User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Furuncular Myiasis in 2 American Travelers Returning From Senegal
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
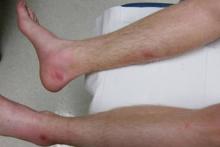
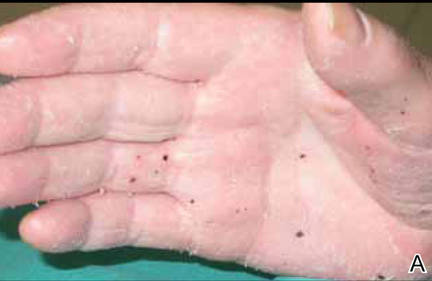
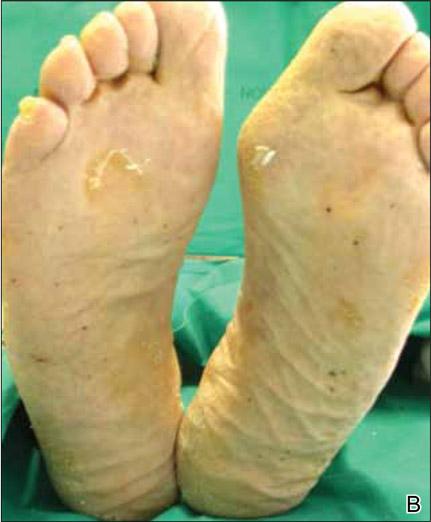
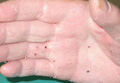
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
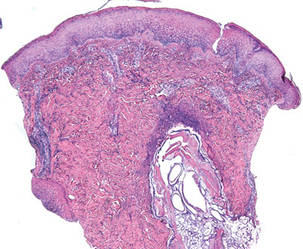
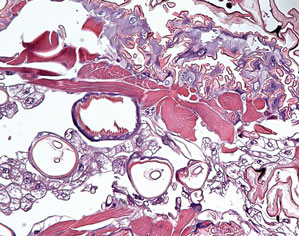
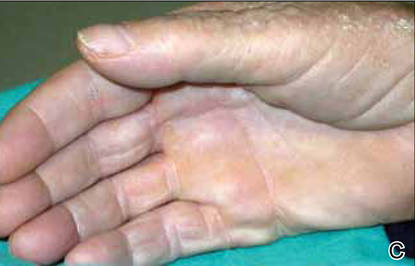
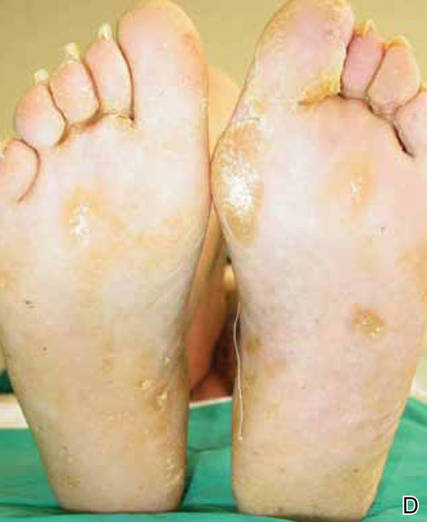
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.
  |
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.
  |
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.
  |
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
Practice Points
- Cutaneous myiasis is caused by an infestation of fly larvae and can present as furuncles (furuncular myiasis), migratory inflammatory linear plaques (migratory myiasis), and worsening tissue destruction in existing wounds (wound myiasis).
- Furuncular myiasis should be included in the differential diagnosis in patients with furuncular skin lesions who have recently traveled to Central America, South America, or sub-Saharan Africa.
- Furuncular myiasis may be treated by both occlusive and extraction techniques.
Nodular Extramammary Paget Disease With Fibroepitheliomatous Hyperplasia
Extramammary Paget disease (EMPD) is an uncommon neoplasm that most commonly occurs in the anogenital region but can arise in any area of the skin or mucosa.1 On clinical examination, EMPD typically presents as a sharply demarcated, erythematous, eczematoid, weeping lesion with varying degrees of induration; it rarely presents as a palpable mass or evenly raised nodule.2 Microscopically, it may be accompanied by varying degrees of epidermal hyperplasia.1 In particular, fibroepitheliomatous hyperplasia contains lacy strands of squamous epithelium resembling fibroepithelioma of Pinkus.3 We report a case of EMPD in a 90-year-old man who presented with a verrucous nodule in the pubic area that histologically demonstrated fibroepitheliomatous hyperplasia with lacy strands of squamous epithelium.
Case Report
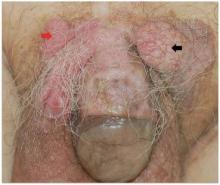
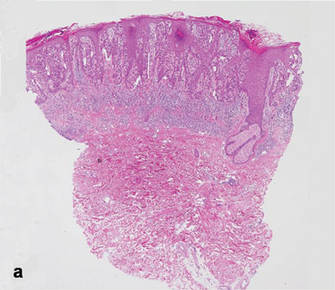
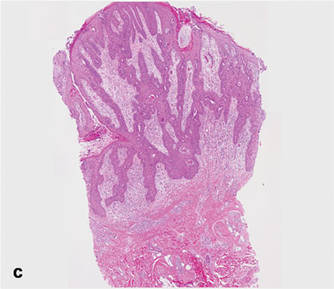
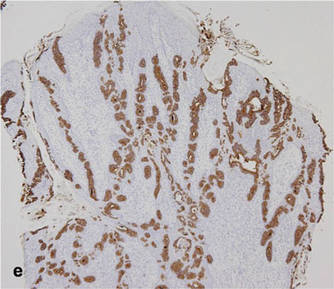
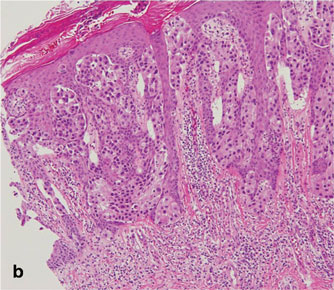
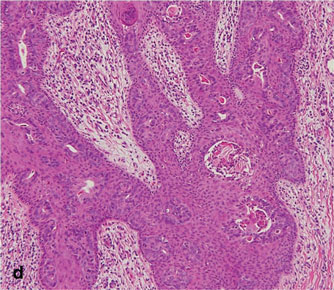
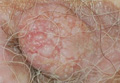
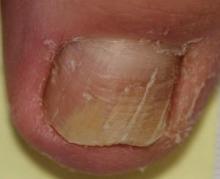
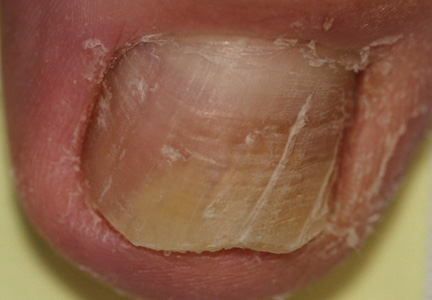
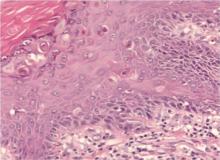
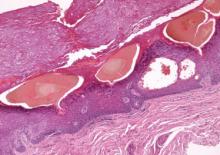
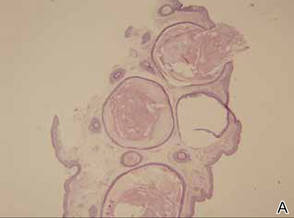
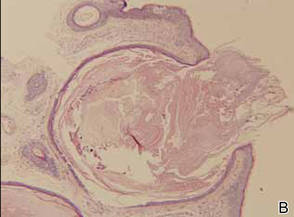
A 90-year-old man presented with asymptomatic, well-demarcated, erythematous plaques in the pubic area of 5 years’ duration, along with a 3.0×2.5-cm nodule on the left side of the pubic area (Figure 1). Laboratory test results including a complete blood cell count, blood chemistry, and routine urinalysis were within reference range. Punch biopsies were taken from each plaque and nodule, as marked with arrows in Figure 1. Histopathologically, the plaques were seen to contain a number of large round cells with abundant pale cytoplasm and pleomorphic hyperchromatic nuclei that were present at various levels of the epidermis where they formed nests and clusters but did not extend into the dermis (Figures 2A and 2B). The nodule contained lacy strands of squamous epithelium extending from the epidermis to the mid dermis as well as many glandular structures (Figures 2C and 2D). The cells in the epidermis stained positively with periodic acid–Schiff (PAS), carcinoembryonic antigen (CEA), and cytokeratin 7 (Figure 2E). We also tested for S-100 protein to rule out malignant melanoma, which was negative.
Based on both the clinical and histological features, a diagnosis of EMPD with fibroepitheliomatous hyperplasia was made. It was recommended that the patient undergo further evaluation and treatment; he declined due to his financial situation and was subsequently lost to follow-up.
Comment
Clinically, EMPD usually presents as a patch of macular erythema, an erythematous eruption, or erythematous papules and plaques.4 The palpable nodule seen in our patient is not a common presentation of EMPD. Pruritus is the most common symptom of EMPD, occurring in 70% of patients.5 Other symptoms include burning, irritation, pain, tenderness, bleeding, and swelling. Ten percent of EMPD cases are asymptomatic.5
Histologically, Paget cells primarily involve the epidermis where they usually form clusters or solid nests. In more than 90% of EMPD cases, the Paget cells contain cytoplasmic mucin that stains positively with mucicarmine and PAS. Immunohistochemical staining for cytokeratin 7, gross cystic disease fluid protein-15, S-100 protein, and CEA sometimes may be needed to differentiate from mimickers such as Bowen disease and superficial spreading melanoma.6 In our patient, the tumor cells stained positive for cytokeratin 7, CEA, and PAS. Malignant melanoma was ruled out with a test for S-100 protein.
|
|
Extramammary Paget disease often is associated with epidermal hyperplasia, which can be classified as squamous, papillomatous, or fibroepitheliomatous.3 Microscopically, squamous hyperplasia is characterized by prominent thickening of the epidermis from diffuse plaquelike hyperplasia and is usually associated with hyperkeratosis. Papillomatous hyperplasia has an exophytic papillary or verrucous architecture and is associated with parakeratosis. Fibroepitheliomatous, or fibroepitheliomalike, hyperplasia generally consists of a discrete, broad, elevated plaque or nodule produced by hyperplasia of keratinocytes that form lacy strands of squamous epithelium.3 The biphasic pattern of proliferating epidermis and entrapped dermis simulates a so-called fibroepithelioma. Paget cells can be seen within the lacy strands of epidermal columns and in the acanthotic surface component.2 The finding of fibroepitheliomatous hyperplasia in anogenital skin should prompt a search for the diagnostic Paget cells to eliminate a fibroepithelioma of Pinkus variant of basal cell carcinoma, though the latter is uncommon and rarely occurs at this site.7
Of the 3 types of epidermal hyperplasia, our case demonstrated the fibroepitheliomatous type. There may be some relationship between EMPD and fibroepitheliomatous hyperplasia because most reported cases of EMPD with fibroepitheliomatous hyperplasia have occurred in the anogenital region. Also, epidermal hyperplasia is more frequent in anogenital Paget disease than in axillary Paget disease.8
Conclusion
Our case showed the unique finding of a verrucous nodular EMPD lesion in which peculiar histological features presented as extensions of the tumor cells forming lacy strands of squamous epithelium from the epidermis to the mid dermis as well as many glandular structures.
1. Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease. J Clin Pathol. 2000;53:742-749.
2. Billings SD, Roth LM. Pseudoinvasive, nodular extramam-mary Paget’s disease of the vulva. Arch Pathol Lab Med. 1998;122:471-474.
3. Brainard JA, Hart WR. Proliferative epidermal lesions associated with anogenital Paget’s disease. Am J Surg Pathol. 2000;24:543-552.
4. Neuhaus IM, Grekin RC. Mammary and extramammary Paget disease. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 1. 7th ed. New York, NY: McGraw-Hill; 2008:1094-1098.
5. Shepherd V, Davidson EJ, Davies-Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273-279.
6. Kim JC, Kim HC, Jeong CS, et al. Extramammary Paget’s disease with aggressive behavior: a report of two cases. J Korean Med Sci. 1999;14:223-226.
7. Rahbari H, Mehregan AH. Basal cell epitheliomas in usual and unusual sites. J Cutan Pathol. 1979;6:425-431.
8. Ishida-Yamamoto A, Sato K, Wada T, et al. Fibroepithelioma-like changes occurring in perianal Paget’s disease with rectal mucinous carcinoma: case report and review of 49 cases of extramammary Paget’s disease. J Cutan Pathol. 2002;29:185-189.
Extramammary Paget disease (EMPD) is an uncommon neoplasm that most commonly occurs in the anogenital region but can arise in any area of the skin or mucosa.1 On clinical examination, EMPD typically presents as a sharply demarcated, erythematous, eczematoid, weeping lesion with varying degrees of induration; it rarely presents as a palpable mass or evenly raised nodule.2 Microscopically, it may be accompanied by varying degrees of epidermal hyperplasia.1 In particular, fibroepitheliomatous hyperplasia contains lacy strands of squamous epithelium resembling fibroepithelioma of Pinkus.3 We report a case of EMPD in a 90-year-old man who presented with a verrucous nodule in the pubic area that histologically demonstrated fibroepitheliomatous hyperplasia with lacy strands of squamous epithelium.
Case Report
A 90-year-old man presented with asymptomatic, well-demarcated, erythematous plaques in the pubic area of 5 years’ duration, along with a 3.0×2.5-cm nodule on the left side of the pubic area (Figure 1). Laboratory test results including a complete blood cell count, blood chemistry, and routine urinalysis were within reference range. Punch biopsies were taken from each plaque and nodule, as marked with arrows in Figure 1. Histopathologically, the plaques were seen to contain a number of large round cells with abundant pale cytoplasm and pleomorphic hyperchromatic nuclei that were present at various levels of the epidermis where they formed nests and clusters but did not extend into the dermis (Figures 2A and 2B). The nodule contained lacy strands of squamous epithelium extending from the epidermis to the mid dermis as well as many glandular structures (Figures 2C and 2D). The cells in the epidermis stained positively with periodic acid–Schiff (PAS), carcinoembryonic antigen (CEA), and cytokeratin 7 (Figure 2E). We also tested for S-100 protein to rule out malignant melanoma, which was negative.
Based on both the clinical and histological features, a diagnosis of EMPD with fibroepitheliomatous hyperplasia was made. It was recommended that the patient undergo further evaluation and treatment; he declined due to his financial situation and was subsequently lost to follow-up.
Comment
Clinically, EMPD usually presents as a patch of macular erythema, an erythematous eruption, or erythematous papules and plaques.4 The palpable nodule seen in our patient is not a common presentation of EMPD. Pruritus is the most common symptom of EMPD, occurring in 70% of patients.5 Other symptoms include burning, irritation, pain, tenderness, bleeding, and swelling. Ten percent of EMPD cases are asymptomatic.5
Histologically, Paget cells primarily involve the epidermis where they usually form clusters or solid nests. In more than 90% of EMPD cases, the Paget cells contain cytoplasmic mucin that stains positively with mucicarmine and PAS. Immunohistochemical staining for cytokeratin 7, gross cystic disease fluid protein-15, S-100 protein, and CEA sometimes may be needed to differentiate from mimickers such as Bowen disease and superficial spreading melanoma.6 In our patient, the tumor cells stained positive for cytokeratin 7, CEA, and PAS. Malignant melanoma was ruled out with a test for S-100 protein.
|
|
Extramammary Paget disease often is associated with epidermal hyperplasia, which can be classified as squamous, papillomatous, or fibroepitheliomatous.3 Microscopically, squamous hyperplasia is characterized by prominent thickening of the epidermis from diffuse plaquelike hyperplasia and is usually associated with hyperkeratosis. Papillomatous hyperplasia has an exophytic papillary or verrucous architecture and is associated with parakeratosis. Fibroepitheliomatous, or fibroepitheliomalike, hyperplasia generally consists of a discrete, broad, elevated plaque or nodule produced by hyperplasia of keratinocytes that form lacy strands of squamous epithelium.3 The biphasic pattern of proliferating epidermis and entrapped dermis simulates a so-called fibroepithelioma. Paget cells can be seen within the lacy strands of epidermal columns and in the acanthotic surface component.2 The finding of fibroepitheliomatous hyperplasia in anogenital skin should prompt a search for the diagnostic Paget cells to eliminate a fibroepithelioma of Pinkus variant of basal cell carcinoma, though the latter is uncommon and rarely occurs at this site.7
Of the 3 types of epidermal hyperplasia, our case demonstrated the fibroepitheliomatous type. There may be some relationship between EMPD and fibroepitheliomatous hyperplasia because most reported cases of EMPD with fibroepitheliomatous hyperplasia have occurred in the anogenital region. Also, epidermal hyperplasia is more frequent in anogenital Paget disease than in axillary Paget disease.8
Conclusion
Our case showed the unique finding of a verrucous nodular EMPD lesion in which peculiar histological features presented as extensions of the tumor cells forming lacy strands of squamous epithelium from the epidermis to the mid dermis as well as many glandular structures.
Extramammary Paget disease (EMPD) is an uncommon neoplasm that most commonly occurs in the anogenital region but can arise in any area of the skin or mucosa.1 On clinical examination, EMPD typically presents as a sharply demarcated, erythematous, eczematoid, weeping lesion with varying degrees of induration; it rarely presents as a palpable mass or evenly raised nodule.2 Microscopically, it may be accompanied by varying degrees of epidermal hyperplasia.1 In particular, fibroepitheliomatous hyperplasia contains lacy strands of squamous epithelium resembling fibroepithelioma of Pinkus.3 We report a case of EMPD in a 90-year-old man who presented with a verrucous nodule in the pubic area that histologically demonstrated fibroepitheliomatous hyperplasia with lacy strands of squamous epithelium.
Case Report
A 90-year-old man presented with asymptomatic, well-demarcated, erythematous plaques in the pubic area of 5 years’ duration, along with a 3.0×2.5-cm nodule on the left side of the pubic area (Figure 1). Laboratory test results including a complete blood cell count, blood chemistry, and routine urinalysis were within reference range. Punch biopsies were taken from each plaque and nodule, as marked with arrows in Figure 1. Histopathologically, the plaques were seen to contain a number of large round cells with abundant pale cytoplasm and pleomorphic hyperchromatic nuclei that were present at various levels of the epidermis where they formed nests and clusters but did not extend into the dermis (Figures 2A and 2B). The nodule contained lacy strands of squamous epithelium extending from the epidermis to the mid dermis as well as many glandular structures (Figures 2C and 2D). The cells in the epidermis stained positively with periodic acid–Schiff (PAS), carcinoembryonic antigen (CEA), and cytokeratin 7 (Figure 2E). We also tested for S-100 protein to rule out malignant melanoma, which was negative.
Based on both the clinical and histological features, a diagnosis of EMPD with fibroepitheliomatous hyperplasia was made. It was recommended that the patient undergo further evaluation and treatment; he declined due to his financial situation and was subsequently lost to follow-up.
Comment
Clinically, EMPD usually presents as a patch of macular erythema, an erythematous eruption, or erythematous papules and plaques.4 The palpable nodule seen in our patient is not a common presentation of EMPD. Pruritus is the most common symptom of EMPD, occurring in 70% of patients.5 Other symptoms include burning, irritation, pain, tenderness, bleeding, and swelling. Ten percent of EMPD cases are asymptomatic.5
Histologically, Paget cells primarily involve the epidermis where they usually form clusters or solid nests. In more than 90% of EMPD cases, the Paget cells contain cytoplasmic mucin that stains positively with mucicarmine and PAS. Immunohistochemical staining for cytokeratin 7, gross cystic disease fluid protein-15, S-100 protein, and CEA sometimes may be needed to differentiate from mimickers such as Bowen disease and superficial spreading melanoma.6 In our patient, the tumor cells stained positive for cytokeratin 7, CEA, and PAS. Malignant melanoma was ruled out with a test for S-100 protein.
|
|
Extramammary Paget disease often is associated with epidermal hyperplasia, which can be classified as squamous, papillomatous, or fibroepitheliomatous.3 Microscopically, squamous hyperplasia is characterized by prominent thickening of the epidermis from diffuse plaquelike hyperplasia and is usually associated with hyperkeratosis. Papillomatous hyperplasia has an exophytic papillary or verrucous architecture and is associated with parakeratosis. Fibroepitheliomatous, or fibroepitheliomalike, hyperplasia generally consists of a discrete, broad, elevated plaque or nodule produced by hyperplasia of keratinocytes that form lacy strands of squamous epithelium.3 The biphasic pattern of proliferating epidermis and entrapped dermis simulates a so-called fibroepithelioma. Paget cells can be seen within the lacy strands of epidermal columns and in the acanthotic surface component.2 The finding of fibroepitheliomatous hyperplasia in anogenital skin should prompt a search for the diagnostic Paget cells to eliminate a fibroepithelioma of Pinkus variant of basal cell carcinoma, though the latter is uncommon and rarely occurs at this site.7
Of the 3 types of epidermal hyperplasia, our case demonstrated the fibroepitheliomatous type. There may be some relationship between EMPD and fibroepitheliomatous hyperplasia because most reported cases of EMPD with fibroepitheliomatous hyperplasia have occurred in the anogenital region. Also, epidermal hyperplasia is more frequent in anogenital Paget disease than in axillary Paget disease.8
Conclusion
Our case showed the unique finding of a verrucous nodular EMPD lesion in which peculiar histological features presented as extensions of the tumor cells forming lacy strands of squamous epithelium from the epidermis to the mid dermis as well as many glandular structures.
1. Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease. J Clin Pathol. 2000;53:742-749.
2. Billings SD, Roth LM. Pseudoinvasive, nodular extramam-mary Paget’s disease of the vulva. Arch Pathol Lab Med. 1998;122:471-474.
3. Brainard JA, Hart WR. Proliferative epidermal lesions associated with anogenital Paget’s disease. Am J Surg Pathol. 2000;24:543-552.
4. Neuhaus IM, Grekin RC. Mammary and extramammary Paget disease. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 1. 7th ed. New York, NY: McGraw-Hill; 2008:1094-1098.
5. Shepherd V, Davidson EJ, Davies-Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273-279.
6. Kim JC, Kim HC, Jeong CS, et al. Extramammary Paget’s disease with aggressive behavior: a report of two cases. J Korean Med Sci. 1999;14:223-226.
7. Rahbari H, Mehregan AH. Basal cell epitheliomas in usual and unusual sites. J Cutan Pathol. 1979;6:425-431.
8. Ishida-Yamamoto A, Sato K, Wada T, et al. Fibroepithelioma-like changes occurring in perianal Paget’s disease with rectal mucinous carcinoma: case report and review of 49 cases of extramammary Paget’s disease. J Cutan Pathol. 2002;29:185-189.
1. Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease. J Clin Pathol. 2000;53:742-749.
2. Billings SD, Roth LM. Pseudoinvasive, nodular extramam-mary Paget’s disease of the vulva. Arch Pathol Lab Med. 1998;122:471-474.
3. Brainard JA, Hart WR. Proliferative epidermal lesions associated with anogenital Paget’s disease. Am J Surg Pathol. 2000;24:543-552.
4. Neuhaus IM, Grekin RC. Mammary and extramammary Paget disease. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 1. 7th ed. New York, NY: McGraw-Hill; 2008:1094-1098.
5. Shepherd V, Davidson EJ, Davies-Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273-279.
6. Kim JC, Kim HC, Jeong CS, et al. Extramammary Paget’s disease with aggressive behavior: a report of two cases. J Korean Med Sci. 1999;14:223-226.
7. Rahbari H, Mehregan AH. Basal cell epitheliomas in usual and unusual sites. J Cutan Pathol. 1979;6:425-431.
8. Ishida-Yamamoto A, Sato K, Wada T, et al. Fibroepithelioma-like changes occurring in perianal Paget’s disease with rectal mucinous carcinoma: case report and review of 49 cases of extramammary Paget’s disease. J Cutan Pathol. 2002;29:185-189.
- Extramammary Paget disease (EMPD) should be considered in the clinical differential diagnosis of verrucous nodules in the pubic area.
- Histopathologically, EMPD in the anogenital area could show fibroepitheliomatous hyperplasia with lacy strands of squamous epithelium.
Multiple Cutaneous Abscesses Revealing Disseminated Nocardiosis in a Patient With Chronic Rheumatoid Arthritis
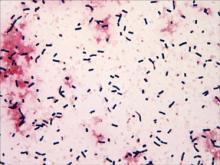
Nocardiosis is a rare human infection that has been reported worldwide but occurs more frequently in patients who reside in tropical areas. Although there is no predilection for age or ethnicity, nocardiosis is slightly more common in males than in females.1,2 The genus Nocardia belongs to the order Actinomycetales and includes more than 50 species of gram-positive, aerobic, filamentous, branching, partially acid-fast bacteria found ubiquitously in soil.3 The bacteria may be present in animals (eg, cattle, dogs),4 but transmission to humans is unusual. Clinical diagnosis of nocardiosis often is difficult because of its nonspecific manifestations and delays in its recognition, especially in western countries where the infection is consistently rare and probably underestimated.5-8 Cutaneous involvement generally manifests as 1 of 4 conditions: mycetoma, lymphocutaneous (sporotrichoid) infection, superficial skin infection, or systemic disease with cutaneous involvement.9 Systemic disseminated disease usually occurs in individuals with cellular immune deficiency, such as patients with human immunodeficiency virus; organ transplant recipients; or patients with a history of long-term use of corticosteroids, configuring an opportunistic infection.10-19 Incidence in these high-risk patients is 140- to 340-fold higher than in the general population.20Nocardia infections usually are acquired via dust inhalation, especially in dry environments. Focal pneumonitis is the first typical manifestation in immunosuppressed patients, followed by skin dissemination and central nervous system involvement.1
We report the case of a 65-year-old man who developed disseminated nocardiosis while undergoing long-term treatment with systemic corticosteroids for rheumatoid arthritis.
Case Report
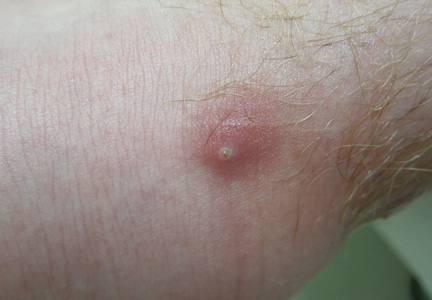
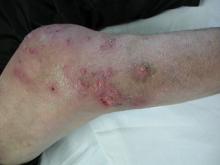
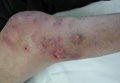
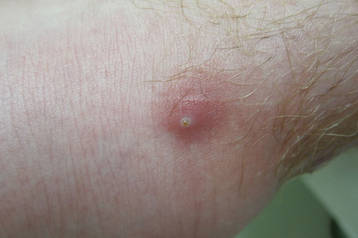
A 65-year-old man presented to the dermatology department for evaluation of multiple papules and nodules with a puruloid discharge on the right leg. The first lesions had appeared on the right ankle approximately 1 month prior to presentation and were treated with systemic antibiotics by the patient’s general practitioner without remarkable benefits. The lesions progressed further on the right leg showing a sporotrichoid disposition.
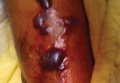
On physical examination major involvement was evident on the right knee with papules, subcutaneous nodules, and sinuses (Figure 1). Isolated lesions also were present on the right thigh. A few days later some lesions were present on the left elbow and arm. Involvement of the popliteal and inguinal lymph nodes was noted, with painful enlarged nodules covered by erythematous skin that were mobile on deep planes. The lesions had a sudden onset while the patient was in good health, causing progressive functional impotency of the leg without general malaise or fever. The patient’s history was remarkable for chronic rheumatoid arthritis of 20 years’ duration that was treated with hydroxychloroquine (400 mg daily) and methylprednisolone (16 mg daily).
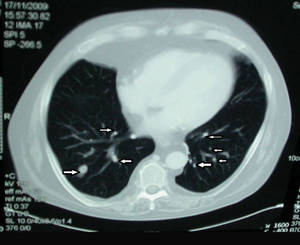
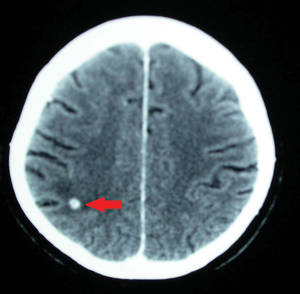
The patient was retired but reported daily tilling and manual labor on his farmland. He denied trauma or insect bites to the leg prior to the onset of lesions. Laboratory examination documented slight neutrophil leukocytosis (white blood cell count, 12.8×103/μL [reference range, 4.5–11.0×103/μL]; neutrophil count, 10.7×103/μL [reference range, 1.8–7.8×103/μL]; 85% neutrophils at formula count [reference range, 56%]), augmented C-reactive protein levels (15.8 mg/L [reference range, 0.08–3.1 mg/L]), a blood sedimentation rate of 74 mm/h (reference range, 0–20 mm/h), and elevated liver enzymes (aspartate aminotransferase, 52 U/L [reference range, 10–30 U/L]; alanine aminotransferase, 89 U/L [reference range, 10–40 U/L]; γ-glutamyltransferase, 68 U/L [reference range, 2–30 U/L]). Rheumatoid factor was 49.7 U/mL (reference range, 0–14.0 U/mL). Skin biopsy of a sample lesion suggested a chronic granulomatous suppuration. Tissue cultures and subsequent polymerase chain reaction assay identified Nocardia asteroides. A chest radiograph revealed multiple opaque nodules disseminated in both lungs, and a computed tomography (CT) scan confirmed multiple pulmonary lesions without involvement of the mediastinal lymph nodes (Figure 2). Computed tomography scans of the brain before and after contrast media perfusion showed the presence of an enhanced 8-mm mass among the right parietal and occipital lobes surrounded by an edematous halo (Figure 3). Neurologic examination was normal. During the radiologic assessment, the patient remembered having been hospitalized 1 year prior in a pulmonology unit at an outside institution for treatment of what was considered to be a multifocal nonspecific lung infection. A 1-month course of levofloxacin (500 mg daily) was administered at that time without any further follow-up. Review of the prior chest radiograph and CT scan confirmed the presence of the same radiologic findings as the current assessment, though of milder entity.
 |
 |
The patient was transferred from the dermatology department to the infectious diseases unit. Fiberoptic bronchoscopy with bronchoalveolar lavage and transbronchial biopsy confirmed pulmonary nocardiosis. Treatment with a combination of endovenous carbapenem and fluoroquinolone antibiotics for 1 month led to complete resolution of both the cutaneous lesions and the single brain abscess. Improvement of pulmonary involvement was noted on radiology, but another course of endovenous treatment with antibiotics was required, followed by oral amoxicillin–clavulanic acid (2 g daily) for 3 months. The patient underwent maintenance antibiotic therapy for 1 year without relapse and is considered to be cured.
Comment
Cutaneous nocardiosis occurs either as part of a disseminated infection or as a primary skin inoculation, usually following trauma or exposure from working outdoors.2,9,21,22 Nocardial mycetoma, also known as actinomycetoma, is a chronic condition that mainly affects the lower extremities but also can affect the hands and forearms; it is frequently reported in tropical regions,23 but disseminated forms are rare and undervalued, especially in European countries. A certain rise in incidence has been reported in Europe as a consequence of immune suppression, and pulmonary disease is the most common presentation in these patients.1,2,6-12 One-third of patients have disseminated disease with high morbidity and a high mortality rate.20 Major case collections in Italy have been studied by a collaborative hospital network of 11 cities,10,11 confirming difficulty of diagnosis and underestimation, prevalence of N asteroides as a pathogen, resistance to several antimicrobials, and a high relapse index. Diagnosis remains challenging, as clinical suspicion is not frequently supported by histology and/or microbiology because of difficulty in bacteria isolation. Molecular methods are not routinely available in Italy, and only stringent efforts and cooperation from different university departments has allowed final identification of nocardiosis.
The sporotrichoid pattern of the lesions on the right leg was unique; it is considered the rarest presentation of cutaneous nocardiosis.24 Our first suspicion was sporotrichosis, an infection that is especially common in farmers in Italy,25,26 but the microbiologist’s evaluation of the bacterial pure cultures growing on skin specimens was negative for deep fungal infections. Further identification and confirmation by polymerase chain reaction assay of N asteroides required several weeks. Meanwhile, the chest radiograph followed by CT scans of the lungs and brain led to the diagnosis of a disseminated infection. Documentation of lung involvement 1 year prior to the development of the cutaneous lesions excluded primary cutaneous nocardiosis with secondary dissemination as reported in other immunocompromised patients, including those undergoing long-term treatment with corticosteroids.15-19 Continuous follow-up and physician awareness of possible unusual infections is mandatory in patients undergoing immunosuppressive therapy. Our patient had a 20-year history of rheumatoid arthritis but was otherwise healthy with no signs of lung distress, fever, or general malaise. His only concern was leg impairment following acute development of painful lesions discharging pus over 1 month.
Conclusion
This case report highlights the role of the dermatologist as the first-line physician involved in the diagnosis of rare and potentially severe infections. Cooperation with the microbiologist, pathologist, and other internal medicine specialists is crucial; however, sometimes it is just the clinical suspicion and the perseverance of the dermatologist that ultimately leads to the correct diagnosis, which often is otherwise unspecific, undervalued, and misdiagnosed as more common diseases.
1. Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
2. Minero MV, Marin M, Cercenado E, et al. Nocardiosis at the turn of the century. Medicine (Baltimore). 2009;88:250-261.
3. McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
4. Ribeiro MG, Salerno T, Mattos-Guaraldi AL, et al. Nocardiosis: an overview and additional report of 28 cases in cattle and dogs. Rev Inst Med Trop Sao Paulo. 2008;50:177-185.
5. Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
6. Boiron P, Provost F, Chevrier G, et al. Review of nocardial infections in France 1987 to 1990. Eur J Clin Microbiol Infect Dis. 1992;11:709-714.
7. Farina C, Boiron P, Ferrari I, et al. Report of human nocardiosis in Italy between 1993 and 1997. Eur J Epidemiol. 2001;17:1019-1022.
8. Farina C, Boiron P, Goglio A, et al. Human nocardiosis in northern Italy from 1982 to 1992. Northern Italy Collaborative Group on nocardiosis. Scand J Infect Dis. 1995;27:23-27.
9. Kalb RE, Kaplan MH, Grossman ME. Cutaneous nocardiosis. case reports and review. J Am Acad Dermatol. 1985;13:125-133.
10. Fontana I, Gasloli G, Rossi AM, et al. Nocardiosis in a kidney-pancreas transplant. J Transplant. 2010;2010:573234.
11. Frank M, Woschnagg H, Mölzer G, et al. Cerebellar nocardiosis and myopathy from long-term corticosteroids for idiopathic thrombocytopenia. Yonsei Med J. 2010;51:131-137.
12. Devi KR, Singh LR, Devi NT, et al. Subcutaneous nocardial abscess in a post-renal transplant patient. Indian J Med Microbiol. 2007;25:279-281.
13. Iona E, Giannoni F, Brunori L, et al. Isolation of Nocardia asiatica from cutaneous ulcers of a human immunodeficiency virus-infected patient in Italy. J Clin Microbiol. 2007;45:2088-2089.
14. Pardo M, Bonifaz A, Valencia A, et al. Actinomycetoma by Nocardia brasiliensis in a girl with Down syndrome. Dermatol Online J. 2008;14:9.
15. Nenoff P, Kellermann S, Borte G, et al. Pulmonary nocardiosis with cutaneous involvement mimicking a metastasizing lung carcinoma in a patient with chronic myelogenous leukaemia. Eur J Dermatol. 2000;10:47-51.
16. Baldi BG, Santana AN, Takagaki TY. Pulmonary and cutaneous nocardiosis in a patient treated with corticosteroids [in English, Portuguese]. J Bras Penumol. 2006;32:592-595.
17. Singh SM, Rau NV, Cohen LB, et al. Cutaneous nocardiosis complicating management of Crohn’s disease with infliximab and prednisone. CMAJ. 2004;171:1063-1064.
18. Dekeyser S, Corroyer-Simovic B, Cachia M, et al. Nocardia otitidiscaviarum, cutaneous infection in a patient receiving long-term corticosteroid treatment [in French]. Ann Biol Clin (Paris). 2003;61:219-222.
19. Hashimoto Y, Hiruma M, Hisamichi K, et al. Primary cutaneous nocardiosis with multiple, subcutaneous abscesses in a patient with sarcoidosis. J Dermatolog Treat. 2002;13:201-203.
20. Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38:89-97.
21. Patil SP, Gautam MM, Sodha AA, et al. Primary cutaneous nocardiosis with craniocerebral extension: a case report. Dermatol Online J. 2009;15:8.
22. Lakshmi V, Sundaram C, Meena AK, et al. Primary cutaneous nocardiosis with epidural abscess caused by Nocardia brasiliensis: a case report. Neurol India. 2002;50:90-92.
23. Bonifaz A, Flores P, Saúl A, et al. Treatment of actinomycetoma due to Nocardia spp. with amoxicillin-clavulanate. Br J Dermatol. 2007;156:308-311.
24. Baradkar VP, Mathur M, Kulkarni SD, et al. Sporotrichoid pattern of cutaneous nocardiasis due to Nocardia asteroids. Indian J Pathol Microbiol. 2008;51:432-434.
25. Barile F, Mastrolonardo M, Loconsole F, et al. Cutaneous sporotrichosis in the period 1978-1992 in the province of Bari, Apulia, Southern Italy. Mycoses. 1993;36:181-185.
26. Alberici F, Paties CT, Lombardi G, et al. Sporothrix schenckii var luriei as the cause of sporotrichosis in Italy. Eur J Epidemiol. 1989;5:173-177.
Nocardiosis is a rare human infection that has been reported worldwide but occurs more frequently in patients who reside in tropical areas. Although there is no predilection for age or ethnicity, nocardiosis is slightly more common in males than in females.1,2 The genus Nocardia belongs to the order Actinomycetales and includes more than 50 species of gram-positive, aerobic, filamentous, branching, partially acid-fast bacteria found ubiquitously in soil.3 The bacteria may be present in animals (eg, cattle, dogs),4 but transmission to humans is unusual. Clinical diagnosis of nocardiosis often is difficult because of its nonspecific manifestations and delays in its recognition, especially in western countries where the infection is consistently rare and probably underestimated.5-8 Cutaneous involvement generally manifests as 1 of 4 conditions: mycetoma, lymphocutaneous (sporotrichoid) infection, superficial skin infection, or systemic disease with cutaneous involvement.9 Systemic disseminated disease usually occurs in individuals with cellular immune deficiency, such as patients with human immunodeficiency virus; organ transplant recipients; or patients with a history of long-term use of corticosteroids, configuring an opportunistic infection.10-19 Incidence in these high-risk patients is 140- to 340-fold higher than in the general population.20Nocardia infections usually are acquired via dust inhalation, especially in dry environments. Focal pneumonitis is the first typical manifestation in immunosuppressed patients, followed by skin dissemination and central nervous system involvement.1
We report the case of a 65-year-old man who developed disseminated nocardiosis while undergoing long-term treatment with systemic corticosteroids for rheumatoid arthritis.
Case Report
A 65-year-old man presented to the dermatology department for evaluation of multiple papules and nodules with a puruloid discharge on the right leg. The first lesions had appeared on the right ankle approximately 1 month prior to presentation and were treated with systemic antibiotics by the patient’s general practitioner without remarkable benefits. The lesions progressed further on the right leg showing a sporotrichoid disposition.
On physical examination major involvement was evident on the right knee with papules, subcutaneous nodules, and sinuses (Figure 1). Isolated lesions also were present on the right thigh. A few days later some lesions were present on the left elbow and arm. Involvement of the popliteal and inguinal lymph nodes was noted, with painful enlarged nodules covered by erythematous skin that were mobile on deep planes. The lesions had a sudden onset while the patient was in good health, causing progressive functional impotency of the leg without general malaise or fever. The patient’s history was remarkable for chronic rheumatoid arthritis of 20 years’ duration that was treated with hydroxychloroquine (400 mg daily) and methylprednisolone (16 mg daily).
The patient was retired but reported daily tilling and manual labor on his farmland. He denied trauma or insect bites to the leg prior to the onset of lesions. Laboratory examination documented slight neutrophil leukocytosis (white blood cell count, 12.8×103/μL [reference range, 4.5–11.0×103/μL]; neutrophil count, 10.7×103/μL [reference range, 1.8–7.8×103/μL]; 85% neutrophils at formula count [reference range, 56%]), augmented C-reactive protein levels (15.8 mg/L [reference range, 0.08–3.1 mg/L]), a blood sedimentation rate of 74 mm/h (reference range, 0–20 mm/h), and elevated liver enzymes (aspartate aminotransferase, 52 U/L [reference range, 10–30 U/L]; alanine aminotransferase, 89 U/L [reference range, 10–40 U/L]; γ-glutamyltransferase, 68 U/L [reference range, 2–30 U/L]). Rheumatoid factor was 49.7 U/mL (reference range, 0–14.0 U/mL). Skin biopsy of a sample lesion suggested a chronic granulomatous suppuration. Tissue cultures and subsequent polymerase chain reaction assay identified Nocardia asteroides. A chest radiograph revealed multiple opaque nodules disseminated in both lungs, and a computed tomography (CT) scan confirmed multiple pulmonary lesions without involvement of the mediastinal lymph nodes (Figure 2). Computed tomography scans of the brain before and after contrast media perfusion showed the presence of an enhanced 8-mm mass among the right parietal and occipital lobes surrounded by an edematous halo (Figure 3). Neurologic examination was normal. During the radiologic assessment, the patient remembered having been hospitalized 1 year prior in a pulmonology unit at an outside institution for treatment of what was considered to be a multifocal nonspecific lung infection. A 1-month course of levofloxacin (500 mg daily) was administered at that time without any further follow-up. Review of the prior chest radiograph and CT scan confirmed the presence of the same radiologic findings as the current assessment, though of milder entity.
 |
 |
The patient was transferred from the dermatology department to the infectious diseases unit. Fiberoptic bronchoscopy with bronchoalveolar lavage and transbronchial biopsy confirmed pulmonary nocardiosis. Treatment with a combination of endovenous carbapenem and fluoroquinolone antibiotics for 1 month led to complete resolution of both the cutaneous lesions and the single brain abscess. Improvement of pulmonary involvement was noted on radiology, but another course of endovenous treatment with antibiotics was required, followed by oral amoxicillin–clavulanic acid (2 g daily) for 3 months. The patient underwent maintenance antibiotic therapy for 1 year without relapse and is considered to be cured.
Comment
Cutaneous nocardiosis occurs either as part of a disseminated infection or as a primary skin inoculation, usually following trauma or exposure from working outdoors.2,9,21,22 Nocardial mycetoma, also known as actinomycetoma, is a chronic condition that mainly affects the lower extremities but also can affect the hands and forearms; it is frequently reported in tropical regions,23 but disseminated forms are rare and undervalued, especially in European countries. A certain rise in incidence has been reported in Europe as a consequence of immune suppression, and pulmonary disease is the most common presentation in these patients.1,2,6-12 One-third of patients have disseminated disease with high morbidity and a high mortality rate.20 Major case collections in Italy have been studied by a collaborative hospital network of 11 cities,10,11 confirming difficulty of diagnosis and underestimation, prevalence of N asteroides as a pathogen, resistance to several antimicrobials, and a high relapse index. Diagnosis remains challenging, as clinical suspicion is not frequently supported by histology and/or microbiology because of difficulty in bacteria isolation. Molecular methods are not routinely available in Italy, and only stringent efforts and cooperation from different university departments has allowed final identification of nocardiosis.
The sporotrichoid pattern of the lesions on the right leg was unique; it is considered the rarest presentation of cutaneous nocardiosis.24 Our first suspicion was sporotrichosis, an infection that is especially common in farmers in Italy,25,26 but the microbiologist’s evaluation of the bacterial pure cultures growing on skin specimens was negative for deep fungal infections. Further identification and confirmation by polymerase chain reaction assay of N asteroides required several weeks. Meanwhile, the chest radiograph followed by CT scans of the lungs and brain led to the diagnosis of a disseminated infection. Documentation of lung involvement 1 year prior to the development of the cutaneous lesions excluded primary cutaneous nocardiosis with secondary dissemination as reported in other immunocompromised patients, including those undergoing long-term treatment with corticosteroids.15-19 Continuous follow-up and physician awareness of possible unusual infections is mandatory in patients undergoing immunosuppressive therapy. Our patient had a 20-year history of rheumatoid arthritis but was otherwise healthy with no signs of lung distress, fever, or general malaise. His only concern was leg impairment following acute development of painful lesions discharging pus over 1 month.
Conclusion
This case report highlights the role of the dermatologist as the first-line physician involved in the diagnosis of rare and potentially severe infections. Cooperation with the microbiologist, pathologist, and other internal medicine specialists is crucial; however, sometimes it is just the clinical suspicion and the perseverance of the dermatologist that ultimately leads to the correct diagnosis, which often is otherwise unspecific, undervalued, and misdiagnosed as more common diseases.
Nocardiosis is a rare human infection that has been reported worldwide but occurs more frequently in patients who reside in tropical areas. Although there is no predilection for age or ethnicity, nocardiosis is slightly more common in males than in females.1,2 The genus Nocardia belongs to the order Actinomycetales and includes more than 50 species of gram-positive, aerobic, filamentous, branching, partially acid-fast bacteria found ubiquitously in soil.3 The bacteria may be present in animals (eg, cattle, dogs),4 but transmission to humans is unusual. Clinical diagnosis of nocardiosis often is difficult because of its nonspecific manifestations and delays in its recognition, especially in western countries where the infection is consistently rare and probably underestimated.5-8 Cutaneous involvement generally manifests as 1 of 4 conditions: mycetoma, lymphocutaneous (sporotrichoid) infection, superficial skin infection, or systemic disease with cutaneous involvement.9 Systemic disseminated disease usually occurs in individuals with cellular immune deficiency, such as patients with human immunodeficiency virus; organ transplant recipients; or patients with a history of long-term use of corticosteroids, configuring an opportunistic infection.10-19 Incidence in these high-risk patients is 140- to 340-fold higher than in the general population.20Nocardia infections usually are acquired via dust inhalation, especially in dry environments. Focal pneumonitis is the first typical manifestation in immunosuppressed patients, followed by skin dissemination and central nervous system involvement.1
We report the case of a 65-year-old man who developed disseminated nocardiosis while undergoing long-term treatment with systemic corticosteroids for rheumatoid arthritis.
Case Report
A 65-year-old man presented to the dermatology department for evaluation of multiple papules and nodules with a puruloid discharge on the right leg. The first lesions had appeared on the right ankle approximately 1 month prior to presentation and were treated with systemic antibiotics by the patient’s general practitioner without remarkable benefits. The lesions progressed further on the right leg showing a sporotrichoid disposition.
On physical examination major involvement was evident on the right knee with papules, subcutaneous nodules, and sinuses (Figure 1). Isolated lesions also were present on the right thigh. A few days later some lesions were present on the left elbow and arm. Involvement of the popliteal and inguinal lymph nodes was noted, with painful enlarged nodules covered by erythematous skin that were mobile on deep planes. The lesions had a sudden onset while the patient was in good health, causing progressive functional impotency of the leg without general malaise or fever. The patient’s history was remarkable for chronic rheumatoid arthritis of 20 years’ duration that was treated with hydroxychloroquine (400 mg daily) and methylprednisolone (16 mg daily).
The patient was retired but reported daily tilling and manual labor on his farmland. He denied trauma or insect bites to the leg prior to the onset of lesions. Laboratory examination documented slight neutrophil leukocytosis (white blood cell count, 12.8×103/μL [reference range, 4.5–11.0×103/μL]; neutrophil count, 10.7×103/μL [reference range, 1.8–7.8×103/μL]; 85% neutrophils at formula count [reference range, 56%]), augmented C-reactive protein levels (15.8 mg/L [reference range, 0.08–3.1 mg/L]), a blood sedimentation rate of 74 mm/h (reference range, 0–20 mm/h), and elevated liver enzymes (aspartate aminotransferase, 52 U/L [reference range, 10–30 U/L]; alanine aminotransferase, 89 U/L [reference range, 10–40 U/L]; γ-glutamyltransferase, 68 U/L [reference range, 2–30 U/L]). Rheumatoid factor was 49.7 U/mL (reference range, 0–14.0 U/mL). Skin biopsy of a sample lesion suggested a chronic granulomatous suppuration. Tissue cultures and subsequent polymerase chain reaction assay identified Nocardia asteroides. A chest radiograph revealed multiple opaque nodules disseminated in both lungs, and a computed tomography (CT) scan confirmed multiple pulmonary lesions without involvement of the mediastinal lymph nodes (Figure 2). Computed tomography scans of the brain before and after contrast media perfusion showed the presence of an enhanced 8-mm mass among the right parietal and occipital lobes surrounded by an edematous halo (Figure 3). Neurologic examination was normal. During the radiologic assessment, the patient remembered having been hospitalized 1 year prior in a pulmonology unit at an outside institution for treatment of what was considered to be a multifocal nonspecific lung infection. A 1-month course of levofloxacin (500 mg daily) was administered at that time without any further follow-up. Review of the prior chest radiograph and CT scan confirmed the presence of the same radiologic findings as the current assessment, though of milder entity.
 |
 |
The patient was transferred from the dermatology department to the infectious diseases unit. Fiberoptic bronchoscopy with bronchoalveolar lavage and transbronchial biopsy confirmed pulmonary nocardiosis. Treatment with a combination of endovenous carbapenem and fluoroquinolone antibiotics for 1 month led to complete resolution of both the cutaneous lesions and the single brain abscess. Improvement of pulmonary involvement was noted on radiology, but another course of endovenous treatment with antibiotics was required, followed by oral amoxicillin–clavulanic acid (2 g daily) for 3 months. The patient underwent maintenance antibiotic therapy for 1 year without relapse and is considered to be cured.
Comment
Cutaneous nocardiosis occurs either as part of a disseminated infection or as a primary skin inoculation, usually following trauma or exposure from working outdoors.2,9,21,22 Nocardial mycetoma, also known as actinomycetoma, is a chronic condition that mainly affects the lower extremities but also can affect the hands and forearms; it is frequently reported in tropical regions,23 but disseminated forms are rare and undervalued, especially in European countries. A certain rise in incidence has been reported in Europe as a consequence of immune suppression, and pulmonary disease is the most common presentation in these patients.1,2,6-12 One-third of patients have disseminated disease with high morbidity and a high mortality rate.20 Major case collections in Italy have been studied by a collaborative hospital network of 11 cities,10,11 confirming difficulty of diagnosis and underestimation, prevalence of N asteroides as a pathogen, resistance to several antimicrobials, and a high relapse index. Diagnosis remains challenging, as clinical suspicion is not frequently supported by histology and/or microbiology because of difficulty in bacteria isolation. Molecular methods are not routinely available in Italy, and only stringent efforts and cooperation from different university departments has allowed final identification of nocardiosis.
The sporotrichoid pattern of the lesions on the right leg was unique; it is considered the rarest presentation of cutaneous nocardiosis.24 Our first suspicion was sporotrichosis, an infection that is especially common in farmers in Italy,25,26 but the microbiologist’s evaluation of the bacterial pure cultures growing on skin specimens was negative for deep fungal infections. Further identification and confirmation by polymerase chain reaction assay of N asteroides required several weeks. Meanwhile, the chest radiograph followed by CT scans of the lungs and brain led to the diagnosis of a disseminated infection. Documentation of lung involvement 1 year prior to the development of the cutaneous lesions excluded primary cutaneous nocardiosis with secondary dissemination as reported in other immunocompromised patients, including those undergoing long-term treatment with corticosteroids.15-19 Continuous follow-up and physician awareness of possible unusual infections is mandatory in patients undergoing immunosuppressive therapy. Our patient had a 20-year history of rheumatoid arthritis but was otherwise healthy with no signs of lung distress, fever, or general malaise. His only concern was leg impairment following acute development of painful lesions discharging pus over 1 month.
Conclusion
This case report highlights the role of the dermatologist as the first-line physician involved in the diagnosis of rare and potentially severe infections. Cooperation with the microbiologist, pathologist, and other internal medicine specialists is crucial; however, sometimes it is just the clinical suspicion and the perseverance of the dermatologist that ultimately leads to the correct diagnosis, which often is otherwise unspecific, undervalued, and misdiagnosed as more common diseases.
1. Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
2. Minero MV, Marin M, Cercenado E, et al. Nocardiosis at the turn of the century. Medicine (Baltimore). 2009;88:250-261.
3. McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
4. Ribeiro MG, Salerno T, Mattos-Guaraldi AL, et al. Nocardiosis: an overview and additional report of 28 cases in cattle and dogs. Rev Inst Med Trop Sao Paulo. 2008;50:177-185.
5. Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
6. Boiron P, Provost F, Chevrier G, et al. Review of nocardial infections in France 1987 to 1990. Eur J Clin Microbiol Infect Dis. 1992;11:709-714.
7. Farina C, Boiron P, Ferrari I, et al. Report of human nocardiosis in Italy between 1993 and 1997. Eur J Epidemiol. 2001;17:1019-1022.
8. Farina C, Boiron P, Goglio A, et al. Human nocardiosis in northern Italy from 1982 to 1992. Northern Italy Collaborative Group on nocardiosis. Scand J Infect Dis. 1995;27:23-27.
9. Kalb RE, Kaplan MH, Grossman ME. Cutaneous nocardiosis. case reports and review. J Am Acad Dermatol. 1985;13:125-133.
10. Fontana I, Gasloli G, Rossi AM, et al. Nocardiosis in a kidney-pancreas transplant. J Transplant. 2010;2010:573234.
11. Frank M, Woschnagg H, Mölzer G, et al. Cerebellar nocardiosis and myopathy from long-term corticosteroids for idiopathic thrombocytopenia. Yonsei Med J. 2010;51:131-137.
12. Devi KR, Singh LR, Devi NT, et al. Subcutaneous nocardial abscess in a post-renal transplant patient. Indian J Med Microbiol. 2007;25:279-281.
13. Iona E, Giannoni F, Brunori L, et al. Isolation of Nocardia asiatica from cutaneous ulcers of a human immunodeficiency virus-infected patient in Italy. J Clin Microbiol. 2007;45:2088-2089.
14. Pardo M, Bonifaz A, Valencia A, et al. Actinomycetoma by Nocardia brasiliensis in a girl with Down syndrome. Dermatol Online J. 2008;14:9.
15. Nenoff P, Kellermann S, Borte G, et al. Pulmonary nocardiosis with cutaneous involvement mimicking a metastasizing lung carcinoma in a patient with chronic myelogenous leukaemia. Eur J Dermatol. 2000;10:47-51.
16. Baldi BG, Santana AN, Takagaki TY. Pulmonary and cutaneous nocardiosis in a patient treated with corticosteroids [in English, Portuguese]. J Bras Penumol. 2006;32:592-595.
17. Singh SM, Rau NV, Cohen LB, et al. Cutaneous nocardiosis complicating management of Crohn’s disease with infliximab and prednisone. CMAJ. 2004;171:1063-1064.
18. Dekeyser S, Corroyer-Simovic B, Cachia M, et al. Nocardia otitidiscaviarum, cutaneous infection in a patient receiving long-term corticosteroid treatment [in French]. Ann Biol Clin (Paris). 2003;61:219-222.
19. Hashimoto Y, Hiruma M, Hisamichi K, et al. Primary cutaneous nocardiosis with multiple, subcutaneous abscesses in a patient with sarcoidosis. J Dermatolog Treat. 2002;13:201-203.
20. Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38:89-97.
21. Patil SP, Gautam MM, Sodha AA, et al. Primary cutaneous nocardiosis with craniocerebral extension: a case report. Dermatol Online J. 2009;15:8.
22. Lakshmi V, Sundaram C, Meena AK, et al. Primary cutaneous nocardiosis with epidural abscess caused by Nocardia brasiliensis: a case report. Neurol India. 2002;50:90-92.
23. Bonifaz A, Flores P, Saúl A, et al. Treatment of actinomycetoma due to Nocardia spp. with amoxicillin-clavulanate. Br J Dermatol. 2007;156:308-311.
24. Baradkar VP, Mathur M, Kulkarni SD, et al. Sporotrichoid pattern of cutaneous nocardiasis due to Nocardia asteroids. Indian J Pathol Microbiol. 2008;51:432-434.
25. Barile F, Mastrolonardo M, Loconsole F, et al. Cutaneous sporotrichosis in the period 1978-1992 in the province of Bari, Apulia, Southern Italy. Mycoses. 1993;36:181-185.
26. Alberici F, Paties CT, Lombardi G, et al. Sporothrix schenckii var luriei as the cause of sporotrichosis in Italy. Eur J Epidemiol. 1989;5:173-177.
1. Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
2. Minero MV, Marin M, Cercenado E, et al. Nocardiosis at the turn of the century. Medicine (Baltimore). 2009;88:250-261.
3. McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
4. Ribeiro MG, Salerno T, Mattos-Guaraldi AL, et al. Nocardiosis: an overview and additional report of 28 cases in cattle and dogs. Rev Inst Med Trop Sao Paulo. 2008;50:177-185.
5. Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
6. Boiron P, Provost F, Chevrier G, et al. Review of nocardial infections in France 1987 to 1990. Eur J Clin Microbiol Infect Dis. 1992;11:709-714.
7. Farina C, Boiron P, Ferrari I, et al. Report of human nocardiosis in Italy between 1993 and 1997. Eur J Epidemiol. 2001;17:1019-1022.
8. Farina C, Boiron P, Goglio A, et al. Human nocardiosis in northern Italy from 1982 to 1992. Northern Italy Collaborative Group on nocardiosis. Scand J Infect Dis. 1995;27:23-27.
9. Kalb RE, Kaplan MH, Grossman ME. Cutaneous nocardiosis. case reports and review. J Am Acad Dermatol. 1985;13:125-133.
10. Fontana I, Gasloli G, Rossi AM, et al. Nocardiosis in a kidney-pancreas transplant. J Transplant. 2010;2010:573234.
11. Frank M, Woschnagg H, Mölzer G, et al. Cerebellar nocardiosis and myopathy from long-term corticosteroids for idiopathic thrombocytopenia. Yonsei Med J. 2010;51:131-137.
12. Devi KR, Singh LR, Devi NT, et al. Subcutaneous nocardial abscess in a post-renal transplant patient. Indian J Med Microbiol. 2007;25:279-281.
13. Iona E, Giannoni F, Brunori L, et al. Isolation of Nocardia asiatica from cutaneous ulcers of a human immunodeficiency virus-infected patient in Italy. J Clin Microbiol. 2007;45:2088-2089.
14. Pardo M, Bonifaz A, Valencia A, et al. Actinomycetoma by Nocardia brasiliensis in a girl with Down syndrome. Dermatol Online J. 2008;14:9.
15. Nenoff P, Kellermann S, Borte G, et al. Pulmonary nocardiosis with cutaneous involvement mimicking a metastasizing lung carcinoma in a patient with chronic myelogenous leukaemia. Eur J Dermatol. 2000;10:47-51.
16. Baldi BG, Santana AN, Takagaki TY. Pulmonary and cutaneous nocardiosis in a patient treated with corticosteroids [in English, Portuguese]. J Bras Penumol. 2006;32:592-595.
17. Singh SM, Rau NV, Cohen LB, et al. Cutaneous nocardiosis complicating management of Crohn’s disease with infliximab and prednisone. CMAJ. 2004;171:1063-1064.
18. Dekeyser S, Corroyer-Simovic B, Cachia M, et al. Nocardia otitidiscaviarum, cutaneous infection in a patient receiving long-term corticosteroid treatment [in French]. Ann Biol Clin (Paris). 2003;61:219-222.
19. Hashimoto Y, Hiruma M, Hisamichi K, et al. Primary cutaneous nocardiosis with multiple, subcutaneous abscesses in a patient with sarcoidosis. J Dermatolog Treat. 2002;13:201-203.
20. Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38:89-97.
21. Patil SP, Gautam MM, Sodha AA, et al. Primary cutaneous nocardiosis with craniocerebral extension: a case report. Dermatol Online J. 2009;15:8.
22. Lakshmi V, Sundaram C, Meena AK, et al. Primary cutaneous nocardiosis with epidural abscess caused by Nocardia brasiliensis: a case report. Neurol India. 2002;50:90-92.
23. Bonifaz A, Flores P, Saúl A, et al. Treatment of actinomycetoma due to Nocardia spp. with amoxicillin-clavulanate. Br J Dermatol. 2007;156:308-311.
24. Baradkar VP, Mathur M, Kulkarni SD, et al. Sporotrichoid pattern of cutaneous nocardiasis due to Nocardia asteroids. Indian J Pathol Microbiol. 2008;51:432-434.
25. Barile F, Mastrolonardo M, Loconsole F, et al. Cutaneous sporotrichosis in the period 1978-1992 in the province of Bari, Apulia, Southern Italy. Mycoses. 1993;36:181-185.
26. Alberici F, Paties CT, Lombardi G, et al. Sporothrix schenckii var luriei as the cause of sporotrichosis in Italy. Eur J Epidemiol. 1989;5:173-177.
- A high index of suspicion by the dermatologist is needed to alert the pathologist, microbiologist, and other clinicians involved in the assessment and diagnosis of nocardiosis.
- Because cultures from skin lesions of nocardiosis often show negative results, several specimens should be collected for analysis.
- Histopathologic analysis of skin biopsies often is necessary to exclude other inflammatory conditions and implicate an infectious process.
- Treatment with empirical broad-spectrum antibiotics should be promptly initiated and adjusted based on antibiotic susceptibility test results.
Onychomycosis: Current and Investigational Therapies
To the Editor:
Onychomycosis is a fungal infection of the nail plate by dermatophytes, yeasts, and nondermatophyte molds. It is a common problem with a prevalence of 10% to 12% in the United States.1,2 The clinical presentation of onychomycosis is shown in the Figure. Although some patients may have mild asymptomatic cases of onychomycosis and do not inquire about treatment, many will have more advanced cases, presenting with pain and discomfort, secondary infection, unattractive appearance, or problems performing everyday functions. The goal of onychomycosis treatment is to eliminate the fungus, if possible, which usually restores the nail to its normal state when it fully grows out. Patients should be counseled that it is a long process that may take 6 months or more for fingernails and 12 to 18 months for toenails. These estimates are based on a growth rate of 2 to 3 mm per month for fingernails and 1 to 2 mm per month for toenails.3 Nails grow fastest during the teenaged years and slow down with advancing age.4 It should be noted that advanced cases of onychomycosis affecting the nail matrix may cause permanent scarring; therefore, the nail unit may still appear dystrophic after the causative organism is eliminated. The US Food and Drug Administration (FDA) defines a complete cure as negative potassium hydroxide preparation and negative fungal culture plus a completely normal appearance of the nail.
Treatment of onychomycosis poses a number of challenges. First, hyperkeratosis and the fungal mass may limit the delivery of topical and systemic drugs to the source of the infection. In addition, high rates of relapse and reinfection after treatment may be due to residual hyphae or spores.5 Furthermore, the extended length of treatment limits patient adherence and many patients are unwilling to forego wearing nail cosmetics during the course of some of the treatments.
There are 4 approved classes of antifungal drugs for the treatment of onychomycosis: allylamines, azoles, morpholines, and hydroxypyridinones.6 The allylamines (eg, terbinafine) inhibit squalene epoxidase.7 Oral terbinafine (250 mg daily) taken for 6 weeks for fingernails and 12 weeks for toenails is considered the current systemic treatment preference in onychomycosis therapy8 with complete cure rates in 12-week studies of approximately 38%9 and 49%.10
The second class of drugs is the azoles, which inhibit lanosterol 14a-demethylase, a step in the ergosterol biosynthesis pathway.6 Two members of this class that are widely used in treating onychomycosis are oral itraconazole11 and off-label oral fluconazole.12 The approved dose for oral itraconazole is 200 mg daily for 3 months (or an alternative pulse regimen) with a reported complete cure rate of 14%.11 Although fluconazole is not FDA approved for the treatment of onychomycosis in the United States, it is used extensively in other countries and to some extent off label in the United States. In a study of 362 patients with onychomycosis treated with oral fluconazole, complete cure rates were 48% in patients who received 450 mg weekly, 46% in those who received 300 mg weekly, and 37% in those who received 150 mg weekly for up to 9 months.12 It should be noted that several oral triazole antifungals, namely albaconazole,13 posaconazole,14 and ravuconazole,15 have undergone phase 1 and 2 studies for the treatment of onychomycosis and have shown some efficacy.
Another class of antifungals are the morpholines including topical amorolfine, which is approved for use in Europe but not in North America.16 Amorolfine inhibits D14 reductase and D7-D8 isomerase, thus depleting ergosterol.17 In one randomized controlled study, the combination of amorolfine nail lacquer and oral terbinafine compared to oral terbinafine alone resulted in a higher clinical cure rate with the combination (59.2% vs 46%); complete cure rate was not reported.16
Finally, the hydroxypyridinone class includes topical ciclopirox, which has a poorly understood mechanism of action but may involve iron chelation or oxidative damage.18,19 Ciclopirox nail lacquer 8% was approved by the FDA in 1999 and has reported complete cure rates of 5.5% to 8.5% with monthly nail debridement.20
Based on the poor efficacy of many of the currently available treatments and time-consuming treatment courses, it is clear that there is a need for alternative and novel therapies. There has been a greater emphasis on topical agents due to their more favorable side-effect profile and lower risk for drug-drug interactions. Although there are many agents for the treatment of onychomycosis currently in development, many are in vitro studies or phase 1 and 2 studies. However, we will focus on drugs that are further along in phase 3 studies and those that were recently FDA approved.
Efinaconazole is a member of the azole class of drugs and has completed 2 phase 3 clinical trials (study 1, N=870; study 2, N=785).21 Patients in these 2 studies were randomized to receive either efinaconazole nail solution 10% or vehicle for 48 weeks followed by a 4-week washout period. Complete cure rates in the 2 studies were 17.8% and 15.2% in the treated group and 3.3% and 5.5% in the control group. The mycological cure rates were 55.2% and 53.4% in the treated group and 16.8% and 16.9% in the control group. The side-effect profile was minimal, with the most common adverse events being application-site dermatitis and vesiculation, which were not significantly higher in the treated group versus the control group.21 Efinaconazole received FDA approval for the treatment of toenail onychomycosis in June 2014.
There are some notable differences between ciclopirox and efinaconazole that may improve patient compliance with the latter. First, treatment with ciclopirox includes monthly nail debridement, which is not required with efinaconazole. Secondly, although ciclopirox lacquer must be removed weekly, efinaconazole is a solution, so no removal is necessary.
Terbinafine nail solution (TNS) is a member of the allylamine class and has completed phase 3 clinical trials.22 Three studies—2 vehicle controlled and 1 active comparator—were performed. The first compared TNS and vehicle, both applied daily for 24 weeks; the second study repeated the same for 48 weeks; and the third study compared TNS to amorolfine nail lacquer 5% daily for 48 weeks. The best results for complete cure were achieved with TNS for 48 weeks in the vehicle-controlled study with a rate of 2.2% versus 0%. The authors also concluded TNS was not more effective than amorolfine, as complete cure rates were 1.2% for TNS and 0.96% for amorolfine. The most common side effects were headache, nasopharyngitis, and influenza.22
Tavaborole is a member of the new benzoxaborole class, which inhibits protein synthesis by forming an adduct with the aminoacyl–transfer RNA synthetase.23 The topical solution was engineered to have improved penetration through the nail plate. In vitro studies showed better penetration than both ciclopirox and amorolfine.24 Two identical phase 3 randomized, double-blind, vehicle-controlled studies were completed involving 1197 patients who were treated with tavaborole topical solution 5% daily compared to vehicle for 48 weeks followed by a 4-week washout period with promising results.25 The incidence of treatment-related side effects was comparable to the vehicle. The most common adverse events were exfoliation, erythema, and dermatitis, all occurring at the application site.25 Tavaborole was approved by the FDA for the treatment of toenail onychomycosis in July 2014.
Luliconazole is a member of the azole class and a phase 2b/3 clinical trial with a 10% solution involving 334 patients was completed in June 2013.26 Results from this trial are expected in early 2015.
Lasers are a developing area for onychomycosis therapy and the appeal stems from their ability to selectively deliver energy to the target tissue, thus avoiding systemic side effects. Since 2010, the FDA has approved numerous laser devices for the temporary cosmetic improvement of onychomycosis, all of which are Nd:YAG 1064-nm lasers.27,28 It was previously thought that the mechanism of action for the fungicidal effect was achieved with heat,29 but newer in vitro studies have shown that the amount of time and level of heat required to kill Trichophyton rubrum would not be tolerable to patients.30 Although the mechanism of action is poorly understood, some clinical trials have shown success using the Nd:YAG 1064-nm laser for treatment of onychomycosis. However, in a study of 8 patients treated with the Nd:YAG 1064-nm laser for 5 treatment sessions, none had a mycological or clinical cure and there was only mild clinical improvement. In addition, most patients had pain and burning during the treatments requiring many short breaks.30 Although not yet FDA approved for the treatment of onychomycosis, other types of lasers are currently being studied, including CO2, near-infrared diode, and femtosecond-infrared laser systems.3
Plasma therapy is a developing area for the treatment of onychomycosis. Plasma was shown to be fungicidal to T rubrum in an in vitro model (MOE Medical Devices, written communication, July 2012), and a clinical trial to evaluate the safety, tolerability, and efficacy of plasma in human subjects is ongoing (registered on March 22, 2013, at www.clinicaltrials.gov with the identifier NCT01819051).
Onychomycosis is a common problem that increases in prevalence with advancing age. Oral terbinafine is considered the first-line treatment at this point in time.31 Two new topical agents, efinaconazole and tavaborole, were recently FDA approved and may be used for the treatment of toenail onychomycosis without the need for nail debridement. The Nd:YAG laser has shown some promise in earlier clinical studies but was ineffective in a more recent study.
1. Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
2. Heikkila H, Stubb S. The prevalence of onychomycosis in Finland. Br J Dermatol. 1995;133:699-703.
3. Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2, suppl 1):S2-S4.
4. Abdullah L, Abbas O. Common nail changes and disorders in older people: diagnosis and management. Can Fam Physician. 2011;57:173-181.
5. Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
6. Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol. 2010;28:151-159.
7. Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. part II. J Am Acad Dermatol. 1994;30:911-933.
8. Gupta AK, Paquet M, Simpson F, et al. Terbinafine in the treatment of dermatophyte toenail onychomycosis: a meta-analysis of efficacy for continuous and intermittent regimens. J Eur Acad Dermatol Venereol. 2013;27:267-272.
9. Drake LA, Shear NH, Arlette JP, et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J Am Acad Dermatol. 1997;37:740-745.
10. Evans EG, Sigurgeirsson B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. the LION Study Group. BMJ. 1999;318:1031-1035.
11. Sporanox [package insert]. Macquarie Park, Australia: Janssen-Cilag Pty Ltd; 2014.
12. Scher RK, Breneman D, Rich P, et al. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38(6, pt 2):S77-S86.
13. Sigurgeirsson B, van Rossem K, Malahias S, et al. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J Am Acad Dermatol. 2013;69:416-425.
14. Elewski B, Pollak R, Ashton S, et al. A randomized, placebo- and active-controlled, parallel-group, multicentre, investigator-blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br J Dermatol. 2012;166:389-398.
15. Gupta AK, Leonardi C, Stoltz RR, et al. A phase I/II randomized, double-blind, placebo-controlled, dose-ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19:437-443.
16. Baran R, Sigurgeirsson B, de Berker D, et al. A multicentre, randomized, controlled study of the efficacy, safety and cost-effectiveness of a combination therapy with amorolfine nail lacquer and oral terbinafine compared with oral terbinafine alone for the treatment of onychomycosis with matrix involvement. Br J Dermatol. 2007;157:149-157.
17. Polak A. Preclinical data and mode of action of amorolfine. Dermatology. 1992;184(suppl 1):3-7.
18. Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3:350-358.
19. Lee RE, Liu TT, Barker KS, et al. Genome-wide expression profiling of the response to ciclopirox olamine in Candida albicans. J Antimicrob Chemother. 2005;55:655-662.
20. Penlac [package insert]. Bridgewater, NJ: sanofi-aventis; 2006.
21. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
22. Elewski BE, Ghannoum MA, Mayser P, et al. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J Eur Acad Dermatol Venereol. 2013;27:287-294.
23. Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
24. Hui X, Baker SJ, Wester RC, et al. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96:2622-2631.
25. Elewski BE, Rich P, Wiltz H, et al. Effectiveness and safety of tavaborole, a novel born-based molecule for the treatment of onychomycosis: results from two phase 3 studies. Poster presented at: Women’s & Pediatric Dermatology Seminar; October 4-6, 2013; Newport Beach, CA.
26. The solution study: Topica’s phase 2b/3 clinical trial. Topica Pharmaceuticals Inc Web site. http://www.
topicapharma.com/phase-2b3. Accessed December 2, 2014.
27. Gupta AK, Simpson FC. Medical devices for the treatment of onychomycosis. Dermatol Ther. 2012;25:574-581.
28. Ortiz AE, Avram MM, Wanner MA. A review of lasers and light for the treatment of onychomycosis. Lasers Surg Med. 2014;46:117-124.
29. Vural E, Winfield HL, Shingleton AW, et al. The effects of laser irradiation on Trichophyton rubrum growth. Lasers Med Sci. 2008;23:349-353.
30. Carney C, Cantrell W, Warner J, et al. Treatment of onychomycosis using a submillisecond 1064-nm neodymium:yttrium-aluminum-garnet laser. J Am Acad Dermatol. 2013;69:578-582.
31. Gupta AK, Daigle D, Paquet M. Therapies for onychomycosis: a systematic review and network meta-analysis of mycological cure [published online ahead of print July 17, 2014]. J Am Podiatr Med Assoc. doi:10.7547/13-110.1.
To the Editor:
Onychomycosis is a fungal infection of the nail plate by dermatophytes, yeasts, and nondermatophyte molds. It is a common problem with a prevalence of 10% to 12% in the United States.1,2 The clinical presentation of onychomycosis is shown in the Figure. Although some patients may have mild asymptomatic cases of onychomycosis and do not inquire about treatment, many will have more advanced cases, presenting with pain and discomfort, secondary infection, unattractive appearance, or problems performing everyday functions. The goal of onychomycosis treatment is to eliminate the fungus, if possible, which usually restores the nail to its normal state when it fully grows out. Patients should be counseled that it is a long process that may take 6 months or more for fingernails and 12 to 18 months for toenails. These estimates are based on a growth rate of 2 to 3 mm per month for fingernails and 1 to 2 mm per month for toenails.3 Nails grow fastest during the teenaged years and slow down with advancing age.4 It should be noted that advanced cases of onychomycosis affecting the nail matrix may cause permanent scarring; therefore, the nail unit may still appear dystrophic after the causative organism is eliminated. The US Food and Drug Administration (FDA) defines a complete cure as negative potassium hydroxide preparation and negative fungal culture plus a completely normal appearance of the nail.
Treatment of onychomycosis poses a number of challenges. First, hyperkeratosis and the fungal mass may limit the delivery of topical and systemic drugs to the source of the infection. In addition, high rates of relapse and reinfection after treatment may be due to residual hyphae or spores.5 Furthermore, the extended length of treatment limits patient adherence and many patients are unwilling to forego wearing nail cosmetics during the course of some of the treatments.
There are 4 approved classes of antifungal drugs for the treatment of onychomycosis: allylamines, azoles, morpholines, and hydroxypyridinones.6 The allylamines (eg, terbinafine) inhibit squalene epoxidase.7 Oral terbinafine (250 mg daily) taken for 6 weeks for fingernails and 12 weeks for toenails is considered the current systemic treatment preference in onychomycosis therapy8 with complete cure rates in 12-week studies of approximately 38%9 and 49%.10
The second class of drugs is the azoles, which inhibit lanosterol 14a-demethylase, a step in the ergosterol biosynthesis pathway.6 Two members of this class that are widely used in treating onychomycosis are oral itraconazole11 and off-label oral fluconazole.12 The approved dose for oral itraconazole is 200 mg daily for 3 months (or an alternative pulse regimen) with a reported complete cure rate of 14%.11 Although fluconazole is not FDA approved for the treatment of onychomycosis in the United States, it is used extensively in other countries and to some extent off label in the United States. In a study of 362 patients with onychomycosis treated with oral fluconazole, complete cure rates were 48% in patients who received 450 mg weekly, 46% in those who received 300 mg weekly, and 37% in those who received 150 mg weekly for up to 9 months.12 It should be noted that several oral triazole antifungals, namely albaconazole,13 posaconazole,14 and ravuconazole,15 have undergone phase 1 and 2 studies for the treatment of onychomycosis and have shown some efficacy.
Another class of antifungals are the morpholines including topical amorolfine, which is approved for use in Europe but not in North America.16 Amorolfine inhibits D14 reductase and D7-D8 isomerase, thus depleting ergosterol.17 In one randomized controlled study, the combination of amorolfine nail lacquer and oral terbinafine compared to oral terbinafine alone resulted in a higher clinical cure rate with the combination (59.2% vs 46%); complete cure rate was not reported.16
Finally, the hydroxypyridinone class includes topical ciclopirox, which has a poorly understood mechanism of action but may involve iron chelation or oxidative damage.18,19 Ciclopirox nail lacquer 8% was approved by the FDA in 1999 and has reported complete cure rates of 5.5% to 8.5% with monthly nail debridement.20
Based on the poor efficacy of many of the currently available treatments and time-consuming treatment courses, it is clear that there is a need for alternative and novel therapies. There has been a greater emphasis on topical agents due to their more favorable side-effect profile and lower risk for drug-drug interactions. Although there are many agents for the treatment of onychomycosis currently in development, many are in vitro studies or phase 1 and 2 studies. However, we will focus on drugs that are further along in phase 3 studies and those that were recently FDA approved.
Efinaconazole is a member of the azole class of drugs and has completed 2 phase 3 clinical trials (study 1, N=870; study 2, N=785).21 Patients in these 2 studies were randomized to receive either efinaconazole nail solution 10% or vehicle for 48 weeks followed by a 4-week washout period. Complete cure rates in the 2 studies were 17.8% and 15.2% in the treated group and 3.3% and 5.5% in the control group. The mycological cure rates were 55.2% and 53.4% in the treated group and 16.8% and 16.9% in the control group. The side-effect profile was minimal, with the most common adverse events being application-site dermatitis and vesiculation, which were not significantly higher in the treated group versus the control group.21 Efinaconazole received FDA approval for the treatment of toenail onychomycosis in June 2014.
There are some notable differences between ciclopirox and efinaconazole that may improve patient compliance with the latter. First, treatment with ciclopirox includes monthly nail debridement, which is not required with efinaconazole. Secondly, although ciclopirox lacquer must be removed weekly, efinaconazole is a solution, so no removal is necessary.
Terbinafine nail solution (TNS) is a member of the allylamine class and has completed phase 3 clinical trials.22 Three studies—2 vehicle controlled and 1 active comparator—were performed. The first compared TNS and vehicle, both applied daily for 24 weeks; the second study repeated the same for 48 weeks; and the third study compared TNS to amorolfine nail lacquer 5% daily for 48 weeks. The best results for complete cure were achieved with TNS for 48 weeks in the vehicle-controlled study with a rate of 2.2% versus 0%. The authors also concluded TNS was not more effective than amorolfine, as complete cure rates were 1.2% for TNS and 0.96% for amorolfine. The most common side effects were headache, nasopharyngitis, and influenza.22
Tavaborole is a member of the new benzoxaborole class, which inhibits protein synthesis by forming an adduct with the aminoacyl–transfer RNA synthetase.23 The topical solution was engineered to have improved penetration through the nail plate. In vitro studies showed better penetration than both ciclopirox and amorolfine.24 Two identical phase 3 randomized, double-blind, vehicle-controlled studies were completed involving 1197 patients who were treated with tavaborole topical solution 5% daily compared to vehicle for 48 weeks followed by a 4-week washout period with promising results.25 The incidence of treatment-related side effects was comparable to the vehicle. The most common adverse events were exfoliation, erythema, and dermatitis, all occurring at the application site.25 Tavaborole was approved by the FDA for the treatment of toenail onychomycosis in July 2014.
Luliconazole is a member of the azole class and a phase 2b/3 clinical trial with a 10% solution involving 334 patients was completed in June 2013.26 Results from this trial are expected in early 2015.
Lasers are a developing area for onychomycosis therapy and the appeal stems from their ability to selectively deliver energy to the target tissue, thus avoiding systemic side effects. Since 2010, the FDA has approved numerous laser devices for the temporary cosmetic improvement of onychomycosis, all of which are Nd:YAG 1064-nm lasers.27,28 It was previously thought that the mechanism of action for the fungicidal effect was achieved with heat,29 but newer in vitro studies have shown that the amount of time and level of heat required to kill Trichophyton rubrum would not be tolerable to patients.30 Although the mechanism of action is poorly understood, some clinical trials have shown success using the Nd:YAG 1064-nm laser for treatment of onychomycosis. However, in a study of 8 patients treated with the Nd:YAG 1064-nm laser for 5 treatment sessions, none had a mycological or clinical cure and there was only mild clinical improvement. In addition, most patients had pain and burning during the treatments requiring many short breaks.30 Although not yet FDA approved for the treatment of onychomycosis, other types of lasers are currently being studied, including CO2, near-infrared diode, and femtosecond-infrared laser systems.3
Plasma therapy is a developing area for the treatment of onychomycosis. Plasma was shown to be fungicidal to T rubrum in an in vitro model (MOE Medical Devices, written communication, July 2012), and a clinical trial to evaluate the safety, tolerability, and efficacy of plasma in human subjects is ongoing (registered on March 22, 2013, at www.clinicaltrials.gov with the identifier NCT01819051).
Onychomycosis is a common problem that increases in prevalence with advancing age. Oral terbinafine is considered the first-line treatment at this point in time.31 Two new topical agents, efinaconazole and tavaborole, were recently FDA approved and may be used for the treatment of toenail onychomycosis without the need for nail debridement. The Nd:YAG laser has shown some promise in earlier clinical studies but was ineffective in a more recent study.
To the Editor:
Onychomycosis is a fungal infection of the nail plate by dermatophytes, yeasts, and nondermatophyte molds. It is a common problem with a prevalence of 10% to 12% in the United States.1,2 The clinical presentation of onychomycosis is shown in the Figure. Although some patients may have mild asymptomatic cases of onychomycosis and do not inquire about treatment, many will have more advanced cases, presenting with pain and discomfort, secondary infection, unattractive appearance, or problems performing everyday functions. The goal of onychomycosis treatment is to eliminate the fungus, if possible, which usually restores the nail to its normal state when it fully grows out. Patients should be counseled that it is a long process that may take 6 months or more for fingernails and 12 to 18 months for toenails. These estimates are based on a growth rate of 2 to 3 mm per month for fingernails and 1 to 2 mm per month for toenails.3 Nails grow fastest during the teenaged years and slow down with advancing age.4 It should be noted that advanced cases of onychomycosis affecting the nail matrix may cause permanent scarring; therefore, the nail unit may still appear dystrophic after the causative organism is eliminated. The US Food and Drug Administration (FDA) defines a complete cure as negative potassium hydroxide preparation and negative fungal culture plus a completely normal appearance of the nail.
Treatment of onychomycosis poses a number of challenges. First, hyperkeratosis and the fungal mass may limit the delivery of topical and systemic drugs to the source of the infection. In addition, high rates of relapse and reinfection after treatment may be due to residual hyphae or spores.5 Furthermore, the extended length of treatment limits patient adherence and many patients are unwilling to forego wearing nail cosmetics during the course of some of the treatments.
There are 4 approved classes of antifungal drugs for the treatment of onychomycosis: allylamines, azoles, morpholines, and hydroxypyridinones.6 The allylamines (eg, terbinafine) inhibit squalene epoxidase.7 Oral terbinafine (250 mg daily) taken for 6 weeks for fingernails and 12 weeks for toenails is considered the current systemic treatment preference in onychomycosis therapy8 with complete cure rates in 12-week studies of approximately 38%9 and 49%.10
The second class of drugs is the azoles, which inhibit lanosterol 14a-demethylase, a step in the ergosterol biosynthesis pathway.6 Two members of this class that are widely used in treating onychomycosis are oral itraconazole11 and off-label oral fluconazole.12 The approved dose for oral itraconazole is 200 mg daily for 3 months (or an alternative pulse regimen) with a reported complete cure rate of 14%.11 Although fluconazole is not FDA approved for the treatment of onychomycosis in the United States, it is used extensively in other countries and to some extent off label in the United States. In a study of 362 patients with onychomycosis treated with oral fluconazole, complete cure rates were 48% in patients who received 450 mg weekly, 46% in those who received 300 mg weekly, and 37% in those who received 150 mg weekly for up to 9 months.12 It should be noted that several oral triazole antifungals, namely albaconazole,13 posaconazole,14 and ravuconazole,15 have undergone phase 1 and 2 studies for the treatment of onychomycosis and have shown some efficacy.
Another class of antifungals are the morpholines including topical amorolfine, which is approved for use in Europe but not in North America.16 Amorolfine inhibits D14 reductase and D7-D8 isomerase, thus depleting ergosterol.17 In one randomized controlled study, the combination of amorolfine nail lacquer and oral terbinafine compared to oral terbinafine alone resulted in a higher clinical cure rate with the combination (59.2% vs 46%); complete cure rate was not reported.16
Finally, the hydroxypyridinone class includes topical ciclopirox, which has a poorly understood mechanism of action but may involve iron chelation or oxidative damage.18,19 Ciclopirox nail lacquer 8% was approved by the FDA in 1999 and has reported complete cure rates of 5.5% to 8.5% with monthly nail debridement.20
Based on the poor efficacy of many of the currently available treatments and time-consuming treatment courses, it is clear that there is a need for alternative and novel therapies. There has been a greater emphasis on topical agents due to their more favorable side-effect profile and lower risk for drug-drug interactions. Although there are many agents for the treatment of onychomycosis currently in development, many are in vitro studies or phase 1 and 2 studies. However, we will focus on drugs that are further along in phase 3 studies and those that were recently FDA approved.
Efinaconazole is a member of the azole class of drugs and has completed 2 phase 3 clinical trials (study 1, N=870; study 2, N=785).21 Patients in these 2 studies were randomized to receive either efinaconazole nail solution 10% or vehicle for 48 weeks followed by a 4-week washout period. Complete cure rates in the 2 studies were 17.8% and 15.2% in the treated group and 3.3% and 5.5% in the control group. The mycological cure rates were 55.2% and 53.4% in the treated group and 16.8% and 16.9% in the control group. The side-effect profile was minimal, with the most common adverse events being application-site dermatitis and vesiculation, which were not significantly higher in the treated group versus the control group.21 Efinaconazole received FDA approval for the treatment of toenail onychomycosis in June 2014.
There are some notable differences between ciclopirox and efinaconazole that may improve patient compliance with the latter. First, treatment with ciclopirox includes monthly nail debridement, which is not required with efinaconazole. Secondly, although ciclopirox lacquer must be removed weekly, efinaconazole is a solution, so no removal is necessary.
Terbinafine nail solution (TNS) is a member of the allylamine class and has completed phase 3 clinical trials.22 Three studies—2 vehicle controlled and 1 active comparator—were performed. The first compared TNS and vehicle, both applied daily for 24 weeks; the second study repeated the same for 48 weeks; and the third study compared TNS to amorolfine nail lacquer 5% daily for 48 weeks. The best results for complete cure were achieved with TNS for 48 weeks in the vehicle-controlled study with a rate of 2.2% versus 0%. The authors also concluded TNS was not more effective than amorolfine, as complete cure rates were 1.2% for TNS and 0.96% for amorolfine. The most common side effects were headache, nasopharyngitis, and influenza.22
Tavaborole is a member of the new benzoxaborole class, which inhibits protein synthesis by forming an adduct with the aminoacyl–transfer RNA synthetase.23 The topical solution was engineered to have improved penetration through the nail plate. In vitro studies showed better penetration than both ciclopirox and amorolfine.24 Two identical phase 3 randomized, double-blind, vehicle-controlled studies were completed involving 1197 patients who were treated with tavaborole topical solution 5% daily compared to vehicle for 48 weeks followed by a 4-week washout period with promising results.25 The incidence of treatment-related side effects was comparable to the vehicle. The most common adverse events were exfoliation, erythema, and dermatitis, all occurring at the application site.25 Tavaborole was approved by the FDA for the treatment of toenail onychomycosis in July 2014.
Luliconazole is a member of the azole class and a phase 2b/3 clinical trial with a 10% solution involving 334 patients was completed in June 2013.26 Results from this trial are expected in early 2015.
Lasers are a developing area for onychomycosis therapy and the appeal stems from their ability to selectively deliver energy to the target tissue, thus avoiding systemic side effects. Since 2010, the FDA has approved numerous laser devices for the temporary cosmetic improvement of onychomycosis, all of which are Nd:YAG 1064-nm lasers.27,28 It was previously thought that the mechanism of action for the fungicidal effect was achieved with heat,29 but newer in vitro studies have shown that the amount of time and level of heat required to kill Trichophyton rubrum would not be tolerable to patients.30 Although the mechanism of action is poorly understood, some clinical trials have shown success using the Nd:YAG 1064-nm laser for treatment of onychomycosis. However, in a study of 8 patients treated with the Nd:YAG 1064-nm laser for 5 treatment sessions, none had a mycological or clinical cure and there was only mild clinical improvement. In addition, most patients had pain and burning during the treatments requiring many short breaks.30 Although not yet FDA approved for the treatment of onychomycosis, other types of lasers are currently being studied, including CO2, near-infrared diode, and femtosecond-infrared laser systems.3
Plasma therapy is a developing area for the treatment of onychomycosis. Plasma was shown to be fungicidal to T rubrum in an in vitro model (MOE Medical Devices, written communication, July 2012), and a clinical trial to evaluate the safety, tolerability, and efficacy of plasma in human subjects is ongoing (registered on March 22, 2013, at www.clinicaltrials.gov with the identifier NCT01819051).
Onychomycosis is a common problem that increases in prevalence with advancing age. Oral terbinafine is considered the first-line treatment at this point in time.31 Two new topical agents, efinaconazole and tavaborole, were recently FDA approved and may be used for the treatment of toenail onychomycosis without the need for nail debridement. The Nd:YAG laser has shown some promise in earlier clinical studies but was ineffective in a more recent study.
1. Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
2. Heikkila H, Stubb S. The prevalence of onychomycosis in Finland. Br J Dermatol. 1995;133:699-703.
3. Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2, suppl 1):S2-S4.
4. Abdullah L, Abbas O. Common nail changes and disorders in older people: diagnosis and management. Can Fam Physician. 2011;57:173-181.
5. Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
6. Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol. 2010;28:151-159.
7. Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. part II. J Am Acad Dermatol. 1994;30:911-933.
8. Gupta AK, Paquet M, Simpson F, et al. Terbinafine in the treatment of dermatophyte toenail onychomycosis: a meta-analysis of efficacy for continuous and intermittent regimens. J Eur Acad Dermatol Venereol. 2013;27:267-272.
9. Drake LA, Shear NH, Arlette JP, et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J Am Acad Dermatol. 1997;37:740-745.
10. Evans EG, Sigurgeirsson B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. the LION Study Group. BMJ. 1999;318:1031-1035.
11. Sporanox [package insert]. Macquarie Park, Australia: Janssen-Cilag Pty Ltd; 2014.
12. Scher RK, Breneman D, Rich P, et al. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38(6, pt 2):S77-S86.
13. Sigurgeirsson B, van Rossem K, Malahias S, et al. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J Am Acad Dermatol. 2013;69:416-425.
14. Elewski B, Pollak R, Ashton S, et al. A randomized, placebo- and active-controlled, parallel-group, multicentre, investigator-blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br J Dermatol. 2012;166:389-398.
15. Gupta AK, Leonardi C, Stoltz RR, et al. A phase I/II randomized, double-blind, placebo-controlled, dose-ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19:437-443.
16. Baran R, Sigurgeirsson B, de Berker D, et al. A multicentre, randomized, controlled study of the efficacy, safety and cost-effectiveness of a combination therapy with amorolfine nail lacquer and oral terbinafine compared with oral terbinafine alone for the treatment of onychomycosis with matrix involvement. Br J Dermatol. 2007;157:149-157.
17. Polak A. Preclinical data and mode of action of amorolfine. Dermatology. 1992;184(suppl 1):3-7.
18. Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3:350-358.
19. Lee RE, Liu TT, Barker KS, et al. Genome-wide expression profiling of the response to ciclopirox olamine in Candida albicans. J Antimicrob Chemother. 2005;55:655-662.
20. Penlac [package insert]. Bridgewater, NJ: sanofi-aventis; 2006.
21. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
22. Elewski BE, Ghannoum MA, Mayser P, et al. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J Eur Acad Dermatol Venereol. 2013;27:287-294.
23. Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
24. Hui X, Baker SJ, Wester RC, et al. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96:2622-2631.
25. Elewski BE, Rich P, Wiltz H, et al. Effectiveness and safety of tavaborole, a novel born-based molecule for the treatment of onychomycosis: results from two phase 3 studies. Poster presented at: Women’s & Pediatric Dermatology Seminar; October 4-6, 2013; Newport Beach, CA.
26. The solution study: Topica’s phase 2b/3 clinical trial. Topica Pharmaceuticals Inc Web site. http://www.
topicapharma.com/phase-2b3. Accessed December 2, 2014.
27. Gupta AK, Simpson FC. Medical devices for the treatment of onychomycosis. Dermatol Ther. 2012;25:574-581.
28. Ortiz AE, Avram MM, Wanner MA. A review of lasers and light for the treatment of onychomycosis. Lasers Surg Med. 2014;46:117-124.
29. Vural E, Winfield HL, Shingleton AW, et al. The effects of laser irradiation on Trichophyton rubrum growth. Lasers Med Sci. 2008;23:349-353.
30. Carney C, Cantrell W, Warner J, et al. Treatment of onychomycosis using a submillisecond 1064-nm neodymium:yttrium-aluminum-garnet laser. J Am Acad Dermatol. 2013;69:578-582.
31. Gupta AK, Daigle D, Paquet M. Therapies for onychomycosis: a systematic review and network meta-analysis of mycological cure [published online ahead of print July 17, 2014]. J Am Podiatr Med Assoc. doi:10.7547/13-110.1.
1. Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
2. Heikkila H, Stubb S. The prevalence of onychomycosis in Finland. Br J Dermatol. 1995;133:699-703.
3. Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2, suppl 1):S2-S4.
4. Abdullah L, Abbas O. Common nail changes and disorders in older people: diagnosis and management. Can Fam Physician. 2011;57:173-181.
5. Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
6. Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol. 2010;28:151-159.
7. Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. part II. J Am Acad Dermatol. 1994;30:911-933.
8. Gupta AK, Paquet M, Simpson F, et al. Terbinafine in the treatment of dermatophyte toenail onychomycosis: a meta-analysis of efficacy for continuous and intermittent regimens. J Eur Acad Dermatol Venereol. 2013;27:267-272.
9. Drake LA, Shear NH, Arlette JP, et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J Am Acad Dermatol. 1997;37:740-745.
10. Evans EG, Sigurgeirsson B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. the LION Study Group. BMJ. 1999;318:1031-1035.
11. Sporanox [package insert]. Macquarie Park, Australia: Janssen-Cilag Pty Ltd; 2014.
12. Scher RK, Breneman D, Rich P, et al. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38(6, pt 2):S77-S86.
13. Sigurgeirsson B, van Rossem K, Malahias S, et al. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J Am Acad Dermatol. 2013;69:416-425.
14. Elewski B, Pollak R, Ashton S, et al. A randomized, placebo- and active-controlled, parallel-group, multicentre, investigator-blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br J Dermatol. 2012;166:389-398.
15. Gupta AK, Leonardi C, Stoltz RR, et al. A phase I/II randomized, double-blind, placebo-controlled, dose-ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19:437-443.
16. Baran R, Sigurgeirsson B, de Berker D, et al. A multicentre, randomized, controlled study of the efficacy, safety and cost-effectiveness of a combination therapy with amorolfine nail lacquer and oral terbinafine compared with oral terbinafine alone for the treatment of onychomycosis with matrix involvement. Br J Dermatol. 2007;157:149-157.
17. Polak A. Preclinical data and mode of action of amorolfine. Dermatology. 1992;184(suppl 1):3-7.
18. Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3:350-358.
19. Lee RE, Liu TT, Barker KS, et al. Genome-wide expression profiling of the response to ciclopirox olamine in Candida albicans. J Antimicrob Chemother. 2005;55:655-662.
20. Penlac [package insert]. Bridgewater, NJ: sanofi-aventis; 2006.
21. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
22. Elewski BE, Ghannoum MA, Mayser P, et al. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J Eur Acad Dermatol Venereol. 2013;27:287-294.
23. Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
24. Hui X, Baker SJ, Wester RC, et al. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96:2622-2631.
25. Elewski BE, Rich P, Wiltz H, et al. Effectiveness and safety of tavaborole, a novel born-based molecule for the treatment of onychomycosis: results from two phase 3 studies. Poster presented at: Women’s & Pediatric Dermatology Seminar; October 4-6, 2013; Newport Beach, CA.
26. The solution study: Topica’s phase 2b/3 clinical trial. Topica Pharmaceuticals Inc Web site. http://www.
topicapharma.com/phase-2b3. Accessed December 2, 2014.
27. Gupta AK, Simpson FC. Medical devices for the treatment of onychomycosis. Dermatol Ther. 2012;25:574-581.
28. Ortiz AE, Avram MM, Wanner MA. A review of lasers and light for the treatment of onychomycosis. Lasers Surg Med. 2014;46:117-124.
29. Vural E, Winfield HL, Shingleton AW, et al. The effects of laser irradiation on Trichophyton rubrum growth. Lasers Med Sci. 2008;23:349-353.
30. Carney C, Cantrell W, Warner J, et al. Treatment of onychomycosis using a submillisecond 1064-nm neodymium:yttrium-aluminum-garnet laser. J Am Acad Dermatol. 2013;69:578-582.
31. Gupta AK, Daigle D, Paquet M. Therapies for onychomycosis: a systematic review and network meta-analysis of mycological cure [published online ahead of print July 17, 2014]. J Am Podiatr Med Assoc. doi:10.7547/13-110.1.
Acitretin-Induced Acral Hemorrhagic Lesions in Darier-White Disease
Darier-White disease (DD), also known as keratosis follicularis, is a rare skin disease that is inherited in an autosomal-dominant fashion. It was first described by both Darier1 and White2 more than 120 years ago. The incidence is 1 to 9 per 100,000 individuals, and onset usually occurs between 8 and 15 years of age. Classic DD is characterized by malodorous, brown, keratotic, warty papules arising mainly in seborrheic areas; palmoplantar pits; and nail dystrophy with typical worsening in summer. Unilateral and segmental variants of DD rarely have been described.3,4
Few cases of hemorrhagic DD involving acral surfaces have been reported.5-8 Most patients develop hemorrhagic bullae at the onset of the disease together with the other characteristic features of DD. The disease also can be associated with salivary gland obstruction, renal and testicular agenesis, bone cysts, and neuropsychiatric disorders.9 Diagnosis is confirmed by hyperkeratosis, papillomatosis, acantholysis, dyskeratosis (corps ronds and grains), and small fissures above the basal layer in the epidermis (suprabasal clefts) noted on biopsy of papular lesions. Darier-White disease is caused by a malfunctioning sarcoplasmic/endoplasmic reticulum calcium pump, SERCA2, encoded by the ATP2A2 (ATPase, Ca++ transporting, cardiac muscle, slow twitch 2) gene, which transports calcium ions from the cytosol into the sarcoplasmic/endoplasmic reticulum, catalyzing the hydrolysis of adenosine triphosphate coupled with the transport of the calcium. Mutations in ATP2A2 have only been identified in up to 50% of reported DD patients.10
Treatment of localized DD usually includes emollients and topical retinoids, and systemic retinoids are recommended as the first-line treatment of diffuse DD.11 Many dermatologic side effects of systemic retinoids have been reported, including frequent dry lips and cheilitis.12 Other skin manifestations include scaling of the palms and soles, excoriations and erosions, alopecia, pruritus, nail fragility, and skin atrophy or fragility. Additionally, common nondermatologic side effects include hepatotoxicity, epistaxis, ophthalmologic effects (eg, loss of eyebrows or eyelashes, redness/swelling of the eyelid, redness of the eyes, sensitivity of the eyes to light, decreased night vision) pancreatitis, bipedal edema, and skeletal alterations.13-15 We report the case of a patient with DD who developed hemorrhagic macules and vesicles in response to the administration of oral retinoids.
Case Report
An 84-year-old woman was admitted to our hospital for treatment of hyperkeratotic papules and plaques on the face, back, groin, submammary folds, and dorsum of the hands and feet that had been present since childhood and typically worsened during the summer. She previously had refused treatment and reported a poor social life due to the cutaneous manifestations of her disease.
Biopsy of a histologic specimen taken from a palmar pit revealed hyperkeratosis, loss of cohesion between suprabasal epidermal cells (acantholysis), and dyskeratotic cells (corps ronds and grains)(Figure 1). On the basis of both clinical and histologic findings, a diagnosis of DD was made. Genetic analysis did not reveal any mutations in the ATP2A2 gene, and a reduced epidermal expression of SERCA2b was demonstrated by immunochemistry.16 Treatment with the oral retinoid acitretin (25 mg daily) for 4 months led to almost complete resolution of the lesions. After 4 months, treatment was reduced to a maintenance dose of 12.5 mg once daily.
The patient returned monthly for follow-up to undergo clinical examination and routine laboratory tests (ie, complete blood cell counts, electrolyte panel, renal and hepatic tests, serum lipid levels, cholesterol level). After beginning the maintenance dose, she returned for follow-up every 2 months.
On follow-up 3 months after beginning the maintenance dose, the patient reported the onset of red and black punctiform macules and vesicles with jagged borders located on the palmoplantar surfaces and dorsal aspect of the fingers on both hands. Lesions were either isolated or confluent. The patient did not report pain or itching, though the skin was severely xerotic, especially on the hands and feet (Figures 2A and 2B). The patient reported no local trauma associated with the lesions, and blood counts and coagulation tests were within reference range. It also was noted that the cutaneous manifestations of DD showed a striking improvement. Histologic analysis of a skin biopsy taken from a hemorrhagic lesion on the palmar surface showed hyperkeratosis, acanthosis, papillomatosis, hypergranulosis, focal dyskeratosis, and hemorrhagic vesicles in the horny layer of the epidermis (Figure 3), leading to a diagnosis of DD.
After 12 months of therapy, the patient spontaneously stopped taking acitretin, and the hemorrhagic lesions spontaneously regressed within 2 weeks (Figures 2C and 2D). Unfortunately, the cutaneous manifestations of DD (eg, papular lesions) progressively reappeared in the previously reported sites. Based on this relapse, the patient was advised to restart treatment with acitretin (25 mg daily). In the follow-up visit 1 month after restarting acitretin, physical examination revealed the reappearance of hemorrhagic lesions on the palmoplantar surfaces and the dorsal aspect of the fingers.
|
|
Comment
We report the case of an 84-year-old woman who developed hemorrhagic lesions on the palmoplantar surfaces and dorsal aspect of the feet as a side effect of oral retinoids for treatment of DD. The first reported case of acral hemorrhagic lesions associated with DD was described 50 years ago in 4 patients with manifestations located mainly on the palms, soles, and dorsal fingers. Local trauma was identified as a triggering factor in the development of the vesicles; however, data regarding the temporal relationship between keratotic papules and hemorrhagic elements and treatment were not specified.5 Twenty-five years later, Coulson and Misch6 described a case of DD in which the hemorrhagic lesions were the first sign of the disease, but correlation with therapy was not reported. A case of retinoid-induced hemorrhagic DD was reported in a female who was treated with etretinate for approximately 10 years after the diagnosis of DD with good clinical response. After 10 years of therapy, she developed hemorrhagic bullae solely on the dorsal aspect of the hands without any direct association with local trauma, along with a small number of nonhemorrhagic bullae.17
In our patient, the onset of hemorrhagic vesicles and red maculae occurred primarily on the palmoplantar surfaces. The lesions were smaller than bullae and contained hemorrhagic elements. Of note, the skin lesions appeared as early as 7 months after the patient started acitretin therapy.
The development of hemorrhagic lesions on different body sites as a consequence of oral retinoid administration also has been reported in patients with psoriasis.15 Emerging evidence indicates that retinoic acid activates vascular endothelial growth factor gene transcription,18 and vascular endothelial growth factor can modify permeability of the endothelial cells. Based on these observations, we propose that DD is characterized by loss of adhesion between epidermal cells (acantholysis) and abnormal keratinization. This defect favors the formation of empty intraepidermal lacunae. An increase in endothelial permeability due to oral retinoid administration promotes the gathering of serum and red blood cells into the lacunae, leading to the onset of hemorrhagic blisters. Moreover, continued microtrauma to the palmoplantar surfaces may account for the peculiar localization of the hemorrhagic lesions described in our patient.
Our unique report of hemorrhagic lesions (vesicles and maculae) presenting in a DD patient treated with acitretin is rare. The first histologic specimen taken from a palmar pit revealed the characteristic histopathologic findings of DD and did not support the diagnosis of the hemorrhagic variant of DD. In the second biopsy, the absence of acantholysis and the poor content of dyskeratotic cells were evident, together with superficial hemorrhagic vesicles. Additionally, the appearance and the disappearance of the cutaneous manifestations were closely correlated with the beginning and suspension of the acitretin therapy, together with an improvement of typical lesions of DD during acitretin treatment. These occurrences indicated a strict causal relationship between treatment with the retinoid and the appearance of hemorrhagic lesions.
Conclusion
We report a rare case of palmoplantar hemorrhagic lesions induced by acitretin for treatment of DD. In our patient, the lesions could have been triggered by a combination of noxious effects of the drug and alterations in keratinocyte physiology due to DD. There is a need for clinicians to be aware of the possible side effects of acitretin and to inform their patients, particularly those presenting with DD, about the possibility of developing hemorrhagic lesions. The latter should be added to the list of potential dermatologic adverse effects of acitretin.
1. Darier J. De la porospermose folliculaire vegetante. Ann Dermatol Syphiligr. 1889;10:597-605.
2. White JC. A case of keratosis (ichtyosis) follicularis. J Cutan Genitourin Dis. 1889;7:201-209.
3. Goldsmith LA, Baden HP. Darier-White disease (keratosis follicularis) and acrokeratosis verruciformis. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 4th ed. New York, NY: McGraw-Hill; 2003:523-528.
4. Sanderson EA, Killoran CE, Pedvis-Leftick A, et al. Localized Darier’s disease in Blaschkoid distribution: two cases of phenotypic mosaicism and a review of mosaic Darier’s disease. J Dermatol. 2007;34:761-764.
5. Jones WN, Nix TE Jr, Clark WH Jr. Hemorrhagic Darier’s disease. Arch Dermatol. 1964;89:523-527.
6. Coulson IH, Misch KJ. Haemorrhagic Darier’s disease. J R Soc Med. 1989;82:365-366.
7. Foresman PL, Goldsmith LA, Ginn L, et al. Hemorrhagic Darier’s disease. Arch Dermatol. 1993;129:511-512.
8. Regazzini R, Zambruno G, DeFilippi C, et al. Isolated acral Darier’s disease with haemorrhagic lesions in a kindred. Br J Dermatol. 1996;135:495-496.
9. Zeglaoui F, Zaraa I, Fazaa B, et al. Dyskeratosis follicularis disease: case reports and review of the literature. J Eur Acad Dermatol Venereol. 2005;19:114-117.
10. Sakuntabhai A, Burge S, Monk S, et al. Spectrum of novel ATP2A2 mutations with Darier’s disease. Hum Mol Genet. 1999;8:1611-1619.
11. Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50.
12. Nikam B, Amladi S, Bingewar G, et al. Acral papular eruption. Indian J Dermatol Venereol Leprol. 2005;71:447-448.
13. Wolverton SE, Remlinger K. Suggested guidelines for patient monitoring: hepatic and hematologic toxicity attributable to systemic dermatologic drugs. Dermatol Clin. 2007;25:195-205.
14. Tey HL, Theng TS. Acitretin induced bipedal edema. J Dermatol. 2006;33:372-374.
15. Aydogan K, Karadogan SK, Tunali S. Acitretin-induced subungual hemorrhage. Int J Dermatol. 2007;46:494-495.
16. Borgogna C, Zavattaro E, Dell’Oste V, et al. No indications for HPV involvement in the hypertrophic skin lesions of a Darier disease case without ATP2A2 gene mutations. J Cutan Pathol. 2009;36:1005-1009.
17. Gebauer K, Holgate C, Navaratnam A. Retinoid-induced haemorrhagic bullae in Darier’s disease. Australas J Dermatol. 1990;31:99-103.
18. Maeno T, Tanaka T, Sando Y, et al. Stimulation of vascular endothelial growth factor gene transcription by all trans retinoic acid through Sp1 and Sp3 sites in human bronchioalveolar carcinoma cells. Am J Respir Cell Mol Biol. 2002;26:246-253.
Darier-White disease (DD), also known as keratosis follicularis, is a rare skin disease that is inherited in an autosomal-dominant fashion. It was first described by both Darier1 and White2 more than 120 years ago. The incidence is 1 to 9 per 100,000 individuals, and onset usually occurs between 8 and 15 years of age. Classic DD is characterized by malodorous, brown, keratotic, warty papules arising mainly in seborrheic areas; palmoplantar pits; and nail dystrophy with typical worsening in summer. Unilateral and segmental variants of DD rarely have been described.3,4
Few cases of hemorrhagic DD involving acral surfaces have been reported.5-8 Most patients develop hemorrhagic bullae at the onset of the disease together with the other characteristic features of DD. The disease also can be associated with salivary gland obstruction, renal and testicular agenesis, bone cysts, and neuropsychiatric disorders.9 Diagnosis is confirmed by hyperkeratosis, papillomatosis, acantholysis, dyskeratosis (corps ronds and grains), and small fissures above the basal layer in the epidermis (suprabasal clefts) noted on biopsy of papular lesions. Darier-White disease is caused by a malfunctioning sarcoplasmic/endoplasmic reticulum calcium pump, SERCA2, encoded by the ATP2A2 (ATPase, Ca++ transporting, cardiac muscle, slow twitch 2) gene, which transports calcium ions from the cytosol into the sarcoplasmic/endoplasmic reticulum, catalyzing the hydrolysis of adenosine triphosphate coupled with the transport of the calcium. Mutations in ATP2A2 have only been identified in up to 50% of reported DD patients.10
Treatment of localized DD usually includes emollients and topical retinoids, and systemic retinoids are recommended as the first-line treatment of diffuse DD.11 Many dermatologic side effects of systemic retinoids have been reported, including frequent dry lips and cheilitis.12 Other skin manifestations include scaling of the palms and soles, excoriations and erosions, alopecia, pruritus, nail fragility, and skin atrophy or fragility. Additionally, common nondermatologic side effects include hepatotoxicity, epistaxis, ophthalmologic effects (eg, loss of eyebrows or eyelashes, redness/swelling of the eyelid, redness of the eyes, sensitivity of the eyes to light, decreased night vision) pancreatitis, bipedal edema, and skeletal alterations.13-15 We report the case of a patient with DD who developed hemorrhagic macules and vesicles in response to the administration of oral retinoids.
Case Report
An 84-year-old woman was admitted to our hospital for treatment of hyperkeratotic papules and plaques on the face, back, groin, submammary folds, and dorsum of the hands and feet that had been present since childhood and typically worsened during the summer. She previously had refused treatment and reported a poor social life due to the cutaneous manifestations of her disease.
Biopsy of a histologic specimen taken from a palmar pit revealed hyperkeratosis, loss of cohesion between suprabasal epidermal cells (acantholysis), and dyskeratotic cells (corps ronds and grains)(Figure 1). On the basis of both clinical and histologic findings, a diagnosis of DD was made. Genetic analysis did not reveal any mutations in the ATP2A2 gene, and a reduced epidermal expression of SERCA2b was demonstrated by immunochemistry.16 Treatment with the oral retinoid acitretin (25 mg daily) for 4 months led to almost complete resolution of the lesions. After 4 months, treatment was reduced to a maintenance dose of 12.5 mg once daily.
The patient returned monthly for follow-up to undergo clinical examination and routine laboratory tests (ie, complete blood cell counts, electrolyte panel, renal and hepatic tests, serum lipid levels, cholesterol level). After beginning the maintenance dose, she returned for follow-up every 2 months.
On follow-up 3 months after beginning the maintenance dose, the patient reported the onset of red and black punctiform macules and vesicles with jagged borders located on the palmoplantar surfaces and dorsal aspect of the fingers on both hands. Lesions were either isolated or confluent. The patient did not report pain or itching, though the skin was severely xerotic, especially on the hands and feet (Figures 2A and 2B). The patient reported no local trauma associated with the lesions, and blood counts and coagulation tests were within reference range. It also was noted that the cutaneous manifestations of DD showed a striking improvement. Histologic analysis of a skin biopsy taken from a hemorrhagic lesion on the palmar surface showed hyperkeratosis, acanthosis, papillomatosis, hypergranulosis, focal dyskeratosis, and hemorrhagic vesicles in the horny layer of the epidermis (Figure 3), leading to a diagnosis of DD.
After 12 months of therapy, the patient spontaneously stopped taking acitretin, and the hemorrhagic lesions spontaneously regressed within 2 weeks (Figures 2C and 2D). Unfortunately, the cutaneous manifestations of DD (eg, papular lesions) progressively reappeared in the previously reported sites. Based on this relapse, the patient was advised to restart treatment with acitretin (25 mg daily). In the follow-up visit 1 month after restarting acitretin, physical examination revealed the reappearance of hemorrhagic lesions on the palmoplantar surfaces and the dorsal aspect of the fingers.
|
|
Comment
We report the case of an 84-year-old woman who developed hemorrhagic lesions on the palmoplantar surfaces and dorsal aspect of the feet as a side effect of oral retinoids for treatment of DD. The first reported case of acral hemorrhagic lesions associated with DD was described 50 years ago in 4 patients with manifestations located mainly on the palms, soles, and dorsal fingers. Local trauma was identified as a triggering factor in the development of the vesicles; however, data regarding the temporal relationship between keratotic papules and hemorrhagic elements and treatment were not specified.5 Twenty-five years later, Coulson and Misch6 described a case of DD in which the hemorrhagic lesions were the first sign of the disease, but correlation with therapy was not reported. A case of retinoid-induced hemorrhagic DD was reported in a female who was treated with etretinate for approximately 10 years after the diagnosis of DD with good clinical response. After 10 years of therapy, she developed hemorrhagic bullae solely on the dorsal aspect of the hands without any direct association with local trauma, along with a small number of nonhemorrhagic bullae.17
In our patient, the onset of hemorrhagic vesicles and red maculae occurred primarily on the palmoplantar surfaces. The lesions were smaller than bullae and contained hemorrhagic elements. Of note, the skin lesions appeared as early as 7 months after the patient started acitretin therapy.
The development of hemorrhagic lesions on different body sites as a consequence of oral retinoid administration also has been reported in patients with psoriasis.15 Emerging evidence indicates that retinoic acid activates vascular endothelial growth factor gene transcription,18 and vascular endothelial growth factor can modify permeability of the endothelial cells. Based on these observations, we propose that DD is characterized by loss of adhesion between epidermal cells (acantholysis) and abnormal keratinization. This defect favors the formation of empty intraepidermal lacunae. An increase in endothelial permeability due to oral retinoid administration promotes the gathering of serum and red blood cells into the lacunae, leading to the onset of hemorrhagic blisters. Moreover, continued microtrauma to the palmoplantar surfaces may account for the peculiar localization of the hemorrhagic lesions described in our patient.
Our unique report of hemorrhagic lesions (vesicles and maculae) presenting in a DD patient treated with acitretin is rare. The first histologic specimen taken from a palmar pit revealed the characteristic histopathologic findings of DD and did not support the diagnosis of the hemorrhagic variant of DD. In the second biopsy, the absence of acantholysis and the poor content of dyskeratotic cells were evident, together with superficial hemorrhagic vesicles. Additionally, the appearance and the disappearance of the cutaneous manifestations were closely correlated with the beginning and suspension of the acitretin therapy, together with an improvement of typical lesions of DD during acitretin treatment. These occurrences indicated a strict causal relationship between treatment with the retinoid and the appearance of hemorrhagic lesions.
Conclusion
We report a rare case of palmoplantar hemorrhagic lesions induced by acitretin for treatment of DD. In our patient, the lesions could have been triggered by a combination of noxious effects of the drug and alterations in keratinocyte physiology due to DD. There is a need for clinicians to be aware of the possible side effects of acitretin and to inform their patients, particularly those presenting with DD, about the possibility of developing hemorrhagic lesions. The latter should be added to the list of potential dermatologic adverse effects of acitretin.
Darier-White disease (DD), also known as keratosis follicularis, is a rare skin disease that is inherited in an autosomal-dominant fashion. It was first described by both Darier1 and White2 more than 120 years ago. The incidence is 1 to 9 per 100,000 individuals, and onset usually occurs between 8 and 15 years of age. Classic DD is characterized by malodorous, brown, keratotic, warty papules arising mainly in seborrheic areas; palmoplantar pits; and nail dystrophy with typical worsening in summer. Unilateral and segmental variants of DD rarely have been described.3,4
Few cases of hemorrhagic DD involving acral surfaces have been reported.5-8 Most patients develop hemorrhagic bullae at the onset of the disease together with the other characteristic features of DD. The disease also can be associated with salivary gland obstruction, renal and testicular agenesis, bone cysts, and neuropsychiatric disorders.9 Diagnosis is confirmed by hyperkeratosis, papillomatosis, acantholysis, dyskeratosis (corps ronds and grains), and small fissures above the basal layer in the epidermis (suprabasal clefts) noted on biopsy of papular lesions. Darier-White disease is caused by a malfunctioning sarcoplasmic/endoplasmic reticulum calcium pump, SERCA2, encoded by the ATP2A2 (ATPase, Ca++ transporting, cardiac muscle, slow twitch 2) gene, which transports calcium ions from the cytosol into the sarcoplasmic/endoplasmic reticulum, catalyzing the hydrolysis of adenosine triphosphate coupled with the transport of the calcium. Mutations in ATP2A2 have only been identified in up to 50% of reported DD patients.10
Treatment of localized DD usually includes emollients and topical retinoids, and systemic retinoids are recommended as the first-line treatment of diffuse DD.11 Many dermatologic side effects of systemic retinoids have been reported, including frequent dry lips and cheilitis.12 Other skin manifestations include scaling of the palms and soles, excoriations and erosions, alopecia, pruritus, nail fragility, and skin atrophy or fragility. Additionally, common nondermatologic side effects include hepatotoxicity, epistaxis, ophthalmologic effects (eg, loss of eyebrows or eyelashes, redness/swelling of the eyelid, redness of the eyes, sensitivity of the eyes to light, decreased night vision) pancreatitis, bipedal edema, and skeletal alterations.13-15 We report the case of a patient with DD who developed hemorrhagic macules and vesicles in response to the administration of oral retinoids.
Case Report
An 84-year-old woman was admitted to our hospital for treatment of hyperkeratotic papules and plaques on the face, back, groin, submammary folds, and dorsum of the hands and feet that had been present since childhood and typically worsened during the summer. She previously had refused treatment and reported a poor social life due to the cutaneous manifestations of her disease.
Biopsy of a histologic specimen taken from a palmar pit revealed hyperkeratosis, loss of cohesion between suprabasal epidermal cells (acantholysis), and dyskeratotic cells (corps ronds and grains)(Figure 1). On the basis of both clinical and histologic findings, a diagnosis of DD was made. Genetic analysis did not reveal any mutations in the ATP2A2 gene, and a reduced epidermal expression of SERCA2b was demonstrated by immunochemistry.16 Treatment with the oral retinoid acitretin (25 mg daily) for 4 months led to almost complete resolution of the lesions. After 4 months, treatment was reduced to a maintenance dose of 12.5 mg once daily.
The patient returned monthly for follow-up to undergo clinical examination and routine laboratory tests (ie, complete blood cell counts, electrolyte panel, renal and hepatic tests, serum lipid levels, cholesterol level). After beginning the maintenance dose, she returned for follow-up every 2 months.
On follow-up 3 months after beginning the maintenance dose, the patient reported the onset of red and black punctiform macules and vesicles with jagged borders located on the palmoplantar surfaces and dorsal aspect of the fingers on both hands. Lesions were either isolated or confluent. The patient did not report pain or itching, though the skin was severely xerotic, especially on the hands and feet (Figures 2A and 2B). The patient reported no local trauma associated with the lesions, and blood counts and coagulation tests were within reference range. It also was noted that the cutaneous manifestations of DD showed a striking improvement. Histologic analysis of a skin biopsy taken from a hemorrhagic lesion on the palmar surface showed hyperkeratosis, acanthosis, papillomatosis, hypergranulosis, focal dyskeratosis, and hemorrhagic vesicles in the horny layer of the epidermis (Figure 3), leading to a diagnosis of DD.
After 12 months of therapy, the patient spontaneously stopped taking acitretin, and the hemorrhagic lesions spontaneously regressed within 2 weeks (Figures 2C and 2D). Unfortunately, the cutaneous manifestations of DD (eg, papular lesions) progressively reappeared in the previously reported sites. Based on this relapse, the patient was advised to restart treatment with acitretin (25 mg daily). In the follow-up visit 1 month after restarting acitretin, physical examination revealed the reappearance of hemorrhagic lesions on the palmoplantar surfaces and the dorsal aspect of the fingers.
|
|
Comment
We report the case of an 84-year-old woman who developed hemorrhagic lesions on the palmoplantar surfaces and dorsal aspect of the feet as a side effect of oral retinoids for treatment of DD. The first reported case of acral hemorrhagic lesions associated with DD was described 50 years ago in 4 patients with manifestations located mainly on the palms, soles, and dorsal fingers. Local trauma was identified as a triggering factor in the development of the vesicles; however, data regarding the temporal relationship between keratotic papules and hemorrhagic elements and treatment were not specified.5 Twenty-five years later, Coulson and Misch6 described a case of DD in which the hemorrhagic lesions were the first sign of the disease, but correlation with therapy was not reported. A case of retinoid-induced hemorrhagic DD was reported in a female who was treated with etretinate for approximately 10 years after the diagnosis of DD with good clinical response. After 10 years of therapy, she developed hemorrhagic bullae solely on the dorsal aspect of the hands without any direct association with local trauma, along with a small number of nonhemorrhagic bullae.17
In our patient, the onset of hemorrhagic vesicles and red maculae occurred primarily on the palmoplantar surfaces. The lesions were smaller than bullae and contained hemorrhagic elements. Of note, the skin lesions appeared as early as 7 months after the patient started acitretin therapy.
The development of hemorrhagic lesions on different body sites as a consequence of oral retinoid administration also has been reported in patients with psoriasis.15 Emerging evidence indicates that retinoic acid activates vascular endothelial growth factor gene transcription,18 and vascular endothelial growth factor can modify permeability of the endothelial cells. Based on these observations, we propose that DD is characterized by loss of adhesion between epidermal cells (acantholysis) and abnormal keratinization. This defect favors the formation of empty intraepidermal lacunae. An increase in endothelial permeability due to oral retinoid administration promotes the gathering of serum and red blood cells into the lacunae, leading to the onset of hemorrhagic blisters. Moreover, continued microtrauma to the palmoplantar surfaces may account for the peculiar localization of the hemorrhagic lesions described in our patient.
Our unique report of hemorrhagic lesions (vesicles and maculae) presenting in a DD patient treated with acitretin is rare. The first histologic specimen taken from a palmar pit revealed the characteristic histopathologic findings of DD and did not support the diagnosis of the hemorrhagic variant of DD. In the second biopsy, the absence of acantholysis and the poor content of dyskeratotic cells were evident, together with superficial hemorrhagic vesicles. Additionally, the appearance and the disappearance of the cutaneous manifestations were closely correlated with the beginning and suspension of the acitretin therapy, together with an improvement of typical lesions of DD during acitretin treatment. These occurrences indicated a strict causal relationship between treatment with the retinoid and the appearance of hemorrhagic lesions.
Conclusion
We report a rare case of palmoplantar hemorrhagic lesions induced by acitretin for treatment of DD. In our patient, the lesions could have been triggered by a combination of noxious effects of the drug and alterations in keratinocyte physiology due to DD. There is a need for clinicians to be aware of the possible side effects of acitretin and to inform their patients, particularly those presenting with DD, about the possibility of developing hemorrhagic lesions. The latter should be added to the list of potential dermatologic adverse effects of acitretin.
1. Darier J. De la porospermose folliculaire vegetante. Ann Dermatol Syphiligr. 1889;10:597-605.
2. White JC. A case of keratosis (ichtyosis) follicularis. J Cutan Genitourin Dis. 1889;7:201-209.
3. Goldsmith LA, Baden HP. Darier-White disease (keratosis follicularis) and acrokeratosis verruciformis. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 4th ed. New York, NY: McGraw-Hill; 2003:523-528.
4. Sanderson EA, Killoran CE, Pedvis-Leftick A, et al. Localized Darier’s disease in Blaschkoid distribution: two cases of phenotypic mosaicism and a review of mosaic Darier’s disease. J Dermatol. 2007;34:761-764.
5. Jones WN, Nix TE Jr, Clark WH Jr. Hemorrhagic Darier’s disease. Arch Dermatol. 1964;89:523-527.
6. Coulson IH, Misch KJ. Haemorrhagic Darier’s disease. J R Soc Med. 1989;82:365-366.
7. Foresman PL, Goldsmith LA, Ginn L, et al. Hemorrhagic Darier’s disease. Arch Dermatol. 1993;129:511-512.
8. Regazzini R, Zambruno G, DeFilippi C, et al. Isolated acral Darier’s disease with haemorrhagic lesions in a kindred. Br J Dermatol. 1996;135:495-496.
9. Zeglaoui F, Zaraa I, Fazaa B, et al. Dyskeratosis follicularis disease: case reports and review of the literature. J Eur Acad Dermatol Venereol. 2005;19:114-117.
10. Sakuntabhai A, Burge S, Monk S, et al. Spectrum of novel ATP2A2 mutations with Darier’s disease. Hum Mol Genet. 1999;8:1611-1619.
11. Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50.
12. Nikam B, Amladi S, Bingewar G, et al. Acral papular eruption. Indian J Dermatol Venereol Leprol. 2005;71:447-448.
13. Wolverton SE, Remlinger K. Suggested guidelines for patient monitoring: hepatic and hematologic toxicity attributable to systemic dermatologic drugs. Dermatol Clin. 2007;25:195-205.
14. Tey HL, Theng TS. Acitretin induced bipedal edema. J Dermatol. 2006;33:372-374.
15. Aydogan K, Karadogan SK, Tunali S. Acitretin-induced subungual hemorrhage. Int J Dermatol. 2007;46:494-495.
16. Borgogna C, Zavattaro E, Dell’Oste V, et al. No indications for HPV involvement in the hypertrophic skin lesions of a Darier disease case without ATP2A2 gene mutations. J Cutan Pathol. 2009;36:1005-1009.
17. Gebauer K, Holgate C, Navaratnam A. Retinoid-induced haemorrhagic bullae in Darier’s disease. Australas J Dermatol. 1990;31:99-103.
18. Maeno T, Tanaka T, Sando Y, et al. Stimulation of vascular endothelial growth factor gene transcription by all trans retinoic acid through Sp1 and Sp3 sites in human bronchioalveolar carcinoma cells. Am J Respir Cell Mol Biol. 2002;26:246-253.
1. Darier J. De la porospermose folliculaire vegetante. Ann Dermatol Syphiligr. 1889;10:597-605.
2. White JC. A case of keratosis (ichtyosis) follicularis. J Cutan Genitourin Dis. 1889;7:201-209.
3. Goldsmith LA, Baden HP. Darier-White disease (keratosis follicularis) and acrokeratosis verruciformis. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 4th ed. New York, NY: McGraw-Hill; 2003:523-528.
4. Sanderson EA, Killoran CE, Pedvis-Leftick A, et al. Localized Darier’s disease in Blaschkoid distribution: two cases of phenotypic mosaicism and a review of mosaic Darier’s disease. J Dermatol. 2007;34:761-764.
5. Jones WN, Nix TE Jr, Clark WH Jr. Hemorrhagic Darier’s disease. Arch Dermatol. 1964;89:523-527.
6. Coulson IH, Misch KJ. Haemorrhagic Darier’s disease. J R Soc Med. 1989;82:365-366.
7. Foresman PL, Goldsmith LA, Ginn L, et al. Hemorrhagic Darier’s disease. Arch Dermatol. 1993;129:511-512.
8. Regazzini R, Zambruno G, DeFilippi C, et al. Isolated acral Darier’s disease with haemorrhagic lesions in a kindred. Br J Dermatol. 1996;135:495-496.
9. Zeglaoui F, Zaraa I, Fazaa B, et al. Dyskeratosis follicularis disease: case reports and review of the literature. J Eur Acad Dermatol Venereol. 2005;19:114-117.
10. Sakuntabhai A, Burge S, Monk S, et al. Spectrum of novel ATP2A2 mutations with Darier’s disease. Hum Mol Genet. 1999;8:1611-1619.
11. Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50.
12. Nikam B, Amladi S, Bingewar G, et al. Acral papular eruption. Indian J Dermatol Venereol Leprol. 2005;71:447-448.
13. Wolverton SE, Remlinger K. Suggested guidelines for patient monitoring: hepatic and hematologic toxicity attributable to systemic dermatologic drugs. Dermatol Clin. 2007;25:195-205.
14. Tey HL, Theng TS. Acitretin induced bipedal edema. J Dermatol. 2006;33:372-374.
15. Aydogan K, Karadogan SK, Tunali S. Acitretin-induced subungual hemorrhage. Int J Dermatol. 2007;46:494-495.
16. Borgogna C, Zavattaro E, Dell’Oste V, et al. No indications for HPV involvement in the hypertrophic skin lesions of a Darier disease case without ATP2A2 gene mutations. J Cutan Pathol. 2009;36:1005-1009.
17. Gebauer K, Holgate C, Navaratnam A. Retinoid-induced haemorrhagic bullae in Darier’s disease. Australas J Dermatol. 1990;31:99-103.
18. Maeno T, Tanaka T, Sando Y, et al. Stimulation of vascular endothelial growth factor gene transcription by all trans retinoic acid through Sp1 and Sp3 sites in human bronchioalveolar carcinoma cells. Am J Respir Cell Mol Biol. 2002;26:246-253.
Practice Points
- The first-line treatment of Darier-White disease (DD) is oral retinoids.
- Numerous side effects of retinoids have been described. Clinicians should take these cutaneous manifestations into consideration in patients affected by DD.
Hemorrhagic Bullous Lesions Due to Bacillus cereus in a Cirrhotic Patient
To the Editor:
A 42-year-old man with hypertension, hypothyroidism, and alcohol-related cirrhosis was admitted for evaluation of rapidly deteriorating mental status. He was referred from a rehabilitation facility where he had been admitted 4 days earlier after a hospitalization for hepatorenal syndrome and pneumonia. He was alert and ambulating until the day of the current admission. On arrival he was hypotensive(54/42 mm Hg); hypothermic (35°C, rectally); and unresponsive, except to painful stimuli. Jaundice, hepatosplenomegaly, ascites, and bilateral lower extremity edema were noted. There were multiple tense and flaccid bullous lesions containing serosanguineous fluid over both tibias and calves, without crepitus (Figure 1).
 |
 |
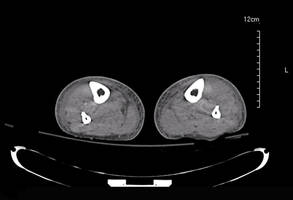 |
Laboratory test results revealed leukocytosis (total leukocytes, 10,900/mm3 [reference range, 4500–10,800/mm3), hypoglycemia (glucose, <20 mg/dL [reference range, 74–106 mg/dL]), renal insufficiency (serum creatinine, 2.5 mg/dL [reference range, 0.66–1.25 mg/dL]), metabolic acidosis (pH, 7.1 [reference range, 7.35–7.45]; bicarbonate, 13 mmol/L [reference range, 22–30 mmol/L]; lactic acid, 11.9 mmol/L [reference range, 0.7–2.1 mmol/L]), liver dysfunction (aspartate aminotransferase, 576 IU/L [reference range, 15–46 IU/L]), and coagulopathy with evidence of diffuse intravascular coagulation (total platelets, 75,000/mm3 [reference range, 150,000–450,000/mm3]; international normalized ratio, 9.5 [reference range, 0.8–1.2]; partial thromboplastin time, 108 seconds [reference range 23.0–35.0 seconds]; fibrinogen, 145 mg/dL [reference range, 228–501 mg/dL]; D-dimer, >20 µg/mL [reference range, 0.01–0.58 μg/mL]). Computed tomography of the pelvis and legs showed ascites, extensive subcutaneous edema, and cutaneous blisterlike lesions superior to the level of the ankles bilaterally. No gas, foreign bodies, collections, asymmetric facial thickening, or evidence of infection across tissue planes was present (Figures 2 and 3).
Specimens of blood and aspirates from the bullae at multiple lower leg sites were sent for microbiologic evaluation. The blood specimens were inoculated at bedside into aerobic and anaerobic blood culture bottles and incubated in an automated blood culture system. The aspirate samples were inoculated to trypticase soy agar with 5% sheep blood, Columbia-nalidixic acid agar, chocolate agar, MacConkey agar, and thioglycollate broth, which were incubated at 37ºC in air supplemented with 5% CO2, and to CDC anaerobic blood agar, which was incubated under anaerobic conditions. Gram-stained smears of the aspirates from the bullae demonstrated few granulocytes and numerous large gram-positive bacilli (Figure 4). By the next day, growth of large gram-positive bacilli was detected in both aerobic and anaerobic blood culture bottles and in pure culture from all the bullae samples. The bacterial colonies on sheep blood agar were opaque and white-gray in color, with a rough surface, undulate margins, and surrounding β hemolysis. The isolate was a motile, catalase-positive, arginine-positive, salicin-positive, lecithinase-positive, and penicillin-resistant organism that was identified as Bacillus cereus.
Antimicrobial susceptibility testing for B cereus has not been standardized, but evaluation by broth microdilution suggested decreased susceptibility to penicillin (minimum inhibitory concentration [MIC], 2 µg/mL) and clindamycin (MIC, 2 µg/mL), but retained susceptibility to ciprofloxacin (MIC, ≤0.25 µg/mL), tetracycline (MIC, ≤1 µg/mL), rifampin (MIC, ≤1 µg/mL), and vancomycin (MIC, ≤2 µg/mL).
The patient was admitted to the intensive care unit and was treated initially with fluid resuscitation; transfusions; ventilatory support; and intravenous vancomycin, clindamycin, and imipenem. This regimen was changed to vancomycin and ciprofloxacin when culture and susceptibility results became available to complete a 14-day course. Signs of sepsis resolved and the mental status and skin lesions improved. Ultimately, the patient died due to complications of hepatic failure.
Bacillus cereus is a rod-shaped, gram-positive, facultative, aerobic organism that is widely distributed in the environment.1 Spore formation makes B cereus resistant to most physical and chemical disinfection methods; as a consequence, it is a frequent contaminant in materials (eg, plants, dust, soil, sediment), foodstuffs, and clinical specimens.1
Traditionally considered in the context of foodborne illness, B cereus is recognized increasingly as a cause of systemic and local infections in both immunosuppressed and immunocompetent patients. Nongastrointestinal infections reported include fulminant bacteremia, pneumonia, meningitis, brain abscesses, endophthalmitis, necrotizing fasciitis, and central line catheter–related and cutaneous infections.1,2
Cutaneous lesions may have a variety of forms and appearance at initial presentation, including small papules or vesicles that progress into a rapidly spreading cellulitis1,2 with a characteristic serosanguineous draining fluid,2 single necrotic bullae,3 and gas-gangrenelike infections with extensive soft tissue involvement resembling clostridial myonecrosis.1,4 Single or multiple papulovesicular lesions can even mimic cutaneous anthrax.1-4 Necrotic or hemorrhagic bullous lesions,3 such as those observed in our patient, are rare.
Exposed areas such as extremities and digits are most often affected, presumably due to entrance of spores from soil, water, decaying organic material, or fomites through skin microabrasions or trauma-induced wounds.1 Once in the tissue, the crystalline surface protein layer (S-layer) of the bacilli promotes adhesion to human epithelial cells and neutrophils,5 followed by release of virulence factors including proteases, collagenases, lecithinaselike enzymes, necrotizing exotoxinlike hemolysins, phospholipases, and most importantly a dermonecrotic vascular permeability factor.1,5 Toxins produced by B cereus are similar to those closely related to Bacillus anthracis, the agent of anthrax.1,2
When large gram-positive bacilli are observed in tissue or wound specimens, initial therapy should address both aerobic (Bacillus species) and anaerobic (Clostridium species) organisms.1,4,6 Once B cereus is recovered, treatment should rely on susceptibility testing of the isolate. Bacillus cereus produces ß-lactamase, thus penicillin and cephalosporin should be avoided.1 Vancomycin, clindamycin, aminoglycosides, and fluoroquinolones are the drugs of choice.1,3,4,6 Daptomycin and linezolid also are active in vitro,1 but clinical experience with these agents is limited. Necrotic infection or deep tissue involvement requires surgical intervention.
Numerous other organisms can cause cellulitis and soft tissue infections with hemorrhagic bullae.1,3,6 Streptococci, particularly Streptococcus pyogenes, and occasionally staphylococci are the primary consideration in normal hosts without trauma.3,6 In immunocompromised patients, including those with cirrhosis, diabetes mellitus, and malignancy, Clostridium perfringens and gram-negative organisms such as Escherichia coli, other enteric bacteria including Pseudomonas aeruginosa, Aeromonas, and halophilic Vibrio species are more frequent.3,6
We describe a patient with underlying cirrhosis who developed bilateral lower extremity hemorrhagic bullous lesions and sepsis due to infection with B cereus, an emerging cause of serious infections in patients with underlying immunocompromising conditions such as cirrhosis, diabetes mellitus, and malignancy. Hemorrhagic bullae in immunocompromised patients are associated with sepsis and rapidly progressive illness, and rapid treatment is essential. Bacillus cereus should be included as a consideration in the differential diagnosis and management of patients presenting with bullous cellulitis and sepsis.
1. Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382-398.
2. Henrickson KJ. A second species of bacillus causing primary cutaneous disease. Int J Dermatol. 1990;29:19-20.
3. Liu BM, Hsiao CT, Chung KJ, et al. Hemorrhagic bullae represent an ominous sign for cirrhotic patients [published online ahead of print November 5, 2007]. J Emer Med. 2008;34:277-281.
4. Meredith FT, Fowler VG, Gautier M, et al. Bacillus cereus necrotizing cellulitis mimicking clostridial myonecrosis: case report and review of the literature. Scand J Infect Dis. 1997;29:528-529.
5. Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000;2:189-198.
6. Lee CC, Chi CH, Lee NY, et al. Necrotizing fasciitis in patients with liver cirrhosis: predominance of monomicrobial gram-negative bacillary infections [published online ahead of print July 23, 2008]. Diagn Microbiol Infect Dis. 2008;62:219-225.
To the Editor:
A 42-year-old man with hypertension, hypothyroidism, and alcohol-related cirrhosis was admitted for evaluation of rapidly deteriorating mental status. He was referred from a rehabilitation facility where he had been admitted 4 days earlier after a hospitalization for hepatorenal syndrome and pneumonia. He was alert and ambulating until the day of the current admission. On arrival he was hypotensive(54/42 mm Hg); hypothermic (35°C, rectally); and unresponsive, except to painful stimuli. Jaundice, hepatosplenomegaly, ascites, and bilateral lower extremity edema were noted. There were multiple tense and flaccid bullous lesions containing serosanguineous fluid over both tibias and calves, without crepitus (Figure 1).
 |
 |
 |
Laboratory test results revealed leukocytosis (total leukocytes, 10,900/mm3 [reference range, 4500–10,800/mm3), hypoglycemia (glucose, <20 mg/dL [reference range, 74–106 mg/dL]), renal insufficiency (serum creatinine, 2.5 mg/dL [reference range, 0.66–1.25 mg/dL]), metabolic acidosis (pH, 7.1 [reference range, 7.35–7.45]; bicarbonate, 13 mmol/L [reference range, 22–30 mmol/L]; lactic acid, 11.9 mmol/L [reference range, 0.7–2.1 mmol/L]), liver dysfunction (aspartate aminotransferase, 576 IU/L [reference range, 15–46 IU/L]), and coagulopathy with evidence of diffuse intravascular coagulation (total platelets, 75,000/mm3 [reference range, 150,000–450,000/mm3]; international normalized ratio, 9.5 [reference range, 0.8–1.2]; partial thromboplastin time, 108 seconds [reference range 23.0–35.0 seconds]; fibrinogen, 145 mg/dL [reference range, 228–501 mg/dL]; D-dimer, >20 µg/mL [reference range, 0.01–0.58 μg/mL]). Computed tomography of the pelvis and legs showed ascites, extensive subcutaneous edema, and cutaneous blisterlike lesions superior to the level of the ankles bilaterally. No gas, foreign bodies, collections, asymmetric facial thickening, or evidence of infection across tissue planes was present (Figures 2 and 3).
Specimens of blood and aspirates from the bullae at multiple lower leg sites were sent for microbiologic evaluation. The blood specimens were inoculated at bedside into aerobic and anaerobic blood culture bottles and incubated in an automated blood culture system. The aspirate samples were inoculated to trypticase soy agar with 5% sheep blood, Columbia-nalidixic acid agar, chocolate agar, MacConkey agar, and thioglycollate broth, which were incubated at 37ºC in air supplemented with 5% CO2, and to CDC anaerobic blood agar, which was incubated under anaerobic conditions. Gram-stained smears of the aspirates from the bullae demonstrated few granulocytes and numerous large gram-positive bacilli (Figure 4). By the next day, growth of large gram-positive bacilli was detected in both aerobic and anaerobic blood culture bottles and in pure culture from all the bullae samples. The bacterial colonies on sheep blood agar were opaque and white-gray in color, with a rough surface, undulate margins, and surrounding β hemolysis. The isolate was a motile, catalase-positive, arginine-positive, salicin-positive, lecithinase-positive, and penicillin-resistant organism that was identified as Bacillus cereus.
Antimicrobial susceptibility testing for B cereus has not been standardized, but evaluation by broth microdilution suggested decreased susceptibility to penicillin (minimum inhibitory concentration [MIC], 2 µg/mL) and clindamycin (MIC, 2 µg/mL), but retained susceptibility to ciprofloxacin (MIC, ≤0.25 µg/mL), tetracycline (MIC, ≤1 µg/mL), rifampin (MIC, ≤1 µg/mL), and vancomycin (MIC, ≤2 µg/mL).
The patient was admitted to the intensive care unit and was treated initially with fluid resuscitation; transfusions; ventilatory support; and intravenous vancomycin, clindamycin, and imipenem. This regimen was changed to vancomycin and ciprofloxacin when culture and susceptibility results became available to complete a 14-day course. Signs of sepsis resolved and the mental status and skin lesions improved. Ultimately, the patient died due to complications of hepatic failure.
Bacillus cereus is a rod-shaped, gram-positive, facultative, aerobic organism that is widely distributed in the environment.1 Spore formation makes B cereus resistant to most physical and chemical disinfection methods; as a consequence, it is a frequent contaminant in materials (eg, plants, dust, soil, sediment), foodstuffs, and clinical specimens.1
Traditionally considered in the context of foodborne illness, B cereus is recognized increasingly as a cause of systemic and local infections in both immunosuppressed and immunocompetent patients. Nongastrointestinal infections reported include fulminant bacteremia, pneumonia, meningitis, brain abscesses, endophthalmitis, necrotizing fasciitis, and central line catheter–related and cutaneous infections.1,2
Cutaneous lesions may have a variety of forms and appearance at initial presentation, including small papules or vesicles that progress into a rapidly spreading cellulitis1,2 with a characteristic serosanguineous draining fluid,2 single necrotic bullae,3 and gas-gangrenelike infections with extensive soft tissue involvement resembling clostridial myonecrosis.1,4 Single or multiple papulovesicular lesions can even mimic cutaneous anthrax.1-4 Necrotic or hemorrhagic bullous lesions,3 such as those observed in our patient, are rare.
Exposed areas such as extremities and digits are most often affected, presumably due to entrance of spores from soil, water, decaying organic material, or fomites through skin microabrasions or trauma-induced wounds.1 Once in the tissue, the crystalline surface protein layer (S-layer) of the bacilli promotes adhesion to human epithelial cells and neutrophils,5 followed by release of virulence factors including proteases, collagenases, lecithinaselike enzymes, necrotizing exotoxinlike hemolysins, phospholipases, and most importantly a dermonecrotic vascular permeability factor.1,5 Toxins produced by B cereus are similar to those closely related to Bacillus anthracis, the agent of anthrax.1,2
When large gram-positive bacilli are observed in tissue or wound specimens, initial therapy should address both aerobic (Bacillus species) and anaerobic (Clostridium species) organisms.1,4,6 Once B cereus is recovered, treatment should rely on susceptibility testing of the isolate. Bacillus cereus produces ß-lactamase, thus penicillin and cephalosporin should be avoided.1 Vancomycin, clindamycin, aminoglycosides, and fluoroquinolones are the drugs of choice.1,3,4,6 Daptomycin and linezolid also are active in vitro,1 but clinical experience with these agents is limited. Necrotic infection or deep tissue involvement requires surgical intervention.
Numerous other organisms can cause cellulitis and soft tissue infections with hemorrhagic bullae.1,3,6 Streptococci, particularly Streptococcus pyogenes, and occasionally staphylococci are the primary consideration in normal hosts without trauma.3,6 In immunocompromised patients, including those with cirrhosis, diabetes mellitus, and malignancy, Clostridium perfringens and gram-negative organisms such as Escherichia coli, other enteric bacteria including Pseudomonas aeruginosa, Aeromonas, and halophilic Vibrio species are more frequent.3,6
We describe a patient with underlying cirrhosis who developed bilateral lower extremity hemorrhagic bullous lesions and sepsis due to infection with B cereus, an emerging cause of serious infections in patients with underlying immunocompromising conditions such as cirrhosis, diabetes mellitus, and malignancy. Hemorrhagic bullae in immunocompromised patients are associated with sepsis and rapidly progressive illness, and rapid treatment is essential. Bacillus cereus should be included as a consideration in the differential diagnosis and management of patients presenting with bullous cellulitis and sepsis.
To the Editor:
A 42-year-old man with hypertension, hypothyroidism, and alcohol-related cirrhosis was admitted for evaluation of rapidly deteriorating mental status. He was referred from a rehabilitation facility where he had been admitted 4 days earlier after a hospitalization for hepatorenal syndrome and pneumonia. He was alert and ambulating until the day of the current admission. On arrival he was hypotensive(54/42 mm Hg); hypothermic (35°C, rectally); and unresponsive, except to painful stimuli. Jaundice, hepatosplenomegaly, ascites, and bilateral lower extremity edema were noted. There were multiple tense and flaccid bullous lesions containing serosanguineous fluid over both tibias and calves, without crepitus (Figure 1).
 |
 |
 |
Laboratory test results revealed leukocytosis (total leukocytes, 10,900/mm3 [reference range, 4500–10,800/mm3), hypoglycemia (glucose, <20 mg/dL [reference range, 74–106 mg/dL]), renal insufficiency (serum creatinine, 2.5 mg/dL [reference range, 0.66–1.25 mg/dL]), metabolic acidosis (pH, 7.1 [reference range, 7.35–7.45]; bicarbonate, 13 mmol/L [reference range, 22–30 mmol/L]; lactic acid, 11.9 mmol/L [reference range, 0.7–2.1 mmol/L]), liver dysfunction (aspartate aminotransferase, 576 IU/L [reference range, 15–46 IU/L]), and coagulopathy with evidence of diffuse intravascular coagulation (total platelets, 75,000/mm3 [reference range, 150,000–450,000/mm3]; international normalized ratio, 9.5 [reference range, 0.8–1.2]; partial thromboplastin time, 108 seconds [reference range 23.0–35.0 seconds]; fibrinogen, 145 mg/dL [reference range, 228–501 mg/dL]; D-dimer, >20 µg/mL [reference range, 0.01–0.58 μg/mL]). Computed tomography of the pelvis and legs showed ascites, extensive subcutaneous edema, and cutaneous blisterlike lesions superior to the level of the ankles bilaterally. No gas, foreign bodies, collections, asymmetric facial thickening, or evidence of infection across tissue planes was present (Figures 2 and 3).
Specimens of blood and aspirates from the bullae at multiple lower leg sites were sent for microbiologic evaluation. The blood specimens were inoculated at bedside into aerobic and anaerobic blood culture bottles and incubated in an automated blood culture system. The aspirate samples were inoculated to trypticase soy agar with 5% sheep blood, Columbia-nalidixic acid agar, chocolate agar, MacConkey agar, and thioglycollate broth, which were incubated at 37ºC in air supplemented with 5% CO2, and to CDC anaerobic blood agar, which was incubated under anaerobic conditions. Gram-stained smears of the aspirates from the bullae demonstrated few granulocytes and numerous large gram-positive bacilli (Figure 4). By the next day, growth of large gram-positive bacilli was detected in both aerobic and anaerobic blood culture bottles and in pure culture from all the bullae samples. The bacterial colonies on sheep blood agar were opaque and white-gray in color, with a rough surface, undulate margins, and surrounding β hemolysis. The isolate was a motile, catalase-positive, arginine-positive, salicin-positive, lecithinase-positive, and penicillin-resistant organism that was identified as Bacillus cereus.
Antimicrobial susceptibility testing for B cereus has not been standardized, but evaluation by broth microdilution suggested decreased susceptibility to penicillin (minimum inhibitory concentration [MIC], 2 µg/mL) and clindamycin (MIC, 2 µg/mL), but retained susceptibility to ciprofloxacin (MIC, ≤0.25 µg/mL), tetracycline (MIC, ≤1 µg/mL), rifampin (MIC, ≤1 µg/mL), and vancomycin (MIC, ≤2 µg/mL).
The patient was admitted to the intensive care unit and was treated initially with fluid resuscitation; transfusions; ventilatory support; and intravenous vancomycin, clindamycin, and imipenem. This regimen was changed to vancomycin and ciprofloxacin when culture and susceptibility results became available to complete a 14-day course. Signs of sepsis resolved and the mental status and skin lesions improved. Ultimately, the patient died due to complications of hepatic failure.
Bacillus cereus is a rod-shaped, gram-positive, facultative, aerobic organism that is widely distributed in the environment.1 Spore formation makes B cereus resistant to most physical and chemical disinfection methods; as a consequence, it is a frequent contaminant in materials (eg, plants, dust, soil, sediment), foodstuffs, and clinical specimens.1
Traditionally considered in the context of foodborne illness, B cereus is recognized increasingly as a cause of systemic and local infections in both immunosuppressed and immunocompetent patients. Nongastrointestinal infections reported include fulminant bacteremia, pneumonia, meningitis, brain abscesses, endophthalmitis, necrotizing fasciitis, and central line catheter–related and cutaneous infections.1,2
Cutaneous lesions may have a variety of forms and appearance at initial presentation, including small papules or vesicles that progress into a rapidly spreading cellulitis1,2 with a characteristic serosanguineous draining fluid,2 single necrotic bullae,3 and gas-gangrenelike infections with extensive soft tissue involvement resembling clostridial myonecrosis.1,4 Single or multiple papulovesicular lesions can even mimic cutaneous anthrax.1-4 Necrotic or hemorrhagic bullous lesions,3 such as those observed in our patient, are rare.
Exposed areas such as extremities and digits are most often affected, presumably due to entrance of spores from soil, water, decaying organic material, or fomites through skin microabrasions or trauma-induced wounds.1 Once in the tissue, the crystalline surface protein layer (S-layer) of the bacilli promotes adhesion to human epithelial cells and neutrophils,5 followed by release of virulence factors including proteases, collagenases, lecithinaselike enzymes, necrotizing exotoxinlike hemolysins, phospholipases, and most importantly a dermonecrotic vascular permeability factor.1,5 Toxins produced by B cereus are similar to those closely related to Bacillus anthracis, the agent of anthrax.1,2
When large gram-positive bacilli are observed in tissue or wound specimens, initial therapy should address both aerobic (Bacillus species) and anaerobic (Clostridium species) organisms.1,4,6 Once B cereus is recovered, treatment should rely on susceptibility testing of the isolate. Bacillus cereus produces ß-lactamase, thus penicillin and cephalosporin should be avoided.1 Vancomycin, clindamycin, aminoglycosides, and fluoroquinolones are the drugs of choice.1,3,4,6 Daptomycin and linezolid also are active in vitro,1 but clinical experience with these agents is limited. Necrotic infection or deep tissue involvement requires surgical intervention.
Numerous other organisms can cause cellulitis and soft tissue infections with hemorrhagic bullae.1,3,6 Streptococci, particularly Streptococcus pyogenes, and occasionally staphylococci are the primary consideration in normal hosts without trauma.3,6 In immunocompromised patients, including those with cirrhosis, diabetes mellitus, and malignancy, Clostridium perfringens and gram-negative organisms such as Escherichia coli, other enteric bacteria including Pseudomonas aeruginosa, Aeromonas, and halophilic Vibrio species are more frequent.3,6
We describe a patient with underlying cirrhosis who developed bilateral lower extremity hemorrhagic bullous lesions and sepsis due to infection with B cereus, an emerging cause of serious infections in patients with underlying immunocompromising conditions such as cirrhosis, diabetes mellitus, and malignancy. Hemorrhagic bullae in immunocompromised patients are associated with sepsis and rapidly progressive illness, and rapid treatment is essential. Bacillus cereus should be included as a consideration in the differential diagnosis and management of patients presenting with bullous cellulitis and sepsis.
1. Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382-398.
2. Henrickson KJ. A second species of bacillus causing primary cutaneous disease. Int J Dermatol. 1990;29:19-20.
3. Liu BM, Hsiao CT, Chung KJ, et al. Hemorrhagic bullae represent an ominous sign for cirrhotic patients [published online ahead of print November 5, 2007]. J Emer Med. 2008;34:277-281.
4. Meredith FT, Fowler VG, Gautier M, et al. Bacillus cereus necrotizing cellulitis mimicking clostridial myonecrosis: case report and review of the literature. Scand J Infect Dis. 1997;29:528-529.
5. Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000;2:189-198.
6. Lee CC, Chi CH, Lee NY, et al. Necrotizing fasciitis in patients with liver cirrhosis: predominance of monomicrobial gram-negative bacillary infections [published online ahead of print July 23, 2008]. Diagn Microbiol Infect Dis. 2008;62:219-225.
1. Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382-398.
2. Henrickson KJ. A second species of bacillus causing primary cutaneous disease. Int J Dermatol. 1990;29:19-20.
3. Liu BM, Hsiao CT, Chung KJ, et al. Hemorrhagic bullae represent an ominous sign for cirrhotic patients [published online ahead of print November 5, 2007]. J Emer Med. 2008;34:277-281.
4. Meredith FT, Fowler VG, Gautier M, et al. Bacillus cereus necrotizing cellulitis mimicking clostridial myonecrosis: case report and review of the literature. Scand J Infect Dis. 1997;29:528-529.
5. Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000;2:189-198.
6. Lee CC, Chi CH, Lee NY, et al. Necrotizing fasciitis in patients with liver cirrhosis: predominance of monomicrobial gram-negative bacillary infections [published online ahead of print July 23, 2008]. Diagn Microbiol Infect Dis. 2008;62:219-225.
Late-Onset Nevus Comedonicus on Both Eyelids With Hypothyroidism
To the Editor:
A 62-year-old woman was referred to the dermatology clinic for papules on both eyelids of 6 months’ duration. She underwent surgery for a thyroid gland adenoma 3 years prior and subsequently experienced hypothyroidism. Levothyroxine sodium was administered daily (100 µg initially; 50 µg over the last 1.5 years). Papules occurred on both eyelids 6 months prior to presentation and gradually increased in number. The center of each papule was filled with a black keratinous plug. The skin lesions became raised after the patient ate fatty foods. The lesions remained entirely asymptomatic and there was no family history of a similar disorder.
Physical examinations showed no systemic abnormalities. Dermatologic examination showed clustered 3- to 4-mm flesh-colored papules on both upper eyelids; the centers of the papules were filled with 1- to 2-mm black keratinous plugs (Figure 1A). Several similar skin lesions existed on the lower eyelids, nasal root, and right side of the nasal dorsum. On laboratory examination, the results of routine blood, urine, and stool tests, as well as renal and hepatic functions, electrolytes, and blood sugar levels, were within reference range. Indicators including triglyceride of 2.50 mmol/L (reference range, 0.40–1.90 mmol/L), total cholesterol of 6.31 mmol/L (reference range, 3.00–5.70 mmol/L), serum total thyroxine (T4) of 5.32 µg/dL (reference range, 6.09–12.23 µg/dL), total triiodothyronine (T3) of 64 ng/dL (reference range, 87–178 ng/dL), serum free thyroxine (FT4) of 0.41 ng/dL (reference range, 0.61–1.12 ng/dL), serum free triiodothyronine (FT3) of 182 pg/dL (reference range, 250–390 pg/dL), and thyrotropin of 33.75 µIU/mL (reference range, 0.34–5.60 µIU/mL) were not within reference range; however, thyroperoxidase antibodies, thyrotropin receptor antibodies, thyroglobulin antibodies, thyroglobulin, and calcitonin were within reference range. Color ultrasonography indicated post–subtotal resection of the bilateral thyroid glands.
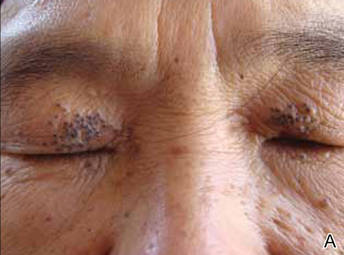  |
Histopathologic analysis of the skin lesions showed that the epidermis became atrophic and thinner, and several atrophic and cyst-dilated follicular structures existed inside the dermis. Some structures opened through the epidermis; the walls were squamous epithelium and keratin filled the structures (Figure 2). The condition was diagnosed as nevus comedonicus (NC).
The patient was referred to the endocrinology department and treated with levothyroxine sodium (100 µg daily). At 5-month follow-up, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions had resolved (Figure 1B).
Nevus comedonicus is an unusual skin lesion with a predilection for the face, neck, shoulders, upper arms, and trunk. The clinical manifestations include comedonelike papules with centers that are characterized by large, black, solid keratinous plugs. When the plugs are peeled off, volcanic craterlike pits will be left. The skin lesions usually are ribbonlike and clustered on 1 side of the body. Pathologic examination often shows that the epidermis is pitted downward, and the dilated follicular ostia are plugged with keratin.1,2 Paige and Mendelson3 divided NC into 2 types: inflammatory and noninflammatory. Approximately half of NC patients experience cysts, abscesses, fistulae, and scars.4
The exact etiology of NC is unclear. Some researchers believe that it is a congenital hair follicle deformity; more specifically, that it is caused by a developmental defect in the hair follicles in the embryonic stage (ie, abnormal differentiation of epithelial stem cells that differentiate into follicles). Most incidences of NC occur at birth or before growth and development. However, few studies have reported late-onset NC.5
|
The relationship between NC and thyroid disease is unique. Clinical research has shown that hypothyroidism can result in hair loss and cracks.6 In animal experiments, hypothyroidism model mice often experienced degenerative changes of their hair follicles and hair papillae as well as changes in the telogen phase, such as thinning of the outer and inner root sheaths.7 Meanwhile, decreased cell proliferation activity in the hair follicles was observed. Therefore, it is reasonable to conclude that thyroid hormones have regulatory effects on the growth and development of hair follicles.7
A study on human hair follicles found that thyroid hormone receptor β1 is expressed in human hair follicles.8 Research on in vitro–cultured human hair follicles showed that thyroid hormones T3 and T4 upregulated the proliferation of hair matrix cells and downregulated their apoptosis. Thyroid hormones also prolonged the duration of the hair growth phase (anagen).9 Furthermore, expression of thyrotropin receptor was detected in human hair follicles. Because increased serum thyrotropin levels can lead to clinical hair loss, thyrotropin may inhibit the growth of hair follicles via thyrotropin receptor.10 In our patient, NC occurred on both eyelids when the patient experienced hypothyroidism following thyroid gland adenoma surgery. Following treatment with levothyroxine sodium, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions resolved. Therefore, the occurrence of NC may be related to hypothyroidism in this patient. The low thyroid hormone levels and elevated thyrotropin level possibly induced degenerative changes and injuries to the hair matrix cells, resulting in hair follicle obstruction and accumulation of keratin, which ultimately led to NC. However, the exact relationship between NC and thyroid diseases requires elucidation in future studies.
1. Engber PB. The nevus comedonicus syndrome: a case report with emphasis on associated internal manifestations. Int J Dermatol. 1978;17:745-749.
2. Kirtak N, Inaloz HS, Karakok M, et al. Extensive inflammatory nevus comedonicus involving half of the body. Int J Dermatol. 2004;43:434-436.
3. Paige TN, Mendelson CG. Bilateral nevus comedonicus. Arch Dermatol. 1967;96:172-175.
4. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: WB Saunders; 2006.
5. Ahn SY, Oh Y, Bak H, et al. Co-occurrence of nevus comedonicus with accessory breast tissue. Int J Dermatol. 2008;47:530-531.
6. Freinkel RK, Freinkel N. Hair growth and alopecia in hypothyroidism. Arch Dermatol. 1972;106:349-352.
7. Tsujio M, Yoshioka K, Satoh M, et al. Skin morphology of thyroidectomized rats. Vet Pathol. 2008;45:505-511.
8. Billoni N, Buan B, Gautier B, et al. Thyroid hormone receptor β1 is expressed in the human hair follicle. Br J Dermatol. 2000;142:645-652.
9. van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388.
10. Bodó E, Kromminga A, Bíró T, et al. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J Invest Dermatol. 2009;129:1126-1139.
To the Editor:
A 62-year-old woman was referred to the dermatology clinic for papules on both eyelids of 6 months’ duration. She underwent surgery for a thyroid gland adenoma 3 years prior and subsequently experienced hypothyroidism. Levothyroxine sodium was administered daily (100 µg initially; 50 µg over the last 1.5 years). Papules occurred on both eyelids 6 months prior to presentation and gradually increased in number. The center of each papule was filled with a black keratinous plug. The skin lesions became raised after the patient ate fatty foods. The lesions remained entirely asymptomatic and there was no family history of a similar disorder.
Physical examinations showed no systemic abnormalities. Dermatologic examination showed clustered 3- to 4-mm flesh-colored papules on both upper eyelids; the centers of the papules were filled with 1- to 2-mm black keratinous plugs (Figure 1A). Several similar skin lesions existed on the lower eyelids, nasal root, and right side of the nasal dorsum. On laboratory examination, the results of routine blood, urine, and stool tests, as well as renal and hepatic functions, electrolytes, and blood sugar levels, were within reference range. Indicators including triglyceride of 2.50 mmol/L (reference range, 0.40–1.90 mmol/L), total cholesterol of 6.31 mmol/L (reference range, 3.00–5.70 mmol/L), serum total thyroxine (T4) of 5.32 µg/dL (reference range, 6.09–12.23 µg/dL), total triiodothyronine (T3) of 64 ng/dL (reference range, 87–178 ng/dL), serum free thyroxine (FT4) of 0.41 ng/dL (reference range, 0.61–1.12 ng/dL), serum free triiodothyronine (FT3) of 182 pg/dL (reference range, 250–390 pg/dL), and thyrotropin of 33.75 µIU/mL (reference range, 0.34–5.60 µIU/mL) were not within reference range; however, thyroperoxidase antibodies, thyrotropin receptor antibodies, thyroglobulin antibodies, thyroglobulin, and calcitonin were within reference range. Color ultrasonography indicated post–subtotal resection of the bilateral thyroid glands.
  |
Histopathologic analysis of the skin lesions showed that the epidermis became atrophic and thinner, and several atrophic and cyst-dilated follicular structures existed inside the dermis. Some structures opened through the epidermis; the walls were squamous epithelium and keratin filled the structures (Figure 2). The condition was diagnosed as nevus comedonicus (NC).
The patient was referred to the endocrinology department and treated with levothyroxine sodium (100 µg daily). At 5-month follow-up, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions had resolved (Figure 1B).
Nevus comedonicus is an unusual skin lesion with a predilection for the face, neck, shoulders, upper arms, and trunk. The clinical manifestations include comedonelike papules with centers that are characterized by large, black, solid keratinous plugs. When the plugs are peeled off, volcanic craterlike pits will be left. The skin lesions usually are ribbonlike and clustered on 1 side of the body. Pathologic examination often shows that the epidermis is pitted downward, and the dilated follicular ostia are plugged with keratin.1,2 Paige and Mendelson3 divided NC into 2 types: inflammatory and noninflammatory. Approximately half of NC patients experience cysts, abscesses, fistulae, and scars.4
The exact etiology of NC is unclear. Some researchers believe that it is a congenital hair follicle deformity; more specifically, that it is caused by a developmental defect in the hair follicles in the embryonic stage (ie, abnormal differentiation of epithelial stem cells that differentiate into follicles). Most incidences of NC occur at birth or before growth and development. However, few studies have reported late-onset NC.5
|
The relationship between NC and thyroid disease is unique. Clinical research has shown that hypothyroidism can result in hair loss and cracks.6 In animal experiments, hypothyroidism model mice often experienced degenerative changes of their hair follicles and hair papillae as well as changes in the telogen phase, such as thinning of the outer and inner root sheaths.7 Meanwhile, decreased cell proliferation activity in the hair follicles was observed. Therefore, it is reasonable to conclude that thyroid hormones have regulatory effects on the growth and development of hair follicles.7
A study on human hair follicles found that thyroid hormone receptor β1 is expressed in human hair follicles.8 Research on in vitro–cultured human hair follicles showed that thyroid hormones T3 and T4 upregulated the proliferation of hair matrix cells and downregulated their apoptosis. Thyroid hormones also prolonged the duration of the hair growth phase (anagen).9 Furthermore, expression of thyrotropin receptor was detected in human hair follicles. Because increased serum thyrotropin levels can lead to clinical hair loss, thyrotropin may inhibit the growth of hair follicles via thyrotropin receptor.10 In our patient, NC occurred on both eyelids when the patient experienced hypothyroidism following thyroid gland adenoma surgery. Following treatment with levothyroxine sodium, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions resolved. Therefore, the occurrence of NC may be related to hypothyroidism in this patient. The low thyroid hormone levels and elevated thyrotropin level possibly induced degenerative changes and injuries to the hair matrix cells, resulting in hair follicle obstruction and accumulation of keratin, which ultimately led to NC. However, the exact relationship between NC and thyroid diseases requires elucidation in future studies.
To the Editor:
A 62-year-old woman was referred to the dermatology clinic for papules on both eyelids of 6 months’ duration. She underwent surgery for a thyroid gland adenoma 3 years prior and subsequently experienced hypothyroidism. Levothyroxine sodium was administered daily (100 µg initially; 50 µg over the last 1.5 years). Papules occurred on both eyelids 6 months prior to presentation and gradually increased in number. The center of each papule was filled with a black keratinous plug. The skin lesions became raised after the patient ate fatty foods. The lesions remained entirely asymptomatic and there was no family history of a similar disorder.
Physical examinations showed no systemic abnormalities. Dermatologic examination showed clustered 3- to 4-mm flesh-colored papules on both upper eyelids; the centers of the papules were filled with 1- to 2-mm black keratinous plugs (Figure 1A). Several similar skin lesions existed on the lower eyelids, nasal root, and right side of the nasal dorsum. On laboratory examination, the results of routine blood, urine, and stool tests, as well as renal and hepatic functions, electrolytes, and blood sugar levels, were within reference range. Indicators including triglyceride of 2.50 mmol/L (reference range, 0.40–1.90 mmol/L), total cholesterol of 6.31 mmol/L (reference range, 3.00–5.70 mmol/L), serum total thyroxine (T4) of 5.32 µg/dL (reference range, 6.09–12.23 µg/dL), total triiodothyronine (T3) of 64 ng/dL (reference range, 87–178 ng/dL), serum free thyroxine (FT4) of 0.41 ng/dL (reference range, 0.61–1.12 ng/dL), serum free triiodothyronine (FT3) of 182 pg/dL (reference range, 250–390 pg/dL), and thyrotropin of 33.75 µIU/mL (reference range, 0.34–5.60 µIU/mL) were not within reference range; however, thyroperoxidase antibodies, thyrotropin receptor antibodies, thyroglobulin antibodies, thyroglobulin, and calcitonin were within reference range. Color ultrasonography indicated post–subtotal resection of the bilateral thyroid glands.
  |
Histopathologic analysis of the skin lesions showed that the epidermis became atrophic and thinner, and several atrophic and cyst-dilated follicular structures existed inside the dermis. Some structures opened through the epidermis; the walls were squamous epithelium and keratin filled the structures (Figure 2). The condition was diagnosed as nevus comedonicus (NC).
The patient was referred to the endocrinology department and treated with levothyroxine sodium (100 µg daily). At 5-month follow-up, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions had resolved (Figure 1B).
Nevus comedonicus is an unusual skin lesion with a predilection for the face, neck, shoulders, upper arms, and trunk. The clinical manifestations include comedonelike papules with centers that are characterized by large, black, solid keratinous plugs. When the plugs are peeled off, volcanic craterlike pits will be left. The skin lesions usually are ribbonlike and clustered on 1 side of the body. Pathologic examination often shows that the epidermis is pitted downward, and the dilated follicular ostia are plugged with keratin.1,2 Paige and Mendelson3 divided NC into 2 types: inflammatory and noninflammatory. Approximately half of NC patients experience cysts, abscesses, fistulae, and scars.4
The exact etiology of NC is unclear. Some researchers believe that it is a congenital hair follicle deformity; more specifically, that it is caused by a developmental defect in the hair follicles in the embryonic stage (ie, abnormal differentiation of epithelial stem cells that differentiate into follicles). Most incidences of NC occur at birth or before growth and development. However, few studies have reported late-onset NC.5
|
The relationship between NC and thyroid disease is unique. Clinical research has shown that hypothyroidism can result in hair loss and cracks.6 In animal experiments, hypothyroidism model mice often experienced degenerative changes of their hair follicles and hair papillae as well as changes in the telogen phase, such as thinning of the outer and inner root sheaths.7 Meanwhile, decreased cell proliferation activity in the hair follicles was observed. Therefore, it is reasonable to conclude that thyroid hormones have regulatory effects on the growth and development of hair follicles.7
A study on human hair follicles found that thyroid hormone receptor β1 is expressed in human hair follicles.8 Research on in vitro–cultured human hair follicles showed that thyroid hormones T3 and T4 upregulated the proliferation of hair matrix cells and downregulated their apoptosis. Thyroid hormones also prolonged the duration of the hair growth phase (anagen).9 Furthermore, expression of thyrotropin receptor was detected in human hair follicles. Because increased serum thyrotropin levels can lead to clinical hair loss, thyrotropin may inhibit the growth of hair follicles via thyrotropin receptor.10 In our patient, NC occurred on both eyelids when the patient experienced hypothyroidism following thyroid gland adenoma surgery. Following treatment with levothyroxine sodium, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions resolved. Therefore, the occurrence of NC may be related to hypothyroidism in this patient. The low thyroid hormone levels and elevated thyrotropin level possibly induced degenerative changes and injuries to the hair matrix cells, resulting in hair follicle obstruction and accumulation of keratin, which ultimately led to NC. However, the exact relationship between NC and thyroid diseases requires elucidation in future studies.
1. Engber PB. The nevus comedonicus syndrome: a case report with emphasis on associated internal manifestations. Int J Dermatol. 1978;17:745-749.
2. Kirtak N, Inaloz HS, Karakok M, et al. Extensive inflammatory nevus comedonicus involving half of the body. Int J Dermatol. 2004;43:434-436.
3. Paige TN, Mendelson CG. Bilateral nevus comedonicus. Arch Dermatol. 1967;96:172-175.
4. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: WB Saunders; 2006.
5. Ahn SY, Oh Y, Bak H, et al. Co-occurrence of nevus comedonicus with accessory breast tissue. Int J Dermatol. 2008;47:530-531.
6. Freinkel RK, Freinkel N. Hair growth and alopecia in hypothyroidism. Arch Dermatol. 1972;106:349-352.
7. Tsujio M, Yoshioka K, Satoh M, et al. Skin morphology of thyroidectomized rats. Vet Pathol. 2008;45:505-511.
8. Billoni N, Buan B, Gautier B, et al. Thyroid hormone receptor β1 is expressed in the human hair follicle. Br J Dermatol. 2000;142:645-652.
9. van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388.
10. Bodó E, Kromminga A, Bíró T, et al. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J Invest Dermatol. 2009;129:1126-1139.
1. Engber PB. The nevus comedonicus syndrome: a case report with emphasis on associated internal manifestations. Int J Dermatol. 1978;17:745-749.
2. Kirtak N, Inaloz HS, Karakok M, et al. Extensive inflammatory nevus comedonicus involving half of the body. Int J Dermatol. 2004;43:434-436.
3. Paige TN, Mendelson CG. Bilateral nevus comedonicus. Arch Dermatol. 1967;96:172-175.
4. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: WB Saunders; 2006.
5. Ahn SY, Oh Y, Bak H, et al. Co-occurrence of nevus comedonicus with accessory breast tissue. Int J Dermatol. 2008;47:530-531.
6. Freinkel RK, Freinkel N. Hair growth and alopecia in hypothyroidism. Arch Dermatol. 1972;106:349-352.
7. Tsujio M, Yoshioka K, Satoh M, et al. Skin morphology of thyroidectomized rats. Vet Pathol. 2008;45:505-511.
8. Billoni N, Buan B, Gautier B, et al. Thyroid hormone receptor β1 is expressed in the human hair follicle. Br J Dermatol. 2000;142:645-652.
9. van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388.
10. Bodó E, Kromminga A, Bíró T, et al. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J Invest Dermatol. 2009;129:1126-1139.
Large Subcutaneous Masses
The Diagnosis: Madelung Disease (Benign Symmetric Lipomatosis)
A 56-year-old man presented for evaluation of massive subcutaneous nodules the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs (Figures 1 and 2) that developed over the last 12 to 18 months and continued to enlarge. In addition, he was beginning to develop symptoms of neuropathy of the bilateral hands. The patient had a long-standing history of alcohol abuse. Biopsies performed by the patient’s primary care physician revealed benign adipose tissue. He was referred to the dermatology clinic and subsequently diagnosed with Madelung disease.
 |
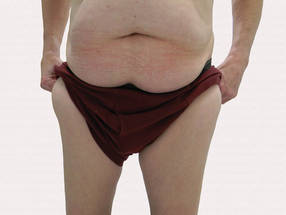 |
Madelung disease, also known as benign symmetric lipomatosis and Launois-Bensaude syndrome, is characterized by multiple large masses of nonencapsulated adipose tissue. These masses are symmetric and most prominent on the head, neck, trunk, and proximal extremities. Classically, a pseudoathletic appearance is described. Madelung disease most frequently affects men aged 30 to 60 years. In more than 90% of cases, it is associated with alcoholism.
In general, the masses of adipose tissue are asymptomatic. However, airway compression and dysphagia requiring surgical intervention has been reported in the otolaryngology literature.1 In addition, neuropathy develops in 84% of cases.2 Nerve biopsies from patients with Madelung disease have revealed a pattern of axonopathy that is distinct from alcohol-induced neuropathy.3 This neuropathy can involve sensory and motor nerves, with the most prominent findings being muscle weakness, tendon areflexia, interosseous muscle atrophy, vibratory sensation loss, and hypoesthesia. Furthermore, dysfunction of the autonomic nervous system can lead to segmental hyperhidrosis, gustatory sweating, and abnormal autonomic cardiovascular reflexes.2 Many patients who develop neuropathy will eventually become incapacitated.
The etiology of Madelung disease is not fully understood. There are several theories on the pathogenesis of this disease, most describing metabolic disturbances induced by alcohol. Specifically, studies have revealed chronic alcohol use causes numerous deletions in mitochondrial DNA.4,5 The mitochondrial DNA damage may explain both the resistance of the lipomatous masses to lipolysis and the nerve-related changes. Comparisons between human immunodeficiency virus/highly active antiretroviral therapy–associated lipodystrophy and Madelung disease lend credence to the metabolic disturbance theory and may help clarify the specific mechanisms involved.6
There have been no cases of spontaneous resolution, even in patients who stop consuming alcohol. For this reason, most patients are referred to a surgeon. Many surgeons prefer open excision for debulking large lipomatous masses, but this technique typically requires general anesthesia. For those in whom this treatment is not an appropriate option, liposuction can be considered with tumescent anesthesia.7 A combination of these surgical modalities also is an option.
Madelung disease is a distinctive disorder typically affecting chronic alcoholics. Recognition of this clinical entity is important, as severe neuropathy and airway compromise may ensue. Although surgical excision is an attractive option for cosmesis and airway compromise, the associated neuropathy can be extremely difficult to treat and can be quite debilitating.
1. Palacios E, Neitzschman HR, Nguyen J. Madelung disease: multiple symmetric lipomatosis. Ear Nose Throat J. 2014;93:94-96.
2. Enzi G, Angelini C, Negrin P, et al. Sensory, motor, and autonomic neuropathy in patients with multiple symmetric lipomatosis. Medicine (Baltimore). 1985;64:388-393.
3. Pollock M, Nicholson GI, Nukada H, et al. Neuropathy in multiple symmetric lipomatosis. Madelung’s disease. Brain. 1988;111:1157-1171.
4. Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862-866.
5. Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102.
6. Urso R, Gentile M. Are ‘buffalo hump’ syndrome, Madelung's disease and multiple symmetrical lipomatosis variants of the same dysmetabolism? AIDS. 2001;15:290-291.
7. Grassegger A, Häussler R, Schmalzl F. Tumescent liposuction in a patient with Launois-Bensaude syndrome and severe hepatopathy. Dermatol Surg. 2007;33:982-985.
The Diagnosis: Madelung Disease (Benign Symmetric Lipomatosis)
A 56-year-old man presented for evaluation of massive subcutaneous nodules the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs (Figures 1 and 2) that developed over the last 12 to 18 months and continued to enlarge. In addition, he was beginning to develop symptoms of neuropathy of the bilateral hands. The patient had a long-standing history of alcohol abuse. Biopsies performed by the patient’s primary care physician revealed benign adipose tissue. He was referred to the dermatology clinic and subsequently diagnosed with Madelung disease.
 |
 |
Madelung disease, also known as benign symmetric lipomatosis and Launois-Bensaude syndrome, is characterized by multiple large masses of nonencapsulated adipose tissue. These masses are symmetric and most prominent on the head, neck, trunk, and proximal extremities. Classically, a pseudoathletic appearance is described. Madelung disease most frequently affects men aged 30 to 60 years. In more than 90% of cases, it is associated with alcoholism.
In general, the masses of adipose tissue are asymptomatic. However, airway compression and dysphagia requiring surgical intervention has been reported in the otolaryngology literature.1 In addition, neuropathy develops in 84% of cases.2 Nerve biopsies from patients with Madelung disease have revealed a pattern of axonopathy that is distinct from alcohol-induced neuropathy.3 This neuropathy can involve sensory and motor nerves, with the most prominent findings being muscle weakness, tendon areflexia, interosseous muscle atrophy, vibratory sensation loss, and hypoesthesia. Furthermore, dysfunction of the autonomic nervous system can lead to segmental hyperhidrosis, gustatory sweating, and abnormal autonomic cardiovascular reflexes.2 Many patients who develop neuropathy will eventually become incapacitated.
The etiology of Madelung disease is not fully understood. There are several theories on the pathogenesis of this disease, most describing metabolic disturbances induced by alcohol. Specifically, studies have revealed chronic alcohol use causes numerous deletions in mitochondrial DNA.4,5 The mitochondrial DNA damage may explain both the resistance of the lipomatous masses to lipolysis and the nerve-related changes. Comparisons between human immunodeficiency virus/highly active antiretroviral therapy–associated lipodystrophy and Madelung disease lend credence to the metabolic disturbance theory and may help clarify the specific mechanisms involved.6
There have been no cases of spontaneous resolution, even in patients who stop consuming alcohol. For this reason, most patients are referred to a surgeon. Many surgeons prefer open excision for debulking large lipomatous masses, but this technique typically requires general anesthesia. For those in whom this treatment is not an appropriate option, liposuction can be considered with tumescent anesthesia.7 A combination of these surgical modalities also is an option.
Madelung disease is a distinctive disorder typically affecting chronic alcoholics. Recognition of this clinical entity is important, as severe neuropathy and airway compromise may ensue. Although surgical excision is an attractive option for cosmesis and airway compromise, the associated neuropathy can be extremely difficult to treat and can be quite debilitating.
The Diagnosis: Madelung Disease (Benign Symmetric Lipomatosis)
A 56-year-old man presented for evaluation of massive subcutaneous nodules the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs (Figures 1 and 2) that developed over the last 12 to 18 months and continued to enlarge. In addition, he was beginning to develop symptoms of neuropathy of the bilateral hands. The patient had a long-standing history of alcohol abuse. Biopsies performed by the patient’s primary care physician revealed benign adipose tissue. He was referred to the dermatology clinic and subsequently diagnosed with Madelung disease.
 |
 |
Madelung disease, also known as benign symmetric lipomatosis and Launois-Bensaude syndrome, is characterized by multiple large masses of nonencapsulated adipose tissue. These masses are symmetric and most prominent on the head, neck, trunk, and proximal extremities. Classically, a pseudoathletic appearance is described. Madelung disease most frequently affects men aged 30 to 60 years. In more than 90% of cases, it is associated with alcoholism.
In general, the masses of adipose tissue are asymptomatic. However, airway compression and dysphagia requiring surgical intervention has been reported in the otolaryngology literature.1 In addition, neuropathy develops in 84% of cases.2 Nerve biopsies from patients with Madelung disease have revealed a pattern of axonopathy that is distinct from alcohol-induced neuropathy.3 This neuropathy can involve sensory and motor nerves, with the most prominent findings being muscle weakness, tendon areflexia, interosseous muscle atrophy, vibratory sensation loss, and hypoesthesia. Furthermore, dysfunction of the autonomic nervous system can lead to segmental hyperhidrosis, gustatory sweating, and abnormal autonomic cardiovascular reflexes.2 Many patients who develop neuropathy will eventually become incapacitated.
The etiology of Madelung disease is not fully understood. There are several theories on the pathogenesis of this disease, most describing metabolic disturbances induced by alcohol. Specifically, studies have revealed chronic alcohol use causes numerous deletions in mitochondrial DNA.4,5 The mitochondrial DNA damage may explain both the resistance of the lipomatous masses to lipolysis and the nerve-related changes. Comparisons between human immunodeficiency virus/highly active antiretroviral therapy–associated lipodystrophy and Madelung disease lend credence to the metabolic disturbance theory and may help clarify the specific mechanisms involved.6
There have been no cases of spontaneous resolution, even in patients who stop consuming alcohol. For this reason, most patients are referred to a surgeon. Many surgeons prefer open excision for debulking large lipomatous masses, but this technique typically requires general anesthesia. For those in whom this treatment is not an appropriate option, liposuction can be considered with tumescent anesthesia.7 A combination of these surgical modalities also is an option.
Madelung disease is a distinctive disorder typically affecting chronic alcoholics. Recognition of this clinical entity is important, as severe neuropathy and airway compromise may ensue. Although surgical excision is an attractive option for cosmesis and airway compromise, the associated neuropathy can be extremely difficult to treat and can be quite debilitating.
1. Palacios E, Neitzschman HR, Nguyen J. Madelung disease: multiple symmetric lipomatosis. Ear Nose Throat J. 2014;93:94-96.
2. Enzi G, Angelini C, Negrin P, et al. Sensory, motor, and autonomic neuropathy in patients with multiple symmetric lipomatosis. Medicine (Baltimore). 1985;64:388-393.
3. Pollock M, Nicholson GI, Nukada H, et al. Neuropathy in multiple symmetric lipomatosis. Madelung’s disease. Brain. 1988;111:1157-1171.
4. Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862-866.
5. Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102.
6. Urso R, Gentile M. Are ‘buffalo hump’ syndrome, Madelung's disease and multiple symmetrical lipomatosis variants of the same dysmetabolism? AIDS. 2001;15:290-291.
7. Grassegger A, Häussler R, Schmalzl F. Tumescent liposuction in a patient with Launois-Bensaude syndrome and severe hepatopathy. Dermatol Surg. 2007;33:982-985.
1. Palacios E, Neitzschman HR, Nguyen J. Madelung disease: multiple symmetric lipomatosis. Ear Nose Throat J. 2014;93:94-96.
2. Enzi G, Angelini C, Negrin P, et al. Sensory, motor, and autonomic neuropathy in patients with multiple symmetric lipomatosis. Medicine (Baltimore). 1985;64:388-393.
3. Pollock M, Nicholson GI, Nukada H, et al. Neuropathy in multiple symmetric lipomatosis. Madelung’s disease. Brain. 1988;111:1157-1171.
4. Klopstock T, Naumann M, Schalke B, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862-866.
5. Mansouri A, Fromenty B, Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102.
6. Urso R, Gentile M. Are ‘buffalo hump’ syndrome, Madelung's disease and multiple symmetrical lipomatosis variants of the same dysmetabolism? AIDS. 2001;15:290-291.
7. Grassegger A, Häussler R, Schmalzl F. Tumescent liposuction in a patient with Launois-Bensaude syndrome and severe hepatopathy. Dermatol Surg. 2007;33:982-985.
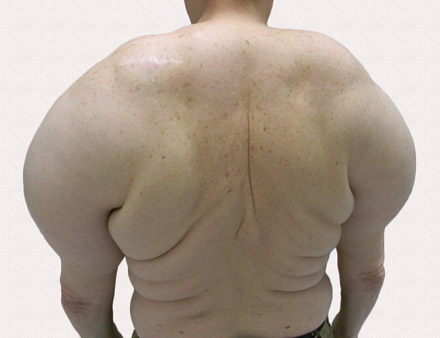
A 56-year-old man presented for evaluation of massive subcutaneous nodules on the bilateral upper arms, shoulders, chest, abdomen, and lateral aspect of the proximal thighs that developed over the last 12 to 18 months and continued to enlarge. His medical history was remarkable for alcoholism, hyperlipidemia, and hypertension.
Manage Your Dermatology Practice: Mastering Communication With Patients About Their Expectations
Communicating expectations to patients at their first visit and at follow-up is an important aspect of managing your dermatology practice. Dr. Gary Goldenberg discusses patient expectations for clinical conditions such as acne or psoriasis and for cosmetic treatments. He also advises what to do if you encounter a patient with unreasonable expectations.
Communicating expectations to patients at their first visit and at follow-up is an important aspect of managing your dermatology practice. Dr. Gary Goldenberg discusses patient expectations for clinical conditions such as acne or psoriasis and for cosmetic treatments. He also advises what to do if you encounter a patient with unreasonable expectations.
Communicating expectations to patients at their first visit and at follow-up is an important aspect of managing your dermatology practice. Dr. Gary Goldenberg discusses patient expectations for clinical conditions such as acne or psoriasis and for cosmetic treatments. He also advises what to do if you encounter a patient with unreasonable expectations.
Cosmetic Corner: Dermatologists Weigh in on Facial Scrubs
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top facial scrubs. Consideration must be given to:
- Facial Fuel Energizing Scrub
Kiehl’s
“This product isn’t terribly expensive and is great for men with all skin types. Patients love the mild stinging the product produces—that’s how they know it’s working!”—Gary Goldenberg, MD, New York, New York
“It is great for women and men. It has menthol and caffeine that gives a refreshing feel to the skin and great exfoliating without excessive irritation.”—Anthony M. Rossi, MD, New York, New York
- NIA24 Physical Cleansing Scrub
Niadyne, Inc.
“It offers a beaded but gentle approach to a facial scrub and is highly rated by our patients and customers.”—Joel Schlessinger, MD, Omaha, Nebraska
- Olay Skin Smoothing Cream Scrub
Proctor & Gamble
“Effective, not expensive, and does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
“This product is inexpensive and great before applying moisturizer or after wearing makeup.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Mineral makeup and eyelash enhancers will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top facial scrubs. Consideration must be given to:
- Facial Fuel Energizing Scrub
Kiehl’s
“This product isn’t terribly expensive and is great for men with all skin types. Patients love the mild stinging the product produces—that’s how they know it’s working!”—Gary Goldenberg, MD, New York, New York
“It is great for women and men. It has menthol and caffeine that gives a refreshing feel to the skin and great exfoliating without excessive irritation.”—Anthony M. Rossi, MD, New York, New York
- NIA24 Physical Cleansing Scrub
Niadyne, Inc.
“It offers a beaded but gentle approach to a facial scrub and is highly rated by our patients and customers.”—Joel Schlessinger, MD, Omaha, Nebraska
- Olay Skin Smoothing Cream Scrub
Proctor & Gamble
“Effective, not expensive, and does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
“This product is inexpensive and great before applying moisturizer or after wearing makeup.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Mineral makeup and eyelash enhancers will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top facial scrubs. Consideration must be given to:
- Facial Fuel Energizing Scrub
Kiehl’s
“This product isn’t terribly expensive and is great for men with all skin types. Patients love the mild stinging the product produces—that’s how they know it’s working!”—Gary Goldenberg, MD, New York, New York
“It is great for women and men. It has menthol and caffeine that gives a refreshing feel to the skin and great exfoliating without excessive irritation.”—Anthony M. Rossi, MD, New York, New York
- NIA24 Physical Cleansing Scrub
Niadyne, Inc.
“It offers a beaded but gentle approach to a facial scrub and is highly rated by our patients and customers.”—Joel Schlessinger, MD, Omaha, Nebraska
- Olay Skin Smoothing Cream Scrub
Proctor & Gamble
“Effective, not expensive, and does not dry the skin.”—Antonella Tosti, MD, Miami, Florida
“This product is inexpensive and great before applying moisturizer or after wearing makeup.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Mineral makeup and eyelash enhancers will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to cutis@frontlinemedcom.com.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.