User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Consider gaps in access and knowledge in diagnosis and treatment in skin of color
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
FDA alert: ‘Substantial’ hypocalcemia risk with denosumab use in dialysis patients
The Food and Drug Administration issued an alert on Nov. 22 that cited preliminary evidence for a “substantial risk” for severe and symptomatic hypocalcemia and serious outcomes related to abnormally low calcium levels in people being treated with dialysis and receiving the osteoporosis medication denosumab (Prolia), including hospitalization and death.
In its alert, the FDA advised clinicians to make sure that people on dialysis who receive Prolia ingest adequate calcium and vitamin D supplementation and undergo frequent blood calcium monitoring, “possibly more often than is already being conducted,” which “may help decrease the likelihood or severity of these risks.”
The agency also called on clinicians to “advise patients on dialysis to immediately seek help if they experience symptoms of hypocalcemia,” such as unusual tingling or numbness in the hands, arms, legs, or feet; painful muscle spasms or cramps; voice box or lung spasms causing difficulty breathing; vomiting; seizures; or irregular heart rhythm.
The FDA had a similar message for people being treated with dialysis who are also receiving Prolia. The alert advised patients to watch for these symptoms and to tell their health care provider if they occur. The agency also advised patients who are undergoing dialysis and receiving Prolia to not stop the agent on their own, without first discussing this step with their care provider.
The FDA also advised providers and patients to contact the agency about episodes of side effects from Prolia (or other medications) via the FDA’s MedWatch program.
Frequent and serious
The FDA explained it issued the alert because of “the frequency and seriousness” of the risk for hypocalcemia and resulting complications. The agency noted that the risk seems most acute for people on dialysis who also receive Prolia, but the risk may also extend to people with advanced kidney disease who are not being treated with hemodialysis.
The alert stemmed from “interim results” in an ongoing safety study of Prolia that the FDA required the agent’s manufacturer, Amgen, to run when the agency first approved denosumab for U.S. marketing in 2010. The FDA said its review of these interim results suggested an increased risk of hypocalcemia with Prolia in patients with advanced kidney disease.
In addition, adverse event reports submitted to the FDA suggested in a separate, internal study that patients on dialysis treated with Prolia are at “substantial risk for severe and symptomatic hypocalcemia, including hospitalization and death.”
The alert explained that “because of the frequency and seriousness of these risks, we are alerting healthcare professionals and patients about them and that we are continuing to evaluate this potential safety issue with Prolia use in patients with advanced kidney disease, particularly those on dialysis.” The FDA added that “we will communicate our final conclusions and recommendations when we have completed our review or have more information to share.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration issued an alert on Nov. 22 that cited preliminary evidence for a “substantial risk” for severe and symptomatic hypocalcemia and serious outcomes related to abnormally low calcium levels in people being treated with dialysis and receiving the osteoporosis medication denosumab (Prolia), including hospitalization and death.
In its alert, the FDA advised clinicians to make sure that people on dialysis who receive Prolia ingest adequate calcium and vitamin D supplementation and undergo frequent blood calcium monitoring, “possibly more often than is already being conducted,” which “may help decrease the likelihood or severity of these risks.”
The agency also called on clinicians to “advise patients on dialysis to immediately seek help if they experience symptoms of hypocalcemia,” such as unusual tingling or numbness in the hands, arms, legs, or feet; painful muscle spasms or cramps; voice box or lung spasms causing difficulty breathing; vomiting; seizures; or irregular heart rhythm.
The FDA had a similar message for people being treated with dialysis who are also receiving Prolia. The alert advised patients to watch for these symptoms and to tell their health care provider if they occur. The agency also advised patients who are undergoing dialysis and receiving Prolia to not stop the agent on their own, without first discussing this step with their care provider.
The FDA also advised providers and patients to contact the agency about episodes of side effects from Prolia (or other medications) via the FDA’s MedWatch program.
Frequent and serious
The FDA explained it issued the alert because of “the frequency and seriousness” of the risk for hypocalcemia and resulting complications. The agency noted that the risk seems most acute for people on dialysis who also receive Prolia, but the risk may also extend to people with advanced kidney disease who are not being treated with hemodialysis.
The alert stemmed from “interim results” in an ongoing safety study of Prolia that the FDA required the agent’s manufacturer, Amgen, to run when the agency first approved denosumab for U.S. marketing in 2010. The FDA said its review of these interim results suggested an increased risk of hypocalcemia with Prolia in patients with advanced kidney disease.
In addition, adverse event reports submitted to the FDA suggested in a separate, internal study that patients on dialysis treated with Prolia are at “substantial risk for severe and symptomatic hypocalcemia, including hospitalization and death.”
The alert explained that “because of the frequency and seriousness of these risks, we are alerting healthcare professionals and patients about them and that we are continuing to evaluate this potential safety issue with Prolia use in patients with advanced kidney disease, particularly those on dialysis.” The FDA added that “we will communicate our final conclusions and recommendations when we have completed our review or have more information to share.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration issued an alert on Nov. 22 that cited preliminary evidence for a “substantial risk” for severe and symptomatic hypocalcemia and serious outcomes related to abnormally low calcium levels in people being treated with dialysis and receiving the osteoporosis medication denosumab (Prolia), including hospitalization and death.
In its alert, the FDA advised clinicians to make sure that people on dialysis who receive Prolia ingest adequate calcium and vitamin D supplementation and undergo frequent blood calcium monitoring, “possibly more often than is already being conducted,” which “may help decrease the likelihood or severity of these risks.”
The agency also called on clinicians to “advise patients on dialysis to immediately seek help if they experience symptoms of hypocalcemia,” such as unusual tingling or numbness in the hands, arms, legs, or feet; painful muscle spasms or cramps; voice box or lung spasms causing difficulty breathing; vomiting; seizures; or irregular heart rhythm.
The FDA had a similar message for people being treated with dialysis who are also receiving Prolia. The alert advised patients to watch for these symptoms and to tell their health care provider if they occur. The agency also advised patients who are undergoing dialysis and receiving Prolia to not stop the agent on their own, without first discussing this step with their care provider.
The FDA also advised providers and patients to contact the agency about episodes of side effects from Prolia (or other medications) via the FDA’s MedWatch program.
Frequent and serious
The FDA explained it issued the alert because of “the frequency and seriousness” of the risk for hypocalcemia and resulting complications. The agency noted that the risk seems most acute for people on dialysis who also receive Prolia, but the risk may also extend to people with advanced kidney disease who are not being treated with hemodialysis.
The alert stemmed from “interim results” in an ongoing safety study of Prolia that the FDA required the agent’s manufacturer, Amgen, to run when the agency first approved denosumab for U.S. marketing in 2010. The FDA said its review of these interim results suggested an increased risk of hypocalcemia with Prolia in patients with advanced kidney disease.
In addition, adverse event reports submitted to the FDA suggested in a separate, internal study that patients on dialysis treated with Prolia are at “substantial risk for severe and symptomatic hypocalcemia, including hospitalization and death.”
The alert explained that “because of the frequency and seriousness of these risks, we are alerting healthcare professionals and patients about them and that we are continuing to evaluate this potential safety issue with Prolia use in patients with advanced kidney disease, particularly those on dialysis.” The FDA added that “we will communicate our final conclusions and recommendations when we have completed our review or have more information to share.”
A version of this article first appeared on Medscape.com.
The right indoor relative humidity could ward off COVID
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF THE ROYAL SOCIETY INTERFACE
Laser and light devices for acne treatment continue to advance
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
HDL cholesterol not linked to CHD risk in Blacks: REGARDS
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Children and COVID: Weekly cases maintain a low-level plateau
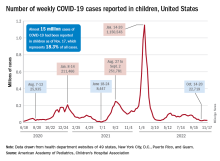
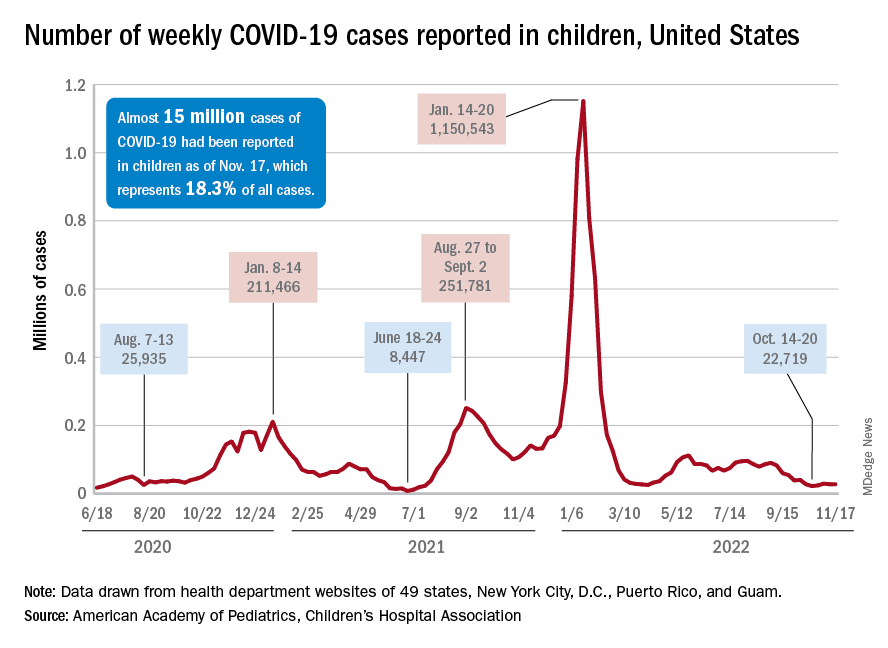
A less-than-1% decrease in weekly COVID-19 cases in children demonstrated continued stability in the pandemic situation as the nation heads into the holiday season.
the American Academy of Pediatrics and the Children’s Hospital Association said in the latest edition of their joint COVID report.
New cases for the week of Nov. 11-17 totaled 27,899, down by 0.9% from the previous week and just 4 weeks removed from the lowest total of the year: 22,719 for Oct. 14-20. There have been just under 15 million cases of COVID-19 in children since the pandemic began, and children represent 18.3% of cases in all ages, the AAP and CHA reported.
Conditions look favorable for that plateau to continue, despite the upcoming holidays, White House COVID-19 coordinator Ashish Jha said recently. “We are in a very different place and we will remain in a different place,” Dr. Jha said, according to STAT News. “We are now at a point where I believe if you’re up to date on your vaccines, you have access to treatments ... there really should be no restrictions on people’s activities.”
One possible spoiler, an apparent spike in COVID-related hospitalizations in children we reported last week, seems to have been a false alarm. The rate of new admissions for Nov. 11, which preliminary data suggested was 0.48 per 100,000 population, has now been revised with more solid data to 0.20 per 100,000, according to the Centers for Disease Control and Prevention.
“We continue to monitor the recent increases in admissions among children. Some of these may be admissions with COVID-19, not because of COVID-19. Co-infections are being noted in our surveillance systems for hospitalizations among children; as much as 10% of admissions or higher have viruses codetected (RSV, influenza, enterovirus/rhinovirus, and other respiratory viruses),” a CDC spokesperson told this news organization.
For children aged 0-17 years, the current 7-day (Nov. 13-19) average number of new admissions with confirmed COVID is 129 per day, down from 147 for the previous 7-day average. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits, are largely holding steady. The latest 7-day averages available (Nov. 18) – 1.0% for children aged 0-11 years, 0.7% for 12- to 15-year-olds, and 0.8% in 16- to 17-year-olds – are the same or within a tenth of a percent of the rates recorded on Oct. 18, CDC data show.
New vaccinations for the week of Nov. 10-16 were down just slightly for children under age 5 years and for those aged 5-11 years, with a larger drop seen among 12- to 17-year-olds, the AAP said in its weekly vaccination report. So far, 7.9% of all children under age 5 have received at least one dose of COVID vaccine, as have 39.1% of 5 to 11-year-olds and 71.5% of those aged 12-17years, the CDC said on its COVID Data Tracker.
A less-than-1% decrease in weekly COVID-19 cases in children demonstrated continued stability in the pandemic situation as the nation heads into the holiday season.
the American Academy of Pediatrics and the Children’s Hospital Association said in the latest edition of their joint COVID report.
New cases for the week of Nov. 11-17 totaled 27,899, down by 0.9% from the previous week and just 4 weeks removed from the lowest total of the year: 22,719 for Oct. 14-20. There have been just under 15 million cases of COVID-19 in children since the pandemic began, and children represent 18.3% of cases in all ages, the AAP and CHA reported.
Conditions look favorable for that plateau to continue, despite the upcoming holidays, White House COVID-19 coordinator Ashish Jha said recently. “We are in a very different place and we will remain in a different place,” Dr. Jha said, according to STAT News. “We are now at a point where I believe if you’re up to date on your vaccines, you have access to treatments ... there really should be no restrictions on people’s activities.”
One possible spoiler, an apparent spike in COVID-related hospitalizations in children we reported last week, seems to have been a false alarm. The rate of new admissions for Nov. 11, which preliminary data suggested was 0.48 per 100,000 population, has now been revised with more solid data to 0.20 per 100,000, according to the Centers for Disease Control and Prevention.
“We continue to monitor the recent increases in admissions among children. Some of these may be admissions with COVID-19, not because of COVID-19. Co-infections are being noted in our surveillance systems for hospitalizations among children; as much as 10% of admissions or higher have viruses codetected (RSV, influenza, enterovirus/rhinovirus, and other respiratory viruses),” a CDC spokesperson told this news organization.
For children aged 0-17 years, the current 7-day (Nov. 13-19) average number of new admissions with confirmed COVID is 129 per day, down from 147 for the previous 7-day average. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits, are largely holding steady. The latest 7-day averages available (Nov. 18) – 1.0% for children aged 0-11 years, 0.7% for 12- to 15-year-olds, and 0.8% in 16- to 17-year-olds – are the same or within a tenth of a percent of the rates recorded on Oct. 18, CDC data show.
New vaccinations for the week of Nov. 10-16 were down just slightly for children under age 5 years and for those aged 5-11 years, with a larger drop seen among 12- to 17-year-olds, the AAP said in its weekly vaccination report. So far, 7.9% of all children under age 5 have received at least one dose of COVID vaccine, as have 39.1% of 5 to 11-year-olds and 71.5% of those aged 12-17years, the CDC said on its COVID Data Tracker.
A less-than-1% decrease in weekly COVID-19 cases in children demonstrated continued stability in the pandemic situation as the nation heads into the holiday season.
the American Academy of Pediatrics and the Children’s Hospital Association said in the latest edition of their joint COVID report.
New cases for the week of Nov. 11-17 totaled 27,899, down by 0.9% from the previous week and just 4 weeks removed from the lowest total of the year: 22,719 for Oct. 14-20. There have been just under 15 million cases of COVID-19 in children since the pandemic began, and children represent 18.3% of cases in all ages, the AAP and CHA reported.
Conditions look favorable for that plateau to continue, despite the upcoming holidays, White House COVID-19 coordinator Ashish Jha said recently. “We are in a very different place and we will remain in a different place,” Dr. Jha said, according to STAT News. “We are now at a point where I believe if you’re up to date on your vaccines, you have access to treatments ... there really should be no restrictions on people’s activities.”
One possible spoiler, an apparent spike in COVID-related hospitalizations in children we reported last week, seems to have been a false alarm. The rate of new admissions for Nov. 11, which preliminary data suggested was 0.48 per 100,000 population, has now been revised with more solid data to 0.20 per 100,000, according to the Centers for Disease Control and Prevention.
“We continue to monitor the recent increases in admissions among children. Some of these may be admissions with COVID-19, not because of COVID-19. Co-infections are being noted in our surveillance systems for hospitalizations among children; as much as 10% of admissions or higher have viruses codetected (RSV, influenza, enterovirus/rhinovirus, and other respiratory viruses),” a CDC spokesperson told this news organization.
For children aged 0-17 years, the current 7-day (Nov. 13-19) average number of new admissions with confirmed COVID is 129 per day, down from 147 for the previous 7-day average. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits, are largely holding steady. The latest 7-day averages available (Nov. 18) – 1.0% for children aged 0-11 years, 0.7% for 12- to 15-year-olds, and 0.8% in 16- to 17-year-olds – are the same or within a tenth of a percent of the rates recorded on Oct. 18, CDC data show.
New vaccinations for the week of Nov. 10-16 were down just slightly for children under age 5 years and for those aged 5-11 years, with a larger drop seen among 12- to 17-year-olds, the AAP said in its weekly vaccination report. So far, 7.9% of all children under age 5 have received at least one dose of COVID vaccine, as have 39.1% of 5 to 11-year-olds and 71.5% of those aged 12-17years, the CDC said on its COVID Data Tracker.
Will ICER review aid bid for Medicare to pay for obesity drugs?
A report from a well-respected nonprofit group may bolster efforts to have Medicare, the largest U.S. purchaser of prescription drugs, cover obesity medicines, for which there has been accumulating evidence of significant benefit.
The Institute for Clinical and Economic Review (ICER) released a report last month on obesity medicines, based on extensive review of research done to date and input from clinicians, drug-makers, and members of the public.
Of the treatments reviewed, the ICER report gave the best ratings to two Novo Nordisk products, a B+ for semaglutide (Wegovy) and a B for liraglutide (Saxenda), while also making the case for price cuts. At an annual U.S. net price estimated at $13,618, semaglutide exceeds what ICER considers typical cost-effectiveness thresholds. ICER suggested a benchmark annual price range for semaglutide of between $7,500 and $9,800.
The ICER report also directs insurers in general to provide more generous coverage of obesity medicines, with a specific recommendation for the U.S. Congress to pass a pending bill known as the Treat and Reduce Obesity Act of 2021. The bill would undo a restriction on weight-loss drugs in the Medicare Part D plans, which covered about 49 million people last year. Sen. Tom Carper (D-Del.) and Sen. Bill Cassidy, MD, (R-La.) have repeatedly introduced versions of the bill since 2013.
“In both chambers of Congress and with bipartisan support, we’ve pushed to expand Medicare coverage of additional therapies and medications to treat obesity,” Sen. Cassidy said in an email. “This report confirms what we’ve worked on for nearly a decade – our legislation will help improve lives.”
The current House version of the bill has the backing of more than a third of the members of that chamber, with 113 Democratic and 40 Republican cosponsors. The Senate version has 22 sponsors.
Changing views
The ICER report comes amid a broader change in how clinicians view obesity.
The American Academy of Pediatrics is readying a new Clinical Practice Guideline for the Evaluation and Treatment of Pediatric Obesity that will mark a major shift in approach. Aaron S. Kelly, PhD, a professor of pediatrics at the University of Minnesota, Minneapolis, described it as a “sea change,” with obesity now seen as “a chronic, refractory, relapsing disease,” for which watchful waiting is no longer appropriate.
But the field of obesity treatment looked quite different in the early 2000s when Congress worked on a plan to add a pharmacy benefit to Medicare.
The deliberate omission of obesity medicine in the Medicare Part D benefit reflected both the state of science at the time and U.S. experience with a dangerous weight-loss drug combo in the late 1990s.
Initial expectations for weight-loss pills were high after the Food and Drug Administration cleared dexfenfluramine HCl (Redux) in 1996, which was part of the popular fen-phen combination. “Newly Approved Diet Drug Promises to Help Millions of Obese Americans – But Is No Magic Bullet,” read a headline about the Redux approval in The Washington Post
When work began in the 2000s to create a Medicare pharmacy benefit, lawmakers and congressional staff had a pool of about $400 billion available to establish what became the Part D program, Joel White, a former House staffer who helped draft the law, told this news organization in an email exchange.
Given the state of obesity research at the time, it seemed to make sense to exclude weight-loss medications, wrote Mr. White. Mr. White is now chief executive of the consulting firm Horizon, which has clients in the drug industry including the Pharmaceutical Research and Manufacturers of America.
“Now we know that obesity is a chronic disease of epidemic proportions. Decades of research have produced a series of advances in the way we understand and treat obesity. While scientists and many who work directly with those impacted by this epidemic understand how treatments have advanced, the law lags behind,” Mr. White said.
XXXCurrent payment policies for obesity treatments are based on “outdated information and ongoing misperception,” he noted. “While Part D has been a resounding success, our Medicare approach to obesity is not.”
“In addition, it makes no sense that Medicare covers the most drastic procedure (bariatric surgery) but not less-invasive, effective treatments,” he added. “We should have long ago lifted restrictions based on advances in science and medicine.”
Overcoming the stigma
Scott Kahan, MD, MPH, agreed and hopes that the new ICER report will help more patients secure needed medications, raising a “call to arms” about the need for better coverage of obesity drugs.
Dr. Kahan is director of the National Center for Weight and Wellness, a private clinic in Washington, and chair of the clinical committee for The Obesity Society. He also served as a member of a policy roundtable that ICER convened as part of research on the report on obesity drugs. Dr. Kahan, who also serves on the faculty at the Johns Hopkins Bloomberg School of Public Health, Baltimore, has received fees from drug makers such as Eli Lilly.
The ICER report may help what Dr. Kahan described as well-founded caution about obesity treatments in general.
“When it comes to weight loss, there are all of these magical treatments that are sold on social media and traditional media. There are a lot of bad actors in terms of people calling themselves experts and gurus and promising all kinds of crazy stuff,” said Dr. Kahan.
And there are long-standing stigmas about obesity, he stressed.
“That underlies a lot of the backward policies, including poor coverage for medications and the noncoverage by Medicare,” Dr. Kahan said. “There’s a societal ingrained set of beliefs and misperceptions and biases. That takes time to unwind, and I think we’re on the way, but we’re not quite there yet.”
Lifestyle changes not enough to tackle obesity
AHIP (formerly America’s Health Insurance Plans) told this news organization its members consider ICER reports when making decisions about which products to cover. “And health plans already cover obesity treatments that they consider medically necessary,” said David Allen, an AHIP spokesperson.
“It is important to note that every treatment does not work for every patient, and many patients experience adverse events and may discontinue treatment,” he added in an email. “Health insurance providers play an important role in helping [health care] providers and patients identify the treatment options that are most likely to be effective as well as affordable.”
Separately, the nonprofit watchdog group Public Citizen cautioned against liraglutide on its Worst Pills, Best Pills website. In its view, the drug is minimally effective and has many dangerous adverse effects, which are even more frequent with the higher-dose weight-loss version (a lower-dose version is approved for type 2 diabetes).
“There is currently no medication that can be used safely to achieve weight loss effortlessly and without dangerous adverse effects,” the group said. “Rather than focus on losing weight by turning to risky drugs, overweight and obese adults seeking to achieve better health should make reasonable and sustainable changes to their lifestyle, such as eating a healthy diet and getting regular exercise.”
Yet, many people find there is little help available for making lifestyle changes, and some patients and physicians say these modifications by themselves are not enough.
“The vast majority of people with obesity cannot achieve sustained weight loss through diet and exercise alone,” said David Rind, MD, chief medical officer of ICER, in an Oct. 20 statement. “As such, obesity, and its resulting physical health, mental health, and social burdens, is not a choice or failing, but a medical condition.”
The focus should now be on assuring that effective medications “are priced in alignment with their benefits so that they are accessible and affordable across U.S. society,” Dr. Rind urges.
‘My own demise with a fork and knife’
ICER sought public feedback on a draft version of the report before finalizing it.
In their comments on ICER’s work, several pharmaceutical researchers and Novo Nordisk questioned the calculations used in making judgments about the value of obesity drugs. In a statement, Novo Nordisk told this news organization that the company’s view is that ICER’s modeling “does not adequately address the real-world complexities of obesity, and consequently underestimates the health and societal impact medical treatments can have.”
Commenters also dug into aspects of ICER’s calculations, including ones that consider quality-adjusted life-years (QALYs). ICER describes QALY as an academic standard for measuring how well all different types of medical treatments can extend or improve patients’ lives. In an explainer on its website, ICER says this metric has served as a fundamental component of cost-effectiveness analyses in the United States and around the world for more than 30 years.
ICER and drug makers have been at odds for some time, with PhRMA having criticized the nonprofit group. A 2020 Reuters article detailed public relations strategies used by firms paid by drug makers to raise questions about ICER’s work. Critics accuse it of allying with insurers.
ICER’s list of its recent financial supporters includes Blue Cross Blue Shield of Massachusetts and the Kaiser Foundation Health Plan, but also many other groups, such as the U.S. Department of Veterans Affairs, the American Academy of Neurology, and the American College of Rheumatology.
The public comments on the ICER report also include one from an unidentified woman who wrote of her past struggles to lose weight.
She said her health plan wouldn’t cover behavioral programs or semaglutide as a weight-loss drug but did cover it eventually because of signs that she had developed insulin resistance. The patient said the drug worked for her, whereas other approaches to control weight had failed.
“To put it simply, I now experience hunger and satiety in a way that I can only assume people with normal metabolism do. I am 49 years old and approaching the age where serious comorbidities associated with obesity begin to manifest,” the patient wrote.
“I no longer worry about bringing about my own demise with a fork and knife because of misfiring hunger cues.”
A version of this article first appeared on Medscape.com.
A report from a well-respected nonprofit group may bolster efforts to have Medicare, the largest U.S. purchaser of prescription drugs, cover obesity medicines, for which there has been accumulating evidence of significant benefit.
The Institute for Clinical and Economic Review (ICER) released a report last month on obesity medicines, based on extensive review of research done to date and input from clinicians, drug-makers, and members of the public.
Of the treatments reviewed, the ICER report gave the best ratings to two Novo Nordisk products, a B+ for semaglutide (Wegovy) and a B for liraglutide (Saxenda), while also making the case for price cuts. At an annual U.S. net price estimated at $13,618, semaglutide exceeds what ICER considers typical cost-effectiveness thresholds. ICER suggested a benchmark annual price range for semaglutide of between $7,500 and $9,800.
The ICER report also directs insurers in general to provide more generous coverage of obesity medicines, with a specific recommendation for the U.S. Congress to pass a pending bill known as the Treat and Reduce Obesity Act of 2021. The bill would undo a restriction on weight-loss drugs in the Medicare Part D plans, which covered about 49 million people last year. Sen. Tom Carper (D-Del.) and Sen. Bill Cassidy, MD, (R-La.) have repeatedly introduced versions of the bill since 2013.
“In both chambers of Congress and with bipartisan support, we’ve pushed to expand Medicare coverage of additional therapies and medications to treat obesity,” Sen. Cassidy said in an email. “This report confirms what we’ve worked on for nearly a decade – our legislation will help improve lives.”
The current House version of the bill has the backing of more than a third of the members of that chamber, with 113 Democratic and 40 Republican cosponsors. The Senate version has 22 sponsors.
Changing views
The ICER report comes amid a broader change in how clinicians view obesity.
The American Academy of Pediatrics is readying a new Clinical Practice Guideline for the Evaluation and Treatment of Pediatric Obesity that will mark a major shift in approach. Aaron S. Kelly, PhD, a professor of pediatrics at the University of Minnesota, Minneapolis, described it as a “sea change,” with obesity now seen as “a chronic, refractory, relapsing disease,” for which watchful waiting is no longer appropriate.
But the field of obesity treatment looked quite different in the early 2000s when Congress worked on a plan to add a pharmacy benefit to Medicare.
The deliberate omission of obesity medicine in the Medicare Part D benefit reflected both the state of science at the time and U.S. experience with a dangerous weight-loss drug combo in the late 1990s.
Initial expectations for weight-loss pills were high after the Food and Drug Administration cleared dexfenfluramine HCl (Redux) in 1996, which was part of the popular fen-phen combination. “Newly Approved Diet Drug Promises to Help Millions of Obese Americans – But Is No Magic Bullet,” read a headline about the Redux approval in The Washington Post
When work began in the 2000s to create a Medicare pharmacy benefit, lawmakers and congressional staff had a pool of about $400 billion available to establish what became the Part D program, Joel White, a former House staffer who helped draft the law, told this news organization in an email exchange.
Given the state of obesity research at the time, it seemed to make sense to exclude weight-loss medications, wrote Mr. White. Mr. White is now chief executive of the consulting firm Horizon, which has clients in the drug industry including the Pharmaceutical Research and Manufacturers of America.
“Now we know that obesity is a chronic disease of epidemic proportions. Decades of research have produced a series of advances in the way we understand and treat obesity. While scientists and many who work directly with those impacted by this epidemic understand how treatments have advanced, the law lags behind,” Mr. White said.
XXXCurrent payment policies for obesity treatments are based on “outdated information and ongoing misperception,” he noted. “While Part D has been a resounding success, our Medicare approach to obesity is not.”
“In addition, it makes no sense that Medicare covers the most drastic procedure (bariatric surgery) but not less-invasive, effective treatments,” he added. “We should have long ago lifted restrictions based on advances in science and medicine.”
Overcoming the stigma
Scott Kahan, MD, MPH, agreed and hopes that the new ICER report will help more patients secure needed medications, raising a “call to arms” about the need for better coverage of obesity drugs.
Dr. Kahan is director of the National Center for Weight and Wellness, a private clinic in Washington, and chair of the clinical committee for The Obesity Society. He also served as a member of a policy roundtable that ICER convened as part of research on the report on obesity drugs. Dr. Kahan, who also serves on the faculty at the Johns Hopkins Bloomberg School of Public Health, Baltimore, has received fees from drug makers such as Eli Lilly.
The ICER report may help what Dr. Kahan described as well-founded caution about obesity treatments in general.
“When it comes to weight loss, there are all of these magical treatments that are sold on social media and traditional media. There are a lot of bad actors in terms of people calling themselves experts and gurus and promising all kinds of crazy stuff,” said Dr. Kahan.
And there are long-standing stigmas about obesity, he stressed.
“That underlies a lot of the backward policies, including poor coverage for medications and the noncoverage by Medicare,” Dr. Kahan said. “There’s a societal ingrained set of beliefs and misperceptions and biases. That takes time to unwind, and I think we’re on the way, but we’re not quite there yet.”
Lifestyle changes not enough to tackle obesity
AHIP (formerly America’s Health Insurance Plans) told this news organization its members consider ICER reports when making decisions about which products to cover. “And health plans already cover obesity treatments that they consider medically necessary,” said David Allen, an AHIP spokesperson.
“It is important to note that every treatment does not work for every patient, and many patients experience adverse events and may discontinue treatment,” he added in an email. “Health insurance providers play an important role in helping [health care] providers and patients identify the treatment options that are most likely to be effective as well as affordable.”
Separately, the nonprofit watchdog group Public Citizen cautioned against liraglutide on its Worst Pills, Best Pills website. In its view, the drug is minimally effective and has many dangerous adverse effects, which are even more frequent with the higher-dose weight-loss version (a lower-dose version is approved for type 2 diabetes).
“There is currently no medication that can be used safely to achieve weight loss effortlessly and without dangerous adverse effects,” the group said. “Rather than focus on losing weight by turning to risky drugs, overweight and obese adults seeking to achieve better health should make reasonable and sustainable changes to their lifestyle, such as eating a healthy diet and getting regular exercise.”
Yet, many people find there is little help available for making lifestyle changes, and some patients and physicians say these modifications by themselves are not enough.
“The vast majority of people with obesity cannot achieve sustained weight loss through diet and exercise alone,” said David Rind, MD, chief medical officer of ICER, in an Oct. 20 statement. “As such, obesity, and its resulting physical health, mental health, and social burdens, is not a choice or failing, but a medical condition.”
The focus should now be on assuring that effective medications “are priced in alignment with their benefits so that they are accessible and affordable across U.S. society,” Dr. Rind urges.
‘My own demise with a fork and knife’
ICER sought public feedback on a draft version of the report before finalizing it.
In their comments on ICER’s work, several pharmaceutical researchers and Novo Nordisk questioned the calculations used in making judgments about the value of obesity drugs. In a statement, Novo Nordisk told this news organization that the company’s view is that ICER’s modeling “does not adequately address the real-world complexities of obesity, and consequently underestimates the health and societal impact medical treatments can have.”
Commenters also dug into aspects of ICER’s calculations, including ones that consider quality-adjusted life-years (QALYs). ICER describes QALY as an academic standard for measuring how well all different types of medical treatments can extend or improve patients’ lives. In an explainer on its website, ICER says this metric has served as a fundamental component of cost-effectiveness analyses in the United States and around the world for more than 30 years.
ICER and drug makers have been at odds for some time, with PhRMA having criticized the nonprofit group. A 2020 Reuters article detailed public relations strategies used by firms paid by drug makers to raise questions about ICER’s work. Critics accuse it of allying with insurers.
ICER’s list of its recent financial supporters includes Blue Cross Blue Shield of Massachusetts and the Kaiser Foundation Health Plan, but also many other groups, such as the U.S. Department of Veterans Affairs, the American Academy of Neurology, and the American College of Rheumatology.
The public comments on the ICER report also include one from an unidentified woman who wrote of her past struggles to lose weight.
She said her health plan wouldn’t cover behavioral programs or semaglutide as a weight-loss drug but did cover it eventually because of signs that she had developed insulin resistance. The patient said the drug worked for her, whereas other approaches to control weight had failed.
“To put it simply, I now experience hunger and satiety in a way that I can only assume people with normal metabolism do. I am 49 years old and approaching the age where serious comorbidities associated with obesity begin to manifest,” the patient wrote.
“I no longer worry about bringing about my own demise with a fork and knife because of misfiring hunger cues.”
A version of this article first appeared on Medscape.com.
A report from a well-respected nonprofit group may bolster efforts to have Medicare, the largest U.S. purchaser of prescription drugs, cover obesity medicines, for which there has been accumulating evidence of significant benefit.
The Institute for Clinical and Economic Review (ICER) released a report last month on obesity medicines, based on extensive review of research done to date and input from clinicians, drug-makers, and members of the public.
Of the treatments reviewed, the ICER report gave the best ratings to two Novo Nordisk products, a B+ for semaglutide (Wegovy) and a B for liraglutide (Saxenda), while also making the case for price cuts. At an annual U.S. net price estimated at $13,618, semaglutide exceeds what ICER considers typical cost-effectiveness thresholds. ICER suggested a benchmark annual price range for semaglutide of between $7,500 and $9,800.
The ICER report also directs insurers in general to provide more generous coverage of obesity medicines, with a specific recommendation for the U.S. Congress to pass a pending bill known as the Treat and Reduce Obesity Act of 2021. The bill would undo a restriction on weight-loss drugs in the Medicare Part D plans, which covered about 49 million people last year. Sen. Tom Carper (D-Del.) and Sen. Bill Cassidy, MD, (R-La.) have repeatedly introduced versions of the bill since 2013.
“In both chambers of Congress and with bipartisan support, we’ve pushed to expand Medicare coverage of additional therapies and medications to treat obesity,” Sen. Cassidy said in an email. “This report confirms what we’ve worked on for nearly a decade – our legislation will help improve lives.”
The current House version of the bill has the backing of more than a third of the members of that chamber, with 113 Democratic and 40 Republican cosponsors. The Senate version has 22 sponsors.
Changing views
The ICER report comes amid a broader change in how clinicians view obesity.
The American Academy of Pediatrics is readying a new Clinical Practice Guideline for the Evaluation and Treatment of Pediatric Obesity that will mark a major shift in approach. Aaron S. Kelly, PhD, a professor of pediatrics at the University of Minnesota, Minneapolis, described it as a “sea change,” with obesity now seen as “a chronic, refractory, relapsing disease,” for which watchful waiting is no longer appropriate.
But the field of obesity treatment looked quite different in the early 2000s when Congress worked on a plan to add a pharmacy benefit to Medicare.
The deliberate omission of obesity medicine in the Medicare Part D benefit reflected both the state of science at the time and U.S. experience with a dangerous weight-loss drug combo in the late 1990s.
Initial expectations for weight-loss pills were high after the Food and Drug Administration cleared dexfenfluramine HCl (Redux) in 1996, which was part of the popular fen-phen combination. “Newly Approved Diet Drug Promises to Help Millions of Obese Americans – But Is No Magic Bullet,” read a headline about the Redux approval in The Washington Post
When work began in the 2000s to create a Medicare pharmacy benefit, lawmakers and congressional staff had a pool of about $400 billion available to establish what became the Part D program, Joel White, a former House staffer who helped draft the law, told this news organization in an email exchange.
Given the state of obesity research at the time, it seemed to make sense to exclude weight-loss medications, wrote Mr. White. Mr. White is now chief executive of the consulting firm Horizon, which has clients in the drug industry including the Pharmaceutical Research and Manufacturers of America.
“Now we know that obesity is a chronic disease of epidemic proportions. Decades of research have produced a series of advances in the way we understand and treat obesity. While scientists and many who work directly with those impacted by this epidemic understand how treatments have advanced, the law lags behind,” Mr. White said.
XXXCurrent payment policies for obesity treatments are based on “outdated information and ongoing misperception,” he noted. “While Part D has been a resounding success, our Medicare approach to obesity is not.”
“In addition, it makes no sense that Medicare covers the most drastic procedure (bariatric surgery) but not less-invasive, effective treatments,” he added. “We should have long ago lifted restrictions based on advances in science and medicine.”
Overcoming the stigma
Scott Kahan, MD, MPH, agreed and hopes that the new ICER report will help more patients secure needed medications, raising a “call to arms” about the need for better coverage of obesity drugs.
Dr. Kahan is director of the National Center for Weight and Wellness, a private clinic in Washington, and chair of the clinical committee for The Obesity Society. He also served as a member of a policy roundtable that ICER convened as part of research on the report on obesity drugs. Dr. Kahan, who also serves on the faculty at the Johns Hopkins Bloomberg School of Public Health, Baltimore, has received fees from drug makers such as Eli Lilly.
The ICER report may help what Dr. Kahan described as well-founded caution about obesity treatments in general.
“When it comes to weight loss, there are all of these magical treatments that are sold on social media and traditional media. There are a lot of bad actors in terms of people calling themselves experts and gurus and promising all kinds of crazy stuff,” said Dr. Kahan.
And there are long-standing stigmas about obesity, he stressed.
“That underlies a lot of the backward policies, including poor coverage for medications and the noncoverage by Medicare,” Dr. Kahan said. “There’s a societal ingrained set of beliefs and misperceptions and biases. That takes time to unwind, and I think we’re on the way, but we’re not quite there yet.”
Lifestyle changes not enough to tackle obesity
AHIP (formerly America’s Health Insurance Plans) told this news organization its members consider ICER reports when making decisions about which products to cover. “And health plans already cover obesity treatments that they consider medically necessary,” said David Allen, an AHIP spokesperson.
“It is important to note that every treatment does not work for every patient, and many patients experience adverse events and may discontinue treatment,” he added in an email. “Health insurance providers play an important role in helping [health care] providers and patients identify the treatment options that are most likely to be effective as well as affordable.”
Separately, the nonprofit watchdog group Public Citizen cautioned against liraglutide on its Worst Pills, Best Pills website. In its view, the drug is minimally effective and has many dangerous adverse effects, which are even more frequent with the higher-dose weight-loss version (a lower-dose version is approved for type 2 diabetes).
“There is currently no medication that can be used safely to achieve weight loss effortlessly and without dangerous adverse effects,” the group said. “Rather than focus on losing weight by turning to risky drugs, overweight and obese adults seeking to achieve better health should make reasonable and sustainable changes to their lifestyle, such as eating a healthy diet and getting regular exercise.”
Yet, many people find there is little help available for making lifestyle changes, and some patients and physicians say these modifications by themselves are not enough.
“The vast majority of people with obesity cannot achieve sustained weight loss through diet and exercise alone,” said David Rind, MD, chief medical officer of ICER, in an Oct. 20 statement. “As such, obesity, and its resulting physical health, mental health, and social burdens, is not a choice or failing, but a medical condition.”
The focus should now be on assuring that effective medications “are priced in alignment with their benefits so that they are accessible and affordable across U.S. society,” Dr. Rind urges.
‘My own demise with a fork and knife’
ICER sought public feedback on a draft version of the report before finalizing it.
In their comments on ICER’s work, several pharmaceutical researchers and Novo Nordisk questioned the calculations used in making judgments about the value of obesity drugs. In a statement, Novo Nordisk told this news organization that the company’s view is that ICER’s modeling “does not adequately address the real-world complexities of obesity, and consequently underestimates the health and societal impact medical treatments can have.”
Commenters also dug into aspects of ICER’s calculations, including ones that consider quality-adjusted life-years (QALYs). ICER describes QALY as an academic standard for measuring how well all different types of medical treatments can extend or improve patients’ lives. In an explainer on its website, ICER says this metric has served as a fundamental component of cost-effectiveness analyses in the United States and around the world for more than 30 years.
ICER and drug makers have been at odds for some time, with PhRMA having criticized the nonprofit group. A 2020 Reuters article detailed public relations strategies used by firms paid by drug makers to raise questions about ICER’s work. Critics accuse it of allying with insurers.
ICER’s list of its recent financial supporters includes Blue Cross Blue Shield of Massachusetts and the Kaiser Foundation Health Plan, but also many other groups, such as the U.S. Department of Veterans Affairs, the American Academy of Neurology, and the American College of Rheumatology.
The public comments on the ICER report also include one from an unidentified woman who wrote of her past struggles to lose weight.
She said her health plan wouldn’t cover behavioral programs or semaglutide as a weight-loss drug but did cover it eventually because of signs that she had developed insulin resistance. The patient said the drug worked for her, whereas other approaches to control weight had failed.
“To put it simply, I now experience hunger and satiety in a way that I can only assume people with normal metabolism do. I am 49 years old and approaching the age where serious comorbidities associated with obesity begin to manifest,” the patient wrote.
“I no longer worry about bringing about my own demise with a fork and knife because of misfiring hunger cues.”
A version of this article first appeared on Medscape.com.
Nurse practitioner fined $20k for advertising herself as ‘Doctor Sarah’
Last month, the San Luis Obispo County, California, District Attorney Dan Dow filed a complaint against Sarah Erny, RN, NP, citing unfair business practices and unprofessional conduct.
According to court documents, California’s Medical Practice Act does not permit individuals to refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
In addition to the fine, Ms. Erny agreed to refrain from referring to herself as a doctor in her practice and on social media. She has already deleted her Twitter account.
The case underscores tensions between physicians fighting to preserve their scope of practice and the allied professionals that U.S. lawmakers increasingly see as a less expensive way to improve access to health care.
The American Medical Association and specialty groups strongly oppose a new bill, the Improving Care and Access to Nurses Act, that would expand the scope of practice for nurse practitioners and physician assistants.
Court records show that Ms. Erny earned a doctor of nursing practice (DNP) degree from Vanderbilt University, Nashville, Tenn., and that she met the state requirements to obtain licensure as a registered nurse and nurse practitioner. In 2018, she opened a practice in Arroyo Grande, California, called Holistic Women’s Healing, where she provided medical services and drug supplements to patients.
She also entered a collaborative agreement with ob.gyn. Anika Moore, MD, for approximately 3 years. Dr. Moore’s medical practice was in another county and state, and the physician returned every 2 to 3 months to review a portion of Ms. Erny’s patient files.
Ms. Erny and Dr. Moore terminated the collaborative agreement in March, according to court documents.
However, Mr. Dow alleged that Ms. Erny regularly referred to herself as “Dr. Sarah” or “Dr. Sarah Erny” in her online advertising and social media accounts. Her patients “were so proud of her” that they called her doctor, and her supervising physician instructed staff to do the same.
Mr. Dow said Ms. Erny did not clearly advise the public that she was not a medical doctor and failed to identify her supervising physician. “Simply put, there is a great need for health care providers to state their level of training and licensing clearly and honestly in all of their advertising and marketing materials,” he said in a press release.
In California, nurse practitioners who have been certified by the Board of Registered Nursing may use the following titles: Advanced Practice Registered Nurse; Certified Nurse Practitioner; APRN-CNP; RN and NP; or a combination of other letters or words to identify specialization, such as adult nurse practitioner, pediatric nurse practitioner, obstetrical-gynecological nurse practitioner, and family nurse practitioner.
As educational requirements shift for advanced practice clinicians, similar cases will likely emerge, said Grant Martsolf, PhD, MPH, RN, FAAN, professor at the University of Pittsburgh School of Nursing.
“Scope of practice is governed by states, [so they] will have to figure [it] out as more professional disciplines move to clinical doctorates as the entry to practice. Pharma, [physical therapy], and [occupational therapy] have already done this, and advanced practice nursing is on its way. [Certified registered nurse anesthetists] are already required to get a DNP to sit for certification,” he said.
More guidance is needed, especially when considering other professions like dentists, clinical psychologists, and individuals with clinical or research doctorates who often call themselves doctors, Dr. Martsolf said.
“It seems that the honorific of ‘Dr.’ emerges from the degree, not from being a physician or surgeon,” he said.
Beyond the false advertising, Mr. Dow alleged that Ms. Erny did not file a fictitious business name statement for 2020 and 2021 – a requirement under the California Business and Professions Code to identify who is operating the business.
A version of this article first appeared on Medscape.com.
Last month, the San Luis Obispo County, California, District Attorney Dan Dow filed a complaint against Sarah Erny, RN, NP, citing unfair business practices and unprofessional conduct.
According to court documents, California’s Medical Practice Act does not permit individuals to refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
In addition to the fine, Ms. Erny agreed to refrain from referring to herself as a doctor in her practice and on social media. She has already deleted her Twitter account.
The case underscores tensions between physicians fighting to preserve their scope of practice and the allied professionals that U.S. lawmakers increasingly see as a less expensive way to improve access to health care.
The American Medical Association and specialty groups strongly oppose a new bill, the Improving Care and Access to Nurses Act, that would expand the scope of practice for nurse practitioners and physician assistants.
Court records show that Ms. Erny earned a doctor of nursing practice (DNP) degree from Vanderbilt University, Nashville, Tenn., and that she met the state requirements to obtain licensure as a registered nurse and nurse practitioner. In 2018, she opened a practice in Arroyo Grande, California, called Holistic Women’s Healing, where she provided medical services and drug supplements to patients.
She also entered a collaborative agreement with ob.gyn. Anika Moore, MD, for approximately 3 years. Dr. Moore’s medical practice was in another county and state, and the physician returned every 2 to 3 months to review a portion of Ms. Erny’s patient files.
Ms. Erny and Dr. Moore terminated the collaborative agreement in March, according to court documents.
However, Mr. Dow alleged that Ms. Erny regularly referred to herself as “Dr. Sarah” or “Dr. Sarah Erny” in her online advertising and social media accounts. Her patients “were so proud of her” that they called her doctor, and her supervising physician instructed staff to do the same.
Mr. Dow said Ms. Erny did not clearly advise the public that she was not a medical doctor and failed to identify her supervising physician. “Simply put, there is a great need for health care providers to state their level of training and licensing clearly and honestly in all of their advertising and marketing materials,” he said in a press release.
In California, nurse practitioners who have been certified by the Board of Registered Nursing may use the following titles: Advanced Practice Registered Nurse; Certified Nurse Practitioner; APRN-CNP; RN and NP; or a combination of other letters or words to identify specialization, such as adult nurse practitioner, pediatric nurse practitioner, obstetrical-gynecological nurse practitioner, and family nurse practitioner.
As educational requirements shift for advanced practice clinicians, similar cases will likely emerge, said Grant Martsolf, PhD, MPH, RN, FAAN, professor at the University of Pittsburgh School of Nursing.
“Scope of practice is governed by states, [so they] will have to figure [it] out as more professional disciplines move to clinical doctorates as the entry to practice. Pharma, [physical therapy], and [occupational therapy] have already done this, and advanced practice nursing is on its way. [Certified registered nurse anesthetists] are already required to get a DNP to sit for certification,” he said.
More guidance is needed, especially when considering other professions like dentists, clinical psychologists, and individuals with clinical or research doctorates who often call themselves doctors, Dr. Martsolf said.
“It seems that the honorific of ‘Dr.’ emerges from the degree, not from being a physician or surgeon,” he said.
Beyond the false advertising, Mr. Dow alleged that Ms. Erny did not file a fictitious business name statement for 2020 and 2021 – a requirement under the California Business and Professions Code to identify who is operating the business.
A version of this article first appeared on Medscape.com.
Last month, the San Luis Obispo County, California, District Attorney Dan Dow filed a complaint against Sarah Erny, RN, NP, citing unfair business practices and unprofessional conduct.
According to court documents, California’s Medical Practice Act does not permit individuals to refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
In addition to the fine, Ms. Erny agreed to refrain from referring to herself as a doctor in her practice and on social media. She has already deleted her Twitter account.
The case underscores tensions between physicians fighting to preserve their scope of practice and the allied professionals that U.S. lawmakers increasingly see as a less expensive way to improve access to health care.
The American Medical Association and specialty groups strongly oppose a new bill, the Improving Care and Access to Nurses Act, that would expand the scope of practice for nurse practitioners and physician assistants.
Court records show that Ms. Erny earned a doctor of nursing practice (DNP) degree from Vanderbilt University, Nashville, Tenn., and that she met the state requirements to obtain licensure as a registered nurse and nurse practitioner. In 2018, she opened a practice in Arroyo Grande, California, called Holistic Women’s Healing, where she provided medical services and drug supplements to patients.
She also entered a collaborative agreement with ob.gyn. Anika Moore, MD, for approximately 3 years. Dr. Moore’s medical practice was in another county and state, and the physician returned every 2 to 3 months to review a portion of Ms. Erny’s patient files.
Ms. Erny and Dr. Moore terminated the collaborative agreement in March, according to court documents.
However, Mr. Dow alleged that Ms. Erny regularly referred to herself as “Dr. Sarah” or “Dr. Sarah Erny” in her online advertising and social media accounts. Her patients “were so proud of her” that they called her doctor, and her supervising physician instructed staff to do the same.
Mr. Dow said Ms. Erny did not clearly advise the public that she was not a medical doctor and failed to identify her supervising physician. “Simply put, there is a great need for health care providers to state their level of training and licensing clearly and honestly in all of their advertising and marketing materials,” he said in a press release.
In California, nurse practitioners who have been certified by the Board of Registered Nursing may use the following titles: Advanced Practice Registered Nurse; Certified Nurse Practitioner; APRN-CNP; RN and NP; or a combination of other letters or words to identify specialization, such as adult nurse practitioner, pediatric nurse practitioner, obstetrical-gynecological nurse practitioner, and family nurse practitioner.
As educational requirements shift for advanced practice clinicians, similar cases will likely emerge, said Grant Martsolf, PhD, MPH, RN, FAAN, professor at the University of Pittsburgh School of Nursing.
“Scope of practice is governed by states, [so they] will have to figure [it] out as more professional disciplines move to clinical doctorates as the entry to practice. Pharma, [physical therapy], and [occupational therapy] have already done this, and advanced practice nursing is on its way. [Certified registered nurse anesthetists] are already required to get a DNP to sit for certification,” he said.
More guidance is needed, especially when considering other professions like dentists, clinical psychologists, and individuals with clinical or research doctorates who often call themselves doctors, Dr. Martsolf said.
“It seems that the honorific of ‘Dr.’ emerges from the degree, not from being a physician or surgeon,” he said.
Beyond the false advertising, Mr. Dow alleged that Ms. Erny did not file a fictitious business name statement for 2020 and 2021 – a requirement under the California Business and Professions Code to identify who is operating the business.
A version of this article first appeared on Medscape.com.
Intermittent fasting diet trend linked to disordered eating
Researchers from the University of Toronto analyzed data from more than 2700 adolescents and young adults from the Canadian Study of Adolescent Health Behaviors, and found that for women, IF was significantly associated with overeating, binge eating, vomiting, laxative use, and compulsive exercise.
IF in women was also associated with higher scores on the Eating Disorder Examination Questionnaire (EDE-Q), which was used to determine ED psychopathology.
Study investigator Kyle Ganson, PhD, assistant professor in the Factor-Inwentash Faculty of Social Work at the University of Toronto, said in an interview that evidence on the effectiveness of IF for weight loss and disease prevention is mixed, and that it’s important to understand the potential harms of IF – even if there are benefits for some.
“If anything, this study shines light on the fact that engagement in IF may be connected with problematic ED behaviors, requiring health care professionals to be very aware of this contemporary and popular dietary trend, despite proponents on social media touting the effectiveness and benefits,” he said.
The study was published online in Eating Behaviors.
Touted for health benefits
The practice of IF has been gaining popularity partly because of reputable medical experts touting its health benefits. Johns Hopkins Medicine, for instance, cited evidence that IF boosts working memory, improves blood pressure, enhances physical performance, and prevents obesity. Yet there has been little research on its harms.
As part of the Canadian Study of Adolescent Health Behaviors, Dr. Ganson and associates analyzed data on 2,700 adolescents and young adults aged 16-30 recruited from social media ads in November and December 2021. The sample included women, men, and transgender or gender-nonconforming individuals.
Study participants answered questions about weight perception, current weight change behavior, engagement in IF, and participation in eating disorder behaviors. They were also administered the EDE-Q, which measures eating disorder psychopathology.
In total, 47% of women (n = 1,470), 38% of men (n = 1,060), and 52% transgender or gender-nonconforming individuals (n = 225) reported engaging in IF during the past year.
Dr. Ganson and associates found that, for women, IF in the past 12 months and past 30 days were significantly associated with all eating disorder behaviors, including overeating, loss of control, binge eating, vomiting, laxative use, compulsive exercise, and fasting – as well as higher overall EDE-Q global scores.
For men, IF in the past 12 months was significantly associated with compulsive exercise, and higher overall EDE-Q global scores.
The team found that for TGNC participants, IF was positively associated with higher EDE-Q global scores.
The investigators acknowledged some limitations with the study – the method of recruiting, which involved ads placed on social media, could cause selection bias. In addition to this, data collection methods relied heavily on participants’ self-reporting, which could also be susceptible to bias.
“Certainly, there needs to be more investigation on this dietary practice,” said Dr. Ganson. “If anything, this study shines light on the fact that engagement in IF may be connected with problematic ED behaviors requiring healthcare professionals to be very aware of this contemporary and popular dietary trend – despite proponents on social media touting the effectiveness and benefits.”
Screening warranted
Dr. Ganson noted that additional research is needed to support the findings from his study, and to further illuminate the potential harms of IF.
Health care professionals “need to be aware of common, contemporary dietary trends that young people engage in and are commonly discussed on social media, such as IF,” he noted. In addition, he’d like to see health care professionals assess their patients for IF who are dieting and to follow-up with assessments for ED-related attitudes and behaviors.
“Additionally, there are likely bidirectional relationships between IF and ED attitudes and behaviors, so professionals should be aware the ways in which ED behaviors are masked as IF engagement,” Dr. Ganson said.
More research needed
Commenting on the findings, Angela Guarda, MD, professor of eating disorders at Johns Hopkins University and director of the eating disorders program at Johns Hopkins Hospital, both in Baltimore, said more research is needed on outcomes for IF.
“We lack a definitive answer. The reality is that IF may help some and harm others and is most likely not healthy for all,” she said, noting that the study results “support what many in the eating disorders field believe, namely that IF for someone who is at risk for an eating disorder is likely to be ill advised.”
She added that “continued research is needed to establish its safety, and for whom it may be a therapeutic versus an iatrogenic recommendation.”
The study was funded by the Connaught New Researcher Award. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers from the University of Toronto analyzed data from more than 2700 adolescents and young adults from the Canadian Study of Adolescent Health Behaviors, and found that for women, IF was significantly associated with overeating, binge eating, vomiting, laxative use, and compulsive exercise.
IF in women was also associated with higher scores on the Eating Disorder Examination Questionnaire (EDE-Q), which was used to determine ED psychopathology.
Study investigator Kyle Ganson, PhD, assistant professor in the Factor-Inwentash Faculty of Social Work at the University of Toronto, said in an interview that evidence on the effectiveness of IF for weight loss and disease prevention is mixed, and that it’s important to understand the potential harms of IF – even if there are benefits for some.
“If anything, this study shines light on the fact that engagement in IF may be connected with problematic ED behaviors, requiring health care professionals to be very aware of this contemporary and popular dietary trend, despite proponents on social media touting the effectiveness and benefits,” he said.
The study was published online in Eating Behaviors.
Touted for health benefits
The practice of IF has been gaining popularity partly because of reputable medical experts touting its health benefits. Johns Hopkins Medicine, for instance, cited evidence that IF boosts working memory, improves blood pressure, enhances physical performance, and prevents obesity. Yet there has been little research on its harms.
As part of the Canadian Study of Adolescent Health Behaviors, Dr. Ganson and associates analyzed data on 2,700 adolescents and young adults aged 16-30 recruited from social media ads in November and December 2021. The sample included women, men, and transgender or gender-nonconforming individuals.
Study participants answered questions about weight perception, current weight change behavior, engagement in IF, and participation in eating disorder behaviors. They were also administered the EDE-Q, which measures eating disorder psychopathology.
In total, 47% of women (n = 1,470), 38% of men (n = 1,060), and 52% transgender or gender-nonconforming individuals (n = 225) reported engaging in IF during the past year.
Dr. Ganson and associates found that, for women, IF in the past 12 months and past 30 days were significantly associated with all eating disorder behaviors, including overeating, loss of control, binge eating, vomiting, laxative use, compulsive exercise, and fasting – as well as higher overall EDE-Q global scores.
For men, IF in the past 12 months was significantly associated with compulsive exercise, and higher overall EDE-Q global scores.
The team found that for TGNC participants, IF was positively associated with higher EDE-Q global scores.
The investigators acknowledged some limitations with the study – the method of recruiting, which involved ads placed on social media, could cause selection bias. In addition to this, data collection methods relied heavily on participants’ self-reporting, which could also be susceptible to bias.
“Certainly, there needs to be more investigation on this dietary practice,” said Dr. Ganson. “If anything, this study shines light on the fact that engagement in IF may be connected with problematic ED behaviors requiring healthcare professionals to be very aware of this contemporary and popular dietary trend – despite proponents on social media touting the effectiveness and benefits.”
Screening warranted
Dr. Ganson noted that additional research is needed to support the findings from his study, and to further illuminate the potential harms of IF.
Health care professionals “need to be aware of common, contemporary dietary trends that young people engage in and are commonly discussed on social media, such as IF,” he noted. In addition, he’d like to see health care professionals assess their patients for IF who are dieting and to follow-up with assessments for ED-related attitudes and behaviors.
“Additionally, there are likely bidirectional relationships between IF and ED attitudes and behaviors, so professionals should be aware the ways in which ED behaviors are masked as IF engagement,” Dr. Ganson said.
More research needed
Commenting on the findings, Angela Guarda, MD, professor of eating disorders at Johns Hopkins University and director of the eating disorders program at Johns Hopkins Hospital, both in Baltimore, said more research is needed on outcomes for IF.
“We lack a definitive answer. The reality is that IF may help some and harm others and is most likely not healthy for all,” she said, noting that the study results “support what many in the eating disorders field believe, namely that IF for someone who is at risk for an eating disorder is likely to be ill advised.”
She added that “continued research is needed to establish its safety, and for whom it may be a therapeutic versus an iatrogenic recommendation.”
The study was funded by the Connaught New Researcher Award. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers from the University of Toronto analyzed data from more than 2700 adolescents and young adults from the Canadian Study of Adolescent Health Behaviors, and found that for women, IF was significantly associated with overeating, binge eating, vomiting, laxative use, and compulsive exercise.
IF in women was also associated with higher scores on the Eating Disorder Examination Questionnaire (EDE-Q), which was used to determine ED psychopathology.
Study investigator Kyle Ganson, PhD, assistant professor in the Factor-Inwentash Faculty of Social Work at the University of Toronto, said in an interview that evidence on the effectiveness of IF for weight loss and disease prevention is mixed, and that it’s important to understand the potential harms of IF – even if there are benefits for some.
“If anything, this study shines light on the fact that engagement in IF may be connected with problematic ED behaviors, requiring health care professionals to be very aware of this contemporary and popular dietary trend, despite proponents on social media touting the effectiveness and benefits,” he said.
The study was published online in Eating Behaviors.
Touted for health benefits
The practice of IF has been gaining popularity partly because of reputable medical experts touting its health benefits. Johns Hopkins Medicine, for instance, cited evidence that IF boosts working memory, improves blood pressure, enhances physical performance, and prevents obesity. Yet there has been little research on its harms.
As part of the Canadian Study of Adolescent Health Behaviors, Dr. Ganson and associates analyzed data on 2,700 adolescents and young adults aged 16-30 recruited from social media ads in November and December 2021. The sample included women, men, and transgender or gender-nonconforming individuals.
Study participants answered questions about weight perception, current weight change behavior, engagement in IF, and participation in eating disorder behaviors. They were also administered the EDE-Q, which measures eating disorder psychopathology.
In total, 47% of women (n = 1,470), 38% of men (n = 1,060), and 52% transgender or gender-nonconforming individuals (n = 225) reported engaging in IF during the past year.
Dr. Ganson and associates found that, for women, IF in the past 12 months and past 30 days were significantly associated with all eating disorder behaviors, including overeating, loss of control, binge eating, vomiting, laxative use, compulsive exercise, and fasting – as well as higher overall EDE-Q global scores.
For men, IF in the past 12 months was significantly associated with compulsive exercise, and higher overall EDE-Q global scores.
The team found that for TGNC participants, IF was positively associated with higher EDE-Q global scores.
The investigators acknowledged some limitations with the study – the method of recruiting, which involved ads placed on social media, could cause selection bias. In addition to this, data collection methods relied heavily on participants’ self-reporting, which could also be susceptible to bias.
“Certainly, there needs to be more investigation on this dietary practice,” said Dr. Ganson. “If anything, this study shines light on the fact that engagement in IF may be connected with problematic ED behaviors requiring healthcare professionals to be very aware of this contemporary and popular dietary trend – despite proponents on social media touting the effectiveness and benefits.”
Screening warranted
Dr. Ganson noted that additional research is needed to support the findings from his study, and to further illuminate the potential harms of IF.
Health care professionals “need to be aware of common, contemporary dietary trends that young people engage in and are commonly discussed on social media, such as IF,” he noted. In addition, he’d like to see health care professionals assess their patients for IF who are dieting and to follow-up with assessments for ED-related attitudes and behaviors.
“Additionally, there are likely bidirectional relationships between IF and ED attitudes and behaviors, so professionals should be aware the ways in which ED behaviors are masked as IF engagement,” Dr. Ganson said.
More research needed
Commenting on the findings, Angela Guarda, MD, professor of eating disorders at Johns Hopkins University and director of the eating disorders program at Johns Hopkins Hospital, both in Baltimore, said more research is needed on outcomes for IF.
“We lack a definitive answer. The reality is that IF may help some and harm others and is most likely not healthy for all,” she said, noting that the study results “support what many in the eating disorders field believe, namely that IF for someone who is at risk for an eating disorder is likely to be ill advised.”
She added that “continued research is needed to establish its safety, and for whom it may be a therapeutic versus an iatrogenic recommendation.”
The study was funded by the Connaught New Researcher Award. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EATING DISORDERS
Could intermittent fasting improve GERD symptoms?
, suggests a small U.S. study.
Twenty-five individuals with suspected GERD symptoms underwent 96-hour pH monitoring. They were asked to follow their normal diet for the first 48 hours; for the second 48 hours, they were asked to switch to a 16-hour fast, which was followed by an 8-hour eating window.
Just over a third of participants were fully compliant with the 16:8 intermittent fasting. But those who followed the regimen experienced a mild reduction in mean acid exposure time and self-reported GERD symptoms scores.
The research was published online by the Journal of Clinical Gastroenterology.
Costly condition
The prevalence of GERD in the United States is estimated at 18%-28%. Annual costs of the condition are more than $18 billion per year, largely through pharmacologic therapies and diagnostic testing, write lead author Yan Jiang, MD, division of gastrointestinal and liver D-diseases, Keck Medicine of University of Southern California, Los Angeles, and colleagues.
Proton pump inhibitor (PPI) therapy is one of the most prescribed classes of medications in the United States, the authors write. But concerns over the long-term safety of the drugs, as well as the fact that half of patients report breakthrough GERD symptoms, have generated interest in non-PPI treatments among patients and providers.
The role of diet in the management of GERD, however, remains poorly understood, despite the fact that obesity and weight gain have been linked to reflux.
The authors note that intermittent fasting has shown benefits in coronary artery disease, inflammatory disorders, obesity, and diabetes. Proposed mechanisms include anti-inflammatory effects, weight loss, and alterations in hormone secretion.
Intervention test in a 96-hour clinical evaluation for GERD
To investigate the effects of intermittent fasting in GERD, the researchers screened patients referred to the Stanford University gastrointestinal clinic for diagnostic 96-hour ambulatory wireless pH monitoring of suspected acid reflux symptoms.
They excluded patients younger than 18 years, pregnant women, those with insulin-dependent diabetes, and those who had used PPIs within the previous 7 days. There were other exclusion criteria as well.
The study was completed by 25 participants. The mean age of the patients was 43.5 years; 52% were women. Just under half (44%) were White, and the mean body mass index was 25.8 kg/m2.
For the first 48 hours of the pH monitoring, the patients followed their baseline diet. For the second 48 hours, they were asked to follow an intermittent fasting regimen.
In that regimen, during a 24-hour period, there was an 8-hour caloric intake window and no caloric intake during the other 16 consecutive hours. Participants who fasted for at least 15 hours, as indicated on a self-report food log, were considered successful.
Only 36% of participants were fully adherent to the fasting regimen; 84% were partially compliant, defined as following the regimen for at least 1 of the 2 days of intermittent fasting.
On intermittent fasting days, the mean acid exposure time was 3.5%, compared with 4.3% on the baseline diet. The team calculated that adhering to the 16:8 intermittent fasting regimen reduced the mean acid exposure time by 0.64%.
Intermittent fasting was also associated with a reduction in total GERD symptom scores, at 9.9 following day 4 versus 14.3 following day 2. There were reductions in heartburn symptoms scores of 2.6 and in regurgitation scores of 1.8.
When the researchers compared individuals who were compliant with intermittent fasting with those who were only partially compliant, they found that there was still an improvement in GERD symptoms, with a reduction in scores of 3.2.
More acid, bigger benefits
There could be several explanations for the findings, Dr. Jiang said in an interview.
In the short-term study, fewer meals during intermittent fasting and more hours between the last meal and bedtime can help with the supine symptoms of GERD, Dr. Jiang said.
Over the longer term, he added, previous studies have suggested that fasting-induced alterations in inflammatory cytokines or cells could be a contributory mechanism, “but it’s not something that we can glean from our study.”
Participants with elevated acid exposure at baseline and who were more likely to have GERD diagnosed by the pH monitoring seemed to experience the greatest benefit from intermittent fasting, Dr. Jiang pointed out.
“This study looked at all comers with GERD symptoms,” he said. “But if you were to do another study with people with proven GERD, they might experience a bigger impact with intermittent fasting.”
Dr. Jiang added, “If a patient is willing to do intermittent fasting, and certainly if they have other reasons [for doing so], I think it doesn’t hurt, and it might actually help them a little bit in their current symptoms.”
Larger scale, longer follow-up studies needed
Luigi Bonavina, MD, department of biomedical sciences for health, University of Milan, IRCCS Policlinico San Donato, Italy, said in an interview that it was a “nice, original study.”
It is “noteworthy that only one previous study explored the effect of Ramadan on GERD symptoms and found a small improvement of GERD symptoms,” Dr. Bonavina said. “Unfortunately, the magnitude of effect [in the current study] was not as one may have expected, due to small sample size and low compliance with intermittent fasting.”
Although the effect was “mild compared to that seen with PPIs,” it would “be interesting to see whether the results of this pilot, proof-of-concept study can be confirmed on a larger scale with longer follow-up to prove that reflux symptoms will not worsen over time,” he said.
“Intermittent fasting may be recommended, especially in overweight-obese patients with GERD symptoms who are poor responders to gastric acid inhibitors,” Dr. Bonavina added. “Reduction of inflammation, reduction of meal intake, and going to bed with an empty stomach may also work in patients with GERD.”
No funding for the study has been declared. The authors and Dr. Bonavina report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a small U.S. study.
Twenty-five individuals with suspected GERD symptoms underwent 96-hour pH monitoring. They were asked to follow their normal diet for the first 48 hours; for the second 48 hours, they were asked to switch to a 16-hour fast, which was followed by an 8-hour eating window.
Just over a third of participants were fully compliant with the 16:8 intermittent fasting. But those who followed the regimen experienced a mild reduction in mean acid exposure time and self-reported GERD symptoms scores.
The research was published online by the Journal of Clinical Gastroenterology.
Costly condition
The prevalence of GERD in the United States is estimated at 18%-28%. Annual costs of the condition are more than $18 billion per year, largely through pharmacologic therapies and diagnostic testing, write lead author Yan Jiang, MD, division of gastrointestinal and liver D-diseases, Keck Medicine of University of Southern California, Los Angeles, and colleagues.
Proton pump inhibitor (PPI) therapy is one of the most prescribed classes of medications in the United States, the authors write. But concerns over the long-term safety of the drugs, as well as the fact that half of patients report breakthrough GERD symptoms, have generated interest in non-PPI treatments among patients and providers.
The role of diet in the management of GERD, however, remains poorly understood, despite the fact that obesity and weight gain have been linked to reflux.
The authors note that intermittent fasting has shown benefits in coronary artery disease, inflammatory disorders, obesity, and diabetes. Proposed mechanisms include anti-inflammatory effects, weight loss, and alterations in hormone secretion.
Intervention test in a 96-hour clinical evaluation for GERD
To investigate the effects of intermittent fasting in GERD, the researchers screened patients referred to the Stanford University gastrointestinal clinic for diagnostic 96-hour ambulatory wireless pH monitoring of suspected acid reflux symptoms.
They excluded patients younger than 18 years, pregnant women, those with insulin-dependent diabetes, and those who had used PPIs within the previous 7 days. There were other exclusion criteria as well.
The study was completed by 25 participants. The mean age of the patients was 43.5 years; 52% were women. Just under half (44%) were White, and the mean body mass index was 25.8 kg/m2.
For the first 48 hours of the pH monitoring, the patients followed their baseline diet. For the second 48 hours, they were asked to follow an intermittent fasting regimen.
In that regimen, during a 24-hour period, there was an 8-hour caloric intake window and no caloric intake during the other 16 consecutive hours. Participants who fasted for at least 15 hours, as indicated on a self-report food log, were considered successful.
Only 36% of participants were fully adherent to the fasting regimen; 84% were partially compliant, defined as following the regimen for at least 1 of the 2 days of intermittent fasting.
On intermittent fasting days, the mean acid exposure time was 3.5%, compared with 4.3% on the baseline diet. The team calculated that adhering to the 16:8 intermittent fasting regimen reduced the mean acid exposure time by 0.64%.
Intermittent fasting was also associated with a reduction in total GERD symptom scores, at 9.9 following day 4 versus 14.3 following day 2. There were reductions in heartburn symptoms scores of 2.6 and in regurgitation scores of 1.8.
When the researchers compared individuals who were compliant with intermittent fasting with those who were only partially compliant, they found that there was still an improvement in GERD symptoms, with a reduction in scores of 3.2.
More acid, bigger benefits
There could be several explanations for the findings, Dr. Jiang said in an interview.
In the short-term study, fewer meals during intermittent fasting and more hours between the last meal and bedtime can help with the supine symptoms of GERD, Dr. Jiang said.
Over the longer term, he added, previous studies have suggested that fasting-induced alterations in inflammatory cytokines or cells could be a contributory mechanism, “but it’s not something that we can glean from our study.”
Participants with elevated acid exposure at baseline and who were more likely to have GERD diagnosed by the pH monitoring seemed to experience the greatest benefit from intermittent fasting, Dr. Jiang pointed out.
“This study looked at all comers with GERD symptoms,” he said. “But if you were to do another study with people with proven GERD, they might experience a bigger impact with intermittent fasting.”
Dr. Jiang added, “If a patient is willing to do intermittent fasting, and certainly if they have other reasons [for doing so], I think it doesn’t hurt, and it might actually help them a little bit in their current symptoms.”
Larger scale, longer follow-up studies needed
Luigi Bonavina, MD, department of biomedical sciences for health, University of Milan, IRCCS Policlinico San Donato, Italy, said in an interview that it was a “nice, original study.”
It is “noteworthy that only one previous study explored the effect of Ramadan on GERD symptoms and found a small improvement of GERD symptoms,” Dr. Bonavina said. “Unfortunately, the magnitude of effect [in the current study] was not as one may have expected, due to small sample size and low compliance with intermittent fasting.”
Although the effect was “mild compared to that seen with PPIs,” it would “be interesting to see whether the results of this pilot, proof-of-concept study can be confirmed on a larger scale with longer follow-up to prove that reflux symptoms will not worsen over time,” he said.
“Intermittent fasting may be recommended, especially in overweight-obese patients with GERD symptoms who are poor responders to gastric acid inhibitors,” Dr. Bonavina added. “Reduction of inflammation, reduction of meal intake, and going to bed with an empty stomach may also work in patients with GERD.”
No funding for the study has been declared. The authors and Dr. Bonavina report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a small U.S. study.
Twenty-five individuals with suspected GERD symptoms underwent 96-hour pH monitoring. They were asked to follow their normal diet for the first 48 hours; for the second 48 hours, they were asked to switch to a 16-hour fast, which was followed by an 8-hour eating window.
Just over a third of participants were fully compliant with the 16:8 intermittent fasting. But those who followed the regimen experienced a mild reduction in mean acid exposure time and self-reported GERD symptoms scores.
The research was published online by the Journal of Clinical Gastroenterology.
Costly condition
The prevalence of GERD in the United States is estimated at 18%-28%. Annual costs of the condition are more than $18 billion per year, largely through pharmacologic therapies and diagnostic testing, write lead author Yan Jiang, MD, division of gastrointestinal and liver D-diseases, Keck Medicine of University of Southern California, Los Angeles, and colleagues.
Proton pump inhibitor (PPI) therapy is one of the most prescribed classes of medications in the United States, the authors write. But concerns over the long-term safety of the drugs, as well as the fact that half of patients report breakthrough GERD symptoms, have generated interest in non-PPI treatments among patients and providers.
The role of diet in the management of GERD, however, remains poorly understood, despite the fact that obesity and weight gain have been linked to reflux.
The authors note that intermittent fasting has shown benefits in coronary artery disease, inflammatory disorders, obesity, and diabetes. Proposed mechanisms include anti-inflammatory effects, weight loss, and alterations in hormone secretion.
Intervention test in a 96-hour clinical evaluation for GERD
To investigate the effects of intermittent fasting in GERD, the researchers screened patients referred to the Stanford University gastrointestinal clinic for diagnostic 96-hour ambulatory wireless pH monitoring of suspected acid reflux symptoms.
They excluded patients younger than 18 years, pregnant women, those with insulin-dependent diabetes, and those who had used PPIs within the previous 7 days. There were other exclusion criteria as well.
The study was completed by 25 participants. The mean age of the patients was 43.5 years; 52% were women. Just under half (44%) were White, and the mean body mass index was 25.8 kg/m2.
For the first 48 hours of the pH monitoring, the patients followed their baseline diet. For the second 48 hours, they were asked to follow an intermittent fasting regimen.
In that regimen, during a 24-hour period, there was an 8-hour caloric intake window and no caloric intake during the other 16 consecutive hours. Participants who fasted for at least 15 hours, as indicated on a self-report food log, were considered successful.
Only 36% of participants were fully adherent to the fasting regimen; 84% were partially compliant, defined as following the regimen for at least 1 of the 2 days of intermittent fasting.
On intermittent fasting days, the mean acid exposure time was 3.5%, compared with 4.3% on the baseline diet. The team calculated that adhering to the 16:8 intermittent fasting regimen reduced the mean acid exposure time by 0.64%.
Intermittent fasting was also associated with a reduction in total GERD symptom scores, at 9.9 following day 4 versus 14.3 following day 2. There were reductions in heartburn symptoms scores of 2.6 and in regurgitation scores of 1.8.
When the researchers compared individuals who were compliant with intermittent fasting with those who were only partially compliant, they found that there was still an improvement in GERD symptoms, with a reduction in scores of 3.2.
More acid, bigger benefits
There could be several explanations for the findings, Dr. Jiang said in an interview.
In the short-term study, fewer meals during intermittent fasting and more hours between the last meal and bedtime can help with the supine symptoms of GERD, Dr. Jiang said.
Over the longer term, he added, previous studies have suggested that fasting-induced alterations in inflammatory cytokines or cells could be a contributory mechanism, “but it’s not something that we can glean from our study.”
Participants with elevated acid exposure at baseline and who were more likely to have GERD diagnosed by the pH monitoring seemed to experience the greatest benefit from intermittent fasting, Dr. Jiang pointed out.
“This study looked at all comers with GERD symptoms,” he said. “But if you were to do another study with people with proven GERD, they might experience a bigger impact with intermittent fasting.”
Dr. Jiang added, “If a patient is willing to do intermittent fasting, and certainly if they have other reasons [for doing so], I think it doesn’t hurt, and it might actually help them a little bit in their current symptoms.”
Larger scale, longer follow-up studies needed
Luigi Bonavina, MD, department of biomedical sciences for health, University of Milan, IRCCS Policlinico San Donato, Italy, said in an interview that it was a “nice, original study.”
It is “noteworthy that only one previous study explored the effect of Ramadan on GERD symptoms and found a small improvement of GERD symptoms,” Dr. Bonavina said. “Unfortunately, the magnitude of effect [in the current study] was not as one may have expected, due to small sample size and low compliance with intermittent fasting.”
Although the effect was “mild compared to that seen with PPIs,” it would “be interesting to see whether the results of this pilot, proof-of-concept study can be confirmed on a larger scale with longer follow-up to prove that reflux symptoms will not worsen over time,” he said.
“Intermittent fasting may be recommended, especially in overweight-obese patients with GERD symptoms who are poor responders to gastric acid inhibitors,” Dr. Bonavina added. “Reduction of inflammation, reduction of meal intake, and going to bed with an empty stomach may also work in patients with GERD.”
No funding for the study has been declared. The authors and Dr. Bonavina report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL GASTROENTEROLOGY