User login
Statins for low CVD risk? Check glucose first
CHICAGO – Statin therapy in individuals who had a low risk of cardiovascular disease was not cost effective when the therapy’s potential to increase the risk of diabetes was taken into account, according to an analysis by researchers at the Centers for Disease Control and Prevention.
Statins were cost effective among patients with a high risk of cardiovascular disease (CVD), but the therapy’s cost effectiveness varied among patients with medium and low CVD risk who were at different levels of diabetes risk, the study investigators found.
"We think that physicians should check patients’ blood glucose levels when prescribing statins for preventing CVD among persons with low or medium CVD risk," explained Xiaohui Zhou, Ph.D., a health economist at the CDC and the lead author of the study.
A recent meta-analysis of existing data suggests that while statins reduce the risk of cardiovascular disease by as much as 38%, they could also increase the risk of diabetes by 8%-25%, said Ping Zhang, Ph.D., a senior health economist at the CDC, who presented the unpublished abstract at the annual meeting of the American Diabetes Association.
To evaluate the cost-effectiveness of statins for the prevention of CVD while accounting for the elevated risk of diabetes, Dr. Zhang and colleagues used a simulation model and assessed the 30-year health outcomes of a low-cost statin therapy among patients at different risk levels for CVD and diabetes. The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
The primary outcomes were the incidences of diabetes and CVD, quality-adjusted life-years (QALYs), and cost per QALY.
The investigators stratified the analysis according to baseline risk, categorizing the CVD risk into low (5-year risk less than 5%), medium (5%-10%), and high risk (more than 10%). The diabetes risk was categorized by normal glucose tolerance, impaired glucose tolerance (IGT), and IGT plus impaired fasting glucose.
When measuring the effect of statin therapy, the researchers made two assumptions: a constant relative CVD risk reduction from statin use across the three levels of baseline CVD risk, and a constant relative diabetes risk increase from statin use across the three levels of baseline diabetes risk.
Statin use reduced CVD events, but it increased diabetes events over a period of 30 years to varying degrees, based on the diabetes risk level.
For instance, for individuals with a 5-year CVD risk greater than 10% and normal glucose tolerance, the risk of diabetes increased by nearly 4%. In those with impaired glucose tolerance, that risk increased by roughly 5%, and in patients with IGT and impaired fasting glucose, the risk rose to more than 6%.
In addition, although the cost-effectiveness of statin use was largely dependent on CVD risk, it was also affected by diabetes risk.
Statin use was not cost effective when the 5-year CVD risk was less than 5%: $101,800/QALY. In patients with a 5-year CVD risk of 5%-10%, the cost-effectiveness of statins varied based on baseline diabetes risk: $8,900-$16,400/QALY for normal glucose tolerance, $16,300-$73,300/QALY for impaired glucose tolerance, and $326,700/QALY for IGT and impaired fasting glucose.
However, in individuals who had a 5-year CVD risk of greater than 10%, statin use was cost effective regardless of the diabetes risk, the researchers found: $4,500-$6,300 cost/QALY for normal glucose tolerance, $6,500-$11,800/QALY for impaired glucose tolerance, and $14,400-$45,800/QALY for IGT and impaired fasting glucose.
So, does the increase in diabetes risk matter when statins are used?
It depends, Dr. Zhang said.
For patients at high risk of CVD, it doesn’t matter, Dr. Zhang noted, and statin use is cost effective across all three levels of diabetes risk. For patients at medium risk of CVD, statin use is cost effective for those who have normal glucose tolerance or impaired glucose tolerance. However, it’s not cost effective for those who have IGT plus impaired fasting glucose.
The increased risk of diabetes does matter in individuals at a low CVD risk, as statin use in those patients was no longer cost effective.
The study had some limitations, Dr. Zhang cautioned. The model did not capture all the possible beneficial or harmful effects of statin use. Also, the results apply to the trial population only, who were mostly middle aged or older, and the authors assumed homogeneity of statin effect in the study population at various baseline risks.
The findings were limited to the population used in the analysis, and the true long-term clinical benefits of statin therapy still remain largely unknown, the authors noted.
Dr. Zhou and Dr. Zhang said they had no relevant financial disclosures.
On Twitter @NaseemSMiller
CHICAGO – Statin therapy in individuals who had a low risk of cardiovascular disease was not cost effective when the therapy’s potential to increase the risk of diabetes was taken into account, according to an analysis by researchers at the Centers for Disease Control and Prevention.
Statins were cost effective among patients with a high risk of cardiovascular disease (CVD), but the therapy’s cost effectiveness varied among patients with medium and low CVD risk who were at different levels of diabetes risk, the study investigators found.
"We think that physicians should check patients’ blood glucose levels when prescribing statins for preventing CVD among persons with low or medium CVD risk," explained Xiaohui Zhou, Ph.D., a health economist at the CDC and the lead author of the study.
A recent meta-analysis of existing data suggests that while statins reduce the risk of cardiovascular disease by as much as 38%, they could also increase the risk of diabetes by 8%-25%, said Ping Zhang, Ph.D., a senior health economist at the CDC, who presented the unpublished abstract at the annual meeting of the American Diabetes Association.
To evaluate the cost-effectiveness of statins for the prevention of CVD while accounting for the elevated risk of diabetes, Dr. Zhang and colleagues used a simulation model and assessed the 30-year health outcomes of a low-cost statin therapy among patients at different risk levels for CVD and diabetes. The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
The primary outcomes were the incidences of diabetes and CVD, quality-adjusted life-years (QALYs), and cost per QALY.
The investigators stratified the analysis according to baseline risk, categorizing the CVD risk into low (5-year risk less than 5%), medium (5%-10%), and high risk (more than 10%). The diabetes risk was categorized by normal glucose tolerance, impaired glucose tolerance (IGT), and IGT plus impaired fasting glucose.
When measuring the effect of statin therapy, the researchers made two assumptions: a constant relative CVD risk reduction from statin use across the three levels of baseline CVD risk, and a constant relative diabetes risk increase from statin use across the three levels of baseline diabetes risk.
Statin use reduced CVD events, but it increased diabetes events over a period of 30 years to varying degrees, based on the diabetes risk level.
For instance, for individuals with a 5-year CVD risk greater than 10% and normal glucose tolerance, the risk of diabetes increased by nearly 4%. In those with impaired glucose tolerance, that risk increased by roughly 5%, and in patients with IGT and impaired fasting glucose, the risk rose to more than 6%.
In addition, although the cost-effectiveness of statin use was largely dependent on CVD risk, it was also affected by diabetes risk.
Statin use was not cost effective when the 5-year CVD risk was less than 5%: $101,800/QALY. In patients with a 5-year CVD risk of 5%-10%, the cost-effectiveness of statins varied based on baseline diabetes risk: $8,900-$16,400/QALY for normal glucose tolerance, $16,300-$73,300/QALY for impaired glucose tolerance, and $326,700/QALY for IGT and impaired fasting glucose.
However, in individuals who had a 5-year CVD risk of greater than 10%, statin use was cost effective regardless of the diabetes risk, the researchers found: $4,500-$6,300 cost/QALY for normal glucose tolerance, $6,500-$11,800/QALY for impaired glucose tolerance, and $14,400-$45,800/QALY for IGT and impaired fasting glucose.
So, does the increase in diabetes risk matter when statins are used?
It depends, Dr. Zhang said.
For patients at high risk of CVD, it doesn’t matter, Dr. Zhang noted, and statin use is cost effective across all three levels of diabetes risk. For patients at medium risk of CVD, statin use is cost effective for those who have normal glucose tolerance or impaired glucose tolerance. However, it’s not cost effective for those who have IGT plus impaired fasting glucose.
The increased risk of diabetes does matter in individuals at a low CVD risk, as statin use in those patients was no longer cost effective.
The study had some limitations, Dr. Zhang cautioned. The model did not capture all the possible beneficial or harmful effects of statin use. Also, the results apply to the trial population only, who were mostly middle aged or older, and the authors assumed homogeneity of statin effect in the study population at various baseline risks.
The findings were limited to the population used in the analysis, and the true long-term clinical benefits of statin therapy still remain largely unknown, the authors noted.
Dr. Zhou and Dr. Zhang said they had no relevant financial disclosures.
On Twitter @NaseemSMiller
CHICAGO – Statin therapy in individuals who had a low risk of cardiovascular disease was not cost effective when the therapy’s potential to increase the risk of diabetes was taken into account, according to an analysis by researchers at the Centers for Disease Control and Prevention.
Statins were cost effective among patients with a high risk of cardiovascular disease (CVD), but the therapy’s cost effectiveness varied among patients with medium and low CVD risk who were at different levels of diabetes risk, the study investigators found.
"We think that physicians should check patients’ blood glucose levels when prescribing statins for preventing CVD among persons with low or medium CVD risk," explained Xiaohui Zhou, Ph.D., a health economist at the CDC and the lead author of the study.
A recent meta-analysis of existing data suggests that while statins reduce the risk of cardiovascular disease by as much as 38%, they could also increase the risk of diabetes by 8%-25%, said Ping Zhang, Ph.D., a senior health economist at the CDC, who presented the unpublished abstract at the annual meeting of the American Diabetes Association.
To evaluate the cost-effectiveness of statins for the prevention of CVD while accounting for the elevated risk of diabetes, Dr. Zhang and colleagues used a simulation model and assessed the 30-year health outcomes of a low-cost statin therapy among patients at different risk levels for CVD and diabetes. The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
The primary outcomes were the incidences of diabetes and CVD, quality-adjusted life-years (QALYs), and cost per QALY.
The investigators stratified the analysis according to baseline risk, categorizing the CVD risk into low (5-year risk less than 5%), medium (5%-10%), and high risk (more than 10%). The diabetes risk was categorized by normal glucose tolerance, impaired glucose tolerance (IGT), and IGT plus impaired fasting glucose.
When measuring the effect of statin therapy, the researchers made two assumptions: a constant relative CVD risk reduction from statin use across the three levels of baseline CVD risk, and a constant relative diabetes risk increase from statin use across the three levels of baseline diabetes risk.
Statin use reduced CVD events, but it increased diabetes events over a period of 30 years to varying degrees, based on the diabetes risk level.
For instance, for individuals with a 5-year CVD risk greater than 10% and normal glucose tolerance, the risk of diabetes increased by nearly 4%. In those with impaired glucose tolerance, that risk increased by roughly 5%, and in patients with IGT and impaired fasting glucose, the risk rose to more than 6%.
In addition, although the cost-effectiveness of statin use was largely dependent on CVD risk, it was also affected by diabetes risk.
Statin use was not cost effective when the 5-year CVD risk was less than 5%: $101,800/QALY. In patients with a 5-year CVD risk of 5%-10%, the cost-effectiveness of statins varied based on baseline diabetes risk: $8,900-$16,400/QALY for normal glucose tolerance, $16,300-$73,300/QALY for impaired glucose tolerance, and $326,700/QALY for IGT and impaired fasting glucose.
However, in individuals who had a 5-year CVD risk of greater than 10%, statin use was cost effective regardless of the diabetes risk, the researchers found: $4,500-$6,300 cost/QALY for normal glucose tolerance, $6,500-$11,800/QALY for impaired glucose tolerance, and $14,400-$45,800/QALY for IGT and impaired fasting glucose.
So, does the increase in diabetes risk matter when statins are used?
It depends, Dr. Zhang said.
For patients at high risk of CVD, it doesn’t matter, Dr. Zhang noted, and statin use is cost effective across all three levels of diabetes risk. For patients at medium risk of CVD, statin use is cost effective for those who have normal glucose tolerance or impaired glucose tolerance. However, it’s not cost effective for those who have IGT plus impaired fasting glucose.
The increased risk of diabetes does matter in individuals at a low CVD risk, as statin use in those patients was no longer cost effective.
The study had some limitations, Dr. Zhang cautioned. The model did not capture all the possible beneficial or harmful effects of statin use. Also, the results apply to the trial population only, who were mostly middle aged or older, and the authors assumed homogeneity of statin effect in the study population at various baseline risks.
The findings were limited to the population used in the analysis, and the true long-term clinical benefits of statin therapy still remain largely unknown, the authors noted.
Dr. Zhou and Dr. Zhang said they had no relevant financial disclosures.
On Twitter @NaseemSMiller
AT ADA 2013
Major finding: In patients with a 5-year CVD risk of 5% and normal glucose tolerance, statins were not cost effective ($101,800 per quality-adjusted life-year).
Data source: The study population included a combined sample of nondiabetic participants in five large-scale trials: ASCOT-LLA, JUPITER, WOSCOPS, MEGA, and AFCAPS/TexCAPS.
Disclosures: Dr. Zhang and Dr. Zhou reported having no relevant financial disclosures.
Transverse myelitis does not share risk factors with MS
ORLANDO – Epstein-Barr virus exposure and vitamin D insufficiency, which are some of the known risk factors for multiple sclerosis in children and adults, appear not to be associated with transverse myelitis, a small study has shown.
"What we found was that MS risk factors are specific for multiple sclerosis, and not necessarily for all autoimmune demyelinating diseases," said Kelley M. Weinfurtner, a third-year medical student at the University of California, San Francisco (UCSF), who presented a poster detailing the study at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis.
Transverse myelitis (TM) has substantial clinical overlap, especially at early stages, with autoimmune diseases such as multiple sclerosis and neuromyelitis optica, according to Ms. Weinfurtner.
Risk factors for TM are not known, and finding what they are "could be helpful in terms of stratifying when patients come in with TM," and deciding whether the patients would be at risk of MS further down the line, she said.
Ms. Weinfurtner and her colleagues collected blood samples from patients with TM (16), neuromyelitis optica (34), and MS (184) at early stages of disease, and from neurologic controls (95) through the Stony Brook Pediatric MS Center and the Accelerated Cure Project for MS Guthy Jackson Biobank.
They measured serum 25-hydroxyvitamin D levels and checked the blood samples for Epstein-Barr virus, cytomegalovirus, herpes simplex virus (HSV), and HLA-DRB1 allele status.
The median ages at disease onset were 12 years for TM, 11 years for neuromyelitis optica, and 14 years for MS; the median ages at sampling were 30, 16, and 16 years, respectively. The median age of healthy controls at the time of sampling was 16 years.
The majority of the patients were female (61%-70%) and white (all TM patients were white).
The results showed that TM patients were less likely to have been exposed to Epstein-Barr virus, compared with MS patients (odds ratio = 0.021; P less than .001), neuromyelitis optica patients (OR = 0.154; P = .054), and controls (P = .001). TM patients were more likely to have been exposed to HSV-1, although the data did not reach statistical significance.
TM patients also had higher levels of 25-hydroxyvitamin D, compared with MS patients (P = .037) and healthy controls (P = .01).
Meanwhile, there were no significant differences in the frequency of the HLA-DRB1*1501 allele or exposure to cytomegalovirus among TM patients, compared with MS and neuromyelitis optica patients and neurologic controls.
TM patients were significantly older and further from disease onset at the time of the blood draw, but the findings were adjusted for age at the time of the draw. The discrepancy in age "could explain the trend toward higher HSV-1 exposure in TM patients, but only strengthens the findings that TM patients have a lower prevalence of [Epstein-Barr virus] exposure than MS patients," she reported in the poster.
The difference in age could also explain the higher levels of 25-hydroxyvitamin D in TM patients, because they may have been on vitamin D supplementation after diagnosis.
The sample size for the study was small, and TM patients were not stratified by etiology or extent of cord involvement, although they all met criteria for TM, Ms. Weinfurtner said. The investigators were not able to adjust for race or ethnicity, because all TM patients were white.
Her study is not published and she said there’s a need for larger studies to confirm the findings.
Her research was supported by a grant from the Dean’s Office Medical Student Research Program at UCSF. The study is an offshoot of a large, multicenter study conducted by the Pediatric MS Network, which is in the third year of its 5-year period.
On Twitter @NaseemSMiller
ORLANDO – Epstein-Barr virus exposure and vitamin D insufficiency, which are some of the known risk factors for multiple sclerosis in children and adults, appear not to be associated with transverse myelitis, a small study has shown.
"What we found was that MS risk factors are specific for multiple sclerosis, and not necessarily for all autoimmune demyelinating diseases," said Kelley M. Weinfurtner, a third-year medical student at the University of California, San Francisco (UCSF), who presented a poster detailing the study at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis.
Transverse myelitis (TM) has substantial clinical overlap, especially at early stages, with autoimmune diseases such as multiple sclerosis and neuromyelitis optica, according to Ms. Weinfurtner.
Risk factors for TM are not known, and finding what they are "could be helpful in terms of stratifying when patients come in with TM," and deciding whether the patients would be at risk of MS further down the line, she said.
Ms. Weinfurtner and her colleagues collected blood samples from patients with TM (16), neuromyelitis optica (34), and MS (184) at early stages of disease, and from neurologic controls (95) through the Stony Brook Pediatric MS Center and the Accelerated Cure Project for MS Guthy Jackson Biobank.
They measured serum 25-hydroxyvitamin D levels and checked the blood samples for Epstein-Barr virus, cytomegalovirus, herpes simplex virus (HSV), and HLA-DRB1 allele status.
The median ages at disease onset were 12 years for TM, 11 years for neuromyelitis optica, and 14 years for MS; the median ages at sampling were 30, 16, and 16 years, respectively. The median age of healthy controls at the time of sampling was 16 years.
The majority of the patients were female (61%-70%) and white (all TM patients were white).
The results showed that TM patients were less likely to have been exposed to Epstein-Barr virus, compared with MS patients (odds ratio = 0.021; P less than .001), neuromyelitis optica patients (OR = 0.154; P = .054), and controls (P = .001). TM patients were more likely to have been exposed to HSV-1, although the data did not reach statistical significance.
TM patients also had higher levels of 25-hydroxyvitamin D, compared with MS patients (P = .037) and healthy controls (P = .01).
Meanwhile, there were no significant differences in the frequency of the HLA-DRB1*1501 allele or exposure to cytomegalovirus among TM patients, compared with MS and neuromyelitis optica patients and neurologic controls.
TM patients were significantly older and further from disease onset at the time of the blood draw, but the findings were adjusted for age at the time of the draw. The discrepancy in age "could explain the trend toward higher HSV-1 exposure in TM patients, but only strengthens the findings that TM patients have a lower prevalence of [Epstein-Barr virus] exposure than MS patients," she reported in the poster.
The difference in age could also explain the higher levels of 25-hydroxyvitamin D in TM patients, because they may have been on vitamin D supplementation after diagnosis.
The sample size for the study was small, and TM patients were not stratified by etiology or extent of cord involvement, although they all met criteria for TM, Ms. Weinfurtner said. The investigators were not able to adjust for race or ethnicity, because all TM patients were white.
Her study is not published and she said there’s a need for larger studies to confirm the findings.
Her research was supported by a grant from the Dean’s Office Medical Student Research Program at UCSF. The study is an offshoot of a large, multicenter study conducted by the Pediatric MS Network, which is in the third year of its 5-year period.
On Twitter @NaseemSMiller
ORLANDO – Epstein-Barr virus exposure and vitamin D insufficiency, which are some of the known risk factors for multiple sclerosis in children and adults, appear not to be associated with transverse myelitis, a small study has shown.
"What we found was that MS risk factors are specific for multiple sclerosis, and not necessarily for all autoimmune demyelinating diseases," said Kelley M. Weinfurtner, a third-year medical student at the University of California, San Francisco (UCSF), who presented a poster detailing the study at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis.
Transverse myelitis (TM) has substantial clinical overlap, especially at early stages, with autoimmune diseases such as multiple sclerosis and neuromyelitis optica, according to Ms. Weinfurtner.
Risk factors for TM are not known, and finding what they are "could be helpful in terms of stratifying when patients come in with TM," and deciding whether the patients would be at risk of MS further down the line, she said.
Ms. Weinfurtner and her colleagues collected blood samples from patients with TM (16), neuromyelitis optica (34), and MS (184) at early stages of disease, and from neurologic controls (95) through the Stony Brook Pediatric MS Center and the Accelerated Cure Project for MS Guthy Jackson Biobank.
They measured serum 25-hydroxyvitamin D levels and checked the blood samples for Epstein-Barr virus, cytomegalovirus, herpes simplex virus (HSV), and HLA-DRB1 allele status.
The median ages at disease onset were 12 years for TM, 11 years for neuromyelitis optica, and 14 years for MS; the median ages at sampling were 30, 16, and 16 years, respectively. The median age of healthy controls at the time of sampling was 16 years.
The majority of the patients were female (61%-70%) and white (all TM patients were white).
The results showed that TM patients were less likely to have been exposed to Epstein-Barr virus, compared with MS patients (odds ratio = 0.021; P less than .001), neuromyelitis optica patients (OR = 0.154; P = .054), and controls (P = .001). TM patients were more likely to have been exposed to HSV-1, although the data did not reach statistical significance.
TM patients also had higher levels of 25-hydroxyvitamin D, compared with MS patients (P = .037) and healthy controls (P = .01).
Meanwhile, there were no significant differences in the frequency of the HLA-DRB1*1501 allele or exposure to cytomegalovirus among TM patients, compared with MS and neuromyelitis optica patients and neurologic controls.
TM patients were significantly older and further from disease onset at the time of the blood draw, but the findings were adjusted for age at the time of the draw. The discrepancy in age "could explain the trend toward higher HSV-1 exposure in TM patients, but only strengthens the findings that TM patients have a lower prevalence of [Epstein-Barr virus] exposure than MS patients," she reported in the poster.
The difference in age could also explain the higher levels of 25-hydroxyvitamin D in TM patients, because they may have been on vitamin D supplementation after diagnosis.
The sample size for the study was small, and TM patients were not stratified by etiology or extent of cord involvement, although they all met criteria for TM, Ms. Weinfurtner said. The investigators were not able to adjust for race or ethnicity, because all TM patients were white.
Her study is not published and she said there’s a need for larger studies to confirm the findings.
Her research was supported by a grant from the Dean’s Office Medical Student Research Program at UCSF. The study is an offshoot of a large, multicenter study conducted by the Pediatric MS Network, which is in the third year of its 5-year period.
On Twitter @NaseemSMiller
AT THE cmsc/actrims ANNUAL MEETING
Major finding: TM patients were less likely to have been exposed to EBV, compared with MS patients (odds ratio = 0.021; P less than .001), neuromyelitis optica patients (OR = 0.154; P = .054), and controls (P = .001).
Data source: Collected blood samples from patients with TM (16), neuromyelitis optica (34), and MS (184) at early stages of disease, and from neurologic controls (95), through the Stony Brook Pediatric MS Centers and the Accelerated Cure Project for MS/Guthy Jackson Biobank.
Disclosures: Ms. Weinfurtner’s research was supported by a grant from the Dean’s Office Medical Student Research Program at UCSF.
HHS issues final rule on contraceptive coverage
The Obama administration issued the final rule on contraceptive coverage June 28, in part simplifying some requirements in response to religious groups that have opposed the health law’s mandated contraceptive coverage.
Officials said the rule has simplified the definition of a "religious employer" and is accommodating the nonprofit organizations such as universities and hospitals that object to providing such coverage on religious grounds.
"The health care law guarantees millions of women access to recommended preventive services at no cost," Health and Human Services Secretary Kathleen Sebelius said in a statement. "Today’s announcement reinforces our commitment to respect the concerns of houses of worship and other non-profit religious organizations that object to contraceptive coverage, while helping to ensure that women get the care they need, regardless of where they work."
The final rule also extends the effective date of its implementation from August 1 this year to January 1, 2014.
But the rule, which received some 400,000 comments since it was proposed in February and has been facing dozens of lawsuits, is unlikely to appease opponents.
The rule leaves out for-profit organizations, some of which have ongoing lawsuits to overturn the health care law’s requirement to provide birth-control coverage on religious grounds. The arts and crafts chain Hobby Lobby received the green light from a federal appeals court June 27 to move forward with its lawsuit to get the law overturned. In a news conference on June 28, Health and Human Services’ officials said they would not comment on ongoing litigation.
The Family Research Council (FRC), which called the proposed rule an "accounting gimmick" in February, continued its strong opposition to the rule.
"A day after the courts issued temporary relief for Hobby Lobby and Geneva College because of the likelihood of success in challenging the antireligious nature of the HHS mandate, this latest rule shows the administration is tone-deaf to religious freedom," Anna Higgins, director of FRC’s Center for Human Dignity, said in a statement. "The extended safe harbor merely grants nonprofits more time to decide whether to violate their fundamental religious beliefs and shows HHS is trying to buy more time as the courts begin to rule against this violation of religious freedom.
"The mandate does not protect women’s health, either. It threatens women’s health by forcing religious employers into the untenable choice of violating their consciences or dropping health coverage for families and the women they employ. That doesn’t help women’s health; it harms it," Ms. Higgins said.
Meanwhile, the proponents of contraceptive coverage continued their support and celebrated the final rule.
"Today's announcement is a win for civil liberties," Sarah Lipton-Lubet, American Civil Liberties Union policy counsel, said in a statement. "With this rule, the administration continues to stand by women and our families and refuses to let employers use religion to discriminate."
Under the final rule, churches and other houses of worship continue to be exempted from contraceptive coverage requirement. In other words, the religious employers may provide health plans to their employees that do not include contraceptive coverage, officials said June 28.
It also provides accommodations for nonprofit organizations including hospitals, universities, and charities that are opposed to providing birth-control coverage based on religious grounds. Under the rule, these organizations won’t have to "contract, arrange, pay for, or refer contraceptive coverage." Instead, the coverage is provided separately by the health plans of the enrolled women, at no cost.
Such nonprofit groups that have insured and self-insured health plans will have to notify the insurer or third-party administrator that they object to contraception coverage, and the insurers in turn will let the enrollees know that they have access to contraceptive coverage under a separate, no-cost payment.
The rule also provides more details on these accommodations for insurers and third-party administrators, officials said.
"We strongly believe that the cost of contraceptive service will be absolutely cost-neutral and will be offset by improvements in women’s health and reduced pregnancy," Ms. Chiquita Brooks-LaSure, deputy director of policy and regulation at the Center for Consumer Information and Insurance Oversight at HHS, said in a news conference.
Coverage of contraceptive services is part of the recommended preventive care under the health care law and includes all the FDA-approved contraceptive services prescribed by health care providers.
On Twitter @NaseemSMiller
The Obama administration issued the final rule on contraceptive coverage June 28, in part simplifying some requirements in response to religious groups that have opposed the health law’s mandated contraceptive coverage.
Officials said the rule has simplified the definition of a "religious employer" and is accommodating the nonprofit organizations such as universities and hospitals that object to providing such coverage on religious grounds.
"The health care law guarantees millions of women access to recommended preventive services at no cost," Health and Human Services Secretary Kathleen Sebelius said in a statement. "Today’s announcement reinforces our commitment to respect the concerns of houses of worship and other non-profit religious organizations that object to contraceptive coverage, while helping to ensure that women get the care they need, regardless of where they work."
The final rule also extends the effective date of its implementation from August 1 this year to January 1, 2014.
But the rule, which received some 400,000 comments since it was proposed in February and has been facing dozens of lawsuits, is unlikely to appease opponents.
The rule leaves out for-profit organizations, some of which have ongoing lawsuits to overturn the health care law’s requirement to provide birth-control coverage on religious grounds. The arts and crafts chain Hobby Lobby received the green light from a federal appeals court June 27 to move forward with its lawsuit to get the law overturned. In a news conference on June 28, Health and Human Services’ officials said they would not comment on ongoing litigation.
The Family Research Council (FRC), which called the proposed rule an "accounting gimmick" in February, continued its strong opposition to the rule.
"A day after the courts issued temporary relief for Hobby Lobby and Geneva College because of the likelihood of success in challenging the antireligious nature of the HHS mandate, this latest rule shows the administration is tone-deaf to religious freedom," Anna Higgins, director of FRC’s Center for Human Dignity, said in a statement. "The extended safe harbor merely grants nonprofits more time to decide whether to violate their fundamental religious beliefs and shows HHS is trying to buy more time as the courts begin to rule against this violation of religious freedom.
"The mandate does not protect women’s health, either. It threatens women’s health by forcing religious employers into the untenable choice of violating their consciences or dropping health coverage for families and the women they employ. That doesn’t help women’s health; it harms it," Ms. Higgins said.
Meanwhile, the proponents of contraceptive coverage continued their support and celebrated the final rule.
"Today's announcement is a win for civil liberties," Sarah Lipton-Lubet, American Civil Liberties Union policy counsel, said in a statement. "With this rule, the administration continues to stand by women and our families and refuses to let employers use religion to discriminate."
Under the final rule, churches and other houses of worship continue to be exempted from contraceptive coverage requirement. In other words, the religious employers may provide health plans to their employees that do not include contraceptive coverage, officials said June 28.
It also provides accommodations for nonprofit organizations including hospitals, universities, and charities that are opposed to providing birth-control coverage based on religious grounds. Under the rule, these organizations won’t have to "contract, arrange, pay for, or refer contraceptive coverage." Instead, the coverage is provided separately by the health plans of the enrolled women, at no cost.
Such nonprofit groups that have insured and self-insured health plans will have to notify the insurer or third-party administrator that they object to contraception coverage, and the insurers in turn will let the enrollees know that they have access to contraceptive coverage under a separate, no-cost payment.
The rule also provides more details on these accommodations for insurers and third-party administrators, officials said.
"We strongly believe that the cost of contraceptive service will be absolutely cost-neutral and will be offset by improvements in women’s health and reduced pregnancy," Ms. Chiquita Brooks-LaSure, deputy director of policy and regulation at the Center for Consumer Information and Insurance Oversight at HHS, said in a news conference.
Coverage of contraceptive services is part of the recommended preventive care under the health care law and includes all the FDA-approved contraceptive services prescribed by health care providers.
On Twitter @NaseemSMiller
The Obama administration issued the final rule on contraceptive coverage June 28, in part simplifying some requirements in response to religious groups that have opposed the health law’s mandated contraceptive coverage.
Officials said the rule has simplified the definition of a "religious employer" and is accommodating the nonprofit organizations such as universities and hospitals that object to providing such coverage on religious grounds.
"The health care law guarantees millions of women access to recommended preventive services at no cost," Health and Human Services Secretary Kathleen Sebelius said in a statement. "Today’s announcement reinforces our commitment to respect the concerns of houses of worship and other non-profit religious organizations that object to contraceptive coverage, while helping to ensure that women get the care they need, regardless of where they work."
The final rule also extends the effective date of its implementation from August 1 this year to January 1, 2014.
But the rule, which received some 400,000 comments since it was proposed in February and has been facing dozens of lawsuits, is unlikely to appease opponents.
The rule leaves out for-profit organizations, some of which have ongoing lawsuits to overturn the health care law’s requirement to provide birth-control coverage on religious grounds. The arts and crafts chain Hobby Lobby received the green light from a federal appeals court June 27 to move forward with its lawsuit to get the law overturned. In a news conference on June 28, Health and Human Services’ officials said they would not comment on ongoing litigation.
The Family Research Council (FRC), which called the proposed rule an "accounting gimmick" in February, continued its strong opposition to the rule.
"A day after the courts issued temporary relief for Hobby Lobby and Geneva College because of the likelihood of success in challenging the antireligious nature of the HHS mandate, this latest rule shows the administration is tone-deaf to religious freedom," Anna Higgins, director of FRC’s Center for Human Dignity, said in a statement. "The extended safe harbor merely grants nonprofits more time to decide whether to violate their fundamental religious beliefs and shows HHS is trying to buy more time as the courts begin to rule against this violation of religious freedom.
"The mandate does not protect women’s health, either. It threatens women’s health by forcing religious employers into the untenable choice of violating their consciences or dropping health coverage for families and the women they employ. That doesn’t help women’s health; it harms it," Ms. Higgins said.
Meanwhile, the proponents of contraceptive coverage continued their support and celebrated the final rule.
"Today's announcement is a win for civil liberties," Sarah Lipton-Lubet, American Civil Liberties Union policy counsel, said in a statement. "With this rule, the administration continues to stand by women and our families and refuses to let employers use religion to discriminate."
Under the final rule, churches and other houses of worship continue to be exempted from contraceptive coverage requirement. In other words, the religious employers may provide health plans to their employees that do not include contraceptive coverage, officials said June 28.
It also provides accommodations for nonprofit organizations including hospitals, universities, and charities that are opposed to providing birth-control coverage based on religious grounds. Under the rule, these organizations won’t have to "contract, arrange, pay for, or refer contraceptive coverage." Instead, the coverage is provided separately by the health plans of the enrolled women, at no cost.
Such nonprofit groups that have insured and self-insured health plans will have to notify the insurer or third-party administrator that they object to contraception coverage, and the insurers in turn will let the enrollees know that they have access to contraceptive coverage under a separate, no-cost payment.
The rule also provides more details on these accommodations for insurers and third-party administrators, officials said.
"We strongly believe that the cost of contraceptive service will be absolutely cost-neutral and will be offset by improvements in women’s health and reduced pregnancy," Ms. Chiquita Brooks-LaSure, deputy director of policy and regulation at the Center for Consumer Information and Insurance Oversight at HHS, said in a news conference.
Coverage of contraceptive services is part of the recommended preventive care under the health care law and includes all the FDA-approved contraceptive services prescribed by health care providers.
On Twitter @NaseemSMiller
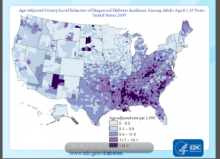
High diabetes incidence in Southern and Appalachian states' counties
CHICAGO – Counties in Southern and Appalachian states have some of the highest incidence rates of diabetes, while counties in large metropolitan areas reported some of the highest numbers of new cases, a government study looking at diabetes incidence rates across the United States revealed.
"We’re hoping that people can use these data to identify high-risk areas, and use it as a tool in implementing and developing diabetes prevention interventions," said Nilka R. Burrows, an epidemiologist at the Centers for Disease Control and Prevention, who presented her abstract at the annual scientific sessions of the American Diabetes Association.
Because changes in incidence rates are one of the first indicators of whether diabetes prevention efforts have been successful, the county-level estimates can help local policy makers, she said.
The interactive maps of diabetes incidence can be found at www.cdc.gov/diabetes/atlas.
Although the study is one of the first of its kind, "the findings don’t tell us anything we didn’t know," said Mercedes R. Carnethon, Ph.D., associate professor at Northwestern University, Chicago, who was not involved in the study. "But [the study] really serves to reemphasize how substantial the burden of the problem is in the high-risk areas," she said.
In 2010, nearly 26 million Americans had diabetes, and 2 million were diagnosed within the past year, said Ms. Burrows.
The authors looked at data for the period of 2004-2009 for adults aged 20 years or older in 3,143 U.S. counties or county equivalents, to identify geographic patterns and high-risk areas.
The county-level incidence estimates incorporated data from the Behavioral Risk Factor Surveillance System (BRFSS) and the U.S. Census.
The sample was age-adjusted to the 2000 U.S. standard population, Ms. Burrows said.
In 2009, the age-adjusted diabetes rate ranged from 4 per 1000 in Colorado to 21 per 1000 in Alabama. Areas that had high diabetes incidence rates (11.7 and higher) were mostly concentrated in Southern and Appalachian states, and in some counties in Arizona, New Mexico, Oklahoma, South Dakota, and Maine, Ms. Burrows reported.
The authors also looked for areas where there were more than 9,000 new diabetes cases in 2009. They identified several major metropolitan areas, including Los Angeles’ LA County with 50,000 new cases, Chicago’s Cook County with 30,000 new cases, Harris County in Houston, and Maricopa County in Phoenix, with 20,000 new cases each.
Urban counties with higher numbers of cases didn’t coincide with counties in rural areas that had the highest incidence rates, since absolute numbers are a function of population size, said Ms. Burrows.
The data "highlighted where we have the highest burden of the problem," said Dr. Carnethon. "So when you think about resources to manage diabetes, including clinical resources, such as people being in those locations, clinicians in particular, we obviously have a higher need for practitioners to deal with diabetes in those parts of the U.S."
The study also showed that the number of counties with a diabetes incidence rate of 11.7 or higher increased from 500 (16%) in 2004 to 870 (28%) in 2009. The median incidence rate was 9.7 in 2004, and 10.1 in 2009.
When calculating the 2009-to-2004 ratio of county-level diabetes incidence rates, the ratios ranged from 0.65 in Indiana to 1.79 in Pennsylvania.
Using a liberal confidence level of 0.1, the 2009-to-2004 incidence rates didn’t change in most of the counties during the 5-year period.
The analysis has some limitations. The diabetes incidence may be underestimated because it was self-reported. Also, the estimates are subject to sampling variability. In addition, during a short time period of 5 years, incidence is not likely to change dramatically, and more years of data are needed.
Ms. Burrows said that surveillance of the information should continue to gauge diabetes prevention efforts.
Ms. Burrows and Dr. Carnethon had no disclosures.
nmiller@frontlinemedcom.com On Twitter @NaseemSMiller
CHICAGO – Counties in Southern and Appalachian states have some of the highest incidence rates of diabetes, while counties in large metropolitan areas reported some of the highest numbers of new cases, a government study looking at diabetes incidence rates across the United States revealed.
"We’re hoping that people can use these data to identify high-risk areas, and use it as a tool in implementing and developing diabetes prevention interventions," said Nilka R. Burrows, an epidemiologist at the Centers for Disease Control and Prevention, who presented her abstract at the annual scientific sessions of the American Diabetes Association.
Because changes in incidence rates are one of the first indicators of whether diabetes prevention efforts have been successful, the county-level estimates can help local policy makers, she said.
The interactive maps of diabetes incidence can be found at www.cdc.gov/diabetes/atlas.
Although the study is one of the first of its kind, "the findings don’t tell us anything we didn’t know," said Mercedes R. Carnethon, Ph.D., associate professor at Northwestern University, Chicago, who was not involved in the study. "But [the study] really serves to reemphasize how substantial the burden of the problem is in the high-risk areas," she said.
In 2010, nearly 26 million Americans had diabetes, and 2 million were diagnosed within the past year, said Ms. Burrows.
The authors looked at data for the period of 2004-2009 for adults aged 20 years or older in 3,143 U.S. counties or county equivalents, to identify geographic patterns and high-risk areas.
The county-level incidence estimates incorporated data from the Behavioral Risk Factor Surveillance System (BRFSS) and the U.S. Census.
The sample was age-adjusted to the 2000 U.S. standard population, Ms. Burrows said.
In 2009, the age-adjusted diabetes rate ranged from 4 per 1000 in Colorado to 21 per 1000 in Alabama. Areas that had high diabetes incidence rates (11.7 and higher) were mostly concentrated in Southern and Appalachian states, and in some counties in Arizona, New Mexico, Oklahoma, South Dakota, and Maine, Ms. Burrows reported.
The authors also looked for areas where there were more than 9,000 new diabetes cases in 2009. They identified several major metropolitan areas, including Los Angeles’ LA County with 50,000 new cases, Chicago’s Cook County with 30,000 new cases, Harris County in Houston, and Maricopa County in Phoenix, with 20,000 new cases each.
Urban counties with higher numbers of cases didn’t coincide with counties in rural areas that had the highest incidence rates, since absolute numbers are a function of population size, said Ms. Burrows.
The data "highlighted where we have the highest burden of the problem," said Dr. Carnethon. "So when you think about resources to manage diabetes, including clinical resources, such as people being in those locations, clinicians in particular, we obviously have a higher need for practitioners to deal with diabetes in those parts of the U.S."
The study also showed that the number of counties with a diabetes incidence rate of 11.7 or higher increased from 500 (16%) in 2004 to 870 (28%) in 2009. The median incidence rate was 9.7 in 2004, and 10.1 in 2009.
When calculating the 2009-to-2004 ratio of county-level diabetes incidence rates, the ratios ranged from 0.65 in Indiana to 1.79 in Pennsylvania.
Using a liberal confidence level of 0.1, the 2009-to-2004 incidence rates didn’t change in most of the counties during the 5-year period.
The analysis has some limitations. The diabetes incidence may be underestimated because it was self-reported. Also, the estimates are subject to sampling variability. In addition, during a short time period of 5 years, incidence is not likely to change dramatically, and more years of data are needed.
Ms. Burrows said that surveillance of the information should continue to gauge diabetes prevention efforts.
Ms. Burrows and Dr. Carnethon had no disclosures.
nmiller@frontlinemedcom.com On Twitter @NaseemSMiller
CHICAGO – Counties in Southern and Appalachian states have some of the highest incidence rates of diabetes, while counties in large metropolitan areas reported some of the highest numbers of new cases, a government study looking at diabetes incidence rates across the United States revealed.
"We’re hoping that people can use these data to identify high-risk areas, and use it as a tool in implementing and developing diabetes prevention interventions," said Nilka R. Burrows, an epidemiologist at the Centers for Disease Control and Prevention, who presented her abstract at the annual scientific sessions of the American Diabetes Association.
Because changes in incidence rates are one of the first indicators of whether diabetes prevention efforts have been successful, the county-level estimates can help local policy makers, she said.
The interactive maps of diabetes incidence can be found at www.cdc.gov/diabetes/atlas.
Although the study is one of the first of its kind, "the findings don’t tell us anything we didn’t know," said Mercedes R. Carnethon, Ph.D., associate professor at Northwestern University, Chicago, who was not involved in the study. "But [the study] really serves to reemphasize how substantial the burden of the problem is in the high-risk areas," she said.
In 2010, nearly 26 million Americans had diabetes, and 2 million were diagnosed within the past year, said Ms. Burrows.
The authors looked at data for the period of 2004-2009 for adults aged 20 years or older in 3,143 U.S. counties or county equivalents, to identify geographic patterns and high-risk areas.
The county-level incidence estimates incorporated data from the Behavioral Risk Factor Surveillance System (BRFSS) and the U.S. Census.
The sample was age-adjusted to the 2000 U.S. standard population, Ms. Burrows said.
In 2009, the age-adjusted diabetes rate ranged from 4 per 1000 in Colorado to 21 per 1000 in Alabama. Areas that had high diabetes incidence rates (11.7 and higher) were mostly concentrated in Southern and Appalachian states, and in some counties in Arizona, New Mexico, Oklahoma, South Dakota, and Maine, Ms. Burrows reported.
The authors also looked for areas where there were more than 9,000 new diabetes cases in 2009. They identified several major metropolitan areas, including Los Angeles’ LA County with 50,000 new cases, Chicago’s Cook County with 30,000 new cases, Harris County in Houston, and Maricopa County in Phoenix, with 20,000 new cases each.
Urban counties with higher numbers of cases didn’t coincide with counties in rural areas that had the highest incidence rates, since absolute numbers are a function of population size, said Ms. Burrows.
The data "highlighted where we have the highest burden of the problem," said Dr. Carnethon. "So when you think about resources to manage diabetes, including clinical resources, such as people being in those locations, clinicians in particular, we obviously have a higher need for practitioners to deal with diabetes in those parts of the U.S."
The study also showed that the number of counties with a diabetes incidence rate of 11.7 or higher increased from 500 (16%) in 2004 to 870 (28%) in 2009. The median incidence rate was 9.7 in 2004, and 10.1 in 2009.
When calculating the 2009-to-2004 ratio of county-level diabetes incidence rates, the ratios ranged from 0.65 in Indiana to 1.79 in Pennsylvania.
Using a liberal confidence level of 0.1, the 2009-to-2004 incidence rates didn’t change in most of the counties during the 5-year period.
The analysis has some limitations. The diabetes incidence may be underestimated because it was self-reported. Also, the estimates are subject to sampling variability. In addition, during a short time period of 5 years, incidence is not likely to change dramatically, and more years of data are needed.
Ms. Burrows said that surveillance of the information should continue to gauge diabetes prevention efforts.
Ms. Burrows and Dr. Carnethon had no disclosures.
nmiller@frontlinemedcom.com On Twitter @NaseemSMiller
AT ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Counties with high incidence rates of diabetes (11.7 and higher) were mostly concentrated in Southern and Appalachian states, and in some counties in Arizona, New Mexico, Oklahoma, South Dakota, and Maine.
Data source: Centers for Disease Control and Prevention’s county-level data between 2004 and 2009 for adults aged 20 years or older, in 3,143 U.S. counties or county equivalents.
Disclosures: Ms. Burrows and Dr. Carnethon had no disclosures.
Acceptance of telecare for MS varied with computer experience, disease duration
ORLANDO – Patients who had multiple sclerosis for a long time or frequently used the Internet were not as accepting of a pilot telecare program as were newer MS patients who didn’t use computers often, according to a small study.
The difference is partly due to the greater body of knowledge that patients with a longer history of MS have gained over the years, sometimes through going online frequently, said Eunme Cha, an epidemiologist at Johns Hopkins University, Baltimore. She presented her poster at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis.
Participants reported that the program had benefits, such as helping them to keep a daily diary of their symptoms and to communicate with their physicians and nurses.
Ms. Cha and her colleagues at the Washington DC VA Medical Center recruited 20 veterans in Washington to use the Home Automated Telemanagement (HAT) system, an online tool they could access via their home computers.
"I wouldn’t say that this is going to replace the entire doctor visit," Ms. Cha said. "But they come pretty far to see the doctor. Traffic is pretty bad in D.C. Some patients travel an entire day to get to their doctor appointment, so they really liked the fact that they could get things done at home."
Mult. Scler.2012;18:472-80A few studies have shown that telemedicine programs can be helpful in the care management of patients with MS. For instance, a 2012 study of 40 patients found that "telecare is a powerful tool for monitoring MS patients at home, carries the potential to improve health care while reducing costs, and should be considered for implementation as part of the management of chronic neurological diseases" (Mult. Scler. 2012;18:472-80).
"I think telemedicine is going to grow in every field of neurology and in every field of medicine that you don’t have to have a hands-on procedure," said Dr. Robert P. Lisak, professor of neurology at Wayne State University, Detroit, and president-elect of the Consortium of Multiple Sclerosis Centers (CMSC). He was not involved in the study.
But there are challenges to overcome. For example, physicians need to be licensed in every state where they are going to be using telemedicine, Dr. Lisak said in an interview. "But if the politics and the economics can be solved, this is very helpful for people and hospitals that are underserved by subspecialists, and even specialists."
For the pilot study, the research staff visited the patients’ homes, provided them with a link to the MS HAT program, and trained them on the system.
"We asked the patients to log in every day and enter "my diary" so that they can report their symptoms on a daily basis," said Ms. Cha. "And then physicians and nurses can log in to the clinician site and see what the patient has put in. Also, patients can put in questions to doctors and nurses. So we try to facilitate communication between patients and physicians."
The 20 veterans (mean age, 54 years) included 14 men and 12 African Americans. They had MS for a mean of 15 years. Sixteen patients said they used a computer at home, and the same number reported using the Internet daily. The patients had a mean Expanded Disability Scale Score (EDSS) of 5.3. Their MS subtypes included secondary progressive (11 patients), relapsing-remitting (6), and primary progressive (3).
A linear regression analysis showed that the length of time patients had MS, how often patients used a computer at home, and English proficiency were all significant predictors of how well the patients accepted the MS HAT program. Meanwhile, race, age, and years of education had no significant relationship with acceptance of the telecare program.
Patients also reported back the benefits of the program, their concerns, and their suggestions. Some said that they liked the ability to report their symptoms right away, keep track of their symptoms, and refresh their memory. They reported concerns with the length of the daily questionnaire and sometimes the redundancy of the content.
Ms. Cha and her colleagues said that "tailoring this technology to patient needs and preferences may improve its acceptance by veterans in MS."
The team is now looking into conducting a clinical trial, comparing a traditional patient group with those who use the telemedicine program, to see if there are any differences in clinical outcomes and patients’ disease management.
Ms. Cha had no disclosures. Dr. Lisak has received research grants from and has been an advisor for several companies, including Avanir, Bayer, Novartis, Questcor, and Teva.
nmiller@frontlinemedcom.com
On Twitter @NaseemSMiller
ORLANDO – Patients who had multiple sclerosis for a long time or frequently used the Internet were not as accepting of a pilot telecare program as were newer MS patients who didn’t use computers often, according to a small study.
The difference is partly due to the greater body of knowledge that patients with a longer history of MS have gained over the years, sometimes through going online frequently, said Eunme Cha, an epidemiologist at Johns Hopkins University, Baltimore. She presented her poster at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis.
Participants reported that the program had benefits, such as helping them to keep a daily diary of their symptoms and to communicate with their physicians and nurses.
Ms. Cha and her colleagues at the Washington DC VA Medical Center recruited 20 veterans in Washington to use the Home Automated Telemanagement (HAT) system, an online tool they could access via their home computers.
"I wouldn’t say that this is going to replace the entire doctor visit," Ms. Cha said. "But they come pretty far to see the doctor. Traffic is pretty bad in D.C. Some patients travel an entire day to get to their doctor appointment, so they really liked the fact that they could get things done at home."
Mult. Scler.2012;18:472-80A few studies have shown that telemedicine programs can be helpful in the care management of patients with MS. For instance, a 2012 study of 40 patients found that "telecare is a powerful tool for monitoring MS patients at home, carries the potential to improve health care while reducing costs, and should be considered for implementation as part of the management of chronic neurological diseases" (Mult. Scler. 2012;18:472-80).
"I think telemedicine is going to grow in every field of neurology and in every field of medicine that you don’t have to have a hands-on procedure," said Dr. Robert P. Lisak, professor of neurology at Wayne State University, Detroit, and president-elect of the Consortium of Multiple Sclerosis Centers (CMSC). He was not involved in the study.
But there are challenges to overcome. For example, physicians need to be licensed in every state where they are going to be using telemedicine, Dr. Lisak said in an interview. "But if the politics and the economics can be solved, this is very helpful for people and hospitals that are underserved by subspecialists, and even specialists."
For the pilot study, the research staff visited the patients’ homes, provided them with a link to the MS HAT program, and trained them on the system.
"We asked the patients to log in every day and enter "my diary" so that they can report their symptoms on a daily basis," said Ms. Cha. "And then physicians and nurses can log in to the clinician site and see what the patient has put in. Also, patients can put in questions to doctors and nurses. So we try to facilitate communication between patients and physicians."
The 20 veterans (mean age, 54 years) included 14 men and 12 African Americans. They had MS for a mean of 15 years. Sixteen patients said they used a computer at home, and the same number reported using the Internet daily. The patients had a mean Expanded Disability Scale Score (EDSS) of 5.3. Their MS subtypes included secondary progressive (11 patients), relapsing-remitting (6), and primary progressive (3).
A linear regression analysis showed that the length of time patients had MS, how often patients used a computer at home, and English proficiency were all significant predictors of how well the patients accepted the MS HAT program. Meanwhile, race, age, and years of education had no significant relationship with acceptance of the telecare program.
Patients also reported back the benefits of the program, their concerns, and their suggestions. Some said that they liked the ability to report their symptoms right away, keep track of their symptoms, and refresh their memory. They reported concerns with the length of the daily questionnaire and sometimes the redundancy of the content.
Ms. Cha and her colleagues said that "tailoring this technology to patient needs and preferences may improve its acceptance by veterans in MS."
The team is now looking into conducting a clinical trial, comparing a traditional patient group with those who use the telemedicine program, to see if there are any differences in clinical outcomes and patients’ disease management.
Ms. Cha had no disclosures. Dr. Lisak has received research grants from and has been an advisor for several companies, including Avanir, Bayer, Novartis, Questcor, and Teva.
nmiller@frontlinemedcom.com
On Twitter @NaseemSMiller
ORLANDO – Patients who had multiple sclerosis for a long time or frequently used the Internet were not as accepting of a pilot telecare program as were newer MS patients who didn’t use computers often, according to a small study.
The difference is partly due to the greater body of knowledge that patients with a longer history of MS have gained over the years, sometimes through going online frequently, said Eunme Cha, an epidemiologist at Johns Hopkins University, Baltimore. She presented her poster at the fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers and the Americas Committee for Treatment and Research in Multiple Sclerosis.
Participants reported that the program had benefits, such as helping them to keep a daily diary of their symptoms and to communicate with their physicians and nurses.
Ms. Cha and her colleagues at the Washington DC VA Medical Center recruited 20 veterans in Washington to use the Home Automated Telemanagement (HAT) system, an online tool they could access via their home computers.
"I wouldn’t say that this is going to replace the entire doctor visit," Ms. Cha said. "But they come pretty far to see the doctor. Traffic is pretty bad in D.C. Some patients travel an entire day to get to their doctor appointment, so they really liked the fact that they could get things done at home."
Mult. Scler.2012;18:472-80A few studies have shown that telemedicine programs can be helpful in the care management of patients with MS. For instance, a 2012 study of 40 patients found that "telecare is a powerful tool for monitoring MS patients at home, carries the potential to improve health care while reducing costs, and should be considered for implementation as part of the management of chronic neurological diseases" (Mult. Scler. 2012;18:472-80).
"I think telemedicine is going to grow in every field of neurology and in every field of medicine that you don’t have to have a hands-on procedure," said Dr. Robert P. Lisak, professor of neurology at Wayne State University, Detroit, and president-elect of the Consortium of Multiple Sclerosis Centers (CMSC). He was not involved in the study.
But there are challenges to overcome. For example, physicians need to be licensed in every state where they are going to be using telemedicine, Dr. Lisak said in an interview. "But if the politics and the economics can be solved, this is very helpful for people and hospitals that are underserved by subspecialists, and even specialists."
For the pilot study, the research staff visited the patients’ homes, provided them with a link to the MS HAT program, and trained them on the system.
"We asked the patients to log in every day and enter "my diary" so that they can report their symptoms on a daily basis," said Ms. Cha. "And then physicians and nurses can log in to the clinician site and see what the patient has put in. Also, patients can put in questions to doctors and nurses. So we try to facilitate communication between patients and physicians."
The 20 veterans (mean age, 54 years) included 14 men and 12 African Americans. They had MS for a mean of 15 years. Sixteen patients said they used a computer at home, and the same number reported using the Internet daily. The patients had a mean Expanded Disability Scale Score (EDSS) of 5.3. Their MS subtypes included secondary progressive (11 patients), relapsing-remitting (6), and primary progressive (3).
A linear regression analysis showed that the length of time patients had MS, how often patients used a computer at home, and English proficiency were all significant predictors of how well the patients accepted the MS HAT program. Meanwhile, race, age, and years of education had no significant relationship with acceptance of the telecare program.
Patients also reported back the benefits of the program, their concerns, and their suggestions. Some said that they liked the ability to report their symptoms right away, keep track of their symptoms, and refresh their memory. They reported concerns with the length of the daily questionnaire and sometimes the redundancy of the content.
Ms. Cha and her colleagues said that "tailoring this technology to patient needs and preferences may improve its acceptance by veterans in MS."
The team is now looking into conducting a clinical trial, comparing a traditional patient group with those who use the telemedicine program, to see if there are any differences in clinical outcomes and patients’ disease management.
Ms. Cha had no disclosures. Dr. Lisak has received research grants from and has been an advisor for several companies, including Avanir, Bayer, Novartis, Questcor, and Teva.
nmiller@frontlinemedcom.com
On Twitter @NaseemSMiller
AT THE CMSC/ACTRIMS ANNUAL MEETING
Major finding: A linear regression analysis showed that the length of time patients had MS, how often patients used a computer at home, and English proficiency were all significant predictors of how well the patients accepted the MS Home Automated Telemanagement program.
Data source: Survey of 20 veterans with MS who participated in a pilot telecare program.
Disclosures: Ms. Cha had no disclosures. Dr. Lisak has received research grants from and has been an advisor for several companies, including Avanir, Bayer, Novartis, Questcor, and Teva.
In esophageal cancer, adenocarcinoma rates increased over four decades
ORLANDO – While esophageal cancer survival rates have improved over the past four decades, the prevalence of adenocarcinoma has also increased significantly, according to an analysis of a national database.
"Our study emphasized the importance of early detection of disease and increased utilization of locoregional therapies," said Dr. Basile Njei of the University of Connecticut, Farmington. He presented his abstract, which is not published, at the annual Digestive Disease Week.
Dr. Njei said that although the rise in the incidence of esophageal cancer in the United States has been well documented, there is a lack of data on trends in long-term survival and prognostic factors associated with esophageal cancer survival.
The authors analyzed the national Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2009. The database included 18 cancer registries representing about 26% of the U.S. population.
The majority of the 93,191 patients included in the analysis were older white males. Patients diagnosed by autopsy or death certificates were excluded.
The number of patients increased from 6,700 in the 1970s to 53,000 in the 2000s. In addition, the median age of patients at baseline increased significantly, from 63 years to 68 years, as did the percentage of white patients, from 74% to 86%, and the rate of adenocarcinoma, from 35% to 61% (P for trend less than .05 for all).
Dr. Njei said that while in the 1990s squamous cell carcinoma (SCC) was the most prevalent type of esophageal cancer, after the 1990s adenocarcinoma became more prevalent. He attributed the rise and fall of the two types of cancer to etiology.
The investigators divided the study population according to histology (adenocarcinoma, AC vs. squamous cell carcinoma, SCC), and by decade of diagnosis. They also analyzed independent predictors of mortality across subgroups. These factors included age, gender, ethnicity, tumor location, stage of disease, and treatment modality.
The results showed that the overall median survival was 9 months. There was a significant increase in overall median survival between the 1970s and the 2000s, from 6 months to 10 months (P less than .001). The overall 5-year survival rate was 15.5%, and there was a significant increase in overall 5-year survival, from 8.1% to 21.3%.
There were also significant improvements in survival for local, regional, and metastatic esophageal cancer during the 40 years, the authors found.
The diagnosis of esophageal cancer at a localized stage increased significantly during the study period, from 11% to 35% (P less than .001).
In addition, there were significant increases in surgical treatment (50% to 64%; P less than .05) and adjuvant radiotherapy (47% to 53%; P less than .05) during the four decades.
Meanwhile, age, sex, tumor histology, stage at diagnosis, node status, adjuvant radiotherapy, and surgery were independently associated with overall survival, the authors reported.
"There’s not much that’s really new in terms of the overall presentation here," said Dr. David C. Metz, professor of medicine at the University of Pennsylvania, Philadelphia. "But it does cement what we’ve been thinking over time, that the shift between squamous cell carcinoma and adenocarcinoma is real, and that we are starting to make an impact in terms of outcome," Dr. Metz said in an interview. "I think there are limitations on how much you can learn from databases, but I think this is a good study."
Although the study had a large sample size and was from a widely validated database, the data are retrospective, there were no chemotherapy data, there is a lead-time and length-time bias, and the findings cannot be generalized to populations in other countries, said Dr. Njei.
Dr. Njei had no disclosures. Dr. Metz is on the advisory committee/review panel for Eisai; has consulted for Novartis, Solesta, Fresenius, and Abbott; and has received grant/research support from Tercica and Lutethera.
On Twitter @NaseemSMiller
ORLANDO – While esophageal cancer survival rates have improved over the past four decades, the prevalence of adenocarcinoma has also increased significantly, according to an analysis of a national database.
"Our study emphasized the importance of early detection of disease and increased utilization of locoregional therapies," said Dr. Basile Njei of the University of Connecticut, Farmington. He presented his abstract, which is not published, at the annual Digestive Disease Week.
Dr. Njei said that although the rise in the incidence of esophageal cancer in the United States has been well documented, there is a lack of data on trends in long-term survival and prognostic factors associated with esophageal cancer survival.
The authors analyzed the national Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2009. The database included 18 cancer registries representing about 26% of the U.S. population.
The majority of the 93,191 patients included in the analysis were older white males. Patients diagnosed by autopsy or death certificates were excluded.
The number of patients increased from 6,700 in the 1970s to 53,000 in the 2000s. In addition, the median age of patients at baseline increased significantly, from 63 years to 68 years, as did the percentage of white patients, from 74% to 86%, and the rate of adenocarcinoma, from 35% to 61% (P for trend less than .05 for all).
Dr. Njei said that while in the 1990s squamous cell carcinoma (SCC) was the most prevalent type of esophageal cancer, after the 1990s adenocarcinoma became more prevalent. He attributed the rise and fall of the two types of cancer to etiology.
The investigators divided the study population according to histology (adenocarcinoma, AC vs. squamous cell carcinoma, SCC), and by decade of diagnosis. They also analyzed independent predictors of mortality across subgroups. These factors included age, gender, ethnicity, tumor location, stage of disease, and treatment modality.
The results showed that the overall median survival was 9 months. There was a significant increase in overall median survival between the 1970s and the 2000s, from 6 months to 10 months (P less than .001). The overall 5-year survival rate was 15.5%, and there was a significant increase in overall 5-year survival, from 8.1% to 21.3%.
There were also significant improvements in survival for local, regional, and metastatic esophageal cancer during the 40 years, the authors found.
The diagnosis of esophageal cancer at a localized stage increased significantly during the study period, from 11% to 35% (P less than .001).
In addition, there were significant increases in surgical treatment (50% to 64%; P less than .05) and adjuvant radiotherapy (47% to 53%; P less than .05) during the four decades.
Meanwhile, age, sex, tumor histology, stage at diagnosis, node status, adjuvant radiotherapy, and surgery were independently associated with overall survival, the authors reported.
"There’s not much that’s really new in terms of the overall presentation here," said Dr. David C. Metz, professor of medicine at the University of Pennsylvania, Philadelphia. "But it does cement what we’ve been thinking over time, that the shift between squamous cell carcinoma and adenocarcinoma is real, and that we are starting to make an impact in terms of outcome," Dr. Metz said in an interview. "I think there are limitations on how much you can learn from databases, but I think this is a good study."
Although the study had a large sample size and was from a widely validated database, the data are retrospective, there were no chemotherapy data, there is a lead-time and length-time bias, and the findings cannot be generalized to populations in other countries, said Dr. Njei.
Dr. Njei had no disclosures. Dr. Metz is on the advisory committee/review panel for Eisai; has consulted for Novartis, Solesta, Fresenius, and Abbott; and has received grant/research support from Tercica and Lutethera.
On Twitter @NaseemSMiller
ORLANDO – While esophageal cancer survival rates have improved over the past four decades, the prevalence of adenocarcinoma has also increased significantly, according to an analysis of a national database.
"Our study emphasized the importance of early detection of disease and increased utilization of locoregional therapies," said Dr. Basile Njei of the University of Connecticut, Farmington. He presented his abstract, which is not published, at the annual Digestive Disease Week.
Dr. Njei said that although the rise in the incidence of esophageal cancer in the United States has been well documented, there is a lack of data on trends in long-term survival and prognostic factors associated with esophageal cancer survival.
The authors analyzed the national Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2009. The database included 18 cancer registries representing about 26% of the U.S. population.
The majority of the 93,191 patients included in the analysis were older white males. Patients diagnosed by autopsy or death certificates were excluded.
The number of patients increased from 6,700 in the 1970s to 53,000 in the 2000s. In addition, the median age of patients at baseline increased significantly, from 63 years to 68 years, as did the percentage of white patients, from 74% to 86%, and the rate of adenocarcinoma, from 35% to 61% (P for trend less than .05 for all).
Dr. Njei said that while in the 1990s squamous cell carcinoma (SCC) was the most prevalent type of esophageal cancer, after the 1990s adenocarcinoma became more prevalent. He attributed the rise and fall of the two types of cancer to etiology.
The investigators divided the study population according to histology (adenocarcinoma, AC vs. squamous cell carcinoma, SCC), and by decade of diagnosis. They also analyzed independent predictors of mortality across subgroups. These factors included age, gender, ethnicity, tumor location, stage of disease, and treatment modality.
The results showed that the overall median survival was 9 months. There was a significant increase in overall median survival between the 1970s and the 2000s, from 6 months to 10 months (P less than .001). The overall 5-year survival rate was 15.5%, and there was a significant increase in overall 5-year survival, from 8.1% to 21.3%.
There were also significant improvements in survival for local, regional, and metastatic esophageal cancer during the 40 years, the authors found.
The diagnosis of esophageal cancer at a localized stage increased significantly during the study period, from 11% to 35% (P less than .001).
In addition, there were significant increases in surgical treatment (50% to 64%; P less than .05) and adjuvant radiotherapy (47% to 53%; P less than .05) during the four decades.
Meanwhile, age, sex, tumor histology, stage at diagnosis, node status, adjuvant radiotherapy, and surgery were independently associated with overall survival, the authors reported.
"There’s not much that’s really new in terms of the overall presentation here," said Dr. David C. Metz, professor of medicine at the University of Pennsylvania, Philadelphia. "But it does cement what we’ve been thinking over time, that the shift between squamous cell carcinoma and adenocarcinoma is real, and that we are starting to make an impact in terms of outcome," Dr. Metz said in an interview. "I think there are limitations on how much you can learn from databases, but I think this is a good study."
Although the study had a large sample size and was from a widely validated database, the data are retrospective, there were no chemotherapy data, there is a lead-time and length-time bias, and the findings cannot be generalized to populations in other countries, said Dr. Njei.
Dr. Njei had no disclosures. Dr. Metz is on the advisory committee/review panel for Eisai; has consulted for Novartis, Solesta, Fresenius, and Abbott; and has received grant/research support from Tercica and Lutethera.
On Twitter @NaseemSMiller
AT DDW 2013
Major finding: During the past 40 years, overall median survival for esophageal cancer significantly increased, from 6 months to 10 months (P less than .001).
Data source: Surveillance, Epidemiology, and End Results (SEER) database, from 1973 to 2009, including 93,191 patients.
Disclosures: Dr. Njei had no disclosures. Dr. Metz is on the advisory committee/review panel for Eisai; has consulted for Novartis, Solesta, Fresenius, and Abbott; and has received grant/research support from Tercica and Lutethera.
Weight loss, exercise didn't affect heart outcomes in Look AHEAD
CHICAGO – A decade of diet and exercise had no effect on cardiovascular morbidity and mortality in overweight and obese adults with type 2 diabetes, according to a randomized, federally funded study that was stopped 2 years early due to futility.
"However, there are many reasons why, I would argue, that you should be encouraging patients with diabetes to lose weight," said Rena R. Wing, Ph.D., the trial’s chair. The results showed that the participants who were in the diet and exercise group, "are less likely to have kidney disease, have less incidence of depression, lower hospital costs, and less medication costs, so there are still many advantages to intense lifestyle interventions," Dr. Wing said during the presentation of the findings at the annual scientific sessions of the American Diabetes Association.
The results also showed that the participants were able to maintain a modest weight loss during a 10-year period.
The Look AHEAD (Action for Health in Diabetes) trial aimed to fill a gap in existing data about whether intensive lifestyle intervention would decrease cardiovascular morbidity and mortality of patients with type 2 diabetes in the long term.
Researchers recruited more than 5,100 patients and randomized them to the intensive lifestyle intervention program, which decreased calorie intake and increased exercise, or to the control group, which provided diabetes support and education. The goal of the intervention was achieving and maintaining weight loss of at least 7% through group and individual counseling.
The trial’s primary outcome was a composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina. The trial was planned to have a greater than 80% probability of detecting an 18% difference in cardiovascular events between the two arms, assuming that two-sided alpha was 0.05, a primary outcome rate of 2% per year in the control group, and the planned maximum follow-up of 13.5 years.
But in September 2012, the trial was stopped at the request of its primary sponsors, based on a futility analysis. The median follow-up at the time was 9.6 years.
By then, 403 patients in the intervention group and 418 patients in the control group had met the trial’s primary outcome, with no statistically significant difference between the two groups (1.83 and 1.92 events per 100 person-years, respectively).
Why didn’t it work?
Researchers listed several possible explanations for the results. The study may have had insufficient power, although that wouldn’t explain the negative results, the authors wrote. It is also possible that a higher sustained weight loss was needed in the intervention group to reduce the risk of cardiovascular disease. Meanwhile, the educational sessions and increased use of statins in the control group may have lessened the difference between the two groups, the authors noted. And intensified medical management of cardiovascular risk factors for both groups may have made the relative benefit of the intensive lifestyle intervention more difficult to demonstrate, they added.
Earlier intervention during the course of diabetes may be needed, they added. The results were published online simultaneously with the presentation (N. Engl. J. Med. 2013 June 24 [doi:10.1056/NEJMoa1212914]).
In an editorial accompanying the published results, Dr. Hertzel C. Gerstein wrote that the inclusion of "the somewhat unreliable outcome of hospitalization for angina in the primary composite outcome may have added noise and further obscured any emerging signal" (N. Engl. J. Med. 2013 June 24 [doi:10.1056/NEJMe1306987]).
During the presentation, an audience member suggested that since the 1-year and 4-year findings of the trial were published and well publicized, the participants in the control group might have taken it upon themselves to take action and lose weight. The panel of investigators said that is also a possibility.
Dr. Peter H. Bennett, who is one of the trial’s investigators and was cochair of eligibility, said that stopping the trial "was a big mistake," and that it should have been carried on at least until the planned termination point. "They stopped this trial because they were projecting that if they carried on for 2 more years, the differences in the primary endpoint were unlikely to be statistically significant. But there are several problems. First, you prejudice the power to analyze any other events, and just as we’ve learned from natural history of diabetes-related mortality, it’s clear that you don’t see excess diabetes-related mortality until they’ve had at least 15 years of diabetes, and that’s just at the point that the trial was stopped," Dr. Bennett, scientist emeritus at the National Institutes of Health – one of the main funders of the trial – said in an interview.
"It is also possible that lifestyle interventions may have a real but modest effect on cardiovascular outcomes akin to that of glucose lowering (e.g., a 10%-15% reduction) that requires more than 10 years to become apparent. If so, this trial was clearly too small to detect such an effect," wrote Dr. Gerstein, an endocrinologist and professor at McMaster University and Hamilton Health Sciences, Hamilton, Ont.
A look at the data
The patients in the intervention group (2,570) and control group (2,575) had similar characteristics at baseline. They were between 45 and 75 years old, were mostly female (60%) and white (63%), and had a mean body mass index of 36.0. Fourteen percent had a history of cardiovascular disease, and the median duration of diabetes was 5 years. Less than 30% were on insulin.
Close to 96% of the patients stayed in the trial.
During months 1 and 6, the intervention group had weekly contact with staff members. The contact was gradually reduced in the following months to one on-site individual session per month, and one phone call or e-mail per month from 2 years onward. The patients’ medications were adjusted by their own physicians.
Patients in the control group had 3-4 meetings per year during years 1 through 4, and after that, they had one meeting per year. They received education on diet, exercise, and social support.
Weight loss was greater in the intervention group compared with the control group, with the greatest difference at 1 year (8.6% vs. 0.7%), but it remained significant through the end of the study (6.0% vs. 3.5%).
Specifically, the intervention group had an initial weight loss of 8.6%, followed by weight regain in the first 5 years, and then gradual weight loss, leading to average weight loss of 6%. The control group had a gradual but consistent weight loss, for an average of 3.5% at the end of the trial.
One audience member suggested that some of the weight loss might have been due to aging. The authors said they are looking at those factors in their ongoing data analysis, which they’re planning to publish in the near future.
Meanwhile, the intervention group had significantly greater weight loss than did the control group throughout the trial. They had significantly greater improvements in fitness, hemoglobin A1c levels, systolic blood pressure, and HDL cholesterol (P less than .05 for all). The control group had significantly greater reductions in LDL cholesterol (P less than .05), although they also had a significantly greater use of medications, including insulin, statins, and antihypertensives, compared with the intervention group, the authors noted.
In the short-term (1-4 years), the intervention group saw improvements in cardiovascular risk factors, preservation of mobility, and improvements in fitness, obstructive sleep apnea, fatty liver disease, urinary incontinence, sexual function, and markers of inflammation.
The intervention also reduced the incidence of high-risk chronic kidney disease by 31%, and reduced the total costs and service utilization, especially related to hospitalization ($2,500 per participant), and medication ($2,500 per participant), the authors reported. Although the intervention didn’t affect neuropathy symptoms, it reduced the incidence of reported retinopathy by 14%, reduced the incidence of symptoms of mild or greater depression by 20%, and slowed age-related decline in reported physical function.
Program reduced burden of diabetes
"Even with no clear evidence of cardiovascular benefit, the Look AHEAD investigators have shown that attention to activity and diet can safely reduce the burden of diabetes and have reaffirmed the importance of lifestyle approach as one of the foundations of modern diabetes care," Dr. Gerstein wrote in his editorial, adding that the data show "intensive lifestyle interventions are unlikely to cause harm and may provide a modest benefit."
Interactions among subgroups – history of cardiovascular disease at baseline, and gender, race/ethnicity – showed nonsignificant interaction with cardiovascular disease history (P = .06).
Summarizing the study’s limitations, the authors said that it’s not clear whether changing the intervention groups’ dietary composition might have yielded different results. Their diet during the intervention was 1,200 to 1,800 kcal/day, with less than 30% of calories from fat, and less than 15% from protein. Authors added that the patients recruited successfully competed the fitness test at baseline, so the results can’t be generalized to all patients with type 2 diabetes.
Although the intervention has stopped, the trial continues as an observational study, said Dr. Wing. And some wonder if longer-term results would reveal new information.
"My prediction is that even after stopping the trial, as long as follow-up is carried on for several more years, you’ll see differences in total mortality," said Dr. Bennett, citing the results of UK Prospective Diabetes Study (UKPDS), which showed no difference in cardiovascular disease at 12 years, but a significant difference at 20 years. "So it does appear that intervention does have a lasting but delayed effect on that endpoint."
"I hope [the results] are not misinterpreted," Dr. Bennett continued. "There’s a danger given the lack of effect of the primary endpoint that the headlines will say weight loss is useless, and that’s a total misinterpretation in my view. There are multiple reasons to encourage weight loss. Other than the fact that it doesn’t appear to affect mortality up to this point, it seems to have benefit for all sorts of other things – people feel better, walk better, they develop advanced renal disease less frequently, their cholesterol and blood pressure are lower. I hope that message gets through rather than the negative one."
Dr. Wing, Dr. Bennett, and Dr. Gerstein had no disclosures relevant to the trial. The trial was supported by the National Institutes of Health, other Department of Health and Human Services agencies, and several companies including FedEx, Johnson & Johnson, Nestle Healthcare Nutrition, and Abbott Nutrition.
On Twitter @NaseemSMiller
I think the trial has a good message: that lifestyle can work, that intervention can work, that education can work, but it's not the only solution. For many people with diabetes, we must use medication. And, in many people with coronary disease, we must use medications. But to call it a failed trial is ridiculous, in my mind. If I can delay complications by 4 or 5 years, then we did well, we saved money, and we improved somebody's life.
We saw a 6% reduction in weight over 10 years. This is awesome and has never been shown before. Actually 3.5% reduction in weight [over 10 years] hasn't been shown before. I think that if you read the study, a lot of good stuff came out of it.
I will tell my patients how well the lifestyle management works. But I still will tell them that lifestyle is important, but many times, it needs to come in combination with medication.
My issue is that the trial was cut [short]. They saw that other aspects improved, and they should have continued to see what it means. It's like anything; we want fast solution to long-term tissues. Maybe we need to see a population study over 25 or 30 years.
Dr. Yehuda Handelsman is the medical director and principal investigator of Metabolic Institute of America, and president-elect of the American College of Endocrinology. He is on the advisory board for Clinical Endocrinology News.
I think the trial has a good message: that lifestyle can work, that intervention can work, that education can work, but it's not the only solution. For many people with diabetes, we must use medication. And, in many people with coronary disease, we must use medications. But to call it a failed trial is ridiculous, in my mind. If I can delay complications by 4 or 5 years, then we did well, we saved money, and we improved somebody's life.
We saw a 6% reduction in weight over 10 years. This is awesome and has never been shown before. Actually 3.5% reduction in weight [over 10 years] hasn't been shown before. I think that if you read the study, a lot of good stuff came out of it.
I will tell my patients how well the lifestyle management works. But I still will tell them that lifestyle is important, but many times, it needs to come in combination with medication.
My issue is that the trial was cut [short]. They saw that other aspects improved, and they should have continued to see what it means. It's like anything; we want fast solution to long-term tissues. Maybe we need to see a population study over 25 or 30 years.
Dr. Yehuda Handelsman is the medical director and principal investigator of Metabolic Institute of America, and president-elect of the American College of Endocrinology. He is on the advisory board for Clinical Endocrinology News.
I think the trial has a good message: that lifestyle can work, that intervention can work, that education can work, but it's not the only solution. For many people with diabetes, we must use medication. And, in many people with coronary disease, we must use medications. But to call it a failed trial is ridiculous, in my mind. If I can delay complications by 4 or 5 years, then we did well, we saved money, and we improved somebody's life.
We saw a 6% reduction in weight over 10 years. This is awesome and has never been shown before. Actually 3.5% reduction in weight [over 10 years] hasn't been shown before. I think that if you read the study, a lot of good stuff came out of it.
I will tell my patients how well the lifestyle management works. But I still will tell them that lifestyle is important, but many times, it needs to come in combination with medication.
My issue is that the trial was cut [short]. They saw that other aspects improved, and they should have continued to see what it means. It's like anything; we want fast solution to long-term tissues. Maybe we need to see a population study over 25 or 30 years.
Dr. Yehuda Handelsman is the medical director and principal investigator of Metabolic Institute of America, and president-elect of the American College of Endocrinology. He is on the advisory board for Clinical Endocrinology News.
CHICAGO – A decade of diet and exercise had no effect on cardiovascular morbidity and mortality in overweight and obese adults with type 2 diabetes, according to a randomized, federally funded study that was stopped 2 years early due to futility.
"However, there are many reasons why, I would argue, that you should be encouraging patients with diabetes to lose weight," said Rena R. Wing, Ph.D., the trial’s chair. The results showed that the participants who were in the diet and exercise group, "are less likely to have kidney disease, have less incidence of depression, lower hospital costs, and less medication costs, so there are still many advantages to intense lifestyle interventions," Dr. Wing said during the presentation of the findings at the annual scientific sessions of the American Diabetes Association.
The results also showed that the participants were able to maintain a modest weight loss during a 10-year period.
The Look AHEAD (Action for Health in Diabetes) trial aimed to fill a gap in existing data about whether intensive lifestyle intervention would decrease cardiovascular morbidity and mortality of patients with type 2 diabetes in the long term.
Researchers recruited more than 5,100 patients and randomized them to the intensive lifestyle intervention program, which decreased calorie intake and increased exercise, or to the control group, which provided diabetes support and education. The goal of the intervention was achieving and maintaining weight loss of at least 7% through group and individual counseling.
The trial’s primary outcome was a composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina. The trial was planned to have a greater than 80% probability of detecting an 18% difference in cardiovascular events between the two arms, assuming that two-sided alpha was 0.05, a primary outcome rate of 2% per year in the control group, and the planned maximum follow-up of 13.5 years.
But in September 2012, the trial was stopped at the request of its primary sponsors, based on a futility analysis. The median follow-up at the time was 9.6 years.
By then, 403 patients in the intervention group and 418 patients in the control group had met the trial’s primary outcome, with no statistically significant difference between the two groups (1.83 and 1.92 events per 100 person-years, respectively).
Why didn’t it work?
Researchers listed several possible explanations for the results. The study may have had insufficient power, although that wouldn’t explain the negative results, the authors wrote. It is also possible that a higher sustained weight loss was needed in the intervention group to reduce the risk of cardiovascular disease. Meanwhile, the educational sessions and increased use of statins in the control group may have lessened the difference between the two groups, the authors noted. And intensified medical management of cardiovascular risk factors for both groups may have made the relative benefit of the intensive lifestyle intervention more difficult to demonstrate, they added.
Earlier intervention during the course of diabetes may be needed, they added. The results were published online simultaneously with the presentation (N. Engl. J. Med. 2013 June 24 [doi:10.1056/NEJMoa1212914]).
In an editorial accompanying the published results, Dr. Hertzel C. Gerstein wrote that the inclusion of "the somewhat unreliable outcome of hospitalization for angina in the primary composite outcome may have added noise and further obscured any emerging signal" (N. Engl. J. Med. 2013 June 24 [doi:10.1056/NEJMe1306987]).
During the presentation, an audience member suggested that since the 1-year and 4-year findings of the trial were published and well publicized, the participants in the control group might have taken it upon themselves to take action and lose weight. The panel of investigators said that is also a possibility.
Dr. Peter H. Bennett, who is one of the trial’s investigators and was cochair of eligibility, said that stopping the trial "was a big mistake," and that it should have been carried on at least until the planned termination point. "They stopped this trial because they were projecting that if they carried on for 2 more years, the differences in the primary endpoint were unlikely to be statistically significant. But there are several problems. First, you prejudice the power to analyze any other events, and just as we’ve learned from natural history of diabetes-related mortality, it’s clear that you don’t see excess diabetes-related mortality until they’ve had at least 15 years of diabetes, and that’s just at the point that the trial was stopped," Dr. Bennett, scientist emeritus at the National Institutes of Health – one of the main funders of the trial – said in an interview.
"It is also possible that lifestyle interventions may have a real but modest effect on cardiovascular outcomes akin to that of glucose lowering (e.g., a 10%-15% reduction) that requires more than 10 years to become apparent. If so, this trial was clearly too small to detect such an effect," wrote Dr. Gerstein, an endocrinologist and professor at McMaster University and Hamilton Health Sciences, Hamilton, Ont.
A look at the data
The patients in the intervention group (2,570) and control group (2,575) had similar characteristics at baseline. They were between 45 and 75 years old, were mostly female (60%) and white (63%), and had a mean body mass index of 36.0. Fourteen percent had a history of cardiovascular disease, and the median duration of diabetes was 5 years. Less than 30% were on insulin.
Close to 96% of the patients stayed in the trial.
During months 1 and 6, the intervention group had weekly contact with staff members. The contact was gradually reduced in the following months to one on-site individual session per month, and one phone call or e-mail per month from 2 years onward. The patients’ medications were adjusted by their own physicians.
Patients in the control group had 3-4 meetings per year during years 1 through 4, and after that, they had one meeting per year. They received education on diet, exercise, and social support.
Weight loss was greater in the intervention group compared with the control group, with the greatest difference at 1 year (8.6% vs. 0.7%), but it remained significant through the end of the study (6.0% vs. 3.5%).
Specifically, the intervention group had an initial weight loss of 8.6%, followed by weight regain in the first 5 years, and then gradual weight loss, leading to average weight loss of 6%. The control group had a gradual but consistent weight loss, for an average of 3.5% at the end of the trial.
One audience member suggested that some of the weight loss might have been due to aging. The authors said they are looking at those factors in their ongoing data analysis, which they’re planning to publish in the near future.
Meanwhile, the intervention group had significantly greater weight loss than did the control group throughout the trial. They had significantly greater improvements in fitness, hemoglobin A1c levels, systolic blood pressure, and HDL cholesterol (P less than .05 for all). The control group had significantly greater reductions in LDL cholesterol (P less than .05), although they also had a significantly greater use of medications, including insulin, statins, and antihypertensives, compared with the intervention group, the authors noted.
In the short-term (1-4 years), the intervention group saw improvements in cardiovascular risk factors, preservation of mobility, and improvements in fitness, obstructive sleep apnea, fatty liver disease, urinary incontinence, sexual function, and markers of inflammation.
The intervention also reduced the incidence of high-risk chronic kidney disease by 31%, and reduced the total costs and service utilization, especially related to hospitalization ($2,500 per participant), and medication ($2,500 per participant), the authors reported. Although the intervention didn’t affect neuropathy symptoms, it reduced the incidence of reported retinopathy by 14%, reduced the incidence of symptoms of mild or greater depression by 20%, and slowed age-related decline in reported physical function.
Program reduced burden of diabetes
"Even with no clear evidence of cardiovascular benefit, the Look AHEAD investigators have shown that attention to activity and diet can safely reduce the burden of diabetes and have reaffirmed the importance of lifestyle approach as one of the foundations of modern diabetes care," Dr. Gerstein wrote in his editorial, adding that the data show "intensive lifestyle interventions are unlikely to cause harm and may provide a modest benefit."
Interactions among subgroups – history of cardiovascular disease at baseline, and gender, race/ethnicity – showed nonsignificant interaction with cardiovascular disease history (P = .06).
Summarizing the study’s limitations, the authors said that it’s not clear whether changing the intervention groups’ dietary composition might have yielded different results. Their diet during the intervention was 1,200 to 1,800 kcal/day, with less than 30% of calories from fat, and less than 15% from protein. Authors added that the patients recruited successfully competed the fitness test at baseline, so the results can’t be generalized to all patients with type 2 diabetes.
Although the intervention has stopped, the trial continues as an observational study, said Dr. Wing. And some wonder if longer-term results would reveal new information.
"My prediction is that even after stopping the trial, as long as follow-up is carried on for several more years, you’ll see differences in total mortality," said Dr. Bennett, citing the results of UK Prospective Diabetes Study (UKPDS), which showed no difference in cardiovascular disease at 12 years, but a significant difference at 20 years. "So it does appear that intervention does have a lasting but delayed effect on that endpoint."
"I hope [the results] are not misinterpreted," Dr. Bennett continued. "There’s a danger given the lack of effect of the primary endpoint that the headlines will say weight loss is useless, and that’s a total misinterpretation in my view. There are multiple reasons to encourage weight loss. Other than the fact that it doesn’t appear to affect mortality up to this point, it seems to have benefit for all sorts of other things – people feel better, walk better, they develop advanced renal disease less frequently, their cholesterol and blood pressure are lower. I hope that message gets through rather than the negative one."
Dr. Wing, Dr. Bennett, and Dr. Gerstein had no disclosures relevant to the trial. The trial was supported by the National Institutes of Health, other Department of Health and Human Services agencies, and several companies including FedEx, Johnson & Johnson, Nestle Healthcare Nutrition, and Abbott Nutrition.
On Twitter @NaseemSMiller
CHICAGO – A decade of diet and exercise had no effect on cardiovascular morbidity and mortality in overweight and obese adults with type 2 diabetes, according to a randomized, federally funded study that was stopped 2 years early due to futility.
"However, there are many reasons why, I would argue, that you should be encouraging patients with diabetes to lose weight," said Rena R. Wing, Ph.D., the trial’s chair. The results showed that the participants who were in the diet and exercise group, "are less likely to have kidney disease, have less incidence of depression, lower hospital costs, and less medication costs, so there are still many advantages to intense lifestyle interventions," Dr. Wing said during the presentation of the findings at the annual scientific sessions of the American Diabetes Association.
The results also showed that the participants were able to maintain a modest weight loss during a 10-year period.
The Look AHEAD (Action for Health in Diabetes) trial aimed to fill a gap in existing data about whether intensive lifestyle intervention would decrease cardiovascular morbidity and mortality of patients with type 2 diabetes in the long term.
Researchers recruited more than 5,100 patients and randomized them to the intensive lifestyle intervention program, which decreased calorie intake and increased exercise, or to the control group, which provided diabetes support and education. The goal of the intervention was achieving and maintaining weight loss of at least 7% through group and individual counseling.
The trial’s primary outcome was a composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina. The trial was planned to have a greater than 80% probability of detecting an 18% difference in cardiovascular events between the two arms, assuming that two-sided alpha was 0.05, a primary outcome rate of 2% per year in the control group, and the planned maximum follow-up of 13.5 years.
But in September 2012, the trial was stopped at the request of its primary sponsors, based on a futility analysis. The median follow-up at the time was 9.6 years.
By then, 403 patients in the intervention group and 418 patients in the control group had met the trial’s primary outcome, with no statistically significant difference between the two groups (1.83 and 1.92 events per 100 person-years, respectively).
Why didn’t it work?
Researchers listed several possible explanations for the results. The study may have had insufficient power, although that wouldn’t explain the negative results, the authors wrote. It is also possible that a higher sustained weight loss was needed in the intervention group to reduce the risk of cardiovascular disease. Meanwhile, the educational sessions and increased use of statins in the control group may have lessened the difference between the two groups, the authors noted. And intensified medical management of cardiovascular risk factors for both groups may have made the relative benefit of the intensive lifestyle intervention more difficult to demonstrate, they added.
Earlier intervention during the course of diabetes may be needed, they added. The results were published online simultaneously with the presentation (N. Engl. J. Med. 2013 June 24 [doi:10.1056/NEJMoa1212914]).
In an editorial accompanying the published results, Dr. Hertzel C. Gerstein wrote that the inclusion of "the somewhat unreliable outcome of hospitalization for angina in the primary composite outcome may have added noise and further obscured any emerging signal" (N. Engl. J. Med. 2013 June 24 [doi:10.1056/NEJMe1306987]).
During the presentation, an audience member suggested that since the 1-year and 4-year findings of the trial were published and well publicized, the participants in the control group might have taken it upon themselves to take action and lose weight. The panel of investigators said that is also a possibility.
Dr. Peter H. Bennett, who is one of the trial’s investigators and was cochair of eligibility, said that stopping the trial "was a big mistake," and that it should have been carried on at least until the planned termination point. "They stopped this trial because they were projecting that if they carried on for 2 more years, the differences in the primary endpoint were unlikely to be statistically significant. But there are several problems. First, you prejudice the power to analyze any other events, and just as we’ve learned from natural history of diabetes-related mortality, it’s clear that you don’t see excess diabetes-related mortality until they’ve had at least 15 years of diabetes, and that’s just at the point that the trial was stopped," Dr. Bennett, scientist emeritus at the National Institutes of Health – one of the main funders of the trial – said in an interview.
"It is also possible that lifestyle interventions may have a real but modest effect on cardiovascular outcomes akin to that of glucose lowering (e.g., a 10%-15% reduction) that requires more than 10 years to become apparent. If so, this trial was clearly too small to detect such an effect," wrote Dr. Gerstein, an endocrinologist and professor at McMaster University and Hamilton Health Sciences, Hamilton, Ont.
A look at the data
The patients in the intervention group (2,570) and control group (2,575) had similar characteristics at baseline. They were between 45 and 75 years old, were mostly female (60%) and white (63%), and had a mean body mass index of 36.0. Fourteen percent had a history of cardiovascular disease, and the median duration of diabetes was 5 years. Less than 30% were on insulin.
Close to 96% of the patients stayed in the trial.
During months 1 and 6, the intervention group had weekly contact with staff members. The contact was gradually reduced in the following months to one on-site individual session per month, and one phone call or e-mail per month from 2 years onward. The patients’ medications were adjusted by their own physicians.
Patients in the control group had 3-4 meetings per year during years 1 through 4, and after that, they had one meeting per year. They received education on diet, exercise, and social support.
Weight loss was greater in the intervention group compared with the control group, with the greatest difference at 1 year (8.6% vs. 0.7%), but it remained significant through the end of the study (6.0% vs. 3.5%).
Specifically, the intervention group had an initial weight loss of 8.6%, followed by weight regain in the first 5 years, and then gradual weight loss, leading to average weight loss of 6%. The control group had a gradual but consistent weight loss, for an average of 3.5% at the end of the trial.
One audience member suggested that some of the weight loss might have been due to aging. The authors said they are looking at those factors in their ongoing data analysis, which they’re planning to publish in the near future.
Meanwhile, the intervention group had significantly greater weight loss than did the control group throughout the trial. They had significantly greater improvements in fitness, hemoglobin A1c levels, systolic blood pressure, and HDL cholesterol (P less than .05 for all). The control group had significantly greater reductions in LDL cholesterol (P less than .05), although they also had a significantly greater use of medications, including insulin, statins, and antihypertensives, compared with the intervention group, the authors noted.
In the short-term (1-4 years), the intervention group saw improvements in cardiovascular risk factors, preservation of mobility, and improvements in fitness, obstructive sleep apnea, fatty liver disease, urinary incontinence, sexual function, and markers of inflammation.
The intervention also reduced the incidence of high-risk chronic kidney disease by 31%, and reduced the total costs and service utilization, especially related to hospitalization ($2,500 per participant), and medication ($2,500 per participant), the authors reported. Although the intervention didn’t affect neuropathy symptoms, it reduced the incidence of reported retinopathy by 14%, reduced the incidence of symptoms of mild or greater depression by 20%, and slowed age-related decline in reported physical function.
Program reduced burden of diabetes
"Even with no clear evidence of cardiovascular benefit, the Look AHEAD investigators have shown that attention to activity and diet can safely reduce the burden of diabetes and have reaffirmed the importance of lifestyle approach as one of the foundations of modern diabetes care," Dr. Gerstein wrote in his editorial, adding that the data show "intensive lifestyle interventions are unlikely to cause harm and may provide a modest benefit."
Interactions among subgroups – history of cardiovascular disease at baseline, and gender, race/ethnicity – showed nonsignificant interaction with cardiovascular disease history (P = .06).
Summarizing the study’s limitations, the authors said that it’s not clear whether changing the intervention groups’ dietary composition might have yielded different results. Their diet during the intervention was 1,200 to 1,800 kcal/day, with less than 30% of calories from fat, and less than 15% from protein. Authors added that the patients recruited successfully competed the fitness test at baseline, so the results can’t be generalized to all patients with type 2 diabetes.
Although the intervention has stopped, the trial continues as an observational study, said Dr. Wing. And some wonder if longer-term results would reveal new information.
"My prediction is that even after stopping the trial, as long as follow-up is carried on for several more years, you’ll see differences in total mortality," said Dr. Bennett, citing the results of UK Prospective Diabetes Study (UKPDS), which showed no difference in cardiovascular disease at 12 years, but a significant difference at 20 years. "So it does appear that intervention does have a lasting but delayed effect on that endpoint."
"I hope [the results] are not misinterpreted," Dr. Bennett continued. "There’s a danger given the lack of effect of the primary endpoint that the headlines will say weight loss is useless, and that’s a total misinterpretation in my view. There are multiple reasons to encourage weight loss. Other than the fact that it doesn’t appear to affect mortality up to this point, it seems to have benefit for all sorts of other things – people feel better, walk better, they develop advanced renal disease less frequently, their cholesterol and blood pressure are lower. I hope that message gets through rather than the negative one."
Dr. Wing, Dr. Bennett, and Dr. Gerstein had no disclosures relevant to the trial. The trial was supported by the National Institutes of Health, other Department of Health and Human Services agencies, and several companies including FedEx, Johnson & Johnson, Nestle Healthcare Nutrition, and Abbott Nutrition.
On Twitter @NaseemSMiller
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: A total of 403 patients in the intervention group and 418 patients in the control group had met the trial’s primary outcome, with no statistically significant difference between the two groups (1.83 and 1.92 events per 100 person-years, respectively).
Data source: Look AHEAD, a study that randomized 5,100 U.S. overweight or obese patients with type 2 diabetes to an intensive weight-loss and physical activity intervention or diabetes support and education.
Disclosures: Dr. Wing, Dr. Bennett, and Dr. Gerstein had no disclosures relevant to the trial.
Low-carb, high-fat diet may not be best for gestational diabetes
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the post-test.
CHICAGO – Women with gestational diabetes on a conventional low-carbohydrate, high-fat diet were more insulin resistant, and their infants had slightly higher rates of adiposity, than did with women who consumed a diet high in complex carbohydrates and low in fat, according to a randomized pilot study of 11 women.
Both diets controlled maternal glucose and weight. "So, they’re both okay, except that tissue data and fasting levels imply that higher fat content is exacerbating insulin resistance during pregnancy," Teri L. Hernandez, Ph.D., said in an interview after presenting her findings at the annual scientific sessions of the American Diabetes Association.
It’s too soon to tell if and when the findings will have implications on practice. "It’s a good study," said Dr. Assiamira Ferrara, senior research scientist at Kaiser Permanente, Oakland, Calif., who was a moderator and not involved in the study. "But we need a bigger sample size and more feasibility studies on whether women will adhere to the diet" before we try this outside of a research environment.
The conventional diet recommended to women who have gestational diabetes (GDM) has mainly focused on carbohydrate (CHO) restriction. But Dr. Hernandez and her colleagues said that the restrictions result in greater fat intake, which in turn could promote insulin resistance and increase fetal adiposity. Meanwhile, owing to patient noncompliance and a lack of controlled designs, evidence remains confounded, she reported.
In a recent unpublished systematic review of prospective, randomized, controlled trials of diet interventions in women with GDM, Dr. Hernandez and her colleagues found that women tolerated higher complex carb/low glycemic index diets and that diets higher in unrefined carbs effectively blunted postprandial glycemia, reduced the need for insulin therapy, and improved insulin sensitivity, hemoglobin A1c, and systolic blood pressure.
Dr. Hernandez of the departments of medicine and nursing at University of Colorado at Denver in Aurora, said that she has a larger number women in her ongoing study, but the findings so far "lend evidence to the idea that women can tolerate more carb than we thought.
"These women are worried about their baby’s outcome, and they’re afraid their babies are going to be born too big, so they become very fearful of carbohydrates. What this says is that they can actually have toast and other carbs in their diet and still have a great outcome, and it could even help improve their insulin resistance," she said in an interview.
Researchers randomized five women to the conventional low-carb/high-fat diet, and 6 women to the high-carb/low-fat diet.
The low-carb/high fat diet comprised 40% CHO, 45% fat, and 15% protein; the high-carb/low fat diet contained 60% CHO, 25% fat, and 15% protein. Simple sugars made up about 18% or less of total daily calories.
The subjects were rather healthy women with mild gestational diabetes, were highly compliant, and were closely matched, with a mean body mass index of 33.5 kg/m2, and were 29-30 years old. They were provided with all the meals.
During the study period, weight gain was similar in both groups, and their glycemic profiles were below target.
However, the high-carb/low-fat diet group had lower fasting glucose and fasting insulin at 6 and 7 weeks, compared with the low-carb/high-fat diet group (P = .007 and .06, respectively)
Results also showed that the postprandial free fatty acids were significantly higher in the low-carb/high-fat diet group (P = .037). And, at week 37, fasting glucose, insulin, and maternal insulin resistance (HOMA-IR) were significantly higher in the low-carb/high-fat diet, compared with the low-fat/high-carb diet (P = .007, .06, and .02, respectively).
Meanwhile, infant adiposity was slightly higher in the infants of the low-carb/high-fat groups, compared with the high-carb/low-fat group (14% v. 11%). And, regardless of diet, higher fasting insulin and HOMA-IR at 37 weeks were associated with greater infant adiposity (P less than .05).
Dr. Hernandez said that both diets are doing a good job, and thus far, there haven’t been any adverse outcomes because of either diet.
Dr. Hernandez had no disclosures. Dr. Ferrara is an employee of Takeda Global Research and Development and has received research support from Takeda Pharmaceutical.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
On Twitter @NaseemSMiller
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the post-test.
CHICAGO – Women with gestational diabetes on a conventional low-carbohydrate, high-fat diet were more insulin resistant, and their infants had slightly higher rates of adiposity, than did with women who consumed a diet high in complex carbohydrates and low in fat, according to a randomized pilot study of 11 women.
Both diets controlled maternal glucose and weight. "So, they’re both okay, except that tissue data and fasting levels imply that higher fat content is exacerbating insulin resistance during pregnancy," Teri L. Hernandez, Ph.D., said in an interview after presenting her findings at the annual scientific sessions of the American Diabetes Association.
It’s too soon to tell if and when the findings will have implications on practice. "It’s a good study," said Dr. Assiamira Ferrara, senior research scientist at Kaiser Permanente, Oakland, Calif., who was a moderator and not involved in the study. "But we need a bigger sample size and more feasibility studies on whether women will adhere to the diet" before we try this outside of a research environment.
The conventional diet recommended to women who have gestational diabetes (GDM) has mainly focused on carbohydrate (CHO) restriction. But Dr. Hernandez and her colleagues said that the restrictions result in greater fat intake, which in turn could promote insulin resistance and increase fetal adiposity. Meanwhile, owing to patient noncompliance and a lack of controlled designs, evidence remains confounded, she reported.
In a recent unpublished systematic review of prospective, randomized, controlled trials of diet interventions in women with GDM, Dr. Hernandez and her colleagues found that women tolerated higher complex carb/low glycemic index diets and that diets higher in unrefined carbs effectively blunted postprandial glycemia, reduced the need for insulin therapy, and improved insulin sensitivity, hemoglobin A1c, and systolic blood pressure.
Dr. Hernandez of the departments of medicine and nursing at University of Colorado at Denver in Aurora, said that she has a larger number women in her ongoing study, but the findings so far "lend evidence to the idea that women can tolerate more carb than we thought.
"These women are worried about their baby’s outcome, and they’re afraid their babies are going to be born too big, so they become very fearful of carbohydrates. What this says is that they can actually have toast and other carbs in their diet and still have a great outcome, and it could even help improve their insulin resistance," she said in an interview.
Researchers randomized five women to the conventional low-carb/high-fat diet, and 6 women to the high-carb/low-fat diet.
The low-carb/high fat diet comprised 40% CHO, 45% fat, and 15% protein; the high-carb/low fat diet contained 60% CHO, 25% fat, and 15% protein. Simple sugars made up about 18% or less of total daily calories.
The subjects were rather healthy women with mild gestational diabetes, were highly compliant, and were closely matched, with a mean body mass index of 33.5 kg/m2, and were 29-30 years old. They were provided with all the meals.
During the study period, weight gain was similar in both groups, and their glycemic profiles were below target.
However, the high-carb/low-fat diet group had lower fasting glucose and fasting insulin at 6 and 7 weeks, compared with the low-carb/high-fat diet group (P = .007 and .06, respectively)
Results also showed that the postprandial free fatty acids were significantly higher in the low-carb/high-fat diet group (P = .037). And, at week 37, fasting glucose, insulin, and maternal insulin resistance (HOMA-IR) were significantly higher in the low-carb/high-fat diet, compared with the low-fat/high-carb diet (P = .007, .06, and .02, respectively).
Meanwhile, infant adiposity was slightly higher in the infants of the low-carb/high-fat groups, compared with the high-carb/low-fat group (14% v. 11%). And, regardless of diet, higher fasting insulin and HOMA-IR at 37 weeks were associated with greater infant adiposity (P less than .05).
Dr. Hernandez said that both diets are doing a good job, and thus far, there haven’t been any adverse outcomes because of either diet.
Dr. Hernandez had no disclosures. Dr. Ferrara is an employee of Takeda Global Research and Development and has received research support from Takeda Pharmaceutical.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
On Twitter @NaseemSMiller
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the post-test.
CHICAGO – Women with gestational diabetes on a conventional low-carbohydrate, high-fat diet were more insulin resistant, and their infants had slightly higher rates of adiposity, than did with women who consumed a diet high in complex carbohydrates and low in fat, according to a randomized pilot study of 11 women.
Both diets controlled maternal glucose and weight. "So, they’re both okay, except that tissue data and fasting levels imply that higher fat content is exacerbating insulin resistance during pregnancy," Teri L. Hernandez, Ph.D., said in an interview after presenting her findings at the annual scientific sessions of the American Diabetes Association.
It’s too soon to tell if and when the findings will have implications on practice. "It’s a good study," said Dr. Assiamira Ferrara, senior research scientist at Kaiser Permanente, Oakland, Calif., who was a moderator and not involved in the study. "But we need a bigger sample size and more feasibility studies on whether women will adhere to the diet" before we try this outside of a research environment.
The conventional diet recommended to women who have gestational diabetes (GDM) has mainly focused on carbohydrate (CHO) restriction. But Dr. Hernandez and her colleagues said that the restrictions result in greater fat intake, which in turn could promote insulin resistance and increase fetal adiposity. Meanwhile, owing to patient noncompliance and a lack of controlled designs, evidence remains confounded, she reported.
In a recent unpublished systematic review of prospective, randomized, controlled trials of diet interventions in women with GDM, Dr. Hernandez and her colleagues found that women tolerated higher complex carb/low glycemic index diets and that diets higher in unrefined carbs effectively blunted postprandial glycemia, reduced the need for insulin therapy, and improved insulin sensitivity, hemoglobin A1c, and systolic blood pressure.
Dr. Hernandez of the departments of medicine and nursing at University of Colorado at Denver in Aurora, said that she has a larger number women in her ongoing study, but the findings so far "lend evidence to the idea that women can tolerate more carb than we thought.
"These women are worried about their baby’s outcome, and they’re afraid their babies are going to be born too big, so they become very fearful of carbohydrates. What this says is that they can actually have toast and other carbs in their diet and still have a great outcome, and it could even help improve their insulin resistance," she said in an interview.
Researchers randomized five women to the conventional low-carb/high-fat diet, and 6 women to the high-carb/low-fat diet.
The low-carb/high fat diet comprised 40% CHO, 45% fat, and 15% protein; the high-carb/low fat diet contained 60% CHO, 25% fat, and 15% protein. Simple sugars made up about 18% or less of total daily calories.
The subjects were rather healthy women with mild gestational diabetes, were highly compliant, and were closely matched, with a mean body mass index of 33.5 kg/m2, and were 29-30 years old. They were provided with all the meals.
During the study period, weight gain was similar in both groups, and their glycemic profiles were below target.
However, the high-carb/low-fat diet group had lower fasting glucose and fasting insulin at 6 and 7 weeks, compared with the low-carb/high-fat diet group (P = .007 and .06, respectively)
Results also showed that the postprandial free fatty acids were significantly higher in the low-carb/high-fat diet group (P = .037). And, at week 37, fasting glucose, insulin, and maternal insulin resistance (HOMA-IR) were significantly higher in the low-carb/high-fat diet, compared with the low-fat/high-carb diet (P = .007, .06, and .02, respectively).
Meanwhile, infant adiposity was slightly higher in the infants of the low-carb/high-fat groups, compared with the high-carb/low-fat group (14% v. 11%). And, regardless of diet, higher fasting insulin and HOMA-IR at 37 weeks were associated with greater infant adiposity (P less than .05).
Dr. Hernandez said that both diets are doing a good job, and thus far, there haven’t been any adverse outcomes because of either diet.
Dr. Hernandez had no disclosures. Dr. Ferrara is an employee of Takeda Global Research and Development and has received research support from Takeda Pharmaceutical.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
On Twitter @NaseemSMiller
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Low-carb, high-fat diet may not be best for gestational diabetes
CHICAGO – Women with gestational diabetes on a conventional low-carbohydrate, high-fat diet were more insulin resistant, and their infants had slightly higher rates of adiposity, than did with women who consumed a diet high in complex carbohydrates and low in fat, according to a randomized pilot study of 11 women.
Both diets controlled maternal glucose and weight. "So, they’re both okay, except that tissue data and fasting levels imply that higher fat content is exacerbating insulin resistance during pregnancy," Teri L. Hernandez, Ph.D., said in an interview after presenting her findings at the annual scientific sessions of the American Diabetes Association.
It’s too soon to tell if and when the findings will have implications on practice. "It’s a good study," said Dr. Assiamira Ferrara, senior research scientist at Kaiser Permanente, Oakland, Calif., who was a moderator and not involved in the study. "But we need a bigger sample size and more feasibility studies on whether women will adhere to the diet" before we try this outside of a research environment.
The conventional diet recommended to women who have gestational diabetes (GDM) has mainly focused on carbohydrate (CHO) restriction. But Dr. Hernandez and her colleagues said that the restrictions result in greater fat intake, which in turn could promote insulin resistance and increase fetal adiposity. Meanwhile, owing to patient noncompliance and a lack of controlled designs, evidence remains confounded, she reported.
In a recent unpublished systematic review of prospective, randomized, controlled trials of diet interventions in women with GDM, Dr. Hernandez and her colleagues found that women tolerated higher complex carb/low glycemic index diets and that diets higher in unrefined carbs effectively blunted postprandial glycemia, reduced the need for insulin therapy, and improved insulin sensitivity, hemoglobin A1c, and systolic blood pressure.
Dr. Hernandez of the departments of medicine and nursing at University of Colorado at Denver in Aurora, said that she has a larger number women in her ongoing study, but the findings so far "lend evidence to the idea that women can tolerate more carb than we thought.
"These women are worried about their baby’s outcome, and they’re afraid their babies are going to be born too big, so they become very fearful of carbohydrates. What this says is that they can actually have toast and other carbs in their diet and still have a great outcome, and it could even help improve their insulin resistance," she said in an interview.
Researchers randomized five women to the conventional low-carb/high-fat diet, and 6 women to the high-carb/low-fat diet.
The low-carb/high fat diet comprised 40% CHO, 45% fat, and 15% protein; the high-carb/low fat diet contained 60% CHO, 25% fat, and 15% protein. Simple sugars made up about 18% or less of total daily calories.
The subjects were rather healthy women with mild gestational diabetes, were highly compliant, and were closely matched, with a mean body mass index of 33.5 kg/m2, and were 29-30 years old. They were provided with all the meals.
During the study period, weight gain was similar in both groups, and their glycemic profiles were below target.
However, the high-carb/low-fat diet group had lower fasting glucose and fasting insulin at 6 and 7 weeks, compared with the low-carb/high-fat diet group (P = .007 and .06, respectively)
Results also showed that the postprandial free fatty acids were significantly higher in the low-carb/high-fat diet group (P = .037). And, at week 37, fasting glucose, insulin, and maternal insulin resistance (HOMA-IR) were significantly higher in the low-carb/high-fat diet, compared with the low-fat/high-carb diet (P = .007, .06, and .02, respectively).
Meanwhile, infant adiposity was slightly higher in the infants of the low-carb/high-fat groups, compared with the high-carb/low-fat group (14% v. 11%). And, regardless of diet, higher fasting insulin and HOMA-IR at 37 weeks were associated with greater infant adiposity (P less than .05).
Dr. Hernandez said that both diets are doing a good job, and thus far, there haven’t been any adverse outcomes because of either diet.
Dr. Hernandez had no disclosures. Dr. Ferrara is an employee of Takeda Global Research and Development and has received research support from Takeda Pharmaceutical.
On Twitter @NaseemSMiller
CHICAGO – Women with gestational diabetes on a conventional low-carbohydrate, high-fat diet were more insulin resistant, and their infants had slightly higher rates of adiposity, than did with women who consumed a diet high in complex carbohydrates and low in fat, according to a randomized pilot study of 11 women.
Both diets controlled maternal glucose and weight. "So, they’re both okay, except that tissue data and fasting levels imply that higher fat content is exacerbating insulin resistance during pregnancy," Teri L. Hernandez, Ph.D., said in an interview after presenting her findings at the annual scientific sessions of the American Diabetes Association.
It’s too soon to tell if and when the findings will have implications on practice. "It’s a good study," said Dr. Assiamira Ferrara, senior research scientist at Kaiser Permanente, Oakland, Calif., who was a moderator and not involved in the study. "But we need a bigger sample size and more feasibility studies on whether women will adhere to the diet" before we try this outside of a research environment.
The conventional diet recommended to women who have gestational diabetes (GDM) has mainly focused on carbohydrate (CHO) restriction. But Dr. Hernandez and her colleagues said that the restrictions result in greater fat intake, which in turn could promote insulin resistance and increase fetal adiposity. Meanwhile, owing to patient noncompliance and a lack of controlled designs, evidence remains confounded, she reported.
In a recent unpublished systematic review of prospective, randomized, controlled trials of diet interventions in women with GDM, Dr. Hernandez and her colleagues found that women tolerated higher complex carb/low glycemic index diets and that diets higher in unrefined carbs effectively blunted postprandial glycemia, reduced the need for insulin therapy, and improved insulin sensitivity, hemoglobin A1c, and systolic blood pressure.
Dr. Hernandez of the departments of medicine and nursing at University of Colorado at Denver in Aurora, said that she has a larger number women in her ongoing study, but the findings so far "lend evidence to the idea that women can tolerate more carb than we thought.
"These women are worried about their baby’s outcome, and they’re afraid their babies are going to be born too big, so they become very fearful of carbohydrates. What this says is that they can actually have toast and other carbs in their diet and still have a great outcome, and it could even help improve their insulin resistance," she said in an interview.
Researchers randomized five women to the conventional low-carb/high-fat diet, and 6 women to the high-carb/low-fat diet.
The low-carb/high fat diet comprised 40% CHO, 45% fat, and 15% protein; the high-carb/low fat diet contained 60% CHO, 25% fat, and 15% protein. Simple sugars made up about 18% or less of total daily calories.
The subjects were rather healthy women with mild gestational diabetes, were highly compliant, and were closely matched, with a mean body mass index of 33.5 kg/m2, and were 29-30 years old. They were provided with all the meals.
During the study period, weight gain was similar in both groups, and their glycemic profiles were below target.
However, the high-carb/low-fat diet group had lower fasting glucose and fasting insulin at 6 and 7 weeks, compared with the low-carb/high-fat diet group (P = .007 and .06, respectively)
Results also showed that the postprandial free fatty acids were significantly higher in the low-carb/high-fat diet group (P = .037). And, at week 37, fasting glucose, insulin, and maternal insulin resistance (HOMA-IR) were significantly higher in the low-carb/high-fat diet, compared with the low-fat/high-carb diet (P = .007, .06, and .02, respectively).
Meanwhile, infant adiposity was slightly higher in the infants of the low-carb/high-fat groups, compared with the high-carb/low-fat group (14% v. 11%). And, regardless of diet, higher fasting insulin and HOMA-IR at 37 weeks were associated with greater infant adiposity (P less than .05).
Dr. Hernandez said that both diets are doing a good job, and thus far, there haven’t been any adverse outcomes because of either diet.
Dr. Hernandez had no disclosures. Dr. Ferrara is an employee of Takeda Global Research and Development and has received research support from Takeda Pharmaceutical.
On Twitter @NaseemSMiller
CHICAGO – Women with gestational diabetes on a conventional low-carbohydrate, high-fat diet were more insulin resistant, and their infants had slightly higher rates of adiposity, than did with women who consumed a diet high in complex carbohydrates and low in fat, according to a randomized pilot study of 11 women.
Both diets controlled maternal glucose and weight. "So, they’re both okay, except that tissue data and fasting levels imply that higher fat content is exacerbating insulin resistance during pregnancy," Teri L. Hernandez, Ph.D., said in an interview after presenting her findings at the annual scientific sessions of the American Diabetes Association.
It’s too soon to tell if and when the findings will have implications on practice. "It’s a good study," said Dr. Assiamira Ferrara, senior research scientist at Kaiser Permanente, Oakland, Calif., who was a moderator and not involved in the study. "But we need a bigger sample size and more feasibility studies on whether women will adhere to the diet" before we try this outside of a research environment.
The conventional diet recommended to women who have gestational diabetes (GDM) has mainly focused on carbohydrate (CHO) restriction. But Dr. Hernandez and her colleagues said that the restrictions result in greater fat intake, which in turn could promote insulin resistance and increase fetal adiposity. Meanwhile, owing to patient noncompliance and a lack of controlled designs, evidence remains confounded, she reported.
In a recent unpublished systematic review of prospective, randomized, controlled trials of diet interventions in women with GDM, Dr. Hernandez and her colleagues found that women tolerated higher complex carb/low glycemic index diets and that diets higher in unrefined carbs effectively blunted postprandial glycemia, reduced the need for insulin therapy, and improved insulin sensitivity, hemoglobin A1c, and systolic blood pressure.
Dr. Hernandez of the departments of medicine and nursing at University of Colorado at Denver in Aurora, said that she has a larger number women in her ongoing study, but the findings so far "lend evidence to the idea that women can tolerate more carb than we thought.
"These women are worried about their baby’s outcome, and they’re afraid their babies are going to be born too big, so they become very fearful of carbohydrates. What this says is that they can actually have toast and other carbs in their diet and still have a great outcome, and it could even help improve their insulin resistance," she said in an interview.
Researchers randomized five women to the conventional low-carb/high-fat diet, and 6 women to the high-carb/low-fat diet.
The low-carb/high fat diet comprised 40% CHO, 45% fat, and 15% protein; the high-carb/low fat diet contained 60% CHO, 25% fat, and 15% protein. Simple sugars made up about 18% or less of total daily calories.
The subjects were rather healthy women with mild gestational diabetes, were highly compliant, and were closely matched, with a mean body mass index of 33.5 kg/m2, and were 29-30 years old. They were provided with all the meals.
During the study period, weight gain was similar in both groups, and their glycemic profiles were below target.
However, the high-carb/low-fat diet group had lower fasting glucose and fasting insulin at 6 and 7 weeks, compared with the low-carb/high-fat diet group (P = .007 and .06, respectively)
Results also showed that the postprandial free fatty acids were significantly higher in the low-carb/high-fat diet group (P = .037). And, at week 37, fasting glucose, insulin, and maternal insulin resistance (HOMA-IR) were significantly higher in the low-carb/high-fat diet, compared with the low-fat/high-carb diet (P = .007, .06, and .02, respectively).
Meanwhile, infant adiposity was slightly higher in the infants of the low-carb/high-fat groups, compared with the high-carb/low-fat group (14% v. 11%). And, regardless of diet, higher fasting insulin and HOMA-IR at 37 weeks were associated with greater infant adiposity (P less than .05).
Dr. Hernandez said that both diets are doing a good job, and thus far, there haven’t been any adverse outcomes because of either diet.
Dr. Hernandez had no disclosures. Dr. Ferrara is an employee of Takeda Global Research and Development and has received research support from Takeda Pharmaceutical.
On Twitter @NaseemSMiller
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: At week 37, fasting glucose, insulin, and maternal insulin resistance (HOMA-IR) were significantly higher in the low carb/high fat diet, compared with the low fat/high carb diet.
Data source: Randomized pilot study of 11 women with mild gestational diabetes.
Disclosures: Dr. Hernandez had no disclosures. Dr. Ferrara is an employee of Takeda Global Research and Development and has received research support from Takeda Pharmaceutical.
Risk score can predict type 1 diabetes
CHIGAGO – In their ongoing quest to refine and identify methods for earlier intervention and prevention of type 1 diabetes, researchers at the University of Miami have developed a risk score, which includes the individual's age, and so far has shown to be highly predictive of symptomatic type 1 diabetes diagnosis. The risk score, or a modification of it, "could potentially provide an additional means for diagnosing type 1 diabetes," said Dr. Jay M. Sosenko, professor of medicine and epidemiology at the University of Miami.
The risk score is based on variables that are most predictive of who would develop symptomatic type 1 diabetes, including body mass index, age, fasting C-peptide levels, a measure of overall C-peptide production, and a measure of overall glucose. So far, the study has shown individuals whose risk scores passed a certain threshold were highly likely to develop symptomatic type 1 diabetes within roughly 2 years.
Dr. Sosenko analyzed the Diabetes Prevention Trial of Type 1 Diabetes (DPT1) database to identify the risk scores. He then applied the DPT1 Risk Score, or DPTRS, to data from the large, multi-center study TrialNet Natural History Study, which is also known as the Pathway to Prevention Study. He spoke about his findings during the annual American Diabetes Association Meeting.
"The DPTRS can identify even those who have normal glucose tolerance but who are nonetheless at risk, in part because it takes age into account," he said in a news release. "Dysglycemia, which is currently used to identify high risk individuals, is based upon the glucose thresholds of adults and these thresholds might not be appropriate for children," said Dr. Sosenko.
In this video, Dr. Sosenko speaks about the clinical applicability of DPTRS. The ongoing developments in the field could ultimately lead to universal childhood screening and further on, immunization of children "to prevent the ongoing process associated with type 1 diabetes from taking hold," said Dr. Richard A. Insel, chief scientific officer for the Juvenile Diabetes Research Foundation.
But in the meantime, "everybody should be aware of the symptoms of diabetes to prevent the onset of ketoacidosis," said Dr. Insel. "The rate of diabetic ketoacidosis hasn't changed over the last 20 years, and many of these individuals are being seen in the health care system and not being diagnosed. So everybody from teachers to parents to health care providers should be aware of symptoms of diabetes so we can prevent ketoacidosis, especially in children."
Dr. Sosenko had no disclosures. TrialNet is sponsored by the National Institute of Diabetes and Digestive and Kidney Disease, and receives support from the ADA and the JDRF.
CHIGAGO – In their ongoing quest to refine and identify methods for earlier intervention and prevention of type 1 diabetes, researchers at the University of Miami have developed a risk score, which includes the individual's age, and so far has shown to be highly predictive of symptomatic type 1 diabetes diagnosis. The risk score, or a modification of it, "could potentially provide an additional means for diagnosing type 1 diabetes," said Dr. Jay M. Sosenko, professor of medicine and epidemiology at the University of Miami.
The risk score is based on variables that are most predictive of who would develop symptomatic type 1 diabetes, including body mass index, age, fasting C-peptide levels, a measure of overall C-peptide production, and a measure of overall glucose. So far, the study has shown individuals whose risk scores passed a certain threshold were highly likely to develop symptomatic type 1 diabetes within roughly 2 years.
Dr. Sosenko analyzed the Diabetes Prevention Trial of Type 1 Diabetes (DPT1) database to identify the risk scores. He then applied the DPT1 Risk Score, or DPTRS, to data from the large, multi-center study TrialNet Natural History Study, which is also known as the Pathway to Prevention Study. He spoke about his findings during the annual American Diabetes Association Meeting.
"The DPTRS can identify even those who have normal glucose tolerance but who are nonetheless at risk, in part because it takes age into account," he said in a news release. "Dysglycemia, which is currently used to identify high risk individuals, is based upon the glucose thresholds of adults and these thresholds might not be appropriate for children," said Dr. Sosenko.
In this video, Dr. Sosenko speaks about the clinical applicability of DPTRS. The ongoing developments in the field could ultimately lead to universal childhood screening and further on, immunization of children "to prevent the ongoing process associated with type 1 diabetes from taking hold," said Dr. Richard A. Insel, chief scientific officer for the Juvenile Diabetes Research Foundation.
But in the meantime, "everybody should be aware of the symptoms of diabetes to prevent the onset of ketoacidosis," said Dr. Insel. "The rate of diabetic ketoacidosis hasn't changed over the last 20 years, and many of these individuals are being seen in the health care system and not being diagnosed. So everybody from teachers to parents to health care providers should be aware of symptoms of diabetes so we can prevent ketoacidosis, especially in children."
Dr. Sosenko had no disclosures. TrialNet is sponsored by the National Institute of Diabetes and Digestive and Kidney Disease, and receives support from the ADA and the JDRF.
CHIGAGO – In their ongoing quest to refine and identify methods for earlier intervention and prevention of type 1 diabetes, researchers at the University of Miami have developed a risk score, which includes the individual's age, and so far has shown to be highly predictive of symptomatic type 1 diabetes diagnosis. The risk score, or a modification of it, "could potentially provide an additional means for diagnosing type 1 diabetes," said Dr. Jay M. Sosenko, professor of medicine and epidemiology at the University of Miami.
The risk score is based on variables that are most predictive of who would develop symptomatic type 1 diabetes, including body mass index, age, fasting C-peptide levels, a measure of overall C-peptide production, and a measure of overall glucose. So far, the study has shown individuals whose risk scores passed a certain threshold were highly likely to develop symptomatic type 1 diabetes within roughly 2 years.
Dr. Sosenko analyzed the Diabetes Prevention Trial of Type 1 Diabetes (DPT1) database to identify the risk scores. He then applied the DPT1 Risk Score, or DPTRS, to data from the large, multi-center study TrialNet Natural History Study, which is also known as the Pathway to Prevention Study. He spoke about his findings during the annual American Diabetes Association Meeting.
"The DPTRS can identify even those who have normal glucose tolerance but who are nonetheless at risk, in part because it takes age into account," he said in a news release. "Dysglycemia, which is currently used to identify high risk individuals, is based upon the glucose thresholds of adults and these thresholds might not be appropriate for children," said Dr. Sosenko.
In this video, Dr. Sosenko speaks about the clinical applicability of DPTRS. The ongoing developments in the field could ultimately lead to universal childhood screening and further on, immunization of children "to prevent the ongoing process associated with type 1 diabetes from taking hold," said Dr. Richard A. Insel, chief scientific officer for the Juvenile Diabetes Research Foundation.
But in the meantime, "everybody should be aware of the symptoms of diabetes to prevent the onset of ketoacidosis," said Dr. Insel. "The rate of diabetic ketoacidosis hasn't changed over the last 20 years, and many of these individuals are being seen in the health care system and not being diagnosed. So everybody from teachers to parents to health care providers should be aware of symptoms of diabetes so we can prevent ketoacidosis, especially in children."
Dr. Sosenko had no disclosures. TrialNet is sponsored by the National Institute of Diabetes and Digestive and Kidney Disease, and receives support from the ADA and the JDRF.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS