the use of fever-reducing medications) or after symptoms have improved (at least 4-5 days after they started) — or, if they do not have a fever, after symptoms improve “for at least 4-5 days after the onset of symptoms.”
Of course, we need to remember that COVID-19 is still with us. With the constant evolution of new strains, it is especially important to avoid exposing patients and colleagues to the disease should you become infected. The most recent advice from the CDC includes the recommendation that those who are mildly ill and not moderately or severely immunocompromised should isolate after SARS-CoV-2 infection for at least 5 days after symptom onset (day 0 is the day symptoms appeared, and day 1 is the next full day thereafter) if fever has resolved for at least 24 hours (without taking fever-reducing medications) and other symptoms are improving. In addition, “a high-quality mask should be worn around others at home and in public through day 10.”
We should also extend these rules to our support staff, starting with providing them with adequate sick leave and encouraging them to use it when necessary. Research has found a direct correlation between preventative health care and the number of paid sick leave days a worker gets. In a study of over 3000 US workers, those with 10 paid sick days or more annually accessed preventative care more frequently than those without paid sick days.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
I began practicing medicine on July 1, 1981. In the 43-plus years since then,
There are several reasons, both good and bad, why this is so: (1) like most physicians, I am a terrible patient; (2) as a solo practitioner, there was (until recently — I’ll get to that in a minute) no one else to see an office full of patients who had waited significant amounts of time for their appointments and in many cases had taken off work themselves to keep them; and (3) there is an unspoken rule against it. Taking sick days is highly frowned upon in the medical world. As a medical student, intern, and resident I was told in so many words not to call in sick, no matter how serious the illness might be.
Apparently, I was not the only doctor-in-training to receive that message. In a survey reported in JAMA Pediatrics several years ago, 95% of the physicians and advanced practice clinicians (APCs) surveyed believed that working while sick put patients at risk — yet 83% reported working sick at least one time over the prior year. They understood the risks, but did it anyway.
There is no question that this practice does put patients’ health at risk. The JAMA study linked numerous reports of outbreaks traceable to symptomatic healthcare workers. Some outbreaks of flu, staph infections, norovirus, and pertussis were shown to originate from a sick physician or supporting staff member. These associations have led to increased morbidity and mortality, as well as excess costs. Those of us who treat immunocompromised patients on a regular basis risk inducing a life-threatening illness by unnecessarily exposing them to pathogens.
The JAMA survey results also confirmed my own observation that many physicians feel boxed in by their institutions or practice situations. “The study illustrates the complex social and logistic factors that cause this behavior,” the authors wrote. “These results may inform efforts to design systems at our hospital to provide support for attending physicians and APCs and help them make the right choice to keep their patients and colleagues safe while caring for themselves.”
What might those efforts look like? For one thing, we can take the obvious and necessary steps to avoid getting sick in the first place, such as staying fit and hydrated, and eating well. We can keep up with routine health visits and measures such as colorectal screening, pap smears, and mammograms, and stay up to date with flu shots and all other essential immunizations.
Next, we can minimize the risk of spreading any illnesses we encounter in the course of our work by practicing the basic infectious disease prevention measures driven home so forcefully by the recent COVID-19 pandemic — washing our hands, using hand sanitizers, and, when appropriate, wearing gloves and masks.
Finally, we can work to overcome this institutional taboo against staying home when we do get sick. Work out a system of mutual coverage for such situations. Two years ago, I merged my solo practice with a local, larger group. I did it for a variety of reasons, but a principal one was to assure that a partner could cover for me if I became ill. Practitioners who choose to remain solo or in small groups should contact colleagues and work out a coverage agreement.
Now, during flu season, it is especially important to resist the temptation to work while sick. The CDC has guidelines for employees specific for the flu, which notes that “persons with the flu are most contagious during the first 3 days of their illness,” and should remain at home until at least 24 hours after their fever subsides (without the use of fever-reducing medications) or after symptoms have improved (at least 4-5 days after they started) — or, if they do not have a fever, after symptoms improve “for at least 4-5 days after the onset of symptoms.”
Of course, we need to remember that COVID-19 is still with us. With the constant evolution of new strains, it is especially important to avoid exposing patients and colleagues to the disease should you become infected. The most recent advice from the CDC includes the recommendation that those who are mildly ill and not moderately or severely immunocompromised should isolate after SARS-CoV-2 infection for at least 5 days after symptom onset (day 0 is the day symptoms appeared, and day 1 is the next full day thereafter) if fever has resolved for at least 24 hours (without taking fever-reducing medications) and other symptoms are improving. In addition, “a high-quality mask should be worn around others at home and in public through day 10.”
We should also extend these rules to our support staff, starting with providing them with adequate sick leave and encouraging them to use it when necessary. Research has found a direct correlation between preventative health care and the number of paid sick leave days a worker gets. In a study of over 3000 US workers, those with 10 paid sick days or more annually accessed preventative care more frequently than those without paid sick days.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Smoking Associated With Increased Risk for Hair Loss Among Men
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Traumatic Brain Injury and CVD: What’s the Link?
The long-term impact of traumatic brain injury (TBI) on neurologic and psychiatric function is well-established, but a growing body of research is pointing to unexpected medical sequalae, including cardiovascular disease (CVD).
A recent review looked at the investigation to date into this surprising connection, not only summarizing study findings but also suggesting potential mechanisms that might account for the association.
“; consequently, they should undergo regular monitoring,” senior author Ross Zafonte, DO, president of Spaulding Rehabilitation Network, Boston, and lead author Saef Izzy, MD, MBChB, a neurologist at the Stroke and Cerebrovascular Center of Brigham and Women’s Hospital, Boston, Massachusetts, told this news organization.
“This holds significant importance for healthcare practitioners, as there exist several strategies to mitigate cardiovascular disease risk — including weight management, adopting a healthy diet, engaging in regular physical activity, and quitting smoking,” they stated.
Leslie Croll, MD, American Heart Association volunteer and assistant professor of clinical neurology at the Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, told this news organization that it’s “extremely important to learn more about the interplay between TBI, neurologic disease, psychiatric complications, and the cardiovascular system.”
Hopefully, she added, “future research will help us understand what kind of cardiovascular disease monitoring and prevention measures stand to give TBI patients the most benefit.”
Chronic Condition
TBI is “a major cause of long-term disability and premature death,” and is “highly prevalent among contact sports players, military personnel (eg, due to injuries sustained during conflict), and the general population (eg, due to falls and road traffic incidents),” the authors wrote.
Most studies pertaining to TBI have “primarily focused on establishing connections between single TBI, repetitive TBI, and their acute and chronic neurological and psychiatric consequences, such as Parkinson’s disease, Alzheimer’s disease, and chronic traumatic encephalopathy (CTE),” Drs. Zafonte and Izzy noted. By contrast, there has been a “notable lack of research attention given to non-neurological conditions associated with TBI.”
They pointed out that recent insights into TBI — particularly the acknowledgment of TBI as an “emerging chronic condition rather than merely an acute aftermath of brain injury” — have come to light through epidemiologic and pathologic investigations involving military veterans, professional American-style football players, and the civilian population. “This recognition opens up an opportunity to broaden our perspective and delve into the medical aspects of health that may be influenced by TBI.”
To broaden the investigation, the researchers reviewed literature published between January 1, 2001, and June 18, 2023. Of 26,335 articles, they narrowed their review down to 15 studies that investigated CVD, CVD risk factors, and cerebrovascular disease in the chronic phase of TBI, including community, military, or sport-related brain trauma, regardless of the timing of disease occurrence with respect to brain injury via TBI or repetitive head impact.
New Cardiovascular Risk
Studies that used national or local registries tended to be retrospective and predominantly conducted in people with preexisting cardiovascular conditions. In these studies, TBI was found to be an independent risk factor for myocardial dysfunction. However, although these studies do provide evidence of elevated cardiovascular risk subsequent to a single TBI, including individuals with preexisting medical comorbidities “makes it difficult to determine the timing of incident cardiovascular disease and cardiovascular risk factors subsequent to brain injury,” they wrote.
However, some studies showed that even individuals with TBI but without preexisting myocardial dysfunction at baseline had a significantly higher risk for CVD than those without a history of TBI.
In fact, several studies included populations without preexisting medical and cardiovascular comorbidities to “better refine the order and timing of CVD and other risk factors in individuals with TBI.”
For example, one study of concussion survivors without preexisting diagnoses showed that cardiovascular, endocrinological, and neuropsychiatric comorbidities occurred at a “significantly higher incidence within 5 years after concussive TBI compared with healthy individuals who were matched in terms of age, race, and sex and didn’t have a TBI exposure.” Other studies yielded similar findings.
Because cardiovascular risk factors and events become more common with age, it’s important to account for age in evaluating the effects of TBI. Although many studies of TBI and subsequent CVD didn’t stratify individuals by age, one 10-year study of people without any known cardiovascular or neuropsychiatric conditions who sustained TBI found that people as young as 18-40 years were more likely to develop hypertension, hyperlipidemia, obesity, and diabetes within 3-5 years following brain injury than matched individuals in the control group.
“Individuals who have encountered TBI, surprisingly even those who are young and in good health with no prior comorbid conditions, face an increased risk of adverse cardiovascular outcomes for an extended duration after the initial event,” Drs. Zafonte and Izzy summarized. “Therefore, it’s imperative that they receive regular and long-term screenings for CVD and associated risk factors.”
Bidirectional Relationship
Brain injury has been associated with acute cardiovascular dysfunction, including autonomic heart-brain axis dysregulation, imbalances between the sympathetic and parasympathetic nervous systems, and excessive catecholamine release, the authors noted.
Drs. Zafonte and Izzy suggested several plausible links between TBI and cardiovascular dysfunction, noting that they are “likely multifaceted, potentially encompassing risk factors that span the pre-injury, injury, and post-injury phases of the condition.”
TBI may induce alterations in neurobiological processes, which have been reported to be associated with an increased risk for CVD (eg, chronic dysfunction of the autonomic system, systemic inflammation, and modifications in the brain-gut connection).
Patients with TBI might develop additional risk factors following the injury, including conditions like posttraumatic stress disorder, depression, and other psychiatric illnesses, which are “known to augment the risk of CVD.”
TBI can lead to subsequent behavioral and lifestyle changes that place patients at an elevated risk for both cardiovascular and cognitive dysfunction when compared to the general population of TBI survivors.
There may be additional as yet undefined risks.
They believe there’s a bidirectional relationship between TBI and CVD. “On one hand, TBI has been associated with an elevated risk of CVD,” they said. “Conversely, cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and sleep disturbances that have been demonstrated to negatively influence cognitive function and heighten the risk of dementia. Consequently, this interplay can further compound the long-term consequences of the injury.”
Their work aims to try and disentangle this “complex series of relationships.”
They recommend screening to identify diseases in their earliest and “most manageable phases” because TBI has been “unveiled as an underappreciated risk factor for CVD within contact sports, military, and community setting.”
An effective screening program “should rely on quantifiable and dependable biomarkers such as blood pressure, BMI, waist circumference, blood lipid levels, and glucose. Additionally, it should take into account other factors like smoking habits, physical activity, and dietary choices,” they recommended.
Heart-Brain Connection
Dr. Croll noted that TBI is “associated with many poorly understood physiologic changes and complications, so it’s exciting to see research aimed at clarifying this chronic disease process.”
In recent years, “we have seen a greater appreciation and understanding of the heart-brain connection,” she said. “Moving forward, more research, including TBI research, will target that connection.”
She added that there are probably “multiple mechanisms” at play underlying the connection between TBI and CVD.
Most importantly, “we are increasingly learning that TBI is not only a discrete event that requires immediate treatment but also a chronic disease process,” and when we “think about the substantial long-term morbidity associated with TBI, we should keep increased risk for CVD on top of mind,” said Dr. Croll.
The review received no funding. Izzy reported receiving grants from the US National Institutes of Health (NIH) and 2023 Stepping Strong Innovator Award. Dr. Zafonte reported receiving grants from the NIH and royalties from Springer and Demos publishing for serving as a coeditor of Brain Injury Medicine. Dr. Zafonte has also served as an adviser to Myomo, Oncare.ai, Nanodiagnostics, and Kisbee. He reported evaluating patients in the Massachusetts General Hospital Brain and Body–TRUST Program, which is funded by the NFL Players Association. The other authors’ disclosures are listed on the original paper. Dr. Croll declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
The long-term impact of traumatic brain injury (TBI) on neurologic and psychiatric function is well-established, but a growing body of research is pointing to unexpected medical sequalae, including cardiovascular disease (CVD).
A recent review looked at the investigation to date into this surprising connection, not only summarizing study findings but also suggesting potential mechanisms that might account for the association.
“; consequently, they should undergo regular monitoring,” senior author Ross Zafonte, DO, president of Spaulding Rehabilitation Network, Boston, and lead author Saef Izzy, MD, MBChB, a neurologist at the Stroke and Cerebrovascular Center of Brigham and Women’s Hospital, Boston, Massachusetts, told this news organization.
“This holds significant importance for healthcare practitioners, as there exist several strategies to mitigate cardiovascular disease risk — including weight management, adopting a healthy diet, engaging in regular physical activity, and quitting smoking,” they stated.
Leslie Croll, MD, American Heart Association volunteer and assistant professor of clinical neurology at the Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, told this news organization that it’s “extremely important to learn more about the interplay between TBI, neurologic disease, psychiatric complications, and the cardiovascular system.”
Hopefully, she added, “future research will help us understand what kind of cardiovascular disease monitoring and prevention measures stand to give TBI patients the most benefit.”
Chronic Condition
TBI is “a major cause of long-term disability and premature death,” and is “highly prevalent among contact sports players, military personnel (eg, due to injuries sustained during conflict), and the general population (eg, due to falls and road traffic incidents),” the authors wrote.
Most studies pertaining to TBI have “primarily focused on establishing connections between single TBI, repetitive TBI, and their acute and chronic neurological and psychiatric consequences, such as Parkinson’s disease, Alzheimer’s disease, and chronic traumatic encephalopathy (CTE),” Drs. Zafonte and Izzy noted. By contrast, there has been a “notable lack of research attention given to non-neurological conditions associated with TBI.”
They pointed out that recent insights into TBI — particularly the acknowledgment of TBI as an “emerging chronic condition rather than merely an acute aftermath of brain injury” — have come to light through epidemiologic and pathologic investigations involving military veterans, professional American-style football players, and the civilian population. “This recognition opens up an opportunity to broaden our perspective and delve into the medical aspects of health that may be influenced by TBI.”
To broaden the investigation, the researchers reviewed literature published between January 1, 2001, and June 18, 2023. Of 26,335 articles, they narrowed their review down to 15 studies that investigated CVD, CVD risk factors, and cerebrovascular disease in the chronic phase of TBI, including community, military, or sport-related brain trauma, regardless of the timing of disease occurrence with respect to brain injury via TBI or repetitive head impact.
New Cardiovascular Risk
Studies that used national or local registries tended to be retrospective and predominantly conducted in people with preexisting cardiovascular conditions. In these studies, TBI was found to be an independent risk factor for myocardial dysfunction. However, although these studies do provide evidence of elevated cardiovascular risk subsequent to a single TBI, including individuals with preexisting medical comorbidities “makes it difficult to determine the timing of incident cardiovascular disease and cardiovascular risk factors subsequent to brain injury,” they wrote.
However, some studies showed that even individuals with TBI but without preexisting myocardial dysfunction at baseline had a significantly higher risk for CVD than those without a history of TBI.
In fact, several studies included populations without preexisting medical and cardiovascular comorbidities to “better refine the order and timing of CVD and other risk factors in individuals with TBI.”
For example, one study of concussion survivors without preexisting diagnoses showed that cardiovascular, endocrinological, and neuropsychiatric comorbidities occurred at a “significantly higher incidence within 5 years after concussive TBI compared with healthy individuals who were matched in terms of age, race, and sex and didn’t have a TBI exposure.” Other studies yielded similar findings.
Because cardiovascular risk factors and events become more common with age, it’s important to account for age in evaluating the effects of TBI. Although many studies of TBI and subsequent CVD didn’t stratify individuals by age, one 10-year study of people without any known cardiovascular or neuropsychiatric conditions who sustained TBI found that people as young as 18-40 years were more likely to develop hypertension, hyperlipidemia, obesity, and diabetes within 3-5 years following brain injury than matched individuals in the control group.
“Individuals who have encountered TBI, surprisingly even those who are young and in good health with no prior comorbid conditions, face an increased risk of adverse cardiovascular outcomes for an extended duration after the initial event,” Drs. Zafonte and Izzy summarized. “Therefore, it’s imperative that they receive regular and long-term screenings for CVD and associated risk factors.”
Bidirectional Relationship
Brain injury has been associated with acute cardiovascular dysfunction, including autonomic heart-brain axis dysregulation, imbalances between the sympathetic and parasympathetic nervous systems, and excessive catecholamine release, the authors noted.
Drs. Zafonte and Izzy suggested several plausible links between TBI and cardiovascular dysfunction, noting that they are “likely multifaceted, potentially encompassing risk factors that span the pre-injury, injury, and post-injury phases of the condition.”
TBI may induce alterations in neurobiological processes, which have been reported to be associated with an increased risk for CVD (eg, chronic dysfunction of the autonomic system, systemic inflammation, and modifications in the brain-gut connection).
Patients with TBI might develop additional risk factors following the injury, including conditions like posttraumatic stress disorder, depression, and other psychiatric illnesses, which are “known to augment the risk of CVD.”
TBI can lead to subsequent behavioral and lifestyle changes that place patients at an elevated risk for both cardiovascular and cognitive dysfunction when compared to the general population of TBI survivors.
There may be additional as yet undefined risks.
They believe there’s a bidirectional relationship between TBI and CVD. “On one hand, TBI has been associated with an elevated risk of CVD,” they said. “Conversely, cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and sleep disturbances that have been demonstrated to negatively influence cognitive function and heighten the risk of dementia. Consequently, this interplay can further compound the long-term consequences of the injury.”
Their work aims to try and disentangle this “complex series of relationships.”
They recommend screening to identify diseases in their earliest and “most manageable phases” because TBI has been “unveiled as an underappreciated risk factor for CVD within contact sports, military, and community setting.”
An effective screening program “should rely on quantifiable and dependable biomarkers such as blood pressure, BMI, waist circumference, blood lipid levels, and glucose. Additionally, it should take into account other factors like smoking habits, physical activity, and dietary choices,” they recommended.
Heart-Brain Connection
Dr. Croll noted that TBI is “associated with many poorly understood physiologic changes and complications, so it’s exciting to see research aimed at clarifying this chronic disease process.”
In recent years, “we have seen a greater appreciation and understanding of the heart-brain connection,” she said. “Moving forward, more research, including TBI research, will target that connection.”
She added that there are probably “multiple mechanisms” at play underlying the connection between TBI and CVD.
Most importantly, “we are increasingly learning that TBI is not only a discrete event that requires immediate treatment but also a chronic disease process,” and when we “think about the substantial long-term morbidity associated with TBI, we should keep increased risk for CVD on top of mind,” said Dr. Croll.
The review received no funding. Izzy reported receiving grants from the US National Institutes of Health (NIH) and 2023 Stepping Strong Innovator Award. Dr. Zafonte reported receiving grants from the NIH and royalties from Springer and Demos publishing for serving as a coeditor of Brain Injury Medicine. Dr. Zafonte has also served as an adviser to Myomo, Oncare.ai, Nanodiagnostics, and Kisbee. He reported evaluating patients in the Massachusetts General Hospital Brain and Body–TRUST Program, which is funded by the NFL Players Association. The other authors’ disclosures are listed on the original paper. Dr. Croll declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
The long-term impact of traumatic brain injury (TBI) on neurologic and psychiatric function is well-established, but a growing body of research is pointing to unexpected medical sequalae, including cardiovascular disease (CVD).
A recent review looked at the investigation to date into this surprising connection, not only summarizing study findings but also suggesting potential mechanisms that might account for the association.
“; consequently, they should undergo regular monitoring,” senior author Ross Zafonte, DO, president of Spaulding Rehabilitation Network, Boston, and lead author Saef Izzy, MD, MBChB, a neurologist at the Stroke and Cerebrovascular Center of Brigham and Women’s Hospital, Boston, Massachusetts, told this news organization.
“This holds significant importance for healthcare practitioners, as there exist several strategies to mitigate cardiovascular disease risk — including weight management, adopting a healthy diet, engaging in regular physical activity, and quitting smoking,” they stated.
Leslie Croll, MD, American Heart Association volunteer and assistant professor of clinical neurology at the Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, told this news organization that it’s “extremely important to learn more about the interplay between TBI, neurologic disease, psychiatric complications, and the cardiovascular system.”
Hopefully, she added, “future research will help us understand what kind of cardiovascular disease monitoring and prevention measures stand to give TBI patients the most benefit.”
Chronic Condition
TBI is “a major cause of long-term disability and premature death,” and is “highly prevalent among contact sports players, military personnel (eg, due to injuries sustained during conflict), and the general population (eg, due to falls and road traffic incidents),” the authors wrote.
Most studies pertaining to TBI have “primarily focused on establishing connections between single TBI, repetitive TBI, and their acute and chronic neurological and psychiatric consequences, such as Parkinson’s disease, Alzheimer’s disease, and chronic traumatic encephalopathy (CTE),” Drs. Zafonte and Izzy noted. By contrast, there has been a “notable lack of research attention given to non-neurological conditions associated with TBI.”
They pointed out that recent insights into TBI — particularly the acknowledgment of TBI as an “emerging chronic condition rather than merely an acute aftermath of brain injury” — have come to light through epidemiologic and pathologic investigations involving military veterans, professional American-style football players, and the civilian population. “This recognition opens up an opportunity to broaden our perspective and delve into the medical aspects of health that may be influenced by TBI.”
To broaden the investigation, the researchers reviewed literature published between January 1, 2001, and June 18, 2023. Of 26,335 articles, they narrowed their review down to 15 studies that investigated CVD, CVD risk factors, and cerebrovascular disease in the chronic phase of TBI, including community, military, or sport-related brain trauma, regardless of the timing of disease occurrence with respect to brain injury via TBI or repetitive head impact.
New Cardiovascular Risk
Studies that used national or local registries tended to be retrospective and predominantly conducted in people with preexisting cardiovascular conditions. In these studies, TBI was found to be an independent risk factor for myocardial dysfunction. However, although these studies do provide evidence of elevated cardiovascular risk subsequent to a single TBI, including individuals with preexisting medical comorbidities “makes it difficult to determine the timing of incident cardiovascular disease and cardiovascular risk factors subsequent to brain injury,” they wrote.
However, some studies showed that even individuals with TBI but without preexisting myocardial dysfunction at baseline had a significantly higher risk for CVD than those without a history of TBI.
In fact, several studies included populations without preexisting medical and cardiovascular comorbidities to “better refine the order and timing of CVD and other risk factors in individuals with TBI.”
For example, one study of concussion survivors without preexisting diagnoses showed that cardiovascular, endocrinological, and neuropsychiatric comorbidities occurred at a “significantly higher incidence within 5 years after concussive TBI compared with healthy individuals who were matched in terms of age, race, and sex and didn’t have a TBI exposure.” Other studies yielded similar findings.
Because cardiovascular risk factors and events become more common with age, it’s important to account for age in evaluating the effects of TBI. Although many studies of TBI and subsequent CVD didn’t stratify individuals by age, one 10-year study of people without any known cardiovascular or neuropsychiatric conditions who sustained TBI found that people as young as 18-40 years were more likely to develop hypertension, hyperlipidemia, obesity, and diabetes within 3-5 years following brain injury than matched individuals in the control group.
“Individuals who have encountered TBI, surprisingly even those who are young and in good health with no prior comorbid conditions, face an increased risk of adverse cardiovascular outcomes for an extended duration after the initial event,” Drs. Zafonte and Izzy summarized. “Therefore, it’s imperative that they receive regular and long-term screenings for CVD and associated risk factors.”
Bidirectional Relationship
Brain injury has been associated with acute cardiovascular dysfunction, including autonomic heart-brain axis dysregulation, imbalances between the sympathetic and parasympathetic nervous systems, and excessive catecholamine release, the authors noted.
Drs. Zafonte and Izzy suggested several plausible links between TBI and cardiovascular dysfunction, noting that they are “likely multifaceted, potentially encompassing risk factors that span the pre-injury, injury, and post-injury phases of the condition.”
TBI may induce alterations in neurobiological processes, which have been reported to be associated with an increased risk for CVD (eg, chronic dysfunction of the autonomic system, systemic inflammation, and modifications in the brain-gut connection).
Patients with TBI might develop additional risk factors following the injury, including conditions like posttraumatic stress disorder, depression, and other psychiatric illnesses, which are “known to augment the risk of CVD.”
TBI can lead to subsequent behavioral and lifestyle changes that place patients at an elevated risk for both cardiovascular and cognitive dysfunction when compared to the general population of TBI survivors.
There may be additional as yet undefined risks.
They believe there’s a bidirectional relationship between TBI and CVD. “On one hand, TBI has been associated with an elevated risk of CVD,” they said. “Conversely, cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and sleep disturbances that have been demonstrated to negatively influence cognitive function and heighten the risk of dementia. Consequently, this interplay can further compound the long-term consequences of the injury.”
Their work aims to try and disentangle this “complex series of relationships.”
They recommend screening to identify diseases in their earliest and “most manageable phases” because TBI has been “unveiled as an underappreciated risk factor for CVD within contact sports, military, and community setting.”
An effective screening program “should rely on quantifiable and dependable biomarkers such as blood pressure, BMI, waist circumference, blood lipid levels, and glucose. Additionally, it should take into account other factors like smoking habits, physical activity, and dietary choices,” they recommended.
Heart-Brain Connection
Dr. Croll noted that TBI is “associated with many poorly understood physiologic changes and complications, so it’s exciting to see research aimed at clarifying this chronic disease process.”
In recent years, “we have seen a greater appreciation and understanding of the heart-brain connection,” she said. “Moving forward, more research, including TBI research, will target that connection.”
She added that there are probably “multiple mechanisms” at play underlying the connection between TBI and CVD.
Most importantly, “we are increasingly learning that TBI is not only a discrete event that requires immediate treatment but also a chronic disease process,” and when we “think about the substantial long-term morbidity associated with TBI, we should keep increased risk for CVD on top of mind,” said Dr. Croll.
The review received no funding. Izzy reported receiving grants from the US National Institutes of Health (NIH) and 2023 Stepping Strong Innovator Award. Dr. Zafonte reported receiving grants from the NIH and royalties from Springer and Demos publishing for serving as a coeditor of Brain Injury Medicine. Dr. Zafonte has also served as an adviser to Myomo, Oncare.ai, Nanodiagnostics, and Kisbee. He reported evaluating patients in the Massachusetts General Hospital Brain and Body–TRUST Program, which is funded by the NFL Players Association. The other authors’ disclosures are listed on the original paper. Dr. Croll declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
How much would you bet on a diagnosis?
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at dermnews@mdedge.com.
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at dermnews@mdedge.com.
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at dermnews@mdedge.com.
EHR Tool Enhances Primary Aldosteronism Screening in Hypertensive Patients
Primary aldosteronism (PA) is a frequently overlooked yet common cause of secondary hypertension, presenting significant risk for cardiovascular morbidity and mortality.
But fewer than 4% of at-risk patients receive the recommended screening for PA, leaving a substantial gap in early detection and management, according to Adina F. Turcu, MD, MS, associate professor in endocrinology and internal medicine at University of Michigan Health in Ann Arbor.
In response to this clinical challenge, Dr. Turcu and her colleagues developed a best-practice advisory (BPA) to identify patients who were at risk for PA and embedded it into electronic health record at University of Michigan ambulatory clinics. Her team found that use of the tool led to increased rates of screening for PA, particularly among primary care physicians.
Over a 15-month period, Dr. Turcu and her colleagues tested the BPA through a quality improvement study, identifying 14,603 unique candidates for PA screening, with a mean age of 65.5 years and a diverse representation of ethnic backgrounds.
Notably, 48.1% of these candidates had treatment-resistant hypertension, 43.5% exhibited hypokalemia, 10.5% were younger than 35 years, and 3.1% had adrenal nodules. Of these candidates, 14.0% received orders for PA screening, with 70.5% completing the recommended screening within the system, and 17.4% receiving positive screening results.
The study, conducted over 6 months in 2023, targeted adults with hypertension and at least one of the following: Those who took four or more antihypertensive medications, exhibited hypokalemia, were younger than age 35 years, or had adrenal nodules. Patients previously tested for PA were excluded from the analysis.
The noninterruptive BPA was triggered during outpatient visits with clinicians who specialized in hypertension. The advisory would then offer an order set for PA screening and provide a link to interpretation guidance for results. Clinicians had the option to use, ignore, or decline the BPA.
“Although we were hoping for broader uptake of this EHR-embedded BPA, we were delighted to see an increase in PA screening rates to 14% of identified candidates as compared to an average of less than 3% in retrospective studies of similar populations, including in our own institution prior to implementing this BPA,” Dr. Turcu told this news organization.
Physician specialty played a crucial role in the utilization of the BPA. Internists and family medicine physicians accounted for the majority of screening orders, placing 40.0% and 28.1% of these, respectively. Family practitioners and internists predominantly used the embedded order set (80.3% and 68.9%, respectively).
“Hypertension often gets treated rather than screening for [causes of] secondary hypertension prior to treatment,” said Kaniksha Desai, MD, clinical associate professor and endocrinology quality director at Stanford University School of Medicine, Stanford, California, who was not involved in the research. But “primary hyperaldosteronism is a condition that can be treated surgically and has increased long term cardiovascular consequences if not identified. While guidelines recommend screening at-risk patients, this often can get lost in translation in clinical practice due to many factors, including time constraints and volume of patients.”
Patients who did vs did not undergo screening were more likely to be women, Black, and younger than age 35 years. Additionally, the likelihood of screening was higher among patients with obesity and dyslipidemia, whereas it was lower in those with chronic kidney disease and established cardiovascular complications.
According to Dr. Turcu, the findings from this study suggest that noninterruptive BPAs, especially when integrated into primary care workflows, hold promise as effective tools for PA screening.
When coupled with artificial intelligence to optimize detection yield, these refined BPAs could significantly contribute to personalized care for hypertension, the investigators said.
“Considering that in the United States almost one in two adults has hypertension, such automatized tools become instrumental to busy clinicians, particularly those in primary care,” Dr. Turcu said. “Our results indicate a promising opportunity to meaningfully improve PA awareness and enhance its diagnosis.”
Dr. Turcu reported receiving grants from the National Heart, Lung, and Blood Institute and Doris Duke Foundation, served as an investigator in a CinCor Pharma clinical trial, and received financial support to her institution during the conduct of the study. Dr. Desai reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Primary aldosteronism (PA) is a frequently overlooked yet common cause of secondary hypertension, presenting significant risk for cardiovascular morbidity and mortality.
But fewer than 4% of at-risk patients receive the recommended screening for PA, leaving a substantial gap in early detection and management, according to Adina F. Turcu, MD, MS, associate professor in endocrinology and internal medicine at University of Michigan Health in Ann Arbor.
In response to this clinical challenge, Dr. Turcu and her colleagues developed a best-practice advisory (BPA) to identify patients who were at risk for PA and embedded it into electronic health record at University of Michigan ambulatory clinics. Her team found that use of the tool led to increased rates of screening for PA, particularly among primary care physicians.
Over a 15-month period, Dr. Turcu and her colleagues tested the BPA through a quality improvement study, identifying 14,603 unique candidates for PA screening, with a mean age of 65.5 years and a diverse representation of ethnic backgrounds.
Notably, 48.1% of these candidates had treatment-resistant hypertension, 43.5% exhibited hypokalemia, 10.5% were younger than 35 years, and 3.1% had adrenal nodules. Of these candidates, 14.0% received orders for PA screening, with 70.5% completing the recommended screening within the system, and 17.4% receiving positive screening results.
The study, conducted over 6 months in 2023, targeted adults with hypertension and at least one of the following: Those who took four or more antihypertensive medications, exhibited hypokalemia, were younger than age 35 years, or had adrenal nodules. Patients previously tested for PA were excluded from the analysis.
The noninterruptive BPA was triggered during outpatient visits with clinicians who specialized in hypertension. The advisory would then offer an order set for PA screening and provide a link to interpretation guidance for results. Clinicians had the option to use, ignore, or decline the BPA.
“Although we were hoping for broader uptake of this EHR-embedded BPA, we were delighted to see an increase in PA screening rates to 14% of identified candidates as compared to an average of less than 3% in retrospective studies of similar populations, including in our own institution prior to implementing this BPA,” Dr. Turcu told this news organization.
Physician specialty played a crucial role in the utilization of the BPA. Internists and family medicine physicians accounted for the majority of screening orders, placing 40.0% and 28.1% of these, respectively. Family practitioners and internists predominantly used the embedded order set (80.3% and 68.9%, respectively).
“Hypertension often gets treated rather than screening for [causes of] secondary hypertension prior to treatment,” said Kaniksha Desai, MD, clinical associate professor and endocrinology quality director at Stanford University School of Medicine, Stanford, California, who was not involved in the research. But “primary hyperaldosteronism is a condition that can be treated surgically and has increased long term cardiovascular consequences if not identified. While guidelines recommend screening at-risk patients, this often can get lost in translation in clinical practice due to many factors, including time constraints and volume of patients.”
Patients who did vs did not undergo screening were more likely to be women, Black, and younger than age 35 years. Additionally, the likelihood of screening was higher among patients with obesity and dyslipidemia, whereas it was lower in those with chronic kidney disease and established cardiovascular complications.
According to Dr. Turcu, the findings from this study suggest that noninterruptive BPAs, especially when integrated into primary care workflows, hold promise as effective tools for PA screening.
When coupled with artificial intelligence to optimize detection yield, these refined BPAs could significantly contribute to personalized care for hypertension, the investigators said.
“Considering that in the United States almost one in two adults has hypertension, such automatized tools become instrumental to busy clinicians, particularly those in primary care,” Dr. Turcu said. “Our results indicate a promising opportunity to meaningfully improve PA awareness and enhance its diagnosis.”
Dr. Turcu reported receiving grants from the National Heart, Lung, and Blood Institute and Doris Duke Foundation, served as an investigator in a CinCor Pharma clinical trial, and received financial support to her institution during the conduct of the study. Dr. Desai reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Primary aldosteronism (PA) is a frequently overlooked yet common cause of secondary hypertension, presenting significant risk for cardiovascular morbidity and mortality.
But fewer than 4% of at-risk patients receive the recommended screening for PA, leaving a substantial gap in early detection and management, according to Adina F. Turcu, MD, MS, associate professor in endocrinology and internal medicine at University of Michigan Health in Ann Arbor.
In response to this clinical challenge, Dr. Turcu and her colleagues developed a best-practice advisory (BPA) to identify patients who were at risk for PA and embedded it into electronic health record at University of Michigan ambulatory clinics. Her team found that use of the tool led to increased rates of screening for PA, particularly among primary care physicians.
Over a 15-month period, Dr. Turcu and her colleagues tested the BPA through a quality improvement study, identifying 14,603 unique candidates for PA screening, with a mean age of 65.5 years and a diverse representation of ethnic backgrounds.
Notably, 48.1% of these candidates had treatment-resistant hypertension, 43.5% exhibited hypokalemia, 10.5% were younger than 35 years, and 3.1% had adrenal nodules. Of these candidates, 14.0% received orders for PA screening, with 70.5% completing the recommended screening within the system, and 17.4% receiving positive screening results.
The study, conducted over 6 months in 2023, targeted adults with hypertension and at least one of the following: Those who took four or more antihypertensive medications, exhibited hypokalemia, were younger than age 35 years, or had adrenal nodules. Patients previously tested for PA were excluded from the analysis.
The noninterruptive BPA was triggered during outpatient visits with clinicians who specialized in hypertension. The advisory would then offer an order set for PA screening and provide a link to interpretation guidance for results. Clinicians had the option to use, ignore, or decline the BPA.
“Although we were hoping for broader uptake of this EHR-embedded BPA, we were delighted to see an increase in PA screening rates to 14% of identified candidates as compared to an average of less than 3% in retrospective studies of similar populations, including in our own institution prior to implementing this BPA,” Dr. Turcu told this news organization.
Physician specialty played a crucial role in the utilization of the BPA. Internists and family medicine physicians accounted for the majority of screening orders, placing 40.0% and 28.1% of these, respectively. Family practitioners and internists predominantly used the embedded order set (80.3% and 68.9%, respectively).
“Hypertension often gets treated rather than screening for [causes of] secondary hypertension prior to treatment,” said Kaniksha Desai, MD, clinical associate professor and endocrinology quality director at Stanford University School of Medicine, Stanford, California, who was not involved in the research. But “primary hyperaldosteronism is a condition that can be treated surgically and has increased long term cardiovascular consequences if not identified. While guidelines recommend screening at-risk patients, this often can get lost in translation in clinical practice due to many factors, including time constraints and volume of patients.”
Patients who did vs did not undergo screening were more likely to be women, Black, and younger than age 35 years. Additionally, the likelihood of screening was higher among patients with obesity and dyslipidemia, whereas it was lower in those with chronic kidney disease and established cardiovascular complications.
According to Dr. Turcu, the findings from this study suggest that noninterruptive BPAs, especially when integrated into primary care workflows, hold promise as effective tools for PA screening.
When coupled with artificial intelligence to optimize detection yield, these refined BPAs could significantly contribute to personalized care for hypertension, the investigators said.
“Considering that in the United States almost one in two adults has hypertension, such automatized tools become instrumental to busy clinicians, particularly those in primary care,” Dr. Turcu said. “Our results indicate a promising opportunity to meaningfully improve PA awareness and enhance its diagnosis.”
Dr. Turcu reported receiving grants from the National Heart, Lung, and Blood Institute and Doris Duke Foundation, served as an investigator in a CinCor Pharma clinical trial, and received financial support to her institution during the conduct of the study. Dr. Desai reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Oncologists Sound the Alarm About Rise of White Bagging
For years, oncologist John DiPersio, MD, PhD, had faced frustrating encounters with insurers that only cover medications through a process called white bagging.
Instead of the traditional buy-and-bill pathway where oncologists purchase specialty drugs, such as infusion medications, directly from the distributor or manufacturer, white bagging requires physicians to receive these drugs from a specialty pharmacy.
On its face, the differences may seem minor. However, as Dr. DiPersio knows well, the consequences for oncologists and patients are not.
That is why Dr. DiPersio’s cancer center does not allow white bagging.
And when insurers refuse to reconsider the white bagging policy, his cancer team is left with few options.
“Sometimes, we have to redirect patients to other places,” said Dr. DiPersio, a bone marrow transplant specialist at Siteman Cancer Center, Washington University, St. Louis.
In emergency instances where patients cannot wait, Dr. DiPersio’s team will administer their own stock of a drug. In such cases, “we accept the fact that by not allowing white bagging, there may be nonpayment. We take the hit as far as cost.”
Increasingly, white bagging mandates are becoming harder for practices to avoid.
In a 2021 survey, 87% of Association of Community Cancer Centers members said white bagging has become an insurer mandate for some of their patients.
A 2023 analysis from Adam J. Fein, PhD, of Drug Channels Institute, Philadelphia, found that white bagging accounted for 17% of infused oncology product sourcing from clinics and 38% from hospital outpatient departments, up from 15% to 28% in 2019. Another practice called brown bagging, where specialty pharmacies send drugs directly to patients, creates many of the same issues but is much less prevalent than white bagging.
This change reflects “the broader battle over oncology margins” and insurers’ “attempts to shift costs to providers, patients, and manufacturers,” Dr. Fein wrote in his 2023 report.
White Bagging: Who Benefits?
At its core, white bagging changes how drugs are covered and reimbursed. Under buy and bill, drugs fall under a patient’s medical benefit. Oncologists purchase drugs directly from the manufacturer or distributor and receive reimbursement from the insurance company for both the cost of the drug as well as for administering it to patients.
Under white bagging, drugs fall under a patient’s pharmacy benefit. In these instances, a specialty pharmacy prepares the infusion ahead of time and ships it directly to the physician’s office or clinic. Because oncologists do not purchase the drug directly, they cannot bill insurers for it; instead, the pharmacy receives reimbursement for the drug and the provider is reimbursed for administering it.
Insurance companies argue that white bagging reduces patients’ out-of-pocket costs “by preventing hospitals and physicians from charging exorbitant fees to buy and store specialty medicines themselves,” according to advocacy group America’s Health Insurance Plans (AHIP).
Data from AHIP suggested that hospitals mark up the price of cancer drugs considerably, charging about twice as much as a specialty pharmacy, and that physician’s offices also charge about 23% more. However, these figures highlight how much insurers are billed, not necessarily how much patients ultimately pay.
Other evidence shows that white bagging raises costs for patients while reducing reimbursement for oncologists and saving insurance companies money.
A recent analysis in JAMA Network Open, which looked at 50 cancer drugs associated with the highest total spending from the 2020 Medicare Part B, found that mean insurance payments to providers were more than $2000 lower for drugs distributed under bagging than traditional buy and bill: $7405 vs $9547 per patient per month. Investigators found the same pattern in median insurance payments: $5746 vs $6681. Patients also paid more out-of-pocket each month with bagging vs buy and bill: $315 vs $145.
For patients with private insurance, “out-of-pocket costs were higher under bagging practice than the traditional buy-and-bill practice,” said lead author Ya-Chen Tina Shih, PhD, a professor in the department of radiation oncology at UCLA Health, Los Angeles.
White bagging is entirely for the profit of health insurers, specialty pharmacies, and pharmacy benefit managers, the middlemen who negotiate drug prices on behalf of payers.
Many people may not realize the underlying money-making strategies behind white bagging, explained Ted Okon, executive director for Community Oncology Alliance, which opposes the practice. Often, an insurer, pharmacy benefit manager, and mail order pharmacy involved in the process are all affiliated with the same corporation. In such cases, an insurer has a financial motive to control the source of medications and steer business to its affiliated pharmacies, Mr. Okon said.
When a single corporation owns numerous parts of the drug supply chain, insurers end up having “sway over what drug to use and then how the patient is going to get it,” Mr. Okon said. If the specialty pharmacy is a 340B contract pharmacy, it likely also receives a sizable discount on the drug and can make more money through white bagging.
Dangerous to Patients?
On the safety front, proponents of white bagging say the process is safe and efficient.
Specialty pharmacies are used only for prescription drugs that can be safely delivered, said AHIP spokesman David Allen.
In addition to having the same supply chain safety requirements as any other dispensing pharmacy, “specialty pharmacies also must meet additional safety requirements for specialty drugs” to ensure “the safe storage, handling, and dispensing of the drugs,” Mr. Allen explained.
However, oncologists argue that white bagging can be dangerous.
With white bagging, specialty pharmacies send a specified dose to practices, which does not allow practices to source and mix the drug themselves or make essential last-minute dose-related changes — something that happens every day in the clinic, said Debra Patt, MD, PhD, MBA, executive vice president for policy and strategy for Texas Oncology, Dallas.
White bagging also increases the risk for drug contamination, results in drug waste if the medication can’t be used, and can create delays in care.
Essentially, white bagging takes control away from oncologists and makes patient care more unpredictable and complex, explained Dr. Patt, president of the Texas Society of Clinical Oncology, Rockville, Maryland.
Dr. Patt, who does not allow white bagging in her practice, recalled a recent patient with metastatic breast cancer who came to the clinic for trastuzumab deruxtecan. The patient had been experiencing acute abdominal pain. After an exam and CT, Dr. Patt found the breast cancer had grown and moved into the patient’s liver.
“I had to discontinue that plan and change to a different chemotherapy,” she said. “If we had white bagged, that would have been a waste of several thousand dollars. Also, the patient would have to wait for the new medication to be white bagged, a delay that would be at least a week and the patient would have to come back at another time.”
When asked about the safety concerns associated with white bagging, Lemrey “Al” Carter, MS, PharmD, RPh, executive director of the National Association of Boards of Pharmacy (NABP), said the NABP “acknowledges that all these issues exist.
“It is unfortunate if patient care or costs are negatively impacted,” Dr. Carter said, adding that “boards of pharmacy can investigate if they are made aware of safety concerns at the pharmacy level. If a violation of the pharmacy laws or rules is found, boards can take action.”
More Legislation to Prevent Bagging
As white bagging mandates from insurance companies ramp up, more practices and states are banning it.
In the Association of Community Cancer Centers’ 2021 survey, 59% of members said their cancer program or practice does not allow white bagging.
At least 15 states have introduced legislation that restricts and/or prohibits white and brown bagging practices, according to a 2023 report by the Institute for Clinical and Economic Review. Some of the proposed laws would restrict mandates by stipulating that physicians are reimbursed at the contracted amount for clinician-administered drugs, whether obtained from a pharmacy or the manufacturer.
Louisiana, Vermont, and Minnesota were the first to enact anti–white bagging laws. Louisiana’s law, for example, enacted in 2021, bans white bagging and requires insurers to reimburse providers for physician-administered drugs if obtained from out-of-network pharmacies.
When the legislation passed, white bagging was just starting to enter the healthcare market in Louisiana, and the state wanted to act proactively, said Kathy W. Oubre, MS, CEO of the Pontchartrain Cancer Center, Covington, Louisiana, and president of the Coalition of Hematology and Oncology Practices, Mountain View, California.
“We recognized the growing concern around it,” Ms. Oubre said. The state legislature at the time included physicians and pharmacists who “really understood from a practice and patient perspective, the harm that policy could do.”
Ms. Oubre would like to see more legislation in other states and believes Louisiana’s law is a good model.
At the federal level, the American Hospital Association and American Society of Health-System Pharmacists have also urged the US Food and Drug Administration to take appropriate enforcement action to protect patients from white bagging.
Legislation that bars white bagging mandates is the most reasonable way to support timely and appropriate access to cancer care, Dr. Patt said. In the absence of such legislation, she said oncologists can only opt out of insurance contracts that may require the practice.
“That is a difficult position to put oncologists in,” she said.
A version of this article appeared on Medscape.com.
For years, oncologist John DiPersio, MD, PhD, had faced frustrating encounters with insurers that only cover medications through a process called white bagging.
Instead of the traditional buy-and-bill pathway where oncologists purchase specialty drugs, such as infusion medications, directly from the distributor or manufacturer, white bagging requires physicians to receive these drugs from a specialty pharmacy.
On its face, the differences may seem minor. However, as Dr. DiPersio knows well, the consequences for oncologists and patients are not.
That is why Dr. DiPersio’s cancer center does not allow white bagging.
And when insurers refuse to reconsider the white bagging policy, his cancer team is left with few options.
“Sometimes, we have to redirect patients to other places,” said Dr. DiPersio, a bone marrow transplant specialist at Siteman Cancer Center, Washington University, St. Louis.
In emergency instances where patients cannot wait, Dr. DiPersio’s team will administer their own stock of a drug. In such cases, “we accept the fact that by not allowing white bagging, there may be nonpayment. We take the hit as far as cost.”
Increasingly, white bagging mandates are becoming harder for practices to avoid.
In a 2021 survey, 87% of Association of Community Cancer Centers members said white bagging has become an insurer mandate for some of their patients.
A 2023 analysis from Adam J. Fein, PhD, of Drug Channels Institute, Philadelphia, found that white bagging accounted for 17% of infused oncology product sourcing from clinics and 38% from hospital outpatient departments, up from 15% to 28% in 2019. Another practice called brown bagging, where specialty pharmacies send drugs directly to patients, creates many of the same issues but is much less prevalent than white bagging.
This change reflects “the broader battle over oncology margins” and insurers’ “attempts to shift costs to providers, patients, and manufacturers,” Dr. Fein wrote in his 2023 report.
White Bagging: Who Benefits?
At its core, white bagging changes how drugs are covered and reimbursed. Under buy and bill, drugs fall under a patient’s medical benefit. Oncologists purchase drugs directly from the manufacturer or distributor and receive reimbursement from the insurance company for both the cost of the drug as well as for administering it to patients.
Under white bagging, drugs fall under a patient’s pharmacy benefit. In these instances, a specialty pharmacy prepares the infusion ahead of time and ships it directly to the physician’s office or clinic. Because oncologists do not purchase the drug directly, they cannot bill insurers for it; instead, the pharmacy receives reimbursement for the drug and the provider is reimbursed for administering it.
Insurance companies argue that white bagging reduces patients’ out-of-pocket costs “by preventing hospitals and physicians from charging exorbitant fees to buy and store specialty medicines themselves,” according to advocacy group America’s Health Insurance Plans (AHIP).
Data from AHIP suggested that hospitals mark up the price of cancer drugs considerably, charging about twice as much as a specialty pharmacy, and that physician’s offices also charge about 23% more. However, these figures highlight how much insurers are billed, not necessarily how much patients ultimately pay.
Other evidence shows that white bagging raises costs for patients while reducing reimbursement for oncologists and saving insurance companies money.
A recent analysis in JAMA Network Open, which looked at 50 cancer drugs associated with the highest total spending from the 2020 Medicare Part B, found that mean insurance payments to providers were more than $2000 lower for drugs distributed under bagging than traditional buy and bill: $7405 vs $9547 per patient per month. Investigators found the same pattern in median insurance payments: $5746 vs $6681. Patients also paid more out-of-pocket each month with bagging vs buy and bill: $315 vs $145.
For patients with private insurance, “out-of-pocket costs were higher under bagging practice than the traditional buy-and-bill practice,” said lead author Ya-Chen Tina Shih, PhD, a professor in the department of radiation oncology at UCLA Health, Los Angeles.
White bagging is entirely for the profit of health insurers, specialty pharmacies, and pharmacy benefit managers, the middlemen who negotiate drug prices on behalf of payers.
Many people may not realize the underlying money-making strategies behind white bagging, explained Ted Okon, executive director for Community Oncology Alliance, which opposes the practice. Often, an insurer, pharmacy benefit manager, and mail order pharmacy involved in the process are all affiliated with the same corporation. In such cases, an insurer has a financial motive to control the source of medications and steer business to its affiliated pharmacies, Mr. Okon said.
When a single corporation owns numerous parts of the drug supply chain, insurers end up having “sway over what drug to use and then how the patient is going to get it,” Mr. Okon said. If the specialty pharmacy is a 340B contract pharmacy, it likely also receives a sizable discount on the drug and can make more money through white bagging.
Dangerous to Patients?
On the safety front, proponents of white bagging say the process is safe and efficient.
Specialty pharmacies are used only for prescription drugs that can be safely delivered, said AHIP spokesman David Allen.
In addition to having the same supply chain safety requirements as any other dispensing pharmacy, “specialty pharmacies also must meet additional safety requirements for specialty drugs” to ensure “the safe storage, handling, and dispensing of the drugs,” Mr. Allen explained.
However, oncologists argue that white bagging can be dangerous.
With white bagging, specialty pharmacies send a specified dose to practices, which does not allow practices to source and mix the drug themselves or make essential last-minute dose-related changes — something that happens every day in the clinic, said Debra Patt, MD, PhD, MBA, executive vice president for policy and strategy for Texas Oncology, Dallas.
White bagging also increases the risk for drug contamination, results in drug waste if the medication can’t be used, and can create delays in care.
Essentially, white bagging takes control away from oncologists and makes patient care more unpredictable and complex, explained Dr. Patt, president of the Texas Society of Clinical Oncology, Rockville, Maryland.
Dr. Patt, who does not allow white bagging in her practice, recalled a recent patient with metastatic breast cancer who came to the clinic for trastuzumab deruxtecan. The patient had been experiencing acute abdominal pain. After an exam and CT, Dr. Patt found the breast cancer had grown and moved into the patient’s liver.
“I had to discontinue that plan and change to a different chemotherapy,” she said. “If we had white bagged, that would have been a waste of several thousand dollars. Also, the patient would have to wait for the new medication to be white bagged, a delay that would be at least a week and the patient would have to come back at another time.”
When asked about the safety concerns associated with white bagging, Lemrey “Al” Carter, MS, PharmD, RPh, executive director of the National Association of Boards of Pharmacy (NABP), said the NABP “acknowledges that all these issues exist.
“It is unfortunate if patient care or costs are negatively impacted,” Dr. Carter said, adding that “boards of pharmacy can investigate if they are made aware of safety concerns at the pharmacy level. If a violation of the pharmacy laws or rules is found, boards can take action.”
More Legislation to Prevent Bagging
As white bagging mandates from insurance companies ramp up, more practices and states are banning it.
In the Association of Community Cancer Centers’ 2021 survey, 59% of members said their cancer program or practice does not allow white bagging.
At least 15 states have introduced legislation that restricts and/or prohibits white and brown bagging practices, according to a 2023 report by the Institute for Clinical and Economic Review. Some of the proposed laws would restrict mandates by stipulating that physicians are reimbursed at the contracted amount for clinician-administered drugs, whether obtained from a pharmacy or the manufacturer.
Louisiana, Vermont, and Minnesota were the first to enact anti–white bagging laws. Louisiana’s law, for example, enacted in 2021, bans white bagging and requires insurers to reimburse providers for physician-administered drugs if obtained from out-of-network pharmacies.
When the legislation passed, white bagging was just starting to enter the healthcare market in Louisiana, and the state wanted to act proactively, said Kathy W. Oubre, MS, CEO of the Pontchartrain Cancer Center, Covington, Louisiana, and president of the Coalition of Hematology and Oncology Practices, Mountain View, California.
“We recognized the growing concern around it,” Ms. Oubre said. The state legislature at the time included physicians and pharmacists who “really understood from a practice and patient perspective, the harm that policy could do.”
Ms. Oubre would like to see more legislation in other states and believes Louisiana’s law is a good model.
At the federal level, the American Hospital Association and American Society of Health-System Pharmacists have also urged the US Food and Drug Administration to take appropriate enforcement action to protect patients from white bagging.
Legislation that bars white bagging mandates is the most reasonable way to support timely and appropriate access to cancer care, Dr. Patt said. In the absence of such legislation, she said oncologists can only opt out of insurance contracts that may require the practice.
“That is a difficult position to put oncologists in,” she said.
A version of this article appeared on Medscape.com.
For years, oncologist John DiPersio, MD, PhD, had faced frustrating encounters with insurers that only cover medications through a process called white bagging.
Instead of the traditional buy-and-bill pathway where oncologists purchase specialty drugs, such as infusion medications, directly from the distributor or manufacturer, white bagging requires physicians to receive these drugs from a specialty pharmacy.
On its face, the differences may seem minor. However, as Dr. DiPersio knows well, the consequences for oncologists and patients are not.
That is why Dr. DiPersio’s cancer center does not allow white bagging.
And when insurers refuse to reconsider the white bagging policy, his cancer team is left with few options.
“Sometimes, we have to redirect patients to other places,” said Dr. DiPersio, a bone marrow transplant specialist at Siteman Cancer Center, Washington University, St. Louis.
In emergency instances where patients cannot wait, Dr. DiPersio’s team will administer their own stock of a drug. In such cases, “we accept the fact that by not allowing white bagging, there may be nonpayment. We take the hit as far as cost.”
Increasingly, white bagging mandates are becoming harder for practices to avoid.
In a 2021 survey, 87% of Association of Community Cancer Centers members said white bagging has become an insurer mandate for some of their patients.
A 2023 analysis from Adam J. Fein, PhD, of Drug Channels Institute, Philadelphia, found that white bagging accounted for 17% of infused oncology product sourcing from clinics and 38% from hospital outpatient departments, up from 15% to 28% in 2019. Another practice called brown bagging, where specialty pharmacies send drugs directly to patients, creates many of the same issues but is much less prevalent than white bagging.
This change reflects “the broader battle over oncology margins” and insurers’ “attempts to shift costs to providers, patients, and manufacturers,” Dr. Fein wrote in his 2023 report.
White Bagging: Who Benefits?
At its core, white bagging changes how drugs are covered and reimbursed. Under buy and bill, drugs fall under a patient’s medical benefit. Oncologists purchase drugs directly from the manufacturer or distributor and receive reimbursement from the insurance company for both the cost of the drug as well as for administering it to patients.
Under white bagging, drugs fall under a patient’s pharmacy benefit. In these instances, a specialty pharmacy prepares the infusion ahead of time and ships it directly to the physician’s office or clinic. Because oncologists do not purchase the drug directly, they cannot bill insurers for it; instead, the pharmacy receives reimbursement for the drug and the provider is reimbursed for administering it.
Insurance companies argue that white bagging reduces patients’ out-of-pocket costs “by preventing hospitals and physicians from charging exorbitant fees to buy and store specialty medicines themselves,” according to advocacy group America’s Health Insurance Plans (AHIP).
Data from AHIP suggested that hospitals mark up the price of cancer drugs considerably, charging about twice as much as a specialty pharmacy, and that physician’s offices also charge about 23% more. However, these figures highlight how much insurers are billed, not necessarily how much patients ultimately pay.
Other evidence shows that white bagging raises costs for patients while reducing reimbursement for oncologists and saving insurance companies money.
A recent analysis in JAMA Network Open, which looked at 50 cancer drugs associated with the highest total spending from the 2020 Medicare Part B, found that mean insurance payments to providers were more than $2000 lower for drugs distributed under bagging than traditional buy and bill: $7405 vs $9547 per patient per month. Investigators found the same pattern in median insurance payments: $5746 vs $6681. Patients also paid more out-of-pocket each month with bagging vs buy and bill: $315 vs $145.
For patients with private insurance, “out-of-pocket costs were higher under bagging practice than the traditional buy-and-bill practice,” said lead author Ya-Chen Tina Shih, PhD, a professor in the department of radiation oncology at UCLA Health, Los Angeles.
White bagging is entirely for the profit of health insurers, specialty pharmacies, and pharmacy benefit managers, the middlemen who negotiate drug prices on behalf of payers.
Many people may not realize the underlying money-making strategies behind white bagging, explained Ted Okon, executive director for Community Oncology Alliance, which opposes the practice. Often, an insurer, pharmacy benefit manager, and mail order pharmacy involved in the process are all affiliated with the same corporation. In such cases, an insurer has a financial motive to control the source of medications and steer business to its affiliated pharmacies, Mr. Okon said.
When a single corporation owns numerous parts of the drug supply chain, insurers end up having “sway over what drug to use and then how the patient is going to get it,” Mr. Okon said. If the specialty pharmacy is a 340B contract pharmacy, it likely also receives a sizable discount on the drug and can make more money through white bagging.
Dangerous to Patients?
On the safety front, proponents of white bagging say the process is safe and efficient.
Specialty pharmacies are used only for prescription drugs that can be safely delivered, said AHIP spokesman David Allen.
In addition to having the same supply chain safety requirements as any other dispensing pharmacy, “specialty pharmacies also must meet additional safety requirements for specialty drugs” to ensure “the safe storage, handling, and dispensing of the drugs,” Mr. Allen explained.
However, oncologists argue that white bagging can be dangerous.
With white bagging, specialty pharmacies send a specified dose to practices, which does not allow practices to source and mix the drug themselves or make essential last-minute dose-related changes — something that happens every day in the clinic, said Debra Patt, MD, PhD, MBA, executive vice president for policy and strategy for Texas Oncology, Dallas.
White bagging also increases the risk for drug contamination, results in drug waste if the medication can’t be used, and can create delays in care.
Essentially, white bagging takes control away from oncologists and makes patient care more unpredictable and complex, explained Dr. Patt, president of the Texas Society of Clinical Oncology, Rockville, Maryland.
Dr. Patt, who does not allow white bagging in her practice, recalled a recent patient with metastatic breast cancer who came to the clinic for trastuzumab deruxtecan. The patient had been experiencing acute abdominal pain. After an exam and CT, Dr. Patt found the breast cancer had grown and moved into the patient’s liver.
“I had to discontinue that plan and change to a different chemotherapy,” she said. “If we had white bagged, that would have been a waste of several thousand dollars. Also, the patient would have to wait for the new medication to be white bagged, a delay that would be at least a week and the patient would have to come back at another time.”
When asked about the safety concerns associated with white bagging, Lemrey “Al” Carter, MS, PharmD, RPh, executive director of the National Association of Boards of Pharmacy (NABP), said the NABP “acknowledges that all these issues exist.
“It is unfortunate if patient care or costs are negatively impacted,” Dr. Carter said, adding that “boards of pharmacy can investigate if they are made aware of safety concerns at the pharmacy level. If a violation of the pharmacy laws or rules is found, boards can take action.”
More Legislation to Prevent Bagging
As white bagging mandates from insurance companies ramp up, more practices and states are banning it.
In the Association of Community Cancer Centers’ 2021 survey, 59% of members said their cancer program or practice does not allow white bagging.
At least 15 states have introduced legislation that restricts and/or prohibits white and brown bagging practices, according to a 2023 report by the Institute for Clinical and Economic Review. Some of the proposed laws would restrict mandates by stipulating that physicians are reimbursed at the contracted amount for clinician-administered drugs, whether obtained from a pharmacy or the manufacturer.
Louisiana, Vermont, and Minnesota were the first to enact anti–white bagging laws. Louisiana’s law, for example, enacted in 2021, bans white bagging and requires insurers to reimburse providers for physician-administered drugs if obtained from out-of-network pharmacies.
When the legislation passed, white bagging was just starting to enter the healthcare market in Louisiana, and the state wanted to act proactively, said Kathy W. Oubre, MS, CEO of the Pontchartrain Cancer Center, Covington, Louisiana, and president of the Coalition of Hematology and Oncology Practices, Mountain View, California.
“We recognized the growing concern around it,” Ms. Oubre said. The state legislature at the time included physicians and pharmacists who “really understood from a practice and patient perspective, the harm that policy could do.”
Ms. Oubre would like to see more legislation in other states and believes Louisiana’s law is a good model.
At the federal level, the American Hospital Association and American Society of Health-System Pharmacists have also urged the US Food and Drug Administration to take appropriate enforcement action to protect patients from white bagging.
Legislation that bars white bagging mandates is the most reasonable way to support timely and appropriate access to cancer care, Dr. Patt said. In the absence of such legislation, she said oncologists can only opt out of insurance contracts that may require the practice.
“That is a difficult position to put oncologists in,” she said.
A version of this article appeared on Medscape.com.
New Federal Rule for Prior Authorizations a ‘Major Win’ for Patients, Doctors
Physicians groups on January 17 hailed a new federal rule requiring health insurers to streamline and disclose more information about their prior authorization processes, saying it will improve patient care and reduce doctors’ administrative burden.
Health insurers participating in federal programs, including Medicare Advantage and Medicaid, must now respond to expedited prior authorization requests within 72 hours and other requests within 7 days under the long-awaited final rule, released on January 17 by the Centers for Medicare & Medicaid Services (CMS).
Insurers also must include their reasons for denying a prior authorization request and will be required to publicly release data on denial and approval rates for medical treatment. They’ll also need to give patients more information about their decisions to deny care. Insurers must comply with some of the rule’s provisions by January 2026 and others by January 2027.
The final rule “is an important step forward” toward the Medical Group Management Association’s goal of reducing the overall volume of prior authorization requests, said Anders Gilberg, the group’s senior vice president for government affairs, in a statement.
“Only then will medical groups find meaningful reprieve from these onerous, ill-intentioned administrative requirements that dangerously impede patient care,” Mr. Gilberg said.
Health insurers have long lobbied against increased regulation of prior authorization, arguing that it’s needed to rein in healthcare costs and prevent unnecessary treatment.
“We appreciate CMS’s announcement of enforcement discretion that will permit plans to use one standard, rather than mixing and matching, to reduce costs and speed implementation,” said America’s Health Insurance Plans, an insurers’ lobbying group, in an unsigned statement. “However, we must remember that the CMS rule is only half the picture; the Office of the Coordinator for Health Information Technology (ONC) should swiftly require vendors to build electronic prior authorization capabilities into the electronic health record so that providers can do their part, or plans will build a bridge to nowhere.”
The rule comes as health insurers have increasingly been criticized for onerous and time-consuming prior authorization procedures that physicians say unfairly delay or deny the medical treatment that their patients need. With federal legislation to rein in prior authorization overuse at a standstill, 30 states have introduced their own bills to address the problem. Regulators and lawsuits also have called attention to insurers’ increasing use of artificial intelligence and algorithms to deny claims without human review.
“Family physicians know firsthand how prior authorizations divert valuable time and resources away from direct patient care. We also know that these types of administrative requirements are driving physicians away from the workforce and worsening physician shortages,” said Steven P. Furr, MD, president of the American Academy of Family Physicians, in a statement praising the new rule.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, called the final rule “ a major win” for patients and physicians, adding that its requirements for health insurers to integrate their prior authorization procedures into physicians’ electronic health records systems will also help make “the current time-consuming, manual workflow” more efficient.
A version of this article first appeared on Medscape.com.
Physicians groups on January 17 hailed a new federal rule requiring health insurers to streamline and disclose more information about their prior authorization processes, saying it will improve patient care and reduce doctors’ administrative burden.
Health insurers participating in federal programs, including Medicare Advantage and Medicaid, must now respond to expedited prior authorization requests within 72 hours and other requests within 7 days under the long-awaited final rule, released on January 17 by the Centers for Medicare & Medicaid Services (CMS).
Insurers also must include their reasons for denying a prior authorization request and will be required to publicly release data on denial and approval rates for medical treatment. They’ll also need to give patients more information about their decisions to deny care. Insurers must comply with some of the rule’s provisions by January 2026 and others by January 2027.
The final rule “is an important step forward” toward the Medical Group Management Association’s goal of reducing the overall volume of prior authorization requests, said Anders Gilberg, the group’s senior vice president for government affairs, in a statement.
“Only then will medical groups find meaningful reprieve from these onerous, ill-intentioned administrative requirements that dangerously impede patient care,” Mr. Gilberg said.
Health insurers have long lobbied against increased regulation of prior authorization, arguing that it’s needed to rein in healthcare costs and prevent unnecessary treatment.
“We appreciate CMS’s announcement of enforcement discretion that will permit plans to use one standard, rather than mixing and matching, to reduce costs and speed implementation,” said America’s Health Insurance Plans, an insurers’ lobbying group, in an unsigned statement. “However, we must remember that the CMS rule is only half the picture; the Office of the Coordinator for Health Information Technology (ONC) should swiftly require vendors to build electronic prior authorization capabilities into the electronic health record so that providers can do their part, or plans will build a bridge to nowhere.”
The rule comes as health insurers have increasingly been criticized for onerous and time-consuming prior authorization procedures that physicians say unfairly delay or deny the medical treatment that their patients need. With federal legislation to rein in prior authorization overuse at a standstill, 30 states have introduced their own bills to address the problem. Regulators and lawsuits also have called attention to insurers’ increasing use of artificial intelligence and algorithms to deny claims without human review.
“Family physicians know firsthand how prior authorizations divert valuable time and resources away from direct patient care. We also know that these types of administrative requirements are driving physicians away from the workforce and worsening physician shortages,” said Steven P. Furr, MD, president of the American Academy of Family Physicians, in a statement praising the new rule.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, called the final rule “ a major win” for patients and physicians, adding that its requirements for health insurers to integrate their prior authorization procedures into physicians’ electronic health records systems will also help make “the current time-consuming, manual workflow” more efficient.
A version of this article first appeared on Medscape.com.
Physicians groups on January 17 hailed a new federal rule requiring health insurers to streamline and disclose more information about their prior authorization processes, saying it will improve patient care and reduce doctors’ administrative burden.
Health insurers participating in federal programs, including Medicare Advantage and Medicaid, must now respond to expedited prior authorization requests within 72 hours and other requests within 7 days under the long-awaited final rule, released on January 17 by the Centers for Medicare & Medicaid Services (CMS).
Insurers also must include their reasons for denying a prior authorization request and will be required to publicly release data on denial and approval rates for medical treatment. They’ll also need to give patients more information about their decisions to deny care. Insurers must comply with some of the rule’s provisions by January 2026 and others by January 2027.
The final rule “is an important step forward” toward the Medical Group Management Association’s goal of reducing the overall volume of prior authorization requests, said Anders Gilberg, the group’s senior vice president for government affairs, in a statement.
“Only then will medical groups find meaningful reprieve from these onerous, ill-intentioned administrative requirements that dangerously impede patient care,” Mr. Gilberg said.
Health insurers have long lobbied against increased regulation of prior authorization, arguing that it’s needed to rein in healthcare costs and prevent unnecessary treatment.
“We appreciate CMS’s announcement of enforcement discretion that will permit plans to use one standard, rather than mixing and matching, to reduce costs and speed implementation,” said America’s Health Insurance Plans, an insurers’ lobbying group, in an unsigned statement. “However, we must remember that the CMS rule is only half the picture; the Office of the Coordinator for Health Information Technology (ONC) should swiftly require vendors to build electronic prior authorization capabilities into the electronic health record so that providers can do their part, or plans will build a bridge to nowhere.”
The rule comes as health insurers have increasingly been criticized for onerous and time-consuming prior authorization procedures that physicians say unfairly delay or deny the medical treatment that their patients need. With federal legislation to rein in prior authorization overuse at a standstill, 30 states have introduced their own bills to address the problem. Regulators and lawsuits also have called attention to insurers’ increasing use of artificial intelligence and algorithms to deny claims without human review.
“Family physicians know firsthand how prior authorizations divert valuable time and resources away from direct patient care. We also know that these types of administrative requirements are driving physicians away from the workforce and worsening physician shortages,” said Steven P. Furr, MD, president of the American Academy of Family Physicians, in a statement praising the new rule.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, called the final rule “ a major win” for patients and physicians, adding that its requirements for health insurers to integrate their prior authorization procedures into physicians’ electronic health records systems will also help make “the current time-consuming, manual workflow” more efficient.
A version of this article first appeared on Medscape.com.
Continued Caution Needed Combining Nitrates With ED Drugs
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.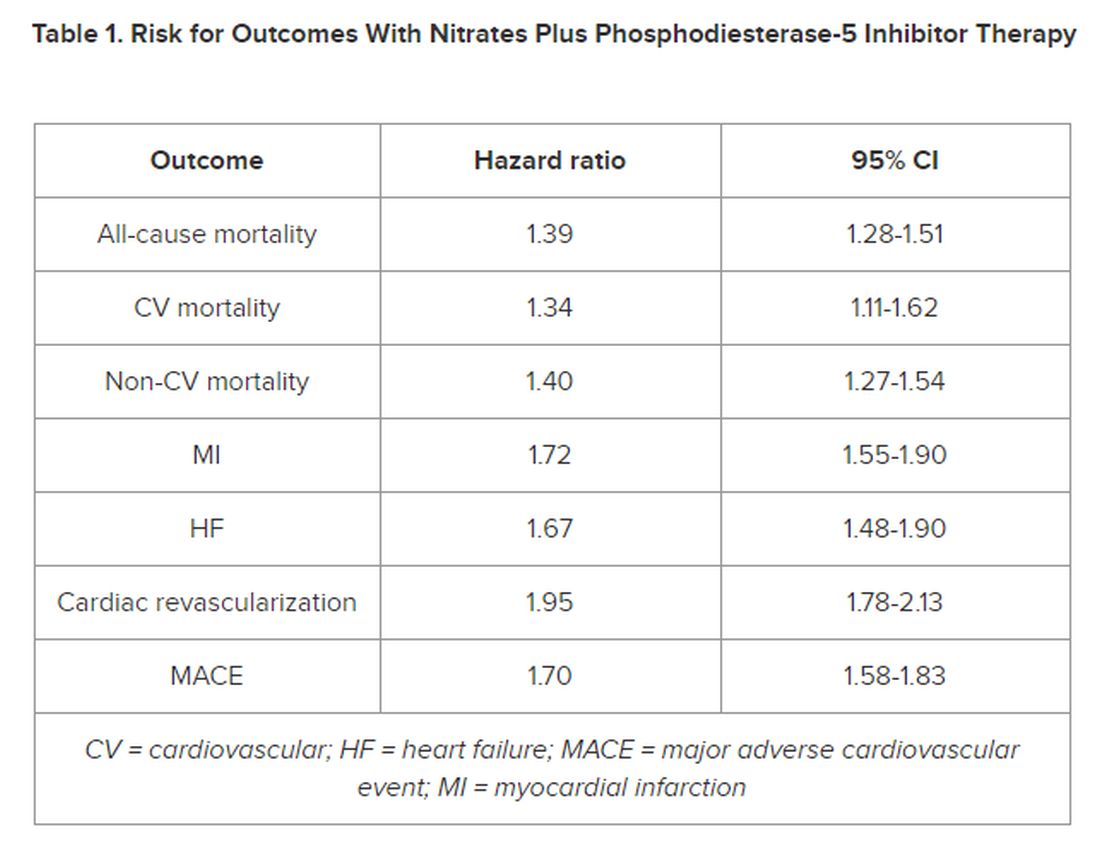
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.



