User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Product News: 11 2014
Effaclar Dermatological Acne System
La Roche-Posay Laboratoire Dermatologique introduces the Effaclar Dermatological Acne System, an over-the-counter system to cleanse, tone, and treat using 3 products: Effaclar Medicated Gel Cleanser, Effaclar Clarifying Solution, and Effaclar Duo. The system is designed to reduce acne with little to no drying or irritation. The Effaclar Dermatological Acne System targets excess sebum, hyperkeratinization, and the main triggers of acne. It can be purchased at select physicians’ offices and pharmacies as well as online. For more information, visit www.laroche-posay.us.
Hydrate Moisturizers
Obagi Medical Products, Inc, introduces 2 moisturizers that provide long-lasting hydration: Hydrate Facial Moisturizer and Hydrate Luxe. Hydrate Facial Moisturizer provides all-day moisture protection for every skin type with immediate and lasting effects on skin barrier function. Hydrate Luxe is engineered with biomimetic peptides to work overnight. It saturates skin with 8-hour moisture protection, and also promotes skin radiance. Both products are dispensed in dermatology, plastic surgery, and other aesthetic physicians’ practices. For more information, visit www.obagi.com.
Stretch Mark Crème
Natural skin care company derma e reveals Stretch Mark Crème to visibly diminish the look of stretch marks, as well as improve texture, color, and overall appearance. Stretch Mark Crème contains argan oil, cocoa butter, coconut oil, and shea butter to intensely condition skin to help increase elasticity and resiliency. It also contains vitamin E to promote self-healing and hyaluronic acid to attract and bind moisture to the skin. For more information, visit www.dermae.com.
Taclonex
LEO Pharma Inc announces a new pediatric indication for Taclonex (calcipotriene 0.005%–betamethasone dipropionate 0.064%) Topical Suspension. Taclonex is a first-line, once-daily combination product indicated for treatment of both scalp and body psoriasis in adults 18 years and older for up to 8 weeks. It is now also indicated for the treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years for the same period. For more information, visit www.taclonex.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com
Effaclar Dermatological Acne System
La Roche-Posay Laboratoire Dermatologique introduces the Effaclar Dermatological Acne System, an over-the-counter system to cleanse, tone, and treat using 3 products: Effaclar Medicated Gel Cleanser, Effaclar Clarifying Solution, and Effaclar Duo. The system is designed to reduce acne with little to no drying or irritation. The Effaclar Dermatological Acne System targets excess sebum, hyperkeratinization, and the main triggers of acne. It can be purchased at select physicians’ offices and pharmacies as well as online. For more information, visit www.laroche-posay.us.
Hydrate Moisturizers
Obagi Medical Products, Inc, introduces 2 moisturizers that provide long-lasting hydration: Hydrate Facial Moisturizer and Hydrate Luxe. Hydrate Facial Moisturizer provides all-day moisture protection for every skin type with immediate and lasting effects on skin barrier function. Hydrate Luxe is engineered with biomimetic peptides to work overnight. It saturates skin with 8-hour moisture protection, and also promotes skin radiance. Both products are dispensed in dermatology, plastic surgery, and other aesthetic physicians’ practices. For more information, visit www.obagi.com.
Stretch Mark Crème
Natural skin care company derma e reveals Stretch Mark Crème to visibly diminish the look of stretch marks, as well as improve texture, color, and overall appearance. Stretch Mark Crème contains argan oil, cocoa butter, coconut oil, and shea butter to intensely condition skin to help increase elasticity and resiliency. It also contains vitamin E to promote self-healing and hyaluronic acid to attract and bind moisture to the skin. For more information, visit www.dermae.com.
Taclonex
LEO Pharma Inc announces a new pediatric indication for Taclonex (calcipotriene 0.005%–betamethasone dipropionate 0.064%) Topical Suspension. Taclonex is a first-line, once-daily combination product indicated for treatment of both scalp and body psoriasis in adults 18 years and older for up to 8 weeks. It is now also indicated for the treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years for the same period. For more information, visit www.taclonex.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com
Effaclar Dermatological Acne System
La Roche-Posay Laboratoire Dermatologique introduces the Effaclar Dermatological Acne System, an over-the-counter system to cleanse, tone, and treat using 3 products: Effaclar Medicated Gel Cleanser, Effaclar Clarifying Solution, and Effaclar Duo. The system is designed to reduce acne with little to no drying or irritation. The Effaclar Dermatological Acne System targets excess sebum, hyperkeratinization, and the main triggers of acne. It can be purchased at select physicians’ offices and pharmacies as well as online. For more information, visit www.laroche-posay.us.
Hydrate Moisturizers
Obagi Medical Products, Inc, introduces 2 moisturizers that provide long-lasting hydration: Hydrate Facial Moisturizer and Hydrate Luxe. Hydrate Facial Moisturizer provides all-day moisture protection for every skin type with immediate and lasting effects on skin barrier function. Hydrate Luxe is engineered with biomimetic peptides to work overnight. It saturates skin with 8-hour moisture protection, and also promotes skin radiance. Both products are dispensed in dermatology, plastic surgery, and other aesthetic physicians’ practices. For more information, visit www.obagi.com.
Stretch Mark Crème
Natural skin care company derma e reveals Stretch Mark Crème to visibly diminish the look of stretch marks, as well as improve texture, color, and overall appearance. Stretch Mark Crème contains argan oil, cocoa butter, coconut oil, and shea butter to intensely condition skin to help increase elasticity and resiliency. It also contains vitamin E to promote self-healing and hyaluronic acid to attract and bind moisture to the skin. For more information, visit www.dermae.com.
Taclonex
LEO Pharma Inc announces a new pediatric indication for Taclonex (calcipotriene 0.005%–betamethasone dipropionate 0.064%) Topical Suspension. Taclonex is a first-line, once-daily combination product indicated for treatment of both scalp and body psoriasis in adults 18 years and older for up to 8 weeks. It is now also indicated for the treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years for the same period. For more information, visit www.taclonex.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com
Efficacy of Cryosurgery and 5-Fluorouracil Cream 0.5% Combination Therapy for the Treatment of Actinic Keratosis
Actinic keratosis (AK) is regarded as a lesion on a continuum of progression to squamous cell carcinoma (SCC).1 Studies have estimated that 44% to 97% of SCCs were associated with AK lesions either in contiguous skin or within the same histologic section and that AK lesions progress to SCCs at a rate of 0.6% at 1 year.2 In 1993-1994 there were 3.7 million reported office visits for AK lesions, while in 2002 alone there were 8.2 million office visits.3,4 As the burden of disease from AKs has increased, so has the associated costs from office-based visits, treatments, and subsequent surveillance.
There are a number of highly effective approaches to AK treatment that are based on several factors such as the number of and extent of the lesions, history of skin cancer, provider practice characteristics (eg, location, appointment availability), patient preferences, cost, and tolerability. Cryosurgery is the most commonly used lesion-directed modality in the treatment of individual AKs based on its effectiveness and relative ease of use. Cryosurgery alone has been shown to have a success rate of 67% on AK lesions.5 Patients often experience erythema, edema, pain, and crusting at treated sites; there also is potential for ulceration, scarring, hypopigmentation, hyperpigmentation, and secondary infection, but these effects are less common. Recurrence may be an indicator of treatment-resistant lesions or new lesions appearing in the field.
A field-directed approach with topical 5-fluorouracil (5-FU) may be preferred in patients with a history of substantial photodamage, AKs that are resistant to cryosurgery, or multiple AKs. Field-directed treatments address multiple AKs simultaneously and treat subclinical lesions. Fluorouracil is a common therapy for AKs that often is implemented by dermatologists due to its efficacy and well-understood mechanism of action. Fluorouracil inhibits thymidylate synthase during DNA synthesis, thereby halting cellular proliferation. 5-Fluorouracil cream 0.5% has been approved for 1-, 2-, and 4-week treatment periods. In one study, resolution of AK lesions was greatest in the 4-week treatment group; however, side effects also were greatest in this group.6 Patients commonly may experience a range of local reactions including erythema, pruritus, erosions, ulcerations, scabbing, crusting, and facial irritation. For patients with substantial photodamage and AKs, a robust response can lead to perceived adverse events (AEs) and considerable downtime, possibly affecting patient satisfaction and treatment compliance.7
Many alternative and combination approaches have been studied to decrease AEs and improve compliance and efficacy in the treatment of AKs. In this study, we examined the efficacy and perceived side effects of cryosurgery and 5-FU cream 0.5% combination therapy in the treatment of AKs.
Methods
Study Design and Participants
This single-blind, single-center, comparator cream–controlled pilot study was parallel designed with a balanced randomization (1:1 frequency). The study protocol and consent form were approved by the Wake Forest University Health Sciences institutional review board (Winston-Salem, North Carolina). Participants were 18 years or older with 8 clinically typical, visible, and discrete AK lesions on the face (forehead and temples) or balding scalp. Typical inclusion and exclusion criteria were observed. No other topical agents or therapies were permitted to be applied to the affected areas at least 4 weeks prior to treatment, depending on the treatment modality.
Assessment
During the screening (baseline) visit, eligible participants provided informed consent, baseline lesion counts and investigator global assessments (IGAs) were performed, and cryosurgery was administered to all visible AK lesions in the study areas. Participants returned at weeks 3, 4, 8, and 26. Three weeks following cryosurgery, participants were randomized according to standard randomization tables into 1 of 2 treatment groups to receive once-daily treatment with either 5-FU cream 0.5% or a moisturizing comparator cream. The cream was applied at bedtime to the affected sites for 1 week. Randomization was investigator blinded, but participants and the study administrators were not blinded. Participants were instructed to record their treatment compliance in daily diary entries, which were reviewed at week 4 using the medication tolerability assessment rating for burning, stinging, and ulceration. Investigator global assessment, IGA of improvement, lesion counts, and quality of life (QOL) survey responses were gathered at weeks 3, 8, and 26. The IGA measured the overall severity of AK disease involvement on a 6-point scale (clear; very severe). The IGA of improvement measured the overall improvement from baseline on a 6-point scale (clear; worse). Adverse events were measured at each visit.
Efficacy End Points
The primary end point was 100% clearance of all AK lesions at the end of the study (week 26) relative to the baseline AK lesion count. Secondary end points included comparisons between the groups for the number of participants with greater than 75% reduction of baseline lesion counts at the end of the study as well as differences at each visit in medication tolerability assessments, QOL measures, IGA improvement scores, and medication adherence based on diary entries at week 4.
Statistical Analysis
An intention-to-treat analysis was performed. The number of participants with 100% or greater than 75% clearance of AK lesions by specified time points were compared using relative risks and risk differences with Poisson regression analysis log and identity link functions, respectively, to obtain robust error variance 95% confidence intervals. Medication tolerability assessment, QOL, and IGA improvement scores were compared between the 2 groups using the Mann-Whitney U test. The significance level was set at α=.05. All analyses were performed using SAS data analysis software.
Results
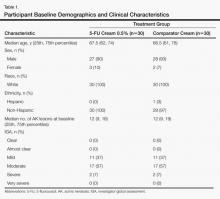
Sixty age-eligible participants were enrolled in the study with 30 participants in each treatment group. All of the participants completed the 26-week study period and were included in the intention-to-treat analysis. All of the participants were white with a median age of 67 years; the median number of baseline AK lesions was 12. Participant baseline demographics and clinical characteristics are provided in Table 1. Treatment compliance in both groups was good with only a few participants reporting missed doses.
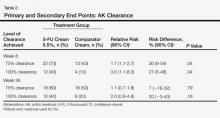
In our evaluation of the rate of change in the number of AK lesions at week 8 compared to baseline, the 5-FU cream 0.5% group showed an 84% reduction in the number of AK lesions versus a 69% reduction in the control group. At week 26, the 5-FU cream 0.5% group showed a 72% reduction in the number of lesions versus 73% in the control group. There was no significant difference between 5-FU cream 0.5% and the comparator cream for either 100% or 75% clearance of AK lesions by the end of the study; however, comparing the AK lesion count from baseline to 8 weeks following the initiation of the study, participants in the 5-FU cream 0.5% group were more likely than the control group to achieve 75% or 100% clearance on the relative risk and risk difference scales (Table 2).
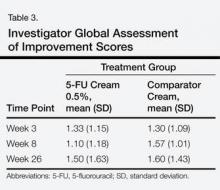
There were no significant differences between the 2 groups for the IGA of improvement at any time point (Table 3). On average, participants in the 5-FU cream 0.5% group experienced more dryness, erosion, fissuring, and redness than the control group but not more ulcerations by the end of week 4 (Table 4). All other QOL measures were statistically comparable between the 2 treatment groups for all time points.
A total of 25 AEs were reported throughout the study but none were considered to be serious. One AE (redness, burning, and itching over the eyebrow) was considered to be related to the study drug. No participants withdrew from the study due to AEs. A total of 12 participants in the 5-FU cream 0.5% group and 10 in the control group reported AEs.
Comment
After a 1-week course of 5-FU cream 0.5% following cryosurgery, a greater reduction in the number of AK lesions for a period of 2 months was noted in the treatment group compared to the control group. These findings are consistent with a similar study from 2006 that used 5-FU cream 0.5% or a vehicle 1 week prior to cryosurgery and then counted the number of AK lesions that remained.8 In the 2006 study, remarkable improvement out to week 26 was noted,8 unlike our study; however, there was insufficient power in our study to demonstrate a continued effect out to week 26.
Both the 2006 study and our current study support the benefit of using a combination treatment to clear AK lesions versus either treatment alone. Of note, these studies also show that combination treatments are equally effective, regardless of the order of treatments, in lowering AK lesion counts compared to cryosurgery alone.
Although participants in the 5-FU cream 0.5% group reported slightly more AEs on average at week 4, the rate of side effects was lower than those reported in a study documenting the side effects of a 4-week course of 5-FU.6 This rate of side effects must be considered in light of the added benefits this combination treatment has demonstrated.
Results of this pilot study suggest that a larger sample size would yield a difference in the study arms for all time periods (weeks 8 and 26). In an effort to maintain exchangeability of the study arms, patients were randomized at baseline treatment, but behaviors of patients in the 6 months following treatment, such as variation in sun exposure or other habits that promote AK lesion development, may have attenuated the results.
Key strengths of this study include no loss to follow-up and high medication adherence rates. The key limitation was the small sample size, which did not demonstrate a statistical advantage of the 5-FU cream 0.5% at 26 weeks; however, our study does show promise for larger future studies in illustrating this difference. A study by Krawtchenko et al9 noted that long-term efficacy of field therapy with 5-FU may ultimately be less than imiquimod cream 5%, suggesting that a possible alteration of the study protocol to compare the efficacy of different forms of field therapy may ultimately achieve better outcomes.
Conclusion
Overall, individuals with AK may benefit from a combination of treatment with cryosurgery and topical 5-FU to resolve lesions for longer periods than with cryosurgery alone. Although prior studies have found statistically significant differences in short-term and long-term treatment efficacy when cryosurgery is combined with an active field therapy versus a placebo vehicle,8,9 the current study aimed to find the best combination of efficacy with the fewest side effects. Therefore, the results of prior literature studies only further the feelings of the authors that with a protocol that looks at a slightly different treatment regimen within the treatment arm, the results can be extremely beneficial to patients. Further studies should be implemented to confirm the longer-term benefits of this combination therapy.
1. Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
2. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
3. Smith ES, Feldman SR, Fleischer AB Jr, et al. Characteristics of office-based visits for skin cancer. dermatologists have more experience than other physicians in managing malignant and premalignant skin conditions. Dermatol Surg. 1998;24:981-985.
4. Shoimer I, Rosen N, Muhn C. Current management of actinic keratoses. Skin Therapy Lett. 2010;15:5-7.
5. Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
6. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(suppl 2):22-29.
7. Jorizzo JL, Carney PS, Ko WT, et al. Treatment options in the management of actinic keratosis. Cutis. 2004;74 (suppl 6):9-17.
8. Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drugs Dermatol. 2006;5:133-139.
9. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
Actinic keratosis (AK) is regarded as a lesion on a continuum of progression to squamous cell carcinoma (SCC).1 Studies have estimated that 44% to 97% of SCCs were associated with AK lesions either in contiguous skin or within the same histologic section and that AK lesions progress to SCCs at a rate of 0.6% at 1 year.2 In 1993-1994 there were 3.7 million reported office visits for AK lesions, while in 2002 alone there were 8.2 million office visits.3,4 As the burden of disease from AKs has increased, so has the associated costs from office-based visits, treatments, and subsequent surveillance.
There are a number of highly effective approaches to AK treatment that are based on several factors such as the number of and extent of the lesions, history of skin cancer, provider practice characteristics (eg, location, appointment availability), patient preferences, cost, and tolerability. Cryosurgery is the most commonly used lesion-directed modality in the treatment of individual AKs based on its effectiveness and relative ease of use. Cryosurgery alone has been shown to have a success rate of 67% on AK lesions.5 Patients often experience erythema, edema, pain, and crusting at treated sites; there also is potential for ulceration, scarring, hypopigmentation, hyperpigmentation, and secondary infection, but these effects are less common. Recurrence may be an indicator of treatment-resistant lesions or new lesions appearing in the field.
A field-directed approach with topical 5-fluorouracil (5-FU) may be preferred in patients with a history of substantial photodamage, AKs that are resistant to cryosurgery, or multiple AKs. Field-directed treatments address multiple AKs simultaneously and treat subclinical lesions. Fluorouracil is a common therapy for AKs that often is implemented by dermatologists due to its efficacy and well-understood mechanism of action. Fluorouracil inhibits thymidylate synthase during DNA synthesis, thereby halting cellular proliferation. 5-Fluorouracil cream 0.5% has been approved for 1-, 2-, and 4-week treatment periods. In one study, resolution of AK lesions was greatest in the 4-week treatment group; however, side effects also were greatest in this group.6 Patients commonly may experience a range of local reactions including erythema, pruritus, erosions, ulcerations, scabbing, crusting, and facial irritation. For patients with substantial photodamage and AKs, a robust response can lead to perceived adverse events (AEs) and considerable downtime, possibly affecting patient satisfaction and treatment compliance.7
Many alternative and combination approaches have been studied to decrease AEs and improve compliance and efficacy in the treatment of AKs. In this study, we examined the efficacy and perceived side effects of cryosurgery and 5-FU cream 0.5% combination therapy in the treatment of AKs.
Methods
Study Design and Participants
This single-blind, single-center, comparator cream–controlled pilot study was parallel designed with a balanced randomization (1:1 frequency). The study protocol and consent form were approved by the Wake Forest University Health Sciences institutional review board (Winston-Salem, North Carolina). Participants were 18 years or older with 8 clinically typical, visible, and discrete AK lesions on the face (forehead and temples) or balding scalp. Typical inclusion and exclusion criteria were observed. No other topical agents or therapies were permitted to be applied to the affected areas at least 4 weeks prior to treatment, depending on the treatment modality.
Assessment
During the screening (baseline) visit, eligible participants provided informed consent, baseline lesion counts and investigator global assessments (IGAs) were performed, and cryosurgery was administered to all visible AK lesions in the study areas. Participants returned at weeks 3, 4, 8, and 26. Three weeks following cryosurgery, participants were randomized according to standard randomization tables into 1 of 2 treatment groups to receive once-daily treatment with either 5-FU cream 0.5% or a moisturizing comparator cream. The cream was applied at bedtime to the affected sites for 1 week. Randomization was investigator blinded, but participants and the study administrators were not blinded. Participants were instructed to record their treatment compliance in daily diary entries, which were reviewed at week 4 using the medication tolerability assessment rating for burning, stinging, and ulceration. Investigator global assessment, IGA of improvement, lesion counts, and quality of life (QOL) survey responses were gathered at weeks 3, 8, and 26. The IGA measured the overall severity of AK disease involvement on a 6-point scale (clear; very severe). The IGA of improvement measured the overall improvement from baseline on a 6-point scale (clear; worse). Adverse events were measured at each visit.
Efficacy End Points
The primary end point was 100% clearance of all AK lesions at the end of the study (week 26) relative to the baseline AK lesion count. Secondary end points included comparisons between the groups for the number of participants with greater than 75% reduction of baseline lesion counts at the end of the study as well as differences at each visit in medication tolerability assessments, QOL measures, IGA improvement scores, and medication adherence based on diary entries at week 4.
Statistical Analysis
An intention-to-treat analysis was performed. The number of participants with 100% or greater than 75% clearance of AK lesions by specified time points were compared using relative risks and risk differences with Poisson regression analysis log and identity link functions, respectively, to obtain robust error variance 95% confidence intervals. Medication tolerability assessment, QOL, and IGA improvement scores were compared between the 2 groups using the Mann-Whitney U test. The significance level was set at α=.05. All analyses were performed using SAS data analysis software.
Results
Sixty age-eligible participants were enrolled in the study with 30 participants in each treatment group. All of the participants completed the 26-week study period and were included in the intention-to-treat analysis. All of the participants were white with a median age of 67 years; the median number of baseline AK lesions was 12. Participant baseline demographics and clinical characteristics are provided in Table 1. Treatment compliance in both groups was good with only a few participants reporting missed doses.
In our evaluation of the rate of change in the number of AK lesions at week 8 compared to baseline, the 5-FU cream 0.5% group showed an 84% reduction in the number of AK lesions versus a 69% reduction in the control group. At week 26, the 5-FU cream 0.5% group showed a 72% reduction in the number of lesions versus 73% in the control group. There was no significant difference between 5-FU cream 0.5% and the comparator cream for either 100% or 75% clearance of AK lesions by the end of the study; however, comparing the AK lesion count from baseline to 8 weeks following the initiation of the study, participants in the 5-FU cream 0.5% group were more likely than the control group to achieve 75% or 100% clearance on the relative risk and risk difference scales (Table 2).
There were no significant differences between the 2 groups for the IGA of improvement at any time point (Table 3). On average, participants in the 5-FU cream 0.5% group experienced more dryness, erosion, fissuring, and redness than the control group but not more ulcerations by the end of week 4 (Table 4). All other QOL measures were statistically comparable between the 2 treatment groups for all time points.
A total of 25 AEs were reported throughout the study but none were considered to be serious. One AE (redness, burning, and itching over the eyebrow) was considered to be related to the study drug. No participants withdrew from the study due to AEs. A total of 12 participants in the 5-FU cream 0.5% group and 10 in the control group reported AEs.
Comment
After a 1-week course of 5-FU cream 0.5% following cryosurgery, a greater reduction in the number of AK lesions for a period of 2 months was noted in the treatment group compared to the control group. These findings are consistent with a similar study from 2006 that used 5-FU cream 0.5% or a vehicle 1 week prior to cryosurgery and then counted the number of AK lesions that remained.8 In the 2006 study, remarkable improvement out to week 26 was noted,8 unlike our study; however, there was insufficient power in our study to demonstrate a continued effect out to week 26.
Both the 2006 study and our current study support the benefit of using a combination treatment to clear AK lesions versus either treatment alone. Of note, these studies also show that combination treatments are equally effective, regardless of the order of treatments, in lowering AK lesion counts compared to cryosurgery alone.
Although participants in the 5-FU cream 0.5% group reported slightly more AEs on average at week 4, the rate of side effects was lower than those reported in a study documenting the side effects of a 4-week course of 5-FU.6 This rate of side effects must be considered in light of the added benefits this combination treatment has demonstrated.
Results of this pilot study suggest that a larger sample size would yield a difference in the study arms for all time periods (weeks 8 and 26). In an effort to maintain exchangeability of the study arms, patients were randomized at baseline treatment, but behaviors of patients in the 6 months following treatment, such as variation in sun exposure or other habits that promote AK lesion development, may have attenuated the results.
Key strengths of this study include no loss to follow-up and high medication adherence rates. The key limitation was the small sample size, which did not demonstrate a statistical advantage of the 5-FU cream 0.5% at 26 weeks; however, our study does show promise for larger future studies in illustrating this difference. A study by Krawtchenko et al9 noted that long-term efficacy of field therapy with 5-FU may ultimately be less than imiquimod cream 5%, suggesting that a possible alteration of the study protocol to compare the efficacy of different forms of field therapy may ultimately achieve better outcomes.
Conclusion
Overall, individuals with AK may benefit from a combination of treatment with cryosurgery and topical 5-FU to resolve lesions for longer periods than with cryosurgery alone. Although prior studies have found statistically significant differences in short-term and long-term treatment efficacy when cryosurgery is combined with an active field therapy versus a placebo vehicle,8,9 the current study aimed to find the best combination of efficacy with the fewest side effects. Therefore, the results of prior literature studies only further the feelings of the authors that with a protocol that looks at a slightly different treatment regimen within the treatment arm, the results can be extremely beneficial to patients. Further studies should be implemented to confirm the longer-term benefits of this combination therapy.
Actinic keratosis (AK) is regarded as a lesion on a continuum of progression to squamous cell carcinoma (SCC).1 Studies have estimated that 44% to 97% of SCCs were associated with AK lesions either in contiguous skin or within the same histologic section and that AK lesions progress to SCCs at a rate of 0.6% at 1 year.2 In 1993-1994 there were 3.7 million reported office visits for AK lesions, while in 2002 alone there were 8.2 million office visits.3,4 As the burden of disease from AKs has increased, so has the associated costs from office-based visits, treatments, and subsequent surveillance.
There are a number of highly effective approaches to AK treatment that are based on several factors such as the number of and extent of the lesions, history of skin cancer, provider practice characteristics (eg, location, appointment availability), patient preferences, cost, and tolerability. Cryosurgery is the most commonly used lesion-directed modality in the treatment of individual AKs based on its effectiveness and relative ease of use. Cryosurgery alone has been shown to have a success rate of 67% on AK lesions.5 Patients often experience erythema, edema, pain, and crusting at treated sites; there also is potential for ulceration, scarring, hypopigmentation, hyperpigmentation, and secondary infection, but these effects are less common. Recurrence may be an indicator of treatment-resistant lesions or new lesions appearing in the field.
A field-directed approach with topical 5-fluorouracil (5-FU) may be preferred in patients with a history of substantial photodamage, AKs that are resistant to cryosurgery, or multiple AKs. Field-directed treatments address multiple AKs simultaneously and treat subclinical lesions. Fluorouracil is a common therapy for AKs that often is implemented by dermatologists due to its efficacy and well-understood mechanism of action. Fluorouracil inhibits thymidylate synthase during DNA synthesis, thereby halting cellular proliferation. 5-Fluorouracil cream 0.5% has been approved for 1-, 2-, and 4-week treatment periods. In one study, resolution of AK lesions was greatest in the 4-week treatment group; however, side effects also were greatest in this group.6 Patients commonly may experience a range of local reactions including erythema, pruritus, erosions, ulcerations, scabbing, crusting, and facial irritation. For patients with substantial photodamage and AKs, a robust response can lead to perceived adverse events (AEs) and considerable downtime, possibly affecting patient satisfaction and treatment compliance.7
Many alternative and combination approaches have been studied to decrease AEs and improve compliance and efficacy in the treatment of AKs. In this study, we examined the efficacy and perceived side effects of cryosurgery and 5-FU cream 0.5% combination therapy in the treatment of AKs.
Methods
Study Design and Participants
This single-blind, single-center, comparator cream–controlled pilot study was parallel designed with a balanced randomization (1:1 frequency). The study protocol and consent form were approved by the Wake Forest University Health Sciences institutional review board (Winston-Salem, North Carolina). Participants were 18 years or older with 8 clinically typical, visible, and discrete AK lesions on the face (forehead and temples) or balding scalp. Typical inclusion and exclusion criteria were observed. No other topical agents or therapies were permitted to be applied to the affected areas at least 4 weeks prior to treatment, depending on the treatment modality.
Assessment
During the screening (baseline) visit, eligible participants provided informed consent, baseline lesion counts and investigator global assessments (IGAs) were performed, and cryosurgery was administered to all visible AK lesions in the study areas. Participants returned at weeks 3, 4, 8, and 26. Three weeks following cryosurgery, participants were randomized according to standard randomization tables into 1 of 2 treatment groups to receive once-daily treatment with either 5-FU cream 0.5% or a moisturizing comparator cream. The cream was applied at bedtime to the affected sites for 1 week. Randomization was investigator blinded, but participants and the study administrators were not blinded. Participants were instructed to record their treatment compliance in daily diary entries, which were reviewed at week 4 using the medication tolerability assessment rating for burning, stinging, and ulceration. Investigator global assessment, IGA of improvement, lesion counts, and quality of life (QOL) survey responses were gathered at weeks 3, 8, and 26. The IGA measured the overall severity of AK disease involvement on a 6-point scale (clear; very severe). The IGA of improvement measured the overall improvement from baseline on a 6-point scale (clear; worse). Adverse events were measured at each visit.
Efficacy End Points
The primary end point was 100% clearance of all AK lesions at the end of the study (week 26) relative to the baseline AK lesion count. Secondary end points included comparisons between the groups for the number of participants with greater than 75% reduction of baseline lesion counts at the end of the study as well as differences at each visit in medication tolerability assessments, QOL measures, IGA improvement scores, and medication adherence based on diary entries at week 4.
Statistical Analysis
An intention-to-treat analysis was performed. The number of participants with 100% or greater than 75% clearance of AK lesions by specified time points were compared using relative risks and risk differences with Poisson regression analysis log and identity link functions, respectively, to obtain robust error variance 95% confidence intervals. Medication tolerability assessment, QOL, and IGA improvement scores were compared between the 2 groups using the Mann-Whitney U test. The significance level was set at α=.05. All analyses were performed using SAS data analysis software.
Results
Sixty age-eligible participants were enrolled in the study with 30 participants in each treatment group. All of the participants completed the 26-week study period and were included in the intention-to-treat analysis. All of the participants were white with a median age of 67 years; the median number of baseline AK lesions was 12. Participant baseline demographics and clinical characteristics are provided in Table 1. Treatment compliance in both groups was good with only a few participants reporting missed doses.
In our evaluation of the rate of change in the number of AK lesions at week 8 compared to baseline, the 5-FU cream 0.5% group showed an 84% reduction in the number of AK lesions versus a 69% reduction in the control group. At week 26, the 5-FU cream 0.5% group showed a 72% reduction in the number of lesions versus 73% in the control group. There was no significant difference between 5-FU cream 0.5% and the comparator cream for either 100% or 75% clearance of AK lesions by the end of the study; however, comparing the AK lesion count from baseline to 8 weeks following the initiation of the study, participants in the 5-FU cream 0.5% group were more likely than the control group to achieve 75% or 100% clearance on the relative risk and risk difference scales (Table 2).
There were no significant differences between the 2 groups for the IGA of improvement at any time point (Table 3). On average, participants in the 5-FU cream 0.5% group experienced more dryness, erosion, fissuring, and redness than the control group but not more ulcerations by the end of week 4 (Table 4). All other QOL measures were statistically comparable between the 2 treatment groups for all time points.
A total of 25 AEs were reported throughout the study but none were considered to be serious. One AE (redness, burning, and itching over the eyebrow) was considered to be related to the study drug. No participants withdrew from the study due to AEs. A total of 12 participants in the 5-FU cream 0.5% group and 10 in the control group reported AEs.
Comment
After a 1-week course of 5-FU cream 0.5% following cryosurgery, a greater reduction in the number of AK lesions for a period of 2 months was noted in the treatment group compared to the control group. These findings are consistent with a similar study from 2006 that used 5-FU cream 0.5% or a vehicle 1 week prior to cryosurgery and then counted the number of AK lesions that remained.8 In the 2006 study, remarkable improvement out to week 26 was noted,8 unlike our study; however, there was insufficient power in our study to demonstrate a continued effect out to week 26.
Both the 2006 study and our current study support the benefit of using a combination treatment to clear AK lesions versus either treatment alone. Of note, these studies also show that combination treatments are equally effective, regardless of the order of treatments, in lowering AK lesion counts compared to cryosurgery alone.
Although participants in the 5-FU cream 0.5% group reported slightly more AEs on average at week 4, the rate of side effects was lower than those reported in a study documenting the side effects of a 4-week course of 5-FU.6 This rate of side effects must be considered in light of the added benefits this combination treatment has demonstrated.
Results of this pilot study suggest that a larger sample size would yield a difference in the study arms for all time periods (weeks 8 and 26). In an effort to maintain exchangeability of the study arms, patients were randomized at baseline treatment, but behaviors of patients in the 6 months following treatment, such as variation in sun exposure or other habits that promote AK lesion development, may have attenuated the results.
Key strengths of this study include no loss to follow-up and high medication adherence rates. The key limitation was the small sample size, which did not demonstrate a statistical advantage of the 5-FU cream 0.5% at 26 weeks; however, our study does show promise for larger future studies in illustrating this difference. A study by Krawtchenko et al9 noted that long-term efficacy of field therapy with 5-FU may ultimately be less than imiquimod cream 5%, suggesting that a possible alteration of the study protocol to compare the efficacy of different forms of field therapy may ultimately achieve better outcomes.
Conclusion
Overall, individuals with AK may benefit from a combination of treatment with cryosurgery and topical 5-FU to resolve lesions for longer periods than with cryosurgery alone. Although prior studies have found statistically significant differences in short-term and long-term treatment efficacy when cryosurgery is combined with an active field therapy versus a placebo vehicle,8,9 the current study aimed to find the best combination of efficacy with the fewest side effects. Therefore, the results of prior literature studies only further the feelings of the authors that with a protocol that looks at a slightly different treatment regimen within the treatment arm, the results can be extremely beneficial to patients. Further studies should be implemented to confirm the longer-term benefits of this combination therapy.
1. Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
2. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
3. Smith ES, Feldman SR, Fleischer AB Jr, et al. Characteristics of office-based visits for skin cancer. dermatologists have more experience than other physicians in managing malignant and premalignant skin conditions. Dermatol Surg. 1998;24:981-985.
4. Shoimer I, Rosen N, Muhn C. Current management of actinic keratoses. Skin Therapy Lett. 2010;15:5-7.
5. Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
6. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(suppl 2):22-29.
7. Jorizzo JL, Carney PS, Ko WT, et al. Treatment options in the management of actinic keratosis. Cutis. 2004;74 (suppl 6):9-17.
8. Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drugs Dermatol. 2006;5:133-139.
9. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
1. Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
2. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
3. Smith ES, Feldman SR, Fleischer AB Jr, et al. Characteristics of office-based visits for skin cancer. dermatologists have more experience than other physicians in managing malignant and premalignant skin conditions. Dermatol Surg. 1998;24:981-985.
4. Shoimer I, Rosen N, Muhn C. Current management of actinic keratoses. Skin Therapy Lett. 2010;15:5-7.
5. Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
6. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(suppl 2):22-29.
7. Jorizzo JL, Carney PS, Ko WT, et al. Treatment options in the management of actinic keratosis. Cutis. 2004;74 (suppl 6):9-17.
8. Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drugs Dermatol. 2006;5:133-139.
9. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
Sulfur Spring Dermatitis
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
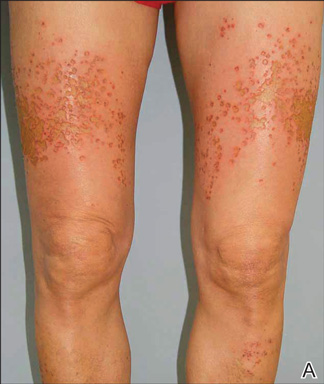
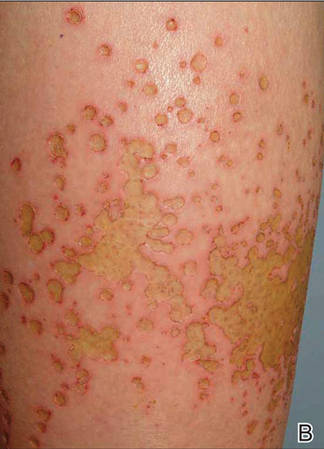
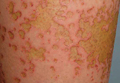
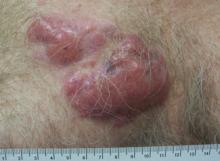
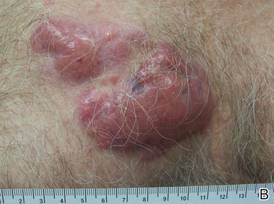
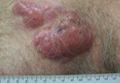
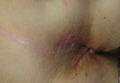
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
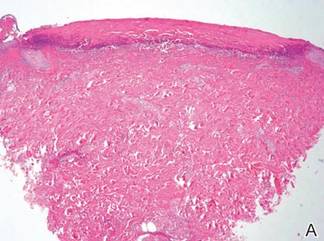
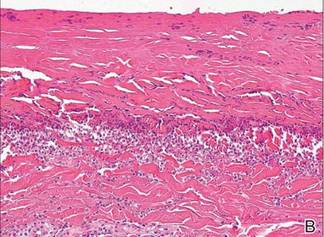
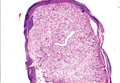
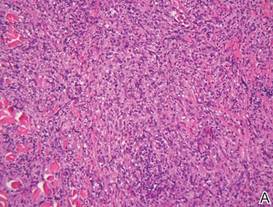
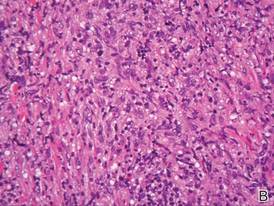
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
Practice Points
- The clinical findings of sulfur spring dermatitis are similar to those of a superficial second-degree burn.
- Careful evaluation of the patient’s clinical history and recognition of characteristic findings are important for correct diagnosis.
- Patients with preexisting skin disorders who engage in thermal sulfur baths should be aware of the potential adverse effect of sulfur spring dermatitis.
The Discount Dilemma
Health care reform has triggered considerable discussion both in print and online about the administrative problems it has created for private practitioners, including decreased cash flow, increased paperwork and business expenses, and an increasing number of high-deductible insurance exchanges with the infamous 90-day “grace periods.” Extending discounts to patients who pay at the time of service or out of pocket may mitigate damage caused by all 3 of these issues; however, caution is necessary, as discounts often can run afoul of federal and state laws, including anti-kickback statutes,1 the anti-inducement provision of the Health Insurance Portability and Accountability Act,2 the Medicare exclusion provision,3 and state insurance antidiscrimination provisions.4
Avoid Kickback Penalties From Patient Discounts
From a legal standpoint, any discount is technically a kickback of sorts because you are returning part of your fee to the patient, and many laws designed to thwart true kickbacks can apply to patient discounts. Take the relatively straightforward case of time-of-service discounts for cosmetic procedures and other services not normally covered by insurance. You would think that these transactions are strictly between you and your patients, but if these discounts appear to be marketing incentives to attract patients, you may face a penalty.5
Patient discounts also may impact third-party payers. Many provider agreements contain “most favored nation” clauses, which require you to automatically give that payer the lowest price you offer to anyone else, regardless of what would be paid otherwise. In other words, the payer could demand the same discount you offer any individual patient. A time-of-service discount is, of course, exactly that: it is offered only when payment is made immediately. Third parties never pay at the time of service and would not be entitled to it, but they may try to invoke their agreement.
If you want to extend discounts for covered services, you must be sure that the discounted fee you charge the patient also is reflected on the claim submitted to the insurer. Billing the insurer more than you charged the patient invites a charge of fraud.6 It is important to avoid discounting so regularly that the discounted fee becomes your usual and customary rate in the eyes of the insurer.
Waiving Costs and Kickbacks
Waiving coinsurance and deductibles can be trouble too, particularly with Medicare and Medicaid. You might intend it as a good deed, but the Centers for Medicare & Medicaid Services (CMS) will see it as an inducement or kickback, especially if you do it routinely, and similar to private carriers, they will consider the discounted fee your new customary fee. The CMS has no problem with an occasional waiver, especially “after determining in good faith that the individual is in financial need,” according to the Office of Inspector General,7 but thorough documentation is necessary in such cases.
Waiving co-pays for privately insured patients can be equally problematic. Nearly all insurers impose a contractual duty on providers to make a reasonable effort to collect applicable co-pays and/or deductibles. They view the routine waiver of patient payments as a breach of contract, and litigation may occur against providers who flout this requirement.8 As with the CMS, accommodating patients with individually documented financial limitations is acceptable, but if there is a pattern of routine waivers and a paucity of documentation, you will have difficulty defending it.
Antidiscrimination Laws
In addition to kickback laws, some states also have antidiscrimination laws that forbid lower charges to any subset of insurance payers or to direct payers.4 Some states make specific exceptions for legitimate discounts, such as individual cases of financial hardship, or if you pass along your lower billing and collection expenditures to patients who pay immediately, but other states do not.
Determining Discount Amounts
The discount amount depends on the physician’s situation and deserves careful consideration. If the amount or percentage that you choose to offer as a discount is completely out of proportion with the administrative costs of submitting paperwork as well as the hassles associated with waiting for third-party payments, you could be accused of running a discount policy that is in effect a de facto increase to insurance carriers, which also could result in charges of fraud.2
In cases of legitimate financial hardship, the most effective and least problematic strategy may be to offer a sliding scale. Many large clinics and community agencies as well as all hospitals have written policies for this system, often based on federal poverty guidelines. To avoid any potential issues, contact your local social service agencies and welfare clinics, learn the community standard in your area, and formulate a written policy with guidelines for determining a patient’s indigence.
Final Thoughts
Consistency of administration, objectivity in policies, and documentation of individual eligibility will ensure that the discounts you offer patients are in line with legal and payer regulations. Before you establish a discount policy, be sure to check your state’s applicable laws, and as always, run everything by your attorney.
1. Guidance on the federal anti-kickback law. Health Resources and Services Administration Web site. http://bphc.hrsa.gov/policiesregulations/policies/pal199510.html. Accessed October 22, 2014.
2. US Department of Health & Human Services. A roadmap for new physicians: fraud & abuse laws. Office of Inspector General Web site.http://oig.hhs.gov/compliance/physician-education/01laws.asp. Accessed October 21, 2014.
3. Exclusion of certain individuals and entities from participation in Medicare and State health care programs, 42 USC §1320a–7 (2011).
4. Non-discrimination in health care, 42 USC §300gg–5 (2014).
5. US Department of Health and Human Services. Offering gifts and other inducements to beneficiaries. http://oig.hhs.gov/fraud/docs/alertsandbulletins/SABGiftsandInducements.pdf. Published August 2002. Accessed October 21, 2014.
6. The challenge of health care fraud. National Health Care Anti-Fraud Association Web site. http://www.nhcaa.org/resources/health-care-anti-fraud-resources/the-challenge-of-health-care-fraud.aspx. Accessed October 21, 2014.
7. US Department of Health & Human Services. Hospital discounts offered to patients who cannot afford to pay their hospital bills. Office of Inspector General Web site. http://oig.hhs.gov/fraud/docs/alertsandbulletins/2004/FA021904hospitaldiscounts.pdf. Published February 2, 2004. Accessed October 16, 2014.
8. Merritt M. Forgiving patient copays can lead to unforgiving consequences. Physicians Practice Web site. http://www.physicianspractice.com/blog/forgiving-patient-copays-can-lead-unforgiving-consequences. Published December 15, 2013. Accessed October 21, 2014.
Health care reform has triggered considerable discussion both in print and online about the administrative problems it has created for private practitioners, including decreased cash flow, increased paperwork and business expenses, and an increasing number of high-deductible insurance exchanges with the infamous 90-day “grace periods.” Extending discounts to patients who pay at the time of service or out of pocket may mitigate damage caused by all 3 of these issues; however, caution is necessary, as discounts often can run afoul of federal and state laws, including anti-kickback statutes,1 the anti-inducement provision of the Health Insurance Portability and Accountability Act,2 the Medicare exclusion provision,3 and state insurance antidiscrimination provisions.4
Avoid Kickback Penalties From Patient Discounts
From a legal standpoint, any discount is technically a kickback of sorts because you are returning part of your fee to the patient, and many laws designed to thwart true kickbacks can apply to patient discounts. Take the relatively straightforward case of time-of-service discounts for cosmetic procedures and other services not normally covered by insurance. You would think that these transactions are strictly between you and your patients, but if these discounts appear to be marketing incentives to attract patients, you may face a penalty.5
Patient discounts also may impact third-party payers. Many provider agreements contain “most favored nation” clauses, which require you to automatically give that payer the lowest price you offer to anyone else, regardless of what would be paid otherwise. In other words, the payer could demand the same discount you offer any individual patient. A time-of-service discount is, of course, exactly that: it is offered only when payment is made immediately. Third parties never pay at the time of service and would not be entitled to it, but they may try to invoke their agreement.
If you want to extend discounts for covered services, you must be sure that the discounted fee you charge the patient also is reflected on the claim submitted to the insurer. Billing the insurer more than you charged the patient invites a charge of fraud.6 It is important to avoid discounting so regularly that the discounted fee becomes your usual and customary rate in the eyes of the insurer.
Waiving Costs and Kickbacks
Waiving coinsurance and deductibles can be trouble too, particularly with Medicare and Medicaid. You might intend it as a good deed, but the Centers for Medicare & Medicaid Services (CMS) will see it as an inducement or kickback, especially if you do it routinely, and similar to private carriers, they will consider the discounted fee your new customary fee. The CMS has no problem with an occasional waiver, especially “after determining in good faith that the individual is in financial need,” according to the Office of Inspector General,7 but thorough documentation is necessary in such cases.
Waiving co-pays for privately insured patients can be equally problematic. Nearly all insurers impose a contractual duty on providers to make a reasonable effort to collect applicable co-pays and/or deductibles. They view the routine waiver of patient payments as a breach of contract, and litigation may occur against providers who flout this requirement.8 As with the CMS, accommodating patients with individually documented financial limitations is acceptable, but if there is a pattern of routine waivers and a paucity of documentation, you will have difficulty defending it.
Antidiscrimination Laws
In addition to kickback laws, some states also have antidiscrimination laws that forbid lower charges to any subset of insurance payers or to direct payers.4 Some states make specific exceptions for legitimate discounts, such as individual cases of financial hardship, or if you pass along your lower billing and collection expenditures to patients who pay immediately, but other states do not.
Determining Discount Amounts
The discount amount depends on the physician’s situation and deserves careful consideration. If the amount or percentage that you choose to offer as a discount is completely out of proportion with the administrative costs of submitting paperwork as well as the hassles associated with waiting for third-party payments, you could be accused of running a discount policy that is in effect a de facto increase to insurance carriers, which also could result in charges of fraud.2
In cases of legitimate financial hardship, the most effective and least problematic strategy may be to offer a sliding scale. Many large clinics and community agencies as well as all hospitals have written policies for this system, often based on federal poverty guidelines. To avoid any potential issues, contact your local social service agencies and welfare clinics, learn the community standard in your area, and formulate a written policy with guidelines for determining a patient’s indigence.
Final Thoughts
Consistency of administration, objectivity in policies, and documentation of individual eligibility will ensure that the discounts you offer patients are in line with legal and payer regulations. Before you establish a discount policy, be sure to check your state’s applicable laws, and as always, run everything by your attorney.
Health care reform has triggered considerable discussion both in print and online about the administrative problems it has created for private practitioners, including decreased cash flow, increased paperwork and business expenses, and an increasing number of high-deductible insurance exchanges with the infamous 90-day “grace periods.” Extending discounts to patients who pay at the time of service or out of pocket may mitigate damage caused by all 3 of these issues; however, caution is necessary, as discounts often can run afoul of federal and state laws, including anti-kickback statutes,1 the anti-inducement provision of the Health Insurance Portability and Accountability Act,2 the Medicare exclusion provision,3 and state insurance antidiscrimination provisions.4
Avoid Kickback Penalties From Patient Discounts
From a legal standpoint, any discount is technically a kickback of sorts because you are returning part of your fee to the patient, and many laws designed to thwart true kickbacks can apply to patient discounts. Take the relatively straightforward case of time-of-service discounts for cosmetic procedures and other services not normally covered by insurance. You would think that these transactions are strictly between you and your patients, but if these discounts appear to be marketing incentives to attract patients, you may face a penalty.5
Patient discounts also may impact third-party payers. Many provider agreements contain “most favored nation” clauses, which require you to automatically give that payer the lowest price you offer to anyone else, regardless of what would be paid otherwise. In other words, the payer could demand the same discount you offer any individual patient. A time-of-service discount is, of course, exactly that: it is offered only when payment is made immediately. Third parties never pay at the time of service and would not be entitled to it, but they may try to invoke their agreement.
If you want to extend discounts for covered services, you must be sure that the discounted fee you charge the patient also is reflected on the claim submitted to the insurer. Billing the insurer more than you charged the patient invites a charge of fraud.6 It is important to avoid discounting so regularly that the discounted fee becomes your usual and customary rate in the eyes of the insurer.
Waiving Costs and Kickbacks
Waiving coinsurance and deductibles can be trouble too, particularly with Medicare and Medicaid. You might intend it as a good deed, but the Centers for Medicare & Medicaid Services (CMS) will see it as an inducement or kickback, especially if you do it routinely, and similar to private carriers, they will consider the discounted fee your new customary fee. The CMS has no problem with an occasional waiver, especially “after determining in good faith that the individual is in financial need,” according to the Office of Inspector General,7 but thorough documentation is necessary in such cases.
Waiving co-pays for privately insured patients can be equally problematic. Nearly all insurers impose a contractual duty on providers to make a reasonable effort to collect applicable co-pays and/or deductibles. They view the routine waiver of patient payments as a breach of contract, and litigation may occur against providers who flout this requirement.8 As with the CMS, accommodating patients with individually documented financial limitations is acceptable, but if there is a pattern of routine waivers and a paucity of documentation, you will have difficulty defending it.
Antidiscrimination Laws
In addition to kickback laws, some states also have antidiscrimination laws that forbid lower charges to any subset of insurance payers or to direct payers.4 Some states make specific exceptions for legitimate discounts, such as individual cases of financial hardship, or if you pass along your lower billing and collection expenditures to patients who pay immediately, but other states do not.
Determining Discount Amounts
The discount amount depends on the physician’s situation and deserves careful consideration. If the amount or percentage that you choose to offer as a discount is completely out of proportion with the administrative costs of submitting paperwork as well as the hassles associated with waiting for third-party payments, you could be accused of running a discount policy that is in effect a de facto increase to insurance carriers, which also could result in charges of fraud.2
In cases of legitimate financial hardship, the most effective and least problematic strategy may be to offer a sliding scale. Many large clinics and community agencies as well as all hospitals have written policies for this system, often based on federal poverty guidelines. To avoid any potential issues, contact your local social service agencies and welfare clinics, learn the community standard in your area, and formulate a written policy with guidelines for determining a patient’s indigence.
Final Thoughts
Consistency of administration, objectivity in policies, and documentation of individual eligibility will ensure that the discounts you offer patients are in line with legal and payer regulations. Before you establish a discount policy, be sure to check your state’s applicable laws, and as always, run everything by your attorney.
1. Guidance on the federal anti-kickback law. Health Resources and Services Administration Web site. http://bphc.hrsa.gov/policiesregulations/policies/pal199510.html. Accessed October 22, 2014.
2. US Department of Health & Human Services. A roadmap for new physicians: fraud & abuse laws. Office of Inspector General Web site.http://oig.hhs.gov/compliance/physician-education/01laws.asp. Accessed October 21, 2014.
3. Exclusion of certain individuals and entities from participation in Medicare and State health care programs, 42 USC §1320a–7 (2011).
4. Non-discrimination in health care, 42 USC §300gg–5 (2014).
5. US Department of Health and Human Services. Offering gifts and other inducements to beneficiaries. http://oig.hhs.gov/fraud/docs/alertsandbulletins/SABGiftsandInducements.pdf. Published August 2002. Accessed October 21, 2014.
6. The challenge of health care fraud. National Health Care Anti-Fraud Association Web site. http://www.nhcaa.org/resources/health-care-anti-fraud-resources/the-challenge-of-health-care-fraud.aspx. Accessed October 21, 2014.
7. US Department of Health & Human Services. Hospital discounts offered to patients who cannot afford to pay their hospital bills. Office of Inspector General Web site. http://oig.hhs.gov/fraud/docs/alertsandbulletins/2004/FA021904hospitaldiscounts.pdf. Published February 2, 2004. Accessed October 16, 2014.
8. Merritt M. Forgiving patient copays can lead to unforgiving consequences. Physicians Practice Web site. http://www.physicianspractice.com/blog/forgiving-patient-copays-can-lead-unforgiving-consequences. Published December 15, 2013. Accessed October 21, 2014.
1. Guidance on the federal anti-kickback law. Health Resources and Services Administration Web site. http://bphc.hrsa.gov/policiesregulations/policies/pal199510.html. Accessed October 22, 2014.
2. US Department of Health & Human Services. A roadmap for new physicians: fraud & abuse laws. Office of Inspector General Web site.http://oig.hhs.gov/compliance/physician-education/01laws.asp. Accessed October 21, 2014.
3. Exclusion of certain individuals and entities from participation in Medicare and State health care programs, 42 USC §1320a–7 (2011).
4. Non-discrimination in health care, 42 USC §300gg–5 (2014).
5. US Department of Health and Human Services. Offering gifts and other inducements to beneficiaries. http://oig.hhs.gov/fraud/docs/alertsandbulletins/SABGiftsandInducements.pdf. Published August 2002. Accessed October 21, 2014.
6. The challenge of health care fraud. National Health Care Anti-Fraud Association Web site. http://www.nhcaa.org/resources/health-care-anti-fraud-resources/the-challenge-of-health-care-fraud.aspx. Accessed October 21, 2014.
7. US Department of Health & Human Services. Hospital discounts offered to patients who cannot afford to pay their hospital bills. Office of Inspector General Web site. http://oig.hhs.gov/fraud/docs/alertsandbulletins/2004/FA021904hospitaldiscounts.pdf. Published February 2, 2004. Accessed October 16, 2014.
8. Merritt M. Forgiving patient copays can lead to unforgiving consequences. Physicians Practice Web site. http://www.physicianspractice.com/blog/forgiving-patient-copays-can-lead-unforgiving-consequences. Published December 15, 2013. Accessed October 21, 2014.
Practice Points
- Discounts to direct and immediate payers (patients) may run afoul of local and national statutes.
- Routine waiving of co-pays and deductibles can be problematic.
- Consistency of administration, objectivity in policies, and documentation of individual eligibility are essential in private practices.
Clear Cell Fibrous Papule
A fibrous papule is a common benign lesion that usually presents in adults on the face, especially on the lower portion of the nose. It typically presents as a small (2–5 mm), asymptomatic, flesh-colored, dome-shaped lesion that is firm and nontender. Several histopathologic variants of fibrous papules have been described, including clear cell, granular, epithelioid, hypercellular, pleomorphic, pigmented, and inflammatory.1 Clear cell fibrous papules are exceedingly rare. On microscopic examination the epidermis may be normal or show some degree of hyperkeratosis and parakeratosis, erosion, ulceration, or crust. The basal layer may show an increase of melanin. The dermis is expanded by a proliferation of clear cells arranged in sheets, clusters, or as single cells (Figure 1). The clear cells show variation in size and shape. The nuclei are small and round without pleomorphism, hyperchromasia, or mitoses. The nuclei may be centrally located or eccentrically displaced by a large intracytoplasmic vacuole (Figure 2). Some clear cells may exhibit finely vacuolated cytoplasm with nuclear scalloping. The surrounding stroma usually consists of sclerotic collagen and dilated blood vessels (Figure 3). Extravasated red blood cells may be present focally. Patchy lymphocytic infiltrates may be found in the stroma at the periphery of the lesion. Periodic acid–Schiff and mucicarmine staining of the clear cells is negative. On immunohistochemistry, the clear cells are diffusely positive for vimentin and negative for cytokeratin AE1/AE3, epithelial membrane antigen, carcinoembryonic antigen, and HMB-45 (human melanoma black 45).2,3 The clear cells often are positive for CD68, factor XIIIa, and NKI/C3 (anti-CD63) but also may be negative. The S-100 protein often is negative but may be focally positive.
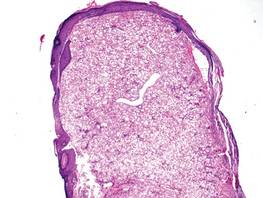
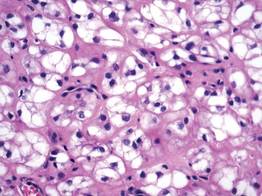
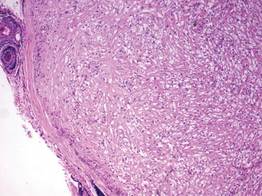
The differential diagnosis for clear cell fibrous papules is broad but reasonably includes balloon cell nevus, clear cell hidradenoma, and cutaneous metastasis of clear cell (conventional) renal cell carcinoma (ccRCC). Balloon cell malignant melanoma is not considered strongly in the differential diagnosis because it usually exhibits invasive growth, cytologic atypia, and mitoses, all of which are not characteristic morphologic features of clear cell fibrous papules.
A balloon cell nevus may be difficult to distinguish from a clear cell fibrous papule on routine hematoxylin and eosin staining (Figure 4); however, the nuclei of a balloon cell nevus tend to be more rounded and centrally located. Any junctional nesting or nests of conventional nevus cells in the dermis also help differentiate a balloon cell nevus from a clear cell fibrous papule. Diffusely positive immunostaining for S-100 protein also is indicative of a balloon cell nevus.
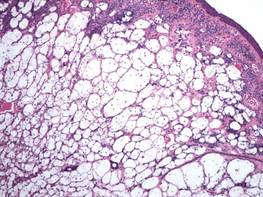
Clear cell hidradenoma consists predominantly of cells with clear cytoplasm and small dark nuclei that may closely mimic a clear cell fibrous papule (Figure 5) but often shows a second population of cells with more vesicular nuclei and dark eosinophilic cytoplasm. Cystic spaces containing hyaline material and foci of squamoid change are common, along with occasional tubular lumina that may be prominent or inconspicuous. Further, the tumor cells of clear cell hidradenoma show positive immunostaining for epithelial markers (eg, cytokeratin AE1/AE3, CAM5.2).
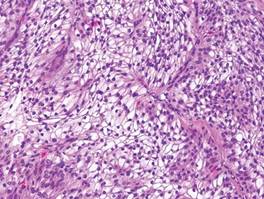
Cutaneous metastasis of ccRCC is rare and usually presents clinically as a larger lesion than a clear cell fibrous papule. The cells of ccRCC have moderate to abundant clear cytoplasm and nuclei with varying degrees of pleomorphism (Figure 6). Periodic acid–Schiff staining demonstrates intracytoplasmic glycogen. The stroma is abundantly vascular and extravasated blood cells are frequently observed. On immunohistochemistry, the tumor cells of ccRCC stain positively for cytokeratin AE1/AE3, CAM5.2, epithelial membrane antigen, CD10, and vimentin.
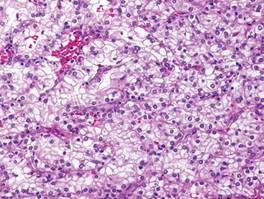
- Bansal C, Stewart D, Li A, et al. Histologic variants of fibrous papule. J Cutan Pathol. 2005;32:424-428.
- Chiang YY, Tsai HH, Lee WR, et al. Clear cell fibrous papule: report of a case mimicking a balloon cell nevus. J Cutan Pathol. 2009;36:381-384.
- Lee AN, Stein SL, Cohen LM. Clear cell fibrous papule with NKI/C3 expression: clinical and histologic features in six cases. Am J Dermatopathol. 2005;27:296-300.
A fibrous papule is a common benign lesion that usually presents in adults on the face, especially on the lower portion of the nose. It typically presents as a small (2–5 mm), asymptomatic, flesh-colored, dome-shaped lesion that is firm and nontender. Several histopathologic variants of fibrous papules have been described, including clear cell, granular, epithelioid, hypercellular, pleomorphic, pigmented, and inflammatory.1 Clear cell fibrous papules are exceedingly rare. On microscopic examination the epidermis may be normal or show some degree of hyperkeratosis and parakeratosis, erosion, ulceration, or crust. The basal layer may show an increase of melanin. The dermis is expanded by a proliferation of clear cells arranged in sheets, clusters, or as single cells (Figure 1). The clear cells show variation in size and shape. The nuclei are small and round without pleomorphism, hyperchromasia, or mitoses. The nuclei may be centrally located or eccentrically displaced by a large intracytoplasmic vacuole (Figure 2). Some clear cells may exhibit finely vacuolated cytoplasm with nuclear scalloping. The surrounding stroma usually consists of sclerotic collagen and dilated blood vessels (Figure 3). Extravasated red blood cells may be present focally. Patchy lymphocytic infiltrates may be found in the stroma at the periphery of the lesion. Periodic acid–Schiff and mucicarmine staining of the clear cells is negative. On immunohistochemistry, the clear cells are diffusely positive for vimentin and negative for cytokeratin AE1/AE3, epithelial membrane antigen, carcinoembryonic antigen, and HMB-45 (human melanoma black 45).2,3 The clear cells often are positive for CD68, factor XIIIa, and NKI/C3 (anti-CD63) but also may be negative. The S-100 protein often is negative but may be focally positive.



The differential diagnosis for clear cell fibrous papules is broad but reasonably includes balloon cell nevus, clear cell hidradenoma, and cutaneous metastasis of clear cell (conventional) renal cell carcinoma (ccRCC). Balloon cell malignant melanoma is not considered strongly in the differential diagnosis because it usually exhibits invasive growth, cytologic atypia, and mitoses, all of which are not characteristic morphologic features of clear cell fibrous papules.
A balloon cell nevus may be difficult to distinguish from a clear cell fibrous papule on routine hematoxylin and eosin staining (Figure 4); however, the nuclei of a balloon cell nevus tend to be more rounded and centrally located. Any junctional nesting or nests of conventional nevus cells in the dermis also help differentiate a balloon cell nevus from a clear cell fibrous papule. Diffusely positive immunostaining for S-100 protein also is indicative of a balloon cell nevus.

Clear cell hidradenoma consists predominantly of cells with clear cytoplasm and small dark nuclei that may closely mimic a clear cell fibrous papule (Figure 5) but often shows a second population of cells with more vesicular nuclei and dark eosinophilic cytoplasm. Cystic spaces containing hyaline material and foci of squamoid change are common, along with occasional tubular lumina that may be prominent or inconspicuous. Further, the tumor cells of clear cell hidradenoma show positive immunostaining for epithelial markers (eg, cytokeratin AE1/AE3, CAM5.2).

Cutaneous metastasis of ccRCC is rare and usually presents clinically as a larger lesion than a clear cell fibrous papule. The cells of ccRCC have moderate to abundant clear cytoplasm and nuclei with varying degrees of pleomorphism (Figure 6). Periodic acid–Schiff staining demonstrates intracytoplasmic glycogen. The stroma is abundantly vascular and extravasated blood cells are frequently observed. On immunohistochemistry, the tumor cells of ccRCC stain positively for cytokeratin AE1/AE3, CAM5.2, epithelial membrane antigen, CD10, and vimentin.

A fibrous papule is a common benign lesion that usually presents in adults on the face, especially on the lower portion of the nose. It typically presents as a small (2–5 mm), asymptomatic, flesh-colored, dome-shaped lesion that is firm and nontender. Several histopathologic variants of fibrous papules have been described, including clear cell, granular, epithelioid, hypercellular, pleomorphic, pigmented, and inflammatory.1 Clear cell fibrous papules are exceedingly rare. On microscopic examination the epidermis may be normal or show some degree of hyperkeratosis and parakeratosis, erosion, ulceration, or crust. The basal layer may show an increase of melanin. The dermis is expanded by a proliferation of clear cells arranged in sheets, clusters, or as single cells (Figure 1). The clear cells show variation in size and shape. The nuclei are small and round without pleomorphism, hyperchromasia, or mitoses. The nuclei may be centrally located or eccentrically displaced by a large intracytoplasmic vacuole (Figure 2). Some clear cells may exhibit finely vacuolated cytoplasm with nuclear scalloping. The surrounding stroma usually consists of sclerotic collagen and dilated blood vessels (Figure 3). Extravasated red blood cells may be present focally. Patchy lymphocytic infiltrates may be found in the stroma at the periphery of the lesion. Periodic acid–Schiff and mucicarmine staining of the clear cells is negative. On immunohistochemistry, the clear cells are diffusely positive for vimentin and negative for cytokeratin AE1/AE3, epithelial membrane antigen, carcinoembryonic antigen, and HMB-45 (human melanoma black 45).2,3 The clear cells often are positive for CD68, factor XIIIa, and NKI/C3 (anti-CD63) but also may be negative. The S-100 protein often is negative but may be focally positive.



The differential diagnosis for clear cell fibrous papules is broad but reasonably includes balloon cell nevus, clear cell hidradenoma, and cutaneous metastasis of clear cell (conventional) renal cell carcinoma (ccRCC). Balloon cell malignant melanoma is not considered strongly in the differential diagnosis because it usually exhibits invasive growth, cytologic atypia, and mitoses, all of which are not characteristic morphologic features of clear cell fibrous papules.
A balloon cell nevus may be difficult to distinguish from a clear cell fibrous papule on routine hematoxylin and eosin staining (Figure 4); however, the nuclei of a balloon cell nevus tend to be more rounded and centrally located. Any junctional nesting or nests of conventional nevus cells in the dermis also help differentiate a balloon cell nevus from a clear cell fibrous papule. Diffusely positive immunostaining for S-100 protein also is indicative of a balloon cell nevus.

Clear cell hidradenoma consists predominantly of cells with clear cytoplasm and small dark nuclei that may closely mimic a clear cell fibrous papule (Figure 5) but often shows a second population of cells with more vesicular nuclei and dark eosinophilic cytoplasm. Cystic spaces containing hyaline material and foci of squamoid change are common, along with occasional tubular lumina that may be prominent or inconspicuous. Further, the tumor cells of clear cell hidradenoma show positive immunostaining for epithelial markers (eg, cytokeratin AE1/AE3, CAM5.2).

Cutaneous metastasis of ccRCC is rare and usually presents clinically as a larger lesion than a clear cell fibrous papule. The cells of ccRCC have moderate to abundant clear cytoplasm and nuclei with varying degrees of pleomorphism (Figure 6). Periodic acid–Schiff staining demonstrates intracytoplasmic glycogen. The stroma is abundantly vascular and extravasated blood cells are frequently observed. On immunohistochemistry, the tumor cells of ccRCC stain positively for cytokeratin AE1/AE3, CAM5.2, epithelial membrane antigen, CD10, and vimentin.

- Bansal C, Stewart D, Li A, et al. Histologic variants of fibrous papule. J Cutan Pathol. 2005;32:424-428.
- Chiang YY, Tsai HH, Lee WR, et al. Clear cell fibrous papule: report of a case mimicking a balloon cell nevus. J Cutan Pathol. 2009;36:381-384.
- Lee AN, Stein SL, Cohen LM. Clear cell fibrous papule with NKI/C3 expression: clinical and histologic features in six cases. Am J Dermatopathol. 2005;27:296-300.
- Bansal C, Stewart D, Li A, et al. Histologic variants of fibrous papule. J Cutan Pathol. 2005;32:424-428.
- Chiang YY, Tsai HH, Lee WR, et al. Clear cell fibrous papule: report of a case mimicking a balloon cell nevus. J Cutan Pathol. 2009;36:381-384.
- Lee AN, Stein SL, Cohen LM. Clear cell fibrous papule with NKI/C3 expression: clinical and histologic features in six cases. Am J Dermatopathol. 2005;27:296-300.
What Is Your Diagnosis? Cutaneous B-cell Lymphoma
A 59-year-old white man presented with 2 large erythematous lesions on the right side of the chest wall that had gradually progressed over the last 1.5 years. The patient denied any fever, night sweats, fatigue, unintentional weight loss, or loss of appetite. Physical examination revealed 2 large, well-circumscribed, nearly contiguous, firm, erythematous tumors. One tumor measured 7.5×4.5 cm and the other measured 4×3.5 cm.
The Diagnosis: Cutaneous B-cell Lymphoma
Biopsies from the right side of the chest wall (Figure 1) revealed an atypical dense and diffuse lymphocytic infiltrate throughout the dermis. There was extensive crush artifact throughout the specimen. However, the findings were consistent with cutaneous B-cell lymphoma (CBCL), and the diffuse large B-cell type was favored (Figure 2). Atypical lymphocytes stained positively for antibodies against CD20 (Figure 3), CD79a, and BCL-6, and stained negatively for antibodies against MUM-1 and BCL-2. Although flow cytometry revealed no definitive immunophenotypic lymphoma population, polymerase chain reaction analysis revealed a monoclonal immunoglobulin heavy chain gene rearrangement. Computed tomography (CT) scans of the chest, abdomen, and pelvis were unremarkable. A preliminary diagnosis of primary CBCL (PCBCL) was formulated. Diffuse large B-cell lymphoma (DLBCL) and follicle center lymphoma subtypes were each considered, which triggered further workup to rule out systemic involvement.
|
|
 |
 |
A bone marrow biopsy from the posterior iliac crest revealed normocellular bone marrow with normal trilineage hematopoiesis. However, whole-body staging with positron emission tomography (PET)–CT scanning revealed osseous disease in the left proximal humerus (Figure 4) as well as a slightly hypermetabolic right axillary lymph node. Magnetic resonance imaging of the brain showed no evidence of intracranial disease. Because of the apparent systemic involvement, stage IV non-Hodgkin lymphoma (DLBCL) became the new suspected diagnosis. The patient was started on the first of 6 cycles of chemotherapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), and the skin lesions quickly dissipated and flattened. A faint pink discoloration remained over a slightly indented area. A repeat PET-CT scan following 4 cycles of R-CHOP chemotherapy also confirmed a complete response to therapy.
In general, CBCL tends to affect adults and presents as relatively firm and plum-colored papules, nodules, tumors, or plaques, which can be either fast or slow growing. Cutaneous B-cell lymphoma may be primary or secondary to systemic involvement. Primary CBCL refers to a group of non-Hodgkin lymphomas that initially present in the skin with no evidence of extracutaneous involvement at the time of diagnosis.1,2 Secondary CBCL (SCBCL) refers to cutaneous disease that occurs secondary to systemic B-cell lymphoma. Detecting systemic involvement and distinguishing between PCBCL and SCBCL is valuable in determining prognosis and therapeutic options, as subtypes of PCBCL often have an improved prognosis and may be treated with local irradiation.
The initial staging techniques that are preferred for cutaneous lymphomas have been debated.3-5 For cutaneous lymphomas, except mycosis fungoides and Sézary syndrome, the International Society for Cutaneous Lymphomas and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer recommends obtaining a complete blood cell count with differential; complete metabolic studies including lactate dehydrogenase; and imaging studies of the chest, abdomen, and pelvis. Bone marrow biopsies and imaging studies of the neck or whole-body PET-CT scanning also may be useful depending on the clinical scenario.3 Although a more limited workup may be sufficient for PCBCLs such as primary cutaneous marginal zone lymphoma,5 a bone marrow biopsy is recommended for cases of primary cutaneous DLBCL (leg type).3 Senff et al5 supported the use of a bone marrow biopsy in the evaluation of follicle center lymphomas first presenting in the skin, though this method is controversial. In our patient, the laboratory results; bone marrow biopsy; and CT scan of the chest, abdomen, and pelvis failed to suggest extracutaneous disease, while the PET-CT scan revealed systemic involvement.
The differential diagnosis of CBCL includes cutaneous lymphoid hyperplasia (pseudolymphoma), which may be the result of insults such as arthropod bites, stings, vaccinations, or trauma. The clinical presentation, histology, and results of molecular studies and immunohistochemistry are essential in differentiating benign versus malignant processes.6 Lymphomas are expected to be larger and more persistent than benign processes, demonstrating an atypical lymphocytic infiltrate and monoclonality; immunohistochemistry will aid in the distinction between B-cell and T-cell processes and can delineate the type of B-cell lymphoma. Histology for CBCL typically reveals an atypical lymphocytic infiltrate showing a CD20+ and CD79a+ immunophenotype. Staining for antibodies against BCL-2, BCL-6, CD10, and MUM-1 also plays an important role in the diagnosis of cutaneous lymphoma and determining where the lesion(s) falls within the classification schemes. For example, to differentiate between primary cutaneous lymphoma subtypes, BCL-2 negativity and BCL-6 positivity in the context of a CD20+ and CD79a+ immunophenotype supports a follicle center lymphoma or a DLBCL (non–leg type). By contrast, CD20, CD79a, BCL-2, and MUM-1 positivity would favor a DLBCL (leg type).7
The natural history and therapeutic options differ greatly between subtypes of CBCL. For example, the prognosis of primary cutaneous follicle center lymphoma is generally favorable with a 5-year disease-specific survival rate of roughly 95%, and radiation therapy is recommended as a first-line therapy for localized disease.2,8 Conversely, primary cutaneous DLBCL (leg type) frequently spreads to extracutaneous sites8 and carries a much lower estimated 5-year disease-specific survival rate of 55%.2 Chemotherapy with R-CHOP is typically included in initial therapy for primary cutaneous DLBCL (leg type).8 The prognosis of systemic B-cell lymphomas also is highly variable and may depend on the type of B-cell lymphoma, the stage of disease at diagnosis, histologic and immunologic characteristics, and the therapy received. Wright et al9 reported that patients with systemic germinal center B cell–like DLBCL had a 5-year survival rate of 62%, whereas patients with activated B cell–like variants of DLBCL had a 5-year survival rate of 26%. Expression of CD40 may be a favorable prognostic factor following treatment with systemic chemotherapy in patients with DLBCL,10 whereas FOXP1 protein overexpression is correlated with poor disease-specific survival in certain DLBCL phenotypes.11
Although it is uncertain whether the cutaneous lesions preceded systemic disease in our patient, the cutaneous lesions could be arbitrarily classified as secondary because extracutaneous disease was discovered within 6 months of the initial diagnosis.1 However, classifying the CBCL as primary or secondary did not alter the course of treatment in our patient, as the presumed systemic disease necessitated treatment with systemic chemotherapy; both PCBCLs that develop systemic involvement and SCBCLs (primary extracutaneous disease) usually are treated with systemic chemotherapy. Our case highlights the importance of whole-body staging, as PET-CT scanning changed the course of care by detecting osseous involvement, necessitating systemic therapy as opposed to local radiation therapy alone. A multidisciplinary team with a focus on the diagnosis and management of cutaneous lymphomas helped streamline our patient’s laboratory testing and imaging studies, diagnostic and therapeutic decision making, and treatment implementation. Open channels and frequent opportunities for communication among dermatologists, dermatopathologists, medical oncologists, hematopathologists, radiologists, and radiation oncologists are needed to optimize and coordinate care for patients with cutaneous lymphoma who require transdisciplinary care.
Acknowledgement—The authors would like to thank Henry Koon, MD (hematology/oncology), for his input and expertise.
1. Willemze R, Kerl H, Sterry W, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the cutaneous lymphoma study group of the European organization for research and treatment of cancer. Blood. 1997;90:354-371.
2. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
3. Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
4. Quereux G, Frot AS, Brocard A, et al. Routine bone marrow biopsy in the initial evaluation of primary cutaneous B-cell lymphoma does not appear justified. Eur J Dermatol. 2009;19:216-220.
5. Senff NJ, Kluin-Nelemans HC, Willemze R. Results of bone marrow examination in 275 patients with histological features that suggest an indolent type of cutaneous B-cell lymphoma. Br J Haematol. 2008;142:52-56.
6. Gilliam AC, Wood GS. Cutaneous lymphoid hyperplasias. Semin Cutan Med Surg. 2000;19:133-141.
7. Burg G, Kempf W, Cozzio A, et al. WHO/EORTC classification of cutaneous lymphomas 2005: histological and molecular aspects. J Cutan Pathol. 2005;32:647-674.
8. Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
9. Wright G, Tan B, Rosenwald A, et al. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9991-9996.
10. Rydström K, Linderoth J, Nyman H, et al. CD40 is a potential marker of favorable prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma. 2010;51:1643-1648.
11. Hoeller S, Schneider A, Haralambieva E, et al. FOXP1 protein overexpression is associated with inferior outcome in nodal diffuse large B-cell lymphomas with non-gerzminal centre phenotype, independent of gains and structural aberrations at 3p14.1. Histopathology. 2010;57:73-80.
A 59-year-old white man presented with 2 large erythematous lesions on the right side of the chest wall that had gradually progressed over the last 1.5 years. The patient denied any fever, night sweats, fatigue, unintentional weight loss, or loss of appetite. Physical examination revealed 2 large, well-circumscribed, nearly contiguous, firm, erythematous tumors. One tumor measured 7.5×4.5 cm and the other measured 4×3.5 cm.
The Diagnosis: Cutaneous B-cell Lymphoma
Biopsies from the right side of the chest wall (Figure 1) revealed an atypical dense and diffuse lymphocytic infiltrate throughout the dermis. There was extensive crush artifact throughout the specimen. However, the findings were consistent with cutaneous B-cell lymphoma (CBCL), and the diffuse large B-cell type was favored (Figure 2). Atypical lymphocytes stained positively for antibodies against CD20 (Figure 3), CD79a, and BCL-6, and stained negatively for antibodies against MUM-1 and BCL-2. Although flow cytometry revealed no definitive immunophenotypic lymphoma population, polymerase chain reaction analysis revealed a monoclonal immunoglobulin heavy chain gene rearrangement. Computed tomography (CT) scans of the chest, abdomen, and pelvis were unremarkable. A preliminary diagnosis of primary CBCL (PCBCL) was formulated. Diffuse large B-cell lymphoma (DLBCL) and follicle center lymphoma subtypes were each considered, which triggered further workup to rule out systemic involvement.
|
|
 |
 |
A bone marrow biopsy from the posterior iliac crest revealed normocellular bone marrow with normal trilineage hematopoiesis. However, whole-body staging with positron emission tomography (PET)–CT scanning revealed osseous disease in the left proximal humerus (Figure 4) as well as a slightly hypermetabolic right axillary lymph node. Magnetic resonance imaging of the brain showed no evidence of intracranial disease. Because of the apparent systemic involvement, stage IV non-Hodgkin lymphoma (DLBCL) became the new suspected diagnosis. The patient was started on the first of 6 cycles of chemotherapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), and the skin lesions quickly dissipated and flattened. A faint pink discoloration remained over a slightly indented area. A repeat PET-CT scan following 4 cycles of R-CHOP chemotherapy also confirmed a complete response to therapy.
In general, CBCL tends to affect adults and presents as relatively firm and plum-colored papules, nodules, tumors, or plaques, which can be either fast or slow growing. Cutaneous B-cell lymphoma may be primary or secondary to systemic involvement. Primary CBCL refers to a group of non-Hodgkin lymphomas that initially present in the skin with no evidence of extracutaneous involvement at the time of diagnosis.1,2 Secondary CBCL (SCBCL) refers to cutaneous disease that occurs secondary to systemic B-cell lymphoma. Detecting systemic involvement and distinguishing between PCBCL and SCBCL is valuable in determining prognosis and therapeutic options, as subtypes of PCBCL often have an improved prognosis and may be treated with local irradiation.
The initial staging techniques that are preferred for cutaneous lymphomas have been debated.3-5 For cutaneous lymphomas, except mycosis fungoides and Sézary syndrome, the International Society for Cutaneous Lymphomas and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer recommends obtaining a complete blood cell count with differential; complete metabolic studies including lactate dehydrogenase; and imaging studies of the chest, abdomen, and pelvis. Bone marrow biopsies and imaging studies of the neck or whole-body PET-CT scanning also may be useful depending on the clinical scenario.3 Although a more limited workup may be sufficient for PCBCLs such as primary cutaneous marginal zone lymphoma,5 a bone marrow biopsy is recommended for cases of primary cutaneous DLBCL (leg type).3 Senff et al5 supported the use of a bone marrow biopsy in the evaluation of follicle center lymphomas first presenting in the skin, though this method is controversial. In our patient, the laboratory results; bone marrow biopsy; and CT scan of the chest, abdomen, and pelvis failed to suggest extracutaneous disease, while the PET-CT scan revealed systemic involvement.
The differential diagnosis of CBCL includes cutaneous lymphoid hyperplasia (pseudolymphoma), which may be the result of insults such as arthropod bites, stings, vaccinations, or trauma. The clinical presentation, histology, and results of molecular studies and immunohistochemistry are essential in differentiating benign versus malignant processes.6 Lymphomas are expected to be larger and more persistent than benign processes, demonstrating an atypical lymphocytic infiltrate and monoclonality; immunohistochemistry will aid in the distinction between B-cell and T-cell processes and can delineate the type of B-cell lymphoma. Histology for CBCL typically reveals an atypical lymphocytic infiltrate showing a CD20+ and CD79a+ immunophenotype. Staining for antibodies against BCL-2, BCL-6, CD10, and MUM-1 also plays an important role in the diagnosis of cutaneous lymphoma and determining where the lesion(s) falls within the classification schemes. For example, to differentiate between primary cutaneous lymphoma subtypes, BCL-2 negativity and BCL-6 positivity in the context of a CD20+ and CD79a+ immunophenotype supports a follicle center lymphoma or a DLBCL (non–leg type). By contrast, CD20, CD79a, BCL-2, and MUM-1 positivity would favor a DLBCL (leg type).7
The natural history and therapeutic options differ greatly between subtypes of CBCL. For example, the prognosis of primary cutaneous follicle center lymphoma is generally favorable with a 5-year disease-specific survival rate of roughly 95%, and radiation therapy is recommended as a first-line therapy for localized disease.2,8 Conversely, primary cutaneous DLBCL (leg type) frequently spreads to extracutaneous sites8 and carries a much lower estimated 5-year disease-specific survival rate of 55%.2 Chemotherapy with R-CHOP is typically included in initial therapy for primary cutaneous DLBCL (leg type).8 The prognosis of systemic B-cell lymphomas also is highly variable and may depend on the type of B-cell lymphoma, the stage of disease at diagnosis, histologic and immunologic characteristics, and the therapy received. Wright et al9 reported that patients with systemic germinal center B cell–like DLBCL had a 5-year survival rate of 62%, whereas patients with activated B cell–like variants of DLBCL had a 5-year survival rate of 26%. Expression of CD40 may be a favorable prognostic factor following treatment with systemic chemotherapy in patients with DLBCL,10 whereas FOXP1 protein overexpression is correlated with poor disease-specific survival in certain DLBCL phenotypes.11
Although it is uncertain whether the cutaneous lesions preceded systemic disease in our patient, the cutaneous lesions could be arbitrarily classified as secondary because extracutaneous disease was discovered within 6 months of the initial diagnosis.1 However, classifying the CBCL as primary or secondary did not alter the course of treatment in our patient, as the presumed systemic disease necessitated treatment with systemic chemotherapy; both PCBCLs that develop systemic involvement and SCBCLs (primary extracutaneous disease) usually are treated with systemic chemotherapy. Our case highlights the importance of whole-body staging, as PET-CT scanning changed the course of care by detecting osseous involvement, necessitating systemic therapy as opposed to local radiation therapy alone. A multidisciplinary team with a focus on the diagnosis and management of cutaneous lymphomas helped streamline our patient’s laboratory testing and imaging studies, diagnostic and therapeutic decision making, and treatment implementation. Open channels and frequent opportunities for communication among dermatologists, dermatopathologists, medical oncologists, hematopathologists, radiologists, and radiation oncologists are needed to optimize and coordinate care for patients with cutaneous lymphoma who require transdisciplinary care.
Acknowledgement—The authors would like to thank Henry Koon, MD (hematology/oncology), for his input and expertise.
A 59-year-old white man presented with 2 large erythematous lesions on the right side of the chest wall that had gradually progressed over the last 1.5 years. The patient denied any fever, night sweats, fatigue, unintentional weight loss, or loss of appetite. Physical examination revealed 2 large, well-circumscribed, nearly contiguous, firm, erythematous tumors. One tumor measured 7.5×4.5 cm and the other measured 4×3.5 cm.
The Diagnosis: Cutaneous B-cell Lymphoma
Biopsies from the right side of the chest wall (Figure 1) revealed an atypical dense and diffuse lymphocytic infiltrate throughout the dermis. There was extensive crush artifact throughout the specimen. However, the findings were consistent with cutaneous B-cell lymphoma (CBCL), and the diffuse large B-cell type was favored (Figure 2). Atypical lymphocytes stained positively for antibodies against CD20 (Figure 3), CD79a, and BCL-6, and stained negatively for antibodies against MUM-1 and BCL-2. Although flow cytometry revealed no definitive immunophenotypic lymphoma population, polymerase chain reaction analysis revealed a monoclonal immunoglobulin heavy chain gene rearrangement. Computed tomography (CT) scans of the chest, abdomen, and pelvis were unremarkable. A preliminary diagnosis of primary CBCL (PCBCL) was formulated. Diffuse large B-cell lymphoma (DLBCL) and follicle center lymphoma subtypes were each considered, which triggered further workup to rule out systemic involvement.
|
|
 |
 |
A bone marrow biopsy from the posterior iliac crest revealed normocellular bone marrow with normal trilineage hematopoiesis. However, whole-body staging with positron emission tomography (PET)–CT scanning revealed osseous disease in the left proximal humerus (Figure 4) as well as a slightly hypermetabolic right axillary lymph node. Magnetic resonance imaging of the brain showed no evidence of intracranial disease. Because of the apparent systemic involvement, stage IV non-Hodgkin lymphoma (DLBCL) became the new suspected diagnosis. The patient was started on the first of 6 cycles of chemotherapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), and the skin lesions quickly dissipated and flattened. A faint pink discoloration remained over a slightly indented area. A repeat PET-CT scan following 4 cycles of R-CHOP chemotherapy also confirmed a complete response to therapy.
In general, CBCL tends to affect adults and presents as relatively firm and plum-colored papules, nodules, tumors, or plaques, which can be either fast or slow growing. Cutaneous B-cell lymphoma may be primary or secondary to systemic involvement. Primary CBCL refers to a group of non-Hodgkin lymphomas that initially present in the skin with no evidence of extracutaneous involvement at the time of diagnosis.1,2 Secondary CBCL (SCBCL) refers to cutaneous disease that occurs secondary to systemic B-cell lymphoma. Detecting systemic involvement and distinguishing between PCBCL and SCBCL is valuable in determining prognosis and therapeutic options, as subtypes of PCBCL often have an improved prognosis and may be treated with local irradiation.
The initial staging techniques that are preferred for cutaneous lymphomas have been debated.3-5 For cutaneous lymphomas, except mycosis fungoides and Sézary syndrome, the International Society for Cutaneous Lymphomas and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer recommends obtaining a complete blood cell count with differential; complete metabolic studies including lactate dehydrogenase; and imaging studies of the chest, abdomen, and pelvis. Bone marrow biopsies and imaging studies of the neck or whole-body PET-CT scanning also may be useful depending on the clinical scenario.3 Although a more limited workup may be sufficient for PCBCLs such as primary cutaneous marginal zone lymphoma,5 a bone marrow biopsy is recommended for cases of primary cutaneous DLBCL (leg type).3 Senff et al5 supported the use of a bone marrow biopsy in the evaluation of follicle center lymphomas first presenting in the skin, though this method is controversial. In our patient, the laboratory results; bone marrow biopsy; and CT scan of the chest, abdomen, and pelvis failed to suggest extracutaneous disease, while the PET-CT scan revealed systemic involvement.
The differential diagnosis of CBCL includes cutaneous lymphoid hyperplasia (pseudolymphoma), which may be the result of insults such as arthropod bites, stings, vaccinations, or trauma. The clinical presentation, histology, and results of molecular studies and immunohistochemistry are essential in differentiating benign versus malignant processes.6 Lymphomas are expected to be larger and more persistent than benign processes, demonstrating an atypical lymphocytic infiltrate and monoclonality; immunohistochemistry will aid in the distinction between B-cell and T-cell processes and can delineate the type of B-cell lymphoma. Histology for CBCL typically reveals an atypical lymphocytic infiltrate showing a CD20+ and CD79a+ immunophenotype. Staining for antibodies against BCL-2, BCL-6, CD10, and MUM-1 also plays an important role in the diagnosis of cutaneous lymphoma and determining where the lesion(s) falls within the classification schemes. For example, to differentiate between primary cutaneous lymphoma subtypes, BCL-2 negativity and BCL-6 positivity in the context of a CD20+ and CD79a+ immunophenotype supports a follicle center lymphoma or a DLBCL (non–leg type). By contrast, CD20, CD79a, BCL-2, and MUM-1 positivity would favor a DLBCL (leg type).7
The natural history and therapeutic options differ greatly between subtypes of CBCL. For example, the prognosis of primary cutaneous follicle center lymphoma is generally favorable with a 5-year disease-specific survival rate of roughly 95%, and radiation therapy is recommended as a first-line therapy for localized disease.2,8 Conversely, primary cutaneous DLBCL (leg type) frequently spreads to extracutaneous sites8 and carries a much lower estimated 5-year disease-specific survival rate of 55%.2 Chemotherapy with R-CHOP is typically included in initial therapy for primary cutaneous DLBCL (leg type).8 The prognosis of systemic B-cell lymphomas also is highly variable and may depend on the type of B-cell lymphoma, the stage of disease at diagnosis, histologic and immunologic characteristics, and the therapy received. Wright et al9 reported that patients with systemic germinal center B cell–like DLBCL had a 5-year survival rate of 62%, whereas patients with activated B cell–like variants of DLBCL had a 5-year survival rate of 26%. Expression of CD40 may be a favorable prognostic factor following treatment with systemic chemotherapy in patients with DLBCL,10 whereas FOXP1 protein overexpression is correlated with poor disease-specific survival in certain DLBCL phenotypes.11
Although it is uncertain whether the cutaneous lesions preceded systemic disease in our patient, the cutaneous lesions could be arbitrarily classified as secondary because extracutaneous disease was discovered within 6 months of the initial diagnosis.1 However, classifying the CBCL as primary or secondary did not alter the course of treatment in our patient, as the presumed systemic disease necessitated treatment with systemic chemotherapy; both PCBCLs that develop systemic involvement and SCBCLs (primary extracutaneous disease) usually are treated with systemic chemotherapy. Our case highlights the importance of whole-body staging, as PET-CT scanning changed the course of care by detecting osseous involvement, necessitating systemic therapy as opposed to local radiation therapy alone. A multidisciplinary team with a focus on the diagnosis and management of cutaneous lymphomas helped streamline our patient’s laboratory testing and imaging studies, diagnostic and therapeutic decision making, and treatment implementation. Open channels and frequent opportunities for communication among dermatologists, dermatopathologists, medical oncologists, hematopathologists, radiologists, and radiation oncologists are needed to optimize and coordinate care for patients with cutaneous lymphoma who require transdisciplinary care.
Acknowledgement—The authors would like to thank Henry Koon, MD (hematology/oncology), for his input and expertise.
1. Willemze R, Kerl H, Sterry W, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the cutaneous lymphoma study group of the European organization for research and treatment of cancer. Blood. 1997;90:354-371.
2. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
3. Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
4. Quereux G, Frot AS, Brocard A, et al. Routine bone marrow biopsy in the initial evaluation of primary cutaneous B-cell lymphoma does not appear justified. Eur J Dermatol. 2009;19:216-220.
5. Senff NJ, Kluin-Nelemans HC, Willemze R. Results of bone marrow examination in 275 patients with histological features that suggest an indolent type of cutaneous B-cell lymphoma. Br J Haematol. 2008;142:52-56.
6. Gilliam AC, Wood GS. Cutaneous lymphoid hyperplasias. Semin Cutan Med Surg. 2000;19:133-141.
7. Burg G, Kempf W, Cozzio A, et al. WHO/EORTC classification of cutaneous lymphomas 2005: histological and molecular aspects. J Cutan Pathol. 2005;32:647-674.
8. Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
9. Wright G, Tan B, Rosenwald A, et al. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9991-9996.
10. Rydström K, Linderoth J, Nyman H, et al. CD40 is a potential marker of favorable prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma. 2010;51:1643-1648.
11. Hoeller S, Schneider A, Haralambieva E, et al. FOXP1 protein overexpression is associated with inferior outcome in nodal diffuse large B-cell lymphomas with non-gerzminal centre phenotype, independent of gains and structural aberrations at 3p14.1. Histopathology. 2010;57:73-80.
1. Willemze R, Kerl H, Sterry W, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the cutaneous lymphoma study group of the European organization for research and treatment of cancer. Blood. 1997;90:354-371.
2. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
3. Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
4. Quereux G, Frot AS, Brocard A, et al. Routine bone marrow biopsy in the initial evaluation of primary cutaneous B-cell lymphoma does not appear justified. Eur J Dermatol. 2009;19:216-220.
5. Senff NJ, Kluin-Nelemans HC, Willemze R. Results of bone marrow examination in 275 patients with histological features that suggest an indolent type of cutaneous B-cell lymphoma. Br J Haematol. 2008;142:52-56.
6. Gilliam AC, Wood GS. Cutaneous lymphoid hyperplasias. Semin Cutan Med Surg. 2000;19:133-141.
7. Burg G, Kempf W, Cozzio A, et al. WHO/EORTC classification of cutaneous lymphomas 2005: histological and molecular aspects. J Cutan Pathol. 2005;32:647-674.
8. Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
9. Wright G, Tan B, Rosenwald A, et al. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9991-9996.
10. Rydström K, Linderoth J, Nyman H, et al. CD40 is a potential marker of favorable prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma. 2010;51:1643-1648.
11. Hoeller S, Schneider A, Haralambieva E, et al. FOXP1 protein overexpression is associated with inferior outcome in nodal diffuse large B-cell lymphomas with non-gerzminal centre phenotype, independent of gains and structural aberrations at 3p14.1. Histopathology. 2010;57:73-80.
Health-Related Quality of Life in Skin Cancer Patients
As the most common form of cancer in the United States,1 dermatologists often focus on treating the physical aspects of skin cancer, but it is equally important to consider the consequences that this disease has on a patient’s quality of life (QOL). Health is a dynamic process, encompassing one’s physical, emotional, and psychosocial well-being. There are a number of ways to measure health outcomes including mortality, morbidity, health status, and QOL. In recent years, health-related QOL (HRQOL) outcomes in dermatology have become increasingly important to clinical practice and may become factors in quality measurement or reimbursement.
Understanding a patient’s HRQOL allows health care providers to better evaluate the burden of disease and disability associated with skin cancer and its treatment. Clinical severity is not always able to capture the extent to which a disease affects one’s life.2 Furthermore, physician estimation of disease severity is not always consistent with patient-reported outcomes.3 As such, clinical questionnaires may be invaluable tools capable of objectively reporting a patient’s perception of improvement in health, which may affect how a dermatologist approaches treatment, discussion, and maintenance.
Nonmelanoma Skin Cancer
Most nonmelanoma skin cancer (NMSC) occurs in readily visible areas, namely the head and neck. Surgical treatment minimizes recurrence and complication rates. Nonmelanoma skin cancer has a low mortality and a high cure rate if diagnosed early; therefore, it may be difficult to assess treatment efficacy on cure rates alone. The amalgamation of anxiety associated with the diagnosis, aesthetic and functional concerns regarding treatment, and long-term consequences including fear of future skin cancer may have a lasting effect on an individual’s psychosocial relationships and underscores the need for QOL studies.
Most generic QOL and dermatology-specific QOL instruments fail to accurately detect the concerns of patients with NMSC.4-6 Generic QOL measures used for skin cancer patients report scores of patients that were similar to population norms,4 suggesting that these tools may fail to appropriately assess unique QOL concerns among individuals with skin cancer. Furthermore, dermatology-specific instruments have been reported to be insensitive to specific appearance-related concerns of patients with NMSC, likely because skin cancer patients made up a small percentage of the initial population in their design.4,7 Nevertheless, dermatology-specific instruments may be suitable depending on the objectives of the study.8
Recently, skin cancer–specific QOL instruments have been developed to fill the paucity of appropriate tools for this population. These questionnaires include the Facial Skin Cancer Index, Skin Cancer Index, and the Skin Cancer Quality of Life Impact Tool.7 The Skin Cancer Index is a 15-item questionnaire validated in patients undergoing Mohs micrographic surgery and has been used to assess behavior modification and risk perceptions in NMSC patients. Importantly, it does ask the patient if he/she is worried about scarring. The Facial Skin Cancer Index and the Skin Cancer Quality of Life Impact Tool do not take into account detailed aesthetic concerns regarding facial disfigurement and scarring or expectations of reconstruction.7 It may be prudent to assess these areas with supplemental scales.
Melanoma
Melanoma, the third most common skin cancer, is highly aggressive and can affect young and middle-aged patients. Because the mortality associated with later-stage melanoma is greater, the QOL impact of melanoma differs from NMSC. There are also 3 distinct periods of melanoma HRQOL impact: diagnosis, treatment, and follow-up. Approximately 30% of patients diagnosed with melanoma report high levels of psychological distress.9 The psychosocial effects of a melanoma diagnosis are longitudinal, as there is a high survival rate in early disease but also an increased future risk for melanoma, affecting future behaviors and overall QOL. The diagnosis of melanoma also affects family members due to the increased risk among first-degree relatives. After removal of deeper melanoma, the patient remains at risk for disease progression, which can have a profound impact on his/her social and professional activities and overall lifestyle. There may be a role for longitudinal QOL assessments to monitor changes over time and direct ongoing therapy.
The proportion of patients with melanoma who report high levels of impairment in QOL is comparable to that seen in other malignancies.10 Generic QOL instruments have found that melanoma patients have medium to high levels of distress and substantial improvement in HRQOL has been achieved with cognitive-behavioral intervention.11 Quality-of-life studies also have shown levels of distress are highest at initial diagnosis and immediately following treatment.12 In a randomized surgical trial, patients with a larger excision margin had poorer mental and physical function scores on assessment.13 Skin-specific QOL instruments have been used in studies of patients with melanoma and found that postmelanoma surveillance did not impact QOL. Also, women experienced greater improvements in QOL over time after reporting lower scores immediately postsurgery.13
The FACT-melanoma (Functional Assessment of Cancer Therapy) is a melanoma-specific HRQOL assessment that has been used in patients undergoing clinical trials. It has been shown to distinguish between early and advanced-stage (stages III or IV) HRQOL issues.14 Patients with early-stage melanoma are more concerned with cosmetic outcome, and those with later-stage melanoma are more concerned with morbidity and mortality associated with treatment.
Comment
Choosing the best QOL instrument depends on the specific objectives of the study. Although generic QOL questionnaires have performed poorly in studies of specific skin diseases and even dermatology-specific tools have shown limited responsiveness in skin cancer, a combination of tools may be an effective approach. However, dermatologists must be cautious when administering these valuable tools to ensure that they do not become a burdensome task for the patient.15 Although no single skin cancer–specific QOL tool is perfect, it is likely that the current questionnaires still allow for aid with appropriate patient management and comparison of treatments.16
It behooves clinicians to recognize and appreciate the value of QOL instruments as an important adjunct to treatment. These tools have shown QOL to be an independent predictor of survival among many types of cancer patients, including melanoma.10 Currently, the psychological and emotional needs of skin cancer patients often go overlooked and undetected by conventional methods. Within one’s own practice, introducing QOL assessments can improve patient self-awareness and physician awareness of matters that may have a greater impact on patient health. On a larger scale, introducing patient-reported outcome measures can affect resource allocation by identifying patient populations that may be most impacted and can give a comprehensive method for physicians to gauge treatment efficacy, leading to improved outcomes.
1. Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
2. Motley RJ, Finlay AY. Practical use of a disability index in the routine management of acne. Clin Exp Dermatol. 1992;17:1-3.
3. Chren MM, Lasek RJ, Quinn LM, et al. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107:707-713.
4. Gibbons EC, Comabella CI, Fitzpatrick R. A structured review of patient-reported outcome measures for patients with skin cancer, 2013. Br J Dermatol. 2013;168:1176-1186.
5. Burdon-Jones D, Thomas P, Baker R. Quality of life issues in nonmetastatic skin cancer. Br J Dermatol. 2010;162:147-151.
6. Lear W, Akeroyd JD, Mittmann N, et al. Measurement of utility in nonmelanoma skin cancer. J Cutan Med Surg. 2008;12:102-106.
7. Bates AS, Davis CR, Takwale A, et al. Patient-reported outcome measures in nonmelanoma skin cancer of the face: a systematic review. Br J Dermatol. 2013;168:1187-1194.
8. Lee EH, Klassen AF, Nehal KS, et al. A systematic review of patient-reported outcome instruments of nonmelanoma skin cancer in the dermatologic population. J Am Acad Dermatol. 2013;69:e59-e67.
9. Kasparian NA. Psychological stress and melanoma: are we meeting our patients’ psychological needs? Clin Dermatol. 2013;31:41-46.
10. Cormier JN, Cromwell KD, Ross MI. Health-related quality of life in patients with melanoma: overview of instruments and outcomes. Dermatol Clin. 2012;30:245-254.
11. Trask PC, Paterson AG, Griffith KA, et al. Cognitive-behavioral intervention for distress in patients with melanoma: comparison with standard medical care and impact on quality of life. Cancer. 2003;98:854-864.
12. Boyle DA. Psychological adjustment to the melanoma experience. Semin Oncol Nurs. 2003;191:70-77.
13. Newton-Bishop JA, Nolan C, Turner F, et al. A quality-of-life study in high-risk (thickness > = or 2 mm) cutaneous melanoma patients in a randomized trial of 1-cm versus 3-cm surgical excision margins. J Investig Dermatol Symp Proc. 2004;9:152-159.
14. Winstanley JB, Saw R, Boyle F, et al. The FACT-Melanoma quality-of-life instrument: comparison of a five-point and four-point response scale using the Rasch measurement model. Melanoma Res. 2013;23:61-69.
15. Swartz RJ, Baum GP, Askew RL, et al. Reducing patient burden to the FACT-Melanoma quality-of-life questionnaire. Melanoma Res. 2012;22:158-163.
16. Black N. Patient-reported outcome measures in skin cancer. Br J Dermatol. 2013;168:1151.
As the most common form of cancer in the United States,1 dermatologists often focus on treating the physical aspects of skin cancer, but it is equally important to consider the consequences that this disease has on a patient’s quality of life (QOL). Health is a dynamic process, encompassing one’s physical, emotional, and psychosocial well-being. There are a number of ways to measure health outcomes including mortality, morbidity, health status, and QOL. In recent years, health-related QOL (HRQOL) outcomes in dermatology have become increasingly important to clinical practice and may become factors in quality measurement or reimbursement.
Understanding a patient’s HRQOL allows health care providers to better evaluate the burden of disease and disability associated with skin cancer and its treatment. Clinical severity is not always able to capture the extent to which a disease affects one’s life.2 Furthermore, physician estimation of disease severity is not always consistent with patient-reported outcomes.3 As such, clinical questionnaires may be invaluable tools capable of objectively reporting a patient’s perception of improvement in health, which may affect how a dermatologist approaches treatment, discussion, and maintenance.
Nonmelanoma Skin Cancer
Most nonmelanoma skin cancer (NMSC) occurs in readily visible areas, namely the head and neck. Surgical treatment minimizes recurrence and complication rates. Nonmelanoma skin cancer has a low mortality and a high cure rate if diagnosed early; therefore, it may be difficult to assess treatment efficacy on cure rates alone. The amalgamation of anxiety associated with the diagnosis, aesthetic and functional concerns regarding treatment, and long-term consequences including fear of future skin cancer may have a lasting effect on an individual’s psychosocial relationships and underscores the need for QOL studies.
Most generic QOL and dermatology-specific QOL instruments fail to accurately detect the concerns of patients with NMSC.4-6 Generic QOL measures used for skin cancer patients report scores of patients that were similar to population norms,4 suggesting that these tools may fail to appropriately assess unique QOL concerns among individuals with skin cancer. Furthermore, dermatology-specific instruments have been reported to be insensitive to specific appearance-related concerns of patients with NMSC, likely because skin cancer patients made up a small percentage of the initial population in their design.4,7 Nevertheless, dermatology-specific instruments may be suitable depending on the objectives of the study.8
Recently, skin cancer–specific QOL instruments have been developed to fill the paucity of appropriate tools for this population. These questionnaires include the Facial Skin Cancer Index, Skin Cancer Index, and the Skin Cancer Quality of Life Impact Tool.7 The Skin Cancer Index is a 15-item questionnaire validated in patients undergoing Mohs micrographic surgery and has been used to assess behavior modification and risk perceptions in NMSC patients. Importantly, it does ask the patient if he/she is worried about scarring. The Facial Skin Cancer Index and the Skin Cancer Quality of Life Impact Tool do not take into account detailed aesthetic concerns regarding facial disfigurement and scarring or expectations of reconstruction.7 It may be prudent to assess these areas with supplemental scales.
Melanoma
Melanoma, the third most common skin cancer, is highly aggressive and can affect young and middle-aged patients. Because the mortality associated with later-stage melanoma is greater, the QOL impact of melanoma differs from NMSC. There are also 3 distinct periods of melanoma HRQOL impact: diagnosis, treatment, and follow-up. Approximately 30% of patients diagnosed with melanoma report high levels of psychological distress.9 The psychosocial effects of a melanoma diagnosis are longitudinal, as there is a high survival rate in early disease but also an increased future risk for melanoma, affecting future behaviors and overall QOL. The diagnosis of melanoma also affects family members due to the increased risk among first-degree relatives. After removal of deeper melanoma, the patient remains at risk for disease progression, which can have a profound impact on his/her social and professional activities and overall lifestyle. There may be a role for longitudinal QOL assessments to monitor changes over time and direct ongoing therapy.
The proportion of patients with melanoma who report high levels of impairment in QOL is comparable to that seen in other malignancies.10 Generic QOL instruments have found that melanoma patients have medium to high levels of distress and substantial improvement in HRQOL has been achieved with cognitive-behavioral intervention.11 Quality-of-life studies also have shown levels of distress are highest at initial diagnosis and immediately following treatment.12 In a randomized surgical trial, patients with a larger excision margin had poorer mental and physical function scores on assessment.13 Skin-specific QOL instruments have been used in studies of patients with melanoma and found that postmelanoma surveillance did not impact QOL. Also, women experienced greater improvements in QOL over time after reporting lower scores immediately postsurgery.13
The FACT-melanoma (Functional Assessment of Cancer Therapy) is a melanoma-specific HRQOL assessment that has been used in patients undergoing clinical trials. It has been shown to distinguish between early and advanced-stage (stages III or IV) HRQOL issues.14 Patients with early-stage melanoma are more concerned with cosmetic outcome, and those with later-stage melanoma are more concerned with morbidity and mortality associated with treatment.
Comment
Choosing the best QOL instrument depends on the specific objectives of the study. Although generic QOL questionnaires have performed poorly in studies of specific skin diseases and even dermatology-specific tools have shown limited responsiveness in skin cancer, a combination of tools may be an effective approach. However, dermatologists must be cautious when administering these valuable tools to ensure that they do not become a burdensome task for the patient.15 Although no single skin cancer–specific QOL tool is perfect, it is likely that the current questionnaires still allow for aid with appropriate patient management and comparison of treatments.16
It behooves clinicians to recognize and appreciate the value of QOL instruments as an important adjunct to treatment. These tools have shown QOL to be an independent predictor of survival among many types of cancer patients, including melanoma.10 Currently, the psychological and emotional needs of skin cancer patients often go overlooked and undetected by conventional methods. Within one’s own practice, introducing QOL assessments can improve patient self-awareness and physician awareness of matters that may have a greater impact on patient health. On a larger scale, introducing patient-reported outcome measures can affect resource allocation by identifying patient populations that may be most impacted and can give a comprehensive method for physicians to gauge treatment efficacy, leading to improved outcomes.
As the most common form of cancer in the United States,1 dermatologists often focus on treating the physical aspects of skin cancer, but it is equally important to consider the consequences that this disease has on a patient’s quality of life (QOL). Health is a dynamic process, encompassing one’s physical, emotional, and psychosocial well-being. There are a number of ways to measure health outcomes including mortality, morbidity, health status, and QOL. In recent years, health-related QOL (HRQOL) outcomes in dermatology have become increasingly important to clinical practice and may become factors in quality measurement or reimbursement.
Understanding a patient’s HRQOL allows health care providers to better evaluate the burden of disease and disability associated with skin cancer and its treatment. Clinical severity is not always able to capture the extent to which a disease affects one’s life.2 Furthermore, physician estimation of disease severity is not always consistent with patient-reported outcomes.3 As such, clinical questionnaires may be invaluable tools capable of objectively reporting a patient’s perception of improvement in health, which may affect how a dermatologist approaches treatment, discussion, and maintenance.
Nonmelanoma Skin Cancer
Most nonmelanoma skin cancer (NMSC) occurs in readily visible areas, namely the head and neck. Surgical treatment minimizes recurrence and complication rates. Nonmelanoma skin cancer has a low mortality and a high cure rate if diagnosed early; therefore, it may be difficult to assess treatment efficacy on cure rates alone. The amalgamation of anxiety associated with the diagnosis, aesthetic and functional concerns regarding treatment, and long-term consequences including fear of future skin cancer may have a lasting effect on an individual’s psychosocial relationships and underscores the need for QOL studies.
Most generic QOL and dermatology-specific QOL instruments fail to accurately detect the concerns of patients with NMSC.4-6 Generic QOL measures used for skin cancer patients report scores of patients that were similar to population norms,4 suggesting that these tools may fail to appropriately assess unique QOL concerns among individuals with skin cancer. Furthermore, dermatology-specific instruments have been reported to be insensitive to specific appearance-related concerns of patients with NMSC, likely because skin cancer patients made up a small percentage of the initial population in their design.4,7 Nevertheless, dermatology-specific instruments may be suitable depending on the objectives of the study.8
Recently, skin cancer–specific QOL instruments have been developed to fill the paucity of appropriate tools for this population. These questionnaires include the Facial Skin Cancer Index, Skin Cancer Index, and the Skin Cancer Quality of Life Impact Tool.7 The Skin Cancer Index is a 15-item questionnaire validated in patients undergoing Mohs micrographic surgery and has been used to assess behavior modification and risk perceptions in NMSC patients. Importantly, it does ask the patient if he/she is worried about scarring. The Facial Skin Cancer Index and the Skin Cancer Quality of Life Impact Tool do not take into account detailed aesthetic concerns regarding facial disfigurement and scarring or expectations of reconstruction.7 It may be prudent to assess these areas with supplemental scales.
Melanoma
Melanoma, the third most common skin cancer, is highly aggressive and can affect young and middle-aged patients. Because the mortality associated with later-stage melanoma is greater, the QOL impact of melanoma differs from NMSC. There are also 3 distinct periods of melanoma HRQOL impact: diagnosis, treatment, and follow-up. Approximately 30% of patients diagnosed with melanoma report high levels of psychological distress.9 The psychosocial effects of a melanoma diagnosis are longitudinal, as there is a high survival rate in early disease but also an increased future risk for melanoma, affecting future behaviors and overall QOL. The diagnosis of melanoma also affects family members due to the increased risk among first-degree relatives. After removal of deeper melanoma, the patient remains at risk for disease progression, which can have a profound impact on his/her social and professional activities and overall lifestyle. There may be a role for longitudinal QOL assessments to monitor changes over time and direct ongoing therapy.
The proportion of patients with melanoma who report high levels of impairment in QOL is comparable to that seen in other malignancies.10 Generic QOL instruments have found that melanoma patients have medium to high levels of distress and substantial improvement in HRQOL has been achieved with cognitive-behavioral intervention.11 Quality-of-life studies also have shown levels of distress are highest at initial diagnosis and immediately following treatment.12 In a randomized surgical trial, patients with a larger excision margin had poorer mental and physical function scores on assessment.13 Skin-specific QOL instruments have been used in studies of patients with melanoma and found that postmelanoma surveillance did not impact QOL. Also, women experienced greater improvements in QOL over time after reporting lower scores immediately postsurgery.13
The FACT-melanoma (Functional Assessment of Cancer Therapy) is a melanoma-specific HRQOL assessment that has been used in patients undergoing clinical trials. It has been shown to distinguish between early and advanced-stage (stages III or IV) HRQOL issues.14 Patients with early-stage melanoma are more concerned with cosmetic outcome, and those with later-stage melanoma are more concerned with morbidity and mortality associated with treatment.
Comment
Choosing the best QOL instrument depends on the specific objectives of the study. Although generic QOL questionnaires have performed poorly in studies of specific skin diseases and even dermatology-specific tools have shown limited responsiveness in skin cancer, a combination of tools may be an effective approach. However, dermatologists must be cautious when administering these valuable tools to ensure that they do not become a burdensome task for the patient.15 Although no single skin cancer–specific QOL tool is perfect, it is likely that the current questionnaires still allow for aid with appropriate patient management and comparison of treatments.16
It behooves clinicians to recognize and appreciate the value of QOL instruments as an important adjunct to treatment. These tools have shown QOL to be an independent predictor of survival among many types of cancer patients, including melanoma.10 Currently, the psychological and emotional needs of skin cancer patients often go overlooked and undetected by conventional methods. Within one’s own practice, introducing QOL assessments can improve patient self-awareness and physician awareness of matters that may have a greater impact on patient health. On a larger scale, introducing patient-reported outcome measures can affect resource allocation by identifying patient populations that may be most impacted and can give a comprehensive method for physicians to gauge treatment efficacy, leading to improved outcomes.
1. Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
2. Motley RJ, Finlay AY. Practical use of a disability index in the routine management of acne. Clin Exp Dermatol. 1992;17:1-3.
3. Chren MM, Lasek RJ, Quinn LM, et al. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107:707-713.
4. Gibbons EC, Comabella CI, Fitzpatrick R. A structured review of patient-reported outcome measures for patients with skin cancer, 2013. Br J Dermatol. 2013;168:1176-1186.
5. Burdon-Jones D, Thomas P, Baker R. Quality of life issues in nonmetastatic skin cancer. Br J Dermatol. 2010;162:147-151.
6. Lear W, Akeroyd JD, Mittmann N, et al. Measurement of utility in nonmelanoma skin cancer. J Cutan Med Surg. 2008;12:102-106.
7. Bates AS, Davis CR, Takwale A, et al. Patient-reported outcome measures in nonmelanoma skin cancer of the face: a systematic review. Br J Dermatol. 2013;168:1187-1194.
8. Lee EH, Klassen AF, Nehal KS, et al. A systematic review of patient-reported outcome instruments of nonmelanoma skin cancer in the dermatologic population. J Am Acad Dermatol. 2013;69:e59-e67.
9. Kasparian NA. Psychological stress and melanoma: are we meeting our patients’ psychological needs? Clin Dermatol. 2013;31:41-46.
10. Cormier JN, Cromwell KD, Ross MI. Health-related quality of life in patients with melanoma: overview of instruments and outcomes. Dermatol Clin. 2012;30:245-254.
11. Trask PC, Paterson AG, Griffith KA, et al. Cognitive-behavioral intervention for distress in patients with melanoma: comparison with standard medical care and impact on quality of life. Cancer. 2003;98:854-864.
12. Boyle DA. Psychological adjustment to the melanoma experience. Semin Oncol Nurs. 2003;191:70-77.
13. Newton-Bishop JA, Nolan C, Turner F, et al. A quality-of-life study in high-risk (thickness > = or 2 mm) cutaneous melanoma patients in a randomized trial of 1-cm versus 3-cm surgical excision margins. J Investig Dermatol Symp Proc. 2004;9:152-159.
14. Winstanley JB, Saw R, Boyle F, et al. The FACT-Melanoma quality-of-life instrument: comparison of a five-point and four-point response scale using the Rasch measurement model. Melanoma Res. 2013;23:61-69.
15. Swartz RJ, Baum GP, Askew RL, et al. Reducing patient burden to the FACT-Melanoma quality-of-life questionnaire. Melanoma Res. 2012;22:158-163.
16. Black N. Patient-reported outcome measures in skin cancer. Br J Dermatol. 2013;168:1151.
1. Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
2. Motley RJ, Finlay AY. Practical use of a disability index in the routine management of acne. Clin Exp Dermatol. 1992;17:1-3.
3. Chren MM, Lasek RJ, Quinn LM, et al. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107:707-713.
4. Gibbons EC, Comabella CI, Fitzpatrick R. A structured review of patient-reported outcome measures for patients with skin cancer, 2013. Br J Dermatol. 2013;168:1176-1186.
5. Burdon-Jones D, Thomas P, Baker R. Quality of life issues in nonmetastatic skin cancer. Br J Dermatol. 2010;162:147-151.
6. Lear W, Akeroyd JD, Mittmann N, et al. Measurement of utility in nonmelanoma skin cancer. J Cutan Med Surg. 2008;12:102-106.
7. Bates AS, Davis CR, Takwale A, et al. Patient-reported outcome measures in nonmelanoma skin cancer of the face: a systematic review. Br J Dermatol. 2013;168:1187-1194.
8. Lee EH, Klassen AF, Nehal KS, et al. A systematic review of patient-reported outcome instruments of nonmelanoma skin cancer in the dermatologic population. J Am Acad Dermatol. 2013;69:e59-e67.
9. Kasparian NA. Psychological stress and melanoma: are we meeting our patients’ psychological needs? Clin Dermatol. 2013;31:41-46.
10. Cormier JN, Cromwell KD, Ross MI. Health-related quality of life in patients with melanoma: overview of instruments and outcomes. Dermatol Clin. 2012;30:245-254.
11. Trask PC, Paterson AG, Griffith KA, et al. Cognitive-behavioral intervention for distress in patients with melanoma: comparison with standard medical care and impact on quality of life. Cancer. 2003;98:854-864.
12. Boyle DA. Psychological adjustment to the melanoma experience. Semin Oncol Nurs. 2003;191:70-77.
13. Newton-Bishop JA, Nolan C, Turner F, et al. A quality-of-life study in high-risk (thickness > = or 2 mm) cutaneous melanoma patients in a randomized trial of 1-cm versus 3-cm surgical excision margins. J Investig Dermatol Symp Proc. 2004;9:152-159.
14. Winstanley JB, Saw R, Boyle F, et al. The FACT-Melanoma quality-of-life instrument: comparison of a five-point and four-point response scale using the Rasch measurement model. Melanoma Res. 2013;23:61-69.
15. Swartz RJ, Baum GP, Askew RL, et al. Reducing patient burden to the FACT-Melanoma quality-of-life questionnaire. Melanoma Res. 2012;22:158-163.
16. Black N. Patient-reported outcome measures in skin cancer. Br J Dermatol. 2013;168:1151.
Combination Therapy for Perianal Squamous Cell Carcinoma In Situ With Imiquimod and Photodynamic Therapy
Perianal squamous cell carcinoma in situ (SCCIS) is an intraepidermal neoplasm with human papillomavirus implicated in its etiology.1 It can present as a raised, scaly, erythematous, fissured, ulcerated, or pigmented lesion; however, perianal SCCIS often is subclinical and therefore requires a high level of suspicion in individuals with risk factors (eg, history of genital or perianal human papillomavirus infection, other sexually transmitted diseases, or cervical dysplasia).2 Although relatively rare, perianal SCCIS is believed to be increasing in frequency and has the potential to progress to invasive squamous cell carcinoma.1,3 The rarity of this neoplasm and its uncertain natural history has made the development of a definitive, evidence-based management strategy difficult and controversial.1,4,5 We present the case of a 61-year-old woman with perianal SCCIS who was treated with a novel combination of 5-aminolevulinic acid–based photodynamic therapy (ALA-PDT) and topical imiquimod cream 5% following 2 unsuccessful surgical excisions. Using this treatment regimen, the neoplasm resolved completely with no evidence of recurrence at 2 years’ follow-up.
Case Report
A 61-year-old woman was referred to our dermatology clinic for management of persistent SCCIS of the perianal region. A colorectal surgeon performed 2 unsuccessful excisions of the neoplasm at 6 months and 1 month prior to presentation. Biopsy results from the second excision demonstrated persistent perianal SCCIS with positive margins (Figure 1). The patient was referred to our clinic to discuss Mohs micrographic surgery versus nonsurgical treatment options. The patient’s medical history was remarkable for an abnormal Papanicolaou test 10 years prior, which resulted in cervical cryotherapy.
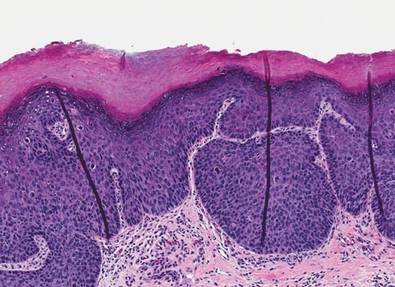 |
|
Physical examination revealed a 2×1-cm pink scar with peripheral scaling on the right anal verge (Figure 2A). Potential therapies discussed with the patient included Mohs surgery, ablative laser treatment, and nonsurgical treatment with ALA-PDT in combination with topical imiquimod cream 5%. To avoid further invasive treatments, we decided to administer a 3-week course of topical imiquimod cream 5% applied 3 times weekly to the entire affected area, followed 1 week later with a treatment of ALA-PDT. The incubation time was 6 hours. After completing 4 cycles of this therapeutic regimen, the erythema and scaling resolved (Figure 2B). The patient did not note any remarkable side effects associated with imiquimod but reported progressively resolving pain in the week following each ALA-PDT treatment, with the worst pain occurring during the first 48 hours after treatment. Three posttreatment scout biopsies revealed no evidence of residual perianal SCCIS. Two years after the negative biopsies, ongoing physical examinations demonstrated no evidence of clinical recurrence. Physicians in the dermatology and gynecology departments are following her closely.
Comment
Wide surgical excision is currently recommended as first-line therapy for perianal SCCIS.1,3,4 Unfortunately, surgical excision has been associated with difficulty in achieving disease-free margins, recurrence rates as high as 31%, and substantial morbidity.1,4 Other nonsurgical treatment modalities have been investigated for treating this neoplasm to simultaneously reduce recurrence rates while minimizing structural and functional damage to the treatment area, including radiotherapy, imiquimod, laser ablation, and ALA-PDT.1,3,4,5
In particular, imiquimod and ALA-PDT have shown promise as monotherapies for the treatment of perianal SCCIS, with several case reports describing complete resolution, low rates of recurrence, and preserved structure and function of the surrounding tissue after therapy1,3,4; however, recurrence of perianal SCCIS is still known to occur after monotherapy with either ALA-PDT or imiquimod.5,6
In contrast to noninvasive monotherapies, the literature is largely devoid of reports of noninvasive combination treatments used for perianal SCCIS. Our case represents successful use of a combination of imiquimod and ALA-PDT to treat persistent perianal SCCIS.
Conclusion
Although further research is necessary, the therapeutic success presented in our case suggests noninvasive combination therapy with imiquimod and ALA-PDT may represent a viable alternative to both surgical excision and noninvasive monotherapies for the treatment of perianal SCCIS.
Acknowledgement—The authors would like to thank Alejandro Gru, MD, Columbus, Ohio, for his review of the pathology slides for this case.
1. van Egmond S, Hoedemaker C, Sinclair R. Successful treatment of perianal Bowen’s disease with imiquimod. Int J Dermatol. 2007;46:318-319.
2. Abbasakoor F, Boulos PB. Anal intraepithelial neoplasia. Br J Surg. 2005;92:277-290.
3. Petrelli NJ, Cebollero JA, Rodriguez-Bigas M, et al. Photodynamic therapy in the management of neoplasms of the perianal skin. Arch Surg. 1992;127:1436-1438.
4. Wietfeldt E, Thiele J. Malignancies of the anal margin and perianal skin. Clin Colon Rectal Surg. 2009;22:127-135.
5. Pineda C, Welton M. Management of anal squamous intraepithelial lesions. Clin Colon Rectal Surg. 2009;22:94-101.
6. Runfola MA, Weber TK, Rodriguez-Bigas MA, et al. Photodynamic therapy for residual neoplasms of the perianal skin. Dis Colon Rectum. 2000;43:499-502.
Perianal squamous cell carcinoma in situ (SCCIS) is an intraepidermal neoplasm with human papillomavirus implicated in its etiology.1 It can present as a raised, scaly, erythematous, fissured, ulcerated, or pigmented lesion; however, perianal SCCIS often is subclinical and therefore requires a high level of suspicion in individuals with risk factors (eg, history of genital or perianal human papillomavirus infection, other sexually transmitted diseases, or cervical dysplasia).2 Although relatively rare, perianal SCCIS is believed to be increasing in frequency and has the potential to progress to invasive squamous cell carcinoma.1,3 The rarity of this neoplasm and its uncertain natural history has made the development of a definitive, evidence-based management strategy difficult and controversial.1,4,5 We present the case of a 61-year-old woman with perianal SCCIS who was treated with a novel combination of 5-aminolevulinic acid–based photodynamic therapy (ALA-PDT) and topical imiquimod cream 5% following 2 unsuccessful surgical excisions. Using this treatment regimen, the neoplasm resolved completely with no evidence of recurrence at 2 years’ follow-up.
Case Report
A 61-year-old woman was referred to our dermatology clinic for management of persistent SCCIS of the perianal region. A colorectal surgeon performed 2 unsuccessful excisions of the neoplasm at 6 months and 1 month prior to presentation. Biopsy results from the second excision demonstrated persistent perianal SCCIS with positive margins (Figure 1). The patient was referred to our clinic to discuss Mohs micrographic surgery versus nonsurgical treatment options. The patient’s medical history was remarkable for an abnormal Papanicolaou test 10 years prior, which resulted in cervical cryotherapy.
 |
|
Physical examination revealed a 2×1-cm pink scar with peripheral scaling on the right anal verge (Figure 2A). Potential therapies discussed with the patient included Mohs surgery, ablative laser treatment, and nonsurgical treatment with ALA-PDT in combination with topical imiquimod cream 5%. To avoid further invasive treatments, we decided to administer a 3-week course of topical imiquimod cream 5% applied 3 times weekly to the entire affected area, followed 1 week later with a treatment of ALA-PDT. The incubation time was 6 hours. After completing 4 cycles of this therapeutic regimen, the erythema and scaling resolved (Figure 2B). The patient did not note any remarkable side effects associated with imiquimod but reported progressively resolving pain in the week following each ALA-PDT treatment, with the worst pain occurring during the first 48 hours after treatment. Three posttreatment scout biopsies revealed no evidence of residual perianal SCCIS. Two years after the negative biopsies, ongoing physical examinations demonstrated no evidence of clinical recurrence. Physicians in the dermatology and gynecology departments are following her closely.
Comment
Wide surgical excision is currently recommended as first-line therapy for perianal SCCIS.1,3,4 Unfortunately, surgical excision has been associated with difficulty in achieving disease-free margins, recurrence rates as high as 31%, and substantial morbidity.1,4 Other nonsurgical treatment modalities have been investigated for treating this neoplasm to simultaneously reduce recurrence rates while minimizing structural and functional damage to the treatment area, including radiotherapy, imiquimod, laser ablation, and ALA-PDT.1,3,4,5
In particular, imiquimod and ALA-PDT have shown promise as monotherapies for the treatment of perianal SCCIS, with several case reports describing complete resolution, low rates of recurrence, and preserved structure and function of the surrounding tissue after therapy1,3,4; however, recurrence of perianal SCCIS is still known to occur after monotherapy with either ALA-PDT or imiquimod.5,6
In contrast to noninvasive monotherapies, the literature is largely devoid of reports of noninvasive combination treatments used for perianal SCCIS. Our case represents successful use of a combination of imiquimod and ALA-PDT to treat persistent perianal SCCIS.
Conclusion
Although further research is necessary, the therapeutic success presented in our case suggests noninvasive combination therapy with imiquimod and ALA-PDT may represent a viable alternative to both surgical excision and noninvasive monotherapies for the treatment of perianal SCCIS.
Acknowledgement—The authors would like to thank Alejandro Gru, MD, Columbus, Ohio, for his review of the pathology slides for this case.
Perianal squamous cell carcinoma in situ (SCCIS) is an intraepidermal neoplasm with human papillomavirus implicated in its etiology.1 It can present as a raised, scaly, erythematous, fissured, ulcerated, or pigmented lesion; however, perianal SCCIS often is subclinical and therefore requires a high level of suspicion in individuals with risk factors (eg, history of genital or perianal human papillomavirus infection, other sexually transmitted diseases, or cervical dysplasia).2 Although relatively rare, perianal SCCIS is believed to be increasing in frequency and has the potential to progress to invasive squamous cell carcinoma.1,3 The rarity of this neoplasm and its uncertain natural history has made the development of a definitive, evidence-based management strategy difficult and controversial.1,4,5 We present the case of a 61-year-old woman with perianal SCCIS who was treated with a novel combination of 5-aminolevulinic acid–based photodynamic therapy (ALA-PDT) and topical imiquimod cream 5% following 2 unsuccessful surgical excisions. Using this treatment regimen, the neoplasm resolved completely with no evidence of recurrence at 2 years’ follow-up.
Case Report
A 61-year-old woman was referred to our dermatology clinic for management of persistent SCCIS of the perianal region. A colorectal surgeon performed 2 unsuccessful excisions of the neoplasm at 6 months and 1 month prior to presentation. Biopsy results from the second excision demonstrated persistent perianal SCCIS with positive margins (Figure 1). The patient was referred to our clinic to discuss Mohs micrographic surgery versus nonsurgical treatment options. The patient’s medical history was remarkable for an abnormal Papanicolaou test 10 years prior, which resulted in cervical cryotherapy.
 |
|
Physical examination revealed a 2×1-cm pink scar with peripheral scaling on the right anal verge (Figure 2A). Potential therapies discussed with the patient included Mohs surgery, ablative laser treatment, and nonsurgical treatment with ALA-PDT in combination with topical imiquimod cream 5%. To avoid further invasive treatments, we decided to administer a 3-week course of topical imiquimod cream 5% applied 3 times weekly to the entire affected area, followed 1 week later with a treatment of ALA-PDT. The incubation time was 6 hours. After completing 4 cycles of this therapeutic regimen, the erythema and scaling resolved (Figure 2B). The patient did not note any remarkable side effects associated with imiquimod but reported progressively resolving pain in the week following each ALA-PDT treatment, with the worst pain occurring during the first 48 hours after treatment. Three posttreatment scout biopsies revealed no evidence of residual perianal SCCIS. Two years after the negative biopsies, ongoing physical examinations demonstrated no evidence of clinical recurrence. Physicians in the dermatology and gynecology departments are following her closely.
Comment
Wide surgical excision is currently recommended as first-line therapy for perianal SCCIS.1,3,4 Unfortunately, surgical excision has been associated with difficulty in achieving disease-free margins, recurrence rates as high as 31%, and substantial morbidity.1,4 Other nonsurgical treatment modalities have been investigated for treating this neoplasm to simultaneously reduce recurrence rates while minimizing structural and functional damage to the treatment area, including radiotherapy, imiquimod, laser ablation, and ALA-PDT.1,3,4,5
In particular, imiquimod and ALA-PDT have shown promise as monotherapies for the treatment of perianal SCCIS, with several case reports describing complete resolution, low rates of recurrence, and preserved structure and function of the surrounding tissue after therapy1,3,4; however, recurrence of perianal SCCIS is still known to occur after monotherapy with either ALA-PDT or imiquimod.5,6
In contrast to noninvasive monotherapies, the literature is largely devoid of reports of noninvasive combination treatments used for perianal SCCIS. Our case represents successful use of a combination of imiquimod and ALA-PDT to treat persistent perianal SCCIS.
Conclusion
Although further research is necessary, the therapeutic success presented in our case suggests noninvasive combination therapy with imiquimod and ALA-PDT may represent a viable alternative to both surgical excision and noninvasive monotherapies for the treatment of perianal SCCIS.
Acknowledgement—The authors would like to thank Alejandro Gru, MD, Columbus, Ohio, for his review of the pathology slides for this case.
1. van Egmond S, Hoedemaker C, Sinclair R. Successful treatment of perianal Bowen’s disease with imiquimod. Int J Dermatol. 2007;46:318-319.
2. Abbasakoor F, Boulos PB. Anal intraepithelial neoplasia. Br J Surg. 2005;92:277-290.
3. Petrelli NJ, Cebollero JA, Rodriguez-Bigas M, et al. Photodynamic therapy in the management of neoplasms of the perianal skin. Arch Surg. 1992;127:1436-1438.
4. Wietfeldt E, Thiele J. Malignancies of the anal margin and perianal skin. Clin Colon Rectal Surg. 2009;22:127-135.
5. Pineda C, Welton M. Management of anal squamous intraepithelial lesions. Clin Colon Rectal Surg. 2009;22:94-101.
6. Runfola MA, Weber TK, Rodriguez-Bigas MA, et al. Photodynamic therapy for residual neoplasms of the perianal skin. Dis Colon Rectum. 2000;43:499-502.
1. van Egmond S, Hoedemaker C, Sinclair R. Successful treatment of perianal Bowen’s disease with imiquimod. Int J Dermatol. 2007;46:318-319.
2. Abbasakoor F, Boulos PB. Anal intraepithelial neoplasia. Br J Surg. 2005;92:277-290.
3. Petrelli NJ, Cebollero JA, Rodriguez-Bigas M, et al. Photodynamic therapy in the management of neoplasms of the perianal skin. Arch Surg. 1992;127:1436-1438.
4. Wietfeldt E, Thiele J. Malignancies of the anal margin and perianal skin. Clin Colon Rectal Surg. 2009;22:127-135.
5. Pineda C, Welton M. Management of anal squamous intraepithelial lesions. Clin Colon Rectal Surg. 2009;22:94-101.
6. Runfola MA, Weber TK, Rodriguez-Bigas MA, et al. Photodynamic therapy for residual neoplasms of the perianal skin. Dis Colon Rectum. 2000;43:499-502.
Practice Points
- Perianal squamous cell carcinoma in situ (SCCIS) is an uncommon malignancy with most cases related to human papillomavirus infection.
- Treatment of perianal SCCIS is important to prevent progression to invasive squamous cell carcinoma.
- Treatment usually is surgical, but nonsurgical modalities such as combination therapy with imiquimod and 5-aminolevulinic acid–based photodynamic therapy should be considered in patients who are poor surgical candidates or in cases of recurrences after excisional surgery.
Risk Factors for Malignant Melanoma and Preventive Methods
Cutaneous melanoma is a malignant tumor of the skin that develops from melanin-producing pigment cells known as melanocytes. The development of melanoma is a multifactorial process. External factors, genetic predisposition, or both may cause damage to DNA in melanoma cells. Genetic mutations may occur de novo or can be transferred from generation to generation. The most important environmental risk factor is UV radiation, both natural and artificial. Other risk factors include skin type, ethnicity, number of melanocytic nevi, number and severity of sunburns, frequency and duration of UV exposure, geographic location, and level of awareness about malignant melanoma (MM) and its risk factors.1
Melanoma accounts for only 1% to 2% of all tumors but is known for its rapidly increasing incidence.2 White individuals who reside in sunny areas of North America, northern Europe, Australia, and New Zealand seem to be at the highest risk for developing melanoma.3 The global incidence of MM from 2004 to 2008 was 20.8 individuals per 100,000 people.4 In Central Europe, 10 to 12 individuals per 100,000 people were diagnosed with melanoma, and 50 to 60 individuals per 100,000 people were diagnosed in Australia. In 2011, the lifetime risk of being diagnosed with melanoma was 1% in Central Europe and 4% in Australia.2 The incidence of melanoma is lower in populations with darker skin types (ie, Africans, Asians). In some parts of the world, the overall incidence and/or severity of melanoma has been declining over the last few decades, possibly reflecting improved public awareness.5
Cutaneous MM is an aggressive skin cancer that has fatal consequences if diagnosed late. Chances of survival, however, increase dramatically when melanoma is detected early. Collecting and analyzing data about a certain disease leads to a better understanding of the condition and encourages the development of prevention strategies. Epidemiologic research helps to improve patient care by measuring the occurrence of an event and by investigating the relationship between the occurrence of an event and associated factors; in doing so, epidemiologic research directly enables a better understanding of the disease and promotes effective preventive and therapeutic approaches.6
Although risk factors for melanoma are well established, current epidemiologic research shows that information on UV exposure and its association with this disease in many parts of the world, including Central Europe, is lacking. The aim of this study was to investigate behavioral and sociodemographic factors associated with the development of MM in the Czech Republic and Germany.
Materials and Methods
This hospital-based, case-control study was conducted in the largest dermatology departments in the Czech Republic (Clinic of Dermatology and Venereology, Third Faculty of Medicine, Charles University, Prague) and Germany (Department of Dermatology and Allergology, Ludwig Maximilian University, Munich). Data from the Czech Republic and Germany were not evaluated separately. These 2 countries were chosen as a representative sample population from Central Europe.
Study Population
The study population included 207 patients (103 men; 104 women) aged 31 to 94 years who were consecutively diagnosed with MM (cases). Patients with acral lentiginous melanoma were excluded from the study due to the generally accepted theory that the condition is not linked to UV exposure. Melanoma diagnosis was based on histopathologic examination. The study population also included 235 randomly selected controls (110 men; 125 women) from the same 2 study centers who had been hospitalized due to other dermatologic diagnoses with no history of any skin cancer. Among patients asked to take part in the study, the participation rates were 83% among cases and 62% among controls.
Assessment
Various sociodemographic factors and factors related to UV exposure were assessed via administration of a structured questionnaire that was completed by all 442 patients.
Four statistical models concerning variables were constructed. The basic model, which was part of all subsequent models, included age, sex, education, and history of skin tumors. Variables included in the biological model were eye color (light vs dark) and Fitzpatrick skin type (I–V). Variables included in the lifestyle model were the use of sunscreen (never and rarely; often; always; always and repetitively), sun exposure during work (yes/no), and seaside vacation (never, rarely, regularly, more than once per year). The variable in the exposure model was the number of sunburns during childhood and adolescence (none, 1–5 times, 6–10 times, ≥11 times).
Sociodemographic characteristics (sex, age, education) and prior incidence of skin tumor were included in each model. Although there were no statistically significant differences in the incidence of melanoma associated with sex and age, those variables were kept in the models to control the impact of other variables by sex and age.
Other variables were added into the model one by one, and the likelihood ratio was tested step-by-step. Only the variables that improved the model fit were kept in the final model. Impact of variables on dependent variables also was tested; variables with no significant impact on dependent variables were left out of the model.
Statistical Analysis
The association between risk factors and MM was assessed using multivariate logistic regression. In total, 4 models were included in the results, which were presented as odds ratios (ORs) and 95% confidence intervals (CIs). A significance level of α=.05 was chosen. The statistical program Stata 11 was used for all analyses.
Results
Descriptive data on the 442 patients surveyed are shown in Table 1. The results of the logistic regression in all studied models are shown in Table 2.
Basic Model
There was no difference in the proportion of men and women in the melanoma and control groups. We observed that more patients in the melanoma group had a university degree than patients in the control group. Patients in the melanoma group with a history of MM showed 4.2 times higher risk for developing another melanoma.
Biological Model
Eye color and Fitzpatrick skin type were the focus of the biological model. The odds of being diagnosed with melanoma were 2.5 times greater in respondents with a light eye color (ie, blue, green, gray) than in respondents with a dark eye color (ie, brown, black). Respondents with Fitzpatrick skin types I and II had a significantly higher association with melanoma (OR, 4.25 and 6.98; 95% CI, 2.13-8.51 and 3.78-12.88) than Fitzpatrick skin type III (OR, 1.0)(P<.001 for both). Respondents with darker skin types (IV and V) also were present in our study population. The numbers were low, and the CI was too wide; nevertheless, the results were statistically significant (P<.001).
Lifestyle Model
The lifestyle model included patients’ use of sunscreen and level of sun exposure at work and on vacation. Respondents who did not use sunscreen were 12 times more likely to develop melanoma than those who always used it (95% CI, 5.56-27.14); however, individuals who used sunscreen always and repetitively (ie, more than once during 1 period of sun exposure) had a higher likelihood of melanoma compared to those who always used it. The incidence of melanoma was lower in respondents who regularly spent their vacations by the sea than those who did not vacation in seaside regions. Respondents who worked in direct sunlight were approximately 2 times more likely to present with melanoma than individuals who did not work outside.
Exposure Model
The number of sunburns sustained during childhood and adolescence was assessed in the exposure model. Respondents with a history of 1 to 5 sunburns during childhood and adolescence did not show a statistically significant increase in the incidence of melanoma diagnosis; however, those with a history of 6 or more sunburns during these periods showed a significant increase in the odds of developing melanoma (OR, 4.95 and 25.52; 95% CI, 2.29-10.71 and 12.16-53.54)(P<.001 for both).
Comment
In this study, we concentrated on UV exposure and various sociodemographic factors that were possibly connected to a higher risk for developing melanoma. We observed that the majority of patients in the melanoma group had achieved a higher level of education than the control group. Most of the melanoma group patients had light-colored eyes and spent more time in direct sunlight at work. Although seaside vacations did not correlate with a higher occurrence of melanoma, it was noted that the melanoma patients used sunscreen much less often than the control group. Major differences among respondents in the melanoma group versus the control group were seen in the reported number of sunburns sustained in childhood and adolescence. More sunburns during these periods seemed to play the most important role in the risk for melanoma. Some of the patient responses to the questionnaire may be biased, as respondents answered the questions by themselves.
Because risk factors for and preventive methods against melanoma are well established, one would assume that general knowledge regarding melanoma is adequate. On the contrary, it has been shown that knowledge about melanoma is insufficient, even among professionals and individuals with higher levels of education. In a study based on a questionnaire administered to plastic surgeons, only 37.5% (27/72) of respondents correctly identified the duration of action of sunscreen to be 3 to 4 hours.7 Approximately half of the respondents (37/72) did not know that geographical conditions such as altitude and latitude as well as shade can alter sunscreen efficacy and also were not aware of the protective action of clothing. These results are alarming and indicate that even medical professionals, who should play a main role in improving the health knowledge of the general population, have an unsatisfactory level of education in prevention of melanoma. Another important part of better education of specialists treating skin disorders is good knowledge of dermatoscopy. In fact, the Annual Skin Cancer Conference 2011 in Australia emphasized the importance of dermatoscopy in primary and secondary prevention of skin cancer.8 Teaching dermatoscopy should be part of melanoma campaigns for professionals.
Our basic model demonstrated that a higher level of education was connected to a higher occurrence of MM, which may seem surprising, considering that most diseases, along with their incidence, prevalence, and mortality, usually are associated with lower levels of education or lower socioeconomic status. A similar trend also was reported in prior studies, with higher socioeconomic groups showing higher incidences of cutaneous melanoma; colon cancer; brain cancer in men; and breast and ovarian cancer in women. Additionally, patients with higher socioeconomic status have been shown to have a survival advantage.9 Individuals with higher socioeconomic status can afford to travel more often for vacation and are more frequently exposed to direct sun. Individuals with higher levels of education also are generally more aware of the importance of disease prevention and therefore go for preventive checkups more often. The detection of melanoma in this socioeconomic group should be higher.
Our biological model demonstrated that respondents with lighter eyes had melanoma almost 3 times more often than individuals with darker eyes. Fitzpatrick skin types I and II also were significantly associated with the development of melanoma (P<.001). These findings are generally confirmed in the literature. In a study of the incidence of melanoma in Spain, statistically significant risk factors included blonde or red hair (P=.002), multiple melanocytic nevi (P=.002), Fitzpatrick skin types I and II (P=.002), and a history of actinic keratosis (P=.021) or nonmelanoma skin cancer (P=.002).10 A group in Italy also has investigated the main risk factors for melanoma. This study suggested dividing patients into high-risk subgroups to help minimize exposure to UV radiation and diagnose melanoma in its early stage.11
The results from our study confirmed the importance of concentrating melanoma prevention campaign efforts on high-risk patients. Dividing these patients into subgroups (eg, individuals who play outdoor sports, individuals with occupations associated with UV exposure, individuals who use indoor tanning beds, individuals with a family history of melanoma) may be helpful. A case-control study on sun-seeking behavior in the Czech Republic showed that the most alarming risk factors were all-day sun exposure during adolescence, frequent holidays spent in the mountains, and inadequate use of sunscreen in adulthood.12 We investigated the effects of sunscreen use on the incidence of melanoma in our lifestyle model and discovered that it decreased the risk for melanoma. Respondents who used it always had a much lower risk for developing melanoma than those who never or rarely applied it. Individuals who used sunscreen always and repetitively (ie, more than once per period of sun exposure) did not show a lower risk than those who used it once per period of sun exposure. This finding could mean that patients who are known to get sunburns or who feel a certain discomfort on direct exposure to the sun tend to use sunscreen always and repetitively.
It is important to note that some investigators disagree with the importance of some generally accepted means of prevention, such as the effect of sunscreen products. Due to insufficient evidence, the role of sunscreen use in reducing the risk for skin cancer, especially cutaneous MM, is controversial.13 Although we could prove there is a considerable difference in the incidence of melanoma in patients who claimed to use sunscreen always versus those who never use it, we agree that more evidence on this topic is needed. Furthermore, it has been reported that risk for melanoma has increased with rising intermittent sun exposure and indoor tanning bed use.14,15
Respondents who regularly traveled to seaside regions showed a surprisingly lower incidence of melanoma than respondents who did not spend their vacations in seaside locations. It is possible that individuals who choose not to spend their vacations at the seaside are more prone to sunburns and therefore do not prefer to spend their free time in direct sunlight. Another possible explanation is that individuals who regularly travel to seaside regions actively try to protect themselves from sunlight and sunburns. A higher incidence of melanoma also was observed in respondents who reported sun exposure during work.
In our exposure model, we demonstrated that a history of sunburns is the strongest risk factor for melanoma. Frequent sunburns during childhood and adolescence were strongly associated with the development of MM. This association has been supported in a systematic review on sun exposure during childhood and associated risks.16
Conclusion
To improve patient knowledge about melanoma prevention, we suggest directing targeted campaigns that address high-risk population groups, such as individuals with red hair and/or light eyes, people with an occupation associated with frequent UV exposure, and individuals with higher levels of education. With regard to younger populations, parents as well as physicians and teachers should be aware that frequent sunburns during childhood and adolescence and use of tanning beds are 2 main risk factors for MM.
1. IARC monographs on the evaluation of carcinogenic risks to humans. solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:1-316.
2. Kunte C, Geimer T, Baumert J, et al. Analysis of predictive factors for the outcome of complete lymph node dissection in melanoma patients with metastatic sentinel lymph nodes. J Am Acad Dermatol. 2011;64:655-662.
3. Parkin D, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
4. SEER Stat Fact Sheets: melanoma of the skin. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/melan.html#incidence-mortality. Accessed October 25, 2014.
5. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
6. Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease [published online ahead of print December 8, 2011]. J Invest Dermatol. 2012;132(3, pt 2):994-1002.
7. Magdum A, Leonforte F, McNaughton E, et al. Sun protection–do we know enough [published online ahead of print February 8, 2012]? J Plast Reconstr Aesthet Surg. 2012;65:1384-1389.
8. Zalaudek I, Whiteman D, Rosendahl C, et al. Update on melanoma and non-melanoma skin cancer. Annual Skin Cancer Conference 2011, Hamilton Island, Australia, 2011. Expert Rev Anticancer Ther. 2011;11:1829-1832.
9. Mackenbach JP. Health inequalities: Europe in profile. http://ec.europa.eu/health/ph_determinants/socio_economics/documents/ev_060302_rd06_en.pdf. Published February 2006. Accessed October 25, 2014.
10. Ballester I, Oliver V, Bañuls J, et al. Multicenter case-control study of risk factors for cutaneous melanoma in Valencia, Spain [published online ahead of print May 22, 2012]. Actas Dermosifiliogr. 2012;103:790-797.
11. Jaimes N, Marghoob AA. An update on risk factors, prognosis and management of melanoma patients. G Ital Dermatol Venereol. 2012;147:1-19.
12. Vranova J, Arenbergerova M, Arenberger P, et al. Incidence of cutaneous malignant melanoma in the Czech Republic: the risks of sun exposure for adolescents. Neoplasma. 2012;59:316-325.
13. Planta MB. Sunscreen and melanoma: is our prevention message correct? J Am Board Fam Med. 2011;24:735-739.
14. Veierød MB, Adami HO, Lund E, et al. Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi. Cancer Epidemiol Biomarkers Prev. 2010;19:111-120.
15. Doré JF, Chignol MC. Tanning salons and skin cancer [published online ahead of print August 15, 2011]. Photochem Photobiol Sci. 2012;11:30-37.
16. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69-82.
Cutaneous melanoma is a malignant tumor of the skin that develops from melanin-producing pigment cells known as melanocytes. The development of melanoma is a multifactorial process. External factors, genetic predisposition, or both may cause damage to DNA in melanoma cells. Genetic mutations may occur de novo or can be transferred from generation to generation. The most important environmental risk factor is UV radiation, both natural and artificial. Other risk factors include skin type, ethnicity, number of melanocytic nevi, number and severity of sunburns, frequency and duration of UV exposure, geographic location, and level of awareness about malignant melanoma (MM) and its risk factors.1
Melanoma accounts for only 1% to 2% of all tumors but is known for its rapidly increasing incidence.2 White individuals who reside in sunny areas of North America, northern Europe, Australia, and New Zealand seem to be at the highest risk for developing melanoma.3 The global incidence of MM from 2004 to 2008 was 20.8 individuals per 100,000 people.4 In Central Europe, 10 to 12 individuals per 100,000 people were diagnosed with melanoma, and 50 to 60 individuals per 100,000 people were diagnosed in Australia. In 2011, the lifetime risk of being diagnosed with melanoma was 1% in Central Europe and 4% in Australia.2 The incidence of melanoma is lower in populations with darker skin types (ie, Africans, Asians). In some parts of the world, the overall incidence and/or severity of melanoma has been declining over the last few decades, possibly reflecting improved public awareness.5
Cutaneous MM is an aggressive skin cancer that has fatal consequences if diagnosed late. Chances of survival, however, increase dramatically when melanoma is detected early. Collecting and analyzing data about a certain disease leads to a better understanding of the condition and encourages the development of prevention strategies. Epidemiologic research helps to improve patient care by measuring the occurrence of an event and by investigating the relationship between the occurrence of an event and associated factors; in doing so, epidemiologic research directly enables a better understanding of the disease and promotes effective preventive and therapeutic approaches.6
Although risk factors for melanoma are well established, current epidemiologic research shows that information on UV exposure and its association with this disease in many parts of the world, including Central Europe, is lacking. The aim of this study was to investigate behavioral and sociodemographic factors associated with the development of MM in the Czech Republic and Germany.
Materials and Methods
This hospital-based, case-control study was conducted in the largest dermatology departments in the Czech Republic (Clinic of Dermatology and Venereology, Third Faculty of Medicine, Charles University, Prague) and Germany (Department of Dermatology and Allergology, Ludwig Maximilian University, Munich). Data from the Czech Republic and Germany were not evaluated separately. These 2 countries were chosen as a representative sample population from Central Europe.
Study Population
The study population included 207 patients (103 men; 104 women) aged 31 to 94 years who were consecutively diagnosed with MM (cases). Patients with acral lentiginous melanoma were excluded from the study due to the generally accepted theory that the condition is not linked to UV exposure. Melanoma diagnosis was based on histopathologic examination. The study population also included 235 randomly selected controls (110 men; 125 women) from the same 2 study centers who had been hospitalized due to other dermatologic diagnoses with no history of any skin cancer. Among patients asked to take part in the study, the participation rates were 83% among cases and 62% among controls.
Assessment
Various sociodemographic factors and factors related to UV exposure were assessed via administration of a structured questionnaire that was completed by all 442 patients.
Four statistical models concerning variables were constructed. The basic model, which was part of all subsequent models, included age, sex, education, and history of skin tumors. Variables included in the biological model were eye color (light vs dark) and Fitzpatrick skin type (I–V). Variables included in the lifestyle model were the use of sunscreen (never and rarely; often; always; always and repetitively), sun exposure during work (yes/no), and seaside vacation (never, rarely, regularly, more than once per year). The variable in the exposure model was the number of sunburns during childhood and adolescence (none, 1–5 times, 6–10 times, ≥11 times).
Sociodemographic characteristics (sex, age, education) and prior incidence of skin tumor were included in each model. Although there were no statistically significant differences in the incidence of melanoma associated with sex and age, those variables were kept in the models to control the impact of other variables by sex and age.
Other variables were added into the model one by one, and the likelihood ratio was tested step-by-step. Only the variables that improved the model fit were kept in the final model. Impact of variables on dependent variables also was tested; variables with no significant impact on dependent variables were left out of the model.
Statistical Analysis
The association between risk factors and MM was assessed using multivariate logistic regression. In total, 4 models were included in the results, which were presented as odds ratios (ORs) and 95% confidence intervals (CIs). A significance level of α=.05 was chosen. The statistical program Stata 11 was used for all analyses.
Results
Descriptive data on the 442 patients surveyed are shown in Table 1. The results of the logistic regression in all studied models are shown in Table 2.
Basic Model
There was no difference in the proportion of men and women in the melanoma and control groups. We observed that more patients in the melanoma group had a university degree than patients in the control group. Patients in the melanoma group with a history of MM showed 4.2 times higher risk for developing another melanoma.
Biological Model
Eye color and Fitzpatrick skin type were the focus of the biological model. The odds of being diagnosed with melanoma were 2.5 times greater in respondents with a light eye color (ie, blue, green, gray) than in respondents with a dark eye color (ie, brown, black). Respondents with Fitzpatrick skin types I and II had a significantly higher association with melanoma (OR, 4.25 and 6.98; 95% CI, 2.13-8.51 and 3.78-12.88) than Fitzpatrick skin type III (OR, 1.0)(P<.001 for both). Respondents with darker skin types (IV and V) also were present in our study population. The numbers were low, and the CI was too wide; nevertheless, the results were statistically significant (P<.001).
Lifestyle Model
The lifestyle model included patients’ use of sunscreen and level of sun exposure at work and on vacation. Respondents who did not use sunscreen were 12 times more likely to develop melanoma than those who always used it (95% CI, 5.56-27.14); however, individuals who used sunscreen always and repetitively (ie, more than once during 1 period of sun exposure) had a higher likelihood of melanoma compared to those who always used it. The incidence of melanoma was lower in respondents who regularly spent their vacations by the sea than those who did not vacation in seaside regions. Respondents who worked in direct sunlight were approximately 2 times more likely to present with melanoma than individuals who did not work outside.
Exposure Model
The number of sunburns sustained during childhood and adolescence was assessed in the exposure model. Respondents with a history of 1 to 5 sunburns during childhood and adolescence did not show a statistically significant increase in the incidence of melanoma diagnosis; however, those with a history of 6 or more sunburns during these periods showed a significant increase in the odds of developing melanoma (OR, 4.95 and 25.52; 95% CI, 2.29-10.71 and 12.16-53.54)(P<.001 for both).
Comment
In this study, we concentrated on UV exposure and various sociodemographic factors that were possibly connected to a higher risk for developing melanoma. We observed that the majority of patients in the melanoma group had achieved a higher level of education than the control group. Most of the melanoma group patients had light-colored eyes and spent more time in direct sunlight at work. Although seaside vacations did not correlate with a higher occurrence of melanoma, it was noted that the melanoma patients used sunscreen much less often than the control group. Major differences among respondents in the melanoma group versus the control group were seen in the reported number of sunburns sustained in childhood and adolescence. More sunburns during these periods seemed to play the most important role in the risk for melanoma. Some of the patient responses to the questionnaire may be biased, as respondents answered the questions by themselves.
Because risk factors for and preventive methods against melanoma are well established, one would assume that general knowledge regarding melanoma is adequate. On the contrary, it has been shown that knowledge about melanoma is insufficient, even among professionals and individuals with higher levels of education. In a study based on a questionnaire administered to plastic surgeons, only 37.5% (27/72) of respondents correctly identified the duration of action of sunscreen to be 3 to 4 hours.7 Approximately half of the respondents (37/72) did not know that geographical conditions such as altitude and latitude as well as shade can alter sunscreen efficacy and also were not aware of the protective action of clothing. These results are alarming and indicate that even medical professionals, who should play a main role in improving the health knowledge of the general population, have an unsatisfactory level of education in prevention of melanoma. Another important part of better education of specialists treating skin disorders is good knowledge of dermatoscopy. In fact, the Annual Skin Cancer Conference 2011 in Australia emphasized the importance of dermatoscopy in primary and secondary prevention of skin cancer.8 Teaching dermatoscopy should be part of melanoma campaigns for professionals.
Our basic model demonstrated that a higher level of education was connected to a higher occurrence of MM, which may seem surprising, considering that most diseases, along with their incidence, prevalence, and mortality, usually are associated with lower levels of education or lower socioeconomic status. A similar trend also was reported in prior studies, with higher socioeconomic groups showing higher incidences of cutaneous melanoma; colon cancer; brain cancer in men; and breast and ovarian cancer in women. Additionally, patients with higher socioeconomic status have been shown to have a survival advantage.9 Individuals with higher socioeconomic status can afford to travel more often for vacation and are more frequently exposed to direct sun. Individuals with higher levels of education also are generally more aware of the importance of disease prevention and therefore go for preventive checkups more often. The detection of melanoma in this socioeconomic group should be higher.
Our biological model demonstrated that respondents with lighter eyes had melanoma almost 3 times more often than individuals with darker eyes. Fitzpatrick skin types I and II also were significantly associated with the development of melanoma (P<.001). These findings are generally confirmed in the literature. In a study of the incidence of melanoma in Spain, statistically significant risk factors included blonde or red hair (P=.002), multiple melanocytic nevi (P=.002), Fitzpatrick skin types I and II (P=.002), and a history of actinic keratosis (P=.021) or nonmelanoma skin cancer (P=.002).10 A group in Italy also has investigated the main risk factors for melanoma. This study suggested dividing patients into high-risk subgroups to help minimize exposure to UV radiation and diagnose melanoma in its early stage.11
The results from our study confirmed the importance of concentrating melanoma prevention campaign efforts on high-risk patients. Dividing these patients into subgroups (eg, individuals who play outdoor sports, individuals with occupations associated with UV exposure, individuals who use indoor tanning beds, individuals with a family history of melanoma) may be helpful. A case-control study on sun-seeking behavior in the Czech Republic showed that the most alarming risk factors were all-day sun exposure during adolescence, frequent holidays spent in the mountains, and inadequate use of sunscreen in adulthood.12 We investigated the effects of sunscreen use on the incidence of melanoma in our lifestyle model and discovered that it decreased the risk for melanoma. Respondents who used it always had a much lower risk for developing melanoma than those who never or rarely applied it. Individuals who used sunscreen always and repetitively (ie, more than once per period of sun exposure) did not show a lower risk than those who used it once per period of sun exposure. This finding could mean that patients who are known to get sunburns or who feel a certain discomfort on direct exposure to the sun tend to use sunscreen always and repetitively.
It is important to note that some investigators disagree with the importance of some generally accepted means of prevention, such as the effect of sunscreen products. Due to insufficient evidence, the role of sunscreen use in reducing the risk for skin cancer, especially cutaneous MM, is controversial.13 Although we could prove there is a considerable difference in the incidence of melanoma in patients who claimed to use sunscreen always versus those who never use it, we agree that more evidence on this topic is needed. Furthermore, it has been reported that risk for melanoma has increased with rising intermittent sun exposure and indoor tanning bed use.14,15
Respondents who regularly traveled to seaside regions showed a surprisingly lower incidence of melanoma than respondents who did not spend their vacations in seaside locations. It is possible that individuals who choose not to spend their vacations at the seaside are more prone to sunburns and therefore do not prefer to spend their free time in direct sunlight. Another possible explanation is that individuals who regularly travel to seaside regions actively try to protect themselves from sunlight and sunburns. A higher incidence of melanoma also was observed in respondents who reported sun exposure during work.
In our exposure model, we demonstrated that a history of sunburns is the strongest risk factor for melanoma. Frequent sunburns during childhood and adolescence were strongly associated with the development of MM. This association has been supported in a systematic review on sun exposure during childhood and associated risks.16
Conclusion
To improve patient knowledge about melanoma prevention, we suggest directing targeted campaigns that address high-risk population groups, such as individuals with red hair and/or light eyes, people with an occupation associated with frequent UV exposure, and individuals with higher levels of education. With regard to younger populations, parents as well as physicians and teachers should be aware that frequent sunburns during childhood and adolescence and use of tanning beds are 2 main risk factors for MM.
Cutaneous melanoma is a malignant tumor of the skin that develops from melanin-producing pigment cells known as melanocytes. The development of melanoma is a multifactorial process. External factors, genetic predisposition, or both may cause damage to DNA in melanoma cells. Genetic mutations may occur de novo or can be transferred from generation to generation. The most important environmental risk factor is UV radiation, both natural and artificial. Other risk factors include skin type, ethnicity, number of melanocytic nevi, number and severity of sunburns, frequency and duration of UV exposure, geographic location, and level of awareness about malignant melanoma (MM) and its risk factors.1
Melanoma accounts for only 1% to 2% of all tumors but is known for its rapidly increasing incidence.2 White individuals who reside in sunny areas of North America, northern Europe, Australia, and New Zealand seem to be at the highest risk for developing melanoma.3 The global incidence of MM from 2004 to 2008 was 20.8 individuals per 100,000 people.4 In Central Europe, 10 to 12 individuals per 100,000 people were diagnosed with melanoma, and 50 to 60 individuals per 100,000 people were diagnosed in Australia. In 2011, the lifetime risk of being diagnosed with melanoma was 1% in Central Europe and 4% in Australia.2 The incidence of melanoma is lower in populations with darker skin types (ie, Africans, Asians). In some parts of the world, the overall incidence and/or severity of melanoma has been declining over the last few decades, possibly reflecting improved public awareness.5
Cutaneous MM is an aggressive skin cancer that has fatal consequences if diagnosed late. Chances of survival, however, increase dramatically when melanoma is detected early. Collecting and analyzing data about a certain disease leads to a better understanding of the condition and encourages the development of prevention strategies. Epidemiologic research helps to improve patient care by measuring the occurrence of an event and by investigating the relationship between the occurrence of an event and associated factors; in doing so, epidemiologic research directly enables a better understanding of the disease and promotes effective preventive and therapeutic approaches.6
Although risk factors for melanoma are well established, current epidemiologic research shows that information on UV exposure and its association with this disease in many parts of the world, including Central Europe, is lacking. The aim of this study was to investigate behavioral and sociodemographic factors associated with the development of MM in the Czech Republic and Germany.
Materials and Methods
This hospital-based, case-control study was conducted in the largest dermatology departments in the Czech Republic (Clinic of Dermatology and Venereology, Third Faculty of Medicine, Charles University, Prague) and Germany (Department of Dermatology and Allergology, Ludwig Maximilian University, Munich). Data from the Czech Republic and Germany were not evaluated separately. These 2 countries were chosen as a representative sample population from Central Europe.
Study Population
The study population included 207 patients (103 men; 104 women) aged 31 to 94 years who were consecutively diagnosed with MM (cases). Patients with acral lentiginous melanoma were excluded from the study due to the generally accepted theory that the condition is not linked to UV exposure. Melanoma diagnosis was based on histopathologic examination. The study population also included 235 randomly selected controls (110 men; 125 women) from the same 2 study centers who had been hospitalized due to other dermatologic diagnoses with no history of any skin cancer. Among patients asked to take part in the study, the participation rates were 83% among cases and 62% among controls.
Assessment
Various sociodemographic factors and factors related to UV exposure were assessed via administration of a structured questionnaire that was completed by all 442 patients.
Four statistical models concerning variables were constructed. The basic model, which was part of all subsequent models, included age, sex, education, and history of skin tumors. Variables included in the biological model were eye color (light vs dark) and Fitzpatrick skin type (I–V). Variables included in the lifestyle model were the use of sunscreen (never and rarely; often; always; always and repetitively), sun exposure during work (yes/no), and seaside vacation (never, rarely, regularly, more than once per year). The variable in the exposure model was the number of sunburns during childhood and adolescence (none, 1–5 times, 6–10 times, ≥11 times).
Sociodemographic characteristics (sex, age, education) and prior incidence of skin tumor were included in each model. Although there were no statistically significant differences in the incidence of melanoma associated with sex and age, those variables were kept in the models to control the impact of other variables by sex and age.
Other variables were added into the model one by one, and the likelihood ratio was tested step-by-step. Only the variables that improved the model fit were kept in the final model. Impact of variables on dependent variables also was tested; variables with no significant impact on dependent variables were left out of the model.
Statistical Analysis
The association between risk factors and MM was assessed using multivariate logistic regression. In total, 4 models were included in the results, which were presented as odds ratios (ORs) and 95% confidence intervals (CIs). A significance level of α=.05 was chosen. The statistical program Stata 11 was used for all analyses.
Results
Descriptive data on the 442 patients surveyed are shown in Table 1. The results of the logistic regression in all studied models are shown in Table 2.
Basic Model
There was no difference in the proportion of men and women in the melanoma and control groups. We observed that more patients in the melanoma group had a university degree than patients in the control group. Patients in the melanoma group with a history of MM showed 4.2 times higher risk for developing another melanoma.
Biological Model
Eye color and Fitzpatrick skin type were the focus of the biological model. The odds of being diagnosed with melanoma were 2.5 times greater in respondents with a light eye color (ie, blue, green, gray) than in respondents with a dark eye color (ie, brown, black). Respondents with Fitzpatrick skin types I and II had a significantly higher association with melanoma (OR, 4.25 and 6.98; 95% CI, 2.13-8.51 and 3.78-12.88) than Fitzpatrick skin type III (OR, 1.0)(P<.001 for both). Respondents with darker skin types (IV and V) also were present in our study population. The numbers were low, and the CI was too wide; nevertheless, the results were statistically significant (P<.001).
Lifestyle Model
The lifestyle model included patients’ use of sunscreen and level of sun exposure at work and on vacation. Respondents who did not use sunscreen were 12 times more likely to develop melanoma than those who always used it (95% CI, 5.56-27.14); however, individuals who used sunscreen always and repetitively (ie, more than once during 1 period of sun exposure) had a higher likelihood of melanoma compared to those who always used it. The incidence of melanoma was lower in respondents who regularly spent their vacations by the sea than those who did not vacation in seaside regions. Respondents who worked in direct sunlight were approximately 2 times more likely to present with melanoma than individuals who did not work outside.
Exposure Model
The number of sunburns sustained during childhood and adolescence was assessed in the exposure model. Respondents with a history of 1 to 5 sunburns during childhood and adolescence did not show a statistically significant increase in the incidence of melanoma diagnosis; however, those with a history of 6 or more sunburns during these periods showed a significant increase in the odds of developing melanoma (OR, 4.95 and 25.52; 95% CI, 2.29-10.71 and 12.16-53.54)(P<.001 for both).
Comment
In this study, we concentrated on UV exposure and various sociodemographic factors that were possibly connected to a higher risk for developing melanoma. We observed that the majority of patients in the melanoma group had achieved a higher level of education than the control group. Most of the melanoma group patients had light-colored eyes and spent more time in direct sunlight at work. Although seaside vacations did not correlate with a higher occurrence of melanoma, it was noted that the melanoma patients used sunscreen much less often than the control group. Major differences among respondents in the melanoma group versus the control group were seen in the reported number of sunburns sustained in childhood and adolescence. More sunburns during these periods seemed to play the most important role in the risk for melanoma. Some of the patient responses to the questionnaire may be biased, as respondents answered the questions by themselves.
Because risk factors for and preventive methods against melanoma are well established, one would assume that general knowledge regarding melanoma is adequate. On the contrary, it has been shown that knowledge about melanoma is insufficient, even among professionals and individuals with higher levels of education. In a study based on a questionnaire administered to plastic surgeons, only 37.5% (27/72) of respondents correctly identified the duration of action of sunscreen to be 3 to 4 hours.7 Approximately half of the respondents (37/72) did not know that geographical conditions such as altitude and latitude as well as shade can alter sunscreen efficacy and also were not aware of the protective action of clothing. These results are alarming and indicate that even medical professionals, who should play a main role in improving the health knowledge of the general population, have an unsatisfactory level of education in prevention of melanoma. Another important part of better education of specialists treating skin disorders is good knowledge of dermatoscopy. In fact, the Annual Skin Cancer Conference 2011 in Australia emphasized the importance of dermatoscopy in primary and secondary prevention of skin cancer.8 Teaching dermatoscopy should be part of melanoma campaigns for professionals.
Our basic model demonstrated that a higher level of education was connected to a higher occurrence of MM, which may seem surprising, considering that most diseases, along with their incidence, prevalence, and mortality, usually are associated with lower levels of education or lower socioeconomic status. A similar trend also was reported in prior studies, with higher socioeconomic groups showing higher incidences of cutaneous melanoma; colon cancer; brain cancer in men; and breast and ovarian cancer in women. Additionally, patients with higher socioeconomic status have been shown to have a survival advantage.9 Individuals with higher socioeconomic status can afford to travel more often for vacation and are more frequently exposed to direct sun. Individuals with higher levels of education also are generally more aware of the importance of disease prevention and therefore go for preventive checkups more often. The detection of melanoma in this socioeconomic group should be higher.
Our biological model demonstrated that respondents with lighter eyes had melanoma almost 3 times more often than individuals with darker eyes. Fitzpatrick skin types I and II also were significantly associated with the development of melanoma (P<.001). These findings are generally confirmed in the literature. In a study of the incidence of melanoma in Spain, statistically significant risk factors included blonde or red hair (P=.002), multiple melanocytic nevi (P=.002), Fitzpatrick skin types I and II (P=.002), and a history of actinic keratosis (P=.021) or nonmelanoma skin cancer (P=.002).10 A group in Italy also has investigated the main risk factors for melanoma. This study suggested dividing patients into high-risk subgroups to help minimize exposure to UV radiation and diagnose melanoma in its early stage.11
The results from our study confirmed the importance of concentrating melanoma prevention campaign efforts on high-risk patients. Dividing these patients into subgroups (eg, individuals who play outdoor sports, individuals with occupations associated with UV exposure, individuals who use indoor tanning beds, individuals with a family history of melanoma) may be helpful. A case-control study on sun-seeking behavior in the Czech Republic showed that the most alarming risk factors were all-day sun exposure during adolescence, frequent holidays spent in the mountains, and inadequate use of sunscreen in adulthood.12 We investigated the effects of sunscreen use on the incidence of melanoma in our lifestyle model and discovered that it decreased the risk for melanoma. Respondents who used it always had a much lower risk for developing melanoma than those who never or rarely applied it. Individuals who used sunscreen always and repetitively (ie, more than once per period of sun exposure) did not show a lower risk than those who used it once per period of sun exposure. This finding could mean that patients who are known to get sunburns or who feel a certain discomfort on direct exposure to the sun tend to use sunscreen always and repetitively.
It is important to note that some investigators disagree with the importance of some generally accepted means of prevention, such as the effect of sunscreen products. Due to insufficient evidence, the role of sunscreen use in reducing the risk for skin cancer, especially cutaneous MM, is controversial.13 Although we could prove there is a considerable difference in the incidence of melanoma in patients who claimed to use sunscreen always versus those who never use it, we agree that more evidence on this topic is needed. Furthermore, it has been reported that risk for melanoma has increased with rising intermittent sun exposure and indoor tanning bed use.14,15
Respondents who regularly traveled to seaside regions showed a surprisingly lower incidence of melanoma than respondents who did not spend their vacations in seaside locations. It is possible that individuals who choose not to spend their vacations at the seaside are more prone to sunburns and therefore do not prefer to spend their free time in direct sunlight. Another possible explanation is that individuals who regularly travel to seaside regions actively try to protect themselves from sunlight and sunburns. A higher incidence of melanoma also was observed in respondents who reported sun exposure during work.
In our exposure model, we demonstrated that a history of sunburns is the strongest risk factor for melanoma. Frequent sunburns during childhood and adolescence were strongly associated with the development of MM. This association has been supported in a systematic review on sun exposure during childhood and associated risks.16
Conclusion
To improve patient knowledge about melanoma prevention, we suggest directing targeted campaigns that address high-risk population groups, such as individuals with red hair and/or light eyes, people with an occupation associated with frequent UV exposure, and individuals with higher levels of education. With regard to younger populations, parents as well as physicians and teachers should be aware that frequent sunburns during childhood and adolescence and use of tanning beds are 2 main risk factors for MM.
1. IARC monographs on the evaluation of carcinogenic risks to humans. solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:1-316.
2. Kunte C, Geimer T, Baumert J, et al. Analysis of predictive factors for the outcome of complete lymph node dissection in melanoma patients with metastatic sentinel lymph nodes. J Am Acad Dermatol. 2011;64:655-662.
3. Parkin D, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
4. SEER Stat Fact Sheets: melanoma of the skin. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/melan.html#incidence-mortality. Accessed October 25, 2014.
5. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
6. Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease [published online ahead of print December 8, 2011]. J Invest Dermatol. 2012;132(3, pt 2):994-1002.
7. Magdum A, Leonforte F, McNaughton E, et al. Sun protection–do we know enough [published online ahead of print February 8, 2012]? J Plast Reconstr Aesthet Surg. 2012;65:1384-1389.
8. Zalaudek I, Whiteman D, Rosendahl C, et al. Update on melanoma and non-melanoma skin cancer. Annual Skin Cancer Conference 2011, Hamilton Island, Australia, 2011. Expert Rev Anticancer Ther. 2011;11:1829-1832.
9. Mackenbach JP. Health inequalities: Europe in profile. http://ec.europa.eu/health/ph_determinants/socio_economics/documents/ev_060302_rd06_en.pdf. Published February 2006. Accessed October 25, 2014.
10. Ballester I, Oliver V, Bañuls J, et al. Multicenter case-control study of risk factors for cutaneous melanoma in Valencia, Spain [published online ahead of print May 22, 2012]. Actas Dermosifiliogr. 2012;103:790-797.
11. Jaimes N, Marghoob AA. An update on risk factors, prognosis and management of melanoma patients. G Ital Dermatol Venereol. 2012;147:1-19.
12. Vranova J, Arenbergerova M, Arenberger P, et al. Incidence of cutaneous malignant melanoma in the Czech Republic: the risks of sun exposure for adolescents. Neoplasma. 2012;59:316-325.
13. Planta MB. Sunscreen and melanoma: is our prevention message correct? J Am Board Fam Med. 2011;24:735-739.
14. Veierød MB, Adami HO, Lund E, et al. Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi. Cancer Epidemiol Biomarkers Prev. 2010;19:111-120.
15. Doré JF, Chignol MC. Tanning salons and skin cancer [published online ahead of print August 15, 2011]. Photochem Photobiol Sci. 2012;11:30-37.
16. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69-82.
1. IARC monographs on the evaluation of carcinogenic risks to humans. solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risks Hum. 1992;55:1-316.
2. Kunte C, Geimer T, Baumert J, et al. Analysis of predictive factors for the outcome of complete lymph node dissection in melanoma patients with metastatic sentinel lymph nodes. J Am Acad Dermatol. 2011;64:655-662.
3. Parkin D, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
4. SEER Stat Fact Sheets: melanoma of the skin. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/statfacts/html/melan.html#incidence-mortality. Accessed October 25, 2014.
5. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917.
6. Nijsten T, Stern RS. How epidemiology has contributed to a better understanding of skin disease [published online ahead of print December 8, 2011]. J Invest Dermatol. 2012;132(3, pt 2):994-1002.
7. Magdum A, Leonforte F, McNaughton E, et al. Sun protection–do we know enough [published online ahead of print February 8, 2012]? J Plast Reconstr Aesthet Surg. 2012;65:1384-1389.
8. Zalaudek I, Whiteman D, Rosendahl C, et al. Update on melanoma and non-melanoma skin cancer. Annual Skin Cancer Conference 2011, Hamilton Island, Australia, 2011. Expert Rev Anticancer Ther. 2011;11:1829-1832.
9. Mackenbach JP. Health inequalities: Europe in profile. http://ec.europa.eu/health/ph_determinants/socio_economics/documents/ev_060302_rd06_en.pdf. Published February 2006. Accessed October 25, 2014.
10. Ballester I, Oliver V, Bañuls J, et al. Multicenter case-control study of risk factors for cutaneous melanoma in Valencia, Spain [published online ahead of print May 22, 2012]. Actas Dermosifiliogr. 2012;103:790-797.
11. Jaimes N, Marghoob AA. An update on risk factors, prognosis and management of melanoma patients. G Ital Dermatol Venereol. 2012;147:1-19.
12. Vranova J, Arenbergerova M, Arenberger P, et al. Incidence of cutaneous malignant melanoma in the Czech Republic: the risks of sun exposure for adolescents. Neoplasma. 2012;59:316-325.
13. Planta MB. Sunscreen and melanoma: is our prevention message correct? J Am Board Fam Med. 2011;24:735-739.
14. Veierød MB, Adami HO, Lund E, et al. Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi. Cancer Epidemiol Biomarkers Prev. 2010;19:111-120.
15. Doré JF, Chignol MC. Tanning salons and skin cancer [published online ahead of print August 15, 2011]. Photochem Photobiol Sci. 2012;11:30-37.
16. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69-82.
Practice Points
- Our study revealed the following common risk factors associated with higher melanoma incidence: light eye color (ie, blue, green, gray), Fitzpatrick skin types I and II, frequent sunburns during childhood and adolescence, and higher level of education.
- Prevention campaigns should be implemented to improve awareness of melanoma to reduce exposure to UV radiation among high-risk patient populations.
Effects of Oral Isotretinoin on Lipids and Liver Enzymes in Acne Patients
Acne is a chronic inflammatory condition of the pilosebaceous unit affecting approximately 79% to 95% of adolescents in the Western world.1 Treatment of acne depends on its severity. Topical tretinoin, adapalene, benzoyl peroxide, azelaic acid, and topical antibiotics generally are used in cases of noninflammatory or mild inflammatory disease. Isotretinoin is recommended for treatment of severe inflammatory acne (eg, nodulocystic or conglobata acne) and for cases of acne that have proven to be resistant to prior treatment with antibiotics or topical agents. Dosages of isotretinoin range from 0.5 to 2 mg/kg daily for 16 to 24 weeks.1 Isotretinoin reduces the activity and size of the sebaceous glands, normalizes keratinization of the sebaceous follicles, and decreases the number of Propionibacterium acnes. Isotretinoin also may cause clinical side effects and laboratory changes, the most important being teratogenicity. It also may cause mucocutaneous side effects including cracked lips, dryness of the skin and nasal mucosa, skin redness, eye dryness, and eye irritation.1 It also may cause blepharoconjuctivitis, photosensitivity, asteatotic dermatitis, pruritus, telogen effluvium, secondary bacterial colonization, nail fragility, periungual pyogenic granuloma, paronychia, myalgia, intracranial hypertension, nausea, headache, vomiting, depression, psychosis, suicide, constipation, and allergic reactions.2 Isotretinoin treatment may increase serum levels of liver enzymes, triglyc-erides (TGs), and low-density lipoprotein (LDL) cholesterol, and reduce the level of high-density lipoprotein (HDL) cholesterol.1 This retrospective study sought to evaluate the effect of isotretinoin on liver enzymes and lipids over 6 months.
Materials and Methods
Our retrospective study was conducted at the Hospital of Atatürk University in Erzurum, a city located in eastern Turkey. All patients who were treated in the department of dermatology and had received oral isotretinoin between June 2009 and June 2012 were included in the study. The study was based on an evaluation of the patients’ medical records. All patients received oral isotretinoin 0.5 to 1 mg/kg daily; the majority of patients received 30 to 40 mg daily. Patient medical records included age; gender; white blood cell (WBC) count; red blood cell (RBC) count; hemoglobin count; and aspartate aminotransferase (AST), alanine aminotransferase (ALT), TG, LDL, and HDL levels at the beginning of treatment. Aspartate aminotransferase, ALT, TG, LDL, and HDL levels also were measured at 3- and 6-month follow-up. Analysis of AST, ALT, TG, LDL, and HDL levels was based on the National Cholesterol Education Program guidelines.3 Aspartate aminotransferase and ALT levels were classified as normal (<40 U/L) and high (≥40 U/L). Triglyceride levels were classified as normal (<150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL), and very high (≥500 mg/dL). Low-density lipoprotein levels were classified as optimal (<100 mg/dL), above optimal (100–129 mg/dL), borderline high (130–159 mg/dL), high (160–189 mg/dL), and very high (≥190 mg/dL). High-density lipoprotein levels were classified as low (<40 mg/dL), normal (40–59 mg/dL), and high (≥60 mg/dL). Normal WBC was defined as 3.5 to 12.5×103/mL. Normal hemoglobin count was defined as 11.5 to 15.0×106/mL for women and 13 to 17×106/mL for men. Normal RBC was defined as 4.0 to 5.2×106/mL for women and 4.5 to 5.9×106/mL for men. Statistical analysis was performed using SPSS version 17.0. Repeated measures analysis of variance was used to compare means between 3 groups (baseline, 3-month, and 6-month values). A paired sample t test was used to compare means between any 2 groups. Results with P<.05 were considered statistically significant.
Results
Treatment with oral isotretinoin was initiated in 349 patients at our institution from June 2009 to June 2012. Twenty-seven of these patients were excluded from the study because their medical records were not available. Medical records from 322 patients were obtained. The study population consisted of 226 (70.2%) women and 96 (29.8%) men. Patients ranged in age from 17 to 64 years, with a mean age of 23.9 years. The mean (standard deviation [SD]) age of the women was 23.9 (5.4) years and the mean (SD) age of the men was 23.8 (7.02) years. Most of the patients were on a regimen of 30 or 40 mg of isotretinoin daily. Differences between dosages and laboratory values were not analyzed because we assumed there would not be a significant difference, as most patients received the same dose. The mean (SD) WBC was 8.4 (3.5)×103/mL. The mean (SD) RBC was 4.9 (0.5)×106/mL. The mean (SD) hemoglobin count was 14.3 (1.7)×106/mL (women, 13.6 [1.5]×106/mL; men, 15.9 [1.1]×106/mL).
The study evaluated the effects of isotretinoin on liver enzymes (AST and ALT) and lipids (TGs, LDL, and HDL). Nearly all of the patients (>95%) had normal AST and ALT levels at baseline. The results are outlined in the Table. Some values were not recorded for all patients at each follow-up.
Aspartate Aminotransferase Analysis
Aspartate aminotransferase levels were classified as normal and high. At baseline, mean (SD) AST levels were 20.2 (6.6) U/L, with normal levels in 311 (96.6%) patients and high in 7 (2.2%) patients. At 3-month follow-up, mean (SD) AST levels were 20.7 (5.2) U/L, with normal levels in 270 (83.9%) patients and high levels in 3 (0.9%) patients. At 6-month follow-up, mean (SD) AST levels were 21.3 (5.7) U/L, with normal levels in 209 (64.9%) patients and high levels in 4 (1.2%) patients. Aspartate aminotransferase levels increased at 3- and 6-month follow-up compared to baseline. Differences between AST levels were statistically significant (F2,416=4.2, P=.016). Differences between AST levels at baseline and 3-month follow-up were not statistically significant (P=.3). Differences between AST levels at 3- and 6-month follow-up were not statistically significant (P=.4). Differences between AST levels at baseline and 6-month follow-up were statistically significant (P=.07). Differences between AST classifications at the 3 time points were not statistically significant (F2,416=0.44, P=.64). Overall, the results indicated that AST levels increased over time in patients treated with isotretinoin, but the increase was not above the normal range and was not statistically significant.
Alanine Aminotransferase Analysis
Alanine aminotransferase levels were classified as normal or high. At baseline, mean (SD) ALT levels were 16.8 (11.2) U/L, with normal levels in 303 (94.1%) patients and high in 19 (5.9%) patients. At 3-month follow-up, mean (SD) ALT levels were 16.2 (9.3) U/L, with normal levels in 263 (81.7%) patients and high in 11 (3.4%) patients. At 6-month follow-up, mean (SD) ALT levels were 17.0 (11.3) U/L, with normal levels in 201 (62.4%) patients and high in 11 (3.4%) patients. Alanine aminotransferase levels at 3-month follow-up were lower than baseline but higher at 6-month follow-up compared to baseline and 3-month follow-up. Overall, ALT levels increased with time, but the differences between baseline and 3- and 6-month follow-up were not statistically significant (F2,416=0.32, P=.72). Differences between ALT classifications at each time point were not statistically significant (F2,418=0.21, P=.54). Overall, the results indicated that ALT levels increased over time in patients treated with isotretinoin, but the increase was not statistically significant.
Triglycerides Analysis
Triglyceride levels were classified as normal, borderline high, high, and very high. At baseline, mean (SD) TG levels were 107 (71) mg/dL, with normal levels in 270 (83.9%) patients, borderline high in 30 (9.3%) patients, high in 20 (6.2%) patients, and very high in 2 (0.6%) patients. At 3-month follow-up, mean (SD) TG levels were 117 (60) mg/dL, with normal levels in 197 (61.2%) patients, borderline high in 38 (11.8%) patients, high in 22 (6.8%) patients, and very high in 1 (0.3%) patient. At 6-month follow-up, mean (SD) TG levels were 122 (65) mg/dL, with normal levels in 145 (45%) patients, borderline high in 36 (11.2%) patients, high in 16 (5%) patients, and very high in 0 (0%) patients. Triglyceride levels increased and differences between TG levels at baseline and 3- and 6-month follow-up were statistically significant (F2,384=17, P<.001). Baseline TG levels compared to 3-month follow-up were statistically signif-icant (P<.001). Differences in TG levels at 6-month follow-up versus baseline were statistically significant (P<.001). However, changes in TG levels from 3- to 6-month follow-up were not statistically significant (P=.21). Differences between TG classifications at each time point were statistically significant (F2,386=6.9, P=.001). Overall, TG levels increased from baseline during isotretinoin treatment at 3- and 6-month follow-up, and these increases were above normal range; however, there was no statistically significant increase from 3- to 6-month follow-up.
Low-Density Lipoprotein Analysis
Low-density lipoprotein levels were classified as optimal, above optimal, borderline high, high, and very high. At baseline, mean (SD) LDL levels were 102 (28) mg/dL, with optimal levels in 162 (50.3%) patients, above optimal in 95 (29.5%) patients, borderline high in 32 (9.9%) patients, high in 11 (3.4%) patients, and very high in 3 (0.9%) patients. At 3-month follow-up, mean (SD) LDL levels were 113 (30) mg/dL, with optimal levels in 89 (27.6%) patients, above optimal in 98 (30.4%) patients, borderline high in 54 (16.8%) patients, high in 12 (3.7%) patients, and very high in 5 (1.6%) patients. At 6-month follow-up, mean (SD) LDL levels were 113 (27) mg/dL, with optimal levels in 60 (18.6%) patients, above optimal in 84 (26.1%) patients, borderline high in 44 (13.7%) patients, high in 8 (2.5%) patients, and very high in 1 (0.3%) patient. Overall, there were statistically significant increases in LDL levels at 3- and 6-month follow-up (F2,382<75, P<.001). Differences between baseline LDL levels and 3-month follow-up were statistically significant (P<.001). Differences between baseline LDL levels and 6-month follow-up were statistically significant (P<.001). However, differences in LDL levels at 3- and 6-month follow-up were not statistically significant (P=.74). Differences between LDL classifications at each time point were statistically significant (F2,382=51.2, P<.001). Overall, statistically significant increases in LDL levels from baseline were noted during isotretinoin treatment and this increase was above normal range; however, LDL levels did not significantly increase from 3- to 6-month follow-up.
High-Density Lipoprotein Analysis
High-density lipoprotein levels were classified as low, normal, and high. At baseline, mean (SD) HDL levels were 52.4 (16) mg/dL, with low levels in 60 (18.6%) patients, normal in 173 (53.7%) patients, and high in 71 (22%) patients. At 3-month follow-up, mean (SD) HDL levels were 48 (12) mg/dL, with low levels in 63 (19.6%) patients, normal in 154 (47.8%) patients, and high in 41 (12.7%) patients. At 6-month follow-up, mean (SD) HDL levels were 47.6 (12) mg/dL, with low levels in 48 (14.9%) patients, normal in 117 (36.3%) patients, and high in 33 (10.2%) patients. Overall, statistically significant decreases were noted in HDL levels (F2,384=19, P<.001). Differences between baseline HDL levels compared to 3-month follow-up were statistically significant (P<.001). Differences between baseline HDL levels compared to 6-month follow-up were statistically significant (P<.001). Differences in HDL levels at 3- and 6-month follow-up were statistically significant (P<.001). Differences between HDL classifications at each time point were statistically significant (F2,384=5.2, P=.006). Overall, there were statistically significant decreases in HDL levels during isotretinoin treatment from baseline and this decrease was above normal range; however, HDL levels did not decrease at 3- and 6-month follow-up.
Comment
Studies in the literature evaluating the effects of isotretinoin on liver enzymes and lipids suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT), TGs, HDL, and LDL in various degrees.1 Zane et al4 studied 13,772 patients with acne undergoing oral isotretinoin therapy between March 1995 and September 2002. The investigators found increased liver transaminase and serum lipid levels. They suggested that these abnormalities were generally transient and reversible.4 Bershad et al5 reported an increase in LDL and TG but a decrease in HDL during isotretinoin therapy. These changes in the lipid profile also appeared to be transient and returned to baseline level 2 months following the end of treatment.5 In another study of 130 patients who were treated with isotretinoin, Vieira et al1 noted an increase in AST, ALT, and TG levels. Most of the studies in the literature that reported effects of isotretinoin on liver enzymes and lipids suggested that the effects were reversible.
Although many studies reported alterations in serum transaminase and lipid levels, other studies reported no effect. In one study of 150 participants, Brito et al2 found no statistically significant changes in liver transaminase, TG, HDL, or LDL levels following treatment with isotretinoin. In another study of 1292 participants by Alcalay et al,6 serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of isotretinoin treatment. In another study of 30 participants, Baxter et al7 reported no significant changes in TG, LDL, or HDL levels measured at baseline or during treatment with isotretinoin.
Some studies suggest that routine laboratory tests are needed when treating patients with isotretinoin due to severe alterations in serum liver transaminase and lipid levels, while other studies conclude that the effects are minimal and laboratory tests are not needed. In the current study, we found that there were statistically significant increases in TG and LDL levels in patients who underwent treatment with isotretinoin. We also found statistically significant decreases in HDL levels. In our study, liver enzymes were less affected than lipids in patients who underwent treatment with isotretinoin. There were statistically significant increases in AST levels, but the clinical classification was not affected. There also were increases in ALT levels, but the changes were not statistically significant.
Overall, we advise dermatologists that isotretinoin can be administered with minimal concern regarding changes in serum transaminase and lipid levels; however, although severe laboratory alterations were not noted in our study, we advise physicians to use caution when administering isotretinoin in patients with a history of abnormal findings.
1. Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglyceride and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
2. Brito MFM, Pessoa IS, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin [in English, Portuguese]. An Bras Dermatol. 2010;85:331-337.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
5. Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
6. Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
7. Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2003;14:216-218.
Acne is a chronic inflammatory condition of the pilosebaceous unit affecting approximately 79% to 95% of adolescents in the Western world.1 Treatment of acne depends on its severity. Topical tretinoin, adapalene, benzoyl peroxide, azelaic acid, and topical antibiotics generally are used in cases of noninflammatory or mild inflammatory disease. Isotretinoin is recommended for treatment of severe inflammatory acne (eg, nodulocystic or conglobata acne) and for cases of acne that have proven to be resistant to prior treatment with antibiotics or topical agents. Dosages of isotretinoin range from 0.5 to 2 mg/kg daily for 16 to 24 weeks.1 Isotretinoin reduces the activity and size of the sebaceous glands, normalizes keratinization of the sebaceous follicles, and decreases the number of Propionibacterium acnes. Isotretinoin also may cause clinical side effects and laboratory changes, the most important being teratogenicity. It also may cause mucocutaneous side effects including cracked lips, dryness of the skin and nasal mucosa, skin redness, eye dryness, and eye irritation.1 It also may cause blepharoconjuctivitis, photosensitivity, asteatotic dermatitis, pruritus, telogen effluvium, secondary bacterial colonization, nail fragility, periungual pyogenic granuloma, paronychia, myalgia, intracranial hypertension, nausea, headache, vomiting, depression, psychosis, suicide, constipation, and allergic reactions.2 Isotretinoin treatment may increase serum levels of liver enzymes, triglyc-erides (TGs), and low-density lipoprotein (LDL) cholesterol, and reduce the level of high-density lipoprotein (HDL) cholesterol.1 This retrospective study sought to evaluate the effect of isotretinoin on liver enzymes and lipids over 6 months.
Materials and Methods
Our retrospective study was conducted at the Hospital of Atatürk University in Erzurum, a city located in eastern Turkey. All patients who were treated in the department of dermatology and had received oral isotretinoin between June 2009 and June 2012 were included in the study. The study was based on an evaluation of the patients’ medical records. All patients received oral isotretinoin 0.5 to 1 mg/kg daily; the majority of patients received 30 to 40 mg daily. Patient medical records included age; gender; white blood cell (WBC) count; red blood cell (RBC) count; hemoglobin count; and aspartate aminotransferase (AST), alanine aminotransferase (ALT), TG, LDL, and HDL levels at the beginning of treatment. Aspartate aminotransferase, ALT, TG, LDL, and HDL levels also were measured at 3- and 6-month follow-up. Analysis of AST, ALT, TG, LDL, and HDL levels was based on the National Cholesterol Education Program guidelines.3 Aspartate aminotransferase and ALT levels were classified as normal (<40 U/L) and high (≥40 U/L). Triglyceride levels were classified as normal (<150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL), and very high (≥500 mg/dL). Low-density lipoprotein levels were classified as optimal (<100 mg/dL), above optimal (100–129 mg/dL), borderline high (130–159 mg/dL), high (160–189 mg/dL), and very high (≥190 mg/dL). High-density lipoprotein levels were classified as low (<40 mg/dL), normal (40–59 mg/dL), and high (≥60 mg/dL). Normal WBC was defined as 3.5 to 12.5×103/mL. Normal hemoglobin count was defined as 11.5 to 15.0×106/mL for women and 13 to 17×106/mL for men. Normal RBC was defined as 4.0 to 5.2×106/mL for women and 4.5 to 5.9×106/mL for men. Statistical analysis was performed using SPSS version 17.0. Repeated measures analysis of variance was used to compare means between 3 groups (baseline, 3-month, and 6-month values). A paired sample t test was used to compare means between any 2 groups. Results with P<.05 were considered statistically significant.
Results
Treatment with oral isotretinoin was initiated in 349 patients at our institution from June 2009 to June 2012. Twenty-seven of these patients were excluded from the study because their medical records were not available. Medical records from 322 patients were obtained. The study population consisted of 226 (70.2%) women and 96 (29.8%) men. Patients ranged in age from 17 to 64 years, with a mean age of 23.9 years. The mean (standard deviation [SD]) age of the women was 23.9 (5.4) years and the mean (SD) age of the men was 23.8 (7.02) years. Most of the patients were on a regimen of 30 or 40 mg of isotretinoin daily. Differences between dosages and laboratory values were not analyzed because we assumed there would not be a significant difference, as most patients received the same dose. The mean (SD) WBC was 8.4 (3.5)×103/mL. The mean (SD) RBC was 4.9 (0.5)×106/mL. The mean (SD) hemoglobin count was 14.3 (1.7)×106/mL (women, 13.6 [1.5]×106/mL; men, 15.9 [1.1]×106/mL).
The study evaluated the effects of isotretinoin on liver enzymes (AST and ALT) and lipids (TGs, LDL, and HDL). Nearly all of the patients (>95%) had normal AST and ALT levels at baseline. The results are outlined in the Table. Some values were not recorded for all patients at each follow-up.
Aspartate Aminotransferase Analysis
Aspartate aminotransferase levels were classified as normal and high. At baseline, mean (SD) AST levels were 20.2 (6.6) U/L, with normal levels in 311 (96.6%) patients and high in 7 (2.2%) patients. At 3-month follow-up, mean (SD) AST levels were 20.7 (5.2) U/L, with normal levels in 270 (83.9%) patients and high levels in 3 (0.9%) patients. At 6-month follow-up, mean (SD) AST levels were 21.3 (5.7) U/L, with normal levels in 209 (64.9%) patients and high levels in 4 (1.2%) patients. Aspartate aminotransferase levels increased at 3- and 6-month follow-up compared to baseline. Differences between AST levels were statistically significant (F2,416=4.2, P=.016). Differences between AST levels at baseline and 3-month follow-up were not statistically significant (P=.3). Differences between AST levels at 3- and 6-month follow-up were not statistically significant (P=.4). Differences between AST levels at baseline and 6-month follow-up were statistically significant (P=.07). Differences between AST classifications at the 3 time points were not statistically significant (F2,416=0.44, P=.64). Overall, the results indicated that AST levels increased over time in patients treated with isotretinoin, but the increase was not above the normal range and was not statistically significant.
Alanine Aminotransferase Analysis
Alanine aminotransferase levels were classified as normal or high. At baseline, mean (SD) ALT levels were 16.8 (11.2) U/L, with normal levels in 303 (94.1%) patients and high in 19 (5.9%) patients. At 3-month follow-up, mean (SD) ALT levels were 16.2 (9.3) U/L, with normal levels in 263 (81.7%) patients and high in 11 (3.4%) patients. At 6-month follow-up, mean (SD) ALT levels were 17.0 (11.3) U/L, with normal levels in 201 (62.4%) patients and high in 11 (3.4%) patients. Alanine aminotransferase levels at 3-month follow-up were lower than baseline but higher at 6-month follow-up compared to baseline and 3-month follow-up. Overall, ALT levels increased with time, but the differences between baseline and 3- and 6-month follow-up were not statistically significant (F2,416=0.32, P=.72). Differences between ALT classifications at each time point were not statistically significant (F2,418=0.21, P=.54). Overall, the results indicated that ALT levels increased over time in patients treated with isotretinoin, but the increase was not statistically significant.
Triglycerides Analysis
Triglyceride levels were classified as normal, borderline high, high, and very high. At baseline, mean (SD) TG levels were 107 (71) mg/dL, with normal levels in 270 (83.9%) patients, borderline high in 30 (9.3%) patients, high in 20 (6.2%) patients, and very high in 2 (0.6%) patients. At 3-month follow-up, mean (SD) TG levels were 117 (60) mg/dL, with normal levels in 197 (61.2%) patients, borderline high in 38 (11.8%) patients, high in 22 (6.8%) patients, and very high in 1 (0.3%) patient. At 6-month follow-up, mean (SD) TG levels were 122 (65) mg/dL, with normal levels in 145 (45%) patients, borderline high in 36 (11.2%) patients, high in 16 (5%) patients, and very high in 0 (0%) patients. Triglyceride levels increased and differences between TG levels at baseline and 3- and 6-month follow-up were statistically significant (F2,384=17, P<.001). Baseline TG levels compared to 3-month follow-up were statistically signif-icant (P<.001). Differences in TG levels at 6-month follow-up versus baseline were statistically significant (P<.001). However, changes in TG levels from 3- to 6-month follow-up were not statistically significant (P=.21). Differences between TG classifications at each time point were statistically significant (F2,386=6.9, P=.001). Overall, TG levels increased from baseline during isotretinoin treatment at 3- and 6-month follow-up, and these increases were above normal range; however, there was no statistically significant increase from 3- to 6-month follow-up.
Low-Density Lipoprotein Analysis
Low-density lipoprotein levels were classified as optimal, above optimal, borderline high, high, and very high. At baseline, mean (SD) LDL levels were 102 (28) mg/dL, with optimal levels in 162 (50.3%) patients, above optimal in 95 (29.5%) patients, borderline high in 32 (9.9%) patients, high in 11 (3.4%) patients, and very high in 3 (0.9%) patients. At 3-month follow-up, mean (SD) LDL levels were 113 (30) mg/dL, with optimal levels in 89 (27.6%) patients, above optimal in 98 (30.4%) patients, borderline high in 54 (16.8%) patients, high in 12 (3.7%) patients, and very high in 5 (1.6%) patients. At 6-month follow-up, mean (SD) LDL levels were 113 (27) mg/dL, with optimal levels in 60 (18.6%) patients, above optimal in 84 (26.1%) patients, borderline high in 44 (13.7%) patients, high in 8 (2.5%) patients, and very high in 1 (0.3%) patient. Overall, there were statistically significant increases in LDL levels at 3- and 6-month follow-up (F2,382<75, P<.001). Differences between baseline LDL levels and 3-month follow-up were statistically significant (P<.001). Differences between baseline LDL levels and 6-month follow-up were statistically significant (P<.001). However, differences in LDL levels at 3- and 6-month follow-up were not statistically significant (P=.74). Differences between LDL classifications at each time point were statistically significant (F2,382=51.2, P<.001). Overall, statistically significant increases in LDL levels from baseline were noted during isotretinoin treatment and this increase was above normal range; however, LDL levels did not significantly increase from 3- to 6-month follow-up.
High-Density Lipoprotein Analysis
High-density lipoprotein levels were classified as low, normal, and high. At baseline, mean (SD) HDL levels were 52.4 (16) mg/dL, with low levels in 60 (18.6%) patients, normal in 173 (53.7%) patients, and high in 71 (22%) patients. At 3-month follow-up, mean (SD) HDL levels were 48 (12) mg/dL, with low levels in 63 (19.6%) patients, normal in 154 (47.8%) patients, and high in 41 (12.7%) patients. At 6-month follow-up, mean (SD) HDL levels were 47.6 (12) mg/dL, with low levels in 48 (14.9%) patients, normal in 117 (36.3%) patients, and high in 33 (10.2%) patients. Overall, statistically significant decreases were noted in HDL levels (F2,384=19, P<.001). Differences between baseline HDL levels compared to 3-month follow-up were statistically significant (P<.001). Differences between baseline HDL levels compared to 6-month follow-up were statistically significant (P<.001). Differences in HDL levels at 3- and 6-month follow-up were statistically significant (P<.001). Differences between HDL classifications at each time point were statistically significant (F2,384=5.2, P=.006). Overall, there were statistically significant decreases in HDL levels during isotretinoin treatment from baseline and this decrease was above normal range; however, HDL levels did not decrease at 3- and 6-month follow-up.
Comment
Studies in the literature evaluating the effects of isotretinoin on liver enzymes and lipids suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT), TGs, HDL, and LDL in various degrees.1 Zane et al4 studied 13,772 patients with acne undergoing oral isotretinoin therapy between March 1995 and September 2002. The investigators found increased liver transaminase and serum lipid levels. They suggested that these abnormalities were generally transient and reversible.4 Bershad et al5 reported an increase in LDL and TG but a decrease in HDL during isotretinoin therapy. These changes in the lipid profile also appeared to be transient and returned to baseline level 2 months following the end of treatment.5 In another study of 130 patients who were treated with isotretinoin, Vieira et al1 noted an increase in AST, ALT, and TG levels. Most of the studies in the literature that reported effects of isotretinoin on liver enzymes and lipids suggested that the effects were reversible.
Although many studies reported alterations in serum transaminase and lipid levels, other studies reported no effect. In one study of 150 participants, Brito et al2 found no statistically significant changes in liver transaminase, TG, HDL, or LDL levels following treatment with isotretinoin. In another study of 1292 participants by Alcalay et al,6 serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of isotretinoin treatment. In another study of 30 participants, Baxter et al7 reported no significant changes in TG, LDL, or HDL levels measured at baseline or during treatment with isotretinoin.
Some studies suggest that routine laboratory tests are needed when treating patients with isotretinoin due to severe alterations in serum liver transaminase and lipid levels, while other studies conclude that the effects are minimal and laboratory tests are not needed. In the current study, we found that there were statistically significant increases in TG and LDL levels in patients who underwent treatment with isotretinoin. We also found statistically significant decreases in HDL levels. In our study, liver enzymes were less affected than lipids in patients who underwent treatment with isotretinoin. There were statistically significant increases in AST levels, but the clinical classification was not affected. There also were increases in ALT levels, but the changes were not statistically significant.
Overall, we advise dermatologists that isotretinoin can be administered with minimal concern regarding changes in serum transaminase and lipid levels; however, although severe laboratory alterations were not noted in our study, we advise physicians to use caution when administering isotretinoin in patients with a history of abnormal findings.
Acne is a chronic inflammatory condition of the pilosebaceous unit affecting approximately 79% to 95% of adolescents in the Western world.1 Treatment of acne depends on its severity. Topical tretinoin, adapalene, benzoyl peroxide, azelaic acid, and topical antibiotics generally are used in cases of noninflammatory or mild inflammatory disease. Isotretinoin is recommended for treatment of severe inflammatory acne (eg, nodulocystic or conglobata acne) and for cases of acne that have proven to be resistant to prior treatment with antibiotics or topical agents. Dosages of isotretinoin range from 0.5 to 2 mg/kg daily for 16 to 24 weeks.1 Isotretinoin reduces the activity and size of the sebaceous glands, normalizes keratinization of the sebaceous follicles, and decreases the number of Propionibacterium acnes. Isotretinoin also may cause clinical side effects and laboratory changes, the most important being teratogenicity. It also may cause mucocutaneous side effects including cracked lips, dryness of the skin and nasal mucosa, skin redness, eye dryness, and eye irritation.1 It also may cause blepharoconjuctivitis, photosensitivity, asteatotic dermatitis, pruritus, telogen effluvium, secondary bacterial colonization, nail fragility, periungual pyogenic granuloma, paronychia, myalgia, intracranial hypertension, nausea, headache, vomiting, depression, psychosis, suicide, constipation, and allergic reactions.2 Isotretinoin treatment may increase serum levels of liver enzymes, triglyc-erides (TGs), and low-density lipoprotein (LDL) cholesterol, and reduce the level of high-density lipoprotein (HDL) cholesterol.1 This retrospective study sought to evaluate the effect of isotretinoin on liver enzymes and lipids over 6 months.
Materials and Methods
Our retrospective study was conducted at the Hospital of Atatürk University in Erzurum, a city located in eastern Turkey. All patients who were treated in the department of dermatology and had received oral isotretinoin between June 2009 and June 2012 were included in the study. The study was based on an evaluation of the patients’ medical records. All patients received oral isotretinoin 0.5 to 1 mg/kg daily; the majority of patients received 30 to 40 mg daily. Patient medical records included age; gender; white blood cell (WBC) count; red blood cell (RBC) count; hemoglobin count; and aspartate aminotransferase (AST), alanine aminotransferase (ALT), TG, LDL, and HDL levels at the beginning of treatment. Aspartate aminotransferase, ALT, TG, LDL, and HDL levels also were measured at 3- and 6-month follow-up. Analysis of AST, ALT, TG, LDL, and HDL levels was based on the National Cholesterol Education Program guidelines.3 Aspartate aminotransferase and ALT levels were classified as normal (<40 U/L) and high (≥40 U/L). Triglyceride levels were classified as normal (<150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL), and very high (≥500 mg/dL). Low-density lipoprotein levels were classified as optimal (<100 mg/dL), above optimal (100–129 mg/dL), borderline high (130–159 mg/dL), high (160–189 mg/dL), and very high (≥190 mg/dL). High-density lipoprotein levels were classified as low (<40 mg/dL), normal (40–59 mg/dL), and high (≥60 mg/dL). Normal WBC was defined as 3.5 to 12.5×103/mL. Normal hemoglobin count was defined as 11.5 to 15.0×106/mL for women and 13 to 17×106/mL for men. Normal RBC was defined as 4.0 to 5.2×106/mL for women and 4.5 to 5.9×106/mL for men. Statistical analysis was performed using SPSS version 17.0. Repeated measures analysis of variance was used to compare means between 3 groups (baseline, 3-month, and 6-month values). A paired sample t test was used to compare means between any 2 groups. Results with P<.05 were considered statistically significant.
Results
Treatment with oral isotretinoin was initiated in 349 patients at our institution from June 2009 to June 2012. Twenty-seven of these patients were excluded from the study because their medical records were not available. Medical records from 322 patients were obtained. The study population consisted of 226 (70.2%) women and 96 (29.8%) men. Patients ranged in age from 17 to 64 years, with a mean age of 23.9 years. The mean (standard deviation [SD]) age of the women was 23.9 (5.4) years and the mean (SD) age of the men was 23.8 (7.02) years. Most of the patients were on a regimen of 30 or 40 mg of isotretinoin daily. Differences between dosages and laboratory values were not analyzed because we assumed there would not be a significant difference, as most patients received the same dose. The mean (SD) WBC was 8.4 (3.5)×103/mL. The mean (SD) RBC was 4.9 (0.5)×106/mL. The mean (SD) hemoglobin count was 14.3 (1.7)×106/mL (women, 13.6 [1.5]×106/mL; men, 15.9 [1.1]×106/mL).
The study evaluated the effects of isotretinoin on liver enzymes (AST and ALT) and lipids (TGs, LDL, and HDL). Nearly all of the patients (>95%) had normal AST and ALT levels at baseline. The results are outlined in the Table. Some values were not recorded for all patients at each follow-up.
Aspartate Aminotransferase Analysis
Aspartate aminotransferase levels were classified as normal and high. At baseline, mean (SD) AST levels were 20.2 (6.6) U/L, with normal levels in 311 (96.6%) patients and high in 7 (2.2%) patients. At 3-month follow-up, mean (SD) AST levels were 20.7 (5.2) U/L, with normal levels in 270 (83.9%) patients and high levels in 3 (0.9%) patients. At 6-month follow-up, mean (SD) AST levels were 21.3 (5.7) U/L, with normal levels in 209 (64.9%) patients and high levels in 4 (1.2%) patients. Aspartate aminotransferase levels increased at 3- and 6-month follow-up compared to baseline. Differences between AST levels were statistically significant (F2,416=4.2, P=.016). Differences between AST levels at baseline and 3-month follow-up were not statistically significant (P=.3). Differences between AST levels at 3- and 6-month follow-up were not statistically significant (P=.4). Differences between AST levels at baseline and 6-month follow-up were statistically significant (P=.07). Differences between AST classifications at the 3 time points were not statistically significant (F2,416=0.44, P=.64). Overall, the results indicated that AST levels increased over time in patients treated with isotretinoin, but the increase was not above the normal range and was not statistically significant.
Alanine Aminotransferase Analysis
Alanine aminotransferase levels were classified as normal or high. At baseline, mean (SD) ALT levels were 16.8 (11.2) U/L, with normal levels in 303 (94.1%) patients and high in 19 (5.9%) patients. At 3-month follow-up, mean (SD) ALT levels were 16.2 (9.3) U/L, with normal levels in 263 (81.7%) patients and high in 11 (3.4%) patients. At 6-month follow-up, mean (SD) ALT levels were 17.0 (11.3) U/L, with normal levels in 201 (62.4%) patients and high in 11 (3.4%) patients. Alanine aminotransferase levels at 3-month follow-up were lower than baseline but higher at 6-month follow-up compared to baseline and 3-month follow-up. Overall, ALT levels increased with time, but the differences between baseline and 3- and 6-month follow-up were not statistically significant (F2,416=0.32, P=.72). Differences between ALT classifications at each time point were not statistically significant (F2,418=0.21, P=.54). Overall, the results indicated that ALT levels increased over time in patients treated with isotretinoin, but the increase was not statistically significant.
Triglycerides Analysis
Triglyceride levels were classified as normal, borderline high, high, and very high. At baseline, mean (SD) TG levels were 107 (71) mg/dL, with normal levels in 270 (83.9%) patients, borderline high in 30 (9.3%) patients, high in 20 (6.2%) patients, and very high in 2 (0.6%) patients. At 3-month follow-up, mean (SD) TG levels were 117 (60) mg/dL, with normal levels in 197 (61.2%) patients, borderline high in 38 (11.8%) patients, high in 22 (6.8%) patients, and very high in 1 (0.3%) patient. At 6-month follow-up, mean (SD) TG levels were 122 (65) mg/dL, with normal levels in 145 (45%) patients, borderline high in 36 (11.2%) patients, high in 16 (5%) patients, and very high in 0 (0%) patients. Triglyceride levels increased and differences between TG levels at baseline and 3- and 6-month follow-up were statistically significant (F2,384=17, P<.001). Baseline TG levels compared to 3-month follow-up were statistically signif-icant (P<.001). Differences in TG levels at 6-month follow-up versus baseline were statistically significant (P<.001). However, changes in TG levels from 3- to 6-month follow-up were not statistically significant (P=.21). Differences between TG classifications at each time point were statistically significant (F2,386=6.9, P=.001). Overall, TG levels increased from baseline during isotretinoin treatment at 3- and 6-month follow-up, and these increases were above normal range; however, there was no statistically significant increase from 3- to 6-month follow-up.
Low-Density Lipoprotein Analysis
Low-density lipoprotein levels were classified as optimal, above optimal, borderline high, high, and very high. At baseline, mean (SD) LDL levels were 102 (28) mg/dL, with optimal levels in 162 (50.3%) patients, above optimal in 95 (29.5%) patients, borderline high in 32 (9.9%) patients, high in 11 (3.4%) patients, and very high in 3 (0.9%) patients. At 3-month follow-up, mean (SD) LDL levels were 113 (30) mg/dL, with optimal levels in 89 (27.6%) patients, above optimal in 98 (30.4%) patients, borderline high in 54 (16.8%) patients, high in 12 (3.7%) patients, and very high in 5 (1.6%) patients. At 6-month follow-up, mean (SD) LDL levels were 113 (27) mg/dL, with optimal levels in 60 (18.6%) patients, above optimal in 84 (26.1%) patients, borderline high in 44 (13.7%) patients, high in 8 (2.5%) patients, and very high in 1 (0.3%) patient. Overall, there were statistically significant increases in LDL levels at 3- and 6-month follow-up (F2,382<75, P<.001). Differences between baseline LDL levels and 3-month follow-up were statistically significant (P<.001). Differences between baseline LDL levels and 6-month follow-up were statistically significant (P<.001). However, differences in LDL levels at 3- and 6-month follow-up were not statistically significant (P=.74). Differences between LDL classifications at each time point were statistically significant (F2,382=51.2, P<.001). Overall, statistically significant increases in LDL levels from baseline were noted during isotretinoin treatment and this increase was above normal range; however, LDL levels did not significantly increase from 3- to 6-month follow-up.
High-Density Lipoprotein Analysis
High-density lipoprotein levels were classified as low, normal, and high. At baseline, mean (SD) HDL levels were 52.4 (16) mg/dL, with low levels in 60 (18.6%) patients, normal in 173 (53.7%) patients, and high in 71 (22%) patients. At 3-month follow-up, mean (SD) HDL levels were 48 (12) mg/dL, with low levels in 63 (19.6%) patients, normal in 154 (47.8%) patients, and high in 41 (12.7%) patients. At 6-month follow-up, mean (SD) HDL levels were 47.6 (12) mg/dL, with low levels in 48 (14.9%) patients, normal in 117 (36.3%) patients, and high in 33 (10.2%) patients. Overall, statistically significant decreases were noted in HDL levels (F2,384=19, P<.001). Differences between baseline HDL levels compared to 3-month follow-up were statistically significant (P<.001). Differences between baseline HDL levels compared to 6-month follow-up were statistically significant (P<.001). Differences in HDL levels at 3- and 6-month follow-up were statistically significant (P<.001). Differences between HDL classifications at each time point were statistically significant (F2,384=5.2, P=.006). Overall, there were statistically significant decreases in HDL levels during isotretinoin treatment from baseline and this decrease was above normal range; however, HDL levels did not decrease at 3- and 6-month follow-up.
Comment
Studies in the literature evaluating the effects of isotretinoin on liver enzymes and lipids suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT), TGs, HDL, and LDL in various degrees.1 Zane et al4 studied 13,772 patients with acne undergoing oral isotretinoin therapy between March 1995 and September 2002. The investigators found increased liver transaminase and serum lipid levels. They suggested that these abnormalities were generally transient and reversible.4 Bershad et al5 reported an increase in LDL and TG but a decrease in HDL during isotretinoin therapy. These changes in the lipid profile also appeared to be transient and returned to baseline level 2 months following the end of treatment.5 In another study of 130 patients who were treated with isotretinoin, Vieira et al1 noted an increase in AST, ALT, and TG levels. Most of the studies in the literature that reported effects of isotretinoin on liver enzymes and lipids suggested that the effects were reversible.
Although many studies reported alterations in serum transaminase and lipid levels, other studies reported no effect. In one study of 150 participants, Brito et al2 found no statistically significant changes in liver transaminase, TG, HDL, or LDL levels following treatment with isotretinoin. In another study of 1292 participants by Alcalay et al,6 serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of isotretinoin treatment. In another study of 30 participants, Baxter et al7 reported no significant changes in TG, LDL, or HDL levels measured at baseline or during treatment with isotretinoin.
Some studies suggest that routine laboratory tests are needed when treating patients with isotretinoin due to severe alterations in serum liver transaminase and lipid levels, while other studies conclude that the effects are minimal and laboratory tests are not needed. In the current study, we found that there were statistically significant increases in TG and LDL levels in patients who underwent treatment with isotretinoin. We also found statistically significant decreases in HDL levels. In our study, liver enzymes were less affected than lipids in patients who underwent treatment with isotretinoin. There were statistically significant increases in AST levels, but the clinical classification was not affected. There also were increases in ALT levels, but the changes were not statistically significant.
Overall, we advise dermatologists that isotretinoin can be administered with minimal concern regarding changes in serum transaminase and lipid levels; however, although severe laboratory alterations were not noted in our study, we advise physicians to use caution when administering isotretinoin in patients with a history of abnormal findings.
1. Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglyceride and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
2. Brito MFM, Pessoa IS, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin [in English, Portuguese]. An Bras Dermatol. 2010;85:331-337.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
5. Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
6. Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
7. Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2003;14:216-218.
1. Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglyceride and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
2. Brito MFM, Pessoa IS, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin [in English, Portuguese]. An Bras Dermatol. 2010;85:331-337.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
5. Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
6. Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
7. Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2003;14:216-218.
Practice Points
- Isotretinoin is recommended for treatment of severe inflammatory acne and for cases resistant to prior treatment with antibiotics or topical agents; however, it may cause alterations in lipids and liver enzymes.
- In our study, liver enzymes were less affected than lipids in patients who were treated with isotretinoin.
- Use caution when administering isotretinoin in patients with a history of abnormal findings.