User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Cutaneous Manifestations of Cocaine Use
The Diagnosis: Levamisole-Induced Cutaneous Vasculopathy
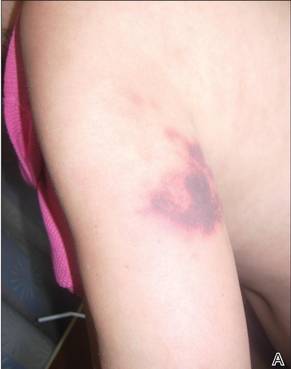
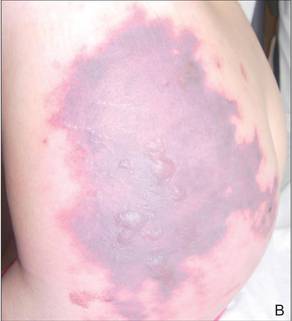
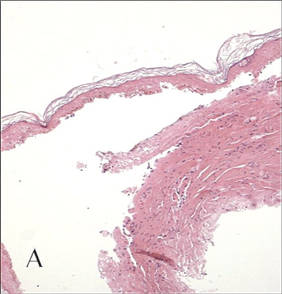
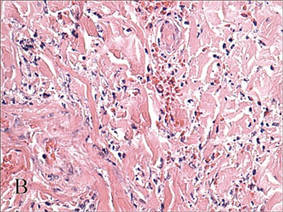
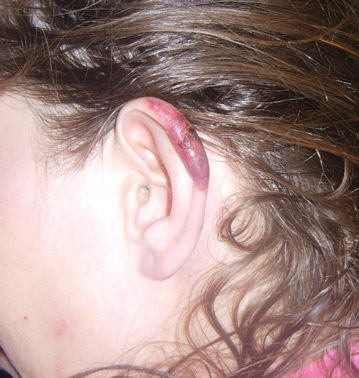
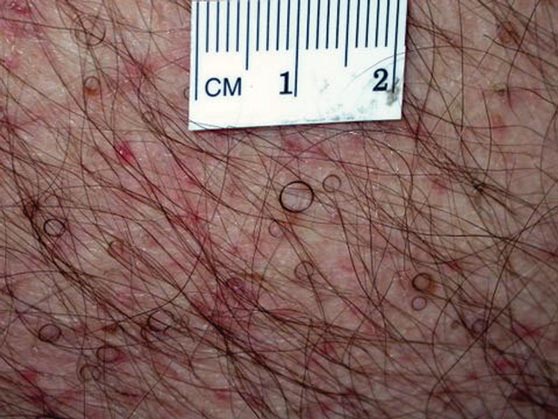
In our patient, tender stellate purpura and occasional bullae were present on the ears, arms and legs, groin, and buttocks (Figure 1). Histopathologic examination revealed subepidermal detachment, perivascular neutrophilic infiltrate, and red blood cell extravasation, consistent with early leukocyctoclastic vasculitis (Figure 2).
|
Levamisole-induced vasculopathy is a condition related primarily to cocaine use. Levamisole is an immunomodulatory agent, historically used as a disease-modifying antirheumatic drug for rheumatoid arthritis and as adjuvant chemotherapy for various types of cancer. However, levamisole for human use was banned from US and Canadian markets in 1999 and 2003, respectively, due to increased risk for agranulocytosis, retiform purpura, and epilepsy.1 Currently, veterinarians use levamisole as an anthelminthic agent to deworm house and farm animals. In Europe, pediatric nephrologists use it as a steroid-sparing agent in children with steroid-dependent nephritic syndrome.
Over the last decade, levamisole has increasingly been used as a cocaine adulterant or bulking agent. This contaminant closely resembles cocaine physically and is theorized to prolong or attenuate cocaine’s “high.” Approximately 69% of cocaine sampled by the US Drug Enforcement Administration is adulterated with levamisole.2 Similarly, levamisole-contaminated cocaine also has been found in Europe, Australia, and other parts of the world. Potential complications include vasculitis, thromboembolism, neutropenia, and agranulocytosis.3
Levamisole-induced vasculopathy appears to affect cocaine users of all ages, ethnicities, and genders. Cocaine can be smoked, snorted, or injected. In nearly all reported cases, patients characteristically present with hemorrhagic bullae of the bilateral ear helix, cheeks, or nasal tip. Any body site can be affected with retiform purpura or necrotic bullae. Along with skin lesions, arthralgia is commonly reported, as are constitutional symptoms (eg, fever, night sweats, weight loss, malaise)4; oral mucosal involvement also has been reported.5 Laboratory investigation can reveal neutropenia, positive antineutrophil cytoplasmic antibodies (ANCAs) in the perinuclear or cytoplasmic pattern, positive proteinase 3, and negative or mildly elevated antimyeloperoxidase.3-5 Acute renal injury and pulmonary hemorrhage are other potentially serious copmlications.4 Antihuman neutrophil elastase antibody testing can help distinguish levamisole-induced vasculopathy from other forms of immune-mediated vasculitis and will be negative in immune-mediated vasculitides such as Churg-Strauss syndrome (allergic granulomatosis), Wegener granulomatosis (granulomatosis with polyangiitis), and polyarteritis nodosa.6 On histology, microvascular thrombosis or leukocytoclastic vasculitis can both, or individually, be seen. Epidermal necrosis, dermal hemorrhage, and endothelial hyperplasia have all been noted in skin biopsied from necrotic bullae.
|
Levamisole’s short half-life (approximately 5–6 hours) makes it difficult to detect on routine blood draws. An astute physician suspecting this diagnosis on initial presentation can ask for levamisole detection on urine toxicology screening.7 Urine samples also can be sent for testing with gas chromatography–mass spectrometry, though this test may only be available at major research centers.8
Differential diagnosis of levamisole toxicity includes different types of vasculitides such as cryoglobulinemia (positive serum IgM and IgG cryoglobulins; possible hepatitis C infection), Wegener granulomatosis (cytoplasmic ANCA positive; associated with upper and lower respiratory tract inflammation, glomerulonephritis), Churg-Strauss syndrome (perinuclear ANCA positive; associated with asthma and eosinophilia), and polyarteritis nodosa (medium vessel involvement only; associated with livedo reticularis, subcutaneous nodules, ulcers).9 Necrotic lesions also may raise the possibility of warfarin necrosis, heparin necrosis, or cholesterol emboli. Cholesterol embolism most frequently presents with small vessel vasculitis and necrosis of distal extremities such as the toes. With large areas of skin involvement and bullae, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis also should be considered.9
Definitive treatment of this condition requires complete and immediate cessation of cocaine use. Levamisole also has been found as a contaminant in heroin.1 Thus, it may be prudent to recommend heroin avoidance to the patient to prevent recurrences. Management of acute levamisole-induced vasculopathy is primarily symptomatic. Some patients with severe neutropenia at risk for infection have been treated with granulocyte colony-stimulating factor, while others have only required pain control, usually with nonsteroidal anti-inflammatory drugs.10 Oral prednisone and colchicine also have been used with reported success.5
Given the increasing incidence of levamisole toxicity and public health implications, clinicians should be aware of this association and the classic clinical and laboratory findings.
1. Aberastury MN, Silva WH, Vaccarezza MM, et al. Epilepsia partialis continua associated with levamisole. Pediatr Neurol. 2011;44:385-388.
2. Nationwide public health alert issued concerning life-threatening risk posed by cocaine laced with veterinary anti-parasite drug [press release]. Rockville, MD: Substance Abuse and Mental Health Services Administration; September 21, 2009. http://beta.samhsa.gov/newsroom/press-announcements/200909211245. Accessed October 9, 2014.
3. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
4. McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasm antibody-associated disease. Clin J Am Soc Nephrol. 2011;6:2799-2805.
5. Poon SH, Baliog CR Jr, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leucopenia. Semin Arthritis Rheum. 2011;41:434-444.
6. Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
7. Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
8. Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2001;35:545-550.
9. Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
10. Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
The Diagnosis: Levamisole-Induced Cutaneous Vasculopathy
In our patient, tender stellate purpura and occasional bullae were present on the ears, arms and legs, groin, and buttocks (Figure 1). Histopathologic examination revealed subepidermal detachment, perivascular neutrophilic infiltrate, and red blood cell extravasation, consistent with early leukocyctoclastic vasculitis (Figure 2).
|
Levamisole-induced vasculopathy is a condition related primarily to cocaine use. Levamisole is an immunomodulatory agent, historically used as a disease-modifying antirheumatic drug for rheumatoid arthritis and as adjuvant chemotherapy for various types of cancer. However, levamisole for human use was banned from US and Canadian markets in 1999 and 2003, respectively, due to increased risk for agranulocytosis, retiform purpura, and epilepsy.1 Currently, veterinarians use levamisole as an anthelminthic agent to deworm house and farm animals. In Europe, pediatric nephrologists use it as a steroid-sparing agent in children with steroid-dependent nephritic syndrome.
Over the last decade, levamisole has increasingly been used as a cocaine adulterant or bulking agent. This contaminant closely resembles cocaine physically and is theorized to prolong or attenuate cocaine’s “high.” Approximately 69% of cocaine sampled by the US Drug Enforcement Administration is adulterated with levamisole.2 Similarly, levamisole-contaminated cocaine also has been found in Europe, Australia, and other parts of the world. Potential complications include vasculitis, thromboembolism, neutropenia, and agranulocytosis.3
Levamisole-induced vasculopathy appears to affect cocaine users of all ages, ethnicities, and genders. Cocaine can be smoked, snorted, or injected. In nearly all reported cases, patients characteristically present with hemorrhagic bullae of the bilateral ear helix, cheeks, or nasal tip. Any body site can be affected with retiform purpura or necrotic bullae. Along with skin lesions, arthralgia is commonly reported, as are constitutional symptoms (eg, fever, night sweats, weight loss, malaise)4; oral mucosal involvement also has been reported.5 Laboratory investigation can reveal neutropenia, positive antineutrophil cytoplasmic antibodies (ANCAs) in the perinuclear or cytoplasmic pattern, positive proteinase 3, and negative or mildly elevated antimyeloperoxidase.3-5 Acute renal injury and pulmonary hemorrhage are other potentially serious copmlications.4 Antihuman neutrophil elastase antibody testing can help distinguish levamisole-induced vasculopathy from other forms of immune-mediated vasculitis and will be negative in immune-mediated vasculitides such as Churg-Strauss syndrome (allergic granulomatosis), Wegener granulomatosis (granulomatosis with polyangiitis), and polyarteritis nodosa.6 On histology, microvascular thrombosis or leukocytoclastic vasculitis can both, or individually, be seen. Epidermal necrosis, dermal hemorrhage, and endothelial hyperplasia have all been noted in skin biopsied from necrotic bullae.
|
Levamisole’s short half-life (approximately 5–6 hours) makes it difficult to detect on routine blood draws. An astute physician suspecting this diagnosis on initial presentation can ask for levamisole detection on urine toxicology screening.7 Urine samples also can be sent for testing with gas chromatography–mass spectrometry, though this test may only be available at major research centers.8
Differential diagnosis of levamisole toxicity includes different types of vasculitides such as cryoglobulinemia (positive serum IgM and IgG cryoglobulins; possible hepatitis C infection), Wegener granulomatosis (cytoplasmic ANCA positive; associated with upper and lower respiratory tract inflammation, glomerulonephritis), Churg-Strauss syndrome (perinuclear ANCA positive; associated with asthma and eosinophilia), and polyarteritis nodosa (medium vessel involvement only; associated with livedo reticularis, subcutaneous nodules, ulcers).9 Necrotic lesions also may raise the possibility of warfarin necrosis, heparin necrosis, or cholesterol emboli. Cholesterol embolism most frequently presents with small vessel vasculitis and necrosis of distal extremities such as the toes. With large areas of skin involvement and bullae, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis also should be considered.9
Definitive treatment of this condition requires complete and immediate cessation of cocaine use. Levamisole also has been found as a contaminant in heroin.1 Thus, it may be prudent to recommend heroin avoidance to the patient to prevent recurrences. Management of acute levamisole-induced vasculopathy is primarily symptomatic. Some patients with severe neutropenia at risk for infection have been treated with granulocyte colony-stimulating factor, while others have only required pain control, usually with nonsteroidal anti-inflammatory drugs.10 Oral prednisone and colchicine also have been used with reported success.5
Given the increasing incidence of levamisole toxicity and public health implications, clinicians should be aware of this association and the classic clinical and laboratory findings.
The Diagnosis: Levamisole-Induced Cutaneous Vasculopathy
In our patient, tender stellate purpura and occasional bullae were present on the ears, arms and legs, groin, and buttocks (Figure 1). Histopathologic examination revealed subepidermal detachment, perivascular neutrophilic infiltrate, and red blood cell extravasation, consistent with early leukocyctoclastic vasculitis (Figure 2).
|
Levamisole-induced vasculopathy is a condition related primarily to cocaine use. Levamisole is an immunomodulatory agent, historically used as a disease-modifying antirheumatic drug for rheumatoid arthritis and as adjuvant chemotherapy for various types of cancer. However, levamisole for human use was banned from US and Canadian markets in 1999 and 2003, respectively, due to increased risk for agranulocytosis, retiform purpura, and epilepsy.1 Currently, veterinarians use levamisole as an anthelminthic agent to deworm house and farm animals. In Europe, pediatric nephrologists use it as a steroid-sparing agent in children with steroid-dependent nephritic syndrome.
Over the last decade, levamisole has increasingly been used as a cocaine adulterant or bulking agent. This contaminant closely resembles cocaine physically and is theorized to prolong or attenuate cocaine’s “high.” Approximately 69% of cocaine sampled by the US Drug Enforcement Administration is adulterated with levamisole.2 Similarly, levamisole-contaminated cocaine also has been found in Europe, Australia, and other parts of the world. Potential complications include vasculitis, thromboembolism, neutropenia, and agranulocytosis.3
Levamisole-induced vasculopathy appears to affect cocaine users of all ages, ethnicities, and genders. Cocaine can be smoked, snorted, or injected. In nearly all reported cases, patients characteristically present with hemorrhagic bullae of the bilateral ear helix, cheeks, or nasal tip. Any body site can be affected with retiform purpura or necrotic bullae. Along with skin lesions, arthralgia is commonly reported, as are constitutional symptoms (eg, fever, night sweats, weight loss, malaise)4; oral mucosal involvement also has been reported.5 Laboratory investigation can reveal neutropenia, positive antineutrophil cytoplasmic antibodies (ANCAs) in the perinuclear or cytoplasmic pattern, positive proteinase 3, and negative or mildly elevated antimyeloperoxidase.3-5 Acute renal injury and pulmonary hemorrhage are other potentially serious copmlications.4 Antihuman neutrophil elastase antibody testing can help distinguish levamisole-induced vasculopathy from other forms of immune-mediated vasculitis and will be negative in immune-mediated vasculitides such as Churg-Strauss syndrome (allergic granulomatosis), Wegener granulomatosis (granulomatosis with polyangiitis), and polyarteritis nodosa.6 On histology, microvascular thrombosis or leukocytoclastic vasculitis can both, or individually, be seen. Epidermal necrosis, dermal hemorrhage, and endothelial hyperplasia have all been noted in skin biopsied from necrotic bullae.
|
Levamisole’s short half-life (approximately 5–6 hours) makes it difficult to detect on routine blood draws. An astute physician suspecting this diagnosis on initial presentation can ask for levamisole detection on urine toxicology screening.7 Urine samples also can be sent for testing with gas chromatography–mass spectrometry, though this test may only be available at major research centers.8
Differential diagnosis of levamisole toxicity includes different types of vasculitides such as cryoglobulinemia (positive serum IgM and IgG cryoglobulins; possible hepatitis C infection), Wegener granulomatosis (cytoplasmic ANCA positive; associated with upper and lower respiratory tract inflammation, glomerulonephritis), Churg-Strauss syndrome (perinuclear ANCA positive; associated with asthma and eosinophilia), and polyarteritis nodosa (medium vessel involvement only; associated with livedo reticularis, subcutaneous nodules, ulcers).9 Necrotic lesions also may raise the possibility of warfarin necrosis, heparin necrosis, or cholesterol emboli. Cholesterol embolism most frequently presents with small vessel vasculitis and necrosis of distal extremities such as the toes. With large areas of skin involvement and bullae, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis also should be considered.9
Definitive treatment of this condition requires complete and immediate cessation of cocaine use. Levamisole also has been found as a contaminant in heroin.1 Thus, it may be prudent to recommend heroin avoidance to the patient to prevent recurrences. Management of acute levamisole-induced vasculopathy is primarily symptomatic. Some patients with severe neutropenia at risk for infection have been treated with granulocyte colony-stimulating factor, while others have only required pain control, usually with nonsteroidal anti-inflammatory drugs.10 Oral prednisone and colchicine also have been used with reported success.5
Given the increasing incidence of levamisole toxicity and public health implications, clinicians should be aware of this association and the classic clinical and laboratory findings.
1. Aberastury MN, Silva WH, Vaccarezza MM, et al. Epilepsia partialis continua associated with levamisole. Pediatr Neurol. 2011;44:385-388.
2. Nationwide public health alert issued concerning life-threatening risk posed by cocaine laced with veterinary anti-parasite drug [press release]. Rockville, MD: Substance Abuse and Mental Health Services Administration; September 21, 2009. http://beta.samhsa.gov/newsroom/press-announcements/200909211245. Accessed October 9, 2014.
3. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
4. McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasm antibody-associated disease. Clin J Am Soc Nephrol. 2011;6:2799-2805.
5. Poon SH, Baliog CR Jr, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leucopenia. Semin Arthritis Rheum. 2011;41:434-444.
6. Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
7. Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
8. Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2001;35:545-550.
9. Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
10. Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
1. Aberastury MN, Silva WH, Vaccarezza MM, et al. Epilepsia partialis continua associated with levamisole. Pediatr Neurol. 2011;44:385-388.
2. Nationwide public health alert issued concerning life-threatening risk posed by cocaine laced with veterinary anti-parasite drug [press release]. Rockville, MD: Substance Abuse and Mental Health Services Administration; September 21, 2009. http://beta.samhsa.gov/newsroom/press-announcements/200909211245. Accessed October 9, 2014.
3. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
4. McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasm antibody-associated disease. Clin J Am Soc Nephrol. 2011;6:2799-2805.
5. Poon SH, Baliog CR Jr, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leucopenia. Semin Arthritis Rheum. 2011;41:434-444.
6. Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
7. Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
8. Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2001;35:545-550.
9. Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
10. Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
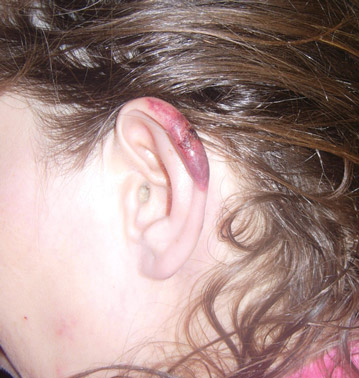
A 43-year-old woman presented to the emergency department with painful skin lesions of 1 day’s duration. Physical examination revealed tender stellate purpura and occasional bullae on the ears, arms and legs, groin, and buttocks. Laboratory results revealed neutropenia and positive lupus anticoagulant; antineutrophil cytoplasmic antibody, antinuclear antibody, and double-stranded DNA antibodies were all negative. Urine toxicology was positive for cocaine and opioids. An incisional biopsy of the left arm was performed.
Checklists to Improve Laser Practices

Hamilton and Dover (Dermatol Surg. 2014;40:1173-1174) discussed the use of checklists for improving laser and light-based procedures in their November 2014 editorial. The authors discussed how the use of checklists has become pervasive in many fields of medicine, including surgery. However, they also highlighted that the utility of checklists and their success do not come from just checking off boxes but rather from actively performing the tasks. The implementation of a laser checklist can seem daunting, but Hamilton and Dover proposed that for a dermatology practice or dermatology department it becomes easier because the dermatologist and staff work closely already, making communication less of a challenge. Also, the procedure lends itself to a systematic approach. In doing so, one can routinely ensure that the necessary practices are being done to ensure both patient and staff safety.
What’s the issue?
The implementation of checklists for medical procedures may seem straightforward; however, to be effective there has to be pertinent information gathered or actions taken. Our department utilizes checklists for our laser procedures. The different steps that we have included help to ensure that the nurses who prepare the patients will communicate the pertinent information to the physician. Our checklist starts with ensuring proper setup, such as connecting the necessary pieces, before starting the laser. Next is making sure all the necessary equipment is in place, such as the correct laser goggles for the patient and staff. We label all of our goggles with the name of the laser on them so it is readily apparent what to use. We also have a check box that ensures the proper signage is outside on the door and the laser shades are drawn. There also is a section relating to the patient. Depending on the type of laser being utilized the checklist may include the following: skin type, recent history of tanning, history of oral herpes, isotretinoin use, and time since last treatment. The next check box ensures consent is signed and pretreatment photographs are taken. Any prior complications also are noted. We have a section for treatment parameters used during the current visit and then another section for posttreatment instructions.
Although a checklist may seem like unnecessary work, we found that it actually helps to enhance productivity and ensures proper laser safety for the physician and patient. We have a different one for each laser, as it also serves as a documentation tool for the procedure. Do you utilize checklists for your laser procedures? If so, what else do you include?

Hamilton and Dover (Dermatol Surg. 2014;40:1173-1174) discussed the use of checklists for improving laser and light-based procedures in their November 2014 editorial. The authors discussed how the use of checklists has become pervasive in many fields of medicine, including surgery. However, they also highlighted that the utility of checklists and their success do not come from just checking off boxes but rather from actively performing the tasks. The implementation of a laser checklist can seem daunting, but Hamilton and Dover proposed that for a dermatology practice or dermatology department it becomes easier because the dermatologist and staff work closely already, making communication less of a challenge. Also, the procedure lends itself to a systematic approach. In doing so, one can routinely ensure that the necessary practices are being done to ensure both patient and staff safety.
What’s the issue?
The implementation of checklists for medical procedures may seem straightforward; however, to be effective there has to be pertinent information gathered or actions taken. Our department utilizes checklists for our laser procedures. The different steps that we have included help to ensure that the nurses who prepare the patients will communicate the pertinent information to the physician. Our checklist starts with ensuring proper setup, such as connecting the necessary pieces, before starting the laser. Next is making sure all the necessary equipment is in place, such as the correct laser goggles for the patient and staff. We label all of our goggles with the name of the laser on them so it is readily apparent what to use. We also have a check box that ensures the proper signage is outside on the door and the laser shades are drawn. There also is a section relating to the patient. Depending on the type of laser being utilized the checklist may include the following: skin type, recent history of tanning, history of oral herpes, isotretinoin use, and time since last treatment. The next check box ensures consent is signed and pretreatment photographs are taken. Any prior complications also are noted. We have a section for treatment parameters used during the current visit and then another section for posttreatment instructions.
Although a checklist may seem like unnecessary work, we found that it actually helps to enhance productivity and ensures proper laser safety for the physician and patient. We have a different one for each laser, as it also serves as a documentation tool for the procedure. Do you utilize checklists for your laser procedures? If so, what else do you include?

Hamilton and Dover (Dermatol Surg. 2014;40:1173-1174) discussed the use of checklists for improving laser and light-based procedures in their November 2014 editorial. The authors discussed how the use of checklists has become pervasive in many fields of medicine, including surgery. However, they also highlighted that the utility of checklists and their success do not come from just checking off boxes but rather from actively performing the tasks. The implementation of a laser checklist can seem daunting, but Hamilton and Dover proposed that for a dermatology practice or dermatology department it becomes easier because the dermatologist and staff work closely already, making communication less of a challenge. Also, the procedure lends itself to a systematic approach. In doing so, one can routinely ensure that the necessary practices are being done to ensure both patient and staff safety.
What’s the issue?
The implementation of checklists for medical procedures may seem straightforward; however, to be effective there has to be pertinent information gathered or actions taken. Our department utilizes checklists for our laser procedures. The different steps that we have included help to ensure that the nurses who prepare the patients will communicate the pertinent information to the physician. Our checklist starts with ensuring proper setup, such as connecting the necessary pieces, before starting the laser. Next is making sure all the necessary equipment is in place, such as the correct laser goggles for the patient and staff. We label all of our goggles with the name of the laser on them so it is readily apparent what to use. We also have a check box that ensures the proper signage is outside on the door and the laser shades are drawn. There also is a section relating to the patient. Depending on the type of laser being utilized the checklist may include the following: skin type, recent history of tanning, history of oral herpes, isotretinoin use, and time since last treatment. The next check box ensures consent is signed and pretreatment photographs are taken. Any prior complications also are noted. We have a section for treatment parameters used during the current visit and then another section for posttreatment instructions.
Although a checklist may seem like unnecessary work, we found that it actually helps to enhance productivity and ensures proper laser safety for the physician and patient. We have a different one for each laser, as it also serves as a documentation tool for the procedure. Do you utilize checklists for your laser procedures? If so, what else do you include?
The Top 100
In the October 2014 issue of the Journal of Clinical and Aesthetic Dermatology (2014;7:10-19), Wu et al published the top 100 most-cited psoriasis articles in clinical dermatologic journals from 1970 to 2012. Given the explosion of literature in this area, I was very excited to rush to find the list online.
The authors conducted a citation analysis of major clinical dermatologic journals from 1970 to 2012 limited to the subject of psoriasis. They used the search term psoriasis in the Science Citation Index from 1970 to 2012 and included articles that have received 100 or more citations. The top 100 articles were further stratified by country, institution, and study type.
The authors found that half of the top 100 cited articles were from the United States; 81 of them were original articles. The majority of the top 100 articles were from dermatology programs in the United States, but institutions in the United Kingdom and Germany also made notable contributions.
There were 2 periods of particular note in their analysis. The high numbers of citations from 1985 to 1989 correlated with the elucidation of the immune-mediated pathogenesis of psoriasis at that time. The high number of top citations from 2000 to 2004 correlated with the development of biologic agents in psoriasis therapy.
What’s the issue?
It is interesting to see which studies have been most influential in this highly active area of dermatology. What articles have most influenced your approach to psoriasis?
In the October 2014 issue of the Journal of Clinical and Aesthetic Dermatology (2014;7:10-19), Wu et al published the top 100 most-cited psoriasis articles in clinical dermatologic journals from 1970 to 2012. Given the explosion of literature in this area, I was very excited to rush to find the list online.
The authors conducted a citation analysis of major clinical dermatologic journals from 1970 to 2012 limited to the subject of psoriasis. They used the search term psoriasis in the Science Citation Index from 1970 to 2012 and included articles that have received 100 or more citations. The top 100 articles were further stratified by country, institution, and study type.
The authors found that half of the top 100 cited articles were from the United States; 81 of them were original articles. The majority of the top 100 articles were from dermatology programs in the United States, but institutions in the United Kingdom and Germany also made notable contributions.
There were 2 periods of particular note in their analysis. The high numbers of citations from 1985 to 1989 correlated with the elucidation of the immune-mediated pathogenesis of psoriasis at that time. The high number of top citations from 2000 to 2004 correlated with the development of biologic agents in psoriasis therapy.
What’s the issue?
It is interesting to see which studies have been most influential in this highly active area of dermatology. What articles have most influenced your approach to psoriasis?
In the October 2014 issue of the Journal of Clinical and Aesthetic Dermatology (2014;7:10-19), Wu et al published the top 100 most-cited psoriasis articles in clinical dermatologic journals from 1970 to 2012. Given the explosion of literature in this area, I was very excited to rush to find the list online.
The authors conducted a citation analysis of major clinical dermatologic journals from 1970 to 2012 limited to the subject of psoriasis. They used the search term psoriasis in the Science Citation Index from 1970 to 2012 and included articles that have received 100 or more citations. The top 100 articles were further stratified by country, institution, and study type.
The authors found that half of the top 100 cited articles were from the United States; 81 of them were original articles. The majority of the top 100 articles were from dermatology programs in the United States, but institutions in the United Kingdom and Germany also made notable contributions.
There were 2 periods of particular note in their analysis. The high numbers of citations from 1985 to 1989 correlated with the elucidation of the immune-mediated pathogenesis of psoriasis at that time. The high number of top citations from 2000 to 2004 correlated with the development of biologic agents in psoriasis therapy.
What’s the issue?
It is interesting to see which studies have been most influential in this highly active area of dermatology. What articles have most influenced your approach to psoriasis?
Practice Question Answers: Allergic Contact Dermatitis, Part 2
1. Which of the following is not a component of fragrance mix?
a. abietic acid
b. α-amylcinnamaldehyde
c. geraniol
d. hydroxycitronellal
e. oakmoss
2. A patient is referred for patch testing for suspected allergic contact dermatitis and is found to have positivity to disperse blue dye 106. The patient should avoid all of the following except:
a. black-colored clothing
b. pure acetate clothing
c. pure polyester clothing
d. purple-colored clothing
e. red-colored clothing
3. A patient with a documented contact allergy to ethylenediamine dihydrochloride should avoid all of the following systemic medications except:
a. aminophylline
b. disulfiram
c. hydroxyzine
d. meclizine
e. promethazine
4. Formaldehyde can cross-react with all of the following except:
a. diazolidinyl urea
b. DMDM hydantoin
c. imidazolidinyl urea
d. para-aminobenzoic acid
e. quaternium-15
5. Colophony can be found in all of the following trees except:
a. cedars
b. firs
c. junipers
d. maples
e. pines
1. Which of the following is not a component of fragrance mix?
a. abietic acid
b. α-amylcinnamaldehyde
c. geraniol
d. hydroxycitronellal
e. oakmoss
2. A patient is referred for patch testing for suspected allergic contact dermatitis and is found to have positivity to disperse blue dye 106. The patient should avoid all of the following except:
a. black-colored clothing
b. pure acetate clothing
c. pure polyester clothing
d. purple-colored clothing
e. red-colored clothing
3. A patient with a documented contact allergy to ethylenediamine dihydrochloride should avoid all of the following systemic medications except:
a. aminophylline
b. disulfiram
c. hydroxyzine
d. meclizine
e. promethazine
4. Formaldehyde can cross-react with all of the following except:
a. diazolidinyl urea
b. DMDM hydantoin
c. imidazolidinyl urea
d. para-aminobenzoic acid
e. quaternium-15
5. Colophony can be found in all of the following trees except:
a. cedars
b. firs
c. junipers
d. maples
e. pines
1. Which of the following is not a component of fragrance mix?
a. abietic acid
b. α-amylcinnamaldehyde
c. geraniol
d. hydroxycitronellal
e. oakmoss
2. A patient is referred for patch testing for suspected allergic contact dermatitis and is found to have positivity to disperse blue dye 106. The patient should avoid all of the following except:
a. black-colored clothing
b. pure acetate clothing
c. pure polyester clothing
d. purple-colored clothing
e. red-colored clothing
3. A patient with a documented contact allergy to ethylenediamine dihydrochloride should avoid all of the following systemic medications except:
a. aminophylline
b. disulfiram
c. hydroxyzine
d. meclizine
e. promethazine
4. Formaldehyde can cross-react with all of the following except:
a. diazolidinyl urea
b. DMDM hydantoin
c. imidazolidinyl urea
d. para-aminobenzoic acid
e. quaternium-15
5. Colophony can be found in all of the following trees except:
a. cedars
b. firs
c. junipers
d. maples
e. pines
Allergic Contact Dermatitis, Part 2
Applications of Lasers in Medical Dermatology
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
Top Dermatology Residency Programs
For more information, access Dr. Wu's article from the October 2014 issue, "US Dermatology Residency Program Rankings."
For more information, access Dr. Wu's article from the October 2014 issue, "US Dermatology Residency Program Rankings."
For more information, access Dr. Wu's article from the October 2014 issue, "US Dermatology Residency Program Rankings."
Hairs With an Irregular Shape
The Diagnosis: Circle Hairs
The patient’s hairs were visualized under dermoscopy (Figure 1). A skin biopsy showed a terminal hair in a horizontal distribution that was located beneath the stratum corneum (Figure 2). The patient was diagnosed with circle hairs.
Circle hairs were first described in 1963.1 These peculiar hairs grow in a circular horizontal distribution beneath the stratum corneum and are considered benign incidental findings. Their exact cause is unknown. If taken out and unrolled, their length and diameter tends to be smaller than surrounding hairs. It has been hypothesized that they are the result of hairs that lack the size necessary to perforate the stratum corneum.2 Others propose that they are vestigial remains that once had a part in preserving body heat.3 Circle hairs tend to grow in elderly, hairy, and obese males, predominantly on the torso and thighs.2,4
It is important to distinguish between circle hairs and rolled hairs. Rolled hairs may be found on the surface or beneath the stratum corneum and are associated with inflammation and keratinization abnormalities.2 If taken together, these latter findings can help differentiate between the two. The importance stands in recognizing that both circle hairs and rolled hairs are benign; however, rolled hairs can be related to other skin disorders that need additional treatment.
 |
 |
| Figure 2. A skin biopsy showed a terminal hair in a horizontal distribution that was located beneath the stratum corneum (A and B)(both Verhoeff-van Gieson, original magnifications ×40). |
1. Adatto R. Poils en spirale (poils enroules). Dermatologica. 1963;127:145-147.
2. Smith JB, Hogan DJ. Circle hairs are not rolled hairs. J Am Acad Dermatol. 1996;35:634-635.
3. Contreras-Ruiz J, Duran-McKinster C, Tamayo-Sanchez L, et al. Circle hairs: a clinical curiosity. J Eur Acad Dermatol Venereol. 2000;14:495-497.
4. Levit F, Scott MJ Jr. Circle hairs. J Am Acad Dermatol. 1983;8:423-425.
The Diagnosis: Circle Hairs
The patient’s hairs were visualized under dermoscopy (Figure 1). A skin biopsy showed a terminal hair in a horizontal distribution that was located beneath the stratum corneum (Figure 2). The patient was diagnosed with circle hairs.
Circle hairs were first described in 1963.1 These peculiar hairs grow in a circular horizontal distribution beneath the stratum corneum and are considered benign incidental findings. Their exact cause is unknown. If taken out and unrolled, their length and diameter tends to be smaller than surrounding hairs. It has been hypothesized that they are the result of hairs that lack the size necessary to perforate the stratum corneum.2 Others propose that they are vestigial remains that once had a part in preserving body heat.3 Circle hairs tend to grow in elderly, hairy, and obese males, predominantly on the torso and thighs.2,4
It is important to distinguish between circle hairs and rolled hairs. Rolled hairs may be found on the surface or beneath the stratum corneum and are associated with inflammation and keratinization abnormalities.2 If taken together, these latter findings can help differentiate between the two. The importance stands in recognizing that both circle hairs and rolled hairs are benign; however, rolled hairs can be related to other skin disorders that need additional treatment.
 |
 |
| Figure 2. A skin biopsy showed a terminal hair in a horizontal distribution that was located beneath the stratum corneum (A and B)(both Verhoeff-van Gieson, original magnifications ×40). |
The Diagnosis: Circle Hairs
The patient’s hairs were visualized under dermoscopy (Figure 1). A skin biopsy showed a terminal hair in a horizontal distribution that was located beneath the stratum corneum (Figure 2). The patient was diagnosed with circle hairs.
Circle hairs were first described in 1963.1 These peculiar hairs grow in a circular horizontal distribution beneath the stratum corneum and are considered benign incidental findings. Their exact cause is unknown. If taken out and unrolled, their length and diameter tends to be smaller than surrounding hairs. It has been hypothesized that they are the result of hairs that lack the size necessary to perforate the stratum corneum.2 Others propose that they are vestigial remains that once had a part in preserving body heat.3 Circle hairs tend to grow in elderly, hairy, and obese males, predominantly on the torso and thighs.2,4
It is important to distinguish between circle hairs and rolled hairs. Rolled hairs may be found on the surface or beneath the stratum corneum and are associated with inflammation and keratinization abnormalities.2 If taken together, these latter findings can help differentiate between the two. The importance stands in recognizing that both circle hairs and rolled hairs are benign; however, rolled hairs can be related to other skin disorders that need additional treatment.
 |
 |
| Figure 2. A skin biopsy showed a terminal hair in a horizontal distribution that was located beneath the stratum corneum (A and B)(both Verhoeff-van Gieson, original magnifications ×40). |
1. Adatto R. Poils en spirale (poils enroules). Dermatologica. 1963;127:145-147.
2. Smith JB, Hogan DJ. Circle hairs are not rolled hairs. J Am Acad Dermatol. 1996;35:634-635.
3. Contreras-Ruiz J, Duran-McKinster C, Tamayo-Sanchez L, et al. Circle hairs: a clinical curiosity. J Eur Acad Dermatol Venereol. 2000;14:495-497.
4. Levit F, Scott MJ Jr. Circle hairs. J Am Acad Dermatol. 1983;8:423-425.
1. Adatto R. Poils en spirale (poils enroules). Dermatologica. 1963;127:145-147.
2. Smith JB, Hogan DJ. Circle hairs are not rolled hairs. J Am Acad Dermatol. 1996;35:634-635.
3. Contreras-Ruiz J, Duran-McKinster C, Tamayo-Sanchez L, et al. Circle hairs: a clinical curiosity. J Eur Acad Dermatol Venereol. 2000;14:495-497.
4. Levit F, Scott MJ Jr. Circle hairs. J Am Acad Dermatol. 1983;8:423-425.
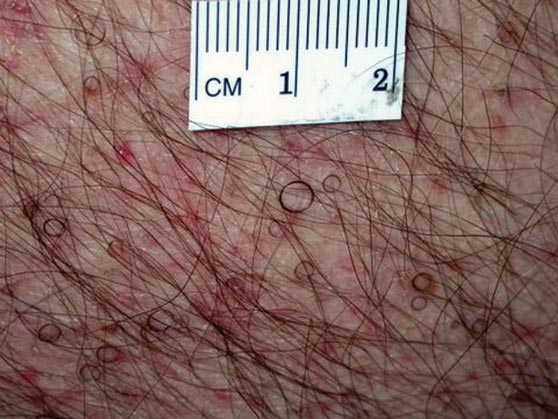
A 74-year-old man was evaluated for numerous peculiar hairs on the back that had been present for several years. He reported no other dermatologic concerns. The patient was obese and led a sedentary lifestyle, spending most of the day sitting or lying down. Physical examination revealed a hairy back with many irregularly shaped hairs.
Hypopigmented Facial Papules on the Cheeks
The Diagnosis: Tumor of the Follicular Infundibulum
Histopathologic findings from a facial papule in our patient revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (Figure). There was no atypia. Gomori methenamine-silver and periodic acid–Schiff stains for fungi were negative. The combined clinical presentation and histopathologic findings supported the diagnosis of multiple tumor of the follicular infundibulum (TFI).
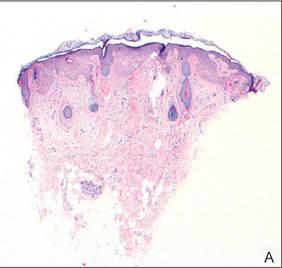 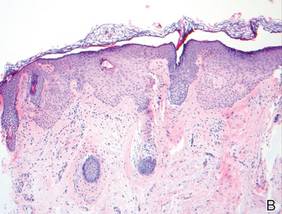 Tumor of the follicular infundibulum was diagnosed based on a biopsy from the right cheek that revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (A and B)(H&E, original magnifications ×40 and ×100). |
Tumor of the follicular infundibulum is an uncommon benign neoplasm that was first described in 1961 by Mehregan and Butler.1 The reported frequency is 10 per 100,000 biopsies.2 The majority of cases have been reported as solitary lesions, and multiple TFI are rare.3 Tumor of the follicular infundibulum affects middle-aged and elderly individuals with a female predominance.4 Multiple lesions generally range in number from 10 to 20, but there are few reports of more than 100 lesions.2,3,5,6 The solitary tumors often are initially misdiagnosed as basal cell carcinomas (BCCs) or seborrheic keratosis. Multiple TFI have been described variably as hypopigmented, flesh-colored and pink, flat and slightly depressed macules and thin papules. Sites of predilection include the scalp, face, neck, and upper trunk.2,3,5
There is no histopathologic difference between solitary and multiple TFI. Tumor of the follicular infundibulum displays a characteristic pale platelike proliferation of keratinocytes within the upper dermis attached to the overlying epidermis. The proliferating cells stain positive with periodic acid–Schiff, diastase-digestible glycogen is present in the cells at the base of the tumor, and a thickened network or brushlike pattern of elastic fibers surrounds the periphery of the tumor.1 Tumor of the follicular infundibulum is occasionally discovered incidentally on biopsy and has been observed in the margin of wide excisions of a variety of neoplasms including BCC.7 Based on the close association of TFI and BCC in the same specimens, Weyers et al7 concluded that TFI may be a nonaggressive type of BCC. Cribier and Grosshans2 reported 2 cases of TFI overlying a nevus sebaceous and a fibroma.
Treatment of TFI includes topical keratolytics, topical retinoic acid,5 imiquimod,8 topical steroids, and oral etretinate,6 all of which result in minimal improvement or incomplete resolution. Destructive treatments include cryotherapy, curettage, electrosurgery, laser ablation, and surgical excision, but all may lead to an unacceptable cosmetic result.
1. Mehregan AH, Butler JD. A tumor of follicular infundibulum. Arch Dermatol. 1961;83:78-81.
2. Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
3. Kolenik SA 3rd, Bolognia JL, Castiglione FM Jr, et al. Multiple tumors of the follicular infundibulum. Int J Dermatol. 1996;35:282-284.
4. Ackerman AB, Reddy VB, Soyer HP. Neoplasms With Follicular Differentiation. New York, NY: Ardor Scribendi; 2001.
5. Kossard S, Finley AG, Poyzer K, et al. Eruptive infundibulomas. J Am Acad Dermatol. 1989;21:361-366.
6. Schnitzler L, Civatte J, Robin F, et al. Multiple tumors of the follicular infundibulum with basocellular degeneration. apropos of a case [in French]. Ann Dermatol Venereol. 1987;114:551-556.
7. Weyers W, Horster S, Diaz-Cascajo C. Tumor of follicular infundibulum is basal cell carcinoma. Am J Dermatopathol. 2009;31:634-641.
8. Martin JE, Hsu M, Wang LC. An unusual clinical presentation of multiple tumors of the follicular infundibulum. J Am Acad Dermatol. 2009;60:885-886.
The Diagnosis: Tumor of the Follicular Infundibulum
Histopathologic findings from a facial papule in our patient revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (Figure). There was no atypia. Gomori methenamine-silver and periodic acid–Schiff stains for fungi were negative. The combined clinical presentation and histopathologic findings supported the diagnosis of multiple tumor of the follicular infundibulum (TFI).
  Tumor of the follicular infundibulum was diagnosed based on a biopsy from the right cheek that revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (A and B)(H&E, original magnifications ×40 and ×100). |
Tumor of the follicular infundibulum is an uncommon benign neoplasm that was first described in 1961 by Mehregan and Butler.1 The reported frequency is 10 per 100,000 biopsies.2 The majority of cases have been reported as solitary lesions, and multiple TFI are rare.3 Tumor of the follicular infundibulum affects middle-aged and elderly individuals with a female predominance.4 Multiple lesions generally range in number from 10 to 20, but there are few reports of more than 100 lesions.2,3,5,6 The solitary tumors often are initially misdiagnosed as basal cell carcinomas (BCCs) or seborrheic keratosis. Multiple TFI have been described variably as hypopigmented, flesh-colored and pink, flat and slightly depressed macules and thin papules. Sites of predilection include the scalp, face, neck, and upper trunk.2,3,5
There is no histopathologic difference between solitary and multiple TFI. Tumor of the follicular infundibulum displays a characteristic pale platelike proliferation of keratinocytes within the upper dermis attached to the overlying epidermis. The proliferating cells stain positive with periodic acid–Schiff, diastase-digestible glycogen is present in the cells at the base of the tumor, and a thickened network or brushlike pattern of elastic fibers surrounds the periphery of the tumor.1 Tumor of the follicular infundibulum is occasionally discovered incidentally on biopsy and has been observed in the margin of wide excisions of a variety of neoplasms including BCC.7 Based on the close association of TFI and BCC in the same specimens, Weyers et al7 concluded that TFI may be a nonaggressive type of BCC. Cribier and Grosshans2 reported 2 cases of TFI overlying a nevus sebaceous and a fibroma.
Treatment of TFI includes topical keratolytics, topical retinoic acid,5 imiquimod,8 topical steroids, and oral etretinate,6 all of which result in minimal improvement or incomplete resolution. Destructive treatments include cryotherapy, curettage, electrosurgery, laser ablation, and surgical excision, but all may lead to an unacceptable cosmetic result.
The Diagnosis: Tumor of the Follicular Infundibulum
Histopathologic findings from a facial papule in our patient revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (Figure). There was no atypia. Gomori methenamine-silver and periodic acid–Schiff stains for fungi were negative. The combined clinical presentation and histopathologic findings supported the diagnosis of multiple tumor of the follicular infundibulum (TFI).
  Tumor of the follicular infundibulum was diagnosed based on a biopsy from the right cheek that revealed multifocal hyperplasia of anastomosing follicular infundibular cells with multiple connections to the overlying epidermis (A and B)(H&E, original magnifications ×40 and ×100). |
Tumor of the follicular infundibulum is an uncommon benign neoplasm that was first described in 1961 by Mehregan and Butler.1 The reported frequency is 10 per 100,000 biopsies.2 The majority of cases have been reported as solitary lesions, and multiple TFI are rare.3 Tumor of the follicular infundibulum affects middle-aged and elderly individuals with a female predominance.4 Multiple lesions generally range in number from 10 to 20, but there are few reports of more than 100 lesions.2,3,5,6 The solitary tumors often are initially misdiagnosed as basal cell carcinomas (BCCs) or seborrheic keratosis. Multiple TFI have been described variably as hypopigmented, flesh-colored and pink, flat and slightly depressed macules and thin papules. Sites of predilection include the scalp, face, neck, and upper trunk.2,3,5
There is no histopathologic difference between solitary and multiple TFI. Tumor of the follicular infundibulum displays a characteristic pale platelike proliferation of keratinocytes within the upper dermis attached to the overlying epidermis. The proliferating cells stain positive with periodic acid–Schiff, diastase-digestible glycogen is present in the cells at the base of the tumor, and a thickened network or brushlike pattern of elastic fibers surrounds the periphery of the tumor.1 Tumor of the follicular infundibulum is occasionally discovered incidentally on biopsy and has been observed in the margin of wide excisions of a variety of neoplasms including BCC.7 Based on the close association of TFI and BCC in the same specimens, Weyers et al7 concluded that TFI may be a nonaggressive type of BCC. Cribier and Grosshans2 reported 2 cases of TFI overlying a nevus sebaceous and a fibroma.
Treatment of TFI includes topical keratolytics, topical retinoic acid,5 imiquimod,8 topical steroids, and oral etretinate,6 all of which result in minimal improvement or incomplete resolution. Destructive treatments include cryotherapy, curettage, electrosurgery, laser ablation, and surgical excision, but all may lead to an unacceptable cosmetic result.
1. Mehregan AH, Butler JD. A tumor of follicular infundibulum. Arch Dermatol. 1961;83:78-81.
2. Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
3. Kolenik SA 3rd, Bolognia JL, Castiglione FM Jr, et al. Multiple tumors of the follicular infundibulum. Int J Dermatol. 1996;35:282-284.
4. Ackerman AB, Reddy VB, Soyer HP. Neoplasms With Follicular Differentiation. New York, NY: Ardor Scribendi; 2001.
5. Kossard S, Finley AG, Poyzer K, et al. Eruptive infundibulomas. J Am Acad Dermatol. 1989;21:361-366.
6. Schnitzler L, Civatte J, Robin F, et al. Multiple tumors of the follicular infundibulum with basocellular degeneration. apropos of a case [in French]. Ann Dermatol Venereol. 1987;114:551-556.
7. Weyers W, Horster S, Diaz-Cascajo C. Tumor of follicular infundibulum is basal cell carcinoma. Am J Dermatopathol. 2009;31:634-641.
8. Martin JE, Hsu M, Wang LC. An unusual clinical presentation of multiple tumors of the follicular infundibulum. J Am Acad Dermatol. 2009;60:885-886.
1. Mehregan AH, Butler JD. A tumor of follicular infundibulum. Arch Dermatol. 1961;83:78-81.
2. Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
3. Kolenik SA 3rd, Bolognia JL, Castiglione FM Jr, et al. Multiple tumors of the follicular infundibulum. Int J Dermatol. 1996;35:282-284.
4. Ackerman AB, Reddy VB, Soyer HP. Neoplasms With Follicular Differentiation. New York, NY: Ardor Scribendi; 2001.
5. Kossard S, Finley AG, Poyzer K, et al. Eruptive infundibulomas. J Am Acad Dermatol. 1989;21:361-366.
6. Schnitzler L, Civatte J, Robin F, et al. Multiple tumors of the follicular infundibulum with basocellular degeneration. apropos of a case [in French]. Ann Dermatol Venereol. 1987;114:551-556.
7. Weyers W, Horster S, Diaz-Cascajo C. Tumor of follicular infundibulum is basal cell carcinoma. Am J Dermatopathol. 2009;31:634-641.
8. Martin JE, Hsu M, Wang LC. An unusual clinical presentation of multiple tumors of the follicular infundibulum. J Am Acad Dermatol. 2009;60:885-886.
A 73-year-old woman presented with multiple mildly pruritic, hypopigmented, thin papules involving both cheeks of 5 months’ duration. The patient had no improvement with ketoconazole cream 2% and hydrocortisone cream 1% used daily for 1 month for presumed tinea versicolor. Physical examination revealed 10 ill-defined, 2- to 5-mm, round and oval, smooth hypopigmented, slightly raised papules located on the lower aspect of both cheeks.
Old Concept, New Drug: Topical Application of Systemic Antineoplastic Agent to Treat Skin Cancer

In a June 29 article published online in Molecular Carcinogenesis, Fenton et al demonstrated that topical dasatinib treatment of UVB-exposed SKH1 hairless mice reduced the total tumor burden (ie, benign tumors, atypical benign tumors, squamous cell carcinomas) per mouse.
Dasatinib is a tyrosine kinase inhibitor currently used to treat imatinib-resistant chronic myeloid leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia. Its mechanism of action is to block the activity of tyrosine kinases by attaching to their adenosine triphosphate–binding site. It can inhibit the activity of the following tyrosine kinases: Src family kinases (SFK), break point cluster region-Abelson (Bcr-Abl), Ephrin type-A receptor 2 (EphA2), platelet-derived growth factor receptor, and mast/stem cell factor receptor (also called CD117 or c-Kit).
Src family kinases are associated with transformation of cells and progression of cancer. Elevated Src family kinases activity is present in the majority of human carcinomas.
Fyn, a nonreceptor tyrosine kinase, is a member of the Src family kinases. Cell growth, cell migration, and protein kinase B (Akt)–mediated inhibition of apoptosis are influenced by Fyn activity. In addition, Fyn activity is overexpressed in cutaneous squamous cell carcinoma.
In conclusion, dasatinib—an Src family kinases inhibitor—was able to inhibit Fyn activity and thereby reduce UV-induced skin carcinogenesis.
What’s the issue?
In 1962, Falkson and Schulz (Br J Dermatol. 1962;74:229-236) noted not only inflammation and subsequent resolution of actinic keratoses after exposure to sunlight in a woman with colon cancer being treated with systemic 5-fluorouracil but also several other patients whose keratoses were seen to disappear during therapy without preceding erythema. Omura and Torre (JAMA. 1969;208:150-151) confirmed these observations in a woman with breast cancer whose actinic keratoses became inflamed after receiving intravenous 5-fluorouracil and subsequently faded. The route of drug administration was modified and 5-fluorouracil was applied topically. Today topical 5-fluorouracil is still used for the treatment of actinic keratoses.
Topical application of nitrogen mustard is used in the treatment of cutaneous T-cell lymphoma. In addition, intralesional administration of systemic antineoplastic agents has been used to treat cutaneous neoplasms: methotrexate for keratoacanthomas and rituximab for primary cutaneous B-cell lymphomas.
The Fenton et al study suggests that topical dasatinib may be a potential therapeutic intervention for the treatment of cutaneous squamous cell carcinoma. The investigators not only demonstrate laboratory data from mouse studies but also provide a potential molecular mechanism for drug-associated tumor suppression. Indeed, dasatinib solution, dasatinib cream, or both may be the next innovative therapy for the suppression of cutaneous squamous cell carcinoma in organ transplant and immunocompromised patients and for the potential management of this skin cancer in immunocompetent individuals. What do you think?

In a June 29 article published online in Molecular Carcinogenesis, Fenton et al demonstrated that topical dasatinib treatment of UVB-exposed SKH1 hairless mice reduced the total tumor burden (ie, benign tumors, atypical benign tumors, squamous cell carcinomas) per mouse.
Dasatinib is a tyrosine kinase inhibitor currently used to treat imatinib-resistant chronic myeloid leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia. Its mechanism of action is to block the activity of tyrosine kinases by attaching to their adenosine triphosphate–binding site. It can inhibit the activity of the following tyrosine kinases: Src family kinases (SFK), break point cluster region-Abelson (Bcr-Abl), Ephrin type-A receptor 2 (EphA2), platelet-derived growth factor receptor, and mast/stem cell factor receptor (also called CD117 or c-Kit).
Src family kinases are associated with transformation of cells and progression of cancer. Elevated Src family kinases activity is present in the majority of human carcinomas.
Fyn, a nonreceptor tyrosine kinase, is a member of the Src family kinases. Cell growth, cell migration, and protein kinase B (Akt)–mediated inhibition of apoptosis are influenced by Fyn activity. In addition, Fyn activity is overexpressed in cutaneous squamous cell carcinoma.
In conclusion, dasatinib—an Src family kinases inhibitor—was able to inhibit Fyn activity and thereby reduce UV-induced skin carcinogenesis.
What’s the issue?
In 1962, Falkson and Schulz (Br J Dermatol. 1962;74:229-236) noted not only inflammation and subsequent resolution of actinic keratoses after exposure to sunlight in a woman with colon cancer being treated with systemic 5-fluorouracil but also several other patients whose keratoses were seen to disappear during therapy without preceding erythema. Omura and Torre (JAMA. 1969;208:150-151) confirmed these observations in a woman with breast cancer whose actinic keratoses became inflamed after receiving intravenous 5-fluorouracil and subsequently faded. The route of drug administration was modified and 5-fluorouracil was applied topically. Today topical 5-fluorouracil is still used for the treatment of actinic keratoses.
Topical application of nitrogen mustard is used in the treatment of cutaneous T-cell lymphoma. In addition, intralesional administration of systemic antineoplastic agents has been used to treat cutaneous neoplasms: methotrexate for keratoacanthomas and rituximab for primary cutaneous B-cell lymphomas.
The Fenton et al study suggests that topical dasatinib may be a potential therapeutic intervention for the treatment of cutaneous squamous cell carcinoma. The investigators not only demonstrate laboratory data from mouse studies but also provide a potential molecular mechanism for drug-associated tumor suppression. Indeed, dasatinib solution, dasatinib cream, or both may be the next innovative therapy for the suppression of cutaneous squamous cell carcinoma in organ transplant and immunocompromised patients and for the potential management of this skin cancer in immunocompetent individuals. What do you think?

In a June 29 article published online in Molecular Carcinogenesis, Fenton et al demonstrated that topical dasatinib treatment of UVB-exposed SKH1 hairless mice reduced the total tumor burden (ie, benign tumors, atypical benign tumors, squamous cell carcinomas) per mouse.
Dasatinib is a tyrosine kinase inhibitor currently used to treat imatinib-resistant chronic myeloid leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia. Its mechanism of action is to block the activity of tyrosine kinases by attaching to their adenosine triphosphate–binding site. It can inhibit the activity of the following tyrosine kinases: Src family kinases (SFK), break point cluster region-Abelson (Bcr-Abl), Ephrin type-A receptor 2 (EphA2), platelet-derived growth factor receptor, and mast/stem cell factor receptor (also called CD117 or c-Kit).
Src family kinases are associated with transformation of cells and progression of cancer. Elevated Src family kinases activity is present in the majority of human carcinomas.
Fyn, a nonreceptor tyrosine kinase, is a member of the Src family kinases. Cell growth, cell migration, and protein kinase B (Akt)–mediated inhibition of apoptosis are influenced by Fyn activity. In addition, Fyn activity is overexpressed in cutaneous squamous cell carcinoma.
In conclusion, dasatinib—an Src family kinases inhibitor—was able to inhibit Fyn activity and thereby reduce UV-induced skin carcinogenesis.
What’s the issue?
In 1962, Falkson and Schulz (Br J Dermatol. 1962;74:229-236) noted not only inflammation and subsequent resolution of actinic keratoses after exposure to sunlight in a woman with colon cancer being treated with systemic 5-fluorouracil but also several other patients whose keratoses were seen to disappear during therapy without preceding erythema. Omura and Torre (JAMA. 1969;208:150-151) confirmed these observations in a woman with breast cancer whose actinic keratoses became inflamed after receiving intravenous 5-fluorouracil and subsequently faded. The route of drug administration was modified and 5-fluorouracil was applied topically. Today topical 5-fluorouracil is still used for the treatment of actinic keratoses.
Topical application of nitrogen mustard is used in the treatment of cutaneous T-cell lymphoma. In addition, intralesional administration of systemic antineoplastic agents has been used to treat cutaneous neoplasms: methotrexate for keratoacanthomas and rituximab for primary cutaneous B-cell lymphomas.
The Fenton et al study suggests that topical dasatinib may be a potential therapeutic intervention for the treatment of cutaneous squamous cell carcinoma. The investigators not only demonstrate laboratory data from mouse studies but also provide a potential molecular mechanism for drug-associated tumor suppression. Indeed, dasatinib solution, dasatinib cream, or both may be the next innovative therapy for the suppression of cutaneous squamous cell carcinoma in organ transplant and immunocompromised patients and for the potential management of this skin cancer in immunocompetent individuals. What do you think?