User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Major life stressors ‘strongly predictive’ of long COVID symptoms
new research suggests.
Major life stressors in the year after hospital discharge for COVID-19 are “strongly predictive of a lot of the important outcomes that people may face after COVID,” lead investigator Jennifer A. Frontera, MD, a professor in the department of neurology at New York University Langone Health, said in an interview.
These outcomes include depression, brain fog, fatigue, trouble sleeping, and other long COVID symptoms.
The findings were published online in the Journal of the Neurological Sciences.
Major stressful events common
Dr. Frontera and the NYU Neurology COVID-19 study team evaluated 451 adults who survived a COVID hospital stay. Of these, 383 completed a 6-month follow-up, 242 completed a 12-month follow-up, and 174 completed follow-up at both time points.
Within 1 year of discharge, 77 (17%) patients died and 51% suffered a major stressful life event.
In multivariable analyses, major life stressors – including financial insecurity, food insecurity, death of a close contact, and new disability – were strong independent predictors of disability, trouble with activities of daily living, depression, fatigue, sleep problems, and prolonged post-acute COVID symptoms. The adjusted odds ratios for these outcomes ranged from 2.5 to 20.8.
The research also confirmed the contribution of traditional risk factors for long COVID symptoms, as shown in past studies. These include older age, poor pre-COVID functional status, and more severe initial COVID-19 infection.
Long-term sequelae of COVID are increasingly recognized as major public health issues.
It has been estimated that roughly 16 million U.S. adults aged 18-65 years ave long COVID, with the often debilitating symptoms keeping up to 4 million out of work.
Holistic approach
Dr. Frontera said it’s important to realize that “sleep, fatigue, anxiety, depression, even cognition are so interwoven with each other that anything that impacts any one of them could have repercussions on the other.”
She added that it “certainly makes sense that there is an interplay or even a bidirectional relationship between the stressors that people face and how well they can recover after COVID.”
Therapies that lessen the trauma of the most stress-inducing life events need to be a central part of treatment for long COVID, with more research needed to validate the best approaches, Dr. Frontera said.
She also noted that social services or case management resources may be able to help address at least some of the stressors that individuals are under – and it is important to refer them to these resources. Referral to mental health services is also important.
“I think it’s really important to take a holistic approach and try to deal with whatever the problem may be,” said Dr. Frontera.
“I’m a neurologist, but as part of my evaluation, I really need to address if there are life stressors or mental health issues that may be impacting this person’s function,” she added.
The study had no commercial funding. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Major life stressors in the year after hospital discharge for COVID-19 are “strongly predictive of a lot of the important outcomes that people may face after COVID,” lead investigator Jennifer A. Frontera, MD, a professor in the department of neurology at New York University Langone Health, said in an interview.
These outcomes include depression, brain fog, fatigue, trouble sleeping, and other long COVID symptoms.
The findings were published online in the Journal of the Neurological Sciences.
Major stressful events common
Dr. Frontera and the NYU Neurology COVID-19 study team evaluated 451 adults who survived a COVID hospital stay. Of these, 383 completed a 6-month follow-up, 242 completed a 12-month follow-up, and 174 completed follow-up at both time points.
Within 1 year of discharge, 77 (17%) patients died and 51% suffered a major stressful life event.
In multivariable analyses, major life stressors – including financial insecurity, food insecurity, death of a close contact, and new disability – were strong independent predictors of disability, trouble with activities of daily living, depression, fatigue, sleep problems, and prolonged post-acute COVID symptoms. The adjusted odds ratios for these outcomes ranged from 2.5 to 20.8.
The research also confirmed the contribution of traditional risk factors for long COVID symptoms, as shown in past studies. These include older age, poor pre-COVID functional status, and more severe initial COVID-19 infection.
Long-term sequelae of COVID are increasingly recognized as major public health issues.
It has been estimated that roughly 16 million U.S. adults aged 18-65 years ave long COVID, with the often debilitating symptoms keeping up to 4 million out of work.
Holistic approach
Dr. Frontera said it’s important to realize that “sleep, fatigue, anxiety, depression, even cognition are so interwoven with each other that anything that impacts any one of them could have repercussions on the other.”
She added that it “certainly makes sense that there is an interplay or even a bidirectional relationship between the stressors that people face and how well they can recover after COVID.”
Therapies that lessen the trauma of the most stress-inducing life events need to be a central part of treatment for long COVID, with more research needed to validate the best approaches, Dr. Frontera said.
She also noted that social services or case management resources may be able to help address at least some of the stressors that individuals are under – and it is important to refer them to these resources. Referral to mental health services is also important.
“I think it’s really important to take a holistic approach and try to deal with whatever the problem may be,” said Dr. Frontera.
“I’m a neurologist, but as part of my evaluation, I really need to address if there are life stressors or mental health issues that may be impacting this person’s function,” she added.
The study had no commercial funding. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Major life stressors in the year after hospital discharge for COVID-19 are “strongly predictive of a lot of the important outcomes that people may face after COVID,” lead investigator Jennifer A. Frontera, MD, a professor in the department of neurology at New York University Langone Health, said in an interview.
These outcomes include depression, brain fog, fatigue, trouble sleeping, and other long COVID symptoms.
The findings were published online in the Journal of the Neurological Sciences.
Major stressful events common
Dr. Frontera and the NYU Neurology COVID-19 study team evaluated 451 adults who survived a COVID hospital stay. Of these, 383 completed a 6-month follow-up, 242 completed a 12-month follow-up, and 174 completed follow-up at both time points.
Within 1 year of discharge, 77 (17%) patients died and 51% suffered a major stressful life event.
In multivariable analyses, major life stressors – including financial insecurity, food insecurity, death of a close contact, and new disability – were strong independent predictors of disability, trouble with activities of daily living, depression, fatigue, sleep problems, and prolonged post-acute COVID symptoms. The adjusted odds ratios for these outcomes ranged from 2.5 to 20.8.
The research also confirmed the contribution of traditional risk factors for long COVID symptoms, as shown in past studies. These include older age, poor pre-COVID functional status, and more severe initial COVID-19 infection.
Long-term sequelae of COVID are increasingly recognized as major public health issues.
It has been estimated that roughly 16 million U.S. adults aged 18-65 years ave long COVID, with the often debilitating symptoms keeping up to 4 million out of work.
Holistic approach
Dr. Frontera said it’s important to realize that “sleep, fatigue, anxiety, depression, even cognition are so interwoven with each other that anything that impacts any one of them could have repercussions on the other.”
She added that it “certainly makes sense that there is an interplay or even a bidirectional relationship between the stressors that people face and how well they can recover after COVID.”
Therapies that lessen the trauma of the most stress-inducing life events need to be a central part of treatment for long COVID, with more research needed to validate the best approaches, Dr. Frontera said.
She also noted that social services or case management resources may be able to help address at least some of the stressors that individuals are under – and it is important to refer them to these resources. Referral to mental health services is also important.
“I think it’s really important to take a holistic approach and try to deal with whatever the problem may be,” said Dr. Frontera.
“I’m a neurologist, but as part of my evaluation, I really need to address if there are life stressors or mental health issues that may be impacting this person’s function,” she added.
The study had no commercial funding. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE NEUROLOGICAL SCIENCES
Experts explain the ‘perfect storm’ of rampant RSV and flu
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
IRONMAN galvanizes case for IV iron repletion in heart failure
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.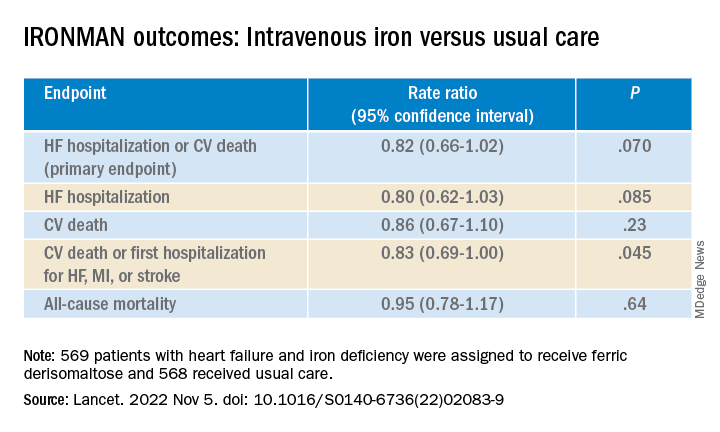
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Intensive gout treatment meets urate goal, lowers tophi burden
PHILADELPHIA – Patients with gout who underwent an intensive treat-to-target regimen of monthly up-titration of urate-lowering therapy (ULT) to reach a target serum urate level were significantly more likely to reach that goal at 1 year than were patients who received conventional gout management in a randomized, controlled trial.
These results came from the TICOG (Tight Control of Gout) trial, one of a handful of recent trials to test a treat-to-target strategy with ULT in the management of gout. Beyond the primary outcome of reaching target serum urate level of < 5 mg/dL (< 300 micromol/L), the results also showed that the tight-control strategy significantly lowered urate to a greater extent than conventional management, reduced tophus size in the first metatarsophalangeal (MTP) joint, and improved gray scale synovitis on ultrasound significantly more than with conventional management, Sarah Black, MBBS, a rheumatology trainee at Musgrave Park Hospital, Belfast, Northern Ireland, reported at the American College of Rheumatology annual meeting.
“Based on these outcomes, we question whether gout is best managed in primary or secondary care. We think there is an argument for establishing specialist gout clinics with more time to focus on patient education to help improve outcomes. These clinics could be led by allied health care professionals, such as specialist nurses and pharmacists,” Dr. Black said at the meeting.
Gout management guidelines issued by the British Society for Rheumatology in 2017 call for a target serum urate level of < 5 mg/dL, whereas the ACR’s 2020 guideline for the management of gout endorses a treat-to-target management strategy that aims for a serum urate level of < 6 mg/dL.
The single-center, nonblinded trial recruited 110 patients aged 18-85 years over a 3-year period to take ULT with allopurinol as first-line therapy starting at 100 mg/day. Everyone received the same advice regarding ULT up-titration, lifestyle changes, and gout education at baseline. The second-line agent for ULT was febuxostat (Uloric) 80 mg daily, with uricosuric drugs as third-line agents. All patients received colchicine or NSAID prophylaxis for gout flares for the first 6 months, depending on their comorbidities.
The trial excluded patients who had been treated with ULT within the past 6 months or had experienced prior hypersensitivity to ULT, severe renal impairment (creatinine clearance < 30 mL/min as measured by estimated glomerular filtration rate), significant liver impairment, or any other significant medical disease affecting life expectancy shorter than 1 year.
Conventional management consisted of urate level review at 0, 6, and 12 months with up-titration at each visit and primary care management of ULT between reviews until the target serum urate level was reached. In the tight-control group, monthly up-titrations occurred at the Musgrave Park Hospital at visits with the study team that were led by a rheumatologist and a specialist pharmacist.
A total of 48 patients in the conventional arm and 47 in the tight-control arm completed the trial. At baseline, monosodium urate crystals were detected in joint aspirates in 56% of patients receiving tight control and in 58.5% of those receiving conventional management. The mean serum urate level was 490 micromol/L (8.24 mg/dL) for tight-control patients and 470 micromol/L (7.9 mg/dL) for conventionally managed patients.
By 1 year, 89.4% of patients in the tight-control group had achieved the target urate level, compared with 39.6% in the conventional-management group (P < .001). At 6 months, serum urate had declined by 37.6% with tight control vs. 18% with conventional management. By the end of the trial, the median allopurinol dose was 400 mg with tight control (range, 200-900 mg) and 200 mg with conventional management (range, 0-400 mg). A total of 89% of patients were taking allopurinol at the end of the trial.
As expected, tight control led to more flares per month on average (0.35 vs. 0.13) in the 79 patients for whom complete data on flare frequency were available.
On blinded ultrasound evaluations, the median diameter of the first MTP tophus declined significantly more with tight control than with conventional management (–4.65 mm vs. –0.30 mm; P = .003). Gray scale synovitis in the knee improved in 63% of patients undergoing tight control, compared with 14% of conventionally managed patients (P = .043). The researchers observed no difference in resolution of the double-contour sign or in the number of erosions between the groups, although the 1-year time frame may not have been long enough to see resolution and improvement, Dr. Black said.
Dr. Black said that a follow-up study is planned with the same patient cohort at 3 years.
When asked about the feasibility of monthly ULT titration visits for gout management, audience member Tuhina Neogi, MD, professor of epidemiology at Boston University and chief of rheumatology at Boston Medical Center, told this news organization, “We don’t have a lot of data to guide us in that regard, and I also think it depends on what the increment of the dose titration is, but we generally do recognize that therapeutic inertia is bad – keeping someone on a dose for a long time. For me, I don’t think monthly is unreasonable if you have good prophylaxis [against acute flares].”
Dr. Neogi also noted that such monthly assessments don’t have to take place at a hospital. “I think there are many different practice models in which it could be implemented [that are not physician-driven].”
The study had no outside funding. Dr. Black has disclosed no relevant financial relationships. Dr. Neogi has received consulting fees from a variety of pharmaceutical companies, including Alnylam, Regeneron, Eli Lilly, EMD Serono, Novartis, Pfizer, and GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Patients with gout who underwent an intensive treat-to-target regimen of monthly up-titration of urate-lowering therapy (ULT) to reach a target serum urate level were significantly more likely to reach that goal at 1 year than were patients who received conventional gout management in a randomized, controlled trial.
These results came from the TICOG (Tight Control of Gout) trial, one of a handful of recent trials to test a treat-to-target strategy with ULT in the management of gout. Beyond the primary outcome of reaching target serum urate level of < 5 mg/dL (< 300 micromol/L), the results also showed that the tight-control strategy significantly lowered urate to a greater extent than conventional management, reduced tophus size in the first metatarsophalangeal (MTP) joint, and improved gray scale synovitis on ultrasound significantly more than with conventional management, Sarah Black, MBBS, a rheumatology trainee at Musgrave Park Hospital, Belfast, Northern Ireland, reported at the American College of Rheumatology annual meeting.
“Based on these outcomes, we question whether gout is best managed in primary or secondary care. We think there is an argument for establishing specialist gout clinics with more time to focus on patient education to help improve outcomes. These clinics could be led by allied health care professionals, such as specialist nurses and pharmacists,” Dr. Black said at the meeting.
Gout management guidelines issued by the British Society for Rheumatology in 2017 call for a target serum urate level of < 5 mg/dL, whereas the ACR’s 2020 guideline for the management of gout endorses a treat-to-target management strategy that aims for a serum urate level of < 6 mg/dL.
The single-center, nonblinded trial recruited 110 patients aged 18-85 years over a 3-year period to take ULT with allopurinol as first-line therapy starting at 100 mg/day. Everyone received the same advice regarding ULT up-titration, lifestyle changes, and gout education at baseline. The second-line agent for ULT was febuxostat (Uloric) 80 mg daily, with uricosuric drugs as third-line agents. All patients received colchicine or NSAID prophylaxis for gout flares for the first 6 months, depending on their comorbidities.
The trial excluded patients who had been treated with ULT within the past 6 months or had experienced prior hypersensitivity to ULT, severe renal impairment (creatinine clearance < 30 mL/min as measured by estimated glomerular filtration rate), significant liver impairment, or any other significant medical disease affecting life expectancy shorter than 1 year.
Conventional management consisted of urate level review at 0, 6, and 12 months with up-titration at each visit and primary care management of ULT between reviews until the target serum urate level was reached. In the tight-control group, monthly up-titrations occurred at the Musgrave Park Hospital at visits with the study team that were led by a rheumatologist and a specialist pharmacist.
A total of 48 patients in the conventional arm and 47 in the tight-control arm completed the trial. At baseline, monosodium urate crystals were detected in joint aspirates in 56% of patients receiving tight control and in 58.5% of those receiving conventional management. The mean serum urate level was 490 micromol/L (8.24 mg/dL) for tight-control patients and 470 micromol/L (7.9 mg/dL) for conventionally managed patients.
By 1 year, 89.4% of patients in the tight-control group had achieved the target urate level, compared with 39.6% in the conventional-management group (P < .001). At 6 months, serum urate had declined by 37.6% with tight control vs. 18% with conventional management. By the end of the trial, the median allopurinol dose was 400 mg with tight control (range, 200-900 mg) and 200 mg with conventional management (range, 0-400 mg). A total of 89% of patients were taking allopurinol at the end of the trial.
As expected, tight control led to more flares per month on average (0.35 vs. 0.13) in the 79 patients for whom complete data on flare frequency were available.
On blinded ultrasound evaluations, the median diameter of the first MTP tophus declined significantly more with tight control than with conventional management (–4.65 mm vs. –0.30 mm; P = .003). Gray scale synovitis in the knee improved in 63% of patients undergoing tight control, compared with 14% of conventionally managed patients (P = .043). The researchers observed no difference in resolution of the double-contour sign or in the number of erosions between the groups, although the 1-year time frame may not have been long enough to see resolution and improvement, Dr. Black said.
Dr. Black said that a follow-up study is planned with the same patient cohort at 3 years.
When asked about the feasibility of monthly ULT titration visits for gout management, audience member Tuhina Neogi, MD, professor of epidemiology at Boston University and chief of rheumatology at Boston Medical Center, told this news organization, “We don’t have a lot of data to guide us in that regard, and I also think it depends on what the increment of the dose titration is, but we generally do recognize that therapeutic inertia is bad – keeping someone on a dose for a long time. For me, I don’t think monthly is unreasonable if you have good prophylaxis [against acute flares].”
Dr. Neogi also noted that such monthly assessments don’t have to take place at a hospital. “I think there are many different practice models in which it could be implemented [that are not physician-driven].”
The study had no outside funding. Dr. Black has disclosed no relevant financial relationships. Dr. Neogi has received consulting fees from a variety of pharmaceutical companies, including Alnylam, Regeneron, Eli Lilly, EMD Serono, Novartis, Pfizer, and GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Patients with gout who underwent an intensive treat-to-target regimen of monthly up-titration of urate-lowering therapy (ULT) to reach a target serum urate level were significantly more likely to reach that goal at 1 year than were patients who received conventional gout management in a randomized, controlled trial.
These results came from the TICOG (Tight Control of Gout) trial, one of a handful of recent trials to test a treat-to-target strategy with ULT in the management of gout. Beyond the primary outcome of reaching target serum urate level of < 5 mg/dL (< 300 micromol/L), the results also showed that the tight-control strategy significantly lowered urate to a greater extent than conventional management, reduced tophus size in the first metatarsophalangeal (MTP) joint, and improved gray scale synovitis on ultrasound significantly more than with conventional management, Sarah Black, MBBS, a rheumatology trainee at Musgrave Park Hospital, Belfast, Northern Ireland, reported at the American College of Rheumatology annual meeting.
“Based on these outcomes, we question whether gout is best managed in primary or secondary care. We think there is an argument for establishing specialist gout clinics with more time to focus on patient education to help improve outcomes. These clinics could be led by allied health care professionals, such as specialist nurses and pharmacists,” Dr. Black said at the meeting.
Gout management guidelines issued by the British Society for Rheumatology in 2017 call for a target serum urate level of < 5 mg/dL, whereas the ACR’s 2020 guideline for the management of gout endorses a treat-to-target management strategy that aims for a serum urate level of < 6 mg/dL.
The single-center, nonblinded trial recruited 110 patients aged 18-85 years over a 3-year period to take ULT with allopurinol as first-line therapy starting at 100 mg/day. Everyone received the same advice regarding ULT up-titration, lifestyle changes, and gout education at baseline. The second-line agent for ULT was febuxostat (Uloric) 80 mg daily, with uricosuric drugs as third-line agents. All patients received colchicine or NSAID prophylaxis for gout flares for the first 6 months, depending on their comorbidities.
The trial excluded patients who had been treated with ULT within the past 6 months or had experienced prior hypersensitivity to ULT, severe renal impairment (creatinine clearance < 30 mL/min as measured by estimated glomerular filtration rate), significant liver impairment, or any other significant medical disease affecting life expectancy shorter than 1 year.
Conventional management consisted of urate level review at 0, 6, and 12 months with up-titration at each visit and primary care management of ULT between reviews until the target serum urate level was reached. In the tight-control group, monthly up-titrations occurred at the Musgrave Park Hospital at visits with the study team that were led by a rheumatologist and a specialist pharmacist.
A total of 48 patients in the conventional arm and 47 in the tight-control arm completed the trial. At baseline, monosodium urate crystals were detected in joint aspirates in 56% of patients receiving tight control and in 58.5% of those receiving conventional management. The mean serum urate level was 490 micromol/L (8.24 mg/dL) for tight-control patients and 470 micromol/L (7.9 mg/dL) for conventionally managed patients.
By 1 year, 89.4% of patients in the tight-control group had achieved the target urate level, compared with 39.6% in the conventional-management group (P < .001). At 6 months, serum urate had declined by 37.6% with tight control vs. 18% with conventional management. By the end of the trial, the median allopurinol dose was 400 mg with tight control (range, 200-900 mg) and 200 mg with conventional management (range, 0-400 mg). A total of 89% of patients were taking allopurinol at the end of the trial.
As expected, tight control led to more flares per month on average (0.35 vs. 0.13) in the 79 patients for whom complete data on flare frequency were available.
On blinded ultrasound evaluations, the median diameter of the first MTP tophus declined significantly more with tight control than with conventional management (–4.65 mm vs. –0.30 mm; P = .003). Gray scale synovitis in the knee improved in 63% of patients undergoing tight control, compared with 14% of conventionally managed patients (P = .043). The researchers observed no difference in resolution of the double-contour sign or in the number of erosions between the groups, although the 1-year time frame may not have been long enough to see resolution and improvement, Dr. Black said.
Dr. Black said that a follow-up study is planned with the same patient cohort at 3 years.
When asked about the feasibility of monthly ULT titration visits for gout management, audience member Tuhina Neogi, MD, professor of epidemiology at Boston University and chief of rheumatology at Boston Medical Center, told this news organization, “We don’t have a lot of data to guide us in that regard, and I also think it depends on what the increment of the dose titration is, but we generally do recognize that therapeutic inertia is bad – keeping someone on a dose for a long time. For me, I don’t think monthly is unreasonable if you have good prophylaxis [against acute flares].”
Dr. Neogi also noted that such monthly assessments don’t have to take place at a hospital. “I think there are many different practice models in which it could be implemented [that are not physician-driven].”
The study had no outside funding. Dr. Black has disclosed no relevant financial relationships. Dr. Neogi has received consulting fees from a variety of pharmaceutical companies, including Alnylam, Regeneron, Eli Lilly, EMD Serono, Novartis, Pfizer, and GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
AT ACR 2022
Be aware, mindfulness training can lower systolic BP: MB-BP
CHICAGO – It’s been said that one can observe a lot just by watching. Turning such observation inward, new evidence suggests, might lead to blood pressure (BP) reductions that approach what’s possible from an antihypertensive agent.
Systolic BP fell over 6 months by almost 6 mm Hg, on average, in people with elevated BP who participated in an 8-week mindful awareness program as part of a randomized trial that included a usual-care control group.
The program taught established mindfulness-training techniques aimed at modifying behaviors regarding diet, exercise, and other controllable influences on the success of antihypertensive therapy.
Participants in the program, called Mindfulness-Based Blood Pressure Reduction (MB-BP), also the name of the single-center study, “showed potentially clinically relevant reductions in systolic blood pressure,” said principal investigator Eric B. Loucks, PhD, Brown University, Providence, R.I.
The phase 2 trial has some limitations, he observed, including on generalizability. For example, it entered about 200 mostly White, college-educated adults from one metropolitan area.
But if these findings are replicated in further studies, “preferably by other research groups, in a larger and broader population, and with longer follow-up,” Dr. Loucks said, the MB-BP intervention could become “an appealing approach to help control blood pressure.”
Dr. Loucks made the comments at a press conference prior to his formal presentation of MB-BP Nov. 6 at American Heart Association (AHA) Scientific Sessions 2022, held in Chicago and virtually.
Mindfulness-based interventions for elevated BP have not been widely studied, “so this is exactly what we need: a well-done trial with a control group to show that it actually works,” Amit Khera, MD, not connected with MB-BP, told this news organization.
The trial is “really important for proof of concept, but it had only 200 people. You need a larger one, and you need longer-term data,” agreed Dr. Khera, who directs the preventive cardiology program at the University of Texas Southwestern Medical Center, in Dallas. “Six months is good, but we want to see if it’s durable.”
Rhian M. Touyz, MBBCh, also not part of MB-BP, agreed that the nearly 6 mm Hg mean systolic BP reduction among program participants is clinically relevant. “I think in the context of global risk and reduction of target organ damage and cardiovascular events, it is significant in terms of events at a population level,” Dr. Touyz, McGill University Health Centre, Montreal, told this news organization.
Many patients on antihypertensive therapy that’s falling short resist the addition of another such agent, she observed, and instead might show further BP reduction from mindfulness training. The intervention probably also “would benefit health in general.” Mindfulness-based approaches could therefore be useful additions to treatment protocols for elevated BP, Dr. Touyz said.
How the training works
The MB-BP program used validated mindfulness-based stress-management techniques, adapted to address elevated BP, that included “personalized feedback and education about hypertension risk factors, mindful awareness training of participants’ relationships with hypertension risk factors, and support for behavior change,” Dr. Loucks and colleagues reported.
Participants were trained in mindfulness skills that included “self-awareness and emotion regulation,” Dr. Loucks said, which they then could apply to their “relationships with the things that we know influence blood pressure, like physical activity, diet, antihypertensive medication adherence, or alcohol consumption.”
One goal is to promote greater “attention control,” he said, “so that there’s some self-awareness that arises in terms of how we feel the next day, after a lot of alcohol consumption, for example, or lack of physical activity.” The process can provide insights that inspire patients to modify behaviors and risk factors that elevate BP, Dr. Loucks explained.
Effects on medication use
Systolic BP responses led some program participants to be managed on fewer or reduced dosages of antihypertensive meds, he told this news organization. Physicians seen outside of the trial could adjust their prescriptions, intensifying or pulling back on meds depending on their assessments of the patient. Any prescription changes would be documented by the researchers at the patient’s next class or trial-clinic visit.
The group that did the training, Dr. Loucks said, was 33% less likely to increase and 30% more likely to decrease their use of BP-lowering medications compared with the control group.
Elevated BP is so common and undertreated that “there is a need for every possible level of intervention, starting from the population level to the individual and everything else in between,” nephrologist Janani Rangaswami, MD, George Washington University, Washington, said at the press conference.
Therefore, “this mindfulness-based approach, in addition to standard of care with pharmacotherapy, is a really welcome addition to the hypertension literature,” said Dr. Rangaswami, who directs her center’s cardiorenal program. The systolic BP reduction seen in the intervention group, she agreed, was “clinically important and meaningful.”
Blinded assessments
The trial entered 201 patients with systolic and diastolic BP greater than 120 mm Hg and 80 mm Hg, respectively; 58.7% were women, 81% were White, and 73% were college-educated, Dr. Loucks reported.
The 100 assigned to the “enhanced usual care” control group received educational materials on controlling high BP. They and the 101 who followed the mindfulness-based program were given and trained on a home BP-monitoring device. They were then followed for the primary endpoint of change in systolic BP at 6 months.
Data management and outcomes assessments were conducted by trialists not involved in the training intervention who were blinded to randomization assignment.
In a prespecified unadjusted analysis by intention-to-treat, systolic BP in the intervention group dropped by a mean of 5.9 mm Hg (P < .001) compared with baseline and 4.5 mm Hg (P = .045), compared with the control group.
A post hoc analysis adjusted for sex and baseline BP showed an average 4.3 mm Hg reduction (P = .056) in those following the MB-BP program, compared with controls.
There were no observed significant effects on diastolic BP.
The study offered clues to how engagement in the MB-BP program might promote reductions in systolic BP, Dr. Loucks observed. For example, it may have led to increased activity levels, reduced sodium intake, and other dietary improvements.
Indeed, program participants averaged about 351 minutes less sedentary time (P = .02) and showed a 0.32-point improvement in Dietary Approaches to Stop Hypertension scores (P = .08), compared with the control group, Dr. Loucks reported. Other modifiable risk factors for elevated BP that could have responded to the mindfulness-based training, he proposed, include obesity, alcohol intake, and reaction to stress.
Dr. Loucks reports that he developed the MB-BP training and was a program instructor but did not receive related financial compensation; he had no other disclosures. Dr. Khera, Dr. Touyz, and Dr. Rangaswami had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – It’s been said that one can observe a lot just by watching. Turning such observation inward, new evidence suggests, might lead to blood pressure (BP) reductions that approach what’s possible from an antihypertensive agent.
Systolic BP fell over 6 months by almost 6 mm Hg, on average, in people with elevated BP who participated in an 8-week mindful awareness program as part of a randomized trial that included a usual-care control group.
The program taught established mindfulness-training techniques aimed at modifying behaviors regarding diet, exercise, and other controllable influences on the success of antihypertensive therapy.
Participants in the program, called Mindfulness-Based Blood Pressure Reduction (MB-BP), also the name of the single-center study, “showed potentially clinically relevant reductions in systolic blood pressure,” said principal investigator Eric B. Loucks, PhD, Brown University, Providence, R.I.
The phase 2 trial has some limitations, he observed, including on generalizability. For example, it entered about 200 mostly White, college-educated adults from one metropolitan area.
But if these findings are replicated in further studies, “preferably by other research groups, in a larger and broader population, and with longer follow-up,” Dr. Loucks said, the MB-BP intervention could become “an appealing approach to help control blood pressure.”
Dr. Loucks made the comments at a press conference prior to his formal presentation of MB-BP Nov. 6 at American Heart Association (AHA) Scientific Sessions 2022, held in Chicago and virtually.
Mindfulness-based interventions for elevated BP have not been widely studied, “so this is exactly what we need: a well-done trial with a control group to show that it actually works,” Amit Khera, MD, not connected with MB-BP, told this news organization.
The trial is “really important for proof of concept, but it had only 200 people. You need a larger one, and you need longer-term data,” agreed Dr. Khera, who directs the preventive cardiology program at the University of Texas Southwestern Medical Center, in Dallas. “Six months is good, but we want to see if it’s durable.”
Rhian M. Touyz, MBBCh, also not part of MB-BP, agreed that the nearly 6 mm Hg mean systolic BP reduction among program participants is clinically relevant. “I think in the context of global risk and reduction of target organ damage and cardiovascular events, it is significant in terms of events at a population level,” Dr. Touyz, McGill University Health Centre, Montreal, told this news organization.
Many patients on antihypertensive therapy that’s falling short resist the addition of another such agent, she observed, and instead might show further BP reduction from mindfulness training. The intervention probably also “would benefit health in general.” Mindfulness-based approaches could therefore be useful additions to treatment protocols for elevated BP, Dr. Touyz said.
How the training works
The MB-BP program used validated mindfulness-based stress-management techniques, adapted to address elevated BP, that included “personalized feedback and education about hypertension risk factors, mindful awareness training of participants’ relationships with hypertension risk factors, and support for behavior change,” Dr. Loucks and colleagues reported.
Participants were trained in mindfulness skills that included “self-awareness and emotion regulation,” Dr. Loucks said, which they then could apply to their “relationships with the things that we know influence blood pressure, like physical activity, diet, antihypertensive medication adherence, or alcohol consumption.”
One goal is to promote greater “attention control,” he said, “so that there’s some self-awareness that arises in terms of how we feel the next day, after a lot of alcohol consumption, for example, or lack of physical activity.” The process can provide insights that inspire patients to modify behaviors and risk factors that elevate BP, Dr. Loucks explained.
Effects on medication use
Systolic BP responses led some program participants to be managed on fewer or reduced dosages of antihypertensive meds, he told this news organization. Physicians seen outside of the trial could adjust their prescriptions, intensifying or pulling back on meds depending on their assessments of the patient. Any prescription changes would be documented by the researchers at the patient’s next class or trial-clinic visit.
The group that did the training, Dr. Loucks said, was 33% less likely to increase and 30% more likely to decrease their use of BP-lowering medications compared with the control group.
Elevated BP is so common and undertreated that “there is a need for every possible level of intervention, starting from the population level to the individual and everything else in between,” nephrologist Janani Rangaswami, MD, George Washington University, Washington, said at the press conference.
Therefore, “this mindfulness-based approach, in addition to standard of care with pharmacotherapy, is a really welcome addition to the hypertension literature,” said Dr. Rangaswami, who directs her center’s cardiorenal program. The systolic BP reduction seen in the intervention group, she agreed, was “clinically important and meaningful.”
Blinded assessments
The trial entered 201 patients with systolic and diastolic BP greater than 120 mm Hg and 80 mm Hg, respectively; 58.7% were women, 81% were White, and 73% were college-educated, Dr. Loucks reported.
The 100 assigned to the “enhanced usual care” control group received educational materials on controlling high BP. They and the 101 who followed the mindfulness-based program were given and trained on a home BP-monitoring device. They were then followed for the primary endpoint of change in systolic BP at 6 months.
Data management and outcomes assessments were conducted by trialists not involved in the training intervention who were blinded to randomization assignment.
In a prespecified unadjusted analysis by intention-to-treat, systolic BP in the intervention group dropped by a mean of 5.9 mm Hg (P < .001) compared with baseline and 4.5 mm Hg (P = .045), compared with the control group.
A post hoc analysis adjusted for sex and baseline BP showed an average 4.3 mm Hg reduction (P = .056) in those following the MB-BP program, compared with controls.
There were no observed significant effects on diastolic BP.
The study offered clues to how engagement in the MB-BP program might promote reductions in systolic BP, Dr. Loucks observed. For example, it may have led to increased activity levels, reduced sodium intake, and other dietary improvements.
Indeed, program participants averaged about 351 minutes less sedentary time (P = .02) and showed a 0.32-point improvement in Dietary Approaches to Stop Hypertension scores (P = .08), compared with the control group, Dr. Loucks reported. Other modifiable risk factors for elevated BP that could have responded to the mindfulness-based training, he proposed, include obesity, alcohol intake, and reaction to stress.
Dr. Loucks reports that he developed the MB-BP training and was a program instructor but did not receive related financial compensation; he had no other disclosures. Dr. Khera, Dr. Touyz, and Dr. Rangaswami had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – It’s been said that one can observe a lot just by watching. Turning such observation inward, new evidence suggests, might lead to blood pressure (BP) reductions that approach what’s possible from an antihypertensive agent.
Systolic BP fell over 6 months by almost 6 mm Hg, on average, in people with elevated BP who participated in an 8-week mindful awareness program as part of a randomized trial that included a usual-care control group.
The program taught established mindfulness-training techniques aimed at modifying behaviors regarding diet, exercise, and other controllable influences on the success of antihypertensive therapy.
Participants in the program, called Mindfulness-Based Blood Pressure Reduction (MB-BP), also the name of the single-center study, “showed potentially clinically relevant reductions in systolic blood pressure,” said principal investigator Eric B. Loucks, PhD, Brown University, Providence, R.I.
The phase 2 trial has some limitations, he observed, including on generalizability. For example, it entered about 200 mostly White, college-educated adults from one metropolitan area.
But if these findings are replicated in further studies, “preferably by other research groups, in a larger and broader population, and with longer follow-up,” Dr. Loucks said, the MB-BP intervention could become “an appealing approach to help control blood pressure.”
Dr. Loucks made the comments at a press conference prior to his formal presentation of MB-BP Nov. 6 at American Heart Association (AHA) Scientific Sessions 2022, held in Chicago and virtually.
Mindfulness-based interventions for elevated BP have not been widely studied, “so this is exactly what we need: a well-done trial with a control group to show that it actually works,” Amit Khera, MD, not connected with MB-BP, told this news organization.
The trial is “really important for proof of concept, but it had only 200 people. You need a larger one, and you need longer-term data,” agreed Dr. Khera, who directs the preventive cardiology program at the University of Texas Southwestern Medical Center, in Dallas. “Six months is good, but we want to see if it’s durable.”
Rhian M. Touyz, MBBCh, also not part of MB-BP, agreed that the nearly 6 mm Hg mean systolic BP reduction among program participants is clinically relevant. “I think in the context of global risk and reduction of target organ damage and cardiovascular events, it is significant in terms of events at a population level,” Dr. Touyz, McGill University Health Centre, Montreal, told this news organization.
Many patients on antihypertensive therapy that’s falling short resist the addition of another such agent, she observed, and instead might show further BP reduction from mindfulness training. The intervention probably also “would benefit health in general.” Mindfulness-based approaches could therefore be useful additions to treatment protocols for elevated BP, Dr. Touyz said.
How the training works
The MB-BP program used validated mindfulness-based stress-management techniques, adapted to address elevated BP, that included “personalized feedback and education about hypertension risk factors, mindful awareness training of participants’ relationships with hypertension risk factors, and support for behavior change,” Dr. Loucks and colleagues reported.
Participants were trained in mindfulness skills that included “self-awareness and emotion regulation,” Dr. Loucks said, which they then could apply to their “relationships with the things that we know influence blood pressure, like physical activity, diet, antihypertensive medication adherence, or alcohol consumption.”
One goal is to promote greater “attention control,” he said, “so that there’s some self-awareness that arises in terms of how we feel the next day, after a lot of alcohol consumption, for example, or lack of physical activity.” The process can provide insights that inspire patients to modify behaviors and risk factors that elevate BP, Dr. Loucks explained.
Effects on medication use
Systolic BP responses led some program participants to be managed on fewer or reduced dosages of antihypertensive meds, he told this news organization. Physicians seen outside of the trial could adjust their prescriptions, intensifying or pulling back on meds depending on their assessments of the patient. Any prescription changes would be documented by the researchers at the patient’s next class or trial-clinic visit.
The group that did the training, Dr. Loucks said, was 33% less likely to increase and 30% more likely to decrease their use of BP-lowering medications compared with the control group.
Elevated BP is so common and undertreated that “there is a need for every possible level of intervention, starting from the population level to the individual and everything else in between,” nephrologist Janani Rangaswami, MD, George Washington University, Washington, said at the press conference.
Therefore, “this mindfulness-based approach, in addition to standard of care with pharmacotherapy, is a really welcome addition to the hypertension literature,” said Dr. Rangaswami, who directs her center’s cardiorenal program. The systolic BP reduction seen in the intervention group, she agreed, was “clinically important and meaningful.”
Blinded assessments
The trial entered 201 patients with systolic and diastolic BP greater than 120 mm Hg and 80 mm Hg, respectively; 58.7% were women, 81% were White, and 73% were college-educated, Dr. Loucks reported.
The 100 assigned to the “enhanced usual care” control group received educational materials on controlling high BP. They and the 101 who followed the mindfulness-based program were given and trained on a home BP-monitoring device. They were then followed for the primary endpoint of change in systolic BP at 6 months.
Data management and outcomes assessments were conducted by trialists not involved in the training intervention who were blinded to randomization assignment.
In a prespecified unadjusted analysis by intention-to-treat, systolic BP in the intervention group dropped by a mean of 5.9 mm Hg (P < .001) compared with baseline and 4.5 mm Hg (P = .045), compared with the control group.
A post hoc analysis adjusted for sex and baseline BP showed an average 4.3 mm Hg reduction (P = .056) in those following the MB-BP program, compared with controls.
There were no observed significant effects on diastolic BP.
The study offered clues to how engagement in the MB-BP program might promote reductions in systolic BP, Dr. Loucks observed. For example, it may have led to increased activity levels, reduced sodium intake, and other dietary improvements.
Indeed, program participants averaged about 351 minutes less sedentary time (P = .02) and showed a 0.32-point improvement in Dietary Approaches to Stop Hypertension scores (P = .08), compared with the control group, Dr. Loucks reported. Other modifiable risk factors for elevated BP that could have responded to the mindfulness-based training, he proposed, include obesity, alcohol intake, and reaction to stress.
Dr. Loucks reports that he developed the MB-BP training and was a program instructor but did not receive related financial compensation; he had no other disclosures. Dr. Khera, Dr. Touyz, and Dr. Rangaswami had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Night lights in the city link to increased risk of diabetes
Higher levels of exposure to outdoor artificial light at night are significantly linked with markers of diabetes and impaired glucose homeostasis, in a new national, cross-sectional study from China.
The results showed a 7% significant increase in diabetes prevalence per quintile exposure to artificial light at night (prevalence ratio, 1.07), report Ruizhi Zheng, PhD, of the Shanghai (China) Jiaotong University School of Medicine, and colleagues. People living in areas with the most exposure to light at night had a 28% higher prevalence of diabetes than those living in places with the lowest exposure (PR, 1.28), the researchers found.
The study was published online in Diabetologia.
Previous animal studies have shown that exposure to light at night may interfere with circadian rhythms and affect glucose homeostasis, the study team note. Other research has demonstrated that chronic exposure to moderate indoor light during sleep elevated the prevalence of diabetes in older adults, compared with those sleeping in a dim setting, the authors add.
“Our findings contribute to the growing literature suggesting that artificial light at night is detrimental to health and demonstrate that artificial light at night may be a potential novel risk factor for diabetes,” they write.
“Considering the coexistence of the diabetes epidemic and the widespread influence of light pollution at night, the positive associations indicate an urgent need for countries and governments to develop effective prevention and intervention policies and to protect people from the adverse health effects of light pollution at night,” the study authors stress.
Gareth Nye, PhD, senior lecturer at the University of Chester, England, agreed that prior research has found an association between metabolic conditions, such as diabetes, and artificial light at night, with most theories as to the cause focusing on the body’s natural circadian cycle.
He said that internal clocks regulate a variety of bodily processes, such as metabolism and hormone synthesis. They also affect sleep patterns by interfering with synthesis of the hormone melatonin, which is essential for sound sleep, Dr. Nye told the UK Science Media Centre.
However, he stressed that much more research is needed before any link can be considered definitive.
Outdoor night light exposure linked to fasting glucose, A1c
The Chinese researchers set out to approximate the relationships between diabetes prevalence and glucose homeostasis with chronic exposure to outdoor light at night.
They assessed 98,658 participants from the China Noncommunicable Disease Surveillance Study across 162 sites. The mean age of participants was 42.7 years. Female participants comprised 49.2% of the study cohort.
Diabetes was defined based on American Diabetes Association criteria. Satellite data were used to determine exposure to outdoor light at night in 2010. The associations between light exposure at night and indicators of glucose homeostasis were investigated.
Prevalence ratios were calculated and adjusted for sex, age, smoking status, education, body mass index, physical activity, household income, family history of diabetes, rural/urban areas, drinking status, and use of lipid-lowering prescription drugs (primarily statins) or antihypertensives.
The findings showed exposure levels to outdoor light at night were positively linked with 2-hour and fasting glucose concentrations, A1c, and insulin resistance (measured using homeostatic model assessment [HOMA]), but negatively related to β-cell function (measured using HOMA).
More research needed
“We advise caution against causal interpretation of the findings and call for further studies involving direct measurement of individual exposure to light at night,” the researchers conclude.
Dr. Nye agreed.
“One issue with this study is that the areas with the highest outdoor artificial light levels are likely to be those in urban areas and bigger cities. It has been known for a long time now that living in an urbanized area increases your risk of obesity through increased access to high-fat and convenience food, less physical activity levels due to transport links, and less social activities. The authors also state this and the fact participants tended to be older,” he noted.
Large datasets are used in this investigation, however, which generally increases the reliability of the data, he observed.
But it is also “unclear as to whether the population here was selected for this study or was retrospectively analyzed, which poses reliability issues, as does the selection of the representative sample, as it is not discussed,” he noted.
Ultimately, there is no confirmed evidence of the link, and until further work is done to directly link light exposure and diabetes in humans, “the link will remain an association only,” he concluded.
A version of this article first appeared on Medscape.com.
Higher levels of exposure to outdoor artificial light at night are significantly linked with markers of diabetes and impaired glucose homeostasis, in a new national, cross-sectional study from China.
The results showed a 7% significant increase in diabetes prevalence per quintile exposure to artificial light at night (prevalence ratio, 1.07), report Ruizhi Zheng, PhD, of the Shanghai (China) Jiaotong University School of Medicine, and colleagues. People living in areas with the most exposure to light at night had a 28% higher prevalence of diabetes than those living in places with the lowest exposure (PR, 1.28), the researchers found.
The study was published online in Diabetologia.
Previous animal studies have shown that exposure to light at night may interfere with circadian rhythms and affect glucose homeostasis, the study team note. Other research has demonstrated that chronic exposure to moderate indoor light during sleep elevated the prevalence of diabetes in older adults, compared with those sleeping in a dim setting, the authors add.
“Our findings contribute to the growing literature suggesting that artificial light at night is detrimental to health and demonstrate that artificial light at night may be a potential novel risk factor for diabetes,” they write.
“Considering the coexistence of the diabetes epidemic and the widespread influence of light pollution at night, the positive associations indicate an urgent need for countries and governments to develop effective prevention and intervention policies and to protect people from the adverse health effects of light pollution at night,” the study authors stress.
Gareth Nye, PhD, senior lecturer at the University of Chester, England, agreed that prior research has found an association between metabolic conditions, such as diabetes, and artificial light at night, with most theories as to the cause focusing on the body’s natural circadian cycle.
He said that internal clocks regulate a variety of bodily processes, such as metabolism and hormone synthesis. They also affect sleep patterns by interfering with synthesis of the hormone melatonin, which is essential for sound sleep, Dr. Nye told the UK Science Media Centre.
However, he stressed that much more research is needed before any link can be considered definitive.
Outdoor night light exposure linked to fasting glucose, A1c
The Chinese researchers set out to approximate the relationships between diabetes prevalence and glucose homeostasis with chronic exposure to outdoor light at night.
They assessed 98,658 participants from the China Noncommunicable Disease Surveillance Study across 162 sites. The mean age of participants was 42.7 years. Female participants comprised 49.2% of the study cohort.
Diabetes was defined based on American Diabetes Association criteria. Satellite data were used to determine exposure to outdoor light at night in 2010. The associations between light exposure at night and indicators of glucose homeostasis were investigated.
Prevalence ratios were calculated and adjusted for sex, age, smoking status, education, body mass index, physical activity, household income, family history of diabetes, rural/urban areas, drinking status, and use of lipid-lowering prescription drugs (primarily statins) or antihypertensives.
The findings showed exposure levels to outdoor light at night were positively linked with 2-hour and fasting glucose concentrations, A1c, and insulin resistance (measured using homeostatic model assessment [HOMA]), but negatively related to β-cell function (measured using HOMA).
More research needed
“We advise caution against causal interpretation of the findings and call for further studies involving direct measurement of individual exposure to light at night,” the researchers conclude.
Dr. Nye agreed.
“One issue with this study is that the areas with the highest outdoor artificial light levels are likely to be those in urban areas and bigger cities. It has been known for a long time now that living in an urbanized area increases your risk of obesity through increased access to high-fat and convenience food, less physical activity levels due to transport links, and less social activities. The authors also state this and the fact participants tended to be older,” he noted.
Large datasets are used in this investigation, however, which generally increases the reliability of the data, he observed.
But it is also “unclear as to whether the population here was selected for this study or was retrospectively analyzed, which poses reliability issues, as does the selection of the representative sample, as it is not discussed,” he noted.
Ultimately, there is no confirmed evidence of the link, and until further work is done to directly link light exposure and diabetes in humans, “the link will remain an association only,” he concluded.
A version of this article first appeared on Medscape.com.
Higher levels of exposure to outdoor artificial light at night are significantly linked with markers of diabetes and impaired glucose homeostasis, in a new national, cross-sectional study from China.
The results showed a 7% significant increase in diabetes prevalence per quintile exposure to artificial light at night (prevalence ratio, 1.07), report Ruizhi Zheng, PhD, of the Shanghai (China) Jiaotong University School of Medicine, and colleagues. People living in areas with the most exposure to light at night had a 28% higher prevalence of diabetes than those living in places with the lowest exposure (PR, 1.28), the researchers found.
The study was published online in Diabetologia.
Previous animal studies have shown that exposure to light at night may interfere with circadian rhythms and affect glucose homeostasis, the study team note. Other research has demonstrated that chronic exposure to moderate indoor light during sleep elevated the prevalence of diabetes in older adults, compared with those sleeping in a dim setting, the authors add.
“Our findings contribute to the growing literature suggesting that artificial light at night is detrimental to health and demonstrate that artificial light at night may be a potential novel risk factor for diabetes,” they write.
“Considering the coexistence of the diabetes epidemic and the widespread influence of light pollution at night, the positive associations indicate an urgent need for countries and governments to develop effective prevention and intervention policies and to protect people from the adverse health effects of light pollution at night,” the study authors stress.
Gareth Nye, PhD, senior lecturer at the University of Chester, England, agreed that prior research has found an association between metabolic conditions, such as diabetes, and artificial light at night, with most theories as to the cause focusing on the body’s natural circadian cycle.
He said that internal clocks regulate a variety of bodily processes, such as metabolism and hormone synthesis. They also affect sleep patterns by interfering with synthesis of the hormone melatonin, which is essential for sound sleep, Dr. Nye told the UK Science Media Centre.
However, he stressed that much more research is needed before any link can be considered definitive.
Outdoor night light exposure linked to fasting glucose, A1c
The Chinese researchers set out to approximate the relationships between diabetes prevalence and glucose homeostasis with chronic exposure to outdoor light at night.
They assessed 98,658 participants from the China Noncommunicable Disease Surveillance Study across 162 sites. The mean age of participants was 42.7 years. Female participants comprised 49.2% of the study cohort.
Diabetes was defined based on American Diabetes Association criteria. Satellite data were used to determine exposure to outdoor light at night in 2010. The associations between light exposure at night and indicators of glucose homeostasis were investigated.
Prevalence ratios were calculated and adjusted for sex, age, smoking status, education, body mass index, physical activity, household income, family history of diabetes, rural/urban areas, drinking status, and use of lipid-lowering prescription drugs (primarily statins) or antihypertensives.
The findings showed exposure levels to outdoor light at night were positively linked with 2-hour and fasting glucose concentrations, A1c, and insulin resistance (measured using homeostatic model assessment [HOMA]), but negatively related to β-cell function (measured using HOMA).
More research needed
“We advise caution against causal interpretation of the findings and call for further studies involving direct measurement of individual exposure to light at night,” the researchers conclude.
Dr. Nye agreed.
“One issue with this study is that the areas with the highest outdoor artificial light levels are likely to be those in urban areas and bigger cities. It has been known for a long time now that living in an urbanized area increases your risk of obesity through increased access to high-fat and convenience food, less physical activity levels due to transport links, and less social activities. The authors also state this and the fact participants tended to be older,” he noted.
Large datasets are used in this investigation, however, which generally increases the reliability of the data, he observed.
But it is also “unclear as to whether the population here was selected for this study or was retrospectively analyzed, which poses reliability issues, as does the selection of the representative sample, as it is not discussed,” he noted.
Ultimately, there is no confirmed evidence of the link, and until further work is done to directly link light exposure and diabetes in humans, “the link will remain an association only,” he concluded.
A version of this article first appeared on Medscape.com.
Hypertension linked to risk of severe COVID
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
FROM PLOS ONE
More weight loss with surgery than new obesity meds: meta-analysis
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK®
Staving off holiday weight gain
Five pounds of weight gain during the holidays is a disproven myth that pops up annually like holiday lights. But before you do a happy dance and pile that extra whipped cream on your pie, you should know two things. One, people do gain weight during the holidays. Two, the extra pounds tend to stick around because most people never lose their holiday weight. Over time, these extra pounds can lead to obesity and weight-related conditions such as diabetes and hypertension.
Let’s be clear. Your weight is one of many markers of your wellness and metabolic health. However, weight changes can indicate that your health is off balance. Holiday weight gain often comes from indulging in increased rich foods, less physical activity, higher stress levels, and sleep disruption.
Optimizing lifestyle factors and trying to lose weight is challenging any time of the year. However, the holiday bustle makes losing weight during this time even more challenging for most people. But maintaining your weight and overall wellness is manageable with three simple shifts in mindset, mindful eating, and meal strategy. Let’s discuss each.
Mindset
From personal and professional experience, I see two primary attitudes regarding holiday eating. They are either “I’ll wait till January to go on a diet” or “I’m on a diet, so I can’t eat anything I like during the holidays.” Both attitude extremes prevent enjoyable and healthy eating during the holidays because they place the focus on food. With both mindsets, food is in control, which leaves you feeling out of control. Rather than having an “all or none” mindset during the holidays, I encourage you to ask yourself:
- “What matters most to me during the holidays?” In a recent survey, 72% of Americans said they look forward to during the holidays. Although food often accompanies family celebrations, it’s the time with family that matters most. Choose to savor sweet time spent with loved ones instead of stuffing yourself with excess sugary sweets.
- “How can I enjoy myself without food or alcoholic beverages?” So often, we eat or drink certain foods out of habit. Shift your mindset from “we always do this” to “what could we do instead?” Asking this question may be the doorway to creating new, non–food-centered traditions.
- “How can I have the foods I love during the holidays and still meet my weight and wellness goals?” This question helps you create opportunities instead of depriving yourself. Rather than depriving yourself, you could cut back on snacking or reduce your sugar intake elsewhere. Or add an extra workout session or stress reduction practice during the holidays.
Mindful eating
The purpose of mindful eating isn’t weight loss. Some studies suggest it may help maintain weight. More importantly, mindfulness can improve your relationship with food and promote wellness. Traditional tips for mindful eating include doing the following as you eat: Being present in the moment, not judging your food, slowing down, and savoring the taste of your food. During the holidays, asking additional questions may enhance mindful eating. For instance:
- “Am I eating to avoid uncomfortable emotions?” The holidays can trigger emotions such as grief, sadness, and anxiety. Also, preexisting can worsen. Decadent foods become a quick fix leading to more emotional eating during this season. Addressing these emotions can help you avoid overeating during the holidays. For mental health resources, visit the
- “What food or drink do I most enjoy during the holidays?” Trying to resist your favorite holiday treats can be an exhausting test of “willpower.” Eventually, and psychological reasons, and you “cheat” on your plan to not eat holiday treats. To prevent this painful battle of treat versus cheat, plan to eat your “indulgence food” in moderation. Savor the foods you enjoy. Then cut out the rest of the food you don’t like or feel you must eat because “Aunty Sarah will feel bad.”
Meal strategy
Many holiday treats and parties are unavoidable unless you plan to hide in a cave for the next few weeks. Rather than torturing yourself nibbling on celery and sipping on sparkling water during your holiday event, create a strategy. For 8 years, I’ve been on my weight loss and wellness journey. I have a holiday strategy that helps my patients, clients, and me maintain our weight and wellness during the holidays. One critical part of the strategy is to anticipate indulgence events. Specifically, look at all the planned holiday events and choose three indulgence events. The rest of the time, do your best to stay on your plan. Knowing your indulgence events to look forward to gives you a sense of control over when you indulge. On non-indulgent days, think, “I can eat it but choose not to” instead of the limiting thought, “I can’t eat that.” Choice is a powerful tool. Once at an indulgence event, I focus on mindful eating and enjoying people around me, which cuts down on overeating just because “I can.”
This holiday season is a reunion time for many people, after enduring long separations from family and friends due to the pandemic. Relishing time with loved ones should be your focus during the holidays – not eating yourself into worse health or worrying about dieting. Even if you choose not to make all the shifts in mindset, mindful eating, and meal strategy mentioned, choosing even one change to focus on can help you both enjoy the holidays and have increased control over your weight and wellness. Whatever you do, may you and your loved ones have a safe, healthy, and enjoyable holiday season.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist who specializes in individualized solutions for emotional and biological overeating. She is CEO and lead physician at Embrace You Weight and Wellness, Telehealth & Virtual Counseling. She has disclosed having no relevant financial relationships. A version of this article first appeared on Medscape.com.
Five pounds of weight gain during the holidays is a disproven myth that pops up annually like holiday lights. But before you do a happy dance and pile that extra whipped cream on your pie, you should know two things. One, people do gain weight during the holidays. Two, the extra pounds tend to stick around because most people never lose their holiday weight. Over time, these extra pounds can lead to obesity and weight-related conditions such as diabetes and hypertension.
Let’s be clear. Your weight is one of many markers of your wellness and metabolic health. However, weight changes can indicate that your health is off balance. Holiday weight gain often comes from indulging in increased rich foods, less physical activity, higher stress levels, and sleep disruption.
Optimizing lifestyle factors and trying to lose weight is challenging any time of the year. However, the holiday bustle makes losing weight during this time even more challenging for most people. But maintaining your weight and overall wellness is manageable with three simple shifts in mindset, mindful eating, and meal strategy. Let’s discuss each.
Mindset
From personal and professional experience, I see two primary attitudes regarding holiday eating. They are either “I’ll wait till January to go on a diet” or “I’m on a diet, so I can’t eat anything I like during the holidays.” Both attitude extremes prevent enjoyable and healthy eating during the holidays because they place the focus on food. With both mindsets, food is in control, which leaves you feeling out of control. Rather than having an “all or none” mindset during the holidays, I encourage you to ask yourself:
- “What matters most to me during the holidays?” In a recent survey, 72% of Americans said they look forward to during the holidays. Although food often accompanies family celebrations, it’s the time with family that matters most. Choose to savor sweet time spent with loved ones instead of stuffing yourself with excess sugary sweets.
- “How can I enjoy myself without food or alcoholic beverages?” So often, we eat or drink certain foods out of habit. Shift your mindset from “we always do this” to “what could we do instead?” Asking this question may be the doorway to creating new, non–food-centered traditions.
- “How can I have the foods I love during the holidays and still meet my weight and wellness goals?” This question helps you create opportunities instead of depriving yourself. Rather than depriving yourself, you could cut back on snacking or reduce your sugar intake elsewhere. Or add an extra workout session or stress reduction practice during the holidays.
Mindful eating
The purpose of mindful eating isn’t weight loss. Some studies suggest it may help maintain weight. More importantly, mindfulness can improve your relationship with food and promote wellness. Traditional tips for mindful eating include doing the following as you eat: Being present in the moment, not judging your food, slowing down, and savoring the taste of your food. During the holidays, asking additional questions may enhance mindful eating. For instance:
- “Am I eating to avoid uncomfortable emotions?” The holidays can trigger emotions such as grief, sadness, and anxiety. Also, preexisting can worsen. Decadent foods become a quick fix leading to more emotional eating during this season. Addressing these emotions can help you avoid overeating during the holidays. For mental health resources, visit the
- “What food or drink do I most enjoy during the holidays?” Trying to resist your favorite holiday treats can be an exhausting test of “willpower.” Eventually, and psychological reasons, and you “cheat” on your plan to not eat holiday treats. To prevent this painful battle of treat versus cheat, plan to eat your “indulgence food” in moderation. Savor the foods you enjoy. Then cut out the rest of the food you don’t like or feel you must eat because “Aunty Sarah will feel bad.”
Meal strategy
Many holiday treats and parties are unavoidable unless you plan to hide in a cave for the next few weeks. Rather than torturing yourself nibbling on celery and sipping on sparkling water during your holiday event, create a strategy. For 8 years, I’ve been on my weight loss and wellness journey. I have a holiday strategy that helps my patients, clients, and me maintain our weight and wellness during the holidays. One critical part of the strategy is to anticipate indulgence events. Specifically, look at all the planned holiday events and choose three indulgence events. The rest of the time, do your best to stay on your plan. Knowing your indulgence events to look forward to gives you a sense of control over when you indulge. On non-indulgent days, think, “I can eat it but choose not to” instead of the limiting thought, “I can’t eat that.” Choice is a powerful tool. Once at an indulgence event, I focus on mindful eating and enjoying people around me, which cuts down on overeating just because “I can.”
This holiday season is a reunion time for many people, after enduring long separations from family and friends due to the pandemic. Relishing time with loved ones should be your focus during the holidays – not eating yourself into worse health or worrying about dieting. Even if you choose not to make all the shifts in mindset, mindful eating, and meal strategy mentioned, choosing even one change to focus on can help you both enjoy the holidays and have increased control over your weight and wellness. Whatever you do, may you and your loved ones have a safe, healthy, and enjoyable holiday season.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist who specializes in individualized solutions for emotional and biological overeating. She is CEO and lead physician at Embrace You Weight and Wellness, Telehealth & Virtual Counseling. She has disclosed having no relevant financial relationships. A version of this article first appeared on Medscape.com.
Five pounds of weight gain during the holidays is a disproven myth that pops up annually like holiday lights. But before you do a happy dance and pile that extra whipped cream on your pie, you should know two things. One, people do gain weight during the holidays. Two, the extra pounds tend to stick around because most people never lose their holiday weight. Over time, these extra pounds can lead to obesity and weight-related conditions such as diabetes and hypertension.
Let’s be clear. Your weight is one of many markers of your wellness and metabolic health. However, weight changes can indicate that your health is off balance. Holiday weight gain often comes from indulging in increased rich foods, less physical activity, higher stress levels, and sleep disruption.
Optimizing lifestyle factors and trying to lose weight is challenging any time of the year. However, the holiday bustle makes losing weight during this time even more challenging for most people. But maintaining your weight and overall wellness is manageable with three simple shifts in mindset, mindful eating, and meal strategy. Let’s discuss each.
Mindset
From personal and professional experience, I see two primary attitudes regarding holiday eating. They are either “I’ll wait till January to go on a diet” or “I’m on a diet, so I can’t eat anything I like during the holidays.” Both attitude extremes prevent enjoyable and healthy eating during the holidays because they place the focus on food. With both mindsets, food is in control, which leaves you feeling out of control. Rather than having an “all or none” mindset during the holidays, I encourage you to ask yourself:
- “What matters most to me during the holidays?” In a recent survey, 72% of Americans said they look forward to during the holidays. Although food often accompanies family celebrations, it’s the time with family that matters most. Choose to savor sweet time spent with loved ones instead of stuffing yourself with excess sugary sweets.
- “How can I enjoy myself without food or alcoholic beverages?” So often, we eat or drink certain foods out of habit. Shift your mindset from “we always do this” to “what could we do instead?” Asking this question may be the doorway to creating new, non–food-centered traditions.
- “How can I have the foods I love during the holidays and still meet my weight and wellness goals?” This question helps you create opportunities instead of depriving yourself. Rather than depriving yourself, you could cut back on snacking or reduce your sugar intake elsewhere. Or add an extra workout session or stress reduction practice during the holidays.
Mindful eating
The purpose of mindful eating isn’t weight loss. Some studies suggest it may help maintain weight. More importantly, mindfulness can improve your relationship with food and promote wellness. Traditional tips for mindful eating include doing the following as you eat: Being present in the moment, not judging your food, slowing down, and savoring the taste of your food. During the holidays, asking additional questions may enhance mindful eating. For instance:
- “Am I eating to avoid uncomfortable emotions?” The holidays can trigger emotions such as grief, sadness, and anxiety. Also, preexisting can worsen. Decadent foods become a quick fix leading to more emotional eating during this season. Addressing these emotions can help you avoid overeating during the holidays. For mental health resources, visit the
- “What food or drink do I most enjoy during the holidays?” Trying to resist your favorite holiday treats can be an exhausting test of “willpower.” Eventually, and psychological reasons, and you “cheat” on your plan to not eat holiday treats. To prevent this painful battle of treat versus cheat, plan to eat your “indulgence food” in moderation. Savor the foods you enjoy. Then cut out the rest of the food you don’t like or feel you must eat because “Aunty Sarah will feel bad.”
Meal strategy
Many holiday treats and parties are unavoidable unless you plan to hide in a cave for the next few weeks. Rather than torturing yourself nibbling on celery and sipping on sparkling water during your holiday event, create a strategy. For 8 years, I’ve been on my weight loss and wellness journey. I have a holiday strategy that helps my patients, clients, and me maintain our weight and wellness during the holidays. One critical part of the strategy is to anticipate indulgence events. Specifically, look at all the planned holiday events and choose three indulgence events. The rest of the time, do your best to stay on your plan. Knowing your indulgence events to look forward to gives you a sense of control over when you indulge. On non-indulgent days, think, “I can eat it but choose not to” instead of the limiting thought, “I can’t eat that.” Choice is a powerful tool. Once at an indulgence event, I focus on mindful eating and enjoying people around me, which cuts down on overeating just because “I can.”
This holiday season is a reunion time for many people, after enduring long separations from family and friends due to the pandemic. Relishing time with loved ones should be your focus during the holidays – not eating yourself into worse health or worrying about dieting. Even if you choose not to make all the shifts in mindset, mindful eating, and meal strategy mentioned, choosing even one change to focus on can help you both enjoy the holidays and have increased control over your weight and wellness. Whatever you do, may you and your loved ones have a safe, healthy, and enjoyable holiday season.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist who specializes in individualized solutions for emotional and biological overeating. She is CEO and lead physician at Embrace You Weight and Wellness, Telehealth & Virtual Counseling. She has disclosed having no relevant financial relationships. A version of this article first appeared on Medscape.com.
Baxdrostat slashes BP in resistant hypertension: BrigHTN
CHICAGO – An investigational aldosterone synthase inhibitor could be an effective new treatment to reduce blood pressure in patients with treatment-resistant hypertension, reslts of a phase 2 study suggest.
The BrigHTN trial showed systolic blood pressure fell by an average of 20.3 mm Hg, 17.5 mm Hg, and 12.1 mm Hg with baxdrostat 2 mg, 1 mg, and 0.5 mg after 12 weeks follow-up in 248 patients unable to achieve target blood pressure on stable doses of at least three antihypertensive agents, including a diuretic.
After adjustment for the –9.4 mm Hg change observed in the placebo group, there was a statistically significant difference of 11.0 mm Hg in the 2-mg baxdrostat group (P = .0001) and of 8.1 mm Hg in the 1-mg baxdrostat group (P = .003).
The adjusted change in diastolic blood pressure was significant only for the 2-mg dose (–5.2 mm Hg; P = .004).
Once-daily oral baxdrostat had an acceptable side-effect profile and no patients died.
The study, which was stopped early after meeting criteria for overwhelming efficacy, was presented in the final late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Threading the needle
For at least 20 years, researchers have tried to create a drug that would lower aldosterone levels directly by inhibiting hormone synthesis rather than blocking the mineralocorticoid receptor.
What’s made this extraordinarily difficult is that the enzyme that makes aldosterone synthase and the enzyme required for cortisol synthase, 11-beta-hydroxylase, are 93% sequence similar. Baxdrostat, however, is able to selectively block aldosterone synthase, and thus the production of aldosterone, without also blocking the production of cortisol, explained Mason W. Freeman, MD, lead author of the study and executive vice president of clinical development at CinCor Pharma, which is developing the agent.
“We have beautiful biomarker evidence of not only blood pressure lowering but the mechanism by which that blood pressure reduction is occurring,” he said.
Over 12 weeks of follow-up in the new study, the use of baxdrostat led to decreases in serum aldosterone levels ranging from 3.0 ng/dL with the 0.5-mg dose to 4.9 ng/dL with the 2-mg dose. The 24-hour urinary aldosterone levels decreased with all three doses tested.
Baxdrostat increased plasma renin activity by 3.6, 5.0, and 13.8 mg/mL per hr with the 0.5, 1.0, and 2.0 mg doses, respectively, an indicator of its effect on lowering salt and fluid retention, Dr. Freeman said. Serum cortisol levels were not reduced in any of the baxdrostat groups throughout the study.
‘A bright future’
“It seems to have a bright future in the area of resistant hypertension, particularly in patients who are producing too much aldosterone,” said Suzanne Oparil, MD, invited discussant for the study and director of the Vascular Biology and Hypertension program at the University of Alabama at Birmingham.
She noted that aldosterone is a major contributor to the pathogenesis of resistant hypertension, which afflicts about 20% of the hypertensive population. Aldosterone antagonists are considered by many to be the best add-on treatment for resistant hypertension and do lower blood pressure.
“But they have major problems,” Dr. Oparil added. “Spironolactone, for example, causes hyperkalemia in many patients and adverse effects such as gynecomastia, erectile dysfunction, and feminization.”
Baxdrostat was well tolerated with no serious adverse events deemed related to treatment, Dr. Freeman reported. A total of 18 serious adverse events occurred in 10 patients, 6 of which were in a patient with urosepsis.
Ten adverse events of special interest occurred in eight patients, including one case of hypotension, three cases of hyponatremia, and six cases of hyperkalemia.
Potassium levels ranged from 6.0 to 6.3 mmol/L (6.0-6.3 mEq/L) in three patients and between 5.5 and 5.9 mmol/L (5.5-5.9 mEq/L) on at least two consecutive occasions in three others. Four of the patients were able to resume baxdrostat and complete the trial, whereas two patients discontinued treatment, one of whom was the patient with urosepsis.
Dr. Freeman pointed out that the study population was relatively diverse, with 33%-48% of participants of Hispanic or Latinx ethnicity and 23%-32% being Black.
At baseline, all patients had a seated blood pressure of at least 130/80 mm Hg (average 147.8/87.9 mm Hg) on a background therapy that included a diuretic in 100%, an agent targeting the renin-angiotensin-aldosterone system in 91%-96%, a beta-blocker in 52%-68%, and a calcium channel blocker in 64%-70%.
The study was not designed to test the benefits and risks of aldosterone synthase inhibition beyond 12 weeks and baxdrostat was not compared to alternative antihypertensives, he said. Additional limitations are that medication adherence was based on pill counts rather than drug analysis and enrolling only patients with an estimated glomerular filtration rate over 45 mL/min per 1.73m2 reduced the likelihood of hyperkalemia and other adverse events.
Nevertheless, “we think that these data suggest that baxdrostat has the potential to treat disorders associated with aldosterone excess, including hypertension and primary hyperaldosteronism,” Dr. Freeman said.
The intention is to carry the drug forward into additional phase 2 studies in chronic kidney disease and to begin a phase 3 study in hypertension in 2023, he noted.
The study was funded by CinCor Pharma. Dr. Freeman and three coauthors are employees of CinCor and receive stock-based compensation. The remaining authors have a financial relationship with CinRx Pharma, which has an equity stake in CinCor. Dr. Oparil reports grant/research support from Bayer, Higi, and Novartis; and serving on the scientific advisory board/expert committee for CinCor Pharma and Preventric Diagnostics.
A version of this article first appeared on Medscape.com.
CHICAGO – An investigational aldosterone synthase inhibitor could be an effective new treatment to reduce blood pressure in patients with treatment-resistant hypertension, reslts of a phase 2 study suggest.
The BrigHTN trial showed systolic blood pressure fell by an average of 20.3 mm Hg, 17.5 mm Hg, and 12.1 mm Hg with baxdrostat 2 mg, 1 mg, and 0.5 mg after 12 weeks follow-up in 248 patients unable to achieve target blood pressure on stable doses of at least three antihypertensive agents, including a diuretic.
After adjustment for the –9.4 mm Hg change observed in the placebo group, there was a statistically significant difference of 11.0 mm Hg in the 2-mg baxdrostat group (P = .0001) and of 8.1 mm Hg in the 1-mg baxdrostat group (P = .003).
The adjusted change in diastolic blood pressure was significant only for the 2-mg dose (–5.2 mm Hg; P = .004).
Once-daily oral baxdrostat had an acceptable side-effect profile and no patients died.
The study, which was stopped early after meeting criteria for overwhelming efficacy, was presented in the final late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Threading the needle
For at least 20 years, researchers have tried to create a drug that would lower aldosterone levels directly by inhibiting hormone synthesis rather than blocking the mineralocorticoid receptor.
What’s made this extraordinarily difficult is that the enzyme that makes aldosterone synthase and the enzyme required for cortisol synthase, 11-beta-hydroxylase, are 93% sequence similar. Baxdrostat, however, is able to selectively block aldosterone synthase, and thus the production of aldosterone, without also blocking the production of cortisol, explained Mason W. Freeman, MD, lead author of the study and executive vice president of clinical development at CinCor Pharma, which is developing the agent.
“We have beautiful biomarker evidence of not only blood pressure lowering but the mechanism by which that blood pressure reduction is occurring,” he said.
Over 12 weeks of follow-up in the new study, the use of baxdrostat led to decreases in serum aldosterone levels ranging from 3.0 ng/dL with the 0.5-mg dose to 4.9 ng/dL with the 2-mg dose. The 24-hour urinary aldosterone levels decreased with all three doses tested.
Baxdrostat increased plasma renin activity by 3.6, 5.0, and 13.8 mg/mL per hr with the 0.5, 1.0, and 2.0 mg doses, respectively, an indicator of its effect on lowering salt and fluid retention, Dr. Freeman said. Serum cortisol levels were not reduced in any of the baxdrostat groups throughout the study.
‘A bright future’
“It seems to have a bright future in the area of resistant hypertension, particularly in patients who are producing too much aldosterone,” said Suzanne Oparil, MD, invited discussant for the study and director of the Vascular Biology and Hypertension program at the University of Alabama at Birmingham.
She noted that aldosterone is a major contributor to the pathogenesis of resistant hypertension, which afflicts about 20% of the hypertensive population. Aldosterone antagonists are considered by many to be the best add-on treatment for resistant hypertension and do lower blood pressure.
“But they have major problems,” Dr. Oparil added. “Spironolactone, for example, causes hyperkalemia in many patients and adverse effects such as gynecomastia, erectile dysfunction, and feminization.”
Baxdrostat was well tolerated with no serious adverse events deemed related to treatment, Dr. Freeman reported. A total of 18 serious adverse events occurred in 10 patients, 6 of which were in a patient with urosepsis.
Ten adverse events of special interest occurred in eight patients, including one case of hypotension, three cases of hyponatremia, and six cases of hyperkalemia.
Potassium levels ranged from 6.0 to 6.3 mmol/L (6.0-6.3 mEq/L) in three patients and between 5.5 and 5.9 mmol/L (5.5-5.9 mEq/L) on at least two consecutive occasions in three others. Four of the patients were able to resume baxdrostat and complete the trial, whereas two patients discontinued treatment, one of whom was the patient with urosepsis.
Dr. Freeman pointed out that the study population was relatively diverse, with 33%-48% of participants of Hispanic or Latinx ethnicity and 23%-32% being Black.
At baseline, all patients had a seated blood pressure of at least 130/80 mm Hg (average 147.8/87.9 mm Hg) on a background therapy that included a diuretic in 100%, an agent targeting the renin-angiotensin-aldosterone system in 91%-96%, a beta-blocker in 52%-68%, and a calcium channel blocker in 64%-70%.
The study was not designed to test the benefits and risks of aldosterone synthase inhibition beyond 12 weeks and baxdrostat was not compared to alternative antihypertensives, he said. Additional limitations are that medication adherence was based on pill counts rather than drug analysis and enrolling only patients with an estimated glomerular filtration rate over 45 mL/min per 1.73m2 reduced the likelihood of hyperkalemia and other adverse events.
Nevertheless, “we think that these data suggest that baxdrostat has the potential to treat disorders associated with aldosterone excess, including hypertension and primary hyperaldosteronism,” Dr. Freeman said.
The intention is to carry the drug forward into additional phase 2 studies in chronic kidney disease and to begin a phase 3 study in hypertension in 2023, he noted.
The study was funded by CinCor Pharma. Dr. Freeman and three coauthors are employees of CinCor and receive stock-based compensation. The remaining authors have a financial relationship with CinRx Pharma, which has an equity stake in CinCor. Dr. Oparil reports grant/research support from Bayer, Higi, and Novartis; and serving on the scientific advisory board/expert committee for CinCor Pharma and Preventric Diagnostics.
A version of this article first appeared on Medscape.com.
CHICAGO – An investigational aldosterone synthase inhibitor could be an effective new treatment to reduce blood pressure in patients with treatment-resistant hypertension, reslts of a phase 2 study suggest.
The BrigHTN trial showed systolic blood pressure fell by an average of 20.3 mm Hg, 17.5 mm Hg, and 12.1 mm Hg with baxdrostat 2 mg, 1 mg, and 0.5 mg after 12 weeks follow-up in 248 patients unable to achieve target blood pressure on stable doses of at least three antihypertensive agents, including a diuretic.
After adjustment for the –9.4 mm Hg change observed in the placebo group, there was a statistically significant difference of 11.0 mm Hg in the 2-mg baxdrostat group (P = .0001) and of 8.1 mm Hg in the 1-mg baxdrostat group (P = .003).
The adjusted change in diastolic blood pressure was significant only for the 2-mg dose (–5.2 mm Hg; P = .004).
Once-daily oral baxdrostat had an acceptable side-effect profile and no patients died.
The study, which was stopped early after meeting criteria for overwhelming efficacy, was presented in the final late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Threading the needle
For at least 20 years, researchers have tried to create a drug that would lower aldosterone levels directly by inhibiting hormone synthesis rather than blocking the mineralocorticoid receptor.
What’s made this extraordinarily difficult is that the enzyme that makes aldosterone synthase and the enzyme required for cortisol synthase, 11-beta-hydroxylase, are 93% sequence similar. Baxdrostat, however, is able to selectively block aldosterone synthase, and thus the production of aldosterone, without also blocking the production of cortisol, explained Mason W. Freeman, MD, lead author of the study and executive vice president of clinical development at CinCor Pharma, which is developing the agent.
“We have beautiful biomarker evidence of not only blood pressure lowering but the mechanism by which that blood pressure reduction is occurring,” he said.
Over 12 weeks of follow-up in the new study, the use of baxdrostat led to decreases in serum aldosterone levels ranging from 3.0 ng/dL with the 0.5-mg dose to 4.9 ng/dL with the 2-mg dose. The 24-hour urinary aldosterone levels decreased with all three doses tested.
Baxdrostat increased plasma renin activity by 3.6, 5.0, and 13.8 mg/mL per hr with the 0.5, 1.0, and 2.0 mg doses, respectively, an indicator of its effect on lowering salt and fluid retention, Dr. Freeman said. Serum cortisol levels were not reduced in any of the baxdrostat groups throughout the study.
‘A bright future’
“It seems to have a bright future in the area of resistant hypertension, particularly in patients who are producing too much aldosterone,” said Suzanne Oparil, MD, invited discussant for the study and director of the Vascular Biology and Hypertension program at the University of Alabama at Birmingham.
She noted that aldosterone is a major contributor to the pathogenesis of resistant hypertension, which afflicts about 20% of the hypertensive population. Aldosterone antagonists are considered by many to be the best add-on treatment for resistant hypertension and do lower blood pressure.
“But they have major problems,” Dr. Oparil added. “Spironolactone, for example, causes hyperkalemia in many patients and adverse effects such as gynecomastia, erectile dysfunction, and feminization.”
Baxdrostat was well tolerated with no serious adverse events deemed related to treatment, Dr. Freeman reported. A total of 18 serious adverse events occurred in 10 patients, 6 of which were in a patient with urosepsis.
Ten adverse events of special interest occurred in eight patients, including one case of hypotension, three cases of hyponatremia, and six cases of hyperkalemia.
Potassium levels ranged from 6.0 to 6.3 mmol/L (6.0-6.3 mEq/L) in three patients and between 5.5 and 5.9 mmol/L (5.5-5.9 mEq/L) on at least two consecutive occasions in three others. Four of the patients were able to resume baxdrostat and complete the trial, whereas two patients discontinued treatment, one of whom was the patient with urosepsis.
Dr. Freeman pointed out that the study population was relatively diverse, with 33%-48% of participants of Hispanic or Latinx ethnicity and 23%-32% being Black.
At baseline, all patients had a seated blood pressure of at least 130/80 mm Hg (average 147.8/87.9 mm Hg) on a background therapy that included a diuretic in 100%, an agent targeting the renin-angiotensin-aldosterone system in 91%-96%, a beta-blocker in 52%-68%, and a calcium channel blocker in 64%-70%.
The study was not designed to test the benefits and risks of aldosterone synthase inhibition beyond 12 weeks and baxdrostat was not compared to alternative antihypertensives, he said. Additional limitations are that medication adherence was based on pill counts rather than drug analysis and enrolling only patients with an estimated glomerular filtration rate over 45 mL/min per 1.73m2 reduced the likelihood of hyperkalemia and other adverse events.
Nevertheless, “we think that these data suggest that baxdrostat has the potential to treat disorders associated with aldosterone excess, including hypertension and primary hyperaldosteronism,” Dr. Freeman said.
The intention is to carry the drug forward into additional phase 2 studies in chronic kidney disease and to begin a phase 3 study in hypertension in 2023, he noted.
The study was funded by CinCor Pharma. Dr. Freeman and three coauthors are employees of CinCor and receive stock-based compensation. The remaining authors have a financial relationship with CinRx Pharma, which has an equity stake in CinCor. Dr. Oparil reports grant/research support from Bayer, Higi, and Novartis; and serving on the scientific advisory board/expert committee for CinCor Pharma and Preventric Diagnostics.
A version of this article first appeared on Medscape.com.
AT AHA 2022