User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
What’s Eating You? Update on the Sticktight Flea (Echidnophaga gallinacea)
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
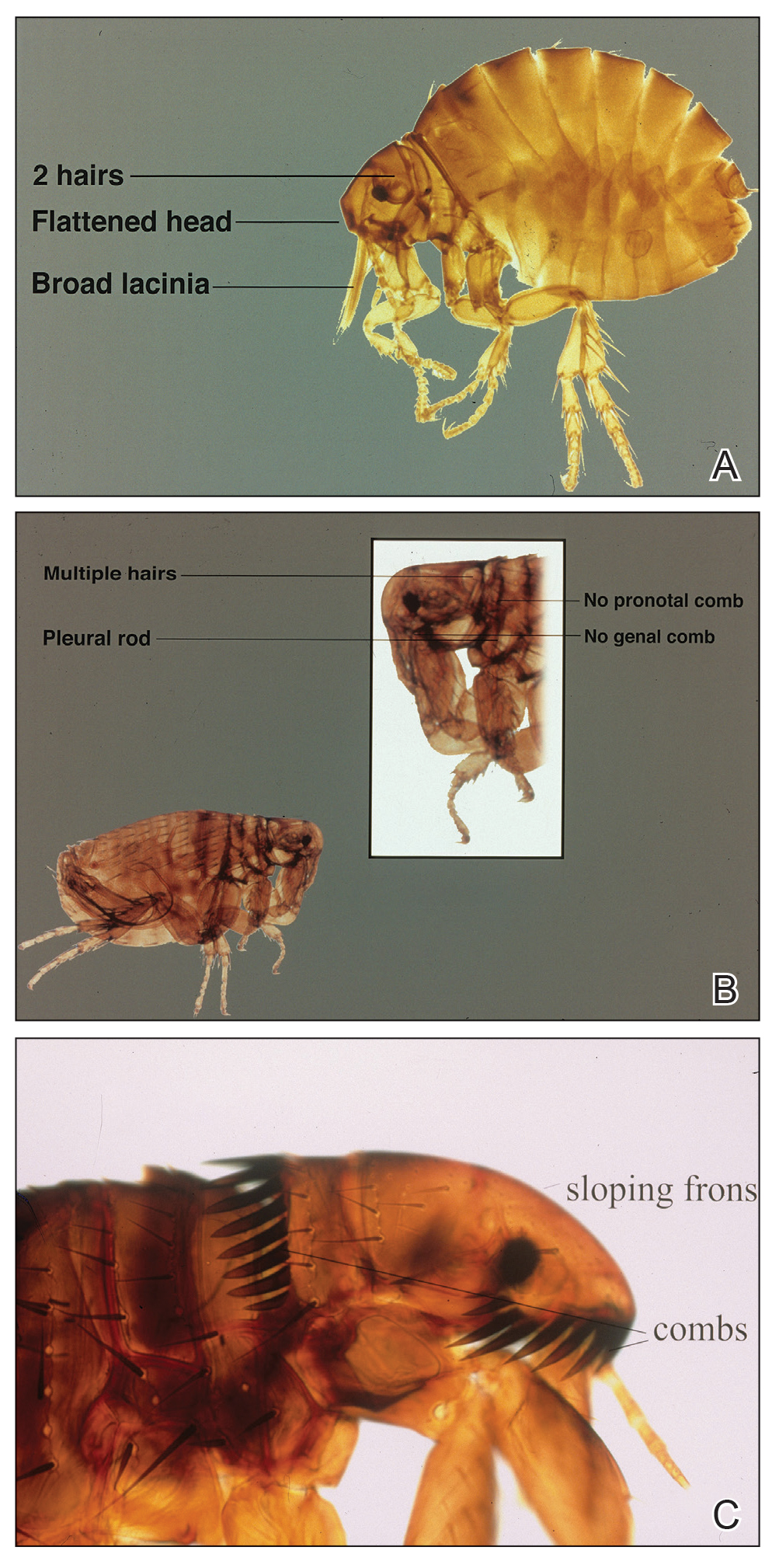
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8
Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8

Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
Fleas (order Siphonaptera) are vectors for various diseases, such as plague (as carriers of Yersinia pestis) and rickettsial infections.1-4 The sticktight flea (Echidnophaga gallinacea) commonly is seen on birds and mammals, including ground squirrels, dogs, cats, and rodents, and can attach to its host for days at a time by burrowing its head into the skin. Similar to other fleas, the sticktight flea needs a blood supply to reproduce.5 Therefore, it is important to study the sticktight flea, its habitat, and infection patterns to improve public health and prevent infestation.
Identification
Echidnophaga gallinacea is named for the female flea’s behavior—it “sticks tight” to the surface of the host by embedding its head into the skin for days at a time.5 The sticktight flea and the rat flea (Xenopsylla cheopis) can be differentiated by the sticktight’s reduced thorax and lack of a pleural rod (the vertical ridge that divides the mesosternum above the second pair of legs)(Figure, A and B). The sticktight flea can be differentiated from the dog flea (Ctenocephalides canis) and the cat flea (Ctenocephalides felis) by its lack of genal ctenidia (horizontal combs in the mustache area) and pronotal ctenidia (vertical combs behind the head)(Figure, B and C).6,7 Other defining features of E gallinacea include 2 pairs of large postantennal setae (hairs) on its anteriorly flattened head; a C-shaped reproductive organ known as the spermatheca; and broad maxillary lacinia (Figure, C).8

Habitat, Seasonality, and Behavior
Echidnophaga gallinacea commonly infests the comb, wattles, and surrounding ears of chickens; the flea also has been found on dogs, cats, rodents, and other species of birds.9 The sticktight flea is more prevalent in summer and autumn, which may explain its predominance in warmer climates, including California, Florida, Mexico, Egypt, Africa, and Iran.1,9-11
When a female sticktight flea begins to feed, it stays on the host for days at a time, waiting for a male.5 The female deposits its fertilized eggs in nests on the host or in lesions caused by infestation. Eventually, eggs hatch and fall into soil, where they lay dormant or grow to adulthood.5
Cutaneous Reaction to Infestation
Flea bites cause a hypersensitivity reaction, with pruritic pustules and erythematous papules that have a central punctum.12 In a reported case in Los Angeles, California, a female sticktight flea buried itself into the cheek of a young boy for more than 12 hours. The lesion was not marked by surrounding erythema, tenderness, pruritus, or swelling; however, several days after the flea was removed, erythema developed at the site then spontaneously resolved.7 In a study of dogs that were infested with E gallinacea, the flea never disengaged to attach to a human; when the flea was deliberately placed on a human, it fed and left hastily.11
Management
Because E gallinacea burrows its head into the skin, the best removal method is applying slow gentle traction under sterile conditions to ensure removal of mouthparts.7 An oral antihistamine can be administered or a topical antihistamine or corticosteroid can be applied to the affected area.12 Flea infestation should be treated with an insecticide. Affected animals should be treated by a veterinarian using a pesticide, such as fipronil, selamectin, imidacloprid, metaflumizone, nitenpyram, lufenuron, methoprene, or pyriproxyfen.13
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
- Hubbart JA, Jachowski DS, Eads DA. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol. 2011;36:117-123. doi:10.1111/j.1948-7134.2011.00148.x
- Jiang J, Maina AN, Knobel DL, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. 2013;13:550-558. doi:10.1089/vbz.2012.1123
- López-Pérez AM, Chaves A, Sánchez-Montes S, et al. Diversity of rickettsiae in domestic, synanthropic, and sylvatic mammals and their ectoparasites in a spotted fever-epidemic region at the western US-Mexico border. Transbound Emerg Dis. 2022;69:609-622. doi:10.1111/tbed.14027
- Ehlers J, Krüger A, Rakotondranary SJ, et al. Molecular detection of Rickettsia spp., Borrelia spp., Bartonella spp. and Yersinia pestis in ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2020;205:105339. doi:10.1016/j.actatropica.2020.105339
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J Parasitol. 2006;92:941-948. doi:10.1645/GE-769R.1
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676. doi:10.1016/j.ijid.2009.11.011
- Linardi PM, Santos JLC. Ctenocephalides felis felis vs. Ctenocephalides canis (Siphonaptera: Pulicidae): some issues in correctly identify these species. Rev Bras Parasitol Vet. 2012;21:345-354. doi:10.1590/s1984-29612012000400002
- Carlson JC, Fox MS. A sticktight flea removed from the cheek of a two-year-old boy from Los Angeles. Dermatol Online J. 2009;15:4. https://doi.org/10.5070/D36vb8p1b1
- Mirzaei M, Ghashghaei O, Yakhchali M. Prevalence of ectoparasites of indigenous chickens from Dalahu region, Kermanshah province, Iran. Turkiye Parazitol Derg. 2016;40:13-16. doi:10.5152/tpd.2016.4185
- Farid DS, Sallam NH, Eldein AMS, et al. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet World. 2021;14:2996-3006. doi:10.14202/vetworld.2021.2996-3006
- Harman DW, Halliwell RE, Greiner EC. Flea species from dogs and cats in north-central Florida. Vet Parasitol. 1987;23:135-140. doi:10.1016/0304-4017(87)90031-8
- Anderson J, Paterek E. Flea bites. StatPearls [Internet]. StatPearls Publishing; 2023. Updated August 8, 2023. Accessed November 27, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541118/
- Gyimesi ZS, Hayden ER, Greiner EC. Sticktight flea (Echidnophaga gallinacea) infestation in a Victoria crowned pigeon (Goura victoria). J Zoo Wildl Med. 2007;38:594-596. doi:10.1638/2007-0062.1
Practice Points
- The sticktight flea (Echidnophaga gallinacea) attaches to its host by embedding its head in the skin for days at a time.
- Unlike other fleas that bite and run, the sticktight flea can be identified dermoscopically.
- The sticktight flea serves as a vector for plague as a carrier of Yersinia pestis, rickettsial infections, and other diseases.
Electronic Health Records — Recent Survey Results
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
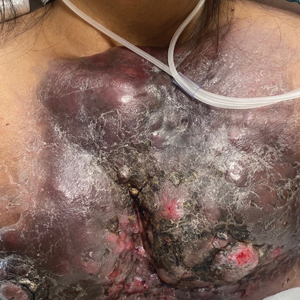
Large Indurated Plaque on the Chest With Ulceration and Necrosis
The Diagnosis: Carcinoma en Cuirasse
Histopathology demonstrated a cellular infiltrate filling the dermis with sparing of the papillary and superficial reticular dermis (Figure 1A). The cells were arranged in strands and cords that infiltrated between sclerotic collagen bundles. Cytomorphologically, the cells ranged from epithelioid with large vesicular nuclei and prominent nucleoli to cuboidal with hyperchromatic nuclei with irregular contours and a high nuclear to cytoplasmic ratio (Figure 1B). Occasional mitotic figures were identified, and cells demonstrated diffuse nuclear positivity for GATA-3 (Figure 1C); 55% of the cells demonstrated estrogen receptor positivity, and immunohistochemistry of progesterone receptors was negative. These findings confirmed our patient’s diagnosis of breast carcinoma en cuirasse (CeC) as the primary manifestation of metastatic invasive ductal carcinoma. Our patient was treated with intravenous chemotherapy and tamoxifen.
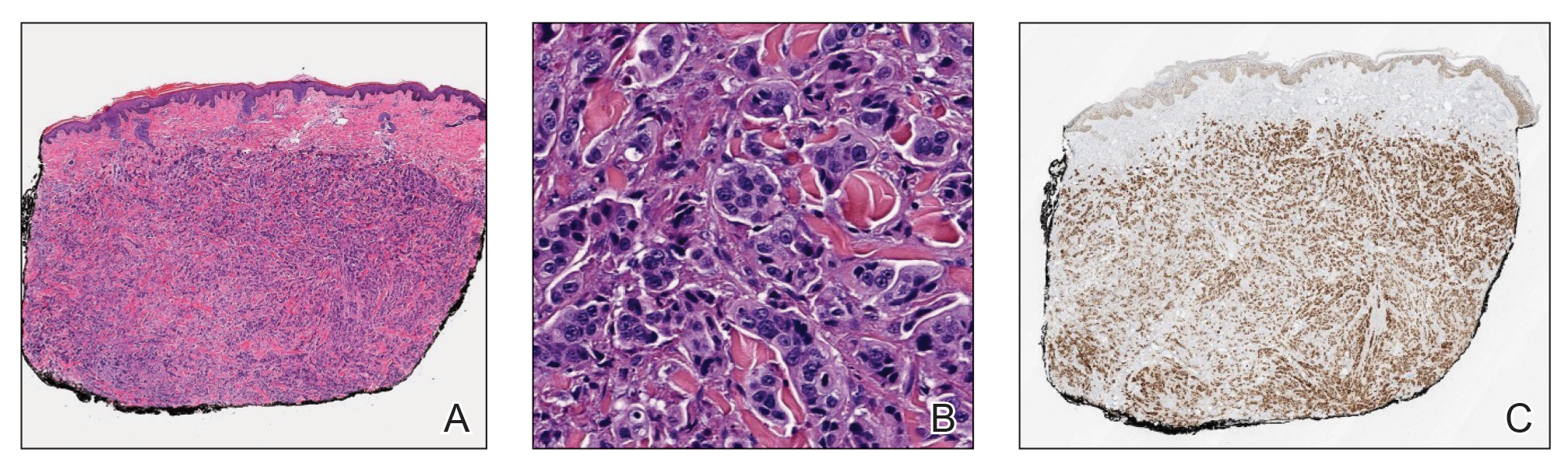
Histopathologic findings of morphea include thickened hyalinized collagen bundles and loss of adventitial fat.1 A diagnosis of chronic radiation dermatitis was inconsistent with our patient’s medical history and biopsy results, as pathology should reveal hyalinized collagen or stellate radiation fibroblasts.2,3 Nests of squamous epithelial cells with abundant eosinophilic cytoplasm and large vesicular nuclei were not seen, excluding squamous cell carcinoma as a possible diagnosis.4 Although sclerosing sweat duct carcinoma is characterized by infiltrating cords in sclerotic dermis, the cells were not arranged in ductlike structures 1– to 2–cell layers thick, excluding this diagnosis.5
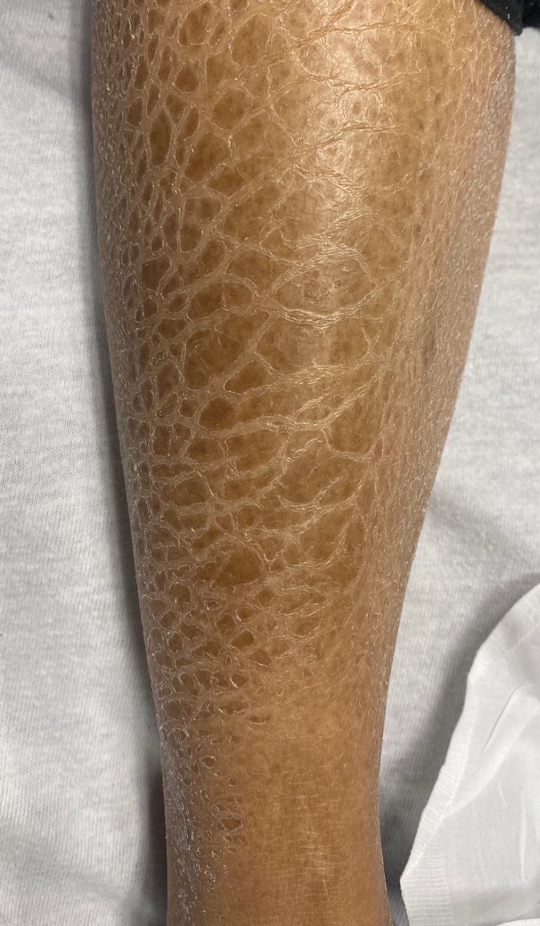
Carcinoma en cuirasse—named for skin involvement that appears similar to the metal breastplate of a cuirassier—is a rare form of cutaneous metastasis that typically presents with extensive infiltrative plaques resulting in fibrosis of the skin and subcutaneous tissue.6,7 Carcinoma en cuirasse most commonly metastasizes from the breast but also may represent metastases from the lungs, gastrointestinal tract, or genitourinary systems.8 In the setting of a primary breast malignancy, metastatic plaques of CeC tend to represent tumor recurrence following a mastectomy procedure; however, in rare cases CeC can present as the primary manifestation of breast cancer or as a result of untreated malignancy.6,9 In our patient, CeC was the primary manifestation of metastatic invasive ductal carcinoma with additional paraneoplastic ichthyosis (Figure 2).
Carcinoma en cuirasse comprises 3% to 6% of cutaneous metastases originating from the breast.10,11 Breast cancer is the most common primary neoplasm displaying extracutaneous metastasis, comprising 70% of all cutaneous metastases in females.11 Cutaneous metastasis often indicates late stage of disease, portending a poor prognosis. In our patient, the cutaneous nodules were present for approximately 3 years prior to the diagnosis of stage IV invasive ductal cell carcinoma with metastasis to the skin and lungs. Prior to admission, she had not been diagnosed with breast cancer, thus no treatments had been administered. It is uncommon for CeC to present as the initial finding and without prior treatment of the underlying malignancy. The median length of survival after diagnosis of cutaneous metastasis from breast cancer is 13.8 months, with a 10-year survival rate of 3.1%.12
In addition to cutaneous metastasis, breast cancer also may present with paraneoplastic dermatoses such as ichthyosis.13 Ichthyosis is characterized by extreme dryness, flaking, thickening, and mild pruritus.14 It most commonly is an inherited condition, but it may be acquired due to malignancy. Acquired ichthyosis may manifest in systemic diseases including systemic lupus erythematosus, sarcoidosis, and hypothyroidism.15 Although acquired ichthyosis is rare, it has been reported in cases of internal malignancy, most commonly lymphoproliferative malignancies and less frequently carcinoma of the breasts, cervix, and lungs. Patients who acquire ichthyosis in association with malignancy usually present with late-stage disease.15 Our patient acquired ichthyosis 3 months prior to admission and had never experienced it previously. Although the exact mechanism for acquiring ichthyosis remains unknown, it is uncertain if ichthyosis associated with malignancy is paraneoplastic or a result of chemotherapy.14,16 In this case, the patient had not yet started chemotherapy at the time of the ichthyosis diagnosis, suggesting a paraneoplastic etiology.
Carcinoma en cuirasse and paraneoplastic ichthyosis individually are extremely rare manifestations of breast cancer. Thus, it is even rarer for these conditions to present concurrently. Treatment options for CeC include chemotherapy, radiotherapy, hormonal antagonists, and snake venom.11 Systemic chemotherapy targeting the histopathologic type of the primary tumor is the treatment of choice. Other treatment methods usually are chosen for late stages of disease progression.10 Paraneoplastic ichthyosis has been reported to show improvement with treatment of the underlying primary malignancy by surgical removal or chemotherapy.14,17 Tamoxifen less commonly is used for systemic treatment of CeC, but one case in the literature reported favorable outcomes.18
We describe 2 rare cutaneous manifestations of breast cancer occurring concomitantly: CeC and paraneoplastic ichthyosis. The combination of clinical and pathologic findings presented in this case solidified the diagnosis of metastatic invasive ductal carcinoma. We aim to improve recognition of paraneoplastic skin findings to accelerate the process of effective and efficient treatment.
- Walker D, Susa JS, Currimbhoy S, et al. Histopathological changes in morphea and their clinical correlates: results from the Morphea in Adults and Children Cohort V. J Am Acad Dermatol. 2017;76:1124-1130. https://doi.org/10.1016/j.jaad.2016.12.020
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skin fibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4 suppl 1):S59-S64. https://doi.org/10.1097/SAP.0000000000002098
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. https://doi.org/10.1111/j .1600-0560.2011.01754.x
- Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-206. https://doi.org/10.1111 /j.0303-6987.2006.00516_1.x
- Harvey DT, Hu J, Long JA, et al. Sclerosing sweat duct carcinoma of the lower extremity treated with Mohs micrographic surgery. JAAD Case Rep. 2016;2:284-286. https://doi.org/10.1016/j.jdcr.2016.05.017
- Sharma V, Kumar A. Carcinoma en cuirasse. N Engl J Med. 2021;385:2562. doi:10.1056/NEJMicm2111669
- Oliveira GM, Zachetti DB, Barros HR, et al. Breast carcinoma en cuirasse—case report. An Bras Dermatol. 2013;88:608-610. doi:10.1590/abd1806-4841.20131926
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393. doi:10.1097 /DAD.0b013e31823069cf
- Glazebrook AJ, Tomaszewski W. Ichthyosiform atrophy of the skin in Hodgkin’s disease: report of a case, with reference to vitamin A metabolism. Arch Derm Syphilol. 1944;50:85-89. doi:10.1001 /archderm.1944.01510140008002
- Mordenti C, Concetta F, Cerroni M, et al. Cutaneous metastatic breast carcinoma: a study of 164 patients. Acta Dermatovenerol Alp Pannonica Adriat. 2000;9:143-148.
- Culver AL, Metter DM, Pippen JE Jr. Carcinoma en cuirasse. Proc (Bayl Univ Med Cent). 2019;32:263-265. doi:10.1080/08998280.2018.1564966
- Schoenlaub P, Sarraux A, Grosshans E, et al. Survival after cutaneous metastasis: a study of 200 cases [in French]. Ann Dermatol Venereol. 2001;128:1310-1315.
- Tan AR. Cutaneous manifestations of breast cancer. Semin Oncol. 2016;43:331-334. doi:10.1053/j.seminoncol.2016.02.030
- Song Y, Wu Y, Fan T. Dermatosis as the initial manifestation of malignant breast tumors: retrospective analysis of 4 cases. Breast Care. 2010;5:174-176. doi:10.1159/000314265
- Polisky RB, Bronson DM. Acquired ichthyosis in a patient with adenocarcinoma of the breast. Cutis. 1986;38:359-360.
- Haste AR. Acquired ichthyosis from breast cancer. Br Med J. 1967;4:96-98.
- Riesco Martínez MC, Muñoz Martín AJ, Zamberk Majlis P, et al. Acquired ichthyosis as a paraneoplastic syndrome in Hodgkin’s disease. Clin Transl Oncol. 2009;11:552-553. doi:10.1007/s12094-009-0402-2
- Siddiqui MA, Zaman MN. Primary carcinoma en cuirasse. J Am Geriatr Soc. 1996;44:221-222. doi:10.1111/j.1532-5415.1996.tb02455.xssss
The Diagnosis: Carcinoma en Cuirasse
Histopathology demonstrated a cellular infiltrate filling the dermis with sparing of the papillary and superficial reticular dermis (Figure 1A). The cells were arranged in strands and cords that infiltrated between sclerotic collagen bundles. Cytomorphologically, the cells ranged from epithelioid with large vesicular nuclei and prominent nucleoli to cuboidal with hyperchromatic nuclei with irregular contours and a high nuclear to cytoplasmic ratio (Figure 1B). Occasional mitotic figures were identified, and cells demonstrated diffuse nuclear positivity for GATA-3 (Figure 1C); 55% of the cells demonstrated estrogen receptor positivity, and immunohistochemistry of progesterone receptors was negative. These findings confirmed our patient’s diagnosis of breast carcinoma en cuirasse (CeC) as the primary manifestation of metastatic invasive ductal carcinoma. Our patient was treated with intravenous chemotherapy and tamoxifen.

Histopathologic findings of morphea include thickened hyalinized collagen bundles and loss of adventitial fat.1 A diagnosis of chronic radiation dermatitis was inconsistent with our patient’s medical history and biopsy results, as pathology should reveal hyalinized collagen or stellate radiation fibroblasts.2,3 Nests of squamous epithelial cells with abundant eosinophilic cytoplasm and large vesicular nuclei were not seen, excluding squamous cell carcinoma as a possible diagnosis.4 Although sclerosing sweat duct carcinoma is characterized by infiltrating cords in sclerotic dermis, the cells were not arranged in ductlike structures 1– to 2–cell layers thick, excluding this diagnosis.5
Carcinoma en cuirasse—named for skin involvement that appears similar to the metal breastplate of a cuirassier—is a rare form of cutaneous metastasis that typically presents with extensive infiltrative plaques resulting in fibrosis of the skin and subcutaneous tissue.6,7 Carcinoma en cuirasse most commonly metastasizes from the breast but also may represent metastases from the lungs, gastrointestinal tract, or genitourinary systems.8 In the setting of a primary breast malignancy, metastatic plaques of CeC tend to represent tumor recurrence following a mastectomy procedure; however, in rare cases CeC can present as the primary manifestation of breast cancer or as a result of untreated malignancy.6,9 In our patient, CeC was the primary manifestation of metastatic invasive ductal carcinoma with additional paraneoplastic ichthyosis (Figure 2).

Carcinoma en cuirasse comprises 3% to 6% of cutaneous metastases originating from the breast.10,11 Breast cancer is the most common primary neoplasm displaying extracutaneous metastasis, comprising 70% of all cutaneous metastases in females.11 Cutaneous metastasis often indicates late stage of disease, portending a poor prognosis. In our patient, the cutaneous nodules were present for approximately 3 years prior to the diagnosis of stage IV invasive ductal cell carcinoma with metastasis to the skin and lungs. Prior to admission, she had not been diagnosed with breast cancer, thus no treatments had been administered. It is uncommon for CeC to present as the initial finding and without prior treatment of the underlying malignancy. The median length of survival after diagnosis of cutaneous metastasis from breast cancer is 13.8 months, with a 10-year survival rate of 3.1%.12
In addition to cutaneous metastasis, breast cancer also may present with paraneoplastic dermatoses such as ichthyosis.13 Ichthyosis is characterized by extreme dryness, flaking, thickening, and mild pruritus.14 It most commonly is an inherited condition, but it may be acquired due to malignancy. Acquired ichthyosis may manifest in systemic diseases including systemic lupus erythematosus, sarcoidosis, and hypothyroidism.15 Although acquired ichthyosis is rare, it has been reported in cases of internal malignancy, most commonly lymphoproliferative malignancies and less frequently carcinoma of the breasts, cervix, and lungs. Patients who acquire ichthyosis in association with malignancy usually present with late-stage disease.15 Our patient acquired ichthyosis 3 months prior to admission and had never experienced it previously. Although the exact mechanism for acquiring ichthyosis remains unknown, it is uncertain if ichthyosis associated with malignancy is paraneoplastic or a result of chemotherapy.14,16 In this case, the patient had not yet started chemotherapy at the time of the ichthyosis diagnosis, suggesting a paraneoplastic etiology.
Carcinoma en cuirasse and paraneoplastic ichthyosis individually are extremely rare manifestations of breast cancer. Thus, it is even rarer for these conditions to present concurrently. Treatment options for CeC include chemotherapy, radiotherapy, hormonal antagonists, and snake venom.11 Systemic chemotherapy targeting the histopathologic type of the primary tumor is the treatment of choice. Other treatment methods usually are chosen for late stages of disease progression.10 Paraneoplastic ichthyosis has been reported to show improvement with treatment of the underlying primary malignancy by surgical removal or chemotherapy.14,17 Tamoxifen less commonly is used for systemic treatment of CeC, but one case in the literature reported favorable outcomes.18
We describe 2 rare cutaneous manifestations of breast cancer occurring concomitantly: CeC and paraneoplastic ichthyosis. The combination of clinical and pathologic findings presented in this case solidified the diagnosis of metastatic invasive ductal carcinoma. We aim to improve recognition of paraneoplastic skin findings to accelerate the process of effective and efficient treatment.
The Diagnosis: Carcinoma en Cuirasse
Histopathology demonstrated a cellular infiltrate filling the dermis with sparing of the papillary and superficial reticular dermis (Figure 1A). The cells were arranged in strands and cords that infiltrated between sclerotic collagen bundles. Cytomorphologically, the cells ranged from epithelioid with large vesicular nuclei and prominent nucleoli to cuboidal with hyperchromatic nuclei with irregular contours and a high nuclear to cytoplasmic ratio (Figure 1B). Occasional mitotic figures were identified, and cells demonstrated diffuse nuclear positivity for GATA-3 (Figure 1C); 55% of the cells demonstrated estrogen receptor positivity, and immunohistochemistry of progesterone receptors was negative. These findings confirmed our patient’s diagnosis of breast carcinoma en cuirasse (CeC) as the primary manifestation of metastatic invasive ductal carcinoma. Our patient was treated with intravenous chemotherapy and tamoxifen.

Histopathologic findings of morphea include thickened hyalinized collagen bundles and loss of adventitial fat.1 A diagnosis of chronic radiation dermatitis was inconsistent with our patient’s medical history and biopsy results, as pathology should reveal hyalinized collagen or stellate radiation fibroblasts.2,3 Nests of squamous epithelial cells with abundant eosinophilic cytoplasm and large vesicular nuclei were not seen, excluding squamous cell carcinoma as a possible diagnosis.4 Although sclerosing sweat duct carcinoma is characterized by infiltrating cords in sclerotic dermis, the cells were not arranged in ductlike structures 1– to 2–cell layers thick, excluding this diagnosis.5
Carcinoma en cuirasse—named for skin involvement that appears similar to the metal breastplate of a cuirassier—is a rare form of cutaneous metastasis that typically presents with extensive infiltrative plaques resulting in fibrosis of the skin and subcutaneous tissue.6,7 Carcinoma en cuirasse most commonly metastasizes from the breast but also may represent metastases from the lungs, gastrointestinal tract, or genitourinary systems.8 In the setting of a primary breast malignancy, metastatic plaques of CeC tend to represent tumor recurrence following a mastectomy procedure; however, in rare cases CeC can present as the primary manifestation of breast cancer or as a result of untreated malignancy.6,9 In our patient, CeC was the primary manifestation of metastatic invasive ductal carcinoma with additional paraneoplastic ichthyosis (Figure 2).

Carcinoma en cuirasse comprises 3% to 6% of cutaneous metastases originating from the breast.10,11 Breast cancer is the most common primary neoplasm displaying extracutaneous metastasis, comprising 70% of all cutaneous metastases in females.11 Cutaneous metastasis often indicates late stage of disease, portending a poor prognosis. In our patient, the cutaneous nodules were present for approximately 3 years prior to the diagnosis of stage IV invasive ductal cell carcinoma with metastasis to the skin and lungs. Prior to admission, she had not been diagnosed with breast cancer, thus no treatments had been administered. It is uncommon for CeC to present as the initial finding and without prior treatment of the underlying malignancy. The median length of survival after diagnosis of cutaneous metastasis from breast cancer is 13.8 months, with a 10-year survival rate of 3.1%.12
In addition to cutaneous metastasis, breast cancer also may present with paraneoplastic dermatoses such as ichthyosis.13 Ichthyosis is characterized by extreme dryness, flaking, thickening, and mild pruritus.14 It most commonly is an inherited condition, but it may be acquired due to malignancy. Acquired ichthyosis may manifest in systemic diseases including systemic lupus erythematosus, sarcoidosis, and hypothyroidism.15 Although acquired ichthyosis is rare, it has been reported in cases of internal malignancy, most commonly lymphoproliferative malignancies and less frequently carcinoma of the breasts, cervix, and lungs. Patients who acquire ichthyosis in association with malignancy usually present with late-stage disease.15 Our patient acquired ichthyosis 3 months prior to admission and had never experienced it previously. Although the exact mechanism for acquiring ichthyosis remains unknown, it is uncertain if ichthyosis associated with malignancy is paraneoplastic or a result of chemotherapy.14,16 In this case, the patient had not yet started chemotherapy at the time of the ichthyosis diagnosis, suggesting a paraneoplastic etiology.
Carcinoma en cuirasse and paraneoplastic ichthyosis individually are extremely rare manifestations of breast cancer. Thus, it is even rarer for these conditions to present concurrently. Treatment options for CeC include chemotherapy, radiotherapy, hormonal antagonists, and snake venom.11 Systemic chemotherapy targeting the histopathologic type of the primary tumor is the treatment of choice. Other treatment methods usually are chosen for late stages of disease progression.10 Paraneoplastic ichthyosis has been reported to show improvement with treatment of the underlying primary malignancy by surgical removal or chemotherapy.14,17 Tamoxifen less commonly is used for systemic treatment of CeC, but one case in the literature reported favorable outcomes.18
We describe 2 rare cutaneous manifestations of breast cancer occurring concomitantly: CeC and paraneoplastic ichthyosis. The combination of clinical and pathologic findings presented in this case solidified the diagnosis of metastatic invasive ductal carcinoma. We aim to improve recognition of paraneoplastic skin findings to accelerate the process of effective and efficient treatment.
- Walker D, Susa JS, Currimbhoy S, et al. Histopathological changes in morphea and their clinical correlates: results from the Morphea in Adults and Children Cohort V. J Am Acad Dermatol. 2017;76:1124-1130. https://doi.org/10.1016/j.jaad.2016.12.020
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skin fibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4 suppl 1):S59-S64. https://doi.org/10.1097/SAP.0000000000002098
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. https://doi.org/10.1111/j .1600-0560.2011.01754.x
- Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-206. https://doi.org/10.1111 /j.0303-6987.2006.00516_1.x
- Harvey DT, Hu J, Long JA, et al. Sclerosing sweat duct carcinoma of the lower extremity treated with Mohs micrographic surgery. JAAD Case Rep. 2016;2:284-286. https://doi.org/10.1016/j.jdcr.2016.05.017
- Sharma V, Kumar A. Carcinoma en cuirasse. N Engl J Med. 2021;385:2562. doi:10.1056/NEJMicm2111669
- Oliveira GM, Zachetti DB, Barros HR, et al. Breast carcinoma en cuirasse—case report. An Bras Dermatol. 2013;88:608-610. doi:10.1590/abd1806-4841.20131926
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393. doi:10.1097 /DAD.0b013e31823069cf
- Glazebrook AJ, Tomaszewski W. Ichthyosiform atrophy of the skin in Hodgkin’s disease: report of a case, with reference to vitamin A metabolism. Arch Derm Syphilol. 1944;50:85-89. doi:10.1001 /archderm.1944.01510140008002
- Mordenti C, Concetta F, Cerroni M, et al. Cutaneous metastatic breast carcinoma: a study of 164 patients. Acta Dermatovenerol Alp Pannonica Adriat. 2000;9:143-148.
- Culver AL, Metter DM, Pippen JE Jr. Carcinoma en cuirasse. Proc (Bayl Univ Med Cent). 2019;32:263-265. doi:10.1080/08998280.2018.1564966
- Schoenlaub P, Sarraux A, Grosshans E, et al. Survival after cutaneous metastasis: a study of 200 cases [in French]. Ann Dermatol Venereol. 2001;128:1310-1315.
- Tan AR. Cutaneous manifestations of breast cancer. Semin Oncol. 2016;43:331-334. doi:10.1053/j.seminoncol.2016.02.030
- Song Y, Wu Y, Fan T. Dermatosis as the initial manifestation of malignant breast tumors: retrospective analysis of 4 cases. Breast Care. 2010;5:174-176. doi:10.1159/000314265
- Polisky RB, Bronson DM. Acquired ichthyosis in a patient with adenocarcinoma of the breast. Cutis. 1986;38:359-360.
- Haste AR. Acquired ichthyosis from breast cancer. Br Med J. 1967;4:96-98.
- Riesco Martínez MC, Muñoz Martín AJ, Zamberk Majlis P, et al. Acquired ichthyosis as a paraneoplastic syndrome in Hodgkin’s disease. Clin Transl Oncol. 2009;11:552-553. doi:10.1007/s12094-009-0402-2
- Siddiqui MA, Zaman MN. Primary carcinoma en cuirasse. J Am Geriatr Soc. 1996;44:221-222. doi:10.1111/j.1532-5415.1996.tb02455.xssss
- Walker D, Susa JS, Currimbhoy S, et al. Histopathological changes in morphea and their clinical correlates: results from the Morphea in Adults and Children Cohort V. J Am Acad Dermatol. 2017;76:1124-1130. https://doi.org/10.1016/j.jaad.2016.12.020
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skin fibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4 suppl 1):S59-S64. https://doi.org/10.1097/SAP.0000000000002098
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. https://doi.org/10.1111/j .1600-0560.2011.01754.x
- Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-206. https://doi.org/10.1111 /j.0303-6987.2006.00516_1.x
- Harvey DT, Hu J, Long JA, et al. Sclerosing sweat duct carcinoma of the lower extremity treated with Mohs micrographic surgery. JAAD Case Rep. 2016;2:284-286. https://doi.org/10.1016/j.jdcr.2016.05.017
- Sharma V, Kumar A. Carcinoma en cuirasse. N Engl J Med. 2021;385:2562. doi:10.1056/NEJMicm2111669
- Oliveira GM, Zachetti DB, Barros HR, et al. Breast carcinoma en cuirasse—case report. An Bras Dermatol. 2013;88:608-610. doi:10.1590/abd1806-4841.20131926
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393. doi:10.1097 /DAD.0b013e31823069cf
- Glazebrook AJ, Tomaszewski W. Ichthyosiform atrophy of the skin in Hodgkin’s disease: report of a case, with reference to vitamin A metabolism. Arch Derm Syphilol. 1944;50:85-89. doi:10.1001 /archderm.1944.01510140008002
- Mordenti C, Concetta F, Cerroni M, et al. Cutaneous metastatic breast carcinoma: a study of 164 patients. Acta Dermatovenerol Alp Pannonica Adriat. 2000;9:143-148.
- Culver AL, Metter DM, Pippen JE Jr. Carcinoma en cuirasse. Proc (Bayl Univ Med Cent). 2019;32:263-265. doi:10.1080/08998280.2018.1564966
- Schoenlaub P, Sarraux A, Grosshans E, et al. Survival after cutaneous metastasis: a study of 200 cases [in French]. Ann Dermatol Venereol. 2001;128:1310-1315.
- Tan AR. Cutaneous manifestations of breast cancer. Semin Oncol. 2016;43:331-334. doi:10.1053/j.seminoncol.2016.02.030
- Song Y, Wu Y, Fan T. Dermatosis as the initial manifestation of malignant breast tumors: retrospective analysis of 4 cases. Breast Care. 2010;5:174-176. doi:10.1159/000314265
- Polisky RB, Bronson DM. Acquired ichthyosis in a patient with adenocarcinoma of the breast. Cutis. 1986;38:359-360.
- Haste AR. Acquired ichthyosis from breast cancer. Br Med J. 1967;4:96-98.
- Riesco Martínez MC, Muñoz Martín AJ, Zamberk Majlis P, et al. Acquired ichthyosis as a paraneoplastic syndrome in Hodgkin’s disease. Clin Transl Oncol. 2009;11:552-553. doi:10.1007/s12094-009-0402-2
- Siddiqui MA, Zaman MN. Primary carcinoma en cuirasse. J Am Geriatr Soc. 1996;44:221-222. doi:10.1111/j.1532-5415.1996.tb02455.xssss
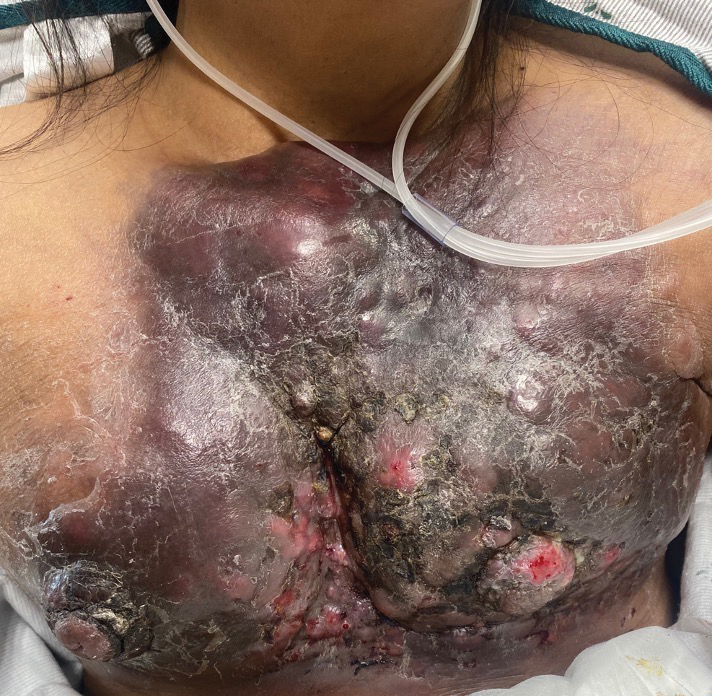
A 47-year-old woman with no notable medical history presented to the emergency department with shortness of breath on simple exertion as well as a large lesion on the chest that had slowly increased in size over the last 3 years. The lesion was not painful or pruritic, and she had been treating it with topical emollients without substantial improvement. Physical examination revealed a large indurated plaque with areas of ulceration and necrosis spanning the mid to lateral chest. Additionally, ichthyotic brown scaling was present on the arms and legs. Upon further questioning, the patient reported that the scales on the extremities appeared in the last 3 months and were not previously noted. She had no recent routine cancer screenings, and her family history was notable for a brother with brain cancer. A punch biopsy of the chest plaque was performed.
Fellowships in Complex Medical Dermatology
Complex medical dermatology has become an emerging field in dermatology. Although a rather protean and broad term, complex medical dermatology encompasses patients with autoimmune conditions, bullous disease, connective tissue disease, vasculitis, severe dermatoses requiring immunomodulation, and inpatient consultations. Importantly, dermatology inpatient consultations aid in lowering health care costs due to accurate diagnoses, correct treatment, and decreased hospital stays.1 A fellowship is not required for holding an inpatient role in the hospital system as a dermatologist but can be beneficial. There are combined internal medicine–dermatology programs available for medical students applying to dermatology residency, but a complex medical dermatology fellowship is an option after residency for those who are interested. I believe that a focused complex medical dermatology fellowship differs from the training offered in combined internal medicine–dermatology residency. My fellow colleagues in combined internal medicine–dermatology programs are exposed to systemic manifestations of cutaneous disease and are experts in the interplay between the skin and other organ systems. However, the focus of their programs is with the intention of becoming double boarded in internal medicine and dermatology with comprehensive exposure to both fields. In my fellowship, I am able to tailor my schedule to focus on any dermatologic disease such as connective tissue disease, pruritus, graft vs host disease, and Merkel cell carcinoma. I ultimately can determine a niche in dermatology and hone my skills for a year under supervision.
Available Fellowships
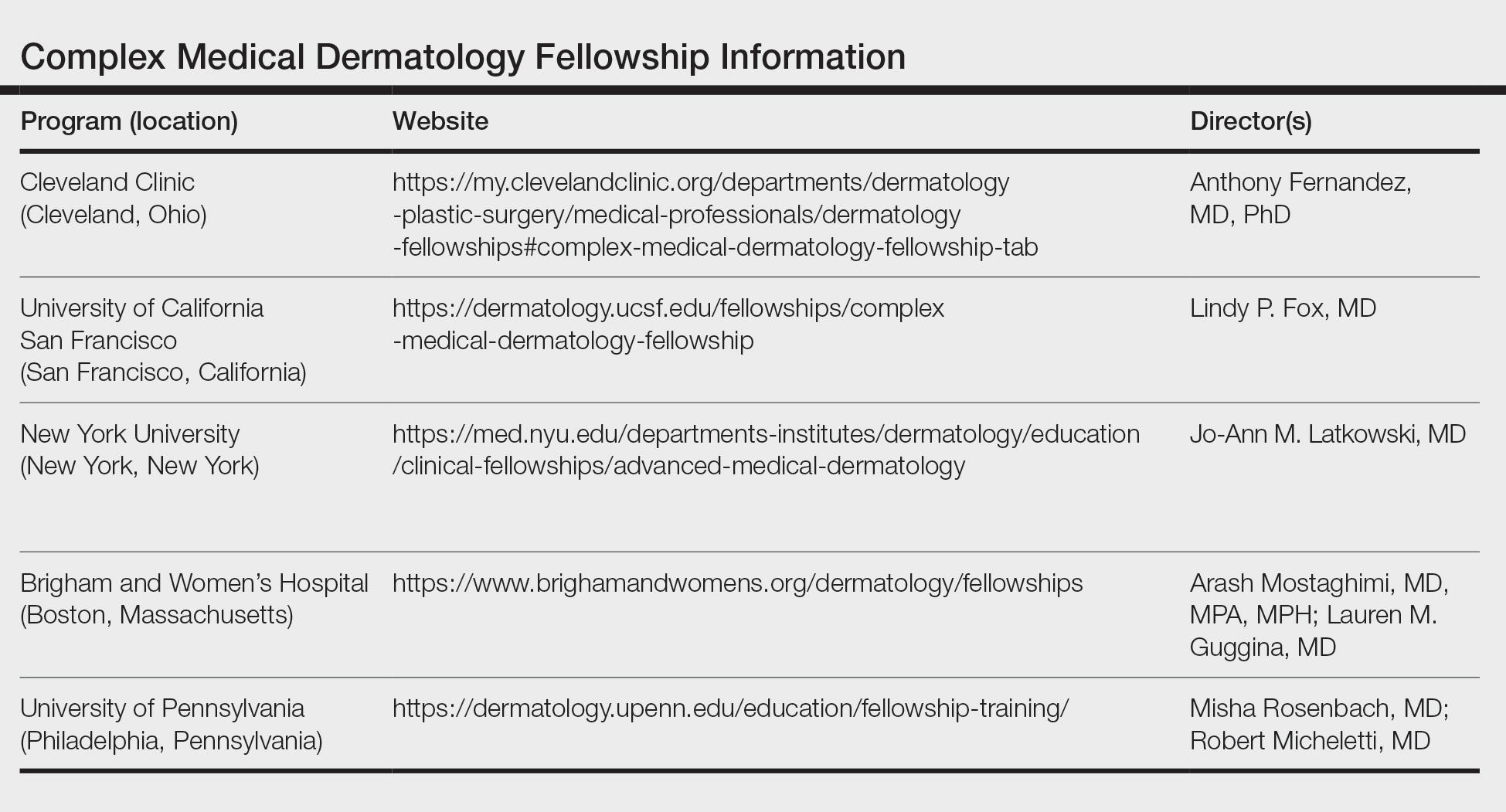
Fellowship Locations—Importantly, the complex medical dermatology fellowship is not accredited by the Accreditation Council for Graduate Medical Education, which can make it difficult to identify and apply to programs. The complex medical dermatology fellowship is different than a rheumatology-dermatology fellowship, cutaneous oncology fellowship, pediatric dermatology fellowship, or other subspecialty fellowships such as those in itch or autoimmune blistering diseases. The fellowship often encompasses gaining clinical expertise in many of these conditions. I performed a thorough search online and spoke with complex medical dermatologists to compile a list of programs that offer a complex medical dermatology fellowship: Brigham and Women’s Hospital (Boston, Massachusetts); University of California San Francisco (San Francisco, California); University of Pennsylvania (Philadelphia, Pennsylvania); Cleveland Clinic (Cleveland, Ohio); and New York University (New York, New York)(Table). Only 1 spot is offered at each of these programs.
Reason to Pursue the Fellowship—There are many reasons to pursue a fellowship in complex medical dermatology such as a desire to enhance exposure to the field, to practice in an academic center and develop a niche within dermatology, to practice dermatology in an inpatient setting, to improve delivery of health care to medically challenging populations in a community setting, and to become an expert on cutaneous manifestations of internal and systemic disease.
Application—There is no standardized application or deadline for this fellowship; however, there is a concerted attempt from some of the programs to offer interviews and decisions at a similar time. Deadlines and contact information are listed on the program websites, along with more details (Table).
Recommendations—I would recommend reaching out at the beginning of postgraduate year (PGY) 4 to these programs and voicing your interest in the fellowship. It is possible to set up an away rotation at some of the programs, and if your program offers elective time, pursuing an away rotation during PGY-3 or early in PGY-4 can prove to be advantageous. Furthermore, during my application cycle I toured the University of California San Francisco, University of Pennsylvania, and Brigham and Women’s Hospital to gain further insight into each program.
Brigham and Women’s Complex Medical Dermatology Fellowship
I am currently the complex medical dermatology fellow at Brigham and Women’s Hospital, and it has been an outstanding experience thus far. The program offers numerous subspecialty clinics focusing solely on cutaneous-oncodermatology, psoriasis, rheumatology-dermatology, skin of color, mole mapping backed by artificial intelligence, cosmetics, high-risk skin cancer, neutrophilic dermatoses, patch testing, phototherapy, psychodermatology, and transplant dermatology. In addition to a wide variety of subspecialty clinics, fellows have the opportunity to participate in inpatient dermatology rounds and act as a junior attending. I appreciate the flexibility of this program combined with the ability to work alongside worldwide experts. There are numerous teaching opportunities, and all of the faculty are amiable and intelligent and emphasize wellness, education, and autonomy. Overall, my experience and decision to pursue a complex medical dermatology fellowship has been extremely rewarding and invaluable. I am gaining additional skills to aid medically challenging patients while pursuing my true passion in dermatology.
1. Sahni DR. Inpatient dermatology consultation services in hospital institutions. Cutis. 2023;111:E11-E12. doi:10.12788/cutis.0776.
Complex medical dermatology has become an emerging field in dermatology. Although a rather protean and broad term, complex medical dermatology encompasses patients with autoimmune conditions, bullous disease, connective tissue disease, vasculitis, severe dermatoses requiring immunomodulation, and inpatient consultations. Importantly, dermatology inpatient consultations aid in lowering health care costs due to accurate diagnoses, correct treatment, and decreased hospital stays.1 A fellowship is not required for holding an inpatient role in the hospital system as a dermatologist but can be beneficial. There are combined internal medicine–dermatology programs available for medical students applying to dermatology residency, but a complex medical dermatology fellowship is an option after residency for those who are interested. I believe that a focused complex medical dermatology fellowship differs from the training offered in combined internal medicine–dermatology residency. My fellow colleagues in combined internal medicine–dermatology programs are exposed to systemic manifestations of cutaneous disease and are experts in the interplay between the skin and other organ systems. However, the focus of their programs is with the intention of becoming double boarded in internal medicine and dermatology with comprehensive exposure to both fields. In my fellowship, I am able to tailor my schedule to focus on any dermatologic disease such as connective tissue disease, pruritus, graft vs host disease, and Merkel cell carcinoma. I ultimately can determine a niche in dermatology and hone my skills for a year under supervision.
Available Fellowships
Fellowship Locations—Importantly, the complex medical dermatology fellowship is not accredited by the Accreditation Council for Graduate Medical Education, which can make it difficult to identify and apply to programs. The complex medical dermatology fellowship is different than a rheumatology-dermatology fellowship, cutaneous oncology fellowship, pediatric dermatology fellowship, or other subspecialty fellowships such as those in itch or autoimmune blistering diseases. The fellowship often encompasses gaining clinical expertise in many of these conditions. I performed a thorough search online and spoke with complex medical dermatologists to compile a list of programs that offer a complex medical dermatology fellowship: Brigham and Women’s Hospital (Boston, Massachusetts); University of California San Francisco (San Francisco, California); University of Pennsylvania (Philadelphia, Pennsylvania); Cleveland Clinic (Cleveland, Ohio); and New York University (New York, New York)(Table). Only 1 spot is offered at each of these programs.

Reason to Pursue the Fellowship—There are many reasons to pursue a fellowship in complex medical dermatology such as a desire to enhance exposure to the field, to practice in an academic center and develop a niche within dermatology, to practice dermatology in an inpatient setting, to improve delivery of health care to medically challenging populations in a community setting, and to become an expert on cutaneous manifestations of internal and systemic disease.
Application—There is no standardized application or deadline for this fellowship; however, there is a concerted attempt from some of the programs to offer interviews and decisions at a similar time. Deadlines and contact information are listed on the program websites, along with more details (Table).
Recommendations—I would recommend reaching out at the beginning of postgraduate year (PGY) 4 to these programs and voicing your interest in the fellowship. It is possible to set up an away rotation at some of the programs, and if your program offers elective time, pursuing an away rotation during PGY-3 or early in PGY-4 can prove to be advantageous. Furthermore, during my application cycle I toured the University of California San Francisco, University of Pennsylvania, and Brigham and Women’s Hospital to gain further insight into each program.
Brigham and Women’s Complex Medical Dermatology Fellowship
I am currently the complex medical dermatology fellow at Brigham and Women’s Hospital, and it has been an outstanding experience thus far. The program offers numerous subspecialty clinics focusing solely on cutaneous-oncodermatology, psoriasis, rheumatology-dermatology, skin of color, mole mapping backed by artificial intelligence, cosmetics, high-risk skin cancer, neutrophilic dermatoses, patch testing, phototherapy, psychodermatology, and transplant dermatology. In addition to a wide variety of subspecialty clinics, fellows have the opportunity to participate in inpatient dermatology rounds and act as a junior attending. I appreciate the flexibility of this program combined with the ability to work alongside worldwide experts. There are numerous teaching opportunities, and all of the faculty are amiable and intelligent and emphasize wellness, education, and autonomy. Overall, my experience and decision to pursue a complex medical dermatology fellowship has been extremely rewarding and invaluable. I am gaining additional skills to aid medically challenging patients while pursuing my true passion in dermatology.
Complex medical dermatology has become an emerging field in dermatology. Although a rather protean and broad term, complex medical dermatology encompasses patients with autoimmune conditions, bullous disease, connective tissue disease, vasculitis, severe dermatoses requiring immunomodulation, and inpatient consultations. Importantly, dermatology inpatient consultations aid in lowering health care costs due to accurate diagnoses, correct treatment, and decreased hospital stays.1 A fellowship is not required for holding an inpatient role in the hospital system as a dermatologist but can be beneficial. There are combined internal medicine–dermatology programs available for medical students applying to dermatology residency, but a complex medical dermatology fellowship is an option after residency for those who are interested. I believe that a focused complex medical dermatology fellowship differs from the training offered in combined internal medicine–dermatology residency. My fellow colleagues in combined internal medicine–dermatology programs are exposed to systemic manifestations of cutaneous disease and are experts in the interplay between the skin and other organ systems. However, the focus of their programs is with the intention of becoming double boarded in internal medicine and dermatology with comprehensive exposure to both fields. In my fellowship, I am able to tailor my schedule to focus on any dermatologic disease such as connective tissue disease, pruritus, graft vs host disease, and Merkel cell carcinoma. I ultimately can determine a niche in dermatology and hone my skills for a year under supervision.
Available Fellowships
Fellowship Locations—Importantly, the complex medical dermatology fellowship is not accredited by the Accreditation Council for Graduate Medical Education, which can make it difficult to identify and apply to programs. The complex medical dermatology fellowship is different than a rheumatology-dermatology fellowship, cutaneous oncology fellowship, pediatric dermatology fellowship, or other subspecialty fellowships such as those in itch or autoimmune blistering diseases. The fellowship often encompasses gaining clinical expertise in many of these conditions. I performed a thorough search online and spoke with complex medical dermatologists to compile a list of programs that offer a complex medical dermatology fellowship: Brigham and Women’s Hospital (Boston, Massachusetts); University of California San Francisco (San Francisco, California); University of Pennsylvania (Philadelphia, Pennsylvania); Cleveland Clinic (Cleveland, Ohio); and New York University (New York, New York)(Table). Only 1 spot is offered at each of these programs.

Reason to Pursue the Fellowship—There are many reasons to pursue a fellowship in complex medical dermatology such as a desire to enhance exposure to the field, to practice in an academic center and develop a niche within dermatology, to practice dermatology in an inpatient setting, to improve delivery of health care to medically challenging populations in a community setting, and to become an expert on cutaneous manifestations of internal and systemic disease.
Application—There is no standardized application or deadline for this fellowship; however, there is a concerted attempt from some of the programs to offer interviews and decisions at a similar time. Deadlines and contact information are listed on the program websites, along with more details (Table).
Recommendations—I would recommend reaching out at the beginning of postgraduate year (PGY) 4 to these programs and voicing your interest in the fellowship. It is possible to set up an away rotation at some of the programs, and if your program offers elective time, pursuing an away rotation during PGY-3 or early in PGY-4 can prove to be advantageous. Furthermore, during my application cycle I toured the University of California San Francisco, University of Pennsylvania, and Brigham and Women’s Hospital to gain further insight into each program.
Brigham and Women’s Complex Medical Dermatology Fellowship
I am currently the complex medical dermatology fellow at Brigham and Women’s Hospital, and it has been an outstanding experience thus far. The program offers numerous subspecialty clinics focusing solely on cutaneous-oncodermatology, psoriasis, rheumatology-dermatology, skin of color, mole mapping backed by artificial intelligence, cosmetics, high-risk skin cancer, neutrophilic dermatoses, patch testing, phototherapy, psychodermatology, and transplant dermatology. In addition to a wide variety of subspecialty clinics, fellows have the opportunity to participate in inpatient dermatology rounds and act as a junior attending. I appreciate the flexibility of this program combined with the ability to work alongside worldwide experts. There are numerous teaching opportunities, and all of the faculty are amiable and intelligent and emphasize wellness, education, and autonomy. Overall, my experience and decision to pursue a complex medical dermatology fellowship has been extremely rewarding and invaluable. I am gaining additional skills to aid medically challenging patients while pursuing my true passion in dermatology.
1. Sahni DR. Inpatient dermatology consultation services in hospital institutions. Cutis. 2023;111:E11-E12. doi:10.12788/cutis.0776.
1. Sahni DR. Inpatient dermatology consultation services in hospital institutions. Cutis. 2023;111:E11-E12. doi:10.12788/cutis.0776.
RESIDENT PEARL
- Complex medical dermatology is a rewarding and fascinating subspecialty of dermatology, and additional training can be accomplished through a fellowship at a variety of prestigious institutions.
Acne stigma persists across social and professional settings
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
FROM JAMA DERMATOLOGY
An 18-month-old male presents with a red mark on the forehead and nose
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
On examination, a faint pink patch was observed on the right forehead, frontal scalp, and nose. The lesion paled under pressure, with small areas of hair loss on the scalp. No atrophy was noted.
CAR T-Cell Therapy: Cure for Systemic Autoimmune Diseases?
A single infusion of autologous CD19-directed CAR T-cell therapy led to persistent, drug-free remission in 15 patients with life-threatening systemic lupus erythematosus, idiopathic inflammatory myositis, or systemic sclerosis, according to research presented at the American Society of Hematology annual meeting.
The responses persisted at 15 months median follow-up, with all patients achieving complete remission, reported Fabian Mueller, MD, of the Bavarian Cancer Research Center and Friedrich-Alexander University of Erlangen-Nuremberg, Bavaria, Germany.
The CAR T-cell treatment appears to provide an “entire reset of B cells,” possibly even a cure, for these 15 patients who had run out of treatment options and had short life expectancies, Dr. Mueller said. “It’s impressive that we have treated these patients.”
Some of the cases have been described previously — including in Annals of the Rheumatic Diseases earlier this year, Nature Medicine in 2022, and the New England Journal of Medicine in 2021.
Now with substantially longer follow-up, the investigators have gained a greater understanding of “the B-cell biology behind our treatment,” Dr. Mueller said. However, “we need longer follow-up to establish how effective the treatment is going to be in the long run.”
All 15 patients included in the analysis were heavily pretreated and had multi-organ involvement. Prior to CAR T-cell therapy, patients had a median disease duration of 3 years, ranging from 1 to as many as 20 years, and had failed a median of five previous treatments. Patients were young — a median age of 36 years — which is much younger than most oncology patients who undergo CAR T-cell therapy, Dr. Mueller said.
The 15 patients underwent typical lymphodepletion and were apheresed and treated with a single infusion of 1 x 106 CD19 CAR T cells per kg of body weight — an established safe dose used in a phase 1 trial of B cell malignancies.
The CAR T cells, manufactured in-house, expanded rapidly, peaking around day 9. B cells disappeared within 7 days and began to reoccur in peripheral blood in all patients between 60 and 180 days. However, no disease flares occurred, Dr. Mueller said.
After 3 months, eight patients with systemic lupus erythematosus showed no sign of disease activity and dramatic improvement in symptoms. Three patients with idiopathic inflammatory myositis experienced major improvements in symptoms and normalization of creatinine kinase levels, the most clinically relevant marker for muscle inflammation. And three of four patients with systemic sclerosis demonstrated major improvements in symptoms and no new disease activity. These responses lasted for a median of 15 months, and all patients stopped taking immunosuppressive drugs.
Patients also tolerated the CAR T-cell treatment well, especially compared with the adverse event profile in oncology patients. Only low-grade inflammatory CAR T-related side effects occurred, and few patients required support for B-cell-derived immune deficiency.
However, infectious complications occurred in 14 patients, including urinary tract and respiratory infections, over the 12-month follow-up. One patient was hospitalized for severe pneumonia a few weeks after CAR T therapy, and two patients experienced herpes zoster reactivations, including one at 6 months and one at 12 months following treatment.
During a press briefing at the ASH conference, Dr. Mueller addressed the “critical question” of patient selection for CAR T-cell therapy, especially in light of the recently announced US Food and Drug Administration investigation exploring whether CAR T cells can cause secondary blood cancers.
Although the T-cell malignancy risk complicates matters, CAR T cells appear to behave differently in patients with autoimmune diseases than those with cancer, he said.
“We don’t understand the biology” related to the malignancy risk yet, Dr. Mueller said, but the benefit for end-of-life patients with no other treatment option likely outweighs the risk. That risk-benefit assessment, however, is more uncertain for those with less severe autoimmune diseases.
For now, it’s important to conduct individual assessments and inform patients about the risk, Dr. Mueller said.
Dr. Mueller disclosed relationships with BMS, AstraZeneca, Gilead, Janssen, Miltenyi Biomedicine, Novartis, Incyte, Abbvie, Sobi, and BeiGene.
A version of this article appeared on Medscape.com.
A single infusion of autologous CD19-directed CAR T-cell therapy led to persistent, drug-free remission in 15 patients with life-threatening systemic lupus erythematosus, idiopathic inflammatory myositis, or systemic sclerosis, according to research presented at the American Society of Hematology annual meeting.
The responses persisted at 15 months median follow-up, with all patients achieving complete remission, reported Fabian Mueller, MD, of the Bavarian Cancer Research Center and Friedrich-Alexander University of Erlangen-Nuremberg, Bavaria, Germany.
The CAR T-cell treatment appears to provide an “entire reset of B cells,” possibly even a cure, for these 15 patients who had run out of treatment options and had short life expectancies, Dr. Mueller said. “It’s impressive that we have treated these patients.”
Some of the cases have been described previously — including in Annals of the Rheumatic Diseases earlier this year, Nature Medicine in 2022, and the New England Journal of Medicine in 2021.
Now with substantially longer follow-up, the investigators have gained a greater understanding of “the B-cell biology behind our treatment,” Dr. Mueller said. However, “we need longer follow-up to establish how effective the treatment is going to be in the long run.”
All 15 patients included in the analysis were heavily pretreated and had multi-organ involvement. Prior to CAR T-cell therapy, patients had a median disease duration of 3 years, ranging from 1 to as many as 20 years, and had failed a median of five previous treatments. Patients were young — a median age of 36 years — which is much younger than most oncology patients who undergo CAR T-cell therapy, Dr. Mueller said.
The 15 patients underwent typical lymphodepletion and were apheresed and treated with a single infusion of 1 x 106 CD19 CAR T cells per kg of body weight — an established safe dose used in a phase 1 trial of B cell malignancies.
The CAR T cells, manufactured in-house, expanded rapidly, peaking around day 9. B cells disappeared within 7 days and began to reoccur in peripheral blood in all patients between 60 and 180 days. However, no disease flares occurred, Dr. Mueller said.
After 3 months, eight patients with systemic lupus erythematosus showed no sign of disease activity and dramatic improvement in symptoms. Three patients with idiopathic inflammatory myositis experienced major improvements in symptoms and normalization of creatinine kinase levels, the most clinically relevant marker for muscle inflammation. And three of four patients with systemic sclerosis demonstrated major improvements in symptoms and no new disease activity. These responses lasted for a median of 15 months, and all patients stopped taking immunosuppressive drugs.
Patients also tolerated the CAR T-cell treatment well, especially compared with the adverse event profile in oncology patients. Only low-grade inflammatory CAR T-related side effects occurred, and few patients required support for B-cell-derived immune deficiency.
However, infectious complications occurred in 14 patients, including urinary tract and respiratory infections, over the 12-month follow-up. One patient was hospitalized for severe pneumonia a few weeks after CAR T therapy, and two patients experienced herpes zoster reactivations, including one at 6 months and one at 12 months following treatment.
During a press briefing at the ASH conference, Dr. Mueller addressed the “critical question” of patient selection for CAR T-cell therapy, especially in light of the recently announced US Food and Drug Administration investigation exploring whether CAR T cells can cause secondary blood cancers.
Although the T-cell malignancy risk complicates matters, CAR T cells appear to behave differently in patients with autoimmune diseases than those with cancer, he said.
“We don’t understand the biology” related to the malignancy risk yet, Dr. Mueller said, but the benefit for end-of-life patients with no other treatment option likely outweighs the risk. That risk-benefit assessment, however, is more uncertain for those with less severe autoimmune diseases.
For now, it’s important to conduct individual assessments and inform patients about the risk, Dr. Mueller said.
Dr. Mueller disclosed relationships with BMS, AstraZeneca, Gilead, Janssen, Miltenyi Biomedicine, Novartis, Incyte, Abbvie, Sobi, and BeiGene.
A version of this article appeared on Medscape.com.
A single infusion of autologous CD19-directed CAR T-cell therapy led to persistent, drug-free remission in 15 patients with life-threatening systemic lupus erythematosus, idiopathic inflammatory myositis, or systemic sclerosis, according to research presented at the American Society of Hematology annual meeting.
The responses persisted at 15 months median follow-up, with all patients achieving complete remission, reported Fabian Mueller, MD, of the Bavarian Cancer Research Center and Friedrich-Alexander University of Erlangen-Nuremberg, Bavaria, Germany.
The CAR T-cell treatment appears to provide an “entire reset of B cells,” possibly even a cure, for these 15 patients who had run out of treatment options and had short life expectancies, Dr. Mueller said. “It’s impressive that we have treated these patients.”
Some of the cases have been described previously — including in Annals of the Rheumatic Diseases earlier this year, Nature Medicine in 2022, and the New England Journal of Medicine in 2021.
Now with substantially longer follow-up, the investigators have gained a greater understanding of “the B-cell biology behind our treatment,” Dr. Mueller said. However, “we need longer follow-up to establish how effective the treatment is going to be in the long run.”
All 15 patients included in the analysis were heavily pretreated and had multi-organ involvement. Prior to CAR T-cell therapy, patients had a median disease duration of 3 years, ranging from 1 to as many as 20 years, and had failed a median of five previous treatments. Patients were young — a median age of 36 years — which is much younger than most oncology patients who undergo CAR T-cell therapy, Dr. Mueller said.
The 15 patients underwent typical lymphodepletion and were apheresed and treated with a single infusion of 1 x 106 CD19 CAR T cells per kg of body weight — an established safe dose used in a phase 1 trial of B cell malignancies.
The CAR T cells, manufactured in-house, expanded rapidly, peaking around day 9. B cells disappeared within 7 days and began to reoccur in peripheral blood in all patients between 60 and 180 days. However, no disease flares occurred, Dr. Mueller said.
After 3 months, eight patients with systemic lupus erythematosus showed no sign of disease activity and dramatic improvement in symptoms. Three patients with idiopathic inflammatory myositis experienced major improvements in symptoms and normalization of creatinine kinase levels, the most clinically relevant marker for muscle inflammation. And three of four patients with systemic sclerosis demonstrated major improvements in symptoms and no new disease activity. These responses lasted for a median of 15 months, and all patients stopped taking immunosuppressive drugs.
Patients also tolerated the CAR T-cell treatment well, especially compared with the adverse event profile in oncology patients. Only low-grade inflammatory CAR T-related side effects occurred, and few patients required support for B-cell-derived immune deficiency.
However, infectious complications occurred in 14 patients, including urinary tract and respiratory infections, over the 12-month follow-up. One patient was hospitalized for severe pneumonia a few weeks after CAR T therapy, and two patients experienced herpes zoster reactivations, including one at 6 months and one at 12 months following treatment.
During a press briefing at the ASH conference, Dr. Mueller addressed the “critical question” of patient selection for CAR T-cell therapy, especially in light of the recently announced US Food and Drug Administration investigation exploring whether CAR T cells can cause secondary blood cancers.
Although the T-cell malignancy risk complicates matters, CAR T cells appear to behave differently in patients with autoimmune diseases than those with cancer, he said.
“We don’t understand the biology” related to the malignancy risk yet, Dr. Mueller said, but the benefit for end-of-life patients with no other treatment option likely outweighs the risk. That risk-benefit assessment, however, is more uncertain for those with less severe autoimmune diseases.
For now, it’s important to conduct individual assessments and inform patients about the risk, Dr. Mueller said.
Dr. Mueller disclosed relationships with BMS, AstraZeneca, Gilead, Janssen, Miltenyi Biomedicine, Novartis, Incyte, Abbvie, Sobi, and BeiGene.
A version of this article appeared on Medscape.com.
FROM ASH 2023
Bimekizumab shows promise for palmoplantar pustular psoriasis
.
PPP is a type of pustular psoriasis that remains a treatment challenge, and available treatments for palmoplantar plaque psoriasis with pustules also “remain unsatisfactory,” according to Thierry Passeron, MD, PhD, of the dermatology service at Centre Hospitalier Universitaire de Nice (France), and colleagues. Bimekizumab, an anti-interleukin (IL)-17A and anti-IL-17F antibody therapy, has been used for psoriasis and psoriatic arthritis (PsA), but its effectiveness for PPP has not been studied, they said. In the United States, bimekizumab (Bimzelx), administered subcutaneously, was recently approved for treating moderate to severe plaque psoriasis in adults; in the European Union, it is approved for treating psoriasis, in addition to psoriatic arthritis, axial spondyloarthritis and ankylosing spondylitis.
In the case series published in JAMA Dermatology, Dr. Passeron and coinvestigators identified 11 adults with PPP and 10 with palmoplantar plaque psoriasis with pustules who were treated at one of seven tertiary dermatology centers in France from September 2022 through June 2023. PPP also has been associated with bone and joint inflammation in SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome.
All patients received bimekizumab for at least 3 months. The patients — 19 women and 2 men — ranged in age from 24 to 68 years (mean age, 46 years). The primary outcome was complete clearance, defined as an Investigator Global Assessment (IGA) score of 0.
A total of 17 patients achieved an IGA score of zero in 1-4 months. Over 3-6 months, three patients achieved an IGA score of 1 (almost clear), and one patient achieved an IGA score of 2 (mild).
Three patients with PPP also had acrodermatitis continua of Hallopeau; in these patients, nail involvement improved by 50%-70% after 4-6 months of bimekizumab use. Two patients with SAPHO experienced complete clearance of skin lesions associated with improvement in joint pain.
Four patients developed oral and genital candidiasis during treatment, but all were treated successfully with antifungals. None of the patients discontinued bimekizumab because of adverse events. “All patients are still receiving treatment, and their psoriatic lesions remain controlled,” the authors wrote.
“The rapid and consistent improvement observed in the present case series supports the effectiveness of bimekizumab therapy in managing PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome,” they said in their discussion.
The study findings were limited by several factors including the small sample size and short follow-up period, and by the inclusion of only patients with severe disease; and prospective, placebo-controlled studies are needed to confirm the results, the researchers noted.
However, the results suggest that bimekizumab could be a treatment approach for PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome, and warrant a prospective, randomized, placebo-controlled, randomized clinical trial to confirm the findings, they concluded.
Dr. Passeron disclosed fees from AbbVie, ACM Pharma, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Calypso, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte, Janssen, LEO Pharma, Eli Lilly, Novartis, Roivant Sciences, Sun Pharmaceuticals, and VYNE Therapeutics outside the current study; he is a cofounder of Yukin Therapeutics. Three authors disclosed receiving personal fees from UCB, manufacturer of bimekizumab, outside of the submitted work, another author disclosed receiving personal fees from UCB during the conduct of the study, and another reported receiving grants from UCB and several other companies, outside the submitted work.
The study findings were also presented at a meeting, Les Journées Dermatologiques de Paris 2023, on December 6, in Paris.
.
PPP is a type of pustular psoriasis that remains a treatment challenge, and available treatments for palmoplantar plaque psoriasis with pustules also “remain unsatisfactory,” according to Thierry Passeron, MD, PhD, of the dermatology service at Centre Hospitalier Universitaire de Nice (France), and colleagues. Bimekizumab, an anti-interleukin (IL)-17A and anti-IL-17F antibody therapy, has been used for psoriasis and psoriatic arthritis (PsA), but its effectiveness for PPP has not been studied, they said. In the United States, bimekizumab (Bimzelx), administered subcutaneously, was recently approved for treating moderate to severe plaque psoriasis in adults; in the European Union, it is approved for treating psoriasis, in addition to psoriatic arthritis, axial spondyloarthritis and ankylosing spondylitis.
In the case series published in JAMA Dermatology, Dr. Passeron and coinvestigators identified 11 adults with PPP and 10 with palmoplantar plaque psoriasis with pustules who were treated at one of seven tertiary dermatology centers in France from September 2022 through June 2023. PPP also has been associated with bone and joint inflammation in SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome.
All patients received bimekizumab for at least 3 months. The patients — 19 women and 2 men — ranged in age from 24 to 68 years (mean age, 46 years). The primary outcome was complete clearance, defined as an Investigator Global Assessment (IGA) score of 0.
A total of 17 patients achieved an IGA score of zero in 1-4 months. Over 3-6 months, three patients achieved an IGA score of 1 (almost clear), and one patient achieved an IGA score of 2 (mild).
Three patients with PPP also had acrodermatitis continua of Hallopeau; in these patients, nail involvement improved by 50%-70% after 4-6 months of bimekizumab use. Two patients with SAPHO experienced complete clearance of skin lesions associated with improvement in joint pain.
Four patients developed oral and genital candidiasis during treatment, but all were treated successfully with antifungals. None of the patients discontinued bimekizumab because of adverse events. “All patients are still receiving treatment, and their psoriatic lesions remain controlled,” the authors wrote.
“The rapid and consistent improvement observed in the present case series supports the effectiveness of bimekizumab therapy in managing PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome,” they said in their discussion.
The study findings were limited by several factors including the small sample size and short follow-up period, and by the inclusion of only patients with severe disease; and prospective, placebo-controlled studies are needed to confirm the results, the researchers noted.
However, the results suggest that bimekizumab could be a treatment approach for PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome, and warrant a prospective, randomized, placebo-controlled, randomized clinical trial to confirm the findings, they concluded.
Dr. Passeron disclosed fees from AbbVie, ACM Pharma, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Calypso, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte, Janssen, LEO Pharma, Eli Lilly, Novartis, Roivant Sciences, Sun Pharmaceuticals, and VYNE Therapeutics outside the current study; he is a cofounder of Yukin Therapeutics. Three authors disclosed receiving personal fees from UCB, manufacturer of bimekizumab, outside of the submitted work, another author disclosed receiving personal fees from UCB during the conduct of the study, and another reported receiving grants from UCB and several other companies, outside the submitted work.
The study findings were also presented at a meeting, Les Journées Dermatologiques de Paris 2023, on December 6, in Paris.
.
PPP is a type of pustular psoriasis that remains a treatment challenge, and available treatments for palmoplantar plaque psoriasis with pustules also “remain unsatisfactory,” according to Thierry Passeron, MD, PhD, of the dermatology service at Centre Hospitalier Universitaire de Nice (France), and colleagues. Bimekizumab, an anti-interleukin (IL)-17A and anti-IL-17F antibody therapy, has been used for psoriasis and psoriatic arthritis (PsA), but its effectiveness for PPP has not been studied, they said. In the United States, bimekizumab (Bimzelx), administered subcutaneously, was recently approved for treating moderate to severe plaque psoriasis in adults; in the European Union, it is approved for treating psoriasis, in addition to psoriatic arthritis, axial spondyloarthritis and ankylosing spondylitis.
In the case series published in JAMA Dermatology, Dr. Passeron and coinvestigators identified 11 adults with PPP and 10 with palmoplantar plaque psoriasis with pustules who were treated at one of seven tertiary dermatology centers in France from September 2022 through June 2023. PPP also has been associated with bone and joint inflammation in SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome.
All patients received bimekizumab for at least 3 months. The patients — 19 women and 2 men — ranged in age from 24 to 68 years (mean age, 46 years). The primary outcome was complete clearance, defined as an Investigator Global Assessment (IGA) score of 0.
A total of 17 patients achieved an IGA score of zero in 1-4 months. Over 3-6 months, three patients achieved an IGA score of 1 (almost clear), and one patient achieved an IGA score of 2 (mild).
Three patients with PPP also had acrodermatitis continua of Hallopeau; in these patients, nail involvement improved by 50%-70% after 4-6 months of bimekizumab use. Two patients with SAPHO experienced complete clearance of skin lesions associated with improvement in joint pain.
Four patients developed oral and genital candidiasis during treatment, but all were treated successfully with antifungals. None of the patients discontinued bimekizumab because of adverse events. “All patients are still receiving treatment, and their psoriatic lesions remain controlled,” the authors wrote.
“The rapid and consistent improvement observed in the present case series supports the effectiveness of bimekizumab therapy in managing PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome,” they said in their discussion.
The study findings were limited by several factors including the small sample size and short follow-up period, and by the inclusion of only patients with severe disease; and prospective, placebo-controlled studies are needed to confirm the results, the researchers noted.
However, the results suggest that bimekizumab could be a treatment approach for PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome, and warrant a prospective, randomized, placebo-controlled, randomized clinical trial to confirm the findings, they concluded.
Dr. Passeron disclosed fees from AbbVie, ACM Pharma, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Calypso, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte, Janssen, LEO Pharma, Eli Lilly, Novartis, Roivant Sciences, Sun Pharmaceuticals, and VYNE Therapeutics outside the current study; he is a cofounder of Yukin Therapeutics. Three authors disclosed receiving personal fees from UCB, manufacturer of bimekizumab, outside of the submitted work, another author disclosed receiving personal fees from UCB during the conduct of the study, and another reported receiving grants from UCB and several other companies, outside the submitted work.
The study findings were also presented at a meeting, Les Journées Dermatologiques de Paris 2023, on December 6, in Paris.
FROM JAMA DERMATOLOGY
Tape strips detect hidradenitis suppurativa biomarkers, novel study shows
, results from a novel study showed.
“Tape strips can provide important clues to when and which drugs to use in HS in patients with both early and late disease, which can change clinical practice,” corresponding study author Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai in New York City, said in an interview. “It is noninvasive and nonscarring,” she added.
Tape stripping has been validated in atopic dermatitis, psoriasis, and other dermatologic conditions in recent years. For the current study, which was published online in the Journal of the American Academy of Dermatology, and is believed to be the first of its kind, Dr. Guttman-Yassky and colleagues performed RNA sequencing from large D-Squame tape strips collected from lesional and nonlesional skin of 22 patients with HS and from 21 age- and sex-matched healthy controls. They correlated the expression of skin biomarkers between tape strips and a previously published gene-signature of HS biopsies. The mean age of patients with HS was 43 years, while the mean age of healthy controls was 35. The average International Hidradenitis Suppurativa Severity Score System (IHS4) score of the HS cohort was 36.
Consistent with published studies, the researchers found that tape strips identified an overall higher inflammatory burden in HS. Specifically, they observed an upregulation of known cytokines within the following pathways: Th1 (such as IFNG, CXCL9/10/11, and CCR5); Th17 (such as interleukin [IL]-17A/F, IL12B, IL23A, CAMP, and CCL20); Th2 (such as IL4R, IL13/IL31/IL10, CCR4, CCL7/CCL13/CCL24, TNFSF4/OX40L, and TNFRSF4/OX40); and Th22 (such as IL22 and IL32).
The researchers also found that the expression of Th17 and tumor necrosis factor (TNF)–alpha pathways were highly correlated between tape strips and biopsies and that HS clinical severity was significantly associated with expression of biomarkers, such as TNF-alpha, IL17A/F, OX40, JAK1-3, and IL4R in HS lesional and/or nonlesional skin.
“It was quite unexpected that we are able to identify, using a minimally invasive approach that samples only the upper layers of the epidermis, products and processes that are considered to be deeper-situated, such as IL-17, and other immune markers,” Dr. Guttman-Yassky said in the interview. “We were also surprised to see how well the tape-stripped–derived skin molecular profile correlated with that of biopsies, as well as how well it correlated with the clinical disease severity of HS.”
Also surprising, she added, was that the biomarkers in nonlesional tape-stripped skin, such as IL-17 and TNF alpha, “show high correlations with disease severity and provide clues to early disease.”
If using tape strips in HS is validated in larger cohort studies, the potential cost implications of using this approach in practice remain unclear, Dr. Guttman-Yassky said. “It is currently not cheap, but we are hoping that one day, we can provide a means to diagnose the disease and treat it early, and appropriately, utilizing this approach,” she commented. “We are excited about the applicability of this study to the early treatment and longitudinal follow up of HS with drugs that are targeting specific immune molecules and pathways,” she said, adding that it will also be useful for helping determine which drug should be used for which patient.
She and her co-authors acknowledged certain limitations of the study, including its small sample size and the fact that tape stripping is limited to the epidermis.
Asked to comment on the study, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, said the findings “have important potential implications for our ability to one day personalize treatments for a patient with early HS in a minimally invasive way.”
As the study authors point out, she added, “tape strips only allow sampling of the epidermis, which is limiting in a disease like HS where much of the disruption is in the dermis with deep nodules and dermal tunnels. However, our overall goal should be to catch patients in the early stages of their disease before the occurrence of irreversible tissue damage such as dermal tunnels. Thus, the ongoing campaign for early diagnosis and early intervention by various stakeholders in the field of HS can help mitigate the impact of this inherent limitation of tape strips. It will be exciting to see larger studies that investigate tape strip results in relation to clinical phenotypes, disease progression, and therapeutic responses.”
The study was funded by an International Dermatology Outcome Measures Hidradenitis Suppurativa Grant. Dr. Guttman-Yassky disclosed that she has been a consultant to, an adviser for, and has received research grants from many pharmaceutical companies. Of the remaining authors, 2 also had multiple disclosures and 11 had no disclosures. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
, results from a novel study showed.
“Tape strips can provide important clues to when and which drugs to use in HS in patients with both early and late disease, which can change clinical practice,” corresponding study author Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai in New York City, said in an interview. “It is noninvasive and nonscarring,” she added.
Tape stripping has been validated in atopic dermatitis, psoriasis, and other dermatologic conditions in recent years. For the current study, which was published online in the Journal of the American Academy of Dermatology, and is believed to be the first of its kind, Dr. Guttman-Yassky and colleagues performed RNA sequencing from large D-Squame tape strips collected from lesional and nonlesional skin of 22 patients with HS and from 21 age- and sex-matched healthy controls. They correlated the expression of skin biomarkers between tape strips and a previously published gene-signature of HS biopsies. The mean age of patients with HS was 43 years, while the mean age of healthy controls was 35. The average International Hidradenitis Suppurativa Severity Score System (IHS4) score of the HS cohort was 36.
Consistent with published studies, the researchers found that tape strips identified an overall higher inflammatory burden in HS. Specifically, they observed an upregulation of known cytokines within the following pathways: Th1 (such as IFNG, CXCL9/10/11, and CCR5); Th17 (such as interleukin [IL]-17A/F, IL12B, IL23A, CAMP, and CCL20); Th2 (such as IL4R, IL13/IL31/IL10, CCR4, CCL7/CCL13/CCL24, TNFSF4/OX40L, and TNFRSF4/OX40); and Th22 (such as IL22 and IL32).
The researchers also found that the expression of Th17 and tumor necrosis factor (TNF)–alpha pathways were highly correlated between tape strips and biopsies and that HS clinical severity was significantly associated with expression of biomarkers, such as TNF-alpha, IL17A/F, OX40, JAK1-3, and IL4R in HS lesional and/or nonlesional skin.
“It was quite unexpected that we are able to identify, using a minimally invasive approach that samples only the upper layers of the epidermis, products and processes that are considered to be deeper-situated, such as IL-17, and other immune markers,” Dr. Guttman-Yassky said in the interview. “We were also surprised to see how well the tape-stripped–derived skin molecular profile correlated with that of biopsies, as well as how well it correlated with the clinical disease severity of HS.”
Also surprising, she added, was that the biomarkers in nonlesional tape-stripped skin, such as IL-17 and TNF alpha, “show high correlations with disease severity and provide clues to early disease.”
If using tape strips in HS is validated in larger cohort studies, the potential cost implications of using this approach in practice remain unclear, Dr. Guttman-Yassky said. “It is currently not cheap, but we are hoping that one day, we can provide a means to diagnose the disease and treat it early, and appropriately, utilizing this approach,” she commented. “We are excited about the applicability of this study to the early treatment and longitudinal follow up of HS with drugs that are targeting specific immune molecules and pathways,” she said, adding that it will also be useful for helping determine which drug should be used for which patient.
She and her co-authors acknowledged certain limitations of the study, including its small sample size and the fact that tape stripping is limited to the epidermis.
Asked to comment on the study, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, said the findings “have important potential implications for our ability to one day personalize treatments for a patient with early HS in a minimally invasive way.”
As the study authors point out, she added, “tape strips only allow sampling of the epidermis, which is limiting in a disease like HS where much of the disruption is in the dermis with deep nodules and dermal tunnels. However, our overall goal should be to catch patients in the early stages of their disease before the occurrence of irreversible tissue damage such as dermal tunnels. Thus, the ongoing campaign for early diagnosis and early intervention by various stakeholders in the field of HS can help mitigate the impact of this inherent limitation of tape strips. It will be exciting to see larger studies that investigate tape strip results in relation to clinical phenotypes, disease progression, and therapeutic responses.”
The study was funded by an International Dermatology Outcome Measures Hidradenitis Suppurativa Grant. Dr. Guttman-Yassky disclosed that she has been a consultant to, an adviser for, and has received research grants from many pharmaceutical companies. Of the remaining authors, 2 also had multiple disclosures and 11 had no disclosures. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
, results from a novel study showed.
“Tape strips can provide important clues to when and which drugs to use in HS in patients with both early and late disease, which can change clinical practice,” corresponding study author Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai in New York City, said in an interview. “It is noninvasive and nonscarring,” she added.
Tape stripping has been validated in atopic dermatitis, psoriasis, and other dermatologic conditions in recent years. For the current study, which was published online in the Journal of the American Academy of Dermatology, and is believed to be the first of its kind, Dr. Guttman-Yassky and colleagues performed RNA sequencing from large D-Squame tape strips collected from lesional and nonlesional skin of 22 patients with HS and from 21 age- and sex-matched healthy controls. They correlated the expression of skin biomarkers between tape strips and a previously published gene-signature of HS biopsies. The mean age of patients with HS was 43 years, while the mean age of healthy controls was 35. The average International Hidradenitis Suppurativa Severity Score System (IHS4) score of the HS cohort was 36.
Consistent with published studies, the researchers found that tape strips identified an overall higher inflammatory burden in HS. Specifically, they observed an upregulation of known cytokines within the following pathways: Th1 (such as IFNG, CXCL9/10/11, and CCR5); Th17 (such as interleukin [IL]-17A/F, IL12B, IL23A, CAMP, and CCL20); Th2 (such as IL4R, IL13/IL31/IL10, CCR4, CCL7/CCL13/CCL24, TNFSF4/OX40L, and TNFRSF4/OX40); and Th22 (such as IL22 and IL32).
The researchers also found that the expression of Th17 and tumor necrosis factor (TNF)–alpha pathways were highly correlated between tape strips and biopsies and that HS clinical severity was significantly associated with expression of biomarkers, such as TNF-alpha, IL17A/F, OX40, JAK1-3, and IL4R in HS lesional and/or nonlesional skin.
“It was quite unexpected that we are able to identify, using a minimally invasive approach that samples only the upper layers of the epidermis, products and processes that are considered to be deeper-situated, such as IL-17, and other immune markers,” Dr. Guttman-Yassky said in the interview. “We were also surprised to see how well the tape-stripped–derived skin molecular profile correlated with that of biopsies, as well as how well it correlated with the clinical disease severity of HS.”
Also surprising, she added, was that the biomarkers in nonlesional tape-stripped skin, such as IL-17 and TNF alpha, “show high correlations with disease severity and provide clues to early disease.”
If using tape strips in HS is validated in larger cohort studies, the potential cost implications of using this approach in practice remain unclear, Dr. Guttman-Yassky said. “It is currently not cheap, but we are hoping that one day, we can provide a means to diagnose the disease and treat it early, and appropriately, utilizing this approach,” she commented. “We are excited about the applicability of this study to the early treatment and longitudinal follow up of HS with drugs that are targeting specific immune molecules and pathways,” she said, adding that it will also be useful for helping determine which drug should be used for which patient.
She and her co-authors acknowledged certain limitations of the study, including its small sample size and the fact that tape stripping is limited to the epidermis.
Asked to comment on the study, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, said the findings “have important potential implications for our ability to one day personalize treatments for a patient with early HS in a minimally invasive way.”
As the study authors point out, she added, “tape strips only allow sampling of the epidermis, which is limiting in a disease like HS where much of the disruption is in the dermis with deep nodules and dermal tunnels. However, our overall goal should be to catch patients in the early stages of their disease before the occurrence of irreversible tissue damage such as dermal tunnels. Thus, the ongoing campaign for early diagnosis and early intervention by various stakeholders in the field of HS can help mitigate the impact of this inherent limitation of tape strips. It will be exciting to see larger studies that investigate tape strip results in relation to clinical phenotypes, disease progression, and therapeutic responses.”
The study was funded by an International Dermatology Outcome Measures Hidradenitis Suppurativa Grant. Dr. Guttman-Yassky disclosed that she has been a consultant to, an adviser for, and has received research grants from many pharmaceutical companies. Of the remaining authors, 2 also had multiple disclosures and 11 had no disclosures. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Global measles deaths increased by 43% in 2022
The number of total reported cases rose by 18% over the same period, accounting for approximately 9 million cases and 136,000 deaths globally, mostly among children. This information comes from a new report by the World Health Organization (WHO), published in partnership with the US Centers for Disease Control and Prevention (CDC).
More Measles Outbreaks
The report also notes an increase in the number of countries experiencing significant measles outbreaks. There were 37 such countries in 2022, compared with 22 the previous year. The most affected continents were Africa and Asia.
“The rise in measles outbreaks and deaths is impressive but, unfortunately, not surprising, given the decline in vaccination rates in recent years,” said John Vertefeuille, PhD, director of the CDC’s Global Immunization Division.
Vertefeuille emphasized that measles cases anywhere in the world pose a risk to “countries and communities where people are undervaccinated.” In recent years, several regions have fallen short of their immunization targets.
Vaccination Trends
In 2022, there was a slight increase in measles vaccination after a decline exacerbated by the COVID-19 pandemic and its impact on global healthcare systems. However, 33 million children did not receive at least one dose of the vaccine last year: 22 million missed the first dose, and 11 million missed the second.
For communities to be considered protected against outbreaks, immunization coverage with the full vaccine cycle should be at least 95%. The global coverage rate for the first dose was 83%, and for the second, it was 74%.
Nevertheless, immunization recovery has not reached the poorest countries, where the immunization rate stands at 66%. Brazil is among the top 10 countries where more children missed the first dose in 2022. These nations account for over half of the 22 million unadministered vaccines. According to the report, half a million children did not receive the vaccine in Brazil.
Measles in Brazil
Brazil’s results highlight setbacks in vaccination efforts. In 2016, the country was certified to have eliminated measles, but after experiencing outbreaks in 2018, the certification was lost in 2019. In 2018, Brazil confirmed 9325 cases. The situation worsened in 2019 with 20,901 diagnoses. Since then, numbers have been decreasing: 8100 in 2020, 676 in 2021, and 44 in 2022.
Last year, four Brazilian states reported confirmed virus cases: Rio de Janeiro, Pará, São Paulo, and Amapá. Ministry of Health data indicated no confirmed measles cases in Brazil as of June 15, 2023.
Vaccination in Brazil
Vaccination coverage in Brazil, which once reached 95%, has sharply declined in recent years. The rate of patients receiving the full immunization scheme was 59% in 2021.
Globally, although the COVID-19 pandemic affected measles vaccination, measures like social isolation and mask use potentially contributed to reducing measles cases. The incidence of the disease decreased in 2020 and 2021 but is now rising again.
“From 2021 to 2022, reported measles cases increased by 67% worldwide, and the number of countries experiencing large or disruptive outbreaks increased by 68%,” the report stated.
Because of these data, the WHO and the CDC urge increased efforts for vaccination, along with improvements in epidemiological surveillance systems, especially in developing nations. “Children everywhere have the right to be protected by the lifesaving measles vaccine, no matter where they live,” said Kate O’Brien, MD, director of immunization, vaccines, and biologicals at the WHO.
“Measles is called the virus of inequality for a good reason. It is the disease that will find and attack those who are not protected.”
This article was translated from the Medscape Portuguese edition.
The number of total reported cases rose by 18% over the same period, accounting for approximately 9 million cases and 136,000 deaths globally, mostly among children. This information comes from a new report by the World Health Organization (WHO), published in partnership with the US Centers for Disease Control and Prevention (CDC).
More Measles Outbreaks
The report also notes an increase in the number of countries experiencing significant measles outbreaks. There were 37 such countries in 2022, compared with 22 the previous year. The most affected continents were Africa and Asia.
“The rise in measles outbreaks and deaths is impressive but, unfortunately, not surprising, given the decline in vaccination rates in recent years,” said John Vertefeuille, PhD, director of the CDC’s Global Immunization Division.
Vertefeuille emphasized that measles cases anywhere in the world pose a risk to “countries and communities where people are undervaccinated.” In recent years, several regions have fallen short of their immunization targets.
Vaccination Trends
In 2022, there was a slight increase in measles vaccination after a decline exacerbated by the COVID-19 pandemic and its impact on global healthcare systems. However, 33 million children did not receive at least one dose of the vaccine last year: 22 million missed the first dose, and 11 million missed the second.
For communities to be considered protected against outbreaks, immunization coverage with the full vaccine cycle should be at least 95%. The global coverage rate for the first dose was 83%, and for the second, it was 74%.
Nevertheless, immunization recovery has not reached the poorest countries, where the immunization rate stands at 66%. Brazil is among the top 10 countries where more children missed the first dose in 2022. These nations account for over half of the 22 million unadministered vaccines. According to the report, half a million children did not receive the vaccine in Brazil.
Measles in Brazil
Brazil’s results highlight setbacks in vaccination efforts. In 2016, the country was certified to have eliminated measles, but after experiencing outbreaks in 2018, the certification was lost in 2019. In 2018, Brazil confirmed 9325 cases. The situation worsened in 2019 with 20,901 diagnoses. Since then, numbers have been decreasing: 8100 in 2020, 676 in 2021, and 44 in 2022.
Last year, four Brazilian states reported confirmed virus cases: Rio de Janeiro, Pará, São Paulo, and Amapá. Ministry of Health data indicated no confirmed measles cases in Brazil as of June 15, 2023.
Vaccination in Brazil
Vaccination coverage in Brazil, which once reached 95%, has sharply declined in recent years. The rate of patients receiving the full immunization scheme was 59% in 2021.
Globally, although the COVID-19 pandemic affected measles vaccination, measures like social isolation and mask use potentially contributed to reducing measles cases. The incidence of the disease decreased in 2020 and 2021 but is now rising again.
“From 2021 to 2022, reported measles cases increased by 67% worldwide, and the number of countries experiencing large or disruptive outbreaks increased by 68%,” the report stated.
Because of these data, the WHO and the CDC urge increased efforts for vaccination, along with improvements in epidemiological surveillance systems, especially in developing nations. “Children everywhere have the right to be protected by the lifesaving measles vaccine, no matter where they live,” said Kate O’Brien, MD, director of immunization, vaccines, and biologicals at the WHO.
“Measles is called the virus of inequality for a good reason. It is the disease that will find and attack those who are not protected.”
This article was translated from the Medscape Portuguese edition.
The number of total reported cases rose by 18% over the same period, accounting for approximately 9 million cases and 136,000 deaths globally, mostly among children. This information comes from a new report by the World Health Organization (WHO), published in partnership with the US Centers for Disease Control and Prevention (CDC).
More Measles Outbreaks
The report also notes an increase in the number of countries experiencing significant measles outbreaks. There were 37 such countries in 2022, compared with 22 the previous year. The most affected continents were Africa and Asia.
“The rise in measles outbreaks and deaths is impressive but, unfortunately, not surprising, given the decline in vaccination rates in recent years,” said John Vertefeuille, PhD, director of the CDC’s Global Immunization Division.
Vertefeuille emphasized that measles cases anywhere in the world pose a risk to “countries and communities where people are undervaccinated.” In recent years, several regions have fallen short of their immunization targets.
Vaccination Trends
In 2022, there was a slight increase in measles vaccination after a decline exacerbated by the COVID-19 pandemic and its impact on global healthcare systems. However, 33 million children did not receive at least one dose of the vaccine last year: 22 million missed the first dose, and 11 million missed the second.
For communities to be considered protected against outbreaks, immunization coverage with the full vaccine cycle should be at least 95%. The global coverage rate for the first dose was 83%, and for the second, it was 74%.
Nevertheless, immunization recovery has not reached the poorest countries, where the immunization rate stands at 66%. Brazil is among the top 10 countries where more children missed the first dose in 2022. These nations account for over half of the 22 million unadministered vaccines. According to the report, half a million children did not receive the vaccine in Brazil.
Measles in Brazil
Brazil’s results highlight setbacks in vaccination efforts. In 2016, the country was certified to have eliminated measles, but after experiencing outbreaks in 2018, the certification was lost in 2019. In 2018, Brazil confirmed 9325 cases. The situation worsened in 2019 with 20,901 diagnoses. Since then, numbers have been decreasing: 8100 in 2020, 676 in 2021, and 44 in 2022.
Last year, four Brazilian states reported confirmed virus cases: Rio de Janeiro, Pará, São Paulo, and Amapá. Ministry of Health data indicated no confirmed measles cases in Brazil as of June 15, 2023.
Vaccination in Brazil
Vaccination coverage in Brazil, which once reached 95%, has sharply declined in recent years. The rate of patients receiving the full immunization scheme was 59% in 2021.
Globally, although the COVID-19 pandemic affected measles vaccination, measures like social isolation and mask use potentially contributed to reducing measles cases. The incidence of the disease decreased in 2020 and 2021 but is now rising again.
“From 2021 to 2022, reported measles cases increased by 67% worldwide, and the number of countries experiencing large or disruptive outbreaks increased by 68%,” the report stated.
Because of these data, the WHO and the CDC urge increased efforts for vaccination, along with improvements in epidemiological surveillance systems, especially in developing nations. “Children everywhere have the right to be protected by the lifesaving measles vaccine, no matter where they live,” said Kate O’Brien, MD, director of immunization, vaccines, and biologicals at the WHO.
“Measles is called the virus of inequality for a good reason. It is the disease that will find and attack those who are not protected.”
This article was translated from the Medscape Portuguese edition.