User login
Apps for your smart phone
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to sknews@frontlinemedcom.com and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
nmiller@frontlinemedcom.com On Twitter @NaseemSMiller
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to sknews@frontlinemedcom.com and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
nmiller@frontlinemedcom.com On Twitter @NaseemSMiller
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to sknews@frontlinemedcom.com and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
nmiller@frontlinemedcom.com On Twitter @NaseemSMiller
Technology is set to change dermatology practice
How can dermatologists cope with increasing patient loads in the face of finite resources and fixed time constraints? Dr. Jeffrey Benabio talks about promising new technology that may improve the efficiency and effectiveness of care, as well as the potential barriers to bringing these new tools into clinical practice.
How can dermatologists cope with increasing patient loads in the face of finite resources and fixed time constraints? Dr. Jeffrey Benabio talks about promising new technology that may improve the efficiency and effectiveness of care, as well as the potential barriers to bringing these new tools into clinical practice.
How can dermatologists cope with increasing patient loads in the face of finite resources and fixed time constraints? Dr. Jeffrey Benabio talks about promising new technology that may improve the efficiency and effectiveness of care, as well as the potential barriers to bringing these new tools into clinical practice.
Technology is set to change dermatology practice
How can dermatologists cope with increasing patient loads in the face of finite resources and fixed time constraints? Dr. Jeffrey Benabio talks about promising new technology that may improve the efficiency and effectiveness of care, as well as the potential barriers to bringing these new tools into clinical practice.
How can dermatologists cope with increasing patient loads in the face of finite resources and fixed time constraints? Dr. Jeffrey Benabio talks about promising new technology that may improve the efficiency and effectiveness of care, as well as the potential barriers to bringing these new tools into clinical practice.
How can dermatologists cope with increasing patient loads in the face of finite resources and fixed time constraints? Dr. Jeffrey Benabio talks about promising new technology that may improve the efficiency and effectiveness of care, as well as the potential barriers to bringing these new tools into clinical practice.
Fingolimod heart effects usually resolve within 6 hours
ORLANDO – The first-dose cardiovascular effects of fingolimod 0.5 mg were transient in most patients with multiple sclerosis and began to resolve within 6 hours after administration, according to analyses of two phase III trials and interim results of a phase IV study sponsored by the drug maker.
The analyses, which were presented in two posters at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers (CMSC) and the Americas Committee for Treatment and Research in Multiple Sclerosis, confirm that the initiation of treatment with fingolimod (Gilenya) is associated with a drop in heart rate (HR) and slowing of atrioventricular conduction, especially in the first 4-6 hours. Previously reported cases of bradycardia and atrioventricular blockhave been mostly transient and self-limited, the authors noted.
Fingolimod’s current prescribing information says that "All patients should be observed and receive hourly pulse and blood pressure measurement for at least 6 hours after first dose and undergo ECG predose and after the 6-hour observation." Its label was revised in May of last year following reports of sudden or unexplained deaths in the United States and Europe after the drug’s 2010 approval for relapsing forms of MS.
In the phase IV EPOC (Evaluate Patient Outcomes, Safety, and Tolerability of Fingolimod) study, 783 patients in the United States and Canada were randomized 3:1 to open-label treatment with once-daily fingolimod 0.5 mg or standard-of-care disease-modifying therapy (DMT) for 6 months.
All patients were fingolimod naive and received at least one dose of the therapy. They had received continual treatment of a single standard-of-care DMT for 6 months or more. The patients’ mean age was 46 years, and most were white (82%) and female (76%). The mean duration of their MS symptoms was 12 years.
Lead investigator Dr. Bruce L. Hughes of Ruan Multiple Sclerosis Center, Des Moines, Iowa, and his colleagues reported that the patients’ mean sitting HR at predose assessment dropped from a mean of 74.1 beats per minute at baseline to a nadir of 65.6 bpm, 5 hours after the therapy’s administration. HR began to recover by the 6th hour.
Most patients (98.6%) were discharged at 6 hours after treatment. Ten required extended observations after the 6th hour; three required a second day of observation and were discharged.
A total of 12 patients (1.5%) had bradycardia during the first-dose observation period. Eight were symptomatic, and four were asymptomatic. None required treatment, the researchers reported. The mean HR in this group decreased to 56.3 plus or minus 8.53 bpm, ranging from 38 to 64 bpm during bradycardia events. The patients recovered to 62.6 plus or minus 9.46 bpm, ranging from 52 to 80 bpm after the symptoms resolved.
Nearly 18% of the patients (137 of 783) had electrocardiography (ECG) performed at 6 hours post dose. In 28, the ECG differed from baseline, and the most common new findings were first-degree atrioventricular block (n = 11), and sinus bradycardia (n = 10). There were no second-degree AV blocks. Other findings included late anterior hemiblock (n = 1), atrial premature complex (n = 2), and biphasic T waves (n = 1).
The two other studies reported at the meeting also analyzed the first-dose cardiovascular effects of fingolimod 0.5 mg. The FIRST trial was a 4-month, open-label, phase IIIb study of 2,417 patients with relapsing MS, and the FREEDOMS II trial was a 2-year, double-blind, placebo-controlled, phase III study of 1,083 patients.
In the FIRST trial, the nadir HR occurred 4-5 hours post dose, and the mean decrease was 7.4 in patients without cardiac factors and 6.5 bpm in those with cardiac factors, the authors reported. The cardiac factors included beta-blocker and/or calcium channel blocker use (n = 120), resting HR of 45-54 bpm, Mobitz type I second-degree atrioventricular block, positive tilt test, or recurrent symptomatic bradycardia.
Dr. Simrat Randhawa and her colleagues at Novartis Pharmaceuticals reported that the mean decrease in HR was 7.2 and 7.3 bpm in patients without and with concomitant beta-blocker and/or calcium channel blocker use, respectively. One patient had a greater than 3-second pause in both screening and post dose ECG results. One patient discontinued the study drug because of second-degree AV block, the authors reported.
In the FREEDOMS II trial, the clinician-observed mean maximal decrease in HR was 8.5 bpm in the fingolimod group. The incidence of Mobitz I second-degree AV block with fingolimod was 3.7%, compared with 2.0% in placebo, while 2:1 AV block occurred in 2% of patients taking fingolimod patients and 0% taking placebo.
Most first-occurrence second-degree AV blocks were observed less than 6 hours after the first dose, the authors reported.
There were no Mobitz II or third-degree AV blocks reported in FIRST and FREEDOMS II.
"This is a good example of ‘I can’t predict who should get fingolimod, but I can say who I should be very careful with and maybe not give [them] fingolimod and give [them] something else,’ " said Dr. Robert P. Lisak, professor of neurology at Wayne State University, Detroit, and president-elect of the CMSC. "But once they’re on it, and if they don’t have other contraindications, they should be OK." Dr. Lisak was not involved in the study.
All the studies were supported by Novartis, which markets fingolimod. Dr. Hughes and another EPOC investigator have served as a speaker and/or advisory board member or received research support from Novartis and other companies involved in MS pharmaceutical research and development. Other investigators involved in the EPOC study are employees of Novartis. Dr. Lisak has received research grants from and has been an adviser for several companies, including, Avanir, Bayer, Novartis, Questcor, and Teva.
On Twitter @NaseemSMiller
ORLANDO – The first-dose cardiovascular effects of fingolimod 0.5 mg were transient in most patients with multiple sclerosis and began to resolve within 6 hours after administration, according to analyses of two phase III trials and interim results of a phase IV study sponsored by the drug maker.
The analyses, which were presented in two posters at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers (CMSC) and the Americas Committee for Treatment and Research in Multiple Sclerosis, confirm that the initiation of treatment with fingolimod (Gilenya) is associated with a drop in heart rate (HR) and slowing of atrioventricular conduction, especially in the first 4-6 hours. Previously reported cases of bradycardia and atrioventricular blockhave been mostly transient and self-limited, the authors noted.
Fingolimod’s current prescribing information says that "All patients should be observed and receive hourly pulse and blood pressure measurement for at least 6 hours after first dose and undergo ECG predose and after the 6-hour observation." Its label was revised in May of last year following reports of sudden or unexplained deaths in the United States and Europe after the drug’s 2010 approval for relapsing forms of MS.
In the phase IV EPOC (Evaluate Patient Outcomes, Safety, and Tolerability of Fingolimod) study, 783 patients in the United States and Canada were randomized 3:1 to open-label treatment with once-daily fingolimod 0.5 mg or standard-of-care disease-modifying therapy (DMT) for 6 months.
All patients were fingolimod naive and received at least one dose of the therapy. They had received continual treatment of a single standard-of-care DMT for 6 months or more. The patients’ mean age was 46 years, and most were white (82%) and female (76%). The mean duration of their MS symptoms was 12 years.
Lead investigator Dr. Bruce L. Hughes of Ruan Multiple Sclerosis Center, Des Moines, Iowa, and his colleagues reported that the patients’ mean sitting HR at predose assessment dropped from a mean of 74.1 beats per minute at baseline to a nadir of 65.6 bpm, 5 hours after the therapy’s administration. HR began to recover by the 6th hour.
Most patients (98.6%) were discharged at 6 hours after treatment. Ten required extended observations after the 6th hour; three required a second day of observation and were discharged.
A total of 12 patients (1.5%) had bradycardia during the first-dose observation period. Eight were symptomatic, and four were asymptomatic. None required treatment, the researchers reported. The mean HR in this group decreased to 56.3 plus or minus 8.53 bpm, ranging from 38 to 64 bpm during bradycardia events. The patients recovered to 62.6 plus or minus 9.46 bpm, ranging from 52 to 80 bpm after the symptoms resolved.
Nearly 18% of the patients (137 of 783) had electrocardiography (ECG) performed at 6 hours post dose. In 28, the ECG differed from baseline, and the most common new findings were first-degree atrioventricular block (n = 11), and sinus bradycardia (n = 10). There were no second-degree AV blocks. Other findings included late anterior hemiblock (n = 1), atrial premature complex (n = 2), and biphasic T waves (n = 1).
The two other studies reported at the meeting also analyzed the first-dose cardiovascular effects of fingolimod 0.5 mg. The FIRST trial was a 4-month, open-label, phase IIIb study of 2,417 patients with relapsing MS, and the FREEDOMS II trial was a 2-year, double-blind, placebo-controlled, phase III study of 1,083 patients.
In the FIRST trial, the nadir HR occurred 4-5 hours post dose, and the mean decrease was 7.4 in patients without cardiac factors and 6.5 bpm in those with cardiac factors, the authors reported. The cardiac factors included beta-blocker and/or calcium channel blocker use (n = 120), resting HR of 45-54 bpm, Mobitz type I second-degree atrioventricular block, positive tilt test, or recurrent symptomatic bradycardia.
Dr. Simrat Randhawa and her colleagues at Novartis Pharmaceuticals reported that the mean decrease in HR was 7.2 and 7.3 bpm in patients without and with concomitant beta-blocker and/or calcium channel blocker use, respectively. One patient had a greater than 3-second pause in both screening and post dose ECG results. One patient discontinued the study drug because of second-degree AV block, the authors reported.
In the FREEDOMS II trial, the clinician-observed mean maximal decrease in HR was 8.5 bpm in the fingolimod group. The incidence of Mobitz I second-degree AV block with fingolimod was 3.7%, compared with 2.0% in placebo, while 2:1 AV block occurred in 2% of patients taking fingolimod patients and 0% taking placebo.
Most first-occurrence second-degree AV blocks were observed less than 6 hours after the first dose, the authors reported.
There were no Mobitz II or third-degree AV blocks reported in FIRST and FREEDOMS II.
"This is a good example of ‘I can’t predict who should get fingolimod, but I can say who I should be very careful with and maybe not give [them] fingolimod and give [them] something else,’ " said Dr. Robert P. Lisak, professor of neurology at Wayne State University, Detroit, and president-elect of the CMSC. "But once they’re on it, and if they don’t have other contraindications, they should be OK." Dr. Lisak was not involved in the study.
All the studies were supported by Novartis, which markets fingolimod. Dr. Hughes and another EPOC investigator have served as a speaker and/or advisory board member or received research support from Novartis and other companies involved in MS pharmaceutical research and development. Other investigators involved in the EPOC study are employees of Novartis. Dr. Lisak has received research grants from and has been an adviser for several companies, including, Avanir, Bayer, Novartis, Questcor, and Teva.
On Twitter @NaseemSMiller
ORLANDO – The first-dose cardiovascular effects of fingolimod 0.5 mg were transient in most patients with multiple sclerosis and began to resolve within 6 hours after administration, according to analyses of two phase III trials and interim results of a phase IV study sponsored by the drug maker.
The analyses, which were presented in two posters at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers (CMSC) and the Americas Committee for Treatment and Research in Multiple Sclerosis, confirm that the initiation of treatment with fingolimod (Gilenya) is associated with a drop in heart rate (HR) and slowing of atrioventricular conduction, especially in the first 4-6 hours. Previously reported cases of bradycardia and atrioventricular blockhave been mostly transient and self-limited, the authors noted.
Fingolimod’s current prescribing information says that "All patients should be observed and receive hourly pulse and blood pressure measurement for at least 6 hours after first dose and undergo ECG predose and after the 6-hour observation." Its label was revised in May of last year following reports of sudden or unexplained deaths in the United States and Europe after the drug’s 2010 approval for relapsing forms of MS.
In the phase IV EPOC (Evaluate Patient Outcomes, Safety, and Tolerability of Fingolimod) study, 783 patients in the United States and Canada were randomized 3:1 to open-label treatment with once-daily fingolimod 0.5 mg or standard-of-care disease-modifying therapy (DMT) for 6 months.
All patients were fingolimod naive and received at least one dose of the therapy. They had received continual treatment of a single standard-of-care DMT for 6 months or more. The patients’ mean age was 46 years, and most were white (82%) and female (76%). The mean duration of their MS symptoms was 12 years.
Lead investigator Dr. Bruce L. Hughes of Ruan Multiple Sclerosis Center, Des Moines, Iowa, and his colleagues reported that the patients’ mean sitting HR at predose assessment dropped from a mean of 74.1 beats per minute at baseline to a nadir of 65.6 bpm, 5 hours after the therapy’s administration. HR began to recover by the 6th hour.
Most patients (98.6%) were discharged at 6 hours after treatment. Ten required extended observations after the 6th hour; three required a second day of observation and were discharged.
A total of 12 patients (1.5%) had bradycardia during the first-dose observation period. Eight were symptomatic, and four were asymptomatic. None required treatment, the researchers reported. The mean HR in this group decreased to 56.3 plus or minus 8.53 bpm, ranging from 38 to 64 bpm during bradycardia events. The patients recovered to 62.6 plus or minus 9.46 bpm, ranging from 52 to 80 bpm after the symptoms resolved.
Nearly 18% of the patients (137 of 783) had electrocardiography (ECG) performed at 6 hours post dose. In 28, the ECG differed from baseline, and the most common new findings were first-degree atrioventricular block (n = 11), and sinus bradycardia (n = 10). There were no second-degree AV blocks. Other findings included late anterior hemiblock (n = 1), atrial premature complex (n = 2), and biphasic T waves (n = 1).
The two other studies reported at the meeting also analyzed the first-dose cardiovascular effects of fingolimod 0.5 mg. The FIRST trial was a 4-month, open-label, phase IIIb study of 2,417 patients with relapsing MS, and the FREEDOMS II trial was a 2-year, double-blind, placebo-controlled, phase III study of 1,083 patients.
In the FIRST trial, the nadir HR occurred 4-5 hours post dose, and the mean decrease was 7.4 in patients without cardiac factors and 6.5 bpm in those with cardiac factors, the authors reported. The cardiac factors included beta-blocker and/or calcium channel blocker use (n = 120), resting HR of 45-54 bpm, Mobitz type I second-degree atrioventricular block, positive tilt test, or recurrent symptomatic bradycardia.
Dr. Simrat Randhawa and her colleagues at Novartis Pharmaceuticals reported that the mean decrease in HR was 7.2 and 7.3 bpm in patients without and with concomitant beta-blocker and/or calcium channel blocker use, respectively. One patient had a greater than 3-second pause in both screening and post dose ECG results. One patient discontinued the study drug because of second-degree AV block, the authors reported.
In the FREEDOMS II trial, the clinician-observed mean maximal decrease in HR was 8.5 bpm in the fingolimod group. The incidence of Mobitz I second-degree AV block with fingolimod was 3.7%, compared with 2.0% in placebo, while 2:1 AV block occurred in 2% of patients taking fingolimod patients and 0% taking placebo.
Most first-occurrence second-degree AV blocks were observed less than 6 hours after the first dose, the authors reported.
There were no Mobitz II or third-degree AV blocks reported in FIRST and FREEDOMS II.
"This is a good example of ‘I can’t predict who should get fingolimod, but I can say who I should be very careful with and maybe not give [them] fingolimod and give [them] something else,’ " said Dr. Robert P. Lisak, professor of neurology at Wayne State University, Detroit, and president-elect of the CMSC. "But once they’re on it, and if they don’t have other contraindications, they should be OK." Dr. Lisak was not involved in the study.
All the studies were supported by Novartis, which markets fingolimod. Dr. Hughes and another EPOC investigator have served as a speaker and/or advisory board member or received research support from Novartis and other companies involved in MS pharmaceutical research and development. Other investigators involved in the EPOC study are employees of Novartis. Dr. Lisak has received research grants from and has been an adviser for several companies, including, Avanir, Bayer, Novartis, Questcor, and Teva.
On Twitter @NaseemSMiller
AT THE CMSC/ACTRMS ANNUAL MEETING
Major finding: Patients’ mean sitting heart rate at predose assessment dropped from a mean of 74.1 bpm at baseline to a nadir of 65.6 bpm 5 hours after the therapy’s administration. HR began to recover by the 6th hour.
Data source: Analysis of two phase III trials, FIRST and FREEDOMS II, and the interim results of a phase IV study, EPOC.
Disclosures: All the studies were supported by Novartis, which markets fingolimod. Dr. Hughes and another EPOC investigator have served as a speaker and/or advisory board member or received research support from Novartis and other companies involved in MS pharmaceuticals research and development. Other investigators involved in the EPOC study report are employees of Novartis. Dr. Lisak has received research grants from and has been an adviser for several companies, including, Avanir, Bayer, Novartis, Questcor, and Teva.
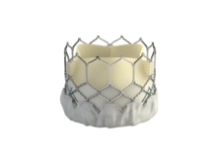
Edwards to start U.S. trial of SAPIEN 3
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
Brain volume loss may be greater in type 1 diabetics with microangiopathy
CHICAGO – Patients with type 1 diabetes and microangiopathy had a greater loss of executive function and brain volume over the course of 4 years compared with healthy controls, a small study showed.
Also, poorer glycemic control and higher systolic blood pressure at the beginning of the study were predictors of alterations in cognition and the brain over time, said Dr. Eelco van Duinkerken of VU University Medical Center, Amsterdam, who presented his abstract at the annual meeting of the American Diabetes Association. He added that the decline was not comparable with mild cognitive impairment.
Dr. van Duinkerken said that studies have shown that cognitive and structural changes in the brain are frequently found in patients with type 1 diabetes, particularly those with peripheral microangiopathy. But scientists don’t know yet how the brain’s structure and function change over time in adult patients with type 1 diabetes.
Dr. van Duinkerken and his colleagues studied 25 patients with type 1 diabetes who had microangiopathy. They were, on average, 46 years old at baseline; 40% were male, with an average IQ of 112 and a hemoglobin A1c level of 7.9. They had diabetes for at least 10 years. They had no disease affecting their brain, no psychiatric comorbidity, and no MRI contraindications.
The patients were compared with 25 closely matched controls (baseline age, 44 years; males, 52%; average IQ, 109; HbA1c, 5.4).
Researchers analyzed the patients’ general cognitive ability, memory, information-processing speed, executive function, attention, and motor and psychomotor speed at baseline and at follow-up, which was 4 years later.
They used a 3D-T1 structural MRI scan at baseline and follow-up to determine whole-brain volume loss.
After 4 years, the results showed that the study group had a significantly greater decline in executive function, compared with the control group (P = .030). Also, the study group showed a larger percentage of whole-brain volume loss (–1.34% vs. –0.68% in controls; P = .036), markedly in the right frontal and central areas.
A larger loss of frontal and central brain volume was related to an accelerated decline in executive function in both groups (P = .025). But a higher baseline HbA1c level was associated with a larger decline in executive performance (P = .003), and a higher baseline systolic blood pressure was correlated with frontal brain volume loss at the time of follow-up (P = .003).
"We need more long-term data on this," said Marjorie Cypress, Ph.D., who is the 2013 president-elect of the ADA’s health care and education committee and serves on the board of directors. "It’s concerning, obviously. And what else do we need to look at that may contribute to it. We need more studies," said Dr. Cypress, who was not involved in the study.
Dr. van Duinkerken has received research support from Novo Nordisk A/S. The study was supported by VU University Medical Center. Dr. Cypress reported having no financial disclosures relevant to the study.
*Correction, 8/23/2013: An earlier version of this story incorrectly reported the percentage of whole-brain volume loss in the Vitals.
On Twitter @NaseemSMiller
CHICAGO – Patients with type 1 diabetes and microangiopathy had a greater loss of executive function and brain volume over the course of 4 years compared with healthy controls, a small study showed.
Also, poorer glycemic control and higher systolic blood pressure at the beginning of the study were predictors of alterations in cognition and the brain over time, said Dr. Eelco van Duinkerken of VU University Medical Center, Amsterdam, who presented his abstract at the annual meeting of the American Diabetes Association. He added that the decline was not comparable with mild cognitive impairment.
Dr. van Duinkerken said that studies have shown that cognitive and structural changes in the brain are frequently found in patients with type 1 diabetes, particularly those with peripheral microangiopathy. But scientists don’t know yet how the brain’s structure and function change over time in adult patients with type 1 diabetes.
Dr. van Duinkerken and his colleagues studied 25 patients with type 1 diabetes who had microangiopathy. They were, on average, 46 years old at baseline; 40% were male, with an average IQ of 112 and a hemoglobin A1c level of 7.9. They had diabetes for at least 10 years. They had no disease affecting their brain, no psychiatric comorbidity, and no MRI contraindications.
The patients were compared with 25 closely matched controls (baseline age, 44 years; males, 52%; average IQ, 109; HbA1c, 5.4).
Researchers analyzed the patients’ general cognitive ability, memory, information-processing speed, executive function, attention, and motor and psychomotor speed at baseline and at follow-up, which was 4 years later.
They used a 3D-T1 structural MRI scan at baseline and follow-up to determine whole-brain volume loss.
After 4 years, the results showed that the study group had a significantly greater decline in executive function, compared with the control group (P = .030). Also, the study group showed a larger percentage of whole-brain volume loss (–1.34% vs. –0.68% in controls; P = .036), markedly in the right frontal and central areas.
A larger loss of frontal and central brain volume was related to an accelerated decline in executive function in both groups (P = .025). But a higher baseline HbA1c level was associated with a larger decline in executive performance (P = .003), and a higher baseline systolic blood pressure was correlated with frontal brain volume loss at the time of follow-up (P = .003).
"We need more long-term data on this," said Marjorie Cypress, Ph.D., who is the 2013 president-elect of the ADA’s health care and education committee and serves on the board of directors. "It’s concerning, obviously. And what else do we need to look at that may contribute to it. We need more studies," said Dr. Cypress, who was not involved in the study.
Dr. van Duinkerken has received research support from Novo Nordisk A/S. The study was supported by VU University Medical Center. Dr. Cypress reported having no financial disclosures relevant to the study.
*Correction, 8/23/2013: An earlier version of this story incorrectly reported the percentage of whole-brain volume loss in the Vitals.
On Twitter @NaseemSMiller
CHICAGO – Patients with type 1 diabetes and microangiopathy had a greater loss of executive function and brain volume over the course of 4 years compared with healthy controls, a small study showed.
Also, poorer glycemic control and higher systolic blood pressure at the beginning of the study were predictors of alterations in cognition and the brain over time, said Dr. Eelco van Duinkerken of VU University Medical Center, Amsterdam, who presented his abstract at the annual meeting of the American Diabetes Association. He added that the decline was not comparable with mild cognitive impairment.
Dr. van Duinkerken said that studies have shown that cognitive and structural changes in the brain are frequently found in patients with type 1 diabetes, particularly those with peripheral microangiopathy. But scientists don’t know yet how the brain’s structure and function change over time in adult patients with type 1 diabetes.
Dr. van Duinkerken and his colleagues studied 25 patients with type 1 diabetes who had microangiopathy. They were, on average, 46 years old at baseline; 40% were male, with an average IQ of 112 and a hemoglobin A1c level of 7.9. They had diabetes for at least 10 years. They had no disease affecting their brain, no psychiatric comorbidity, and no MRI contraindications.
The patients were compared with 25 closely matched controls (baseline age, 44 years; males, 52%; average IQ, 109; HbA1c, 5.4).
Researchers analyzed the patients’ general cognitive ability, memory, information-processing speed, executive function, attention, and motor and psychomotor speed at baseline and at follow-up, which was 4 years later.
They used a 3D-T1 structural MRI scan at baseline and follow-up to determine whole-brain volume loss.
After 4 years, the results showed that the study group had a significantly greater decline in executive function, compared with the control group (P = .030). Also, the study group showed a larger percentage of whole-brain volume loss (–1.34% vs. –0.68% in controls; P = .036), markedly in the right frontal and central areas.
A larger loss of frontal and central brain volume was related to an accelerated decline in executive function in both groups (P = .025). But a higher baseline HbA1c level was associated with a larger decline in executive performance (P = .003), and a higher baseline systolic blood pressure was correlated with frontal brain volume loss at the time of follow-up (P = .003).
"We need more long-term data on this," said Marjorie Cypress, Ph.D., who is the 2013 president-elect of the ADA’s health care and education committee and serves on the board of directors. "It’s concerning, obviously. And what else do we need to look at that may contribute to it. We need more studies," said Dr. Cypress, who was not involved in the study.
Dr. van Duinkerken has received research support from Novo Nordisk A/S. The study was supported by VU University Medical Center. Dr. Cypress reported having no financial disclosures relevant to the study.
*Correction, 8/23/2013: An earlier version of this story incorrectly reported the percentage of whole-brain volume loss in the Vitals.
On Twitter @NaseemSMiller
AT ADA 2013
Major finding: The study group showed a larger percentage of whole-brain volume loss (–1.34%* vs. –0.68% in controls; P = .036), markedly in the right frontal and central areas.
Data source: A total of 25 patients with type 1 diabetes who had microangiopathy, compared with 25 closely matched healthy participants.
Disclosures: Dr. van Duinkerken has received research support from Novo Nordisk A/S. The study was supported by VU University Medical Center. Dr. Cypress reported having no financial disclosures relevant to the study.
Analysis: Healthy babies born after accelerated elimination of teriflunomide
ORLANDO – When individuals with multiple sclerosis who were treated with teriflunomide and were using contraception, but became pregnant and followed the clinical program by stopping treatment and flushing the drug out of their systems, they had healthy babies, according to the drug maker’s analysis of several phase II and III trials.
The authors, who presented their findings as a poster at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis, said that there’s need for more prospective data on pregnancy outcomes, and a registry is planned.
The Food and Drug Administration approved teriflunomide, a once-daily oral medication for treating relapsing forms of multiple sclerosis, last year. The drug is currently classified as "X" and is contraindicated in pregnant women and women of childbearing age. Animal studies have shown that teriflunomide is teratogenic and embryo lethal, the authors wrote, although there has been no evidence that it affected the genes or fertility.
For those who worry about using the drug in women, "This is reassuring that if you follow the guidelines, it’s not going be an issue for mommies and daddies for teratogenicity," said Dr. Robert P. Lisak, professor of neurology at Wayne State University, Detroit, and president-elect of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Lisak, who was not involved in the analysis, but has been a part of one of the trials used in the analysis, said that his concern, as a practicing physician, is that women outside of clinical trials are not monitored as closely.
Women who are using the drug are advised to use contraception. Those who want to become pregnant are advised to discontinue treatment and undergo an elimination procedure with cholestyramine or activated charcoal until the drug’s plasma concentrations are less than 0.02 mcg/mL. Men should also stop treatment and undergo accelerated elimination.
Researchers summarized the results of nine phase II and III clinical trials in the MS clinical development program, which included pregnancy outcomes in female patients and partners of men who were exposed to teriflunomide.
The patients had received 7 mg or 14 mg teriflunomide, interferon-beta, placebo, or a combination of treatments. In all trials, reliable contraception was required, but pregnancies were reported.
From a total of more than 4,000 patients, there were 81 pregnancies, 63 of which occurred in women taking teriflunomide, and 18 in women who were on placebo or interferon-beta.
Of those 63, 20 were live births, 26 were induced abortions, 12 were miscarriages, and 5 pregnancies were ongoing when the data collection stopped on April 2013.
The live births in patients treated with teriflunomide resulted in healthy babies. Most of the women had discontinued the drug a few days to 11 weeks after becoming pregnant and underwent an accelerated elimination procedure after discontinuing treatment. Two refused to undergo the procedure. Seven became pregnant after completing the elimination procedure.
The rate of miscarriage in the teriflunomide group was 19%, which is within the range reported in the non–MS population (Eur. J. Obstet. Gynecol. Reprod. Biol. 2002;102:111-9), the authors wrote.
There were 20 pregnancies in partners of 17 men in the teriflunomide clinical trials. In 16, the father was exposed to the drug. There were 12 live births of healthy babies, 1 induced abortion, 1 miscarriage, and 2 ongoing pregnancies when data collection stopped.
Researchers said that planned teriflunomide pregnancy registries will provide more information, but data so far haven’t shown the teratogenic signal seen in the leflunomide registry.
Teriflunomide is the main active metabolite in rheumatoid arthritis drug leflunomide. Animal studies for leflunomide have shown embryo lethality and teratogenicity. But in small human studies, there was no significant difference in teratogenicity and spontaneous abortion rates, compared with the general population (Arthritis Rheum. 2010;62:1494-503; Arthritis Rheum. 2012;64:2085-94), the authors noted.
Teriflunomide also has been detected in human semen. Although there have been no animal studies, researchers said that the estimated female exposure via semen of men treated with the drug is expected to be 100 times lower than in those who actually take the drug with 14-mg dosing.
"Pregnancy outcomes among women who received teriflunomide, including rates of spontaneous abortion, and gestational age and weight at birth, are consistent with those for non–MS populations," the authors wrote. The treatment "is a therapeutic option for women of childbearing potential and for male patients with female partners of childbearing potential, when using reliable contraception."
Some of the authors were employees of Sanofi or had received research support from the company. The study was supported by Genzyme, a Sanofi company, which is marketed as teriflunomide (Aubagio).
On Twitter @naseemsmiller
ORLANDO – When individuals with multiple sclerosis who were treated with teriflunomide and were using contraception, but became pregnant and followed the clinical program by stopping treatment and flushing the drug out of their systems, they had healthy babies, according to the drug maker’s analysis of several phase II and III trials.
The authors, who presented their findings as a poster at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis, said that there’s need for more prospective data on pregnancy outcomes, and a registry is planned.
The Food and Drug Administration approved teriflunomide, a once-daily oral medication for treating relapsing forms of multiple sclerosis, last year. The drug is currently classified as "X" and is contraindicated in pregnant women and women of childbearing age. Animal studies have shown that teriflunomide is teratogenic and embryo lethal, the authors wrote, although there has been no evidence that it affected the genes or fertility.
For those who worry about using the drug in women, "This is reassuring that if you follow the guidelines, it’s not going be an issue for mommies and daddies for teratogenicity," said Dr. Robert P. Lisak, professor of neurology at Wayne State University, Detroit, and president-elect of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Lisak, who was not involved in the analysis, but has been a part of one of the trials used in the analysis, said that his concern, as a practicing physician, is that women outside of clinical trials are not monitored as closely.
Women who are using the drug are advised to use contraception. Those who want to become pregnant are advised to discontinue treatment and undergo an elimination procedure with cholestyramine or activated charcoal until the drug’s plasma concentrations are less than 0.02 mcg/mL. Men should also stop treatment and undergo accelerated elimination.
Researchers summarized the results of nine phase II and III clinical trials in the MS clinical development program, which included pregnancy outcomes in female patients and partners of men who were exposed to teriflunomide.
The patients had received 7 mg or 14 mg teriflunomide, interferon-beta, placebo, or a combination of treatments. In all trials, reliable contraception was required, but pregnancies were reported.
From a total of more than 4,000 patients, there were 81 pregnancies, 63 of which occurred in women taking teriflunomide, and 18 in women who were on placebo or interferon-beta.
Of those 63, 20 were live births, 26 were induced abortions, 12 were miscarriages, and 5 pregnancies were ongoing when the data collection stopped on April 2013.
The live births in patients treated with teriflunomide resulted in healthy babies. Most of the women had discontinued the drug a few days to 11 weeks after becoming pregnant and underwent an accelerated elimination procedure after discontinuing treatment. Two refused to undergo the procedure. Seven became pregnant after completing the elimination procedure.
The rate of miscarriage in the teriflunomide group was 19%, which is within the range reported in the non–MS population (Eur. J. Obstet. Gynecol. Reprod. Biol. 2002;102:111-9), the authors wrote.
There were 20 pregnancies in partners of 17 men in the teriflunomide clinical trials. In 16, the father was exposed to the drug. There were 12 live births of healthy babies, 1 induced abortion, 1 miscarriage, and 2 ongoing pregnancies when data collection stopped.
Researchers said that planned teriflunomide pregnancy registries will provide more information, but data so far haven’t shown the teratogenic signal seen in the leflunomide registry.
Teriflunomide is the main active metabolite in rheumatoid arthritis drug leflunomide. Animal studies for leflunomide have shown embryo lethality and teratogenicity. But in small human studies, there was no significant difference in teratogenicity and spontaneous abortion rates, compared with the general population (Arthritis Rheum. 2010;62:1494-503; Arthritis Rheum. 2012;64:2085-94), the authors noted.
Teriflunomide also has been detected in human semen. Although there have been no animal studies, researchers said that the estimated female exposure via semen of men treated with the drug is expected to be 100 times lower than in those who actually take the drug with 14-mg dosing.
"Pregnancy outcomes among women who received teriflunomide, including rates of spontaneous abortion, and gestational age and weight at birth, are consistent with those for non–MS populations," the authors wrote. The treatment "is a therapeutic option for women of childbearing potential and for male patients with female partners of childbearing potential, when using reliable contraception."
Some of the authors were employees of Sanofi or had received research support from the company. The study was supported by Genzyme, a Sanofi company, which is marketed as teriflunomide (Aubagio).
On Twitter @naseemsmiller
ORLANDO – When individuals with multiple sclerosis who were treated with teriflunomide and were using contraception, but became pregnant and followed the clinical program by stopping treatment and flushing the drug out of their systems, they had healthy babies, according to the drug maker’s analysis of several phase II and III trials.
The authors, who presented their findings as a poster at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis, said that there’s need for more prospective data on pregnancy outcomes, and a registry is planned.
The Food and Drug Administration approved teriflunomide, a once-daily oral medication for treating relapsing forms of multiple sclerosis, last year. The drug is currently classified as "X" and is contraindicated in pregnant women and women of childbearing age. Animal studies have shown that teriflunomide is teratogenic and embryo lethal, the authors wrote, although there has been no evidence that it affected the genes or fertility.
For those who worry about using the drug in women, "This is reassuring that if you follow the guidelines, it’s not going be an issue for mommies and daddies for teratogenicity," said Dr. Robert P. Lisak, professor of neurology at Wayne State University, Detroit, and president-elect of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Lisak, who was not involved in the analysis, but has been a part of one of the trials used in the analysis, said that his concern, as a practicing physician, is that women outside of clinical trials are not monitored as closely.
Women who are using the drug are advised to use contraception. Those who want to become pregnant are advised to discontinue treatment and undergo an elimination procedure with cholestyramine or activated charcoal until the drug’s plasma concentrations are less than 0.02 mcg/mL. Men should also stop treatment and undergo accelerated elimination.
Researchers summarized the results of nine phase II and III clinical trials in the MS clinical development program, which included pregnancy outcomes in female patients and partners of men who were exposed to teriflunomide.
The patients had received 7 mg or 14 mg teriflunomide, interferon-beta, placebo, or a combination of treatments. In all trials, reliable contraception was required, but pregnancies were reported.
From a total of more than 4,000 patients, there were 81 pregnancies, 63 of which occurred in women taking teriflunomide, and 18 in women who were on placebo or interferon-beta.
Of those 63, 20 were live births, 26 were induced abortions, 12 were miscarriages, and 5 pregnancies were ongoing when the data collection stopped on April 2013.
The live births in patients treated with teriflunomide resulted in healthy babies. Most of the women had discontinued the drug a few days to 11 weeks after becoming pregnant and underwent an accelerated elimination procedure after discontinuing treatment. Two refused to undergo the procedure. Seven became pregnant after completing the elimination procedure.
The rate of miscarriage in the teriflunomide group was 19%, which is within the range reported in the non–MS population (Eur. J. Obstet. Gynecol. Reprod. Biol. 2002;102:111-9), the authors wrote.
There were 20 pregnancies in partners of 17 men in the teriflunomide clinical trials. In 16, the father was exposed to the drug. There were 12 live births of healthy babies, 1 induced abortion, 1 miscarriage, and 2 ongoing pregnancies when data collection stopped.
Researchers said that planned teriflunomide pregnancy registries will provide more information, but data so far haven’t shown the teratogenic signal seen in the leflunomide registry.
Teriflunomide is the main active metabolite in rheumatoid arthritis drug leflunomide. Animal studies for leflunomide have shown embryo lethality and teratogenicity. But in small human studies, there was no significant difference in teratogenicity and spontaneous abortion rates, compared with the general population (Arthritis Rheum. 2010;62:1494-503; Arthritis Rheum. 2012;64:2085-94), the authors noted.
Teriflunomide also has been detected in human semen. Although there have been no animal studies, researchers said that the estimated female exposure via semen of men treated with the drug is expected to be 100 times lower than in those who actually take the drug with 14-mg dosing.
"Pregnancy outcomes among women who received teriflunomide, including rates of spontaneous abortion, and gestational age and weight at birth, are consistent with those for non–MS populations," the authors wrote. The treatment "is a therapeutic option for women of childbearing potential and for male patients with female partners of childbearing potential, when using reliable contraception."
Some of the authors were employees of Sanofi or had received research support from the company. The study was supported by Genzyme, a Sanofi company, which is marketed as teriflunomide (Aubagio).
On Twitter @naseemsmiller
AT CMSC/ACTRIMS ANNUAL MEETING
Major finding: Women and partners of men treated with teriflunomide who became pregnant and underwent accelerated elimination procedure had healthy babies.
Data source: Pregnancy outcomes in female patients and partners of men who were exposed to teriflunomide in nine phase II and III clinical trials.
Disclosures: Some of the authors were employees of Sanofi or had received research support from the company. The study was supported by Genzyme, a Sanofi company, which is marketed as teriflunomide (Aubagio).
Analysis: Estrogen therapy after hysterectomy may have saved lives
Thousands of women in their 50s who had a hysterectomy may have died prematurely since 2002 because they did not use estrogen-only therapy, according to a mathematical analysis of data from the Women’s Health Initiative.
The use of estrogen therapy (ET) has been on a steady decline since 2002, when the Women’s Health Initiative (WHI) halted its trial of estrogen plus progestin due to adverse events, which sent shockwaves among women and the medical community. The therapy’s decline has continued even after recent WHI studies showed mortality benefits from estrogen therapy.
"We felt a sense of urgency about this project," said Dr. David L. Katz, director of the Yale University Prevention Research Center at Griffin Hospital, Derby, Conn., who developed the formula for the analysis.
"Our calculation is simple and robust, and there was really nothing aggressive about our assumptions. The urgency we feel is in getting the word out about the fact that women were dying every year as a result of unwillingness to talk about estrogen therapy," he said in an interview.
"The Mortality Toll of Estrogen Avoidance," part of the title of the study, is a mathematical analysis of the 2011 WHI-ET (Women’s Health Initiative Estrogen-Alone Trial) data, showing that a minimum of 18,600 and as many as 91,600 excess deaths occurred between 2002 and 2011 among hysterectomized women aged 50-59 years due to ET avoidance (Am. J. Public Health 2013 [doi: 10.2105/AJPH.2013.301295]).
In the 1990s, more than 90% of women in their 50s who had a hysterectomy used ET. It was the standard treatment. Research has consistently shown that ET is cardioprotective and bone protective, and relieves menopausal symptoms.
But all that came to a screeching halt in July 2002, when the WHI published the results of the Estrogen Plus Progestin Trial, and terminated the study because of the adverse effects of the therapy, which was the combination drug Prempro. The results were quickly generalized to all forms of hormone therapy, including ET, the authors of the analysis said.
In less than 2 years, half of the women who were using systemic hormone therapy stopped the treatment. Compared with 2001, use of oral estrogen-only among women aged 50-59 years with no uterus dropped by almost 60% in 2004, 71% by 2006, and 79% in 2010 and 2011, the authors noted.
The decline continued despite the positive findings of WHI-ET, first published in 2004, then in 2011, showing that the absolute total mortality risk was reduced by 13 per 10,000 women per year among hysterectomized women aged 50-59 years who were using estrogen during the 10-year follow-up (JAMA 2011;305:1305-14).
"I said to everyone that this is the most important paper in the last 10 years," said Dr. Philip M. Sarrel, one of the authors of the analysis, and emeritus professor of obstetrics and gynecology and psychiatry at Yale University, New Haven, Conn. "I said it’s got to have an impact. It hit the news, and 24 hours later it was gone. There was no impact. They came out and said here’s a lifesaving set of data, and the message just wasn’t heard."
The authors offered several reasons for why the study did not gain traction, but they pointed to clear communication as one of the main pitfalls.
"We’re not criticizing the WHI investigators," Dr. Sarrel said in an interview. "We’re critical of how the nuanced findings were presented."
"We believe that a mortality toll will better communicate the meaning and significance of the WHI-ET findings to women, health care providers, and the media," the authors wrote.
Despite repeated requests, WHI investigators said they were not available to comment.
For their analysis, the researchers used the WHI’s 13 per 10,000 women per year as a point estimate for the mortality burden associated with not using estrogen among this specific group of women. Dr. Katz developed a formula that would apply the excess mortality in women aged 50-59 years who had a hysterectomy to the entire population of comparable women in the United States.
There were more than 49,000 excess deaths over 10 years when the researchers applied the lower estimate for hysterectomy rate in the population. The extreme low estimate showed nearly 22,700 deaths; a higher estimated rate showed almost 59,500 excess deaths, and the extreme high estimate approximately 91,600.
They also calculated the mortality toll of estrogen avoidance for women whose ovaries were retained. When the lower hysterectomy estimates were applied, the sum of excess mortality for both groups was 40,300, the low-end estimate was 18,600, the higher estimate 48,800, and the high-end estimate 75,100.
The range of excess deaths was estimated to be approximately 40,300-48,800, when the researchers used the best available point estimate values with year-by-year adjustment, and adjustment for differential rates of estrogen use with and without retaining ovaries at hysterectomy.
"If you choose to believe that the formula is correct, then this is a very impressive paper," said Dr. David M. Jaspan, a pelvic surgeon at Einstein Medical Center in Philadelphia, who was not involved in the study. "Others may say you’re looking at a paper where the authors came up with their own calculation using ‘extrapolated’ data to generate their own results, so it’s garbage in, garbage out.
"In my opinion, this paper was successful in making the reader think about what we’re doing and think about the data we have, and think, ‘Are we extrapolating the information to patients who do not fit the WHI model?’ This paper should allow the reader to think about the postmenopausal patient population as individuals rather than all postmenopausal women as a group," he said in an interview.
The study authors emphasized that they were not being prescriptive, and that discussions about hormone therapy should be individualized.
"We’re not saying that the WHI harmed anybody," said Dr. Katz. "The only reason we know that there’s a survival advantage with estrogen is because of the WHI. What we’re lamenting is the oversimplified translation of WHI findings into headlines, which have characterized hormone replacement as all bad. Where medicine meets media, we have lumped together baby and bathwater. That’s the problem we’re trying to fix."
The study had several limitations. The estimates may be lower than they actually are because some of the hysterectomies are now done laparoscopically outside of the hospitals and were not taken into account in the calculations. The authors noted that they used the decline in use of oral ET-only for their estimates. They also did not include transdermal ET use, which was included in the WHI studies, and is found to be more effective than oral estrogen in preventing cardiovascular events. Meanwhile, the use of vaginal estrogen has increased between 2001 and 2009, but its effect on mortality needs further study, they said.
Dr. Katz had no disclosures. Dr. Sarrel has been a medical consultant for Noven Therapeutics, which is the maker of transdermal estrogen patches. Dr. Jaspan had no relevant disclosures. The article was funded by the Centers for Disease Control and Prevention.
nmiller@frontlinemedcom.com
On Twitter @naseemsmiller
Thousands of women in their 50s who had a hysterectomy may have died prematurely since 2002 because they did not use estrogen-only therapy, according to a mathematical analysis of data from the Women’s Health Initiative.
The use of estrogen therapy (ET) has been on a steady decline since 2002, when the Women’s Health Initiative (WHI) halted its trial of estrogen plus progestin due to adverse events, which sent shockwaves among women and the medical community. The therapy’s decline has continued even after recent WHI studies showed mortality benefits from estrogen therapy.
"We felt a sense of urgency about this project," said Dr. David L. Katz, director of the Yale University Prevention Research Center at Griffin Hospital, Derby, Conn., who developed the formula for the analysis.
"Our calculation is simple and robust, and there was really nothing aggressive about our assumptions. The urgency we feel is in getting the word out about the fact that women were dying every year as a result of unwillingness to talk about estrogen therapy," he said in an interview.
"The Mortality Toll of Estrogen Avoidance," part of the title of the study, is a mathematical analysis of the 2011 WHI-ET (Women’s Health Initiative Estrogen-Alone Trial) data, showing that a minimum of 18,600 and as many as 91,600 excess deaths occurred between 2002 and 2011 among hysterectomized women aged 50-59 years due to ET avoidance (Am. J. Public Health 2013 [doi: 10.2105/AJPH.2013.301295]).
In the 1990s, more than 90% of women in their 50s who had a hysterectomy used ET. It was the standard treatment. Research has consistently shown that ET is cardioprotective and bone protective, and relieves menopausal symptoms.
But all that came to a screeching halt in July 2002, when the WHI published the results of the Estrogen Plus Progestin Trial, and terminated the study because of the adverse effects of the therapy, which was the combination drug Prempro. The results were quickly generalized to all forms of hormone therapy, including ET, the authors of the analysis said.
In less than 2 years, half of the women who were using systemic hormone therapy stopped the treatment. Compared with 2001, use of oral estrogen-only among women aged 50-59 years with no uterus dropped by almost 60% in 2004, 71% by 2006, and 79% in 2010 and 2011, the authors noted.
The decline continued despite the positive findings of WHI-ET, first published in 2004, then in 2011, showing that the absolute total mortality risk was reduced by 13 per 10,000 women per year among hysterectomized women aged 50-59 years who were using estrogen during the 10-year follow-up (JAMA 2011;305:1305-14).
"I said to everyone that this is the most important paper in the last 10 years," said Dr. Philip M. Sarrel, one of the authors of the analysis, and emeritus professor of obstetrics and gynecology and psychiatry at Yale University, New Haven, Conn. "I said it’s got to have an impact. It hit the news, and 24 hours later it was gone. There was no impact. They came out and said here’s a lifesaving set of data, and the message just wasn’t heard."
The authors offered several reasons for why the study did not gain traction, but they pointed to clear communication as one of the main pitfalls.
"We’re not criticizing the WHI investigators," Dr. Sarrel said in an interview. "We’re critical of how the nuanced findings were presented."
"We believe that a mortality toll will better communicate the meaning and significance of the WHI-ET findings to women, health care providers, and the media," the authors wrote.
Despite repeated requests, WHI investigators said they were not available to comment.
For their analysis, the researchers used the WHI’s 13 per 10,000 women per year as a point estimate for the mortality burden associated with not using estrogen among this specific group of women. Dr. Katz developed a formula that would apply the excess mortality in women aged 50-59 years who had a hysterectomy to the entire population of comparable women in the United States.
There were more than 49,000 excess deaths over 10 years when the researchers applied the lower estimate for hysterectomy rate in the population. The extreme low estimate showed nearly 22,700 deaths; a higher estimated rate showed almost 59,500 excess deaths, and the extreme high estimate approximately 91,600.
They also calculated the mortality toll of estrogen avoidance for women whose ovaries were retained. When the lower hysterectomy estimates were applied, the sum of excess mortality for both groups was 40,300, the low-end estimate was 18,600, the higher estimate 48,800, and the high-end estimate 75,100.
The range of excess deaths was estimated to be approximately 40,300-48,800, when the researchers used the best available point estimate values with year-by-year adjustment, and adjustment for differential rates of estrogen use with and without retaining ovaries at hysterectomy.
"If you choose to believe that the formula is correct, then this is a very impressive paper," said Dr. David M. Jaspan, a pelvic surgeon at Einstein Medical Center in Philadelphia, who was not involved in the study. "Others may say you’re looking at a paper where the authors came up with their own calculation using ‘extrapolated’ data to generate their own results, so it’s garbage in, garbage out.
"In my opinion, this paper was successful in making the reader think about what we’re doing and think about the data we have, and think, ‘Are we extrapolating the information to patients who do not fit the WHI model?’ This paper should allow the reader to think about the postmenopausal patient population as individuals rather than all postmenopausal women as a group," he said in an interview.
The study authors emphasized that they were not being prescriptive, and that discussions about hormone therapy should be individualized.
"We’re not saying that the WHI harmed anybody," said Dr. Katz. "The only reason we know that there’s a survival advantage with estrogen is because of the WHI. What we’re lamenting is the oversimplified translation of WHI findings into headlines, which have characterized hormone replacement as all bad. Where medicine meets media, we have lumped together baby and bathwater. That’s the problem we’re trying to fix."
The study had several limitations. The estimates may be lower than they actually are because some of the hysterectomies are now done laparoscopically outside of the hospitals and were not taken into account in the calculations. The authors noted that they used the decline in use of oral ET-only for their estimates. They also did not include transdermal ET use, which was included in the WHI studies, and is found to be more effective than oral estrogen in preventing cardiovascular events. Meanwhile, the use of vaginal estrogen has increased between 2001 and 2009, but its effect on mortality needs further study, they said.
Dr. Katz had no disclosures. Dr. Sarrel has been a medical consultant for Noven Therapeutics, which is the maker of transdermal estrogen patches. Dr. Jaspan had no relevant disclosures. The article was funded by the Centers for Disease Control and Prevention.
nmiller@frontlinemedcom.com
On Twitter @naseemsmiller
Thousands of women in their 50s who had a hysterectomy may have died prematurely since 2002 because they did not use estrogen-only therapy, according to a mathematical analysis of data from the Women’s Health Initiative.
The use of estrogen therapy (ET) has been on a steady decline since 2002, when the Women’s Health Initiative (WHI) halted its trial of estrogen plus progestin due to adverse events, which sent shockwaves among women and the medical community. The therapy’s decline has continued even after recent WHI studies showed mortality benefits from estrogen therapy.
"We felt a sense of urgency about this project," said Dr. David L. Katz, director of the Yale University Prevention Research Center at Griffin Hospital, Derby, Conn., who developed the formula for the analysis.
"Our calculation is simple and robust, and there was really nothing aggressive about our assumptions. The urgency we feel is in getting the word out about the fact that women were dying every year as a result of unwillingness to talk about estrogen therapy," he said in an interview.
"The Mortality Toll of Estrogen Avoidance," part of the title of the study, is a mathematical analysis of the 2011 WHI-ET (Women’s Health Initiative Estrogen-Alone Trial) data, showing that a minimum of 18,600 and as many as 91,600 excess deaths occurred between 2002 and 2011 among hysterectomized women aged 50-59 years due to ET avoidance (Am. J. Public Health 2013 [doi: 10.2105/AJPH.2013.301295]).
In the 1990s, more than 90% of women in their 50s who had a hysterectomy used ET. It was the standard treatment. Research has consistently shown that ET is cardioprotective and bone protective, and relieves menopausal symptoms.
But all that came to a screeching halt in July 2002, when the WHI published the results of the Estrogen Plus Progestin Trial, and terminated the study because of the adverse effects of the therapy, which was the combination drug Prempro. The results were quickly generalized to all forms of hormone therapy, including ET, the authors of the analysis said.
In less than 2 years, half of the women who were using systemic hormone therapy stopped the treatment. Compared with 2001, use of oral estrogen-only among women aged 50-59 years with no uterus dropped by almost 60% in 2004, 71% by 2006, and 79% in 2010 and 2011, the authors noted.
The decline continued despite the positive findings of WHI-ET, first published in 2004, then in 2011, showing that the absolute total mortality risk was reduced by 13 per 10,000 women per year among hysterectomized women aged 50-59 years who were using estrogen during the 10-year follow-up (JAMA 2011;305:1305-14).
"I said to everyone that this is the most important paper in the last 10 years," said Dr. Philip M. Sarrel, one of the authors of the analysis, and emeritus professor of obstetrics and gynecology and psychiatry at Yale University, New Haven, Conn. "I said it’s got to have an impact. It hit the news, and 24 hours later it was gone. There was no impact. They came out and said here’s a lifesaving set of data, and the message just wasn’t heard."
The authors offered several reasons for why the study did not gain traction, but they pointed to clear communication as one of the main pitfalls.
"We’re not criticizing the WHI investigators," Dr. Sarrel said in an interview. "We’re critical of how the nuanced findings were presented."
"We believe that a mortality toll will better communicate the meaning and significance of the WHI-ET findings to women, health care providers, and the media," the authors wrote.
Despite repeated requests, WHI investigators said they were not available to comment.
For their analysis, the researchers used the WHI’s 13 per 10,000 women per year as a point estimate for the mortality burden associated with not using estrogen among this specific group of women. Dr. Katz developed a formula that would apply the excess mortality in women aged 50-59 years who had a hysterectomy to the entire population of comparable women in the United States.
There were more than 49,000 excess deaths over 10 years when the researchers applied the lower estimate for hysterectomy rate in the population. The extreme low estimate showed nearly 22,700 deaths; a higher estimated rate showed almost 59,500 excess deaths, and the extreme high estimate approximately 91,600.
They also calculated the mortality toll of estrogen avoidance for women whose ovaries were retained. When the lower hysterectomy estimates were applied, the sum of excess mortality for both groups was 40,300, the low-end estimate was 18,600, the higher estimate 48,800, and the high-end estimate 75,100.
The range of excess deaths was estimated to be approximately 40,300-48,800, when the researchers used the best available point estimate values with year-by-year adjustment, and adjustment for differential rates of estrogen use with and without retaining ovaries at hysterectomy.
"If you choose to believe that the formula is correct, then this is a very impressive paper," said Dr. David M. Jaspan, a pelvic surgeon at Einstein Medical Center in Philadelphia, who was not involved in the study. "Others may say you’re looking at a paper where the authors came up with their own calculation using ‘extrapolated’ data to generate their own results, so it’s garbage in, garbage out.
"In my opinion, this paper was successful in making the reader think about what we’re doing and think about the data we have, and think, ‘Are we extrapolating the information to patients who do not fit the WHI model?’ This paper should allow the reader to think about the postmenopausal patient population as individuals rather than all postmenopausal women as a group," he said in an interview.
The study authors emphasized that they were not being prescriptive, and that discussions about hormone therapy should be individualized.
"We’re not saying that the WHI harmed anybody," said Dr. Katz. "The only reason we know that there’s a survival advantage with estrogen is because of the WHI. What we’re lamenting is the oversimplified translation of WHI findings into headlines, which have characterized hormone replacement as all bad. Where medicine meets media, we have lumped together baby and bathwater. That’s the problem we’re trying to fix."
The study had several limitations. The estimates may be lower than they actually are because some of the hysterectomies are now done laparoscopically outside of the hospitals and were not taken into account in the calculations. The authors noted that they used the decline in use of oral ET-only for their estimates. They also did not include transdermal ET use, which was included in the WHI studies, and is found to be more effective than oral estrogen in preventing cardiovascular events. Meanwhile, the use of vaginal estrogen has increased between 2001 and 2009, but its effect on mortality needs further study, they said.
Dr. Katz had no disclosures. Dr. Sarrel has been a medical consultant for Noven Therapeutics, which is the maker of transdermal estrogen patches. Dr. Jaspan had no relevant disclosures. The article was funded by the Centers for Disease Control and Prevention.
nmiller@frontlinemedcom.com
On Twitter @naseemsmiller
FROM THE AMERICAN JOURNAL OF PUBLIC HEALTH
Major finding: A minimum of 18,600 and as many as 91,600 excess deaths occurred between 2002 and 2011 among hysterectomized women aged 50-59 years due to estrogen therapy avoidance
Data source: The 2011 Women’s Health Initiative Estrogen-Only trial
Disclosures: Dr. Katz had no disclosures. Dr. Sarrel has been a medical consultant for Noven Therapeutics, which is the maker of transdermal estrogen patches. Dr. Jaspan had no relevant disclosures. The article was funded by the Centers for Disease Control and Prevention.
Glucose management program reduced hospital costs
CHICAGO – A centralized glucose management program at Johns Hopkins Hospital had a significant impact on hospital costs, and reduced the in-hospital mortality rate and length of stay for patients with diabetes or hyperglycemia, a 3-year analysis showed.
"This policy is important ... to make the hospital more efficient and less costly," said Dr. Elias K. Spanakis of Johns Hopkins University, Baltimore, who presented his abstract at the annual scientific sessions of the American Diabetes Association.
Studies have shown that it is associated with increased mortality, higher risk of infections, and prolonged length of stay (J. Clin. Endocrinol. Metab. 2002;87:978-82 and Diabetes Care 1999;22:1408-14).
Many hospitals now have hypoglycemia policies in place, said Dr. Spanakis, and there have been other studies on the topic. But what sets this study apart from previous research is the fact that it is long term and also considers multiple glycemia-related policies. Most of the previous studies have been done on isolated policies, and over a short period of time, he said.
In 2006, Johns Hopkins Hospital, a 1,000-bed tertiary referral center, developed an institutional glucose management committee led by an endocrinologist and a diabetes nurse practitioner.
The program was implemented between January 2006 and December 2009 and included ongoing staff education. Its individual elements were developed and implemented gradually: hospital-wide hypoglycemia policies, diabetes nursing super-user program, hospital-wide hyperglycemia order set and policy, and hyperglycemia order sets.
Specifically, the policies included a subcutaneous insulin order set, guidelines for calculation and management of insulin doses, and a transition protocol from intravenous to subcutaneous insulin, Dr. Spanakis said. The computer-based smart hyperglycemia order set gave dosing recommendations based on four clinical questions: including the patient’s type of diabetes, type of insulin, total daily dose of insulin, and the nutrition source.
By the end of 2009, the hospital showed a sustained reduction in hyperglycemic episodes. However, to know whether the program had any impact on in-hospital mortality rate, length of stay and total and daily hospital costs required a closer look.
Dr. Spanakis and colleagues conducted a retrospective cohort study of 16,537 unique admissions for patients who had a diagnosis code for diabetes or hyperglycemia during the program’s 4-year stint. Patients who were nonpregnant adults in non–critical care units were excluded.
Researchers compared the results of four consecutive intervention periods with the baseline (January to June 2006). The intervention periods included the implementation of hypoglycemia policy at the hospital (July to Dec. 2006), the diabetes nursing super-user program (January to November 2007), the hyperglycemia order set and policy (December 2007 to November 2008), and finally the smart hyperglycemia order set (December 2008 to December 2009).
They used three models:
• Model 1 adjusted for age, sex, and race.
• Model 2 adjusted for model 1 plus hyperglycemia.
• Model 3 adjusted for model 2 plus mortality risk index, severity of illness index, and hospital unit.
In all three models, researchers found more than $3,000 in reductions in hospital costs per admission in the final period compared with baseline, which was statistically significant in all models. The researchers are still collecting data in this area, Dr. Spanakis said.
For mortality rate ratio, model 1 showed a significant 68% reduction in mortality in the final period of the study versus baseline. The significance persisted in model 2.
However, in model 3, the mortality rate ratio became statistically nonsignificant (0.53; P = .08), showing a 47% reduction in the final period, compared with baseline.
Length of stay was also significantly reduced in models 1 and 2, but lost its significance in model 3 (0.54 fewer days; P = .15).
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress."
Dr. Spanakis said that although the mortality rate and length of stay did not reach statistical significance after the full adjustment in model 3, the policies did have some effect.
The study had some limitations, including its retrospective design. Also, the interventions were not randomized and "secular trends in other inpatient practices may have impacted the outcomes," Dr. Spanakis said.
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress," Thomas J. Hoerger, Ph.D., a health economist and senior fellow at RTI International, Research Triangle Park, N.C., said in an interview. He was not involved in the study.
Dr. Spanakis said further studies could determine which specific program components and policies are most effective in reducing hospital costs, in-patient mortality, and length of stay in patients with diabetes and hyperglycemia.
Dr. Spanakis had no disclosures.
On Twitter @NaseemSMiller
CHICAGO – A centralized glucose management program at Johns Hopkins Hospital had a significant impact on hospital costs, and reduced the in-hospital mortality rate and length of stay for patients with diabetes or hyperglycemia, a 3-year analysis showed.
"This policy is important ... to make the hospital more efficient and less costly," said Dr. Elias K. Spanakis of Johns Hopkins University, Baltimore, who presented his abstract at the annual scientific sessions of the American Diabetes Association.
Studies have shown that it is associated with increased mortality, higher risk of infections, and prolonged length of stay (J. Clin. Endocrinol. Metab. 2002;87:978-82 and Diabetes Care 1999;22:1408-14).
Many hospitals now have hypoglycemia policies in place, said Dr. Spanakis, and there have been other studies on the topic. But what sets this study apart from previous research is the fact that it is long term and also considers multiple glycemia-related policies. Most of the previous studies have been done on isolated policies, and over a short period of time, he said.
In 2006, Johns Hopkins Hospital, a 1,000-bed tertiary referral center, developed an institutional glucose management committee led by an endocrinologist and a diabetes nurse practitioner.
The program was implemented between January 2006 and December 2009 and included ongoing staff education. Its individual elements were developed and implemented gradually: hospital-wide hypoglycemia policies, diabetes nursing super-user program, hospital-wide hyperglycemia order set and policy, and hyperglycemia order sets.
Specifically, the policies included a subcutaneous insulin order set, guidelines for calculation and management of insulin doses, and a transition protocol from intravenous to subcutaneous insulin, Dr. Spanakis said. The computer-based smart hyperglycemia order set gave dosing recommendations based on four clinical questions: including the patient’s type of diabetes, type of insulin, total daily dose of insulin, and the nutrition source.
By the end of 2009, the hospital showed a sustained reduction in hyperglycemic episodes. However, to know whether the program had any impact on in-hospital mortality rate, length of stay and total and daily hospital costs required a closer look.
Dr. Spanakis and colleagues conducted a retrospective cohort study of 16,537 unique admissions for patients who had a diagnosis code for diabetes or hyperglycemia during the program’s 4-year stint. Patients who were nonpregnant adults in non–critical care units were excluded.
Researchers compared the results of four consecutive intervention periods with the baseline (January to June 2006). The intervention periods included the implementation of hypoglycemia policy at the hospital (July to Dec. 2006), the diabetes nursing super-user program (January to November 2007), the hyperglycemia order set and policy (December 2007 to November 2008), and finally the smart hyperglycemia order set (December 2008 to December 2009).
They used three models:
• Model 1 adjusted for age, sex, and race.
• Model 2 adjusted for model 1 plus hyperglycemia.
• Model 3 adjusted for model 2 plus mortality risk index, severity of illness index, and hospital unit.
In all three models, researchers found more than $3,000 in reductions in hospital costs per admission in the final period compared with baseline, which was statistically significant in all models. The researchers are still collecting data in this area, Dr. Spanakis said.
For mortality rate ratio, model 1 showed a significant 68% reduction in mortality in the final period of the study versus baseline. The significance persisted in model 2.
However, in model 3, the mortality rate ratio became statistically nonsignificant (0.53; P = .08), showing a 47% reduction in the final period, compared with baseline.
Length of stay was also significantly reduced in models 1 and 2, but lost its significance in model 3 (0.54 fewer days; P = .15).
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress."
Dr. Spanakis said that although the mortality rate and length of stay did not reach statistical significance after the full adjustment in model 3, the policies did have some effect.
The study had some limitations, including its retrospective design. Also, the interventions were not randomized and "secular trends in other inpatient practices may have impacted the outcomes," Dr. Spanakis said.
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress," Thomas J. Hoerger, Ph.D., a health economist and senior fellow at RTI International, Research Triangle Park, N.C., said in an interview. He was not involved in the study.
Dr. Spanakis said further studies could determine which specific program components and policies are most effective in reducing hospital costs, in-patient mortality, and length of stay in patients with diabetes and hyperglycemia.
Dr. Spanakis had no disclosures.
On Twitter @NaseemSMiller
CHICAGO – A centralized glucose management program at Johns Hopkins Hospital had a significant impact on hospital costs, and reduced the in-hospital mortality rate and length of stay for patients with diabetes or hyperglycemia, a 3-year analysis showed.
"This policy is important ... to make the hospital more efficient and less costly," said Dr. Elias K. Spanakis of Johns Hopkins University, Baltimore, who presented his abstract at the annual scientific sessions of the American Diabetes Association.
Studies have shown that it is associated with increased mortality, higher risk of infections, and prolonged length of stay (J. Clin. Endocrinol. Metab. 2002;87:978-82 and Diabetes Care 1999;22:1408-14).
Many hospitals now have hypoglycemia policies in place, said Dr. Spanakis, and there have been other studies on the topic. But what sets this study apart from previous research is the fact that it is long term and also considers multiple glycemia-related policies. Most of the previous studies have been done on isolated policies, and over a short period of time, he said.
In 2006, Johns Hopkins Hospital, a 1,000-bed tertiary referral center, developed an institutional glucose management committee led by an endocrinologist and a diabetes nurse practitioner.
The program was implemented between January 2006 and December 2009 and included ongoing staff education. Its individual elements were developed and implemented gradually: hospital-wide hypoglycemia policies, diabetes nursing super-user program, hospital-wide hyperglycemia order set and policy, and hyperglycemia order sets.
Specifically, the policies included a subcutaneous insulin order set, guidelines for calculation and management of insulin doses, and a transition protocol from intravenous to subcutaneous insulin, Dr. Spanakis said. The computer-based smart hyperglycemia order set gave dosing recommendations based on four clinical questions: including the patient’s type of diabetes, type of insulin, total daily dose of insulin, and the nutrition source.
By the end of 2009, the hospital showed a sustained reduction in hyperglycemic episodes. However, to know whether the program had any impact on in-hospital mortality rate, length of stay and total and daily hospital costs required a closer look.
Dr. Spanakis and colleagues conducted a retrospective cohort study of 16,537 unique admissions for patients who had a diagnosis code for diabetes or hyperglycemia during the program’s 4-year stint. Patients who were nonpregnant adults in non–critical care units were excluded.
Researchers compared the results of four consecutive intervention periods with the baseline (January to June 2006). The intervention periods included the implementation of hypoglycemia policy at the hospital (July to Dec. 2006), the diabetes nursing super-user program (January to November 2007), the hyperglycemia order set and policy (December 2007 to November 2008), and finally the smart hyperglycemia order set (December 2008 to December 2009).
They used three models:
• Model 1 adjusted for age, sex, and race.
• Model 2 adjusted for model 1 plus hyperglycemia.
• Model 3 adjusted for model 2 plus mortality risk index, severity of illness index, and hospital unit.
In all three models, researchers found more than $3,000 in reductions in hospital costs per admission in the final period compared with baseline, which was statistically significant in all models. The researchers are still collecting data in this area, Dr. Spanakis said.
For mortality rate ratio, model 1 showed a significant 68% reduction in mortality in the final period of the study versus baseline. The significance persisted in model 2.
However, in model 3, the mortality rate ratio became statistically nonsignificant (0.53; P = .08), showing a 47% reduction in the final period, compared with baseline.
Length of stay was also significantly reduced in models 1 and 2, but lost its significance in model 3 (0.54 fewer days; P = .15).
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress."
Dr. Spanakis said that although the mortality rate and length of stay did not reach statistical significance after the full adjustment in model 3, the policies did have some effect.
The study had some limitations, including its retrospective design. Also, the interventions were not randomized and "secular trends in other inpatient practices may have impacted the outcomes," Dr. Spanakis said.
"People are trying to get better at safety within hospitals, and this seems to be an area where they’re making progress," Thomas J. Hoerger, Ph.D., a health economist and senior fellow at RTI International, Research Triangle Park, N.C., said in an interview. He was not involved in the study.
Dr. Spanakis said further studies could determine which specific program components and policies are most effective in reducing hospital costs, in-patient mortality, and length of stay in patients with diabetes and hyperglycemia.
Dr. Spanakis had no disclosures.
On Twitter @NaseemSMiller
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Hospital costs were reduced by $3,000 per admission during a glucose management program.
Data source: A retrospective cohort study of 16,537 unique admissions that were nonpregnant adults who were not in critical care units and had a diagnosis code for diabetes or hyperglycemia between January 2006 and December 2009.
Disclosures: Dr. Spanakis reported having no financial conflicts of interest.
Statins for low CVD risk? Check glucose first
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the post-test.
CHICAGO – Statin therapy in individuals who had a low risk of cardiovascular disease was not cost effective when the therapy’s potential to increase the risk of diabetes was taken into account, according to an analysis by researchers at the Centers for Disease Control and Prevention.
Statins were cost effective among patients with a high risk of cardiovascular disease (CVD), but the therapy’s cost effectiveness varied among patients with medium and low CVD risk who were at different levels of diabetes risk, the study investigators found.
"We think that physicians should check patients’ blood glucose levels when prescribing statins for preventing CVD among persons with low or medium CVD risk," explained Xiaohui Zhou, Ph.D., a health economist at the CDC and the lead author of the study.
A recent meta-analysis of existing data suggests that while statins reduce the risk of cardiovascular disease by as much as 38%, they could also increase the risk of diabetes by 8%-25%, said Ping Zhang, Ph.D., a senior health economist at the CDC, who presented the unpublished abstract at the annual meeting of the American Diabetes Association.
To evaluate the cost-effectiveness of statins for the prevention of CVD while accounting for the elevated risk of diabetes, Dr. Zhang and colleagues used a simulation model and assessed the 30-year health outcomes of a low-cost statin therapy among patients at different risk levels for CVD and diabetes. The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
The primary outcomes were the incidences of diabetes and CVD, quality-adjusted life-years (QALYs), and cost per QALY.
The investigators stratified the analysis according to baseline risk, categorizing the CVD risk into low (5-year risk less than 5%), medium (5%-10%), and high risk (more than 10%). The diabetes risk was categorized by normal glucose tolerance, impaired glucose tolerance (IGT), and IGT plus impaired fasting glucose.
When measuring the effect of statin therapy, the researchers made two assumptions: a constant relative CVD risk reduction from statin use across the three levels of baseline CVD risk, and a constant relative diabetes risk increase from statin use across the three levels of baseline diabetes risk.
Statin use reduced CVD events, but it increased diabetes events over a period of 30 years to varying degrees, based on the diabetes risk level.
For instance, for individuals with a 5-year CVD risk greater than 10% and normal glucose tolerance, the risk of diabetes increased by nearly 4%. In those with impaired glucose tolerance, that risk increased by roughly 5%, and in patients with IGT and impaired fasting glucose, the risk rose to more than 6%.
In addition, although the cost-effectiveness of statin use was largely dependent on CVD risk, it was also affected by diabetes risk.
Statin use was not cost effective when the 5-year CVD risk was less than 5%: $101,800/QALY. In patients with a 5-year CVD risk of 5%-10%, the cost-effectiveness of statins varied based on baseline diabetes risk: $8,900-$16,400/QALY for normal glucose tolerance, $16,300-$73,300/QALY for impaired glucose tolerance, and $326,700/QALY for IGT and impaired fasting glucose.
However, in individuals who had a 5-year CVD risk of greater than 10%, statin use was cost effective regardless of the diabetes risk, the researchers found: $4,500-$6,300 cost/QALY for normal glucose tolerance, $6,500-$11,800/QALY for impaired glucose tolerance, and $14,400-$45,800/QALY for IGT and impaired fasting glucose.
So, does the increase in diabetes risk matter when statins are used?
It depends, Dr. Zhang said.
For patients at high risk of CVD, it doesn’t matter, Dr. Zhang noted, and statin use is cost effective across all three levels of diabetes risk. For patients at medium risk of CVD, statin use is cost effective for those who have normal glucose tolerance or impaired glucose tolerance. However, it’s not cost effective for those who have IGT plus impaired fasting glucose.
The increased risk of diabetes does matter in individuals at a low CVD risk, as statin use in those patients was no longer cost effective.
The study had some limitations, Dr. Zhang cautioned. The model did not capture all the possible beneficial or harmful effects of statin use. Also, the results apply to the trial population only, who were mostly middle aged or older, and the authors assumed homogeneity of statin effect in the study population at various baseline risks.
The findings were limited to the population used in the analysis, and the true long-term clinical benefits of statin therapy still remain largely unknown, the authors noted.
Dr. Zhou and Dr. Zhang said they had no relevant financial disclosures.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
On Twitter @NaseemSMiller
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the post-test.
CHICAGO – Statin therapy in individuals who had a low risk of cardiovascular disease was not cost effective when the therapy’s potential to increase the risk of diabetes was taken into account, according to an analysis by researchers at the Centers for Disease Control and Prevention.
Statins were cost effective among patients with a high risk of cardiovascular disease (CVD), but the therapy’s cost effectiveness varied among patients with medium and low CVD risk who were at different levels of diabetes risk, the study investigators found.
"We think that physicians should check patients’ blood glucose levels when prescribing statins for preventing CVD among persons with low or medium CVD risk," explained Xiaohui Zhou, Ph.D., a health economist at the CDC and the lead author of the study.
A recent meta-analysis of existing data suggests that while statins reduce the risk of cardiovascular disease by as much as 38%, they could also increase the risk of diabetes by 8%-25%, said Ping Zhang, Ph.D., a senior health economist at the CDC, who presented the unpublished abstract at the annual meeting of the American Diabetes Association.
To evaluate the cost-effectiveness of statins for the prevention of CVD while accounting for the elevated risk of diabetes, Dr. Zhang and colleagues used a simulation model and assessed the 30-year health outcomes of a low-cost statin therapy among patients at different risk levels for CVD and diabetes. The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
The primary outcomes were the incidences of diabetes and CVD, quality-adjusted life-years (QALYs), and cost per QALY.
The investigators stratified the analysis according to baseline risk, categorizing the CVD risk into low (5-year risk less than 5%), medium (5%-10%), and high risk (more than 10%). The diabetes risk was categorized by normal glucose tolerance, impaired glucose tolerance (IGT), and IGT plus impaired fasting glucose.
When measuring the effect of statin therapy, the researchers made two assumptions: a constant relative CVD risk reduction from statin use across the three levels of baseline CVD risk, and a constant relative diabetes risk increase from statin use across the three levels of baseline diabetes risk.
Statin use reduced CVD events, but it increased diabetes events over a period of 30 years to varying degrees, based on the diabetes risk level.
For instance, for individuals with a 5-year CVD risk greater than 10% and normal glucose tolerance, the risk of diabetes increased by nearly 4%. In those with impaired glucose tolerance, that risk increased by roughly 5%, and in patients with IGT and impaired fasting glucose, the risk rose to more than 6%.
In addition, although the cost-effectiveness of statin use was largely dependent on CVD risk, it was also affected by diabetes risk.
Statin use was not cost effective when the 5-year CVD risk was less than 5%: $101,800/QALY. In patients with a 5-year CVD risk of 5%-10%, the cost-effectiveness of statins varied based on baseline diabetes risk: $8,900-$16,400/QALY for normal glucose tolerance, $16,300-$73,300/QALY for impaired glucose tolerance, and $326,700/QALY for IGT and impaired fasting glucose.
However, in individuals who had a 5-year CVD risk of greater than 10%, statin use was cost effective regardless of the diabetes risk, the researchers found: $4,500-$6,300 cost/QALY for normal glucose tolerance, $6,500-$11,800/QALY for impaired glucose tolerance, and $14,400-$45,800/QALY for IGT and impaired fasting glucose.
So, does the increase in diabetes risk matter when statins are used?
It depends, Dr. Zhang said.
For patients at high risk of CVD, it doesn’t matter, Dr. Zhang noted, and statin use is cost effective across all three levels of diabetes risk. For patients at medium risk of CVD, statin use is cost effective for those who have normal glucose tolerance or impaired glucose tolerance. However, it’s not cost effective for those who have IGT plus impaired fasting glucose.
The increased risk of diabetes does matter in individuals at a low CVD risk, as statin use in those patients was no longer cost effective.
The study had some limitations, Dr. Zhang cautioned. The model did not capture all the possible beneficial or harmful effects of statin use. Also, the results apply to the trial population only, who were mostly middle aged or older, and the authors assumed homogeneity of statin effect in the study population at various baseline risks.
The findings were limited to the population used in the analysis, and the true long-term clinical benefits of statin therapy still remain largely unknown, the authors noted.
Dr. Zhou and Dr. Zhang said they had no relevant financial disclosures.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
On Twitter @NaseemSMiller
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the post-test.
CHICAGO – Statin therapy in individuals who had a low risk of cardiovascular disease was not cost effective when the therapy’s potential to increase the risk of diabetes was taken into account, according to an analysis by researchers at the Centers for Disease Control and Prevention.
Statins were cost effective among patients with a high risk of cardiovascular disease (CVD), but the therapy’s cost effectiveness varied among patients with medium and low CVD risk who were at different levels of diabetes risk, the study investigators found.
"We think that physicians should check patients’ blood glucose levels when prescribing statins for preventing CVD among persons with low or medium CVD risk," explained Xiaohui Zhou, Ph.D., a health economist at the CDC and the lead author of the study.
A recent meta-analysis of existing data suggests that while statins reduce the risk of cardiovascular disease by as much as 38%, they could also increase the risk of diabetes by 8%-25%, said Ping Zhang, Ph.D., a senior health economist at the CDC, who presented the unpublished abstract at the annual meeting of the American Diabetes Association.
To evaluate the cost-effectiveness of statins for the prevention of CVD while accounting for the elevated risk of diabetes, Dr. Zhang and colleagues used a simulation model and assessed the 30-year health outcomes of a low-cost statin therapy among patients at different risk levels for CVD and diabetes. The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
The primary outcomes were the incidences of diabetes and CVD, quality-adjusted life-years (QALYs), and cost per QALY.
The investigators stratified the analysis according to baseline risk, categorizing the CVD risk into low (5-year risk less than 5%), medium (5%-10%), and high risk (more than 10%). The diabetes risk was categorized by normal glucose tolerance, impaired glucose tolerance (IGT), and IGT plus impaired fasting glucose.
When measuring the effect of statin therapy, the researchers made two assumptions: a constant relative CVD risk reduction from statin use across the three levels of baseline CVD risk, and a constant relative diabetes risk increase from statin use across the three levels of baseline diabetes risk.
Statin use reduced CVD events, but it increased diabetes events over a period of 30 years to varying degrees, based on the diabetes risk level.
For instance, for individuals with a 5-year CVD risk greater than 10% and normal glucose tolerance, the risk of diabetes increased by nearly 4%. In those with impaired glucose tolerance, that risk increased by roughly 5%, and in patients with IGT and impaired fasting glucose, the risk rose to more than 6%.
In addition, although the cost-effectiveness of statin use was largely dependent on CVD risk, it was also affected by diabetes risk.
Statin use was not cost effective when the 5-year CVD risk was less than 5%: $101,800/QALY. In patients with a 5-year CVD risk of 5%-10%, the cost-effectiveness of statins varied based on baseline diabetes risk: $8,900-$16,400/QALY for normal glucose tolerance, $16,300-$73,300/QALY for impaired glucose tolerance, and $326,700/QALY for IGT and impaired fasting glucose.
However, in individuals who had a 5-year CVD risk of greater than 10%, statin use was cost effective regardless of the diabetes risk, the researchers found: $4,500-$6,300 cost/QALY for normal glucose tolerance, $6,500-$11,800/QALY for impaired glucose tolerance, and $14,400-$45,800/QALY for IGT and impaired fasting glucose.
So, does the increase in diabetes risk matter when statins are used?
It depends, Dr. Zhang said.
For patients at high risk of CVD, it doesn’t matter, Dr. Zhang noted, and statin use is cost effective across all three levels of diabetes risk. For patients at medium risk of CVD, statin use is cost effective for those who have normal glucose tolerance or impaired glucose tolerance. However, it’s not cost effective for those who have IGT plus impaired fasting glucose.
The increased risk of diabetes does matter in individuals at a low CVD risk, as statin use in those patients was no longer cost effective.
The study had some limitations, Dr. Zhang cautioned. The model did not capture all the possible beneficial or harmful effects of statin use. Also, the results apply to the trial population only, who were mostly middle aged or older, and the authors assumed homogeneity of statin effect in the study population at various baseline risks.
The findings were limited to the population used in the analysis, and the true long-term clinical benefits of statin therapy still remain largely unknown, the authors noted.
Dr. Zhou and Dr. Zhang said they had no relevant financial disclosures.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
On Twitter @NaseemSMiller
AT ADA 2013