User login
Acitretin-Induced Acral Hemorrhagic Lesions in Darier-White Disease
Darier-White disease (DD), also known as keratosis follicularis, is a rare skin disease that is inherited in an autosomal-dominant fashion. It was first described by both Darier1 and White2 more than 120 years ago. The incidence is 1 to 9 per 100,000 individuals, and onset usually occurs between 8 and 15 years of age. Classic DD is characterized by malodorous, brown, keratotic, warty papules arising mainly in seborrheic areas; palmoplantar pits; and nail dystrophy with typical worsening in summer. Unilateral and segmental variants of DD rarely have been described.3,4
Few cases of hemorrhagic DD involving acral surfaces have been reported.5-8 Most patients develop hemorrhagic bullae at the onset of the disease together with the other characteristic features of DD. The disease also can be associated with salivary gland obstruction, renal and testicular agenesis, bone cysts, and neuropsychiatric disorders.9 Diagnosis is confirmed by hyperkeratosis, papillomatosis, acantholysis, dyskeratosis (corps ronds and grains), and small fissures above the basal layer in the epidermis (suprabasal clefts) noted on biopsy of papular lesions. Darier-White disease is caused by a malfunctioning sarcoplasmic/endoplasmic reticulum calcium pump, SERCA2, encoded by the ATP2A2 (ATPase, Ca++ transporting, cardiac muscle, slow twitch 2) gene, which transports calcium ions from the cytosol into the sarcoplasmic/endoplasmic reticulum, catalyzing the hydrolysis of adenosine triphosphate coupled with the transport of the calcium. Mutations in ATP2A2 have only been identified in up to 50% of reported DD patients.10
Treatment of localized DD usually includes emollients and topical retinoids, and systemic retinoids are recommended as the first-line treatment of diffuse DD.11 Many dermatologic side effects of systemic retinoids have been reported, including frequent dry lips and cheilitis.12 Other skin manifestations include scaling of the palms and soles, excoriations and erosions, alopecia, pruritus, nail fragility, and skin atrophy or fragility. Additionally, common nondermatologic side effects include hepatotoxicity, epistaxis, ophthalmologic effects (eg, loss of eyebrows or eyelashes, redness/swelling of the eyelid, redness of the eyes, sensitivity of the eyes to light, decreased night vision) pancreatitis, bipedal edema, and skeletal alterations.13-15 We report the case of a patient with DD who developed hemorrhagic macules and vesicles in response to the administration of oral retinoids.
Case Report
An 84-year-old woman was admitted to our hospital for treatment of hyperkeratotic papules and plaques on the face, back, groin, submammary folds, and dorsum of the hands and feet that had been present since childhood and typically worsened during the summer. She previously had refused treatment and reported a poor social life due to the cutaneous manifestations of her disease.
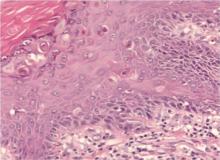
Biopsy of a histologic specimen taken from a palmar pit revealed hyperkeratosis, loss of cohesion between suprabasal epidermal cells (acantholysis), and dyskeratotic cells (corps ronds and grains)(Figure 1). On the basis of both clinical and histologic findings, a diagnosis of DD was made. Genetic analysis did not reveal any mutations in the ATP2A2 gene, and a reduced epidermal expression of SERCA2b was demonstrated by immunochemistry.16 Treatment with the oral retinoid acitretin (25 mg daily) for 4 months led to almost complete resolution of the lesions. After 4 months, treatment was reduced to a maintenance dose of 12.5 mg once daily.
The patient returned monthly for follow-up to undergo clinical examination and routine laboratory tests (ie, complete blood cell counts, electrolyte panel, renal and hepatic tests, serum lipid levels, cholesterol level). After beginning the maintenance dose, she returned for follow-up every 2 months.
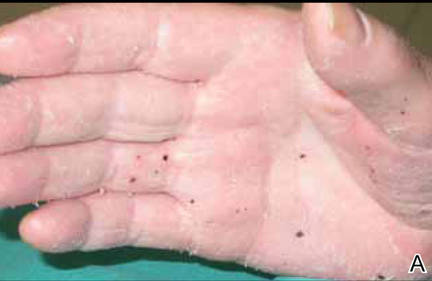
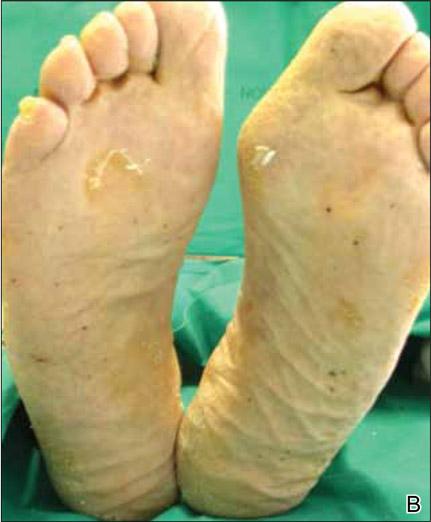
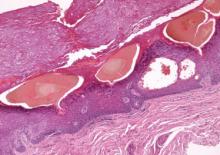
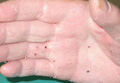
On follow-up 3 months after beginning the maintenance dose, the patient reported the onset of red and black punctiform macules and vesicles with jagged borders located on the palmoplantar surfaces and dorsal aspect of the fingers on both hands. Lesions were either isolated or confluent. The patient did not report pain or itching, though the skin was severely xerotic, especially on the hands and feet (Figures 2A and 2B). The patient reported no local trauma associated with the lesions, and blood counts and coagulation tests were within reference range. It also was noted that the cutaneous manifestations of DD showed a striking improvement. Histologic analysis of a skin biopsy taken from a hemorrhagic lesion on the palmar surface showed hyperkeratosis, acanthosis, papillomatosis, hypergranulosis, focal dyskeratosis, and hemorrhagic vesicles in the horny layer of the epidermis (Figure 3), leading to a diagnosis of DD.
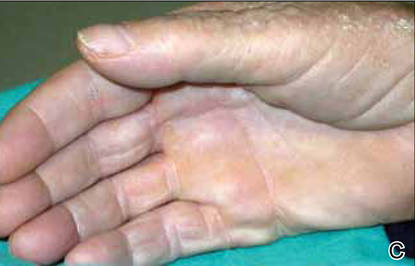
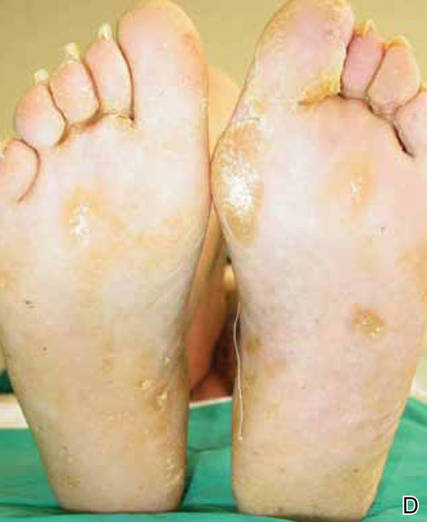
After 12 months of therapy, the patient spontaneously stopped taking acitretin, and the hemorrhagic lesions spontaneously regressed within 2 weeks (Figures 2C and 2D). Unfortunately, the cutaneous manifestations of DD (eg, papular lesions) progressively reappeared in the previously reported sites. Based on this relapse, the patient was advised to restart treatment with acitretin (25 mg daily). In the follow-up visit 1 month after restarting acitretin, physical examination revealed the reappearance of hemorrhagic lesions on the palmoplantar surfaces and the dorsal aspect of the fingers.
|
|
Comment
We report the case of an 84-year-old woman who developed hemorrhagic lesions on the palmoplantar surfaces and dorsal aspect of the feet as a side effect of oral retinoids for treatment of DD. The first reported case of acral hemorrhagic lesions associated with DD was described 50 years ago in 4 patients with manifestations located mainly on the palms, soles, and dorsal fingers. Local trauma was identified as a triggering factor in the development of the vesicles; however, data regarding the temporal relationship between keratotic papules and hemorrhagic elements and treatment were not specified.5 Twenty-five years later, Coulson and Misch6 described a case of DD in which the hemorrhagic lesions were the first sign of the disease, but correlation with therapy was not reported. A case of retinoid-induced hemorrhagic DD was reported in a female who was treated with etretinate for approximately 10 years after the diagnosis of DD with good clinical response. After 10 years of therapy, she developed hemorrhagic bullae solely on the dorsal aspect of the hands without any direct association with local trauma, along with a small number of nonhemorrhagic bullae.17
In our patient, the onset of hemorrhagic vesicles and red maculae occurred primarily on the palmoplantar surfaces. The lesions were smaller than bullae and contained hemorrhagic elements. Of note, the skin lesions appeared as early as 7 months after the patient started acitretin therapy.
The development of hemorrhagic lesions on different body sites as a consequence of oral retinoid administration also has been reported in patients with psoriasis.15 Emerging evidence indicates that retinoic acid activates vascular endothelial growth factor gene transcription,18 and vascular endothelial growth factor can modify permeability of the endothelial cells. Based on these observations, we propose that DD is characterized by loss of adhesion between epidermal cells (acantholysis) and abnormal keratinization. This defect favors the formation of empty intraepidermal lacunae. An increase in endothelial permeability due to oral retinoid administration promotes the gathering of serum and red blood cells into the lacunae, leading to the onset of hemorrhagic blisters. Moreover, continued microtrauma to the palmoplantar surfaces may account for the peculiar localization of the hemorrhagic lesions described in our patient.
Our unique report of hemorrhagic lesions (vesicles and maculae) presenting in a DD patient treated with acitretin is rare. The first histologic specimen taken from a palmar pit revealed the characteristic histopathologic findings of DD and did not support the diagnosis of the hemorrhagic variant of DD. In the second biopsy, the absence of acantholysis and the poor content of dyskeratotic cells were evident, together with superficial hemorrhagic vesicles. Additionally, the appearance and the disappearance of the cutaneous manifestations were closely correlated with the beginning and suspension of the acitretin therapy, together with an improvement of typical lesions of DD during acitretin treatment. These occurrences indicated a strict causal relationship between treatment with the retinoid and the appearance of hemorrhagic lesions.
Conclusion
We report a rare case of palmoplantar hemorrhagic lesions induced by acitretin for treatment of DD. In our patient, the lesions could have been triggered by a combination of noxious effects of the drug and alterations in keratinocyte physiology due to DD. There is a need for clinicians to be aware of the possible side effects of acitretin and to inform their patients, particularly those presenting with DD, about the possibility of developing hemorrhagic lesions. The latter should be added to the list of potential dermatologic adverse effects of acitretin.
1. Darier J. De la porospermose folliculaire vegetante. Ann Dermatol Syphiligr. 1889;10:597-605.
2. White JC. A case of keratosis (ichtyosis) follicularis. J Cutan Genitourin Dis. 1889;7:201-209.
3. Goldsmith LA, Baden HP. Darier-White disease (keratosis follicularis) and acrokeratosis verruciformis. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 4th ed. New York, NY: McGraw-Hill; 2003:523-528.
4. Sanderson EA, Killoran CE, Pedvis-Leftick A, et al. Localized Darier’s disease in Blaschkoid distribution: two cases of phenotypic mosaicism and a review of mosaic Darier’s disease. J Dermatol. 2007;34:761-764.
5. Jones WN, Nix TE Jr, Clark WH Jr. Hemorrhagic Darier’s disease. Arch Dermatol. 1964;89:523-527.
6. Coulson IH, Misch KJ. Haemorrhagic Darier’s disease. J R Soc Med. 1989;82:365-366.
7. Foresman PL, Goldsmith LA, Ginn L, et al. Hemorrhagic Darier’s disease. Arch Dermatol. 1993;129:511-512.
8. Regazzini R, Zambruno G, DeFilippi C, et al. Isolated acral Darier’s disease with haemorrhagic lesions in a kindred. Br J Dermatol. 1996;135:495-496.
9. Zeglaoui F, Zaraa I, Fazaa B, et al. Dyskeratosis follicularis disease: case reports and review of the literature. J Eur Acad Dermatol Venereol. 2005;19:114-117.
10. Sakuntabhai A, Burge S, Monk S, et al. Spectrum of novel ATP2A2 mutations with Darier’s disease. Hum Mol Genet. 1999;8:1611-1619.
11. Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50.
12. Nikam B, Amladi S, Bingewar G, et al. Acral papular eruption. Indian J Dermatol Venereol Leprol. 2005;71:447-448.
13. Wolverton SE, Remlinger K. Suggested guidelines for patient monitoring: hepatic and hematologic toxicity attributable to systemic dermatologic drugs. Dermatol Clin. 2007;25:195-205.
14. Tey HL, Theng TS. Acitretin induced bipedal edema. J Dermatol. 2006;33:372-374.
15. Aydogan K, Karadogan SK, Tunali S. Acitretin-induced subungual hemorrhage. Int J Dermatol. 2007;46:494-495.
16. Borgogna C, Zavattaro E, Dell’Oste V, et al. No indications for HPV involvement in the hypertrophic skin lesions of a Darier disease case without ATP2A2 gene mutations. J Cutan Pathol. 2009;36:1005-1009.
17. Gebauer K, Holgate C, Navaratnam A. Retinoid-induced haemorrhagic bullae in Darier’s disease. Australas J Dermatol. 1990;31:99-103.
18. Maeno T, Tanaka T, Sando Y, et al. Stimulation of vascular endothelial growth factor gene transcription by all trans retinoic acid through Sp1 and Sp3 sites in human bronchioalveolar carcinoma cells. Am J Respir Cell Mol Biol. 2002;26:246-253.
Darier-White disease (DD), also known as keratosis follicularis, is a rare skin disease that is inherited in an autosomal-dominant fashion. It was first described by both Darier1 and White2 more than 120 years ago. The incidence is 1 to 9 per 100,000 individuals, and onset usually occurs between 8 and 15 years of age. Classic DD is characterized by malodorous, brown, keratotic, warty papules arising mainly in seborrheic areas; palmoplantar pits; and nail dystrophy with typical worsening in summer. Unilateral and segmental variants of DD rarely have been described.3,4
Few cases of hemorrhagic DD involving acral surfaces have been reported.5-8 Most patients develop hemorrhagic bullae at the onset of the disease together with the other characteristic features of DD. The disease also can be associated with salivary gland obstruction, renal and testicular agenesis, bone cysts, and neuropsychiatric disorders.9 Diagnosis is confirmed by hyperkeratosis, papillomatosis, acantholysis, dyskeratosis (corps ronds and grains), and small fissures above the basal layer in the epidermis (suprabasal clefts) noted on biopsy of papular lesions. Darier-White disease is caused by a malfunctioning sarcoplasmic/endoplasmic reticulum calcium pump, SERCA2, encoded by the ATP2A2 (ATPase, Ca++ transporting, cardiac muscle, slow twitch 2) gene, which transports calcium ions from the cytosol into the sarcoplasmic/endoplasmic reticulum, catalyzing the hydrolysis of adenosine triphosphate coupled with the transport of the calcium. Mutations in ATP2A2 have only been identified in up to 50% of reported DD patients.10
Treatment of localized DD usually includes emollients and topical retinoids, and systemic retinoids are recommended as the first-line treatment of diffuse DD.11 Many dermatologic side effects of systemic retinoids have been reported, including frequent dry lips and cheilitis.12 Other skin manifestations include scaling of the palms and soles, excoriations and erosions, alopecia, pruritus, nail fragility, and skin atrophy or fragility. Additionally, common nondermatologic side effects include hepatotoxicity, epistaxis, ophthalmologic effects (eg, loss of eyebrows or eyelashes, redness/swelling of the eyelid, redness of the eyes, sensitivity of the eyes to light, decreased night vision) pancreatitis, bipedal edema, and skeletal alterations.13-15 We report the case of a patient with DD who developed hemorrhagic macules and vesicles in response to the administration of oral retinoids.
Case Report
An 84-year-old woman was admitted to our hospital for treatment of hyperkeratotic papules and plaques on the face, back, groin, submammary folds, and dorsum of the hands and feet that had been present since childhood and typically worsened during the summer. She previously had refused treatment and reported a poor social life due to the cutaneous manifestations of her disease.
Biopsy of a histologic specimen taken from a palmar pit revealed hyperkeratosis, loss of cohesion between suprabasal epidermal cells (acantholysis), and dyskeratotic cells (corps ronds and grains)(Figure 1). On the basis of both clinical and histologic findings, a diagnosis of DD was made. Genetic analysis did not reveal any mutations in the ATP2A2 gene, and a reduced epidermal expression of SERCA2b was demonstrated by immunochemistry.16 Treatment with the oral retinoid acitretin (25 mg daily) for 4 months led to almost complete resolution of the lesions. After 4 months, treatment was reduced to a maintenance dose of 12.5 mg once daily.
The patient returned monthly for follow-up to undergo clinical examination and routine laboratory tests (ie, complete blood cell counts, electrolyte panel, renal and hepatic tests, serum lipid levels, cholesterol level). After beginning the maintenance dose, she returned for follow-up every 2 months.
On follow-up 3 months after beginning the maintenance dose, the patient reported the onset of red and black punctiform macules and vesicles with jagged borders located on the palmoplantar surfaces and dorsal aspect of the fingers on both hands. Lesions were either isolated or confluent. The patient did not report pain or itching, though the skin was severely xerotic, especially on the hands and feet (Figures 2A and 2B). The patient reported no local trauma associated with the lesions, and blood counts and coagulation tests were within reference range. It also was noted that the cutaneous manifestations of DD showed a striking improvement. Histologic analysis of a skin biopsy taken from a hemorrhagic lesion on the palmar surface showed hyperkeratosis, acanthosis, papillomatosis, hypergranulosis, focal dyskeratosis, and hemorrhagic vesicles in the horny layer of the epidermis (Figure 3), leading to a diagnosis of DD.
After 12 months of therapy, the patient spontaneously stopped taking acitretin, and the hemorrhagic lesions spontaneously regressed within 2 weeks (Figures 2C and 2D). Unfortunately, the cutaneous manifestations of DD (eg, papular lesions) progressively reappeared in the previously reported sites. Based on this relapse, the patient was advised to restart treatment with acitretin (25 mg daily). In the follow-up visit 1 month after restarting acitretin, physical examination revealed the reappearance of hemorrhagic lesions on the palmoplantar surfaces and the dorsal aspect of the fingers.
|
|
Comment
We report the case of an 84-year-old woman who developed hemorrhagic lesions on the palmoplantar surfaces and dorsal aspect of the feet as a side effect of oral retinoids for treatment of DD. The first reported case of acral hemorrhagic lesions associated with DD was described 50 years ago in 4 patients with manifestations located mainly on the palms, soles, and dorsal fingers. Local trauma was identified as a triggering factor in the development of the vesicles; however, data regarding the temporal relationship between keratotic papules and hemorrhagic elements and treatment were not specified.5 Twenty-five years later, Coulson and Misch6 described a case of DD in which the hemorrhagic lesions were the first sign of the disease, but correlation with therapy was not reported. A case of retinoid-induced hemorrhagic DD was reported in a female who was treated with etretinate for approximately 10 years after the diagnosis of DD with good clinical response. After 10 years of therapy, she developed hemorrhagic bullae solely on the dorsal aspect of the hands without any direct association with local trauma, along with a small number of nonhemorrhagic bullae.17
In our patient, the onset of hemorrhagic vesicles and red maculae occurred primarily on the palmoplantar surfaces. The lesions were smaller than bullae and contained hemorrhagic elements. Of note, the skin lesions appeared as early as 7 months after the patient started acitretin therapy.
The development of hemorrhagic lesions on different body sites as a consequence of oral retinoid administration also has been reported in patients with psoriasis.15 Emerging evidence indicates that retinoic acid activates vascular endothelial growth factor gene transcription,18 and vascular endothelial growth factor can modify permeability of the endothelial cells. Based on these observations, we propose that DD is characterized by loss of adhesion between epidermal cells (acantholysis) and abnormal keratinization. This defect favors the formation of empty intraepidermal lacunae. An increase in endothelial permeability due to oral retinoid administration promotes the gathering of serum and red blood cells into the lacunae, leading to the onset of hemorrhagic blisters. Moreover, continued microtrauma to the palmoplantar surfaces may account for the peculiar localization of the hemorrhagic lesions described in our patient.
Our unique report of hemorrhagic lesions (vesicles and maculae) presenting in a DD patient treated with acitretin is rare. The first histologic specimen taken from a palmar pit revealed the characteristic histopathologic findings of DD and did not support the diagnosis of the hemorrhagic variant of DD. In the second biopsy, the absence of acantholysis and the poor content of dyskeratotic cells were evident, together with superficial hemorrhagic vesicles. Additionally, the appearance and the disappearance of the cutaneous manifestations were closely correlated with the beginning and suspension of the acitretin therapy, together with an improvement of typical lesions of DD during acitretin treatment. These occurrences indicated a strict causal relationship between treatment with the retinoid and the appearance of hemorrhagic lesions.
Conclusion
We report a rare case of palmoplantar hemorrhagic lesions induced by acitretin for treatment of DD. In our patient, the lesions could have been triggered by a combination of noxious effects of the drug and alterations in keratinocyte physiology due to DD. There is a need for clinicians to be aware of the possible side effects of acitretin and to inform their patients, particularly those presenting with DD, about the possibility of developing hemorrhagic lesions. The latter should be added to the list of potential dermatologic adverse effects of acitretin.
Darier-White disease (DD), also known as keratosis follicularis, is a rare skin disease that is inherited in an autosomal-dominant fashion. It was first described by both Darier1 and White2 more than 120 years ago. The incidence is 1 to 9 per 100,000 individuals, and onset usually occurs between 8 and 15 years of age. Classic DD is characterized by malodorous, brown, keratotic, warty papules arising mainly in seborrheic areas; palmoplantar pits; and nail dystrophy with typical worsening in summer. Unilateral and segmental variants of DD rarely have been described.3,4
Few cases of hemorrhagic DD involving acral surfaces have been reported.5-8 Most patients develop hemorrhagic bullae at the onset of the disease together with the other characteristic features of DD. The disease also can be associated with salivary gland obstruction, renal and testicular agenesis, bone cysts, and neuropsychiatric disorders.9 Diagnosis is confirmed by hyperkeratosis, papillomatosis, acantholysis, dyskeratosis (corps ronds and grains), and small fissures above the basal layer in the epidermis (suprabasal clefts) noted on biopsy of papular lesions. Darier-White disease is caused by a malfunctioning sarcoplasmic/endoplasmic reticulum calcium pump, SERCA2, encoded by the ATP2A2 (ATPase, Ca++ transporting, cardiac muscle, slow twitch 2) gene, which transports calcium ions from the cytosol into the sarcoplasmic/endoplasmic reticulum, catalyzing the hydrolysis of adenosine triphosphate coupled with the transport of the calcium. Mutations in ATP2A2 have only been identified in up to 50% of reported DD patients.10
Treatment of localized DD usually includes emollients and topical retinoids, and systemic retinoids are recommended as the first-line treatment of diffuse DD.11 Many dermatologic side effects of systemic retinoids have been reported, including frequent dry lips and cheilitis.12 Other skin manifestations include scaling of the palms and soles, excoriations and erosions, alopecia, pruritus, nail fragility, and skin atrophy or fragility. Additionally, common nondermatologic side effects include hepatotoxicity, epistaxis, ophthalmologic effects (eg, loss of eyebrows or eyelashes, redness/swelling of the eyelid, redness of the eyes, sensitivity of the eyes to light, decreased night vision) pancreatitis, bipedal edema, and skeletal alterations.13-15 We report the case of a patient with DD who developed hemorrhagic macules and vesicles in response to the administration of oral retinoids.
Case Report
An 84-year-old woman was admitted to our hospital for treatment of hyperkeratotic papules and plaques on the face, back, groin, submammary folds, and dorsum of the hands and feet that had been present since childhood and typically worsened during the summer. She previously had refused treatment and reported a poor social life due to the cutaneous manifestations of her disease.
Biopsy of a histologic specimen taken from a palmar pit revealed hyperkeratosis, loss of cohesion between suprabasal epidermal cells (acantholysis), and dyskeratotic cells (corps ronds and grains)(Figure 1). On the basis of both clinical and histologic findings, a diagnosis of DD was made. Genetic analysis did not reveal any mutations in the ATP2A2 gene, and a reduced epidermal expression of SERCA2b was demonstrated by immunochemistry.16 Treatment with the oral retinoid acitretin (25 mg daily) for 4 months led to almost complete resolution of the lesions. After 4 months, treatment was reduced to a maintenance dose of 12.5 mg once daily.
The patient returned monthly for follow-up to undergo clinical examination and routine laboratory tests (ie, complete blood cell counts, electrolyte panel, renal and hepatic tests, serum lipid levels, cholesterol level). After beginning the maintenance dose, she returned for follow-up every 2 months.
On follow-up 3 months after beginning the maintenance dose, the patient reported the onset of red and black punctiform macules and vesicles with jagged borders located on the palmoplantar surfaces and dorsal aspect of the fingers on both hands. Lesions were either isolated or confluent. The patient did not report pain or itching, though the skin was severely xerotic, especially on the hands and feet (Figures 2A and 2B). The patient reported no local trauma associated with the lesions, and blood counts and coagulation tests were within reference range. It also was noted that the cutaneous manifestations of DD showed a striking improvement. Histologic analysis of a skin biopsy taken from a hemorrhagic lesion on the palmar surface showed hyperkeratosis, acanthosis, papillomatosis, hypergranulosis, focal dyskeratosis, and hemorrhagic vesicles in the horny layer of the epidermis (Figure 3), leading to a diagnosis of DD.
After 12 months of therapy, the patient spontaneously stopped taking acitretin, and the hemorrhagic lesions spontaneously regressed within 2 weeks (Figures 2C and 2D). Unfortunately, the cutaneous manifestations of DD (eg, papular lesions) progressively reappeared in the previously reported sites. Based on this relapse, the patient was advised to restart treatment with acitretin (25 mg daily). In the follow-up visit 1 month after restarting acitretin, physical examination revealed the reappearance of hemorrhagic lesions on the palmoplantar surfaces and the dorsal aspect of the fingers.
|
|
Comment
We report the case of an 84-year-old woman who developed hemorrhagic lesions on the palmoplantar surfaces and dorsal aspect of the feet as a side effect of oral retinoids for treatment of DD. The first reported case of acral hemorrhagic lesions associated with DD was described 50 years ago in 4 patients with manifestations located mainly on the palms, soles, and dorsal fingers. Local trauma was identified as a triggering factor in the development of the vesicles; however, data regarding the temporal relationship between keratotic papules and hemorrhagic elements and treatment were not specified.5 Twenty-five years later, Coulson and Misch6 described a case of DD in which the hemorrhagic lesions were the first sign of the disease, but correlation with therapy was not reported. A case of retinoid-induced hemorrhagic DD was reported in a female who was treated with etretinate for approximately 10 years after the diagnosis of DD with good clinical response. After 10 years of therapy, she developed hemorrhagic bullae solely on the dorsal aspect of the hands without any direct association with local trauma, along with a small number of nonhemorrhagic bullae.17
In our patient, the onset of hemorrhagic vesicles and red maculae occurred primarily on the palmoplantar surfaces. The lesions were smaller than bullae and contained hemorrhagic elements. Of note, the skin lesions appeared as early as 7 months after the patient started acitretin therapy.
The development of hemorrhagic lesions on different body sites as a consequence of oral retinoid administration also has been reported in patients with psoriasis.15 Emerging evidence indicates that retinoic acid activates vascular endothelial growth factor gene transcription,18 and vascular endothelial growth factor can modify permeability of the endothelial cells. Based on these observations, we propose that DD is characterized by loss of adhesion between epidermal cells (acantholysis) and abnormal keratinization. This defect favors the formation of empty intraepidermal lacunae. An increase in endothelial permeability due to oral retinoid administration promotes the gathering of serum and red blood cells into the lacunae, leading to the onset of hemorrhagic blisters. Moreover, continued microtrauma to the palmoplantar surfaces may account for the peculiar localization of the hemorrhagic lesions described in our patient.
Our unique report of hemorrhagic lesions (vesicles and maculae) presenting in a DD patient treated with acitretin is rare. The first histologic specimen taken from a palmar pit revealed the characteristic histopathologic findings of DD and did not support the diagnosis of the hemorrhagic variant of DD. In the second biopsy, the absence of acantholysis and the poor content of dyskeratotic cells were evident, together with superficial hemorrhagic vesicles. Additionally, the appearance and the disappearance of the cutaneous manifestations were closely correlated with the beginning and suspension of the acitretin therapy, together with an improvement of typical lesions of DD during acitretin treatment. These occurrences indicated a strict causal relationship between treatment with the retinoid and the appearance of hemorrhagic lesions.
Conclusion
We report a rare case of palmoplantar hemorrhagic lesions induced by acitretin for treatment of DD. In our patient, the lesions could have been triggered by a combination of noxious effects of the drug and alterations in keratinocyte physiology due to DD. There is a need for clinicians to be aware of the possible side effects of acitretin and to inform their patients, particularly those presenting with DD, about the possibility of developing hemorrhagic lesions. The latter should be added to the list of potential dermatologic adverse effects of acitretin.
1. Darier J. De la porospermose folliculaire vegetante. Ann Dermatol Syphiligr. 1889;10:597-605.
2. White JC. A case of keratosis (ichtyosis) follicularis. J Cutan Genitourin Dis. 1889;7:201-209.
3. Goldsmith LA, Baden HP. Darier-White disease (keratosis follicularis) and acrokeratosis verruciformis. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 4th ed. New York, NY: McGraw-Hill; 2003:523-528.
4. Sanderson EA, Killoran CE, Pedvis-Leftick A, et al. Localized Darier’s disease in Blaschkoid distribution: two cases of phenotypic mosaicism and a review of mosaic Darier’s disease. J Dermatol. 2007;34:761-764.
5. Jones WN, Nix TE Jr, Clark WH Jr. Hemorrhagic Darier’s disease. Arch Dermatol. 1964;89:523-527.
6. Coulson IH, Misch KJ. Haemorrhagic Darier’s disease. J R Soc Med. 1989;82:365-366.
7. Foresman PL, Goldsmith LA, Ginn L, et al. Hemorrhagic Darier’s disease. Arch Dermatol. 1993;129:511-512.
8. Regazzini R, Zambruno G, DeFilippi C, et al. Isolated acral Darier’s disease with haemorrhagic lesions in a kindred. Br J Dermatol. 1996;135:495-496.
9. Zeglaoui F, Zaraa I, Fazaa B, et al. Dyskeratosis follicularis disease: case reports and review of the literature. J Eur Acad Dermatol Venereol. 2005;19:114-117.
10. Sakuntabhai A, Burge S, Monk S, et al. Spectrum of novel ATP2A2 mutations with Darier’s disease. Hum Mol Genet. 1999;8:1611-1619.
11. Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50.
12. Nikam B, Amladi S, Bingewar G, et al. Acral papular eruption. Indian J Dermatol Venereol Leprol. 2005;71:447-448.
13. Wolverton SE, Remlinger K. Suggested guidelines for patient monitoring: hepatic and hematologic toxicity attributable to systemic dermatologic drugs. Dermatol Clin. 2007;25:195-205.
14. Tey HL, Theng TS. Acitretin induced bipedal edema. J Dermatol. 2006;33:372-374.
15. Aydogan K, Karadogan SK, Tunali S. Acitretin-induced subungual hemorrhage. Int J Dermatol. 2007;46:494-495.
16. Borgogna C, Zavattaro E, Dell’Oste V, et al. No indications for HPV involvement in the hypertrophic skin lesions of a Darier disease case without ATP2A2 gene mutations. J Cutan Pathol. 2009;36:1005-1009.
17. Gebauer K, Holgate C, Navaratnam A. Retinoid-induced haemorrhagic bullae in Darier’s disease. Australas J Dermatol. 1990;31:99-103.
18. Maeno T, Tanaka T, Sando Y, et al. Stimulation of vascular endothelial growth factor gene transcription by all trans retinoic acid through Sp1 and Sp3 sites in human bronchioalveolar carcinoma cells. Am J Respir Cell Mol Biol. 2002;26:246-253.
1. Darier J. De la porospermose folliculaire vegetante. Ann Dermatol Syphiligr. 1889;10:597-605.
2. White JC. A case of keratosis (ichtyosis) follicularis. J Cutan Genitourin Dis. 1889;7:201-209.
3. Goldsmith LA, Baden HP. Darier-White disease (keratosis follicularis) and acrokeratosis verruciformis. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 4th ed. New York, NY: McGraw-Hill; 2003:523-528.
4. Sanderson EA, Killoran CE, Pedvis-Leftick A, et al. Localized Darier’s disease in Blaschkoid distribution: two cases of phenotypic mosaicism and a review of mosaic Darier’s disease. J Dermatol. 2007;34:761-764.
5. Jones WN, Nix TE Jr, Clark WH Jr. Hemorrhagic Darier’s disease. Arch Dermatol. 1964;89:523-527.
6. Coulson IH, Misch KJ. Haemorrhagic Darier’s disease. J R Soc Med. 1989;82:365-366.
7. Foresman PL, Goldsmith LA, Ginn L, et al. Hemorrhagic Darier’s disease. Arch Dermatol. 1993;129:511-512.
8. Regazzini R, Zambruno G, DeFilippi C, et al. Isolated acral Darier’s disease with haemorrhagic lesions in a kindred. Br J Dermatol. 1996;135:495-496.
9. Zeglaoui F, Zaraa I, Fazaa B, et al. Dyskeratosis follicularis disease: case reports and review of the literature. J Eur Acad Dermatol Venereol. 2005;19:114-117.
10. Sakuntabhai A, Burge S, Monk S, et al. Spectrum of novel ATP2A2 mutations with Darier’s disease. Hum Mol Genet. 1999;8:1611-1619.
11. Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50.
12. Nikam B, Amladi S, Bingewar G, et al. Acral papular eruption. Indian J Dermatol Venereol Leprol. 2005;71:447-448.
13. Wolverton SE, Remlinger K. Suggested guidelines for patient monitoring: hepatic and hematologic toxicity attributable to systemic dermatologic drugs. Dermatol Clin. 2007;25:195-205.
14. Tey HL, Theng TS. Acitretin induced bipedal edema. J Dermatol. 2006;33:372-374.
15. Aydogan K, Karadogan SK, Tunali S. Acitretin-induced subungual hemorrhage. Int J Dermatol. 2007;46:494-495.
16. Borgogna C, Zavattaro E, Dell’Oste V, et al. No indications for HPV involvement in the hypertrophic skin lesions of a Darier disease case without ATP2A2 gene mutations. J Cutan Pathol. 2009;36:1005-1009.
17. Gebauer K, Holgate C, Navaratnam A. Retinoid-induced haemorrhagic bullae in Darier’s disease. Australas J Dermatol. 1990;31:99-103.
18. Maeno T, Tanaka T, Sando Y, et al. Stimulation of vascular endothelial growth factor gene transcription by all trans retinoic acid through Sp1 and Sp3 sites in human bronchioalveolar carcinoma cells. Am J Respir Cell Mol Biol. 2002;26:246-253.
Practice Points
- The first-line treatment of Darier-White disease (DD) is oral retinoids.
- Numerous side effects of retinoids have been described. Clinicians should take these cutaneous manifestations into consideration in patients affected by DD.