User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
FDA approves first-ever agent to delay type 1 diabetes onset
“Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” said John Sharretts, MD, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “The drug’s potential to delay clinical diagnosis of type 1 diabetes may provide patients with months to years without the burdens of disease.”
The agent, which interferes with T-cell-mediated autoimmune destruction of pancreatic beta cells, is the first disease-modifying therapy for impeding progression of type 1 diabetes. It is administered by intravenous infusion once daily for 14 consecutive days.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In type 1 diabetes staging, adopted in 2015, stage 1 is defined as the presence of beta cell autoimmunity with two or more islet autoantibodies with normoglycemia, stage 2 is beta-cell autoimmunity with dysglycemia yet asymptomatic, and stage 3 is the onset of symptomatic type 1 diabetes.
Stage 2 type 1 diabetes is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
The FDA had previously rejected teplizumab for this indication in July 2021, despite a prior endorsement from an advisory panel in May 2021.
Now, with the FDA approval, Provention Bio cofounder and CEO Ashleigh Palmer said in a statement, “This is a historic occasion for the T1D community and a paradigm shifting breakthrough ... It cannot be emphasized enough how precious a delay in the onset of stage 3 T1D can be from a patient and family perspective; more time to live without and, when necessary, prepare for the burdens, complications, and risks associated with stage 3 disease.”
T1D onset delayed by 2 years
In 2019, a pivotal phase 2, randomized, placebo-controlled trial involving 76 at-risk children and adults aged 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes compared with 32 who received placebo.
Those “game changer” data were presented at the American Diabetes Association (ADA) annual meeting in June 2019 and simultaneously published in the New England Journal of Medicine.
Three-year data were presented at the June 2020 ADA meeting and published in March 2021 in Science Translational Medicine, by Emily K. Sims, MD, department of pediatrics, Indiana University, Indianapolis, and colleagues.
At a median follow-up of 923 days, 50% of those randomly assigned to teplizumab remained diabetes free, compared with 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01). The teplizumab group had a greater average C-peptide area under the curve compared with placebo, reflecting improved beta-cell function (1.96 vs. 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“The mid-range time from randomization to stage 3 type 1 diabetes diagnosis was 50 months for the patients who received Tzield and 25 months for those who received a placebo. This represents a statistically significant delay in the development of stage 3 type 1 diabetes,” according to the FDA statement.
The most common side effects of Tzield include lymphopenia (73% teplizumab vs. 6% placebo), rash (36% vs. 0%), leukopenia (221% vs. 0%), and headache (11% vs. 6%). Label warnings and precautions include monitoring for cytokine release syndrome, risk for serious infections, and avoidance of live, inactivated, and mRNA vaccines.
This approval is likely to accelerate discussion about universal autoantibody screening. Currently, most individuals identified as having preclinical type 1 diabetes are first-degree relatives of people with type 1 diabetes identified through the federally funded TrialNet program. In December 2020, the type 1 diabetes research and advocacy organization JDRF began offering a $55 home blood test to screen for the antibodies, and other screening programs have been launched in the United States and Europe.
Previous studies have examined cost-effectiveness of universal screening in children and the optimal ages that such screening should take place.
In October, Provention Bio announced a co-promotion agreement with Sanofi for the U.S. launch of Tzield for delay in onset of clinical T1D in at-risk individuals. Provention Bio offers financial assistance options (e.g., copay assistance) to eligible patients for out-of-pocket costs.
A version of this article first appeared on Medscape.com.
“Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” said John Sharretts, MD, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “The drug’s potential to delay clinical diagnosis of type 1 diabetes may provide patients with months to years without the burdens of disease.”
The agent, which interferes with T-cell-mediated autoimmune destruction of pancreatic beta cells, is the first disease-modifying therapy for impeding progression of type 1 diabetes. It is administered by intravenous infusion once daily for 14 consecutive days.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In type 1 diabetes staging, adopted in 2015, stage 1 is defined as the presence of beta cell autoimmunity with two or more islet autoantibodies with normoglycemia, stage 2 is beta-cell autoimmunity with dysglycemia yet asymptomatic, and stage 3 is the onset of symptomatic type 1 diabetes.
Stage 2 type 1 diabetes is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
The FDA had previously rejected teplizumab for this indication in July 2021, despite a prior endorsement from an advisory panel in May 2021.
Now, with the FDA approval, Provention Bio cofounder and CEO Ashleigh Palmer said in a statement, “This is a historic occasion for the T1D community and a paradigm shifting breakthrough ... It cannot be emphasized enough how precious a delay in the onset of stage 3 T1D can be from a patient and family perspective; more time to live without and, when necessary, prepare for the burdens, complications, and risks associated with stage 3 disease.”
T1D onset delayed by 2 years
In 2019, a pivotal phase 2, randomized, placebo-controlled trial involving 76 at-risk children and adults aged 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes compared with 32 who received placebo.
Those “game changer” data were presented at the American Diabetes Association (ADA) annual meeting in June 2019 and simultaneously published in the New England Journal of Medicine.
Three-year data were presented at the June 2020 ADA meeting and published in March 2021 in Science Translational Medicine, by Emily K. Sims, MD, department of pediatrics, Indiana University, Indianapolis, and colleagues.
At a median follow-up of 923 days, 50% of those randomly assigned to teplizumab remained diabetes free, compared with 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01). The teplizumab group had a greater average C-peptide area under the curve compared with placebo, reflecting improved beta-cell function (1.96 vs. 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“The mid-range time from randomization to stage 3 type 1 diabetes diagnosis was 50 months for the patients who received Tzield and 25 months for those who received a placebo. This represents a statistically significant delay in the development of stage 3 type 1 diabetes,” according to the FDA statement.
The most common side effects of Tzield include lymphopenia (73% teplizumab vs. 6% placebo), rash (36% vs. 0%), leukopenia (221% vs. 0%), and headache (11% vs. 6%). Label warnings and precautions include monitoring for cytokine release syndrome, risk for serious infections, and avoidance of live, inactivated, and mRNA vaccines.
This approval is likely to accelerate discussion about universal autoantibody screening. Currently, most individuals identified as having preclinical type 1 diabetes are first-degree relatives of people with type 1 diabetes identified through the federally funded TrialNet program. In December 2020, the type 1 diabetes research and advocacy organization JDRF began offering a $55 home blood test to screen for the antibodies, and other screening programs have been launched in the United States and Europe.
Previous studies have examined cost-effectiveness of universal screening in children and the optimal ages that such screening should take place.
In October, Provention Bio announced a co-promotion agreement with Sanofi for the U.S. launch of Tzield for delay in onset of clinical T1D in at-risk individuals. Provention Bio offers financial assistance options (e.g., copay assistance) to eligible patients for out-of-pocket costs.
A version of this article first appeared on Medscape.com.
“Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” said John Sharretts, MD, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “The drug’s potential to delay clinical diagnosis of type 1 diabetes may provide patients with months to years without the burdens of disease.”
The agent, which interferes with T-cell-mediated autoimmune destruction of pancreatic beta cells, is the first disease-modifying therapy for impeding progression of type 1 diabetes. It is administered by intravenous infusion once daily for 14 consecutive days.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In type 1 diabetes staging, adopted in 2015, stage 1 is defined as the presence of beta cell autoimmunity with two or more islet autoantibodies with normoglycemia, stage 2 is beta-cell autoimmunity with dysglycemia yet asymptomatic, and stage 3 is the onset of symptomatic type 1 diabetes.
Stage 2 type 1 diabetes is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
The FDA had previously rejected teplizumab for this indication in July 2021, despite a prior endorsement from an advisory panel in May 2021.
Now, with the FDA approval, Provention Bio cofounder and CEO Ashleigh Palmer said in a statement, “This is a historic occasion for the T1D community and a paradigm shifting breakthrough ... It cannot be emphasized enough how precious a delay in the onset of stage 3 T1D can be from a patient and family perspective; more time to live without and, when necessary, prepare for the burdens, complications, and risks associated with stage 3 disease.”
T1D onset delayed by 2 years
In 2019, a pivotal phase 2, randomized, placebo-controlled trial involving 76 at-risk children and adults aged 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes compared with 32 who received placebo.
Those “game changer” data were presented at the American Diabetes Association (ADA) annual meeting in June 2019 and simultaneously published in the New England Journal of Medicine.
Three-year data were presented at the June 2020 ADA meeting and published in March 2021 in Science Translational Medicine, by Emily K. Sims, MD, department of pediatrics, Indiana University, Indianapolis, and colleagues.
At a median follow-up of 923 days, 50% of those randomly assigned to teplizumab remained diabetes free, compared with 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01). The teplizumab group had a greater average C-peptide area under the curve compared with placebo, reflecting improved beta-cell function (1.96 vs. 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“The mid-range time from randomization to stage 3 type 1 diabetes diagnosis was 50 months for the patients who received Tzield and 25 months for those who received a placebo. This represents a statistically significant delay in the development of stage 3 type 1 diabetes,” according to the FDA statement.
The most common side effects of Tzield include lymphopenia (73% teplizumab vs. 6% placebo), rash (36% vs. 0%), leukopenia (221% vs. 0%), and headache (11% vs. 6%). Label warnings and precautions include monitoring for cytokine release syndrome, risk for serious infections, and avoidance of live, inactivated, and mRNA vaccines.
This approval is likely to accelerate discussion about universal autoantibody screening. Currently, most individuals identified as having preclinical type 1 diabetes are first-degree relatives of people with type 1 diabetes identified through the federally funded TrialNet program. In December 2020, the type 1 diabetes research and advocacy organization JDRF began offering a $55 home blood test to screen for the antibodies, and other screening programs have been launched in the United States and Europe.
Previous studies have examined cost-effectiveness of universal screening in children and the optimal ages that such screening should take place.
In October, Provention Bio announced a co-promotion agreement with Sanofi for the U.S. launch of Tzield for delay in onset of clinical T1D in at-risk individuals. Provention Bio offers financial assistance options (e.g., copay assistance) to eligible patients for out-of-pocket costs.
A version of this article first appeared on Medscape.com.
Bepirovirsen: Is a ‘functional cure’ for HBV on the horizon?
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING
Celiac disease linked to higher risk for rheumatoid arthritis, juvenile idiopathic arthritis
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Higher metal contact allergy rates found in metalworkers
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS
Optimize HF meds rapidly and fully after hospital discharge: STRONG-HF
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Flu vaccination associated with reduced stroke risk
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM LANCET PUBLIC HEALTH
‘A huge deal’: Millions have long COVID, and more are expected
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
Clear toe lesion
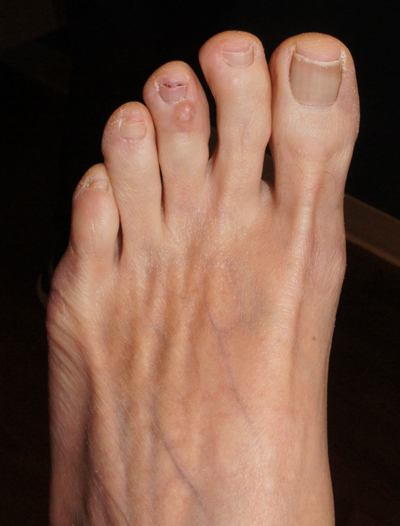
This is a digital mucous cyst, also known as a myxoid cyst. The clear to translucent appearance over a finger or toe joint is usually diagnosed clinically. If uncertain, a biopsy can confirm the diagnosis.
Digital mucous cysts are a type of ganglion cyst that is associated with trauma or arthritis in the toe joint. A microscopic opening in the joint capsule results in a fluid filled cyst in the surrounding tissue. If the cyst is ruptured, thick, gelatinous (sometimes blood-tinged) hyaluronic acid–rich fluid may escape. Sometimes, the cyst applies pressure to the nail matrix, causing a scooped out longitudinal nail deformity.
Digital mucous cysts more commonly affect the fingers than the toes. Although benign, patients may be bothered by the appearance of these cysts and their effect on nails. Observation is a reasonable approach. Rarely, digital mucous cysts resolve spontaneously.
Treatment options include cryotherapy, needle draining and scarification, and surgical excision with flap repair. Surgical excision may be performed quickly in the office and offers the highest cure rate of 95% in 1 study on fingers.1 Cryotherapy is successful in 70% of cases and needle drainage is successful in 39% of cases, but these modalities are quick and require minimal downtime.1
In this case, the patient was not significantly bothered by the lesion and was happy to forego treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Jabbour S, Kechichian E, Haber R, et al. Management of digital mucous cysts: a systematic review and treatment algorithm. Int J Dermatol. 2017;56:701-708. doi: 10.1111/ijd.13583

This is a digital mucous cyst, also known as a myxoid cyst. The clear to translucent appearance over a finger or toe joint is usually diagnosed clinically. If uncertain, a biopsy can confirm the diagnosis.
Digital mucous cysts are a type of ganglion cyst that is associated with trauma or arthritis in the toe joint. A microscopic opening in the joint capsule results in a fluid filled cyst in the surrounding tissue. If the cyst is ruptured, thick, gelatinous (sometimes blood-tinged) hyaluronic acid–rich fluid may escape. Sometimes, the cyst applies pressure to the nail matrix, causing a scooped out longitudinal nail deformity.
Digital mucous cysts more commonly affect the fingers than the toes. Although benign, patients may be bothered by the appearance of these cysts and their effect on nails. Observation is a reasonable approach. Rarely, digital mucous cysts resolve spontaneously.
Treatment options include cryotherapy, needle draining and scarification, and surgical excision with flap repair. Surgical excision may be performed quickly in the office and offers the highest cure rate of 95% in 1 study on fingers.1 Cryotherapy is successful in 70% of cases and needle drainage is successful in 39% of cases, but these modalities are quick and require minimal downtime.1
In this case, the patient was not significantly bothered by the lesion and was happy to forego treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This is a digital mucous cyst, also known as a myxoid cyst. The clear to translucent appearance over a finger or toe joint is usually diagnosed clinically. If uncertain, a biopsy can confirm the diagnosis.
Digital mucous cysts are a type of ganglion cyst that is associated with trauma or arthritis in the toe joint. A microscopic opening in the joint capsule results in a fluid filled cyst in the surrounding tissue. If the cyst is ruptured, thick, gelatinous (sometimes blood-tinged) hyaluronic acid–rich fluid may escape. Sometimes, the cyst applies pressure to the nail matrix, causing a scooped out longitudinal nail deformity.
Digital mucous cysts more commonly affect the fingers than the toes. Although benign, patients may be bothered by the appearance of these cysts and their effect on nails. Observation is a reasonable approach. Rarely, digital mucous cysts resolve spontaneously.
Treatment options include cryotherapy, needle draining and scarification, and surgical excision with flap repair. Surgical excision may be performed quickly in the office and offers the highest cure rate of 95% in 1 study on fingers.1 Cryotherapy is successful in 70% of cases and needle drainage is successful in 39% of cases, but these modalities are quick and require minimal downtime.1
In this case, the patient was not significantly bothered by the lesion and was happy to forego treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Jabbour S, Kechichian E, Haber R, et al. Management of digital mucous cysts: a systematic review and treatment algorithm. Int J Dermatol. 2017;56:701-708. doi: 10.1111/ijd.13583
1. Jabbour S, Kechichian E, Haber R, et al. Management of digital mucous cysts: a systematic review and treatment algorithm. Int J Dermatol. 2017;56:701-708. doi: 10.1111/ijd.13583
OSA raises risk of atrial fibrillation and stroke
compared with controls, based on data from 303 individuals.
OSA has become a common chronic disease, and cardiovascular diseases including AFib also are known independent risk factors associated with OSA, Anna Hojager, MD, of Zealand University Hospital, Roskilde, Denmark, and colleagues wrote. Previous studies have shown a significant increase in AFib risk in OSA patients with severe disease, but the prevalence of undiagnosed AFib in OSA patients has not been explored.
In a study published in Sleep Medicine, the researchers enrolled 238 adults with severe OSA (based on apnea-hypopnea index of 15 or higher) and 65 with mild or no OSA (based on an AHI of less than 15). The mean AHI across all participants was 34.2, and ranged from 0.2 to 115.8.
Participants underwent heart rhythm monitoring using a home system or standard ECG for 7 days; they were instructed to carry the device at all times except when showering or sweating heavily. The primary outcome was the detection of AFib, defined as at least one period of 30 seconds or longer with an irregular heart rhythm but without detectable evidence of another diagnosis. Sleep was assessed for one night using a portable sleep monitoring device. All participants were examined at baseline and measured for blood pressure, body mass index, waist-to-hip ratio, and ECG.
Overall, AFib occurred in 21 patients with moderate to severe OSA and 1 patient with mild/no OSA (8.8% vs. 1.5%, P = .045). The majority of patients across both groups had hypertension (66%) and dyslipidemia (77.6%), but the severe OSA group was more likely to be dysregulated and to have unknown prediabetes. Participants who were deemed candidates for anticoagulation therapy were referred for additional treatment. None of the 22 total patients with AFib had heart failure with reduced ejection fraction, and 68.2% had normal ejection fraction and ventricle function.
The researchers noted that no guidelines currently exist for systematic opportunistic screening for comorbidities in OSA patients, although the American Academy of Sleep Medicine recommends patient education as part of a multidisciplinary chronic disease management strategy. The high prevalence of AFib in OSA patients, as seen in the current study, “might warrant a recommendation of screening for paroxysmal [AFib] and could be valuable in the management of modifiable cardiovascular risk factors in patients with OSA,” they wrote.
The study findings were limited by several factors including the observational design and absence of polysomnography to assess OSA, the researchers noted. However, the study has the highest known prevalence of silent AFib in patients with moderate to severe OSA, and supports the value of screening and management for known comorbidities of OSA.
The study received no outside funding. The researchers had no financial conflicts to disclose.
compared with controls, based on data from 303 individuals.
OSA has become a common chronic disease, and cardiovascular diseases including AFib also are known independent risk factors associated with OSA, Anna Hojager, MD, of Zealand University Hospital, Roskilde, Denmark, and colleagues wrote. Previous studies have shown a significant increase in AFib risk in OSA patients with severe disease, but the prevalence of undiagnosed AFib in OSA patients has not been explored.
In a study published in Sleep Medicine, the researchers enrolled 238 adults with severe OSA (based on apnea-hypopnea index of 15 or higher) and 65 with mild or no OSA (based on an AHI of less than 15). The mean AHI across all participants was 34.2, and ranged from 0.2 to 115.8.
Participants underwent heart rhythm monitoring using a home system or standard ECG for 7 days; they were instructed to carry the device at all times except when showering or sweating heavily. The primary outcome was the detection of AFib, defined as at least one period of 30 seconds or longer with an irregular heart rhythm but without detectable evidence of another diagnosis. Sleep was assessed for one night using a portable sleep monitoring device. All participants were examined at baseline and measured for blood pressure, body mass index, waist-to-hip ratio, and ECG.
Overall, AFib occurred in 21 patients with moderate to severe OSA and 1 patient with mild/no OSA (8.8% vs. 1.5%, P = .045). The majority of patients across both groups had hypertension (66%) and dyslipidemia (77.6%), but the severe OSA group was more likely to be dysregulated and to have unknown prediabetes. Participants who were deemed candidates for anticoagulation therapy were referred for additional treatment. None of the 22 total patients with AFib had heart failure with reduced ejection fraction, and 68.2% had normal ejection fraction and ventricle function.
The researchers noted that no guidelines currently exist for systematic opportunistic screening for comorbidities in OSA patients, although the American Academy of Sleep Medicine recommends patient education as part of a multidisciplinary chronic disease management strategy. The high prevalence of AFib in OSA patients, as seen in the current study, “might warrant a recommendation of screening for paroxysmal [AFib] and could be valuable in the management of modifiable cardiovascular risk factors in patients with OSA,” they wrote.
The study findings were limited by several factors including the observational design and absence of polysomnography to assess OSA, the researchers noted. However, the study has the highest known prevalence of silent AFib in patients with moderate to severe OSA, and supports the value of screening and management for known comorbidities of OSA.
The study received no outside funding. The researchers had no financial conflicts to disclose.
compared with controls, based on data from 303 individuals.
OSA has become a common chronic disease, and cardiovascular diseases including AFib also are known independent risk factors associated with OSA, Anna Hojager, MD, of Zealand University Hospital, Roskilde, Denmark, and colleagues wrote. Previous studies have shown a significant increase in AFib risk in OSA patients with severe disease, but the prevalence of undiagnosed AFib in OSA patients has not been explored.
In a study published in Sleep Medicine, the researchers enrolled 238 adults with severe OSA (based on apnea-hypopnea index of 15 or higher) and 65 with mild or no OSA (based on an AHI of less than 15). The mean AHI across all participants was 34.2, and ranged from 0.2 to 115.8.
Participants underwent heart rhythm monitoring using a home system or standard ECG for 7 days; they were instructed to carry the device at all times except when showering or sweating heavily. The primary outcome was the detection of AFib, defined as at least one period of 30 seconds or longer with an irregular heart rhythm but without detectable evidence of another diagnosis. Sleep was assessed for one night using a portable sleep monitoring device. All participants were examined at baseline and measured for blood pressure, body mass index, waist-to-hip ratio, and ECG.
Overall, AFib occurred in 21 patients with moderate to severe OSA and 1 patient with mild/no OSA (8.8% vs. 1.5%, P = .045). The majority of patients across both groups had hypertension (66%) and dyslipidemia (77.6%), but the severe OSA group was more likely to be dysregulated and to have unknown prediabetes. Participants who were deemed candidates for anticoagulation therapy were referred for additional treatment. None of the 22 total patients with AFib had heart failure with reduced ejection fraction, and 68.2% had normal ejection fraction and ventricle function.
The researchers noted that no guidelines currently exist for systematic opportunistic screening for comorbidities in OSA patients, although the American Academy of Sleep Medicine recommends patient education as part of a multidisciplinary chronic disease management strategy. The high prevalence of AFib in OSA patients, as seen in the current study, “might warrant a recommendation of screening for paroxysmal [AFib] and could be valuable in the management of modifiable cardiovascular risk factors in patients with OSA,” they wrote.
The study findings were limited by several factors including the observational design and absence of polysomnography to assess OSA, the researchers noted. However, the study has the highest known prevalence of silent AFib in patients with moderate to severe OSA, and supports the value of screening and management for known comorbidities of OSA.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM SLEEP MEDICINE
Scaly forearm plaque
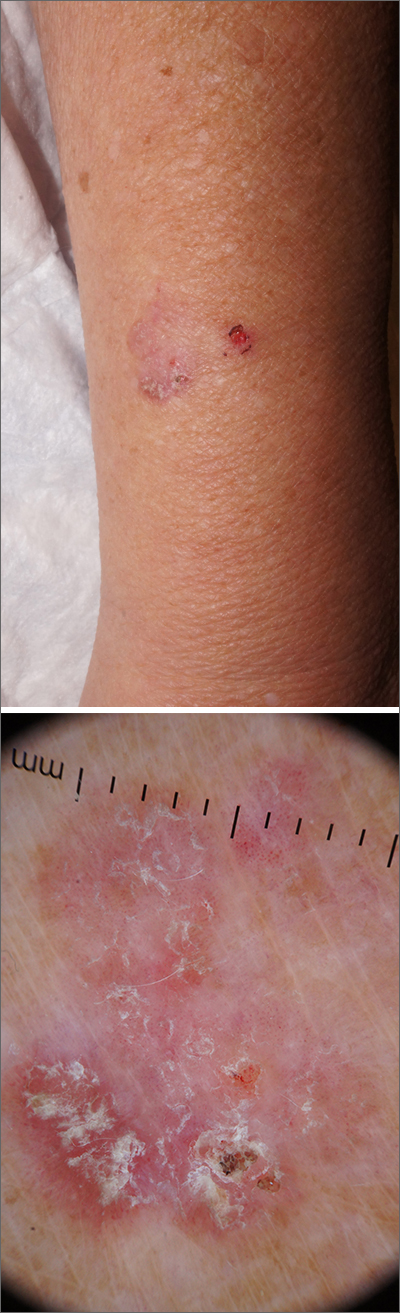
Dermoscopy revealed a keratotic, 2.5-cm scaly plaque with linearly arranged dotted vessels, ulceration, and shiny white lines. A shave biopsy was consistent with a squamous cell carcinoma in situ (SCC in situ)—a pre-invasive keratinocyte carcinoma.
SCC in situ, also known as Bowen’s disease, is a very common skin cancer that can be easily treated. Lesions may manifest anywhere on the skin but are most often found on sun-damaged areas. Actinic keratoses are a pre-malignant precursor of SCC in situ; both are characterized by a sandpapery rough surface on a pink or brown background. Histologically, SCC in situ has atypia of keratinocytes over the full thickness of the epidermis, while actinic keratoses have limited atypia of the upper epidermis only. With this in mind, suspect SCC in situ (over actinic keratosis) when a lesion is thicker than 1 mm, larger in diameter than 5 mm, or painful.1
Treatment options include surgical and nonsurgical modalities. Excision and electrodessication and curettage (EDC) are both effective surgical procedures, with cure rates greater than 90%.2 Nonsurgical options include cryotherapy, 5-fluorouracil (5FU), imiquimod, and photodynamic therapy. Treatment with 5FU or imiquimod involves the application of cream to the lesion for 4 to 6 weeks. Marked inflammation during treatment is to be expected.
In the case described here, the patient underwent EDC in the office and was counseled to continue with complete skin exams twice a year for the next 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012;148:1422-1423. doi: 10.1001/archdermatol.2012.3104
2. Reschly MJ, Shenefelt PD. Controversies in skin surgery: electrodessication and curettage versus excision for low-risk, small, well-differentiated squamous cell carcinomas. J Drugs Dermatol. 2010;9:773-776.

Dermoscopy revealed a keratotic, 2.5-cm scaly plaque with linearly arranged dotted vessels, ulceration, and shiny white lines. A shave biopsy was consistent with a squamous cell carcinoma in situ (SCC in situ)—a pre-invasive keratinocyte carcinoma.
SCC in situ, also known as Bowen’s disease, is a very common skin cancer that can be easily treated. Lesions may manifest anywhere on the skin but are most often found on sun-damaged areas. Actinic keratoses are a pre-malignant precursor of SCC in situ; both are characterized by a sandpapery rough surface on a pink or brown background. Histologically, SCC in situ has atypia of keratinocytes over the full thickness of the epidermis, while actinic keratoses have limited atypia of the upper epidermis only. With this in mind, suspect SCC in situ (over actinic keratosis) when a lesion is thicker than 1 mm, larger in diameter than 5 mm, or painful.1
Treatment options include surgical and nonsurgical modalities. Excision and electrodessication and curettage (EDC) are both effective surgical procedures, with cure rates greater than 90%.2 Nonsurgical options include cryotherapy, 5-fluorouracil (5FU), imiquimod, and photodynamic therapy. Treatment with 5FU or imiquimod involves the application of cream to the lesion for 4 to 6 weeks. Marked inflammation during treatment is to be expected.
In the case described here, the patient underwent EDC in the office and was counseled to continue with complete skin exams twice a year for the next 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Dermoscopy revealed a keratotic, 2.5-cm scaly plaque with linearly arranged dotted vessels, ulceration, and shiny white lines. A shave biopsy was consistent with a squamous cell carcinoma in situ (SCC in situ)—a pre-invasive keratinocyte carcinoma.
SCC in situ, also known as Bowen’s disease, is a very common skin cancer that can be easily treated. Lesions may manifest anywhere on the skin but are most often found on sun-damaged areas. Actinic keratoses are a pre-malignant precursor of SCC in situ; both are characterized by a sandpapery rough surface on a pink or brown background. Histologically, SCC in situ has atypia of keratinocytes over the full thickness of the epidermis, while actinic keratoses have limited atypia of the upper epidermis only. With this in mind, suspect SCC in situ (over actinic keratosis) when a lesion is thicker than 1 mm, larger in diameter than 5 mm, or painful.1
Treatment options include surgical and nonsurgical modalities. Excision and electrodessication and curettage (EDC) are both effective surgical procedures, with cure rates greater than 90%.2 Nonsurgical options include cryotherapy, 5-fluorouracil (5FU), imiquimod, and photodynamic therapy. Treatment with 5FU or imiquimod involves the application of cream to the lesion for 4 to 6 weeks. Marked inflammation during treatment is to be expected.
In the case described here, the patient underwent EDC in the office and was counseled to continue with complete skin exams twice a year for the next 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012;148:1422-1423. doi: 10.1001/archdermatol.2012.3104
2. Reschly MJ, Shenefelt PD. Controversies in skin surgery: electrodessication and curettage versus excision for low-risk, small, well-differentiated squamous cell carcinomas. J Drugs Dermatol. 2010;9:773-776.
1. Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012;148:1422-1423. doi: 10.1001/archdermatol.2012.3104
2. Reschly MJ, Shenefelt PD. Controversies in skin surgery: electrodessication and curettage versus excision for low-risk, small, well-differentiated squamous cell carcinomas. J Drugs Dermatol. 2010;9:773-776.